Note: Descriptions are shown in the official language in which they were submitted.
CA 02347858 2001-04-24
WO 01/27353 PCT/US00/27936
-1-
CATHODE COLLECTOR BAR WITH SPACER
FOR IMPROVED HEAT BALANCE
This invention relates to electrolytic cells. In one aspect, this
invention relates to cathode collector bars of electrolytic reduction smelting
cells
used in the production of aluminum.
Aluminum is produced by an electrolytic reduction of alumina in an
electrolyte. The aluminum produced commercially by the electrolytic reduction
of
alumina is referred to as primary aluminum.
Electrolysis involves an electrochemical oxidation-reduction
associated with the decomposition of a compound. An electrical current passes
between two electrodes and through molten Na3AlF6 cryolite bath containing
dissolved alumina. Cryolite electrolyte is composed of a molten Na3A1F6
cryolite
bath containing alumina and other materials, e.g., such as fluorspar,
dissolved in the
electrolyte. A metallic constituent of the compound is reduced together with a
correspondent oxidation reaction.
Electrical current is passed between the electrodes from an anode to a
cathode to provide electrons at a requisite electromotive force to reduce the
metallic
constituent which usually is the desired electrolytic product, such as in the
electrolytic smelting of aluminum. The electrical energy expended to produce
the
desired reaction depends on the nature of the compound and the composition of
the
electrolyte.
Hall-Heroult aluminum reduction cells are operated at low voltages
(e.g. 4-5 volts) and high electrical currents (e.g. 70,000-325,000 amps). The
high
electrical current enters the reduction cell through the anode structure and
then
passes through the cryolite bath, through a molten aluminum metal pad, and
then
enters a carbon cathode block. The electrical current is carried out of the
cell by
cathode collector bars.
As the electrolyte is traversed by electric current, alumina is reduced
electrolytically to aluminum at the cathode, and carbon is oxidized largely to
carbon
dioxide at the anode. The aluminum, thus produced, accumulates at the molten
aluminum pad and is tapped off periodically. Commercial aluminum reduction
cells
are operated by maintaining a minimum depth of liquid aluminum in the cell,
the
CA 02347858 2001-04-24
WO 01/27353 PCT/US00/27936
-2-
surface of which serves as the actual cathode. The minimum aluminum depth is
about 2 inches and may be 20 inches.
The alumina-cryolite bath is maintained on top of the molten
aluminum metal pad at a set depth. The current passes through the cryolite
bath at
a voltage loss directly proportional to the length of the current path, i.e.,
the
interpolar distance gap between the anode and molten aluminum pad. A typical
voltage loss is about 1 volt per inch. Any increase of the anode to cathode
spacing
restricts the maximum power efficiency and limits the efficiency of the
electrolytic
cell operation.
Much of the voltage drop through an electrolytic cell occurs in the
electrolyte and is attributable to electrical resistance of the electrolyte,
or electrolytic
bath, across the anode-cathode distance. The bath electrical resistance or
voltage
drop in conventional Hall-Heroult cells for the electrolytic reduction of
alumina
dissolved in a molten cryolite bath includes a decomposition potential, i.e.,
energy
used in producing aluminum, and an additional voltage attributable to heat
energy
generated in the inter-electrode spacing by the bath resistance. This latter
heat
energy makes up 35 to 45 percent of the total voltage drop across the cell,
and in
comparative measure, as much as twice the voltage drop attributable to
decomposition potential.
An adverse result from reducing anode-cathode distance is a
significant reduction in current efficiency of the cell when the metal
produced by
electrolysis at the cathode is oxidized by contact with the anode product. For
example, in the electrolysis of alumina dissolved in cryolite, aluminum metal
produced at the cathode can be oxidized readily back to alumina or aluminum
salt
by a close proximity to the anodically produced carbon oxide. A reduction in
the
anode-cathode separation distance provides more contact between anode product
and
cathode product and significantly accelerates the reoxidation or "back
reaction" of
reduced metal, thereby decreasing current efficiency.
The high amperage electrical current passing through the electrolytic
cell produces powerful magnetic fields that induce circulation in the molten
aluminum pad leading to problems such as reduced electrical efficiency and
"back
reaction" of the molten aluminum with the electrolyte. The magnetic fields
also
CA 02347858 2001-04-24
WO 01/27353 PCT/US00/27936
-3-
vary the depths in unequal distance between the molten aluminum pad and the
anode. The motion of the metal pad increases, sometimes violently stirring the
molten pad and generating vortices, and causing localized electrical shorting.
Metal pad depth variations restrict the reduction of the anode to
cathode gap and produce a loss in current efficiency. Power is lost to the
electrolyte interposed between the anode and cathode blocks. Movement of the
molten aluminum metal pad also contributes to uneven wear on the carbon
cathode
blocks and can accelerate cell failure.
Metal pad turbulence also increases the "back reaction," or
reoxidation, of cathodic products, thereby lowering cell efficiency. Metal pad
turbulence accelerates distortion and degradation of the cathode bottom liner
through
attrition and penetration of the cryolite.
In the conventional cathode today, steel cathode collector bars extend
from the external bus bars through each side of the electrolytic cell into the
carbon
cathode blocks. The steel cathode collector bars are attached to the cathode
blocks
with cast iron, carbon glue, or rammed carbonaceous paste to facilitate
electrical
contact between the carbon cathode blocks and the steel cathode collector
bars.
The flow of electrical current through the aluminum pad and the
carbon cathode follows the path of least resistance. The electrical resistance
in a
conventional cathode collector bar is proportional to the length of the
current path
from the point the electric current enters the cathode collector bar to the
nearest
external bus. The lower resistance of the current path starting at points on
the
cathode collector bar closer to the external bus causes the flow of current
through
the molten aluminum pad and carbon cathode blocks to be skewed in that
direction.
The horizontal components of the flow of electric current interact with the
vertical
component of the magnetic field, adversely affecting efficient cell operation.
Existing Hall-Heroult cell cathode collector bar technology is limited
to rolled or cast mild steel sections. The high temperature and aggressive
chemical
nature of the electrolyte combine to create a harsh operating environment. The
high
melting point and low cost of steel offset its relatively poor electrical
conductivity.
In comparison, potential metallic alternatives such as copper or silver have
high
electrical conductivity but low melting points and high cost. Copper is used
in the
CA 02347858 2001-04-24
WO 01/27353 PCT/US00/27936
-4-
apparatus and process of the present invention because it provides a preferred
combination of electrical conductivity, melting point, and cost. Other high
conductivity materials could be used based on their combinations of electrical
conductivity, melting point, and cost relative to the aluminum smelting
process.
The electrical conductivity of steel is so poor relative to the
aluminum metal pad that the outer third of the collector bar, nearest the side
of the
pot, carries the majority of the load, thereby creating a very uneven cathode
current
distribution within each cathode block. Because of the chemical properties,
physical
properties, and, in particular, the electrical properties of conventional
anthracite
cathode blocks, the poor electrical conductivity of steel had not presented a
severe
process limitation until recently.
Conventional cathodes contained either 100% Gas Calcined
Anthracite (GCA) or 100% Electrically Calcined Anthracite (ECA). These cathode
blocks had poor thermal shock resistance. These cathode blocks swelled badly
under electrolysis conditions, i.e., under the influence of cathodic current,
reduced
sodium, and dissolved aluminum. These cathode blocks had poor electrical
conductivity (relative to graphite). In their favor, these cathode blocks had
low
erosion or wear rates (relative to graphite).
To overcome the shortcomings of 100% anthracite cathodes, cathode
manufacturers added an increasing proportion of graphite to the raw cathode
block
mix. A minimum of 30% graphite seems to be sufficient to avoid thermal shock
cracking and to provide reasonable electrical properties and sodium resistance
in
most instances. Further additions up to 100% graphite aggregate or 100% coke
aggregate graphitized at 2,000-3,000 C provide preferred operating and
productivity
conditions.
As the graphite content or degree of graphitization increases, the rate
increases at which the cathode blocks erode or are worn away.
In pursuit of economies of scale, aluminum smelting pots have
increased in size as the operating amperage has increased. As the operating
amperage has been increased, the percentage of graphite in cathodes has
increased to
take advantage of improved electrical properties and maximize production
rates. In
many cases, this has resulted in a move to graphitized cathode blocks.
CA 02347858 2005-06-09
50989-9
The operation of the pot is most typically
terminated when the aluminum metal is contaminated by
contact with the steel collector bars. This can happen when
the cathode to seam mix joints leak, when the cathode blocks
5 crack or break because of thermal or chemical effects or the
combined thermochemical effects, or when erosion of the top
surface of the block exposes the collector bar. In the
application of higher graphite and graphitized cathode
blocks, the dominant failure mode is due to highly localized
erosion of the cathode surface, eventually exposing the
collector bar to the aluminum metal.
In a number of pot designs, higher peak erosion
rates have been observed for these higher graphite content
blocks than for 30% graphite/ECA blocks or 100% ECA blocks.
Operating performance is therefore traded for operating
life.
There is a link between the rapid wear rate, the
location of the area of maximum wear, and the non-uniformity
of the cathode current distribution. The higher graphite
content and graphitized cathodes are more electrically
conductive and as a result have a much more non-uniform
cathode current distribution pattern and hence higher wear
rate.
Accordingly, there is a need to develop and
provide a more even cathode current distribution so that the
cathode wear rate will be decreased, the pot life will be
increased, and the operating benefits of the higher graphite
and graphitized cathode blocks can be realized.
Therefore, embodiments of the present invention
provide an electrolytic reduction cell apparatus and
CA 02347858 2005-06-09
50989-9
5a
method utilizing a novel cathode collector bar, including a
solid, ferrous metal spacer for maintaining a controlled
heat balance in the pot.
These and other objects of the present invention
will become more apparent from reference to the following
detailed description of our invention.
In one aspect of the present invention, there is
provided an electrolytic reduction cell for the production
of aluminum, comprising: a cell having a first cell wall, a
second cell wall opposite said first cell wall, and a cell
center between said first cell wall and said second cell
wall; a first external bus bar external to said first cell
wall; at least one anode; a carbonaceous cathode block
positioned below said anode; a ferrous cathode collector bar
positioned in electrically conductive contact with said
cathode block, extending from said first cell wall to at
least toward said cell center, and electrically connected to
said first external bus bar; and a copper insert inside said
cathode collector bar, said copper insert having a first
portion spaced apart from an external end of said cathode
collector bar toward said cell center and terminating at a
first interior end between said first cell wall and said
cell center.
In a second aspect of the present invention, there
is provided a method of producing aluminum in an
electrolytic reduction cell, comprising: providing a cell
having a first cell wall, an opposite second cell wall, and
a cell center between said first cell wall and said second
cell wall; providing a first external bus bar external to
said first cell wall; providing at least one anode;
CA 02347858 2005-06-09
50989-9
5b
providing a carbonaceous cathode block positioned below said
anode; providing a ferrous cathode collector bar having a
longitudinal axis positioned in electrically conductive
contact with said cathode block and extending from said
first cell wall to at least near to said cell center and
electrically connected to said first external bus bar; said
cathode collector bar having a copper insert, said copper
insert having a first portion spaced apart from an external
end of said cathode collector bar toward first cell wall and
terminating at a first interior end between said first cell
wall and said cell center; and passing an electric current
between said anode and said cathode block, said copper
insert providing a more uniform cathode current
distribution.
In a third aspect of the present invention, there
is provided an electrolytic reduction cell for the
production of aluminum, comprising: a cell having a first
cell wall, a second cell wall opposite said first cell wall,
and a cell center between said first cell wall and said
second cell wall; a first external bus bar external to said
first cell wall; at least one anode; a carbonaceous cathode
block positioned below said anode; a ferrous cathode
collector bar, having a top side and a bottom side,
positioned in electrically conductive contact with said
cathode block, extending from said first cell wall to at
least toward said cell center, and electrically connected to
said first external bus bar; a copper insert inside said
cathode collector bar, said copper insert having a first
portion spaced apart from an external end of said cathode
collector bar toward said cell center and terminating at a
CA 02347858 2006-05-08
50989-9
5c
first interior end between said first cell wall and said
cell center, wherein said copper insert cross-section is
between 0.042 to 0.125 times the cross-sectional area of
said cathode collector bar; a melting allowance slot having
sufficient volume to accept an increased copper volume
associated with melting of said copper insert; a thermal
expansion allowance in said collector bar, said thermal
expansion allowance having a dimension of 0.25-0.97 inches
(0.635-2.464 cm) or 0.37-1.44% more than a length dimension
of said copper insert; and a top plate welded to said top
side to contain said copper insert.
In accordance with another aspect of the present
invention, there is provided an apparatus and method for
production of aluminum. The apparatus of the invention
comprises an electrolytic cell for reducing alumina
dissolved in a molten salt bath to aluminum metal. An
electric current passes between an anode and a cathode
through the molten bath, producing aluminum metal adjacent
the cathode.
The electrolytic cell has cell walls including a
first cell wall and a
CA 02347858 2001-04-24
WO 01/27353 PCT/US00/27936
-6-
second cell wall, an anode, a carbonaceous cathode block separated from the
anode,
a bus bar external to the first cell wall, and a collector bar connecting the
bus bar
with the cathode block. The cathode block preferably defines a slot in which
the
collector bar is seated. The cell walls define a chamber containing a molten
salt
bath.
The collector bar includes a ferrous metal body and a copper insert.
As used herein, the term "ferrous metal" refers to iron and steel, including
mild
steel, low carbon steel, and stainless steel. The term "copper" includes
alloys of
copper with various other metals including silver. For practice of the present
invention we prefer relatively pure forms of copper containing at least 99
wt.%
copper because of their excellent electrical conductivity.
The collector bar has a ferrous metal body comprising a solid, ferrous
metal spacer having an external end portion connected with the bus bar and an
internal end portion spaced inwardly of the first cell wall. The spacer
improves
heat balance in the cell by preventing excessive heat transfer between the
copper
insert and the bus bar. The ferrous metal body also includes a ferrous metal
sheath
integral with the spacer and defining a cavity containing the copper insert.
The
cavity extends between an external end adjacent the internal end portion of
the
spacer, and an internal opening. The cavity and the copper insert may be
polygonal
or circular in transverse cross-section. We prefer a cylindrical copper insert
inside a
cavity having a circular transverse cross-section.
The slot in the cathode block preferably contains means for joining
the collector bar to the cathode block, preferably an electrically conductive
material.
This material may be cast iron, carbonaceous glue or rammed carbonaceous paste
and is preferably cast iron.
The cathode assembly of the present invention is useful for producing
aluminum by electrolysis. The cathode assembly is spaced downwardly of an
anode
in a chamber containing a molten salt bath. An electric current passes from
the
anode to the cathode assembly, reducing alumina dissolved in the molten salt
bath
to aluminum deposited in a pad above the cathode block. The copper insert in
the
collector bar distributes electric current more evenly than in prior art cells
having
collector bars containing only steel or other ferrous metal. The ferrous metal
spacer
CA 02347858 2001-04-24
WO 01/27353 PCT/[JS00/27936
-7-
in the collector bar body reduces heat losses, compared with collector bars
having a
copper insert connected directly to the bus bar.
Figure 1 is a schematic cross-sectional view of a prior art electrolytic
cell for aluminum production.
Figure 2 is a schematic cross-sectional view of an electrolytic cell for
aluminum production in accordance with the present invention.
Figure 3 is a schematic view of a collector bar in the electrolytic cell
of Figure 2.
Figure 4 is a cross-sectional view taken along the lines 4-4 of Figure
3.
Figure 5 is a schematic depiction of current paths in a prior art
electrolytic cell for aluminum production.
Figure 6 is a schematic depiction of current paths in an electrolytic
cell of the present invention.
The apparatus and method of the present invention provide a novel
collector bar that minimizes horizontal electrical currents while controlling
heat
losses. The novel collector bar of our invention may be incorporated into
existing
aluminum production cells having standard carbon cathode blocks.
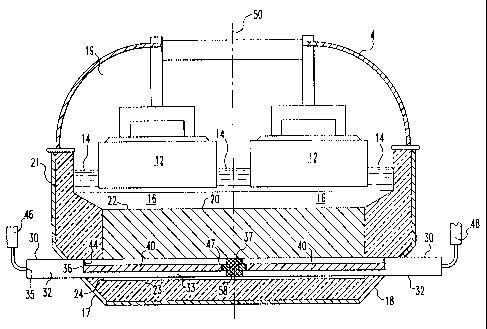
Referring now to Figure 1, there is shown a prior art electrolytic cell
2 fox aluminum production, having a pair of conventional cathode collector
bars 10.
The collector bars 10 have a rectangular transverse cross-section and they are
fabricated from mild steel. Electrical current enters the cell through anodes
12,
passes through an electrolytic bath 14, and a molten metal pad 16, and then
enters a
carbon cathode block 20. Current is carried out of the cell by the cathode
collector
bars 10. As shown in Figure 5, electrical current lines 70 are non-uniformly
distributed and concentrated more toward ends of the collector bar closest to
an
external bus bar (not shown).
Referring now to Fig. 2, there is shown an electrolytic cell 4 of the
present invention. The cell 4 includes a first cell wall 17 and a second wall
18.
The cell walls define a chamber 19 lined on its bottom and sides with
refractory
bricks 21 and containing the molten electrolyte 14. A cathode block 10 has two
half-width cathode collector bars 30. Each collector bar 30 extends from a bus
bar
CA 02347858 2001-04-24
WO 01/27353 PCT/USOO/27936
-8-
46,48 outside the cell walls 17,18, and inwardly toward a center line 50. The
collector bars 30 are separated by a gap 58 in the middle of the cell filled
by a
crushable material or by a piece of carbon or by tamped seam mix or by a
mixture
of such materials. Preferably, the gap 58 is filled by a mixture of materials.
The cathode block 20 has an upper surface 22 supporting the metal
pad 16 and a lower surface 23 defining a slot or groove 24 extending between
opposed lateral ends of the block 20. The steel collector bars 30 are received
in the
slot 24 and secured there by a layer of electrically conductive material,
preferably
cast iron, joining the collector bars 30 to the block 20.
Referring now to Figures 2-4, the collector bars 30 each include a
ferrous metal body comprising a solid spacer 32 and a sheath 33 defining a
cavity
34. Each spacer 32 has an external end portion 35 connected with a bus bar 46
and
an internal end portion 36 spaced inwardly of the external end portion 35.
Preferably, the internal end portion 36 is inside the cell wall 17, as shown
in Figure
2.
Referring now to Figures 3 and 4, a cavity or slot 34 is drilled into
an internal side 37 of each collector bar 30. The cavities 34 are machined to
have a
diameter of about 1.628 inches so that their cross-sectional area is about
2.08 in2, in
a collector bar having a transverse cross-sectional area of about 24 in2. The
cavities
34 extend longitudinally, between openings in the collector bar internal sides
37, and
internal end portions 36 of the spacers 32. The cavities 34 are positioned
centrally
inside the collector bars 30, to minimize distortion accompanying heating and
cooling.
A copper insert 40 comprising a cylindrical rod is placed inside each
cavity 34. The copper insert has a diameter of approximately 1.625 .0025
inches
so that its transverse cross-sectional area is about 2.07 in2. For practice of
our
invention we prefer copper inserts composed of a high conductivity grade of
copper
containing about 99.95 - 99.99 wt% copper.
An air vent 44 is drilled into each collector bar, between the cavity
34 and a top side. The air vent 44 vents air from the cavity 34 when the
copper
insert 40 is positioned inside. After the insert 40 is in place, the vent 44
is filled
with refractory mortar. If sufficient pressure develops in the cavity 34 to
expel the
CA 02347858 2001-04-24
WO 01/27353 PCT/US00/27936
-9-
mortar, the vent 44 provides a path for pressure relief.
A steel plug 45 is placed in the cavity 34 to enclose the copper insert
40. A small space 47 between the plug 45 and the insert 40 allows for
expansion
upon heating to operating temperature. The plug 45 is welded to the internal
side
37 of the collector bar 20. In particularly preferred embodiment, the space 47
provides an expansion allowance of about 0.65 inch and the steel plug 45 has a
length of about 1 inch.
The apparatus and method of our invention redirect current in a
Hall-Heroult cell to reduce or eliminate inefficiencies attributable to non-
uniform
electrical currents and horizontal electrical currents.
The cathode current path and current distribution are influenced by
the differential between electrical conductivity of the aluminum metal pad and
the
cathode assembly. With a high electrical conductivity differential in favor of
the
aluminum pad, the preferred current path will be sideways through the metal
pad
toward a side wall of the pot and then downwardly through the cathode block to
the
collector bar.
In Figure 5 there is shown a current gradient 70 in a prior art pot 2
having an anode 12, a molten metal pad 16, a cathode block 20, and a collector
bar
10. The highest current concentration is found directly over the steel
collector bar
10 close to an outer end 72 of the cathode block 20. The lowest current
distribution
is found in the middle of the cathode block 20, adjacent an internal end
portion of
the collector bar 10. Localized wear patterns observed on the cathode block 20
are
deepest in the area of highest electrical current density.
As electrical conductivity of the cathode block 20 increases to reflect
a change to higher graphite content or graphitized cathode blocks, the cathode
current distribution 70 becomes more concentrated at the outer end 72 of the
block.
At constant amperage, the localized wear rate will increase near the outer end
72 of
the block 20 as the graphite content increases and reaches a maximum for
graphitized cathode blocks.
The apparatus and method of our invention redirect current in a
Hall-Heroult cell to reduce inefficiencies attributable to non-uniform and
horizontal
electric currents. As shown in Figure 6, our cell 2 includes an anode 12,
molten
CA 02347858 2005-06-09
50989-9
metal pad 16, cathode block 20, and a collector bar 30
having a copper insert 40. The current gradient 90 extends
from the anode 12 to the metal pad 16 and along the entire
length of the cathode block 20. The current distribution
5 pattern 90 is more uniform than the pattern 70 shown in
Figure 5. The copper insert 40 increases electrical
conductivity of the collector bar 30 by reducing the
conductivity differential in favor of the aluminum pad 16,
thereby distributing current more evenly across the
10 block 20.
We have observed that placing a copper insert in a
steel collector bar significantly increases its overall
electrical conductivity, corresponding to a lower overall
resistance in the collector bar. The result is more uniform
current distribution in the cathode and reduced localized
wear rates.
The cathode voltage drop is also reduced by up
to 50 mv. This voltage drop can be utilized to reduce the
cost of electrical power at a constant production rate, or
to increase the tonnage of aluminum produced at constant
power.
Ends of the collector bars extending through side
walls of the pot act as fins or heat sinks. Integrating
copper inserts into the collector bars increases pot heat
losses. Accordingly, the length of the copper insert must
be carefully controlled to prevent excessive heat losses and
adverse pot life effects. Preferably the copper inserts
should not extend outside the pot side walls 17, 18. We
also combine the copper insert collector bar with additional
insulation material in the pot to offset the extra heat loss
caused by the copper insert.
CA 02347858 2005-06-09
50989-9
11
Having thus described the presently preferred
embodiments of our invention, it is to be understood that
the invention may be otherwise embodied without departing
from the spirit and scope of the following claims.