Note: Descriptions are shown in the official language in which they were submitted.
14 fl7 2flfJfl ~~ i lt'7~C991QL~4~2 ' p~SC
WO 00/06079 , , , , .. . ~ ~ ~ ~ ~ . . ~ Y .t.
C ~4h9~~G~~_
1 . . . . . . . ..
.... » . . . . . . .
..
... . .. . .. ..
A bag or a bag system for collecting and storing blood
and a method therefor
The present invention relates to a bag or a bag system
for collecting and storing blood and a method for col
lecting and storing blood.
It is well mown to extract blood from human beings and
store this blood in a frozen or refrigerated condition
for later use during operations, blood transfusions, and
the like. For this purpose a number of different blood
bags have been provided.
In the past few years it has become known that blood from
the umbilical cord and the placenta at birth contains
stem cells, cells that are only present in the blood at
this specific time of a person's life. These cells have
shown a considerable potential curative value, e.g. for
use in bone marrow replacement procedures in cancer
treatment and other immunodeficiencv disorders.
In order to preserve these cells, blood from the umbili-
cal cord and/or the placenta, which would otherwise just
have been discarded, has been collected and stored at low
temperatures. The containers used in this procedure are
known to be conventional blood bags, as those mentioned
above_
However, in order to ta~~e a sample from a bag with frozen
blood, it is necessary to defrost the entire amount of
blood, and once defrosted it must either be used shortly
after or discarded, since it is not refreezable. The
problem regarding the conventional bags and methods is
therefore that the chance of using the stem cells to cure
a patient is limited to only once.
~~ 5e a ~~ ~l a:.
AMENDED SHEET;
R~'~~"~t~~ ~CA 02338604 2001-O1-25
'~1t
14 ~ ~ ?fl(~fl ~~T1~~9~14fl~:22 .. . . . . .. . .
:: .: .: : . . . . . . ,
. . . . . . . . . . .. ,
.... . . . . . . . .
. . . . . . . ..
... . .. . .. ..
la
EP 0 790 051 ~:2 discloses a flexible plastic container.
The container has at least four compartments. Each
compartment has its own inlet opening. An outlet feeds
the liquids into the body into the intestines or via any
other way. The compartments can be interconnected by
valves, which can be opened from the outside. By
selectively opening these valves, liquids in the
compartments may be completely mixed.
AMENDED SHEE'~,
~~"~~~~ CA 02338604 2001-O1-25
WO 00/06079 PCT/DK99/00422
2
JP 10000229A discloses a bag system for isolating stem
cells and precursor cells from umbilical cord blood by
centrifugation. This system consisting of 6 bags equipped .
with connectors and tubes to lead the blood from bag to
S bag, comprises one bag wherein the obtained leucocyte ,
component containing the above mentioned cells are fro-
zen. The problem relating to this bag is, however, the
same, as it is not possible to defrost part of the cells
without destroying the rest.
It is therefore the object of the present invention to
provide a solution to the problem defined above, and in '-
particular to provide a bag or a bag system for collect-
ing and storing blood, in particular umbilical cord blood
and/or blood from the placenta, designed to make it pos
sible to defrost only a part of the stored blood.
The invention relates to a bag or a bag system for col-
lecting and storing blood as defined in claim l, a bag or
a bag system for collecting and storing blood in combina-
tion with a mixing bag as defined in. claim 4, and a bag
or a bag system for collecting, mixing, and storing blood
as defined in claim 5. ,.
A bag or a bag system according to the present invention
may contain a system of storage chambers alone, equipped
only with an opening for receiving blood, or the opening
for receiving blood may comprise an inlet channel. This
inlet channel can be a small tube suitable to be con-
nected to a mixing chamber adapted with e.g. a snap lock,
a clamp or a similar easy operational closing device.
Another aspect of the invention is a bag or a bag system
for collecting and storing blood in combination with a
mixing bag, where the mixing bag comprises at least one
mixing chamber, an opening for receiving blood and an
CA 02338604 2001-O1-25
WO 00/06079 PCT/DK99/00422
3
opening for discharging blood. Regarding the mixing bag,
the opening for receiving blood and the opening for dis-
charging blood may be the same. 'flue opening for discharg-
ing blood is adapted to be connected to the opening for
receiving blood in the bag or the bag system for collect-
ing and storing blood, e.g. by use of suitable connec-
tors.
A further aspect of the invention is a bag or a bag sys-
tem for collecting and storing blood comprising a mixing
chamber, where said mixing chamber can be sealed off from
the storage chambers by closing e.g. a snap loci: or by
using a clamp or by welding. In order to minimise the
amount of equipment to be stored, it is preferred that
the mixing chamber is arranged in such a relation to the
storage chambers so that it can be ~>ealed off therefrom
and removed before storage.
A mixing chamber of a bag or a bag system according to
o-
the invention should have a volume which corresponds to
or is bigger than the sum of the volumes of the primary
and secondary storage chambers. In order to use the mix-
ing chamber for mixing the blood with anticoagulation
agent and/or preserving agent, the mixing bag should
preferably have a volume at least twi~~e as big as the sum
of tine volumes of the primary and secondary storage cham-
bers. The mixing chamber may a.g. h<~ve a volume in the
range of 100 to 500 ml, preferably ;?25 to 900 ml, more
preferably 250 to 300 mI. In a preferred embodiment the
mixing chamber may e.g. have a volume in the range of 100
to 500 ml, preferably 125 to 900 ml, more preferably 150
to 250 ml.
The mixing chamber may further comprise at least one port
for injection of anticoagulation and/or preserving agents
CA 02338604 2001-O1-25
WO 00/06079 PC'T/DK99/00422
4
and/or at least one port for extracting blood in order to
perform tests, e.g. to detect contamination by vira.
In all of the above mentioned bags the primary and secon-
dary chambers are in liquid coaTUnunication witin each
other. This liquid communication may preferably be pro
vided by pipe connections with a length in the range of
10-20 mm, preferably 12-15 mm. In another embodiment the
pipe connections has a length in the range of 10-300 mm,
preferably about 100 mm.
The inner diameter of said pipe connections may be in the 'v
range of 2-10 mm, preferably 3-8 mm, more preferably 9-6
mm when the pipes are in a non collapsed condition.
During the process of sealing off each of the storage
chambers, e.g. by welding (heat welding, impulse weldincJ
or other) or by closing a snap lock or a clamp, prefera
bly shortly after the filling is completed, it is impor
o
tant to ensure that only a minimal air,ount of the obtained
blood is destroyed. The bag or the bag system according
to the invention, which is provided with the above men-
tioned pipe connections containing only a minimum of
blood, ensures that only a very small percentage of the
total amount of blood is damaged in connection with the
closing of these channels by e.g. heat.
By using a bag or a bag system according to the present
invention for storing umbilical cord bl_oed it is possible
to obtain at least two, preferably at least 5 primary
storage chambers, more preferably at least 8 primary
storage chambers, and most preferably at least 10 primary
storage chambers, all containing blood. Of course the
number of chambers filled with blood depends on how much
blood it is possible to extract froru the umbilical cord
CA 02338604 2001-O1-25
WO 00/06079 PCT/DK99/00422
and the placenta as well as how the blood bag is de-
signed.
The volume of the primary storage chambers is usually in
5 the range of 10 to 40 ml, preferably 25 to 35 ml, more
preferably 25 to 30 ml. In a another embodiment the vol-
ume of the primary storage chambers i'> in the range of 10
to 80 ml, preferably 25 to 70 ml, more preferably 25 to
50 ml.
It is however preferred to obtain as many storage cham-
bers as possible, since this provides the patient with
the largest flexibility in using the stored blood. Since
any number of chambers filled with blood can be removed
from the bag, i t is possible to defrost only the amount
of blood needed far the intended treatment.
The system of chambers also comprises a number of secon
dary storage chambers, preferably equal to at least the
a
number of primary storage chambers, all of which may be
sealed off and removed individually as the primary cham-
bers. The object of these secondary chambers is to pro-
vide small amounts of blood for test purposes. Therefore
the ratio between the volumes of the primary and secon-
dary storage chambers is preferably between 40:1 and
60:1, more preferably about 50:1. In another embodiment
the ratio between the volumes of the primary and secon-
dary storage chamber is in between 90:1 and 150:1, pref-
erably between 50:1 and 80:1.
having secondary storage chambers with a volume in the
range of 0, 2 to l, S ml, preferably 0, 3 to 0, 7 ml, more
preferably 0,4 to 0,6 ml, eliminates the need for de-
frosting more than one cr two secondary storage chambers
to obtain the necessary amount, e.g. 0,5 ml, of blood in
order to test it and see if it is suitable for the in-
CA 02338604 2001-O1-25
WO 00/06079 PC'T/DK99/00422
6
tended use. This particular feature is of great impor-
tance, since it is essential to determine the guality of
the blood in order to find out the right amount of blood
needed for the treatment, to check Cor contamination of
the blood (e. g. bacteria) or to perform a gene test.
In a preferred embodiment all the primary storage cham-
bers are marked or adapted to be marked individually,
each with at least one corresponding secondary storage
chamber. Further, the bag or the bag system itself is
preferably marked or adapted to be marked with a date
and/or a number in order to be able to identify the bag.
The primary storage chambers can have various shapes,
preferrea embodiments, however, are those where the shape
is substantially rectangular or subst=antially round when
the bag or the bag system is in a collapsed condition.
Since long term storage of blood typically includes Bub-
o-
jecting the bag or the bag system to temperatures below -
170 °C, it is very important that the bag or the bag sys-
tem is made of a suitable material, e.g. a material of
medical or pharmaceutical grade. Lxamples of suitable ma-
terials are e.g. a polyethylen, an ethylene vinyl acetate 'J
copolymer, a fluorine resin, a polyimide, silicone, an
ABS polymer (acrylonitrile/butadien/styrene polymer) or a
polycarbonate.
The bag or the bag system may consist of one or more fo-
lio layers, e.g. joined by welding, and in cases regard-
ing more than one folio layer, the mai=erial of the layers
may be selected among the above-mentioned materials and
may be identical or different.
In a preferred e~c~bodiment according to tine invention, the
bag system comprises primary and secc>ndary storage cham-
CA 02338604 2001-O1-25
WO 00/06079 PCT/DK99/00422
7
tiers made of one of the above mentioned materials, said
bag system being arranged or being able to be arranged in
a second bag e.c~. surrounding flue ba<1 system by the sec-
ond bag made of a suitable material. The second bag may
be divided into a number of compartments.
The invention also relates to a method for collecting and
storing umbilical cord blood and/or blood from a pla-
centa, comprising the steps of:
a) extracting blood from the umbilica l cord and/or the
placenta,
b) leading it into a mixing chamber, ~e.g. by injection,
c) mixing the blood with a preserving agent and/or an an-
ticoagulation agent,
d) optionally storing it for no longer than 29 hours,
o-
e) optionally adding further chemicals to prepare the
blood for cryo-preservation,
f) providing a liquid communication between the mixing
chamber and a number of storage chambers, allowing the
blood mixture to flow into said storage chambers,
g) closing the bag or the bag system witt: a clamp and op-
tionally removing the mixing chamber,
h) closing the channels between the various chambers e.g.
by welding or by closing a snap loch or a clamp, thereby
providing a number of primary and secondary storage cham-
bers sealed off from each other, and
CA 02338604 2001-O1-25
WO 00/06079 PCT/DK99/00422
8
i) immediately placing the bag or the bag system in a
freezer bringing the temperature below about -70 °C,
wherein said primary and said secondary storage chambers
are arranged in a bag or a bag system wherein the chan-
nels are linked to each other with intermediate seals,
and the volume of the primary storage chambers is larger
than the volume of the secondary stor<3ge chambers.
It should be observed that step e)-i) may be carried out
after storing the blood for no longer than 29 hours under
conditions with no bacteria growth, e.g. in a refrigera-
tor.
The extracting of blood from an umbilical cord and lead-
ing it into a mixing chamber may be done in any conven-
tional way. Preferably the extracting should be done in a
way accepted by those skilled in the art.
The anticoagulation agent and/or the preserving agent may
be added after the blood has been l.ed into the mixing
chamber, however, it is preferred that they are already
present in the chamber at the time the blcod is led,
1
which simplifies the method, since the mixing chambers
can be prepared for use in advance.
However, in special cases where the amount of extracted
blood is small it may be preferred to add the anticoagu-
lation agent and/or the preserving agent after the col-
lecting of the blood, in order to ensure that t=he ratio
between the blood and the above ment=Toned agents is in
the best possible range. Therefore, in a preferred em-
bodiment, the mixing chamber is provided with at least
one extra port to facilitate the addition of anticoagula-
tion agent and/or preserving agent.
CA 02338604 2001-O1-25
WO 00/06079 PCT/DK99/00422
9
After mixing the blood with the above mentioned agents,
the mixing chamber is brought into liquid communication
with the storage chambers of the bag or the bag system
according to the invention. Depending on which type of
bag is being used, the connection can be made by use of
tubes and connectors, or in a preferred embodiment of the
invention simply by opening a snap lock between the mix-
ing chamber and the storage chambers.
This allows the blood to flow into the primary and secon-
dary storage chambers, and after filling of as many cham-
bers as possible with blood, the opening for receiving
blood is closed, e.g. by use of a clamp er a snap lock.
Since there is no reed to store the attached empty mixing
chamber, it is preferred to remove the mixing chamber,
e.g. by cutting it of or removing the tubes and connec-
tors that have been used.
o-
In order to obtain a number of chambers that can be re-
moved individually, the liquid conununication bet=ween the
chambers has to be sealed off. This may preferably be
done by use of heat.
Finally the bag or the bag system is i:rozen to a tempera-
ture below about -70 °C to make sure that the blood can
be stored until the need for use thereof arises.
Various embodiments of the invention will be described
below with reference to the drawings.
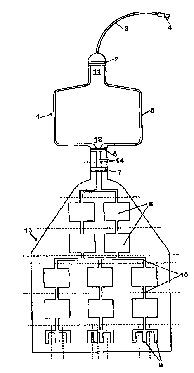
Fig. 1 illustrates a preferred embodiment of a bag ac-
cording to the invention comprising a mixing chamber.
Fig. 2 illustrates a bag according to the invention
adapted to be connected with a mixing bag.
CA 02338604 2001-O1-25
WO 00/06079 PCT/DK99/00422
Fig. 3 illustrates a bag in combination with a mixincJ bag
according to tl~e invention.
5 Fig. 9 illustrates a bag system acct>rding to the inven-
tion comprising a mixing chamber.
Fig. 5 illustrates another embodiment of storage chambers
as a part of a bag system according to the invention.
Fig. 1 illustrates a bag according to tine invention com-
prising a mixing chamber (1) with a double welding (S), w'
and an inlet (11) equipped with a tube (3), which tube
(3) at one end is equipped with a ~~onnector (4) to be
connected with a needle for harvesting blood. Two snap
locks (2) and (6) are placed at the inlet (11) and at the
outlet (12) of the mixing chamber (1). The storage bag
(13), which is connected with the mixing chamber (1)
through a tube (14) equipped with a clamp (7), comprises
o-
ten primary storage chambers (8) and ten secondary stor-
age chambers (9) in liquid communication with each other
via pipe connections (10). The dottf=d lines show where
the bag or the bag system is welded in order to seal off
each chamber after the bag has been filled with blood.
When using a bag according to Fig. 1 the blood is led
into the mixing chamber (1), where the above mentioned
necessary agents are preferably already present, then the
snap loch (2) is closed, and the bag is turned upside
down or shaken iu order to mix the ingredients properly.
Then the snap lock (6) is opened and the blood mixture
flows into the storage bag (13) filling up as many pri-
mary (8) and secondary (9) storage chambers as possible.
To make sure that the blood mixture flows properly into
the chambers, the person handling the bag can preferably
CA 02338604 2001-O1-25
WO 00/06079 PCT/DK99/00422
11
squeeze or shal>e the bag a little to ease the flow of the
blood mixture.
When all of the blood mixture contained in the mixing
chamber (1) has flown into the storage bag (l.3), the
clamp (7) is closed and the mixing chamber (1) is cut
off. Then the pipe connections (10) are closed e.g. by
welding, either shortly after the bag has been filled or
at the latest just before the bag is frozen. The welding
can be performed at the site, if the proper welding
equipment is available or the bag can be placed at a tem-
perature where substantially no bacteria can grow for up
to 24 1-rours, and during that time be transported to a fa-
cility at which the welding can be performed.
Fig. 1 illustrates a preferred embod-invent of a bag ac-
cording to the invention wherein the mixing chamber con-
stitutes part of the bag. In such a bag according to the
invention, where the mixing chamber is included as a part
of the bag, the risk of infection has been minimised,
since there are no unnecessary contact with the surround-
ings, such as transferring the blood from one bag to an-
other, once tlne blood has been led into t1e bag. This bag
also ensures that only a minimum of the blood is lost or
destroyed during the process of collecting and storing
the blood. In cases where the amount of blood obtained
from the umbilical cord and the placenta is very small,
this is particularly important.
As shown in F'ig . 1 the primary storage chambers and the
pipe connections form a certain patteriu. However, this is
merely one example of a pattern and many others may be
appropriate, as e.g. shown in Figs. 2 and 3. The impor-
tant aspect is that the pattern is designed to snake it
possible to fill as many storage chambers as possible,
even with only a small amount of blood available.
CA 02338604 2001-O1-25
WO 00/06079 PCT/DK99/00422
12
Fig. 2 shows a storage bag according to the invention
with an opening for receiving blood (21) eauipped with a
snap lock (22) and a clamp (23). 1'he bag comprises 12
primary storage chambers (24) and 7_2 secondary storage
chambers (25) in liquid cormnunicatiori with each other via
pipe connections (26). The dotted lines show where the
bag is welded in order to seal off each chamber after the
bag has been filled with blood.
This bag is adapted to be connected with a mixing bag,
wherein the extracted blood is mixed with agents men- '--f
boned above.
Fig. 3 shows a mixing bag (31) and a storage bag (35) ac
cording to the invention. The mixing bag (31) consists of
a mixing chamber (32) surrounded by a double welding (33)
and is equipped with a connectcr (39) adapted to be con
nected with the storage bag (35). The storage bag (35),
o
which is provided with an opening for receiving blood
(36) equipped with a snap lock (37) and a clamp (28),
comprises ten primary storage charnbe=rs (39) and ten sec-
ondary storage chambers (40) in liquid corrununication with
each other via pipe connections (41). The dotted lines
show where the bag is welded in order to seal off each
chamber after the bag has been filled with blood.
Fig. 9 shows a bag system according to tlae invention com-
prising a mixing chamber (91) provided with means (51)
for hanging the mixing chamber on a suitable stand or
similar means, an opening for receiving and discharging
blood (42) and a further port (43) for injection of addi-
tives and/or extraction of blood for tests. The bag sys-
tem further comprises 4 primary storage chambers (98) in
liquid communication with each ether and the mixing cham-
ber via pipe connectior_s (46) and connectors (95, 99) and
CA 02338604 2001-O1-25
WO 00/06079 PCT/DK99/00422
13
for each primary storage chamber two secondary storage
chambers (50). L,ach primary storage chamber may be sealed
off from each other and from the mixing bag by using
clamps (97).
When using a bag according to Figs. ~?, 3 and 9 the blood
is led into a mixing bag and treated as described above,
the difference being that the mixing chamber does not
constitute part of the storage bag, but is provided as a
separate bag. After the separate mixing bag has been con-
nected to the storage bag, the blood flows into the pri-
mary and secondary storage chambers as already described.
After the storage chambers have been filled the mixing
bag is disconnected from the storage bag, and the storage
bag is handled as explained above.
When using a bag system according to fig. 4 the primary
and secondary storage chambers are preferably placed in a
separate bag after being disconnected from the mixing
chamber and optionally disconnected from the pipe connec
tion by simply cutting them off. This bag may consist of
one or more compartments each suitable for containing one
ore more primary storage chambers and secondary storage
chambers connected thereto. The compartments may be sepa
rated by weldings.
In all four figures the secondary storage chambers have a
substantial rectangular shape. However, this is only one
example, and other shapes may be ~llst as appropriate.
Even though it appears from the figu=res that the primary
and secondary storage chambers of a bag, respectively,
have equal sizes and shapes, it should be understood that
the sizes and shapes of the primary and secondary storage
chambers of a bag, respectively, can differ from each
other.
CA 02338604 2001-O1-25
WO 00/06079 PCT/DK99/00422
14
As shown in figs. 1 and 3 the mixing chamber is prefera-
bly provided with a double welding, but other embodiments
may be just as appropriate, e.g. a triple welding, or a
double folio and a single welding.
Iin alternative embodiment of the storage chambers is
shown in fig. 5, where the primary storage chamber (61)
via a connector (63) is connected to the secondary stor-
age chambers (65) which are sealed off from each other
and the primary storage chamber by talurites (62) after
being filled with blood. The clamp (69) is for sealing
off the storage chamber from the pipe connection.
o-
CA 02338604 2001-O1-25