Note: Descriptions are shown in the official language in which they were submitted.
CA 02342248 2001-02-28
WO 00/12672 PCT/US99/15514
Title: FOAMING DRAIN CLEANER
BACKGROUND OF THE INVENTION
1. Field of The Invention
The present invention relates to foaming cleaning compositions, and in
particular to
an in-situ foaming cleaning composition incorporating a bleach and which is
formulated to have utility as a drain cleaner, or as a hard surface cleaner.
2. Descr~tion of Related Art
United States Patent 5,084,546 to Hall discloses a personal care product,
specifically a foaming shower gel. As such, the foam-generating components
must
be biologically compatible. Accordingly, Hall teaches only citric acid and a
carbonate or bicarbonate to generate carbon dioxide gas. Nothing is mentioned
which would suggest a peroxide/hypochlorite system, nor is use in surface
cleaning
or drain opening disclosed. Published Japanese applications to Ishimatsu et al
JP
59-24798 and JP 60-32497; JP 59-164399, to Miyano et al; and Sakuma, JP
57-74379 all disclose, describe and claim a binary foaming cleaner having
utility as
a drain opener. Miyano et al specifically describes the advantages of a foam
in
drain opening. Ishimatsu_et al and Miyano et al both describe an aqueous
peroxide
solution containing 0.25-25% active, paired with an aqueous solution of 0.25-
6%
hypochlorite, and both references teach the inclusion of surfactants with
either or
both solutions to enhance foaming. None of these references, however, teach,
suggest or disclose a thickened formulation, nor any of the advantages and
foam
characteristics associated therewith.
-1-
CA 02342248 2001-02-28
WO 00/12672 PCT/US99/15514
A hypochlorite composition paired with a chelating agent/builder solution in a
dual
chamber container is disclosed in U.S. Pat. No. 5,767,055 to Choy et al.
Drain cleaners of the art have been formulated with a variety of actives in an
effort
to remove the variety of materials which can cause clogging or restriction of
drains.
Such actives may include acids, bases, enzymes, solvents, reducing agents,
oxidants
and thioorganic compounds. Tobiason, U.S. 5,264,146, Steer, et al, U.S:
5,630,833
and Taylor, Jr. et al., U.S. 4,664,836 all disclose dry compounds which
generate
foam when mixed with water in a drain. Kuehn, U.S. 4,691,710 describes a dry
in-sink garbage disposal cleaning composition which uses adipic acid and
sodium
bicarbonate to generate gas upon contact with water. This composition requires
mechanical shearing from the disposal to assist in foam generation. Davis,
U.S.
4,206.068 describes an exothermic drain opening composition comprising an
1 S oxidant and a reducing agent in a compartmentalized container.
SUMM,A.RY OF THE PRESENT INVENTION
In view of the prior art, there remains a need for a foam generating cleaning
composition capable of delivering a high percentage of active and possessing a
long
contact time on non-horizontal surfaces. There further remains a need for an
in-situ
foam-generating composition which is stable during storage and can be
economically formulated.
It is another object of the present invention to provide a composition capable
of
forming an active-carrying foam in-situ
It is another object of the present invention to provide a composition capable
of
generating a stable foaming active cleaner.
-2-
CA 02342248 2001-02-28
WO 00/I2672 PCTIUS99115514
it is another object of the present invention to provide a dual component
composition and containment means which isolates each component during
storage.
It is another object to provide a drain opening composition which is
formulated to
S be safe to store and use.
It is another object of the present invention to provide a composition capable
of
producing an active-containing foam which can reach all affected parts of a
drain.
It is another object of the present invention to provide a foaming cleaning
composition having utility as a drain cleaner by virtue of a viscoelastic
rheology.
It is yet another object of the present invention to provide a drain cleaning
composition which is highly effective.
1S
It is yet another object of the present invention to provide a cleaning
composition
which is stable during normal storage, and at elevated or very low
temperatures.
It is yet another object of the present invention to provide a composition
having a
viscoelastic rheology and a long relaxation time to provide beneficial flow
properties during dispensing.
More specifically, the composition is a product of two liquids which are
separately
maintained prior to forming an admixture during delivery to a surface to be
treated,
2S whereupon the admixture generates a foam sufficient for cleaning efficacy
and
stability. A first liquid includes an oxidant, preferably a hypohalite or a
hypohalite
generating agent (hereinafter "hypohalite") and a second liquid includes a gas
generating agent, preferably a peroxygen containing or releasing agent. As the
two
liquids are initially separated, the oxidant can be maintained in an
environment free
of gas generating agent and otherwise conducive to their cleaning activity and
-3-
CA 02342248 2001-02-28
WO 00/12692 PCTIUS99/15514
stability up to the time of use. When the two liquids are allowed to mix, for
example, by simultaneously pouring into a drain, the hypohalite and peroxygen
react to liberate oxygen gas in accordance with the following reaction
equation:
NaOCI + HZOZ -~ OZ(g) + NaCI + Hz0
The liberated gas contacts surfactant in the solution, creating foam which
expands to
completely fill the drain pipe. The expanded foam contains an excess of the
hypohalite, which acts to clean the drain. The resulting foam is stable, and
preferably characterized by a density of greater than about 0.1 g/ml, a
percentage
active of about 0.5 - 15, a half life of greater than about 30 minutes; a
volume of
greater than about 500 ml; and an initial foam development rate of about 10-50
ml/sec. Foam stability is defined as the foam's resistance to a force tending
to
collapse or displace the foam. The foam is further characterized by a ratio of
foam:liquid of at least l:I, preferably 2:1, more preferably 3:1; and a foam
height
sufficient to yield a greater than twelve cm. column in the drain (as measured
from
the center, or lowest point of the P-trap, and for a 3.2 cm. diameter drain),
more
preferably greater than seventeen cm. and most preferably seventeen to thirty-
one
cm. Most preferred in terms of foam volume and height in the drain, is an
amount
sufficient to reach the drain's stopper mechanism, a site of frequent hair
and/or soap
contamination. Such stopper mechanisms are typically positioned about twenty
cm.
up the vertical pipe. The foam would preferably contain greater than 0.1 %
active,
more preferably greater than 0.5% active, and most preferably between about
0.75
and 3% active. An active contact time, or foam half life, should be between
0.5 and
4.0 hours preferably between 1 and 8 hours. Foam half life is the time elapsed
between maximum foam volume development and a 50% volume reduction thereof,
absent any external forces (other than gravity) acting upon the foam. Further,
the
foam is self generating, produced by reaction of composition components, and
requires no mechanical agitation or other forms of physical activation.
-4-
CA 02342248 2001-02-28
WO 00/12672 PCT/US99115514
In a preferred embodiment of the present invention, either or both of the
liquids
include a thickening agent or system, present in an amount such that when the
liquids form an admixture during delivery to a surface, the admixture results
in a
dense, stable foam sufficient for cleaning efficacy and stability. Thus, when
the
initially separated liquids are allowed to interact, the resulting liquid
cleaning
composition being delivered to the surface will have the cleaning or bleaching
activity and stability appropriate for the cleaning or bleaching of that
surface. The
term "liquid" as used herein may include homogeneous liquids, solutions and
suspensions. Preferably an aqueous liquid is contemplated; however, nonaqueous
liquids are within the scope of the invention. The thickening agent or system
should
impart both a viscous component and an elastic component to the corresponding
liquid; both components are most preferred for attaining the desired foam
characteristics. Most preferably the thickening agent or system imparts a
viscoelastic rheology to the corresponding liquid; however, thickened,
nonelastic or
1 S slightly elastic systems can provide performance benefits and are thus
within the
scope of the present invention. The composition of the thickening system is
less
important than the attainment of at least one of the desired foam qualities as
defined
herein.
The present invention also relates to a container which maintains the two
liquids
separately until delivery and provides for such delivery, during which the
pH-maintained admixture is formed and delivered to a surface to be treated.
The
container includes one compartment for the hypohalite containing liquid and
another compartment for the peroxygen-containing liquid. Either or both of
these
two compartments may contain the thickening system or agent which, is present
in
an amount sufficient to thicken and for stability of the liquid, as described
above.
According to one aspect of the invention, the container may have separate
delivery
channels for the two liquid components for delivering the two liquids,
whereupon
the admixture is formed. These delivery channels may be constructed to provide
for
the contemporaneous delivery of the two liquids to the exterior of the
container,
-5-
CA 02342248 2004-05-13
whereupon the two liquids meet to form the admixture. Alternately, the
separate
delivery channels may communicate with an admixing space in which the two
liquids form the admixture and from which the admixture is delivered to the
exterior of the container. One example of such a container is that disclosed
in U.S.
Pat. 5,767,055 Choy et al.
The present invention further includes a method of cleaning drains which
comprises
the step of:
pouring into a drain at least one liquid which generates foam in situ, the
foam
characterized by a volume of at least 1.0 times the liquid volume; a density
of at
least about O. l g/ml, a half life of greater than about thirty minutes, and
wherein the
foam contains a cleaning-effective amount of a drain cleaning active. It is
also
within the scope of the present invention to provide a single solution capable
of
generating the foam upon release from its container, as by pouring into the
drain.
Briefly, a first embodiment of the present invention comprises a stable
cleaning
composition comprising, in aqueous solution:
(a) a first liquid containing an oxidizing agent; and
(b) a second liquid containing a gas generating agent; and wherein
the oxidizing agent and gas generating agent react to generate a foam
characterized by a density of at least about 0.1 g/ml, a volume of at
least 1.0 times the liquid volume, a half life of greater than about
thirty minutes, and wherein the foam contains a cleaning-effective
amount of a drain cleaning active.
It should be noted that as used herein the term "cleaning" refers generally to
a
chemical, physical or enzymatic treatment resulting in the reduction or
removal of
unwanted material, and "cleaning composition" specifically includes drain
openers,
hard surface cleaners and bleaching compositions. The cleaning composition may
-6-
CA 02342248 2004-05-13
consist of a variety of chemically, physically or enzymatically reactive
active
ingredients, including solvents, acids, bases, oxidants, reducing agents,
enzymes,
detergents and thioorganic compounds. Unless otherwise specified, all
ingredient
percentages are weight percentages.
For purposes of the discussion of the invention disclosed herein, a typical
household
sink drain comprises four sections: a vertical section, thence to a U-bend (or
P-trap),
thence to a 90-degree elbow, and finally a horizontal sewer arm.
A viscous rheology, preferably one with an elastic component, most preferably
a
viscoelastic rheology, may be imparted to a single liquid, or to both liquids
of the
composition, preferably by a binary system including a betaine or sulfobetaine
having a C,4.,g alkyl group, or a C,o_,a alkylamino or alkylamido group, and
an
anionic organic counterion that is thought to promote elongated micelles. Such
systems are more fully described in U.S. 4,900,467 and 5,389,157 to Smith, and
assigned to the assignee of the invention herein. Preferably the betaine is a
C,4_,8
alkyl betaine and the counterion is a CZ_~ alkyl carboxylate, aryl
carboxylate, CZ_,~
alkyl sulfonate, aryl sulfonate, sulphated aryl or CZ_,o alkyl alcohols, and
mixtures
thereof. Most preferably the counterion is an aryl sulfonate, e.g. sodium
xylene
sulfonate. The counterion may include substituents which are chemically stable
with the active cleaning compound. Preferably, the substituents are alkyl or
alkoxy
groups of 1-4 carbons, halogens and nitro groups, all of which are stable with
most
actives, including hypochlorite. The viscosity of the formulations of the
present
invention can range from slightly greater than that of water, to several
thousand
centipoise (cP). Preferred from a consumer standpoint is a viscosity range of
about
20 cP to 2500 cP. A preferred viscosity range for the first (oxidant-
containing)
liquid is about 100 to 2500 cP, more preferred is 500 to 2200 cP. A preferred
viscosity for the second (gas generating) liquid is about 50-1000 cP, more
preferred is 100 - 800 cP.
_7_
CA 02342248 2001-02-28
WO 00112672 PCTIUS99/15514
A second embodiment of the present invention is a composition and method for
cleaning drains, the composition comprising separately maintained aqueous
solutions of:
(a) a first liquid comprising a hypohalite compound; and;
(b) a second liquid comprising a peroxygen compound; and wherein
at least one of liquids (a) or (b) is viscous.
The liquids (a) and {b} are maintained separately during storage, and combined
concurrently with, or immediately prior to use. Preferably, the liquids (a)
and (b)
are maintained in a dual chamber or compartment bottle, and poured
simultaneously
into the drain wherein the foam generation occurs. The resulting foam is
stable and
dense, and contains a high percentage of cleaning active, especially
hypohalite,
which coats the vertical and upper P-trap portions of a drain. The rheology of
each
composition provides a favorable rate of foam generation and residence time,
resulting in excellent cleaning efficacy. The rate of foam generation should
be
relatively slow, preferably less than about SO ml/sec and the foam should
remain
stable for an extended period of time. The rheology also facilitates filling
of the
container, e.g., during manufacturing, and affords consumer-acceptable pouring
properties during dispensing and use. The preferred viscoelastic rheology may
be
imparted by a thickener, preferably a surfactant thickener. While only one
solution
may be viscoelastic, it is preferred that both are viscoelastic, and the same
or
different thickening agents or systems can -be used. Most preferably
viscoelasticity
is imparted to both liquids (a) and (b) by the same thickening agent or
system.
It is therefore an advantage of the present invention that the composition is
chemically and phase-stable, and retains such stability at both high and low
temperatures.
It is another advantage of the present invention that, when formulated as a
drain
cleaner the foaming composition provides an elevated contact time, improving
the
efficacy of the cleaner.
-g_
CA 02342248 2004-05-13
It is another advantage of the present invention that the improved efficacy
resulting from the increased
contact time allows for safer drain cleaning formulations.
It is yet another advantage of the present invention that the composition
generates a stable, ;active-
containing foam in situ.
It is a further advantage of the composition of the present invention that the
rheology of the
composition facilitates container filling, and dispensing.
In another aspect, the present invention provides a composition for cleaning
comprising:
(a) a first aqueous liquid comprising an oxidant; and
(b) a second aqueous liquid comprising a gas-generating agent;
wherein at least one of the first and second liquids includes a betaine
surfactant and an aryl sulfonate surfactant, wherein at least one of the
liquids is
viscoelastic; and
wherein the first and second aqueous liquids are separately maintained prior
to forming an admixture during delivery to a surface to be treated, whereupon
the
admixture generates a foam sufficient for cleaning efficacy and stability.
In another aspect, the present invention provides a composition for cleaning
comprising:
(a) a first thickened liquid comprising an oxidant; and
(b) a second thickened liquid comprising a gas-generating agent;
wherein both of the first and second liquids include a betaine surfactant and
an aryl sulfonate surfactant, wherein both liquids are viscoelastic; and
wherein the first and second thickened liquids are separately maintained prior
to
forming an admixture during delivery to a surface to be treated, whereupon the
admixture
generates a foam sufficient for cleaning efficacy and stability.
In another aspect, the present invention provides a composition for cleaning
comprising:
(a) a first thickened liquid comprising an oxidant; and
(b) a second thickened liquid comprising a gas-generating agent;
-9-
CA 02342248 2004-05-13
wherein the first and second aqueous liquids are separately maintained prior
to
forming an admixture during delivery to a surface to be treated, whereupon the
admixture
generates a foam sufficient for cleaning efficacy and stability; and
wherein an initial phase of foam is generated at an initial rate of about 10-
50 ml/sec,
said initial phase lasting no longer than about 60 seconds.
In another aspect, the present invention provides a composition for cleaning
comprising:
(a) a first thickened liquid comprising an oxidant; and
(b) a second thickened liquid comprising a gas-generating agent,
wherein the first and second aqueous liquids are separately maintained prior
to
forming an admixture during delivery to a surface to be treated, whereupon the
admixture
generates a foam sufficient for cleaning efficacy and stability, and
wherein at least one of the first and second liquids is characterized by a
rheology
wherein a shear viscosity is at about 50-2500 cP, a relative elasticity is at
about 1-300 seclPa
and a relaxation time is at least about 0.1 seconds.
In another aspect, the present invention provides an in situ foaming drain
cleaner comprising:
(a) a first aqueous liquid, having a viscosity of at least about 100 cP, a
relative elasticity
of at least about 1 sec/Pa and a relaxation time of at least about 0.3 sec,
the first liquid
comprising an oxidant, and a surfactant;
(b) a second aqueous liquid, having a viscosity of at least about 50 cP, a
relative elasticity
of at least about 1 sec/Pa and a relaxation time of at least about 0.1 sec,
the second
liquid comprising a gas-generating agent and a surfactant; and
wherein the second aqueous liquid is denser than the first aqueous liquid and
the first
and second aqueous liquids are disposed in a dual chamber container such that
they are
separately maintained prior to forming an admixture during delivery to a drain
to be treated,
whereupon the admixture generates a foam sufficient for cleaning efficacy and
stability.
In another aspect, the present provides a method for clearing restrictions
caused by organic mal:erials
in drain pipes comprising:
(a) introducing a first thickened liquid comprising an oxidant and a second
thickened
liquid comprising a gas-generating agent into a drain where the liquids
generate foam
-9a-
CA 02342248 2004-05-13
in situ, the foam characterized by a density of at least about 0.1 g/ml, a
volume of at
least 1.0 times the liquid volume, and a half life of greater than about
thirty minutes,
and wherein the foam contains a cleaning-effective amount of a drain cleaning
active;
and
(b) allowing the composition to remain in contact with the organic restriction
material to
react therewith.
In another aspect, the present invention provides an in situ foaming drain
cleaner comprising:
(a) a first aqueous liquid, having a viscosity of at least about 100 cP and a
relaxation time
of at least about 0.1 seconds, said first liquid comprising an oxidant; and
(b) a second aqueous liquid, the second liquid comprising a gas-generating
agent;
wherein said first and second aqueous liquids are disposed in a dual chamber
container such that they are separately maintained prior to forming an
admixture .during
delivery to a drain to be treated, whereupon the admixture generates a foam
sufficient for
cleaning efficacy and stability;
wherein an initial phase of foam is generated at an initial rate of at least
about 25
ml/sec, said initial phase lasting no longer than about 60 seconds;
wherein said foam is characterized by a density of at least about 0.1 g/ml, a
volume of a.t least
1.0 times the liquid volume, and a half life of greater than about thirty
minutes; and
wherein the oxidant is present in a molar excess over the gas-generating agent
in a
range of about 5:1 to 2:1, wherein said excess oxidant acts as a drain-opening
active.
These and other objects and advantages of the present invention will no doubt
become apparent to
those skilled in the art after reading the following Detailed Description of
the Preferred Embodin-~ents.
DESCRIPTION OF THE DRAWING
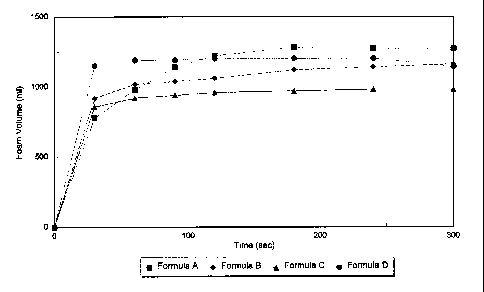
Fig. 1 is a graph comparing foam generation rates of a composition of the
present invention 'to the
other compositions.
-9b-
CA 02342248 2004-05-13
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Oxidizing Agent
The oxidizing agent, or oxidant, may preferably be selected from various
hypohalite-producing
species, for example, halogen bleaches selected from the group consisting of
the alkali metal and
alkaline earth salts of hypohalite, haloamines, haloimines, haloimides and
haloamides. All of the°se are
believed to produce hypohalous bleaching species in situ. Preferably, the
first oxidizing agent is a
hypohalite or a hypohalite generator capable of generating hypohalous
bleaching species. As used
herein, the term "hypohalite" is used to describe both a hypohalite or a
hypohalite generator, unless
otherwise indicated. Hypochlorite and compounds producing hypochlorite in
aqueous solution are
preferred, although hypobromite is also suitable. Representative hypochlorite-
producing compounds
-9c-
v
CA 02342248 2004-05-13
include sodium, potassium, lithium and calcium hypochlorite, chlorinated
trisodium
phosphate dodecahydrate, potassium and sodium dicholoroisocyanurate and
trichlorocyanuric acid. Organic bleach sources suitable for use include
heterocyclic
N-bromo and N-chloro imides such as trichlorocyanuric and tribromo- cyanotic
acid, dibromo- and dichlorocyanuric acid, and potassium and sodium salts
thereof,
N-brominated and N-chlorinated succinimide, malonimide, phthalimide and
naphthalimide. Also suitable are hydantoins, such as dibromo and dichloro
dimethyl- hydantoin, chlorobromodimethyl hydantoin, N-chlorosulfamide
(haloamide) and chloramine (haloamine). Particularly preferred in this
invention is
sodium hypochlorite having the chemical formula NaOCI, in an amount ranging
from about 0.1 weight percent to about IS weight percent of the first liquid,
more
preferably about 0.1 to 10 weight percent, and most preferably about 1 to 8
weight
percent. The oxidizing agent may be present in an stoichiometric amount to the
gas
generating agent for the generation of foam. If so, it is preferred that a
separate
cleaning active be included with either or both the first and second liquids.
More
preferred is that the oxidizing agent be present in a stoichiometric excess,
to both
generate foam and provide cleaning and drain opening activity.
Gas Generating Agent
The gas generating agent is a compound which can react with the oxidizing
agent to
generate a gas and is preferably a peroxide or peroxide-generator, such as
hydrogen
peroxide, or a peracid or persalt, including both organic and inorganic
peracids and
persalts, such as peracetic acid and monoperoxysulfate, respectively. A number
of
peroxides, peracids and persalts are disclosed in U.S. Patent No. 4,964,870,
to
Fong, et al. Hydrogen peroxide is normally supplied as a liquid, although
other
hydrogen peroxide sources may also function satisfactorily. For example,
perborate and percarbonate also supply HZOz in solution. The gas generating
agent
is present in an amount of about 0.01 to 8 weight percent of the second
liquid,
preferably about 0.1 to 5 weight percent, more preferably about 0.2 to 3
weight
percent.
- 10 -
CA 02342248 2001-02-28
WO 00/12672 PCTlUS99115514
Where peroxide is the gas generating agent and a hypohalite is the oxidizing
agent,
a preferred weight ratio (to provide a stoichiometric excess) of hypohalite to
peroxide is about 20:1 to 3:1, more preferred is about 15:1 to 7:1, and most
preferred is 12:1 to 5.1. A preferred mole ratio (to provide a stoichiometric
excess)
of hypohalite to peroxide is about 10:1 to 1:1, more preferred is about 7:1 to
5:4,
and most preferred is about 5:1 to 2:1.
ElectrolyteBuffer
An electrolyte/buffer may be included with either the first or second liquids
and
preferably is included in the first, oxidant-containing liquid in a buffering-
effective
amount.
According to the present invention, suitable electrolytes/buffers may be
selected
from the group consisting of a carbonate, a phosphate, a pyrophosphate, an
amino
carboxylate, a polycarboxylate, a polyacrylate, a phosphonate, an amino
phosphonate, a polyphosphonate, a salt thereof, and a mixture thereof. The
electrolyte/buffer is present in an amount ranging from 0 to about 5 weight
percent
of the first liquid, preferably from about 0.01 to about 4 weight percent of
the first
liquid.
pH Adjusting Agents
A pH-adjusting agent may be present in either one or both of the two liquids,
i.e.,
with the oxidant and/or gas generating agent. According to the present
invention,
the pH-adjusting agent maintains the pH of the liquid such that the active
agent
therein is stable and efficacious. The pH adjusting agent can be either
alkaline or
acidic in solution, and correspondingly serve to adjust and/or maintain either
solution to an alkaline or acidic pH. In the present invention, each solution
is
maintained at a pH which is appropriate for the activity and stability of the
oxidizing or gas generating agent and/or cleaning active therein. For an
alkaline
- 11 -
CA 02342248 2001-02-28
WO 00112672 ... X50.26 PCT/US99/15514
oxidizing agent, such as a hypohalite, the solution pH is alkaline. When the
gas
generating agent is peroxygen, the pH is acidic. The pH-adjusting agent may be
present in a pH adjusting effective amount, such as between about 0 and about
10
weight percent of one of the liquids.
S
Suitable acidic pH adjusting agents include: organic acids, especially
carboxylic
acids such as citric, giycolic, or acetic acids, weak inorganic acids such as
boric acid
or sodium bisulfate, and dilute solutions of strong inorganic acids such as
sulfuric
acid, hydrochloric acid, pyrophosphates, triphosphates, tetraphosphates,
silicates,
metasilicates, polysilicates and borates and mixture of the foregoing. When
the
gas-generating agent is peroxide, a preferred acidic pH adjusting agent is
sulfuric
acid. For a peroxygen-containing liquid, especially hydrogen peroxide, it is
preferred the pH be maintained below about 7, more preferably between 3 and 6
to
maintain stability and efficacy of the peroxygen compound. An acidic
1 S pH-adjusting agent is present in an amount of from 0 to 5 weight percent
to the
second liquid, preferably from 0.001 to 2 weight percent.
Preferred alkaline pH adjusting agents include: carbonates, bicarbonates,
hydroxides, hydroxide generators and mixtures of same. When the oxidant is a
hypohalite, a preferred alkaline pH-adjusting agent is an alkali metal
hydroxide,
especially sodium hydroxide. For example, when a hypohalite oxidizing agent is
used, the pH of the solution is preferably maintained at above about 10,
preferably
above about 10.5, and more preferably above about 11, An solution pH of above
about 11 is believed to be sufficient for both the cleaning efficacy and the
stability
of hypohalite. More particularly, this solution pH is believed to be
sufficient to
protect against the autocataiytic destruction of the hypohalite that might
otherwise
occur when the solution is formed. An alkaline pH-adjusting agent is present
in an
amount of from 0 to 20 weight percent, to the first liquid, preferably from
0.1 to 1 S
weight percent.
- 12 -
CA 02342248 2004-05-13
THICKENER
Either or both of the first oxidant and second gas-generating solutions or
liquids is
thickened, preferably with a surfactant thickener. Suitable thickener are as
described in previously referenced Smith patents. Other suitable systems may
be
found in the disclosures of U.S. 5,055,219 and U.S. 5,011,538 to Smith; U.S.
5,462,689 and U.S. 5,728,665 to Choy, et al., all commonly owned with the
invention herein. Additional thickeners such as polymers and gums are suitable
as
long as the desired foam characteristics andlor rheology is attained. Most
preferred is a binary surfactant viscoelastic thickener comprising a betaine
and
anionic counterion.
Betaine
Operative betaines include the C,4_,e alkyl betaines and C,0. ,a alkyl
sulfobetaines.
Especially preferred is a cetyl dimethyl betaine (CEDB) such as Amphosol CDB
(a
trademarked product of the Stepan Company), which is about 95% or greater C,6,
less than 5% C,v" and less than 1% C,a. It is noted that when referring to
carbon
chain lengths of the betaine or any other compound herein, the commercial,
polydisperse forms are contemplated (but not required). Thus, a given chain
length
within the preferred C"_" range will be predominately, but not exclusively,
the
specified length. As used herein in reference to the betaine or sulfobetaine,
the term
"alkyl" includes both saturated and unsaturated groups. Fully saturated alkyl
groups
are preferred in the presence of hypochlorite. C,0." alkylamido and alkylamino
betaines, and sulfobetaines having C,~,a alkyl, or C,o.,a alkylamino or
alkylamido
groups, are also suitable for use in the compositions of the present
invention.
The betaine is added at levels, which, when combined with the counterion, are
thickening effective. Generally about 0.1 to 10.0 weight percent of the
betaine is
utilized per each of the first and/or second liquid, preferred is to use about
0.1 to
5.0% betaine, and most preferred is about 0.15-2.0 percent betaine.
- 13 -
CA 02342248 2001-02-28
WO 00/12672 PCTIUS99/I5514
Counterion
The counterion is an anionic organic counterion selected from the group
consisting
of CZ_6 alkyl carboxylates, aryl carboxylates, CZ_,o alkyl sulfonates, aryl
sulfonates,
sulfated CZ_~o alkyl alcohols, sulfated aryl alcohols, and mixtures thereof.
The aryl
compounds are derived from benzene or napthalene and may be substituted or
not.
The alkyls may be branched or straight chain, and preferred are those having
two to
eight carbon atoms. The counterions may be added in acid form and converted to
the anionic form in situ, or may be added in anionic form. Suitable
substituents for
the alkyls or aryls are C,_4 alkyl or alkoxy groups, halogens, nitro groups,
and
mixtures thereof. Substituents such as hydroxy or amine groups are suitable
for use
with some non-hypochlorite cleaning actives, such as solvents, surfactants and
enzymes. If present, a substituent may be in any position on the rings. If
benzene is
used, the para (4) and meta (3) positions are preferred. In some circumstances
the
cleaning active itself may be within the class of thickening-effective
counterions.
For example, some carboxylic acid cleaning actives may be present in both the
acid
and conjugate base forms, the latter which could serve as the counterion. The
CZ_6
alkyl carboxylates may act in this manner. The counterion is added in an
amount
sufficient to thicken and result in a viscoelastic rheology, and preferably
between
about 0.01 to 10 weight percent. A preferred mole ratio of betaine to
counterion
depends on the chain length and concentration of the betaine, type of
counterion,
and the ionic strength of the solution, as well as whether the primary object
of the
composition is phase stability or viscosity. Using CEDB and sodium xylene
sulfonate (SXS), a preferred mole ratio is about 10:1 to 1:3, and more
preferred is
about 2:1 to 1:2. A preferred weight ratio of CEDB to SXS is about 15:1 to
1:2,
and more preferred is 3:1 to 1:1.
The viscoelasticity of the thickener advantageously imparts unusual flow
properties
to the cleaning composition. Elasticity causes the stream to break apart and
snap
back into the bottle at the end of pouring instead of forming syrupy
streamers.
Further, elastic fluids appear more viscous than their viscosity indicates.
The
- 14 -
CA 02342248 2001-02-28
WO 00/12672 PCT/US99/I5514
viscoelastic properties of a fluid can be measured with instruments such as a
Bohlin
VOR rheometer. A frequency sweep with a Bohlin rheometer can produce
oscillation data which, when applied to a Maxwell model, result in parameters
such
as relaxation time (Tau) and static shear modulus (GO). The relaxation times
of the
formulations of the present invention are between about 0.1-50 seconds,
preferably
between about 0.3-45 seconds more preferably between about 1-30 seconds and
most preferably between about 5-25 seconds. The ratio of relaxation time to
static
shear modulus (Tau/GO), previously defined as relative elasticity by Smith,
should
be between about 1-300 sec/Pascal (Pa,) preferred between about S-150 sec/Pa.,
and
more preferred between about 10-100 sec/Pa. While the thickeners described
herein
are effective to develop viscoelasticity over a range of solution ionic
strengths, the
ionic strength does influence rheology to some extent. Accordingly, unless
otherwise stated, the relaxation times relative elasticities and viscosity
values used
herein are calculated for a first (hypohalite-containing) liquid having an
ionic
strength of about 2.4 molal and a second {peroxygen-containing) liquid having
an
ionic strength of about 3.4 molal. Examples of such liquids are shown in Table
III
as formulas (b) and (e), respectively.
ADJUNCTS
A number of classes of adjunct compounds are known and are compatible with the
first and second liquids and components thereof. One such class are adjunct
cleaning actives, which interact with their intended target materials either
by
chemical or enzymatic reaction or by physical interactions, hereinafter
collectively
referred to as reactions. It is noted that either the oxidant or gas
generating agent
can function as the cleaning active, particularly when one is present in a
stoichiometric excess over the other. Preferably, the oxidant is present in a
stoichiometric excess over the gas generating agent; however, a cleaning
active may
be additionally included. Useful active compounds thus include acids, bases,
oxidants, reductants, solvents, enzymes, thioorganic compounds, surfactants
- 15 -
CA 02342248 2001-02-28
8
WO 00/12672 PCTNS99/i5514
(detergents) and mixtures thereof. Examples of enzymes include lipases,
keratinases, proteases, amylases, and cellulases. Useful solvents include
saturated
hydrocarbons, ketones, carboxylic acid esters, terpenes, glycol ethers, and
the like.
Thioorganic compounds such as sodium thioglycolate can be included to help
break
S down hair and other proteins. Various nonionic, anionic, cationic or
amphoteric
surfactants can be included, as known in the art, for their detergent
properties.
Examples include taurates, sarcosinates and phosphate esters. Other
noncleaning
active adjuncts as known in the art, such as corrosion inhibitors, dyes and
fragrances, may also be included.
While compositions having a viscous rheology, especially a viscoelastic
rheology,
provide a benefit when applied to drains having porous or partial clogs, the
full
benefit is obtained when the composition is thickened and possesses a density
greater than water. This density may be attained without the need for a
densifying
I S material, however, when necessary to increase the density, a salt such as
sodium
chloride is preferred and may be added at levels of 0 to about 25 weight
percent to
the liquid, preferably 12 - 2S weight percent. It is preferred that the second
liquid,
i.e., that including the gas generating agent, be denser than the first
liquid, i.e., that
containing the oxidant. By so doing, the gas generating agent will fill the
lowest
portion of the P-trap, and the first liquid, containing the oxidant, will
"cap" the
second liquid on either side of the P-trap, i.e., in the 90-degree elbow and
in the
vertical pipe. Gas generation thus occurs principally at the interface of the
two
liquids, and within the lowest portions of the P-trap, permitting the foam to
expand
upwards to contact fully the clogged portions of the drain, especially the
vertical
2S pipe. The expanding gas passes through the oxidant, entraining it into the
foam and
distributing it throughout the pipe. The rate of foam generation additionally
is
slowed by the rheology of the first and second liquids, so that the foam is
long
lasting and a greater percentage of actives is delivered. The rheology of the
oxidant-containing first liquid specifically controls foam generation in at
least two
respects. First the viscosity and elasticity of the first solution acts to cap
the denser
- 16-
CA 02342248 2001-02-28
WO 00!12672 PCT/US99I15514
second solution, especially on the vertical side of the P-trap, providing a
slow,
continuous foaming reaction. Second, the rheology of the first liquid which
remains
in the 90-degree elbow of the pipe acts to physically plug the pipe,
preventing the
liquids and/or foam from being siphoned off into the sewer arm of the drain.
For
S the foregoing reasons; it is most preferred the first liquid have a specific
gravity of
about 1.10 or greater, and the second liquid have a specific gravity greater
than that
of the first, more preferably about 1.12 or greater. A preferred ratio of
specific
gravities of second to first liquids is about 1.01:1 to 1.5:1.
Figure 1 shows four foam generation/decornposition curves for four different
thickening systems in conjunction with the preferred hypochlorite/peroxide
oxygenlgas generating system of the present invention. Formula A (curve A)
utilizes the preferred betaine plus sodium xylene sulfonate thickener in both
the
hypochlorite and peroxide solutions. The composition used to generate curve A,
is
1 S as shown in Table III examples (b) and (e) combined. Curve/formula B
utilizes the
preferred betaine plus sodium xylene sulfonate in the hypochlorite solution
Table III
example (b) arid the ethoxylated alcohol thickener of Table III (f) in the
peroxide
solution. The Formula C (curve C) utilizes the preferred thickening system
with the
peroxide (Table III (e)), and the amine oxide/soap (Table III (c)) with the
hypochlorite. Finally, curve D utilizes the amine oxide/soap thickener (Table
III(c))
for the hypochlorite and the ethoxylated alcohol thickener of Table iII (f) in
the
peroxide solution. Note that all thickeners used to generate the curves of
Figure 1
are within the scope of the present invention.
2S The following table (Table I) illustrates the important rheological
characteristics of
the hypochlorite and peroxide components for each formula shown in Figure 1.
- 17 -
CA 02342248 2001-02-28
WO 00/12672 PCT/US99/!55l4
Table I
Formula Viscosity Relative Relaxation
{cP) Elasticity Time (sec.)
(sec/Pa)
S A hypochlorite 1,232 26 32.1
(b)
peroxide (e) 456 50.3 33.6
B hypochlorite 1,232 26 32.1
(b)
peroxide (fJ 2,112 0.002 0.13
C hypochlorite 1,880 0.02 0.36
{c)
peroxide (e) 456 50.3 33.6
D hypochlorite 1,880 0.02 0.36
(c)
peroxide (fj 2,112 0.002 0.13
In the Figure, the foam volume was measured by pouring about 500 ml of a
composition according to Example 10, Table V, into a 2 L graduated cylinder.
Viscosities were measured on a Brool~eld Rheometer, model DV-II+, with a
teflon~-coated number 2 spindle at Srpm after two minutes. Tau, GO and
relaxation
times were measured on a Bohlin VOR at 25°C in the oscillatory mode.
Foam
volume was visually measured at various intervals. An initial phase (or phase
I) of
foam generation begins when the first and second liquids are combined, for
example
in a drain or on a surface, at time zero {to). The initial phase generally
lasts about 60
seconds, preferably about 50 seconds, from to. A secondary phase (or phase II)
2S begins at the end of the initial phase and extends from about 20 to S00
seconds,
preferably about 30 to 300 seconds, after the initial phase ends. Following
the end
of the secondary phase, a tertiary phase (or phase III) lasts for another 80
to 3600
seconds, preferably 90 to 1000 seconds.
- 18 -
CA 02342248 2001-02-28
WO OOI12672 PCT/US99/15514
At the completion of the tertiary phase, the foam is essentially dissipated;
therefore,
an exact end point is not critical. Further, the duration of both the
secondary phase
and the tertiary phase is less important than the duration of the initial
phase, as the
initial phase defines the initial foam generation kinetics which are important
in
treatment efficacy.
At greater than about 60 ml/second generation rate, the foam tends to siphon
into
the sewer arm resulting in minimal contact time. At less than about 20
ml/seconds
there will be insufficient foam "moment" to attack the clog. The first two
phases
define foam development: an initial rapid increase (phase I) and a second,
slow
increase (phase II). The tertiary phase defines a very slow increase to a slow
decrease. It is preferred that phase I occur at about 10 - 50 ml/sec, more
preferably
- 45 mllsec, and most preferably 25 - 40 ml/sec. Phase II preferably occurs at
0.01 - 6 mi/sec, more preferably at 0.1 - 5 ml/sec, and most preferably 1 -
3.5
15 ml/sec. In phase III, the rate of foam development should be about 0.001
ml/sec to
negative 0.2 ml/sec.
It can be seen from Fig 1, that curve A displays the most preferred
performance,
showing a rapid initial phase; a slower second phase; and a final slow
degradation
phase. The remaining curves: B, C and D, while all displaying performance
within
the scope of the present invention, do not employ the most preferred
thickening
system with both the hypochlorite and the peroxide components, therefore do
not
yield the same curve.
Table II below gives preferred viscosity, relative elasticity and relaxation
time
ranges for each of the preferred oxidizing agent and gas generating agent.
- 19 -
CA 02342248 2001-02-28
WO 00112672 PCT/US99/15514
Table II
Oxidizing Agent Gas Generating Agent
Viscosity (cPs.) 100 - 2500 50 - 2500
Relative elasticity (Tau/GO) I - 300 I - 300
Relaxation time (sec.) >0.3 >0.1
The aforementioned parameters of viscosity, relaxation time and relative
elasticity
I O influence the effective performance of compositions of the present
invention, and it
most preferred that each of the f rst and second liquids possess all three of
the
desired properties. However, there are a number of variables which influence
theology, thus it is understood that the theological properties herein are not
necessarily exclusive in defining a composition within the scope of the
present
invention, which is preferably defined by the functional foam characteristics
and
cleaning efficacy. Compositions are within the scope of the present invention
if
they include one liquid which has at least one of the theological properties
as long
as foam generation and cleaning efficacy is attained. Preferably one liquid
has at
least one of the properties and the remaining liquid possesses at least two
such
theological properties. More preferably, one of the liquids has at least two,
and the
other three, theological properties.
A third embodiment of the present invention comprises a drain opening
formulation
and method of use. The formulation includes a f rst liquid comprising:
(i) a hypohalite;
(ii) a corrosion inhibitor;
(iii) a buffer;
(iv) a pH adjusting agent, and
(v) a thickener
-20-
CA 02342248 2001-02-28
WO 00/12672 PCTIUS99/15514
and a second liquid comprising:
(i) a peroxide;
(ii) a pH adjusting agent;
(iii) a densifying agent;
(iv) a thickener
and wherein the first and second liquids are separately maintained, for
example, in
separate chambers of a dual chambered bottle, and admix upon, concurrently
with or
shortly after dispensing into a drain. A most preferred method of opening
drains
involves pouring a first and a second liquid, simultaneously from a dual
chamber
bottle, into a drain to be cleaned, and allowing a period of time for the
active-entrained foam to decompose the obstruction.
A preferred example of a drain cleaning formulation includes a first
composition
comprising:
(i) a C,4_,g alkyl betaine or sulfobetaine;
(ii) an anionic organic counterion;
(iii) an alkali metal hydroxide;
(iv) an alkali metal silicate;
(v) an alkali metal carbonate; and
(vi) an alkali metal hypochlorite
and a second composition comprising
(i) a C,4_,8 alkyl betaine or sulfobetaine;
{ii) an anionic organic counterion;
(iii) hydrogen peroxide;
(iv) sulfuric acid; and
(v) sodium chloride.
Components (i) and (ii) comprise the viscoelastic thickener and are as
described
previously. The alkali metal hydroxide is preferably potassium or sodium
- 21 -
CA 02342248 2001-02-28
WO 00/12672 PCTIUS99/15514
hydroxide, and is present in an amount of between about 0.5 and 20% percent.
The
preferred alkali metal silicate is one having the formula MZO(Si0)~ where M is
an
alkali metal and n is between 1 and 4. Preferably M is sodium and n is 3.2.
The
alkali metal silicate is present in an amount of about 0 to 5 percent. The
preferred
alkali metal carbonate is sodium carbonate, at levels of between about 0 and 5
percent. About 1 to 15 percent hypochlorite is present, preferably about 4 to
8.0
percent.
Generally, the preferred betaine for use with hypochlorite is an alkyl
dimethyl
betaine or sulfobetaine compound having a 12 to 18 carbon alkyl group, and
most
preferably the betaine is CEDB. The alkylamido betaines and alkylamino
betaines
are not preferred in the presence of hypochlorite. Also when hypochlorite is
present, the composition is most stable with no more than about 1.0 weight
percent
betaine, although up to about 10 weight percent betaine can be used.
Substituted
benzene sulfonic acids are preferred as the caunterion with xylene sulfonic
acid
being most preferred.
While the hypochlorite/perioxide foam generating system is preferred, other
systems can be used to generate foam as long as the desired foam
characteristics are
attained. Most preferably such foam characteristics are attained when one or
both
solutions are viscous, and more preferably when one or both solutions are
viscoelastic, having a Tau/GO of 1-300 and relaxation time of at least about
0.3 sec.,
preferably at least about 5 sec.
30
-22-
CA 02342248 2001-02-28
WO 00/12672 PCT/US99115514
FORMULATION EXAMPLES
Formulation Example 1:
i . iT ~'. ' 4, ..
:~'.''0:;
< >~: .- ~I;..,x
.... 9
...... . . ..''::'.'24 . ~.,r... . ~..;.~.
. -'::.:':'.' :::,'.'.,'.:::::2. .. '.~ r.... .: ...fib
:. <':.. . :' . .: .:..... ...: ..:
.. ,....~'
: ::.. 2.... .C. .
::~::<::O~c~#t F, ;: ; ..En~rntor. ,ae lrtrt'~''
:'~'~~:>. ~~... lrl..:. . . .
::. ...... ......,.......................~.. ...... ......
. ...:;.. ...................... , ..~>
.. .. ..~,. . .:..'4Y ...
... :: .:: : .::: .::...:... , . . . ,.....:............,
.......: .................:.. ...:.
.. . ...... ...................... ......,~.. : ...
..,.. ... :...:..:: :: ... , ......, ..........,...
:..::..:::::.:::.: ::.::::.:, ... . . ...:.
: :.. ....: ................:. . ... ~....
:.........................: .: :..: ~~:...:
..._ ~ . ...:.. :::...:::., .. .: .........,~.:....:.,
..::::.::: :. . .. , ...:, 0.01 -
_:.. .......... .... .. 5.0
......... .~.. ~'' ._'".:. ~~ ~~ ~..,.:::"~",~"";~,~..:~~.
.. :.....:...: . .. ..~ ~. ~:
Sodium hypochlorite I Hydrogen peroxide
- I S
Sodium hydroxide 0.1 Sodium chloride0 - 30
- 20
Sodium carbonate 0 - Sulfuric acid O.OOI
5 - 5
Sodium silicate 0-5 Betaine 0.1 -
j 10
~
betaine 0.1 - I0 SXS 0.1 -
10
SXS 0.1 -10
EXPERIMENTAL
Table III
Foam Generation l/sec)
Rate
{m
Example Formula phase Phase Phase
I II III
1 b + a 27 3.0516 -0.0759
2 b + a 26 3.2432 -0.0584
3 b + f 31 0.7841 -0.0574
4 c + a 29 0.0177 n/a
5 c + f 38 0.1765 -0.651
(b) - 5.80% sodium hypochlorite, 1.85% sodium hydroxide, 0.0578% sodium
carbonate, 0.1128%
sodium silicate, 0.78% betaine, 0.39% SXS.
(c) = 5.57% sodium hypochlorite, 2.50% sodium hydroxide, 1.10% sodium
silicate, 1.00% C,4
amine oxide, 0.18% C,6 amine oxide, 0.58% C,° fatty acid soap, 0.34%
C,2 fatty acid soap.
(e) = 0.50% hydrogen peroxide, 20% sodium chloride, 0.015% sulfuric acid,
0.374% betaine,
0.262% SXS.
(~ - 0.51% hydrogen peroxide, 10% sodium chloride, 10% ethoxylated alcohol
sulfate (sodium
salt).
- 23 -
CA 02342248 2001-02-28
w0 00/I2672 PCT/US99/15514
Table III above shows the midpoint foam generation rate for phases I and II,
and the
midpoint for the foam degradation rate, for 5 different formulations of
hypochlorite/peroxide, having the thickening systems noted. Examples l and 2
illustrate performance of the most preferred embodiments, wherein both
components are thickened with the preferred system. Examples 3 and 4 include
one
binary component thickened with the most preferred thickener, and the other
binary
component thickened with a less preferred thickener, as indicated in the
Table.
Example 5 is an amine oxide and ethoxylated alcohol sulfate thickened binary
system, which is still within the scope of the present invention.
Table IV below shows the chemical stability at various storage temperatures of
both
the bleach and peroxide compositions of the present invention. The numbers
reported are percentage active remaining. Actives stability is very good,
especially
for the peroxide composition which contains 20% NaCI. High ionic strength
tends
to destablize peroxides, thus the peroxide stability is surprising, and
thought to be
due to the thickening system acting to immobilize the ions (as well as any
residual
metals) in the composition. The Bleach Composition of Table IV comprises the
following weight percent of ingredients: 5.80% sodium hypochlorite, 1.85%
sodium hydroxide, 0.0578% sodium carbonate, 0.1128% sodium silicate, 0.78%
betaine, 0.39% SXS. The Peroxide Composition comprises 0.51% hydrogen
peroxide, 20% sodium chloride, 0.015% sulfuric acid, 0.3742% betaine, and
0.2616% SXS.
30
-24-
CA 02342248 2001-02-28
WO 00/12672 PCT/US9911551a
Table IV. Percent Actives Remaining
Bleach Peroxide
Composition Composition
Time (weeks) 2 21 38 2 C 21 38
C C C C C
4 99 95 66 99 99 90
8 97 88 48 100 97 77
12 94 84 38 100 98 74
16 93 79 3i 100 95 61
26 87 72 23 100 88 31
( ( ~ ~ ~
Table V shows the effect of thickener type and rheology on clog remover
performance. While thickened formulas alone may provide benefits, it has been
found that the combination of thick, viscoelastic solutions of the present
invention
provide the greatest clog remover performance. All tests were performed on
typical
household sink drains comprising of 3.8 cm diameter pipe with a vertical
section, a
U-bend or P-trap, a 90° elbow and a horizontal sewer arm. Foam
volume and
bleach delivery were measured 5 minutes after pouring. Examples 1 and 2 show
that non-thickened formulas do not produce enough foam in the drain pipe.
Examples 3 and 4 show results when at least one formula is viscoelastic. The
next
examples show the effects of a combination of a simple thickened formula with
a
thick, viscoelastic solution. Examples 5 and 7 utilize a hydrogen peroxide
formula
thickened with an alternative thickener, e.g., an ethoxylated alcohol sulfate.
Examples 6 and 7 illustrate the use of another thickener, e.g:, C,4_~6 amine
oxides, a
bleach stable thickening surfactant commonly known to those skilled in the
art.
Examples 8-10 illustrate the beneficial performance of the preferred
embodiments
of the present invention.
- 25 -
CA 02342248 2001-02-28
WO OOI12672 PCT/US99115514
Table V. Surfactant Effect on Drain Opener Performance
Hypochlorite Peroxide
Composition Composition
Example% ActiveAdditional% ActiveAdditionalVertical Bleach
Ingredients IngredientsPipe Delivery
(~ %) (~ %) Foam Volume{wt %)
(ml)
1 5.80 ~" 0.45 $ 0* 0
2 5.56 # 0.49 $ 203 1.37
3 5.80 {a) 0.51 $ 376 0.15
4 5.66 # 0.50 {e) 188 2
I0
S 5.80 (b) 0.51 (0 304 0.07
6 5.57 (c) 0.51 (g) 232 1.46
7 S.S7 (c) 0.48 (f] 246 0.88
8 5.43 (d) 0.42 (h) 333 1.06
9 5.80 (a) 0.51 (g) 333 1.21
1$ 10 5.80 (b) O.SO {e) 362 1.13
*foam was generated but immediately siphoned off
(a) = 5.80% sodium hypochlorite, 1.85% sodium hydroxide, 0.0578% sodium
carbonate, 0.1128%
sodium silicate, 0.78% betaine, 0.35% SXS.
(b) = 5.80% sodium hypochlorite, 1.85% sodium hydroxide, 0.0578% sodium
carbonate, 0.1128%
20 sodium silicate, 0.78% betaine, 0.39% SXS.
(c) = 5.57% sodium hypochlorite, 2.50% sodium hydroxide, 1.10% sodium
silicate, 1.00% C,4
amine oxide, 0.18% C,6 amine oxide, 0.58% C,° fatty acid soap, 0.34%
C,z fatty acid soap.
(d) = 5.43% sodium hypochlorite, 1.85% sodium hydroxide, 0.0578% sodium
carbonate, 0.1128%
sodium silicate, 0.77% betaine, 0.35% SXS.
(e) = 0.50% hydrogen peroxide, 20% sodium chloride, 0.015% sulfuric acid,
0.374% betaine,
25 0.262% SXS.
(fj = 0.51% hydrogen peroxide, 10% sodium chloride, 10% ethoxylated alcohol
sulfate (sodium
salt).
(g) = 0.51% hydrogen peroxide, 20% sodium chloride, 0.015% sulfuric acid,
0.374% betaine,
0.262% SXS.
(h) = 0.42% hydrogen peroxide, 20% sodium chloride, 0.015% sulfuric acid,
0.4675% betaine,
30 0.3275% SXS.
-26-
CA 02342248 2001-02-28
WO 00/12672 PCTIUS99/15514
'f' = 5.80% sodium hypochlorite, i.85% sodium hydroxide, 0.0578% sodium
carbonate, 0.1128%
sodium silicate.
= 0.50% hydrogen peroxide, 20% sodium chloride, 0.015% sulfuric acid.
#, = non-thickened bleach containing surfactant: 5.47% sodium hypochlorite,
1.82% sodium
hydroxide, 0.0569% sodium carbonate, 0.1111 % sodium silicate, 0.7681 %
betaine, 0.9946% SXS.
$ = non-thickened peroxide containing surfactant: 0.48% hydrogen peroxide,
19.7% sodium
chloride, 0.0148% sulfuric acid, 0.368% betaine, 0.9239% SXS.
Other foam properties of interest include foam density and stability. A dense,
stable
foam will allow longer contact time between cleaning actives and organic clog
materials. Foam stability is defined as the foam's resistance to a force
tending to
collapse or displace the foam. For the present invention, foam stability is
determined by measuring the rate of travel of a standard object through a
column of
foam. The object used in this experiment is a black, phenolic screw cap found
on
typical laboratory sample jars. The cap has a 5 cm diameter, a 1.2 cm lip, and
weighs 11 grams. The inverted cap is placed on top of the column of foam and
the
time to completely travel through the foam is measured. A foam displacement
rate
is calculated by dividing the height of the foam column by the total time
required to
travel through it. A preferred foam displacement rate is less than about 10
cm/min;
more preferred is less than about 6 cm/min. The ratio of foam displacement
rate to
density can also be determined for combinations of thickened gas generating
and
oxidizing agents. A preferred ratio is about 50:1 to l:l, more preferred is
about
30:1 to 10:1. Table VI lists these foam properties.
30
-27-
CA 02342248 2001-02-28
WO 00/12672 PCT/US99/15514
Table VI. Foam Properties
Ratio of
HypochloritePeroxide Foam DensityFoam Foam
ExampleCompositionComposition(g/ml) DisplacementDisplacement
(wt %) (wt %) Rate {cm/min)Rate to
Density
1 (a) (b) 0.21 4.3 20.5
2 (a) (c) 0.31 1.5 4.8
3 (d) (b) 0.41 0.6 1.5
4 (d) (C) 0.33 1.5 4:5
(a) = 5.78% sodium hypochlorite, 1.85% sodium hydroxide, 0.0578% sodium
carbonate, 0.1128%
sodium silicate, 0.7800% betaine, 0.3900% SXS.
(b) = 0.49% hydrogen peroxide, 20% sodium chloride, 0.015% sulfuric acid,
0.3742% betaine,
0.2612% SXS.
{c) = 0.48% hydrogen peroxide, 10% sodium chloride, 10% ethoxylated alcohol
sulfate (sodium
salt).
(d) = 5.57% sodium hypochlorite, 2.50% sodium hydroxide, 1.10% sodium
silicate, 1.00% C,4
amine oxide, 0.18% C,6 amine oxide, 0.58% C,o fatty acid soap, 0.34% C,Z fatty
acid soap.
Table VII shows performance of the present invention on hair restrictions in
drains. For this test, 4 grams of human hair was mixed with about 2 grams of a
20% soap solution, and the resulting hair ball was suspended in the drain at
the
approximate location of the stopper rod mechanism. The time for 3.785 liters
of
water to drain from the sink was recorded as the initial flowrate. Non-
thickened
and thickened commercially available clog removers were used in the tests
according to label instructions. Tests were also conducted with unthickened
oxidizing and gas generating composition, along with compositions of the
present
invention. About 500 ml of each of the drain opening compositions was poured
into the drain. The time for 3.785 liters of water to drain from the sink was
again
measured and recorded as the final flowrate. After the completion of each test
the
remaining hair was rinsed, dried overnight at 38°C, and weighed. The
present
- 28 -
CA 02342248 2001-02-28
w0 00112672 PCTIUS99/15514
invention dissolved an average of 71.8% of the hair while the non-thickened
and
thickened commercial products dissolved an average of only 20.1 % and 52.9%,
respectively. The unthickened combination of oxidizing and gas generating
liquids
dissolved an average of only 13.8% of hair. Final flowrates for drains treated
with
either the thickened commercial product or the present invention are
comparable to
flows found in sinks with unobstructed drains. The unthickened compositions
did
not result in significantly improved flowrates.
Table VII Performance On Hair Clogs
. .~r,
:~:, . ~: .. ..~ ~ , ~,.~ .
..: ". ~p ~ .~ ~ ~. ..
:~, ~ ~ ~. . .. ~.. :: :
. . ~ ~ ;. :. ~r . ~4 .1::1.~~:al=
:. . . h.:. 1 . ~......................ay>
. . .......~ ~ .at:x, a ~ :-
,.~~can~~ '~.~::.:;r h ,:.::::....1...........:.
. ... tt.::.>:-,:::;..::.. .::. ~~ w....:....... :...::
~ :..,............~.L.. ....... ., ~d .....
.:::.::..". .. .~ f . ...............: ~~,
.::..t~....:.:. . *~..:........ .::; ...
:. ..;: ...~ ".. :.:., ..~::::..:~..,....
<.:::::,:.::::.::. ... ~. . . .:.:.::.~::::....
.....:...:: .. .... .....>:...,..:.~. ....
T........:.....,..:::..w~,..~.~.~ _....:: ~:::.:. ::
~ :....... . ...:::
:~ x .. .:: :::~f;._~ .
.. .: ~....: .... :...,
' ~ ... .,z.::...t,: : ' ..;
~!ii::Y'::::- :: .. ry i:::. ,..:
:::!:::::-nl ~ : t, ~
: Y ;: ..., . .,Ba
~ . , a
;= .f: o ::::':
a ~ ,x ...,o
~ . '
, ~ t
.
,"
::
a
~
~
. . . , ,
...................... . : .. Il :l i~r:r.;:cap:>:1~'.:.
s...:,.,.
...................... . ..r ~::~:; i$S o::.:::,:x~::-:x;:>
. ... i .... ...~..,...- 3!f ....................
.::::::::::.....~:..?..:::..:.-.S .. ... .: ..... ..:......~. .
. :::::::.:........,x.,.......$ .. .. ...............:.....
.....,.
: :::::-..::::..:::.. ::::...o,... .. .....
...............,....5...................:: : : . .
_.:..:.:..:..,.....t. ...................
r . . ....,.....4,:..:.:.:..a,.;.::...:.......:::....: ,
.. ::::::.:..,.:.:1 .... .::.:::.....;s. .:.
:::.::..:..,:::: , : .:. ;. :. ,
.,.:.....n.i..n..:..................~ :::: . ,...a<.,
~ ~ ::,~..:_,.., ..:...3.""
'~ 3
~:
.................,.................... .:.... . ,
. ...................:...........: . ... .....,... .. .
:..
..............:............:,.,........,:.......:....... . ..
.:..:: ,.::..
: ::::.~.:,.........,...........:..........?..............
...:......",,...:.:. .:, : '~>.....
. :::: ...............:r,...........,,.........,...."...... :.. : :
. ...
:E .::::.....~:.,:::::.~..:::::.....:.::,~............ ~... . .
....:
.................;5....... .............. .: ,................ ....
......
. . ::::::.~...:..:...; . : . . ."..
::::::........r.;..:....::.:~::.,. ;..".;:.;.,.:.... .. ~:.d.::
~: :~:. , .......... .... . .. . . .::
~-::::::::..:::::. .......:.~:.:..,.:.,,.:.;....;., ....f ......
F .:: :.: ....: ..., . . . :.
:::::::::: .r:.. y.:. . . .. ..
.:: : . .... .. .
: ~~. , : .....,~.~
: r.. v . . . ~
g ~ v::: ~
...Y:. ~ . ~
'W ~' .
- .
.: ~
..
3
. . :. .. : .: . . ... .
5 . a . : ....., : ". vn.. ..:
f :.::;..~:~:::::U: .. :: . v:. , ,.:Y-~.. .. .
.........
. .... f~ : w: :::.a.~:. ~"a.v::r:w:.:x...
.,.........:..... . :. .,..u.:,:...,:.,.,n. ...... a ..
Y,:....... .::..~,...... . . . .
.. ..~":.?...:::..;::::::,......;;..~,. > ",.. ..:
..... ... ... ~ ~ , . . -.:1...
. :... . .:: .... .;t .n.,o..7
. . ... .. .......,.."..
..:....... ~: ..#.5:..: :.:..".
. r;." .
1 (a) 5.59 4 3.1 22.5 3.5 7.3
2 (a) 5.66 4 3.3 17.8 20.1 2.4 3.7
3 (b) 5.49 4.1 2 51.2 3 23.5
4 (b) 5.53 4 1.8 54.6 52.9 3.6 26.5
S (c) 5.66 4 3.2 19.8 3 3.8
6 {c) 5.66 4 3.7 7.8 13.8 3.8 3.8
7 (d) 5.57 4 I.I 72.5 4.1 19.7
8 (d) 5.57 4 L2 70.3 4.2 18.9
9 (d) 5.57 4 L 1 72.8 71.8 4.2 22.7
(a) = an unthickened, commercially available liquid drain opener
(b) = a thickened, commercially available liquid drain opener
(c) = an unthickened combination of oxidizing and gas generating agents,
made according to Example 2 of Table V
(d) _ a formulation of the present invention, made according to Example 10 of
Table V
Examples 7-9 which are formulation of the present invention, show a much
greater
average hair dissolved than any of the other examples. This improvement is
thought
to be due to the increased contact time afforded by the present invention. It
can be
seen that the present invention also yield a better initial flow rate, and the
final
flowrates were better than all but product (b).
-29-
CA 02342248 2001-02-28
WO 00/12672 PCT/US99/15514
A most preferred method of opening drains involves pouring a first and a
second
liquid, as illustrated by Formulation Example 1, simultaneously from a dual
chamber bottle. A most
preferred dual chamber bottle comprises one having side-by-side, equal
capacity
chambers and a single dispensing orifice.
Table VIII
Percent
Improvement
Number of Number with Number withflows < all drains
11.4
Drains Testedflows < 11.4 flows < 1/min
l/min 11.4
before treatment1/min after
treatment
38 18 9 105.5 53.9
Table VIII illustrates the specific improvement in slow-flowing drains, i.e.
Those
having flows of less than about 11.4 liters per minute (1/min), following
treatment by a
formulation of the present invention made according to Example 10 of Table V.
The
test protocol called for measuring the amount of time taken for 4 liters of
cold tap
water to drain from the sink. This was performed three times and an average
flowrate
was calculated. The present invention was then applied to the drain. After one
hour
the drain was flushed with hot tap water. Again, the amount of time taken for
4 liters
of cold tap water to drain from the sink was measured three times and an
average
flowrate determined. A percent flow improvement was calculated for each drain
using
the average flowrates obtained before and after application of the present
invention.
A preferred bottle orientation during pouring results in both liquids exiting
the dual
chambered container such that optimum foam generation occurs in the drain
pipe.
While described in terms of the presently preferred embodiment, it is to be
understood
that such disclosure is not to be interpreted as limiting. Various
modifications and
- 30 -
CA 02342248 2001-02-28
WO 00/12672 PCT/US99/lSSl4
alterations will no doubt occur to one skilled in the art after having read
the above
disclosure. Accordingly, it is intended that the appended claims be
interpreted as
covering all such modifications and alterations as fall within the true spirit
and scope
of the invention.
10
20
30
- 31 -