Note: Descriptions are shown in the official language in which they were submitted.
CA 02343554 2001-04-05
MULTI-PROBE SYSTEM
This invention relates to an apparatus and method for catheterization of the
tissues and fluid spaces, including blood vessels, ofthe human body: The
invention
also relates to the method by which diagnostic and therapeutic agents and/or
procedures may be delivered to any of those body parts or regions. In
particular, the
present invention relates to the design and use of a mufti-lumen catheter for
providing
multiple, and not necessarily complimentery functions, such as sampling of the
fluids
within the extracellular and interstitial spaces ofthe braiin, spinal cord, or
other body
tissues, concurrently with drug delivery, electrical recording / stimulating,
or the
delivery of any other type of therapy into the same tissues in accordance with
the need
for such therapies.
Surgical procedures, especially neurosurgical procedures that involve open
craniotomy, carry an intrinsically high level of risk of infection and
hemorrhage. A
variety of new techniques aimed at minimizing the invasiveness of
interventional
procedures have been introduced in the hope ofreducin.g the surgical risk and
shorten
a patient's hospital stay and overall rehabilitation. Placement of probes and
catheters
into the brain using stereotactic and image-guided procedures provides one
means of
minimizing these risks. However, many types of interventional procedures,
including
those that require drug delivery into the brain, sometimes require either
catheterization at multiple target points, or subsequent .re-implantation
ofthe catheter
to optimize the therapy being delivered to the brain.
Current methods of catheterization of the parenchyma) tissues of the brain
make it possible to measure intracranial pressure (U.S. Patent No. 5,107,847),
deliver
drugs in a rate-controlled manner (U.S. Patent No. 5,836,935), infuse various
substances into the brain (U.S. Patent No. 5,720,720), and convey fluids out
of the
brain (U.S. Patent No. 5,772,625). Very recent technological developments are
now
leading to intraparenchymal catheterization systems that can be positioned
within the
brain by magnetic stereotaxis (U.S. Patent Nos. 5, 125,888; 5,707,335;
5,779,694), that
are visible under magnetic resonance (MR) imaging (U.S. Patent No. 6,026,316),
and
that contain mufti-purpose electrodes (U.S. Patent No. 5,843,093). In
addition, there
are several types of implantable neurostimultor devices; that have been
disclosed.
1
_. -. _. ; ~ _ ,.a.. ~. _ : __ _ --~,~ ,., ~_ -
__; ~ -s- ~_ -' - :y _ ; _ _ ~ ~ -' _ ~_- _~ ___
CA 02343554 2001-04-05
These include those described by Otten (U.S. Patent No. 5,344,439), Hess et
al. (U.S.
Patent No., 4,800,898), and Tarjan et al. (U.S. Patent No. 4,S49,SS6) as three
examples thereof. However, none of the available methods of intraparenchymal
catheterization can carry out multiple input-output functions at the same time
with
the same implanted device. With the exception of the method taught by Otten
(U.S.
Patent No. 5,344,439), an already implanted device or part ofan implanted
device
must be withdrawn before another probe is subsequently inserted into the
tissue to
perform additional functions. U.S. patent No. 5,788,713 describes the
availability of
both a delivery lumen and sampling lumen on a single catheter system.
The inventors have determined that it is increasingly important to determine
other local characteristics of the region where active materials are delivered
that can
effect the efficacy or optimization of the treatment, such as pH, osmololity,
viscosity,
electrolyte content, temperature, fluid flow rates, and concentrations of
specific
ingredients. No present systems enable both the delivery of therapeutic
materials and
the measurement of significant local properties (except for the single noted
instance
oif delivery and physical sampling).
U.S. Pat. 4,114,606 discloses a monitoring app<~ratus for intracranial
pressure
measurement, wherein electromagnetic radiation is imposed on a passive circuit
implanted in the cranium, the frequency at which the radiation is absorbed
reflecting
intracranial pressure. U.S. Pat. 4,147,161 to Ikebe, et a.l. discloses a
system for
measuring or monitoring intracranial pressure which comprises a non-elastic
detecting
pouch inserted between the skull and the brain, wherein a pressure measuring
device
in the liquid in the pouch indirectly measures the intracranial pressure. U.S.
Pat.
4,156,422 to Hildebrandt discloses an apparatus for treating hydrocephalus
comprising a housing which contains subcutaneously implantable components for
measuring and controlling fluid pressure, wherein a second housing outside the
patient contains measuring and control components whereby an intracerebral
space
may be automatically drained in response to a predetermined adjustable ICP.
U.S. Pat. 4,210,029 to Porter discloses a difFerential sensor unit utilizing
fiber
optic light guides, wherein three light guides pass within a pneumatic line
into one
end of a rigid cylindrical envelope implanted in the skull. Detectors are
arranged to
actuate pressure display and pneumatic controls to adjust the internal
pressure of the
2
CA 02343554 2001-04-05
envelope to match the ICP and thereby measure the ICP. U.S. Pat. 4,265,252 to
Chubbuck discloses an implantable transensor device containing a passive RF
resonant circuit which is responsive to changes in ICP. US Pat. 4,385,636 to
Cosman
discloses an implantable telemetric differential pressure sensing device
comprising a
planar closed conductive loop which moves with a flexible diaphragm, wherein
the
resonant frequency of the conductive loop is detected i:elemetrically to
determine
pressure in a body compartment.
Guidewires for the catheter or drug delivery system are usually made of
radiopaque material so that their precise location can be identified during a
surgical
procedure through fluoroscopic viewing. Exemplary of guidewires used under X-
ray
viewing is the guidewire disclosed by LeVeen, U.S. Pat. No. 4,448,195, in
which a
radiopaque wire can be identified on fluoroscopic ima~;es by metered bands
placed at
predetermined locations. U.S. Pat. No. 5,375,596 to 'fvviss et al. discloses a
method
for locating catheters and other tubular medical devices implanted in the
human body
using an integrated system of wire transmitters and receivers.
U.S. Pat. No. 5,325,865 to Beckman, et al. discloses a catheter assembly for
measuring fluid pressure in a body cavity, comprising an optical converter
responsive
to an electrical power source for energizing a light-emitting diode which has
drift
characteristics which vary in response to temperature, and a detection
circuit.
U.S. Pat. No. 5,843,093 to Howard discloses a dual purpose
neuron-monitoring electrode assembly particularly suited for performing
magnetic
pallidotomy for the treatment of Parkinson's disease. However, unlike the
present
invention, the patent to Howard does not provide for an MR-compatible, mufti-
lumen
probe which is capable of containing several different internal devices that
can sample
the extracellular environment and react to it.
U.S. Pat. No. 5,843,150 to Dreessen, et al. discloses a system and method for
providing electrical and/or fluid treatment within a patient's brain, wherein
the device
is an implantable device comprising a lumen, a catheter, an electrode, and a
pump.
However, unlike the present invention, the patent to Dreessen, et al. does not
provide
for an MR-compatible, mufti-lumen probe capable of containing several
different
internal devices that can sample the extracellular environment and react to
it.
.:_ __~ . , - .--
CA 02343554 2001-04-05
U.S. Pat. No. 5,843,148 to Gijsbers, et al. discloses a brain stimulation lead
and multiple electrodes for precise delivery of electrical stimuli to a
patient's brain.
U.S. Pat. No. 5,820,589 to Torgerson, et al. discloses an implantable medical
pump comprising a fluid reservoir, a passive regulator assembly, an
electromechanical
controls means, and a means for receiving radio frequency signals to operate
the
electromechanical control means.
U.S. Pat. No. 5,858,009 to Jonkman discloses a mufti-lumen cannula for
conducting fluid to and from a body region, especially in left-heart and right-
heart
assist cardiac surgical procedures, wherein the septum separating the first
and second
catheter lumens is wire-reinforced to resist deflection ofthe septum.
A mufti-lumen catheter system with novel forms and functions is disclosed. A
desirable design feature for practice of this invention comprises an elongate
element,
such as a central barrel of a catheter which is surrounded by (or including
within a
major lumen) additional lumens which perform or transport various functions.
In one
embodiment that is particularly useful for therapy of th<; parenchyma) tissues
of the
brain, one or more of the plurality of lumens around the central barrel are
configured
for sampling of fluids within the interstitial space (e.g., semiconductor
based,
microdialysis-based, electronically based, electrically based, interactance
and
transmittal, etc. sampling). Other lumens ofthe mufti-lumen probe can be used
for
the delivery of drugs, therapeutic agents, diagnostic agents or view-enhancing
agents
into the parenchyma) tissues, either via ei~lux from a single drug delivery
lumen or
via a mufti-port configuration to facilitate broad spatial distribution ofthe
drug within
the tissue. In this embodiment, the central lumen can b~e used for any
treatment or
function, but especially either microdialysis or drug deliivery, or it can be
configured
to accommodate a recording or stimulating electrode, such as a mufti-purpose
stereotactically placed electrode (e.g., U.S. Pat. No. 5,843,093). In a method
of the
invention, additional probes or devices that might be passed through either
the central
barrel of the catheter or through one of the surrounding ports include
intracranial
pressure probes, optical fibers and/or optical fiber bundles configured for
conveying
illumination and/or optical signals to and from the target tissues,
iontophoresis probes,
thermometry probes, blood-flow-sensing probes, chemical probes, sensing
devices
CA 02343554 2001-04-05
(even audio sensing devices, pressure-sensing devices, pH sensing devices,
viscosity
or osmololity sensing devices, radiation-sensing device, light-sensing device,
etc.),
vacuum lines, fluid delivery tubes and conduits, guidewires, fixed and
adjustable-tipped steering probes and wires, electric field and magnetic field-
sensing
probes, electrodes and applicators, gene analysis chips and "lab-on-a-chip"
structures,
biopsy devices, tissue and cell implantation devices, cryogenic probes,
thermal
probes, ablation probes, cauterizing probes and tips, stems, coils,
angioplasty balloons
and devices, radioactive sources, magnetic and electric field sources,
integrated
circuits and electronic devices.
The unique compound nature of the catheter invention makes it possible to
carry out several diagnostic and therapeutic tasks concurrently, with or
without
additional functional coupling between the processes. An important attribute
of the
medical device disclosed by this invention is to optimize the therapeutic
response to
the patient's clinical condition by using the sampling capabilities ofthe
microdialysis
or other diagnostic components ofthe catheter to provide information on the
metabolic state of the target tissue (especially the brain) via analysis of
the fluids
within the extra-cellular matrix. Drug delivery into the parenchyma) tissues
can then
be carried out via positive pressure infusion, or by dif;r'u~sion-based
delivery of
pharmacological agents via the microdialysis process, using available lumens
within
the catheter to carry out either form of treatment. In parallel with drug
delivery,
electrostimulation of the same or nearby target tissues/neurons can be carried
out via a
recording/stimulating electrode passed down a central or radially disposed
barrel of
the device.
In a method ofthe invention, a feedback mechanism may be used to automate and
optimize the entire process, wherein a number of physiological variables can
be taken
into account by an algorithm that governs the therapeutic response of the
catheter
system. In another embodiment, physiological and metabolic data on the status
ofthe
patient (derived form other sensors on/in the body, such as, for example,
probes which
monitor tissue oxygen levels, blood pH, concentration ofmaterials in the
blood, blood
flow, and other physiologic parameters} can be incorporated into the
algorithm's
treatment optimization process. Thus, for example, if a. stroke or brain-
injury patient
'°~ - _ - _ ,~ -. _ . _~
- _ _ ; __ _ _ - ~ >.-~:
CA 02343554 2001-04-05
were in an intensive care unit or other hospital bed setting, the vital-signs
data from
patient monitoring systems (including, in particular, intracranial pressure
measurements) could be monitored by the system's signal processor, wherein the
resulting information provided feedback control of the rates of drug flow into
the
brain. In another embodiment ofthe method ofthe invention, the microdialysis
systems ofthe catheter device are used to sample endorphin levels, wherein the
catheter's signal processor could then provide feedback control of the
electrostimulation process so as to attenuate the effects of chronic pain.
In another embodiment of the method of the invention, the algorithm
governing the patient's therapy preferably utilizes proportional-integral-
derivative
(PID) control functions, adaptive control functions, nonlinear control
functions,
mufti-variable/state-space control functions, stochastic control functions
and/or any
other functional approach deemed appropriate for the implementation of the
therapy.
In all such cases, the controller could be designed to respond to changes in
the
patient's condition using artificial intelligence techniques that would let
the feedback
mechanism "learn" the best way to respond to changes in the patient's
physiological or
anatomical status. Such techniques might employ, among otlher things, "fuzzy
logic"
algorithms that can function in the presence of incomplete or indeterminate
data.
A summary of the present invention includes at least a mufti-lumen,
mufti-functional catheter system comprising a plurality of axial lumens, at
least one
lumen supporting a functionality other than material delivery and material
removal.
At least two individual lumens may be parallel to a central barrel of the
catheter, and
at least one of the at least two lumens may be used for sampliing fluids in a
body part
into which the catheter is inserted. At least one of the axial lumens may be
used for
infusion, injection or other mechanism of delivery of diagnostic and/or
therapeutic
agents into a body part in which the catheter is inserted. The central lumen
ofthe
catheter may contain an electrode, such as a neurostimulator or
radiofrequency-ablation lead.
An outer surface ofthe catheter may comprise a continuous sheath inside
ofwhich are
located individual lumens, a central barrel and other functional components of
the
catheter system. At least some of said other function. components may comprise
electrical leads. There does not have to be an envelope around the catheter
system, so
Af~?.t~5-2001 12-32 MAP,4~LITMAt~IR~~ °2343554 2ooi-o4-o5 , 952 83~
9191 P.02
there nod not be any exterior covering element aver the at least two lumens, a
central
barrel and other functional components of the callletGr sy;;tCtn. Il is
expected that at
least one biological or physiological measuring device is present rw'rthin at
least one
lumen.
Such a biological or physiological 111Ca5171'ill~, d~Yil:G WVIIhI bC CXI7GCa~d
tv be
connected to a signal receiving device by an electrical lecud associated with
the
catheter system. The at least one biological or physiological measuring device
may
connected to a signal 1'1:L~IVLBI~ tICVtGC by Iln Gtectrical iea~d permanently
attached to
the catheter system.
The at least ore diagnostic component may rrnvide any u~seiirl information,
such as
information about metabolic, physiologic a:uctJur anatumil; status of a
patient.
The information from said at least one biological or physivlogieal measuring
device
would ot~dirlsri>.v be received by a hn~t cc~mruter connected to said device.
More than one component or sensing alrry~cut ur cnC~tsuruz#1 device would be
among
components that provides iuaformation other thatl information from said at
Icast ono
biological or physiological measuring device_ Ail ar most ofthat information
should
be received by a host computer to evaluate a ucatlnem pruccllurC yr patient
conditions
around the locality of treatment, The additional conapon~ents may measure, for
example, vttat signs ofa patient. Information from more than one information
source
may received by the hose cuu~puter tend a treatmern planning and control
algorithm
could be present in said host computer to process that information from more
than one
source_
A method according to the invention for treatimig a patient according to a
treatment plan Could Comprise inserting the catheter system the invention into
a
patient, delivering therapy to the patielit tleruugh al least a~ne lumen of
said catheter
system, taking biological or physiological measurements oftissuc or fluids
within the
patient, repot<ltlg said information tn a. enmrnter, and evaluating
performance of the
treatment plan with the cornputcr based upon cumpurirxg Said information to
expected
biological o'r physiological information. The method could include information
relatm~ to at least two different biological or physiological measurements
being
eleetricalJy transmitted to the computer fur evaluating performance ofthe
treatment
./
TOTHL P.02
CA 02343554 2001-04-05
plan. Upon evaluation of the performance of the treatment plan, the computer
could
indicate a) a deviation for a range of acceptable levels ofperformance ofthe
treatment
plan; and b) an alteration of an existing treatment plan its identified. The
method
could have the computer signal the catheter system to actively modify the
existing
treatment plan. The method could also include having the computer signal an
operator to actively modify the existing treatment plan.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a representation of a layout of one embodiment of a multi-lumen,
multi-function catheter system disclosed by the present invention.
Figure 2 shows a cross-sectional view of one embodiment of a multi-lumen
catheter device.
The present invention has enabled the combinatiion of multiple functions into
a
catheter or probe system, enabling the performance of complete procedures by
catheter along with additional, multiple or complete capability for ancillary
or
essential analytical procedures, diagnostic procedures, quantitative and
qualitative
analyses, operational environmental determinations, and any other task or
information
providing mechanism that provides information useful to the operation
procedure.
For example, a catheter system according to the present invention may provide
two,
three, four or more separate and distinct functions that can be performed
distally (at
the site ofthe catheter) without the necessity for removal of distal elements
or
replacement ofthe catheter. Information from the distal location can then be
transmitted to a proximal intelligence source (e.g., processor,
microprocessor,
computer, hardware, software, etc.) for reading, visualization, analyzing,
comparing,
evaluation, and the like. This information can then be used to evaluate the
ongoing
procedure on a periodic, episodic, near real time, or real time basis to
suggestion
continuation of the existing procedure or alteration of the procedure. For
example,
the catheter may have separate biological and physiological delivery and
evaluation
systems for in vivo activity during a procedure. This ca.n be important as
ambient or
induced biological activity and ambient or induced physiological activity can
have
direct results on the efficacy of the treatment or may indicate problems with
the
-~-:~,_-~-_-_, -- -~. - :_- ~ -.,.~. - _.._:~." _.__ ~: , .:e
_ __ . _..W - :w _ _ : . - _ _ ._:
CA 02343554 2001-04-05
procedure. Determination of the status of these activities can assist in the
assurance
that an optimal or satisfactory procedure is being performed, and suggest
methodology on improving the procedure while it is being administered.
Multiple
procedures or ancillary procedures, and multiple forms of operation
environment
evaluations can be performed through a single catheter with a single insertion
of a
catheter, with many or all of the functional elements needed for the
procedures
originally delivered with the catheter insertion or fed through the catheter.
For
example, tissue can be removed from a surface, therapeutic material delivered
to the
site, and conditions monitored on a local level to assure; that at least
minimal
performance or operation standards are being maintained within the operational
environment. A prophetic example of a multiple function procedure supported by
a
catheter according to techniques of the invention would be treatment of an
area of
tissue in the cranium where infection has damaged tissue over a targetable
surface
area. A catheter would be designed to provide fiber optic viewing capability
with a
multiplicity of fiber optic viewing fibers (and an optional light piping
fiber), an
ablative or irrigating device to remove irreparably damaged surface tissue, a
removal
lumen would be provided to assist in the physical removal of detritus from the
removed damaged tissue, a delivery lumen would provide therapeutic agent to
assist
in the rapid healing or protection of newly exposed tissue that had previously
been
under the damaged tissue, and a solid state (e.g., semiconductor) pH sensing
element
extending from its own lumen can assist in measuring real time pH in the
region of
the therapeutic material delivery to assure that the pH in the region does not
vary to
such a degree that would indicate some deviation from the desired rate of
delivery or
loss of fluid from the newly exposed tissue. Other sensing systems (e.g.,
pressure
measuring, thermometric, chemometric, etc.) can also be present. These sensing
systems would transmit electrical signals through conductive elements (e.g.,
lines,
wires, coils, etc.) to reading or recording systems. This information, either
directly
from the electrical conductors or through the reading or recording systems,
would be
evaluated (either by an operator or by artificial intelligf,nce) and the
procedure
evaluated (by an operator or computer) on the basis of the information
provided. The
evaluation, based on known parameters for measuring the compliance of the
procedure to acceptable standards, would dictate or indicate continuation of
the
CA 02343554 2001-04-05
procedure, modification ofthe procedure, or cessation ofthe procedure.
Evaluation ofthe performance of the procedure by artificial intelligence
(e.g.,
hardware, software, computer, processor, microprocessor; chips, circuit
boards, and
the like) can be performed by any acceptable format. ~~pecific or modified
programs
(for individual patients, for ranges or parameters within. operable
conditions, or
specifically identified conditions being treated) can be provided to the
artificial
intelligence that suggest or dictate the progression of the procedure
depending upon
the readings. Although some general programs for the procedures my be
developed,
the system is particularly useful for increased automation of procedures where
unusual circumstances might be present, such as patients with particular
toxicity
sensitivities, patients with underlying conditions (e.g., F'arkinsonism, Type
I diabetes,
alcoholism, drug dependency, PKU, allergies, and the like) or patients on
separate
drug therapies where changed conditions in their blood chemistry from the
operational procedure could affect the underlying condition. The software for
the
operational procedure could include special commands. and attributes for the
unique
conditions or patients undergoing the procedure. As local conditions that
could
contribute to complications in operations are presently not easily considered
and
measured on a real time basis, this type of procedure, and especially
tailoring the
software to,_procedures on patients with unique requirements provides a
significant
advance within the field. As an example of this, the pH~ level of patients
suffering
from long term drug dependency (including excessive alcohol use) may have
blood
pH levels that are not within a normally accepted range. As some drugs and
therapies
may have pH sensitivities themselves, and the therapy rnay itself alter local
pH even
further, special modifications in the rates and/or concentrations of delivery
may have
to be modified in the program for a particular patient. A standard software
procedure
for otherwise healthy patients would not consider this underlying condition,
even to
the extent that a modification suggested by the 'normal" program could worsen
the
procedure under the special conditions of the underlying condition.
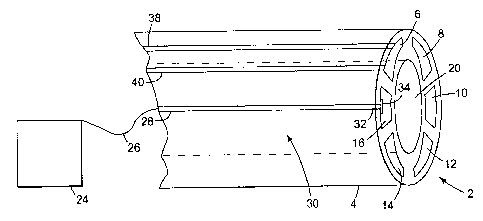
Figure 1 is a representation of a layout of one embodiment of a mufti-lumen,
mufti-function catheter system disclosed as an aspect oiPthe present
invention. The
catheter superstructure or manifold 2 comprises an external guide-tube 4 that
contains
__ _.--_: .:,_._ _ ,....:~. ._...... -:::. _.:. :: _-~~ -. _:._ . . _ -- ~
..~_ - --m _ ' ~°
__ ---_:, -_ ..... __ - ~ ,:.. ~. . _.. _ _.:_ , _._ -..- _. ~ _-~~- x ~_ ~-
:..:. --_'.._.__.
' . _ ..':: :- ~-' _.__ =:_ -.. _ __'---_-- ;:.:: _ ~_, 'r _, ~~.. .....--_.,
_ - ,. _ _ :.. -.. -..x..~ ..=--~-.-... :,~;. __~W-::.-.
__ .__ _ _ _._ __. . __ ,. -.. ;. 1.-~.~-.~''~'- _ ._ _--.~ ~ _.~.z~ ~;.
CA 02343554 2001-04-05
the six individual internal lumens 6, 8, 10, 12, 14 and 16 of the catheter, or
of a
skeleton-like framework (shaped in cross section like a star washer) to which
are
affixed the individual lumens, including the central bawe120 ofthe catheter.
(Same
specific configurations of the assembly of lumens that fbrmulate the catheter
are
described later in Figure 2.) There may be several such individual lumens (for
instance, up to "n" of the type of internal lumens such as 6, 8, 10, 12, 14
and 16)
inside of or otherwise integral to (e.g., distinct, self supporting extruded
tubes carried
on an exterior surface or interior surface) the overall device. Some ofthe
internal
lumens can be configured as microdialysis devices, e.g.,, designed to sample
the
interstitial fluids. Other internal lumens might be infusion probes or drug
delivery
membranes. An online, real-time assessment of the dia.lysates gathered by such
microdialysis devices can be accomplished by a variety of methods and systems
that
might range from microscale liquid chromatograph systems that are part ofthe
physical structure of the device, to sophisticated, stand-alone genetic
material
analyzers and processors. Concurrently with the microdialysis analyses, a data
acquisition system 24 can be added when needed to read in obtained and
transmitted
signals. The signals may be transported to the data acquisition system 24, for
example, through a lead wire 26. That lead wire 26 is connected to a
conductive
element 28 embedded or affixed to the surface 30 ofthe: guide tube 4. The
conductive
element 30 is electrically connected at a distal end 32 to a contact plate 34.
The
contact plate 34 is provided so that any functional device or microcatheter
(not
shown) can be electrically connected to the data acquisition system 24 easily,
merely
by being in electrical contact with the contact plate 34. In this manner, a
mufti-probe
component (a component catheter) ofthe system could be produced as a catheter,
and
a selection of electrically driven or signaling elements rnay be inserted into
the
component catheter. Snaps and other securing fitting may be provided, and
these
snaps and fittings may themselves be remotely controlled to secure and then
release
specific lumen fed devices on the catheter. Where there are both active and
signaling
functions within a single lumen {as in 6), there may be multiple electrical
leads 38 and
40. There may be three, four or more electrical leads at each lumen, as is
needed.
These electrical leads may be manufactured by any convenient manner such as
microlithographic etching of a metal coating into a circuit or conductor
pattern,
11
;:-_ _:_: -- , _ _._ _ _ . __ . T~. . _~ . _ .. ..
_ - - _ __ -- ~ ~ -_ __
CA 02343554 2001-04-05
extruding conductors along with the extrusion of the guide tube 4, and the
like. The
availability of multiple electrical leads 38 and 40 allows for the possibility
of systems
wherein at a single lumen, a device may be secured, released, activated,
signaled to,
signaled from, and programmed {if it contains memory). This offers unique
functional capability to the system and the ability of the; operator to
control the local
events of a procedure.
Figure 2 shows a cross-section of a mufti-probe catheter 60 of the invention.
A central barrel 64 is shown carrying a microcatheter 66 that can deliver
material
within a patient. Lumen 68 is shown carrying a solid state sensor 70 with an
electrical
conductor 72 carrying away signals. Another lumen 741 is shown carrying away a
sampling tube 76. A third lumen 78 is shown carrying an abrading tip 80 that
may be
distally or remotely controlled. A fourth lumen 82 carries a pH sensor 84
which is
electrically connected to a conductor or wire 86 that carries signals from the
pH
sensor 84 to a computer (not shown). It is to be noted that lumens may be of
different
diameters, unevenly distributed, and as with lumen 88, may be located outside
of the
main surface 90 of the catheter 60.
The lumens, or at least some of the lumens, may carry vital signs sensors
(e.g., pH
sensors, pulse sensors, electrolyte concentration sensor:>, pressure sensors,
specific
solute sensors and any other type ofmedical monitors) that might be needed or
desirable to successfully treat the patient. The data coll'.ection acquisition
device 24
(e.g., a host computer) may collect and process these data via a treatment
planning
algorithm that the patient=s physician deems approprial:e for the therapy
being given,
such as, for example the delivery of electrostimulation via a probe (not
shown)
through lumen 8 within the central barrel ZO of the catheter 2. The strength
of the
stimulation signal can be regulated a controller upon in:>truction from the
host
computer or the computer can directly institute a modification of the
procedure,
particularly with coincident notification to the controller. An infusion and
dialysis
drug delivery controller electrically connected to the computer or manually
operated
can also be used to regulate the influx of drugs or other therapeutic agents
into the
patient via syringe drivers and infusion and output dialysis membrane probes
passed
through other available lumens (e.g., 10 and 12) in the catheter 2 . In other
12
~. , -_~_ ...___~...~ _._... .. :~.~. -.. _,__ --..: _ :~_.~ ._ _. _.-:- -:- --
_ .__--~-- _ . ~~__._
.. . _ _ _ ... .: . ._ _ _ -_ __ __ ,_.-_,_ _ . . ,_ . _ ,. . ~w .".~.,~ : -
.:_ .~.
-. --~~ : - _ r -=a ~.~-~-3. : _ <_ ' °._ . _
n __~~ ~ . ; : '.. - --:-:_ - _-__ v _ . ___:_. . ,,.._ -,: _ __.. :: :---. ;
~_ _--l-_.-_~-
CA 02343554 2001-04-05
embodiments of this invention, the treatment planning process might be carried
out by
the physician without recourse to an automated mechanism (computer, etc.). In
such
circumstances, the physician or therapist could simply monitor the results of
the
dialysis process and manually regulate the flow rates, pressures, and other
variables of
the infusion, dialysis drug-delivery, and electrostimulation processes. In
still other
embodiments of this invention, the computer and possibly other components of
the
therapy control system can be located at a remote site, perhaps a hospital or
a
care-provider=s office. The input and output signals to and from the therapy
control
system can be mediated via transmission over wired or wireless telephone
systems or
by other types of data telemetry including satellite-based and other kinds of
telemedicine linkages. As shown in Figure 1, individual lumens can be made in
a~
variety of sizes to suit the application at hand for each ofthem. They might
also have
a variety of different port structures, for microdialysis, infusion, and other
therapy
delivery techniques.
One embodiment of the present invention also m~:ay comprise an integrated
electronic personal health care monitoring system. The personal health care
system
center could comprise, for example, a computer for rec<;iving or acquiring,
storing,
processing, and transmitting information, and a plurality ofinterfacing ports.
Each
interfacing port is adapted to accept a plurality of dif~'ere;nt patient
monitoring
modules, a plurality of different accessory modules for transactions, and a
plurality of
other interactive modules. Each module would be electrically interconnected to
the
data processor via the computer=s bus to exchange information with the
computer=s
CPU. The data processor includes means for providing operating instructions to
the
sensor modules, accessory modules, and therapy-providing modules. Each module
provides information on a condition or vital sign of the patient. The data
processor
monitors the information provided by the modules and v~ould act on it via an
algorithm run by the computer.
The specific design of any feedback system disclosed by the present invention
will depend on the application for which it is to be used. In general, it will
have the
arrangement shown in Figure 1. Dialysates sampled by the microdialysis probes
will
be analyzed by an online monitor, and the resulting data (following the
necessary
signal conditioning and, where appropriate, analog-to-digital conversion) will
be input
13
CA 02343554 2001-04-05
to a dedicated computer (CPU) that can also monitor the patient's vital signs,
ICP
readings, etc. The CPU can use any of a number of different feedback and
control
algorithms ("Patient Treatment Plans" or PTPs) selected by the physician as
being the
most appropriate for the needs of the patient at the timf; of treatment. Such
PTPs
might incorporate straightforward PID laws or any other feedback control
mechanism
that effectively regulates drug delivery and electrostimu~lation levels as
needed. In the
method of the invention, the CPU that processes the feedback control mechanism
can
be in the patient's home, at the hospital bedside, in the physician's office,
or at a
location remote to the patient. In the latter case, given that the data rates
for most
physiological processes can be made slow, it would be possible to transmit /
receive
information to/from the processing computer using either ground-based or
cellular
phone systems and a modem. 'thus, in a preferred embodiment ofthe invention, a
physician or other appropriate health care professional can monitor and adjust
the
therapy provided to the patient, eg., the stimulation level, drug delivery
rate, etc., via
a telemedicine link, rather than requiring a visit by the patient to a
conventional
clinical care setting.
The system may include testing and measuring instruments to monitor the
patient=s vital physiologic information, and may be adapted to a home
healthcare and
maintenance environment, even on a specific (unique) I>atient basis. The
system may
further include control devices having health care and maintenance functions
monitored by the testing and measuring instruments in the system. In one
embodiment wherein the system is arranged in the centralized network
configuration,
the testing and measuring instruments and the control devices are connected
via a
local area network with a data controller wherein all the vital information
obtained in
the system is stored. Instruments and devices are permitted controlled access
to the
controller through the network to retrieve necessary vital information
therefrom. In
another embodiment, the system is arranged in the distributed network
configuration,
with the vital information obtained by respective measuring instruments stored
therein.
Figure 2 shows two examples of cross-sectional views of an actual
mufti-lumen mufti-function catheter device. Each of the. lumens has nominally
the
same internal diameter, although they can be of different internal diameters,
as
14
CA 02343554 2001-04-05
dictated by design considerations. The central barrel 52 ofth~e catheter
contains an
electrostimulation lead 50 centered within it. The structural elements 53 keep
the
individual internal lumens 54, 56, 58; and 60 and the central barrel from
collapsing
upon each other during catheter insertion, withdrawal, or during use.
These techniques may be used alone or in combination with the techniques
described in U.S. Pat. No. 6,026,316 wherein problems, such as the following
are
addressed by real time or near real time observation of material concentration
changes
are observed by MR imaging techniques and acted upon after observation. One of
the
significant difficulties with delivery of materials such as drugs, hormones,
or
neurotransmitters to living tissue is assuring that the materials are
delivered to the
target receptor location in the intended amount. Many materials which are
delivered
to a patient, even though beneficial in the treatment of a speciific
condition, may be
moderately or even strongly noxious or damaging to healthy tissue. It is
therefore one
object of conventional materials application treatment to maximize dosage to a
desired location and to minimize dosage to locations which do. not require the
delivery
of the material. Additionally, newer medical treatments may include procedures
which remove unwanted deposits of materials with an expectation that the
removal
will be assisted by physical removal (by a withdrawal system). or natural
bodily
function removal (e.g., the circulatory system), or which may attempt to
stimulate the
body to produce natural chemicals (of which a patient may bc~ deficient) at an
increased rate (e.g., electrical stimulation to increase the production of
dopamine).
Because these procedures are usually highly invasive, it would be extremely
desirable
to have a real time indication of immediate, transient and persistent
effectiveness of
the procedure. Where undesired deposits or collections of materials are being
dispersed, it would be desirable to visualize the actual movement ofmaterials
to assist
in collecting them (e.g., through catheters) or tracking them to assure that
they are not
again depositing or collecting (as in intravenous or cerebrospinal fluid
blockage), or
moving in segments which are too large and could cause blockage in other parts
of the
body as they are carried about. Unfortunately, with in vivo delivery of
materials,
particularly extremely small doses in small volumes delivered! by small
instrumentation into tissue regions protected by the blood-brain barrier, or
the brain-
MAR-20-X001 11-53 MRRKLITMfahl~~ °?34s554 2ooi-o4-o5 95~ 83~ 9191 P.03
cerebrospinal fluid barrier, or into visually inaccessilale areas, it krac not
hP.e~n roscihoe
to observe real time distribution vfthe material delivery, ur thr ctlSpG~i~rt~
ur
distribution ofthc material at the injection or infusion site within the
tissue. Where
even small variations ar raiscalculations about the location of the target
sight and the
delivery device can significantly afl'bct tl;~e delivery of material and the
c;~'ectivrnass
of the delivered material, real time observation of the mat<;rial delivery is
even more
critical than in topical or gross (e.g., massive systemic injection) delivery
Pventc.
'there has been no truly effective observation system for such dalivery prior
to the
invention of'U.S. Fatcnt No. 6,026,316, which specifcatio~n is incorporated
herein, in
its entirety, by reference.
The basic operation oftlte U.S. Patent No, 6,026,316 involves the initial M1~.
imaging observation of a molecular environment of a patient (c.g., a
particular arcs or
region of a patient, such as tyssue, particularly such tissue :a~s that
present in organs or
systems of animal bodies and especially the human body, including" but not
limited to
the uztracranial compartrrrerrt and the various anatomic rc,~;ions ofthc
brain, including
the cerebral ventricles, cisterns, epidural and subdural spa~:es, sinuses, and
blood
vessels, the spinal cord, including disks, nerves and assocutted r~asc~tlar
system, the
heart and flat coronary vascular circulation, liver and the hepatic vascular
circulation,
kidney and the renal vascular circulation, spleen and the splenic vascular
system,
gastrointestipal system, special senses, including the visual' system,
auditory system,
and olfactory system endocrine system uicIudutg the pituitary gland, dlyruid
gland,
adrenal gland, testes, and ovaries, with observation of an 14ZR image signal
intensity at
a given time andlor state (e.g., prior to material introducticm or at some
defined wage
ofmaterial difl'usion into the molecular cnvimnment. In an cxarnplc oftlm
u~ethud of
the invention, the distribution ofthe material in the tissu~ is determined by
releawing
an amount of the material through a drug delivery device positioned m the
tissue.
allowing the material to diffuse in tho tissue, and analyain~; tlae rcsultir~
MR signal
intensity, On a continual basis or at some subsequent time interval later
(e,g,, n
pulsed interval, preselected interval, random interval, freqyaent or sporadic
intervals),
the MR usage of the uiolecular stay wittein tlrC same gcnGnsl tug W VbsCrVCd.
Changes in the characteristics, properties or quality of the :image, such as
the image
signal intensity within the area are presumptivpiy (and in mast cases
detinrttvely) the
16
MAP-20-2001 11:57 MARt<LITMAhIA~ ,°343554 2001-04-05 952 83~ 9191
P.0~1.~0~1
rrssull of tht~ inlruductiun of rrratGrial iuw the urigirrt~l raulcuuhsr
envu~rtunent,ar~d
nlteration of the MR r~sponse for regions of the enviro>rament where delivered
material
concentration has changed. >f3y rereating nhcenraticm nfttie MTt image signet
intensity within an area at ICa,.yl vnvc (a.g.,1"urE ttdcirr~ the initial
vbsCrvatiuu at a
material concentration state at s time Ti, and at least one subsequent
observation of
MRf-nhsenrahle changes such ac in the signal intensity qualities at a time'1
Z), the
c;irtrngc in MR irrragc ~iguat ifrterysity qualisies can be relat~cd to the
change in material
coaccntration between times T, and T2, whether that oltarrl;e is from a
starting paint of
Bern cnncentratinn nr tmm an existing cnncentratinn levell. The ch.~ervatinns
tlrCrGfurc rclatG to tlrG actual delivery ofmaterial iota the anolecular
envu~unrnent in au
observable, and to some lesser degree, quanti~'rable rnwnma~.
A medical device used in the nreterred practice ofthe present invention fir
delivery of materials may vary widely with respect to its structure, being
highly
dependent upon the particular procedural use to which it i,s being intended.
However,
there fire rrratiy ~:ahrres which can be common to ail ofth~P dPV1(:PF nr
which shauld at
least be considered in the various cvnsiruc,~tiuns. The simplest device could
be a,
single delivery tube (catheter) having multiple lumens (odthe same or
difh'erent size),
the GathPter having MR responsive material in nr nn the rmmpncitinn ofthe
hrbing 1 q,
prGfGrrrbly rrGar thG distal Gntl ur outlet ufltiG delivery lubG fur ~sisturg
in dCtCUliun
by the MR imaging system. The next lcrcl of simplified construction would be
the
presence nfMR coils nr mic:racc~ilx at ~r near the distal end ~fthe ~srtheter,
' his
a'~~'cYlil~ a9 CI~t:W11G1'G dcx:ritrml, uupruvC~ tlrG visibility uftlrC
viCwrzblG sigrrrtl
observable by the MPJ system. More than one coil or m icxocoil toes be
present, as
the distrihutinn nt micrncnils along a Length nt the cathetaer helps define
the region
within which Iocai signals are detected at efficlettt irtteztsiltles, As
di>~Teni medical
procedures are performed in dif~ererrt environments, wills different shapes
and
different variations in densities, the c~il5 may he located, dyed, angled, r,r
~therwiae
designed to provide specific MR signals and/or responses tailored to the
anticipated
needs of a particular procedure. In general, the inventions is best practiced
by
employing an array of RF miarpcc~iis, such that an image; is obtained for any
uriGntatiun of the dru,~ dGlivcry devie~C.
The device may alt include numerous catheter elements and/or ports andAor
17
TfiTAI P. Gtd
CA 02343554 2001-04-05
supplemental or independent functional elements. For example, at least two
ports
may be needed, one to carry in on chemical material, one to deliver a specific
medical
device or sensor and/or another port to deliver a second distinct chemical
material
which is or may become desirable during a medical procedure. For example, in
addition to a primary treatment chemistry being delivered, saline solutions or
specifically tailored solutions to dilute potential oversized deliveries could
be
desirable. Some treatments may require sequences of drug delivery or delivery
of
various drugs which may not be storage stable prior to delivery to a patient.
Separate
ports would be desirable in those events. Additionally, ports may be used to
evacuate
undesirable materials directly or indirectly introduced by the medical
procedure. A
withdrawal port may comprise a tube with a port attached to negative pressure
with
respect to the opening in such a withdrawal port, thus being able to reduce
liquid or
small particle solids volumes within the area ofthe procedure. Where the
liquid
volume or solids are MR viewable, the MR viewable device may be directed
towards
specific locations or areas and the ports targeted towards those specific
areas. In
addition, the various ports may be marked or designed to provide distinct
signals
when viewed by MR systems so that they may be distinguished during performance
of the procedures. For example, MR insensitive materialls may be used to line
a port
or materials with different distributions or intensities of MR response may be
used in
the various ports to differentiate the elements while being observed during
performance of procedures. For example, where a withdfrawal tube has openings
through which materials may be withdrawn, the orientation ofthat opening
within the
device becomes important. By lining the edges ofthe opening with material
having
unique MR responsiveness within the device, the position and orientation ofthe
opening can be readily determined. Particularly preferred is a 2,000-5,000
angstrom
thick coating of MR-visible material along the distal shaft of the device.
Where multiple catheters or ports or functional elements are combined into a
single device, the configuration of the different components should be
tailored for a
particular procedure. The different components may be associated by various
orientations. An MR-visible guidewire may be inserted 'within the device to
assist in
positioning the device at a target anatomical location. Particularly preferred
is a
guidewire or other structural support made of NitinoITM or other MR-compatible
18
-___ _ _: _ _-_-. ~ ___-_ . = _ -_ -_ . -_ -.~ - .- ,- -= ~, -_
_;~.. .__ _. _~ ~____ _._:.. . _ _'.'. ._ . __._ .,_ __~- __ ~-_ -._ -_~.--
..,~, -:
_ '._~.~-_ -~
-.-..~-~.-.r.-~ _. _ _ -, _, _ - . _ . . ~ :v ::-_ ___ ~_ . _ _ w._ -:. - _-
_~ _ : ' -.~ . -=_.
.._- _. __ _ -:.. _:..>____ .__-~ . __--.... ____...... _ , _ ---_ ... -..: . -
_ ~__.. ~ ~~~ ~~.~.-~. _--~-:-~.--=:~.~sr,~...._~.
__- :---~: -_ . _.. :.-. __ . _..__-_ , ~.--_.~....,. ~ _:~ -. .._ ._._ __.
~~.~-~~- __.~_
-___ __ _ ____ ___ _ -:: . _ _ ~_ - - __ :- ___ -' T-~ -----T=.-- -:-
_ _.. __._ ~ T_ _- _ _.._ ___ _ _ ., n _ .: , __. .. . ...._ _r
_~_ . _ :::.. ..____-_ _. _ ~ - _~ , ~~-. _- ' ----_.~._ ~:
_ :- _.= .__ ,~~. __-_ _ _ _._ _ __
.', _: ~ _ _ __._ _ _ s.~, "' .=:~"'.""'"' _.._.
CA 02343554 2001-04-05
shape memory metal. This is the simplest geometry and provides for smallest
diameter sizing of the device. Other configurations suclh as parallel
alignment of the
elements in a strip-like orientation, stacking of elements in rows and
columns, or
mixtures of these and other configurations may also be useful. Other elements
which
may be included within the device, in addition to or separate from the use of
delivery
and/or withdrawal tubes, include thermal elements (for providing heat),
radiation
carrying elements (e.g., ultraviolet radiation, visible radiation, infrared
radiation, and
even hard radiation carrying elements, such as optical fiibers or other
internal
reflection radiation carrying systems), detection elements (e.g., pH
indicators,
electronic activity indicators, pressure detectors, ion detectors, thermal
detectors,
solid-state chemical/gas detectors, cryogenic delivery, sonic delivery systems
(for
sonic disruption of material), radiation delivery systems,, light delivery
devices (e.g.,
UV for treatment or stimulation, infrared for ablation), cell delivery
mechanisms,
nutrient delivery systems, implantation systems, robotic element delivery
systems;
etc.), and any other sensing, treatment or detection elennent which would be
useful
during medical procedures. These individual elements are each (preferably
independently) extendable to permit optimal positioning within the tissue
would be
configured as desired or needed for the particular procedure intended for the
device.
Procedurally inert elements such as structural supports, reinforcing elements
or
coatings, back-up elements, and the like, may also be present within the
device.
Particularly preferred as structural supports or reinforcing elements are
circumferential bands of Nitinol~ or other MR-compatible shape memory metals
which, when activated, can facilitate accurate directed placement of the
functional tip
of the device.
One type of configuration which is presently considered as the preferred
embodiment of the invention is the use of a core of elements) surrounded by a
sheath
or distribution of additional elements. A central core element my comprise a
single
tube for delivery of a material, a pair of tubes for delivery of two
chemicals, a
delivery and withdrawal tube, or a procedurally inert structural support
element.
Around the central core element may be disposed multiple additional elements,
usually seeking as near to a circular distribution about t:he central core as
geometries
allow. The attempt at the circular distribution is primarily for purposes of
optimizing
19
CA 02343554 2001-04-05
a small size for the diameter ofthe article, and is not necessarily a
functional aspect to
the performance of the device. MR responsive material:>, including MR
microcoils,
may be located within the central core, around the central core (beneath any
next
layering of elements), or over the elements surrounding the central core.
Where one
or more of the elements receive, transmit or are powered by electrical
signals, it is
desirable that these elements be electrically separated by either or both of
physical
separation or additional insulation to prevent mixing or cross-transmission of
signals
between the distinct elements. Carrying and withdrawing tubes (as well as
other
elements) may also secondary functions. For example, a carrying tube may be
conductive (by being naturally conductive or by having a conductive coating in
or
outside of the tube) and the electrical connection may be associated with an
electronic
element or component at the distal end ofthe device. The tube may thereby act
as a
carrying tube and electrical connection to the electronic component or
element.
Structural or adhesive support materials between different elements may also
provide
such functions.
The various individual elements within the device; must be structurally
associated, especially away from the distal end, and during insertion, may
need
structural association at the distal end. The structural support or structural
integrity
may be provided by some physical means of attaching the various elements. This
may be done by adhesive materials between the individual elements (which
adhesive
should be MR compatible), fusion ofthe various elementa, or by coextrusion
ofthe
tubes into a single unit (or single component of a multiple element device).
The
adhesive may be an organic or inorganic adhesive. The distal end of the device
may
have the ends ofthe elements temporarily or controllably bonded during
insertion.
This may be beneficial because it may be desirable to have the individual
elements
fan out or separate during a medical procedure, for example, as in the case
ofa target
tissue or area of pathology that is anatomically extensive. The adhesive could
be
water soluble (which would dissolve in a timely manner after insertion),
solvent
soluble (with solvent delivered into the distal end during o~ preliminary
procedure, or
radiation disruptable (e.g., a positive-acting resist adhesive composition
which is
sensitive to LTV, visible or IR radiation which may be delivered through a
radiation
CA 02343554 2001-04-05
carrying port). Many other variations and combinations of these considerations
and
constructions may be used within the practice of the prf;sent invention. Iin
another
embodiment the dialysis probe is replaced by an MR-visible microcatheter,
which is a
single extrusion catheter made from one of several possible sizes of a
polyethylene
terephthalate proximal shaft. A 12 mm distal segment of the microcatheter drug
delivery device is made of elastomeric hydrogel or similar soft material which
minimizes tissue damage during insertion. A plurality of semipermeable
membranes
are placed circumferentially at regular intervals along the distal segment of
the
microcatheter, thus enabling wide dispersion of an injected agent,
semipermeable
membrane consisting of a 0. 1 8-0.22 ml millipore filter. The companion
microguidewire in this example is made ofNitinol or similar memory metal which
enables directed placement of the tip of the catheter. A, microguidewire may
be
threaded into a clear hub luek-lock cap made of poly-rnethel-pentene or
similar MR-
compatible plastic. Both the catheter and guidewire have a linearly arranged
array of
radiopaque and MR-visible markers disposed at the distal end to provide easily
identifiable reference points for trackability and localization under MR
imaging and
X-ray fluoroscopy guidance. The microcatheter can also be made from any of the
well known soft, biocompatible plastics used in the catheter art such as
Percuflex, a
trademarked plastic manufactured by Boston Scientific Corporation of
Watertown,
Massachusetts. When the delivery device is positioned intracranially, the
distal
markers will be identifiable in an MR image and by X-rays. In another
preferred
embodiment, two or more R-F microcoils are placed along the distal shaft of
the
m icrocatheter.
The delivery device can be employed to deliver pharmacologic therapies in
order to reduce morbidity and mortality associated with cerebral ischemia,
intracranial
vasospasm, subarachnoid hemorrhage, and brain tumors. In the method ofthe
invention the distal tip ofthe mufti-lumen catheter assembly is typically
positioned a
few millimeters above the intracranial target structure using MR imaging. In
one
embodiment of the invention, surface modifications ofthe material components
of the
dialysis probe enable timed-release kinetics of MR-visible biologic response
modifiers, including peptide macromolecules. In another embodiment ofthe
invention, a pump or other infusion or injection device circulates a solution
containing
21
-. __ - ..~ _. . S _...
_ -. ~ __
_ -= ~._- _.~... - ,_ --~._. _ ..- _ __:.:: _ _' .. _, _.._._ _..-_ .'._~_ -e-
~.:::_.m~-_-. _.~---.. i ~ _..._':-.~.
_ .u---a asp
CA 02343554 2001-04-05
a therapeutic drug or an MR-visible contrast agent through the walls of the
dialysis
fiber into the brain at rates between 0.01 microliter/min to 10
microliter/min. In
another preferred embodiment of the invention, pressure ejection techniques
well
described in the medical literature are used to deliver a ;predetermined
amount of a
therapeutic drug agent or MR-visible contrast through one or more of the
tubular
components of the multi-lumen device. In one specific preferred embodiment of
the
invention, the catheter is backfilled with the drug or contrast agent, which
is
functionally connected to a PicospritzerTM (General Valve Corp, Fairfield, NJ)
or a
similar instrument that is able to deliver pulses of nitrogen or compressed
air with a
duration ranging from a few milliseconds to several seconds at a pressure of
10-50
psi. Using such a pressure ejection mode of drug delivery, the concentration
of the
released substance in the vicinity ofthe tip is accurately defined by the
concentration
of the material in the delivery device. A binary solution can also be
released, in that
two therapeutic or diagnostic compounds can be delivered at the same time by
pressure ejection oftwo materials from two or more separate microcatheters.
Any material delivery system may be used in combination with the multiprobe
catheter system ofthe invention. The material delivery device, for example,
may
comprise at least one device selected from the group consisting of
A) a catheter assembly comprising at least two lumens;
B) a catheter assembly of from 2 to 10 mass transporting elements;
C) at least one light carrying element connected to a light
reading system so that light projected into said area provides a signal
through said
light carrying device to said light reading system;
D) a light transmitting element associated with said material delivery
device;
E) at least one thermally responsive element connected to a reading
system for said thermal response so that temperatures or temperature changes
within
said area provide a signal to said reading system for said thermal response.
The system may contain an element capable of providing a charge is part of
said material delivery device, said charge when provided being at a location
on said
material delivery device which assists in orienting of ionic material being
delivered by
said material delivery device within an area electrostatically near a point of
release of
22
CA 02343554 2001-04-05
said material from said material delivery device. The charge-providing element
may,
for example, be present to deliver electrical charge onto said material
delivery device
electrostatically near a point of release of said material from said material
delivery
device. The method may include observing the increase of material within
aqueous
environments or tissue in a living patient comprising the steps of
a) observing by Magnetic Resonance Imaging a visible image within an
area or volume comprising tissue of a living patient, said area or volume
including a
medical device which can be observed by Magnetic Resonance Imaging,
b) causing by said medical device at least some material which causes an
alteration in the magnetic response of water in which said material is
dispersed or
dissolved to increase its concentration within said area or volume comprising
an
aqueous environment or tissue of a living patient,
c) observing a change in a property of said visible image of an area or
volume comprising tissue of a living patient while said medical device is
still present
within said volume,
d) observing a change in a property of said visible image after said
medical device has been moved from within said area or volume of tissue.
The medical device may stimulate a part ofthe patient to increase or decrease
its
production of a chemical whose presence in water causes a change in a property
of
said visible image. The method may include observing a different rate of
passage of a
chemical through structural material within the body of a living patient, said
structural
material having a delivery side and a distribution side, said method
comprising the
steps of
a) observing by Magnetic Resonance Imaging a visible image within an
area to volume comprising tissue of said living patient, said area or volume
including
a delivery device which can be observed by Magnetic Resonance Imaging,
b) causing by said delivery device to deliver at least some material which
causes an alteration in the magnetic response of water in which said material
is
dispersed or dissolved,
c) observing movement of said material within said patient through said
material from said delivery side to said distribution side,
d) observing a change in a property of a visible image in an area or
23
CA 02343554 2001-04-05
volume on said distribution side of said material said medical device is still
present
within said area or volume, and
e) observing differences in rates of penetration of said chemical material
through said structural material at different areas of said structural
material which are
indicative of different properties in said structural material at said
different areas
which are evidence of a clinical condition in said structural material. The
changes in
property of said visible image of an area or volume may, for example, comprise
tissue
of a living patient while said material delivery device is still present
within said area
or volume are caused by at least one change selected from the group consisting
of
a) a change in the apparent diffusion coefficient of tissue water protons;
b) a change in tissue magnetic susceptibility;
c) a change in T, tissue relativity;
d) a change in T2 tissue relativity;
e) a change in tissue magnetization transfer coefficients;
f) a change in tissue chemical shift frequency; and
g) a change in tissue temperature.
24
= ._._. _. _~._-_~.~.~~...~__ __T_ :~_:_._ _~:-_ --: _:..._ ~ ;;~ ~~ v _:~..
,..~ _., _~~..~.-..;:~. ,
.~..~~- _~ _- ___ .~ __ _ _ -~" ._,... .~. ~-~. -; _ _: _,.,~.. .~.z = . . .
r
_ ~ ~- --- --- -- _- __ ~__.~=y __: __~:~:-- _.. ~__. -.__ -:-:- ___
°_:.~,._-- -:.- ~:_~-_ _:_~.~ _--= ~:~ s,...d _ __.- ~. _ :.:~ __:~' "
_ .._ _.,:: ~:;---_~..-~_,.
- - :~,--_