Note: Descriptions are shown in the official language in which they were submitted.
CA 02337256 2001-O1-12
a
' ~- " WO 00/03707 PCT/FR99/01715
ISOFLAVONOID-BASED THERAPEUTIC COMPOSITION INTENDED TO
j BE USED TN THE TREATMENT OF TUMOURS WITH CYTOTOXIC
AGENTS
The present invention relates to the use of
compounds of the isoflavonoid type in the treatment of
cancers with cytotoxic agents.
A cancer is a disorder of the somatic genes in
which genetic dysfunctions become amplified as the
tumour process progresses from the state of a
precancerous lesion to that of a malignant
transformation, the cancerous tumour becoming
metastatic and often resistant to cytotoxic
medicaments.
In spite of major efforts made in all developed
countries, in particular through experimental and
clinical research programmes, mortality due to the
various cancers (solid tumours and haematological
neoplasias) remains unacceptably high. In many
countries, the mortality caused by cancer is ranked
second, just after cardiovascular diseases.
In terms of newly diagnosed cancers, the
distribution between solid tumours and haematological
neoplasias (bone marrow, blood, lymphatic system) shows
that 9 cancers out of 10 are solid tumours. Contrary to
what is observed in haematological oncology
(therapeutic success in 40 to 900 of the cancers of the
blood cells), only a small number of advanced or
disseminated solid tumours respond to chemotherapy
treatments alone. It is partly for this reason that the
overall mortality caused by cancer increased in the USA
between 1973 and 1992.
It is unfortunately not certain that this trend
can be reversed solely by the appearance, besides the
established chemotherapy arsenal, of novel antitumour
medicaments such as taxanes (paclitaxel and docetaxel)
which interfere with the formation of the microtubules
(W.P. McGuire et al., Am. Intern. Med., 1989), the
inhibitors of topoisomerases I derived from
camptothecin (topotecan and irinotecan), vinorelbine
CA 02337256 2001-O1-12
_ 2 _
(novel alkaloid derived from periwinkle), gemcitabine
(novel cytotoxic antimetabolic agent), raltitrexed
(inhibitor of thymidylate synthetase) and miltefosine
(first representative of the alkylphosphocholine
family). These treatments are in addition, either as a
first line treatment, or as a second line treatment, to
the medicaments whose specific activity is now well
recognized such as doxorubicin, cisplatin, vincristine,
methotrexate, 5-fluorouracil.
One of the most difficult current problems of
anticancer chemotherapy is due to the fact that many
populations of malignant cells exhibit substantial
resistance to the established cytotoxic substances.
Most often, this situation results from the existence
of multiresistance genes or from the frequency of
genetic mutations in certain types of tumours. Thus,
the treatment of cancers requires novel approaches,
complementary to those currently used, and intended for
better combating the extension and heterogeneity of the
tumour load and the acquisition of "multi-cytotoxic
drug" resistance.
Among these novel approaches, some are already
promising. That is the case for the induction of
apoptosis, the inhibition of tumour angiogenesis and of
metastatic processes, not to mention gene therapy or
immunotherapy.
The inventors were interested in a different
approach. The objective sought was to make the
population of tumour cells more sensitive to the
reference anticancer treatments in order to achieve a
double beneficial effect:
1) to increase the cytotoxic activity and
therefore the efficacy, and
2) to reduce the frequency and the severity of
certain side effects by virtue of the reduction of the
dosage which might follow the induction of the increase
in the antitumour efficacy.
It is this strategy which is at the origin of
the discovery of an innovative mechanism caused by
CA 02337256 2001-O1-12
_ 3 _
substances having a low antitumour power or even
lacking this power, but capable of inducing a very
significant increase in the cytotoxic activity of
proven anticancer medicaments. This innovative
mechanism results from the possibility for these
substances either to stimulate the recruitment of
clonogenic cells inside the tumour, making it more
sensitive to conventional treatment with cytotoxic
agents, or to inhibit the proliferation of clonogenic
cells, thus contributing to the regression of the
tumour.
The subject of the present invention is thus
the use, in the treatment of cancers with at least one
antitumour agent chosen from cytotoxic agents, of a
compound having an activity on the proliferation of
clonogenic cells, chosen from isoflavonoids and
', analogous compounds of the chromone type and in
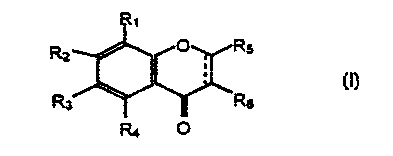
' particular the compounds of formula:
R,
Rx R~
~~)
R3 Rs
in which formula:
- R1, R2, R3 and R4 are chosen, independently of each
other, from H, OH, a C1-C4 alkoxy group, an -OCOR7
group, R7 being a C1-CQ alkyl group, at least one of the
substituents Rl, R2, R3 or R4 being other than H and it
being possible for R2 and Rj to form together a
methylenedioxy group,
- RS is chosen from H, OH, a C1-C4 alkoxy group, an
O-glycosyl group and a cyclohexyl group,
- R6 is chosen from a cyclohexyl group, a phenyl group
and a phenyl group substituted 1 to 3 times with groups
chosen from H, OH and a C1-CQ alkoxy group,
CA 02337256 2001-O1-12
' - 4 _
- and ____.__, denotes either a double bond, or a single
bond.
A preferred class of compounds of formula I are
those in which R6 is chosen from the phenyl group, the
4-hydroxyphenyl group and the 4-(C1-C4alkoxy)phenyl
groups.
.:i
The cytotoxic agents may be used at their usual
dose and, in this case, their efficacy is enhanced, or
at lower doses taking into account the increase in
their antitumour efficacy if the desired objective is
first to enhance the patient's tolerance to the
treatment.
The subject of the present invention is also a
composition having an activity on the proliferation of
clonogenic cells by interfering with the generation of
clonogenic cells, either by stimulating the
proliferation and recruitment, or by inhibiting the
proliferation, comprising a therapeutically effective
quantity of an isoflavonoid or of an analogous compound
of the chromone type, and in particular of a compound
chosen from the compounds of formula:
R2 Rs
~E)
R3 Rs
in which formula:
- R1, R2, R3 and R4 are chosen, independently of each
other, from H, OH, a C1-C4 alkoxy group, an -OCOR7
group, R7 being a C1-C4 alkyl group, at least one of the
subs tituents Rl, R2, R3 or RQ being other than H and it
being possible for R2 and R3 to form together a
methylenedioxy group,
R~
CA 02337256 2001-O1-12
- 5 -
- R5 is chosen from H, OH, a C1-C4 alkoxy group, an
O-glycosyl group, and a cyclohexyl group,
', - R6 is chosen from a cyclohexyl group, a phenyl group
and a phenyl group substituted 1 to 3 times with groups
chosen from H, OH and a C1-C4 alkoxy group,
- and .___._._ denotes either a double bond, or a single
bond.
The subject of the present invention is also
the use of an isoflavonoid, in particular of a compound
of formula I as defined above, for the manufacture of a
medicament intended to interfere (by induction or
inhibition) with the generation of clonogenic cells in
tumours during a treatment with at least one cytotoxic
agent.
In the chemotherapeutic treatment of cancers
with cytotoxic agents, the isoflavonoids and in
particular the compounds of formula I may be
administered at the beginning of the chemotherapy
treatments either once, or over several days at the
beginning of these treatments (for example for 5 to
7 days) and, depending on the chemotherapy protocol, at
the beginning of each treatment cycle (for example for
2 to 5 days) during each cure. -
The isoflavonoids and in particular the
compounds of formula I are advantageously administered
by infusion (generally over 1 to 3 hours) at doses of 5
to 50 mg/kg/day or 200 to 2000 mg/m2/day.
In order to obtain a maximum effect on the
production of clonogenic cells, the isoflavonoids
should be administered such that the tissue
concentrations obtained are the highest which can be
possibly envisaged.
For the treatment protocols in the acute phases
of the cures, the intravenous route is to be preferred
using:
- ready-to-use infusion solutions (bags, vials
and the like) intended to be administered as they are
by intravenous infusion with the aid of an infusion
line and using the recommended flow rate:
CA 02337256 2001-O1-12
_ 6 _
- lyophilizates to be resuspended in solution
for intravenous infusion with the aid of pharmaceutical
solutions known to persons skilled in the art;
- for the maintenance treatments, it is also
possible to envisage the oral route when the
chemotherapy treatment preferably uses the
administration of cytostatic agents by the oral route.
For this purpose, oral lyophilizates (for oral or
perlingual absorption), instant or delayed release
tablets, oral solutions, suspensions, granules,
gelatine capsules and the like may be used.
The compounds of formula (I) are, for the
majority, compounds of natural origin or are
derivatives of compounds of natural origin. As
examples, there may be mentioned:
- genistein,
- biochanin A,
- daidzein,
- formononetin,
- 7-acetylformononetin,
- glycetein,
- orobol or 5,7,3',4'-tetrahydroxyisoflavone,
- irizolone or 6,7-methylenedioxy-
4'-hydroxyisoflavone,
- irigenin or 3',5,7-trihydroxy-
4',5',6-methoxyisoflavone,
- tectorigenin or 4',5,7-trihydroxy-
6-methoxyisoflavone,
- 2-hydroxy-8-methoxy-2,3-dihydroisoflavone,
- 4',7-dihydroxy-5-methoxyisoflavone.
Other isoflavones which can be used are
described by Donnelly et al. in Natural Product
Reports, 1995, 321, or can be prepared by the methods
described in this article.
The cytotoxic agents may be chosen from:
i) intercalating agents, in particular doxorubicin
(adriamycin), daunorubicin, epirubicin,
idarubicin, zorubicin, aclarubicin,
CA 02337256 2001-O1-12
7 _
j
pirarubicin, acridine, mitoxanthrone,
actinomycin D, eptilinium acetate;
ii) alkylating agents chosen from platinum
derivatives
(cisplatin, carboplatin,
oxaliplatin and the like),
iii) a compound chosen from the other groups of
i
alkylating agents:
- cyclophosphamide, ifosfamide, chlormetrin,
melphalan, chlorambucil, estramustine,
- busulfan, mitomycin C,
- nitrosoureas: BCNU (carmustine), CCNU
(lomustine), fotemustine, streptozotocin,
- triazines or derivatives, procarbazine,
dacarbazine,
- pipobroman,
- ethyleneimines: altretamine, triethylene-
thiophosphoramide,
iv) a compound chosen from the other groups of
antimetabolic agents:
- antifolic agents: methotrexate, raltitrexed,
- antipyrimidines: 5-fluorouracil (5-FU),
cytarabine (Ara-C),
- hydroxyurea
- antipurines: purinethol, thioguanine
,
pentostatin, cladribine
- inductors of the synthesis of cytotoxic
nucleosides: gemcitabine,
v) a compound chosen from the other groups of
agents with high affinity for the tubules:
- vinca alkaloids which disorganize the mitotic
spindle: vincristine, vinblastine, vindesine,
navelbine
- agents blocking the depolymerization of the
mitotic spindle: paclitaxel, docetaxel
_
- agents inducing breaks in the DNA by
inhibition of topoisomerase II: etoposide,
teniposide
- inhibitors of topoisomerase I inducing breaks
in DNA: topotecan, irinotecan,
CA 02337256 2001-O1-12
- 8 _
vi) an agent breaking, fragmenting DNA, such as
. j
bleomycin,
vii) one of the following compounds: plicamycin,
L asparaginase, mitoguazone, dacarbazine,
viii) an anticancer progestogenic steroid:
medroxyprogesterone, megestrol,
ix) an -- anticancer oestrogenic steroid:
diethylstilbestrol; tetrasodium fosfestrol,
x) an antioestrogen: tamoxifen, droloxifen,
raloxifen, aminogluthetimide,
xi) a steroidal antiandrogen (e.g. cyproterone) or
a nonsteroidal antiandrogen (flutamide,
nilutamide).
In particular, the compounds of formula I may
be combined with all the treatments with the
major
cytotoxic agents used in polychemotherapy of solid
tumours such as:
- doxorubicin
- alkylating agents: oxazophorines
(cyclophosphamide, ifosfamide, chlorambucil, melphalan)
- nitrosoureas
- mitomycin C
- antimetabolites such as methotrexate, 5-FU,
Ara-C, capecitabine
- agents which interfere with tubulin: vinca
alkaloids (vincristine, vinblastine, vindesine,
navelbine), taxoids (paclitaxel, docetaxel),
derivatives of epipodophyllotoxins (etoposide,
teniposide)
- bleomycin
- inhibitors of topoisomerase I: topotecan,
irinotecan.
Likewise, the compounds of formula I may be
combined with the treatment with the major cytotoxic
agents used in oncohaematology for the treatment of
blood cancers:
- Hodgkin's disease: cyclophosphamide,
mechlorethamine, chlorambucil, melphalan, ifosfamide,
etoposide, doxorubicin, daunorubicin;
CA 02337256 2001-O1-12
a
_ g -
- acute leukaemias: methotrexate,
6-mercaptopurine, cytarabine, vinblastine, vincristine,
doxorubicin, daunorubicin, L-asparaginase;
- non-Hodgkin's malignant lymphomas,
mechlorethamine, chlorambucil, cyclophosphamide,
melphalan, ifosfamide, methotrexate, cytarabine,
vinblastine, vincristine, etoposide, doxorubicin
,
daunorubicin, carmustine, lomustine, cisplatin;
- chronic lymphoid leukaemias: mechloretamine
,
chlorambucil, cyclophosphamide, melphalan, ifosfamide.
Results of pharmacological trials demonstrating
the effects obtained will be given below.
1 - Interaction (stimulation or inhibition of
proliferation) with the generation of clonogenic cells
(clonogenic test)
The test used is that described by Hamburger et
al. (Science, 1977; 197, 461-463) and Salmon et al.
(New England J. Med., 298, 1321-1327). A cell i
s
considered to be clonogenic if it
possesses the
capacity to proliferate and to give rise to a c
ll
e
colony. The "human tumour stem cells" are the cells
which are at the origin of the neoplastic cells which
constitute a given tumour. These tumour stem cells
are
responsible for the recidivation
processes which can be
observed after surgical resection of the
i
pr
mary
tumours and are also responsible fo
th
r
e formation of
metastases. At the level of a tumour or a tumour cell
line, these clonogenic stem cells are distinguishable
from the other cells of the tumour or the neoplastic
cell line considered, by the fact that the
r
t
i
y
e
a
n
their capacity to proliferate in th
e absence of any
solid support.
In this test, the tumour cells are cultured
on
a semisolid support. Only the cells which do
not
require a solid support for their growth (that i
s to
say the highly tumorigenic cells called "anchorage-
independent cells" by M.I. Dawson et al
Can
.,
cer Res.
1995; 55: 4446-4451; also called clon
i
ogen
c cells with
CA 02337256 2001-O1-12
_ Z.~ _
reference to "clonal growth") are capable of developing
on such an agar-based support. Indeed, on such a
medium, the normal cells - which grow in "adherent
mode" ("anchorage-dependent cells" according to the
terminology of M.I. Dawson) - such as for example the
fibroblasts, do not survive. Within a tumour cell
population, cultured on such a support, it is these
clonogenic cells (associated with an unlimited number
of cell divisions and whose proliferation is called
"anchorage-independent [clonal] growth" by M.I. Dawson)
which are capable of growing. The percentage of these
clonogenic cells within a tumour or a cell line
i
var
es
between 0.1% and 0.001%. The nonclonogenic cells
(associated with a limited number of cell divisions) do
'; 15 not develop in this test because they require a solid
support for their growth which should occur i
n
"adherent mode" ("anchorage-dependent [adherent]
growth", according to M.I. Dawson et al., Cancer Res.
1995; 55: 4446-51)".
The influence of compounds of formula (I) on
the growth of the cell colonies obtained by culturing,
for example, the mammary tumour lines MCF7 and MXT and
the colorectal line HT-29 on the semiliquid culture
medium called "soft agar" was measured. On such
a
medium, only the clonogenic cells called "anchorage-
independent (clonal) cells" by M
I
Dawson s
i
.
.
urv
ve and
develop. The growth of these cells in such
a
"nonadherent" mode reflects their degree of
tumorigenicity. The inhibition of the growth of the
size of a tumour in which a larger number of clonogenic
cells have developed then becomes the control for
a
reinforced cytotoxic activity.
By contrast, this test can also reveal that
a
compound is capable of inhibiting the
generation/proliferation of clonogenic cells, which
makes the tumour less capable of developing, and
therefore reduces the population of tumour cells.
The tumour cell lines studied are maintained in
culture in 25 cm2 falcon flasks. They are then
CA 02337256 2001-O1-12
- 11 -
trypsinized and the cells well dissociated from each
other. The percentage of living cells is determined
after staining with trypan blue. A cellular suspension
at the concentration 5 104 to 15 104 cells/ml (depending
on the cell type considered) is prepared in a 0.3% agar
solution. Next, 200 ~,l of this suspension are
inoculated into Petri dishes 35 mm in diameter, in
which 3 ml of a bottom layer consisting of a 0.5% agar
solution are deposited. The 200 ~1 of cellular
suspension are in turn covered with 1.8 ml of a top
layer consisting of a 0.3% agar solution. The dishes
are then placed in an incubator at 37°C, 5% C02 and 70%
humidity until the treatment. The latter is performed
about 1 to 2 hours after inoculation. The compounds to
be tested are prepared at a concentration 100-fold
greater than the desired concentration and 50 ~tl of
these treating solutions are deposited on the agar top
layer of the corresponding dishes. In the present
study, the final concentration of the products tested
is 10-5, 10-7 and 10-9 M. The dishes are then maintained
in the incubator for 21 days. On the 21st day, the
dishes are treated by depositing on the top layer
100 ~1 of a solution of MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolinium
bromide) at 1 mg/ml prepared with RPMI 1640 medium for
3 h at 37°C. After this period of time, the cell
colonies are fixed by adding 2 ml of formalin per dish.
After fixing for 24 hours, the formalin is evaporated
and a number of coloured cell colonies, therefore
consisting of metabolically active cells, and whose
surface area is greater than 100 umz, is determined
with the aid of an inverted microscope.
The average number of clonogenic cell clones
determined for each experimental condition studied is
expressed as a percentage relative to the average
number of clonogenic cell clones counted under the
control condition and posed as equal to 100%. These
values, expressed as the percentage relative to the
control condition, are presented in Table I.
CA 02337256 2001-O1-12
- 12 -
TABLE I
CELL Genistein (in
moll-1)
LINES 10-5 10-7 10-s
MCF7 66.9 t 29 74.2 t 4.7 89.2 0.9
** * NS
HT-29 118.2 t 2.8 108.9 2.3 104.6 2.5
** * NS
~T 71 t 2.5 118.5 2.2 117.5 2.2
** ** **
- The results summarized in this table represent Lne
mean values t standard error of the mean (SEM)
established on at least 6 wells.
- Control condition = 100
- (NS: p>0.05; *: p<0.05; **: p<0.01; ***: p<0.001).
Depending on the cell line studied, genistein
can:
- recruit the clonogenic cells inside the tumour (cell
lines HT-29 at the concentrations of 10 5 M and 10-7,
and MXT at the concentrations of 10-7 M and 10-9 M) ,
that is to say induce a significant increase in the
number of colonies of these cells compared with that
obtained under the control condition, and then makes
them more sensitive to the conventional treatment with
cytotoxic agents, or
- be capable of directly inhibiting the proliferation
of these clonogenic cells (MCF7 cell line at the
concentrations of 10-5 M and 10-7 M) .
2 - Cytotoxic activity at the level of the
nonclonogenic cells: "MTT test"
The influence of the compounds of formula (I)
on the nonclonogenic cells was evaluated with the aid
of the MTT colorimetric test.
The principle of the MTT test is based on the
mitochondrial reduction by metabolically active living
cells of the product MTT (3- (4, 5-dimethylthiazol-2-yl)
2,5-diphenyltetrazolium bromide), which is yellow in
CA 02337256 2001-O1-12
- 13 -
colour, to a product which is blue in colour, formazan.
The quantity of formazan thus obtained is directly
proportional to the quantity of living cells present in
the culture well(s). This quantity of formazan is
measured by spectrophotometry.
The cell lines are maintained in monolayer
culture at 37°C in closed-stopper culture dishes
containing basal medium MEM 25 MM HEPES (Minimum
Essential Medium). This medium is quite suitable for
the growth of a range of varied mammalian diploid or
primary cells. This medium is then supplemented:
- with a quantity of 5% of decomplementized SVF
(Foetal Calf Serum) at 56°C over 1 hour,
- with 0.6 mg/ml of L-glutamine,
- with 200 IU/ml of penicillin,
- with 200 ~g/ml of streptomycin,
- with 0.1 mg/ml of gentamicin.
The 12 human cancer cell lines which were used
were obtained from the American Type Culture Collection
(ATCC, Rockville, MD, USA). These 12 cell lines are:
- U-373MG (ATCC code: HTB-17) and U-87MG (ATCC
code: HTB-14) which are two glioblastomas,
- SW1088 (ATCC code: HTB-12) which is an
astrocytoma,
- A549 (ATCC code: CCL-185) and A-427 (ATCC
code: HTB-53) which are two non-small-cell
lung cancers,
- HCT-15 (ATCC code: CCL-225) and LoVo (ATCC
code: CCL-229) which are two colorectal
cancers,
- T-47D (ATCC code: HTB-133) and MCF7 (ATCC
code: HTB-22) which are two breast cancers,
- J82 (ATCC code: HTB-1) and T24 (ATCC code:
HTB-4) which are two cancers of the bladder, _
- PC-3 (ATCC code: CRL-1435) which is a
prostate cancer.
From the experimental point of view, 100 ~l of
a cellular suspension containing 20,000 to 50,000
(according to the cell type used) cells/ml of culture
CA 02337256 2001-O1-12
- 14 -
medium are inoculated into flat-bottomed 96-well
multi-well plates and are incubated at 37°C, under an
atmosphere comprising 5% C02 and 70% humidity. After
24 hours of incubation, the culture medium is replaced
with 100 ~1 of fresh medium containing either the
various compounds to be tested at concentrations
varying from ~0-5 to 10-1° M, or the solvent which served
for the dissolution of the products to be tested
(control condition). After 72 hours of incubation under
the preceding conditions, the culture medium is
replaced with 100 ~l of a yellowish solution of MTT
dissolved in an amount of 1 mg/ml in RPMI 1640. The
microplates are incubated for 3 hours at 37°C and then
centrifuged for 10 minutes at 400 g. The yellowish
solution of MTT is removed and the blue formazan
crystals formed in the cell are dissolved in 100 ~1 of
DMSO. The microplates are then placed under stirring
for 5 minutes. The intensity of the resulting blue
colour, and therefore of the conversion of the yellow
MTT product to blue formazan by the cells still alive
at the end of the experiment, is quantified by
spectrophotometry with the aid of a DYNATECH
IMMUNOASSAY SYSTEM type apparatus at the wavelengths of
570 nm and 630 nm corresponding to the wavelengths for
maximum absorption of formazan and to the background
noise, respectively. A software integrated into the
spectrophotometer calculates the mean optical density
values as well as the standard deviation (Std. Dev.)
and standard error of the mean (SEM) values.
By way of nonlimiting example, the results of
the mean optical density, expressed as a percentage
relative to the mean optical density measured under the
control condition (posed equal to 100%), obtained with
an isoflavonoid: genistein, on the 5 tumour cell lines
U-87MG, J82, HCT-15, T-47D and A549, will be given in
Table II.
CA 02337256 2001-O1-12
. i _ 15 _
TABLE II
CELL G enistein (in mo_l.l'1)
LINES 10 5 10 6 10 ' 10 a 10 9 10 io
U-87MG 83.8 98.1 94.3 100.1 98.2 108.6
t t
3.5 4.4 3.7 6.6 3.5 2.3
** NS NS NS NS
J82 87.0 99.3 101.6 101.8 102.8 104.2
1.0 1.1 0.8 1.8 &.5[sick 1.5
*** NS NS NS NS NS
HCT-15 96.8 100.9 97.5 89.2 89.4 t 90.5 t
t
5.3 6.0 5.2 3.5 4.0 3.3
NS NS NS
T-47D 92.3 98.9 95.1 97.8 100.0 102.4
2.2 3.3 1.6 3.0 3.4 1.7
* NS NS NS NS NS
A-549 81.4 105.0 101.6 106.0 108.9 103.6
t t
4.8 4.1 5.4 3.2 2.1 3.9
** NS NS NS * NS
- xx ~ yy = mean value ~ standard error of the mean
- control condition = 100
- (NS: p >0.05; *: p <0.005; **; p <0.01; ***: p c
0.001).
Genistein has a low antitumour power. This
nontoxic product induces, when it is the case,
inhibition of the overall cell proliferation of these
lines only at the concentration of 10-5 M and this
inhibition does not exceed 20%. At the other
concentrations tested, only a few marginal effects can
be demonstrated.
3. - Determination of the maximum tolerated
dose (MTD)
The evaluation of the maximum tolerated dose
was carried out in 4- to 6-week old B6D2F1/Jico mice.
The compounds were administered by the intraperitoneal
route in increasing doses ranging from 2.5 to
CA 02337256 2001-O1-12
- 16 -
160 mg/kg. The value of the MTD (expressed in mg/kg) is
determined from the observation of the rate of survival
of the animals over a period of 14 days after a single
administration of the product considered. The variation
of the weight of the animals is also monitored over
-I
this period. When the MTD value is greater than
160 mg/kg, the MTD value is considered to be 160 mg/kg
by default.
Genistein is by default associated with an MTD
equal to 160 mg/kg. This result suggests that the
products of the isoflavonoid family do not exhibit any
direct toxicity and can be used in high tissue
concentrations, and therefore in high dosages.
4. - Antitumour activity in vivo in combination
with a cytotoxic agent
The trials were carried out on the models of:
- hormone-sensitive murine mammary
adenocarcinoma MXT (HS-MXT),
- lymphoma P 388,
in the presence or otherwise of cytotoxic agents such
as cyclophosphamide, etoposide, doxorubicin or
vincristine. _
When the MTD value for a product was
determined, its in vivo antitumour activity was
characterized at the doses of MTD/2, MTD/4 and MTD/8 on
the model of mammary adenocarcinoma of murine origin
HS-MXT and on the lymphoma P388 model) . It is the dose
which exhibited the best antitumour activity on these
different models which was selected and used in the
context of the treatments combined with the cytotoxic
agents.
In all the examples presented below, whatever
the model (mammary adenocarcinoma HS-MXT or lymphoma
P 388), the control condition is represented by a group
of 9 mice to which a volume of 0.2 ml of physiological
saline containing the solvent used to dissolve the
different compounds of formula (I) used is administered
for 5 consecutive weeks and at the rate of
CA 02337256 2001-O1-12
_ 17
administrations (Monday, Tuesday, Wednesday, Thursday
and Friday) per week.
The following were determined during these
trials:
5 i) - rate of survival the mice
of
', This rate o f survival was calculated in the
form of a ratio T/C:
(Number of days (Treated (Number of mice which
of survival of median - died during the
days
the median mouse mouse) which preceded that
of the treated for the treated median
mouse group) mouse)
T - + _________________________________
(Number of mice which died on the
same day as the treated median
mouse)
(Number of days (Treated (Number of mice which
of survival of median - died during the days
the median mouse mouse) which preceded that
of the treated for the control median
mouse group) mouse)
C = + _________________________________
(Number of mice which died on the
same day as the control median
mouse)
This ratio represents the mean survival time for the
median mouse of the treated mouse group relative to the
mean survival time for the median mouse of the control
mouse group. Thus, a molecule induces a significant
increase (P <0.05) in the survival of the animals when
the T/C ratio exceeds 130%. On the other hand, it
exhibits a toxic effect when this T/C value is less
than 70%.
ii) - tumour growth by measuring, twice per
week (Monday and Friday), the surface area of the
transplanted HS-MXT and P388 tumours. This surface area
CA 02337256 2001-O1-12
- 18 -
is calculated by taking the product of the value of the
two largest perpendicular axes of the tumour. The value
of these axes is measured with the aid of a slide
calliper.
4.1. Murine mammary adenocarcinoma (HS-MXT)
The model of murine mammary adenocarcinoma MXT
which is hormone-sensitive (HS-MXT) transplanted in 4-
to 6-week old B6D2F1/..Tico mice is a model derived from
the galactophorous ducts of the mammary gland (Watson
C. et al. Cancer Res. 1977; 37: 3344-48).
The results obtained using genistein either
alone or in combination with the cytotoxic agents will
be given by way of example.
Treatment 1
Genistein is administered alone. The first
injection of the product is carried out on the seventh
day post-transplantation (D7) for four consecutive
weeks at the rate of 5 injections per week (Monday,
Tuesday, Wednesday, Thursday and Friday) and at the
dose of 20 mg/kg.
Treatment 2
Cyclophosphamide is administered alone. The
first injection of the product is carried out on the
fourteenth day post-transplantation (D14) for three
consecutive weeks at the rate of 3 injections per week
(Monday, Wednesday, and Friday) and at the dose of
10 mg/kg.
Treatment 3
Vincristine (VCR) is administered alone. The
first injection of the product is carried out on the
fourteenth day post-transplantation (D14) for three
consecutive weeks at the rate of 3 injections per week
(Monday, Wednesday, and Friday) and at the dose of
0.63 mg/kg.
Treatment 4
Etoposide (ETO) is administered alone. The
first injection of the product is carried out on the
CA 02337256 2001-O1-12
19
fourteenth day post-transplantation (D14) for three
consecutive weeks at the rate of 3 injections per week
(Monday, Wednesday, and Friday) and at the dose of
mg/kg.
5 Treatment 5
Genistein is coadministered with
cyclophosphamide. In this case, the first injection of
genistein is carried out on the seventh day post-
transplantation (D7) for four consecutive weeks at the
10 rate of 5 injections per week (Monday, Tuesday,
Wednesday, Thursday and Friday) at the dose of 20 mg/kg
and the first injection of cyclophosphamide is carried
out on the fourteenth day post-transplantation (D14)
for three consecutive weeks at the rate of three
injections per week (Monday, Wednesday and Friday) at
the dose of 10 mg/kg.
Treatment 6
Genistein is coadministered with vincristine.
In this case, the first injection of genistein is
carried out on the seventh day post-transplantation
(D7) for four consecutive weeks at the rate of
5 injections per week (Monday, Tuesday, Wednesday,
Thursday and Friday) at the dose of 20 mg/kg and the
first injection of vincristine is carried out on the
fourteenth day post-transplantation (D14) for three
consecutive weeks at the rate of three injections per
week (Monday, Wednesday and Friday) at the dose of
0.63 mg/kg.
Treatment 7
Genistein is coadministered with etoposide. In
this case, the first injection of genistein is carried
out on the seventh day post-transplantation (D7) for
four consecutive weeks at the rate of 5 injections per
week (Monday, Tuesday, Wednesday, Thursday and Friday)
at the dose of 20 mg/kg and the first injection of
etoposide is carried out on the fourteenth day post-
transplantation (D14) for three consecutive weeks at
the rate of three injections per week (Monday,
Wednesday and Friday) at the dose of 10 mg/kg.
CA 02337256 2001-O1-12
- 20 -
The results obtained for the survival period
(Table III) for genistein will be given below.
TABLE III
Treatments T/C (expressed in %)
1 (genistein) 100
2 1CPA) 107
3 (VCR) 105
4 (ETO) 116
(genistein + CPA) 131
6 (genistein + VCR) 135
7 (genistein + ETO) 131
5
These results show that the coadministration of
genistein with the cytotoxic agents: cyclophosphamide,
vincristine or etoposide, significantly increases the
mean survival time for the median mouse of the
different groups of mice thus treated compared with the
mean survival time for the median mouse of the control
mouse group. Furthermore, this increase in the mean
survival time for the median mouse of the different
groups of mice treated with these coadministrations is
_ 15 significantly longer than that obtained with the
treatments involving genistein or these cytotoxic
agents used alone.
The study of tumour growth moreover showed the
following results. In Table IV below are indicated, in
per cent, the decreases (-) or the increases (+) in the
surface area of the HS-MXT tumours induced with the
different treatments 1, 2, 3, 4, 5, 6 and 7 compared
with the control condition on the 28th day after the
tumour transplantation, that is after
15 administrations of genistein and 6 administrations
of the different cytotoxic agents used or otherwise in
coadministrations with genistein. On the 28th day post-
transplantation, 89% of the control animals are still
alive (that is 8 animals out of 9).
CA 02337256 2001-O1-12
- 21
Table IV
Treatments Variation in the tumour
surface area
(expressed in ~)
1 (genistein) + 2.6
2 (CPA) - 25
3 (VCR) - 32
4 (ETO) - 22
5 (genistein + CPA) - 20
6 (genistein + VCR) - 45
7 (genistein + ETO) - 41
These results show that the coadministration of
genistein with the cytotoxic agents: vincristine and
etoposide, significantly induces a decrease in the
growth of the HS-MXT tumours which is greater than that
induced by the treatments involving genistein alone
(which has no relevant clinical effect) or the latter
two cytotoxic agents used alone.
4.2. Lymphoma P 388:
The 4- to 6-week old CDF1 mice receive a
transplant consisting of a piece of P388 tumour
(obtained from a bank of tumours maintained in the
laboratory) subcutaneously on the right side on day D0.
In order to be in a situation similar to the clinical
reality, we wait for the 5th day post-transplantation
(D5) before starting the treatment. This was because,
after this period of time, the subcutaneous P388
tumours are palpable.
By way of example, the results obtained with
genistein alone or in combination with vincristine are
reported below.
Treatment 1
Genistein is administered alone. The first
injection of the product is carried out on the fifth
day post-transplantation (D5) at the rate of
5 injections per week (Monday, Tuesday, Wednesday,
CA 02337256 2001-O1-12
- 22 -
Thursday and Friday) for five consecutive weeks and at
the dose of 40 mg/kg.
Treatment 2
Vincristine (VCR) is administered alone. The
first injection of the product is carried out on the
fifth day post-transplantation (D5) at the rate of
3 injections per week (Monday, Wednesday and Friday)
for three consecutive weeks and at the dose of
0.63 mg/kg.
Treatment 3
Genistein is coadministered with vincristine.
In this case, the first injection of genistein is
carried out on the fifth day post-transplantation (D5)
at the rate of 5 injections per week (Monday, Tuesday,
Wednesday, Thursday and Friday) for five consecutive
weeks at the dose of 40 mg/kg and the first injection
of vincristine is carried out on the fifth day post-
transplantation (DS) at the rate of 3 injections per
week (Monday, Wednesday and Friday) for three
consecutive weeks at the dose of 0.63 mg/kg.
The results obtained with treatments 1, 2 and 3
on the survival times for the mice are presented below
in Table 5.
Table V
2S
Treatments T/C (expressed in %)
1 (genistein) 125
2 (VCR) 122
3 (genistein + VCR) 169
These results show that the coadministration of
genistein with vincristine increases in a very highly
significant manner the mean survival time for the
median mouse of the different groups of mice thus
treated compared with the mean survival time for the
median mouse of the control mouse group. Furthermore,
this increase in the mean survival time for the median
mouse of the different groups of mice thus treated is
highly significant compared with the mean survival time
CA 02337256 2001-O1-12
23 -
for the median mouse of the different groups of mice
treated with genistein or vincristine which are used
alone.
Examples of the modality of using the compounds
of formula I in mono- or polychemotherapy protocols
with cytotoxic agents will be given below.
A. Solid tumours
1/ Lung cancers
l.l. Non-small-cell type (advanced stage):
- to the recommended protocol (T. Le
Chevalier et al., J. Clin. Oncol. 1994; 12.
360-367), the intravenous infusions of
genistein or of another isoflavonoid are
added:
Dose Route Days
isoflavonoid 200-2000 mg/m'/day D1, De, Dls,
or 5 - 50 mg/kg/day i .v. D22, Dz9 and
D3s
infusion of 1 h
navelbine 30 mg/m2/day i .v. D1, De, D15,
Dzz, Dzs and
D3s
cisplatin 120 mg/m' i.v. D1 and Dz9
this cure is repeated 8 times.
1.2. Small-cell type (advanced stage):
- to the recommended CAV or VAC protocol
(B.J. Roth et al., J. Clin. Oncol. 1992; 10:
282-291), the isoflavonoid infusions are
added:
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D1
infusion of 1 h
cyclophophamide 1000 mg/m2 bolus i.v. D1
doxorubicin ~ 40 to 50 mg/m2 bolusi.v. D1
vincristine 1 to 1.4 mg/m2 bolusi.v. D1
(max 2 mg)
CA 02337256 2001-O1-12
- 24 -
this cure is to be repeated 6 times every
21 days.
- to the recommended Pt-E protocol (B. J. Roth et al.,
J. Clin. Oncol. 1992; 10: 282-291) the genistein
infusions are added
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v. D1-Ds
infusion of 1 h
cisplatin 20 mg/m2/day
infusion of 20 to i.v. Dl-Ds
60 minutes
etoposide 80 mg/m2/day
infusion of i.v. Dl - Ds
60 minutes
each cycle is repeated every 21 days and the
cure comprises 6 cycles.
1.3. Non-small-cell bronchial cancer, locally advanced
or metastatic:
~ monochemotherapy:
Dose Route Days
isoflavonoid 200-2000 mg/m'/day Dm Da- Dis
or 5 - 50 mg/kg/day i.v. then 1
infusion of 1 h week/rest
gemcitabine 1000 mg/m~/day D1, Da. Dis
infusion of i.v. then
0.5 hour 1 week/rest
it being possible for the cure to comprise the
repetition of the cycle of 4 weeks.
~ gemcitabine/cisplatin combination:
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D1-Ds, Da-Dls
infusion of 1 h
CA 02337256 2001-O1-12
gemcitabine 1000 mg/m2/day
infusion of i.v. Dl, D8, Dls
0.5 hour
cisplatin 20 mg/m2/day i.v.
infusion of 20-60
minutes
the cure comprising the repetition of this
cycle every 21 days.
2/ Breast cancers
- CMF protocol as adjuvant treatment for operable
breast cancer (G. Bonnadonna et al., N. Engl.
J. Med.; 1976; 294: 405-410):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. Dl to
infusion of 1 h Dla
cyclophosphamide 100 mg/m~/day oral D1 to
D14
methotrexate 40 mg/m' bolus i.v. D1 and
DB
S-FU 600 mg/m2 i.v. D1 and
De
each cycle is repeated every 28 days and the
cure comprises 6 cycles.
- AC protocol (B. Fisher et al., J. Clin.
Oncol.; 1990; 8: 1483 - 1496) as adjuvant
treatment:
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v. D1
infusion of 1 h
doxorubicin 60 mg/m2 i.v. D1
bolus
cyclophosphamide 600 mg/mz i.v. D1
bolus
each cycle is repeated every 21 days and the
cure comprises 4 cycles.
- Breast cancers with metastases:
CA 02337256 2001-O1-12
- 26 -
- in the FAC protocol (A. U. Buzdar et al.,
Cancer 1981; 47: 2537-2542) and its different
adaptations, the isoflavonoid infusions are
added according to the following scheme
(nonlimiting):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day Dl-DS and
D$-
or 5 - 50 mg/kg/dayi.v. D12 or Di-DS
infusion of 1 h
5-FU 500 mg/mz/day i.v. Dl and D8
or
bolus Dl-D2
doxorubicin 50 mg/mz i.v. D1 or D1 and
bolus DZ
cyclophos- 500 mg/m2 bolus D1
phamide i.v.
or
oral D1
each cycle is repeated every 3 weeks until a
new progression of the disease is diagnosed.
- in the CAF protocol (G. Falkson et al.,
Cancer 1985; 56: 219-224):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/dayi.v. D1-Dla
infusion of 1 h
cyclophos- 100 mg/m2/day oral Dl-D14
phamide
doxorubicin 30 mg/m' i.v. D1 and D8
5-FU 500 mg/m' i.v. D1 and Da
bolus
each cycle is repeated every 28 days until a
new progression of the disease is diagnosed.
- in the CMF protocol:
CA 02337256 2001-O1-12
- 27 _
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v. Dl-DS and
infusion of 1 h Dg-Dlz
cyclophos- 600 mg/mz/day i.v. D1 and Da
phamide bolus
methotrexate 40 mg/mz/day i .v. D1 and Da
bolus
5-FU 600 mg/mz/day i.v. D1 and DB
bolus
this cycle is to be repeated every 3 to 5 weeks
and the cure comprises 6 cycles.
- in the CMF-VP protocol:
Dose Route Days
isoflavonoid 200-2000 mg/mz/day D1-DS
or 5 - 50 mg/kg/day i.v. D8-Dlz
infusion of 1 h Dis-Di9
Dzz-Dzs
cyclophos- 2 to 2.5 mg/kg/day oral daily
phamide
methotrexate 25 to 50 mg/mz/day i.v. D1, Dg, D15,
Dzz
5-FU 300 to 500 mg/mz/dayi.v. D,, D8, Dls,
Dzz
vincristine 0.6 to 1.2 mg/mz/dayi.v. D,, D~, D15,
Dzz
prednisone 30 mg/mz/day oral from D1 to Dlo
this cure is to be repeated every 4 weeks.
- in the FEC protocol:
Dose Route Days
isoflavonoid 200-2000 mg/mz/day D1-DS
or 5 - 50 mg/kg/day i.v. and Da-Dlz
infusion of 1 h
5-FU 600 mg/mz/day i.v. D1 and D8
epirubicin 50 mg/mz i.v. D1
cyclophos- 600 mg/mz i.v. D1
phamide
this cure is to be repeated every 3 weeks.
CA 02337256 2001-O1-12
- 28 -
- in the MMC-VBC protocol (C. Brambilla et al.,
Tumori, 1989; 75: 141-144):
Dose Route Days
isoflavonoid 200-2000 mg/mz/day D1-Ds
or 5 - 50 mg/kg/day i.v. and Dls-Dls
infusion of 1 h
mitomycin C 10 mg/mz i.v.
bolus
vinblastine 50 mg/m2/day i .v. Dl and Dls
bolus
this cure is to be repeated every 28 days until
progression of the disease is diagnosed.
- in the NFL protocol (S.E. Jones et al., J.
Clin. Oncol. 1991; 9: 1736 - 1739):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. Dl-Ds
infusion of 1 h
mitoxantrone 10 mg/m2 i.v.
bolus
5-FU 1000 m 2
g/m as an
infusion of i.v. Dl-D3
24 hours
leucovorin 100 mg/m2 i.v. D1
bolus
the cure comprises two cycles 21 days apart and
then requires evaluation.
The isoflavonoid infusions may also be combined
with the treatment of breast cancers with metastases
when a taxoid is used, for example:
- with paclitaxel (F. A. Holmes et al., J. Natl
Cancer Inst. 1991; 83: 1797 - 1805) in the
treatment of the forms with metastases which
may be resistant to anthracyclines:
CA 02337256 2001-O1-12
- 29 -
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v.
infusion of 1 h
paclitaxel 175 mg/m2 as an i.v.
infusion of 3 to
24
hours
This cycle is repeated every 21 days until a
new progression of the disease is diagnosed.
- with docetaxel (C. A. Hudis et al., J. Clin.
Oncol. 1996; 14: 58-65), in locally advanced
or metastatic breast cancer, resistant or in
relapse after cytotoxic chemotherapy (which
comprised an anthracycline) or in relapse
during an adjuvant treatment:
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v. D1-DS
infusion of 1 h
docetaxel 100 mg/m2 or i.v.
60-100 mg/m2 as an
infusion of 1 hour
(or of 24 hours)
This cycle is repeated every 21 days for a cure
of 2 cycles or until a progression of the disease
appears.
in the dose intensification protocols
combining a transplantation of autologous
medullary cells and peripheral blood stem
cells as a consolidation of the first line
treatment, for example:
- CPB protocol (W. P. Peters et al., J. Clin.
Oncol. 1993; 11: 132 - 1143), in which the
i.v. infusion of stem cells takes place on
days D_1, Do and Dl:
CA 02337256 2001-O1-12
i
- 30 -
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v. D_6 to
D_1
infusion of 1 h
cyclophosphamide 1875 mg/mz as an .v. D_6 to
i
infusion of 1 hour
cisplatin 55 mg/m2/day i.v. D_6 to
D_4
as a continuous
infusion of
i
24 hours
carmustine 600 mg/m2/day as i.v.
(BCNU) an
infusion of 2 hours
- CTCb protocol al., J. Clin.
(K. Amman et
Oncol. 1 992; 10: 102-110), which
infusion in the
of stem cells takes i.v.
p lace
on
day
Do:
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D_, to
infusion of 1 h D_1
cyclophosphamide 1500 mg/m2 as a
- continuous infusion i.v. D_, to
of 24 hours (4 D_3
doses)
thiotepa 125 mg/m2
as a continuous i.v. D_~ to
infusion of D_3
24 hours (4 doses)
carboplatin 200 mg/m2
as a continuous i.v. D_, to
D_3
infusion of
24 hours (4 doses)
- CTM protocol (L. E. Damon et al., J. Clin.
Oncol. 1989; 7: 560-571 and I.C. Henderson et
al., J. Cellular Biochem. 1994 (Suppl 18B): 95)
in which the i.v. infusion of haematopoietic
stem cells takes place on Do:
CA 02337256 2001-O1-12
- 31 -
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v. D_6 to
D_1
infusion of 1 h
cyclophosphamide .1500 mg/mz/day as i
an
.v. D to
-6 D
_3
infusion of 1 hour
thiotepa 150 mg/m2/day
as an infusion of i .v. D_6 to
D_3
2 hours
mitoxantrone 10 - 15 mg/m2 as i
a
n .v. D_6 to
D_3
infusion of 1 hour
Gynaecological cancers
3.1. Ovarian cancer:
- for the treatment of in particular metastatic
ovarian carcinomas:
i) PAC protocol (G. A. Omura et al. J. Clin.
Oncol. 1989; 7: 457 - 465): the infusions of
isoflavonoids are administered according to the
following scheme:
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or i.v. D1-DS
5
-
50
mg/kg/day
infusion
of
1
h
cisplatin 50
mg/mz
(or i.v. D1
40-90
mg/m2)
infusion
of
1
to
2
hours
doxorubicin 50
mg/m~
bolus
(or i.v. D
30
to
50
mg/m2)
cyclophosphamide 1000
mg/m2
infusion
of i.v. Dl
1
to
2
hours
(or
200
to
600
mg/m2)
this cycle is to 28 days and
repeated
every
21
the cure comprises 8
cycles.
CA 02337256 2001-O1-12
- 32 -
ii) altretamine protocol, according to
A. Marietta et al. (Gynecol. Oncol. 1990; 36:
93-96)
Dose Route Days
~ isoflavonoid 200-2000 mg/mz/day Dl-Ds
or 5 - 50 mg/kg/day i.v. DB-Dlz
infusion of 1 h
~ altretamine 200 mg/mz/day
divided into 4 oral Dl-Dls
doses
the cure comprising two cycles, 28 days apart.
ii) paclitaxel protocol: the isoflavonoids may
be added to the paclitaxel protocol as has been
described by 4J. P. McGuire et al. (Ann. Intern.
Med. 1989; 111: 273-279):
Dose Route _Days _
~ isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v.
infusion of 1 h
~ paclitaxel 135 mg/mz
infusion of 3 hours i.v. D1
or of 24 hours
the cure comprising two of these cycles,
28 days apart (with evaluation at the end).
- for the treatment of metastatic and refractory
ovarian carcinomas, the isoflavonoids may be
added to the second line protocol, based on
topotecan:
D_os_e Route Days
~ isoflavonoid 200-2000 mg/m
or 5 - 5 0 mg/kg/day i , v . D1 _DS
infusion of 1 h
~ topotecan 1.5 mg/mz/day
infusion of 0.5 i.v.
hour
CA 02337256 2001-O1-12
- 33 -
the cure comprising two cycles, 21 days apart
(with evaluation at the end)
according to A.P. Kudelka et al. (J. Clin.
Oncol. 1996: 14: 1552-1557).
3.2 Trophoblastic tumours:
- in low-risk patients, the isoflavonoids may be
combined with the protocol described by
H. Takamizawa et al. (Semin. Surg. Oncol. 1987;
3: 36 - 44):
i
Dose Route Days
~ isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D1-Ds
infusion of 1 h
~ methotrexate 20 mg/day i.m. D1-Ds
(MTX)
~ dactinomycin 0.5 m da as a
g/ Y i.v. Dl-Ds
(DACT) bolus
(MTX-DATC protocol).
3.3 Uterine cancers:
- the isoflavonoids may also be combined with
the CAV (or VAC) protocol according to the
scheme below:
Dose Route Days
~ isoflavonoid 200-2000 mg/mz/day
or S - 50 mg/kg/day i.v. D1-D3
infusion of 1 h
~ cyclophosphamide 750 - 1200 mg/m2 i.v. D
as an infusion
doxorubicin 45-50 m
g/m i.v.
as an infusion
~ vincristine 1.4 mg/mz
i.v.
the cure comprising a repetition of this cycle
every 21 days.
- in the FAP protocol:
CA 02337256 2001-O1-12
- 34 -
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. Dl-DS
infusion of 1 h
fluorouracil 600 mg/mz/day i.v.
(5-FU)
doxorubicin 30 m m3
g/ i.v.
cisplatin 75 mg/m2 i.v.
Di
the cure comprising the rep etitionof this
cycle every 21 or 8 days.
2
4/ Testicular and rostate cancers
- the isoflavonoids may also be combined with
the testicular cancer protocols:
BEP protocol- Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D1-DS
infusion of 1 h
bleomycin 30 mg/m2 i.v.
as an infusion
etoposide 100 mg/m2/day i.v.
as an infusion
cisplatin 20 mg/m2/day i.v. D1-D
the cure comprising three cycles, at the rate
of one cycle every 21 days.
5/ Bladder cancers
- the isoflavonoids may be combined with the
CISCA2 (also called PAC) protocol
Dose Route Days
isoflavonoid 200-2000 mg/mZ/day
or 5 - 50 mg/kg/day i.v. D1-DS
infusion of 1 h
cisplatin 50 m m2
g/ i . D1
v .
cyclophosphamide 600 mg/m3 i.v. D1
as an infusion
doxorubicin ~ 75 mg/mz i v
CA 02337256 2001-O1-12
I ~ as an infusion
the cycle having to be repeated every 3 weeks.
- in the MVAC protocol (according to CN Sternberg et
al., J. Urol. 1988; 139: 461-469):
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/dayi.v. Dls-Dle
infusion of 1 h Dzz-Dzs
methotrexate 30 mg/mz bolus i.v. Dl, Dls,
Dzz
vinblastine 3 mg/mz i.v. Dz or Dz,
Dis~ Dzz
doxorubicin 30 mg/mz bolus i.v. Dz
cisplatin 70-100 mg/mz i.v. DI or Dz
infusion of 1 h
5 this cycle being repeated every 4 to 5 weeks,
at least for 2 cycles.
6/ Nasopharyngeal carcinomas/head and neck cancers
- The isoflavonoids may be legitimately
10 combined with the polychemotherapy protocols
used in the treatment of these cancers:
6.1 Nasopharyngeal cancers:
- ABVD protocol:
Dose Route Days
isoflavonoid 200-2000 mg/mz/da D -D
Y ~ 3
or 5 - 50 mg/kg/day i .v. D8-Dlo
infusion of 1 h or Dls-Dl,
doxorubicin 30 mg/mz/day i.v. D1 and
De
or Dls
bleomycin 10 mg/mz/day i.v. D1 and
DB
or Dls
vinblastine 6 mg/mz/day i.v. D1 and
De
or Dls
dacarbazine ~ 200 mg/mz/day i.v. D1 and
DB
o r Dls
CA 02337256 2001-O1-12
36 -
the cure comprising 1 to 6 cycles repeated at
the rate of 1 cycle every 4 weeks.
6.2 Head and neck cancers with metastases:
- in the Pt-FU protocol (e.g.. for cancers of the
pharynx): according to the DVAL Study Group
(New Engl. J.M. 1991; 324: 1685 - 1690):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D1-DS
infusion of 1 h
cisplatin 100 mg/m2 i.v.
infusion of 1 h
fluorouracil 1000 mg/m2/day i.v.
(5-FU) continuous infusion
the cure comprising two cycles, at the rate of
1 cycle every 3 weeks.
7/ Carcinomas of the soft tissues
- The isoflavonoids may be introduced in a
protocol such as the CYVADIC protocol:
- according to H.M. Pinedo et al. (Cancer 1984;
53 : 1825)
Dose Route Days
--.-
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. DB-Dlo
infusion of 1 h Dls-D1~
cyclophosphamide 500 mg/m2 bolus i.v.
(Cy)
vincristine (V) 1.5 mg/m~/day bolus i.v. D1, De,
Dis.
doxorubicin (A) 50 mg/m2 bolus i.v.
dacarbazine 250 mg/m2/day i.v.
(DIC) infusion of
15 minutes
CA 02337256 2001-O1-12
_ 37 _
the cure comprising the repetition of this
cycle every 4 weeks, first for 2 cycles.
8/ Hormone-refractory prostate cancer, with
metastases
- in the VBL-estramustine, according to
G.R. ~ dis et al. (J. Clin. Oncol. 1992; 10:
1754:1761):
Dose Route Days
isoflavonoid 200-2000 mg/mz/day D1-D3,
or 5 - 50 mg/kg/day i.v. D8-Dlo
infusion of 1 h Dls-Dm.
Dzz _Dza,
Dzs-D3m
D36-D38
vinblastine 4 mg/mz/day bolus i.v. D1, ~De,Dls,
Dzz~ D29
D36
estramustine 200 mg/mz/day tid oral every day
(600 mg/mz/day) for 6
weeks
a treatment cycle lasting for 6 weeks and being
followed by 2 weeks of free interval.
9/ Cancers of the germ cells
i) for tumours with a favourable prognosis:
- Pt-E protocol, according to G.J. Bosl et al.
(J. Clin. Oncol. 1988: 6: 1231-1238)
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or S - SO mg/kg/day i.v. D1-DS
infusion of 1 h
cisplatin 20 mg/mz/day
(Pt) infusion of 20 to i.v. D1-DS
60 minutes
etoposide 100 mg/mz/day
(E) infusion of 1 hour i.v. D~-DS
CA 02337256 2001-O1-12
- 38 -
the cure comprising 4 cycles, at the rate of
1 cycle every 21 or 28 days.
ii) for tumours with metastases:
- PEB protocol, according to S.D. Williams et al.
(N. Eng. J. Med. 1987; 316: 1435-1440):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D1-DS
infusion of 1 h D9-Dli
Dis-Dia
cisplatin 20 mg/m2/day
(P) infusion of 20 to i.v. D1-DS
1 h
etoposide 100 mg/m2/day
(E) infusion of 1 h i.v. D2, D9, Dls
bleomycin 30U (or mg)/day i.v. Dl-DS
(B) bolus
the cure comprising 4 cycles, at the rate of
1 cycle every 21 days.
10/ Kidney cancers
- metastatic renal carcinoma: the isoflavonoids
may be introduced in the protocol described by
M.J. Wilkinson et al. (Cancer 1993; 71:
3601-3604):
Dose Route Days
isoflavonoid 200-2000 mg/m~/day
or 5 - 50 mg/kg/day i.v. D1-DS
infusion of 1 h Da-Dis
floxuridine 0.075 mg/kg/day i.v. Dl-Dla
continuous infusion
the cure comprising two cycles 28 days apart.
- nephroblastoma: the isoflavonoids may be
introduced in the DAVE protocol:
CA 02337256 2001-O1-12
- 39 -
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. Dl-D3
infusion of 1 h De-Dlo
dactinomycin 0 . 6 mg/m2/day i .v. D1, D8
doxorubicin 30 mg/mz/day i .v. D1, D8
cyclophosphamide 200 mg/mz/day i.v. D
infusion of 1 hour
at the rate of one cycle every 3 to 4 weeks.
11/ Cancers of the digestive tube
11.1 Cancers of the oesophagus:
- the isoflavonoids may be introduced in the
FAP protocol according to:
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v. D1-D3
infusion of 1 h D
5-fluorouracil 600 mg/m2 i.v. D1, De
(5-FU)
doxorubicin 30 mg/m2 i.v. D1
cisplatin 75 mg/mz i.v. D1
this cycle being repeated every 3 to 4 weeks.
11.2 Stomach cancers
- in advanced gastric carcinomas and/or with
metastases:
- EAP protocol (according to P. Preusser et al.,
J. Clin. Oncol. 1989; 7: 1310):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D1-D5,
infusion of 1 h Da-Dlo
etoposide 120 mg/m2/day i.v. D3, D4,
DS
infusion of 1 hour or DQ-D6
doxorubicin 20 mg/m~/day bolus i.v. D1, D~
CA 02337256 2001-O1-12
- 40 -
~ cisplatin 40 mg/mz/day i.v. Dz,De
infusion of 1 hour
at the rate of 1 cycle every 28 days.
i
- FAMtx protocol: according to J.A. Wils et al.
(J. Clin. Oncol. 1991; 89; g2~):
r-_
Dose Route Days
~ -~-~-------_---_
isoflavonoid I 200-2000 mg/mz/day
i ~ or 5 - 50 mg/kg/day i.v. D1-D3
infusion of 1 h
~ fluorouracil 1500 mg/mz bolus
i.v.
(5-FU) (F) 1 hour after
methotrexate
~ doxorubicin (A) 30 mg/mz bolus
i.v. I Dls
' ~
methotrexate ~ 1500 mg/mz infusion i.v.
I (Mtx) of 30 minutes
the cure first comprising two cycles, 28
apart. days
- in certain patients, the protocol or its
variant (epirubicin replacing doxorubicin) may
be used according to the following scheme:
----~-- _
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
I
or 5 - 50 mg/kg/day i.v. D1-D;
infusion of 1 h
~ fluorouracil 1500 mg/mz D
i.v.
(5-FU)
I ~ doxorubicin (A) 30 mg/mz bolus i.v. D1 = FAMTX
or
60 mg/mz bolus
~ epirubicin (A)
i . v . Dl = FEMTX
~ methotrexate 1500 mg/mz i.v.
(to be infused
before 5-FU)
leucovorin ~ 15 mg/mz/day oral
---~-- Dz-D4
12/ Colorectal cancers
- the isoflavonoids may be introduced in the
protocol for FU-Levamisole adjuvant treatment
CA 02337256 2001-O1-12
i _ 41 _
of colorectal cancer (according to C.G. Moertel
et al., N. Eng. J. Med. 1990; 322: 352):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
i
or 5 - 50 mg/kg/dayi.v. D1-Ds
infusion of 1 h
5-fluorouracil 450 mg/m2/day bolusi.v. D1-Ds
5-fluorouracil 450 mg/mz bolus i.v,
levamisole 50 mg tid oral 3 days/week
one week
out of two
the treatment with 5-FU
in the form induction
of a bolus
being repeated
every week after
the D1-DS
phase, for 52 s; that with an isoflavo noid being
week me rate, the day of 5-FU bolus
repeated at the the
sa
and then the days.
next 2
- for the treatment of colorectal cancer which is
refractory to treatment with 5-fluorouracil
(5-FU) and with metastases:
- according to M.L. Rothenberg et al. (J. Clin.
Oncol. 1996; 14: 1128-1135):
Dose Route Days
isoflavonoid 200-2000 mg/m~/day
or 5 - 50 mg/kg/day i.v. D1-Dj,
infusion of 1 h DB-Dlo,
Dis-Dm
Dz2 -D29
irinotectan 125 mg/m2/day i.v. Dl, D8,
Dls
the cure comprising two cycles, 42 days apart.
13/ Kaposi's sarcomas
- the isoflavonoids may be combined with the two
protocols using antracyclines formulated in the
form of liposomes:
CA 02337256 2001-O1-12
- 42 -
i) protocol described by P.S. Gill et al.
(J. Clin. Oncol. 1995; 13: 996-1003) and
C.A. Presant et al. (Lancet 1993; 341:
1242-1243):
10
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v. D1-D3
infusion of 1 h and D15-D~,
liposomal 20 mg/m2/day i.v. D1, Dls
daunorubicin infusion of 1 hour
the cure comprising two cycles repeated at an
interval of 28 days before evaluating the effects.
ii) protocol of M. Harrison et al. (J. Clin. Oncol.
1995; 13: 914-920):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D1-D3
infusion of 1 h
liposomal 20 mg/m2 i.v. D
doxorubicin infusion of 30
minutes
the cure comprising two cycles repeated at an
interval of 28 days before evaluating the effects.
14/ Metastatic melanomas
- The isoflavonoids may also be incorporated into
the combined protocols for treating metastatic
malignant melanomas:
- DTIC/TAM protocol: according to G. Cocconi et
al. (N. Eng. J. Med. 1992; 327: 516), the cure
comprising the repetition of 4 cycles, at the
rate of 1 cycle every 21 days, according to the
following scheme:
CA 02337256 2001-O1-12
.
- 43 -
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/dayi.v. Dl-Ds
infusion of 1 h
dacarbazine 250 mg/m2/day i.v. Dl-Ds
(DTIC) infusion [15 to
30 min if central
catheter] or
[30 min if
peripheral infusion
in 250 ml]
tamoxifen (TAM) 20 mg/m~/day oral D1-Ds
the cure comprising 4 cycles at the rate of
1 cycle every 21 days.
15/ Neuroendocrine carcinoma
- the isoflavonoids may be combined with the
protocol described by C.G. Moertel et al.
(Cancer 1991; 68: 227):
- Pt-E protocol:
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. Dl-D3
infusion of 1 h
etoposide 130 mg/m2/day i.v. D~-D3
infusion of 1 hour
cisplatin 45 mg/mz/day i.v. Dz, D3
infusion of 1 hour
the cure comprising two cycles repeated every
28 days.
16/ Pancreatic cancer
- advanced-stage pancreatic adenocarcinoma: the
isoflavonoids may be combined with the treatment
with gemcitabine according to the protocol of
M. Moore et al. (Proc. Am. Soc. Clin. Oncol. 1995;
14: 473):
I CA 02337256 2001-O1-12
- 44 -
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. Dl-D3, D$_lo,
infusion of 1 h Dis. Dza.
D29r D36i
D43 , Ds~
gemcitabine 1000 mg/m2 i.v. Dl, De, Dis.
infusion of 0 . 5 D2z ~ Dz9.
hour D3s ~ D43
~
then Ds,
then
once/week
for 3 weeks
then 1 week
rest and
evaluation
B. Oncohaematology
1/Acute adult leukaemias
1.1. Acute lymphoblastic leukaemia:
1.1.1. Linker protocol
The isoflavonoids may be added to the Linker
protocols - induction chemotherapy and consolidation
chemotherapy (see C.A. Linker et al. Blood 1987; 69:
1242-1248 and C.A. Linker et al. Blood 1991; 78:
2814-2822) according to the following schemes:
i) induction chemotherapy:
Dose Route Days
~ isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D1-Ds,
infusion of 1 h Da-Di2.
Dls-Di9
CA 02337256 2001-O1-12
_ A C
daunorubicin 50 mg/mz bolus everyi.v. Dl, Dz, D3
2 4 hours ( 3 0 mg/mz
in patients of over
50 years)
vincristine 2 mg bolus i.v. Dl, D8, Dls
Dzz
prednisone 60 mg/mz/day oral D1-Dzs
L-asparaginase 6000 U/mz i.m. D1,-Dza
ii) consolidation chemotherapy (regime A):
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v. D1-Ds, De-Dlz
infusion of 1 h
daunorubicin 50 mg/mz bolus everyi.v. Dl, Dz
24 hours
vincristine 2 mg bolus i .v. D1, De,
prednisone 60 mg/mz/day dividedoral
into 3 doses
L-asparaginase 12, 000 U/mz i.m. Dz, D4, D,,
D9 and Dla
the consolidation cure A comprises
4 consecutive cycles as that described above - cycles
1, 3, 5 and 7.
iii) consolidation chemotherapy (regimes B and C):
The regimes described below correspond to the
consolidation cycles 2, 4, 6 and 8 (regime B) and
9 (regime C), described by C.A. Linker et al.:
regime B: Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v. D1-Ds, DB-Dlz
infusion of 1 h
Ara-C 300 mg/mz infusion i.v. D1, D4,
of 2 hours D11
CA 02337256 2001-O1-12
- d~ -
teniposide 165 mg/mz infusion i.v. Dl, Dq, De,
of 2 hours D11
(4 cycles)
regime C: Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/dayi.v. D1-Ds
infusion of 1 h
methotrexate 690 mg/mz continuousi.v. D1-Dz
infusion of
42 hours
leucovorin 15 mg/mz every oral Dz-Ds
6 hours
1.1.2. Hoelzer protocol
The claimed products may be added to the cytotoxic
agents of this polychemotherapy protocol (D. Hoelzer et
al., Blood 1984; 64: 38-47, D. Hoelzer et al., Blood
1988; 71: 123-131) according to the following scheme:
i) induction chemotherapy/Phase 1:
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i .v. Dl-Ds, De-Dlz
infusion of 1 h D~s-D~s
daunorubicin z
25 mg/m i.v. D1, D8, Dls,
Dzz
vincristine 1.5 mg/mz (maximum i.v. D1, D8, Dls,
2 mg) D
z2
prednisone 60 mg/mz oral Dl-Dze
L-asparaginase 5000 U/mz i .m. D1-D14
(maximum 2 mg)
ii) induction chemotherapy/phase 2:
The phase 2 of the induction may be carried out
as follows:
CA 02337256 2001-O1-12
- 47 -
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/dayi.v. Dzg-D33, Dj6-D4o,'
infusion of 1 h D43-Da7
cyclo- 650 mg/mz i.v. Dz9, D43, Ds7
phosphamide (maximum 1000 mg)
cytarabine 75 mg/mz/day i.v. D31-Dj4,D38-D41,
infusion of 1 h Dqs-Dgg, Ds2-Dss
mercapto- 60 mg/mz oral D -D
29 s7
purine
methotrexate 10 mg/mz/day i .v. D31, Daa, Das,
Dsz
(maximum 15 mg)
iii) reinduction chemotherapy/phase 1:
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/dayi.v. Dl-Ds, De-Dlz,
infusion of 1 h Dls-D~9, Dzz-Dzs
doxorubicin 25 mg/mz/day i.v. D1, D8, Dls,
Dzz
dexamethasone 10 mg/mz/day oral D1-Dze
vincristine ~ 1.5 mg/mz/day oral D1, De, D1s
(maximum 2 mg) and
Dzz
iv) reinduction chemotherapy/phase 2:
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/dayi.v. D31-D3s, D3s-Daz
infusion of 1 h
cyclophos- 650 mg/mz i .v. Dz9
phamide (maximum: 1000 mg)
cytarabine 75 mg/mz i.v. D31-Dj4, D38-D41
thioguanine 60 mg/mz oral Dz9-D4z
CA 02337256 2001-O1-12
- 48 -
1.2. Acute myeloid leukaemias:
1.2.1. Treatment of adults of any age
The isoflavonoids may be added, according to
the scheme below, to the treatment incorporating the
standard dose of cytarabine previously described by
R.O. Dilleman et al. (Blood, 1991; 78: 2520-2526),
Z.A. Arlin et al. (Leukemia 1990; 4: 177-183) and
P.H. Wiernik et al. (Blood 1992; 79: 313-319):
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v. D1-D12
infusion of 1 h
cytarabine 100-200 mg/m~/day i.v. D1-D~
as a continuous
infusion
daunorubicin 45 mg/m2/day as a i.v. D1-D3, or
bolus De-Dlo
(30 mg/m2/day if
age
>_ 60)
or
mitoxantrone 12 mg/m2 i.v. D1-D3
as a daily bolus
or
idarubicin 13 mg/m2 i.v. D -D
1 3
as a daily bolus
1.2.2. Treatment of adults below 60 years of age
i) induction chemotherapy:
This induction cycle incorporates the
administration of cytarabine in a high dose
according to the following scheme: --
Dose Route Days
isoflavonoid 200-2000 mg/mZ/day
or 5 - 50 mg/kg/day i .v. Dl-Dlo
infusion of 1 h
CA 02337256 2001-O1-12
- 49 -
Ara-C 2000 mg/mz/day i.v. D1-D6
(cytarabine) as an infusion of
2 hours, every
12 hours
daunorubicin 60 mg/m2/day i.v.
as a continuous
infusion of
24 hours
or
cytarabine 3000 mg/mz/day i.v. D1-D6
as an infusion of
1 hour, every
12 hours
daunorubicin 45 mg/m2 bolus everyi .v. D.,-D9
24 hours
(in order to reduce the risk of S.N.S.
toxicity, in the event of renal insufficiency,
adjust the cytarabine dosage to the clearance
of creatinine)
according to L.E. Damon et al. (Leukemia 1994;
8: 535-541), G.L. Phillips et al. (Blood 1991;
77: 1429-1435) and G. Smith et al. (J. Clin.
Oncol. 1997; 15: 833-839).
ii) consolidation chemotherapy:
The cycle, described below, will be repeated
8 times, at the rate of 1 cycle every
4 to 6 weeks (according to R.J. Mayer et al.,
N. Engl J. Med. 1994; 331: 896-903):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D1-DS
infusion of 1 h
CA 02337256 2001-O1-12
- 50 -
cytarabine 3000 mg/m2 i.v. Dl, D3, DS
as an infusion of
3 hours, every
12 hours (4 cycles)
then 100 mg/m2/day s . D1-DS
c .
cytarabine every 12 hours
daunorubicin 45 mg/m~ bolus i.v. D1
(4 cycles)
iii) consolidation chemotherapy (with a high dose of
cytarabine):
The cycle, described below, will have to be
repeated twice and is adapted according to
G.L. Phillips et al, (Blood 1991; 77:
1429-1435); S.N. Wolff et al, (J. Clin. Oncol.
1989; 7: 1260-1267); R.J. Mayer et al. (N. Engl
J. Med. 1994; 331: 896-903):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D1-Dlo
infusion of 1 h
cytarabine 3000 mg/m2 i.v. D1-D6
1 hour every
12 hours
daunorubicin 30-45 mg/mz/day i.v. D~-D9
bolus
once/day
1.2.3. Treatment of adults aged 60 or above
The claimed substances may be added to the
consolidation chemotherapy protocols below:
i) according to R.O. Dilman et al, (Blood 1991;
78; 2520-2526), Z.A. Arlin et al. (Leukemia
1990; 4: 177-183), P.H. Wiernik et al. (Blood
1992; 79: 313-319):
CA 02337256 2001-O1-12
- 51 -
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/dayi.v. D1-D6
infusion of 1 h
cytarabine 100-200 mg/mz i.v. D1-DS
continuous infusion
of 24 hours
daunorubicin 30-45 mg/mz/day i .v. D1, Dz,
bolus
or
mitoxantrone 12 mg/mz/da i.v. D D
Y m z
bolus
or
idarubicin 13 mg/mz/day i.v. D,, Dz
bolus
ii) according to R.J. Mayer et al. (N. Engl. J.
Med, 194; 331: 896-903):
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v. D1-D6
infusion of 1 h
cytarabine 100 mg/mz i.v. Dl-DS
continuous infusion
of 24 hours
(4 cycles)
then
cytarabine s . Dl, DS
c .
100 mg/mz
every 12 hours
daunorubicin 45 mg/mz/day i.v. D1
bolus (4 cycles)
CA 02337256 2001-O1-12
- 52 -
iii) according to C.A. Linker et al. (Blood 1993; 81:
311-318), N. Chao et al. (Blood 1993; 81: 319-323)
and A.M. Yeager et al. (N. Eng. J. Med. 1986; 315:
145-147):
This protocol comprises an autologous bone marrow
transplant (performed on day Do)
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D_,-D_2
infusion of 1 h
busulfan 1 mg/kg qid oral D_., to D_4
(in total 16 doses)
etoposide 60 mg/kg/day i.v. D_3
infusion of
10 hours
or
Dose Route Days
isoflavonoid 200-2000 mg/m'/day
or 5 - 50 mg/kg/day i.v. D_9-D_1
infusion of 1 h
busulfan 1 mg/kg qid oral D_9 to D_6
cyclo- 50 mg/kg/day i.v. D_5 to D_2
phosphamide infusion of 1 hour
iv) in the case of HLA-compatible allogeneic bone
marrow transplant according to:
P.J. Tutscha et al. Blood 1987; 70: 1382-1388,
F.R. Applebaum et al., Ann. Int. Med. 1984;
101: 581-588:
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D_~-D_1
infusion of 1 h
CA 02337256 2001-O1-12
busulfan 1 mg/kg qid oral D_., to D_
(in total 16 doses)
cyclo- 60 mg/kg/day i.v. D_3 to D_
phosphamide infusion of 1 hour
2/ Chronic adult leukaemias
2.1 Chronic myeloid leukaemia
In the myeloblastic phase, the isoflavonoids
may be added to the HU-Mith treatment, described by
C.A. Koller et al. (N. Engl. J. med. 1986; 315:
1433-1438):
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/dayi .v. D1-Ds
infusion of 1 h Ds-Dlz
Dis-Dis
Dzz-Dzs
hydroxyurea 500 mg/day oral every day
mithramycin 25 ~g/kg/day i.v. daily for
infusion of 2-4 3 weeks then
hours 3 times/week
2.2 Chronic lymphocytic leukaemia
2.2.1 FCG-CLL protocol
The isoflavonoids may be added to the "pulsed
chlorambucil" combinations as described by E. Kimby et
al. (Leuk. Lymphoma 1991; 5 (Suppl.) 93-96) and by
FCGCLL (Blood 1990; 75: 1422-1425):
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/dayi.v. D1-Ds,
infusion of 1 h D_a-D_lz,
Dis-Dzz
CA 02337256 2001-O1-12
- 54 -
chlorambucil 0.1 mg/kg/day oral once/day
or
chlorambucil 0.4 mg/kg/day oral D1
every 14 days
and
prednisone 75 mg/day oral D1-D3
2.2.2 Fludarabine-CdA protocol
according to H.G. Chun et al. (J. Clin. Oncol.
1991; 9: 175-188), M.J. Keating et al. (Blood 1989; 74:
19-25 / J. Clin. Oncol. 1991; 9: 44-49) and A. Saven et
al. (J. Clin. Oncol. 1995; 13: 570-574):
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v. D1-De
infusion of 1 h (once/month
for 6 to
12 cycles)
fludarabine 25-30 mg/m2/day i.v. D1-DS
infusion of
30 minutes
[every 4 weeks for
6 to 12 cycles]
or
cladibrine 0.09 mg/kg/day as i.v. D1-D~
a
continuous infusion
[1 cycle every 28
to 35 days for 1
to
9 cycles (median:
4 cycles)] -
CA 02337256 2001-O1-12
- 55 -
3/ Lymphoproliferative diseases
3.1 Hodgkin's disease
The isoflavonoids may be incorporated into the
polychemotherapy protocols conventionally used
for the treatment of Hodgkin's lymphoma:
3.1.1 AVDB protocol
according to G. Bonnadonna et al. (Cancer Clin.
Trials 1979; 2: 217-226) and G.P. Canellos et al.
(N. Engl. J. Med. 1993; 327: 1478-1484):
Dose Route Days
isoflavonoid 200-2000 mg/m~/day D1-D3,
or 5 - 50 mg/kg/dayi.v. Dls-Dla
infusion of 1 h
doxorubicin (A) 25 mg/mz bolus i.v. D1, Dls
bleomycin (B) 10 U/m2 bolus i.v. D1, Dls
vinblastine (V) 6 mg/mz bolus i.v. D;, Dls
dacarbazine (D) 375 mg/m2 bolus i.v. D1, Dls
the cure comprising 6 to 8 cycles, at the rate
of 1 cycle every 28 days.
3.1.2 MOPP/ABVD protocol
according to G. Bonnadonna et al. (Ann. Intern.
Med. 1986; 104: 739-746) and G.P. Canellos et al.
(N. Engl. J. Med. 1993; 327: 1478-1484):
The MOPP protocol should be alternated with the
ABVD protocol (cf. ~ 3.1.1) every 28 days and the cure
comprises 6 cycles:
MOPP protocol: _ Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v. D1-D3,
infusion of 1 h De-D11
and
Di4-D1,
CA 02337256 2001-O1-12
- 56 -
mechlorethamine 6 mg/mz bolus i.v. Dl, De
(M)
vincristine (0) 1.4 mg/mz bolus i.v. D
(no maximum)
procarbazine (P) 100 mg/mz/day oral Dl-Dln
prednisone (P) 40 mg/mz/day oral Dl-D14
3.1.3 Stanford V protocol
according to N.L. Bartlett et al. (J. Clin.
Oncol. 1995; 13: 1080-1088):
Dose Route Days
isoflavonoid 200-2000 mg/mz/day D1-Ds
or 5 - 50 mg/kg/day i.v. De-Dlz
infusion of 1 h Dls-D19
Dzz-Dzs
doxorubicin 25 mg/mz i.v. D1, Dls
vinblastine 6 mg/mz bolus i . D, , Dls
v.
(4 mg/mz during
cycle 3 if age
50)
mechlorethamine 6 mg/mz bolus i.v. D1
(M)
vincristine 1.4 mg/mz bolus i.v. D1, Dzz
(max. dose: 2 mg)
[1 mg/mz during
cycle 3 if age
>_ 50)
bleomycin 5 U/mz i.v. D8, Dzz
etoposide 60 mg/mz oral Dls, Dls
prednisone 40 mg/mz/day oral once/week
(weeks
1-9)
the cure comprising 3 cycles, at the rate of
1 cycle every 28 days.
3.1.4 EVA protocol
CA 02337256 2001-O1-12
- 57 -
according to G.P. Canellos et al. (Proc. Am.
Soc. Clin. Oncol. 1991; 10: 273):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/dayi.v.
infusion of 1 h
etoposide (E) 100 mg/m2 infusion oral D1, D2,
of 2 hours D3
vinblastine (V) 6 mg/m2 bolus i.v.
doxorubicin (A) 50 mg/m2 bolus ~ i.v. D1
the cure comprising 6 cycles, at the rate of
1 cycle every 28 days.
3.1.5 B-CAVe protocol
according to W.G. Harker et al. (Ann. Intern.
Med. 1984; 101: 440-446):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/dayi.v.
infusion of 1 h
bleomycin (B) 5 U/mz bolus i.v. D1
lomustine (CCNU) 100 mg/m2 oral D1
doxorubicin (A) 60 mg/m2 bolus i.v. D1
vinblastine (Ve) 5 mg/m~ bolus i.v. D1
the cure comprising 8 cycles, at the rate of
1 cycle every 28 days.
3.2. Non-Hodgkin's lymphomas
3.2.1. of low grade of malignancy
i)-CVP protocol
- according to C.M. Bagley et al. (Ann. Intern.
Med. 1972; 76: 227-234) and C.S. Portlock et
al. (Blood 1976; 47: 747-756)
CA 02337256 2001-O1-12
_ 58 _
Dose Route Days
isoflavonoid 200-2000 mg/mz/day D1-DS
or 5 - 50 mg/kg/day i.v.
infusion of 1 h
cyclophosphamide 300-400 mg/m2/day oral D
(c)
vincristine (V) 1.4 mg/mz bolus i.v. D1
(max: 2 mg)
prednisone (P) 100 mg/m~ day oral
This cycle is repeated every 21 days up to the
maximum response
ii)- I-COPA protocol
- according to RV Smalley et al. (N. Eng. J. Med. 1992;
327: 1336-1341)
Dose Route Days
isoflavonoid 200-2000 mg/m2/day DI-DS
or 5 - 50 mg/kg/day i.v.
infusion of 1 h
cyclophosphamide 600 mg/mz/day i.v. D1
(C)
vincristine (O) 1.2 mg/mz bolus i.v. D1
(max: 2 mg)
prednisone (P) 100 mg/m~/day i.v. D1-DS
doxorubicin (A) 50 mg/m2 bolus i.v. D1
interferon-alpha 6 MU/m2 i.m. D22-D26
(I)
The cure comprises 8 to 10 cycles, at the rate
of one cycle every 28 days.
iii)- Fludarabine-CdA protocol
- according to P. Solol-Celigny et al. (Blood
1994; 84 (Supp. 1): 383a), H. Hoeschster et
al.; (Blood 1994; 84 (Suppl. 1): 564a and A.C.
Kay (J. Clin. Oncol. 1992; 10: 371-377)
CA 02337256 2001-O1-12
- 59 -
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/dayi.v. Dl-D,
infusion of 1 h
fludarabine 25 mg/m2/day i.v. D1-DS
--- infusion of 0.5
hour
or
fludarabine 20 mg/m2/day i.v. D
and cyclophos- 600 - 1000 mg/m2/dayi.v. D1
phamide
or cladribine 0.1 mg/m2/day i.v. D1-D.,
infusion of
24 hours
For fludaribine, each cycle is repeated every
28 days; for cladribine, each cycle is repeated every
35 days.
3.-2.2. of intermediate malignancy grade
i)-CHOP or CNOP protocol
- according to EM McKelvey et al. (Cancer 1976;
38: 1484 - 1493), J.O. Armitage et al.
(J. Clin. Oncol. 1984; 2: 898-902, S. Paulovsky
et al. (Ann. Oncol. 1992; 3: 205-209)
Dose Route Days
isoflavonoid 200-2000 mg/m2/day i.v. D1-DS
or 5 - 50 mg/kg/day
infusion of 1 h
cyclophosphamide 750 mg/m2/day i.v. D1
(c)
doxorubicin (H) 50 mg/m2 bolus i.v. D1
vincristine (O) 1.4 mg/m2 bolus i.v. D1
(max. 2 mg)
CA 02337256 2001-O1-12
- 60 -
prednisone (P) 100 mg/mz/day (as oral D1-DS
1 dose/day)
for the CHOP protocol
The mitoxantrone (N) may be used to replace
(CNOP protocol) the doxorubicin in patients over 60
(dose: 12 mg/m2 as an i.v. bolus on day D1 of each
cycle) .
The cure by the CHOP or CNOP protocol comprises
6 to 8 cycles at the rate of 1 cycle every 21 days.
ii) - MACOP-B protocol
- according to P. Klimo et al. (Ann. Intern.
Med. 1985; 102: 596-602) and I.A. Cooper et al.
(J. Clin. Oncol. 1994; 12: 769-778)
Dose Route Days
isoflavonoid 200-2000 mg/m2/day Dl-Ds,
or 5 - 50 mg/kg/dayi.v. Da-D12,
infusion of 1 h D~s-Daz,
D29-D33
D43-D47
i
Ds,-Dsi
D~mD~s
methotrexate (M) 100 mg/m2/bolus i.v. Da, D3s,
then
300 mg/mz infusion D64
of 4 hours
leucovorin 15 mg qid oral D9, D3.,,
Dss
doxorubicin (A) 50 mg/mz bolus i.v D1, Dls.
D29~ D43~
Ds~ ~ Dm
cyclo- 350 mg/m2 bolus i.v. D1, Ds,
phospshamide (c) DZ9, D43,
Ds7 ~ Dm
vincristine (D) 1.4 mg/m2 bolus i.v. Da, Dz2.
(max: 2 mg) D3s, Dso
Ds4 ~ D
CA 02337256 2001-O1-12
- 61 -
prednisone (P) 75 mg/day oral Every day
for 12
weeks
bleomycin 10 U/mz bolus i.v. Dzz, Dso
Die
This treatment protocol extends over 12 weeks
and corresponds to 1 cycle.
iii) - VACOP-B protocol
S - according to J.M. Connors et al. (Proc. Am.
Soc. Clin. Oncol. 1990; 9:254):
Dose Route Days
isoflavonoid 200-2000 mg/mz/day D1-Ds,
or 5 - 50 mg/kg/day i .v. De-Dlz,
infusion of 1 h Des-Dzz,
D29-D39
r
D4a -Day
Ds~-Dsi
Dm -D~s
etoposide (V) 50 mg/mz i.v. Dls D43,
Dm
etoposide 100 mg/mz oral Dls, D1~,
Daa~ Das.
D~z . D73
doxorubicin (A) 50 mg/mz bolus ' Dl, Dls,
i.v.
Dzg~ Da3.
i
Ds~ ~ Dm
cyclophosphamide 30 mg/mz day bolus De, Dzz,
i i.v.
( C ) D3s Dso,
Dsa ~ Die
vincristine (O) 1.2 mg/mz bolus i.v. De, Dzz,
Das ~ Dso
Ds4 ~ Die
CA 02337256 2001-O1-12
- 62 -
prednisone (P) 45 mg/mz/day oral 1/day for
1 week,
then
4/day the
next
11 weeks
Each cycle lasting for 12 weeks.
iv)- m-BACOD/M-BACOD protocol
- according to M.A. Shipp et al. (Ann. Int.
Med. 1986; 140: 757-765) and A.T. Skarin et al.
(J. Clin. Oncol. 1983; 1:91-98)
Dose Route Days
isoflavonoid 200-2000 mg/mz/day D1-Ds,
or 5 - 50 mg/kg/day i.v. D8-D12
infusion of 1 h Dls-D19
methotrexate 200 mg/m2 i .v De, Dls
(m) infusion of 4 hours
or
or
(M) 3000 mg/m2 infusion i.v. Dls
of 4 hours
leucovorin 10 mg/m2 qid oral D9, D16
or
(6 doses in total) Dls
bleomycin (B) 4 U/m2 bolus i.v. D1
doxorubicin (A) 45 mg/m2 day bolus i.v.
cyclophosphamide 600 mg/m2 bolus i.v. D1
(C)
vincristine (O) 1 mg/mz bolus i.v. D1
dexamethasone 6 mg/m2/day oral D3-Ds
(D)
The cure comprising 10 cycles, at the rate of
1 cycle every 21 days.
v)- ProMACE/CytaBOM protocol
- according to D.L. Longo et al. (J. Clin.
Oncol. 1991; 9: 25-38):
CA 02337256 2001-O1-12
- 63 -
Dose Route Days
isoflavonoid 200-2000 mg/m2/day Dl-D5,
or 5 - 50 mg/kg/day i.v. D8-D12
infusion of 1 h
cyclophosphamide 650 mg/m2 infusion i.v.
(C) of 0.5 hour
doxorubicin (A) 25 mg/mz bolus i.v.
etoposide 120 mg/mz infusion i.v.
of 1 hour
prednisone (P) 60 mg/day oral D1-D14
cytarabine 300 mg/m2 bolus i.v D8
bleomycin (B) 5 U/m2 bolus i.v
vincristine (O) 1.4 mg/mz bolus i.v Da
methotrexate 120 mg/mz bolus i.v D8
leucovorin 25 mg/m2 qid oral D9
(4 doses in total)
The cure comprising 6 to 8 cycles, at the rate
of one cycle every 14 days.
3.2.3. of low or intermediate malignancy grade
i)- ESHAP rescue protocol
- in case of recidivation or in case of failure
of the first line treatment, according to
W.S. Velasquez et al. (J. Clin. Oncol. 1994;
12: 1169-1176)
Dose Route Days
isoflavonoid 200-2000 mg/m2/day D1-DS
or 5 - 50 mg/kg/day i.v.
infusion of 1 h
etoposide (E) 40 mg/mz infusion i.v. D1-Dq
of
2 hours
methyl- 500 mg/day infusion i.v. D1, D4
prednisolone (S) of 15 minutes
CA 02337256 2001-O1-12
- 64 -
cytarabine (HA) 2000 mg/mz infusion i.v. DS
of 3 hours
cisplatin (P) 25 mg/m2/day bolus i.v.
continuous infusion
of 24 hours
The cure comprising 6 cycles, at the rate of
1 cycle every 28 days.
ii)- MINE rescue protocol
- in case of recidivation or in the case of failure
of the first line treatment, according to
F. Cabanillas et al. (Semin. Oncol. 1990; 17
(Suppl. 10): 28-33)
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/dayi.v. D1-DS
infusion of 1 h
ifosfamide (I) 1330 mg/m' infusioni.v. D1-D3
of 1 hour
mesna (M) 1330 mg/mz i.v. D1-D3
in the ifosfamide
i
infusion then
266 mg/m2 bolus
4 and 8 hours after
each dose of
ifosfamide
mitoxantrone (M) 8 mg/m2 infusion i.v. D1
of
15 minutes
etoposide (E) 65 mg/m2/day i.v. Dl-D3
infusion of l hour
This cycle to be repeated every 21 days.
3.3. Non-Hodgkin's lymphomas: Burkitt's lymphoma,
small cell lymphoma, lymphoblastic lymphoma
3.3.1 Magrath protocol
CA 02337256 2001-O1-12
- 65 -
- The claimed products may be combined with the
Magrath protocols according to the following
schemes:
i) - cycle 1
- according to I.T. Magrath et al. (Blood 1984;
63: 1T02-1111)
Dose Route Days
isoflavonoid 200-2000 mg/mz/day D1-D5,
or 5 - 50 mg/kg/dayi.v. De-Dlz
infusion of 1 h
cytarabine 30 mg/mZ intra- D1, D2,
thecal Dj, D,
cyclophosphamide 1200 mg/m2 bolus i.v. D1
methotrexate 12.5 mg/m2 intra- Dlo
(max: 12.5 mg) thecal
methotrexate 300 mg/mz/day i.v. Dlo-D11
infusion of 1 hour
then 60 mg/mz/h
infusion of
41 hours
leucovorin 15 mg/m2 bolus qid i.v. to be
(8 successive started
doses) 42 hours
after
the
start
of
the
admini-
stration
of
metho-
trexate
ii) - cycles 2 to 15
- according to I.T. Magrath et al. (1984) also
CA 02337256 2001-O1-12
- 66 -
Dose Route Days
isoflavonoid 200-2000 mg/m2/day Dl-DS
or 5 - 50 mg/kg/day i.v. Dlo, D11
infusion of 1 h
cytarabine 45 mg/m2 intra- D1-DZ
thecal (cycles
2
and 3 )
D1
(cycles
4
and 6)
cyclophosphamide 1200 mg/mz bolus i.v. D1
(C)
doxorubicin 40 mg/m2 bolus i.v. D1
vincristine 1.4 mg/mz bolus i.v. D1
(max: 2 mg)
methotrexate 12.5 mg/mz intra- D3, Dlo
(max: 12.5 mg) thecal (cycles
2
and 3)
Dio
(cycles
4, 5, 6)
methotrexate 300 mg/m2 infusion i.v. Dlo, D1~
of 1 hour then (cycles
2
60 mg/m2 continuous and 6)
infusion of D14, Dls
41 hours (cycles
7-15)
leucovorin 15 mg/m2 bolus qid i.v. start at
(8 consecutive the 42na
doses) hour of
the
treatment
with
metho-
trexate
the cure comprising 14 cycles, at the rate of
one cycle every 28 days.
3.4 Waldenstrom macroglobulinaemia
3.4.1 CVP protocol
CA 02337256 2001-O1-12
- 67 -
according to the CVP protocol described by
M.A. Dimopoulous et al, (Blood 1994; 83: 1452-1459) and
C.S. Portlock et al. (Blood 1976; 47: 747-756):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D1-DS
infusion of 1 h
cyclo- 300-400 mg/m2/day oral D1-DS
phosphamide (C)
vincristine (V) 1.4 mg/rn2/day bolusi.v. D1
(max: 2 mg)
prednisone (P) 100 mg/mz/day oral
the cure to be continued indefinitely (1 cycle
every 21 days).
3.4.2 Fludarabine-CdA protocol
according to H.M. Kantarjian et al. (Blood 1990;
75: 1928-1931) and M.A. Dinopoulous et al. (Ann.
Intern. Med. 1993; 118: 195-198):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D1-DS
infusion of 1 h
fludarabine 25-30 mg/mz infusioni.v. D1-DS
of 0.5 hour
or
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i.v. D1-D,
infusion of 1 h
cladribine 0.09 mg/m~/day i.v. D1-D~
(CdA) continuous infusion
the cure comprising 6 to 12 cycles 28 days
apart in the case of fludarabine and 2 cycles 28 days
apart also in the case of cladribine.
CA 02337256 2001-O1-12
- 68 -
3.5 Multiple myeloma
3.5.1 MP protocol
according to R. Alexanian et al. (JAMA 1969:
208: 1680-1685), A. Belch et al. (Br. J. Cancer 1988;
57: 94-99) and F. Mandelli et al. (N. Engl. J. med.
1990; 322: 1430-1434):
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/day i .v. D1-DS
infusion of 1 h
melphalan (M) 0.25 mg/kg/day oral D1-DQ
prednisone (P) 100 mg/day oral D1-D4
or
Dose Route Days
isoflavonoid 200-2000 mg/m2/day
or 5 - 50 mg/kg/dayi .v. Dl-DS
infusion of 1 h
melphalan (M) 9 mg/m2/day oral D1-Dq
prednisone (P) 100 mg/day oral D1-DQ
the cure comprising at least 12 cycles, at the
rate of 1 cycle every 4 to 6 weeks.
3.5.2 VAD protocol
according to B. Barlogie et al, (N. Engl. J.
Med. 1984; 310: 1353-1356):
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/dayi .v. D1-DS
infusion of 1 h
vincristine (V) 0.4 mg/day i.v. D1-D4
continuous infusion
of 24 hours
CA 02337256 2001-O1-12
- 69 -
doxorubicin (A) 9 mg/mz/day i.v. Dl-D4
continuous infusion
of 24 hours
dexamethasone 40 mg/day i.v. D1-D4,
(D)
D9-Diz ,
Dl~-Dzo
3.5.3 MP-interferon a protocol
according to 0. Osterborg et al. (Blood 1993;
81: 1428-1434):
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/day i.v. Dl-DS
infusion of 1 h
melphalan (M) 0.25 mg/kg/day oral D1-D4
prednisone (P) 2 mg/kg/day oral D1-D4
interferon- 7 MU/mz /day s . D1-DS and
alpha c . Dzz-Dzs
the cure comprising the indefinite repetition
of this cycle, at the rate of 1 cycle every 42 days.
3.5.4 VCAP or VBAP protocol
according to S.E. Salmon et al. (J. Clin.
Oncol. 1983; 1: 453-461):
VCAP protocol:
Dose Route Days
isoflavonoid 200-2000 mg/mz/day
or 5 - 50 mg/kg/dayi.v. D1-DS
infusion of 1 h
vincristine (V) 1 mg/mz bolus (max:i.v. D1
1.5 mg)
doxorubicin 30 mg/mz bolus i.v. D1
prednisone (P) 60 mg/mz/day oral
cyclophosphamide 125 mg/mz oral D1-D4
(C)
VBAP protocol: the cyclophosphamide is replaced
with carmustine (BCNU), the remainder being identical:
CA 02337256 2001-O1-12
- 70 -
-- Dose Route Days
carmustine 30 mg/mz infusion i.v.
of
1 hour
C. CHILDHOOD TUMOURS - Paediatric oncology
The isoflavonoids may also be incorporated into
the polychemotherapy protocols for treating paediatric
tumours in order to enhance the antitumour efficacy
while reducing the severity of the side effects by
means of the action on the recruitment and mobilization
of clonogenic cells and the possibility of reducing the
active doses.
1/ Ewing sarcoma/primitive neuroectodermal tumour
The isoflavonoids may be introduced in the
VCR-Doxo-CY-Ifos-Mesna-E (E. D. Bergert et al., J. Clin.
Oncol. 1990; 8: 1514-1524; W.H. Meyer et al., J. Clin.
Oncol. 1992; 10: 1737-1742):
Dose Route Days
isoflavonoid 100-200 mg/m2/day D1-Ds,
or
2 - 50 mg/kg/day i.v. Da-D11,
infusion of 1 h Dls-Dla,
Dza-Da, ,
vincristine 2 mg/m2 bolus i.v. D,, De, Dls,
(maximum dose = D43
2 mg )
doxorubicin 30 mg/m~/day i.v. D1-D3,
as an infusion of Da3-D4s
24 hours
cyclo- 2.2 g/m' as an i.v. D
phasphamide infusion of 0.5
hour
ifosfamide 1800 mg/m2/day as i .v. Dzz-Dzs
an
infusion of 1 hour D63-D6.,
CA 02337256 2001-O1-12
- 71 -
mesna 360 mg/mz as an i.v. admini-
infusion of stered with
15 minutes at the cyclophos-
rate of S doses phamide and
every 3 hours ifosfamide
etoposide 100 mg/m2 as an i .v. D22-D2s
infusion of 1 hour D63-D6.,
the cure comprises 6 to 10 of these cycles
depending on the initial severity of the sarcoma and
the extent of the response.
2/ Childhood acute lymphoblastic leukaemia
2.1. Induction chemotherapy (days Dl-D_3o)
The isoflavonoids may be added to the
recommended protocols (P. S. Gaynon et al., J. Clin.
Oncol., 1993, 11, 2234-2242; J. Pullen et al., J. Clin.
Oncol. 1993; 11: 2234-2242; J. Pullen et al., J. Clin.
Oncol. 1993; 11: 839-849; VJ Land et al., J. Clin.
Oncol. 1994; 12: 1939-1945):
Dose Route Days
isoflavonoid 100-200 mg/m2/day D1-Ds and
or
2 - 50 mg/kg/day i.v. D22-D2.,
and
infusion of 1 h D1, De,
Dls and Dz2
vincristine 1 .5 mg/mz bolus i .v. D1, D8, Dls.
(maximum dose = Dz2
2 mg )
L-asparaginase 6000 IU/m2 i.m. 3
times/week
for 3 weeks
prednisone 60 mg/m2 in oral D1 to
3 doses/day
daunorubicin 25 mg/m2day as an i.v. D1, D8, Dls
infusion of and D22
15 minutes
methotrexate depending on age intra- Dls,
the thecal
CA 02337256 2001-O1-12
- 77 -
cytarabine depending on age intra-
thecal
depending on the result of the examination of
the bone marrow, the passage to the consolidation phase
is made on day Dza of the treatment protocol.
2.2. Consolidation/maintenance chemotherapy
The isoflavonoids may be introduced in the
maintenance protocol (P. S. Gaynon et al., J. Clin.
Oncol. 1993; 11: 2234-2242; J. Pullen et al., J. Clin.
Oncol. 1993; 11: 839-849; V.J. Land et al., J. Clin.
Oncol. 1994; 12: 1939-1945) according to the following
scheme:
Dose Route Days
isoflavonoid 100-200 mg/mz/day D1-Ds, Dls-Dzo
or i.v. and D94-D9g,
2 - 50 mg/kg/day DiomDios.
infusion of 1 hour
D122-D127
cyclophos- 1000 mg/mz as an i.v. Dl, D15, Dlzz
phamide infusion of
0.5 hour
L-asparaginase 6000 U/mz i.m. 3 times/week
between D9~
and Dlzz
cytarabine 75 mg/mz/day as i.v./s.c. a sequence
an of
infusion of 4 days
15 minutes starting Dz,
Ds. Disc D23i
Diz3 ~ Di
doxorubicin 25 mg/mz/day as i.v.
an
infusion of 15
minutes
mercaptopurine 60 mg/mz/day oral D1-D93, D143
at
the end of
the treatment
CA 02337256 2001-O1-12
- 73 -
methotrexate 20 mg/mz/day oral once/week
between D3s
and D.,z and
between D143
and the end
of the
treatment
prednisone 40 mg/mz/day oral 5 consecutive
(divided into days per
3 doses/day) month between
Dlq3 and the
end of the
treatment
thioguanine 60 mg/m2/day oral D122-Dl3s
vincristine 1.5 mg/mz bolus i.v. D94, Dlol~
D
(maximum dose = then
2 mg) once/month
between D143
and the end
of the
treatment
methotrexate depending on age intra- D1, D8, Dls,
thecal D22, D123i
D130
then
once/3 months
between Dla3
and the end
of the
treatment
3/ Childhood acute myeloid leukaemia
The isoflavonoids are added to the induction
and consolidation/maintenance protocols according to
the following schemes:
3.1. Induction chemotherapy
According to Y. Ravindranath et al., J. Clin.
Oncol. 1991; 9: 572-580; M.E. Nesbit et al., J. Clin.
CA 02337256 2001-O1-12
- 74 -
Oncol. 1994; 12: 127-135; RJ Wells et al., J. Clin.
Oncol. 1994; 12: 2367-2377):
Dose Route Days
isoflavonoid 100-200 mg/m2/day
or i .v. Dl-D5,
2 - 50 mg/kg/day Dlo-D13
infusion of 1 h
cytarabine according to age intra- D1
thecal
daunorubicin 20 mg/mz/day as i.v. D1-D4,
an
infusion of Dlo-D13
24 hours
cytarabine 200 mg/m2/day as i .v. Dl-D4,
an
infusion of
24 hours
thioguanine 100 mg/m2/day oral D1-D9,
divided into
2 doses/day
etoposide 100 mg/m2/day as i.v. D1-D4,
an
infusion of Dlo-D13
24 hours
dexamethasone 6 mg/m2 divided i.v./ D1-D4,
into 3 doses/day oral Dlo-D13
this cycle being repeated from D28.
3.2. Consolidation/maintenance chemotherapy
According to Y. Ravidranath et al., J. Clin.
Oncol. 1991; 9: 572-580; M.E. Nesbit et al., J. Clin.
Oncol. 1994; 12: 127-135; R. J. Wells et al, J. Clin.
Oncol. 1994; 12: 2367-2377):
Dose Route Days
cytarabine according to age intra- D1, Dza, Dse
thecal
isoflavonoid 100-200 mg/m2/day D1-D5, D8-D13
or i.v. and D28-Dj3,
2 - 50 mg/kg/day Dss-Dsi.
infusion of 1 h D89_D94
CA 02337256 2001-O1-12
- 7r, -
cytarabine 3000 mg/mz as an i .v. Dl-Dz, and
infusion of Da-D9
3 hours every
12 hours
L-asparaginase 6000 IU/mz i.m. Dz, D
3 hours after
cytarabine
vincristine 1.5 mg/mz bolus i.v. Dza, Dss
(maximum dose =
2 mg )
thioguanine 75 mg/mz/day oral Dza-Da4
cytarabine 25 mg/mz/day bolus i.v. D
Dss-Ds9
cyclophos- 75 mg/mz/day as i.v. D
an
phamide infusion of 0.5 Dss-Ds9
hour
cytarabine 25 mg/mz/day bolus sc/i.v Da9-D93
thioguanine 50 mg/mz/day oral
etoposide 100 mg/mz/day as i.v. Dg9, D9z
an
infusion of 1 hour
dexamethasone 2 mg/mz/day oral
daunorubicin 30 mg/m' as an i.v.
infusion of
15 minutes
4/ Childhood Hodgkin's disease
The isoflavonoids may be addeed to the
MOPP-ABVD protocol according to EA Gehan et al. (Cancer
1990; 65: 1429-1437), SP Hunger et al. (J. Clin. Oncol.
1994; 12: 2160-2166) and MM Hudson et al. (J. Clin.
Oncol. 1993; 11: 100-108):
Dose Route Days
isoflavonoid 100-200 mg/mz/day
or i . D1-Ds and
v .
2 - 50 mg/kg/day De-Diz
infusion of 1 h
CA 02337256 2001-O1-12
- 76 -
mechlorethamine 6 mg/m2 bolus i.v.
(M)
vincristine (O) 1.5 mg/m2 bolus i.v. D1, DB
(maximum 2 mg)
procarbazine 100 mg/mz/day oral D1- D14
(P)
prednisone (P) 40 mg/m2/day oral
(divided into
3 doses/d)
doxorubicin (A) 25 mg/m2/day as i.v. D29, D_43
an
infusion of
15 minutes
bleomycin (B) 10 U/m2 as an i .v. D29, D43
infusion of
15 minutes
vinblastine 6 mg/mz bolus i .v. Dz9, D93
(maximum 2 mg)
dacarbazine (D) 375 mg/m2 as an i .v. D29, D4a
infusion of
15 minutes
This cycle should be repeated 6 times at the
_ rate of 1 cycle every 8 weeks, the cure comprising
6 cycles.
If an autologous bone marrow transplant
(autograft) is prescribed, the CVB protocol described
by R. Chopra et al. (Blood 1993; 81: 1137-145),
C. Wheeler et al. (J. Clin. Oncol. 1990; 8: 648-656)
and RJ Jones et al (J. Clin Oncol 1990, 8, 527-537) may
be used according to the following scheme (the
allograft taking place on day Do):
Dose Route Days
isoflavonoid 100-200 mg/m2/day
or i.v. D_~, D_1
2 - 50 mg/kg/day
infusion of 1 h
cyclo- 1800 mg/m2/day as i .v. D_,, D_6
phosphamide 2 infusions of D_5, D_Q
1 hour
CA 02337256 2001-O1-12
_ 77 _
carmustine 112 mg/mz/day as i.v. D_,, D_6
an
(BCNU) infusion of 0.5 D_5, D_Q
hour
etoposide 500 mg/mz/day as i.v. D_7, D_6
2 infusions of D-s. D-4
1 hour
5/ Childhood lymphoblastic lymphoma
The isoflavonoids may also be combined with the
induction chemotherapy protocols (A. T. Meadows et al.,
J. Clin. Oncol. 1989; 7: 92-99 - C. Patte et al . , Med.
Ped. Oncol. 1992; 20: 105-113 and A. Reiter et al.,
J. Clin. Oncol. 1995; 13: 359-372) and the maintenance
chemotherapy protocols:
5.1 Induction chemotherapy
Dose Route Days
isoflavonoid 100-200 mg/mz/day
or i .v. D1-Ds, D1.,-Dzz
2 - 50 mg/kg/day Dza-Dz9
infusion of 1 h
cyclo- 1200 mg/mz as an i.v. D1
phosphamide infusion of 0.5
hour
cytarabine according to age intra- D1
thecal
vincristine 1.5 mg/mz bolus i.v. D3, Dlo, D1~,
(maximum 2 mg) Dz4
prednisone 60 mg/mz/day oral D3-Dzs
divided into
3 doses/day
daunorubicin 60 mg/mz i.v.
as an infusion of.
15 minutes
L-asparaginase 6000 U/mz/day im D1,-Das
as an infusion of 3 times/week
15 minutes
CA 02337256 2001-O1-12
-
methotrexate according to age intra- D1,, D_3~
thecal
5.2 Maintenance chemotherapy
according to the following scheme:
Dose Route Days
isoflavonoid 100-200 mg/m2/day
or i .v. D1-D5, D15-D2o,
2 - 50 mg/kg/day Dz9-D3a
infusion of 1 h
cyclo- 1000 mg/m2 as an i.v. D1
phosphamide infusion of 0.5
hour
vincristine 1.5 mg/mz bolus oral Dl, D
(maximum 2 mg) (cycles 2 to
10)
methotrexate 300 mg/m2/day (60% i.v. Des
as an infusion of
15 minutes and 400
as an infusion of
4 hours) _
leucovorin 10 mg/m2/every 4 oral
h
daunorubicin 30 mg/m~ i.v. D29
as an infusion of
0.5 hour
methotrexate according to the intra- D1, De,
age thecal (cycle 1),
then
once/month
(cycles 2 to
10)
the cure comprising 10 cycles
6/ Paediatric neuroblastoma
The recommended polychemotherapy Doxo-E-Cy-Pt
protocol is adapted from R.P. Castleberry et al.
(J. Clin. Oncol. 1992; 10: 1299-1304), A. Garaventa et
CA 02337256 2001-O1-12
_ 79 _
al. (J. Clin. Oncol. 1993; 11: 1770-1779) and D.C. West
et al. (J. Clin. Oncol. 1992; 11: 84-90):
Dose Route Days
isoflavonoid 100-200 mg/mz/day
or i.v. Dl-Ds, Dzs-D3s,
2 - 50 mg/kg/day Dss-Dss
infusion of 1 h
doxorubicin 25 mg/m2/day as i.v. D2, D3o, Dse
an infusion of
15
minutes
etoposide 100 mg/m2 as an oral/ D2, Ds, D3o.
infusion of naso- D33, Dss, Dsl
1 hour gastric
cyclo- 1000 mg/mz as a i.v. D3, D4, Djl,
phosphamide infusion of 0.5 D3z, Dss. Dso
hour
cisplatin 60 mg/m2 as an i.v. D1, D28, Dss
infusion of
6 hours
The evaluation of the therapeutic response is
made after 9 weeks in order to decide on the attitude:
surgical resection, radiotherapy or new chemotherapy.
7/ Paediatric osteosarcoma
The isoflavonoids may be added to the
Doxo-Pt-Mtx-Lcv protocol as described by M. Hudson et
al. (J. Clin. Oncol. 1990; 8: 1988-1997), PA Meyers
(J. Clin. Oncol. 1992; 10: 5-15), and V.H.C. Bramwell
et al. (J. Clin. Oncol. 1992; 10: 1579-1591):
Dose Route Days
isoflavonoid 100-200 mg/m2/day
or i . v D1-Ds , D21-Dzs
.
2 - 50 mg/kg/day Das-D33
infusion of 1 h
doxorubicin 25 mg/m2/day as i.v. D1-D3
an
infusion of
24 hours
CA 02337256 2001-O1-12
cisplatin 120 mg/mz as an i.v. D,
infusion of
6 hours
methotrexate 12 mg/mz/day as i .v. Dzl, Dza
an
infusion of 1 hour
leucovorin 100 mg/mz oral Dzz, Dz9
every 6 hours
8/ Childhood rhabdomyosarcoma
The Vcr-Dact-CY-Mesna protocol (H. Maurer et
al., Cancer 1993; 71: 1904-1922 and LR Mandell et al.,
Oncology 1993; 7: 71-83) may include i.v. infusion of
the isoflavonoids according to the following scheme:
Dose Route Days
isoflavonoid 100-200 mg/mz/day
or i.v. D1-Ds, Da-Dlz~
2 - 50 mg/kg/day Dzz-Dz~,
infusion of 1 h D43-D4.,
vincristine 1.5 mg/mz/day i.v. D1, Da, Dls.
(bolus maximum Dzz, Dz9.
D36.
2 mg) D43, Dso.
Ds7
dactinomycin 0.015 mg/kg bolus i.v. Dl-Ds, Dzz-Dz,,
(max daily dose: D43-D4~
0.5 mg
cyclo- 2.2 g/mz as an i.v. Dl, Dzz, D43
phosphamide infusion of 1 hour
mesna 360 mg/mz as an i.v. D1, Dzz, DQa
infusion of 1 hour
every 3 hours for
5 doses
At the end of the 9th week of treatment, the
efficacy should be evaluated in order to decide on the
future course of action (surgery, radiotherapy,
continuation of the chemotherapy).
CA 02337256 2001-O1-12
- 81 -
9/ Childhood Wilms tumour
In the Vcr-Dact protocol as described by
GJ D'Angio et al. (Cancer, 1989; 64: 349-360) and DM
Green et al. (J. Clin. Oncol. 1993; 11: 91-95):
Dose Route Days
isoflavonoid 100-200 mg/m2/day
or i .v. Dl-D5, Dg-D12,
2 - 50 mg/kg/day then every
infusion of 1 h week
vincristine 2 mg/m2 bolus (max i.v. D., then every
dose: 2 mg) week
dactinomycin 0.045 mg/kg bolus i.v. D1, then
(P<_30 kg) every 3 weeks
1.35 mg/m2
(P>30 kg)
(max dose: 3 mg
This protocol being started, after the surgical
resection.
In case of autologous bone marrow transplant
(autograft) according to A. Garaventar et al. (Med.
Pediatr. Oncol. 1994; 22: 11-14), the E-Thio-Cy
protocol may be modified as follows
Dose Route Days
isoflavonoid 100-200 mg/m2/day
or i.v. D_e-D_1
2 - 50 mg/kg/day
infusion of 1 h
etoposide 1800 mg/m2 i.v. D_e
(infusion of
24 hours)
thiotepa 300 mg/m2/day as i.v. D_,, D_6, D_5
an
infusion of
2 hours
cyclo- 50 mg/kg/day as i.v. D_4, D_3, D_z,
an
phosphamide infusion of D_1
1 hour'
CA 02337256 2001-O1-12
- 82 -
the bone marrow transplant taking place on Do.