Note: Descriptions are shown in the official language in which they were submitted.
CA 02344293 2001-04-18
Title: Gas Phase .~lkylation Wethod and Catalyst
Inventors: James R. Butler and ~lshim Kumar Ghosh
l Backsround
2 This invention relates to an aromatic alkylation process and catalyst
involving
w
3 vapor phase alkvlation of an aromatic subsuate over an improved silicalite
aromatic
a alkylation catalyst. The improved catalyst and method provide alkylation
products with
decreased impurities and undesirable side reaction products.
6 Aromatic conversion processes which are carried out over molecular sieve
7 catalysts are well known in the chemical processing industry. Such aromatic
conversion
8 reactions include the alkylation of aromatic substrates such as benzene to
produce alkyl
9 aromatics such as ethvlbenzene, ethvltoluene, cumene or higher aromatics and
the
to transalkylation of polyalkvl benzenes to monoalkyl benzenes. Typically, an
alkylation
11 reactor which produces a mixture of mono- and poly- alkyl benzenes may be
coupled
12 through various separation sra°es to a downstream transalkylation
reactor. Such
13 alkylation and transalkvlation conversion processes can be carried out in
the liquid phase,
t~ in the vapor phase or under conditions in which both liquid and vapor
phases are present.
In efforts to improve commercial alkylation operations, emphasis is placed not
16 only on the conversion efFiciency of the catalyst but also on the
byproducts that are
17 generated. For example, in the manufacture of ethylbenzene, ethylene and
benzene are
18 introduced into an ally lation reactor in the presence of various
catalysts. Some of the
19 measured byproducts include diethylbenzene, xylene, propylbenzene, cumene,
butvlbenzene and other components referred to collectively as heavies. These
byproducts
1 have a negative effect on the purification of the desired product.
Additionally. even when
February 18, 1999 1 spec.doc
CA 02344293 2001-04-18
t separated. these byproducts have to be removed from the system. Proper
disposal adds to
Z the cost of the intended product.
3 An example of vapor phase alkylation is found in U.S. Patent No. 4,107,224
to
4 Dwyer. Here, vapor phase ethvlation of benzene over a zeolite catalyst is
accomplished
in a down flow reactor having four series-connected catalyst beds. The output
from the
6 reactor is passed to a separation system in which ethylbenzene product is
recovered. with
7 the recycle of polvethylbenzenes to the alkylation reactor where they
underjo
8 transalkylation reactions with benzene. The Dwyer catalysts include ZSM-~,
ZSVI-11,
9 ZSVI-12. ZSM-3~. ZSVI-38. and similar materials.
to The molecular sieve silicalite is a well-known alkvlation catalyst. For
example,
t t U.S. Patent Vo. ~..~20.?'_'0 to ~,Vatson et al. discloses the use of
silicalite catalysts having
t2 an average crystal size of less than 8 microns and a silicaialumina ratio
of at least about
13 200 in the ethy-lation of an aromatic substrate such as benzene or toluene
to produce
t~ ethylbenzene or ethvltoluene, respectively. As disclosed in Watson et al.,
the alkylation
is procedure can be carried out in a mufti-bed alkylation reactor at
temperatures ranging
t6 from about 3~0° - 47~'C, with or without a steam co-feed. The
reactor conditions in
17 Watson et al. are such as to provide generally for vapor phase alkylation
conditions.
18 Another procedure employing silicalite and involving the ethylation of
benzene
19 under vapor phase reaction conditions coupled with the recycle of
polyethylbenzene
3o containing products back to the alkylation reactor is disclosed in U.S.
Patent ~lo.
21 4,92?.0~3 to V'asuespack. Here, alkylation is carried out at temperatures
generally in the
33 range of 370°C. to about -~-0°C. and pr;.ssures ranking from
atmospheric up to about 2~
23 atmospheres over a catalyst such as silicalite or ZS1~I-~. The catalysts
are described as
February t 8, 1999 2 spec.doc
CA 02344293 2001-04-18
being moisture sensitive and care is taken to prevent the presence of moisture
in the
2 reaction zone. The alk~~lation/transalkvlation reactor comprises four series-
connected
3 catalyst beds. Benzene and ethylene are introduced into the top of the
reactor to the first
~t catalyst bed coupled by recycle of a polyethylbenzene fraction to the top
of the first
catalyst bed as well as the interstate injection of polyethylbenzene and
benzene at
6 different points in the reactor.
Another process involving the use of a silicalite as an alkylation catalyst
involves
8 the alkylation of an alkylbenzene substrate in order to produce
dialkylbenzene of a
9 suppressed ortho isomer content. Thus, as disclosed in U.S. Patent No.
=1,489,21=~ to
1o Butler et al., silicalite is employed as a catalyst in the alkylation of a
monoalkylated
I I substrate, toluene or ethvlbenzene, in order to produce the corresponding
dialkylbenzene,
13 such as ethyl toluene or dieth;~lb~nzene. Specifically disclosed in Butler
et al. is the
13 ethylation of toluene to produce ethyltoluene under vapor phase conditions
at
la temperatures ranging from 3~0°- X00°C. As disclosed in
Butler, the presence of ortho
ethyltoluene in the reaction product is substantially less than the
thermodynamic
16 equilibrium amount at the vapor phase reaction conditions employed.
17 U.S. Patent No. ~,18~,040 to Ward et a1. discloses an alkylation process
18 employing a molecular sieve catalyst of low- sodium content which is said
to be especially
19 useful in the production of eth;-lbenzene from benzene and ethylene and
cumene from
3o benzene and propylene. The ~a~0 content of the zeolite should be less than
0.~ wt.%.
21 Examples of suitable z~olites include molecular sieves of the X, Y, L, B,
ZSVI-~, and
?? omega crystal types, wth steam stabilized hydrogen Y' zeolite being
preferred.
33 Sgeciricall;~ disclosed is a steam stabilized ammonium Y zeolite containing
about 0.2%
February 18, 1999 3 spee.doc
CA 02344293 2001-04-18
l Na~O. Various catalyst shapes are disclosed in the Ward et al. patent. While
cylindrical
2 extrudates maybe employed. a particularly preferred catalyst shape is a so-
called
3 "trilobal" shape which is configured as something in the nature of a three
Leaf clover.
~t The surface are3,'volume ratio of the extrudate should be within the range
of 8~-160 in.'s.
'The alkvlation process may be carried out with either upward or downward
flow, the
6 latter being preferred, and preferably under temperature and pressure
conditions so that at
7 least some liquid phase is present, at least until substantially all of the
olefin alkylating
8 went is consumed. Ward et al. states that rapid catalyst deactivation occurs
under most
9 alkylating conditions when no Liquid phase is present.
to U.S. Patent Vo. x.169.111 to Wight discloses an alkylation,'transalkylation
t t process for the manufacture of ethvlbenzene employing crystalline
aluminosilicates in the
t2 alkylation and transalkvlation reactors. The catalysts in the alkylation
and transalkylation
13 reactors may be the same or different and include low sodium zeolites
having
to silicalalumina mole ratios between ? and 80, preferably beriveen 4-12.
Exemplary
zeolites include molecular sieves of the X, Y, L, B. ZSVI-~, and omega crystal
types with
16 steam stabilized Y zeolite containing about 0.2% Na~O being preferred. The
alkytation
17 reactor is operated in a dow-nflow mode and under temperature and pressure
conditions in
is which some liquid phase is present.. The output from the alkylating reactor
is cooled in a
t9 heat exchanger and supplied to a benzene separation column from which
benzene is
2o recovered overhead and recycled to the alkvlation reactor. The initial
higher boiling
31 bottoms fraction from the benzene column comprising ethylbenzene and
2~ polvethlvbenzene is supplied to an initial ethvlbenzene column from which
the
23 ethylbenzene is reco~-ered ~ the process product. The bottoms product from
the
Februay 18, 1999 :l spec.doc
CA 02344293 2001-04-18
l ethylbenzene column is supplied to a third column which is operated to
provide a
3 substantially pure dizthybenzene overheads fraction which contains tiom 10
to 90%,
3 preferably 20 to 60° ° of diethvlbenzene. The diethylbenzene
overheads fraction is
recycled to the alk~~lation reactor while a side cut containing the remaining
diethylbenzene and triethvlbenzene and higher molecular weight compounds is
supplied
6 to the reactor along wah benzene. The effluent from the reactor is recycled
through the
7 heat e:cchanger to the benzene column.
8 U.S. Patent Vo. .~,77~,377 to Barger et al. discloses an
alkylation/transalkylation
9 process which involves the use of separate alkylation and transalkylation
reaction zones,
to with recycle of the tra~nsalkylated product to an intermediate separation
zone. In the
t I BarUer process. the te.:lperature and pressure conditions are adjusted so
that the
t? alkylation and tran.;aLs,; lation reactions take place in essentially the
liquid phase. The
l3 transalkylation catalyst is an aluminosilicate molecular sieve including X-
type, Y-type,
t:~ ultrastable-Y, L-t<pe. omega type and mordenite type zeolites with the
latter being
I5 preferred. The catalyst employed in the alkylation reaction zone is a solid
phosphoric
16 acid containing material. 3luminosilicate alkylation catalysts may also be
employed and
17 water varying from 0.01 to 6 volume percent is supplied to the alkylation
reaction zone.
18 The output from the alkylation reaction zone is supplied to first and
second separation
t9 zones. Water is recovered in the first separation zone. In the second
separation zone,
?o intermediate aromatic products and trialkvlaromatic and heavier products
are separated to
? 1 provide an input to the transalkylation reaction zone having only dialkyl
aromatic
?? components. or dieth~:lbe:L?ene in the case of an ethvlbenzene
manufacturing procedure
33 or diisopropylbenze.~.e in the case of cumzne production. :~ benzene
substrate is also
February 18, 1999 5 spec.doc
CA 02344293 2001-04-18
t supplied to the transalkvlation Zone for the transalkylation reaction and
the output from
the transalkylation zone is recycled to the Erst separation zone. The
alkylation and
3 transalkylation zones may be operated in downtlow, upElow, or horizontal
Ilow
~t configurations.
Accordingly. the art provides for various transalkylation processes to handle
some
6 of the alkylation bG~products such as diethylbenzene. It would be desirable
to have a
7 catalyst that would reduce the amount of byproducts that are not easily
handled in an
s alkylation/transallcylation process. It is desirable to provide a catalyst
that results in
9 reduced amounts of ~~~lene and propylbenzene in an ethylene/benzene
alkylation process.
to
l t Summary of the In~~ention:
t. In accordanc: W th the present invention, there is provided a process for
the
t3 vapor-phase alkvlation of an aromatic substrate. This is accomplished by
introducing a
t~ feedstock comprising an aromatic substrate in a gaseous phase and an
alkylation agent
15 into contact with a molecular sieve aromatic alkylation catalyst to produce
a reaction
16 product containing a tnonoalkylated aromatic product and reduced
byproducts. The
17 catalyst is characterized in having bimodal acidity with the weak acid
sites concentration
is of less than ~0 micromoles per gram of catalyst (~0 umoles/g).
19 The allvlation reaction may be carried out in a reaction zone having a
single or a
2o plurality of series-connected catalyst beds. The monoclinic silicalite
catalysts may also
21 be characterized b~- a crystal size of less than one micron, prefereably
about O.~ microns.
32 A feedstoch of an rematic substrate, such as benzene, and an alkvlating
agent. such as
33 ethylene. prop;.::r.~. or alpha-olefin, is introduced into the reaction
zone and the
February ( 8, l 999 6 spec.doc
CA 02344293 2001-04-18
t alkylation reaction zone is operated at temperature and pressure conditions
in which the
aromatic substrate is in a gaseous phase to cause gas-phase alkylation of the
aromatic
3 substrate in the presence of the monoclinic silicalite catalysts having
bimodal acidity to
produce an alley Iation product. The alkylation product is then withdrawn from
the
reaction zone. The fe:dstock used may have an aromatic substrate,~alkylatin~
agent
6 weight ratio of between about I O to 2~.
7 In the production of ethylbenzene or other alkylated aromatics, the
alkvlation
s product from the reaction zone may be supplied to an intermediate recovery
zone for the
9 separation and recovery of ethylbenzene from the alkylation product and for
the
t0 separation and recovery of polvalkylated aromatic components. At least a
portion of the
1 t polyalkylated aromatic component is supplied to a transalkylation reaction
zone of the
13 intermediate recover; zone. Benzene is supplied to the transalkylation
reaction zone and
l3 the transalkyiation reaction zone is operated under temperature and
pressure conditions to
t cause disproportionation of the polyalkyiated aromatic fraction to produce a
is disproportionation product having an enhanced ethylbenzene content and a
reduced
16 polyalky(ated aromatic components content. To effect the transalkylation
reaction, the
17 transalkylation zone may contain a zeolite Y transalkylation catalyst and
be operated
18 under temperature and pressure conditions effective to maintain the
feedstock in the
19 transalkylation zone in the liquid phase.
2 t Brief Description of the Draw nas:
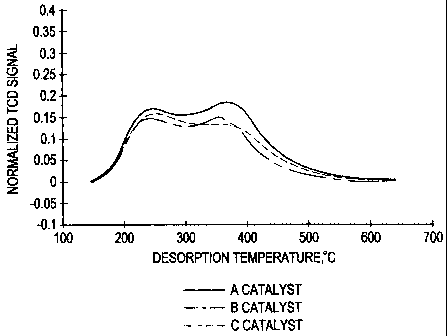
'-'? Fisure 1 shows the bimodal acidin- of the catalysts.
'-3 Figure 2 shows the deconvolution of the bimodal acidity curve for catalyst
C.
February 18, 1999 7 spec.doe
CA 02344293 2001-04-18
t Figure 3 is a Graph showing the xylenes formation in an zthylbenzene
alkylation
3 process in relation to the wzak acid sites concentration in the catalysts.
Figure .~ is a _raph showin' the propylbenzene formation in an ethylbenzene
-~ alkylation process in relation to the weak acid sites concentration in the
catalysts.
6 Detailed Description of the Invention:
7 The present invention relates to the vapor-phase alkylation of benzene over
8 compatible silicalite alkvlation catalvsts having a weak acid sites
concentration of less
9 than ~0 micromoles per dam of catalyst. The process results in improved
product
to quality and reaction erFciency. In the formation of ethylbenzene in vapor-
phase
1 l alkylation From a feedstock of ethylene and benzene, other impurities and
undesirable
12 side products may be formed in addition to the desired ethylbenzene. These
undesirable
13 products include such compounds as ~ylene, cumene, n-propylbenzene and
burylbenzene,
1~ as well as polvethvlbenzenes, and high boiling point alkyl aromatic
components,
sometimes referred to as "heavies," having a boilin' point at or above
13~°C. As can be
16 well understood. reduction of these impurities and side products is
important. This is
17 especially true in the case of xylenes. The orthoxylene (o-xylene) is a
styrene
18 contaminant that cannot be removed by distillation. The meta and para
~cylenes have
19 distillation points that are in close pro~cimity to that of ethylbenzene,
or styrene and can
2o make product separation and purification difficult. The presence of these
isomers, meta
t and para xylenes, require high reflex and a large number of distillation
stakes. The
33 propylbznzenes requi:e additional retlw or allowing more smrene to sta~~ in
the residue,
?3 causinV a decr:a:e in ~: isld of the desired product and an increase in
residue volume. It
February 18, 1999 8 spec.doc
CA 02344293 2001-04-18
1 should be pointed out that although the present invention has particular
application to the
formation of ethvlbenzene through the alkylation of benzene with ethylene, the
method
3 may also be used to produce other alkylated aromatics, such as the formation
of
a propylbenzene with the use of propylene as the alkylating agent. Other
olefins may also
be used as the alkvlatina went.
6 Zeolite catalysts are widely used for acid-catalyzed reactions such as
alkylation,
7 cracking, isomerization, disproportionation, and so on. The number of acid
sites, their
8 strengths and accessibility, to reactant molecules, play a significant role
in catalyst
9 activity. selectivit<- and catalyst deactivation. It is generally accepted
that only a fraction
to of the total number of sites is involved in a catalytic process. Often
unwanted reactions
11 occur due to the presence of acid sites of different strengths.
12 Three different catalyst samples were tested. The properties on these
catalysts are
13 outlined below in Table I.
14
Table I: Catalysts
16
C atal st ~ A B C
Forms CDS or Smooth Same Same
Ext.
Size 1/10" CDS Same Same
1/16" Smooth
Composition ~ Pentasil ZeolitePentasil Pentasil
Alumina Zeolite Zeolite
Alumina Alumina
LOI. 1000 F ~ < 6.0 w~t. < ?.0 wt. < 6.0 wt.
~ Bulk Density- ~CBD). CDS = 26.=1 CDS = 27
Lbs'fr' i CDS = ?7 . 3
'.-_ 3
i Smooth = 33 = 3
i
Surface Area. m-; 276 j 226
~ min. I 276
Pore Volume. cc:'s 0.69 0.48 (min.)
> ''9.? ~ j 0.60
(min.)
Crush Stren~h. Lbs~~mm 1.1 > 0.9
t > 0.9
t~
is
19 .-X11 ~=atalvst samples wsre sized to ?0--IO mesh. .~,pproximatelv 0.=16
grams of
'0 catalyst sample was taken in a samplz tube. The thermocouple was placed at
the mid-
February 13,1999 9 spec.doc
CA 02344293 2001-04-18
l height of the catalyst bed. The sample tube was placed with TPD/TPR
instrument and
was tightened. The TPD (temperature programmed desorption) instrument measures
the
3 temperature required to remove ammonia from a catalyst. The higher the
temperature,
a the stronger is the acid site. Leakage was checked by flowing an inert gas
through the
tube and by blocking the outlet of the vent line. If no leaks e:cisted,
blocking the end of
6 e~cit line would completely stop flow of the gas. The sample temperature was
raised to
7 6~0 °C at p degrees centigrade per minute (~'C/min) under an inert
gas flow of (~0
8 ml/min) at which temperature the catalyst was dried for .~ hours. The sample
was cooled
9 down to I00 °C. The catalyst sample was saturated with ammonia
(i~lH3) by flowing
to NH3 gas at I00 ~C. The phvsisorbed or weekly held VH3 was desorbed by
flowing an
1 t inert gas through the sample tube (catalyst bed) at 1 ~0 °C for 2
hr. NH3 was desorbed at
12 a temperature ramp ~ =Cmin to a maximum 6~0 °C. Flow rate of
sweeping gas was ~0
13 ml/min. The e~pe:imznts were repeated by changing the NH3 desorption ramp
rate from
1=~ ~ °Gmin to 10 'C. min. This allowed us to see the effect of ramp
rate on the peak shape
and position.
16 Pore geometry of a catalyst, particularly zeolite catalyst, is known to
control
17 ''traff c" of reactant and product molecules and thereby controls the
characteristics of the
18 products formed. The subject catalysts are silicalite based catalysts.
Silicalite has an
i9 isotypic framework st<ucture of ZSVI-~ zeolite with five membered rims of
Si-O or Al-O
tetrahedra in the tetn,.hedral framework. The framework outlines a three
dimensional
21 system of intersecting channels defined by I O-rims of oty~en in all three
directions with
2' width about 6 _~ in diarr!~te:. Table ? describes the catalyst samples.
These catalysts
2~ were prepared :gm siiicalite powder and their pore size distribution was
the same.
Fzbruarv l8. 1999 10 spec.doc
CA 02344293 2001-04-18
t
'_ Table ~. Catalyst description and Va content.
3
CatalystVa. ppm Si02/A1203
_Ratio
~
~ <IOO ??J
B j 1 ~0 320
C I~o ~2a
The acidiy fvr each of the alkylation catalyst samples was determined by NH3-
6 TPD after drying at 6~0 °C. Acid sites were saturated with VH3 by
flowing NH3 at 100
7 °C. Physisorbed and weakly chemisorbed NH3 were then desorbed at I~0
°C for 2 hr.
3 Desorption at 1~0'C was completed in the 2 hr as indicated by TCD signal.
NH3 was
9 then desorbed by tlo«ing an inert 'as through the catalyst bed at a ramp of
~ °C/min or
to 10 °C.!min to a ma.~imum 6~0'C. Unless stated otherwise acidity
results obtained at
11 desorption ramp rate ~ 'C: min were taken to make comparison or to e~camine
correlation
1' between the acidit<- and catalyst activity and selectivity.
13 TCD (thermal conductiviy detector) signals (milliamp) were converted to
mmole
1a ofNH3 per Gram of catalyst sample as an indication of the acid sites
concentration. The
results are showy in Table 3. Figure 1 shows NH3-TPD profile of the catalyst
samples.
16 All three catalyst samples showed t~vo iVH3-desorption peaks, one with peak
maximum
l at around 240 °C and the other Leith peak maximum at around 3~0
°C. These values are
1s for the ramp rate of ~ =C,'min. A slight variation in the peak temperature
may be
t9 attributed to a variation of the location of catalyst bed in the sample
tube. The peak
3o temperatures shown in Table 3 for the three samples were obtained by using
sample
2t weights of 0.-1~6-0.00 Q. T'ne numeer of sites reported in Table 3 were
averaged from
ti~ree runs and the=. ~wr: reproducible within 3° o error. The acid
sites showing? peak at
<300 ~C and at > :00 =C are c!assitied as weak and strong acid sites. This
classification is
February 1 s, 1999 1 l spec.doc
CA 02344293 2001-04-18
t an arbitrary and relative classification and is utilized to distinguish the
bimodal peaks for
these catalysts. The terms should not be used to compare these catalysts with
different
3 types of catalysts of compounds. NH3-desorption peaks were separated by
using the
~t Peak-Fit deconvolution technique. The number of weak and strong acid sites
was
calculated from the integrated areas of peaks after peak deconvolution. As an
example,
5 the deconvoluted peaks of VH3-TPD of catalyst sample C is shown in Figure 2.
Thus the
7 term weak acid sites is used to define the acidity attributable to the first
peak of a bimodal
s acidity curve. This is the peak at the lower temperature values in a bimodal
acidity curve
9 as shown in figures 1 and 2.
t o An increased desorption ramp rate ( 10 'C/min) shifted peak maxima to
higher
1 t temperatures. The number of total acid sites was found to be in an
excellent agreement
t2 (~0.001 mmole; ~).
13 Catalysts B and C were shown to possess decreased amounts of total acid
sites
i~t compared to that of catalyst A. The smaller amounts of acid sites of
catalysts B and C
are attributed to lower aluminum contents (or higher SiO~/A1~0; ratio) of the
silicalite
t6 powders used for making these catalysts. In addition, catalysts B and C
powder samples
17 have sli?htly higher sodium (~1a) contents than catalyst A. Sodium ions are
known to
18 reduce zeolite acidity. It is important to note that the three catalyst
samples show a
19 smaller variation in weak acid sites compared to strong acid sites. The
results suggest the
?o decrease of framework aluminum attributes to preferential reduction of
strong acid sites
3 t with less of an effect on the weak acid sites.
February 15,1999 1? spec.doc
CA 02344293 2001-04-18
! Table 3. Acidity of catalysts determined by NH3-TPD.
Catalyst~ _ Acid
Sites,
mmol~~a
_
weak (Tmax.Strong (Tmax,Total NH3-Des Ramp
C) C) Rate
A 0.00 (?37) o.ias (3~6) 0.193 ~ C/min
0.0-1: (?~2)0.1:19 (377)0.19 10 C/min
B i 0.06 (?>j) o.ios(;~16) 0.1>j ~ C/min
I
~ 0.010 (2~1)0.113 (363) 0.13 10 C/min
C 0.0~~ (?~1)o.os(3~1) 0.18 ~ C/min
I
0.033 ('?-1~)0.12:1 (3:17)0.136 10 C/min
3
Table 4 summarizes some of the byproducts formation for each of the tested
catalysts for benzene alkvlation with ethylene at gas phase conditions (single
pass, Tinlet
6 = :100 °C. LHSV 70 hr-1 ). Product selectivities to total ~cylenes,
propylbenzenes.
7 butylbenzenes and hea~~ies are given in Table -1. Figures 3 and -1 show
plots of catalyst
8 selectivities to xylenes. and propylbenzenes as a function of catalyst weak
acid sites.
9 Linear lines were drawn and R' values are shown in Table 3.
to As indicated earlier, the three catalyst samples showed a smaller
difference in
11 number of weak acid sites. However. a small increase of the weak acid sites
was found
12 to increase the formation of ~cylenes and propylbenzenes.
13
Table ~. Catalyst deactivation and by-product selectivity of benzene
alkylation.a
Catal PPyf
st Selectivity.
Relative
to EB
Xvlenes Pro vIBZ ButvlBZ
A ~ 8~ 34~ 3
B ~6 ?37 3?2
C .18 X07 ?66
16
l7
February 18, 1999 13 spec.doc
CA 02344293 2001-04-18
Table ~. R''-Values
R'
Weak Acid Strong Acid Correlation e:cists with
Sites Sites
Xvlene I 0.9997 ~ 0.77?6 ~ Weak acid sites
Pro vIBZ I 0.9997 0.771 ~ ( Weak acid sites
ButvlBZ I 0.607 0.1601 I No 'ood correlation
3
In one embodiment of the present invention, a mufti-stage alkylation reactor
6 having a pluralit'- of alkvlation catalyst beds in series is employed. One
or more of the
catalyst beds contain the silicalite alkylation catalyst of the present
invention having a
8 weak acid site conc~._~.tration of less than fifty micromoles per gram (<~0
umoles/~).
9 Preferably. the catalyst of the present invention has a weak acid site
concentration of less
to than =18 umoles. a. « wile the catalyst of the present invention can be
utilized alone, it is
11 also contemplated that one or more of the catalyst beds contain other
alkylation catalysts.
12 The combination of catalysts is utilized to optimize the catalyst life and
yield.
t3 Silicalite, as is well known in the art. is a molecular sieve catalyst
which is similar
t~ to the ZSVi-~ zeolites but is n-pically characterized by a higher
silicaialumina ratio
15 providing an aluminum%unit cell ratio of less than 1, and in addition, is
normally
16 characterized as having a somewhat larder than average crystal size than is
commonly
17 associated ~-ith the ZS~I zeotites. As is well known in the art,
silicalite, which in the as-
18 synthesized form is characterized by orthorhombic symmetry. can be convened
to
19 monoclinic svmmet~: b~~ a c~lcination procedure as disclosed. for example,
in U.S. Patent
Zo No. -1.99.-ti; to DzBras et al. As described in detail in DeBras et al..
"Phvsico-chemical
I characterization of pe~tasil t~~pe materials, I. Precursors arid calcined
z;.olites, and II.
2? Thermal analwis of :e precursors," Zeulit~s. 193. ~'ol. ~, pp. X69-~8~, the
silicalite
February 18. 1999 l-1 spzc.doc
CA 02344293 2001-04-18
t typically has a relatively lame crystal size. Thus, at an average of less
than one
2 aluminum atom per unit cell (a silica/alumina ratio of about 200) silicalite
typically has
3 an average crystal size of perhaps ~-10 microns or more. The aforementioned
Patent No.
a 4,:89,? 1:~ to Butler et al. discloses e~cperimental work involving the
ethyiation of toluene
~ over silicalite of a crystal size greater than one micron, ranging from 1-?
microns up to 8
6 microns. Thz silicalize is further characterized in terms of a variable
aluminum gradient
7 such that the aluminum Gradient is positive when going from the interior to
the surface of
8 the molecular sieve crystal. That is, the silicalite can be characterized by
a core portion
9 which is relatively aluminum deficient with an outer shell portion which is
relatively
t0 aluminum rich. It is to be understood that the term "aluminum rich" is a
relative term and
1 t that for silicalite even the out;,r shell portion of the crystallite has a
Iow aluminum
12 content.
t3 In another embodiment, vapor-phase alkylation using silicalite catalyst as
to described herein is followed by a liquid phase transalkylation procedure in
which the
15 alkylation and transalkylation reactors are integrated with an intermediate
recovery zone,
16 preferably involving a plurality of separation zones operated in a manner
to effectively
17 provide feed streams to the reactors with recycle of the output from the
transalkylation
is reactor to a benzene recover- zone downstream of the alkylation reactor. In
this
19 integrated mode of operation, the transalkylation product is applied to an
initial stage of a
?0 benzene recovetw zone. Subsequent separation steps are carried out in a
manner to apply
't a split feed to the transalk~-lation reactor. The preferred catal~~st uszd
in the
?'_' transalkUation reactor is a molecular sieve having a pore size greater
than the pore size
33 of the silicalite caal~~st. Prefzrablv, the transalkylation catalyst is
zeolite Y. The
February 13. 1999 l ~ spec.doc
CA 02344293 2001-04-18
l alkylation reactor is preferably operated at substantially higher
temperature conditions
2 than the transalkvlation reactor. In one embodiment of the invention, the
recycled output
3 from the transalkvlation reactor is passed in a heat e:cchan~e relationship
with the
.t alkylation reactor product feed to the initial separation zone.
Preferably:. the alkvlation reactor comprises at least four catalyst beds as
described
6 above. More beds can be provided, and it will sometimes be advantageous to
provide at
7 least five or six catalyst beds in the alkylation reactor. The reactor is
operated so as to
8 provide vapor phase alkylation (both the aromatic substrate and the
alkylatin' agent are
9 in the vapor phase ) at temperatures ranain~ from about 630°F-
800°F at the inlet to about
l0 700°F-8~0°F at he outlet. The pressure may be within the
range of about ?~0 to -1~0 psia
11 with the pressure decrea.sina from one bed to the next as the temperature
increases. By
12 w-ay of example. the benzene and ethylene supplied to the top of the
reactor may enter the
13 reactor at a temperatt:re of about 7=10°F and a pressure of about
:130 psia. The alkylation
14 reaction is exotlhermic so that the temperature progressively increases
from the first to the
1~ last catalyst bed by way of example. The interstage temperatures may
increase from
16 7~0°F for the first catalyst bed to 76~°F after the second
catalyst bed to 820°F after the
17 third catalyst bed to a temperature of about 8=10°F after the last
catalyst bed.
18 ~iormallv in the operation of mufti-stage reaction zone, a benzene-ethylene
19 mixture is introduce' ;o the first catalyst bzd at the initial stage of the
reaction zone and
?o also in benve:n the _~~wal successive stages of catalyst beds. In the
examples presented.
?1 ethylene is supplied slung with benzene to the first catalyst bed locatzd
at the top or
?2 uppzr end of th: reac_.~:. In vddition, int;.rstage injection of ethylene
and benzene is
February 18, 1999 16 spec.doc
CA 02344293 2001-04-18
l provided for between the subsequent catalyst beds. The feedstock benzene-to-
ethylene
2 weight ratio injected into the top of the alkylation reactor may be between
about 18 to 22.
3 The silicalite alkvlation catalysts, as referred to herein, are molecular
sieves from
the pentasil family of high silica molecular sieves. Such pentasil molecular
sieves are
s described, for example, in Kokotailo et al., wPentasil Family of High Silica
Crystalline
6 Materials," Chem. Soc. Special Publ. 33, 133-139 (1980). The silicalite
molecular sieve
7 alkylation catalysts have a somewhat smaller pore size than the preferred
zeolite-Y
8 employed in the transalkylation reactor. The silicalite crystals have an
effective pore size
9 or window within the range of ~-6 angstroms. Zeolite Y has a pore size of
about 7
t0 angstroms.
11 Preferred silicalites for the catalysts used in the present invention are
ertruded
13 with an alumina binder in a "trilobe" shape having a nominal diameter of
about 1/16" and
13 a length of the extrudate of about I/6-1/4.'' The "trilobe" cross sectional
shape is
to something on the order of a three leaf clover. The purpose of this shape is
to increase the
15 surface area of the extruded catalyst beyond what one would expect with a
notTnal
16 cylindrical e~ctrudate. The silicalite catalysts used are characterized as
monoclinic
17 silicalite. Monoclinic silicalite may be prepared as disclosed in U.S.
Patent Nos.
18 x,781,906 to Cahen et al. and 4,772,-1~6 to DeClippeleir et al. The
silicalite catalyst
19 typically contains small amounts of sodium and iron.
?o As noted previously. the silicalite alkylation catalysts ha~~e a crystal
structure
21 characterized by 3n aluminum rich outer shell and an aluminum deficient
interior portion
?? when compared with the outer shell. The silicalite catalysts are dw and
have no
February 18,1999 I? spec.doc
CA 02344293 2001-04-18
I appreciable or intended water content. Speciticallv, the silicalite
catalysts preferably
contain no more than about '00 ppm sodium. preferably no more than about 100
ppm
sodium, and no more thin about X00 ppm iron, preferably no more than about 300
ppm
~+ iron. The alumina binder is a high purity alumina such as "catapal
alumina." Preferably,
the alumina binder is ~;.aracterized in terms of an unusually high pore size
and unusually
6 low sodium content. .~s noted previously. the silicalite itself has a low
sodium content in
7 its crystalline structure. 8v maintaining a low sodium content in the
alumina, a high
3 portion of the catal~~st sites in the silicalite structure are maintained in
the active hydrogen
9 form-that is. the low ;odium content of the binder tends to minimize
neutralization of
(o the cr,~stalline catalwst situ due to ion exchange between sodium in the
binder and the
1 l acid sites in the ;,ataiwt. Thz alumina binder is further charactzrized in
terms of a
12 relatively large average pore size after the catalyst is extruded and
divided into particles.
t3 Specifically, the average pore size of the binder, which can be termed the
"maximum''
l~s pore size to avoid confusion with the pore size of the silicalite itself,
is about 1,000
angstroms or more. preferably within the range of 1000 to X000 angstroms. A
preferred
16 pore size range is within the range of about 1,000 to about 1,800
angstroms. This
17 relatively lame pore size binder can enhance the efficiency of the catalyst
by avoiding, or
18 at least minimizing, an aIumina-diffusing mechanism as applied to the
catalyst particles
19 themselves, thus enhancing access to the silicalite molecular sieve within
the catalyst
particles. The pore siz: of the molecular siese structure itself normally can
be e~cpected
21 to be on the order of about ~-6 angstroms. The silicalitz catal~~sts
preferably contain only
3? a small amount of ~oc:~:::. shout 70-?00 ppm sodium, and contain only a
small amount
February 18, 1999 13 sp~c.doc
CA 02344293 2001-04-18
t of iron, about 200-X00 ppm. The catalyst need not contain any additional
''promoter"
2 metals incorporated during the synthesis of the catalyst.
Having described specific embodiments of the present invention, it will be
-~ understood that modifications thereof may be su~aested to those skilled in
the art, and it
is intended to cover all such modifications as fall within the scope of the
appended
6 claims.
February l8. 1999 19 spec.doc