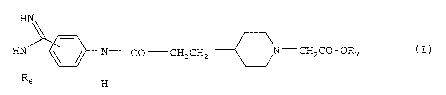Note: Descriptions are shown in the official language in which they were submitted.
CA 02337413 2001-O1-12
Boehringer Ingelheim Pharma KG Case 5/1242-FL
55216 Ingelheim :E'oreign filing text
Substituted phenylamidines, pharmaceutical compositions con-
taming these compounds and processes for preparing them
The scope of protection of WO 96/33970 includes phenylamidines
of general formula
R2
O
N C X Y--Z CO-ORS
~4
wherein inter alia R1 denotes a Cl_4-alkyloxycarbonyl group, an
aryl-Cl_3-alkyloxycarbonyl group or a group of formula
O 0
Rb C// ~C- ,
\O- (HCRa) -O
wherein
Ra denotes a hydrogen atom or an alkyl group and
Rb denotes an alkyl group or a 3- to 7-mE:mbered cycloalkyl
group, although no such compound is described in so many words
in this published application.
It has now been found that the phenylamiaines of general
formula
CA 02337413 2001-O1-12
- 2 -
HN
H ~ ~ / N CO CH2 CH2 N-- CH2 CO - OR7 ( I ) ,
R
s H
wherein
R6 denotes a Cl_1g-alkyloxycarbonyl or phenyl-Cl_4-alkyloxycar-
bonyl group,
R~ denotes a hydrogen atom, a Cl_8-alkyl, C4_.,-cycloalkyl,
phenyl-Cl_4-alkyl or Re-CO-OCHR9-group wherein
Re denotes a Cl_4-alkyl, Cl_4-alkoxy, C3_.,-cycloalkyl or
C4_,-cycloalkoxy group and
R9 denotes a hydrogen atom or a Cl_4-alkyl. group,
the tautomers, stereoisomers and salts thereof, particularly
the physiologically acceptable salts thereof with inorganic or
organic acids or bases, have even more valuable pharmacological
properties, preferably antithrombotic effects.
The present invention relates to the compounds of the above
general formula I, the tautomers, stereoisomers and salts
thereof, particularly the physiologically acceptable salts
thereof with inorganic or organic acids or bases, pharmaceu-
tical compositions containing these compounds, their use and
processes for preparing them.
Preferred compounds of the above general formula I are those
wherein
the substituted amidino group is in the 4 position,
CA 02337413 2001-O1-12
- 3 -
particularly those compounds wherein
R6 denotes a Cl_18-alkyloxycarbonyl or phenyl-Cl_4-alkyloxycar-
bonyl group and
R, denotes a hydrogen atom, a Cl_8-alkyl, CS_~-cycloalkyl or phe-
nyl-Cl_4-alkyl group,
the tautomers, stereoisomers and salts thereof.
Particularly preferred compounds of the <~bove general formula z
are those wherein
R6 denotes a C1_lz-alkyloxycarbonyl or phenyl-C1_2-alkyloxycar-
bonyl group and
R., denotes a Cl_$-alkyl or CS_,-cycloalkyl group,
the tautomers, stereoisomers and salts thereof.
Most particularly preferred compounds of the above general for-
mula I are those wherein
R6 denotes a CS_12-alkyloxycarbonyl or benzyloxycarbonyl group
and
R., denotes a Cl_4-alkyl or CS_6-cycloalkyl group,
the tautomers, stereoisomers and salts thereof.
The following are mentioned as examples of particularly
preferred compounds:
(1) 4- [2- [ [4- (octyloxycarbonylamidino) phenyl] amino-carbonyl] -
ethyl]-1-[(ethoxycarbonyl)methyl]-piperidine,
CA 02337413 2001-O1-12
- 4 -
(2) 4- [2- [ [4- (hexyloxycarbonylamidino)phenyl] amino-carbonyl] -
ethyl] -1- [ (ethoxycarbonyl)methyl] -piperidine,
( 3 ) 4 - [ 2 - [ [ 4 - ( hexyloxycarbonyl amidino ) phf~nyl I amino -
carbonyl ] -
ethyl] -1- [ (methoxycarbonyl) methyl] -piper:idine and
(4) 4- [2- [ [4- (octyloxycarbonylamidino)phenyl] amino-carbonyl] -
ethyl]-1-[(methoxycarbonyl)methyl]-piperidine
and the salts thereof.
According to the invention the new compounds of general formula
I are obtained, for example, by the following method:
reacting a compound of general formula
HN
HN \ ~ NH2 ( I I ) ,
R6
wherein
R6 is as hereinbefore defined, with a compound of general for-
mula
HO- CO CH CH N-CHZCO-OR7 ( I I I ) ,
2 2
wherein
R., is as hereinbefore defined, or a reactive derivative thereof
and
optionally subsequently converting the croup R7 into a hydrogen
atom.
CA 02337413 2001-O1-12
- 5 -
Examples of reactive derivatives of a compound of general
formula III include the acid chlorides, acid azides, mixed
anhydrides with aliphatic or aromatic carboxylic acids or
monocarbonates, imidazolides and esters 'thereof such as the
alkyl, aryl and aralkyl esters, e.g. the methyl, ethyl,
isopropyl, pentyl, phenyl, nitrophenyl o:r benzyl esters.
The reaction is expediently carried out in a solvent or mixture
of solvents such as methylene chloride, dimethylformamide, di-
methylsulphoxide, benzene, toluene, chlorobenzene, tetrahydro-
furan, pyridine, pyridine/methylene chloride, pyridine/dime-
thylformamide, benzene/tetrahydrofuran or dioxane, optionally
in the presence of a dehydrating agent, e.g. in the presence of
isobutyl chloroformate, thionylchloride, trimethylchlorosilane,
sulphuric acid, methanesulphonic acid, p-toluenesulphonic acid,
phosphorus trichloride, phosphorus pentoxide, N,N'-dicyclohe-
xylcarbodiimide, N,N'-dicyclohexylcarbodiimide/N-hydroxysuc-
cinimide, 2-(1H-benzotriazolyl)-1,1,3,3-tetramethyl-uronium
salts, N,N'-carbonyldiimidazole, N,N'-thionyldiimidazole,
2-chloro-1-methylpyridinium iodide or triphenylphosphine/carbon
tetrachloride, optionally in the presence of dimethylaminopyri-
dine or 1-hydroxy-benzotriazole and/or a base such as triethyl-
amine, N-ethyl-diisopropylamine, pyridine or N-methyl-morpho-
line, expediently at temperatures between -10 and 180°C, prefe-
rably at temperatures between 0 and 120°C.
The subsequent conversion of the group R., into a hydrogen atom
is expediently carried out either in the presence of an acid
such as hydrochloric acid, sulphuric acid, phosphoric acid,
acetic acid, trichloroacetic acid, trifluoroacetic acid or
mixtures thereof or in the presence of a. base such as lithium
hydroxide, sodium hydroxide or potassium hydroxide in a sui-
table solvent such as water, water/metha.nol, water/ethanol,
water/isopropanol, methanol, ethanol, wa.ter/tetrahydrofuran or
water/dioxane at temperatures between -1.0 and 120°C, e.g. at
temperatures between ambient temperature: and the boiling tem-
perature of the reaction mixture.
CA 02337413 2001-O1-12
- 6 -
Moreover, the compounds of general formula I obtained may op-
tionally be resolved into their enantiomers and/or diastereo-
mers, as mentioned hereinbefore. Thus, f:or example, cis/trans
mixtures may be resolved into their cis and traps isomers, and
compounds with at least one optically acaive carbon atom may be
separated into their enantiomers.
Thus, for example, the cis/trans mixturE:s obtained may be re-
solved by chromatography into the cis and traps isomers
thereof, the compounds of general formula I obtained which
occur as racemates may be separated by methods known per se
(cf. Allinger N. L. and Eliel E. L. in "Topics in Stereochemi-
stry", Vol. 6, Wiley Interscience, 1971) into their optical
antipodes and compounds of general formula I with at least 2
asymmetric carbon atoms may be resolved into their diastereo-
mers on the basis of their physical-chemical differences using
methods known per se, e.g. by chromatography and/or fractional
crystallisation, and, if these compound~~ are obtained in race-
mic form, they may subsequently be resolved into the enantio-
mers as mentioned above.
The enantiomers are preferably separated by column separation
on chiral phases or by recrystallisation from an optically
active solvent or by reacting with an optically active sub-
stance which forms salts or derivatives such as e.g. esters or
amides with the racemic compound, particularly acids and the
activated derivatives or alcohols thereof, and separating the
diastereomeric mixture of salts or derivatives thus obtained,
e.g. on the basis of their differences i.n solubility, whilst
the free antipodes may be released from the pure diastereomeric
salts or derivatives by the action of suitable agents. Opti-
cally active acids in common use are e.c~. the D- and L-forms of
tartaric acid or dibenzoyltartaric acid, di-o-tolyltartaric
acid, malic acid, mandelic acid, camphox-sulphonic acid, glu-
tamic acid, aspartic acid or quinic acid. An optically active
alcohol may be for example (+) or (-)-menthol and an optically
CA 02337413 2001-O1-12
- 7 _
active acyl group in amides, for example:, may be a (+)- or
(-)-menthyloxycarbonyl.
Furthermore, the compounds of formula I obtained may be con-
s verted into the salts thereof, particularly for pharmaceutical
use into the physiologically acceptable salts with inorganic or
organic acids. Acids which may be used f:or this purpose include
for example hydrochloric acid, hydrobromic acid, sulphuric
acid, methanesulphonic acid, phosphoric acid, fumaric acid,
succinic acid, lactic acid, citric acid, tartaric acid or
malefic acid.
Moreover, if the new compounds of formula I thus obtained con-
tain a carboxy group, they may subsequently, if desired, be
converted into the salts thereof with inorganic or organic
bases, particularly for pharmaceutical use into the physiologi-
cally acceptable salts thereof. suitable: bases for this purpose
include for example sodium hydroxide, potassium hydroxide,
arginine, cyclohexylamine, ethanolamine, diethanolamine and
triethanolamine.
The compounds used as starting material: are known from the
literature in some cases or may be obtained by methods known
from the literature (cf. the Examples).
As already mentioned, the new phenylamidines of general formula
I and the salts thereof, particularly the physiologically ac-
ceptable salts thereof with inorganic ox- organic acids or
bases, have valuable properties. Thus, when administered oral-
1y, the new compounds of general formula I produce high and
long-lasting plasma levels compared with the aggregation-inhi-
biting compound 4-[2-[(4-amidinophenyl)aminocarbonyl]ethyl]-
1-carboxymethyl-piperidine (compound A) described in
WO 96/33970. Thus, the new phenylamidine s of general formula I
and the salts thereof have valuable pharmacological properties,
not only an anti-inflammatory effect and an inhibitory effect
CA 02337413 2001-O1-12
on bone degradation but particularly antithrombotic, antiag-
gregatory and tumour- or metastasis-inhibiting effects.
For example, the plasma concentration of compound A after oral
administration of the compounds of Examples 1 (compound B) and
1(1) (compound C) of the present invention was measured and
compared with the plasma concentration of compound A after oral
administration of compound A.
After the oral administration of 1 mg/kg~ of the test compounds
to Rhesus monkeys, the concentration of compound A in the
plasma was measured 4, 8, 12 and 24 hours after the admini-
stration of the substance. To do this th.e Rhesus plasma was
incubated with a suspension of human thrombocytes in plasma and
3H-BIBU 52 (cf. DE-A-4,214,245) as ligand. The free and bound
ligand is separated by centrifuging and quantitatively determi-
ned by scintillation counting. The concentration of compound A
is calculated from the quantity of bound. ligand using a cali-
bration curve.
For this purpose donor blood is taken from an anticubital vein
and anticoagulated with trisodium citrate (final concentration:
13 mmol/1). The blood is centrifuged for 10 minutes at 170 x g
and the supernatant platelet-rich plasma (PRP) is removed. The
.. 25 residual blood is sharply centrifuged off again at 3200 x g and
the supernatant platelet-poor plasma (PPP) is removed.
For the calibration curve for calculating the concentration,
5 ~.l of a solution of compound A is added to 995 ~.l PPP (final
concentration 5000 nmol/1). Further samples of this plasma are
diluted with PPP to a final concentration of 2.5 nmol/1.
To 150 ~.1 of plasma sample from Rhesus monkeys or calibration
curve plasma are added 10 ~.l of 3H-BIBU 52 (final concentration
10 nmol/1) , 10 ~.1 of 14C-sucrose (370 Bq) and 80 ~Cl of PRP and
the mixture is incubated at ambient temperature for 20 minutes.
Then the samples are centrifuged at 2000 x g for 5 minutes and
CA 02337413 2001-O1-12
_ 9 _
the supernatant is removed. 100 ~.l of the supernatant are mixed
with 100 ~.1 of NaOH 0.2 moll, 15 ~Cl of HCl 5 mol/1 and 2 ml of
scintillator and the 3H and 14C radioactivity is measured quan-
titatively. The pellet is dissolved in 200 ~Cl of NaOH 0.2
moll. 180 ~.l thereof are mixed with 15 ~,l of HCl 5 mol/1 and
2 ml of scintillator and the 3H and 14C radioactivity is mea-
sured. The residual plasma remaining in the pellet is deter-
mined from the 14C content and removed. The quantity of bound
ligand is determined from the 3H content. The quantity of bound
ligand is plotted against the concentration of the calibration
curve plasma. The concentration of compound A in the Rhesus
plasma is calculated from the quantity of bound ligand in the
relevant plasma sample compared with the calibration curve.
The following Table contains the result::
Substance cone. of cone. of A cone. of cone. of
A in [nM] , A A
in [nM] , 8h in [.nM] in [nM] ,
4h , 24h
12h
A 174 38 25 3
B 133 127 81 51
C 170 123 80 52
In view of their biological properties the new compounds of
general formula I according to the invention and their physio-
logically acceptable salts are suitable for fighting or preven-
ting diseases in which larger or smaller cell aggregations
occur or in which cell-matrix interactions are involved, e.g.
in combating or preventing venous and arterial thromboses,
cerebrovascular diseases, pulmonary embolisms, cardiac infarct,
arteriosclerosis, osteoporosis and tumour metastasis and for
treating genetically caused or acquired disorders of cell in-
teractions with one another or with solid structures. Moreover,
they are suitable for parallel therapy in thrombolysis using
fibrinolytics or vascular interventions such as transluminal
CA 02337413 2001-O1-12
- 10 -
angioplasty or in the treatment of states of shock, psoriasis,
diabetes and inflammation.
For fighting or preventing the abovement.ioned diseases the
dosage is between 0.1 ~.g and 30 mg/kg of body weight, prefe-
rably 1 ~,g to 15 mg/kg of body weight, taken up to 4 times a
day. For this, the compounds of formula I prepared according to
the invention, optionally in conjunction with other active sub-
stances such as thromboxane receptor antagonists and thrombo-
xane synthesis inhibitors or combinations thereof, ADP-receptor
antagonists, clopidogrel, ticlopidine, ~;erotonin antagonists,
a-receptor antagonists, alkylnitrates such as glycerol trini-
trate, phosphodiesterase inhibitors, prostacycline and the ana-
logues thereof, fibrinolytics such as tPA, prourokinase, uroki-
nase, streptokinase, or anticoagulants ~>uch as heparin, derma-
tane sulphate, activated protein C, vitamin K antagonists,
hirudine, inhibitors of thrombin or other activated clotting
factors, may be incorporated, together with one or more inert
conventional carriers and/or diluents, e.g. with corn starch,
lactose, glucose, microcrystalline cellulose, magnesium
stearate, polyvinylpyrrolidone, citric acid, tartaric acid,
water, water/ethanol, water/glycerol, water/sorbitol, water/
polyethyleneglycol, propyleneglycol, stearylalcohol, carboxy-
methylcellulose or fatty substances such as hardened fat or
suitable mixtures thereof, in conventional galenic preparations
such as plain or coated tablets, capsules, powders, suspen-
sions, solutions, sprays or suppositoriE~s.
The following Examples are intended to illustrate the invention
more fully:
CA 02337413 2001-O1-12
- 11 -
Preparation of the starting compounds:
~~y~yca_rbon~r.l_amidino) -ani1in~
5.1 g of 4-(hexyloxycarbonylamidino)-nitrobenzene are hydro-
genated in 100 m1 of tetrahydrofuran in the presence of 0.5 g
of palladium on activated charcoal at ambient temperature under
a hydrogen pressure of 50 psi. Then the catalyst is removed by
suction filtering and the filtrate is concentrated by evapora-
tion.
Yield: 4.5 g 100 0 of theory,
Rf value: 0.23 (silica gel; cyclohexane/ethyl acetate = 1:1)
The following compounds are obtained analogously to Example I:
(1) 4-(octyloxycarbonylamidino)-aniline
Rf value: 0.25 (silica gel; cyclohexane/ethyl acetate = 1:1)
(2) 4-(methoxycarbonylamidino)-aniline
Rf value: 0.35 (silica gel; methylene chloride/methanol - 9:1)
(3) 4-(benzyloxycarbonylamidino)-aniline
Rf value: 0.35 (silica gel; methylene ch=Loride/methanol/conc.
aqueous ammonia = 9:1:0.1)
F~xamp 1 P T T
4- (hex~rl o~~rcarhonyl_ami i no) -ni rob n ,
2.8 ml of hexyl chloroformate in 80 ml of tetrahydrofuran are
added dropwise to 3.5 g of 4-nitrobenzam~idine-hydrochloride and
7.2 g of potassium carbonate in a mixture of 350 ml tetrahydro-
furan and 70 ml water, whilst cooling with ice. After 1 hour's
stirring in an ice bath the mixture is left to stand overnight
at ambient temperature. The organic pha"e is separated off,
CA 02337413 2001-O1-12
- 12 -
washed twice with saturated saline solution, dried and concen-
trated by evaporation.
Yield: 5.1 g (100 % of theory),
Rf value: 0.72 (silica gel; cyclohexane/ethyl acetate = 1:1)
The following compounds are obtained analogously to Example II:
(1) 4-(octyloxycarbonylamidino)-nitrobenzene
mass spectrum: M+ - 321
(2) 4-(methoxycarbonylamidino)-nitrobenzene
melting point: 183-185°C
(3) 4-(benzyloxycarbonylamidino)-nitrobe:nzene
Rf value: 0.88 (silica gel; methylene chloride/methanol/conc.
aqueous ammonia = 9:1:0.1)
4- [2- (chlorocarbonyl) ethyl] -1- [ (ethoxycarbonyl) methyl] -
To 1.46 g of 4-(2-carboxyethyl)-1-[(ethoxycarbonyl)methyl]-
piperidine in 10 ml of methylene chloride is added 1 m1 of
saturated ethereal hydrochloric acid. 1.2 g of thionyl chloride
are added and the mixture is stirred for 3 hours at ambient
temperature. The reaction mixture is concentrated by evapora-
tion and the residue is mixed twice with toluene and again
concentrated by evaporation. The crude product is reacted
further in Example 1 without purification.
The following compounds are obtained analogously to Example
III:
( 1 ) 1- [2 - ( chlorocarbonyl ) ethyl ] -4 - [ ( methoxycarbonyl ) -methyl ] -
piperidine-hydrochloride
CA 02337413 2001-O1-12
- 13 -
(2) 4- [2- (chlorocarbonyl) ethyl] -1- [ (cyclohexyloxycarbonyl) -
methyl]-piperidine-hydrochloride
(3) 4- [2- (chlorocarbonyl) ethyl] -1- [ (isopropoxycarbonyl) -
methyl]-piperidine-hydrochloride
10 g of 4- [2- (benzyloxycarbonyl) ethyl] -1.- [ (ethoxycarbonyl-
)methyl]-piperidine are hydrogenated in 150 ml of tetrahydro-
furan for 4 hours at ambient temperature: under a hydrogen
pressure of 50 psi in the presence of 1.3 g of palladium on
activated charcoal. The reaction mixture: is concentrated by
evaporation and crystallised with dieth~.~lether and a little
acetone.
Yield: 5.8 g of (79 % of theory),
melting point: 65-67°C
The following compounds are obtained analogously to Example IV:
(1) 4-(2-carboxyethyl)-1-[(cyclohexyloxycarbonyl)methyl]-pipe-
ridine
_ 25 melting point: 85-88°C
(2) 4-(2-carboxyethyl)-1-[(isopropoxycarbonyl)methyl]-piperi-
dine
Rf value: 0.41 (Reversed Phase silica gel; methanol/5o saline
- 6:4)
(3) 4- (2-carboxyethyl) -1- [ (methoxycarbonyl)methyl] -piperidine
melting point: 82-83°C
CA 02337413 2001-O1-12
- 14 -
4- [2- (benzyloxycarbonyl) ethyl] -1- [ (ethoxycarbonyl) methyl] -
piperidine
6.35 g of ethyl bromoacetate in 20 ml of acetonitrile are added
dropwise, with stirring, to 9.0 g of 4-[2-(benzyloxycarbonyl)-
ethyl]-piperidine and 5.2 g of N-ethyl-<iiisopropylamine in
70 ml of acetonitrile, in an ice bath, <~nd the mixture is stir-
red for 18 hours at ambient temperature.. The reaction mixture
is concentrated by evaporation and the residue is quickly dis-
tributed between tert.butylmethyl ether,, ice water and 10 ml of
2N sodium hydroxide solution. The organ_Lc phase is separated
off, washed with ice water and saturated saline, dried and con-
centrated by evaporation.
Yield: 10.05 g of (83 % of theory),
Rf value: 0.84 (silica gel; methylene ch.loride/methanol/conc.
aqueous ammonia = 95:5:1)
The following compounds are obtained analogously to Example V:
(1) 4- [2- (benzyl oxycarbonyl) ethyl] -1- [ (cyclohexyloxycarbonyl) -
methyl]-piperidine
Rf value: 0.47 (silica gel; methylene ch.loride/methanol/conc.
aqueous ammonia = 98:2:0.5).
(2) 4- [2- (benzyloxycarbonyl) ethyl] -1- [ (isopropoxycarbonyl) -me-
thyl]-piperidine
mass spectrum: M* - 347
(3) 4- [2- (benzyloxycarbonyl) ethyl] -1- [ (rnethoxycarbonyl) -me-
thyl]-piperidine
Rf value: 0.40 (silica gel; methylene ch.loride/methanol/conc.
aqueous ammonia = 9:1:0.1)
CA 02337413 2001-O1-12
- 15 -
4- r2- rb~yl oxyca_rbonyl ~ ethyl ] -~i_ ri i
9.7 g of 4-(2-carboxyethyl)piperidine-hvydrochloride (melting
point: 240-250°C, prepared by hydrogenating 3-(4-pyridyl)-
acrylic acid in glacial acetic acid in the presence of platinum
oxide and subsequently treating with hydrochloric acid), 30 ml
of benzylalcohol, 3 g of p-toluenesulphonic acid and 50 ml of
toluene are heated for 75 minutes using a water separator. The
reaction mixture is concentrated by evaporation in vacuo, the
residue is mixed with 50 ml of ice water and extracted three
times with tert.butylmethyl ether. The .aqueous phase is made
alkaline and extracted with tert.butylm~=thyl ether. The extract
is washed with saline, dried and concentrated by evaporation.
Yield: 9.0 g of (73 % of theory),
Rf value: 0.18 (silica gel; methylene chloride/methanol/conc.
aqueous ammonia = 95:5:1)
Pre= ara i on of h . n omno in
4- [2- [ [4- (octyloxycarbonylamidinophenyll -aminocarbonyl] ethyl] -
l- f (ethox~rc~arhon~rl m hurl ] -~i z .ri di n
5.7 g of 4-[2-(chlorocarbonyl)ethyl]-1-[(ethoxycarbonyl)methyl-
piperidine-hydrochloride in 30 ml of methylene chloride are ad-
ded dropwise, within 30 minutes, to 5.1 g of 4-(octyloxycar-
bonylamidino)aniline and 100 mg of 4-dimethylaminopyridine in
30 ml of pyridine whilst cooling with i~~e. After standing over-
night at ambient temperature the reaction mixture is concentra-
ted by evaporation and the residue is purified by chromatogra-
phy over a silica gel column with methylene chloride/methanol/
conc. aqueous ammonia (9:1:0.1).
Yield: 2.9 g (32 0 of theory),
melting point: 151-153°C
mass spectrum: (M+H)' - 517
CA 02337413 2001-O1-12
- 16 -
The following compounds are obtained analogously to Example l:
( 1 ) 4 - [ 2 - [ [4 - ( hexyloxycarbonylamidino ) phenyl ] -aminocarbonyl ] -
ethyl] -1- [ (ethoxycarbonyl) methyl] -piperidine
melting point: 151-153°C
mass spectrum: M+ - 489
(2) 4- [2- [ [4- (hexyloxycarbonylamidino) phenyl] -aminocarbonyl] -
ethyl]-1-[(isopropoxycarbonyl)methyl]-piperidine
melting point: 161-162°C
Rf value: 0.20 (silica gel; methylene chloride/methanol/conc.
aqueous ammonia = 9:1:0.1)
(3) 4- [2- [ [4- (octyloxycarbonylamidino)phenyl] -aminocarbonyl] -
ethyl] -1- [ (isopropoxycarbonyl) methyl] -pi_peridine
melting point: 151-152°C
mass spectrum: M+ - 531
2 0 ( 4 ) 4 - [ 2 - [ [4 - ( hexyloxycarbonylamidino ) phenyl ] -aminocarbonyl
] -
ethyl]-1-[(cyclohexyloxycarbonyl)methyl]-piperidine
melting point: 149-151°C
mass spectrum: (M+H)+ - 543
2 5 ( 5 ) 4 - [ 2 - [ [ 4 - ( octyloxycarbonylamidino ) phenyl ] -
aminocarbonyl ] -
ethyl]-1-[(cyclohexyloxycarbonyl)methyl]-piperidine
melting point: 157-162°C
mass spectrum: (M+H)~ - 571
3 0 ( 6 ) 4 - [ 2 - [ [ 4 - ( hexyloxycarbonylamidino ) phenyl ] -
aminocarbonyl ] -
ethyl] -1- [ (methoxycarbonyl) methyl] -piperidine
melting point: 153-155°C
Rf value: 0.32 (silica gel; methylene chloride/methanol/conc.
aqueous ammonia = 9:1:0.1)
CA 02337413 2001-O1-12
- 17 -
( 7 ) 4 - [ 2 - [ [4 - ( octyloxycarbonylamidino ) phenyl ] -aminocarbonyl ] -
ethyl]-1-[(methoxycarbonyl)methyl]-piperidine
melting point: 149-150°C
mass spectrum: (M+H)+ - 503
(8) 4- [2- [ [4- (methoxycarbonylamidino)phe:nyl] -aminocarbonyl] -
ethyl] -1- [ (ethoxycarbonyl) methyl] -piperi.dine
melting point: 175-177°C
mass spectrum: (M+H)+ - 419
(9) 4- [2- [ [4- (benzyloxycarbonylamidino)~>henyl] -aminocarbonyl] -
ethyl]-1-[(methoxycarbonyl)methyl]-piperidine
melting point: 150-152°C
Instead of the carboxylic acid chloride the corresponding car-
boxylic acid is used in the presence of N-methyl-morpholine and
2-chloro-1-methylpyridinium iodide in di.methylformamide.
(10) 4- [2- [ [4- (decyloxycarbonylamidino)phenyl] -ami.nocarbonyl] -
ethyl] -1- [ (ethoxycarbonyl)methyl] -piperi.dine
(11) 4- [2- [ [4- (dodecyloxycarbonylamidino) phenyl] aminocar-
bonyl] -ethyl] -1- [ (ethoxycarbonyl)methyl] -piperidine
(12) 4- [2- [ [4- (tetradecyloxycarbonylamic~ino) phenyl] -amino-
carbonyl]-ethyl]-1-[(ethoxycarbonyl)methyl]-piperidine
(13) 4- [2- [ [4- (hexadecyloxycarbonylamidi.no)phenyl] -amino-
carbonyl]-ethyl]-1-[(ethoxycarbonyl)methyl]-piperidine
(14) 4- [2- [ [4- (octadecyloxycarbonylamidi_no)phenyl] -amino-
carbonyl] -ethyl] -1- [ (ethoxycarbonyl)methyl] -piperidine
CA 02337413 2001-O1-12
- 18 -
4- [2- [ [4- (hexyloxycarbonylamidino) phenyl] aminocarbonyl] -
1,228 ml of a 1-molar solution of methanesulphonic acid in ace-
tone are added dropwise to 600 mg of 4-[2-[[4-(hexyloxycar-
bonylamidino)phenyl]aminocarbonyl]-ethyl]-1-[(ethoxycarbonyl)-
methyl]-piperidine in 30 ml of acetone. After standing over-
night and triturating with a glass rod a solid is obtained
which is suction filtered and washed twice with acetone. The
solid is then dried in vacuo at 40°C.
Yield: 560 mg (78 a of theory),
melting point: 117-120°C
Calc.: C 55.46 H 7.58 N 9.58 S 5.48
Found: 55.56 7.85 9.72 5.54
The following compound is obtained analogously to Example 2:
(1) 4- [2- [ [4- (octyloxycarbonylamidino) phenyl] -aminocar-
bonyl]ethyl]-1-(ethoxycarbonyl)methyl]-;piperidine methane-
sulphonate
melting point: 125-127°C
Calc.: C 56.84 H 7.90 N 9.14 S 5.23
Found: 56.94 7.88 9.32 5.27
30 Composition:
(1) Active substance 50.0 mg
(2) Lactose 98,0 mg
(3) Maize starch 50.0 mg
(4) Polyvinylpyrrolidone ' 15.0 mg
(5) Magnesium stearate 2.
215.0 mg
CA 02337413 2001-O1-12
- 19 -
Preparation:
(1), (2) and (3) are mixed together and granulated with an
aqueous solution of (4). (5) is added to the dried granulated
material. From this mixture tablets are pressed, biplanar,
faceted on both sides and with a dividing notch on one side.
Diameter of the tablets: 9 mm.
Exam= le 4
Preparation:
(1) Active substance 350.0 mg
( 2 ) Lactose 13 ~5 . 0 mg
(3) Maize starch 80.0 mg
(4) Polyvinylpyrrolidone 30.0 mg
(5) Magnesium stearate 4.b mg
600.0 mg
(1), (2) and (3) are mixed together and granulated with an
aqueous solution of (4). (5) is added to the dried granulated
_ 25 material. From this mixture tablets are pressed, biplanar,
faceted on both sides and with a dividing notch on one side.
Diameter of the tablets: 12 mm.
Composition:
(1) Active substance 50.0 mg
(2) Dried maize starch 58.0 mg
CA 02337413 2001-O1-12
- 20 -
(3) Powdered lactose 50.0 mg
(4) Magnesium stearate
160.0 mg
Preparation:
(1) is triturated with (3). This trituration is added to the
mixture of (2) and (4) with vigorous mixing.
This powder mixture is packed into size 3 hard gelatine capsu-
les in a capsule filling machine.
Composition:
(1) Active substance 350..0 mg
(2) Dried maize starch 46..0 mg
(3) Powdered lactose 30..0 mg
4) ium stearate ~.~ mg
M
( agnes
430.0 mg
Preparation:
(1) is triturated with (3). This tritu:ration is added to the
mixture of (2) and (4) with vigorous mixing.
This powder mixture is packed into size 0 hard gelatine
capsules in a capsule filling machine.