Note: Descriptions are shown in the official language in which they were submitted.
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-1-
Tit e: Apparatus and Method for Atmospheric Pressure
3-Dimensional Ion Trapping
FIELD OF THE INVENTION
The present invention relates to an apparatus and method for
trapping ions, at atmospheric pressure, within a defined 3-dimensional space,
based
on the ion focussing principles of high field asymmetric waveform ion mobility
spectrometry.
BACKGROUND OF THE INVENTION
High sensitivity and amenability to miniaturization for field-portable
applications have helped to make ion mobility spectrometry an important
technique
for the detection of many compounds, including narcotics, explosives, and
chemical
warfare agents (see, for example, G. Eiceman and Z. Karpas, Ion Mobility
Spectrometry (CRC. Boca Raton, FL. 1994); and Plasma Chromatography, edited by
T.W. Carr (Plenum, New York, 1984)). In ion mobility spectrometry, gas-phase
ion
mobilities are determined using a drift tube with a constant electric field.
Ions are
gated into the drift tube and are subsequently separated based upon
differences in
their drift velocity. The ion drift velocity is proportional to the electric
field strength
at low electric fields (e.g., 200 V/cm) and the mobility, K, which is
determined from
experimentation, is independent of the applied field. At high electric fields
(e.g.
5000 or 10000 V/cm), the ion drift velocity may no longer be directly
proportional to
the applied field, and K becomes dependent upon the applied electric field
(see G..
Eiceman and Z. Karpas, Ion Mobility Spectrometry (CRC. Boca Raton, FL. 1994);
and
E.A. Mason and E.W. McDaniel, Transport Properties of Ions in Gases (Wiley,
New
York, 1988)). At high electric fields, K is better represented by Kh, a non-
constant
high field mobility term. The dependence of Kh on the applied electric field
has been
the basis for the development of high field asymmetric waveform ion mobility
spectrometry (FAIMS), a term used by the inventors throughout this disclosure,
and
also referred to as transverse field compensation ion mobility spectrometry,
or field
ion spectrometry (see I. Buryakov, E. Krylov, E. Nazarov, and U. Rasulev, Int.
J.
Mass Spectrom. Ion Proc. 128. 143 (1993); D. Riegner, C. Harden, B. Carnahan,
and
S. Day, Proceedings of the 45th ASMS Conference on Mass Spectrometry and
Allied
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-2-
Topics, Palm Springs, California, 1-5 June 1997, p. 473; B. Carnahan, S. Day,
V.
Kouznetsov, M. Matyjaszczyk, and A. Tarassov, Proceedings of the 41st ISA
Analysis Division Symposium, Framingham, MA, 21-24 April 1996, p. 85; and B.
Carnahan and A. Tarassov, U.S. Patent Number 5,420,424). Ions are separated in
FAIMS on the basis of the difference in the mobility of an ion at high field
Kh relative
to its mobility at low field K. That is, the ions are separated because of the
compound dependent behaviour of Kh as a function of the electric field. This
offers
a new tool for atmospheric pressure gas-phase ion studies since it is the
change in ion
mobility and not the absolute ion mobility that is being monitored.
One application of this tool as realized by the present inventors is in
the area of ion trapping. To the inventors' knowledge, there are no previously
known
devices or methods that produce any sort of a 3-dimensional ion trap at
atmospheric
pressure (about 760 torr). While other 3-dimensional ion trapping mechanisms
do
exist, these known ion traps are typically designed to operate below 1 torr,
in near-
vacuum conditions. The efficiency of these ion traps degrades extremely
rapidly as
the pressure increases beyond 10 torr, and there is no experimental or
theoretical
basis to suggest that any trapping oecurs, using these known methods, at 760
torr.
SUMMARY OF THE INVENTION
In one aspect, the present invention provides an apparatus for
selectively transmitting ions and trapping said ions within a defined 3-
dimensional
space, comprising:
a) at least one ionization source for producing ions;
b) a high field asymmetric waveform ion mobility spectrometer,
comprising an analyzer region defined by a space between at least
first and second spaced apart electrodes for connection, in use, to an
electrical controller capable of supplying an asymmetric waveform
voltage and a direct-current compensation voltage for selectively
transmitting a selected ion type in said analyzer region between said
electrodes at a given combination of asymmetric waveform voltage
and compensation voltage, said analyzer region having a gas inlet and
a gas outlet for providing, in use, a flow of gas through said analyzer
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-3-
region, said analyzer region further having an ion inlet for introducing
a flow of ions produced by said ionization source into said analyzer
region; and
c) a curved surface terminus provided on at least one of said electrodes,
said terminus being a part of said one of said electrodes which part is
closest to said gas outlet, said defined 3-dimensional space being
located near said terminus, whereby, in use, said asymmetric
waveform voltage, compensation voltage and gas flow are adjustable,
so as to trap said transmitted ions within said 3-dimensional space.
Said first and second electrodes may comprise curved electrode
bodies to provide a non-constant electric field therebetween, whereby, in use,
said
ions are selectively focussed in a focussing region created between said
curved
electrode bodies in said analyzer region.
In another embodiment, said first and second electrodes comprise
outer and inner generally cylindrical coaxially aligned electrode bodies
defining a
generally annular space therebetween, said annular space forming said analyzer
region, and said terminus being provided at an end of said inner cylindrical
electrode
body.
In another aspect, the present invention provides a method for
selectively transmitting and trapping ions within a defined 3-dimensional
space, said
method comprising the steps of:
a) providing at least one ionization source for producing ions;
b) providing an analyzer region defined by a space between at least first
and second spaced apart electrodes, said analyzer region being in
communication with a gas inlet, a gas outlet and an ion inlet, said ions
produced by said ionization source being introduced into said
analyzer region at said ion inlet;
c) providing an asymmetric waveform voltage and a direct-current
compensation voltage, to at least one of said electrodes;
d) adjusting said asymmetric waveform voltage and said compensation
voltage to selectively transmit a type of ion within said analyzer
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-4-
region;
e) providing a curved surface terminus on at least one of said electrodes,
said defined 3-dimensional space being located near said terminus;
and
f) providing a gas flow within said analyzer region flowing from said gas
inlet to said gas outlet and adjusting said gas flow to trap said
transmitted ions within and near said defined 3-dimensional space,
said gas outlet being located near said terminus.
Advantageously, said analyzer region is operable substantially at
atmospheric pressure and substantially at room temperature.
The method may further comprise the step of providing an ion outlet
and supplying an extraction voltage at said ion outlet for extracting said
trapped
ions, said ion outlet being substantially aligned with said terminus and said
defined
3-dimensional space.
In yet another aspect, the present invention provides an apparatus for
selectively focussing ions and trapping said ions within a defined 3-
dimensional
space, comprising:
a) at least one ionization source for producing ions;
b) a segmented high field asymmetric waveform ion mobility
spectrometer, comprising an analyzer region defined by spaces
between a plurality of corresponding pairs of first and second spaced
apart electrodes, for connection, in use, to an electrical controller
capable of supplying an asymmetric waveform voltage, a direct
current compensation voltage and a direct current segment offset
voltage, each of said plurality of corresponding pairs of first and
second spaced apart electrodes forming a segment and said segments
being aligned in a row immediately adjacent to and electrically
isolated from each other, said analyzer region having an ion inlet for
introducing a flow of ions produced by said ionization source into
said analyzer region.
In yet another aspect, the present invention provides a method of
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-5-
selectively focussing ions and trapping said ions within a defined 3-
dimensional
space, comprising the steps of:
a) providing at least one ionization source for producing ions;
b) providing an analyzer region defined by spaces between a plurality of
corresponding pairs of first and second spaced apart electrodes and
providing a non-constant electric field between said first and second
electrodes, each of said plurality of corresponding pairs of first and
second spaced apart electrodes forming a segment and said segments
being aligned in a row immediately adjacent to and electrically
isolated from each other, said analyzer region being in communication
with an ion inlet, and introducing said ions produced by said
ionization source into said analyzer region at said ion inlet;
c) supplying an asymmetric waveform voltage to one of said first and
second spaced apart electrodes in each of said segments;
d) supplying a direct current compensation voltage to said one of said
first and second spaced apart electrodes in each of said segments,
said direct current compensation voltages supplied to each of said
segments being independently adjustable;
e) supplying a direct current segment offset voltage to another of said
first and second spaced apart electrodes in each of said segments,
said direct current segment offset voltages supplied to each of said
segments being independently adjustable; and
f) adjusting said direct current compensation voltages and said direct
current segment offset voltages substantially equally, thereby
providing a constant direct current potential across each
corresponding pair of first and second electrodes in each of said
segments, so as to focus desired ions between each corresponding pair
of first and second electrodes in each of said segments at a given
combination of said asymmetric voltage, direct current compensation
voltage, and direct current segment offset voltage.
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-6-
BRIEF DESCRIPTION OF THE DRAWINGS
For a better understanding of the present invention, and by way of
example, reference will now be made to the accompanying drawings, which show
preferred embodiments of the present invention in which:
Figure 1 shows three possible examples of changes in ion mobility as a
function of the strength of an electric field;
Figure 2 illustrates the trajectory of an ion between two parallel plate
electrodes under the influence of the electrical potential V(t);
Figures 3A and 3B show schematically an embodiment of a modified
FAIMS device;
Figure 4 illustrates two opposite waveform modes which may be used
with the apparatus of Figures 3A and 3B;
Figures 5A and 5B show schematically the coupling of the FAIMS
apparatus of Figures 3A and 3B together with a mass spectrometer;
Figures 6A and 6B shows schematically a FAIMS apparatus for
measuring the ion distribution in the analyzer region;
Figures 7 illustrates the high voltage, high frequency asymmetric
waveform applied to the FAIMS apparatus shown in Figures 6A and 6B;
Figure 8 illustrates varying ion arrival time profiles at the innermost
ion collector electrode of the FAIMS apparatus in Figures 6A and 6B;
Figures 9A and 9B show schematically a first embodiment of a 3-
dimensional atmospheric pressure high field asymmetrical waveform ion trap,
referred to as the FAIMS-R2-prototype;
Figures 10A through 101 show the experimental results for extraction
of ions trapped using the FAIMS apparatus of Figures 9A and 9B, with voltages
ranging from +1 V to +30 V;
Figures 11A-11C show a second embodiment of a 3-dimensional
atmospheric pressure high field asymmetrical waveform ion trap, referred to as
the
FAIMS-R3-prototype;
Figure 11D shows a timing diagram for a voltage applied to the
FAIMS apparatus of Figures 11A-11C;
Figure 12 shows an alternative embodiment of the FAIMS apparatus
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-7-
of Figures 11A-11C, having a simplified electrospray ionization chamber, and
using
the sampler cone as an extraction grid;
Figure 13A is a schematic view of a system comprising an apparatus
similar to the FAIMS apparatus disclosed in Figure 12, and a time-of-flight
(TOF)
mass spectrometer;
Figure 13B shows a timing diagram for control of the FAIMS
apparatus and the TOF mass spectrometer of Figure 13A;
Figure 13C illustrates a TOF mass spectrum acquired using the system
shown in Figure 13A;
Figure 13D illustrates a compensation voltage spectrum of an ion with
a TOF flight time of 27.0 gs;
Figure 13E shows graphically the results of an experiment designed to
determine the overall response time of the system shown in Figure 13A;
Figures 13F and 13G illustrate experimental verification of the 3-
dimensional ion trap using the system of Figure 13A;
Figures 13H, 131 and 13J, show the intensity of the TOF peak for
variable ion trapping periods from 1 ms to 60 ms, at three compensation
voltages;
Figures 14A-14C show schematically an alternative embodiment of a
3-dimensional atmospheric pressure high field asymmetric waveform ion trap;
Figure 15 shows the relevant dimensions of a FAIMS apparatus
required for calculation of the voltage within the FAIMS analyzer region;
Figure 16 shows the change in the Kh/K ratio for (H2O)nH+ as a
function of electric field E;
Figure 17 provides a portion of the original data that was used to
calculate the high field mobility Kh of (H2O)nH+;
Figures 18A-18D show the trajectory of an ion with the high electric
field properties shown by the curves in Figure 16;
Figures 19A-19D illustrate ion trajectory calculations near the
terminus of an inner electrode, calculated using the FAIMS apparatus and
method
described in Figures 11A-11D;
Figures 19E-19I illustrate the results of ion trajectory calculations near
the terminus of an inner electrode, using various sampler cone voltages in the
FAIMS
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-8-
apparatus shown in Figure 13A;
Figure 20 shows an example of an unusual shape of a FAIMS device
designed to establish conditions for ion trapping or focussing;
Figure 21 is a graph plotting the optimum combinations of CV and DV
for (H2O)nH+, based on data collected by a series of CV scans as shown in
Figure 17;
Figure 22A illustrates the electric field due to DV radially across the
FAIMS analyzer region for a given FAIMS apparatus;
Figure 22B is a graph showing the electric fields due to DV and CV
plotted against each other at several radial locations in the FAIMS analyzer
region;
Figure 22C is a graph showing the intersection of the actual conditions
and optimum conditions for DV and CV;
Figure 23 shows a segmented FAIMS apparatus for transporting ions
along the FAIMS analyzer region; and
Figure 24 shows a schematic of a segmented FAIMS apparatus for
trapping ions within the FAIMS analyzer region.
DETAILED DESCRIPTION OF THE INVENTION
As an important preliminary note, the discussion below uses the term
"ion" to mean a charged atomic or molecular entity. The "ion" can be any
electrically charged particle, solid or liquid, of any size. The discussion
always refers
to the "ion" as positively charged. However, all of the discussion in this
document is
equally applicable to negative ions, but with the polarity of applied voltages
being
reversed.
The principles of operation of FAIMS have been described in
Buryakov et. al. (see I. Buryakov, E. Krylov, E. Nazarov, and U. Rasulev, Int.
J. Mass
Spectrom. Ion Proc. 128. 143 (1993)) and are summarized here briefly. The
mobility
of a given ion under the influence of an electric field can be expressed by:
Kh(E) =
K(1+f(E)), where Kh is the mobility of an ion at high field, K is the
coefficient of ion
mobility at low electric field and "f(E)" describes the functional dependence
of the
ion mobility on the electric field (see E.A. Mason and E.W. McDaniel,
Transport
Properties of Ions in Gases (Wiley, New York, 1988); and I. Buryakov, E.
Krylov, E.
Nazarov, and U. Rasulev, Int. J. Mass Spectrom. Ion Proc. 128. 143 (1993)).
SUBSTTTUTE SHEET (RULE 26)
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-9-
Referring to Figure 1, three examples of changes in ion mobility as a
function of the strength of an electric field are shown: the mobility of type
A ions
increases with increasing electric field strength; the mobility of type C ions
decreases; and the mobility of type B ions increases initially before
decreasing at yet
higher fields. The separation of ions in FAIMS is based upon these changes in
mobility at high electric fields. Consider an ion 1, for example a type A ion
shown in
Figure 1, that is being carried by a gas stream 6 between two spaced apart
parallel
plate electrodes 2, 4 as shown in Figure 2. The space between the plates 2, 4
defines
an analyzer region 5 in which the separation of ions may take place. The net
motion
of the ion 1 between the plates 2, 4 is the sum of a horizontal x-axis
component due
to a flowing stream of gas 6 and a transverse y-axis component due to the
electric
field between the plates 2, 4. (The term "net" motion refers to the overall
translation
that the ion 1 experiences, even when this translational motion has a more
rapid
oscillation superimposed upon it.) One of the plates is maintained at ground
potential (here, the lower plate 4) while the other (here, the upper plate 2)
has an
asymmetric waveform, V(t), applied to it. The asymmetric waveform V(t) is
composed of a high voltage component, Vl, lasting for a short period of time
t2 and a
lower voltage component, V21 of opposite polarity, lasting a longer period of
time tl.
The waveform is synthesized such that the integrated voltage-time product
(thus the
field-time product) applied to the plate during a complete cycle of the
waveform is
zero (i.e., Vl t2 + V2 tl = 0 ); for example +2000 V for 10 s followed by -
1000 V for
20 s. Figure 2 illustrates the ion trajectory 8 (as a dashed line) for a
portion of the
waveform shown as V(t). The peak voltage during the shorter, high voltage
portion
of the waveform will be called the "dispersion voltage" or DV in this
disclosure.
During the high voltage portion of the waveform, the electric field will cause
the ion 1
to move with a transverse velocity component vl = KhEhigh, where Ehigh is the
applied field, and Kh is the high field mobility under ambient electric field,
pressure
and temperature conditions. The distance travelled will be d 1= vi t2 =
KhEhight2,
where t2 is the time period of the applied high voltage. During the longer
duration,
opposite polarity, low voltage portion of the waveform, the velocity component
of
the ion will be v2 = KEIoNõ where K is the low field ion mobility under
ambient
pressure and temperature conditions. The distance travelled is d2 = vA =
KE]oN,tl.
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-10-
Since the asymmetric waveform ensures that (Vl t2) +(V2 tl) = 0, the field-
time
products Ehight2 and E1oN,tl are equal in magnitude. Thus, if Kh and K are
identical,
dl and d2 are equal, and the ion 1 will be returned to its original position
along the
y-axis during the negative cycle of the waveform (as would be expected if both
portions of the waveform were low voltage). If at Ei;gh the mobility Kh > K,
the ion 1
will experience a net displacement from its original position relative to the
y-axis.
For example, positive ions of the type A shown in Figure 1 will travel further
during
the positive portion of the waveform (i.e., dl > d2) and the type A ion 1 will
migrate
away from the upper plate 2 (as illustrated by the dashed line 8 in Figure 2).
Similarly, ions of type C will migrate towards the upper plate 2.
If an ion of type A is migrating away from the upper plate 2, a
constant negative dc voltage can be applied to this plate 2 to reverse, or
"compensate" for this transverse drift. This dc voltage, called the
"compensation
voltage" or CV in this disclosure, prevents the ion 1 from migrating towards
either
plate 2, 4. If ions derived from two compounds respond differently to the
applied
high electric fields, the ratio of Kh to K may be different for each compound.
Consequently, the magnitude of the compensation voltage CV necessary to
prevent
the drift of the ion toward either plate 2, 4 may also be different for each
compound.
Under conditions in which the compensation voltage CV is appropriate for
transmission of one compound, the other will drift towards one of the plates
2, 4
and subsequently be lost. The speed at which the compound will move to the
wall of
the plates 2, 4 depends on the degree to which its high field mobility
properties differ
from those of the compound that will be allowed to pass under the selected
condition. A FAIMS instrument or apparatus is an ion filter capable of
selective
transmission of only those ions with the appropriate ratio of Kh to K.
The term FAIMS, as used in this disclosure, refers to any device which
can separate ions via the above described mechanism, whether or not the device
has
focussing or trapping behaviour.
Improvements to FAIMS
The FAIMS concept was first shown by Buryakov et. al. using flat
plates as described above. Later, Carnahan et. al. improved the sensor design
by
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-11-
replacing the flat plates used to separate the ions with concentric cylinders
(see B.
Carnahan, S. Day, V. Kouznetsov, M. Matyjaszczyk, and A. Tarassov, Proceedings
of the 41st ISA Analysis Division Symposium, Framingham, MA, 21-24 April 1996,
p. 85; U.S. Patent No. 5,420,424 issued to Carnahan et al.). The concentric
cylinder
design has several advantages including higher sensitivity than the flat plate
configuration (see R.W. Purves, R. Guevremont, S. Day, C.W. Pipich, and M.S.
Matyjaszczyk, Rev. Sci. Instrum., 69, 4094 (1998)).
As mentioned earlier, an instrument based on the FAIMS concept has
been built by Mine Safety Appliances Company (MSA). The MSA instrument uses
the concentric cylinder design and is described further below. (For the
purposes of
this disclosure, the MSA instrument is referred to as FAIMS-E, where E refers
to an
electrometer or electric current detection device.)
One previous limitation of the cylindrical FAIMS technology (see D.
Riegner, C. Harden, B. Carnahan, and S. Day, Proceedings of the 45th ASMS
Conference on Mass Spectrometry and Allied Topics, Palm Springs, California, 1-
5
June 1997, p. 473; and B. Carnahan, S. Day, V. Kouznetsov, M. Matyjaszczyk,
and
A. Tarassov, Proceedings of the 41st ISA Analysis Division Symposium,
Framingham, MA, 21-24 April 1996, p. 85) was that the identity of the peaks
appearing in the FAIMS-E CV spectra could not be unambiguously confirmed due
to
the unpredictable changes in Kh at high electric fields.
Thus, one way to extend the capability of instruments based on the
FAIMS concept, such as the FAIMS-E instrument, is to provide a way to
determine
the make-up of the FAIMS-E CV spectra more accurately, for example, by
introducing ions from the FAIMS-E device into a mass spectrometer for mass-to-
charge (m/z) analysis.
Electrospray Ionization
ESI is one of several related techniques that involves the transfer of
ions (which can be either positively or negatively charged) from liquid phase
into the
gas-phase. Kebarle has described four major processes that occur in
electrospray
ionization (intended for use in mass spectrometry): (1) production of charged
droplets, (2) shrinkage of charged droplets by evaporation, (3) droplet
disintegration
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-12-
(fission), and (4) formation of gas-phase ions (Kebarle, P. and Tang, L.
Analytical
Chemistry, 65 (1993) pp. 972A-986A). In ESI, a liquid solution (e.g. 50/50 w/w
water/methanol) is passed through a metal capillary (e.g., 200 m outer
diameter
and 100 m ID) which is maintained at a high voltage to generate the charged
droplets, say +2000 V (50 nA) for example. The liquid samples can be pumped
through at, say, 1 L/min. The high voltage creates a very strong, non-constant
electric field at the exit end of the capillary, which nebulizes the liquid
exiting from
the capillary into small charged droplets and electrically charged ions by
mechanisms
described by Kebarle and many others. Several related methods also exist for
creating gas-phase ions from solution phase. Some examples of these methods
include ionspray, which uses mechanical energy from a high velocity gas to
assist in
nebulization; thermospray, which applies heat instead of a voltage to the
capillary;
and nanospray, which uses small ID capillaries. In this disclosure, the term
ESI is
used to encompass any technique that creates gas-phase ions from solution.
Modified FAIMS-E
As a first step, the FAIMS-E device designed and built by Mine Safety
Appliances Company was modified to permit the introduction of ions using ESI.
The inventors believe that the coupling of an ESI source together with a FAIMS-
E
device is not obvious as it is known that ions produced by ESI have a high
degree of
solvation, and that a FAIMS-E device may not function properly when exposed to
high levels of solvent vapour. The inventors have developed various practical
embodiments of an apparatus that combines an ESI source together with a FAIMS
device to show that such coupling is possible.
One example is the modified FAIMS-E device 10 shown schematically
in 3-dimensional view in Figure 3A and in cross section in Figure 3B. The
FAIMS-E
apparatus 10 is composed of two short inner cylinders or tubes 11, 12 which
are
axially aligned and positioned about 5 mm apart, and a long outer cylinder 13
which
surrounds the two inner cylinders 11, 12. The inner cylinders 11, 12 (12 mm
inner
diameter, 14 mm outer diameter), are about 30 mm and 90 mm long, respectively,
while the outer cylinder 13 (18 mm inner diameter, 20 mm outer diameter) is
about
125 mm long. Ion separation takes place in the 2 mm annular space of FAIMS
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-13-
analyzer region 14 between the long inner cylinder 12 and the outer cylinder
13. To
produce ions using electrospray ionization (ESI), for introduction into the
FAIMS
analyzer region 14 of the FAIMS device, the metal capillary of the ESI needle
15 was
placed along the central axis of the shorter inner cylinder 11, terminating
about 5 mm
short of the gap or ion inlet between the two inner cylinders 11, 12. The
positioning
of the ESI needle 15 shown in Figures 3(A) and 3(B) differs from the
positioning of
the ionization source found in the MSA FAIMS-E device in that the ESI needle
15
does not extend through the long inner cylinder 12 to which the asymmetric
waveform V(t) is typically applied. By introducing the ESI needle 15 from the
opposite end of the FAIMS-E, i.e. through the short inner cylinder 11, and not
positioning the tip of the ESI needle 15 too close to the long inner cylinder
12, the
performance of the ESI needle 15 is not compromised by the asymmetric waveform
V(t), which would be the case if the ESI needle 15 was positioned within the
long
inner cylinder 12 (as disclosed in U.S. Patent No. 5,420,424).
As explained above, the FAIMS-E device 10 can be considered as an
ion "filter", with the capability of selectively transmitting one type of ion
out of a
mixture. If a mixture of ions is presented continuously to the entrance of the
FAIMS
analyzer region 14, for example by an ESI needle 15, and the ions are carried
along
the length of the analyzer 14 by a flowing gas under conditions in which no
voltages
are applied to either the inner cylinder 12 or outer cylinder 13 (i.e. the
electrodes are
grounded), some finite level of transmission for every ion is expected, albeit
without
any separation.
It might be expected that the detected current of any selected ion in
this mixture should never exceed the current for that ion when it is
transmitted
through the device 10 in the no-voltages condition. It might also be expected
that
application of high voltages (i.e. application of transverse fields,
perpendicular to
the gas flows) designed to yield ion separation should not increase the ion
transmission, but should decrease transmission through collisions with the
walls of
the cylinders 12, 13. That is, the asymmetric waveform might effectively
narrow the
"width" of the FAIMS analyzer region 14, and therefore should decrease the ion
transmission. However, contrary to this prediction, experiments conducted by
the
inventors and described in this disclosure have shown that the sensitivity of
ion
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-14-
detection in the cylindrical geometry FAIMS-E 10 increases as the voltage
amplitude
of the asymmetric waveform V(t) is increased. As will be explained below,
these
unusual observations suggest that atmospheric pressure ion focussing is
occurring in
the FAIMS analyzer region 14.
Still referring to Figures 3A and 3B, four gas connections to the
FAIMS-E apparatus 10 are shown. Compressed gas (e.g. air or nitrogen) is
passed
through a charcoal/molecular sieve gas purification cylinder (not shown) into
the
FAIMS-E 10 through carrier in (Cin) and/or sample in (Sin) ports. The gas
exits the
FAIMS-E 10 via the carrier out (Cout) and/or sample out (Soõt) ports. All four
gas
flow rates can be adjusted. Non-volatile analytes are typically introduced
into the
FAIMS-E 10 using an ESI needle 15. Alternatively, volatile analytes may be
introduced into the FAIMS-E 10 through the S;n line, and a portion may be
ionized as
the compound(s) pass by a corona discharge needle.
Still referring to Figures 3A and 3B, the outer cylinder 13 of the
FAIMS-E apparatus 10, and the shorter inner cylinder 11, are typically held at
an
adjustable electrical potential (VFAIMS). VFAIMS is usually ground potential
in
FAIMS-E. During operation, a high frequency high voltage asymmetric waveform
is
applied to the long inner cylinder 12 to establish the electric fields between
the inner
and outer cylinders 12, 13. In addition to this high frequency (e.g., 210 kHz)
high
voltage waveform a dc offset voltage (i.e. the compensation voltage CV added
to
FAIMS) is applied to the long inner cylinder 12. This leads to the separation
of ions
in the FAIMS analyzer region 14 in the manner discussed earlier.
Still referring to Figures 3A and 3B, some of the ions produced by the
ionization source are carried by the gas stream along the length of the
annular space
between the outer cylinder 13 and the long inner cylinder 12, also referred to
as the
FAIMS analyzer region 14. If the combination of DV and CV are appropriate, and
the ion is not lost to the tube walls, a series of openings or ion outlets 16
near the
downstream end of the outer cylinder 13 allow the ions to be extracted to an
electrical current detector 17 which is biased to about -100 V. (Note that
here the
carrier gas also exits from the ion outlet 16.)
In practice, the simplified square wave version of V(t) shown in
Figure 2 cannot be used because of the electrical power demands that such a
wave
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-15-
would place on the waveform generator. The actual waveforms V(t) appear in
Figure
4. These waveforms are produced by the electronic addition of a sine wave and
its
harmonic of twice the frequency. As shown in Figure 4, the FAIMS-E apparatus
10
operates using one of the two waveform modes (with the waveform applied to the
inner cylinder). These reversed polarity waveform modes do not yield "reversed
polarity" CV spectra as might be expected. This is because the reversal of
polarity
in this manner also creates a mirror image effect of the ion focussing
behaviour of
FAIMS. The result of such polarity reversal is that the ions are not focussed,
but
rather collide with the walls of the cylinders 12, 13. The mirror image of a
focussing
valley is a hill-shaped potential surface. (This characteristic, and the
various
"modes" of operation of FAIMS, is discussed further below.)
FAIMS MS
As discussed earlier, one way to extend the functionality of FAIMS
devices is to couple them together with a mass spectrometer. The use of a mass
spectrometer together with a FAIMS device is advantageous because the mass
spectrometer facilitates a mass-to-charge (m/z) analysis to determine the make-
up
of CV spectra more accurately. One possible FAIMS-MS embodiment is described
here.
Referring to Figures 5A and 5B, the coupling of FAIMS and a mass
spectrometer (FAIMS-MS 20) is shown schematically. The FAIMS-MS 20 of Figures
5A and 5B, and the FAIMS-E 10 shown in Figures 3A and 3B, differ significantly
only at the detection end of the instrument. In accordance with the invention,
the
electrometer 17 has been replaced by a sampler cone 18, placed at the end of
the
FAIMS cylinders 12, 13 as is shown in a simplified form in Figure 5B. The
diameter
of the orifice 19 in the sampler cone 18 is approximately 250 m. The gas
flows in
the FAIMS-MS 20 are analogous to those in the FAIMS-E 10 except that the Cout
is
divided into two components, namely the original Cout and the flow through the
orifice 19 into the mass spectrometer. The electrical waveforms applied to the
long
inner cylinder 12 are identical to those used in the FAIMS-E apparatus 10. The
sampler cone 18 may be electrically insulated from the other components so a
separate voltage OR can be applied to it. Furthermore, a voltage can be
applied to
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-16-
the cylinders of the entire FAIMS unit (VFAIMS) for the purpose of enhancing
the
sensitivity of the FAIMS-MS.
Figure 5B shows the FAIMS cylinders 12, 13 at a 45 degree angle in
relation to the sampler cone 18 of the mass spectrometer. Figure 5A showed the
FAIMS cylinders 12, 13 at a 90 degree angle in relation to the sampler cone
18. The
way (i.e., the angle between the two tubes of the FAIMS and the sampler cone
18) in
which the ions are extracted from the cylinders 12, 13 of the FAIMS-MS 20 into
the
mass spectrometer is not limited to these angles. Furthermore, the location in
which
the ions are extracted from the two tubes can also be changed. That is, the
ions can
be extracted anywhere along the separation region of the FAIMS.
Ion Focussing/FAIMS-R1-prototype
Referring now to Figures 6A and 6B, to demonstrate the focussing
effect referred to above, a special FAIMS instrument was designed by the
inventors
and constructed to measure the ion distribution between the two cylinders
(outer and
inner cylinders) of a FAIMS device. This instrument will be referred to in
this
disclosure as the FAIMS-R1-prototype 30 and is illustrated schematically in
Figures
6A and 6B. Ions were generated inside of an electrically grounded cylinder 31
approximately 35 mm long and 20 mm i.d.. The tip of an ionization needle 15
was
typically located near the center of this tube, and at least 15 mm from the
end of the
FAIMS analyzer region 34. The FAIMS analyzer region 34 in this embodiment is
composed of an outer tube 32 which is 70 mm long and 6 mm i.d., and which
surrounds a 2 mm o.d. inner shield electrode 33. The inner shield electrode 33
is an
electrically grounded stainless steel tube which is closed at the end that
faces the
ionization needle 15. This inner electrode 33 surrounds, and shields, an
electrically
isolated conductor 35 passing into its center. This innermost conductor 35
(i.e the
ion collector electrode) is a collector for ions, and is connected to a fast
current
amplifier or electrometer 36 (e.g. Keithly model 428) and a digital storage
oscilloscope 37 (e.g. LeCroy model 9450).
In the system shown in Figures 6A and 6B, the ions which surround
the inner electrode 33 are forced inwards by a pulsed voltage. These ions
travel from
the FAIMS analyzer region 34 to the innermost conductor 35 through a series of
50
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-17-
m holes 38 drilled through the inner shield electrode 33. The holes drilled in
the
inner shield electrode 33 are positioned about 2 cm from the end facing the
ionization
needle 15, and are spaced about 0.5 mm apart for a distance of 10 mm on one
side
of the inner shield electrode 33. The holes 38 drilled in the inner shield
electrode 33
are located in this manner to minimize the variability in distance between the
inner
shield electrode 33 and the outer cylinder 32 in the vicinity of these holes
38. It was
the inventors' objective to measure the ion abundance radial profiles of the
ions
located in the annular space (i.e. the FAIMS analyzer region 34) between the
inner
shield electrode 33 and the outer electrode 32 by pulsing the ions toward the
inner
shield electrode 33 and through the holes 38 and against the innermost ion
collector
electrode 35. The time-dependent distribution of ions arriving at the
innermost
conductor 35 is related to the physical radial distribution of ions around the
inner
electrode 33. Excessive variation in the distance between the two cylinders
32, 33
would have increased the uncertainty of the ion arrival times at the innermost
conductor 35, thus decreasing the spatial resolution of the measurements made
with
this device.
Now referring to Figure 7, the high voltage, high frequency asymmetric
waveform V(t), applied to the FAIMS-Rl-prototype of Figures 6A and 6B, is
shown.
The waveform is divided into two parts, the focussing period and the
extraction
period. The waveform was synthesized by an arbitrary waveform generator (e.g.
Stanford Research Systems model DS340, not shown) and amplified by a pulse
generator (e.g. Directed Energy Inc., model GRX-3.OK-H, not shown). The
frequency
of the waveform, and the relative duration of the high and low voltage
portions of
the waveform could easily be modified. Because of the high voltages, and steep
rise-times of the square waves applied to this FAIMS-Ri-prototype 30, the
power
consumption limits were severe, and waveforms in excess of about 1330 pulses
(16
ms at 83,000 Hz) could not be delivered by this system without overheating
electronic components of the high voltage pulse generator.
Note that, in the case of the FAIMS-R1-prototype 30, the high voltage,
high frequency asymmetric waveform was applied to the outer cylinder 32 of the
FAIMS-Rl-prototype 30 shown in Figures 6A and 6B. Since all other forms of
FAIMS discussed in this disclosure have the waveform applied to the inner tube
or
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-18-
electrode, confusion may arise from the "polarity" of the waveform and the
polarity
of CV. In the FAIMS-Ri-prototype 30 shown in Figures 6A and 6B, ions of type A
(shown in Figure 1) are focussed during application of the opposite polarity
waveform and CV than that shown for the devices in Figures 3A, 3B, 5A and 5B.
Nevertheless, for simplification, the polarity will be written to be the same
as if the
device was constructed in the same way as those of the more conventional
configuration. In other words the ions transmitted during application of
waveform
#1 will appear with DV positive and with CV negative. (Please note, however,
that
the actual voltages used on the device in Figures 6A and 6B are DV negative
and CV
positive).
As was observed in the conventional parallel plate FAIMS apparatus
described earlier (Figure 2), the application of a high voltage asymmetric
waveform
V(t) will cause ions to migrate towards one of the FAIMS electrodes 2, 4
because of
the changes in ion mobility at high electric fields (shown in Figures 1 and
2). This
migration can be stopped by applying an electric field or compensation voltage
CV in
a direction to oppose the migration. For the FAIMS-R1-prototype 30 of Figures
6A
and 6B, this CV was applied to the same electrode as the high voltage
asymmetric
waveform (i.e. the outer electrode 32), and was added to the waveform as a
small dc
bias (up to 50 V). At an appropriate combination of DV, and compensation
voltage CV, a given ion will pass through the FAIMS device 30. The unit
therefore
acts like an ion filter. It is possible to fix conditions such that a single
type of ion is
isolated in the FAIMS analyzer 34 although a mixture flows uniformly out of
the exit
of the FAIMS device 30 although a mixture of ions are presented to the inlet
of the
FAIMS analyzer region 34.
The second part of the waveform shown in Figure 7 (i.e. the extraction
period) was used to pulse the ions out of the FAIMS analyzer region 34 between
the
outer electrode 32, and the inner shield electrode 33 (shown in Figures 6A and
6B).
At the end of the focussing period, i.e. after 16 ms of waveform, the
asymmetric
waveform was replaced by a constant dc bias of approximately +30 V. This
caused
the ions from the annular space 34 between the outer electrode 32 and the
inner
shield electrode 33 to move in the direction of the inner shield electrode 33.
A
detector bias of -5 V, applied to innermost ion collector electrode 35, helped
to carry
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-19-
the ions from the vicinity of the holes 38 in the inner shield electrode 33,
through the
holes 38 and into contact with the innermost ion collector electrode 35. The
+30 V
bias created an electric field of approximately 150 V/cm across the FAIMS
analyzer
region 34 and most ions located within this region 34 travelled across the 2
mm
space in about 1 ms. The ion current due to the arrival of ions at the center
inner
shield electrode 33 can be predicted. For example, if only one type of ion,
with
mobility of 2.3 cm2/V-s, e.g., (H2O)nH+ at ambient temperature and pressure
conditions, was located in the FAIMS analyzer region 34, and if this ion was
distributed evenly in the space, an approximately square-topped signal lasting
approximately 0.6 ms should be observed. Deviation from this expected ion
arrival
profile would suggest that the ions were distributed in non-uniform profile
across the
FAIMS analyzer region 34 between the outer and inner cylinders of the FAIMS
device 30.
Still referring to Figures 6A, 6B, and 7, the FAIMS-R1-prototype 30
was operated as follows. A 2L/min flow of purified air, Carrier Gas In (Cin),
was
passed into the cylinder 31 housing the ionization needle 15. Approximately
2000
V was applied to the needle 15, and the voltage was adjusted to produce a
stable
ionization current. The high voltage asymmetric waveform V(t) was applied to
the
outer FAIMS cylinder 32 for approximately 16 ms; this was followed by a 2 ms
extraction pulse (Figure 7). The ion current striking the innermost ion
collecting
electrode 35 was detected and displayed on a digital oscilloscope 37. A
measurement would typically consist of 100 averaged spectra, collected at a
rate of
approximately 5 Hz. Many experimental parameters were varied, including gas
flow
rates, the voltages of the asymmetric waveform V(t), the dc voltage applied to
the
outer electrode CV, and the extraction voltage.
Figure 8 illustrates the ion arrival times at the innermost ion collector
electrode 35 observed by conducting these experiments. Each trace was recorded
with 2500 V applied DV, but with variable CV voltages. As can be seen, during
application of DV and CV, the radial distribution of ions is not uniform
across the
annular space of the FAIMS analyzer region 34. For example, at CV near -11 V,
the
ions are focussed into a narrow band near the inner electrode 33, and
therefore are
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-20-
detected as a high intensity pulse occurring very early after the extraction
voltage has
been applied. At low CV, for example at -5.6 V, the ions are much more
uniformly
distributed between the walls of the concentric cylinders 32 33 making up the
FAIMS
analyzer region 34. When no electrical voltages are applied to the cylinders
32, 33,
the radial distribution of ions should be approximately uniform across the
FAIMS
analyzer region 34 (data for this no-voltage experimental condition is not
shown in
this document). The experimental data shown in Figure 8 is evidence that the
ion
focussing is indeed occurring in FAIMS instruments. This focussing results in
the ions
being focussed in a uniform "sheet" or band around the inner cylinder 33
within the
FAIMS analyzer region 34. As mentioned previously, to the inventors'
knowledge,
this focussing effect has never been observed or explained previously.
The 3-Dimensional Atmospheric Pressure Ion Trap
The gas flows between the cylinders of the FAIMS devices described
above serve to carry the ions from one end of the device to the other end. In
every
case the action of the electric fields is perpendicular to the transporting
motion of the
gas flow. This is the reason the early devices were referred to as transverse
field
compensation ion mobility spectrometers. The present invention is the result
of
attempts to convert the 2-dimensional ion focussing action of the FAIMS-E 10
and
FAIMS-Rl-prototype 30 into a 3-dimensional trap by ensuring that the ions are
caught in a physical location in which the gas flows and the electrical fields
are not
perpendicular, but rather act in opposition to each other. This creates a
3-dimensional atmospheric pressure ion trap.
Note that, in this disclosure, the term "ion focussing" is restricted to a
2-dimensional configuration. That is, if the ions are "focused", they will be
restricted to a sheet-like structure, and the thin, flat sheet can extend in
any
direction, for any distance. For example, if ions are "focused" around the
external
surface of a long metallic cylinder, this will mean that they are restricted
to be within
a cylindrical space (composed of the ions) which is coaxial to, or surrounding
the
metallic cylinder. This sheet of ions will extend as far as the cylinder, and
all
around it continuously. On the other hand, in this disclosure the term "ion
trapping"
is restricted to the condition that an ion cannot move freely in any direction
in
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-21-
3-dimensional space. This is more restrictive than "focussing", in which the
ion is
free to move anywhere in the 2-dimensions e.g. along the length of the
cylinder
described in the example noted above or around the cylinder at a fixed radius.
3-dimensional ion traps for operation in vacuum chambers of mass
spectrometers are well known, and several geometry's exist. However, the
mechanism and operation of these vacuum-ion-traps is vastly different from
that of
the atmospheric pressure (760 torr) version of the ion trap described in this
document. The physical geometry, the layout of the hardware components, and
the
electrical voltages applied in known 3-dimensional ion traps are in no way
related to
the present atmospheric version of the ion trap. Several embodiments of the
3-dimensional atmospheric pressure ion trap of the present invention will be
considered below.
FAIMS-R2-Prototype
Referring to Figures 9A and 9B, the device which will be referred to as
the FAIMS-R2-prototype 40 is shown. Here, the asymmetric waveform V(t) and the
compensation voltage CV are applied to the inner, solid, electrode 42, having
a
diameter of about 2 mm. The outer, electrically grounded electrode 43 has an
inner
diameter of about 6 mm, thereby allowing an annular space of about 2 mm
between
the electrodes. This annular space has been referred to as the FAIMS analyzer
or
FAIMS analyzer region 14, 34, 44 in the discussion above, and for simplicity
we will
continue to use this terminology. The ions are created by corona discharge
using a
corona needle 15 in a closed cell (not shown) located adjacent to a 0.5 mm
hole
through the wall of the outer cylinder. As shown in Figure 9A, ions are driven
by the
high electric field generated by the corona discharge needle 15 (held at about
+ 2000
V), through the 0.5 mm hole 45, and into the FAIMS analyzer region 44 (only
those
ions travelling directly toward the hole 45 are shown for simplicity). Inside
the
FAIMS analyzer region 44, near this hole 45, the electric fields and the gas
flow
(shown to be flowing from right to left in Figures 9A and 9B) are
perpendicular to
each other and the ions experience the 2-dimensional focussing effect
described in the
sections above in relation to the FAIMS-R1-prototype 30. However, the inner
electrode 42 in the device shown in Figure 9A, terminates about 1 - 4 mm from
the
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-22-
end of the outer electrode 43. The inner surface of the outer electrode 43 at
the
downstream end is contoured in such a way as to maintain approximately the
same
electric fields (i.e. created by the application of DV and CV) as would be
experienced along the length of the FAIMS analyzer region 44. The end of the
outer
electrode 43 has an exit grid 46 comprising a hole (about 2 mm) which is
covered
with a fine, high transmission metallic screen. The gas flowing through the
device 40
also flows freely through the grid 46 and exits from the space between the
outer
electrode 43 and a collector plate 47. In the absence of any applied voltages
(i.e.
DV and CV = 0) the ions will travel through the device very much as shown in
Figure
9A. The ions enter the analyzer region 44, flow with the gas out through the
exit grid
46 of the outer electrode 43, and the few remaining ions are attracted to an
ion
collector plate 47 biased at about -5 V. The collector plate 47 was connected
to a
high gain current amplifier or electrometer 36 (e.g. Keithly 428) and an
oscilloscope
37.
The application of an asymmetric waveform of the type shown in
Figure 7 resulted in the ion focussing behavior described above for the
conventional
FAIMS-E 10 and FAIMS-R1-prototype 30, except that the focussing action extends
around the generally spherically shaped terminus 42T of the inner electrode
42, as
shown in Figure 9B. This means that the ions cannot escape from the region
around
the terminus 42T of the inner electrode 42. This will only occur if the
voltages
applied to the inner electrode 42 are the appropriate combination of CV and DV
as
described in the discussion above relating to 2-dimensional focussing. If the
CV and
DV are suitable for the focussing of an ion in the FAIMS analyzer region 44,
and the
physical geometry of the inner surface of the outer electrode 43 shown in
Figures 9A
and 9B does not disturb this balance, the ions will collect near the terminus
42T as
shown in Figure 9B. Several contradictory forces are acting on the ions in
this region
near the terminus 42T of the inner electrode 42. The ion cloud shown near the
terminus 42T of the inner electrode 42 in Figure 9B would like to travel from
right to
left to the exit grid 46 in the manner shown in Figure 9A, because of the
force of the
gas flow. This also means that the ions cannot migrate back from left to
right,
toward the ionization source 15. The ions that get too close to the inner
electrode 42
are pushed back away from the electrode 42, and those near the outer electrode
43
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-23-
will migrate back towards the inner electrode 42, because of the application
of the
negatively polarized CV. The ions are captured in every direction, either by
forces
of the flowing gas, or by the electric fields (electric potential well) of the
FAIMS
mechanism.
Note that, while the above discussion refers to the ions as being
"captured" or "trapped", in fact, the ions are subject to 'diffusion'.
Diffusion
always acts contrary to focussing and trapping. The ions will always require
an
electrical, or gas flow force to reverse the process of diffusion. This means
that
although the ions may be focused into an imaginary cylindrical zone in space
(with
almost zero thickness), or within a 3-dimensional ion trap, in reality it is
well known
that the ions will actually be dispersed in the vicinity of this idealized
zone in space
because of diffusion. This means that ions will always be "distributed" over
some
region, rather than all precisely located in the same place. This is
important, and
should be recognized as a global feature superimposed upon all of the ion
motions
discussed in this document. This means that, for example, a 3-dimensional ion
trap
will actually have real spatial width, and leak for several physical, and
chemical
reasons.
Expanding on the chemical effects in FAIMS, if an ion collides with a
neutral molecule and temporarily forms a stable complex, this complex may
drift out
of the FAIMS focussing or trapping region because this new complex has high
field
mobility properties which are different from the original ion. This means that
the
complex may have behavior at high electric field (see Figure 1) which differs
from the
original simple parent ion. For example (at the extreme) the original ion may
be of
type A, and the new complex of type C shown in Figure 1. If this is the case,
the new
complex will not be trapped at the prevailing DV and CV conditions. The
collision
of any of these ions with the walls of the device will soon result in loss of
the ions
from the trap. Although the original ion itself may continue to be trapped,
the
removal of this ion via "chemical" effects is entirely possible, and is the
reason the
FAIMS analyzer will fail in the presence of significant water vapor or
contaminants
in the gas flows. The FAIMS analyzer works best in very clean conditions.
During
operation in P2 mode, the requirement for a high purity gas is somewhat
relaxed.
Now referring to Figures 10A through 101, experimental results with
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-24-
the FAIMS-R2-prototype 40 are shown. The dimensions of the electrodes were
described above, for Figures 9A and 9B. The DV was approximately 2000 V, CV
was -12 V, and the gas flow through the device was 0.9 L/min. The DV and CV
were applied to the inner electrode for about 16 ms, then these voltages were
replaced by an extraction voltage applied to the inner electrode 42. The DC
extraction voltage applied to the inner electrode 42 pushes the ions away from
the
inner electrode 42 towards the exit grid 46, whereby the gas flow carries
these ions
through the grid 46 (with some percentage of the ions lost in collisions with
this grid
46). The traces in Figure 10A through 101 represent results for the ions
extracted
with voltages ranging from +1 V (Figure 10A) to +30 V (Figure 10I). The
extraction
of trapped ions results in a positive pulse 48 recorded in Figures 10A-10I.
The
negative pulse 49 shown in the figures is the electronic transient noise that
occurs
when the DV and CV are removed and replaced by the extraction voltage. It is
clear
from the data shown in Figures 1OA-10I that an increase in the extraction
voltage will
yield a shorter, more intense ion signal 48. This occurs since the ions are
pulsed out
of the trap more vigorously with the +30 V than the +1 V. The experimental
results
shown in Figures 1OA-10I verify the hypothesis that a cloud of ions
accumulates near
the terminus 42T of the inner electrode 42. A pulse of ions, as shown in
Figure 10I,
could not be extracted from the FAIMS-R2-prototype 40 unless some ions were
available near the terminus 42T of the inner electrode 42.
FAIMS-R3-Prototype
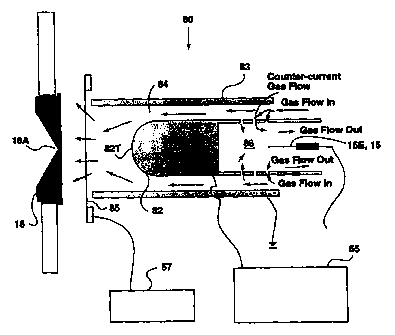
Now referring to Figures 11A through 11C, the FAIMS-R3-prototype
50 is shown. This device is configured for detection by mass spectrometry, and
a
sampler cone 18, through which gas and ions are pulled into the vacuum chamber
of
a mass spectrometer is shown on the left side of Figures 11A-11C. The right
side of
the vacuum housing, and sampling cone 18, is substantially at atmospheric
pressure.
The left side of those components is labelled "Mass Spectrometric Vacuum
Chamber", and is typically below 1 torr pressure. In most systems a second
orifice
(not shown) leads to the mass analyzer region of the mass spectrometer which
is
usually below 10-5 torr pressure.
The FAIMS-R3-prototype analyzer 50 shown in Figure 11A consists of
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-25-
an inner, solid, cylindrical electrode 52 of about 2 mm diameter, and an outer
electrode 53 which is about 6 mm inner diameter. The center electrode 52 is
powered, through an electrical connection, by an asymmetric waveform generator
power supply 55. Both DV and CV are supplied by this generator 55. The
waveforms, and the timing diagram are shown in Figure 11D. As shown in Figure
11D, the asymmetric waveform is applied continuously to the inner electrode
52.
Referring back to Figure 11A, gas enters the FAIMS-R3-prototype 50
from the right side and flows along the annular space comprising the FAIMS
analyzer
region 54, and out through the open end of the outer electrode 53. Adjacent to
the
open end (left side) of the outer cylinder 53 is an exit grid 56 comprising a
fine,
thin-wired metallic grid which is electrically isolated from the outer
electrode 53, and
has an electrical connection to a grid electric pulse generator power supply
57. The
voltage on the grid 56 can be changed step-wise using this power supply. The
grid
voltage and timing diagram is shown in Figure 11D. The grid is typically
maintained
between -5 and +5 V during the ion storage time (e.g. 0 V) shown in Figure
11D. The
grid will then be stepped (100 ns transition) to between -5 V and -50 V (e.g. -
15 V in
Figure 11D) in order to extract the ions from the 3-dimensional atmospheric
pressure
trap which is located at the spherical terminus 52T of the inner electrode.
Figure 11B
shows schematically the approximate location of the ions during the storage
period.
It should be kept in mind that the ions trapped here must have the correct
high field
ion mobility (see Figure 1) so that their "net" motion is zero at the
combination of CV
and DV being applied to the storage device (the term "net" is used because the
ion is
constantly moving back-and-forth due to the application of the asymmetric
waveform: if the ion returns to the same location repeatedly, then the "net"
motion
caused by the application of DV and CV is zero). For example, the (H2O)nH+
ions
will be stored in the geometry shown in Figure 11A-11C at a DV of about +2000
V
and a CV of approximately -10 V (typical of P1 mode ). At conditions very
different (e.g. at DV 2000 and CV +10 V) from this combination of DV and CV,
the
(H2O)nH+ ions will not assemble into one physical location as shown in Figure
11B.
Instead, these ions will collide with the walls of the cylinders 52, 53. At a
second set
of DV and CV conditions, such as the DV 2500 and CV -5 V, another ion (e.g.
(Leucine)H+ ) may be able to collect at the tip 52T of the inner electrode 52
as shown
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-26-
in Figure 11B.
Near the terminus 52T of the inner electrode 52 shown in Figure 11B,
the ions are restricted in motion because of several contrary forces. The gas
flowing
along the FAIMS analyzer region 54 applies a force which will prevent
migration of
ions from the left to right (Figure 11B) back toward the ion trap gas inlet,
and this
force will also tend to pull the ions out of the trap towards the exit grid 56
shown at
the left end of the outer electrode. The electrical forces characteristic of
FAIMS
maintain the ions at a fixed distance from the sides of the inner electrode
52: (1) the
ions which are too distant from the inner electrode 52 are attracted to the
inner
electrode 52 because of the negative polarity of the applied dc offset, i.e. a
negative
CV; and (2) the ions close to the inner electrode 52 are pushed away because
of the
increase of the ion mobility at high field (see Figure 1) assuming the ions
are of type
Pl. Details of the ion motions are presented below.
Figure 11C illustrates the removal of ions from the 3-dimensional
atmospheric pressure trap via a stepwise change to the voltage applied to the
grid
electrode 56. If the voltage applied to the grid 56 is decreased from, say, 0
V to -15
V as shown in the timing diagram Figure 11D, the well depth of the ion trap is
reduced or eliminated, and the ions are free to escape under the influence of
the gas
flow, or by the electric field which might pull the ions toward the exit grid
56.
The FAIMS-R3-prototype 50 shown in Figures 11A-11C is
appropriate for detection of ions produced by electrospray ionization (ESI).
FAIMS
is highly sensitive to moisture and contaminants in the gas entering the
analyzer
region. It is common that contaminants, or too much water vapor, will result
in
complete loss of signal, and failure of the FAIMS to function in the manner
described
in this document. Since electrospray ionization involves the
high-voltage-assisted-atomization of a solvent mixture, the amount of water
and
other volatile solvents is far too high to be tolerated in the FAIMS analyzer
region 54.
This will mean that the ESI-FAIMS combination will always require a type of
gas-isolation, curtain gas, or counter-current gas flow, to prevent neutral
solvent
molecules from entering the FAIMS analyzer region 54. One method to accomplish
this is shown in Figures 11A-11C. The FAIMS is separated from the ESI chamber
60
by a small chamber 61 which has provision for gas inlets 62 and gas outlets
63. If a
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-27-
flow of gas enters this intermediate chamber 61, and a portion of the gas
flows
toward the ESI chamber, then the neutral solvent molecules will exit via the
port on
the ESI chamber, and will be prevented from entering the ion trap aperature.
The
electrospray needle 15E, shown in Figures 11A-11C is more likely to be in a
horizontal plane or lower than the FAIMS analyzer region 54, rather than the
higher,
vertical position shown. This minimizes the tendency for very large droplets
to fall
via gravity, into the FAIMS analyzer region 54. In a horizontal or lower
configuration the large droplets will fall into the bottom of the ESI chamber
60, which
could (optionally) have a drain for removal of excess solvent. Alternatively,
if the
ion trap gas inlet is closed off, gas entering the purge gas inlet could be
used to both,
help in desolvation, and a portion of this gas flow would be used to carry the
ions
along the FAIMS analyzer region 54 from right to left in Figure 12.
The counter-current of gas can be achieved in a second way shown in
Figure 12 (gas flows are emphasized, and most of the ions are omitted). If the
FAIMS analyzer gas flow is adjusted so that some of the gas will exit the
FAIMS
analyzer region 54 into the ESI chamber 60, the entrance of neutral
contaminants can
be avoided. This may result in higher ion transmission than that for the
device
shown in Figures 11A-11C. Note also that the exit grid electrode 56 (Figures
11A-
11C) has not been shown in Figure 12. In this embodiment the 'extraction'
pulse that
destroys the ion trap is applied to the mass spectrometer sampling cone 18.
FAIMS Ion Trapping Mass Spectrometry Experiments: Instrumental Overview
Now referring to Figures 13A-13J, a system of using a time-of-flight
(TOF) mass spectrometer, in conjunction with the FAIMS-R3-prototype 50, is
discussed. As shown in Figure 13A, the assembly of the FAIMS, the ion
production,
and gas controls are similar to that shown in Figure 12. For the purposes of
further
elucidation of the operating details of this system, and the experimental
results, the
diagram has been extended to show the internal components of the time-of-
flight
(TOF) mass spectrometer 70 used for this work. The timing diagram for control
of
the dispersion voltage, compensation voltage, sampler cone voltage VOR, and
the
TOF acceleration pulse appears in Figure 13B.
Figure 13A shows that there is an electrical connection to the sampler
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-28-
cone. This electrical connection is used to control the sampler cone voltage
VOR, the
voltage used to gate the ions out of the ion trap. For example, in a typical
experiment, the VOR may be set at +40 V during trapping of the ions and at +1
V for
ion extraction (e.g., at FAIMS offset voltage +20 V, and compensation voltage -
3 V).
These voltages would be applied for time periods, for example, 40 ms for
trapping
and 10 ms for ion extraction. After the initiation of the ion extraction, a
cloud of
ions, which was located near the terminus 52T of the inner electrode 52, would
move
towards the sampler cone 18. Because of the electric field between the sampler
cone
18 and the FAIMS 50, and the high flow of gas through the sampler cone orifice
18A
and into the vacuum system, some ions would be transported into the low
pressure
(1 torr) region between the sampler cone 18 and the skimmer cone 71. The
skimmer
71 is typically held at ground potential. The 1 V difference between the
sampler
cone 18 and the skimmer 71 is sufficient to draw the ions through the skimmer
71,
after which they enter a low pressure region (9x10-5 torr) and are transported
to the
entrance of the TOF acceleration region 72 via an octopole ion guide 73. The
octopole guide 73 is operated at low pressure so that the delay and broadening
of
the pulse during transport of the pulse through the octopole 73 is minimized.
The
octopole 73 is typically operated using a DC offset of -4 V, and a 1.2 MHz
applied
waveform of 700 V (peak-to-peak) to confine the ions. An exit aperture lens of
the
octopole ion guide (not shown) is held at -5.5 V. The ions pass through the
octopole exit lens and through a series of grids which compose the ion
acceleration
region 72 of the TOF mass spectrometer 70.
Still referring to Figure 13A, the acceleration region 72 of the TOF is
connected to two high voltage pulse generators 74A, 74B, and operates as
follows.
The device includes 3 fine mesh metal grids 72A, 72B, 72C. The grid 72C which
is
located closest to the flight tube is held at constant ground potential. The
other two
grids 72A, 72B are each connected to a high voltage pulse generator 74A, 74B
respectively. The grids 72A, 72 Bare found at two possible voltage states,
controlled
by external pulse generator digital logic 75. In one voltage state both of the
grids
72A, 72B are held at one voltage, our experiments used -5.5 V. In this state,
the ions
that travel from the exit lens of the octopole ion guide 73, will pass through
the grids
72A, 72B. The grids 72A, 72B are also held at a second, high voltage condition
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-29-
obtained by applying a pulse (less than 0.1 s risetime) of about 50 gs
duration.
There will be some ions which are located in the regions between these grids
72A,
72B when the pulse is applied, and these ions will be accelerated in the
direction of
the flight tube 76 and the detector 77 shown in Figure 13A. These ions pass
through
the second grid 72B and are further accelerated because of the high electric
field
between the second grid 72B and the third grid 72C, which is at ground
potential.
Once these ions have passed out of the acceleration region 72, and are
travelling
along the flight tube 76, the voltages on the two variable grids 72A, 72B in
the
acceleration region 72 are returned to the low voltage condition, and new ions
can
enter the space between the grids 72A, 72B. The grids 72A, 72B are typically
maintained at high voltage for about 50 s.
In principle, the ions which pass down the flight tube 76 separate
according to mass (for this discussion, we assume charge (Z) = +1) because all
of the
ions have the same energy (as a first approximation), defined by the voltage
drop
between the pulsed voltage grids 72A, 72B and the fixed grounded grid 72C. The
ion
energy is defined by Ei = mv2/2, therefore ions of different mass, m, hade
different
ion velocities, v, so that Ei, the energy, is constant. The ions arrive at the
TOF
detector 77 in sequence of ion mass. The lowest mass ions have the highest
velocity
and arrive at the detector 77 first, and the highest mass ions arrive last.
In practice, however, not the all of the ions leaving the acceleration
region 72 have identical voltages. The ions have energy in part dictated by
their
starting location between the two pulsed acceleration grids. This difference
in
energy allows the device the capability of 'spatial' focussing, which means
that all of
the ions with a given m/z, regardless of their starting position in the
acceleration
region 72, will reach the detector 77 simultaneously. This use of the word
'focussing'
in this context is much like the focussing of light in an optical system
(e.g., camera).
Ions accelerated from between the second grid 72B and the grounded grid 72C
have a
wide range of energy and contribute to unwanted 'background' noise. This is
minimized by locating the second grid 72B very close (2 mm) to the grounded
grid
72C.
The TOF acceleration region 72 is pulsed at fixed delay times
following the pulse applied to the sampler cone 18 (VOR). There will be a
finite delay
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-30-
time for the pulse of ions to be extracted from FAIMS 50, pass through the
vacuum
interface 18, 71, through the octopole 73, and into the acceleration region
72. TOF
mass spectra are collected at a series of delay times after the extraction
pulse is
applied to the sampler cone 18. The arrival of the pulse of ions is
characterized by
the appearance of a strong, transient signal, followed by a decay of signal
intensity
down to a constant level which corresponds to the uniform signal which would
be
detected if the sampler cone was held at the low voltage (i.e., +1 V) state
continuously.
TOF Mass Spectra, and CV Spectra for the Study of Ion Trapping
The low mass ions produced by corona discharge ionization,
particularly protonated water ions were at very high ion density (abundance),
and
were therefore expected to either fill the trap too rapidly, or have too short
lifetimes
for the present study. Therefore, it was decided to look for higher mass ions
in P2
mode. The abundance of these ions were expected to be low since they were only
formed from trace contaminants in the carrier gas. No additional sample
compounds or gases were added to the system. The ions studied here were formed
by corona discharge ionization in the "clean" nitrogen atmosphere. The ion
source,
and the FAIMS device were operated in as clean a condition as possible.
Figure 13C illustrates a typical mass spectrum acquired for this study.
The exact mass was not determined, since this would require a known
calibration
compound, however the approximate mass was determined using the flight times
for
some lower mass ions including the protonated water ions. Several impurity
ions 81,
82 appear in the spectrum, however only the ion 83 of highest abundance
(flight time
27.0 s) was considered for the present study. This ion 83 has a m/z of about
380
( 10 m/z).
Figure 13D illustrates the compensation voltage scan for detection of
the ion 83 with flight time of 27.0 s (m/z about 380) at an applied
dispersion
voltage of -3500 V. This polarity of DV is referred to as P2 mode, and the
ions
typically passing through FAIMS in P2 mode usually have mass above m/z 300.
The
ions that are usually present in P2 mode have ion mobilities that decrease as
the
electric field increases (ion type C, Figure 1). One limitation of P2 mode is
that the
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-31-
ions are typically found at low CV, and therefore the strength of the ion
trapping is
weak. On the other hand, one advantage of higher mass ions is that the ion
mobility
is usually lower, and therefore the distances travelled during the application
of the
high voltage asymmetric waveform are reduced, and the rate of ion loss to the
walls
via diffusion is expected to be minimized.
The ion intensity at each experimental point in Figure 13D was
acquired by averaging the spectra recorded from 5000 repeat TOF acceleration
pulses. The compensation voltages were adjusted manually, with a digital
voltmeter
used to read out the voltages set by a power supply. Three traces appear in
Figure
13D, corresponding to the collection of compensation voltage sweeps in three
operating methods including: (1) pulsed sampler cone 18 with detection at 4.5
ms
after the 'down' edge (transition) of the VOR; (2) continuous ion transport
from
FAIMS through to the TOF with the VOR set at +1 V; and (3) continuous ion
transport from FAIMS to the TOF with VOR at +15 V. The compensation voltage
corresponding to the maximum detected ion transmission was comparable for
these
three methods of data acquisition. From Figure 13D, the ion with flight time
20.0 s
was transmitted through the FAIMS- device 50 at DV = -3500 V and a
compensation
voltage between about -2.5 V and -4 V.
Ion Transport Delays within the Ion Optics of the TOF
Now referring to Figure 13E, the results of an experiment designed to
determine the response time of the entire system are shown. The VOR was
stepped
between two values, one (+15 V) which was suitable for ion transmission
through the
FAIMS and into the vacuum system, and the second voltage (-10 V) which was
unsuitable for either trapping or ion transmission. Figure 13E illustrates the
intensity
of mass spectra collected at a series of time delays between the VOR
transitions of
both possible types, namely from high to low voltage, and also from low to
high
voltage. For the discussion below, these transitions will be considered the
'down'
and 'up' edges of the change in VOR, respectively. The origin of the delay,
and the
length of delay, is different for the two cases. The reasons are considered
next.
In the case of high to low voltage transition, 'down', which occurs at
ms in Figure 13E, the low voltage applied to sampler cone 18 will prevent any
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-32-
(positively charged) ions from passing between the sampler cone 18 (i.e., VOR
at -10
V) and the skimmer cone 71 (at 0 V), and thus the "down" transition will
create an
extremely abrupt decrease in ion flux passing into the octopole ion guide 73.
At one
extreme it might therefore be expected that the intensity of spectra taken by
the TOF
might decrease to zero abruptly. Experimentally, the abrupt decrease in ion
density
will be 'blurred' due to ions moving back into the low ion density region.
This
broadening is expected: (a) because not every ion will have identical kinetic
energy,
and those ions with slightly less energy will fall behind, and (b) because
collisions
between the ions and the residual gas within the octopole housing 73 will
affect the
kinetic energy of some fraction of the ions. Since the octopole 73 is an ion
guide, this
longitudinal spreading will be accentuated since ions which have undergone
collision
with the residual gas will remain contained within the octopole 73. Because of
their
lowered kinetic energy, these ions will travel through the octopole 73 and
arrive at
the acceleration region 72 of the TOF with long delay times. Figure 13E shows
that
the ions continue to arrive at the TOF acceleration grids for about 2 ms after
the VOR
is shifted from +15 V down to -10 V. Note that this 'down' transition occurs
at 40
ms on Figure 13E.
The 'up' voltage transition of the sampler cone from low to high
voltage has a slightly different effect. This transition occurs at time 0 ms
in Figure
13E. As shown in Figure 13E, the time required for the intensity of the TOF
spectra
to reach a plateau is about 10 ms. Several delays are expected. When VOR is
raised, the relatively low density of ions which are located in front of the
terminus
52T of the inner cylinder 52 of FAIMS 50 must be augmented by newly arriving
ions
which have been passing along the annular region 54 between the FAIMS
cylinders
52, 53. Secondly those ions must start to pass through the sampler cone 18 to
the
skimmer 71 region, and subsequently through the octopole 73. From the
discussion
of the 'down' edge of the VOR pulse described above, it requires a minimum of
2 ms
for changes in ion density (of the ion which is monitored) to be transmitted
through
the octopole 73. The additional time delays required prior to ion abundance
increases in the TOF spectra (i.e., the difference between 2 ms and 10 ms) are
therefore attributed to delays in appearance of ions in front of the sampler
cone 18,
and secondly due to the transmission through the sampler cone 18 to the
skimmer 71
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-33-
region.
Experimental Verification of Ion Trapping in FAIMS
Now referring to Figures 13F and 13G, the experimental verification of
the 3-dimensional ion trap located near the spherical terminus 52T of the
inner
electrode 52 of the FAIMS 50 is illustrated. The experimental conditions for
collection of the data for Figure 13F and 13G were identical, except that the
carrier
gas flow into FAIMS 50 was decreased for collection of Figure 13G. The data
for
these plots were collected in independent experiments, about 1 week apart.
The plots in Figure 13F and 13G show the measured intensity of the
ion with flight time 27.0 s (about m/z 380) collected at various times after
the
'down' transition of the sampler cone 18. The timing of these pulses is shown
at the
bottom of Figure 13F. Time zero represents the time at which the sampler sone
18 is
pulsed from the high voltage state (VOR = +40 V) to its low voltage state (VOR
= +1
volt), thereby extracting ions from the FAIMS trap. The ions require about 5
ms to
travel through the system to the TOF acceleration region 72. The pulse of ions
is
widened during passage, and appears to be about 3 ms wide (at half height)
when
detected by the present system.
Figures 13F and 13G also include two horizontal lines corresponding
to collection of non-pulsing mode data at two different settings of VOR. The
lower
intensity data was collected with VOR = +1 V, which corresponds to the 'low'
state
of the sampler cone 18 when operating in pulsed mode. The higher intensity,
horizontal trace, was collected at an experimentally optimized setting for the
sampler cone 18 (at VOR = +15 V). At this setting the dc level of the sampler
cone 18
resulted in the maximum possible TOF spectrum intensity for the non-pulsing
mode.
Note that the intensity of signals for VOR =+15 V for Figures 13F and 13G are
comparable although the data was collected on different occasions and with
different FAIMS gas flow conditions. Ion trajectory modelling has shown that
the
ions passing around the terminus 52T of the inner electrode 52 can be focused
towards the center channel as they pass by the end of the electrode 52. In
this way
the ions will tend to be transmitted into the sampler cone orifice 18A leading
to the
vacuum with maximum sensitivity. An example of this trajectory calculation
that
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-34-
indicates that this ion focussing will occur is shown in Figure 19C and 19D
(below).
Ion Storage Time Period
An experiment was performed to determine the effect of the ion
storage period on the intensity of the detected pulse of ions resulting from
extracting
the ions from the storage zone near the end of the inner FAIMS electrode. The
intensity of the TOF peak for the ion at flight time 27.0 s, plotted as a
function of
the length of the storage time period is shown in Figures 13H, 131 and 13J.
The
waveform applied to the sampler cone 18 was composed of a constant period of
time (10 ms) at low voltage (VOR =+1 V) during which the ions were permitted
to
enter the TOF. The signal intensity was measured by activating the TOF
acceleration
grids 72A, 72B about 4.5 ms after the sampler cone grid voltage VOR was
lowered.
VOR was held at a high value (VOR = +40 V) for periods of time shown on the x-
axis
of Figures 13H-13J. Three traces appear in Figures 13H, 131 and 13J,
corresponding
to data collected at various settings of compensation voltage CV. The ion
intensity
for a non-optimum compensation voltage, CV = -4 V (Figure 13J), suggests that
the
ion trap is relatively inefficient, and the maximum number of ions which can
be held
in the trap is reached relatively rapidly, i.e., about 10 ms. On the other
hand, at
CV = -3 V (Figure 13H) and -3.5 V (Figure 131), the intensity rises for over
30 ms.
This suggests that the lifetime of the ion, i.e., with drift time 27.0 s,
within the
FAIMS ion trap is at least 5 ms. At high trapping times it is assumed that the
trap
has filled and that the influx of ions is balanced by losses by diffusion and
gas
flows. This experiment can be considered to be a simple kinetics problem. The
influx of ions is X ions/sec. The loss of ions, Y ions/sec, is proportional to
the
number of ions in the trap. The increase in the number of ions in the trap, Z
ions
total, will continue until steady state is reached and X = Y = kZ, where k is
the rate
constant for the function describing the rate of ion loss from the trap. At a
short
delay time Z is small and kZ is small. It therefore can be assumed that Z = Xt
where
t is the time. The solution to the differential equation dZ/dt = X - kZ is
Z(t) =
X(1-e-kt)/k, if Z at time zero is zero. The data set can be fit to this
function to
determine X and k. Figures 13H, 131 and 13J show the experimental data, and
calculated curves based on the equation above used to fit the data. The k
values
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-35-
were 0.06, 0.12 and 0.34 for the CV curves -3, -3.5 and -4 V respectively. The
corresponding X values were 0.4, 0.72 and 0.8 respectively. High values of k
represent conditions wherein the rate of ion loss is high. High values of X
correspond to a high rate of ion input into the trap.
FAIMS-R4 prototype
Now referring to an alternative embodiment shown in Figures 14A-
14C, referred to as FAIMS-R4-prototype 80, a FAIMS 3-dimensional atmospheric
pressure ion trap is shown in which the electrospray (or other ionization)
occurs
within the radius of the inner electrode 82. This is the configuration
preferred in the
Mine Safety Appliances Company version of the FAIMS. A modified version of
this
device is shown schematically in Figures 3A and 3B. In general, ions may be
introduced to the FAIMS analyzer region 84 either from outside (external) to
the
outer electrode 83, or from inside (internal) the inner electrode 82. The
latter is less
convenient because the dimensions are small, and the radius of the inner
electrode 82
must be much larger than can be used in devices using the external ion source.
Moreover, the ionization source (e.g. corona discharge needle) may be
susceptible to
the influence of the high voltages applied in the asymmetric waveform. The
electrode
immediately surrounding the ion source is electrically grounded in the FAIMS
shown
schematically in Figure 3A and 3B.
In the device shown in Figures 14A-14C, the inner electrode 82 would
be about 14 mm outer diameter, and the outer electrode 83 about 18 mm inner
diameter, with about 2 mm annular space (FAIMS analyzer region 84) between
these
two concentric cylinders 82, 83. The end of the inner cylinder 82T (left end
in
Figures 14A-14C) is closed, and shaped as appropriate to maintain the electric
fields
suitable for FAIMS ion trapping in all locations near the end of the electrode
82T.
The inside of the outer cylinder electrode 83 is shown to be uniform in
diameter in Figures 14A-14C, but with wide diameter inner electrodes 82 such
as
shown in Figure 14A-14C, it is very likely that the FAIMS analysis conditions
will be
better maintained if the inner surface of the outer electrode 83 is contoured
very
much like that shown in Figures 9A and 9B. This will maintain substantially
constant distance between the inner electrode 82, and the outer electrode 83
near the
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-36-
spherically shaped (or conical etc.), closed end 82T of the inner electrode
82. While
the spacing between the inner and outer electrodes near the end of the
electrode 82T
may be substantially uniform, it will be understood that the spacing may also
be
non-uniform, as long as equilibrium conditions can be maintained at loations
near the
end of the electrde 82T.
Gas flows enter the end of the FAIMS analyzer region 84 shown in
Figures 14A-14C (right hand side of the FAIMS in the figure), and flow toward
the
closed end or terminus 82T of the inner electrode 82. Beyond the terminus 82T
of
the inner electrode 82 the gas flow passes through an exit grid 85 comprising
a high
transparency, fine-wire grid, and exits through the space between the mass
spectrometer sampler cone 18 and the exit grid 85. A portion of the gas flows
into
the orifice of the sampler cone 18, drawn by the vacuum of the mass
spectrometer.
Some of the ions which have passed through the exit grid 85 during the
extraction
time period will also be drawn into the mass spectrometer, by gas flows and by
electrical fields.
Some of the gas entering the FAIMS analyzer region 84 shown in
Figures 14A-14C must be permitted to flow inwards (i.e. the counter current
gas
flow) from the analyzer region 84 into the ionization region 86, thereby
preventing
neutral molecules, large liquid droplets and other unwanted non-charged
components
from passing into the FAIMS analyzer region 84. These components would
contaminate the gas in the FAIMS analyzer region 84, and the ion focussing and
trapping described elsewhere in this document may be degraded. The device
therefore may fail if the gas flow from the FAIMS analyzer into the ionization
region
is reversed during electrospray experiments. If the ionization occurs in a
very clean
non-contaminated gas, then this restriction on the gas flow direction may be
relaxed (
e.g. ionization of clean gas with radioactive 63Ni foil, corona discharge
ionization,
ionization by UV light radiation etc.). During operation in P2 mode the
requirement
for high purity gas is somewhat relaxed.
The device shown in Figures 14A-14C operates in a manner analogous
to that described previously. The ions pass radially out of the ionization
region 86,
transported by electric fields against the radially inward flowing gas. Having
passed into the FAIMS analyzer region 84 the electric fields will either
confine the
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-37-
ions inside the analyzer region 84 (focussing or trapping) or the ions,
because of
application of DV and CV which are not appropriate, will collide with the
walls of
the device. Assuming that the DV and CV are appropriate for one of the ions in
the
sample, that ion will be focused in the FAIMS analyzer region 84, and flow
with the
gas (since in the FAIMS analyzer region 84 the gas and electric fields act
perpendicularly to each other) toward the closed, dome-shaped terminus 82T of
the
inner electrode 82. If the trapping fields (electrical potential well) remain
appropriate, the ions will assemble near the terminus 82T of the inner
electrode 82 as
shown in Figure 14B. This will occur because the ions cannot return toward the
ion
source against the flow of gas, and the ions cannot flow with the gas out of
the grid
85 because of the confining action of the electric fields near the terminus
82T of the
inner electrode. As long as the following conditions are maintained, this trap
will
exist: (1) the DV and CV must be applied, and the voltages remain appropriate
for
the ion being trapped; (2) the voltages on the outer electrode and the grid
remain
fixed, e.g. near 0 V, as appropriate for the ion being trapped; and (3) the
gas flow is
maintained. If any condition changes the ions may leave the trap. If it is
desired to
have the ions travel to the sampler cone 18 of the mass spectrometer after
.passing
out of the trapping region, and through the grid 85 as shown in Figure 14C,
then one
of the above conditions may be optionally changed to achieve this result. This
could
occur in a number of ways:
(1) The grid 85 voltage may be lowered (from its value during trapping)
relative to
the inner electrode 82, and relative to the outer electrode 83. This will have
the
effect of attracting (positively charged ions) away from the FAIMS trapping
region (near the terminus 82T), and thereby breaking the hold of the trap. The
ions will leave the trap, and travel toward the grid 85. Some ions will strike
the
grid wires, and some will travel through (assisted by the gas flow). Since all
of the voltages in the device must be considered relative to each other, the
same
effect can be achieved by changes in the voltages applied to the outer
electrode
83, and to the inner electrode 82. For example, an increase in voltage applied
to both the outer electrode 83 and to the inner electrode 82, will have
exactly
the same effect as a decrease in the voltage applied to the grid 85.
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-38-
(2) The DV or CV can be changed in many ways which alter the ion motion in the
vicinity of the FAIMS trapping region. If the CV is made more negative the
ions
(positive ions) will tend to collide with the inner electrode 82, and if the
CV is
more positive the ions will be positioned farther from the inner electrode 82,
and at some voltage the FAIMS trap will no longer exist for this ion and the
ion
will travel with the gas flow and under the influence of the average dc
electric
field, to the grid 85, as noted in (1) above. If DV is removed the trap will
no
longer function. If CV is altered, e.g. more positive, and DV is removed,
(positively charged) ions will be repelled from the inner electrode 82, and
may
travel to the grid 85.
(3) The gas flow can be changed. If the gas flow is sufficiently high to
overcome
the trapping action of the electric fields near the closed end of the inner
electrode 82T, the ions will be pushed out of the trap and toward the grid 85,
as described above. If the gas flow is decreased, or stopped, the ions will
move via diffusion, and via chemical changes. The diffusion will permit the
ions to return back toward the ion source, thereby de-populating the FAIMS
trapping region near the terminus 82T of the inner electrode 82. Even in the
presence of gas flows the ions may soon de-populate the trap because of
chemical effects. If the ion collides with a neutral molecule and temporarily
forms a stable complex, this complex may drift out of the FAIMS trapping
region because this new complex has high field mobility properties which were
different from the original ion.
Other Versions of FAIMS-Rx-prototypes
The primary objective of the atmospheric pressure FAIMS ion trap is
to collect, confine and increase the concentration of ions in some location in
space.
This can be achieved using the devices described in the paragraphs above.
Several
simple variations on these devices can be visualized.
(1) The geometry of the end of the inner electrode has been assumed to be
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-39-
spherical, but the surface may be conical, or some variation on these shapes.
The shape will be selected to establish the non-uniform electric fields which
are
necessary to create the FAIMS focussing of ions and FAIMS trapping of ions
that is described above.
(2) The geometry of the inside of the outer electrode may be varied. Most of
the
examples shown have simple cylindrical geometry, for ease in mechanical
fabrication. A non uniform surface is more difficult to fabricate, but will be
advantageous in some cases, especially if the inner electrode has an outer
diameter in excess of approximately 4 mm.
(3) The inner and outer electrodes have been shown to have walls that are
parallel
to the central longitudinal axis, but this is not essential. The inner
electrode
may have an outer diameter which varies linearly or non-linearly along its
length. The outer electrode may have an inner diameter which varies along its
length. This will be advantageous in those geometries in which the ionization
source is located within the radial distance of the inner electrode, for
example,
as shown in Figures 3A, 3B, 14A, 14B and 14C.
(4) The gas flows shown in the devices illustrated in this document serve two
independent and identifiable purposes. First, the gas flow serves to carry the
ions along the length of the FAIMS analyzer region since the electric fields
are
acting perpendicularly to the length of the region, and therefore cannot help
to
transport the ions along the length of the device. Secondly the gas flows are
always arranged to maintain the FAIMS analyzer and FAIMS trapping regions
clean, and relatively free of gas phase water and chemical contaminants.
Where possible, the ions must travel upstream, counter-current, to the flowing
gas prior to entering the FAIMS analyzer region, in order to avoid entrance of
neutrals and droplets into the FAIMS analyzer region. The embodiments of the
atmospheric pressure, 3-dimensional ion traps that have been described above
may permit the replacement of one or another function of the gas flow. For
example, the transport of ions along the length of the FAIMS could be
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-40-
accomplished via electrical means. For example, if an electrical gradient is
established along the length of the FAIMS analyzer which will serve to carry
the
ions along its length, this would replace one of the functions of the gas flow
noted above. The electrical gradient can be created in two ways. First, the
inner and/or outer electrodes can be segmented, with a slightly different
constant, dc, voltage applied to each segment in such a way as to create
another voltage gradient from one end of the device to the other. This is
entirely feasible if the device can simultaneously maintain the DV and CV and
geometric conditions which are necessary to maintain the ion focussing or
trapping conditions. Secondly, one or more of the electrodes may be fabricated
in such a way as to permit a voltage gradient to be established along the
length
of the device. The has been accomplished using insulating electrodes coated
with a semiconductive layer. If a different voltage is applied to each end of
such an electrode, the electrode acts like a resistive device, and the voltage
gradient sits along its length. The voltage gradient can be linear, or non-
linear
depending on the application of the semi-conducting layer. The methods for
ion motion modelling described below permit evaluation of such approaches
without construction of prototypes. Modelling has shown that these devices
are feasible.
(5) The electrode to which the asymmetric waveform is applied has in most
cases
been the so-called "inner electrode" in the discussion above. The "outer
electrode" surrounds the inner electrode, and is usually held at VFAIMS. As
explained earlier, in one case (Figures 6A and 6B), the asymmetric waveform
was applied to the "outer electrode". There is no theoretical reason for
applying the DV and/or CV to the inner electrode. In all of the configurations
described in this disclosure, it is possible to apply the asymmetric waveform
and/or the offset CV to either the inner or outer electrodes. In some cases,
including the case illustrated in Figures 14A-14C, there is significant
advantage
to application of the waveform to the outer electrode.
(6) DV and CV need not be applied to the same electrode. For example in order
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-41-
to achieve a CV of -11 V, the -11 V is either applied to the inner electrode,
or
+11 V is applied to the outer electrode. Exactly the same logic applies to DV.
If a condition for trapping an ion requires DV 2500 V (applied to the inner
electrode), then exactly the same behavior can be expected when DV -2500 V is
applied to the outer electrode. As was discussed in the text describing the
hardware shown in Figures 6A and 6B, it was understood that these changes in
voltage polarity are required, therefore the text for Figures 6A and 6B was
simplified and the polarity was described as if the waveform DV and CV was
applied to the inner electrode. This was done to simplify comparisons amongst
the devices described in other sections.
(7) The geometry of the mass spectrometer sampler cone has not been discussed.
The sampler cone 18 shown in Figures 11A-11C for example has been drawn
(for simplicity) as flat on the side facing the FAIMS device. There is some
advantage to be gained by use of a sampler cone which has a raised (pointed)
.15 front surface, with the orifice itself at the apex of the cone. The ions
are
generally attracted toward the pointed surface, and the ion transmission
across
the space between the grid, and through the orifice may be improved.
(8) The applied asymmetric waveform may be operated with small transient
changes in voltage, phase shifts, and polarity. For example, if an ion is
focused or trapped with DV 2500 V, and CV -11 V in a certain geometry, short
(ms) changes of DV will affect the capability for ion separations. The voltage
of DV may be changed for millisecond periods, the polarity reversed for
millisecond periods, and the relative time periods of high and low voltage can
be changed for small periods of time. This will create conditions whereby ions
which are focused or trapped in a marginal way will be rejected from the
FAIMS. For example, two ions which have almost the same high field ion
mobility properties, my co-exist in the FAIMS analyzer region or the FAIMS
trapping region. Unless steps are taken to selectively remove one of the ions,
both will reach the detector (electrometer or mass spectrometer). Small
voltage
changes to DV or CV, and transient changes in voltages and phases of the
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-42-
waveform may help to eject one of the ions.
(9) The exit grid electrode can take many forms, and in some cases may not be
necessary. The exit grid electrode serves 3 functions, including (1)
completing
the electric fields around the inner electrode, so that the ion trap is
formed, (2)
forming the electrode described in (1) but simultaneously permitting the gas
flow to pass through this region substantially unimpeded and (3) allowing a
mechanism by which to form, and destroy the trap without modification to the
voltages applied to the inner electrode. Clearly these functions can be
carried
out by other parts of the device. For example in Figure 9, the ion trap is
controlled via the voltages applied to the inner electrode. The extraction
voltage used to eliminate the ion trap may be applied to the outer electrode,
or
to the inner electrode. Moreover, the grid can be totally eliminated if the
sampler cone of the mass spectrometer is placed substantially near the end of
the outer cylindrical of the FAIMS. This is the case shown in Figure 13.
Modelling the Ion Motion in the FAIMS-E, FAIMS-MS, 2-dimensional and
3-dimensional ion traps:
The ion motion in the FAIMS was modeled using a combination of
experimental and theoretical considerations. First, consider the two cylinders
used
in FAIMS shown in Figure 15. When a voltage is applied to the inner cylinder,
the
voltage at any point between the two cylinders can be calculated using the
following
formula: Vr = V(ln(r/b)/ln(a/b)) where Vr is the potential at radial distance
r
(assuming that r falls in the space between the two cylinders), V is the
potential
applied to the inner electrode, the outer diameter of the inner cylinder is
"a" (cm),
and the inner diameter of the outer cylinder is "b" (cm). The outer electrode
is
electrically grounded, i.e. 0 V applied. The annular space (called the FAIMS
analyzer region) falls in the radial distance between a and b. This is shown
in Figure
15. The voltage between the tubes is not linear, and the electric field (which
is the
derivative of the voltage i.e. dV/dr) is also non-linear. The electric field
between the
tubes (at location r) can be shown to be: E = -V (1/(r In (a/b)) where E is
the
electric field (V/cm) and V is the voltage applied to the inner electrode,
while the
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-43-
outer electrode is at 0 V. Variables a and b (cm) are defined above, and shown
in
Figure 15.
The motion of an ion in an electric field at atmospheric pressure is
described by: v = KE where v is the ion driftvelocity (cm/sec) and E is the
electric
field (V/cm). The "constant" of proportionality for a given set of conditions
is called
the "ion mobility constant" K. Note however that many changes in conditions
can
change the value of K. The obvious conditions that change the velocity of an
ion in
an electric field include: (1) temperature and (2) gas pressure. As discussed
above,
K also varies with the electric field.
Although it will not be shown here, the ion mobility at high field
(called Kh in the discussion above) can be estimated using the modified FAIMS-
E 10
instrument shown in Figures 3A and 3B. Figure 16 shows the change in ion
mobility
of one type of ion, (H2O)nH+, at high electric field. The word "terms" in
Figure 16
refers to the cyclic refinement of correction factors for the ion mobility
during the low
field portion of the asymmetric waveform. In practice, during a waveform (e.g.
Figure 4) at DV 3000 V, the low voltage portion of the waveform is at about -
3000/2
or -1500 V. Even at this lower voltage, the electric field is sufficiently
high that the
ion mobility cannot be assumed to be at it's "low field" value that is shown
at the
left axis of Figure 1. This requires a correction, that can be repeated in a
cyclic
manner to get the best estimates of the ion mobility ratio Kh/K at very high
electric
field. Figure 17 provide a portion of the original data that was used to
calculate the high field mobility that was used to produce the curves shown in
Figure
16. The details will not be discussed here. Note also that the calculations
are based
on a square asymmetric waveform (e.g. V(t) in Figure 2) while the actual
asymmetric
waveform is shown in Figure 4 (waveform 1).
Assuming that the high electric field change in the ion mobility of
(H2O),,H+ is represented by the curve shown in Figure 16, the trajectory of
this ion
within the cylindrical geometry shown in Figure 15 can be calculated. As a
first
approximation, it can be shown that: Rfiõal = sqrt(2tK(V/ln(a/b)) + Rinitiat2)
where
Rfinal is the radial location of the ion after a time period of length t, and
Rinitial was
the radial location before the time period t. The sqrt() is the square root
function.
Again, this equation only applies if the ion spends all of its time between
the radial
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-44-
distances a and b shown in Figure 15. Moreover the equation only gives useful
values
of final radial distance if the electric field does not vary significantly
between RiI,;til
and Rfina,. The voltage applied to the inner electrode is V, and the ion
mobility is K.
For this calculation K is assumed to be constant for the trajectory distance
(distance
the ion travels), but recall that K is calculated from the high field behavior
shown in
Figure 16. For example, if the ion is located at a distance r, and at some
selected
time (during application of the asymmetric waveform) that the voltage applied
to the
inner electrode results in an electric field of about 10,000 V/cm, then the
ion mobility
is calculated to be about K*1.01 where the 1.01 is the value taken from Figure
16.
The value of K is about 2.3 cmz/V-s for (H2O)nH+ at room temperature. This
mobility, K, cannot be easily determined using the FAIMS instrument, but can
be
found in the conventional ion mobility spectrometry (IMS) literature.
Figures 18A-18D show the trajectory of an ion with the high field
properties shown by the curve in Figure 16. Figure 18A shows very few
oscillation
motions caused by the applied asymmetric waveform of the type shown in Figure
2.
By way of illustration, the cylindrical geometry shown in Figure 15
may have an inner cylinder having an outer radius of 0.1 cm and an outer
cylinder
having an inner radius of 0.3 cm. This means that all of the calculations
giving rise
to the trajectory must be done with a=0.1 and b=0.3 cm, and the trajectory
must not
extend past these limits. The ion trajectory shown in Figure 18A is calculated
with
the ion initially at 0.11 cm radial distance. This is shown as the left-most
point in
Figure 18A. The ion will oscillate as a result of the applied waveform and
this is
shown as an increase and decrease in the radial distance of the ion. The gas
flow
which transports the ions in the FAIMS analyzer region is simulated (for
figure
clarity) by showing the trajectory as a function of time (x-axis) in Figure
18A. The
applied voltages for the trajectory simulation were: CV=O V, DV=2500 V,
frequency=83000 Hz, relative ratio of low voltage to high voltage (tl and t2
in Figure
2) was 5 to 1. Figure 18A shows that the ion does not travel exactly the same
distances during the low field, and high field portions of the waveform, and
the ion
experiences a "net" drift. The "net" drift refers to the general motion of the
ion
radially outward (in the case of Figure 18A). The simulation was repeated
several
times, and the results shown in Figures 18B through 18D. Figure 18B was
simulated
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-45-
in exactly the same manner as Figure 18A, except that the number of
oscillations of
the waveform, and thus of the ion motion, are significantly higher in Figure
18B.
This shows that the ion will eventually cross over the FAIMS analyzer region,
and
collide with the outer wall which is located in Figure 18B at the top of the
Figure, at
radial distance of 0.3 cm. Therefore, the DV and CV conditions that were used
to
simulate the motion of (H2O),,H+ ion in Figures 18A and 18B, would not be
suitable
for the focussing or trapping in an FAIMS with the physical geometry described
above. The condition which would be suitable for ion storage is shown in
Figure
18C. The conditions are: CV=-11 V, DV=2500 V, frequency=83000 Hz, relative
ratio of low voltage to high voltage (tl and t2 in Figure 2) of 5:1. This
could have
been predicted from Figure 18B, since the outward drift of the ion might be
expected
to be retarded by the application of a negative dc potential to the inner
electrode.
Figure 18C shows that the ion will experience a net drift from its starting
position of
0.1 cm radial distance outwards, but quickly the drift stops (note the ion
oscillates
because of application of the asymmetric waveform), and the ion progresses
neither
inward nor outward. Figure 18D shows the calculated ion trajectory for the
same
conditions as Figure 18C except that the original radial starting point for
the ion
motion was selected to be about 0.26 cm. The ion experiences a drift toward
the
inner electrode, and stabilizes at exactly the same radial distance as the ion
shown in
Figure 18C. This means that an ion, irrespective of its starting position will
fall into
the ion focussing region. The focussing characteristics of the FAIMS are
therefore
demonstrated by ion trajectory calculations.
The radial location of the optimum focussing of an ion depends on the
high field mobility properties of the ion, and the DV and CV, and geometry of
the
FAIMS analyzer region. For the example shown above, the (H2O)õH+ ion was
selected because the high field ion mobility behavior of this ion had
previously been
established. The optimum combination of DV and CV for the (H2O)nH+ ion can be
calculated for various FAIMS hardware geometries. The trajectory of the ion
can be
calculated based upon the principles described in the paragraphs above.
Figures 19A-19D show the ion trajectory for a geometry that is shown
in Figures 11A-11C, the device referred to as the FAIMS-R3-prototype (one of
the
3-dimensional, atmospheric pressure ion trapping devices). Because the
geometry is
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-46-
not a simple cylinder the ion trajectory calculation is more complex. The
calculation
is composed of two independent calculations. In the first, the mechanical
geometry
of the device is entered into a computer program which then calculates the
strength
of the electric fields around the components. This is done by a method called
"relaxation" (Jacobi iteration Richardson method), and involves a repetitive
series of
approximations of the field at every point in the physical space. The field at
a given
point is calculated as the 'average' of the points in each direction around
it. This is
repeated for every point in the space. Once this calculation has been
completed for
every point in the entire space, then the process is started again at the
first point,
now using the estimations from the previous calculation. This is shown below
in 1-
dimension. Let us assume that the following are the voltages at several
adjacent
points in an imaginary 1-dimensional world (before the 'relaxation'
calculation has
begun). The point at the left most of the array is an electrode at 100 V, and
that at
the right most point is an electrode at 0 V. We begin by assuming every point
is at 0
V, except the electrode at 100 V. The array is shown in the next line:
100 0 0 0 0 0 0 0
Consider the result if we make each point the average of its neighbors:
100 50 0 0 0 0 0 0
And again:
100 50 25 0 0 0 0 0
100 62.5 25 12.5 0 0 0 0
100 62.5 37.5 12.5 6.25 0 0 0
This calculation must be repeated until no further change in the data points
is
occurring, or at least until the changes in the data array are within
specified error
limits. The 2 and 3-dimensional versions of the calculation are analogous.
The "relaxation" and "successive over relaxation" methods for numerically
solving Laplace and Poisson equations are described in most text books of
fluid
dynamics (see M.B. Abbot and D.R. Basco, Computational Fluid Dynamics, An
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-47-
Introduction for Engineers (Longmans, London, 1989), Chap. 8). The calculation
for
a cylindrical geometry must include a small correction for the fact that the
points in
the radial direction cannot be used with equal weight in the 'average' used to
calculate the new value of a point in space. The points in the axial direction
(along
the length of this cylindrical geometry) are equivalent to each other, but are
not
equivalent to a point at smaller or larger radial dimension. The surrounding 4
points
used for the average of a point in cylindrical geometry must therefore be
weighted,
two axial points are identical, and the inner and outer points in the radial
direction
are weighted independently from each other, and from the axial points.
Nevertheless the overall method of calculation of electric potential using the
'relaxation' method is the same for all geometries.
The second calculation which is necessary to determine the ion trajectory in
an arbitrary geometry is the calculation of the motion itself, given that the
electric
fields have been established as discussed above. The trajectory is calculated
by
breaking the ion motion down into small steps in time. At each step in time
the ion
location, the electric field, phase of the applied asymmetric waveform, etc.
are
determined. From the strength of the electric field at the point in
space/time, the ion
mobility at high electric field is calculated (as was demonstrated for
(H2O)nH+
above). The ion velocity is estimated to be v = KE (or v=KhE), in the manner
described above, and the distance travelled is distance = (velocity)(duration
of time
step). The distance (cm) travelled for the single time step is determined from
the
velocity (cm/sec) multiplied by the time duration (sec). The new ion location
is
calculated from the old location, and the distance travelled in the time step.
This is
repeated, now beginning at the new ion location just calculated in the
previous
iteration. The iterations are repeated, with the strength of the electric
fields due to
the asymmetric waveform being constantly adjusted (as appropriate for the
frequency of the waveform, and the relative times of the high and low voltage
periods in the waveform). The calculation may also include an adjustment of
the
ion location due to the external forces of a gas flow, or if necessary to
clarify the
motion of the ion (since a simple back and forth motion doesn't demonstrate
the
motion very clearly).
Figure 19A illustrates an ion trajectory calculated in the method described
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-48-
above, for the geometry shown in Figures 11A-11C. The inner electrode is about
2
mm outer diameter, and the outer electrode is about 6 mm inner diameter. This
is
the same size as the cylinders used in the trajectory calculations shown in
Figures
18A-18D. The conditions were: CV=-11 V, DV=2500 V, frequency=83000 Hz,
relative ratio of low voltage to high voltage (tl and t2 in Figure 2) was 5:1,
outer
electrode =OV, grid electrode =OV. Three electrodes appear in Figure 19A,
exactly
corresponding to the hardware illustrated in Figures 11A-11C. The inner
electrode
52 is solid and ends in a spherical shape 52T near the center of the Figure
19A. The
top and bottom edges are the outer electrode 53, and the left edge of the
Figure is the
grid electrode 56 shown in Figures 11A-11C. The ion trajectory was initiated
near
the inner electrode, and an artificial (gas flow) horizontal motion was added
to carry
the ion from right to left on the Figures 11A-11C. The ion oscillated because
of the
applied asymmetric waveform, and two types of net motion are observed in
Figure
19A. The ion initially moves away from the inner electrode 52, then the
distance
from the electrode becomes constant. This is exactly the condition shown in
Figure
18C where the net motion in the radial direction soon becomes zero. The ion
also
drifts because of the added 'gas flow', an artificially imposed longitudinal
velocity.
Note that the ion progresses along the electrode at constant distance from the
inner
electrode 52, then follows its curvature 52T. The ion will not leave the
location near
the terminus 52T of the electrode even with the applied artificial axial
direction 'gas
flow' velocity. The ion has become trapped near the terminus 52T of the
electrode.
Figure 19B shows the motion if the ion trajectory is initiated at larger
radial distance
(as in Figure 18D). As explained before, the ion cannot escape the 3-
dimensional
ion trap near the tip of the electrode 52T.
Figures 19C and 19D represent the same physical geometry as Figure 19A
and 19B, and the ion trajectory begins in analogous locations in radial and
axial
directions. The conditions are: CV=-11 V, DV=2500 V, frequency=83000 Hz,
relative ratio of low voltage to high voltage (tl and t2 in Figure 2) was 5 to
1, outer
electrode =OV, grid electrode =-7 V. The only difference between the Figures
19A-
19D is that the latter two (Figures 19C and 19D) were calculated with the exit
grid
56 voltage negative relative to the outer electrode 53. Under these conditions
the ion
trap has been removed and the ions will travel toward the grid 56. Initially,
the
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-49-
trajectory takes the same form as shown in Figures 19A and 19B as the ion will
first
move toward the optimum balance point for the DV and CV and geometry being
used. However, the conditions are not maintained in the vicinity of the
spherical
terminus 52T of the inner electrode 52, and the exit grid 56 voltage modifies
the ion
motion, and attracts the (positively charged) ions away from the inner
electrode 52,
and toward the exit grid 56. Both the attractive force of the grid 56 voltage,
and the
artificially applied 'gas flow' axial motion will contribute to the ion
trajectory as it
leaves the vicinity of the inner electrode 52, and approaches the exit grid
56. Note
also that the magnitude of the 'oscillation' of the ion due to the asymmetric
waveform decreases significantly as the ion moves away from the inner
electrode 52.
While 3-dimensional trapping is not achieved in Figures 19C and 19D, the
behaviour of the ions shown may nevertheless be very useful. When operated in
a
compromised condition, i.e., non-trapping, but very near trapping conditions,
the
ions follow the curved surface of the spherical end 52T of the electrode and
tend to
move toward the center axis. If they are not completely trapped, the ions will
essentially escape from the end 52T of the electrode, but they are confined to
a small
radial distance along the center axis of the inner electrode 52. If the flow
of ions is
directed into the sampler cone orifice 18A leading to the vacuum chamber, the
signal
sensitivity will be greatly enhanced in conditions where this 'partial
focussing' takes
place. It is possible to visualize a commercial version of FAIMS wherein this
signal
enhancing behavior of the spherical terminus 52T of the electrode is the only
part of
FAIMS which is exploited. All of the embodiments of the 3-dimensional ion trap
of
FAIMS described above might be used for this signal enhancement even if the
"3-dimensional trapping" is not used per se.
Signal enhancement using ion focussing at the spherical terminus of the inner
electrode of FAIMS
Figures 19E-191 illustrate the results of ion trajectory calculations using a
FAIMS consisting of a cylindrical outer electrode 93 of about 6 mm id, and an
inner
electrode 92 of about 2 mm o.d.. The annular FAIMS analyzer region 94 is about
2
mm wide along the sides of the device. The inner electrode 92 terminates in a
spherical shape 92T which is about 2 mm from the flat, front plate of the
sampler
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-50-
cone 18. At the center of the sampler cone 18 is a small orifice 18A leading
into the
vacuum system. In Figure 19E, the sampler cone 18 is held at 0 V, i.e., VOR =
0 V.
The conditions used for the ion trajectory simulation appear in Figure 19E.
Figures
19F through 191 were prepared in exactly the same manner as Figure 19E, except
that
the VOR was changed to -2.5, -5, -7.5 and -15 V respectively. This low applied
VOR
had the effect of drawing the ions out of the 3-dimensional trapping region.
If this
extraction occurs at voltages very close to the normal 'trapping' conditions
(i.e.,
indefinite ion trapping), then the ions tend to be focused to near the center
axis of the
inner electrode 92, and therefore are focused to regions very close to the
exit orifice
18A. The detected signal intensity will be maximized at the VoR which confines
the
ions as closely as possible to the center axis.
Although not shown in the Figures, it is possible that further improvements to
the 'compactness' of the ion beam can be achieved by modification of the
sampler
cone 18. This might involve addition of extra lenses with voltages applied, or
the
modification of the shape of the front of the sampler cone 18. Additional
improvements might also be achieved by 'shaping' the inside surfaces of the
outer
FAIMS cylinder 93 at the end of the cylinder that is adjacent to the sampler
cone 18.
A previous version of the trapping experiments, shown in Figures 9A and 9B,
did use
a device with an outer cylinder which had a curved inner surface to maintain
an
(approximately) constant distance between the outer cylinder and the inner
electrode
at the spherical end of the inner electrode.
Several experimental parameters will affect the focussing described above,
and are shown to be optimized near VOR = -5 V in Figure 19G. These include the
gas
flow rate, the spacing between the spherical end of the inner electrode 92 and
the
sampler cone 18, and the applied DV and CV. It is expected that optimization
of
the detected ion intensity will depend mainly on these parameters. The gas
flow
will control at least two factors, namely the rate that ions flow into the
trapping
region from the length of the FAIMS analyzer region 94, and secondly the
turbulence
at the end of the inner electrode. The simulations shown in Figures 19E
through 191
do not take into account gas turbulence and ion diffusion. The effectiveness
of the
focussing action will require a gas flow that maximizes the ion transport rate
into the
'trapping region', and simultaneously minimizes ion loss through turbulence.
The
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-51-
trajectory calculations shown in Figures 19E through 191 also do not account
for gas
flows in directions non-parallel to the x-axis. If the experimental system
includes
gas flowing, for example, radially outward from the FAIMS trapping region, as
would occur in the system shown schematically in Figure 13A, the locations of
the
maximum ion intensity would have to be determined experimentally. The
modelling
serves to suggest that this ion focussing can enhance sensitivity in some set
of
optimized experimental conditions.
Several possible hardware designs can achieve the effects shown in Figures
19E through 191. These embodiments require some essential components:
(1) the electrodes must have curved surfaces, including cylindrical, or
spherical, but
also including surfaces that do not simply fall into one of these categories.
An
example of an unusual shape which would serve to establish conditions for
trapping or focussing, is a cylindrical rod which has a bend in it, somewhat
like
a hairpin turn, shown in Figure 20. With appropriate gas flows, a trapping
region can be created.
(2) the ions must be transported to the trapping region by gas flows, or by
electric
field gradients. All of the previously described embodiments of FAIMS take
advantage of gas flows, since these function quite independently of the
voltages
applied, especially DV and CV. The use of gas flows is relatively simple to
visualize, and easy to create experimentally.
Qualitative, Simple Method for the Understanding of Ion Focussing and Ion
Trapping
There exists an optimum condition of DV and CV at which an ion is
transmitted through the FAIMS analyzer. Referring back to Figure 17A, a set of
repeat sweeps of the CV at a series of DV values ranging from 2100 V to 3000 V
is
shown. The location of the peak maximum for some ion (in this case (H2O)nH+ )
represents the condition where the compensation voltage CV is just strong
enough to
balance the net ion drift towards the wall of the FAIMS analyzer. Consider
therefore that the (H2O)nH+ ion can be transmitted through the FAIMS analyzer
region at a number of ideal combinations of CV and DV. If the ion experiences
a
combination of CV and DV that is different from the ideal, then the ion
collides with
SUBSTTTUTE SHEET (RULE 26)
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-52-
a surface. A plot showing the ideal combinations of CV and DV for (H2O)nH+ is
shown in Figure 21. The ideal combination of DV and CV might be called
"balanced" because the ion experiences no 'net' motion. The plot shown in
Figure 21
illustrates this balanced condition in terms of electric field (based on DV),
rather
than the applied voltage DV and CV, moreover each data point on the Figure is
an
experimentally acquired combination of DV and CV, collected much as shown in
the
traces of Figure 17. Each point is the CV with the maximum efficiency of
transmission (peak maximum shown for each trace in Figure 17) for that setting
of
DV. Since the annular FAIMS analyzer region 14 of the FAIMS-E 10 is about 2 mm
wide, a voltage of DV 2000 will result in a field of about 2000/0.2= 10,000
V/cm.
Similarly an applied CV of -10 V will result in a field of about -10/0.2 = -50
V/cm.
The x- and y- axes of Figure 21 are displayed as electric field (V/cm)
(absolute
values, unsigned).
Figure 21 also shows a trace for the best fit third order regression to
this data. This regression will help to determine the best combination of CV
and DV
under conditions which fall between the experimentally determined points. The
fit to
the data shows only an appearing for the last points at high DV field
(electric field
which results from the application of DV, at the maximum applied voltage).
Note
that the 'DV field' is intermittent, since a part of the asymmetric waveform
has a
lower, opposite polarity time period. This maximum will be used as a
'reference
point', for the purposes of this description.
We will address the following question. Assume that an ion is
located in the center of the FAIMS analyzer region (radially) and assume it is
at a
balanced condition at optimum DV and CV for the given hardware geometry. This
means that the electric fields due to CV and DV fall directly on the line
drawn in
Figure 21. The cylindrical geometry shown in all of the FAIMS diagrams in this
document will have electric fields that are not constant along the radial
direction in
the FAIMS analyzer region. (The field may or may not be constant in the
longitudinal direction, depending on the geometry of the particular device. )
If the
electric field is not constant, will the optimum conditions shown by the curve
in
Figure 21 be maintained everywhere in the FAIMS analyzer region?
Figure 22A illustrates the actual fields due to DV (2500 V) radially
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-53-
across the FAIMS analyzer region 14 of the modified FAIMS-E 10 shown in
Figures
3A and 3B. Figure 22B illustrates both the actual fields due to DV (2500 V)
and
CV (about -13 V) that are found radially across the FAIMS analyzer region 14
of the
FAIMS-E 10, but unlike Figure 22A, the fields will be plotted against each
other in
the manner used in Figure 21. The points corresponding to some of the
physical,
radial positions are noted on the diagram. At the right side of Figures 22A-
22B, the
field is highest, and corresponds to the surface of the inner electrode 12, at
radial
distance of 0.7 cm. Similarly the left side of the Figure 22B corresponds to
the inner
edge of the outer electrode 13.
Compare Figures 21 and 22B. During a FAIMS experiment DV and
CV are applied to the inner electrode. The DV field is not constant, but
rather falls
within a small range of values (Figure 22B, x-axis), which in turn is only a
small
portion of the range of fields described by Figure 21. The curve in Figure 22B
can be
superimposed on the graphic shown in Figure 21 to give Figure 22C. Figure 22C
shows that the real, physical conditions of electric fields within the FAIMS
analyzer
region do not all correspond to points with a balance of DV and CV. Recall
that the
short curve of 'actual' conditions reflects the conditions at a set of
different radial
distances (i.e. the left most point of the short curve is the condition of
fields at 0.9
cm, near the outer electrode, and the right most point is physically located
near the
inner electrode surface). Naturally at least at one point, corresponding to
the center
of the FAIMS analyzer region in this 'selected' combination of DV and CV,
there
exists the so-called balance where the ion migrates (net drift) neither toward
the
inner or outer electrode. Note also, there are many combinations of DV and CV
in
which the entire line shown for 'actual conditions' in the FAIMS analyzer will
not
cross the optimum balance curve at any point. For example if the DV is reduced
to
50% of that shown in Figure 22B, the short trace for 'actual conditions' in
Figure 22C
will move left along the x-axis to fall at a much lower x-axis value of 'DV
Electric
Field'. If the CV voltage is unchanged the short trace in Figure 22C will not
cross
over the 'optimum' balance trace, and the ions will not be able to be
transmitted
through the FAIMS.
The comparison of the two traces in Figure 22C also introduces one
further question. If the ions, which are at the radial distance wherein the
'optimum'
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-54-
and 'actual' traces intersect, experience no 'net' motion radially, i.e. are
at a balance
point, what is the behavior of ions at larger radial distances, and at smaller
radial
distances? They must experience a net drift. For the conditions shown in
Figure
22C, the ions at radial distances larger than the crossing point, i.e. to the
left of the
intersection, will drift towards the intersection point i.e. toward smaller
radial
distances. The ions which are at small radial distances, will also drift
toward the
intersection point i.e. toward larger radial distances. If ions from every
radial
location other than the 'balance' or focus point (intersection of the traces
in Figure
22C) drift toward this focus point, then the device has the FAIMS ion
focussing
property that was described above. If the motions are divergent, i.e. away
from the
'balance' point, then no ions can pass through the FAIMS. In mode 1(P1,
positive
ions) the ions drift toward the focus point when DV is positive, and CV is
negative
polarity (CV and DV applied to the inner electrode). If both of these polarity
values
are reversed, then the ion motion (type A, Figure 1) is divergent instead of
convergent. This is the reason that the ions of the two types are
automatically
separated in the FAIMS. This is the reason the spectra of modes 1 and 2 are
always
different, and must be always considered as independent spectra. The ions (to
a
first approximation) which appear in Pl do not appear in P2 type spectra, and
vice
versa. The same applies to Nl and N2 type spectra.
Now referring to Figure 23, it is possible to visualize the transport of
ions in FAIMS using electric fields. A possible embodiment would require that
the
FAIMS unit be segmented, much in the same way that Javahery and Thomson U. Am.
Soc. Mass Spectrom. 1997, 8, 697-702) used a segmented rf-only quadrupole to
create a longitudinal electric field to draw ions along the length of a set of
quadrupole rods which were operating with the usual applied high frequency,
high
voltage ac voltage applied to them. The segments in either the case of
segmented
quadrupole rods, or FAIMS, are held at slightly different dc potentials, which
creates
a field superimposed on the other non-constant fields. A possible way to do
this is
shown in Figure 23.
Based on a similar concept, a device for 3-dimensional trapping using
only electric fields in a segmented FAIMS may be developed, and is described
below.
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-55-
3-Dimensional Trapping using only Electric Fields in a Segmented FAIMS
This segmented FAIMS version of the 3-dimensional trap is novel
because it does not use gas flows as one of the trapping components. In the
trapping of ions at the spherical end of the inner electrode, as previously
described,
the ions are held by a combination of the motion of the gas sweeping the ions
towards the end of the inner electrode, and the FAIMS focussing action caused
by
the asymmetric waveform. In that device, upon stopping of the gas flows, the
ions
can begin to make their way back along the length of the FAIMS inner cylinder.
The
driving force for this migration would be diffusion and ion-ion repulsion
which
creates a so-called space-charge in the zone where the ions are congregated.
In the present description of a segmented FAIMS ion trap, the ions are
held entirely because of the combination of the asymmetric waveform and the
gently
rising dc voltages applied to the adjacent segments which prevents ion motion
in
either direction along the length of the segmented FAIMS device. The stopping
of the
gas flow will have only a minor affect on the ions caught in the ion trap, and
there
exists no escape even if the gas flow is zero. Consider this new version of
3-dimensional trapping in more detail.
Figure 24 shows the segmented cylindrical outer 113 and inner 112
electrodes of FAIMS device 110. The high voltage asymmetric waveform of FAIMS
is applied to the inner electrode 112. The ions will be focused between these
cylinders 112, 113 given the correct combination of DV and CV, and the
cylindrical
geometry. In normal operation all of the segments 112A or 113A would be at
identical voltages, i.e., the inner electrode 112 is one conductor, and the
outer
electrode 113 is also one conductor. Assume that typical conditions for some
ion to
be focused between the cylinders are DV=2500 V and CV= -12 V. This is the
condition shown in Figure 24. If the electrodes were not segmented, the outer
electrode would be at e.g., 0 V. Similarly the inner electrode would be only
at one
condition e.g., asymmetric waveform DV=2500 V with -12 V offset compensation
voltage. Under these conditions if the ions were carried into the annular
space
between the cylinders by a gas flow, they would proceed longitudinally,
carried from
one end of the FAIMS analyzer to the other end of the device by the flowing
gas,
simultaneously being focused at some radial distance and between the inner and
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-56-
outer electrodes 112, 113.
Still referring to Figure 24, this situation can be changed substantially
once the inner and outer electrodes 112, 113 are segmented 112A-112E, 113A-
113E.
If all of the new dc voltages added to the outer electrodes 113A-113E are 0 V,
and
all of the new dc voltages added to the inner electrodes 112A-112E are 0 V,
then the
conditions described in the paragraph above are returned, and no 3-dimensional
trap
exists, only the 2-dimensional focussing between the cylinders. Visualize
next, that a
set of new, small, dc voltages are applied to each segment 112A-112E, 113A-
113E
of the FAIMS device 110, such that the middle segment of the inner and outer
electrodes 112C, 113C have the lowest applied voltage. Note, however, that
each
voltage applied to the outer electrode 113 must be matched by the same
(approx.)
voltage added to the inner electrode 112. This means that if +5 V extra are
added
to the first segment 113A of the outer electrode, then +5 must be also added
to the
same segment 112A of the inner electrode. If that segment already had -12 V
compensation voltage added to it, then the new +5 is added to that CV to give
a net
dc voltage of -7 V on that segment. This approach is used to add voltages to
the
other segments 112B-112E, 113B-113E, in such a way that the middle segment
112C,
113C (in the Figure) has the lowest applied dc voltage. This means that
positive
ions caught somewhere in this assembly will fall to the lowest voltage region,
i.e.,
between the inner and outer electrodes of the middle segment 112C, 113C. Since
the
normal FAIMS conditions continue to apply within each segment 112A-112E, 113A-
113E, namely the inner electrode has an asymmetric waveform with DV=2500 V and
the difference between the dc applied to the inner electrode 112 and the dc
applied
to the outer electrode 113 within that segment continues to be 12 V (a
required
compensation voltage in this example), then the ions will be focused in the
normal
way in the annular space between the inner and outer electrodes 112, 113. The
flow
of gas along the length of this FAIMS will not (at low gas flows, 1 L/min) be
able to
remove the ions which are located in the space within the middle segment 112C,
113C of this trap. For the ions to escape they must climb up the dc potential
walls
(of about +5 V in the Figure). At high gas flows, and at high ion density in
which
space charge is high, this escape might be possible. Nevertheless, there
exists a
trapping region which is totally electrical in nature, and the ions are held
in place
CA 02339548 2001-02-05
WO 00/08457 PCT/CA99/00718
-57-
only by electric fields.