Note: Descriptions are shown in the official language in which they were submitted.
CA 02347977 2002-O1-09
WO pp~3~pg PCTNS99124467
APPARATUS FOR PERCUTAI~OtIS IhITERPOSITI0Ii BALLOOR ARTHROPLASTY
FIELD OF TIC INVENTION
This invention relates to a method and apparatus for performing arthroplastic
surgery
for the interposition of balloons within joints. Specifically, this invention
relates to a method
and apparatus for performing percutaneous interposition balloon arthroplasty
for the repair of
movable and mixed articulating joints in the body.
BACKGROUND OF THE INVENTION
There are three basic classifications of joints of the human body:
synarthroidal,
amphiarthroidal, and diarthroidal. Synarthroidal joints provide immovable
articulations;
amphiarthroidal joints provide mixed articulations; and diarthroidal joints
provide movable
articulations. )EIealthy fibrocartilage and hyaline cartilage within the joint
provide a weight
bearing function and allow painless articulation of amphiarthroidal and
diarthroidal joints.
Primary osteoarthritis is a debilitating disease that affects amphiarthroidal
and
diarthroidal joint.5. The changes that occur with primacy osteoarthritis
involve aiteral
biomechanical, biochemical, histologie and metabolic characteristics of the
cartilage, synovial
fluid and bone. Initially, these changes affect the articular cartilage and
eventually affect the
surrounding peric.hondral tissues in a cascade of events. Articular cartilage
comprises 70-
8096 water and functions as a weight bearing surface by its unique interxtion
between the
water and cartilage matrix. There are many theories concerning how articular
cartilage
functions as a weight bearing surface which include hydrodynamic, boundary,
CA 02347977 2002-O1-09
wo oon3oo9 rcrnrs~n~s~
elastohydrodynamic and squeeze film Iubricadon. However, it is known that the
viscoelastic
properties contribute to the multiple functions of articular cartilage,
including its weight
bearing function. The viscoelastic properties of cartilage are due to an
intricate tight
meshwork of interlacing collagen fibers that physically ensnare the large
macromolecules of
proteoglycan.
For example, in a typical case of osteoarthritis of the hip joint, the femoral
head
remains covered with fibrocartilage over a third to two-thirds of its surface,
but in the
superior weight bearing region, the liaing tissue becomes considerably
thinner, although
intact throughout. Where thin, the lining tissue is partly fibrocartilage, and
partly fibrous,
displaying focal areas of cystic degeneration. However, where fibrocartilage
is present, the
thickness of the membrane between the femoral cup, cacetabulum, and the bone
does not
generally exceed 2 nu-n.
To treat osteoarthritis effectively, procedures are needed for repairing
amphiarhroidal
and diarthroidal joints that prevent the disintegration of fibrocartilage and
that restore the
viseoelastic properties of articular cartilage for an indefinite period of
time.
Historically, repair of the joint was conducted by disintegration of the
diseased tissue
followed by fibrous repair. However, this method has significant
disadvantages, even when
accompanied by conventional arthreplasty.
Orthopedic surgeons who specialize in total joint arthroplasties have been
uncomfortable with performing resections of an entire joint. 1n the hip joint,
for example, the
entire acetabulum down to the intra-pelvic bone and the proximal femur would
have to be
resected. Sections of the proximal femur that are resected in this procedure
include the
femoral head, neck and intrarnedullary bone of the upper half of the femur.
However, the
2
CA 02347977 2002-O1-09
WO tNIlZ3009 PCT/US99/Z4467
pathology in grimary osteaarthritis is initially and primarily isolated to the
articular cartilage.
Thus, the resected tissue is many times greater than the surface area actually
responsible for
the patient's symptoms.
Traditionally, osteoarthritis of the hip has been treated in one of two ways:
arthroplasty utilizing foreign substances of non-animal origin, and other
methods that
ameliorate pain and disability in osteoaethritis of the hip. Arthroplastic
procedures that
consist of interposing membranes, metallic cups, or other inserts to sustain
the joint space
until new joint spaces can regenerate have been extensively used in the prior
art.
Cup or mould arthroplasty has commonly been used to treat degenerative
arthritis of
the hip joint. This procedure consists of denuding the femoral head and the
acetabulum to
bleeding bone, and reshaping them into a ball and socket joint with a metallic
cup interposed
between the two surfaces. The aim of mould arthroplasty is the formation of
smooth
glistening fibrocartilage around the periphery of the articular surface and
hyaline cartilage in
the central portion. Smith-Petersen concluded that, in response to physiologic
stresses of
friction and intermittent pressure of movement and supported weight-bearing,
the repairing
tissue will mold into smooth fibre-cartilage and in some instances to hyaline
cartilage. M.N.
Smith-Petersen; 21 ~J. Bone Bt Joint Surg: 2b9 ( 1939). Other inventors used
mould '' '
arthroplasty with varying degrees of success. P'ean and Chlumsky were the
first to utilize
foreign materials in arthroplasty, the former in human joints, while tlar
latter experimented
with an array of metal plates and films of celluloid, rubber, and collodion;
Sir Robert Jones
successfully utilized a strip of gold foil to cover the reconstructed head of
the femur, Hrpovac
used magnesium plates; and Rehn was the first to use cup arthroplasty when he
inserted a
previously molded cap-like appliance of steel into the acetabular side of the
hip joint. Paul H.
CA 02347977 2002-O1-09
WO 00/23009 PCTNS99/24469
Hanmon, 76 Surg. Gyn. Obst. 347 (1943). Smith-Perterson utilized cups of
various materials:
glass, viscaloid, pyrex glass, bakelite, and vitallium. M.N. Snuth-Peterson,
J. M. J. Bone
Surg., 18: 869 (1936). Vitatlium was the most successful material used in cup
arthroplasty.
However, cup arthrvplasty caused severe trauma in patients and showed poor
formation of hyaline cartilage.
Other approaches have been used to repair disease of joints in the human body.
For
instance, in the vertebral column, a collapsible plastic bladder-like
prosthesis with the same
shape as the nucleus pulposis of an intervertebral disc is delivered via a
stem into the space
between the vertebrae. U.S. Pat. No. 3,875,595 (Froning). A method and
apparatus has been
described for the repair of tissue in the vertebral column, such as
fibrocartilage, using a
bladder-like prosthesis device that can be inserted into the disc space and
thereby infused
with biomaterial to distract the space and provide a permanent replacement
disc. However,
this method addresses only prosthetic placement in the vertebral column. PCT
Pat. App. No.
WO 97/26847 (Felt, et al). In yet another case, an arthroscopically
implantable prosthetic
device consisting of a pair of multi-compartment rings shaped to ftt into a
joint and filled
with a polymeric substance is used to restore function to a diseased joint.
U.S. Pat. No.
5,344,459 (SwarTZ).
What is needed is a method and apparatus for restoring the function of movable
and
mixed articulating joints and for repairing fibrocartiiaginous tissue and
restoring the
viscoeiastic properties of articular cartilage in amphiarthroidal joints such
as the hip for an
indefinite period of time.
4
CA 02347977 2002-O1-09
WO 00/23009 PCTNS99/24467
SUr~I~IARY OF THE INVENTION
The current invention provides a method and related materials for percutaneous
interposition arthroplasty, comprising the steps of entering the joint with a
probing device,
introducing a deflated balloon within the joint, and inflating the balloon.
Moreover, this
method may further comprise the step of distending and debriding the joint
prior to
introducing the deflated balloon, the step of keeping ligaments (e.g., the
ligamentum teres)
intact, the step of reseeting the ligaments, the step of closing the puncture
wound, and the step
of removing the balloon. Combinations of these steps are considered part of
this invention.
In addition, this invention is a balloon suitable for percutaneous
interposition
atthroplasty and comprising an outer shell and a filler solution. The filler
solution may be a
condensed phase composition, a gas composition, or mixtures of gases and
condensed phases.
Condensed phase compositions include, but are not limited to, polymers,
curable condensed
compositions, gels, resins, liquids, and solutions. This group further
includes, for example,
silicone-gel, saline solution, and soybean oil. Gas phase compositions may
include, but are
not limited to, air, nitrogen, oxygen, argon, carbon dioxide, and mixtures
thereof. The
balloon may be any shape, may have multiple compartments, may be capable of
withstanding
,~~ificant-pressures and may be relatively impenetrable:Combinations of filler
solution
materials are considered part of this invention.
The interposition of a balloon may also facilitate the repair or reformation
of the
cartilage tissue on the surfaces of the bones of the joint.
5
CA 02347977 2002-O1-09
WO 00123009 PCTNS991~4467
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows primary osteoarthritis of the hip joint.
Figure 2 shows hip joint distention and arthroseopic placement.
Figure 3 shows balloon introduction.
Figure 4 shows balloon inflation.
Figure 5 shows interposition balloon arthroplasty.
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides a method and related devices for repairing
joints and
surrounding tissues by minimally invasive means. In particular, the invention
provides a
method and related materials for using minimally invasive means to repair and
reconstruct
tissue such as fibrocartilage, particularly fibrocartilage associated with
diarthroidal and
amphiarthroidal joints. The method involves using minimally invasive means to
prepare the
site of pathology, and distending a joint site in situ in order repair the
damaged joint.
The method comprises the steps of:
a. performing surgery to enter the joint;
...,._: :~ :. ... .. _ . b" -. using a minimahy invasive means~tn remove
darn~ged~or~diseased
material from a narrowing joint space and nearby tissues;
c. introducing at least one deflated balloon to a cavity; and
d. inflating the balloon.
Arthroscopy has revolutionized knee surgery but has been of minimal use, for
example, when it comes to hip pathology. However, arthroscopy is applicable to
the hip
joint. The hip joint is well suited for balloon arthroplasty because the hip
is accessible
6
CA 02347977 2002-O1-09
WO 0~/~3009 PCT/US99114467
pereutaneously without disturbing its blood supply. Furthermore, the joint can
be
significantly distended by releasing its inherent negative pressure, resulting
in a relatively
uncomplicated surgical process as the space between the aeetabular fossa and
the head of the
femur can serve as a suitable cavity for the delivery and inflation of a
balloon. The surfaces
of the acetabular fossa, the femoral head and surrounding tissues can be
treated or covered
with a suitable material in order to enhance their integrity and use as a
cavity. Moreover, the
ligamentum teres may be preserved or resected to ensure a permanent
replacement procedure.
The ligamentum teres is vital only in infancy and childhood. By adulthood, the
primary blood supply to the femoral head is through the anterior and posterior
femoral
circumflex arteries. As a result, the ligamentum teres can be resected with
balloon insertion
while maintaining the blood supply through the circumflex arteries. The
advantage of
preserving the ligamentium teres is to add to the stability of the femur.
The procedure consists of, first, making an appropriate incision. Then,
through a
probing device such as an orthoscope, the hip joint is entered, distended and
debrided.
Depending on the circumstances, the ligamentum teres may be preserved. The
debriding step
is optional. Then, through a robing device, a deflated balloon may be
introduced within the
joint and distended similar~towthe fashion in which a bre8ist itttplant is
filled with saline: °The
artluoscopic introducer-inflator may be removed and the puncture wounds)
closed.
Rehabilitation for such a procedure is relatively minimal. The advantages
include joint space
restoration with restoration of viscoelastic dynamics. Additionally, a total
hip reglacement
would remain viable if the procedure should fail. Moreover, a surgeon could
wait for a
predetermined period of time and then remove the balloon implant if desired.
7
CA 02347977 2002-O1-09
WOr OO/Z3009 PCTNS99124467
This procedure permits the femoral head to be resurfaced (with the balloon or,
subsequently, by repair or reformation of the cartilage) without dislocating
the hip which in
some circumstances would disrupt the blood supply, thereby eausiag avascular
necrosis. The
procedure, therefore, allows full access to the femoral head for complete
resurfacing while
maintaining the primary blood supply.
Surgeons could reduce total hip arthroplasty with its inherent risks and
complications
to a brief, percutaneous, out-patient procedure with minimal risk plus
maintenance of
anatomy should a formal hip resection and replacement be required later on.
Similar
procedures could be used for balloon arthroplasty in other joints.
PREPARING TIC JOINT SPACE AND INSERTING A BALLOON
The description of the preparation of the joint space and insertion of a
balloon are
described for the hip joint. Similar procedures can be used for balloon
arthroplasty of other
joints. Figure 1 shows primary osteoatthritis of the hip joint. This may be
characterized, in
part, by a narrowing joint space 10, cartilage loss 20, and thickening joint
capsule 30. The
narrowing joint space 10 is the space between the head of the femur 40 and the
acetabular
fossa 50:-- op~her syrttptoms may exist-and are welt known to those in'the'
art:' "
The procedure for balloon arthroplasty contains the following steps as
appropriate.
ti) Assessment of the extent of osteoarthritis.
(ii) Distension of the,joint.
(iii) Orthoscopy into the joint space.
(iv) Optional removal and/or cleaning of destroyed tissue.
(v) Delivery of a deflated balioon.
8
CA 02347977 2002-O1-09
WO 00/23009 PCT1IJS99/2446~
(vi) Inflation of the balloon.
(vii) Optional deflation/removal of the balloon.
Performing the surgery for entering the hip joint can be carried out using
techniqrres
well within the skill of those in the art. The narrowing joint space 10 may be
viewed, for
instance, by remote visualization techniques such as fiberoptic visualization.
The integrity of
the hip and femur can be assessed, and optionally, repaired, for example, by
the application of
a biocompatible patching material, such as a fibrin glue.
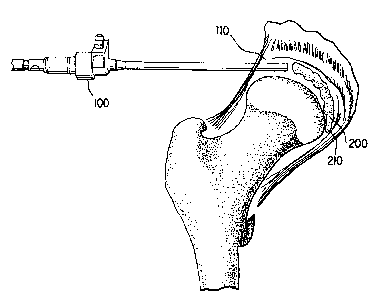
Figure 2 shows hip joint distention and arthroscopic placement. In some
patients,
joint distention is unnecessary. However, if a surgeon desires, distention may
be achieved by
mechanical displacement of the femur with respect to the acetabular fossa. The
joint space
10 can be distended prior to andlor during either the preparation andlor
delivery of the
balloon. Distension can be accomplished by any suitable means, including by
mechanical
andlor hydrostatic means. A surgeon can employ external traction. An
otthoscope 100 may
be inserted into the cavity 110 formed by distending the narrowed joint space
10. The
deflated balloon may then be placed into the distended cavity.
IS If required, the destroyed pelvic and femur related tissue such as
fibrocartilage may be
. ... :omovdd and cleaned prioF tar inserting the defl~ecl balloon: The
remaining, repaired tissue T
and bone matter serve as a support for an inflated balloon. The head of the
femur 40 engages
a balloon 300 like a penile condom. The ligamentum tares may be preserved, so
as to allow
any balloon to occupy the cavity,110, except for the void around the
ligamentum tares. See,
for example, Figure 5. Once the damaged material has been removed and the
remaining joint
tissues repaired, the joint space 10 may be used as a cavity 110 to contain a
delivered balloon.
The joint space 10, including any repaired portions is created to be of
sufficient dimension to
9
CA 02347977 2002-O1-09
WO 00/231109 PCTNS99124467
allow a deflated balloon to be delivered and distended. By the use of
distension, the joint
space 10 can be sufficiently re-established to achieve any desired final
dimension and
position. The means usod to accomplish distension (for example, another
balloon or other
mechanical devices) may also form at least one barrier (for example, a cavity
110) for the
balloon.
The narrowed joint space 10 may be easily distended by the use of one or more
inflatable balloons. When inflated, a balloon provides rigid walls that are
capable of
expanding the joint space 10. An inflatable balloon provides sufficient
strength and
dimensions and can be prepared using conventional materials. In use, the
deflated balloon
can be delivered to the narrowed joint space ZO and inflated to separate the
space 10. For
example, a balloon may be inserted with an orthoscope. Under certain
circumstances,
distension prior to the insertion of the inflatable balloon is unnecessary.
Once positioned within the cavity 110, Figure 4 shows that the orthroscope 100
may
be used to inflatE: a balloon by injecting a suitable filler solution (not
shown) to create an
inflated balloon 300. Depending on the application, the same or different
orthoscope may be
used for insertion of the deflated balloon 200, and for inflating the deflated
balloon 200. For
~~ example, an orthroscope 100W ay be insbtted into the sp~e-10 fb deliver an
iieflated balloon
and a second probing device used to inflate the deflated balloon.
A suitable gas (for example, nitrogen, carbon dioxide, oxygen, argon, ere.)
may be
delivered in order to inflate the balloon in situ. Positioning of the balloon
may be facilitated
by the use of ancillary means, such as using a C-arm tine , or by self-
effecting means
embodied within the balloon or the delivery apparatus.
CA 02347977 2002-O1-09
WO 00/23009 PCTNS9912146?
Suitable materials for preparing balloons of the present invention, for
example, are
those that may be used for balloon angioplasty. Suitable materials provide an
optimal
combination of such properties as compliance, biostability and
biocompatability, and
mechanical characteristics such as elasticity and strength. Balloons can be in
any suitable
form, including those having at least one layer and having at least one
compartment when
expanded. A useful balloon device will include the balloon (optionally having
a plurality of
lumens), a delivery probing device, and fluid or gas pressure means. An
orthoscope may be
used as a probing device.
Examples of suitable balloon outer shell 210 materials include, but are not
limited to,
polyolefm copolymers, polyethylene, polycarbonate, and polyethylene
terephthalate. Such
polymeric materials can be used in either unsupported form, or in supported
form, for
example, by the integration of polyethylene terephthalates or other fibers.
Balloons can also take several fornns, depending on how the balloon is to be
delivered
and inflated. A single, thin walled balloon can be used, for instance, to
contact and form a
barrier along the joint surface. A balloon can be provided that occupies less
than the entire
volume of the cavity 110. The balloon may be, for instance, in the shape of a
cylinder or a
colxlapsed, bell tent.
Any portion, region or surface of the outer shell of the balloon 210 may be
treated
with friction modifying coatings or other materials to improve or otherwise
alter the physical
or chemical properties.
A balloon of the present invention can be inflatably attached (far example,
provided
in an releasable and deflated ar inflated configuration) within or upon the
end of a probing
device, in order to be inserted into the space 10 or cavity 110. Moreover, the
balloon may be
11
CA 02347977 2002-O1-09
wp pp~3ppg PCTNS991Z1167
inserted using minimally invasive means, and remote visualization methods
including
fiberoptic visualization.
Once within the space 10 or cavity 110, the balloon can be finally positioned
and
delivered. The balloon may be self-venting, in that whatever volume of gas may
be present
within the balloon and shaft at the time of insertion can be displaced by the
filler solution and
vented through the balloon walls, for example, to the surrounding tissue. The
balloon may be
ev~uated by the application of suction or vacuum to the shaft. Some or all of
the gas present
within the shaft andlor balloon may be vented through the balloon material by
the deliver of a
filler solution. As the filler solution fills the balloon and displaces the
gas the filler solution
also serves to inflate the balloon to a desired extent, and in a suitable
position within ttte
cavity 110 sufficiently distended, the 611er solution may be cured, or
permitted to fully cure,
in situ in order to retain the balloon and filler solution permanently in
place. This step is
optional and depends upon the filler solution.
The balloon may be fabricated from natural or synthetic materials, including
but not
limited to, polymeric materials, such as films or membranes, and woven or
nonwoven fabrics
or meshes. Balloons that will not permit the effusion or diffusion of liquids,
gels, solids,
other condensed compositions and gases can be fabricated as one or more layers
comprising
such materials, andlor with one or more regions or portions of differing
properties.
The materials used to fabricate balloons may provide an optimal combination of
such
properties as biocompatability, biodurability, strength, wall thickness,
wettability with a filler
solution, puncture resistance, compliance, flexibility, modulus of elasticity,
stress/strain curve
yield point, burst pressure, maximum inflation, Young's modulus, shear
modulus, and the
ability to be easily fabricated and sterilized.
12
CA 02347977 2002-O1-09
WO 023009 t'CTNS99l1146?
Examples of suitable balloon materials include, but are not limited to, solid
polymeric
materials such as membranes. Polymeric materials may be provided with suitable
venting
holes. Suitable polymeric materials include, but are not limited to,
elastomeric and other
materials commonly used for angioplasty and related application, and include
polyurethanes,
polyolefins, polyamides, polyvinyl chlorides, and polyethylene tercphthalates,
as well as
various copolymers, combinations and permutations thereof.
Balloon materials are available commercially for use in filtration and other
applications, and include cloth and mesh formed of polymeric materials such as
polyester,
polypropylene and nylon threads. A material may be reinforced, for example,
with woven
glass or hne fibers of other materials, to provide added strength or other
desirable properties.
Such materials can be selected to provide an optimal combination of such
properties as
strength, mesh opening, thread diameter, mesh count, percent open area, and
cost. Examples
of suitable materials are eomrnercially available and include, but are not
limited to, nylon
screen cloth, such a nylon mesh.
The balloons themselves may be fabricated by a variety of means. The balloon
may
be formed as a continuous (for example, unitary) and non-interrupted (for
example, seamless)
form. :The balloon may bo fabricated from ~ plurality of generally sheet-like
portions; whieh-
can be assembled and sealed together. Scaling may be accomplished by any
suitable means,
including by the use of adhesives, sewing, RF bonding, heat scaling, impulse
sealing, and any
combination thereof. Once sealed, the balloon may be turned inside out in
order to provide
the sealed seam on the interior of the resultant balloon.
The balloon may be fabricated to assume any desired shape upon inflation, for
example, a generally oval shape, or the shape of a kidney bean, in order to
approximate the
13
CA 02347977 2002-O1-09
wo ooa3oo9 Prrrus~r~aab~
natural anatomical shape of the space 10. The balloon may provide major
surfaces for
contxting the principal surfaces of the joint, fox example, the head of the
femur 40 and
aeetabular fossa 50. The balloon may further provide wal I portions for
contact with other
tissue.
A balloon may be provided with one or more orientation markers, in order to
permit
the surgeon to determine the optimal orientation of the balloon in situ.
Suitable orientation
markers include, but are not limited to, the placement of detectable markers
or indications
within or upon the balloon material and/or probing device, the marking or
indications
themselves being detectable by minimally invasive means, for example, by
ftberoptic
visualization, interoperative magnetic resonance ig (MRl], ultrasound, and
laser
radiation.
The deflated balloon may be positioned within the space 10 or cavity 110
following
preparation. As described hereinabove, mechanical distension of the space can
be used as
well, for example, either while inserting and/or positioning a balloon and/or
during inflation
of a balloon with filler solution.
Once in place within the cavity 110, the balloon may be filled by having the
probing
.,.,. . ,. .deviee.eonnected to a filler solution delivery device capai~la~of
delivering filler solution ' '"
through the probing device and into the balloon under sufficient pressure.
When in the form
of a curable polymer, the filler solution may begin to cure as it leaves the
mixing chamber of
the delivery device. Saline, as a filler solution, will never cure. if a
curing filler is chosen,
the cure rate of the biopolymer may lx controlled, in combination with the
dimensions and
other conditions of the distension means, in order to provide sufficient time
for the filler
solution to expand the balloon before final curing occurs. Noncuring materials
may change
14
CA 02347977 2002-O1-09
' WO pp~3pp9 PCTNS99/?A46?
viscosity upon entering the cavity 110. Such changes may result from different
pressures,
temperatures or both. In some cases, the phase of filler solution may change,
for example,
from gas to liquid or solid to liquid or gel, ere. The progress of inflating
may be monitored,
for example, by C-arm cine or interoperative MRI.
"Cure" and inflections thereof, will refer to any change in the physical
properties of a
material by chemical reaction or wlcanization. Curing may occur with the aid
of any
combination of heat, chemicals, catalysts, and energy, such as, but not
limited to, light and
ultrasound. When used with regard to the method of the invention, for
instance, "curable"
can refer to uncured biomaterial, having the potential to be cured in vivo (as
by the
application of a suitable energy source), as well as to a biomaterial that is
in the process of
curing, as with a biomaterial formed at the time of delivery by the concurrent
mixing of a
plurality of biomaterial components.
Figure 5 shows interposition balloon arthroplasty upon completion. The balloon
300
becomes semi-circular shaped balloon 400, which encompasses the head of the
femur 40.
The balloon can be left in place for a fixed period of time, and then removed
by a similar
procedure, or the balloon may be permanent. The balloon was conswcted so that
it would
. . . ., , , t::.:=: ~fomn a semicircular shape and~as it was distended would
obtain stabilityby "lockiag'~ av~x the
expanse of the head-neck angle. The balloon may leave the iigamentum teres
undisturbed.
FILLER SOLUTIONS
Natural cartilage is a non-vascular structure found in various parts of the
body.
Articular cartilage tends to exist as a finely granular matrix forming a thin
iacrustation on the
surfaces of joints. The natural elasticity of articular cartilage enables it
to break the force of
IS
CA 02347977 2002-O1-09
WO 002'i009 PCT/US99/24467
concussions, while its smoothness affords ease and freedom of movement. Filler
solutions
are intended to mimic many of the physical-chemical characteristics of natural
tissue.
Filler solutions can be provided as one component systems, or as two or more
component systems that can be mixed prior to or during delivery, or at the
site of repair.
Generally, fillers are flowable, meaning they are of sufficient viscosity to
allow their delivery
into the balloon. The fillers may be heated or subject to pressure changes to
aid flowing.
Moreover, the fillers may be solvated in a liquid, gel, or other condensed
phase composition
to aid flowing into the balloon with or without temperature or pressure
changes. Suitable
fillers may comprise gas phase compositions which include, but are not limited
to, air,
nitrogen, oxygen, argon, carbon dioxide, other inert gases, and mixtures
thereof.
Filler solutions may be homogeneous (i.e., providing the same chemical-
physical
parameters throughout), or they can be heterogeneous. Filler solutions may be
used that
provide implants having varying regions of varying or different physical-
chemical properties.
Common polymeric materials for use in medical devices include, but are not
limited
to, polyvinyl chlorides, polyethylenes, styrenic resins, polypropylene,
thermoplastic
IS polyesters, thermoplastic elastomers, po~ycarbonates,
acrylonitrilebutadiene-styrene ("ABS")
r~sins,.aerylics, polyurethanes; .nylons; styrene acrylonitriles, and
cellu~~sic.
Suitable filler solutions are those polymeric materials that provide a
suitable
combination of properties relating to their device application and in vivo
use. Such properties
include, but are not limited to, processability and the ability to be stably
sterilized and stored.
In the course of applying such material, such properties include in vivo
flowability and
moldability.
16
CA 02347977 2002-O1-09
wo oon3oo9 Pcrms99n~~
The filler solution rnay comprise a thermosetting polyurethane polymer based
on a
suitable combination of isocyanates, lang chain polyols, and short chain (low
molecular
weight) extenders andlor crosslinkers. Suitable components are available
commercially and
are each may be used in the highest possible grade, for example, reagent or
analytical grade or
higher. Examples of suitable isocyanates include, but are not limited to, 4,4'-
Biphenyl
methane diisocyanate ("MDI"), and 4,2'-diphenylmethane diisocyanate, including
mixtures
thereof, as well as toluene diisocyanate ("TDP'). Examples of suitable long
chain polyols
include, but are not limited to, tetrahydrofuran polymers such as
poly(tetramethylene oxide)
("PTMO"). Examples of suitable extenders/crosslinkers include, but are not
limited to, 1,4-
butanediol and trimethylo! propane, and blends thereof.
Such performance may be evaluated using procedures commonly accepted for the
evaluation of natural tissue and joints. Curing is unnecessary, for example,
for oils (carbon or
silicon based), water, saline solution, gels, resins and other condensed
phases which may
possess an optimal combination of physical chenucal properties. Suitable gels,
for example,
include silicone gels. Suitable oils include, for example, soybean oils.
Filler solutions of the present invention may further include adjuvants and
additives,
.. . . . .such. as tabilizers.~Ftllers,antioxiBants, catalysts; plasticizers,
pigments, and lubricants, Lo the
extent such ingredients do not diminish the utility of the composition for its
intended purpose.
Filler solutions may be stable under conditions used for sterilization and
stable on
storage and in the course of delivery. They may be capable of flowing through
a delivery
device to an in vivo location, andlor being cured in situ, as by exposure to
an energy source
such as light or by chemical reaction. Thereafter, a cured filler solution may
be amenable to
17
CA 02347977 2002-O1-09
wo oonaoo9 pcrrus99n4a6~
shaping and contouring. Uncured filler solutions may be shaped and contoured
to the extent
that the balloon and filler solution will allow.
One or more catalysts may be incorporated into one or more components of the
curable filler solutions in order to cure the filler solution in the
physiological environment
within a desired length of time. Curable filler solutions may be able to cure
within about 5
minutes or less.
Means may be employed to improve the biostability, for example, the oxidative
andlor
hydrolytic stability, of the filler solution, thereby extending the Iife of
the implant. Suitable
means for improving biostability include the use of aliphatic macrodiol such
as hydrogenated
polybutadiene (HPDn. By judicious choice of the corresponding diisoeyanate
(far example,
MDn and chain extender (for example, ethylenediamine), those skilled in the
art will be able
to achieve the desired packing density or crystallinity of the hard segments,
thereby
improving the hydrolytic stability of the cured polyurethane.
Filler solutions may be provided as a plurality of components, for example, a
two-part
polyurethane system, may be mixed at the time of use using suitable mixing
techniques, such
1S as those commonly used for the delivery of two-part adhesive formulations.
A suitable
. ... . . . , mixing device i~nvolvES,.for.instanc~,, a-static mixer having a
hollow tube having a segmented,. . . , " , .: ," ,,
helical vein running through its lumen. A two-part polyurethane system can be
mixed by
forcing the respective components through the lumen under pressure.
The foregoing description is intended to be illustrative of the invention, but
is not to
- be considered as comprehensive or limiting of its scope.
18