Note: Descriptions are shown in the official language in which they were submitted.
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
SOLAR RADIATION PROTECTION COMPOSITION
FIELD OF THE INVENTION
10
The invention relates to novel compositions that protect humans and objects
from damaging exposure to solar radiation. The invention also relates to
active
compounds which occur naturally in plants, algae and cyanobacteria as well as
to
the chemical derivatives of these compounds that protect against solar
radiation.
BACKGROUND OF THE INVENTION
The various layers of the earth's atmosphere absorb most of the solar
radiation reaching earth with the more energetic ultraviolet radiation (UV)
filtered out
by the ozone layer. Of the solar radiation reaching the earth's surface that
is of
biological interest, only about 2% of the radiation is UV-B with its
characteristic
wavelength range of 280 to 315 nm. The remainder consists mainly of UV-A with
the
characteristic wavelength range of 315 to 400 nm, visible light of 400 to 700
nm and
infrared radiation (> 740 nm). Anthropogenic depletion of the stratospheric
ozone
layer (Madronich et al., 1995, Ambio 24: 143) has resulted in elevated levels
of UV
radiation reaching the earth and these levels are expected to increase in the
future.
The higher levels of radiation have been linked to a significant rise in the
incidence of
skin lesions and skin cancer in humans (Dagger, 1985, Solar-UV Actions on
Living
Cells, Praeger Pub., N.Y.). Skin cancer now occurs more frequently than all
other
cancers combined. In 1998, there will be approximately one million new cases
of skin
cancer in North America alone. According to the American Cancer Society,
diagnosed cases of skin cancer have increased at a rate of about 4% per year
in the
U.S. since 1973 (Science News 1997, 151: 383). Thus, increased incidence of
skin
cancer has become a serious health concern.
Skin damage is caused by both UV-A and UV-B radiation. Although damage
caused by UV-B radiation has been extensively studied (Motoyoshi et al., 1998,
Cosmetics and Toiletries 113: 51 ), only a few published papers describe the
effects
of UV-radiation on human hair or quantify photodamage (Noting et al., 1995, J.
Soc.
Cosm. Chem. 46: 85). Erythma is the most apparent result of the sunburn
reaction
1
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
but sunburn can also disturb DNA and RNA structure and metabolism. The most
serious results of chronic sun exposure are photoaging and
photocarcinogenesis.
The natural human skin pigment, melanin, has evolved to provide a certain
level of protection against the damaging effects of solar radiation (Kollias
et al.,
1991, J. Photochem. Photobiol. B: Biol. 9:135). The epidermis has the ability
to
interfere with the transmission of UV-radiation. Only about 20% of the
incident
radiation between 300 and 350 nm reaches the dermis. This penetration rises
gradually to about 80% at or near 550 nm. Evidently, the cells of the stratum
corneum are a remarkably effective block against UV-B radiation. Keratinocytes
are,
however, exposed to UV-A radiation even though the more energetic UV-B
wavelengths are excluded. Moreover, UV-A radiation has been shown to induce
mutations and to be potentially lethal (Dagger, 1985, Solar-UV Actions on
Living
Cells, Praeger Pub., N.Y.).
Strategies to protect skin against solar radiation have most often involved
the
application of compositions that either block the radiation or absorb the
harmful UV
component (Gasparro et al., 1998, Photochem. Photobiol. 68:243). Although
sunscreens on the market today protect people from sunburn to varying degrees,
they do not prevent skin cancer. The use of sunscreens has increased
significantly
in the last five to ten years but cases of melanoma have also continued to
rise (ASP
News, 1998, 27:7). Typical sunscreen preparations block UV-B but UV-A passes
through very easily. It has been reported that although UV-B causes sunburn,
UV-A
plays a major role in inducing skin cancer (ASP News, 1998, 27:7). We believe
that
there is a clear need to devise strategies to supplement the body's natural
protective
mechanisms against both UV-A and UV-B radiation. A suitable composition must
have high activity against UV-A as well as UV-B, be safe for application to
human
skin and provide good cosmetic application and appearance.
Cosmetic chemists have incorporated sunscreen activities into formulations
in order to achieve the desired protection. Formulating products with an
improved
UV screening capacity is a challenge for cosmetic chemists due to concerns
over the
use of high levels of organic actives (Dromgoole et al., 1990, Sunscreens:
development, evaluation and regulatory aspects. Marcel Dekker, N.Y., pp 313-
317).
Sunscreen compounds are most effective when they remain on the surface of the
epidermis but unfortunately, they are often absorbed to deeper layers of the
skin,
2
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
thereby limiting their efficacy and causing irritation. Cosmetic chemists use
organic
compounds in their sunscreen formulations. For example, combinations of nylon,
butyl methoxydibenzoylmetan and octylmethoxycinnamate have been used as UV-
screens in many solar Potions. (Cosmetics and Toiletries, 1998, 113: 83;
Gasparro et
al., 1998, Photochem. Photobiol. 68:243) (see Table 1 ).
Table 1. Example of a Sunscreen Lotion/Cream Formulation (Cosmetics &
Toiletries, 1998, 113:84)
Composition
Carbomer 0.15
A.
B. Water 70.70
C. Propylene glycol 2.0
methylparaben 0.20
D. Sodium cocoyl lactylate 0.50
Octoaryl alcohol 1.60
Octyl methoxycinnamate 7.00
Benzophenone-3 2.00
Octyl salicytate 3.50
C12-15 alkyl benzoate 5.00
isopropyl palmitate 2.00
Propylparaben 1.10
E. DMDM hydantoin 0.20
This formulation provides some protection against UV-B but provides minimal
protection against UV-A.
Based on recent reports showing the harmful effect of UV-A exposure on skin
tissue, it is imperative that the cosmetic industry develops as soon as
possible
protective lotions by broadening the light absorption range of skin lotions
(Gasparro
et al., 1998, Photochem. Photobiol. 68:243).
Photoautotrophic plants and microorganisms such as green algae and
cyanobacteria have evolved to protect the sensitive photosynthetic process
against
the damaging effects of UV-radiation. Photosynthetic organisms as well as
heterotrophic organisms such as fungi and bacteria produce naturally occurring
3
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
compounds that protect them from the damaging effects of solar radiation. To
date,
these organisms have not been exploited as sources for sunscreen compounds
that
can be applied to human skin. This is probably due in part, to the scientific
background of the industrial scientists involved in the cosmetic industry.
These
scientists are typically synthetic organic chemists with limited knowledge and
expertise in photosynthesis research and the biology and biochemistry of
terrestrial
plants, green algae and cyanobacteria. It would be helpful if the naturally
occurring
sunscreens from these organisms could be adapted to protect humans from solar
radiation.
SUMMARY OF THE INVENTION
We identified several naturally occurring compounds which: {1 ) act as natural
sunscreening agents; (2) protect not only against visible light and UV-B
radiation but
also protect against UV-A radiation; (3) can be produced in large quantities;
(4) are
easily isolated; (5) are easily incorporated in sunscreen lotions which will
offer
protection to humans against both harmful forms of ultraviolet radiation (UV-A
and
UV-B); (6) and as a consequence, should reduce the development of skin
cancers,
sunburn, photoaging and photodamage to hair and eyes upon exposure to
sunlight.
The invention includes several main components: First, we showed that
cyanobacteria principally use a combination of three different types of
compounds to
protect themselves from the harmful affects of solar radiation: (i)
carotenoids (ii)
scytonemin and (iii) mycosporine amino acids. These compounds may be used to
formulate sunscreen compositions that provide significant protection from
solar
radiation. The compositions are non-toxic, resistant to absorption by the
skin, non-
irritating to the skin and capable of application to the skin in compositions
that create
a uniform, continuous film. In addition, the compounds are chemically stable
and
resistant to chemical and photodegradation when on the skin. The formulations
will
not only protect human skin from photoaging and photodamage but also will
provide
protection to human hair and other surfaces. In addition, these compounds can
be
integrated into or coated on objects such as windows, contact lenses,
sunglasses,
paints and films for protection against the damaging effects of prolonged
exposure to
solar radiation. The invention also relates to a method of protecting human
skin and
other surfaces against the deleterious effects of solar radiation by topically
applying
4
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
thereto an effective amount of a composition of the invention.
Second, we have shown a novel method to induce overproduction of these
natural screening compounds. For example, these compounds are very easy to
produce in cyanobacteria. Cyanobacteria are amenable to manipulation by
5 controlling culturing conditions. Since these compounds are produced
naturally by
photosynthetic organisms, the formulations are "eco-friendly" and can be
extracted
from organisms easily and efficiently. The compounds may also be chemically
synthesized.
Next, based on the results of research described above, we developed a
10 sensitive, relevant biological assay to test the potential of various
screening
compounds to protect against UV-radiation. This is accomplished by exploiting
the
sensitivity of cyanobacterial photosystems to UV-radiation.
Finally, we identify useful naturally occurring sunscreen compounds from
other organisms and methods for their isolation and use.
15 The sunscreen agents, in particular those from cyanobacteria, provide a
synergistic effect. Compositions containing the three compounds isolated from
cyanobacteria with a carrier are superior to those which may be obtained, with
an
equal amount of sunscreen compound and a carrier identical in nature,
employing
either of the compounds alone. The three compounds are preferably present in
the
20 final composition in relative proportions chosen so that the synergistic
effect, at the
level of the sun protection factor conferred by the resulting composition, is
optimal.
In one embodiment, the invention is a sunscreen composition including a
carotenoid or a carotenoid derivative and a carrier. In an alternate
embodiment, the
invention is a sunscreen composition including a polyphenolic compound or a
25 polyphenolic compound derivative and a carrier. In another embodiment, the
invention is a sunscreen composition comprising a mycosporine amino acid or a
mycosporine amino acid derivative and a carrier. In yet another embodiment,
the
invention is a sunscreen composition comprising a carotenoid or a carotenoid
derivative, a polyphenolic compound or a polyphenolic compound derivative, a
30 mycosporine amino acid or a mycosporine amino acid derivative and a
carrier. The
compositions protect skin, hair and other surfaces from solar radiation. The
carriers
are compatible (cosmetically and otherwise) with the surface to which the
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
compositions to which they will be applied, for example, human skin or hair.
The
carriers are preferably at least one of either water, a gas, a water-based
liquid, an oil,
a gel, an emulsion, a dispersion or a mixture thereof.
The carotenoid preferably comprises a cyanobacterial carotenoid, such as a
compound selected from the group consisting of f3-carotene, lutein,
neoxanthin,
zeaxanthin, violaxanthin, caloxanthin, nostoxanthin, echinenone,
canthexanthin,
oscillaxanthin and myxoxanthophyll. The polyphenolic compound is preferably a
cyanobacterial polyphenolic compound such as scytonemin. The mycosporine
amino acid is preferably a compound selected from the group consisting of
rnycosporine-glycine, palythine, asterina-330, palythinoi, palythene, porphyra-
334,
mycosporine-glycine:valine and shinorine. The carotenoid is preferably present
in an
amount of about 2 mg by weight. The poiyphenolic compound is preferably
present
in an amount of about 1 mg by weight. The mycosporine amino acid is preferably
present in an amount of about 1 mg by weight.
Suitable compositions include an oil-in-water emulsion or a water-in-oil
emulsion. In a variation, the sunscreen composition further includes at least
one
cosmetically acceptable adjuvant or additive, such as a preservative, organic
solvent,
browning agent, antioxidant, stabilizer, emollient, silicone, alpha-hydroxy
acid,
demulcent, anti-foaming agent, moisturizing agent, vitamin, fragrance, ionic
or
nonionic thickener, surfactant, filler, thickener, sequestrant, polymer,
propellant,
alkalinizing or acidifying agent, opacifier, fatty compound or colorant.
Other useful compositions are a nonionic vesicle dispersion, emulsion,
cream, milk, gel, ointment, suspension, dispersion, powder, solid stick, foam
or
spray. The composition may also include a makeup or an anhydrous or aqueous
solid or paste. The composition may also include a hair rinse, spray, mist,
gel,
mousse, shampoo, conditioner, lotion, emulsion and colouring product.
In another embodiment, the invention relates to a method of protecting
human skin human hair or another surface from solar radiation, by topically
applying
thereto an effective amount of the compositions of the invention.
An alternate embodiment of the invention is a sunscreen composition
including a photoautotrophic cell extract and a carrier. Carriers are
compatible
(cosmetically and otherwise) with the surface to which the compositions to
which
6
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
they will be applied, for example, human skin or hair. The carriers are
preferably at
least one of either water, a gas, a water-based liquid, an oil, a gel, an
emulsion, a
dispersion or a mixture thereof. The extract is preferably present in an
amount of
about 0.1 to 25% by weight or about 0.1 to 10% by weight. The sunscreen
composition including a photoautotrophic cell extract and a carrier, wherein
the
photoautotrophic cell extract is preferably obtained by extraction of
photoautotrophic
cells with methanol and acetone.
In another embodiment, the invention is a kit for assaying a test compound to
determine its sunscreen efficacy, comprising: a photoautotrophic cell culture,
a
chlorophyll tluorometer and an artificial filter for containing the test
compound. The
invention also includes a method for protecting the human skin, human hair or
another surface from solar radiation, comprising topically applying thereto an
effective amount of the sunscreen composition of the invention, The invention
also
includes a method of inducing cyanobacteria to produce myxoxanthophyll,
scytonemin andlor mycosporine amino acid, the method comprising culturing the
cyanobacteria under conditions of high excitation pressure. The invention also
includes a method of producing an extract having an increased concentration of
at
least one of myxoxanthophyli, scytonemin andlor mycosporine amino acid, the
method preferably including: culturing cyanobaeteria under conditions of high
excitation pressure and isolating an extract including at least one of
myxoxanthophyll, scytonemin and mycosporine amino acid. In the methods the
conditions of high excitation pressure are preferably about 5oC and a light
intensity
of about 150 ~mol m 2 s 1 or about 29oC and a light intensity of about 750
wmol m 2
s 1. The methods preferably further comprising isolating at least one of
myxoxanthophyll, scytonemin andlor mycosporine amino acid. The invention also
includes a method of assaying a compound to determine its sunscreen efficacy,
including extracting photoautotrophic cells to produce a solution; producing
an
artificial filter; determining whether the artificial filter protects
photosystem II
photochemical efficiency from UV radiation. The protection of photosystem II
photochemical efficiency from UV radiation is determined by measuring
chlorophyll a
fluorescence.
7
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
The invention also includes method for protecting human eyes from solar
radiation, comprising applying at least one of a carotenoid, a polyphenolic
compound
andlor a mycosporine amino acid or a derivative of a carotenoid, a
polyphenolic
compound or a mycosporine amino acid to an eye wear lens or a window.
In another embodiment, the sunscreen composition includes a light absorbing
amino acid or a light absorbing amino acid derivative and a carrier.
The invention also includes a sunscreen composition comprising: a
carotenoid or a carotenoid derivative, a polyphenolic compound or a
polyphenolic
compound derivative, a light absorbing amino acid or a light absorbing amino
acid
derivative and a carrier. The amino acid or amino acid derivative is
preferably
selected from the group consisting of tyrosine, tryptophan, a tyrosine
derivative and
a tryptophan derivative. The derivatives have sunscreen activity which means
that
they are capable of absorbing light (preferably ultraviolet radiation) and are
useful in
the sunscreen compositions of the invention to reduce damage caused by light.
The invention relates to a sunscreen composition comprising a carrier and at
least one compound selected from the group consisting of: a carotenoid, a
carotenoid derivative having sunscreen activity, a polyphenolic compound, a
polyphenolic compound derivative having sunscreen activity, sunscreen amino
acid
and a sunscreen amino acid derivative. Another aspect of the invention relates
to a
sunscreen composition including: a) a carotenoid or a carotenoid derivative
having
sunscreen activity; b) a polyphenolic compound or a polyphenolic compound
derivative having sunscreen activity; and c) a light absorbing amino acid
having
sunscreen activity and a light absorbing amino acid derivative having
sunscreen
activity. The amino acid or amino acid derivative may be one of tyrosine,
tryptophan, a tyrosine derivative having sunscreen activity and a tryptophan
derivative having sunscreen activity.
The invention also includes a method of reducing degradation of a chemical
that is sensitive to ultraviolet light comprising applying a composition of
the invention
to the chemical. The .chemical is a herbicide, a pesticide, an auxin, a
gibberellin,
abscisic acid, a cytokinin. derivative of a carotenoid, a polyphenolic
compound, a
mycosporine amino acid and or a derivative of any of the foregoing (mixtures
or pure
preparations).
8
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/009$1
The invention also includes a method of determining the sunscreen activity of
an extract, including: extracting photoautotrophic cells to produce a
solution;
producing an aqueous filter; determining whether the aqueous filter protects
photosystem I or II from UV radiation wherein improved protection from UV
radiation
indicates that the compound has sunscreen activity. Compounds may also be
purified from the extracts for use in the method.
The invention includes a system for determining the sunscreen activity of a
test compound including:
a) light means or light member for generating ultraviolet radiation;
10 b) container means or container member, coupled to the light means, for
containing
a photoautotrophic bacterial culture, homogenate or extract thereof having PSI
or
PSII activity;
c) sample means or sample member for holding a test compound, interposed
between the light means and the container means.
15 The system may further include a scoring means or a scoring member for
assaying the culture, homogenate or extract to determine PSI or PSII activity,
wherein the amount of decrease in PSI or PSII activity caused by ultraviolet
radiation
indicates the sunscreen activity of the test compound.
The invention also includes a method for determining the sunscreen activity
20 of a test compound comprising the steps of:
(a) generating ultraviolet radiation for passing through the test compound;
(b) exposing a photoautotrophic bacterial culture, homogenate or extract
thereof
having PSII activity to the ultraviolet radiation being passed through the
test
compound, the test compound being spaced from the culture, homogenate or
25 extract;
(d) assaying the culture, homogenate or extract for PSI or PSII activity; and
(e) correlating the PSI or PSII activity to the sunscreen activity of the test
compound.
Purified compounds (synthetic or isolated from culture, homogenate or
extract),
photoautotrophic bacterial culture, homogenate or extract may be tested in the
30 systems or methods of the invention.
The method may further include determining the sun protection factor of the
test compound.
9
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
The invention also includes a method for reducing ultraviolet light damage to
a surface, including applying to the surface an effective amount of an extract
from a
photoautotroph, wherein the extract has sunscreen activity. The photoautotroph
may
be selected from the group consisting of photoautrotrophic bacteria,
photoautrotrophic plants, photoautrotrophic fungi or heteroautotrophic
bacteria. The
surface may be skin (eg human skin). In the method, the extract includes at
least
one compound from the group consisting of: a carotenoid, a carotenoid
derivative
having light absorption activity, a polyphenolic compound, a polyphenolic
compound
derivative having light absorption activity, a light absorbing amino acid and
a light
absorbing amino acid derivative.
BRIEF DESCRIPTION OF THE DRAWINGS
Preferred embodiments of the invention will be described in relation to the
drawings in which:
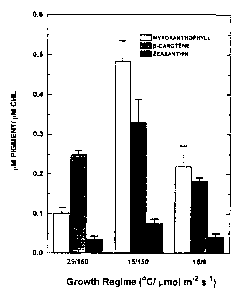
Figure 1. Effects of exposure to excessive radiation on the carotenoid
composition
of Plectonema boryanum. Conditions of excessive radiation (15/150) were
created
by exposing cell cultures to low temperature (15o C) and moderate light
intensity
(150 pmol m 2 s 1 ). Control cultures (29 / 150) were exposed to high
temperature
(29 oC) and moderate light intensity (150 p,mol m 2 s 1). Cell cultures
relieved from
exposure to excessive radiation (15 / 6) were exposed to low temperature (15
oC)
but low light intensity (6 pmol m 2 s 1 ). Mean values t were calculated from
7 to 9
measurements from 3 to 5 independent experiments.
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
Figure 2. Effect of UV-A treatment on the efficiency of photosystem II of
Plectonema boryanum cultures. Cells were grown either under control conditions
(29 / 150) or exposed to excessive radiation (15 / 150). Both cultures were
then
exposed to UV-A radiation and photosystem II efficiency, estimated as the
chlorophyll a fluorescence ratio Fv / Fm, was measured as a function of time.
All
data are presented as a percentage of Fv / Fm of non-treated control cultures.
Mean
values are calculated from 5 to 7 measurements in 3 to 5 independent
experiments.
Figure 3. Effect of UV-A + UV-B treatment on the efficiency of photosystem II
of
Plectonema boryanum cultures. Cells were grown either under control conditions
(29 I 150) or exposed to excessive radiation (15 I 150). Both cultures were
then
exposed to UV-A + UV-B radiation and photosystem II efficiency, estimated as
the
chlorophyll a fluorescence ratio Fv / Fm, was measured as a function of time.
All
data are presented as a percentage of Fv / Fm of non-treated control cultures.
Mean
values are calculated from 5 to 7 measurements in 3 to 5 independent
experiments.
Figure 4. Chemical structures of scytonemin, mycosporine and myxoxanthophyll.
Figure 5. Absorption spectra of commercial UV sunscreen creams (BRAND 1 and
BRAND 2) and 0.5% CYANOS CREAM.
Figure 6. A. Effect of CYANOS CREAM (0.5%) on the UV light - induced
deterioration
of PSII photochemistry measured as Fv/Fm in P. boryanum cell culture. B.
Exposure
time effects of UV light on the Fv/Fm in control sample and sample protected
by
CYANOS CREAM. The data are presented as a percentage of non-UV treated
samples.
C. Protection effect of CYANOS CREAM calculated as a difference between the
Fv/Fm
values of cells protected by CYANOS CREAM (+ CYANOS CREAM) and control
sample.
11
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
Figure 7. Exposure time dependent protection against UV light of commercial UV
sunscreen creams BRAND1 and BRAND 2 and the CYANOS CREAM. All creams were
applied by spreading 0.3 gm of cream over a fixed area ( 9.45 cm2) of the
polystyrene
culture flask. This resulted in a standardized application of 0.032 gm of
cream I cmz.
The amount of cream used and the surface area may be varied.
DETAILED DESCRIPTION OF THE INVENTION
Preferred embodiments of the invention will be described in relation to the
data from our photosynthetic experiments.
Light is a fundamental form of energy that enters our biosphere through the
process of photosynthesis and ultimately sustains all living organisms. In
photosynthetic organisms, this light energy is absorbed by pigments such as
chlorophyll which are present in essential structures called photosystems.
With the
help of photosystem I and photosystem II, the solar radiation absorbed by the
pigments is subsequently converted to chemical energy that is used to make
complex sugars from relatively simple carbon dioxide molecules present in our
air.
Photosynthetic organisms face an important conundrum: although they require
light
for photosynthesis and, thus, require light to survive, exposure to excessive
solar
radiation containing UV-A and UV-B can destroy the photosystems. Thus, it is
imperative that plants, green algae and cyanobacteria protect these
photosystems
from damaging UV radiation.
Identification of Sunscreen Compounds
We have shown that when the cyanobacterium, Plectonema boryanum, is
exposed to conditions whereby the photosystems are exposed to too much light
energy, the cultures increase their production of three important sunscreening
agents: the carotenoid, myxoxanthophyll and scytonemin and mycosporine amino
acids. When this cyanobacterium is exposed to excessive radiation (Figure 1,
15/150), this organism produces about 5 times more myxoxanthophyll compared to
control, untreated cells (Figure 1, 291150). This response appears to be
specific for
myxoxanthophyll since the other major carotenoids, 13-carotene and zeaxanthin,
12
CA 02350056 2001-04-23
WO 00/Z4369 PCT/CA99/00981
exhibit minimal increases. Furthermore, when these cells were relieved from
exposure to excessive radiation (Figure 1, 15/6), the levels of
myxoxanthophyll
decreased to levels close to that of controls (Figure 1: compare 15/6 with
29/150). In
addition to the accumulation of the carotenoid myxoxanthophyll, these cells
(Table 2,
151150) doubled the levels of the UV-absorbing compounds scytonemin (Scy) and
mycosporine amino acids (MAA) relative to control, untreated cells (Table 2,
29/150).
As observed with myxoxanthophyll, the levels of scytonemin and mycosporine
returned to levels found in controls when the cells were relieved from
exposure to
excessive radiation (Table 2, compare 15/6 with 29/150).
Table 2. Effects of growth regime (temperature and light (photosynthetic
photon flux
density) on the content of UV-absorbing compounds scytonemin (Scy) and
mycrosporine amino acids (MAA). Mean values tSE are calculated from 3
independent experiments.
Growth Regime mg Scy I mg chlorophyll mg MAA / mg chlorophyll
(oC, mol photons m 2 s 1 )
29/150 0.6870.010 3.84310.125
15/150 1.14510.090 8.45010.213
15/6 0.72410.010 4.96910.270
To show that these cyanobacterial cultures were indeed protected against UV
radiation when they had accumulated high levels of myxoxanthophyll, scytonemin
and mycosporin, we examined changes in the efficiency of photosystem II when
the
cyanobacterial cultures were exposed to excessive radiation (Figure 2).
Changes in
the efficiency of photosystem ll ("PS II") is the most sensitive measure of
photodamage to photosynthetic organisms exposed to excessive radiation and can
be measured conveniently as changes in the chlorophyll fluorescence ratio,
Fv/Fm
(Long et al., 1994, Ann. Rev. Plant Physiol. Plant Mol. Biol. 45: 633).
Changes in the
efficiency of photosystem I ("PSI", for example, as determined by
spectroscopy) may
also be used as a measure. The results in Figure 2 illustrate that the
efficiency of
13
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
photosystem II, measured as FvIFm, in control, cyanobacterial cells (closed
symbols) with low levels of the three protective compounds (29/150), decreased
gradually over time of. exposure to UV-A. After 90 minutes of exposure to UV-
A, the
efficiency of photosystem II had decreased to about 50% of initial values and
to
5 about 30% after 90 minutes exposure to UV-A + UV-B (Figure 3, closed
symbols).
Cells which had been relieved from exposure to excessive radiation (1516) and
hence also accumulated low levels of the three compounds, also exhibited
similar
sensitivities to UV-A and UV-A + UV-B radiation as controls cultures (data not
shown). In contrast, photosystem II efficiency decreased by 5% or less during
10 exposure to either UV-A (Figure 2, open symbols) or UV-A + UV-B (Figure 3,
open
symbols) indicating that the cyanobacterial cultures which accumulated high
levels of
myxoxanthophyll, scytonemin and mycosporine were virtually completely
resistant to
UV radiation. Thus, the presence of increased levels of myxoxanthophyll,
scytonemin and mycosporine amino acids protects the highly sensitive
15 photosynthetic photosystems of these cyanobacterial cells from excessive UV-
A and
UV-B radiation. The general chemical structures of these compounds is
illustrated in
Figure 4. '
These natural compounds and their derivatives make excellent sunscreen
agents to protect humans as well as objects from exposure to excessive UV
20 radiation. The mechanism by which we have induced these cyanobacterial
cultures
to accumulate these compounds appears to be due to the unique interaction of
environmental parameters such as light intensity and temperature and not to UV
radiation. This is consistent with published data indicating that terrestrial
plants as
well as algae sense changes in temperature and light through changes in the
redox
25 status of PSII, that is, PSII excitation pressure (Maxwell et al., 1995,
Plant Physiol.
107:687; Maxwell et al., 1995, Plant Physiol. 109:787; Gray et al., 1997,
114:467;
Escoubas et al., 1995, Proc. Nat. Acad. Sci. USA 92: 10237; Dumford and
Falkowski, 1997, Photosyn. Res. 53:229). We have also invented a novel method
to
induce the accumulation of these natural screening compounds in an organism
that
30 is very easy to grow and amenable to manipulation by controlling culturing
conditions.
In a preferred embodiment, the sunscreen agents that may be used
individually, or together, in the compositions include: 1 ) a cyanobacterial
carotenoid,
14
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
preferrably myxoxanthophyll, 2) a polyphenolic compound, preferrably
scytonemin
and 3) at least one type of amino acid derivative, preferably a mycosporine
amino
acid. However, the greatest efficacy for protection against high light and UV
radiation occurs when these compounds are present in combination. Each class
of
compound in this composition absorbs a specific range of light ie. carotenoids
in
general absorb in the visible range, mycosporine absorbs primarily UV-B and
scytonemin absorbs both UV-B and UV-A. These compounds may be isolated from
any species of cyanobacteria, such as Plectonema boryanum, Gloeocapsa sp.,
Chlorogloeopsis sp. Scytonema sp. and Nostoc commune. The compositions also
preferably include a cosmetically acceptable carrier compatible with human
skin.
(1) Cyanobacterial Carotenoids
The preferred carotenoids of this invention are the product of cyanobacterial
biosynthesis activated under conditions of excessive visible radiation (Ehling-
Schulz
et. AL, 1997, J. Bacteriol. 179:1940). Typical carotenoids include: f3-
carotene, lutein,
neoxanthin, zeaxanthin, violaxanthin, caloxanthin, nostoxanthin, echinenone,
canthexanthin, antheraxanthin, and oscillaxanthin. The preferred compounds
are,
among others, echinenone and myxoxanthophyll (Figure 4). In this application,
the
term carotenoid includes both single species of carotenoid and a mixture of
several
carotenoids.
(2) Polyphenoiic Compounds
Scytonemin or one of its conjugates is the preferred polyphenolic compound
(Figure 4). It is a yellow, lipid-soluble pigment with an in vivo absorption
maximum of
370 nm and has a structure based on indolic and phenolic subunits (Protean et
al.,
1993, Experentia 49:825; Ehling-Schuiz et al., 1997, 179: 1940; Garcia-Pichel
et al.,
1992, Photochem. Photobiol. 56: 17). In this application, the term
polyphenolic
compound includes both a single species of polyphenolic compound and a mixture
of
several polyphenolic compounds.
(3) Mycosporine Amino Acids
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99100981
Mycosporine is a general term for a group of about 10 to 12 compounds that
exhibit the general structure shown in Figure 4. All mycosporines have the
central
ring structure shown with various amino groups modifying this ring structure.
Mycosporine amino acids represent a relatively broad class of water-soluble
substituted cyclohexenes that are linked to amino acids and iminoalcohols and
have
absorption maxima between 310 and 360 nm. The term mycosporine in this
application includes both a single species of mycosporine compound and a
mixture
of several mycosporines (Garcia-Pichel et al., 1992, J. Bacteriol. 56: 17;
Garcia-
Pichel et al., 1993, Applied Environ. Microbiol. 59: 170; Ehling-Schilz et
al.,1997, J.
Bacteriol. 179: 1940; Xiong et al., 1997, Physioi. Plant. 100: 378; Karsten et
al.,
1998, Phycological Res. 46: 271 ). All of the compounds commonly referred to
as
mycosporine are included within the scope of the invention. Typical
mycosporine
amino acids include: mycosporine-glycine, palythine, asterina-330, palythinol,
palythene, porphyra-334, mycosporine-glycine:valine, shinorine and MAA 357.
(4) Aitemative Compositions
Alternative compositions of the invention include sunscreen agents from other
organisms, such as plant, algal, fungal and bacterial sunscreen agents.
Carotenoids, polyphenolic compounds and mycosporine amino acids from
plants, green algae and cyanobacteria may also be used together or
individually in
the compositions. Carotenoids, as a general class of pigments, are found in
all
photosynthetic organisms including plants, green algae, cyanobacteria and
photosynthetic bacteria. Although the poiyphenolic scytonemin is particular to
cyanobacteria, plants as well as green algae produce other polyphenolic
compounds
that act as potential UV screening compounds. Mycosporine amino acids are
found
in a myriad of Antarctic marine organisms and are of similar structure
independent of
its biological source (Karentz et al., 1991, Mar. Biol. 108: 157). Amino acids
that
absorb solar radiation adequately, such as tyrosine and tryptophan, are also
useful
in the compositions of the invention.
Certain species of heterotrophic organisms (such as certain fungi and
bacteria) produce carotenoids to protect themselves from light. However, these
organisms do not produce these pigments in response to excitation pressure.
They
16
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/009$1
may produce these compounds in response to UV exposure or other
photobiological
signals. The invention also includes carotenoids or pigments from either
photosynthetic or heterotrophic (ie. non-photosynthetic) organisms, for
example
Deinococcus radiodurans, which produces a carotenoid which is a xanthin,
called
deinoxanthin. It is also possible to use extracts of these organisms in
sunscreen
compositions.
Modification of cvanobacterial sunscreen agents and related sunscreen agents
Compounds similar to the carotenoid, polyphenolic compounds, mycosporine
amino acids (and light absorbing amino acids such as tryptophan and tyrosine)
and
other sunscreen agents of the invention described in this application may also
be
used in the cornpositions.« Derivatives may be prepared by the following
general
methods and procedures as well as other procedures known in the art. For
example,
carbonyl groups on scytonemin and mycosporine may be reduced or reductively
aminated to give an alcohol or an amine compound. Optionally, these alcohol or
amine compounds can be further derivatized by reaction with, for example, acyl
halides, acyl anhydrides, halo formates and isocyanates to afford esters,
amides,
carbonates, ureas and the like. Amine compounds can also be reductively
alkylated
with aldehydes and ketones to from secondary amines. Such derivatization
reactions of alcohols and amines are well known and can be accomplished using
known procedures (Organic Chemistry, 1967, Morrison and Boyd, Second Edition).
The alcohol groups on mycosporine, scytonemin and myxoxanthophyll may be
modified using similar techniques. The water or lipid solubility of the
compounds
may be changed by either removing or conjugating the compounds with sugars,
oligosaccharides, fatty acids, fatty alcohols, amino acids, peptides and
protein
modifications. These derivatives can be assayed using known techniques and
techniques described i~ this application to determine their ability to absorb
light and
their usefulness in the compositions of the invention. When the compounds are
intended for use on mammalian skin (e.g. humans) they may be tested for
compatibility with skin using known methods.
Extracts
17
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
The present invention shows that extracts of photoautotrophs, such as
photoautotrophic bacteria (eg. cyanobacteria) can be incorporated into
sunscreen
compositions. Heteroautotrophic bacteria may also be used. The extracts may be
aqueous extracts or hydroalcoholic extracts. The extracts are preferably
present in
amount necessary to give the desired protection from solar radiation. These
amounts would be apparent to one skilled in the art. For example, the amounts
of
extracts in the formulated sunscreen composition may range from 0.001 to 25%
by
weight, 0. 1 % to 25% by weight, 0.1 % to 10%, 0.1 % to 5%, 0.1 % to 1 % or
0.5% to
5%. For example, the cyanobacterial extracts can be made by treating the
photoautotrophic cells, such as cyanobacteria, with extraction agents by
methods
known in the art. Useful extraction agents may include water, mineral oil,
hydrocarbons, silicones, fatty acids, fatty acid derivatives, waxes, oils,
ketenes and
mixtures thereof. For example, water and an aliphatic alcohol, such as ethanol
may
be used. Hydrophobic extraction agents, as well as hydrophilic extraction
agents
may be employed. Such agents can include fatty acids, esters, diesters,
triesters,
hydrocarbons, waxes or silicones. Hydrophilic agents can include water and iow
molecular weight aliphatic alcohols. Other extraction agents will be apparent
to
those skilled in the art.
Preferably, in the present invention, the cyanobacterial extract is derived by
extraction in methanol and acetone. Useful extracts may also be obtained from
photosynthetic bacteria, plants and algae. The foregoing procedure may be
adapted
as necessary and applied to these organisms. The invention includes methods of
preparing sunscreen compositions by producing extracts from photoautotrophs,
photoautotrophic bacteria (such as cyanobacteria), heteroautotrophic bacteria
and
other organisms and formulating the extracts in a sunscreen composition (the
active
ingredients may be separated from the extracts). The invention also includes a
method of identifying a composition or an agent with sunscreen activity by
preparing
an agent or a composition, such as an extract, from one of the aforementioned
organisms and determining the ability of the agent or composition to act as a
sunscreen by measuring its sunscreen activity. Techniques described in this
application may be used to identify sunscreen activity.
Formulation of Sunscreen Compositions
A large number of efficient formulation methods for sun care products have
1$
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
been reported during the past twenty years (Flick, 1984, Cosmetic and Toiletry
Formulations, pp 513, Noyes Pub., New Jersey; Gasparro et al., 1998,
Photochem.
Photobiol. 68:243). Sunscreen products may include a wide range of ingredients
that do not absorb solar radiation but help to control characteristics such as
film
thickness, opacity, rub resistance, water proofing and uniformity. The
sunscreens
agents of the invention, carotenoids, such as myxoxanthophyll, andlor
polyphenolic
compounds, such as scytonemin and/or mycosporine amino acids or derivatives of
these compounds are formulated with known compounds in order to obtain
sunscreens with desired properties (Sun Care Products Formulary, 1998,
Cosmetics
and Toiletries 113:83).
The preferred concentrations for myxoxanthophyll are 0.02 to 20 mg by
weight and more preferably 0.5 to 5 mg by weight. The preferred concentrations
for
scytonemin are 0.01 to 10 mg by weight and more preferably 0.1 to 2 mg by
weight.
The preferred concentrations for mycosporine amino acids or other light
absorbing
amino acids are in the range 0.01 to 10 mg by weight and more preferably 0.1
to 2
mg by weight. Suitable masses and concentrations for other sunscreen agents
disclosed may be determined by those skilled in the art using known techniques
The compounds of the invention are also useful for integration into other
articles exposed to sunlight to prevent photodestruction and photobleaching.
The
following specific examples are presented to illustrate more particularly the
invention
and are not to be construed as limitations.
(1) Formulation of a UV Blocking Cream or other Composition
The formulated sunscreens preferably containing myxoxanthophyll,
scytonemin and mycosporine amino acids are preferably prepared in a phosphate
based emulsifying system combined with long chain esters such as lignoceryl
erucate to confer water resistance and controlled spreading when applied to
the skin.
Compositions are also formulated according to other techniques well known in
the
art, in particular techniques for preparation of oil-in-water or water-in-oil
emulsions.
The specific amount of sunscreen compounds needed to obtain a desired sun
protection factor (SPF) can be determined by those skilled in the art. The SPF
may
be determined according to known techniques. The preferred SPF is at least 2.
19
CA 02350056 2001-04-23
WO 00/243b9 PCT/CA99/00981
Other useful carriers include any of gases, water, water-based liquids,
lotions,
dispersions, oils, oil-based solutions, powder, gels, emulsions, dispersions
or
mixtures thereof. The appropriate amount of carrier can readily be determined
by
those skilled in the art according to the SPF desired. Hydrophobic carriers as
well as
hydrophilic carriers may be employed with the sunscreen compositions. Carriers
to
be applied to the skin or hair are compatible with human skin or hair,
respectively.
Additional sunscreen agents known in the ark may also be added to the
compositions, for example at least one additional hydrophilic or lipophilic
organic UV-
A and/or UV-B sunscreen agent. The compositions of the invention may also
include, in addition, conventional cosmetic adjuvants and additives such as
preservatives, organic solvents, browning agents, antioxidants, stabilizers,
emollients, silicones, alpha-hydroxy acids, demulcents, anti-foaming agents,
moisturizing agents, vitamins, fragrances, ionic or nonionic thickeners,
surfactants,
fillers, thickeners, sequestrants, polymers, propellants, alkalinizing or
acidifying
agents, opacifiers, fatty compounds (eg. oil, wax, aicohols, esters, fatty
acids),
colorants, or mixtures thereof or any other ingredient that may be used in
cosmetics
and in particular for the production of sunscreen compositions.
The invention also relates to a method of protecting human skin or hair
against the deleterious effects of solar radiation by topically applying
thereto an
effective amount of a composition of the invention.
(2) Formulation of the 'Cyanos-cream'
Plectonema boryanum was cultured under conditions of high excitation
pressure ie. low temperature (15°C) and moderate irradiance (150 Nmol
m'2 s'') to
maximize the production of myxoxanthophyll, scytonemin and mycosporine.
Subsequently, the cells were concentrated by centrifugation and extracted.
The steps to obtain the crude pigment extract consist of: break cells using
dry
ice or liquid nitrogen; extract the lysate using 100% methanol; isolate the
pigments
from the methanol solution; evaporate the methanol. The remaining material is
called crude pigment extract. One may also formulate purified compounds.
Compounds are separated and purified using techniques known in the art,
such as high pressure liquid chromatography (HPLC).
A suitable formulation base is Glaxal base (Roberts; Dermatological Base-
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
Emollient). Glaxal base is a water.miscible, nongreasy, nonmedicated,
hypoallergenic, lanolin free base used for the preparation or dilution of
dermatological creams and ointments. The active ingredients include: 4-chloro-
m-
cresol 0.1 %, cetomacrogol 1 000 1.8%, cetostearyf alcohol 7.2%, paraffin 15%,
petrolatum liquid 6%. Any emulsion, cream, lotion, spray that is either an oil
water or a water / oil base is a suitable base may be used. Prefered bases are
compatible with human skin and/or hair. Other suitable bases will be apparent.
0.5% weightlweight of the crude pigment extract were added to the base to form
the
"sunscreen lotion". The preferred masses described elsewhere in this
application
may also be used.
(3) Formulation of a personal-care product with UV screening compounds.
Compositions for hair or other personal care may be prepared by adding UV
screening compounds in hair rinses, aerosol sprays, mists, gels, mousses,
shampoos, conditioners, lotions, films, emulsions and colouring products to
reduce
photodamage to hair and photobleaching of hair and hair dyes. The invention
also
relates to a method of protecting human hair against the deleterious effects
of solar
radiation by topically applying thereto an effective amount of a composition
of the
invention.
Makeup products such as foundation, lipstick, eyeshadow, blush, nail polish,
mascara, moisturizing creams and lotions or eyeliner may also contain the
compounds of the invention. These are formulated according to known methods
for
makeup products such as those for preparation of an anhydrous or aqueous solid
or
paste, emulsion, suspension or dispersion.
(4) Formulation of car windshields, solar panels, solarium and building
windows with
UV screening compounds.
Car windshields, solar panels, solarium and building windows and other
glass, plexi-glass, transparent polymer, plastic or similar products may be
impregnated with UV-screening compounds or covered by a membrane impregnated
with UV-screening compounds to protect biological organisms, plants and
objects
from exposure to UV radiation.
21
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99100981
(5) Formulation of eyewear products with UV screening compounds.
Eye glasses, contact lenses and lens implants may be impregnated with UV
screening compounds or covered with a membrane impregnated with UV screening
compounds to protect eyes from exposure to UV-radiation.
(6) Formulation of transparent shields with UV screening compounds for use in
space travel.
Transparent shields and windows for use in space vehicles and space
stations may be impregnated with UV-screening compounds to protect biological
organisms, plants and objects from exposure to UV-radiation.
(7) Formulation of protective coatings With UV screening compounds.
The sun screen compounds may be incorporated into a variety of paints,
stains, coloring compositions, lacquers and similar coatings to prevent
premature
photodamage and photobleaching to surfaces of objects.
(8) Formulation of foliar sprays with UV screening compounds.
The sun screen compounds may be mixed with foliar sprays containing either
herbicides, pesticides or plant growth substances such as hormones, auxins,
gibberellins, abscisic acid, cytokinins and / or their derivatives to prevent
photodegradation of the herbicides, pesticides or plant growth substances such
as
auxins, gibberellins, abscisic acid and / or their derivatives. Similar
compositions
may be prepared for animals, such as mammals.
Overproduction of Screening Co J~ounds
The invention also relates to methods for inducing cyanobacterial cultures to
over-produce the screening compounds of the invention by growing the cultures
under high excitation pressure. This refers to the manipulation of photosystem
II
efficiency by altering its inherent oxidation-reduction state. We have shown
that
22
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
myxoxanthophyll is the primary carotenoid accumulated in Plectonema boryanum
which is localized almost exclusively in the cell wall of this filamentous
cyanobacterium. We have shown for the first time that the accumulation of
myxoxanthophyll, scytonemin and mycosporine amino acids are a response to
growth at high excitation pressure rather than UV-radiation. This high
excitation
pressure can be created for example either by keeping the growth irradiance
constant and lowering the growth temperature or by keeping the growth
temperature
constant and increasing the growth irradiance in the absence of UV-radiation.
The
accumulation of these compounds makes the cultures totally resistant to UV-A
and
UV-B radiation based on the measurement of photosystem II efficiency (Figures
2
and 3). Where typical or preferred process conditions (e.g., reaction
temperatures,
times, mole ratios of reactants, solvents, pressures, etc.) are given in this
application, other process conditions may also be used. Optimum conditions may
vary with the particular cultures, nutrients or reactants used, but such
conditions can
be determined by one skilled in the art by routine optimization procedures.
Sunscreen compound production may be induced in plants (preferably higher
plants), algae and other organisms containing chlorophyll a or b, using
similar
methods and known methods in the art.
Cyanobacteria, such as Plectonema boryanum, may be induced to
accumulate high levels of myxoxanthophyll, scytonemin and mycosporine by
simply
growing the cultures at either moderate irradiance (150 wmol m 2 s 1 ) and a
temperature of 15oC or at a moderate temperature of 29oC and high light (750
~mol
m 2 s 1 ). Thus, we exploit these rapidly growing cyanobacteria as a natural
source
of these screening compounds. We culture the cyanobacteria under conditions
which maximize production of myxoxanthophyll, scytonemin and mycosporine in
the
shortest possible time.
One important factor that constrains the efficiency and capacity to produce
these compounds biologically is growth temperature. The lower the growth
temperature, the slower is the rate of growth of the cells and, therefore, the
slower
the rate of production of the compounds of interest. In fact, growth
temperature and
not growth irradiance is the major factor limiting growth rates of Plectonema
23
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
boryanum (Miskiewicz and Huner, unpublished). However, low growth temperature
is required for maximum production of the screening compounds. Since the
growth
rate of Plectonema boryanum, measured as dry matter production, has a Q10 of
about 2 between 15oC and 30oC, an increase of only 1oC in growth temperature
has a potential to increase cell dry matter production by about 10%. We may
determine the optimum growth temperature for the maximum production of
myxoxanthophyll, scytonemin and mycosporine with the batch culture method
presently used in the laboratory. Growth irradiance (150 ~,mol photons m 2 s 1
) and
inoculum concentrations remain constant and growth temperature varied between
a
high temperature of 29oC and a low temperature of 15oC. Pigments are
preferably
extracted in acetone, separated, quantified on a per gram cell dry weight
basis by
HPLC as described in detail previously (Maxwell et al., 1995, Plant Physiology
107:687). Scytonemin and mycosporine amino acids are extracted in methanol and
then quantified spectrophotometrically. Subsequently, mg screening compound
produced per gram cell dry weight is plotted against growth temperature to
determine optimum growth temperature for maximum production of the screening
compounds.
The accumulation of the screening compounds in Plectonema boryanum is a
response to increased PSII excitation pressure. Thus, we increase further the
production of these screening compounds at the optimum temperature by
increasing
the growth irradiance. After the optimum growth temperature for production is
established, the optimum irradiance for production is assessed. The growth
temperature is held constant at the optimum determined above and growth
irradiance varied between 150 and 750 p.mol photons m 2 s 1. Subsequently, mg
quantities of screening compound produced per gram cell dry weight is plotted
against growth irradiance to show optimum growth irradiance for the maximum
production of the screening compounds. Thus, the optimum growth temperature
and
growth irradiance conditions allow us to maximize production of the compounds
of
interest. From the known growth time, the rate of production is calculated as
mg
screening compound per gm cell dry weight per hour. From the known photon flux
24
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
used for the production of the screening compounds, we calculate the
efficiency of
production as mg screening compound accumulated per gm cell dry weight per
hour
per photon absorbed.
We also scale up culture production to maximize the volume of the culture
vessels and to maximize dry weight production. Larger culture vessels maintain
a
homogeneous distribution of light of sufficient intensity to create sufficient
excitation
pressure to induce the cells to produce the screening compounds. Second, the
vessel includes a water jacket to maintain constant low temperature (l5oC in
the
case of P. boryanum).
Scytonemin and mycosporine are not specific to the filamentous
cyanobacterium, Plectonema boryanum. Other species of cyanobacteria known to
produce scytonemin and mycosporine are useful sources of the screening
compounds when exposed to high PSII excitation pressure. Employing the same or
similar growth conditions for the production of the screening compounds in P.
boryanum, we produce sunscreening compounds in the following filamentous
cyanobacteria Gloeocapsa sp.(Garcia-Pichel et al., 1993, Applied Environ.
Microbiol.
59:170), Chlorogloeopsis sp. (Garcia-Pichel et al., 1992, Photochem.
Photobiol.
56:17), Scytonema sp (Protean et al., Experentia 49:825) and Nosl'oc commune
(Ehling-Schulz et al., 1997, J. Bacteriol. 179:1940). Since the tatter
flourishes under
extremely cold conditions, this species is ideal for the production of the
screening
compounds under high PSII excitation pressure. In addition, we produce
sunscreen
compounds in the unicellular cyanobacterial species Synechocystis and
Synechococcus sp. Concomitantly, we also assess the resistance of these
species
to UV-radiation relative to P. boryanum as described above (Figures 2 and 3).
These procedures are adapted for overproduction of screening compounds in
other organisms that produce sunscreen agents described in this application.
Biological Assay for Sunscreen Protection
Sun protection factor (SPF) is used generally as a measure of protection
against skin burn due to UV-B radiation. SPF is defined as the ratio of skin
burn due
to sun exposure that skin can tolerate with and without sunscreen protection.
Thus,
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/009$1
SPF is a measure of protection against sunburn due to UV-B exposure but does
not
reflect the damaging effects of exposure to UV-A. As a consequence, the
inherent
value of this measure to estimate sunscreen efficacy has come into question
recently
{Gasparro et al., 1998, Photochem. Photobiol. 68: 243). We use a sensitive,
relevant, biological assay to assess the efficacy of various compounds to
protect
against UV-radiation. This is accomplished by exploiting the sensitivity of
either
cyanobacterial, alga! or plant photosystem II to UV-radiation. The sensitivity
of
cyanobacterial photosystem II to UV-radiation (see Figures 2 and 3) is useful
in an
assay to assess the protective qualities of a potential sunscreen agent or a
formulated UV-screening cream. The agents are preferably tested using an
artificial
filter of cyanobacterial extract (or an extract from a plant, algae or
photosynthetic
bacteria). Protection from UV damage provided by the artificial filter is
assessed by
monitoring changes in the photosystem II efficiency of the cyanobacterial
culture
using the chlorophyll fluorescence parameter, Fv/Fm. This test is useful to
cosmetic
or pharmaceutical companies to test the efficiency of UV screening agents.
The advantages of the cyanobacterial or alga! culture assays include speed,
simplicity, reproducibility and low cost. The cyanobactecial or alga! culture
assays
will replace or supplement more expensive and complex animal models currently
in
use in the cosmetic and pharmaceutical industries. Although cyanobacterial
cultures
are likely to be the easiest organism to use to assay the effects of UV-
screening
compounds by monitoring the efficiency of photosystem using chlorophyll
fluorescence ratio (Fv/Fm), these assays are conducted using algae, lichens,
higher
plants and any other organism containing chlorophyll a.
Assessment of the Extracted ScreenincLCompounds to Protect Against UV-A and
UV-B Radiation
To show efficacy of extracts containing myxoxanthophyll, scytonemin and
mycospocine and the other compounds of the invention to protect against UV-
radiation, we create an artificial filter using a cyanobacterial extract. For
example,
cyanobacteria grown under conditions where the screening compounds do not
accumulate (either 29/150 or 15/6) are exposed to UV-A + UV-B radiation as
performed in experiments illustrated in Figures 2 and 3 above. Between this
culture
and the UV source, an artificial filter consisting of the base only is placed.
26
CA 02350056 2001-04-23
WO 00124369 PCT/CA99/009$1
Concomitantly, an artificial filter containing the extracted screening
compounds at a
known concentration is placed between an identical culture and the UV source.
The
effects of exposure to the UV radiation at 29oC is assessed by monitoring
changes
in photosystem II efficiency through changes in the ratio of Fv/Fm as a
function of
exposure time as illustrated in Figures 2 and 3. Thus, the cells under the
artificial
filter containing the screening compounds are protected from the UV-radiation
compared with identical cells associated with the artificial filter containing
only the
base. UV damage is quantified by the decrease in Fv/Fm andJor by changes in
other
chlorophyll fluorescence parameters such as Fo, Fo', Fm', Fv', Fv'IFm', qp and
qn,
during or following exposure to UV-radiation. We used a similar approach
successfully to test the capacity of plant anthocyanin, a red flavonoid
accumulated
specifically in leaf upper epidermal cells, to protect leaves from excess
irradiance
(Krol et al., 1995, Can. J. Bot. 73: 1119). The optimum concentration and
ratios of
myxoxanthophyil: scytonemin: rnycosporine required for maximum protection
against
UV-radiation are determined. Similar assessments are completed for other
sunscreen compounds described in this application.
We also determine the stability of the extracted compounds to visible light as
well as UV-radiation as a function of time. In our work with plant
anthocyanins (Krol
et al., 1995, 73: 1119 ), we stabilized the extract by altering the solvent
and pH. The
breakdown of myxoxanthophyll, scytonemin and mycosporine is monitored first by
changes in their visible and UV absorption spectra as well as by a decrease in
the
ability of the liquid filter containing these compounds to protect cells
against the UV-
radiation as indicated by increased photoinhibition of cyanobacterial cultures
that do
not contain these screening compounds. We now stabilize the extracted
screening
compounds initially by altering solvent, solvent concentrations and pH.
Bioassay to Assess UV Protection
Control cultures of Plectonema boryanum were grown at high temperature
(29°C) and moderate irradiance (150 ~mol m-2 s'') to ensure minimal
production of
myxoxanthophyll, scytonemin and mycosporine and thus maximal sensitivity to UV
radiation. Control cultures were transferred axenically to disposable Corning
polystyrene culture flasks with a stirring bar to keep the cells suspended.
The
27
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
surface of the culture flask was covered in black tape except for a small area
A
known amount (0.3 gm) of either a commercially available sunscreen, the 0.5%
'cyanos-cream' or the FORMULATION blank consisting of the carrier only was
applied and spread evenly over this area of the culture flask. The UV-visible
absorption spectra of a commercially available sunscreen (Brand 1 ), the 0.5%
'cyanos-cream' and the formulation blank (the carrier used for the 'cyanos-
cream')
on the surface of the culture flask are illustrated in Figure 5. Even though
Brand 1
had a reported SPF of 15, its absorption of UVB and UVA radiation between 280
and
400 nm is comparable to that of the carrier employed in the 'cyanos-cream'.
The
active UV absorbing agent in Brand 1 was present at a concentration of 8%. In
contrast to Brand 1, the 0.5% 'cyanos-cream exhibited significantly greater
absorption not only in the UVB-UVA region of the spectrum (280 to 400nm) but
also
in the visible region of the spectrum (400 to 650 nm). Thus, the 0.5% 'cyanos-
cream'
exhibited greater UV absorption than the commercially available cream (Brand 1
)
even though the active absorbing agents in the 'cyanos-cream' were present at
a 16-
fold lower concentration than the active ingredient in Brand 1.
Purification of Screening Compounds
UV screening compounds are preferably used as crude extracts or partially
purified extracts obtained from chlorophyll containing organisms. These
screening
compounds may alsa~ be purified. Extracts and sunscreen compounds are
preferably
further purified preferably by preparative HPLC. For exmaple, semi-purified
extracts
are dissolved in chloroform/methanol and injected onto preparative HPLC
columns
(30 x 100cm) containing high-resolution silica gel ( 200-400 mesh) particles.
Solvent
is pumped through the column using a Kiloprep Waters preparative HPLC system.
Fractions containing myxoxanthophyll, scytonemin and microsporine are detected
using a UV/visible flow detector, collected and the solvent evaporated in
vacuo.
Purified material is tested on cyanobacterial cultures as a UV screen using
the liquid filter method as described above. Because myxoxanthophyil is by far
the
most abundant material produced by the cyanobacteria, it is extensively
purified and
used as a UV screen. Fractions containing mixtures of scytonemin and
mycosporine
are also used to screen out UV radiation separately as well as in combination
with
myxoxanthophyll. Similar procedures are used to purify the other sunscreen
28
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
compounds described in this application.
Further experiments {1) show the validity of the biological assay to assess
protection against UV-induced damage and (2) test the ability of a formulation
('cyano-cream'), derived from a crude extract of Plecfonema boryanum cultured
under high excitation pressure (15°C / 150 Nmol m-Z s'), to protect
control cultures of
cyanobacteria against UV-induced damage relative to commercially available
sunscreens of known SPF.
These culture flasks with the control suspensions of Plectonema boryanum
and containing either no cream (control sample) or 0.3 gm of 'cyanos-cream'
spread
over a surface area of 9.45 cm2 were subsequently exposed to UV radiation at
25°C
for up to 2 hours. Samples of the cell suspension were removed every 30
minutes to
assess PSII photochemical efficiency measured as Fv/Fm (Figure ~6A). In the
absence of any cream on the surface of the culture flask (Figure ~6A, closed
squares), the FvIFm ratio decreased by about 50% after 2h exposure to UV
radiation
indicating damage to the photosynthetic apparatus. In contrast, in the
presence of
the 0.5% 'cyanos-cream' on the surface of the culture flask (Figure ~6A,
closed
circles), the Fv/Fm ratio of the Plectonema suspension decreased by only 10%
after
2 h exposure to UV radiation indicating a significant protection against UV
radiation-
induced damage. When 0.3 gm of the carrier (formulation blank) was spread on
the
surface of the culture flask, no protection against UV radiation was observed.
This
shows that the protection against the UV radiation imparted by the 0.5%
'cyanos-
cream' is due to the cyano-extract incorporated into the formulation.
Figure 6B illustrates the same data as illustrated in Figure 6A but normalized
to the initial FvIFm value of the Plectonema suspension prior to exposure to
the UV
radiation. From the normalized data we calculated the % protection imparted by
the
0.5% 'cyanos-cream' relative to the control, unprotected sample during the 2 h
exposure to the UV radiation (Figure 6C). The % protection was calculated by
subtracting the FvIFm value of the control samples (Figure 6B, closed squares)
from
the Fv/Fm value of the sample with the 0.5% 'cyanos-cream' (Figure 6B, closed
circles) at each time of exposure to the UV radiation. Thus, after 2h exposure
to UV
radiation, the 0.3 gm of the 0.5% 'cyanos-cream' provided 30% greater
protection
than either no cream or 0.3 gm of the carrier. We conclude that the 0.5%
'cyanos-
cream' does indeed protect the cyanobacterial culture against damage due to UV
29
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/009$1
radiation. Furthermore, we conclude that our bioassay which assesses the UV-
induced damage to the photosynthetic apparatus via the standard chlorophyll
fluorescence ratio, FvIFm, is a sensitive and useful assay for UV damage. An
added
advantage of this bioassay is that it is relatively inexpensive, easy to
perform and
circumvents the use of animals to assess UV damage. The assay may be used to
test other compositions in addition to sunscreen, such as makeup and the other
compositions listed in this application.
The protection against UV-induced damage provided by the 0.5% 'cyanos-
cream' is superior to that provided by commercially available sunscreens of
known
SPF. To show this, we purchased two sunscreen creams available from two
different
manufacturers. Both Brand 1 and Brand 2 were labelled as exhibiting an SPF of
15.
In both cases, the active UV absorbing ingredient was present at a
concentration of
8%. Using the same bioassay as described above, we compared the capacity of
0.3
gm of either Brand 1, Brand 2 or the 0.5% 'cyanos-cream' to protect a
suspension of
Plectonema boryanum against UV damage. The results illustrated in Figure 37
indicate that the 0.5% 'cyanos-cream' imparted 2 to 3 times greater protection
after 2
h of exposure to V radiation than either Brand 1 or Brand 2 respectively.This
higher
level of protection was provided by the 'cyanos-cream' despite the fact that
the
concentration of the active ingredients in the 'cyanos-cream' (0.5%) is 16-
fold lower
than that present in either Brand 1 or Brand 2 (8%). These data are consistent
with
the absorption spectra presented in Figure 1. Furthermore, we conclude that
the
0.5% 'cyanos-cream' exhibits an SPF greater than 15. A 0.5% 'cynaos-cream'
pravides protection against UV-induced damage that is greater than
commercially
available sunscreen preparations purported to exhibit an SPF value of 15.-
Other
systems and methods for determining the sunscreen activity of a compound are
taught, for example in US 5,691,158.
in addition, when we apply the commercial cream to the polystyrene culture
flasks container holding the cyanobacteria during exposure to UV radiation,
the
polystyrene becomes translucent. This reaction does nat occur unless the
container
is exposed to UV radiation. A photochemical reaction induces free radicals in
the
commercial formulations which subsequently react with the polystyrene. The
commercial creams do not have sufficient protection from free radicals induced
by
exposure to UV radiation. This reaction does not occur with the creams of the
invention. The carotenoids present in the formulations of invention quench any
free
CA 02350056 2001-04-23
WO 00/24369 PCT/CA99/00981
radicals produced by the UV radiation. The carotenoids will also quench
similar free
radicals produced by UV light on skin.
The present invention has been described in terms of particular embodiments
found or proposed by the present inventors to comprise preferred modes for the
practice of the invention. It will be appreciated by those of skill in the art
that, in light
of the present disclosure, numerous modifications and changes can be made in
the
particular embodiments exemplified without departing from the intended scope
of the
invention. All such modifications are intended to be included within the scope
of the
appended claims.
All publications, patents and patent applications including Canadian patent
application no. 2,251,x57 ("Composition Including Naturally Occuring compunds
from Plants, Algae and Cyanobacteria for Protectiton Against Solar
Radiation.") are
incorporated by reference in their entirety to the same extent as if each
individual
publication, patent or patent application was specifically and individually
indicated to
be incorporated by reference in its entirety.
31