Note: Descriptions are shown in the official language in which they were submitted.
CA 02341458 2001-02-21
1
DESCRIPTION
NEW COMPOUND
TECHNICAL FIELD
This invention relates to new aminoalcohol derivatives
and salts thereof which are ~3 adrenergic receptor agonists
and useful as a medicament.
DISCLOSURE OF INVENTION '
This invention relates to new aminoalcohol derivatives
which are ~3 adrenergic receptor agonists and salts thereof.
More particularly, it relates to new aminoalcohol
derivatives and salts thereof which have gut selective
sympathomimetic, anti-ulcerous, anti-pancreatitis, lipolytic,
anti-urinary incontinence and anti-pollakiuria activities, to
processes for the preparation thereof, to a pharmaceutical
composition comprising the same and to a method of using the
same therapeutically in the treatment and/or prevention of
gastro-intestinal disorders caused by smooth muscle
contractions in human beings or animal.
One object of this invention is to provide new and
useful aminoalcohol derivatives and salts thereof which have
gut selective sympathomimetic, anti-ulcerous, lipolytic,
anti-urinary incontinence and anti-pollakiuria activities.
Another object of this invention is to provide processes
for the preparation of said aminoalcohol derivatives and
salts thereof.
A further object of this invention is.to provide a
pharmaceutical composition comprising, as an active
ingredient, said aminoalcohol derivatives and salts thereof.
Still further object of this invention is to provide a
therapeutical method for the treatment and/or prevention of
aforesaid diseases in human beings or animals, using said
aminoalcohol derivatives and salts thereof.
CA 02341458 2001-02-21
2
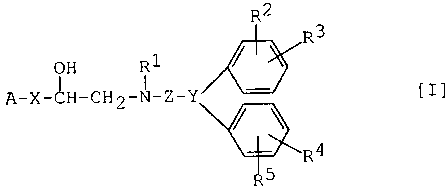
The object aminoalcohol derivatives of this invention
are new and can be represented by the following general
formula [I] .
R2
R3
i
OH R1
A-X-~H-CH2-N-Z-Y [I~
~R4
5
wherein
A is a heterocyclic group or aryl, each of which may have
1 to 3 same or different substituent(s) selected from a
group consisting of halogen, hydroxy, amino, lower
alkyl, lower alkylsulfonylamino, phenyl(lower)alkoxy and
phenyl(lower)alkoxycarbonylamino,
-X- is bond, -CH2-, -CH2-CH2-, -NH-CH2-, -0-CH2-, -S-CH2-,
-SO-CH2- or -S02-CH2-,
-Y~ is -C ' (in which R11 is hydrogen, hydroxy,
~11
lower alkoxy or acyloxy) and
R6 R8
-Z- is -(CH2)n-~-(CH2)m-~-(CH2)k-, -(CH2)n ~ C.\ (CH2)m-,
~7 ~9 (CH2) r
30
/( CH2 ) p.~
-(CH2)n-Q-(CH2)m-, -(CH2)n-CH CH-(CH2)m-~
~( CH2 ) q~
-(CH2)n-CH=CH-(CH2)m- or -(CH2)n-C=C-(CH2)m- (in which
-Q- is -O-, -S-, -SO-, -S02-, -N-CO-, -CO-N-,
~10 R10
-CH-CO-N-, -CH-CH2-N-, -S02-N-,
R11 R10 R11 R10 R10
CA 02341458 2001-02-21
3
-N-S02-, -CO- or -N-, wherein R10 is hydrogen or
~10 ~10
lower alkyl, and R11 is lower alkyl,
R6, R~, Rg and R9 are each independently hydrogen,
hydroxy, lower alkyl, lower alkenyl, lower alkoxy,
lower alkoxy(lower)alkyl or aryl which may have 1
to 3 lower alkoxy,
n, m and k is each independently 0 to 6,
p is 0 to 4,
q is 1 to 4, and
r is 2 to 7) and
-Z-Y~ is -(CH2)i-CH=C~ (in which i is 0 to 6),
R1 is hydrogen or an amino protective group, and
R2, R3, R4 and R5 are each independently hydrogen;
lower alkyl; lower alkylthio; lower alkylsulfonyl;
hydroxy; lower alkoxy;
amino; lower alkylamino; acylamino; N-(lower
alkyl)acylamino; carboxy; lower alkoxycarbonyl;
carbamoyl optionally substituted with one or two lower
alkyl; hydroxy(lower)alkyl; lower alkoxy(lower)alkyl;
N-acylamino(lower)alkyl; N-(lower alkyl)-N-
acylamino(lower)alkyl; carboxy(lower)alkyl;
lower alkoxycarbonyl(lower)alkyl; carbamoyl(lower)alkyl
optionally substituted with one or two lower alkyl; or
R12
-(CH2)j-N ~R13 (in which R12 and R13 are each
independently hydrogen or lower alkyl, or R12 and R13
may be bonded to form a lower alkylene chain, and j is 0
to 6) .
The object. compound [I] or a salt thereof can be
prepared by the following processes.
CA 02341458 2001-02-21
4
Process 1
R2
R3
o R1
A-X-C CH2 + HN-Z-Y
[II]
R4
or a salt thereof R5
[III]
or a salt thereof
R2
R3
i
OH R1
A-X-~H-CH2-N-Z-Y
R4
R5
[I]
or a salt thereof
Dr~~occ '7
R2
R3
i
OH Ra
A-X-CH-CH2-N-Z-Y
~R4
R5
[Ia]
or a salt thereof
R2
elimination reaction R3
~i
of amino protective OH H
group A-X-~H-CH2-N-Z-Y
~R4
R5
[Ib]
or a salt thereof
CA 02341458 2001-02-21
wherein A, X, Y, Z, R1, R2, R3, R4 and R5 are each as defined
above, and
Ra is an amino protective group.
5 In the above and subsequent description of the present
specification, suitable examples of the various definition to
be included within the scope of the invention are explained
in detail in the following.
The term "lower" is intended to mean a group having 1 to
6 carbon atom(s), unless otherwise provided.
Suitable example of "lower alkyl" and "lower alkyl"
moiety may include straight or branched one having 1 to 6
carbon atom(s), such as methyl, ethyl, propyl, isopropyl,
butyl, isobutyl, sec-butyl, tert-butyl, pentyl,
1-methylpentyl, tert-pentyl, neo-pentyl, hexyl, isohexyl and
the like.
Suitable "lower alkenyl" may include vinyl, 1-(or
2-)propenyl, 1-(or 2- or 3-)butenyl, 1-(or 2- or 3- or
4-)pentenyl, 1-(or 2- or 3- or 4- or 5-)hexenyl, methylvinyl,
ethylvinyl, 1-(or 2- or 3-)methyl-1-(or 2-)propenyl, 1-(or 2-
or 3-)ethyl-1-(or 2-)propenyl, 1-(or 2- or 3- or 9-)methyl-1-
(or 2- or 3-)butenyl, and the like, in which more preferable
example may be C2-C4 alkenyl.
Suitable "lower alkoxy" and "lower alkoxy" moiety may be
a straight or branched one such as methoxy, ethoxy, propoxy,
isopropoxy, 1-ethylpropoxy, butoxy, sec-butoxy, tert-butoxy,
pentyloxy, neopentyloxy, tert-pentyloxy, hexyloxy, and the
like, in which the preferred one may be C1-C4 alkoxy, and the
most preferred one may be methoxy.
Suitable example of "halogen" may be fluoro, chloro,
CA 02341458 2001-02-21
6
bromo and iodo.
Suitable example of "aryl" and "ar" moiety may include
phenyl, naphthyl, anthryl, and the like, in which the
preferred one may be phenyl.
Suitable example of "heterocyclic group" may include
unsaturated 3 to 8-membered (more preferably 5 or 6-
membered) heteromonocyclic group containing 1 to 4 nitrogen
atom(s), for example, pyrrolyl, pyrrolinyl, imidazolyl,
pyrazolyl, pyridyl, dihydropyridyl, pyrimidyl, pyrazinyl,
pyridazinyl, triazolyl (e. g., 4H-1,2,4-triazolyl, 1H-1,2,3-
triazolyl, 2H-1,2,3-triazolyl, etc.), tetrazolyl (e.g., 1H-
tetrazolyl, 2H-tetrazolyl, etc.), etc.;
saturated 3 to 8-membered (more preferably 5 or 6-
membered) heteromonocyclic group containing 1 to 4 nitrogen
atom(s), for example, pyrrolidinyl, imidazolidinyl,
piperidyl, piperazinyl, etc.;
unsaturated condensed heterocyclic group containing 1 to
4 nitrogen atom(s), .for example, indolyl, isoindolyl,
indolinyl, indolizinyl, benzimidazolyl, quinolyl,
isoquinolyl, indazolyl, benzotriazolyl, etc.;
unsaturated 3 to 8-membered (more preferably 5 or 6
membered) heteromonocyclic group containing 1 to 2 oxygen
atoms) and 1 to 3 nitrogen atom(s), for example, oxazolyl,
isoxazolyl, oxadiazolyl (e. g., 1,2,4-oxadiazolyl, 1,3,4-
oxadiazolyl, 1,2,5-oxadiazolyl, etc.), etc.;
saturated 3 to 8-membered (more preferably 5 or 6-
membered) heteromonocyclic group containing 1 to 2 oxygen
atoms) and 1 to 3 nitrogen atom(s), for example,
morpholinyl, sydnonyl, etc.;
unsaturated condensed heterocyclic group containing 1 to
2 oxygen atoms) and 1 to 3 nitrogen atom(s), for example,
benzoxazolyl, benzoxadiazolyl, etc.;
unsaturated 3 to 8-membered (more preferably 5 or 6-
CA 02341458 2001-02-21
7
membered) heteromonocyclic group containing 1 to 2 sulfur
atoms) and 1 to 3 nitrogen atom(s), for example, thiazolyl,
isothiazolyl, thiadiazolyl (e. g., 1,2,3-thiadiazolyl,
1,2,4-thiadiazolyl, 1,3,4-thiadiazolyl, 1,2,5-thiadiazolyl,
etc.), dihydrothiazinyl, etc.;
saturated 3 to 8-membered (more preferably 5 or 6-
membered) heteromonocyclic group containing 1 to 2 sulfur
atoms) and 1 to 3 nitrogen atom(s), for example,
thiazolidinyl, etc.;
unsaturated 3 to 8-membered (more preferably 5 or 6-
membered) heteromonocyclic group containing 1 to 2 sulfur
atom(s), for example, thienyl, dihydrodithiinyl,
dihydrodithionyl, etc.;
unsaturated condensed heterocyclic group containing 1 to
2 sulfur atoms) and 1 to 3 nitrogen atom(s), for example,
benzothiazolyl, benzothiadiazolyl, imidazothiadiazolyl, etc.;
unsaturated 3 to 8-membered (more preferably 5 or 6-
membered) heteromonocycli.c group containing an oxygen atom,
for example, furyl, etc.;
saturated 3 to 8-membered (more preferably 5 or 6-
membered) heteromonocyclic group containing an oxygen atom,
for example, tetrahydrofuran, tetrahydropyran, etc.;
unsaturated 3 to 8-membered (more preferably 5 or 6-
membered) heteromonocyclic group containing an oxygen atom
and 1 to 2 sulfur atom(s), for example, dihydrooxathiinyl,
etc.;
unsaturated condensed heterocyclic group containing 1 to
2 sulfur atom(s), for example, benzothienyl, benzodithiinyl,
etc.;
unsaturated condensed heterocyclic group containing an
oxygen atom and 1 to 2 sulfur atom(s), for example,
benzoxathiinyl, etc.;
2-oxo-2,3-dihydro-1H-benzimidazolyl; and the like.
Suitable example of "amino protective group" moiety may
CA 02341458 2001-02-21
8
be common amino protective group such as acyl, for example,
substituted or unsubstituted lower alkanoyl [e. g. formyl,
acetyl, propionyl, trifluoroacetyl, etc.], phthaloyl, lower
alkoxycarbonyl [e. g. tert-butoxycarbonyl, tert-amyloxy-
carbonyl, etc.], substituted or unsubstituted aralkyloxy-
carbonyl [e. g. benzyloxycarbonyl, p-nitrobenzyloxycarbonyl,
etc.], substituted or unsubstituted arenesulfonyl [e. g.
benzenesulfonyl, tosyl, etc.], nitrophenylsulfenyl,
ar(lower)alkyl [e.g. trityl, benzyl, etc.], and the like, in
which preferable one is phenyl(lower)alkyl such as benzyl.
Suitable "acyl" and "acyl" moiety may be carboxy;
esterified carboxy; carbamoyl optionally substituted with one
or two lower alkyl; lower alkylsulfonyl [e. g. methylsulfonyl,
ethylsulfonyl, propylsulfonyl, butylsulfonyl, pentylsulfonyl,
hexylsulfonyl, etc.]; substituted or unsubstituted lower
alkanoyl [e. g. formyl, acetyl, trifluoroacetyl, propanoyl,
butanoyl, 2-methylpropanoyl, pentanoyl, 2,2-
dimethylpropanoyl, hexanoyl, etc.]; and the like.
The esterified carboxy may be substituted or
unsubstituted lower alkoxycarbonyl [e. g. methoxycarbonyl,
ethoxycarbonyl, propoxycarbonyl, butoxycarbonyl,
hexyloxycarbonyl, 2-iodoethoxycarbonyl,
trifluoromethoxycarbonyl, 2,2,2-trichloroethoxycarbonyl, .
etc.], substituted or unsubstituted aryloxycarbonyl [e. g.
phenoxycarbonyl, 4-nitrophenoxycarbonyl,
2-naphthyloxycarbonyl, etc.], substituted or unsubstituted
ar(lower)alkoxycarbonyl [e. g. benzyloxycarbonyl,
phenethyloxycarbonyl, benzhydryloxycarbonyl,
4-nitrobenzyloxycarbonyl, etc.] and the like, in which the
preferred one may be lower alkoxycarbonyl, and the most
preferred one may be ethoxycarbonyl.
Suitable example of "lower alkylsulfonylamino" may
include methylsulfonylamino, ethylsulfonylamino,
propylsulfonylamino, butylsulfonylamino, pentylsulfonylamino,
CA 02341458 2001-02-21
9
hexylsulfonylamino, and the like, in which the preferred one
may be (Cl-C4)alkylsulfonylamino, and the most preferred one
may be methylsulfonylamino.
Suitable example of "heterocyclic group" in A can be
referred to aforementioned "heterocyclic group", in which the
preferred one may be unsaturated 3 to 8-membered (more
preferably 5 or 6-membered) heteromonocyclic group containing
1 to 4 nitrogen atoms) or unsaturated condensed heterocyclic
group containing 1 to 4 nitrogen atom(s), and the most
preferred one may be pyridyl, indolyl or 2-oxo-2,3-dihydro-
1H-benzimidazolyl.
The lower alkylene chain formed by R~ and R8 is a
straight or branched chain alkylene having 1 to 6 carbon
atoms and is exemplified by methylene, ethylene,
trimethylene, propylene, butylene, 1,2-dimethylethylene,
pentamethylene and hexamethylene.
Preferred embodiments of the object compound [I] are as
follow:
A is pyridyl, indolyl, 2-oxo-2,3-dihydro-1H-benzimidazolyl or
phenyl, each of which may have 1 to 3 same or different
substituent(s) selected from a group of hydroxy, amino
lower alkyl (more preferably methyl), lower
alkylsulfonylamino (more preferably
methanesulfonylamino), phenyl(lower)alkoxy (more
preferably benzyloxy) and
phenyl(lower)alkoxycarbonylamino (more preferably
benzyloxycarbonylamino),
-X- is bond, -CH2-, -CH2-CH2-, -O-CH2- or -S02-CH2-,
-Y~ is -C ~ (in which R11 is hydrogen or hydroxy) and
R11
R6 R8
-Z- is -(CH2)n-i-(CH2)m-i-(CH2)k-, -(CH2)ri ~C ~ (CH2)m- or
R7 R9 (CH2 ) r
CA 02341458 2001-02-21
-(CH2)n-Q-(CH2)m- (in which
-Q- is -CH-CO-N- or -CH-CHZ-N-, wherein
~11 ~10 ~11 ~10
5
R10 is hydrogen or lower alkyl (more preferably
methyl) and
R11 is lower alkyl (more preferably methyl),
R6, R~, R8 and R9 are each independently hydrogen,
10 lower alkyl (more preferably methyl) or aryl (more
preferably phenyl) which may have 1 to 3 lower
alkoxy (more preferably methoxy),
n, m and k is each independently 0 to 6, and
r is 2 to 7) and
-Z-Y~ is -(CH2)i-CH=C~ (in which i is 0 to 6),
R1 is hydrogen or ar(lower)alkyl (more preferably benzyl),
and
R2, R3, R4 and R5 are each independently hydrogen;
lower alkyl (more preferably methyl); lower alkylthio
(more preferably methylthio); lower alkylsulfonyl (more
preferably methanesulfonyl); hydroxy; lower alkoxy (more
preferably methoxy or ethoxy);
amino; lower alkylamino (more preferably methylamino);
acylamino (more preferably lower alkoxycarbonylamino,
lower alkylsulfonylamino, lower alkanoylamino, ureido or
trifluoroacetylamino, most preferably
methoxycarbonylamino, ethoxycarbonylamino,
methanesulfonylamino, formylamino, acetylamino or
propionylamino); N-(lower alkyl)acylamino [more
preferably N-(lower alkyl)-[(lower)alkoxycarbonyl]amino,
most preferably N-methyl-methoxycarbonylamino]; carboxy;
or lower alkoxycarbonyl (more preferably
methoxycarbonyl).
More preferred embodiments of the object compound [I]
CA 02341458 2001-02-21
11
are as follow:
A is phenyl each of which may have 1 to 3 same or different
substituent(s) selected from a group of hydroxy, amino,
lower alkylsulfonylamino (more preferably
methanesulfonylamino) and phenyl(lower)alkoxy (more
preferably benzyloxy),
-X- is bond, -CH2-, -CH2-CH2-, -0-CH2- or -S02-CH2-,
-Y~ is -C ~ (in which R11 is hydrogen or hydroxy),
R11
R6 Rg
-Z- is -(CH2)n-~-(CH2)m-~-(CH2)k- (in which
R~ R9
R6, R~, R8 and R9 are each independently hydrogen,
lower alkyl (more preferably methyl) or phenyl
which may have 1 to 3 lower alkoxy (more preferably
methoxy),
n, m and k is each independently 0 or 1),
R1 is hydrogen or phenyl(lower)alkyl (more preferebly
benzyl), and
R2, R3, R4 and R5 are each independently hydrogen; lower
alkyl (more preferably methyl); lower alkylthio (more
preferably methylthio); lower alkylsulfonyl (more
preferably methanesulfonyl); hydroxy; lower alkoxy (more
preferably methoxy or ethoxy); amino; lower alkylamino
(more preferably methylamino); lower alkoxycarbonylamino
(more preferably methoxycarbonylamino or
ethoxycarbonylamino); lower alkylsulfonylamino (more
preferably methanesulfonylamino); lower alkanoylamino
(more preferably formylamino, acetylamino or
propionylamino); ureido; trifluoroacetylamino; N-(lower
alkyl)-[(lower)alkoxycarbonyl]amino (more preferably N-
methyl-methoxycarbonylamino); carboxy; or lower
alkoxycarbonyl (more preferably methoxycarbonyl).
CA 02341458 2001-02-21
12
Suitable salts of the object arninoalcohol derivatives
[I] are pharmaceutically acceptable salts and include
conventional non-toxic salts such as an inorganic acid
addition salt [e. g. hydrochloride, hydrobromide, sulfate,
phosphate, etc.], an organic acid addition salt [e. g.
formate, acetate, trifluoroacetate, oxalate, maleate,
fumarate, tartrate, methanesulfonate, benzenesulfonate,
toluenesulfonate, etc.], an alkali metal salt [e. g. sodium
salt, potassium salt, etc.] or the like.
The processes for preparing the object compound [I] are
explained in detail in the following.
Process 1
The object compound [I] or a salt thereof can be
prepared by reacting a compound [II] with a compound [III] or
a salt thereof.
Suitable salt of the compound [III] may be the same as
those exemplified for the compound [I].
The reaction is preferably carried out in the presence
of a base such as an alkali metal carbonate [e. g. sodium
carbonate, potassium carbonate, etc.], an alkaline earth
metal carbonate [e. g. magnesium carbonate, calcium carbonate,
etc.], an alkali metal bicarbonate [e. g. sodium bicarbonate,
potassium bicarbonate, etc.], tri(lower)alkylamine [e. g.
trimethylamine, triethylamine, etc.], picoline or the like.
The reaction is usually carried out in a conventional
solvent, such as an alcohol [e. g. methanol, ethanol,
propanol, isopropanol, etc.], diethyl ether, tetrahydrofuran,
dioxane, or any other organic solvent which does not
adversely influence the reaction.
The reaction temperature is not critical, and the
reaction can be carried out under cooling to heating.
Process 2
CA 02341458 2001-02-21
13
The object compound [Ib] or a salt thereof can be
prepared by subjecting a compound [Ia] or a salt thereof to
elimination reaction of the amino protective group.
Suitable salts of the compounds [Ia] and [Ib] may be the
same as those exemplified for the compound [I].
This reaction is carried out in accordance with a
conventional method such as hydrolysis, reduction or the
like.
The hydrolysis is preferably carried out in the presence
of a base or an acid including Lewis acid.
Suitable base may include an inorganic base and an
organic base such as an alkali metal [e. g. sodium, potassium,
etc.], an alkaline earth metal [e. g. magnesium, calcium,
etc.], the hydroxide or carbonate or bicarbonate thereof,
hydrazine, trialkylamine [e. g. trimethylamine, triethylamine,
etc.], picoline, 1,5-diazabicyclo[4.3.0]non-5-ene,
1,4-diazabicyclo[2.2.2]octane, 1,8-diazabicyclo[5.9.0]undec-
7-ene, or the like.
Suitable acid may include an organic acid [e. g. formic
acid, acetic acid, propionic acid, trichloroacetic acid,
trifluoroacetic acid, etc.], an inorganic acid [e. g.
hydrochloric acid, hydrobromic acid, sulfuric acid, hydrogen
chloride, hydrogen bromide, hydrogen fluoride, etc.] and an
acid addition salt compound [e. g. pyridine hydrochloride,
etc. ] .
The elimination using trihaloacetic acid [e. g.
trichloroacetic acid, trifluoroacetic acid, etc.] or the like
is preferably carried out in the presence of cation trapping
agents [e.g. anisole, phenol, etc.].
The reaction is usually carried out in a solvent such as
water, an alcohol [e. g. methanol, ethanol, etc.], methylene
chloride, chloroform, tetrachloromethane, tetrahydrofuran, a
mixture thereof or any other solvent which does not adversely
influence the :reaction. A liquid base or acid can be also
used as the solvent. The reaction temperature is not
CA 02341458 2001-02-21
14
critical and the reaction is usually carried out under
cooling to heating.
The reduction method applicable for the elimination
reaction may include chemical reduction and catalytic
reduction.
Suitable reducing agents to be used in chemical
reduction are a combination of metal (e. g. tin, zinc, iron,
etc.] or metallic compound [e. g. chromium chloride, chromium
acetate, etc.] and an organic or inorganic acid [e. g. formic
acid, acetic acid, propionic acid, trifluoroacetic acid,
p-toluenesulfonic acid, hydrochloric acid, hydrobromic acid,
etc.].
Suitable catalysts to be used in catalytic reduction are
conventional ones such as platinum catalysts [e. g. platinum
plate, spongy platinum, platinum black, colloidal platinum,
platinum oxide, platinum wire, etc.], palladium catalysts
[e. g. spongy palladium, palladium black, palladium oxide,
palladium on carbon, colloidal palladium, palladium on barium
sulfate, palladium on barium carbonate, etc.], nickel
catalysts [e. g. reduced nickel, nickel oxide, Raney nickel,
etc.], cobalt catalysts [e. g. reduced cobalt, Raney cobalt,
etc.], iron catalysts [e.g. reduced iron, Raney iron, etc.],
copper catalysts [e. g. reduced copper, Raney copper, Ullman
copper, etc.] and the like.
In case that the amino protective group is benzyl, the
reduction is preferably carried out in the presence of a
combination of palladium catalysts [e. g. palladium black,
palladium on carbon, etc.] and formic acid or its salt [e. g.
ammonium formate, etc.].
The reduction is usually carried out in a conventional
solvent which does not adversely influence the reaction such
as water, an alcohol [e. g. methanol, ethanol, propanol,
etc.], chlorobenzene, N,N-dimethylformamide, or a mixture
thereof. Additionally, in case that the above-mentioned
acids to be used in chemical reduction are in liquid, they
CA 02341458 2001-02-21
can also be used as a solvent. Further, a suitable solvent
to be used in catalytic reduction may be the above-mentioned
solvent, and other conventional solvent such as diethyl
ether, dioxane, tetrahydrofuran, etc. or a mixture thereof.
5 The reaction temperature of this reduction is not
critical and the reaction is usually carried out under
cooling to heating.
The compounds obtained by the above processes can be
10 isolated and purified by a conventional method such as
pulverization, recrystallization, column chromatography,
reprecipitation, or the like, and converted to the desired
salt in conventional manners, if necessary.
It is to be noted that the compound [I] and the other
15 compounds may include one or more stereoisomers due to
asymmetric carbon atoms, and all of such isomers and mixture
thereof are included within the scope of this invention.
It is further to be noted that isomerization or
rearrangement of the object compound [I] may occur due to the
effect of the light acid, base or the like, and the compound
obtained as the result of said isomerization or rearrangement
is also included within the scope of the present invention.
It is also to be noted that the solvating form of the
compound [I] (e. g. hydrate, etc.) and any form of the crystal
of the compound [I] are included within the scope of the
present invention.
The object compound [I] or a salt thereof possesses gut
selective sympathomimetic, anti-ulcerous, anti-pancreatitis,
lipolytic and anti-pollakiuria activities, and are useful for
the treatment and/or prevention of gastro-intestinal
disorders caused by smooth muscle contractions in human
beings or animals, and more particularly to methods for the
treatment and/or prevention of spasm or hyperanakinesia in
case of irritable bowel syndrome, gastritis, gastric ulcer,
CA 02341458 2001-02-21
16
duodenal ulcer, enteritis, cholecystopathy, cholangitis,
urinary calculus and the like; for the treatment and/or
prevention of ulcer such as gastric ulcer, duodenal ulcer,
peptic ulcer, ulcer causes by non steroidal anti-inflammatory
drugs, or the like; for the treatment and/or prevention of
dysuria such as pollakiuria, urinary incontinence or the like
in case of nervous pollakiuria, neurogenic bladder
dysfunction, nocturia, unstable bladder, cystospasm, chronic
cystitis, chronic prostatitis, prostatic hypertrophy or the
like; and for the treatment and/or prevention of
pancreatitis, obesity, diabetes, glycosuria, hyperlipidemia,
hypertension, atherosclerosis, glaucoma, melancholia,
depression or the like; and for the treatment and/or
prevention of diseases as the result of insulin resistance
(e. g. hypertension, hyperinsulinemia, etc.), and the like.
In order to show the usefulness of the compound [I] for
the prophylactic anc therapeutic treatment of above-mentioned
disease in human beings or animals, the pharmacological test
data of a representative compound thereof are shown in the
following.
Test
Effect on the increase in intravesical pressure induced
by carbachol in anesthetized dog
Test Compouund
(1) (2S)-1-[[(2RS)-4,9-bis(4-hydroxyphenyl)-2-butyl]amino]-
3-phenoxy-2-propanol hydrochloride
Test Method
Female Beagle dogs weighing 8.0-15.0 kg were fasted for
24 hours and maintained under halothane anesthesia. A 12F
Foley catheter was lubricated with water soluble jelly,
inserted into the urethral orifice and advanced approximately
CA 02341458 2001-02-21
17
cm until the balloon tip was placed well inside the
bladder. The balloon was then inflated with 5 ml of room air
and catheter slowly withdrawn just part the first resistance
that is felt at the bladder neck. Urine was completely
5 drained out through the catheter, and 30 ml of biological
saline was infused. The catheter was connected to pressure
transducer, and intravesical pressure was continuously
recorded. The test compound was injected intravenously at 5
minutes before the administration of carbachol (1.8 ~g/kg).
Test Results
Treatment Increase in intravesical pressure (mmHg)
Control 4.5 ~ 0.3
Test Compound (1) **
2.5 ~ 0.3
(0.01 mg/kg)
**P<0.01 vs Control (ANOVA) (N=3)
The following Preparations and Examples are given for
the purpose of illustrating this invention.
Example 1
A solution of (2S)-3-phenoxy-1,2-epoxypropane (195 mg)
(IL FARMACO, 50 (10), 643 (1995)) and 4,4-bis(4-
hydroxyphenyl)-2-butylamine (257 mg) in ethanol (2 ml) was
stirred under reflux for 24 hours and evaporated in vacuo.
The residue was chromatographed (chloroform-methanol) over
silica gel (9 g) and the eluate was treated with 4N hydrogen
chloride in ethyl acetate to afford a crude oil, which was
powdered from diethyl ether to afford (2S)-1-[[(2RS)-4,4-
bis(4-hydroxyphenyl)-2-butyl]amino]-3-phenoxy-2-propanol
hydrochloride (117 mg) as a pale brown powder.
mp . 73°C (dec.)
IR (Nujol) . 3600-3100, 2700-2400, 1230 cm-1
NMR (DMSO-d6, d) . 1.21-1.27 (3H, m, CH3), 1.94 (1H, m,
CA 02341458 2001-02-21
18
CH2), 2.60 (1H, m, CH2), 2.85-3.2 (3H, m, CH2NCH),
3.95 (1H, m, CHAr2), 4.01-4.05 (2H, m, ArOCH2),
4.16 (1H, m, CH_OH), 5.85 (1H, br s, OH), 6.64-6.72
(4H, m, aromatic H), 6.92-7.16 (7H, m, aromatic H),
7.26-7.35 (2H, m, aromatic H), 8.62 (1H, br, NH),
8.92 (1H, br, HC1), 9.23 (1H, br s, OH), 9.28 (1H,
br s, OH)
MS m/z . 408 (M++1)
Example 2
(2R)-N-Benzyl-4,4-bis(4-methoxyphenyl)-2-butylamine
hydrochloride (412 mg) was converted to the corresponding
free base in a usual manner. 1.OM Solution of tin(IV)
chloride in dichlo.romethane (1.5 ml) was added dropwise to a
stirred solution of the free base and (2S)-3-phenoxy-1,2-
epoxypropane (225 mg) (IL FARMACO, 50 (10), 643 (1995)) in
dichloromethane (4 ml) at -10 - -5°C under a nitrogen
atmosphere over 10 minutes and the resulting mixture was
stirred for 1.5 hours at the same temperature. The reaction
mixture was poured into 1N hydrochloric acid and the mixture
was stirred under ice cooling for 20 minutes. The organic
layer was separated, washed with an aqueous solution of
sodium fluoride and a saturated aqueous solution of sodium
bicarbonate, dried over sodium sulfate, and evaporated in
vacuo. The residue was chromatographed (ethyl acetate) over
silica gel (11 g) and the eluate was treated with 4N hydrogen
chloride in ethyl acetate to afford (2S)-1-[N-benzyl-[(2R)-
4,4-bis(4-methoxyphenyl)-2-butyl)amino]-3-phenoxy-2-propanol
hydrochloride (136 mg) as an oil.
IR (Film) . 3292, 2850-2400, 1243 cm-1
NMR (CDC13, ~) . 1.27 and 1.47 (3H, each d, J=6.2 and
6.6Hz), 2.08 (1H, m), 2.9-3.5 (4H, m), 3.68-4.0
(2H, m), 3.74, 3.75 and 3.76 (6H, each s), 4.02-
4.08 (2H, m), 4.10-4.26 (1H, m), 4.3-4.6 (1H, m),
6.72-7.43 and 7.64-7.68 (18H, m)
CA 02341458 2001-02-21
19
MS m/z . 526 (M++1)
_Example 3
(2S)-N-Benzyl-9,4-bis(4-methoxyphenyl)-2-butylamine
hydrochloride (412 mg) was converted to the corresponding
free base in a usual manner. A solution of the free base and
(2S)-3-phenoxy-1,2-epoxypropane (195 mg) (IL FARMACO, 50
(10), 643 (1995)) in ethanol (4 ml) was stirred under reflux
for 10 hours, cooled to room temperature, and evaporated in
vacuo. The residue was chromatographed (chloroform) over
silica gel and the eluate was treated with 4N hydrogen
chloride in ethyl acetate to afford (2S)-1-[N-benzyl-[(2S)-
4,4-bis(4-methoxyphenyl)-2-butyl]amino]-3-phenoxy-2-propanol
hydrochloride (549 mg) as an amorphous powder.
[a]D4 . -22.59° (c=0.54, MeOH)
IR (KBr) . 3300 (br), 2850-2400, 1248 cm-1
NMR (CDC13, d) . 1.41 and 1.56 (3H, each d, J=6.6Hz),
1.64 and 2.05 (1H, m), 2.94-3.6 (4H, m), 3.74,
3.75, 3.76 and 3.77 (6H, each s), 3.87-3.96 (2H,
m), 4.05-4.25 (3H, m), 4.5-4.65 and 4.8 (1H, m),
5.9 (1H, br), 6.69-6.98 (8H, m), 7.08-7.17 (4H, m),
7.22-7.41 (4H, m), 7.65-7.73 (2H, m)
MS m/z . 526 (M++1)
Example 4
A mixture of (2S)-1-[N-benzyl-[(2R)-4,4-bis(4-
methoxyphenyl)-2-butyl]amino]-3-phenoxy-2-propanol
hydrochloride (96 mg) and 10% Pd/C (50o wet, 60 mg) in
methanol (4 ml} was stirred at room temperature in the
presence of hydrogen at an atmospheric pressure for 6 hours
and filtered. The filtrate was evaporated in vacuo and the
residue was partitioned between dichloromethane and an
aqueous solution of sodium bicarbonate. The organic layer
was separated, dried over sodium sulfate, and evaporated in
vacuo. The residue was chromatographed (dichloromethane-
CA 02341458 2001-02-21
methanol) over silica gel (2 g) to afford an oil, which was
converted to the corresponding oxalate in a usual manner to
afford (2S)-1-[[(2R)-4,4-bis(4-methoxyphenyl)-2-butyl]amino]-
3-phenoxy-2-propanol oxalate (1:1) (26 mg) as a colorless
5 powder.
mp . 121-123°C (from diethyl ether)
IR (KBr) . 3423 (br), 2850-2650, 1734, 1701, 1633,
1603, 1250 cm-1
NMR (DMSO-d6, d) . 1.23 (3H, d, J=6.3Hz), 1.99 (1H, m),
10 2.58 (1H, m), 2.85-3.01 (2H, m), 3.11-3.17 (1H, m),
3.69 (3H, s), 3.71 (3H, s), 3.9-4.6 (8H, m), 6.81-
6. 99 ( 7H, m) , 7 . 16-7 . 35 ( 6H, m)
MS m/z . 436 (M++1)
15 Example 5
The following compound was obtained according to a
similar manner to that of Example 4.
(2S)-1-[[(2S)-4,4-Bis(4-methoxyphenyl)-2-butyl]amino]-3-
20 phenoxy-2-propanol oxalate (l: l)
[a]D4 . 11.06° (c=0.515, MeOH)
mp . 79-94°C (from diethyl ether)
IR (KBr) . 3423 (br), 2750-2400, 1739-1691 (m), 1643,
1608, 1247 cm-1
NMR (DMSO-d6, d) . 1.25 (3H, d, J=6.3Hz), 1.96 (1H, m),
2.66 (1H, m), 2.86 (1H, m) 2.95-3.15 (2H, m), 3.69
(3H, s) , 3.70 (3H, s) , 3.94-4.15 (4H, m) , 5. 1 (4H,
br), 6.81-6.88 (4H, m), 6.92-6.99 (3H, m), 7.17-
7 . 35 ( 6H, m)
MS m/z . 436 (M++1)
Preparation 1
A mixture of methyl 3-aminobutyrate (4.3 g), (2S)-3-
phenoxy-1,2-epoxypropane (4.59 g), ytterbium(III)
trifluoromethanesulfonate (1.8 g) and dichloromethane (25 ml)
CA 02341458 2001-02-21
21
was stirred at 40°C for 2. hours and at room temperature
overnight, worked up in the usual manner and purified by
silica gel column chromatography (toluene: ethanol:
concentrated ammonia water = 9:1:0.1) to give (3RS)-3-[((2S)-
2-hydroxy-3-phenoxypropyl)amino]butyric acid methyl ester
(2.59 g) .
IR (Neat): 3400 (br m), 1739 (s), 1599 (m), 1495 (m),
1458 (m), 1298 (m), 1246 (s), 1041 (m),
756 (m) cm-1
NMR (CDC13, d): 1.16 (3H, d, J=5.2Hz), 2.41-2.46 (2H,
m), 2.6-3.0 (2H, m), 3.14 (1H, quartet, J=6.4Hz),
3.68 (3H, s), 3.9-4.1 (3H, m), 6.90-6.99 (3H, m),
7.24-7.33 (2H, m)
MS m/z: 268 (M++1)
_P_reparation 2
A mixture of (2S)-N-benzyl-(2-hydroxy-3-
phenoxypropyl)amine (142 mg), 5-bromopentanoic acid ethyl
ester (173 mg), potassium carbonate (153 mg) and N,N-
dimethylformamide (2 ml) was stirred at 80°C for 4.5 hours,
worked up in the usual manner and purified by silica gel
column chromatography (20o ethyl acetate-hexane) to give
(2S)-5-[N-benzyl-(2-hydroxy-3-phenoxypropyl)amino]pentanoic
acid ethyl ester (93 mg).
NMR (CDC13, b): 1.25 (3H, t, J=7.lHz), 1.5-1.7
(5H, broad), 2.2-2.4 (2H, m), 2.4-2.8 (4H, m), 3.5-
3.7 (1H, m), 3.7-3.9 (1H, m), 3.94 (2H, t,
J=3.9Hz), 4.0-4.2 (1H, m), 4.12 (2H, quartet,
J=7.lHz), 6.86-6.98 (4H, m), '7.2-7.4 (6H, m)
MS m/z: 386 (M++1)
_Pre aration 3
The following compound was obtained according to a
similar manner to that of Preparation 2.
CA 02341458 2001-02-21
22
(2S)-4-[N-Benzyl-(2-hydroxy-3-phenoxypropyl)amino]-
butanoic acid methyl ester
IR (Neat) : 3482 (br m) , 2949 (m) , 1736 (s) , 1599 (w) ,
1495 (m), 1456 (m), 1246 (s), 1041 (m), 754 (m),
696 (m) cm-1
NMR (CDC13, b): 1.88 (2H, quintet, J=7.lHz), 2.32 (2H,
t, J=7.2Hz), 2.5-2.8 (4H, m), 3.5-3.7 (1H, m), 3.64
(3H, s) , 3. 84 (1H, d, J=13.OHz) , 3.89-4.0 (2H, m) ,
4.0-4.2 (1H, m), 6.86-6.99 (4H, m), 7.2-7.4 (6H, m)
MS m/z: 358 (M++1)
Preparation 4
The following compounds were obtained according to a
similar manner to that of Example 6.
(1) 1-[2,2-Bis(4-methoxyphenyl)-2-hydroxyethyl]cyclopentanol
IR (KBr): 3347 (s), 3240 (m), 2958 (s), 1608 (m), 1510
(s), 1466 (m), 1248 (s), 1174 (m), 1034 (s), 835
(m) cm-1
NMR (CDC13, b): 1.2-1.7 (8H, m), 2.39 (1H, br s), 2.70
(2H, s) , 3.78 (6H, s) , 4.87 (1H, br s) , 6. 82 (4H,
d, J=8.9Hz), 7.37 (4H, d, J=8.9Hz)
(2) 3-(Dibenzylamino)-1,1-bis(4-bromophenyl)-1-propanol
NMR (CDC13, ~): 2.3-2.4 (2H, m), 2.6-2.7 (2H, m), 3.51
(4H, s), 7.07 (2H, d, J=8.5Hz), 7.1-7.4 (16H, m)
MS m/z: 564, 566 (M++1), 568
(3) (3RS)-3-(Dibenzylamino)-1,1-bis(9-bromophenyl)-1-butanol
NMR (CDC13, b): 1.10 (3H, d, J=6.7Hz), 2.09 (1H, d,
J=14.8Hz), 2.45 (1H, dd, J=11.2 and 14.8Hz), 3.1-
3.3 (1H, m), 3.18 (2H, d, J=12.8Hz), 3.91 (2H, d,
J=12.7Hz), 6.82 (2H, d, J=8.7Hz), 7.11 (2H, d,
J=8.7Hz), 7.2-7.3 (12H, m), 7.40 (2H, d, J=9.9Hz)
CA 02341458 2001-02-21
23
(4) (2S)-1-Phenoxy-3-[N-benzyl-[(3RS)-1,1-bis(4-
bromophenyl)-1-hydroxy-3-butyl]amino]-2-propanol
MS m/z: 638, 690 (M++1), 642
(5) N-Benzyl-[4,4-bis(4-methoxyphenyl)butyl]amine
NMR (CDC13, b): 1.4-1.7 (2H, m), 2.00 (2H, quartet,
J=7.8Hz), 2.64 (2H, t, J=7.lHz), 3.76 (6H, s),
3.72-3.79 (3H, m), 6.80 (4H, d, J=8.7Hz), 7.11 (4H,
d, J=8.7Hz), 7.28 (5H, s)
MS m/z: 376 (M++1)
(6) N-Benzyl-[3,3-bis(4-ethoxyphenyl)propyl]amine
MS m/z: 390 (M++1)
Preparation 5
1-[2,2-Bis(4-methoxyphenyl)-2-hydroxyethyl]cyclopentanol
(0.79 g) was hydrogenated in the usual manner to give 1-[2,2-
bis(4-methoxyphenyl)ethyl]cyclopentanol (0.76 g).
IR (Neat): 3563 (m), 3448 (m), 2956 (s), 1610 (w), 1510
(s), 1460 (m), 1246 (s), 1178 (m), 1036 (s), 829
(m) cm-1
NMR (CDC13, g): 1.5-1.9 (9H, m), 2.40 (2H, d, J=7.3Hz),
3 . 77 ( 6H, s ) , 4 . 17 ( 1H, t, J=7 . 2Hz ) , 6 . 81 ( 4H, d,
J=6.6Hz), 7.21 (4H, d, J=6.6Hz)
Preparation 6
To a mixture of 1-[2,2-bis(4-methoxyphenyl)ethyl]-
cyclopentanol (0.76 g), azidotrimethylsilane (0.32 g) and
benzene (5 ml), boron trifluoride diethyl etherate (0.34 ml)
was added dropwise at 0°C. The reaction mixture was stirred
at room temperature for 30 minutes and worked up in the usual
manner. The crude product was hydrogenated in the usual
manner to give [1-[2,2-bis(4-methoxyphenyl)ethyl]-
cyclopentyl]amine (0.74 g).
IR (Neat): 2949 (s), 1610 (m), 1510 (s), 1460 (m), 1248
CA 02341458 2001-02-21
24
(s), 1178 (m), 1038 (s), 829 (s) cm-1
NMR (CDC13, ~): 1.3-1.8 (lOH, m), 1.20 (2H, t, J=6.8Hz),
3.77 (6H, s), 4.09 (1H, quartet, J=6.8Hz), 6.81
(4H, dd, J=2.2 and 6.6Hz), 7.14 (4H, d, J=6.5Hz)
MS m/z: 326 (M++1)
Preparation 7
To a solution of dibenzylamine (1.14 g) and
tetrahydrofuran (4 ml), butyllithium (1.54M in hexane, 3.7
ml) was added dropwise at -78°C under a flow of nitrogen.
After 30 minutes, a solution of 3-(3,4-dimethoxyphenyl)-
acrylic acid methyl ester (1.06 g) in tetrahydrofuran (3 ml)
was added dropwise, stirred for 1 hour and worked up in the
usual manner. The crude product was purified by silica gel
column chromatography (hexane: ethyl acetate = 5:1) to give
3-(dibenzylamino)-3-(3,4-dimethoxyphenyl)propionic acid
methyl ester (0.53 g).
IR (Neat): 1739 (s), 1514 (s), 1458 (m), 1261 (m), 1146
(m) , 1028 (m) , 744 (m) cm-1
NMR (CDC13, b): 2.72 (1H, dd, J=7.3 and 14.4Hz), 3.07
(1H, dd, J=8.6 and 14.4Hz), 3.21 (1H, d, J=13.7Hz),
3.65 (3H, s), 3.73 (2H, s), 3.79 (2H, s), 3.89 (6H,
s), 4.25 (1H, t, J=7.4Hz), 6.75-6.90 (3H, m), 7.1-
7.4 (lOH, m)
Preparation 8
A mixture of 3-(dibenzylamino)-3-(3,4-dimethoxyphenyl)-
propionic acid methyl ester (0.53 g), acetic acid (3 ml),
methanol (3 ml), and 20% palladium hydroxide on charcoal
(0.05 g) was stirred under hydrogen (1 atm) at room
temperature for 6 hours, filtered and evaporated to give 3-
amino-3-(3,4-dimethoxyphenyl)propionic acid methyl ester
acetate (0.24 g).
IR (KBr): 1729 (s), 1539 (s), 1523 (s), 1398 (m), 1265
(m), 1203 (m), 1155 (m), 1020 (m) cm-1
CA 02341458 2001-02-21
NMR (MeOH-d4, b): 1.90 (3H, s), 2.92 (2H, dd, J=5.4 and
6. 6Hz) , 3. 63 (3H, s) , 3. 82 (3H, s) , 3.84 (3H, s) ,
4.52 (1H, t, J=7.5Hz) , 6. 95 (2H, s) , 7.02 (1H, s)
5 Preparation 9
To a solution of 3-aminopropionic acid methyl ester
hydrochloride (1.12 g) in methanol (10 ml), 28% sodium
methoxide-methanol solution (1.60 g) was added, filtered and
evaporated. To the crude product, (2S)-2-phenoxy-1,2-
10 epoxypropane (901 mg) and methanol (10 ml) were added and
stirred under reflux for 2.5 hours. The reaction mixture was
evaporated and purified by silica gel column chromatography
to give 3-[((2S)-2-hydroxy-3-phenoxypropyl)amino]propionic
acid methyl ester (0.76 g).
15 IR (KBr): 1734 (s), 1601 (m), 1498 (m), 1435 (m),
1252 (s), 1196 (m), 1043 (m), 752 (m) cm-1
NMR (CDC13, b): 2.54 (2H, t, J=6.4Hz), 2.72-2.98 (4H,
m), 3.69 (3H, s), 3.97-4.07 (3H, m), 6.90-6.99 (3H,
m), 7.25-7.32 (2H, m)
20 MS m/z: 254 (M++1)
Preparation 10
The following compound was obtained according to a
similar manner to that of Preparation 9.
(3RS)-3-[((2S)-2-Hydroxy-3-phenoxypropyl)amino]-3-
phenylpropionic acid methyl ester
IR (KBr): 1724 (s), 1599 (m), 1495 (m), 1435 (m),
1246 (s), 1126 (m), 1038 (m), 756 (m), 698 (m).cm-1
NMR (CDC13, b): 2.54-2.75 (4H, m), 3.66 (1.5H, s), 3.67
(1.5H, s), 3.9-4.0 (2H, m), 4.0-4.2 (2H, m), 6.85-
6.98 (3H, m), 7.2-7.4 (7H, m)
MS m/z: 330 (M++1)
Preparation 11
CA 02341458 2001-02-21
26
To a mixture of N-carbobenzyloxy-D-alanine (0.81 g),
[bis(4-methoxyphenyl)methyl]amine (0.80 g),
1-hydroxybenzotriazole (0.58 g) and N,N-dimethylformamide (5
ml), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
hydrochloride (0.76 g) was added at 0°C and stirred at room
temperature for 30 minutes. After a usual workup,
N-carbobenzyloxy-D-alanine [bis(4-methoxyphenyl)methyl]amide
(1.38 g) was obtained.
IR (KBr): 3296 (s), 1689 (m), 1647 (s), 1539 (s), 1512
(s), 1257 (s), 1176 (rn), 1031 (m) cm-1
NMR ( DMSO-d6, ~ ) : 1 . 21 ( 3H, d, J=7 . 1Hz ) , 3 . 33 ( 6H, s ) ,
4.17 (1H, t, J=7.2Hz), 5.01 (2H, s), 5.96 (1H, d,
J=8.4Hz), 6.86 (4H, d, J=8.7Hz), 7.1-7.2 (4H, m),
7.3-7.5 (5H, m), 8.60 (1H, d, J=8.5Hz)
Preparation 12
To a solution of N-carbobenzyloxy-D-alanine [bis(4-
methoxyphenyl)methyl]amide (0.33 g) in methanol and
tetrahydrofuran (1:2, 10 ml), 50o wet 10°s palladium on
charcoal was added and stirred under hydrogen atmosphere (1
atm) for 30 minutes. After a usual workup, D-alanine [bis(4-
methoxyphenyl)methyl]amide (0.25 g) was obtained.
IR (Neat): 3357 (br s), 1678 (s), 1649 (s), 1538 (s),
1513 (s), 1259 (m), 1176 (m), 1032 (s), 831 (m),
812 (m) cm-1
NMR ( DMSO-d6, b ) : 1 . 13 ( 3H, d, J=6 . 9Hz ) , 3 . 33 ( 6H, s ) ,
3.3-3.4 (3H, br) , 5.96 (1H, d, J=8.2Hz) , 6.87 (4H,
d, J=8.7Hz), 7.15 (4H, d, J=8.4Hz), 8.44 (1H, d,
J=8Hz)
Preparation 13
To a solution of 4-methoxyphenylmagnesium bromide (1M in
tetrahydrofuran, 35 ml) a solution of 3-(dibenzylamino)-
propionic acid ethyl ester (4.87 g) in terahydrofuran (2 ml)
was added, stirred under reflux for 1 hour, worked up in the
CA 02341458 2001-02-21
27
usual manner and purified by silica gel column chromatography
(hexane: ethyl acetate = 5:1) to give 3-dibenzylamino-1,1-
bis(4-methoxyphenyl)-1-propanol (3.45 g).
Preparation 14
3-(Dibenzylamino)-1,1-bis(4-methoxyphenyl)-1-propanol
(2.0 g) was hydrogenated by the usual manner to give
N-benzyl-[3,3-bis(9-methoxyphenyl)propyl]amine, which was
further hydrogenated by heating with 20o palladium on
charcoal and ammonium formate in methanol to give [3,3-bis(4-
methoxyphenyl)propyl]amine (165 g).
Preparation 15
To a solution of 4-benzyloxy-3-nitrophenyl acetate (4.20
g) in methanol (20 ml), 28o sodium methoxide-methanol
solution (2.96 g) was added and evaporated. To the crude
residue, N,N-dimethylformamide (20 ml) and (2S)-3-[(3-
nitrophenyl)sulfonyloxy]-1,2-epoxypropane (3.80 g) were
added. The mixture was stirred at room temperature
overnight, and worked up in the usual manner to give (2S)-3-
(4-benzyloxy-3-nitrophenoxy)-1,2-epoxypropane (4.30 g).
NMR (CDC13, ~): 2.72-2.77 (1H, m), 2.92 (1H, quintet,
J=4.8Hz), 3.32-3.37 (1H, m), 3.91 (1H, quartet,
J=5.9Hz), 4.27 (1H, dd, J=2.8 and 11.4Hz), 5.18
(2H, s), 7.07-7.15 (2H, m), 7.34-7.46 (6H, m)
Preparation 16
A mixture of 3-(dibenzylamino)-1,1-bis(4-bromophenyl)-1-
propanol (8.42 g), benzophenone imine (10.8 g),
tris(dibenzylideneacetone)dipalladium (546 mg), (RS)-2,2'-
bis(diphenylphosphino)-1,1'-binaphthyl (l.ll g), sodium
tert-butoxide (5.7 g) and toluene (90 ml) was stirred at
100°C for 6 hours. The reaction mixture was added to
tetrahydrofuran (300 ml), and 3N hydrochloric acid (300 ml)
and stirred at room temperature for 1.5 hours. The aqueous
CA 02341458 2001-02-21
28
phase was separated, neutralized by sodium hydroxide and
extracted with ethyl acetate. The ethyl acetate solution was
evaporated and purified by silica gel column chromatography
(hexane: ethyl acetate =1:1) to give 3-(dibenzylamino)-1,1-
bis(4-aminophenyl)-1-propanol (1.76 g).
MS m/z: 438 (M++1)
Preparation 17
The following compound was obtained according to a
similar manner to that of Preparation 16.
(3RS)-3-(Dibenzylamino)-l,1-bis(4-aminophenyl)-1-butanol
IR (Neat): 3356 (m), 3219 (m), 2964 (m),.1622 (s), 1512
(s), 1454 (m), 1383 (w), 1271 (m), 1246 (m), 1176
(m), 1142 (m), 831 (m) cm-1
NMR (CDC13, b): 1.03 (3H, d, J=6.7Hz), 2.02 (1H, d,
J=11.7Hz), 2.50 (1H, dd, J=11.2 and 14.7Hz), 3.10-
3.2 (1H, m), 3.21 (2H, d, J=13.OHz), 3.92 (2H, d,
J=12.9Hz), 6.35 (2H, d, J=6.5Hz), 6.55 (2H, d,
J=6.6Hz), 6.76 (2H, d, J=6.6Hz), 7.13 (2H, d,
J=6.5Hz), 7.24 (lOH, s)
MS m/z: 452 (M++1)
Preparation 18
To a mixture of 3-(dibenzylamino)-1,1-bis(4-
aminophenyl)-1-propanol (0.64 g), pyridine (0.5 ml) and
dichloromethane (10 ml), methyl chlorocarbonate (0.34 ml) was
added at 0°C and the reaction mixture was worked up in a
usual manner. The crude product was dissolved in methanol
(10 ml), followed by addition of 4N hydrogen chloride in 1,4-
dioxane (0.5 m:L) and 20o palladium hydroxide on charcoal.
The mixture was stirred under hydrogen (1 atm) at room
temperature overnight, worked up in a usual manner and
purified by silica gel column chromatography
(dichloromethane:methanol:concentrated ammonia water =
CA 02341458 2001-02-21
29
20:1:0.1) to give N-benzyl-[3,3-bis[4-[(methoxycarbonyl)-
amino]phenyl]propyl]amine (466 mg).
MS m/z: 448 (M++1)
Preparation 19
A mixture of (3RS)-3-aminobutyric acid methyl ester
hydrochloride (5.0 g), benzyl bromide (11.7 g), potassium
carbonate (18 g) and N,N-dimethylformamide (40 ml) was
stirred at room temperature overnight, worked up in the usual
manner and purified by silica gel column chromatography
(hexane:ethyl acetate = 15:1) to give (3RS)-3-
(dibenzylamino)butyric acid methyl ester (7.27 g).
IR (Neat): 2968 (m), 1741 (s), 1454 (m) 1259 (m),
1196 (m), 1146 (m), 1072 (m), 1022 (m), 744 (m),
698 (m) cm-1
NMR (CDC13, ~): 1.10 (3H, d, J=6.7Hz), 2.28 (1H, dd,
J=6.9 and 13.9Hz), 2.64 (1H, dd, J=8.01 and
13.9Hz), 3.30 (1H, dd, J=6.8 and 14.7Hz), 3.49 (2H,
d, J=13.7Hz), 3.60 (3H, s), 3.66 (2H, d, J=13.7Hz),
7.18-7.35 (10H, m)
MS m/z: 298 (M++1)
Preparation 20
The following compound was obtained according to a
similar manner to that of Preparation 19.
(3RS)-3-[N-Benzyl-((2S)-2-hydroxy-3-phenoxypropyl)-
amino]butyric acid methyl ester
MS m/z: 358 (M++1)
Preparation 21
To a mixture of (3RS)-3-(dibenzylamino)-l,l-bis(4-
aminophenyl)-1-butanol (360 mg), triethylamine (0.44 ml) and
dichloromethane (4 ml), methanesulfonyl chloride (0.20 ml)
was added dropwise at 0°C. The reaction mixture was stirred
CA 02341458 2001-02-21
at room temperature for 30 minutes and worked up in the usual
manner. The crude product was dissolved in methanol followed
by addition of loo palladium on charcoal (50o wet) and
ammonium formate, heated under reflux for 1.5 hours,
5 filtrated and extracted by water. The aqueous solution was
treated with benzyl chlorocarbonate (136 ~1) in the usual
Shotten method, worked up in the usual manner and purified by
silica gel column chromatography (hexane: ethyl acetate = 2:3)
to give (3RS)-[3,3-bis[4-(methanesulfonylamino)phenyl]-1-
10 methylpropyl]carbamic acid benzyl ester (138 mg).
IR (Neat): 3259(m), 1680 (s), 1512 (s), 1331 (m),
1153 (s) , 974 (m) cm-1
NMR (CDC13, d): 1.13 (3H, d, J=6.8Hz), 2.4-2.6 (2H, m),
2.98 (6H, s), 3.7-3.9 (1H, m), 4.6-4.8 (1H, m),
15 6.52 (2H, s), 7.07-7.15 (5H, m), 7.2-7.5 (8H, m)
Preparation 22
(3RS)-1,1-Bis[4-(methanesulfonylamino)phenyl]-3-
butylamine (77.4 mg) was obtained from (3RS)-[3,3-bis[4-
20 (methanesulfonylamino)phenyl]-:L-methylpropyl]carbamic acid
benzyl ester (1.01 mg) by hydrogenation in the usual manner.
IR (KBr): 3425 (br s), 1635 (m), 1506 (m), 1325 (m),
1151 (s), 1103 (m), 980 (w) cm-1
NMR (MeOH-d4, d): 1.13 (3H, d, J=6.4Hz), 2.05 (2H,
25 quartet, J=7.7Hz), 2.73 (1H, quartet, J=6.5Hz),
2.89 (6H, s), 4.06 (1H, dd, J=7.8Hz and 14.8Hz),
7.16 (4H, d, J=8.5Hz), 7.26 (9H, d, J=8.5Hz)
MS m/z: 412 (M++1)
30 Preparation 23
To a mixture of (3RS)-3-(dibenzylamino)-l,l-bis(4-
aminophenyl)-1--butanol (0.40 g), triethylamine (0.37 ml) and
dichloromethane (4 ml), acetic anhydride (0.18 ml) was added
at 0°C, followed by addition of additional triethylamine (0.1
ml) and acetic anhydride (0.09 ml). The reaction mixture was
CA 02341458 2001-02-21
31
worked up in the usual manner to give (3RS)-3-
(dibenzylamino)-1,1-bis[4-(acetylamino)phenyl]-1-butanol
(0.49 g) .
IR (Neat): 2300 (m), 1666 (s), 1623 (m), 1539 (s), 1514
(m) , 1319 (m) , 1265 (m) , 1142 (w) , 837 (m) cm-1
NMR (CDC13, b): 1.06 (3H, d, J=6.6Hz), 2.12 (3H, s),
2.16 (3H, s), 2.0-2.2 (1H, m), 2.45-2.58 (1H, m),
3.0-3.2 (1H, m), 3.20 (2H, d, J=12.9Hz), 3.92 (2H,
d, J=13.OHz), 6.72 (2H, d, J=6.8Hz), 7.09-7.36
(16H, m)
MS m/z: 536 (M++1)
Preparation 24
(3RS)-3-Amino-1,1-bis[4-(acetylamino)phenyl]-1-butanol
(0.23 g) was obtained from (3RS)-3-(dibenzylamino)-1,1-bis[4-
(acetylamino)phenyl]-1-butanol (0.32 g) by the usual
hydrogenation.
IR (KBr): 3294 (m), 1666 (s), 1623 (s), 1537 (s), 1514
(m), 1406 (m), 1321 (n), 1014 (m) cm-1
NMR (MeOH-d6, b): 1.10-1.15 (3H, m), 2.07 (3H, s), 2.11
(3H, s), 2.5-3.1 (3H, m), 4.23 (2H, d, J=10.6Hz),
4.55 (2H, d, J=10.6Hz), 7.21-7.58 (8H, m)
Preparation 25
The following compound was obtained according to a
similar manner to that of Example 33.
(3RS)-3-(Dibenzylamino)-1,1-bis[4-(methoxycarbonyl-
amino)phenyl]-1-butanol
MS m/z: 568
Preparation 26
(3RS)-1,1-Bis[4-(methoxycarbonylamino)phenyl]-3-
butylamine hydrochloride (191 mg) was obtained from (3RS)-3-
(dibenzylamino)-l,l-bis(4-methoxycarbonylaminophenyl)butanol
CA 02341458 2001-02-21
32
(173 mg) by hydrogenation in the usual manner.
MS m/z: 372 (M++1) (free)
Preparation 27
Methanesulfonyl chloride (1.4 ml) was added dropwise to
a solution of 3-amino-4-benzyloxyphenyl acetate (4.3 g) in
pyridine (20 ml) under ice cooling over 10 minutes and the
mixture was stirred at room temperature for a further 1 hour.
To the reaction mixture was added water (100 ml) and was
stirred at the same temperature for 1 hour. The precipitates
were collected by filtration and dissolved into chloroform
(100 ml), followed by its dryness over magnesium sulfate and
its evaporation in vacuo. The residue was chromatographed
(hexane-ethyl acetate) over silica gel to afford 4-benzyloxy-
3-(methanesulfonylamino)phenyl acetate (1.6 g).
NMR (CDC13, d): 2.27 (3H, s), 2.95 (3H, s), 5.09 (2H,
s), 6.80-7.03 (3H, m), 7.25-7.95 (6H, m)
MS m/z: 336 (M++1)
_Preparation 28
A solution of 4-benzyloxy-3-(methanesulfonylamino)phenyl
acetate (1.6 g) and potassium hydroxide (2.67 g) in methanol
(10 ml) was stirred for 18 hours at room temperature. The
reaction mixture was acidified with 1N hydrochloric acid to
pH 5-7 and extracted with ethyl acetate. The organic layer
was washed with brine, dried over magnesium sulfate, and
evaporated in vacuo. The residue was chromatographed
(hexane-ethyl acetate) over silica gel to afford N-(2-
benzyloxy-5-hydroxyphenyl)methanesulfonamide (750 mg).
NMR (CDC1 j, b) : 2.90 (3H, s) , 5.04 (2H, s) , 6.58 (1H,
dd, J=2.9 arid 8.8Hz), 6.80-6.90 (2H, m), 7.09 (1H,
d, J==2.9Hz), 7.30-7.50 (6H, m)
MS m/z: 2 94 (M++1)
Preparation 29
CA 02341458 2001-02-21
33
Under nitrogen, to a solution of N-(2-benzyloxy-5-
hydroxyphenyl)methanesulfonamide (740 mg) and sodium hydride
(92.4 mg) in N,N-dimethylformamide (30 ml) was added (2S)-
glycidyl tosylate (616 mg) at 0°C and the mixture was stirred
at the same temperature for 0.5 hour. The mixture was
allowed to warm to room temperature and stirred for 2.5 hours
at the same temperature. The resulting mixture was poured
into 10% aqueous ammonium chloride solution, and extracted
with ethyl acetate. The organic layer was washed with brine,
dried over magnesium sulfate, and evaporated in vacuo. The
residue was chromatographed (hexane-ethyl acetate) over
silica gel to afford (2S)-3-[4-benzyloxy-3-
(methanesulfonamino)phenoxy]-1,2-epoxypropane (440 mg).
NMR (CDC13, ~): 2.75 (1H, dd, J=2.7 and 4.9Hz),
2.84-2.95 (4H, m), 3.30-3.37 (1H, m), 3.90 (1H, dd,
J=5.8 and 11.08Hz), 4.07-4.25 (1H, m), 5.05 (2H,
s ) , 6. 63-7 . 4 8 ( 9H, m)
MS m/z: 350 (M++1)
Preparation 30
Under nitrogen, a solution of N-benzyl-[3,3-bis(4-
methoxyphenyl)-1-methylpropyl]amine (480 mg), N-[2-benzyloxy-
5-[(1R)-2-iodo-1-(triethylsilyloxy)ethyl]phenyl]-
methanesulfonamide (600 mg) and N,N-diisopropylethylamine
(0.74 ml) in tetrahydrofuran (6 ml) was sealed with stirring
at 110°C for 58 hours, followed by at 160°C for 92 hours.
The resulting mixture was poured into aqueous sodium hydrogen
sulfite and extracted with ethyl acetate. The organic layer
was washed successively with saturated aqueous sodium
bicarbonate and brine, dried over anhydrous sodium sulfate,
and evaporated in vacuo. The residue was purified by column
chromatography on silica gel (hexane:ethyl acetate = 10:1 to
5:1) to give N-[5-[(1R)-2-[N-benzyl-[(1RS)-3,3-bis(4-
methoxyphenyl)-1-methylpropyl]amino]-1-(triethylsilyloxy)-
ethyl]-2-(benzyloxy)phenyl]methanesulfonamide (226 mg).
CA 02341458 2001-02-21
34
NMR (CDC13, b): 0.25-0.5 (6H, m), 0.7-0.95 (12H, m),
1.5-2.25 (2H, m), 2.35-2.9 (6H, m), 3.45-3.9 (8H,
m), 4.25-4.4 (1H, m), 5.0-5.1 (2H, m), 6.65-7.75
(21H, m)
Preparation 31
N-Benzyl-[(1S)-3,3-bis(4-methoxyphenyl)-1-methylpropyl]-
amine hydrochloride (800 mg) was dehydrochlorinated with
saturated aqueous sodium bicarbonate and extracted with ethyl
acetate. A mixture of the above obtained product and l00
palladium on activated carbon (50o wet, 300 mg) in methanol
(10 ml) was stirred at room temperature in the presence of
hydrogen at an atmospheric pressure for 5.5 hours. After
filtration, the filtrate was evaporated in vacuo to give
[(1S)-3,3-bis(4-methoxyphenyl)-1-methylpropyl]amine
(568 mg).
NMR (CDC13, ~): 1.10 (3H, d, J=6.3Hz), 1.95-2.1 (2H,
m), 2.7-2.9 (1H, m), 3.76 (6H, m), 3.98 (1H, t,
J=8.OHz), 6.75-6.9 (4H, m), 7.1-7.2 (4H, m)
Preparation 32
The following compound was obtained according to a
similar manner to that of Preparation 31.
[(1R)-3,3-Bis(4-methoxyphenyl)-1-methylpropyl]amine
NMR (CDC13, b): 1.19 (3H, d, J=6.3Hz), 1.9-2.1 (2H, m),
2 . 7-2 . 85 ( 1H, m) , 3 . 76 ( 6H, m) , 3 . 98 ( 1H, t,
J=7.9Hz), 6.75-6.9 (4H, m), 7.15 (4H, d, J=8.OHz)
Preparation 33
A mixture of N-benzyl-[3,3-bis(4-hydroxyphenyl)-1-
methylpropyl]amine (300 mg) and loo palladium on activated
carbon (50o wet, 100 mg) in methanol (5 ml) was stirred at
room temperature in the presence of hydrogen at an
atmospheric pressure for 4 hours. After filtration, the
CA 02341458 2001-02-21
filtrate was evaporated in vacuo to give [3,3-bis(4-
hydroxyphenyl)-1-methylpropyl]amine (230 mg).
NMR (DMSO-d6, ~): 0.96 (3H, d, J=5.3Hz), 1.75-1.9 (2H,
m), 2.4-2.6 (1H, m), 3.87 (1H, t, J=7.9Hz), 6.63
5 (4H, d, J=8.5Hz), 7.03 (4H, d, J=8.2Hz)
Preparation 34
The following compound was obtained according to a
similar manner to that of Preparation 33.
N-Benzyl-[3,3-bis(4-methoxyphenyl)-1-methylpropyl]amine
Preparation 35
A mixture of 6-[(4-nitrophenyl)azo]pyridin-3-of (J. Am.
Chem. Soc. 1959, 81, 6049, 300 mg) and 20o palladium
hydroxide on carbon (60 mg) in a mixture of acetic acid (30
ml) and methanol (30 ml) was stirred at room temperature in
the presence of hydrogen at an atmospheric pressure for 70
minutes. After filtration, the filtrate was evaporated in
vacuo. Under nitrogen, to a mixture of the residue in
dichloromethane (10 ml) was added bis(trimethylsilyl)-
acetamide (6.0 ml) at 5°C. After being stirred at room
temperature for_ 30 minutes, to the resulting mixture was
added benzyl chloroformate (0.54 ml) at 5°C, and the mixture
was stirred at the same temperature for 3 hours. The
resulting mixture was poured into saturated aqueous sodium
bicarbonate, and extracted with ethyl acetate. The organic
layer was washed with brine, dried over anhydrous magnesium
sulfate, and evaporated in vacuo. To the residue was added
chloroform and insoluble materials were filtered off. After
the filtrate was evaporated in vacuo, the residue was
purified by column chromatography on silica gel
(chloroform:methanol = 50:1 to 20:1), followed by
crystallization from toluene-methanol to give (5-
hydroxypyridin-2-yl)carbamic acid benzyl ester (159 mg).
CA 02341458 2001-02-21
36
MS m/z: 245 (M++1)
Preparation 36
Under nitrogen, a suspension of sodium hydride (60% in
oil, 189 mg) in N,N-dimethylformamide (20 ml) was dropwise
added (5-hydroxypyridin-2-yl)carbamic acid benzyl ester (1.1
g) in N,N-dimethylformamide (12 ml) at 5°C, and the mixture
was stirred at room temperature for 1 hour. To this one was
added (2S)-glycidyl tosylate (1.1 g) at 5°C, and the mixture
was stirred at room temperature for 7 hours. The resulting
mixture was poured into saturated aqueous sodium bicarbonate,
and extracted with ethyl acetate. The organic layer was
washed with brine, dried over anhydrous magnesium sulfate,
and evaporated in vacuo. The residue was purified by column
chromatography on silica gel (chloroform: ethyl acetate = 9:1)
to give (2S)-3-[2-(benzyloxycarbonylamino)pyridin-5-yloxy]-
1,2-epoxypropane (780 mg).
MS m/z: 301 (M++1)
Preparation 37
To a suspension of but-3-enylbenzene (3 ml) and sodium
bicarbonate (2.5 g) in a mixture of dichloromethane (200 ml)
and water (60 ml) was added small portions of
m-chloroperbenzoic acid (3.5 g) at room temperature, and the
mixture was stirred at the same temperature for 4 hours.
After separation, the organic layer was washed successively
with saturated aqueous sodium bicarbonate and brine, dried
over anhydrous magnesium sulfate and evaporated in vacuo.
The residue was purified by column chromatography on silica
gel (hexane: ethyl acetate = 100:3) to give phenethyloxirane
( 970 mg) .
NMR (CDC13, b): 1.7-1.9 (2H, m), 2.45-2.5 (1H, m),
2.7-3.0 (4H, m), 7.1-7.4 (5H, m)
Preparation 38
CA 02341458 2001-02-21
37
Under nitrogen, to a solution of (2R)-glycidyl tosylate
(3.0 g) in tetrahydrofuran (30 ml) were added N,N-
diisopropylethylamine (2.5 ml) and thiophenol (1.3 ml) at
5°C, and the mixture was stirred at room temperature for 12
hours. The resulting mixture was poured into water and
extracted with ethyl acetate. The organic layer was washed
successively with saturated aqueous sodium bicarbonate and
brine, dried over anhydrous magnesium sulfate and evaporated
in vacuo. The residue was purified by column chromatography
on silica gel (hexane: ethyl acetate = 5:1 to 3:1) to give
toluene-4-sulfonic acid (2S)-2-hydroxy-3-(phenylthio)propyl
ester (3.9 g) .
NMR (CDC13, ~): 2.44 (3H, m), 2.75-3.25 (3H, m),
3.85-4.3 (3H, m), 7.15-7.4 (7H, m), 7.7-7.8 (2H, m)
Pr~ration 39
Under nitrogen, to a solution of toluene-4-sulfonic acid
(2S)-2-hydroxy-3-(phenylthio)propyl ester (3.9 g) in ethanol
(40 ml) was added 20o sodium methoxide in ethanol (4.7 ml) at
5°C, and the mixture was stirred at the same temperature for
minutes. After being filtrated off to remove
precipitates, the filtrate was concentrated in vacuo. The
residue was dissolved into a mixture of aqueous O.1N sodium
hydroxide and diethyl ether. After separation, the organic
25 layer was washed successively with water and brine, dried
over anhydrous magnesium sulfate and evaporated in vacuo.
The residue was purified by column chromatography on silica
gel (hexane:ethyl acetate = 20:1 to 10:1) to give (2S)-3-
(phenylthio)-1,2-epoxypropane (1.5 g).
30 MS m/z: 167 (M++1)
Example 6
To a solution of 4-bromothioanisole (508 mg) in
tetrahydrofuran, butyllithium (1.54M in hexane, 1.62 ml) was
added at -78°C. After 30 minutes, (3RS)-3-[((2S)-2-hydroxy-
CA 02341458 2001-02-21
38
3-phenoxypropyl)amino]butyric acid methyl ester (131 mg) was
added and warmed to 0°C. The reaction mixture was worked up
in the usual manner and purified by a silica gel column
chromatography to give (2S)-1-phenoxy-3-[(3RS)-1,1-bis[4-
(methylthio)phenyl]-1-hydroxy-3-butyl]amino-3-propanol (58.5
mg).
IR (Neat): 3400 (br s), 2921 (s), 1594 (s), 1492 (s),
1243 (s), 1093 (m), 1043 (m), 817 (s), 754 (s) cm-1
NMR (CDC13, ~): 1.14 (3H, d, J=6.3Hz), 2.4-2.6 (2H,
m), 2.43 (3H, s), 2.46 (3H, s), 2.7-2.8 (2H, m),
2.88 (1H, dd, J=11.9 and 8.OHz), 3.9-4.1 (3H, m),
6.9-7.0 (3H, m), 7.1-7.4 (12H, m)
Example 7
The following compounds were obtained according to a
similar manner to that. of Example 6.
(1) (2S)-1-Phenoxy-3-[(3RS)-1,1-bis(3-methoxyphenyl)-1-
hydroxy-3-butyl]amino-2-propanol
IR (Neat) : 3296 (br m) , 2933 (s) , 1599 (s) , 1491 (s) ,
1464 (m), 1246 (s), 1171 (m), 1045 (s), 756 (m),
694 (m) cm-1
NMR (CDC13+D20, b): 1.09 (3H, d, J=6.3Hz), 2.04 (1H,
dd, J=11.9 and 14.2Hz), 2.39-2.49 (2H, m), 2.6-2.8
(1H, m), 2.83 (1H, dd, J=8.3 and 11.9Hz), 3.74 (3H,
s), 3.76 (3H, s), 3.8-3.9 (2H, m), 3.9-4.1 (1H, m),
6.72-6.75 (2H, m), 6.8-7.1 (7H, m), 7.12-7.31 (4H,
m)
MS m/z: 452 (M++1)
(2) (2S)-1-Phenoxy-3-((3RS)-1,1-diphenyl-1-hydroxy-3-butyl)-
amino-2-propanol
IR (Neat): 3292 (br m), 2925 (m), 1597 (m), 1495 (s),
1456 (m) , 1244 (s) , 1043 (m) , 754 (s) , 698 (s) cm-1
MS m/z: 392 (M++1)
CA 02341458 2001-02-21
39
(3) (2S)-1-Phenoxy-3-[(3RS)-1,1-bis(4-methoxyphenyl)-1-
hydroxy-3-butyl]amino-2-propanol
IR (Neat) : 3292 (br m) , 2929 (m) , 1604 (m) , 1508 (s) ,
1460 (m), 1248 (s), 1176 (m), 1038 (m), 833 (m),
756 (m) cm-1
NMR (CDC13, ~): 1.12 (1.5H, d, J=5.lHz), 1.16 (1.5H, d,
J=6.2Hz), 2.0-2.5 (2H, m), 2.6-3.2 (3H, m), 3.74
(1.5H, s) , 3.75 (1.5H, s) , 3.78 (3H, s) , 3.8-4.2
(3H, m), 6.77-7.01 (7H, m), 7.2-7.4 (6H, m)
MS m/z: 452 (M++1)
(4) (2S)-1-Phenoxy-3-[(3RS)-l,l-bis(3,4-dimethoxyphenyl)-1-
hydroxy-3-butyl]amino-2-propanol
IR (Neat) : 3294 (br m) , 2933 (m) , 1597 (w) , 1510 (s) ,
1460 (m), 1257 (s), 1146 (m), 1028 (m),
760 (m) cm-l
MS m/z: 512 (M++1)
(5) (2S)-1-Phenoxy-3-[(3RS)-1,1-bis(4-methylphenyl)-1-
hydroxy-3-butyl]amino-2-propanol
IR (Neat): 3400 (br s), 2923 (s), 1596 (m), 1498 (s),
1457 (s) , 12.43 (s) , 1087 (m) , 1043 (m) , 817 (s) ,
754 (s) cm 1
NMR (CDC13, ~): 1.13 (2H, d, J=6.2Hz), 2.16 (3H, s),
2.31 (3H, s), 2.4-2.5 (2H, m), 2.6-2.9 (3H, m),
3.9-4.1 (3H, m), 6.9-7.4 (13H, m)
MS m/z: 420 (M++1)
(6) 5-[N-Benzyl-((2S)-2-hydroxy-3-phenoxypropyl)amino]-1,1-
bis(4-methoxyphenyl)-1-pentanol
IR (Neat): 3446 (br m), 2943 (m), 1604 (m), 1508 (s),
1458 (m), 1248 (s), 1176 (m), 1036 (m), 831 (m),
752 (m) cm-1
NMR (CDC13, ~): 1.2-1.3 (2H, m), 1.4-1.7 (2H, m), 2.1-
2.2 (2H, m), 2.4-2.7 (4H, m), 3.4-3.6 (1H, m), 3.78
CA 02341458 2001-02-21
(6H, s), 3.7-3.8 (1H, m), 3.8-3.9 (2H, m), 3.9-4.1
(1H, rn), 6.77-6.97 (7H, m), 7.2-7.4 (11H, m)
MS m/z: 556 (M++1)
5 (7) 4-[N-Benzyl-((2S)-2-hydroxy-3-phenoxypropyl)amino]-1,1-
bis(4-methoxyphenyl)-1-butanol
IR (Neat): 3446 (br m), 2951 (m), 1604 (m), 1508 (s),
1458 (m), 1248 (s), 1176 (m), 1036 (s), 831 (m),
752 (m) cm-1
10 NMR (CDC13, ~): 1.5-1.7 (2H, m), 2.1-2.4 (2H, m), 2.4-
2.7 (4H, m), 3.4-3.5 (1H, m), 3.77 (6H, s), 3.7-3.8
(1H, m), 3.8-4.0 (2H, m), 4.1-4.3 (1H, m), 6.78-
6.98 (7H, m), 7.2-7.4 (11H, m)
MS m/z: 542 (M++1)
(8) (2S)-1-Phenoxy-3-[(1RS)-3,3-bis(4-methoxyphenyl)-3-
hydroxy-1-(3,4-dimethoxyphenyl)propyl]amino-2-propanol
IR (Neat): 3361 (br m), 2929 (m), 1602 (m), 1512 (s),
1459 (m), 1298 (s), 1032 (s), 833 (m), 756 (m) cm-1
NMR (CDC13, b): 2.3-2.8 (5H, m), 3.74 (3H, s), 3.82
(3H, s), 3.86 (6H, s), 3.9-4.1 (3H, m), 6.6-7.1
(8H, m), 7.2-7.5 (8H, m)
MS m/z: 574 (M++1)
(9) (2S)-1-Phenoxy-3-[(1RS)-3,3-bis(4-methoxyphenyl)-3-
hydroxy-1-phenylpropyl]amino-2-propanol
IR (Neat): 3361 (br m), 2929 (m), 1603 (m), 1506 (s),
1458 (m), 1296 (s), 1176 (m), 1035 (m), 833 (m),
756(m), 698 (m) cm-1
NMR (CDC13, ~): 2.3-2.8 (4H, m), 3.6-3.7 (1H, m), 3.74
( 3H, s ) , 3 . 82 ( 3H, s ) , 3 . 8-4 . 2 ( 4H, m) , 6 . 7-7 . 0
(9H, m), 7.1-7.2 (2H, m), 7.2-7.4 (5H, m), 7.4-7.5
(2H, m)
MS m/z: 514 (M++1)
CA 02341458 2001-02-21
41
(10) (2S)-1-Phenoxy-3-[3,3-bis(4-methoxyphenyl)-3-
hydroxyphenyl]amino-2-propanol
IR (Neat): 3313 (br m), 2931 (m), 1602 (s), 1508 (s),
1462 (m), 1248 (s), 1176 (m), 1036 (m), 831 (m),
756 (m) cm-1
NMR (CDC13+D20, b): 2.3-2.5 (2H, m), 2.7-2.9 (4H, m),
3.77 (6H, s), 3.9-4.2 (4H, m), 6.80-7.01 (7H, m),
7 . 2-7 . 4 ( 6H, m)
MS m/z: 938 (M++1)
Example 8
The following compounds were obtained according to a
similar manner to that of Preparation 33.
(1) (2S)-1-Phenoxy-3-[5,5-bis(4-methoxyphenyl)pentyl)-
amino-2-propanol hydrochloride
IR (Neat) : 3322 (br m) , 1602 (m) , 1510 (s) , 1460 (m) ,
1246 (s), 1178 (m), 1036 (m), 831 (m) cm-1
NMR (MeOH-d4, ~): 1.2-1.4 (2H, m), 1.6-1.8 (2H, m),
2.04 (2H, quartet, J=7.5Hz), 2.99 (2H, t, J=8.OHz),
3.09-3.22 (2H, m), 3.73 (6H, s), 3.6-3.85 (1H, m),
3.9-4.0 (2H, m), 4.1-4.3 (1H, m), 6.84 (4H, d,
J=8.7Hz), 6.9-7.0 (3H, m), 7.14 (4H, d, J=8.6Hz),
7.28 (2H, t, J=7.9Hz)
MS m/z: 450 (M++1) (free)
(2) (2S)-1-Phenoxy-3-[4,4-bis(4-methoxyphenyl)butyl]amino-2-
propanol hydrochloride
IR (Neat): 3355 (br m), 1602 (m), 1510 (s), 1960 (m),
1246 (s) 1178 (m), 1036 (m), 831 (m) cm-1
NMR (MeOH-d4, b): 1.6-1.8 (2H, m), 2.08 (1H, quartet,
J=7.9Hz), 2.55 (1H, quartet, J=7.6Hz), 3.0-3.2 (4H,
m) , 3. 73 ( 6H, s) , 3. 7-4 . 3 (4H, m) , 6. 8-7 . 0 (7H, m) ,
7.1-7.4 (6H, m)
MS m/z: 436 (M++1) (free)
CA 02341458 2001-02-21
42
Example 9
A mixture of (2S)-1-phenoxy-3-[(3RS)-1,1-bis[4-
(methylthio)phenyl]-1-hydroxy-3-butyl]amino-2-propanol (47
mg), water (2 ml), methanol (3 ml) and OXONE~ (potassium
peroxymonosulfate) (180 mg) was stirred at room temperature
overnight and worked up in the usual manner to give (2S)-1-
phenoxy-3-[(3RS)-1,1-bis(4-methanesulfonylphenyl)-1-hydroxy-
3-butyl]amino-2-propanol (52 mg).
IR (Neat): 3521 (br m), 2927 (m), 1595 (m), 1494 (s),
1309 (s), 1244 (m), 1149 (s), 1091 (m), 958 (m),
771 (s), 694 (m) cm-1
NMR (CDC13, d): 1.19 (3H, d, J=6.3Hz), 2.3-2.8 (4H, m),
2.9 (1H, m), 3.00 (3H, s), 3.05 (3H, s), 3.9-4.1
(3H, m), 6.9-7.1 (3H, m), 7.2-7.4 (1H, m), 7.5-7.74
(5H, m), 7.8-7.9 (4H, m)
MS m/z: 548 (M++1)
Example 10
A mixture of (2S)-3-phenoxy-1,2-epoxypropane (40 mg),
3-amino-3-(3,4-dimethoxyphenyl)propionic acid methyl ester
acetate (80 mg), triethylamine (0.5 ml) and methanol (3 ml)
was heated under reflux, evaporated and purified by silica
gel column chromatography (hexane:ethyl acetate:methanol =
1:1:0.07) to give (3RS)-3-((2S)-2-hydroxy-3-phenoxypropyl)-
amino-3-(3,4-dimethoxyphenyl)propionic acid methyl ester (92
mg ) .
IR (Neat): 2925 (m), 1738 (s), 1597 (m), 1514 (s), 1460
(m), 1263 (m), 1138 (m), 1027 (s), 758 (m) cm-1
NMR (CDC13, ~): 2.6-2.8 (4H, m), 3.67 (3H, s), 3.87
(6H, s), 3.9-4.0 (2H, m), 4.0-4.1 (2H, m), 6.8-7.0
(7H, m), 7.26 (1H, t, J=8.9Hz)
MS m/z: 390 (M++1)
Example 11
The following compound was obtained according to a
CA 02341458 2001-02-21
43
similar manner to that of Example 10.
(2S)-1-Phenoxy-3-[1,1-bis(4-methoxyphenyl)-3-methyl-3-
butyl]amino-2-propanol
IR (Neat): 3350 (br m), 2962 (m), 1606 (m), 1508 (s),
1460 (m), 1248 (s), 1178 (m), 1036 (m), 829 (m),
756(m) cm-1
NMR (CDC13, 0 :1.03 (3H, s), 1.05 (3H, s), 2.22 (2H, d,
J=6.8Hz), 2.55 (1H, dd, J=7.0 and 11.7Hz), 2.68
(1H, dd, J=3.6 and 11.7Hz), 3.73 (6H, s), 3.8-3.9
(3H, m) , 4.04 (1H, t, J=6.7Hz) , 6.7.7-6. 99 (7H, m) ,
7.1-7.4 (6H, m)
MS m/z: 450 (M++1)
Example 12
A mixture of (2S)-3-phenoxy-1,2-epoxypropane (0.12 g),
1-[2,2-bis(4-methoxyphenyl)ethyl]cyclopentylamine (0.24 g)
and methanol (5 ml) was heated under reflux, evaporated and
purified by silica gel column chromatography (hexane: ethyl
acetate:methanol = 1:1:0.07) to give N-((2S)-2-hydroxy-3-
phenoxypropyl)-[1-[2,2-bis(4-methoxyphenyl)ethyl]-
cyclopentyl]amine (40.9 mg).
IR (Neat): 2954 (m), 1606 (w), 1510 (s), 1460 (m),
1246 (m), 1176 (m), 1038(s), 825 (m), 754 (m) cm-1
NMR (CDC13, b): 1.2-1.8 (8H, m), 2.25 (2H, t, J=6.8Hz),
2.46 (1H, dd, J=6.9 and 12.OHz), 2.61 (1H, dd,
J=4.4 and 12.OHz), 3.73 (6H, s), 3.8-3.9 (2H, m),
4.0-4.1 (2H, m), 6.78 (4H, d, J=8.2Hz), 6.9-7.0
(3H, m), 7.19 (4H, d, J=8.7Hz), 7.24-7.33 (2H, m)
MS m/z: 476 (M++1)
_Example 13
The following compounds were obtained according to a
similar manner to that of Example 12.
CA 02341458 2001-02-21
44
(1) (1R)-1-(3-Pyridyl)-2-[[(3RS)-1,1-bis(4-methoxyphenyl)-3-
butyl]amino]ethanol
MS m/z: 407 (M++1)
(2) (2S)-1-(3-Pyridyloxy)-3-[(3RS)-1,1-bis(4-methoxyphenyl)-
3-butyl]amino-2-propanol dihydrochloride
MS m/z: 437 (M++1) (free)
(3) (2S)-1-(1H-Indol-4-yloxy)-3-[3,3-bis(4-methoxyphenyl)-
propyl]amino-2-propanol
MS m/z: 461 (M++1)
(4) (2RS)-1-(2-Oxo-2,3-dihydro-1H-benzimidazol-4-yloxy)-3-
[(3RS)-1,1-bis(4-methoxyphenyl)-3-butyl]amino-2-propanol
MS m/z: 492 (M++1)
(5) (2R)-3-[4-Benzyloxy-3-(methanesulfonylamino)phenyl]-1-
[(3RS)-1,1-bis(4-methoxyphenyl)-3-butyl]amino-2-propanol
(6) (2S)-1-Phenoxy-3-[(3RS)-l,l-bis[4-(methanesulfonyl-
amino)phenyl]-3-butyl]amino-2-propanol
IR (KBr): 3440 (br s), 1603 (m), 1508 (m), 1325 (m),
1242 (m) , 1151 (s) , 1103 (m) , 974 (m) , 758 (m) cm-1
NMR (CDC13, ~): 1.11 (3H, d, J=6.2Hz), 2.0-2.2 (2H, m),
2.2-2.9 (3H, m), 2.97 (6H, s), 3.9-4.0 (3H, br s),
4.1-4.2 (1H, m), 6.88-7.00 (4H, m), 7.10-7.33 (9H,
m)
MS m/z: 562 (M++1)
(7) (2S)-1-Phenoxy-3-[(3RS)-1,1-bis[4-(acetylamino)phenyl)-
1-hydroxy-3-butyl]amino-2-propanol
MS m/z: 506 (M++1)
(8) (2S)-1-Phenoxy-3-[(3RS)-1,1-bis[4-(acetylamino)phenyl]-
3-butyl]amino-2-propanol
CA 02341458 2001-02-21
MS m/z: 490 (M++1)
Example 14
A mixture of (1S)-1-phenoxy-3-[3,3-bis(4-methoxyphenyl)-
5 3-hydroxypropyl]amino-2-propanol (93 mg), p-toluenesulfonic
acid hydrate (53 mg) and toluene was heated under reflux for
1.5 hours, evaporated and purified (preparative TLC, silica
gel, loo methanol-dichloromethane) to give (1S)-1-phenoxy-3-
[3,3-bis(4-methoxyphenyl)-2-propenyl]amino-2-propanol (80.5
10 mg).
IR (Neat): 3359 (br m), 1604 (s), 1510 (s), 1460 (m),
1248 (s), 1176 (m), 1034 (m), 835 (m), 756 (m), 686
(m) cm-1
NMR (CDC13+D20, b): 2.9-3.1 (2H, m), 3.58 (2H, d,
15 J=7.OHz), 3.77 (3H, s), 3.82 (3H, s), 3.9-4.0 (2H,
m), 9.2-4.3 (1H, m), 6.07 (1H, t, J=7.lHz), 6.73-
7.29 (13H, m)
Example 15
20 To a mixture of (2S)-1-phenoxy-3-[3,3-bis(4-
methoxyphenyl)-3-hydroxypropyl]amino-2-propanol (34 mg),
triethylsilane (0.5 ml) and dichloromethane (1 ml),
trifluoroacetic acid (0.1 ml) was added dropwise at room
temperature. The reaction mixture was worked up immediately
25 in the usual manner and purified by silica gel preparative
TLC (eluent; lOs methanol/dichloromethane) to give (2S)-1-
phenoxy-3-[3,3-bis(4-methoxyphenyl)propyl]amino-2-propanol
trifluoroacetat.e (21 mg).
IR (Neat): 3900 (br m), 2933 (m), 1680 (s), 1604 (m),
30 1508 (s), 1248 (s), 1203 (m), 1180 (s), 1134 (m),
1036 (m), 829 (m), 756 (m) cm-l
NMR (CDC13, ~): 2.3-2.5 (2H, m), 2.7-2.9 (2H, m), 2.9-
3.1 (2H, m), 3.74 (6H, s), 3.8-4.0 (3H, m), 4.1-4.3
(1H, m), 6.7-6.9 (6H, m), 6.95 (2H, t, J=7.9Hz),
35 7.10 (4H, d, J=8.5Hz), 7.26 (1H, t, J=7.9Hz)
CA 02341458 2001-02-21
46
MS m/z: 422 (M++1)
Example 16
A mixture of (2S)-3-phenoxy-1,2-epoxypropane (0.11 g),
D-alanine bis(4-methoxyphenyl)methylamide (0.25 g) and
methanol (4 ml) was heated under reflux overnight, evaporated
and purified by silica gel column chromatography to give N-
((2S)-2-hydroxy-3-phenoxypropyl)-D-alanine [bis(4-
methoxyphenyl)methyl]amide (217 mg).
IR (KBr): 3290 (s), 1643 (s), 1606 (m), 1512 (s), 1642
(m), 1250 (s), 1176 (m), 1034 (s), 831 (w), 812
(m) , 752 (m) cm-1
NMR (CDC13, ~): 1.27 (3H, d, J=7.2Hz), 2.66 (1H, dd,
J=3.8 and 12.OHz), 2.80 (1H, dd, J=7.6 and 12.OHz),
3.24 (1H, quartet, J=6.9Hz), 3.74 (3H, s), 3.78
(3H, s), 3.8-4.0 (3H, m), 6.14 (1H, d, J=8.6Hz),
6.8-6.9 (6H, m), 6.98 (1H, t, J=7.3Hz), 7.13 (4H,
dd, J=2.0 and 8.6Hz), 7.29 (2H, t, J=7.4Hz), 7.71
(1H, d, J=8.6Hz)
MS m/z: 465 (M++1)
Example 17
To a suspension of lithium aluminum hydride (10 mg) in
tetrahydrofuran (0.5 ml), a solution of N-((2S)-2-hydroxy-3-
phenoxypropyl)-D-alanine [bis(4-methoxyphenyl)methyl]amide
(52.4 mg) in tetrahydrofuran was added dropwise at 0°C under
a flow of nitrogen. The reaction mixture was heated under
reflux. After 2 hours, additional lithium aluminum hydride
(50 mg) was added to the reaction mixture under a flow of
nitrogen at 0°C. The reaction mixture was heated under
reflux for 2.5 hours, worked up in the usual manner and
purified (preparative TLC, 10% methanol-ethyl acetate) to
give (2S)-1-phenoxy-3-[(2R)-1-[[bis(4-methoxyphenyl)methyl]-
amino]-2-propyl]amino-2-propanol (31.4 mg).
IR (Neat): 3316 (br s), 2931 (m), 1606 (m), 1508 (s),
CA 02341458 2001-02-21
47
1458 (m), 1292 (s), 1174 (m), 1036 (s), 820 (m),
756 (m) cm-1
NMR (CDC13, d): 1.06 (3H, d, J=6.3Hz), 2.46 (1H, dd,
J=8.7 and 12.OHz), 2.62 (1H, dd, J=9.2 and 12.OHz),
2. 80 (2H, d, J=4.7Hz) , 3.76 (6H, s) , 3. 9-4. 1 (3H,
m), 4.71 (1H, s), 6.82 (4H, dd, J=2.0 and 6.7Hz),
6.9-7.0 (3H, m), 7.2-7.3 (6H, m)
MS m/z: 451 (M++1)
Example 18
A mixture of (2R)-2-[9-benzyloxy-3-
(methanesulfonylamino)phenyl]-2-(triethylsilyloxy)-1-
iodoethane (156 mg), [3,3-bis(4-methoxyphenyl)propyl]amine
(75 mg), N,N-diisopropylethylamine (0.19 ml) and
dimethylacetamide (0.75 ml) was heated at 110°C overnight and
worked up in the usual manner. The crude product was treated
with 4N hydrogen chloride in ethyl acetate (2 ml), worked up
in the usual manner and purified by preparative TLC (10%
methanol-dichloromethane) to give (1R)-1-[4-benzyloxy-3-
(methanesulfonylamino)phenyl]-2-[3,3-bis(4-methoxyphenyl)-
propyl]aminoethanol (47 mg).
IR (Neat): 3310 (br m), 1608 (w), 1510 (s), 1460 (m),
1329 (m), 1248 (s), 1157 (s), 1120 (s); 1034 (m),
818 (m), 739 (m) cm-1
NMR (CDC13, b): 2.16 (2H, quartet, J=7.lHz), 2.5-2.7
(3H, m), 2.81 (1H, dd, J=3.6 and 12.2Hz), 2.90 (3H,
s), 3.79 (6H, s), 3.95 (1H, t, J=7.9Hz), 4.55 (1H,
dd, J=3.5 and 8.9Hz), 5.09 (2H, s), 6.81 (4H, d,
J=8.6Hz), 6.95 (1H, d, J=8.5Hz), 7.13 (5H, d,
J=8.6Hz), 7.35 (5H, s), 7.47 (1H, d, J=2.OHz)
MS m/z: 591 (M++1)
Example 19
The following compound was obtained according to a
similar manner to that of Example 18.
CA 02341458 2001-02-21
48
(1R)-1-[4-Benzyloxy-3-(methanesulfonylamino)phenyl]-2-
[[(3RS)-1,1-bis[4-(methoxycarbonylamino)phenyl]-3-butyl]-
amino]ethanol
Example 20
(1R)-1-[4-Benzyloxy-3-(methanesulfonylamino)phenyl]-2-
[[3,3-bis(4-methoxyphenyl)propyl]amino]ethanol (35 mg) was
hydrogenated by the usual manner to give (1R)-1-[4-hydroxy-3-
(methanesulfonylamino)phenyl]-2-[[3,3-bis(4-methoxyphenyl)-
propyl]amino]ethanol (19.3 mg).
IR (KBr): 3430 (br m), 1608 (w), 1510 (s), 1319 (m),
1304 (m) , 1248 (s) , 1.153 (m) , 1034 (m) ,
825 (m) cm-1
NMR (CDC13, d): 2.1-2.3 (2H, m), 2.5-2.7 (2H, m),
2.7-2.9 (2H, m), 2.90 (3H, s), 3.74 (6H, s), 3.83
(1H, t, J=7.8Hz), 4.5-4.7 (1H, m), 6.79 (5H, d,
J=8.3Hz), 7.01 (1H, d, J=8.lHz), 7.13 (4H, d,
J=8.4Hz), 7.13 (1H, br s)
MS m/z: 501 (M++1)
Example 21
A mixture of (2S)-3-(4-benzyloxy-3-nitrophenoxy)-1,2-
epoxypropane (197 mg), N-benzyl-[3,3-bis(4-methoxyphenyl)-
propyl]amine (236 mg) and ethanol (3 ml) was heated under
reflex for 12 hours. Iron powder, ammonium chloride and
water were added to the reaction mixture and heating was
continued for 1. hour. The reaction mixture was filtrated and
worked up in the usual manner to give (2S)-1-(3-amino-9-
benzyloxyphenoxy)-3-[N-benzyl-[3,3-bis(4-methoxyphenyl)-
propyl]amino]-2-propanol (412.'7 mg).
NMR (CDC13, ~): 2.1-2.3 (2H, m), 2.4-2.7 (4H, m), 3.50
(1H, d, J=l4Hz), 3.75 (6H, s), 3.7-4.0 (5H, m),
5.01 (2H, s), 6.15-6.4 (2H, m), 6.71-6.80 (5H, m),
7.03-7.08 (4H, m), 7.2-7.4 (10H, m)
MS m/z: 633 (M+~-1)
CA 02341458 2001-02-21
49
Example 22
The following compounds were obtained according to a
similar manner to that of Example 21.
(1) (2S)-1-(3-Amino-4-benzyloxyphenoxy)-3-[N-benzyl-[4,4-
bis(4-methoxyphenyl)butyl]amino]-2-propanol
NMR (CDC13, ~): 1.45 (2H, quintet, J=7.5Hz), 1.93 (2H,
quintet), 2.3-2.6 (4H, m), 3.44 (1H, d, J=13.5Hz),
3.69-9.1 (4H, m), 3.76 (6H, s), 5.00 (2H, s), 6.17
(1H, dd, J=2.9 and 8.8Hz), 6.31 (1H, d, J=2.8Hz),
6.73 (1H, d, J=8.8Hz), 6.79 (4H, d, J=8.7Hz), 7.07
(4H, d, J=7.7Hz), 7.2-7.4 (lOH, m)
MS m/z: 647 (M++1)
(2) (1RS)-1-(3-Amino-4-benzyloxyphenyl)-2-[N-benzyl-[4,9-
bis(4-methoxyphenyl)butyl]amino]ethanol
NMR (CDC13, ~): 1.4-1.6 (2H, m), 1.8-2.1 (2H, m),
2.4-2.7 (4H, m) , 3.41 (1H, d, J=13. 5Hz) , 3.76 (6H,
s ) , 3 . 7-3 . 9 ( 2H, m) , 4 . 51 ( 1H, t ) , 5 . 05 ( 2H, s ) ,
6.50-6.65 (2H, m), 6.75-6.85 (5H, m), 7.05-7.15
(4H, m), 7.2-7.5 (lOH, m)
MS m/z: 617 (M++1)
(3) (2S)-1-Phenoxy-3-[[3,3-bis(4-ethoxyphenyl)propyl]-
amino]-2-propanol
IR (Neat): 3305 (br m), 1604 (m), 1510 (s), 1392 (w),
1300 (w), 1246 (s), 1176 (m), 1047 (s), 822 (m),
756 (m) cm-1
NMR (MeOH-d6, b): 1.34 (6H, t, J=7.OHz), 2.2-2.4 (2H,
m), 2.6-2.9 (4H, m), 3.97 (4H, quartet, J=7.OHz),
3. 9-4. 1 (4H, m) , 6. 80 (4H, d, J=8. 6Hz) , 6. 89-7. 0
(3H, m), 7.14 (4H, d, J=8.6Hz), 7.26 (2H, t,
J=7.9Hz)
MS m/z: 450 (M++1)
CA 02341458 2001-02-21
Example 23
To a mixture of (2S)-1-(3-amino-4-benzyloxyphenoxy)-3-
[N-benzyl-[3,3-bis(4-methoxyphenyl)propyl]amino]-2-propanol
(59 mg), pyridine (0.1 ml) and dichloromethane (1 ml),
5 methanesulfonyl chloride (27 ~1) were added at 0°C and
stirred for 30 minutes. The reaction mixture was worked up
in the usual manner. The crude product was hydrogenated in
the usual manner to give (2S)-1-[4-hydroxy-3-
(methanesulfonylamino)phenoxy]-3-[[3,3-bis(4-methoxyphenyl)-
10 propyl]amino]-2-propanol (17.2 mg).
IR (KBr): 3440 (br s), 1610 (w), 1510 (s), 1460 (m),
1325 (m), 1248 (s), 1176 (m), 1151 (m), 1115 (w),
1034 (m), 816 (m) cm-1
NMR (MeOH-d4, d): 2.1-2.3 (2H, m), 2.5-2.8 (4H, m),
15 2. 91 (3H, s) , 3.74 (6H, s) , 3.8-4.1 (4H, m) , 6.6-
6 . 7 ( 1H, m) , 6 . 7-6 . 9 ( 5H, m) , 6 . 97 ( 1H, d,
J=2.7Hz), 7.1-7.2 (9H, m)
MS m/z: 531 (M++1)
20 Example 24
The following compounds were obtained according to a
similar manner to that of Example 23.
(1) (1R)-1-[4-Hydroxy-3-(methanesulfonylamino)phenyl]-2-
25 [[3,3-bis[4-[(methoxycarbonyl)amino]phenyl]propyl]-
amino]ethanol
MS m/z: 587 (M++1)
(2) (2S)-1-[(4-Hydroxy-3-(methanesulfonylamino)phenoxy]-3-
30 [[3,3-bis[4-[(methoxycarbonyl)amino]phenyl]propyl]-
amino]-2-propanol
MS m/z: 617 (M++1)
(3) (2S)-1-[4-Hydroxy-3-(methanesulfonylamino)phenoxy]-3-
35 [4,4-bis(4-methoxyphenyl)butyl]amino-2-propanol
CA 02341458 2001-02-21
51
IR (KBr) : 3480 (br m) , 1612 (m) , 1512 (s) , 1460 (m) ,
1321 (w), 1248 (s), 1178 (m), 1113 (m), 1039 (m),
826 (m) cm-1
NMR (MeOH-d4, b): 1.4-1.6 (2H, m), 1.9-2.1 (2H, m),
2.6-2.8 (4H, m), 2.89 (3H, s), 3.73 (6H, s), 3.8-
4.1 (4H, m), 6.61 (1H, dd, J=2.9 and 8.8Hz), 6.76
( 1H, d, J=8 . 5Hz ) , 6 . 80 ( 4H, d, J=8 . 6Hz ) , 6 . 96 ( 1H,
d, J=2.9Hz), 7.13 (4H, d, J=8.6Hz)
MS m/z: 545 (M++1)
(4) (1RS)-1-[4-Hydroxy-3-(methanesulfonylamino)phenyl]-2-
[[4,4-bis(4-methoxyphenyl}butyl]amino]ethanol
IR (KBr): 3420 (br m), 1560 (m), 1512 (s), 1321 (m),
1248 (s), 1153 (m), 1113 (w), 1039 (m), 817(m) cm-1
NMR (MeOH-d4, b): 1.4-1.7 (2H, m), 1.9-2.1 (2H, m),
2.7-2.9 (4H, m), 2.90 (3H, s), 3.73 (6H, s), 3.7-
3.9 (1H, m), 4.6-4.8 (1H, m), 6.81 (4H, d,
J=8.7Hz), 6.85 (1H, m), 7.05 (1H, m), 7.14 (4H, d,
J=8.6Hz), 7.34 (1H, s)
MS m/z: 515 (M++1)
Example 25
To a solution of (2S)-1-phenoxy-3-[N-benzyl-[3,3-bis(4-
methoxyphenyl)propyl]amino]-2-propanol (97 mg) and
dichloromethane (1 ml), 1M boron tribromide-dichloromethane
solution (0.28 ml) was added at -78°C. The reaction mixture
was stirred at 0°C for 50 minutes and worked up in the usual
manner to give (2S)-1-phenoxy-3-[N-benzyl-[3,3-bis(4-
hydroxyphenyl)propyl]amino]-2-propanol (44 mg).
MS m/z: 484 (M++1)
Example 26
To a mixture of (2S)-1-phenoxy-3-[N-benzyl-[3,3-bis(4-
hydroxyphenyl)propyl]amino]-2-propanol (40 mg), N,N-
diisopropylethylamine (43 ~l) and dichloromethane (1 ml),
CA 02341458 2001-02-21
52
trifluoromethanesulfonic anhydride (31 ~1) was added at
-78°C. The reaction mixture was worked up in the usual
manner. A mixture of the obtained crude product, palladium
acetate (5.6 mg), 1,3-bis(diphenylphosphino)propane (10.2
mg), triethylamine (46 ~,1), N,N-dimethylformamide (1 ml) and
methanol (0.5 ml) was stirred at 100°C under carbon monoxide
atmosphere (1 atm) for 3.5 hours, worked up in the usual
manner and purified by preparative TLC (hexane: ethyl acetate
- 3:1) to give (2S)-1-phenoxy-3-(N-benzyl-[3,3-bis[4-
(methoxycarbonyl)phenyl]propyl]amino]-2-propanol (21 mg).
MS m/z: 568 (M++1)
Example 27
N-[5-[(2S)-3-[N-Benzyl-[(1RS)-3,3-bis(4-hydroxyphenyl)-
1-methylpropyl]amino]-2-hydroxypropoxy]-2-benzyloxyphenyl]-
methanesulfonamide (120 mg) and 10o palladium on activated
carbon (50% wet, 30 mg) in methanol (10 ml) was stirred at
room temperature in the presence of hydrogen at an
atmospheric pressure for 3 hours, and filtered. The filtrate
was evaporated in vacuo and treated with 4N hydrogen chloride
in ethyl acetate to afford N-[5-[(2S)-3-[[(1RS)-3,3-bis(4-
hydroxyphenyl)-1-methylpropyl]amino]-2-hydroxypropoxy]-2-
hydroxyphenyl]methanesulfonamide hydrochloride (50 mg).
MS m/z: 516 (M++1) (free)
Example 28
The following compound was obtained according to a
similar manner to that of Example 27.
N-[5-[(2S)-3-[[(1RS)-3,3-Bis(9-methoxyphenyl)-1-
methylpropyl]amino]-2-hydroxypropoxy]-2--hydroxyphenyl]-
methanesulfonamide hydrochloride
MS m/z: 544 (M++1) (free)
Example 29
CA 02341458 2001-02-21
53
(2S)-1-Phenoxy-3-[N-benzyl-[3,3-bis[4-(methoxycarbonyl)-
phenyl]propyl]amino]-2-propanol (14 mg) was treated with
sodium hydroxide in the usual manner and hydrogenated in the
usual manner to give (2S)-1-phenoxy-3-[3,3-bis(4-
carboxyphenyl)propyl]amino-2-propanol (12 mg).
MS m/z: 450 (M++1)
Example 30
A mixture of (2S)-3-phenoxy-1,2-epoxypropane (0.36 g),
N-benzyl-[3,3-bis[4-[(methoxycarbonyl)amino]phenyl]propyl]-
amine (0.97 g) and ethanol (10 ml) was heated under reflux
for 12 hours and cooled to room temperature. To the reaction
mixture, loo palladium on charcoal (50o wet, 0.4 g), 4N
hydrogen chloride in 1,4-dioxane (1.1 ml) and methanol (5 ml)
was added. The mixture was stirred under hydrogen (1 atm)
for 3.5 hours, filtrated, diluted with ethyl acetate,
neutralized by washing with aqueous sodium bicarbonate
solution and the organic layer was evaporated. The crude
product was purified by silica gel column
chromatography(dichloromethane:methanol:concentrated ammonia
water = 20:1:0.05) to give (2S)-1-phenoxy-3-[[3,3-bis[4-
[(methoxycarbonyl)amino]phenyl]propyl]amino]-2-propanol,
which was converted to the corresponding hydrochloride salt
(0.71 g) in a usual manner.
IR (KBr): 3400 (br m), 1711 (s), 1599 (m), 1537 (s),
1317 (m), 1238 (s), 1072 (m), 758 (m) cm-1
NMR (MeOH-d4, ~): 2.3-2.5 (2H, m), 2.9-3.3 (4H, m),
3 . 65 ( 3H, s ) , 3 . 71 ( 3H, s ) , 3 . 9-4 . 00 ( 3H, m) , 4 . 1
4 . 3 ( 1H, m) , 6. 91-6. 98 ( 3H, m) , 7 . 18-7 . 39 ( lOH, m)
MS m/z: 508 (M++1) (free)
Example 31
The following compound was obtained according to a
similar manner to that of Example 30.
CA 02341458 2001-02-21
54
(2S)-1-(4-Hydroxyphenoxy)-3-[(3RS)-1,1-bis(4-
methoxyphenyl)-3-butyl]amino-2-propanol
MS m/z: 452 (M++1)
Example 32
The following compound was obtained according to a
similar manner to that of Preparation 16.
(2S)-1-Phenoxy-3-[N-benzyl-[(3RS)-1,1-bis(4-
aminophenyl)-1-hydroxy-3-butyl]amino]-2-propanol
MS m/z: 512 (M++1)
Example 33
To a mixture of (2S)-1-phenoxy-3-[N-benzyl-[(3RS)-1,1-
bis(4-aminophenyl)-1-hydroxy-3-butyl]amino]-2-propanol (100
mg), pyridine (48 ~1) and dichloromethane (2 ml), methyl
chlorocarbonate (33 ~l) was added at 0°C. The reaction
mixture was worked up in the usual manner to give (2S)-1-
phenoxy-3-[N-benzyl-[(3RS)-1,1-bis[4-[(methoxycarbonyl)-
amino]phenyl]-1-hydroxy-3-butyl]amino]-2-propanol (125 mg).
MS m/z: 628 (M++1)
Example 34
The following compound was obtained according to a
similar manner to that of Example 33.
(2S)-1-Phenoxy-3-[N-benzyl-[(3RS)-1.,1-bis[4-[N-methyl-
(methoxycarbonyl)amino]phenyl]-1-hydroxy-3-butyl]amino]-2-
propanol
MS m/z: 656 (M++1)
Example 35
(2S)-1-Phenoxy-3-[N-(benzyl-[(3RS)-1,1-bis[4-
[(methoxycarbonyl)amino]phenyl]-1-hydroxy-3-butyl]amino]-2-
propanol (99 mg) was hydrogenated in the usual manner to give
CA 02341458 2001-02-21
(2S)-1-phenoxy-3-[(3RS)-1,1-bis[4-[(methoxycarbonyl)amino]-
phenyl]-3-butyl]amino-2-propanol (58 mg).
MS m/z: 522 (M++1)
5 Example 36
The following compounds were obtained according to a
similar manner to that of Preparation 18.
(1) (2S)-1-Phenoxy-3-[(3RS)-l,l-bis[9-[(ethoxycarbonyl)-
10 amino]phenyl]-3-butyl]amino-2-propanol hydrochloride
MS m/z: 550 (M++1) (free)
(2) (2S)-1-Phenoxy-3-[(3RS)-l,l-bis[4-[(trifluoroacetyl)-
amino]phenyl]-1-hydroxy-3-butyl]amino-2-propanol
15 hydrochloride
MS m/z: 614 (M++1) (free)
(3) (2S)-1-Phenoxy-3-[(3RS)-l,l-bis[4-(propionylamino)-
phenyl]-3-butyl]amino-2-propanol
20 MS m/z: 518 (M++1)
Example 37
To a mixture of (2S)-1-phenoxy-3-[N-benzyl-[(3RS)-l,l
bis(4-aminophenyl)-1-hydroxy-3-butyl]amino]-2-propanol (120
25 mg), acetic acid (3 ml) and water (0.6 ml), potassium cyanate
(77 mg) was added. The reaction mixture was worked up in the
usual manner to give (2S)-1-phenoxy-3-[N-benzyl-[(3RS)-1,1-
bis(4-ureidophenyl)-1-hydroxy-3-butyl]amino]-2-propanol (65
mg ) .
30 MS m/z: 598 (M++1)
Example 38
Formic acid (650 ~~1) and acetic anhydride (540 ~1) were
mixed and started at room temperature for 30 minutes. The
35 mixture was added to a solution of (2S)-1-phenoxy-3-[N-
CA 02341458 2001-02-21
56
benzyl-[(3RS)-1,1-bis(4-aminophenyl)-1-hydroxy-3-butyl]-
amino]-2-propanol (325 mg) in dichloromethane (2 ml) at 0°C,
warmed to room temperature and worked up in the usual manner.
The crude product was stirred with potassium carbonate (0.62
g) in methanol (4 ml) at room temperature for 4 hours and
worked up in the usual manner to give (2S)-1-phenoxy-3-[N-
benzyl-[(3RS)-1,1-bis[4-(formylamino)phenyl)-1-hydroxy-3-
butyl)amino]-2-propanol (342.4 mg).
MS m/z: 568 (M++1)
Example 39
To a mixture of lithium aluminum hydride (0.1 g) and
tetrahydrofuran (1 ml), a solution of (2S)-1-phenoxy-3-[N-
benzyl-[(3RS)-1,1-bis[4-(formylamino)phenyl]-1-hydroxy-3-
butyl]amino]-2-propanol (280 mg) in tetrahydrofuran (2 ml)
was added dropwise at 0°C. The reaction mixture was stirred
for 2.5 hours and worked up in the usual manner to give (2S)-
1-phenoxy-3-[N-benzyl-[(3RS)-1,1-bis[4-(methylamino)phenyl]-
1-hydroxy-3-butyl]amino]-2-propanol (273 mg).
MS m/z: 540 (M++1)
Example 40
(2S)-1-Phenoxy-3-[(3RS)-l,l-bis[4-[N-methyl-
(methoxycarbonyl)amino]phenyl]-1-hydroxy-3-butyl]amino-2-
propanol (30 mg) was obtained from (2S)-1-phenoxy-3-[N-
benzyl-[(3RS)-l,l-bis[4-[N-methyl-(methoxycarbonyl)amino]-
phenyl]-1-hydroxy-3-butyl]amino)-2-propanol (60 mg) by the
usual hydrogenation.
MS m/z: 566 (M++1)
Example 41
(1R)-1-[4-Hydroxy-3-(methanesulfonylamino)phenyl]-2-
[[(3RS)-l,l-bis[4-[(methoxycarbonyl)amino]phenyl]-3-butyl)-
amino]ethanol (14.3 mg) was obtained from (1R)-1-[4-bezyloxy-
3-(methanesulfonylamino)phenyl]-2-[[(3RS)-l,l-bis[4-
CA 02341458 2001-02-21
57
[(methoxycarbonyl)amino]phenyl]-3-butyl]amino]ethanol (46.1
mg) by hydrogenation in the usual manner.
MS m/z: 601 (M++1)
Example 42
The following compound was obtained according to a
similar manner to that of Example 41.
(2R)-3-[4-Hydroxy-3-(methanesulfonylamino)phenyl]-1-
[(3RS)-1,1-bis(4-methoxyphenyl)-3-butyl]amino-2-propanol (5.0
mg)
MS m/z: 529 (M++1)
Example 43
A mixture of (R)-(4-benzyloxy-3-nitrophenyl)oxirane
(34.4 mg), N-benzyl-[3,3-bis[4-(methoxycarbonylamino)phenyl]-
propyl]amine (56.7 mg) and ethanol (2 ml) was heated under
reflux for 12 hours. Iron powder, ammonium chloride and
water was added to the reaction mixture and heating was
continued for 1 hour. The reaction mixture was filtrated and
worked up in the usual manner to give crude (1R)-1-(3-amino-
4-benzyloxyphenyl)-2-[N-benzyl-[3,3-bis[4-[(methoxy-
carbonyl)amino]phenyl]propyl]amino]ethanol (111.7 mg).
MS m/z: 689 (M++1)
Example 44
The following compound was obtained according to a
similar manner to that of Example 43.
(2S)-1-(3-Amino-4-benzyloxyphenoxy)-3-[N-benzyl-[3,3-
bis[4-[(methoxycarbonyl)amino]phenyl]propyl]amino]-2-propanol
MS m/z: 719
Example 45
Under nitrogen, to a solution of 4,4-bis(4-
CA 02341458 2001-02-21
58
methoxyphenyl)-2-butanone (187 mg) and (1RS)-2-amino-1-(2-
methylpyridin-6-yl)ethanol (100 mg) prepared from 6-
methylpyridin-2-carboxaldehyde and trimethylsilylcyanide
catalized with zinc iodide followed by reduction with lithium
aluminum hydride, in 1,2-dichloroethane (10 ml) was added
sodium triacetoxyborohydride (257 mg) at room temperatue, and
the mixture was stirred at the same temperature for 24 hours.
The resulting mixture was poured into saturated aqueous
sodium bicarbonate solution, and extracted with ethyl
acetate. The organic layer was washed with brine, dried over
magnesium sulfate, and evaporated in vacuo. The residue was
treated with 4N hydrogen chloride in 1,4-dioxane to afford
(1RS)-2-[[(1RS)-3,3-bis(4-methoxyphenyl)-1-methylpropyl]-
amino]-1-(6-methylpyridin-2-yl)ethanol (140 mg)
dihydrochloride.
MS m/z: 421 (M++1) (free)
Example 46
Under nitrogen, to a solution of (2S)-3-[4-benzyloxy-3-
(methanesulfonylamino)phenoxy]-1,2-epoxypropane (198 mg) and
N-benzyl-[(1RS)-3,3-bis(4-hydroxyphenyl)-1-methylpropyl]amine
(200 mg) in methanol (20 ml) was refluxed for 18 hours. The
resulting mixture was poured into saturated aqueous sodium
bicarbonate solution, and extracted with ethyl acetate. The
organic layer was washed with brine, dried over magnesium
sulfate, and evaporated in vacuo. The residue was
chromatographed (hexane-ethyl acetate) over silica gel to
afford N-[5-[(2S)-3-[N-benzyl-[(1RS)-3,3-bis(4-
hydroxyphenyl)-1-methylpropyl]amino]-2-hydroxypropoxy]-2-
(benzyloxy)phenyl]methanesulfonamide (120 mg).
MS m/z: 696 (M++1)
Example 47
Under nitrogen, to a solution of (2S)-3-[4-benzyloxy-3-
(methanesulfonylamino)phenoxy]-1,2-epoxypropane (200 mg) and
CA 02341458 2001-02-21
59
N-benzyl-[(IRS)-3,3-bis(4-methoxyphenyl)-1-methylpropyl]amine
(163 mg) in dichloromethane (10 ml) was added ytterbium(III)
trifluoromethanesulfonate (1.0 g) at room temperature, and
the mixture was stirred at the same temperature for 3 hours.
The resulting mixture was poured into saturated aqueous
sodium bicarbonate solution, arid extracted with ethyl
acetate. The organic layer was washed with brine, dried over
magnesium sulfate, and evaporated in vacuo. The residue was
chromatographed (hexane-ethyl acetate) over silica gel to
afford N- [5- [ (2S) -3- [N-benzyl- [ (1RS) -3, 3-bis (4-
methoxyphenyl)-1-methylpropyl]amino]-2-hydroxypropoxy]-2-
(benzyloxy)phenyl]methanesulfonamide (50 mg).
MS m/z: 724 (M++1)
_Example 48
Under nitrogen, to a solution of N-benzyl-[3,3-bis(4-
hydroxyphenyl)-1-methylpropyl]amine (300 mg) and
phenethyloxirane (130 mg) in a mixture of ethyl acetate (5
ml) and tetrahydrofuran (5 ml) was added ytterbium(III)
trifluoromethanesulfonate (110 mg) at room temperature, and
the mixture was stirred at the same temperature for 96 hours.
The resulting mixture was poured into saturated aqueous
sodium bicarbonate and extracted with ethyl acetate. The
organic layer was washed with brine, dried over anhydrous
magnesium sulfate, and evaporated in vacuo. The residue was
purified by column chromatography on silica gel
(chloroform: methanol ==20:1) to give 1-[N-benzyl-[3,3-bis(4-
hydroxyphenyl)-1-methylpropyl]amino]-4-phenyl-2-butanol (240
mg ) .
NMR (CDC13, d): 0.95-1.10 (3H, m), 1.45-2.9 (9H, m),
3.2-3.75 (3H, m), 3.75-3.9 (1H, m), 6.55-6.8 (4H,
m), 6.85-7.3 (14H, m)
Example 49
The following compound was obtained according to a
similar manner to that of Example 48.
CA 02341458 2001-02-21
(2S)-1-[N-Benzyl-[(1RS)-3,3-bis(4-hydroxyphenyl)-1-
methylpropyl]amino]-3-(phenylthio)-2-propanol
NMR (CDC13, ~): 0.85-1.1 (3H, m), 1.7-3.1 (7H, m), 3.3-
3.75 (3H, m), 3.75-3.9 (1H, m), 6.55-6.75 (4H, m),
5 6.8-7.25 (14H, m)
Example 50
Under nitrogen, to a solution of N-[5-[(1R)-2-[N-benzyl-
[(1RS)-3,3-bis(4-methoxyphenyl)-1-methylpropyl]amino]-1-
10 (triethylsilyloxy)ethyl]-2-(benzyloxy)phenyl]methane-
sulfonamide (221 mg) in tetrahydrofuran (3 ml) were added
acetic acid (63 ~tl) and tetrabutylammonium fluoride (1M
solution in tetrahydrofuran, 0.68 ml) at room temperature,
and the mixture was stirred at the same temperature for 4.5
15 hours. The resulting mixture was poured into saturated
aqueous sodium bicarbonate and extracted with ethyl acetate.
The organic layer~was washed with brine, dried over anhydrous
magnesium sulfate, and evaporated in vacuo. The residue was
purified by column chromatography on silica gel (hexane: ethyl
20 acetate = 2:1) to give N-[5-[(1R)-2-[N-benzyl-
[(1RS)-3,3-bis(4-methoxyphenyl)-1-methylpropyl]amino]-1-
hydroxyethyl]-2-(benzyloxy)phenyl]methanesulfonamide (164
mg ) .
NMR (CDC13, d) : 0. 95-1. 1 (3H, m) , 1.7-2. 85 (5H, m) ,
25 2.88 (3H, m), 3.35-4.05 (8H, m), 4.25-4.5 (1H, m),
5.08 (2H, m), 6.7-7.5 (21H, m)
Example 51
A mixture of N-[5-[(1R)-2-[N-benzyl-[(1RS)-3,3-bis(4-
30 methoxyphenyl)-1-methylpropyl]amino]-1-hydroxyethyl]-2-
(benzyloxy)phenyl]methanesulfonamide (149 mg) and 10°s
palladium on activated carbon (50°s wet, 50 mg) in methanol (5
ml) was stirred at room temperature in the presence of
hydrogen at an atmospheric pressure for 6 hours. After
35 filtration, the filtrate was evaporated in vacuo, followed by
CA 02341458 2001-02-21
61
treatment with 9N hydrogen chloride in ethyl acetate to give
N-[5-[(1R)-2-[(1RS)-3,3-bis(4-methoxyphenyl)-1-methylpropyl]-
amino-1-hydroxyethyl]-2-hydroxyphenyl]methanesulfonamide
hydrochloride (90 mg).
NMR (DMSO-d6, b): l.l-1.35 (3H, m), 1.9-2.2 (1H, m),
2.55-3.1 (7H, m), 3.70 (6H, m), 3.95-4.1 (1H, m),
4.7-4.9 (1H, m), 6.8-7.4 (11H, m)
Example 52
The following compounds were obtained according to a
similar manner to that of Example 51.
(1) 1-[3,3-Bis(4-hydroxyphenyl)-1-methylpropyl]amino-4-
phenyl-2-butanol hydrochloride
NMR (CD30D, ~): 1.1-1.5 (3H, m), 1.7-1.9 (2H, m), 1.95-
2.2 (1H, m), 2.45-3.2 (6H, m), 3.6-4.0 (1H, m),
6.5-6.8 (4H, m), 7.0-7.35 (9H, m)
(2) (2S)-1-Benzenesulfonyl-3-[(1RS)-3,3-bis(4-hydroxy-
phenyl)-1-methylpropyl]amino-2-propanol hydrochloride
NMR (CD30D, d): 1.25-1.4 (3H, m), 1.95-2.2 (1H, m),
2.45-2.7 (1H, m), 2.9-3.55 (5H, m), 3.85-4.0 (1H,
m), 9.25-4.4 (1H, m), 6.65-6.85 (4H, m), 7.05-7.2
(4H, m), 7.6-7.8 (3H, m), 7.95-8.05 (2H, m)
(3) (2S)-1-Phenoxy-3-[(3RS)-l,l-bis(4-ureidophenyl)-3-
butyl]amino-2-propanol hydrochloride
MS m/z: 492 (M++1) (free)
Example 53
Under nitrogen, a solution of [(1S)-3,3-bis(4-
methoxyphenyl)-1-methylpropyl]amine (0.55 g), N-[2-benzyloxy-
5-[(1R)-2-iodo-1-(triethylsilyloxy)ethyl]phenyl]-
methanesulfonamide (1.1 g) and N,N-diisopropylethylamine (1.4
ml) in N,N-dimethylacetamide (5 ml) was stirred at 110°C for
CA 02341458 2001-02-21
62
24 hours. The resulting mixture was poured into saturated
aqueous sodium bicarbonate and extracted with ethyl acetate.
The organic layer was washed successively with water and
brine, dried over anhydrous sodium sulfate and evaporated in
vacuo. Under nitrogen, to the residue in ethyl acetate (10
ml) was added 4N hydrogen chloride in ethyl acetate (2 ml) at
5°C, and the mixture was stirred at room temperature for 45
minutes. The resulting mixture was poured into saturated
aqueous sodium bicarbonate and extracted with ethyl acetate.
The organic layer was washed with brine, dried over anhydrous
magnesium sulfate and evaporated in vacuo. The residue was
purified by column chromatography on silica gel
(chloroform: methanol = 50:1 to 20:1) to give N-[2-benzyloxy-
5-[(1R)-2-[(1S)-3,3-bis(4-methoxyphenyl)-1-methylpropyl]-
amino-1-hydroxyethyl]phenyl]methanesulfonamide (0.65 g).
NMR (CDC13, ~): 1.09 (3H, d, J=6.3Hz), 1.85-2.3 (2H,
m), 2.35-2.6 (2H, m), 2.9-3.2 (4H, m), 3.76 (6H,
s), 4.0-4.1 (1H, m), 4.45-4.6 (1H, m), 5.10 (2H,
m), 6.82 (4H, d, J=8.lHz), 6.96 (1H, d, J=8.5Hz),
7.1-7.2 (5H, m), 7.35-7.5 (6H, m)
Example 54
The following compounds were obtained according to a
similar manner to that of Example 53.
(1) N-[2-Benzyloxy-5-[(1R)-2-[(1R)-3,3-bis(4-methoxyphenyl)-
1-methylpropyl]amino-1-hydroxyethyl]phenyl]-
methanesulfonamide
NMR (CDC13, b): 1.08 (3H, d, J=6.2Hz), 1.9-2.2 (2H, m),
2.5-2.85 (3H, m), 2.90 (3H, s), 3.76 (6H, s), 4.03
(1H, t, J=8.2Hz), 4.47 (1H, dd, J=3.6 and 8.5Hz),
5.10 (2H, s), 6.8-6.9 (4H, m), 6.96 (1H, d,
J=8.5Hz), 7.1-7.2 (5H, m), 7.35-7.5 (6H, m)
(2) N-[2-Benzyloxy-5-[(1R)-2-[(1RS)-3,3-bis(4-hydroxy-
CA 02341458 2001-02-21
63
phenyl)-1-methylpropyl]amino-1-hydroxyethyl]phenyl]-
methanesulfonamide
NMR (DMSO-d6, ~): 1.0-1.1 (3H, m), 1.7-1.95 (1H, m),
2.1-2.85 (4H, m), 2.90 (3H, s), 3.8-3.95 (1H, m),
4 .5-4. 6 (1H, m) , 5. 17 (2H, s) , 6. 6-6.75 (4H, m) ,
6.95-7.2 (6H, m), 7.25-7.6 (6H, m)
_Example 55
A mixture of N-(2-(benzyloxy)-5-[(1R)-2-[(1S)-3,3-bis(4-
methoxyphenyl)-1-methylpropyl]amino-1-hydroxyethyl]phenyl]-
methanesulfonamide (620 mg) and loo palladium on activated
carbon (50°s wet, 300 mg) in methanol (10 ml) was stirred at
room temperature in the presence of hydrogen at an
atmospheric pressure for 7.5 hours. After filtration, the
filtrate was evaporated in vacuo. The residue was purified
by column chromatography on silica gel (chloroform:methanol =
20:1 to 10:1), followed by treatment with 4N hydrogen
chloride in ethyl acetate to give N-[5-[(1R)-2-[(1S)-3,3-bis(
4-methoxyphenyl)-1-methylpropyl]amino-1-hydroxyethyl]-2-
hydroxyphenyl]methanesulfonamide hydrochloride (290 mg)
NMR (DMSO-d6, b): 1.15-1.4 (3H, m), 1.85-2.2 (1H, m),
2.4-3.2 (7H, m), 3.70 (6H, s), 3.95-4.1 (1H, m),
4.7-4.9 (1H, m), 6.7-7.4 (11H, m)
_Example 56
The following compounds were obtained according to a
similar manner to that of Example 54.
(1) N-[5-[(1R)-2-[(1R)-3,3-Bis(4-methoxyphenyl)-1-
methylpropyl]amino-1-hydroxyethyl]-2-
hydroxyphenyl]methanesulfonamide hydrochloride
NMR (DMSO-d6, b): 1.15-1.4 (3H, m), 1.9-2.15 (1H, m),
2.4-3.15 (7H, m), 3.70 (6H, m), 3.95-4.1 (1H, m),
4.75-4.9 (1H, m), 6.8-7.4 (11H, m)
CA 02341458 2001-02-21
64
(2) N-[5-[(1R)-2-[[3,3-Bis(4-hydroxyphenyl)-1-methylpropyl]-
amino]-1-hydroxyethyl]-2-hydroxyphenyl]-
methanesulfonamide hydrochloride
NMR (DMSO-d6, ~): 1.15-1.3 (3H, m), 1.85-2.1 (1H, m),
2.55-3.2 (7H, m), 3.8-4.0 (1H, m), 4.7-4.9 (1H, m),
6.6-6.75 (4H, m), 6.9-7.3 (7H, m)
(3) (2S)-1-(6-Aminopyridin-3-yloxy)-3-[[(1RS)-3,3-bis(4-
methoxyphenyl)-1-methylpropyl]amino]-2-propanol
trihydrochloride, starting from the objective compound
of Example 57.
NMR (DMSO-d6, d): 1.05-1.4 (3H, m), 1.9-2.2 (1H, m),
2.5-3.2 (4H, m), 3.55-3.85 (7H, m), 3.85-4.3 (3H,
m), 6.9-7.4 (9H, m), 7.5-7.9 (2H, m)
Example 57
A mixture of [3,3-bis(4-methoxyphenyl)-1-methylpropyl]-
amine and (2S)-3-[2-(benzyloxycarbonylamino)pyridin-5-yloxy]-
1,2-epoxypropane (98 mg) in methanol (5 ml) was refluxed for
19 hours. After removal of the solvent in vacuo, the residue
was purified by column chromatography on silica gel
(chloroform:methanol = 30:1 to 20:1) to give [5-[(2S)-3-
[(1RS)-3,3-bis(4-methoxyphenyl)-1-methylpropyl]amino-2-
hydroxypropoxy]pyridin-2-yl]carbamic acid benzyl ester (110
mg).
NMR (CDC13, b): 1.1-1.2 (3H, m), 1..7-2.3 (2H, m), 2.45-
2.6 (2H, m), 2.7-2.75 (1H, m), 3.76 (6H, s), 3.85-
3.95 (3H, m), 4.0-4.1 (1H, m), 5.22 (2H, s), 6.8
(4H, d, J=8.6Hz), 7.1-7.45 (lOH, m), 7.9-7.95 (2H,
m)
Example 58
To a solution of (2S)-1-[N-benzyl-[(1RS)-3,3-bis(4-
hydroxyphenyl)-1-methylpropyl]amino]-3-phenylthio-2-propanol
(300 mg) in methanol (10 ml) was added OXONE~ (potassium
CA 02341458 2001-02-21
peroxymonosulfate) (710 mg) in water (2 ml) at room
temperature, and the mixture was stirred at the same
temperature for 4 hours. The resulting mixture was poured
into a mixture of ethyl acetate and water, and was made basic
5 with saturated aqueous sodium bicarbonate. After separation,
the organic layer was washed with brine, dried over anhydrous
magnesium sulfate and evaporated in vacuo. The residue was
purified by column chromatography on silica gel
(chloroform: methanol = 20:1) to give (2S)-1-benzenesulfonyl-
10 3-[N-benzyl-[(1RS)-3,3-bis(4-hydroxyphenyl)-1-methylpropyl]am
ino]-2-propanol (220 mg).
NMR (CDC13, ~): 0.9-1.1 (3H, m), 1.75-2.3 (2H, m),
2.35-2.7 (3H, m), 2.9-3.25 (2H, m), 3.3-4.0 (4H,
m), 6.65-6.8 (4H, m), 6.9-7.35 (9H, m), 7.5-7.7
15 (3H, m), 7.75-7.9 (2H, m)
Example 59
A mixture of (2S)-1-phenoxy-3-[N-benzyl-[3,3-bis[4-
(methoxycarbonyl)phenyl]propyl]amino]-2-propanol (103 mg),
20 methanol (2 ml), 1,4-dioxane (2 ml) and 1N aqueous sodium
hydroxide solution (1 ml) was stirred at 50°C for 2 hours.
The reaction mixture was acidified with 3N hydrochloric acid
(1 ml) and worked up in a usual manner to give (2S)-1-
phenoxy-3-[N-benzyl-[3,3-bis(4-carboxyphenyl)propyl]amino]-2-
25 propanol (75.1 mg).
Example 60
A mixture of (2S)-1-phenoxy-3-[N-benzyl-[3,3-bis(4-
carboxyphenyl)propyl]amino]-2-propanol (75 mg),
30 diphenylphosphoryl azide (96 mg), triethylamine (58 ~l),
toluene (1 ml) and 1,4-dioxane (1 ml) was stirred at 50°C for
0.5 hour, then at 100°C for 45 minutes. Methanol (1 ml) was
added to the reaction mixture, and the heating was continued
for 15 hours. The reaction mixture was worked up in a usual
35 manner followed by purification by preparative thin-layer
CA 02341458 2001-02-21
66
chromatography to afford (2S)-1-phenoxy-3-[N-benzyl-
[3,3-bis[4-[(methoxycarbonyl)amino]phenyl]propyl]amino]-2-
propanol (21.5 mg).
MS m/z: 598 (M++1)
_Example 61
(2S)-1-Phenoxy-3-[N-benzyl-[3,3-bis[4-[(methoxy-
carbonyl)amino]phenyl]propyl]amino]-2-propanol (18.8 mg) was
hydrogenated in a usual manner to give (2S)-1-phenoxy-3-[[3,
3-bis[4-[(methoxycarbonyl)amino]phenyl]propyl]amino]-2-
propanol (8.1 mg).
IR (KBr): 1710 (s), 1601 (m), 1537 (s), 1315 (w),
1238 (s), 1070 (m) cm-1
NMR (MeOH-d4, d): 2.2-2.3 (2H, m), 2.6-2.9 (4H, m),
3.72 (6H, s), 3.9-4.1 (4H, m), 6.9-7.0 (3H, m),
7.2-7.4 (lOH, m)
MS m/z: 508 (M++1)
25
35