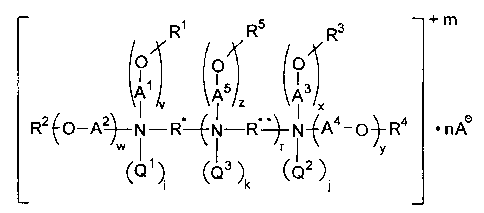Note: Descriptions are shown in the official language in which they were submitted.
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
BACKGROUND OF THE INVENTION
The present invention relates to amine and quaternary ammonium compounds
and formulations thereof useful as, for instance, fabric softeners, paper
debonders, hair
conditioners, skin conditioners, paper deinking and ink floatation agents,
asphalt emulsion
agents, corrosion inhibitor agents, ore floatation agents, pesticide emulsion
agents, car drying
aid sprays, drilling fluid additives, and the like.
Heretofore quaternary ammonium compounds and a very few dialkyl
ammonium compounds ("conventional quats") have found widespread use in many
applications. For example, a variety of conventional quats have been proposed
for many
uses, for example, in fabric softeners for home use or for industrial and
institutional use. In
general, such compounds exhibit properties which present some difficulty in
the manufacture,
formulation use, aesthetic properties, biodegradability, and environmental
compatibility of
these compositions. For example, many of the conventional compositions used
for these
functions, even if completely biodegradable with time, do not biodegrade as
rapidly as could
be desired and are thus not considered readily biodegradable. In addition,
several of the
commercial readily biodegradable softeners, conditioners, and debonders do not
function as
effectively as the conventional products that are less biodegradable. Thus, to
maintain
effective levels of performance, increased amounts of such less effective,
more readily
biodegradable products (such as softeners) must be employed and, as will be
readily
apparent, this factor decreases the cost-effectiveness of the product. In
addition, the color
and the odor of the products using conventional quats also pose problems with
many
biodegradable raw materials. Light color and low odor are essential to
obtaining customer
acceptance and to achieving stable and acceptable long-term product aesthetic
properties.
Such properties are difficult to achieve with conventional quats. Moreover,
there is
increasing interest in obtaining fabric softener and personal care
formulations which are clear
(translucent or transparent) liquids, even to the point of obtaining a crystal-
clear dispersion
when the formulation is dispensed and dispersed into rinse water (even at
levels of 50-100
pprn actives in water). Clear formulations may also offer several performance
advantages,
depending on the application, for example, clear fabric softeners offer
reduced staining of the
fabric, improved dispersibility, and greatly improved rewetting of the fabric
or other
substrate. Discovery of such clear compositions requires careful
identification of proper
quaternary and/or polyquaternary ammonium compounds, together with appropriate
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
additives, such as solvents and cosolvents, which act together to achieve the
desired
appearance. The relatively poor solubility of conventional quats also
contributes to certain
difficulties that will vary, depending on the application. For example, when
such
conventional quats are used in fabric softeners, their poor solubility
inhibits the dispersibility
of the fabric softener actives into water and the dispersibility of the
formulated fabric softener
product into the washing machine.
Thus, there remains a need for identification of new amine and ammonium
derivatives, and particular diquaternary and polyquaternary derivatives, which
are useful as
fabric softeners and which are also biodegradable, highly effective in
softening, debonding,
conditioning, and the like, and yet avoid these problems upon manufacture,
formulation and
use. It is also desirable for the active agents used in hair and skin
conditioners, paper
debonding compositions, textile softeners, and the like, to be readily
biodegradable and to
exhibit a satisfactorily high activity. Conventional products have to date not
been able to
exhibit both properties to a high degree, thus necessitating acceptance of
reduced
biodegradability or reduced activity. There is thus still a need for compounds
exhibiting
levels of activity as conditioners, paper debonders, and so on, as the case
may be, which are
comparable or superior to conventionally employed actives, such as
conventional quats, while
also exhibiting ready biodegradability.
Certain polyquaternary ammonium compounds have been disclosed. For
example, Conbere et al., U.S. Patent No. 2,87$,144, discloses the use of
certain
diquaternary ammonium compounds for use as softening and antistatic agents for
use in
aqueous textile resin treating baths (see, in particular, col. 2, lines 50-
65). Conbere et al. ,
however, discloses no actual examples of the use of diquaternary compounds in
aqueous
textile resin treating baths, does not provide any formulations incorporating
such
diquaternary compounds, nor mentions or suggests the use of such diquaternary
compounds
in any application other than for textile fabric treatment using aqueous
textile resin treating
baths, which use is very different from the applications disclosed and claimed
herein.
Moreover, the disclosure of Conbere et al. is limited to diquaternary
compounds, whereas
the instant application and claims include amines and triquats and tetraquats.
Schroeder et
al., U.S. Patent No. 5,725,736, discloses the incorporation of certain
silicone betaines into
tissue to improve the softness thereof. Schroeder et al. also discloses the
optional use of
certain polyquaternary ammonium compounds in combination with the silicone
betaines of
the invention. Schroeder et al. , however, discloses no actual examples of the
use of
polyquaternary compounds in the method of the invention, does not provide any
formulations
incorporating such polyquaternary compounds, nor mentions or suggests the use
of such
2
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
polyquaternary compounds in any application other than tissue softening and
only in
combination with certain silicone betaines.
As can be appreciated, the chemistry of fabric softeners, paper debonders,
hair conditioners, skin conditioners, textile softeners, car wax sprays, and
the like is
challenging. Each of these applications presents its own complications,
because the
interactions between the various components of the compositions must be
considered in
addition to the individual chemistry of each component. For example,
considering the fabric
softening application, the detergent compounds with the widest range of
cleaning properties
are generally anionic (negatively charged) surfactants. Such anionic
surfactants, for
example, may include the alkylbenzene sulfonates, a,-olefin sulfonates, and
xylene sulfonates
available from Witco Corporation under the WITCONATE~ trademark. In contrast,
as
exemplified by the amine and ammonium compounds discussed above, fabric
softening
compounds are generally cationic (positively charged). Thus, when the anionic
detergent
ingredients and cationic softening ingredients are present in the same aqueous
solution, they
have a natural tendency to complex together or even precipitate out of
solution. This
complexation or precipitation reaction interferes with the performance of both
the detergent
compounds and the softening compounds and is therefore undesirable. It can be
readily
appreciated that this undesirable complexation or precipitation reaction may
occur if both
detergent and softener compounds are added together in a wash cycle; however,
as North
American washing machines typically rinse the clothes only once before fabric
softener is
added to the washload, even if the fabric softener is added during a rinse
cycle (as is typically
done), residual anionic detergent compounds (including builders) present in
the fabric
complexes with the cationic softener compounds.
SUMMARY OF THE INVENTION
The present invention achieves these objectives and also exhibits the
properties and advantages described herein.
One aspect of the present invention comprises compounds of the following
structural formula (hereinafter "structural formula (1)"):
R~ R5 s + m
R
O O O
~5 ~3
A z A X
RZ~O-q~--N-R--~N-R"~-N-~Aa-O~-R4 ~ nA~
v
W~QS ~. ~Q3 ~ ~Qz ~.
i k
3
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
wherein each of R* and R** is independently a linear, branched or cyclic
alkylene group
containing 2 to 12 carbon atoms, wherein no two nitrogen atoms are separated
by fewer than
2 carbon atoms;
each of A1, A2, A3, A4, and AS is independently a straight or branched
alkylene
containing 2 to 4 carbon atoms;
each of R', R2, R3, R4, and RS is independently -H or RAC(O)- wherein RA is
straight or branched alkyl or alkenyl containing 7 to 21 carbon atoms and 0 to
4 carbon-
carbon double bonds; provided that at least one of R' , RZ, R3, R°, or
RS is RAC(O)-;
each of Q~, QZ and Q3 is independently -H, -CH3, -CZHS, -C3H7, -C4H5, benzyl,
CH2COOH, or -CHzCOOA-; or, if R* is a -CHZCHz- group, Q1 and Q3 together or Q'
and
QZ together may be a -CH2CH2- group to form a six-membered piperazine ring;
or, if R** is
a -CHZCHZ- group, Q3 and Q3 together may be a -CH~CHZ- group to form a six-
membered
piperazine ring;
m is 0 to 4; r is 0 to 2; each of v, w, x, y, and z is independently 1 to 8;
i is 0 to 1, j is 0 to 1, and each k is 0 to 1, and the sum of (i + j +k) is 0
to 4;
each A- is independently an anion as defined below; and n is the number of
moles of
A- needed to give the compound of structural formula ( 1 ) a zero net charge.
In a preferred embodiment of the invention, the composition includes water
in the formulation. In another preferred embodiment of the invention, the
formulation does
not contain a significant amount of silicone betaines or any silicone
betaines. In a further
preferred embodiment of the invention, the formulation does not contain a
significant amount
of silicone compounds or any silicone compounds. In yet another preferred
embodiment of
the invention, the formulation does not contain a significant amount of
textile resin treating
compounds or any textile resin treating compounds.
In another preferred embodiment of the invention, the composition comprises
a mixture of two or mare different compounds of structural formula (1). In a-
preferred
embodiment of the invention, m is from about 1 to 4, more preferably 2 to 4,
and most
preferably 3 to 4.
Another embodiment of the invention comprises compounds of structural
formula (1) is compounds wherein at least one of v, w, x, y, and z is greater
than 1. In a
preferred embodiment of the invention, each of v, w, x, y, and z is greater
than 1. Another
embodiment of the invention includes compounds of structural formula (1)
wherein
substituents Q', QZ and Q3 are not present, or there is one or more
substituents selected from
Q', QZ and Q3 and any such substituent present is -H. Another embodiment of
the present
invention further comprises an amine salt, polyamine salt, or mixture thereof.
Yet another
CA 02347036 2001-04-11
WO 00/21918 PCTNS98/21683
embodiment of the present invention comprises liquid compositions, useful for
instance as
fabric softeners, comprising (a) a component selected from the group
consisting of
compounds of structural formula (1) and mixtures thereof; (b) water; and (c)
optionally, one
or more solvents or cosolvents.
Another aspect of the present invention comprises a composition comprising:
(a) a compound of structural formula (1); and (b) a second surfactant selected
from
the group consisting of anionic surfactants, cationic surfactants,
zwitterionic surfactants,
nonionic surfactants, amphoteric surfactants, and blends thereof.
In an embodiment of the present invention, the composition further comprises
water. In an embodiment of the present invention, the second surfactant
comprises a
conventional quaternary compound. In another embodiment of the present
invention, the
composition does not contain a significant amount of silicones. In yet another
embodiment of
the present invention, the composition comprises a mixture of two or more
different
compounds of structural formula (1). In a further embodiment of the present
invention, the
composition comprises a mixture of two or more different conventional
quaternary
compounds. In an embodiment of the present invention, the secondary surfactant
is selected
from the group consisting of: alkylbenzene sulfonates, a-olefin sulfonates,
and xylene
sulfonates. In another embodiment of the present invention, the secondary
surfactant is
selected from the group consisting of: nonylphenol ethoxylates; CS-CZO linear
or branched
alcoxylates using EO, PO, iPO, BO, or mixtures thereof; amine ethoxylates;
fatty amide
ethoxylates; fatty acid ethoxylates; carboxylated nonionics; a-polyglucosides;
and mixtures
thereof. In yet another embodiment of the present invention, the secondary
surfactant is an
alkanolamide. In an embodiment of the present invention, the secondary
surfactant is an
amine oxide.
A further aspect of the present invention comprises a composition
comprising: (a) a compound of structural formula (1); (b) a solvatrope or
coupling agent or
blends thereof; and (c) an oil or hydrophobic organic component and blends
thereof.
In an embodiment of the present invention, the composition further comprises
water. In another embodiment of the present invention, the amount of the
compound of
structural formula (I) is about 0. I wt. % to about 65 wt. % of the
formulation; the amount of
solvatrope or coupling agent is about 0.1 wt. % to about 65 wt. % of the
formulation, the
amount of oil or hydrophobic organic component is about 0.1 wt. % to 65 wt. %
of the
formulation, and the amount of water is about 20 to 99.7 wt. % of the
formulation, preferably
about 35 wt. % to about 99.7 wt. % . In yet another embodiment of the present
invention, the
oil or hydrophobic organic component is selected from the group consisting of:
fatty acids;
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
fatty amides; fatty alcohols; fatty oils; fatty esters made from a Cg-CZZ
fatty acid and a CI-Cg
alcohol; dialkyl esters; mineral oil; mineral seal. oils; silicone oils;
petrolatums;
monoglycerides; diglycerides; triglycerides; aliphatic, paraffinic, and
naphthalinic
hydrocarbons; oils and spirits; and mixtures thereof. In other embodiments of
the present
invention, the composition comprises a mixture of two or more different
compounds of
structural formula (1) or a mixture of two or more different solvents or
coupling agents. In
another embodiment of the present invention, the iodine value of the fatty
acid used to make
the compound of structural formula (1) ranges from about 30 to about 140,
preferably from
about 80 to about 120, most preferably, from about 90 to about 110.
In an embodiment of the present invention, the composition is emulsified into
a microemulsion. In another embodiment of the present invention, the
composition is clear.
In yet another embodiment of the present invention, the amount of the compound
of
structural formula (1) is about 5 wt. % to about 50 wt. % of the formulation,
preferably about
10 wt. % to about 30 wt. % , and most preferably about 15 wt. % to about 25
wt. % ; the
amount of solvatrope or coupling agent is about 2 wt. % to about 15 wt. % of
the formulation,
preferably about 3 wt. % about 10 wt. %, and most preferably about 6 wt. % to
about 10
wt. %; the amount of oil or hydrophobic organic component is about 5 wt. % to
50 wt. % of
the formulation, preferably about 10 wt. % to about 30 wt. % , and most
preferably about 15
wt. % to about 20 wt. % ; and the amount of water is about 10 to 70 wt. % of
the formulation,
preferably about 20 wt. % to about 60 wt. %, and most preferably about 30 wt.
% to about 50
wt. % .
In an embodiment of the present invention, the composition is used as a
personal care formulation, wherein the amount of the compound of structural
formula (1) is
about 0.1 wt. % to about 65 wt. % of the formulation, preferably about 0. I
wt. % to about 25
wt. % , and most preferably about 0.1 wt. % to about 5 wt. % ; the amount of
solvatrope or
coupling agent is about 0.1 wt. % to about 65 wt. % of the formulation,
preferably about 0.1
wt. % about 25 wt. %, and most preferably about 0.1 wt. % to about 0.5 wt. %;
the amount of
oil or hydrophobic organic component is about 0.1 wt. % to 65 wt. % of the
formulation,
preferably about 0.1 wt. % to about 25 wt. % , and most preferably about 0.1
wt. % to about 5
wt. %; and the amount of water is about 20 to 99.7 wt. % of the formulation,
preferably about
wt. % to about 99.7 wt. % , and most preferably about 65 wt. % to about 99.7
wt. % .
In another embodiment of the present invention, the composition is a
pesticide emulsion agent formulation further comprising water. In yet another
embodiment
of the present invention, the composition is a pesticide formulation
comprising the pesticide
35 emulsion agent formulation and an effective amount of pesticide. In another
embodiment of
CA 02347036 2001-04-11
WO 00/21918 PCTNS98/21683
the present invention, the pesticide formulation comprises an amount of
compound of
structural formula ( 1 ) ranging from about 5 wt. % to about 50 wt. % ,
preferably from 10
wt. % to about 40 wt. % , of the pesticide formulation.
Other aspects of the instant invention include using the compounds of
structural formula (1) either alone or in formulations, as a fabric softener
(either alone or in a
combination detergent/fabric softener), as a car spray microemulsion, as a
paper debonder,
as a hair or skin conditioner, as a corrosion inhibitor, as an asphalt
emulsifier, as an
organoclay ingredient, or as an agricultural product emulsifier. The
polyethoxylated
compounds of structural formula (1) may also find uses as textile treatments,
particularly
when used in conjunction with a resin bath. Other aspects of the present
invention include
such methods and processes using the compounds of structural formula (1)
either alone or in
formulations.
It can be seen from the above, that ranges in amounts given for each
ingredient or component of a composition or formulation set forth herein in
certain
circumstances may be theoretically capable of adding up to a sum of greater
than 100% . As
would be appreciated by those of skill in the art, it is understood that such
impossible
formulations (that is, those formulations whose component amounts add to a sum
greater than
100%) are excluded from the claims and disclosure. For example, a formulation
having
components A and B, where the amount of A is said to range from 25 % to 75 %
and the
amount of B is said to range from 25 % to 55 % , if containing 65 % of A, is
understood to
have 35 % or less of B in that formulation, so that the sum of A and B does
not exceed 100 % .
Thus, all formulations or compositions presented herein whose component
amounts add to a
sum less than or equal to 100% are understood as being part of the claims and
disclosure.
DETAILED DESCRIPTION OF THE INVENTION
The present invention is directed to novel polyester amine compounds and
polyester polyquaternary compounds, compositions and formulations containing
such novel
compounds, and uses thereof.
A. PREFERRED COMPOUNDS AND COMPOSITIONS
As will be appreciated, a particularly preferred embodiment of the present
invention comprises mixtures of compounds corresponding to structural formula
(1), such
that degrees of quaternization or protonation (including unquaternized,
unprotonated
compounds), degrees of esterification, and chain lengths and molecular weights
of different
molecules within the mixture will differ such that the respective properties
can be represented
as an average over all the molecules present in the mixture. In such mixtures,
then,
7
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
properties such as the degree of quaternization can be expressed as average
values which may
lie between integer whole numbers.
Referring to structural formula ( 1 ), the groups depicted as Q' , QZ and Q3
represent substituents which, when present, protonate (if they are -H) or
quaternize (if they
are any other group) the respective nitrogen atoms to which they are bonded.
While the
structure of structural formula ( 1 ) embraces compounds ranging from
compounds wherein no
nitrogen atom is quaternized or protonated with a corresponding Q substituent,
through
compounds wherein every nitrogen atom is quaternized or protonated with a Q
substituent,
one preferred embodiment is compounds wherein the compound of structural
formula ( 1 )
should be partially quaternized. The term "partially" is meant to convey that
at least one
nitrogen atom on the molecule as depicted in structural formula ( 1 ) does not
have a Q
substituent attached to it. In particular, in mixtures of compounds which are
diamines (that
is, in structural formula (1), the subscript r is zero), there is on average
in the mixture
preferably 1-2, and more preferably I.5-2, quaternizing groups Q per molecule.
Another preferred embodiment is compounds, and mixtures thereof, in which
there are either no Q substituents or the only Q substituents present are -H.
Such Q
substituents can be present in a degree such that all nitrogen atoms are
protonated, or fewer
than all nitrogen atoms are protonated ("full" and "partial" protonation,
respectively).
Although many of the teachings and disclosure below uses the terms "quats",
"ester quats",
"polyester quats", and similar language with respect to applications and
formulations
including compounds of structural formula (1), such disclosures should be
understood to
apply equally to the non-quaternized compounds according to structural formula
(1), unless
specifically and unambiguously excluded.
The compounds of structural formula ( 1 ) may also have any of a range of
degrees of esterification, depending on the application. As shown in
structural formula (1),
the degree of esterification can range from 1 to 4 for diamines, arid up to 5
or 6 for- triamines
or tetramines, respectively. The preferred degree of esterification, that is,
the total number
of RA groups present, preferably ranges from about 2 to about 4. This is true
especially for
diamines, wherein a preferred degree of esterification is from 1 to about 3
for clear,
translucent formulations and about 2 to 3.5 for easily dispersible
formulations, and the more
preferred degree of esterification is about 2.0 to about 2.5; for triamines
and tetramines the
preferred degree of esterification should be adjusted accordingly; that is,
approximately one
additional ester group per additional amine group. In emulsifier applications,
the compound
of structural formula (1) is prepared having only about 1 to about 2 ester
groups per molecule
to ensure that the molecule as a whole is less hydrophobic than those that
would typically be
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
employed in other applications. Any desired degree of esterification can be
attained by
reaction between the polyhydroxy-substituted precursor. and a
stoichiometrically appropriate
amount of the corresponding carboxylic acid(s). For instance, even a
noninteger target
degree of esterification such as 2.5 can be attained by reaction to form a
mixture containing
S equal amounts of triester and diester.
The respective RAC(O)- acyl groups can all have the same chain lengths.
More preferably they have several chain lengths and degrees of carbon-carbon
unsaturation,
reflecting the fact that the fatty acyl groups can be derived from naturally
occurring sources
which contain mixtures of fatty acids with differing chain lengths and
differing degrees of
carbon-carbon unsaturation. Examples of such sources include fatty acids
derived from the
following sources: tallow, fish oils, canola (including fatty acids derived
from partially
hydrogenated canola), jojoba, palm, coconut, avocado, wheat germ, rapeseed,
olive, orange,
corn, linseed, neem, peanut, safflower, sesame seed, soybean, sunflower seed,
and cocoa
butter. Preferred materials are tallow, canola, and palm. If a clear
formulation is desired,
the use of an unsaturated fatty acid is preferred to make the compound of
structural formula
( 1 ) or at least a portion of the compound of structural formula ( 1 ) used
in the formulation.
The degree of unsaturation of a compound is typically expressed as the iodine
value (LV.).
If a clear formulation is desired, the I. V . of the fatty acid used to make
the compound of
structural formula ( 1 ) ranges from about 30 to about 140, more preferably
the I. V . ranges
from about 80 to about 120, most preferably the LV. ranges from about 90 to
about 110.
Such clear formulations have an additional advantage: they generally exhibit
excellent
freeze/thaw recovery.
Referring to structural formula (I), the molecules shown can be diamines,
triamines, or tetramines. For many purposes, the diamines are preferred. The
R* bridge
linking the two nitrogen atoms of the diamine can contain 2 to 12 carbon
atoms, and
preferably contains 3 to 12 carbon atoms, most preferably 6 to 10 carbon
atoms. One
particularly preferred R* group is an alkylene containing 6 to 12 carbon
atoms, such as linear
hexamethylene. Other useful groups can contain up to 10 carbon atoms between
the nitrogen
atoms, or anywhere from 3 to 10 carbon atoms presenting straight or branched
alkylene
structures. The various moieties depicted at structural formula (I) as A~-S
can each,
independently, be ethyl, propyl (straight or branched) or butyl (straight or
branched).
Preferably, each of the A1-5 groups present is the same, for ease of
synthesis. It is also
preferred that each of A1-5 is ethyl or propyl (especially isopropyl). Further
preferred
embodiments of compounds of structural formula (1) are described herein with
respect to the
various formulation capabilities of the products of this invention. The
compounds of
9
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
structural formula (1), when quaternized and/or protonated to any degree,
include an anion
A- which is present in a number of moles equal to the total positive charge of
the nitrogen-
containing canon. The anion A- can represent any anion which is not
deleterious to the
properties of the overall compound. Non-limiting examples of A- include
fluoride, chloride,
bromide, iodide, chlorite, chlorate, hydroxide, hypophosphite, phosphite,
phosphate,
carbonate, formate, acetate, lactate, and other carboxylates, oxalate, methyl
sulfate, ethyl
sulfate, benzoate, and salicylate, and the like. Preferred examples are
chloride, bromide,
methyl sulfate, ethyl sulfate, and salicylate. If the anion is monovalent (has
a charge of -I),
A- represents the anion group, if the anion is divalent (has a charge of -2),
A- represents half
of the anion group, if the anion is trivalent (has a charge of -3), A-
represents a third of the
anion group, and so on. As will be appreciated given these definitions, m will
always equal
n in structural formula (1). The compounds of structural formula (I) include
structures that
incorporate a piperazine ring. For example, if R* is a -CHZCHz- group, Q' and
Q3 together
or Q' and QZ together may be a -CHzCH2- group to form a six-membered
piperazine ring;
or, if R** is a -CHZCHZ- group, Q3 and Q3 together may be a -CHZCHZ- group to
form a
six-membered piperazine ring.
As used in this application, the term "monoalkoxylated compound" or similar
term means a compound of structural formula (1) wherein none of v, w, x, y,
and z is greater
than i . Thus, a monoalkoxylated compound does not contain any polymeric
chains of alkoxy
groups (that is, two or more alkoxy groups attached together) attached
directly to a nitrogen
atom of a compound of structural formula (1). In contrast, the term
"polyalkoxylated
compound" or similar term means a compound of structural formula (1) wherein
any of v, w,
x, y, and z is greater than 1. Thus, a polyalkoxylated compound contains at
least one
polymeric chain of alkoxy groups (that is, two or more alkoxy groups attached
together)
attached directly to a nitrogen atom of a compound of structural formula (1).
The instant invention involves compounds of structural formula (1) and
formulations thereof, and uses thereof. In certain parts of this application,
however,
distinctions will be made between monoalkoxylated compounds of structural
formula ( 1 ) and
polyalkoxylated compounds of structural formula (1) and such distinctions will
be explicitly
made. If no such distinction is made, it is be understood that the statement
applies both the
monoalkoxylated compounds of structural formula ( 1 ) and the polyalkoxylated
compounds of
structural formula (1). Thus, the term "alkoxylated compounds" includes both
monoethoxylated compounds and polyethoxylated compounds as defined above.
Similarly,
the term "ethoxylated compounds" includes both monoethoxylated compounds and
polyethoxylated compounds.
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21b83
The compounds of structural formula ( 1 ) can be used alone or in mixtures,
used in combination with other compounds or additives, or used as a
formulation with other
compounds or additives, depending on the intended use and the advantages and
disadvantages
attendant with each alternative application method. Some examples of compounds
or
additives that may be used in conjunction with compounds of structural formula
(1) or made
into formulations with compounds of structural formula ( 1 ) . include
surfactants or detergents,
especially quaternary ammonium compounds, perfumes, preservatives, insect and
moth
repelling agents, polymeric soil release agents, antistatic agents, dyes and
colorants, viscosity
control agents, antioxidants, silicones, mineral oils and petrolatums,
synthetic lubricants,
defoaming agents, antifoaming agents, emulsifiers, brighteners, opacifiers,
freeze-thaw
control agents, shrinkage control agents, and mixtures thereof. Many examples
of these
additives are set forth in detail and are intended to demonstrate the scope of
the invention.
Other compounds or additives familiar to those of skill in the art and
appropriate to a
particular use, however, may also be used with or formulated with the
compounds of
structural formula ( 1 ).
The compounds of structural formula (1) (either alone or in combination with
other compounds) have many potential applications. For example, the compounds
of
structural formula (1) may be used as detailed herein, without limitation, as
a fabric softener
(either alone or in a combination detergent/fabric softener), as a car spray
microemulsion, as
a paper debonder, as a hair or skin conditioner, as a corrosion inhibitor, as
an asphalt
emulsifier, as an organoclay ingredient, or as an agricultural product
emulsifier. The
polyethoxylated compounds may also fmd uses as textile treatments,
particularly when used
in conjunction with a resin bath.
B. APPLICATIONS
The present disclosure shows that the compounds and formulations of the
present invention may be used for many purposes and suitable additives may be
incorporated
therein based on the ultimate application. Such ingredients, for example, may
contribute
significantly to the ease of formulation, stability, dispersibility, fluidity,
and the performance
properties of the compositions.
In one aspect, the present invention provides compounds and formulations
that have the ability to impart to fabric (that is, articles of clothing,
textiles, and so forth),
properties including softness to the touch, ease of handling, increased
lubricity, and a
reduced tendency to carry or pick up static electricity. One form in the
compounds and
formulations of the present invention are provided is as a liquid, for
instance, as an emulsion
or as a solution/suspension of the desired components. During use, an
appropriate controlled
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
amount of the liquid formulation is employed, for example, by pouring the
formulation
directly into the washing machine. Typically, the formulation is dispensed
during the rinse
cycle of the washing machine, either poured in by hand or metered in by an
appropriate
automatic metering device with which the washing machine is equipped. In
addition, the
S present invention also provides compounds and formulations that may find use
as textile
treatments to add lubricity and finishing to the fabric prior to shipping the
textile to market,
particularly when used in conjunction with a resin bath. In such an
application, the textile
mill would typically apply the formulation in dilute emulsions and rapidly dry
the excess
water from the fabric to lubricate the fibers and give it a surface finish.
Moreover, the
present invention provides compounds and formulations that have the ability to
impart to
paper and paper products softness, lubricity, and antistatic properties, and
improve ease of
handling of the substrate and surface appearance; in the papermaking process,
such
compounds of the present invention are termed debonders. Debonders are usually
added to
the paper fibers in the head tank or headbox of a papermaking machine where
the paper
fibers are pulped as an aqueous slurry just prior to feeding the resulting
slurry onto the
papermaking or dewatering screen. These debonders condition the fibers to give
improved
softness feeling to the paper fibers that is valuable for their use in tissue
and towelmaking.
The compositions and formulations of the present invention can also be
incorporated into the
paper or tissue by any suitable means such as spraying or printing onto the
surface of the
paper or tissue.
The present invention also provides compounds and formulations that are
useful in personal care products such as hair or skin conditioners. In this
application, the
present invention provides formulations that impart softness, lubricity, and
improve the
surface appearance of the skin or hair. The hair conditioners additionally
reduce the
tendency for tangling, improve the manageability, and impart a soft feel to
the hair strands.
Such hair conditioners are applied as dilute emulsions to the hair following
its wash or may
be incorporated into a combined conditioner and shampoo composition, also
known as a
conditioning shampoo, two-in-one shampoo, or two-in-one. Such hair and skin
conditioning
formulations typicaily incorporate effective amounts, for example, 0.1 wt. %
to 10 wt. % or
more, of emollients, humectants, and/or slip and conditioning agents, such as
organopolysiloxanes and the like, to create formulations that are monophasic
and can be
made to be translucent or even clear. Compounds suitable for use as
emollients, humectants
and conditioners in formulations for skin care or hair care can be found in
the CTFA
Cosmetic Ingredient Dictionary, 3d Edition, and the CTFA Cosmetic Ingredient
Handbook.
~z
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
The compositions of the present invention are particularly useful in
applications that take advantage of their ability to disperse hydrophobic
material, to stabilize
foam, and to enhance the penetration and wetting exhibited by the
compositions. Examples
of such compositions and applications are set forth below, each revealing an
additional aspect
of the present invention. For example, the compounds and formulations of the
present
invention may be used as oil dispersants and oil slick dispersant formulations
for application
onto oil, for example, onto a film of oil, to disperse the oil. The compounds
and
formulations of the present invention may also be used as oil well stimulation
and oil
recovery aids for injection into oil wells in order to penetrate into the
surface of the borehole
and assist liberation of crude oil from the matrix material into the borehole,
from which it
can be brought to the surface. In addition, the compounds and formulations of
the present
invention may be used as vehicles for hydrophobic sheeting agents such as
mineral oil and
silicone oil. Such oils can readily be dispersed in compositions, according to
the present
invention, and the resulting formulations are highly satisfactory when sprayed
or otherwise
applied to a surface, such as a freshly washed automobile surface, to impart a
lustrous,
water-repellent film to the surface. The compounds and formulations of the
present invention
may also be used as rinse aids, such as used in automatic dishwashers, wherein
application of
the composition of the present invention disperses residual hydrophobic
matter, including
cleaner residues and films. Furthermore, the compounds and formulations of the
present
invention may be used as paper deinking and ink flotation agents for treating
waste inked
paper by addition to the pulp slurry such that the ink is liberated from the
paper and
prevented from redepositing onto the paper. In this application, the ink is
typically dispersed
or even fully solubilized in the resulting solution when the ink particles are
floated from the
fibers. The compounds and formulations of the present invention may also be
used as asphalt
emulsion agents for emulsifying finely divided asphalt (at loadings of
typically 1-20 wt. % ),
with or without particulate filler such as sand, in an aqueous phase which
comprises the
composition according to the present invention. Moreover, the compounds and
formulations
of the present invention may be used as corrosion inhibitor agents for
application to any
surface to which one desires to apply a film that protects against corrosion.
The composition
would typically contain an effective amount of a hydrophobic corrosion
inhibiting material,
such as liquid or waxy-solid fatty ester, paraffinic hydrocarbon, silicone, or
the like,
dispersed in a composition according to the present invention. In addition,
the compounds
and formulations of the present invention may be used with ore flotation
agents for separating
ore from rock. Such floatation agents might include, for example, the agent
available from
Witco Corporation under the tradename WITCAMINE~ AIA2-12. Typically, the ore
~3
CA 02347036 2001-04-11
WO 00121918 PCT/US98/21683
floatation agent (a collector or frother, depending on the characteristics of
the particular
separation desired in the flotation cell) or mixture thereof, which is a
relatively hydrophobic
material, is dispersed in a composition according to the invention and an
effective amount is
added (on a batch or continuous basis) to the ore separation cell. This
permits the formulator
to improve the dispersibility of the hydrophobic ore floatation agent, which
often improves
the performance of the mineral separation by improving the efficiency of the
floatation
agent's dispersibility. This can enable the operator to use smaller amounts of
the ore
floatation agent to achieve the desired purpose because there is a higher
concentration of
active ingredients available. In addition, the compounds and formulations of
the present
invention may be used as suspension concentrates and emulsifiable concentrates
of
herbicides, pesticides, miticides, fungicides, or bactericides, wherein one or
more liquid or
solid, generally hydrophobic, active ingredients are dispersed in a
composition according to
the present invention. The resulting concentrate can be applied as a
concentrate on or around
desired vegetation, but is more often mixed with water (for example, at the
point of use) to
form a final dilute formulation having the desired concentration of active
ingredient(s). This
application takes advantage of the noteworthy property of this invention that
addition of the
water does not disrupt the monophasic state, nor the fluidity, of the
formulation.
As noted above, the compositions and formulations of the present invention
can also optionally contain other components, depending on the additional
properties one may
wish to provide in the finished composition. Such additional components
include, but are not
limited to, additional coupling agents and solvents, additional quaternary
ammonium
compounds, additional surfactants, hydrocarbon actives, perfumes,
preservatives including
bacteriocides and fungicides, insect and moth repelling agents, polymeric soil
release agents,
antistatic agents, dyes and colorants, especially bluing agents, viscosity
control agents,
antioxidants, silicones, defoaming agents, antifoaming agents, emulsifiers,
brighteners,
opacifiers, freeze-thaw control agents, shrinkage control agents, aloe,
humectants, skin
protectants, feel modifiers, and mixtures thereof.
C. SYNTHESIS
Compounds of structural formula (1) can easily be synthesized from readily
available starting materials using reaction procedures and conditions quite
familiar to those of
ordinary skill in this art. The preferred procedure begins with a diamine,
triamine or
tetramine of structural formula HZN-R*-(NH-R**)~ NHS. Preferred compounds
having the
required structural formula include hexamethylenediamine (HMDA),
bis(hexamethylene)triamine (BHMT), 1,2-diaminocyclohexane; 1,3-pentanediamine,
and 2
methylpentamethylenediamine (MPMD). This polyamine compound is next
alkoxylated
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
using an alkoxylating agent. The alkoxylating agents may be ethylene oxide
(EO), propylene
oxide (PO), isopropylene oxide (iP0), or butylene oxide (BO), or (less
preferred) a mixture
thereof, depending on the desired choice of alkyl groups to become attached to
the respective
nitrogen atoms. The number of moles of alkylene oxide reactant and the
reaction conditions
are established such that the terminal primary amine hydrogen atoms are
disubstituted with
hydroxyalkyl, and each of the secondary amines, if any, is monosubstituted
with
hydroxyalkyl. It should be noted that polyethoxylated polyamine products
typically are quite
dark (4-8 Gardner units) after only 8 to 12 moles of EO are added. If
colorless
polyalkoxylated products are desired, it is often necessary to react the
polyamine with a
sufficient amount of PO, iPO, or BO or a mixture thereof to convert the amine
groups into
tertiary amine groups before addition of EO, usually with the addition of
sodium borohydride
and an alkaline catalyst. Although such product is not a pure ethoxylated
amine, it is
desirably colorless.
Next, the alkoxylated product is esterified by reaction of fatty acids with
the
respective hydroxyl groups resulting from the alkoxylation. As indicated
above, the
molecule may be completely esterified, but it is preferred for formulation
reasons to achieve
only partial esterification. There should, however, be at least one ester
group per molecule
of structural formula (1). The esterification is carried out with carboxylic
acids of structural
formula RAC(O)OH, wherein RA is straight or branched alkyl or alkenyl
containing 9 to 21
carbon atoms and 0 to 4 carbon-carbon double bonds. While the esterification
can be carried
out with an appropriate quantity of one such carboxylic acid, it is preferred
for reasons of
economy, product performance, and convenience to employ mixtures of carboxylic
acids
each corresponding to structural formula RAC(O)OH. For instance, mixtures of
such fatty
acids from various animal and vegetable origins are conveniently commercially
available.
One example of such material is tallow fatty acids which, as is generally
known in this field,
is a mixture of fatty acids predominantly composed of fatty acids containing
14, 16, and 18
carbon atoms, and 0, 1, and 2 degrees of unsaturation. Other preferred sources
include
coconut fatty acids and canola fatty acids, although any suitable fatty acid
may be used.
Esterification is carried out under conventional conditions, well-known to the
chemist in this
field, allowing for the withdrawal of byproduct water. The number of moles of
fatty acid is
selected to provide the desired average degree of esterification in the
mixture of products
formed upon esterification.
Next, if desired, the esterified product or mixture of esterified products is
quaternized and/or protonated. As recognized hereinabove, the esterified
product can be
completely quaternized, but it is preferred to carry out partial
quaternization only, if any.
,s
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
Quaternization is carried out under conditions well known in this field for
quaternization of
amines, by reaction of the esterified amine with a . suitable quaternizing
agent. The
quaternizing agents have the formulas Q1A and QUA, which can of course be the
same and
preferably are the same. With triamines and tetramines a second (or third)
quaternizing
agent Q3A can be used; it is likewise preferably the same as Q1A and QzA.
Preferably, only
one particular quaternizing agent is employed, in which case all of the
quaternizing
substituents Q will be the same. Preferred quaternizing agents include methyl
chloride,
dimethyl sulfate (DMS), and diethyl sulfate (DES).
Protonation, if desired, can be carried out by reacting the esterified product
or mixture of products with an acid of the formula HA, such as hydrochloric
acid, or other
strong acids, such as sulfuric acid or phosphoric acid. Each of the foregoing
reactions can be
carried out in solvent or in solvent-free conditions, in each case employing
conditions well
established for the respective reactions in this field.
Preferred diamine compounds of the present invention include
hexamethylenediamine (HMDA) alkoxylated ester diamines or quats, most
preferably HMDA
alkoxylated ester quats, which are made according to the above method.
Accordingly,
HMDA may be reacted with 4 moles of alkoxylating agent per mole of HMDA. If a
polyalkoxylated HMDA ester compound is desired, more than 4 moles of
alkoxylating agent
would be used, for example, 4.1 to 32 moles of ethylene oxide. The resulting
HMDA
alkoxylate is then esterified by reaction with 1 to 4 moles of fatty acid, for
example, tallow
fatty acid. The resulting HMDA alkoxylated ester may then be quaternized with
up to 2
moles of dimethyl sulfate (DMS) per mole of HMDA. Example 1 below details the
synthesis
of a particular HMDA monoalkoxylated ester quat. Preferred triamine compounds
of the
present invention include bis(hexamethylene)triamine (BHMT) alkoxylated ester
quats, most
preferably BHMT ethoxylated ester quats, which are made according to the above
method.
Accordingly, BHMT may be reacted with 5 moles of ethylene oxide per mole of
BHMT. If
a polyalkoxylated BHMT ester compound is desired, more than 5 moles of
alkoxylating agent
would be used, for example, 5.1 to 40 moles of ethylene oxide. The resulting
BHMT
alkoxylate is then esterified by reaction with 5 moles of fatty acid, for
example, canola fatty
acid. The resulting BHMT aikoxylated ester may then be quaternized with up to
3 moles of
dimethyl sulfate (DMS) per mole of BHMT.
D. FORMULATIONS AND PROPERTIES
The products as described herein exhibit a number of desirable properties
making them particularly suitable for formulation into commercial products
such as fabric
softeners and other commercial products, as mentioned above.
/~a
CA 02347036 2001-04-11
WO 00121918 PCT/US98/21683
Most notably, the compounds of structural formula (1) can readily be
formulated into useful compositions such as aqueous compositions, which
achieve the desired
functionality and which are clear, that is, transparent or translucent. This
property can be
realized at a variety of concentrations of active ingredient, with or even
without special
solvents or coupling agents. In addition, microemulsion formulations can be
made that
improve the ease of application and effectiveness of the resulting
formulation.
Emulsion or microemulsion formulations according to the present invention
have many applications, for example, as car "cheater" wax spray technology to
improve
beading and act as a drying aid, as fabric softeners, and as personal care
products, for
example, as a moisturizer/conditioner if the compound of structural formula
(1) is not skin
irritating. In addition, such emulsions or microemulsions may fmd uses in
paper
debonding/finishing, as emulsifiers for silicone oils and pesticides, and as
emulsifiers/softeners for textile finishing. Generally such formulations
include three
components: (a) compound of structural formula ( 1 ), (b) a solvatrope or
coupling agent and
1S blends thereof, and (c) an oil or hydrophobic organic component and blends
thereof, which
are blended in water. These microemulsion formulations give stable, clear
(translucent)
products that do not go through thick or viscous gel phases but disperse
readily and quickly
into very fme particle size microemulsions when diluted or dispersed in water.
These
microemulsions have many additional advantages over conventional
microemulsions, such as
the products sold by Witco Corporation under the tradename CARSPRAYTM: reduced
particle size, improved beading and sheeting over, and improved
biodegradability, especially
when used with methyl ester oils.
If the resulting formulation is intended to be used as a personal care
formulation, the amount of the compound of structural formula (1) is about 0.1
wt.% to
about 65 wt. % of the formulation, preferably about 0.1 wt. % to about 25 wt.
%, and most
preferably about 0.1 wt. % to about 5 wt. % ; the amount of solvatrope or
coupling agent is
about 0.1 wt. % to about 65 wt. % of the formulation, preferably about 0.1 wt.
% about 25
wt. % , and most preferably about 0.1 wt. % to about 0.5 wt. % ; the amount of
oil or
hydrophobic organic component is about 0.1 wt. % to 65 wt. % of the
formulation, preferably
about 0.1 wt. % to about 25 wt. %, and most preferably about 0.1 wt. % to
about 5 wt. % ; and
the amount of water is about 20 to 99.7 wt. % of the formulation, preferably
about 35 wt.
to about 99.7 wt. % , and most preferably about 65 wt. % to about 99.7 wt. % .
A further
example of personal care microemulsions according to the invention is provided
below as
Example 29. If the resulting formulation is intended to be used in a car
drying aid
formulation or other application, the amount of the compound of structural
formula (1) is
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
about 5 wt. % to about 50 wt. % of the formulation, preferably about 10 wt. %
to about 30
wt. % , and most preferably about 15 wt. % to about 25 wt. % ; the amount of
solvatrope or
coupling agent is about 2 wt. % to about 15 wt. % of the formulation,
preferably about 3 wt.
about 10 wt. % , and most preferably about 6 wt. % to about 10 wt. % ; the
amount of oil or
hydrophobic organic component is about 5 wt. % to 50 wt. % of the formulation,
preferably
about 10 wt. % to about 30 wt. % , and most preferably about 15 wt. % to about
20 wt. % ; and
the amount of water is about 10 to 70 wt. % of the formulation, preferably
about 20 wt. % to
about 60 wt. % , and most preferably about 30 wt. % to about 50 wt. % .
Other properties are realized as well. For instance, as noted above, the
products are biodegradable. Surprisingly, the embodiments which have no Q
substituents, or
wherein the only Q substituents are -H, exhibit particularly high
biodegradability. Also, the
compounds of structural formula (1) exhibit advantageous stability,
solubility, and freedom
from excessively objectionable color and odor. The color and odor stability is
exhibited in
formulations wherein the compound or compounds of structural formula (1) are
the only
nitrogenous compounds present. Color and order stability is also exhibited in
formulations
wherein one or more compounds of structural fornmla ( 1 ) is present with one
or more
additional conventional quaternary ammonium compounds. Thus, the compounds of
the
present invention improve the color and odor stability of the other
conventional quaternary
ammonium compounds present. The color stability property is shown in the
following data,
in which color stability was assessed by heating samples of each product shown
in Table I to
110°C in open tubes in an air circulation oven, periodically measuring
the color in Gardner
units and calculating the color change over time:
TABLE
I
ProductColor Rate of color
(Gardner change (Gardner
units) units)/day
start 1 day 2 days 6 daysAt 2 days At 6 days
1 2.5 4.5 6.0 14.5 1.75 2.0
2 1 4.5 6.5 16 2.75 2.5
3 1 1.5 4 8.5 1.5 1.3
4 4 -- - 11.5* - 1.9*
5 2.5 5.5 6.5 12.5 2.0 1.7
6 3 4.5 5.5 5.5 1.25 0.4
7 2.5 3.5 3.5 3.5 0.5 O.I7
8 1 1 2 3.5 0.5 0.4
9 2.5 2.5 2.5 2.5 0 0
/g
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
1.5 1.5 1.5 1.5 0 0
11 1.5 1.5 1.5 2.5 0 0.17
12 2.5 5.5 7.5 11.5 2.5 1.5
13 1 - - 3.5* - 0.62*
14 1.5 - - 5.5* - 1.0*
* denotes
4 day
readings
and
averages
Key
to
product
numbers:
1: Soft
tallow-derived,
triethanolamine-ester
quaternary
2: Soft
tallow-derived,
methyl
diethanolamine-ester
quaternary
3: Canola-derived,
triethanolamine-ester
quaternary
4: Soft
tallow-derived,
diamidoamine
quaternary
5: HMDA
+ 4
moles
ethylene
oxide
+ 2.5
moles
soft
tallow
fatty
acids
+ 1
mole
DMS
6: Same
as
5 except
2 moles
DMS
used
7: Same
as
6 except
2 moles
of
canola
fatty
acid
used
8: BHMT
+ 5
moles
ethylene
oxide
+ 4
moles
soft
tallow
fatty
acid
+ 3
moles
DMS
9: Same
as
8 except
1 mole
soft
tallow
fatty
acid
used
10:
Same
as
8 except
2 moles
soft
tallow
fatty
acid
used
11:
Same
as
8 except
3 moles
canola
fatty
acid
used
12:
Same
as
8 except
2 moles
soft
tallow
fatty
acid
used
and
product
not
quaternized
13:
50/50
(wt.
%)
mixture
of
1 and
8
14:
50/50
(wt.
% )
mixture
of
1 and
BHMT
+ 5
moles
ethylene
oxide
+ 3.5
moles
soft
tallow
fatty
acid
+ 3
moles
DMS
Although the compounds of structural formula (1) have many potential uses,
in particular, they exhibit highly satisfactory fabric softening capabilities.
Thus, the
compounds of structural formula (1), as well as mixtures of such compounds,
can be
5 advantageously formulated appropriately into products useable as fabric
softeners. It has
been found that, regardless of the other components that may be present in the
fabric softener
formulation, the pH of the formulation as a whole should be below 5, and
preferably 2.5 to
4.0, in order to maintain low susceptibility of the ester functionality to
hydrolysis in water.
The preferred method for providing or adjusting the desired pH value is adding
small
10 amounts of an acid, such as HCl or H2S04, consistent with appropriate
adjustment of the
average degree of esterification and average degree of quaternization mixture
of compounds
corresponding to structural formula (1). Preferred emulsions useful as fabric
softener
compositions can contain about 2 wt. % to about 80 wt. % , preferably 5 wt. %
to 30 wt. % ,
and more preferably 6 wt. ~ to 25 wt. %, of one or more compounds
corresponding to
structural formula (1). In general, higher solids contents can be provided
more easily with
lower degrees of quaternization. On the other hand, higher degrees of
quaternization (such
as a degree of quaternization approaching 2.0 for a diamine), lead to aqueous
formulations
wherein the maximum acceptable solids content without excessive solubility
problems is
lower.
~9
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
The compounds of structural formula ( 1 ) can be formulated into
compositions, including clear fabric softener compositions. Such fabric
softening
composition would typically include water and one or more of the solvents
which are
conventionally used in formulating fabric softeners. Examples of such solvents
include
ethanol, isopropanol, hexylene glycol, propylene glycol, diethylene glycol, or
similar solvent
of mixture thereof, as a concentrate or more dilute form, depending on the
application.
Selection of a suitable solvent for a particular application is well-known to
those of skill in
the art. Such formulations generally comprise about 10 wt. % up to about 50
wt. % of one
compound of structural formula (1) or a mixture thereof.
The compounds of structural formula ( 1 ) can be formulated with greater ease
than is encountered with conventional quaternary ammonium fabric softener
actives. Most
conventional quaternary ammonium compounds exhibit a tendency to form a gel
during
dilution with water when formulated in clear formulations. When formulated in
clear
formulations, however, the compounds of structural formula (1) show very
little gelation, or
no gelation at all, during dilution with water, even cold water, which would
be expected to
provoke gelation. This freedom from a tendency to gel means that compositions
including
compounds of structural formula (1) can be prepared at active concentrations
of 40 wt.% or
higher, for example, even 50 wt. % or higher. The preparation itself of such
formulations is
much simpler: the freedom from gelation means that when a formulator chooses
to
manufacture a more concentrated product for resale to the consumer, there is
no need for
special treatment to deal with gel formation. Also, the freedom from gel
formation means
that the consumer can use the more concentrated product directly without
concern that a
gelled byproduct would form. For example, the consumer could add a
concentrated fabric
softener composition according to the present invention directly into the wash
water in the
wash or rinse cycle, without any apprehension that a gel will form that that
would reduce
softening efficiency or leave a deposit on the clothes.
It should be noted that the ability of the compounds of structural formula (1)
to form a clear formulation, at lower and at higher concentrations, extends
also to
formulations which also contain one or more other quaternary ammonium
compounds as
fabric softener co-actives, as well as other additives as disclosed herein.
E. ADDITIONAL QUATERNARY AMMONIUM COMPOUNDS
Additional conventional quaternary ammonium compounds or salts may be
present with the compound or compounds of structural formula (1) in accordance
with the
present invention. The compounds presented below are only examples of
conventional
quaternary compounds that are suitable for use in the formulations of the
present invention.
,Z 0
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
As with the compounds of structural formula (1), these conventional quaternary
ammonium
compounds (quits or salts) may have an anion to provide electrical neutrality
and, in general,
such anion may be any anion which is not deleterious to the properties of the
overall
compound. Thus, in the structural formulas (i) to (xxii) below, the
counteranion, whether
designated as A- or not shown but understood, may be selected, without
limitation, from the
group consisting of fluoride, chloride, bromide, iodide, chlorite, chlorate,
hydroxide,
hypophosphite, phosphate, phosphate, carbonate, formate, acetate, lactate, and
other
carboxylates, oxalate, methyl sulfate, ethyl sulfate, benzoate, and
salicylate, and the like.
Preferred examples of the anions are chloride, bromide, methyl sulfate, ethyl
sulfate, and
salicylate. If the anion is monovalent (has a charge of -1), A- represents the
anion group, if
the anion is divalent (has a charge of -2), A- represents half of the anion
group, if the anion
is trivalent (has a charge of -3), A- represents a third of the anion group,
and so on.
The conventional quits that may be formulated with the compounds of
structural formula (1) in accordance with the present invention include, but
are not limited to,
nitrogenous compounds selected from the group consisting of quaternized or
acid salt
derivatives of:
(i) alkylenediamines, including compounds of the formula:
R~ N~, ,N R~
~J _ R3 ,~
O 0 1 O 0-1
wherein each Rt is an acyclic alkyl or alkylene C,,-Cz, hydrocarbon group,
each Z is
-(R20)o_4H, or -RZH, and RZ and R3 are divalent C~-C6 alkylene groups;
(ii) substituted imidazoline compounds having the formula:
/N
R~ --~
HO''R2
(iii) reaction products of higher fatty acids with alkylenetriamines in, for
example, a
molecular ratio of about 2:1, the reaction products containing compounds of
the formula:
hi H H
O~N. ,N. ~N~O
R2 R '~3
R~ R~
wherein R~, Rz and R3 are defined as above; and
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
(iv) substituted imidazoline compounds having the formula:
,N
R2 ~\/
R N
G~ R2
O
wherein G is -O- or -NH- and R~ and Rz are defined as above; and mixtures
thereof.
Preferred examples of compounds of structural formula (i) are those derived
from hydrogenated tallow fatty acids and the hydroxyalkylalkylenediamine is N
2-
hydroxyethylethylenediamine, such that Ri is an aliphatic C,s-Cz, hydrocarbon
group, and Rz
and R3 are divalent ethylene groups. A preferred example of a compound of
structural
formula (iii) is stearic hydroxyethyl imidazoline, wherein R1 is an aliphatic
Czl hydrocarbon
group and Rz is a divalent ethylene group. A preferred example of compounds of
structural
formula (iii) is N,N"-ditallowalkanoyldiethylenetriamine where R1 is an
aliphatic Cls-Czi
hydrocarbon group and Rz and R3 are divalent ethylene groups. A preferred
example of
compounds of structural formula (iv) is 1-tallowamidoethyl-2-tallowimidazoline
wherein R1 is
an aliphatic Cls-Cz~ hydrocarbon group and Rz is a divalent ethylene group.
Both N,N"-
ditallowalkanoyldiethylenetriamine and 1-tallowethylamido-2-tallowimidazoline
are reaction
products of tallow fatty acids and diethylenetriamine, and are precursors of
the cationic fabric
softening agent methyl-I-tallowamidoethyl-2-tallowimidazolinium methylsulfate.
N,N"-
ditallowalkanoyldiethylenetriamine and 1-tallowamidoethyl-2-tallowimidazoline
can be
obtained from Witco Corporation. Methyl-1-tallowamidoethyl-2-
tallowimidazolinium
methylsulfate is available from Witco Corporation under the tradename
VARISOFTO 475.
Other suitable quats are those containing one long chain acyclic aliphatic Cg-
Czz hydrocarbon group, selected from the group consisting of:
(v) acyclic quaternary ammonium salts having the formula:
AO
R5
Ra- NO Rs
R6
wherein R4 is an acyclic aliphatic Cs-Czz hydrocarbon group, alkyl, benzyl or
(C,~-
C1$ alkyl)-(OCHZCHz)z-3-, Rs and R6 are C,-C4 saturated alkyl or hydroxyalkyl
groups, and
A- is an anion as defined above;
(vi) substituted imidazolinium salts having the formula:
az
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
N
R~ ~ ~ AO
ON
H -'~
R~
wherein R~ is an acyclic alkyl or alkylene C~2-C2, hydrocarbon group, R7 is
hydrogen or a C1-C4 saturated alkyl or hydroxyalkyl group, and A- is an anion
as defined
above;
5 (vii) substituted imidazolinium salts having the formula:
N
R //
SON
HOR2 R
wherein Rl, R,, R5, and A- are as defined above;
(viii) alkylpyridinium salts having the formula:
AO
O
R4 N \
10 wherein R4 is an acyclic aliphatic Cg-C22 hydrocarbon group and A- is an
anion as
defined above; and
(ix) alkanamide alkylene pyridinium salts having the formula:
AO
O
O R2-N \
R~ H
wherein R1 is an acyclic aliphatic C,z-C21 hydrocarbon group, Rz is a divalent
C~-C6 aikylene
group, and A- is an anion as defined above; and mixtures thereof.
Examples of compounds of structural formula (v) are the
monoalkyltrimethylammonium salts such as monotallowtrimethylammonium chloride,
mono(hydrogenated tallow)-trimethylanunonium chloride,
palmityltrimethylammonium
chloride and soyatrimethylammonium chloride, available from Witco Corporation
under the
20 tradenames ADOGEN~ 471, ADOGEN~ 441, ADOGEN~ 444, and ADOGEN~ 415,
respectively. In these compounds, R4 is an acyclic aliphatic C,6-C1g
hydrocarbon group, and
RS and R6 are methyl groups. Mono(hydrogenated tallow)trimethylammonium
chloride and
monotallowtrimethylammonium chloride are preferred. Other examples of
compounds of
~3
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
structural formula (v) are behenyltrimethylammonium chloride wherein R4 is a
Czz
hydrocarbon group, which is available from the Humko Chemical Division of
Witco
Corporation under the tradename KEMAMINE~ Q2803-C; soyadimethylethylammonium
ethylsulfate wherein R4 is a C16-C~$ hydrocarbon group, RS is a methyl group,
R6 is an ethyl
group, and A- is an ethylsulfate anion; and methyl bis(2-
hydroxyethyl)octadecylammonium
chloride wherein R4 is a C,8 hydrocarbon group, RS is a 2-hydroxyethyl group
and R6 is a
methyl group.
An example of a compound of structural formula (vii) is 1-ethyl-1-(2-
hydroxyethyl)-2-isoheptadecylimidazolinium ethylsulfate wherein R, is a C17
hydrocarbon
group, Rz is an ethylene group, Rs is an ethyl group, and A- is an
ethylsulfate anion. Other
quats useful in the present invention include cationic nitrogenous salts
having two or more
long chain acyclic aliphatic C$-Czz hydrocarbon groups or one long chain
acyclic aliphatic
C$-Czz hydrocarbon group and an arylalkyl group. Examples include:
(x) acyclic quaternary ammonium salts having the formula;
AO
R4-N R5
I
wherein each R4 is an acyclic aliphatic Cg-Czz hydrocarbon group, RS is a C1-
C4
saturated alkyl or hydroxyalkyl group, Rg is selected from the group
consisting of R4 and RS
groups, and A- is an anion as defined above;
(xi) diamido quaternary ammonium salts having the formula:
ACO
O
R~ R2 ~~
R5 R2
~O -
R~
wherein each R, is an acyclic alkyl or alkylene Clz-Czi hydrocarbon group,
each Rz
is a divalent alkylene group having 1 to 3 carbon atoms, R5 and R9 are CI-C4
saturated alkyl
or hydroxyalkyl groups, and A- is an anion as defined above;
(xii) alkoxylated diamido quaternary ammonium salts having the formula:
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
AO
O
~Nv p+ ,~CH2CH20)nH
R~ R2 ~~
R1 O
R5 R2
wherein n is equal to 1 to about 5, and R,, R~, R5, and A- are as defined
above;
(xiii) quaternary ammonium compounds having the formula:
AO Ra~O. Rs
Rs.
wherein R4 is an acyclic aliphatic C8-C~Z hydrocarbon carbon group, each RS is
a C~-
C4 saturated alkyl or hydroxyalkyl group, and A- is an anion as defined above;
(xiv) amide-substituted imidazolinium salts having the formula:
N\
R~--
i- \O A
R~ Rs
R~ ~N~H
O
wherein each Rl is an acyclic aliphatic CIZ-C~, hydrocarbon group, Rz is a
divalent
alkylene group having 1 to 3 carbon atoms, and RS and A- are as defined above,
or RS is -H;
and
(xv) ester-substituted imidazolinium salts having the formula:
,N\
R~ --
R2~~o A
R5
R~ \ /O
~O
wherein Rl, R2, R5, and A- are as defined above; and mixtures thereof.
Examples of compounds of structural formula (x) are the well-known
dialkyldimethylammonium salts such as ditallowdimethylammonium chloride,
ditallowdimethylammonium methylsulfate, di(hydrogenated
tallow)dimethylammonium
chloride, distearyldimethylammonium chloride, dibehenyldimethylammonium
chloride.
Di(hydrogenated tallow)dimethylamrnonium chloride and ditallowdimethylammonium
chloride are preferred. Examples of commercially available
dialkyldimethylammonium salts
ZS
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
usable in the present invention are di(hydrogenated tallow)dimethylammonium
chloride
(available from Witco Corporation under the tradename ADOGEN~ 442);
ditallowdimethylammonium chloride (available from Witco Corporation under the
tradename
ADOGEN~ 470); distearyldimethylammonium chloride (available from Witco
Corporation
under the tradename AROSURF~ TA-100); dicocodimethyl ammonium chloride
(available
from Witco Corporation under the tradename ADOGEN~ 462), and
dibehenyldimethylammonium chloride, wherein R4 is an acyclic aliphatic Cz2
hydrocarbon
group (available from the Humko Chemical Division of Witco Corporation under
the
tradename KEMAMINE~ Q-2802C). Examples of compounds of structural formula (xi)
are
methylbis(tallowamidoethyl)(2-hydroxyethyl)ammonium methylsulfate and
methylbis(hydrogenated tallowamidoethyl)(2-hydroxyethyl)ammonium
methylsulfate, wherein
R1 is an acyclic aliphatic C15-C17 hydrocarbon group, RZ is an ethylene group,
RS is a methyl
group, R9 is a hydroxyalkyl group and A- is a methylsulfate anion; both of
these materials
are available from Witco Corporation under the tradenames VARISOFT~ 222 and
VARISOFT~ 110, respectively. An example of a compound of structural formula
(xiii) is
dimethylstearylbenzylamrnonium chloride, wherein R4 is an acyclic aliphatic
C1g hydrocarbon
group, RS is a methyl group and A- is chloride, which is available from Witco
Corporation
under the tradename VARISOFT~ SDC. Examples of compounds of structural formula
(xiv) are 1-methyl-1-tallowamidoethyl-2-tallowimidazolinium methylsulfate and
1-methyl-1-
(hydrogenated tallowamidoethyl)-2-(hydrogenated tallow)imidazolinium
methylsulfate
wherein RI is an acyclic aliphatic C15-C17 hydrocarbon group, RZ is an
ethylene group, RS is
a methyl group and A- is a chloride anion; available from Witco Corporation
under the
tradenames VARISOFT~ 475 and VARISOFT~ 445, respectively.
Additional examples of quaternary ammonium compounds useful in the
present invention include:
(xvi) compounds having the formula:
O
q oCH 3
R» - N
Ry0
O ZmRi2
wherein Rll is selected from the group consisting of: (a) -CH3, -CHzCH3, -
CHZCH20H, or straight chain aliphatic hydrocarbon groups each of which
contains from 12
through 24 carbon atoms, (b) ether groups each of which has the structure:
R~30(CH~O)y-,
(c) amide groups each of which has the structure:
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
R, 4~r- r
N
O (CH2)y
and (d) ester groups each of which has the structure:
R14
O
O (CH2)y
R12 is a straight chain aliphatic hydrocarbon group containing from 8 to 32
carbon
atoms, R13 is a straight chain aliphatic hydrocarbon group containing from 8
to 21 carbon
atoms, R14 is a straight chain aliphatic hydrocarbon group containing from 7
to 17 carbon
atoms, Z is an alkoxy group containing one oxygen atom and either two or three
carbon
atoms, A-~ is an anion as defined above, m is an integer from 1 through 12,
and y is an
integer which is either 2 or 3.
Yet additional examples of fabric softening compounds useful in the present
invention include:
(xvii) compounds having the formula:
O
R1s
RI.c~N~(CHz)~ N ~ ~R1~
(R1~)y H
wherein R~5 is hydrogen or C,-C4 alkyl,
R, s
R~6 N~(CHZ)n
each Ri6 is C1-C4 alkyl or (R18)y ,
each R,7 is a C8-Cz8 alkyl or alkenyl group, R1$ is hydrogen or C1-C4 alkyl,
each y is 0 or I,
x is 0 or 1, and each n is from 1 to 6;
(xviii) amides represented by the structural formula:
N
R19\
R2o R21
wherein R19 and RZO are selected independently from the group consisting of
C,_22
alk(en)yl aryl or alkyl aryl groups, R21 is hydrogen or a C1_22 alk(en)yl,
aryl or alkyl-aryl
group or is O-RZZ, wherein RZZ is a Cl.z2 alk(en)yl, aryl or alkyl-aryl group,
and R21 and R2~
optionally contain I to 10 alkylene oxide units or functional groups selected
from hydroxy,
amine, amide, ester, and ether groups; the aryl groups being possibly derived
from
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
heterocyclic compounds; at least one of the R19 and RZO groups contains 10 or
more carbon
atoms; and where the sum of carbon atoms in RI9+RZO+R21 is equal to or greater
than 14.
Preferably, the sum of carbon atoms in R,9+R2o is equal to or greater than 16.
Examples of
compounds of structural formula (xviii) include N,N ditallow acetamide, N,N
dicoconut
acetamide, N,N dioctadecyl propanamide, N dodecyl-N octadecyl acetamide, N
hexadecyl-N
dodecyl butanamide, N,N ditallow benzamide, N,N dicoconut benzamide, and N,N
ditallow
2-phenyl acetamide.
Additional fabric softening compounds useful in the present invention include
all ester quaternaries, including but not limited to:
(xix) compounds of the following structural formulas:
AO
Q21
R21 AOI k2 ~ N+ AI k? 1 R21
Q21 O
14
1-a and
Q21
R21 O O-CH~CH2~ ~ Q21 AO
0-3 Q21
R21 ~~CH2
1-3
wherein
each RZ, is independently a saturated or unsaturated alkyl or alkylene radical
containing 12 to 22 carbon atoms; each Q2' is independently an alkyl group
containing 1 to 4
carbon atoms, benzyl, -CHzCH20H, -CHZCH(OH)CH3, or R2~-C(O)-(O-(Alk2~)),-4-;
each
Alkz~ is independently C2H4, C3H6 or C4Hg; and A- is an anion as defined
above;
(xx) compounds of the formula:
A22 (cH ~-Xz2 +~;+;)
2 2-4
N--~CH2~-N- f D ~i ~ nA0
22
CCH2~-X22
2-4
wherein each A22 is the same or different and each is alkyl containing up to 3
carbon
atoms, benzyl, or H-(A1k22-O)i-3-Alk2'- wherein each Alk2'' represents -
CHzCH(CH3)-,
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
-CH(CH3)CH2-, or -CHZCHZ-, provided further that one of the Az2 can be
hydrogen; D is
methyl, ethyl, propyl, -(CHZ),_3C00-, benzyl or hydrogen; i is 0 or 1 and j is
0 or 1,
provided that the sum of (i + j) is 1 or 2; each X2~ is a straight or branched
saturated or
unsaturated aliphatic group containing up to 3 carbon-carbon double bonds and
containing 11
to 23 carbon atoms; n is two minus the number of -(CH2),_3C00- substituents
present; and
A- is an anion as defined above;
(xxi) compounds of the formula:
Rzs-CC(O)O(CHZ)i-slo-~-C(O)NH(CH2)2-s-N(R23a)(R23b)-(CHz)2-s-OC(O)R23A-
wherein each Rz3 is independently straight or branched alkyl or alkenyl
containing 8 to 22
10 carbon atoms; RZSa is straight or branched alkyl or hydroxyalkyl containing
1 to 3 carbon
atoms, benzyl, or -CZH40C(O)Rzb, wherein R26 is straight or branched alkyl or
alkenyl
containing 8 to 22 carbon atoms; R23n is -H, -CH3, -CzHs or benzyl; and A- is
an anion as
defined above; and
(xxii) compounds of the following structural formulas:
AO
A ~ YR2s
~R24)4-rWN'E(CH2)n ~'-R2~ (R24)3-N-CHz-CH
'" and CH2YR25
wherein
each R24 is independently straight or branched alkyl or alkenyl containing 1
to 8
carbon atoms and 0 to 3 hydroxyl groups; each Rz5 is straight or branched
alkyl or alkenyl
containing 10 to 22 carbon atoms and 0 to 3 hydroxyl groups; each Y is -O-C(O)-
or -
20 C(O)-O-; each m is 1 to 3; each n is from 1 to 8; and A- is an anion as
defined above.
Preferred examples of compounds of structural formulas (xxii) include methyl
diethanolamine
(MDEA) ester quats, triethanolamine (TEA) ester quats, for example, di-(tallow
carboxyethyl) hydroxyethyl methylammonium methosulfate, available from Witco
Corporation under the tradenarne REWOQUATT"' WE 16, or epichlorohydrin-based
ester
25 quats, all of which are used and accepted as fabric softeners worldwide
because of their
favorable biodegradation profiles, but usually lack the optimum softening
performance of
other quats.
F. DIOL AND DIOL ALKOXYLATE COUPLING AGENT ADDITIVES
In a preferred embodiment of this invention, fabric softener formulations and
30 other formulations which may be clear (translucent or transparent) and
easily dispersed in
water can be provided by including an appropriate amount of one or more
straight or
branched alkyl diols containing 4 to 12 carbon atoms, and/or alkoxylates of
such diols with
~-9
CA 02347036 2001-04-11
WO 00/21918 PCTNS98/21683
up to 40 alkoxy units per diol moiety, wherein the alkoxylate chains are
composed of alkoxy
units which are ethoxy, propoxy or butoxy or mixtures thereof, and preferably
ethoxy or
isopropoxy. These diol and diol alkoxylate hydrotropes or coupling agents are
added to the
formulations to increase the amount of the relatively water-insoluble
surfactants that can be
S solubilized into the system. In most cases, they do not act as surfactants
to lower surface
tension but they often allow surfactants in the presence of salts or
electrolytes to be added and
subsequently dispersed into water at higher concentrations or at lower
viscosities of the
formulation than is otherwise achieved using only surfactant and water. These
coupling
agents assist surfactants by increasing the surfactant's solubility in water
and its stability in
the formulation, especially in the presence of salts, electrolytes and/or pH
agents.
These diols and alkoxylates correspond to structural formula (T)
HO-(X-O)x-RT-(O-Y)y-OH (T)
wherein each X and each Y is ethylene (that is, -CZH4-), propylene (that is, -
C3H6-), or
butylene (that is, -C4H8-); x is 0-40; y is 0-40; the sum (x+y) is 0-40; and
RT is straight,
1S branched or cyclic alkyl containing 4 to 12 carbon atoms. Preferably, RT
contains 7-12 or
even 7-9 carbon atoms.
The alkylene residue RT in structural formula (T) represents a saturated,
straight-chain, branched-chain, or cyclic moiety containing 4 to 12 carbon
atoms. It is
preferred that RT is branched, wherein the term "branched" is intended to
encompass
structures having one side alkyl chain, more than one side alkyl chain, or one
or more side
alkyl chains, one or more of which is itself branched. Branched structures
include cyclic
structures substituted with one or more alkyl groups, the alkyl groups being
straight or
branched. Examples of suitable RT groups include such groups as -CHZC(CH3)ZCHZ-
,
-CHZCH(CHZCH2CHZCH3)-, -CHzC(CH3)zCH(CH(CH3)Z)-, -CHZCHZCHZ-,
2S -C(CH3)ZCH2-, -(CHz)6-, -CH2CH(CH3)CHz-,-CHZCH(CHZCH3)CHZCH2CHzCH2-, and
-CH(CH2)2CH(CHZ)2
I
In the alkoxylated diols, the number of repeating units in each poly(alkoxy)
chain can be up to 40 but it is preferred that each chain contains 1 to 10
repeating alkoxy
units or more preferably 1 to S alkoxy units. The preferred alkoxy chains are
poly(ethoxy),
or are composed of 1 to 2 ethoxy units capped with a chain of 1 to S propoxy
units.
Compounds of structural formula (T) defined above are in many instances
commercially
available. Compounds of structural formula (T) can be prepared in
straightforward manner
familiar to those of ordinary skill in this art by obtaining or preparing the
corresponding
3~
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
precursor diol of structural formula HO-RT-OH, and then alkoxylating the
precursor diol
with a stoichiometrically appropriate number of moles of the desired
corresponding aIkylene
oxide, such as ethylene oxide, propylene oxide, andlor butylene oxide. In
those cases where
it is desired to alkoxylate only one of the hydroxyl groups on the precursor
diol, in some
5 embodiments the alkoxylation will preferentially occur at only one of the
hydroxyl groups,
particularly where one of them is a primary hydroxyl and the other is a
secondary hydroxyl.
However, in those cases where both hydroxyl groups on the precursor diol might
tend to
alkoxylate but alkoxylation at only one of the hydroxyl groups is desired, the
hydroxyl group
at which alkoxylation is desired not to occur can be protected by
preliminarily reacting it
10 with a suitable protecting group such as a lower alkyl moiety or an
esterifying substituent.
Thereafter, following the alkoxylation, the protecting group is removed in a
known manner.
Preferred examples of compounds of the foregoing structural formula (T)
include any one, or mixtures, of 2,2,4-trimethyl-1,3-pentanediol (TMPD) and/or
2-
ethylhexane-1,3-diol, and/or the reaction product of TMPD and/or 2-ethylhexane-
1,3-diol
15 with I to 10 moles of ethylene oxide, and preferably with 1 to 5 moles of
ethylene oxide, as
well as analogs alkoxylated with other C3 or C4 alkyl oxides or mixtures of
any of Cz, C3
and/or C4 alkyl oxides. Since the diol which is alkoxylated includes one
primary hydroxyl
group and one secondary hydroxyl group, the alkoxylation proceeds
predominantly at the
primary hydroxyl group.
20 The compositions which contain one or more compounds of structural
formula ( 1 ) can also contain one or a mixture of compounds of structural
formula (E)
Rs~'-C(O)O-R$z-(OC(O)Res)o.i (E)
wherein RE1 is straight, cyclic or branched alkyl containing 1-15 carbon
atoms, and RE1 is
substituted with 0 to 3 hydroxyl groups; and wherein R~z is straight, cyclic
or branched alkyl
25 containing 1 to 10 carbon atoms, and REZ is substituted with 0 to 3
hydroxyl groups, and REz
can optionally be substituted with a group of the structure -OC(O)-RE3 wherein
RE3 is
straight, cyclic or branched alkyl containing 1 to 15 carbon atoms and is
optionally
substituted with a hydroxyl group. Preferred compounds of structural formula
(E) include
those wherein REZ contains 2 or 3 carbon atoms, for example, glycol and
glyceryl derivative,
30 or REZ contains about 8 carbon atoms, for example, derivatives of 2,2,4-
trimethylpentanediol
or of 2-ethylhexanediol. Preferred compounds of structural formula (E) include
2,2,4-
trimethyl-1,3-pentanediol monoisobutyrate, hydroxypivalyl hydroxypivalate, and
the
monoester of TMPD with hydroxypivalic acid.
Formulations can also contain what may be termed aesthetic additives to
35 provide properties such as fragrance, preservative, viscosity control, and
color. Such
31
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
additives are discussed below. The formulations according to the present
invention generally
exhibit highly satisfactory viscosities, generally as pourable and even
sprayable fluids.
G. ADDITIONAL SURFACTANTS
Other suitable non-quaternary compound surfactants, whether anionic,
cationic, zwitterionic, nonionic, or amphoteric, may be used in combination
with the
compounds and formulations of the invention, depending on the application. For
example, in
a fabric softening application, suitable anionic surfactants may include,
without limitation, the
alkylbenzene sulfonates, a-olefin sulfonates, and xylene sulfonates available
from Witco
Corporation under the WITCONATEC~7 trademark. While these surfactants may be
unsuitable for personal care applications because they may cause skin and eye
irritation,
surfactants suitable for personal care applications may be used in other non-
personal care
applications. For personal care applications, suitable anionic surfactants
would include,
without limitation, ammonium lauryl sulfate, sodium lauryl sulfate, any a-
olefin sulfonate,
ammonium laureth sulfate (2 or 3 moles), sodium laureth sulfate (2 or 3
moles), sodium
myristyl sulfate, sodium myristeth sulfate (1-4 moles), ammonium xylene
sulfonate, sodium
xylene sulfonate, TEA dodecylbenzene sulfonate, TEA lauryl sulfate, ammonium
pareth
sulfate, sodium pareth sulfate, sodium oleth sulfate, derivatives of any of
the forgoing, and
similar compounds known to those of skill in the art, and mixtures thereof.
For personal
care applications, suitable amphoteric surfactants or non-ionic surfactants
include betaines,
sulfosuccinates, mono- and diglycerides, glycinates, sugars and derivatives
thereof,
hydroxysultaines, mono- and diacetates, ethoxylated derivatives of any of the
forgoing, and
similar compounds known to those of skill in the art, and mixtures thereof.
Other surfactants
that may be added to these systems include, but are not limited to,
alkanolamides, many of
which are available from Witco Corporation under the WITCAMIDE~ tradename. In
addition, various amine oxides, several of which are available from Witco
Corporation under
the VAROX~ tradename, may be used in these systems.
H. PERSONAL CARE EMOLLIENTS AND EMULSIFIERS
Emollients and emulsifiers are also typically used in personal care
formulations in combination with the polyester amine compounds and
polyquaternary
compounds of the invention, depending on the application. The preferred
embodiment of this
invention may also be used in emulsions that can be used as skin or hair
conditioners which
can take the form of lotions, creams, leave-on products, and rinse-off
products. These
systems may also include additional products that may improve the feel and
conditioning, or
the emolliency of skin and hair. Some of these products are available from
Witco
3~.
CA 02347036 2001-04-11
WO 00/21918 PCTNS98/21683
Corporation under the KEMSTRENE~, WITCONOLT"', STARFOL~; and KEMESTER~
tradenames. Such emulsions usually include emulsifiers to form and preserve
the emulsion.
I. OTHER ADDITIVES
Other additives and adjuvants can be optionally added to the compounds and
formulations of the present invention for their known purposes. Such additives
and adjuvants
include, but are not limited to, perfumes, preservatives including
bacteriocides and
fungicides, insect and moth repelling agents, polymeric soil release agents,
antistatic agents,
dyes and colorants, especially bluing agents, viscosity control agents,
antioxidants, silicones,
defoaming agents, antifoarning agents, emulsifiers, brighteners, opacifiers,
freeze-thaw
control agents, shrinkage control agents, aloe, humectants, skin protectants,
feel modifiers,
waxes, glycerine, vitamins and extracts, and mixtures thereof. The identity
and amounts of
the additives and adjuvants used would depend on the application of the
formulation and its
desired properties. The additives and adjuvants are well-known to those of
skill in the art
and the additives and adjuvants listed below are not meant to be an exhaustive
list but merely
a guide to the types of additives that would typically be used.
I. Perfumes
As noted above, perfumes or fragrance materials may be added to the
compositions and formulations of the present invention. The selection of the
perfume or
perfumes is based upon the application, the desired effect on the consumer,
and preferences
of the formulator. Such perfume is preferably present at a level of from about
0.01 % to
about 5 % , preferably from about 0.05 % to about 3 % , more preferably from
about 0.1 % to
about 2%, by weight of the total composition. Preferably, the perfume is
composed of
fragrance materials selected from the group consisting of aromatic and
aliphatic esters having
molecular weights from about 130 to about 250; aliphatic and aromatic alcohols
having
molecular weights from about 90 to about 240; aliphatic ketones having
molecular weights
from about 150 to about 260; aromatic ketones having molecular weights from
about 150 to
about 270; aromatic and aliphatic lactones having molecular weights from about
130 to about
290; aliphatic aldehydes having molecular weights from about 140 to about 200;
aromatic
aldehydes having molecular weights from about 90 to about 230; aliphatic and
aromatic
ethers having molecular weights from about 150 to about 270; and condensation
products of
aldehydes and amines having molecular weights from about 180 to about 320; and
mixtures
thereof. The selection of such perfumes and fragrance materials are well-known
to
those of skill in the art, both for desired scent and appropriate scent
impact.
2. Preservatives
33
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
Optionally, solubilized, water-soluble preservatives can be added to the
present invention. Preservatives are especially preferred when organic
compounds that are
subject to microorganisms are added to the compositions of the present
invention, especially
when they are used in aqueous compositions. When such compounds are present,
long term
5 and even short term storage stability of the compositions and formulations
becomes an
important issue since contamination by certain microorganisms with subsequent
microbial
growth often results in an unsightly and/or malodorous solution. Therefore, it
is preferable
to include a solubilized water-soluble, antimicrobial preservative, which is
effective for
inhibiting and/or regulating microbial growth in order to increase storage
stability of the
10 preferably clear and often aqueous compositions and formulations of the
present invention. It
is preferable to use a broad spectrum preservative, for example, one that is
effective on both
bacteria and fungi. A limited spectrum preservative, for example, one that is
only effective
on a single group of microorganisms, for example, fungi, can be used in
combination with a
broad spectrum preservative or other limited spectrum preservatives with
complimentary
15 and/or supplementary activity. Antimicrobial preservatives useful in the
present invention
can be biocidal or biostatic compounds. If the antimicrobial preservative is
included in the
compositions and formulations of the present invention, it is preferably
present in an effective
amount, wherein an "effective amount" means a level sufficient to prevent
spoilage or
prevent growth of inadvertently added microorganisms for a specific period of
time.
20 Preferred levels of preservative are from about 0.0001 % to about 0.5 % ,
more preferably
from about 0.0002 % to about 0.2 %, most preferably from about 0.0003 % to
about 0.1 % , by
weight of the composition. Bacteriostatic effects can sometimes be obtained
for aqueous
compositions by adjusting the composition pH to an acid pH, for example, less
than about pH
4, preferably less than about pH 3. Low pH for microbial control is not a
preferred
25 approach in the present invention because the low pH can cause chemical
degradation of the
cyclodextrins. Therefore, aqueous compositions of the present invention should
have a pH
greater than about 3.0, preferably greater than about 4.0, more preferably
greater than about
4.5. As stated above, it is preferable to use the preservative at an effective
amount, as
defined hereinabove. Optionally, however, the preservative can be used at a
level which
30 provides an antimicrobial effect on the treated fabrics.
3. Antistatic Agents
The composition of the present invention can optionally contain an effective
amount of antistatic agent to provide the treated clothes with in-wear static.
Preferred
antistatic agents are those that are water soluble in at least effective
amount, such that the
35 composition remains a clear solution. An example of these antistatic agents
is ethyl
3Y
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
bis(polyethoxyethanol) alkylammonium ethylsulfate (available from Witco
Corporation under
the tradename VARIQUAT~ 66). It is preferred that a. no foaming, or low
foaming, agent is
used, to avoid foam formation during fabric treatment. It is also preferred
that
polyethoxylated agents such as polyethylene glycol or VARIQUAT~ 66 are not
used when
a-cyclodextrin is used. When an antistatic agent is used it is typically
present at a level of
from about 0.05% to about LO%, preferably from about 0.1 % to about 5%, more
preferably
from about 0.3 % to about 3 %, by weight of the composition.
4. Dyes and Colorants
Colorants and dyes, especially bluing agents, can be optionally added to the
compositions of the present invention for visual appeal and performance
impression. When
colorants are used, they are used at extremely low levels to avoid fabric
staining. Preferred
colorants for use in the present compositions are highly water-soluble dyes,
for example,
LIQUITINT~ dyes available from Milliken Chemical Company.
S. Insect and Moth Repelling Agents
The composition of the present invention can optionally contain an effective
amount of insect or moth repelling agents. Typical insect and moth repelling
agents are
pheromones, such as anti-aggregation pheromones, and other natural and/or
synthetic
ingredients. When an insect and/or moth repellent is used it is typically
present at a level of
from about 0.005 wt. % to about 3 wt. % of the composition.
6. Polymeric Soil Release Agents
Soil release agents, usually polymers, are especially desirable additives at
levels of from about 0.05 wt. % to about S wt. %, preferably from about 0.1
wt. % to about 4
wt. % , more preferably from about 0.2 wt. % to about 3 wt. % . Suitable soil
release agents
are disclosed in U.S. Patent Nos. 4,702,857; 4,711,730; 4,713,194; 4,877,896;
4,956,447;
and 4,749,596. Especially desirable optional ingredients are polymeric soil
release agents
comprising block copolymers of polyalkylene terephthalate and polyoxyethylene
terephthalate, and block copolymers of polyalkylene terephthalate and
polyethylene glycol.
The polymeric soil release agents useful in the present invention can include
anionic and
cationic polymeric soil release agents. Suitable anionic polymeric or
oligomeric soil release
agents are disclosed in U.S. Patent No. 4,018,569. Other suitable polymers are
disclosed in
U.S. Patent No. 4,808,086. Suitable cationic soil release polymers are
described in U.S.
Patent No. 4,956,447.
7. Viscosity Control Agents
Viscosity control agents can be organic or inorganic in nature and may either
lower or raise the viscosity of the formulation. Examples of organic viscosity
modifiers
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
(lowering) are aryl carboxylates and sulfonates (for example, benzoate, 2-
hydroxybenzoate,
2-aminobenzoate, benzenesulfonate, 2-hydroxybenzenesulfonate, 2-
aminobenzenesulfonate,
etc.), fatty acids and esters, fatty alcohols, and water-miscible solvents
such as short chain
alcohols. Examples of inorganic viscosity control agents are water-soluble
ionizable salts. A
wide variety of ionizable salts can be used. Examples of suitable salts are
the halides and
acetates of ammonium ion and the group IA and IIA metals of the Periodic Table
of the
Elements. The amount of ionizable salts used depends on the amount of active
ingredients
used in the compositions and can be adjusted according to the desire of the
formulator.
Typical levels of salts used to control the composition viscosity are from 0
to about 10 wt. %,
10 preferably from about 0.01 wt. % to about 6 wt. % , and most preferably
from about 0.02
wt. % to about 3 wt. % of the composition.
Viscosity modifiers (raising) or thickening agents can be added to increase
the ability of the compositions to stably suspend water-insoluble articles,
for example,
perfume microcapsules. These viscosity raisers (thickeners) are typically used
at levels from
15 about 0.5 wt. % to about 30 wt. % , preferably from about 1 wt. % to about
5 wt. % , more
preferably from about 1.5 wt. % to about 3.5 wt. % , and most preferably from
about 2 wt.
to about 3 wt. % , of the composition.
8. Pearlizing and Opacifying Agents
Examples of pearlizing or opacifying agents that can be added to the
20 compositions of this invention include, but are not restricted to, glycol
distearate, propylene
glycol distearate, and glycol stearate. Some of these products are available
from Witco
Corporation under the KEMESTER~ tradename.
9. Vitamins and Extracts
In personal care applications, vitamins and extracts are often used in the
25 formulations thereof.
10. Antioxidants
Examples of antioxidants that can be added to the compositions of this
invention are propyl gallate, available from Eastman Chemical Products, Inc.
under the
tradenames TENOX~ PG and TENOX~ S-1, and dibutylated hydroxytoluene, available
30 from UOP Inc. under the tradename SUSTANE~ BHT.
Il. Silicones
The present compositions can contain silicones to provide additional benefits,
for example, in a fabric application they may provide ease of ironing and
improved fabric
absorbency. As used herein, the term "silicones" comprises cationic and
amphoteric
35 silicones, polysiloxanes, and polysiloxanes having hydrogen-bonding
functional groups
36
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
consisting of amino, carboxyl, hydroxyl, ether, polyether, aldehyde, ketone,
amide, ester,
and thiol groups. Such polysiloxanes include, but are not limited to,
polyether-modified
polysiloxanes, amino-modified polysiloxanes, epoxy-modified polysiloxanes,
polyhydrido-
modified polysiloxanes, phenol-derivative-modified polysiloxanes, ABA-type
polysiloxanes,
S [AB]N-type polysiloxanes, amino [AB]N-type polysiloxanes, including those
available from
OSi Specialties, Inc. (a division of Witco Corporation), under the SILWET~,
NUWET~,
NUDRYT"', NUSOFTT"', MAGNASOFT~ tradenames. Suitable silicones may include
polydimethylsiloxanes of viscosity of from about 100 centistokes (cs) to about
100,000 cs,
preferably from about 200 cs to about 60,000 cs and/or silicone gums. These
silicones can
be used in emulsified form, which can be conveniently obtained directly from
the suppliers.
Examples of these preemulsified silicones are the 60% emulsion of
polydimethylsiloxane (350
cs) sold by Dow Corning Corporation under the tradename DOW CORNING~ 1157
Fluid
and the 50% emulsion of polydimethylsiloxane {10,000 cs) sold by General
Electric
Company under the tradename GENERAL ELECTRIC~ SM 2140 silicones. The optional
silicone component can be used in an amount of from about 0.1 wt. % to about 6
wt. % of the
composition.
Silicone foam suppressants can also be used. These are usually not
emulsified and typically have viscosities of from about 100 cs to about 10,000
cs, preferably
from about 200 cs to about 5,000 cs. Very low levels are used, typically from
about 0.01 %
to about 1 % , preferably from about 0.02 % to about 0.5 % . Another preferred
foam
suppressant is a silicone/silicate mixture, for example, Dow Corning's
ANTIFOAMT"' A.
The pH { 10 % solution) of the compositions of this invention is generally
adjusted to be in the range of from about 2 to about 7, preferably from about
2.4 to about
6.5, more preferably from about 2.6 to about 4. Adjustment of pH is normally
carried out
by including a small quantity of free acid in the formulation. Because no
strong pH buffers
are present, only small amaunts of acid are required. Any acidic material can
be used; its
selection can be made by anyone skilled in the softener arts on the basis of
cost, availability,
safety, etc. Among the acids that can be used are methyl sulfonic,
hydrochloric, sulfuric,
phosphoric, citric, malefic, and succinic. For the purposes of this invention,
pH is measured
by a glass electrode in a 10% solution in water of the softening composition
in comparison
with a standard calomel reference electrode.
12. Lubrication and Slip Additives
Compositions and formulations of the present invention can contain additives
such as water, insoluble organics such as fatty acids, fatty esters,
triglycerides, oils, alcohols,
3~
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
fatty alcohols, fatty amines and derivatives, amides, hydrocarbons, mineral
oils, waxes, and
the like, and mixtures thereof, as lubrication and slip agents.
13. Dye Transfer Inhibitors
Compositions and formulations of the present invention can contain
5 ethoxylated amines, amphoterics, betaines, polymers such as
polyvinylpyrrolidone, and other
ingredients that inhibit dye transfer.
14. Papermaking and Tissuemaking Additives
Paper and tissue softening or debonding compositions of the present invention
would typically contain other chemicals commonly used in papermaking or
tissuemaking, or
to the paper or tissue furnish so long as they do not significantly and
adversely affect the
softening, absorbency of the fibrous material, and softness enhancing actions
of the amine
and quaternary ammonium softening compounds of the present invention.
A. Wettine Aeents
The present invention may contain as an optional ingredient from about
15 0.005 % to about 3.0 % , more preferably from about 0.03 % to 1.0 % by
weight, on a dry
fiber basis of a wetting agent. Such wetting agents may be selected from
polyhydroxy
compounds, nonionic surfactants such as alkoxylated compounds and linear
alkoxylated
alcohols. Examples of water soluble polyhydroxy compounds that can be used as
wetting
agents in the present invention include glycerol, polyglycerols having a
weight-average
20 molecular weight of from about 150 to about 800, and polyoxyethylene
glycols and
polyoxypropylene glycols having a weight-average molecular weight of from
about 200 to
about 4000, preferably from about 200 to about 1000, most preferably from
about 200 to
about 6U0. Polyoxyethylene glycols having an weight-average molecular weight
of from
about 200 to about 600 are especially preferred. Mixtures of the above-
described
25 polyhydroxy compounds may also be used. A particularly preferred
polyhydroxy compound
is polyoxyethylene glycol having an weight average molecular weight of about
400, available
from Union Carbide Corporation under the tradename PEG-400. Suitable nonionic
surfactants can be used as wetting agents in the present invention. These
include addition
products of alkoxylating agents such as ethylene oxide (EO), propylene oxide
(PO),
30 isopropylene oxide (iP0), or butylene oxide (BO), or a mixture thereof,
with fatty alcohols,
fatty acids, fatty amines, etc. Any of the alkoxylated materials of the
particular type
described hereinafter can be used as the nonionic surfactant. Suitable
compounds are
substantially water-soluble surfactants of the general formula:
Rio-1'-(CZH40)Z-C2HaOH
3g
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
wherein Rlo for both solid and liquid compositions is selected from the group
consisting of
primary, secondary and branched chain alkyl and/or acyl hydrocarbyl groups;
primary,
secondary and branched chain alkenyl hydrocarbyl groups; and primary,
secondary and
branched chain alkyl- and alkenyl-substituted phenolic hydrocarbyl groups; the
hydrocarbyl
5 groups having a hydrocarbyl chain length of from about 8 to about 20,
preferably from about
to about 18 carbon atoms. More preferably the hydrocarbyl chain length for
liquid
compositions is from about 16 to about 18 carbon atoms and for solid
compositions from
about 10 to about 14 carbon atoms. In the general formula for the ethoxylated
nonionic
surfactants herein, Y is typically -O-, -C(O)O-, -C(O)N(R,1)-, or -
C(O)N(Rll)Rll-, in which
10 Rlo, and Rl,, when present, have the meanings given hereinbefore, and/or
Rl, can be
hydrogen, and z is at least about 8, preferably at least about 10-11.
Performance and,
usually, stability of the softener composition decrease when fewer ethoxylate
groups are
present.
Examples of nonionic surfactants according to the above formula follow,
wherein the integer in parenthesis identifies the number of EO groups in the
molecule. In
particular, the deco-, under-, dodder-, terraced-, and pentadecaethoxylates of
n-hexadecanol
and n-octadecanol are useful wetting agents in the context of this invention.
Exemplary
ethoxylated primary alcohols useful herein as the viscosity/dispersibility
modifiers of the
compositions are n-octadecanol EO(10); and n-decanol EO(11). The ethoxylates
of mixed
20 natural or synthetic alcohols in the "oleyl" chain length range are also
useful herein. Specific
examples of such materials include oleyl alcohol EO(11), oleyl alcohol EO(18),
and oleyl
alcohol EO(25). In addition, the deco-, undeca-, dodeca-, tetradeca-,
pentadeca-, octadeca-,
and nonadecaethoxylates of 3-hexadecanol, 2-octadecanol, 4-eicosanol, and 5-
eicosanol can
be used as wetting agents in the present invention. As in the case of the
alcohol alkoxylates,
25 the hexa- through octadecaethoxylates of alkylated phenols, particularly
monohydric
alkylphenols, are useful as the viscosity/dispersibility modifiers of the
instant compositions.
In particular, the hexa- through octadeca-ethoxylates of p-tridecylphenol, m-
pentadecylphenol, and the like, are useful herein. Exemplary ethoxylated
alkylphenols useful
as the wetting agents of the mixtures herein are: p-tridecylphenol EO(11) and
p-
30 pentadecylphenol EO(18). As used herein and as generally recognized in the
art, a
phenylene group in the nonionic formula is the equivalent of an alkylene group
containing
from 2 to 4 carbon atoms. It should also be noted that the alkenyl alcohols,
both primary and
secondary, and alkenyl phenols corresponding to those disclosed immediately
hereinabove
can be ethoxylated and used as wetting agents in the present invention.
Furthermore,
39
CA 02347036 2001-04-11
WO 00/21918 PC'T/US98/21683
branched-chain primary and secondary alcohols, usually synthesized using the
well-known
Oxo Process, can be ethoxylated and can be used as wetting agents in the
present invention.
The above ethoxylated nonionic surfactants are useful in the present
compositions alone or in combination, and the term "nonionic surfactant"
encompasses
mixed nonionic surface active agents. The level of surfactant, if used, is
preferably from
about 0.01 % to about 2.0% by weight, based on the dry fiber weight of the
tissue paper.
The surfactants preferably have alkyl chains with eight or more carbon atoms.
Exemplary
anionic surfactants are linear alkyl sulfonates and alkylbenzene sulfonates.
Exemplary
nonionic surfactants are alkylglycosides including alkylglycoside esters,
alkylglycoside
ethers, and aikylpolyethoxylated esters.
B. r ngt_h_ Additives
Other types of chemicals which may be added, include the strength additives
to increase the dry tensile strength and the wet burst of the tissue webs. The
present
invention may contain as an optional component from about 0.01 wt. % to about
3.0 wt. % ,
more preferably from about 0.3 wt. % to about 1.5 wt. % , on a dry fiber
weight basis, of a
water-soluble strength additive resin. Such water-soluble strength additive
resins may include
dry strength additives, permanent wet strength resins, temporary wet strength
resins, or a
compatible mixture thereof.
As used herein, the term "permanent wet strength resin" refers to a resin
which allows the paper sheet, when placed in an aqueous medium, to keep a
majority of its
initial wet strength for a period of time greater than at least two minutes.
Permanent wet
strength resins useful herein can be of several types. Generally, those resins
which have
previously found and which will hereafter find utility in the papermaking art
are useful
herein. Numerous examples are described by Westfelt in Cellulose Chemistry and
Technology, Volume 13, at pages 813-825 (1979). Usually, the wet strength
resins are
water-soluble, cationic materials. That is to say, the resins are water-
soluble at the time they
are added to the papermaking furnish. It is quite possible, and even to be
expected, that
subsequent events such as cross-linking will render the resins insoluble in
water. Further,
some resins are soluble only under specific conditions, such as over a limited
pH range. Wet
strength resins are generally believed to undergo a cross-linking or other
curing reactions
after they have been deposited on, within, or among the papermaking fibers.
However, such
cross-linking or curing does not normally occur so long as substantial amounts
of water are
present. Of particular utility are the various polyamide-epichlorohydrin
resins. These
materials are low molecular weight polymers provided with reactive functional
groups such
as amino, epoxy, and azetidinium groups. These materials are available from
the Monsanto
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
Company under the tradename SANTO-REST"', such as SANTO-REST"' 31. Other water-
soluble cationic resins useful herein are the polyacrylamide resins. Other
types of water-
soluble resins useful in the present invention include acrylic emulsions and
anionic styrene-
butadiene latexes. Still other water-soluble cationic resins finding utility
in this invention are
5 the urea formaldehyde and melamine formaldehyde resins. These
polyfunctional, reactive
polymers have molecular weights on the order of a few thousand. The more
common
functional groups include nitrogen containing groups such as amino groups and
methylol
groups attached to nitrogen. Although less preferred, polyethylenimine-type
resins fmd
utility in the present invention. More complete descriptions of the
aforementioned water-
10 soluble resins, including their manufacture, can be found in TAPPI
Monograph Series No.
29, Wet Strength In Paper and Paperboard, Technical Association of the Pulp
and Paper
Industry (New York: 1965).
The above-mentioned permanent wet strength additives are those that produce
paper products with permanent wet strength, that is, paper which when placed
in an aqueous
15 medium retains a substantial portion of its initial wet strength over time.
However,
permanent wet strength in some types of paper products can be an unnecessary
and
undesirable property. Paper products such as toilet tissues, etc., are
generally disposed of
after brief periods of use into septic systems and the like. Clogging of these
systems can
result if the paper product permanently retains its hydrolysis-resistant
strength properties.
20 Thus, manufacturers use temporary wet strength additives to paper products
for which wet
strength is sufficient for the intended use, but which then decays upon
soaking in water.
Decay of the wet strength facilitates flow of the paper product through septic
systems.
Examples of suitable temporary wet strength resins include modified starch
temporary wet
strength agents, such as that available from the National Starch and Chemical
Corporation
25 under the tradename NATIONAL STARCHT'" 78-0080. This type of wet strength
agent can
be made by reacting dimethoxyethyl-N methyl-chloroacetamide with cationic
starch
polymers. Modified starch temporary wet strength agents are also described in
U.S. Patent
Nos. 4,675,394 and 4,981,557.
C. Other Additives
30 Other suitable additives may be used in paper and tissuemaking
applications,
depending on the application. For example, glycerin may also be used in the
composition
and formulations thereof. If used, the amount of glycerin in the aqueous
softening
composition can be from about 0.1 wt. % to about 98 wt. % , more preferably
from about 60
to about 80 wt. % , and still more preferably from about 40 to about 60 wt. %
, of the
35 composition. In addition, the compositions and formulations of the instant
invention can
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
contain glycols, such as propylene glycol or polyethylene glycol, instead of
or in addition to
glycerin in such formulations.
Other optional ingredients include aloe, humectants, skin protectants, and
feel
modifiers. Suitable humectants include lactic acid and its salts, sugars,
ethoxylated glycerin,
ethoxylated lanolin, corn syrup, hydrolyzed starch hydrolysate, urea, and
sorbitol. Suitable
skin protectants include allantoin, kaolin, and zinc oxide. Suitable feel
modifiers include
corn starch, oat flour, talc, boron nitride, and cyclodextrin.
J. EXAMPLES
The following examples are but a few examples of more particular
formulations embodying the compositions of the present invention. The
following examples
are provided for purposes of further description of the present invention and
are not intended
to limit the scope of that which is regarded as the invention.
Basic Fabric Softener Formulations
Hexamethylenediamine was reacted with 4 moles of ethylene oxide per mole
of HMDA. The HMDA ethoxylate was esterified in two different runs with a
mixture of
C~4-C18 fatty acids (2 moles of fatty acid per mole of ethoxylate) following
which the HMDA
ethoxylated ester was quaternized with 2 moles of DMS per mole of HMDA. In Run
(1-A)
the fatty acids were a commercial mixture of tallow fatty acids and in Run (1-
B) the fatty
acids were obtained from canola oil.
This example illustrates formulations of compounds according to structural
formula (1) in conventional solvent systems. A product was made by thoroughly
blending
the quaternary and solvent, then blending in the fragrance and then the water.
The
components and the amounts thereof were:
EXAMPLE 2
Component Amount (wt. %)
Product of Run 1-B (85 wt. % in isopropanol) 23.5
Solvent (hexylene glycol) 18-20
Fragrance, dye, preservative, and other as needed
additives
Deionized water to 100
The resulting formulations had a cloud point of 45°C, a melting
point above
10°C, and a Gardner color of above 0.5. The quaternary product of Run 1-
B exhibits little
~2
CA 02347036 2001-04-11
WO 00/21918 PCTNS98/21683
or no gelation upon addition to water. The above formulation was repeated
except using a
50:50 (wt.) mixture of products prepared in accordance with Runs 1-A and 1-B.
The
resulting formulation has a cloud point of 50-55°C and exhibits
satisfactory properties.
EXAMPLE 3
This example illustrates that clear formulations can be made which contain a
conventional quaternary ammonium fabric softener compound together with one or
a mixture
of compounds of structural formula (1) as defined herein. This discovery is
particularly
unexpected in that many conventional quaternary ammonium fabric softener
actives, such as
the di(Cg-C22 alkyl/alkenyl) di(C,-C4 alkyl) quaternary ammonium compounds,
like the one
10 employed in this example, cannot readily be formulated into a clear
composition in an
acceptable amount using existing solvent systems or solvent/coupling agent
systems.
The composition of Example 3 was made using the procedure used in
Example 2. The components and the amounts thereof were:
EXAMPLE 3
Component Amount (wt. % )
Product of Run 1-B, 85 wt. % in isopropanol14.1
Ditallow dimethyl ammonium chloride (available10.7
from Witco
Corporation as ADOGEN~ 470), 75 wt. %
in isopropanol
Hexylene glycol 24.0
Fragrance, dye, preservative, and other as needed
additives
Deionized water to 100
15 The resulting product has a cloud point of 45-50°C, and a melting
point of
27°C. Its color is less than 0.5 Gardner.
EXAMPLES 4 and 5
Additional formulations employing another solvent system include Example 4
and Example 5, both made by the procedure disclosed in Example 2.
EXAMPLE 4
Component Amount (wt. % )
Product of Run 1-B 23.5
TMPD 7.5
Hexylene glycol 7.5
Fragrance, dye, preservative, and other as needed
additives
Deionized water to 100
~3
CA 02347036 2001-04-11
WO 00/21918 PCTNS98/21683
EXAMPLE 5
Component Amount (wt. % )
Product of Run 1-A 11.8
Product of Run 1-B 11.8
TMPD 7.5
Hexylene glycol 7.5
Fragrance, dye, preservative, and other as needed
additives
Deionized water to 100
As noted above, fabric softening actives, such as amines and ammonium
derivatives, are often rendered ineffective by reaction with the anionic
detergent compounds
present in the wash water. Although this is most obviously a problem if both
detergent and
5 softener compounds are added together in a wash cycle, even if the fabric
softener is added
during a rinse cycle (as is typically done), residual detergent compounds
present in the fabric
interferes with the softener.
More generally, exemplary formulation guidelines for fabric softener
compositions which include polyester polyquaternary compounds conforming to
structural
formula (1), include Examples 6 and 7. As noted above, when a clear
formulation is desired,
the use of an unsaturated fatty acid is preferred to make the compound of
structural formula
( 1 ) or at least a portion of the compound of structural formula ( 1 ) used
in the formulation. If
a clear formulation is desired, the I.V. of the fatty acid used to make the
compound of
15 structural formula ( 1 ) ranges from about 30 to about 140, more preferably
the I . V . ranges
from about 80 to about 120, most preferably the I. V . ranges from about 90 to
about 110.
Examples of some fatty acids that would be preferred may be derived from the
following fats
and oils: tallow, fish oils, canola (including fatty acids derived from
partially hydrogenated
canola), palm, wheat germ, rapeseed, olive, corn, neem, peanut, safflower,
sesame seed,
20 soybean, sunflower seed, and cocoa butter.
EXAMPLE 6
Clear Polyester Polyquaternary in Conventional
Solvents)
Component Amount (wt. %)
Compound of structural formula ( 1 ) 15-20
Solvents) (for example, isopropanol, hexylene20-30
glycol)
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
Fragrance, dye, preservative, and other as needed
additives
Deionized water to 100
EXAMPLE 7
Clear Polyester Polyquaternary with Coupling
Agent
Component Amount (wt. % )
Compound of structural formula ( 1 ) 15-25
Coupling agents) (for example, diol or 10-15
alkoxylate of
structural formula (T) and/or hydroxyester
of structural
formula (E))
Acid (for example, HCl) to pH 2.5-4
Fragrance, dye, preservative, and other as needed
additives
Deionized water to 100
EXAMPLES 8 TO 13
As disclosed above, the compounds of structural formula ( 1 ) may be used
either alone or in combination with other compounds. Such compounds may
include
conventional quats such as those set forth herein above (see E. ADDITIONAL
QUATERNARY AMMONIUM COMPOUNDS). Indeed, some of these formulations
provide advantageous formulations providing and synergistic or unexpected
properties. For
example, as explained above, detergent compounds are generally anionic
surfactants and
naturally tend to complex with or even precipitate out of solution when
cationic softening
ingredients are present in the same aqueous solution. This complexation or
precipitation
reaction interferes with the performance of both the detergent compounds and
the softening
compounds and is therefore undesirable. Detergents and softeners are therefore
generally not
added simultaneously in laundry operations to avoid this undesirable
complexation or
precipitation reaction; however, as North American washing machines typically
rinse the
clothes only once before fabric softener is added to the washload, even if the
fabric softener
is added during a rinse cycle (as is typically done), residual anionic
detergent compounds
present in the fabric complexes with the cationic softener compounds, often
rendering about
15% to 30% or more of the softener inactive. It has been found that certain
conventional
quats, particularly the ester quats of structural formula (xxii), in
combination with
compounds of structural formula {1) provide an advantageous fabric softening
formulation
with surprising resistance to complexation with anionic surfactants.
~s
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
EXAMPLE 8
High Solids HMDA Fabric Softener Formulation
Component Amount (wt. % )
HMDA + 4 moles EO + 2.5 moles tallow fatty10
acid + 2
moles DMS
Tallow-based TEA ester quat (DMS-based 15
quat)
Fragrance, preservative, dye, and other as needed, to 100
additives
EXAMPLE 9
High Solids BHMT Fabric Softener Formulation
Component Amount (wt. % )
BHMT + 4 moles EO + 3 moles tallow fatty 10
acid + 3 moles
DMS
Tallow-based TEA ester quat (DMS-based 15
quat)
Fragrance, preservative, dye, and other as needed, to 100
additives
EXAMPLE 10
High Solids BHMT Fabric Softener Formulation
Component Amount (wt. %)
BHMT + 5 moles EO + 2.5 moles tallow fatty5
acid + 3
moles DMS
Tallow-based MDEA ester quat (DMS-based 20
quat)
Fragrance, preservative, dye, and other as needed, to 100
additives
EXAMPLE 11
High Solids BHMT Fabric Softener Formulation
Component Amount (wt. % )
BHMT + 5 moles EO + 3.5 moles tallow fatty5
acid + 3
moles DMS
Ditallow ester of 3-(dimethylamino)-1,2-propanediol10
(methyl
chloride-based quat)
Fragrance, preservative, dye, and other as needed, to 100
additives
EXAMPLE 12
Low Solids HMDA Fabric Softener Formulation
Component Amount (wt. %)
CA 02347036 2001-04-11
WO 00/21918 PCTNS98/21683
HMDA + 4 moles EO + 2 moles tallow fatty 2.5
acid + 2 moles
DMS
Tallow-based TEA ester quat (DMS-based 5
quat)
Fragrance, preservative, dye, and other as needed, to 100
additives
E~PLE 13
Low Solids BHMT Fabric Softener Formulation
Component Amount (wt. %)
BHMT + 5 moles EO + 2.5 moles tallow fatty2
acid + 2
moles DMS
Tallow-based MDEA ester chloride quat 5
Fragrance, preservative, dye, and other as needed, to 100
additives
There are many unexpected benefits of the above formulations. Most
importantly, there is improved softening performance of the formulation over
an equivalent
amount of dialkyl ester quat formulation absent compounds of structural
formula (1). In
addition, however, the formulations above disperse readily into water, even
cold water,
without a viscosity increase as with conventional quats, and provide a finer
final particle size
when so dispersed, for example, at 100 ppm concentration. Moreover, the
formulations have
improved fluidity and viscosity, even in high solids formulations, over
conventional non-
blended formulations, exhibit reduced staining, and provide high color and
odor stability. It
should also be noted that certain of the formulations may be made clear, the
provide clear
compositions more readily (that is, over a greater concentration range with
lower solvent
concentrations) when the fatty acid levels of the polyester quat component is
lower, for
example, 3 or fewer moles of fatty acid per mole of BHMT, while it is
difficult to obtain
clear formulations with higher fatty acid levels of the polyester quat
component, 3.5 or
greater moles of fatty acid per mole of BHMT, even at low concentrations.
This example illustrates formulations of compounds according to the present
invention that are formulated into a microemulsion. As noted above, such
formulations
generally include three components: (a) compound of structural formula (1),
(b) a solvatrope
or coupling agent and blends thereof, and (c) an oil or hydrophobic organic
component and
blends thereof, which are blended in water. Suitable solvatropes or coupling
agents may be
selected from the diols and alkoxylates corresponding to structural formulas
(T) or (E),
TMPD, TMPD alkoxylates, ethanol, isopropanol, butanol, 1,2-
cyclohexanedimethanol (1,2-
CHDM), 1,4-cyclohexanedimethanol (1,4-CHDM), HPHP glycol, isopentyldiol, 1,2-
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
hexanediol, ethylene glycol butyl ether, hexylene glycol, 2-butoxyethanol
(sold by Union
Carbide under the tradename butyl CELLOSOLVE~), C6-C12 diols/triols and ester
diols/triols, glycol ethers, and the like. Oils and hydrophobic organic
components may be
selected from the fatty C8-C22 methyl esters, such as methyl oleate, mineral
seal oils, silicone
oils, fatty acids, monoglycerides, diglycerides, triglycerides, dialkyl
esters, and the like,
depending on the application. The methyl esters are the preferred oil based on
performance
and biodegradability, although mineral seat oil is preferred in car drying aid
applications.
An example of such a microemulsion is the following formulation.
EXAMPLE 14
Microemulsion Formulation
Component Amount (wt. % )
HMDA + 4 moles EO + 2 moles tallow fatty 15
acid + 2 moles
DMS
Isopropanol 2.6
TMPD/CHDM (80%/20%) 11.7
Methyl oleate 18.0
Fragrance, dye, preservative, and other as needed
additives
Deionized water to 100
This example illustrates formulations of compounds according to structural
formula (1) for use as emulsifiers, for example, for agricultural emulsifiers
or asphalt
emulsifiers. In a typical formulation, HMDA is ethoxylated using 4 moles of
ethylene oxide
per mole of HMDA. The resulting HMDA ethoxylate is esterified with 1.5 to 2
moles of
tallow fatty acid per mole of HMDA and the resulting HMDA ethoxylated ester is
quaternized with 2 moles of DMS. The HMDA ethoxylated ester quat therefore
contains
only 1.5 to 2 ester groups per HMDA molecule. This HMDA compound is therefore
less
hydrophobic than many of the other examples of compounds according to
structural formula
( 1 ) given in other Examples for other applications. Thus, this compound is
useful as an
emulsifier for organic compounds, for example, when formulated with
pesticides, other
surfactants and dispersants, and water, the resulting formulation would make a
useful
agricultural pesticide spray, the HMDA ethoxylated ester quat encouraging the
organic
components to remain dispersed in the water, allowing efficient transfer and
coverage in
treating plants. Examples 16 to 18 provide farther examples of this use of the
compounds of
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
structural formula (1) and fotmtulations thereof. As can be appreciated, for
these applications
the polyquaternary compound is prepared having only about 1 to about 2 ester
groups per
molecule to ensure that the molecule as a whole is less hydrophobic than those
that would
typically be used in other applications.
EXAMPLE 16
This example illustrates formulations of compounds according to structural
formula (1) for use as a herbicide emulsion agent. A compound according to
structural
formula (1), for example, HMDA + 12 moles EO + 1.2 moles canola fatty acid, or
mixture
thereof is added to a solvent or solvent mixture and water and a herbicide is
incorporated
therein and an emulsion formed. The amount of the polyquaternary compound is
generally
from about 5 wt. % to about 50 wt. % , preferably from about 10 wt. % to about
40 wt. % ,
most preferably from about 15 wt. % to about 30 wt. % , of the herbicide
concentrate
composition. A typical formulation might be:
EXAMPLE 16
Herbicide Concentrate Emulsion Formulation
Component Amount (wt. %)
HMDA + 12 moles EO + 1.2 moles canola 20.0
fatty acid
Propylene glycol 4.0
Water 2.0
Clo-C1, alcohol + 9 moles EO 3.0
Glufosinate herbicide 71.0
EXAMPLE 17
This example illustrates formulations of compounds according to structural
formula (1) for use as a pesticide emulsion agent formulation. Unlike Example
16, Example
17 does not incorporate the pesticide itself, instead an appropriate amount of
pesticide must
be added to the pesticide emulsion agent formulation to make a pesticide
emulsion
concentrate, which is diluted with water by the user and applied in the dilute
form. In
general, only 10-30 wt. % of the pesticide emulsion agent formulation is used
to in the
pesticide emulsion concentrate, that is, there is 70-90 wt. % pesticide in the
pesticide
emulsion concentrate, which is the form it will generally be commercialized.
The final
customer will then dilute the pesticide emulsion concentrate
(pesticidelemulsifier package)
into water for actual application of pesticide.
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
In such a pesticide emulsion agent formulation, a compound according to
structural formula (1), for example, BHMT + 15 moles EO + 1.2 motes canola
fatty acid,
or mixture thereof is added to a solvent or solvent mixture and water and a
pesticide is
incorporated therein and an emulsion formed. The amount of the polyquaternary
compound
is generally from about 20 wt. % to about 70 wt. %, preferably from about 15
wt. % to about
50 wt. %, most preferably from about 20 wt. % to about 40 wt. % , of the
pesticide emulsion
agent composition. A typical emulsion agent formulation might be:
EXAMPLE 17
Pesticide Emulsion Agent Formulation
Component Amount (wt. %)
BHMT + 15 moles EO + 1.2 moles canola 40.0
fatty acid
Phosphate ester 6.0
Water 2.0
C,o-C1~ alcohol + EO/PO block copolymer 52.0
EXAMPLE 18
As noted above, the compounds of structural formula (1) may also be used as
an asphalt emulsifier as an additive for asphalt. As with the previous
emulsifier formulations,
the compound of structural formula (1) is prepared having only about 1 to
about 2 ester
groups per molecule to ensure that the molecule as a whole is less hydrophobic
than those
that would typically be employed in other applications. If the resulting
compound is not
quaternized or quaternized to a limited extent, for example, only 1 mole of
DMS per mole of
HMDA, the possible applications in asphalt products are as a cationic rapid
set (CRS)
emulsion for chip seal, as a cationic medium set (CMS) for mixing grade
applications, in a
slurry seal or microsurfacing application, or in a roofing and driveway
sealer. The amount
of the compound of structural formula (1) in such an application would likely
include from
about 0.15 wt. % to about 2.0 wt. %, preferably about U.20 wt. % to about 0.75
wt. %, and
most preferably about 0.25 wt. % to about 0.65 wt. % , of the asphalt.
Generally the fatty
amine should be protonated in order to dissolve in the water. The pH of the
emulsifier
solution should be less than about 6.5, and may be adjusted with any strong
acid to have a
pH of between about 1.0 and about 5.0, preferably between about 2.0 to about
4.0, most
preferably between about 2.0 and about 3Ø
SO
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
If the compound is quaternized, for example, with 2 or more moles of DMS
per mole of HMDA, the possible applications in asphalt products are as a
cationic slow set
emulsion, cationic quick set emulsion, tack coat, fog seal, base
stabilization, prime coat,
slurry seal, microsurfacing, industrial asphalt emulsion, or filled asphalt
emulsion. The
amount of the compound of structural formula (1) in such an application would
likely include
from about 0.1 wt. % to about 8.0 wt. %, preferably about 0.20 wt. % to about
5.0 wt. % , and
most preferably about 0.5 wt. % to about 2.0 wt. % , of the asphalt. Generally
the fatty amine
should be protonated in order to dissolve in the water. The pH of the
emulsifier solution
should be less than about 7.0, and may be adjusted with any strong acid to
have a pH of
between about 1.0 and about 5.0, preferably between about 2.0 to about 4.0,
most preferably
between about 2.0 and about 3Ø
EXAMP ,E 19
This example illustrates formulations of compounds according to structural
formula (1) for use in corrosion inhibition, for example, for lubricating oil
or oil field use. A
compound according to structural formula (1), for example, HMDA + 4 moles EO +
2
moles canola fatty acid, or mixture thereof is added to lubricating or other
oils as a corrosion
inhibitor. The polyamine may be used alone or in combination with a surfactant
and/or
coupling agent, which may be incorporated with the polyamine in a formulation
or applied
separately. When used, an effective amount is applied to the oil or oil
mixture that will come
in contact with the metal. The term "effective amount" denotes the amount of
polyamine
compound that would be effective to inhibit corrosion. In general, the amount
of the
polyamine compound ranges from about 0.001 wt. %o to about 5 wt. %, preferably
from about
0.01 wt. % to about 1 wt. %, most preferably from about 0.01 wt. % to about
0.5 wt. %, of the
oil mixture in which it is used.
EXAMPLE 20
This example illustrates formulations of compounds according to structural
formula (1) for use in a lubricant and anti-balling agent for silicate muds
and other water-
based muds, for example, to lubricate drill strings to prevent stuck pipe, bit-
balling, or string
balling associated with drilling wells. The apparatus for drilling and general
lubrication
processes in well-known to those of skill in the art, and are disclosed, for
example, in U.S.
Patent Nos. 5,586,608; 5,593,954; and 5,639,715. In this lubrication process,
a compound
according to structural formula (1), for example, BHMT + 5 moles EO + 2.5
moles canola
fatty acid, or mixture thereof is added to lubricating or other oils effective
to inhibit stuck
pipe, bit balling, or string balling. The polyamine may be used alone or in
combination with
a surfactant and/or coupling agent, for example, propylene glycols or
ethoxylated glycols,
sr
CA 02347036 2001-04-11
WO 00/21918 PCT/US98I21683
which may be incorporated with the polyamine in a formulation or applied
separately. Of
particular use are the oilfield products available from Witco Corporation
under the tradename
WITBREAKT"', such as WITBREAKT"' DPG-484. When used, an effective amount of
polyamine is applied to the lubricant mixture as used in the drilling
operation. The term
"effective amount" denotes the amount of polyamine compound that would be
effective to
inhibit stuck pipe, bit balling, or string balling. In general, the amount of
the polyamine
compound ranges from about 0.001 wt. % to about 5 wt. % , preferably from
about 0.01 wt. %
to about 2 wt. % , most preferably from about 0.05 wt. % to about 0.5 wt. %,
of the lubricating
mixture in which it is used. If used in a formulation, a typical formulation
might be:
EXAMPLE 20
Lubricant and Anti-Balling Agent Formulation
Component Amount (wt. % )
BHMT + 5 moles EO + 2.5 moles canola fatty50
acid
Surfactant or coupling agent 50
This example illustrates formulations of compounds according to structural
formula (1) for use as a mineral and coal dewatering agent, for example, for
removal of
water by means of a filtration dewatering apparatus known to those of skill in
the art. In this
process, a compound according to structural formula ( 1 ), for example, HMDA +
4 moles
EO + 3 moles tallow fatty acid + 2 moles DMS, or mixture thereof is added to
the mineral
slurry prior to mechanical dewatering, the process becoming more effective as
a result. The
amount of the polyquaternary compound is generally from about 20 wt. % to
about 95 wt. %,
preferably from about 30 wt. % to about 80 wt. % , most preferably from about
50 wt. % to
about 75 wt. % , of the dewatering agent formulation. A typical formulation
might be:
EXAMPLE 21
Mineral and Coal Dewatering Agent Formulation
Component Amount (wt. %)
HMDA + 4 moles EO + 3 moles tallow fatty 70.0
acid + 2 moles
DMS
TMPD + 1 mole EO 20.0
Nonionic surfactant 10.0
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
These examples illustrate formulations of compounds according to structural
formula (1) for use as softener/debonding agents, for example, in tissue or
paper products.
Examples 22 to 24 are specific examples, while Example 24 presents a general
softener/debonder formulation according to the present invention. Example 22
was a thick
liquid while Examples 23 and 24 were both clear liquids; all of Examples 22 to
24 were easy
to disperse in water.
EXAMPLE 22
Softening/Debonding Agent Formulation
for Paper
Component Amount (wt. %)
HMDA + 4 EO + 1.5 moles tallow fatty acid80
+ 1.5 moles
canola fatty acid + 1.75 moles DMS
Propylene glycol 5
Alkoxylated fatty acid (nonionic surfactant)15
_ - EXAMPLE 23
Softening/Debonding Agent Formulation
for Paper
Component Amount (wt. %)
HMDA + 4 EO + 3 moles canola fatty acid 80
+ 1.5 moles
DMS
Propylene glycol 5
Alkoxylated fatty acid (nonionic surfactant)15
EXAMPLE 24
Softening/Debonding Agent Formulation
for Paper
Component Amount (wt. % )
HMDA + 10 EO + 1.5 moles tallow fatty 80
acid + 1.5 moles
canola fatty acid + 1.5 moles DMS
Propylene glycol 5.0
Alkoxylated fatty acid (nonionic surfactant)15
In general, the softener/debonder formulation of the present invention would
include the components set forth in Example 25 in the amounts shown.
EXAMPLE 25
General Softener/Debonder Concentrate Formulation
5,~
CA 02347036 2001-04-11
WO 00/21918 PCTNS98/21683
Component Amount
(wt. %)
typical preferred most preferred
Compound of structural formula10-90 30-80 60-80
(1)
Propylene glycol 0-3U 0-20 0-10
Polyethylene glycol 0-30 0-20 0-10
Nonionic surfactant such 10-80 10-60 10-40
as alkoxylated
fatty acid or alkoxylated
nonionic
surfactant
In the following tests, the performance of formulations of the present
invention were compared to the performance of a commercial product available
from Witco
Corporation under the tradename AROSURF~ PA-801, at dosages corresponding to
1, 3,
and 5 Ib. (#) debonder/ton of fiber using a softwood (SW) fiber furnish.
Standard
preparation and test methods were employed to prepare handsheets and to
conduct the
comparative tests against AROSURF PA-801; they are as follows: handsheet
preparation
(TAPPI test method T-205); dry tensile (TAPPI test method T-492); sorptive
rate and
capacity (TAPPI test method T-561); paper conditioning (TAPPI test method T-
402); and
grammage and thickness (TAPPI test method T-220). Softness was evaluated using
paired
comparison softness panels.
In each case, a dispersion of the appropriate formulation was prepared in
water at 25-30°C. An aqueous slurry of SW fibers was treated with the
dispersion of the
respective formulation at dosages corresponding to 1, 3, and 5 lb. (#)
debonder/ton of fiber.
Tissue weight handsheets, approximately 60 g/m2, were prepared according TAPPI
procedure T-205. The handsheets were equilibrated under conditions specified
in TAPPI
Method T-402. The handsheets were tested for tensile and sorptive rate and
capacity
according to TAPPI Methods T-492 and T-561, respectively. The results are
presented in
Table II. In each case the softness of the product treated with Examples 22 to
24 was better
than the product treated with a comparable amount of AROSURF~ PA-801 debonder.
TABLE
II
Testing
Results
Debonder Tensile Absorbency Absorbency
used strength Capacity Capacity
(kNm/kg) at at
0-5 20
sec sec.,
(g/g/sec) (gH,O/g
fiber)
1#/ton3#/ton5#lton1#/ton3#/ton5#/ton1#/ton3#/ton5#/ton
Blank 15.28 15.2815.28 0.19 0.19 0.19 2.08 2.08 2.08
AROSURF~ 13.61 10.057.67 0.16 0.13 0.13 1.80 1.39 1.34
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
PA-801
Example 13.27 9.83 7.99 0.16 0.13Ø11 1.83 1.40 1.26
22
Example 13.30 10.327.83 0.15 0.13 0.13 1.76 1.51 1.43
23
Example 12.20 10.118.61 0.16 0.13 0.13 1.68 1.40 1.36
24
From these tests and the results presented in Table II, it can be seen that
HMDA ester quats are effective debonders and compare favorably in performance
to an
industry standard. Indeed, the HMDA ester quats afford debonded tissue
products with good
absorbency rates and capacities and hand panels confirm that the formulations
of the instant
invention give better softness than AROSURF~ PA-801 debonder. In addition, the
HMDA
ester quat formulations have low odor and each of the above formulations was
easy to
disperse in warm water. As with all of the Examples given, Examples 22 to 25
are only
exemplary and, although applied here to softwood fibers, may be used with
hardwood fiber,
recycled fiber, baggasse fibers, fluff pulp, and all natural papermaking
fibers and blends
thereof.
formula (1) for use as deinking agents, for example, in recycled paper
products. Generally
such formulations are added to the pulper and/or to the slurry passing into
the flotation cell.
Furnishes that may be deinked with the compounds of the instant invention
include waste
paper with laser print, printed waste paper for a newsprint mill, ink fixed on
pulp fiber of the
printed waste paper product such as newspaper and magazines, and flexo
news/magazine/newsprint, and the like. The apparatus and processes for
deinking operations
in well-known to those of skill in the art. Typical apparatus and processes
are disclosed in
U.S. Patent Nos. 5,622,597; 5,346,543; and 5,696,292; additional apparatus and
methods
are disclosed in PCT WO/95/12026 and EP 0 726 246 A1. In such a deinking
process, the
amount of the compound of structural formula ( i ) is about 0.01 wt. % to
about 2.0 wt. % ,
preferably about 0.03 wt. % to about 1.0 wt. %, and most preferably about 0.05
wt. % to
about 0.5 wt. % of the dry fiber. Other additives typically used include an
ethylene
oxide/propylene oxide nonionic (for example, alcohol or fatty acid alkylphenol
derivatives)
surfactant for frothing and foam control, sodium silicate, sodium hydroxide,
hydrogen
peroxide or sodium hypochlorite, and an EDTA or DTPA chelant. In addition,
such cationic
deinking agents can be combined with fatty acids, alkylene oxide adducts, and
other solvents.
Deinking is usually conducted on fiber slurries of 1 % consistency at 40-
50°C.
EXAMPLES 27 TO 31
ss
CA 02347036 2001-04-11
WO 00/21918 PCT/U598/21683
These examples illustrate formulations of compounds according to structural
formula (1) for use in personal care formulations, for example, for hair
conditioners and skin
conditioners. Example 29 is a microemulsion example comparable to Example 14.
EXAMPLE 27
HMDA Hair Conditioner Formulation
Component Amount (wt. %)
HMDA + 4 moles EO + 2 moles canola fatty 1.18
acid + 2 moles
DMS
Cetyl Alcohol 2.00
Ceteareth-20 (cetearyl alcohol ethoxylated1.00
with 20 moles of
EO), available from Witco Corporation
under the tradename
VARONIC~ 63-E20
Fragrance, dye, preservative, and other as needed
additives
Deionized water to 100
EXAMPLE 28
BHMT Hair Conditioner Formulation
Component Amount (wt. %)
BHMT + S moles EO + 3.5 moles canola fatty1.18
acid + 2.5
moles DMS
Cetyl Alcohol 2.00
Ceteareth-20 (cetearyl alcohol ethoxylated1.00
with 20 moles of
EO), available from Witco Corporation
under the tradename
VARONIC~ 63-E20
Fragrance, dye, preservative, and other as needed
additives
Deionized water to 100
EXAMPLE 29
BHMT Microemulsion Hair Conditioner Formulation
Component Amount (wt. % )
BHMT + 5 moles EO + 3.5 moles canola fatty1.8
acid + 2.5
moles DMS
Mineral oil (available from Witco Corporation1.8
under the
tradename KAYDOL~)
TMPD + 1 EO 1.2
Fragrance, dye, preservative, and other as needed
additives
Deionized water to 100
s~
CA 02347036 2001-04-11
WO 00/21918 PCTNS98/21683
IJS98/21683
P.57
NOT TO BE TAKEN INTO ACCOUNT FOR THE
PURPOSES OF INTERNATIONAL PROCESSING
5 ~-
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
US98/21683
P.58
NOT TO BE TAI~.EN INTO ACCOUNT FOR THE
PURPOSES OF INTERNATIONAL PROCESSING
5$
CA 02347036 2001-04-11
WO 00/21918 PCT/US98/21683
be replaced by other cations, such as potassium ion or ammonium ion, without
appreciably
affecting the performance of the anionic surfactant. Furthermore, with regard
to the other
components that may be salts that are be added to the composition or
subsequently added to
the composition, for example, viscosity modifiers, the component includes all
similar
compounds, that is, compounds where the ions are substituted by any other ion
which is not
significantly deleterious to the desired chemical or physical properties of
the overall
compound in its intended use. It is therefore understood that such ion
substitution is well-
known in the art and all such possibilities and equivalents are intended to be
embraced within
the appended claims.
As noted above, the examples provided are intended to further describe the
aspects of the present invention. The examples are illustrative only and are
not to be
construed as limiting the scope of that which is regarded as the invention.
Therefore, the
scope of the present invention is only to be limited by the following claims
and the
equivalents thereto. In the specification and claims, the terms "comprise",
"comprisi ", or
"comprises" are intended to convey that the composition or formulation has or
include the
recited components, but does not exclude other non-recited components.