Note: Descriptions are shown in the official language in which they were submitted.
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
o PEPTIDES USEFUL FOR REDUCING SYMPTOMS
OF TOXIC SHOCK SYNDROME AND SEPTIC SHOCK
RELATED APPLICATIONS
This is a continuation-in-part application of co-
pending U.S. Application Serial No. 09/168,303 filed
October 7, 1998, which is in turn a continuation-in-part
of co-pending U.S. Application Serial No. 08/838,413
filed April 7, 1997. Pursuant to 35 USC 365, U.S.
APPlication Serial No. 09/168,303 is also a
continuation-in-part of co-pending International
Application PCT/US98/06663, filed April 1, 1998. The
entire disclosure of U.S. Application Serial No.
08/838,413 and U.S. Application Serial No. 09/168,303
are incorporated herein by reference.
FIELD OF THE INVENTION
This invention relates to compositions and methods
for protecting against, or reducing the severity, of
toxic shock syndrome and septic shock from bacterial
infections. More particularly it relates to peptides,
which may be polymeric, and carrier-conjugates thereof,
derived from homologous sequences of the family of
staphylococcal and streptococcal pyrogenic toxins. The
peptides of the invention are useful to prevent, treat,
or protect against the toxic effects of bacterial
toxins, including most, if not all, of the
staphylococcal and streptococcal pyrogenic toxins.
These are also useful to induce serum antibodies and may
also be useful in diagnostic assays.
The invention also relates to antibodies induced by
the peptides and/or carrier-conjugates and their use to
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-2-
o prevent, treat, or protect against the toxic effects of
bacterial toxins, including most, if not all, of the
staphylococcal and streptococcal pyrogenic toxins.
The invention also relates to compositions and
methods to protect against, or ameliorate the effects
of, autoimmune diseases which are associated with, or
are the result of, the presence of staphylococcal or
streptococcal toxins.
The invention also relates to diagnostic assays and
kits to detect the presence of staphylococcal and
streptococcal pyrogenic toxins, or antibodies thereto.
The invention also relates to isolated and purified
nucleic acids encoding the peptides of the invention and
transformed host cells containing those nucleic acids.
BACKGROUND OF THE INVENTION
The pyrogenic exotoxins of Group A streptococci and
the enterotoxins of Staphylococcus aureus, which are
also pyrogenic exotoxins, constitute a family of
structurally related toxins which share similar
biological activities (I1, 13). The staphylococcal and
streptococcal pyrogenic exotoxins also share significant
amino acid homology throughout their sequences (11, 19,
40). This pyrogenic exotoxin family contains nine main
toxin types, and several allelic variants (subtypes)
have been described. Several studies have shown that
the toxins share common motifs based on immunologic
cross reactivity between the toxins (26, 27). They
stimulate CD4+, CD8+ and y8+ T cells by a unique
mechanism. These toxins share the ability to bind the ~3
chain variable region (Vp) elements on the lateral face
of the T cell receptor (TCR) and simultaneously bind to
CA 02345023 2001-04-06
WO 00/Z0598 PCT/US99/22180
-3-
o the lateral face of the class II major
histocompatibility complex (MHC) of antigen presenting
cells (Figure 1), causing an aberrant proliferation of
specific T-cell subsets (3, 4, 12). This property of
the toxins has labeled them as "superantigens" (36)
since they do not interact with the MHC and TCR
molecules in the manner of conventional antigens (14,
18) and produce a massive proliferation of T cells.
The variability of the sequences in the TCR-binding
region and within the MHC-II-binding regions most likely
provides the different superantigen toxins their
specificities for different Va molecules and variable
affinities for MHC-II types.(69-70)
The cross-linking of TCR with MHC-II molecules by
superantigens causes a profound blastogenesis of
lymphocytes and antigen-presenting cells. The resulting
stimulation of leukocytes leads to a significant
increase in cytokine production.
Monocytes stimulated with bacterial superantigens
produce the Thl cytokines IL-2 and IFN-y and the anti-
inflammatory cytokines IL-10 and IL-1 receptor
antagonist. (71). T cells activated by superantigen
stimulation produce IL-12. (72). Whole preparations of
peripheral blood mononuclear cells containing
lymphocytes and antigen-presenting cells elicited a wide
range of inflammatory cytokines in significant amounts.
The generation of monocyte cytokines such as IL-1, IL-6,
TNF-a, and TNF-(3 was dependent on the presence of T
cells. (73).
Costimulatory molecules important in conventional
immune responses also play a significant role in the
CA 02345023 2001-04-06
WO 00/20598 PCTNS99/Z2180
-4-
o response of immune cells to superantigens. The
costimulatory T cell antigen, CD28, and its
corresponding ligand on MHC-II-bearing cells, B7,
contribute to superantigen mitogenicity. (74, 75).
Other costimulatory molecules, such as LFA-1/ICAM-1 and
VLA-4/VCAM-1, also contribute to the activation of
immune cells by superantigens.(76, 77). These
immunostimulatory activities of superantigens are
crucial to their ability to cause injury to the host.
The bacterial toxins cause a variety of syndromes
in humans. Staphylococcal enterotoxins have been
implicated in staphylococcal food poisoning (26), as
well as toxic shock like syndromes (1). The gene
sequences and deduced amino acid sequences of at least
six staphylococcal enterotoxins ("SE"): A, B, C, D, E
and H, are known, i.e., SEA, SEB, SEC, SED, SEE, and SEH
(19, 23). The streptococcal pyrogenic exotoxins ("SPE")
have been implicated in causing the symptoms of scarlet
fever and toxic shock like syndrome (8, 20, 30). The
sequences of three members of this family are known:
SPEA, SPEC, and SSA (5, 23, 35).
Toxic shock syndrome toxin (TSST-1) from S. aureus
shares similar biological activity with the SE's and
SPE's, however amino acid sequences of this toxin are
significantly different from these two classes of toxins
(2). Structural analysis suggests that, despite the
differences in amino acid composition, the overall
topology of TSST-1 and the SE/SPE family of toxins is
similar (41). The molecular structure of SE's and SPE's
has been determined by various methods. Reviews
concerning the molecular structures are available (19,
62). Molecular evolution studies of the SE/SPE family of
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-5-
a toxins suggests that the toxins can be grouped into two
main clades(34). All these toxins are highly resistant
to denaturation by heat and to proteases. With the
exception of TSST-1, they are soluble proteins of
approximately 230 amino acids and have a central
disulphide loop. In contrast TSST-1 has only 194 amino
acids and does not possess any cysteines.
It has been suggested that the conservation of
amino acids is important to maintaining the structure
necessary for the biological activity of the toxins
(32). Mutations constructed in various positions
throughout the SPEA and SEB molecules were sufficient to
inactivate biological activity (6, 15). Mutations at
various points throughout the molecules often had
different effects, su
ggesting that functional activities
could not be attributed to any one region of the toxins
(7). These results suggested that a functional tertiary
structure must be maintained. Chemical modifications of
highly conserved histidine residues inactivated biologic
activity (29). The high conservation of the disulfide
loop in the SE's and SPE's suggests an important role in
the structure of the SE/SPE family of toxins. Studies
show the disulfide loop is required for mitogenic
activity of SEA and SEB. Reduction of the disulfide
loop inactivated T cell stimulatory activity, but did
not affect MHC-II binding and stimulation of monocytes
(54). Peptide cleavages within the loop had no effect on
T-cell mitogenicity, however cleavage of conserved
sequences outside the loop of SEA resulted in loss of
mitogenic activity. The loop and conserved adjacent
sequences appear to be associated with avidity of the
toxins to the TCR, and do not contribute to the
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-6-
specificity of toxins for a particular Vp type (6) .
Residues determining TCR V~ specificity appear to be
located within the carboxy-terminus of the SE/SPE toxins
(59), while residues critical for MHC-II binding appear
to be located in the amino-terminal region, and the
central portion of the molecule near the disulfide loop
(53). The disulfide loop and adjacent highly conserved
sequences contribute to the structural integrity of the
toxins, and serve to bring the TCR and MHC binding
regions in functional proximity to each other (65).
The SEs are named for their ability to induce
gastrointestinal illnesses upon oral intake of a few
micrograms of the toxin. The clinical effect appears in
IS 2 to 4 hours and is manifested by nausea and diarrhea.
These symptoms appear to be caused by leukotrienes and
histamine released from mast cells. Additionally, both
the staphylococcal and streptococcal exotoxins are
implicated in gram-positive shock. Although
superantigen-related septic shock appears to be
primarily mediated by tumor necrosis factor (TNF)-a and
interleukin (IL) 12, the contribution of other cytokines
cannot be discounted. (78, 79, 80).
The physiologic response to superantigens is
similar to septic shock induced by gram-negative
endotoxin (lipopolysaccharide, LPS). In fact, LPS and
superantigens can work synergistically to produce lethal
toxic shock. (81, 82, 83). Toxic shock syndrome can be
exacerbated by the synergistic effects of TSST-1 with
the SE/SPE family of toxins. (84, 85). Superantigen
stimulation of immune cells can exacerbate autoimmune
syndromes by causing the expansion of autoreactive T
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/Z2180
o cell subsets, upregulation of MHC-II expression, and the
potentiation of cytotoxic T cell response (86, 87, 88,
89, 90, 91).
Toxic shock syndrome is a specific syndrome caused
by either the Stapylococcal or Streptococcal organisms.
It is specifically caused by the toxins produced by
these bacteria. Clinically it often occurs in young
women and children and is characterized by a raised
temperature, low blood pressure, a rash that eventually
leads to skin loss especially on the palms and the soles
and multi-organ involvement.
Septic Shock on the other hand involves both gram
negative as well as gram positive organisms, occurs in
all groups of patients especially the elderly and post-
surgical. It has similar symptoms except for the lack
of a skin losing rash. Both diseases have a high
mortality-however there are many more cases of septic
shock as compared to toxic shock. The term "septic
shock" is used herein to describe hypotension and organ
failure associated with bacterial infections.
"Toxic shock like syndrome" is the term previously
used to describe the syndromes caused by staphyloccal
and streptococcal pyrogenic bacterial exotoxins other
than toxic shock syndrome toxin (TSST-1) from S. aureus.
Currently, the term "toxic shock syndrome" is used to
describe the syndromes caused by TSST-1 and the other
pyrogenic exotoxins, and is the terminology used
hereinafter.
Toxic shock syndrome can be exacerbated by the
synergistic effects of TSST-1 with the
enterotoxin/pyrogenic toxin family of toxins (9, 25).
Gram negative bacterial endotoxin and the pyrogenic
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
_g_
o toxins can work synergistically to produce intractable
shock ( 17 , 3 0 ) .
With respect to septic shock, lipopolysaccharide
(LPS) is an integral part of the cell wall of Gram-
negative bacteria and is a potent inducer of cytokine
release by macrophages (52). During the induction phase
of septicemia, LPS binds to the CD14 receptors of
macrophages and triggers the release of a number of
cytokines including Interleukin-1 (IL-1), and Tumor
Necrosis Factor-a(TNF- a) (49). Accordingly, therapeutic
strategies for septic shock have centered on the
neutralization of LPS or LPS-induced cytokines (64).
Unfortunately, trials using either monoclonal antibodies
directed against part of the LPS molecule or the use of
CD14 soluble receptors have riot been very promising
(45). The reasons for these failures might be: 1. The
type of patient selected (many were already in
irreversible shock). 2. The monoclonal antibody did not
block all sites of LPS. 3. Soluble CD14 receptors did
not block all LPS molecules.
Toxic shock syndrome and septic shock are still
among the most life threatening syndromes affecting
humans. It is estimated that approximately 20,000 cases
of toxic shock syndrome occur each year of with a 10%
mortality rate (66). With respect to septic shock
approximately 400,000-500,000 cases occur each year with
a 50% mortality (63). Present therapy is primarily
symptomatic with administration of fluids, antibiotics,
pressor agents and occasionally steroids (56). There is
no vaccine available for toxic shock syndrome since all
of the superantigens are antigenically distinct even
though there is some sequence homology present in all
CA 02345023 2001-04-06
WO 00!10598 PCT/US99/22180
-9-
o the superantigens. There have been numerous vaccine
trials for septic shock none of which have been
successful.
With respect to the failed vaccine trials for
septic shock, we believe that there was a failure to
recognize that the interaction between the superantigens
described above and LPS enhances the lethal potency of
both these antigens by about 1000 fold. In contrast,
each antigen when given alone requires a much higher
dose for lethal septic shock (46).
Hence, it is proposed that at least two independent
pathways of lethal septic shock can occur. LPS and
peptidoglycan interact with macrophages. The
superantigens interact with T cells. In both cases
target cells are induced to release large amounts of
cytokines. There is increasing evidence that gram-
positive infections frequently accompany gram-negative
infections in patients with septic shock (see article by
Range!-Frausto, pages 299-312) (96). Exposure to gram-
negative endotoxin produces a state of macrophage
hyperesponsiveness on subsequent stimulation (92). A
similar state is seen with monocytes in septic shock.
Our group and others have shown that LPS and
superantigens can act synergistically to produce lethal
septic shock in animal models (93). It is our
hypothesis that a significant amount of septic shock
involves an early gram-negative infection that causes
significant symptoms of vasodilation and hypotension.
This is then treated with fluids and antibiotics,
leading to early recovery by the patient. Some days
later, a gram-positive insult either via a line sepsis
°f the skin or gastrointestinal flora may cause severe
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-10-
o irreversible shock in a previously LPS-sensitized
patient. This model is depicted graphically in Figure 2
herein (94).
In other words, since Gram-negative and Gram-
positive organisms can be recovered from patients with
sepsis, it appears that it is the "two hit" hypothesis
that is operative and the interaction between LPS and
the superantigens markedly enhances the lethal
properties of both molecules. In this model, the
interruption of the toxin pathway by anti-peptide
antibody(ies) or by peptides) of the invention prevents
the onset of lethal shock induced by the combination of
the LPS and one or more of the superantigens.
SUMMARY OF THE INVENTION
The present invention relates to the identification
of consensus sequences derived from two conserved
regions of the staphylococcal enterotoxins and
streptococcal pyrogenic toxins (hereinafter called
"region 1" and "region 2") and the discovery that
compositions comprising amino acid sequences based on
these two conserved regions of the staphylococcal
enterotoxins and streptococcal pyrogenic exotoxins are
capable of inducing antibodies which react with a
variety of staphylococcal and streptococcal pyrogenic
exotoxins and are also capable of ameliorating or
preventing diseases related to the deleterious effects
of these toxins.
The invention also relates to compositions and
methods for preventing and treating diseases related to
the release of certain pyrogenic exotoxins from
bacteria.
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
o This invention provides peptides comprising amino
acid sequences which reduce, inhibit or eliminate the
deleterious effects of bacterial toxins and/or axe
capable of inducing antibodies that reduce, inhibit or
eliminate the deleterious effects of bacterial toxins,
S such as those of staphylococcus and a variety of
streptococci. Antibodies may be induced by
administration of a pharmaceutical composition and/or
vaccine containing a composition comprising a peptide
1~ derived from one or both of the two conserved regions
described herein, or a structurally and/or
immunologically related antigen.
The amino acid sequences provided by this invention
are sufficiently common to all members of this family of
1S
pyrogenic exotoxins to be useful for eliciting
antibodies which are cross-reactive with toxins derived
from various bacteria.
The amino acid sequences provided by this invention
20 are also useful for new methods of preventing and
treating symptoms associated with the bacterial release
of the staphylococcal enterotoxins and the streptococcal
pyrogenic exotoxins. Such methods include, for example,
administering to an individual who is suspected of
2S
having an infection or developing and/or having a toxic
or septic reaction, a compound comprising at least one
of the consensus amino acid sequences of this invention
in an amount sufficient to inhibit superantigen
30 stimulation of T-cells, preferably an amount sufficient
to reduce, inhibit or eliminate the deleterious effects
of the exotoxins. Such methods also include
administering to an individual at risk of infection or
3S developing a toxic reaction to the exotoxins at least
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-12-
0 one of the consensus amino acid sequences of this
invention in an amount sufficient to elicit the
production of antibodies to the exotoxins.
In a preferred embodiment of this invention, an
individual at risk for developing toxic or septic shock
syndrome or an individual with symptoms of toxic shock
syndrome or septic shock may be treated by administering
to such individual a composition comprising at least one
of the peptides of this invention and/or carrier-
conjugate thereof.
In another preferred embodiment of this invention,
an individual at risk for developing toxic shock
syndrome or septic shock, or an individual with symptoms
of toxic shock syndrome or septic shock, may be treated
by administering to such individual antibodies which
have been generated in a mammal immunized with at least
one of the compositions of this invention.
Vaccines and pharmaceutical compositions comprising
at least one of the consensus amino acid sequences and a
physiologically acceptable carrier and optionally an
adjuvant are also part of this invention.
Another object of the invention is to provide
antibodies induced by the peptides and carrier-
conjugates thereof. These antibodies may be used to
prevent, treat, or protect against the toxic effects of
most, if not all, of the staphylococcal and
streptococcal pyrogenic exotoxins. The antibodies may
also be useful to protect against, or ameliorate the
effects of, autoimmune diseases which are associated
with, or are the result of, the presence of
staphylococcal or streptococcal pyrogenic exotoxins.
These antibodies are also useful in diagnostic assays
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-13-
o and kits to detect the presence of staphylococcal and
streptococcal pyrogenic exotoxins and to aid in the
diagnosis of diseases related to the presence of those
toxins.
Another object of the invention is to provide
isolated and purified nucleic acids encoding the amino
acid sequences of the invention, as well as suitable
expression systems, vector components and transformed
host cells containing those nucleic acids.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1. Schematic diagram of the interaction
between a T cell receptor, superantigen, and a class II
MHC molecule. Superantigens bind to common sequences in
class II MHC molecules and T cell receptors that lie
outside the normal antigen-binding sites. T cell
activation by superantigens is not limited by the
antigenic specificity of the T cell.
Figure 2. Diagram of the "two hit" model of septic
shock.
Figure 3. Comparison of the synthetic peptide
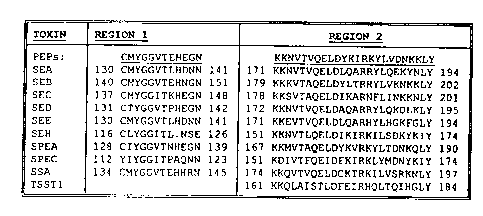
sequences to conserved regions 1 and 2 of the
staphylococcal enterotoxins (SEA, SEB, SEC, SED, SEE,
and SEH), and sire tococcal
p pyrogenic exotoxins (SPEA,
SPEC, and SSA). Staphylococcal toxic shock syndrome
toxin 1 (TSST-1) was compared with the region 2 peptide.
Numbers represent the residue positions as a reference
to where these regions exist in the whole toxin
molecules. Sequences are from either the Swiss protein
or GenBank databases under the following accessian
numbers. Swiss protein: SPEA, P08095; SPEC, P13380;
SEA, P13163; SEB, P01552; SEC, P01553; SED, P20723; SEE,
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/2Z180
-14-
o P12993. GenBank: SEH, U11702; SSA, L29565; TSST1,
J02615.
Figure 4. ELISA titers of antibodies from rabbits
immunized with polymeric peptide #6348. The peptide was
diluted so that it was delivered to each well to give a
final concentration of 2 ~,g/100 ~1. The serum was then
diluted to 1:1,000; 1:10,000; 1:100,000; 1:500,000; and
1:1,000,000 and 100 ~1 of each dilution of serum was
placed in each well. Experiments were run in triplicate
for each dilution of serum. Note the 1 log higher
titers of rabbit #443 serum as compared to rabbit #442
serum. Cut off readings were at O.D. 0.6.
Figure 5. 12% SDS PAGE gel immunoblot of a variety
of staphylococcal and streptococcal toxins developed
with the anti-peptide 6348 antibody. Note bands of
correct molecular weight (M. W.) of each toxin identified
by the anti-peptide antibody. Lane 1: SPEA, lane 2:
SEA, lane 3: SEB, lane 4: SED, lane 5: SEE, lane 6: SEC
and lane 7 TssT-1. Note bands at appropriate M.W. in
lanes 1-4. Fainter bands are seen in lanes 5 and 7.
Figure 6. Bar graphs of blastogenesis assays of
human mononuclear cell populations stimulated by various
toxins in the presence of normal rabbit serum and
anti-peptide 6348 serum. Note the marked inhibition of
SEB, SEC, SEE, SPEA and SPEC by the anti-peptide
antibody. Less, but definite, inhibition of SEA by the
anti-peptide antibody was also seen.
Fi ure 7. Bar
g graphs of blastogenesis assays of
human mononuclear cell populations stimulated by SEB in
the presence of (A) peptide 6343 (i.e., CMYGGVTEHEGN,
SEQ ID N0:3),(B) peptide 6346 (i.e.,
CGKKNVTVQELDYKIRKYLVDNKKLYGC, SEQ ID NO: 6)) and (C)
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-15-
o peptide 6348 (i.e.,
CMYGGVTEHEGNKKNVTVQELDYKIRKYLVDNKKLYGC, SEQ ID N0:8).
Figure 8. Inhibition of SEB, SEC, SED, SPEC, SPEA
and TSST-1 toxin blastogenesis of peripheral blood
mononuclear cells (PBMC) by the 6343 peptide. 2 x 105
PBMC were stimulated with either 2~g of the indicated
toxin or a combination of 2~,g of the toxin with 150~.g of
the 6343 peptide. These were incubated for 72 hours and
the results were measured via tritiated thymidine
incorporation. CPM represents counts per minute. Note
that the single peptide (6343) inhibited all of the
superantigens tested.
Figure 9. Inhibition of SPEG, SPEH, and SPEZ toxin
blastogenesis of peripheral blood mononuclear cells
(PBMC) by the 6343 peptide. 2 x 105 PBMC were stimulated
with either 2~.g of the indicated toxin or a combination
of 2~g of the toxin with the indicated amount of the
6343 peptide. These were incubated for 72 hours and the
results were measured via tritiated thymidine
incorporation. CPM represents counts per minute. Normal
represents normal media. Note that the single peptide
(6343) inhibited the superantigens SPEG, SPEH and SPEZ.
Figure Z0. (A). Binding of peptide 6343 to the
MHC complex as measured by ELISA. (B). Inhibition of
binding of SEB toxin biding by peptide 6343 as measured
by decreased anti-SEB binding at increased
concentrations of added peptide 6343.
Figure 11. Confocal microscope pictures. (A)
Binding of peptide 6343 is indicated by the green color.
(B) Binding of anti-MHC peptide is indicated by the red
color. (C) Combined picture showing stippled pattern of
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-16-
o red and green color. Binding of peptide 6343 is
indicated by the green color and binding of anti-MHC
peptide is indicated by the red color.
DETAILED DESCRIPTION OF THE INVENTION
It is to be understood that both the foregoing
general description and the following detailed
description are exemplary and explanatory only, and are
not restrictive of the invention, as claimed. The
accompanying drawings, which are incorporated in and
constitute a part of the specification, illustrate an
embodiment of the invention and, together with the
description, serve to explain the principles of the
invention.
Two consensus patterns, corresponding to conserved
region 1 and region 2, respectively, are identified as
common to members of the staphylococcal enterotoxin and
streptococcal pyrogenic toxin family of toxins when the
Program "Motifs" in a software package from the Genetics
Computer Group, Inc. ("GCG") is run using the
streptococcal SPEC toxin as an example. "Program Manual
for the Wisconsin Package, Version 8, September 1994,
Genetics Computer Group, 575 Science Drive, Madison,
Wisconsin USA 53711"
incorporated herein by reference.
The first consensus sequence ("GCG consensus #1")
identified by the Motifs program has the amino acid
sequence YGG(LIV)TXXXXN, which is rewritten herein as
YGGX1TX2X3X4XSN (SEQ ID NO:1) , wherein X1 is selected from
the group consisting of L, I, or V; and X2, X3, X4 and XS
are each independently selected from the group
consisting of any amino acid. This pattern is present
in the staphylococcal enterotoxins and streptococcal
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-17-
o pyrogenic exotoxins, but not in TSST-1. The sequence
begins immediately at the COOH-terminal side of the
cysteine loop. The second consensus sequence ("GCG
consensus #2") identified by the Motifs program has the
amino acid sequence
KXX(LIV)XXXX(LIV)DXXXRXXLXXXXX(LIV)Y, rewritten herein
aS KX6X~XgXgX1pX11X12X13DX14X15X16RX17X18LX19X20X21X22X23X24Y (SEQ
ID NO: 2) , wherein X8, X13 and X24 are each independently
selected from the group consisting of L, I and V, and
X6r X7r X9r XlOr Xllr Xl2r X14 XlSr Xl6r Xl7r XlBr Xl9r X20r
Xzl, X22 and X23 are each independently selected from the
group consisting of any amino acid. This pattern is
present in the staphylococcal enterotoxins,
streptococcal pyrogenic exotoxins, and TSST-1.
One object of the invention is to provide
compositions comprising peptides comprising amino acid
sequences based on these two conserved regions of the
staphylococcal enterotoxins and streptococcal pyrogenic
toxins. These peptides may be used for eliciting an
immunogenic response in mammals, including responses
which provide protection against, or reduce the
severity, of toxic shock or septic shock from
staphylococcal or streptococcal infections. These
peptides may also be useful to protect against, or
ameliorate the effects of, autoimmune diseases which are
associated with, or are the result of, the presence of
staphylococcal or streptococcal pyrogenic exotoxins.
These peptides are also useful in diagnostic assays and
kits to detect the presence of antibodies to
staphylococcal and streptococcal pyrogenic exotoxins and
to aid in the diagnosis of diseases related to the
Presence of those toxins.
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-18-
o The peptides of the invention are those derived
from either one or both of the following two consensus
sequences:
YGGX1TX2X3X4X5N (SEQ ID NO:l) , wherein X1 is selected from
the group consisting of L, I, or V; and X2, X3, X4 and XS
are each independently selected from the group
consisting of any amino acid.
KXX(LIV)XXXX(LIV)DXXXRXXLXXXXX(LIV)Y, rewritten herein
aS KX6X7XgX9X1pX11X12X13DX14X15X16RX17X18LX19X20X21X22X23X24Y ( SEQ
ID NO: 2) , wherein X8, X13 and X24 are each independently
selected from the group consisting of L, I and V, and
X6i X7i X9i XlOi Xlli Xl2i Xl4i XlSi Xl6i Xl7i XlBi Xl9i X201
X21, X2z and X23 are each independently selected from the
group consisting of any amino acid.
A preferred consensus sequence of the invention
from Region 1 (consensus #la) has the amino~acid
sequence X25X2sYGGXITX2X3X4X5N (SEQ ID NO: 28) , wherein X1
is selected from the group consisting of L, I, and V;
X2. X4 and XS are each independently selected from the
group consisting of any amino acid; and X3, X25 and X26
are each independently selected from the group
consisting of any amino acid and of no amino acid; but
preferably X1 is selected from the group consisting of I
and V; X2 is selected from the group consisting of L, E,
K, P and N; X3 is selected from the group consisting of
H and A and no amino acid; X4 is selected from the group
consisting of D, N, E, Q, and H; XS is selected from the
group consisting of N, G, S, and R; X25 is selected from
the group consisting of C and Y and no amino acid; and
X26 is selected from the group consisting of M, T, L, I,
and no amino acid.
A Preferred consensus sequence of the invention
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-19-
o from region 2 (consensus ##2a) has the amino acid
sequence : KX6X~XgXgX1pX11X12X13DX14Xi5X16RX17X18X27Xy9X20X21
X22X23X24Y (SEQ ID NO: 29) , wherein Xa, X13 and X24 are each
independently selected from the group consisting of L, I
and V; X6, X~, Xs. Xlo~ X11. X12 Xla~ Xis~ Xls. Xl~. Xls Xls.
X2o, X21. X22, and X23 are each independently selected from
the group consisting of any amino acid; and X2~ is
selected from the group consisting of L and Y; but
preferably X6 is selected from the group consisting of K
and D; X, is selected from the group consisting of N, K,
S, E, M, I and Q; X$ is selected from the group
consisting of L and V; X9 is selected from the group
consisting of T and A; Xlo is selected from the group
consisting of V, A, L, F and I; X11 is selected from the
group consisting of Q and S; X12 is selected from the
group consisting of E and T; X13 is selected from group
consisting of L and I; X14 is selected from the group
consisting of L, Y, I, A, F and C; Xls is selected from
the group consisting of Q, L, K and E; X16 is selected
from the group consisting of A, T, I and V; X1-, is
selected from the group consisting of R, H, N and K; X18
is selected from the group consisting of Y, F, I, L and
Q; X19 is selected from the group consisting of Q, V, I,
H, S, T and M; X2o is selected from the group consisting
of E, K, N, G, D, S and Q; X21 is selected from the group
consisting of K, N, D, R and I; X22 is selected from the
group consisting of Y, K, L, F and H; X23 is selected
from the group consisting of N, K, G and Q; X24 is
selected from the group consisting of L and I; and X2~ is
L.
The following Table 1 lists the amino acids that
are found at each of the variable positions in the
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-20-
o sequences shown in Figure 3, and the number of times
they appear at that position:
10
20
30
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-21-
Table
1
Frequency of the ids in riablepositions
amino the va
ac
in
the sectuences shown in gure 3
Fi
X1 6V 3I
Xz 3L 2E 1K 2P 1N
X3 7H lA one deletion (no amino acid)
X4 2D 2N 3E 1Q 1H
Xs 3N 4G 1S 1R
Xs 9K 1D
X~ 3N 1K 1S lE 1M lI 1Q
Xa 9V 1L
X9 9T lA
Xlo 4V 3A 1L 1F lI
X11 9Q 1s
X12 9E 1T
X13 9L lI
X14 2L 2Y 2I 1A 2F 1C
Xls 3Q 1L 5K lE
X16 4A 2T 3I 1V
Xl~ 2R 3H 1N 4K
X18 5Y 1F 2I 1L 1Q
X19 2Q 2V lI 1H 1S 2T 1M
X2o lE 2K 1N 1G 3D 1S 1Q
X21 4K 3N 1D 1R lI
X22 3Y 4K 1L 1F 1H
X23 3N 4K 2G 1Q
Xz4 8L 2I
X2s 8C lY
X26 5M 2I 1L 1T
X2~ 9L lY
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/Z2180
-22-
o In the peptides of the present invention, X1, Xe,
X13 and X24 may each independently be selected from the
group consisting of L, I and V; X2, X3, X4, Xs, X6, X7,
X9. X10. X11. X12. X14. Xls. X16. X17. Xle X19. X201 X21. X22.
Xz3. Xzs and X26 may each independently be any amino acid;
X3. Xzs and X26 may also each independently be no amino
acid; and X27 is selected from the group consisting of L
and Y. However, in general, the amino acids present at
the positions X1 to X27 in the toxins shown in Figure 3
(and listed in Table 1) are preferred for those
positions, and the amino acids present most often at
those positions in the toxins shown in Figure 3 (and
listed in Table 1) are more preferred. For example,
from Figure 3, and Table 1, it can be determined that H
(histidine) is present in seven toxins at position X3
and A (alanine) is present in one toxin at position X3,
and there is no amino acid present in one toxin at X3.
These are the preferred amino acids for position X3.
The more preferred amino acid for position X3 in a
peptide of the invention is H (histidine). The more
preferred amino acids for X1 through X26 are: X1 =
valine; X2 = leucine; X3 = histidine; X4 = glutamic acid;
Xs = glycine; X6 = lysine; X7 = asparagine; X8 = valine;
Xs = threonine; Xlo = valine; X11 = glutamine; X12 =
glutamic acid; X13 - leucine; X14 = leucine, tyrosine,
isoleucine or phenylalanine; X15 = lysine; X16 = alanine;
X17 = lysine; X18 = tyrosine; X19 = glutamine, valine or
threonine; X2o = aspartic acid; Xzl = lysine; X22 =
lysine; X23 = lysine; X2g = leucine; X25 = cysteine; X26 =
methionine; and X27 = leucine. But note that in the
exemplified peptides of the invention described
hereinbelow, i.e., SEQ ID NOS: 6, 7 and 8, inosine (I)
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-23-
o is used at position X16 instead of the more frequently
found alanine (A).
As is evident from Figure 3 and the above Table 1,
some amino acid residues are much more highly conserved
than suggested by the GCG package data provided by the
"Motifs" program.
In region 1, the preferred consensus is larger
(consensus #la), and usually includes a C in the first
position (X25) . The second residue (X26) is most often a
M, but this can vary. In the ninth position (X3), H is
the most highly conserved. The eleventh residue (XS) is
most often a G.
In region 2, the preferred consensus (consensus
#2a) is much more highly conserved than suggested by the
GCG program, especially if one excludes TSST-1 sequences
from consideration, as follows: The second position
(X6) is more highly conserved than suggested, being
almost exclusively a K; the fourth residue (X8) is
always a V followed exclusively by a T in the fifth
position (X9); the sixth position (Xlo) is somewhat
variable; but the seventh position (X11) is always a Q,
followed by E (X12). The next position is almost always
an L (X13) , and the second to last position (X24) is
almost always an L.
Thus, additional modified consensus sequences for
region 1 and region 2, which are of narrower scope than
the GCG consensus sequences #1 and #2 and the modified
consensus sequences #la and #2a, are as follows:
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-24-
o Consensus #lb:
CMYGGX1TX2HX4GN (SEQ ID NO: 30)
wherein
X1 is V or I, preferably V;
X2 is L, E, K, P or N, preferably E or L;
and
X4 is D, N, E, Q or H, preferably E.
Consensus #2b:
KKX~VTXIOQELDX14X1sX1sRXI~XleXz~XzsX2oXz1Xa2Xa3LY (SEQ ID
N0:31)
wherein
X~ is N, K, S, E, M, I or Q, preferably N;
X10 is V, A, L, F or I, preferably V;
X14 is L, Y, I, A, F or C, preferably Y;
Xls is Q, L, K or E, preferably K;
Xls is A, T, I or V, preferably I;
Xl~ is R, H, N or K, preferably K;
X18 is Y, F, I, L or Q, preferably Y;
X19 is Q, V, I, H, S, T or M, preferably V;
X2o is E, K, N, D, G, S or Q, preferably D;
X21 is K, N, D, R or I, preferably N;
X22 is Y, K, L, F or H, preferably K;
X23 is N, K, G or Q, preferably K; and
X2~ is L or Y, preferably L.
Peptides exemplified herein are CMYGGVTEHEGN (SEQ
ID NO: 3), CMYGGVTEHEGNGC* (SEQ ID NO: 5),
KKNVTVQELDYKIRKYLVDNKKLY (SEQ ID N0: 4),
CGKKNVTVQELDYKIRKYLVDNKKLYGC* (SEQ ID NO: 6),
CMYGGVTEHEGNKKNVTVQELDYKIRKYLVDNKKLY (SEQ ID NO: 7) and
CMYGGVTEHEGNKKNVTVQELDYKIRKYLVDNKKLYGC* (SEQ ID NO: 8),
wherein an asterisk indicates that the peptide is a
randomly cross-linked polymer. The exemplified polymer
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-25-
o peptides are at least 6,000 to 8,000 daltons. The
average size of the exemplified polymer peptides is
about 12,000 to 15,000 daltons. Small peptides and/or
contaminants may be removed by dialysis or other methods
available in the art. Similarly, larger aggregates may
be removed usin
g, e.g., a 0.25 micron filter, which can
also be used to sterilize the peptides.
Note that the amino acids cysteine and methionine,
"CM", are present at the amino terminus of the
exemplified region 1 peptides since those amino acids
are most often found in that position in nature. Note
also that the amino acids cysteine and glycine, "CG" and
"GC", are used at the amino and/or carboxy- termini of
some of the exemplified region 2 peptides. The amino
acid cysteine "C" is used to facilitate cross-linking
through the formation of disulfide bonds. The amino
acid glycine, "G", is used as a spacer residue.
The preferred peptides of the invention are those
which exclude full length native toxin molecules. The
preferred peptides of this invention are not toxic, but
toxic peptides maybe useful in this invention, for
example, in eliciting antibodies in a non-human system.
The most preferred peptides of the invention do not
contain amino acid sequences in the sequence in which
they are found in any particular native toxin molecule.
The present invention encompasses monomers of the
peptides derived from either one or both of the two
consensus regions described herein. These monomers may
comprise one or more sequences derived from either
region 1 or region 2 or both, such as consensus
sequences #1 and #2, preferably consensus sequences #la
and/or #2a, more preferably consensus sequences #lb
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-26-
o and/or #2b, most preferably one or more of the
exemplified consensus sequence peptides. If the monomer
contains more than one consensus sequence, these
sequences may be immediately adjacent to each other or
separated by a linker. In addition, different
orientations of the peptides are within the scope of
this invention. Furthermore, the order of the consensus
peptides within the full peptide may be variable.
The present invention also encompasses homogeneous
or heterogeneous polymers of the peptides disclosed
herein (e. g., concatenated, cross-linked and/or fused
identical peptide units or concatenated, cross-linked
and/or fused diverse peptide units), and mixtures of the
peptides, polymers, and/or conjugates thereof.
Linkers useful in the invention may, for example,
be simply peptide bonds, or may comprise amino acids,
including amino acids capable of forming disulfide
bonds, but may also comprise other molecules such as,
for example, polysaccharides or fragments thereof.
In the peptides exemplified herein, sequences
derived from consensus region 1 and consensus region 2
may be immediately adjacent to each other, linked by
peptide bonds, (see, era., SEQ ID N0:7) and/or connected
via amino acid linkers capable of forming di-sulfide
bonds via cysteine residues (see, e-a., SEQ ID N0: 8).
In the native toxin molecules, the sequences of region 1
and region 2 are separated by about 27 amino acids.
When the linkers are additional amino acids, they are
most preferably 1 to 27 amino acids in length, although
longer linkers may also be used in accordance with this
invention.
The linkers for use with this invention may be
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-27-
o chosen so as to contribute their own immunogenic effect
which may be either the same, or different, than that
elicited by the consensus sequences of the invention.
For example, such linkers may be bacterial antigens
which also elicit the production of antibodies to
infectious bacteria. In such instances, for example,
the linker may be a protein or protein fragment of an
infectious bacteria, or a bacterial polysaccharide or
polysaccharide fragment.
A peptide of the invention includes any substituted
analog or chemical derivative of a peptide derived from
one or both of the two consensus regions described
herein, most preferably of the exemplified peptides
described herein, so long as the peptide is capable of
inhibiting binding of staphylococcal and streptococcal
pyrogenic exotoxins to the MHC complex; inhibiting
blastogenesis of human mononuclear cells in the presence
of any one of the toxins; eliciting the production of
antibodies capable of binding to most of the
staphylococcal and streptococcal pyrogenic exotoxins; or
reacting with (i.e., specifically binding to) antibodies
that react with most of the staphylococcal and
streptococcal pyrogenic exotoxins. Therefore, a peptide
can be subject to various changes that provide for
certain advantages in its use. For example, D amino
acids can be substituted for L amino acids to increase
in vivo stability of the peptides, while still retaining
biological activity. See, e~a., Senderoff et al. (1998)
(95). Likewise, retro-inverso peptides, which contain
NH-CO bonds instead of CO-NH peptide bonds, are much
more resistant to proteolysis than L-peptides.
Moreover, they have been shown to mimic natural L-
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-28-
o peptides with respect to poly- and monoclonal antibodies
(48). Therefore, peptides having at least one D amino
acid on the amino terminal and/or carboxy terminal end
of the molecule and which retain biological activity are
considered part of the invention. In addition, retro-
inverso peptides which contain one or more of the amino
acid sequences of the invention and which retain
biological activity are also considered part of the
invention.
The peptides of the invention are useful for
providing active immunization for the prevention of
disease related to the deleterious effects of
staphylococcal and streptococcal pyrogenic exotoxins and
for preparation of antibodies as a passive immunization
therapy. When used to prepare antibodies, the peptides
are designed to induce antibodies which react with a
variety of staphylococcal and streptococcal pyrogenic
exotoxins (preferably with at least two, more preferably
with at least four, and most preferably with at least
seven of the pyrogenic exotoxins) for use in therapy to
increase resistance to, prevent and/or treat toxic shock
syndrome and septic shock.
The peptides may also be useful to protect against,
or ameliorate the effects of, autoimmune diseases which
are associated with, or are the result of, the presence
of staphylococcal or streptococcal exotoxins.
The peptides of the invention will also be useful
in diagnostic tests for detecting antibodies to
staphylococcal and streptococcal pyrogenic exotoxins.
The peptide may be mixed with an adjuvant. The
peptide also may be bound to a non-toxic non-host
Protein carrier to form a conjugate or it may be bound
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-29-
o to a saccharide carrier and/or a non-toxic non-host
protein carrier to form a conjugate.
The molecular weight of the peptide monomers having
one consensus sequence of the invention range from about
1000 to 5000 daltons. Such lower molecular weight
species of the invention are useful themselves to
inhibit superantigen induced T cell proliferation and/or
reduce, inhibit or eliminate the deleterious effects of
bacterial exotoxins in vivo, either when used alone or
in combination with another form of therapy, e.g.,
anticytokine antibodies.
Such lower molecular weight species of the
invention may also be useful as immunogens themselves
or, more preferably, may be used as haptens conjugated
to a larger carrier molecule, such as, for example, a
protein. As with other peptides, the molecular weight
of the peptide alone, or when conjugated to a carrier,
or in the presence of an adjuvant, is related to its
immunogenicity. Thus, the peptide may vary in molecular
weight in order to enhance its antigenicity or
immunogenicity. In an exemplified embodiment, the
molecular weight of the peptide, in polymeric form, is
greater than about 6000 to 8000 daltons, with an average
weight of 12,000 to 15,000 daltons. The total size of
the peptide is only limited to its ability to be
physiologically tolerated.
The invention also relates to isolated and purified
nucleic acid molecules which code for the peptides of
the invention to produce the encoded peptides. The
encoded peptides may be monomers, polymers or linked to
other peptide sequences (e. g., they may be fusion
proteins). Other features of the invention include
CA 02345023 2001-04-06
WO 00/20598 PC'T/US99/22180
-30-
o vectors which comprise the nucleic acid molecules of the
invention operably linked to promoters, as well as cell
lines, such as prokaryotic (e.g., E. coli) and
eukaryotic (e. g., CHO and COS) cells transfected with
the nucleic acid molecules of the invention. Vectors
and compositions for enabling production of the peptides
in vivo, i.e., in the individual to be treated or
immunized, are also within the scope of this invention.
The nucleic acids encoding the peptides of the
invention can be introduced into a vector such as a
plasmid, cosmid, phage, virus or mini-chromosome and
inserted into a host cell or organism by methods well
known in the art. In general, the vectors containing
these nucleic acids can be utilized in any cell, either
eukaryotic or prokaryotic, including mammalian cells
(e. g., human (e. g., HeLa), monkey (e. g., COS), rabbit
(e.g., rabbit reticulocytes), rat, hamster (e.g., CHO
and baby hamster kidney cells) or mouse cells (e.g., L
cells), plant cells, yeast cells, insect cells or
bacterial cells (e.g., E. coli). The vectors which can
be utilized to clone and/or express these nucleic acids
are the vectors which are capable of replicating and/or
expressing the nucleic acids in the host cell in which
the nucleic acids are desired to be replicated and/or
expressed. See, e.g., F. Ausubel et al., Current
Protocols in Molecular Biolocrv, Greene Publishing
Associates and Wiley-Interscience (1992) and Sambrook et
al. (1989) for examples of appropriate vectors for
various types of host cells. Strong promoters
compatible with the host into which the gene is inserted
may be used. These promoters may be inducible. The
host cells containing these nucleic acids can be used to
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-31-
o express large amounts of the protein useful in
pharmaceuticals, diagnostic reagents, vaccines and
therapeutics.
The nucleic acids could be used, for example, in
the production of peptides for diagnostic reagents,
vaccines and thera ies for
p pyrogenic exotoxin related
diseases. For example, vectors expressing high levels
of peptide can be used in immunotherapy and
immunoprophylaxis, after expression in humans. Such
vectors include retroviral vectors and also include
direct injection of DNA into muscle cells or other
receptive cells, resulting in the efficient expression
of the peptide, using the technology described, for
example, in Wolff et al., Science 247:1465-1468 (1990),
~"~olff et al. , Human Molecular Genetics 1 (6) :363-369
(1992) and Ulmer et al., Science 259:1745-1749 (1993).
See also, for example, WO 96/36366 and WO 98/34640.
In another embodiment of this invention antibodies
are provided which react with peptides of the invention,
as well as a variety of staphylococcal and streptococcal
pyrogenic exotoxins (preferably with at least two, more
preferably with at least four, and most preferably with
at least seven of the pyrogenic exotoxins). These
antibodies will be useful for passive immunization
therapy to increase resistance to or prevent toxic shock
syndrome or septic shock or other diseases related to
the presence of bacterial pyrogenic exotoxin. The
antibodies may also be useful to protect against, or
ameliorate the effects of, autoimmune diseases which are
associated with, or are the result of, the presence of
staphylococcal or streptococcal pyrogenic exotoxins.
The antibodies of the invention will also be useful in
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-32-
o diagnostic tests and kits for detecting the presence of
staphylococcal and streptococcal pyrogenic exotoxins.
These uses are discussed in more detail below.
Methods for t~reparincr peptides of the invention
The peptides of the invention may be prepared by
synthetic methods or by recombinant DNA methods, as
known in the art and as described herein.
Pharmaceutical Compositions
The pharmaceutical compositions of this invention
contain a pharmaceutically and/or therapeutically
effective amount of at least one peptide and/or carrier
thereof, antibody, or nucleic acid encoding a peptide of
this invention. In one embodiment of the invention, the
effective amount or peptide per unit dose is an amount
Sufficient to inhibit T-cell proliferation by
staphylococcal and/or streptococcal pyrogenic exotoxins.
In another embodiment of the invention, the effective
amount of peptide per unit dose is an amount sufficient
to prevent, treat or protect against the toxic effects
of bacterial toxins, including diarrhea and/or
cardiopulmonary depression or lethal shock. The
effective amount of peptide per unit dose depends, among
other things, on the species of mammal inoculated, the
body weight of the mammal and the chosen inoculation
regimen, as is well known in the art.
In such circumstances, inocula for a human or
similarly sized mammal typically contain peptide
concentrations of 100 to 500 mgs/kg, body weight of the
mammal per inoculation dose.
Preferably, the route of inoculation of the peptide
will be subcutaneous or intravenous. The dose is
administered at least once.
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-33-
o When the peptide of the invention is used as
immunogen, the pharmaceutical composition contains an
effective, immunogenic, amount of peptide of the
invention. The effective amount of peptide per unit
dose sufficient to induce an immune response depends,
among other things, on the species of mammal inoculated,
the body weight of the mammal and the chosen inoculation
regimen, as well as the presence or absence of an
adjuvant, as is well known in the art. Inocula
typically contain peptide concentrations of about 1
microgram to about 1000 micrograms per inoculation
(dose), preferably about 3 micrograms to about 100
micrograms per dose, most preferably about 5 micrograms
to 50 micrograms. The use of higher amounts is
envisaged. In Example 1, rabbits were injected twice
with 500 micrograms of polymeric peptide in the presence
of adjuvant. In Example 5,an example in which the
peptide is administered directly to prevent toxic or
septic shock, which may not be dependent on the
production of antibodies, mice were injected twice with
1.5 mg of monomer peptide for a total of 3 mgs.
Standard procedures to determine dose response
relationships known to those skilled in the art may be
used to determine optimum doses of peptide to be used
either to prevent or treat toxic or septic shock, or to
raise antibodies for its prevention or treatment.
The term "unit dose" as it pertains to the inocula
refers to physically discrete units suitable as unitary
dosages for mammals, each unit containing a
predetermined quantity of active material (e. g.,
peptide, antibody or nucleic acid) calculated to produce
the desired immunogenic effect in association with the
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-34-
o required diluent.
Inocula are typically prepared as a solution in a
physiologically acceptable carrier such as saline,
phosphate-buffered saline and the like to form an
aqueous pharmaceutical composition.
The peptides of the invention are generally
administered with a physiologically acceptable carrier
or vehicle therefor. A physiologically acceptable
carrier is one that does not cause an adverse physical
reaction upon administration and one in which the
antibodies are sufficiently soluble and retain their
activity to deliver a therapeutically effective amount
of the compound. The therapeutically effective amount
and method of administration of a peptide of the
invention may vary based on the individual patient, the
indication being treated and other criteria evident to
one of ordinary skill in the art. A therapeutically
effective amount of a peptide of the invention is one
sufficient to attenuate the dysfunction without causing
significant side effects such as non-specific T cell
lysis or organ damage. The routes) of administration
useful in a particular application are apparent to one
or ordinary skill in the art.
Routes of administration of the peptides include,
but are not limited to, parenteral, and direct injection
into an affected site. Parenteral routes of
administration include but are not limited to
intravenous, intramuscular, intraperitoneal and
subcutaneous. The route of inoculation of the peptides
of the invention is typically parenteral and is
preferably intramuscular, sub-cutaneous and the like.
The present invention includes compositions of the
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-35-
o peptides described above, suitable for parenteral
administration including, but not limited to,
pharmaceutically acceptable sterile isotonic solutions.
Such solutions include, but are not limited to, saline
and phosphate buffered saline for nasal, intravenous,
intramuscular, intraperitoneal, subcutaneous or direct
injection into a joint or other area.
A system for sustained delivery of the peptides of
the invention may also be used. For example, a delivery
system based on containing a peptide in a polymer matrix
of biodegradable microspheres may be used (57). One
such polymer matrix includes the polymer poly(lactide-
co-glycolide) (PLG). PLG is biocompatible and can be
given intravenously or orally. Following injection of
the microspheres into the body, the encapsulated protein
is released by a complex process involving hydration of
the particles and drug dissolution. The duration of the
release is mainly governed by the type of PLG polymer
used and the release of modifying excipients (4~).
The dose is administered at least once. When a
composition of the invention is used to induce
antibodies, at least one booster dose may be
administered after the initial injection, preferably at
about 4 to 6 weeks after the first dose, in order to
increase the antibody level. Subsequent doses may be
administered as indicated.
To monitor the antibody response of individuals
administered the compositions of the invention, antibody
titers may be determined. In most instances it will be
sufficient to assess the antibody titer in serum or
plasma obtained from such an individual. Decisions as
.to whether to administer booster inoculations or to
CA 02345023 2001-04-06
WO 00/20598 PCT/US99I22180
-36-
o change the amount of the composition administered to the
individual may be at least partially based on the titer.
The titer may be based on either an immunobinding
assay which measures the concentration of antibodies in
the serum which bind to a specific antigen, i.e. peptide
or toxin; or bactericidal assays which measure the
ability of the antibodies to participate with complement
in killing bacteria. The ability to neutralize in
vitro and in vivo biological effects of the pyrogenic
exotoxins may also be assessed to determine the
effectiveness of the treatment. See, e.g., the examples
herein.
Antibodies
The term "antibodies" is used herein to refer to
immunoglobulin molecules and immunologically active
portions of immunoglobulin molecules. Exemplary
antibody molecules are intact immunoglobulin molecules,
substantially intact immunoglobulin molecules and
portions of an immunoglobulin molecule, including those
portions known in the art as Fab, Fab', F(ab')z and F(v)
as well as chimeric antibody molecules.
An antibody of the present invention is typically
produced by immunizing a mammal with an immunogen or
vaccine containing one or more peptides of the
invention, or a structurally and/or antigenically
related molecule, to induce, in the mammal, antibody
molecules having immunospecificity for the immunizing
peptide or peptides. The peptides) or related
molecules) may be monomeric, polymeric, conjugated to a
carrier, and/or administered in the presence of an
adjuvant. The antibody molecules may then be collected
from the mammal if they are to be used in immunoassays
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-37-
0 or for providing passive immunity.
The antibody molecules of the present invention may
be polyclonal or monoclonal. Monoclonal antibodies may
be produced by methods known in the art. Portions of
immunoglobulin molecules may also be produced by methods
known in the art.
The antibody of the present invention may be
contained in various carriers or media, including blood,
plasma, serum (e. g., fractionated or unfractionated
serum), hybridoma supernatants and the like.
Alternatively, the antibody of the present invention is
isolated to the extent desired by well known techniques
such as, for example, by using DEAE Sephadex, or
affinity chromatography. The antibodies may be purified
so as to obtain specific classes or subclasses of
antibody such as IgM, IgG, IgA, IgGl, IgG2, IgG3, IgG4
and the like. Antibody of the IgG class are preferred
for purposes of passive protection.
The presence of the antibodies of the present
invention, either polyclonal or monoclonal, can be
determined by various assays. Assay techniques include,
but are not limited to, immunobinding,
immunofluorescence (IF), indirect immunofluorescence,
immunoprecipitation, ELISA, agglutination and Western
blot techniques.
The antibodies of the present invention have a
number of diagnostic and therapeutic uses. The
antibodies can be used as an in vitro diagnostic agent
to test for the presence of various staphylococcal and
streptococcal pyrogenic exotoxins in biological samples
in standard immunoassay protocols and to aid in the
diagnosis of various diseases related to the presence of
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-38-
o bacterial pyrogenic exotoxins. Preferably, the assays
which use the antibodies to detect the presence of
bacterial pyrogenic exotoxins in a sample involve
contacting the sample with at least one of the
antibodies under conditions which will allow the
formation of an immunological complex between the
antibody and the toxin that may be present in the
sample. The formation of an immunological complex if
any, indicating the presence of the toxin in the sample,
is then detected and measured by suitable means. Such
assays include, but are not limited to,
radioimmunoassays, (RIA), ELISA, indirect
immunofluorescence assay, Western blot and the like.
The antibodies may be labeled or unlabeled depending on
the type of assay used. Labels which may be coupled to
the antibodies include those known in the art and
include, but are not limited to, enzymes,
radionucleotides, fluorogenic and chromogenic
substrates, cofactors, biotin/avidin, colloidal gold and
magnetic particles. Modification of the antibodies
allows for coupling by any known means to carrier
proteins or peptides or to known supports, for example,
polystyrene or polyvinyl microliter plates, glass tubes
or glass beads and chromatographic supports, such as
paper, cellulose and cellulose derivatives, and silica.
Such assays may be, for example, of direct format
(where the labelled first antibody reacts with the
antigen), an indirect format (where a labelled second
antibody reacts with the first antibody), a competitive
format (such as the addition of a labelled antigen), or
a sandwich format (where both labelled and unlabelled
antibody are utilized), as well as other formats
CA 02345023 2001-04-06
WO 00/20598 PC'T/US99/22180
-39-
o described in the art. In one such assay, the biological
sample is contacted to antibodies of the present
invention and a labelled second antibody is used to
detect the presence of staphylococcal and streptococcal
pyrogenic exotoxins, to which the antibodies are bound.
The antibodies of the present invention are also
useful as therapeutic agents in the prevention and
treatment of diseases caused by the deleterious effects
of staphylococcal and streptococcal pyrogenic exotoxins.
The antibodies are generally administered with a
physiologically acceptable carrier or vehicle therefor.
A physiologically acceptable carrier is one that does
not cause an adverse physical reaction upon
administration and one in which the antibodies are
sufficiently soluble and retain their activity to
deliver a therapeutically effective amount of the
compound. The therapeutically effective amount and
method of administration of the antibodies may vary
based on the individual patient, the indication being
treated and other criteria evident to one of ordinary
skill in the art. A therapeutically effective amount of
the antibodies is one sufficient to inhibit superantigen
stimulation of T-cells and/or attenuate the dysfunction
caused by the presence of bacterial toxins without
causing significant side effects such as non-specific T
cell lysis or organ damage. The routes) of
administration useful in a particular application are
apparent to one or ordinary skill in the art.
Routes of administration of the antibodies include,
but are not limited to, parenteral, and direct injection
into an affected site. Parenteral routes of
administration include but are not limited to
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-40-
o intravenous, intramuscular, intraperitoneal and
subcutaneous.
The present invention includes compositions of the
antibodies described above, suitable for parenteral
administration including, but not limited to,
pharmaceutically acceptable sterile isotonic solutions.
Such solutions include, but are not limited to, saline
and phosphate buffered saline for nasal, intravenous,
intramuscular, intraperitoneal, subcutaneous or direct
injection into a joint or other area.
Antibodies for use to elicit passive immunity in
humans are preferably obtained from other humans
previously inoculated with compositions comprising one
or more of the consensus amino acid sequences of the
invention. Alternativel
y, antibodies derived from other
species may also be used. Such antibodies used in
therapeutics suffer from several drawbacks such as a
limited half-life and propensity to elicit an immune
response. Several methods have been proposed to
overcome these drawbacks. Antibodies made by these
methods are encompassed by the present invention and are
included herein. One such method is the "humanizing" of
non-human antibodies by cloning the gene segment
encoding the antigen binding region of the antibody to
the human gene segments encoding the remainder of the
antibody. Only the binding region of the antibody is
thus recognized as foreign and is much less likely to
cause an immune response. An article describing such
antibodies is Reichmann et al., "Reshaping Human
Antibodies for Therapy", Nature 332:323-327 (1988),
which is incorporated herein by reference. See also,
Queen et al., US Patent 5,585,089, which is incorporated
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-41-
o herein by reference.
In providing the antibodies of the present
invention to a recipient mammal, preferably a human, the
dosage of administered antibodies will vary depending
upon such factors as the mammal's age, weight, height,
sex, general medical condition, previous medical history
and the like.
In general, it is desirable to provide the
recipient with a dosage of antibodies which is in the
range of from about 5 mg/kg to about 20 mg/kg body
weight of the mammal, although a lower or higher dose
may be administered. In general, the antibodies will be
administered intravenously (IV) or intramuscularly (IM).
Intravenous immunoglobulin (IVIG) can generally be given
with a loadin dose of 200 m k
g g/ g, with monthly
injections of 100 mg/kg. High-dose IVIG may be given at
400-800 mg/kg, for antibody-deficient patients. See,
e.g., The Merck Manual of Diagnosis and Therapy, 16th
Edition, (Berkow R and Fletcher AJ, Eds.), Merck
Research Laboratories, Rahway, NJ (1992).
The peptides and/or antibodies of the present
invention are intended to be provided to the recipient
subject in an amount sufficient to prevent, or attenuate
the severity, extent or duration of the deleterious
effects of staphylococcal and streptococcal pyrogenic
exotoxins.
The administration of the agents including peptide
and antibody compositions of the invention may be for
either "prophylactic" or "therapeutic" purpose. When
provided prophylactically, the agents are provided in
advance of any symptom. The prophylactic administration
of the agent serves to prevent or ameliorate any
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-42-
o subsequent deleterious effects of staphylococcal and
streptococcal pyrogenic exotoxins. When provided
therapeutically, the agent is provided at (or shortly
after) the onset of a symptom of infection with bacteria
expressing staphylococcal or streptococcal pyrogenic
exotoxins. The a ent of the
g present invention may,
thus, be provided either prior to the anticipated
exposure to bacteria expressing staphylococcal or
streptococcal pyrogenic exotoxin (so as to attenuate the
anticipated severity, duration or extent of disease
symptoms) or after the initiation of the infection. The
agent may also be provided to individuals at high risk
for getting an infection with bacteria expressing
staphylococcal or streptococcal pyrogenic exotoxins.
Also envisioned are therapies based upon vectors,
such as viral vectors containing nucleic acid sequences
coding for the peptides described herein. These
molecules, developed so that they do not provoke a
pathological effect, will stimulate the immune system to
respond to the peptides.
For all therapeutic, prophylactic and diagnostic
uses, the peptide of the invention, alone or linked to a
carrier, as well as antibodies and other necessary
reagents and appropriate devices and accessories may be
provided in kit form so as to be readily available and
easily used.
Where immunoassays are involved, such kits may
contain a solid support, such as a membrane (e. g.,
nitrocellulose), a bead, sphere, test tube, rod, and so
forth, to which a receptor such as an antibody specific
for the target molecule will bind. Such kits can also
include a second receptor, such as a labelled antibody.
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-43-
o Such kits can be used for sandwich assays to detect
toxins. Kits for competitive assays are also
envisioned.
The following examples illustrate certain
embodiments of the present invention, but should not be
construed as limiting its scope in any way. Certain
modifications and variations will be apparent to those
skilled in the art from the teachings of the foregoing
disclosure and the following examples, and these are
intended to be encompassed by the spirit and scope of
the invention.
EXAMPLE 1
Peptides whose sequences are based on the two
highly conserved regions of the staphylococcal and
streptococcal pyrogenic exotoxins described herein were
constructed. The sequences were based on alignments of
the streptococcal pyrogenic exotoxins with the
staphylococcal enterotoxins, and the amino acids used in
positions with possible degeneracy were the amino acids
most frequently found in these positions. Three of the
peptides were then catenated and polymerized to produce
peptides of greater than 8000 daltons (i.e., peptides
6343, 6345 and 6348, described below). As described
further below, peptide 6348 was used to immunize
rabbits, which produced high titer antibodies to this
peptide. These antibodies were tested for the ability
to recognize the streptococcal and staphylococcal
pyrogenic exotoxins. Immunological assays (immunoblots)
revealed that these antibodies recognized regions common
to all the pyrogenic exotoxins. These antibodies were
also tested for the ability to neutralize in vitro and
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-44-
o in vivo biological activity of the pyrogenic exotoxins.
These antibodies protected against the biological T-cell
proliferation of these toxins in an in vitro
blastogenesis assay using human mononuclear cell
populations. The lethal effects of staphylococcal toxin
SEB and streptococcal pyrogenic toxin SPEA in vivo were
also completely blocked by mixing the antibodies with
the toxin prior to injection.
Materials and Methods
Construction of Synthetic Peptides:
Peptides were constructed by solid phase synthesis
(20) using the modifications described by Houghton (10).
1 . GCG Consensus #1 YGGXITXzX3X4XSN ( SEQ ID NO : 1 )
Peptide #1 CMYGGVTEHEGN (SEQ ID N0:3)
2. GCG Consensus #2 KXsX~XeX9XlaX11X1zX13DX14X1sX1sRXmXls
LXlgX2pX21X22X23X24Y ( SEQ ID NO : 2 )
peptide #2 KKNVTVQELDYKIRKYLVDNKKLY (SEQ
ID N0:4)
As is evident above, synthetic peptides #1 and #2
are not native peptides, i.e., their sequences differ
from those found in native toxins. Variations of these
peptides have also been constructed in order to generate
concatenated polymers of the peptides. These polymers
were constructed by the addition of glycine and of
additional cysteine residues to the amino- and/or
carboxyl- termini of the initial 2 peptides, thus
facilitating concatenation via disulfide bond formation
(37, 38, 39) . The polvmerizec~ mn1 Pr."1 A~ ,.,o,.,o +-~,o.,
dialyzed to remove molecules with molecular weights less
than 6000-8000 daltons. One polymeric construct is
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-45-
o composed of the monomer: CMYGGVTEHEGNGC (SEQ ID N0:5).
An additional polymer is composed of the peptide:
CGKKNVTVQELDYKIRKYLVDNKKLYGC (SEQ ID N0:6).
In the native toxin molecules, consensus region #1
precedes consensus region #2 by 27 amino acid residues
(e.g. [consensus region 1] x27 [consensus region 2]).
We have constructed the peptide:
CMYGGVTEHEGNKKNVTVQELDYKIRKYLVDNKKLY (SEQ ID N0:7).
Like the native toxin molecule, this peptide is
representative of the two consensus regions joined
together in the proper order (region 1 in the N terminal
half, and region 2 in the C-terminal half of the
molecule), however they are not separated by an
additional 27 residues as they are in the native toxins.
We have also constructed concatenated polymers based on
the monomer: CMYGGVTEHEGNKKNVTVQELDYKIRKYLVDNKKLYGC (SEQ
ID N0:8) .
ID Peptide
6343 CMYGGVTEHEGN (SEQ ID N0:3)
6344 CMYGGVTEHEGNGC* (SEQ ID N0:5)
6345 KKNVTVQELDYKIRKYLVDNKKLY (SEQ ID N0:4)
6346 CGKKNVTVQELDYKIRKYLVDNKKLYGC* (SEQ ID N0:6)
6347 CMYGGVTEHEGNKKNVTVQELDYKIRKYLVDNKKLY (SEQ ID
N0:7)
6348 CMYGGVTEHEGNKKNVTVQELDYKIRKYLVDNKKLYGC* (SEQ ID
N0:8)
Peptides with an (*) are cross-linked polymers composed
of the described sequence. It is expected that monomers
of these peptides will also be useful in the present
invention.
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-46-
o Generation of anti-peptide sera.
New Zealand White rabbits were immunized by
subcutaneous injection with 500 ~.g of peptide in
complete Freund's adjuvant. Additional booster
injections of 500 ~,g in incomplete adjuvant was
administered 4 weeks after the primary injections. Ten
days after booster injections, the rabbits were bled,
and the anti-peptide titers were determined by ELISA.
Staphylococcal enterotoxins, TSST-1, and
j0 streptococcal pyrogenic exotoxins were purchased from
Toxin Technology Inc. (Sarasota, FL).
Immunoblots
Each of the staphylococcal and streptococcal
pyrogenic exotoxins were electrophoresed through 10°s SDS
15 pAGE gels (16) and transferred to nitrocellulose for
western blots (33). The western blots were developed
using the rabbit anti-peptide 6348 serum (anti-pep 6348
or AP6348) diluted 1:5000, followed by goat anti-rabbit
20 (IgG) alkaline phosphate conjugate (Sigma).
Inhibition of blastoaenesis
Human peripheral blood mononuclear cell (PBMC)
preparations were stimulated by each of the
staphylococcal enterotoxins and streptococcal pyrogenic
exotoxins. 100 ng of toxin was used to stimulate PBMC
preparations at cell concentrations of 105 cells per
well in 96 well microliter plates. Phytohemagglutinin
(PHA) was used in place of the toxins as a positive
mitogenic control. Cell culture medium was supplemented
with either 10% normal rabbit serum (NRS) or AP6348
serum. Blastogenesis was assayed by incorporation of
tritiated thymidine after 5 days of culture (22). All
experiments were performed in triplicate.
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-47-
o Passive protection of rabbits
Female New Zealand White rabbits >lyr old were
obtained from Hazelton Dutchland Labs, Inc. (Denver,
PA). Rabbits were challenged with staphylococcal or
streptococcal toxins at doses ranging from 50 to 100
~Cg/kg, as previously described (24). Briefly, pyrogenic
toxins were incubated with either 200 ul of normal
rabbit serum or 200 ul of anti-pep #6348 serum for one
hour prior to challenge. Toxin-serum mixtures were
administered intravenously through the marginal ear
veins. Normal control rabbits were treated in an
identical manner, with isotonic saline substituted for
the pyrogenic toxin. Four hours later, rabbits were
given a sub-lethal dose (5 ~g/kg) of endotoxin (E. coli
LPS, List Biological Laboratories, Inc., Campbell, CA).
Rabbits were monitored 72 h for clinical signs of toxic
shock. These included elevated temperature, diarrhea,
cardiopulmonary distress, and conjunctival injection.
Rabbits with severe toxic shock exhibiting cyanosis and
temperatures less than 97°F were declared moribund.
Moribund rabbits were euthanized by administration of 5
ml pentobarbital sodium. All animal protocols were
reviewed by the Laboratory Animal Research Center at the
Rockefeller University.
Results
ELISA assays
As seen in Figure 4, rabbits raised significant
antibody titers to peptide 6348. Similarly, rabbits
receiving immunizations with peptides 6344 and 6346 also
developed high titers.
CA 02345023 2001-04-06
wo oonos9s Pc r~s99n2~so
-4$-
o Recognition of staphylococcal and
streptococcal toxins by anti-pep 6348 serum
Western blots of the staphylococcal and
streptococcal toxins were developed with anti-peptide
6348 serum followed by an anti-rabbit IgG alkaline
phosphatase conjugate (Sigma). The results of the
western blot shown in Figure 5 indicate the anti-peptide
6348 serum recognizes the conserved regions of the
bacterial toxin molecules; SEA, SEB, SED, SEE, SPEA, and
TSST-1. SEC did not show a significant reaction with
anti-peptide 6348.
Blastogenesis inhibition
The percentage of inhibition, of toxin mediated
blastogenesis, by AP6348 was assayed. Tritiated
thymidine incorporation by human PBMC stimulated with
staphylococcal and streptococcal pyrogenic toxins was
significantly inhibited by the addition of AP6348
compared to normal rabbit serum (NRS) (Figure 6). This
suggests blastogenesis of PBMC in response to the toxins
was inhibited by AP6348. The AP6348 serum did not
affect the blastogenesis of human PBMC in response to
PHA, suggesting a specific inhibition of toxin biologic
activity.
In vivo protection of rabbits
We tested the ability of AP6348 serum to prevent
severe toxic shock in rabbits challenged with SEB and
NRS. Rabbits challenged intravenously with a mixture of
SEB and NRS developed symptoms of severe toxic shock
(Table 2). One rabbit receiving 50 ~g/kg SEB with NRS,
and two receiving 100 ~g/kg of SEB with NRS, developed
severe toxic shock and were declared moribund within 30
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-49-
o hrs. In contrast, two rabbits challenged with 50 ~g/kg
and 100 ~g/kg SEB with AP6348 developed fever, but this
returned to normal by 32 hours. No diarrhea or
cardiopulmonary depression was observed. Rabbits were
followed for a total of 5 days (data not shown) and
appeared fully recovered.
15
25
35
CA 02345023 2001-04-06
WO 00/20598 PCT/US99I22180
-50-
0
Table 2
Passive Protection of Rabbits Challenged with SEB SPEA and
LPS
Toxin LPS Diarrhea
Temperature F
~.g/kg\Serum ~g/kg 0 hr 4 hr 24 hr 32 hr 48 hr
SEB
nsf\NRS 5 - 100.4 102 101.4 101.2 NT
50\NRS 5 + 101 104.4 102.8 960
100\NRS S + 102 104.6 103 970
100\NRS 5 + 101 104.5 102.6 970
50\APS 5 - 101.4 103.8 103 102 101
100\APS 5 - 100.4 104.4 103 102 101
IS SPEA
50\NRS 5 + 101 104.2 NTO
100\NRS 5 + 102 104.8 NT0
50\APS 5 102 104 103 102 102
100\APS 5 + 101.6 104.4 104 100 970
ns =control rabbit isotonic saline in place of SEB or
given SPEA
NRS=Normal rabbit serum
APS=Anti-peptide 6348
serum
0=animals were declaredmoribund
NT=not taken
Discussion
Our results demonstrate
that antibodies rabbit
antiserum generated to peptides representative of two
regions with highly conserved amino acid sequences
(AP6348) are capable of recognizing most of the
staphylococcal enter otoxins and streptococcal pyrogenic
exotoxins (e. g. SEA, SEB, SEC, SEE, SPEA, SPEC), as well
as TSST-1, using Wes tern blots. We expect that other
,
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-51-
o more sensitive assays, will result in the demonstration
of binding of these antibodies to additional members,
probably all members, of the staphylococcal and
streptococcal pyrogenic toxin family.
Since recognition of the toxins by AP6348 was
successful, we tested this serum for the ability to
inhibit the biological effects of these pyrogenic
toxins. AP6348 was capable of inhibiting in vitro
blastogenesis of human PBMCs by many of the pyrogenic
toxins (e. g., SEA, SEB, SEC, SEE, SPEA, and SPEC).
AP6348 was also able to provide passive in vivo
protection of animals challenged with lethal doses of
SEB and SPEA. These animals developed fever, however
the fever returned to normal within 30 hours and
remained normal. Rabbits appeared to be fully recovered
within days of challenge.
In contrast, rabbits receiving similar doses of SEB
and SPEA pre-incubated with NRS developed severe toxic
shock as evidenced by high fevers, diarrhea, and
cardiopulmonary distress. The illness progressed and
these animals were declared moribund.
The therapeutic and biological implications of
these observations are as follows: (i) antibodies
prepared against this peptide may be administered during
the early stages of toxic shock or septic shock
irrespective of the toxin causing the symptoms and (ii)
the peptide may be used as an immunogen to block the
toxic effects of this family of superantigens.
EXAMPLE 2
PBMCs were isolated via Ficoll-Hypaque Solution.
The appropriate concentration of nonpolymeric
peptide
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-52-
o and 2x105 cells in 200~,L of RPMI solution was plated in
each well. The cells were incubated for one hour at 37
degrees Centigrade, with mild agitation every 15
minutes. After one hour, 2~,g of SEB was added in each
well. The PBMCs were incubated for 72 hours and the
results were measured via tritiated thymidine
incorporation. The cells were collected and read on a
beta counter. The results are shown in Figure 7. Note
the dose-response inhibition of blastogenesis
demonstrated in Figure 7A. Peptide 6343 (i.e.,
CMYGGVTEGEGN, SEQ ID N0:3)(Figure 7A) showed more
inhibitory activity of SEB than peptide 6346 (i.e.,
CGKKNVTVQELDYKIRKYLVDNKKLYGC, SEQ ID N0:6) (Figure 7B)
or peptide 6348 (i.e.,
CMYGGVTEHEGNKKNVTVQELDYKIRKYLVDNKKLYGC, SEQ ID N0:8)
(Figure 7C).
EXAMPLE 3
PBMCs were isolated via Ficoll-Hypaque Solution.
150~.g of nonpolymeric 6343 peptide (i.e., CMYGGVTEGEGN,
SEQ ID N0:3) and 2x105 cells in 200~L of RPMI solution
was plated in each well. The cells were incubated for
one hour at 37 degrees Centigrade, with mild agitation
zs
every 15 minutes. After one hour, 2~.g of either SEB,
SEC, SED, SPEC, SPEA, or TSST-1 was added to each well.
The PBMCs were incubated for 72 hours and the results
were measured via tritiated thymidine incorporation.
The cells were collected and read on a beta counter.
All experiments were run in triplicate. The results are
shown in Figure 8. Note that peptide 6343 inhibited
blastogenesis of PBMCs by all of the superantigens
tested.
CA 02345023 2001-04-06
WO 00/20598 PCTNS99/22180
-53-
0
EXAMPLE 4
Two-hit Septic Shock Model
Based on the two hit septic shock hypothesis
described in the background and Figure 2 we have created
a model of septic shock. While the amounts of either
SPEA or SEB superantigens used in the rabbit model were
relatively high, the amounts used in the mouse model
were much lower due to D-galactosamine priming, size of
animals and synergy between the toxins and LPS. BALB/c
mice challenged intra-peritoneally after priming with D-
galactosamine (20 mg/mouse) concurrently with LPS
followed by SEB, showed that extremely small amounts of
LPS and SEB were needed to effect lethality (46). The
synergy between these two mediators of shock was
extremely impressive and extended for at least an 18
hour period. We chose an 8 hour delay between the two
toxins for our model. We established and optimized doses
of toxin for SEA, SEB, SPEA, SPEC, and TSST-1 that would
lead to 100% lethality. The doses of the various toxins
are shown in Table 3.
Table 3: Doses of various toxins and LPS/ D-
galactosamine used in the lethal two hit septic shock
model
Toxin (~.g) SEA SPEA SEB SPEC TSST-1
2.5 2.0 0.02 2.5 2.0
LPS (~.~g) 0.001 0.001 0.001 0.001 0.001
D-galactosamine 20 20 20 20 20 mg
(mg)
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-54-
EXAMPLE 5
All mice were sensitized with 0,001 ~,g
Lipopolysaccharide (LPS) and 20 mg of D-Galactosamine
via intraperioneal injection. The results are shown in
Table 4. After six hours, saline or 1.5 mg of the
non of meric
p y peptide 6343 was administered to the
experimental mice by subcutaneous injection. One hour
later, the mice were injected again with either saline
or 1.5 mg peptide (3.0 mg total). One hour later, all
mice were challenged with 0.02 ~.g SEB, SPEA or TSST-1
(via intraperitoneal injection) and the mice were
observed overnight. In this model, it has been observed
that peptide 6343, given one and two hours before
administration of the toxic dose of the indicated toxin,
protected 5 out of 6 mice exposed to toxin SEB; 2 out of
2 mice exposed to the toxin SPEA and 2 out of two mice
exposed to the toxin TSST-1.
Table 4
The Use of Peptide 6343 to Block the Superantigen
Induction of Septic Shock in a Mouse Model
Mice Dose A me Tota A me Tota A me Tota
and AdministrationSEB SPEA TSST-1
ControlSaltine 0 6 (0% 0 2 0%) 0 2 (0%)
subcutaneous
injection
Peptide 5 6 83%) 2 2 (100% 2 2 (100%
3.0
mg
6343
subcutaneous
injection
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-55-
0
EXAMPLE 6
Two streptococcal antigens SEG and SEH have
recently been synthesized and three new streptococcal
exotoxins, i.e., SPEG, SPEH and SPEZ, have recently been
described by Dr. Fraser and colleagues (61).
In order to determine whether 6343 peptide is
capable of inhibiting the toxic effects of the
streptococcal exotoxins SPEG, SPEH and SPEZ, experiments
similar to those described above were conducted.
In a similar manner as above, PBMCs were isolated
via Ficoll-Hypaque Solution. Either O~g, 75~Cg, 100~g or
150~,g of nonpolymeric 6343 peptide (i.e., CMYGGVTEGEGN,
SEQ ID N0:3) and 2x205 cells in 200~L of RPMI solution
IS was plated in each well. The cells were incubated for
one hour at 37 degrees Centigrade, with mild agitation
every 15 minutes. After one hour, 2~.g of the
recombinant forms of either SPEG, SPEH, SPEZ, which were
provided by Dr. Fraser of the University of Auckland,
New Zealand, was added to each well. The PBMCs were
incubated for 72 hours and the results were measured via
tritiated thymidine incorporation. The cells were
collected and read on a beta counter. The results are
shown in Figure 9. Note that peptide 6343 inhibited
blastogenesis of PBMCs by the bacterial superantigens
SPEG, SPEH and SPEZ.
EXAMPLE 7
To determine the nature of the inhibition of
superantigen stimulation by the inhibitory peptides and
antigens, purified MHC class II molecules (obtained from
J. Strominger, Harvard University) were used in a
competitive ELISA to determine binding to this molecule.
CA 02345023 2001-04-06
WO 00/20598 PCT/U599/22I80
-S 6-
o The purified MHC were immobilized on a 95 well plate and
4~.g SEB was added. After appropriate washing a rabbit
monoclonal anti-SEB antibody (Toxin Tech) was added to
the ELISA followed by a colorimetric reagent. The plate
was read on an ELISA plate reader. In similar
experiments various concentrations of the peptide 6343
( i . a . , O~g, 50~.g, 75~.g, 100~,g, or 150~Cg) were added to
the immobilized MHC before the addition of SEB. Binding
of the peptide to the MHC was demonstrated by the
decreased amount of SEB bound to the MHC, as indicated
by a decreased amount of antibody binding measured by a
decreased color reaction. The results are shown in
Figure 11. The results indicate that peptide 6343 binds
very strongly to the MHC complex and is able to compete
effectively for the site of SEB binding.
The binding of the peptide to the MHC complex of
monocytes was supported by con-focal microscopy results
of experiments using a method described in Ojcius et al.
(68) using fluorinated peptides constructed for us by
NEN LifeSciences, antibodies directed against MHC class
II proteins, and a phycoerythrin (PE) antibody directed
to the anti-MHC antibody. Various doses of the peptide
and various concentrations of the cells were titrated to
achieve optimum binding of the peptide. Confocal
microscope pictures (Figure 11) show similar patterns of
binding for anti-MHC antibodies and for the flourinated
peptides, supporting the determination that the peptide
binds to the cells' MHC complex.
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-57-
° DISCUSSION
In the experiments described herein, the dose of
peptide 6343 used directly for the prevention of toxic
and septic shock was 3 mgs per mouse. Thus, the direct
use of peptides in septic or toxic shock would be
expected to involve doses in the range of several grams
for the acute treatment of shock in humans. Peptide
6343, possibly because of its small size, does not
induce detectable antibodies in rabbits and mice, yet it
still has all the therapeutic properties described for
the anti-peptide antibodies. Therefore, if desired, it
is expected that the peptide can be used repeatedly in
the same individual without raising anti-peptide
antibodies.
20
30
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-58-
o References
1. Bergdoll, M. S. 1985. The
staphylococcal enterotoxins-an update., 247-254. In J.
Jeljaszewicz (ed.). The staphylococci. Gustav Fischer
Verlag, New York, NY.
2. Blomster-Hautamaa, D. A., B. N.
Kreiswirth, J. S. Kornblum, R. P. Novick and P. M.
Schlievert. 1986. The nucleotide and partial amino
acid sequence of toxic shock syndrome toxin-1. Journal
of Biological Chemistry. 261:15783-15786.
3. Choi, Y., B. Kotzin, L. Herron, J.
Callahan, P. Marrack and J. Kappler. 1989. Interaction
of staphylococcus aureus toxin superantigens with human
IS T cells. Proc. Natl. Acad. Sci. USA. 86:8941.
4. Fleischer, B. and H. Schrezenmeier.
1988. T cell stimulation by staphylococcal
enterotoxins. Clonally variable response and requirement
for major histocompatibility complex class II molecules
on accessory or target cells. Journal of Experimental
Medicine. 167:1697.
5. Goshorn, S. C. and P. M. Schlievert.
1988. Nucleotide sequence of streptococcal exotoxin
tYPe C. Infect. Immun. 56:2518-2520.
6. Grossman, D., M. Van, J. A. Mollick, S.
K. Highlander and R. R. Rich. 1991. Mutation of the
disulfide loop in staphylococcal enterotoxin A.
Consequences for T cell recognition. J. Immunol.
147:3274-3281.
7. Hartwig, U. F. and B. Fleisher. 1993.
Mutations affecting MHC class II binding of the
superantigen streptococcal erythrogenic toxin A.
International Immunology. 5:869-875.
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-59-
8. Hauser, A. R., D. L. Stevens, E. L.
Kaplan and P. M. Schlievert. 1991. Molecular analysis
of pyrogenic exotoxins from streptococcus pyogenes
isolates associated with toxic shock-like syndrome.
Journal of Clinical Microbiology. 29:1562-1567.
9. Hensler, T., M. Koller, C. Geoffroy, J.
E. Alouf and W. Konig. 1993. Staphylococcus aureus
toxic shock syndrome toxin 1 and streptococcus pyogenes
erythrogenic toxin A modulate inflammatory mediator
release from human neutrophils. Infect. Immun.
61:1055-1061.
10. Houghten, R.A. 1985. General method for
the rapid solid phase synthesis of large numbers of
peptides: specificity of antigen-antibody interactions
at the level of individual amino acids. Proc. Natl.
Acad. Sci. USA. 82:5131-5135.
11. Hynes, W. L., C. R. Weeks, J. J. Iandolo
and J. J. Ferretti. 1987. Immunologic cross-reactivity
of type A streptococcal exotoxin (erythrogenic toxin)
and staphylococcal enterotoxins B and C1. Infect.
Immun. 55:837-840.
12. Janeway, C. A. J., J. Yagi, P. J. Conrad,
M. E. Katz, B. Jones, S. Vroegop and S. Buxser. 1989.
T-cell responses to Mls and to bacterial proteins that
mimic its behavior. Immunology Reviews. 107:61.
13. Johnson, L. P., J. J. L~Italien and P. M.
Schlievert. 1986. Streptococcal pyrogenic exotoxin
type A (scarlet fever toxin) is related to
staphylococcus aureus enterotoxin B. Molecular and
General Genetics. 203:354-356.
14. Kappler, J., B. L. Kotzin, L. Herron, E.
W- Gelfand, R. D. Bigler, A. Boylston, S. Carrell, D. N.
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-60-
° Posnett, Y. Choi and P. Marrack. 1989. V(3-specific
stimulation of human T-cells by staphylococcal toxins.
Science. 248:705.
15. Kappler, J. W., A. Herman, J. Clements
and P. Marrack. 1992. Mutations defining functional
regions of the superantigen staphylococcal enterotoxin
B. Journal of Experimental Medicine. 175:387-396.
16. Laemmli, U. K. 1970. Cleavage of
structural proteins during the assembly of the head of
bacteriophage T4. Nature. 227:680.
17. Leonard, B. A. and P. M. Schlievert.
1992. Immune cell lethality induced by streptococcal
pyrogenic exotoxin A and endotoxin. Infect. Immun.
60:3747-3755.
18. Marrack, P., M. Blackman, E. Kushnir and
J. Kappler. 1990. The toxicity of staphylococcal
enterotoxin B in mice is mediated by T cells. Journal
of Experimental Medicine. 171:455.
19. Marrack, P. and J. Kappler. 1990. The
staphylococcal enterotoxins and their relatives.
Science. 248:705-711.
20. Merrifield, R.B. 1963. Solid-phase
peptide synthesis I. The synthesis of a tetrapeptide. J.
Am. Chem. Soc. 85:2149-2154.
21. Musser, J. M., A. R. Hauser, M. H. Kim,
P. M. Schlievert, K. Nelson and R. K. Selander. 1991.
Streptococcus pyogenes causing toxic-shock-like syndrome
and other invasive diseases: clonal diversity and
pyrogenic exotoxin expression. Proceedings of the
National Academy of Sciences of the United States of
America. 88:2668-2672.
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-61-
22. Read, S. E., H. F. M. Reid, V. A.
Fischetti, T. Poon-King, R. Ramkissoon, M. McDowell and
J. B. Zabriskie. 1986. Serial studies on the cellular
immune response to streptococcal antigens in acute and
convalescent rheumatic fever patients in Trinidad.
Journal of Clinical Immunology. 6:433-441.
23. Reda, K. B., V. Kapur, J. A. Mollick, J.
G. Lamphear, J. M. Musser and R. R. Rich. 1994.
Molecular characterization and phylogenetic distribution
of the streptococcal superantigen gene (ssa) from
streptococcus pyogenes. Infect. Immun. 62:1867-1874.
24. Ren, K., J. D. Barman, V. Pancholi, A. L.
Cheung, J. C. Robbins, V. A. Fischetti and J. B.
Zabriskie. 1994. Characterization and biological
properties of a new staphylococcal exotoxin. Journal of
Experimental Medicine. 180:1675-1683.
25. Smith, R. J., P. M. Schlievert, I. M.
Himelright and L. M. Baddour. 1994. Dual infections
with staphylococcus aureus and streptococcus pyogenes
causing toxic shock syndrome. Possible synergistic
effects of toxic shock syndrome toxin 1 and
streptococcal pyrogenic exotoxin C. Diagnostic
Microbiology & Infectious Disease. 19:245-247.
26. Spero, L., B. Morlock and J. Metzger.
1978. On the cross-reactivity of staphylococcal
enterotoxins A, B, and C. J. Immunol. 120:86-89.
27. Spero, L. and B. A. Morlock. 1978.
Biological activities of the peptides of staphylococcal
enterotoxin C formed by limited tryptic hydrolysis.
Journal of Biological Chemistry. 253:8787-8791.
28. Spero, L. and B. A. Morlock. 1979.
Cross-reactions between tryptic polypeptides of
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-62-
o staphylococcal enterotoxins B and C. J. Immunol.
122:1285-1289.
29. Stelma, G. N., Jr. and M. S. Bergdoll.
1982. Inactivation of staphylococcal enterotoxin A by
chemical modification. Biochemical and Biophysical
Research Communications. 105:121-126.
30. Stevens, D. L., M. H. Tanner, J. Winship,
R. Swarts, K. M. Ries, P. M. Schlievert and E. Kaplan.
1989. Severe group A streptococcal infections
associated with a toxic shock-like syndrome and scarlet
fever toxin A. The New England Journal of Medicine.
321:1-7.
31. Sugiyama, H., E. M. J. McKissic, M. S.
Bergdoll and B. Heller. 1964. Enhancement of bacterial
endotoxin lethality by staphylococcal enterotoxin. J.
Infect. Dis. 114:111-118.
32. Swaminathan, S., W. Furey, J. Pletcher
and M. Sax. 1992. Crystal structure of staphylococcal
enterotoxin B, a superantigen. Nature. 359:801-806.
33. Towbin, H., T. Staehlin and J. Gordon.
1979. Electrophoretic transfer of proteins from
polyacrylamide gels to nitrocellulose sheets. Procedure
and some applications. Proceedings of the National
Academy of Sciences, USA. 76:4350.
34. Van den Bussche, R. A., J. D. Lyon and G.
A. Bohac. 1993. Molecular evolution of the
staphylococcal and streptococcal pyrogenic toxin gene
family. Molecular Phylogenetics and Evolution.
2:281-292.
35. Weeks, C. R. and J. J. Ferretti. 1986.
Nucleotide sequence of the type A streptococcal exotoxin
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-63-
o (erythrogenic toxin) gene from streptococcus pyogenes
bacteriophage T12. Infect. Immun. 52:144-150.
36. White, J., A. Herman, A. M. Pullen, R.
Kubo, J. W. Kappler and P. Marrack. 1989. The V
beta-specific superantigen staphylococcal enterotoxin B:
stimulation of mature T cells and clonal deletion in
neonatal mice. Cell. 56:27-35.
37. Patarroyo, M.E., R. Amador, P. Clavijo,
A. Moreno, F. Guzman, P. Romero, R. Tascon, A. Franco,
L.A. Murillo, G. Ponton and G. Trujillo. 1988. A
synthetic vaccine protects humans against challenge with
Plasmodium falciparum malaria. Nature. 332:158-161.
38. Lopez, M.C., Y. Silva, M.C. Thomas, A.
Garcia, M.J. Faus, P. Alonso, F. Mart:inez, G. Del Real
and C. Alonso. 1994. Characterization of SPf(66)n: a
chimeric molecule used as a malaria vaccine. Vaccine.
12:585-591.
39. Rodriguez, R., A. Moreno, F. Guzman, M.
Calvo and M.E. Patarroyo. 1990. Studies in owl monkeys
leading to the development of a synthetic vaccine
against the asexual blood stages of Plasmodium
falciparum. Am. J. Trop. Med. Hyg. 43:339-354.
40. Hoffman, M.L., L.M. Jablonski, K.K. Crum,
S.P. Hackett, Y.-I. Chi, C.V. Stauffacher, D.L. Stevens
and G.A. Bohach. 1994. Predictions of T-cell receptor
and Major Histocompatibility Complex-binding sites on
staphylococcal enterotoxin C1. Infection and Immunity.
62:3396-3407.
41. Acharya, K.R., E.F. Passalacqua, E.Y.
Jones, K. Harlos, D.I. Stuart, R.D. Brehm and H.S.
Tranter (1994). ~~Structural basis of superantigen action
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-64-
o inferred from crystal structure of toxic-shock syndrome
toxin-l." Nature 367: 94-97.
42. DELETED
43. Barman, J.D., F. Mingo, A. Viteri and
J.B. Zabriskie (1997). "Neutralization of streptococcal
pyrogenic exotoxins and staphylococcal enterotoxins by
antisera to synthetic peptides representing conserved
amino acid motifs." Adv Exp Med Biol 418: 903-907.
44. Bartus, R.T., M.A. Tracy, D.F. Emerich
IO and S.E. Zale (1998). "Sustained delivery of proteins
for novel therapeutic agents." Science 281(5380): 1161
2.
45. Baumgartner, J.D. (1990). "Monocolonal
anti-endotoxin antibodies for the treatment of gram-
negative bacteremia and septic shock." Eur J Clin
Microbiol Infect Dis 9(10): 711-6.
46. Blank, C., A. Luz, S. Bendigs, A.
Erdmann, H. Wagner and K. Heeg (1997)."Superantigens and
endotoxin synergize in the induction of lethal shock."
Eur J Immunol 27(4): 825-833.
47. Bohach, G.A., C.J. Hovde, J.P. Handley
and P.M. Schlievert (1988). "Cross-Neutralizaton of
staphylococcal and streptococcal pyrogenic toxins by
monoclonal and polyclonal antibodies." Infect Immun
56 (2) : 400-404.
48. Chorev, M. and M. Goodman (1995). "Recent
developments in retro peptides and proteins--an ongoing
topochemical exploration." Trends Biotechnol 13(10):
438-45.
49. Cohen, L., B. David and J.M. Cavaillon
(1991). "Interleukin-3 enhances cytokine production by
LSP-stimulated macrophages." Immunol Lett 28(2): 121-6.
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-65-
50. Edwin, C., S.R. Tatini and S.K.
Maheswaran (1986). "Specificity and cross-reactivity of
staphylococcal enterotoxin A monoclonal antibodies with
enterotoxins B, Cl, D, and E." App Environ Micro 52(6):
1253-7.
51. Fleischer, B., D. Gerlach, A. Fuhrmann
and K.H. Schmidt (1995). "Superantigens and
pseudosuperantigens of gram-positive cocci." Med Micro
Immunol 184(1): 1-8.
52. Glauser, M.P. (1996). "The inflammatory
cytokines. New developments in the pathophysiology and
treatment of septic shock." Drugs 52 Suppl 2: 9-17.
53. Griggs, N.D., C.H. Pontzer, M.A. Jarpe
and H.M. Johnson (1992). "Mapping of multiple binding
domains of the superantigen staphylococcal enterotoxin A
for HLA." J Immunol 148(8): 2516-2521.
54. Grossrnan, D., R.G. Cook, J.T. Sparrow,
J.A. Mollick and R.R. Rich (1990). "Dissociatioin of the
stimulatory activities of staphylococcal enterotoxins
for T cells and monocytes." J Exp Med 172(6): 1831-1841.
55. Hoffmann, M.L., L.M. Jablonski, K.K.
Crum, S.P. Hackett, Y.-I. Chi, C.V. Stauffacher, D.L.
Stevens and G.A. Bohach (1994). "Predictions of T-cell
receptor and major histocompatibility complex-binding
sites on staphylococcal enterotoxin C1." Infect Immun
62: 3396-3407.
56. Howe, L.M. (1998). "Treatment of
endotoxic shock: glucocorticoids, lazaroids,
nonsteroidals, others." Vet Clin North Am Small Anim
Pract 28(2): 249-67.
57. Jeong, B., Y.H. Bae, D.S. Lee and S.W.
Kim (1997). "Biodegradable block copolymers as
CA 02345023 2001-04-06
WO OOI20598 PCT1US99/22180
-66-
o injectable drug-delivery systems." Nature 388(6645):
860-2.
58. Jett, M., R. Neill, C. Welch, T. Boyle,
E. Bernton, D. Hoover, G. Lowell, R.E. Hunt, S.
Chatterjee and P. Gemski (1994). "Identification of
staphylococcal enterotoxin B sequences important for
induction of lymphocyte proliferation by using synthetic
peptide fragments of the toxin." Infect Imun 62(8):
3408-3415.
59. Mollick, J.A., R.L. McMasters, D.
Grossman and R.R. Rich (1993). "Localization of site on
bacterial superantigens that determines T cell receptor
beta chain specificity." J Exp Med 177(2): 282-293.
60. Pontzer, C.H., N.D. Griggs and H.M.
Johnson (1993). "Agonist properties of a microbial
superantigen peptide." Biochem Biophys Res Commun
193(3): 1191-1197.
61. Proft, T., S.L. Moffat, C.J. Berkahn and
J.D. Fraser (1999). "Identification and Characterization
of Novel Superantigens from Streptococcus pyogenes." J
Exp Med. 189(1):89-102.
62. Schlievert, P.M., G.A. Bohach, D.H.
Ohlendorf, C.V. Stauffacher, D.Y. Leung, D.L. Murray,
C.A. Earhart, L.M. Jablonski, M.L. Hoffmann and Y.I. Chi
(1995). "Molecular structure of staphylococcus and
streptococcus superantigens." J Clin Immnol
15(69supppl.)): 4s-lOs.
63. Schoenberg, M.H., M. Weiss and P.
Radermacher (1998). "Outcome of patients with sepsis and
septic shock after ICU treatment." Langenbecks Arch Surg
383 (1) : 44-8.
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-67-
0 64. Wang, M.H., H.D. Flad, W. Feist, J.
Musehold, S. Kusumoto, H. Brade, J. Gerdes, H.T.
Rietschel and A.J. Ulmer (1992). "Inhibition of
endotoxin or lipid A-induced tumor necrosis factor
production by synthetic lipid A partial structures in
human peripheral blood mononuclear cells." Lymphokine
Cytokine Res 11(1): 23-31.
65. Warren, J.R., L. Spero and J.F. Metzger
(1974). "Stabilization of native structure by the closed
disulfide loop of staphylococcal enterotoxin B." Bioch
Biophys Acta 359: 351-363.
66. Weiss, K.A. and M. Laverdiere (1997).
"Group A Streptococcus invasive infections: a review."
Can J Surg 40(1): 18-25.
67. Woodley, J.F. (1994). "Enzymatic barriers
for GI peptide and protein delivery." Crit Rev Ther Drug
Carrier Syst 11(2-3): 61-95.
68. Ojcius, D.M., F. Niedergang, A. Subtil,
R. Hellio, and A. Dautry-Varsat (1996). "Immunology and
the confocal microscope." Research in Immunology
147(3):175-188.
69. Soos, JM, Johnson HM: Multiple binding
sites on the superantigen, staphylococcal enterotoxin B,
imparts versatility in binding to MHC class II
molecules. Biochem biophys Res commun 201:596-602,
1994.
70. Yagi J, Rath S, Janeway CA, Jr: Control
of T cell responses to staphylococcal enterotoxins by
stimulator cell MHC class II polymorphism. J Immunol
147:13998-11405,1991.
71. Muller-Alouf H, Alouf JE, Gerlach D, et
al: Human pro- and anti-inflammaotry cytokine patterns
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-68-
o induced by Streptococcus pyogenes erythrogenic
(pyrogenic) exotoxin A and C superantigens. Infect Immun
64:1450-1453, 1996.
72. Leung DY, Travers JB, Giorno R, et al:
Evidence for a streptococcal superantigen-driven process
in acute guttate psoriasis. J Clin Invest 96:2106-2112,
1995.
73. Muller-Alouf H, Alouf JE, Gerlach D, et
al: Comparative study of cytokine release by human
peripheral blood mononuclear cells stimulated with
Streptococcus pyogenes superantigenic erythrogenic
toxins, heat killed streptococci, and
lipopolysaccharide. Infect Immun 62:4915-4921, 1994.
74. Blankson JN, Morse SS: The CD28/B7
pathway cosimulates the response of primary murine T
cells to superantigens as well as to conventional
antigens. Cell Immunol 157:306-312, 1994.
75. Fleischer B, Schrezenmeier H: T cell
stimulation by staphylococcal enterotoxins. Clonally
variable response and requirement for major
histocompatibility complex class II molecules on
accessory or target cells. J Exp Med 167:1697, 1988.
76. Krakauer T: Cell adhesion molecules are
co-receptors for staphylococcal enterotoxin B-induced T-
cell activation and cytokine production. Immunol Lett
39:121-125, 1994.
77. van Seventer GA, Newman W, Shimizu Y, et
al: Analysis of T cell stimulation by superantigen plus
major histocompatibility complex class II molecules or
by CD3 monoclonal antibody: Costimulation by purified
adhesion ligands VCAM-1 ICAM-1 but not ELAM-1. J Exp Med
174:901-913, 1991.
CA 02345023 2001-04-06
WO 00/20598 PCf/US99/22180
-69-
c 78. Chapes SK, Beharka AA, Hart ME, et al:
Differential RNA regulation by staphylococcal
enterotoxins A and B in murine macrophages. J Leukoc
Biol 55:523-529, 1994.
79. Hackett SP, Stevens DL: Superantigens
associated with staphylococcal and streptococcal toxic
shock syndrome are potent inducers of tumor necrosis
factor beta synthesis. J Infect Dis 1.68:232-235, 1993.
80. Imanishi K, Akatsuka H, Inada K, et al:
IFN-gamma-stimulated human vascular endothelial cells
function as accessory cells for superantigen-induced TNF
production in human T-cells. Int Arch Allergy Immunol
106:163-165, 1995.
81. Blank C, Luz A, Bendigs S, et al:
superantigens and endotoxin synergize in the induction
of lethal shock. Eur J Immunol 27:825-833, 1997
82. Leonard BA, Schlievert PM: Immune cell
lethality induced by streptococcal pyrogenic exotoxin A
and endotoxin. Infect Immun 60:3747-3755, 1992.
83. Sugiyama H, McKissic EMJ, Bergdoll MS, et
al: Enhancement of bacterial endotoxin lethality by
staphylococcal enterotoxin. J Infect Dis 114:111-118,
1964.
84. Hensler T, Koller M, Geoffroy C, et al:
Staphylococcus aureus toxic shock syndrome toxin 1 and
Streptococcus pyogenes erythrogenic toxin A modulate
inflammatory mediator release from human neutrophils.
Infect Immun 61:1055-1061, 1993.
85. Smith RJ, Schlievert PM, Himelright IM,
et al: Dual infections with Staphylococcus aureus and
Streptococcus pyogenes causing toxic shock syndrome.
Possible synergistic effects of toxic shock syndrome
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-70-
o toxin 1 and streptococcal pyrogenic exotoxin C. Diagn
Microbiol Infect Dis 19:245-247, 1994.
86. Brocke S, Gaur A, Piercy C, et al:
Induction of relapsing paralysis in experimental
autoimmune encephalomyelitis by bacterial superantigen.
Nature 365:642-644, 1993.
87. Kotzin BL, Leung DY, Kappler J, et al:
Superantigens and their potential role in human disease.
Adv Immunol 54:99-166, 1993.
88. Li S, Quayle AJ, Thoen JE, et al:
Superantigen-mediated proliferation and cytotoxicity of
T cells isolated from the inflammatory tissues and
peripheral blood of arthritis patients. Clin Immunol
Immunopathol 79:278-287, 1996.
89. Schiffenbauer J, Johnson HM, Butfiloski
EJ, et al: Staphylococcal enterotoxins can reactivate
experimental allergic encephalomyelitis. Proc Natl Acad
Sci USA 90:8543-8546, 1993.
90. Schleivert PM: Role of superantigens in
human disease. J Infect Dis 167:997-1002, 1993.
91. Schwab J, Brown R, Anderle S, et al:
Superantigen can reactivate baacterial cell wall-induced
arthritis. J Immunol 150:4151-4159, 1993.
92. Astiz M, Saha D, Lustbader D, et al:
Monocyte response to bacterial toxins, expression of
cell surface receptors, and release of anti-inflammatory
cytokines during sepsis. J Lab Clin Med 128:594-600,
1996 .
93. Schleivert PM, Bohach GA, Ohlendorf DH,
et al: Molecular structure of staphylococcus and
streptococcus superantigens. J Clin Immunol 15:4s-lOs,
1995.
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-71-
0 94. Barman J, Visvanathan K, and Zabriskie
JB. "Structure and function of streptococcal and
staphylococcal superantigens in Septic Shock,"
Infectious Disease Clinics of North America 13(2):387-
396, 1999.
95. Senderoff RI, Kontor KM, Kreilgaard L,
Chang JJ, Patel S, Krakover J, Heffernan JK, Snell LB,
Rosenberg GB. Consideration of conformational
transitions and racemization during process development
of recombinant glucagon-like peptide-1. Journal of
Pharmaceutical Sciences. 87(2):183-9, 1998
96. Rangel-Frausto MS, Pittet D, Costigan M,
Hwang T, Davis CS, Wenzel RP. The natural history of
the systemic inflammatory response syndrome (SIRS). A
prospective study. JAMA 273(2):117-23, 1995
Every reference cited hereinbefore is hereby
incorporated by reference in its entirety.
Modifications of the above described modes for
carrying out the invention that are obvious to those of
skill in the fields of immunology, protein chemistry,
microbiology, medicine, and related fields are intended
to be within the scope of the following claims.
The data suggest a theory for a possible mechanism
of action which is discussed in the application.
However, the application describes how to make and use
the invention. This description is not dependent upon
theory and, accordingly, the claims are not bound by
theory.
The following table shows the correspondence
between peptides in Figure 3 and their sequence
identification numbers:
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-72-
Table 5
Correspondence between Sequence
Identification Numbers and Peptides in Figure 3
Figure 3 Sequence ID
Nos.
Region
1
PEP CMYGGVTEHEGN SEQ ID N0:3
SEA 130 CMYGGVTLHDNN 141 SEQ ID N0:9
SEB 140 CMYGGVTEHNGN 151 SEQ ID NO:10
SEC 137 CMYGGITKHEGN 148 SEQ ID NO:11
SED 131 CTYGGVTPHEGN 142 SEQ ID N0:12
SEE 130 CMYGGVTLHDNN 141 SEQ ID N0:13
SEH 116 CLYGGITL.NSE 126 SEQ ID N0:14
SPEA 128 CIYGGVTNHEGN 139 SEQ ID N0:15
SPEC 112 YIYGGIT'PAQNN 123 SEQ ID N0:16
SSA 134 CMYGGVTEHHRN 145 SEQ ID N0:17
Region
2
PEP KKNVTVQELDYKIRKYLVDNKKLY SEQ ID N0:4
SEA 171 KKNVTVQELDLQARRYLQEKYNLY 194 SEQ ID N0:18
SEB 179 KKKVTAQELDYLTRHYLVKNKKLY 202 SEQ ID N0:19
SEC 178 KKSVTAQELDIKARNFLINKKNLY 201 SEQ ID N0:20
SED 172 KKNVTVQELDAQARRYLQKDLKLY 195 SEQ ID N0:21
SEE 171 KKEVTVQELDLQARHYLHGKFGLY 194 SEQ ID N0:22
SEH 151 KKNVTLQELDIKIRKILSDKYKIY 174 SEQ ID N0:23
SPEA 167 KKMVTAQELDYKVRKYLTDNKQLY 190 SEQ ID N0:24
SPEC 151 KDIVTFQEIDFKIRKLYMDNYKIY 174 SEQ ID N0:25
SSA 174 KKQVTVQELDCKTRKILVSRKNLY 197 SEQ ID N0:26
TSST1 161 KKQLAISTLDFEIRHQLTQIHGLY 184 SEQ ID N0:27
30
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-73-
o SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT:
(A) NAME: The Rockefeller University
(B) STREET: 1230 York Avenue
(C) CITY: New York
(D) STATE OR PROVINCE: New York
(E) COUNTRY: UNITED STATES OF AMERICA
(F) POSTAL CODE: 10021-6399
(ii) TITLE OF INVENTION: PEPTIDES USEFUL FOR
REDUCING SYMPTOMS OF TOXIC SHOCK
SYNDROME AND SEPTIC SHOCK
(iii) NUMBER OF SEQUENCES: 31
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: MORGAN & FINNEGAN
(B) STREET: 345 PARK AVENUE
(C) CITY: NEW YORK
(D) STATE: NEW YORK
(E) COUNTRY: USA
(F) ZIP: 10154
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: FLOPPY DISK
(B) COMPUTER: IBM PC COMPATIBLE
(C) OPERATING SYSTEM: MS-WINDOWS
(D) SOFTWARE: MS WORD 95
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER: TO BE ASSIGNED
(B) FILING DATE: 24 SEPTEMBER 1999
(C) CLASSIFICATION:
(vii) PRIOR APPLICATION DATA:
(A) APPLICATION NUMBER: 09/335,581
(B) FILING DATE: 18 JUNE 1999
(C) CLASSIFICATION:
(vii) PRIOR APPLICATION DATA:
(A) APPLICATION NUMBER: 09/168,303
(8) FILING DATE: 07 OCTOBER 1998
(C) CLASSIFICATION:
(vii) PRIOR APPLICATION DATA:
CA 02345023 2001-04-06
WO 00!20598 PCT/US99/22180
o (A) APPLICATION NUMBER: 08/838,413
(B) FILING DATE: 07 APRIL 1997
(C) CLASSIFICATION:
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: MORRY, MARY J.
(B) REGISTRATION NUMBER: 34,398
S (C) REFERENCE/DOCKET NUMBER: 2016-4010PC2
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: (212)758-4800
(B) TELEFAX: (212)751-6849
(2) INFORMATION FOR SEQ ID NO: 1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 10
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTIONS:SEQ ID NO: 1:
Tyr Gly Gly Xaa Thr Xaa Xaa Xaa Xaa Asn
5 10
(3) INFORMATION FOR SEQ ID NO: 2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTIONS:SEQ ID NO: 2:
Lys Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Asp Xaa Xaa
5 10
Xaa Arg Xaa Xaa Leu Xaa Xaa Xaa Xaa Xaa Xaa Tyr
15 20
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-75-
0
(4) INFORMATION FOR SEQ ID NO: 3:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 12
(B) TYPE: AMINO ACID
{C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTIONS:SEQ ID NO: 3:
Cys Met Tyr Gly Gly Val Thr Glu His Glu Gly Asn
5 10
{5) INFORMATION FOR SEQ ID NO: 4:
(i) SEQUENCE CHARACTERISTICS:
{A) LENGTH: 24
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
{D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTIONS:SEQ ID NO: 4:
Lys Lys Asn Val Thr Val G:ln Glu Leu Asp Tyr Lys
Ile Arg Lys Tyr Leu Val Asp Asn Lys Lys Leu Tyr
20
(6) INFORMATION FOR SEQ ID NO: 5:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 14
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTIONS:SEQ ID NO: 5:
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-76-
o Cys Met Tyr Gly Gly Val Thr Glu His Glu Gly Asn
10
Gly Cys
(7) INFORMATION FOR SEQ ID NO: 6:
5
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 28
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 6:
Cys Gly Lys Lys Asn Val Thr Val Gln Glu Leu Asp
5 10
Tyr Lys Ile Arg Lys Tyr Leu Val Asp Asn Lys Lys
15 20
Leu Tyr Gly Cys
20
(8) INFORMATION FOR SEQ ID NO: 7:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 36
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
25 (D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 7:
CYs Met Tyr Gly Gly Val Thr Glu His Glu Gly Asn
5 10
Lys Lys Asn Val Thr Val Gln Glu Leu Asp Tyr Lys
15 20
Ile Arg Lys Tyr Leu Val Asp Asn Lys Lys Leu Tyr
25 30 35
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
_77_
0
(9) INFORMATION FOR SEQ ID NO: 8:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 38
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 8:
Cys Met Tyr Gly Gly Val Thr Glu His Glu Gly Asn
5 10
Lys Lys Asn Val Thr Val Gln Glu Leu Asp Tyr Lys
20
IS Ile Arg Lys Tyr Leu Val Asp Asn Lys Lys Leu Tyr
30 35
Gly Cys
(10) INFORMATION FOR SEQ ID NO: 9:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 12
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE 'DESCRIPTION: SEQ ID NO: 9:
Cys Met Tyr Gly Gly Val Thr Leu His Asp Asn Asn
5 10
(11) INFORMATION FOR SEQ ID NO: 10:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 12
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
_78_
o (D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 10:
Cys Met Tyr Gly Gly Val Thr Glu His Asn Gly Asn
(12) INFORMATION FOR SEQ ID NO: 11:
(i) SEQUENCE CHARACTERISTICS:
10 (A) LENGTH: 12
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 11:
Cys Met Tyr Gly Gly Ile Thr Lys His Glu Gly Asn
(13) INFORMATION FOR SEQ ID NO: 12:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 12
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 12:
Cys Thr Tyr Gly Gly Val Thr Pro His Glu Gly Asn
5 10
(14) INFORMATION FOR SEQ ID NO: 13:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 12
(B) TYPE: AMINO ACID
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-79-
o (C) STRANDEDNESS: UNKNOWN
(D} TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 13:
S Cys Met Tyr Gly Gly Val Thr Leu His Asp Asn Asn
(15) INFORMATION FOR SEQ ID NO: 14:
(i) SEQUENCE CHARACTERISTICS:
10 (A) LENGTH: 11
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 14:
Cys Leu Tyr Gly Gly Ile Thr Leu Asn Ser Glu
5 10
(16) INFORMATION FOR SEQ ID NO: 15:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 12
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 15:
CYs Ile Tyr Gly Gly Val Thr Asn His Glu Gly Asn
5 10
(17) INFORMATION FOR SEQ ID NO: 16:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 12
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/2Z180
-80-
o (B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 16:
Tyr Ile Tyr Gly Gly Ile Thr Pro Ala Gln Asn Asn
5 10
(18) INFORMATION FOR SEQ ID NO: 17:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 12
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 17:
Cys Met Tyr Gly Gly Val Thr Glu His His Arg Asn
5 1. 0
(19) INFORMATION FOR SEQ ID NO: 18:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 18:
LYs Lys Asn Val Thr Val Gln Glu Leu Asp Leu Gln
Ala Arg Arg Tyr Leu Gln Glu Lys Tyr Asn Leu Tyr
20
35 (20) INFORMATION FOR SEQ ID NO: 19:
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-81-
0
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
{xi) SEQUENCE DESCRIPTION: SEQ ID NO: 19:
Lys Lys Lys Val Thr Ala Gln Glu Leu Asp Tyr Leu
5 10
Thr Arg His Tyr Leu Val Lys Asn Lys Lys Leu Tyr
20
(21) INFORMATION FOR SEQ ID NO: 20:
is (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 20:
Lys Lys Ser Val Thr Ala Gln Glu Leu Asp Ile Lys
5 10
Ala Arg Asn Phe Leu Ile Asn Lys Lys Asn Leu Tyr
15 2 0
(22) INFORMATION FOR SEQ ID NO: 21:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 21:
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-82-
0
Lys Lys Asn Val Thr Val Gln Glu Leu Asp Ala Gln
5 10
Ala Arg Arg Tyr Leu Gln Lys Asp Leu Lys Leu Tyr
20
(23) INFORMATION FOR SEQ ID NO: 22:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH:
(B) TYPE: AMINO ACID
10 (C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 22:
15 LYs Lys Glu Val Thr Val Gln Glu Leu Asp Leu Gln
5 10
Ala Arg His Tyr Leu His Gly Lys Phe Gly Leu Tyr
15 20
(24) INFORMATION FOR SEQ ID NO: 23:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 23:
Lys Lys Asn Val Thr Leu Gln Glu Leu Asp Ile Lys
5 10
Ile Arg Lys Ile Leu Ser Asp Lys Tyr Lys Ile Tyr
15 20
(25) INFORMATION FOR SEQ ID NO: 24:
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-83-
o (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 24:
Lys Lys Met Val Thr Ala Gln Glu Leu Asp Tyr Lys
5 10
Val Arg Lys Tyr Leu Thr Asp Asn Lys Gln Leu Tyr
15 20
(26) INFORMATION FOR SEQ ID NO: 25:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 25:
Lys Asp Ile Val Thr Phe Gln Glu Ile Asp Phe Lys
Ile Arg Lys Leu Tyr Met Asp Asn Tyr Lys Ile Tyr
20
(27) INFORMATION FOR SEQ ID NO: 26:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24
3O (B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 26:
CA 02345023 2001-04-06
WO 00/20598 PCf/US99/22180
-$4-
o Lys Lys Gln Val Thr Val Gln Glu Leu Asp Cys Lys
10
Thr Arg Lys Ile Leu Val Ser Arg Lys Asn Leu Tyr
20
5 (28) INFORMATION FOR SEQ ID NO: 27:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
10 (D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 27:
Lys Lys Gln Leu Ala Ile Ser Thr Leu Asp Phe Glu
15 5 10
Ile Arg His Gln Leu Thr Gln Ile His Gly Leu Tyr
15 20
(29) INFORMATION FOR SEQ ID NO: 28:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 12
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 28:
Xaa Xaa Tyr Gly Gly Xaa Thr Xaa Xaa Xaa Xaa Asn
5 10
(30) INFORMATION FOR SEQ ID NO: 29:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24
(B) TYPE: AMINO ACID
CA 02345023 2001-04-06
WO 00/20598 PCT/US99/22180
-$5-
o (C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 29:
Lys Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Asp Xaa Xaa
Xaa Arg Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Tyr
20
(31) INFORMATION FOR SEQ ID NO: 30:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH; 12
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 30:
Cys Met Tyr Gly Gly Xaa Thr Xaa His Xaa Gly Asn
ZO
(32) INFORMATION FOR SEQ ID NO: 31:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24
(B) TYPE: AMINO ACID
(C) STRANDEDNESS: UNKNOWN
(D) TOPOLOGY: UNKNOWN
(ii) MOLECULE TYPE: PEPTIDE
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 31:
Lys Lys Xaa Val Thr Xaa Gln Glu Leu Asp Xaa Xaa
Xaa Arg Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Leu Tyr
20