Note: Descriptions are shown in the official language in which they were submitted.
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
INHIBITORS OF a4(3, MEDIATED CELL ADHESION
Background of the Invention
Field of the Invention
The present invention relates to small molecules that are potent inhibitors of
a4(3,
mediated adhesion to either VCAM or CS-l and which 2ure useful for the
treatment of
inflammatory diseases.
Description of Related Art
The extracellular matrix (ECM) is he major component of connective tissue
which
provides structural integrity, and promotes cell migration and
differentiation. As part of
these functions, extracellular matrix molecules such as :fibronectin,
collagen, Iaminin, won
Willebrand factor, thrombospondin; fibrinogen, and ten<~scin have been shown
to support
adhesion of cells in vitro. This adhesive interaction is critical for a number
of biological
processes including hemostasis, thrombosis, wound healing, tumor metastasis,
immunity and
inflammation.
Fibronectin (FN) is the prototype ECM molecule. The major cell attachment site
in
the fibronectin molecule has been reproduced synthetically with the amino acid
sequence
arginine-glycine-aspartic acid, or RGD using single letter nomenclature.
Peptides containing
the RGD sequence which either inhibit or promote cell .adhesion have been
described (US
Patent Nos. 4,589,881; 4,661,111; 4,SI7,686; 4,683.,291; 4,578,079; 4,614,517;
and
4,792,525}. Changes in the peptide as small as the e:cchange of alanine for
glycine or
glutamic acid for aspartic acid, which constitute the addition of a single
methyl or methylene
group to the tripeptide, eliminates these activities (Piersch6acher et al.,
Proc. Natl. Acad Sci:
USA 81:5985 (1984)). Recently, a second- FN cell binding domain has been
identified within
the alternatively spliced region of the A chain of the molecule, known as the
connecting
segment 1 (CS-1). The most active cell-binding site within this alternatively
spliced region
is composed of 25 amino acids where the carboxy terminus contains the sequence
CA 02342778 2000-12-20
WO 99167230 PCTIUS99114233
-2-
EILDVPST. The amino acid sequence EILDVPST forms a recognition motif on FN for
cell
surface receptors. (Wayner et al., J. Cell Biol. 109:1321 (1989); Guan et al.,
Cell 60:53
( 1990}).
The receptors which recognize these sites on FN belong to a gene superfamily
called
integrins which consist of heterodimeric complexes of non-covalently
associated alpha and
beta subunits. A common ~3 subunit combines with unique I subunits to form an
adhesion
receptor of defined specificity. To date, 8 (3 subunits have been identified
which can
dimerize with 16 distinct I subunits forming 22 distinct integrins. The (31
subfamily, also
known as the VLA family (Very Late Activation Antigen:>), binds to ECM
molecules such as
FN, collagen and laminin. For reviews, see, Hynes, Cell 48:549 (1987); Hemier,
Annu. Rev.
Immunol. 8:365 (1990). Leukocyte interaction with FN at the two spatially
separate binding
domains is mediated by twa distinct integrins. The RGI) site is recognized by
the integrin
ash,, while, EILDV is recognized by aA(i, (Pytela et al., Cell 40:191 (I985);
Wayner et al., J.
Cell Biol. 109:1321 (1989); Guan et al, Cel160:53 (1990)).
Vascular endothelial cells form the interface between blood and tissues and
control
the passage of leukocytes as well as plasma fluid into tissues. A variety of
signals generated
at the site of inflammation can activate both endothelial cells as well as
circulating
leukocytes so that they become more adhesive to onc: another. Following this
initial
adhesion the leukocytes migrate into the tissues to perform host defense
functions. Several
adhesion molecules have been identified which are iinvolved in leukocyte-
endothelial
interactions.
In the Vii, subfamily; in addition to binding to fibronectin, a4(3, interacts
with a
cytokine inducible protein on endothelial cells termed vascular cell adhesion
molecule
(VCAM). Further involved in the leukocyte-endothelial adhesion process is the
biz integrin
subfamily. (32 integrins include CD 11 a/CD 18, CD 11 b/CD I 8, and CD 11 c/CD
18. In
addition, the X37 subunit associates with a4 to form a unique x4(37
heterodimer which binds to
FN, to VCAM, and to Mucosal Addressin Cell Adhesion lVlolecule-1 (MAdCAM)
(Ruegg et
al, J. Cell.Biol. 117:179 (1992); Andrew et al., J. Immunol. 153:3847 (I994);
Briskin et al.,
Nature 363:461 (1993); Shyjan et al, J. Immunol. 156:28:11 (1996)). a4
integrins are widely
expressed on different cell types including hematopoietic progenitors,
lymphocytes, natural
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-3-
killer cells, monocytes, eosinophils, basaphils, and mast: cells {Hehner, M.
E., Annu. Rev.
Immunol. 8:365 (1990)). Other molecules on endothelial cells which bind to the
leukocytes
include ICAM-1, ICAM-2, E-selectin and P-selectin ((:arlos and Harlan, Immunol
Rev.
114:1 (1990); Osborn, L., Cell 62:3 (1990); Springer T., lJature 346:425
(1990); Geng et- - al.,
Nature 347:757 (1990); Stoolman, Cell 56:907 {1989)).
A number of in vitro and in vivo studies indicate 'chat a4(3, plays a critical
role in the
pathogenesis of a variety of diseases. Monoclonal antibodies directed against
a4 have been
tested in a variety of disease models. Anti-a4 antibodies block adhesion of
lymphocytes to
synovial endothelial cells; this adhesion plays a potential role in rheumatoid
arthritis (van
Dinther-Janssen et al, J. Immunol. 147:4207 (1991)). a4 has also been
implicated with
respect to rheumatoid arthritis in separate studies {Laffon <~t al, J. Clin.
Invest. 88:546 (1991);
Morales-Ducret et al, J. Immunol. 149:1424 (1992)). A significant number of
studies have
evaluated the role of a4 in allergy and asthma. For example, monoclonal
antibodies to a4
block adhesion of basophils and eosinophils to cytokine activated endothelial
cells {Walsh et
al, J. Immunol. 146:3419 ( 1991 ); Bochner et- aI, J. Exp. Med. 173:1553 (
1991 )).
Monoclonal antibodies to a4 were also effective in several lung antigen
challenge models
(Abraham et al, J. Clin. Invest. 93:776 (1994); Weg et ;~1, J. Exp. Med.
177:561 (1993)).
The cotton-top tamarin, which experiences spontaneous clhronic colitis, showed
a significant
attenuation of their colitis when anti-as antibody was administered (Podolsky
et al, J. Clin.
Invest. 92:372 (1993); Bell et al, J. Immunol. 151:4790 (1.993)). In a rat and
mouse model,
autoimmune encephalomyelitis was blocked by anti-a4 antibody (Yednock et al,
Nature
356:63 (1992); Baron et a1, J. Exp. Med. 177:5? (1993)). Anti-a4 monoclonal
antibodies
also inhibit insulitis and delay the onset of diabetes in the non-obese
diabetic mouse (Baron
et al, J. Clin. Invest. 93:1700 (1994); Yang et al, Proc. Natl. Acad. Sci. USA
90:10494
(1993); Burkly et al, Diabetes 43:529 {1994)). a4 is also implicated in
atherosclerosis due to
its endothelial expression during atherogenesis (Cybulsk;~ et al, Science
251:7$8 (1991)).
The migration of leukocytes to an inflammatory site can also be blocked by
anti-a,4
antibodies. In addition to the blocking of migration, inhibitors of leukocyte
endothelial
adhesion may block the costimulatory signals mediated by integrins and thus
inhibit
overproduction of inflammatory cytokines. In a sepaxate set of experiments not
using anti-a4
CA 02342778 2000-12-20
WO 99/67230 PCT/US99It4233
antibodies, the peptides GRDGSP or EILDV were tested against contact
hypersensitivity
response. The contact hypersensitivity response was found to be blocked by
GRDGSP or
EILDV suggesting that both a4(3, and as(3, are involved in this inflammatory
response.
Other ailments which may involve a4~i,-mediated conditions include the
inflammatory disorders rheumatoid arthritis, allergic disorders, asthma,
spontaneous chronic
colitis, insulitis, contact hypersensitivity response, atheroselerosis and
autoimmune
encephalomyelitis. These studies illustrate that small molecules that are
potent inhibitors of
a4~3, mediated adhesion to either VCAM-1 or CS-I may be used as a form of
treatment in
numerous inflammatory diseases. However, these inflammatory conditions could
be
expanded to include adult respiratory distress syndrome, AIDS, cardiovascular
diseases,
thrombosis or harmful platelet aggregation, reocclusion following
thrombolysis, aliograft
rejection, reperfusion injury, psoriasis, eczema, contact dermatitis and other
skin
inflammatory diseases, osteoporosis, osteoarthritis, atherosclerosis,
neoplastic diseases
including metastasis of neoplastic or cancerous growl;h, wound healing
enhancement,
treatment of certain eye diseases such as detaching retina, 'Type I diabetes,
multiple sclerosis,
systemic lupus erythematosus (SLE), inflammatory and immunoinflammatory
conditions
including ophthalmic inflammatory conditions and inflammatory bowel diseases,
ulcerative
colitis, regional enteritis and other autoimmune diseases. Accordingly, a
compound which
could inhibit these conditions is desirable.
Summary of the Invention
The present invention particularly provides:
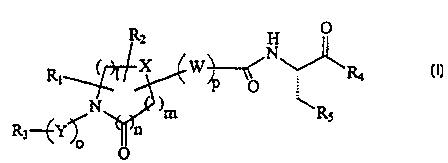
A compound of the formula:
Rz H O
C 1 X W N~
R! _ R4 C I )
v
N )m ~ Rs
R3-"~y~ n .
o O
CA 02342778 2000-12-20
WO 99!67230 PCT/US99/14233
-5-
In the above formula (I), R; may occur one to four times and each occurrence
is
independently hydrogen or C,~ alkyl. Also in the above formula (I), RZ is
hydrogen,
pyridyl, C,_6 alkyl, (C,_6 alkyl)-COZ-R", or -COZ-R". In ;addition, R, and RZ
may be
attached to the same carbon atom and form a carbocycIic; ring of 5-8 atoms
together with
the carbon atom to which they are attached, or they may be attached to the
same carbon
atom and form a ring of 5-8 atoms of the formula:
~ /(CH2)1~
C NR~~
\(CHz)l
together with the carbon atom to which they are attached.
In the above formula (I), R3 is hydrogen, phenyl, C,_6 alkyl, C3_6 alkenyl,
C,_"
arylalkyi , (C,_6 alkyl)-COZ-R", (CZ_6 alkenyl)-COZ-R", (C,_6 alkyl)-CO-C,_6
alkyl, (C,.~
alkyl)-O-C,_6 alkyl, (C,~ alkyl)-OH, {C,~ alkyl)-CN, adarnantyl or one of the
following:
N~N ~~,, OR~~ ~ /~ \
-(C 1 _ 6 aikyl~ y ~ ~ -(C I -6 a~Y~ ~Z -N Z
~N p 'i--' ~/
H q 9 N
/ / / / ~
COz-R~ t --E~COz-R \
\ ~ ~ ~ \ ~ -COz-R> > '-(C I-6 alkYl~-~
N
N
/ / N\ O /
\ 10(Cy-3a~Y!))~-s COz-Rit \ ~ ~ '~CF3
/ N
OH
S \
O NOz I-IN-N \ ~ /
N
N~ v / \
H C~z-R~ i H COz-R" '~ ~COz-R~ t
N(C,_3a1kY~2
In addition, RZ and R3-(Y)o may combine with each other at the terminal
thereof to
form a ring of the following formula together with the carbon atom and the
nitrogen atom
to which they are attached:
CA 02342778 2000-12-20
WO 99167230 ' PCT/US99/14233
-6-
Z N.:r"
O
In the above formula {I), R4 is -O-R", NH2, NHOH, -O-(C7_,o arylalkyl), or is
of the
formula:
!°-c"2 ~ .
'N
i
In the above formula (I), R5 is a formula of the following:
R6
j Ra-Ri2
In the above, R~ is N or CH, R7 is hydrogen or halogen, R8 is -NH-Y,-, -OCHZ-,
or
-CONH-, R9 may occur one to three times and is a halogen, C,~ alkoxy, C,_6
alkyl or
trifluoromethyl, R,fl is C,~ alkyl, or (C,~ alkyl)-OH, or hydrogen, R" is
hydrogen or C,~
alkyl, R,, is C,~ alkyl or the following formula:
Rt3
-{CH2~. R9
and R,3 is N or CH.
In the above formula (I), W is (C,_6 alkyl), X is S, O, or CH2, Y and Y, are
independently -CO-, -C(=O)O-, -SOZ-, or -C(=O)N(R,o)- and Z is O, CHz, or N-
R".
In theabove,lis 1,2,or3,mis l or2,nis0or l,ois0or l,pis0or l,qis0or
l,andris0, l,2or3.
The above formula {I) has the provisos that:
(l) when Y is -C{=O)O-, R3 cannot be hydrogen;
(2) when R4 is equal to O-(C4 alkyl), C4 alkyl is not equal to tent-butyl;
(3) in those pyrrolidine structures (1 is 1; m is. 2; n is 0; o is 0; p is 1;
X is
CHZ), W is equal to CH2; and
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
(4) the compound is not
H
N
H
In another embodiment of the present invention, RS is a formula of the
following:
cl
~~a
C J RB
CI
wherein R, is hydrogen or CI.
In another embodiment of the present invention, R Z is hydrogen or C,~ alkyl:
Additionally, R, and Rz may be attached to the same carbon atom and may form a
carbocyclic ring of 5-8 atoms together with the carbon atom to which they are
attached or
be attached to the same carbon atom and form a ring of S-8 atoms of the
formula:
~ /(CH2)l~
C NRn
~ ~(CH2)~
together with the carbon atom to which they are attached,. Also, in this
embodiment, n is
O,mis2andpis0.
In yet another embodiment of the present invention, R, is hydrogen or C,_3
alkyl,
and RZ is hydrogen or C,~, alkyl. Additionally, R, and R~ may be attached to
the same
carbon atom and may form a carbocyclic ring of 5-8 atoms together with the
carbon atom
to which they are attached or be attached to the same atom and form a ring of
5-8 atoms of
the formula:
~ /(CH2)l~
C NRIi
~ ~(CH2)~
CA 02342778 2000-12-20
WO 9916?230 PCT/US99/14233
-$_
together with the carbon atom to Which they are attached. Also in this
embodiment, R3 is
hydrogen, C,_6 alkyl, C,_,~ arylalkyl, {C,_b alkyl)-OH, (C,_b alkyl)-CO,-R",
(C,_6 alkyl}-CN,
adamantyl, phenyl, or one of the following:
N1N
~(C 1-6 ~Y~ ~'N _.(C 1-6 alkyl)
'N N
H N
-(CI_6 allcyl)-N Z --N~ Z
(~ (~ w
N(CH3)2
Additionally, in this embodiment, R4 is -O-R" , NHZ, N:HOH, ar R4 is of the
formula:
-o-cH2 ' %
N , R" is hydrogen or CH3, and X is S or O.
In another embodiment, R, and RZ are hydrogen" and R3 is (CZ~ alkenyl)-COZ-R",
(C,.~ alkyl)-O-C,_3 alkyl, (C,_6 alkyl}-COzR", or one of tile following:
N
l -
N N
OH
S
O NOZ H~N-N~~
H~COZ-Rtt ' ~ ~COZ-Rat
Also, in this embodiment, R4 is O-R,,, R~ is CH, R" is hydrogen, and R7 is
hydrogen.
Additionally, X is S, Y is -CO-, and 1 is 1.
In another embodiment of the present invention, R, and RZ are hydrogen, and R3
is
C,_6 alkyl, (C,_6 alkyl)-COZ-R", (C,_6 alkenyl}-COz-R", (C,.~ alkyl)-CO-C,_6
alkyl,
(C,_6 alkyl}-O-C,_3 alkyl, {C,_6 alkyl)-CN, or one of the following:
CA 02342778 2000-12-20
WO 99!67230 PCT/US99/14233
-9-
/ / / / \
\ COz-Rt t \N~COz-Rt t -~--COz-Rt t -(C 1-6 ally
\\ O
N
/ / Nw / O
COz-Rt t ~ -~- CF3 \
OCH3 \ / sN
OCH3 OH
S S p ~~ ORt t
NOz HN-N
~ \/ , ~
N' \ ~;~ _...~~ ~.,~
H COz-Ri t H COz-Rt t ~COz-Rt t O
Additionally, R4 is OH, R6 is CH, R" is hydrogen, R, is hydrogen, X is CH2,
and Y is -
CO- or -C(=O)NH-.
In another embodiment of the present invention, W is (C,~3 alkyl), X is CHz, Y
is
-C(=O)O-, R, is hydrogen, RZ is hydrogen, (C,_3 alkyl)-C;Oz-R", or -COZ-R", R3
is
hydrogen, C,_,o arylalkyl, C,_6 alkyl, or (C,~ alkyl)-COz-R", R4 is OH, R~ is
CH, R" is
hydrogen, R, is hydrogen, l is I or 3, and n is 0,
In another embodiment of the present invention, W is C,_3 alkyl, X is CH,, R,
is
hydrogen, RZ is (Ct_4 alkyl)-COZ-R", or COZ-R", R3 is hydrogen, C,_3 alkyl, or
CZ_, alkeilyl,
R4 is OH, R6 is CH, R" is hydrogen, R, is hydrogen, l is 1, m is l, n is l, o
is 0, and p is 1.
In another embodiment, the compound of the preaent invention is represented by
the following formula
/---S
H O
R3--~Y~ N y
W _ Ra
p O
\Rs
In another embodiment of the,present invention, R, and R~ are hydrogen, and R3
is
C,_6 alkyl, {C,~ alkyl)-COZR", (CZ_6 alkenyl)-C02R", (C,_6 alkyl)-O-C,_3
alkyl, or one ofthe
following:
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-10-
N
'N J CO2-Rtt
OH
O N~ HlN-N
H~C02-Rtt , ~ ~COz-Rtt
Additionally, in this embodiment, R4 is O-R", R6 is CH,, R" is hydrogen or
C,_6 alkyl, R, is
hydrogen, X is S, Y is -C(=O)O-, and I is 1.
In another embodiment of the present invention, R, is hydrogen or C,_3 alkyl
and RZ
is hydrogen or C,~, alkyl. Additionally, R, and R, may be attached to the same
carbon
atom and may form a carbocyciic ring of 5-8 atoms, or be attached to the same
atom and
form a ring of S-8 atoms of the formula:
~ /ECH2)1~
C NRIi
~ ~(CH2)~
together with the carbon atom to which they are attached. In this embodiment,
R3 is
hydrogen, C,_6 alkyl, C7_" arylalkyl, (C,~ alkyl)-OH, (C,..~ alkyl)-COZ-R",
(C,_6 alkyl}-CN,
adamantyl, phenyl, or one of the following:
~(C ~ _6 alkyn--C ~N -(C ~ _6 ally ~ \ -(C 1-b a~Yn!
N
H N ,N (
3
(~ \ ~ \ \
~. N
N(C E-3a~Y1)2
H
S
'N IN
H
COzRt, OH C02R> >
Additionally, in this embodiment, R4 is -O-R" , NH2, NHOH, or R4 is of the
formula
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-11-
0 CH \
~N . Also, R" is hydrogen or C,~ alkyl, and X is S or O.
in yet another embodiment, the compound of the present invention is
represented
by the following formula {I-a):
R2 H O
X ~ N
RI 1 ~ { I-a )
N )m SRS
R3~y'~ n
In the above formula (I-a), R, may occur one to :four times and each
occurrence is
independently hydrogen or C,~ alkyl, and RZ is hydrogen, pyridyl, C,~ alkyl,
(C,_6 alkyl)-
CO2-Ri,, or -COZ-R". Additionally, R, and RZ may be attached to the same
carbon atom
and form a carbocyclic ring of 5-8 atoms together with t:he carbon atom to
which they are
attached, or they may be attached to the same carbon atom and form a ring of 5-
8 atoms of
the formula:
~ ~(CH2)l~
/X11
~ ~(CH2)~
together with the carbon atom to which they are attached. Additionally, in
this
embodiment, R3 is hydrogen, phenyl, C,~ alkyl, C3_6 alks~nyl, C7_" arylalkyl ,
(C,~ alkyl)-C02-R", {C2~ alkenyl)-COZ-R", (C,.6 alkyl)-~CO-C,_6 alkyl, (C,_6
alkyl)-O-C,_6
alkyl, (C,.~ alkyl)-OH, (C,_6 alkyl)-CN, adamantyl or onf; of the following:
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-I2-
N''N ~'~',~, ORt t I~ \
-(C I _ 6 alky)~ ~N -(C I -6 a~Y~- ~ -N Z
(~ N
N, O
H
/ /
w
/ / ~ ~\
\ COz-Rt t \ COz-Rt 1 \ \ --~ COz-Rt t '-(C I -6 alkYlr~
N
N
/ / N ,' O /
\ [O(Ct-3alkyO~t-a COz-Rt i I ~ -~CF3
\ i
N
OH
~ O NO~ HN-N \ I /
N'
N~ ~ / \
H COz-Rt t H COz-Rt t ~'~ ~COz-Rt t
N(C t-aa~Y~z
In addition, RZ and R3-(Y)o may combine with each other at the terminal
thereof to form a
ring of the following formula together with the carbon atom and the nitrogen
atom to
which they are attached:
Z IV ~,
O
Additionally, R4 is -O-Rtt, NH2, NHOH, -O-(C,_to arylalkyl), or is of the
formula
-o-cH2 ; ~
LH
Also, in this embodiment, RS is a formula of the following:
I R8-'_-RI2
R~
Additionally, in this embodiment, R.6 is N or CH, R, is hydrogen or halogen,
Rg is
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-13-
-NH-Y,-; -OCHZ-, or -CONH-, R, may occur one to three times and is a halogen,
C,~
alkoxy, C,_6 alkyl or trifluoromethyl, R,a is C,_6 alkyl, or (C,_6 alkyl)-OH,
or hydrogen, R"
is hydrogen or C,_6 alkyl, R,2 is C,~ alkyl or the following formula:
Rt 3
-(CH2~. I
R,3 is N or CH, W is (C,_6 alkyl), X is S, O, or CHZ, Y and Y,
are independently -CO-, -C(=O)O-, -SOz-, or -C(=O)N(R,o)-, Z is O, CH2, or N-
R",1 is I,
2,or3,mislor2,nis0orl,ois0orl,pis0orl,qi:>0orlandris0,i,2or3. This
particular embodiment of the present invention has the provisos that
{ 1 ) when Y is -C(=O)O-, R3 cannot be hydrogen;
(2) when R4 is equal to O-(C4 alkyl), C~ alkyl. is not equal to tent-butyl;
(3) in those pyrralidine structures (I is 1; m is 2; n is 0; o is 0; p is 1; X
is
CHZ), W is equal to CH2;
(4) the compound has an IC~o value of less than Sp,M in a Jurkat CS-1 assay
and/or an ICso value of less than SOpM in a Jurkat EC assay; and
(5) the compound is not
C1
O
O COzH
H .,~ ;
N '
C1
N
In yet another embodiment of the compound of formula {I), R, may occur one to
four times and each occurrence is independently hydrogen or C,_6 alkyl. Also,
RZ is
hydrogen, pyridyl, C,_6 alkyl, (C,_6 alkyl)-COZ-R", or -COZ-R". Additionally,
R, and RZ
may be attached to the same carbon atom and form a carbocyclic ring of 5-8
atoms
together with the carbon atom to which they are attached, or they may be
attached to the
same carbon atom and form a ring of 5-8 atoms of the formula:
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99114233
-14-
~ ~{CH2)l~
p1
~(CHZ)~
together with the carbon atom to which they are attachedl. In this embodiment,
R3 is
hydrogen, phenyl, Ct,b alkyl, C3_6 alkenyl, C,_" arylalkyl , (C,~ alkyl)-COZ-
Rtt, (CZ_6
alkenyl)-COZ-R", (Ct_6 alkyl)-CO-C,_6 alkyl, (Ct_6 alkyl}-O-C,~ alkyl, (Ct_6
alkyl)-OH, {C,~
alkyl)-CN, adamantyl or one of the following:
N..N ~'''4,, ORtt
-(C 1-6 alkyl N -(C 1-6 alkyl?-. ~ _ ~ \
N.. O (
H a (~q N
/ / /
CO -R ~\
2 t t CO -R
\ ~ z t t \ \ -COz-Rt t '-(C 1-6 alkylp
N
N
/ / N' O /
\ 10(Ct-3alkynlt-s COz-Rit ~ __~~CF3
\ / N
OH
S s \
'''~~ O NOz HN-N \ ~ /
N' \ ~ ,..
N~ / \
H COz-Rt t H COz-R" ~~ ~COz-Rt t
N(C t-3a~Y~z
In addition, RZ and R3-(Y)o may combine with each other at the terminal
thereof to form a
ring of the following formula together with the carbon atom and the nitrogen
atom to
which they are attached:
Z N ..,
O
In this embodiment, R4 is -O-R", NH2, NHOH, -O-(C7_,o arylalkyl), or R4 is of
the formula:
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-15-
L i
N
i
Alsa, in this embodiment, RS is a formula of the following:
R6
w
~-Riz
R~
Additionally, in this embodiment, R.6 is N or CH, R, is hydrogen or halogen,
R8 is
-NHCO-, R, may occur one to three times and is a halogen, C,_6 alkoxy, C,_b
alkyl or
trifluoromethyl, R,o is C,~ alkyl, or (C,_6 alkyl)-OH, or hydrogen, R" is
hydrogen or C,_6
alkyl, R,2 is C,_6 alkyl or the following formula:
Ri3
-(CHz~. R9
/
R,3 is N or CH, W is (C,_6 al:kyl), X is S, O; or CHz, Y is -CO-,
-C(=O)O-, -C(=Q)N(R,a)-, Z is O, CH2, or N-R",1 is 1, :?, or 3, m is 1 or 2, n
is 0 or 1, o is
0 or l, p is 0 or 1, q is 0 or 1, and r is 0, 1, 2 or 3. In this embodiment,
the following
provisos apply:
(1) when Y is -C(=O)O-, R3 cannot be hydrogen;
(2) when R4 is equal to O-(C4 alkyl), C4 alkyl is not equal to tent-butyl; and
(3) in those pyrrolidine structures (1 is 1; m is~ 2; n is 0; o is 0; p is l;
X is
CHZ), W is equal to CH2.
In yet another embodiment of the campound of formula (I-a), R, may occur one
to
four times and each occurrence is independently hydrogen or C,~ alkyl, and Rz
is
hydrogen, pyridyI, C,.~ alkyl, (C,~ alkyl)-COz-R", or -Ci7z-R". Additionally,
R, and RZ
may be attached to the same caxbon atom and form a carlbocyclic ring of 5-8
atoms
together with the carbon atom to which they are attached, or they may be
attached to the
same carbon atom and form a ring of 5-8 atoms of the formula:
CA 02342778 2000-12-20
W0 99/67230 PCT/US99/14233
-16-
~ yCH2)l~
C NRtt
~ ~~CHa)~
together with the carbon atom to which they are attached. Additionally, in
this
embodiment, R3 is hydrogen, phenyl; Ct.b alkyl, C;_6 alkc~nyl, C,_t, arylalkyl
,
{Ct_6 alkyl)-COz-Rtt, (C2~ alkenyl)-COZ-R", (Ct_6 alkyl)-CO-C,_6 alkyl,
(Ct_6 alkyl)-O-C,.~ alkyl, (Ct_6 alkyl)-OH, (C,~ alkyl)-CI\f, adamantyl or one
of the
following:
ORt t
-(CI_6 alkyl \N ~~'~,'' -(CI-6 alkyl}-- ~Z _N~ \
N ~ O (~
H q (~ N
/ / / \
\ COz-Rt t \ CO_rRt i \ \ -COz-Rt i -(C I _( alky'1]I-
N N
/ / N :, O '/~
I~(CI-3a~~~1-3 COz-Rtt I y~CF3
\ \ / ~N
OI~f
S S \
O NOz HN N \ ~ /
N ~ ~ ,,, ~~v
H COz-Rt t H COz-Rt t '''~~ ~COz-Rt z
N(C t _3alkylyz
In addition, RZ and R3-(Y)o may combine with each other at the terminal
thereof to form a
ring of the following formula together with the carbon atom and the nitrogen
atom to
which they are attached:
Z N ,",
Additionally, R4 is -O-R", NH2, NHOH, -O-(C7_to arylalkyl), or is of the
formula
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/I4233
-17-
-O-CIi2 ' ~
N
i
Also, in this embodiment, RS is a formula of the following:
Rs"-Ri 2
R~
Additionally, in this embodiment, R fi is N or CH, R7 is hydrogen or halogen,
R8 is
-NHCO-, It9 may occur one to three times and is a halogen, C,_6 alkoxy, C,_6
alkyl or
trifluoromethyl, R,o is C,~ alkyl, or (C,_~ alkyl)-OH, or hydrogen, R" is
hydrogen or C,_6
a.lkyl,R,2 is C,~ alkyl or the following formula:
R13
R9
R" is N or CH, W is (C,~ alkyl), X is S, O, or CHZ, Y is
-CO-, -C(=O)O- or -C(=O)N(R,o)-, Z is O, CH2, or N-R,,, l is I, 2, or 3, m is
1 or 2, n is 0
or 1, o is 0 or I, p is 0 or I, q is 0 or l; and r is 0, 1, 2 or :3. This
particular embodiment of
the present invention has the provisos that
(I) when Y is -C(=O)O-, R3 cannot be hydrogen;
(2) when R4 is equal to O-(C4 alkyl), C4 alkyl is not equal to tent-butyl;
(3) in those pyrrolidine structures (I is 1; m is 2; n is 0; o is 0; p is 1; X
is
CHZ), W is equal to CH2; and
(4) the compound has an ICso value of less than Sp.M in a Jurkat CS-1 assay
and/or an ICso value of less than 50pM in a Jurkat EC assay.
In another embodiment of the compound of formu.'la (I), R, may occur one to
four
times and each occurrence is independently hydrogen or C,_6 alkyl, R, is
hydrogen,
pyridyl, C,_b alkyl, (C,_6 alkyl)-COZ-R", or -COZ-R". Additionally, R, and RZ
may be
attached to the same carbon atom and form a carbocyclic ring of 5-8 atoms
together with
the carbon atom to which they are attached, or they may be attached to the
same carbon
atom and form a ring of 5-8 atoms of the formula:
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-18-
~C/(CHz)I~NR
/ a
~(CH2)~
together with the carbon atom to which they are attached.. Also, in this
embodiment, R3 is
hydrogen, phenyl, C,_6 alkyl, C,_6 alkenyl, C,_,7 arylalkyl , (Ct_6 alkyl)-COz-
R", (CZ_6
alkenyl)-COZ-R,t, (C,_6 alkyl)-CO-C,_6 alkyl, {C,_6 alkyl)-O-C,_6 alkyl, (Ct_6
alkyl)-OH, (Ct.e
alkyl)-CN, adamantyl or one of the following:
N~ ORtt
-(C t _6 alkyl-< N ~ -(C t-6 alkylr-- ~Z - ~Z \
,N O
N (~ (~ N
H
/ / / \
\ COz-Rt t \ COz-Rt t --.COz-Rt t '-"(C 1-6 a)kY
N \ \ NJ
/ / N~ ~ ~/
IO(C t-3a~Ynl r-s COz-Rt t ~ --~~CF3
\ \ / ~N/
OHL
S \
~S -~ ~ O ~ NOz HN N \ j /
N' \ _ ~'"'~CO~-R
H COz-Rt t H COz Rt t _ t t
N(C t _3alkyi)z
In addition, RZ and R3-(Y)o may combine with each other at the terminal
thereof to form a
ring of the following formula together with the carbon ai:om and the nitrogen
atom to
which they are attached:
Z N ...,
O
In this embodiment, R4 is -O-R", NH,, NHOH, -O-(C7_,~, arylalkyl), or R4 is of
the formula:
-o-cH2 l
N
s
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-19-
In this embodiment, RS is a formula of the following:
(- / Rs_"Ri2
R~
Also, in this particular embodiment, Rd is N or CH, R, is hydrogen or halogen,
R8 is
-OCHZ-, R9 may occur one to three times and is a halogen, C,~ alkoxy, C,.~
alkyl or
trifluoromethyl, R,o is C,_6 alkyl, (C,_6 alkyl)-OH, or hydrogen, R" is
hydrogen or C,_6
alkyl, R,z is C,~ alkyl or the following formula:
Ri3
-(CH2~ R9
R,3 is N or CH, W is (C,.~ alkyl), X is S, O, or CH2, Y is -CO-,
-C(=O)O- or -C(=O)N(R,o)-, Z is O, CH2, or N-R", I is 1, 2, or 3, m is 1 or 2,
n is 0 or 1; o
is 0 or 1, p is 0 or 1, q is 0 or l, and r is 0, 1, 2 or 3. This particular
embodiment has the
following provisos:
(1) when Y is -C(=O)O-, R3 cannot be hydro3;en;
(2) when R4 is equal to O-(C, alkyl), C4 alkyl is not equal to tert-butyl;
(3) in those pyrrolidine structures (1 is 1; m is. 2; n is 0; o is 0; p is 1;
X is
CH,), W is equal to CH2;
(4) when R3 is phenyl, C,_6 alkyl, C,_" arylalk;yl, (C,.~ alkyl)-COZ R",
(C,.~ alkyl)-O- C,~ alkyl or (C,_6 alkyl)-OH, o is 0; and
{5) the compound is not
Cl
O ~ /
H O C02H ~ \
N I-
N S / Cl
H
CA 02342778 2000-12-20
WO 99/6723U PCT/US99/14233
-20-
In yet another embodiment of the compound of formula (I-a), R, may occur one
to
four times and each occurrence is independently hydrogs~n or Ct_6 alkyl, and
Rz is
hydrogen, pyridyl, C,.6 alkyl, (C,~ alkyl)-COZ-Rtt, or -COZ-R". Additionally,
R, and RZ
may be attached to the same carbon atom and form a carbocyclic ring of 5-8
atoms
together with the carbon atom to which they are attached, or they may be
attached to the
same carbon atom and form a ring of 5-8 atoms of the formula:
~ ~f CH2)l~
C NR~ 1
/ ~(CH2)r~
together with the carbon atom to which they are attached. Additionally, in
this
embodiment, R3 is hydrogen, phenyl, C,_6 alkyl, C3~ alke;nyl, C,_,~ arylalkyl
,
(C,~ alkyl)-COZ-R", (C2~ alkenyl)-COZ-R", (C,_6 alkyl)-~CO-C,_6 alkyl,
{C,~ alkyl)-O-C,_6 alkyl, {C,_6 alkyl)-OH, (C,_6 alkyl)-CN, adamantyl or one
of the
following:
N'N ~~''~,, ORtt
-(C~-6 alkyl}--~ ~~ -(C1-6 alkyl)-- ~Z - ~Z \
,N O
N ~ ~ (~ (~ N
H
,~' / / / \
\ CO~-R" -~CO~-R~ ~ -CO~-R" -(C l_6 alkyl
\ \
N N
/ / N :, O /
[O(C,-3alky4lt-s COZ-Rt t W-~~CF3
\ \ / N
OHf
S \
S ---~ O NO~ HN~N \ ( /
N ~ I -~' \ ' CO -R
H COZ_Rt t H COZ-Rt t '~ ~ 2 t t
N(C t-a~Y~2
In addition, RZ and R3-(I~a may combine with each othE;r at the terminal
thereof to form a
ring of the following formula together with the carbon atom and the nitrogen
atom to
which they are attached:
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-21-
Z N ...."
O
Additionally, R4 is -O-R", NHz, NHOH, -O-(C,_,o arylalk:yl), or is of the
formula
-O-CHy-',',
i
N
Also, in this embodiment, RS is a formula of the foilowin.g:
Rg_.Riz
R7
Additionally, in this embodiment, R.6 is N or CH, R7 is hydrogen or halogen,
Rg is -OCHZ-,
R, may occur one to three times and is a halogen, C,~ alk:oxy, C,~ alkyl or
trifluoromethyl,
R,o is C,~ alkyl, or (C,_6 alkyl)-OH, or hydrogen, R" is hydrogen or C,_6
alkyl, R,z is C,~
alkyl or the following formula:
Ri3
-(CHz}r Rg
R,3 is N or CH, W is (C,~ all;yl), X is S, O, or CH,, Y is -CO-,
-C(=O)O- or -C(=O)N(R,o}-, Z is O, CHZ, or N-R", l is 1, 2, or 3, m is 1 or 2,
n is 0 or 1, o
is 0 or 1, p is 0 or l, q is 0 or l, and r is 0, l, 2 or 3. This particular
embodiment of the
present invention has the provisos that:
(1) when Y is -C(=O)O-, R3 cannot be hydrok;en;
(2) when R4 is equal to O-(C4 alkyl), C4 alkyl is not equal to tent-butyl;
(3} in those pynrolidine structures (1 is 1; m is 2; n is 0; o is 0; p is 1; X
is
CHZ), W is equal to CH2;
(4) the compound has an ICSO value of less than Sp,M in a Jurkat CS-1 assay
and/or an ICSO value of less than SOp.M in a Jurkat EC assay;
(5) when R3 is phenyl, C,_6 alkyl, C~_" arylalk;yl, (C,_6 alkyl)-COZ-R",
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-22-
(C,_6 alkyl)-O- C,.6 alkyl or (C,~ alkyl)-OH, o is 0; and
{6) the compound is not
Cl .'w
o co2H ~ ~
H '/~ ;
N s ; ~ s / C1
In the above formula (I) the absolute stereochemistry for the bond leading to
RS is
shown, however the absolute stereochemistry has not been shown for alI
examples which
follow. It is understood that all other formulas also follow this type of
absolute
stereochemistry unless otherwise stated. Additionally, it is to be understood
by those of skill
in the art that the present invention embodies stereochemical configurations
other than those
shown. Specifically, the present invention embodies all configurations
including the various
stereoisomers. Compounds which do not meet the absolute stereochemistry in
formula (I)
should meet an activity threshold in various assays, to be explained below,
which can ensure
their efficacy as useful molecules. The present invention includes mixtures,
such as racemic
mixtures, which contain molecules having the claimed stereochemistry.
The desired compound of the present invention may be clinically used either in
a free
form or in the form of pharmaceutically acceptable salts thereof.
Pharmaceutically
acceptable salts include acid-addition salts with inorganic acid or organic
acid (e.g.,
hydrochloride, sulfate, nitrate, hydrobromide, methanesul#onate, p-
toluenesulfonate, acetate),
salt with inorganic base, organic base or amino acid (e.g., triethylamine
salt, a salt with
lysine, an alkali metal salt, an alkali earth metal salt and the like).
The compound may also be formulated into a pharmaceutical composition
comprising a therapeutically effective amount of the compound as defined above
and a
pharmaceutically acceptable carrier or diluent.
The compound can also be used for treating or preventing a4(3, adhesion
mediated
conditions in a mammal such as a human. This method. may comprise
administering to a
CA 02342778 2000-12-20
WO 99!67230 PCT/US99/14233
-23-
mammal- or a human patient an effective amount of the compound or composition
as
explained above.
This method can be used to treat such inflammatory conditions as rheumatoid
arthritis, asthma, allergy conditions, adult respiratory distress syndrome,
AIDS,
cardiovascular diseases, thrombosis or harmful platelet aggregatian,
reocclusion following
thrombolysis, allograft rejection, reperfusion injury, psori~~sis, eczema,
contact dermatitis and
other skin inflammatory diseases, osteoporosis, osteoa~.~thritis,
atherosclerosis, neoplastic
diseases including metastasis of neoplastic or cancerous growth, wound healing
enhancement, treatment of certain eye diseases such as detaching retina, Type
I diabetes,
multiple sclerosis, systemic lupus erythematosus (SLE), inflammatory and
immunoinflammatory conditions including ophthalmic inflammatory conditions and
inflammatory bowel diseases, ulcerative colitis, atheroscllerosis, regional
enteritis and other
autoimmune diseases.
As mentioned above, the compounds and compositions containing the compounds
according to the present invention are particularly useful in treating or
preventing cc4[3,
adhesion mediated conditions in a mammal such as a hi.iman. The present
inventors have
found that the compounds and compositions containing; the compounds according
to the
present invention are most useful in the treatment of asthma.
The desired compound of the present invention or pharmaceutically acceptable
salts
thereof may be administered either orally or parenterally, and it may be used
as a suitable
pharmaceutical preparation, for example, a tablet, a l;ranule, a capsule, a
powder, an
injection, and an inhalation by a conventional process.
The dose of the desired compound of the present invention or a
pharmaceutically
acceptable salt thereof varies depending on an administration method, age,
body weight, and
state of a patient, but, in general, the daily dose is preferably about 0.1 to
100 mg/kg/day,
however, 1 to 100 mglkg/day may also be suitable.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-24-
Preferred routes of administration for asthma:
It is preferred that the compound of the present invention be administered in
the form
of an Aerosol. However, other routes of administration include intravenous,
oral,
intramuscular, and subcutaneous.
S In the case of aerosol administration, compositions containing the compounds
of the
present invention can be prepared to provide for an excellent means for
administering in
aerosol form for inhalation therapy. Accordingly, the present invention will
provide far self-
propelling compositions containing the compounds of the present invention.
Propellants employed should be non-toxic and have a vapor pressure suitable
for the
conditions under which administration occurs. These ;propellants can be
fluorinated or
fluorochlorinated lower saturated aliphatic hydrocarbons. The preferred
propellants of this
type are the halogenated aikanes containing not more than two carbon atoms and
at least one
fluorine atom. Illustrative of these are trichloromonofluoromethane,
dichlorodifluoromethane, monochlorotrifluoromethane, dichloromonofluoromethane
and
1S 1,2-dichloro-1,1,2,2-tetrafluoroethane. These compounds are available from
E.I. duPont de
Nemours and Company under the trade name "Freon". These propellants may be
employed
singularly or in admixture.
In addition to the propellant, an organic solvent may also be employed. The
organic
solvent must be non-toxic and without undesirable effects on inhalation in the
amount
present in the aerosol produced. in addition, the solvent should be
substantially anhydrous,
completely miscible with the propellant or mixture of propellants employed and
have a
suitable boiling point. Examples of such solvents included non-toxic aliphatic
alcohois such
as ethanol; ethers such as ethyl ether and vinyl ether; ketones such as
acetone; and suitable
halogenated lower alkanes.
2S In addition to the organic solvent, the composition may also optionally
contain a non-
toxic hygroscopic glycol. The glycol must be suhstantiall3r miscible with the
organic solvent
and the propellant employed. Satisfactory glycols include propylene glycol,
triethylene
glycol, glycerol, butylene glycol and hexylene glycol.
The above indicated methods of administration and formulation of aerosol
compositions should not be viewed as limiting. The compounds of the present
invention can
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-25-
be formulated in anyway deemed suitable to one of ordinary skill in the art so
as to obtain the
desired effects.
Pharmaceutical Compositions
As indicated previously, the compounds of formula {I) can be formulated into
pharmaceutical compositions. In determining when a compound of formula (I} is
indicated
for the treatment of a given disease, the particular disease in question, its
severity, as well as
the age, sex, weight, and condition of the subject to be treated, must be
taken into
consideration and this perusal is to be determined by the slkill of the
attendant physician.
For medical use, the amount of a compound of formula (I) required to achieve a
therapeutic effect will, of course, vary both with the particular compound,
the route of
administration, the patient under treatment, and the particular disorder or
disease being
treated. A suitable daily dose of a compound of Formula (I), or a
pharmaceutically
acceptable salt thereof, for a mammalian subject suffering; from, or likely to
suffer from, any
1 S condition as described hereinbefore is 0.1 mg to 100 mg of the compound of
formula I, per
kilogram body weight of the mammalian subject. In the case of systematic
administration,
the dose may be in the range of 0.5 to 500 mg of the compound per kilogram
body weight,
the most preferred dosage being 0.5 to SO mglkg of mammal body weight
administered two
to three times daily. In the case of topical administration, e.g., to the skin
or eye, a suitable
dose may be in the range of 0.1 p.g to 100 pg of the compound per kilogram,
typically about
0.1 p,glkg.
In the case of oral dosing, a suitable dose of a compound of Formula (I), or a
physiologically acceptable salt thereof, may be as specified in the preceding
paragraph, but
most preferably is from 1 mg to 10 mg of the compound per kilogram, the most
preferred
dosage being from 1 mg to 5 mglkg of mammal body weight, for example, from 1
to 2
mg/kg. Most preferably, a unit dosage of an orally admitiistrable composition
encompassed
by the present invention contains less than about 1.0 g of a formula (I)
compound.
It is understood that formulation, both for human. and veterinary use, of the
present
invention may be presented to the mammal by inhalation. To achieve therapeutic
effect, the
dose may be in the range of 0.5 to 500 rng of the compound, per kg body
weight. The most
CA 02342778 2000-12-20
WO 99/67230 PCTlUS99/I4233
-26-
preferred -dosage being 0.5 to 50 mglkg of mammal body weight administered two
to three
times daily.
It is understood that the ordinarily skilled physician or veterinarian will
readily
determine and prescribe the effective amount of a compound of formula (I) to
prevent or
arrest the progress of the condition for which treatment is administered. In
so proceeding,
the physician or veterinarian could employ relatively low doses at first,
subsequently
increasing the dose until a maximum response is obtained.
The compounds and compositions of the present invention can be administered to
patients suffering from a condition listed herein in an amount which is
effective to fully or
partially alleviate undesired symptoms of the condition. The symptoms may be
caused by
inappropriate cell adhesion mediated by a4(3, integrins. Such inappropriate
cell adhesion
would typically be expected to occur as a result of increased VCAM-1 and/or CS-
1
expression on the surface of endothelial cells. Increased VCAM-1 and/or CS-1
expression
can be due to a normal inflammation response or due to abnormal inflammatory
states. In
either case, an effective dose of a compound of the invention may reduce the
increased cell
adhesion due to increased VCAM-1 expression by endothelial cells. Reducing the
adhesion
observed in the disease state by 50% can be considered an effective reduction
in adhesion.
More preferably, a reduction in adhesion by 90%, is achieved. Most preferably
adhesion
mediated by VCAM-1/a4(3, and/or CS-1 interaction is abolished by an effective
dose.
Clinically. in some instances, effect of the compound can be observed or a
decrease in white
cell infiltration into tissues or a site of injury. To achieve a therapeutic
effect, then, the
compounds or compositions of the present invention are administered to provide
a dose
effective to reduce or eliminate inappropriate cell adhesion or to alleviate
undesired
symptoms.
While it is possible for an active ingredient to be administered alone, it is
preferable
to present it as a pharmaceutical formulation comprising a compound of formula
(I) and a
pharmaceutically acceptable carrier thereof. Such formulations constitute a
further feature of
the present invention.
The formulations, both for human and veterinary medical use, of the present
invention comprise an active ingredient of formula (I), in association with a
pharmaceutically
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/I4233
-27-
acceptable carrier thereof and optionally other therapeutic; ingredient(s),
which are generally
known to be effective in treating the disease or condition encountered. The
carriers) must
be "acceptable" in the sense of being compatible v~rith the other ingredients
of the
formulations and not deleterious to the recipient thereof.
The formulations include those in a form suitable for oral, pulmonary,
ophthalmic,
rectal, parenteral (including subcutaneous, intramuscular, and intravenous),
infra-articular,
topical, nasal inhalaxion (e.g., with an aerosol) or buccal administration:
Such formulation
are understood to include long-acting formulations known in the art.
The formulations may conveniently be presented in unit dosage form and may be
prepared by any of the methods well known in the art: of pharmacy. All methods
may
include the step of bringing the active ingredient into association with the
earner which
constitutes one or more accessory ingredients. In general, the formulations
are prepared by
uniformly and intimately bringing the active ingredient into association with
a liquid carrier
or a finely divided solid carrier or both; and then, if necessary, shaping the
product into the
desired form.
Formulations of the present invention suitable for oral administration may be
in the
form of discrete units such as capsules, cachets, tablets, or lozenges, each
containing a
predetermined amount of the active ingredient in the form of a powder or
granules; in the
form of a solution or suspension in an aqueous liquid. Formulations for other
uses could
involve a nonaqueous liquid; in the form of an oil-in-water emulsion or a
water-in-oil
_ emulsion; in the form of an aerosol; or in the form of a cream or ointment
or impregnated
into a transdermal patch for use in administering the a<;tive ingredient
transdermally, to a
patient in need thereof. The active ingredient of the present inventive
compositions may also
be administered to a patient in need thereof in the form of a bolus,
electuary, or paste.
The practitioner is referred to "Remington: The Science and Practice of
Pharmacy,"
19th Edition, - c. 1995 by the Philadelphia College of Pharmacy and Science,
as a
comprehensive tome on pharmaceutical preparations.
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99114233
-28-
Abbreviations
Ac20: Acetic anhydride
EtOAc: Ethyl acetate
BCECF-AM: 2',T-bis-(2-carboxyethyl)-5-(and 6-) carboxyfluorescein
acetoxy-
methyl ester
BOP-Cl: Bis (2-oxo-3-oxazolidinyl) phosphinic chloride
BOP Reagent: Benzotriazol-1-yloxy-tris (dimethylamino)-phosphonium
hexafluoro-
phosphate
DMEM: Dulbecco's Minimal Eagle's Media
DMF: Dimethyl formamide
DIEA: Diisopropylethylamine
EDC: 1-(3-Dimethylaminopropyl)-3-eth;ylcarbodiimide
hydrochloride
Et: Ethyl
EtOH: Ethanol
HATU: N [(Dimethylarnino)-1H 1,2,3-txi~~zolo[4,5-b~-pyridin-1-
ylmethylene]-N methylmethanaminium hexafluorophosphate
N oxide
HBSS: Hank's Balanced Salt Solution
HBTU: O-Benzotriazol-1-yl-N,N,N',N'-tetxamethyluronium
hexafluorophosphate
HOBT (HOBt): 1-Hydroxybenzotriazole
HSA: Human serum albumin
LDA: Lithium diisopropylamide
Me: Methyl
meq: milliequivalent
MeOH: Methanol
n-Bu: n-Butyl
NMP: 1-Methyl-2-pyrrolidinone
PBS: Phosphate buffered saline
Pd-C: Palladium on charcoal
Ph: Phenyl
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-29-
SPDP: - 3-(2-pyridyldithio}propionic acid N'-hydroxysuccinimide
ester
t-Bu: t-butyl
THF: Tetrahydrofuran
TFA: Trifluoroacetic acid
DMSO: dimethyl sulfoxide
HOAt: 1-hydroxy-7-azabenzotriazole
DMAP: 4-dimethylaminopyridine
FMOC: 9-fluorenylmethoxycarbonyl
Bn: benzyl
PyBOP: (benzotriazol-1-yloxy}tripynrolidinophosphonium
hexafluoro-
phosphate
BOC: tert-butoxycarbonyl
The representative
compounds
according
to the present
invention
are prepared
as described
below. The
compounds
of the present
invention
are prepared
in a similar
manner.
i
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-30-
Scheme A
HS RA-t
RA 2 A_1
HZN~'OH
O
RA-~
C ~OH q_2
H O
R-
S Rq2
C~OH A-
~[O
R3_O~O
R5
O A-4
H2N ~ w
O RA-t
RAa S Rp_y O
S Rpz O ~
C NH ~ ---~- Cn~NH pH
O ,~~ ~O~
~O O ~ A-5 Ra-O' O Rs A_9
R3_O R5
1 1
RE"~ Rp_t
S Rp_2 O S Rqz O
N~NH O~ C , NH
H A~ N ~ OH
O ~ H O A-10
R5
R5
RA-1
S Rp_2 O
N~NH Oi
O ~ A-7
R3 5
Rp_~
S Rp_2 O
C~NH OH
~y O
R3 s
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-3 I -
RA_, and RA_2 are defined independently as R,.
Scheme A describes a general method for the preparation of examples of the
formula A-5,
A-6, A-7, A-B, A-9 and A-10. A commercially available; or readily prepared
sulfur
containing amino acid of structure A-1 (for the synthesis of ~3,~i-
disubstituted cysteine
amino acids see: Stanfield, G.F.; Hruby; V.J. Synth. Cornmun.1988, 18, 531 and
references therein) is condensed with formaldehyde to afford the thiazolidine-
4-carboxylic
acid of general formula A-2 (for the condensation of alde;hydes with cysteine
or similar
sulfur containing amino acids see for example: (a) Ratner, S.; Clark, H.T. .J
Am. Chem.
Soc. 1937, 59, 200. (b) Lewis, N.J.; Inloes, R.L.; Hes, J.; Matthews, R.H.;
Miio, G. J. Med.
IO Chem. 1978, 21, 1070. (c) Oya, M.; Baba, T.; Kato, E.; l~:awashima, Y.;
Watanbe, T.
Chem. Pharm. Bull. 1982, 30, 440.}. Standard protection affords carbamate A-3
which is
readily condensed with amino acid derivative A-4 under standard peptide
synthesis
conditions to provide the psuedodipeptide A-S (for a reviiew of procedures of
peptide
synthesis see: Bodansky, M.; Bodansky, A. The Practice of Peptide Synthesis;
Springer-
Verlag: Berlin, 1984). Deprotection of the carbamate firom A-5 provides the
useful
intermediate A-6. The amine group may be reacted with a variety of
electrophilic reagents
such as: ( I ) commercially available or readily prepared sulfonyl chlorides
(for the
synthesis of sulfonyl chlorides see for examples: (a} Roblin, R.O.; Clapp,
J.W. J. Am.
Chem. Soc. 1950, 72, 4890. (b) Gilbert, E.E. in Sulfonation and Related
Reactions Olah,
G.A., Ed. John Wiley and Sons, New York; 1965. (c} Park, Y.J.; Shin, H.H:;
Kin, Y.H.
Chem Lett. 1992, 1483. (d) Kim, D.; Ko, Y.K.; Kim, S.Ff. Synthesis, 1992,
1203.) to afford
sulfonamides of general structure A-7 where Y is SOZ- (preparation S); (2)
carbonates or
chloroformates to afford carbamates of general structure A-7 where Y is COz
(preparations 2, 7, 8}; (3) isocyanates to afford areas of general structure A-
7 where Y is
CONHR3 (preparation 9); (4} phosgene or a suitable equivalent and an amine to
afford
areas of general structure A-7 where Y is CON{C,_6 alkyli)R3- (preparation 10,
also see for
example: Nowick, J.S.; Homes, D.L.; Noronha, G.; Smith, E.M.; Tram, M.N.;
Huang, S.
J. Org. Chem. 1996, 61, 3929.); (5) acid chlorides and c~~rboxylic anhydrides
to provide
amides of structure A-7 where Y is CO- (preparation 1 I}. Mild base hydrolysis
of
monoesters of general structure A-7 (preparation 6) or diesters of general
structure A-7
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-32-
(preparation 12) affords the acids' of general structure A-8. Mild base
hydrolysis of the
ester of general structure A-S provides acid A-9 (preparation 6 or 13) which
may be further
deprotected to afford the amino acid A-10 (preparation a 4).
Preparation 1
(Scheme A, A-2: where RA_, and RA_z are the same and equal to H and
stereochemistry is
s-.
~N~OH
H [~O
D-Cysteine hydrochloride monohydrate (A-1, where RA_, and RA_2 are the same
and equal
to H and stereochemistry is (,S~) (35.04 g, 0.19 mol) was dissolved in
formaldehyde (40
wt% solution in water, 38 mL) and the reaction mixture allowed to stir for 18
h at ambient
temperature. The mixture was cooled (0-5°C ) and absolute ethanol {93
mL} and pyridine
{57 mL) were added. After one hour, the precipitate was collected by
filtration, washed
with cold absolute ethanol followed by diethyl ether and dried in vacuo to
afford the title
compound {24.6 g) as a white crystalline solid: mp 181-184 °C (Lit. 194-
196 °C; Lewis,
N.J.; Tnoles, R.L.; Hes, 3. J. Med. Chem. 1978, 21, 1070.};'H NMR (DMSO-db) 8
4.22
(1 H), 4.04 (1 H), 3.86 {1 H), 3.09 (1 H), 2.24 (1 H); MS (ESI+) for C4H,NOZS
m/z 134.0
(M+H)+.
Preparation 2
(Scheme A, A-3: where RA_, and RA_2 are the same and equal to H, R3 is t-butyl
and
stereochemistry is (S})
s-.
~OH
I~N
~O~O O
zs
A solution of A-2 (Scheme A where RA_, and RA_2 are th.e same and equal to H
and
stereochemistry is (f~) (24.6 g, 0.185 mol) and di-t-butyl dicarbonate (44.4
g, 0.2 mol) in
THF (1 L) was heated to reflux for 18 h. Volatiles were; removed in vacuo and
the residue
CA 02342778 2000-12-20
WO 9916723U PCTIUS99114233
-33-
partitioned between ethyl acetate and 0.1 N NaOH. The aqueous layer was washed
with
ethyl acetate, made acidic with I .0 N HCl {pH 3-4) and i:hen extracted with
ethyl acetate.
The combined organic extracts were washed with brine, dried (Na2S04), filtered
and
concentrated in vacuo. Crystallization of the white solid. from
hexane/methylene chloride
provided the title compound (31.8 g) as white crystals: mp 132-134 °C;
[a]ZSD = 117° {c
fl.66, ethanol); IR (mull) 3002, 1747, 1635, I421, 1404, 1393, 1310, 1215,
1198, 1166,
1144, 1 I22 , 894, 862, 774 cm'';'H NMR (DMSO-d6) 8 4.57 (2 H), 4.28 {1 H),
3.09 (1 H},
1.35 (9 H); "C NMR (DMSO-db) 8 171.8, 152.5, 79.8, 60.9, 48.4, 47.7, 33.8,
32.6,27.7;
MS (ESI+) for C9H,SN04S mlz 234.2 (M+H)+; MS (EST-) for C9H,SN04S mlz 232.1 (M-
H)'
; Anal. Calcd for C9H,SNO,S: C, 46.34; H, 6.48; N, 6.00. Found: C, 46.27; H,
6.48; N,
6.03.
Preparation 3, Example 1
[S-~R*,R*)]-4-[[[I-[[4-[(2,6--Dichlorobenzoyl)amino]phenyl]methyl]-2-methoxy-2-
oxoethyl]amino]carbonyl]-3-thiazolidinecarboxylic acid 3-(I,1-dimethylethyl)
ester
1 S (Scheme A, A-5: where RA_, and RA_z are the same and equal to H, R3 is t-
butyl, RS is 4-
[(2,6-dichlorobenzoyl)amino]phenyl, and stereochemist>iy is {S,S)).
S-. H O
,~ C~'lf N.J~o
0 0° ~ ~ o cl
H~I ~
CI
To a cooled (0-5°C ) suspension of A-3 (Scheme A where RA_, and R.A_2
are the same and
equal to H, R3 is t-butyl and stereochemistry is (S)) (8.6 i' g, 37.2 mmol)
and HOBT (5.69
g, 37.2 mmol) in CHZCIZ (60 mL) was added a solution of EDC (7.12 g, 37.2
mmol) in
CHZC12 (I40 mL). After 30 min at 0-5°C , A-4 (where P:5 is 4-[(2,6-
dichlorobenzoyl)-
amino]phenyl, and stereochemistry is (S)) (10 g, 24.8 mmol) was added followed
by 4-
methylmorpholine (2.72 mL, 24.8 mmoI). The reaction mixture was gradually
warmed to
ambient temperature, stirred an additional l8 h and diluted with CHZCl2 and
0.1 N HCI.
The organic layer was separated and washed with 0.1 N HCI, sat. aqueous
NaHC03, brine,
CA 02342778 2000-12-20
WO 99/67230 PCT/US99l14233
-34-
dried (NaZS04), filtered and concentrated in vacuo. Flash. chromatography of
the residue
using hexane/ethyl acetate (50%) as eluant afforded the title compound (13.9
g) as a white
solid. Recrystallization from acetone/hexane afforded a crystalline solid: mp
222-224°C;
IR (mull) 3282, 3254, 1738, 1714, 1707, 1678, 1662, 1610, I562, 1545, 1431,
1414, 1287,
1256, 784 crri';'H NMR {CDCl3) b 7.57 (2 H), 7.34 (5 H:), 7.14 (2 H}, 4.74 (3
H), 4.30
(1 H), 3.74 {3 H), 3.37 (1 H), 3.15 (3 H), 1.45 {9 H);'3C 1VMR (DMSO-db) 5
172.1, 162.3,
153.2, 137.6, 136.9, 133.2, 131.8, 131.7, 130.1, 128.7, 119.8, 80.4, 62.2,
53.7, 52.4, 36.7,
28.3; MS (ESI+) for CZ6H2~C1zN3O6S m/Z 604.3 (M+Na)+:; Anai. Calcd for
CZ6Hz9C13N3O6S:
C, 53.6I ; H, 5.02; N, 7.2I . Found C, 53.82; H, 4.81; N, 7.22.
Preparation 4
(Scheme A, A-6: where RA_, and RA_z are the same and equal to H, RS is 4-[(2,6-
dichlorobenzoyl)amino]phenyl, and stereochemistry is (S,S)).
s-. H o
CN~N~Oi
H ISO
O CI
H/ II
C ~I
To a cooled (5-10°C ) solution of A-5 (Scheme A where RA_, and RA_z are
the same and
equal to H, R3 is t-butyl, R5 is 4-[(2,6-dichlarobenzoyl)annino]phenyl, and
stereochemistry
is (S,S)) (3.3 g, 5.67 mmol) in dioxane (34 mL) was added a solution of HCl in
dioxane (4
M, 140 mL) in a dropwise manner over 30 min. After an additional 30 min at 0-
5°C , the
ice bath was removed and the reaction mixture stirred 1 h at ambient
temperature. The
volatiles were removed in vacuo to afford the title compound (2.94 g) as a
light yellow
solid: 'H NMR (DMSO-db) 8 10.70 (I H), 9.1 (1 H), 7.52 (5 H), 7.I8 {2 H), 4.64
{1 H},
4.37 (1 H), 4.2I (2 H), 3.67 (3 H), 3.10 (1 H), 2.89 (1 H)., 2.70 (1 H);'3C
NMR {DMSO-
db) b 172.1, 162.3, 153.2, 137.6, 136.9, 133.2, 131.8, 131.7, 130.1, 128.7,
119.8, 80.4,
62.2, 53.7, 52.4, 36.7, 28.3; MS (ESI+) for CZ,HZ,C12N3O'aS m/z 482.I (M+H)+.
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-35-
Preparation 5 arid Example 2.
4-[(2,6-Dichlorobenzoyl)amino]-N-[ j(4S}-~3-(methyisulfonyl}-4
thiazolidinylJcarbonyl]-L phenylalanine methyl ester
(Scheme A, A-7: where RA_, and RA_2 are the same and equal to H, R3 is methyl,
Y is SOz,
RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (S, S)).
0
c~.~r "~.~~
i o <:I
H ~~
C ~I
To a cooled (0-5°C ) solution of A-6 (Scheme A, where RA_, and RA_Z are
the same and
equal to H, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is
(S, S)) (2.0
g, 3.86 mmol) in anhydrous THF (50 mL) was added meahanesulfonyl chloride
(2.99 mL,
38.6 mrnol} followed by pyridine (6.24 mL, 77.2 mmol}. After 1 h at 0-
5°C , the ice bath
was removed and the solution stirred at ambient temperature for 2 h then
diluted with ethyl
acetate and 0.25 N HCI. The layers were separated and the organic layer washed
with sat.
aqueous NaHC03, brine, dried (MgS04), filtered and concentrated in vacuo.
Purif canon
of the residue by flash chromatography using ethyl acetate/methylene
chloride/hexane
( 1:1:1 ) and isopropanol {0.1 %) as eluant afforded the title compound ( 1.99
g) as an
amorphous powder: IR {mull) 1742, 1666, 1605, 1562, :1534, 1515, 1432, 1413,
1344,
1327, 1269, 1218, 1195, 1156, 780 cm'; 'H NMR (300 rvlHz, CDC13) b 7.55 (3 H),
7.33
(3 H), 7.12 {3 H}, 4.84 { 1 H), 4.69 ( 1 H), 4.61 ( 1 H), 4.29 ( 1 H), 3.74 (3
H), 3.50 ( 1 H),
3.29 (1 H), 3.14 (2 H), 2.92 (3 H);'3C NMR {75 MHz, C'DC13) 8 171.2, 168.1,
162.4,
136.5, 135.9, 132.3, 132.2, 130.8, 130.0, 128.1, 120.6, 64.9, 53.2, 52.5,
51.8, 37.2, 34.1;
MS {ESI+) for CZZHzsC1zN306S2 mlz 559.8 (M+H)T; HRMS {FAB) calcd for
CZZH23CLZN3O6Sz+H, 560.0483, found 560.0504; Anal. (:alcd for CzZHz3CIzNsObSx~
C,
47.15; H, 4.14; N, 7.50. Found: C, 46.88; H, 4.32; N, 7.'16.
CA 02342778 2000-12-20
WO 99167230 PCT/US99J14233
-36-
Preparation 6 and Example 3.
4-[(2,6-Dichlorobenzoyl)amino]-N-[[{4S}-3--(methylsulfonyl~4-
thiazolidinyl]carbonyl]-~-phenylalanine
(Scheme A, A-8: where RA_, and RA_Z are the same and equal to H, R3 is methyl,
Y is SO2,
RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (S, S)).
S-. H O
~N~N v -OH
O S 00
O (:I
H
CI
To a cooled (0-5°C ) solution of A-7 (Scheme A, where :R,,_, and R,,_2
are the same and
equal to H, R3 is methyl, Y is SO2, RS is 4-[(2,6-dichIorobenzoyl)amino]phenyl
and
stereochemistry is (S, S)) ( 1.75 g, 3 . I 2 mmol) in THF ( 100 mL) and water
( 10 mL) was
added an 0.1 N aqueous solution of NaOH (34.3 mL, 3.4.3 mmol) via a syringe
pump over
1 h. After an additional 45 min at 0-5°C, the reaction mixture was
diluted with ethyl
acetate and acidified with 0.25 N HCI to a pH of ca. 3. The organic layer was
separated,
washed with water and concentrated in vacuo. Purification of the residue by
flash
chromatography using methylene chloride and methanol (0-5%) as eluant provided
a solid
which was lyophilized from glacial acetic acid to provide the title compound
(1.42g) as an
amorphous powder: [a]ZSO = 103°(c 0.97, ethanol); IR (mull} 3291, 1736,
1666, 1605,
1562; 1534, I516, 1432, 1414, 1339, 1270, 1195, 1 I54, '799, 780 cm'. 'H NMR
(300 MHz, CD3OD) b 7.58 (2 H), 7.45 (3 H), 7.25 (2 H},, 4.72 (2 H), 4.37 (1
H), 3.17
(S H), 2.99 (3 H); '3C NMR (75 MHz, CD3OD) 8 174.1, 171.6, 165.2, 138.3,
I37.7, 134.8,
133.4, 132.4, 131.2, 129.4, 121.6, 66.2, SS.O, 52.9, 37.9, 37.5, 35.7; MS
(ESI+) for
C2lH2EC12N3O6S2 ~z 545.8 (M+H)*; HRMS (FAB) calcd for C,,HZ,CLZN306S2+H,
546.0327, found 546.0358. Anal. Calcd for CZ,HZ,C12N3O6Sz: C, 46.16; H, 3.87;
N, 7.69.
2S Found: C, 46.24; H, 4.04; N, 7.33.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-37-
Preparation 7 and Example 4.
[S-(R*,R*)]-4-[[[1-j[4-[{2,6-Dichlorobenzoyl)amino]phenyl]methyl]-2-methoxy-2
oxoethyl]amino]carbonyl]-3-thiazolidinecarboxylic acid 3-ethyl ester
(Scheme A, A-7: where RA_, and RA_2 are the same and equal to H, R3 is ethyl,
Y is COZ-,
RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereoc.hemistry is (S, S)).
S-. H O
~N~ N~Oi
/'~O~O O ~ \ ~ C7
N \
H
Cite
To a cooled (0-5°C ) solution of A-6 (where RA_, and R,~_Z are the same
and equal to H, RS
is 4-[{2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (S, S)) (1.25
g, 2.40
mmol) in anhydrous THF (30 mL) was added ethyl chloroformate (340 p,L, 3.60
mmol)
followed by triethylamine (810 ~L, 5.79 mmoi). After 1 h at 0-5°C , the
ice bath was
removed and the solution stirred at ambient temperature. for 2 h then diluted
with ethyl
acetate and 0.25 N HCI. The layers were separated and the organic layer washed
with sat.
aqueous NaHC03, brine, dried (MgS04), filtered and concentrated in vacuo.
Purif ration
of the residue by flash chromatography using ethyl acet;ate/methylene
chloride/hexane
( 1:1:1 ) and isopropanol {0.1 %) as eluant afforded the title compound ( 1.10
g) as an
amorphous powder: 'H NMR (300 MHz, CDC13) 8 7.59 (2 H), 7.30 (3 H), 7.10 {2
H),
4.81 (1 H), 4.72 {2 H), 4.38 (I H), 4.11 (2 H), 3.I9 (4 H), 1.25 (3 H);'3C NMR
(75 MHz, CDCl3) 8 171.6, 163.0, 136.9, 136.2, 132.3, I32.1, 130.7, 129.8,
128.0, 120.3,
63.0, 62.8, 57.1, 53.2, 52.5, 37.0, 14.4; MS (ESI+) for C:z4HzsC1aNa06S m/Z
SS4.2 {M+H)+;
MS {FAB) m/z (rel. intensity) 554 (MH+, 99), 557 (29), 556 (76), 555 (45), 554
(99), 349
(35), 245 (27), 175 (35), 173 (52), 160 (93), 88 (38); HFtMS (FAB) calcd for
Cz4Hz5CL2N306S +H, 554.0919, found 554.0908. Anal. Calcd for C24HzsClaN306S:
C,
51.99; H, 4.55; N, 7.58.
Found: C, 52.05; H, 4.67; N, 7.44.
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
_3 8-
Preparation 8 and Example S .
[S-(R*,R*)]-4-[[ji-j4-[(2,6-Dichlorophenyl)methoxy]phenyl]methyl]-2-methoxy-2--
oxoethyi]amino]carbonyl]-3-thiazolidinecarboxyiic acrid 3-j2-(1-
piperidinyi)ethyl] ester
(Scheme A, A-7: where R"_, and R"_z are the.same and equal to H, R3 is 2-(1-
S piperidinyl)ethyl, Y is COZ-, R5 is 4-((2,6-dichlorophenyl)methoxy]phenyl
and
stereochemistry is (S, S)).
S- H OI'
~N~N~Oi
~N~'O~O ~ ~ CI
CI
The title compound was prepared by a modification of i:he literature procedure
of Ghosh,
A.K.; Duong, T.T.; McKee, S.P.; Thompson, W.J. Tetrahedron Lett. 1992, 33,
2781. To a
solution of 1-(2-hydroxyethyl)piperidine (S.I1 g, 39.6 rnmol) in CH3CN (220
rnL) at
ambient temperature was added N,N-disuccinimidyl carbonate (10.13 g, 39.6
mmol) and
triethylamine (16.6 mL, I I8.8 mmol). The solution was stirred at room
temperature for 4
1 S h and concentrated in vacuo to give a viscous oil. The oil was dissolved
in a minimal
amount of methylene chloride (SO mL) and added to a solution of A-b (Scheme A,
where
RA_, and RA_2 are the same and equal to H, RS is 4-[(2,6-
~dichlorophenyl)methoxy]phenyl
and stereochemistry is (S, S)) (2.0 g, 3.96 rnmol), trieth~ylamine (0.60 mL)
and DMAP (S
mg) in CHZCIZ (10 mL). The solution was stirred overnight and an additional S
equivalents
of carbonate in methylene chloride (10 mL) [prepared as described above from
N,N-
disuccinimidyl carbonate (S.6 g, 19.8 mmol), triethylamine (8.3 mL, 59.4
mmol), and I-
(2-hydroxyethyl)piperidine (2.56 g, I9.8 mmol)] were aldded . After 3 h at
room
temperature, propylamine (30 mL, 0.71 mol) was slowly added (exothermic) and
the
solution diluted with CHZCIz. The resulting solution w~us stirred vigorously
for IS min.
and diluted with water. The organic layer was separated and washed with 0.1 M
HCI, sat.
aqueous NaHC03, dried (MgS04), filtered and concentrated in vacuo.
Purification of the
residue by flash chromatography using ethyl acetate/meahylene chloride (3:1 )
as eluant
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-39-
followed by trituration in hexanes afforded the title compound ( 1.54 g, 62%)
as an white
powder: IR (mull) 1745, 1704, 1660, 1553, 1512, 1435;, 1425, 1397, 1303, L245,
1227,
1212, I 173, 1019, 765 cm -';'H NMR (300 MHz, DMS~D-db) 8 8.42 (I H), 7.54 (2
H),
7.44 (1 H), 7.13 (2 H}, 6.94 {2 H), 5.17 (2 H), 4.59 (2 H}, 4.48 (I H), 4.26
(1 H), 4.02
{2 H), 3.63 (3 H), 3.19 {2 H), 3.19 (I H), 2.82 (3 H), 2.35 (4 H), 1.36 (6 H);
'3C NMR (75
MHz, DMSO-db) 8 172.2; 170.0, 157.7, 153.9, 136.5, 132.2, 132.0, 130.8, 130.1,
129.2,
114.8, 65.3, 63.6, 57.3, 54.6, 53.9, 52.4, 36.4, 26.0, 24.3; MS (ESl+) for
Cz9H35CI2N3O6S
m/z 623.9 (M+H}+; Anal. Calcd for C2gH35C12N3O6S: C, :55.77; H; 5.65; N, 6.73.
Found: C,
55.48;H,5.73;N,6.9I.
Preparation 9 and Example 6.
4-[(2,6-Dichlorobenzoyl)amino]-N-[[{4S)--3-[({ 1,1-
d.imethylethyl)amino)carbonyl]-4
thiazolidinyl]carbonyl]-1.-phenylalanline methyl ester
(Scheme A, A-7: where RA_, and RA_2 are the same and equal to H, R3 is t-
butyl, Y is
CONH-, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (S,
S)).
S-. H O
~~~ N ~Oi
O O ' ~ ~ CI
N
H
CI ~
To a cooled (0-5°C ) solution of A-6 (Scheme A, where RA_, and RA.2 are
the same and
equal to H, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is
(S, S)) (140
mg, 0.27 mmoI) in anhydrous THF (6 mL) was added tent-butyl isocyanate (0.62
mL, 5.4
mmol} followed by 4-dimethylaminopyridine {5 mg, 0.04 mmol). After 0.5 h at 0-
5°C, the
ice bath was removed and the solution stirred at ambienl: temperature for 16
h. Additional
tent-butyl isocyanate (0.62 mL, 5.4 mmol} was added and the solution warmed to
50°C for
4 h. The reaction mixture was cooled to room temperature and diluted with
ethyl acetate
and 0.25 N HCI. The layers separated and the organic layer washed with sat.
aqueous
NaHC03, brine, dried (MgS04), filtered and concentrated in vacuo. Purification
of the
residue by flash chromatography using ethyl acetate/mel:hylene chloride
(1:1:1) and
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-40-
isopropanol (0.1%) as eluant afforded the title compound) {150 mg) as an
amorphous
powder: 'H NMR (300 MHz, CDC13) 8 7.93 (1 H), 7.54 (2 H}, 7.26 (4 ~I), 7.09 (2
H}, 4.74
(2 H), 4.66 (1 H), 4.41 (1 H), 4.23 (I H), 3.70 (3 H); 3.28 (1 H), 3.09 (3 H),
1.31 (9 H);
'3C NMR (75 MHz, CDCl3) S 171.4, 170.6, 162.4, 155.5,; 136.3, 135.9, 132.5,
132.3,
130.8, 129.9, 128.1, 120.6, 62.7, 53.2, 52.4, 51.5, 49.0, 37.0, 32.9, 29.2; MS
(ESI+) for
Cz6H3oCI2N4OsS mlZ 581.0 (M+H}+, 603.0 (M+Na)'; MS (FAB) mlz (rel. intensity)
581
{MH+, 23), 482 (SO), 97 (36}, 88 (36}, 83 (4S), 69 {99), 57 (81), 55 {79), 43
(SO), 43 (69),
41 (50). HRMS (FAB) calcd for CZ6H3oCLzN4O5S +H1 581.1392, found 581.1376.
Preparation 10 and Example 7.
4-[(2,fi-Dichlorobenzoyl)amino]-N-[[(453-[(<iiethylamino)carbonyl]-4-
thiazolidinyl]carbonyl]-t,-phenylalaniine methyl ester
{Scheme A, A-7: where RA_, and RA_2 are the same and equal to H, R; is ethyl,
Y is
CON(CHZCH3)-, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry
is (S,
S-. H O'I
~NI~N~Oi
~N~O O l ~ O CI
J ~N
H ~~
CI
The title compound was prepared by a modification of the literature procedure
of Majer,
P.; Randad, R.S. J. Org. Chem. 1994, S9, 1937. A cooled (0-5°C )
solution of A-6
(Scheme A, where RA_, and RA_2 are the same and equal to H, RS is 4-[{2,6-
dichlorobenzoyl)arnino]phenyl and stereochemistry is (S, S)) (200 mg, 0.39
mrnol} and
triethylamine (56 p,L, 0.40 mmoI) in anhydrous methylene chloride (10 mL) was
added to
triphosgene (47 mg; 0. i 6 mmol) followed by additional t:riethylarnine (56
P,L, 0.40 mmol).
After 0.5 h at 0-5°C , the ice bath was removed and the solution
stirred at ambient
temperature for 2 h. The solution was re-cooled (0-5°C ;E and
diethylamine (1.20 mL,
11.70 mmol} and 4-dimethylarninopyridine {S mg, 0.04 mmol} were added. After
0.5 h at
0-S°C , the ice bath was removed and the solution stirred at ambient
temperature for 16 h.
CA 02342778 2000-12-20
WO 99167230 PCTlUS99/14233
-41-
The solution was concentrated in vacuo and the residue ;partitioned between
ethyl acetate
and 0.25 N HCI. The layers were separated and the orgamic layer washed with
sat.
aqueous NaHC03, brine, dried (MgS04), filtered and co~;~centrated in vacuo.
Purif cation
of the residue by flash chromatography using ethyl acetate/methylene
chloride/hexane
(1:1:1) and isopropanol (0.1%) as eluant afforded the tithe compound (200 mg)
as an
amorphous solid: 'H NMR (300 MHz, CDC13} 8 7.86 (1 H), 7.55 (2 H), 7.30 (3 H),
7.03
(2 H), 6.90 (1 H), 5.09 (1 H), 4.83 (1 H), 4.33 (2 H), 3.76 (3 H}, 3.34 (3 H),
3.1 I (5 H),
1.08 {6 H);'3C NMR (CDCl3) 8 171.1, 169.6, 162.3, 162.2, 136.5, 135.9, 132.3,
132.1,
130.9, 129.9, 128.1, 120.3, 64.7, 53.4, 52.7, 52.5, 42.0, :37.0; 32.4, 13.1;
MS (ESI+) for
IO CZ6H,°N4OSS m/z 580.9 (M+I-i)+; ~IR1VIS (EI) calcd for
C:Z6H3oCL2NøOSS 580.1314, found
580.1297. Anal. Calcd for CZ6H3°C12N4O5S: C, 53.70; H, 5.20; N, 9.63.
Found: C. 53.63;
H, 5.33; N, 9.36.
Preparation 11 and Examl>le 8.
[S-{R*,R*)j~1-[[[ 1-[[4-[(2,6-dichlorobenzoyl)aminajphenyljmethylj-2-methoxy-2-
I5 oxoethylJaminojcarbonyl]-y-oxo-3-thiazolidinebutanoic acid 3-methyl ester
(Scheme A, A-7: where RA_, and RA.2 are the same and equal to H; R3 is
GHZCHZCOzCH3,
Y is CO, RS is 4-[(2,6-dichlorobenzoyl}aminojphenyl, and stereochemistry is
(S,S)).
S-. H O
N N O~
°~o ° I ~ p ci
0
H I ~
CII
To a cooled (0-5°C ) solution of A-6 (Scheme A, where RA_, and RA_Z are
the same and
equal to H, RS is 4-[(2,6-dichlorobenzoyl)aminoj phenyl and stereochemistry is
(S, S))
(1.03 g, 1.72 mmol) in anhydrous CHZCIz (25 mL) was added triethylamine (460
p,L, 3.27
mmol) followed by methyl succinyl chloride (320 ~L, 2,.5$ mmol). After 1 h at
0-5°C,
the ice bath was removed and the solution stirred at ambient temperature for 2
h then
diluted with 1 N HCI. The organic layer was separated, washed with sat.
aqueous
NaHC03, brine, dried (Na2S04), filtered and concentrated in vacuo.
Crystallization of the
CA 02342778 2000-12-20
w0 99/67230 PCT/US99I14233
-42-
yellow solid from ethanol/water provided the title compound (824 mg) as a
light yellow
solid: mp 221-223 °C; IR (mull) 3275, 1748, 1731, 1687, 1626, 1610,
156I, 1542, 1517,
1430, 1416, 1326 , 1268, 1224, I 193 cm'; 'H NMR (DMSO-db) S 10.64 (1 H), 8.59
(I H),
8:24 (1 H}, 7.50 (5 H), 7.16 (2 H), 4.75 (2 H), 4.51 (2 H;I, 4.23 (1 H); 3.63
(3 H), 3.56
(3 H), 2.87 (5 H); "C NMR (DMSO-db) 8 172.9, 171.6, 169.9, 169.5, 161.9,
137.2, 136.5,
133.0, 132.8, 13I.3, 129.8, 128.3, 119.4, 61.6, 53.6, 52.11, 51.4, 48.6, 36.4,
35.7, 35.2,
33.1, 29.0, 28.9, 28.5; MS (ESI+) for CZ6Hz7C12N307S m,~z 596.0 (M+H}+; MS
(ESI-) for
CZ6Hz7C12N3O7S mlZ 593.9 (M-H)'; Anal. Calcd for CZbHz7C1zN3O7S: C, 52.35; H,
4.5d; N,
7.04. Found: C, 52.1 I; H, 4.47; N, 6.96.
Preparation 12 and Example 9.
[S-(R*,R*)j-~--[[[ 1-Carboxy-2-[4-[(2,Ei-dichlorobenzoyl)
aminojphenyljethyljaminojcarbonylj-y-oxa-3-thiazolidinebutanoic acid
(Scheme A, A-8: where RA_, and R"_2 are the same and equal to H, R3 is
CHzCH2COZCOZH, Y is CO, RS is 4-[(2,6-dichiorobenzo~yl)amino)phenyl, and
I S stereochemistry is (S,S)).
S, H O
~N~ N ~OH
HO~a IO! I ~ O~ CI
O
H I /
CI
To a cooled (0-5°C ) solution of A-7 (Scheme A where RA_, and RA_z are
the same and
equal to H, R3 is CHZCHzCOZCH3, Y is CO, RS is 4-[{2,6~-
dichlorobenzoyl)aminojphenyl,
and stereochemistry is (S,S)) (130 mg, 0.22 mmol) in anhydrous THF (5 mL) and
MeOH
( 1 mL) was added an aqueous (2 mL) solution of lithium hydroxide monohydrate
{23 mg,
0.55 mmol) via a syringe pump over I h. After an additional 1 h at 0-
5°C, the ice bath was
removed and the solution stirred 2 h at ambient temperature. The reaction
mixture was
diluted with ethyl acetate and 0.1 N HCl and the organic layer was separated,
washed with
water, dried (Na2S04), filtered and concentrated in vacuc~. Lyophilization of
the residue
from glacial acetic acid afforded the title compound (101. mg) as a white
amorphous
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-43-
powder: ~'H NMR (CD3CN) 8 8.87 (1 H), 7.SS (2 H), 7.~E2 (3 H), 7.22 {2 H),
7.I6 (1 H),
4.95 (1 H), 4.52 (3 H), 3.12 (S H), 2.SS (S H);'3C NMR (CD3CN} 8 173.8, 171.7,
171.1,
169.8, 162.5, I36.7, 136.0, 133.5, 131.7, I31.3, 130.1, 129.2, 128.2, 119.7,
62.4, 53.6,
48.8, 36.2, 32.4, 29:1, 28.6; MS (ESI-) for Cz4Hz3C12N30~,S m/z 566.1 (M-H)';
Anal. Calcd
S for C24HZSCIaN3O7S: C, 50.31; H, 4.13; N, 7.33. Found: C, 50.13; H, 4.3?; N,
6.93.
Preparation 13 and Example I0.
[S-(R*,R*)]-4-[[[1-Carboxy-2-[4-[(2,6-dichlorobenzoyl)amino]-
phenyl)ethyl]aminoJcarbonyl]-3-thiazolidinecarboxylic; acid 3-(1,1-
dimethylethyl) ester
(Scheme A, A-9: where RA_, and RA_2 are the same and equal to H, R3 is t-
butyl, Y is COZ-,
RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl, and stereochemistry is (S,S)).
/S-. H O
~~~N~OH
O.~O O ' ~ O CI
H~I ~
CI
To a cooled (0-S°C ) of A-5 (Scheme A where RA., and RA_Z are the same
and equal to H,
1S R3 is t-butyl, Y is C02-, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl, and
stereochemistry
is (S,S)) (1 SO mg, 0.26 mmol) in anhydrous THF (S mL;l and MeOH (I mL) was
added an
aqueous (2 mL) solution of lithium hydroxide monohydrate (14 mg, 0.325 mmol)
via
syringe pump over I h. After an additional i h at 0-S°C , the ice bath
was removed and
the solution stirred 2 h at ambient temperature. The reaction mixture was
diluted with
ethyl acetate and 0.1 N HCI, the organic layer separated .and washed with
water, dried
(Na2S04), filtered and concentrated in vacuo. Lyophilization of the residue
from glacial
acetic acid afforded the title compound (I42 mg) as an amorphous powder: IR
(mull)
3285, i 735, 1666, 1606, 1562, 1539, 1 S I6, 1432, 1413, 1394, 1326, 1259 ,
12I9, 1195,
i 161 cm'; 'H NMR (DMF-d7) S 10.71 (1 H), 8.36 (1 H), 7.91 (2 H}, 7.72 (3 H),
7.47
2S (2 H}, 4.88 (3 H), 4.51 (1 H), 3.40 (3 H), 3.22 (2 H), i.S7 (9 H);'3C NMR
(DMF-d7) 8
173.3, 163.1, 162.9, 162.7, 162.3, 154.0, 138.3, 137.5, 1:34.2, 132.3, 131.9,
130.6, 128.9,
120.0, 80.9, 63.1, S4.S, 49.9, 37.4, 28.3; MS {FAB) m/z (:rel. intensity) 568
(MH+, 23),
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-44-
570 (14}-, 568 (23}, 471 (13), 470 ( 65), 469 (23), 468 (99), 466 (23), 175
{19}, 88 (17), 57
{42); HRMS (FAB) calcd for Cz5Hz7CIzN306S+H, 568.1075, found 568.1071; MS (ESI-
)
for CZSH27C12N3O6S m/Z 565.8 (M-H)-; Anal. Calcd for CZSH27C1zN3O6S ~ 0.26
HzO: C,
52.38; H, 4.84; N, 7.33. Found: C, 52.07; H, 5.12; N, 7..46; % Water (KF):
0.83.
Preparation 14 and Example i 1.
4-[(2,6-Dichlorobenzoyl)amino]-N-[[(4S}-4-thiazolidinyl]carbonyl]-1,-
phenylalanine
monohydrochloride salt
(Scheme A, A-10: where RA_, and RA_2 are the same and equal to H, RS is 4-
[(2,6-
dichlorobenzoyl)aminojphenyl, and stereochemistry is (S ,S~).
/S-. H O
_OH
O
O GI
To a cooled (5-10°C ) solution of A-9 (Scheme A where RA_, and RA_Z are
the same and
equal to H, R3 is t-butyl, Y is COZ-, RS is 4-[(2,6-
dichlorobenzoyl)aminojphenyl, and
stereochemistry is (S,S)) (193 mg, 0.34 mmol) in dioxane (2 mL) was added a
solution of
HCl in dioxane (4 M, 8 mL) in a dropwise manner over :30 min. After an
additional 3.5 h
at 0-5°C, the reaction mixture was concentrated in vacuo.
Lyophilization of the residue
from water afforded the title compound {158 mg) as an amorphous powder: IR
(mull)
3248, 3191, 3048, 1731, 1664, 1605, 1577, 1562, 1541, 1516, 1431, 1414, 1327,
1195,
799 cm''; 'H NMR (DMF-d,) 8 9.11 (1 H), 7.76 (2 H), 7.60 (3 H), 7.:35 (2 H),
4.66 (2 H),
4.46 (2 H}, 3.55 (3 H), 3.24 (2 H}, 3.10 {2 H); '3C NMR (DMF-d7) 8 172.8,
167.6, 163.1,
162.9, 162.7, 162.3, 138.5, 137.5, 133.8, 132.3, 131.9, 1:30.6, 128.9, 120.0,
63.8, 54.8,
50.0, 37.3; MS (FAB) mlz (rel. intensity} 468 (MH+, 99), 544 (18), 528 (15),
472 (13),
471 ( 16), 470 (70), 469 (24), 468 (99), 175 (14), 173 (lfi), 88 (18); HRMS
(FAB) calcd
for CZOH,9CIzN304S+H, 468.0551, found 468.0556.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99I14233
-45-
Example 12.
(S-(R*,R*)]-4-((( I-Carboxy-=~>_[4-[(2,6-
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]~-3-thiazolidinecarboxylic
acid 3-
ethyl ester
(Scheme A, A-8: where RA_, and RA.Z are the same and equal to H, R3 is ethyl,
Y is COZ, RS
is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (S, S)).
,S-. H O'I
~NI~ N ~OH
~O~O O I ~ ~ CI
N
H i ~ /
CI
Example I2 was prepared from example 4 by the procedure described in
preparation 6.
Physical properties as follows: mp 118-121 °C; [cc]ZSD = I06° (c
0.88, ethanol); IR (mull)
3283, 3196, 1665, 1606, 1561, 1539, 1516, 1431, 1414, 1345, 1327, ~ 271, 1219,
I 195,
799 crri';'H NMR (300 MHz, CD30D) 8 7.59 (2 H), 7.44 {3 H), 7.22 (2 H), 4.69
(1 H),
4.64 (1 H), 4.41 (1 H); 3.24 (3 H), 2.95 (2 H), 1.26 (3 H);'3C NMR (75 MHz,
CDCl3) 8
172.4, 17I .5, i 63.0, 154.8, 136.7, 136.6, 132.4, I32.1, 1130.6, 129.9,
127.9, 120.3, 63.0,
62.7, 53.1; 36.7, 14.3; MS {FAB) mlz (rel. intensity) 540 (MH+, 59}, 544 {12),
543 {17),
542 (53), 540 (59), 160 (32), 123 (15); l I8 (20), 107 (9'9), 95 (11), 23
(2I); HRMS (FAB)
calcd for C,3H23CLZN306S +H, 540.0762, found 540.07:30. Anal. Calcd for
C23H23C12N3O6"~: C, 51.12; H, 4.29; N, 7.78. Found: C, 50.77; H, 4.43; N,
7.68.
Example 13.
[R-(R*,S * )]-4-([( I-[[4-[(2,b-Dichlorobenzoyi)amino]phenyl]methyl]-2-methoxy-
2-
oxoethyi]amino]carbonyl]-3-thiazolidinecarboxylic acid 3-(l,l-dimethylethyl)
ester
(Scheme A, A-7: where RA_, and RA_z are the same and equal to H, R3 is t-
butyl, Y is CO2,
RS is 4-((2,6-dichlorobenzoyl)amino]phenyl and stereoc:hemistry is (R, S)}.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-46-
0
N N Oi
O~
~~O C ' ~ ~ O CI
_N
H I ~
C1
Example 13 was prepared as described in Scheme A fronn L-cysteine using di-t-
butyl
dicarbonate to form the requisite carbamate. Physical data as follows: IR
(mull) 1746,
S 1666, 1606, 1562, 1538, 1 S 16, 1432, 1413, 1324, 1267, 1260, I2I6, 1195,
1162, 799 cm'';
'H NMR (CDCl3) 8 7.56 (2 H), 7.46 (1 H}, 7.33 (3 H), 7.13 (2 H), 6.94 (1 H),
4.75 (3 H),
4.25 (I H), 3.75 (3 H), 3.39 (I H), 3.14 (3 H), 1.43 (9 H);'3C NMR (DMSO-db} b
171.6,
171.5,170.7, 170.1, 161.7, 152.6, 137.0, 136.9, 136.2,1:32.6, 132.6,
131.2,131.0, 129.3,
128.1, 119.1, 79.7, 78.2, 61.5, 53.4, 53.3, 51.8, 49.3, 49.1, 35.8, 27.6; MS
(ESI+) for
C26Hz9C12NsO6S mlz 604 (M+Na)*; MS (ESI-) for Cz6H29'C12N306S mlz 580 (M-H)-;
Anal.
Calcd for Cz6Hz9C12N3O6S ~ 0.17 HzO: C, 53.34; H, 5.05; N, 7. i 8. Found: C,
53.47; H,
5.14; N, 7. I S. % Water (KF): 0.51.
Example 14.
ER-tR* ~ S * )]-4-UL I-Carboxy-2._I~~(2~~
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]-~3-thiazolidinecarboxylic
acid 3-
(1,1-dimethylethyl) ester
(Scheme A, A-8: where RA., and R,,_Z are the same and equal to H, R3 is t-
butyl, Y is COZ,
R5 is 4 j{2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (R, f~).
0
-~I' H- ~
N N v 'OH
O''~
~O O ~ ~ ~ CI
~N
H ~
CI
Example 14 was prepared from example 13 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 3285, 1665, 1607, 1562, 1538, 1516, 1432,
1413, 1394,
1327, 1259, 1217, 1195, 1162, 799 crri';'H NMR (DMSO-db) 8 12.70 (1 H); 10.67
(1 H),
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-47-
8.14 (1 H), 7.51 (5 H), 7.20 (2 H); 4.56 (1 H), 4.35 (3 H), 2.98 (3 H), 1.22
{9 H);'3C NMR
(DMSO-db) 8 172.5, 170:1, 169.9, 161.6, 152.6, 136.9, 136.3, 133.0, 131.2,
131.0,129.4,
128.1, 119.1, 79.8, 79.7, 61.6, 53.4, 49.2, 48.3, 35.9, 27.6, 20.9; HRMS (FAB)
calcd for
CZSH27C12N3O6S+H, 568.1075, found 568.1058; MS (ESI+) for CzSHz,C12N3O6S m/z
567.8
(M+H)+; MS (ESI-) for CZSH27C12N3O6S m/Z 565.8 (M-H)'; Anal. Calcd for
CZSH27CIZN3O6S
~ 0.24 H20: C, 52.43; H, 4.84; N, 7.34. Found: C, 52.23; H, 4.76; N, 7.24. %
Water (KF):
0.75.
Example 15.
[R-(R*,S*)]~4-[[[1-[[4-[(2,6-Dichlorobenzoyl)amino]phenyl]methyl]-2-methoxy-2-
oxoethyl]amino]carbonyl]-3-thiazolidinecarboxylic acid 3-ethyl ester
(Scheme A, A-7: where RA_, and RA_2 are the same and equal to H, R3 is ethyl,
Y is COz, R5
is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochennistry is (R, f)).
0
N~N~Oi
~ ~O C I ~ ~ CI
'N
Ci
Example 15 was prepared as described in Scheme A from L-cysteine using ethyl
chloroformate to form the requisite carbamate. Physical data as follows: IR
(mull) 1744,
1666, 1606, 1561, 1538, 1515, 1445, 1431, 1414, 1345, 1325; 1270, 1216, 1194,
1184 cm
'; 'H NMR (CDC13) 8 7.55 (2 H), 7.36 {4 H), 7.13 (2 H), 6.95 (1 H), 4.74 (3
H), 4.21 (3
H), 3.75 {3 H), 3.40 (1 H), 3.13 (3 H), 1.26 (3 H); '3C N:MR (CDCl3) 8 171.2,
169.4, 162.3,
136.2, 135.8, 132.4, 132.2, 131.0, 130.3, 130.1, 129.9, 128.2, 128.0, 127.9,
120.2, 62.9,
62.7, 53.2, 52.5, 37.1, 14.5, 14.3; MS (ESI+) for C24H25(..12N3O6S m/z 553.8
(M+H)+; MS
(ESI-) for Cz4H25C12N3~6S ~Z 551.8 {M-H)'; Anal. Calcd for C24H2sC1zN3O6S ~
0.24 H20:
C, 51.59; H, 4.60; N, 7.52. Found: C, 51.89; H, 4.62; N, 7.51. % Water (KF):
0.77.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-48-
Example 16.
[R-(R*~S*)]-4-j[L1-Carboxy-2._jq_[(2~6
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]-3-thiazolidinecarboxylic
acid 3
ethyl ester
(Scheme A, A-8: where RA_, and RA_2 are the same and equal to H, R3 is ethyl,
Y is CO2, RS
is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereocherra<istry is (R, S}).
S H O
y _OH
~O~O O I ~ O CI
H 11 /
C /'I
1 fl Example 16 was prepared from example 15 by the procedure described in
preparation 6.
Physical data as follows: IR {mull) 3287, 1664, 1606, 1561, 1539, 1516, 1445,
1431,
1414, 1346, 1327, 1271, 1217, 1195, 799 crri'; 'H NMR (DMSO-db) 8 12.80 (1 H),
10.69
(1 H), 8.26 (1 H}, 7.51 (5 H), 7.19 {2 H), 4.62 (2 H), 4.37 (2 H), 3.94 (2 H),
2.96 (3 H},
1.11 {3 H); "C NMR {DMSO-db) 8 i 72.5, 169.6, 161.7, 153.5, 136.9, 136.3,
133.0, 13I.2,
131.0, 129.5, 128.1, 119.1, 61.2, 53.4, 52.5, 35.9, 22.3, 14.3; MS (ESI+) for
C23H23C12N3O6S YYllZ 540.0 (M+H)+; MS (ESI-) for Cz3Hz.'C1zN306S mlz 538.0 (M-
H)-;
HRMS (FAB) calcd for C23H~3C12N3ObS+HI 540.0762, found 540.0775; Anal. Calcd
far
C23H23C12N3O6S ~ 0.34 H20: C, 50.54; H, 4.37; N, 7.69. Found: C, 50.53; H,
4.48; N, 7.59.
Water {KF): 1.13.
z0 Example 17.
jR-(R*,S*)]-4-[jj 1-[[4-j{2,6--Dichlorobenzoyl}amino]phenyl]methyl]-2-methoxy-
2
oxoethyl]amino]carbonyl]-5,5-dimethyl-3-thiazolidinecarboxylic acid 3--(l,I
dimethylethyl) ester
(Scheme A, A-7: where RA_1 and RA_z are the same and equal to CH3, R3 is t-
butyl, Y is
CO2, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and ste;reochemistry is (R,
,S~).
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-49-
<s o
N~pi
O'''~
~O O I ~ ~ CI
~ w
ti
CI
Example 17 was prepared as described in Scheme A from L-penicillamine using di-
t-butyl
dicarbonate to form the requisite carbamate. Physical data as follows: IR
(mull) 1747,
1666, 1606, 1562, 1537, 1516, 1432, 1413, 1324, 1268, 1259, 1213, 1195, l 161,
1142 cm'
';'H NMR (CDCl3) 8 7.56 (2 H), 7.34 {4 H), 7.21 (2 H), 6.44 (1 H), 4.94 (1 H),
4.60 (2 H),
4.08 (1 H), 3.70 (3 H), 3.10 (2 H), 1.53 (3 H), 1.42 (9 H:), 1.25 (3 H);'3C
NMR (CDCI,) 8
171.4, 162.3, 136.3, 135.8, 132.6, 132.4, 131.0, 130.2, 130.0, 128.2, 120.5,
120.3, 120.2,
72.7, 63.9, 60.4, 52.9, 52.3, 48.3, 38.0, 30.3; 28.1, 23.9, 21.0, 14.2; MS
(ESI+) for
CZ$H33C12N3O6S m/z 630.7 (M+Na)+; Anal. Calcd for CZ8H33CIZN3O6S ~ 0.13 HzO:
C,
54.87; H, 5.47; N, 6.86. Found: C, 54.54; H, 5.55; N, 6.54. % Water (KF):
0.38.
Example 18.
[S--(R*,R*)]-4-[[[1-[[4-[(2,6-Dichiorobenzoyl)amino]phenyl]methyl]-2-methoxy-2-
oxoethylJamino]carbonyl]-3 thiazolidinecarboxylic acid 3-[(9H-fluoren-1-
yl)methyl]
ester
(Scheme A, A-7: where RA_, and RA_2 are the same and equal to H, R, is 9-
fluorenylmethyl,
Y is CO2, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is
(S, S)).
S- : H O
_ CN~N~Oi
/ O~O ~O ~ ~ O CI
~r~ w
Ea I /
cl
Example 18 was prepared as described in Scheme A from D-cysteine using 9-
fluorenylmethyl chloroformate to form the requisite carlbamate. Physical data
as follows:
IR {mull) 3280, 1750, 1692, 1671, 1604, 1560, 1538, 1515, 1441, 1430, 1422,
1346,
1320, 1222, 1118 cm'';'H NMR (DMSO-db) 8 8.59 (1 H), 7.87 (2 H), 7.49 (12 H),
4.65
CA 02342778 2000-12-20
WO 99167230 PCT/US99/I4233
-50-
(3 H), 4.26 (4 H), 3.52 (3 H), 2.96 (3 H); t3C NMR (DMSO-db) b 171.4, 161.8,
143.5,
140.6, 137.0, 136.3, 132.5, 131.2,131.1, 129.5, 128.1, 12;7.6, 127.1, 125.2,
125.1, 120.0,
119.2, 70.6, 70.0, 63.8, 63.2, 53.3, 53.1, 46.4, 36.2, 25.4; MS (ESI+} for
C36H3,C12N3O6S
m/z 703.9 (M+H)+; Anal. Calcd for C36H3,C12N3O6S ~ O.I HZO: C,,6I.23; H,
4.:45; N, 5.95.
Found: C, 61.18; H, 4.56; N, 5.89. % Water (KF): 0.22.
Example 19.
[S-(R*,R*)]-4-[[[ I-Carboxy-2--[4-[(2,6-
dichlorobenzoyl)amino]phenyl]ethyl]arrZino]carbonyl]-:3-thiazolidinecarboxylic
acid 3-
[(9H-fluoren-I-yl)methyl] ester
(Scheme A, A-8: where RA_, and RA_2 are the same and equal to H, R3 is 9-
fluorenylmethyl,
Y is CO2, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl arnd stereochemistry is
(S, S)).PNU-
/S-.. H O
~N~N~OH
/ O~O O I ~ ~J CI
~N~
H
Gt
Example 19 was prepared from example I 8 by the procedure described in
preparation 6.
Physical data as follows: IR {mull) 1672, 1606, 1561, 1533, 1517, 1431, 1413,
1347,
1324; 1269, 1218, 1195, 1 i 16, 760, 742 cm'';'H NMR (:DMSO-db) 8 10.71 (1 H),
8.32
(1 H), 7.87 (2 H), 7.47 (13 H), 7.16 (2 H), 4.62 (2 H), 4.15 (5 H), 2.90 (4
H); '3C NMR
(DMSO-d6} S 172.4, 169.2, i 61.8, 143.6, 140.6, 137.0, 136.3, I33.0, 131.3,
131.1, 129.7,
129.6. 128.9, 128.2, 127.7, 127.1, 126.8, 125.2, 121.3, 12O.I, 120.0, 119.2,
53.4, 48.4,
46.4, 36.4, 29.5; 20.0; MS (ESI+) for C35H29CIZN3O6S mlz 690.1 (M+H)+; Anal.
Calcd for
C35H29CIZN3O6S ~ 0.4 H20: C, 60.25; H, 4.30; N, 6.02. Found: C, 59.88; H,
4.47; N, 5.75.
Water (KF): 1.02.
Example 20.
[S--(R*,R*)]-4-[[[1-[[4-[(2,6-Dichlorobenzoyl)amino~~phenyl]methyl]-2-methoxy-
2-
oxoethyl]amino]carbonyl]-3-thiazolidinecarboxylin acid 3-phenylmethyl ester
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-S 1-
(Scheme A, A-7: where RA_, and RA_2 are the same and adual to H, R3 is
phenylmethyl, Y is
CO2, RS is 4-[(2,6-dichlorobenz°yl)amino]phenyl and ste;reochemistry is
(S, S)).
s-. w o
CN~ N~°i
O~O l° ~ % ~> CI
N W
H
CI
Example 20 was prepared as described in Scheme A from D-cysteine using benzyl
chloroformate to form the requisite carbamate. Physical data as follows: IR
(mull) 1748,
1694, 1690, 1673, 1610, 1561, iS42, 1517, 1441, 1430, 1408, 1355, 1324, 1269,
1217 cm
-'; 1H NMR (CDCl3) 8 7.50 (2 H), 7.33 (9 H), 7.09 (2 H), 6.75 (1 H), S.I9 (2
H), 4.78
(3 H), 4.38 (3 H), 3.73 (3 H), 3.20 (3 H);'3C NMR (DMSO-db) 8 171.4, 162.3,
136.3,
135.9, 135.7, 132.5, 132.4, 131.0, 130.0, 128.7, 128.4, 128.2, 128.1, 120.4,
68.2, 63.3,
53.2, S2.S, 37.2; MS (ESI+) for CZ9H27CIzN3O6S mlz 637.8 (M+Na)+; MS (ESI-)
for
CZ9Hz7CI,N306S m/z 613.8 (M-H)'; Anal. Calcd for C29Hz7C12N3O6S ~ 0.1 H20: C,
56.39; H,
4.43; N, 6.80. Found: C, 56.31; H; 4.67; N, 6.71. % Water (KF): 0.19.
1 S Example 21.
[S--(R*,R*)]-4-[[[1-Carboxy-2-[4-[(2,6-
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]--3-thiazolidinecarboxylic
acid 3-
phenylmethyl ester
(Scheme A, A-8: where RA_, and RA_z are the same and equal to H, R3 is
phenylmethyl, Y is
COZ, RS is 4-[(2;6-dichlorobenzoyi)amino phenyl and st<~reochemistry is (S,
S)).
s-. H_ o
N~OH
0 0 ° ( % p cl
n
cl
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-52-
Example 2I was prepared from example 20 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 3290, 3034, 1666, 1606, 1562, 1537, 1516,
1431,
1413, 1351, 1326, 1270, 1215, 1195, 799 ciri';'H NMR (DMSO-d6) & 12.85 (1 H),
10.65
( 1 H), 7.39 { 10 H), 7.18 (2 H), 4.98 (2 H), 4.65 (2 H), 4.5 5 ( 1 H), 4.33 (
1 H), 3.06 ( I H),
2.83 {2 H); '3C NMR (CD30D) 8 172.6, 163.7, 154.4, 13.6:8, 136.2, 136.1,
131.9, 130.9,
129.6, 128.1, 127.9, 127.8, 127.7, 127.6, 120.0, 67.5, 66.7, 53.4, 36.5; MS
(ESI-) for
Cz8H25C12N3O6S mlz 599.7 (M-H)'; MS (FAB) mlz (rel. intensity) 602 (MH+, 99),
678
(37), 604 {74), 603 (33), 602 ( 99), 560 (32), 558 (48), 468 (35),466 (51),
371 (50); 91
(73); HRMS (FAB) calcd for CZgHz5C12N306S+H' 602.0'x19, found 602.0913; Anal.
Calcd
for CZBHZSCI,N3O6S ~ 0.23 H20: C, 55.45; H, 4.23; N, 6.x)3. Found: C, SS.S3;
H, 4.46; N,
6.88. % Water {KF): 0.67.
Example 22.
[S-{R*,R*)]-4-[[[I-[[4-[{2,6-Dichlorobenzoyl)amino]phenyl]methyl]-2-methoxy-2-
oxoethyl]amino]carbonyl]-3-thiazoIidinecarboxylic acid 3-
{tricyclo[3.3.1.13~7]dec-1-yl)
ester
(Scheme A, A-7: where RA_, and RA_2 are the same and equal to H, R3 is 1-
adamantyl, Y is
CO2, RS is 4-[(2,6-dichlorabenzoyl)amino]phenyl and st~ereochemistry is (S,
S)).
S-. H O
CN~N~Oi
~p~p ~O1 I ~ CJ CI
~N~
H I
GI
Example 22 was prepared as described in Scheme A froam D-cysteine using I-
adamantyl
fluoroformate to form the requisite carbamate. Physical data as follows: IR
(mull) 3284,
3271, 1747, 1690, 1684, 1666, 1557, 1532, 1436, 1412, 1355, 1298, 1194, 1053,
799 crri ';
'H NMR (DMSO-db) b 10.67 {1 H), 8.41 {1 H}, 7.53 (5 :H), 7.1? (2 H), 4.51 (3
H), 4.23
( I H), 3.63 (3 H}, 3.22 { 1 H}, 3.04 ( I H), 2.90 { 1 H), 2.75 ( 1 H), 2.01
(9 H), 1.56 (6 H);
'3C NMR (DMSO-db) 8 172.1, 162.3, 152.7, I37.b, 136.9, 133.2, I31.8, 131.6,
130.0,
128.7, 119.8, 53.8, 52.4, 36.7, 36.1, 30.6; MS (ESI+) fo:r C32H35C12N3~6'S ~Z
659.7
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-53-
(M+H)+;~MS (ESI-) for C32H35C12N3~6's ~Z 657.7 (M-H;I'; MS (FAB) m/z (rel.
intensity)
660 (MH+, 8), d62 (5), 660 (8), 618 (6), 616 (8), 480 (5;1, 173 (7), I36 {11),
135 (99); 123
(14), 93 (8); Anal. Calcd for CgZH35C12N3O6S ~ 0.04 HZO: C, 58.12; H, 5.35; N,
6.35..
Found: C, 58.19; H, 5.62; N, 6.25. % Water (KF): 0.10.
Example 23.
[S-(R*,R*)J-4-[[[ 1-Carboxy-2--[4--[(2,b-
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]-3 thiazolidinecarboxylic
acid 3
(tricyclo[3.3.1.137]dec-1-yll) ester
(Scheme A, A-8: where RA_, and RA_Z are the same and equal to H, R3 is 1-
adamantyl, Y is
CO2, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and ste:reochemistry is (S,
S)).
S-. H O
~~~N~OH
~O O O ! ~ O'i C1
~N~
H 1 ~
Ci'
Example 23 was prepared from example 22 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 3287, 1667, 1606, 1562, 1537, 1516, 1431,
1412,
1353, 1326, 1299, 1274, 1220, 1194, 1049 cm ';'H NMR (DMSO-db) 8 12.70 {1 H),
10.67 (1 H), 8.21 (1 H), 7.58 (5 H), 7.17 (2 H), 4.51 {3 I=t), 4.22 (1 H),
3.20 (1 H), 3.04
(1 H}, 2.88 (1 H}, 2.76 (1 H), 2.07 (9 H), 1.12 (6 H);'3C NMR (DMSO-db} 8
173.1, 162.3,
152.7, 137.5, 136.9, 133.8, 131.8, 131.6, 130.1, 128.7, 119.7, 80.0, 53.8,
36.8, 36.1, 30.6;
MS (ESI+) for C3,H33C12N3O6S m/Z 645.8 (M+H)'; HRIVIS (FAB) calcd for
C3,H3,C12N~O6S+H~ 646.1545, found 646.1564; Anal. C;~lcd for C3'H33C12N3ObS ~
0.29
H20: C, 57.13; H, 5.19; N, 6.45. Found: C, 56.82; H, 5.21; N, 6.32. % Water
{KF): 0.80.
Example 24.
[S-(R*,R*)]~--[[[1-[[4-[(2,6-Dichlorobenzoyi)amino]phenylJmethyl]-2-methoxy-2-
oxoethyi]amino]carbonyl]-3-thiazalidinecarboxylic acid 3-[2--(4-
morpholinyl)ethyl]
ester
CA 02342778 2000-12-20
WO 99167230 PCTIUS99114233
-54-
(Scheme-A, A-7: where RA_, and RA_Z are the same and equal to H, R3 is 2-(4-
morpholinyl)ethyl, Y is COz-, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and
stereochemistry is (S, S)).
S-. O
~N~N~Oi
~N~'~Q~p 'O' I ~ O CI
H~ I /
Ca
Example 24 was prepared as described in Scheme A from D-cysteine using 4-(2-
hydroxyethyl)morpholine to form the requisite carbamat<:. Physical data as
follows: IR
(mull) 1745, 1705, 1679, 1605, 1536, 1515, 1431, 1414, 1344, 1323, 1269, 1216,
1194,
1183, 1117 cm' 1H NMR (300 MHz, CDCl3} 8 7.82 (1 H}, 7.56 (2 H), 7.31 (3 H),
7.12
(2 H), 6.95 (1 H}, 4.65 (3 H), 4.39 (3 H), 3.74 (3 H), 3.69 {3 H), 3.34 (1 H),
3.18 (3 H),
2.763 (5 H); "C NMR (7S MHz, CDCl3} $ 171.5, 169.8, 162.9, 154.4, 136.8,
136.1,
132.1, 130.6, 129.7, 127.9, 120.2, 66.3, 62.7, 57.0, 53.4, 53.2, 52.4, 36.7,
29.5; MS {ESI-)
for CZ8H32C1zN4O7S mlZ 636.8 {M-H}'; HRMS (FAB) calcd for CZ$H3zCLZN407S +H'
639.1447, found 639.1419. Anal. Calcd for CZ8H32CI,N4O7S: C, 52.58; H, 5.04;
N, 8.76.
Found: C, 52.47; H, 5.17; N, 8.69.
Example 25.
[S-(R*,R* )]4-[[[ 1-Carboxy-2-[4-[{2,6-
dichlorobenzoyl)amino]phenyljethyl]amino]carbonyl]-3-thiazolidinecarboxylic
acid 3-
[2-(4-morpholinyl)ethyI] ester
(Scheme A, A-8: where R,~_, and RA_2 are the same and equal to H, R3 is 2-(4-
morpholinyl)ethyl, Y is COZ-, Rs is 4-[(2,6-dichiorobenzoyl)amino]phenyl and
stereochemistry is (S, S)}.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-55-
O
O~ ~N~N~OH
~N~O~° O I ~ p Ci
H
~G
Example 2S was prepared from example 24 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 3278, 1667, 1606, 1.562, 1541, 1515, 1431,
1413,
1351, 1326, 1270, 1195, 1 I34, 11 I8, 799 cni';'H NMR {300 MHz, DMSO-db) 8
10.64
( I H), 8.25 ( 1 H), 7.50 (5 H), 7.16 (2 H), 4.60 (2 H), 4.44 ( 1 H}, 4.27 ( 1
H), 4.06 (2 H),
3.51 (4 H), 3.43 (2 H), 3.29 (4 H), 2.42 (4 H);'3C NMR (75 MHz, DMSO-d6) 8
173.0,
172.4, 169.8, 162.3, 137.5, 136.8, 133.5, 131.7, 130.1, 128.6, 119.7, 66.6,
63.3, 57.0, 53.7,
36.7, 21.5; MS (FAB) m/z (rel. intensity) 625 (MH+, 5:5), 629 (9), 628 (14),
627 (39),
626 (21}, 62S (SS), 308 (7}, 141 (19), I 14 (99), 113 (24), 100 (7); HRMS
(FAB) calcd for
C27H3oCLZN407S +H, 625.1290, found 625.1309.
Example 26.
[S-{R*,S*)]-lt-[[[1-[[4-[(2,6-Dichlorobenzoyl)amino]phenyl]methyl]-2-methoxy 2-
oxoethyi]aminojcarbonyl]-3-thiazolidinecarboxylic acid 3-{1,1-dimethylethyl)
ester
(Scheme A, A-7: where RA_, and RA_Z are the same and equal to H, R3 is t-
butyl, Y is CO2,
RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (S, R)).
s-. ~ o
~~~ N °/
0 0° ~ ~ o ct
CI
Example 26 was prepared as described in Scheme A from D-cysteine using di-t-
butyl
dicarbonate to form the requisite carbamate. Physical data as follows: IR
(mull) 3293,
1746, 1666, 1606, 1562, 1538, 1516, 1432, 1413, 1324, 1260, 1216, I 195, 1162,
799 cm'';
'H NMR (CDCl3) 8 7.55 (2 H), 7.37 {4 H), 7.14 {2 H), 4.89 {1 H), 4.66 (2 H),
4.25 (I H),
3.75 (3 H), 3.39 (1 H), 3.24 (3 H), 1.44 (9 H); '3C NMR {CDCI3) 8 17I .2,
169.8, 162.3,
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-56-
136.3, 135.8, 132.4, 132.3, 131.0; 130.2, 130.1, 129.9; 128.2, 128.1, 127.9,
120.5, 120.2,
120.1, 82.2, 53.2, 52.5, 37.3, 31.0, 28.4, 28.2; MS (ESI+~) for CZ6Hz9C1zN306S
mlZ 603.9
(M+Na)+; MS {ESI-) for CZ6Ha9C1zN30eS m/Z 580.0 (M-l~)'; HRMS (FAB) calcd for
C26HZ9C12N3O6S+H~ 582.1232, found 582:/231. Anal. C'.alcd for CZ6HxgC12N3O6S ~
0.26
HZO: C, 53.18; H, 5.07; N; 7.16. Found: C, 52.78; H, 5.14; N, 6.91. % Water
(KF): 0.66.
Example 27.
[s-{R*,s*)]-4-[[[1-c~rboxy-2-[4-[~2,6
dichlorobenzayl)amino]phenyl]ethyl]amino]carbonyl]--3-thiazolidinecarboxylic
acid 3
{i,l-dirnethylethyl) ester
(Scheme A, A-8: where RA_, and RA_z are the same and e~~ual to H, R, is t-
butyl, Y is COZ,
RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (S, R)).
H . O
OH
O OO I ~ O CI
H~I /
CI
Example 27 was prepared from example 26 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 3286; 1665, 1606, 1562, 1538, 1516, /432,
1413,
1394, 1326, 1259, 1216, 1195, 1161, 799 cm''; 'H NMR (DMSO-db) 8 12.70 (I H),
10.63
(1 H), 8.13 (1 H), 7.51 (5 H), 7.20 (2 H), 4.45 (4 H), 2.915 (3 H), 1.23 {9
H);'3C NMR
(DMSO-db) 8 173.1,170.5, 162.3, 153.2, 137.5, 136.9, 13 / .8, 131.6,130.0,
128.7, 119.7,
80.4, 62.2, 54.0, 49.8, 36.5, 28.3, 21.5; MS (ESI+) for CZSH27C12N3O6S m/Z
567.9 (M+H)+;
MS (ESI-) for Cz5Hz7C12Ns06S m/z 565.9 (M-H)'; HRMS (EI) calcd for
Cz5H27C1zN3O6S
567.0997, found 568.1096. Anal. Calcd for CZSHZ,C12N306S ~ 0.34 HzO: C, 52.82;
H,
4.79; N, 7.39. Found: C, 52.17; H, 4.90; N, 7.25. % Wz~ter (KF): 1.07.
Example 28.
jS-{R*,R*)]-4-[[[1-[[4-[(2,6-Dichlorobenzoyl)amino]phenyl]methyl]-2-rnethoxy-2-
oxoethyl]amino]carbonyl]-5,5-dimethyl-3 thiazollidinecarboxylic acid 3-(1,1-
dimethylethyl) ester
CA 02342778 2000-12-20
WU 99/67230 PCT/US99/14233
-57-
(Scheme-A, A-7: where RA_, and RA_2 are the same and equal to CH3, R3 is t-
butyl, Y is
CO2, R5 is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stE;reochemistry is (S,
S)).
s-l- H o
0 0° ~ ~ o c~
H~I ~
C1
Example 28 was prepared as described in Scheme A from D-penicillamine using di-
t-butyl
dicarbonate to form the requisite carbamate. Physical data as follows: IR
(mull) 1744,
1707, 1688, 1678, 1657, 1606, 1562, 1541, 1516, 1431, 1414, 1326, 1253,1161,
1140 crn
';'H NMR (CDC13) 8 7.58 (2 H), 7.36 (3 H), 7.14 (2 H), 6.55 (1 H}, 4.87 (I H),
4.56 (2 H),
4.10 (1 H), 3.72 (3 H), 3.08 (2 H), 1.53 (3 H), I.44 {9 H), 1.40 (3 H);'3C NMR
(CDCl3) 8
171.6, 162.3, 153.6, 136.4, 135.8, 132.6, 132.4, 131.0, 1:30.1, 129.9, 129.8,
128.2, 120.7,
120.5, 120.3, 8I.7, 73.0, 52.8, 52.6; 52.4, 48.4, 39.8, 39.5, 37.d, 30.3,
28.3, 28.0,23.5;
MS (ESI-) for CZ$H33C12N3O6S mlz 607.9 (M-H)'; MS (F.AB) mlz (rel. intensity)
610
(MH+, 6), 512 (26), 510 (44), 117 (30), 1 L5 (1 6), 99 (16), 87 {16), 59 (99),
57 {27), 57
{20}, 41 (23); HRMS {FAB) calcd for CZ8H33C12N3O6S+H, 610.1545, found
610.1501;
Anal. Calcd for CZ8H33C12N3O6S ~ 0.07 HzO: C, 54.97; H., 5.46; N, 6.87. Found:
C, 54.92;
H, 5.54; N, 7.11. % Water (KF): 0.2I.
Example 29.
[S--(R*,R*)]-4-[[[1-Carboxy-2--[4-[(2,6-
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]-5,5-dimethyl-3-
thiazolidinecarboxylic acid 3--(I,1-dirnethylethyl) ester
(Scheme A, A-8: where RA_, and R~_2 are the same and equal to CH3, R3 is t-
butyl, Y is
CO2, RS is 4-[{2,6-dichlorobenzoyI)amino]phenyl and ste,reochemistry is (S,
S}).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-S 8-
S~ H~ O
~N~N~OH
~O~O O ~ ~ O CI
H ~~
Ct
Example 29 was prepared from example 28 by the procedure described in
preparation 6.
Physical data as follows: IR {mull) 1739, 1666, 1606, 1.'i62, 1535, 1516,
1432, 14I3,
S 1394, 1325, 1270, 1260, 1194, 1160, 799 czri';'H NMR (DMSO-db} 8 12.62 (I
H), 10.62
( 1 H), 8.18 ( 1 H), 7.51 (S H), 7.19 (2 H), 4.50 (3 H), 4.24 ( 1 H), 2.91 (2
H), I .33 { 12 H),
1.04 (3 H); '3C NMR (DMSO-db) 8 172.8, 168.7, 161.9, 153.2, 137.2, 136.5,
133.1, 131.4,
131.3, 129.6, 128.3, I 19.5, 80.0, 70.9, 53.7; 48.5, 37.0, 3Ø7, 28.1, 27.9,
24.6; MS (ESI+)
for C27H3'C12N3O6S mlz S9S.9 (M+H}+; MS (ESI-) for C~,,H3,C12N304S mlz 593.8
(M-H)';
MS (FAB} m/z (rel. intensity) 596 (MH+, 19), 672 (17), 596 (19}, 499 (15), 498
{ 60}, 497
(26), 496 (99), 494 (3S); 173 (20), 1 I6 (27), 57 (48); An~~l. Calcd for
C27H3,CIZN3O6S
0.27 HZO: C, 53.93; H, 5.29; N, 6.99. Found: C, 53.73; '.H, 5.39; N, 7.10. %
Water (KF):
0.80.
Example 30.
1 S [S~R*,R*)]-4-[[[1-[[4-[(2,6-Dichlorobenzoyl)amino]phenyl]methyl]-2-methoxy-
2-
oxoethyl]amino]carbonyl]-S,S-dimethyl-3-thiazolidinecarboxylic acid 3-ethyl
ester
(Scheme A, A-7: where RA_' and RA_Z are the same and equal to CH3, R3 is
ethyl, Y is CO2,
RS is 4-[(2,6-dichlorobenzoyl}amino]phenyl and stereochemistry is (S, S)).
s--~- H o
CN~N~Oi
~O~O ~ ~ ~ O CI
_N
H~I ~
cl
Example 30 was prepared as described in Scheme A from D-penicillamine using
ethyl
chioroformate to form the requisite carbamate. Physical data as follows: IR
(mull} 3292,
1748, 1666, 1606, 1562, 1538, 1516, 1445, 1431, 1414, 1341, 1325, 1271, 1212,
1194 cm
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-59-
'; 'H NMR (CDGl3) 8 7.57 (2 H), 7.37 {4 H), 7.I 1 {2 H), 6.49 (1 H), 4.86 (1
H), 4.59 (2 H),
4. I4 (3 H), 3.75 (3 H), 3.09 (2 H), 1.60 (3 H), 1.54 (3 H), 1.23 (3 H); "C
NMR (CDC13) 8
171.6, 162.3, 154.5, 136.3, 135.8, 132.6, 132.4, 131.1, 1:30.2, 130.1, 129.8,
128.2, 120.5,
120.4, 72.7, 62.5, 52.7, 52.5, 37.3, 30.2, 23.7, I4.6, 14.I:, MS (ESI+) for
C26HZ9CIZN3O6S
m/z 581.9 (M+H)+; MS (ESI-) far CZ6H29C1zN3O6S m/Z 5'79.8 (M-H)'; HRMS (EI)
calcd for
Cz6Hz9ChNa06S SBI.I IS4, found 581.1132; Anal. Calcd fox CZ6Hz9C12N3O6S ~ O.I6
H20:
C, 53.35; H, 5.05; N, 7.18. Found: C, 53.74; H, 5.12; N, 7.12. % Water (KF):
0.49.
Example 31.
(S--(R*,R*)]-4-[[[I--Carboxy-2--[4-[{2,6-
I 0 dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]-5,5-dimethyI-3-
thiazaiidinecarboxylic acid 3-ethyl ester
{Scheme A, A-8: where RA_, and RA_2 are the same and equal to CH3, R3 is
ethyl, Y is CO2,
RS is 4-[(2,6-dichlorobenzoyl)arnino]phenyl and stereochemistry is (S, S)).
s--L H o
~NI~N~OH
~O~O a I ~ O CI
~N ~
IS H
CI
Example 31 was prepared from example 30 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 3287, 3070, 1666; IEi06, 1562, 1538, 1516,
1431,
1414, 1342, 1328, 1271, 1213, I 194, 799 crri';'H NMR (DMSO-d6) S 12.58 (1 H),
10.63
20 (I H), 8.22 (I H), 7.51 (5 H), 7.20 (2 H), 4.51 {3 H), 3.95 (3 H), 3.04 {I
H), 2.86 (1 H),
1.35 (3 H), 1.16 (6 H);'3C NMR (DMSO-db) 8 223.3, 184.1, I83.9, 172.8, 168.4,
I61.7,
136.9, 136.3, 133.1, I3I.2, 131.0, 129.4, 128.1, I I9.2, 70.6, 61.1, 53.6,
53.4, 48.6, 36.2,
30.I, 25.4, 24.3, 21.0, 14.1; MS (ESI+) for CZSHZ~CIZN3O~6S mlZ 568.0 (M+H)+;
MS (ESI-)
for CZSH27C12N3O6S mlZ 565.9 (M-H)'; MS (FAB) mlz (rei. intensify) 568 (MH+,
86), 644
25 (18), 571 {19), 570 (61), 569 (30), 568 (86), 335 (16), 188 {99), I73 (19),
141 {S3), 116
(23); HRMS (FAB) calcd for CZSHz7CI2N306S+H, 568.1075, found 568.1096; Anal.
Calcd
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-60-
for C25Hz;C12N3O6S ~ 0.4 HzO: C, 52.16; H, 4.87; N, 7.30. Found: C, 52.46; H,
4.90; N,
7.15. % Water (KF): 1.25.
Example 32.
[S-{R*,R*)]~-[[[1-[4-[(2,6-Dichlorophenyi)methoxy]phenyl]methyl]-2-methoxy-2-
oxoethyl]amino]carbonyl]-3-thiazoIidinecarboxylic acid 3-(1,1-dimethylethyl)
ester
(Scheme A, A-7: where RA_, and RA_2 are the same and equal to H, R3 is t-
butyl, Y is C02-,
RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl and stereochemistry is (S, S)).
IO
/s-. ~ oIf
~~~N~Oi
O O O ~ ~ CI
O~ %
CI
Example 32 was prepared as described in Scheme A from D-cysteine using di-t-
butyl
dicarbonate to form the requisite carbamate. Physical data as follows: IR
(Iiq.) 1745,
15 1702, 1565, 15I l, 1467, 1439, 1368, 1299, 1241, 1197, 1177, 1162, 1017,
778, 768 cm';
'H NMR (CDCl3} 8 7.36 (2 H), 7.25 {1 H), 7.06 {2 H}, 6.94 (2 H), 5.23 (2 H},
4.75 (3 H),
4.12 (1 H), 3.72 (3 H), 3.37 (1 H), 3.14 (3 H), 1.45 (9 H); '3C NMR {CDCi3) 8
171.5,
157:9, 136.9, 132.0, 130.3, 128.4, 128.2, 115.0, 81.9, 65.:1, 62.8, 53.2,
52.3, 49.2, 36.9,
28.1, 27.9; MS (ESI+) for CZ6HsoClzNzO6S ~Z 568.9 (M-~-H)+; MS (ESI-} for
20 Cz6HaoC1zNz06S m/z 566.7 (M-H)'; Anal. Calcd for C26H3~,C12NZObS ~ 0.09
H20: C, 54.68;
H, 5.33; N, 4.91. Found: C, 54.62; H, 5.41; N, 4.73. % Water {KF): 0.28.
Example 33.
[S-{R*,R*)]-4-[[[1-Carboxy-2_.[4-[(2,6
dichlorophenyl)methoxy]phenyl]ethyl]amino]carbonyl]-3 thiazolidinecarboxylic
acid 3
25 ( 1,1-dimethylethyl) ester
(Scheme A, A-8: where RA_, and RA_2 are the same and equal to H, R3 is t-
butyl, Y is COZ-,
RS is 4-[(2,6-dichlorophenyl}methoxy]phenyl and stereoc:hemistry is (S, S)).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-61-
S'~ H O
~~~N~OH
O O O I ~ CI
0~1I w
CI~
Example 33 was prepared from example 32 by the procedure described in
preparation 6.
Physical data as follows: IR (mull} 1734, 1704, 1676, 1612, 1565, 1511, 1439,
1393,
1300, 1241, 1196, i I78, 1162, 777, 769 crri';'H NMR (DMSO-ds} 8 8.24 (1 H),
7.50
(3 H), 7.13 (2 H); b.93 (2 H), S.I6 (2 H}, 4.52 {3 H), 4.21 (I H}, 2:91 (4 H),
I.31 (9 H);
"C NMR (DMSO-db) 8 172.7, 169.7, 157.1, 152.6, 135.9, 131.7, 131.5, 130.2,
129.8,
128.7, 1 I4.2, 79.8, 64.8, 6i.6, 53.2, 49.2, 36.0, 34.8, 27.8, 21.0; MS (ESI+)
for
C25HZgC12NzO6S mlz 554.9 (M+H)+; MS (ESI-) for CZSHT,8C12NZO6S mlz 552.8 (M-
H)';
Anal. Calcd for CZSHZ8C12NZO6S ~ 0.15 H20: C, 53.79; H:, 5.11; N, 5.02. Found:
C, 54.17;
H, 5.17; N, 5.00. % Water {KF): 0.50.
Example 34.
[S-{R*,R* )]-4-[[[ 1-[4-[(2,6-Dichlorophenyi)methox;y]phenyl]methyl]-2-methoxy-
2-
IS oxoethyl]amino]carbonyl]-3 thiazolidinecarboxylic acid 3-ethyl ester
(Scheme A, A-7: where RA_I and RA_z are the same and equal to H, R3 is ethyl,
Y is COz-,
RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl and stereochemistry is (S, S)).
/S-. H O
~N~N~Oi
~O~O 'O' I ~ CI
'O~
C'/I .i
Example was prepared as described in Scheme A from L)-cysteine using ethyl
chloroformate to form the requisite carbamate. Physical data as follows: IR
{mull) 3282,
1742, 1705, 1692, 1664, 1562, 1509, 1436, 1352, 1343, 1236, 1196, 1175, 1015,
786 cm ';
'H NMR (CDCl3) b 7.37 (2 H), 7.25 (1 H), 7.05 (2 H), 6.,94 {2 H}, 6.74 (I H},
5.23 (2 I-i),
CA 02342778 2000-12-20
WO 9916723b PCTIUS99/14233
-62-
4.77 (3 H), 4.34 ( 1 H), 4. I 8 (2 H); 3.74 (3 H), 3.37 ( 1 H;f, 3.13 (3 H),
1.31 (3 H); '3C NMR
(CDCl3) s 171.6, 171.4, 158.0, 137.0, 132.1, 130.5, 130.4, 128.5, 128.2,115.0;
65.2, 63.6,
63.2, 62.7, 53.6, 53.2, 52.4, 36.9, 14.5; MS (ESI+) for C24H26C12N2~6'~ nilz
540.9 (M+H)+;
HRMS (EI) calcd for C24HzeClaNzO6S 540.0889, found 540.0878; Anal. Calcd for
Cz4HZ6C12NZO6S ~ 0.26 H20: C, 52.79; H, 4.89; N, 5.13. Found: C, 52.41; H,
4.82; N, 4.96.
Water (KF): 0.85.
Example 3S.
[S-~R*,R*)]-4-~y l-carboxy-2._~4-~(2,6
dichlorophenyl)methoxy]phenyl]ethyl]aminojcarbonyl]-3-thiazolidinecarboxylic
acid 3
ethyl ester
(Scheme A, A-8: where RA_, and RA_Z are the same and equal to H, R3 is ethyl,
Y is CO2-,
i5 RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl and stereochemistry is (S, S)).
s-. ~ o
~N~N~OH
~O~O ° I ~ CI
cl
Example 35 was prepared from example 34 by the procedure described in
preparation 6.
24 Physical data as follows: IR (mull) 1709, 1675, 1612, 1.565, 1511, 1439,
1416, 1346,
1300, 1241, I I96, 1179, 1115, 1018, 768 cm'; IH NMR {CDCI~) S 7.34 (2 H),
7.23 (1 H),
7.12 (2 H), 6.94 (2 H), 6.85 (I H), 5.22 (2 H), 4.77 (4 H), 4.34 (1 H), 4.16
(2 H), 3.33
(4 H), 1.26 (3 H);'3C NMR (CDC13) ~ 174.0, 170.2, 15f,.1, 155.1, 137.0, 132.0,
130.5,
I28.5, 128.1, 115.0, 65.2, 63.9, 63.0, 62.9, 53.3, 36.4, 21.9, 14.5; MS (ESI+)
for
25 C23H24C12Nz06S mlz 527.0 (M+H)+; MS (ESI-) for Cz3H24C1zN2O6S mlz 524.9 (M-
H)';
HRMS {EI) calcd for C23H24C1zNz06S 526.0732, found 526.0726; Anal. Calcd fox
CA 02342778 2000-12-20
WO 991b7230 PCTIUS99I14233
-63-
C23H24C12N2~6S ~ 0.20 H20: C, 52.02; H, 4.63; N, 5.27. Found: C, 52.12; H,
4.73; N, 5.34.
Water (KF): 0.69.
Example 36.
[S-{R*,R*)]-4-[[[ 1-[4-[(2,6-Dichlorophenyl)methox;y]phenyl]methyl]-2-methoxy-
2-
S oxoethyl]amino]carbonyl]-3 thiazolidinecarboxylic acid 3-[2-(4-
morpholinyl)ethyl]
ester
(Scheme A, A-7: where RA_, and RA_2 are the same and equal to H, R3 is 2-(4-
morpholinyl)ethyl, Y is COZ-, RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl and
stereochemistry is (S, S~).
S-- H O
0..~~ CN~.N~Oi
~N~'O~O ~O~ I ~ CI
v 'O
CI
Example 36 was prepared as described in Scheme A from D-cysteine using 4-(2-
hydroxyethyl)morpholine to form the requisite carbamat:e. Physical data as
follows: mp
1S I38-I40°C;1R (mull) 3286, 1743, 1705, 1660, 1559, 1513, 1435, 1428,
1302, 1245, 1226,
12I S, 1176, 1015, 764 cm ''; 1 H NMR (300 MHz, DMS~O-db) S 8.43 (1 H), 7.48
(3 H),
7.I3 (2 H), 6.94 (2 H), 5.16 {2 H), 4.59 (2 H), 4.48 (1 H;), 4.26 (I H), 4.07
(2 H), 3.63
(3 H), 3.51 {4 H), 3.23 (1 H), 3.01 (1 H), 2.84 (I H), 2.71 (1 H), 2.41 (6
H);'3C NMR (75
MHz, DMSO-db) b I72.2, 157.7, 153.9, 136.5, 132.2, 132.0, 130.8, I30.1, 129.2,
114.8,
66.6, 65.4, 63.4, 57.0, 53.9, 53.8, 52.4, 36.4; MS (ESl+) for CZ$H33C12N3O,S
m/z 625.8
(M+I-I)+; Anal. Calcd for CZgH33CIzN3O,S: C, 53.67; H, :i.3I; N, 6.71. Found:
C, 53.69; H,
5.27; N, 6.69.
Example 37.
[S-{R*,R*)]~-[[[ 1-Carboxy=~;-[4-[(2,6_
dichlorophenyl)methoxy]phenyl]ethyl]amino]carbonyl]~-3-thiazolidinecarboxylic
acid 3-
[2-(4-morpholinyl)ethyI] ester
CA 02342778 2000-12-20
WO 99/G723U PCT/US99114233
_(~-
(Scheme A, A-8: where RA_, and RA_z are the same and equal to H, R3 is 2-(4-
morpholinyl)ethyl, Y is COz-, RS is 4-[{2,6-dichlorophenyl)methoxy]phenyl and
stereochemistry is (S, S)).
S-. H O
O~ ~N~N~ON
~N~O~O O ~ \ CI
~O. \
(/
S c,l
Example 37 was prepared from example 36 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 1710, 1610, 1S8S, 1565, 1511, 1439, 1408,
1351,
1301, 1240, 1196, 1179, 1116, 1017, 767 cm ''; 1 H NMR (300 MHz, DMSO-db) 8
8.27
( 1 H), 7. S S (2 H), 7.45 ( 1 H), 7.13 (2 H), 6.94 (2 H), S. I ti (2 H), 4.59
{2 H), 4.40 ( I H),
4.27 (1 H}, 4.04 (2 H), 3.52 (4 H); 3.2I (1 H), 2.86 (3 H), 2.44 (6 H}, '3C
NMR (7S MHz,
DMSO-db) 8 173.2, I72.S, 157.6, 136.5, 132.2, 132.0, 130.8, 130.4, 129.2,
114.7, 66.5,
65.3, 63.2, 57.0, 53.9, 53.7, 36.5, 2i.S; MS (ESI+) for C,z,H3,C12N307S m/z
611.9 (M+H);;
Anal. Calcd for CZ,H3,CIZN307S ~ 1.0 CZH402 ~ 0.63 Hz0 ~ 0.28 HCI: C, 50.13;
H, 5.31; N,
1 S 6.03; Cl, 11.59. Found: C, 49.80; H, 5.30; N, 6.OS; CI, 11.20. % Water
(KF): 1.58.
Example 38.
[S-(R*,R*)]-4-j[[1-[4-[(2,6--Dichlorophenyl)methoxy]phenyl]methyl]-2-methoxy-2-
oxoethyl]amino]carbonyl]-3-thiazolidinecarboxylic acrid 3-[(2-
pyridinyl)methyl] ester
(Scheme A, A-7: where RA_, and R,,_2 are the same and equal to H, R3 is 2-
pyridinylmethyl,
Y is COZ-, RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl and stereochemistry is
(S, S}).
s-. H o
CN~ N~Oi
I Nw O~O ~Ot ~ ~ Cf
/
CI
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-65-
Example 38 was prepared as described in Scheme A from D-cysteine using 2-
pyridinemet:hanol to form the requisite carbamate. Physical data as follows:
mp 123-
125°C; IR (muil) 3334, 1728, 1709; I668, 1531, 1511, 1441, 1405. 1345,
1294, 1286,
1236, 1228, 1015, 762 cm ''; ~H NMR (300 MHz, DMSO-db) 8 8.57 (2 H), 7.79 (1
H),
7.54 (2 H}, 7.42 (1 H), 7.27 (2 H), 7.12 (2 H), 6.92 (2 H.), 5.13(4 H), 4.69
(2 H), 4.49
( 1 H), 4.34 ( 1 H), 3.59 (3 H), 3.24 ( 1 H), 2.89 (3 H); '3C; NMR (75 MHz,
L7MS0-db 8
172.2, 169.9, 157.7, 156.5, 153.6, 137.3, 136.5, 132.2, ',132.0, 130.8, 130:1,
129.2, I23.2,
121.0, I I4.8, 67.7, 65.3, 62.0, 54.0, 52.4, 50.3, 36.3, 35.3; HRMS (EI} calcd
for
C~8Hz7C1,N306S 603.0997. found 603.0992; Anal. Calc<i for C,gH,7ChN306S: C,
55.63; H,
4.50; N, 6.95. Found: C, 55:56; H, 4.59; N, 6.93.
Example 39.
[S-(R*,R*)]-4-[[[1-[4-[(2,6-Dichlorophenyi)methoxy]phenyl]methyl]-2-methoxy-2-
oxoethyl]amino]carbonyl]-3-thiazolidinecarboxylic acid 3-j2-(I-
pyrrolidinyl)ethyl] ester
(Scheme A, A-7: where RA_, and RA_Z are the same and equal to H, R3 is 2-(1-
pyrrolidinyl}ethyl, Y is COz-, RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl and
stereochemistry is (S, S)).
/S-. H OII
~N~N~,Oi
° I ~ ci
~o
I~
ci
Example 39 was prepared as described in Scheme A from D-cysteine using I-{2-
hydroxyethyl)pyrrolidine to form the requisite carbamat:e. Physical data as
follows: mp
130-I32°C; IR (mull) 1745, 1702. 1661, 1556, 1513, 1435, 1426, 1303,
1245, 1226, 1214,
I 176, 1017, 825, 7b5 cm ~~y'H NMR (300 MHz, DMSC>-db) 8 8.42 (1 H), 7.48 (3
H}, 7.I3
(2 H), 6.94 (2 H), 5.16 (2 H), 4.59 (2 H), 4.48 ( I H), 4.27 ( 1 H), 3.99 (2
H), 3.63 (3 H),
3.24 (1 H), 2.68 {9 H), 1.62 (4 H); "C NMR (75 MHz, I7MS0-d~) cS 172.2, 170.0,
157.7,
136.5, I32.2, 132.0, 130.8, I30.1, 129.2, I 14.8, 65.3, 65.I, 54.4, 53.9.
52.42, 36.35, 23.6;
CA 02342778 2000-12-20
WO 99/62230 PCT/U899/14233
-66-
MS (ESI+) for C,gH33C12N306S m/z 609.8 (M+H)'; Anal. Calcd for CzgH33CI2N,O6S:
C,
55.08; H. 5.45; N, 6.88. Found: C, 54.72; H, 5.58; N, 6.60.
Example 40.
[R-(R*;S*)J-4-[[[1-[4-[(2,6-Dichlorophenyl)methoxyJphenyl]methyl)-2-methoxy-2-
oxoethyl]amino]carbonylJ-3-thiazolidinecarboxylic acid 31,1--dimethylethyl}
ester
(Scheme A, A-7: where RA., and RA_Z are the same and equal to H, R3 is t-
butyl, Y is C02-,
R5 is 4-[(2,6-dichlorophenyl)methoxy]phenyl and stereochemistry is {R, ,S~).
~s o
H ~~
N~ N ~Oi
0''~
O 0 I ~ CI
O~
i
CI
Example 40 was prepared as described in Scheme A frorn L-cysteine using di-t-
butyl
dicarbonate to form the requisite carbamate. Physical data as follows: IR
(mull) 1746,
1702, 1611. 1565, 1511, 1439,1299, 1241, 1197, 1177, i 162, 1118, 1016, 777,
768 cm'';
'H NMR (CDCl3} b 7.39 (2 H), 7.25 (1 H), 6.93 (2 H), 6.93 (2 H), 5.24 (2 H),
4.72 (3 H),
4.20 ( 1 H), 3.74 (3 H), 3.35 ( 1 H), 3.12 (3 H}, 1.44 (9 H); "C NMR (CDC13) 8
171.7,
170.0, 169.5, 158.3, 137.3, 132.4, 130.7, 130.6, 128.8, 128.5, I 15.2, 115.1,
82.4. 65.5,
53.5, 52.6. 50.3. 37.3, 28.4; MS (ESI+) for C,~H3oCI,N,C>6S m/z 554.9 (M+I-
I)'; MS (ESI-)
for C,6H~oCI~N,O6S m/z 552.8 (M-H)'; Anal. Calcd for C,bH3oCIZNZO6S ~ 0.1 H,O:
C,
54.65; H, 5.33; N, 4.90. Found: C, 54.59; H, 5.30; N, 4.88. % Water (KF):
0.33.
Example 41.
[R-(R*,S*}J-4--[[[ 1-Carbox~J-2__[q-[{2,6-.
dichlorophenyl)methoxyjphenylJethyljaminoJcarbonyl]--3-thiazoiidinecarboxylic
acid 3-
( 1,1-dimethyIethyl) ester
(Scheme A. A-8: where R"_, and RA.2 are the same and equal to H, R3 is t-
butyl, Y is CO,-,
RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl and stereoc;hemistry is (R, S~).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-67-
H
<S~ ~ ~°
N Nv 'oH
O
~o ° ~ ~ ct
~ o
Gt
Example 4I was prepared from example 40 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 1737, 1705, 1679, Ifil2, 1565, 1512, 1439,
1300,
1241, I 196, 1178, 1 i63, 1117, 777, 769 crri';'H NMR (DMSO-db) 8 8.07 (1 H),
7.54
{2 H), 7.44 (1 H), 7.16(2 H), 6.93 (2 H), 5.16 (2 H), 4.5Ei (1 EI), 4.39 (3
H), 3.56 (I H),
2.84 (3 H), 1.23 (9 H);
"C NMR (DMSO-db) 8 I72.7, 169.9, 157.0, 152.6, 135.9, I3I.6, 131.4, I30.1,
I29.9,
129.8, I28.7, 114.1, 79.8, 66.9, 64.7, 61.7, 53.5, 49.3, 35.6, 34.8, 27.9,
27.6; MS (ESI+)
for CZSH,gC12N206S mlz 554.9 (M+H)+; MS (ESI-) for CZSHZgCI,N,ObS mlz 552.8 (M-
H)';
Anal. Calcd for CzSHzgCI,N,O6S ~ 0.27 HZO: C, 53.59: H., 5.13; N, 5.00. Found:
C, 53.97;
H, 5.14; N, 4.96. % Water (KF): 0.86.
Example 42.
4-[(2,6-Dichlorobenzoyl)amino]-N-[[(4S~-3--(ethylsulfonyl~4-
thiazolidinyl]carbonyl]-
~-phenyialanine methyl ester
(Scheme A, A-7: where R"_, and RA_2 are the same and equal to H, R, is ethyl,
Y is SO2, RS
is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereocherrtistry is (S, S)).
S. H O
~N~N~Oi
O=5=OIOI y O C:I
Et
H
..O Gt
Example 42 was prepared as described in Scheme A from D-cysteine using
ethanesulfonyl
chloride to form the requisite sulfonamide. Physical data as follows: IR
(mull) 1743,
1666; 1605, 1561, 1535, 1515. 1432, 1413, 1328, 1269, 1219. 1195, I I46, 799,
782 cm -';
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-68-
'H NMR (CDCI3) 8 7.58 (2 H), 7.46 (I H), 7.32 (3 H), '1.17 (2 H), 7.07 (I H),
4.85 (I H),
4.73 (2 H), 4.28 ( I H), 3.76 (3 H), 3.54 ( 1 H), 3.26 ( 1 Hf), 3.05 (4 H), I
.40 (3 H); "C NMR
(CDCl3) b 171.3, 168.2, 162.4, 136.4, 135.8, 132.4, 13f..3, 131.0, 130.1,
128.2. 120.6,
64.9, 53.2, 52.6, 51.5, 45.8, 37.1, 34.2, 31.0, 29.3, 7.7; IVIS (ESI+) for
C,3H~SCI~N306S2 m/z
573.9 {M+H)+; MS (ESI-) for Cz3H25C1,N;O6S2 m/z 571..7 {M-H)'; HRMS (FAB)
calcd for
C~3HzsC1,N3O6S2+H, 574.0640, found 574.0634; Anal. Calcd for Cz3H,5CI,N3O6Sz ~
0.1
H20: C, 47.97; H, 4.40; N, 7.30. Found: C, 48.36; H, 4.59; N, 6.80.
Example 43.
4-[{2,b--Dichlorobenzoyl)amino]-N-[[(453-(ethylsu.lfonyl)-4--
thiazolidinyl]carbonyl]-
~-phenylalanine
(Scheme A, A-8: where RA_, and R,,_2 are the same and equal to H, R~ is ethyl,
Y is SOz, RS
is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (S, S)).
S-. H OII
CN~N~ON
O=S=O 4 w O CI
Et
IS
cl
Example 43 was prepared from example 42 by the procedure described in
preparation 6..
Physical data as follows: IR (mull) 1734, I 664, 1605, 1562, I 536, I S I6,
1432, 1414,
1330, I272, 1234, I I95, I 146, 799, 78I cm''; IH NMR (DMSO-db) 8 12.91 {I H),
10.65
( 1 H), 8.15 ( 1 H), 7.51 (5 H), 7. I 7 (2 H), 4.77 {2 H), 4.43 { 1 H), 4.29 (
1 H), 3.94 (6 H),
1.20 (3 H); 13C NMR (DMSO-db) 8 172.5, 168.9, 161.9, 137.1, I3d.5, I33.2,
131.4, I3I.3,
129.8, 128.3, 1 I9.3, 63.7, 53.6, S 1.4, 45.3, 36.1, 34.7; NIS (ESI+) for
C"H,,CI,N3O6Sz m/z
559.9 (M+H)'; MS (ESI-) for C,~H,3C1,N~O6S, m/z 557.8 (M-H)-; HRMS {FAB) calcd
for
C"H,~CI,N;O6S2+H, 560.0483, found 560.0488; Anal. C;alcd for C,~H,3Cl,NzO6S2 ~
0.72
H,O: C, 46.08: H, 4.30; N, 7.33. Found: C, 46.42; H. 4.37; N, 7.01. % Water
(KF): 2.26.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-69-
Example 44.
4-[(2,6-Dichlorobenzoyl)amino]-N-[[{4S~3--[[5--(trifluoromethyl}-2-
pyridinyl]sulfonyl]-4-thiazolidinyI]carbonyl]-~--phenylalanine methyl ester
(Scheme A, A-7: where R"_, and RA_Z are the same and e~quaI to H, R3 is 2-(5-
triflouromethylpyridyl}, Y is SO~, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl
and
stereochemistry is (S, S)).
S-. H 0
CN~N~Oi
0=S=O O
O C!
i N
w I H
CI
CF3
Example 44 was prepared as described in Scheme A from D-cysteine using 2-(5-
triflouromethyipyridyl)sulfonyl chloride to form the requisite sulfonamide.
Physical data
as follows: IR (mull) 1745, 1668, 1603; 1535, 1515, 14:32, 1413, 1327, 1219,
1179, 1142,
1108, 1073, 1016, 616 cm ' '; 'H NMR (CDCI3) 8 8.76 ( i H), 8.17 (2 H}, 7.90 (
1 H), 7.51
(2 H), 7.32 (4 H}, 7.17 (2 H), 5.18 (1 H), 4.96 (1 H), 4.66 (1 H), 4.31 (1 H),
3.78 (3 H),
1 S 3.52 {1 H}, 3.15 (3 H); "C NMR (CDCI,) 8 171.3, 168.4, 162.2, 147.2,
136.2, 136.0,
132.4, 131.0, 130.3. 128.2, 123.0, 120.4, 120.3, 65.7, 53.6. 52.5, 51.4, 37.3,
34.0; MS
(ESI+) for CZ,H,aCl,F3N~O6S2 mlz d90.8 (M+H)+; MS (ESI+} for C27H23C12F3N4O6Sz
mlz
712.9 {M+Na)+; Anal. Caled for C27HZ3C12F3N4O6S~ ~ 0.2 HBO: C, 46.68; H, 3.39;
N, 8.06.
Found: C, 46.60; H, 3.52; N, 7.92. % Water (KF): 0.47.
Example 45.
4-[(2,6-Dichlorobenzoyl)amino]-N-[[(4S~3--[[5--(trifluoromethyl)-2-
pyr~idinyl]sulfonyi]-4-thiazolidinyl]carbonyl]=L-phenylalanine
(Scheme A, A-8: where RA_, and RA_z are the same and equal to H, R3 is 2-(5-
triflouromethylpyridyl), Y is SO,, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl
and
stereochemistry is (S, S)}.
CA 02342778 2000-12-20
WO 99167230 PCT/US99/I4233
-70-
S~ H- O.
~N~N~OH
O=S=O O ~ O (:I
N ~ i N w
H
CI
CF3
Example 45 was prepared from example 44 by the procedure described in
preparation 6.
Physical data as follows: IR {mull) 1740, 1666, 1602, 1:>62, 1533, 1517, 1432,
1354,
1327, I 179, 1 I43, 1108, 1074, 1016. 613 cni';'H NMR (DMSO-dh) 8 10.63 (I H),
9.24
( 1 H), 8.54 ( 1 H), 8.46 ( 1 H), 8.18 ( 1 H), 7.50 (5 H), 7. I '~ (2 H), 5
.00 { 1 H), 4:74 { 1 H);
4.42 (2 H), 3.04 (2 H), 2.90 ( 1 H), 2.78 ( 1 H); '3C NMR (DMS4-db) ~ 172.8,
168.9, 162.3,
159.0, 147.8, 137.5, 137.4, 136.9, 133.5, 131.8, 131.7, 1:30.2, 128.7, 123.8,
119.7, 64.7,
53.9, 52.3, 36.7, 35.1; MS (ESI+) for C~6HZ,C12F,N406SZ mlz 676.5 (M+H)*; MS
(ESI-) for
IO C_sH,,Cl,F3N4O6S2 m/z 674.5 (M-H)'; Anal. Calcd for CZ6Hz,C12F3N4O6Sz ~
0.33: C, 45.69;
H, 3.20; N, 8.20. Found: C, 45.81; H, 3.38; N, 8.13. % Water (KF): 0.88.
Example 46.
4-[(2,6-Dichlorobenzoyl)amino]-N-[[(4S}-3-(phenylsulfonyl}-4-
thiazolidinyl]carbonyl]-L-phenylalan:ine methyl ester
(Scheme A, A-7: where RA_, and R;,_z are the same and e(lual to H, Rj is
phenyl, Y is SO2,
RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (S, S)).
S-. H O
CN~N~Oi
O-S-O CIO
O C;i
I~
H IW
C1
Example 46 was prepared as described in Scheme A fronn D-cysteine using
benzenesulfonyl chloride to form the requisite sulfonamide. Physical data as
follows: IR
(mull) 1744, 1668, 1604, 1531, 1515. 1432, 1413, 1355, 1324, 1268, 1220. 1195.
1167,
1090, 730 em -';'H NMR (CDCI,) S 7.84 (2 H), 7.65 (5 lH), 7.45 (I H}, 7.30 (6
H), 4.90
(1 H), 4.63 (2 H), 4.37 (I H}, 3.75 (3 H), 3.32 (1 H), 3.1 '_> (2 H), 2.53 (I
H); "C NMR
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-7I -
(CDC13) 8 171.2, 168.2, 162.4, 136.4, 136.3. 134.1. 132.4, 131.0, 130.2,
129.6. 128.2,
128.1, 127.9, 120.6. 65.3, 53.3, 52.6, 51.8, 37.4. 33.3: M.S (ESI+) far
C,7HZSChN3ObS2 m/z
621.8 (M+H)'; MS (ESI-) for C,,HzsCIzN,O6S, rrrlz 619.8 (M-H)'; Anal. Calcd
for
C27H23C1zN;O6S~ ~ 0.2 H20: C, 51.84; H, 4.18; N, 6.72. lFound: C, 51.72; H,
4.18; N, 6.52.
% Water (KF): 0.48.
Example 47.
4-[(2,6-Dichlorobenzoyl)amino]-N-[[(4S}-3-(phenylsulfonyl}-4-
thiazolidinyl]carbonyl]-t -phen.ylalanine
(Scheme A, A-8: where RA., and R,,_Z are the same and edual to H, R3 is
phenyl, Y is S02,
I0 RS is 4-[(2,6-dichlorobenzoyl)aminoJphenyl and stereocl~emistry is (S, S)).
S-. H ~O[I
CN~N~OH
O=S=O 0
O C:I
~ 1 I ~ N w
H I ,,
Ct
Example 47 was prepared from example 46 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 1735, 1666, 1605, 1:162, 1533, 1516, 1432,
1414,
1352, 1328, 1195. 1180, 1167, 1090, 731 crri';'H NMR (CDC13) 8 8.11 (1 H),
7.82 (2 H),
7.66 (3 H), 7.56 (2 H), 7.23 (6 H), 4.91 ( 1 H), 4.66 ( 1 H), 4.60 ( 1 H),
4.35 ( 1 H), 3.30
(I H), 3.19 (2 H), 2.59 (1 H);'3C NMR (DMSO-db) 8 I72.2, 168.2, 161.7, 137.0,
136.9,
136.3, 133.6, 133.0, 131.2, 131.1; 129.6, 129.3, 128.1, 1;27.6, 119.2, 63.9,
53.4, 51.5, 48.4,
36.0, 33.8; MS (ESI+) far CZ6H23C12N3~6S2 ~z 607.9 (1V1+H)+; MS (FAB) m/z
(rel.
intensity} 608 (MH+, g5}, 6I0 (67), 608 (85), 466 (30}, >i71 ( 41), 228 (38),
193 (38), 149
(30), 129 (31), 118 (99), 63 (35}; HRMS (FAB) calcd fo:r CzbH,3C12N3O6Sz+H~
608.0483,
found 608.0491: Anal. Calcd for C,6Hz3ChN3O6S~~ 0.27 I-i,0: C, 50.9I; H, 3.87;
N. 6.85.
Found: C, 50.68; H, 4.05; N, 6.65. % Water (KF): 0.79.
Example 48.
4-[(2,6-Dichlorobenzoyl)amino] N-[[(453-~[[5-(dirnethylamino}-1
naphthalenyl]sulfonyl]--0-thiazolidinyl]carbonyl]-mphenylalanine methyl ester
CA 02342778 2000-12-20
WO 99167230 PCT/US99114233
-72-
(Scheme A, A-7: where RA_, and R~.2 are the same and equal to H, R3 is 5-
dimethylamino-
1-naphthyl, Y is SO,, RS is 4-[(2,6-dichlorobenzoyl)aminoJphenyl and
stereochemistry is
(S~ ~)~
/S-. H OI'
~N~N~Oi
O=S=O O
CI
I w I ~ i N
w ~ . ~ i
CI
N
Fi C~ ,Chi
Example 48 was prepared as described in Scheme A from D-cysteine using 5-
dimethylamino-1-napthalenesuifonyl chloride to form the requisite sulfonamide.
Physical
data as follows: 1R (rnulI) 1744, 1684, 1605, 1562, 153:3, 1515. 1431, 1412,
1350, 1324,
1231, 1202, 1163, I 145, 798 cm -'; 'H NMR (CDCl3) 8 8.64 ( 1 H), 8.34 (2 H),
7.53 {5 H),
7.29 (4 H), 6.87 (3 H), 4.93 ( 1 H), 4.75 (1 H), 4.64 ( 1 H}, 4.31 ( I H),
3.69 (3 H), 3.47
(1 H), 2.84 {8 H), 2.46 (1 H); "C NMR (CDCl3) S 171.2., 167.7, 162.5, 136.3,
135.9,
132.4, 132.3, 131.9, 131.5, 131.0, 130.1, 129.7, 129.2, 128.2, 124.4, 120.4,
65.1, 53.3,
52.5, 50.1, 45.9; 37.1, 33.3; MS (ESI+) for C3~H32C1ZN4t~6Sz m/z 736.8
(M+Na)+; Anal.
Calcd for C33H32C12N4~6S2 ' 0.17 H,O: C, 55.15; H, 4.54: N, 7.79. Found: C,
55.20; H,
4.73; N, 7.49. % Water (KF}: 0.43.
Example 49.
4--[(2,6--Dichlorobenzoyl)amino]-N-[[(4S}-3--[[5-(dimethylamino)-1-
naphthalenyl]suIfonyl]~--thiazolidinyl]carbonyl]-L-phenylalanine
(Scheme A, A-8: where RA., and RA_z are the same and equal to H, R; is 5-
dimethylamino-
1-naphthyl, Y is S4~, RS is 4-[(2,6-dichlorobenzoyl)aminoJphenyl and
stereochemistry is
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-73-
S- H O
CN~N~OH
O=S=O O
CI
I ~~ ~ ~ ~ N w
iw H !~
cr
N
H3c 'cH3
Example 49 was prepared from example 48 by the proce;dare described in
preparation 6.
Physical data as follows: IR (mull) 1666,1605, 1587, 1577, 1562, 1532, 1516,
1431,
1412, 1395, 1325, 1163, 114S, 798, 631 cm -';'H NMR (DMSO-db) 8 10.60 (1 H),
8.54
( 1 H), 8.28 (2 H), 7.54 (8 H), 7.25 ( 1 H), 6.93 (2 H), 4.95 ( 1 H), 4.84 ( 1
H), 4.39 ( 1 H),
4. I 7 ( 1 H), 2.95 (2 H), 2.80 (7 H), 2.54 ( 1 H); ' lC NMR (DMSO-db) b
167.2; 161.7, I 51.4,
136.6, 136.3, 133.6, 133.1, 131.1, 130.8, 130.3, 129.5, 129.3, 129.0, 128.8,
128.1, 123.6,
118.9, 118.3, 115.3, 63.6, 54.2, 50.3, 44.9, 36.7, 33.9, 2:l .0; MS (ESI+) for
lO C3zH3oC1,N4ObS2 m~Z 700.8 (M+H)+; HRMS (FAB) calcd for C3iH,oChN,O6Sz+H,
701.1062, found 701.1039
Example 50.
U--[(2,6-Dichlorophenyl)methyl]-N-[[(4S~3-{rnethylsulfonyl}-4-
thiazolidinyljcarbonylj-1,-tyrosine. methyl ester
(Scheme A. A-7: where RA_~ and RA_2 are the same and equal to H, R3 is methyl,
Y is SO2,
RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl and stereochemistry is (S, S)).
S-. H O
CN N O~
O S OO ~ cl
CH3
O~ i
C ~I
Example 50 was prepared as described in Scheme A from D-cysteine using
methanesulfonyl chloride to form the requisite sulfonamide. Physical data as
follows: IR
(mull) 1742, 1680, 1611, 1564, 15 i 0, 1439, 1345, 1299, 1240, 1179, 1158,
1016, 976,
779, 768 cm~';'H NMR (CDCl3) 8 7.36 (2 H), 7.24 (1 H), 7.08 (3 H), 6.97 {2 H),
5.25
(2 H), 4.77 (3 H), 4.29 ( 1 H), 3.74 (3 H). 3.43 ( 1 H), 3.5:3 ( 1 H), 3.1 ~
(2 H), 2.93 (3 H);
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-74-
'3C NMR (CDCI,) 8 171.4, 168.0; 158.2, .137.0, 132.1, 1.30.3, 128.5, 127.9,
115.3, 65.2,
65.0, 59.4, 53.5, 53.3, 52.5. 5I.9, 42.2, 37.3, 36.9, 34.2; MS (ESI+) for
CZZH~4C12NZO6Sz
mlz 546.8 (M+H)'; MS (ESi+) for C~ZH24C12NZO6S2 mlz 568.8 (M+H)'; HRMS (EI)
calcd
for CZZH,,C12N2O6Sz 546.0453, found 546.0448; Anal. C'.alcd for C"H24C1,N,O6Sz
~ 0.07
H,O: C, 48.15; H, 4.43; N, 5.10. Found: C, 48.17; H, 4.51; N, 5.02. % Water
(KF): 0.24.
Example 51.
O-[(2,6-Dichlorophenyl)methylJ-N-([(4S)--3-(methylsulfonyl)--4-
thiazolidinyl]carbonyl]-t.-tyrosine
(Scheme A, A-8: where R,,_, and RA_Z are the same and equal to H, R, is
methyl, Y is SO2,
RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl and stereochemistry is (S, S)).
S- H O
~N~N~OH
O CH~ O ~ CI
O ~ w
CI
Example 5I was prepared from example 50 by the procedure described in
preparation 6.
Physical data as follows: IR {mull) 1737, 1675, 161 I, 1:565, 1511, 1439,
1345, 1300,
1241, 1197, 1179, 1 I57, 1016, 778, 769 crn''; 'H NMR (CDC1,) b 7.34 (2 H),
7.16 (5 H),
6.99 (2 H), 5.23 (2 H), 4.85 ( 1 H), 4.68 (2 H), 4.27 (1 H); 3.51 ( 1 H), 3.32
{ 1 H), 3. I 5
(2 H), 2.93 (3 H);'3C NMR (CDCl3) b 174.8, 168.7, 158.3, 137.0, 132.1, 130.5,
130.4,
128.5, I27.6, 1 I5.4, 115.2, 65.2, 64.9, 53.1, 52.0, 37.1. ?~6.4, 34.3; MS
{ESI+) for
CZ,Hz,ChN,O6S, mlz 532.8 (M+H)'; MS {ESI-) for C~,H,~CI,N,O6S2 mlz 530.7 (M-
H)';
HRMS (FAB) calcd for C~,H,~Cl,N206Sz+H, 533.0374, iPound 533.0386; Anal. Calcd
for
C,,H~ZC1,N,06S, ~ 0.06 HBO: C, 47.19; H, 4.17; N, 5.24. Found: C, 47.58; H,
4.35; N,
5.10. % Water (KF): 0.20.
Example 52.
4-[(2,6-Dichlorobenzoyl)aminoJ-N-[((4S)~3-
[[(I,l~li,methyiethyl)amino]carbonylJ-4-
thiazolidinyl]carbonyl]-t,-phenylalanine
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-75-
(Scheme A, A-8: where RA_, and R~_~ are the same and equal to I-I, R, is t-
butyl, Y is
CONH-, R5 is 4-((2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (S,
S)).
5-.. H O
C~~N~OH
0 0 l ~ O CI
H
C ~'I
Example 52 was prepared from example 6 by the procedure described in
preparation 6.
Physical properties as follows: IR (mull) 3289. 1728, 1664, 1607, I 580, 1561,
1536,
I432, 1414, 1394, 1326, 1270, 1242, 1213, I 195 cm'IH: NMR (300 MHz, CD30D) b
7.47 (2 H), 7.24 (3 H), 7.04 (2 H), 4.63 (2 H), 4.37(1 H)., 4.17 (I H), 3.09
(4 H), 1.22
(9 H);'3C NMR (75 MHz, CDCl3) 8 176.9, 174.8, 167.2, 159.9, 140.5, 144.0,
136.4,
I36.I, I34.6, 133.8, 131.8, 124.5, 66.6, 57.I, 55.3, 53.0, 40.1, 37.I, 32.9;
MS (ESI+) for
CzSH,gCl,N405S mlz 566.9 (M+I-i)', Sgg.9 M+Na)+; MS (:ESI+) for C,sHzgC1,N405S
mlz
566.9 (M+H)+; HRMS (FAB) calcd for C,SHZBCL,N405S +HI 567.1235, found
567.1253.
Example 53.
1 S 4-[{2,6-Dichiorobenzoyl)amino]-N-([{4S}--3-[(dliethylamino)carbonyl)-4-
thiazolidinyl]carbonyl]-L-phenylalanine
(Scheme A, A-8: where RA., and RA_, are the same and equal to H, R, is ethyl,
Y is
CON(CH,CH3)-, R5 is 4-[(2,6-dichlorobenzoyl)amino] phenyl and stereochemistry
is (S,
S-. H O
~N~N~OH
~N~O O I ~ 0 CI
J ~N
H ~~
CI
Example S3 was prepared from example 7 by the procedure described in
preparation 6.
Physical properties as follows: IR (mull) 3269, 1734, 16fi3, 1607. 1562. 1535,
15I5,
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-76-
1431, 1415, 1348, 1325, 1269, 1213, 1195, 799 cm-';'I-1 NMR (300 MHz, CD30D) 8
7.57
{2 H), 7.35 (3 H), 7.09 {2 H), 5.09 (1 H), 4.76 (1 H), 4.?~8 (2 H), 3.31 (3
H), 3.13 (5 H),
1.05 (6 H); '3C NMR (75 MHz, CD30D) b 176.6, 173.9, 167.1, 166.3, 140.7,
140.1, 136.1,
134.6, 133.8, 131.8, 124.1, 68.5, 57.4, 56:6, 45.9. 40.4, 36.6, 16.8; MS
(ESI+) for
CZSHZ8C12N~O5S m/z 567.1 (M+H)+; Anal. Calcd for C,S:H~gCI2N405S: C, 52.91; H,
4.97; N,
9.87.
Found: C, 52.60; H, 5.13; N, 9.47.
Example 54.
4-((2,6-Dichlorobenzoyi)amino]-N-[[{4S}-3-[[methyl [2-{2-
pyridinyl)ethyl]amino]carbonyl]-4-thiazolidinyl]carbonyl]-E.-phenylalanine
methyl ester
(Scheme A, A-7: where RA_, and RA_Z are the same and equal to H, R3 is 2-(2-
pyridyl}ethyl,
Y is CONCH;)-, Rs is 4-((2,6-dichlorobenzoyl)amino]phenyl and stereochemistry
is (S,
S-- H OI(
CN : N~Oi
~N ~ N~O O I ~ O CI
I
~NI ''.
HI ~ ,
CI
Example 54 was prepared as described in Scheme A from D-cysteine using 2-{2-
methylaminoethyl)pyridine to form the requisite urea. Physical data as
follows: mp 80-
90°C (dec); IR (mull) 1743, 1665, 1606, 1561, 1538, 15~14, 1489, 1432,
1413, 1395, 1323,
1268, 1216. 1195, 799 cm''; IH NMR (300 MHz, DMSO-d6} 8 8.44 (I H), 8.34 (1
H),
7.66 ( 1 H), 7.5 I {5 H), 7.19 (4 H), 4.72 ( 1 H), 4.48 ( 1 H); 4.40 ( 1 H),
4.20 ( 1 H), 3.58
{4 H), 3.42 { I H), 3.89 ( I0 H); '3C NMR (75 MHz: DMISO-db) S 172.1, 170.3,
162.3,
161.8, 159.4, 149.4, 137.6, 137.0, 136.8, 133.3, 131.8, 1131.7, 130.0; 128.7,
123.8, 122.0,
119.8, 64.58. 53.8, 52.7, 52.5, 49.7, 36.5, 36.2, 35.7. 33.4; MS (ESI+) for
C3oH3,ChNSOSS
m/z 643.9(M+H)'; Anal. Calcd for C3oH~,C1zN50sS: C. :>5.90; H, 4.85; N; 10.86.
Found:
C, 55.52; H, 5.09; N, I0.b4
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-77-
Example SS.
4-[(2,6-Dichlorobenzoyl)amino]-N-[[(4S}-3-[[methyl[2-{2-
pyridinyl)ethyl]amino]carbonyl]-4-thiazolidinyl]carbonyl]-L-phenylalanine
S (Scheme A, A-8: where R~_, and RA_2 are the same and equal to H. R, is 2-(2-
pyridyl)ethyl,
Y is CON(CH3)-, R5 is 4-[(2,6-dichiorobenzoyl)amino]phenyl and stereochemistry
is (S,
~)~
S-. H O'I
w ( ~~~N~Oi-!
N N O I ~ O Cl
I
H I/
Ct
Example SS was prepared from example S4 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 1682, 1656, 1606, 1561, 1540, 1 S 13,
1432, 1413,
1398, 1323, 1268, 1242, 1195, 799, 780 cm ''; 'H NMR (300 MHz, DMSO-db) S
10.66
( 1 H), 8.45 ( 1 H), 8.09 ( 1 H), 7.66 ( 1 H), 7.S 1 (S H), 7.f.0 (4 H), 4.72
( 1 H), 4.40 (2 H),
IS 4.23 (1 H), 3.61 (1 H), 3.40 (1 H), 3.04 (7 H), 2.79 (3 H); MS (ESI+) for
C,9H29C1zN505S
m/z 629.9 (M+I-i)~; Anal. Calcd for C,9H29C12NSOSS ~ 0.~6I H,O: C. 54:29; H,
4.75; N,
10.92. Found: C, 54.29; H, 5.00; N, 10.32. % Water (k;F): 1.72.
Example S6.
4-[(2,6-Dichlorobenzoyl)amino]-N-[[(453-(4--morpholinylcarbonyl~4--
thiazolidinyl]carbonyl]-t,--phenylalanine methyl ester
(Scheme A, A-7: where RA_, and RA_Z are the same and equal to H, R~ and Y
together form
CO-morpholino, R5 is 4-[(2,6-dichlorobenzoyl)amino]p:henyl and stereochemistry
is (S,
~).
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-78-
S.,. H O
~N N °i
M ,r
Example 56 was prepared as described in Scheme A from D-cysteine using
morpholine to
form the requisite urea. Physical data as follows: mp 223-225°C; I H
NMR (300 MHz,
DMSO-db) 8 10.66 ( I H), 8.25 ( 1 H), 7.5 I (5 H}, 7.16 (2 H), 4.81 ( l H),
4.60 ( 1 H), 4.50
(1 H), 4.28 (1 H), 3.64 (3 H), 3.53 (4 H), 3.09 (8 H}; "C NMR (75 MHz, DMSO-
d6)
172.1, 170.3, 162.3, 161.4, 137.6, 136.8, 133.3, 131.8, 131.7, 130.0, 128.7,
119.8, 66.2,
64.3, 53.8, 52.8, 52.5, 46.8, 36.1, 33.7; HRMS (FAB) cz~lcd for
C,6H2gCL,N406S+H,
595.1185, found 595.1189; Anal. Calcd for C26HZ$CI,N,~D6S: C, 52.44; H, 4.74;
N, 9.41.
Found: C, 52.42; H, 4.96; N, 9:23.
Example 5 7.
N-[[(4S~3-[[Bis(2-hydroxyethyl)aminojcarbonylj-4--thiazolidiny ljcarbonyl]-4-
[(2,1r
dichlorobenzoyl)amino]-~-phenylalanine methyl ester
(Scheme A, A-7: where RA_, and RA_Z are the same arid equal to H, R3 is 2-
hydroxyethyl
and Y is CON{CH,CH,OH), RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and
stereochemistry is (S, S)).
5-.. H O
CN~ N~Oi
HO~N~° I° ! ~ ~ G
HoJ ~N
i
Example 57 was prepared as described in Scheme A from D-cysteine using
diethanolamine to form the requisite urea. Physical data as follows: mp I05-
107°C; IR
(mull) 3284. 1743, 1662, 1608, 156I, 1539, 1516, 1432, 1414, 1355, 1326, 1270,
1217,
1 I96, 799 cm ~'; ~H NMR (300 MHz, DMSO-d~) 8 10.69 (l H), 8.28 (1 H), 7.56 (4
H),
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-79-
7.47 ( I H}, 7.16 (2 H), 4.87 (3 H),~ 4.66 { I H}, 4:47 ( 1 H}, 4.25 ( I H),
3.63 (3 H), 3.47
(6 H), 3.02 (6 H); MS (ESI+) for Cz6H3oCIZN4O7S m/z 612.9 (M+H)*; Anal. Calcd
for
C26H30C1?N4O7S ~ 0.47 H,O: C, 50.21; H, 5.01: N, 9.Oi. :Found: C, 50.02; H,
5.00; N, 8.93.
Water (KF): 1.36.
Example 58.
N-[[(4S}-3-[[Bis(2-hydroxyethyl)amino]carbonyl]-4-thiazolidinyl]carbonyl]-4-
[(2,6
dichlorobenzoyl)amino]-L-phenylalanine
(Scheme A, A-8: where RA_, and R~_z are the same and equal to H, R, is 2-
hydroxyethyl
and Y is CON(CH,CH,OH), RS is 4-[(2,6-dichiorobenzoyl)amino]phenyl and
stereochemistry is (S, S)).
S. H~ O
~N~N~OH
HO~N~O O I ~ ~ CI
HOJ ~N
H
cl-
Example 58 was prepared from example 57 by the procedure described in
preparation 6.
Physical data as follows: IR (mull} 3281, 3196, 1724, 1660, 1608, 1580, 1561,
1542,
1515, 1431, 1415, 1354, 1328, 1271, 1196 cm ~'; 'H NMR (300 MHz, DMSO-db) 8
10.65
(1 H), 8.13 (1 H), 7.55 (4 H), 7.47 (1 H), 7.16 {2 H), 4.85 (2 H}, 4.66 (1 H),
4.40 (i H),
4.27 ( 1 H), 3.48 {6 H), 3.01 (6 H); MS (ESI+) for CZSHz$(:1,N~07S mlz 598.9
(M+H}+;
Anal. Calcd for Cz5H~8C1~N,~O,S ~ 1.04 HBO: C, 58.58; H, 4.90; N, 9.06. Found:
C, 48.88;
H, 5.05; N, 8.79. % Water (KF): 3.02.
Example 59.
[S-(R*,R*)]-4-jj[ 1-[j4-[(2,6--dichlorobenzoyl)amino]phenyl]methyl]-2-methoxy-
2
oxoethyl]amino]carbonyl]-S-oxo-3-thiazolidinepe~ntanoic acid 3-methyl ester
{Scheme A, A-'7: where RA_, and RA_, are the same and equal to H, R~ is
CH~CH,CH,CO~CH3, Y is CO, R$ is 4-[(2,6-dichioroben2:oyl)amino]phenyl, and
stereochemistry is (S,S)}.
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
_80_
S-. H OI'
O ~N~N~Oi
~O~O O l ~ O CI
N' w
h~ l /
iCl
Example 59 was prepared as described in Scheme A from D-cysteine using methyl
glutaryl chloride to form the requisite amide. Physical dlata as follows: IR
(mull) 3266,
I741, 1734, 1685, 1678, 1630, 1610. 1560, 1545, 1441, 1435, 1414, 1327, 1268,
1227 cm'
' ; IH NMR (DMSO-db} 8 10.65 (1 H), 8.43 (I H), 7.50 (5 H}, 7.15 (2 H), 4.72
(2 H), 4.44
(2 H), 3.63 (3 H), 3.56 (3 H), 3.10 (4 H), 2.15 (4 H), 1. i'0 (2 H}; "C NMR
(CDC13) 8
171.6, 170.6, 170.1, 169.9, I69.6, 161.9, 137.2, 136.5, 132.9, 131.3, 129.7,
128.3, 119.4,
61.4, 53.7, 53.5, 52.0, 51.3, 48.7, 36.4, 35.8, 35.1, 33.I, 32.8, 32.5, 25.4,
19.8; MS (ESI+)
for C,7HZ9N3O7SC12 mlz 610.0 (M+H)'; MS (ESI-) for C,7Hz9N30,SCh mlz 608.0 (M-
H)';
Anai. Calcd fox C,7H29C12N30,S: C, 53.12; H, 4.79; N, 6.88. Found: C, 52.81;
H, 4.90; N,
6.88
Example 60.
[S-(R *,R* )]--4-[[[ I -Carboxy-2-[4-[(2,6-
dichlorobenzoyl)amino]phenylJethyl]amino]carbonyl]-b--oxo-3-
thiazolidinepentanoic
acid
(Scheme A, A-8: where RA_, and RA_z are the same and equal to H, R3 is
CHZCH~CH,COZH, Y is CO, RS is 4-[(2,6-dichlorobenzo~yl}amino]phenyl, and
stereochemistry is (S,S)}.
S-. H OI'
~N~OH
HO O O l ~ O CI
H~ I /
C;i
Example 60 was prepared from example 59 by the procedure described in
preparation 12.
Physical data as follows: IR (mull} 3271, 3193, 3124, 1'.125, 1661, 1607,
I56I. 1539,
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-81-
1516, 1432, 1414, 1327, 1271, i 195, 799 cm -';'H NMR (DMSO-db) S 12.40 (1 H),
10.63
( l H), 8.31 ( 1 H). 7.50 (5 H), 7.16 (2 H), 4.75 (2 H), 4:34 {2 H), 2.95 {4
H), 2.15 {4 H),
1.66 (2 H); "C NMR (CD~CN) 8 175.0, i 72.7, 172.6, 17I Ø 163.5, 137.8,
137.0, 134.4,
132.7, 132.3, 131.1, 130.9, 129.1, 120.7, 63.2, 54.5, 49.9, 37.2, 34.1, 33.3,
20.7; MS
(ESi+) for C,SH~sCI,N30,S mlz 582.0 (M+H)+; MS fESI~-) far C,SHz5C12N30,S mlz
579.9
(M-H)-; Anal. Calcd for CZSH2sChN30,S ~ H20: C. 50.01. ; H, 4.53; N, 7.00.
Found: C,
49.61; H, 4.38; N. 6.61.
Example 61.
N-[[(4S~3-Acetyl-thiazolidinyl]carbonyl]-~i--[{f.,6-dichlorobenzoyl)amino]-~-
i 0 phenylalanine methyl ester
(Scheme A, A-7: where R.4:1 and RA.z are the same and equal to H, R3 is CH3, Y
is CO, RS
is 4-[(2,6-dichlorobenzoyl)amino]phenyl, and stereochelmistry is (S,S)).
S-. H t7
O t:l
H II ,
C ~I
Example 61 was prepared as described in Scheme A from D-cysteine using acetyl
chloride
to form the requisite amide. Physical data as follows: l:R (mull) 3260, 3067,
1748, 1686,
1623, 1608, 156 I , 1542, I' S 1 S, 1445, 1429, 1419, 1324, 1267, 1221 cm '';
' H NMR
(DMSO-db) 8 8.44 ( 1 H), 7.52 (5 H), 7.17 (2 H), 4.78 (2 H), 4.37 (2 H), 3.63
(3 H), 3.06
(4 H), 1.94 (3 H); "C NMR (DMF-d,) b 172.4, 170.6, 1:38.4, 137.5, 133.8,
132.3. 131.9,
130.6, 128.9, 120.0, 70.9, 63.6, 62.4, 54.7, 52.3, 50.2, 4!x.8, 49.2, 37.2,
36.6, 22.7; MS
(ESI+) for C,3H,3C1zN~O5S mlz 523.9 (M+H)+; MS (ESI+) for Cz3Hz3C12N30sS mlZ
545.8
(M+Na)'; HRMS (FAB) calcd for Cz3H,,C12N305S+H, 524.0814, found 524.0812;
Anal.
Calcd for C,3H,3Cl,N~OSS ~ 0.1 H,O: C, 52.46; H, 4.45; N, 7.98. Found: C,
52.85; H,
4.42; N, 8.00. % Water (KF): 0.24.
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-8z-
Example 62.
N-[[(4S}-3-Acetyl-4-thiazolidinyl]carbonyl]-~-[(2;6-dichlorobenzoyl)amino]-t,-
phenylalanine
(Scheme A, A-8: where R"_, and RA_Z are the same and equal to H. R, is CH3, Y
is CO, RS
is 4-[(2,6-dichlorobenzoyl)amino]phenyl, and stereochemistry is (S,S)).
S-. H O
'OH
Q O f ~ O CI
H
CI
Example 62 was prepared from example 61 by the procecture described in
preparation 6.
Physical data as follows: IR (mull) 3070, 1747, 1682, 1663, 1625, 1608, 1580,
1561,
1548, 1514, 1443, 1431, 1416, 1278, 1220 cm'';'H NMR (DMSO-d~) 8 12.48 (1 H),
10.63 (I H), 8.29 (1 H}, 7.50 (5 H), 4.73 (2 H), 4.34 (2 H;), 2.97 (4 H), 1.93
(3 H);
'3C NMR (DMSO-db) 8 172.4, 169.6, 169.2, 168.5, 168.0, 161.7, 136.9, 136.3,
133.2,
133.0, 131.2, 129.6, 128.1, I 19.2, 62.2, 61.0, 53.5, 53.3, 49.3, 48.4, 36.3,
35.7; 35.0, 33.1,
22.4, 20.9; MS (ESI-) for C"H2,C12N305S m/z 507.9 (M-l~)'; Anal. Calcd for
C~~H~,C1,N305S ~ 0.1 HZO: C, 51.57; H, 4.18; N, 8.20. Found: C, ~ 1.49: H,
4.36; N, 8.07.
°,% Water (KF): 0.40.
Example 63.
[S-(R*,R*)]-4-CCCI-carboxy-2_.[4-C(2,6-
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]-5,5-dimethyl-$-oxo-3-
thiazolidinebutanoic acici
(Scheme A, A-8: where RA_, and RA_z are the same and equal to CH3, R, is
CH,CHZCOZH,
Y is C0, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl, and stereochemistry is
(S,S)).
CA 02342778 2000-12-20
WO 99/6?230 PCT/US99114233
-83-
/Sy H~ O
~PI~N =' OH
HO~O O I ~ ~ CI
~0 i
H I~
Ct'
Example 63 was prepared as described in Scheme A from D-penicillamine using
methyl
succinyl chloride to form the requisite amide. Physical data as follows: IR
(mull) 3264,
S 3198, 3071, 1721, 1660, 1608, 1562, 1541, 1516, 1432, 1415, 1327, 1270,
1195, 799 cm'';
'H NMR (DMSO-db) 8 10.62 (I H), 8.1 S (i H), 7.52 (5 H), 7.18 (2 H), 4.72 (2
H), 4.43
(3 H), 2.91 {4 H}, 2.17 (2 H), 1.36 (3 H), 1.07 (3 H);'3C NMR (DMSO-db) 8
173.5, 172.6,
170.2, 169.7, 168.4, 168.1, 161:7, 136.9, 136.3, 133.3, 1:31.1, 129.6, 129.4,
128.1, 119.2,
70.6, 53.8, 53.5, 51.6, 48.4, 47.7, 35.9, 30.7, 30.5, 28.9, :?8.6, 24.1; MS
(ESI+) for
CZ6Hz7ChN307S mlz 596.0 (M+H)+; MS (ESI+) for C,6H~,~C12N30,S mlz 6I7.9
(M+Na)+.
MS (ESI-) for C~6Hz7C1,N30,S mla 593.8 (M-H)'; MS (FAB) mlz (rel. intensity)
596
(MH+, 20), S98 (1S), S96 (20), 331 (1 I), 193 ( 13), 141 (IS), 139 (99), 116
(16), 107 (13),
105 (S0), 89 (2S); HRMS {FAB) calcd for Cz6H27C1,N30.,S+H, 596.1025, found
596.1036.
Example 64.
1 S [S-{R*,R* )]-4-[[[ 1-Carboxy-2--[4-[(2,6-
dichlorophenyi)methoxy]phenyl]ethyl]amino]carbonyl] y-oxo-3-
thiazolidinebutanoic
acid
(Scheme A, A-$: where RA_, and RA_2 are the same and equal to H, R3 is
CHZCH~COzH, Y
is CO, RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl, and stereochemistry is
(S,S)).
z0
S-: H O
~N~N~OH
HO~O O I ~ CI
[O~ - i O~
CI
Example 64 was prepared as described in Scheme A from D-cysteine using methyl
succinyl chloride to form the requisite amide. Physical data as follows: IR
(rnull) 3073,
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-84-
3031, 1725, 1640, 1612, 1585, 1565, 1535, 1511, 1439, 1300, 1241, 1196, 1179,
768 cm';
'H NMR (DMSO-db) 8 8.26 (I H), 7.53 (2 H), 7.44 (I H), 7.I2 (2 H), 6.93 (2 H),
5.16
(2 H), 4.75 (2 H), 4.40 (2 H), 4.20 (I H), 2.81 (4 H), 2.3:2 (2 H), 2,07 (1
H);'3C NMR
(DMSO-db) 8 173.8, 172.6. 170.0, 169.3,157.I, 136.0; 131.5, 130.4, 130.0,
129.8, 128.7,
114.2, 64.8, 61.5, 53.6, 48.5, 36.I, 35.5, 35.0, 33.1, 28.9, 28.6, 21.0; MS
(ESI+) for
C24H24C12N2~7S ~Z 554.8 (M+H)~; MS (ESI-) for Cz4HzaC1zN,07S m/z 552.7 (M-H)';
HRMS (FAB) calcd for Cz4Hz4C1zN20,S+Ht 555.0759, found 555.0750.
Example 65.
N-[[(4S}-3-Acetyl-4--thiazolidinyl]carbonyl]-O-[(?.,6-dichlorophenyl)methyl]-
1.-
I0 tyrosine methyl ester
(Scheme A, A-7: where R~_, and R,,_z are the same and equal to H, R, is CH3, Y
is CO, RS
is 4-[(2,6-dichlorophenyl)methoxyJphenyl, and stereochemistry is (S,S~).
s--.. ~ oI,
CN~N~Oi
H3C~0 C ~ ~ Ct
'O
Ct
IS
Example 65 was prepared as described in Scheme A frorn D-cysteine using acetyl
chloride
to form the requisite amide. Physical data as follows: I1z (mull) 1744, 1657,
1612, 1585,
1564, 1511, 1438, 1405, 1352, 1299, I240, 1197, 1 I79, 1016, 768 cm'; 'H NMR
{CDCl3)
b 7.36 (2 H), 7.24 ( 1 H), 6.97 (5 H), 5.24 (2 H), 5.04 ( 1 H), 4.78 ( 1 H),
4.50 (2 H), 3.74
20 (3 H}, 3.45 ( 1 H), 3. I 7 (3 H), 2.02 (3 H); '3C NMR (CDC:1,) 8 172.7,
171.6, 171.4, 169.8,
168.9, 168.7, 157.8, 136.9, I3I.9, 130.3, 129.9, 128.6, 128.4, 114.9, 65.1,
61.6, 56.0,
53.6, 53.3, 49.6, 36.7, 3 I.7, 22.5; MS (ESI+) for Cz3H24C:IzN,OSS m/z 532.9
(M+Na)+; MS
(Ei) mlz (rel. intensity) 510 (M+, I), 338 (42), 337 (I2), 336 (63), 267 (12
), 265 (18), 163
(10), 161 (63), 159 (99), 130 (9), 88 (43); Anal. Calcd for
Cz3HzaCIzN,OSS~0.19 H,O: C,
25 53.66; H, 4.77; N, 5.44. Found: C. 53.81; H, 4.75; N, 5.:33. % Water (KF):
0.66.
CA 02342778 2000-12-20
WO 99/67230 PCT/LTS99/14233
-85-
Example 66.
N-[[(4S}-3-Acetyl-4-thiazolidinyl]carbonyl]-0-[(~!,6-dichlorophenyl)methyi]-~-
tyrosine
(Scheme A, A-8: where R,,_, and RA_2 are the same and equal to H, R3 is CH,, Y
is CO, R,
is 4-[(2,6-dichlorophenyl)methoxy]phenyl, and stereochemistry is (S,S)). .
S-. H O
~~~ N ~OH
O
H3C O I ~ Ct
Example 66 was prepared from example 65 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 1730, 1646, T6I2, 1585, 1565, 151 I, 1439,
14I4,
1299, 1240, 1196, 1179, 1016, 779, 768 cm''; ' H NMR (DMSO-db) 8 8.26 ( 1 H),
7.54
(2 H), 7.44 ( 1 H), 7.13 (2 H), 6.92 (2 H), 5. i 6 (2 H), 4.7:? (2 H), 4.31 (2
H), 2.91 (5 H),
1.92 (3 H);'3C NMR (DMSO-db) 8 172.6, 169.2, 168.0, 157.1, 136.0, 131.7,
131.5, 130.3,
128.7, 114.2, 64.$, 62.2, 6I.0, 53.7, 49.4, 48.5, 36.1, 35..5, 35.1, 33.2,
22.4, 21.0; MS
(ESI+) for CZZH~ZC12NZOSS mlz 496.9 (M+H)+; MS (ESI-) for C~HZZCIzN,OSS mlz
494.8
(M-H)'; MS (FAB) m/z (reI. intensity} 497 (MH+, 99), 6I7 (29), 573 (12), 539
(25), 500
11 ), 499 (78), 498 (38), 497 (99), 496 ( 11 ), 225 (62}, 130 ( 14); HRMS
{FAB} calcd for
C,~H"CI,NZOSS+H, 497.0705, found 497.0713; Anal. Calcd for CZ,H~,C1,N,05S ~
0.41
H20: C, 52.35; H, 4.56; N, 5.55. Found: C, 52.65; H, 4.51; N, 5.50. % Water
(KF): 1.46.
Example 67.
[R-(R*,S*)]-4-[[[ I-Carboxy-2.-[4-[(2,6-
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]-y-oxo-3-
thiazolidinebutanoic acid
(Scheme A, A-8: where RA_~ and R4_2 are the same and equal to H, Ra is
CH,CH,CO,H, Y
is CO, RS is 4-[{2,6-dichlorobenzoyl)amino]phenyl, and stereochemistry is
{R,S)).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-86-
~s~ o
.~~~,H- ~
'OH
O-
0 O I ~ ~) Ci
HO
H I ~
CI
Example 67 was prepared as described in Scheme A from L-cysteine using methyl
succinyl chloride to form the requisite amide. Physical data as follows: ' H
NMR
(CD3CN) 8 8.86 ( 1 H), 7.55 (2 H), 7.42 (3 H}, 7.24 {2 H), 7.11 ( 1 H), 4.90 (
1 H}, 4.65
(2 H), 4.33 (I H), 3.14 {3 H), 2.47 (6 H), 1.80 (1 H); "C NMR (CD3CN) 8 174.7,
172.6,
172.3, 170.7, 163.4, 137.7, 137.0, 134.4, 132.7, 132.2, 130.9, 129.1, 63.2,
60.9, 54.4, 49.7,
37.1, 36.7, 32.7, 30.6, 30.0, 29.5, 21.1, 14.4; MS (FAB} m/Z (rel. intensity)
S68 {MH+,
99), 646 (11); 644 (16), 572 (13), 571 { 12), 570 (73}, 5Ei9 (38), 568 (99),
567 (15), 216
(22), 88 (27); Anal. Calcd for C,4H23CIzN307S ~ 0.5 H,O: C, 49.92; H, 4.19; N,
7.23.
Found: C, 50.01; H, 4.54; N, 7.05.
Example 68.
[R-{R*,S*)]-4-[[[1-Carboxy-2-[4-[(2,6-
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]-8-oxo-3-
thiazolidinepentanoic
acid
(Scheme A, A-8: where R.4_, and R,~_z are the same and equal to H, R3 is
CHZCH~CH~CO,H, Y is CO, RS is 4-[(2,6-dichlorobenzoyi)amino]phenyl, and
stereochemistry is {R,S~).
H_ J~
~s~ o
'ON
O O I ~ O CI
HO-
O N' w
~ I ~
cl
Example 67 was prepared as described in Scheme A from L-cysteine using methyl
glutaryl
chloride to form the requisite amide. Physical data as follows: 'H NMR (CD3CN)
8 8.85
(1 H), 7.55 (2 H), 7.44 (3 H), 7.17 (3 H), 4.86 (1 H), 4.64 {2 H), 4.34 (1 H),
3.64 (1 H),
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
_87-
3.13 (3 H), 2.27 (4 H}, 1.79 (4 H):; '3C NMR (CD3CN) 8 175.0, 172.9. 172.7,
170.6, 163.4,
I37.7, 137.0, 134.2, 132.7, 132.2, 131.0, 129.1, 120.7, 68.2, 63.1, 54.2,
49.8, 37.1, 36.9,
34.0, 33.2, 32.6, 26.2, 20.6; MS (FAB) m/z (rel. intensity) 582 {MH+, 99), 585
(12), 584
{68}, 583 (35), 582 ( 99), 581 (11), 88 (23}, 69 {8), 57 (9;1, 55 (11), 43
(11); Anal. Calcd for
C,SH,SC1~N~O,S ~ 0.2 HZO: C, 51.24; H, 4.37; N, 7.17. Found: C, 51.25; H,
4.68; N, 6.92.
Example 69.
N-[[{4R}-3-Acetyl-4.-thiazolidinyl]carbonyl]-~-[(2,6-dichlorobenzoyi)amino]-~.-
phenylalanine methyl ester
(Scheme A, A-7: where RA_, and RA_Z are the same and equal to H, R~ is CH3; Y
is CO, RS
is 4-[(2,6-dichlorobenzoyl)amino]phenyl, and stereochernistry is (R,S~).
0
H I'
N~N~Oi
H3C~0 ~ I ~ O CI
~N
H~N
CI
Example 69 was prepared as described in Scheme A from L-cysteine using acetyl
chloride
to form the requisite amide. Physical data as follows: IR. (mull) 3268, 1743,
1662, 1607,
1561, 1538, 1515, 1431, 1413, 1354, 1324, 1270, I217, 1195, 799 crri ';'H NMR
(CDC13)
8 7.56 (3 H), 7.31 (4 H}, 7.14 (2 H), 4.92 (2 H), 4.49 ( 1 H), 4.29 ( 1 H),
3.77 (3 H), 3.54
(1 H), 3.26 {1 H}, 3.00 (2 H), 2.11 (3 H);'~C NMR (CDC13) b 171.5, 170.3,
168.9, 152.5,
135.9, 135.7, 132.9, 132.3, 131.0, 130.1, 129.9, 128.2, 1:28.0, 127.9, 120.4,
63.9, 61.4,
53.1, 52.5, 49.7, 37.0, 3 i.2, 22.6; MS {ESI+) for Cz3Hz3C=1,N305S mlz 523.8
(M+H)+.
Example 70.
N-[[(4R)-3 Acetyl-4-thiazolidinyl]carbonyl]-4-[(2,6-dichlorobenzoyl)amino]-~-
phenylalanine
{Scheme A, A-8: where RA_, and RA_2 are the same and equal to H, R~ is CH,, Y
is CD, RS
is 4-[(2,6-dichlorobenzoyl)amino]phenyl, and stereochemistry is {R,,S~).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-88-
S H O
C ~ N~OH
HgC~O O 1 ~ O CI
H '
CI
Examp1e70 was prepared from example 69 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 3276, 3068, 1727, 1661, 1607, 1561, 1540,
1516,
1444, 1431, 1414, 1327, 1271, 1218, 1 I95 cm '; 'H NMR (DMSO-db) 8 12.78 (1
H), 10.67
(1 H), 8.27 (1 H), 7.49 (4 H), 7.19 {2 H), 4.75 {2 H), 4.415 (3 H), 3.01 (3
H), I.96 (3 H);
'3C NMR {DMSO-db) b 172:6, 172.3, 169.4, 168.6, 168.:2, 161.7, 136.9, 136.3,
133.3,
132.8, 131.1, 129.6, 128.1, 119.1, 62.2, 60.9, 53.4, 49.3, 48.7, 35.9, 35.1,
32.7, 22.4; MS
(ESI+) fox C,zH2,Cl~N305S mlz 509.8 (M+H)+; MS (ESI-.) for CZZHZIC1,N305S mlz
507.$
(M-H)'; HRMS (FAB) calcd for CzzH21C12N305S+HI 5It1.0657, found 510.0667;
Example 7I .
4-[(2,6-Dichiorobenzoyl)amino]-N-[[(4R}-4-thiazolidinyI]carbonyl]-L-
phenylalanine
monohydrochloride salt
(Scheme A, A-10: where RA_, and RA_2 are the same and equal to H, RS is 4-
[(2,6-
i S dichlorobenzoyl)amino]phenyl, and stereochemistry is (n,.S'~).
<s~ a
H_ ~
N N v 'OH
H
O I ~ O CI
CI
Example 7I was prepared as described in Scheme A from L-cysteine. Physical
data as
follows: IR (mull) 3249, 3190, 3036, 1729. 1662, 1605, 1578, 1562, 1541, 1516,
1432,
1414, 1328, 1271, 1195 crri';'H NMR (DMSO-db) 8 10.71 (1 H), 8.83 (1 H), 7.56
(5 H),
7.24 (2 H), 4.50 ( 1 H), 4.25 ( I H), 4.2I (2 H), 3.62 ( 1 H), 3.01 (4 H); '3C
NMR (DMSO-
db) 8 172.0, 166.8, 161.8, 137.7, 132.7, 131.3, 131Ø 12SL5, 128.1, I 19.4,
72.0, 70.4, 62.3,
53.8, 49.0, 35.7, 33.4; MS (ESI+) for C,oH,9Cl,N;O,S rrva 468.1 (M+H)';
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-89-
Scheme B
HS R9.1
Re z 8-1
HZN~OH.
nO
O
B-2
R8 ~Re~
Rg_~
Rg~ Re-2
~OH 8.3
Rs~ H O
RB_~
RB~~' R8.2 B-4
~OH
[[[[N
RB~a y O
R~
3
rR5
!3-5
Hy ' ~N
RB_~
O
RB.3 S Re_2 O
R Res
0 ~ 8-8
/Y R5
R3
RB_~
Rea S Rs_2 O
R ~NH OH
O ~ B-7
/Y R5
R3
Re_~
Rss S RB 2 O
~NH OH &8
Rep H 0
R5
CA 02342778 2000-12-20
WO 99!67230 PCTIUS99/14233
-90-
where RB_,, RR_,, RH_,, and RBA are defined as R, and in addition RB_, and Rep
may be
attached to the same carbon atom and form a cyclic ring of 5-8 atoms of the
formula:
~(CH~)l~
C NR~~
~ ~(CHZ)t/
together with the carbon atom to which they are attached; Rs_5 is defined as
OH or O-(C,~
alkyl)
Scheme B describes a general method for the preparation of thiazolidine-4-
carboxylic acid derivatives of general structure B-6, B-7 and B-8 which are
disubstituted
at the 2 position (i.e., RB_3 and RBA are not equal to H). VVithin this class
of structures, the
nitrogen is derivatized immediately after forming the heterocyclic ring.
Accordingly, a
I O commercially available or readily prepared sulfur-containing amino acid of
structure B-I
(which is the same structure as A-1) is condensed with a commercially
available or readily
prepared ketone to afford the thiazolidine-4-carboxylic acid of general
formula B-3
(preparation 15) (for a general discussion of the condensation of aldehydes
and ketones
with cysteine or similar sulfur containing amino acids see: Coppola, G.M.;
Schuster, H.F.
I 5 Asymmetric Synthesis: Construction of Chiral Molecules Using Amino Acids;
.Iahn Wiley:
New York, 1987; Chapter 6, I7I.). The amine group may be reacted with a
variety of
electrophilic reagents such as sulfonyl chlorides, carbonates, chlaroformates.
isocyanates,
phosgene (or a suitable equivalent) and an amine, acid chilorides, and
carboxylic acid
anhydrides as described in Scheme A far the reaction of A-6. Preparation 16 is
provided
20 as a specific example of the synthesis of a compound of l;eneral structure
B-4.
Condensation of B-4 with amino acid derivative B-5 under standard peptide
synthesis
conditions provides the compound of general structure B-6 (preparation 17).
Mild base
hydrolysis of the methyl ester of general structure B-6 (where RB_5 is OCH3)
may be
effected as described and exemplified in Scheme A (preparation 6 or i3) to
afford
25 compounds of the general structure B-7. Alternatively, in those cases where
RB_5 is O-t-
Bu, mild acidolysis can also provide compounds of the general structure B-7
(by the
procedure described in preparation 4 of Scheme A). In the case of t-
butoxycarbonyl
derivatised analogs of general structure B-7 (i.e. where R, is t-butyl and Y
is CO,), mild
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-91-
acidolysis affords compounds of general structure B-8 (by the procedure
described in
preparation 4 of Scheme A).
Preparation 15.
(Scheme B, B-3: where Rg_, and RB_2 are the same and c;qual to H, RB_~ and R~~
are the
S same and equal to CH,. and stereochemistry is (R)).
H3G,,./S
H3C/~H~OH
O
A suspension of L-cysteine hydrochloride monohydrate (Scheme B. B-1: where
R,~_, and
RB_Z are the same and equal to H and stereochemistry is (R)) {20 g, 0.11 mol)
in acetone
(Scheme B, B-2: where RB_3 and Rg~ are equal to CH3) (800 mL) was heated to
reflux for
8 h. Cooling to room temperature resulted in precipitation of a solid which
was collected
by filtration, washed with acetone and dried in vacuo to afford the title
compound (17.46
g) as a white solid: mp 165-167°C; (Lit. 165-I68°C: Sheehan,
3.C.; Yang, D-D.H. J. Am.
Chem. Soc. 1957, 80, i 1 S 8) ~ H NMR (DSO) 8 4.75 ( 1 H), 3.59 ( 1 H), 3.44 (
1 H), 1.73
(3 H), 1.71 (3 H); MS (ESi-) for C6H"NOZS m/z 159.9 (:M-H)'.
Preparation 16.
(Scheme B, B-4: where Ra_, and Ra_z are the same and equal to H. RB_3 and RB~
are the
same and equal to CH,, Y is COz, R3 is t-butyl and stereochemistry is (R)).
H3G,~S
H3C N~OH
O~ ''O
O
To a solution of B-3 (Scheme B where Rs:, and RB_2 are the same and equal to
H, RB_3 and
RB~ are the same and equal to CH3, and stereochemistry is (R)) ( 17.46 g, 0.11
mol) in
acetonitrile (250 mL) at ambient temperature was added di-t-butyl dicarbonate
(25.64 g,
0.117 mol) followed by N,N-diisopropylethylamine (16.9 mL, 0.097 mol). The
reaction
mixture was stirred for ? days and volatiles remove in vcrcuo. The residue was
slurried in
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-92-
diethyl ether and filtered through a pad of celite. The filtrate was washed
with 0.1 N HCI,
water, brine, dried (Na2S04), filtered and concentrated ir! vacuo:
Crystallization of the
clear oil from hexane provided the title compound (3.85 g) as a white solid:
mp 125-
126°C; {Lit. 114°C: Woodward, R.B.; Heusler, K.; Go:>teli, J.;
Naegeli, P.; Oppolzer, W.;
Ramage, R.; Ranganathan, S.; Vorbruggen, H. J. Am. Cliem. Soc. 1966, 88,
8S2)'H NMR
(CDCI~) & 8.70 (1 H), 4.89 (1 H), 3.27 (2 H), 1.81 (6 H); MS (ESI-) for CI
IH,9N04S m/z
260.1 (M-H)-.
Preparation 17 and Example 72.
IO [R-(R*,S*)]-4-[[[1-[[4-[(2,6-Dichlorobenzoyl)amino]phenyl]methyl]-2-methoxy-
2-
oxo-ethyl]amino]carbonyl]-2.2-dimethyl-3-thiazolidinecarboxylic acid 3-{1;1-
dimethyiethyl)ester
(Scheme B, B-6: where RB_, and Re_2 are the same and Equal to H, RB_~ and RBA
are the
same and equal to CH3, Y is COz, R, is t-butyl, RB_5 is OCH3, RS is 4-[(2,6-
dichlorobenzoyl)amino]phenyl and stereochemistry is (R, S)).
H3CH.. S Q
H3~~~N~Oi
O
~O O ~ ~ ~ CI
CI'
To a cooled (0-5°C) suspension of B-4 {Scheme B where Rs_, and RB_2 are
the same and
equal to H, RB_3 and Re~, are the same and equal to CH3, Y is CO2, R~ is t-
butyl and
stereochemistry is (R)) (1.0 g, 3.83 mmol) and HOBt (6a8 mg, 4.17 mmol) in
CH,CIz (20
mL) was added a solution of EDC (799 mg, 4.17 mmol) in CI-IZCI~ (20 mL). After
30 min
at 0-5°C, B-5 (Scheme B where RB_5 is OCH3, RS is 4-[(2,6-
dichlorobenzoyl)amino]phenyl
and stereochemistry is (S)) ( 1.93 g, 4.77 mmol) was added followed by 4-
methyimorpholine (520 p.L, 4.77 mmol). The reaction mixture was gradually
warmed to
room temperature, stirred an additional 18 h and diluted with CH,CI,. The
organic layer
was separated and washed with 0.1 N HCi, sat. aqueous NaHCO;, brine. dried
(Na,SO,),
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-93-
filtered and concentrated in vacuo: Flash chromatography of the residue using
CHZCl2lacetone (3%) as eluant afforded the title compowzd (2.05 g) as a white
solid: IR
(mull) 1746, 1666, 1606, 1562, I53?, 1515, 1432, 1413, 1347, 1325, 1259, I2I4,
I 195,
1169, 799 cni';'H NMR (CDCl3) b 7.54 (2 H}, 7.32 (4 hf), 7.20 (2 H), 6.90 (1
H), 4.78
(2 H), 3.74 (3 H), 3.17 (4 H), 1.74 (3 H), 1:43 (9 H); "C NMR (CDCl3) 8 171.6,
136.7,
136.2, 135.9, 132.7, 132.4, 132.3, 131.0, 130.6, 130:4, 1~f0.0, 129.9, 128.2,
127.9, 120.5,
120.2, 120.1, 67.3, 53.4, 52.4, 42.8, 37.3, 36:9, 34.9, 30.9, 28.3; MS (ESI+)
for
Cz$H33C12N3O6S mlz 610 {M+H)+; MS (ESI-) for CzgH33C:I2N3O6S mlz 607.9 (M-H)';
Anal.
Calcd for CZgH3~Cl,N3O6S ~ 0.13 HZO: C, 54.88; H, 5.47; N, 6.86. Found: C,
54.66; H,
I0 5.57; N, 6.73. % Water (KF): 0.37.
Example 73:
[R--{R*,S*)]-4-[[[I-car6oxy-2--[4-[(2,6
dichlorobenzoyI)amino]phenyl]ethyl]amino]carbonyl]-2,2-dimethyl-3
thiazolidinecarboxylic acid 3-(I,1-dirnethylethyl}ester
(Scheme B, B-7: where RB_, and RB_2 are the same and equal to H, RB_3 and RB~,
are the
same and equal to CH3, Y is CO2, R3 is t-butyl, RS is 4-[(2,6-
dichlorobenzoyl)amino]phenyl and stereochemistry is (R, ,f~).
HgCv~s H O
H3C~/~N~ N ~OH
~O~O O ~ ~ O CI
w
H~I
2~ CI
Example 73 was prepared from example 72 by the procedure described in
preparation 6.
Fhysicai data as follows: IR (mull) 1738, 1'665, 1606, 1562, 1535, 1516, 1432,
1413, 1347,
i2S9, 1213, 1194, 1167, 799, 777 crri';'H NMR (CD,CN) b 8.89 (I H), 7.54 (2
H), 7.41
2S {3 H), 7.25 (2 H), 4.66 (2 H), 3.I5 (4 H), 1.72 (3 H), 1.7CI (3 I-I), I.35
(9 H); "C NMR
(CD3CN) 8 177.3. 171.8, 163.4, /37.9, I37.I, 133.9, 132.7, 132.3, 131.3,
129.1, 120.7,
8I.2, 79.3, 68.0, 54.5. 37.4, 28.4; MS (ESI-) for C"H"CI,N,O6S m/z 593.9 (M-
H)'; Anal.
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-94-
Calcd for C27H3,CIZN3O6S ~ 0.5 H,O: C, 53.56; H, 5.33; N, 6.94. Found: C,
53.77; H,
5.39; N, 6.70.
Example 74.
4-[(2,6-Dichlorobenzoyl)amino]-N-[[(4R~2,2-dimethyl-4-thiazolidinyl]carbonyl]-
L-
phenylalanine
(Scheme B, B-8: where Ra_, and RB_2 are the same and equal to H, R~_3 and RB.~
are the
same and equal to CH3, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and
stereochemistry is
(R, ~).
' HgC~~s O
/~ ~H
H3C H~N~~H
O CI
w
H ~~
I 0 CI
Example 74 was prepared from example 73 by the procedure described in
preparation 4.
Physical data as follows: IR (mull) 3244, 3192, 3049, 1720, 1664, 1605, 1578,
1562,
1541, 1516, 1432, 1414, 1327, 1195, 799 cni l;'H NMR (DMSO-db) 8 13.00 (1 H),
10.69
( 1 H), 7.52 (6 H), 7.24 (2 H), 4.52 (2 H), 3.12 (2 H), 2.93 ( 1 H), 1.62 (9
H); 13C NMR
(CD,OD) & 166.9, 136.8, 136.0, 133.5, 131.8, 130.9, 129..5, 129.4, 127.9,
120.4, 120.2,
120.1, 60.8, 54:3, 54.0, 36.2, 36.0, 25.1; MS (ESI+) for CZZH23CI,N3O~S rnlz
496.2
(M+H)+; MS (ESI-) for C,zH,3CI,N304S m/z 494.2 (M-H)-; Anal. Calcd for
Cz,H23C1zN3O,S
~ HCI ~ 0.50 H20: C, 48.76; H, 4.65; N, 7:75. Found: C, 48.56; H. 4.72; N,
7.49.
Example 75.
[S-(R*,R*)]-4-[[[I-[[4-[(2,6-Dichlorobenzoyl)amino]phenyl]methyl]-2-methoxy-2-
oxo-ethyl]amino]carbonyl]-2,2-dimethyl-3-thiazol.idinecarboxylic acid 3-( 1,1-
dimethylethyl)ester
(Scheme B, B-6: where RB_, and R$_Z are the same and equal to H, Rg_3 and R~~
are the
same and equal to CH,, Y is CO,, R, is t-butyl, RS is 4-[(~!,6-
dichlorobenzoyl)amino]phenyl and stereochemistry is (S, S~).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-95-
S_ H O.
N N Oi
~O~O O I ~ 0 CI
H
CI
Example 75 was prepared as described in Scheme B froml D-cysteine and acetone
using di-
t-butyl dicarbonate to form the requisite carbamate. Physical data as follows:
IR (mull)
1745, 1686, 1666, 1605, 1537, 1515, 1432, 1413, 1349, 1325, 1259, 1213, 1206.
I I95,
1169 cni';'H NMR (CDCl3) 8 7.56 (2 H), 7.32 (3 H), 7.16 (2 H), 6.92 (I H),
4.83 (2 H);
3.73 (3 H), 3.20 (4 H), 1.78 (6 H), 1.45,(9 H);'3C NMR (CDCl3) 8 171.6, 170.0,
136.2,
132.6, 132.4, 131.0, 130.9, 130.1, 129.9, /28.2, 120.5, 12Ø4, 67.3, 53.3,
52.5, 52.4,37.4,
28.4; MS (ESI+) for C~gH33CI,N3O6S m/z 610.0 (M+H)'; :MS (ESI-) for
C,8H33C1,N3O6S
m/z 607.9 (M-H)'; Anal. Calcd for Cz$H33C1zN3O6S: C, 55.08; H, 5.45; N, 6.88;
Cl, 1 i.61;
S, 5.25. Found: C, 54.87; H, 5.47; N, 6.78
Example 76.
(S-(R*,R*)j-~4-[[[ 1-Carboxy-2-~(4-[(2,6-
dichlorobenzoyI)aminojphenyl]ethyljaminojcarbonylj-2,2--dimethyl--3-
thiazolidinecarboxylic acid 3-( 1,1--dim~ethyiethyl) ester
(Scheme B, B-7: where RB_, and RB_Z are the same and equal to H, Rr,_3 and RB~
are the
same and equal to CH3, Y is CO2, R3 is t-butyl, RS is 4-[(2l,6-
dichlorobenzoyl)aminojphenyl and stereochemistry is (S, S)).
S~ H~ O
H~~~OH
~O~O 0 ~ ~ O CI
H
CI
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-96-
Example 76 was prepared from example 75 by the procedure described in
preparation 6.
Physical data as follows: IR {mull) 3280, 1739, 1665, 1fi06, 1562, 1535, 1516,
1432,
I4I3, 1348, 1272, 1259. 1195, 1167, 799 crri';'H NMR (CD3CN) 8 8.83 (I H),
7.55
(2 H), 7.43 (3 H), 7.22 (2 H), 6.83 (1 H), 4.68 (2 H), 3.07 (5 H), I.73 (6 H),
1.40 (9 H);
S '3C NMR (CD3CN) b 172.6, 163.3, 137.9, 133.4, 133.3, 132.7, 132.1, 131.1,
130.2, 130.1,
129.1, 128.1, 128.0, 120.6, 119.5, 54.1, 37.4, 29.4, 28.5, 27.6; MS (ESI+) for
C~,H3,C12N3O6S m/z 595.9 (M+H)'; MS (ESI-) for CZ,H3,CIZN~O6S »~/z 593.7 (M-
H)'; MS
(FAB) m/z {rel. intensity) 596 (MH+, 16), 598 (12), 596 ( 16), 500 ( 16), 499
( 19), 498
(7I ), 497 {32), 496 (99), 173 ( 16), 1 I6 { 19), 57 {51 }; HRMS (FAB) calcd
for
C27H3,CI,N306S+H, 596.1389, found 596.1364; Anal. Calcd for C,7H3,CIZN306S ~
0.5
H20: C, 53.56; H, 5.33; N, 6.94. Found: C, 53.86; H, 5.35; N, 6.90.
Example 77.
[S-(R*,R*)]-3-[[[ 1-[ [4-[(2,6-Dichlorobenzoyl)amino] phenyl]methyl]-2-methoxy-
2
oxoethyl]amino]carbonyl] -1-thia~-azaspiro[4.4Jnonane-4-carboxylic acid 4-
ethyl ester
I S (Scheme B, B-b: where RB., and Rg_Z are the same and equal to H, RB.3 and
Ray together
form a carbocyclic ring of 5 atoms, Y is CO2, R3 is ethyl, RS is 4-[(2,6-
dichlorobenzoyl)amino]phenyl and stereochemistry is (S, S)).
5-.. H O
~N~N~°~
~o'~o ° f ~ o ci
H
Ct
Example 77 was prepared as described in Scheme B from D-cysteine and
cyclopentanone
using ethyl chloroformate to form the requisite carbamate. Physical data as
follows: IR
(mull) 1760, 1739, 1694, 1656, 1607, 1560, 1543, 1517, 1445. 1429, 1411, 1334,
1273,
1253, I 1I6 crri';'H NMR (CDCIz) b 7.56 (2 H), 7.44 {1 H), 7.34 (3 H}, 7.11 (2
H), 6.70
( 1 H), 4.84 (2 H), 4.14 (2 H), 3.74 (3 H), 3.12 (4 H), 2.6 i' ( 1 H), 2.51 (
1 H), 1.73 (6 H),
1.25 (3 H); '3C NMR (CDCI~) 8171.4, 170.7, 162.3, 136.3. 135.8, 132.5, 132.4,
131.0,
130.1, 128.2, 120.2, 66.4, 62.1. 53.1, 52.5, 37.3, 32.3. 31.9, 25.1, 24.6,
14.5, 14.1; MS
CA 02342778 2000-12-20
WO 99167230 PCTIUS99/14233
-97-
(ESI+) for CzgH3~CI2N3ObS m/Z 630.0 (M+Na)'; HRMS (EI) calcd for
C,gH~,C1~N306S
607.1310, found 607.131 S; Anal. Calcd for C,$H3,C1~N3(J6S ~ 0.75 H20: C,
54.06; H, 5.27;
N, 6.90. Found: C, 53.98; H, 5.16; N, 6.72.
Example 78.
S [S-(R*,R* ) j-3-[ [[ 1-Carboxy-2-[4--[(2,6-
dichlorobenzoyl)amino]phenyl)ethyl]amino]carbonyl]-I-this-4-
azaspiro[4.4]nonane-4-
carboxylic acid 4-ethyl ester
(Scheme B, B-7: where Rs_, and RH_2 are the same and equal to H, RB_3 and RH-0
together
form a carbocyclic ring of S atoms, Y is CO~, R3 is ethyl., RS is 4-[(2,6-
dichlorobenzoyI)amino]phenyl and stereochemistry is {S', f~).
S-- H O
C~N~N " OH
~O~O O I ~ O Ci
H~I ~
Ct
Example 78 was prepared from example 77 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 3276, 1664, 1606, 1.'i62, 1537, 1 S 1 S,
1445, 1432,
1413, 1335, 1273, 1239, 1195, 1116, 799 cni';'H NMR (CDC13) 88.08 (1 H), 7.62
{2 H),
7.24 (3 H), 7.14 (2 H), 6.72 (1, H), 4.85 (2 H), 4.13 (2 H), 3.21 (4 H), 2.53
(2 H), 1.75
{6 H), 1.22 (3 H); '3C NMR (CDCl3) b 175.2, 171.2, 162.6, 136.7, 135.7, 132.3,
132.0,
130.7, 130.2, 127.9, 120.2, 66.3, 62.2, 53.2, 37.3, 36.6, 32.3, 25.2, 24.6,
20.5. 14.5; MS
{ESI+) for C,7H29C1zN3O6S mlz 593.8 (M+H)+; MS {ESI-) for Cz,H,gChN3O~S mlZ
591.8
(M-H)-; HRMS (FAB) caicd for C27H~~C1,N306S+H~ 594.1232, found 594.1226; Anal.
Calcd for C,7Hz9CIzN3O6S ~ 0.37 H,O: C, S3.9S; H, 4.99; N, 6.99. Found: C.
54.28; H,
5.10; N, 7.03. % Water (KF): 1.10.
Example 79.
2S [S-{R*,R*)]-3-[[[1-[[4-[{2,6-Dichlorobenzoyl)amino~]phenyljmethylj-2-
methoxy-2-
oxoethyl]amino]carbonylj-1-thia-4-azaspiro[4.S]decan;e-4-carboxylic acid 4-
ethyl ester
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
_98-
(Scheme B, B-6: where R8_, and RB_Z are the same and equal to H, RB_3 and RBA
together
form a carbocyclic ring of 6 atoms, Y is CO2, R3 is ethyl, RS is 4-[{2,6-
dichlorobenzoyl)amino]phenyl and stereochemistry is (,~~, S~).
S H OI'
~N~ N~Oi
CI
w
H iI
$ CI
Example 79 was prepared as described in Scheme B from D-cysteine and
cyclohexanone
using ethyl chloroformate to form the requisite carbamate. Physical data as
follows: IR
(mull) 1745, 1704, 1683, 1668, 1607, 1561, 1538, 1514, I43I, I4I3, 1327, 1269,
1213,
1196, I 117 crri'; 'H NMR (CDCl3) S 7.57 (2 H), 7.46 (1 H), 7.33 (3 H), 7.11
(2 H), 6.72
( 1 H), 4.88 (2 H), 4.14 (2 H), 3.74 (3 H), 3. I 3 (4 H), 2.51 ( 1 H), 1.69 (8
H), 1.22 (5 H);
'3C NMR (CDCl3) 8 171.4, 170.7, 168.1, 162.3, 136.3, 135.7, 132.4, 131.0,
128.2, 120.3,
66.9, 62.8, 62.0, 55.1, 53.1, 52.5, 42.0, 39.9, 37.3, 36.9, 31.1, 29.6, 27.6,
27.1, 26.0, 25.3,
24.7, 23.1; 14.5; MS (ESI-) for Ci9H3~CI2N3O6S m/Z 621.9 (M)'; HRMS {FAB)
calcd for
C29HssC1zN30eS+HI 622.1545, found 622.1536.
Example 80.
[S--(R*,R*)]--3-[[[1-Carboxy-2-[4-{(2,6-
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]-~I-thia-4-
azaspiro[4.5]decane-4-
carboxylic acid 4~thy1 a ter
(Scheme B, B-7: where Rs_, and Rs_Z are the same and equal to H, RB.3 and Ray
together
form a earboeyclic ring of 6 atoms, Y is CO,, R3 is ethyl: R5 is 4-[(2,6-
dichlorobenzoyl)~mino]phenyl and stereochemistry is (~~, f~).
s ~ o
N~ N~OH
~O~O O ~ ~ CI
/ H I ~
Cir /
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-99-
Example 80 was prepared from example 79 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 3276, 1710, i 664, I X606, 1562. 1537,
1515, 1432,
1413, 1329, 1272. 1256, 1195, i I 17, 800 cm ';'H NMR (CDCl3) b 8.07 (I H),
7.62 (2 H),
7.24 {3 H), 7.14 (2 H), 6.75 (I H), 4.88 (2 H), 4.12 (3 H;I, 3.14 (4 H), 2.74
{I H), 2.50
( I H), 1.69 (6 H), 1.19 (5 H); ' 3C NMR (CDC13) 8 175.4, 175.1. 171.3, 162.6,
136.7,
135.7, 132.3, 132.0, 130.7, 130.3, 130./, 128.0, 120.2, 66.8, 62.1, 53.2,
42.0, 36.9, 30.5,
27.0, 26.0,25.3, 25.0, 24.6, 20.5. 14.4, 3.7; MS {ESI+) for C28H3,C1~N306S mlz
608.1
(M+H)'; MS (ESI-) for CZgH3~CIzN3O6S m/Z 605.9 (M-H)'; HRMS (EI) calcd for
C,gH3'C12N306S 607.1310, found 607.1309; Anal. Calcd fvr CZgH3~C1,N3O6S ~ 0.3
HBO: C,
54.78; H, 5.19; N, 685. Found: C, 54.56; H. 5.24; N, 6.S>0. % Water (KF):
0.87.
Example 81.
[S-(R*,R*)]-4-[[[1-[{4-[(2,6-Dichlorobenzoyl)aminojphenyl)methyl]-2-methoxy 2-
oxoethyl]amino)carbonyl]-2,2,S,S-tetramethyl-3-thiazolidinecarboxylic acid 3-
ethyl
ester
(Scheme B, B-6: where RB_,, RB.2, R$_3 and Re~, are the same and equal to CH3,
Y is COZ,
R3 is t-butyl, RS is 4-[(2,6-dichiorobenzoyl)amino]phenyl and stereochemistry
is (S, S)).
S~ H O
~CN~ NJ~o
~O~O O I ~ O CI
'N
H~I ~
CI
Example 81 was prepared as described in Scheme B from D-penicillamine and
acetone
using ethyl chloroformate to form the requisite carbamate. Physical data as
follows: IR
(mull) 1748, 1666, 1606, 1562, 1538, 1 S 16, 1432, 1413, 1327, 1275, 1233,
1215, I 195,
1080, 799 cm'; 'H NMR (CDCl3) b 7.55 (2 H), 7.33 (4 H), 7.09 (3 H), 6.59 (1
H), 4.91
( 1 H), 4.47 { I H), 4.16 {2 H), 3.75 (3 H), 3.13 (2 H), 1.9:? (3 H), 1.75 (3
H), 1.67 (3 H),
1.60 (3 H), 1.21 (3 H);'jC NMR (CDC13) 8 171.5, 170.0, 162.2, 136.1, 135.7,
132.5,
132.3. 131.9. 130.9, 130.7, 129.7, 128.1. 120.3. 120.1, 1:20.0, 61.6. 61.4,
52.9, 52.4, 52.2,
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-100-
49.3, 37.4, 37.3, 34.1, 31.4, 30.8, 29.5, 24:9; MS (ESI-) :for C28H33C1zN3O6S
mlz 607.9 (M-
H)'; HRMS (EI) calcd for CZ8H33C12N306S 609.1467, found 609.1461; Anal. Calcd
for
CZ8H3~CIzN3O6S ~ O.I9 H~O: C, 54.77; H, 5.48; N, 6.84. Found: C, 55.00; H,
5.48; N, 6.78.
Water {KF): 0.56.
Example 82.
[S-(R*,R*)]-4-[[[1-Carboxy-2~-[4-((2,6-
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]-2,2,5,5-tetrarnethyl-3-
thiazolidinecarboxylic acid 3-ethyl ester
(Scheme B, B-7: where RB_,, RB_Z, Rs_, and RBA are the same and equal to CH,,
Y is CO2,
Rz is t-butyl, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry
is (S, S)).
S~ H OI'
(v[~'~~OH
~O~O O ~ ~ O CI
N !~
H
CI
Example 82 was prepared from example 81 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 3275,1750, 1735, 1678, 1666, 1609, 1562,
1543,
1516, 1432, 1413, 1333, 1276, 1195, 1077 crri';'H NMR (DMSO-d~} 8 12.48 (1 H),
10.63
( 1 H), 8.36 { 1 H), 7.53 (5 H), 7.19 (2 H}, 4.49 {2 H), 3.9fi (2 H), 3.03 ( 1
H), 2.79 ( 1 H),
1.78 (6 H}, 1.48 (3 H), 1.15 (3 H), 0.90 (3 H); "C NMR (DMSO-db) b 172.9,
168.7, 161.7,
152.3, 137.0, 136.3, 132.9, 131.2, 131:1, 129.5, 128.1, 119.2, 74.7, 71.3,
60.5, 53.5, 49.0,
48.4, 38.3, 36.7, 33.5, 31.8, 28.1, 24.7, 21.0, 14.0; MS (F?SI+) fox
Cz7H3,CI2N306S m/z
595.8 {M+H)'; MS (ESI-) for C27H3,C1zN3O6S m/z 593.8 {M-H)'; HRMS (FAB) calcd
for
C,7H3,C1,N306S+H, 596.1389, found 596.1362: Anal. Calcd for C2,H3,C1,N,O6S ~
0.56
HzO: C, 53.46; H, 5.34; N, 6.93. Found: C, 53.73, H, S.aS; N, 6.73. % Water
(KF): 1.67.
Example 83.
N-[[(4S}-3-Acetyl-2,2-dimethyl-4-thiazolidinyl]carbonyl]-4-[(2,6-
dichlorobenzoyl)amino]-!r-phenylalan.ine methyl ester
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-101-
(Scheme B, B-6: where R~_, and RB_2 are the same and equal to H; RB_3 and R~~
are the
same and equal to CH;, Y is CO, R3 is methyl, R, is 4-j(2,6-
dichlorobenzoyl)amino]phenyl
and stereochemistry is (S, S)).
s-. H o
H3C~p O f ~ O CI
H
CI
Example 83 was prepared as described in Scheme B from D-cysteine and acetone
using
acetyl chloride to form the requisite amide. Physical data as follows: IR
(mull) 1745,
1682, 1662, 1628, 1610, 1579,1561, 1541, 1515, 1431, 1412, 1326, 1270, 1240,
i2I 1 cm'
I0 ';'H NMR (CDCl3} b 7.55 (3 H); 7.32 (3 H), 7.19 (1 H), 7.11 (1 H), 6.66 (1
H), 4.89 (1 H),
4.60 (1 H), 3.78 (3 H), 3.24 (4 H), 2.04 (3 H}, 1.87 (3 H), 1.79 (3 H); I'C
NMR (DMSO-
db) b I7I.6, 171.5, 169.9, 169.4, 167.9, 161.8, 137.1, I3fi.3, 132.8, 132.4,
131.1, 129.5,
128.1, 119.2, 72.6, 66.5, 53.7, 53.3, 52.0, 51.9, 35.8, 31.'7, 29.0, 26.9,
24.7, 24.5; MS
(ESI+) for CZSHZ,C1zN305S m/z 551.9 (M+H)+; HRMS (l::I) calcd for
CZ5H27CIZN3OSS
551.1049, found 551.1053; MS {EI ) m/z (rel. intensity) 551 (M+, 7), 351 (46),
349 (68),
278 {16), 186 (14 ), 175 (63), 173 (98), 158 (23), 116 (99), 100 (20), 99
(69).
Example 84.
N-j[(4S}-3-Acetyl-2,2-dimethyl-4-thiazolid.inyl]carbonyl]-4-[(2,6-
dichlorobenzoyl)amino]-L-phenylalanine
(Scheme B; B-7: where RB_, and RB_2 are the same and equal to H. RB_3 and Re~,
are the
same and equal to CH3, Y is CO, R3 is methyl, RS is 4-[{:?,6-
dichlorobenzoyl)amino]phenyl
and stereochemistry is (S, S)).
S-. H O
~~N~pH
O
H3C O I ~ O CI
CI
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
- I 02-
Example 84 was prepared from example 83 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 3279, 1723, 1661, I 608, 1562, 1542, 1516,
1432,
1413, 1349, 1329, 1270, 1238, 1207, 1195 crri';'H NMI~t (DMSO-d6) 8 12.47 (1
H), 10.62
( I H), 7.5 I (5 H), 7. i 8 (2 H), 4.80 ( 1 H), 4.67 ( 1 H), 4.4'7 { i H),
2.98 (3 H), 1.68 (9 H);
'3C NMR (DMSO-db) 8 184.2, 172.6, 171.9, 169.8, 169.2, 167.8, 136.9, 136.3,
133.1,
131.2, 131.0, 129.5, 128.1, 119.2, 72.6, 66.6, 53.7, 53.3, 3b.2, 31.7, 29.0,
27.0, 24.8, 24.6,
21.0; MS (ESI+) for CZ4HzsC1zN30sS ~Z 538.0 (M+H}'; MS (ESI-) for
C~4HZSCl,N305S
m/z 535.9 (M-H)'; HRMS (FAB) calcd for C,4HZSC1ZN3CtSS+H, 538.0970, found
538.0961.
Example 85..
[S-(R*,R*}]-4-[[[1-Carboxy-2-[4-[{2,6-
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]-2,2-dimethyl-y-oxo-3-
thiazolidinebutanoic acid
(Scheme B, B-7: where RB~, and RB_2 are the same and <:qual to H, RB_3 and
RH,~ are the
I5 same and equal to CH3, Y is CO, R3 is CH,CH~CO,H, RS is 4-[(2,6-
dichlorobenzoyl}amino]phenyl and stereochemistry is (S., S"}).
~N~N~OH
HO~O O ~ w ~ CI
[O~ - ~N
H
CI
Example 85 was prepared as described in Scheme B from D-cysteine and acetone
using
methyl succinyl chloride to form the requisite amide. Physical data as
follows: IR (mull)
3264, 3125. 3071, 1724, 1658, /607, 1562, 1537, 1517, 1432, 1414, 1326, 1241.
1195,
I I 81 crri'; 'H NMR (DMSO-db) 8 12.31 ( 1 H), 10.65 ( 1 H), 8.20 ( I H), 7.50
(5 H), 7.19
{2 H), 5.81 (1 H), 4.50 {1 H), 3.02 (3 H), 2.30 (3 H), 1.71 (6 H); '3C NMR
(DMSO-d6) 8
/74.1. 172.8, 169.4, 161.9, 137.1, 136.5. 133.2, 131.4, 131.3; 129.7, 128.3,
119.3, 73.1,
65.7, X3.5, 36.3, 32.0, 30.5, 29.1, 29.0, 27:1; MS (ESI+) for C,6H27C1,N3O,S
m/z 595.9
(M+H)'; MS (ESI-) for C~6Hz,CI,N30,S m/z 593.8 (M-H)'; Anal. Calcd for
C,6H,7C1,N307S
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-103-
~ 0.51 H,O: C, 51.55; H, 4.66; N, 6.94. Found: C, 51.71. ; H, 4.85; N, 6.93. %
Water (KF):
I .53.
Example 86.
[S--(R*,R*)]-4-{[[1-[[4-[(2,6-Dichlorobenzoyl)amino]phenyl]methylJ-2-methoxy-2-
oxoethyl]amino]carbonylJ-2,2,5,5-tetramethyl-y-oxo-3-thiazolidinebutanoic acid
methyl
ester
(Scheme B, B-6: where Ra_,, RB_Z, Rs_3 and RB~ are the same and equal to CH3,
Y is CO,
R3 is CHZCH,CO,CH~, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and
stereochemistry is
I O (S, S~}.
s-'-~ H o
~N~N~O~
i0 0 ~ ~0 C!
0
O I i N. ~
H I ,
C:I
Example 86 was prepared as described in Scheme B from D-penicillamine and
acetone
using methyl succinyl chloride to form the requisite amiide. Physical data as
follows: IR
(mull) 3287, 1741, 1660, 1608, 1562, 1540, 1516, 1432, 1413, 1323; 1267, 1241,
1225,
1196, i 168 cm '; ' H NMR (CDC13) S 7.55 (2 H), 7.29 (=i H), 7. I 3 (2 H),
6.94 ( 1 H), 4.95
1 H), 4.39 ( 1 H), 3.74 (3 H}, 3.67 (3 H), 3.27 ( I H), 3.10 ( I H), 2.80 ( 1
H), 2.57 (3 H),
2.18 (1 H), 1.95 (3 H), 1.77 (3 H), 1.72 (3 H), 1.68 (3 H) 1.63 (3 H);'3C NMR
(CDC13) b
173.3, 171.6, 170.3, 169.9, 162.4, 136.3, 132.5, 132.4, 1.31Ø I30.2, 129.8,
128.2, 120.8,
120.7, 120.5, 73.8, 53.1, 52.6, 51.8, 49.9, 40.0, 37.4, 33.9. 31.6, 31.4,
29.4, 29.1, 24.3; MS
(ESI+) for C3oH35C1~N~07S m/z 652.1 (M+H)~; Anal. Ca.lcd for
C3°H35CI,N307S ~ 0.3I
H,O: C, 54.75; H, 5.45: N. 6.38. Found: C, 55.04; H, 5.,50; N, 6.69. % Water
(KF): 0.84.
Example 87.
N-[[(4S)-3-Acetyl-2,2,5,5-tetramethyl-4-thiaz;oiidinyl]carbonylJ-4-[(2,6-
dichlorobenzayl)amino]-~-phenylalanine methyl ester
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-I04-
(Scheme~B, B-6: where RB_" Rp_,; RB.3 and RB.~ are the s,~ne and equal to CH;,
Y is CO,
R3 is methyl, R, is 4-[(2,6-dichlorobenzoyl)amino]phenyi and stereochemistry
is (S, S)).
s~- H o'I
~N~N~Oi
li3C~0 C ~ ~ 0 CI
_N
CI
Example 87 was prepared as described in Scheme B fronn D-penicillamine and
acetone
using acetyl chloride to form the requisite amide. Physical data as follows:
IR {mull)
1741, 1692, 1676, 1629, 1606, 1563, 1538, 1516, 1431, 1410, 1352, 1320, 1276,
1251,
1223 cm'; 'H NMR (DMSO-db) 8 10.70 (I H), 8.57 (1 I;f), 7.56 (4 H), 7.22 (2
H), 4.63
( 1 H), 4.49 ( I H), 3.65 (3 H), 3.30 ( 1 H), 3.12 { I H), 2.84 ( 1 H), 1.92
(3 H), 1.82 (3 H),
I .78 (3 H), 1.51 (3 H), 0.80 (3 H); "C NMR (DMSO-db) 8 184.2, 17I.9, 168.9,
168.1,
161.8, I37.I, 136.3, 132.4, 131.3, 131.2, 131.0, 129.4, 1:?8.1, 119.3, 75.9,
72.8, 53.5, 52.0,
49.2, 48.4, 36.0, 33.5, 31.9, 27.6, 24.9, 24.5; MS (ESI+) for Cz7H3,CI,N305S
mlz 580.1
{M+I-I)'; MS (ESI-) for CZ,H3,C1zN3O5S m/Z 577.9 (M-H;f; Anal. Calcd for
C,,H3,C1ZN305S
I5 ~ 0.12 HBO: C, 55.66; H, 5.40; N, 7.21. Found: C, 55.68:, H, 5.39; N. 7.16.
% Water (KF):
0.36.
Example 88.
[R-(R*,S*)]--4-[[I I-Carboxy-2__[4-[(2,6-
dichlorobenzoyl}amino]phenyl]ethyl]amino]carbonylJ~-2,2,5,5-tetramethyl-S-oxo-
3-
thiazolidinepentanoic acid
(Scheme B, B-6: where RB.,, RB_2, RB_3 and Ra~, are the same and equal to CH3,
Y is CO,
R~ is CH,CH,CH,COZH, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and
stereochemistry
is (R, S)).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-1 OS-
0
H~ ,~
'OH
O O ' ~ O CI
HD-~ i
O N' w
H
C;i
Example 88 was prepared as described in Scheme B fronn D-penicillamine and
acetone
using methyl glutaryl chloride to form the requisite amide. Physical data as
follows:
'H NMR (DMSO-db) 8 10.65 (1 H), 8.45 (1 H}, 7.53 (5 H), 7.22 (2 H), 4.51 (1
H), 3.11
(3 H), 2.77 (1 H), 2.21 {4 H), 1.89 (3 H), 1.82 {3 H), 1.11 (2 H), 1.48 (3 H),
0.77 (3 H};
'3C NMR (DMSO-db) 8 184.2, 174.2, 173.1, 170.1, 168.'x, 161.7, 137.0, 136.3,
133.1,
131.3, 131.1, 129.4, 128.1, 119.2, 74.9, 73.0, 53.8, 49.3, 36.1, 34.8, 33.5,
32.7, 31.9, 27.7,
24.5, 20.0; MS (ESI+) for C29H33C1zN3O7S m/Z 638.0 (M+H)+; MS (ESI-) for
C2qH33C1zN~O,S mlz 635.9 {M-H)'; HRMS (FAB) calcd for CZ9H3,Cl,N~O,S+H,
638.1494,
found 638.1481; Anal. Calcd for C29H33C1ZN3O7S ~ 0.751Ei20: C, 53.42; H, 5.33;
N, 6.44.
Found: C, 53.20; H, 5.26; N, 6.45.
CA 02342778 2000-12-20
WO 99!67230 PCT/US99/14233
-106-
Scheme C
NH-Fmoc
i w
I , ~ .~ ~~~NH-Fmoc C-1
~O O O
O
Rink-amide-MBHA resin
1
~NHz C-2
O
Fmoo-HN- ~
~OH C-3
R5
O
~N~NH-Fmoc
C-4
Rs
O
~ NHp
"' H C-5
R5
RC-t
S R~_z
Rc.3~~OH C-6
RCS N
Fmoc O
O Rc-2y! C ~Rc-s
/~H
~.N N II N Rc-a C-7
H O Fmoc
Rs
Rc-2 J ~~s
O \/R-
H N~H~RC.a C-8
R O
s
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-107-
Scheme C (continued)
RC.2 RC.S
O H \' RC.a
N N~RC~ C:-8
H O H
R5
R .2 RC-t
RC-3
C-9
~N N~1J~RC-4
H 0 Y'R3
R5
RC_~
RCS ~ RC.z O
NH
RCS Y O ~HH2 C:-10
R3 R5
Where: Rc-,, Rc_2, and Rc_3 are defined independently as R,. Rc~ is defined as
R2.
Scheme C describes a method for the preparation of examples of the formula C-
10.
Commercially available Rink Amide MBHA resin is de~protected under standard
solid-
phase peptide synthesis conditions (Atherton. E.; Sheppa~rd R.C. Solid Phase
Peptide
Synthesis: A Practical Approach; IRL Press at Oxford Llniversity Press:
Oxford, 1989) to
afford the amine of formula C-2. Acylation with a comnnercially available or
readily
prepared amino acid residue of general C-3 affords the r<ain bound derivative
of formula
C-4. Removal of the Fmoc group under standard conditiions provides amine of
general
structure C-5 which is acylated with a commercially available or readily
prepared
thiazolidine-4-carboxylic acid of general formula C-6 to afford the resin
bound
intermediate C-7. Standard Fmoc deprotection affords the resin bound amine of
general
formula C-8 which may be reacted with a variety of electrophilic reagents as
described in
Scheme A to afford resin bound amides, ureas. sulfonamides and carbamates of
general
structure C-9. Preparation 18 details an example of the reaction of a mixed
carbonate to
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-108-
afford a carbamate of general structure C-9 (where Y is equal to CO~).
Standard acidolysis
affords the amide of general structure C-10.
Preparation I8 and Example 89.
[S-(R*,R*)]-4-[[[ 1-[[4-[(2,6-Dichlorophenyl)methoxy]phenyl]methyl]-2-amino-2-
oxoethyl]amino]carbonyl]-3-thiazolidinecarboxylic acid 3-[2-(4-
morpholinyl)ethyl]
ester
(Scheme C, C-10: where R~_,, Rc_2, Rc_3 and ltc~, are the same and equal to
proton, R3 is 2-
(4-morpholinyl}ethyl, RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl, Y is COZ
and
stereochemistry is (S, S)).
S-. H O
O~ ~~~N~NHZ
O I ~ C1
~, ~ w
I 5 To a mixture of Rink Amide MBHA resin (Scheme C, C-1 ) (Nova Biochem., 1.2
g, ca. 0.59 mmol) in methylene chloride (20 mL) was added a solution of
piperidine in
DMF (30%, 20 mL}. A slow stream of nitrogen was bubbled through the mixture to
effect
mixing for 20 min. The resin was filtered and washed vvith DMF. The resin was
suspended in a solution of piperidine in DMF (30%, 40 ml) and mixed for 40
min. The
resin was filtered and washed with DMF, methylene chloride, methanol and
methylene
chloride to afford resin C-2, which was diluted with DMF (40 mL}. To this
mixture was
added Fmoc-Tyr{2,6-Cl,-Bn) (Scheme C, C-3: where R,S is 4-[(2,6-
dichiorophenyl)methoxy]phenyl and stereochemistry is (S)) (Advanced Chemtech,
I.32 g,
2.35 mmol), HOBt (0.36 g, 2.35 mmol), PyBOP (1.20 g, 2.35) and D1EA (1.03 mL,
5.90
mmol). The reaction was mixed for 4 h and the resin filtered and washed with
DMF,
methylene chloride, MeOH and methylene chloride to afford the intermediate
resin-bound
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-109-
amino acid derivative C-4 (Scheme C, where R5 is 4-[(2,6-
dichlorophenyl)methoxy]phenyl
and stereochemistry is (S)) which was used without characterization.
To a mixture of resin C-4 described above in methylene chloride (20 mL) was
added a solution of piperidine in DMF (30%, 20 mL). A slow stream of nitrogen
was
bubbled through the mixture to effect mixing for 20 min.. The resin was
filtered and
washed with DMF. The resin was suspended in a solution of piperidine in DMF
(30%, 40
ml) and mixed for 40 min. The resin was filtered and washed with DMF,
methylene
chloride, methanol and methylene chloride to afford the resin of structure C-5
(Scheme C,
where RS is 4-[(2;6-dichlorophenyl)methoxy]phenyl and stereochemistry is (S))
, which
I0 was diluted with DMF (40 mL). To this mixture was added Fmoc-D-thiazolidine-
4-
carboxylic acid (Scheme C, C-6: where Rc_,, Rc_2, Rc.3 and Rc~ are the same
and equal to
proton and stereochemistry is (S}) (Advanced Chemtech, 832 mg, 2.35 mmol),
HOBt (0.36
g, 2.35 mmol}, PyBOP (1.20 g, 2.35) and DIEA (I.03 mL, 5.90 mmol). The
reaction was
mixed for 4 h and the resin filtered and washed with DMF, methylene chloride,
MeOH and
methylene chloride to afford the intermediate resin-bour,~d derivative C-7
{Scheme C,
where R~_,, Rc_~, Rc_3 and Rc~, are the same and equal to proton, Rs is 4-
[(2,6-
dichlorophenyl}methoxy]phenyl and stereochemistry is (S, S)) which was used
without
characterization.
To a mixture of resin C-7 described above in methylene chloride (20 mL) was
added a solution of piperidine in DMF (30%, 20 mL}. A slow stream of nitrogen
was
bubbled through the mixture to effect mixing for 20 min: The resin was
filtered and
washed with DMF. The resin was suspended in a solution of piperidine in DMF
(30%, 40
ml) and mixed for 40 min. The resin was filtered and washed with DMF,
methylene
chloride, methanol and methylene chloride to provide the intermediate of
structure C-8
(Scheme C, where Rc_,, Rc_z, Rc_3 and Rc~, are the same and equal to proton,
R5 is 4-[(2,6-
dichlorophenyl)methoxy]phenyl and stereochemistry is I;S, S)) , which was
diluted with
methylene chloride ( 10 mL). To this mixture was added a solution of the mixed
carbonate
prepared from 4-{2-hydroxyethyl)morpholine (2.14 mL, 17.7 mmol) and N,N-
disuccinimidyl carbonate (4.53 g, 17.7 mmol) as described in preparation 8 in
methylene
chloride (20 mL} followed by triethyIamine (0.33 mL, 2"36 rnmol). The reaction
was
CA 02342778 2000-12-20
WO 99/b7230 PCT/US99/14233
-110-
mixed for 24 h and the resin filtered and washed extensively with DMF,
methylene
chloride, MeOH, and anhydrous ethyl ether. The resin was dried in vacuo to
afford the
resin-bound carbamate C-9 (Scheme C, where R~_,, Rc_2, Rc._3 and Rc~ are the
same and
equal to proton, R~ is 2-(4-morpholinyl)ethyl, RS is 4-[(2,6-
dichlorophenyl)methoxy]phenyl, Y is COZ and stereoche;mistry is (S, S)).
Resin C-9 was swelled with a minimum of methvlene chloride (ca. 2 mL) and
suspended with 95 % aqueous TFA (20 mL). The mixture was mixed by magnetic
stirring
for lh and filtered. The resin was washed with additional TFA (2 X 5 mL),
followed by
methylene chloride and methanol. The combined filtrates were evaporated in
vacuo, and
partioned between ethyl acetate and saturated aqueous sodium bicarbonate. The
arganic
layer was separated and washed with brine, dried (MgSC>4), filtered and
evaporated in
vacuo. The residue was purified by flash chromatography using methylene
chloride/methanol ( 1 to 3%) as eluant to afford the title compound (215 mg)
as an
amorphous powder: IR (mull) 3288, 1676, 1657, 161 l, 1564, 15I l, 1439, 1424,
1346,
1302, 1237, 1179, 1116, 1021, 767 ctri';'H NMR (CDC13, 300 MHz) 8 7.24 (5 H),
b.93
(2 H), 5.20 (2 H), 4.63 (3 H), 4.34 (1 H), 4.22 (2 H), 3.63 (4 H), 3.11 (4 H),
2.50 (6 H); "C
NMR (CDCl3, 75 MHz) 8 173.4, 170.1, 157.9, 154.5; 1x6.9, 132.0, 130.4,129.1,
128.4,
I 15.1, 66.8, 65.2, 63.4, 57.1, 54.0, 53.7, 53.5, 49.4, 35.2.; MS {EI ) m/z
(rel. intensity) 610
(M+, 1 ), 323 ( 13), 321 (20), 161 {34), 159 (53}, 114 (31 ), 113 {98), 100
(99}, 88 {13), 70
(8), 56 ( 11 ); MS (FAB) m/z (rel. intensity) 611 (MH', T 1 }, 614 ( I 8), 613
(49), 612 (27),
611 (71), 123 (60), 114 (99), 113 (76), 1I2 (19); 107 (22), 100 (28}; HRMS
(FAB) calcd
for CZ,H32C12N4O6S +H, 6I 1.1498, found 611.1494. An<~1. Calcd for
Cz7H32C12N,O6S: C,
53.03; H, 5.27; N, 9.16. Found: C, 52.74; H, 5.17; N, 9.01.
Example 90.
[S-(R*,R*)]-4-([[1-[[4-[(2,6-Dichlorophenyl)metho:xy]phenyl]methyl]-2-amino-2-
oxoethyl]amino]carbonyl]-3 thiazolidinecarb~oxylic acid 3--ethyl ester
(Scheme C, C-10: where It~_,, Rc_2, Rc_3 and R~~ are the same and equal to
proton, R3 is
ethyl, RS is 4-((2,6-dichlorophenyl)methoxy]phenyl, Y is CO, and
stereochemistry is (S,
S)).
CA 02342778 2000-12-20
w0 99lb7230 PCT/US99l14233 .
-111-
/S-. H O
~N~N~Nii2
~O~O O 1 ~ CI
'O
CI
Example 90 was prepared as described in Scheme C. Plhysical data as follows:
IR (mini)
3369, 3308, 3192, 1713, 1667, 1650, 1629, 1539, 1513, 1441, 1344, 1290, 1240,
1016,
768 cni';'H NMR (CDC13, 300 MHz) b 7.33 (3 H), 7,16 (2 H), 6.95 (2 H), 5.25 (2
H),
4.48 (4 H), 4.20 (2 H), 3.03 (4 H), 1:26 (3 H); "C NMR (CDCl3, 75 MHz) 8
173.6, 170.5,
157.8, 154.7, 136.8, 313.9, 130.4, 130.2, I29.0, 128.4, 114.9, 67.9, 65.1,
63.1, 62.5, 53.8,
36.5, 14.3; MS (EI ) mlz (reH. intensity) 525 (M', 1), 32.3 (44), 322 {i3),
321 (68), 267 (9),
265 (I4), 163 (I2), 161 (65), 160 (35), I59 (99), 88 (30~); MS (FAB) mlz (rel.
intensity}
526 (MH~, 58), 528 (40}, 527 (19), 526 (58), 32I (27), 188 (29), 161 (37), 160
(99), 159
(48), 107 (26), 88 (39); HRMS (FAB) calcd for CZ3HzsCIZN345S +H, 526.0970,
found
526.0942. Anal. Calcd for C23HzsChN305S: C, 52.47; ~C, 4.79; N, 7.98. Found:
C, 52.34;
H, 4.81; N, 7.90.
ii
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-112-
Scheme D
R5
~ D-t
Fmoc.N~OH
H (~O
H2NOt Bu D-2
R5
H
Fmoc~N~N,O~ D-3
H O
Rs
H ~
H N ~ N'0I \ D-4
z
O
RD-t
~'z D-5
R~
N OOH
R~ FmocO
F,moc O RsH
Ro3~(N N~N,O~ D-6
Rw~S~H O
Rp-z
R5
H O H
RD3 N N~N~O~ D-7
R ~S~H IiOII
RO-z
R3 R
~Y 0 sH
R~ N N~N~O~ D-$
~~../-R I'H
R XS ~R~p.~ O
R02
1
R3 R
,Y 0 sH
Roy N N~N~OH D-9
Rp S'1 RDH IIO
Ro_2
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-113-
R0.,, Ra2, and R~3 are defined.independentiy as R,. RD.~ is defined as RZ.
Scheme D describes a method for the preparation of examples of general formula
D-9. Commercially available or readily prepared N-a-Fmoc protected amino acids
of
general structure D-1 are coupled with O-(tent-butyl)hydroxyiamine (D-Z) under
standard
coupling conditions as previously referenced to afford tlhe t-butyl
hydroxamate of general
structure D-3. Standard Fmoc deprotection affords the iintermediate amine of
formula D-4.
Coupling of this amine with a commercially available or readily prepared N-a-
Fmoc-
thiazolidine-4-carboxylic acid of general structure D-5 affords the
pseudodipeptide
intermediate of general structure D-6. Standard Fmoc d:eprotection affords the
intermediate amine of general structure D-7 which may be reacted under the
variety of
conditions described in Scheme A to afford amides, carlbamates, sulfonamides
and areas of
general structure D-8. Preparation 22 provides a specific example of the
reaction of an
amine of general structure D-7 with a carbonate to afford a carbamate of
general structure
D-8. Mild acidolysis affords the hydroxamate of general structure D-9.
I S Preparation 19.
(Scheme D, D-3: where RS is 4-[(2,6-dichlorophenyl)mE;thoxy~phenyl and
stereochemistry
is (,f~).
0
Fmoc'N~H'O
Ct
,.
ci
To a cooled (0-5 °C) solution of Fmoc-Tyr(2,6-CI,-Bn) (Scheme D, D-1:
where RS is 4-
[(2,6-dichlorophenyl)methoxy]phenyl and stereochemistry is (,S~) (Advanced
Chemtech,
6.0 g, I0.7 mmol), HOBt (1.63 g, 10.7 mumol), O-(tert-butyl)hydroxylamine
hydrochloride
(Scheme D, D-2) (1.61 g, 12.80 mmol) in methyIene ch'.ioride (30 mL) was added
PyBOP
(6.66 g, 12.80m~mol) followed by DIEA (6.5I mL, 37.35 mmol). The mixture was
stirred
at 0-5 °C for 1 hour, gradually allowed to warm to rooms temperature
and stirred an
additional 2 h. The mixture was diluted with methylene; chloride and 0.25 N
HCI, the
organic layer separated and washed with saturated aqueous NaHC03, and brine,
dried
CA 02342778 2000-12-20
WO 99/67230 PCTJUS99/14233
-114-
(MgSO,), filtered and evaporated in vacuo. The residue was purified by flash
chromatography using methylene chloridelmethanol (0-~2.5%) as eluant to afford
the title
compound (5.87 g) as an amorphous powder: 'H NMR (CDCI,, 300 MHz ) b 8.08 (1
H},
7.76 (2 H), 7.54 (2 H), 7.29 (9 H), 6.95 (2 H), 5.43 (1 Hl), 5.30 {2 H), 4.28
(4 H), 3.06 (2
H), 1.19 (9 H); "C NMR (CDCI3, 75 MHz) 8 169.7, 157.8, 156.3, 143.6, 141.2,
136.8,
132.0, I 30.4, 128.9, 128.4, 127.7, 127.0, 125.0, I 19.9,_ I 15.0, 82.3, 67. I
, 65. I , 54.0, 46.9,
37.6, 26.0; MS (ESI+) for C35H34CIZNZOS m/z.632.9 (M-~H)'. MS (ESI+) for
C3sH3aCI,N,Os m/z 654.9 (M+Na}+.
Preparation 20.
(Scheme D, D-6: where RD_,, Rfl_2, Rti_3 and Rfl~ are the same and equal to
proton, RS is 4-
[(2,6-dichlorophenyl)methoxy]phenyl and stereochemistry is (S, S)).
S-. H OI[
C ~N~N.O~
N H
Fmoc O k w CI
cl l
To a solution of D-3 (Scheme D, where RS is 4-[(2,6-
dichlorophenyl)methoxy]phenyl and stereochemistry is (S)) (5.87 g, 9.28 mmol)
in
anhydrous DMF (94 mL) was added diethylamine (9.4CI mL, 90.84 mmol) at ambient
temperature. The solution was stirred for 90 rnin and volatiles were removed
in vacuo to
afford the intermediate amine D-4 (Scheme D, where RS is 4-[(2,6-
dichlorophenyl}methoxy]phenyl and stereochemistry is (S)) as an oil which was
used
without further purification.
To a cooled (0-5 °C) solution of, Fmoc-D-thiazolidine-4-carboxylic acid
(Scheme
D, D-5: where Rp_,, RD-2, Rp_3 and Ro.~ are the same andl equal to proton and
stereochemistry is (S)) (Advanced Chemtech. 3.93 g, i I .10 mmol) and HOAt ( I
.5 I g,
11. IO mmol) in methylene chloride/DMF {4:1,-30 mL) 'was added EDC (2.12 g, i
1.10
mmol). The reaction mixture was allowed to stir for 15 min and a solution of
the amine
(D-4 described above) in methylene chloride/DMF (4:1, 30 mL) was added
followed by
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-115-
DIEA ( 1:61 mL, 9.28 mmol). After 1 h at 0-5 °C, an adcfitional
equivalent of DIEA ( 1.61
mL, 9.28 mmol) was added and the mixture allowed to warm to room temperature.
After
stirring overnight, volatiles were removed in vacuo and the residue
partitioned between
ethyl acetate and 0.25 N aqueous HCI. The organic layer was separated and
washed with
water, saturated aqueous NaHC03, brine, dried {Na~S04), filtered and
concentrated in
vacuo. The residue was purified by flash chromatography using CHZClzlacetone
(3%)
containing isopropanol (0.1 %) as eluant to afford the titlc: compound (2.4 g)
as an
amorphous solid: 'H NMR (300 MHz, CDC13) S 7.77 (2 :H), 7.55 (2 H), 7.32 (7
H), 7.12 (2
H), 6.92 (2 H), 6.70 (1 H), 5.19 (3 H), 4.55 (5 H), 4.26 {2 H), 3.30 (1 H),
3.11 {3 H), 1.I4
(9 H);'3C NMR (75 MHz, CDC13) 8 170.0, 168.8, 158.0" 143.4, 141.3, 137.0,
132.1,
130.4, 128.5, 127.9, 127.2, 124.9, 120.1, 115.3, 82.5, b8.4, 65.3, 52.$; 47.1,
36.5, 2b.2.
MS (ESI+) for C,9H39C1,N;O6S m/Z 747.9 (M+H)'; MS (IESI+) for C39H39CI,N3O6S
m/Z
769.8 {M+Na)~; MS (ESI-) for C39H39C1zN3O6S mlz ?45.'r (M-H)'.
Preparation 2I .
{Scheme D, D-7: where Ra,, Ro_Z, Rp_3 and R~ are the s~rzne and equal to
proton, RS is 4-
[(2,6-dichlorophenyi)methoxy]phenyl and stereochemist~ry is (S, S)).
S-. H O
CN~N~N.O~
H
~ Cn
v 'o
To a solution of D-6 (Scheme D, where R~_,, Ro_Z, Ro_3 and Rpm are the same
and equal to
proton, RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl and stereochemistry is (S,
S}) (500
mg, 0.67 mmol) in anhydrous DMF (7 mL} was added di.ethylamine (0.70 mL, 6.55
mrnol)
at ambient temperature. The solution was stirred for 90 min and volatiles were
removed in
vacuo. The residue was washed with ethyl ether/hexane (3:2) to afford the
title compound
(352 mg) as an amorphous solid which was used without further purification: 'H
NMR
(300 MHz, DMSO-d6) s 10.61 (1 H), 8.27 (1 H), 7.54 (2 H), 7.44 (1 H), 7.15 (2
H), 6.94
(2 H}, 5.15 {2 H), 4.50 ( 1 H), 4.03 (2 H), 3.75 { 1 H). 3.19 ( 1 H), 2.82 {3
H}, 2.57 ( 1. H),
CA 02342778 2000-12-20
WO 99167230 PCT/US99114233
-I 16-
1.06 (9 H); MS (ESI+) for C24Hz9C1zN3O4S m/Z 526.1 (M:+H)+; MS (ESI-) for
Cz4H29CI2N304S m~2 524.1 {M-H)'.
Preparation 22.
(Scheme D, D-8: where Rp_,, RD_z, RD_3 and RDA are the :>ame and equal to
proton, R3 is 2-
(4-morpholinyl)ethyl, RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl, Y is COZ
and
stereochemistry is (S, S)).
O~ CN~N~N.O~~
I~ - ~ H
~N~O~O O ~ ~ CI
O' \
i/y~\ i i
To a solution of 4-{2-hydroxyethyl)morpholine (1.22 mL, 10.05 mmol) in CH3CN
(55 mL) at ambient temperature was added N,N-disuccinimidyl carbonate (2.49 g,
10.05
mmol) and triethylamine (4.20 mL, 30.15 mmol). The solution was stirred at
room
temperature for 4 h and concentrated in vacuo to give a viscous oil. The oil
was dissolved
in a minimal amount of methylene chloride ( 15 mL) and. added to a solution of
D-7
(Scheme D, where Rte,, Rp_2, RD_3 and Ro_4 are the same and equal to proton,
RS is 4-[(2;6-
dichlorophenyl)methoxy]phenyl and stereochemistry is {S, S) (350 mg, 0.67
mmol),
triethylamine (0.10 m, 0.74 mmol) and DMAP (1 mg) in CH2Clz (4 mL). The
reaction
mixture was stirred overnight and diluted with CHzCl2 ('l5 mL). Propylamine
(8.6 mL,
100.5 mmol) was slowly added (exothermic) and the solution stirred vigorously
fox 15
min, then diluted with water. The organic layer was separated and washed with
0.1 M
HCI, saturated aqueous NaHC03, and brine, dried (MgS04), filtered and
concentrated in
vacuo. Purification of the residue by flash chromatography using ethyl
acetatelacetone
{3:1) as eIuant afforded the title compound (251 mg) as .an white powder: IR
(mull) 3264,
1709, 1661, 1564, 1531, 1512, 1439, 1419, 1345, 1301, 1241, l I81, 1118, 1016,
767 cm';
'H NMR (300 MHz, CDCI3) 8 8.48 (1 H), 7.36 (2 H), 7.:24 (1 H), 7.17 {2 H),
6.96 (2H),
5.23 {2 H), 4.60 (3 H), 4.31 {3 H), 3.71 {4 H}, 3.33 {4 H ), 2.59 (6 H), 1.15
(9 H); '3C
NMR {75 MHz, CDCl3) S 168.9, 158.0, 137.0, 132.1, 130.5, 128.7, 128.5, 115.3,
82.5,
CA 02342778 2000-12-20
WO 99167234 PCT/US99/14233
-117-
66.8, 65:3, 63.4, 57.2, 53.7, 52.9, 36.4, 30.6, 29.3, 26.2, 19.1, 13.7; MS
(ESI+) for
C31H40C12N4O7S mlZ 682.9 (M+H)*, MS (ESI+) for C3lHaoC12N407S mlz 705.0
(M+Na)*;
Anal. Calcd for C3,HaoC12N4O7S ~ 0.35 HzO: C, 53.97; H:, 5.95; N, 7.95. Found:
C, 54.22;
H, d.l 1; N, 7.95. % Water {KF): 0.91.
Preparation 23 and Example 91.
(Scheme D, D-9: where Ro_,, RD_Z, RD_3 and Rpm are the same and equal to
proton, R3 is 2-
{4-morpholinyl)ethyl, RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl, Y is COZ
and
stereochemistry is (S, S)).
o~~ CNi~r"'~~"~LN.ol~l
~ - H
~N~O~O C ~ ~ C!
CI
Hydroxamate D-8 (Scheme D, where RD_,; RD_2, Ro_3 and. RDA are the same and
equal to
proton, R3 is 2-(4-morphoiinyl)ethyl, RS is 4-[(2,6-
dichlorophenyl)methoxy]phenyl, Y is
COZ and stereochemistry is (S, S)) ( 1 SO mg, 0.22 mmol) was dissolved in
anhydrous TFA
( 12 mL) at ambient temperature and gradually warmed to 40 °C. After 5
h at 40 °C,
volatiles were removed in vacuo and the residue partitioned between ethyl
acetate and
saturated aqueous NaHC03. The organic layer was separated and washed with
brine, dried
(MgSOa), filtered and concentrated in vacuo. Purification of the residue by
flash
chromatography using methylene chloride/methanol (S°r'o} as eluant
afforded the title
compound (51 mg) as an amorphous solid: IR (mull} 32 73, 3229, 1708, 1652,
1564, 1546,
151 l, 1439, 1422, 1346, 1236, 1180, 1114, 1022, 768 cm'';'H NMR (300 MHz,
DMSO-
d6) 8 10.70 (1 H), 8.93 (1 H}, $.32 (1 H), 7.54 (2 H}, 7.4E5 (1 H), 7.12 (2
H), 6.93 {2 H),
5.16 (2 H), 4.56 (2 H}, 4.36 (I H}, 4.25 (1 H), 4.06 (2 H}, 3.51 (4 H), 3.15
(1 H), 2.76 (3
H), 2.36 (4 H); "C NMR (75 MHz, DMSO-d6) b 169.8, 167.9, 157.6, 136.5, 132.2,
132.0,
130.8, 130.5, 129.2, i 14.7, 66.7, 65.3, 63.3, 59.1, 57.1, :53.8, 52.1, 37.8,
31.3, 30.1; MS
(ESI+) for Cz7H32C12N4O7S mlz 627.0 (M+H}+; MS (ESI-} for Cz7H32C12NaO7S mlz
624.9
CA 02342778 2000-12-20
WO 99/6'7230 PCT/US99/I4233
-118-
{M-H)'; Anal. Calcd for C27H3ZCIZN40,S ~ 0.46 H20: C, S 1.00; H, 5.22; N,
8.81. Found: C,
51.34; H, 5.23; N, 8.67. % Water (KF): 1.31.
Example 92.
(Scheme D, D-9: where RD_,, Raz, RD_3 and Rpm are the same and equal to
proton, R3 is
S ethyl, RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl, Y is CO~ and
stereochemistry is (S,
S).
S_ H O
~N~N~N,OH
I H
~O~O O I ~ CI
'O~
CI
Example 92 was prepared as described in Scheme D from Fmoc-Tyr(2,6-C12-Bn)
using
ethyl chloroformate to provide the requisite carbamate. Physical properties as
follows: IR
(mull) 3278, 1654, 1612, 1585, 1564, 1547,1 S 11, 1439, 1347, 1237, 1 I95, 1 I
79, 1022,
782, 769 cm';'H NMR (300 MHz , CDC13) 8 7.39 (3 H;), 7.I7 (2 H), 6.94 (2 H),
5.24 (2
H), 4.53 (4 H), 4.18 (2 H), 3.15 {2 H), 2.87 (2 H), I .28 (:3 H); '3C NMR (75
MHz ,
IS CD30D) 8 172.7, 170.1, 159.4, 156.2, 138.1, 133.7, 132.1, 131.6, 130.8,
129.8, 115.9,
66.3, 64.4, 63.6, 38.6, 36.8, 35.1, 15.0; HRMS (FAB) ca.lcd for C23HzsCL,N3O6S
+H,
542.0919, found 542.0921; Anal. Calcd for C23H2sC1zN3O6S: C, 50.93; H, 4.64;
N, ?.75.
Found: C, 50.79; H, 4:79; N, 7.52.
Example 93.
(Scheme D, D-9: where Rp_,, Rp_,, RD_3 and Rp~ are the same and equal to
proton, R3 is 2-
(1-piperidinyl)ethyl, RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl, Y is COZ
and
stereochemistry is (S, S)).
S-. H O
~N~N~N.OH
I ~I H
~N~O~O O ~ CI
O:
C:I
CA 02342778 2000-12-20
WO 99/67230 PCT/US99I14233
-I 19-
Example 93 was prepared as described in Scheme D from Fmoc-Tyr(2,6-Cl2-Bn)
using 1-
(2-hydroxyethyl)piperidine to provide the requisite cart~arnate. Physical
properties as
follows: IR (mull} 3276, 1707, 1653, 161 I, 1584, IS64., 1511, 1439, 1237, l
I9S, I 179,
S 1144, I 1 I3, 1093, 1021 cm -';'H NMR (300 MHz, DM:SO-d6) 8 7.54 (2 H), 7.44
(1 H),
7.12 (2 H), 6.93 (2 H), 5.16 (2 H), 4.58 (2 H), 4.36 (I Hf), 4.25 {1 H), 4.00
(3 H), 2.76 (3
H), 2.31 (3 H), 1.62 {1 H), I.42 (3 H), 1.26 (8 H};."C NMR (7S MHz, DMSO-db) 8
172.2,
168.9, 167.3, I57.0, 135.9, 131:7, 131.4, 130.2, 129.9,a28.7, 1I4.1, 64.7,
63.0, 54.0, 51.6,
36.2, 30.9, 2S.S, 24.7, 23.8, 22.0, 21.5, I3.9; MS (ESI+) for CZ8H34CIZN406S
m/z 624.9
IO (M+H)+.
CA 02342778 2000-12-20
W(3 99/67230 PCT/US99/I4233
-120-
Scheme E
0,~
i~s E-1
°b
O
O/
O N E-2
W
O
O O/
N
O E-3
/
O
'O~r-~~OH
O N E-4
Rs
H2N~0'~ E-5
O
Rs
O
O N Ow
N H O
O
y E-6
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-121-
Scheme E (continued.)
R5
O
~O ~~~~~ Ow
N H
O O
I ~ E-6
O R'
~O ~r~N~OH
N H
Q ~ O E_7
I,
R5
O
HO~r~~ N~OH
l N H O E-8
O
I
Preparation 24.
{Scheme E, E-2 where stereochemistry is (S)).
5- (2-Methoxy-2-oxoethylidene)-1- (phenylmethyl)-L-Proline 1,1-dimethylethyl
ester
0
°~L...~o~
0
I
To a stirring solution of E-1 (Scheme E where stereochemistry is (S)) (3.628,
12.4mmo1), prepared by the method of Rapoport (J. Am. Chem. Soc. 1984,106,
4539), in
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-122-
CH3CN~ (IOrnL) was added methyl bromoacetate (1.4rnL, I4.9rnmol). After
stirring for
70h, CH2Cl2 (70mL) was added. The solution stirred i:or IO minutes before Ph3P
(4.89g, 18.6mmoi) was added, and after 2 minutes Et3N (5.2mL, 37.3mmo1) was
added.
After stirring for 20h, the solution was washed with 1 M NaH2P04 ( 100mL), and
the
aqueous phase was extracted with CH2Ci2 (SOmL). 'The combined organic phases
were
washed with brine, dried (Na2SO4), filtered, and evaporated in vacuo. The
resulting
yellow oillwhite solid was dissolved in CHC13 and chromatographed on silica
gel (300g,
230-400 mesh, 70mm OD column, packed CHC13, eluted with CHC13, 3L, then 10:90
EtOAc-CHC13, 250mL fractions) using the flash technique. Fractions i 9-23
provided the
title compound (3.23g) as a pale yellow oil. IH-NMR: (300MHz, CDCl3): 8 = 7.17-
7.36
(5 H), 4. 75 ( 1 H), 4.54 ( 1 H), 4.20 ( 1 H), 3 .96 ( 1 H), 3 .61 (3 H), 3.3
6-3.47 ( 1 H), 3 .08
(1 H), 2.04-2.28 (2 H), I.4I (9 H); EI/MS (70eV) mlz (rel. intensity): 33I
(M+, 17.3),
275 (1 I.9), 230 (95.0), 170 (26.0), 91 (base); IR (neat): :?979, 2948, 1735,
1692, 1600,
1454, 1435, 1414, 1369, 1299, 1277, 1184, I I37, 1059, 964, 843, and 789cm-I;
HRMS:
IS Calcd. for ClgH2SNI04: 331.1783. Found: 33I.I771; [a]D25: +p07~ ( c= 0.939,
CH2C12).
Preparation 25.
(Scheme E, E-3 where stereochemistry is (2R,SS)).
(2R-cis)-5-[ (1;1-dimethylethoxy)carbonyl]-1- {phenyimethyl)-2-
pyrrolidineacetic
acid methyl ester ( (2R,SS))
~o~l"~~"~,~o~
°b
Raney-Nickel (20g of a 50% slurry in H20) was washed with abs. EtOH (3x25mL)
and
suspended in abs. EtOH (SOmL), and a solution of E-2 (Scheme E, where
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-123-
stereochemistry is (S)) {9.34g, 28.2mmo1) in abs. EtOH (SOmL) was added. After
stirring for 3h, the Ra-Ni was removed by filtration, and the filtrate was
evaporated in
vacuo. The residue was dissolved in EtOAc ( 1 OOmL), ;5% PdC (3.Og) was added,
and the
mixture was hydrogenated under 50 psi Hz for 12 h. The catalyst was removed by
S filtration and the filtrate was evaporated in vacuo. The residue was
chromatographed on
silica gel (300g, 230-400 mesh, 70mm OD column, packed and eluted with 15:85
EtOAc-hexanes, 270mL fractions) using the flash technique. Fractions S-8
provided the
title compound (6.SSg) as a clear, colorless oil. 1H-NMR: (300MHz, CDC13): & =
7.22-7.34 (S H), 3:86 (1 H), 3.79 (I H), 3.62 (3 H), 3.2I~-3.29 (2 H), 3.57 (I
H), 2.33 (1 H),
1.64-2.OS (4 H), 1.37 (9 H); EI/MS (70eV) m/z (rel. intensity):232 (base), 91
(39.8); IR
(nujol): 2977, 1739, 1454, 1437, 1367, 1295, I2S l, 1 I9'l, 11 S3, 1074, 844,
753, and
699cm-1; Anal: Calcd. for C19H27N104: C, 68.44; H, 8.16; N, 4.20. Found: C,
68.39;
H, 8.1 S; N, 4.11; Ia]D2S; _22° (c = 1.OS I, CH2Cl2).
Preparation 26.
1S (Scheme E, E-4 where stereochemistry is (2R,SS)).
(2R-cis)-5-[ (1,1-dimethylethoxy)carbonyl]-1- (phenylmethyl)-2-
pyrrolidineacetic
acid ( (2R,SS})
y .~~y ~,~'OH
~o~l N
~b
To a stirring solution of E-3 (Scheme E, where stereochemistry is (2R,SS))
{2.OOg,
6.OOmmoI) in MeOIi {60mL) was added 1 M K2C03 (2~OmL). After stirring for 12h,
the
reaction mixture was evaporated in vacuo, the residue was dissolved in H20
(0.1 L), the
pH was adjusted to ca. 6 with 1M HCI, and the mixture was extracted with CHCI3
2S (2XO.IL). The combined extracts were washed with H2~D, brine, dried
(Na2S04), and
evaporated in vacuo to afford the title compound (I.89g) as a white solid. MP:
9S-96°C
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-124-
(lit. 98-101 °C); 1H-NMR: (300MHz, CDCl3}: & = 7.30-7.37 (5 H), 4.00 (1
H), 3.68 (1 H),
3.45 (1 H), 3.20 (i H), 2.59 (1 H), 2.46 (1 H), 1.76-2.21 (4 H), 1.31 {9 H);
El/MS (70eV)
m/z (rel. intensity):218 (base), 91 (86.7); IR (nujol): 1719, 1497, 1451,
1367, 1296, 1285,
1260, 1160, 1153, 1079, 965, and 757cm-1; Anal: Calc~d. for C18H25N104:C,
67.69; H,
7.89; N, 4.39. Found: C, 67.55; H, 7.97; N, 4.15; [a]D25: +27° ( c =
0.795, CH2C12).
Preparation 27.
{Scheme E, E-6 where RS is 4-[ (2,6-dichlorophenyl)methoxy]phenyl, and
stereochemistry
of the pyrrolidine ring is (2R,SS) and the amino acid is (S~).
(SR)-5-[2-[( (1S)-1-Methoxycarbonyl-2-[4-[ (2,6-
dichlorophenyl)methoxyJphenyl]ethyl]amino]-2-axo~ahylJ-1- (phenyimethyl}-L-
proline 1,1-dirnethylethyl ester ( (1S, SR, L))
~ o w i
ci
o
°.
0 0
To a stirring solution of E-4 (Scheme E, where stereochernistry is (2R,SS))
(0.48g,
1.50mrn0I) in CH2Cl2 (lOmL) was added 1- (3-dimethyIaminopropyl)-3-
ethylcarbodiimide hydrochloride (0.29g, 1.50mmol), 1-hydroxybenzotriazole
hydrate
(0.20g, l.SOmmo1), 4-dimethylarninopyridine (0.05g, 0.45mmol), and 2,6-
dichlorobenzyl-L-tyrosine methyl ester hydrochloride (Scheme E, E-S: where RS
is 4-[
(2,6-dichlorophenyl)methoxy]phenyl and stereochemistry is (f~) (0.59g,
I.SOmmol) to
give a heterogeneous mixture. Upon addition of triethylamine (0.3mL) the
reaction
CA 02342778 2000-12-20
WO 99167230 PCTIUS99/I4233
-125-
mixture became homogeneous and stirred for 12h. The reaction mixture was
partitioned
between CH2C12 (SOmL) and IN HCl (SOmL). The organic phase was washed with
sat'd
aq. NaHC03, H20, brine, dried (Na2S04), filtered and evaporated in vacuo, The
resulting yellow oil was chromatographed on silica gei (1508, 230-400 mesh,
70mm OD
column, packed and eluted acetone/CH2C12 5:95, 40mL fractions). Fractions 36-
48
furnished the title compound (0.90g) as a glass. 1H-NM:R: (300MHz, CDCl3): 8 =
9.09 (1
H), 7.38 (2 H), 7.10-7.30 (8 H), 6.97 (2 H), 5.22 (2 H), 4..75 (1 H), 3.81 (1
H), 3.68 (3 H),
3.60 (1 H), 3.i5-3.35 (3 H), 3.04 (1 H), 2.42 (I H), 2.21 (1 H), 1.86-2.07 {4
H), 1.37 (9 H);
FAB/MS m/z (rel. intensity): 655 {M+H, 46.4), 599 (11.3), 553 (23.6), 260
(18.8), 204
(91.4), 91 (base); IR {nujol):3262, 3001, 1733, 1665, 16 L2, 1585, 1565, 1512,
1439, 1392,
1240, 1226, 1 I97, 1177, 1 IS3, 1018, and 768 cm-1; Anal: Calcd. for
C35H40N206C12:
C, 64.12; H, 6.15; N, 4.27; Cl, 10.82. Found: C, 63.751H, 6.29; N, 4.11; Cl,
10.88;
[a]D25; +6~ ( c = 0.863, CHCI3).
Preparation 28.
1S (Scheme E, E-7 where RS is 4-[ (2,6-dichlorophenyi)methoxy]phenyl, and
stereochemistry
of the pyrrolidine ring is (2R,SS) and the amino acid is (S~).
(5R)-5-(2-[[ (1S)-I-Carboxy-2-[4-[ (2,6-
dichlorophenyl)methoxy)phenyl]ethyl]amino]-2-oxoethyl]-1- {phenylmethyl)-L-
proline 1,1-dimethyiethyl ester ( (1S, 5R, L))
ca
~ o. w i
w I c~
o
o~l '.~.H OH
ob o
To a stirring solution of E-6 (Scheme E, where Rs is 4-[ (2,6-
dichlorophenyl)methoxy]-
phenyl, and stereochemistry of the pyrrolidine ring is (21f~,5S) and the amino
acid is (S))
CA 02342778 2000-12-20
WO 9916230 PCT/LTS99/14233
-126-
(I.00g, 1.53mmo1) in MeOH (50mL) was added 1M K2,C03 (IOmL). After stirnng for
12h, the reaction mixture was evaporated in vacuo, the residue was dissolved
in H20
(0.1 L), the pH was adjusted to ca. 6 with 1 M HCI, and the mixture was
extracted with
CHCl3 (2X0.1 L}. The combined extracts were washed with H20, brine, dried
(Na2S04), and evaporated in vacuo to afford the title compound (0.85g) as a
white solid.
MP: 80-83°C; 1H-NMR: (300MHz, CDCl3): 8 = 9.87 (1 H}, 7.35 {2 H), 7.21-
7.26 (7 H),
7.13 (2 H}, 6.94 (2 H), 5.22 (2 H}, 4.64 (1 H), 3.80 (1 H), 3.55 (1 H}, 3.29-
3.37 (3 H}, 3.04
1 H), 2.48 { 1 H}, 2.26 ( 1 H}, 1.94 ( 1 H}, 1.76 ( 1 H}, 1.59 ( 1 H), 1.41 (
1 H), 1.34 {9 H);
FAB/MS m/z (rel. intensity): 641 (M+H, 45.8), 585 (13.0}, 260 (6.1), 204
(base}, 91
{96.8); IR (nujol): 1732, 1642, 1612, 1585, 1565, 1534, 1511, 1439, 1240,
1230, 1196,
1178, 1153, 1018, 779, and 767cm-1; HRMS: Calcd. for C3qH38C12N~O6: 641.2185.
Found. 641.2164; [a]D25: +5° ( c = 0.795, CHCl3).
Preparation 29 and Example 94.
{Scheme E, E-8 where RS is 4-[ (2,6-dichlorophenyl)methoxy]phenyl, and
stereochemistry
of the pyrrolidine ring is (2R,5S) and the amino acid is (S~).
(SR)-5-[2-[[ (1S)-1-Carboxy-2-(4-( (2,6-
dichlorophenyi)methoxy]phenyl]ethyl]amino]-2-oxoethyl]-1- (phenylmethyl)-L-
proline ( (1S, 5R, L))
ci. ~
°~ ~ I
I c~
°
HO~j, ,~,",.~H OH
°b °
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-127-
To a solution of E-7 (Scheme E, where RS is 4-[ (2,6-
diichlorophenyl)methoxy]phenyl,
and stereochemistry of the pyrrolidine ring is (2R,SS) and the amino acid is
(S)) (0.85g,
1.32mmoi) in H20/n-PrOH ( 1:1, 0.1 L) was added HOAc {6mL), and the solution
refluxed for Sh, then stirred at RT for 12h. Evaporation. in vacuo afforded
the title
compound (0.78g) as a white solid. MP: 198-202°C; 1 H-NMR: (300MHz,
DMSO}: b
=8.56 (1 H), 7.56-7.59 (2 H), 7.45-7.50 (1 H), 7.25-7.29 (5 H), 7.21 (2 H),
6.96 (2 H), 5.18
(2 H), 4.41 ( 1 H}, 3.89 { 1 H), 3.74 ( 1 H), 3.31 ( 1 H), 3.03-3.09 (2 H),
2.85 ( 1 H), 2.18-2.34
(2 H), 1.88-2:00 (I H), 1.63-1.84 (2 H}, 1.44-I.58 (1 H); FAB/MS m/z (rel.
intensity): 585
(M+H, 21.6}, 539 (2.1), 246 (14.5), 204 (60.9), 159 (12..6), 91 (base); IR
(nujol): 3309,
3083, 3037, 3014, 1662, 1644, 1562, 1514, 1440, 1377; 1348, 1241, 1197, 1178,
1018,
998, 815, and 771cmI; Anal: Calcd. for C3oH3oNZO6C1zO.38H20: C, 60.83; H,
5.24; N,
4.73. Found: C, 60.83; H, 5.33; N, 4.69; Karl Fischer water: 0.42%.
Example 95.
(Scheme E, E-8 where RS is 4-( (2,6-dichlorophenyl)methoxy]phenyl, and
stereochemistry
of the pyrroiidine ring is (2S,SR} and the amino acid is (S)).
(5S)-5-[2-([ (1S)-1-Carboxy-2-[4-[ (2,6-
dichlorophenyl)methaxy]phenyl]ethyl]amino]-
2-oxoethyl]-1- (phenylmethy!)-D-proline ( (1S, 5S, I)))
G
~I
G
O
HO~~~~N OH
I N H O
ob
I,
Example 95 was prepared as described in Scheme E from E-1 (Scheme E where
stereochemistry is (R) prepared by the method of Rapoport (.7. Am. Chem. Soc.
1984, 106,
4539). Physical data as follows: 198-204°C; IH-NMR: (300MHz; DMSO): cS
= 8.60 (1
H}, 7.54-7.68 (3 H), 7.26-7.41 (8 H), 7.05 (2 H), 5.27 (2, H), 4.57 (I H),
3.96 (1 H), 3.82 (1
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-I28-
H), 3.40 ( I H), 3.14 (2 H), 2.86-2.94 ( 1 H), 2.27-2.47 (2 H), 1.96-2:10 ( 1
H), 1.72-1.88 ( 1
H), I.SS-1.72 (1 H), I.30-1.42 (1 H}; FAB/MS m/z (rel. intensity): S8S (M+H,
SO.S), S39
(4.S), 332 (32.1), 331 (18.1}, 246 (6.2}, 244 (6.0), 204 ('77.2), 91 (base);
IR (nujol): 3211,
3033, 3006, 1724, 1647, 1610, 1S6S, 1512, 1438, i3S4, 1301, 1273, 1240, 1196,
1018,
S 871, and 767ctri'; Anal: Calcd. for C30H30N246C12~0.43HZO:C, 60.77; H, 5.24;
N, 4.72.
Found: C, 60.76; H, 5.37; N, 4.59; Karl Fischer water analysis: 1.71%.
Scheme F
$ RF-t
~RF~2 F-1
~OH
[~N~
~O.-~O O
R5
O. F-2
H2N~ RF-a
O
RF.t
S RF-2 O $ RF-1
RF_Z O
N~NM O.Rp.~ C NH
//O N~ OH
F-3
O O R5 ~ O~O O ~ F-7
R5
$ R
RF.2 O $ F-~
~ RF_p O
N~ NH O, RF_3 NN
[O~ ~ F-4 H [~ OH
R5 O ~ F-j
R5
~$ RF RF-2 ~ $ Rf'_t
NH R ~ ~'RF_y O
N ~O ~ N ~NH pH
R3 Y O R5 F-5 R ,Y O ~ F-6
s R5
Where RF_3 is defined as proton or C,-6 alkyl.
CA 02342778 2000-12-20
WO 99/67230 PCT/IJS99/14233
-129-
Scheme F describes a general method for the prf;paration of examples of the
formula F-4, F-S, F-6, F-7, and F-8. A commercially available or readily
prepared sulfur
containing amino acid of structure F-1 is condensed with amino acid derivative
F-2 under
standard peptide synthesis conditions as described in Scheme A. Deprotection
of the
carbamate from F-3 provides the useful intermediate F-4. The amine group may
be
reacted with a variety of electrophilic reagents as described in Scheme A to
provide esters
of general structure F-S. Mild base hydrolysis provides acids of structure F-
6. Mild
hydrolysis of esters of general structure F-3 provides acid of formula F-7. In
those cases
in which R~_3 is equal to t-butyl, mild acidolysis of compounds of general
structure F-3
afford the amino acid of general structure F-8.
Preparation 30 and Example 96.
(Scheme F, F-3: where RF_, and RF_2 are the same and equal to proton, RF_3 IS
CH3, RS is 4-
[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (R, S})
s o
N N O~
~O~O O ~ ~ O~~ CI
~N~
cl
To a cooled (0 °C) solution of Boc-L-thiomorpholine-3-carboxylic acid
((a) Van Der
Auwera, C.; Anteunis, M.J.O. Int. J. Peptide Protein Res. 1987, 29, 574: (b)
Kogami, Y.;
Okawa, K. Bull. Chem. Soc. Jpn. 1987, 60, 2963: (c) L.~rsson U.; Carlson R.
ACTA
Chemica. Scared. 1994, ~8, 517: (d) Carson 3.F.; Wong F.F. J. Org. Chem. 1964,
29,
2203.} (Scheme F, F-1: where RF_, and RF_2 are the same and equal to proton
and
stereachemistry is (R)) (6.7 g, 27 mmol) in CHZCIz (100 mL) was added HOBt
(4.0 g, 29.7
mmol), DMAP (700 mg}, EDC (5.7 g, 29.7 rnmol) and l:riethylamine ( 13.5 mL, 97
mrnol).
The reaction mixture was stirred for IO min, then the amino acid derivative F-
2 (Scheme
F, where RS is 4-[(2,6-dichlorobenzoyl}amino]phenyl, R.H_31$ CH3, and
stereochemistry is
(S)) (10.0 g, 24.7 mmol) was added. After 20 h, volatile;s were removed in
vacuo and the
residue partitioned between 2.5% aqueous HCl (100 mL,) and H20 (I00 mL). The
organic
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-130-
layer was separated and washed saturated aqueous NaHC03 (100 mL), dried and
concentrated in vacuo. Purification of the residue by cl>J-omatography on Si02
(500 g)
using CHZC12 /ethyl acetate (10%) as eluent afforded thE; title compound
(12.31 g) as a
solid:'H NMR (CDCl3) S 1.44 (9 H}, 2.35 (I H), 2.70 (3 H), 3.13 (2 H), 3.33 (1
H), 3.77
{3 H), 4.22 (1 H), 5.00 (1 H), 6.48 ( I H), 7.18 {2 H), 7.3~ 1 (3 H), 7.44 (I
H), 7.56 (2 H);
'3C NMR (CDC13) 8 171.6, 168.9, 162.5, 136.5, 135.9, 132.4, 131.0, 130.2,
128.2, 120.5,
81.7, 77.3, 53.3, 52.6, 37.0, 28.2, 26.5; IR {mull) 3296, 2924, 1744, 1685,
1668, 1605,
IS36, 15I5, 1432, 1412, I321, I294, 1260, 1244, 1213, 1195, 1161, 798 cm'; MS
(FAB)
mlz (rel. intensity) 598 (M+H, 3), 596 (M+H, 5). Anal. Calcd for
C27H3,C12N3O6S : C,
54.36; H, 5.24; N, 7.04. Found: C, 54.23; H, 5.24; N, 6.86. Corrected for
0.60% HZO,
found by Karl Fischer analysis.
Preparation 31 and Example 97.
(Scheme F, F-4: where RF_, and RF_z are the same and equal to proton, RF_31S
CH3, RS is 4-
[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (R, ,f})
s o
r~ N~:o
H o
o c;l
~ HCI I ~ N
H ~~
Acetyl chloride (1.75 mL, 24 mmol) was slowly added to MeOH (26 mL} at 0-5
°C. After
I5 min, a solution of the carbamate F-3 (Scheme F, where RF_, and RF.2 are the
same and
equal to proton, RF.3 is CH3, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and
stereochemistry is (R, S~) (2.4 g, 4.0 mmol) in methanol (8 mL) was added.
After 50.5 h at
0 °C, the solvent was removed in vacuo to yield the title compound
(2.11 g): 'H NMR
(300 MHz, DMSO-d6) s 2.68 (I H), 3.00 (6 H), 3.47 (1 H), 3.64 (3 H), 4.02 (1
H), 4,52
(I H), 7.28 (2 H), 7.54 (5 H), 9.15 (1 H), 9.3 (I H}, 9.70 (1 H), 10.7 (I H);
IR {mull) 3191,
3031, 1742, 1664, 1604, 1577, 1561, 1540, 1516, 1432, 1414, 1326, 1271, 1210,
799 cm'
'; MS (EI} m/z (rel. intensity) 495 (M+, 1).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-131-
Preparation 32 and Example 98.
(Scheme F, F-5: where RF_, and Rr_2 are the same and equal to proton, RF.a is
CH3, R3 is
CHZCHZCOZCH3, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl, Y is CO-, and
stereochernistry is (R, S~).
s o
°.
-°~o ° ~ ~ cl
I
H I ~
CI
To a solution of amine F-4 (Scheme F, where RF_, and R,:.2 axe the same and
equal to
proton, Rr_3 is CH3, RS is 4-[(2,6-dichlorobenzoyi}amino=phenyl and
stereochemistry is (R,
,S~) (650 mg, 1.2 mmol) was added mono-methyl succinate (320 mg, 2.4 mmol),
EDC
(460 mg, 2.4 mmol), pyridine (10 mL) and DMAP at ambient temperature. After 27
h, the
mixture was diluted with 25 mL of saturated NaHC03 was extracted with
methylene
chloride. The combined organic extracts were dried and concentrated in vacuo.
Purification of the residue by flash chromatography using methylene
chloride/ethyl acetate
{3:2) as eluant followed by lyophilization afforded the title compound (600
mg} as an
amorphous solid: IR {mull) 1742, 1659, 1657, 1608; 1537, 1516, 1432, 14/4,
1324, /269,
1259, 1228, 1214, 1195, 1176 crri';'H NMR (300 MHz, CDCl3) S 2.63 (7 H), 3.21
(3 H),
3.68 (3 H), 3.78 (3 H), 3.90 (I H), 4.80 (2 H), 5.50 (1 H), 6.56 (1 H); 7.29
{5 H), 7.57
(2 H);'3C NMR (75 MHz, CDCl3) 8 173.9, 172.0, 171.7., 168.5, 162.7, 136.1,
135.9,
133.3, 132.3, 13x.0, 130.1, 128.2, 121.0, 120.7, 53.1, 52.5, 52.3, 52.0, 44.4,
36.7, 29.4,
27.7, 26.9, 26.4; MS (EI) m/z (rel. intensity} 609 (M+, 5). Anal. Calcd for
CZ7HZ9C12N3O7S
. C, 53.12; H, 4.79; N, 6.88. Found: C, 53.04; H, 4.81; N, 6.83. Corrected for
0.74%
Hz0 found by Karl Fischer analysis.
Preparation 33 and Example 99.
[R-(R*,S*)]- 3-[[jl-Carboxy-2--[4-[(2,6-
dichlbrobenzoyl)amino]phenyl]ethyl]amino]carbonyl] ~-oxo-4-
thiomorpholinebutanoic
acid
CA 02342778 2000-12-20
WO 9916'1230 PCTlUS99/14233
-132-
(Scheme F, F-6: where RF_, and RF_2 are the same and equal to proton, RF_3 IS
proton, R3 is
CHZCH2COZH, RS is 4-[(2,6-dichlorobenzoyl)amino]ph.enyl, Y is CO-, and
stereochemistry is (R, S)).
0
N
HO
O
O
S
To a solution of the diester F-5 (Scheme F, where RF_, a'nd RF_2 are the same
and equal to
proton, Rg_g IS CH,, R3 is CHZCHZCOZCH3, RS is 4-[(2,6-
dichlorobenzoyl)amino]phenyl, Y
is CO-, and stereochemistry is (R, ,S~) {490 mg, 0.80 mrnol) in THF (20 mL)
and MeOH (6
mL) was added a solution of LiOH~H20 ( 178 mg, 4.25 mmol) in H20 (6 mL). After
22 h,
the mixture was concentrated in vacuv. The residue ways partially dissolved in
10% HCI
(20 mL) and the resulting solid collected by filtration. 'The solid was washed
with water
and lyophilized from aqueous acetonitrile to afford the title compound (400
mg) as an
amorphous solid: IR {mull) 3267, 3 L93, 3058, 3034, 2924, 1725, 1658, 1607,
1562, 1537,
1516, 1432, 1414, 1326, 1195, 1178, 800 cm'; 'H NMR {300 MHz, DMSO-d6) S 2.62
(7 H), 3.17 (3 H), 3.95 (1 H), 4.57 (2 H), 5.26 {1 H), 7.18 (2 H), 7.55 (5 H);
8.02 (1 H),
10.64 (1 H), 12.34 ( 1 H); MS (FAB) m/z (rel. intensity;} 582 (M+H, 18).
Preparation 34 and Example 100.
[R-(R*,S*)]-3-[[[1-Caurboxy-.'.'?-[4-[(2,6-
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]~-4--
thiomorpholinecarboxylic acid
4-{ 1,1-dimethylethyl) ester
(Scheme F, F-7: where RF_, and RF_z are the same and equal to proton, RF_3 is
proton, RS is
4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (R, S)).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-133-
s
0
N NH v 'OH
~0~0 O ~ O CI
r~ H
CI
To a solution of ester F-3 (Scheme F, where RF_, and R,:_2 are the same and
equal to proton,
Rr_3 is CH3, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is
(R, S})
(656 mg, 1.02 mmoI) in MeOH (25 mL) was added KZCO~ (554 mg, 4 mmol) and H20
(13
mL). After 3 h, volatiles were partially removed and the solution diluted with
10% HCI
(20 mL) causing precipitation of a solid. The product cvas collected by
filtration, washed
with H20 and dried in a vacuum oven to afford the product (610 mg): IR {mull)
1736,
1665, 1606, 1562, 1537, 1516, I432, 1413, 1323, 1294., 1260, 1244, 1211, I
195, 1160 cm-
~; 'H NMR (300 MHz, DMSO-d6) 8 1.35 (9 H), 2.45 (:2 H}, 2.74 (5 H), 4.00 (I
H), 4.47
{ 1 H}, 4.70 ( 1 H), 7.19 {2 H), 7.52 (5 H), 7.92 ( 1 H), I 0.60 ( 1 H), 12.75
( 1 H); MS (FAB)
m/z (rel. intensity) 582 {M+H, 12). Anal. Calcd for CZ6Hz9CIzN3O6S : C, 53.61;
H, 5.02;
N, 7.21;. Found: C, 53.20; H, 5.12; N, 7.10. Corrected for 2.30% HZO found by
Karl
Fischer analysis.
Preparation 35.
(Scheme F, F-3: where RF_t and RF_2 are the same and equal to proton, RF_3 is
t-butyl, RS is
4-[(2,6-dichlorobenzoyl}amino]phenyl and stereochemi.stry is (R, f~).
s
N N O
O 00 ( ~ OIt CI
~N
H
CI
To a cooled {0-5 °C) solution of Boc-L-thiomorpholine-3-carboxylic acid
(Scheme F, F-
1: where Rr_= and RF_Z are the same and equal to proton and stereochemistry is
(R}) ( 1.34 g,
S.4 mmol) in methylene chloride (20 mL) was added HOBt (800 mg, 5.94 mmol),
DMAP
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-134-
(140 mg; EDC (1.14 g, 5.94 mmol) and triethylamine (2.7 mL, 19.4 mmol). After
10
min, F-2 (Scheme F where RF_, and RF_Z are the same and. equal to proton, RF_3
IS t-butyl, RS
is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (~) (2.02 g,
4.94 mmoi)
was added, the reaction allowed to warm to ambient temperature and stirred for
24 h.
Volatiles were removed in vacuo and the residue partitioned between methylene
chloride
and 2.5% aqueous HCI. The organic layer was separated and washed with sat.
aqueous
NaHC03, dried and concentrated in vacuo: Purification of the residue by flash
chromatography using methylene chloride/ethyl acetate ( 5%) as eluant afforded
the title
compound (1.64 g): IR (mull) 1730, 1687, 1667,1606, I538, 1515, 1431, 1412,
1395,
1320, 1294, 1258, 1250, 1194, 1158 cm''; 'H NMR (300 MHz, CDC13) 8 I.44 (9 H),
1.46
(9 H), 2.3 5 ( 1 H), 2.67 (3 H), 3.22 (3 H), 4.25 ( 1 H), 4.73 ( 1 H), 4.97 (
1 H), 6.52 ( I H),
7.29 (5 H), 7.53 (3 H); MS (FAB) mlz (rel. intensity) 638 {M+H, 2). Anal.
Calcd for
C3oH37C12N,O6S: C, 56.42; H, 5.84; N, 6.58. Found: C, 56.13; H, 5.98; N, 6.58.
Preparation 36 and Example 101.
4-[(2,6-Dichlorobenzoyl)amino]-N-[[(3R)-3-thiomorpholinyl]carbonyl]-t,-
phenyIalanine monohydroch.loride
{Scheme F, F-8: where RF_, and RF_z are the same and equal to proton,. RF.31s
proton, R5 is
4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (R, S)).
0
NH_ ~
N~ v 'OH
H I'
i I O Cl
\ NHS / I
Cl \
To a solution of HC1 saturated in ethyl ether (5 mL) at ambient temperature
was added
carbamate F-3 (Scheme F, where RF_, and RF_z are the sanne and equal to
proton, RF_3 IS t-
butyl, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (R,
S)) (100 mg,
0.15 mmol) with vigorous stirring. After 43.5 h, the precipitate was collected
by filtration
and washed with ethyl ether to afford the title compound {90 mg): IR (mull)
3241, 3189,
3033, 2731, 1725, 1661, 1605, 1578, 1562, 1542, 1515, :1432, 1414, 1328, 1195
cm'';'H
CA 02342778 2000-12-20
WO 99/67230 PCTlUS99/14233
-135-
NMR (300 MHz, DMSO-d6) 8 2:66 (1 H), 2.99 {6 H), 3.50 (1 H), 3.98 (1 H), 4.45
(I H),
7.26 (I H), 7.52 (5 H), 9.00 (2 H), 9.30 (1 H), 10.69 (1 H:); MS (FAB) m/z
(rel. intensity)
482 (M+H, 83), 540 (32), 539 (9), 538 (42), 486 (13), 485 (16), 484 (60), 483
(24), 482
(83), 173 {11), 102 (99). Anal. Calcd for CZ,HZ,C12N304S ~ HCI: C, 48:61; H,
4.27; N,
8.I0; Cl, 20.50; S, 6.18. Found: C, 48.92; H, 4.27; N, 7.'79; Cl, 19.68.
Corrected for
6.53% HZO found by Karl Fischer analysis.
Example 102.
4-{(2,b-Dichlorobenzoyl}amino]--N-[[{3R)-4--[ I-oxo-3-( I H-tetrazol-S-
yl)propyl]-3
thiomorpholinyl]carbonyl]-r.-phenylalanine methyl ester
(Scheme F, F-5: where RF_, and RF_, are the same and equal to proton, RF_3 is
CH3, R3 is 2-
(5-1H-tetrazolyl)ethyl, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl, Y is CO-,
and
stereochemistry is {R, S~).
g O .
CN II N~Oi
N~C p ~ OII Ct
NN.N L / N~ W
H , I r
Ci
i5
Example I02 was prepared as described in Scheme F using 1H-tetrazole-5-
propanoic acid
(Hutchinson, D. W.; Naylor, M. Nucleic Acids Res. 1985,13, 8519) to form the
requisite
amide.,.Physical data as follows: IR {mull) 3264, 3047, 11742, 1659, 1607,
1561, 1537,
1516, 1432, 1415, 1324, 1268, 1219, 1195, 799 cxri';'H NMR (300 MHz, DMSO-d6)
8
2.26 (1 H), 2.66 (2 H), 3.02 (8 H), 3.61 (3 H}, 3.87 (I H), 4.56 (1 H), 5.25
(1 H), 7.21
. (2 H), 7.50 (5 H), 8.18 ( 1 H), 8.45 ( 1 H), 8:64 { 1 H); MS (FAB) mlz (rel.
intensity) 620
(M+H, 61 ).
Example 103.
4-[(2,6-Dichlorobenzoyl)amino]-N-[[(3R)-4-[ 1-oxo-:3-( 1 H-tetrazol-5-
yl)propyI]-3
thiornorpholinyl]-carbonyl]-L-phe;nylalanine
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-136-
(Scheme F, F-6: where RF_, and RF_2 are the same and equal to proton, RF_31S
proton, R3 is
2-(S-1H-tetrazolyI)ethyl, RS is 4-[(2,6-dichlorobenzoyl)arnino]phenyl, Y is CO-
, and
stereochemistry is (R, S)).
S
O
H
N N v 'OH
.N O
N ~ 'O .~ I O Cl
N-N
N
H
Cl
Example I03 was prepared from example 102 by the procedure described in
preparation
34. Physical data as follows: IR (mull) 3376, 3296, 3267, 3127, i 746, 1683,
1669, 1641,
1623, 1610, 1542, 1522, 1444, 1436, 1411 cm' ~ MS (E;iI+) for CZSHzsC12N705S
m/z 605.8
(M+H)~; MS (FAB) m/z (rel. intensity) 606 (MH~, S l )., 682 (17), 608 (40),
607 (28), 606
(S1), 605 (16), 254 (99), 226 (23), 175 (17), 137 (20), 1!02 (33); HRMS (FAB)
calcd for
C,SHz5C1zN705S +Hi 606.1093, found b06.1105.
Example 104.
N-[[(3R)-4-(3-Cyano-1-oxopropyl~3-thiomo:rphoiinyl]carbonyl]-4-[[2,6
dichlorobenzoyl)amino]-L-phenylalanine
(Scheme F, F-6: where RF_, and RF_z are the same and equal to proton, RF_~ is
proton, R3 i5
CHZCHZCN, RS is 4-[(2,6-dichlorobenzoyl)amino)phenyl, Y is CO-, and
stereochemistry is
(R, S)).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-137-
S
O
H
N N OH
N=C O ~ ~ ~ O Cl
N
H
C1
Example 104 was prepared as described in Scheme F u;>ing 3-cyanopropanoic acid
(readily
prepared from commercially available 3-cyanopropanoi.c acid) to form the
requisite amide.
Physical data as follows: IR (mull) 2251, 1735, / 655, I6I2, 1585, 1565, 1512,
1439,
1298, /240, 1 i96, 1179, 1016, 1000, 779, 768 cm'; 'I-I NMR (300 MHz, CDCl3) 8
2.40
(12 H), 4.88 (1 H), 5.22 (2 H), 6.72 (I H), 6.96(2 H), 7.23 (5 H).
Example 105.
[R-(R*,S*)]-3-[[[I-Carboxy-2-[4-[(2,6-dichlorophenyl)methoxy]-
phenyl]ethyl]amino]carbonyl]-y-oxo-4-thiomorpholinebutanoic acid
I O (Scheme F, F-6: where RF., and RF_Z are the same and eq~,ual to proton,
RF_3 iS proton, R3 is
CHzCH2COzH, RS is 4-[{2,6-dichlorophenyl)methoxy]p:henyl , Y is CO-, and
stereochemistry is (R, S)).
S
H O
N N~OH
HO O~ _
~O
O
Example 105 was prepared as described in Scheme F using mono-methyl succinate
to form
the requisite amide. Physical data as follows: IR (mull;t 3031, 1726, 1646,
/612, 1585,
1565, 1511, 1439, 1421, 1297, 1240, 1 I96, 1179, 1016, 768 crri'; 'H NMR (300
MHz,
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99114233
-138-
DMSO-d6) 8 2.62 (8 H), 3.64 (3 H), 4.39 (2 H), 5.20 (2 H), 6.92 (2 H), 7.15 (2
H), 7.50
(3 H), 7.98 (2 H); "C NMR {75 MHz, DMSO-d6) 8 174.5, 173.2, 171.9, 168.9,
136.5,
132.3, 132.0, 130.8, 130.7, 129.2, 114.8, 65.3, 54.3, 52,.5, 36.0, 29.7, 28.0,
27.0; MS
(FAB) m/z (rel. intensity) 569 {M+H, 24). Anal. Calcd for CZSHa6C1zN207S: C,
52.73; H,
4.60; N, 4.92; Cl, 12.45. Found: C, 52.51; H, 4.60; N, 4.94; Cl, 12.78.
Corrected for
3.37% Hz0 found by Karl Fischer analysis.
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-139-
Scheme G
0
O NH
G-1
RG_2_O g ll O
O RG_t
O
O N'RG-~
Gi-2
R~,.~-O 9 O
O R~_t
O
O N-RG-~ G..3
RGa'O ~ ~9 ~-OH
O
Rs
HZN~O~ G-4
'IO
O
O N-RG_s
H~ O
RG.~'O g N ~O~ G-5
~O
R5
O
O N RG-3 O
H ti-6
HO 9~N~OH
O
R5
Where R~_, and Re_2 are defined independently as H or CH 3; R~-3 is defined as
H, C,~ alkyl
or C3_6 alkenyl; and g is defined as 0 or 2.
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-140-
Scheme G describes a general method to prepare lactam examples of general
structures G-5 and G-6. Readily prepared iactams of general structure G-1 may
be
aIkylated by the reaction of an appropriate alkylating in the presence of a
suitable base as
described in preparation 37 to provide intermediates of general structure G-2.
Mild
hydrolysis provides the monoacid of general structure G-3 which may be
condensed with
an amino acyl intermediate of structure G-4 as describef. in Scheme A. Full
hydrolysis of
the diester of general structure G-5 affords the diacid of structure G-6.
Preparation 37.
(Scheme G, G-2: where R~_, and RG_z are CHI, RG_3 is CH3 and g is equal to 2).
0
N-CH3
O O
O O
To a cooled (0-5 °C) solution of dimethyl ester G-1 {Sch.eme G, where
R~_, and R.~_2 are
CHI, R~_3 is H and g is equal to 2) (Thomas, E.T.; Rynbrandt, R.H; Zimmermann,
D.C.;
IS Bell, L.T.; Muchmore, C.R.; Yankee, E.W. J. Org. Chem. 1989, 54, 4535)
(25.7 g, 0.1
mole) and iodomethane {30 mL, 0.48 mol) in THF {200 ~mL) was added NaH (4.8 g,
0.12
mmol, 60% in oil dispersion). After 22 h, the reaction was quenched by the
addition of
HZO ( 100 mL) and diluted with CHZC12 (50 mL). The organic layer was
separated, dried
and dried in vacuo. The crude brown oil was triturated v~rith hexanes {200 mL)
and the
residue concentrated in vacuo to afford the crude produce: ( 18.44 g) which
was used
without further purification: 'H NMR (300 MHz, CDCI3;! 8 2:03 (I2 H), 2.64 (3
H), 3.64
(6 H).
Preparation 38.
(Scheme G, G-3: whereR~_2 is CH3, RG_3 is CH3 and g is f:qual to 2).
0
N-CH3
O OH
O O
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-141-
To a cooled (0-5 °C) solution of diester G-2 (Scheme G,, where RG_, and
RG_2 are CH3, R~3
is CH3 and g is equal to 2) (10.0 g, 36.9 mmol) in aqueous methanol (66%, 75
mL) was
added LiOH~H20 (1.55 g, 36.9 rnmol). After 22 h, the mixture was partially
concentrated
in vacuo and diluted with water. The aqueous layer was washed with methylene
chloride
and acidified (pH ca. 2) with 1Q% HCI. The aqueous layer was extracted with
methylene
chloride and the combined organic extracts dried and concentrated in vacuo to
afford the
title compound (3.63 g) as a yellow solid: IR (mull) 1735, 1630, 1442, 1429,
1418, 1405,
1330, 1308, 1287, 1255, 1217, I 185, 1122, 1016, 642 crri';'H NMR (300 MHz,
CDCl3) 8
1.90 (6 H), 2.24 (4 H), 2.43 (2 H}, 2.68 (3 H), 3.67 (3 H}; '3C NMR (75 MHz,
CDCl3) 8
176.3, 175.5, 173.2), 64.6, 52:0, 33.1, 32.9, 29.9, 28.41, 28.38, 26.0, 24.9;
MS (EI) m/z
(rel: intensity) 257 (M+, 1 ).
Preparation 39 and Example 106.
2-j3-j[( 1 S)-1-[[4-[(2,6-Dichlorophenyl)methoxy]phenyl]methyi]-2-rizethoxy-2-
oxoethyl]amino]-3-oxopropyl]-1--methyl-5-oxo-2-pyrrolidinepropanoic acid
methyl
ester
(Scheme G, G-5: where Rc_2 and R~~ are both equal to C:H3, RS is 4-[(2,6
dichlorophenyl)methoxy]phenyl, g is equal to 2 and ster~eochemistry is (S~).
0
N-CH3 O~~
% N~Oi
O O
CI
Or \
Cs
To a solution of acid G-3 (Scheme G, whereRG_2 and R~3 are equal to CH3 and g
is equal to
2) (1.0 g, 3.9 mmol), amine G-4 (Scheme G, where RS is 4-[(2,6-dichlorophenyl)-
methoxy]phenyl and stereochemistry is (S'~) (1.82 g, 4.67 mmol), and DMAP (100
mg, 0.8
mmol} in pyridine (15 mL) was added EDC (895 mg, 4.iS7 mmol). After 21 h, the
reaction was diluted with saturated aqueous NaHC03 and methylene chloride. The
organic
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-142-
layer was separated and washed with 10% aqueous HCI, dried and concentrated i~
vacuo.
Purification of the residue by flash chromatography using methylene
chloride/ethyl acetate
(20%) as eluant followed by lyophiiization from aqueous acetonitrile afforded
the title
compound (i.71 g} as an amorphous solid: IR (mull} 3284, 2924, 1738, 1666,
1665, 1564,
1539, 1511, 1439, 1398, 1299, 1240, 1198, 1178, 1117., 1016, 768 cm'';'H NMR
{300
MHz, CDC13) 8 2.10 (12 H), 2.66 (3 H), 3:07 (2 H), 3.66 (3 H}, 3.73 (3 H),
4.83 {1 H),
5.24 (2 H), 5.98 (1 H), 6.95 (2 H), 7:02 ( 2 H), 7.25 (1 H), 7.36(2 H);'3C NMR
(75 MHz,
CDCI3) S 174.7, 1?4.66, 173.2, 172.0, 171.2), 158.1, 137.0), 132.1, 130.5,
130.3, 128.5,
128:3, 115.2, 65.2, 64.3, 53.3, 52.4, SI.9, 37.0, 33.2, 30.2, 30.17, 28.4,
26.0, 24.7; MS (EI)
mlz (rel. intensity) 592 (M+, 4). Anal. Calcd for CZ9H34t~IzNzO,: C, 58.69; H,
5.77; N, 4.72;
CI, 11.95: Found: C, 58.33; H, 5.65; N, 4.76; Cl, 11.89.
Preparation 40 and Example 107.
2-[3-[[( I S)-1--Carboxy-2-[4-[(2;6-dichlorophenyl}methoxy]phenyl]ethyl]amino]-
3
oxo-propyl]-1-methyl-5-oxo-2-pyrrolidinepropanoic acid
(Scheme G, G-6: where RG_3 is equal to CHI, RS is 4-[{2,6-
dichlorophenyl)methoxy]phenyl, g is equal to 2 and stelreochemistry is (,f~).
0
N-CHg O
H~ ~
HO N~OH
O O
~ CI
O'
CI
To a solution of diester G-5 (Scheme G, where R~z and RG_3 are both equal to
CHa, RS is 4-
[(2,6-dichlorophenyl)methoxy]phenyl, g is equal to 2 arwd stereochemistry is
(S) (1.0 g,
1.68 mmol), in THF (30 mL) and MeOH (9 mL) was added LiOH~H20 (370 mg, 8.8
mmol). After 22.5 h, the reaction mixture was acidified with 10% aqueous HCI
(30 rnL)
causing precipitation of a solid. The solid was collected) by filtration and
lyophilized from
aqueous acetonitrile to afford the title compound (0.91 ~;) as an amorphous
solid: IR
(mull) 3031, 2925, 1727, 1637, 1585, 1564, 1543, 1511', 1439, 1424, 1404,
1299, 1240,
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-143-
1196, 1 I79, 768 crn''; 'H NMR (300 MHz, DMSO-d6) ~8 1.77 (12 H), 2.76 (1 H),
3.97
( 1 H), 4.36 ( 1 H), 5.16 (2 H); 6.94 (2 H), 7.1 S (2 H), 7.4.9 (3 H), 8.16 (
1 H); ' 3C NMR (75
MHz, CDCI3) 8 174.6, 174.1, 173.6, 172.2, 157.6; 136.~i, 132.2, 132.0, 130.7,
130.6,
129.2, 114.7, 65.3, 64.1, 54.0,.36.5, 33.7, 33.1, 30.1, 30.05, 29.8, 28.7,
25.7, 24.6; MS
S {FAB) nz/z (rel. intensity) S6S (M+H, 99). Anal. Calcd for C27H3oC12N2O7: C,
S7.3S; H,
S.3S; N, 4.95; Cl, 12.54. Found: C, 56:93; H, S.1 S; N, 5.02; CI, 12.42.
Corrected for
1.03% H20 found by Karl Fischer analysis.
Example 108.
2-[3-[[(1S)-1-[[4-[(2,6-Dichlorophenyl)methoxy]phenyl]methyl]-2-methoxy-2-
oxoethyl]amino]-3-oxopropyI]-1-(3-methyl-2-butenyl}--S-oxo-2-
pyrrolidinepropanoic
acid methyl ester
(Scheme G, G-5: where RG_Z is CH3, Re_3 is 1-(3-methyl-2-butenyl), RS is 4-
[(2,6-
dichlorophenyl)methvxyJphenyl, g is equal to 2 and stereochemistry is (S'~).
1S
o~
0
0
0 0
ci
I ~ o'
I~
c:'
Example 108 was prepared as described in Scheme G using 1-bromo-3-methyl-2-
butene to
form the requisite N-alkyl lactam. Physical data as follows: IR (mull) 32.95,
29.52, 1740,
1678, 1662, 1564, 1538, 1512, 1439, 1414, 1300, 1279, 1240, 1199, 1178, 1017,
768 cm'';
'H NMR (300 MHz, CDC13) 8 I.64 (3 H), 1.70 (3 H), 2.06 (12 H), 3.09 (2 H),
3.66 (3 H),
3.74 (2 H), 3.74 (3 H), 4.85 ( 1 H), 5.20 ( 1 H), 5.24 (2 H;y, 6.95 (2 H),
7.02 (2 H), 7.25
{1 H), 7.36 (2 H);'3C NMR (7S MHz, CDCI3) 8 174.7, 1.73.3, 172.1, 172.0,
171.3, 158.1,
137.0, 135.1, 132.1, 130.5, 130.3, 128.5, 128.3, 119.7, I 15.2, 65.2, 65.1,
53.2, 52.4, 51.9,
37.5, 37.0,. 34.4, 34.2, 30.6, 29.9, 28.6, 26.6, 25.6, 2S.S, I7.9; MS (FAB)
mlz (rel.
intensity) 647 (M+H, 24).
CA 02342778 2000-12-20
WO 99/6'7230 PCT/US99/1a233
-144-
Example 109.
2-[3-[[( I S)-1-Caxboxy-2-[4-[{2,6~iichlorophenyl)rnethoxy]phenyl]ethyl]amino]-
3
oxo-propyl]-1-(3-methyl-2-butenyl)--5-oxo-2;-pyrroIidinepropanoic acid
(Scheme G, G-b: where RG_3 is I-(3-methyl-2-butenyl), RS is 4-[(2,6-
dichlorophenyl)methoxy]phenyl, g is equal to 2 and stereochemistry is (S~).
o~
N
H O
HO N~OIi
O O.
CI
0' I \
(:I
Example 109 was prepared from example 108 by the procedure described in
preparation
40. Physical data as follows: IR (mull) 3290, 2921,1726, 1635, 1585, 1565,
1545, 1511,
1439, I4I9, 1341, 1299, 1240, 1197, I 179, 780, 768 cm-'; 'H NMR (300 MHz,
DMSO-
d() b 1.57 (3 H}, 1.62 (3 H), 1.72 (6 H); 2.02 (6 H), 2.77 (I H), 2.98 (1 H),
3.55 (2 H),
5.05 (I H), S.I6 (2 H), 6.94 (2 H), 7.15 (2 H), 7.49 (2 H;), 7.54 (2 H), 8.15
(1 H); '3C
NMR (75 MHz, DMSO-d6) $ 174.6, 174.3, 173.6, 173..'i6, 172.2, 157.6, 136.5,
134.0,
IS 132.2, 132.0, I30.6, 129.2, 121.1, 121.0, 114.7, 65.2, 65.0, 54.1, 54.0,
37.1, 36.5, 34.5;
34.1, 30.0, 29.9, 28.8, 26.3, 25.84), 25.81, 18.1; MS (FA~B) mlz (rel.
intensity} 619 (M+H,
99).
Example 1 I0.
2-[3-[[( 1 S)-1-[[4-[(2,6-Dichlorobenzoyl)amino]plhenyl]methyl]-2-methoxy-2-
oxoethyl]aminoJ-3-oxopropyl]-1-methyl-5--oxo-2-pyrralidinepropanoic acid
methyl
ester
{Scheme G, G-S: where R~_2 and RG_3 are equal to CH3, R$ is 4-[(2,6-
dichlorobenzoyl)-
amino]phenyl, g is equal to 2 and stereochemistry is (S~).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-14S-
O
v
N-CH3 O
H
O NJL,Oi
O O
0 C!
N' W
H I
C:f
Example 110 was prepared as described in Scheme G using iodomethane to form
the
requisite N-alkyl Iactam. Physical data as follows: IR (mull} 3258, 2922,
1738, 1662,
S 1606, 1561, 1539, 1SIS, 1432, 1414; 1401, 1323, 1268, 1196, 1177, 799
cm:';'H NMR
(300 MHz, CDCl3} 8 2.01 (I2 H), 2.61 (3 H), 3.09 (2 H)~; 3.64 (3 H), 3.75 (3
H}, 4.84
(1 H), 6.15 (1 H), 7.09 (2 H), 2.31 (3 H), 7.58 (2 H), 7.99 {1 H);MS (EI) mlz
(rel. intensity)
607 (M+, 4), 60S {M+, 6); Anal. CaIcd for C29H33C12N3O7: C, 57.43; H, 5.48; N,
6.93; CI,
11.69. Found: C, 57.18; H, S.S6; N, 6.85; Cl, 11.68. Corrected for 0.93% HzO,
found by
Karl Fischer analysis.
Example 111.
2-[3-[[( 1 S)-1-Carboxy-2-[4-[(2;6-dichlorobenzoyl)aminoJphenyl)ethyl]amino]--
3-oxo
propyl]-1-methyl-S-oxo-2-pyrroIidinepropanoic acid
(Scheme G, G-6: where R.~_3 is CH3, RS is 4-[(2,6-
dichlorobenzoyl)amino]phenyl; g is
1 S equal to 2 and stereochemistry is {,S~).
0
N-CH3 O
H~ J~
HO ~N~OH
O O
O CI
i
1 ~
c;!
Example 111 was prepared from example 110 by the procedure described in
preparation
40. Physical data as follows: IR (mull) 3265, 3056, 29:>.S, 1724, 1658, 1609,
IS79, 1561,
1542, 1516, 1432, 1414, 1327, 1271, 1217, 1195, 800 cW ';'H NMR (300 MHz, DMSO-
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-i46-
d6) 8 i.89 (12 H), 2.49 (3 H), 2.78 (1 H), 2.99 (1 H), 4.:38 (1 H), 7.18 (2
H}, 7.51 (S H),
8.17 (1 H), 10.64 {1 H); MS (FAB) mlz (rel. intensity) :580.5 (M+H, 68).
Example 112.
2-[3-[[(1 S)-1-[[4-[{2,6-Dichloraphenyl)methoxy]phenyl]methyl]-2-methoxy-2-
oxoethyi]amino]-3-oxopropyl]-S-oxo--2-pyrrolid:inepropanoic acid methyl ester
(Scheme G, G-5: where R~_z is CH,, R~_31S proton, RS is 4-[(2,6-
dichlorophenyl)
methoxy]phenyl; g is equal to 2 and stereochemistry is (,S~).
0
NH
O
N Oi
O O
Cl
O
C:I
Example 112 was prepared as described in Scheme G. Physical data as follows:
IR (mull)
3276, 3029, 1738, 1686, 1564, 1538, 1 S I 1, 1439, 1299, 1279, 1239, 1197,
1178, 1016,
767, cm'; 'H NMR (300 MHz, CDCl3) 8 1.8S {6 H}, 2.2.4 (2 H), 2.35 (4 H), 3.03
(2 H),
3.66 (3 H), 3.74 (3 H), 4.82 {1 H), 5.24 (2 H), 6.50 (2 Hj, 6.95 (2 H), 7.05
(2 H), 7.27
I5 (1 H}, 7.37 (2 H); MS (EI) mlz (rel. intensity) 578 (M+, 0.2); Anal. Calcd
fox
Cz$H3zC12N2O7: C, 58.04; H, 5.57; N, 4.83. Found: C, ST.93; H, 5.43; N, 4.97.
Corrected
for 1.14 % H20 found by Karl Fischer analysis.
Example i 13.
2-[3-[[(1 S)-1-Carboxy-2-[4-[{2,6-dichlorophenyl)methoxyjphenyl]ethyl]amino]-3-
oxo-propyl]-5-oxo--2 pyrrolidinepropanoic acid
(Scheme G, G-6: where RG_3 is proton, RS is 4-[(2,6-
dichlorophenyl)methoxy]phenyl, g is
equal to 2 and stereochemistry is (,S~).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/I4233
-147-
0
NH O
H ~
HO ~N~OH
O O
I ~ CI
o- w
I~
(.~
Example 113 was prepared from example 112 by the procedure described in
preparation
40. Physical data as follows: IR (mull) 3294, 3033, 1716, 1647, 1585, 1565,
1544, 1511,
1439; 1420, 1299, 1240, 1197, 1179, 768 crri'; 'H NMR; (300 MHz, DMSO-d6) 8
1.60
(6 H), 2.12 (6 H), 2.78 ( I H), 2.99 ( 1 H), 4.36 ( i H), 5.19 (2 H), 6.97 (2
H), 7.17 (2 H),
7.45 (1 H), 7.58 (2 H), 7.70 (1 H}, 8.22 (1 H); MS {FAE~} m/z (rel. intensity)
551 (M+H,
99); Anai. Calcd for C26HZgCIzNZO7: C, 56.63; H, 5.12; Cl, 12.86; N, 5.08.
Found: C,
56.28; H, 5.01; Cl, 13.08; N, 5.24. Corrected for 1.47% Hz0 found by Karl
Fischer
analysis.
Example 114.
2-[3-[[( 1 S}- I -[ [4-[(2, 6-Dichlorobenzoyl)amino]phenyl]methyl]-2-methoxy-2-
oxoethyl]amino]-3--~xopropyl]-5-oxo-2-pyrrolidiinepropanoic acid methyl ester
{Scheme G, G-5: where Ro_z is CHI, RG_3 is proton, RS is 4-[{2,6-
dichlorobenzoyi)-
amino]phenyl, g is equal to 2 and stereochemistry is (S}}.
0
~H O
O ~N~Oi
O O
O CI
~N~
H I
(:I
Example 114 was prepared as described in Scheme G. Physical data as follows:
'H NMR
(300 MHz, CDCI3) 8 1.81 (6 H), 2.27 (6 H), 3.10 (2 H), 3.63 (3 H); 3.75 (3 H),
4.89 (I H),
6.46 (I H), 6.58 (1 H), 7.10 (2 H), 7.26 (3 H), 7.58 (2 H), 8.14 (1 H); MS
(FAB) m/z 592
(M+H)+, 568, 367, 349, 307, 278, 226, 194, 173.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-I48-
Example 115.
2-[3-[j( 1 S)- I-Carboxy-1-[4-j(2,6-
dichlorobenzolyl).amino]phenyl]ethyl]amino]-3-
oxopropyl]-5-oxo-2-pyrrolidinepropanoic acid
(Scheme G, G-6: where R.~_3 is proton, RS is 4-[(2,6-
dichlorobenzoyl)amino]phenyl, g is
equal to 2 and stereochemistry is (f~).
0
NH
H O
HO N""OH
O O I W O C1
N~
H ( ,
C;I
I0
Example i 15 was prepared from example 1 I4 by the procedure described in
preparation
40. Physical data as follows: IR (mull) 3272, 3195, 3 /:21, 3063, 2953, 2923,
286$, 2855,
/715, 1659, 1608, /579; 1561, 154/, 15/6, 1456, /432, 1414, 1377, 1367, 1326,
1271,
1221, i 195, 800 cm-I; 'H NMR (300 MHz, DMSO-ds ~i 1.63 {6 H), 2.13 (6 H),
2.79 (1
I 5 H), 3 .00 ( I H), 4.3 8 ( I H), 7.19 (2 H), 7.51 (5 H), 7.64 ( 1 H), 8.16
( I H), 10.5 8 ( 1 H),
12.37 {2 H); MS (FAB) mlz 564 (M+H)+; 546, 519, 33'.i, 280, I94, 173. Anal.
Calc'd for
CZ6H27C12N3O7: C, 55.33; H, 4.82; Cl, 12.56; N, 7.44. Found: C, 55.10; H,
4.76; Cl,
12.56; N, 7.36. Corrected for 2.49% H20, found by Karl Fischer analysis.
Example I16.
20 2-[3-jj(1ST-1-j[4-[(2,6-Dichlorophenyl)methoxy]phenyl]methyl]-2-methoxy-2-
oxoethyl]amino]-3-oxopropyl]-5-oxoproline
(Scheme G, G-5: where R~_2 and RG_3 are equal to proton, RS is 4-[(2,6-
dichlorophenyl)-
mathoxy]phenyl, g is equal to 0 and stereochemistry is (~).
CA 02342778 2000-12-20
WO 99167230 PCTlUS99/14233
-149-
0
NH
HO O N~Or
O
I ~ CI
O~
rl
CI
Example I 15 was prepared as described in Scheme G from 2-carboxy-5-oxo-2-
pyrrolidinepropanoic acid (Majer, Z.; Kajtar, M.; Tichy, M.; Blaha, K. Coll.
Czech. Chem.
Commun. 1982, 47, 950). Physical data as follows: IR (mull) 3302, 1736, 1671,
1612,
1S8S, 1564, 1S3S, 1511, 1439, 1298, 1240, I 197, 1179, 1016, 768 crn';'H NMR
(300
MHz, CDC13} S 2.12 (8 H), 3.02 (2 H), 3.65 (3 H}, 4.81 ('1 H), 5.21 (2 H),
6.94 (2 H), 7.07
(2 H), 7.22 (1 H}, 7.35 (2 H), 7.86 (1 H), 8:34 (I H); MS (FAB) m/z {rel.
intensity) 537
(M+H, 99).
Example 117.
2-[3-[[( 1 S)- I-Carboxy-2-[4-[(2,6-
dichlorophenyl)m:ethoxy]phenyl]ethyl]amino]-3
oxo-propyl]-5-oxoproline
{Scheme G. G-6: where R~.3 is proton, RS is 4-[(2,6-
dichlorophenyl)methoxy]phenyl, g is
equal to 0 and stereochemistry is (,S~).
0
NH 0
HO ~N
OH
O
C~
"' 'O
CI
Example 116 was prepared from example 1 I 5 by the procedure described in
preparation
40. Physical data as follows: IR (mull} 3292, 3059, 3029, 1718, 1650, 1612,
1585, 1565,
IS37, 1511, 1439, 1240, 1196, 1179, 768 crri';'H NMR (300 MHz, CDC13) 8 2.I 1
(8 H),
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/I4233
-150-
2.83 ( 1 H), 3.20 ( 1 H), 4.74 ( 1 H), 5.24 (2 H), 6.95 (2 H), 7.15 (2 H),
7.25 ( 1 H), 7.36 (2
H); MS (FAB) m/z (rel. intensity) 523 (M+H, 99); Anal: Calcd for
Cz4HzaC12Nz07: C,
55.08; H, 4.62; N, 5.35, Cl, 13.55. Found: C, 54.68; H, 4.66; N, 5.13: Cl,
13.70.
Corrected for 1.59% H20 found by Karl Fischer analysis.
Scheme H.
off
1 ~ H-~
N CI
OH
I i H_2
i N CI
CI
I
~I CI H_3
ICI
CI
w O w I
I N "CI CI
Boc~N O~ H-4
H O
CI
w 0 w I
I N "Ct CI
H-5
H2N Ow
O ~ 2 HCI
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-151-
Scheme H teaches a genexal method for the preparation of 6-chloroazatyrosine
examples
of structures H-4 and H-S, by adaption of he methodology for the preparation
of protected
azatyrosine reagents as described by Kawata, S.; Ashizawa, S.; Hirama, M. J.
Am. Chem.
Soc. 1997, 119, 12012-12013 and references cited therein. Thus regioselective
iodination
of 2-chloro-3-pyridinol gives the chloro-iodopyridinol ~3f-2, that is O-
alkylated as
exemplified by the synthesis of H-3. Palladium-catalyzed reaction of H-3 with
the
organozinc derived from a suitably protected (3-iodoalanine, provides the
protected 5-
chloroazatyrosine H-4. N deprotection of H-4 gives the ;aminoester H-S, that
is used (as
I O exemplified by reagent A-4 of Scheme A, and by reagent B-5 of Scheme B)
for the
synthesis of Examples of this invention
Preparation 4I .
(Scheme H: H-2)
2-Chloro-G-iod0-3-pyridinoi (CSH3C1IN0).
To a solution of 2-ehloro-3-pyridinol H-1 (10.2, g, 78.7 mmol) and K2C03 (38.9
g, 0.274 mol) in H20 (120 mL) is added I2 (24.3 g, 95.8 mmol), and the
reaction mixture
is stirred at rt for 4 h. The reaction mixture is quenched by the addition of
aq satd
Na2S203~5H20, and its pH is lowered to pH 2 with the addition of I2 M aq HCi.
The
mixture is extracted with EtOAc. The combined EtOAc extracts are dried,
filtered and
concnetrated to a yellow solid, that is crystallized from 120:25 heptane/EtOAc
(145 mL)
to give, as a yellow solid, 11.2 g of the title compound: 1 H NMR (CD3 SOCD3,
300
MHz) 8 9.87 (IH), 7.59 {1H), 7.06 (1H); 13C NMR (CD3SOCD3, 75 MHz) 8 150.68,
138.07, 134.98, 127.02, 101.18.
Preparation 42.
(Scheme H: H-3)
2-ChlorO-3-((2,6-dichlorophenyl)methoxy]~-iodop;yridine (C12H7C13IN0).
To a solution of H-2 (5.11 g, 20.0 mmol), PPh3 (5.30 g, 20.0 mmol), and 2,6-
dichlorobenzylalcohol (3.54 g, 20.0 mmol) in dry THF ( 100 mL) at 0 °C
under Ar is added
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-152-
DEAD (3.15 mL, 20.0 mmol). The reaction mixture is kept at 0 °C for 1.5
h and at rt for
1.5 h. It is concentrated to a residue, that is purifed by silica flash
chromatography (17:3
hexanesIEtOAc) to give 7.61 g of the title compound: T'LC (17:3 hexanes/EtOAc)
R f0.57;
IH NMR (CD3SOCD3, 300 MHz) b 7.85 (1H}, 7.62 (1H), 7.58-7.45 (3H), 5.34 (2H}.
Preparation 43.
{Scheme H: H-4}
(S)-2--Chloro-3-[(2,G-dichlorophenyl)methoxy]-a-~;[(1,1-dimethylethoxy)-
carbonyI]amino)-6-pyridinepropanoic acid methyl ester (C21H23C13N205)~
To an amberized flask containing activated Zn dust (0.777 g, 1 i .89 mmol)
under
I0 Ar is added sequentially N-[(l,l-dimethylethoxy)carbonylJ-3-iodo-t,-alanine
methyl
ester [93267-04-OJ (3.91 g, I.89 mmol), THF (11.9 mL,), and dimethyiacetamide
(11.9
mL). The reaction mixture was purged of 02 by the bubbling of Ar through the
mixture
for 5 min. It is stirred at 655 °C for 2 h, and is cooled t:o rt. To
this mixture is added
PdCl2(PPh3)2 (0.412 g), followed immediately afterward by a degassed solution
of H-3
15 {2.46 g, 5.94 mmol} in 1:1 THF/dimethylacetamide (1 I..8 mL). The reaction
mixture is
stirred at 655 °C for S h. it is cooled to 0 °C and quenched
with sat'd aq NH4Cl {100
mL). The reaction mixture is extracted with EtOAc. The: combined extracts are
washed
with brine, dried, filtered and concentrated to a green-yellow oiI; that is
purified by silica
flash chromatography to give 1.90 g of the title compound: TLC (7:3
hexanes/EtOAc) R f
20 0.32; I H NMR (CDCl3, 300 MHz) b 7.76 ( i H), 7.57 (2H), 7.48 (1 H), 7.29 (
1 H}, 7.27
( 1 H); 5.30 (2H), 4.32 ( 1 H), 3.b0 (3 H), 3.0 I ( 1 H), 2.98 ( 1 H), 1.31
(9H).
Preparation 44.
(Scheme H: H-4)
(S')--2-Chloro-3-[(2,6-dichlorophcnyl)methoxy]-~c-amino-6-pyridinepropanoic
acid
25 methyl ester dihydrochloride salt (CI6HISC13N203'~ZHCI).
A solution of H-4 (1.90 g, 3.88 mmol) in 4 M H:CI in dioxane (35 mL) is
stirred at
rt under Ar for 20 h. The reaction mixture is concentrated in vacuo. The
residue is taken up
in H20 (40 mL), and extracted with Et20. The aqueous solution is frozen and
lyophilized
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-153-
to give I .39 g of the title compound: I H NMR {CD3SO~CD3, 300 MHz) 8 8.62
(3H), 7.81
{1H), 7.58 {2H); 7.48 (IH), 7.38 (1H), 5.32 (2H), 4.37 (IH), 3.72 (3H), 3.27
(2H).
Example 118.
(Scheme A, A-5)
S [S-(R*,R*)]-4-[[[1-[[2-Chloro-3-[(2,6-dichloropheruyl)methoxy]-6-
pyridyl]methyl] Z-methoxy-2-oxoethyi]amino]carbonyl] 3-thiazolidinecarboxylic
acid {l,l-dimethylethyl) ester (C30H37C13N306S).
Example 118 was prepared as described in Scherne A from D-cysteine using the
product of preparation 44 as amino acid intermediate A-.4. Physical properties
as follows:
TLC (1:1 hexanes/EtOAc) R f0.27; 1H NMR {CDC13, 300 MHz) b 8.43 {1H), 7.74
(IH),
7.58 (2H), 7.48 {1H); 7.29 {1H), 5.29 (2H), 4.67 (1H), 4..53 (1H), 4.44 (IH),
4.23 (1H),
3.62 (3H), 3.06 (3 H), 2.82 ( 1 H), 1.27 (9H}.
Example 119.
(Scheme A, A-9)
(S-{R';R*)]-4-((1-Carboxy-2-[[2-Chloro-3-[(2,6-dichlorophenyl)methoxy]-&-
pyridyi]ethyl]amino]carbonyl]-3-thiazolidinecarbox;ylic acid (1,1-
dimethylethyl)
ester (C24H26CI3N306S).
Example 119 was prepared from example 118 by the pra~cedure described in
preparation
12. Physical data as follows: mp 93-95 °C; TLC (600:400:2
EtOAc/hexanes/HC02H) R f
0.38; IH NMR (CD3SOCD3, 300 MHz) 8 8.30 (1H}, 7.'74 (1H), 7.5'7 (2H), 7.48
(1H),
7.27 ( 1 H), 5.29 (2H), 4.67-4.36 (2H); 4.53 ( I H), 4.23 ( 1 H), 3.37-3.11 (3
H), 2.97 ( 1 H),
1.27 {9H).
CA 02342778 2000-12-20
WO 99167230 PCTIUS99/14233
-154-
Scheme i.
OH
I I N CI I 1
O O
i ~ N Ci v
0 O
N "CI
Boc.N O~ I-3
H O
0
~NJ
Boc.H O~ I~d
O
OH
I N'
Boc,N O~ I-S
H O
CI
O
~ NJ
Boc.N O~ t-6
H O
CI
O ~ I
~NJ
O I=7
HyN
O ~2HCI
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-155-
Scheme I teaches a general method for the preparation o:P azatyrosine reagents
I-3, I-4, I-5,
I-6 and I-7, by adaptation of the methodology for the preparation of protected
azatyrosine
reagents as described by Kawata, S.; Ashizawa, S.; Hira~na, M. J. Am. Chem.
Soc. 1997,
119, 12012-12013 and references cited therein. Thus O-protection of
chloroiodopyridinoi
S I-1 (identical to H-2) is followed by reaction with the or;ganozinc, derived
from a suitably
protected j3-iodoalanine, to provide the protected 6-chloroazatyrosine I-3.
Reductive
dehalogenation of I-3 gives I-4, that is D-deprotected to give I-5. Reagent I-
5 is O-
alkylated, as exemplif ed by the preparation of I-6. N de;protection of I-6
gives the
aminoester I-7, that is used (as exemplified by reagent A-4 of Scheme A, and
by reagent
B-5 of Scheme B) for the synthesis of Examples of this invention.
Preparation 45.
(Scheme I, I-2)
(f}-2-Chloro-3-[(2-tetrahydropyranyI)oxyj--6--iodopyridine (C I OH I 1
C1IN02).
To a solution of chloroiodopyridinol I-1 (same as H-2, the product of
preparation 41 )
IS (1.00 g, 3.91 mmol) and dihydropyran (1.0 mL, 10.6 mrnol) in CH2C12 (10 mL}
under Ar
at rt is added pyridinium chloride (0.050 g). The reactiorA mixture is stirred
for 72 h. It is
diluted with CH2Cl2, and is washed with satd aq NaHCO3 and brine. The CH2Cl2
solution is dried, filtered and concentrated to an oii, that is purified by
silica flash
chromatography (19:1 hexanes/EtOAc) to give 1.06 g of the title product: TLC
(19:1
hexanes/EtOAc) R f0.24; 1H NMR (CDC13, 300 MHz) Ci 7.55 (1H), 7.17 (IH), 5.50
(1H),
3.77 (1H), 3.61 (1H), 2.07-1.57 (6H).
Preparation 46.
(Scheme I, I-3)
(S~-2-Chloro-a-[[(1,1-dimethylethoxy)carbonyl]amino] 3-[(2-tetrahydro-
pyranyl)oxy)-6-pyridinepropanoic acid methyl ester (CI9H27C1N206).
To an amberized flask containing activated Zn dust (0.349 g, 5.51 mmol) under
Ar is
added THF (2 mL) and 1,2-dibromoethane (0.018 mL, 0.2I rnmol). The suspension
is
CA 02342778 2000-12-20
WO 99!67230 PCT/US99/14233
-156-
brought to reflux for several minutes, cooled to approximately 30 °C,
and TMSCI (0.17
mL of a 1 M solution in THF) is added. The reaction mixture is stirred at 405
°C for 30
min and then is cooled to below rt. A solution of N-j(l,lL-
dimethylethoxy)carbonyl]-3-
iodo-t,-alanine methyl ester [93267-04-0] (1.81 g, S.SO mmol) in 11:7
dimethyiacetamide/THF (9.0 mL) is added, and the resulting reaction mixture is
stirred at
45 °C for 5 h. The reaction mixture is cooled to below rt, and solid
PdCl2(PPh3)2 (0.192
g) is added, followed immediately by addition of a degassed solution of the
iodopyridine
(0.936 g, 2.76 mmol) in I :1 THF/dimethylacetamide (5.6 mL). This reaction
mixture is
stirred for 4 h at 45 °C. It is cooled to 0 °C, quenched with
sat'd aq NH4Cl, and extracted
with EtOAc. The combined EtOAc portions are washed with sat'd aq NH4C1 and
brine.
The EtOAc solution is dried, filtered and concentrated to give a green-yellow
colored
foam, that upon purification by silica flash chromatography (7:3
hexanes/EtOAc) gives
0.879 g (I.85 mmol, 60%) of the title product: TLC (7:3 hexaneslEtOAc} R
f0.21; IH
NMR (CDC13, 300 MHz) d 7.39 ( 1 H), 7.00 ( 1 H), 5.46 ( 1 H), 4.61 ( I H),
4.13 ( I H), 3.80
I S (3 H), 3.62 ( 1 H), 3.20 ( I H), 2.13-1.53 (6H), I .42 {9H}.
Preparation 47.
(Scheme I, I-4)
(S)-a.-[[(I,I-Dimethylethoxy)carbonyl]amino]-3-[(2-.tetrahydropyranyl)oxy]-6-
pyridinepropanoic acid methyl ester {C19H28N2O6).
A suspension of pre-reduced Pd/CaC03 (3.5 g) and I-31;1.15 g, 2.77 mmol) in
EtOH (40
mL) is hydrogenated (30 psi H2) for I9 h at rt. The mixtv:~re is filtered, and
the filtrate is
evaporated to give a yellow-colored foam, that is purified by silica flash
chromatography
(600:400:I hexanes/EtOAc/iPrOH) to give 0.367 g ofthe title compound: TLC (I:1
hexanes/EtOAc) R j0.27; 1H NMR (CDCl3, 300 MHz) 8 8.30 (1H), 7.29 (1H), 7.03
(1H),
5.81 (1H}, 5.39 (1H), 4.65 {1H), 3.86 {IH); 3.73 (3H), 3.fi2 (1H}, 3.21 (2H),
1.96-1.53
(6H), 1.42 (9H).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-157-
Preparation 48.
(Scheme I, I-5)
(Sj--a,-[[{l,l-Dimethylethoxy)carbonyl]amino]-5.-hydroxy-2-pyridinepropanoic
acid
methyl ester (C14H20N205)~
A solution of I-4 (0.346 g, 0.91 mmol) and pyridinium ~rtoluenesulfonate
(0.031 g, 0.12
mmol) in EtOH (8 mL) is stirred at 555 °C for 20 h. The reaction
mixture is cooled to rt,
and concentrated in vacuo. The residue is taken up in Et~~Ac. The solution is
washed with
brine, and dried, f ltered and concentrated to a pale yellow-colored oil that
is purified by
silica flash chromatography (500:500:1 hexanes/EtOAc/iPrOH). Evaporation of
the
column fractions gives 0.132 g of the title compound: TLC (1:1 hexanes/EtOAc)
R f0.18;
1H NMR (CDCI3, 300 MHz) 8 8.I3 (1H), 7.13 (1H), 7.03 (1H), 5.71 (1H}, 4.65
(1H),
3.70 (3H), 3.20 (2H), 1.39 (9H).
Preparation 49.
(Scheme I, I-6)
(.S'}~-5-((2,G-Dichiorophenyl)methoxy)-oc-(((1,1-
dimethylethoxy)carbonyl)amino] 2-
pyridinepropanoic acid methyl ester (C21H24C12N205).
To a solution of I-5 (0.126 g, 0.43 mmol}; 2,6~iichlorobenzylalcohol (0.075 g,
0.43
mmol) and PPh3 (0.113 g, 0.43 mmol) in dry THF (4 ml.) at 0 °C under Ar
is added
DEAD (0.068 mL). The reaction mixture is permitted to warm to rt, and is
stirred for 18
h. It is concentrated, and the residue is purif ed by silica flash
chromatography (700:300:1
hexanes/EtOAc/iPrOH) to give 0.149 g of the title compound: TLC (7:3
hexanes/EtOAc)
R f0.34; 1 H NMR (CDCl3, 300 MHz) 8 8.31 ( 1 H), 7.37 (2H), 7.25 (2H), 7.08 (
1 H), 5.81
{ 1 H), 5.29 (2H), 4.65 ( 1 H), 3.70 (3H), 3.24 (2H), i .63 ( 1 H), 1.43 (9H).
Preparation 50.
(Scheme I, I-7)
(S}-a,-Amino-a-[(2,6-dichlorophenyl)methoxy] 2-p;yridinepropanoic acid methyl
ester dihydrogen chloride salt (C16H16C12N203~2HC.'1).
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-158-
A solution of carbamate I-6 (0.546 g, 1:20 mmol) in 4 M HCl in dioxane (12 mL)
is
stirred at rt under Ar for 16 h. The reaction mixture is concentrated ih
vacuo. The residue
is dissolved in H20 (40 mL), and this solution is extracted with Et20. The
aqueous
solution is frozen and lyophilized to give, as a light yellow-colored solid,
0.485 g of the
title compound: 1H NMR (CD3SOCD3, 300 MHz) 8 8.'75 (3H), 8.47 (1H), 7.81 (1H),
7.57 (3H), 7.48 (1H), 5.35 (2H), 4.49 (1H), 3.67 (3H), 3.42 (2H).
Example 120.
(Scheme A, A-5)
[S-{R*,R*)]-4-[[[I-[[3-[{2,6-Dichlorophenyl)methoxy]-6-pyridyl]methyl] 2-
methoxy-2-oxoethyi]amino]carbonyl]-3-thiazolidinecarboxyiic acid (I,I-
dimethylethyl) ester (C25H30C12N306S).
Example 120 was prepared as described in Scheme A from D-cysteine using the
product of
preparation 49 as amino acid intermediate A-4. Physical properties as follows:
TLC (1:1
hexanes/EtOAc) R~ 0.22; 1H NMR (CDCl3, 300 MHz) 8 8.28 {2H), 7.38 (2H), 7.28
(2H),
7.09 (1H), 5.29 (2H), 4.90--4.74 (3H), 4.40 (IH), 3.67 (3H), 3.38-3.22 (3H),
1.6I (2H),
1.40 (9H).
Example 121.
(Scheme A; A-9)
[S-{R*,R*)]-4-[[[1-Carboxy 2-(3-[(2,6-dichlorophenyi)methoxy]-6-
pyridyl]ethyl]amino]carbonyl]-3-thiazolidinecarbox;ylic acid (1,1-
dimethylethyl)
ester (C24H2gC12N3O6S).
Example 121 was prepared from example 120 by the procedure described in
preparation
12. Physical data as follows: mp 92-94°; TLC (500:500:3
hexanes/EtOAc/HC02H) R f
0.10; 1H NMR (CDC13, 300 MHz) 8 8.31 (1H), 8.26 (1H), 7.55 (2H), 7.28 (2H),
7.46
( 1 H), 7.2I ( 1 H), 5.25 (2H}, 4.72-4.38 (2H), 4.60 ( 1 H), 4.23 ( 1 H), 3.21-
3.12 (2H), 3.09-
2.94 ( 1 H), 2.74 ( 1 H), I .29 (9H).
CA 02342778 2000-12-20
WO 99/6'7230 PCT/US99/14233
-159-
Scheme J.
NHZ
I ~ ~ N J-1
CI
O CI
N O CI J-2
I ~ ~ CI / ~
CI /
O ' CI
i N N O CI J_3
CI /
Boc~N O~
N O
J-4
H2N
lHti1
Scheme J teaches a general method for the preparation ofpara-acylamino
derivatives of
aza-phenylalanine. Thus bis-acylation of 2-amino-5-iodopyridine J-1 gives the
imide J-2,
that is reacted with the organozinc, derived from a suita>:dy protected ~3-
iodoalanine, to
provide the protected acylamino azaphenylalanine J-3. l~J deprotection of J-3
gives the
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-160-
aminoester J-4, that is used (as exemplified by reagent E~-4 of Scheme A, and
by reagent
B-5 of Scheme B) for the synthesis of Examples of this;invention.
Preparation 51.
{Scheme J: J-2)
2-[[Bis(2;trDichlorobenzoyl)]amino]-5-iodopyridina (CI9H9C1;IIN202).
To a solution of 2-amino--5-iodopyridine J-I (2.20 g, 10.0 mmol) and Et3N
(2.I2 mL,
I5.0 rnmoi) in dry THF (I00 mL) at rt under Ar, is added dropwise 2,6-
dichlorobenzoylchloride (1.60 mL, 1 I.0 mmoi) over 45 min. The reaction
mixture is
stinted, for 15 h. It is diluted with EtOAc (300 mL), and its washed with cold
aq 1 M NaOH
and brine. The solution is dried, filtered and concentrated to give a yellow-
colored waxy
solid, that is purified by silica flash chromatography (3:1 hexanes/EtOAc) to
give 2.60 g of
the title compound: TLC (7:3 hexanes/EtOAc) Rf 0.60; 1H NMR (CDCl3, 300 MHz) 8
8. S 9 ( I H), 8 .03 ( 1 H), 7.99 ( 1 H), 7.44-7.26 (6H).
Preparation 52.
(Scheme J: J-3)
(S~-2-[[Bis--(2,6-dichlorobenzoyl)]amino]-oc-[[(1,1-
dimethylethoxy)carbonyl]amino]-5--pyridinepropanoic acid methyl ester
{C28H25C14N3~6)~
To an amberized flask containing activated Zn dust (0.8ti5 g, 13.23 mmol)
under Ar is
_ added sequentially N-[(I,1-dimethylethoxy)carbonyl]-3-iodo-~-alanirie methyl
ester
(4.36 g; I3.23 mmol), THF (13 mL) and N,N-dimethylacetamide {13 mL). The
reaction
mixture is purged of 02 by the bubbling Ar through the mixture for 5 min, and
then is
warmed to 4515 °C for 7 h. It is cooled to rt. To this mi~;ture is
added PdCl2(PPh3)2
{0.461 g) followed immediately by a degassed solution of iodide J-2 (2.60 g,
4.59 mmol)
in 1:I THF/N,N-dimethylacetamide (I8 mL). The reaction mixture is stirred at
455 °C
under Ar for 13 h. It is cooled to 0 °C and quenched with satd aq NH4CI
( 1 SO mL). The
mixture is extracted with EtOAc. The combined EtOAc extracts are washed with
brine,
dried, filtered and concentrated to a green-yellow~olore;d paste, that is
purified by silica
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/I4233
-161-
hash chromatography (700:300:1 hexanes/EtOAc/iPrOH) to give 1.43 g of the
title
compound: TLC (7:3 hexanes/EtOAc) R~0.29; 1H NMR (CDC13, 300 MHz) 8 8.13 (1H),
7.57 { 1 H), 7.46 ( 1 H), 7.26 (6H), 4.80 (1 H), 4.50 { 1 H), 3.67 (3H), 3.05
(2H), 1.46 (9H).
Preparation 53.
(Scheme J: J-4}
{S)-a.-Amino-2-[[bis-(2,6--dichlorobenzoyi}]amino]-.5--pyridinepropanoic acid
methyl ester dihydrochloride salt (C23H1~C14N304~HfC1).
A solution of J-3 (0.69 g, 1.08 mmol) in 4 M HCI in dio:Kane (15 mL) is
stirred under Ar
for 20 h. The reaction mixture is concentrated in vacuo, dliiuted with H20,
and extracted
with Et20. The aqueous solution is frozen and lyophilized to give, as a pale
yellow-
colored solid, 0.627 g of the title compound: I H NMR (C;D3SOCD3, 300 MHz) 8
8.80
(2H), 8.27 ( 1 H), 7.82 ( 1 H), 7.67-7.26 (7H), 4.25 ( 1 H), 3.52 (3 H}, 3.16
(2H), 3.04 ( 1 H);
MS {ESl+) m/z 541.7.
Example 122.
1 S {Scheme A, A-5)
(S-(R *,R*)J-4-[[[1-[(2-[[Bis-{2,6~iichlorobenzoyl)]a~minoJ-5-pyridyl]methyl]-
2-
methoxy-2-oxoethyl]amino]carbonylJ-3-thiazolidinecarboxylic acid (1,1-
dimethylethyl) ester (C~SH30C12N306S).
Example 122 was prepared as described in Scheme A from D-cysteine using the
product of
preparation 52 as amino acid intermediate A-4. Physical properties as follows:
TLC (l:l
hexanes/EtOAc) R f0.22; 1H NMR (CDC13, 300 MHz) 8 8.28 {2H), 7.38 (2H), 7.28
(2H),
7.09 (1H), 5.29 (2H), 4.90--4.74 {3H}, 4.40 (IH), 3.67 (3H), 3.38-3.22 (3H),
1.61 (2H),
1.40 (9H).
Example 123.
(Scheme A, A-9)
[S-{R*,R*)]--4-(([[1-Carboxy-2-(2-[[Bis-{2,6-tiichloro~benzoyl)]amino]-5-
pyridyl]]ethyl]amino]carbonyl] 3-thiazolidinecarboxyiic acid (1,1-
dimethylethyl)
ester (C31 H'8C14N407S).
CA 02342778 2000-12-20
WO 99/67230 PCTIUS9911~233
-162-
Example 123 was prepared from example 122 by the procedure described in
preparation
12. Physical data as follows: mp 158-160°; TLC (50:SC1:2
hexanes/EtOAc/HC02H) Rf
0.18: 1 H NMR (CD3 SOCD3, 300 MHz) 8 8.42 ( 1 H), 8.22 ( 1 H), 7.75 ( 1 H);
7.69-7.17
(5H), 7.53 ( 1 H); 4.59 ( 1 H), 4.40 (2H), 4.19 ( 1 H), 3.19-3 .02 (2H), 2.84
( 1 H), 2.56 ( 1 H),
1.34 (9H).
Scheme K.
~os~
C
N K_1
,Y O
R3
R5
HzN~O~ !<-2
O
O O
O~
O ~ K-3
Rg Y R5
O-.,, H O
L ~N~ON
N K-4
R Y O Rs
Scheme K teaches a general method for the preparation of oxazolidinecarboxylic
acid
Examples K-3 and K-4, where R,, RS and Y are identical to the definitions of
Scheme B.
Thus coupling of oxazolidinecarboxylic acid K-1 and aminoester K-2 (as
exemplified by
the reaction of reagents A-3 and A-.~ of Scheme A, and B-4 and B-5 of Scheme
B)
provides Examples K-3, that are hydrolyzed to Examples K-4 of this invention.
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-163-
Example 124.
(Scheme K: K-3, where R3 is {phenyl)methyl, Rs is 4-[(2,6-
dichlorobenzoyl)amino]phenyl,
Y is CO, and the stereochemistry is [S-(R *, R *)])
[S (R*,R*)]-4-[([Z-[[4-((2,6-Dichlorobenzoyl)amino]pHenyl]methyl]-2-methoxy-2-
oxoethyl]amino]carbonyl]-3-oxazolidinecarboxylic acid 3-phenylmethyl ester
(C29Hz~C1zN30,}.
Example 124 was prepared as by coupling commercially available (S)-3,4-
oxazolidinedi-
carboxylic acid 3-{phenylmethyl) ester to amino acid K-a (Scheme K, where RS
is 4-[(2,6-
dichlorobenzoyl)amino]phenyl stereochemistry is (S)) under the conditions
described by
preparation 3. Physical properties as follows: TLC (3:2 Heptane/EtOAc) Rf=
0.17; UV
(MeOH) lmax 225 (e 12600, sh), 251 (17900); "C NMR (db dimethylsulfoxide) 8
188.75,
171.96, 169.86, 162.40, 162.30, 152.99, 137.59, 137.47, 136.87, 136.81,
133.23, 131.79,
131.65; 130.08, 128.80, 128.67, 128.28, 127.76, I 19.84, 119.75, 79.82, 66.77,
57.85,
53.85, 52.47, 36.62 (23 lines expected; 26 lines observed); MS (FAB) m/z 602,
600, 558,
556, 531; 466, 371, 351, 349, 280, 278, 175, 173; MS (F,AB} m/z 600:1312
(calcd [M+H]+
600.1304; Anal. C, 57.75; H, 4.75; N, 6.80; Cl, 11.86 (calcd for 0.42% H20: C
57.77, H
4.56, N 6.97, CI 11.76).
Example 125:
(Scheme K: K-4 where R3 FS (phenyl)rnethyl, RS is 4-[(2.,6-
dichlorobenzoyl)amino]phenyl,
Y is CO, and the stereachemistry is [S (R*,R*)])
[S (R*,R*)]-4-[[[1-Carboxy-2-[4-[(2,6-
dichlorobenzoy!!)amino]phenyt]ethyl]amino]-
carbonyl]-3-oxazolidinecarboxylic acid 3-phenylmeth;yl ester (CzgH25C1zN30~).
Example 125 was prepared from example 124 by the procedure described in
preparation
12.
Physical properties as follows: TLC {950:50:1 CH,CI,/MeOH/HCOzH} Rf= 0.34;'H
NMR (db-dimethylsulfoxide) 8 IO.bS (1H); 8.31 (1H), 7.61-7.42 (SH), 7.40-7.20
(SH),
7.15 (2H), 5.19-4.85 (2H), 4:90 ( 1 H), 4.76 ( 1 H), 4.43 ( 1 H), 4.36 ( 1 H),
4.11 ( 1 H), 3 .65
(1H), 3.04 (1H), 2.87 (1H); MS (FAB) m/z 588, 586 544,. 542, 532; 391, 337,
335, 327,
269, 267, 161, 147.133, 129, 117, 1 I5, 103, 101, 91: MS (FAB) 586.1132 (calcd
586.1147).
CA 02342778 2000-12-20
WO 99/67230 PCTIU599/14233
-164-
Example 126.
{Scheme K: K-3 where R, is benzyl, RS is 4-[(2,6-dichlorobenzoyl)amino)phenyl,
Y is
C02 and the stereochemistry is [R-(R*.S*)])
[R-(R*,S*)]-4-[[[1-[[4-~(2,6-Dichlorobenzoyi)amino]pHenyl]methyl]-2-methoxy-2-
oxoethyl]amino]carbonyl]-3-oxazolidinecarboxylic acid 3-phenylmethyl ester
(C29H27C12N3~7)~
Example 126 was prepared as described in Scheme K from commercially available
(R)-
3,4-oxazolidinedicarboxylic acid 3-(phenyimethyl) ester. Physical properties
as follows:
TLC (3:2 heptane/EtOAc) R f= 0.19; UV (MeOH) ~.,"~ 2:2,5 (e 12400, sh), 252 (
17700),
284 {2960, sh);'3C NMR (CDCI3) & 171.23, 169.14, 162.43, 154.26, 136.47,
135.93,
135.65, 132.37, 132.28, 130.91, 130.00, 128.62, 128.39, 128.1 l, 120.30,
79.66, 67.94,
58.38, 53.14, 52.49, 37.19 (23 lines expected; 2I lines observed); MS (FAB)
m/z 602, 600,
558, 556, 466, 351, 349, 280, 278, 175, 173; MS (FAB) m/z 600.1299 (calcd for
[M+H]+
600.1304); Anal. C 57.69, H 4.90, N 6.71, CI 11.49 (calcd for 0.35% H20: C
57.81, H
4.56, N 6.97, Cl I 1.77).
Example 127.
(Scheme K: K-4 where R~ is benzyl, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl,
Y is
C02 and the stereochemistry is [R-(R*,S*)])
[R-(R*,S*)}-4-[[[1-Carboxy-2-[4-[(2,6-
dichlorobenzoyl)amino}phenyl]ethyl]amino]-carbonyl]-3-oxazolidinecarboxylic
acid
3-(phenylmethyl) ester (Cz$Hz5C1zN30,). ..
Example 127 was prepared from example 126 by the procedure described in
preparation
12.
Physical properties as follows: TLC (950:50:1 CH2C12/MeOHIHCO2H) Rf= 0.31;'H
NMR (dQ-methanol} 8 7.60 (2H),7.49-7.23 (8H), 7.21 (2H), 5.23-4.97 (2H), 4.95
{1H),
4.88 (1H), 4.70 (1H), 4.36 (1H), 4.14 (1H), 3.85-3.73 (1H), 3.23 (1H), 3.00
(1H); MS
(FAB) m/z 588, 586, 544, 542, 532, 391, 371, 337, 335, '<?45, 177, 173, 149,
123, 105, 103,
91; MS (FAB) m/z 586.1163 (calcd 586:1147).
CA 02342778 2000-12-20
WO 99167230 PCTIUS99/14233
-I 65-
Scheme L.
RL-2 ~ Rl-t
O N O
-O~L~O- L_1
R~-2 ~ RL-t
O N O L_2
-O~~~OH
R5
HyN~O~ L-3
O
RL-2~ RL-~
O~~-~O
O
-O HN~Oi
ll,,~~ L-4
R5
R1.2 ~ R~-t
I ~~''/~.~~/ O
HO HN ~ L-
OH
R$
S Scheme L teaches a general method for the preparation of N alkylaryl
azetidinedi-
carboxylic acid Examples L-4 and L-5, where R~-, is C,~, alkyl, RL-Z is C~,o
aryl and RS is
defined as in Scheme B. The N phenylethyl-2.4-azetidinedicarboxylic acid
dimethyl ester
stereoisomers of general structure L1 were prepared as described (Hoshino,
:1.; Hiraoka, J.;
Hata, Y.; Sawada. S.; Yamamoto, Y. J. Chem. Soc. Perkin Trans. I 1995, 693-
697) and
separated by silica flash chromatography. Thus partial saponification of
diester L-1 gives
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/I4233
-166-
the half acid L-2, that is coupled with reagent L-3 (as exemplified by the use
of reagents
A-3 and A-4 of Scheme A, and B-4 and B-5 of Scheme 13) to provide Examples L-
4, that
are then hydrolyzed to Examples L-5 of this invention.
Preparation S4.
S (Scheme L: L-1 where R~_, is methyl, R~_Z is phenyl and stereochemistry is
[2S
[ 1 (R*),2a,4(3]]).
[2S-[1(R*),2a,4(3]]-1-(1-Phenylethyl)-2,4-azetidinedic,arboxylic acid dimethyl
ester
[168647-92-S] (C,SH,9NO4).
Physical properties as follows: TLC (4:1 Hexanes/EtOA,c) R f= 0.42; "C NMR
(CDC13) 8
173.61, 142.41, 128.31, 127.39, 127.35, 60.95, 60.67, 51.64, 24.96, 21.72.
Preparation SS.
(Scheme L: L-1 where R~_, is methyl, R, _z is phenyl and stereochemistry is
[2R-
[ 1 (S'''')~2a~4 ~]])~
[2R-[I(S*),2a,4ji]]-1-(1-Phenylethyl)-2,4-azetidinedicarboxylic acid dimethyl
ester
I S (C,SH,9NO,).
Physical properties as follows: TLC (4:1 Hexanes/EtOAc) R f= 0.31; '3C NMR
(CDC13) S
i 72.97, 141.21, 128.44, 128.07, 127.68, 61.54, 60.69, S 1.61, 24.91, 19.55.
Preparation S6.
(Scheme L: L-1 where RL_, is methyl, RL_z is phenyl and stereochemistry is
jl(S~-cis]).
jI(S')-cu]-1-(1-Phenylethyl)-2,4-azetidinedicarboxylic acid dimethyl ester
[168753-32-
0] (CisHi9N~a)~
Physical properties as follows: TLC (4:1 Hexanes/EtOA,c) Rf= 0.21; '3C NMR
(CDC13) S
172.58, 172.08, 140.84, 128.22, 128.15, 127.65, 66.32. 60.10, 59.65, 52.06,
S1.S9, 24.26,
19.91.
2S Preparation 57.
(Scheme L: L-I where RL_, is methyl, R, _z is phenyl and stereochemistry is
[2R-
[1 (R*),2a,4[3]]).
[2R-[1(R*),2a,4[3]]-1-(1-Phenylethyl)-2,4-azetidinedicarboxylic acid dimethyl
ester
(C~sH~eNOa)~
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-167-
Physical properties as follows: TLC (8:2 hexanesIEtOAc) R, f= 0.42; '3C NMR
(CDCI3) b
173.61, 142.41, 128.31, 127.39, 127.35, 60.95, 60.67, 51.64, 24.96, 21.72; MS
(+ESI) mJz
278.3.
Preparation 58.
(Scheme L: L-1 where R~_, is methyl, RL_2 ss phenyl and stereochemistry is [2S
[1 (S*),2a,4(3]].
[2S [1(S*),2a,4(3]]-1-(I-Phenylethyl)-2,4-azetidinedicairboxylic acid dimethyl
ester
(CisH~9NOa)~.
Physical properties as follows: TLC (8:2 hexanes/EtOA.c) R f= 0.31; "C NMR
(CDC13) 8
172.97. 141.21, 128.44, 128.07, 127.68, 61.54. 60.69, 51.61,24.91. 19.55; MS
(+ESI). »tlz
278.3.
Preparation 59.
(Scheme L: L-1 where R~_, is methyl, R~_z is phenyl and stereochemistry is [ 1
(R)-cis]).
[1(R)-cis]-1-(1-Phenylethyl)-2,4-azetidinedicarboxylic acid dimethyl ester
(C,SH19N04)~
Physical properties as follows: TLC (8:2 hexanes/EtOAc) R f= 0.2 I ; ' 3C NMR
(CDC13) F
172.58, 172.08, 140.84, 128.22, 128:15, 127.65, 66:32, 60.10, 59.65, 52.06,
51.59, 24.26,
19.91; MS (+ESI) m/z 278.3.
Preparation 60.
(Scheme L: L-2 where R,~, is methyl, RL_2 is phenyl and stereochemistry is [2S-
[ 1 (R*),2a,4(3]]).
[2S-(1(R*),2a,4(3]]-1-(1-Phenylethyi)-2,4-azetidinedicarboxylic acid
monomethyl ester
(C~aHpN04)~
A mixture of L-1 (Scheme L, where RL_, is methyl, R, _Z is phenyl and
stereochemistry is
[2S-[I(R*),2a,4(i]], the product of preparation 54) (7.95 g; 28.7 mmol) and
LiOH (30
mmol) in 1:1 MeOHIH20 (240 mL) is stirred at rt for 42 h. The reaction mixture
is
adjusted to pH 5 with HOAc, and is concentrated. The resulting concentrate is
diluted with
brine and extracted repeatedly with CHCI,. The combined CHCI~ extracts are
dried,
filtered and concentrated to give a yellow foam (6.61 g), that is purified by
preparative
CI8 reverse phase chromatography to give the title compound as a crystalline
solid: mp
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
_ 168-
112-113 °C; TLC (6S0:3S0:1 hexanes/EtOAc/HC02H) lei= 0.17; MS (FAB) m/z
527, 264,
248, 218, 204, 192, 186, 177, 160, 114, 105; Anal. C 64.04, H 6.57, N 5.37
(calcd C
63.87, H 6.S 1, N 5.32).
Preparation 61 and Example 128.
S (Scheme L: L-4 where R~_, is methyl, R~_2 is phenyl, Rs is 4-[(2,6-
dichlorobenzoyl}-
amino]phenyl and stereochemistry is [2S [1(R*),2a,4(3(iQ*)]])
j2S-[1(R*),2a,4(3(R*)j]-4-([[I-[(4-[(2,6-Dichlorobenzoyl)amino]phenyl]methyl]-
2-
methoxy-2-oxoethyl]amino[carbonyl[-1-(1-phenylethyl)-2-azetidinecarboxylic
acid
methyl ester (C" H3 'C12N3O6).
A mixture of L-2 (Scheme L, where RL_, is methyl, RL_2 its phenyl and
stereochemistry is
[2S-[1(R*),2a,4(3]], the product of preparation 60) (0.62 g, 2.4 mmol), L-3
(Scheme L
where RS is 4-[(2,6-dichlorobenzoyl)amino)phenyl and stereochemistry is (S))
(0.95 g, 2.4
mmole), and BOP-CI (0.68 g; 2.7 mmol) in CH2Cl2 (10 rnL) is treated with (i-
Pr)2NEt (1.7
mL, 9.8 mmol). The reaction mixture is stirred at rt under N2 for 19 h. It is
diluted with
1 S half saturated NaHC03 and extracted with CH,Cl2. The CH2C12 extracts are
dried, filtered
and concentrated to give a beige-colored foam ( I .40 g), that is purified by
silica flash
chromatography to give the title compound: TLC ( 1:1 Hexanes/EtOAc) Rf= 0.23;
"C
NMR (CDCl3) 8 173.25, 172.73, 171.63, 162.57, 141.8d, 136.76, 136.01, 132.44,
132.39,
130.90, 130.00, 128.57, 128.12, 127.SS, 127.08, 120.37, 63.25, 60.06, 59.17,
52.41, 52.12,
51.47, 37.65, 26.06, 21.25; MS (FAB) m/z 612.1658: Anal. C 59.66, H 5.14, N
6.64 (calcd
C 60.79, H 5.10, N, 6.86).
Preparation 62 and Example 129.
(Scheme L: L-S where RL_, is methyl, RL_2 is phenyl, RS is 4-[(2,6-
dichlorobenzoyl)-
amino]phenyl and stereochemistry is [2S [1(R*),2a,4[i(R*)]]}
2S (2S-[1(R*),2a,4[3(R*))]-4-[([1-Carboxy-2-[4-[(2,6-
dichlorobenzoyt)amino)phenyl]-
ethyl)-amino]carbonyl]-1-(1-phenylethyl)-2-azetidine~carboxylic acid dilithium
salt
(C29H25CI2L1,N3O6).
A mixture of L-4 (Scheme L, where R~_, is methyl, R, _2 is phenyl, RS is 4-
[(2,6-
dichlorobenzoyl)amino]phenyl and stereochemistry is [2S-[1(R*),2a,4ji(R*)]],
the product
of preparation 61 ) (0.684 g, 1.12 mmol) and LiOH~H,O 1;0.25 g, 6.0 rnmol) is
dissolved by
CA 02342778 2000-12-20
WO 99/b7230 PCT/US99/14233
-169-
warming in MeOH ( 10 mL). Thin solution is diluted with 1:1 H~O/THF (20 mL),
and the
reaction mixture is stirred at rt for 22 h. The mixture is adjusted with 1 N
HCI to pH 6. The
solution is concentrated to give a white solid, that is purified by
preparative C I 8 reverse
phase chromatography (MeCN/H,O gradient). Evaporatiion of the column fractions
gives a
S white solid, that is dissolved in warm HzO. The solution is frozen and
lyophilized to give,
as a white solid, the title compound: mp 270 °C; TLC (850:150:1
CHCI,/MeOH/HC02H}
Rf= 0.21-0.36; "C NMR (CD30D) S 178:05, 174.59, 174.01, 162.20, 142.33,
135.09,
134.91, 133.04, 130.54, 129.24, 128.61, 126.46, 126.41,126.17, 124:94,
118.33,82.70,
61.70, 60.50, 53.30, 36.23. 25.85, 18.65; MS (FAB) m/z 584.1350: Anal. C
SS.I7, H 5.01,
I 0 N 6.63, Cl 11.16 (calcd for 7.52% H~O: C 54.02, H 4.75, N 6.52. Cl, I 1:21
).
Example 130.
(Scheme L: L-4 where RL_, is methyl, RL_2 IS phenyl, RS is 4-[(2,6-
dichiorabenzoyl)-
amino]phenyl and stereochemistry is [2R-[I(S*),2a,4[i(S*)]]).
[2R-[1(S*),2a,4(i(S*}]]-4-[[(1-[[4-[(2,6-Dichlorobenzo~yl)amino]phenyl]methyl]-
2-
I5 methoxy-2-oxoethyl]amino]carbonyl]-1-(i-phenyleth;yl)-2-azetidinecarbaxylic
acid
methyl ester (C3,H3,ChN306).
Example 130 was prepared as described in Scheme L usiing the product of
preparation SS
as intermediate L-1. Physical properties as follows: TLC (3:2 EtOAc/Hexanes)
Rf=
0.43; [a]ZSp +58 (c 0.91, MeOH); "C NMR (CDCl3} 8 1'3.24, 172.15,172.05,
162.58,
20 140.75, 136.45, 136.01, 132.89, 132.38, 130.91, 129.60, 128.67, 128.39,
128.11, 127.97,
120.39, 63.54, 60.19, S9.S4, 53.15, 52.16, 51.91, 37.86, 26.12, 18.3 ~, MS
(EI) mlz 613,
61 I, 598, 596, 554, 552, 527, 525, 508, 506, 450, 448, 351, 349, 218, 191,
175, 173, 160,
131, I I4, 105; Anal. C 60.68, H 5.18, N 6.67, Cl 11.24 (calcd for C 60.79, H
5.10, N 6.86,
Cl 11.58).
25 Example 131.
(Scheme L: L-5 where RL_, is methyl, RL_2 is phenyl, RS is 4-[(2,6-
dichlorobenzoyl}-
amino]phenyl and stereochemistry is [2R-[1(S*),2a,4[i(S*)]]).
[2R-[1{S*),2a,4[3(S*)]]-4-[[[1-Carboxy-2-[4-[(2,6-dichllorobenzoyl}-
amino]phenyl]ethyl]-amino]carbonyl]-1-(1-phenylethyl)-2-azetidinecarboxylic
acid,
30 dilithium salt (C,9H,SChLi,N,06).
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-170-
Example 131 was prepared from Example 130 by the procedure described in
preparation
62. Physical properties as follows: [aJ 'Sp +84 (c 0.95, :MeOH); '3C NMR
(CD30D) S
179.18. 176.55, 174.13, 163.58, I4I.88, 136.32, 136.29, 134.96, 131.90,
130.86, 129.47,
128.49, 128.07,127.87, 127.16, 119.74, 63.70, 63.49, 59,.77, 56.27, 38.24,
26.25, 18.70;
MS (FAB) m/z 598, 596, 592, 590, 552, 550, 546. 544, 161; Anal. C 55.20, H
5.52, N,
6.59 (calcd for 10.98% H20: C 52.00, H 4.99, N 6.27}.
Example 132.
(Scheme L: L-4 where R~_, is methyl, R~_Z is phenyl, RS is 4-[(2,6-
dichlorobenzoyl)-
amino]phenyl and stereochemistry is [I (S~,2a,4a(,S~], a single diastereomer
having a cis
I 0 relative conf guration but unknown absolute configuraticrn at C-2 and C-4
of the
azetidine.)
[1(,f~,2a,4a(,5~]-4-(([1-[[4-[(2,6-Dichlorobenzoyl)amino]phenyl]methyl]-2-
methoxy-2-
oxoethyl]amino]carbonyl]-1-(1-phenylethyl)-2-azetidinecarboxylic acid 2-methyl
ester (Cg'H3tC12N3O6}~
I S Example 132 was prepared as described in Scheme L using the product of
preparation 56
as intermediate L-1. Physical properties as follows: TLC (9:1 CHC131acetone) R
f = 0.29;
'3C NMR (CDC13) 8 172.70, 172.29, 171.65, 162.49, 144.97, 136.71, 136.12,
132.47,
I32.39, 130.81, 130.22, 128.35, 128.07, 127.82, 120.17, 66.61, 61.52, 59.99,
52.38, 52.24,
51.79. 37.73. 25.16, 20.09.
20 Example 133.
{Scheme L: L-5 where R~., is methyl, R,~_2 is phenyl, RS i.s 4-[{2,6-
dichlorobenzoyl)- --
amino]phenyl and stereochemistry is [1(S');2a,4a], a single diastereomer
having a cis
relative configuration but unknown absolute configuration at C-2 and C-4 of
the
azetidine.)
25 [1(S'),2a,4a(,S~]-4-([[1-Carboxy-2-(4-((2,6-
dichlorobenzoyi)amino]phenyl]ethyl]-
amino]-carbonyl]-1-(1-phenylethyl)-2-azetidinecarboxylic acid (C,9H,,CI,N3O6}.
Example 133 was prepared from Example 132 by the procedure described in
preparation
62. Physical properties as follows: [aJ-'SO +20 (c 0.88, MaeOH); '3C NMR
(CD,OD) 8
I73.70. 173.05, 172.61, 163.69, 140.09, 136.93, 136.20, 133.18, I3I.89,
130.89, 129.95,
30 128.09, 127.89,127.88, 127.65, 120.03, 66.07, 61.29, 59.86, 52.70, 36.98.
25.03, 18.81;
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-171-
MS {FAB) m/z 662, 660, 586, 584, 539, 482, 480, 436, 434, 204, I75, 173, 160,
133, 109,
105; Anal. C 57.90, H S.OI, N 6.93 (calcd for 4.98% H,O: C 56.63, H 4.98, N
6.83).
Example 134.
(Scheme L: L-4 where RL_, is methyl, RL_z is phenyl, RS is 4-[(2,6-
dichlorobenzoyl)-
amino]phenyl and stereochemistry is [2R-[1(R*),2a,4[i('S*)]])
[2R-(1(R*),2a,4[3(S*)]]-4-[[[1-(j4-[(2,6-Dichlorobenzoyl)amino]phenyl]methyl]-
2-
methoxy-2-oxoethyl]amino]carbonyl]-1-(1-phenyleth;yl}-2-azetidinecarboxylic
acid
methyl ester (C3,H3,ChN,06).
Example 134 was prepared as described in Scheme L using the product of
preparation 57
as intermediate L-1. Physical properties as follows: TLC; ( 1:1 EtOAc/Hexanes)
R~= 0.30;
'3C NMR (CDCl3) 8 173.78, 172.76, 172.10, 162.47, 141.81, 136.70, 135.96,
132.43,
132.38, 130.88, 129.79, 128.55, 128.10, 127.58, 127.00, 120.58, 63.32, 60.06,
59.27,
52.70, 52.50, 51.44, 37.08, 25.76, 21.52; MS (+ESI, 200:1 MeOH/HCO,H solution)
m/z
613.8, 611.8.
Example 135.
{Scheme L: L-5 where RL_, is methyl, R, _2 is phenyl, RS is 4-[{2,6-
dichlorobenzoyl)-
amino]phenyl and stereochemistry is [2R-[1(R*),2a,4[3(S*)]])
[2R-[1(R*),2a,4(3(S*)]]-4-[([1-Carboxy-2-(4-[(2,6-
dichlorobenzoyi)amino]phenyt]-
ethyl]-amino]carbonyl]-1-(1-phenylethyl)-2-azetidinecarboxylic acid dilithium
salt
(Cz9H25C12L1~N3O6).
Example 135 was prepared from Example 134 by the procedure described in
preparation
62. Physical properties as follows: [a]ZSD +76 (c 0.89, MeOH); '3C NMR (CD30D)
8
179.44, 176.71, 175.90, 163.55, 143.81, 136.54, 136.26. 134.47, I3I.90,
120.81, 129.70,
127.83, 127.34, 126.33, 119.88, 63.06, 62.1 l, 58.71, 55.46, 37.17, 26.76,
20.56; MS
{FAB) m/z 598, 596, 592, 590, 552, 550, 546, 544, 237. 305; Anal. C 55.12, H
5.24, N
6.59, Cl 10.56 (calcd for 6.21 % H,O: C 54.78, H 4.66, N 6.61, Cl 11.15).
Example 136.
(Scheme L: L-4 where R,: , is methyl, R~_2 1S phenyl, RS is 4-[{2,6-
dichlorobenzoyl)-
amino]phenyl and stereochemistry is [2S-[1(S*),2a,4[i{R*)]])
CA 02342778 2000-12-20
WO 99167230 PCTIUS99114233
-172-
[2S-[1(S*),2a,4(3(R*)]]-4-[[(I-[(4-((2,6-Dichlorobenzoy!)amino]phenyl]methyl]-
2
methoxy-2-oxoethyl]amino]carbonyl]-1-(1-phenyleth~~l)-2-azetidinecarboxylic
acid
methyl ester (C3,H3,CIzN3O6)
Example 136 was prepared as described in Scheme L using the product of
preparation 58
as intermediate L-1. Physical properties as follows: TLC (85: I5
CHCI,/acetone) Rf= 0.54;
"C NMR (CDCl3) 8 173.44, 172.62, 171.72, 162.79, 14CL46, 136.69, 136.34, 1
x3.12,
132.79, 131.29, 130.40, 129.08, 128.52, 128.45, 128.23, 120.57, 64.I5, 60.51,
60.09,
53.11, 52.59, 52.19, 37.73, 26.70, 18.75; Anal. C 60.70, H 5.39, N 6.62 (calcd
C 60.79, H
5.10, N 6.86).
Example 137.
(Scheme L: L-S where RL_, is methyl, RL_2 is phenyl, RS is 4-[(2,6-
dichlorobenzoyl)-
amino]phenyl and stereochemistry is [2S-[1(S*),2a,4(3{R*)]])
(2S (I(S*),2a,4[3(R*)]]-4-[[(I-Carboxy-2-(4-[{2,6-
dichllorobenzoyl)amino]phenyl]-
ethyl]-amino]carbonyl]-1-(I-pheny!ethyl)-2-azetidinecarboxylic acid
(C~9H,7ChN3Ob).
Example 137 was prepared from Example 136 by the procedure described in
preparation
62. Physical properties as follows: [a]ZSD +2 (c 1.00, Me;OH);'3C NMR (CD30D)
8
172.31, 169.43, 167.37, 163.72, 161.39, 160.93, 136.98, 136.11, 134.40,
133.20, 131.84,
130.93, 129.42, 129.39, 128.60, 127.89, 120.1 I; 62.68, 61.41, 53.51,
36.35,25.00, 16.17;
MS (FAB) m/z 586, 584, 482, 480, 204, 175. 173, 106, I ~05; Anal. C 53.07. H
4.52, N
6.15, CI 10.46 (cakd for 0.80 equiv TFA and 2.13% HZO: C 53.20, H 4.30, N
6.08, CI
10.26).
Example 138.
(Scheme L: L-4 where RL_, is methyl, R,.2 is phenyl, RS i.s 4-[{2,6-
dichlorobenzoyl)-
aminoJphenyl and stereochemistry is [ 1 (R);2a,4a{S)], a s~ingie diastereomer
having a cis
relative configuration but unknown absolute configuration at C-2 and C-4 of
the
azetidine.)
[I(R),2a,4a(,S~]-4-([(1-((4-[(2,6-Dichlorobenzoyl)amino]phenyl]methyl]-2-
methoxy-2-
oxoethyi]amino]carbonyl]-I-(I-pheny!ethyl)-2-azetidinecarboxyiic acid 2-methyl
ester
(C,~H3IC13N306)~
CA 02342778 2000-12-20
WO 99/G7230 PCT/US99/14233
-I73-
Example 138 was prepared as described in Scheme L using the product of
preparation 59
as intermediate L-1. Physical properties as follows: TLC Rf= 0.47 (85:15
CHC13/acetone); "C NMR (CDCl3) 8 173.42, 172.40, 171.74. 162.46, 140.80,
136.76,
136.03, 132.64, 132.35, 130.80, 129.82, 128.38, 128.04., 127.85, 127.69,
120.39, 66:11,
61.50, 59.78, 52.83, 52.42. 51.72, 37.12, 25.03, 20.20; MS {+ESI, 200:1
MeOH/HCO2H
solution) mlz 6 i 4.2, 612.2; Anal. C 60.66, H 5.18, N 6.80, Cl l 1.42 (calcd
C 60.79, H
5.10, N 6.86, CI 11.58).
Example 139.
(Scheme L: L-5 where RL_, is methyl, R~_2 is phenyl, Ra is 4-[(2,6-
dichlorobenzoyl)-
amino]phenyl and stereochemistry is [I(R),2a,4a(,S~]. a single diastereomer
having a cis
relative configuration but unknown absolute configuration at C-? and C-4 of
the
azetidine)
[1(R),2a,4a(,f)]-4-[[[1-Carboxy-2-[4-[(2,6-dichlorobenzoyi)amino]phenyl]ethyl]-
amino]-carbonyl]-1-(I-phenylethyl)-2-azetidinecarba~xylic acid
(CZ9H,SC12N3O6).
Example 139 was prepared from Example I38 by the procedure described in
preparation
62. Physical properties as follows: [a]z5D -30 (c 0.90, MeOH); '3C NMR (CD30D)
8
172.71, 172.44, 171.81, 163.71, 138.03, 136.99, 136:15, 133.31, 131.89,
130.89, 129.45,
128.33, 128.29,128.02, 127.86, 120.23, 65.62, 61.51. 59'.60, 53.47, 36.04,
24.91, 17.68;
MS (FAB) m/z 586. 584, 371, 298, 204, 177; 175. 173. 133. 105, 100; Anal. C
55.69, H
4.49, N 6.55, Cl 11.81 (calcd for 0.38 equiv TFA and 2.06% HZO: C 55.80, H
4.54, N
6.56, Cl 11.07).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-174-
Scheme M.
RM.2 ~ RM-1
OQ N O
-O, v 'O- M-1
O, N ,.O
-O~--~--l~O- M-2
R3'Y
O' N O
~ ~ M-3
-O~~O._
R3,Y
O N ,O
~~''' M~4
-O " OH
R5
HzN~O~ M-5
[JO
R3'Y
O N O
\'~~''~ O
-Ol' ' HN Oi NI-6
R5
R3'Y
O ~ O
\'~~''~ O
HO~~~N OH M-7
RS
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
- I 75-
Scheme M teaches 'a general method for the preparation of N acyl
azetidinedicarboxylic
acid Examples M-6 and M-7, where RM_, is C,_b alkyl, RM.2 is Cb_,°
aryl, and R~, RS and Y are
defined as in Scheme B. Thus removal of the N alkylaryl substituents RM_, and
RM_2 of M-1
gives aminodiester M-2, that is acylated to provide M-3. Partial
saponification of diester
S M-3 gives half acid M-4, that is coupled with reagent M-5 (as exemplified by
the use of
reagents A-3 and A-4 of Scheme A, and B-4 and B-5 of Scheme B) to provide
Examples
M-6, that are then hydrolyzed to Examples M-7 of this invention.
Preparation 63.
(Scheme M: M-2 where stereochemistry is (2R-traps))
(2R-traps)-2,4-Azetidinedicarboxylic acid dimethyi diester (C,H"N04).
To an N,-purged solution of freshly chromatographed Preparation M-1 (Scheme M
where
RM_, is methyl, RM_2 IS phenyl and stereochernistry is [2R-[I(R*),2a,4[3]]
prepared as
described by preparation 57) (656 mg, 2.37 mmol) in Me;OH (20 mL) is added 20%
Pd(OH)z/C ( 120 mg), and this mixture is hydrogenated for 19 h under an H~
atmosphere
IS (approximately 42 psi pressure). The mixture is filtered and concentrated
to give the title
compound as a colorless oil: TLC (4:1 HexaneslEtOAc) Rf= 0.04;'H NMR (CDC13) 8
4.33 (2H), 3.77 (6H), 3.38 (1H), 2.71 (2H); MS {+ESI) m/z I74.2.
Preparation 64.
(Scheme M: M-3 where R3 is ethyl, Y is CO,-, and the stereochemistry is (2R-
traps))
(2R-traps}-1,2,4-Azetidinetricarboxylic acid i-ethyl-2,4-dirnethyl triester
(C,°H,SN06).
To a mixture of freshly prepared amine M-2 (Scheme M where stereochemistry is
(2R-
trans)} (14 mrnol} in CHZCl2 {20 mL) under N, at 0 °C is added Et3N
(3.0 mL, 22 mmol),
followed by the dropwise addition of C1CO,Et ( 1.5 mL, :l 8 mmol). After 22 h
the reaction
is quenched with saturated NaHC03, diluted with H,O, and extracted with EtOAc.
The
combined organic extracts are dried, filtered and concentrated to give 2.79 g
of the
carbamate, that is purified by silica flash chromatography: TLC (4:1
Hexanes/EtOAc) R f=
0.17; [a]'-SD + 183 (c 0.83, MeOH); 1 H NMR (CDCIz) S 4.77 (2H), 4.15 ( 1 H),
4.10 ( 1 H),
3.80 (6H), 2.58 (2H), 1.23 {3H); "C NMR (CD,OD) b 173.11, 173.05, 157.47,
63.30,
61.00, 60.18, 53.41, 26.43, 15.27; MS (EI) miz 245. 186, 172, 142, 114; Anal.
C 48.90, H
6.21, N 5.73 (calcd C 48.98, H 6:16, N 5.71 ).
CA 02342778 2000-12-20
WO 99!67230 PCTIUS99l14233
-176-
Preparation 65.
(Scheme M: M-4 where R, is ethyl, Y is COZ-, and the stereochemistry is (2R-
traps))
(2R-traps)-1,2,4-Azetidinetricarboxylic acid 1-ethyl-2.-methyl diester
(C9H,3N06).
A mixture of M-3 (Scheme M, where R3 is ethyl, Y is CO,-, and the
stereochemistry is
(2R-traps)) (1.68 g, 6.85 mmol) and LiOH (7.00 mmol) iin I :1 MeOH/H,O} (40
mL) is
stirred at rt for 45 h, and then is concentrated. The residue is dissolved in
half saturated
NaHC03, and the solution is extracted with Et20. The Et,O solution is
discarded. The
aqueous solution is adjusted with concentrated HCI to pH 4, and is
concentrated to a
yellow solid. This solid is triturated with CHCl3. The CH;CI, solution is
filtered and
concentrated to give, as a light brown oil, the title compound: TLC (600:400:1
HexaneslAcetoneIHCO,H) Rf= 0.21-0.45;'H NMR (CD~C13) S 4.87 (1H), 4.77-4.68
(1H),
4.64-4.50 (1H), 4.18-3.97 (2H), 3.76 (3H), 2.52-2.33 (2H), 1.26-1.13 (3H}; MS
(-ESI,
MeOH solution) m/z 230.1. This maternal is used without: purification.
Preparation 66 and Example 140.
(Scheme M: M-6 where R3 is ethyl, Rs is 4-[{2,6-dichlorobenzoyl)amino]phenyl,
Y is
COZ-, and the stereochemistry is [2R-(2a,4[i(S*)])
[2R-(2a,4/3(S*)]-4-[ [[1-[ [4-[(2,6-Dichlorobenzoyl)amino]phenyl] methyl]-2-
methoxy-2-
oxoethyl]amino]carbonyl]-1,2-azetidinedicarboxyiic acid i-ethyl-2-methyl
diester
(C26H27C12N3~8)~
To a mixture of Preparation M-4 {1.08 g, 4.67 mmol) and HOBt+H~O (0.63 g, 4.7
mmoI)
in CHzCI, {10 mL} at 0 OC is added a solution of EDC (1.04 g, 5.42 mmol) in
CHzCh (15
mL}. This mixture is stirred at 0°C for 30 min. It is then treated with
M-S (Scheme M
where RS is 4-[(2,6-dichiorobenzoyl)amino)phenyl and stereochemistry is (S))
(1.88 g,
4.66 mmol) and N methylmorpholine (0.52 mL, 4.7 mmol). The resulting solution
was
stirred at 0°C for 2 h and at rt for 2 h. The reaction mixture is
diluted with 10% IGIiS04
and extracted with CH,Ch. The combined CH~CI, extracts are washed with satd
NaHC03
and brine, and are combined, dried, filtered and concentrated to give a yellow
foam (2.58
g), that is purified by flash chromatography to give, as a white solid, the
title compound:
mp 97-99°C; TLC (9:1 CHCl,/acetone) Rf= 0.30; [a]''Sp -+-79 {c 1.02.
MeOH); UV
(MeOH) ~m~ 225 (E 12200: sh), 251 {17500);'3C NMR (C17,OD) S 171.50, 171.30,
CA 02342778 2000-12-20
WO 99167230 PCT/US99l14233
-177-
171.08, 163.67, 156.23, 155.96, 136.92, 136.19, 133.21" 133.03, 131.87,
130.93, 129.64,
129.44, 127.92, 119.97, 61.61, 60.56, 59.82, 59.1 I, 58.41, 53.45, SL.58,
SI.52. 36.57,
36.29. 24.91. 13.43 (22 lines expected; 28 lines observed); MS (FAB) m/z
580.1260
(calcd for [M+HJ+, 580.1253); Anal. C S 1.95, H 4.56, N 6.89, Ci 15.18 (calcd
for 0.61
H,O: C 53.48, H 4.73, N 7.20, Cl 12.14).
Preparation 67 and Example 141.
(Scheme M: M-7 where R3 is ethyl, RS is 4-[(2,6-dichlorobenzoyl)arnino)phenyl,
Y is
CO,-, and the stereochemistry is [2R-(2oc,4[3(S*)])
[2R-(2oc,4~(S*}]-4-[[[I-Carboxy-2-[4-[(2,6-dichlorobenzoyl)aminoJphenyllethyl]-
amino]-carbonylJ-1,2-azetidinedicarboxylic acid 1-etlhyl ester
(C24H,3C1,N,08).
To a suspension of M-6 (Example 140, Scheme M where R3 is ethyl, R~ is 4-[(2,6-
dichlorobenzoyl)aminojphenyl, Y is CO,-; and the stereochemistry is [2R-
(2a,4[i(S*)])
(0.201 g, 0.347 mmol) in MeOH (5 mL) is added HBO (4.3 mL}and1.00 M LiOH (0.70
mL}. The reaction mixture is stirred at rt for 23 h. It is concentrated in
vacuo. The aqueous
I5 concentrate is diluted with H20, and the solution is adjusted to
approximately pH 12 with
1 N NaOH. It is extracted with Et,O, and the Et,O extract is discarded. The
aqueous
solution is adjusted to approximately pH 3 with 1N HCI.. It is extracted
repeatedly with
EtzO. The combined Et,O extracts were dried, filtered and concentrated to
give, as a white
solid, the title compound: TLC (500:500:1 MeOH/CH~CIi/HCO,H) Rf= 0.20; 'H NMR
(CD;OD) 8 7.59 (2H), 7.50-7.37 (3H), 7.23 (2H), 4.78-4.64 (2H), 4.61-4.51
(1H), 4.22-
3.89 (2H), 3.30-3.185 (1H), 3.07-2.93 (1H), 2.42-2.23 (2H), 1.21 and 1.1 l (3H
total); MS
(FAB) mlz 552.0946 (calcd for [M+HJ' 552.0940); Anal. C 50.4$, H 4.61, N 6.64,
Cl
12.95 (calcd for 1.60% H20: C 51.35, H 4.31, N 7.49, Cl 12.63).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-178-
Scheme N.
0
OH
X N-1
m
O
n OH
R3-Y-X~~ N-2
m
R5
HzN~O~ N-3
O
R5
O
n N~'O~ N~1
R3 Y X~~H 'IO
m
Rs
O r
N~OH N_5
R3-Y-xr~H JO~
m
Rs
O ~OH
n N [J N~
H-X~~~H O
m
Scheme N teaches a general method for the preparation of N acyl
azetidinecarboxylic acid
Examples N-S where n equals 0, 1 or 2, rn equals 0, 1 or 2. (m + n} equals 2,
X is nitrogen
and R3, Rs and Y are defined as in Scheme B, and azetidine-carboxylic acid
Examples N-6
where n equals 0, 1 or 2. m equals 0, 1 or 2, (m + n) equals 2, X is nitrogen
and RS is
defined as in Scheme B. Thus acyiation of aminoacid N-I gives Itr'-acylacid N-
2, that is
CA 02342778 2000-12-20
WO 99!67230 PCT/US99/14233
-179-
coupled-with reagent N-3 (as exetnplified by the use of reagents A-3 and A-4
of Scheme
A, and B-4 and B-5 of Scheme B) to provide Examples N-4. Ester hydrolysis of N-
4
provides Examples N-5. N deacylation of Examples N-~> provides Examples N-b.
Preparation 68.
{Scheme N: N-2 where n is 2, m is 0. X is N, Y is -COZ-, Ra is ( 1,1-
dimethyl)ethyl, and
the stereochemistry is (S~).
(S)-1,2-Azetidinedicarboxylic acid 1-(1,1-dimethyle#hyl) ester (C~H,SN04).
To a mixture of (S')-(-)-2-azetidinecarboxylic acid (I 10 mg, i. l mmol),
Boc40 (290 mg,
1.30 mmol), and DMAP (0.017 g, 0.14 mmol) in 4:1 DMF/H,O (10 mL) is added Et3N
(0.30 mL, 2.2 mmol). The reaction mixture is stirred at i~t for 68 h, and then
is
concentrated. The concentrate is diluted with EtOAc, and the EtOAc solution is
washed
with cold 10% KHS04. The combined organic extracts are dried, filtered and
concentrated
to give the title compound as a colorless oil: TLC (750:250:1
Hexanes/acetone/HCOZH)
R~ 0.26; 1 H NMR (CD30D) ~ 4.97 ( 1 H), 4.57 ( 1 H), 3:~~8 ( 1 H), 3.87 ( 1
H), 2.57 (H), 2.13
( 1 H), 1.42 (9H); MS (-ESI) m/z 200.3.
Preparation 69 and Example 142.
(Scheme N: N-4 where n is 2, m is 0, X is N, Y is -COz-, R3 is { l , l -
dimethyl)ethyl, RS is
4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is (,S~).
[2S-(R*,R*)]-2-[[[1-[[4-[(2,6-Dichlorobenzoyl)amino]phenyl]methyl]-2-methoxy-2-
oxoethyl]amino]carbonyl]-1-azetidinecarboxyiic acid 1-(1,1-dimethyl)ethyl
es#er
(C26H29C1zN3O6)~
To a mixture of acid N-2 (Scheme N. where n is 2, m is t), X is N; Y is -CO,-,
R3 is (1,1-
dimethyl)ethyl, and the stereochemistry is (.S'~) (1.04 g; 5.I7 rnmol) and
HOBt~H,O (0.71
g, 5.3 mmol) in CHzCIz (10 mL) at 0 °C is added a mixture of EDC~HC1
(1.00 g, 5.22
mmol) in CHZC12 (20 mL). The reaction mixture is stirred at 0 °C for 30
min, and then N-3
(Scheme N where R5 is 4-[(2,6-dichlorobenzoyl)amino)phenyl and stereochemistry
is (S})
(2.10 g, 5.20 mmol) and N methylmorpholine (0.60 mL, 5.46 mmol) are added. The
reaction mixture is stirred at 0°C for 30 min and at rt for 3 h. The
reaction mixture is
partitioned between 10% KHSO, and CH,C1,. The aqueous phase is extracted twice
more
with CH,CI,. The combined organic extracts are washed with saturated NaHCO,
and brine,
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-180-
and then are dried, filtered and concentrated to a yellow oiI (2.54 g) that is
purified by
silica flash chromatography to give the title compound as a white foam: mp i06-
108 °C;
TLC (l :l Hexanes/EtOAc); Rf= 0.21; [a]ZSD -38 (c 1.01., MeOH); "C NMR (CD,OD)
8
172.37, 171.58, 163.64, IS6.68, 136.98, 136.22. 133.11, 131:87; 130.90,
129.49, 127.91;
S 120.00, 80.42, 61.87, 53.31, S 1.45, 36.40, 27.16. 20.08; MS (EI) m/z SS 1,
549, 478; 476,
451; 449, 396, 394, 351, 349, 280, 278, 175, 173; Anai. C 56.33, H 5.48, N
7.23, CI 12.43
(calcd for 0.S2% H,O: C 56.44, H 5.34, N 7.59, Cl 12.82).
Preparation 70 and Example 143.
{Scheme N: N-S where n is 2, m is 0, X is N, Y is -COZ-, R3 is (I,I-
dimethyl)ethyl, RS is
4-[(2,6-dichlorobenzoyl)aminojphenyl and stereochemi stry is [2S-(R*,R*}])
[2S (R*,R*)]-2-[[[1-Carboxy-2-[4-[(2,6-
dichlorobenzoyl)amino]phenyl]ethyl]amino]-
carbonyl]-1-azetidinecarboxylic acid 1-{1,1-dimethylethyl) ester
(CZSHZ,Cl,N306).
A solution of N-4 (Scheme N, where n is 2, rn is 0. X is N, Y is -COZ-, R~ is
(1,1-
dimethyl)ethyl, RS is 4-[(2,6-dichlorobenzoyl)arnino]phenyl and
stereochemistry is (S))
1S (S07 mg, 0.92 mmol) and LiOH (1.25 mmol) in 1:1 MeOH/H20 (10 mL) is stirred
at rt for
18 h. The reaction mixture is diluted with cold 10% KHS04 and extracted with
CH2CI2.
The organic extracts are dried, f Itered and concentrated to a white foam (498
rng), that is
purifed by silica flash chromatography to give the title compound: TLC
(7S0:2S0:1
hexanes/acetone/HCO~H) R f= 0.12; [a]z5D -27 (c 0.94, CHC33); "C NMR (CD30D) 8
210.OS, 172.70, 172.27, 163.63, 1S6.7S, 136.89, 136.22, 133.36, 131.88,
130.90, 129.56,
127.91, 119.95, 80.47, 61.93, 53.18, 36.47, 27.1 S, 20.09; MS (-ESI) nr/z
533.8; MS (EI)
m/z 435, 419, 417, 401, 399, 373, 371, 280, 278, 175, 1 i'3, 147, 145; Anal. C
SS.23, H
S.2S, N 7.42, CI 12.87 (calcd for 1.10% H20: C SS:36, H 5.14, N 7.75, CI
13.07).
Preparation 7 i and Example 144.
2S (Scheme N: N-6 where n is 2, m is 0, X is N, RS is 4-[(2,6-
dichlorobenzoyl)amino]phenyl
and stereochernistry is {2S), ~)
N [[(2S~-2-Azetidinyi]carbonyl]-4-[(2, 6-dichlorobenz;oyl)amino]-L-
phenylalanine
trifluoroacetic acid salt (C,°H~9CI,N3O6 .C,HF~O=).
A solution of N-5 (Scheme N, where n is 2, m is 0. X is N, Y is -CO~-, R3 is
(1,1-
dimethyl)ethyi, RS is 4-[(2,6-dichlorobenzoyl}amino]phemyl and stereochemistry
is [2S-
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-181-
(R*,,R*)]) (900 mg; 1.7 mmol) in 1:1 TFA/ CHzCl2 (5 mL) is stirred at rt for
1.5 h, and is
concentrated. The residue is thrice diluted with CHCIj anal re-concentrated.
This residue is
dissolved in MeOH and concentrated to a white foam, that is dissolved in 1:1
MeOH/H20
and then concentrated to remove most of the MeOH. The; solution is frozen and
lyophilized to give the product as a white powder: [a]zsp -6 (c 0.72, MeOH);
'3C NMR
(CD30D) 8 172.62, 167.54, 163.78, 136.82, 136.13, 133.43, 131.84, 130.97,
I29.38,
127.93, 120.15, 58.38,.54.03, 43.73, 36.27, 23.33; MS (+ESI) mlz 436.0; MS
(FAB) mlz
438, 436; Anal. C 46.77, H 3.75, N 7.24, Cl 12.44 (calcd for a l:l TFA salt
with 1.68%
H20: C 47.21, H 3.79, N 7.S 1, Cl 12.67).
Example 145.
{Scheme N: N-4 where n is 1, m is 1, X is N, R3 is (l,l-dimethyl)ethyl, RS is
4-[{2,6-
dichlorobenzoyl)amino]phenyl, Y is COZ- and the stereochemistry is {,S'~)
(S]-3-[[[1-( [4-[{2,6-Dichlorobenzoyl)amino]phenyl] methyl)-2-methoxy-2-
oxoethyl]amino]-carbonyl]-I-azetidinecarboxylic acid I-{l,I-dimethyl)ethyl
ester
I S (C26H29C12N3~6O
Example 14S was prepared as described in Scheme N from 3-azetidinecarboxyIic
acid.
Physical properties as follows: TLC ( 1:1 EtOAc/hexanes) R f = 0.22; [a]'SD
+18 (c 0.92,
MeOH);'3C NMR (CD,OD) b 172.92, 171.91, 163.68, 1S6.SS, 136.87, 136.20,
133.27,
131.89, 130.91, 129.36, 127.90. 120.04, 79.80, 53.85; S 1.45. 36.43, 32.26,
27.24; MS (EI)
m/z 469, 467, 4S I, 359, 3S 1, 349. 280, 278, 175, 173, 57., Anal: C 56.82, H
5.39, N 7.52,
Cl 12.81 (calcd for 0.06% HBO: C 56.70, H 5.31, N 7.63, Cl 12.87).
Example 146.
(Scheme N: N-5 where h is 1, m is 1, X is N, R3 is (1,1-dimethyl)ethyl, RS is
4-[(2,6-
dichlorobenzoyl)amino]phenyl, Y is COZ- and the stereoc:hemistry is (~)
2S {S~-3-[[[1-Carboxy-2-[4-[(2,6-
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]]-
I-azetidinecarboxylic acid 1-(1,I-dimethylethyl) ester (C,SH,7Cl;N306).
Example i46 was prepared from example 14S by the procedure described in
preparation
70. Physical properties as follows: TLC (600:400:1 Hexanes/Acetone/HCO,H) Rf=
O.I7;
[a]'gyp +33 {c 0.92, MeOH); "C NMR (CD30D) 8 I73Ø'i, 172.89, 163.69, 1S6.S6,
136.78,
136.19, 133.59, 131.89, 130.91. 129.40, 127.90. 120.00, 79.78, 53.67, S1.72,
36.47, 32.31,
CA 02342778 2000-12-20
WO 99/67230 PCT1US99/14233
-182-
27.24; MS (-ESi) m/z 533.9; MS (FAB) m/z 538, 536; 438, 436, 337, 335. 280,
278, 175,
173. 57; Anal. C 55.03, H 5.21, N 7.52, CI 12.81 (calcd for 1.22% H20: C
55.30, H 5.15,
N 7.74. Cl 13.06}.
Example 147.
(Scheme N: N-b where n is l, m is l, X. is NH, RS is 4-[(2,6-
dichlorobenzoyl)amino]-
phenyl. and the stereochemistry is (L))
N [[3-Azetidinyl]carbonyl]-4-[(2,6-dichlorobenzoyl)amino[-1,-phenylalanine,
trifluoroacetic acid salt {CZ°H~gCI2N3O6 .C,HF30z).
Example 147 was prepared from Example 146 by the procedure described in
preparation
71. Physical properties as follows: [a.]-'SD +32 (c 0.87, MeOH); "C NMR
(CD,OD) 8
i 73.32, 170.57, 163.80, 136.70, 136.13, 133.76, 131.84, 130.97, 129.45,
127.93, 120.17,
54.03, 36.59, 35.32); MS (FAB} m/z 438, 436, 391, 331, 175, 173, 101, 55;
Anal. C
46.85, H 4.07, N 7.33, CI 12.39 (calcd for a 1:1 TFA salt with 3.34% H,O: C
46.41, H
3.92, N 7.38, CI 12.45).
Example 148.
[S--(R*,R*)]-4-[[[1-[[4-[(2,6-Dichlorobenzoyl)amino]phenyl]methyl]-2-methoxy 2-
oxoethyl]amino]carbonyl]-3-thiazolidinecarboxyiic acid 3-{2-pyridinyImethyl)
ester
(Scheme A, A-7: where RA_, and RA_2 are the same and equal to proton, R3 is 2-
pyridinylmethyl, Y is CO,-, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and
stereochemistry is (S, S)).
S-- H O
" " O~
~ "~ oho ° ~ ~ p cl
"
H I ~
CI
Example 148 was prepared as described in Scheme A from D-cysteine using 2-
pyridinemethanol to form the requisite carbarnate. Physical data as follows:
'H NMR
(300 MHz, CDCI,) b 8.37 (1 H), 7.70 (1 H), 7.51 (2 H), 7.27 (6 H), 7.08 (2 H),
6.92 (1 H},
5.24 (2 H), 4.77 (3 H). 4.40 (1 H), 3.74 {3 H). 3.37 (1 H;), 3.15 (3 H); "C
NMR (75 MHz,
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-183-
CDC13) b 171.4, 162.5, 155.2, 149.0, 137.1, 136.5, 136.01, 132.4, 130.7,L29.9,
128.0,
123.1, 120.6, 63.0, 53.4, 52.5, 36.8; MS (ESI+) for C~$H,~CI,N,O~S mlz 616.8
(M+H),.;
HRMS (EI) calcd for C,$Hz6Cl,N406S 616.0950, found 1516.0946. Anal. Calcd for
Cz8H26CI2N4O6S: C, 54.46; H, 4.24; N, 9.07. Found: C, 54.61; H, 4.32; N, 8.97.
Example 149.
[S-{R*,R*)]-4-[[[I-Carboxy--2-[4-[(2,6-dichlorobenzoyl)-
amino]phenyl]ethyl]amino]carbonyl]-3-thiazolidinecarboxylic acid 3-(2-
pyridinylrnethyi) ester
(Scheme A, A-8: where RA_, and RA_z are the same and equal to proton, R3 is 2-
pyridinyl-
methyi, Y is CO,-, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and
stereochemistry is (S,
/S-. H~ O
~N~'N~OH
I Nw O~p O ~ ~ ~1 CI
Nl' W
H
I S CI
Example 149 was prepared from example 148 by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 1713, 1666, 1605, 1576, 1561, 1539, 1515,
1442,
1431, 1413, 1351, 1325, 1271, 1194, 766 cni';'H NMR (300 MHz, CD30D) 8 8.80 (1
H),
8.56 (1 H), 7.99 (2 H), 7.58 (2 H), 7.45 (3 H), 7.25 (2 H), 5.43 (2 H). 4.60
(3 H), 3.30 (3
H), 2.93 (2 H); 130 NMR (75 MHz, CD,OD) 8 172.7, 171.5, 163.7, 152.8, 151.3,
146.6,
141.9, 136.8, 136.1, 133.6, 131.8,,130.9, 129.6, 127.9, 126.1, 125.4, 120.1,
63.2, 62.8,
59.4, 53.5, 36.4, 35.1; MS (ESI+) for C27H24C1zN~O6S nv'z 602.9 (M+H)+; MS
(FAB) mlz
(rel. intensity) 603 (MH+, 59), 605 {43), 603 (59), 154 (5I ), 139 (99), 137
(46), 136 (47),
123 (67), 105 (58), 103 (61); 93 (38); HRMS (FAB) calcd for C,7H,aCl,N406S +H,
603.0872, found 603.0876; Anal. Calcd for C,~H,,CI,NaO~S ~ 0.3 HBO: C, 53.26;
H, 4.07;
N, 9.20. Found: C. 52.97; H, 4.23; N, 9.04.
CA 02342778 2000-12-20
WO 99/67230 PGTlUS99/14233
-184-
Example 150.
[S~R*,R*)]-4-[[[1-[[4-[(2,6-Dichlorobenzoyl}amino]phenyl]methyl]-2-methoxy-2
oxoethyl]amino]carbonyl]-~-oxo-3-thiazolidinepentanoic acid
{Scheme A, A-?: where RA_, and RA.Z are the same and equal to proton, R, is
(CHZ)3COzH,
Y is CO-, RS is 4-[(2,6-dichlorobenzoyl)amino]phenyl and stereochemistry is
(S, S)).
S- H OII
O CN~N~Oi
HO'~O O I ~ O CI
/ w
H, I
CI
Example 150 was prepared as described in Scheme A from D-cysteine using
glutaric
anhydride to form the requisite amide. Physical data as follows: IR (mull)
3077, 3053,
3040, 1738, 1728, 1696, 1682, 1641, 1557, 1437, 1430, 1414, 1307, 1232, 1209
crri '; 'H
NMR (300 MHz, CDC13) 8 7.56 (2 H), 7.30 (3 H), 7.08 (2 H), 4.96 ( 1 H), 4.62
(3 H), 3.72
(3 H), 3.28 (4 H), 2.37 (4 H), 1.90 (2 H); '3C NMR (75 MHz, CDC13) 8 179.5,
176.0,
175.6, 173.6, 167.2, 140.6, 140.0, 136.1, 134.5, 133.7, 133.4, 131.8, 124.4,
66.7, 65.9,
57.2, 56.3, 40.3, 39.3, 37.3, 36.7, 36.2, 33.5, 23.6; MS (FAB) m/z {rel.
intensity) 596
(MH*, 90), 598 (63), 597 {40), 596 {90), 341 {25), 263 (.?S}, 230 (32), 225
(31}, 193 (31),
141 (99), 88 (36); HRMS (FAB) calcd for CZ6H,7C12N3O7S +H, 596.1025, found
596.1036.
Anal. Calcd fox Cz fiH27C12N307S ~ 0.3 H,O: C, 51.88; H, 4.62; N, 6.98. Found:
C, 51.69;
H, 4.69; N, 6.59.
Example 151.
[S-{R*,R*)]-4-[[[I-Carboxy-2-[4-[(2,6-dichlorobenzoyl)amino]
phenyl]ethyl]amino)carbonyl]-S-oxo-3-thiazolidinepentanoic acid methyl ester
(Scheme A, A-8: where RA_, and R"_Z are the same and equal to proton, R3 is
(CH,)3CO,CH3, Y is CO-, RS is 4-j(2,6-dichlorobenzoyl)amino]phenyl and
stereochemistry is (S, S)).
CA 02342778 2000-12-20
WO 99167230 PCT/US99114233
-185-
S--. H O
O ~N~N~OH
~O~O O I ~ O Ci
N. ~
H I /
CI
Example 151 was prepared as described in Scheme A from D-cysteine using methyl
glutaryl chloride to form the requisite amide. Physical data as follows: IR
(mull) 3287,
3196, 1724, 1662, 1607, 1562, 1540, 1516, 1431, 1414, 1326, 1268, 1217, 1195,
799 crri';
MS (FAB) m/z (rel. intensity} 596 (MH+, 72), 598 (52), 596 (72), 229 (37), 193
(37), 167
(34), 133 (44), 129 (69), I21 (48), 103 (83}, 89 (99); ' H NMR (300 MHz,
CD30D} 8 7.56
(2 H), 7.43 (3 H), 7.23 (2 H), 4.53 (3 H), 3.63 (3 H), 2.915 (4 H), 2.45 (3
H), 2.24 (2 H},
I.93 (2 H); "C NMR (75 MHz, CDC13) 8 174.0, 172.1, 1.69.9, I62.9, 162.8,
136.1, 132.2,
130.6, 130Ø 127.9, 120.3, 62.9, 54.5, 51.6, 49.8, 36.8, ?.3.5, 33:0, 32.7;
29.6, 19.6; HRMS
(FAB) calcd for C,6H~,C1,N30,S +H~ 596.1025, found 596.1047. MS {FAB) mlz
(rel.
intensity) 596 (MH', 72), 598 {52), 596 (72), 229 (37), 193 (37), 167 (34},
133 (44), 129
{69), 121 (48), 103 (83}, 89 (99).
Example 152.
[S-{R*,R*)]-4-[[[1-Carboxy-2-[4-[(2,6--
dichlorophenyl)methoxyJphenyl]ethyl]amino]-
carbonyl]-3-thiazolidinecarboxylic acid 3-{2--{1-piperidinyl)ethyl] ester
(Scheme A, A-8: where RA., and RA.2 are the same and equal to proton; R3 is 2-
(1-
piperidinyl)ethyl, Y is CO,-, RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl and
stereochemistry is (S, S)).
S-- H O
~N~N " OH
~N~O~O O ( ~ CI
~O~
I/
CI
Example I52 was prepared from example S by the procedure described in
preparation 6.
Physical data as follows: IR (mull) 3254, 2654. 171 I , 1565, 1547, 15I2,
1438, 1344,
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-186-
1300, 1240, 1196, 1179, 1 I 19, 1014, 767 crri ';'H NMR (300 MHz, CD30D) 8
7.45 (2 H),
7.36 (1 H), 7.19 (2 H), 6.97 (2 H), 5.26 (2 H), 4.50 (5 H), 3.60 {11 H), 1.83
(6 H); MS
(ESI+} for C38H33CIZN3O6S m/Z 610.0 (M+H)T; Anal. Caicd for C,8H33C1,N306S ~
1.5 HZO
HCI: C, 49.90; H, 5.53; N, 6.24; Cl, 15.78. Found: C, 49:86; H, 5.43; N, 6.29;
C1, 15.65.
% Water (ICF): 3.99.
Example i 53.
[S-{R *,R* )]-4-[[[ 1-Carboxy-2-[4-[(2,6-
dichlorophenyl)methoxy]phenyl]ethyl]aminoJ
carbonyl]-N-methyl-N-[2-(2-pyridinyi}ethyl]-~3-thiazolidinecarboxamide
(Scheme A; A-8: where R,,_, and RA_Z are the same and equal to proton, R3 is 2-
(2-pyridyl)-
ethyl, Y is CON(CH,)-, RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl and
stereochemistry
is {S, S)).
s-. H~ o
~N~OH
N N O I ~ CI
O
I 5 Example 153 was prepared as described in Scheme A using 2-(2-
methylaminoethyl)-
pyridine to form the requisite urea and hydrolysis according to the procedure
described in
preparation 6. Physical data as follows: mp 80°C (softens),
125°C; IR {mull) 1661, 1611,
1585, 1565, 1511, 1489, 1439, 1394, 1300, 1240, 1196, 1179, 1017. 779, 768
cni';'H
NMR (300 MHz, CD30D) 8 8:44 (1 H), 7.75 (1 H), 7.35 (5 H), 7.I2 (2 H), 6.93 (2
H),
5.22 (2 H), 4.83 ( I H), 4.65 ( 1 H), 4.32 (2 H), 3.77 ( I H;?, 3.45 ( 1 H),
3.20 ( 1 H), 3.00 (5
H), 2.84 (3 H); "C NMR (75 MHz. CD30D) 8 173.0, 1'70.8, 162.4. 158.4, I57.9,
147.9,
138.0, 136.6, 132.2, 130.6, 130.1, I29.3, 128.3, 124.2, 122.0; 114.5, 64.8,
64.7, 53.3, 52.5,
49.7, 35.7, 34.8, 32.8; MS (ESI+) for C,9H3°ClzN4O5S rrxlz 617.0
(M+H}'; Anal. Calcd for
C,9H3oC12N405S: C, 56.40; H, 4.90; N, 9.07. Found: C, :56.31; H, 5.07; N,
8.98.
Preparation 72 and Example 154.
[S-{R*,R*)]-4-j[[ 1-[[4-[(2,6-Dichlorobenzoyl)amino]phenyl]methyl]-2-[(4-
pyridinyl}
methoxy]-2-oxoethyl]amino]carbonyl]-3-thiazolidinecarboxylic acid 3~thy1 ester
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-187-
S- O
~N : NH~ / .
O
/ ~ CI
NH %
C1
To a solution of Example 12 (Scheme A, A-8: where RA_, and RA_z are the same
and
equal to H, R3 is ethyl, Y is COZ, RS is 4-[{2,6-dichlorobenzoyl)amino]phenyl
and
stereochemistry is (S, S)) (400 mg, 0.74 mmol) in dimetlhylformamide (4 mL)
was added
tetramethylguanidine (204 ~L, I.63 mmol) follovi~ed by 4-picolyl chloride (138
mg, 0.81
mmol). The solution was heated to 65 °C for 3 h and volatiles removed
in vacuo.
Purification of the residue by flash chromatography using methylene
chloride/methanol
(2%) as eluant afforded the title compound (320 mg) as ~an amorphous solid: IR
(mull}
3275, 1748, 1677, 1608, 1561, 1539, 1515, 1431, 1415, /344, 1325, 1271, 1222,
1194,
799 crri':'H NMR (300 MHz, CDCl3) S 8.87 (1 H), 8.48 (2 H), 7.50 (2 H), 7.25
(3 H),
7.10 (2 H), 6.93 (2 H), 5.08 (2 H), 4.79 (2 H), 4.61 (1 H), 4.28 (1 H), 4.13
(2 H), 3.16 (4
H), 1.21 (3 H); "C NMR {75 MHz, CDC13) 8 174.6, 170.7, 170.6, 162.6, 149.2,
144.5,
136.9, 136.0, 132.3, 131.7, 130.7, 129.8, 128.0, 122.5, 120.3, 65.0, 63.0,
62.7, 53.3, 37.3,
20.9, 14.5; MS {ESI+) for Cz9Hz8C12N4O6S m/z 630.8 (M:+H)'; Anal. Calcd for
CZ9HZ8C12N4O6S: C, 55.15; H, 4.47; N, 8.87. Found: C, 54.85; H, 4.58; N, 8.74.
Anal. Calcd for CZ9HZ8C12N4O6S: C, 55.15; H, 4.47; N, 8.87; Ci, 11.23; S,
5.08.
Found: C, 54.85; H, 4.58; N, 8.74.
Preparation 73 and Example 155.
[S-(R*,R*)]-3-[[[1-Carboxy-2-[4--[(2,6-
dichlorophenyl}methoxy]phenyl]ethyl]amino]-
carbonyl]-8-methyl-I-thia-4,8-diazaspiro[4.5]decane~-carboxylic acid 4-ethyl
ester
(Scheme B, B-7: where RB_, and RB_z are the same and equal to H, RB_3 and R~,~
together
form a cyclic ring of 6 atoms of the formula -CH,CH,N(CH3)CH,CH,-, Y is CO~,
R3 is
ethyl, RS is 4-[{2,6-dichlorophenyl)methoxy]phenyl and stereochemistry is (S,
S)).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-188-
S H O
sC N N~tJ~OH
~O~O O ( ~ CI
i O~ I w
C
t-Butyl ester B-6 (Scheme B where RB_, and RB_Z are the same and equal to H,
Ra_3 and RH_
together form a cyclic ring of 6 atoms of the formula -CH,CHZN(CH3)CHZCH2-, Y
is
CO2, R3 is ethyl, Ra_5 is O-t-butyl, R5 is 4-[(2,6-
dichlorophenyl)rnethoxyjphenyl and
stereochemistry is (S, S), prepared according to Scheme I3 from 1-methyl-4-
piperidone and
D-cysteine) (681 mg, 1.02 mmol) was dissolved in a solultion of HCI in dioxane
(4 M, 28
mL) at ambient temperature. After 18 h, volatiles were removed in vacuo to
afford a
residue (650 mg) which was lyophilized from water. Further purification of a
portion of
this product (200 mg) was effected by chromatography on a Biotage Flash
40~'~"'' system
using a 40 g KP-C 18-HS (35-70 p,m) silica gel cartridge using aqueous
acetonitrile (40%)
as eluant to afford the title compound (94 mg) as an amorphous powder: IR
(mull) 1696,
1611, 1585, 1565, 1511, 1439, 1404, 1335, 1303, 1271, 1239, 1196, I 178, 1017,
769 cm
'; 'H NMR (DMSO-d6, 300 MHz) S 8.25 (I H), 7.54 (2 H}, 7.43 (1 H), 7.13 (2 H),
6.94 (2
H), 5.16 (2 H), 4.84 ( 1 H), 4.41 ( 1 H), 4.00 (2 H), 3.07 (4 H), 2.73 (4 H),
2.53 (3 H), 1.96
(1 H), 1.69 (1 H), 1.11(3 H);'3C NMR (CDCI3, 75 MHz;I 8 173.9, 169.2, 157.7,
153.2,
136.9, 132.1, 130.7, 130.5. 129.3, 128.5, I 14:7, 73.2, 66.'7, 65.2, 62.8,
54.0, 43.5, 36.5,
I4.6; MS (ESI+) for C~gH33C1,N3O6S m/Z 610.0 (M+H)'; :MS (ESI-) for
CzgH33C~I2N3O6S
n~lz 607.9 (M-H)'; HRMS (FAB) calcd for CZ8H,3C1,N3OE,S+H~ 610.1545, found
610.1561.
Anal. Calcd for CZ8H33ChN3O6S ~ 0.6 HC1 ~ H20: C, 51.70; H, 5.52; N, 6.46; Cl,
14.17.
Found: C, 51.28; H, 5.49; N, 6.50; Cl, 14.57. % Water (I1F): 2.72.
Example 156.
[S~R*,R*)J-4-[[[1-[[4-[(2,6-Dichlorophenyl)methoxy]phenylJmethylJ-2-methoxy-2-
oxoethylJamino]carbonylJ-3-thiazolidinecarboxylic aced 3-(3-tetrahydrofuranyl)
ester
(Scheme A, A-7: where RA_, and RA_Z are the same and equal to proton, R3 is 3-
tetrahydrofuranyl; Y is CO,-, R~ is 4-[(2,6-dichlorobenzoyi)amino]phenyl and
stereochemistry is (S, S)).
CA 02342778 2000-12-20
WO 99!67230 PCT/US99I14233
-189-
S-- H O
O O O I ~ CI
.~ O~' ~ w
C
Example 156 was prepared as described in Scheme A from D-cysteine using 3-
hydroxytetrahydrofuran to form the requisite carbamate. Physical properties as
follows:
mp 125-126:5°C. IR (mull) 3311, 1750, 1744, 1708, 1661, 1549, 1515,
1439, 1408, 1307,
1243, 1227, 1212, 1 173, 1019 cm''; 'H NMR (300 MHz;, CD30D) & 7.38 (3 H),
7.14 (2 H);
7.96 (2 H), 5.20 (i H), 5.25 (2 H}, 4.61 (4 H), 3.79 (4 H); 3.74 (3 H), 3.19
(2 H}, 2:84 (2
H), 2.29 {2 H); '3C NMR ( 75 MHz, CDCIj, spectra cornpIicated via the presence
of
diastereomers) 8171.?, I7I.6, 158.1, 137.0, 132.1, 130.'.>, 130.4, 130.3,
128.5, 128.2,
115.1, 77.2, 77.2, 73.2, 67.0, 65.2, 63.1, 63.0, 53.3, 52.5, 52.4, 36.9, 32.9;
MS (ESI+) for
C,6H;gChN,O,S mlz 582.8 (M+H)*; Anal. CaIcd for C,6HZ8C12NZO,S: C, 53.52; H,
4.84; N,
4.80. Found: C, 53.34; H, 4.87; N, 4.86.
Example 157.
[S-(R*,R*)]-2-[[[1-Carboxy-2-[4-[(2,6-
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]~-hexahydro- y-oxo-1 H-
azepine-1-
butanoic acid
0
N NH~OH
HO O
O r ~ O CI
O
~ ~NH
Cl ~
Example 157 was prepared as described for the preparation of Example 167.
Physical
properties as follows: IR {mull) 1781, 1709, 1651, 1625, 1612, 1550, 1537,
I5I5, 1444,
1431, I4I8, 1398, 1331, 1193, 798 crri '; 'H NMR (300 MHz, DMSO-d6) 8 1.45 (8
H),
2.30 (4 H), 2.90 {3 H), 3.80 { 1 H), 4.50 (2 H), 7. I 6 (2 H), 7.50 (5 H),
7.94 ( I H), 10.62
CA 02342778 2000-12-20
WO 99167230 PCT/US99/i4233
-190-
(1 H), 7.71 (1 H); MS (FAB) mla {rel. intensity) 578 (M+H. 43), 581 {9), 580
{29), 579
(19), 578 (43), 577 {10), 227 {11), 226 (99), 198 (18), 173 (9), 98 {46).
Preparation 74 and Example 158.
(Scheme N, N-6: where n is 2, m is 0, X is N, RS is 4-[(2,6-
dichlorobenzoyl)arnino]phenyl and stereochemistry is 2~~-(R*,R*))
(2S-(R*,R*)j-2-(([1-[(4-[(2,6-Dichlorobenzoyl)amino]phenyijmethylj-2-methoxy-2-
oxoethyljamino[carbonyljazetidine (CZ,HZ,CiZN304).
A solution of the product of example 142 (Scheme N: N'-4 where n is 2, m is 0,
X is N, Y
is -COZ-, R3 is (l,l-dimethyl)ethyl, RS is 4-[(2,6-
dichlorobenzoyl)amino]phenyl and
stereochemistry is (,S~) (512 mg; 0.93 mmol) in 1:1 TFA.ICH,Ch ( 10 mL) is
stirred at rt for
1 h. The reaction mixture is concentrated under reduced pressure. The residue
is taken up
in a mixture of CH~CI, and satd aqueous NaHCO,. The aqueous phase I extracted
twice
additionally with CHZCI,. The combined CHZCI, portions are dried, filtered and
concentrated to give a yellow oil (440 mg); that is purifce;d by silica flash
chromatography
(95:5 CHZC12lMeOH) to afford the title compound (324 mg) as a white foam: mp
113-
1150C; TLC (95:5 CHZCI,/MeOH) Rf= 0.10; [a]'-Sp -34 (c 0.96, MeOH); UV (MeOH)
7v",~ 224 (sh, s 12100), 251 ( 17700), 284 (sh, 2880); IR (mineral oil mull)
3260, 1744, .
1664, 1606. 1561, 1537, 15 i 5, 1431, 1414, 1323, 1270, 1223, 1195, 799, 782
cm '; 'H
NMR (CD30D) b 7.61 (2H), 7.50-7.38 (3H), 7.25 {2H), 4.86 (1H), 4.75 (1H), 4.2I
(1H),
3.73 (3H), 3.61 (2H), 3.39-3.28 (1H), 3.23 (1H), 3.05 (lI-I), 2.63-2.50 (1H),
2.21-2.08
(1H); MS (+ESI) mlz 450.0; MS (EI) mlz 451, 449, 396, 394, 351, 349, 278, 211,
175,
173; 96, 70, 56; Anal. C 55.68, H 4.79, N 8.96, CI 15.43 (calcd for +1.08%
HzO: C 55.41,
H 4.77, N 9.23, Cl 15.58).
Example 159:
O-[{2,6-Dichlorophenyl)methyl]-N-[[(4S~:3-(methylsulfonyl)-4-
thiazolidinyl]carbonyl]-L-tyrosinamide
{Scheme C, C-10: where R~_,_ R~_z, R.c_3 and R~~ are the same and equal to
proton, R3 is
methyl , Y is SO,-, RS is 4-[(2,6-dichlorophenyl)methoxy]phenyl and
stereochemistry is
).
CA 02342778 2000-12-20
WO 99/6'I230 PCTIUS99/14233
-191-
S-. H O~~
CN~N~NH2
O=S=O O
I G
O
G I r
Example 159 was prepared as described in Scheme C using methanesulfonyl
chloride to
form the requisite sulfonamide. Physical properties as follows: mp 228-
230°C; 'H NMR
(300 MHz, DMSO-db) 8 8.03 (1 H), 7.54 (2 H), 7.44 {2 H), 7.14 (3 H), 6.92 (2
H), 5.16 (2
H), 4.69 (2 H), 4.41 ( 1 H), 4.31 ( 1 H), 3.2 I ( 1 H}, 3.01 (3 H), 2.87 (3
H); ' 3C NMR (75
MHz, DMSO-d6) 8172.9, 169.1, 157.5, 136.5, 132.3, 132.0, 130.8, 130.6. 129.2,
114.6,
65.3, 64.4, 54.3, 52.0, 37.3, 35.0; MS (ESI-} fox CZ,Hz3C12N3O5S2 m/z 530.2 (M-
H)'; Anal.
Calcd for C,,H23C1ZN305S2: C, 47.37; H, 4.35; N, 7.89. Found: C, 47.43; H,
4.46; N, 7.81.
Example 160.
[S-{R*,R*)]-3-[[[I-[[4-[(2,6-Dichlorophenyl)metho~:y]phenyl]methyl]-2-methoxy
2-
oxoethyl]amino]carbonyl] -I-this-4-azaspiro[4.4]nonane-~4-carboxylic acid 4-
ethyl ester
(Scheme B, B-6: where RB_, and RB_z are the same and equal to H, RB_3 and RB.~
together
form a carbocyclic ring of 5 atoms, RH_5 is OCH3, Y is CO2, R3 is ethyl, RS is
4-[(2,6-
dichlorophenyl)methoxy]phenyl and stereochemistry is (S, S)).
S-- H O
~N~N~O~
~O~O O I ~ CI
O~
Ir
CI
Example 160 was prepared as described in Scheme B. Physical properties as
follows: IR
(mull) 1746, 1705, 1681, 1510, 1439, 1399, I336, 1301" 1276, 1241, 1203, 1178,
1110,
1017, 769 cm ';'H NMR (CDCl3) 8 7.36 (2 H), 7.25 (I JEI), 7.03 (2 H), 6.93 (2
H), 6.64
( 1 H), 5.22 (2 H), 4.85 (2 H}, 4.15 (2 H), 3.73 (3 H), 3.15 (4 H), 2.69 ( 1
H), 2.48 { 1 H),
1.76 (6 H), 1.23 (3 H); '3C NMR (CDC13) b 171.5, 170. 7, 158.0, 152.5, 137.0,
132.0,
130.5, 130.3, 128.5, 128.2, 1 I5.0, 66.4, 65.2, 6I.9, 53.1, 52.4, 37.1,32.3,
25.1. 24.6, 14.5;
MS (ESI+) for CZ8H3zC1,N,ObS mlz 594.9 (M+H)+. MS (ESI-) for C,8H3,Cl,N,O6S
mlz
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-192-
592.8 (M-H)'; Anal. Calcd for CZ8H32Cl~N,O6S ~ 0.10 H'O: C, 56:30: H, 5.43; N;
4.69.
Found: C, 56.20; H, 5.24; N, 4.69. % Water {KF): 0.31.
Example 161.
[S-(R*,R*)]-3-[[[ 1--Carboxy-2-[4-[(2,6-
dichlorophenyl)methoxy]phenyl]ethyl]amino]carbonyl]--I thia-4-
azaspiro[4.4]nonane-4--
carboxylic acid 4-ethyl ester
(Scheme B, B-7: where R$_, and RB_2 are the same and equal to H, RB_3 and RB.~
together
form a carbocyclic ring of 5 atoms, Y is COZ, R3 is ethyl, RS is 4-[{2,6-
dichiorophenyl)-
methoxy]phenyl and stereochemistry is (S, S)).
S- H~ O
~N~~~OH
~O~O O I ~ CI
~ o
Cl
Example 161 was prepared ftom example 160 by the procedure described in
preparation 6.
Physical properties as follows: IR (mull) 1737, 1708, 1675, 1612, 1511; 1439,
1402,
1338, 130I, 1241, 1197, 1179, 1115, 1018, 769 cm'';'H NMR (DMSO-db) 8 8.07 (1
H),
7.54 (2 H), 7.45 ( 1 H), 7.12 (2 H), 6.94 (2 H), 5.15 (2 H), 4.63 ( 1 H), 4.3
3 ( 1 H), 3.91
(2 H), 3.05 (2 H), 2.79 {I H), 2.60 (1 H), I,60 (6 H), 1.07 (3 H); "C NMR
(DMSO-d~) b
172.7, I69.4, 157.1, 155.7, I35.9, 131.6,, 131:4, 130.3, l:?9.6, 128.7, 114.1,
83.9, 64.7,
60.5, 53.1, 38.0, 36.2, 31.8, 24.2, 24.1, I4.1; MS (ESI+) for C27H3oC12N206S
m/z 580.8
(M+H)+; MS (ESI-) for Cz,H3oCI,N206S mlz 578.8 (M-H)'; HRMS (EI) calcd for
Cz7H3flC1zN,O6S 580.1202, found 580.1172; Anal. Calcd for CZ,H3oC12NzO6S ~ 0,
i 9 HzO:
C, 55.44; H, 5.24; N, 4.79. Found: C, 55.24; H, 5.32; N, 4.79. % Water (KF):
0.59.
Preparation 75.
0
HZN~a.cH3 . Hc'
~ o ci
N W
H
CI
The aminoester product of preparation 75 i useful as a synthetic intermediate
(for
example, reagent A-4 of Scheme A).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-193-
To a cold (0-5°C) solution of anhydrous methanolic HCl was added i 00 g
of L-4-
nitrophenylalanine (Advanced ChemTech) portionwise over 15 min. The
mechanically
stirred mixture was heated to gentle reflex for 48 h. The mixture was allowed
to cool and
then filtered through a sintered glass filter funnel, washing the collected
solids with hot
MeOH until only insoluble residues remained. The filtrate was concentrated in
vacuo to
afford the methyl ester ( 120 g) as waxy off white solid which was used
without further
purification.
To a suspension of methyl ester described above (87 g, 0:33 mole) in CH2CI,
(1500 mL) at
ambient temperature was added di-t-butyldicarbonate ( 109 g, 0.50 mole)
followed by the
dropwise addition of Et3N (51 mL, 0.37 mole). After 15 min additional Et3N (40
mL, 0.29
mol) was added to maintain a slightly basic mixture (ca.. pH 8). The reaction
mixture was
stirred 18 h and additional CH~Ch (1400 mL) and Et3N {15 mL, 0.11 mol) were
added.
After an additional 2 h the reaction mixture was quenched by the slaw addition
of MeOH
{100 mL), stirred for I h and then partitioned between CHZCI, and cold 10%
aqueous
KHS04. The organic layer was washed with saturated NaHC03 and brine, dried
(Na2S04),
filtered and concentrated in vacuo. Flash chromatography of the residue using
hexane and
a gradient of a 1:1 mixture of EtOA~/CHZCIz (25-33%) afforded the Boc-methyl
ester (69
g) as a white solid. Physical properties as follows: ~H NMR (300 MHz; CDCl3) S
8.16
(2H), 7.31 (2H), 5.04 ( 1 H), 4.63 ( 1 H), 3.73 (3H), 3.18 (:ZH), 1.41 (9H);
MS (ES+) for
2O C,SHZ°N2O6 m/z 325.2 (M+H)'.
Palladium on carbon ( 10% w/w, 1.25 g) was added to a Parr hydrogenation flask
under a
N, atmosphere and carefully wetted with 100 mL of MeOH/THF ( 1:1 ). A solution
of the
Boc-methyl ester described above (25 g, 77 mmol) in 400 mL of MeOH/THF ( 1: i
) was
added and the mixture shaken on a hydrogenation apparatus under a hydrogen
atmosphere
{20 psi) for 1 h at ambient temperature. The reaction mixture was filtered
through a pad of
Celite and the solids washed several times with MeOH. The combined filtrates
were
concentrated in vacuo to afford the 4-aminophenylalanyl derivative (22.7 g)
which was
used without further purification. Physical properties as. follows: 'H NMR
{300 MHz,
CDC1,) 8 6.89 (2H), 6.61 (2H). 4.96 {1H), 4.50 {IH), 3.59 {3H). 2.95 (2H),
1.4I (9H); MS
(ES+) far C,SH"N,O, m/~ ?95.2 (M+H)'.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-194-
A cold (0-5°C) solution of 2,6-dichlorobenzoyl chloride (l I.1 mL, 77.5
mural) in 125 mL
of ~THF was treated dropwise with a solution of the 4-alminophenylalanyl
derivative
described above (22.7 g, 77.1 mmol) and Et,N ( 16 mL, 1:15 mmol) in 125 mL of
THF.
The reaction mixture was allowed to warm to temperature and stir an additional
18 h. The
mixture was diluted with EtOAc (2 L) and then washed with 1 N HCI, H~O, 1 N
NaOH and
brine. The organic extract was dried (Na,S04), filtered, and concentrated in
vacuo to give
the crude product as a pale yellow solid. This material r~ras recrystallized
from
acetone/hexanes (ca. I : I ) to afford the amide (30.8 g} as a crystalline
solid. Physical
properties as follows: mp 192.2-193.1°C; IR (mull) 3305, 1747, 1736,
1690, 1665,1609,
I548, I S I2, 1433, 1414, 1325, 1277, 1219, 1 I 7I. 781 cni'; 'H NMR (300 MHz;
CDCI3) 8
7.57 {2H), 7.34 (4H), 7. I4 (2H), 4.98 ( 1 H), 4.60 ( 1 H), 3.74 (3H), 3.1 i
(2H), 1.42 (9H);
MS (ES+) for C,zH24C12Nz05 mlz 467:0 {M+H)+:
To 650 mL of anhydrous 4M HCI in dioxane at ambient temperature was added the
amide
described above (30.6 g, 65.5 mmol) portionwise and the; resulting mixture was
stirred
until all the solids had dissolved (ca. 1 h). Volatiles were removed in vacuo
to give a light
yellow solid which was partitioned between water (500 mL) and ether ( I L).
The water
layer was separated and concentrated in vacuo to approximately 200 mL. The.
aqueous
solution was then frozen and lyophilized to afford the aminoester product
(25.6 g) as a
light yellow solid. Physical properries as follows: [a]25,~ _ +5 (c 1, MeOH);
IR (mull)
3244, 3186, 3112, 1747, 1660, 1604, 1562, 1539, 1516, u431, I4I6, 1327, 1273;
1243,
799 crri';'H NMR (300 MHz; CD30D) 8 7.69 (2H), 7.45 (3H), 7.29 (2H), 4.34
(IH), 3.83
(3H), 3.2I (2H); '3C NMR (300 MHz; CD30D) 8 169.0, 163.9, 137.8, 136.08,
131.8,
131.0, 130.3, 129.7, 127.9, 120.6, 53.8, 52.3, 35.4; MS (13S+} for
C"H,6Cl,N,O, m/z 367.1
(M+H)'.
Preparation 76.
0
li2N~a.CHg ~ HC!
CI
a w
i
CI
CA 02342778 2000-12-20
WO 99J6'1230 PCT/US99114233
-195-
The aminoester product of preparation 75 is useful as a synthetic intermediate
(for
example, reagent A-4 of Scheme A).
To a cold {0-5°C) solution of anhydrous methanolic HCI (200 mL) was
added 25 g of N
a-t-Boc-O-2,6-dichlorobenzyl-L-tyrosine (Sigma) portionwise over 15 min. After
30
minutes at 0-5°C, the mixture was heated to 50°C for 2 h. The
solution was cooled to
room temperature and the volatiles removed in vacuo. T'he solid was suspended
in ethyl
ether and collected by filtration to afford the title compound (21.4 g) which
was used
without further purification. Physical properties as follows: [a]ZSp _ +I6 (c
1.00,
ethanol};'H NMR (300 MHz, CD30D) b 7.44 (2 H}, 7.35 (1 H), 7.21 (2 H), 7.02 (2
H),
5.28 (2 H), 4.29 {1 H), 3.81 (3 H), 3.18 (2 H); MS (ESI-+~) for C"H"C1,N03 mlz
354.1
{M+H}+; Anal. Calcd for C"H"C12N03~ HCI: C, 52.26; l~, 4.64; N, 3.59. Found:
C,
52.17; H, 4.74; N, 3.6I.
Example 162
2-[[(( 1 S)-1-[[4-[(2,6-Dichlorobenzoyl)amino]phenyl]methyl] -2-methoxy-2-
oxoethyl]amino]carbonyl-1-piperidinecarboxylic acid l-((1,1-dimethyl)ethyl]
ester
0
O
OH N
O
O. ~ w O d > Vibe O ' ~ ~ 0 d
N
d li ~ w
Ex.162 HG
Example 162: HC1 gas was bubbled through a solution of N (tert-butoxycarbonyl)-
4-(2,6-
dichlorobenzoylamino}-L-phenylalanine (2.51 g, 5.53 mrnol) in MeOH (20 mL) for
10
minutes. The solution was stirred for additional 2 hours at room temperature.
The solvent
was removed in vacuo and the excess HGl was removed by the addition of Et20 (3
x 15
mL) and evaporation under reduced pressure. The resultant gum was dissolved in
THF
( 10 mL) and N-ten-butoxycarbonyl-pipecolinic acid ( 1.28 gm, 5.59 mmol), BOP-
reagent
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/I4233
-196-
(2.69 grri, 6.09 mmol) and DIEA (2.9 mL, 16.6 mmol) were added and the
reaction
mixture was stirred overnight. EtOAc (25 mL) was added and the mixture was
extracted
with 1N HCl (20 rnL). The organic phase was washed vvith saturated LiCI (20
mL) then
saturated NaHCO3 (30 mL). The organic layer was dried over Na2S04, filtered
and
evaporated. Chromatography of the residue (silica gel, Hexanes -> 50%
EtOAcIHexanes
gradient elution) provided Example 162 as a solid (1.45 gm, 45 %): ESMS (m/z)
578, 580
(MH+).
Example 163
2..[[[(1 S)-1-[[4-[(2,6-Dichlorobenzoyl)amino]phenyljmethyl]-2-methoxy-2-
oxoethyl]aminoJcarbonyl]-y-oxo-1-piperidinebutanoic acid
F~Ya ~ O N~NH~,O/
pi
Example ~~ I ~~~~p ~~ O CI
162 D C1 ---r CfI))'
I ~ NH
Example 163 CI
Q
HCI gas was bubbled through a MeOH (20 mL) solution of Example 162 (1.27 gm,
2.20 mmol) for IO min. Stirring was continued overnight at room temperature.
The
solvent was removed irr vacuo and the excess HCl was removed by washing with
Et20 (3
x 10 mL) on a vacuum filter. The HCl salt was completely dried under
highvacuum to
provide Compound 1 (1.09 gm, 97 %) as a solid: ESMS (m/z) 478; 480 {MH+).
Example 163: Compound 1 (147 mg, 0.285 mmol) was dissolved in DMF~(5 mL)
containing DIEA {I50 pL, 0.88 mmol). To this solution was added succinic
anhydride (59
rng, 0.59 mmol) and the mixture was stirred at 50°C for :S hr under dry
nitrogen. The
solvent was evaporated and the residue was purified by column chromatography
{silica
gel, Hexanes -> EtOAc gradient elution) to provide Exannple 163 as a solid (
164 rng):
ESMS (m/z) 578, 580 (MH+)
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-197-
Examples 164-166
The following mono methyl esters were prepared in a similar manner as Example
163
0
NH~O~
O
R3 / O - C1
~NH~ /
CI
ex# R3 MS
164 646
Hooc (MH'~
165 ~ 620
Hooc (MH+)
166 Hp0~ 574
([M=H]-)
Example 167
2-[[[(1S)-1-Carboxy-2-[4-[(2,6-
dichlorobenzoy!)amino]phenyl]ethyl]amino]carbonyl]-y-
axo-1-piperidinebutanoic acid
0
N NHv 'OH
O
HO~~~~ / ( p C!
O ~~,;H /
CI
Example 167
Example 163 ( 154 mg, 0.266 mmol) was treated with LiOH {26 mg, 1.07 mmol) in
H20 (5 mL) for 3 hours. The product was then precipitated by the addition of 3
N HCI.
CA 02342778 2000-12-20
WO 99/6723b PCT/US99/14233
-19$-
The product was collected by vacuum filtration and washed with cold H20 (2 x 3
mL).
Drying under high vacuum provided Example 167 as a solid ( 109 mg): ESMS (m/z)
562
([M-HI )~
Example 168-170
The following compounds were prepared in a similar manner as 167.
0
N NN~nu
O
R3
ex# Rs MS
( )
168 630
HOOC"'
([M-HJ->
169 604
HOOC"'
( [M-H)-)
170 Hood 560
([M-HJ-)
Preparation 77
P ~~~OH .1, ~~H~O~Re
C ~ / +
Rs-OI HC ' ~ HC ~
llerrifield resin fntertnediate-1
p
O = polymer
CA 02342778 2000-12-20
WO 99167230 PCT/US99I14233
-199-
Intermediate-1: Attachment of N-tent-butoxycarbonyl-~[4-(2,6;
dichlorobenzoylamino)]-
L-phenylalanine to Merrifield resin was done using Horiki's method (Horiki et
al., Chem.
Lett. 1978(2) 165-168). In a 250 mL round bottom flask fitted with a drying
tube,
Merrifield resin (Biorad, 10.0 g, 13.5 mmollg) and anhydrous potassium
fluoride (Aldrich,
1.57g, 27.0 mmol) were added to a solution of N-tert-butoxycarbonyl-[4-(2,6,-
dichlorobenzoylamino)]-L-phenylalanine {Bachem California, 6:13 g, 13.5 mmol}
in dry
DMF ( 100 mL). The reaction mixture was stirred at 80QC in an oil bath for 24
hr. The
cooled resin was then filtered and washed thoroughly with DMF (2 x 250 mL),
50%
aqueous DMF (3 x 250 mL), methanol (3 x 250 mL}, dichioromethane (3 x 250 mL),
and
fnally methanol {3 x 250 mL). The resin was then dried under reduced pressure
to
constant weight to give intermediate-1. Incorporation ofN-tent-butoxycarbonyl-
[4-(2,6,-
dichlorobenzoylamino)]-L-phenylalanine onto the resin was estimated to be
0.045 mmol/g
from the increase in resin mass.
Example 171
N [[2-(I,3-Benzodioxol-5-yl)-1-methyl-5-oxo-3-pyrrolidinyljcarbonyl]-4-[(2,6-
dichlorobenzoyi)amino]-L-phenylalanine
° ~ \ /
O N ~°~ Rg 1V
O~ N' ~
O CI "..~, v 'OH
0
~ ~ O CI
Intem~ediate-1 CI ~ Examq~le 171 ~ N /
H
CI
Example 171: The Intermediate-1 (0.15 g, 0.1065 tnmol/g) was pretreated with
CH2C12
(2x 3 mL). The swollen resin was then deprotected with 50% TFA/CH2Cl2 (3mL,
30min). The resin was rinsed in the following order: CH2Cl2 (2 x 3 mL), CH30H
{2 x 3
mL), CH~CI~ {2 x 3 mL). The resin was swollen with DMF (2 x 3 mL). 2-(3,4-
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99114233
-200-
methylenedioxyphenyl)-1-methyl=5-oxo-3-pyrrolidine carboxylic acid (84 mg,
0.32 mmol}
in DMF (1.0 mL) was activated with 0.5 M HBTU/HOBT in DMF (0.7 mL) and DIEA
(0.139 mL, 0.799 mmol), and then added to the swollen resin. The mixture was
vortexed
for 2 hr at room temperature. The resin was filtered and washed in the
following order:
DMF(2 x 3 mL}, CH2Cl2 {2 x 3 mL), CH30H {2 x 3 mL), CH2Cl2 (2 x 3 mL),
respectively. If a Kaiser test on a small quantity of the resin is positive
(blue) then repeat
the coupling procedure until a negative result is obtained. The resulting
resin was then
dried in vacuo to constant weight. The resin was placed in the polypropylene
column and
pretreated with 3 mL of THF. Then to the preswollen resin 1.6 mL of THF, 0.48
mL of
I 0 CH30H, and 0.160 mL of 2N LiOH were added. The mixture was vortexed for I5
min
and filtered to a clean and preweighed test tube. The resin was next washed
with 2mL of
THF/S% CH30H (2x) and the combined filtrates were evaporated. The resulting
gum was
dissolved in I mL of water. The solution was then acidified with I N HCl to pH
2Ø The
precipitate was centrifuged, washed with water (SmL, 2x) and dried in vacuv to
furnish
15.4 mg of Example 17I as a solid. ESMS (m/z): 596, {[M-H]-).
Preparation 78
0
0
O NH~O~RB R
Bac~x~NH~O~ B
t
/ O CI
--i / O CI
NH ./
NH
Lrtctmadiate-1 ~ ~ Irttertrtedeic-2
CI
CA 02342778 2000-12-20
WO 99167230 PCT/US99114233
-201-
~ ~ E O F G
~~ N'~(' ~ ~O
I O I ~ _ N
O
X,I~ I
H ~ J _ K
'i 10 ~~O N~O ~N~~O
I. I
Intermediate-2F: The resin bound N-tert-butoxycarbonyl-[4-(2,b-
dichlorobenzoylamino)J-L-phenylaianine (Intermediate-1), (250 mg, 0.1 I25
mmollg) was
placed in a 8.0 mL, polypropylene filter column fitted with a 2-way
polypropylene
stopcock. The resin was pretreated with CH2C12 (2 x 3 mL). The swollen resin
was then
deprotected with 50% TFA/CH2Cl2 (3-4 mL, 30min) with shaking. The resin was
rinsed
in the following order: CH2C12 (2 x 3 mL), CH30H (2 x 3 mL), CH2C12 (2 x 3
mL).
The resin was swollen with DMF {2 x 3 mL). N-Tert-butoxycarbonyl-nipecotic
acid (103
mg, 0.45 mmol) in DMF ( I .0 mL) was activated with 0:5 M HBTU/HOBT in DMF
(0.9I 0
mL) and DIEA (0.195 mL), then added to the swollen resin. The mixture was
vortexed for
2 hr at room temperature. The resin was washed in the fallowing order: DMF (2
x 3 mL),
CH2Cl2 (2 x 3 mL), CH30H (2 x 3 mL), CH2Cl2 (2 x 3 mL) and dried (Intermediate-
ZF). If a Kaiser test on a small quantity of the resin is positive (blue) then
repeat the
coupling procedure until a negative result is obtained.
The intermediate resins Intermediate-2D, 2E, 2G, 2H; 2I, 23 & 2K were each
produced
following this procedure.
Example I72
S-[[(2S)-2-[ [[( 1 S )- I -Carboxy-2-[4-[(2,6-
dichlorobenzoyl)amino]phenyl]ethyl)amino]carbonyl]-1-piperidinyl]carbonyl]-3-
pyridinecarboxylic acid
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-202-
~N~~s N O
.b O U' ~ ~ _'~O H
O O ~ O C I -~,- O
I~ N i' N~ O I~ O CI
i ~ N
COON I~ I i
Intermediate-2D
Example 172
Example i 72: Resin bound Intermediate-2D (2.0 g, 1.~ mrnollg) was pretreated
with
CH2C12 (2 x 20 mL). The swollen resin was then deprotected with 50% TFA/CH2Cl2
(20
mL, 30min). The resin was rinsed in the following order: CH2C12 (2 x 20 mL),
CH30H
(2 x 20 mL), CH2Cl2 (2 x 20 mL). The resin was swollen with DMF (2 x 20 mL).
3;5-
Pyridine dicarboxylic acid (652 mg, 3.9 mmol) in 20 mL of DMF was activated
with 0.5
M HBTUIHOBT in DMF (8.0 mL) and DIEA ( 1.7 mL, 9.75 mmol), then added to the
swollen resin. The mixture was vortexed for 2 hr at room temperature. The
resin was
fltered and washed in the following order: DMF(2 x 20 mL), CH2C12 (2 x 20 mL),
CH30H (2 x 20 mL), CH~CI2 (2 x 20 mL), respectively.. If a Kaiser test (Kaiser
et al.,
Anal. Biochem. 1970, 3-~, 594-598) on a small quantity of the resin is
positive (blue) then
repeat the coupling procedure until a negative result is obtained. The
resulting resin was
then dried in vacuo to constant weight (2.2 g). The resin was treated with 25
mL of liquid
HF by stirring for 60 min at 0°C in an HF-reaction apparatus (Peninsula
Laboratories Inc.,
I S Belmont, CA). The HF was rapidly evaporated off by vacuum aspiration at
0°C. Then
100 mL of dry ethyl ether was added. The resin and the resulting precipitates
were filtered
off and washed three times with 50 mL of ethyl ether, and dried in vacuo. The
mixture
was then treated with 25 mL of 1N NaOH (4x), and the combined solutions were
Lyophilized. The crude product was purified by HPLC using a C-18 column and a
linear
acetonitrile/0. l % HCI gradient. The gradient was run from 60% solvent A (D.1
% HC1) to
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-203-
80% solvent B (80% acetonitrile in 0.1 % HCi) in 20 mina Lyophilization
furnished 20 mg
(2.5%) of Example 172. ESMS (m/z): 612 ([M-H]').
Examples I73-267
0 0
Boc~ X~NH~O~ Rg Q~ X~ NH~ OH
\ ~ O C~ ~,. ~ ~ O Cl
NH ~ ~~
Cl~ CI \
Method-A (Example 173)
4-[{2,6-Dichiorobenzoyl}amino]-N [[1-(3-methoxy-1-oxopropyl)-3-
piperidinyl]carbonyl]-
1.-phenylalanine
0
iO~ N ~N'~OH
O o
O CI
i
Hi I ~
CI
Example 173
The Intermediate-2F (0.25 g, 0.1125 mmol/g) was pretreated with CH2C12 (2 x 3
mL}.
The swollen resin was then deprotected with 50% TFA/CH2Cl2 (3mL. 30min). The
resin
was rinsed in the following order: CH2C12 (2 x 3 mL), CH30H (2 x 3 mL), CH2Cl2
(2 x
3 mL). The resin was swollen with DMF (2 x 3 mL). 3-methoxypropionic acid (53
mg,
0.45 mmol) in DMF ( 1.0 mL) was activated with 0.5 M HBTU/HOBT in DMF (0.910
mL) and DIEA (0. i 95 mL), then added to the swollen resin . The mixture was
vortexed for
2 hr at room temperature. The resin was filtered and washed in the following
order: DMF
CA 02342778 2000-12-20
WO 991b7230 PCT/US99/14233
-204-
(2 x 3 mL), CH2C12 {2 x 3 mL), CH30H (2 x 3 mL), CH2C12 (2 x 3 mL),
respectively. If
a Kaiser test on a small quantity of the resin is positive (blue) then repeat
the coupling
procedure until a negative result is obtained. The resulting resin was then
dried in vacuo
to constant weight. The resin was placed in the polypropylene column and
pretreated with
THF (3 mL). Then THF (3.5 mL), CH30H ( 1.0 mL) and 2N LiOH (0.175 mL) were
added. The mixture was vortexed for 15 min and filtered to a clean and
preweighed test
tube. The resin was next washed with THF/5% CH30H (2mL) and the combined
filtrates
were evaporated. The resulting gum was dissolved in H?O (1 mL). The solution
was then
acidified with 1N HCl to pH 2Ø The precipitate was centrifuged, washed with
water (2 x
5 mL} and dried in vacuo to furnish 38.3 mg of Example; 173 as a solid: ESMS
(m/z} 548
([M-H]-).
Method B (Example 174):
3-[[[( 1 S}-1-Carboxy-2-[4-[(2,6-
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]-y-
oxo-1-piperidinebutanoic acid
O H O
HO~'N~N'~OH
O O ~ ~ O CI
H 1~
CI
Example 174
The Intermediate-2F (0.25g, 0.1125 mmollg) was pretreated with CH2C12 (2 x 3
mL). The swollen resin was then deprotected with 50% 'rFA/CH2CI2 (3mL. 30min}.
The
resin was rinsed in the following order: CH2C12 (2 x 3 mL), CH30H (2 x 3 mL),
CH2Cl2
(2 x 3 mL). The resin was then swollen with DMF (3mL). Succinic anhydride (45
mg,
0.45 mmol} dissolved in DMF (4 mL) was added to the swollen resin and stirred
at 50°C
for 2hr. The resin was filtered arid washed in the following order: DMF (2 x 3
mL),
CH2Cl2 (2 x 3 mL), CH30H (2 x 3 mL), CH2Ch (2 x 3 mL), respectively. if a
Kaiser
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-205-
test on a small quantity of the resin is positive (blue} then repeat the
coupling procedure
until a negative result is obtained. The resulting resin was then dried in
vacuo to constant
weight: The resin was placed in the polypropylene cohunn and pretreated with
THF (3
mL). Then to the swollen resin THF (3.5 mL), CH30H ( 1.0 mL) and 2N LiOH
(0.175
mL} were added. The mixture was vortexed for 15 min and f ltered to a clean
and
preweighed test tube. The resin was next washed with 'THFIS% CH30H (2 x 2 mL)
and
the combined fprates were evaporated. The resulting gum was dissolved in H20
(1 mL).
The solution was then acidified with 1 N HC1 to pH 2.0~. The precipitate was
centrifuged,
washed with H20 (2 x 5 mL} and dried in vacuo to furnish 30.5 mg of Example
174 as a
solid: ESMS (m/z} 562 ([M-H]-).
Method C (Example 175}:
N [[1-[[(4-Carboxyphenyl)amino]carbonyl]-4-piperidinyl]carbonyl]-4-[(2,6-
dichlorobenzoyl)amino]-t,-phenylalanine
O~ O
H N '~OH
w NON
O Ct
HO ( i O ~ 1 ,~ N
O H I~
Cl
Example 175
The Intermediate-2G (0.25g, 0.1 I25 mmol/g) was pretreated with CH2Cl2 (2 x 3
mL). The swollen resin was then deprotected with 50% TFAlCH2C12 (3mL, 30min).
The
resin was rinsed in the following order: CH2Cl2 (2 x 3 mL), CH30H (2 x 3 mL),
CH2C12
(2 x 3 mL). The resin was then swollen with DMF (3mL). Ethyl 4-
isocyanatobenzoate
(22 mg, 0.108 mmol} dissolved in DMF (3 mL) and DIEA (471zL 0.27 mmol) were
added
to the swollen resin. This reaction mixture was vortexed for 6-8 hr at room
temperature.
The resin was filtered and washed in the following order: DMF (2 x 3 mL),
CH2CI2 (2 x
3 mL), CH30H (2 x 3 mL), CH2Cl2 (2 x 3 mL), respectively. if a Kaiser test on
a small
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-206-
quantity-of the resin is positive (blue) then repeat the coupling procedure
until a negative
result is obtained. The resulting resin was then dried in vacuo to constant.
The resin was
placed in the polypropylene coiumn and pretreated with THF (3 mL). Then to the
swollen
resin THF (3.5 mL}, CH30H (1.0 mL) and 2N LiOH (0.175 mL) were added
respectively.
The mixture was vortexed for 15 min and filtered to a clean and preweighed
test tube.
The resin was next washed with THF/S% CH30H (2mL) and the combined filtrates
were
evaporated. The resulting gum was dissolved in H20 ( 1 rnL}. The solution was
then
acidified with 1N HCl to pH 2Ø The precipitate was centrifuged, washed with
H20 (2 x
5 mL) and dried irr vacuo to furnish 18 mg of Example 175 as a solid: ESMS
(m/z) 625,
(~M-H]-)
Examples 176-266
The following compounds were prepared in a similar manner as described above.
0
H
N
~ X;
~ \ o cl
H
CI
ex# Method Q X, MS
(~z)
176 A "-:~ ~ 560
UM-H]-)
177 A '~co~ ~ 548
.a
(LM-Hl
)
178 A o 616
C'~' -
i-
o (fM-Hl
)
179 A 560
( [M-H]-)
~i
CA 02342778 2000-12-20
WO 99167230 PCTILTS99/14233
-207-
1-80 A ~c° \ ° ~ 626
"~~° i N o ([M-H)-)
181 A ° 610
~ ' cooH ~ ([M-H _
182 A ° ) )
610
' ' ' N o ([M-H)-)
cooH
183 A ~ I ° ~'~ 660
~ o ( [M-H)-)
HOOC '
184 A ° 611
H00 ' ~
" ~ o ([M-H)-)
185 A ' i ° ~ 567
N ~ o ([M-H)-)
186 A ° 611
~~ ~
~ " ~ o ~[M-H)-)
HOOC
187 A ° ~ 611
Hooc ~ " N ° ([M-H)-)
188 A °" ~ 633
~ ' H ([M-H)-)
0
189 A HOOC ' NN ~~ 600
N o ([M-H)-)
O
190 A "°°c~.,,S 621
HN..( N ° M-H '
o/\j~
19I A °~" 603
° C~
° ([M-H)-)
192 A 560
0
M-H _
193 A ~ ~ ([ ) )
560
o ~ ([M-H)-)
194 B Hood ~~° 562
' QM-H)-)
195 B ~~° 630
Hooc ° '~ ([M-H)-)
196 B ~ ~°
604
HOOC ° ~N
( [M-H)-)
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-208-
19? B \ "~~y ~ S60
' ([M-Hl-
198 A "c.~ ~~ 560
0 N
~ ([M-H]-)
199 A ~,co a S48
('~.~
N
' ([M-H]-)
200 A 616
'
([M-H]
0 )
201 A ~ ~~ S60
o % ( [M-H]-)
202 C , HOOC~N~ ~ S63
' ([M-H]-)
203 A ,~co~ (~-.~ 626
rtco ~ ~ ~ ([M-H]-)
204 A 0
~-~..~ 610
cooH ~ (~M-H]-)
20S A ~ 610
~
' ([M-H]-)
CooH
206 A 660
.
i
i ' ' ([M-H]-)
HOOC
20? A "~ ~~ 611
N
' ( [M-H]-)
2os c "~ ~ . ~~ 62s
" ' ([M-HI-)
209 B "~,~ _ ~~ S62
' ([M-H]-)
210 B 630
_ H~
Hooc ([M-H]-)
211 B _ ~ 604
HpOC' "
([M H]
)
212 B Hoocw _ S60
N
( [M-H]-)
213 A "c.~ -N~ S60
o ~
_
(~M
] )
214 A ~co~ ' ~o g
O ~N
([M-H]
)
i;,
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-209-
215 A _ ~° 616
N
° ([M-HJ-)
216 A ~ _ N~° 560
([M-HJ-)
217 C "ooc~N~ _h~° 563
o ([M-HJ-)
218 A ° ~ ~~° 626
h6C0~ - h~
~,co /''~~ ~/ ~ ([M-HJ-)
219 A ° _ ~° 610
i. N
' co°H ([M-HJ-)
220 A ° _ ° 610
N
cooH ([M HJ )
22 I A ° _ N~° 660
~ ~ 1
"ooc ~ ~ ([M-HJ-)
222 A ° ° 611
HOOC~ - N
L~ ~9T ~ ([M-HJ )
N
223 C "°°c ~ ~ ~ _ ~~° 625
N
" ([M-HJ )
224 B ° 0 548
"~.~ «..~
([M-HJ-)
225 B ~0 616
HOO~ W
([M-HJ-)
226 B ~0 590
"~~ ~~ -
([M-Hl-)
227 B "o~~° .- ~~0 546
a
228 A "°°c~ ° ([M ~J )
229 A ~,co ([M-HJ-)
'~1~ ~~0 534
0 N
([M-HJ-}
230 A ~0 602
~N
° ( [M-HJ-)
231 A ~ C~o 546
o .~i
([M-HJ-)
232 C HOOCvN~ ~0 549
'°1 ~ ~ ~ ([M-H]")
i.,
CA 02342778 2000-12-20
WO 99/67230 pCT/US99/14233
-210-
233 A ~c ~ (~0 6I 2
~
' ' ( CM-H]-)
~CO
234 A (~o S96
N
' Coo" i ([M-HJ-)
23S A ' ( o S96
(CM-H]-)
coo"
236 A (~0 646
'
~ ; ~ ' (fM-H]-)
HOOC
237 A "oo S97
'
I ([M-H] )
N
238 C "ooc ~ 0 6I1
I ~
JL
/ N N
~
" ~ (CM-HJ-)
239 A 5 S7I
~
~
~ N ~ ([M_H]-)
240 A ~'''~''~~ 615
r I '~
~N~O
' N y LM-H]-)
Nooc
241 A ~~ 61S
~ N~
"ooC ' " ~ ~ (CM-HJ-)
242 A " ~ 637
I ' ~ N~O
' N ([M-H]-)
0
243 A HOOC N, ~S~ 604
([M-HJ-)
O
244 A roo ~S ~ 62S
N~ (CM-H] )
'
24S A S S64
<
o
i~~ (CM-HJ-)
246 A ~ ~~ S64
~ ([M-H]-)
'
247 A ~ j~ C = S62
"OC
r~ ([M-H]-)
248 A ~ J~ C~ S60
HOOC
" (CM-HJ )
249 A C~ S48
~CO''~
(CM-HJ-)
i,,
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-21I-
250 A H~ ~~ 611
~~ "~ (LM-HJ-)
251 A i ~ _ 611
~
'1~
Hooc N ~ (fM-Hl-)
252 A ~a 567
i N ; ~ O ((M-HJ
)
253 A a 560
~'1t' M-H _
o (f l )
254 A 548
H~
' (fM-HI'>
255 A ~~ ~ ~~"~ 546
HOOC~
N (f M-HI-)
'
256 A ,~. 534
~co'~
UM-~~-
257 A H~ ~~ 597
'
)
258 A I ~ ~~"y 597
HOOC N
o N ( fM-HJ-)
259 A 553
I N ~~ (fM-Hl-)
260 A 546
N''
o
~ (fM-Hl')
"
261 A 'i ~-s 564
~ <
HOOC N -
.
(fM-H1
)
262 A c~ ~ y 552
~ ~ -
(fM-Hl
)
263 A Hooc ~'' 615
(LM-Hl
)
264 A J~ 615
I <
HOOC N ([M-H]
N )
0
265 A '~ 571
C
I N~O
N ' (fM-HI
266 A ('~ 564
0 ~
" (fM-HJ-)
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-212-
Example 267
5-[[[( 1 S)-1-Carboxy-2-[4-[(2,6-
dichlorobenzoyl)aminoJphenylJethylJamino]carbonyl]
tetrahydro-y-oxo-1,4-thiazepine-4{SIB-butanoic acid
O S O
H H
O N~O~Rg ~ N~ FRB
N O
O ~ O
/ O CI ~,~ o 'O / O CI
/ ~ \ H /
Intermediate-1 Cl \ Irnem~ediate-3 Cl
S
H O
N N ~~0~ RB
O
HO O ~ / O CI
O \
H
~ 2b7 CI \
The Intermediate-1 (0.3g, 0. i 95mmo1/g) was pretreated with CH2CIz (2x 3 mL).
The
swollen resin was then deprotected with 50% TFAICH,Cl2 (3mL, 30 min). The
resin was
rinsed in the following order: CHZC12 (2x 3 mL), CH~OH (2x 3mL), CH,C12(2x
3mL). The
resin was swollen with DMF (2x 3mL). N-Tert-butoxycarbonyl-1,4-thiazoline-5-
carboxylic acid (204 mg, 0.78 mmol) in DMF (2.0 mL) was activated with 0:5 M
HBTUIHOBT in DMF (1.6 mL) and DIEA (0.340 mL, 1.95 mmol), then added to the
swollen resin. The mixture was vortexed for 2 hr at room temperature. The
resin was
filtered and washed in the following order: DMF (2x 3 mL), CH,C12 (2x 3mL),
CH30H
(2x 3mL), CHzCI,(2x 3mL), respectively. If a Kaiser test on a small quantity
of the resin is
positive (blue) then repeat the coupling procedure until a negative result is
obtained. The
resin (intermediate-3, 0.2 g, 0..13mmollg) was pretreated with CH~C12 (2x 3
mL). The
swollen resin was then deprotected with 50% TFA/CH,CI, (3mL. 30 min). The
resin was
rinsed in the following order: CH~C12 (2x 3mL), CH30H (2x 3mL), CH~CIZ (2x
3mL). The
resin was then swollen with DMF (3mL). Succinic anhydride (78 mg, 0.78 mmol)
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-213-
dissolved in DMF (4mL) was added to the swollen resin and stirred at
50°C for 2hr. Then
the resin was filtered and washed in the following order: DMF (2x 3mL}, CH,C12
(2x
3mL), CH30H (2x 3mL), CH,CI, (2x 3mL) respectively. If a Kaiser test on a
small
quantity of the resin is positive (blue) then repeat the coupling procedure
until a negative
result is obtained. The resulting resin was then dried in vacuo to constant
weight. The resin
was placed in the polypropylene column and pretreated with THF (3 mL). Then
THF (3.9
mL), CH,OH (1.2 mL} and 2N LiOH (0.195 mL) were added to the swollen resin.
The
mixture was vortexed for 15 min and filtered to a clean and preweighed test
tube. The
resin was next washed with THF/S% CH30H (2x 2mL) and the combined filtrates
were
evaporated. The resulting gum was dissolved in H20 (1 mL). The solution was
then
acidified with 1N HCl to pH 2Ø The precipitate was centrifuged, washed with
HBO (2 x
SmL} and dried in vacuo to furnish 80 mg of Example 267 as a solid: ESMS (m/z)
594([M-H]').
CA 02342778 2000-12-20
WO 99167230 PCTIUS99114233
-zl4-
Scheme O
~~~OH O-1
Wang resin
~ o
Fmoc-HN OH
O-2
.w
o i .~ NOZ
O NH-Fmoc
\ O-3
02N I
O
O NH-Fmoc
\ O-4
HpN I /
O
O NH-Fmoc
O-5
Ro-f YtN
H
O
O NH2
1~' O-6
i\
RO_i Y~.H /
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-215-
Scheme O (continued)
0
O NH
w O-6
Ro-f/Y'.N I /
H Ro-3
Ro~~L
H0, / S,Ro.S
N~R~ O-7
O
O~O~Ro-2
O Roy R S3RO-5 O RO.a R S 80.5
H
-O N~N~Ro'e HO N N~R0.6
Ro-2
Ro-, Yt.N / O-8 4R ~YtN I /
\ O O~O' -.-.. \ O O~O~Ro-2
H o.~ H 0.9
Ro3
O Ros~ SVRo_5
O N~N~R0.6
~O H
Y
Ro-i ~~H / O-10
Ro-a
~ S Ro-5
O N~N~RO~
\ O Y2-Ra
Y I
Ro-i LH / O-11
Ro-3
O Ho~~ S Ro-5
HO N~N~~~
D Y2-Ra
Y I
Ro-i ~~H / O-12
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-2I 6-
Where: Rq_, is defined as R,2; Ro_2 is defined as C,~ alkyl or C,_,7
arylalkyl; Ro_3,
Rte, and Ro_5, are defined independently as R,. Ro_6 is defined as Rz. Y, and
YZ are
defined independently as Y.
Scheme O describes a method for the preparation. of examples of the formula O-
9
and O-12. Commercially available Wang resin (O-1} is acyiated with
commercially
available N a-Fmoc-Phe(NOZ)-OH {O-2) under standard conditions to afford the
resin of
formula O-3. Reduction of the aromatic nitro group (Meyer et al., Mol.
Diversity 1995, 1,
13-20} affords the resin bound aniline (O-4) which may be reacted v~~ith a
variety of
electrophilic reagents to afford resin bound amides (O-5 where Y, is C(=O)),
ureas (O-5
where Y, is C{=O)NH), sulfonamides (O-5 where Y, is SOz), and carbamates (O-5
where
Y, is C(=O)O). Removal of the Fmoc group under standard conditions provides
amine of
general structure O-6 which is acylated using standard solid-phase peptide
synthesis
conditions (Atherton, E.; Sheppard R.C. Solid Phase Peptide Synthesis: A
Practical
Approach; IRL Press at Oxford University Press: Oxford, 1989) with a
commercially
available or readily prepared thiazolidine-4-carboxylic acid of general
formula O-7 to
afford the resin bound intermediate O-8. Mild cleavage under standard
conditions
(Atherton, E.; Sheppard R.C. Solid Phase Peptide Synthesis: A Practical
Approach; IRL
Press at Oxford University Press: Oxford, 1989) affords the acid of general
structure O-9.
In those cases where Ro_2 is a 9-fluorenylmethyl group, standard Fmoc group
removal
affords the amine of general structure O-10, which may be reacted with a
variety of
electrophilic reagents as described in Scheme A to afford resin bound amides,
ureas,
sulfonamides and carbamates of general structure O-11. Mild cleavage under
standard
conditions affords the acid of general structure O-12.
Preparation 79 and Example 268.
(4S)-4-[[[( 1 S)-1-Carboxy-2-[4-[(benzoyl)amino]phenyl]ethyl]amino]carbonyl]-3
thiazolidinecarboxylic acid 3-ethyl ester
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-217-
<s, o
H_ ~
N~N~OH
/.~0~0 O I ~ O
N
H I
To a mixture of Wang resin (I% DVB, Advanced Chemtech, 2.75 g, 2.20 mmol
based on manufacture's loading of 0.8 rnmol/g resin) in DMF (12 mL) was added
N oc-
Fmoc-Phe(NOz)-OH, O-2 {Advanced Chemtech, 1.90 g; 4.40 mrnol) at room
temperature.
Aftex mixing for 10 min {by passing a slow stream of nitrogen through the
mixture)
pyridine (587 ~.L, 7.26 mmol) and 2,6-dichlorobenzoyl chloride (630 ~,L, 4.40
mmol) were
added. The mixture was agitated overnight via nitrogen bubbling, filtered,
washed with
DMF, methylene chloride and methanol and dried in va~:uo. In order to cap any
unreacted
hydroxymethyl groups, the resin was suspended in dichloroethane (5 mL} and to
this
mixture was added benzoyl chloride (0.75 mL) and pyridine (0.75 mL). The
mixture was
agitated for 2 h, filtered, washed with DMF, methyIene chloride, methanol,
methylene
chloride and methanol, and dried in vacuo to afford the resin O-3 (3.30 g). IR
(diamond
anvil) 1733, 1606 (resin), 1520 (resin), 1494 (resin), 1452 (resin), 1347,
1247, 1174, 1029
cm'.
To the prewashed (2 X 20 mL DMF) resin O-3 (1.0 g, ca. 0.6 mmol based on an
adjusted loading of 0.6 mmol/g) was added SnCl2~ 2 Hz0 (6 mL of a 2M solution
in DMF,
I2 mmol). The viscous-suspension was agitated for 4 hours by nitrogen
bubbling, filtered
and washed with DMF (2 X 20 mL). The resin was resospended with SnCIZ~ 2 HZO
(6
mL of a 2M solution in DMF, 12 mmol), agitated overnight via nitrogen
bubbling, filtered,
washed extensively with DMF, water, 2-propanol, methylene chloride and
methanol and
dried in vacuo to afford the resin O-4. Examination of tlhe FTIR spectra of a
small sample
of resin O-4 failed to exhibit an absorption at 1347 crri'.
To a mixture of prewashed (2 X 20 mL CHZC12) resin O-4 (0.30 g, ca. 0.18 mmol
based on an adjusted loading of 0.6 mmol/g) in 1,2-dichlioroethane (3 mL) was
added
benzoyl chloride (174 pL, 1.50 mmol) and DIEA (313 p,L, 1.80 mmol). The
mixture was
agitated overnight via nitrogen bubbling, filtered, washed with methylene
chloride, DMF,
methanol, and methylene chloride and dried in vacuo to afford resin O-5. To a
mixture of
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-218-
resin O-S in methylene chloride (S mL} was added a solution of piperidine in
DMF (30%,
mL). A slow stream of nitrogen was bubbled through the mixture to effect
mixing for 20
min. The resin was filtered, washed with DMF and resuspended in a solution of
piperidine
in DMF (30%, 10 ml). After gentle mixing for 40 min, the resin was filtered
and washed
5 with DMF, rnethylene chloride, methanol and methylene chloride and diluted
with DMF
(40 mL). To this mixture was added N ethoxycarbonyl-r>-thiazolidine-4-
carboxylic acid
(O-7, 0.15 g, 0.72 mmol}, HOBt (0.11 g, 0.72 mmol), PyBOP (0.37 g, 0.72 mmol)
and
DIEA (313 pL, 1.80 mmol). T'he reaction was mixed for 4 h at which point the
qualitative
Kaiser test was negative. The resin was filtered and washed with DMF,
methylene
chloride and MeOH and dried in vacuo to afford resin O-8. After swelling with
a
minimum of methylene chloride (ca. 0.5 mL), the resin O-8 was suspended with
95
aqueous TFA (5 mL). The mixture was mixed by magnetic stirring for 1 h,
filtered and
washed with TFA (2 X 3 mL) and methylene chloride. T'he combined filtrates
were
evaporated in vacuo to afford a residue that was purified by flash
chromatography using
1 S methylene chloride/methanol (2%) containing glacial acetic acid (0.1 %) as
eluant to afford
the title compound (80 mg). Lyophilization from glacial acetic acid afforded
an
amorphous powder: IR (drift) 331 I, 3298; 1670, 1601, 11579, 1531, 1487, 1412,
1380,
1345, 1324, 1265, 1205, 1190, 709 czri';'H NMR (300 MHz, DMSO-d6) b 10.39 (1
H},
8.39 (1 H), 8.14 {2 H), 7.86 {2 H), 7.74 (3 H), 7.36 (2 H), 4.83 (2 H), 4.59
(1 H), 4.48 (1
H), 4.22 (2 H), 3.48 (2 H), 3.26 {1 H), 3.06 (2 H), 1.35 (3 H);'3C NMR (75
MHz, DMSO-
d6} b 173.4, 169.8; 165.8, 154.0, 138.0, 135.4, 133.3, 131.9, 129.8, 128.8,
128.0, 120.4,
61.8, 54.2, 48.8, 36.7, 14.8; MS (ESI+) for C~H25N306S m/z 472.0 (M+H)+; MS
{ESI-) for
C2sHzsNaObS ~Z 470.1 (M-H)-; MS (FAB)' mJz (rei. intensity) 472 (MH+, 99), 472
(99),
371 (31), 160 (31), 8I (31), 71 (45), 69 (46), 57 (71}, 55 (58), 43 (47), 41
(42); HRMS
(FAB) caIcd for C~HZSN30~S +H~ 472.1542, found 472.1563; Anal. Calcd for
C23H25N3~6S ~ 1.5 H20: C, 55.41; H, 5:66; N, 8.43. Found: C, 55.47; H, 5.21;
N, 8.00.
Example 269.
(4S}-4-[[[{ 1 S')-1-Carboxy-2-[4-[(acetyl)aminojphenyijethyljaminojcarbonylj-3-
thiazolidinecarboxylic acid 3-ethyl ester
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-219-
~s~ o
H~ ~
N~N~OH
~O~O O I ~ O
~hf~CH3
H
The title compound was prepared as described in Scheme O using acetyl chloride
to form the requisite amide. Physical data as follows: IR (drift) 331 I ,
1709, 1667, 1602,
1536, 1517, 1412, 1378, 1344, 1321, 1266, 1218; 1 I 85, 1116, 769 cni'; 'H NMR
(300
MHz, DMSO-d6) 8 9.79 (1 H), 8.15 (1 H), 7.37 (2 H}, 7.02 (2 H), 4.54 (2 H),
4.31 (1 H),
4.19 ( I H), 3.95 (2 H), 3. I 2 ( 1 H), 2.93 { 1 H); 2. 75 (2 H), 1.82 (3 H),
I .09 (3 H); ' 3C NMR
(75 MHz, DMSO-d6} b 173.2, 169.8, 168.5, 154.0, 138.2, 132.4, 129.8, 119.1,
62.1, 61.7,
53.9, 36.7, 24.4, 21.5, 14.$; MS (ESI+) for C,8Hz3N3O6S m/Z 410.0 (M+H)+; MS
(ESI-) for
C,8Hz3N3O6S mlZ 408.0 (M-H}-; MS (FAB) mlz (rel. intensity) 410 (MH+, 99), 486
(20),
411 (22), 410 (99), 409 (9), 205 (22), 188 (9), 177 (9}, 160 (35), 148 (9), 88
(14}; HRMS
(FAB) calcd for C,8Hz3N3O6S +H' 410.1385, found 410.I379. Anal. Calcd for
C,gH23N306S ~ 0.3 H20: C, 52.1 I; H, 5.73; N, 10.13. Found: C, 51.73; H, 5.73;
N, 9.82.
Example 270.
(4,5~-4-[[[{1S)-1-Carboxy-2-[4-[(3-
phenylpropanoyl)amino]phenyl]ethyl]amino]carbonyl]-
3-thiazolidinecarboxylic acid 3-ethyl ester
~s~ o
H_ ~
H~N~OH
/~O~O O I \ O
~O.N~. ,
H I/
The title compound was prepared as described in Scheme O using hydrocinnamoyl
chloride to form the requisite amide. Physical data as follows: IR (drift) 331
I, 2978,
2930, 1665, 1601, 1534, 1517; 1413, 1379, 1344, 1252, 1216, 1187, 1115, 700
crri';'H
NMR (300 MHz, CDC13/CD30D (10%)) 8, 7.34 (2 H), 7.21 (5 H), 7.02 {2 H), 4.67
(3 H),
4.30 (1 H), 4.09 (2 H), 3.I 1 (4 H), 2.97 (2 H), 2.58 (2 H), 1.19 (3 H);'3C
NMR {75 MHz,
CDCl3) S 172.7, 171.3, 170.0, 154.8, 140.7, 137.0, 131.6, 129.7, 128.4, 128.2,
126.2,
119.9, 63.0, 62.6, 53.I, 38.9, 36.7, 31.5, 29.6, 14.2; MS (ESI+) for
Cz5Hz9N3O6S m/z 500.2
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-220-
(M+H)+;~MS (ESI-) for Cz5H2g1V3O6S m/z 498.3 (M-H)'; Anal. Calcd for
CZSHz9N30sS~ C,
60.10; H, 5.85; N, 8.41. Found: C, 59.85; H, 6.07; N, 8.09.
Example 271.
(4S)-4-[[[(1,5'~-1-Carboxy-2-[4-[(3-pyridinylcarbonyl)amino]phenyl]
ethyl]amino]carbonyl]-3-thiazolidinecarboxylic acid 3-ethyl ester
H_ ~
N~N~OH
~O~O O ! W O
v _N ~N
H
The title compound was prepared as described in Scheme O using nicotinoyl
chloride to form the requisite amide. Physical data as follows: IR {drift)
3301, 3061,
2983, 2935, 1709, 1675, 1603, 1535, 1SI7, 1415, 1380, 1345, 1326, 1204, 1140
crri';'H
NMR (300 MHz, DMSO-d6) 8 12.74 (1 H), 10.42 (1 IT), 9.11 (1 H), 8.76 (1 H),
8.29 (2
H), 7.67 (2 H), 7.55 ( 1 H), 7.19 (2 H), 4.62 (2 H), 4.44 ( 1 H), 4.29 ( 1 H),
4.OS (2 H), 3.24
(1 H), 3.04 (1 H), 2.88 {2 H), 1.15 (3 H);'3C NMR (75 MHz, DMSO-d6) 8 173.1,
170.0,
164.3, 154,0, 152.4, 149.0, 137.7,135.9, 133.5, 131.1, 129.8, 123.9, 120.5,
62.4, 61.8,
53.8, 36.7, 14.8; MS (ESI+) for C22H24N4~6'S m/z 473.3 {M+H)+; MS (ESI-) for
C22Hz4N406S m/z 471.3 {M-H)'; HRMS (FAB) cakd for CzZHzaNaOaS +H, 473.1494,
found
473.1509.
Example 272.
(4~-4-[[[(1S')-1-Carboxy-2-[4-[(4-
methoxybenzoyl)amino]phenyl]ethyl]amino]carbonyl]-
3-thiazolidinecarboxylic acid 3-ethyl ester
~s~ o
H_ ~
NI~N~OH
~O~O O ~ ~ O
v 'N
H ~
OCH3
The title compound was prepared as described in Scheme O using p-anisoyl
chloride to form the requisite amide. Physical data as follows: IR (drift)
1709, 1667,
CA 02342778 2000-12-20
WO 99!67230 PCT/U599/14233
-22I-
1604, 1532, 1514, 1439, 1412, 1379, 1343; 1323, 1255, 1221, 1178, 1027, 763
crri';'H
NMR (300 MHz, CDC13/CD30D (10%)) 8 7.79 (2 H), 7.47 {2 H), 7.05 {2 H), 6.87 (2
H),
4.68 {2 H}, 4.58 (1 H), 4.28 (1 H), 4.07 (2 H), 3.79 (3 H}, 3.66 (2 H), 3.04
(4 H), 1.17 (3
H);'3C NMR (7S MHz, CDCl3) 8 177.9, 174.0, 170.1, 166.3, 158.8, 141.1, 135.8,
133.7,
I33.0, 130.8, 124.6, 117.7, 66.9, 66.d, 59.3, 40.7, 33.5, 24.5, 18.2; MS
(ESI+) for
C24H2TN3~7S ~Z 502.0 (M+H)+; MS (ESI-) for Cz4H27N3O7S m/z 500.1 (M-H)-; MS
(FAB)
m/z (rel. intensity) 502 (MH+, 52), 503 (I7), S02 (52), 297 (12), 240 (12),
160 (21), 135
(99), 88 (12), 73 {20), 69 (13), 57 (12); HRMS (FAB) calcd for C24H27N3O7S +H,
502.1648, found 502.1657. Anal. Calcd for C24H27N3O,S ~ 0.3 H20: C, 56.86; H,
5.49; N,
8.29. Found: C, 56.65; H, 5.34; N, 7.92.
Example 273.
(4f~-4-[[[( LSD-1-Carboxy-2-[4-[(4-methylbenzoyl)amino
jphenyl]ethyl]amino]carbonylj-3-
1 S thiazolidinecarboxylic acid 3-ethyl ester
0
H_ ~
N~N~OH
~O~O O I ~ O
~N
H/~\
CH3
The title compound was .prepared as described in Scheme O using p-tolouyl
chloride to form the requisite amide. Physical data as follows: IR {drift)
3310, 2981,
2929, 1671, 1608, 1599, 1531, 1517, 1413, 1379, 1344, 1324, 1265, 1210, 1188
cni';'H
NMR (300 MHz, DMSO-d6) 8 10.10 {1 H), 8.24 {1 H), 7.85 (2 H), 7.67 (2 H}, 7.33
(2 H),
7.17 (2 H), 4.62 (2 H), 4.4I (1 H), 3.28 {2 H), 4.02 {2 H), 3.23 (1 H), 3.04
(I H}, 2.87 (2
H), 2.38 (3 H), 1.1 S (3 H); "C NMR (75 MHz, DMSO-db) b I73.2, 169.8, 165.6,
154.0,
141.9, 138.1, 133.1, I32.S, 129.7, 129.7, 128.1, 120.4, 62.4, 61.8, 54.0,
36.7, 21.4, 14.8;
MS (ESI+) for Cz4Hz7N3O6S mlZ 486.2 {M+H)+; HRMS (FAB) calcd for C24H27N306S
+H,
486.1699, found 486.1713; Anal. Calcd for C24Hz,N~O6S ~ 0.3 HzO: C, 58.71; H,
5.67; N,
8.56. Found: C, 58,37; H, 5.67; N, 8.35.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99I14233
-222-
Example 274.
(4,5~-4-[[[{ 1,5~-1-Carbaxy-2-[4-[[2-
(trifluoromethyl)benzoyl]amino]phenyl]ethyl]amino]
carbonyl]-3-thiazolidinecarboxylic acid 3-ethyl ester
~s~ o
H~ ~
N~N~OH
O CF3
N W
H
S
The title compound was prepared as described in Scheme O using (2
trifluoromethyl)benzoyl chloride to form the requisite amide. Physical data as
follaws:
IR (drift) 3295, 1709, 1663, 1603, 1533, 1518, 1414, 1380, 1344, 1316, 1269, l
176, 1 I32,
1108, 769 crri';'H NMR (300 MHz; DMSO-d6) b 10.48 (1 H), 8.23 (1 H), 7.77 (2
H),
7.S 8 (2 H), 7.17 (2 H), 4.64 (2 H), 4.44 ( I H), 4.29 ( I H), 3.99 (2 H);
3.24 { 1 H), 3.OS (2
H), 1.09 {3 H); '3C NMR (7S MHz, DMSO-d6) 8 173.2, 169.2, I6S.8, 154.1, 137.8,
I36.7,
133.0, 130.4, 129.9, 128.9, 126.7, 126.4, 126.0, 119.8, 62.2, 61.8, 54.0,
37.1, I4.7; MS
(ESI+) for C24H25F3N3~6's ~Z 540.0 (M+H)+; MS (ESI-) for CZ4HZSF3Na06S m/z
538.1 (M-
H)-; HRMS (FAB) calcd for Cz4H24F3N3O6S +H~ 540.1416, found 540.1423; Anal.
Calcd
for Cz4H24F3N3O~S ~ O.S HZO: C, S I .49; H, 4.70; N, 7.83. Found: C, S 1.42;
H, 4.42; N,
7.45.
Example 275.
(4S'~-4-[[[(15~-1-Carboxy-2-[4-[(2,4,6-
trichlorobenzoyl)amino]phenyl]ethyl]amino)-
carbonyl]-3-thiazolidinecarboxylic acid 3-ethyl ester
~s~ o
H-~ ~
N~N~OH
~O O O ' ~ O C1
N
H I ~
CI CI
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-223-
The title compound was prepared as described in Scheme O using 2,4,6-
trichlorobenzoyl chloride to form the requisite amide. Physical data as
follows: IR (drift)
3286, 2926, 1709, 1664, 1604, 1578, 1542, 1 S 17, I 413, 1379, i 345, 1325,
1269, 12I 8,
1187 crri';'H NMR (300 MHz, CDC13/CD30D {10%)) ~i 7.58 (2 H), 7.41 (2 H), 7.20
(2
H), 4.84 (2 H), 4.b9 (I H}, 4.41 (1 H), 4.19 (2 H), 3.27 (4 H), 1.26 (3 H);'aC
NMR (75
MHz, DMSO-dø) 8 173.0, I69.8, 161.5, 154.0, 137.2, 1:35.9, 135.1, 133:9,
132.6, 130.1,
128.5, 119.6, 62.2, 61.7, 54.0, 36.7, 14.8; MS (FAB) mOz (rel. intensity) 574
(MH+, 95),
576 {96), 574 {95}, 160 (99), 91 (79), 88 (40), 69 (64), 5'7 (59), 55 (59), 43
(S6), 41 (39};
HRMS (FAB) calcd for C2gH22CI3N3O6s +H, 574.0373, :found 574.0364. Anal. CaIcd
for
lO C23HZZC13N3O6S: C, 48.06; H, 3.86; N, 7.31; Cl, 18.50. 1~ound: C, 48.52; H,
4.13; N, 7.08.
Example 276.
(4S}-4-((((1,5~-1-Carboxy-2-[4-[[(2,5-dichlorophenyl;isulfonyl]amino)
phenyl]ethylJ
amino]carbonyl)-3-thiazolidinecarboxylic acid 3-ethyl ester
IS
~s~ o
H ''
N~N~OH
~O~O O ~ Cl
~ N 6 _
H n
O
CI
The title compound was prepared as described in Scheme O using 2,5-
dichlorobenzene sulfonyl chloride to form the requisite sulfonamide. Physical
data as
20 follows: IR (drift) 1709, 1676, I531, 1512, 1450, 1428" 1409, 1378, 1344,
1221, 1167,
1143, 1113, 1101, 1041 crri'; 'H NMR (300 MHz, DMSO-d6) S 8.36 (1 H}, 7.90 (3
H),
7.20 (2 H), 7.12 (2 H), 4.70 (2 H), 4.47 ( 1 H), 4.34 ( I H}, 4.10 (2 H}, 3.19
(4 H), 1.27 (3
H); '3C NMR (75 MHz, DMSO-d6) 8 173.0, 169.8, 153.9, 138.7, 135.5, 134.8,
134.1,
132.6, 131.0, 130.5, 130.0, 120.0, 62.4, 61.7, 53.5, 36.3, 14.8; MS (FAB) m/z
(rel.
25 intensity) 576 (MH+, 99), 652 (27), S78 (83), 577 (31), 576 (99), 160 (98),
106 (47), 88
(40), 81 (32), 69 (31 }, 57 (28); HRMS (FAB) calcd for C:zZHz3CI2N3O7S2+H~
576.0433,
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-224-
found 576.0400. Anal. Calcd for Cz2H23C1zN30,Sz ~ 0.1 H20: C, 45.70; H, 4.04;
N, 7.27.
Found: C, 45.94; H, 4.04; N, 6.87.
Example 277.
(4~'}-4-[[[{1S?-1-Carboxy-2-[4-[[[(2,6-dichlorophenyl}amino]carbonyl]
amino]phenyl]-
ethyl]amino]carbonyl]-3-thiazolidinecarboxylic acid 3-ethyl ester
<s~ o
H' ~
~ v 'OH
OCI~ /
I / ~LN ~ I
H H OI
The title compound was prepared as described in Scheme O using 2,6-
dichlorophenyl isocyanate to form the requisite urea. Physical data as
follows: IR (drift)
3284, 3277, 1709, 1655, 1600, 1569, 1544, 1452, 1431, 1415, 1347, 1238, 1217,
1 i95,
771 crri';'H NMR (300 MHz, DMSO-d6) 8 9.09 {1 H), 8.41 (1 H}, 8.09 (1 H), 7.52
(2 H),
7.31 (3 H), 7.08 (2 H), 4.62 (2 H), 4.35 ( 1 H), 4.28 ( 1 H;), 3.27 ( 1 H),
3.02 ( 1 H), 2.85 {2
H), 1.10 (3 H);'3C NMR (75 MHz, DMSO-d6) S 173.6, 169.8, 154.0, 152.8, 138.7,
134.4,
133.9, I31.3, 130.0, 128.8, 128.7, 118.1, 62.4, 61.8, 54.:3, 36.7, 14.8; HRMS
(FAB} calcd
for Cz3H,4C12N406S +H, 555.0872, found SS5.0877. Anal. Calcd for
Cz3HzaC12NQO6S ~ 2
H20: C, 46.71; H, 4.77; N, 9.47. Found: C, 47.08; H, 4.53; N, 9.06.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-225-
Scheme P
~~~-OH p-1
Wang resin
0
Boc-HN OH
P-2
y
O I I
~O NH-8oc
P-3
I ( /
O
NH-Boc
P-4
N
R~~
O
O
H2N OH
t'-5
\ H
/ N.
Rp-y
O
O
H2N O~CH3
P-6
H
N,
RP-1
O
CA 02342778 2000-12-20
WO 99167230 PCT/US99I14233
-226-
Scheme P (continued;)
Rp.~
Rpm~~..
HO' / S'Rps
~N~RPS P_7
O
O~O~Rp_z
O HRp~ R S O Rpa Rps
w0 N /\Rps N~S Rp5
RP.s P'8 -----~ HO N~R P-9
O p.s
rN I / O~O.Rp.2 N I \ O O~O.Rp.2
Rv.t Rwi w
O HRp~ R S
N~,~- ~-~'/Rps
~/~Rps P-10
\ O H
i/
R
O
O Rpm Rpa
H S RP.S
O ~~RP~6 P-~ 1
\ O Y
N I / wRa
R~lr
O
O ~ pa R S
N Rps
HO ~~Rps P-'12
O
( Y2wR3
R r
a-~
O
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-227-
Where: RP_, is defined as R,2; RP_Z is defined as C:,_6 alkyl or C,_,~
arylaIkyl; RP_3, Rr_
4, and RP_5, are defined independently as R,. Rp_6 is defined as RZ.
Scheme P describes a method for the preparation of examples of the formula P-9
S and P-12. Commercially available Wang resin (P-1) is a.cylated with
commercially
available N a-Boc-Phe(I)-OH (P-2) under standard conditions to afford the
resin of
formula P-3. Carbonylation of the resin bound aryl iodide with carbon monoxide
and an
amine in the presence of a source of palladium(0) affords the resin bound
amide of general
formula P-5 (for a general review of carbonylation chemistry, see: C'~lquhoun,
H.M.;
Thompson, D.3.; Twigg, M.V. Carbonylation Plenum Press: New York, 1991}. Mild
cleavage under standard conditions affords the amino acid of general structure
P-5 which
is esterified under mild acid catalysis to afford the amino ester of general
structure P-6.
Condensation with a commercially available or readily prepared thiazolidine-4-
carboxylic
acid of general formula P-7 under conditions described in Scheme A affords the
pseudodipeptide of general structure P-8. Mild base hydrolysis of the ester of
general
structure P-8 affords the acid of general structure P-9. In the case where
RP_Z is a 9-
fluarenylmethyl group, standard Fmoc group removal (A.therton, E.; Sheppard
R.C. Solid
Phase Peptide Synthesis: A Practical Approach; IRL Press at Oxford University
Press:
Oxford, 1989) affords the amine of general structure P-10, which may be
reacted with a
variety of electrophilic reagents as described in Scheme A to afford amides,
areas,
sulfonamides and carbamates of general structure P-11. Mild base hydrolysis of
the ester
in general structure P-11 affords the acid of general structure P-12.
Preparation 80 and Example; 278.
(4,5~-4-[[[(IS}-I-Carboxy-2-[4-[[(2,4,6-trichlorophenyl;)amino]carbonyl)
phenyl]ethylJ-
aminoJcarbonyl]-3-thiazolidinecarboxylic; acid 3-ethyl ester
CA 02342778 2000-12-20
WO 99167230 PCTIUS99114233
-228-
S- H' O.
~NI~N~OH
'~O~O ~ , ~ H CI
N
0 I
CI CI
To a cooled (0-SOC) mixture of Wang polystyrene resin P-1 {Advanced Chemtech,
2.0 g, ca. 1.5 mmol), N Boc-4-iodo-L-phenylalanine P-:! {Bachem, 4.OO g, 10
mmol), and
PPh3 (1.30 g, 5.0 mmoI) in THF (20 mL) was added diethyl azodicarboxylate
(0.80 mL,
5.0 mmol) in 4 approximately equal portions at 5 min intervals. When the
orange color
had discharged, the mixture was warmed to ambient temperature and stirred for
5 h. The
mixture was diluted with THF (30 mL) and f ltered. Thf; resin was washed with
DMF,
THF and MeOH and dried in vacuo to afford the esterifind resin P-3 (2.68 g) as
a colorless
powder: "C NMR (100 MHz, CDZC12, 4 mm MAS probe) 8 171.86, 155.33, 137.85,
136.40, 131.87, 128.00, 92.74, 80.09, 54.05, 38.05, 28.51.
N2 was bubbled through a mixture of N Boc-4-iodo-L-phenylalanine
functionalized
Wang resin P-3 (500 mg, ca. 0.3 mmol), PPh3 (0.21 g, 0.8 mmol), 2,4,6-
trichloroaniline
(0.98 g, 5.0 mmol) and DIEA (3.48 mL, 20 mmol) in NMP (20 mL) for 10 min.
Pd2dba3
(0.18 g; 0.2 mmol) was added and the reaction mixture was placed under a CO
atmosphere
and heated (bath temp. 70 °C) for 18 h. Upon cooling to ambient
temperature, the mixture
was diluted with 3% (w/v) sodium diethyldithiocarbamate in 95:5 NMP:DIEA (10
mL).
After an additional 10 min, the mixture was filtered and the resin washed with
NMP, THF
and MeOH and dried in vacuo to afford the functionalize;d resin P-4 as a
colorless powder.
Resin P-4 was swollen with methylene chloride (0.5 mL) and diluted with 95:5
TFA:H20 ( 10 mL). After 90 min the mixture was filtered and the resin washed
with TFA
(3 x 5 mL) and CHZC12. The combined filtrates were concentrated in vacuo and
the residue
lyophilized from glacial acetic acid to provide the amino acid P-5 ( 152 mg)
as a powder
which was used without purification. Physical data as follows: MS (FAB) rnlz
(rel.
intensity) 387 (M+H, 42), 427 (26), 426 (80); 389 (46), 387 {42), 366 (33),
279 (99), 177
(54), 146 (18), 119 (26), 23 (26); HRMS (FAB) calcd for C,6H~3C13NZO3+HI
387.0070,
found 387.0084.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99I14233
-229-
The amino acid P-5 was dissolved in methanolic HCI (20 mL) and stirred at room
temperature for 18 h. Concentration in vacuo afforded tire methyl ester P-6
which was
used without purification. Physical data as follows: MS (ES+) for
C=,H,SC13NZOa m/z
400.9 (M+H)+,
To a cooled (0-5 °C) solution of N ethoxycarbonyl-~-thiazolidine-4-
carboxylic
acid P-7 (82 mg, 0.4 mmol) and HOAt (54 rng, 0.4 mmol) in CHZCIz/DMF (1:I, 4
mL)
was added EDC (76 rng, 0.4 rnmol). After stirring fox 10 min, the solution was
added to
the amino ester P-6 described above at 0-5 °C followed by DIEA (208
p,L, 1.2 mmol).
After an additional 30 min at 0-5 °C, the solution was allowed to warm
to room
temperature and stirred an additional hour. Volatiles were removed in vacuo
and the
residue partitioned between ethyl acetate and 0.1 N aqueous HCI. The organic
layer was
separated, washed with 0.l N aqueous HCl, saturated aqueous NaHC03, brine;
dried
(MgS04}, filtered and concentrated in vacuo. Purification of the residue by
flash
chromatography using CHZCIZ/ethyl acetate/hexanes (1:1:2} containing 2-
propanol (0.1 %)
IS as eluant afforded the ester P-8 as a powder: 'H NMR {300 MHz, CDC13} 8
7.99 (I H),
7.87 (2 H), 7.40 (2 H), 7.24 (2 H), 4.92 ( 1 H}, 4.70 (2 H), 4.34 ( I H), 4. I
0 (2 H), 3:74 {3
H), 3.20 (4 H), 1.25 (3 H);'3C NMR (75 MHz, CDCl3) 8 171.2, 169.7, 165.4,
154.9,
140.6, 134.3, 133.4, 132.2, 131.3, 129.7, 128.4, 127.9, 6:3.1, 62.7, 60.4,
53.0, 52.6, 37.6,
2I.0, 14.5; MS (ESI+) for C24H2aCI3N3O6S m/z 589.9 (M~+H)+; MS (ESI-) for
2O Cz4Hz4C13N3O6S m/Z 588.0 (M-H}'.
To a cooled (0-5°C ) solution of the ester P-8 (72 mg, 0.12 mmol) in
THF (5 mL)
and water (0.5 mL) was added an 0.1 N aqueous solution of NaOH { 1.3 mL, 0.13
mmol)
via a syringe pump over 1 h. After an additional 45 min at 0-5°C, the
reaction mixture
was diluted with ethyl acetate and acidified with 0.25 N 1HC 1 to a pH of ca.
3. The organic
25 layer was separated, washed with water and concentrated) in vacuo.
Purification of the
residue by flash chromatography using methylene chloride and methanol {0-5%)
as eluant
provided a solid which was crystallized from ethyl acetat:e/CHZCI2/hexanes to
afford the
title compound (45 mg) as colorless solid: IR (drift) 1743, 1726, 1709, 1691,
1675, 1663,
1553, 1521, 1490, 1428, 1415, 1379, 1345, 1290, 1189 crri';'H NMR (300 MHz,
DMSO-
30 d6) 8 10.27 (1 H), 8.35 (1 H), 7.92 (, 2 H}, 7.81 (2 H), 7.:37 (2 H), 4.62
(2 H), 4.53 (1 H},
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-230-
4.29 (2 H), 4.00 (2 H), 3.11 (3 H), 2.77 (1 H), I.12 (3 H);'3C NMR (75 MHz,
DMSO-d6)
8 172.9, 170.0, 165.4, 154.0, 142.5, 135.5, 133.9, 133.0, 131.6, 129.8, 128.7,
128.0, 62.0,
61.7, 53.4, 37.0, 14.8; MS (FAB) m/z (rel: intensity) 574 (MH+, 80), 576 (80),
574 {80),
379 (99), 160 (82), 91 (95), 81 (72), 69 (93), 57 (86), 551;86), 43 (90); HRMS
(FAB) calcd
S for CZ~HZZC13N3O6S +H, 574.0373, found 574.0358.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-231-
Scheme Q
Hs ~-~
:2 Q-1
O.
HyN Rp-3
O
Rp-~
~5 Rq '"N Rq.O. O-2
H ~-s
O
Rp_~
H~Rq~ Rq-20, Q-3
Rq-a
O
Rp-~
~~2
(/~N~O_Rp-a Q-4
Z- < O
O
Rq-~
S'~ Rp-2
~OH p 5
Z--~ O
O
O Q-6
HZN w
O
Rp.t
N NH O~
Z--~ O ~ 4-7
O 5
Rp_1
S Rp_Z O
NH OH q-g
Z-~ (?
O Rs
CA 02342778 2000-12-20
WO 99/67230 PCTlUS99/14233
-232-
Rø, and RQ_Z are defined independently as R,. ~RQ_, is deoned as C,~ alkyl or
C,_"
arylalkyl. RQd is defined as oxygen or N-R". ItQ.s is defined as a suitable
protecting group
for a nitrogen (such as Boc or Fmoc) or oxygen (such as t-butyldimethylsilyl)
(Greene,
T.W.; Wuts, P.G.M.. Protective Groups in Organic Synthesis, John Wiley and
Sons, New
York, 1991 ).
Scheme Q describes a general method for the preparation of examples of the
formula Q-7. A commercially available or readily prepared sulfur containing
amino acid
of structure Q-1 is condensed with a suitably protected aldehyde to afford the
thiazolidine-4-carboxylic acid of general formula Q-2. ;>tandard deprotection
(Greene,
T.W.; Wuts, P.G.M.. Protective Groups in Organic Synthesis, John Wiley and
Sons, New
York, 1991 ) affords intermediate Q-3 which is readily cyclized to the bicycle
Q-4 using
1,1'-carbonyldiimidazole or phosgene or a suitable equivalent. For the
preparation of
bicycles of general structure Q-4 in which Z is CHz, see as examples: (a)
Aszodi, 3.;
Bonnet, A.; Teutsch, G. Tetrahedron 1990, 46, 1579. (b) Baldwin, J.E.; Lee,
V.;
Schofield, C.J. Heterocycles 1992, 34, 903. (c) Genin, NLJ.; Johnson, R.L. J.
Am. Chem.
Soc. 1992.114, 8778. (d) Siddiqui, M.A.; Preville, P.; Tarazi, M.; Warder,
S.E.; Eby, P.;
Gorseth, E.; Puumalay K.; DiMaio; J. Tetrahedron Lett. 11997, 38, 8807.
(e)Subasinghe,
M.L.; Bontems, R.J.; McTntee, E.; Mishra, R.K.; Johnson, R.L. J. Med. Cherry.
1993, 36,
2356. Removal of the ester protecting group affords the acid of general
structure Q-5
which is condensed with amino acyl derivative Q-G under standard peptide
synthesis
conditions to provide Q-7 (for a review of procedures of peptide synthesis
see: Bodansky,
M.; Bodansky, A. The Practice of Peptide Synthesis; Springer-Verlag: Berlin,
1984).
Mild base hydrolysis of the ester of general structure Q-'7 provides acid Q-8.
Preparation 81.
(Scheme Q, Q-2: where RQ_, and Ro_, are equal to hydrogen, RQ_3 is ethyl, RQ~,
is
NH, R~5 is Boc and stereochemistry is (S)).
S~
Boc~N
H HN
--OEt
O
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-233-
To a suspension of D-cysteine 1.5 hydrate hydro~;hloride (Q-1, Scheme Q where
RQ_, and RQ_, are equal to hydrogen, R~3 is hydrogen and stereochemistry is
(S')) {5 g, 27.1
mmol) in absolute ethyl alcohol (50 mL) was added triethylorthoformate (13.5
mL, 81.2
mmol) at ambient temperature. A stream of anhydrous HCl gas was bubbled
through the
solution for 30 min. The stream of anhydrous HCl gas was maintained as the
mixture
heated to 70°C for 2 h. The reaction mixture was concentrated in vacuo
and the resulting
residue triturated with diethyl ether to afford D-cysteine ethyl ester (4.43
g) as a white
solid which was used without further purification. Physical data as follows:
'H NMR
(300 MHz, D20) 8 4.36 (1 H), 4.27 (2 H), 3.12 (2 H), 1.25 (3 H); MS (ESI+) for
CSH"NOZS m/z 150.0 (M+H)*.
To a solution of ~-cysteine ethyl ester (1.89 g, Ii).2 mmol} in HZO {46 mL)
was
added potassium acetate ( 1.22 g, 12.4 mmol) and t-butyl N (2-
oxoethyl)carbamate
(Aldrich, 2.38 g, 12.0 mmol based on 80% purity as detE;rznined by'H NMR) in
ethyl
1 S alcohol (46 mL) at ambient temperature. After 8 h, the reaction mixture
was concentrated
in vacuo and the residue purified by flash chromatography using methylene
chloride/methanol (1%) as eluant to afford the title corn;pound (1.9 g) as an
oil: 'H NMR
(300 MHz, CDC13) b 5.01 {1 H), 4.75 (1 H), 4.26 (2 H), :3.93 (1 H), 3.37 (2
H), 3.12 (1 H),
2.91 (1 H), 1.47 (9 H), 1.32 (3 H); MS (ESI+) for C,zHZ2N204S mlz 291.1
(M+H)+. MS
(ESI-) for C,ZHZZNZO4S m/Z 288.9 (M-H)'.
Preparation 82.
(Scheme Q, Q-3: where RQ_, and R~,, are equal to hydrogen, RQ.3 is ethyl; RQ~
is
NH, and stereochemistry is (S~).
~..~S
H2N
HN
--OFt
O
CA 02342778 2000-12-20
WO 99/6'7230 PCT/US99I14233
-234-
To a cooled (10-15°C) solution of Q-2 (Scheme Q where RQ_, and RQ_, are
equal to
hydrogen, RQ_3 is ethyl, ItQ~, is NH, RQ_5 is Boc and stereochemistry is (S~)
(1.9 g) in
dioxane (38 mL) was added dropwise anhydrous 4 M HC:I in dioxane {156 mL). The
solution was allowed to warm to ambient temperature and stirred -for 2 h. The
reaction
S mixture was concentrated in vacuo and azeotroped three times with methanol
which
afforded the title compound (1.72 g) as a tacky yellow solid: 'H NMR (300 MHz,
CDC13)
8 8.57 (2 H}, 5.80 {1 H), 5.25 (1 H), 4.39 (2 H), 4.17 {1 I-I), 3.79 (4 H),
1.37 {3 H); MS
(ESI+) for C7H,4NZOzS mlZ 191.1 (M+H)+. MS (ESI-) for C,H,4NZO2S ~ 2HCI mlz
261.0
(M_H)_.
Preparation 83.
(Scheme Q, Q-4: where R.Q_, and R~_, are equal to hydrogen, RQ_~ is ethyl, Z
is NH,
and stereochemistry is (,S~).
~S~
HN/~'N
--OEt
0 0
To a cooled (0-5°C) solution of Q-3 (Scheme Q vvhere RQ_, and RQ_, are
equal to
hydrogen, RQ_3 is ethyl, RQ~ is NH, and stereochemistry i s (,S'}) (1.72 g,
6.54 mrnol) in THF
{650 mL) was added triethylamine (2.83 mL, 20.3 mmol) and 1,1'-
carbonyldiimidazole
(1.11 g, 6.87 mmol). After 3 d at ambient temperature, the mixture was
recooled (0-5°C),
treated with additional 1,1'-carbonyldiimidazole (S30 mg, 3.27 mmol) and
allowed to
warm to ambient temperature. After 18 h, the reaction mixture was concentrated
in vacuo
and the resulting residue was partitioned between ethyl acetate and 0.25 N
HCI. The
organic layer was separated, washed with brine, dried (MgS04), filtered and
concentrated
in vacuo. The diastereorneric mixture was separated by chiral chromatography
[5 x 25 cm
(R,R) Whelk-O I, SO mL/min 40% Isopropanol/heptane, :210 nm,]. Further
purification of
each isolated diastereomer by flash chromatography using methyiene
chloride/ethyl
acetate (25%) as eluant afforded the diastereomers (255 mg, 464 mg) as oils.
Physical
data for the faster eluting diastereomer (analytical column conditions 0.46 x
25 cm (R,R)
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-235-
Whelk-O I, 0.5 mL/min 40% IPA/heptane; 210 nm) as follows: 'H NMR (300 MHz,
CDCl3} 8 5.51 ( 1 H), 5.15 ( 1 H), 4.32 (2 H), 3.96 ( 1 H), 3.86 ( 1 H), 3.53
( 1 H), 3.26 ( 1 H},
3.13 (1 H), I.34 (3 H); MS (ESI+) for CBH,ZN203S m/z 217.1 (M+H)+. MS (ESI+)
for
CBH,ZNZO3S mlZ 239.0 (M+Na)+. MS (ESI-) for CgH,2N20jS mlz 215.1 (M-H}'.).
Preparation 84.
(Scheme Q, Q-5: where RQ_, and RQ_, are equal to hydrogen, and Z is NH).
~S
HNI~1N
OH
O O
To a cooled (0-5°C) solution of the faster eluting diastereomer of
general structure
Q-4 (Scheme Q where RQ_, and RQ_, are equal to hydrogen, R~~ is ethyl, Z is
NH, and
stereochemistry is (,S~) (100 mg, 0.46 mmol) in THF (13 mL) and H20 (1.5 mL)
was added
via syringe pump over 1 h 0.1 N NaOH (9.7 mL, 0.97 m.mol). The reaction
mixture was
stirred for 2 h at 0°C, acidified with 1.0 N HCl (0.97 mL,} and
concentrated in vacuo. The
resulting residue was dried over P205 in a vacuum desiccator to afford the
title compound
(87 mg} as a glassy solid which was used without further purification: MS
(ESI+) for
C6H8NZO3S mlZ 189.0 (M+H}+. MS (ESI+) for C6H8NZC~3S mlz 211.0 (M+Na)+. MS
(ESI-)
for C6H$Nz03S mlz 187.0 (M-H}'.
Preparation 85.
(Scheme Q, Q-7: where RQ_, and RQ_, are equal to hydrogen, Z is NH, RS is 4-
[(2,6-
dichIorobenzoyl)amino]phenyl, and stereochemistry of the C-terminal amino acid
is (~).
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-236-
S 0
HN N
OMe
O O
O CI
H I ~
C!
To a cooled {0-5°C) suspension of Q-5 (Scheme Q where RQ_, and RQ_, are
equal to
hydrogen, and Z is NH) (87 mg, 0.46 mmol) in methyh;ne chloride ( 10 mL) was
added
O-(7-azabenzotriaol-1-yi)-N,N,N',N'-tetramethyluronium hexafluorophosphate
{HATU)
(I75 mg, 0.46 mmol), Q-6 (Scheme Q where R5 is 4-[(:?,6-
dichlorobenzoyl)amino]phenyl,
and stereochemistry is (,f}) (204 mg, 0.51 mmol) and N,N diisopropylethylamine
(0.24
mL, 1.38 mmol). After 7 h, the reaction mixture was diluted with methylene
chloride,
washed with 0.1 N HCl and brine, dried (MgSC?4), filtered and concentrated in
vacuo.
Purification of the residue by flash chromatography using methylene
chloride/ethyl acetate
(2S%) as eluant afforded the title compound (89 mg} as a white solid: ~H NMR
(300
MHz, CDC13) 8 7.57 (2 H), 7.31 (3 H), 7.13 (2 H), 4.97 (1 H), 4.82 (1 H), 4.47
(1 H), 3.80
(4 H), 3.55 (1 H), 3.46 (1 H), 3.34 (1 H}, 3.08 (2 H); MS (ESI+) for
Cz3H22C12N4~5S m/z
537.0 (M+H)+. MS (ESI+) for C,3HZZCIZN405S m/z 558.9 (M+Na)+.
Preparation 86 and Example 279.
4-[(2,6-Dichlorobenzoyl)amino]-N [[(7a,S'~-hexahydro-~5-oxoimidazo[5,1-
b]thiazol-3-yl]
carbonyl]-~-phenylalanine
(Scheme Q, Q-8: where Rte, and RQ_, are equal to hydrogen, Z is NH, RS is 4-
[(2,6-
dichlorobenzoy!)amino]phenyl, and stereochemistry of the C-terminal amino acid
is (S'~).
O
H N N_ ~~
'ONI
O O
O CI
H 1
cl
CA 02342778 2000-12-20
WO 99/6'7230 PCT/US99/14233
-237-
To a cooled (0-S°C} solution of Q-7 (Scheme Q where RQ_, and RQ_, are
equal to
hydrogen, Z is NH, RS is 4-[(2,6-dichlorobenzoyl)amir~o]phenyl, and
stereochemistry of
the C-terminal amino acid is (,S~) {88 mg, 0.I6 mmol) in THF (S mL) and HZO
(O.S mL)
was added via syringe pump over 1 h 0.1 N NaOH (3.4 mL, 0.34 mmol). After 2 h,
the
reaction mixture was partitioned between ethyl acetate and 0.1 N HCl (7 mL)
and diluted
with H20 (20 mL). The organic layer was separated, washed with H20, dried
(MgS04),
f ltered and concentrated in vacuo. The resulting white solid was lyophilized
from glacial
acetic acid to afford the title compound (22 mg) as white solid: IR (drift)
2924, 1726,
1720, 1663, 1657, 1608, 1 S 1 S, 1456, 1431, 1402, 1398, 1243, 1194, 797, 780
cm'' : 'H
NMR (300 MHz, CD30D) 8 8.OS ( I H), 7.61 {2 H), 7.46 (3 H), 7.25 (2 H), 4.93
(2 H),
4.74 (2 H), 4.47 (1 H), 3.77 (1 H), 3.39 (2 H), 3.25 (1 H), 3.04 {2 H);'3C NMR
{7S MHz,
CD30D) 8 171.8, 165.2, 164.7, 138.2, 137.7, I3S:1, 13=1.4, 132.4, 130.9,
129.4, 121.7,
66: 3, 64.4, S4.S, 44.9, 37.4, 33.9; HRMS (FAB) calcd fbr Cz2HZOCL2N40$S +H,
523.0610,
found 523.0629. MS (ESI+) for CZZHZOCIzN4O5S m/Z 523.0 (M+H)+. MS (ESI-) for
CZZHZaC12N4O5S mla 521.1 (M-H)'.
Example 280.
4-[(2,6-Dichlorobenzoyl)amino]-N [[(7aR}-hexahydro~~S-oxoimidazo[S,1-b]thiazol-
3-yl]-
carbonyl]-L-phenylalanine
(Scheme Q, Q-8: where RQ_, and RQ_, are equal to hydrogen, Z is NH, RS is 4-
[(2,6-
dichlorobenzoyl)amino]phenyl, and stereochemistry of 'the C-terminal amino
acid is (S}).
2S
O
H N N,
OH
O O
O CI
CI
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-23 8-
Example 280 was prepared as described in Schenne Q from the slower eluting
diastereomer of general structure Q-4 {Scheme Q where RQ_, and RQ_, are equal
to
hydrogen, RQ-3 is ethyl, and Z is NH). Physical data as follows: IR {drift)
3251, 3079,
1730, 1662, 1611, 1549, 1516, 1482, 1431, 1333, 1306, 1269, 1229, 1196, 792
cni';'H
NMR (300 MHz, CD30D) b 7.59 (2 H), 7.44 (3 H), 7.24 (2 H), 4.65 (1 H), 3.81 (1
H),
3.44 ( I H), 3.23 (2 H), 3.08 (2 H); '3C NMR (75 MHz, CD30D} 8 175.3, I 72.22,
164.6,
155.3, 139.7, 139.1, 136.1, 134.1, 133.9, 132.3, 131.8,130.9, 122.0, 68.4,
64.6, 45,6, 44.0,
36.3; HRMS (FAB) calcd for CzzHzoClzNaOsS +H, 523.0610, found 523.0629. MS
(ESI+)
for CzzHzoC1zN405S mlz 523.0 (M+H)+. MS (ESI-) for CzzHzoClzN4O5S mlZ 52I .0
(M-H)-.
Anal. Calcd for CzzHzoClzNaOsS ' 0.13 HzO: C, 50.26; H:, 3.88; N, 10.66.
Found: C,
50.72; H, 3.96; N, 10.13. % Water (KF): 0.45.
Example 281.
4-j(2,6-Dichlorobenzoyl)amino]-N [(tetrahydro-5-oxo-~5H thiazolo[3,2-c]oxazol-
3-yl}
carbonyl]-L-phenylalanine (less polar diastereomer)
(Scheme Q, Q-8: where RQ_, and RQ_, are equal to hydrogen, Z is O, RS is 4-
[(2,6-
dichlorobenzoyl)amino]phenyl, and stereochemistry of the C-terminal amino acid
is (S~).
S O
N N
OH
0 o w c~ cl
J
H
CI
CA 02342778 2000-12-20
WO 99/67230 PCTIUS99/14233
-239-
Example 281 was prepared as described in Scheme Q using commercially available
(t-butyldimethylsilyloxy)acetaldehyde to form the requisite thiazolidine-4-
caroxylic acid.
Physical data as follows: IR (drift) 3293,3194, 1754, 1667, 1603, 1533, 1517,
1431,
1411, 1392, 1324, 1266, 1206, 798, 78I cni';'H NMR (300 MHz, DMSO-d6) 8 8.44
(1
H), 7.53 (5 H), 7.2I (2 H), 5.00 (1 H), 4.84 (I H), 4.65 (1 H), 4.42 (2 H),
3.08 (2 H), 2.91
(1 H);'3C NMR (75 MHz, CD30D) 8 169.4, 163.8, 160.6, 136.7, 136.2, 133.9,
131.9,
130.9, 129.5, 127.9, 120.4, 68.1, 64.4, 63.2, 53.4, 36.I, :33.7; MS (ESI+) far
CZZH~9C12N3O6S mlZ 524.0 (M+H)+. MS (ESI-) for CzzH.~9C12N306S mlz 522.0 (M-H)-
.
Anal. Calcd for CZZH,9C1zN3O6S ~ 0.31 HZO: C, 49.86; Ii, 3.73; N, 7.93. Found:
C, 49.61;
H, 3.82; N, 7.54. % Water (KF): I.06.
Example 282.
4-[(2,6-Dichlorobenzoyl)amino]-N [(tetrahydro-5-oxo-SH thiazolo[3,2-c}oxazol-3-
yl)
carbonylJ-1.-phenylalanine (more polar diastereomer)
(Scheme Q, Q-8: where RQ_, and RQ.~ are equal to hydrogen, Z is O, RS is 4-
[(2,6-
dichlorobenzoyl)amino]phenyl, and stereochemistry of t:he C-terminal amino
acid is (,S~).
N v 'O
H
O O
I ~ ~ CI
H I ~
CI~ i
Example 282 (diastereomer of Example 281 ) was prepared as described in Scheme
Q. Physical data as follows: IR (drift) 3296, 1753, 1666, 1603, 1579, 1535,
1517, 1431,
I41 i, 1390, 1324, 1267, 1207, 798, 781 cm''; 'H NMR (300 MHz, DMSO-d6) 8 8.57
(1
H), 7.54 (5 H), 7.20 (2 H), 5.23 (1 H), 4.86 (I H), 4.66 (1 H), 4.45 (2 H),
3.22 {l H), 3.09
(1 H), 2.93 (m, 2 H);'3C NMR (75 MHz, CD30D) 8 172.8, 169.7, 163.8, 160.6,
136.8,
CA 02342778 2000-12-20
WO 99J67230 PCT/US99/14233
-240-
136.2, 133.7, 131.9, 130.9, 129.5; 127.9, 120.2, 68.1, 69.9, 63.1, 53.6, 37.5,
36.2; 34.2;
MS (ESI+) for CZZH,9C12N3O6S mlz 523.9 (M+H)+. MS (ESI-) for C2zH~9C12N3O6S
mlz
521.9 (M-H)'; HRMS (EI) calcd for CZZHL9CL,zN306S 5:?3.0372, found 523.0366.
.Anal.
Calcd for CZZH19C1zN3O6S ~ 0.35 H20: C, 49.79; H, 3.74E; N, 7.92. Found: C,
50.14; H,
4.08; N, 8.13. % Water (KF): 1.19.
Exairiple 283.
(45')-4-[[[( I,f)-1-Carboxy-2-[4-[(2,6-
dichlorobenzoyl)amino]phenyl]ethyl]amino]carbonyl]-2-(4-pyridinyl)-3
thiazolidinecarboxylic acid 3-ethyl ester
N~ ~ 5 : ~ 0
~NI~ v 'OH
~O~O ~ I ~ O CI
H I
CI
Example 283 was prepared as described in Scheme B using ~-cysteine and 4-
pyridinecarboxaldehyde to from the requisite thiazolidine carboxylic acid.
Physical data
as follows: IR (drift) 3055, 2981, 2928, 1679, 1604, 1535, 1515, 1450,
1431,1406, 1378,
1331, 1 I94, 797, 778 cnri I; 'H NMR (300 MHz, CD30D~) b 8.68 (2 H), 8.28 {2
H), 7.55 (2
H), 7.42 (4 H), 7.25 (2 H), 6.28 ( 1 H), 4.70 ( 1 H), 4.09 (2 H), 3.4G ( 1 H),
3.16 ( 1 H), 2.94
(I H), 2.73 (1 H), 1.I7 (3 H);'3C NMR (75 MHz, CD30D) 8 190.2, 171.1; 162.1,
153.8,
141.4, 135.3, 134.6, 131.9, 130.3, 129.4, 128.2; 126.4, 122.4, I 18.5, 61.0,
51.7, 35.5, 26.6,
11.7; MS (FAB) mlz (rel. intensity) 617 (MH+, 99), 621 (35), 620 (72), 619
(99), 618
(91 ), 6I 7 (99), 371 (22), 179 (23), 173 {28), 124 (27), 5 ;~ (24); HRMS
(FAB) calcd for
CZgH26CI2N4O6S +HI 617.1028, found 617.1019. Anal. Calcd for CZgH26C1zN4O6S ~
0.9
HCl ~ I.1 H20: C, 50.19; H, 4.38; N, 8.36; CI, 15.34. Found: C, 49.79; H,
4.49; N, 8.11;
CI, 15.05. % Water (KF): 2.96.
CA 02342778 2000-12-20
w0 99/6723U PCT/US99/14233
-24I -
Example 284.
4-[[[(1,5~-2-Amino-1-[4-[(2,6-dichlorophenyl)methoxy]phenyl]methyl]-2
oxoethyl)aminoJ-carbonyl]-3-thiazolidinecarboxylic acid 3-[2-(I-
pyrrolidinyl)ethyl] ester
S- H O
CNN,
n~u_
cN~o~o O
Example 284 was prepared as described in Scheme C. Physical data as follows:
IR (drift) 1709, I675, 1511, 1458, 1435, 1421, 1390, 1380, I354, 1299, 1240,
119
5, 1179, 1114, 765 cm -1;'H NMR {300 MHz, CD30D;) & 8.40 (1 H), 7.4I (3 H),
7.22 (2
H}, 6.99 (2 H}, 5.29 (2 H), 4.46 (6 H}, 3.77 {2 H); 3.50 (2 H), 3.19 (4 H},
2.89 (1 H), 2.04
(1 H), 2.I2 (4 H);'3C NMR (75 MHz, CD30D) b 174.5, 171.6, 171.1, 157.9, 153.3,
136.6,
132.2, 130.6, 130.0, 129.7, 128.3, 114.5, 64.9, 63.2, 62.1, 60.9, 54.4, 53.6,
50.0, 49:0,
37.0, 36.5, 35.2, 22.6; MS (ESI+) for Cz7H32C12N4O5S m/z 595. i (M+H)+. HRMS
(FAB)
calcd for C~,H32C12N4OSS +H~ 595.1548, found 595.1531.
Example 285.
E4s~-4-[[[(IS}-I-Carboxy-2-[4-[(2,6
dichlorophenyl)methoxyjphenyl]ethyl]amino]carbonyl]-3-thiazolidinecarboxylic
acid 3
(3-tetrahydrofuranyl) ester
/s- w ~o
~N~N~/~OH
O~O~O O \ /
O CI
Cr /
CA 02342778 2000-12-20
WO 99/67230 PCT/US991I4233
-242-
Example 285 was prepared as described in Scheme A. Physical data as follows:
'H NMR (300 MHz, CD30D) S 7.41 (3 H), 7.18 (2 H), 6.98 (2 H), 5.28 (2 H), 5.15
(I H)
4.69 (2 H), 4.44 {1 H), 3.82 (4 H); '3C NMR (75 MHz, DMSO-d6) 8 172.8, 157.9,
136.7,
132.2, 130.6, 130.1, 129.5, 128.3, 114.5, 76.8, 72.7, 66.5, 64.9, 62.4, 53.5,
36.3, 34.9,
32.4; MS (FAB) m/z (rel. intensity) 569 (MH+, 7S), 571 (51), 570 (29), 569
(75), 322
(22), 161 {20), 159 (30), 89 {2S}, 73 (31), 71 (99), 43 (41); HRMS (FAB) calcd
for
Cz5Hz6CLzN207S +H' 569.0916, found 569.0939. Anal. Calcd for CZSHZeCI2NzO7S:
C,
52.73; H, 4.60; N, 4.92;. Found: C, 52.41; H, 4.80; N, 4.62.
Example 286.
(45~-4-[[[(I,f)-2-Amino-I-[[4-[{2,6-dichlorophenyl)mei:boxy]phenyI]methyl]-2-
oxoethyl]-
amino]carbonylJ-3-thiazolidinecarboxylic acid 3-[2-(I-piperidinyi)ethyl] ester
S' H O
~N~N~~NH2
O
~O~O
Example 286 was prepared as described in Scheme C. Physical data as follows:
'H NMR {300 MHz, CD30D) 8 7.43 (3 H); 7.22 (2 H), 6.98 (2 H), 5.28 (2 H), 4.66
(2 H},
4.59 (1 H}, 4.47 (I H), 4.47 (2 H}, 3.05 (IO H), 1.78 {4 H), 1.62 (2 H); '3C
NMR (75 MHz,
CD30D) 8 175.9, 172.9, 159.3, 138.1, 133.6, 132.0, 131.4, 131.1, 129.7, 120.1,
115.9,
66.3, 64.6, 63.7, 62.3, 61.9, 57.8, 55.8, 55.1, S 1.3, 50.3, 38.5, 37.8, 36.6,
25.0, 23.5; MS
{ESI+) for Cz$H34CIZN4:OSS m/z 609.0 (M+H}+.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-243-
Example 287.
(4S~-4-[[[( 1 S~-2-Amino-1-[[4-[(2;6-dichlorophenyl)meiboxy]phenyl]methyl]-2-
oxoethyl]-
amino]carbonyl]-3-thiazolidinecarboxylic acid 3-[2-{4-methyl-1-
piperazinyi)ethyl] ester
S- H O
\N~ ~N~N~'~NH2
~N~O~O O
O CI
C!
Example 287 was prepared as described in Scheme C. Physical data as follows:
'H NMR (300 MHz, CD30D) 8 7.41 (3 H), 7.21 {2 H), 6.98 (2 H), 5.28 {2 H), 4.67
(I H),
4.58 {I H), 4.46 (1 H), 4.23 (2 H), 3.20 (2 H), 2.7i {13 H), 2.50 (3 H); MS
{ESI+) for
C2gH35CIzN505S m/z 624.0 (M+H)+; Anal. Calcd for Cz81-i35C12N5O5S ~ 0.5 CZH402
~ 0.5
H20: C, 49.82; H, 5.54; N, 10.02. Found: C, 49.82; H, :5.77; N, 9.65.
Example 288.
(4S)-4-[[[( 1 S~-2-Amino-1-[[4-[(2,6-dichlorobenzoyl)amino]phenyl]methyl]-2-
oxoethyl]-
amino]carbonyl]-3-thiazolidinecarboxylic acid 3-[2-{4-morpholinyl)ethyI] ester
S- H O
O~ ~N~N~~NH2
N ~O~O O I ~
NH CI
~ HCI CI /
O
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-244-
Example 288 was prepared' as described in Scheme C. Physical data as follows:
IR (drift) 1671, 1603, 1536, 1518, 1430, 1415, 1361, 1349, 1324, 1269, 1194, 1
I34, 1118,
1 I04, 799 crri';'H NMR (300 MHz, CD30D) 8 7.62 (1 H), 7.46 (3 H), 7.31 (2 H),
4.68 (3
H), 4.41 (3 H), 3.95 {4 H), 3.48 (5 H), 3.22 (3 H), 2.90 (2; H); '3C NMR {75
MHz, CD30D)
8 175.8, 173.1, 165.3, 154.7, 138.2, 137.6, 135.2, /33.3, 132.4, /30.9, 129.4,
121.7, 121.5,
65.0, 64.8, 63.7, 60.6, 57.4, 55.8, 53.6, 51.4, 50.4, 38.6, 38.1, 36.6, 35.0;
MS (ESI+) for
C27H3'C12NSO6S m/Z 623.9 (M+H)+; HRMS (FAB) calcd for C27H3'C1zN506S+I-I
624.1450,
found 624.1452.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-245-
Scheme R
J-2
R-i R~ R8 I
i \
R~ _ Rx
O \
O~~ OCH3
R-2
s0
~ R9
R~ R.~ I
\
R-3 OCH3
H2N ~ ~2HC1
O
~ R9
o R~
R-4 R~ N O
R2~ ~m _ ,OCH3
X ~ HN
O~ O
R3
o R' ~ Rg
R_5 R1 N O
.OH
R2 X HN
O In O
CA 02342778 2000-12-20
WO 99/67230 PCT/US99114233
-24b-
Scheme R
Scheme R teaches a general method for the preparation of Examples
corresponding
to structures R-4 and R-5, where R6 is nitrogen. Thus reaction of the amide R-
1 (obtained
from the imide J-2), with the organozinc derived from a suitable protected (3-
iodoalanine,
provides the acylamino azaphenylalanine R-2. N deprotection of R-2 gives the
aminoester
R-3, that is used (as exemplified by reagent A-4 of Scheme A, and by reagent B-
5 of
Scheme B) far the synthesis of Examples R-4 and R-5 of this invention.
Preparation 87
(Scheme R: R-1 where R6 is N, R, is H, R8 is -NHC{O)-, and R~ is 2,6-dichloro)
2,6-Dichloro-N (S-iodo-2-pyridinyl)benzamide (C,ZH7ChIN20). A mixture of J-2
and NHZNHZ~H20 in MeOH is refluxed for 6 h under Ar. The reaction mixture is
cooled,
and the MeOH is removed in vacuo. The residue is partitioned between H20 and
EtOAc.
The EtOAc extracts are dried, filtered and concentrated ~to give a brown-
colored solid, that
is purified by silica flash chromatography (99:1 toluene~'EtOAc) to provide
Preparation
87: TLC (98:2 toluene/EtOAc) Rf= 0.43; 'H NMR (CDCI3, 300 MHz) b 10.23 (1H),
8.26
( 1 H), 7.99 ( I H), 7.57 ( 1 H}, 7.41-7.31 (3H}; '3C NMR (CDC13, 75 MHz) 8
163.40, 153.22,
150.95, 147.16, 135.83, 132.72, 131.90, 130.34, 128.65,, 128.50, i 17.09,
86.55; MS (ESI)
393, 391.
Preparation 88
(Scheme R: R-2 where R6 is N, R7 is H, R8 is -NHC(O)-, R, is 2,6-dichloro and
the
stereochemistry is S~
(S)-6-[(2,6-Dichlorobenzoyl)amino]-a-[[(1,1-dimethylethoxy)carbonyl]amino]-3-
pyridinepropanoic acid methyl ester (CI,H23C1ZN3O5). To an amberized flask
containing
activated Zn dust (0Ø802 g, 12.27 mmol) under Ar is added dry THF (b mL) and
1,2-
dibromoethane (0.045 mL). This suspension is brought briefly to a gentle
reflux, and then
is cooled to rt. A solution of TMSCI (1 M in THF, 0.39 mL) is added. The
reaction
mixture is stirred at 45 t 5 °C for 30 min, and then is cooled to rt.
To this mixture is added
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-247-
a degassed solution of N-[(1,1-dimethylethoxy)carbonyl]-3-iodo--L alanine
methyl ester
(4.04 g, 12.27 mmol) in 2:1 N,N-dimethylacetamide/THF (18 mL). The reaction
mixture
is stirred at 45 ~ 5 °C for 5 h, and then is cooled to 0 °t',.
To this mixture is added
PdCl2(PPh3)Z (0.428 g) followed immediately by a degassed solution of
Preparation 87 in
1:1 N,N~iimethylacetamide/THF (19 mL). This reaction mixture is stirred at 45
~ 5 °C for
44 h. It is cooled to 0 °C, and is quenched with cold aqueous satd
NH4Cl. This mixture is
extracted with EtOAc. The combined EtOAc extracts are washed with aqueous satd
NH4Cl
and brine. The EtOAc extracts are dried, filtered and concentrated to provide
a green-
brown colored oil, that is purified by silica flash chromatography (steps of
750:250:1,
700:300:1 and 650:350:1 heptane/EtOAc/iPrOH) to provide Preparation 88: TLC
R~=
0.28 (7:3 hexanesIEtOAc).
Preparation 89
(Scheme R: R-3 where Itb is N, R7 is H, R8 is -NHC(O)-, R9 is 2,6-dichloro and
the
stereochemistry is S)
{S'~-a-Amino-6-[(2,6-dichiorobenzoyl)amino]-3-p~yridinepropanoic acid methyl
ester dihydrochloride salt (C,6H,SC1ZN303~2HCl, R-3). A solution of
Preparation 88
(0.812 g, 1.73 mmol) in 4 M HCl in dioxane (20 mL) is stirred under Ar at rt
for 20 h. The
reaction mixture is concentrated in vacuo, and the residue is taken up in H20
(60 mL).
This aqueous mixture is extracted with Et20 (3 60 rnL), and the Et20 extracts
are
discarded. The aqueous solution is frozen and lyophilized to provide
Preparation 89: IR
(diffuse reflectance) 3021, 2995, 2953, 2893, 2884, 2866, 2853, 2844, 2341,
2015, 1916,
1749, 1646, 1569, 1252 cm '; MS (EI) 367 (M+), 282, 280, 262, 175, 173, 147,
145, 109,
107, 88.
30
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-248-
Preparation 90 & Example 289
[S-(R *, R *)]-4-[ [[ 1-[[2-[{2,6-Dichiorobenzoyl}amino)-5-pyridinyl]methyl]-2-
methoxy-2-oxoethyl]amino]carbonyl]-3-thiazolidinecarboxylic acid ethyl ester
(C23H24C12N4~6'rl}
(Scheme R: R-4 where R, is H, RZ is H, R3 is -Et, X is S., (Y) is -C(O)O, m is
2, n is 0, o is
1, Itb is N, R7 is H, R8 is -NHC{O)-, R, is 2,6-dichloro and the
stereochemistry is [S
{R * R *)I )
C1
NH
EtC O Cl
To a mixture of the N acylthiazolidinecarboxylic acid' (0.292 g, 1.42 mmol),
HOAt
(0.193 g, 1.42 mmol) in 4:1 CH2CIzlDMF (5.25 mL) at 0 °C is added EDC
(0.272 g, I .42
mmol}. This reaction mixture is stirred at 0 °C for 20 miry. Solid
Preparation 89~ (0.568 g,
1.29 mmol) and NMM (0.316 mL, 3.27 mmol) are added.. The resulting reaction
mixture is
stirred at 0 °C for 4 h, and then is kept at 4°C for 40 h. Tlle
mixture is concentrated in
vacuo, and the residue is taken up in CHZC12. The CHZCI? mixture is extracted
with HzO,
aq satd NaHC03, and H20. The combined aqueous washes are back-extracted with
CHZCIZ. The combined CHZCIz extracts are dried, filtered and concentrated to a
pale
yellow-colored foam, that is purified by silica flash chromatgraphy (600:400:1
EtOAc/heptane/iPrOH) to give Preparation 90 (Examplle 289): IR (diffuse
reflectance)
3275, 1742, 1697, 1665, 1586, 1559, 1532, 1476, 1427, 1.398, 1382, 1351, 1344,
1311,
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-249-
1284, 1279, 1242, 1223, 1 I96, 1100, 1023, 802, 787, 7 70, 697 cm '; MS (EI)
SS4 (M+),
495, 422, 395, 352, 315, 293, 280, 172, 160, 144, 116, 107, 88, 60.
Example 290
S [S-(R *,R *)]-4-[[[ I-Carboxy-2-[2-[(2,6-dic:hlorobenzoyl)amino]-S-
pyridinyl]ethyl]amino]carbonyl)-3-thiazolidine;carboxylic acid ethyl ester
{C22H22C12N4~6"~~ EXa1T1p1f: 290)
(Scheme R: R-4 where R, is H, RZ is H, R3 is -Et, X is S~, (Y) is -C(O)O, m is
2, n is 0, o is
l, R6 is N, R~ is H, R8 is -NHC(O}-, R9 is 2,6-dichloro and the
stereochemistry is [S
to (R*.R*)D
Cl
NH ~ /
Et0 O
O
N
S
To a solution of Preparation 289 (0.400 g, 0.72 mmol) in 6:1 THF/H20 (25.6
rnL)
15 at 0 °C under Ar is added slowly over 4 h (via syringe pump} an
aqueous solution of
NaOH ( 1 M, 7.92 mL). The reaction mixture is stirred a:n additional 1.S h.
The reaction
mixture is partitioned between aqueous HCl and EtOAc" The aqueous solution is
separated, and is extracted further with EtOAc. The combined EtOAc extracts
are dried,
filtered and concentrated to a beige-colored foam. This foam is taken up in
1:1
20 MeCN/H20. This solution is frozen and lyophilized to give Example 290 as a
beige-
colored solid: rnp I42-144 °C; IR (diffuse reflectance) 3169, 3094,
3031, 2980, 2964,
2935, 1735,1691, 1591, 1556, 1531, 1480, 1431, 1400, 1379, 1344, 1308, 1288,
1266,
1216, 1194, 1148, 799, 782, 772 cm '; Anal. C 48.72, H 4.29, Cl 12.26, N 9.95,
S 5.62
{calcd C 48.81, H 4.10, Cl 13.10, N I0.3S, S 5.92).
CA 02342778 2000-12-20
WO 99!67230 PCT/US99114233
-250-
BioIo;gicai Assays
Jurkat-Endothelial Cell Adhesion Assay
The following assay established the activity of the present compounds in
inhibiting [3,-mediated cell adhesion in a representative in vitra system.
This assay
measures the adhesive interactions of a T-cell line, Jurkat, known to express
the
a4~3, integrin, to endothelial monolayers in the presence of test compounds.
The
test compounds were added in increasing concentrations to T-cells and then the
T-
ceIl compound mixture was added to IL-1 stimulated endothelial cell
monolayers.
The plates were incubated, washed and the percentage oi' attached cells was
quantitated. The present assay directly demonstrates the cell adhesion
inhibitory
activity and adhesion modulatory activity of the compounds.
Human umbilical vein endothelial cells were pur<:hased from Clonetics
(San Diego, CA.) at passage number 2. The cells were grown on 0.5% porcine
skin gelatin pre-coated flasks (Sigma, St. Louis MO.) in EGM-UV media
(Clonetics, San Diego, CA) supplemented with I O% fetal bovine serum. Cells
are
refed every 2-3 days reaching confluence by day 4 to 6. The cells are
monitored
for factor VIII antigen and results show that at passage l:Z, the cells are
positive for
this antigen. The endothelial cells are not used following passage 6.
The T-cell line Jurkat was obtained from American Type Tissue Culture
Collection {Rockville, MD) and the cells were cultured in RPMI containing 10
fetal calf serum. The cells were washed twice in Hank's :Balanced Salt
Solution
(HBSS) and resuspended in Dulbecco's Minimal Eagle's Media (DMEM)
containing 2.5 mg/ml Human Serum Albumin (HSA). Jurkat cells ( 1 x 106
cells/ml)
were stained with 10 nglml BCECF-AM (Molecular Probes, Eugene, OR)) in
HBSS without phenol red. The cells were loaded with BCECF for 60 minutes in
the dark at 370C, washed 2 times, and resuspended in DMEM-HSA solution.
Confluent endothelial monolayers, grown in 96-well tissue culture plates,
were stimulated for 4 hr. at 37 °C with 0.1 ng/ml (~50 U/ml)
recombinant IL-1
(Amgen, Thousand Oaks, CA). Following this incubation, the monolayers were
washed twice with HBSS and 0.1 ml of DMEM-HSA solution was added. Jurkat
CA 02342778 2000-12-20
WO 99167230 PCT/US99l14233
-2sI-
cells (s X 105 cells} were combined with the appropriate concentration of the
test
compound and 0:1 ml of the Jurkat cell-compound mixture was added to the
endothelial cell monolayers. Generally, 100, 20, s and I.2s p,M compound
concentrations were tested. These concentrations are adjusted downward for
s analogs found or thought to be more potent. The plates were placed on ice
for s
minutes to allow for Jurkat cell settling and the plates were incubated at 37
°C for
20 minutes. Following this incubation, the monolayers were washed twice with
PBS containing 1 mM calcium chloride and I mM magnesium chloride and the
plates were read using a Millipore Cytofluor 2300 (Marllboro, MA.}.
Fluorescence
in each well was measured as Arbitrary Fluorescence Units and percent adhesion
in
the absence of compound was adjusted to 100% and the % adhesion in the
presence of compound was calculated. Monolayers were also fixed in 3%
paraformaldehyde and evaluated microscopically to verify the adhesion. This
procedure is a modification of a previously published method (Cardarelli et
al., J.
1 s Biol. Chem. 269:18668-1$673 (1994)}.
Jurkat-CS-1 assay
The CS-1 derived peptide, CLHPGEILDVPST, and the scrambled control
peptide, CLHGPIELVSDPT, were synthesized on a Beckman 990 synthesizer
using t-Boc methodology. The peptides were immobili2:ed onto microtiter plates
using the heterobifunctional crosslinker 3-(2-pyridyldithio)propionic acid N-
hydroxysuccinimide ester (SPDP) as reported by Pierschbacher et al., Proc.
Nall.
Acad. USA, 80:1224-1227 (1983). Microtiter plates were coated with 20- pg/ml
HSA for 2 hr. at room temperature, washed once with Pl3S and derivatized with
10
2s p.g/ml SPDP for 1 hr. After washing, 100 ~1 of a 100 pg/ml cysteine
containing
peptide solution which had been recently dissolved was added to the wells and
allowed to crosslink to the plates overnight at 4 °C. Unbound peptide
was removed
from plates by washing with PBS. To block non-reactecE sites, the plates are
coated
with 100 ~l of a 2.s mg/ml BSA solution in PBS for I hr. at 37 °C. 100
pl of
Jurkat cells (2.s x 106 cells/ml) in DMEM plus BSA (2.s mg/ml) was mixed with
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-252-
an appropriate concentration of the compound to be tested and the mixture was
added to peptide coated dishes and incubated fox 1 hr. at 37 °C.
Generally 100 20,
and I.25 ~M concentrations of the compound were tested. The concentrations of
the compound were adjusted downward for compounds thought or found to be
5 more potent.
Following this incubation the plates werewashed. once with PBS and the
attached cells were fixed with 3% paraformaidehyde in fBS and stained with
0.5%
toluidine blue in 3.7% formaldehyde. The cells were stained overnight at room
temperature and the optical density at 590 nm of toluidin.e blue stained cells
was
determined using a vertical pathway spectrophotometer to quantitate attachment
(VMAX Kinetic Microplate Reader, Molecular Devices, Menlo Park, CA). This
procedure is a modification of a previously published method (Cardarelli et
al, J.
Biol. Chem., 269:18668-18673 (1994) and Cardarelli et al, Proc. Natl. Acad.
Sci.
USA, 83:2647-2651 (1986)).
I S The preferred compounds are those which have low ICS° values
in the
Jurkat EC assay or the Jurkat-CS-1 assay described above or which have at
least
moderate activity in both assays. AlI of the compounds of the present
invention
have an activity of less than 50 uM in the Jurkat CS-1 assay or less than 500
p,M in
the 3urkat EC assay. Compounds with activity in the Jurkat CS-1 assay
preferably
have ICso values of less than 1 pM, more preferably less than 0.5 ACM, most
preferably less than or equal to 0.08 p.M. Compounds with activity in the
Jurkat
EC assay preferably have ICS° values of less than 10 ~,M,, more
preferably less than
5 pM, most preferably less than or equal to 0.8 ~xM.
In the Jurkat EC Assay, ICS° value ranges (p,M) are depicted by A,
B, and C
and in the Jurkat CS-1 Assay, ICS° value ranges are depicted by D, E,
and F. These
ranges are as follows:
In vitro data:
EC: A >_ 1 1ZM; I p,M > B > 0.25 p,M; C <_ 0:25 p,M
CS-1 D >_ 0.75 pM; 0.75 p.M > E > 0.05 wM; F s 0.05 N.M.
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-253-
Ex. JK/EC JK/CS-I
,.-~.
1 A ~ E
2 C E
__
3
4 C F
_
B E
6 A D
7 A E
8 B E
9 C F
C F
11 A E
__
12 C F
I3 A D
14 B E
I5 A D
16 C E
17 A D
I8 D
19 C E
A D
21 C E
22 A D
23 C F
24 B E
C F
26 A D
27 A D
28 A D.
29 B E
A E
31 C F
32 A D
33 C E
34 A E
B E
36 C
37 C _ F
38 A D
39 C E
41 A D
42 ~ B ~ E
CA 02342778 2000-12-20
WO 99/67234 PCT/US99I14233
-254-
Ex. JK/EC JK/CS-1
43 C F
44 A __._._ D
45 B E
46 A D
47 C F
48 A D
49 A D
50 A E
51 B E
52 C E
53 C - F
54 A E
SS C F
56 A E
57 A D
58 C F
59 A E
60 C F
61 g E
62 C _. - _ F
63 C F
64 C _ F
65 A E
66 C E
67 A E
68 A - E
__.-
69 A D
__
70 B F
71 A
72 A D
73 A E
74 B E
75 A D
76 C E
77 A D
78 A E
-
79 , A D
80 A D
_
82 B E
83 A E
_
8 C F
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-255-
E~. JK/EC JKICS-1
85 C F
86 A D
A D
88 A E
89 A E
90 A,
91 A D
92 A D
93 A D
94 A
95 A
96 A D
97 A _ D
99 C F
100 g E
lOT B E
I02 A D
i03 B F
104 A E
I05 A E
107 A E
109 A D
110 A _
lil B E
112 A
113 A _ E
I14 A D
i15 A E
ii6 A D
ill A E
119 A D
121 A E
123 A D
124 A D
125 B E
_
126 A D
127 C E
129 B E
131 B E
133 A D
135 B E
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-256-
Ex. JK/EC JK/CS-1
~
137 B E
I39 A E
140 A D
141 A E
142 A D
143 A E
144 A E
145 A D
146 A D
147 A D
148 C F
149 C F
150 B E
151 C F
I52 C F
I53 A D
154 B F
I55 A D
156 A E
157 C E
158 A
159 A D
I6I A D
I63 A
164 A
165 C
166 A
167 B E
168 A,
169
170 A
171 A E
I72 C F
173 C E
174 B
i75 A
176 A E
177 A F
178 A F
179 B
180 A
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-257-
Ex. JKlEC JK/CS-1
_. _ ~
181 A -~ E
182 B E
183 A
184 C F
185 C F
186 C F
187 i A E
188 A E
189 A E
190 B E
191 A _ E
192 C F
193 C
195 A E
196 A E
197 A D
298 B
200 A E
_ _
201 A E
202 A
203 A E
204 B E
205 B
206 B g
207 A E
208 A
209 A
210 A
211 A
212 A
213 A
214 A
215 A
216 A E
217 A
218 A
219 A
220 A E
221 A
222 A E
224 A - E
CA 02342778 2000-12-20
WO 99167230 PCT/C1S99/14233
-2S8-
Ex. JK/EC JK/CS-3
225 A E
226 A E
227 A E
228 A E
229 A
230 A - E
231 A E
232 A D
233 A E
234 A E
23S A E
___ 236 A D
237 A E
238 A
_
239 __ A E
240 A _ E
241 A E
242 A E
243 B _ E
244 B g
24S C E
246 B _ E
247 B E
248 B E
249 B E
2S0 C E
2S1 C F
252 C F
253 C E
254 B E
2SS A __. E
2S6 C F
ZS7 B E
258 C F
259 C E
260 B E
261 B E
262 C - F
263 B E
264 C _ F
26S C F
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-259-
Ex. JKIEC JK/CS-1
266 C F
267 A E
268 D
269
270 E
271 - E
272 E
_ 273 D
274 E
275 F
276 . D
277 D
278 E
279 E
280 E
281 E
282 F
283 E
284 E
285 E
286 E
287 E
288 D
290 F
Bioloeicai Activity in Dextran Pleurisy Model
Certain compounds of the present invention were tested in a Dextran~ pleurisy
model.
Rationale for Developin an a,~i, Inte~rin Antagonist to 'Treat Inflammatory
Diseases
VLA-4, a member of the (31 integrin family of adhesion molecules, is thought
to
play a critical role in several types of inflammatory disease processes by
promoting
leukocyte adhesion to vascular cell adhesion molecule (VCAM-1 ) and the CS-1
domain of
fibronectin in extracellular tissue matrix (Elices MJ, Osborn L, Takada Y,
Crouse C,
Luhowskyj S. Hemler M, Lobb RR. VCAM-1 on activated endothelium interacts with
the
leukocyte intesrin VLA-4 at a site distinct from the VLA-4-fibronectin binding
site. Cell;
60: 577-584. 1990. Humphries MJ. Akiyama SK. Komoriya A, Olden K, Yamada KM.
CA 02342778 2000-12-20
WO 99/67230 PCTNS99/14233
-260-
Identification of an alternatively-stiIiced site in human plasma f bronectin
that mediates
cell type-specific adhesion. J Cell Biol; 103: 2637-2647. 1986, Wayner EA,
Garcia-Pardo
A, Humphries MJ, McDonald JA, Carter WG. Identification and characterization
of the T
lymphocyte adhesion receptor for an alternative cell attachment domain CS-1 in
plasma .
fibronectin. J Cell Biol; 109: 1321-1330, 1989, Guan J-L, Hypes RO. Lymphoid
cells
recognize an alternatively-spliced segment of fibronectin via the inte~rin
a~(3, . Cell; 60:
53-6I, 1990) Of the cell types expressing VLA-4, the major emphasis has been
on
eosinophils, lymphocytes, and monocytes. Validation of the role of VLA-4 has
relied
predominantly on the use of anti-VLA-4 antibodies which have been shown to
suppress
delayed-type hypersensitivity responses {Issekutz TB. Dual inhibition of VLA-
4. and
LFA-1 maximally inhibits cutaneous delayed-type hyper<~ensitivitv-induced
inflammation.
Am J Pathol; 143: 1286-1293, 1993, Scheynius A, Camp RL, Pure E. Reduced
contact
sensitivity reactions in mice treated with monoclonal antibodies to leukocyte
function
associated molecule-1 and intercellular adhesion molecule~l . J Immunol; 150:
655-663,
1993, Ferguson TA, Kupper TS. Antigen-independent processes in antigen-
specific
immuni . J Immunol; 150: 1172-1182, 1993, Chisholm PL, Williams CA, Lobb RR.
Monoclonal antibodies to the rote ~; 'ran a-4 subunit inhibit the murine
contact
hypersensitivity response. Eur J Immunol; 23: 682-688. 1993, Elices MJ, Tamraz
S,
Tollefson V, Vollger LW. The integrin VLA-4 mediates leukocyte recruitment to
skin
inflammatory sites in vivo. Clip Exp Rheumatol; 1 I (Suppl 8) S77-80}, 1993,
experimental allergic encephalomyelitis (Yednock TA, Cannon C, F:itz LC,
Sanchez-
Madrid F, Steinman LM, Karin N. Prevention of exnerirr~ental autoimmune
encephalomyelitis by antibodies a~ a~inst aa~3, rote rip. Nature; 356: 63-66.
1992, CaneIla
B, Raine CS. The VCAM-1/VLA-4 pathway is involved in chronic lesion expression
in
multiple sclerosis (MSI. J Neuropathol Exp Neurol; 52: 311, 1993), HIV-induced
encephalitis (Sasseville VG, Newman W, Brodie 5.1. Hesterberg P, Pauley D,
Ringler DJ.
Monocvte adhesion to endothelium in simian immunodeficiencv virus-induced AIDS
encephalitis is mediated by vascular cell adhesion rrtolecule-1/a,~, integrin
reactions. Am
J Pathol; 144: 27-40, 1994), pulmonary inflammation and airway hyperreactvity
in asthma
(Abraham WM, Sielczak MW. Ahrned A, Cortes A. Lauredo IT, Kim J. Pepinsky, B,
et al.
CA 02342778 2000-12-20
WO 99167230 PCT/US99114233
-261-
a~-inte~rms mediate antigen-induced late bronchial responses and prolonged
airw~
hvperresponsiveness in sheen. J Clin Invest: 93: 776-787, 1994, Pretolani M.
Ruffle C,
Roberto LapaeSilva J, Joseph D, Lobb RR. Vargaftig BB. Antibody to very late
activation
antigen 4 prevents antigen-induced bronchial hyperreactivitv and cellular
infiltration in the
rub. inea-p~rwa~. J Exp Med; I 80: 795-805; 1994), experimental models of
autoimmune-mediated diabetes (Yang X-D, Karin N, Tisch R, Steinman L, McDevitt
HO.
Inhibition of insulitis and prevention of diabetes in non-obese diabetic mice
by blocking
L-selectin and very late antigen 4n 4 adhesion receptors. Proc Natl Acad Sci
USA; 90:
10494-10498, 1993, Burkly LC, Jakubowski A, Hattori Ivl. Protection aeainst
adoptive
transfer of autoimmune diabetes medicated through very late antigen-4
integrin. Diabetes;
43: 529-534, 1994), and experimental colitis {Podolsky I)K, Lobb R, King N,
Benjamin
CD, Pepinsky B, Sehgal P, et al. Attentuation of colitis in the cotton-top
Tamarin by anti-
4 inteirrin monoclonal antibody. 3 Clin Invest; 92: 372-380, 1993). Since
eosinophils
represent a major component of the inflammatory cell influx in asthmatic lung
tissue we
developed a simple acute inflammatory model of VLA-4 integrin-dependent
eosinophil
infiltration which could be used to identify VLA-4 antagonists; such compounds
would be
of potential value in the treatment of asthma as well as other diseases in
which VLA-4
played a role.
Materials and Methods
Animals, housing and viral testing:
C57BL/6 mice (Jackson, Bar Harbor, ME; ), 6-8 weeks old, weighing 20-25g were
used
throughout. All mice were acclimated for at least 7-14 days after arrival and
maintained
under controlled temperature {20-22oC) and a 12 hr daily light cycle (6.00
A.M. - 6.00
P.M.). Mice were housed in laminar flow racks and checked biweekly for viral
infections
(mouse hepatitis virus, minute virus of mice. rodent orphan parvovirus,
Sendai) with kits
obtained from Oreganon Teknika {Durham. NC) using established enzyme-linked
immunoabsorbent assays. Mice testing positive for any of the above were
omitted from the
study. All mice were fed standard laboratory chow (Upjohn Lab Rodent
Irradiated Mouse
Chow. #5011-3, PMI Feeds, St. Louis. MO) and acidified drinking water (pH 5.0)
ad
libitum.
CA 02342778 2000-12-20
WO 99167230 PCT/US99/14233
-262-
Induction of Inflammation by Intrapleural Injection of Dextran:
Intrapleural injections were made using a 27G needle cut to 3-4 mm and blunted
by
filing. Injections were made by inserting the needle between the mid-
intercostaI ribs on
the right side of the thoracic cavity.
Dextran (MW S-40x106, St Louis; MO.) was injected as a I0% solution in saline
in a volume of 100 ul/mouse. Care was taken to avoid bleeding at the site of
injection at
which the intercostal muscles were cut to facilitate smooth insertion of the
needle.
Ouantitation of Pleural Inflamrnatory Leukocyte Responses:
Pleural leukocytes were collected as follows: 4h post-induction, pleural
inflammatory exudate was removed by washing with 2 x 1.0 mi Ca'~/Mg'~"~ free
HBSS
{Gibco, Grand Island, NY) containing 45 mg EDTA/100 rnl HBSS, 4oC. Total
leukocyte
counts were made by hemocytometer following erythrocyte Iysis in 2% acetic
acid in PBS
buffer; exudate leukocyte pellets were resuspended in serum for cytospin
preparations arid
stained (Diff Quik, Baxter Healthcare, McGraw Park, IL) for differential
leukocyte counts
{neutrophils, eosinophils, and mononuclear leukocytes}.
The pleural cavities of mice receiving either no intrapleural injection, or
saline
were washed and the cells counted in the same way to estimate baseline or
saline-induced
pleural leukocyte counts respectively.
Administration of compounds'
All drugs were dissolved in PBS and the pH adjusted to 7.5 with NaOH. Each
compound was administered intravenously through the retroorbital sinus at
hourly
intervals (0-3h) starting from time "0" as indicated. Mice were carefully
monitored for
side effects; none were noted for the series of compounds reported herein.
The following compounds were tested for their inhibitory effects on dextran-
induced leukocyte infiltration: Examples 3, 8, 9, 10, 12, 16, 37, 62, 66, 67,
99, 100, 11 I,
113, 1 I5, 127. 131, 141, I84, 185, 192, PBS (saline} was administered iv. as
a control.
CA 02342778 2000-12-20
WO 99/67230 PCTlUS99/14233
-263-
Inhibition of eosinophii infiltration. which was suppressed by anti-alpha-4
Mab {PS/2,
s0%}, was used as a readout of VLA-4 antagonist activity of the compounds
tested. Data
for neutrophils are also reported.
Results:
s Dextran pleural leukocyte response. The total pleural leukocyte counts were
255x104(+/-
16 SEM) cells in the normal pleural cavity; of the normail pleural leukocyte
population, all
cells were mononuclear (a similar response was observed following intrapleural
saline
injection). Four hours after intrapleuraI injection ofdextaran total pleural
leukocyte counts
increased to 719x104(+/-67 SEM) and comprised 36.8x104(+/-4.1 SEM}
eosinophils,
292x104(+/-2s SEM) neutrophils and 391x104(+L-48 SEM) mononuclear leukocytes.
inhibition of Eosinophil inf ltration
A>40; B: 20-39; C < 19
Example Dose Eos
3 sOx2iv A
8 100 x 1 C
po
9 SOx2iv A
10 100 x I B
po
12 sOx2iv A
16 sOx2iv A
37 sOx2iv A
62 s0x2iv B
66 SOx2iv A
67 SOx2iv A
99 s0 x 2 iv A
loo soxziv B
111 sOx2iv C
113 s0 x 2 iv C
CA 02342778 2000-12-20
WO 99/67230 PCT/US99/14233
-264-
Example Dose Eos
I15 SOx2iv B
127 50x2iv B
I31 50x2iv C
141 SOx2iv A
185 50 x 2 iv B
184 50 x 2 iv B
192 SOx2iv B