Note: Descriptions are shown in the official language in which they were submitted.
Ot- 3-14;13;03 ;t"v:~*T~~ CA023445322001-03-15 ;03-3609-9000 H 7i 41
SPECIFICATIONS
PTC ELEMENT AND METHOD OF MANUT~'AC'1'UltINU THE SAME
[Technical Field]
6 The present invention relates to a PTC element
composed of a conductive composition material (hereinafter
referred to PTC composition material) showing positive
temperature properties, so-called PTC (Positive Temperature
Coefficient) properties that mean its resistance value
suddenly rises when a fixed temperature (hereinafter referred
to as a switching temperature) region is reached.
[Technical Background]
Up to now, being employed in electrical equipment and
electronic equipment as well as secondary batteries, PTC
elements are used as an protective element of electrical
circuits to prevent over-current that flows when an abnormal
state is happen in these apparatus.
In general, a PTC element is composed of a PTC
composition material obtained by blending and kneading
conductive powder with a crystalline polymer and of electrodes
formed on said PTC composition material, and shows sudden
increase in its resistance value when the switching
temperature is reached. That is, a PTC composition material
:~5 produces heat in accordance with joule heat (I'R heat)
generated by resistance value R peculiar to the material and
electric current value I flowed in the element through the
above-mentioned electrodes. Like this, if relatively high
1
01- 3-14:13.63 ::'~*$3~ CA 02344532 2001-03-15 ;03-3609-9000 # Bi 4t
current flows in the PTC composition material, heat
generation occurs to make its resistance value increase. In
general, PTC elements are used as a sheet heat generation
material using the generation of above-mentioned joule heat,
an over-current protective element using increase in
resistance value, and the like.
As former PTC elements, especially from the viewpoint
of the method of forming their electrodes, for example, the
following 3 elements are known.
First, an element in which the surface of a metal plate of
stainless steel, or nickel and the like is agglutinated on the
surface of a PTC composition material and the metal plate is
used as electrodes.
Second, an element in which in order to improve the
16 adhesion of such electrodes and a PTC composition material,
the surface of a metal plate is further roughened physically or
chemically and the roughened surface is agglutinated on the
surface of a PTC composition material to make electrodes.
Third, an element in which a PTC composition material
is directly metal plated to make electrodes.
However, in the first former example in which electrodes
are made by agglutinated the surface of a metal plate on the
surface of a PTC composition material, the contact resistance
value between the PTC composition material and the electrode
becomes high and good ohmic contact cannot be obtained. As
a result, in the PTC composition material relating to this first
former example, because resistance value becomes high at
room temperature, it is difficult to use the PTC composition
2
01- 3-14:13::,3 :~t,'°~'"sr~*~~ ~ 02344532 2001-03-15 ;03-3609-9000 al
9/ 41
material as an over-current protective element and the like.
In addition, because the adhesion between the PTC
composition material and the electrodes is insufficient,
resistance value increases greatly in cases where the PTC
6 element is repeatedly operated (turning on electricity).
Moreover, in the second former example in which the
surface of a metal plate roughened physically or chemically is
agglutinated on the surface of a PTC composition material to
make electrodes, the contact resistance value between the PTC
1o composition material and the electrode becomes lower than
that in the above-mentioned first former example and the
adhesion between the two also becomes better, but it is not yet
reached to obtain good ohmic contact. In association with
the second former example, it is also proposed to increase the
15 amount of conductive powder to be dispersed in a PTC
composition material up to 45 volume percent or more in order
to improve the stability of the PTC element against its
repeated operation as well as lowering its resistance value at
room temperature. However, also in this case, it is difficult
20 not only to lower the resistance value at room temperature to a
certain value or less but also to retard completely the increase
in the resistance value for every operation when operations
are repeated.
Furthermore, in the third former example in which a
26 PTC composition material is directly metal plated to make
electrodes, the adhesion between the PTC composition
material and the plated coat is not sufficient and the contact
resistance value between the two becomes high. And great
3
01- 3-14; 13: G3 ;~~JyS:~*$f~ CA 02344532 2001-03-15 ; 03-3609-9000 ~f 1 Oi 41
increase in resistance value due to repeated operation is also
inevitable.
As a controversial point common in the first to third
former examples, there is a problem that resistance values at
b room temperature increase due to the deterioration of PTC
composition materials themselves in cases where those PTC
elements are repeatedly operated. As a cause for this
problem, it is presumed that crystalline polymer components
deteriorate due to heat shock for every operation when
operations are repeated_
On the other hand, since a PTC composition material is a
complex material of an organic matter and an inorganic
substance, there is also such a problem that the composition
material is greatly affected by environmental humidity during
16 being stocked and used and the change of its resistance
becomes to be great as the passage of time when switching
operation is repeatedly carried out. In particular, in order to
obtain an element having good conductivity in a PTC
composition material using metallic conductive filler, it fs
needed to fill up the conductive fillex in large amount. But
when a conductive filler of inorganic substance is filled in
large amount, the resulting composition material is easily
affected by environmental humidity during being stocked and
used. And, as mentioned above, since the element itself
becomes to be apt to peel off from the electrodes as the passage
of time when switching operation is repeatedly carried out, the
composition material could not obtain sufficient reliability for
the long time usage (repeated usage).
4
01- 3-14:13:63 ::#*3~~ CA 02344532 2001-03-15 :03-3609-9000 ~I 11/ 41
It is therefore the first object of the present invention to
provide a PTC element that is excellent in stability to
repeated operation and has sufficient adhesion with the PTC
composition material and electrodes with low contact
resistance value, and to provide its production method.
It is the second object of the present invention, in a PTC
element and its production method to achieve the above-
mentioned first object, to provide a PTC element that can
effectively prevent the PTC element itself from peeling off
from the electrodes due to the effect of environmental
humidity and is good in stability and reproducibility to
repeated usage and has a high reliability.
[Disclosure of the Invention]
In order to achieve the above-mentioned first object, in
the PTC element and its production method of the present
invention, conductive powder filler composed of at least one
out of TiC, WC, WzC, ZrC, VC, NbC, TaC, and Mo$C is blended
and kneaded with a crystalline polymer component by 36 to 60
volume percent to form a formed composition material, a
conductive material is pressure sealed and buried so that the
material is partly exposed from the surface of said formed
composition material, and then electrodes are foamed by
plating on said surface of said formed composition material.
That is, according to an aspect of the present invention,
a PTC element can be obtained, and the PTC element is
characterized in that it has a formed composition material in
which conductive powder filler is blended and kneaded with a
5
01- 3-14:13:03 ;,~~*v:~*~~ CA 02344532 2001-03-15 ;03-3009-9000 N 12i 41
crystalline polymer component by 35 to 60 volume percent, a
conductive material pressure sealed and buried so that the
material is partly exposed from the surface of said formed
composition material, and electrodes formed by plating on said
surface of said formed composition material, and in that at
least one out of TiC, WC, WaC, ZrC, VC, NbC, TaC, and MoZC is
used as said conductive powder filler.
Said conductive material is preferable to include Ni
powder, Al powder, Cu powder, Fe powder, Ag powder, or
graphite powder.
And, it is preferable to form a formed composition
material by blending and kneading said conductive powder
filler with a crystalline polymer component by 45 to 60 volume
percent.
1.5 Further, according to another aspect of the present
invention, it is possible to obtain a production method of a
PTC element that is characterized in that the method has a
process in which a formed composition material is obtained by
means of blending and kneading at least one out of TiC, WC,
WaC, ZxC, VC, NbC, TaC, and Mo2C with a crystalline polymer
component by 36 to 60 volume percent as a conductive powder
filler, a process in which after coated said formed composition
material with a conductive paste containing a conductive
powder, said conductive powder is treated by pressure sealing
2G to bury said conductive powder so that part of said conductive
powder is exposed from the surface of said formed composition
material, and a process in which said surface of said formed
composition material is plated to form electrodes.
6
01- 3-14713:03 t~'(,~.~*~~ CA 02344532 2001-03-15 703-3609-9000 M 13/ 41
It is preferable that Ni powder, A1 powder, Cu powder, Fe
powder, Ag powder, or graphite powder is used as said
conductive powder.
And, it is preferable that said conductive powder filler is
6 blended and kneaded with a crystalline polymer component by
45 to 60 volume percent to form a formed composition
m aterial.
In order to achieve the above-mentioned second object,
in the PTC element and its production method of the present
1U invention, conductive powder filler composed of at least one
out of TiC, WC, W2C, ZrC, VC, NbC, TaC, and Mo~C is blended
and kneaded with a crystalline polymer component by 35 to 60
volume percent to form a formed composition material, a
conductive material is pressure sealed and buried so that the
7.5 material is partly exposed from the surface of said formed
composition material, then electrodes are formed by plating on
said surface of said formed composition material, moreover, a
steam barrier layer i~s formed in the part other than said
plated electrode of the PCT element.
2U That is, according to further another aspect of the
present invention, it is possible to obtain a PTC element that
is characterized in that the PTC element has a formed
composition material in which a conductive powder filler using
at least one out of TiC, WC, WQC, ZrC, VC, NbC, TaC, and MoaC
25 is blended and kneaded with a crystalline polymer component
by 35 to 60 volume percent, a conductive material which is
pressure sealed and buried so that it is partly exposed from
the surface of said formed composition material, electrodes
7
01- 3-14;13:63 :~'"st~*~~ CA 02344532 2001-03-15 ;03-3x09-9066 ~1 14/ 41
formed by plating on said surface of said formed composition
material, and a steam barrier layer formed in the part other
than said plated electrode_
It is preferable that said crystalline polymer component
is a polymer alloy in which at least one kind of thermoplastic
polymer is mixed.
Moreover, according to a further different aspect of the
present invention, it is possible to obtain a production method
of a PTC element that is characterized in that the method has
i0 a process in which a formed composition material is obtained
by means of blending and kneading at least one out of TiC, WC,
WaC, ZrC, VC, NbC, TaC, and Mo2C with a crystalline polymer
component by 35 to 60 volume percent as a conductive powder
filler, a process in which after coated said formed composition
material with a conductive paste containing a conductive
powder, said conductive powder is treated by pressure sealing
to bury said conductive powder so that part of said conductive
powder is exposed from the surface of said formed composition
material, a process in which said surface of said formed
composition material is plated to form electrodes, and a
process in which a steam barrier treatment is carried out in
the part other than said plated electrodes.
(Brief Description of the Drawings]
Fig_ 1 is a sectional view showing the PTC element
related to the first embodiment of the present invention;
Fig. 2 indicates drawings to explain the production
method of the PTC element related to the first embodiment of
8
07- 3-14: 13: vS ;,~Jhl~R3 ~O'~*~f5~ CA 02344532 2001-03-15 ; 03-3609-9060 ll
t Gi 41
the present invention, they are sectional views showing (a) the
formed composition material, (b) a state where a conductive
material is pressure sealed and buried so that it is partly
exposed from the surface of the formed composition material,
b and (c) a state where said formed composition material is
plated to form electrodes, respectively;
Fig. 3 is a graphical representation showing the
temperature - resistivity property i.n a PCT element in an
example of the present invention;
l0 Fig. 4 is a graphical representation showing resistivity
properties after current of l0A (60V) is repeatedly impressed
on the PTC element of the first example of the present
invention and on the PTC elements of comparative examples;
Fig. 5 is a partial sectional view showing the PTC
16 element related to the second embodiment of the present
invention; and
Fig. G indicates drawings to explain the production
method of the PTC element related to the second embodiment
of the present invention, (a) is a sectional view of a formed
20 composition material, (b) is a sectional view showing a state
where the conductive material is pressure sealed and buried so
that it is partly exposed from the surface of the formed
composition material, (c) is a sectional view showing a state
where the composition material is plated to form electrodes,
25 (d) is a schematic view showing steam barrier treatment, (e) is
a partial sectional view showing the PTC element after steam
barrier treatment is performed, respectively.
9
Ot- 3-t4:t3:G3 :~,'~*~~ CA023445322001-03-15 :03-3009-9000 ~I tOi 41
[Prefexred Embodiment for carrying out the Present Invention]
The present invention will be explained in more detail
according to attached drawings.
At first, referring to Figs from 1 to 4, the PTC element
G and its production method related to the first embodiment of
the present invention is explained.
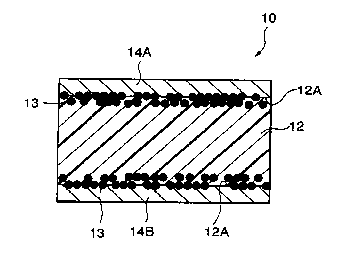
As shown in Fig. 1, PTC element 10 related to the first
embodiment or the present invention has formed composition
material 12 in which conductive powder filler (not shown in
the figure) was blended and kneaded with a crystalline
polymer component by 35 to 60 volume percent, conductive
material 13 pressure sealed and buried so that it is partly
exposed from surface 12A of formed composition material I2,
and electrodes 14A and 14B formed by means of plate
16 treatment on surface 12A of formed composition material 12.
As conductive powder fillex, one or two kinds or more out of
TiC, WC, W$C, ZrC, VC, NbC, TaC, and MoQC can be used.
Still more, conductive material 13 is preferable to include Ni
powder, A1 powder, Cu powder, Fe powder, Ag powder, or
20 graphite powder.
In order to produce PTC element 10 in embodiments of
the present invention, it is necessary to have at least a process
in which as shown in Fig. 2 (a), at least one out of TiC, WC,
WZC, ZrC, VC, NbC, TaC, and MoZC is blended and kneaded
25 with a crystalline polymer component by 36 to 60 volume
percent as a conductive powder filler to obtain formed
composition material 12, a process in which after conductive
paste containing conductive powder 13 is coated on surface
Ot- 3-t 4; t 3: G3 ;~t,~~va~*1~~ CA 02344532 2001-03-15 :03-3609-9000 H 17i 4t
12A of formed composition material 12, pressure sealing
treatment of conductive powder 13 is carried out and, as shown
in Fig. 2 (b), conductive powder 13 is buried so that it is partly
exposed from surface 12A of formed composition material, and
a process in which as shown in Fig. 2 (c), surface 12A of formed
composition material 12 is plated to form (plated) electrodes
14A and 14B.
And, an example of the production methods of above-
mentioned PTC element 10 will be explained more concretely.
At first, crystalline high-density polyethylene having
softening point of about 130°C as a polymer component and
conductive powder filler of 1 to 5 ~ m in particle size were
blended and kneaded on a roller mill heated about 140 to 200°C
so that the content of the conductive powder was 35 to 60
16 volume percent, and kneaded polymer material was obtained.
Further, for example, one or two kinds or more out of TiC, WC,
W2C, ZrC, VC, NbC, TaC, and MoaC can be used as conductive
powder [ See Fig. 2(a)]
Next, after the above-mentioned kneaded high polymer
material was powdered, the powder was press molded to make
a sheet and a sheet of kneaded polymer material was obtained
as a result. Then, both sides of the kneaded polymer
material were coated with conductive paste composed of Ni
powder, polyvinylbutyral, and a solvent and dried at room
temperature fox 5 hours or more to make a dried sheet. This
dried sheet was pressed at temperatures of about 140 to 200°C
fox about 5 t;o 15 minutes to make Ni powder pressure aealed.
As a .result, a PTC composition material sheet, in which most
01- 3-14:13;r3 :'~*~~ CA023445322001-03-15 :03-3009-9000 ~I 18i 41
of Ni powder was buried in the sheet and part of the powder
was exposed on the surface of the sheet, was obtained C See Fig.
2 (b)]
After the PTC composition material sheet pressure
sealed as mentioned above was defatted in succession, Ni
electroless plating was carried out on the sheet to form
electrodes C See Fig. 2(c)]
A test piece of 1 cma in area was punched out from the Ni
plated sheet obtained as mentioned above and used as a
l0 sample for assay (hereinafter, this sample for assay is referred
to as "the example" ). Furthermore, as conductive powder
buried in the sheet by pressuxe sealing, it is acceptable to use
A1 powder, Cu powder, Fe powder, Ag powder, or graphite
powder, as well as Ni powder.
Then, to compare with the example, comparative sample
1 was prepared as in the following (hereinafter, this
comparative sample is referred to as "comparative example
1" )
In this comparative example 1, the same treatment as in
above-mentioned example was carried out until the kneaded
polymer material was sheeted. After that, metal plates were
agglutinated on both sides of the kneaded material sheet by
heat pressing at temperatures of about 140 to 200°C to form
electrodes. And, a test piece of 1 cm2 in area was punched out
from this sheet and a PTC element was obtained (Comparative
example 1).
Moreover, comparative sample 2 was prepared as in the
following (hereinafter, this comparative sample is referred to
12
Ot- 3-14;13:,r,3 :*(1~~"V~*~~ CA 02344532 2001-03-15 ;03-3609-9000 # 19i 41
as "comparative example 2" ). Also in this comparative
example 2, the same treatment as in the above-mentioned
example was carried out until kneaded polymer material was
sheeted. After that, metal plates, their one side to be
contacted with the kneaded material sheet (one side of each
metal plate) were roughened with electrolyte, were
agglutinated on both sides of the kneaded material sheet by
heat pressing at temperatures of about 140 to 200°C to form
electrodes. And, a test piece of 1 cm' in axea was punched out
from this sheet and a PTC element was obtained (comparative
example 2).
Further, comparative sample 3 was prepared as in the
following (hereinafter, this comparative sample is referred to
as "comparative example 3" ). Also in this comparative
16 example 3, the same treatment as in the above-mentioned
example was carried out until kneaded polymer material was
sheeted. After that, the kneaded material sheet was defatted
and then Ni electroless plating was carried out on the sheet to
form electrodes. And, a test piece of 1 cm' in area was
punched out from this sheet and a PTC element was obtained
(comparative example 3).
Furthermore, comparative sample 4 was prepared as in
the following (hereinafter, this comparative sample is referred
to as " comparative example 4" ). Crystalline high-density
polyethylene having softening point of about 130°C as a
polymer component and conductive powder of 1 to S a m in
particlo size were blended and kneaded on a roller mill heated
about 140 to 200°C so that the content of the conductive
is
01- 3-14:13:63 :~fiv':~*$f~ CA 02344532 2001-03-15 :03-3609-9066 ~I 20i 41
powder was 34 volume percent, and kneaded polymer material
was obtained. Still more, TiC, WC, W2C, ZrC, VC, NbC, TaC,
and MoaC was used as conductive powder. The same
treatment as in the above-mentioned example was carried out,
6 then a test piece of 1 cma in area was punched out from this
sheet and a PTC element was obtained (comparative example
4).
Characteristic properties were tested on the example
and comparative example 1 to 3 obtained as mentioned above.
Well, as target properties of the PTC element were decided as
follows: bonding strength of the electrode is 500 gf/cm2 or more,
which means sufficient reliability as an electrode, resistance
at room temperature is 2 S~ ~ cm or less, and the ratio of
resistance value after resistance value was suddenly increased
1s to temperatures (after switching) to resistance value at room
temperature (after switching R/room temperature R) is 104 or
more, which means that the PTC element sufficiently operates
as over-current protective element and can be fully used as a
sheet heater. Further, the target of resistance value at room
2U temperature in cases of repeated switching of the PTC was
decided to be 2 t2 ~ cm or less even after 60 times switching.
At first, lead wires were connected by soldering on the
surfaces of electrodes of PTC elements (example and
comparative example 1 to 3) obtained as mentioned above and
25 surroundings of the electrodes were coated with epoxy resin.
Samples for measuring bonding strength were thus prepared.
Then, bonding strength of each electrode was measured by
pulling a lead wire of each sample for measuring
14
01- 3-14:13:63 :#"o:~*.~~ CA 02344532 2001-03-15 :03-3609-9006 I~ 21i 41
corresponding to the example and comparative example 1 to 3.
Measurements are shown in Table 1.
16
0 1 - 3 - 1 4 ; 1 3 . G 3 :'~J,FI~t~*~~ CA 02344532 2001-03-15 ; 0 3 -3 G 0 9 -
9 0 G G 8 2 2 i 4 1
(Table 11 Bnndin~ atrPn~rth of r.~lprtrndpA
Sample Bonding
stren th f/cma
Exam le 800 to 2300
Com arative exam le 1 Metal late) 25 to 150
Comparative example 2 850 to 2400
Rou hened metal late
Comparative example 3 (Plating only) 30 to 170
As clearly shown in Table 1, the bonding strength of
electrodes in the example was higher than those in
comparative example 1 where a metal plate not roughened was
used as electrodes and comparative example 3 where only
plating was performed to form electrodes, and was about the
same value as that in comparative example 2 where a
roughened metal plate was used as electrodes. And the
1o bonding strength of electrodes of the example was confirmed to
be more than 500 gf/cma, the value is possible to sufficiently
holding reliability as an electrode.
In the next place, resistance values at room temperature
after 500 times of switching were measured on example and
comparative example 1 to 3. Measurements are shown in
Table 2. Well, a digital multimeter with direct current 4
short needles was used for measuring resistivity at room
temperature.
16
01- 3-14:13:03 ;,'~J~,S~~r"F*1fi~ CA 02344532 2001-03-15 :03-3009-9000 ~1 23i
41
fTahlo 21 Rpcictivitv at room temperature
Sample Resistivity at room
temperature (~ cm)
Exam le TiC 0.4
Exam le WC 0.5
Example (WZC) 0.4
_-._-- _-_ , _._ 0.4
Exam le ZrC
Exam le VC _
0.6
Exam le (NbC 0.5
Exam le TaC 0.4
Exam le Mo C 0.5
Comparative example 1 (Metal 110.0
late)
Comparative example 2 1.3
Rou hened metal late
Comparative example 3 (Plating 320.0
onl )
As clearly shown in Table 2, in the examples, in cases
where any of TiC, WC, WaC, ZrC, VC, NbC, TaC, and Mo,C is
used, resistivity at room temperature was confirmed to be as
low as target values of 2 S2 ~ cm or less.
To the contrary, in comparative example 1 where a metal
plate not roughened was used as electrodes and comparative
example 3 where only plating was performed to form electrodes,
resistivity at room temperature was high, and it was found
that it was quite difficult to obtain target values of 2 ~ ~ cm or
less. It is considered that this is because of high contact
resistance between electrodes and kneaded material sheets.
On the other hand, in comparative example 2 where a
i6 roughened metal plate was used as electrodes, resistivity at
room temperature was found to be as low as target values of 2
SZ ~ cm or less but higher than those in the examples. It is
considered that this is because ohmic contact between the
m
Ot- 3-t4:t3:G3 ::~*~~ CA 02344532 2001-03-15 703-3009-9000 8 24i 4t
electrodes and the kneaded material sheet is not so good as in
the examples.
In the next place, a relationship between temperature
and resistivity was measured on example_ Measurements are
shown in Fig. 3. Well, measurements were made by using a
four short needle method in an oil bath and a digital
multimeter was used to determine resistivity.
As clearly shown in Fig. 3, in the example, resistivity at
room temperature is as low as target value of 2 S2 ~ cm or less
and the curve of temperature-resistivity greatly increases at
temperatures roughly corresponding to the softening point
(about 130°C) of crystalline high-density polyethylene used in
the example. And the ratio of resistance value after
resistance value was suddenly increased to temperatures
1s (after switching) to resistance value at room temperature
(after switching R/room temperature R) is higher than 108 and
greatly exceeds the target value of 104 which means that the
PTC element sufficiently operates as over-current protective
element and can be fully used as a sheet heater.
Moreover, current of 10A (50V) was repeatedly caused to
flow through PTC elements (in the example and comparative
example 1 to 4) obtained as mentioned above, respectively, and
the changes of resistivity after operations were measured.
Measurements are shown in Fig. 4.
26 As clearly shown in Fig. 4, in the example, initial
resistivity at room temperature was as low as target value of 2
S~ ~ cm or less and continued to keep target values of 2 S2 ~ cm
or less after repeated current flow. And after several times
IS
Ot- 3-t4it3:G3 ~~t~*~S~ CA 02344532 2001-03-15 '.03-3609-9000 1f 2vi 4t
of repeated current flow, increase in resistivity at room
temperature was found to be saturated.
To the contrary, in comparative example 1 where a metal
plate not roughened was used as electrodes and comparative
6 example 3 where only plating was performed to form electrodes,
initial resistivity at room temperature greatly exceeded the
target value of 2 S~ ~ cm, and further, it is known that
resistivity at room temperature greatly increases nearly in
proportion to repeated current flow. On the other hand, in
l0 comparative example 2 where a roughened metal plate was
used ~as electrodes, initial resistivity at room temperature was
as low as target values of 2 S? ~ cm or less but exceeded the
target value of 2 S2 ~ cm by repeated current flow, and no
saturation is seen in the increase in resistivity at room
15 temperature.
Furthermore, in comparative example 4 whexe
conductive powder was 34 volume percent, initial resistivity at
room temperature was as low as target values of 2 ST ~ cm or less
but exceeded the target value of 2 SZ ~ cm by repeated current
20 flow, and it was found that stability to repeated current flow
would not be obtained.
Now, in cases where metal powder is used as conductive
powder, powder itself flocculates partly to form conductive
routes and dielectric strength is decreased accordingly. And
25 in cases where any powder of carbon series, including carbon
black and graphite, is used as a conductive powder, resistivity
at room temperature exceeds the target value of 2 S7 ~ cm
because conductivities of powder materials are higher than
I9
O1- 3-14:13:63 :*JF1,'~ti~*~~ CA 02344532 2001-03-15 :03-3009-90GG If 2Gi 41
those of metal carbide powders.
Further, in cases where the filling amount of conductive
powder is less than 35 volume percent, as mentioned above,
stability to repeated current flow decreases and resistivity at
6 room temperature may exceed the target value of 2 S2 ~ cm
according as the frequency of operations. On the other hand,
in cases where the filling amount of conductive powder is more
than 60 volume percent, it will be actually difficult to
manufacture elements because of decrease in their
1o manufacturing efficiency.
In addition; in cases where electrodes are formed by a
method other than those of burying conductive powders and
plating metal as in the example, as mentioned above, stability
to repeated current flow decreases and resistivity at room
15 temperature may exceed the target value of 2 S2 ~ cm according
as the frequency of operations. Well, when the filling
amount of conductive powder is 45 volume percent or more,
stability to repeated current flow was found to be further
improved.
20 As mentioned above, in the first embodiment of the
present invention, a conductive material was pressure sealed
and buried on the surface of a formed composition material, in
which conductive powder filler was blended and kneaded with
a crystalline polymer component by 35 to 60 volume percent, so
25 that the conductive material is partly exposed, and electrodes
were formed by plating treatment on the surface of the formed
composition material where the conductive material was
partly exposed, and further, as conductive powder filler, at
01- 3-14:13: r3 ;;~J,~~*~$~ CA 02344532 2001-03-15 ; 03-3609-90GG ~I 27i 41
least one out of TiC, WC, WaC, ZrC, VC, NbC, TaC, and MoaC
was used. Consequently, the adhesion between the PTC
composition material and electrodes becomes good and the
contact resistance between the two can be lowered. And it is
possible to obtain a PTC element excellent in stability to
repeated current flow.
In the next place, the second embodiment of the present
invention will be explained in detail with reference to
drawings.
Fig. 5 is a partial sectional view showing the PTC
element related to the second embodiment of the present
invention. As shown in Fig. 5, the PTC element 10 in the
embodiment has formed composition material 12, conductive
material 13 which is pressure sealed and buried so that the
i5 material is partly exposed from the surface 12A of formed
composition material 12, and electrodes 14A and 14B formed
by plating treatment on the surface 12A of formed composition
material 12. And on the part where formed composition
material 12 other than (plated) electrodes 14A and 14B is
exposed, steam barrier layer 16 is coated.
Formed composition material 12 is quite the same as
that in the above-mentioned embodiment 1 and was formed as
follows: conductive powder filler using at least one out of TiC,
WC, WZC, ZrC, VC, NbC, TaC, and MoaC was blended and
26 kneaded with a crystalline polymer component by 35 to 60
volume percent and then the mixture was formed. The
crystalline polymer component in formed composition material
12 is composed of a polymer alloy in which one kind or two or
21
01- 3-14;13;63 ;*(h,~~*~~ CA 02344532 2001-03-15 :03-3009-9000 N 28/ 41
more kinds of thermoplastic polymers, for example, modified
polyethylene or modified polypropylene, are mixed.
Nickel (Ni) foil is used in each of electrode 14A and 14B.
Steam barrier layer 16 is formed by conducting steam barrier
treatment, for example, as mention later, such as coating with
PVDC latex and others_
In the following, one example of the production method
of PTC element 10 in the above-mentioned embodiment will be
explained concretely with reference to Fig. 6. Fig. 6
indicates drawings to explain the production method of the
PTC element of the example, (a) shows the formed composition
material, (b) shows a state in which a conductive material is
pressure sealed and buried so that the material is partly
exposed from the surface of the formed composition material,
(c) shows a state in which electrodes are formed by plating
treatment on the formed composition material, (d) shows
steam barrier treatment, and (e) shows the PTC element
treated with steam barrier treatment, respectively.
In Fig. 6, each process of (a), (b), and (c) is quite the
same as each pxocess of (a), (b), and (c) in the first embodiment
shown in Fig. 2.
In this example, steam barrier layer 16 was formed by
coating PVCD latex 16a on the part except for plated
electrodes (nickel foil) 14A and 14B in formed composition
material 12, that is, on part 12 C where the surface of formed
composition material 12 is exposed, as shown in Fig. 6 (d), and
PTC (resistant) element 10 of the example shown Fig. 6 (e) was
consequently made.
22
01- 3-14: 13: 03 :'~°9'"v~*~~ CA 02344532 2001-03-15 ; 03-3009-9000 ~
29i 41
Like this, in the PTC element of the example in which
the steam barrier layer was formed by coating YVCD latex on
the part except for plated electrodes (nickel foil) 14A and 14B,
for example, even if 500 hours are passed after the PTC
F element is put in a constant-temperature bath with 85°C x 90%
RH, resistivity after switching is stable and reliability is
increased by 8 times compared to elements not formed steam
barrier layer , and it was confirmed that more stable repeated
current breaking can be achieved. Consequently, even if
switching operation is repeatedly performed at a state of high
environmental humidity during stock and usage, stable
resistance can be obtained.
As mentioned above, the present invention has been
described in particular embodiments. However, the present
invention should not be limited to these embodiments but
should be applied to other embodiments within the scope of the
invention as defined by the appended claims.
For example, in the above-mentioned first and second
embodiments, high-density polyethylene resin was used as a
main component of the foxmed composition matexial, but the
main component is not limited to the resin. As main
components of formed composition materials, polypropylene
type, low-density polyethylene type and other type resins can
be used, as well as high-density polyethylene resin.
Furthermore, in the above-mentioned second
embodiment, steam barrier was formed by coating with PVCD
latex, but in addition to this, methods of steam barrier
treatment using substances with low steam permeability, for
28
01- 3-ia:i3;03 :~55~~v:~*~~ CA023445322001-03-15 :03-3009-9oc0 ~ 30i ai
example, polyvinylidene chloride and the like are considered.
[Industrial Usability]
As mentioned above, in the first embodiment of the
present invention, on the surface of the formed composition
material in which a conductive powder filler is blended and
kneaded with a crystalline polymer component by 36 to 60
volume percent, a conductive material is pressure sealed and
buried so that part of the conductive material is exposed, and
the surface of the formed composition material with partly
exposed conductive material is plated to form electrodes.
And at least one out of TiC, WC, WaC, ZrC, VC, NbC, TaC, and
MoaC is used as conductive powder filler. Consequently, the
adhesion between the PTC composition material and the
electrodes becomes good and contact resistance value between
the two can be reduced. And a PTC element with excellent
stability to repeated turning on electricity and its production
method can be obtained.
Besides, according to the second embodiment of the
2o present invention, in the PTC element and its production
method of the above-mentioned first embodiment, it is further
possible to effectively prevent the PTC element itself from
peeling from electrodes due to the effect of environmental
humidity. And a high reliable PTC element with good
26 stability and reproducibility to repeated use and its
production method can be provided.
24