Note: Descriptions are shown in the official language in which they were submitted.
CA 02343444 2008-05-14
3 1264-17
-1-
PRINTED CONDIJCTORS MADE OF POLY ALKYLENE DIOXYTHIOPHENE
Tlle invention relates to a process for coating substrates, such as paper or
plastic
films, with open, electrically conductive structures, by ink-jet printing or
using
X,Y plotters. A polymer solution made from ,vater-dispersible
polvalkylenedioxy-
tlvophenes is used for the printing process.
Printed circtiit boards used for electrical eircuitry are' ~vell known
substrates which
have an electrically conductive structure_ Printed circuit boards are composed
of a
rigid or flexible plastic substrate to which conductor tracks made from copper
have
been applied. The copper tracks are applied by photogr-aphic printing or by
sereen
printing.
In photo~raphic printin~ by the positive process the first step isfull-surface
1 S application of copper to the substrate. Photoresist is distributed over
the full surface
of the copper. The photoresist is irradiated throu2h a masl: at those
locations where
conductor tracks are to run. The irradiation cures the photoresist.
Subseqtient
development removes the uncured photoresist areas. T'he copper which is now
uncovered is removed by etching in the next step. Once the cured photoresist
has
been removed (strippecl) it is only the desired copper conductor tracks which
renlain.
In the screen printing process an iinage of the desired coriductor structure
is prirlted
as an etch resist, onto a substrate with a full-surface covering of copper. jn
the
etching which follows the copper between the desired conductor tracks is
removed by
etching, and then the etch resist is removed.
In the direct coating of a nietal deposit onto nonconducting substrates ther e
can be
probleins with the adhesion of the nletal to the substrate. In this case a
screen-printed
paste based on an electrically conductive polymer can produce a good bond
between
substrate and superimposed layer. The conductor track structures made from the
polynler are, for example, printed using screen printirig onto a
nonc.onducting
substrate and then coppered chernically (DE 36 25 5S7, DE 36 27 256).
T}Ze use of electrically conductive polymers as an electrically conductive
structure on
substrates is also l:.no-,vn in connection with polymer-based
electroluminescence
indicators (Science, 17 October 1997, p. 383). To apply the electrically
conductive
polti-:ncrs, a solution oFthe polyni:;rs wa~ introduced into tlie cartrid~~e
of an ink-jet
CA 02343444 2008-05-14
31264-17
- 2 -
printer, and printed onto the substrate by the printer. A
major problem with this process was that the organic solvent
for the polymers, generally a halogenated hydrocarbon or
tetrahydrofuran, attacked the plastic of the printer
cartridge by salvation or swelling.
To avoid this disadvantage Y. Yang and J. Bharathan (Science,
Vol. 279, 20 February 1998) used a water-soluble polymer
from the polythiophene class of compounds. These water-
soluble polymers did not attack the printer cartridges.
Since structures of water-soluble polymers are also altered
by atmospheric moisture, they are suitable only if they have
no further contact with water after the water has been
removed in an annealing step. In polymer-based luminescence
indicators this condition poses no problem, since, after the
water has been removed and after any further operations
which may be required, conductive structures are
encapsulated under inert conditions excluding water
completely. However, the water-soluble polythiophenes are
unsuitable for open conductor track structures, i.e. those
exposed to the atmosphere.
The object of the invention was to find a process for
producing open electrically conductive structures on
substrates which is simpler and faster to carry out than the
known processes of structuring using copper conductor tracks,
and gives stable conductor track structures under normal
conditions.
Thus, the present invention provides such a process by
printing, onto a substrate, the conductive structures using
an ink-jet printer or X,Y plotter in the cartridge of which
there is an aqueous dispersion of
polyalkylenedioxythiophenes with a suitable polyanion as a
counter anion.
CA 02343444 2008-05-14
31264-17
- 2a -
The substrate used may be paper or plastic film.
The polyalkylenedioxythiophenes have been cationically
charged and are composed of structural units of the
formula (I)
CA 02343444 2001-03-09
Le A 33 106-Foreign Countries
-3-
A20 O-A
I 1 {I)
s
in which
A' and A2, independently of one another, represent substituted or
unsubstituted
CI-C4-alkyl or together form a substituted or unsubstituted Cl-C4-alkylene
group, and
n represents an integer from 2 to 10,000, preferably from 5 to 5000,
in the presence of polyanions.
Preferred cationic polyalkylenedioxythiophenes are composed of structural
units of
the formula (Ia) or (Ib)
R2Ri
O O
(Ia)
s
n
RR3
O O
\ {Ib)
s
n
where
R, and R2, independently of one another, represent hydrogen, substituted or
unsubstituted C1-C18-alkyl, preferably C1-Ci,o-alkyl, in particular CI -C6-
alkyl,
C2-C]Z-alkenyl, preferably C2-C8-alkenyl;, C3-C7-cycloalkyl, preferably
cyclopentyl or cyclohexyl, C7-CI5-aralkyl, preferably phenyl-Cl-C4-alkyl,
C6-C10-aryl, preferably phenyl or naphth.yl, Ct-C18-alkyloxy, preferably
CA 02343444 2001-03-09
Le A 33 106-Foreign Countries
-4-
C1-Clo-alkyloxy, such as methoxy, ethoxy, n-propoxy or isopropoxy, or
C2-Clg-alkyloxy esters, and
R3 and R4, independently of one another, represent hydrogen, but not both
simultaneously, or at least singly siul:phonate-substituted C1-C1%-alkyl,
preferably Cl-Clo-alkyl, in particular CI-C6-alkyl, C2-C12-alkenyl, preferably
C2-C8-alkenyl, C3-C7-cycloalkyl, preferably cyclopentyl or cyclohexyl,
C7-Ci5-aralkyl, preferably phenyl-CI-C4-allcyl, C6-Clo-aryl, preferably phenyl
or naphthyl, C1-C18-alkyloxy, preferably Ci-Cto-alkyloxy, such as methoxy,
ethoxy, n-propoxy or isopropoxy, or C2-C1 g-alkyloxy esters, and
n represents a number from 2 to 10,000, preferably from 5 to 5000.
Particular preference is given to cationic or neutral
polyalkylenedioxythiophenes of
the formulae (Ia-1) and/or (lb-1)
0 0
(Ia-1)
S
n
R 3
O O
(Ib-1)
s
n
where
R3 is as defined above, and
n represents an integer from 2 to 10,000, preferably from 5 to 5000.
The polyanions comprise anions of polymeric carboxylic acids, for example
polyacrylic acids, polymethacrylic acids or polymaleic acids, and of polymeric
sulphonic acids, such as polystyrenesulphonic acids and polyvinylsulphonic
acids.
These polycarboxylic and polysulphonic acids may also be copolymers of
CA 02343444 2001-03-09
Le A 33 106-Foreign Countries
-5-
vinylcarboxylic and vinylsulphonic acids with other polymerizable monomers,
such
as acrylates and styrene.
The gegenion particularly preferably comprises tlie anion of
polystyrenesuiphonic
acid (PSA).
The molecular weight of the polyacids supplying the polyanions is preferably
from
1000 to 2,000,000, particularly preferably from 2000'ao 500,000. The polyacids
or
their alkali metal salts are available commercially, e.g. polystyrenesulphonic
acids
and polyacrylic acids, or else can be prepared by known processes (see, for
example,
Houben Weyl, Methoden der organischen Chemie [Methods in Organic Chemistry],
Vol. E 20 Malcromolekulare Stoffe, Part 2, (1987), pp. 1141 et seq.).
Instead of the free polyacids required for forming the dispersions of
polyalkylenedioxythiophenes and polyanions, it is also possible to use
mixtures of
alkali metal salts of the polyacids and appropriate amounts of monoacids.
In the case of formula (Ib-1) the polyalkylenedioxythiophenes carry positive
and
negative charge in the structural unit.
The preparation of the polyalkylenedioxythiopheries is described, for example,
in
EP-A 0 440 957 (= US-A 5 300 575). The polyalkylenedioxythiophenes are
prepared
by oxidative polymerization. This gives them positive charges which are not
represented in the formulae, since their number and position cannot be
established
beyond question.
An advantage of the novel process is that open, electrically conductive
structures can
be produced on printed circuit boards with quality comparable to that of the
known
copper conductor tracks, but in a small number of' steps which are easy to
carry out.
All that is needed for production is an ink-jet printer with an appropriately
prepared
printer cartridge and a computer to control the printer. The desired conductor
track
structure can be designed on the computer screen and immediately printed out
on a
suitable substrate.
The water-dispersible polyalkylenedioxythiophene is not water-soluble and,
even
under normal conditions, forms a conductive structure which has long-term
stability.
I'.
CA 02343444 2001-03-09
Le A 33 106-Foreign Countries
-6-
Fitwes and examples
The figures show:
Fig.l Bayer cross symbols printed on paper using poly-(3,4-ethylenedioxy-
thiophene) (PEDT) and polystyrene sulphonate (PSS).
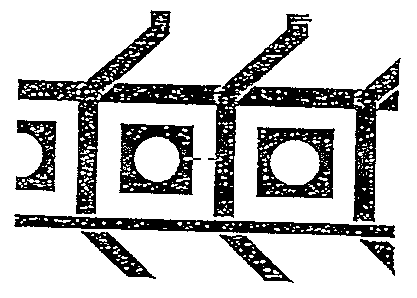
Fig. 2 Conductor track system printed onto paper using PEDT/PSS.
Fig. 3 Conductor track system printed onto polyethylene terephthalate (PET)
film
using PEDT/PSS.
Example 1
Preparation of the 3,4-polyalkylenedioxythiophene dispersion
g of free polystyrenesulphonic acid (Mn about 40,000), 21.4 g of potassium
15 peroxodisulphate and 50 mg of iron(III) sulphate were stirred in 2000 ml of
water.
8.0 g of 3,4-ethylenedioxythiophene were added, with stirring. The dispersion
was
stirred for 24 h at room temperature. 100 g of anion exchanger (commercially
available product Lewatit MP 62 from Bayer AG) and 100 g of cation exchanger
(commercially available product Lewatit S 100 from Bayer AG) were added, both
in
20 moistened form, followed by stirring for 8 hours.
The ion exchangers were removed by filtering tl:n-ough a polyacrylonitrile
fabric of
pore size 50 m. This gave a ready-to-use dispersion of 3,4-polyalkylenedioxy-
thiophenes (PEDT) with polystyrenesulphonate (PSS) as gegenanion (see Il) with
a
solids content of about 1.2% by weight.
The dispersion could easily be filtered through a 0.45 m filter and was used
after
filtration to prepare the colours for the ink-jet printer.
a o a o O o
s S
s H s
0 o c) o
CA 02343444 2001-03-09
Le A 33 106-Foreign Countries
-7-
Tn
m
SO~ S 3 H
Example 2
The aqueous dispersion of the PEDT/PSS as in (I][) from Example 1 was
introduced
into an empty ink-jet printer cartridge for an HP-]Desk-Jet PLUS (Hewlett-
Packard)
ink-jet printer. The cartridge had been cut open and thoroughly cleaned and
after the
PEDT/PSS dispersion as in (II) had been introduced it was sealed again using a
polyethylene hot-melt adhesive from Henkel. An ink-jet printer cartridge
prepared in
this way was inserted into the HP-Desk-Jet PLI;rS ink j et printer and served
as a
reservoir for the printing liquid for applying the PEDT/PSS dispersion using
the
printer under computer control. The pattern to be printed was designed on the
computer using a conventional software progran;i. An image of three Bayer
cross
symbols placed under one another (Fig. 1) was selected. This image was printed
onto
paper using computer control. The result was a;print on paper of the three
Bayer
cross symbols placed one underneath the other and composed of conductive
PEDT/PSS, which had a blue intrinsic colour.
Example 3
The procedure followed was similar to that of Exzunple 2, except that a
polyethylene
terephthalic (PET) film of 0.1 mm thickness was used instead of the paper and
printed with the Bayer cross symbols.
Example 4
The procedure followed was similar to that of Example 2, except that the
pattern was
a section for a printed circuit board designed using EAGLE layout software
(Fig. 2).
This conductor track pattern was printed onto paper using PEDT/PSS as in
Example 3.
CA 02343444 2001-03-09
Le A 33 106-Foreign Countries
-8-
Example 5
In a manner similar to that of Example 4, the conductor track system of Fig. 3
was
printed onto a PET film of 0,1 nun thickness. The electrical conductivity of
the
printed conductor tracks was demonstrated using a continuity tester.