Note: Descriptions are shown in the official language in which they were submitted.
CA 02347469 2003-11-12
wo oom~o rcrnJS9gm6oa
FIRS OF (+~ALPHA-( 2,3- DI~'fiOXYP~fYYL) -1-[2-(4- FLUOROPFIEIVYL) EniYLI-4
PIpERIHINEM1THANOL AND TIiE~ USE AS PRODRUGS OF TIC SHTZA RFCEPZ'OR ANTAGONIST
s MDL 100,907
The compound (+)-a-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-
piperidinemethanol, also known as MDL 100,907, is a potent SHTz,, receptor
antagonist which
is being evaluated in the clinic for treatment of schizophrenia. J. Pharm.
Exp. Ther. 277:968-
9881 (1996). It was described in U.S. patent 5,134;149.
MDL 100,907 antagonizes the effects of serotonin at the SHT," receptor and
thus is
useful for treating a variety of conditions. However, this compound may also
act directly or
indirectly act to achieve therapeutic effects other than by its SHT~
antagonism. For example,
see European Journal of Pharmacology 273: 273-279 ( 1995} where MDL 100,907
has been
shown to exert a tonic inhibitory influence on dopamine efflux in the medial
prefrontal cortex.
Some of the uses for M100,907 have been disclosed in patents or patent
applications U. S.
Patent 5,169,096 claimed compounds having a generic scope which encompassed
the
M100,907 and disclosedwses of the treatment of anorexia nervosa, variant
angina, ltaynaud's
phenomenon, coronary vasospasms, prophylactic treatment of migraine,
cardiovascular
diseases such as hypertension, peripheral vascular disease, thrombotic
episodes,
2, cardiopulmonary emergencies and arrythmias, and has anesthetic properties.
See also U.S.
Patent nos. 4,783,471; 4,912,117; and 5,021,428, which are divisions of U. S.
Patent
5,169,096. See also U. S. Patent nos. 4,877,798 (fibromyalgia}, 4,908,369
(insomnia};
5,106,855 {glaucoma); EP 319 962 (anxiety}; EP 337 136 {extrapyramidal
symptoms).
The M100,907 was then specifically claimed in U. S. Patent no. 5,134,149 which
disclosed uses of antagonizing serotonin at the SHt2 receptor, treating
anxiety, variant angina,
CA 02347469 2003-11-12
WO OO1Z1930 PCTNS981Z1608
-2-
anorexia nervosa, Raynaud's phenomenon, intermittent claudication, coronary or
peripheral
vasospasms, fibromyalgia, extrapyramidal symptoms, arrytlunias, thrombotic
illness, transient
ischemic attacks, drug abuse, and psychotic illness such as schizophrenia and
mania. See also
U. S. Patent nos. 5,561,144; 5,700,812; 5,700,813; 5,721,249- divisionals of
U. S. Patent no.
5,134,149- and also U. S. Patent nos.5,618,824 (obsessive compulsive disorder)
aad
PCT/US97102597 (depressive disorders including major depressive episode and
dysthymia,
and bipolar disorders), and insomnia and sleep apnea as described in U.S.
Patent
No. 6,277,864 and U.S. Patent No. 6,613,T79.
An object of the present invention is to provide a new compound which after
,o administration, releases a therapeutically effective amount of MDL 100,907
over an extended
period of time. The extended period of time means a time longer than a single
dose of MDL
100,907, and would last for several days, several weeks, about one month up to
about 6 to
about 8 weeks , and preferably from about 2 weeks to about one month.
There are many advantages to administering a single dose of a compound to a
paticnt
,s which lasts over an extended period of time. It can avoid compliance
problems which can be
particularly important in patients suffering from psychoses or addictive
behaviors such as
schizophrenia, obsessive compulsive behavior, depression, anxiety, anorexia
and drug
addiction. Other advantages include an absence of the typical oscillations of
drug level
achieved with multiple dose therapy through which the patient should
experience an
improved efficacy in treatment with lower peak drug concentrations.
While the concept of sustained release formulations is not new, not all
compounds are
capable of being chemically altered to produce a new compound capable of being
metabolized
into the active ingredient at a desirable rate and over the desired length of
time. Other factors
contribute to the difficulty of preparing a sustained release formulation such
as protein binding
Zs and other physiological processes which can affect the therapeutic effect
of the active
ingredient, see for example Biochemical Pharmacology, Vol. 3b, No. 10 pp1715-
1722 (19877.
Also, the chemically altered compound must be compatible with pharmaceutically
acceptable carriers and be stable enough not to substantially degrade on the
shelf to the
active ingredient. In short, the design of an acceptable sustained release
formulation is a
difficult, unpredictable task.
CA 02347469 2001-04-12
WO 00/21930 PCT/US98/21608
-3
SUMMARY OF THE INVENTION
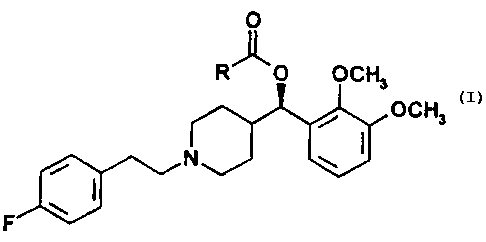
The present invention is a compound of Formula I:
O
R"O OCH
3
OCH3
N J ~J
F
FORMULA I
wherein R is C4-Czo alkyl, or a stereoisomer or a pharmaceutically acceptable
salt thereof. The
,o present invention also comprises:
a pharmaceutical composition comprising the compound of formula I and a
pharmaceutically acceptable carrier;
a method of antagonizing the effects of serotonin at the SHTzA receptor
comprising
administering a compound of formula 1 to a patient in need thereof;
,5 a method of treating a patient for a disease state comprising administering
to the
patient in need of such therapy a therapeutically effective amount of a
compound of formula I
wherein the disease state is psychoses (including schizophrenia), obsessive
compulsive
disorder, thrombotic illness, coronary vasospasm, anxiety, anorexia nervosa ,
Raynaud's
phenomenon, fibromyalgia, extra-pyramidal side effects, anxiety, arrhythmia,
depression,
Za bipolar depression, insomnia, sleep apnea, Raynaud's phenomenon, or drug
abuse; and
a method of making the compounds comprising reacting alcohol of structure (5)
shown
hereafter with an acid halide, acid anhydride or carboxylic acid in the
presence of a sufficient
amount of an appropriate base.
CA 02347469 2001-04-12
WO 00/21930 PCTNS98/21608
-4
DETAILED DESCRIPTION OF THE INVENTION
Terms used herein have the following meanings:
a) "Pharmaceutically acceptable salts" means either an acid addition salt or a
basic
addition salt whichever is possible to make with the compounds of the present
invention.
"Pharmaceutically acceptable acid addition salt" is any non-toxic organic or
inorganic acid
,o addition salt of the base compounds represented by Formula I. Illustrative
inorganic acids
which form suitable salts include hydrochloric, hydrobromic, sulfuric and
phosphoric acid and
acid metal salts such as sodium monohydrogen orthophosphate and potassium
hydrogen
sulfate. Illustrative organic acids which form suitable salts include the mono-
, di- and tri-
carboxylic acids. Illustrative of such acids are, for example, acetic,
gIycolic, lactic, pyruvic,
,5 malonic, succinic, glutaric, fumaric, malic, tartaric, citric, ascorbic,
malefic, hydroxymaleic,
benzoic, hydroxybenzoic, phenylacetic, cinnamic, salicyclic, 2-phenoxybenzoic,
p-
toluenesulfonic acid and sulfonic acids such as methanesulfonic acid and 2-
hydroxyethanesulfonic acid. Either the mono- or di-acid salts can be formed,
and such salts
can exist in either a hydrated or substantially anhydrous form. In general,
the acid addition
zo salts of these compounds are more soluble in water and various hydrophilic
organic solvents
and which in comparison to their free base forms, generally demonstrate higher
melting
points.
"Pharmaceutically acceptable basic addition salts" means non-toxic organic or
inorganic basic
zs addition salts of the compounds of Formula (I) or any of its intermediates.
Examples are
alkali metal or alkaline-earth metal hydroxides such as sodium, potassium,
calcium,
magnesium or barium hydroxides; ammonia, and aliphatic, alicyclic, or aromatic
organic
amines such as methylamine, trimethylamine and picoline. The selection of the
appropriate
salt may be important so that the ester is not hydrolyzed. The selection
criteria for the
3o appropriate salt will be known to one skilled in the art.
CA 02347469 2001-04-12
WO 00/21930 PGT/IJS98l21608
-5-
b) "Stereoisomers" is a general term for all isomers of the individual
molecules that differ
only in the orientation of their atoms iri space. It includes mirror image
isomers
(enantiomers), geometric (cis/trans) isomers, and isomers of compounds with
more than one
chiral center that are not mirror images of one another (diastereoisomers).
c) "Alkyl" means a branched or straight chain alkyl group specified by the
amount of
carbons in the alkyl group, e.g., C4-C20 alkyl means a four, five, six, seven,
eight, nine, ten,
eleven, twelve, thirteen, fourteen, fifteen, sixteen, seventeen, eighteen,
nineteen, or twenty
carbon branched or straight chain alkyl or any ranges thereof, for example,
but not limited to
,o CS-C20, C 1-C 15, C3-C 15, CS-C 15, C7-C 15, and C7-C9.
d) "Patient" means a warm blooded animal, such as for example rat, mice, dogs,
cats,
guinea pigs, and primates such as humans.
e) "Treat" or "treating" means to alleviate symptoms, eliminate the causation
of the
,5 symptoms either on a temporary or permanent basis, or to prevent or slow
the appearance of
symptoms of the named disorder or condition.
f) "Therapeutically effective amount" means an amount of the compound which is
effective in treating the named disorder or condition.
g) "Pharmaceutically acceptable carrier" is a non-toxic solvent, dispersant,
excipient,
zo adjuvant or other material which is mixed with the compound of the present
invention in order
to permit the formation of a pharmaceutical composition, i.e., a dosage form
capable of
administration to the patient. One example of such a carrier is a
pharmaceutically acceptable
oil typically used for parenteral administration.
h) The term "Restorative Sleep" means sleep which produces a rested state upon
waking;
zs i) the term "Sleep Disorder" means Insomnia and Obstructive Sleep Apnea;
j) the term "Insomnia" means Primary Insomnia, Insomnia related to another
Mental
Disorder, and Substance-Induced Insomnia;
k) The term "Primary Insomnia" means difficulty in initiating sleep, in
maintaining sleep
or having restorative sleep which is not caused by a Mental Disorder or due to
CA 02347469 2001-04-12
WO 00/Z1930 PCT/US98/21608
-6-
physiological effects of taking or withdrawing from certain substances
(substance-
induced). As used herein, it also includes Circadian Rhythm Insomnia which.is
insomnia due to a change in the normal sleep-wake schedule (shift changes, jet
lag,
etc.);
1) The term "Insomnia related to another Mental Disorder" means difficulty in
initiating
sleep, in maintaining sleep or having restorative sleep which is caused by an
underlying Mental Disorder such as, for example, depression, anxiety or
schizophrenia;
m) The term "Substance-Induced Insomnia" means difficulty in initiating sleep,
in
,o maintaining sleep or having restorative sleep which is caused by
physiological effects
of taking or withdrawing from certain substances such as caffeine, alcohol,
amphetamine, opioids, sedatives, hypnotics and anxiolytics; and
n) The term "Obstructive Sleep Apnea" means repeated episodes of upper-airway
obstruction during sleep and is normally characterized by loud snores or brief
gasps
,5 that alternate with episodes of silence.
The (+)-isomer of a-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-
piperidinemethanol can be prepared by methods described in U.S. patent no.
5,134,149. One
suitable method follows.
CA 02347469 2001-04-12
WO 00/Z1930 PCT/US98/21608
SCHEME I- Starting Materials
w
HO i
(+, _ ) * OCH3
OCH3
0cH3
N + ~ I ~ ~COOH Step A
esterification
i
F 1 2
0CH3 OCH3
OCH
0CH3 0 0CH3 \ ~ 0 3 0 OCH3
* 0 (+) * (+) ~
(_,+)
(+)
Step B
C ---.-r N
N + chromotography
i
i
3'(+,+)
F diastereomer
F 3
4
i
Step C H0 * ~
(+)~ ~OCH3
Hydrolysis OCH3
~N-
F
CA 02347469 2001-04-12
WO 00/21930 PCTNS98/21608
_g_
In Step A of Reaction Scheme I, an esterification reaction is carried out
between
racemic alpha-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-
piperidinemethanol
(structure 1 ) and the (+)-isomer of alphamethoxyphenylacetic acid (structure
2). This
esterification produces the diastereomeric mixture identified as structure 3.
These
diastereomers are subjected to silica gel chromatography which separates the
two
diastereomers, thereby isolating the (+,+) diastereomer as is depicted in Step
B. In Step C, the
(+,+) diastereomer is hydrolyzed which produces the (+)-isomer of alpha(2,3-
dimethoxy-
phenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol.
The esterification reaction can be carried out using techniques known in the
art.
Typically approximately equivalent amounts of racemic alpha-(2,3-
dimethoxyphenyl)-1-[2-(4-
fluorophenyl)ethyl]-4-piperidinemethanol and the (+)-isomer of alpha-
methoxyphenylacetic
acid are contacted in an organic solvent such as methylene chloride, THF,
chloroform, or
toluene and heated to reflux for a period of time ranging from 5 to 24 hours.
The
esterification is typically carried out in the presence of an equivalent
amount of
dicyclohexylcarbodiimide (DCC) and a catalytic amount of 4-
dimethylaminopyridine
(DMAP). The resulting diastereomers can be isolated by filtration of the
dicyclohexylurea
and evaporation of the filtrate.
zo
The diastereomers are then subjected to silica gel chromatography which
separates the
(+,+) and the (-,+) diastereomers. This chromatographic separation may be
carried out as is
known in the art. A 1:1 mixture of hexane and ethyl acetate is one suitable
eluent.
zs The resulting (+,+) diastereomer is then subjected to a hydrolysis reaction
which
produces the (+)-en~tiomer of alpha-(2,3-dimethoxyphenyl)-1-[2-(4-
fluorophenyl)ethyl]-4-
piperidinemethanol. The hydrolysis is carried out by contacting the
diastereomer with an
excess of a base such as potassium carbonate in an aqueous alcoholic solution.
The hydrolysis
is carried out at a temperature of about 15 to 30°C for a period of
time ranging from 2 to 24
3o hours. The resulting (+)-isomer of alpha-(2,3-dimethoxyphenyl)-1-[2-(4-
fluorophenyl)ethyl]
4-piperidinemethanol rnay then be recovered by dilution with water and
extraction with
CA 02347469 2001-04-12
WO 00121930 PCT/US98r11608
-9-
methylene chloride. It is then purified by recrystallization from a solvent
system such as
cyclohexane/hexane or ethyl acetate/hexane.
Methods for producing the starting materials of Reaction Scheme I are known in
the
art. For example, United States Patent No. 4,783,471 teaches how to prepare
racemic alpha-
(2,3-dimethoxyphenyl)-I-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol. This
patent is
hereby incorporated by reference. Examples No. 1 and 2 of this application
also teach suitable
methods. Alternatively, racemic alpha-(2,3-dimethoxyphenyl)-I-[2-(4-
fluorophenyl)ethyl]-4-
piperidinemethanol can be prepared in the following manner. Initially 4-
hydroxypiperidine is
,o subjected to an N-alkylation reaction with p-fluorophenylethyl bromide
which produces 4-
hydroxy-1-[2-(4-fluorophenyl)ethyl]-piperidine. This compound is brominated
with Ph3P ~ Br,
which produces 4-bromo-1-[2-(4-fluorophenyl)ethyl]piperidine. This compound is
contacted
with Mg thereby forming a Grignard Reagent which is then reacted with 2,3-
dimethoxybenzaldehyde which produces the desired product (t)-alpha-(2,3-
,5 dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol. The (+)-
isomer of
alphamethoxyphenylacetic acid is known in the art.
SCHEME II
OH OCH3
OCH3 RCOX or base
(RCO)20 or
i ~ RC02H
O
R' _O OCH3
OCH3
N J ~J
Zo
CA 02347469 2001-04-12
WO 00/21930 PCT/US98/21608
-10-
FORMULA I
Referring to Scheme II, X is chloro or bromo, with chloro being preferred and
R is as
previously defined. This reaction scheme shows the making of the sustained
release
compounds of Formula I from the alcohol (5).
The alcohol (5) is reacted with an acid halide (RC(O)X), RCOzH or acid
anhydride
(RCO),O in the presence of an a sufficient amount of an appropriate base.- An
appropriate
base is one that permits ester formation from the acid halide or anhydride.
Examples of
appropriate bases are trialkylamines, pyridine such as dimethylamino pyridine,
diisopropyl
ethyl amines, N-methyl morpholines, with triethylamine being preferred. A
sufficient amount
,o of the base can be ascertained by one skilled in the art which permits the
formation of the
compounds of Formula I.
Preferably the base is added to the alcohol (5) and that mixture added
dropwise to the
acid halide or acid anhydride in an appropriate solvent. Examples of
appropriate solvents are
chloroform, methylene chloride, or toluene, all of which are readily
available, with chloroform
,5 being preferred.
The temperature of the reaction may be at a range of about 0-25 ° C.
The reaction
mixture may be stirred for from a few hours to overnight to enhance the
reaction. Catalysts
may also be added for enhancement of reaction times, e.g., 4-
dimethylaminopyridine or the
like.
Zo The starting materials for the acid halide {RCOX) are readily available for
those skilled
in the art. For example, Aldrich Chemical company provides stearoyl chloride,
heptadecanoyl
chloride, palmitoyl chloride, myristoyl chloride, isovaleryl chloride, valeryl
chloride, hexanoyl
chloride, hexanoyl chloride, heptanoyl chloride, octanoyl chloride, nonanoyl
chloride,
decanoyl chloride, undecanoyl chloride and lauroyl chloride. For those acid
halides not
25 readily available, one skilled in the art may prepare the acid halide
desired. For example, a
carboxylic acid may be mixed with a halide donor to produce the desired acid
halide. One
example of this is to mix carboxylic acid (0.17 mol), methylene chloride (660
mL) and
dimethylformamide (0.5 mL) under a nitrogen atmosphere. Add oxalyl chloride
(0.2 mol)
CA 02347469 2003-11-12
WO 00121930 PCTNS98IZ1608
_11_
over about 5 minutes with stirring. Stir at ambient temperature for 3 hours
and evaporate the
solvent in vacuo to the acid chloride. Another method is to dissolve the
carboxylic acid (10
mmol) in methylene chloride (50 mL). Cool to 0 ° C, place under a
nitrogen atmosphere and
add, by dropwise addition, thionyl chloride (11 mmol). Stir at room
temperature for several
hours and evaporate the volatiles in vacuo to give the acid chloride. The
carboxylic acids are
readily available or can be easily made by those skilled in the art.
The starting materials for the acid anhydrides (RCO)20 are readily available
for those
skilled in the art. For example, Aldrich Chemical company provides butryic
anhydride,
isobutyric anhydride, valeric anhydride, 2-2,dimethylglutaric anhydride, and
phthalic
,o anhydride. Alternatively, acid anhydrides may be synthesized by methods
well known in the
art.
'The starting materials for the acids (RCOzH) are readily available or may be
synthesized by methods well know in the art. For example, see Advanced Organic
Chemistry,
Reactions, Mechanisms, and Structure, 4th ed., John Wiley & Sons" New York
1992.
,s Aldrich Chemical Company also provides isovaleric acid,
valeric acid, tert-butylacetic acid , 2, 2dimethylbutyric acid, 2-ethylbutyric
acid, hexanoic
acid, 3-methylvaleric acid, 4-methylvaleric acid, heptanoic acid, octanoic
acid, 2-
propylpentanoic acid, nanoic acid, decanoic acid, undecanoic acid, lauric
acid, tridecanoic
acid, myristoleic acid, myristic acid, pentadecanoic acid, palmitic acid,
heptadecanoic acid,
,o stearic acid, nonadecanoic acid, eicosanoic acid, as well as others wherein
R is between four
and twenty alkyl groups.
The following examples are being present to further illustrate the invention.
However,
they should not be construed as limiting the invention in any manner.
zs EXAMPLE 1 - starting material
Example 1, Steps A-D, demonstrates the preparation of the starting material
(t)-alpha(2,3-
dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol, structure
l, Scheme I.
CA 02347469 2001-04-12
WO 00/21930 PCT/US98/21608
A) 1-[2-(4-Fluorophenyl)ethyl]-4-piperidinecarboxamide
A solution of isonipecotamide (10.9 g, 85.0 mmol), 2-(4-fluorophenyl)ethyl
bromide (15.7 g,
77.3 mmol), and KzC03 (2.3 g, 167 mmol) was prepared in DMF (280 mL) and
stirred under
argon at 90-95°C overnight. The cooled solution was concentrated to a
white oily solid. The
solid was partitioned between water and CH2C12. The layers were separated and
the aqueous
layer was extracted with CH2Cl2. The combined organic layers were washed 2x
with water,
dried (MgS04), filtered, and evaporated to a oily solid. The solid was
recrystallized from
,o EtOAc to afford 1-[2-(4-fluorophenyl)ethyl]-4-piperidinecarboxamide as a
white powder, m.p.
177-178°C (decomp.). Anal. Calcd for C,4H,~FN20: C, 67.18; H, 7.65; N,
11.19. Found: C,
67.25; H, 7.67; N, 11.13.
B) 4-Cyano-1-[2-(4-fluorophenyl)ethyl]piperidine
~s
To stirred phosphorus oxychloride (25 mL, 41.12 g, 268 mmol) and sodium
chloride (5.1 g,
87.3 mmol) was added 1-[2-(4-fluorophenyl)ethyl]-4-piperidinecarboxamide (8.9
g, 35.6
mmol) portionwise. After complete addition, the solution was refluxed for 2
hours. The
cooled solution was carefully poured into dilute NH40H to destroy the POCl3.
The aqueous
zo solution was cooled to 0°C, then extracted 2x with CHzCl2. The
combined organic layers were
dried (MgS04), filtered, and evaporated to afford 8.1 g of an oily solid. The
solid was
distilled, (b.p. 150°C, 0.1 mm Hg), to afford a clear, colorless oil
that solidified. This material
was crystallized from hexane to afford 4-cyano-1-[2-(4-
fluorophenyl)ethyl]piperidine as white
needles, m.p. 47-48°C. Anal. Calcd for C~4H,7FN2: C, 72.39; H, 7.38; N,
12.06. Found: C,
2s 72.62; H, 7.49; N, 12.12.
C) 1 j2-(4-Fluorophenyl)ethyl]-4-piperidinecarboxaldehyde
To a stirred solution of 4-cyano-1-[2-(4-fluorophenyl)-ethyl]piperidine ( 1.00
g, 4.3 mmol) in
3o THF (20 mL) under argon at 0°C was added DIBAL-H (4.6 mL of a 1.0 M
solution in THF,
4.6 mmol) via syringe. After stirring overnight at room temperature, 10%
aqueous HCI (25
CA 02347469 2001-04-12
WO 00121930 PCT/US98/21608
-13-
mL) was added and the solution was stirred for 3 hours. The entire mixture was
then poured
into 10% aqueous NaOH (50 mL), then extracted 2x with ether. The combined
organic layers
were washed with brine, dried (MgS04), filtered, and evaporated to afford a
pale yellow oil.
The oil was chromatographed on silica gel , eluting with EtOAc. The
appropriate fractions
were combined and evaporated to afford an oil. This oil was distilled (b.p.
166°C, 0.05 mm
Hg) to afford 1-(2-(4-fluorophenyl)ethyl]-4-piperidinecarboxaldehyde, obtained
as a colorless
oil. Anal. Calcd for C~4H,8FN0: C, 71.46; H, 7.71; N, 5.95. Found: C, 71.08,
H, 7.81; N,
5.86.
,o D) (t)-alpha(2,3-Dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl-4-
piperidinemethanol
To a stirred solution of veratrole (0.93 g, 6.7 mmol) in THF (20 mL) under
argon at 0°C was
added n-BuLi (2.7 mL of a 2.5 M solution in hexane, 6.75 mmol). After stirring
2.5 h, the
solution was cooled to -78°C and treated with 1-[2-(4-
fluorophenyl)ethyl]-4-
,5 piperidinecarboxaldehyde (1.30 g, 5.5 mmol) in THF (25 mL) via an addition
funnel. The
cooling bath was removed and the solution was allowed to stir for 2 hours.
Water was added,
the layers separated, and the aqueous layer was extracted with EtOAc. The
combined organic
layers were washed with brine, dried (MgS04), filtered, and chromatographed on
silica gel,
eluting with acetone. The appropriate fractions were combined and evaporated
to afford a
zo white solid. The solid was recrystallized from hexane to afford racemic
alpha(2,3-
dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol as shiny
white needles,
m.p. 126-127°C.
Anal. Calcd for CzzHz$FN03: C, 70.75; H, 7.56; N, 3.75..
Found: C, 70.87; H, 7.65; N, 3.68.
zs
EXAMPLE 2-starting material
Example 2, Steps A-F, demonstrate an alternative manner of preparing (~)-
alpha(2,3-
dimethoxyphenyl)-1-[2-(4-fluorophenyl)-ethyl)-4-piperidinemethanol, structure
1.
CA 02347469 2001-04-12
WO 00/21930 PCT/US98/21608
-14-
A) 1-( 1, I -Dimethylethyl)-1,4-piperidinedicarboxylic acid
To isonipecotic acid (I07.5 g, 832 mmol) stirred in 1N NaOH,(40 g NaOH in 900
mL H20)
and tert-butanol ( 1800 mL) was added di-tert-butyl dicarbonate (200 g, 916
mmol) in portions.
After stirnng overnight, the solution was concentrated and the resulting water
layer was
acidified with aqueous HCI. This acidic aqueous layer was extracted 3x with
ether. The
combined organic layers were washed with water, brine, dried (MgS04),
filtered, and
evaporated to a white solid, which was recrystallized from EtOAc/hexane (300
mL/200 mL)
,o to afford 1-(1,1-dimethylethyl)-1,4-piperidinedicarboxylic acid as white
needles, m.p. 147-
149°C.
B) 4-(N-Methoxy-N-methylcarboxamido}-1-piperidinecarboxylic acid 1,1-
dimethylethyl ester
,$ To a stirred solution of 1-(1,1-dimethylethyl)-1,4-piperidinedicarboxylic
acid (50.0 g, 218
mmol) in anhydrous CH2C12 (S00 mL) under N2 in a 2L flask was added 1,1'-
carbonyldiimidazole (38.9 g, 240 mmol) portionwise. After stirring for 1 hour,
N,O-
dimethylhydroxylamine hydrochloride (23.4 g, 240 mmol) was added in one
portion. After
stirring overnight, the solution was washed twice with 1N HCI, twice with
saturated NaHC03,
20 once with brine, dried (MgS04), filtered, and evaporated to an oil.
Distillation afforded 4-(N-
methoxy-N-methylcarboxamido)-1-piperidinecarboxylic acid 1,1-dimethylethyl
ester as a
clear oil, b.p. I20-140°C, 0.8 mm.
C) 4-(2,3-Dimethoxybenzoyl)-1-piperidinecarboxylic acid 1,1-dimethylethyl
ester
n-Butyl lithium (14.5 mL of a 2.5 M solution in hexane, 36.3 mmol) was added
via syringe to
a stirred solution of veratrvle (5.00 g, 36.2 mmol) in THF (50 mL, anhydrous)
under argon at
0°C. The ice bath was removed and the mixture was allowed to stir for
90 minutes. The
mixture was cooled to -78°C and treated with 4-(N-methoxy-N-
methylcarboxamido)-1-
3o piperidinecarboxylic acid 1,1-dimethylethyl ester (9.20 g, 33.8 mmol) in
THF (50 mL,
anhydrous) via syringe. The cooling dry ice-acetone bath was removed and the
mixture was
CA 02347469 2001-04-12
WO 00/21930 PCT/US98/21608
-15-
allowed to come to room temperature. After stirring for 3 hours, saturated
aqueous NH4C1
was added and the mixture was allowed to stir overnight. The layers were
separated and the
aqueous layer was extracted with ether. The combined organic layers were
washed with brine,
dried (MgS04), filtered, and evaporated to afford an amber oil. The oil was
chromatographed
on silica gel, eluting with 20% EtOAc in hexane. The appropriate fractions
were combined
and evaporated to an amber oil. The oil was distilled to afford 4-(2,3-
dimethoxybenzoyl)-1-
piperidinecarboxylic acid 1,1-dimethylethyl ester as a colorless oil.(b.p. 225-
250°C, .OS mm).
Anal. Calcd for C,9Hz,N05: C, 65.31; H, 7.79; N, 4.01. Found: C, 65.04; H,
7.92; N, 4.11.
,o D) 4-(2,3-Dimethoxyphenyl)-4-piperidinylmethanone
4-(2,3-Dimethoxybenzoyl)-1-piperidinecarboxylic acid 1,1-dimethylethyl ester
(7.75 g, 22.2
mmol) was dissolved in trifluoroacetic acid (50 mL, 650 mmol) and stirred for
45 minutes.
The entire solution was poured into ether (900 mL) and allowed to stand
overnight. Filtration
,5 yielded 4-(2,3-dimethoxyphenyl)-4-piperidinylmethanone trifluoroacetate as
fine white
needles, m.p. 123°C. Anal. Calcd for C~4H~9N03.CF3CO,H: C, 52.89; H,
5.55; N, 3.86.
Found: C, 52.77; H, 5.62; N, 3.82.
The resulting 4-(2,3-dimethoxyphenyl)-4-piperidinylmethanone trifluoroacetate
was dissolved
zo in water, treated with NaOH ( 10% aqueous) until basic, and extracted three
times with
dichloromethane. The combined organic layers were washed with brine, dried
(MgS04),
filtered, and evaporated to afford 4-(2,3-dimethoxyphenyl)-4-
piperidinylmethanone as an oil.
E) X2,3-Dimethoxyphenyl)[1- 2-(4-fluorophenyl)ethyl-4-piperidinyl~methanone
zs monohydrochloride
A solution of 4-(2,3-dimethoxyphenyl)-4-piperidinylmethanone (8.00 g, 32.1
mmol) and 2-(4-
fluorophenyl)ethyl bromide (6.52 g, 32.1 mmol) was prepared in DMF (90 mL),
treated with
ICzC03 (7.0 g, 50.7 mmol), then stirred and heated at 80°C under argon
overnight. The cooled
so solution was poured into a partition of 2/1 EtOAc/toluene and water. The
layers were
separated and the aqueous layer was extracted with 2/1 EtOAc/toluene. The
combined organic
CA 02347469 2001-04-12
WO 00/21930 PCT/US98/21608
-16-
layers were washed 2x with water, lx with brine, dried (MgS04), filtered, and
evaporated to
afford 11.0 g of an oil. The oil was chromatographed on silica gel, eluting
with EtOAc. The
appropriate fractions were combined, concentrated, dissolved in ethyl acetate
and treated with
HCl/ethyl acetate. (2,3-dimethoxyphenyl)[1-[2-(4-fluorophenyl)ethyl]-4-
piperidinyl]-
methanone monohydrochloride was obtained as a precipitate, m.p. 225-
227°C (decomp). Anal
Calcd for CzzHz6FN03.HC1: C, 64.78; H, 6.67; N, 3.43. Found: C, 64.44; H,
6.73; N, 3.41.
F) (f)-alpha-(2,3-Dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl-4-
piperidinemethanol
,o To a stirred solution of (2,3-dimethoxyphenyl)[1-[2-(4-fluorophenyl)ethyl]-
4-
piperidinyl]methanone (6.0 g, 16.2 mmol) in MeOH (100 mL) at 0°C was
added NaBH4 (1240
mg, 32.8 mmol) in two portions, over a one hour period. After stirring
overnight, the solution
was concentrated to a solid. The solid was partitioned between water and
ether. The layers
were separated and the aqueous layer was extracted with ether. The combined
organic layers
,5 were washed with brine, dried (MgS04), filtered, and evaporated to a solid.
The solid was
chromatographed on silica gel, eluting with acetone. The appropriate fractions
were combined
and evaporated to afford a white solid. The solid was recrystallized from
cyclohexane to
afford (~)-alpha-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)-ethyl]-4-
piperidinemethanol as
white needles, m.p. 126-127°C.
zo Anal. Calcd for CzzH,gFN03: C, 70.75; H, 7.56; N, 3.75.
Found: C, 70.86; H, 7.72; N, 3.93.
EXAMPLE 3 - starting material
zs This example demonstrates the preparation of the alcohol, structure 5.
Preparation of (+)-alpha-(2,3-Dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl~-4-
piperidinemethanol
CA 02347469 2001-04-12
WO OO/Z1930 PCT/US98/21608
_ 17_
A) Preparation of diastereomers.
A solution of 3.90 g (10.4 mmol) of (t)-alpha-(2,3-dimethoxyphenyl)-1-[2-(4-
fluorophenyl)ethyl]-4-piperidinemethanol, 1.74 g ( 10.4 mmol) of S-(+)-alpha-
methoxyphenylacetic acid, 2.15 g (10.4 mmol} of 1,3-dicyclohexylcarbodiimide
and O.l g of
4-dimethylaminopyridine in chloroform (75 mL) was refluxed for I 7 hours,
allowed to cool to
room temperature and filtered. The filtrate was concentrated and
chromatographed on a silica
gel column eluting with ethyl acetate/hexane ( 1:1 ) to afford two
diastereomers, Rf = 0.1 and
0.2 (TLC EtOAc/hexane, I :1 ). Intermediate fractions were rechromatographed
to give
,o additional material. Those fractions with Rf = 0.2 were combined to give a
single
diastereomeric ester, (+,+)-(2,3-dimethoxyphenyl)[1-[2-(4-fluorophenyl)ethyl]-
4-
piperidinylJmethyl-alpha-methoxybenzeneacetate.
B) Preparation of (+)-alpha-(2,3-Dimethoxyphenyl)-1- 2-(4-fluorophenyl)ethyl~-
4-
,5 piperidinemethanol
To a stirred solution of 0.97 g ( I .9 mmol) of the above mentioned
diastereomeric ester, Rf =
0.2, in 25 mL of methanol was added 0.5 g (3.6 mmol) of potassium carbonate
and S.0 mL of
water. After stirring 17 hours at room temperature the reaction mixture was
diluted with water
and extracted twice with methylene chloride. The combined extracts were washed
with water,
2o brine and dried over MgS04. After filtering, the filtrate was concentrated
to an oil and
crystallized from 40 mL of cyclohexane/hexane ( I : I ) to give (+)-alpha-(2,3-
dimethoxy-
phenyl)-I-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol, m.p.112-I
13°C, [a]p ° =+13.9°.
CA 02347469 2001-04-12
WO 00/21930 PCT/US98/21608
-18_
EXAMPLE 4
(+)-a-(2,3-Dimethyoxyphenyl)-1-[2-(4-fluorophenyl)ethyl)-4-piperidinementhanol
decanoate
O
O OCH3
OCH3
N J ~J
F
A mixture of 49.0 g (0.131 mol) of (+)-a-(2,3-dimethoxyphenyl)-1-[2-(4-
fluorophenyl)ethyl]-4-piperidinemethanol, 500 mL of CHC13 and 16.0 g (0.158
mol) of
triethylamine was charged into a one liter, 3-necked flask fitted with a
stirrer, thermometer,
dropping funnel, and a continuous nitrogen purge. A solution of 27.4 g (0.144
mol) of
decanoyl chloride in 25 mL of CHCI, was added over 5 minutes while maintaining
a reaction
,o temperature of 20-25 ° C. The resulting solution was stirred at 20-
25 ° C for two hours. The
progress of the reaction was monitored by TLC (5/95 methanol/CHZCIz; Merck 60F-
254
plates; UV; Rf of (+)-a-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyI)ethyl]-4-
piperidinemethanol - 0.23; Rf of titled compound - 0.55). An additional 2.8 g
(0.015 mol) of
decanoyl chloride (Aldrich) and 1.6 g (0.016 mol) of triethylamine was added
to the reaction
,5 mixture and stirring was continued for two hours. The reaction mixture was
diluted with 500
mL of CH2Clz and washed with 250 mL of 5% KzC03, 250 mL of Hz0 and 250 mL of
saturated NaCI. The organic phase was dried over 500 g of MgS04 and filtered.
The filter
cake was washed with 200 mL of CHZCIz. The filtrate was concentrated at 40
° C/50 torn to
give an oil.
zo The crude product was purified by flash chromatography ( 14 x 29 cm column,
2.035
kg of 230-400 mesh silica gel). The crude product was loaded onto the column
by dissolving
it in 75 mL of CHZCIzcHZC~z~ The column was eluted with 24 L of 1/4
EtOAc/CHZCIz collecting
CA 02347469 2001-04-12
WO 00/21930 PCTNS98/21608
-19-
24 x one liter fractions. The fractions which were homogenous by TLC were
combined and
concentrated at 35 ° C/50 torr followed by 70 ° C/0.5 torn for
one hour to give a colorless oil.
MS(M+=528)
Anal sis:
Calc. for C32H46~~4 (527.73): 72.83 %C 8.79 %H 2.65 %N
Found: 72.25 %C 8.88 %H 2.63 %N
EXAMPLE 5
+)-a-(2,3-Dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl)-4-piperidinemethanol
hexanoate
O
H3C O OCH3
OCH3
F
To a stirred solution of 2.0 g (5.37 mmol) of (+)-a-(2,3-dimethyloxyphenyl)-1-
[2-(4-
fluorophenyl)ethyl]-4-piperidinemethanol in 6 mL of dry methylene chloride was
added 0.74
mL (0.537 g, 5.32 mmol) of triethylamine. The solution was cooled in an ice
bath after which
was added by syringe 1.07 mL (5.89 mmol) of hexanoic anhydride. The solution
was stirred
,s for several minutes at ice bath temperature and allowed to warm to room
temperature. To the
solution was then added 66 mg (0.541 mmol) of 4-dimethylaminopyridine. The
mixture was
stirred overnight at room temperature, poured into ice/water/0.5 M NaOH,
extracted with
ether, and filtered and concentrated to an oil. The oil was dissolved in
methylene chloride, and
2% methanol/methylene chloride, respectively. The pure product-containing
fractions were
Zo combined and concentrated to provide an oil which was dried overnight at 60
° C, under high
CA 02347469 2001-04-12
WO 00/21930 PCTNS98/21608
-20-
vacuum to give the titled compound. The compound was homogenous by TLC. IR
(Kbr),
NMR (CDC13) and MS (MH+ = 472) were consistent with the proposed structure.
Analysis:
Calc. for Cz8H38FN04: 71.31 %C 8.12 %H 2.97 %N
Found: 70.94 %C 8.07 %H 2.88 %N
EXAMPLE 6
~+)-a-(2,3-Dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl-4-piperidinemethanol
octanoate
io
O
H3C O OCH3
~ OCH3
N J ~J
F
To a stirred solution of 2.0 g (5.37 mmol) of (+)-a-(2,3-dimethyloxyphenyl)-1-
[2-(4-
fluorophenyl)ethyl]-4-piperidinemethanol in 6 mL of dry methylene chloride was
added 0.74
mL (0.537 g, 5.32 mmol) of triethylamine. The solution was cooled in an ice
bath after which
,s was added by syringe 1.75 mL (5.89 mmol) of octanoic anhydride. The
solution was stirred
for several minutes at ice bath temperature and allowed to warm to room
temperature. To the
solution was then added 66 mg (0.541 mmol) of 4-dimethylaminopyridine. The
mixture was
stirred, washed with water and saturated sodium chloride. The organic extract
was dried
(Na2S04), filtered and concentrated to an oil. The oil was dissolved in
methylene chloride and
2o purified by flash chromatography on silica gel, eluting with methylene
chloride, 1 % and 2%
methanol/methylene chloride, respectively. The pure product-containing
fractions were
CA 02347469 2001-04-12
WO 00/21930 PCTlUS98/21608
-21-
combined and concentrated to provide an oil which was dried overnight at
60°C under high
vacuum to provide the titled compound. The compound was homogenous by TLC. The
IR
(KBr), NMR (CDC13) and MS (MH+ = 501) were consistent with the proposed
structure.
Analysis:
Calc. for C3oH4zFN04: 72.11 %C 8.47 %H 2.80 %N
Found: 71.94 %C 8.63 %H 2.83 %N
RY A 1~~1DT ~ 7
,o ~+)-a-(2,3-Dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl)-4-
piperidinemethanol
hexadecanoate
v v v v v 'O OCH3
OCH3
N J ~J
CH3
F
To a stirred solution of 2.00 g (5.36 mmol) of (+)-a-(2,3-dimethyloxyphenyl}-1-
[2-(4-
fluorophenyl)ethyl]-4-piperidinemethanol in 6 mL of dry methylene chloride was
added 0.76
mL (5.46 mmol) of triethylamine via syringe after which the solution was
chilled in an ice
bath. To the solution was added 2.92 g (5.89 mmol) of hexadecanoic acid
anhydride after
which the mixture was stirred at 0 ° C for 15 minutes. The reaction
mixture was allowed to
warm to ambient temperature and to the reaction mixture was added 65 mg (0.536
mmol) of 4-
zo dimethylaminopyridine after which the solution was stirred under nitrogen
overnight. The
reaction mixture was poured into 50 mL of 0.5 N sodium hydroxide and SO mL of
diethyl
CA 02347469 2001-04-12
WO 00/21930 PCT/US98/21608
-22-
ether. Some precipitate was observed and the resultant suspension was
extracted with
methylene chloride. The methylene chloride layer was washed with water and
brine, and dried
over sodium sulfate, filtered, and concentrated to a yellow oil. The oil was
dissolved in
methylene chloride and purified using a flash chromatography column packed
with silica gel
and methylene chloride and eluted with the same solvent system followed by 1 %
and 2.0%
methanol/methylene chloride, respectively. The appropriate fractions were
combined and
concentrated to a yellow oil . The oil was dried twice overnight at 60
° C under high vacuum
to provide the titled compound. The compound was homogenous by TLC. IR (film),
NMR
(CDC13) and MS (MH+ = 612) were consistent with the proposed structure.
,o Analysis:
Calc. for Cz4HsoFN04: 74.59 %C 9.55 %H 2.29 %N
Found: 74.34 %C 9.45 %H 2.29 %N
EXAMPLE 8
~+)-a-(2,3-Dimethoxyphenyl)-1- 2-(4-fluorophenyl)ethyl-4-piperidinemethanol
2,2-
dimethyloctanoate
O
H3C O OCH3
OCH3
N J ~J
F
2-Rexene-1-mesylate is prepared by adding N,N-diisopropyl ethylamine (12.9g,
O.lm)
to a solution of trans-2-hexene-1-of (lO.Og, O.lm) (Aldrich) in 100 mL of
methylene chloride.
zo Methanesulfonyl chloride (12.6g, 0.1 lm) in methylene chloride (50 mL) is
added dropwise to
the solution with stirring at room temperature for 4 hours. The reaction
mixture is transferred
to a separatory funnel and washed with cold 1N HCl (2 times) and then with
saturated
CA 02347469 2001-04-12
WO 00/21930 PCT/US98/21608
-23-
NaHC03 solution (2 times). The solution is dried over anhydrous MgS04,
filtered and
concentrated, under vacuum at 40 ° C to give 2-hexene-1-mesylate.
2,2-Dimethyl-3-octene nitrite is prepared by adding 2-hexene-1-mesylate
(11.02g,
O.OSm) in anhydrous THF (1 OOmL) to a solution prepared by the addition of
isobutyro nitrite
(3.7g, 0.053m) in THF (25mL) after treatment with NaH (60% in mineral oiI)(2.
i g, 0.053m)
in dry THF (SOmL). The resulting reaction mixture is stirred at reflux for 5
hours, cooled and
stirred with cold ethanol (95%) and concentrated under vacuum to remove
solvents. After
adding water (SOmL), the mixture is extracted with diethyl ether (3x40mL). The
extracts are
washed with water and then saturated NaCI solution and dried over MgS04,
filtered and
,o concentrated to give 2,2-dimethyl-3-octene nitrite.
2,2-Dimethyl-3-octenoic acid is prepared by adding 2,2-dimethyl-3-octene
nitrite
(l.Slg, O.OIm) to a solution prepared from 15 % NaOH in butanol/H20 (2:3)
(30mL). The
reaction mixture is stirred and refluxed for 7 hours, is cooled and made
acidic with 10%
hydrochloric acid. The reaction mixture is extracted with diethyl ether and
the extract washed
,5 with saturated NaCI and dried over MgS04, filtered and concentrated to give
2,2-dimethyl-3-
octenoic acid.
2,2-Dimethyl octanoic acid is prepared by dissolving 2,2-dimethyl-3-octenoic
acid
(0.20g, 1.1 mmol) in absolute ethanol blanketed with Nz and 10% palladium on
carbon which
is then hydrogenated for 6 hours. The catalyst is removed by filtration and
the filtrate
zo concentrated under vacuum to give 2;2-dimethyl octanoic acid.
To a stirred solution of (+)-a-(2,3-dimethyloxyphenyl)-1-[2-(4-
fluorophenyl)ethyl]-4-
piperidinemethanol (3.9g, 10.4 mmol) in 70 ml of methylene chloride is added
dicyclohexylcarbodiimide (2.1 Sg, I 0.4 mmol), 4-dimethylamino pyridine 0.1 g
and 2,2-
dimethyloctanoic acid (1.70g, 10.4mmo1). The resulting solution is stirred and
refluxed for 16
zs hours. The cooled reaction solution is filtered and concentrated to an oil
The resulting oil is
chromatographed on silica gel and eluted with ethyl acetate/hexane (1:1).
Appropriate
fractions are collected, warmed (40 ° C) and concentrated at reduced
pressure to give the titled
compound.
CA 02347469 2001-04-12
WO 00/21930 PCTNS98l21608
-24-
FXAMPT.R 9
This example demonstrates one pharmaceutical composition of the present
invention.
In a suitable 100 mL volumetric vessel, place 70 mL of Sesame Oil, NF (Sigma),
1.2g of
Benzyl Alcohol, NF and 14.129g of (+)-a-(2,3-Dimethyoxyphenyl)-1-[2-(4-
fluorophenyl)ethyl]-4-piperidinementhanol decanoate. To this solution add
sufficient Sesame
Oil, NF to bring the volume to 100m1 and mix until homogeneous. This solution
may be
sterilized and packaged for parenteral injection.
EXAMPLE 10
This example describes a behavioral test (antagonism of DOI-induced behaviors)
designed to identify compounds which possess antagonist activity at the SHT,A
receptor. The
compound of the present invention used in this test was (+)-a-(2,3-
Dimethyoxyphenyl)-1-[2-
(4-fluorophenyl)ethyl]-4-piperidinemethanol decanoate - Example 4 herein. The
5-HT2A/2C
~s agonist (~}-DOI HCl (1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane
hydrochloride)
induces several quantifiable behaviors in rats. These behaviors include
"shakes" (a quick head
and body shake, a.k.a. wet dog shakes), "forepaw tapping" (rapid forepaw
treading) and "skin-
jerks" (paraspinal muscle contractions or dorsal skin-shrugging). The 5-HT2
antagonists
mianserin, ritanserin and methysergide as well as the selective S-HT2A
antagonist MDL
zo 100,907 have been demonstrated to dose-dependently block the behavioral
effects of DOI
(Pranzatelli, 1990, Neurosci. Let. 11: 74-80; Wettstein et al., 1996, Soc.
Neurosci. Abs. 22:
481 ). Significantly, drugs with S-HT2,, antagonist activity have been
proposed to have
atypical antipsychotic properties in schizophrenic patients (Meltzer et al.,
1989, JPET. 251:
238-246), as well as potential therapeutic activity in a number of other CNS
disorders
zs including depression, dysthymia, and anxiety (Stefanski & Goldberg, 1997,
CNS Drugs, 7:
3 99-409)
CA 02347469 2001-04-12
WO 00/21930 PCT/US98/21608
-25-
Methods
Subjects and housing
Male Sprague-Dawley rats ( 180 ~ 50 g) were housed seven per cage and allowed
1 week to
acclimate to the vivarium. Food and water were freely available. The
temperature and light
cycle ( 12 h on- 12 h off)were automatically maintained. Individual rats were
tested once.
Each testing group contained seven animals. Experiments took place in the
vivarium room
where the animals were housed.
,o Drug preparation and administration
(+)-a-(2,3-DimethyoxyphenyI)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol
decanoate
(120 mg/kg equivalent to MDL 100,907) was dissolved in sesame oil and
administered
intramuscularly to separate groups of rats on Day 0 in a volume equal to 60
mL/1 OOg body
weight. Vehicle control animals were injected with sesame oil alone. (~)-DOI
HCl (3.0
,5 mg/kg, 1mL/kg body weight) was dissolved in distilled water with the aid an
ultrasonic bath,
and injected intraperitoneally on appropriate testing days.
Observation and behavioral assessment
(+)-a-(2,3-Dimethyoxyphenyl)-1-(2-(4-fluorophenyl)ethyl]-4-piperidinementhanol
decanoate
zo -treated rats were tested for antagonism of DOI-induced behaviors 1, 5, 7,
14, 21, 28 and 40
days after the single (+)-a-(2,3-Dimethyoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-
4-
piperidinementhanol decanoate intramuscular injection. Each rat was tested
once.
Immediately after DOI injection rats were placed under inverted clear plastic
boxes (28L x
25W x 25H cm) which were arranged on top of clean absorbent paper. Rats were
zs continuously watched by trained observers (blind to treatment) for 30
minutes for the
occurrence of DOI-induced behaviors (shakes, skin jerks and forepaw tapping
bouts) and then
returned to their home cages. Rats were later used for pharmacokinetic
studies. The
frequencies of DOI-induced behaviors were recorded and then summed to provide
a single
behavioral score for each animal.
CA 02347469 2001-04-12
WO 00/Z1930 PCT/US98/21608
-26-
Data Analysis
The mean and standard error of the behavioral scores of each group was
determined. Each
treatment group's mean was then compared separately to the mean of the vehicle-
control
group using a one-way analysis of variance test (ANOVA), followed by a
Bonferroni/Dunn
post-hoc comparison. Differences between groups were considered statistically
significant if
p values were less than or equal to 0.05.
Results
,o Significantly antagonized DOI-induced behavior in the rats was observed for
a full 28 days.
The effect was no longer significant at day 40.
EXAMPLE 11
,5 This example demonstrates the single dose absorption of MDL 100,907
following
intramuscular administration (i.m.) of (+)-a-(2,3-Dimethyoxyphenyl)-1-[2-(4-
fluorophenyl)ethyl]-4-piperidinemethanol decanoate, a compound of the present
invention,
over time.
zo A total of ninety male Wistar rats weighing approximately I 50-200 grams
each are
dosed i.m. with (+)-a-(2,3-Dimethyoxyphenyl)-I-[2-(4-fluorophenyl)ethyl]-4-
piperidinemethanol decanoate in sesame oil (equivalent to 120mg/kg of MDL
100,907) on day
0. Rats are anesthetized with a lethal i.p. dose of nembutal and blood
collected at 3 and 6
hours, and various days post-dose (n=5 for each timepoint) in heparinized
vacutainers. Blood
zs samples are centrifuged for 30 minutes at 5 ° C and at approximately
2700rpm. Plasma is
removed and stored at -20 ° C until assay. The plasma samples are
analyzed by an appropriate
HPLC method. Brains will also be collected at the above timepoints and stored
at -80 ° C until
analyzed by the appropriate HPLC method. The results are shown in Table 1.
CA 02347469 2001-04-12
WO 00/21930 PCT/US98/2160$
-27-
TABLE 1
Time (days) (+)-a-(2,3- (+)-a-(2,3-
Dimethyoxyphenyl)-1-[2-(4- Dimethyoxyphenyl)-1-[2-(4-
fluorophenyl)ethyl]-4- fluorophenyl)ethyl]-4-
piperidinemethanol piperidinemethanol
(ng/mL)- MDL 100,907 decanoate- Example 4 herein
(ng/mL)
0.125 21.48 8.00 54.18 13.33
0.25 24.30 7.65 53.79 17.13
1 32.00 10.48 54.08 17.98
2 25.59 ~ 8.90 21.61 7.39
3 25.48 4.65 38.95 5.64
4 31.82 10.22 52.02 12.92
6 32.84 11.83 41.87 14.64
8 34.93 11.36 28.70 9.16
12 19.89 5.65 21.00 12.79
15 11.67 6.18 52.36 15.20
19 6.37 2.19 40.06 8.44
22 15.93 2.45 46.30 9.31
26 15.51 7.15 38.929.35
29 13.22 4.54 43.71 10.81
41 9.883.81 24.129.15
The dosage range at which the compounds of Formula I exhibits their ability to
block
the effects of serotonin at the SHTZA receptor can vary depending upon the
particular disease
or condition being treated and its severity, the patient, the formulation,
other underlying
disease states that the patient is suffering from, and other medications that
may be
concurrently administered to the patient. Generally, the compounds of Formula
I will exhibit
their serotonin SHTZA antagonist properties at dosages of between about 0.001
mg/kg of
,o patient body weight/day to about 100 mg/kg of patient body weight/day. The
sustained
CA 02347469 2003-11-12
WO 00121930 PC'fNS98lZ1608
-28-
release formulations may contain multiples of the foregoing dosages depending
upon over
what period the active ingredient is released. The dosage of the compounds of
the present
invention may be determined by administering the compound to an animal and
determining
the plasma level of the active ingredient.
The compounds of the present invention may be mixed with a pharmaceutically
acceptable carrier capable of being administered by the preferred route in
order to produce a
sustained release of the compound of the present invention so that a
therapeutically effective
amount of the compound (+)-a-(2,3-Dimethoxyphenyl~l-[2-(4-fluorophenyl)ethyl]-
4-
,o piperidinemethanol can be supplied to the patient over a period of days or
weeks. Preferably
the sustained release formulation comprises a compound of Formula I and a
pharmaceutically
acceptable carrier for parenteral administration as either an aqueous
suspension, oil solution,
oil suspension or emulsion. Some oils which may be used for intramuscular
injection are
sesame, olive, arachnis, maize, almond, cottonseed, peanut and castor oil,
with sesame oil
being preferred. A pharmaceutically acceptable preservative such as benzyl
alcohol may also
be added. The sustained release formulation is preferably administered
intramuscularly or
subcutaneously, with intramuscular administration preferred although other
routes of
administration such as oral, transdermal, nasal spray, etc. could be used if
appropriate to the
needs of the patient.
Since the compounds of the present invention release (+)-a-(2,3-
Dimethoxyphenyl)-1-
[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol ("Active Ingredient") into the
patient for the
therapeutic effect, the compounds of the present invention are useful for all
indications of use
for which the Active Ingredient is useful. Some of these indications of use
have been
described in the patents issued generically encompassing the Active Ingredient
(U.S. Patent
a no.4,783,471) or specifically covering the Active Ingredient (LJ.S. Patent
nos. 5,134,149;
5,561,144; 5,618,824; ~d p~/US97/02597. These references disclose uses of
psychosis
(including schizophrenia), obsessive compulsive disorder, thrombotic illness,
coronary
vasospasm, intermittent claudication, anorexia nervosa, Raynaud's phenomenon,
fibromyalgia, extra-pyramidal side effects, anxiety, arrhythmia, depression
and bipolar
depression, or drug abuse (e.g., cocaine, nicotine, etc.). Some of these
ao
CA 02347469 2003-11-12
WO OOI21930 PCTNS98~L1608
-29-
indications have been disclosed in the patents described above and in U.S.
Patent nos.
5,561,144; 5,618,824; 4,877,798; 5,134,149; and 5,021,428.
Psychoses as used herein are a conditions where the patient experiences a
major mental
disorder of organic and/or emotional origin characterized by derangement of
the personality
and loss of contact with reality, often with delusions, hallucinations or
illusions.
Representative examples of psychotic illnesses which can be treated with the
compounds of
the present invention include schizophrenia, schizophreniform disorder,
schizoaffective
disorder, delusional disorder, brief psychotic disorder, shared psychotic
disorder, psychotic
,o disorder not otherwise specified, and substance-induced psychotic disorder.
See Diagnostic
and Statistical Manual of Mental Disorders, 4"' ed., American Psychiatric
Association
(1994). The Active Ingredient is currently in clinical trials for the
treatrnent of
schizophrenia.
Patients with obsessive-compulsive disorders (OCD) fail to inhibit or "gate"
intrusive,
,5 distressing thought or images. Since OCD is characterized by deficient
"cognitive gating" and
by aberrant metabolic activity in circuitry linking the orbital cortex and
straitum, it has been
predicted that OCD patients might exhibit deficient PPI (prepulse inhibition).
The Active
ingredient has been found to restore disrupted PPI. See Psychopharmacology
124: 107-116
(1996), R. A. Padich, et al., "SHT modulation of auditory and visual
sensorimotor gating: II.
Effects of SHT,~ antagonist MDL 100,907 on disruption of sound and light
prepluse inhibition
produced by SHT agonists in Wistar rats."
The Active Ingredient is also effective in the prevention of acute thrombosis,
especially those of the coronary arteries. This compound decreases the rate at
which platelets
aggregate as the result of minor alterations in the endothelial lining of the
vasculature and
therefore prevent the formation of acute pathological thrombi. See U.S. Patent
no. 5,561,144
for description.
Anxiety, variant angina, anorexia nervosa, Raynaud's phenomenon and coronary
vasospams are used in the manner defined in the 27th edition of Dorland's
Illustrated Medical
Dictionary, W.B. Saunders Co.,1988.
CA 02347469 2001-04-12
WO 00/21930 PCT/US98121608
-30-
Fibromyaligia is a chronic disease state wherein the patient suffers from
numerous
'symptoms such as, for example, widespread generalized musculoskeletal pains,
aching,
fatigue, morning stiffness and a sleep disturbance which can be characterized
as inadequacy of
stage 4 sleep.
Extra-pyramidal side effects often accompany the administration of neuroleptic
agents
such as haloperidol and chlorpromazine. Patient often experiences a
parkinsonian-like
syndrome, wherein they experience muscular rigidity and tremors. Others
experience
akathisia and acute dystonic reactions.
The Active Ingredient increases the duration of the action potential of
myocardial
,o tissue producing an increase in the refractory period of that tissue, which
under the
classification system of Vaughan Williams, exhibits Class III anti-arrhythmic
activity.
The compounds of the present invention may be used to treat drug abuse in the
patient.
See T. F. Meert, et al., European Journal of Pharmocology 183: 1924 where SHTz
antagonist
,5 abolished preference for both alcohol and cocaine in the rodent model of
the drug abuse.
Other animal models such as the rodent self stimulation model described in R.
A. Frank, et.
al., Behavioral Neuroscience 101: 546-559 (1987) may be used to demonstrate
the ability of
the compounds of the present invention to treat drug abuse.
zo The compounds of the present invention are useful in treating patients with
Depressive
Disorders and Bipolar Disorders. In the Diagnostic and Statistical Manual of
Mental
Disorders (Third Edition-Revised) ("DSM-III-R"), incorporated herein by
reference,
Depressive Disorders are defined as Major Depression, Dysthymia and Depressive
Disorder
NOS. We also include in this category Major Depressive Episode including
Chronic Type,
zs Melancholia, and Seasonal Pattern. Bipolar Disorders include Bipolar
Disorder, Cyclothymia
and Bipolar Disorder NOS.
A feature of Depressive Disorders is one or more periods of depression without
a
history of either Manic or Hypomanic episodes. A feature of Bipolar Disorders
is the presence
CA 02347469 2001-04-12
WO 00/21930 PCT/US98/21608
-31-
of one or more Manic or Hypomanic Episodes usually accompanied by one or more
Major
Depressive Episodes. A Manic or Hypomanic Episode is a distinct period during
which the
predominant mood is either elevated, expansive or irritable and there are
associated symptoms
of the Manic Syndrome as defined in DSM-III-R. The disturbance is severe
enough to cause
marked impairment in occupational or social functioning.
Major Depression has one or more Major Depressive Episodes. A Major Depressive
Episode is characterized by: (1) at least five of the following) depressed
mood, loss of interest
in pleasure (anhedonia), significant weight loss or weight gain when not
dieting, insomnia or
,o hypersomnia, psychomotor agitation or retardation, fatigue or loss of
energy, feelings of
worthlessness or excessive or inappropriate guilt, diminished ability to think
or concentrate, or
recurrent thoughts of death including suicide; (2) it cannot be established
that an organic
factor initiated and maintained the disturbance; (3) there are no delusions or
hallucinations for
as long as two weeks in the absence of prominent mood symptoms; and (4) it is
not
,5 superimposed on Schizophrenia, Schizophreniform Disorder, Delusional
Disorder, or
Psychotic Disorder NOS.
Dysthymia has a history of a depressed mood more days than not for at least
two years
and during the first two years of the disturbance, the condition does not meet
the criteria for a
zo Major Depressive Episode. The depressed mood in children and adolescents
can be exhibited
as irritability. Also present is at least two of the following: poor appetite
or overeating,
insomnia or hypersomnia, low energy or fatigue, low self esteem, poor
concentration or
difficulty making decisions or feeling of hopelessness. These symptoms are not
superimposed
on a chronic psychotic disorder such as Schizophrenia or Delusional Disorder.
Also it cannot
zs be determined that an organic factor initiated and maintained the
disturbance.
There are many ways to show that the compound of the present invention is
useful in
treating Depressive Disorders and Bipolar Disorders such as in animal models.
See for
example, "Animal Models as simulations of depression" by Paul Willner, TIPS
12:131-136
so (April 1991 ); "Animal Models of Depression: An overview" by Paul Willner,
Pharmac. Ther.
CA 02347469 2003-11-12
WO OOIZ1930 PC'TNS98l11608 -
-32-
45:425-455 (1990). One such model is the Chronic Mild Stress Model of
Depression
("CMS").
CMS uses mild stressors, such as food and water deprivation, small temperature
changes, changes of cage mates, etc. Over a period of weeks of exposure to the
mild stressors,
the animals gradually reduce their consumption of a highly preferred sucrose
solution which
persists (in untreated animals) for several weeks following the cessation of
stress. This
decreased sensitivity to reward (the sucrose solution) reflects anhedonia, a
symptom of a
Major Depressive Episode (see for example, Behavioral Pharmacol.5: Suppl.l, p.
86 (1994}
where lithium, carbamazepine and ketoconazole were evaluated in CMS;
_Psychopharmacology 93:358-364 (1987} where a tricyclic antidepressant was
evaluated in
CMS; Behavioral Pharmacology:5:344-350 (1994) where a catechol-O-methyl
transferase
inhibitor was evaluated in CMS).
~s The following CMS study was performed using the Active Ingredient of the
compounds of the present invention (hereafter "MDL 100,907") in comparison to
known anti-
depressant compound Imipramine.
Male Wistar rats were brought into the laboratory two months before the start
of the
z° experiment at which time they weighed approximately 300 grams.
Except as described below,
the animals were singly housed, with food a water freely available, and
maintained on a 12
hour lightldark cycle (lights on at 8AM) at a temperature of 22~°C.
The animals were first trained to consume a 1% sucrose solution; training
consisted of
zs eight 1 hour baseline tests in which sucrose was presented, in the home
cage, following 14
hours food and water deprivation; intake was measured by weighing pre-weighed
bottles
containing the sucrose solution at the end of the test. Subsequently, sucrose
consumption was
monitored, under similar conditions, at weekly intervals throughout the whole
experiment.
3° On the basis of their sucrose intakes in the final baseline test,
the animals were divided
into two matched groups. One group of animals was subjected to a chronic mild
stress
CA 02347469 2001-04-12
WO 00/21930 PCT/US98/21608
-33-
procedure for a period of 9 consecutive weeks. Each week of stress regime
consisted of: two
periods of food or water deprivation (12 and 14 hour), two periods of 45
degree cage tilt (12
and 14h), two periods of intermittent overnight illumination (lights on and
off every 2 hours),
two 14 hour periods of soiled cage (200 ml water in sawdust bedding), two 14
hour periods of
paired housing, two 14 hour periods of low intensity stroboscopic illumination
(150
flashes/min). Stressors were applied continuously throughout the day and
night, and
scheduled randomly. Control animals were housed in a separate room and had no
contract
with the stressed animals. They were deprived of food and water for the 14
hours preceding
each sucrose test, but otherwise food and water were freely available in the
home cage. On the
,o basis of their sucrose intake scores following 3 weeks of stress, both
stressed and control
animals were each divided further into matched subgroups (n=8), and for
subsequent five
weeks they received daily administrations of vehicle (lml/kg, intraperineally
(ip)) imipramine
(lOmg/kg, ip) or MDL 100,907 (0.002, 0.02 and 0.2 mg/kg orally). All drug
injections were
in a volume of 1 ml/kg body weight. Drugs were administered at 1 OAM and
sucrose tests were
,5 carried out 24 hours following the last drug treatment. After five weeks,
the treatments were
terminated and after one week of withdrawal a final sucrose test was carried
out. Stress was
continued throughout the period of treatment and withdrawal.
Results were analyzed by multiple analysis of variance, followed by Fisher's
LSD test
Zo for post hoc comparisons of means.
Chronic mild stress caused a gradual decrease in the consumption of 1 %
sucrose
solution, in the final baseline test, sucrose intake was approximately 13 gram
in both groups.
Following three weeks of stress (Week 0), intakes remained at 12.4 (~0.4)
grams in controls
is but fell to 7.2 (~0.2) grams in stressed animals (p<0.001 ). Such a
difference between control
and stressed animals treated with vehicle, persisted at similar level for the
remainder of the
experiment.
Imipramine had no significant effect on the sucrose intake in control animals
[F(1,84)=0.364; NS]. However, the drug caused a gradual increase of sucrose
intake in
stressed animals (F(1,84)=16.776; p<0.001]. Sucrose intake in imipramine-
treated stressed
CA 02347469 2001-04-12
WO 00/21930 PCTNS98/21608
-34-
animals was significantly increased from Week 0 scores after four weeks of
treatment
(p=0.05) and after five weeks of treatment there were no significant
differences between drug-
treated stressed animals and drug- and saline-treated controls. The increase
of sucrose intake
in imipramine-treated stressed animals was maintained at similar level one
week after
withdrawal from the drug.
MDL 100,907 had no significant effect on the sucrose intake in control animals
[Treatment effect:F(3,168)=0.821; NS Treatment x Weeks interaction:
F(15,168=0.499; NS).
In stressed animals, MDL 100,907 gradually reversed the CMS-induced deficit in
sucrose
,o intake, resulting in a signif cant Treatment effect [F(3,168)=22.567;
p<0.001 ) and Treatment x
Weeks interaction (F(15,158}=1.559; p=0.05).
In stressed animals treated with two higher doses of MDL 100,907 (0.02 and 0.2
mg/kg), sucrose intakes were significantly increased from initial scores (Week
0) after two
,5 (0.02 mg/kg) and three (0.2mg/kg) weeks of treatment (p=0.03 and p=0.04,
respectively).
This effect was increased further during next weeks, and at the end of
treatment period (Week
5) the amount of sucrose solution drunk by these animals was comparable to
that of vehicle-
treated controls and significantly higher than that of vehicle-treated
stressed animals (0.02
mg/kg: p<0.001, 0.2mg/kg: p-0.002).
zo
At the lowest dose of 0.002mg/kg., MDL 100,907 had no significant effect on
the
sucrose intake throughout the whole treatment period. In consequence, after
five weeks of
treatment the sucrose consumption of stressed animals treated with this dose
did not differ
from the intakes of the vehicle-treated stressed animals (p=0.860) and was
significantly lower
zs than the intakes of vehicle-treated controls (p<0.01). One week after
withdrawal from the
treatment, the sucrose intakes were not significantly changed in all of MDL
100,907 -treated
control (0.002mg/kg:p=0.2, 0.02mg/kg: p=0.9, 0.2mg/kg: p=0.4) and stressed
animals
(0.002mg/kg: p=0.6, 0.02mg/kg: p=0.8, 0.2mg/kg: p=0.6).
3o Of course, clinical trials on humans may also be used to show the
usefulness of the
compound of the present invention in treating depression such as using the
Abbreviated
CA 02347469 2001-04-12
WO 00/21930 PCT/US98/Z1608
-35-
Hamilton Psychiatric Rating Scale for Depression. This comprises a series of
17 categories in
which the individual is rated, e.g., for depressed mood, guilt, suicide
tendencies, insomnia,
anxiety, etc., to reach a score which indicates to the clinician whether or
not the patient is
suffering depression.