Note: Descriptions are shown in the official language in which they were submitted.
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
CHARACTERIZATION OF LUMINESCENCE IN A SCATTERING MEDIUM
10
BACKGROUND
The present invention relates to spectroscopic techniques involving
luminescence,
and more particularly, but not exclusively relates to the determination of
lifetime of a
luminophore in a light scattering medium.
There has been significant development of fluorescent and phosphorescent dyes
or
probes with decay kinetics dependent upon the presence or concentration of an
analyte or
metabolite. Accordingly, lifetime of these dyes can be measured to detect
corresponding
analyte(s) and/or metabolite(s) concentration. Fluorescent probes in the near-
infrared
range appear particularly promising for in vivo biomedical diagnostic
techniques that
involve external, noninvasive measurements or minimally invasive, endoscopic
measurements of emitted light.
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
Unfortunately, quantitative lifetime measurements for such probes are often
difficult to obtain in the light scattering environment typically encountered
with in vivo
diagnostics. Light scattering also hampers other applications of luminophore
probes both
inside and outside the biomedical field. Consequently, lifetime measurements
are usually
restricted to dilute, nonscattering solutions. Notably, even equipment used in
this manner,
such as a curvette to contain the dilute solution, tends to scatter light to
some degree
introducing an attendant inaccuracy.
Moreover, current lifetime measurement approaches have other limits -
especially.
for fluorophore probes. For example, deconvolution of instrument function
often hampers
1o accurate time-domain measurement of lifetimes. In another example,
frequency-domain
approaches generally require a reference fluorophore with known lifetime
characteristics
in the environment of interest and at the appropriate excitation and emission
wavelengths.
Thus, there is a need for further contributions that address these limits
and/or other
drawbacks confronting this technology.
2
CA 02346728 2009-07-31
SUMMARY OF INVENTION
Certain exemplary embodiments may provide a method, comprising: interrogating
a light scattering medium including an amount of a selected luminophore with
light at an
emission wavelength of the luminophore; sensing multiply scattered light at
the emission
light wavelength in response to the interrogating to provide a first optical
characterization
of the medium; exposing the medium to light at an excitation wavelength of the
luminophore; and illuminating the medium with the excitation light wavelength
and
detecting multiply scattered light at the excitation wavelength in response to
illuminating
to establish a second optical characterization of the medium and determining a
value
corresponding to lifetime of the luminophore from the first optical
characterization, the
second optical characterization, and the luminescence.
Certain other exemplary embodiments may provide a method, comprising:
exposing a light scattering medium including an amount of a luminophore to a
number of
different light wavelengths associated with characteristics of the
luminophore;
establishing a plurality of optical characteristics of the medium by sensing
multiply
scattered light at each of the different wavelengths; and determining a value
corresponding to lifetime of the luminophore from the optical characteristics
and a
multiply scattered emission at a first one of the light wavelengths caused by
exposure of
the medium to a second one of the light wavelengths.
Yet another exemplary embodiment may provide a method, comprising:
evaluating a light scattering medium including an amount of a luminophore with
a light
interrogation system, the system including a first device optically coupled to
a first site of
the medium and a second device optically coupled to a second site of the
medium spaced
apart from the first site; illuminating the medium to a first light wavelength
associated
with a characteristic of the luminophore from the first and second devices;
optically
coupling the first device to the second site and the second device to the
first site;
illuminating the medium with an excitation light wavelength from the first and
second
devices after the optically coupling; and determining a value corresponding to
lifetime of
the luminophore from a first light output detected in response to the first
light wavelength
and a second light output detected in response to the excitation light
wavelength.
3
CA 02346728 2009-07-31
Still certain other exemplary embodiments may provide a system comprising:
light source instrumentation to illuminate a light scattering medium including
a
luminophore; detection instrumentation to detect multiply scattered light
output from the
medium in response to illumination by the light source instrumentation; and a
processor
operatively coupled to the detection instrumentation to determine a first
optical
characterization of the medium from a first multiply scattered light output of
a first
illumination light wavelength and a second optical characterization of the
medium from a
second multiply scattered light output of a second illumination light
wavelength different
than the first illumination light wavelength, the processor being operable to
calculate a
value corresponding to lifetime of the luminophore from the first optical
characterization,
the second optical characterization, and a multiply scattered emission of the
luminophore
from the medium in response to excitation.
Still certain other exemplary embodiments may provide a system, comprising:
means for illuminating a light scattering medium including a luminophore; and
means for
characterizing light scattering behavior of the medium for an excitation
wavelength of the
luminophore and an emission wavelength of the luminophore; means for
determining
lifetime of the luminophore from the characterizing means and a multiply
scattered light
emission from the medium at the emission wavelength in response to
illumination by
light at the excitation wavelength.
Other embodiments provide a technique to evaluate a medium including a
luminophore. Other forms include unique systems and methods to measure optical
properties of a luminophore in a light scattering medium.
In another form, a light scattering medium includes a luminiphore that is
exposed
to a number of different wavelengths. Multiply scattered light from exposure
to these
wavelengths is measured, and one or more optical characteristics of the medium
are
determined relative to the different wavelengths.
In still another form, an optical characteristic of a luminophore in a light
scattering
medium is determined. For this form, the medium is illuminated by light at a
first
wavelength corresponding to an emission wavelength of the luminophore and at a
second
wavelength corresponding to an excitation wavelength of the luminophore.
Multiply
4
CA 02346728 2009-07-31
scattered light in response to illumination by the different wavelengths is
detected to
optically characterize the medium and/or luminophore.
A further form is a technique to determine the lifetime of a fluorophore. This
technique includes both a method and instrumentation directed to fluorescence
lifetime
measurements. Preferably, this technique does not utilize a reference
fluorophore and is
not adversely impacted by any light scattering and absorption that might occur
in a
scattering medium.
Still a further form includes a frequency-domain approach to measuring
fluorescence lifetime of one or more fluorophores. This approach may include
exciting a
fluorophore in a light scattering sample with a modulated excitation light and
detecting
phase shift information. The fluorescence lifetime may be determined from the
phase
shift information through relationships characterizing light scattering by the
sample.
In yet a further form, a light scattering sample containing a fluorophore is
exposed
to an intensity modulated light at a first wavelength selected to cause the
fluorophore to
fluoresce at a second wavelength of light. Scattered light at the first
wavelength is
detected and scattered light at the second wavelength is detected. Optical
properties are
determined from the detected scattered light to provide fluorescence lifetime
based on
relationships that characterize photon migration in a light scattering medium.
This form
may include characterizing the detected scattered light in terms of phase
shift in the
frequency domain.
In an additional form, a system includes an intensity modulated excitation
light
source configured to deliver an excitation light of a first wavelength to a
light scattering
substance containing a fluorophore of interest. The fluorophore responds to
the excitation
light to provide a light emission at a second wavelength. Scattered light is
detected at two
locations spaced apart from one another. A processor gathers information
corresponding
to the detected scattered light and processes this information to determine
fluorescence
lifetime by applying relationships that characterize photon migration of
scattered light.
For this form, a first detector may be used for detection of light at a first
one of the
locations that includes a first optical fiber coupled to a first sensor. Also
a second detector
may be used for detection of light at a second one of the locations that
includes a second
4a
CA 02346728 2009-07-31
optical fiber coupled to a second sensor. The first and second detectors may
also include
corresponding first and second optical filters to selectively detect the first
wavelength of
light with the first sensor and the second wavelength of light with the second
sensor. The
coupling arrangement of the first and second fibers to the first and second
sensors may be
interchanged to obtain comparative measurements for the minimization of
equipment
inaccuracies.
Further forms, embodiments, objects, features, aspects, advantages, and
benefits
of the present invention shall become apparent from the detailed description
and drawings
of the present application.
4b
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a schematic view of a system according to one embodiment of the
present
invention.
Figs. 2A and 2B depict a flow chart illustrating one process that may be
performed
with the system shown in Fig. 1.
Fig. 3 is a schematic view of a system of another embodiment of the present
invention.
Fig. 4 depicts normalized excitation and emission spectra for DTTCI (top) and
ICG (bottom) in a comparative format pertinent to experimental examples.
to Fig. 5 plots phase shift versus modulation frequency for two different
concentrations of DTTCI in a comparative format pertinent to experimental
examples.
Fig. 6 plots phase shift versus modulation frequency for two different
concentrations of ICG in a comparative format pertinent to experimental
examples.
Fig. 7 plots lifetime versus modulation frequency for DTTCI and ICG
corresponding to the plots of Figs. 5 and 6.
Fig. 8 plots relative modulation attenuation versus modulation frequency for
two
different concentrations of DTTCI in a comparative format pertinent to
experimental
examples.
Fig. 9 plots relative modulation attenuation versus modulation frequency for
two
different concentrations of ICG in a comparative format pertinent to
experimental
examples.
Fig. 10 plots lifetime versus modulation frequency for DTTCI and ICG
corresponding to the plots of Figs. 8 and 9.
5
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
DESCRIPTION OF PREFERRED EMBODIMENTS
For the purpose of promoting an understanding of the principles of the
invention,
reference will now be made to the embodiments illustrated in the drawings and
specific
language will be used to describe the same. It will nevertheless be understood
that no
limitation of the scope of the invention is thereby intended. Any alterations
and further
modifications in the described embodiments, and any further applications of
the principles
of the invention as described herein are contemplated as would normally occur
to one
skilled in the art to which the invention relates.
As used herein, "lifetime" refers to the mean survival time of an activated
to luminophore or the mean time between the absorption of an excitation photon
and
emission of a photon. Further, as used herein "multiply scattered light"
refers to light that
travels at least five (5) times the mean isotropic scattering length [(1-g)
sj't; where g is
the mean cosine of angular scatter and s is the scattering coefficient of the
medium.
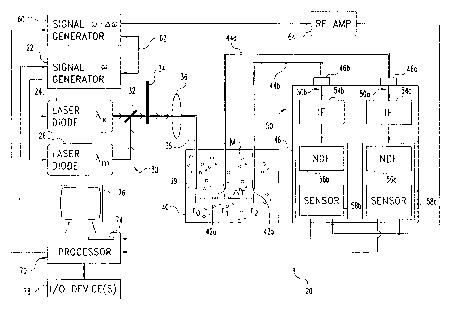
Fig. 1 illustrates evaluation system 20 of one embodiment of the present
invention.
System 20 includes light source instrumentation 30, container 40, detection
instrumentation 50, and processor 70. Light source instrumentation 30 includes
modulation signal generator 22 having a range of selectable output Radio
Frequencies
(RF). Generator 22 drives two monochromatic light sources in the form of laser
diodes
24, 26. Laser diodes 24, 26 are intensity modulated at a selected RF frequency
((o) input
from generator 22 to provide light at an excitation wavelength (),.,,) and
emission
wavelength Q,,,,), respectively. Instrumentation 30 also includes kinematic
mirror 32 that
is operable to successively select between the two laser beams at wavelengths
X,,,
output by the respective laser diodes 24, 26. Light from mirror 32 encounters
continuously variable neutral density filter wheel 34 of instrumentation 30 to
selectively
adjust intensity. Lens assembly 36 of instrumentation 30 collects the light
output by
neutral density filter wheel 34 for input to optical source fiber 38.
Source fiber 38 enters container 40. Container 40 holds a light scattering
medium
M including a selected amount of a luminophore as a constituent. Source fiber
38
discharges light at site 39 of medium M corresponding to the origin position
ro (r=0).
Detection instrumentation 50 includes optical detector fibers 44a, 44b having
respective
light input sites 42a, 42b in medium M. Sites 42a, 42b are spaced apart from
each other
6
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
and correspond to radial distances r1 and r2 relative to r0; where this
spacing is represented
by&(&r=r2-r1).
Interchangeable connectors 46a, 46b couple fibers 44a,44b to detector 48 of
detection instrumentation 50. Detector 48 includes two optic channels 50a, 50b
coupled
by connectors 46a, 46b to receive light from detector fibers 44a, 44b,
respectively. Each
channel 50a, 50b has a corresponding interchangeable/removable interference
filter (IF)
54a, 54b; adjustable neutral density filter (NDF) 56a, 56b; and light sensor
58a, 58b.
Light sensors 58a, 58b are coupled to processor 70 to provide one or more
output
signals corresponding to light detected from medium M. Sensors 58a, 58b are
arranged in
a standard heterodyne configuration and may be of any form such as
Photomultiplier
Tubes (PMTs), photodiodes, or image intensified Charge Coupled Devices (CCDs)
to
name just a few. For the heterodyne configuration, RF signal generator 60 is
phase locked
to generator 22 at a slightly different frequency w + iw as represented by
coupling 62;
where Oco is the frequency difference. The output of generator 60 is amplified
by RF
amplifier 64 and mixed at sensors 58a, 58b to provide a corresponding
differential output
signal from which information corresponding to phase and modulation magnitude
of the
detected light can be determined with processor 70. Accordingly, processor 70
is also
operatively coupled to generators 22, 60.
Processor 70 includes a port for insertion and removal of a portable memory
device
such as an electromagnetically or optically encoded disk, cartridge, or tape.
In addition to
being coupled to light source instrumentation 30 and detection instrumentation
50,
processor 70 is also operatively coupled to visual display 76 and one or more
Input/Output
(I/O) devices 78, including for example a keyboard, mouse. light pen, acoustic
loudspeakers, microphone, and/or printer just to name a few. Processor 70 may
be
comprised of one or more components configured as a single unit, or when of a
multi-
component form, processor 70 may have one or more components remotely located
relative to the others, or otherwise have its components distributed
throughout system 20.
Processor 70 may be programmable, a state logic machine or other type of
dedicated
hardware, or a hybrid combination of programmable and dedicated hardware. One
or
more components of processor 70 may be of the electronic variety including
digital
circuitry, analog circuitry, or both. As an addition or alternative to
electronic circuitry,
processor 70 may include one or more optical elements.
7
CA 02346728 2001-04-09
WO 00/22414 PCTIUS99/23709
Processor 70 includes an integrated and/or remote storage capability in the
form of
one or more types of memory. By way of nonlimiting example, this memory may
include
one or more of the solid-state. magnetic, and/or optical memory types. Such
memory
types may include Random Access Memory (RAM), Sequential Accessible Memory
(SAM) (such as the First-In, First-Out (FIFO) variety, or the Last-In, First-
In LIFO
variety), Programmable Read Only Memory (PROM), Electrically Programmable Read
Only Memory (EPROM), flash memory or Electrically Erasable Programmable Read
Only
Memory (EEPROM); an optical disc memory (such as a CD ROM); a magnetically
encoded hard disc, floppy disc, tape, or cartridge; another variety of storage
device as
would occur to those skilled in the art, or a combination of any of these
types.
Furthermore, the memory may be volatile, nonvolatile, or a hybrid combination
of volatile
and nonvolatile varieties. Also. memory may be permanently installed, in a
portable form
that may be readily removed and reinstalled, or a combination of these types.
In one embodiment including electronic circuitry, processor 70 is of a
standard
personal computer configuration with a common solid-state digital integrated
processing
unit operatively coupled to solid-state memory. For this personal computer
embodiment,
appropriate interfaces are installed to facilitate control of generators 22,
60 and receipt of
data from detector 48. The memory of this embodiment contains programming to
be
executed by the processing unit, and is arranged for reading and writing of
data in
accordance with one or more routines executed by processor 70. Besides memory,
processor 70 may include any oscillators, control clocks, interfaces, signal
compensators/conditioners, filters, limiters, Analog-to-Digital (A/D)
converters, Digital-
to-Analog (D/A) converters, communication ports, or other types of circuits as
would
occur to those skilled in the art to implement the present invention.
Processor 70 is configured to execute one or more routines to perform selected
calculations with data received from detector 48 for lifetime evaluation
process 120.
Referring additionally to the flow chart of Figs. 2A and 2B, evaluation
process 120 is
illustrated. Evaluation process 120 utilizes Frequency Domain Photon Migration
(FDPM)
techniques to extract the characteristic optical properties of medium M. These
properties
are obtained for photons of two wavelengths: the wavelength used to optically
excite a
selected luminophore and the wavelength of the luminescence resulting from
this
excitation. Lifetime measurements are determined from these wavelength-
dependent
8
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
characterizations of the medium and comparative luminescence measurements, as
described hereinafter.
In stage 122 of process 120, a luminiphore probe with known excitation
wavelength kr and emission wavelength Xm is selected for evaluation and placed
in light
scattering medium M. This light scattering medium M may include, for example,
living
biologic tissue for which metabolites/analytes are being interrogated in terms
of lifetime of
the selected luminophore probe. In other examples, the light scattering medium
M may be
a cell culture, flow cytometry stream, a chemical reaction medium or other
light scattering
environment as would occur to those skilled in the art. In one alternative,
endogenous
luminophores in medium M are interrogated without introduction of an exogenous
probe
in stage 122.
In stage 130, intensity modulated light at frequency-(o with the excitation
wavelength k, of the designated luminophore is provided by light source
instrumentation
30 to source site 39 of medium M. Accordingly, light from laser diode 24 is
provided
from light source instrumentation 30 through fiber 38 to site 39. The
illuminating light is
subsequently scattered and/or absorbed by medium M. As multiply scattered
light sourced
from site 39 reaches sites 42a, 42b; it can be sensed with detection
instrumentation 50 and
quantitized in terms of frequency domain parameters of relative phase shift
and/or
modulation magnitude. Filters of detector 48 are removed and/or adjusted to
facilitate
detection of the excitation light wavelength with sensors 58a, 58b for this
stage.
Photon transport in a light scattering medium M may be modeled as a diffusive
process. In the frequency domain, the photon density U(r,w) in a homogeneous
medium
at a vector position r can be related to optical properties of the medium by
the diffusion
equation (1) as follows:
-cDV2U(r,w) + (c a + i(o)U(r,w) = q(r,w); (1)
where: D = [3( 'S+ 11a)]'' is the diffusion coefficient of the medium, c is
the speed of light
in the medium, q(r,w) describes properties of the light source, and CO is the
modulation
angular frequency of the light source (generator 22). The output signal from
detection
instrumentation 50, V(r,w), depends on the complex responsivity R(X,(o)
expressed as:
V(r,w) = R(?.,w)U(r,w). The magnitude of R(X,co) represents gain and
conversion
9
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
efficiency of the photon transport and the angle represents the phase delay of
the
modulated light.
For the diffusion equation model, the absorption coefficient p.a and the
isoptropic
scattering coefficient 'S characterize pertinent light scattering properties
of the medium at
a given light wavelength. The isotropic scattering coefficient is related to
the scattering
coefficient g, by: 'S = (1-g)p. ; where g is the mean cosine of angular
scatter. As used
herein, the subscripts "x" and "m" are used to designate various optical
parameters
specific to the excitation and emission wavelengths, respectively. It has been
found that
lifetime measurements in a multiply scattering medium may be performed by
accounting
for the optical characteristics of the medium at two light wavelengths
commonly
associated with the luminophore: (a) the excitation wavelength Xx and (b) the
emission
wavelength km. This characterization can be in terms of the absorption and
isotropic
scattering coefficients for each of the two wavelengths: (a) .Lax , 'S,, for
?s and (b) am ,
for k
g',. m=
Accordingly, in stage 130 medium M is characterized at X beginning with
operation 132. In operation 132, medium M is exposed to modulated light of
wavelength
).,r at site 39 as sourced from laser diode 24 of light source instrumentation
30. This source
light can be modeled as an isotropic point source of the form: q,,(r,(O) =
P,,((0)8(r); where
P.(a) is a complex number that represents the source magnitude and phase and
6(r) is the
Dirac delta function. Furthermore, by modeling the light behavior in terms of
the
diffusion equation with infinite boundary conditions, the solution to the
diffusion equation
takes the form of spherical photon density waves described by the following
expression
(2):
Ui(r, w) P'M e-k.(w) r
47rcD=r (2)
where: Ur(r,co) is the frequency domain excitation photon density in the
medium M and
the complex wave vector k,(w) is given by expression (3) as follows:
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
kr2(w) _ Aar (3)
Ds C/.as
On substitution of expression (3) into expression (2), the excitation photon
density can be
modeled as follows in expression (4):
U.(r,w) = 4 Pc(D) r (cos(ryr(w)r) + i sin(-yx(w)r)) (4)
r
1 o where:
lz r w is 1/2
ar(w) l Dr J + `C D= /
i3 (w) = ar(w) cos (! tan-1(_ _)
)
C /lax
ryr(w) = ar(w) sin 1 tan-1 w
2 CA= The photon density is related to the observed modulation phase 0(r,(0)
by
expression (5a) as follows:
ImU(r, w) (5a)
tan 9(r, c,>) = ReU(r, w)
The modulation M(r,co) of the photon density waves a distance r away from and
normalized to unity at the source (r = 0) is related to photon density by
expression (5b) as
follows:
M(r, w) _ Reg U(r, w) + 1m2 U(r, w) (5b)
7Ze2 U(r,0)+Im2U(r,0)
For injection of source light at the excitation wavelength into the medium M
in operation
132, the substitution of expression (4) into equations (5a) and (5b) results
in expressions
11
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
(6a) and (6b), respectively, as follows:
0,(r,w) = yc(co)r (6a)
P=(w)
=(o)
The relative modulation phase and magnitude are observed with detection
instrumentation 30 at the two radial distances r1 and r2; where Ar = r2 - rl,
r2> r1. The
resultant phase difference is expressed as: AO,,(Ar,(o) = y,,(co)Ar; and the
resultant
modulation magnitude may be as follows in expression (7)
M., (Or, w) - M. (r2, w) = e Grlas(~) - ~s(~)l (7)
M.(r1, w)
where the actual modulation information observed with detection
instrumentation 50 is as
follows:
m=(r,w) = Ai-(r, W) m,., md,~ (r,w) (8)
and is related to M,(r,cw) by the modulation of source m,, and the modulation
response
m4j (r,w) of detection instrumentation 50. The ratio of the observed
modulation signals is
provided by expression (9) as follows:
s( , ) - ms(r2, LI;)
m ~rw - mds(r2, w) a Gr(a=(0) - /3 (~)1 /9)
ms(ri,w) _ md=(rl,w)
This ratio is generally independent of source modulation.
12
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
In operation 132, data is collected and stored in processor 70 corresponding
to
detected multiply scattered light output from medium M. It should be
appreciated that
only one of relative phase and modulation attenuation information needs to be
observed to
provide the desired characterization of the medium M at the excitation
wavelength. In
order to calculate both the absorption and isotropic scattering coefficients
ar , '5x with
expression (4) from relative phase or magnitude observations, a regression or
other
iterative estimation can be employed. To facilitate this calculation,
operation 132 detects
multiply scattered light outputs for a number of different RF modulation
frequencies.
While relative measures of phase or modulation attenuation are indicative of
1o photon-migration through medium M, differences in the response function of
channels 50a
and 50b of detector 48 may result in inaccuracy that reaches an undesirable
level for some
applications. It has been found that instrumentation response function effects
may be
reduced by switching from the illustrated (first) configuration with sites
42a, 42b
respectively coupled to channels 50a, 50b to a second configuration with sites
42a, 42b
coupled to channels 50b, 50a; and repeating the phase and/or modulation
magnitude
measurements of operation 132 in operation 134 with the second configuration.
The
measurements of operation 134 are conducted with the same relative spacing
between sites
39, 42a, and 42b. Connectors 46a, 46b provide a convenient way to manually
perform this
reconfiguration. In an alternative embodiment, an optical multiplexer may be
utilized to
automatically accomplish this reconfiguration under the control of processor
70.
In an example based on relative phase measurements, the effective response
function of the detection instrumentation is designated O;r,str . Letting
AOõ(r2rl) = 0õ(r2) -
0,,(ri) + Oi,str represent measurements during operation 132 and AOY(rlr2) =
0õ(ri) - O,,(r2) +
9;,,str represent measurements during operation 134, the instrument effects
can be removed
by taking one half of the difference between these two relative measurements.
The desired
phase difference, AO, is then expressed as: AO,, = 0.5[AO. (r2rj) -
AOõ(rlr2)].
Measurements gathered with detection instrumentation 50 in operations 132, 134
of stage 130 for a desired number of different modulation frequencies are
recorded with
processor 70. Stage 140 is next encountered to obtain an optical
characterization of
medium M at the emission wavelength Xm in terms of absorption and isotropic
scattering
coefficients Pam, '5n,. In operation 142, emission wavelength light is
provided to source
site 39 with laser diode 26 of light source instrumentation 30, instead of
excitation
13
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
wavelength light from laser diode 24. Filtering of detector 48 is
removed/adjusted to
facilitate detection of multiply scattered light output from medium M at the
emission
wavelength ?m and measurement of relative modulation phase or magnitude over a
selected range of modulation frequencies in the manner described for stage
130. The
emission photon density is characterized in stage 140 in accordance with
expressions (2)-
(9) substituting the emission wavelength for the excitation wavelength (and
correspondingly substituting subscript "x" with "m" in these expressions). In
operation
144, the measurements are repeated with coupling of site 42a being switched to
sensor 58b
of channel 50b and coupling of site 42b being switched to sensor 58a of
channel 50a. The
measurements of operations 142, 144 are recorded with processor 70 to
determine
absorption and isotropic scattering coefficients a,,,,
In stage 150, luminescence data is gathered. Excitation wavelength light is
provided to site 39 of medium M from laser diode 24 of light source
instrumentation 30 in
operation 152 of stage 150 and frequency domain measurements are gathered. For
stage
150, detection instrumentation 50 is configured with filtering of channel 50a
adjusted/removed to detect the excitation wavelength with sensor 58a and
filtering of
channel 50b adjusted/removed to detect the emission wavelength with sensor
58b. For a
fluorescent type of luminophore, fluorescence photon density may be modeled in
accordance with expression (10) as follows ( where the subscript "f' denotes
fluorescence
optical parameters):
/ of P(w) k)* - e'k"'(") 1 +
wT (10)
Uf(r,w = 47rcDxD,..r (!Lkjm(w) - k2(w) ) (1 + (wr)2
The quantum efficiency of the fluorophore is denoted by 0 and af describes
absorption of
excitation light due to fluorescence. Fluorescence decay is assumed to be of
the
monoexponential type with lifetime i; however, the principles of the present
invention can
be applied to multiexponential decays using techniques known to those skilled
in the art.
Using expression (3), the fluorescence photon density of expression (10) can
be written as
presented in the following expression (11):
14
CA 02346728 2001-04-09
WO 00/22414 PCTIUS99/23709
-i,F(w) (II)
U1(r, w) = '([4,(r, w) - K(r, w)w7]
47rcD,Dm[1 + (wT) ]r
+ i[K(r, w) + t41(r, w)wT]},
where:
&(r, w)E + r(r, w)p(w)
E2 + [p(w)]2
K(r, W) = I(r, w)E - S(r, w)p(w)
E2 + [p(w)]2
5(r, w) = exp[-R,(w)r)cos[y,(w)r]
- exp[-pm(w)r)cos[ym(w)r],
E(r, w) = exp[-R:(w)r)sin[y,(w)r]
- exp[-O,(w)r]Sin['Ym(w)r],
D,,, D,,
w(l 1
p(w)CDtD,
The fluorescence photon density is related to the observed fluorescence
modulation phase
ei(r,co) by expression (5a). Substituting expression (11) into expression
(5a), expression
(12) results as follows:
K(r, w) + t,(r, w)wi (12)
tan 81(r, w) = Cr, w) - K(r, w)WT
and the fluorescence decay lifetime can be written per expression (13) as
follows:
1 tan 8f(r, w) - r(r, w) (13)
T = "
w r(r, w)tan 8f(r, w) + 1'
where rj(r,co) = K(r, w)/yr(rp) and determinations of yf(r,w) and K(r, (0) are
based on
measured phase shifts AO (&r,co) and AO (4r,o)).
Alternatively, the fluorescence photon density is related to fluorescence
modulation Mf(r,w) by expression (5b). Substituting expression (11) into
expression (5a)
and noting that x(r, 0) = 0 yields expressions (14) and (15) as follows:
w - P(w) c(r2,w) (14)
MI (r, ) - p(0) (1 + (wr)2)
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
where:
E(r2,W) - ti'2(r2iGJ) + K2(r2,W) (15)
02(r2, 0)
is based upon the optical coefficients of the sample. Comparable expressions
can be
derived for other types of luminescence and different boundary conditions.
After measurements of relative modulation phase or magnitude are made in
operation 152, the measurements are repeated in operation 154 with sites 42a,
42b being
switched relative to channels 50a, 50b as described in connection with
operation 134 and
144. As part of the reconfiguration for stage 154, the filtering of channels
50a, 50b is also
adjusted to facilitate detection of the excitation wavelength with sensor 58b
and the
emission wavelength with sensor 58a. Measurements of operations 152, 154 are
stored
with processor 70.
In calculation stage 160 (Fig. 2B) of evaluation process 120, processor 70
performs
calculations with the data collected during stages 130, 140, 150. In operation
162,
processor 70 performs regression analysis of measurements from excitation
characterization stage 130 to determine the absorption and isotropic
scattering coefficients
8x, '5X at the excitation wavelength. In operation 164, processor 70 performs
regression
analysis of measurements from emission characterization stage 140 to determine
the
absorption and isotropic scattering coefficients am, '5m at the emission
wavelength.
In operation 166, processor 70 calculates lifetime based on the measurements
obtained from luminescence characterization stage 150 and the coefficients
obtained from
operations 162, 164. For calculations of lifetime based on relative phase
measurements in
stage 150, the measured phase shift 06,{ir,w) = 01(r2 ca) - 9X(rl co) is
adjusted by the
addition of phase change OX,(ri ca) associated with the excitation propagation
from site 39
to site 42a at ri. This phase value 0XJri ,.o) can be calculated from
expression (6a) and the
optical characteristics determined in operation 162 from measurements of
excitation
wavelength characterization stage 130. The resultant modulation phase 0c(r2
,co) _
16
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
O6KOr,co) + 0, (r, co) is the phase at the source (r = 0) and can be used in
expression (13)
to determine lifetime z.
For calculation of lifetime based on modulation attenuation, the modulation
magnitude at radial distance r2 is referenced relative to the excitation
modulation
magnitude at radial distance r1. The ratio of expression (14) to expression
(6b) yields
expression (16) and (17) as follows:
M Or w MI(r2,w) = 1 e(r2iw)
M=(r1,w) eI" [ax(O) -as(W)l (1+(wr)2) (16)
from lifetime i is derived:
E(r2, w) (17)
r W Mj (Or, w) e2r,(a:(0) - As(w)l
The modulation magnitude detected with detection instrumentation 50 is given
by
expression (18) as follows:
mj(r2,w) = Mj(r2,w) m3 mdm(r2,w) (18)
which is related to modulation Mf(r,co) by modulation in,, of the source at
the excitation
wavelength, and the modulation md,,,(r2 co) ,of the detection instrumentation
50 at the
emission wavelength. The ratio of expression (18) and the excitation
modulation of
expression (8) is provided by expression (19) as follows:
mf (r2, W) = M/(r2,w) mdm(r2,w)
MI (Ar, w) =
rn (ri, w M=(r1, w md=(rl, w (19)
From the ratio of expression (19), the following expression (20):
17
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
Mf(Ar,w) = mf(Or,w) mdX(ri,w) (20)
mdm(r2, w
is found to depend upon the ratio of the detection instrumentation 50 response
functions at
excitation and emission wavelengths. This information may be expressed as a
single ratio
of modulations of the sources; where given the optical coefficients of the
medium M from
operations 162 and 164, excitation MXc(r,(o) and emission M,,(r,co) can be
determined
from expression (7). The product of source modulation and detection instrument
response
function can be determined from expression (8) at the excitation:
rn,= mdc(ri,w) = M= ((rl)) (21)
ri,w
and emission:
M. (r2) (22)
m,m mdm (r2, w) _
Mmc(r2, w)
wavelengths. The resulting ratio of detection instrument response functions is
given by
expression (23) as follows:
mda(ri, w) m,m ms(ri, w) Mmc(r2, w) (23)
mdm(r2,w) m,r mm(r2,w) Mic(ri,w)
which depends upon a comparison between measured and calculated modulation
information and a constant ratio of modulations of the sources at emission and
excitation
wavelengths. Varying the source modulation ratio, the lifetime measured at
multiple
modulation frequencies can be regressed to obtain a unique source modulation
ratio
associated with a vanishing slope, i.e. minimized x2 of the lifetime
distribution, and to
obtain the resulting lifetime i. After lifetime is determined in stage 160
from relative
phase or modulation measurements, it is output in stage 170, concluding
process 120.
18
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
It should be understood that only one possible sequence of the measurement
stages
130, 140, 150 is illustrated. Indeed, the various measurements may be
performed in many
other sequences with respect to selected light wavelengths, modulation
frequencies, and
coupling configuration of sites 42a, 42b relative to channels 50a, 50b. Also,
the
calculations of stage 160 may be performed any time by processor 70 in
relation to the
measurement stages 130, 140, 150 to the extent measurement data has been
provided to
processor 70.
In other embodiments. the arrangement of system 20 may differ. For example,
light source instrumentation may include a source of excitation and/or
emission
wavelength light such as a different type of laser, lamp, or other device as
would occur to
those skilled in the art. In another example, detection instrumentation 50 may
include an
optical multiplexer controlled by processor 70 to provide for the switching of
optical
couplings relative to detector 48. In one alternative embodiment, the
measurements may
be based on two spaced apart light source sites 39 in medium M with just one
detection
site 42a or 42b and corresponding sensor 58a or 58b. In one form of this
arrangement,
light source instrumentation may be configured with another set of the
components
described for instrumentation 30 to provide two light source channels; while
detection
instrumentation 50 could be modified to include only a single channel 50a or
50b. The
lifetime calculations may be readily adapted to this dual source using
techniques known to
those skilled in the art. Also, instrument function that might lead to
calculation
discrepancies relative to the two source sites can be addressed by switching
the equipment
supplying light to each source; thereby providing the first and second
measurement
configurations described in connection with stages 130, 140, 150.
Fig. 3 depicts medical diagnostic system 220 of another embodiment of the
present
invention. System 220 includes diagnostic instrument 225 to evaluate a
patient's medical
condition based on lifetime readings of an endogenous, exogenous, or
immobilized
exogenous fluorophore in a patient's tissue 280. Instrument 225 includes light
source
apparatus 230, detection apparatus 250, and processor 270 that are
operationally
configured like light source instrumentation 30, detection instrumentation 50,
and
processor 70, respectively, but are packaged in a manner convenient for in
vivo
interrogation of tissue 280.
The fluorophore in tissue 280 is typically an exogenous probe introduced into
tissue 280 that has a known excitation and emission wavelength to which the
optics of
19
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
instrument 225 are matched. Such exogenous probes may be immobilized in a
subcutaneous implant, by oral ingestion, or any other means that would occur
to those
skilled in the art. Alternatively or additionally, the present application may
be applied to
monitor endogenous fluorophores for which excitation emission wavelengths are
known.
Light source apparatus 230 includes one or more sources to provide light to
tissue
280 at excitation and emission wavelengths for the selected fluorophore in
accordance
with evaluation process 120. The source light is provided to site 239 of
tissue 280 as
represented by arrow IL. The source light is multiply scattered by tissue 280
and received
by detection apparatus at sites 242a, 242b as represented by arrows MS 1, MS2.
Instrument 225 is configured to maintain a generally constant relative spacing
between
sites 239, 242a, and 242b, corresponding to ro, ri, and r2, respectively.
Processor 270 may
be configured to control selection of the appropriate source light wavelength
and
modulation frequencies for light source apparatus 230 and the corresponding
filter
configuration for detection apparatus 250 to automatically perform the stages
and
operations of process 120 with instrument 225.
For external interrogations, instrument 225 may include a moveable hand-held
wand or optical probe coupled to a base unit; where the wand is arranged for
placement
proximate to surface 282 of tissue 280. In this case, surface 282 may be the
patient's skin,
with instrument 225 being arranged to make percutaneous measurements. In other
instances, instrument 225 may include an endoscope coupled to a base unit that
is arranged
to perform interrogations through a body lumen, cavity, or small surgical
incision. For
these instances, surface 282 can be the boundary of the body lumen or an organ
selected
for interrogation, to name just a few examples. Depending on the configuration
of
instrument 225, the calculations performed in stage 160 may be adjusted to
account for
finite boundary conditions to improve performance. In another embodiment,
instrument
225 is arranged to place sites 239, 242a, 242b in the tissue, more closely
approximating
infinite boundary conditions. Furthermore, in alternative embodiments,
instrument 225
may be completely contained in a portable device that is battery powered.
Yet another embodiment includes interrogating a light scattering medium
including
an amount of a selected luminophore with light at an emission wavelength of
the
luminophore and sensing multiply scattered light at the emission light
wavelength in
response to this interrogation to provide a first optical characterization of
the medium.
The medium is also exposed to light at an excitation wavelength of the
luminophore and a
CA 02346728 2001-04-09
WO 00/22414 PCTIUS99/23709
multiply scattered luminescence at the emission wavelength is detected in
response to this
exposure. A value corresponding to lifetime of the luminophore is determined
from the
first optical characterization and the luminescence.
A further embodiment includes exposing a light scattering medium including an
amount of a luminophore to a number of different light wavelengths selected
relative to
the luminophore. A plurality of optical characteristics of the medium are
established by
sensing multiply scattered light at each of the different wavelengths. A value
corresponding to lifetime of the luminophore is determined from the optical
characteristics
and a multiply scattered emission at a first one of the light wavelengths
caused by
exposure of the medium to a second one of the light wavelengths.
Still a further embodiment provides for an evaluation of a light scattering
medium
including an amount of a luminophore with a light interrogation system. This
system
includes a first device optically coupled to a first site of the medium and a
second device
optically coupled to a second site of the medium that is spaced apart from the
first site.
Also included are subjecting the medium to a first light wavelength selected
relative to the
luminophore, optically coupling the first device to the second site and the
second device to
the first site, and exposing the medium to the first light wavelength after
coupling. A
value corresponding to lifetime of the luminophore is determined from a first
light output
and a second light output.
Another embodiment includes means for illuminating a light scattering medium
including a luminophore; means for characterizing light scattering behavior of
the medium
for an excitation wavelength of the luminophore and an emission wavelength of
the
luminophore; and means for determining lifetime of the luminophore from the
characterizing means and a multiply scattered light emission from the medium
at the
emission wavelength in response to illumination by light at the excitation
wavelength.
Also, because luminophores may have different activated states, they may have
multiple lifetimes corresponding to these states. While the examples described
herein are
directed to the lifetime of luminophores that are excited to a single
activated state to
preserve clarity, in other embodiments the principles of the present invention
can be
applied to luminophores with multiple activated states and lifetimes using
techniques
known to those skilled in the art. Such multiple lifetime probes are also
within the scope
of the present invention.
21
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
EXPERIMENTAL EXAMPLES
The present invention will be further described with reference to the
following
specific examples. These experiments and results are intended to be
illustrative of the
present invention and should not be considered limiting or restrictive with
regard to the
scope of the present invention. Further, any theory, mechanism of operation,
proof, or
finding stated herein is meant to further enhance understanding of the present
invention
and is not intended to make the present invention in any way dependent upon
such theory,
mechanism of operation, proof, or finding.
Several experiments were conducted with a test setup comparable to system 20.
FDPM measurements according to process 120 were conducted in a light
scattering tissue-
simulating phantom form of medium M. For these experiments the lifetimes of
different
micromolar concentrations of two luminophores of the fluorescent type where
evaluated:
(1) 3,3'-Diethylthiatricarbocyanine Iodide (DTTCI) and (2) Indocyanine Green
(ICG or
IR-125) (both from AR-COS Organics, Fair Lawn, N.J.). Container 40 for the
phantom
was a cylindrical acrylic vessel 11.3 cm in diameter and 15 cm in height. The
phantom
was prepared as an aqueous (DUIF water; Fisher Scientific, Fair Lawn, N.J.)
intralipid
(20%; Pharmacia & Upjohn Company, Clayton, N.C.) solution to mimic tissue-like
scattering properties. A DTTCI stock solution was prepared with ethanol; and
ICG was
dissolved directly in water. Appropriate quantities of stock solutions were
suspended in
1.0% Intralipid to yield the final fluorophore concentrations in the phantoms
as listed in
Table 1 that follows:
Dye Conc. ar Par isr Am 11am ftem T
[yM] [nm] [1/cm] [1/cm] [nm] [1/cm] [1/cm] [ns]
DTTCI 0.5 749 0.054(8) 9.2(12) 828 0.032(3) 10.2(8) 1.:34(3)
1.0 749 0.07(2) 6.8(15) 828 0.031(:3) 8.4(7) 1.34(4)
ICG 0.0625 778 0.039(8) 9.5(13) 828 0.033(4) 8.0(8) 0.54(3)
0.125 778 0.05(1) 7.9(20) 828 0.051(9) 9.2(15) 0.56(4)
22
CA 02346728 2001-04-09
WO 00/22414 PCT/US99/23709
Additional samples were prepared in water for spectral analysis. Figure 4
shows the
concentrations of the analyzed dyes and presents the relevant excitation and
emission
spectra measured with a spectroiluorometer (Fluorolog-2; SPEX Industries,
Inc., Edison,
N.J.).
The lifetime of DTTCI dissolved in ethanol was previously reported as 1.33
0.02
ns at 790-nm excitation and 820-nm emission wavelengths. The lifetime of ICG
was
reported to be 0.57 0.02 ns at 780-nm excitation and 830-nm emission
wavelengths
measured without scattering by use of time-domain techniques as well as by
conventional
frequency-domain fluorimetry referenced against the known lifetime of a well-
characterized fluorophore.
For the experimental examples, excitation wavelengths were provided by laser
diodes of the 56 DFS series; Melles Griot, Boulder, Colorado with
power/wavelengths of
3 mW at 749nm for ICG excitation and 25 mW at 778nm for DTTCI excitation. A 30
mW 830 rim laser diode from Melles Griot was used for emission wavelengths.
Kinematic
mirror 32 was a model 9891 from New Focus, Santa Clara and neutral density
filter wheel
34 was from Newport of Irvine. California. Source fiber 38 was a 1000- m-core
source
fiber (3M FT SILICA/0.39-NA TECS multimode fiber type). Also, fibers 44a, 44b
were
provided by two additional I000-pm-core fibers of identical length placed in
medium M at
equal depth and parallel to source fiber 38. Detector fibers 44a, 44b were
positioned
within the sample at distances r: = 1.0 cm and r2 = 2.5 cm away from source
fiber 38. The
radial separation distance (Or = 1.5cm) of the two detection fibers remained
unchanged
during all measurements. The light sensed by the fibers was delivered to
sensors 58a, 58b
in the form of gain-modulated photomultiplier tube (PMT) detectors (Model
H6573;
Hammamatsu, Bridgewater, N.J.). The detector fibers 44a, 44b were terminated
with
connectors 46a, 46b in the form of SMA fiber-optic connectors that could be
interchanged
conveniently to perform the reconfigurations of stages 130, 140, 150.
Detector 48 included neutral-density filters 56a, 56b from CVI Laser
Corporation,
Albuquerque, N.M. and interference filters 54a, 54b of a narrow-bandpass form
(10nm
FWHM; CVI Laser Corporation). Also included were lens assemblies that focused
the
collected light onto the PMTs. The gain settings of the PMTs remained
unchanged during
all measurements. The three neutral-density filters in the setup aided in
maintaining
constant dc levels of the detected PMT signals. The filters 34, 54a, 54b were
used to adapt
the setup to different output power levels of the two source laser diodes 24,
26 and to
23
CA 02346728 2009-07-31
facilitate acquisition of light intensity signals over a large dynamic range.
The PMTs were
gain modulated at the modulation frequency of the laser diodes plus an offset
frequency of
100 Hz (ow). The heterodyned PMT signals were then digitized, Fourier
transformed, and
analyzed for phase shift and amplitude attenuation by use of LabVIEWTM
software (National
Instruments Corporation, Austin, Tex.) executed by a personal computer form of
processor
70.
The experimental results illustrated in Figs. 5-7 are based on relative phase
measurements in stages 130, 140, 150. To obtain these results the stages were
performed
in a different order than illustrated in the flow chart of Figs. 2A and 2B.
Namely, Stage
150 was performed first, followed by stage 130, and then stage 140. The
measurement
protocol for the lifetime determination comprises the three stages 130, 140,
150 for each
concentration; where each stage includes measurements for two different
configurations
over a range of modulation frequencies from about 60 to about 140 MegaHertz
(MHz).
The kinematic mirror placed in the experimental setup expedited execution of
the three
stages of each measurement. Optical parameters of the sample at excitation and
emission
wavelengths were obtained from two-parameter least-squares fits of expression
(5) to the
excitation and the emission data, respectively. These optical coefficients
together with the
fluorescence phase shift then permitted the deduction of the lifetime of the
fluorescent dye
from expression (13).
The top graph (a) of Fig. 5 provides experimentally determined plots of phase
shift
versus modulation frequency for a 0.5 w1 concentration of DTTCI with the
excitation
characterization measurements of stage 130, the emission characterization
measurements
of stage 140, and the fluorescence characterization measurements of stage 150
shown in
ascending order. The bottom graph (b) of Fig. 5 provides plots of the same
type and in
the same order as top graph (a) for a 1.0 M concentration of DTTCI. The top
graph (a)
and the bottom graph (b) of Fig. 6 provide excitation, emission, and
fluorescence
characterization plots in the same order as the graphs of Fig. 5 for 0.0625 M
and
0.125 M concentrations of ICG, respectively. Fig. 7 plots the lifetime
calculation results
of stage 160 for the two concentrations for DTTCI (hollow symbols) and ICG
(solid
symbols) as based on the measurements illustrated in Figs. 5 and 6. It should
be
understood that optical parameters of the samples were not known a priori, and
lifetime
determinations were made without a reference luminophore, being based instead
on the
phase-shift measurements shown. Results of the two-parameter fits of
expression (5) to
24
CA 02346728 2011-07-11
the phase-shift data are indicated by curves that join the data symbols
obtained at the
excitation and the emission wavelengths. The optical coefficients extracted
for the
samples containing the various concentrations of DTTCI or ICG are summarized
in
Table 1.
Experiments were also conducted determining lifetime based on relative
modulation attenuation measurements with detector 48. FIG. 8 provides relative
modulation magnitude versus modulation frequency plots of fluorescence,
emission, and
excitation characterization for the two DTTCI concentrations as illustrated by
different
curve-fitted symbols. FIG. 9 provides relative modulation magnitude versus
modulation
frequency plots of fluorescence, emission, and excitation characterizations
for the two
ICG concentrations as illustrated by different curve-fitted symbols. FIG. 10
provides the
lifetime calculations for the two concentrations of DTTCI and ICG based on the
measurements plotted in FIGS. 8 and 9.
The following provide additional details related to characterization of
luminescence in a scattering medium: U.S. Patent No. 5,865,754 to Sevick-
Muraca et al;
U.S. Patent No. 5,818,583 to Sevick-Muraca et al.; U.S. Patent No. 7,187,441;
U.S.
Patent No. 7,328,059 and WO 00/22414. While the invention has been illustrated
and
described in detail in the drawings and foregoing description, the same is to
be considered
as illustrative and not restrictive in character, it being understood that
only the preferred
embodiment has been shown and described and that all changes, equivalents, and
modifications that come within the spirit of the inventions defined by
following claims
are desired to be protected.