Note: Descriptions are shown in the official language in which they were submitted.
CA 02340757 2001-02-16
WO 01/03669 PCT/CAOOI00806
DELIVERY OF LIPOSOMAL-ENCAPSULATED ANTIOXIDANTS
AND APPLICATIONS THEREOF
FIELD OF THE INVENTION
The present invention is related to the field of liposome-encapsulation of
hydrophilic and hydrophobic agents. More specifically, the present invention
relates to
the use of liposome-encapsulated antioxidants in the amelioration of pulmonary
and
hepatic damage in multiple organ dysfunction syndrome (MODS), as well as
respiratory
distress syndromes of various types and etiologies.
BACKGROUND OF THE INVENTION
Massive hemorrhage is one of the leading causes of mortality in cases of
penetrating trauma. For example, the hypoxia within tissues which is
associated with
hemorrhage and hemorrhagic shock can cause serious damage to endothelial
cells.
I S Reperfusion of such ischemic tissue can actually exacerbate the condition,
by further
promoting the generation of reactive oxygen species. initially by the
enzymatic action of
xanthine oxidase on xanthine, and later by the recruitment of neutrophils.
See, e.g.,
Granger, 1988. Am. J. Physiol. 255: H1269-Hi275; Weiss, 1989. Nei,.~ Engl. J.
Med
320: 365-376. Although the body is equipped with an anti-oxidant system which
possesses the ability to counteract a limited oxidative insult, if the
oxidative burden is
overwhelming (e.g., in the case of extensive reperfusion injury) the
endogenous
antioxidant defense simply cannot cope with the extraordinary damaging
oxidative load.
This condition commonly referred to as oxidant stress.
In addition, in cases of penetrating trauma (e.g., such as those encountered
in
accident victims or combat casualties), microbial- and toxin-based
contamination is
frequently encountered. This contamination causes a "double-insult" type of
clinical
scenario, namely concomitant massive hemorrhage and microbial toxic
contamination.
Despite inten~ention with blood replacement and aggressive antibiotic
administration,
the patient may still develop an uncontrolled, systemic inflammatory response.
the
severity of mhich appears to correlate with the development of multiple organ
dysfunction syndrome (MODS). See, Marshall and Sweeney, 1990. Arch. Surg. 125:
17-
23. The lung is among the earliest and most frequently affected organ in
critically ill
patients developing MODS. The severity of dysfunction ranges from mild
hypoxemia to
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
wo oiro3ss9 rcricwooroosob
a profound respiratory failure designated - Adult Respiratory Distress
Syndrome
CARDS).
ARDS is clinically-characterized by hypoxemia, reduced lung compliance. and
diffuse alveolar infiltrates. The syndrome usually manifests itself within 2-3
days of the
initial underlying disease process, where pro-inflammatory mediators and cells
are
known to be involved. For example, infiltrating alveolar neutrophils may
release
reactive oxygen species (i.e., free radicals) and various proteolvtic enzymes,
causing
damage to the endothelium and the epithelium. The invading neutrophils may
also
either directly release, or initiate the release of, a large number of pro-
inflammatory
molecules, thus promoting further cellular sequestration and injury. See.
Murray, et al.,
1988. Am. Rev. Respir. Dis. 138: 720-723.
ARDS is a frequent complication of sepsis and trauma. The mortality rate
associated with this acute lung injury is in the range of 50-70%, and the
overall annual
incidence of ARDS within the United States has been reported to be no less
than
150,000. See, Kirkpatrick, et al., 1996. Shock 6: S 17-S22, 1996. The terminal
mediators of the pathophysiological changes associated with ARDS are believed
to be
reactive oxygen species, which are either generated by de novo synthesis at
injured tissue
sites or released in large concentrations by infiltrating neutrophils. In
humans, the extent
of neutrophil influx and the presence of neutrophil products in the alveolar
lavage fluid
have been correlated with the severity of the lung injury. See. Hanson, et
al., 1984. Fed
Proc. 43 : 2 799.
These aforementioned observations have Ied to the development of several
therapeutic strategies which are designed to both reduce the influx of
neutrophiIs and to
counteract the damaging effects of reactive oxygen species. N-acetyl cysteine
(NAC), a
known free-radical scavenger and anti-oxidant (see, e.g., Dressier, et al.,
1994. Methods
Find Fxp. Clin. Pharmacol. 16: 9-13) has been shown to confer protective
effects in
endotoxemia (see, e.g., Zhang, et al., 1994. Am. J. Phvsiol. 266: H1746-
H1754); to
reduce neutrophil influx and lung Ieaks (see, e.g., Leff. et al., 1993. Am. J.
Phvsiol. 265:
L501-L506; and attenuate LPS-induced acute lung injury (see, e.g.. Daweux, et
al..
1997. Shock 6: 432-438) in animals. In each of the above-referenced scenarios,
however, a very high dose of N-acetyl cysteine (i.e.. 1 ~0 mg/kg
intravenously, or 1 gikg
intraperitoneally) was required in order to achieve a demonstrable biological
effect.
Moreover, the potential therapeutic application of N-acetylceyteine in humans
has been
2
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
WO 01/03669 PCTlCA00100806
examined in at least two clinical trials, with markedly different experimental
r'~sults.
The results of one human trial showed no beneficial treatment effect (see,
Jepsen, et al..
1992. Crit. Care. Med 20: 918-923), whereas the other trial demonstrated that
the
administration of NAC significantly reduced the extent of pulmonary
dysfunction (see,
S Suter, et al., 1994. Chest 105: 190-194).
Another therapeutic strategy has been to use a very potent lipophilic
antioxidant
(e.g., a-tocopherol) to quench reactive oxygen species, which mediate the
injury at the
site of inflammation. It has been demonstrated that a-tocopherol protects
against
oxidant-induced tissue injury by inhibiting membrane lipid peroxidation and
lipid
peroxide formation; via scavenging singlet oxygen and other reactive oxygen
species
and by exerting a stabilizing effect on membranes. See, e.g., Burton and
Ingold, 1989.
.4nn. N. Y. Acad Sci. 570: 7-22. However, a-tocopherol, in its free-form, is
too viscous
for parenteral administration and emulsifiers which are utilized to
solublilize this anti-
oxidant are generally found to be toxic to tissues.
Thus, there remains an, as yet, unfulfilled need, for the development of a
therapeutic for the treatment of Adult Respiratory Distress Syndrome (ARDS) by
mitigating the influx of neutrophils and the damaging effects of pro-
inflammatory
mediators (e.g., reactive oxygen species and various proteolytic enzymes).
SUMMARY OF THE INVENTION
The invention features a therapeutic liposomal formulation, which is useful to
ameliorate oxidative tissue damage. Such pathological conditions may be
induced by
hemorrhagic shock and endotoxin insult. The liposomal formulation of the
invention is
suitable for circulatory (e.g., intravenous) or intratracheal administration,
and has been
quantitatively demonstrated to ameliorate lung and liver injuries caused by
neutrophil
infiltration and reactive oxygen species. The compositions and methods are
also useful
for ieducing the extent of complications associated with the clinical
condition of ARDS
and liver injuries.
Accordingly, the invention provides a Iiposomal composition containing a
hydrophilic sulfhydryl agent and a lipophilic antioxidant. Preferably the
composition
contains at least 1 %, more preferably at Least 10%, more preferably at least
20%, more
preferably at least 25% by weight of the hydrophilic agent. For example, the
composition contains 28% by weight of a hydrophilic sulfhydryl agent. The
amount of
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
wo oiro~9 rc-ricAOOrooso6
hydrophilic sulfhydn~l agent in the composition does not exceed SO% by
wleight.
The composition is characterized as having free radical scavenging activity
and
antioxidant activity. The term "free-radical", as utilized herein, is defined
as a reactive
chemical intermediate form of an oxygen molecule. For example, a free-radical
is [O,]-
which, due to its high reactivity, can irreversibly damage organic compounds
within
cells. The term "antioxidant", as utilized herein, is defined as a chemical
compound
which possesses the ability to mitigate oxidation. For example, an antioxidant
reduces
the level of oxidation of biological tissues by highly reactive free radicals
by neutralizing
free radicals. Antioxidants fall into at least two classes: (i) endogenously-
produced
enzyme anti-oxidants (e.g., superoxide dimutase (SOD); glutathione peroxidase)
which
can catalytically alter or destroy free radicals; and (ii) exogenously-
ingested nutrients or
agents (e.g., a-tocopherol (vitamin E); vitamin C, and ~3-carotene (vitamin
A)) which
function to bind and sequester free radicals. Each of the antioxidants listed
above may
be incorporated into the therapeutic compositions described herein. The
antioxidants to
I S be administered are substantially pure, i.e., purified from substances
with which they
naturally occur.
The compositions contain liposomes, e.g., in the form of unilamellar and
oligolamellar liposome vesicles. The range of size of liposomes in the
composition is
within 25% of the mean size of the liposomes. The liposomes of the composition
are
relatively uniform in size. For example, the range of size of liposomes in the
composition is preferably within 20%, more preferably within I S%, more
preferably
within 10%, and most preferably within 5% of the mean size of the liposomes.
For
example, at least 85% (more preferably 90%, more preferably 95%, and most
preferably
99-100%) of the liposomes in the composition are with a defined size range,
e.g.,
between 100-400 nm in size. To produce uniformly-sized liposomes, the vesicles
are
produced by extrusion rather than sonication. Liposomes are extruded to be
approximately 150 nm in size. In another example, the Iiposomes are extruded
to be
approximately 450 nm in size. Unlike other methods of liposome manufacture
(e.g.,
sonication which method yields a heterogeneous population of iiposomes which
vary
widely in size), extrusion yields a population of liposomes that are
relatively uniform in
size. Uniformity of size allows more reproducible pharmacokinetics than other
methods
in the art. The hydrophilic sulfhydryl agent is encapsulated in an aqueous
interior of a
liposomal vesicle and the lipophilic antioxidant is incorporated an outer
membrane of
4
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
WO 01/03669 PCT/CA00/00806
the liposomal vesicle.
The hydrophilic sulfhydryi agent preferably is an antioxidant such as N-acetyl
cysteine, and the lipophilic antioxidant is preferably ct-tocopherol. The
composition
contains at least 1%, more preferably at least ~%, more preferably at least
7%, and most
preferably at least 9% by weight of the lipophilic antioxidant. The
composition may
also contain a phospholipid such as a phosphatidylcholine, a
dipalmitoylphosphatidylcholine, a lysophosphatidylcholine, a
phosphatidylserine, a
phosphatidyl-ethanolanune, a phosphatidylglyceroh or a phosphatidylinositol.
Cholesterol may also be present in the composition. However, the composition
preferably does not contain a metal such as Zn, Se, Cr, Cu, or Mn. For
example, the
composition is substantially free of such metals which may contaminate a
liposomal
preparation as a consequence of the sonication process. Since the liposomal
compositions described herein are prepared by extrusion rather than
sonication,
contamination by trace amounts of metals is avoided.
l~ The Iiposomal composition contains a hydrophilic suifhydryl agent, a
phospholipid, and cholesterol, and the approximate molar ratio of
dipalmitoylphosphatyidylcholine:cholesterol is 7:3, 6:4, or 9:I. For example,
the
phospholipid is dipalmitoylphosphatyidylcholine and the approximate molar
ratio of
dipalmitoylphosphatyidylcholine:cholesterol is 7:3. In another example, the
the
approximate molar ratio of phospholipid:cholesterol:hydrophilic sulfhydryl
agent is
7:3:15. The liposomal composition is formulated to contain hydrophilic
sulfhydryi
agent, a phospholipid, cholesterol, and a lipophilic antioxidant, with an
approximate
molar ratio of phospholipid:lipophilic antioxidant:cholesterol:hydrophilic
sulfhydryl
agent of 7:2:1:15.
The approximate molar ratio of phospholipid to cholesterol is altered to
achieve a
desired pharmacokinetic effect. The rate of antioxidant release from the
composition is
indirectly proportionate to the concentration of cholesterol in the
composition, i.e., a
higher percentage of cholesterol yields a composition with a slower
phannacokinetic
release profile compared to a composition with a lower percentage of
cholesterol.
Increasing the amount of cholesterol in the composition results in production
of
liposomes with a more rigid membrane. A more rigid membrane indicates a
relatively
more stable liposome. A composition formulated with an approximate molar ratio
of
dipalmitoylphosphatyidylcholine:cholesterol of 7:3 is systemically released
over a
5
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
WO 01/03669 PCT/CA00/00806
longer period of time compared to fatirtulations with a lower relative amount
of
cholesterol. The compositions contain at least I 0% cholesterol. To tailor the
kinetics of
drug release, the composition is formulated to contain at least 20%, 25%, 30%,
35% or
40% cholesterol. Preferably, the percentage of cholesterol in the composition
does not
exceed 45%.
The compositions are therapeutically active and have been demonstrated to
produce clinical benefits in subjects suffering from oxidative tissue damage.
Accordingly, the invention provides a method of delivering an antioxidant to a
vertebrate
(e.g., a mammal) by contacting a pulmonary tissue of the mammal with a
liposomal
composition containing a hydrophilic sulfhydryl agent and a lipophilic
antioxidant as
described above. Preferably, the mammal is a human. The subjects to be treated
include
those which have been identified as suffering from or at risk of developing a
pulmonary
injury, a hepatic injury, hemorrhagic shock, endotoxic insult, reperfusion
injury, or adult
respiratory distress syndrome. Methods of diagnosing such ailments are known
in the
art. The compositions are administered orally or parenterally, e.g., by an
intratracheal,
intravenous, intraarterial, intraperitoneal, or intratissue route. The
invention also
includes a method of treatment for insults of oxidative stress and neturophil
infiltration
induced by hemorrhagic shock and bacterial Iipopolysaccharide challenge. The
methods
result in a demonstrable reduction of lung and liver injuries.
Other features and advantages of the invention will be apparent from the
following
detailed description, and from the claims.
DESCRIPTIOV OF THE DRAWI1V'GS
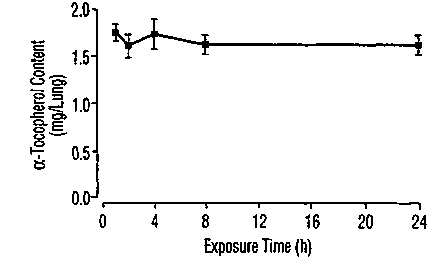
FIGS. l A-B are line graphs showing the recov ery of a-tocopherol and N-acetyl
cysteine (NAC) from lung homogenates following the intratracheal instillation
of
liposomal a-tocopherol (FIG. 1 A) or free NAC, liposomal NAC (L-NAC) or
liposomal
a-tocopherol and NAC (L-aT-NAC) (FIG. 1B). The liposomal preparations were
formulated as described in "Preparation of iiposome-associated antioxidants",
and lungs
of treated animals were removed at various time periods after intratracheal
instillation as
indicated in the figure. Each point represents the mean percentage of
recovered dose +
SEM of 4 animals.
FIGS. 2 A-B are bar graphs showing the effects of free N-acetyl cysteine
(NAC),
6
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
WO 01/03669 PCT/CA00/00806
liposomal NAC (L-NAC), liposomaI a-tocopherol (L-a,T), or Iiposomal a-
tocopherol
and NAC (L-aT-NAC), administered intratracheally to the lungs of shocked
animals.
FIG. 2A shows changes on the LPS-induced changes in lipid peroxidation, an
indicator
of oxidative stress. and FIG. 2B shows changes in non-protein thiol
concentration, a
group of protective agents against oxidant-induced injury. Animals were
maintained in
the hemorrhagic shock state for 60 minuets, followed by reperfusion with shed
blood
over a ? hour period. Thirty minutes after reperfusion, animals were
intratracheally
instilled with saline or different antioxidant preparations. Following a
period of 18
hours after the initiation of shock. animals were challenged intratreacheally
with LPS
(300 pgvxg body weight) and killed 4 hours later. Each data point represents
the mean
SEM of 6 animals. The symbol "*" represents significantly different (p<0.05)
from the
corresponding value obtained from shocked animals treated with saline and
challenged
with LPS.
1 ~ FIGS. 3A-B are bar graphs showing the effects of free N-acetyl cysteine
(NAC),
liposomal NAC (L-NAC), liposomal a-tocopheroI (L-aT), or liposomal a-
tocopherol
and NAC (L-aT-NAC), administered intratracheally to the lungs of shocked
animals.
FIG. 3A shows changes on the LPS-induced changes in pulmonary myeloperoxidase
concentration, and FIG. 3B shows the number of polymorphonuclear leukocytes
(PMN)
in the bronchoalveolar lavage (BAL) fluid. Animals were maintained in the
hemorrhagic shock state for 60 minuets, followed by reperfusion with shed
blood over a
hour period. Thirty minutes after reperfusion, animals were intratracheallv
instilled
with saline or different antioxidant preparations. Following a period of 18
hours after
the initiation of shock, animals were challenged intratracheally with LPS (300
g/kg
body weight) and killed 4 hours later. Each data point represents the mean +
SEM of 6
animals. The symbol "*" represents significantly different (p<0.05) from the
corresponding value obtained from shocked animals treated with saline and
challenged
with LPS.
FIG. 4 is a bar graph showing the effects of free N-acetyl cysteine (NAC),
Iiposomal NAC (L-NAC), liposomal a-tocopherol (L-aT), or liposomal a-
tocopherol
and NAC (L-a.T-NAC), administered via the circulation to shocked animals, on
the LPS-
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
WO 01/03669 PCT/CAOOI00806
induced changes in the number of polvmorphonuclear leukocytes (PMN) in the
bronchoalveolar lavage (BAL) fluid. Animals were maintained in the hemorrhagic
shock state for 60 min, followed by reperfusion with shed blood and an equal
volume of
Ringer's lactate with or without liposomal antioxidants, over a 2-h period.
Following a
period of 18 h after the initiation of shock, animals were challenged
intratracheally with
LPS (300 micrograms/kg body weight) and killed 4 h later. Each data point
represents
the mean ~ SEM of 3 animals. The symbol "*" represents significantly different
(p<0.05) from the corresponding value obtained from shocked animals treated
with
saline and challenged with LPS.
FIGS. SA-B are photographs showing the results of a Northern blot assay. FIG.
SA shows expression of cytokine-induced neutrophil chemoattractant (CINC)
mRNA,
and FIG. 5 B shows expression of G3PDH mRNA (as a control). FIGS. ~C-D are bar
graphs showing the effects of free N-acetyl cysteine (NAC), liposomal NAC (L-
NAC),
liposomal u-tocopherol (L-aT}, or liposomal a-tocopherol and NAC (L-aT-NAC),
administered to shocked animals, on the LPS-induced changes in cytokine-
induced
neutrophil chemoattractant (CINC) expression in lung tissue. Thirty minutes
after
reperfusion, animals were intratracheally instilled with saline or different
antioxidant
preparations (FIG. SC). Alternatively, the saline or antioxidant preparations
were
administered via the circulation during the 2 hour reperfusion period (FIG.
SD}. Animals
were maintained in the hemorrhagic shock state for 60 min, followed by
reperfusion
with shed blood over a 2-h period. Thirty minutes after reperfusion, animals
were
intratracheally instilled with saline or different antioxidant preparations
(Panel A).
Alternatively, the saline or antioxidant preparations were administered via
the circulation
during the 2-h reperfusion period (Panel B). Following a period of 18 h after
the
initiation of shock, animals were challenged intratreacheally with LPS (300
micrograms/kg body weight) and 4 h later, animals were killed and their iun~
tissues
harvested for the Northern blot procedure. Corresponding G3PDH mRNA bands (in
lanes 1 - 6, each representing the corresponding treatment group shown in
FIGS. SC-D)
are shown as evidence of comparable loading. Scanning densitometry of Northern
blots
for CINC mRNA was normalized by densitometry of corresponding G3PDH mRNA
bands and expressed as mean ~SEM of 4 animals per group. The symbol "*"
represents
significantly different (p<p.01 ) from the corresponding value obtained from
shocked
8
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
WO 01/03669 PCT/CA00/00806
animals treated with saline and challenged with LPS.
FIGS. 6A-B are bar graphs showing the effects of free N-acetyl cysteine (NAC),
liposomal \ AC (L-NAC), liposomal a-tocopherol (L-aT), or liposomal a-
tocopherol
S and NAC (L-aT-NAC), administered to shocked animals, on the LPS-induced
changes
in angiotensin converting enzyme (ACE) activity, an injury marker of pulmonary
endothelial cells. Thirty minutes after reperfusion. animals were
intratracheallv instilled
with saline or different antioxidant preparations (FIG. 6A). Alternatively,
the saline or
antioxidant preparations were administered via the circulation during the 2
hour
reperfusion period (FIG. 6B). Animals were maintained in the hemorrhagic shock
state
for 60 min, followed by reperfusion with shed blood over a 2-h period. Thirty
minutes
after reperfusion, animals were intratracheally instilled with saline or
different
antioxidant preparations (FIG. 6A). Alternatively. the saline or antioxidant
preparations
were administered via the circulation during the 2-h reperfusion period (FIG.
6B).
I S Following a period of I 8 h after the initiation of shock, animals were
challenged
intratracheally with LPS (300 micrograms/kg body weight) and killed 4 h later.
Each
data point represents the mean ~ SEM of 6 animals. The symbol "*" represents
significantly different (p<0.05) from the corresponding value obtained from
shocked
animals treated with saline and challenged with LPS.
FIGS. 7A-B are bar graphs showing the effects of free N-acetyl cysteine (NAC).
liposomal \ aC (L-NAC), liposomal a-tocopherol (L-aT), or liposomal u-
tocopherol
and NAC (L-aT-NAC), administered intratracheally to the lungs of shocked
animals, on
the LPS-induced changes in transpulmonary albumin flux. Animals were
maintained in
the hemorrhagic shock state for 60 min, followed by reperfusion with shed
blood over a
2-h period. Thirty minutes after reperfusion, animals were intratrachealIy
instilled with
saline or different antioxidant preparations. Following a period of I8 h after
the
initiation of shock, animals were challenged intratracheally with LPS (300
micrograms. kg body weight) and 4 h later, their transpulmonary albumin flux
was
assessed as described in the text. Each data point represents the mean + SEM
of 3
animals in each group. The symbol "*" represents significantly different
{p<O.OI ) from
the corresponding value obtained from shocked animals treated with saline and
challenged with LPS.
9
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
~WO 01/03669 PCT/CA00/100806
FIG. 8 is a bar graph showing the effects of free N-acetyl cysteine (ATAC),
liposomal NAC (L-NAC), Iiposomal a-tocopherol (L-aT), or liposomal a-
tocopheroI
and NAC (L-aT-NAC), administered via the circulation to shocked animals, on
the LPS-
induced changes in the plasma levels of alanine aminotransferase (ALT), an
indicator of
liver damage. Animals were maintained in the hemorrhagic shock state for 60
min,
followed by reperfusion with shed blood and an equal volume of Ringer's
lactate with or
without liposomaI antioxidants, over a 2-h period. Following a period of 18 h
after the
initiation of shock, animals were challenged intratracheally with LPS (300
micrograms/kg body weight) and killed 4 h later. Each data point represents
the mean _+
SEM of 6 animals. The symbol "*" represents significantly different (p<0.05)
from the
corresponding value obtained from shocked animals treated with saline and
challenged
with LPS.
DETAILED DESCRIPTION OF THE INVENTION
Methods and compositions are disclosed herein for the production of a
therapeutic
agent for the treatment of inflammatory complications associated with
pulmonary and
hepatic injuries, induced by hemorrhagic shock and endotoxemia. The
compositions
comprise bifimctional liposomal vesicles containing a hydrophilic sulfhydryl
agent
encapsulated in the aqueous interior of the vesicle and a lipophilic
antioxidant
incorporated in the vesicle membrane. One of the novel characteristic of the
present
invention resides in its sustained release property, which enables the initial
delivery and
subsequent retention of the active therapeutic agents) at the injured tissue
"target site".
For example, antioxidants are release shortly after administration (e.g,
within 30
minutes) and continue to be released for a prolonged period of time, e.g., for
6 hours. 12
hours, 24 hours, and up to several days post-administration.
The compositions are prepared by producing liposomes with a specific
combination of bilayer-forming lipids, which are compatible with, and non-
toxic to,
pulinonary tissues. A wide variety of lipids including, but not limited to,
phosphatidyl
esters and ethers (e.g., phosphatidylcholine, phosphatidylethanolamine, etc.);
glycerides;
cerebrosides; sphingomyelin; gangliosides; steroids (e.g., cholesterol); and
the like, may
be utilized in the production of the liposomes disclosed herein. One or more
lipid
entities may be present in the liposome, with a bilayer-forming lipid
constituting the
major liposomal component and the other lipid (e.g., cholesterol) constituting
the minor
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
WO 01/03669 PGT/CA00/00806
component.
The biologically-active, therapeutic ingredients are incorporated in the
liposomal
microcapsules and do not interfere with the integrity, nor the stability of
the lipid carrier.
Moreover, the therapeutic agents comprising the liposomal formulations of the
present
invention may also serve to increase the overall structural and/or chemical
stability of
said formulations. Typically, the hydrophilic component of the liposomal
preparation is
comprised of a compound possessing a suifhydryl group with free-radical
scavenging
and antioxidant properties. The hydrophobic therapeutic agent, also possessing
strong
antioxidant properties, is incorporated into the liposomal bilayer. The
vesicles were
prepared by combining the selected lipids, in appropriate ratios, in the
presence of the
lipophilic antioxidant, followed by the subsequent entrapment of the
hydrophilic
antioxidant. The procedure is conducted in such a manner so as not to
denature,
macnvate, or compromise the therapeutic efficacy of said antioxidants.
\tumerous
methodologies may be utilized for liposome production, including, but not
limited to,
1 ~ Shek, et al.. 1985. Irnmunologv ~7: 153-157, 1985; Jurima-Romet and Shek,
1991. J.
Phar»r. Pharmacol. 43: 6-10; Suntres and Shek, 1994. J. Pharm. Pharmacol. 46:
23-28,
whose disclosures are incorporated herein by reference in their entirety.
The liposomal antioxidant formulation may be administered to a vertebrate host
by
acceptable conventional methods, including, but not limited to, intratracheal,
intravenous, intraarterial, and intraperitoneal procedures. The administered
dose will
vary depending upon the specific antioxidant composition and the recipient. A
therapeutic regimen can be established by determining the antioxidant
retention time at
the target body-site and the extent of local inflammation and injury. The
liposomal
antioxidant preparation may be used for the treatment of inflammatory
complications
associated with sepsis, trauma, and adult respiratory distress syndrome.
In contrast to the aforementioned results for NAC, the administration of
liposome-
entrapped a-tocopherol, alone. has been reported to significantly attenuate
endotoxin-
induced tissue injury in the liver and the lung. See, Suntres and Shek, 1996.
Shock 6:
S57-64, 1996; Suntres and Shek, 1996. J. Endotoxin. Res. 3: SOS-512. Liposome-
entrapped a-tocopherol also has been shown to reduce the toxic effects of
reactive
oxygen species released from phorbol myristate acetate-stimulated pulmonary
target
cells and infiltrating neutrophils. See, Suntres and Shek, 1995. J. Drug
Targeting. 3_:
201-208. The doses of NAC administered according to the invetion are at least
1-2 logs
11
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
wo oiio3~9 PcricAOO~ooso6
less than the doses administered using previously described therapeutic
methods. The
methods described herein are therefore safer than earlier methods.
DETAILED DESCRIPTION OF THE INVENTION
S I. Preparation of Liposome-Associated Anti-Oxidants
Liposome preparations consisted of either DPPC:cholesterol 7:3 with NAC
entrapped; DPPC:a-tocopherol:cholesterol 7:2:1 with NAC entrapped; or DPPC:a-
tocopherol:cholesterol 7:2:1 without NAC. The lipids were dissolved in
chloroform:methanol (2:1, v/v) and the lipid solution was dried in a water-
bath at 40'C
under a stream of helium gas to a thin film, coating the interior surface of a
round-
bottomed glass vessel. Any residual solvent was removed by placing the vessel
under
vacuum for at least 18 hours. The dried lipid was then hydrated with either I
ml of 200
mgiml NAC for every I 00 mg of lipid or 1 ml of phosphate-buffered saline for
every
I00 mg of lipid at S I °C. The glass vessel was vortexed periodically
and kept at this
temperature for one hour to form multilamellar vesicles. The multilamellar
vesicles
were subjected to a total of 5 freezelthaw cycles using liquid nitrogen and a
40'C water-
bath. The multilamellar vesicles were then extruded a total of 10-times with
ail extruder
(Lipex Biomolecules; Vancouver, BC) fitted with two, stacked polycarbonate
filters of
various pore sizes (e.g., 100 nm, 400 nm) under a helium pressure of 100 to
200 p.s.i.
Non-entrapped NAC was removed by washing the liposomes twice in phosphate-
buffered saline (PBS) and pelleting by centrifugation at 105,000 x g for 1
hour at 5°C in
a Beckman L8-70 ultracentrifuge. Supernatant and resuspended pellet fractions
were
then assayed to determine overall NAC entrapment, and liposomal vesicle size
was
determined with the use of a Coulter N4SD particle size analyser. The final
NAC
liposome preparations were diluted to a concentration of 25.5 mgiml before
use.
12
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
WO 01/03669 PCT/CA00/00806
Table 1 illustrates particle sizing and the entrapment efficiencies of the
~osomal
antioxidants of the present invention.
Table 1
Liposome Composition Vesicle Size Entrapment Efficiency
(molar ratio) (mean ~ MEM) V-acetvl cysteine a-Tocopherol
DPPC:Chol (7:3) 337.3 ~ 1 S.0 27.9 ~ 4.5% 100°,'°
DPPC: u-T:Chol (7:2:1 ) 477.0 + 7.00 21.2 + 0.5% 100%
Data represent Mean + SEM of three experimental determinations.
Abbreviations: Chol = cholesterol; a-T = a-tocopherol
As shown in Table 1, a-tocopherol incorporated with a high degree of
efficiency
into the liposomal bilayers, with 100% entrapment at the lipid molar ratio
used.
The anti-oxidant, a-tocopherol is an extremely viscous and highly insoluble
liquid,
which renders it very difficult, if not impossible, to administer
parenterally. The
liposomal formulation disclosed herein provides a vehicle to facilitate the
incorporation
of a-tocopherol and its parenteral delivery. Additionally, the same liposome
vehicle
also enables the encapsulation of N-acetyl cysteine (NAC) for concomitant
delivery of
both anti-oxidants. N-acetyl cysteine was encapsulated in the vesicles at an
entrapment
efficiency of about 21-28%. Unlike previously described methods which describe
liposomes which entrap a hydrophilic agent or those which entrap a lipophilic
agent, the
methods described herein co-entrap a hydrophilic and lipophilic agent such as
tocopherol
and NAC.
Preferred Liposomal Antioxidant Composition
For intratrac6eal administration:
Formula 1
Dipalmitoylphosphatidylcholine 7.56 mg
a-Tocopherol 1.27 mg
Cholesterol 0.57 mg
Isotonic saline 94 pl
13
SUBSTITUTE SHEET (RCTLE 26)
CA 02340757 2001-02-16
WO 01/03669 PCTICA00/00~6
Formula 2
Dipalmitoylphosphatidylcholine7.67 mg
Cholesterol 1.73 mg
N-acetylcysteine 3.76 mg
Isotonic saline 94 ~1
Formula 3
Dipalmitoylphosphatidylcholine7.56 mg
a-Tocopherol 1.27 mg
Cholesterol 0.57 mg
N-acetylcysteine 3.76 mg
Isotonic saline 94 pl
For intravenous administration:
Formula 4
Dipalmitoylphosphatidylcholine37.82 mg
a-Tocopherol 6.34 mg
Cholesterol 2.85 mg
Isotonic saline 470 p1
Formula 5
Dipahnitoylphosphatidylcholine38.34 mg
Cholesterol 8.66 mg
N-acetylcysteine 18.8 mg
Isotonic saline 470 pl
Formula 6
Dipalmitoylphosphatidylcholine37.82 mg
a-Tocopherol 6.34 mg
Cholesterol 2.85 mg
N-acetylcysteine 18.8 mg
Isotonic saline 470 pl
Preferred Lipid and Antioxidant
Molar Ratio
Formula 7
Dipalmitoylphosphatidylcholine7.00
Cholesterol 3.03
N-acetylcysteine 15.45
14
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
WO 01/03669 PCTICA00/00806
Formula 8
Dipalmitoylphosphatidylcholine 7.00
a-Tocopherol 1.97
Cholesterol I .02
N-acetylcysteine 15.59
Preferred Liposome Size (prepared by the extrusion method)
a) For intratracheal administration:
Liposomes containing a-tocopherol and N-acetylcysteine 477 + 7.0 nm
b) For intravenous administration:
Liposomes containing a-tocopherol and N-acetylcysteine 149 + 0.3 nm
Preferred Liposomal Antioxidant Dosage for Effective Therapy
a) For intratracheal administration:
\'-acetylcysteine in a-tocopherol liposomes 9.=1 mg/kg body weight
b) For intravenous administration:
I S V-acetylcysteine in a-tocopherol Iiposomes 47.0 mg/kg body weight
II. Prolonged Anti-Oxidant Retention in the Lung
The therapeutic efficacy of an antioxidant in treating oxidant stress-induced
lung
injury, to a large extent, depends upon the availability of the antioxidant in
sufficient
quantities in the pulmonary milieu. Most, if not all of the published studies
have
disclosed very limited (e.g., 0.5-5% of initial dose) pulmonary uptake of a-
tocopherol
following intragastric and parenteral administration. In contrast, the results
disclosed
herein demonstrate that the intratracheal administration of liposomal a-
tocopherol
resulted in a total pulmonary level of about 1.5 mg or a retention of about
79% of the
administered a-tocopherol dose at 24 hours post-administration (see, FIG. 1
A).
The retention of NAC within the lungs of normal rats treated intratracheally
with
free ~1AC; liposomal NAC; or a-tocopherol liposomal NAC is shown in FIG. 1 B.
Recovery of NAC in the lung was approximately 1 % of the initial dose 1 hour
after the
administration of free NAC, and subsequently declined to 0.2% of initial dose
approximately 3 hours, later. In direct contrast, the recovery of NAC after
the
administration of L-NAC was found to be approximately 8% and 3% of initial
dose at 1
hour and 24 hours post-administration, respectively. The pulmonary retention
of NAC
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
wo oiro~9 rcricnooiooso6
following the administration of a-tocopherol liposomal NAC followed a similar
retention characteristics to that of liposomal NAC, but it was lower.
III. Animal Model of Hemorrhagic Shock and Lung Injuy
Male Sprague-Dawley rats (300-350 grams in weight) were anesthetized with
intraperitoneally administration of ketamine (80 mg/kg) and xylazine (8
mg/kg). The
right carotid arten~ was cannulated with a 22-gauge angiocath (Becton
Dickinson;
Franklin Lakes, NJ) for monitoring of mean arterial pressure (MAP), blood
sampling
and resuscitation. Hemorrhagic shock was then initiated by blood urithdrawal
and
reduction of the MAP to 40 mm Hg within 15 minutes. This blood pressure was
subsequently maintained by further blood withdrawal if the MAP rose to a level
of
greater-than 45 mm Hg, and by infusion of 0.5 ml of Ringer's Lactate (IRL) if
the MAP
dropped to a level greater-than 35 mm Hg. Withdrawn blood was collected into a
solution of 0.1 ml citrate/ml of blood, to prevent clotting. After a
hypotensive period of
60 minutes, animals were resuscitated by transfusion of the withdrawn blood
and RL in a
volume equal to that of withdrawn blood, over a period of two hours. The
catheter was
then removed. the carotid artery ligated, and the cervical incision sutured.
Control
(sham) animals underwent the same surgical procedures, but hemorrhage was not
induced. NAC delivery occurred in the control animals at an equiv alent time
to that
received by the experimental animals in which shock was induced.
Thirty minutes after resuscitation, the endotracheal intubation of liposome-
associated NAC and/or a-tocopheroi was performed. The animal was placed on a
slanted board (20° from the vertical) and was supported by an elastic
band under its'
upper incisors. A microscope lamp, with its beam directed at the neck area,
provided
transillumination during the procedure. By opening the mouth of the animal and
depressing the tongue, the larynx could be easily visualized. The liposomal
suspension
was delivered to the lung, via the intratracheal administration, using PE-50
polythylene
tubing (6.5 cm) connected to a 25-gauge epidural catheter. The endotracheal
tube was
introduced into the trachea using gentle pressure. All animals received 1 SO
pl of a
liposomal preparation which contained 9.4 mg/kg body weight of I'TAC and/or a-
tocopherol, followed by 20 mechanically-ventilated breaths using a rodent
ventilator.
Eighteen hours after hemorrhage-resuscitation, lipopolysaccharide (LPS;
Escherichia
colt strain O 11 B4; at a concentration of 300 pglkg in 200 ml saline) was
administered
16
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
WO 01/03669 PCT/CA00/00806
intratrachealy. Animals were sacrificed in 4-6 hours by a pentobarbital
overdose.
IV. Lung Tissue Preparation
The lungs were removed from animals immediately after decapitation and rinsed
with ice-cold saline to remove residual blood. All subsequent steps were
carried out at
0-4°C. Approximately 1 g of lung sample was homogenized with a
Brinkmann Poivtron
in a sufficient volume of ice-cold 50 mM potassium phosphate buffer. pH 7.4,
to
produce a 20% homogenate.
V . Lipid Peroxidation Determination
Lipid peroxidation products in lung homogenates (i.e., malonaldehyde (MDA) and
4-hydroxyalkenals (4-HNE)), were measured by the use of an assay kit (R&D
Systems;
Minneapolis. MN). This assay is based upon the reaction of a chromogenic
reagent (IV-
methyl-2-phenylindole), with MDA and 4-HNE at 4~°C. One molecule of
either MDA
or 4-HNE reacts with 2 molecules of the chromogenic reagent to yield a stable
chromophore with maximal absorbance at 586 nm. The concentration of MDA and 4-
HNE is then quantitated by the absorbance at this wavelength.
VI. Determination of Pulmonary Non-Protein Sulphydryl Concentration
The non-protein sulphydryl concentration, which includes glutathione and NAC,
in
pulmonary homogenates was determined as described by Suntres and Shek ( I 994.
J.
Pharm. Pharmacol. 46: 23-28). Briefly, the tissue was homogenized in 20% (wiv)
trichloroacetic acid and centrifuged at 600 x g for 20 minutes in a
refrigerated Beckman
GS-6R centrifuge. An aliquot of the deproteinized supernatant fraction was
added to 2
ml of 0.3 M ~Ia,HPO, solution followed by addition of 0.5 ml of 0.04% 5,5-
dithiobis-[2-
nitrobenzoic acid) (NbS,) dissolved in 10% sodium citrate. The absorbance at
412 nm
was measured immediately after mixing.
VII. Calculation of Pulmonary NAC Content
Since the assay performed above measures the total non-protein sulphydryl
content
(GSH + NAC) in the lungs of normal animals, the I~TAC values were obtained by
subtracting the values for GSH (460 pg/Iung) from the total non-protein
sulfhydryl
values.
VIII. Enz~~me Measurements
17
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
WO 01/03669 PCT/CA00/00806
The activity of angiotensin converting enzyme (ACE) in lung hoz~ogenate~ ~tras
determined by using a kit (Sigma Chemical Company, St. Louis, MO) according to
the
manufacturer's protocol. The activity of myeloperoxidase (MPO) in sonicated
whc:
lung homogenates was performed using an assay kit (R&D Systems; Minneapolis,
MN)
according to the manufacturer's directions. Plasma alanine aminotransferase
(ALT)
activity, expressed as Sigma Frankel (SF) units/ml, was determined with a
diagnostic kit
(No. 505; Sigma Chemical Company; St. Louis, MO).
IX. Reduction of Pulmonary Oxidant-Stress After Treatment with Liposomal
Anti-Oxidants
Pulmonary Lipid Peroxidation:
The level of lipid peroxidation has been used as an indicator of oxidative
stress. --
Challenge of shocked with LPS produced a significant increase in lipid
peroxidation in
pulmonary homogenates (i.e., an 18-fold increase), as measured by the
formation of
MDA and 4-HNE. Pre-treatment of rats with NAC did not significantlw alter the
LPS-
induced increases in lipid peroxidation (see, FIG. 2A). Conversely, pre-
treatment of rats
with NAC- or a-T-containing liposomes or liposomes containing both u-T and
NAC,
were found to partially protect against the LPS-induced lipid peroxidation at
levels of
55%, 38%, and 62%, respectively.
Pulmonary Non-Protein TTiiols:
As the non-protein sulphydryls (NP-SH), glutathione and ~1AC, are known to
play
an important role in protecting cells against oxidant-induced tissue injun~,
the
concentration of non-protein sulphydryls in lung tissues were also measured.
The
administration of LPS in shocked animals was found to result in a significant
reduction
(43%) in NP-SH concentration (see, FIG. 2B). Intratracheal administration of
NAC did
not significantly increase the NP-SH content of the lung.
In contrast, intratracheal administration instillation of liposomes containing
NAC
or NAC and a-tocopherol was shown to result in a significant increase in the
pulmonary
NP-SH content, which may be attributed to the retention of NAC within the
lung.
X. Bronchoalveolar Lavage Preparation
For Bronchoalveolar Lavage Preparation (BAL), the lungs were lavaged with cold
18
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
WO 01/03669 PCT/CA00~06
phosphate-buffered saline (PBS; 8 mM sodium phosphate. ? mM potassium
phosphate,
0.14 M sodium chloride, 0.01 M potassium chloride, pH 7.4 with 0.1 mM EDTA)
using
an intratracheal angiocath. The PBS was instilled in 10 ml aliquots, and
gently
withdrawn with a 10 ml syringe, so as to provide a total administered volume
of 40 mi.
The collected BAL fluid was then centrifuged at 300 x g for 10 minutes to
pellet cells.
The supernatant was discarded, and the peileted cells were resuspended in a
small
volume of serum-free DMEM culture medium (Gibco; Burlington, Ontario). Total
cell
counts were determined on a grid hemocytometer. Differential cell counts were
enumerated on cytospin-prepared slides that were stained with Wright-Giemsa
stain. A
total of 500 cells were counted in cross-section per sample and the number of
polymorphonuclear leukocytes (PMN) and alveolar macrophages were calculated as
the
total cell count multiplied by the percentage of the respective cell type in
the BAL fluid
(BALF) sample.
XI. Quantitation of CINC mR.~IA Expression by Northern Blot Analysis
1 ~ Total RNA from lungs was obtained using the guanidium-isothiocyanate
method.
See. Chomczynski and Sacchi, 1987. Anal. Biochem. 162: 156-160. In brief, the
lungs
were harvested from treated animals and immediately frozen in liquid nitrogen.
The
lungs were then thawed and homogenized in 10 ml of 4 M guanidine-
isothiocyanate
containing 25 mM sodium citrate, 0.5% sarcosyl, and 100 mM (3-mercaptoethanol.
RNA was denatured, eiectrophoresed through a 1.2% formaldehyde-agarose gel and
transferred to nylon membrane. Hybridization was carried out using a [3ZP]ATP-
end-
labeled 30-base oligonucleotide probe for the cytokine-induced neutrophil
chemoattractant (CINC) possessing the with the nucleotide sequence
s'-GCGGCATCACCTTCAAACTCTGGATGTTCT-3', [SEQ ID NO:1 ] which is
complementary to nucleotides 134 to 164 of CINC cDNA (see, Balckwell, et aL.
1994.
Am. J. Respir. Cell Mol. Biol. 11: 464-472), kindly provided by Dr. Timothy S.
Blackwell; ~'anderbilt University School of Medicine, Nashville, TN. Blots
were then
washed under conditions of high stringency and specific mRNA bands were
detected by
autoradiography in the presence of intensifying screens as previously
reported. Blots
were stripped and reprobed for glyceraldehyde 3-phosphate dehydrogenase
(G3PDH),
which is a ubiquitously expressed housekeeping gene to control for loading
(see, Tso, et
al.. Nucl. Acids Res. 13: 2485-2490). Expression of mRNA was quantitated using
a
19
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
wo oiro~9 PcTicAOOioo8o6
phosphoimager and accompanying ImageQuant software (Molecular Dyi~mics;
Sunnyvale, CA) and was non~nalized to the G3PDH signal.
XII. Reduction of Neutrophil Infiltration in the Lung After Treatment with
Liposomal Anti-Oxidants
Lung hTreloperoxidase .4ctiviy:
Lung injury in shocked animals subsequently challenged with LPS, is generally
associated with the infiltration and activation of neutrophils. This
neutrophilic
infiltration in the lungs of shocked animals challenged with LPS, was assessed
by
measuring the activity of myeloperoxidase (MPO), an enzyme localized primarily
in
neutrophils. As shown in FIG. 3A, the MPO activity in shocked animals was
increased
by 16-fold, following LPS administration. This increase is suggestive of
neutrophil
infiltration within the lungs. A very similar increase in MPO activity was
also observed
in LPS-challenged animals pretreated with NAC. Although
L-aT liposomal treatment prevented some neutrophil infiltration (i.e., 16%
reduction),
L-NAC and L-aT-NAC had a more pronounced suppressive effect against neutrophil
infiltration in the lung, with a 30% and 35% reduction, respectively.
Neutrophil Infrltration:
Hemorrhage-resuscitation followed by LPS administration caused a 14-fold
increase in polvmorphonuclear leukocyte (PMI~ ) infiltration, in comparison to
that in the
control (sham) animals (see, FIG. 3B). The increase in PMN in the shock/LPS
animal
group was attenuated to 71.2%, 80.0%, and 58.9% by liposome-associated NAC;
liposome-associated a-tocopherol, and liposome-associated NAC/a-tocopherol,
respectively. Empty liposome alone, did not alter PMN infiltration. Similarly,
intratracheal administration of NAC alone, did not decrease PMN influx after
shock/LPS
administration. A very similar pattern of Iiposomal antioxidant-mediated
reduction in
PMN infiltration was also evident upon anti-oxidant administration via the
circulation
(see. FIG. 4).
CINC mRNA E.rpressiorr:
In order to determine whether the alteration in poIymorphonuclear leukocyte
(PMN) infiltration is associated with changes in cytokine-induced neutrophil
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
WO 01/03669 PGT/CA00/00806
chemoattractant {CINC) expression, total RNA was extracted from whore lung
tissue 4
hours after LPS administration. Northern blot analysis for CINC mRNA was then
performed.
As shown in FIGS. SA-D, it was determined that antecedent shock primed the
S increase in CINC mRNA in response to a subsequent LPS challenge. However.
the
administration of liposome-associated NAC with or without a-tocopherol via the
trachea
(see, FIG. SC) or the circulation (see, FIG. SD), significantly decreased the
CINC
mRNA expression. in comparison to those mRNA expression levels found in
shocked
animals treated with saline and challenged with LPS. Since the inventors of
the present
invention have previously shown that CINC is the major chemokine, which
contributes
to PMN influx into alveoli in a two-hit model (see, Fan, et al., 1998. J.
Immunol. 161:
4.40-447), the sustained effect of NAC on preventing PMN infiltration may be
mediated
by a decrease in CINC expression.
XIII. Assessment of Transpulmonary Albumin Flux
ias
Transpulmonary albumin flux was assessed by injecting 1 mCi of I-albumin, in a
total volume of 0.2 ml saline, into the tail vein of the rat immediately
following
intratracheal administration of LPS or saline (see, Nathens, et al., 1996.
Surgew 120:
360-366). Six hours after LPS administration, 1 ml of blood was withdrawn by
cardiac
puncture for scintillation counting by the following procedure. Following
exsanguination, the lungs were perfused via a cannula in sitzr with 10 ml of
PBS. The
perfused PBS was withdrawn gently and a volume of I ml/tube was aliquoted for
counting. The transpulmonary albumin llux was normalized to blood cpm using
the
following formula:
Transpulmonary Albumin Flux - BALF cpm/ml
Blood cpm/ml
XIV. Evidence of Reduced Lung Damage by Treatment with Liposomai
Anti-Oxidants
Lung Angiotensin Converting Enzyme:
Due to the fact that angiotensin converting enzyme (ACE) has been used as an
injury marker of pulmonary endothelial cells, the effect of LPS on the
activity of this
21
SUBSTITUTE SHEET (RI: LE 26)
CA 02340757 2001-02-16
WO 01/03669 PCT/CA00/00806
enzyme in lung homogenates of shocked animals pre-treated with saline, NAC, L-
NAC,
La-T, or L-aT-NAC, was measured. As shown in FIGS. 6A-B, the challenge of
shocked animals with LPS produced a significant reduction in ACE (3~%) in Lung
homogenates of saline-pretreated animals. Treatment of animals with :SAC
failed to
attenuate the LPS-induced decreases in ACE activity, whereas treatment of
animals with
NAC-containing liposomes conferred a protective effect ( 19% of saline-pre-
treated
animals). Additionally, pre-treatment of animals with L-a.T or L-aT-NTAC also
ameliorated the LPS-induced changes in ACE activity, to approximately the same
level
as that which was observed following L-NAC treatment. The administration of
the
liposomal preparations via the tracheal (see. FIG. 6A) or the circulation
(see, FIG. 6B)
were effective in maintaining ACE activities in the lung, and therefore in
reducing the
extent of associated pulmonary endothelial cell damage.
Transpulmonan~ Albtimtn Fhcr:
In order to evaluate whether liposome-associated NAC and/or a-tocopherol could
prevent lung injury, transpulmonary albumin flux was meausred 24 hours after
hemorrhage-resuscitation and 6 hours after intratracheal administration of
LPS. As
shown in FIGS. 7A-B, the antecedent shock and the subsequent challenge with
LPS,
markedly increased lung permeability index (PI). The intratracheal
administration of
liposome-associated NAC, a-tocopherol, and NAC/a-tocopherol significantly
attenuated
the increase in PI to 27.7°%, 50.9%, and 20.4%, respectively, as
compared to that of
shocked animals treated with saline and challenged with LPS.
XV. Reduced Hepatic Damage in Animals Treated with Liposomal
Anti-Oxidants
Plasma Alanine ~IminotransJ'erase (ALT) Enzyme:
The measurement of hepatic enzymes such as plasma alanine aminotransferas
(ALT) released into the blood has been shown to be a reliable indicator of
hepatic injury.
Plasma ALT activities were found to be elevated by greater than 8-fold in
shocked
animals subsequently challenged with LPS, thus indicating a rather substantial
hepatic
injury (see. FIG. 8). The administration of free antioxidant reduced the ALT
down to
about 6-fold, but the most effective treatment was mediated by the
administration of
liposomal antioxidants, which essentially prevented hepatic injury as
indicated by the
22
SUBSTITUTE SHEET (RULE 26)
CA 02340757 2001-02-16
WO 01/03669 PCT/CA00/0080b
presence of normal plasma ALT activities.
It is evident from the above observations that the said bifi.tttctional
liposome
formulation containing both a-tocopheroi and N-acetyl cysteine is effective in
providing
a therapeutic benefit for treating lung and liver injuries. Furthermore, the
concept of
bifunctional liposomes can be further exploited to coentrap other pairs of
related
antioxidants in therapeutic applications.
Equivalents
From the foregoing detailed description of the specific embodiments of the
present
invention, it should be readily apparent that a unique compositions and
methods of
1 U treatment involving the use of uni- and mufti-lamellar liposomes as a
vehicle to provide
systemic delivery of an antioxidant, via administration to the pulmonary
system, have
been disclosed. Although particular embodiments have been disclosed herein in
detail,
this has been done by way of example for purposes of illustration only, and is
not
intended to be limiting with respect to the scope of the appended claims which
follow.
1 ~ In particular, it is contemplated by the inventor that various
substitutions, alterations,
and modifications may be made to the invention without departing from the
spirit and
scope of the invention as defined by the claims.
Other embodiments are within the following claims.
23
SUBSTITUTE SHEET (RULE 26)