Note: Descriptions are shown in the official language in which they were submitted.
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
METHOD AND APPARATUS FOR SCREENING CATALYST LIBRARIES
This invention relates to rapid screening for activities and selectivities of
heterogeneous and homogeneous catalyst libraries by mass spectrometry. This
invention
provides very rapid screening of gaseous, liquid or solid products from all
catalyst sites
in a catalyst library by mass spectrometry and its combination with selective
resonance
enhanced multiphoton ionization (REMPI).
Solid and liquid catalysts are used in the manufacture of a vast array of
chemicals
and fuels, and in this manner significantly contribute to the economy and high
living
standards. National Research Council, "Catalysis Looks to the Future",
National
Academy Press, Washington, D.C., 1992. Catalysts also provide important
environmental benefits, such as in catalytic converters for internal
combustion engines.
However, in spite of their significance and broad utility, the development of
new and
improved catalysts continues to be an arduous and rather unpredictable trial
and error
process. Conventionally, an individual catalyst is prepared using a large
variety of
tedious and time consuming methods, characterized and tested for catalytic
activity,
modified, again characterized and tested again, until no further improvements
are
justified. This approach, although time consuming, has been successful for the
discovery
of a significant number of solid state catalysts, Heinemann, H., "A Brief
History of
Industrial Catalysts", Catalysis: Science and Technology, Anderson, J.R. and
Boudart,
M. Eds., Chapter 1, Springer-Verlag, Berlin, 1981, and homogeneous, liquid-
state
catalysts, Montreus, A. and Petit, F., "Industrial Applications of Industrial
Catalysts"
Kluwer Publishing, New York, 1988.
Combinatorial chemistry, in which a large number of chemical variants are
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
produced rapidly and a chemical library generated which is then screened for
desirable
properties using a suitable technique, is a particularly attractive approach
for the
discovery of new catalysts. Chem. Eng. News, 12 Feb. 1996. Combinatorial
synthesis
was initially used to synthesize large libraries of biological oligomers, such
as peptides
S and nucleotides, however, the creation of small molecule libraries which can
be used for
drug testing is growing. Nielsen, J., Chem. & Indus., 902, 21 Nov. 1994.
Recently,
combinatorial diversity synthesis has been extended to solid-state compounds
used in
superconducting, Xiang, X-D., Sun, X., Briceno, G., Lou, Y., Wang, K-A.,
Chang, H.,
Wallace-Freedman, W.G., Chen, S-W. and Schultz, P.G., "A Combinatorial
Approach to
Materials Discovery", Science, 268, 1738, 1995, magnetoresistivity, Briceno,
G., Chang,
H., Sun, X., Schultz, P.G. and Xiang, X-D., "A Class of Cobalt Oxide
Magnetoresistance Materials Discovered With Combinatorial . Synthesis"
Science, 270,
273, 1995 and luminescence, Wang, J., Yoo, Y., Takeuchi, I, Sun X-D., Chang,
H.,
Xiang, X-D. and Schultz, P.G., "Identification of Blue Photoluminescent
Composite
Material from a Combinatorial Library", Science 279, 1712, 1998, Danielson,
E.,
Golden, J.H., McFarland, E.W., Reaves, C.M., Weinberg, W.H., and Wu, X-D., "A
Combinatorial Approach to the Discovery and Optimization of Luminescent
Materials",
Nature, 398, 944, 1997, Sun, X-D, Gao, C., Wang, J. and Xiang, X-D.,
"Identification
and Optimization of Advanced Phosphors using Combinatorial Libraries",
App.Phy.Lett.,
70, 3353, 1997 and Sun, X-D., Wang, K.A., Yoo, Y., Wallace-Freedman, W.G.,
Gao, C,
Xiang, X-D. and Schultz, P.G., "Solution-Phase Synthesis of Luminescent
Materials
Libraries", Adv.Mater, 9, 1046, 1997. In these cases physically masked
individual
specimens were each measured using contact probes with a computer-controlled
multichannel switching system. Microprobe sampling coupled to mass
spectrometry,
Kassem, M., Qum, M. and Senkan, S.M., "Chemical Structure of Fuel-Rich 1,2-
C2H4C 1~/CH~/O~JAr Flames: Effects of Microprobe Cooling on Sampling of Flames
of
Chlorinated Hydrocarbons", Combust. Sci. Tech., 67, 147, 1989, and in situ IR,
Moates,
F.C., Somani, M., Annamalai, J., Richardson, J.T., Luss, D. and Wilson, R.C.,
"Infrared
Thermographic Screening of Combinatorial Libraries of Heterogeneous
Catalysts", Ind.
Eng. Chem. Res., 35, 4801, 1996, have been proposed for catalyst screening,
but suffer
serious deficiencies in not having sufficient sensitivity, selectivity,
spatial resolution or
high throughput capacity to screen large catalyst libraries, as well as the
lack of ability to
2
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
test the activity of hundreds or thousands of compounds simultaneously.
Service, R.F.,
"High Speed Materials Design", Science, 277, 474, 1997. Microprobe mass
spectrometry requires sampling and transfer of very small quantities of gases
containing
low concentrations of product species from each site rendering the process
impractical
for rapid screening. In situ infrared techniques cannot provide information on
product
selectivity which is crucial for catalyst identification.
Mass spectrometry is a well established and broadly applicable method for
determining mass of gaseous species. The technique involves the ionization of
gaseous
molecules by a number of methods, such as, for example, by electron impact or
light
photoionization followed by separation of ions using techniques, such as, for
example,
quadrupole mass spectrometry or time of flight mass spectrometry and detection
of
selected ions by a suitable detector. Capillary probe sampling mass
spectrometry has
recently been reported for screening of catalyst libraries by Cong, P.;
Giaquinta, D.;
Guan, S.; McFarland, E.; Self, K.; Turner, H.; and Weinberg, W. H., "A
combinatorial
Chemistry Approach to Oxidation Catalyst Discovery and Optimization", Process
Miniaturization Section, 2nd Intl. Conf. Micro Technol., March 9-12, 1998, New
Orleans, La., pg. 118. Cong, et al teach introduction of reactant gas to an
individual
library site through an annular space surrounding a capillary tube through
which product
gas flows from that library site to the ionization zone of a mass
spectrometer. Cong. et al
report measurement of 144 library sites in about 2 hours. Sample transfer
rates by
capillary in the Cong, et al method are limited by the pumping speed tolerated
by the
mass spectrometer chamber. Another disadvantage of capillary probe sampling is
the
potential of adsorption and catalysis induced by relatively long transfer line
surfaces.
There remains a large unexplored universe of binary, ternary, quaternary and
higher-
order solid state materials, organometallic species and other complex metal
compounds
that could have superior catalytic properties. Prior conventional approaches
have been
inadequate to rapidly synthesize and screen this vast universe of catalytic
compounds.
There is clearly a need for development or more efficient and systematic
methods to
produce heterogeneous and homogeneous state libraries and to screen them for
desired
catalytic properties. Combinatorial solid state synthesis techniques have not
been applied
to the discovery of new and/or improved catalysts. A significant impediment
for this has
been the lack of a broadly applicable, sensitive, selective and high
throughput
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
measurement technique which could be used to rapidly screen large catalysts
libraries.
Catalyst screening requires the unambiguous detection of the presence of a
specific
product molecule in the vicinity of a small catalyst site on a large library,
unlike
superconductivity or magnetoresisitivity which can both be easily tested by
conventional
contact probes, or luminescence that can be tested by light emission.
This invention provides a high-throughput method to rapidly screen the
activities
and selectivities of homogeneous and heterogeneous catalyst libraries
generated by
combinatorial synthesis. Solid and liquid state catalyst libraries can be
generated using a
variety of techniques and can involve the combination of a large number of
chemical
elements and compounds.
In one embodiment, catalyst libraries may be screened for both activity and
selectivity by high throughput screening using mass spectrometry. Catalyst
libraries of
microreactors and direct transfer of reaction products to a mass spectrometer
for analysis
according to this invention provides rapid screening of catalyst libraries.
The technique
and apparatus of this invention using catalyst libraries of an array of
microreactors in
monolithic structures with free jet sampling probes passing reaction products
to a mass
spectrometer makes it possible to screen each site in about one to five
seconds, a
significant improvement over the teachings of the Cong, et al reference cited
above,
while eliminating potential wall effects inherent in capillary microprobe
sampling.
In another embodiment, the mass spectrometric analysis may also be used in
combination with resonance-enhanced ionization of product gases and
microelectrode
screening. In cases where both screening methods are feasible, radiation
activation may
be used to rapidly identify promising sites and then mass spectrometry may be
used to
quantify yields and selectivities in greater detail. In instances in which the
identification
of radiation frequencies over which unique resonance enhanced multiphoton
ionization
signals of reaction products may not be feasible, the mass spectrometric
method may be
used to rapidly screen. catalyst libraries.
Detection methods in situ in the reactor use the high sensitivity, specificity
and
real-time features of resonance-enhanced multiphoton ionization, REMPi, in
which
pulsed and tunable ionizing light sources are used to selectively photoionize
desired
reaction products without ionizing reactants and/or other background species.
Photoions or photoelectrons generated by a tunable light beam in a reaction
product
4
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
plume from reactants in contact with a specific catalyst library site are
detected by an
array of microelectrodes positioned in close proximity to the library sites.
While this
invention will be described using a tunable ionizing beam, any radiation beam
of an
energy level to promote formation of specified photoions and photoelectrons
may be
used. When reaction products are solids or liquids, they can be ablated using
a pulsed
laser beam followed by selective photoionization of the products' using a
suitable UV
laser. The process of this invention can provide information on catalyst
selectivity by
detecting several reaction product species. This can be done using different
light
frequencies to sequentially generate specific ions of different products and
the REMPI
signals can then be converted into absolute concentrations by use of
calibration
standards.
Internal calibration standards introduced with the reactant feed can be used
to
quantify reaction products, as will be readily apparent to one skilled in the
art. The
process of this invention is broadly applicable and can be used to
simultaneously screen
an entire catalyst library. The process of this invention can also be used to
study
operational lifetimes, resistance to poisoning, regeneration and loss of
catalysts in tests
or in full scale chemical plant processes.
The process of this invention for rapid screening of potential catalyst
libraries for
catalytic properties broadly comprises; forming a potential catalyst library
having
potential catalysts at a plurality of addressable sites, passing reactant gas
in contact with
the potential catalysts at the plurality of addressable sites, and screening
gas plumes of
products of reaction from the addressable sites, the screening comprising at
least one of
translating one of the addressable sites into a position in proximity to a
sampling probe
orifice followed by passing products of reaction through a free jet sampling
probe to a
mass spectrometer for analysis and passing a radiation beam of an energy level
to
promote formation of specified ions and electrons in the product stream, such
as, for
example, a laser beam of a frequency to promote formation of specified
photoions or
photoelectrons and detecting the formed photoions or photoelectrons by
microelectrode
collection in situ in proximity to the addressable sites.
The above advantages and other features of this invention will be better
understood upon reading specific embodiments of the invention with reference
to the
figures wherein:
5
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
Fig. 1 is a schematic showing the principles of REMPI microelectrode detection
of product species;
Fig. 2 is a schematic showing REMPI microelectrode detection of products
formed by reactant contact of a catalyst library with physical masking;
Fig. 3 is a schematic showing REMPI microelectrode detection of products
formed by reactant contact of a catalyst library through a dedicated reactant
feed tube;
Fig. 4 is a schematic showing similar to Fig. 3 having a tilted test site;
Fig. 5 is a schematic showing REMPI microelectrode detection of products
formed by reactant contact of a catalyst library with flow through porous
sites;
Fig. 6 is a schematic showing REMPI microelectrode detection of products
formed by reactant contact of a catalyst library of catalyst coating on a
monolithic
structure;
Fig. 7 is a schematic showing of a monolithic catalyst library with expansion
cooling of products for REMPI microelectrode detection;
Fig. 8 is a schematic showing of a reactor with a flat plate solid catalyst
library
with row REMPI microelectrode detection;
Fig. 9 is a schematic showing of a reactor with a flat plate catalyst library
having
reactant flow through porous sites and row REMPI microelectrode detection;
Fig. 10 is a schematic top view of a reactor as shown in Fig. 9 having
simultaneous REMPI detection of all sites;
Fig. 11 is a schematic showing of a reactor with a monolith solid catalyst
library
having reactant flow through with row REMPI microelectrode detection;
Fig. 12 is a schematic showing of a reactor with a monolith catalyst library
having
simultaneous REMPI detection of all sites;
Fig. 13 is a schematic showing of a catalyst library with reactant contact for
homogeneous catalyst sites with REMPI microelectrode detection of products;
Fig. 14 is a schematic showing of a reactor with a homogeneous catalyst
library
with reactant flow through with row REMPI microelectrode detection of
products;
Fig. 15 is a schematic showing of a catalyst library using solid catalyst
particles
for gas distribution and for catalyst contact with REMPI microelectrode
detection of
products;
Fig. 16 is a schematic showing of a heterogeneous catalyst library with
reactant
6
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
flow through with expansion cooling of products for REMPI microelectrode
detection;
Fig. 17 is a schematic showing of a catalyst library using an ablation laser
for
gasification of solid and/or liquid products for REMPI microelectrode
detection of
products;
Fig. 18 is a molecular beam REMPI spectra for benzene and cyclohexane by
TOF-MS;
Fig. 19 is a microelectrode REMPI spectra for benzene and cyclohexane;
Fig. 20 is microelectrode REMPI signals from screening of catalyst library
site
activity for benzene production.
Fig. 21 is a schematic showing of one embodiment of a single microreactor
system of this invention;
Fig. 22 is a schematic showing of another embodiment of a single microreactor
system of this invention suitable for solution deposition;
Fig. 23 is a schematic showing of an array of microreactors in a single body;
Fig. 24 is a schematic showing of another embodiment of an array of
microreactors in a single body with a cover wafer;
Fig. 25 is a schematic showing of a catalyst library in vertical stacked
arrays of
microreactors as shown in Fig. 24;
Fig. 26 is a schematic showing a microreactor array as shown in Fig. 24
fitting
into a frame;
Fig. 27 is a schematic showing of arrays of microreactors in frames as shown
in
Fig. 26 arranged in adjacent side-by-side configuration;
Figs. 28A and 28B are diagrams summarizing combinatorial catalyst library
preparation and screening according to one embodiment of this invention;
Fig. 29 is a cross sectional schematic showing of a sampling probe having a
conical orifice in sampling mode for transfer of reaction products of one site
in a catalyst
library in an array of microreactors to a mass spectrometer for analysis;
Fig. 30 is a schematic showing of a sampling probe similar to Fig. 29 having a
capillary orifice in a translation mode;
Fig. 31 is a perspective schematic showing of an array of microreactors with a
sampling probe for passage of a portion of reaction products to a mass
spectrometer in
combination with an activating energy beam for REMPI measurement on a
translation
7
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
table for translation in a single dimension; and
Fig. 32 is a perspective schematic showing of horizontally stacked arrays of
microreactors on a translation table for translation in two dimensions for
combined mass
spectrometer and REMPI measurement of reaction products from a catalyst
library.
Generation of combinatorial solid state libraries has been achieved by
sputtering
with physical masking for measurement of superconducting, fang, et al, 1995,
supra,
magnetoresistivity, Briceno, et al, 1995, supra, and luminescence, Wang, et
al, 1998,
supra and Sun, et al, 1997, supra. Other thin film deposition techniques are
known to
the art, such as, electron beam evaporation, Danielson, et al, 1997, supra,
thermal,
Miyao, T., Shishikura, L, Matsuoka, M. and Nagai, M., "CVD Synthesis of
Alumina-
Supported Molybdenum Carbide Catalyst", Chem. Lett., 121, 561, 1996, and
plasma,
Kizling, M.B. and Jaras, S.G., "A Review of the Use of Plasma Techniques in
Catalyst
Preparation and Catalytic Reactions", Appl. Catalysis - A General, 147, 1,
1996,
chemical vapor deposition, molecular beam epitaxy, Kim, Y.J., Gao, Y. and
Chambers,
S.A., "Selective Growth and Characterization of Pure Epitaxial a-Fe203(0001)
and
Fe304(001) Films by Plasma-Assisted Molecular Beam Epitaxy, Surf. Sci., 371,
358,
1997, and pulsed-laser deposition, Gorbunov, A.A., Pompe, W., Sewing, A.,
Gapanov,
S.V., Akhsakhalyan, A.D., Zabrodin, LG., Kaskov, LA., Klyenkov, E.B., Mozorov,
A.P., Salaschenko, N.N., Dietsch, R., Mai, H. and Vollmar, S., "Ultrathin Film
Deposition by Pulsed Laser Ablation Using Crossed Beams", App. Surf. Sci., 96-
98,
649, 1996 and Russo, R.E., Mao, X.L. and Perry, D.L., "Make Catalytic Coatings
by
Pulsed-Laser Deposition", Chemtech, 12, 14, 1994, can be used to create large
solid
state catalyst libraries. These techniques provide good control of surface
chemistry and
are ideally suited to generate a wide spectrum of solid materials. Other well
established
preparation techniques, such as co-precipitation and impregnation, can also be
used to
generate catalyst libraries. Satterfield, C.N., "Heterogeneous Catalysts in
Practice", 2nd
Ed., Chap. 4, 87, McGraw Hill, New York, 1991. For example, a large variety of
co-
precipitates can be synthesized in parallel and the resulting slurries/pastes
can be applied
on suitable substrates using, for example, multichannel pipettes or solenoid
inkjet valves
to generate spatially addressable sites. Lemmo, A.V., Fisher, J.T., Geysen,
H.M. and
Rose, D.J., "Characterization of an Inkjet Chemical Microdispenser for
Combinatorial
Library Synthesis", Anal. Chem., 69, 543, 1997. Catalyst libraries can also be
prepared
8
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
by impregnating suitable carrier materials, such as, for example, porous
silica or alumina,
that were previously applied to addressable sites on a substrate by a suitable
liquid
solution containing a catalyst. The slurries/pastes and impregnation solutions
applied on
the substrates can then be dried and treated to produce suitable catalyst
materials.
Porous catalyst libraries can also be prepared by coating porous corners, for
example,
silica or alumina, with thin films of catalytic materials using various film
deposition
techniques described above. An important aspect of this approach is prevention
of
excessive deposition to prevent pores becoming plugged by the catalytic
materials.
Reactant contact with the porous libraries can be accomplished either by
passing
reactants over or through the catalyst sites.
In testing for catalysis, however, chemical composition is not the sole
determinator of activity. Physical properties of the surface, such as edges,
corners and
defects, as well as pore size can have an influence in determining activity.
Satterfield,
C.N., 1991, supra and Smith, J.M., "Chemical Engineering Kinetics", Chap. 8,
327-358,
McGraw Hill, New York, 1981. These properties are determined to a large extent
by the
catalyst preparation procedure. Therefore, thin film combinatorial libraries
may be
subjected to a variety of treatment methods to generate suitable catalytic
materials, such
as, for example, oxidation, reduction, calcination, leaching, the subsequent
addition of
dopants and other treatments well known to the art. These different
preparation
processes also substantially increase the number of combinations of catalyst
formulations
which must be tested in order to obtain the best catalyst.
Heterogeneous catalyst libraries can also be prepared by using monolithic, or
honeycomb, structures. Satterfield, C. N., 1991, supra. These materials
provide parallel,
uniform, straight and nonconnecting channels, thereby providing a convenient
matrix for
creating large catalyst libraries. A variety of cell shapes and sizes with
cell densities
varying from about 10 to about 500 cells per square inch can be produced with
catalyst
library sites. However, a wide variety of desired custom cell densities can be
fabricated
within and beyond the above ranges. Monolithic structures can be made from
metals or
they can be extruded from inorganic dough, such as magnesia-alumina silicate,
through a
die followed by drying and firing. Catalyst libraries can also be prepared by
coating
metal monoliths with inorganic substrates, wherein the metal inlay serves as a
barrier to
prevent intercell diffusion of species. Catalysts can then be incorporated
into the library
9
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
substrate using any of the variety of methods described above. Monolith
structures can
also be machined for optical access and placement of microelectrodes.
Homogeneous catalyst libraries comprising, for example, organometallic and
inorganometallic compounds and other complex molecules such as enzymes, can be
similarly generated using multichannel pipettes, Burgess, K., Lim H-J., Porte,
A.M. and
Sulikowski, G.A., "New Catalyst and Conditions for a C-H Insertion Reaction
Identified
by High Throughput Catalyst Screening", Angew. Chem. Int. Ed. Engl., 5, 220,
1996,
and solenoid inkjet valves. These libraries may have arrays of microtubes
bundled
together with reactant gas bubbled through them. Homogeneous liquid catalysts
can also
be held or immobilized in the pores of porous carriers which can be in the
form of
particles or can be coated on the walls of monolithic structures. Since the
screening
method of this invention can be readily miniaturized, the physical dimensions
of catalyst
sites that determine library density are primarily dependent upon the nature
of the
catalyst, liquid or solid phase, the method of preparation of the library,
diffusional mixing
of gases in the library, heat conduction through the library substrate, the
objectives of the
screening process and other relevant factors. For example, when the objective
of
screening is to evaluate catalytic materials for gas-phase reactions using
flat catalytic
sites, library densities will be limited by the gas-phase diffusion because at
high library
densities intersite diffusion can result in signal crossovers between sites.
However, the
evaluation of catalyst operating temperature windows requires the fabrication
of libraries
in which each site is thermally insulated to maintain different temperatures.
In this case,
the library density will be limited by the thermal conductivity of the wafer
of substrate.
For liquid phase homogeneous catalysts, surface tension and viscosity play a
significant
role in determining gas dispersion, and thus in establishing the minimum
dimensions of
the library sites and hence the library density.
In this invention, the catalyst sites must be sufficiently separated from each
other
so that product formation from each site and its unambiguous detection can be
achieved.
Monolithic, or honeycomb, structures offer advantages by providing clear
physical
separation of the library sites. These and other catalyst library design
factors will be
further discussed in descriptions of screening methods. Unambiguous and rapid
screening of solid catalyst sites of 0.5 cm by 0.5 cm have been demonstrated
using the
present invention. These site dimensions provide catalyst libraries having
densities of
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
about 10 sites per square inch which permits creation of over 900 sites on a
substrate
having dimensions of 8.5 inches by 11 inches, the size of a sheet of letter
paper. Higher
library densities are clearly practical using smaller site dimensions or by
the use of
monolithic structures. The pattern of the sites should be designed to expedite
the
generation and screening of the libraries, libraries having rows of catalyst
sites offering
distinct advantages both for generation and screening of the sites. Any method
of
production of chemical libraries having sites of the above mentioned
characteristics is
suitable for production of catalyst libraries for use in the rapid screening
process for
catalyst evaluation according to this invention.
In one embodiment of this invention, sampling of reaction products emanating
from individual sites in a library is accomplished by passing the reaction
products through
a small orifice, placed in close relationship to the source of the reaction
products, to a
significantly larger cross section area chamber for passage to a detection
device, such as,
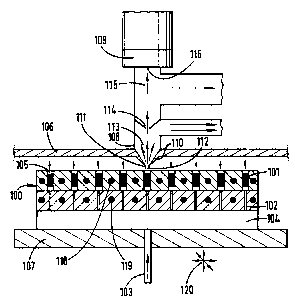
a mass spectrometer. Shown schematically in Fig. 29 is a catalyst library
having
individual sites configured in microreactors, as will be described below in
fiuther detail.
Briefly, inert microreactor body 100 has reactant feed passages 102 leading to
enlarged
catalyst zones with catalyst beds 101. Reactant gases are supplied through
reactant gas
supply passage 103 to reactant gas distribution plenum 104 for distribution to
reactant
feed passages 102. Reaction products exit the microreactors through reaction
products
exit passages 105 and pass from reactor enclosure 106 or a portion may pass
from an
individual library site through a micro sampling probe to a detection device.
The reactor
enclosure can be pressurised to provide the desired reaction pressure.
Alternatively,
each microreactor can be individually pressurised to test catalysts under
different
pressures, or each array of microreactos can be individually pressurised. As
shown in
Fig. 29, the catalyst library is fixidly mounted on translation table 107 for
positioning of
sampling probe 108 over a single library site for detection of reaction
products from that
site. Translation table 107 may be moved in x-y-z directions by computer
controlled
stepper motors, as well known by one skilled in the art, to rapidly move
single library
sites into position for sampling from a single site by sampling probe 108
fixidly mounted
in reactor enclosure 106. It is also possible to translate the sampling probe
and the
detection system while maintaining the library stationary, or both the library
and the
sampling probe may be simultaneously moved by translators. As shown in Fig.
29, a
11
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
single library site has been moved to a sampling position in proximity to
sampling probe
108 to pass a portion of reaction product gases through the sampling probe to
mass
spectrometer 109. Reactants may be passed through all library sites may be
operated
simultaneously and product gases from other library sites may be withdrawn
from reactor
enclosure 106. Following product gas analysis at a particular library site,
the library may
be translated into position for evaluation of another catalyst site. Since
several or all of
the sites in a library may be under reaction conditions simultaneously,
analysis of the
reaction products may take place immediately after positioning the library
without the
necessity of waiting for equilibrium conditions and without transfer line
delays
encountered by use of capillary tube sampling probes, as described by Cong, et
al, supra.
The tip of sampling probe 108 must be made from material which can be
machined and withstand the pressure and temperature of the reaction chamber,
in the
event that the reactor enclosure lOb is pressurised, as well as being inert to
the reactants
and reaction products. As shown in Fig. 29, reactor enclosure wall 106 has
sampling
cone 110 which may be integral with or attached with proper sealing to the
reactor
enclosure wall. When the microreactors in the reactor array are internally
pressurised
and products discharged into atmospheric pressure, the sampling cone can be
directly
attached to the mass spectrometer. As shown, sampling cone 110 has a sampling
probe
extension 111 to minimally perturb the reaction product stream and allow
positioning of
the sampling probe very close to the catalyst reaction site without hindering
product gas
venting. Sampling cone 110 should have a half cone angle of about 15 to about
45
degrees to allow free jet expansion of the gas samples into a vacuum chamber
while
sampling probe extension 111 may have a smaller cone angle. Free jet expansion
in the
sampling probe leads to substantial cooling and quenching of all possible
homogeneous
and heterogeneous reactions and provides direction to the molecules towards a
mass
spectrometer positioned downstream of the sampling cone. Sampling cone orifice
112,
at the small end of the cone, is sized so that reaction chamber pressures and
vacuum
pump capacities of all stages can be accommodated. Suitable sampling cone
orifice
diameters are about 1 micrometer to about 200 micrometers, typically about S
micrometers to about 50 micrometers for use with modest size vacuum pumps. The
expanding reaction product sample from the sampling cone passes through first
vacuum
stage 113 and skimming cone 114 to ensure that only the central portion of the
reaction
12
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
product sample jet enters the mass spectrometer chamber, eliminating any
surface
induced reactions that may occur in the sampling probe. The cone angle and
diameter of
the opening at the tip of the skimmer must be suitable to meet reaction
chamber pressure
and sampling probe pumping speed requirements, as can be readily determined by
one
skilled in the art. The reaction product sample jet passing through the
skimming cone
then passes through second vacuum stage 115 and is directly introduced into a
mass
spectrometer through mass spectrometer inlet orifice 116. The mass
spectrometer can
be a quadrupole mass spectrometer or a time of flight spectrometer with fast
electronics
to acquire and process the data, as well known to the art. Electron impact or
radiation
can be used to ionize species. Tunable lasers can also be used to selectively
ionize
reaction products under REIvIPI conditions. When catalyst Libraries are
screened at
atmospheric pressure, or when microreactor arrays are internally pressurised
and
products discharged to atmospheric pressure, only one pump down stage may be
necessary to prepare the sample for mass spectrometer pressure conditions,
while
catalyst libraries screened at high pressures may require more than two pump
down
stages, as will be apparent to one skilled in the art. The staged pump down
process
rapidly brings the pressure of the reaction products from high pressures, in
some
applications from about 20 to about 50 atmospheres, to a small fraction of an
atmosphere so that the reaction product samples can be directly introduced
into the mass
spectrometer where pressures are typically maintained at about 10-5 to about
10'6 Torr.
When microreactors in an array are internally pressurised and discharged into
atmospheric pressure, this will constitute a pump down stage. The pressure of
the last
pump down stage, second stage in Fig. 29, and the mass spectrometer inlet
orifice
diameter must be compatible with this pressure limitation in view of the
pumping speed
attainable by the vacuum system. Typically, pressure in the first and second
stages of a
two stage system should be maintained at about 760 to about 10'2 and 10'2 to
about 10'5
Ton, respectively. The pressures in all stages should be maintained the same
during
calibration and screening processes to quantify the results of catalyst
evaluation.
The distance from the sampling probe orifice to the mass spectrometer should
be
kept as short as possible to maximize detection sensitivity since the gas
concentration
decreases upon expansion into vacuum according to 1/r2, where r is the
distance from the
tip of the sampling probe. However, shorter sampling probe orifice to mass
13
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
spectrometer distance decreases the pumping speed provided by the vacuum
pump(s),
thereby adversely affecting the free jet sampling process. In view of these
conflicting
results, the spacing between the sampling probe orifice and the mass
spectrometer is
determined to balance signal detection and pumping speed needs. Typically,
spacing
between the sampling probe orifice and the mass spectrometer is about 7.5 to
about 25
cm. In the limit, the sampling system performance approaches mo 1 ecu 1 ar
beam
sampling conditions as described by Chang, W.D.; Karra, S.B.; and Senkan,
S.M.,
Molecular Beam Mass Spectroscopic Study of Trichloroethylene Flames, Environ.
Sci.
Technol., 20, 12, 1243, (1986) where the expanding sample jet velocities in
the first
stage can reach supersonic levels and the jet stream entering the mass
spectrometer is a
directed molecular beam.
Another embodiment of the invention is shown in Fig. 30 wherein the
microreactor array catalyst library is shown in translation mode withdrawn
from the
sampling position shown in Fig. 29 and sampling orifice 117 is a short
capillary inert to
reactants and reaction products having a diameter of about 1 to about 500
micrometers,
typically about 5 to about 100 micrometers, and lengths of about 1 micrometer
to about
20cm, typically about S to about 100 micrometers. The capillary orifice used
in this
invention is significantly shorter than those used by Kassem, M., Qum, M., and
Senkan,
S. M., supra, and by Cong, P., Giaquinta, D., Guan, S., McFarland, E., Self,
K., Turner,
H. and Weinberg, W.H., supra. To maximize product sample signals and to
minimize
pumping speed requirements, capillary diameters of about 5 to about 20
micrometers and
capillary lengths of about 50 to about 100 micrometers are compatible with
small
commercial vacuum pumps. The capillary orifice of this embodiment passes
directly into
first vacuum stage 113 of sampling microprobe 108. In other respects, the
apparatus and
process shown in Fig. 30 is similar to those described above with respect to
Fig. 29.
In the sampling probe configurations shown in Figs. 29 and 30, the time
required
to transfer reaction products from the reaction zones of the microreactors to
the mass
spectrometer can be in the order of microseconds to tens of milliseconds.
Acquisition of
mass spectrometric data can be accomplished in a time scale of the order of
several
hundred milliseconds, especially when specific mass ions are monitored.
Therefore, the
time limiting step of the screening process is the time to mechanically
position individual
sites in the library in sampling position, in proximity to the sampling
orifice of the
14
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
sampling probe, by the stepper motor driven translation apparatus. All
microreactor sites
in the catalyst library can be simultaneously operated to simultaneously
generate reaction
products. In this mode, the product stream from any site in the library can be
sampled at
any time without the requirement of waiting for establishment of steady state
operating
S conditions in each site. Alternatively, reactant gas flows to individual
sites in a library
may be independently controlled by flow controllers in each reactant feed
passage so that
reactant flow to a specific library can be turned on early enough for
establishment of
steady state operating conditions while screening another site and then turned
off after
the screening process of that site, as more fully explained below. This mode
of operation
is necessary when it is important to screen the library sites at the same on-
stream time
conditions.
The catalyst library shown in Figs. 29 and 30 represent a cross section of an
array
of packed bed microreactors in a high thermal conductivity metal microreactor
body.
Catalyst powders, particles, or any other form of solid catalysts, can be
placed into
cylindrical, or other shaped, cartridges which can be inserted into the
catalyst zones of
the microreactor body. Other methods of catalyst loading of microreactors, as
more
fully explained below, are also suitable. Reactor heating elements 118 are
shown
embedded in microreactor body 100 to provide uniform temperature control of
the entire
library. Individual library sites also may be insulated from each other and
each have an
individually controlled heating element to provide different temperature
control of each
site. In a similar manner, each site may be provided with an individual flow
control
regulator to provide different residence times in each site. A similar
reactant preheat
zone in the reactant feed zone may be provided, as shown by reactant
preheating
elements 119, to heat reactant gases to a desired temperature prior to contact
with the
catalyst. These microreactor configurations are more fully described below.
The entire
library is attached to translation table 107 in a fixed relation to provide
precision x-y-z
three dimensional movement, as indicated by translation arrows 120. Two
dimensional
translation in the x and y axes moves the library into position for sampling a
specific site
while movement in the third dimensional z axis positions reaction product exit
passage
I21 into proximity to sampling cone orifice 112 of sampling microprobe 108.
The mass spectrometric screening method described above can be used with
other catalyst library designs, such as, for example, those described herein
as well as
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
other types of libraries which may include homogeneous catalyst libraries,
fluidized bed
(gas and liquid) libraries, and combinations thereof. The mass spectrometric
screening
method may be combined with the resonance-enhanced multiphoton ionization
method,
REMPI, described in greater detail herein. The REMPI method for screening
catalyst
libraries has been more fully described in Senkan, S.M.; High-Throughput
Screening of
Solid-State Catalyst Libraries, Nature, 394, 350, 23 July 1998. The
combination of mass
spectrometric screening, as described above, with a microreactor array as
shown in and
described further in respect to Fig. 24 is shown in Fig. 31. As shown in Fig.
3 I,
microreactor array 122 has activating radiation beam 77 passing through the
reaction
product streams from the individual sites with microelectrodes 87 in proximity
thereto
with internal wiring leads 88 for powering each electrode and for passage of
detection
signals from each electrode to a detection device. In the manner as described
above with
respect to Fig. 29, sampling cone tip 111 with a sampling orifice is placed in
proximity to
the reaction product stream at the exit of an individual microreactor by
movement of the
microreactor array on translation table 107 in an x direction and placed in
sampling
position by movement of the microreactor array in a z direction, as indicated
by
translation arrows 120.
Stacked arrays of microreactors for combined mass spectrometric and REMPI
screening methods may be formed using multiple microreactor arrays, as more
fully
shown in and described with respect to Fig. 25. As in the case of reactor
arrays shown in
Figs 29 and 30, heating elements can be embedded in thermal conducting walls
between
individual microreactors. In similar manner as shown and described with
respect to Fig.
31, REMPI measurements and/or mass spectrometric measurements may be made by
positioning the arrays to a single site for mass spectrometric sampling by
movement of
translation table in the x-y-z axes indicated by translation arrows 120. Fiber
optics
facilitates mounting laser light sources on translation table 107 to provide
laser beams 77
to all of the library sites simultaneously for rapid REMPI microelectrode
screening. In
cases where both methods of screening are feasible, radiation activation may
be used to
rapidly identify promising sites and mass spectrometric analysis used to
quantify yields
and activities more precisely.
It will be apparent to one skilled in the art upon reading of this description
that
any of the microreactor configurations, microreactor arrays, and stacked
arrays of
16
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
microreactors disclosed with respect to the REMPI microelectrode screening
method
may be readily adapted to the mass spectrometric screening method by mounting
the
microreactors on a suitable translation table and provision of a free jet
expansion
sampling probe leading to a mass spectrometer.
Screening of large libraries for desired catalytic activity according to this
invention is based upon the fact that when a laser frequency is tuned to a
real electronic
intermediate state of a gaseous molecule, the cross section for ionization of
that molecule
is significantly enhanced. This process is resonance-enhanced multiphoton
ionization, or
REMPI. When the laser wavelength is not tuned to a real electronic state, the
probability
for photoionization is very small. Thus, the ionization cross section reflects
the
absorption-excitation spectrum of the intermediate electronic state of the
molecule.
Using REMPI, specific catalytic reaction products can be selectively ionized
with high
efllciency using a suitable laser frequency, while avoiding the simultaneous
photoionization of reactants and/or background gases. While preferred
embodiments of
this invention are described using laser beams, any radiation beam of a
suitable energy
level to promote formation of specified ions and electrons from reaction
products may be
used, thereby allowing detection of the formed ion and/or electrons by
microelectrode
collection in downstream proximity to the radiation beam.
In cases where the catalytic reaction products) do not provide easy generation
of
REMPI photoions, the process of this invention may be used in detection of
directly
related products. For example, reaction product molecules may be fragmented
into
smaller daughter products by a suitable energy source, such as, for example, a
pulsed
laser beam or by a plasma arc. The fragments may be stable molecules, radicals
or ionic
species. Following fragmentation of a catalytic reaction product molecule to a
daughter
product which can be uniquely attributed to a catalytic reaction product
molecule that is
desired to be detected, the daughter product can be selectively photoionized
using the
REMPI process and detected by a microelectrode as described herein.
Quantification of
reaction products by detection of their fragmentation products requires
additional
calibration to account for the efficiencies of fragmentation.
It may also be possible that upon irradiation of reaction products by specific
light
frequency, the reaction products or their fragmentation products may emit
unique
radiation signatures involving, for example, luminescence, fluorescence or
17
CA 02347697 2001-04-24
WO OOI29844 PCT/GB99/03767
phosphorescence. These emissions can then be used to rapidly screen the
catalyst
libraries using, for example, monochromators and diode array and charge
coupled device
(CCD) detectors.
For example, selective identification of ethylene oxide (C2H40) and
acetaldehyde
(CH3CH0) as a consequence of the reaction of ethylene (C2H4) and (02) can be
performed on fragmentation products which may be described by the following
equations:
CzH40 + by -~ CHZO + CH2
C2HaO + hV -~ C2Ha + O
C2H4O + by -~ C2Hs+OH
In the case of acetaldehyde, the fragmentation may be described by the
following:
CH3CH0 + by -~ CH3 + CHO
Although it may be possible to detect the catalytic product molecules directly
by
their REMPI ions, information about their presence in a reactant-product
mixture can
I 5 also be obtained by measuring the REMPI characteristics of their
fragmentation
products. Thus, the formation of fragmentation products CH20, CH2, CZH3, O and
OH
can be uniquely attributed to ethylene oxide while the formation of CH3 and
CHO can be
uniquely attributed to acetaldehyde. In this manner, the selective detection
of any one of
the fragmentation products, except ethylene which is present abundantly as a
reactant,
can signify the level of the parent ethylene oxide and/or acetaldehyde in a
mixture of
these chemicals.
As another example, acrylonitrile (C2H3CN) when produced by the reaction of
propane (C3H8), ammonia (NH3) and oxygen may be detected by detection of
either of
the products resulting from the fragmentation C2H3CN + hv --~ C2H2+ CN which
gives
unique information about the level of acrylonitrile in a product mixture.
There are several means for inducing REMPI, the most common is the resonant
2-photon ionization, R2PI, in which one photon, hv,, energizes the molecule to
an
excited electronic state and the second photon, hv2, ionizes the molecule.
Lubman, D.M.,
"Lasers and Mass Spectrometry", Oxford Univ. Press, New York, 1990, Chap. 16,
Lubman, D.M. and Li, L., "Resonant Two-Photon Ionization Spectroscopy of
Biological
Molecules in Supersonic Jets Volitalized by Pulsed Laser Desorption", 353.
However,
depending upon circumstances, the absorption of two or more photons in each
step can
18
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
also be used for REMPI. Ionization occurs if (hvi+hv2)>IP, where IP is the
ionization
potential. The two photons used can have the same or different energies and
can be
obtained from the same or different lasers. Higher energy UV photons may also
be used
to photoionize species in a single photon process. The two photon REMPI
process can
be described for selective photoionization of a product P by the following
equations: P +
hvl = P* and P* + hv2= P+ + e, wherein P is the product, P* is the real
electronic excited
state of the product, P+ is the photoion of the product and a is the
photoelectron. By
varying the photon energies, which can be accomplished using tunable lasers,
the
ionization spectrum of the target molecule P can be mapped to determine a
suitable laser
frequency which ca.n be used to exclusively ionize it without simultaneously
ionizing
other molecules in the mixture. Since the REMPI process involves the
participation of
two or more photons, the laser light wavelengths used must take this into
account. As a
crude approximation, each photon in a successful REMPI must possess an energy
of
about 1/2 the IP in the R2PI process using a single laser beam. Similarly, if
a single laser
beam is used, each photon energy must be about 1/3 the IP in a 2 + 1 process
and 1/4 the
IP in a 2 + 2 process, etc. When two or more laser beams are used, each photon
energy
can be independently selected to optimize the resulting REMPI signals. Laser
wavelengths covering the range from deep ultraviolet, UV, such as 150
nanometers, nm,
to visible light, such as 700 nm, can be used to induce REMPI using a variety
of
multiphoton processes.
REMPI is inherently a high resolution technique in which ion absorption
features
of any molecule can be determined with high precision. Also, molecules are
ionized from
a vibrational level of an electronically excited state, thereby providing
specific
photoionization of only target molecules. This can be used to distinguish
between
isomers, for example dichlorotoluenes, due to their different electronic
structures.
Zimmerman, R., Lerner, Ch., Schramm, K.W., Kettrup, A. and Boesl, U., "Three-
dimensional Trace Analysis: Combination of Gas Chromatography, Supersonic Beam
UV Spectroscopy and Time-of Flight Mass Spectrometry", Euro. Mass Spectrom.,
1,
341, 1995. The REMPI process can be sequentially used to detect different
products
using different laser frequencies, thus also providing the determination of
catalyst
selectivities. REMPI is a high sensitivity technique with real-time detection
of species at
low parts per billion, Gittins, C.M., Castaldi, M.J., Senkan, S.M. and
Rohlfing, E.A.,
19
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
"Real-Time Quantitative Analysis of Combustion Generated Polycyclic Aromatic
Hydrocarbons by Resonance Enhanced Multiphoton Ionization Time of Flight Mass
Spectometry", Anal. Chem., 69, 287, 1997, and high parts per trillion already
demonstrated. Castaldi, M.J. and Senkan, S.M., "Real-time Ultrasensitive
Monitoring of
Air Toxics by Laser Photoionization Time of Flight Mass Spectrometry, J. Air
and
Waste Mgmnt. Assoc., 48, 77, 1998.
Fig. 1 is a generalized illustration of the REMPI method of selective
detection of
a gaseous product generated by contacting a catalytic site with reactants.
According to
the present invention, gaseous reaction products form a gaseous plume 22 when
catalyst
21 mounted on substrate 20 is contacted by the reactants. The gaseous products
are
photoionized by pulsed UV laser beam 23 formed from tunable laser source 24
and/or
using second tunable laser source 25 directed by minor 26 through the central
portion of
gaseous product plume 22 generating photoions, P+ , and photoelectrons a , as
indicated
in Fig. 1. Microelectrode 27 is positioned a few millimeters above laser beam
23 to
collect the photoelectrons or photoions, depending upon the voltage bias
applied by DC
power source 30 to cathode 28 and anode 29. The electrical signal collected by
microelectrode 27 is then amplified and detected by detector 31, such as a
digital
oscilloscope. If the measured electrical signal is higher than reference sites
which do not
have catalysts, the site can be tagged catalytically active. Otherwise, the
site must be
considered catalytically inactive. It is apparent that selection of a suitable
laser
frequency, or frequencies for detection of multiple products, is critical to
ensure that the
electrical signals generated by the laser beam are exclusively due to
photoionization of
the specified product gas and not from the reactants and/or background gases.
The
suitable laser frequency for a specific material may be identified by laser
photoionization
mass spectrometry studies, using for example, a tunable laser and time-of
flight mass
spectrometer. Castaldi, M.J. and Senkan, S.M., 1997, supra and Gittins, C.M.,
Castaldi,
M.J., Senkan, S.M. and Rohlfing E.A., 1998, supra. Using this approach, a gas
mixture
containing the species of interest is introduced into a vacuum chamber using,
for
example, a pulse valve. The expanding gas jet is then intercepted by UV
photons at a
specific energy from a tunable laser generator. The resulting REN1PI signals
are then
recorded by the time-of flight mass spectrometer system. By scanning the UV
laser
frequency range, the photoionization spectra of the reactants, products, by-
products and
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
background gases can be determined. In the case of molecular isomers, the
photoionization spectra of each isomer must be determined individually.
Following the
determination of the photoionization spectra for all of the relevant species,
specific LTV
frequencies can be identified which would lead to the exclusive generation of
the REMPI
ions of specific product isomers desired to be evaluated.
It should be recognized that the REMPI spectra broadens at elevated
temperatures due to the overlapping transitions from a large number of
rovibronic levels.
However, it is generally possible to identify a laser frequency that
selectively
photoionizes the desired products without interferences from the reactants,
other
products and the Garner gas, due to the availability of broadly tunable UV
lasers. This
identification process is expedited when the product gases are structurally
different from
the reactant and background gases, for example, in the production of benzene,
an
aromatic compound, from hexane, an aliphatic compound, in Ar carrier gas with
H2 as
the only by-product. Potential problems associated with spectral congestion of
REMPI
signals can be effectively solved by the use of supersonic jet expansion.
Parker, D.H.,
"Laser Ionization Spectrometry and Mass Spectrometry" in "Ultrasensitive Laser
Spectroscopy" Kliger, D.S. Ed., Academic Press, New York, 1983 and Trembreull,
R.,
Sin, C.H., Li, P., Pang, H.M. and Lubman, D.M., "Applicability of Resonant Two-
Photon Ionization in Supersonic Beam Mass Spectrometry to Halogenated Aromatic
Hydrocarbons", Anal. Chem., 57, 1186, 1985. Jet expansion, which can be
achieved by
expanding the product gases into a vacuum through a small orifice, leads to
transitional,
rotational and vibrational cooling resulting in significant simplification of
the REMPI
spectra. This method permits selective detection of specific species in a
similar
background.
The product photoions and photoelectrons generated above the catalyst site can
be collected using a microelectrode, which can be either anode or cathode or
both. The
substrate upon which the catalyst library is deposited can also serve as the
cathode or the
anode, or another microeIectrode can be placed within the substrate for this
purpose.
High temperature REMPI-electrode approach has previously been used to
determine the
concentration of gaseous species containing only a few atoms, such as PO, NO,
H and
O. Smyth, K.C. and Mallard, W.G., "Two Photon Ionization Processes of PO in a
C2H2/air Flame", J. Chem. Phys., 77, 1779, 1982; Cool, T.A., "Quantitative
21
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
Measurement of NO Density by Resonance Three-Proton Ionization", App. Optics,
23,
10, 1559, 1984; Goldsmith, J.E.M., "Resonant Multiphoton Optogalvanic
Detection of
Atomic Oxygen in Flames", J. Chem. Phys., 78(3), 1610, 1983; and Bjorklund,
G.C.,
Freeman, R.R. and Storz, R.H., "Selective Excitation of Rydberg Levels in
Atomic
Hydrogen by Three Photon Absorption", Optics Comm., 31 ( 1 ), 47, 1979. These
earlier
studies, which exhibit problems of spectral congestion and broadening of REMPI
signals,
implicitly teach against use of the REMPI-electrode approach when larger
molecule
species are involved. However, it has now been discovered that larger
molecules can be
measured by this technique for catalyst screening. Significant broadening of
the REMPI
spectra can be tolerated in catalyst screening where the REMPI features of the
reactants
and products are generally separated. When the REMPI spectra overlap, which
should
be rare in catalyst screening where reactants and products have distinct
electronic
structures, this problem can be solved by jet cooling the products by
expanding them into
a vacuum chamber through small orifices.
The REMPI microelectrode technique can also be used to detect liquid and solid
products. In these cases, the reaction products must be gasified first using
an ablation
laser, for example, a pulsed C02 or another type of laser. The gasified
products then can
be photoionized by REMPI and detected by a microelectrode, as described above.
The
REMPI method can also be used to monitor reaction intermediates involved in
the
catalytic process, which cannot be detected by analysis of product gases
collected at the
exit of the reactor. This can be particularly useful in developing insights
into reaction
pathways associated with catalytic reactions, and thereby can significantly
accelerate the
catalyst development process
No literature is known to the inventor which suggests the use of REMPI and
microelectrodes for the high speed screening of heterogeneous and homogeneous
catalyst libraries. A multitude of approaches for the rapid screening of large
libraries for
catalytic activity may be followed, and the following presently preferred
approaches are
set forth as exemplary and should not be considered as limiting the invention.
For heterogeneous catalyst libraries, the solid state catalysts may be
arranged in
rows of catalyst clusters on a flat sheet to expedite the screening process.
In addition,
monolithic, or honeycomb, structures with well defined channels can also be
used to
generate suitable catalyst libraries. The catalyst sites can also be created
to be porous or
22
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
non-porous depending upon the catalyst and method of preparation. Fig. 2
illustrates a
non-porous, flat sheet catalyst library with reactant contact with the
catalyst achieved by
flowing reactant gases over the library followed by row screening of product
plumes.
The same numerals have the same meaning throughout this disclosure and in the
figures.
Test catalyst site 21 with upstream catalyst site 21u and downstream catalyst
site 21d are
shown on substrate 20 with mask 32 shielding upstream catalyst site 21u from
the
reactant gas stream indicated by reactant velocity profile 33. The gases
containing
products must be removed from the library aRer their emanation from the sites
to
minimize product circulation in the reactor. In the arrangement shown in Fig.
2, the
catalyst sites upstream from the test catalyst site, 21, must be masked to
prevent signal
crossover from different sites. If the upstream sites are not masked and if
some of these
sites are catalytic, products formed at these sites would be transported
downstream and
interfere with the row screening process. Masking can be accomplished by using
a
physical mask to cover upstream catalysts sites, as shown in Fig. 2, or by
introducing
1 S reactant gases directly onto the catalyst sites using dedicated gas
reactant feed tubes,
shown as 34 in Fig. 3. Fig. 4 shows tilted catalyst test site 21t to promote
transport of
products away from the catalyst surface. This arrangement improves signal
detection of
products from the test site.
When reactant molecules pass over the test sites with catalytic properties,
products will be formed at the surface. These products will then diffuse into
the flowing
gas stream and establish a product concentration boundary layer, or product
plume 22,
as shown in Figs. 2-4. Assuming a constant catalyst surface concentration for
the
product, the product concentration layer thickness 8~(x) = 3.3(DxL/Uo)1~,
where x is the
distance from the leading edge of the catalyst site as shown in Figs. 2-4, D
is the
molecular diffusion coefficient of the product, Uo is a characteristic gas
velocity as shown
in Figs. 3-4 and L is a characteristic dimension in the vertical direction,
such as the height
of the reactor or the diameter of the reactant feed tube shown in Figs. 3-4 as
2R.
To illustrate some of the design issues involved, consider the solid state
library of
catalyst sites 5 mm long by 5 mm wide. Assuming a gas feed line diameter of
0.5 cm and
a mean reactant gas velocity of 1.0 cm/sec and a diffusion coefRcient of 0.1
cmz/sec
which is typical for most gases at 1 atm, the concentration boundary layer
thickness at 5
nun from the leading edge of the catalytic site can be estimated to be:
23
CA 02347697 2001-04-24
WO 00129844 PCT/CB99/03767
8(0.5) = 3.3[(0.1)(0.5)(0.25}/LOJ'~ = 0.767cm or 7.67mm
This boundary layer is thick enough to pass a laser beam through and to
photoionize products, if present. The diameter of the gas feed tube 2R, the
gas velocity
Uo and the catalyst site dimension x can be altered to further control the
thickness of the
concentration boundary layer. Additionally, test sites 21t can be tilted, as
shown in Fig.
4, during the screening process to promote the transport of products away from
the
catalyst surface.
When porous catalyst libraries are generated, reactant gases can also be
passed
through the sites in the library generating a product plume above the test
catalyst sites, as
shown in Fig. S. In this embodiment, the reactants pass through all of the
catalyst sites
thereby rendering simultaneous screening of all sites on the library feasible.
As shown in
Fig. 5, reactants are passed through reactant plenum 36 to and through porous
test sites
21p forming product plumes 35 which are measured in the same manner as
described
above.
Catalyst libraries may also be created, as shown in Fig. 6, using monolithic
structures 40 wherein reactant gases will also pass through channels 37 over
catalyst
coatings 38 forming product gases which pass through laser beam 23 and over
microelectrodes 27. In this embodiment, simultaneous screening of the entire
library is
readily accomplished. Microelectrodes 27 may be inserted into channels 37, as
shown in
Fig. 6, to significantly reduce signal crossover between catalytic sites.
Optical access to
the product gases in each channel must be provided through small windows 39
for the
laser beam, as shown in Fig. 6. As a consequence of good spatial resolution
and site
separation provided, monolith structures provide a good framework for high
throughput
and simultaneous screening of high density catalytic libraries.
When the high temperature microelectrode REMPI spectra of the product
molecules do not have distinguishing features or have features exhibiting
overlap,
products must be cooled to improve REMPI spectra. This can be readily
accomplished,
as shown in Fig. 7, by expanding a portion of the product gas plumes 41
emanating from
the library sites 33 into vacuum chamber 42 through small orifices 43.
Portions of the
product gas directed through orifices 43 undergo adiabatic expansions forming
24
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
supersonic jets in vacuum chamber 42 thereby decreasing the gas temperature
resulting
in significant simplification of the REMPI spectra. In addition, as shown in
Fig. 7,
precooling thermal exchanger may be located upstream of orifices 43 to reduce
the
temperature of the product gases prior to passage through orifices 43. Gas
flow into a
vacuum chamber can also be pulsed to improve the pumping requirements. For an
ideal
gas with a heat capacity ratio y, that is y= c~/c", the temperature of the gas
is related to
pressure by the following relationship under adiabatic conditions: Tz=
T1(P1lPz}~''~~'
wherein T~, P1 and Tz, Pz are the initial and final temperatures and
pressures, respectively.
For example, for y = 1.4 and an initial temperature of 800 K and 760 Torr
pressure, the
temperature of the adiabatically cooled gas expanded into a vacuum at I0'3
Torr will be:
Tz = 800 (10-3/760)~l~a-iy.a = 16.7 K
This temperature is suitable for generation of an excellent REMPI spectra.
Castaldi, M.J. and Senkan, S.M., 1998, supra. Simultaneous product screening
of the
catalyst library can be achieved by photoionizing the products using laser
beams) 23,
followed by detection of photoelectrons or photoions using microelectrodes 27
placed
inside vacuum chamber 42 in close proximity to the expanding jet.
Fig. 8 schematically shows a flat plate solid state catalyst library
containing
seventy two test sites 21, arranged 8 rows wide by 9 rows axially which are
sufficiently
separated from one another to result in minimal intersite diffusion of product
gases,
within reactor 45. Contact of reactants with the catalytic test sites is
achieved by use of
reactant feed tubes 34, as described with reference to Fig. 3, which
effectively mask the
upstream catalyst sites. Each of the test sites in a row being screened has a
dedicated
microelectrode 27 for product gas detection, eight as shown in Fig. 8, for
screening by
row. Arranging the test sites in rows expedites screening in a row-by-row
fashion using
a single laser beam and provides simultaneous screening of eight sites. Any
row size can
be accommodated using this invention. However, any library pattern having
specific
addresses for individual test sites can be screened by moving the library with
a computer
controlled two-dimensional translation device. The smallest site size,
providing the
highest library density, is determined by the gas phase dispersion rate of
product gas
between test sites. Consequently, different products can allow the generation
and testing
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
of different library densities. In the row screening process, as exemplified
in Fig. 8, laser
beams 23 pass through window 39 of reactor 45 and through the product gases
above
the test sites 21, perpendicular to the reactant gas flow from reactant feed
tubes 34 and
passes through the product gas plumes of all of the sites in a row, as
indicated by the
dotted line, and exits reactor 45 to laser beam dump 46. Reactant feed tubes
34 are
supplied by reactor gas supply manifold 48. In Fig. 8, two lasers are
indicated, however,
any number of lasers may be used in a given application. Based upon the
numerical
design example given above, positioning of the laser beam about S mm above the
substrate surface should be adequate for the laser beam to intercept the
product plume
and generate photoions, if a product is formed. Product gas exits reactor 45
through gas
outlet 49. However, the laser beam may be placed anywhere in the product plume
to
maximize signal generation. It is apparent that if the test site is not
catalytic, no product
formation and therefore no photoionization will take place. Photoions and
photoelectrons generated are collected by the microelectrodes 27 positioned in
close
position above the laser beam. Based upon the above numerical design example,
microelectrodes can be positioned anywhere beyond 5 mm above the test site
surface and
close to the laser beam to maximize signal intensity. However, microelectrodes
can be
placed at different positions above the test sites to maximize signal
collection in
conjunction with the local fluid dynamics of the product plume. As noted
above, the
library substrate can also serve as the ground or cathode, or a microelectrode
can be
placed through a nonconductive substrate, if necessary, or microelectrodes can
include
both the anode and cathode as shown in Fig. 8. The microelectrodes are powered
from
DC power source 30 through a multichannel switch and the measured signal of
each
microelectrode fed to detector 31. After testing of a particular row, the
library can be
moved either upstream or downstream, using library translator 47, to position
the next
row of sites for catalytic screening.
Another embodiment of this invention to exemplify the row screening process is
shown in Fig. 9. The embodiment shown in Fig. 9 is similar to Fig. 8 except
that porous
catalyst libraries having porous test sites 21p are fed reactant gas from a
plenum beneath
them which is supplied reactant gas through reactant gas supply inlet 50. The
reactant
gas passes through porous test sites 21 p forming a plume above each test site
simultaneously as indicated by the arrows. The reactor can be rotated
180° around the
26
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
x-axis, if desired, to enhance product detection by altering the natural
convection
processes in the reactor vessel. As shown in Fig. 9, screening is done on a
row-by-row
in similar manner as described with respect to Fig. 8. Alternatively,
screening of all sites
simultaneously may be done by equipping each site with a dedicated
microelectrode and
providing the ionizing laser beam 23 to pass all sites simultaneously using
turning mirrors
26, as shown in the top view of Fig. 10. Optical fibers may also be used to
direct the
laser beam to all sites simultaneously. Signals from the microelectrodes are
then
detected and recorded by a dedicated detector for each site on catalyst
library 51 or by
use of a computerized multichannel switching system 65 to rapidly and
sequentially
detect the signal coming from each site. It is apparent that any catalyst
library size and
shape can be accommodated and operated in this simultaneous screening mode as
long as
each site is individually addressable.
Another embodiment of this invention is shown in Fig. 11 which schematically
shows a 16 by 16 or 256 site monolith structure 40 as described with respect
to Fig. 7
forming a solid state catalyst library. Any monolith cell density can be used.
Reactant
gases are provided through reactant gas supply inlet 50 to a manifold beneath
the library
and pass upwardly through the channels, passing over or through catalysts,
generating
product plumes which may be measured within the channels as shown in Fig. 6,
above
the exit from the channels as shown in Fig. 9, or following cooling by a
supersonic jet
into a vacuum chamber as shown in Fig. 7. Catalyst screening may be
accomplished
using row-by-row method as shown in Fig. I 1 or by screening all sites
simultaneously as
described with respect to Fig. 10.
Another embodiment of a.monolith supported catalyst library screening
structure
within a reactor is shown in Fig. 12, generally using the arrangement as
described with
respect to Fig. 6. As shown in Fig. 12, a separate catalyst library monolith
55 having 72
sites and a separate catalyst screening monolith 56 forms the catalyst
screening structure
within reactor 45. A dedicated microelectrode 27 is provided inside of each
monolith
channel. Upstream of each microelectrode 27, optical access to each channel is
provided
by laser access windows 39. Reactant gases are introduced by reactant gas flow
distributor and enter each of the individual library channels, as indicated by
the arrows,
to pass over the catalyst sites. Products are detected downstream inside the
screening
monolith 56. Lasers emanating from tuneable laser sources 24 and/or 25 are
directed to
27
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
each row of the screening monolith 56 via beam splitters 52 and through laser
windows
39 to pass through each of the channels in the row through the internal laser
windows as
shown. This arrangement provides simultaneous screening of all sites in the
library.
Different laser beams can be directed to different rows in the screening
monolith 56 to
screen for different products. This technique can also be applied for
screening other
library configurations. Fiber optic lines 53 can also be used to direct the
laser beam to
the library sites. If product cooling is desirable, this can be accomplished
by adiabatically
expanding the product gas plumes into a vacuum chamber through small orifices,
as
shown in Fig. 7.
In the above description of catalyst screening apparatus and techniques the
temperature has been the same at all catalyst sites, which would be
appropriate for
screening for new catalysts or to modify catalysts. It is possible, according
to this
invention, to construct catalyst libraries having individually temperature
controlled sites
wherein different sites would be maintained at different temperatures or their
temperatures could be programmed to follow a specified temperature-time
program.
Such differing temperatures generates information on the effects of reaction
temperatures
on catalyst activity and selectivity. Using micromachining, individually
temperature
controlled and programmable sites may be economically constructed, such as
done for
thermal inkjet printer heads. It is readily apparent that the amount of
insulation provided
by the substrate and the temperature programming demands influence the
intersite
spacing and the density of the catalyst libraries with temperature controlled
sites.
It is also possible to screen an entire catalyst library using a batch mode
operation. In the batch mode, the entire catalyst library is first isolated
from the reactant
gases by a physical mask. The test chamber is then purged and filled with
fresh reactant
gases. The chamber contents are allowed to reach thermal equilibrium which can
be
monitored by thermocouples placed within the test chamber. The physical mask
is then
removed exposing either a specified section or the entire catalyst library to
reactant
gases. Since there is no forced convection, diffusion and natural convection
are the
major modes of gas transport in the test chamber. The sites that are catalytic
then
generate reaction products which diffuse into the bulk gas phase generating a
product
concentration plume. For a constant concentration of the product, the
concentration
penetration depth, 8~(t), can be approximated by the relation:
28
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
8~(t) _ (l2Dt)'nwhere D is the diffusivity and t is the time. The
concentration
penetration depth must be kept less than the intersite spacing to prevent
overlapping of
concentration plumes from adjacent sites resulting in signai crossover. For a
flat plate
catalyst library, assuming 1 cm intersite spacing, 8~ = 1 and 0.1 cm2/sec for
gas
diffusivity, the REMPI measurements for the entire library must be completed
in about 1
second to avoid overlap of concentration boundary layers. Available fast
electronic
equipment can meet these requirements. Larger site dimensions and/or placing
physical
barriers between sites can significantly decrease intersite diffusion-mixing
rates, thereby
providing longer times for measurements. In the case of monolith structures,
physical
walls existing between the sites substantially decrease intersite diffusion,
thereby allowing
acquisition of data for longer periods of time by microelectrodes placed near
or inside the
channels for detection of photoions and/ or photoelectrons created by the
laser beam.
An advantage of the batch system is that it can be used to simultaneously
screen all sites
in the solid state catalyst library.
One embodiment of a homogeneous catalyst library which can be synthesized, as
described earlier, and screened according to this invention is shown in Fig.
13 wherein
catalyst solution 57 is maintained in container 58 and reactant gases are
bubbled through
the liquid. Gas dispersion through the liquid catalyst can be achieved in any
suitable
fashion as will be apparent to one skilled in the art, for example,
pressurised reactant
gases can be fed through reactant plenum 36 and forced through a controlled
porosity
distribution plate at the bottom of the sample site, as shown on the left in
Fig. 13.
Alternatively, reactant gases from reactant plenum 36 may be bubbled through a
capillary
sparger 60 at each sample site, as shown on the right in Fig. 13. Gaseous
products 22
formed leave the liquid catalyst solution, as indicated by the arrows in Fig.
13, and
product gas detection performed in any of the manners described earlier. The
minimum
diameter of container 58, which controls the library density, must be
established from
considerations of the surface tension and viscosity of catalyst solution 57
which influence
the extent of gas dispersion and liquid carryover.
Fig. 14 is a schematic showing of catalyst library screening using a
homogeneous
liquid catalyst library, as described with respect to Fig. 13, within reactor
45. REMPI
catalyst screening can be either on a.row by row basis, as illustrated in Fig.
14, or the
entire catalyst library can be screened simultaneously, using the method as
described with
29
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
respect to Fig. 10. The reactor system exemplified in Fig. 14 may also be used
to screen
solid catalyst powders which can be placed in the container, as will be
described in
further detail with respect to Fig. 15.
Solid particles may be incorporated into the liquid catalyst library to
achieve three
phase, gas-liquid-solid, operating conditions. The introduction of solid
particles 60 to
the liquid in container 58 enhances gas dispersion, forms smaller gas bubbles
61 to
provide better gas-liquid contact and improves reactant conversion, thereby
increasing
the speed of library screening, is shown in the left hand portion of Fig. 1 S.
The bed can
also be fluidized, partially or fully, under the screening conditions. Product
gases 22,
indicated by arrows, emanate from container 58 and may be analyzed by any of
the
REMPI methods previously described. The solid particles used may be catalytic,
thereby
providing the opportunity of screening multi-phase catalytic reactions.
Homogeneous
liquid catalysts may also be placed into porous particles, for example to
immobilize
proteins or molten salt catalysts, in the systems as shown in Fig. 15. Solid
catalytic
particles 62 may be introduced into container 58, without liquid, to achieve
gas-solid
operating conditions, as shown in the right hand portion of Fig. 15. Catalyst
powders,
prepared in a number of different manners, can be placed within the container
shown in
Fig. 15 to create a micro packed bed reactor library. Reactant gases may be
introduced
to the packed bed reactors through plenum 36 and products formed detected
using the
REMPI microelectrode systems previously described.
Fig. 16 is a schematic showing of another catalyst screening method using
catalyst particles in a monolithic library. Catalyst particles or powders 62,
prepared in a
number of different ways, can be placed into the cells of monolithic structure
40. The
reactant gases are then passed through the packed bed of catalyst particles 62
and are
discharged through a small channel/orifice 43 into vacuum chamber 42. The
product jets
then undergo expansion cooling and are subjected to laser beam 23 for the
generation of
photoions and photoelectrons. The photoions or photoelectrons generated are
then
detected by microelectrodes 27, as described above.
The magnitude of the REMPI signals produced by the photoionization of product
species will be proportional to their concentration. In addition, the
generated signals are
also influenced by the operational parameters, such as, the power of the W
laser used,
the DC bias voltage applied to collect the photoions/photoelectrons, the
separation
CA 02347697 2001-04-24
WO 00129844 PCT/GB99/03767
distance of the anode and cathode and the position of the microelectrode
relative to the
laser beam. Once optimized for the particular system to be used for catalyst
library
screening, the operational variables can be fixed so that the measured REMPI
signals can
be directly attributed to produce concentrations generated by the catalyst
sites.
S Consequently, in addition to the qualitative, active versus inactive,
screening of catalyst
libraries, the REMPI microelectrode technique of this invention can be used to
quantitatively rank the activities and selectivities of catalysts.
Catalytically more active
sites will produce higher concentration of products in the product plume and
thereby
generate larger REMPI signals, and likewise, less active catalyst sites will
generate lower
concentrations of products and thereby lower REMPI signals. In catalyst
library
quantitative screening, gas mixtures containing known concentrations of
product gases
are first passed sequentially over the library under conditions at which no
reactions take
place and the microelectrode responses noted. Using microelectrode responses
to
known product concentrations, calibration of each site and microelectrode may
be
achieved. These calibration functions are then used to determine the
quantitative
concentrations of products formed during the active catalyst screening
process. If the
catalyst loading is dii~erent at dii~erent library sites, this also must be
accounted for in
ranking ofthe catalytic activity of the sites. Alternatively, internal
standards can be
added to the reactant feed stream during the screening process to expedite the
quantification of the activities and selectivities of catalyst sites.
The catalyst screening techniques disclosed can be utilized to obtain a
greater
spectrum of objectives. Two or more laser beam energies can be used
sequentially to
monitor two or more reaction products in a product plume, which is important
to
establish catalyst selectivity and to discover multifunctional catalysts. For
example, the
development of catalysts which not only maximizes the formation of specific
products
but also minimizes the formation of by-products or pollutants is an
increasingly important
objective in environmentally conscious manufacturing. In the practice of this
invention, a
series of laser pulses, each pulse specifically photoionizing a selected
molecule, can be
used to sequentially monitor different products. Since laser photoionization
and product
detection are fast processes, having time scales in microseconds, rapid
screening of large
potential catalyst libraries for multifunctional catalytic activity can be
accomplished even
with the sequential detection of a large number of species.
31
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
In some applications, the products formed by the catalytic reaction may be in
the
liquid or solid state, for example, reactions of high molecular biomolecules
catalysed by
enzymes, thus the direct application of REMPI is not suitable to screen
catalytic activity
and selectivity. The REMPI method, however, can be applied if the reaction
products
are first gasified. This can be accomplished by using a pulsed ablation laser,
such as a
pulsed C02 or excimer laser, to rapidly gasify product molecules from a liquid
or solid
surface. One embodiment using an ablation laser is shown in Fig. 17 wherein
ablation
laser source 63 generates ablation laser beam 64 to rapidly gasify product
molecules
from the surface of liquid catalyst solution 57 into gaseous product plume 22
which may
be intercepted by ionization laser beam 23 and produced photoions and
photoelectrons
detected by any of the microelectrode methods described above.
It is evident from the above disclosure, that it is also possible to monitor
reaction
intermediates as well as reaction products using the REMPI microelectrode
methods of
this invention. The ability to monitor reaction intermediates, as well as
products, greatly
1 S enhances the range of applicability of the methods of this invention. In
addition, because
measurements according to this invention can be undertaken in real time
without any
delay, fast transient processes can be monitored. This capability then leads
to better
understanding of the catalyst fianction and thus aids in the development of
new and
improved catalysts.
The following specific example is set forth in detail to specifically
demonstrate
this invention and should not be taken to limit the invention in any way.
The catalyst screening method of this invention was used in the catalytic
dehydrogenation of cyclohexane into benzene according to the reaction C6H~2 --
~ C6Hb +
3H2. This is a well established reaction which is catalyzed by transition and
precious
metals in the temperature range of 250° to 350°C. Rebhan, D.M.
and Haensel, V., "A
Kinetic and Mechanistic Study of Cyclahexane Disproportionation: An Example of
Irreversible Hydrogen Transfer', J. Catalysis, 111, 397, 1988.
Supported Pt and Pd catalysts, 0.5% and 1.0% Pt and Pd on activated carbon,
were obtained from Precious Metals Corp. These catalysts, as well as several
inert
carrier materials, silica and alumina, were then incorporated into one row in
a library
substrate in 5 mm by S mm cells similar to Fig. 5. The addresses for the
catalysts and
inert carrier materials were:
32
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
Site No. 1 2 3 4 5 6 7 8
Material Inert 0.5%Pt Inert 1.0%Pd Inert Inert 1.0%Pt 0.5%Pd
The catalyst library was then placed into a reactor and heated to 300°C
in the
presence of an argon gas flow. Following establishment of the steady state
operating
S temperature, which was determined by thermocouples inside the reactor, a
cyclohexane
reactant stream was introduced. The reactant stream composition was 13%
cyclohexane
in argon gas which was prepared by bubbling argon gas through cyclohexane
liquid at
about 25°C by using a sparger.
The library screening process demands the unambiguous detection of benzene in
a cyclohexane, hydrogen and argon mix. A suitable UV laser wave length for
selectively
producing benzene REMPI ions was identified in separate tests using a laser
photoionization time of flight mass spectrometer, TOF-MS. Gas pulses of
cyclohexane
and benzene, each at a concentration of about SOOppm in argon, were expanded
into the
vacuum chamber of the TOF-MS using a pulsed valve and the resulting
jet/molecular
beam was crossed by a pulsed UV laser beam in the 258-262 nm range to generate
their
photoionization and mass spectra. The UV laser had about 100pJ/pulse energy
and was
obtained from the dye laser using Coumarin 500 dye. These measurements led to
the
conclusion that the REMPI ions produced by the 258-262 nm UV laser were
exclusively
due to photoionization of benzene, mass 78, with no photoions detected at
masses 84 for
cyclohexane or 40 for argon or 2 for hydrogen. No peaks other than the benzene
parent
at mass 78 were detected. Fig. 18 shows the REMPI spectrum of benzene and
cyclohexane as determined by the TOF-MS technique. It is evident from Fig. 18
that
benzene exhibits a major REMPI peak starting at 259.7 nm, where there is no
contribution from cyclohexane.
REMPI spectra of benzene and cyclohexane were also determined at 1 atm and
ambient temperature using the microelectrode process. Cyclohexane and benzene
in
argon carrier gas were photoionized by passage of a pulsed W laser beam in the
258-
262 nm range within I-2 mm of the probe tip. A DC bias of +500 V from a power
source was applied to the anode to collect the photoelectrons. The resulting
REMPI
spectra are shown in Fig. 19 and are similar to the spectra obtained by the
TOF-MS
33
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
shown in Fig. 18, with expected spectral broadening observed in the ambient
temperature
and 1 atm. pressure conditions. This shows that use of the 259.7 laser results
in the
exclusive and effcient production of benzene REMPI ions in the presence of
cyclohexane, argon and hydrogen in the reactor system.
The reactor system shown in Fig. 9 was used passing cyclohexane in argon
carrier gas through the eight library sites in a row, as identified above. The
259.7 nm
laser beam was passed through the product plume from the library sites and the
benzene
REMPI signals detected in the vicinity of each of the eight sites are shown in
Fig. 20.
These measurements correspond to data acquired by one laser shot and the
signals
exhibited fast rise and decay time, in the order of microseconds. As evident
from Fig.
20, microelectrodes located at sites 2, 4, 7 and 8 picked up appreciable
benzene signals,
consistent with the presence of Pt and Pd catalysts at these sites. While some
REMPI
signals were also detected at sites l, 3, 5 and 6, they were significantly
lower, consistent
with the absence of catalysts at these sites. Evidently some benzene was
present in the
reactor bulk gas due to low gas flow rates and recirculation patterns present
in the
reactor, both of which reduce the rapid removal of reaction products from the
reactor. A
smaller reactor chamber, use of monolithic structures or other library designs
would
reduce this problem. Nevertheless, Fig. 19 shows that the method of this
invention
rapidly and clearly distinguished between active and inactive sites in the
library. The
reactor exhaust gases were also analyzed by the TOF-MS using the 259.7 nm
laser beam
during screening to ascertain whether species other than benzene could have
contributed
to the measured microelectrode signals. No photoions other than those with
mass 78
were detected.
Based upon the magnitude of the REMPI signals measured, as shown in Fig. 20,
the relative activities of the catalytic sites appear to be 7>2>4>8. These
results are
consistent with the relative loadings of the Pd and Pt commercial catalysts at
these sites,
and also suggest that Pt is a more active cyclohexane dehydrogenation catalyst
than Pd.
These findings are in agreement with results using conventional catalytic
reactor systems.
Rehbon, D.M. and Haensel, V., 1988, supra and Ahmed, K. and Chowdhury, H.M.,
"Dehydration of Cyclohexane and Cyclohexene over Supported Nickel and Platinum
Catalysts", Chem. Eng. J., 50, 165, 1992.
It should be recognized that the conditions specified in the above description
and
34
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
example are meant to illustrate the application of the catalyst screening
technique of this
invention. One skilled in the art can infer from this description and example
that the
method of this invention can be used to screen any catalyst for any reaction.
The
reaction conditions can be broadly varied without change in the screening
method. For
S example, the reaction temperature can easily be varied from room
temperature, such as
25°C., to higher temperatures, such as 1000°C. Similarly, the
pressure can be varied
from vacuum, such as 10'~ Torr, to high pressures, such as 500 atmospheres.
The
screening process can easily accomodate a wide range of reactant feed
concentrations
from pure components, 100%, to very dilute streams, such as a few hundred
parts per
million, 100 ppm.
Combinatorial catalyst libraries can also be generated by machining miniature
reactors using integrated circuit manufacturing steps such as thin film
deposition,
lithography, etching, plasma processing and the tike. This approach has been
used
recently to make a reactor on a chip for the catalytic oxidation of ammonia,
as described
in Srinivasan, R., Hsing, LM., Berger, P.E., Jensen, K.F., Firebaugh, S.L.,
Schmidt,
M.A., Harold, M.P., Lerou, J.J. and Ryley, J.F., "Micromachined Reactor for
Catalytic
Partial Oxidation Reactions", AIChE Journal, 43, 3059-3069, 1997. Unlike
monolithic
or honeycomb structures which are passive, the micromachined reactors can also
incorporate flow and temperature sensors, heating elements and actuators for
the control
of operating conditions. In this invention, a large number of microreactors
are prepared
in parallel using any suitable integrated circuit manufacturing sequence. Each
microreactor system includes passages for reactant feed, catalytic reaction,
product exit,
and radiation access. These passages can be machined by either wet or dry
etching of an
inert wafer substrate, such as silica or alumina, or materials which are
coated by such
inert films, for example, metals coated by inert materials. The exit passage
of each
reaction zone should be large enough to accommodate a microelectrode for
detection of
product REMPI ions. Sensing, flow and temperature controllers can also be
embedded
into the individual reactor sites on the wafer. In addition, electricai
circuitry can be
embedded to electrochemically control the catalytic reactions. Different
catalytic
materials can be deposited into different reactor passages of the library by a
variety of
techniques, such as, for example, sputtering, laser ablation, thermal or
plasma enhanced
chemical vapor deposition, and the like, with the use of masks. Alternatively,
catalysts
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
can be deposited into the reactor passages using solution techniques with the
aid of
micro jet or micro-drop dispensers. These dispensers can also be used to
deposit slurnes
containing catalyst particles. When using solution techniques, the reactor
passages can
be modified in the reaction zone to contain the necessary amounts of liquid
and/or slurry
catalyst precursors. This can be accomplished, for example, by machining a
reservoir in
a central region of the reactor passage for collection of liquid or slurry
catalyst precursor
mixtures. These reservoirs may be of any shape and can also have internal
battles,
actuators and sensors to better control the preparation of catalysts and
operation of the
reactors during the screening process. The reservoirs can also be placed at
different
locations along the microreactors to control pressure drop, reactant preheat
and product
quench conditions. Liquid and/or slurry mixtures of catalyst precursors may be
introduced into the reservoirs using micro jet or micro-drop dispensers and
robotics.
Following the addition of the liquids, agitation may be induced, for example,
by
mechanical vibration, micro-activators or sonication, to assure mixing of the
liquid or
slurry mixtures. After dispensing the catalyst precursors, the resulting
mixtures are
thermally and chemically treated for the formation of catalysts. These
treatment
processes may include drying, calcining, oxidation, reduction and activation.
Figs. 21 and 22 are simplified schematic showings of bases for single
microreactor systems according to this invention. Fig. 21 shows a microreactor
suitable
for thin film or solid particle catalyst deposition processes and Fig. 22
shows a
microreactor additionally suitable for solution based catalyst deposition
processes. In the
figures, inert microreactor body 70 has reactant feed passage 71 leading to a
catalyst
zone, shown in Fig. 21 as zone 72 and in Fig. 22 as enlarged reservoir
catalyst zone 73.
As shown in Fig. 22, baffle structure 74 may be located in reservoir 73. Such
baffle
structures may have a number of effects, such as, providing additional exposed
surface
area for the catalyst and for inducing mixing to benefit some reactions.
Products exit the
reaction volume through exit passage 75. Reactant feed and product flow are
shown by
the arrows. Activating radiation passages 76, having optical access windows
for
isolation of exit passage 75, are provided to direct passage of activating
radiation beam
77 through the product stream passing through exit passage 75. Fig. 21 shows
external
microeiectrode 78 and Fig. 22 shows internal microelectrode positioned in exit
passage
75 in proximity to the activating radiation beam 77 to collect photoelectrons
or
36
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
photoions for detection, as previously described. Internal microelectrode 84
is attached
to microreactor body 70, such as embedded to the bottom, side or top walls of
the
product exit passage, and is thus an integral part of the microreactor body.
These
internal microelectrodes may be flush with the product exit passage walls or
may
protrude from them. The internal microelectrodes are provided with suitable
wiring for
powering the microelectrodes and for passage of detected signals to a
detection
measurement device. These wirings and connections are embedded in the
microreactor
body during fabrication using established microelectronics manufacturing
techniques.
Fig. 23, wherein the numerals referred to above have the same meaning,
schematically shows an array of microreactors in a single inert microreactor
body 70.
Any number of microreactors may be present in the array, depending upon the
size of the
microreactors and the physical characteristics of the substrate wafer. Each
microreactor
72 can be of any size, however, reactor channels in the order of about 0.1 to
2
millimeters wide are most suitable for fabrication and subsequent screening
processes.
Reactant plenum 79 is in fluid communication with each reactant feed
passageway 72 to
distribute reactants to each microreactor. Reactant plenum 79 is sufficiently
large to
insure the establishment of similar fluid flow rates through each
microreactor, provided
that the pressure drop characteristics of the microreactors are similar.
Alternatively, flow
sensors and actuators may be fabricated in each microreactor to independently
control
fluid flow through each of the microreactors. A different catalyst may be
placed in each
microreactor using, for example, any of the techniques described. The physical
forms of
these catalysts can be films, as indicated by numeral 86, or powders, as
indicated by
numeral 85. Fabrication of a microreactor array from a single base wafer
insures good
alignment of the activating radiation passages 76 and microelectrodes 84,
thereby
expediting the screening process. Internal electrodes 84 make possible
internal wiring for
powering the microelectrodes and for passage of detection signals to a
detection
measurement device. Alternatively, separate and different electrodes, one for
the anode
and one for the cathode, can be embedded to different walls of the reactor for
powering
and signal detection. Suitable connectors may be located on the exterior of
the array for
easy connection of the entire array to a power source and a detection
measurement
device through selective switching. Reactant feed and product flows are shown
by the
arrows.
37
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
Following fabrication of the microreactor base layer, an inert cover wafer 80
is
bonded to inert microreactor base 70 to cover the microreactor array, as shown
in Fig.
24, to isolate each microreactor system while allowing the flow of reactant in
and the
flow of products out of the microreactor array. Fig. 24 shows internal
microelectrodes
S 87 attached to or embedded in cover wafer 80 in similar manner as described
with
respect to internal microelectrodes 84 attached to microreactor body 70, as
disclosed
above. Internal wiring 88 leads from each microelectrode 87 to external
connector 89 for
powering each microelectrode and for passage of detection signals from each
microelectrode to a detection device. Alternatively, separate electrodes can
be
embedded to the base 70 for signal detection and/or for power supply. Heating
elements
may be embedded in thermal conducting microreactor body 70 between
microreactor
chambers 72 and/or in thermal conducting cover wafer 80 and/or in thermal
conducting
sheets between stacked reactor arrays to provide desired temperature control
to the
microreactors and/or reactant feed channels. As shown in Fig. 2S, individual
flat
1 S microreactor arrays, as shown in Fig. 24, may be stacked vertically to
obtain three
dimensional structures of a plurality of flat microreactor arrays, thereby
providing rapid
analysis of a large number of samples in the manner similar to that shown in
Fig. 12. The
microreactor arrays may have any suitable fasteners for maintaining adjacent
arrays in
fixed relationship with each other. The microelectrodes are powered by DC
power
source and the signal from each microelectrode fed through a mufti-channel
selector to
measurement device.
Fig. 26 shows a microreactor array 91, as shown in Fig. 24, may be placed in
microreactor array frame 92 for easy handling and connection for catalyst
screening.
The microreactor array fits into an opening in the frame as indicated by the
reversible
2S arrow. Reactant feed is provided through the frame to the reactant feed
manifold of the
microreactor array, as indicated by the arrows, and product exits through the
frame, as
indicated by the arrows. Radiation passages 93 are provided through frame 92
for entry
and exit of radiation beam 77 aligned to pass through radiation passages 76 in
microreactor body 70, as described above. The frame also has internal wiring
94 for
connection at one end to internal wiring 88 of the microreactor array and at
the opposite
end to a power source and a detection measurement device. The internal wiring
of a
plurality of microreactor array frames may connect through a single connector
to
38
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
external wiring. The frames may also have reactant feed manifolds arranged so
that a
single feed supply can provide reactant to a plurality of microreactor array -
frame
assemblies. The frames may also provide temperature control for the
microreactor array
through heating elements built into the frames. Microreactor array - frames
may have
any suitable means for connection of adjacent microreactor array frame
assemblies.
A plurality of microreactor - frame assemblies may be joined in a vertical
fashion
similar to that shown in Fig. 25. In another embodiment, shown in Fig. 27,
microreactor
array - frame assemblies 95 may be joined horizontally in side-by-side
relation.
Alignment of radiation passages 93 makes it possible to use one radiation beam
77 in the
evaluation of large catalyst libraries.
Screening is accomplished by passing a known amount of reactant gases through
the microreactor array in contact with the potential catalysts forming
reaction products
which are activated by passing a suitable tunable radiation beam through
activating
radiation passages 76, having access windows providing fluid isolation, to
form product
REMPI ions in the product exit passages 75. These product REMPI ions are
detected
by the microelectrodes within the exit passages and.measured in manners
described
above. During screening, the microreactor arrays may be placed in a furnace
for
temperature control of the entire array or the temperature of each
microreactor may be
independently controlled using sensors and heating elements built into the
microreactors
during the microreactor fabrication process. Alternatively, temperature
control can be
provided by the frame.
Figures 28A and 28B summarize another example of combinatorial catalyst
library preparation and screening method using a different microreactor array
and
microdrop/microjet technology according to this invention. Step 1 shows
preparation of
the catalyst library inert substrate using a plug to form desired passageways
and to retain
liquid during solution deposition. Step 2 shows catalyst precursor solution
deposition
into reservoirs of catalyst reaction zones. Step 3 shows drying and calcining
of the
catalyst by methods well known in the art. Step 4 shows opening of the product
exit
passages by removal of the plugs used to form the passages. Step S shows
formation
and/or activation of the catalyst by passage of a suitable gas through the
microreactor
array. Step 6 shows screening of the catalysts within the array of
microreactors by
passing reactant gas(s) in contact with the catalyst in each microreactor,
passing a
39
CA 02347697 2001-04-24
WO 00/29844 PCT/GB99/03767
radiation beam of an energy level to promote formation of specified ions
through each
reaction product stream, and detecting the formed fans or electrons by
microelectrode
collection in proximity to the activating radiation beam.
While in the foregoing specification this invention has been described in
relation
to certain preferred embodiments thereof, and many details have been set forth
for
purpose of illustration it will be apparent to those skilled in the art that
the invention is
susceptible to additional embodiments and that certain of the details
described herein can
be varied considerably without departing from the basic principles of the
invention.
15
25
40