Note: Descriptions are shown in the official language in which they were submitted.
CA 02339371 2002-11-25
ELECTRICALLY MEDIATED ANGIOGENESIS
Technical Field
The present application relates to angiogenesis, and more particularly to the
electrically
mediated upregulation of angiogenic factors to promote revascularization of
ischemic body
tissue.
Background
Current medical practices call for diagnosing, testing and treating certain
maladies and
injuries with various agents. In the past few years there have been great
strides in the
development of agents that have improved therapeutic and diagnostic
application. For example,
scientists and medical researchers are rapidly developing genetic materials
and other
1
CA 02339371 2001-02-02
WO 00/27466 PCT/US99/26834
agents that cause cells to participate in the generation of new blood vessels;
a process called
angiogenesis. There are believed to be two main types of vascular disease
which are
especially suitable for treatment by angiogenesis therapy, namely coronary
artery disease and
peripheral vascular disease.
Coronary artery disease is a disease that restricts the flow of blood to the
myocardial
tissue of the heart. This restricted blood flow is commonly caused by a
blockage or blockages
resulting from a disease process known as arteriosclerosis. The blockages can
cause an
infarction where the flow of blood to a certain part of the myocardium or
cardiac muscle is
interrupted, generally resulting in a localized area of dead myocardial tissue
that is
surrounded by an area of myocardial tissue receiving reduced blood flow. This
area of
reduced blood flow is called a zone ofischemia. Other people suffer from
diffuse coronary
disease, which is the blockage of many coronary arteries. By-passing or
reopening all of
these arteries is not an option because of the extreme procedural difficalties
and trauma that
such a procedure would cause. As a result, there exists a need to provide an
adequate flow of
blood to ischemic areas of the heart without resorting to by-pass surgery or
efforts to reopen
the blocked vessels. Ischemia in the heart is generally present in those with
coronary vessel
blockage which results in a heart attack.
Peripheral vascular disease is indicated when blood flow is restricted to
areas other
than the myocardium. These ischemic areas are often induced by vascular blood
clots or
degenerative diseases. One example is the ischemic limb. The ischemic limb
often occurs in
patients having diseases such as diabetes. In a diabetic patient, the small
vessels are often
destroyed causing certain tissue areas to be oxygen and nutrient deficient, or
ischemic. Areas
2
CA 02339371 2001-02-02
WO 00/27466 PCTIUS99/26834
of ischemic tissue also result from strokes. In the case of a stroke, the
cerebral blood flow is
impaired due to a thrombosis, hemorrhage or embolism.
One way to address the need for improved blood flow to ischemic tissues in the
body
is to treat such tissues in such a way that the tissue or tissues generate new
blood vessels. As
stated above, the process of creating or generating new blood vessels is
called angiogenesis.
One method to promote angiogenesis is by direct injection of an angiogenic
agent . One
technique for delivery of such an agent to an ischemic area involves the
direct injection of
genetic material in or near the ischemic area to promote angiogenesis.
In practice, however, direct injection also has many shortcomings. One
shortcoming
is the inefficiency in transferring the genetic material into the cells and
relatively low level of
stable transfection of the genetic material within target cells. The
transfection efficiency of
.such local such delivery by direct injection is generally believed to be
about 1% to 2%.
Another method of direct injection employs electroporation, or a treatment of
tissue
with a series of high-energy electrical pulses to porate the tissue and allow
the genetic
material to enter. One problem with this approach is that many healthy cells
are frequently
killed in the process and overall transfection is still not very high.
The inability to effectively deliver the angiogenic agent to the targeted
area, therefore
is one of the major limitations of the use of such agents. During delivery of
such agents, large
amounts are often destroyed or lost to general circulation. This is
inefficient, expensive, and
can promote toxicity in certain regions. Other side effects are also possible
in healthy tissue
due to the inefficiency of such local delivery methods.
Therefore, there is a need for improved methods for enhancing angiogenesis and
the
c;ellular expression of agents to promote angiogenesis. There is also a need
for a treatment
3
CA 02339371 2001-02-02
WO 00/27466 PCT/US99/26834
apparatus that is cost effective and reduces the risk of side effects. There
is also a need for a
method and/or device that utilizes the body's natural healing mechanisms to
promote
angiogenesis, while avoiding the neeci for any introduction of foreign agents.
Summarv of the Invention
The present invention provides an electrical stimulation apparatus for
delivering an
electrical field to a targeted body tissue over a predetermined period of time
in order to stimulate
a cell-initiated angiogenic response in living cells within the targeted body
tissue. The electrical
stimulation apparatus having an electrical field generating unit including a
power supply and a
control mechanism interconnected with the power supply; and a plurality of
electrodes designed
to deliver an electrical field to the targeted body tissue. The plurality of
electrodes are in
electrical communication with the power supply and the control mechanism
controls an
amplitude and a duration of a period of delivery of electrical pulse from the
power supply to the
respective electrodes and through the targeted body tissue when the electrodes
are in contact
with the targeted body tissue at a plurality of first locations. The amplitude
of the electrical field
delivered to the targeted body tissue and the duration of the period of
delivery is sufficient to
stimulate angiogenesis in the targeted body tissue; preferably by causing
living cells within the
-targeted body tissue to increase vascular endothelial growth factor (VEGF)
expression.
In one embodiment of the present invention, the electrical field generating
unit is a
constant current delivery device and the electrical field is generated by the
constant current
delivery device. The amplitude of the electrical current delivered to the
targeted body tissue by
t.he constant current delivery device is preferably from about 0.1mA to about
250mA and the
duration of the period of delivery is preferably equal to or greater than
about I ms.
4
;
CA 02339371 2002-11-25
In another embodiment of the present invention, the electrical field
generating unit is a
constant voltage delivery device and the electrical field is generated by the
constant voltage
delivery device. The amplitude of the electrical voltage delivered to the
targeted body tissue by
the constant voltage delivery device is preferably a generally constant
voltage of from about
50V/cm to about 300V/cm. In further preferred embodiments, the electrical
field is produced by
a number of pulses in the range of from 1 to about 1000 pulses with a
frequency between about
0.1 Hz to about 5 Hz and the electrical field is preferably generated for a
duration betweenabout
0.0001 seconds to several days.
In other preferred embodiments, the control mechanism includes a computer
processing
unit in electronic communication with the power supply, the computer
processing unit being
programmed to cause the electrical stimulation apparatus to deliver a
predetermined amount of
electrical current or voltage over a predetermined period of delivery to the
plurality of electrodes
such that the electrical stimulation apparatus can deliver such electrical
current or voltage to the
targeted body tissue. In other preferred embodiments, the plurality of
electrodes are configured
in a manner selected from the group consisting of unipolar, bipolar, and
multiple electrode
configurations and the apparatus is preferably designed and configured to be
implantable.
The present invention also includes use of electrical field stimulation in the
treatment
of targeted body tissues by: (1) provided living cells, preferably autologous
or heterologous
living cells, more preferably autologous or heterologous myocardial cells for
treatment of
myocardial tissue, which, in the case of autologous cells, have been removed
from the
prospective patient, which are biologically compatible with the targeted body
tissue; (2)
stimulating the living cells with an electrical field sufficient in a manner
described herein to
increase VEGF expression by the living
5
CA 02339371 2001-02-02
WO 00/27466 PCT/US99/26834
cells, wherein the amplitude of the electrical fieid delivered to the targeted
body tissue and the
duration of the period of delivery is sufficient to cause the living to
increase VEGF expression;
and (3) injecting the stimulated cells into the targeted body tissue.
The present invention has several advantages. For example, angiogenesis can be
promoted without the delivery of foreign agents, which allows the body to heal
naturally and
minimizes potential for side effects. T'he procedure provides minimum
discomfort and may
be performed on an outpatient basis. The main power supply can be reused,
while the
electrodes are disposable. A combination of a reusable power supply and
disposable
sterilized electrodes reduces both the expense and the chance of
contamination. Yet another
advantage is that electrical energy can be applied for extended periods of
time with minimal
risk of killing the target cells.
Another advantage is that the present invention can be used to treat deep
tissues, as
ivell as superficial tissue. Certain techniques may be either invasive,
minimally invasive, or
rioninvasive. Furthermore, the treatment of the ischemic tissue can be
targeted while
exposure to healthy tissue is minimized.
The above described features and advantages along with various other
advantages and
features of novelty are pointed out with various other advantages and features
of novelty are
pointed out with particularity in the claims of the present application.
However, for a better
understanding of the invention, its advantages, and objects attained by its
use, reference should
be made to the drawings which form a further part hereof and to the
accompanying descriptive
matter in which there is illustrated and described preferred embodiments of
the invention.
Brief Description of the Drawings
6
CA 02339371 2001-02-02
WO 00/27466 PCT/US99/26834
Figures lA-1C, taken in series, diagrammatically illustrate initial in vitro
capillary
formation overtime between untreated free cells and free cells induced in
accordance with a
preferred embodiment of the present invention;
Figure 2A schematically illustrates an electrical stimulation apparatus for
generating
an electrical field to enhance angiogenesis in ischemic, and other targeted
body tissues;
Figure 2B is an enlarged broken away side view of a portion of electrical
stimulation
apparatus shown in circle 2B-2B of Figure 2A.
Figure 3 schematically illustrates an alternative embodiment of the electrical
stimulation apparatus shown in Figure 2A;
Figure 4A illustrates an alternate electrical stimulation apparatus having a
single
needle having two electrodes having opposite polarity in a "bipolar" needle
configuration;
Figure 4B is an enlarged broken away side view of a portion of the electrical
stimulation apparatus shown in circle 4B-4B of Figure 4A
Figure 5 schematically illustrates an alternative application of the apparatus
shown in
Figure 2;
Figure 6 schematically illustrates an alternative application of the
electrical
stimulation apparatus shown in Figure 2;
Figure 7 schematically illustrates another alternative application of the
electrical
stimulation apparatus shown in Figure 2;
Figure 8A schematically illustrates an alternative embodiment of an
application of an
alternate electrical stimulation device having two electrodes, both of which
are patch-type
electrodes;
7
CA 02339371 2001-02-02
WO 00/27466 PCT/US99/26834
Figure 8B is a perspective view of the contact surface of the alternate patch-
type
electrode 130 shown in Figure 8A having a plurality of pins which can insert
into the body
tissue;
Figure 8C is a perspective view of an alternate patch-type electrode 142
having a
plurality of electrode surfaces 144 which can contact the surface of a
targeted body tissue to
deliver an electrical current;
Figure 9A-9B schematically illustrate an alternative embodiment of the
electrical
stimulation apparatus shown in Figures 4A and 4B in a similar manner;
Figure 10 schematically illustrates an alternate application of a further
embodiment of
the electrical stimulation apparatus of the present invention; and
Figures 11A-11D schematically illustrate circuits which are representative of
alternate
circuits which can be employed when certain of the alternate electrical
stimulation apparatti
Df the present invention are employed to deliver an electrical field to
various targeted body
tissues.
Detailed Description of the Preferred Embodiments
Various embodiments of the present invention are described below in detail
with
reference to the drawings, wherein like reference numerals represent like
parts and assemblies
throughout the several views. Reference to the various embodiments is not
intended to limit
the scope of the invention.
In general, the present invention relates to an apparatus for generating an
electrical
field proximate to or within a targeted body tissue and methods of treatment
of such targeted
body tissues with such an apparatus to stimulate an angiogenic response within
living cells in
8
CA 02339371 2001-02-02
WO 00/27466 PCT/US99/26834
such body tissues. In these methods, electrical energy is delivered directly
to cells in the
targeted body tissue which located in an electrical path between at least two
electrodes of
such an apparatus. Such delivery is believed to promote a cell-initiated
angiogenic response
that promotes angiogenesis in targeted body tissues which can include body
tissues in
ischemic zones. The cell-initiated angiogenic response is believed to include
a cellular
process of capillary formation which is initiated or accelerated following
application of
electrical stimulation of body tissues.
Referring now to the drawings, the cellular process of capillary formation
during
electrically-mediated angiogenesis is illustrated in Figures lA-1C. Figure lA
illustrates an
array of epithelial cells 4 in culture distributed in a normal growth pattern
before the
application of an electric current. Figure 1B illustrates the beginning stages
of angiogenesis
following electrical stimulation in which the cells 4 begin to organize and
align. Figure 1 C
illustrates what is believed to be the initial formation of the tube
structures 6 in such cell
culture. It is believed that these tube structures 6 are believed to develop
into new capillaries.
A. discussion of the aggregation of these cells will follow below.
Figures 2A and 2B schematically illustrate an electrical stimulation apparatus
10 of
the present invention useful for stimulation of ischemic tissue and other
targeted body tissues
by the delivery of low amperage electric current. The apparatus 10 includes a
needle 100
having a proximal portion 112 and a distal portion 110. The distal portion 110
includes an
electrically conductive shafl 102 which forms a primary electrode, an
insulating material 104
partially covering the shaft 102, a delivery zone 106, and a distal tip 108.
The shaft 102 has a
lumen 115 and a plurality of delivery ports 116. The diameter of the proximal
portion 112 is
greater than the diameter of the distal portion 110. A radially oriented
surface 114 defines a
9
CA 02339371 2001-02-02
WO 00/27466 PCT/US99/26834
distal end of the proximal portion 112 and forms a depth guide 114 that
generally limits the
distance that the proximal end 112 of the needle 100 can be inserted into the
patient's body
tissue. The distance between the distal tip 108 of the needle 100 and the
depth guide 114 can
vary depending on how deep the intended target area is from the surface of the
body tissue
into which the needle 100 is to be inserted.
The needle 100 also defines a lumen 115 and defines delivery ports 116 in the
distal
portion 110. The lumen 115 and delivery ports 116 enable injection of a liquid
into the
targeted body tissues, including agents such as an angiogenic agents and/or
cooling mediums
to minimize heating of the targeted body tissue. In alternative embodiments
such as that
illustrated in Figure 3, the needle 100' is solid and does not include
delivery ports, so that
fluids cannot be injected through the distal portion of the needle 100'.
Referring now again to Figure 2A, the needle 100 is in electrical
communication via
electrical lead A with an electrical field generating unit (EFGU) 117. The
EFGU 117 is in
electrical communication with a control mechanism 119. In preferred
embodiments, the
control mechanism 119 is a computer processing unit which is programmed to
generate a
preferred electrical field within a proximate to a targeted body tissue. In an
alternate
embodiment of the present invention shown in the circuit diagram in Figure 1
lA, the EFGU
117 will include an electrical power supply 117a, a switch 11 7b and a
variable resistor so that
the current may be varried and the circuit can be broken. In other embodiments
the EFGU
117 is a constant current delivery device, such as an iotophoretic electrical
current generation
clevice such as the constant current delivery device (CCDD) sold by EMPI, Inc.
with a system
sold under the tradename DUPEL , or the CCDD sold by IOMED, Inc. which is DC
powered
"'dose controller." In order to effectively provide computer controls for the
CCDDs,
11 j i 1
CA 02339371 2002-11-25
appropriate modifications are made to provide for programmed control of these
devices by a
CPU 119'. If the EFGU 117 is a constant voltage delivery device (CVDD), then a
device similar
to the PA-4000 sold by CYTOPULSE, Inc. will be used.
Referring again to Figure 2A, a flexible, path-type electrode 120 is also in
electrical
communication with the source of current 117 via electrical lead B so that it
has an opposite
polarity from the delivery zone 106 of the needle 100. In alternative
embodiments an additional
needle can be used in place of the patch-type electrode 120 or, conversely, an
additional patch-
type electrode (hereinafter patch) can be used in place of the needle 100. The
source of current
117 will generally include a signal generator, a variable resistor, a switch,
orother circuitry that
is electrically connected to the source of current 117 in certain embodiments
in order to shape or
otherwise control the signal used to pass electric current through the
electrodes. In preferred
embodiments, the source of current 117 is controlled by a microprocessor or
other computer
processing unit (CPU) 119 which is preferably programmed to cause the
electrical stimulation
apparatus to deliver a predetermined amount of electrical current over a
predetermined period of
delivery to the plurality of electrodes such that the electrical stimulation
apparatus can deliver
such electrical field to the body tissue when the plurality of electrodes are
in contact with the
body tissue. Electrodes of all types can be used.
Alternative configurations of the electrical stimulation apparatus (not shown)
also
include multiple needles. In a further alternate embodiment, for example,
there are two
needles, two patches, or a needle and a patch having the same polarity and a
further needle or
patch having the opposite polarity. In another possible configuration, there
is an array of
electrodes, possibly needles with at least one electrode, or needle having one
polarity and at
least one other electrode or needle, preferably a plurality, having an
opposite polarity similar
to that illustrated in the schematic circuit drawing shown in Figure 11 D
where there are five
electrodes of one polarity and a single electrode of opposite polarity. In yet
another
11
I I ii
CA 02339371 2002-11-25
embodiment, illustrated in Figure 10 and discussed below, there is a sensing
electrode 138
separate from the positively and negatively charged electrodes 130 and 120,
respectively,
which is especially useful for cardiovascular applications. In this
embodiment, the sensing
electrode 138 is used to sense the electrical activity of the heart 128 and
pace delivery of the
electrical energy. In this regard, it is noted that the heart muscle is in a
state of general
relaxation during a "refractory period" which follows each contraction of the
heart muscle. In
a preferred embodiment, treatments of ischemic zones of the myocardium are
synchronized
so that pulses of electrical energy are generated to deliver an electrical
field to the heart
during these refractory periods in order to reduce the risk of creating an
arrhythmia. In
preferred embodiments, the apparatus will monitor the heart 128 with a sensing
lead 136 so
that the CPU 118 can provide the programmed synchronization necessary to
provide the
appropriate timing to deliver pulses during the refractory period. In further
embodiments, the
sensing lead 136 in coordination with the CPU 118 will also have heart
pacemaking
capabilities to allow it to pace the heart 128 to facilitate the
synchronization of the pulsed
electrical field generation with the occun ence of the refractory period.
12
CA 02339371 2001-02-02
WO 00/27466 PCTIUS99/26834
Another alternative embodiment of the electrical stimulation apparatus 10
shown in
Figure 2 is shown in Figures 4A-4B. In this embodiment, the apparatus 10"
includes a bipolar
needle 101 having a proximal portion 1 l2"and a distal portion 110". The
proximal portion
112" has a depth guide 114" and the distal portion 110' has a shaft 102', an
insulating material
104', a delivery zone 106', a distal tip 108', and a first electrode 103 that
extends around the
circumference of the needle 101. A second electrode 105 is spaced apart from
the first
electrode 103 and also extends around the circumference of the needle 101. In
one possible
embodiment, the needle 101 is formed from a nonconductive material, such as a
ceramic
material or hard polymer such a polycarbonate, high density polyethylene and
the like, so that
the first and second electrodes 103, 105 are electrically isolated from one
another. In an
alternative embodiment (not shown), a nonconductive material or substrate is
positioned
between the needle and the first and second electrodes. The electrodes and can
be formed as
described herein including coils wrapped around the needle, electrically
conductive ink, and
electrically conductive bands or foil.
Electrical leads B", A" provide electrical communication between the EFGU 117"
and
the first and second electrodes 103 and 105 such that the first electrode 103
has an opposite
polarity from the second electrode 105. In this configuration, one of the
electrodes is an
anode and the other electrode is a cathode. An alternative configuration (not
shown) includes
multiple anodes and/or multiple cathodes mounted on a needle. Other
alternative
configurations include multiple needles. Furthermore, the polarity of the
first and second
electrodes 103 and 105 can be switched by programming the CPU 119" to switch
the polarity
of the respective electrodes when the EFGU 117" permits such switching.
13
I' 1'I H
CA 02339371 2002-11-25
In one possible application, as shown in Figure 5, the needle 100 is attached
to a syringe
122 for injection of an agent or a cooling liquid and is then inserted into or
proximal a target area
in a diseased limb, such as a lower leg 124, until the depth guide 114 is
against the surface of the
delivery area. The patch-type electrode 120 is attached to the surfxe ofthe
patient's body 129 in
a convenient location such as the thigh 126. Next, current delivery is
initiated between the
needle 100 and the patch-type electrode 120. A single or multiple insertions
may be used. The
bipolar needle 101 may also be used in as somewhat similar application. The
primary difference
when using the bipolar needle 101 is that there is no need to attach a patch-
type electrode to the
patient's skin.
In an alternative embodiment as shown in Figure 6, a patch-type electrode 121
defines an
opening 123 that passes therethrough. The patch-type electrode 121 is
positioned against the
surface of the skin at a site that is adjacent or over the target area of
tissue. The caregiver then
inserts the needle 100 through the opening 123 and into the target area of
tissue until the depth
guide 114 is against the surface of the skin. In this position, the needle 100
is not in direct
electrical contact with the electrically conductive portions of the patch-type
electrode 121.
Current is then generated between the needle 100 and the patch-type electrode
121.
The current in any of the alternate applications can have different waveforms
including
direct current, alternating current and pulsed. Any wellknown waveforms can be
used including
those which are described in United States Patent 5,499,971, which issued on
March 19, 1996
and is entitled INTERNAL IONTOPHORESIS DRUG DELIVERY APPARTUS AND
METHOD. In one possible embodiment, a low level of current between about 0.1
mA and
about 50 mA, preferably between about 0.2 mA and about 25 mA, more preferably
between
14
CA 02339371 2001-02-02
WO 00/27466 PCT/I3S99/26834
about 0.4 mA and about l OmA, and more preferably between about 0.5 and about
5 mA is
preferabiy conducted between the electrodes. In one possible embodiment that
uses direct
current, the amplitude is between about 0.5 to 5 mA although other current
amplitudes can be
used.
In an embodiment that uses pulsed or alternating waveform, the amplitude of
the
current can be adjusted in relation to the pulse width and duty cycle, which
allows control
over the overall density of the current being emitted from the electrode. In
one possible
embodiment using pulsed or alternating waveforms, the amplitude of the signal
is in the range
from about 5 mA to about 250 mA, and the pulse width is in the range from
about 0.1 ms to
about 100 ms. In certain embodiments, the treatment may generating pulses
having a current
of 1mA for a period of 1 minute in one or more, perhaps 5 of more, locations.
Alternately, a
5mA pulse can be delivered for 5 seconds in 5 second intervals for an extended
period of 1
minute and repeated a one to five different locations. Alternately, 250 mA
pulses can be
delivered for 15 msec every second for one minute. In another preferred
embodiment of the
present invention, the preferred apparatus allows for the delivery of constant
voltages
generally in the range of from about 1 V/cm to about 500 V/cm (applied voltage
divided by
the distance in cm separating the respective electrodes); preferably from
about 5 V/cm to
about 250 V/cm, and more preferably from about 10 V/cm to about 100 V/cm. The
voltage
may be delivered in a variety of waveforms, pulse durations, frequencies,
pulse widths, and
number of pulses. If a CVDD is used the pulses can be from about 1 V/cm to
about
500V/cm, preferably from about 10 V/cm to about 300 V/cm, more preferably
about 50
V/cm to about 100 V/cm. In one alternate treatment 50 V/cm is generated by
CVDD for 20
CA 02339371 2002-11-25
msec, at 1 Hz for 1 minute. In another 300V/cm is generated for 1 msec at 1 Hz
for 1 to
about 60 seconds.
In another possible application as shown in Figure 7, the needle 100 can be
inserted in
to the myocardium of the heart 128. In this application, the needle 100 is
typically inserted
into or proximal to a zone of ischemia. In this application, the location of
the patch-type
electrode 120 depends on whether the procedure is used with minimally invasive
techniques
such as an orthoscopic incision, or whether an open heart surgery is
performed. If a
minimally invasive technique is used, the patch-type electrode is placed
against the surface of
the patient's body 150, such as the abdomen or thigh. If open heart surgery is
performed, the
patch-type electrode 120 can be placed near or against the surface of the
myocardium. Again,
the bipolar needle 101 may also be use in this application in place of the
needle 100.
In yet another possible embodiment, multiple needles could be inserted into
the target
area and polarized. These needles could be configured to all have the same
polarity.
Alternatively, some of the needles have one polarity and the other needles
have an opposite
polarity to form a bipolar electrode configuration. Such a bipolar
configuration may not
include the patch-type electrode.
In yet another possible embodiment (not shown), the needle 100 is mounted on a
trans-vascular catheter that can be introduced into a patient's vascular
system and threaded
to a target area. In one possible procedure, a trans-vascular catheter is used
to introduce
the needle 100 into one of the chambers of the heart and then to deploy the
needle 100 into
the myocardium. The bipolar needle 101 also can be used with a trans-vascular
catheter.
Referring now to Figures 8A, 8B and 8C, an alternative embodiment of the
present
invention uses a second patch-type electrode 130 as the primary electrode. The
second
patch-type electrode 130, which is for application proximal to or over the
target area, has a
delivery surface 132 configured to be placed against a bodily surface. An
advantage of
using a patch for the primary electrode 130 is that its size is adjustable,
which allows for
the entire target area to be incorporated into the electrical field. This
adjustability also
allows the size to be adjusted so that otherwise healthy tissue is not covered
by the primary
electrode 130, which maximizes the current density in the target tissue.
16
CA 02339371 2002-11-25
In one possible configuration of the second patch-type electrode 130', there
are a
plurality of projecting structures 134' that can penetrate at least the outer
surface of the
tissue. An example of such a structure is small pin points 134'. These
projecting
structures have several advantages. One such advantage is that they increase
the surface
area of the electrode which permits a greater density of the current being
radiated from the
electrodes. Another advantage is that the outer layer of the skin is more
resistive than
internal tissue. Thus piercing this outer layer will permit electrical current
delivery at
lower resistance to the target area. In yet another possible configuration,
there are not any
projecting structures that pierce the tissue surface. An advantage of not
having any
piercing structures is that the procedure becomes noninvasive during
applications to the
outer surface of the patient's body, which is more comfortable for the patient
and reduces
the risk of infection.
17
CA 02339371 2001-02-02
WO 00/27466 PCT/US99/26834
Another possible embodiment of the needle 100"' is shown in Figures 9A-9B. In
this
embodiment, an electrode support meinber 131 is connected to the needle 100"'
adjacent to
the insulating material 104 and projects outward therefrom. An electrode 133
is positioned
on the electrode support member 131 and faces the tip 108"' of the needle
100"'. In one
possible embodiment (not shown), the electrode 133 is spaced apart from the
insulating
material 104. In another possible embodiment (not shown), the electrode 133
extends over
the surface of the electrode support member 131 and is directly adjacent to
the insulating
rnaterial 104"'. In these configurations the insulating material 104"' and the
electrode support
inember 131 insulate the electrode 133 from the needle 100"' and prevents a
short circuit. As
shown in Figure 9B, the support member 131 can also be formed as a part of the
insulating
inaterial 104"' In use, the needle 100"' is inserted into the myocardium until
the electrode 133
is in contact with the surface of the myocardium. An advantage of this
configuration is that
the current conducted between the needle 100"' and the electrode 133 can be
controlled to a
relatively discrete area.
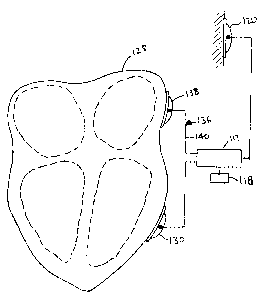
Referring now to Figure 10, yet another possible embodiment includes a sensing
lead
136 that includes a sensing electrode 138 and a lead 140 that is preferably in
electrical
communication with the source of current 117 and the CPU 118. In use, both the
sensing lead
136 and the second patch-type electrode 130 are placed against the myocardium
of the heart
128. The sensing lead 136 can be placed into electrical contact with any
portion of the heart
where a strong signal from the heart's intrinsic electrical activity can be
detected. Examples
include portions of the myocardium such as the epicardial surface, the
myocardium that forms
the left or right ventricles, the sinoatrial node, the atrioventricular node
and the like.
18
CA 02339371 2002-11-25
The sensing lead 136 is then used to sense the electrical impulses in the
cardiac
conduction system, which causes the heart to beat. In response to sensing
these electrical
impulses, the circuitry in the source of cun-ent 117 and the CPU 118
synchronizes delivery of
electrical current with the refactory period of the heart beat, which is the
period between
depolarization and repolarization of the heart. Synchronization is
advantageous because the
heart is least susceptible to the inducement of arrhythmia during the
refactory period.
In alternative configurations, the circuitry in the source of current 117 and
the CPU
118 paces the heart 128 if the heart beat is irregular. Such pacing is
accomplished by sending
an electric pulse into the heart 128 that causes it to depolarize. The
caregiver can then more
accurately synchronize the cutrent to the refactory period of the heart.
Cardiac pacing is
disclosed in United States Patent 5,634,899.
Yet another altenYative embodiment that is useful in cardiac applications does
not
have any type of synchronizing or pacing circuitry in the source of current
117 and the CPU
118. Choice of electric pulse amplitude, pulse width, pulse frequency, and
number of pulses,
is tailored to avoid stimulation of arrhythmia. In one possible embodiment,
the electrical
signal has pulses with a constant voltage amplitude between about 50 V/cm to
about 300
V/cm or a constant current aznplitude between about 5 mA to about 250 mA, with
a fi-equency
between about 0.1 Hz to about 2 Hz. Although various numbers of pulses can be
applied in a
treatment, one possible treatment is in the range from about I pulse to about
60 pulses.
Envisioned in a further alternate embodiment (not shown) is a dedicated
electrode
system designed specifically for implantation, allowing chronic administration
of electric
current to target tissue for purposes of stimulating angiogenesis. In
principal, any conductor,
such as metal or electrically conducting organic polymer (or combination of
the two), can
19
CA 02339371 2001-02-02
WO 00/27466 PCT/US99/26834
serve as the electrode material. Design of the electrode can take on a number
of different
shapes, and sizes, depending on the nature of the target tissue. In the case
of heart muscle or
other tissue, the electrode(s) can consist of a straight pin, a screw, a
helix, or a patch. The
patch can be further divided into mechanisms for delivery either to a smooth
surface for
contact with the heart, or with various barbs, hooks, needles, clamps,
stapels, and the like for
penetration into some portion of the heart muscle. Penetrating electrodes
could be made
hollow, with one or more terminal or side ports, enabling delivery of water,
saline, or
pharmaceutical agent solutions into or to the surface of target tissue. Drug
delivery, however,
:is not a requisite for electrically mediated angiogenesis. Some advantage
might be achieved
by use of electrical insulation on some portion of an electrode, which can
provide a useful
inechanism for directing electric energy in a most desired manner within or to
a target tissue.
Similar electrode arrangements are envisioned for other target tissues. In
addition,
strap type electrodes can be used with applications to target tissues such as
bone, where it
rnight be desired to wrap the electrode around the bone or other body tissue.
Electrical leads from the electrodes would be connected to a power source
similar to
tlhose disclosed herein or commonly used in other implantable battery driven
devices. Most
convenient would be a source which is implanted into a location which does not
interfere
vtith the patient, and can be generally ignored until such time that a battery
power source
would require replacement.
The electrode systems or needles used with the present invention may be
monopolor
or bipolar. A mono electrode system has an electrode of one polarity
positioned on one
structure and an electrode of an opposite polarity positioned on a different
structure. In a
biipolar electrode, electrodes of both polarities are mounted on a single
structure such as a
CA 02339371 2001-02-02
WO 00/27466 PCT/US99/26834
needle, catheter or probe and are electrically isolated from one another.
Additionally, a single
f;lectrode may be used for each polarity or a group of electrodes might be
used. For example,
there might be two or more electrodes placed over a diseased area of a limb
where it is
desired to stimulate the growth of new vasculature. Additionally, the
materials used to form
the electrodes may be either sacrificial or nonsacrificial. Examples of
sacrificial materials
include silver/silver chloride, copper, tin, nickel, iron, lithium, and
amalgams thereof.
I?xamples of nonsacrificial materials include platinum, gold, and other noble
metals. The
electrodes also can be fonned with zirconium, iridium, titanium, certain
carbons, and stainless
steel, which may oxidize under certain circumstances. The polarity of the
delivering as well
as the return electrode may be in either direction as long as the circuit is
closed.
The circuits diagrammed in Figures 11 A-11 D are circuits which are
representative of
a number of applications described herein. In each case the resistance RL is
provided by the
targeted body tissue. In each case any of the previously described EFGUS 117
can be
employed in the respective circuit. Similarly, any appropriate CPU 119 can
provide computer
processing central for the EFGU 117.
In addition to the in vivo and in vitro method described above, an alternative
embodiment can be used with an ex vivo process. In an ex vivo process, cells
such as muscle
cells, endothelial cells and the like, preferably autologous cells in culture,
are treated with
electrical current and then injected into an ischemic zone. The process
includes providing
living cells, preferably autologous living cells which have been removed form
the prospective
patient, which are biologically compatible with the targeted body tissue;
stimulating the living
cells with an electrical field sufficient in a manner describe herein to
increase VEGF expression
by the living cells, wherein the amplitude of the electrical field delivered
to the targeted body
21
CA 02339371 2001-02-02
WO 00/27466 PCT/US99/26834
tissue and the duration of the period of delivery is sufficient to cause the
living to increase
VEGF expression; and injecting the stimulated cells into the targeted body
tissue. This process
eliminates the need for in vivo stimulation by electric energy.
As described above, the use of low levels of electrical energy stimulates the
target
tissue's natural ability to heal or revascularize in an ischemic area. The
delivery of electrical
current generally improves blood pressure and increases capillary density in
both ischemic
tissue and in other body tissues as well. It also has been shown to cause
upregulation of
various cellular materials resulting in increased angiogenesis. In particular,
passing low
amperage electrical current through body tissues causes cells to increase
overall expression of
vascular endothelial growth factor (VEGF), which is believed to promote
revascularization of
body tissues, as well as the expression of the tyrosine kinase receptor (KDR)
receptor on
endothelial cells, also believed to be important in promoting
revascularization in body tissue.
This treatment can, under certain conditions, also cause cells to modulate
their expression of
either acidic or basic fibroblast growth factors (FGFs) which is also believed
to promote
revascularization or angiogenesis. This enhancement is demonstrated with the
following
experimental examples.
Example 1
In Vitro Cellular VEGF Induced Mitiration
Figure 10 illustrates equipment for demonstrating in vitro cellular VEGF
induced
migration. Cells are grown on a Coming Costar Transwell System. The
transwells are then
inserted into the holding chamber containing a conductive media. An electrode
is placed in
the lower chamber and one in the transwell. The bottom of the transwell is a
microporous
membrane which allows media and current to pass through while cells remain in
the upper
(transwell) chamber. This system is advantageous for modeling human systems.
It allows for
22
CA 02339371 2001-02-02
WO 00/27466 PCTIUS99/26834
the collection of data that relates to upregulation of such genetic factors as
vascular
endothelial growth factor (VEGF), known to have an active roll in
angiogenesis.
During the experiment the epithelial cells are first grown to confluency in
the
transwell system and then serum-starved for 24 hours. The cells are then
placed into the
holding chamber and stimulated with :5 mA DC current for 3 minutes. The
negative electrode
is placed in the top well containing PBS and the cells. The positive electrode
is placed in the
lower chamber with M 199 low serum media. The cells are then allowed to
recover in whole
serum for 24 hours. They are then starved again for another 24 hours to mimic
ischemic
conditions. At the end of this starvation period the cells are trypsinized,
counted, and equal
amounts of the cells are mounted in a modified Boyden chamber, which is well
known in the
art. Approximately 20,000 cells are placed into the upper well. VEGF as a
chemoattractant
is placed in the lower well. After 4 hours of migration the membrane is fixed
and stained,
-md the migration patterns of cells in each condition are evaluated. All
conditions are
repeated in triplicate.
Western Blot analysis showed an increase in proteins for both the KDR receptor
and
VEGF with electrical stimulation. The Boyden chamber results indicated that
there was an
increase in migration, as shown in Figures 1 A-1 C, with current alone verses
that with no
current. This supports the hypothesis that the electrical stimulation
activated the cells.
23
CA 02339371 2001-02-02
WO 00/27466 PCT/US99/26834
Example 2
Effect of Electric Current Deliverv on ahVEGF165 Treatment in Rabbit Model
A well established rabbit ischemic hind limb model was studied. Twenty-one
rabbits
were treated 10 days after surgical intervention to promote ischemia. Control
rabbits (n=9)
received saline or water injection together with electrical stimulation (Group
1). Six rabbits
were treated with a gene plasmid coding for VEGF (500 ug) alone without any
electrical
stimulation (Group 2). Six additional rabbits received iontophoretic delivery
of VEGF
(Group 3) VEGF delivered along with electrical stimulation. After 30 days the
effect on
blood pressure (BP) ratio (ischemic/nortnal) and angiogenic scores (AS) were
evaluated. The
angiogenic scores relate to an increase in capillary density. The results
(mean+ SEM) are
shown below in Table 1.
All values were significantly higher at follow-up compared to baseline. No
differences between the groups were present. Electric current alone had a
remarkably positive
effect on blood pressure recovery and angiogenesis in the ischemic limb.
Addition of VEGF
gene plasmid to treatment with electric current did not further improve
angiogenesis.
Detectable quantities of VEGF were found in the blood of the animals which
received
electrical stimulation, whereas this result was not the case for non-
electrical controls.
Table 1
Group BP ratio BP ratio AS AS
Baseline Follow up d) Baseline Follow up d)
1 0.45f0.02 0.91f0.03 0.54f0.02 0.67t0.02
2, 0.48 0.02 0.94 0.03 0.54 0.02 0.73 0.01
3 0.48 0.03 0.84 0.07 0.51 0.02 0.74 0.03
While the invention has been described in conjunction with a specific
embodiments
thereof, it is evident that other alternatives, modifications, and variations
can be made in view of
24
CA 02339371 2001-02-02
WO 00/27466 PCT/US99/26834
the foregoing description. For example, features of one of the embodiments or
methods
described above can be combined with features of any of the other embodiments
or methods.
Alternatively there can be modifications that are not explicitly taught
herein, but still embody
the spirit of the inventions described herein. Accordingly, the invention is
not limited to these
embodiments or the use of elements having specific configurations and shapes
as presented
herein.