Note: Descriptions are shown in the official language in which they were submitted.
CA 02337240 2001-O1-12
WO 00/03637 PCT/CA99/00652
1
DISTURBANCE-FREE ELECTROMYOGRAPHIC PROBE
BACKGROUND OF THE INVENTION
1. Field of the invention:
The present invention relates to an apparatus for reducing
disturbances induced in a signal measurement or recording, in particular,
but not exclusively, by movement of the electrodes or changes in the
pressure applied to the electrodes.
2. Brief description of the prior art:
Oesophageal recording of diaphragm electromyogram
(EMG) has traditionally been problematic due to the low amplitude of the
EMG signal relative to the artifactual disturbances such as, in particular,
the so-called electrode motion artifacts. At high gain settings, large
electrode motion artifacts lead to saturation of the output of the
preamplifier, thereby causing a temporary loss of the EMG signal. This
problem of the prior art makes EMG recording very difficult during
dynamic manoeuvres, such as for example rapid shallow breathing or
. panting.
CA 02337240 2001-O1-12
WO 00/03637 PCT/CA99/00652
2
OBJECTS AND SUMMARY OF THE INVENTION
A first object of the present invention is to provide a
technology capable of reducing disturbances induced in a measurement
or recording by:
- movements of detecting electrodes;
- changes in the pressure applied to these electrodes; or
- other mechanical influence on the electrodes, generally
referred to as motion artifacts.
Another object of the present invention is to reduce the
amplitude of motion artifacts relative to the amplitude of the EMG signal
to thereby reduce the possibility for saturation of the preamplifier.
A third object of the present invention is to overcome the
problems of the prior art related to low signal-to-artifact ratio.
A further object of the present invention is to improve bipolar
electrode measurements of diaphragm electromyogram (EMG).
In a preferred embodiment of the invention, there is
provided a measurement apparatus for detecting an electrical signal
produced by a muscle while reducing signal disturbances caused by
motion artifacts, the measurement apparatus comprises:
a) a probe;
b) at least one electrode mounted on said probe; and
c) a disturbance reducing interface attached to said probe
CA 02337240 2001-O1-12
WO 00/03637 PCT/CA99/00652
3
and covering said at least one electrode, the interface being
ion permeable and segregating said at least one electrode
from the muscle.
The objects, advantages and other features of the present
invention will become more apparent upon reading of the following non
restrictive description of preferred embodiments thereof, given by way of
example only with reference to the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
In the appended drawings:
Figure 1 is a schematic representation of a set-up of an
EMG analysis system;
Figure 2 is a side elevation view of the free end section of
an oesophageal catheter on which an array of electrodes of the EMG
analysis system of Figure 1 is mounted;
Figure 3 is a longitudinal, partial cross sectional view of the
free end section of the oesophageal catheter of Figure 2, showing an
individual matrix of permeable material applied to each separate
electrode of the array;
Figure 4 is a longitudinal, partial cross sectional view of the
free end section of the oesophageal catheter of Figure 2, showing a
CA 02337240 2001-O1-12
WO 00/03637 PCT/CA99/00652
4
continuous matrix of permeable material applied to and spanning the
entire electrode array;
Figure 5 is a partial perspective view of the free end section
of an oesophageal catheter, showing an array of semicircular electrodes
and a continuous matrix of permeable material applied to and spanning
the entire array of semicircular electrodes;
Figure 6 is a partial perspective view of the free end section
of an oesophageal catheter, showing an array of button electrodes which
can be circular, square, rectangular, or of any other shape, and a
continuous matrix of permeable material applied to and spanning the
entire array of button electrodes;
Figure 7 is a longitudinal, partial cross sectional view of the
free end section of an oesophageal catheter, showing an electrode
embedded in the material of the catheter, and a matrix of permeable
material applied to the embedded electrode;
Figure 8 is a longitudinal, partial cross sectional view of the
free end section of an oesophageal catheter, showing a stud electrode
and a matrix of permeable material applied to the stud electrode;
Figure 9 is a partial perspective view of the free end section
of the oesophageal catheter of Figure 7, showing an array of electrodes
such as shown in Figure 7, embedded into the material of the catheter;
Figure 10 is a partial perspective view of the free end
CA 02337240 2001-O1-12
WO 00/03637 PCT/CA99/00652
section of an oesophageal catheter, showing an array of button
electrodes covered by a matrix of permeable material applied to the outer
surface of the oesophageal catheter;
5 Figure 11 is a partial perspective view of the free end
section of an oesophageal catheter, showing an array of button
electrodes as well as an array of grounding electrodes; and
Figure 12 is an end cross sectiorial view of the array of
button electrodes of Figure 10 covered by the matrix applied to the outer
surface of the oesophageal catheter.
DETAILED DESCRIPTION OF THE PREFERRED
EMBODIMENTS
The present invention relates to a technology capable of
reducing disturbances induced in an electrical signal measurement and/or
recording by movement of detecting electrodes or changes in the
pressure applied to these electrodes. The electrodes are conductive
elements used to detect electrical activity. The range of applications of
the present invention includes electrical signal measurement and/or
recording wherein electrodes are immersed in an eleclectrolyte (so-called
wet electrodes). A typical example is the measurement and/or recording
of diaphragm electromyogram (EMG), oesophageal peristalsis, or ECG
with electrodes positioned on a catheter which in turn is introduced in the
oesophagus.
CA 02337240 2001-O1-12
WO 00/03637 PCT/CA99/00652
6
Although the preferred embodiments will be described
hereinafter with reference to oesophageal catheters and an application
to the measurement of diaphragm electromyogram (EMG), it should be
kept in mind that it is within the scope of the present invention to envisage
other applications for this technology using other types of catheters or
probes.
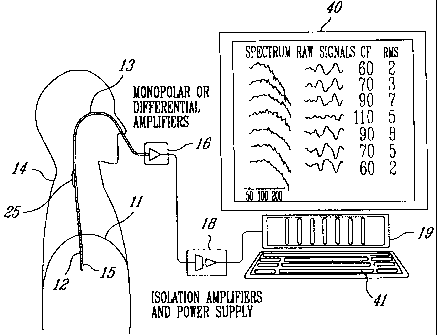
Referring to Figures 1 and 2, to measure EMG activity of the
diaphragm 11 of a human patient 14, an array of electrodes such as 12
are mounted on the free end section 15 of an oesophageal catheter 13,
with an inter-electrode distance d (Figure 2). The distance d is adjusted
in relation to body size; distance d will be larger for an adult than for an
infant. The catheter 13 is a hollow tube having a diameter related to body
size; the diameter will be smaller for infants than for adults. The catheter
diameter, electrode size as well as the inter-electrode distance d may
also vary in relation to the purpose of the catheter use.
As shown in Figure 1, the catheter 13 is introduced into the
patient's oesophagus through one nostril or the mouth until the array of
electrodes 12 is situated at the level of the gastro-oesophageal junction.
Of course, positioning of the electrode array comprising a series of
differentially and axially arranged electrode pairs (for example electrode
pairs 1-7 of Figure 2) is guided by the electrocardiographic (ECG)
recordings and the diaphragm EMG. Alternatively, the electrodes 12 are
monopolar electrodes differentiated in a computer, for example computer
19 of Figure 1. When required, ground is obtained through a separate
grounding electrode structure 25 (Figure 1).
CA 02337240 2001-O1-12
WO 00/03637 PCT/CA99/00652
7
Positioning of an electrode at the oesophageal hiatus
(where the oesophagus passes through the diaphragm is guided by visual
inspection and/or computer algorithms studying the intensity, shape and
polarity of ECG and diaphragm EMG signals. When the electrode is
close to the oesophageal hiatus, i.e. next to the heart, ECG signal
amplitude is high. If the electrode array is positioned close to the mouth
(away from the heart), ECG signals present lower amplitudes at the
proximate electrodes, and higher amplitudes at the distal electrodes. If
the electrode array is positioned too far in the stomach, ECG has a high
amplitude at the proximate electrodes of the array and a low amplitude at
the distal electrodes. If the electrode array spans the region of the heart,
ECG signals will show a time shift along the electrode array. If the
electrodes are positioned away from the heart, ECG signals show no time
lag. Diaphragm EMG signals obtained through electrode pairs located
above and below the diaphragm have opposite polarities {with no time
shift). EMG signals obtained on the same side of the diaphragm show
the same polarity {and no time shift). The characteristics described in this
paragraph will help the operator to adequately position the array of
electrodes.
According to a preferred embodiment, an electrode 12 is
mounted on the free end section 15 of the catheter 13 by winding
stainless steel wire (not shown) around catheter 13. The wound stainless
steel wire presents a rough surface smoothed out by solder, which in tum
is electroplated with nickel, copper and then gold or silver. Use of other
metallic elements such as semicylindrical electrodes 21 (Figure 5), button
electrodes 22 and 23 (Figure 6), etc., could be contemplated. The button
electrodes can be arranged into a longitudinal linear array {electrodes
CA 02337240 2001-O1-12
WO 00/03637 PCT/CA99/00652
8
22), or at least one button electrode (see 23) can be angufarly offset from
the electrodes 22 about the longitudinal axis of the catheter section 15.
For larger diameter feeding tubes or catheters, electrodes
such as electrode 26 in Figure 7 can be embedded into the material 27
of the feeding tube or catheter 28. Figure 9 shows a longitudinal array of
electrodes 26 embedded into the material 27 of the free end section of
the oesophageal catheter 28. figure 9 also shows the electric wires such
as 30, embedded in the material 27 of the catheter 28, and individually
connecting each electrode 26 to the amplifiers 16 of Figure 1. In the
example of Figures 7 and 9, the electrodes 26 are oval. The electric
wires such as 30 in Figure 9 individually connect each electrode such as
26 with a respective input of the monopolar or differential (depending on
the monopolar or differential arrangement of the electrodes 12 or 26)
amplifiers 16 (Figure 1 ). Obviously, these electric wires 30 follow the
catheter such as 28 from the respective electrodes such as 26 to the
corresponding amplifiers 16; the electric wires 30 can be embedded in the
material such as 27 of the catheter such as 28 or passed separately
outside (see for example 45 in Figure 10) or inside (see for example 46
in Figure 10) the catheter lumen 47 depending on the intended
application. The electric wires such as 30 transmitting the EMG signals
collected by the various electrodes such as 26 are necessarily electrically
insulated from each other and preferably surrounded by a conductive
mesh constituting a shield against external disturbances.
Referring now to Figure 8, a stud electrode 31 is illustrated.
Each stud electrode 31 is mounted in a hole 32 made through the wall of
an oesophageal catheter 33.
CA 02337240 2001-O1-12
WO 00/03637 PCT/CA99/00652
9
The electrodes such as 34 in Figure 10 can also be applied
by means of glue or any other suitable adhesive material or compound,
including double adhesive tape.
In the example of Figures 10 and 12, a linear array of oval
electrodes 34 is mounted on the outer surface 44 of a catheter 36
comprising two longitudinal lumens 47 and 48. Referring to Figure 12,
each electrode 34 is applied to the catheter surface 44. As described in
the foregoing description, the electric wires (see 45 and 46) for
individually connecting the electrodes 34 to the amplifiers 16 will extend
either inside lumen 47 (see 46 in Figure 10), inside lumen 48, outside the
catheter 36 (see 45 in Figure 10), or embedded in the material of the
catheter 36.
Figure 11 is a partial perspective view the free end section
of an oesophageal catheter 37, comprising a longitudinal, linear array of
button electrodes 38. Figure 11 also shows an example of grounding
electrode structure (see 25 in Figure 1). In the example of Figure 11, the
grounding electrode structure comprises a helical array of grounding
electrodes 39 mounted on the outer surface 40 of the catheter 37. Of
course, the array of grounding electrodes 39 is centered on the
longitudinal axis of the catheter 37 and presents the general configuration
of a cylindrical helix.
Pressure sensors, pH sensors, thermistors and other
detector devices can be added onto the catheter in accordance with the
requirements of the intended application.
CA 02337240 2001-O1-12
WO 00/03637 PCT/CA99/00652
Referring back to Figure 1, the group of amplifiers 16
amplifies and band-pass filters each EMG signal. The amplified EMG
signals are sampled by a personal computer 19 through respective
isolation amplifiers of a unit 18, to form signal segments of fixed duration.
5 Unit 18 supplies electric power to the various electronic components of
the amplifiers 16 and isolation amplifiers while ensuring adequate
isolation of the patient's body from such power supply. The unit 18 also
incorporates bandpass filters included in the respective EMG signal
channels to eliminate the effects of aliasing. The successive EMG signal
10 segments are then digitaAy processed into the personal computer 19 after
analog-to-digital conversion thereof. This analog-to-digital conversion is
conveniently carried out by an analog-to-digital converter implemented in
the personal computer 19. The personal computer 19 includes a monitor
40 and a keyboard 41.
It is believed to be within the capacity of those of ordinary
skill in the art to construct suitable amplifiers 16 and an adequate isolation
amplifiers and power supply unit 18. Accordingly, the amplifiers 16 and
the unit 18 will not be further described in the present specification.
To eliminate the problems related to motion of the electrode,
changes in the pressure applied to the electrode, andlor intermittent
contact with surrounding tissue, a motion artifact reducing interface is
applied to the electrode surface. The problems listed above can grouped
as disturbances; the motion artifact reducing interface may therefore also
be referred to as a disturbance reducing interface. The motion artifact
reducing interface advantageously consists of a matrix of permeable
material comprising, for example, a mesh, foam or other porous material,
CA 02337240 2001-O1-12
WO 00/03637 PCT/CA99/00652
11
e.g. a fine filament matrix of nylon. The principle of operation is that the
matrix of permeable material creates an interface that hosts ions and
electrodes and prevents direct contact between the metal surface of the
electrode and the surrounding body tissue. The type of permeable
material and thickness thereof is not crucial for performance as long as
it forms an ion saturated interface producing no direct contact between
the electrode and body tissue. However, excessive thickness may cause
increased distance between the electrode and muscle, which will weaken
the signal strength and lower the frequency content of this signal.
As illustrated in Figures 3-8 and 10, the matrix of permeable
material is applied to the exposed surface of the electrodes where the ion
concentration gradients are largest to reduce mechanically-caused
movements of ions.
The matrix can be formed by separate single matrices 17
{Figure 3), 29 {Figure 7) or 42 (Figure 8) individually applied to or
integrated in the exposed surface of each electrode 12 (Figure 3), 26
(Figure 7) or 31 (Figure 8). For example, each individual matrix 17, 29 or
42 can be glued on, or adhere to by other means, the outer surface of the
catheter to cover the associated electrode. However no adhesive material
may cover the electrode surface.
The matrix can also take the form of a continuous matrix 20
(Figures 4, 5 and 6) or 35 (Figure 10 and 12). For example, the
continuous matrix may form a tube that can be pulled over the catheter
to cover the entire span of the array of electrodes 12 (Figure 4), 21
(Figure 5), 22 and 23 (Figure 6), and 34 (Figures 10 and 12). In the case
CA 02337240 2001-O1-12
WO 00/03637 PCT/CA99/00652
12
of a continuous matrix spanning the entire electrode array, the
conductivity of the material constituting the matrix, when dry, has to
present a conductivity lower than the conductivity of the metal forming the
electrodes, whereby electrical conduction is carried out across the matrix,
i.e., through the electrolyte. These matrices provide a much more stable
voltage with a reduction of the so-called electrode motion induced
artifacts on the diaphragm EMG signal.
Also, the matrix can either cover the entire circumference
of the catheter (see matrices 20 and 17 of Figures 3-6) or a portion of the
circumference of the catheter (see matrices 29, 42 and 35 of Figures 7,
8, 10 and 12). Again these matrices can be adhered to the outer surface
of the catheter to cover the electrodes; no adhesive material may cover
the electrode surface.
Other alternatives (not shown) are ( a) to wind or wrap the
matrix around the catheter and the electrodes, and ( b) to host or embed
the electrodes into the matrix.
The electrode structure according to the invention can be
applied to measurement of the diaphragm electromyogram (EMG)
exclusively or in combination with a device for providing
feedinglmedication/liquid supply to the patient, and emptying of gastric
liquids, common to the treatment of patients in need of ventilatory
support. The electrode structure is usable to provide diaphragm EMG
signals from a plurality of conductive elements which in turn can be used
to:
CA 02337240 2001-O1-12
WO 00/03637 PCT/CA99/00652
13
- monitor diaphragm EMG (frequency, amplitude or power);
- trigger and control gas flow, gas volume or gas pressure
delivered by a mechanical lung ventilator; and
- control a closed loop ventilator system that will
automatically adjust the level of inspiratory support in
proportion to changes in the neuro-ventilatory efficiency
such that the neural drive remains stable at a desired target
level.
The closed loop ventilator system control can further use the
intensity of the diaphragm electromyogram (EMG) obtained immediately
before inspiratory flow occurs to quantify pre-inspiratory breathing efforts.
The catheter including the array of electrodes is aimed to be
disposed of after a single use; however, when desired, conventional
sterilization techniques can be applied in view of re-using the catheter.
The catheter can stay in the same patient for extensive periods of time;
it is therefore important that the electrodes and matrix be made out of a
non-allergen material.
Retrocardiac recording of electrocardiogram and
oesophageal peristalsis are other possible applications.
The electrode structure according to the invention is
applicable in all patients on ventilatory support and will enhance the
possibility of obtaining spontaneous breathing and of optimizing patient
ventilator interaction. There exists also a utility for this electrode
structure
during anaesthesia for monitoring vital fonctions of the patient. The
CA 02337240 2001-O1-12
WO 00/03637 PCT/CA99/00652
14
electrode structure can be used in connection with all kinds of ventilator
systems in intensive care unit settings or other wards where assisted
ventilation is required.
Although the present invention has been described
hereinabove with reference to preferred embodiments thereof, these
embodiments can be modified at will, within the scope of the appended
claims, without departing from the spirit and nature of the subject
invention.