Note: Descriptions are shown in the official language in which they were submitted.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-I-
ACETAL BENZYLMALTOSIDES AS INHIBITORS OF SMOOTH
MUSCLE CELL PROLIFERATION
BACKGROUND OF THE INVENTION
This invention relates to the use of substituted 4',6'-acetal benzylmaltosides
as smooth muscle cell proliferation inhibitors and as therapeutic compositions
for
treating diseases and conditions which are characterized by excessive smooth
muscle
proliferation such as restenosis.
All forms of vascular reconstruction such as angioplasty and vein bypass
procedures effect a response to injury that ultimately leads to smooth muscle
cell
(SMC) proliferation and subsequently, deposition of profuse amounts of
extracellular matrix (Clowes, A. W.; Reidy, M. A. J. Vasc. Surg 1991, 13,
885).
These events are also central processes in the pathogenesis of atherosclerosis
(Raines
E.W.; Ross R. Br. Heart J. 1993, 69 (Supplement), S. 30) as well as transplant
arteriosclerosis (Isik, F.F.; McDonald, T. O.; Ferguson, M.; Yamanaka, E.;
Gordon
Am. J. Pathol. 1992, 141, 1139). In the case of restenosis following
angioplasty,
clinically relevant solutions for controlling SMC proliferation through
pharmacological intervention have remained elusive to date (Herrman, J. P. R.;
Hermans, W.R.M.; Vos, J.; Serruys P. W. Drugs 1993, 4, 18 and 249). Any
successful approach to selective SMC proliferation inhibition must not
interfere with
endothelial cell repair or the normal proliferation and function of other
cells
(Weissberg, P.L.; Grainger, D.J.; Shanahan C.M.; Metcalfe, J.C. Cardiovascular
Res. 1993, 27, I 191 ).
The glycosaminoglycans heparin and heparan sulfate are endogenous
inhibitors of SMC proliferation, yet are able to promote endothelial cell
growth
(Castellot, J.J. Jr.: Wright, T. C.; Karnovsky, M.J. Seminars in Thrombosis
and
Hemostasis 1987, 13, 489). However, the full clinical benefits of heparin,
heparin
fragments, chemically modified heparin, low molecular weight heparins, and
other
heparin mimicking anionic polysaccharides may be compromised due to other
pharmacological liabilities (excessive bleeding arising from anticoagulation
effects, in
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-2-
particular) coupled with heterogeneity of the various preparations (Borman, S.
Chemical and Engineering News', 1993, June 28, 27).
WO 96/14325 discloses acylated benzylglycosides as smooth muscle cell
proliferation inhibitors. The compounds of the present invention differ in
that the
substituents on the carbohydrate backbone are different.
Zehavi, U.; Herchman, M. in Carbohyd. Res. 1986, 151, 371, disclosed 4-
carboxy-2-nitrobenzyl 4-O-a-D-glucopyranosyl-~i-D-glucopyranoside which is
attached to a polymer for study as an acceptor in the glycogen synthase
reaction. The
compounds of the present invention differ in that the substituents on the
benzyl
groups are different and the use (smooth muscle antiproliferation) is
different.
Patent numbers US 5,498,775, W096/14324, and US 5,464,827 describe
polyanionic benzylglycosides or cyclodextrins as smooth muscle cell
proliferation
inhibitors for treating diseases and conditions which are characterized by
excessive
smooth muscle proliferation. (3-cyclodextrin tetradecasulfate has been
described as a
smooth muscle cell proliferation inhibitor and as an effective inhibitor of
restenosis
(Reilly, C.F.; Fujita, T.; McFall, R. C.; Stabilito, I. L; Wai-se E.; Johnson,
R. G.
Drceg Development Research 1993, 29, 137). US 5019562 discloses anionic
derivatives of cyclodextrins for treating pathological conditions associated
with
undesirable cell or tissue growth. WO 93/09790 discloses antiproliferative
polyanionic derivatives of cyclodextrins bearing at least 2 anionic residues
per
carbohydrate residues. Meinetsberger (EP 312087 A2 and EP 312086 A2) describes
the antithrombotic and anticoagulant properties of sulfated bis-aldonic acid
amides.
US 4431637 discloses polysulfated phenolic glycosides as modulators of the
complement system. The compounds of the present invention differ from all of
the
prior art in that the compounds (a) are benzylglycosylamides which bear no
structural
resemblance to heparin, sulfated cyclodextrins, or to sulfated lactobionic
acid dimers,
(b) contain no more than two contiguous sugar residues (disaccharide), (c) are
of a
defined structure, (d) and are not sulfated.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-3-
DESCRIPTION OF THE INVENTION
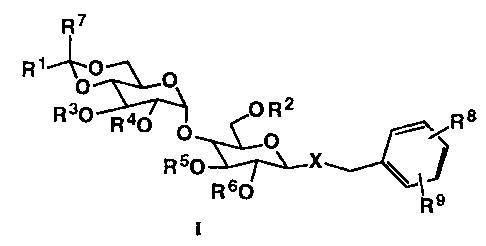
This invention provides benzylmaltosides of formula I
7
R~ O
O
2
R30 R40 O R ~-/Rs
RsO
R 60 ~ s
R
I
wherein
XisOorS;
Rl is alkyl of 1-6 carbon atoms, haloalkyl of 1-6 carbon atoms, nitroalkyl of
1-6
carbon atoms, cyanoalkyl of 1-6 carbon atoms, alkoxyalkyl of 2-12 carbon
atoms, phenyl mono-, di-, or tri-substituted with Rg, phenylalkyl of 7-10
carbon atoms, wherein the phenyl ring is mono-, di-, or tri-substituted with
Rg, pyridyl substituted with Rg, furyl substituted with Rg, thienyl
substituted
with Rg, and thiazolyl substituted with Rg;
R2 is hydrogen, acyl of 2-6 carbon atoms, haloacyl of 2-6 carbon atoms,
nitroacyl of
2-7 carbon atoms, cyanoacyl of 2-7 carbon atoms, or trifluoromethylacyl of 3-
8 carbon atoms, carboalkoxyacyl of 4-12 carbon atoms,
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-4-
O
Rio R» O
O I~~~~ R12 ~' I HIV Rio ~ - jR~o ~~ iRio
, , ~ ,
n
O
Rio R1~ ~~02 -~--fCH2 Rio ~~N
O ~~~~ R~2 ~'~ I ~ Rio n ~~1 R»
~' , ~r
%~ R1~ R12 '
13
R R
O O O
~~ N .~~ s .~ ,O
~/ ~ ~ ~ .
or N ,
Rio Rio
R3, R4, R5, and R6 are each, independently, hydrogen, acyl of 2-7 carbon
atoms,
benzoyl, wherein the phenyl moiety is mono-, di-, or tri-substituted with Rg,
haloacyl of 2-7 carbon atoms, nitroacyl of 2-7 carbon atoms, cyanoacyl of 2-
7 carbon atoms, or trifluoromethylacyl of 3-8 carbon atoms;
R~ is hydrogen, methyl, or phenyl;
Rg is hydrogen, alkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, phenyl,
-CN,
-N02, halogen, or -CF3;
R9 is hydrogen, alkyl of I-6 carbon atoms, alkoxy of 1-6 carbon atoms, phenyl,
-CN,
-N02, halogen, -CF3, -NHR3, -NR3R3, -NR3R1~, -NHC02R14, -NHS02R14,
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-5-
~N R1o -~-H ~'%R1o _~-H ~ ~ jRlo
-~_H ~ -Cl _ _H (.~ _~_H
~N ,
RION
R1o
R14
-~-NH P ' -~-NH
NHRIs
.N R1o R11
or ~\ /~ R12
R1o N _~_NH n
Rlo, R11, and R12, are each, independently, hydrogen, alkyl of 1-6 carbon
atoms,
alkoxy of 1-6 carbon atoms, phenyl, -CN, -N02, halogen, -CFA, acyl of 2-7
carbon atoms, or benzoyl, wherein the phenyl group or the phenyl moiety of
the benzoyl group is optionally mono-, di-, or tri-substituted with alkyl of 1-
6
carbon atoms, alkoxy of 1-6 carbon atoms, -CN, -N02, halogen, or -CFg;
R13 is hydrogen, alkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, -CN, -
N02,
halogen, -CF3, or phenyl group, wherein the phenyl moiety is optionally
mono-, di-, or tri-substituted with alkyl of 1-h carbon atoms, alkoxy of 1-6
carbon atoms, or halogen;
R14 is alkyl of 1-b carbon atoms;
R1$ is hydrogen, acyl of 2-7 carbon atoms, benzoyl, or -C02R16;
R16 is alkyl of 1-6 carbon atoms, benzyl, phenyl, or fluorenyl;
n = 0-3;
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-6-
p = 0-6;
or a pharmaceutically acceptable salt thereof.
Alkyl includes both straight chain as well as branched moieties. Halogen
means bromine, chlorine, fluorine, and iodine. When a compound of this
invention
contains a group that contains the same moiety more than once (i.e., when Ry
is
-NR3R3), each of the moieties may be the same or different.
Pharmaceutically acceptable salts can be formed from organic and inorganic
acids, for example, acetic, propionic, lactic, citric, tartaric, succinic,
fumaric, malefic,
malonic, mandelic, malic, phthalic, hydrochloric, hydrobromic, phosphoric,
nitric,
sulfuric, methanesulfonic, napthalenesulfonic, benzenesulfonic,
toluenesulfonic,
camphorsulfonic, and similarly known acceptable acids. Salts may also be
formed
from organic and inorganic bases, preferably alkali metal salts, for example,
sodium,
lithium, or potassium. Acid addition salts can be prepared when Y is nitrogen
or the
compound of formula I contains a basic nitrogen, and base addition salts can
typically
be prepared when the compound of formula I contains a hydroxyl group.
The compounds of this invention may contain an asymmetric carbon atom and
some of the compounds of this invention may contain one or more asymmetric
centers and may thus give rise to optical isomers and diastereomers. While
shown
without respect to stereochemistry in Formula I, the present invention
includes such
optical isomers and diastereomers; as well as the racemic and resolved,
enantiomerically pure R and S stereoisomers; as well as other mixtures of the
R and S
stereoisomers and pharmaceutically acceptable salts thereof.
Preferred compounds of this invention are benzylmaltosides of formula I
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
7
R~ O
O
R30 R40 O R2 ~/R8
R50
R 60 r s
R
I
wherein
XisOorS;
R1 is alkyl of 1-6 carbon atoms, haloalkyl of 1-6 carbon atoms, nitroalkyl of
1-6
carbon atoms, cyanoalkyl of 1-6 carbon atoms, alkoxyalkyl of 2-12 carbon
atoms, phenyl mono-, di-, or tri-substituted with Rg, phenylalkyl of 7-10
carbon atoms, wherein the phenyl ring is mono-, di-, or tri-substituted with
Rg, pyridyl substituted with Rg, furyl substituted with Rg, thienyl
substituted
with Rg, and thiazolyl substituted with Rg;
R2 is hydrogen, acyl of 2-6 carbon atoms, carboalkoxyacyl of 4-12 carbon
atoms,
O
Rjo R» O O
O \~/~ ~~'~IV ~ ,O~ Rio ~ _S~ Rjo
-R~2 -Rio ~~
n
R~~ R» ,502 -~~CH2 n Rio
O ~1 R~2 '~ ~'J Rio /~ R»
' R1~ ~ or ~ ~R~2
Rya
R3, R4, R5, and R6 are each, independently, hydrogen, or acyl of 2-7 carbon
atoms;
R~ is hydrogen, methyl, or phenyl;
Rg is hydrogen, alkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, phenyl,
-CN,
-N02, halogen, or -CF3;
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
_g_
R9 is hydrogen, -N02, halogen, -CF3, -NHR3, -NR3R3, -NR3R14, -NHC02R14,
-NHS02R14,
R1a
y-NH P ~ -~-NH
NHRIs
'~ N R, o -~'H ~ '%R ~ o
i1
_N~,S. Rio
H I ~ ~ ~ R,2
or
' -~-N H n
5
Rlo~ R11, ~d R12, ~.e each, independently, hydrogen, alkyl of 1-6 carbon
atoms,
alkoxy of 1-6 carbon atoms, phenyl, -CN, -N02, halogen, -CF3, acyl of 2-7
carbon atoms, or benzoyl, wherein the phenyl group or the phenyl moiety of
the benzoyl group is optionally mono-, di-, or tri-substituted with alkyl of 1-
6
10 carbon atoms, alkoxy of 1-6 carbon atoms, -CN, -N02, halogen, or -CF3;
R13 is hydrogen, alkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, -CN, -
N02,
halogen,' -CF3, or phenyl group, wherein the phenyl moiety is optionally
mono-, di-, or tri-substituted with alkyl of 1-6 carbon atoms, alkoxy of 1-6
carbon atoms, or halogen;
R14 is alkyl of 1-6 carbon atoms;
R15 is hydrogen, acyl of 2-7 carbon atoms, benzoyl, or -C02R16;
R16 is alkyl of 1-6 carbon atoms, benzyl, phenyl, or fluorenyl;
n = 0-3;
p = 0-6;
or a pharmaceutically acceptable salt thereof.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-9-
More preferred compounds of this invention are benzylmaltosides of formula I
R1 O
O
R3O R40 O R2 ~/R8
R 50
R 60 t s
R
I
wherein
XisO;
Rl is alkyl of 1-6 carbon atoms, haloalkyl of 1-6 carbon atoms, nitroalkyl of
1-6
carbon atoms, cyanoalkyl of 1-6 carbon atoms, alkoxyalkyl of 2-12 carbon
atoms, phenyl mono-, di-, or tri-substituted with Rg, phenylalkyl of 7-10
carbon atoms, wherein the phenyl ring is mono-, di-, or tri-substituted with
Rg, or pyridyl substituted with Rg;
R2 is hydrogen, acyl of 2-6 carbon atoms, carboalkoxyacyl of 4-12 carbon
atoms,
R10 R11 -~~CH2 p10
//~' R 12 ~ ' ~ ~ N R 10 ' ' ~ \/~' R 11
, \ 12 ,
R
~~/R11 ' .502
R12 ~ -R1o
o r , ~~ ,
' - R11
R13
R3, R4, R~, and R6 are each, independently, hydrogen, or acyl of 2-7 carbon
atoms;
R~ is hydrogen;
Rg is hydrogen, alkyl of 1-6 carbon atoms, -CN, or halogen;
R9 is hydrogen, -N02, halogen, -CF3, -NHR3, -NR3R3, -NR3R14, -NHCO2R14,
-NHSO~R14,
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
- 10-
R~4
-~-NH P~ ' -~-NH
NHRIs
O
_N~'~N _N _O~ Rio
-~ H I ! Rto -~ H
O Rio R»
_~_H~~ jR~o O ~~~~~ R12
' -~-N H n ,
Rlo, R11, and R12, are each, independently, hydrogen, alkyl of 1-6 carbon
atoms,
alkoxy of 1-6 carbon atoms, phenyl, -CN, -N02, halogen, -CF3, acyl of 2-7
carbon atoms, or benzoyl, wherein the phenyl group or the phenyl moiety of
the benzoyl group is optionally mono-, di-, or tri-substituted with alkyl of 1-
6
carbon atoms, alkoxy of 1-6 carbon atoms, -CN, -N02, halogen, or -CFg;
R13 is hydrogen, alkyl of 1-6 carbon atoms, halogen, or phenyl group, wherein
the
phenyl moiety is optionally mono-, di-, or tri-substituted with alkyl of 1-6
carbon atoms, alkoxy of 1-6 carbon atoms, or halogen;
R14 is alkyl of 1-6 carbon atoms;
R15 is hydrogen, acyl of 2-7 carbon atoms, benzoyl, or -C02R16;
R16 is alkyl of 1-6 carbon atoms, or fluorenyl;
n = 0-3;
p = 0-6;
or a pharmaceutically acceptable salt thereof.
Specifically preferred compounds of this invention are:
N-(2-Chloro-5-[(4',6'-O-ethylidene)-p-D-maltosyloxymethylJ-phenyl}-acetamide
or
a pharmaceutically acceptable salt thereof;
CA 02350066 2001-05-09
WO 00/31096 PCTNS99/27828
-11-
(R)-N [5-[[[6-O-Benzoyl-4-O-(4,6-O-ethylidene-a-D-glucopyranosyl)-(i-D-
glucopyranosyl]oxy]methyl]-2-chlorophenyl]acetamide or a pharmaceutically
acceptable salt thereof;
(R)-N-[2-Chloro-5-[[[2,3-di-O-acetyl-6-O-benzoyl-4-O-(2,3-di-O-acetyl-4,6-O-
ethylidene-«-D-glucopyranosyl)-p-D-glucopyranosyl]oxy]methyl] phenyl]acetamide
or a pharmaceutically acceptable salt thereof;
N-{ 2-Chloro-S-[(4',6'-O-propylidene-13-D-maltosyl)-oxy-methyl]-phenyl }-
acetamide
or a pharmaceutically acceptable salt thereof;
N-{ 5-[(6-O-Benzoyl-4',6'-O-propylidene-~-D-maltosyl)-oxy-methyl)-2-chloro-
phenyl }-acetamide or a pharmaceutically acceptable salt thereof;
N (5-{[4',6'-O-Benzylidene-6-D-(4-toluenesulfonyl)-~-D-maltosyl]-oxy-methyl}-2-
chloro-phenyl)-acetamide or a pharmaceutically acceptable salt thereof;
N-(5-{ [2,3,2',3'-Tetra-O-acetyl-4',6'-O-benzylidene-fi-O-(4-toluenesulfonyl)-
p-D-
maltosyl]-oxy-methyl}-2-chloro-phenyl)-acetamide or a pharmaceutically
acceptable
salt thereof;
N {S-[(6-O-Benzyl-4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-chloro-
phenyl }-acetamide or a pharmaceutically acceptable salt thereof;
N-{ 5-[(6-O-Benzyl-4',6'-O-ethylidene-p-D-maltosyl)-oxy-methyl]-2-chloro-
phenyl }-
acetamide or a pharmaceutically acceptable salt thereof;
N (2-Chloro-5-{[4',6'-O-(4-nitro)-benzylidene-p-D-maltosyl]-oxy-methyl}-
phenyl)-
acetamide or a pharmaceutically acceptable salt thereof;
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
- 12-
N (5-{ [6-O-Benzoyl-4',6'-O-(4-nitro)-benzylidene-~-D-maltosyl]-oxy-methyl }-2-
chloro-phenyl)-acetamide or a pharmaceutically acceptable salt thereof;
N { 2-Chloro-5-[(4',6'-O-(4-chloro)-benzylidene-p-D-maltosyl)-oxy-methyl]-
phenyl }-acetamide or a pharmaceutically acceptable salt thereof;
N- { 5-[ (6-O-Benzoyl-4' ,6'-O-(4-chloro)-benzylidene-p-D-maltosyl)-oxy-
methyl]-2-
chloro-phenyl }-acetamide or a pharmaceutically acceptable salt thereof;
N-{ 2-Chloro-5-[(4',6'-O-isobutylidene-p-D-maltosyl}-oxy-methyl]-phenyl }-
acetamide or a pharmaceutically acceptable salt thereof;
N {5-[(6-O-Benzoyl-4',6'-O-isobutylidene-p-D-maltosyl)-oxy-methyl]-2-chloro-
phenyl}-acetamide or a pharmaceutically acceptable salt thereof;
N {5-[(4',6'-O-((1R)-2-Phenyl-ethylidene)-p-D-maltosyloxy)-methyl]-2-chloro-
phenyl }-acetamide or a pharmaceutically acceptable salt thereof;
N-{ 5-[(6-O-Benzoyl-4',6'-O-(( 1R)-2-phenyl-ethylidene)-~-D-maltosyloxy)-
methyl]-
2-chloro-phenyl}-acetamide or a pharmaceutically acceptable salt thereof;
N {2-Chloro-5-[(4',6'-O-((1R)-3-cyano-propylidene)-p-D-maltosyloxy)-methyl]-
phenyl}-acetamide or a pharmaceutically acceptable salt thereof;
N {5-{[6-O-Benzoyl-4',6'-O-((1R)-3-cyanopropylidene)-p-D-maltosyloxy]-methyl}-
2-chloro-phenyl }-acetamide or a pharmaceutically acceptable salt thereof;
N- { 2-Chloro-5-[(4',6'-O-(( 1 R)-3-ethoxy-propylidene)-p-D-maltosyloxy)-
methyl]-
phenyl}-acetamide or a pharmaceutically acceptable salt thereof;
N {S-{[6-O-Benzoyl-4',6'-D-((1R)-3-ethoxypropylidene)-p-D-maltosyloxy]-methyl}-
2-chloro-phenyl }-acetamide or a pharmaceutically acceptable salt thereof;
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
- 13-
N (2-Chloro-5-{[4',6'-O-(4-pyridinemethylidene)-p-D-maltosyl]-oxy-methyl}-
phenyl)-acetamide or a pharmaceutically acceptable salt thereof;
Benzoic acid 6-(3-acetylamino-4-chloro-benzoyloxy)-3-(7,8-dihydroxy-2-pyridin-
4-
yl-hexahydro-pyrano[3,2-d] [ 1,3] dioxin-6-yloxy)-4,S-dihydroxy-tetrahydro-
pyran-2-
ylmethyl ester or a pharmaceutically acceptable salt thereof;
N-{ 5-[(4',6'-O-Benzylidene-p-D-maltosyloxy)-methyl]-2-chloro-phenyl }-
acetamide
or a pharmaceutically acceptable salt thereof;
N {5-[(4',6'-O-Benzylidene-2,2',3,3',6-penta-O-acetyl-p-D-maltosyl-axy)-
methyl]-2-
chloro-phenyl }-acetamide or a pharmaceutically acceptable salt thereof;
N { 5-[(6-O-Benzoyl-4',6'-O-benzylidene-p-D-maltosyl-oxy)-methyl]-2-chloro-
phenyl}-acetamide or a pharmaceutically acceptable salt thereof;
(R)-N-[2-Chloro-5-[[[2,3-di-O-acetyl-6-O-benzoyl-4-O-[2,3-di-O-acetyl-4,6-O-
(phenylmethylene)-a-D-glucopyranoysl]-p-glucopyranosyl]-oxy]methyl]-
phenyl]acetamide or a pharmaceutically acceptable salt thereof;
(R)-N [2-Chloro-5-[[[6-O-(5-methoxy-1,5-dioxopentyl)-4-O-[4,6-O-
(phenylmethylene)-a-D-glucopyranoysl]-p-D-glucopyranosyl]oxy]methyl]-
phenyl]acetamide or a pharmaceutically acceptable salt thereof;
4-Chloro-3-nitro-benzyl-4',6'-O-benyzlidene-p-D-maltoside or a
pharmaceutically
acceptable salt thereof;
4-Chloro-3-nitro-benzyl-6-O-benzoyl-4',6'-O-benyzlidene-p-D-maltoside or a
pharmaceutically acceptable salt thereof;
CA 02350066 2001-05-09
WO 00!31096 PCT/US99/27828
- 14-
(R)-(4-Chloro-3-nitrophenyl)methyl-2,3-di-O-acetyl-6-O-benzoyl-4-O-(2,3-di-O-
acetyl-4,6-O-(phenylmethyleve)-a-D-glucopyranosyl]-[i-D-glucopyranoside or a
pharmaceutically acceptable salt thereof;
Nicotinic acid 6-(4-chloro-3-vitro-benzyloxy)-3-(?,8-dihydroxy-2-phenyl-
hexahydro-
pyrano[3,2-d][1,3]dioxin-6-yloxy)-4,5-dihydroxy-tetrahydro-pyran-2-ylmethyl
ester
or a pharmaceutically acceptable salt thereof;
(R)-(4-Chloro-3-nitrophenyl)methyl 4-[2,3-di-O-acetyl-4,6-D-(phenylmethylene)-
a-
D-glucopyranosyl]-p-D-glucopyranoside 2,3-diacetate 6-(3-pyridinecarboxylate)
or a
pharmaceutically acceptable salt thereof;
4-Methoxy-benzoic acid 6-(3-acetylamino-4-chIoro-benzyloxy)-3-(?,8-dihydroxy-2-
phenyl-hexahydro-pyrano[3,2-d] [ 1,3] dioxin-6-yloxy)-4,5-dihydroxy-tetrahydro-
pyran-2-ylmethyl ester or a pharmaceutically acceptable salt thereof;
4-Methoxy-benzoic acid 4,5-diacetoxy-6-(3-acetylamino-4-chloro-benzyloxy)-3-
(?,8-
diacetoxy-2-phenyl-hexahydro-pyrano[3,2-d] [ 1,3]dioxin-6-yloxy)-tetrahydro-
pyran-
2-ylmethyl ester or a pharmaceutically acceptable salt thereof;
4-Chloro-benzoic acid 6-(3-acetylamino-4-chloro-benzyloxy)-3-(7,8-dihydroxy-2-
phenyl-hexahydro-pyrano[3,2-d][ 1,3]dioxin-6-yloxy)-4,5-dihydroxy-tetrahydro-
pyran-2-ylmethyl ester or a pharmaceutically acceptable salt thereof;
4-Chloro-benzoic acid 4,5-diacetoxy-6-(3-acetylamino-4-chloro-benzyloxy)-3-
(?,8-
diacetoxy-2-phenyl-hexahydro-pyrano[3,2-d] [ 1,3]dioxin-6-yloxy)-tetrahydro-
pyran-
2-ylmethyl ester or a pharmaceutically acceptable salt thereof;
(R)-N-[2-Chloro-5-[[[6-O -(4-chloro-3-nitrobenzoyl)-4-O-[4,60-
(phenylmethylene)-
«-D-glucopyranosyl]-p-D-glucopyranosyl]oxy]methyl]phenyl]acetamide or a
pharmaceutically acceptable salt thereof;
CA 02350066 2001-05-09
WO 00/31096 PCTNS99/Z7828
-15-
N-{5-[(2,2',3,-Tri-O -Acetyl-6-O -(4-chloro-3-nitrobenzoyl)-4',6'-O-
(benzylidene)-
p-D-maltosyl)-oxymethyl]-2-chloro-phenyl}-acetamide or a pharmaceutically
acceptable salt thereof;
(R)-N-[2-Chloro-5-[[[6- O -(4-cyanobenzoyl)-4-O-[4,60-(phenylmethylene)-«-D-
glucopyranosyl]-p-D-glucopyranosyl]oxy]methyl]phenyl]acetamide or a
pharmaceutically acceptable salt thereof;
(R}-N-[2-Chloro-S-[[[6-O -(4-nitrobenzoyl)-4-O-[4,60-(phenylmethylene)-a-D-
glucopyranosyl]-p-D-glucopyranosyl]oxy]methyl]phenyl]acetamide or a
pharmaceutically acceptable salt thereof;
(R)-N [2-Chloro-5-[[[6-O -(3-trifluoromethylbenzoyl}-4-O-[4,60-
(phenylmethylene)-«-D-glucopyranosyl]-p-D-glucopyranosyl]oxy]
methyl]phenyl]acetamide or a pharmaceutically acceptable salt thereof;
N-{ S-[(4',6'-O-Benzylidene-6-O-(2-iodo)-benzoyl-p-D-maltosyl)-oxy-methyl]-2-
chloro-phenyl)-acetamide or a pharmaceutically acceptable salt thereof;
N {5-[(4',6'-O-Benzylidene-6-O-(3-iodo)-benzoyl-p-D-maltosyl)-oxy-methyl]-2-
chloro-phenyl }-acetamide or a pharmaceutically acceptable salt thereof;
N { 5-[(4',6'-O-Benzylidene-6-(4-iodo-benzoyl)-oxy-p-D-maltosyl)-oxy-methyl]-2-
chloro-phenyl }-acetamide or a pharmaceutically acceptable salt thereof;
(R)-N [-2-Chloro-5-[[[6-O-(phenylacetyl)-4-O-[4,6-O-(phenylmethylene)-«-D-
glucopyranosyl]-p-D-glucopyranosyl]oxy]methyl]phenyl]acetamide or a
pharmaceutically acceptable salt thereof;
(R)-N [2-Chloro-5-[[[2,3-di-O-acetyl-4-O-[2,3-di-O-acetyl-4,6-O-(phenyl-
methylene)-«-D-glucopyranosyl]-6-O-(phenylacetyl)-p-D-glucopyranosyl]-
oxy]methyl]phenyl]acetamide or a pharmaceutically acceptable salt thereof;
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
- 16-
N-{ 5-[(4',6'-O-Benzylidene-6-O-phenyl-ethyl-carboxyl-p-D-maltosyl}-oxy-
methyl]-
2-chloro-phenyl }-acetamide or a pharmaceutically acceptable salt thereof;
N {5-[(4',6'-O-Benzylidene-6-O-phenyl-propyl-carboxyl-p-D-maltosyl)-oxy-
methyl]-2-chloro-phenyl }-acetamide or a pharmaceutically acceptable salt
thereof;
biphenyl-acetic acid 6-(3-acetylamino-4-chloro-benzyloxy)-3-(7,8-dihydroxy-2-
phenyl-hexahydro-pyrano[3,2-d] [ 1,3] dioxin-6-yloxy)-4,5-dihydroxy-tetrahydro-
pyran-2-ylmethyl ester or a pharmaceutically acceptable salt thereof;
biphenyl-acetic acid 4,S-diacetoxy-6-(3-acetylamino-4-chloro-benzyloxy)-3-(7,8-
diacetoxy-2-phenyl-hexahydro-pyrano[3,2-d] [ 1,3] dioxin-6-yloxy}-tetrahydro-
pyran-
2-ylmethyl ester or a pharmaceutically acceptable salt thereof;
(3,4-Dimethoxy-phenyl)-acetic acid 6-(3-acetylamino-4-chloro-benzyloxy)-3-(7,8-
dihydroxy-2-phenyl-hexahydro-pyrano[3,2-d][1,3]dioxin-6-yloxy)-4,5-dihydroxy-
tetrahydro-pyran-2-ylmethyl ester or a pharmaceutically acceptable salt
thereof;
(3,4-Dimethoxy-phenyl)-acetic acid 4,5-diacetoxy-6-(3-acetylamino-4-chloro-
benzyloxy)-3-(7,8-diacetoxy-2-phenyl-hexahydro-pyrano[3,2-d] [ 1,3]dioxin-6-
yloxy)-
tetrahydro-pyran-2-ylmethyl ester or a pharmaceutically acceptable salt
thereof;
Nicotinic acid 6-(3-acetylamino-4-chloro-benzyloxy)-3-(7,8-dihydroxy-2-phenyl-
hexahydro-pyrano[3,2-d][1,3]dioxin-6-yloxy)-4,5-dihydroxy-tetrahydro-pyran-2-
ylmethyl ester or a pharmaceutically acceptable salt thereof;
Nicotinic acid 4.5-diacetoxy-6-(3-acetylamino-4-chloro-benzyloxy)-3-(7,8-
diacetoxy-
2-phenyl-hexahydro-pyrano[3,2-d] [ 1,3] dioxin-6-yloxy)-tetrahydro-pyran-2-
ylmethyl
ester or a pharmaceutically acceptable salt thereof;
CA 02350066 2001-05-09
WO 00/31096 PCTNS99/27828
-17-
(R)-N [5-[[[6-O-(4-Benzoylbenzoyl)-4-O-[4,6-O-(phenyimethylene)-a-D-
glucopyranosyl]-p-D-glucopyranosyl]oxy]methyl]-2-chiorophenyl]acetamide or a
pharmaceutically acceptable salt thereof;
N { 5-[(4',6'-O-Benzylidene-p-maltosyl)-oxy-methyl]-2-methyl-phenyl }-
acetamide or
a pharmaceutically acceptable salt thereof;
N Acetyl-{ 5-[(2,2',3,3',6-penta-D-acetyl-4',6'-O-benzylidene-p-D-maltosyl)-
oxy-
methyl]-2-methyl-phenyl}acetamide or a pharmaceutically acceptable salt
thereof;
N-(S-{ [4',6'-O-Benzylidene-6-O-(4-toluenesulfonyl)-~3-D-maltosyl]-oxy-methyl
}-2-
methyl-phenyl)-acetamide or a pharmaceutically acceptable salt thereof;
N-{ 5-[(4',6'-O-Benzylidene-6-O-phenyl-p-D-maltosyl)-oxy-methyl]-2-chloro-
phenyl}-acetamide or a pharmaceutically acceptable salt thereof;
(R)-N [2-Chloro-5-[[[4-O-[4',6'-O-(phenylmethylene)-a-D-glucopyranosyl]-p-D-
glucopyranosyl]oxy]methyl] phenyl]-3-pyridinecarboxamide or a pharmaceutically
acceptable salt thereof;
(R)-N-[5-[[[6-O-Benzoyl-4-O-[4',6'-O-(phenylmethylene)-a-D-glucopyranosyl]-~-D-
glucopyranosyl]oxy]methyl]-2-chlorophenyl]-3-pyridinecarboxamide or a
pharmaceutically acceptable salt thereof;
Furan-2-carboxylic acid {5-[(4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-
chloro-phenyl }-amide or a pharmaceutically acceptable salt thereof;
Furan-2-carboxylic acid {5-[(6-O-benzoyl-4',6'-O-benzylidene-p-D-maltosyl)-oxy-
methyl]-2-chloro-phenyl }-amide or a pharmaceutically acceptable salt thereof;
N-{ 2-Chloro-5-[(4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-phenyl }-pent-4-
enamide or a pharmaceutically acceptable salt thereof;
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
- 18-
N { 2-Chloro-5-[(6-O-benzoyl-4',6'-O-benzilidene-p-D-maltosyl)-oxy-methyl]-
phenyl }-pent-4-enamide or a pharmaceutically acceptable salt thereof;
5-(6-O-Benzoyl-4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl-2-chloro-
phenylamine or a pharmaceutically acceptable salt thereof;
(4-Chloro)-benzyl-4',6'-O-benzylidene-p-D-maltoside or a pharmaceutically
acceptable salt thereof;
Benzoic acid 1-O-(4-chloro)-benzyl-4',6'-O-benzylidene-6-deoxy-p-D-malto-6-yl
ester or a pharmaceutically acceptable salt thereof;
4-Benzoyl-N- { 5-[(4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-chloro-
phenyl }-
benzamide or a pharmaceutically acceptable salt thereof;
4-Benzoyl-N {5-[(6-benzoyl-oxy-4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-
chloro-phenyl}-benzoic acid amide or a pharmaceutically acceptable salt
thereof;
4-Benzoyl-N {5-[(4',6'-O-benzylidene-6-O-(2-iodo)-benzoyl-p-D-maltosyl)-oxy-
methyl]-2-chloro-phenyl }-benzamide or a pharmaceutically acceptable salt
thereof;
4-Benzoyl-N {5-[(4',6'-O-benzylidene-6-O-(3-iodo-benzoyl)-p-D-maltosyl)-oxy-
methyl]-2-chloro-phenyl }-benzamide or a pharmaceutically acceptable salt
thereof;
4-Benzoyl-N { 5-[(4',6'-O-benzylidene-6-(4-iodo-benzoyl)-oxy-p-D-maltosyl)-oxy-
methyl]-2-chloro-phenyl }-benzoic acid amide or a pharmaceutically acceptable
salt
thereof;
(1-{5-[(4',6'-O-Benzylidene-p-D-maltosyl}-oxy-methyl]-2-chloro-phenyl-
carbamoyl}ethyl)-carbamic acid 9H-fluoren-9ylmethyl ester or a
pharmaceutically
acceptable salt thereof;
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
- 19-
N-(9H-Fluoren-9ylmethoxycarbonyl)-N'-{ 5-[(6-O-benzoyl-4',6'-O-benzylidene-p-D-
maltosyl)-oxy-methyl]-2-chloro-phenyl }-L-alaninamide or a pharmaceutically
acceptable salt thereof;
N'-{ 5-[(6-O-benzoyl-4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-chloro-
phenyl }-L-alaninamide or a pharmaceutically acceptable salt thereof;
N {5-[(4',6'-O-Benzylidene-p-D-maltosyl)-oxymethyl]-2-chloro-phenyl}-N methyl-
acetamide or a pharmaceutically acceptable salt thereof;
N { 5-[(6-O-Benzoyl-4',6'-O-benzylidene-p-D-maltosyl)-oxymethyl]-2-chloro-
phenyl }-N-methyl-acetamide or a pharmaceutically acceptable salt thereof;
N {5-[(4',6'-O-Benzylidene-p-D-maltosyl)-oxy-methyl]-2-chloro-phenyl}-carbamic
acid methyl ester or a pharmaceutically acceptable salt thereof;
N {5-[(6-O-Benzoyl-4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-chloro-
phenyl }-carbamic acid methyl ester or a pharmaceutically acceptable salt
thereof;
N-{ 5-((6-O-(3-Benzyl-1-oxo-propyl)-4',6'-O-benzylidene-p-D-maltosyl)-oxy-
methyl]-2-chloro-phenyl }-carbamic acid methyl ester or a pharmaceutically
acceptable salt thereof;
N {5-[(4',6'-D-Benzylidene-p-D-maltosyl)-oxy-methyl]-2-chloro-phenyl}
methanesulfonamide or a pharmaceutically acceptable salt thereof;
N-{ 5-[(6-O-Benzoyl-4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-cyano-
phenyl }-acetamide or a pharmaceutically acceptable salt thereof;
N-{ 5-[(6-O-Benzoyl-4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-methyl-
phenyl }-acetamide or a pharmaceutically acceptable salt thereof;
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-20-
6-[-6-(4-Chloro-3-nitro-benzylsulfanyl)-4,5-dihydroxy-2-hydroxymethyl-
tetrahydro-
pyran-3-yloxy]-2-phenyl-hexahydro-pyrano[3,2-d][1,3]dioxine-7,8-diol or a
pharmaceutically acceptable salt thereof;
S
(4-Chloro-3-nitro-benzyl) 6-O-benzoyl-4',6'-O- benzoyl-4',6',-O-benzylidene-1-
thio-p-D-maltoside or a pharmaceutically acceptable salt thereof.
The compounds of this invention were be prepared according to the following
schemes from commercially available starting materials or starting materials
which
can be prepared using literature procedures. This scheme shows the preparation
of
representative compounds of this invention.
Acetobromomaltose 1 is coupled with a benzyl alcohol 2 in the presence of a
catalyst such as a mercuric bromide, mercuric cyanide, silver triflate, or
silver
perchlorate in an aprotic solvent such as acetonitrile, dichloromethane,
ether, toluene
or nitromethane at temperatures ranging from -40 °C to reflux to yield
glycoside 3
(Scheme 1). This glycosidation can also be accomplished using Schmidt's
trichloroacetimidate coupling with zinc bromide in a solvent such as
dichloromethane. Reduction of the nitro group of 3 can be accomplished with a
reducing agent such as stannous chloride in a polar aprotic solvent such as
ethyl
acetate at ambient temperature to reflux to afford an anilino compound 4.
Coupling of
4 with an acid chloride can be completed in the presence of an amine base such
as
triethylamine or diisopropylethylamine or using a stronger base such as sodium
hydride (for sterically hindered systems) in an aprotic solvent such as
dichloromethane or tetrahydrofuran at temperatures ranging from 0 °C to
ambient
temperature to yield the target compound 5. The peracetylated compound 5 can
be
converted to the heptahydroxy compound 6 with catalytic sodium methoxide in
methanol or aqueous sodium hydroxide in methanol at temperatures ranging from
ambient temperature to reflux.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-21-
As illustrated in Scheme 2, an acetal (7) was formed at the C-4',6' positions
of the disaccharide of heptahydroxy compound 6 using an appropriate aldehyde
dimethyl or diethyl acetal and an acid source such as p-toluenesulfonic acid
monohydrate or camphorsulfonic acid in a polar solvent such as N,N-
dimethylformamide at 60 °C. In difficult cases, an aldehyde and
sulfuric acid can be
used in DMF at higher temperatures to obtain the product acetal. At this
point, the 6-
position primary alcohol was selectively acylated using an acid chloride in a
1:1
mixture of tetrahydrofuran and the hindered base 2,4,6-collidine at -40
°C initially to
ambient temperature overnight to generate compound 8. The remaining four
secondary alcohols of the disaccharide can then be protected with acetic
anhydride
and triethylamine in a solvent such as dichloromethane to afford the
peracetylated
compound 9.
On the other hand, acetal 7 can first be convened to a tosylate using tosyl
chloride and pyridine in a solvent such as dichloromethane (Scheme 3); the
resulting
intermediate is then peracetylated as mentioned above to generate compound 10.
Through overall displacement of the 6-position tosylate (alcohol formation
with
sodium formate followed by ether formation with benzyl 2,2,2-
trichloroacetimidate),
an ether linkage is incorporated at this site on the molecule. The benzylidene
acetal is
then removed under strongly acidic conditions such as 1M ethereal ~ HCl to
afford
compound 11. A new acetal is then formed at the C-4',6' positions using the
conditions mentioned previously or just an aldehyde and the acid source in
benzene at
elevated temperature (60 °C). Finally, the four secondary acetates are
removed with
catalytic sodium methoxide in methanol or aqueous sodium hydroxide in methanol
at
temperatures ranging from ambient temperature to reflux to obtain the hydroxy
compound 12.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-22-
Scheme 1
c
A c0-'!~ CI
Ac0 Ac0 Ac
O + HO
Ac 2 N02
Ac0 Br
glycosidation
OAc
Ac0
A c0~ Ac
O O '' CI
Ac0 O
Ac0 N02
3
reduction
Ac
Ac0'~'
AcOA-'cO~ ~Ac
~~°_r, ~-a
Aco~o
A c0
4
acylation
)A c
AcOa~
Ac Ac0 Ac
o a
Aco 0
5 Ac0 HN-
hydrolysis
H
HO O
H H
HO O a
HO O
HO
H
CA 02350066 2001-05-09
WO 00/31096 ~ PCT/US99/27828
-23-
If R, = Bz, then:
Scheme 2
H
HO-,'~
HO HO O H ~ CI
HO O
HO
H
G4',6'-acetal formation
R4
l r
R1~0 O
O
HO H
HO O CI
HO ~ O
7 HO HN
~6 acyiation
R4
R 1~0
O
HO R2
HO O
CI
HO O
HO H
R4 ~ peracetylation
R ~~O 1'
Rg0 R 2
R30 O _ CI
R30 O
9 R 30 H N
If RZ = Bn, then:
CA 02350066 2001-05-09
WO 00/31096 PCT/U599/27828
-24-
R4 Scheme 3
R'' \'O O
O
HO H
HO O ~ CI
7 H O
HO HN
1 ) tosylatio ~n
2) peracetylation
CH3
R
R ~~O O OJ
O
O' T
Ac0
Ac0 O -' CI
Ac0 O
Ac0
10 H
1 ) ether formation
2) acetal removal
H
HO
Ac0 R2
A c0 O ~ CI
Ac0 O
A o0 HN
11
1 ) new G4',6'-acetal formation
2) hydrolysis
R4
R ~~O
R~O R2
R30 O ''~ CI
R30 O
R30 H
12
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-25-
The compounds of this invention are useful as antiproliferative agents. The
following procedures show the evaluation of representative compounds of this
invention in standard pharmacological test procedure which measured ability of
the
evaluated compound to inhibit smooth muscle cell proliferation.
Effects of Compounds on Cell Proliferation Usin '~H Thymidine Incorporation
Human and porcine smooth muscle cells were tested in early passage (generally
passage 3 - 7) at sub-confluent conditions. Cultures were grown in 16 mm (24
well)
mufti-well culture dishes in medium 199 supplemented with 10% fetal bovine
serum
and 2% antibiotic / antimycotic. At sub-confluence, the cells were placed in a
defined serum free medium (AIM-V; Gibco) for 24 - 48 h prior to initiating the
experimental protocol.
Although compounds were found to be more effective with longer pre-
incubations,
in general, the procedures were initiated with the addition of compound, 3H
thymidine
and serum / growth factor to serum deprived synchronized cells and results are
reported accordingly.
Compounds were added to each well at 50 fold dilution (20 ~ZL / well) and the
plates
were incubated for 24 - 36 h at 37 °C in 5% CO2. Compounds were
initially
dissolved in 50% ethanol and serially diluted into media. Compounds were
routinely
evaluated at concentrations from 1 to 100 pM. As a control, grade II porcine
intestinal mucosal heparin (sodium salt) was routinely evaluated in all cell
preparations at concentrations from 0.1 to 100 pg/mL.
At the completion of the test procedure, plates were placed on ice, washed
three
times with ice cold phosphate buffered saline (PBS) and incubated in ice cold
10%
trichloroacetic acid (TCA) got 30 min to remove acid soluble proteins.
Solution was
transferred to scintillation vials containing 0.4 N HCl (500 ~L,/ vial to
neutralize
NaOH) and each well was rinsed two times with water (500 pL) for a total
volume of
2 mL / vial.
Data was obtained, in triplicate, for both control and experimental samples.
Control
(100%) data was obtained from maximally stimulated cells, as the result of
growth
factor or serum stimulation. Experimental data was obtained from cells
maximally
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-26-
stimulated with growth factor or serum and treated with compound. Data are
expressed as an ICsp or percent inhibition in Table I below.
Table 1.
Porcine Smooth Muscle
Com ound of Exam Cell
le Anti r_o_liferation IC50
1 32% @ 100 M
2 0.103
3 2.92
4 16% @ 50 M
5 0.037 M
6 0.133
7 0.088
8 I).001 M
9 0.083
10 19.2
11 0.003
12 29.4
13 0.023
15 I).003
16 16.3 M
17 I).035
18 43% @ SO
19 t).001 M
20 48% @ 50
21 t).062 M
22 5.53
23 0.003 M
24 6.60
25 0.700
26 0.010-0.030 M
27 0.070
28 I).400
29 44.1
30 t).351
31 t).380
32 t).405
33 0.312
34 0.061 M
35 1).851
36 0.089 M
37 I).588
38 0.187 M
39 2.53 M
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-27-
Table 1 (Continued)
Porcine Smooth Muscle
Com ound of Exam Cell
le Anti roliferation IC50
40 0.092
41 0.273
42 0.027 M
43 0.008 M
44 0.062 M
45 6.44 M
46 0.032 M
47 0.078 M
48 0.007 M
49 0.104
50 0.084 M
51 0.354
52 0.048 M
53 0.266
54 0.211 M
55 0.304 M
S6 0.530
57 8.90 M
58 0.600 M
59 0.490
60 0.038 M
61 13.7 M
62 0.023 M
63 7.73
64 0.050 M
65 17.8 M
66 0.180 M
67 1.29 M
68 25.3 M
69 1.53
70 9.94
71 0.050
72 0.016 M
73 0.132 M
74 1.22
75 4.69 M
76 0.156
77 0.081 M
78 8% @ SO
79 2.36 ~M
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-28-
Table 1 (Continued)
80 7.57 M
~
81 0.014 M
82 0.352 M
83 18% @ 50 M
84 0.250
85 0.418 M
86 16.3 M
87 0.031 M
The compounds of this invention are useful in treating or inhibiting diseases
which are characterized by excessive smooth muscle cell proliferation (smooth
muscle cell hyperproliferation). The compounds are particularly useful in
treating
hyperproliferative vascular diseases which are characterized by smooth muscle
cell
hyperproliferation, such as restenosis, which most frequently arises from
vascular
reconstructive surgery and transplantation, for example, balloon angioplasty,
vascular
graft surgery, coronary artery bypass surgery, and heart transplantation.
Other
disease states in which there is unwanted "cellular" vascular proliferation
include
hypertension, asthma, and congestive heart failure. The compounds of this
invention
are also useful as inhibitors of angiogenesis. Angiogenesis
(neovascularization), the
process by which new capillaries are formed, is of principal importance for a
number
of pathological events including chronic inflammation and malignant processes.
The
compounds of this invention are therefore useful as antineoplastic agents.
The compounds of this invention can be. formulated neat or with a
pharmaceutical carrier for administration, the proportion of which is
determined by
the solubility and chemical nature of the compound, chosen route of
administration
and standard pharmacological practice. The pharmaceutical carrier may be solid
or
liquid.
A solid carrier can include one ar more substances which may also act as
flavoring agents, lubricants, solubilizers, suspending agents, fillers,
glidants,
compression aids, binders or tablet-disintegrating agents; it can also be an
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-29-
encapsulating material. In powders, the carrier is a finely divided solid
which is in
admixture with the finely divided active ingredient. In tablets, the active
ingredient is
mixed with a carrier having the necessary compression properties in suitable
proportions and compacted in the shape and size desired. The powders and
tablets
preferably contain up to 99% of the active ingredient. Suitable solid carriers
include,
for example, calcium phosphate, magnesium stearate, talc, sugars, lactose,
dextrin,
starch, gelatin, cellulose, methyl cellulose, sodium carboxymethyl cellulose,
polyvinylpyrrolidine, low melting waxes and ion exchange resins.
Liquid carriers are used in preparing solutions, suspensions, emulsions,
syrups, elixirs and pressurized compositions. The active ingredient can be
dissolved
or suspended in a pharmaceutically acceptable liquid carrier such as water, an
organic
solvent, a mixture of both or pharmaceutically acceptable oils or fats. The
liquid
carrier can contain other suitable pharmaceutical additives such as
solubilizers,
emulsifiers, buffers, preservatives, sweeteners, flavoring agents, suspending
agents,
thickening agents, colors, viscosity regulators, stabilizers or osmo-
regulators.
Suitable examples of liquid earners for oral and parenteral administration
include
water (partially containing additives as above, e.g. cellulose derivatives,
preferably
sodium carboxymethyl cellulose solution), alcohols (including monohydric
alcohols
and polyhydric alcohols, e.g. glycols) and their derivatives, lethicins, and
oils (e.g.
fractionated coconut oil and arachis oil). For parenteral administration, the
carrier
can also be an oily ester such as ethyl oleate and isopropyl myristate.
Sterile liquid
earners are useful in sterile liquid form compositions for parenteral
administration.
The liquid carrier for pressurized compositions can be halogenated hydrocarbon
or
other pharmaceutically acceptable propellant.
Liquid pharmaceutical compositions which are sterile solutions or suspensions
can be utilized by, for example, intramuscular, intraperitoneal or
subcutaneous
injection. Sterile solutions can also be administered intravenously. T'he
compounds
of this invention can also be administered orally either in liquid or solid
composition
form.
The compounds of this invention may be administered rectally or vaginally in
the form of a conventional suppository. For administration by intranasal or
intrabronchial inhalation or insufflation, the compounds of this invention may
be
formulated into an aqueous or partially aqueous solution, which can then be
utilized
in the form of an aerosol. The compounds of this invention may also be
administered
transdermally through the use of a transdermal patch containing the active
compound
and a carrier that is inert to the active compound, is non toxic to the skin,
and allows
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27$28
-30-
delivery of the agent for systemic absorption into the blood stream via the
skin. The
carrier may take any number of forms such as creams and ointments, pastes,
gels, and
occlusive devices. The creams and ointments may be viscous liquid or semisolid
emulsions of either the oil-in-water or water-in-oil type. Pastes comprised of
absorptive powders dispersed in petroleum or hydrophilic petroleum containing
the
active ingredient may also be suitable. A variety of occlusive devices may be
used to
release the active ingredient into the blood stream such as a semipermeable
membrane covering a reservoir containing the active ingredient with or without
a
carrier, or a matrix containing the active ingredient. Other occlusive devices
are
known in the literature.
The dosage requirements vary with the particular compositions employed, the
route of administration, the severity of the symptoms presented and the
particular
subject being treated. Based on the results obtained in the standard
pharmacological
test procedures, projected daily dosages of active compound would be 0.1 to 10
mg/kg administered parenterally (intravenous preferred), with projected daily
oral
dosage being approximately ten-fold higher. Anticipated intravenous
administration
would last for approximately 5-30 days following acute vascular injury (i.e.,
balloon
angioplasty or transplantation) and for a longer duration for the treatment of
chronic
disorders. Treatment will generally be initiated with small dosages less than
the
optimum dose of the compound. Thereafter the dosage is increased until the
optimum effect under the circumstances is reached; precise dosages for oral,
parenteral, nasal, or intrabronchial administration will be determined by the
administering physician based on experience with the individual subject
treated.
Preferably, the pharmaceutical composition is in unit dosage form, e.g. as
tablets or
capsules. In such form, the composition is sub-divided in unit dose containing
appropriate quantities of the active ingredient; the unit dosage forms can be
packaged
compositions, for example, packaged powders, vials, ampoules, pre filled
syringes or
sachets containing liquids. The unit dosage form can be, for example, a
capsule or
tablet itself, or it can be the appropriate number of any such compositions in
package
form.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/Z7828
-31-
The following provides the preparation of representative compounds of this
invention.
Example 1
N-(2-Chloro-5-f(4'.6'-O-ethylidene)-I~-D-maltosyloxymethvll-phenyl}-acetamide
step 1
4-Chloro-3-nitro-benzyl-p-D-maltoside heptaacetate
To a stirred solution of 4-chloro-3-nitrobenzyl alcohol (6.70 g, 35.7 mmol)
and .HgBr~ ( 14.2 g, 39.3 mmol) in freshly distilled C'.H3CN (239 mL) was
added in
one portion Hg(CN)2 (9.02 g, 35.7 mmol). After 0.5 h, acetobromomaltose (25.0
g,
35.7 mmol) was added, and the mixture stirred for 18 h at rt. The reaction was
then
quenched with a mixture of HZO:brine (1:1, 100 mL) and extracted with 10%
CH2C12:EtOAc. The combined organic extracts were dried (MgS04) and
concentrated.
Purification by flash chromatography (10:90 to 80:20 EtOAc:petroleum ether
gradient) gave 51.9 g (90%) of the title compound as a glassy oil which was
recrystallized from EtZO:petroleum ether to afford a glassy white solid, mp
107-111
°C; 'H NMR (CDCl3) s 2.00 (s, 3H), 2.02 (s, 3H), 2.03, (s, 3H), 2.04
(s, 6H), 2.11 (s,
3H), 2.15 (s, 3H), 3.70 (ddd, J = 2.9, 4.2, 9.7 Hz, 1H), 3.94-3.98 (m, 1H),
4.01-4.07
(m, 2H), 4.20-4.28 (m, 2H), 4.54 (dd, J = 2.9, 12.3 Hz, 1H), 4.63-4.68 (m,
2H), 4.84-
4.94 (m, 3H), 5.06 (t, J = 10.1 Hz, 1H), 5.26 (t, J = 9.2 Hz, 1H), 5.36 (dd, J
= 9.7,
10.3 Hz, 1 H), 5.42 (d, J = 4.2 Hz, 1H), 7.43 (dd, J = 2.2, 8.3 Hz, 1 H), 7.53
(d, J = 8.3
Hz, 1H), 7.83 (d, J = 2.0 Hz, 1H); IR (KBr) 3450, 2950, 1755, 1550, 1375, 1230
and
1050 cm-'; mass spectrum [(+) ESI], m/z 823/825 (M + NH,+), 828/830 (M + Na)';
Anal. Calcd. for C33H,oC1NO2o: C, 49.17; H, 5.00; N, 1.74, Found: C, 49.16; H,
4.88; N, 1.71.
step 2
2-Chloro-5-(hepta-O-acetyl-p-D-maltosyl-oxymethyl)-phenylamine
A solution containing 4-chloro-3-nitro-benzyl- ~3-D-maltoside heptaacetate
( 19.3 g, 23.9 mmol) and tin (II) chloride dihydrate (37.7 g, 167 mmol) in
EtOAc (479
mL) was refluxed for 2 h. The reaction was cooled to rt, carefully quenched
with sat.
aq. NaHCO, (until basic), diluted with EtOAc (250 mL), stirred for 0.5 h and
filtered.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-32-
The biphasic filtrate was separated and the aqueous phase extracted with
EtOAc. The
combined organic extracts were dried (NazS04) and concentrated. Purification
by
flash chromatography (0 to 12% acetone/CHC13 gradient) gave 17.8 g (96%) 2-
Chloro-5-(hepta-O-acetyl-p-D-maltosyl-oxymethyl)-phenylamine as a glassy
solid,
mp 78-79 °C; 'H NMR (CDC13) s 2.00 (s, 9H}, 2.026 (s, 3H), 2.032 (s,
3H), 2. I 1 (s,
3H), 2.16 (s 3H), 3.00-5.00 (bs, 2H), 3.64-3.68 (m, 1 H), 3.97 (ddd, J = 2.4,
4.2, 10.1
Hz, 1H), 4.02-4.07 (m, 2H), 4.24 (dd, J = 2.2, 3.7, 1H), 4.27 (dd, J = 2.6,
4.0 Hz,
1H), 4.50-4.57 (m, 3H), 4.74 (d, J = 12.1 Hz, 1H), 4.83-4.90 (m, 2H), 5.05 (t,
J =
10.1 Hz, 1H), 5.22 (t, J = 9.2 Hz, 1H), 5.35 (dd, J = 9.7, 10.5 Hz, 1H), 5.42
(d, J =
4.0 Hz, 1H), 6.62 (dd, J = 2.0, 8.1 Hz, 1H), 6.76 (d, J = 2.0 Hz, IH), 7.21
(d, J = 8.1,
1H); IR (KBr) 3450, 3350, 2950, 1755, 1650, 1425, 1375, 1230 and 1050 cm-';
mass
spectrum [(+) ESI], m/z 776/778 (M + H)', 798/800 (M + Na)+; Anal. Calcd. for
C33Hy,CIN0,8: C, 51.07; H, 5.45; N, 1.80, Found: C, 50.94; H, 5.52; N, 1.60.
step 3
N-[2-Chloro-5-(hepta-O-acetyl-p-D-maltosyl-oxymethyl)-phenyl]-acetamide
To a stirred solution of 2-chloro-5-(hepta-O-acetyl-p-D-maltosyl-oxymethyl)-
phenylamine (20.6 g, 26.5 mmol) and triethylamine (8.13 mL, 58.3 mmol) in THF
(265 mL) at 0 °C was added dropwise acetyl chloride (2.26 mL, 31.8
mmol). After
0.5 h at this temperature, it was warmed to rt and stirred an additional 6 h.
At this
point, the reaction was concentrated and taken up in EtOAc (700 mL). This
organic
solution was washed with 1 N HCl (70 mL), sat. aq. NaHC03 (70 mL), and brine
(70
mL) and then dried (MgS04). After concentration, the residue was purified by
flash
chromatography (20:80 to 100:0 EtOAc:petroleum ether gradient) to afford the
product (16.2 g, 75%) as a glassy solid, mp 84-86 °C; 'H NMR (CDCl3) s
2.00 (s,
6H}, 2.020 (s, 3H}, 2.027 (s, 3H), 2.03 (s, 3H), 2.11 (s, 3H), 2.16 {s, 3H),
2.24 (s,
3H), 3.66-3.69 (m, 1H), 3.94-3.98 (m, 1H), 4.00-4.06 (m, 2H), 4.22-4.28 (m,
2H},
4.50-4.61 (m, 3H), 4.80-4.91 (m, 3H), 5.05 (t, J = 10.1 Hz, 1H), 5.22 (t, J =
9.2 Hz,
1H), 5.35 (dd, J = 9.4, 10.5 Hz, 1H), 5.41 (d, J = 4.0 Hz, 1H), 6.99 (dd, J =
2.0, 8.1
Hz, IH), 7.34 {d, J = 8.1 Hz, 1H), 7.62 (s, 1H), 8.32 (s, 1H); IR (KBr) 3400,
2950,
1750, 1690, 1600, 1540, 1425, 1375, 1230 and 1050 cm-'; mass spectrum [(+)
ESI],
CA 02350066 2001-05-09
WO 00/31096 PCT/U599/2'7828
-33-
m/z 818/820 (M + H)', 840 (M + Na)+; Anal. Calcd. for C35HQ,C1N0,9: C, 51.38;
H,
5.42; N, 1.71, Found: C, 51.03; H, 5.36; N, 1.59.
step 4
N-[2-Chloro-5-(~-D-maltosyl-oxymethyl)-phenyl]-acetamide
A solution containing N-[2-chloro-5-(hepta-O-acetyl-p-D-maltosyl-
oxymethyl)-phenyl]-acetamide (0.945 g, 1.I2 mmol) and 25 weight % NaOMe in
MeOH (19.2 ~L, 0.336 mmol) in MeOH (27.6 ml) was refluxed for 2.5 h. The
reaction was cooled to room temperature and concentrated, and the resulting
residue
was triturated with EtrO to afford the product (0.583 g, 99°10) as a
foam; 'H NMR
(DMSO-db) 8 2.07 (s, 3H), 3.03-3.16 (m 2H), 3.19-3.49 (m, 7H), 3.55-3.62 (m,
2H),
3.67-3.73 (m, 1H), 4.28 (d, J = 7.7 Hz, 1H), 4.33-5.76 (bs, 7H), 4.67 {ABq, J
= 12.5
Hz, es = 0.22, 2H), 5.01 (d, J = 3.7 Hz, 1H), 7.21 (dd, J = 1.8, 8.1 Hz, 1H),
7.44 (d, J
= 8.1 Hz, 1H), 7.64 (d, J = 1.5 Hz, IH), 9.33-9.69 (bs, 1H); IR (KBr) 3400,
2900,
1680, 1600, 1540, 1430, 1375, 1310, 1150 and 1035 cm~', mass spectrum [(+)
ESI],
m/z 524/526 (M + H)', 546 (M + Na)+; Anal. Calcd. for CZ,H3oC1N0,2 ~ 1.0 MeOH:
C, 47.53; H, 6.16; N, 2.52. Found: C, 47.94; H, 6.34; N, 2.42.
step 5
N-{2-Chloro-5-[(4',6'-O-ethylidene)-[i-D-maltosyloxymethyl]-phenyl}-acetamide
To a stirred solution of N [2-chloro-5-(~-D-maltosyl-oxymethyl)-phenyl]-
acetamide (0.500 g, 0.954 mmol) in DMF (12.5 mL) at rt was added acetaldehyde
dimethyl acetal (0.202 mL, 1.91 mmol) dropwise followed by TsOH~HzO (0.0907 g,
0.477 mmol). The reaction mixture was heated to 60 °C for 6 h and then
quenched
with KzC03 (0.0659 g, 0.477 mmol) with an additional 0.5 h heating at this
temperature. At this point, the solution was filtered hot, and the solvent was
distilled
off using the high vac. The residue was purified by flash chromatography
(80:2:1
EtOAc:EtOH:H.,O) to afford the product (0.323 g, 62°!0) as an off-white
powder, mp
144-146 °C; 'H NMR (DMSO-d6) s 1.22 (d, J = 5.1 Hz, 3H), 2.07 (s, 3H),
3.05-3.11
(m, 1H), 3.11 (t, J = 9.4 Hz, 1H), 3.25-3.37 (m, 3), 3.39-3.58 (m, 5H), 3.65-
3.71 (m,
1H), 3.92 (dd, J = 4.8, 9.9 Hz, 1H), 4.28 (d, J = 7.7 Hz, 1H), 4.65 (t, 3 =
5.7 Hz, 1H),
4.67 (ABq, J = 12.3 Hz, e~ = 0.22, 2H), 4.69 (dd, J = 4.8, 9.9 Hz, 1H), 5.09
(d, J =
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-34-
4.0 Hz, 1H), 5.23 (t, J = 5.7 Hz, 2H), 5.47 (d, J = 3.5 Hz, 1H), 5.57 (d, J =
6.6 Hz,
1H), 7.22 (dd, J = 1.8, 8.3 Hz, 1H), 7.44 (d, J = 8.3 Hz, 1H), 7.65 (s, 1H),
9.52 (s,
1H); IR (KBr) 3400, 2910, 2880, 1675, 1600, 1535, 1450, 1420, 1375, 1310,
1150,
1120, 1060, and 1020 cm-'; mass spectrum [(+) FAB], m/z 550/552 (M + H)+,
572/574 (M + Na)'; Anal. Calcd. for C23H,~C1NO,2 ~ 1.0 H20: C, 48.64; H, 6.03;
N,
2.47, Found: C, 48.55; H, 5.90; N, 2.41.
Example 2
LR)-N-f5-f f f6-O-Benzoyl-4-O-(4.6-O-ethylidene-oc-D-glucopvranosvl)-a-D-
~lucopyranosyllox l~yll-2-chlorophenyllacetamide
To a stirred solution of N-{2-chloro-5-[(4',6'-O-ethylidene)-p-D-maltosyloxy-
methyl]-phenyl }-acetamide (0.323 g, 0.587 mmol) in THF (4.0 mL) at -40
°C was
added collidine (4.0 mL, 30.3 mmol) dropwise followed by dropwise addition of
BzCl (0.0818 mL, 0.704 mmol). After 2 h at this temperature, it was warmed to
rt
and stirred an additional 18 h. At this point, the solvent was distilled off
using the
high vac, and the residue was diluted with EtOAc (200 mL). This layer was
washed
with 1 N HCl (20 mL), sat. NaHC03 (20 mL), and brine (20 mL) and then dried
(MgSO,). After concentration, the oilly residue was purified by flash
chromatography
( 1 % to 11 % MeOH:CHC13 gradient) and recrystallization (EtOAc:EtzO) to
afford the
product (0.209 g, 54%) as a white glassy solid, mp 166-169 °C; 'H NMR
(DMSO-db)
8 1.19 (d, J = 5.1 Hz, 3H), 2.04 (s, 3H), 3.09 (t, J = 9.4 Hz, 1H), 3.14-3.21
(m, 1H),
3.27-3.36 (m, 2H), 3.45-3.52 (m, 2H), 3.52-3.60 (m, 2H), 3.73 (ddd, J = 1.5,
5.1, 9.4
Hz, 1H), 3.89 (dd, J = 4.8, 9.9 Hz, 1H), 4.30 (dd, J = 5.5, 12.1 Hz, 1H), 4.38
(d, J =
7.7 Hz, 1H), 4.53-4.61 (rn, 2H), 4.65 (q, J = 5.1, 1H), 4.73 (d, J = 12.5 Hz,
1H), 5.07
(d, J = 4.0 Hz, 1H), 5.28 (d, J = 5.3 Hz, 1H), 5.34 (d, J = 5.3 Hz, 1H), 5.55
(d, J = 2.9
Hz, 1H), 5.77 (d, J = 5.9 Hz, 1H), 7.18 (dd, J = 2.0, 8.1 Hz, 1H), 7.40 (d, J
= 8.1 Hz,
1H), 7.50-7.55 (m, 2H), 7.62-7.68 (m, 2H), 7.96-8.00 (m, 2H), 9.50 (s, 1H); IR
(KBr) 3450, 3360, 2990, 2910, 2860, 1725, 1750, 1600, 1520, 1450, 1420, 1385,
1375, 1260, 1230, 1130, 1110, 1075, 1055, and 1020 cm~'; mass spectrum [(+)
FAB],
m/z 654/656 (M + H)', 676/678 (M + Na)', 692/694 (M + K)'; Anal. Calcd. for
C30H36C1NO~3: C, 55.09; H, 5.55; N, 2.14, Found: C, 54.76; H, 5.40; N, 2.00.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-35-
Example 3
(R)-N f 2-Chloro-5-f f f 2.3-di-O-acetyl-6-O-benzovl-4-O-(2 3-di-D-acetyl-4 6
O
ethvlidene-«-D-elucopyranos 1 -a-D- lucopyranosvlloxvlmethyll phen~lacetamide
To a stirred solution of (R)-N [5-[[(6-D-benzoyl-4-O-(4,6-O-ethylidene-a-D
glucopyranosyl)-p-D-glucopyranosyl]oxy]methyl]-2-chlorophenyl]acetamide (0.086
g, 0.131 mmol) and triethylamine (0.161 mL, 1.15 mmol) in CHZC12 (6 mL) at rt
was
added dropwise acetic anhydride (0.0544 mL, 0.576 mmol) followed by a
catalytic
amount of DMAP (0.0064 g, 0.0524 mmol). After 18 h, the mixture was diluted
with
EtOAc (200 mL). This layer was washed with 1 N HCl (20 mL), sat. aq. NaHC03
(20
mL), and brine (20 mL) and then dried (Na2S04). After concentration, the
residue was
purified by preparatory plate chromatography (10:9() MeOH:CHC13) to afford the
product (0.071 g, 66%) as a white, mp >87 °C (decomp.); 'H NMR (DMSO-
d6) s 1.13
(d, J = 4.8 Hz, 3H), 1.92 (s, 3H), 1.95 (s, 3H), 1.97 (s, 3H), 1.99 (s, 3H),
2.05 (s, 3H),
3.31-3.39 (m, 1H), 3.55-3.67 (m, 3H), 4.10-4.19 (m, 2H}, 4.41 (dd, J = 3.1,
12.3 Hz,
1H), 4.54 (d, J = 12.7 Hz, 1H), 4.64-4.74 (m, 3H), 4.77 (dd, J = 8.3, 9.4 Hz,
1H},
4.82 (dd, J = 4.0, 10.1 Hz, 1H), 4.89 (d, J = 7.9 Hz, 1H), 5.18 (t, J = 9.7
Hz, 1H),
5.29 (d, J = 4.2 Hz, 1H), 5.33 (t, J = 9.0 Hz, 1H), 7.04 (dd, J = 1.5, 8.1 Hz,
1H), 7.41
(d, J = 8.1 Hz, 1H), 7.55 (t, J = 7.7 Hz, 2H), 7.61 (s, 1H), 7.68 (t, J = 7.5
Hz, 1H),
8.01-8.07 (m, 2H), 9.45 (s, 1H); IR (KBr) 3410, 2940, 2850, 1755, 1690, 1590,
1530,
1440, 1420, 1370, 1240, 1135, 1060, and 1030 cm-'; mass spectrum [(+) FAB),
m/z
822/824 (M + H)', 844/846 (M + Na)'; Anal. Calcd. for C38H"C1N0" ~ 1.5 H20: C,
53.74; H, 5.58; N, 1.65, Found: C, 53.69; H, 5.14; N, 1.57.
Example 4
N 12-Chloro-5-f (4' 6'-O-oropvlidene-a-D-malto~l -oxy-methylLphenyl l
acetamide
The title compound was prepared as a white solid (0.309 g, 57%) from N [2-
chloro-5-([i-D-maltosyl-oxymethyl)-phenyl]-acetamide using propionaldehyde
diethyl
acetal and a procedure similar to step 5 of Example 1, mp >64 °C
(decomp.); 'H
NMR (DMSO-db) 8 0.86 (t, J = 7.5 Hz, 3H}, 1.48-1.58 (m, 2H), 2.07 (s, 3H),
3.04-
3.14 (m, 2H), 3.25-3.58 (m, 8H), 3.68 (dd, J = 6.2, 10.5 Hz, I H), 3.95 (dd, J
= 4.6,
9.9 Hz, 1H), 4.28 (d, J = 7.7 Hz, 1H), 4.48 (t, J = 5.1 Hz, 1H), 4.65 (t, J =
5.9 Hz,
1H), 4.67 (ABq, J = 12.5 Hz, es = 0.22, 2H), 5.08 (d, J = 4.0 Hz, 1H), 5.20
(d, J = 5.3
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-36-
Hz, 1H), 5.24 (d, J = 5.3 Hz, 1H), 5.48 (d, J = 3.3 Hz, 1H), 5.57 (d, J = 6.6
Hz, 1H),
7.22 (dd, J = 1.8, 8.3 Hz, 1H), 7.44 (d, J = 8.3 Hz, 1H), 7.65 (s, 1H), 9.52
(s, IH); IR
(KBr) 3400, 2980, 2920, 2840, 1675, 1580, 1530, 1460, 1425, 1375, 1310, 1275,
1150, 1060, and 1020 cm~'; mass spectrum [(+) FAB], m/z 586/588 (M + Na)a;
Anal.
Calcd. for CuH3aC1N0,2 ~ 1.0 H20: C, 49.53; H, 6.23; N, 2.41, Found: C, 49.89;
H,
6.38; N, 2.19.
Example 5
N-t 5-((6-O-Benzovl-4' 6'-O-nropvlidene-a-D-maltos 1)-oxv methyll 2 chloro
phenyl )-acetamide
The title compound was prepared as a white solid (0.140 g, 47%) from N {2-
chloro-5-[(4',6'-O-propylidene-p-D-maltosyl)-oxy-methyl]-phenyl}-acetamide
using
a procedure similar to Example 2, mp >88 °C (decomp.); 'H NMR (DMSO-db)
s 0.84
(t, J = 7.5 Hz, 3H), 1.45-1.57 (m, 2H), 2.04 (s, 3H), 3.08 (t, J = 5.1 Hz,
1H), 3.17 (dd,
J = 8.3, 13.2 Hz, IH), 3.26-3.37 (m, 2H), 3.45-3.59 (m, 4H), 3.70-3.77 (m,
1H), 3.91
(dd, J = 4.6, 9.7 Hz, 1H), 4.30 (dd, J = 5.3, 12.1 Hz, IH), 4.38 (d, J = 7.7
Hz, 1H),
4.43 (t, J = 4.8 Hz, 1H), 4.53-4.6I (m, 2H), 4.73 (d, J = 12.3 Hz, 1H), 5.07
(d, J = 3.5
Hz, 1H), 5.25 (d, J = 5.3 Hz, 1H), 5.34 (d, J = 5.1 Hz, 1H), 5.56 (d, J = 2.2
Hz, 1H),
5.77 (d, J = 5.9 Hz, 1H), 7.18 (d, J = 8.1 Hz, 1H), 7.39 (d, J = 8.3 Hz, 1H),
7.53 (t, J
= 7.7 Hz, 2H), 7.61-7.68 (m, 2H), 7.98 (d, J = 7.5 Hz, 2H), 9.45 (s, 1H); IR
(KBr)
3410, 2970, 2920, 2860, 1720, 1675, 1590, 1530, 1450, 1420, 1375, 1270, 1060,
1020, and 720 cm~': mass spectrum [(-) FAB], m/z 666 (M - H)'; Anal. Calcd.
for
C3,H38C1NO,3: C, 55.73; H, 5.73; N, 2.09, Found: C, 55.67; H, 5.71; N, 2.09.
Example 6
N (5-( f4',6'-O-Benzvlidene-6-O-(4-toluenesulfonyl)-a D maltosvll oxv methvll
2
chloro-phenyl)-acetamide
step 1
N-{5-[(4',6'-O-Benzylidene-a-n-maltosyloxy)-methyl]-2-chloro-phenyl}-
acetamide
To a stirred solution of N-[2-chloro-5-(p-D-maltosyl-oxymethyl)-phenyl]-
acetamide ( 14.1 ~ g, 27.0 mmol) in DMF (325 mL) at rt was added benzaldehyde
CA 02350066 2001-05-09
WO 00/31096 PCT/U599/27828
-37-
dimethyl acetal (8.11 mL, 54.0 mmol) dropwise followed by TsOH~H~O (2.57 g,
13.5
mmol). The reaction mixture was heated to 60 °C for 6 h and then
quenched with
K,CO, (1.87 g, 13.5 mmol) with an additional 0.5 h heating at this
temperature. At
this point, the solution was filtered hot, and the solvent was distilled off
using the
high vac. The residue was purified by flash chromatography (80:2:1 to 20:2:1
EtOAc:EtOH:H20 gradient) to afford the product (10.8 g, 65%) as a white solid,
mp
143-147 °C; 'H NMR (DMSO-d6) 8 2.08 (s, 3H), 3.07-3.12 (m, 1H), 3.28-
3.50 (m,
5H), 3.51-3.60 (m, 2H), 3.64-3.75 (m, 3H), 4.10-4.12 (m, 1H), 4.30 (d, J = 7.9
Hz,
1H), 4.67 (t, 5.9 Hz, 1H), 4.68 (ABq, J = 12.5 Hz, as = 0.22, 2H), 5.14 (d, J
= 4.0 Hz,
1H), 5.25 (d, J = 5.1 Hz, 1H), 5.30 (d, J = 5.3 Hz, 1H), 5.51 (d, J = 3.3 Hz,
1H), 5.57
(s, 1H), 5.63 ( d, J = 6.8 Hz, 1H), 7.22 (dd, J = 1.5, 8.3 Hz, 1H), 7.35-7.38
(m, 3H),
7.42-7.46 (m, 3H), 7.66 (s, 1H), 9.53 (s, 1H); IR (KBr) 3500, 3410, 2910,
2850,
1700, 1600, 1550, 1440, 1425, 1375, 1310, 1230, 1150, 1070, and 1030 cm-';
mass
spectrum [(+) FAB], m/z 634 (M + Na)'; Anal. Calcd. for CZ8H34C1N0,z ~ 1.0
H20:
C, 53.38; H, 5.76; N, 2.22, Found: C, 53.58; H, 5.62; N, 2.25.
step 2
N-(5-{[4',6'-O-Benzylidene-6-O-(4-toluenesulfonyl)-p-D-maltosyl]-oxy-methyl}-2-
chIoro-phenyl)-acetamide
At 0 °C, to a stirred solution of N { 5-[(4',6'-O-benzylidene-p-D-
maltosyloxy)-methyl]-2-chloro-phenyl }-acetamide ( 1.81 g, 2.96 mmol) in
pyridine
(6.0 mL) was added a solution of p-toluenesulfonyl chloride (0.686 g, 3.60
mmol) in
CH~C12 (3.75 mL). After 2 h, additional p-toluenesulfonyl chloride (0.686 g,
3.60
mmol) in CHZC12 (3.75 mL) was added and the solution was stirred at 0
°C for 2h.
The reaction was quenched with ice cold H20 (50 mL) and extracted with EtOAc.
The combined organic extracts were washed successively with sat. aq. NaHC03
(2x),
sat. aq. CuSO, (2x), brine (2x), dried (NazS04) and concentrated. Purification
by flash
chromatography (5-10% MeOH:CHZCIz gradient) gave 0.930 g, (41 %) of a
colorless
solid, mp 105-120 °C; 'H NMR (DMSO-d6) s 2.08 (s, 3H), 2.33 (s, 3H),
3.04-3.09
(m, 1H), 3.27-3.45 (m, 4H), 3.49-3.53 (m, 1H), 3.60-3.65 (m, 3H), 3.95 (d,
1H), 4.13
(dd, 1H), 4.29-4.33 (m, 2H), 4.46 (d, 1 H), 4.62 (d, 1 H), 5.05 (d, 1H), 5.33-
5.35 (m,
2H), 5.55 (d, IH), 5.57 (s, 1H), 5.75 (d, 1H), 7.18 (d, 1H), 7.35-7.47 (m,
8H), 7.78
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/Z7828
-38-
(d, 2H}, 9.53 (s, 1H); mass spectrum [(+) ESI], m/z 766/768 (M + H);, 783/785
(M +
NH4)+; Anal. Calcd. for C35H,oNC10,4S ~ H20: C, 53.60; H, 5.40; N, 1.79,
Found: C,
53.46; H, 5.18; N, 1.80.
S Example 7
N-(5-f (2.3,2',3'-Tetra-O-acetyl-4' 6'-O-benzvlidene 6 O (4 toluenesulfony~
maltosvll-oxv-methvll-2-chloro- henyl)-acetamide
The title compound was prepared as a colorless solid (0.942 g, 99%) from N-
(5-{ [4',6'-O-benzylidene-6-O-(4-toluenesulfonyl)-p-D-maltosyl]-oxy-methyl }-2-
chloro-phenyl)-acetamide using a procedure similar to Example 3, mp 116-122
°C;
'H NMR (DMSO-db) s 1.91 (s, 3H), 1.92 (s, 3H), 1.96 (s, 3H), 2.00 (s, 3H),
2.08 (s,
3H), 2.29 (s, 3H), 3.68 (dd, 1H), 3.77 (t, 1H), 3.85 (t, 1H), 3.90 (t, 1H),
3.97-4.00
(m, 1H), 4.21 (dd, 1H), 4.32 (s, 2H), 4.39 (d, 1H), 4.56 (d, 1H), 4.60 (d,
1H), 4.78 (d,
1H), 4.86 (dd, 1H), 5.17-5.30 (m, 3H), 5.65 (s, 1H), 7.03 (d, 1H), 7.34-7.41
(m, 7H),
7.46 (d, 1H), 7.59 (s, 1H), 7.80 (d, 2H), 9.52 (s, 1H}; mass spectrum [(+)
ESI], m/z
934/936 {M + H)'; Anal. Calcd. for C,3H,8NCIO,gS: C, 55.27; H, 5.17; N, 1.50,
Found: C, 55.07; H, S.OS; N, 1.47.
Example 8
N (5-f(6-O-Benzvl-4' 6'-O-benzvlidene-a D maltosvl) oxv methyll 2 chloro
shenyl 1-acetamide
step 1
N {5-[(2,2',3,3'-Tetra-O-acetyl-4',6'-O-benzylidene-p-n-maltosyl)-oxy-methyl]-
2-
chioro-phenyl)-acetamide
A solution containing N (5-{ [2,3,2',3'-tetra-O-acetyl-4',6'-O-benzylidene-6-
O-(4-toluenesulfonyl)-p-D-maltosyl]-oxy-methyl}-2-chloro-phenyl)-acetamide
(1.021
g, 1.093 mmol) and sodium formate (0.1858 g, 2.732 mmol) in EtOH:DMSO:H20
(2:2:1, 21.9 mL) was heated at 100°C for 2 days. The reaction was
cooled to ambient
temperature, diluted with 10% CH2CIz:EtOAc (100 mL), washed with brine (3x),
dried (MgSOa) and concentrated in vacuo. Purification by flash chromatography
(1,2
and 3% MeOH:CHCl3 gradient) gave 0.446 g (52%) of title compound as a
colorless
solid; 'H NMR (DMSO-db) s 1.93 (s, 3H), 1.95 (s, 3H), 1.98 (s, 3H), 2.01 (s,
3H),
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-39-
2.08 (s, 3H), 3.68-3.99 (m, 7H), 4.19-4.22 (m, 1H), 4.57 (d, 3 = 12.7 Hz, 1H),
4.64-
4.87 (m, 4H), 5.00 (br.s, 1H), 5.21-5.33 (m, 3H), 5.63 (s, 1H), 7.08 (dd, J =
8.3, 1.8
Hz, 1H), 7.38 (s, 5H), 7.47 (d, J = 8.2 Hz, 1H), 7.64 (s, IH), 9.53 (s, 1H).
S step 2
N-{5-[(2,2',3,3'-Tetra-O-acetyl-6-O-benzyl-4',6'-O-benzylidene-p-n-maltosyl)-
oxy-methyl]-2-chloro-phenyl}-acetamide
At ambient temperature, to a stirred solution containing N {5-[(2,2',3,3'-
tetra-
O-acetyl-4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-chloro-phenyl }-
acetamide (0.221 g, 0.283 mmol) and benzyl 2,2,2-trichloroacetimidate (0.105
mL,
0.567 mmol) in 10% CHzCIZ:benzene ( 10 mL) was added trifluoromethane sulfonic
acid (1 drop). After 16 h, the reaction was diluted with 5% MeOH:CHC13 (10
mL),
filtered through a 1" silica gel pad eluting with 5% MeOH:CHC13 and
concentrated in
vacuo. Purification by flash chromatography (1 and 2% MeOH:CHC13 gradient)
gave
0.134 g (54%o) of title compound as a colorless solid; 'H NMR (DMSO-db) s 1.94
(s,
3H), 1.96 (s, 3H), 1.98 (s, 3H), 2.01 (s, 3H), 2.07 (s, 3H), 3.69-4.07 (m,
8H), 4.54-
4.59 (m, 3H), 4.69-4.77 (m, 2H), 4.82-4.86 (m, 2H), 5.23-5.34 (m, 3H), 5.61
(s, 1H),
7.09 (dd, J = 8.3, 1.7 Hz, IH), 7.27-7.37 (m, lOH), 7.46 (d, J = 8.2 Hz, 1H),
7.65 (s,
1H), 9.52 (s, 1H).
step 3
N-{5-[(6-O-Benzyl-4',6'-O-benzylidene-p-n-maltosyl}-oxy-methyl]-2-chloro-
phenyl}-acetamide
The title compound was prepared as a colorless solid (0.085 g, 65%) from N
{5-[(2,2',3,3'-tetra-O-acetyl-6-O-benzyl-4',6'-O-benzylidene-p-o-maltosyl)-oxy-
methyl)-2-chloro-phenyl }-acetamide using a procedure similar to step 4 of
Example
1, mp 98-105 °C; 'H NMR (DMSO-d6) s 2.06 (s, 3H), 3.09-3.11 (m, 1H),
3.28-3.75
(m, l OH), 3.99 (dd, 1 H), 4.33 (d, J = 7.7 Hz, 1 H), 4.50 (s, 1 H), 4.51 (s,
1 H), 4.66
(ABq, J = 12.6 Hz, os = 0.08, 2H), 5.14 (d, J = 3.7 Hz, 1H), 5.30 (d, J = 9.0
Hz, IH),
5.31 (d, J = 9.0 Hz. 1H), 5.56-5.57 (m, 2H), 5.70 (d, J = 6.6 Hz, 1H), 7.21-
7.38 (m,
9H), 7.42 - 7.45 (m, 3H), 7.66 (s, 1H), 9.52 (s, 1H); mass spectrum [(+) FAB],
m/z
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-40-
724 (M + H)'; Anal. Calcd. for C,SH4oNCl0,, ~ 0.5 H.,O: C, 59.11; H, 5.81; N,
1.97,
Found: C, 59.12; H, 5.76; N, 1.98.
Example 9
N !5-f!6-O-Benzyl-4'.6'-O-ethvlidene-p-D-maltosyl)-oxy-methyll-2-chloro phenyl
acetamide
step 1
N-{5-[(2,2',3,3'-Tetra-O-acetyl-6-O-benzyl-p-D-maltosyl)-oxy-methyl]-2-chloro-
phenyl }-acetamide
At ambient temperature, to a stirred solution of N { 5-[(2,2',3,3'-tetra-O-
acetyl
-6-O-benzoyl-4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-chloro-phenyl }-
acetamide (0.202 g, 0.232 mmol) in MeOH (5 mL) was added 1 M ethereal ~ HCI.
After 2 h, the reaction was quenched with sat. aq. NaHC03 (25 mL), diluted
with H20
(25 mL), extracted with EtOAc, dried (NazS04) and concentrated. Purification
by
flash chromatography (5% MeOH:CHCl3) gave 0.136 g (75%o) of title compound; 'H
NMR (DMSO-d6) s 1.92 (s, 3H), 1.95 (s, 6H), 2.00 (s, 3H), 2.07 (s, 3H), 3.49-
3.56
(m, 4H), 3.75-3.94 (m, 4H), 4.50-4.59 (m, SH), 4.67-4.76 (m, 2H), 4.83 (d, J =
7.9
Hz, 1H), 5.06-5.13 (m, 1H), 5.21-5.29 (m, 2H), 5.44 (d, J = 6.0 Hz, 1H), 7.08
(dd, J
= 8.1, 1.5 Hz, 1H), 7.26-7.36 (m, SH), 7.45 (d, J = 8.2 Hz, 1H), 7.64 (s, 1H),
9.52 (s,
1 H).
step 2
N-{5-[(2,2',3,3'-Tetra-O-acetyl-6-O-benzyl-4',6'-O-ethylidene-~-~-maltosyl)-
oxy-
methyl]-2-chioro-phenyl}-acetamide
A stirred solution containing N-{5-[(2,2',3,3'-tetra-O-acetyl-6-O-benzyl-R-D-
maltosyl)-oxy-methyl]-2-chloro-phenyl }-acetamide {0.274 g, 0.350 mmol),
priopionaldehyde (45.5 NL,, 0.630 mmol) and camphor sulfonic acid (18.3 mg,
0.0787
mmol) in benzene (6.3 mL) was ret7uxed with Dean-Stark azeotxopic removal of
water. After 2.5 h, The reaction was cooled to room temperature, quenched with
NaHCO, (25 mL), extracted with EtOAc and dried (NazS04). Purification by flash
chromatography (1, 2 and 3% MeOH:CHC13 gradient:) gave 0.346 g (96%) of title
compound; 'H NMR (DMSO-d6) s 0.82 (t, 3H), 1.48-1.,53 (m, 2H), 1.92 (s, 3H),
1.95
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-41 -
(s, 3H), 1.97 (s, 3H), 2.01 (s, 3H), 2.07 (s, 3H), 3.41-3.48 (m, 1H), 3.60-
3.64 (m,
2H), 3.71-4.03 (m, SH), 4.49-4.69 (m, 4H), 4.70-4.85 (m, 4H), S.I4-5.32 (m,
3H),
7.07-7.36 (m, 6H), 7.46 (d, J = 8.2 Hz, 1 H), 7.64 (s, 1 H), 9.52 (s, 1 H).
step 3
N-{5-[(6-O-Benzyl-4',6'-O-ethylidene-p-~-maltosyl)-oxy-methyl]-2-chloro-
phenyl}-acetamide
A solution containing N { 5-[(2,2',3,3'-tetra-O-acetyl-6-O-benzyl-4',6'-O-
ethylidene-p-n-maltosyl)-oxy-methyl]-2-chloro-phenyl }-acetamide (0.217 g,
0.264
mmol) and 25 weight% NaOMe in MeOH (0.0285 g, 0.132 mmol) in MeOH (5.3
mL) was refluxed for 3h. The reaction was cooled to room temperature and
concentrated. Purification by flash chromatography (10% MeOH/CHZCl2 gradient)
gave the product (O.100g, 58%) as a white solid, mp 182-185 °C; 'H NMR
(DMSO-
db) s 0.85 (t, J = 7.5 Hz, 3H), 1.21-1.55 (m, 2H), 2.05 (s, 3H), 3.05-3.13 (m,
2H),
1 S 3.24-3.70 (m, 9H), 3.83 (dd, J = 9.8, 4.7 Hz, 1 H), 4.30 (d, J = 7.7 Hz, 1
H), 4.43-4.49
(m, 3H), 4.63 (ABq, J = 12.4 Hz, es = 0.07, 2H), 5.07 (d, J = 3.5 Hz, 1H),
5.24 (d, J
= 5.3 Hz, 1H), 5.31 (d, J = 5.3 Hz, 1H), 5.55 (d, J = 2.6 Hz, 1H), 5.65 (d, J
= 6.6 Hz,
1H), 7.17-7.34 (m, 6H), 7.41 (d, J = 8.3 Hz, 1H), 7.63 (s, IH), 9.55 (s, 1H).
mass
spectrum [(+) ESI] 671 (M + NH4)'; Anal. Calcd. for C3,H,oNCIO,~: C, 56.92; H,
6.16; N, 2.14, Found: C, 56.69; H, 6.33; N, 1.99.
Example 10
N (2-Chlora-5-( f4' 6'-O-(4-nitro)-benzylidene-a-D-maltosyll-oxv methyl l
phenyl)
acetamide
To a stirred solution of N [2-chloro-5-(p-D-maltosyl-oxymethyl)-phenyl]-
acetamide (0.500 g, 0.954 mmol) in DMF (30 mL) at rt was added 3-nitro-
benzaldehyde dimethyl acetal (0.752 g, 3.82 mmol) followed by CSA (0.111 g,
0.477
mmol). The reaction mixture was heated to 60 °C for 18 h and was about
35%
complete by TLC. Another 0.5 eq. CSA (0.111 g) added and heated at 90
°C for 3 h.
The reaction was then quenched with KZC03 (0.132 g, 0.954 mmol) with an
additional 0.5 h heating at 60 °C. At this point, the solution was
filtered hot, and the
solvent was distilled off using the high vac. The residue was purified by
flash
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-42-
chromatography (40:2:1 to 20:2:1 EtOAc:EtOH:H20 gradient) to afford the
product
(0.262 g, 42%) as a white solid, mp 221-223 °C; 'H NMR (DMSO-d6) s 2.07
(s, 3H),
3.06-3.12 (m, 1H), 3.26-3.49 (m, SH), 3.49-3.62 (m, 2H), 3.68-3.77 (m, 3H),
4.11-
4.20 (m, 1H), 4.30 (d, J = 7.7 Hz, 1H), 4.67 (t, J = 5.7 Hz, IH), 4.68 (ABq, J
= 12.3
Hz, es = 0.22, 2H), 5.16 (d, J = 3.7 Hz, 1H), 5.25 (d, J = 4.8 Hz, 1H), 5.36
(d, J = 4.8
Hz, 1 H), 5.49 (d, J = 3.1 Hz, 1 H), 5.62 (d, J = 6.6 Hz, 1 H), 5.74 (s, I H),
7.22 (dd, J =
1.8, 8.1 Hz, 1 H), 7.44 (d, J = 8.3 Hz, 1 H), 7.65 (s, 1 H), 7.73 (d, J = 8.8
Hz, 2H), 8.25
(dt, J = 2.2, 9.0 Hz, 2H), 9.52 (s, 1H); IR (KBr) 3410, 2910, 2870, 1670,
1610, 1590,
1530, 1440, 1420, 1355, 1320, 1265, 1140, 1075, and 1035 cm~'; mass spectrum
[(-)
FAB], m/z 655 (M - H)-; Anal. Calcd. for Cz8H33C1N2U": C, 51.19; H, 5.06; N,
4.26,
Found: C, 50.87; H, 4.87; N, 4.32.
Example 11
N (5-lf6-O-Benzoyl-4',6'-O-(4-nitro)-benzvlidene-a-~-maltosvll-oxy-methyl?-2-
chl. oro-nhenyl)-acetamide
The title compound was prepared as a white solid (0.080 g, 35%) from N-(2-
chloro-S-{ [4',6'-O-(4-nitro)-benzylidene-p-D-maltosyl)-oxy-methyl }-phenyl)-
acetamide using the procedure of Example 2, mp >167 °C (decomp.); 'H
NMR
(DMSO-d6) s 2.04 (s, 3H), 3.15-3.22 (m, 1H), 3.37-3.43 (m, 2H), 3.51 (td, J =
2.9,
8.8 Hz, IH), 3.57-3.64 (m, 3H), 3.70-3.78 (m, 2H), 4.09 (dd, J = 4.6, 9.9 Hz,
1H),
4.35 (dd, J = 5.1, I2.3 Hz, 1H), 4.39 (d, J = 7.7 Hz, 1H), 4.57-4.63 (m, 1H),
4.65
(ABq, J = 12.3 Hz, es = 0.14, 2H), 5.15 (d, J = 4.0 Hz, 1H), 5.36 (d, J = 5.3
Hz, 1H),
5.41 (d, 3 = 5.3 Hz, 1H), 5.57 (d, J = 3.1 Hz, 1H), 5.70 (s, 1H), 5.81 (d, J =
6.2 Hz,
1H), 7.19 (dd, J = 1.8, 8.1 Hz, 1H), 7.40 (d, J = 8.1 Hz, 1H), 7.50-7.56 (m,
2H), 7.62-
7.72 (m, 4H), 7.97-8.01 (m, 2H), 8.24 (dt, J = 2.4, 9.2 Hz, 2H), 9.45 (s, 1H);
IR
(KBr) 3410, 2850, 1725, 1660, 1610, 1590, 1530, 1445, 1420, 1355, 1270, 1120,
1075, 1025, and 715 cm~'; mass spectrum [(+) FAB], m/z 783 (M + Na)+; Anal.
Calcd. for C35H3,C1NZO~5 ~ 1.0 H.,O: C, 53.95; H, 5.05; N, 3.60, Found: C,
53.86; H,
4.75; N, 3.51.
CA 02350066 2001-05-09
WO 00/31096 PCTNS99/27828
-43-
Example 12
N (2-Chloro-5-f(4'.6'-O-(4-chloro)-benzvlidene-a-D-maltos 1)-oxy-methvll
phenyl ?-acetamide
The title compound was prepared as a white glass (0.315 g, 51 %) from N [2-
chloro-5-(p-D-maltosyl-oxymethyl)-phenyl]-acetamide using p-chloro-
benzaldehyde
dimethyl acetal and the procedure of Example 10, mp >97 °C (decomp.);
'H NMR
(DMSO-d~) s 2.07 (s, 3H), 3.09 (t, J = 8.8 Hz, 1H), 3.28-3.48 (m, SH}, 3.49-
3.61 (m,
2H), 3.61-3.75 (m, 3H), 4.11 (d, J = 5.3 Hz, 1H), 4.3(1 (d, J = 7.7 Hz, 1H),
4.63-4.72
(bs, 1H), 4.68 (ABq, J = 12.3 Hz, os = 0.22, 2H), 5.14 (d, J = 4.0 Hz, 1H),
5.21-5.36
(bs, 2H), 5.47-5.53 (bs, 1H), 5.57-5.66 (bs, 1H), 5.59 (s, 1H), 7.22 (dd, J =
2.0, 8.3
Hz, 1 H), 7.42-7.48 (m, SH), 7.65 (s, 1 H), 9.52 (s, 1 H); IR (KBr) 3390,
2920, 2850,
1670, 1590, 1530, 1500, 1450, 1420, 1365, 1300, 1140, 1070, 1030, and 815 cm-
';
mass spectrum [(+) FAB], m/z 668 (M + Na)"; Anal. Calcd. for CZ8H33C12NO,2 ~
0.5
HZO: C, 51.31; H, 5.23; N, 2.14, Found: C, 51.13; H, 5.44; N, 1.92.
Example 13
N-!5-f(6-O-Benzovl-4' 6'-O-(4-chloro)-benz~lidene-a-D-maltosvl)-oxy-meth lv 1
2 ,
chloro phenyl l-acetamide
The title compound was prepared as a white solid (0.158 g, 50%) from N-{ 2-
chloro-5-[(4',6'-O-(4-chloro)-benzylidene-p-D-maltosyl)-oxy-methyl]-phenyl}-
acetamide using a procedure similar to Example 2, mp ,182 °C (decomp.);
'H NMR
(DMSO-d6) s 2.05 (s, 3H), 3.15-3.22 (m, 1H), 3.28-3.43 (m, 2H), 3.48-3.63 (m,
4H),
3.67-3.73 (m, 1H), 3.73-3.78 (m, 1H), 4.03-4.07 (m, 1H), 4.32-4.37 (m, 1H),
4.39 (d,
J = 7.9 Hz, 1H), 4.57-4.63 (m, IH), 4.65 (ABq, J = 12.5 Hz, 08 = 0.14, 2H),
5.14 (d,
J = 4.0 Hz, IH), 5.36 (dd, J = 2.6, 5.1 Hz, 2H), 5.54 (s, 1H), 5.56 (d, J =
3.1 Hz, 1H),
5.79 (d, J = 6.4 Hz. 1H), 7.19 (dd, J = 2.0, 8.3 Hz, 1H), 7.40 (d, J = 8.3 Hz,
IH), 7.43
(s, 4H), 7.53 (t, J = 7.9 Hz, 2H), 7.63-7.68 (m, 2H), 7.99 (dd, J = 0.9, 7.9
Hz, 2H),
9.SU (s, 1H); IR (KBr) 3450, 3380, 2960, 2900, 2860, 1730, 1700, 1665, 1590,
1530,
1495, 1440, 1415. 1365, 1310, I28U, 1140, 1075, 1050, 1035, 1015, 820, and 720
cm-
'; mass spectrum [(+) FAB], m/z 75U (M + H)", 772 (M + Na)"; Anal. Calcd. for
C35H3~C12NO,3: C. 56.01; H, 4.97; N, 1.87, Found: C, 55.67; H, 4.91; N, 1.87.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
Example 14
N !2-Chloro-S-ff4',6'-O-isobutvlidene-a-D-maltosyl)-oxv-methvll_phenyll
acetamide
The title compound was prepared as a white solid (0.250 g, 45%) from N-[2
chloro-5-(p-D-maltosyl-oxymethyl)-phenyl]-acetamide using isobutyraldehyde
diethyl acetal and the procedure of Example 10, mp >122 °C (decomp.);
'H NMR
(DMSO-d6) s 0.87 (dd, J = 5.5, 6.6 Hz, 6H), 1.68-1.78 (m, 1H), 2.07 (s, 3H),
3.04
3.13 (m, 2H), 3.24-3.55 (m, 8H), 3.68 (dd, J = 6.2, 10.5 Hz, 1H}, 3.97 (dd, J
= 4.6,
9.7 Hz, 1 H), 4.27 (s, 1 H), 4.28 (d, J = 3.7 Hz, 1 H), 4.65 (t, J = 5.9 Hz, 1
H), 4.67
(ABq, J = 12.5 Hz, es = 0.22, 2H), 5.06 (d, J = 4.0 Hz, I H), 5.17 (d, J = 5.3
Hz, 1 H),
5.24 (d, J = 5.3 Hz, 1H), 5.50 (d, J = 3.3 Hz, 1H), 5.59 (d, J = 6.6 Hz, 1H),
7.22 (dd,
J = 1.5, 7.9 Hz, 1H), 7.44 (d, J = 8.3 Hz, 1H), 7.65 {s, 1H), 9.52 (s, 1H); IR
(KBr)
3420, 2960, 2910, 2830, 1670, 1590, 1530, 1460, 1420, 1370, 1310, 1255, 1145,
1080, 1055, and 1030 cm-'; mass spectrum [(-) ESI], m/z 576 (M - H)-; Anal.
Calcd.
for C,SH36C1N0,~ ~ 0.5 H20: C, 51.15; H, 6.35; N, 2.39, Found: C, 51.06; H,
6.56; N,
2.45.
Example 15
N f 5-f(6-O-Benzovl-4',6'-D-isobutvlidene-a-D-maltosyl)-oxv-methyll-2-chloro-
phenyl ~-acetamide
The title compound was prepared as a white solid (0.079 g, 41%o) from N-{2-
chloro-S-[ (4' ,6'-O-isobutylidene-p-D-maltosyl)-oxy-methyl]-phenyl }-
acetamide
using the procedure of Example 2, mp >123 °C (decomp.); 'H NMR (DMSO-
d6) s
0.85 (t, J = 6.4 Hz, 6H), 1.66-1.75 (m, 1H), 2.05 (s, 3H), 3.07 (t, J = 9.4
Hz, IH),
3.15-3.21 (m, IH), 3.26-3.36 (m, 2H), 3.46-3.60 (m, 4H), 3.72-3.77 (m, 1H),
3.92
(dd, J = 4.8. 10.1 Hz, 1H), 4.23 (d, J = 4.8 Hz, 1H), 4.30 (dd, J = 5.1, 12.1
Hz, 1H),
4.38 (d, J = 7.7 Hz, 1H), 4.57 (d, J = 10.5 Hz, 1H), 4.64 (ABq, J = 12.5 Hz,
es =
0.14, 2H), 5.06 (d, J = 4.0 Hz, 1H), 5.21 (d, J = 4.8 Hz, 1H), 5.34 (d, J =
5.1 Hz, 1H),
5.57 (d, J = 2.2 Hz, 1H), 5.76 (d, J = 5.9 Hz, IH), 7.18 (dd, J = 1.5, 8.3 Hz,
1H), 7.40
(d, J = 8.3 Hz. 1H), 7.53 (t, J = 7.9 Hz, 2H), 7.61-7.68 (m, 2H), 7.96-8.01
(m, 2H),
9.50 (s, 1H); IR (KBr) 3410, 2960, 2910, 2840, 1725, 1660, 1610, 1590, 1530,
1440,
1425, 1375. 1270, 1080, 1055, 1025, and 715 cm-'; mass spectrum [(-) APCI],
m/z
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-45-
681.0/683.1 (M)~, Anal. Calcd. for C32H,oC1NO,3 ~ 1.0 HZO: C, 54.90; H, 6.05;
N,
2.00, Found: C, 54.95; H, 5.90; N, 1.94.
Example 16
N-15-f(4',6'-O-((1R)-2-Phenyl-ethylidenel-f~-y-maltosvloxv)-methyll-2-chloro-
phenyl 1-acetamide
The title compound was prepared as a white solid (0.210 g, 35%) from N [2-
chloro-5-(p-D-maltosyl-oxymethyl)-phenyl]-acetamidE: using phenylacetaldehyde
dimethyl acetal and the procedure of Example 10, mp >106 °C (decomp.);
'H NMR
(DMSO-db) s 2.07 (s, 3H), 2.81 (dd, J = 6.4, 14.1, 1H), 2.91 (dd, J = 4.0,
14.1 Hz,
1H), 3.04-3.11 (m, 1H), 3.16 (t, J = 9.4 Hz, 1H), 3.26-3.54 (m, 7H), 3.58 (td,
J = 5.1,
10.1 Hz, 1H), 3.65-3.72 (m, 1H), 3.93 (dd, J = 4.8, 9.9 Hz, 1H), 4.29 (d, J =
7.7 Hz,
1H), 4.65 (t, J = 5.9 Hz, 1H), 4.67 (ABq, J = 12.3 Hz, n8 = 0.22, 2H), 4.75
(dd, J =
4.2, 6.2 Hz, 1H), 5.09 (d, J = 3.7 Hz, 1H), 5.23 (dd, J = 5.3, 10.1 Hz, 2H),
5.47 (d, J
= 3.5 Hz, 1H), 5.57 (d, J = 6.6 Hz, 1H), 7.17-7.30 (m, 6H), 7.44 (d, J = 8.1
Hz, 1H),
7.65 (s, 1H), 9.52 (s, 1H); IR (KBr) 3400, 2920, 2850, 1670, 1590, 1530, 1450,
1420,
1375, 1310, 1250, 1150, 1130, 1060, 1025, and 750 cm-'; mass spectrum [(+)
FAB],
m/z 648 (M + Na)'; Anal. Calcd. for Cz9H36C1NO12 ~ 2.75 H20: C, 51.56; H,
6.19; N,
2.07, Found: C, 51.47; H, 5.52; N, 2.13.
Example 17
N-15-f(6-O-Benzovl-4'.6'-O-((1R)-2-phenyl-eth~,lidene)-f3-D-maltosylox )-y
methyll
2-chloro-phe~l )-acetamide
The title compound was prepared as a white solid (1.25 g, 63%) from N {5-
[(4',6'-O-((1R)-2-phenyl-ethylidene)-p-D-maltosyloxy)-methyl]-2-chloro-phenyl}-
acetamide using the procedure of Example 2, mp 153-156°C; 'H NMR (DMSO-
d6) s
2.05 (s, 3H), 2.78 (dd, J = 6.2, 14.1 Hz, 1H), 2.89 (dd, J = 4.2, 14.3 Hz,
1H), 3.10-
3.21 (m, 2H), 3.26-3.37 (m, 2H), 3.47-3.64 (m, 4H), 3.71-3.77 (m, iH), 3.89
(dd, J =
4.8, 9.9 Hz, 1H), 4.31 (dd, J = 5.3, 12.1 Hz, 1H), 4.38 (d, J = 7.7 Hz, 1H),
4.53-4.60
(m, 2H), 4.68-4.77 (m, 2H), 5.07 (d, J = 4.0 Hz, 1H), 5.27 (d, J = 5.5 Hz,
1H), 5.34
(d, J = 5.3 Hz, 1H), 5.55 (d, J = 2.9 Hz, 1H), 5.77 (d, J = 6.2 Hz, 1H), 7.16-
7.28 (m,
6H), 7.40 (d, J = 8.1 Hz, 1H), 7.52 (t, J = 7.7 Hz, 2H), 7.62-7.67 (m, 2H),
7.98 (dd, J
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-46-
= 0.7, 7.9 Hz, 2H), 9.50 (s, 1H); IR (KBr) 3480, 3370, 2910, 1725, 1695, 1590,
1525,
1450, 1425, 1380, 1355, 1310, 1275, 1250, 1235, l 140, 1120, 1075, 1050, 1035,
and
715 cni'; mass spectrum [(-) FAB], m/z 728 (M - H)-; Anal. Calcd. for
C36H,oC1N0,3:
C, 59.22; H, 5.52; N, 1.92, Found: C, 58.93; H, 5.45; N, 1.86.
Example 18
N= (2-Chloro-5-f(4',6'-O-((1R)-3-cyano-propvlidene)J3-D-maltosvloxy)-methyll
henyl l-acetamide
The title compound was prepared as a tan solid (0.101 g, 18%) from N-[2-
chloro-5-((3-D-maltosyl-oxymethyl)-phenyl)-acetamide using 3-cyanopropionalde-
hyde dimethyl acetal and the procedure of Example 10, mp 185-188 °C; 'H
NMR
(DMSO-d~) s 1.82-1.89 (m, 2H), 2.07 (s, 3H), 2.47-2.55 (m, 2H), 3.04-3.11 (m,
1H),
3.15 (t, J = 9.2 Hz, 1 H), 3.24-3.59 (m, 8H), 3.65-3.71 (m, 1 H), 3.99 (dd, J
= 4.6, 9.9
Hz, 1H), 4.28 (d, J = 7.7 Hz, IH), 4.62-4.67 (m, 2H), 4.67 (ABq, J = 12.5 Hz,
es =
0.22, 2H), 5.10 (d, J = 3.7 Hz, 1H), 5.24 (dd, J = 5.3, 7.2 Hz, 2H), 5.48 (d,
J = 3.3
Hz, 1H), 5.58 (d, J = 6.6 Hz, 1H), 7.22 (d, J = 1.8, 8.1 Hz, 1H), 7.44 (8.1
Hz, 1H),
7.65 (s, 1H), 9.52 (s, 1H); IR (KBr) 3540, 3410, 3120, 2930, 2850, 2230, 1685,
1590,
1540, 1450, 1425, 1420, 1370, 1320, 1255, 1150, 1130, 1100, 1065, 1050, 1020,
995,
and 890 cm-'; mass spectrum [(+) FAB], m/z 611 (M + Na)'; Anal. Calcd. for
C~H3,C1Nz0,2 ~ 0.5 H=O: C, 50.21; H, 5.73; N, 4.68, Found: C, 50.32; H, 5.51;
N,
4.84.
Example 19
N (5-If6-O-Benzovl-4' 6'-O-((1R)-3-cyanopropylidene)-Q-D-malto~yloxyl-methyll
2-chloro-phenvll-acetamide
The title compound was prepared as a white solid (0.038 g, 46%) from N { 2-
chloro-5-[(4',6'-O-(( 1R)-3-cyano-propylidene)-p-D-maltosyloxy)-methyl]-phenyl
}-
acetamide using the procedure of Example 2, mp 164-:166 °C; 'H NMR
(DMSO-d6) s
1.79-1.86 (m, 2H), 2.04 (s, 3H), 2.48-2.52 (m, 2H), 3.13 {t, J = 9.2 Hz, 1H),
3.14-
3.20 (m, 1H), 3.27-3.40 (m, 2H), 3.46-3.60 (m, 4H), 3.71-3.77 (m, 1H), 3.93
(dd, J =
4.6, 9.9 Hz, 1H), 4.30 (dd, J = 5.3, 12.1 Hz, 1H), 4.38 (d, J = 7.9 Hz, 1H),
4.55-4.62
(m, 2H), 4.64 (ABq, J = 12.5 Hz, os = 0.14, 2H), 5.08 (d, J = 3.7 Hz, 1H),
5.28 (d, J
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-47-
= 5.1 Hz, 1H), 5.35 (d, J = 5.3 Hz, 1H), 5.55 (d, J = 2.9 Hz, 1H), 5.77 (d, J
= 6.2 Hz,
1H), 7.18 (dd, J = 1.5, 8.1 Hz, 1H), 7.40 (d, J = 8.1 Hz, 1H), 7.53 (t, J =
7.9 Hz, 2H),
7.62-7.68 (m, 2H}, 7.98 (dd, J = 1.5, 8.3 Hz, 2H), 9.50 (s, 1H); IR (KBr)
3450, 3380,
3320, 2920, 2880, 2240, 1725, 1710, 1670, 1610, 1590, 1530, 1440, 1420, 1370,
1310, 1275, 1125, 1100, 1060, 1030, 1020, and 720 cm~'; mass spectrum [(-)
FAB],
m/z 691 (M - H}-; Anal. Calcd. for C3~H3,C1NZO,3: C, 55.45; H, 5.38; N, 4.04,
Found: C, 55.25; H, 5.44; N, 3.90.
Example 20
N-f2-Chloro-5-f(4'.6'-O-((1R)-3-ethoxy-nropvlidene)-a-D-maltos~y)-meth3r11-
phenyl 1-acetamide
The title compound was prepared as a white solid (0.080 g, 14%) from N [2-
chloro-5-(p-D-maltosyl-oxymethyl)-phenyl]-acetamide using 3-chloropropionalde-
hyde diethyl acetal and the procedure of Example 10, mp 149.5-153 °C;
'H NMR
(DMSO-d6) s 1.08 (t, J = 7.0 Hz, 3H), 1.70-1.81 (m, 2H), 2.07 (s, 3H), 2.44-
2.54 (m,
2H), 3.04-3.17 (m, 2H), 3.24-3.60 (m, lOH), 3.64-3.71 (m, 1H), 3.95 (dd, J =
4.8, 9.9
Hz, 1H), 4.28 (d, J = 7.7 Hz, 1H), 4.63 (dd, J = 5.7, 9.7 Hz, 2H), 4.67 (ABq,
J =
12.3 Hz, ~s = 0.22, 2H), 5.09 (d, J = 3.7 Hz, 1H), 5.19 (d, J = 5.3 Hz, 1H),
5.23 (d, J
= 5.3 Hz, 1H), 5.46 (d, J = 3.3 Hz, 1H), 5.56 (d, J = 6.6 Hz, 1H), 7.22 (dd, J
= 1.8,
8.1 Hz, 1H), 7.44 (d, J = 8.3 Hz, 1H), 7.65 (s, 1H), 9.51 (s, 1H); IR (KBr)
3500,
3420, 2970, 2920, 2840, 1690, 1590, 1530, 1440, 1420, 1370, 1320, 1250, 1110,
1070, and 1020 cm-'; mass spectrum [(-) FAB], m/z 606 (M - H)~; Anal. Calcd.
for
C26H38C1NO" ~ 1.5 H=O: C, 49.17; H, 6.51; N, 2.21, Found: C, 48.89; H, 5.93;
N,
2.27.
Example 21
N t5-(f6-O-Benzoyl-4',6'-O-((1R)-3-ethoxypropvlidene)-(~-D-malto~lox3rl-
methyll-
2-chloro-phenyl~-acetamide
The title compound was prepared as a off-white solid (0.015 g, 26%) from N-
{2-chloro-5-[(4',6'-O-((1R)-3-ethoxy-propylidene)-p-D-maltosyloxy)-methyl]-
phenyl}-acetamide using the procedure of Example 2, mp >94 °C
(decomp.); 'H
NMR (DMSO-db) s 1.07 (t, J = 7.0 Hz, 3H), 1.69-1.78 (m, 2H), 2.04 (s, 3H),
2.44-
CA 02350066 2001-05-09
WO 00/31096 PCTNS99/27828
-48-
2.54 (m, 2H), 3.09 (t, J = 9.7 Hz, 1H), 3.14-3.21 (m, IH), 3.26-3.42 (m, 4H),
3.45-
3.60 (m, 4H), 3.71-3.76 (m, 1H), 3.91 (dd, J = 4.4, 9.7 Hz, 1H), 4.30 (dd, J =
5.3,
12.3 Hz, 1H), 4.37 (d, J = 7.7 Hz, 1H), 4.54-4.61 (m, 2H), 4.64 (ABq, J = 12.5
Hz, es
= 0.14, 2H), 5.07 (d, J = 4.0 Hz, IH), 5.25 (d, J = 5.3 Hz, 1H), 5.34 (d, J =
5.3 Hz,
1H), 5.55 (d, J = 2.6 Hz, 1H), 5.77 (d, J = 6.2 Hz, 1H}, 7.18 (d, J = 8.3 Hz,
1H}, 7.40
(d, J = 8.1 Hz, 1H), 7.53 (t, J = 7.9 Hz, 2H), 7.62-7.68 (m, 2H), 7.98 (d, J =
7.2 Hz,
2H), 9.50 (s, 1H); IR (KBr) 3410, 2910, 2850, 1720, 1670, 1590, 1530, 1440,
1420,
1370, 1280, 1115, 1060, 1025, and 720 cm-'; mass spectrum [(+) FAB], m/z 734
(M
+ Na)r; Anal. Calcd. for C33H42C1NO,4 ~ 4.0 H20: C, 50.54; H, 6.43; N, 1.79,
Found:
C, 50.22; H, 5.28; N, 1.77.
Example 22
N-(2-Chloro-5-( f4',6'-O-(4-pyridinemethylidene)-Q-D-maltosyll-ox_y meth,
phenvll-acetamide
To a stirred solution of N [2-chloro-5-(p-D-maltosyl-oxymethyl)-phenyl]-
acetamide (0.500 g, 0.954 mmol) in DMF (25 mL) at rt was added 4-
pyridinecarboxaldehyde (0.446 mL, 4.67 mmol) followed by concentrated HZSO,
(0.105 mL, 3.78 mmol). The reaction mixture was heated to 110 °C for 18
h. The
reaction was then quenched with KZC03 ( 1.40 g, 10.1 mmol) with an additional
0.5 h
heating at 60 °C. At this point, the solution was filtered hot, and the
solvent was
distilled off using the high vac. The residue was purified by flash
chromatography
(80:6:3 to 5:2:1 EtOAc:EtOH:H20 gradient) to afford the product (0.050 g, 9%)
as a
yellow solid, mp >112 °C (decomp.); 'H NMR (DMSO-d6) s 2.07 (s, 3H),
3.05-3.13
(m, 1H), 3.27-3.49 (m, 4H), 3.49-3.62 (m, 3H), 3.66-3.75 (m, 3H), 4.14 (dd, J
=
12.5, 17.5 Hz, 1H), 4.29 (d, J = 7.7 Hz, 1H), 4.67 (t, J = 5.9 Hz, 1H), 4.67
(ABq, J =
12.3 Hz, es = 0.22, 2H), 5.16 (d, J = 3.7 Hz, 1H), 5.25 (d, J = 5.3 Hz, 1H),
5.35 (d, J
= 4.8 Hz, 1H), 5.50 (d, J = 3.3 Hz, 1H), 5.61-5.65 (m, 2H), 7.22 (dd, J = 1.8,
8.3 Hz,
1H), 7.42-7.47 (m, 3H), 7.65 (s, 1H), 8.60 (d, J = 5.9 Hz, 2H), 9.52 (s, 1H);
IR (KBr)
3390, 2920, 2830, 1670, 1620, 1590, 1530, 1450, 1420, 1380, 1310, 1270, 1245,
1180, 1145, 1075, 1055, 1030, and 755 cm-'; mass spectrum [(-) FAB], m/z 611
(M -
H)-; Anal. Calcd. for C,,H"ClNz0,2 ~ 4.25 H,O: C, 47.03; H, 6.07; N, 4.06,
Found:
C, 46.63; H, 5.00; N, 3.60.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-49-
Example 23
Benzoic acid 6-(3-acetvlamino-4-chloro-benzoyloxy)-3-(7 8 dihydroxy 2 pvridin
4
-hexahvdro-nvranof3 2-dlf 1 3Tdioxin-6- r~loxv)-4 5-dihydroxv tetrahvdro
n_vran 2
S ylmethvl ester
The title compound was prepared as a off-white solid (0.025 g, 46%) from N
(2-chloro-S-{ [4',6'-O-(4-pyridinemethylidene)-p-D-maltosyl]-oxy-methyl }-
phenyl)-
acetamide using the procedure of Example 2, mp 260-261.5 °C; 'H NMR
(DMSO-d6)
s 2.04 (s, 3H), 3.15-3.22 (m, 1H), 3.25-3.43 (m, 2H), 3.51 (td, J = 2.9, 8.8
Hz, 1H),
3.55-3.64 (m, 3H), 3.68-3.77 (m, 2H), 4.07 (dd, J = 4.6, 9.7 Hz, 1H), 4.34
(dd, J =
5.3, 12.3 Hz, 1H), 4.39 (d, J = 7.7 Hz, 1H), 4.56-4.62 (m, 1H), 4.65 (ABq, J =
12.3
Hz, es = 0.14, 2H), 5.15 (d, J = 4.0 Hz, 1 H), 5.35 (d, J = 5.3 Hz, 1 H), 5.39
(d, J = 5.1
Hz, 1H), 5.56 (d, J = 3.1 Hz, 1H), 5.58 (s, 1H), 5.79 (d, J = 6.2 Hz, 1H),
7.18 (dd, J =
2.0, 8.3 Hz, 1 H), 7.38-7.45 (m, 3H), 7.53 (t, J = 7.9 Hz, 2H), 7.62-7.68 (m,
2H),
7.97-8.01 (m, 2H), 8.59 (d, J = 4.6 Hz, 2H), 9.49 (s, 1H); IR (KBr) 3460,
3310, 3240,
2910, 2830, 1725, 1665, 1620, 1590, 1530, 1420, 1375, 1275, 1140, 1075, 1055,
1030, and 715 cm~'; mass spectrum [(+) FAB], m/z 717 (M + H)', 739 (M + Na)';
Anal. Calcd. for C3;H3,C1NZO,3 ~ 1.5 H.,O: C, 54.88; H, 5.42; N, 3.76, Found:
C,
54.55; H, 4.98; N, 3.68.
Example 24
N 15-f(4'.6'-O-Benzvlidene-a-D-maltosyloxy)-methyll-2-chloro~henvll acetamide
To a stirred solution of N [2-chloro-5-(~i-D-maltosyl-oxymethyl)-phenyl]-
acetamide ( 14. i 5 g, 27.0 mmol) in DMF (325 mL) at rt was added benzaldehyde
dimethyl acetal (8.11 mL, 54.0 mmol) dropwise followed by TsOH~HzO (2.57 g,
13.5
mmol). The reaction mixture was heated to 60 °C for 6 h and then
quenched with
K',C03 (1.87 g, 13.5 mmol) with an additional 0.5 h heating at this
temperature. At
this point, the solution was filtered hot, and the solvent was distilled off
using the
high vac. The residue was purified by flash chromatography (80:2:1 to 20:2:1
EtOAc:EtOH:H20 gradient) to afford the product (10.8 g, 65%) as a white solid,
mp
143-147 °C; 'H NMR (DMSO-d6) b 2.08 (s, 3H}, 3.07-3.12 (m, 1H}, 3.28-
3.50 (m,
SH), 3.51-3.60 (m, 2H), 3.64-3.75 (m, 3H), 4.10-4.12 (m, 1H), 4.30 (d, J = 7.9
Hz,
CA 02350066 2001-05-09
WO 00/31096 PCTNS99/27828
- 50 -
1H), 4.67 (t, 5.9 Hz, 1H), 4.68 (ABq, J = 12.5 Hz, es = 0.22, 2H), 5.14 (d, J
= 4.0 Hz,
1H), 5.25 (d, J = 5.1 Hz, 1H), 5.30 (d, J = 5.3 Hz, 1H), 5.51 (d, J = 3.3 Hz,
1H), 5.57
(s, IH), 5.63 ( d, J = 6.8 Hz, 1H), 7.22 (dd, J = I.S, 8.3 Hz, 1H), 7.35-7.38
(m, 3H),
7.42-7.46 (m, 3H), 7.66 {s, 1H), 9.53 (s, 1H); IR (KBr) 3500, 3410, 2910,
2850,
1700, 1600, 1550, 1440, 1425, 1375, 1310, 1230, 1150, 1070, and 1030 cm-';
mass
spectrum [(+) FAB], m/z 634 (M + Na)'; Anal. Calcd. for CZ8H34CINO,2 ~ 1.0
H20:
C, 53.38; H, 5.76; N, 2.22, Found: C, 53.58; H, 5.62; N, 2.25.
Example 25
N ( 5-f (4'.6'-O-Benzvlidene-2 2' 3 3' 6-penta-O-aced-p-D-maltosyl-oxy)
methyll 2
chloro-phenvl ~-acetamide
To a stirred solution of N-{5-[(4',6'-O-benzylidene-~3-D-maltosyloxy)-
methyl]-2-chloro-phenyl }-acetamide (0.230 g, 0.376 mmol) and triethylamine
(0.576
mL, 4.I4 mmol) in DMF (5 mL) at rt was added dropwise acetic anhydride (O.I95
I5 mL, 2.07 mmol) followed by a catalytic amount of DMAP (0.023 g, 0.188
mmol).
After 18 h, the mixture was concentrated, and the resulting residue was
diluted with
EtOAc (100 mL). This layer was washed with 1 N HCl (10 mL), sat. aq. NaHC03
(10
mL), and brine ( 10 mL) and then dried (NazSO,). After concentration, the
residue was
purified by flash chromatography (10:90 to 80:20 EtOAc:petroleum ether
gradient) to
afford the product (0.181 g, 59%) as an off-white solid, mp 99-102 °C;
'H NMR
(DMSO-d6) s 1.92 (s, 3H), 1.94 (s, 3H), 1.97 (s, 3H), 1.98 (s, 3H), 1.99 (s,
3H), 2.08
(s, 3H), 3.67-3.75 (m, 1H), 3.89 (t, J = 9.4 Hz, 1H), 3.95-4.04 (m, 3H), 4.12-
4.19 (m,
2H), 4.39 (dd, J = 1.5, 11.9 Hz, 1 H), 4.54 (d, J = 12.7 Hz, 1 H), 4.68-4.74
(m, 2H),
4.85-4.89 (m, 2H), 5.24 {t, J = 10.1 Hz, 1H), 5.28 (d, J = 4.0 Hz, 1H), 5.32
(d, J = 9.4
Hz, IH), 5.62 (s, IH), 7.07 (dd, J = 1.8, 8.1 Hz, 1H), 7.36 (s, 5H), 7.45 (d,
J = 8.3
Hz, 1 H), 7.62 (s, 1 H), 9.50 (s, 1 H); IR (KBr) 3390, 2920, 2850, 1755, 1690,
1600,
1530, 1410, 1375, 1230, and 1050 cm-'; mass spectrum [(+) FAB], m/z 822 (M +
H)',
844 (M + Na)+; Anal. Calcd. for C,$H,4CINO" ~ 1.0 Hz,O: C, 54.32; H, 5.52; N,
1.67,
Found: C, 54.68; H. 5.44: N, 1.57.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-51
Example 26
N-( 5-f l6-O-Benzovl-4' 6'-O-benzylidene-D-D-maltosyl-~xy)-methyll 2 chloro
phenyl 1-acetamide
The title compound was prepared as a white solid (4.04 g, 69%) from N-{5
[(4',6'-O-benzylidene-R-~-maltosyloxy)-methyl]-2-chloro-phenyl}-acetamide
using a
procedure similar to Example 2, mp 185-187 °C;'H NMR (DMSO-d6) s 2.05
(s, 3H),
3.16-3.22 (m, 1 H), 3.32-3.42 (m, 2H), 3.48-3.64 (m, 4H), 3.71 (dd, J = 4.8,
9.7 Hz,
1H), 3.74-3.79 (m, 1H), 4.05 (dd, J = 4.8, 10.3 Hz, 1H), 4.35 (dd, J = 5.3,
12.3 Hz,
1H), 4.39 (d, J = 7.7 Hz, 1H), 4.58-4.63 (m, 1H), 4.65 (ABq, J = 12.5 Hz, es =
0.14,
2H), 5.14 (d, 4.0 Hz, 1H), 5.34 (t, J = 5.1 Hz, 2H), 5.52 (s, 1H), 5.57 (d, J
= 3.1 Hz,
1H), 5.79 (d, J = 6.2 Hz, 1H), 7.19 (dd, J = 2.0, 8.3 Hz, 1H), 7.32-7.38 (m,
3H), 7.38-
7.45 (m, 3H), 7.51-7.55 (m, 2H), 7.63-7.68 (m, 2H), 7.98-8.01 (m, 2H), 9.49
(s, 1H);
IR (KBr) 3380, 3290, 2890, 2870, 1730, 1670, 1600, 1540, 1440, 1420, 1375,
1275,
1070, 1050, 1025, 975, and 710 cm-'; mass spectrum [(+) FAB], m/z 716/718 (M +
H)', 738/740 (M + Na)'; Anal. Calcd. for C35H38C1NO,3: C, 58.70; H, 5.35; N,
1.96,
Found: C, 58.53; H, 5.36; N, 1.94.
Example 27
~R)-N f 2-Chloro-5-f f f 2 3-di-O-acetyl-6-O-benzoyl-4-O-f 2 3-di O acetyl 4 6
O
(nhenvlmethvlene)-a-D-~lucopvranoysll-li-D- lucopyranosylloxyl methvll
phenyllacetamide
The title compound was prepared as a glassy white solid (0.048 g, 86%) from
N-{ 5-[(6-O-benzoyl-4',6'-O-benzylidene-p-D-maltosyl-oxy)-methyl]-2-chloro-
phenyl}-acetamide using a procedure similar to Example 25, mp 101-104
°C; 'H
NMR (DMSO-db) s 1.94 (s, 3H), 1.95 (s, 3H), 1.98 (s, 3H), 1.99 (s, 3H), 2.05
(s, 3H),
3.61 (t, J = 9.7 Hz, 1H), 3.70-3.76 (m, 1H), 3.80 (dd, :f = 4.6 Hz, 1H), 3.86
(t, J = 9.4
Hz, 1H), 4.13-4.22 (m, 2H), 4.46 (dd, J = 3.7, 12.5 Hz, 1H), 4.64 (ABq, J =
12.7 Hz,
es = 0.14, 2H), 4.68 (d, J = 10.5 Hz, 1H), 4.79 (dd, J = 8.1, 9.4 Hz, 1H),
4.87-4.91
(m, 2H), 5.27 (t, J = 9.9 Hz, 1H), 5.33-5.38 (m, 2H), 5.54 (s, 1H), 7.04 (dd,
J= 1.8,
8.1 Hz, 1H), 7.28-7.36 (m, SH), 7.41 (d, J = 8.3 Hz, 1H), 7.52-7.57 (m, 2H),
7.61 (s,
1H), 7.65-7.71 (m, 1H), 8.03-8.07 (m, 2H), 9.48 (s, 1H); IR (KBr) 3400, 2950,
2850,
1755, 1600, 1540. 1440, 1420, 1375; 1240, 1070, and 1030 cm-'; mass spectrum
[(+)
CA 02350066 2001-05-09
WO 00/31096 PCTNS99/27828
-52-
FAB], m/z 884 (M + H)', 906 (M + Na)'; Anal. Calcd. for C,3H,6C1N~": C, 58.41;
H, 5.24; N, 1.58, Found: C, 58.24; H, 5.31; N, 1.59.
Example 28
(R)-N-12-Chloro-5-fl(6-O-(5-methoxv-1 5-dioxo~entyl)-4-O-f4 6-O-
~henvlmethvlene)-«-D-glucopvranoysll-f~-D- lg ucopyranosvlloxvlmethvll-
phenyllacetamide
The title compound was prepared as a white foam (0.149 g, 50%) from N-{5-
[(4',6'-O-benzylidene-p-D-maltosyloxy)-methyl]-2-chloro-phenyl}-acetamide
using
methyl-4-(chloroformyl)-butyrate as the acid chloride and a procedure similar
to
Example 2, mp 79-81 °C; 'H NMR (CDC13) s 1.90-1.98 (m, 2H), 2.13 (s,
3H), 2.35-
2.43 (m, 4H}, 3.41 (t, J = 9.4 Hz, 1H), 3.47-3.73 (m, 6H), 3.65 (s, 3H), 3.82-
3.89 (m,
2H), 3.93 (t, d = 9.4 Hz, 1H), 4.06-4.26 (bs, 1H), 4.20 (dd, J = 4.6, 12.3 Hz,
1H),
4.28 (q, J = 5.1, 10.5 Hz, 1H), 4.34-4.40 (m, 2H), 4.70 (ABq, J = 12.5 Hz, es
= 0.23,
2H), 4.76-4.94 (bs, 1H), 5.04 (d, J = 4.0 Hz, 1H), 5.20-5.36 (bs, 1H}, 5.47
(s, 1H),
7.01 (dd, J = 1.8, 8.6 Hz, 1H), 7.30 (d, J = 8.3 Hz, 1H), 7.33-7.35 (m, 3H),
7.46-7.48
(m, 2H), 7.64 (s, 1H), 8.32 (s, 1H); IR (KBr) 3400, 2930, 2880, 1735, 1600,
1540,
1450, 1420, 1375, 1310, 1250, 1200, 1160, 1070, and 1025 cm''; mass spectrum
[(+)
FAB], m/z 740 (M + H)', 762 (M + Na)'; Anal. Calcd. for C34H4,C1N0,5 ~ 1.0
H.,O:
C, 53.86; H, 5.85; N, 1.85, Found: C, 53.51; H, 5.80; N, 1.73.
Example 29
4-Chloro-3-nitro-benz,~rl-4' 6'-O-benyzlidene-a-D-maltoside
step 1
4-Chloro-3-nitro-benzyl-~-D-maltoside
The title compound was prepared as a yellow powder (1.U4 g, 97%) from 4-
chloro-3-nitro-benzyl- p-D-maltoside heptaacetate using a procedure similar to
step 4
of Example 1, mp 168-169 °C; 'H NMR (DMSO-d6) s 3.03-3.13 (m, 2H), 3.20-
3.38
(m, 4H), 3.41-3.49 (m, 3H), 3.55-3.64 (m, 2H), 3.68-3.75 (m, 1H), 4.00-5.50
(bs,
7H), 4.31 (d, J = 7.7 Hz, 1H), 4.79 (ABq, J = 13.6 Hz, es = 0.17, 2H), 5.00
(d, J = 3.7
Hz, 1H), 7.70-7.78 (m, 2H), 8.09 (d, J = 1.8 Hz, 1H); IR (KBr) 3380, 2900,
1720,
1625, 1550, 1365, 1140, 1080, and 1030 cm-'; mass spectrum [(+) FAB], m/z
533/535
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-53-
(M + Na)+; Anal. Calcd. for C,9H26C1NO~3 ~ 1.0 H20: C, 43.07; H, 5.33; N,
2.64,
Found: C, 43.11; H, 5.23; N, 2.58.
step 2
4-Chloro-3-nitro-benzyl-4',6'-O-benyzlidene-p-n-maltoside
The title compound was prepared as a yellow solid (0.869 g, 74%) from 4-
chloro-3-nitro-benzyl-p-D-maltoside using a procedure similar to Example 24,
mp
>122 °C (decomp.); 'H NMR (DMSO-d6) s 3.11 (dd, J = 4.8, 8.8 Hz, 1H},
3.30-3.42
(m, 4H), 3.46 (dd, J = 3.3, 9.0 Hz, 1H), 3.49-3.60 (m, 2H), 3.64-3.75 (m, 3H),
4.12
(dd, J = 2.9, 8.1 Hz, 1H), 4.33 (d, J = 7.7 Hz, IH), 4.65 (t, J = 5.7 Hz, 1H),
4.80
(ABq, J = 13.6 Hz, es = U.16, 2H), 5.I4 (d, J = 4.0 Hz, 1H), 5.29 (d, J = 5.1
Hz, 1H),
5.34 (d, J = 5.1 Hz, 1H), 5.53 (d, J = 3.1 Hz, 1H), 5.57 (s, 1H), 5.62 (d, J =
6.6 Hz,
1H}, 7.34-7.38 (m, 3H), 7.42-7.46 (m, 2H), 7.70-7.77 (m, 2H), 8.10 (d, J = 1.6
Hz,
1H); IR (KBr) 3390, 2920, 2870, 1625, 1610, 1590, 1550, 1440, 1420, 1360,
1200,
1140, 1070, and 1030 cm-'; mass spectrum [(+) FAB], m/z 600/602 (M + H)',
622/624 (M + Na)'; Anal. Calcd. for CZ6H3oC1NO,3 ~ 0.5 H20: C, 51.28; H, 5.13;
N,
2.30, Found: C, 51. I 3; H, 5.21; N, 2.30.
Example 30
4-Chloro-3-nitro-benzvl-6-O-benzovl-4' 6'-O-benyzlidene-(~-D-maltoside
The title compound was prepared as a off-white glass (0.155 g, 49%) from 4-
chloro-3-nitro-benzyl-4',6'-O-benyzlidene-p-~-maltoside using a procedure
similar to
Example 2, mp 111-114 °C;'H NMR (DMSO-d6) s 3.19-3.26 (m, 1H), 3.28-
3.43 (m,
2H), 3.51-3.65 (m, 4H), 3.68-3.75 (m, 1H), 3.77-3.81 (m, 1H), 4.04 (dd, J =
4.6, 9.9
Hz, 1H), 4.34 (dd, J = 5.1, 12.3 Hz, 1H), 4.45 (d, J = 7.9 Hz, 1H), 4.59 (d, J
= 12.3
Hz, 1H), 4.79 (ABq, J = I3.6 Hz, es = 0.10, 2H), 5.14 (d, J = 3.7 Hz, IH),
5.35 (d, J
= 5.3 Hz, 1H), 5.46 (d, J = 5.1 Hz, IH), 5.52 (s, 1H), 5.60 (d, J = 3.1 Hz,
IH), 5.80
(d, J = 6.2 Hz, IH), 7.33-7.37 (m, 3H), 7.39-7.43 (m, 2H), 7.50-7.55 (m, 2H),
7.63-
7.68 (m, IH), 7.68-7.73 (rn, 2H), 7.97 (dd, J = I.I, 8.1 Hz, 2H), 8.08 (s,
1H); IR
(KBr} 3400, 2910, 2860, 1725, 1610, 1540, 1440, 1360, 1325, 1275, 1070, and
1025
cm-'; mass spectrum [(+) FAB], m/z 704 (M + H)', 726 (M + Na)'; Anal. Calcd.
for
CA 02350066 2001-05-09
WO 00/31096 PCTNS99/27828
-54-
~33~34~1NOt~ ~ 1.0 HZO: C, 54.89; H, 5.03; N, 1.94, Found: C, 54.72; H, 4.56;
N,
1.91.
Example 31
(R)-(4-Chloro-3-nitroohenvl)methvl-2 3-di-O-acetyl-6-O-benzoyl-4-O-f 2 3 di O
acetyl-4,6-O-lnhenvlmethylene)-a-D-~lucopyranos I~ lucopyranoside
The title compound was prepared as a off-white solid (0.0773 g, 84%) from 4-
chloro-3-nitro-benzyl-6-O-benzoyl-4',6'-O-benyzlidene-p-D-maltoside using a
procedure similar to Example 25, mp 138-140 °C; 'H NMR (DMSO-d6) s 1.95
(s,
3H), 1.96 (s, 3H), 1.98 (s, 3H), 1.99 (s, 3H), 3.6I (t, J = 9.4 Hz, 1H}, 3.69-
3.77 (m,
1 H), 3.79 (dd, J = 4.6, 9.4 Hz, 1 H), 3. 86 (t, J = 9.4 Hz, 1 H), 4.13-4.25
(m, 2H), 4.44
(dd, J = 3.5, 12.3 Hz, 1 H), 4.66-4.69 (m, 1 H), 4.71 (d, J = 13.6 Hz, 1 H),
4.80-4.84
(m, 2H), 4.89 (dd, J = 4.2, 10.3 Hz, 1H), 4.96 (d, J = 7.9 Hz, 1H), 5.27 (t, J
= 9.9 Hz,
1H), 5.34-5.40 (m, 2H), 5.54 (s, 1H), 7.27-7.37 (m, 5H), 7.51-7.56 (m ZH),
7.57 (dd,
J = 2.0, 8.3 Hz, 1H), 7.65-7.70 (m, 1H), 7.72 (d, J = 8.3 Hz, 1H), 7.93 (d, J
= 2.0 Hz,
1H), 8.01-8.05 (m, 2H); IR (KBr) 3440, 2950, 2830, 1755, 1620, 1550, 1440,
1410,
1370, 1320, 1240, 1160, 1120, 1070, 1030, and 990 cm-'; mass spectrum [(+)
FAB],
m/z 872 (M + H)', 894 (M + Na)'; Anal. Calcd. for C"H,ZCINO,$ ~ 0.5 HZO: C,
55.88; H, 4.92; N, 1.59, Found: C, 55.90; H, 4.80; N, 1.56.
Example 32
Nicotinic acid 6-(4-chloro-3-nitro-benzyloxy)-3-(7 8-dihvdroxy-2=phen 1
hexahvdro
nyranof3.2-dlf 1.31dioxin-6-vloxy)-4 5-dihydroxv-tetrahydro-p, ran-2-ylmethyl
ester
The title compound was prepared as a white foam (0.211 g, 45%} from 4
chloro-3-nitro-benzyl-4',6'-O-benyzlidene-p-D-maltoside using nicotinoyl
chloride
hydrochloride and a procedure similar to Example 2 (except compound purified
directly with flash chromatography), mp >105 °C (decomp.); 'H NMR (DMSO-
d6) s
3.21-3.28 (m, 1H), 3.32-3.42 (rn, 2H), 3.51-3.60 (m, 3H}, 3.65 (t, J = 9.2 Hz,
1H),
3.67-3.74 (m, 1H), 3.78-3.83 (m, 1H), 4.01-4.06 (m, iH), 4.38 (dd, J = 5.1,
12.3 Hz,
1H), 4.45 (d, J = 7.9 Hz, 1H), 4.58-4.64 (m, 1H), 4.79 (ABq, J = 13.6 Hz, es =
0.09,
2H), 5.17 (d, J = 3.7 Hz, 1 H), 5.35 (d, J = 5.3 Hz, 1 H), 5.46 (d, J = 5.3
Hz, 1 H), 5.52
(s, 1H), 5.6I (d, J = 3.1 Hz, 1H), 5.82 (d, J = 6.2 Hz, 1H), 7.34-7.37 (m,
3H), 7.39-
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-55-
7.43 (m, 2H), 7.54-7.59 (m, 1H), 7.68-7.71 (m, 2H), 8.08 (d, J = 1.3 Hz, 1H),
8.31
(d, J = 7.9 Hz, 1H), 8.78-8.88 (bs, IH), 9.05-9.19 (bs, 1H); IR (KBr) 3400,
2900,
2870, 1725, 1600, 1540, 1440, 1410, 1360, 1285, 1070, 1030, 740, and 690 cm~';
mass spectrum [(+) FAB], m/z 705/707 (M + H)'; Anal. Calcd. for C3zH33CINZO,4
~ 1.0
H20: C, 53.15; H, 4.88; N, 3.87, Found: C, 53.33; H, 4.78; N, 3.72.
Example 33
LR)-(4-Chloro-3-nitronhenvl)methvl 4-f2 3-di-O-acetyl-4 6-O-(phenylmethylene)
a
D-eluconvranosvll-D-D-~lucopvranoside 2 3-diacetate 6-(3-pvridinecarboxlate)
The title compound was prepared as a white foam (0.123 g, 95%) from
nicotinic acid 6-(4-chloro-3-nitro-benzyloxy)-3-(7,8-dihydroxy-2-phenyl-
hexahydro-
pyrano[3,2-d][1,3]dioxin-6-yloxy)-4,5-dihydroxy-tetrahydro-pyran-2-ylmethyl
ester
using a procedure similar to Example 25, mp ,101 °C (decomp.); 'H NMR
(DMSO-
d6) s 1.95 (s, 3H), 1.96 (s, 3H), 1.98 (s, 3H), 1.99 (s> 3H), 3.64 (t, J = 9.4
Hz, 1H),
3.69-3.81 (m, 2H), 3.87 (t, J = 9.4 Hz, 1H), 4.17 (dd, J = 3.1, 5.7 Hz, 1H),
4.27 (t, J =
9.4 Hz, 1H), 4.47 (dd, J = 4.0, 12.5 Hz, 1H), 4.69-4.75 (m, 2H), 4.80-4.91 (m,
3H),
4.96 (d, J = 8.1 Hz, IH), 5.26 (t, J = 10.1 Hz, 1H), 5.33-5.39 (m, 2H), 5.55
(s, 1H),
7.29-7.36 (m, SH)> 7.55-7.59 (m, 2H), 7.72 (d, J = 8.3 Hz, 1H), 7.93 (d, J =
2.0 Hz,
1H), 8.36 (dt, J = 2.2, 7.9 Hz, 1H), 8.84 (dd, J = 1.8, 4.8 Hz, 1H), 9.16 (dd,
J = 0.9,
2.2 Hz, 1H); IR (KBr) 3440, 2930, 2860, 1755, 1600, 1540, 1420, 1375, 1280,
1240,
1140, 1070, 1060, 1030, and 995 cm~'; mass spectrum [(+) FAB], m/z 873/875 (M
+
H)', 895/897 (M + Na)'; Anal. Calcd. for CQ°HQ,C1N20,8 ~ 1.25 H20: C,
53.64; H,
4.89; N, 3.13, Found: C, 53.46; H, 4.51; N, 2.96.
Example 34
4-Methoxv-benzoic acid 6-(3-acetylamino-4-chloro-benzvloxv)-3-l7 8-dihvdroxv 2
phenvl-hexahvdro-nvranof3 2-dlf 1 3ldioxin-6-~v)-4 5-dihvdroxv-tetrahydro
pyran-2-ylmethyl ester
The title compound was prepared as a white foam (0.284 g, 47%) from N { 5-
[(4',6'-O-benzylidene-p-D-maltosyloxy)-methyl]-2-chloro-phenyl}-acetamide
using
p-anisoyl chloride and a procedure similar to Example 2, mp >I 17 °C
(decomp.); 'H
NMR (DMSO-d6) s 2.05 (s, 3H), 3.15-3.21 (m, IH), 3.35 (d, J = 9.4 Hz, 1H),
3.35-
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-56-
3.42 (m, 1 H), 3.48-3.61 (m, 4H), 3.67-3.76 (m, 2H), 3.82 {s, 3H}, 4.05 (dd, J
= 4.8,
9.9 Hz, 1H), 4.29 (dd, J = 5.5, 12.3 Hz, 1H), 4.38 (d, J = 7.9 Hz, 1H), 4.SS-
4.60 (m,
1 H), 4.65 (ABq, J = 12.5 Hz, es = 0.14, 2H), 5.13 (d, J = 3.7 Hz, 1 H), 5.34
(dd, J =
4.0, 5.3 Hz, 2H), 5.52 (s, 1H), 5.56 (d, J = 2.9 Hz, 1H), 5.77 (d, J = 6.2 Hz,
1H),
S 7.02-7.06 (m, 2H), 7.19 (dd, J = 2.0, 8.3 Hz, 1H), 7.33-7.37 (m, 3H), 7.39-
7.44 (m,
3H), 7.64 (s, 1H), 7.94 (dt, J = 2.9, 9.9 Hz, 2H), 9.50 (s, 1H); IR (KBr)
3410, 3000,
2910, 2880, 1720, 1610, 1580, 1530, 1515, 1450, 1420, 1375, 1255, / 160, 1070,
and
1025 cm-'; mass spectrum [(+) FAB], m/z 746/748 {M + H)', 768/770 (M + Na)+,
784/786 (M + K)'; Anal. Calcd. for C36H4oC1N0" ~ 2.0 H,O: C, 55.28; H, 5.67;
N,
1.79, Found: C, 55.38; H, 5.28; N, 1.72.
Example 35
4-Methoxv-benzoic acid 4,5-diacetoxy-6-(3-acetylamino-4-chloro-benzvloxv) 3 (7
8
diacetoxv-2-nhenvl-hexahvdro-nvranof3 2-dlfl 3ldioxin-6~ylox -tetrahvdro nvran
2-vlmeth ly ester
The title compound was prepared as a white foam (0.142 g, 81 %) from 4-
methoxy-benzoic acid 6-(3-acetylamino-4-chloro-benzyloxy)-3-(7,8-dihydroxy-2-
phenyl-hexahydro-pyrano[3,2-d] [ 1,3] dioxin-6-yloxy}-4,5-dihydroxy-tetrahydro-
pyran-2-ylmethyl ester using a procedure similar to Example 25, mp >110
°C
(decomp.); 'H NMR (DMSO-d6) s 1.94 (s, 3H), 1.95 (s, 3H), 1.98 (s, 3H), 1. 99
(s,
3H), 2.06 (s, 3H), 3.61 (t, J = 9.7 Hz, 1 H), 3.68-3.75 (m, 1 H), 3.80 (dd, J
= 4.6, 9.4
Hz, 1H), 3.82 (s, 3H), 3.82-3.89 (m, 1H), 4.11-4.16 (m, 1H), 4.17 (q, 9.7 Hz,
1H),
4.40 (dd, J = 3.3, 12.3 Hz, IH), 4.63 (ABq, J = 12.7 Hz, es = 0.15, 2H), 4.64
(d, J =
10.8 Hz, 1H), 4.77 (dd, J = 7.9, 9.4 Hz, 1H), 4.86-4.91 (m, 2H), 5.26 (t, J =
9.9 Hz,
1H), 5.32-5.38 (m, 2H), 5.54 (s, 1H), 7.06 (dt, J = 2.9, 9.7 Hz, 3H), 7.29-
7.37 (m,
SH), 7.42 (d, J = 8.1 Hz, 1H), 7.61 (s, 1H), 7.99 (dt, J = 2.9, 9.9 Hz, 2H),
9.49 (s,
1H); IR (KBr) 3400, 2950, 2840, 1755, 1700, 1600, 1580, 1535, 1515, 1450,
1420,
1375, 1240, 1165, 1110, 1070, 1055, and 1030 cm-'; mass spectrum [(+) FAB],
m/z
914/916 (M + H)', 936/938 (M + Na)'; Anal. Calcd. for C"HQ8C1N0,8 ~ 1.0 H~O:
C,
56.68; H, 5.41; N, 1.50, Found: C, 56.37; H, 5.08; N, 1.48.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-57-
Example 36
4-Chloro-benzoic acid 6-(3-acetvlamino-4-chloro-benz~~)-3-l7 8-dihydroxy-2-
phenyl-hexahvdro-pyranof3,2-d111.31dioxin-6-vloxy -4 5-dihydroxv-tetrahydro-
pyran-2- ly methyl ester
The title compound was prepared as a white foam (0.372 g, 61 %) from N { 5-
((4',6'-O-benzylidene-p-D-maltosyloxy)-methyl]-2-chloro-phenyl}-acetamide
using
4-chlorobenzoyl chloride and a procedure similar to Example 2, mp >113
°C
(decomp.); 'H NMR (DMSO-db) s 2.05 (s, 3H), 3.16-3.21 (m, 1H), 3.28-3.41 (m,
2H), 3.48-3.62 (m, 4H), 3.69 (dd, J = 5.1, 9.9 Hz, 1 H), 3.76 (ddd, J = 1.5,
4.6, 9.4
Hz, 1H), 4.04 (dd, J = 4.8, 9.9 Hz, 1H), 4.33-4.40 (m, 2H), 4.55-4.60 (m, 2H),
4.73
(d, J = 12.5 Hz, IH), 5.13 (d, J = 4.0 Hz, 1H), 5.35 (t, J = 5.3 Hz, 2H), 5.52
(s, IH),
5.58 (d, J = 2.9 Hz, 1H), 5.81 (d, J = 6.2 Hz, 1H), 7.19 (dd, J = 2.0, 8.3 Hz,
1H),
7.33-7.37 (m, 3H), 7.38-7.43 (m, 3H), 7.59 (dt, J = 2.4, 9.2 Hz, ZH), 7.64 (s,
1H),
7.99 (dt, J = 2.4, 9.0 Hz, 2H), 9.50 (s, 1H); IR (KBr) 3410, 2910, 2870, 1725,
1590,
1530, 1440, 1420, 1375, 1270, 1070, 1025, and 760 cm~'; mass spectrum [(+)
FAB],
m/z 750/752/754 (M + H)', 772 (M + Na)~, 788/790/792 (M + K)'; Anal. Calcd.
for
C,SH3,C12NO,3 ~ 1.5 H.,O: C, 54.06; H, 5.18; N, 1.80, Found: C, 53.76; H,
4.78; N,
1.77.
Example 37
4-Chloro-benzoic acid 4,5-diacetoxv-6-(3-acet~rlamina-4-chloro-benzylo~)-3-(7
8-
diacetoxy-2-phenyl-hexahvdro-pyranof3 2-dlfl 3ldioxin-6-yloxy)-tetrahydro
Dvran
2- l~thvl ester
The title compound was prepared as a white foam (0.225 g, 72%) from 4-
chloro-benzoic acid 6-(3-acetylamino-4-chloro-benzyloxy)-3-(7,8-dihydroxy-2-
phenyl-hexahydro-pyrano[3,2-d] [ I,3]dioxin-6-yloxy)-4,5-dihydroxy-tetrahydro-
pyran-2-ylmethyl ester using a procedure similar to Example 25, mp 114-115
°C; 'H
NMR (DMSO-d6) s 1.94 (s, 3H), 1.95 (s, 3H), 1.98 (s, 3H), 1.99 (s, 3H), 2.05
(s, 3H),
3.62 (t, J = 9.2 Hz, 1H), 3.68-3.74 (m, 1H), 3.79 (dd, J = 4.2, 9.2 Hz, 1H),
3.86 (t, J =
9.4 Hz, 1 H), 4.13-4.19 (m, 1 H), 4.18 (q, J = 9.4, 1 H), 4.47 (dd, J = 3.5,
12.3 Hz, 1 H),
4.63 (ABq, J = 12.7 Hz, os = 0.14, 2H), 4.66 (d, J = 10.8 Hz, 1H), 4.79 (dd, J
= 8.1,
9.2 Hz, 1H), 4.86-4.91 (m, 2H), 5.26 (t, J = 9.9 Hz, :lH), 5.32-5.38 (m, 2H),
5.54 (s,
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-58-
1H), 7.05 (dd, J = 1.8, 8.3 Hz, 1H), 7.27-7.31 (m, 2H), 7.32-7.36 (m, 3H),
7.42 (d, J
= 8.3 Hz, 1H), 7.58-7.63 (m, 3H), 8.02-8.06 (m, 2H), 9.49 (s, 1H); IR (KBr)
3410,
2950, 2860, 1755, 1690, 1600, 1530, 1450, 1420, 1375, 1260, 1140, 1070, 1055,
and
1030 cm-'; mass spectrum [(+) FAB], m/z 918/920/922 (M + H)', 940/942/944 (M +
S Na)'; Anal. Calcd. for C,3H45C12NO" ~ 1.0 H20: C, 55.13; H, 5.06; N, 1.50,
Found:
C, 54.77; H, 4.73; N, 1.45.
Example 38
(R)-N-12-Chloro-5-f f ~6-O -(4-chloro-3-nitrobenzoyl)-4-O-14 60-lphenvlmeth
lene)-
~.-D-~luconvranosvll-a-n-glucop ra~nos, l~oxvlmeth,~llphenvllacetamide
The title compound was prepared as a white solid (0.158 g, 52%) from N-{S-
[(4',6'-O-benzylidene-p-D-maltosyloxy)-methyl]-2-chloro-phenyl}-acetamide
using
4-chloro-3-nitrobenzoyl chloride and a procedure similar to Example 2; 'H NMR
(DMSO-db) s 2.13 (s, 3H), 3.41 (apparant t, J = 9.4 Hz, 1H), 3.55-3.69 (m,
6H), 3.77
(apparant t, J = 9.0 Hz, 1H), 3.87-3.97 (m, 3H), 4.29 (dd, J = 10.5, 4.8 Hz,
1H), 4.41
(d, J = 7.7 Hz, 1H), 4.47 (dd, J = 12.1, 5.3 Hz, 1H), 4.60 (d, J = 12.7 Hz,
2H), 4.72
(dd, J = 12.0, 2.0 Hz, 1 H), 4.84 (d, J = 12.5 Hz, 1 H), 5.08 (d, J = 3.7 Hz,
1 H), 5. I 4
(bs, 1H), 5.47 (s, 1H), 6.99 (dd, J = 8.3, 2.0 Hz, 1H), 7.29 (d, J = 8.3 Hz,
1H), 7.32-
7.44 (m, 3H), 7.45-7.47 (m, 2H), 7.60 (s, 1H), 7.64 (d, J = 8.6 Hz, 1H), 8.16
(dd, J =
8.3, 2.0 Hz, 1H), 8.34 (s, 1H), 8.51 (d, J = 2.0 Hz, 1H); IR (KBr) 3400, 2900,
1750,
1660, 1275 and 1075 cm-'; mass spectrum [(+) FAB], m/z 795/797/799 (M + H)',
817/819/821 (M + Na)'; Anal. Calcd. for C35H36C1zN2O,5 ~ 1.0 H20: C, 51.67; H,
4.71;
N, 3.44, Found: C, 51.87; H, 4.84; N, 3.60.
Example 39
N-(5-f(2.2',3.-Tri-O -Acetvl-6-O -(4-chloro-3-nitrobenzovl)-4' 6'-O-
(benzvlidene)-
~-D-maltosvl)-oxymethvll-2-chloro-phenyl)-acetamide
The title compound was prepared as a white solid from (R)-N-[2-chloro-5-
[[[6-O -(4-chloro-3-nitrobenzoyl)-4-D-[4,60-(phenylmethylene)-«-D-
glucopyranosyl]-p-D-glucopyranosyl]oxy]methyl]phenyl]acetamide using a
procedure
similar to Example 25; 'H NMR (CDCl3) s 2.04 (s, 3H), 2.05 (s, 3H), 2.06 (s,
3H),
2.22 (s, 3H), 3.11 (d. J = 3.7 Hz, 1H), 3.62 (m, 4H), 3.81-3.99 (m, 2H), 4.29
(dd, 3 =
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-59-
10.3, 4.8 Hz, 1H), 4.48-4.61 (m, 3H), 4.98 (dd, J = 10.3, 3.7 Hz, 1H), 5.35
(d, J = 4.0
Hz, 1H), 5.44 - 5.52 (m, 2H), 6.99 (dd, J = 8.1, 2.0 Hz, 1H), 7.31-7.42 (m,
6H), 7.58-
7.59 (m, 2H), 7.63 (d, J = 8.3 Hz, 1H}, 8.14 (dd, J = 8.3, 2.0 Hz, 1H), 8.31
(bs, 1H),
8.51 (d, J = 2.0 Hz, 1H); IR (KBr) 3400, 2900, 1750, 1660, 1275 and 1075 cm';
mass spectrum [(+) FAB), m/z 921/923/925 (M + H)', 943/945/947 (M + Na)';
Anal.
Calcd. for C4,H,~C12NZO~8: C, 53.43; H, 4.59; N, 3.04, Found: C, 52.88; H,
5.11; N,
2.59.
Example 40
(R)-N-f2-Chloro-5-ff f6- O -(4-cyanobenzovl)-4-O-f4 60-(phen l~ylene)-«-D-
Qlucopvranosyll-a-D- lg_ucopyranos l~x~lmethvllphenyllacetamide
The title compound was prepared as a white solid from N-{5-[(4',6'-O-
benzylidene-p-D-maltosyloxy)-methyl]-2-chloro-phenyl }-acetamide using 4-
cyanobenzoyl chloride and a procedure similar to Example 2, mp 143-145
°C; 'H
NMR (DMSO-db) s 2.04 (s, 3H), 3.17-3.22 (m, 2H), 3.28-3.41 (m, 3H), 3.48-3.80
(m, 5H), 4.03 (dd, J = 9.4, 5.1 Hz, 1H), 4.38-4.42 (m, 2H), 4.62 (d, J = 10.8
Hz, 1H},
4.65 (ABq, J = 12.5 Hz, es = 0.13, 2H), 5.14 (d, J = 4..80 Hz, 2H), 5.35
(apparant t, J
= 5.3 Hz, 2H), 5.52 (s, 1H), 5.59 (d, J = 2.9 Hz, 1H), 5.83 (d, J = 6.0 Hz,
1H), 7.19
(dd, J = 8.3, 2.0 Hz, 1H), 7.34-7.39 (m, 3H}, 7.39-7.42 (m, 2H), 7.63 (s, IH),
8.0 (d,
J = 8.8 Hz, 2H), 8.13 (d, J = 8.8 Hz, 2H), 9.49 (s, 1H); IR (KBr) 3400, 2900,
1725,
1660, 1275 and 1075 cm '; mass spectrum [(-) FAB], m/z 739/741 (M - H)~; Anal.
Calcd. for C36H3,C1N2O,3 ~ 0.5 H20: C, 57.64; H, 5.11; N, 3.73, Found: C,
57.47; H,
5.08; N, 3.57.
Example 41
(R)-N-f2-Chloro-5-fff6-O -(4-nitrobenzoyl)-4-O-f4 60-(phenylmethylene)-a,-D-
~lucop ry anosyll-a-D glucopvranosylloxylmethyl~~phenyllacetamide
The title compound was prepared as a white solid from N-{5-[(4',6'-O
benzylidene-p-D-maltosyloxy)-methyl]-2-chloro-phenyl}-acetamide using 4
nitrobenzoyl chloride and a procedure similar to Example 2; 'H NMR (CDC13) s
2.14
(s, 3H), 3.41 (apparant t, J = 9.2 Hz, 1H), 3.54-3.69 (m, 5H), 3.84-3.99 (m,
5H), 4.30
(dd, J = 10.I, 5.1 Hz, 1H), 4.41 (d, J = 7.7 Hz, 1H), 4.50 (dd, J = 7.5, 4.6
Hz, 2H),
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
- 60 -
4.72 (dd, J = 12.1, 1.3 Hz, 1H), 4.73 (ABq, J = 12.5 Hz, es = 0.21, 2H), 4.85
(d, J =
3.4 Hz, 2H), 5.47 (s, 1H), 6.97-7.0 (m, 1H), 7.29 (d, J = 2.9 Hz, 1H), 7.32-
7.36 (m,
3H), 7.44-7.52 (m, 2H), 7.60 (bs, 1 H), 8.20 (d, J = 9.0 Hz, 2H), 8.29 (d, J =
9.0 Hz,
2H), 8.36 (bs, 1 H); IR (KBr) 3400, 2900, 1725, 1660, 1275 and 1075 cm~'; mass
spectrum [(+) FAB], m/z 761/763 (M + H)', 783/785 (M + Na)'; Anal. Calcd. for
C,SHj,CIN2O,5 ~ 2.0 HzO: C, 52.74; H, 5.18; N, 3.51, Found: C, 52.92; H, 5.07;
N,
3.45.
Example 42
(R)-N-f2-Chloro-5-ff~6-O -(3-trifluoromethvlbenzoyl)-4-O-f4 60-(phenvl-
methylene)-«-~-~lucopvranosvll-a-D-glucopyranos)rlloxylmethyllphenvllacetamide
The title compound was prepared as a white solid from N-{5-[(4',6'-O-
benzylidene-p-D-maltosyloxy)-methyl]-2-chloro-phenyl }-acetamide using 3-
trifluoro-
methylbenzoyl chloride and a procedure similar to Example 2, mp 194 °C;
'H NMR
(CDC13) s 2.11 (s, 3H), 3.40 (apparant t, J = 9.4 Hz, 1H), 3.53-3.68 (m, 5H),
3.77
(apparant t, J = 8.8 Hz, 1H), 3.88-3.93 (m, 2H), 3.97 (apparant t, J = 9.4 Hz,
1H),
4.30 (dd, J = 10.3, 5.1 Hz, 1H), 4.40 (d, J = 7.7 Hz, 1H), 4.71 (ABq, J = 12.5
Hz, es
= 0.22, 2H), 4.73 (d, J = 11, Hz, 1H), 5.08 (d, J = 3.7 Hz, 2H), 5.46 (s, 1H),
6.95 (dd,
J = 8.34, 1.8 Hz, 1H), 7.26 (d, J = 8.3, Hz, 1H), 7.30-7.34 (m, 3H), 7.43-7.47
(m,
2H), 7.60 (bt, 7 Hz, 2H), 7.81 (bd, J = 7.7 Hz, 1H), 8.22 (bd, J = 8.0 Hz,
1H), 8.32
(bd, J = 9.2 Hz, 2H); IR (KBr) 3400, 2900, 1725, 1660, 1250 and 1075 cm-';
mass
spectrum [(+) FAB], m/z 784/786 (M + H);, 806/808 (M + Na)'; Anal. Calcd. for
C36H39Cllrr3013 ~ 1.0 H20: C, 53.91; H, 4.90; N, 1.75, Found: C, 54.19; H,
4.67; N,
1.75.
Example 43
N-( 5-f (4'.6'-O-Benzylidene-6-O-(2-iodo)-benzovl-p-D-maltosyl)-oxv-methvll-2-
chloro-phenyl)-acetamide
The title compound was prepared as a white solid from N-{5-[(4',6'-O-
benzylidene-p-D-maltosyloxy)-methyl]-2-chloro-phenyl }-acetamide using o-
iodobenzoyl chloride and a procedure similar to Example 2, mp 140-143
°C; 'H
NMR (DMSO-d6) s 2.05 (s, 3H), 3.15-3.17 (m, 1 H), 3.28-3.65 (m, 6H), 3.75-3.79
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-61-
(m, 3H), 4.14 (dd, J = 9.1 Hz, 1H), 4.35 (dd, J = 12.1, 5.7 Hz, 1H), 4.40 (d,
J = 7.9,
1H), 4.62 (d, J = 10.8, Hz, 1H), 4.66 (ABq, J = 12.3 Hz, es = 0.14, 2H), 5.15
(d, J =
4.0 Hz, 1H), 5.36 (t, J = 5.3 Hz, 2H), 5.55 (s, 1H), 5.60 (d, J = 2.64 Hz,
1H), 5.87 (d,
J = 6.2 Hz, 1H), 7.18 (dd, J = 8.1, 2.00 Hz, 1H), 7.26-7.30 (m, 1H), 7.34-7.50
(m,
6H), 7.51-7.53 (m, IH), 7.63 (s, 1H), 7.78 (dd, J = 7.9, 1.5 Hz, 1H), 8.02
(dd, J = 7.9,
1.1 Hz, 1H), 9.50 (s, 1H); IR (KBr) 3400, 2930, 1750, 1550, 1245 and 1075 cm-
';
mass spectrum [(+) ESI], m/z 842/844 (M + H)', 859/861 (M + NH,)'; Anal.
Calcd.
for C35H3,C1IN0,3' 1.0 H~O: C, 48.84; H, 4.53; N, 1.66, Found: C,48.59.; H,
4.28; N,
1.58.
Example 44
N (5-f(4',6'-O-Benzylidene-6-O-(3-iodo)-benzoyl-a-D-maltosvl)-ox -y meth 1~~1-
2-
chloro~henvl 1-acetamide
The title compound was prepared as a white solid from N { 5-[(4',6'-0
benzylidene-R-D-maltosyloxy)-methyl]-2-chioro-phenyl }-acetamide using rrc-
iodo
benzoyl chloride and a procedure similar to Example 2, mp 175-177 °C;
'H NMR
(DMSO-d6) s 2.05 (s, 3H), 3.15-3.20 (m, 2H), 3.32-3.42 (m, 2H), 3.50-3.61 (m,
4H),
3.70-3.77 (m, 2H), 4.00-4.09 (m, 2H), 4.34 (dd, J = 12.1, 5.7 Hz, 1H), 4.40
(d, J =
7.9 Hz, 1H), 4.62 (d, J = 10.5, Hz, 1H), 4.66 (ABq, J = 12.3 Hz, es = 0.14,
2H), 5.14
(d, J = 4.0 Hz, 1 H), 5.35 (apparant t, J = 5.7 Hz, 2H), 5.53 (s, 1 H), 5.57
(d, J = 2.9
Hz, 1H), 5.79 (d, J = 6.4 Hz, 1H), 7.01 (dd, J = 8.3, 1.8 Hz, 1H), 7.32-7.37
(m, 3H),
7.40-7.42 (m, 2H), 7.65 (s, 1H), 7.99-8.03 (m, 2H), 8.26 (t> J = 1.8 Hz, 1H),
9.50 (s,
1H); IR (KBr) 3400, 2930, 1700, 1250 and 1075 cm~'; mass spectrum [(-) FAB],
m/z
840 (M - H)~; Anal. Calcd. for C35H3~C1N0,3~ 1.0 HZO: C, 48.88; H, 4.57; N,
1.63,
Found: C, 49.02; H, 4.49; N, 1.54.
Example 45
N f 5-f (4',6'-O-Benzvlidene-6-(4-iodo-benzovl)-oxv-~-p-maltosvl)-oxv-methvll-
2-
chloro-phenyl ~-acetamide
The title compound was prepared as a white solid (0.410 g, 60°/o)
from N-{5-
[(4',6'-O-benzylidene-p-D-maltosyloxy)-methyl]-2-chloro-phenyl}-acetamide
using
p-iodobenzoyl chloride and a procedure similar to Example 2, mp >187 °C
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-62-
(decomp.); 'H NMR (DMSO-d6) s 2.05 (s, 3H), 3.15-3.22 (m, 1H), 3.28-3.42 (m,
2H), 3.48-3.62 (m, 4H), 3.66-3.73 (m, 1H), 3.75 (ddd, J = 1.5, 4.8, 9.7 Hz,
IH), 4.01-
4.06 (m, IH), 4.35 (dd, J = 5.3, 12.3 Hz, 1H), 4.39 (d, J = 7.7 Hz, 1H), 4.57
(d, J =
10.3 Hz, 1H), 4.65 (ABq, J = 12.5 Hz, es = 0.14, 2H), 5.12 (d, J = 4.0 Hz,
1H), 5.35
(t, J = 5.3 Hz, 2H), 5.52 (s, 1H), 5.58 (d, J = 2.9 Hz, IH), 5.81 (d, J = 6.2
Hz, 1H),
7.19 (dd, J = 1.8, 8.1 Hz, 1H), 7.33-7.38 (m, 3H), 7.38-7.43 (m, 3H), 7.64 (s,
1H),
7.73 (dt, 3 = 2.0, 8.8 Hz, 2H), 7.91 (dt, J = 2.2, 8.8 Hz, 2H), 9.50 (s, 1H);
IR (KBr)
3420, 3270, 2920. 2880, 1725, 1660, 1590, 1530, 1450, 1425, 1385, 1375, 1280,
1140, 1110, 1070, 1050, 1030, 1005, and 750 cm-'; mass spectrum [(+) FAB], m/z
864/866 (M + Na)'; Anal. Calcd. for C35H3,ClIN0,3: C, 49.93; H, 4.43; N, 1.66,
Found: C, 49.65; H, 4.51; N, 1.77.
Example 46
~R)-N f-2-Chloro-5-f f f 6-O-(phenylacetyl)-4-O-f4,6-O-lphenvlmethylene)-a-D-
lg ucopvranosvll-D-D-glucopyranosvlloxvlmethvllphenvllacetamide
The title compound was prepared as a white glass (0.249 g, 42%) from N { 5-
[(4',6'-O-benzylidene-p-D-maltosyloxy)-methyl]-2-chloro-phenyl}-acetamide
using
phenylacetyl chloride and a procedure similar to Example 2, mp >98 °C
(decomp.);
'H NMR (DMSO-d6) s 2.08 (s, 3H), 3.08-3.13 {m, 1H), 3.27-3:49 (m, 4H), 3.54
(dd,
J = 5.3, 9.2 Hz, 1H), 3.56-3.63 (m, 2H), 3.66 (dd, J = 4.4, 9.4 Hz, 1H), 3.71
(s, 2H),
4.06 (dd, J = 4.4, 9.4 Hz, 1H), 4.12 (dd, J = 5.7, 12.3 Hz, 1H), 4.32 (d, J =
7.7 Hz,
1H), 4.37 (d, J = 11.0 Hz, 1H), 4.58 (ABq, J = 12.5 Hz, es = 0.16, 2H), 5.02
(d, J =
3.7 Hz, 1H), 5.32 (d, J = 5.3 Hz, 1H), 5.35 (d, J = 5.3 Hz, 1H), 5.55 (s, 1H),
5.57 (d,
J = 2.9 Hz, IH), 5.83 (6.2 Hz, 1H), 7.18-7.31 (m, 6H), 7.34-7.36 (rn, 3H),
7.41-7.46
(m, 3H), 7.65 (s, 1H), 9.53 (s, 1H); IR (KBr) 3390, 2910, 2880, 1740, 1670,
1590,
1535, 1460, 1420, 1375, 1310, 1250, 1140, 1070, and 1025 cm~'; mass spectrum
[(+)
FAB], m/z 730/732 (M + H)', 752/754 (M + Na)'; 768/770 (M + K)'; Anal. Calcd.
for C36H40C1NO~3 ~ 2.0 H,,O: C, 56.43; H, 5.79; N, 1.83, Found: C, 56.62; H,
5.35; N,
1.79.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-63-
Example 47
(R)-N f2-Chloro-5-fff2.3-di-O-acetyl-4-O-f2.3-di-O-acetyl-4 6-O-(phenvl-
methvlene)-a-D-~luconvranosyll-6-O-(phen l~yl)-f3-D- luco~vranosvll-
oxylmethvllphenyllacetamide
The title compound was prepared as a white foam (0.125 g, 73%) from (R)-N-
[-2-chloro-5-[[[6-O-(phenylacetyl)-4-O-[4,6-O-(phenylmethylene)-«-D-
glucopyranosyl]-p-D-glucopyranosyl]oxy]methyl]phenyl]acetamide using a
procedure
similar to Example 25, mp >98 °C (decomp.); 'H NMR (DMSO-db) s 1.93 (s,
3H),
1.94 (s, 3H), 1.97 (s, 3H), 1.99 (s, 3H), 2.08 (s, 3H), 3.69-3.78 (m, 4H),
3.86-3.94
(m, 2H), 4.01 (ddd, J = 2.6, 4.4, 9.7 Hz, 1H), 4.10 (d, J = 5.3 Hz, 1H), 4.20
(dd, J =
5.1, 12.3 Hz, 1H), 4.44-4.50 (m, 2H), 4.63-4.70 (m, 2H), 4.83 (d, J = 8.1 Hz,
1H),
4.88 (dd, J = 4.2, 10.3 Hz, 1H), 5.21-5.27 (m, 2H), 5.30 (t, J = 9.2 Hz, 1H),
5.61 (s,
1H), 7.05 (dd, J = 1.8, 8.1 Hz, 1H), 7.19-7.30 (m, 5H), 7.35 (s, 5H), 7.45 (d,
J = 8.3
Hz, 1H), 7.62 (s, 1H), 9.51 (s, 1H); IR (KBr) 3400, 3030, 2940, 2840, 1755,
1690,
1600, 1530, 1445, 1420, 1375, 1240, 1140, 1060, and 1030 cm'; mass spectrum
[(+)
FAB], m/z 898/900 (M + H)', 920/922 (M + Na)'; Anal. Calcd. for C"H,xClNO"
1.75 H~O: C, 56.84; H, 5.58; N, 1.51, Found: C, 56.44; H, 5.11; N, 1.59.
Example 48
N-(5-f(4'.6'-O-Benzylidene-6-O-phenyl-ethyl-carboxyl-t3-D-maltosyl)-ox -y
meth~l-
2-chloro=phenyl 1-acetamide
The title compound was prepared as a white solid (0.352 g, 63%) from N {5-
[(4',6'-O-benzylidene-p-n-maltosyloxy)-methyl]-2-chloro-phenyl }-acetamide
using
hydrocinnamoyl chloride and a procedure similar to Example 2, mp 192-193; 'H
NMR (DMSO-d6) s 2.06 (s, 3H), 2.66 (t, J = 7.7 Hz, 2H), 2.84 (t, J = 7.7 Hz,
2H),
3.08-3.16 (m, 1H), 3.33-3.49 (m, 4H), 3.53-3.59 (m, 2H), 3.61-3.72 (m, 2H),
4.05-
4.12 (m, 2H). 4.32-4.37 (m, 2H), 4.62 (ABq, J = 12.3 Hz, e8 = 0.16, 2H), 5.09
(d, 3 =
4.0 Hz, 1H), 5.35 (d, J = 5.1 Hz, 1H), 5.33 (d, J = 5.3 Hz, 1H), 5.54-5.58 (m,
1H),
5.56 (s, 1H), 5.82 (d, J = 6.2 Hz, 1H), 7.12-7.18 (m, 1H}, 7.18-7.26 (m, 5H),
7.34
7.40 (m, 3H), 7.42-7.46 (m, 3H), 7.65 (s, 1H), 9.51 (s, 1H); IR (KBr) 3560,
3390,
3260, 3080, 2900, 2880, 1745, 1660, 1590, 1540, 1450, 1425, 1370, 1320, 1280,
1200, 1180, 1140, 1070, 1050, 1025, 970, 755, and 695 cm''; mass spectrum [(+)
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-64-
FAB], m/z 744 (M + H)', 766 (M + Na)'; Anal. Calcd. for C3,H,=C1NO,3: C,
59.72;
H, 5.69; N, 1.88, Found: C, 59.75; H, 5.75; N, 2.03.
Example 49
N ( 5-f (4',6'-O-Benzvlidene-6-O-phenyl-propyl-carboxvl-a-D-maltosyl)-oxv-
methyll-2-chloro-phenyl )-acetamide
The title compound was prepared as a white solid (0.210 g, 68%) from N-{ 5-
[(4',6'-O-benzylidene-R-D-maltosyloxy)-methyl]-2-chloro-phenyl }-acetamide
using
4-phenylbutyryl chloride (prepared from 4-phenylbutyric acid and oxalyl
chloride)
and a procedure similar to Example 2, mp 184-185 °C; 'H NMR (DMSO-d6) s
1.77-
1.86 (m, 2H), 2.07 (s, 3H), 2.34 {t, J = 7.2 Hz, 2H), 2.58 (t, J = 7.5 Hz,
2H), 3.08-
3.16 (m, 1H), 3.29-3.51 (m, 4H), 3.53-3.73 (m, 4H), 4.07-4.14 (m, 2H), 4.33-
4.38
(m, 2H), 4.62 (ABq, J = 12.3 Hz, os = 0.16, 2H), 5.09 (d, J = 4.0 Hz, 1H),
5.28-5.37
(bs, 2H), 5.56 (s, 2H), 5.82 (d, J = 5.3 Hz, 1H), 7.13-7.19 (m, 4H), 7.21-7.27
(m,
2H), 7.33-7.37 (m, 3H), 7.40-7.46 (m, 3H), 7.63 (s, 1H), 9.51 (s, 1H); IR
(KBr)
3560, 3390, 3260, 3080, 2930, 2900, 2880, 1745, 1665, 1590, 1540, 1450, 1420,
1370, 1320, 1275, 1175, 1140, 1070, 1050, 1025, and 690 cm-'; mass spectrum
[(+)
FAB], m/z 780/782 (M + Na)'; Anal. Calcd. for C38H44C1NO,3. C, 60.20; H, 5.85;
N,
1.85, Found: C, 60.13; H, 5.73; N, 1.99.
Example SO
Diphenvl-acetic acid 6-l3-acetylamino-4-chloro-benzyloxv)-3-(7 8-dihvdroxy-2-
~henvl-hexah~pvranof3.2-dlf 1.31dioxin-6-vloxy)-4 5-dihydroxy-tetrahydro-
pyran-2-vlmethvl ester
The title compound was prepared as a white foam (0.510 g, 77%) from N { 5-
[(4',6'-O-benzylidene-p-D-maltosyloxy)-methyl]-2-chloro-phenyl}-acetamide
using
diphenylacetyl chloride and a procedure similar to Example 2, mp >106
°C
(decomp.); 'H NMR (DMSO-d6) s 2.08 (s, 3H), 3.03-3.09 (m, 1H), 3.26-3.38 (m,
3H), 3.41-3.47 (m, 1H), 3.51-3.62 (m, 3H), 3.66-3.7:3 (m, 1H), 4.02-4.07 (m,
1H),
4.15 {q, J = 6.2 Hz, 1 H), 4.27 (d, J = 7.7 Hz, 1 H), 4.46 (ABq, J = 12.5 Hz.
os = 0.15,
2H), 4.50 {d, J = 10.8 Hz, 1 H), 4.90 (d, J = 4.0 Hz, 1 H), 5.27 (s, 1 H),
5.31 (d, J = 5.3
Hz, 1H), 5.35 (d, J = 5.3 Hz, 1H), 5.54-5.56 (m, 2H), 5.81 (d, J = 6.4 Hz,
1H), 7.14
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-65-
(dd, J = 1.8, 8.3 Hz, 1H), 7.19-7.25 (m, 2H), 7.25-7.34 (m, 8H), 7.34-7.38 (m,
3H),
7.40-7.45 (m, 3H), 7.61 (s, 1H), 9.52 (s, IH); IR (KBr) 3400, 3050, 2900,
2860,
1730, 1680, 1600, 1530, 1500, 1450, 1420, 1375, 1310, 1275, 1240, 1150, 1070,
1025, 750, and 690 cm-'; mass spectrum [(+} FAB], m/z 806/808 (M + H)+,
828/830
(M + Na}', 844/846 (M + K)'; Anal. Calcd. for C,ZH4,C1NO,3 ~ 1.0 H20: C,
61.20; H,
5.63; N, 1.70, Found: C, 61.17; H, 5.48; N, 1.59.
Example 51
Diphenvl-acetic acid 4.5-diacetoxy-6-(3-acetylamino-4-chloro-benzyloxy)-3-(7,8-
diacetoxy-2-phenyl-hexahydro-pvranol3,2-dlfl.3ldioxin-6~yloxv)-tetrahvdro-
pyran-
2- l~methvl ester
The title compound was prepared as a white foam (0.289 g, 76%) from
diphenyl-acetic acid 6-(3-acetylamino-4-chloro-henzyloxy)-3-(7,8-dihydroxy-2-
phenyl-hexahydro-pyrano [3,2-d] [ 1,3] dioxin-6-yloxy)-4,5-dihydroxy-
tetrahydro-
pyran-2-ylmethyl ester using a procedure similar to Example 25, mp >99
°C
(decornp.); 'H NMR (DMSO-db) s I.92 (s, 3H), 1.94 (s, 3H), 1.96 (s, 3H), 1.99
(s,
3H), 2.08 (s, 3H), 3.66 (t, J =9.9 Hz, 1 H), 3.72-3.78 (m, 1 H), 3.78 (t, J =
9.0 Hz, 1 H),
3.87 (t, J = 9.7 Hz, 1H), 3.98-4.05 (m, 2H), 4.18-4.24 (m, 1H), 4.44 (ABq, J =
12.7
Hz, es = 0.16, 2H), 4.61-4.67 (m, 2H), 4.80 (d, J = 7.9 Hz, IH), 4.88 (dd, J =
4.2,
10.3 Hz, 1H), 5.16 (d, J = 4.0 Hz, 1H), 5.22 (t, J = 9.9 Hz, 1H), 5.29 (t, J =
9.2 Hz,
1H), 5.31 (s, 1H), 5.60 (s, 1H), 6.99 (dd, J = 1.5, 8.1 Hz, 1H), 7.20-7.25 (m,
2H),
7.27-7.35 (m, 8H), 7.35 (s, SH), 7.43 (d, J = 8.1 Hz, 1H), 7.59 (s, 1H), 9.51
(s, 1H);
IR (KBr) 3410, 3070, 3025, 2930, 2860, 1755, 1690, 1600, 1525, 1450, 1410,
1375,
1240, 1140, 1055, and 1030 cm~'; mass spectrum [(+) FAB], m/z 974 (M + H)',
996
(M + Na)'; Anal. Calcd. for CsoH52C1N0" ~ 1.25 HZO: C, 60.24; H, 5.51; N,
1.40,
Found: C, 59.97: H. 5.10: N, 1.37.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-66-
Example 52
(3,4-Dimethoxv-phenyl)-acetic acid 6-l3-acetylamino-4-chloro-benzvloxv)-3-(7 8-
dihydroxv-2-nhenvl-hexahvdro-pyranof3.2-d1f1.31dioxin-6-vloxv)-4 5-dihydroxv-
tetrahydro-pvran-2- l~h ly ester
The title compound was prepared as a white foam (0.316 g, 49%} from N { 5-
[(4',6'-O-benzylidene-~-D-maltosyloxy)-methyl]-2-chloro-phenyl}-acetamide
using
3,4-dimethoxyphenylacetyl chloride and a procedure similar to Example 2, mp
>116
°C (decomp.); 'H NMR (DMSO-db) s 2.07 (s, 3H), 3.07-3.14 (m, 1H), 3.28-
3.50 (m,
6H), 3.52-3.65 (m, 4H}, 3.67 (s, 3H), 3.68 (s, 3H), 4.07 (dd, J = 4.4, 9.7 Hz,
1H),
4.11 (dd, J = 5.5, 12.3 Hz, 1H), 4.31-4.38 (m, 2H), 4.58 (ABq, J = 12.3 Hz, 08
=
0.16, 2H), 4.99 (d, J = 3.7 Hz, 1H}, 5.34 (dd, J = 5.3, 9.2 Hz, 2H), 5.55 (s,
1H), 5.57
(d, J = 2.9 Hz, 1H), 5.82 (d, J = 6.2 Hz, 1H), 6.76 (dd, J = 2.0, 8.3 Hz, 1H),
6.82 (d, J
= 8.3 Hz, 1H), 6.85 (d, J = 2.0 Hz, 1H), 7.18 (dd, J = 2.0, 8.3 Hz, 1H), 7.34-
7.37 (m,
3H), 7.41-7.46 (m, 3H), 7.64 (s, 1H), 9.53 (s, 1H); IR (KBr) 3410, 2920, 1735,
1675,
1600, 1520, 1450, 1420, 1375, 1265, 1230, 1140, 1070, and 1025 cm-'; mass
spectrum [(+) FAB], m/z 790/792 (M + H)'; Anal. Calcd. for C38H,~C1N0,5 ~ 0.5
H20:
C, 57.11; H, 5.68; N, 1.75, Found: C, 56.95; H, 5.55; N, 1.71.
Example 53
(3,4-Dimethox~phenvl)-acetic acid 4.5-diacetoxv-6-(3-acetylamino-4-chloro-
benzvloxy)-3-(7.8-diacetoxy-2-phenyl-hexahvdro-pvran~3,2-dlf 1.31dioxin-6-
yloxv)-
tetrahydro-pyran-2-ylmethyl ester
The title compound was prepared as a white foam (0.105 g, 89%) from (3,4-
dimethoxy-phenyl)-acetic acid 6-(3-acetylamino-4-chloro-benzyloxy)-3-(7,8-
dihydroxy-2-phenyl-hexahydro-pyrano(3,2-d][1,3]dioxin-6-yloxy)-4,5-dihydroxy-
tetrahydro-pyran-2-ylmethyl ester using a procedure similar to Example 25, mp
>98
°C (decomp.); 'H NMR (DMSO-d6) s 1.92 (s, 3H), 1.94 (s, 3H), 1.97 (s,
3H), 1.99 (s,
3H), 2.07 (s, 3H), 3.62-3.77 (m, 4H), 3.66 (s, 3H), 3.67 (s, 3H), 3.89 (t, J =
9.2 Hz,
2H), 3.99-4.03 (m, 1H), 4.10 (dd, J = 10.5, 16.0 Hz, 1H), 4.19 (dd, J = 4.4,
11.9 Hz,
1H), 4.44-4.50 (m, 2H), 4.64-4.70 (m, 2H), 4.84 (d, J = 8.1 Hz, 1H), 4.87 (dd,
J =
4.0, 10.1 Hz. 1H), 5.21 (d, J = 3.7 Hz, 1H), 5.25 (d, J = 9.9 Hz, 1H), 5.30
(t, J = 9.2
Hz, 1H), 5.62 (s, 1H), 6.77 (dd, J = 1.8, 8.1 Hz, 1H), 6.81 (d, J = 8.3 Hz,
1H), 6.86
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-67-
(d, J = 1.8 Hz, 1H), 7.04 (dd, J = 1.8, 8.3 Hz, 1H), 7.35 (s, 5H), 7.44 (d, J
= 8.1 Hz,
1H), 7.61 (s, 1H), 9.51 (s, 1H); IR (KBr) 3370, 2930, 2860, 1755, 1690, 1600,
1520,
1450, 1420, 1370, 1240, 1140, 1055, and 1030 cm-'; mass spectrum [(+) FAB],
m/z
958/960 (M + H)', 980/982 (M + Na)'; Anal. Calcd. for C46H52C1N0,9 ~ 1.75 HZO:
C,
55.81; H, 5.65; N, 1.41, Found: C, 55.60; H, 5.14; N, 1.38.
Example 54
Nicotinic acid 6-l3-acetylamino-4-chloro-benzYloxy)-3-(7.8-dihydroxv-2-phenyl-
hexahydro=pyranof3.2-dlfl,3ldioxin-6-yloxv)-4.5-dih d~ -t~hydro-p,r
vlmeth 1 e~ster_
The title compound was prepared as a white solid (0.278 g, 47%) from N-t5-
[(4',6'-O-benzylidene-p-D-maltosyioxy)-methyl]-2-chloro-phenyl}-acetamide
using
nicotinoyl chloride hydrochloride and a procedure similar to Example 2, mp
>133 °C
(decomp.); 'H NMR (DMSO-d6) s 2.04 (s, 3H), 3.16-3.23 (m, 1H), 3.36 (d, J =
9.4
Hz, 1H), 3.36-3.42 (m, 1H), 3.48-3.61 (m, 3H), 3.63 (t, J = 9.4 Hz, 1H), 3.68-
3.75
(m, 1H), 3.76-3.80 (m, 1H), 4.04 (dd, J = 4.6, 9.9 Hz, 1H), 4.36-4.41 (m, 2H),
4.64
(d, J = 10.5 Hz, 1H), 4.65 (ABq, J = i2.5 Hz, es = 0.14, 2H), 5.16 (d, J = 4.0
Hz,
1H), 5.35 (dd, J = 4.0, 5.1 Hz, 2H), 5.52 (s, 1H), 5.58 (d, J = 2.9 Hz, 1H),
5.82 (d, J =
6.2 Hz, 1H), 7.19 (dd, J = 1.8, 8.3 Hz, 1H), 7.34-7.38 (m, 3H), 7.39-7.43 (m,
3H),
7.57 (ddd, J = 0.7, 4.8, 7.9 Hz, 1H), 7.64 (s, 1H), 8.33 (dt, J = 2.0, 7.9 Hz,
1H), 8.82
(dd, J = 1.8, 4.8 Hz, 1H), 9.12 (d, J = 2.2 Hz, 1H), 9.49 (s, 1H); IR (KBr)
3410, 2910,
2870, 1730, 1625, 1600, 1530, 1455, 1425, 1380, 1291), 1130, 1110, 1070, 1025,
740,
and 690 cm'; mass spectrum [(+) FAB], m/z 717/719 (M + H)'; Anal. Calcd. for
C~,H"C1N20" ~ 1.0 H20: C, 55.55; H, 5.35; N, 3.81, Found: C, 55.55; H, 5.30;
N,
3.78.
Example 55
Nicotinic acid 4.5-diacetoxy-6-(3-acetvlamino-4-chloro-benzyloxy)-3-(7,8-
diacetox~
2-phenyl-hexahvdro-pvranof3.2-dlf 1.31dioxin-6-yloxy)-tetrahydro-pvran-2-
vlmeth~
a ter
The title compound was prepared as a white foam (0.157 g, 73%) from
nicotinic acid 6-(3-acetylamino-4-chloro-benzyloxy)-3-(7,8-dihydroxy-2-phenyl-
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-68-
hexahydro-pyrano[3,2-d] [ I,3]dioxin-6-yloxy)-4,5-dihydroxy-tetrahydro-pyran-2-
ylmethyl ester using a procedure similar to Example 25, mp >1I2 °C
(decomp.); 'H
NMR (DMSO-d6) s 1.94 (s, 3H), 1.95 (s, 3H), 1.98 (s, 3H), 1.99 (s, 3H), 2.05
(s, 3H),
3.64 (t, J = 9.4 Hz, 1 H), 3.69-3.76 (m, 1 H), 3.79 (dd, J = 4.2, 9.2 Hz, 1
H), 3.87 (t, J =
9.4 Hz, 1H), 4.13-4.19 (m, 1H), 4.26 (t, J = 9.4 Hz, 1H), 4.48 (dd, J = 4.0,
12.3 Hz,
1H), 4.64 (ABq, J = 12.7 Hz, es = 0.14, 2H), 4.71-4.76 (m, 1H), 4.82 (dd, J =
8.1, 9.2
Hz, 1H), 4.87-4.92 (m, 2H), 5.26 (t, J = 10.1 Hz, 1H), 5.32-5.38 (m, 2H), 5.55
(s,
IH), 7.04 (dd, J = 1.8, 8.1 Hz, 1H), 7.29-7.36 (m, 5H), 7.42 (d, J = 8.1 Hz,
1H), 7.58
(dd, J = 4.8, 8.1 Hz, 1H), 7.61 (s, 1H), 8.39 (dt, J = 2.0, 7.9 Hz, IH), 8.84
(dd, J =
1.5, 4.6 Hz, 1H), 9.17-9.19 (m, 1H), 9.49 (s, 1H); IR (KBr) 3410, 2940, 2860,
1755,
1690, 1600, 1530, 1450, 1420, 1380, 1290, 1240, 1140, 1055, 1030, and 995 cm-
';
mass spectrum [(+) FAB], m/z 885 (M + H)r, 907 (M + Na)'; Anal. Calcd. for
CQZH,SC1N20" ~ 1.0 H~O: C, 55.85; H, 5.24; N, 3.10, Found: C, 55.61; H, 4.89;
N,
2.99.
Example 56
(R)-N-f 5-f f f 6-O-(4-Benzoylbenzoyl)-4-O-f 4,6-O-(nhenvlmethylene)-a-D-
lucop r~ anosvll-Q-D- lg ucopyranosvlloxvlmethyll-2-chlorophenvllacetamide
The title compound was prepared as a white powder (0.347 g, 52%) from N-
{5-[(4',6'-O-benzylidene-p-D-maltosyloxy)-methyl]-2-chloro-phenyl}-acetamide
using p-benzoylbenzoyl chloride (prepared from p-benzoylbenzoic acid and
oxalyl
chloride) and a procedure similar to Example 2, mp ,117 °C (decomp.);
'H NMR
(DMSO-d6) 8 2.03 (s, 3H), 3.17-3.23 (m, 1H), 3.27-3.43 (m, 2H), 3.49-3.66 (m,
4H),
3.70-3.82 (m, 2H), 4.08 (4.8, 10.1 Hz, 1 H), 4.39-4.44 (m, 2H), 4.64 (d, J =
10.5 Hz,
1H), 4.66 (ABq, J = 12.3 Hz, es = 0.14, 2H), 5.16 (d, J = 4.0 Hz, 1H), 5.36
(t, J = 5.3
Hz, 2H), 5.53 (s, 1H), 5.60 (d, J = 2.9 Hz, 1H), 5.83 (d, J = 6.2 Hz, 1H),
7.20 (dd, J =
1.8, 8.1 Hz, 1H), 7.33-7.37 (m, 3H), 7.39-7.43 (m, 3H), 7.54-7.59 (m, 2H),
7.65 (s,
1H), 7.67-7.73 (m, IH), 7.73-7.77 (m, 2H), 7.82-7.86 (m, 2H), 8.13-8.17 (m,
2H),
9.49 (s, 1H); IR (KBr) 3410, 3080, 2910, 2850, 1725, 1660, 1600, 1530. 1450,
1420,
1400, 1365, 1270, 1140, 1070, and 1025 cm~'; mass spectrum [(-) ESI]; n~/z
818.1 (M
- H)-; Anal. Calcd. for C,~H,zCIN0,4 ~ 0.5 HBO: C, 60.83; H, 5.23; N, 1.69,
Found:
C, 60.71; H, 5.28; N, 1.61.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-69-
Example 57
N- ( 5-f (4',6'-O-Benzvlidene-a-D-maltosvl)-oxv-methvll-2-meth~_phenvl 1-
acetamide
step 1
5-[(Hepta-O-acetyl-~-D-maltosyl)-oxy-methyl]-2-methyl-1-nitrobenzene
The title compound was prepared as a colorless solid (8.02 g, 53%) from 4-
methyl-3-nitrobenzyl alcohol and acetobromomaltose using a procedure similar
to
step 1 of Example 1, mp 68-74 °C; 'H NMR (DMSU-d6) s 1.93 (s, 3 H),
1.94 (s, 3
H), 1.95 (s, 3 H), 1.96 (s, 3 H), 1.97 (s, 3 H), 2.012 (s, 3 H), 2.07 (s, 3
H), 3.93 - 4.01
(m, 4 H), 4.13 - 4.21 (m, 2 H), 4.37 (d, 2 H), 4.64 - 4.90 (m, 5 H), 4.97 (t,
1 H), 5.20
(dd, 1 H), 5.27 - 5.33 (m, 2 H), 7.48 (d, 1 H), 7.52 (d, 1H), 7.88 (s, I H).
IR (KBr)
2950, 1750, 1230 and 1050 cm', mass spectrum [(+FAB)], m/z 808 (M + H)'. Anal.
Calcd. for C"H,3NOZO: C, 51.98; H,5.52; N, 1.78. Found: C, 51.59; H, 5.45; N,
1.86.
step 2
5-(Hepta-O-acetyl-p-D-maltosyloxymethyl)-2-methylphenylamine
The title compound was prepared as a white foam (5.39 g, 79%) from 5-
(hepta-O-acetyl-p-D-maltosyloxymethyl)-2-methyl-1-nitrobenzene using a
procedure
similar to step 2 of Example 1; 'H NMR (DMSO-d6) s 1.93 (s, 3 H), 1.94 (s, 3
H),
1.95 (s, 3 H), 1.97 (s, 3 H), 1.98 (s, 3 H), 2.03 (s. 6 H), 2.10 (s, 3 H),
3.93 - 4.03 (m,
4 H), 4.14 - 4.23 (m, 2 H), 4.32 - 4.41 (m, 2 H), 4.58 (d, 1 H), 4.68 (t, 1
H), 4.76 -
4.88 (m, 4 H), 4.98 (t, 1 H), 5.22 (t, 1 H), 5.28 -5.31 (m, 2 H), 6.37 (d, 2
H), 6.49 (s,
1 H), 6.87 (d, 1 H).
step 3
N-[5-(Hepta-O-acetyl-p-~-maltosyloxymethyl)-2-methylphenyl]acetamide
The title compound was prepared as a white foam (6.60 g, 91%) from 5-
(hepta-O-acetyl-p-D-maltosyloxymethyl)-2-methylphenylamine using a procedure
similar to step 3 of Example 1; 'H NMR (DMSO-db) s 1.93 (s, 3 H), 1.94 (s, 3
H),
1.95 (s, 3 H), 1.979 (s, 3 H), 1.984 (s, 3 H), 2.03 (s, 3 H), 2.10 (s, 3 H),
2.18 (s, 3 H),
3.94 - 4.02 (m, 4 H), 4.14 - 4.24 (m, 2 H), 4.40 (d, 1 H), 4.48 (d, 1 H), 4.67
- 4.74 (m
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-70-
2 H), 4.81 - 4.89 (m 2 H), 4.98 (t, 1 H), 5.19 - 5.32 (m, 3 H), 6.98 (d, 1 H),
7.17 (d, 1
H), 7.33 (s, 1 H), 9.27 (s, 1 H).
step 4
N-[5-(p-D-Maltosyloxy-methyl)-2-methyl-phenyl]-acetamide
A solution containing N-[5-(hepta-O-acetyl-p-D-maltosyloxymethyl)-2-
methylphenyl]acetamide (6.60 g, 8.27 mmol) and 25 weight% NaOMe in MeOH
(0.893 g, 4.14 mmol) in MeOH (198 mL) was refluxed for 2.Sh. The reaction was
cooled to room temperature and concentrated to give 4.09 g (98%) of the
product as a
white foam. This material was used without any additional purification.
An analytical sample was obtained by reverse phase HPLC (C18, IS%
CH3CN/H20) to give a white solid, mp 115 °C; 'H NMR (DMSO-db) 8 2.03
(s, 3 H),
2.16 (s, 3 H), 3.04 - 3.09 (m, 2 H), 3.21 - 3.56 (m, 7 H), 3.57 - 3.62 (m, 2
H), 3.70 -
3.73 (m, 1 H), 4.26 (d, 1 H), 4.48 - 4.54 (m, 3 H}, 4.76 (d, 1 H), 4.86 - 4.89
(m, 2 H),
5.01 (d, 1 H), 5.17 (d, 1 H), 5.42 (d, 1 H), 5.49 (d, 1 H), 7.10 (d, IH), 7.15
(d, I H),
7.35 (s, 1 H), 9.28 (s, 1 H). IR (KBr) 3375, 2900, 1670 and 1025 cm ', mass
spectrum
[(+) FAB], m/z 504 (M + H)+, 526 (M + Na)+. Anal. Calcd. for CZZH33N012 ~ 0.5
H20:
C, 51.56; H, 6.67; N, 2.73. Found: C, 51.78; H, 6.81; N, 2.75.
step 5
N-{5-[(4',6'-O-Benzylidene-p-D-maltosyl)-oxy-methyl]-2-methyl-phenyl}-
acetamide
A solution containing N {5-[(~i-D-maltosyl)-oxy-methyl]-2-methyl-phenyl}-
acetamide (1.88 g, 3.83 mmol), benzaldehyde dimethyl acetal (0.807 mL, 5.36
mmol)
and p-toluenesulfonic acid monohydrate (72.7 mg, 0.383 mmol) was heated at 60
°C.
After 4h, additional benzaldehyde dimethyl acetal (0.403 mL, 2.68 mmol) and p-
toluenesulfonic acid monohydrate (36.4 mg, 0.192 mmol) was added and the
reaction
was heated at 60 °C for 16 h. To the reaction was added K=C03 and
heating was
continued for O.Sh. The hot solution was filtered and the filtrate
concentrated.
Purification by reverse phase HPLC (C18, 15% CH3CN:H~O) gave 1.26 g (56%) of
the title compound as a white solid, mp I90-197 °C; ''H NMR (DMSO-d6) s
2.04 (s,
3H), 2.16 (s, 3H}, 3.08 (t, 1H), 3.35=3.40 (m, 3H), 3.45 (t, 1H), 3.53-3.59
(m, 2H},
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-71 -
3.64-3.75 (m, 3H), 4.11 (dd, J = 5.1, 2.4 Hz, 1H}, 4.28 (d, 1H), 4.50 (d,IH),
4.67 (t,
IH), 4.77 (d, 1H), 5.13 (d, 1H), 5.21 (br. s, IH), 5.29 (br. s, IH), 5.49 (br.
s, 1H),
5.57 (s, IH), 5.61 (br. s, 1H), 7.10 (d, 1H), 7.16 (d, 1H), 7.34-7.38 (m, 4H),
7.42-
7.45 (s, 2H), 9.28 (s, IH); IR (KBr) 3400, 2900, 1650 and1075 cm-'; mass
spectrum
[(+) ESI], m/z 609 (M + NH,)', 614 (M + Na)'; Anal. Calcd. for C29H3,NO,z ~
0.5 H20:
C, 57.99; H, 6.30; N, 2.37, Found: C, 57.80; H, 6.39; N, 2.50. Found: C,
57.85; H,
6.33; N, 2.27.
Example 58
N Acetyl-I5-f(2.2'.3,3'.6-penta-O-acetyl-4',6'-O-benzylidene_(3-D-maltos~l)-
oxy_
methvll-2-methyl-phenyl 1 acetamide
At 0 °C, to a stirred solution containing N-{5-[(4',6'-O-
benzylidene-p-D-
maltosyl)-oxy-methyl]-2-methyl-phenyl }-acetamide (0.406 g, 0.686 mmol),
pyridine
(1.66 mL, 20.6 mmol) and 4-dimethylaminopyridine (0.768 g, 6.86 mmol) was
added
dropwise acetic anhydride (1.28 mL, 13.7 mmol). After 6h, with reaction
eventually
warmed to room temperature, the solution was diluted with diethyl ether ( 100
mL),
washed successively with H20 (2x), sat. aq. NaHC03 (2x), sat. aq. CuSO, (2x),
brine
(2x), dried (NazS04) and concentrated. Purification by flash chromatography
(3, 4 and
5%o MeOH:CHCl3 gradient) gave 0.194 g, (34%), of a white solid after
cryatallization
from CHZCl2:petroleum ether, mp 97 °C; 'H NMR (DMSO-d6) s 1.90 (s, 3H),
1.92 (s,
3H), 1.98 (s, 3H), 1.99 (s, 3H), 2.06 (s, 3H), 2.08 (s, 3H), 2.13 (s, 3H),
2.17 (s, 3H),
3.71-3.80 (m, 2H), 3.95-4.02 (m, 2H), 4.12-4.18 (m, 2H), 4.39 (dd, J = 9.9,
2.2 Hz,
IH), 4.56 (d, IH), 4.67-4.75 (m, 2H), 4.86-4.90 (m, 2H), 5.22-5,33 (m, 3H),
5.61 (s,
1H), 7.11 (s, 1H), 7.24 (d, 1H), 7.34 (d, 1H), 7.36 (s, 5H); IR (KBr) 3450,
2900,
1750 and 1240 cm~'; mass spectrum [(+) FAB], m/z 844 (M + H)', 866 (M + Na)';
Anal. Calcd. for C"H,9N0,8: C, 58.36; H, 5.85; N, 1.66, Found: C, 57.99: H,
5.76; N,
1.67.
CA 02350066 2001-05-09
WO 00/31096 PCTNS99/27828
-72-
Example 59
N (5-1f4'.6'-O-Benzvlidene-6-O-(4-toluenesulfonvl)-Q-D-maltos lv 1-o~-methyl}-
2-
methyl-phen~)-acetamide
At 0 °C, to a stirred solution of N-{5-[(4',6'-O-benzylidene-p-D-
maltosyl)-
oxy-methyl]-2-methyl-phenyl}-acetamide (0.711 g, 1.20 mmol) in pyridine (2.4
mL)
was added a solution of p-toluenesulfonyl chloride (0.275 g, 1.44 mmol) in
CHZCl2
(1.5 mL). After 2 h, additional p-toluenesulfonyl chloride (0.275 g, 1.44
mmol) in
CHZC12 (1.5 mL) was added and the solution was stirred at 0 °C for 2h.
The reaction
was quenched with ice cold H,O (50 mL) and extracted with EtOAc. The combined
organic extracts were washed successively with sat. aq. NaHC03 (2x), sat. aq.
CuS04
(2x), brine (2x), dried (NaZSO,) and concentrated. Purification by reverse
phase
HPLC (C18, 50% CH3CN:H20) gave 0.421 g, (47%) of a colorless solid, mp 115-121
°C; 'H NMR (DMSO-db) s 2.05 (s, 3H), 2.17 (s, 3H), 2.33 (s, 3H), 3.05
(t, 1H), 3.24-
3.44 (m, 4H), 3.52 (t, 1H), 3.58-3.62 (m, 3H), 3.95 (d, 1H), 4.13 (dd, 1H),
4.28 (d,
1H), 4.33 (d, 1H), 4.41 (d, 1H), 4.59 (d, 1H), 5.05 (d, 1H), 5.57 (s, 1H),
7.06 (d, 1H),
7.16 (d, 1H), 7.33-7.47 (m, 8H), 7.78 (d, 2H), 9.29 (s, 1H); IR (KBr) 3375,
2900,
1650, 1350, 1175 and 1075 cm-'; mass spectrum [(+) FAB], m/z 746 (M + H)', 768
(M + Na)'; Anal. Calcd. for C36H43N0'4S ~ H20: C, 56.61; H, 5.94; N, 1.83,
Found:
C, 56.61; H, 5.77; N, 1.80.
Example 60
N-~ 5-f (4'.6'-O-Benz~idene-6-O-phenyl-Q-D-maltosvl)-oxv-methyll-2-chloro-
phenyl)-acetamide
At ambient temperature, to a stirred solution of phenol (0.0784 g, 0.833
mmol) in DMF (10 mL) was added potassium-t-butoxide (0.0982 g, 0.833 mmol).
After 0.5 h, to the reaction was added a solution of N (S-{[2,3,2',3'-tetra-O-
acetyl-
4',6'-O-benzylidene-6-O-(4-toluenesulfonyl)-p-D-maltosyl]-oxy-methyl }-2-
chloro-
phenyl)-acetamide (0.389 g, 0.417 mmol) in DMF (4 mL) and the reaction was
heated at 65 °C for 3 h. The reaction was cooled to ambient
temperature, quenched
with H20 (40 mL), extracted with EtOAc, dried (NaZSO,) and concentrated. The
crude product was dissolved in MeOH ( 10 mL) and treated with 25 weight% NaOMe
in MeOH (45 mg) at 65 °C for 3 h. The reaction was cooled to ambient
temperature
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-73-
and concentrated. Purification by flash chromatography (5 and 10% MeOH:CHCl3
gradient) gave 0.149 g (39%) of title compound as a solid; 'H NMR (DMSO-d6)
s 2.05 (s, 3H), 3.13-3.19 (m, 1H), 3.27-3.40 (m, 2H), 3.44-3.66 (m, 5H), 3.70-
3.73
(m, 2H), 4.14 (dd, J = 10.8, 4.4 Hz, 1H), 4.23 (d, J = 10.9 Hz, 1H), 4.39 (d,
J = 7.7
Hz, 1H), 4.64 (ABq. J = 12.3 Hz, es = 0.08, 2H), 5.16 (d, J = 3.7 Hz, 1H),
5.28 (d, J
= 5.1 Hz, 1H), 5.33 (d, J = 5.3 Hz, 1H), 5.47 (s, 1H), 5.56 (d, J = 3.3 Hz,
1H), 5.67
(d, J = 6.4 Hz, 1H), 6.90-6.97 (m, 3H), 7.20 (dd, J = 8.2, 1.9 Hz, 1H), 7.25-
7.29 (m,
2H), 7.32-7.36 (m, 5H), 7.42 (d, J = 8.3 Hz, IH), 7.b5 (s, 1H), 9.50 (s, 1H);
IR (KBr)
3400, 2900, 1650 and 1070 cm-'; mass spectrum [(+) FAB], m/z 710 (M + Na)';
Anal.
Calcd. for C3,H3gNCIO,~: C, 59.34; H, 5.57; N, 2.03, Found: C, 58.96; H, 5.78;
N,
2.16.
Example 61
(R)-N-12-Chloro-5-fff4-O-f4'.6'-O-lphenylmethylene)-oc-D- lg_ucopvranosvll-a-D-
lg ucopvranosvlloxylmethyll phen 1~-3-pyridinecarboxamide
step 1
N-[2-Chloro-5-[[[2,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-a-n-
glucopyranoysl)-p-D-glucopyranosyl]oxy]methyl]phenyl]-3-pyridinecarboxamide
To a stirred solution of 2-chloro-5-(hepta-O-acetyl-p-D-maltosyl-oxymethyl)-
phenylamine (0.200 g. 0.258 mmol) and triethylamine (0.119 mL, 0.851 mmol) in
THF (3 mL) at 0 °C was added nicotinoyl chloride hydrochloride (0.0551
mg, 0.310
mmol). After 0.5 h at this temperature, it was warmed to rt and stirred an
additional
18 h. At this point, the solid was filtered off and washed with additional THF
( 10
mL). The filtrate was then concentrated and taken up in EtOAc (100 mL). This
organic solution was washed with H20 ( 10 mL) and brine ( 10 mL) and then
dried
(NazS04). After concentration, the residue was purified by preparatory plate
chromatography ( 10:90 MeOH:CHC13) to afford the product (0.183 g, 80%) as a
white foam, mp 83-86 °C; 'H NMR (CDCl3) 8 1.99 (s, 3H), 2.00 (s, 3H),
2.02 (s, 3H),
2.03 (s, 3H), 2.04 (s, 3H), 2.10 (s, 3H), 2.16 (s, 3H), 3.67-3.72 (m, 1H),
3.93-3.98
(m, 1H), 4.04 (dd, J = 2.2, 11.9 Hz, 2H), 4.25 (dt, J = 3.7, 12.5 Hz, 2H),
4.53 (dd, J =
2.9, 12.3 Hz, 1H), 4.60 (d, J = 7.7 Hz, 1H), 4.64 (d, J = 12.5 Hz, 1H), 4.83-
4.93 (m,
3H), 5.05 (t, J = 10.1 Hz, 1 H), 5.23 (t, J = 9.4 Hz, 1 H), 5.34 (dd, J = 9. 7,
10.5 Hz,
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27$28
-74-
1H), 5.41 (d, J = 4.2 Hz, 1H), 7.07 (dd, J = 2.0 Hz, 1H), 7.41 (d, J = 8.1 Hz,
1H},
7.48 (ddd, J = 0.9, 4.8, 7.9 Hz, 1H), 8.23 (ddd, J = 1.5, 2.2, 7.9 Hz, 1H),
8.43 (s, 1H),
8.48 (d, J = 2.0 Hz, 1H), 8.82 (dd, J = 1.5, 4.8 Hz, 1H), 9.15 (dd, J = 0.7,
2.2 Hz,
1H); IR (KBr) 3400, 2950, 1755, 1675, 1600, 1550, 1420, 1375, 1235, and 1050
cm~';
S mass spectrum [(+) FAB], m/z 881 (M + H)+, 903 (M + Na)+; Anal. Calcd. for
C39H,SC1NZO~9 ~ 2.0 H20: C, 51.07; H, 5.38; N, 3.05, Found: C, 50.80; H, 4.83;
N,
2.89.
step 2
N-[2-chloro-5-[[(4-O-a-v-glucopyranosyl-p-D-glucopyranosyl)oxy]methyl]
phenyl]-3-pyridinecarboxamide
The title compound was prepared as a white foam (1.97 g, 57%) from N [2-
chloro-5-[[[2,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-«-D-glucopyranoysl)-
p-D-
glucopyranosyl]oxy]methyl]phenyl]-3-pyridinecarboxamide using a procedure
similar to step 4 of Example 1, mp >106 °C (decomp.); 'H NMR (DMSO-d6)
s 3.02-
3.13 (m, 2H), 3.19-3.29 (m, 2H), 3.31-3.39 (m, 1H), 3.39-3.50 (m, 3H), 3.55-
3.63
(m, 2H), 3.70-3.76 (m, 1H), 4.09 (q, J = 5.3 Hz, 1H), 4.31 (d, J = 7.9 Hz,
1H), 4.49-
4.55 (m, 2H), 4.60 (d, J = 12.5 Hz, 1H}, 4.84-4.91 (m, 3H), 5.01 (d, J = 3.7
Hz, 1H),
5.26 (d, J = 5.1 Hz, 1H}, 5.43 (d, J = 6.4 Hz, 1H), 5.52 (d, J = 3.1 Hz, 1H),
7.35 (dd,
J = 2.0, 8.3 Hz, 1 H), 7.54 (d, J = 8.1 Hz, I H), 7.56-7.60 (m, 2H), 8.31 (dt,
J = 2.0,
7.9 Hz, 1H), 8.77 (dd, J = 1.5, 4.8 Hz, 1H), 9.12-9.14 (m, 1H), 10.34 (s, 1H);
IR
(KBr) 3390, 2910, 2320, 1660, 1590, 1525, 1475, 1450, 1420, 1360, 1310; 1190,
1140, 1080, and 1030 cm'; mass spectrum [(+) FAB], m/z 587 (M + H)', 609 (M +
Na)', Anal. Calcd. for CZSH3,C1NZO,2 ~ 1.5 HzO: C, 48.90; H, 5.58; N, 4.56,
Found:
C, 49.18; H, 5.52; N, 4.32.
step 3
(R)-N-[2-Chloro-5-[([4-D-(4',6'-O-(phenylmethylene)-a-D-glucopyranosyl]-~-D-
glucopyranosyl]oxy]methyl] phenyl]-3-pyridinecarboxamide
The title compound was prepared as a white solid (1.25 g, 57%) from N [2-
chloro-5-[[(4-0-o~.-D-glucopyranosyl-p-D-glucopyranosyl)oxy]methyl] phenyl]-3-
pyridinecarboxamide using a procedure similar to Example 24, mp 208-210
°C; 'H
CA 02350066 2001-05-09
WO 00/31096 PCTNS99/27828
-75-
NMR (DMSO-db) s 3.08-3.15 (m, 1H), 3.30-3.42 (m, 4H), 3.42-3.51 (m, 1H), 3.51-
3.60 (m, 2H), 3.64-3.76 (m, 3H), 4.12 (dd, J = 3.3, 8.6 Hz, 1H), 4.33 (d, J =
7.7 Hz,
1H), 4.68 (t, J = 5.7 Hz, 1H), 4.74 (ABq, J = 12.5 Hz, es = 0.22, 2H), 5.14
(d, J = 3.7
Hz, 1H), 5.30 (t, J = 4.4 Hz, 2H), 5.52 (d, J = 3.3 Hz, 1H), 5.57 (s, 1H),
5.63 (d, J =
6.6 Hz, 1H), 7.34-7.38 (m, 4H), 7.42-7.46 (m, 2H), 7.54 (d, J = 8.3 Hz, 1H),
7.55-
7.60 (m, 2H), 8.31 (dt, J = 1.8, 7.9 Hz, 1H), 8.77 (dd, J = 1.8, 4.8 Hz, 1H),
9.13 (dd, J
= 0.7, 2.2 Hz, 1H), 10.34 (s, 1H); IR (KBr) 3530, 3400, 2920, 2830, 1680,
1590,
1540, 1460, 1420, 1380, 1320, 1275, 1150, 1120, 1070, and 1025 cm-'; mass
spectrum [(+) FAB], m/z 675/677 (M + H)+, 697/699 (M + Na)'; Anal. Calcd. for
C32H35C1N20,z ~ 0.5 HzO: C, 56.18; H, 5.30; N, 4.09, Found: C, 56.31; H, 5.13;
N,
4.19.
Example 62
(R)-N f5-ffl6-O-Benzo~-4-O-f4',6'-O-(phenylmethylene)-a-D-glucopyranosyll-a-~-
luconvranosvlloxylmethvll-2-chlorophenyll-3-pvridinecarboxamide
The title compound was prepared as a off-white glassy solid (0.628 g, 44%)
from (R)-N-[2-chloro-5-[[[4-D-[4,6-O-(phenylmethylene)-a-D-glucopyranosyl]-p-D-
glucopyranosyl]oxy]methyl] phenyl]-3-pyridinecarboxamide using a procedure
similar to Example 2, mp 190-193 °C; 'H NMR (DMSO-d6) s 3.17-3.24 (m,
1H),
3.30-3.43 (m, 2H), 3.50-3.64 (m, 4H), 3.68-3.76 (m, 1H), 3.78 (ddd, J = 1.5,
5.1, 9.7
Hz, 1H), 4.50 (dd, J = 4.8, 9.9 Hz, IH), 4.36 (dd, J = 5.1, I2.1 Hz, 1H), 4.43
(d, J =
7.7 Hz, 1H), 4.59-4.64 (m, 1H), 4.71 (ABq, J = 12.7 Hz, es = 0.14, 2H), 5.14
(d, J =
3.7 Hz, 1H), 5.35 (d, J = 5.1 Hz, 1H), 5.39 (d, J = 5.3 Hz, 1H), 5.52 (s, 1H),
5.59 (d,
J = 3.1 Hz, 1H), 5.80 (d, J = 6.4 Hz, 1H), 7.31-7.37 (m, 4H), 7.39-7.43 (m,
2H), 7.51
(dd, J = 5.9, 7.9 Hz, 3H), 7.54-7.58 (m, 2H), 7.60-7.66 (m, 1H), 7.98 (dd, J =
l.l, 8.1
Hz, 2H), 8.29 (dt, J = 1.8, 7.9 Hz, 1H), 8.77 (dd, J = 1.8, 4.8 Hz, 1H), 9.11
(d, J = 2.0
Hz, 1H), 10.31 (s, 1H); IR (KBr) 3410, 3080, 2900, 2850, 1720, 1680, 1590,
1530,
1440, 1420, 1375, 1320, 1275, 1070, 1025, 755, and 710 cm~'; mass spectrum
[(+)
FAB], m/z 779/781 (M + H)', 801/803 (M + Na)'; Anal. Calcd. for C39H39C1NZO,3
'
0.5 HZO: C, 59.43; H, 5.12; N, 3.55, Found: C, 59.34; H, 4.91; N, 3.45.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-76-
Example 63
Furan-2-carboxylic acid (5-f(4'.6'-O-benzylidene-a-D-maltosyl)-oxy-methyll-2-
chloro-phenyl l-amide
step 1
Furan-2-carboxylic acid {5-[(2,2',3,3',4',6,6'-hepta-O-acetyl-p-D-maltosyl)-
oxymethyl]-2-chloro-phenyl}-amide
The title compound was prepared as a white foam (1.47 g, 94%) from 2-
chloro-5-(hepta-O-acetyl-p-D-maltosyl-oxymethyl)-phenylamine using a procedure
similar to step 3 of Example 1, mp >85 °C (decomp.); 'H NMR (DMSO-d6) s
1.93 (s,
6H), 1.94 (s, 3H), 1.969 (s, 3H), 1.972 (s, 3H), 2.01 (s, 3H), 2.07 (s, 3H),
3.92-4.02
(m, 4H), 4.12-4.23 (m, 2H), 4.38 (dd, J = 2.2, 12.1 Hz, 1H), 4.67 (ABq, J =
13.0 Hz,
es = 0.15, 2H), 4.73 (dd, J = 7.9, 9.4 Hz, 1H), 4.83-4.90 (m, 2H), 4.97 (t, J
= 9.7 Hz,
1H), 5.21 (t, J = 10.1 Hz, 1H), 5.27 (d, J = 4.2 Hz, 1H), 5.31 (d, J = 9.2 Hz,
1H), 6.70
(q, J = 1.8 Hz, 1H), 7.18 (dd, J = 2.0, 8.3 Hz, 1H), 7.32 (d, J = 3.5 Hz, 1H),
7.53 (d, J
= 8.1 Hz, 1H), 7.57 (d, J = 2.0 Hz, 1H), 7.93-7.95 (m, 1H), 9.84 (s, 1H); IR
(ICBr)
3390, 3130, 2950, 1755, 1690, 1590, 1530, 1445, 1420, 1375, 1320, 1230, 1140,
and
1040 cm '; mass spectrum [(+) FAB], m/z 870 (M + H)', 892 (M + Na)', Anal.
Calcd.
for C3gH,4C1NO2o' 1.0 H,O: C, 51.39; H, 5.22; N, 1.58, Found: C, 51.00; H,
4.93; N,
1.51.
step 2
Furan-2-carboxylic acid {2-chloro-5-[(~-n-maltosyl)-oxy-methyl]-phenyl}-amide
A solution containing furan-2-carboxylic acid {5-[(2,2',3,3',4',6,6'-hepta-O
acetyl-(3-D-maltosyl)-oxymethyl]-2-chloro-phenyl}-amide (1.36 g, 1.56 mmol)
and 25
weight % NaOMe in MeOH (26.8 ~L, 0.468 mmol) in MeOH (41 ml) was stirred at rt
for 18 h. At this point, the mixture was concentrated, and the resulting
residue was
triturated with Et~O to afford the product (0.890 g, 99%) as a white foam, mp
>127
°C (decomp.); 'H NMR (DMSO-db) s 3.01-3.12 (m, 2H), 3.21 (dd, J = 3.7,
9.7 Hz,
1H), 3.24-3.29 (m, 1H), 3.29-3.38 (m, 2H), 3.38-3.50 (m, 3H), 3.54-3.63 (m,
2H),
3.73 (d, J = 12.3 Hz, 1H), 4.30 (d, J = 7.7 Hz, 1H), 4.45-4.58 (m, 2H), 4.71
(ABq, J =
12.5 Hz, es = 0.22, 2H), 4.83-4.93 (bs, 2H), 5.01 (d, J = 4.0 Hz, 1H), 5.18-
5.32 (bs,
1H), 5.34-5.58 (bs, 2H), 6.69 (dd, J = 1.5, 3.3 Hz, 1H), 7.26-7.33 (m, 2H),
7.50 (d, J
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-77-
= 8.3 Hz, 1 H), 7.62 (d, J = 2.0 Hz, 1 H), 7.92-7.94 (m, 1 H), 9.82-9.94 (bs,
1 H); IR
{ICBr) 3400, 2920, 2880, 1675, 1590, 1530, 1445, 1425, 1365, 1315, 1140, 1080,
1030, and 755 cm''; mass spectrum [(+) FAB], m/z 598/600 (M + Na);, Anal.
Calcd.
for C~,H3oC1N0,3 ~ 0.5 H.=O: C, 49.28; H, 5.34; N, 2.39, Found: C, 49.06; H,
5.34; N,
2.21.
step 3
Furan-2-carboxylic acid {5-[(4',6'-O-benzylidene-~-D-maltosyl)-oxy-methyl]-2-
chloro-phenyl}-amide
The title compound was prepared as a white solid (0.352 g, 63°l0) from
furan-
2-carboxylic acid { 2-chloro-5-[(p-D-maltosyl)-oxy-methyl]-phenyl }-amide
using a
procedure similar to Example 24, mp 224-226 °C; 'H NMR (DMSO-db) s 3.07-
3.14
(m, 1H), 3.27-3.42 (m, 4H), 3.42-3.49 (m, 1H), 3.51-3.59 (m, 2H), 3.b3-3.76
(m,
3H), 4.11 (dd, J = 2.4, 7.9 Hz, 1H), 4.32 (d, J = 7.7 Hz, 1H), 4.67 (t, J =
5.9 Hz, 1H),
4.72 (ABq, J = 12.5 Hz, es = 0.22, 2H), 5.14 (d, J = 3.7 Hz, 1H), 5.29 (t, J =
6.2 Hz,
2H}, 5.51 (d, J = 3.3 Hz, 1 H), 5.57 (s, 1 H), 5.62 (d, J = 6.8 Hz, 1 H), 6.68-
6.72 (M,
1H), 7.3U-7.34 (m, 2H), 7.34-7.38 (m, 3H), 7.41-7.46 (m, 2H), 7.52 (d, J = 8.1
Hz,
1H), 7.62 (s, 1H), 7.94 (t, J = 0.9 Hz, 1H), 9.88 (s, 1H); IR (ICBr) 3390,
2920, 2850,
1680, 1600, 1590. 1530, 1455, 1430, 1385, 1320, 1280, 1140, 1070, 1050, 1025,
and
750 cm~'; mass spectrum [(-) FAB], m/z 662 (M - H)-; Anal. Calcd. for
C3,H3,C1NO,3'
1.0 H20: C, 54.59; H, 5.32; N, 2.05, Found: C, 54.82; H, 4.91; N, 2.03.
Example 64
Furan-2-carboxylic acid ( 5-f (6-O-benzoyl-4' .6'-O-benz~lidene-a-D-maltosvl)-
oxv-
methyll-2-chloro-~hen~l-amide
The title compound was prepared as a white solid (0.130 g, 47%) from furan-
2-carboxylic acid { 5-[(4',6'-O-benzylidene-~-D-maltosyl)-oxy-methyl]-2-chloro-
phenyl}-amide using a procedure similar to Example 2, mp >142 °C
(decomp.); 'H
NMR (DMSO-db) s 3.16-3.23 (m, 1H), 3.27-3.42 (m, 2H), 3.49-3.64 (m, 4H), 3.71
(dd, J = 5.1, 10.1 Hz, 1H), 3.75-3.80 (m, 1H), 4.05 (dd, J = 4.8, 9.9 Hz, 1H),
4.36
(dd, J = 5.3, 12.3 Hz, 1H), 4.42 (d, J = 7.9 Hz, 1H), 4.58-4.63 (m, 2H), 4.78
(d, J =
12.7 Hz, 1H), 5.14 (d, J = 3.7 Hz, 1H), 5.34 (d, J = 5.3 Hz, 1H), 5.38 (d, 3 =
5.3 Hz,
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
_78_
1H), 5.52 (s, 1H), 5.58 (d, J = 2.9 Hz, 1H), 5.79 (d, J = 6.2 Hz, IH), 6.69
(q, J = 1.8
Hz, 1H), 7.26-7.32 (m, 2H), 7.33-7.38 (m, 3H), 7.38-7.44 (m, 2H), 7.47 (d, J =
8.3
Hz, 1 H), 7.51 (t, J = 7.9 Hz, 2H), 7. 6 I (d, J = 2.0 Hz, 1 H), 7.61-7.66 (m,
1 H), 7.93
(dd, J = 0.7, 2.6 Hz, 1H), 7.99 (dd, J = 5.3, 7.0 Hz, 2H), 9.85 (s, 1H); IR
(KBr) 3460,
3380, 3140, 3080, 2880, 1730, 1660, 1590, 1535, 1445, 1425, 1375, 1320, 1275,
1140, 1120, 1075, 1025, 980, and 715 cm-'; mass spectrum [(+) ESI], m/z 768 (M
+
H)', 790 (M + Na)'; Anal. Calcd. for C38H38C1NO" ~ 1.0 HZO: C, 58.05; H, 5.13;
N,
1.78, Found: C, 57.96; H, 4.93; N, I.76.
Example 65
N-( 2-Chloro-5-f (4',6'-O-benzylidene-a-D-maltosyl)-oxv-methyh~phenvl 1-pent-4-
enamide
step 1
N-[2-Chloro-5-[[[2,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-a-D-
glucopyranosyl)-p-n-glucopyranosyl]oxy)methyl]phenyl]-4-pentenamide
To a stirred solution of 4-pentenoic acid (57.9 wL, 0.567 mmol) and DMF
(cat. amt.) in CHZC12 (3 mL) at rt was added oxalyl chloride (49.4 wL, 0.567
mmol)
dropwise. After 5 min. at this temperature, it was heated to 40 °C for
an additional 10
min. This completed the preparation of the acid chloride starting material. At
this
point, to a second stirred solution of NaH (0.0206 g, 0.515 mmol) and CHzCl2
(3 mL)
at rt was added 2-chloro-5-(hepta-O-acetyl-p-D-maltosyl-oxymethyl)-phenylamine
(0.400 mg, 0.515 mmol). After 10 min., the acid chloride solution was added to
this
solution dropwise. The reaction was stirred at rt for 1 h and then diluted
with EtOAc
( 100 mL). This layer was washed with 1 N HCl ( 10 mL), sat. NaHC03 ( 10 mL),
and
brine ( 10 mL) and then dried (MgSO,). After concentration, the oilly residue
was
purified by flash chromatography ( I0:90 to 70:30 EtOAc:petroleum ether
gradient) to
afford the product (0.321 g, 73%) as a white foam, mp >68 °C (decomp.);
'H NMR
(DMSO-d6) s 1.93 (s, 3H), 1.94 (s, 6H), 1.969 (s, 3H), 1.972 (s, 3H), 2.01 (s,
3H),
2.08 (s, 3H}, 2.29-2.36 (m, 2H), 2.44-2.49 (m, 2H), 3.92-4.02 (m, 4H), 4.13-
4.22 (m,
2H), 4.38 (d, J = 10.1 Hz, 1H), 4.53 (d, J = 12.7 Hz, 1H), 4.69-4.75 (m, 2H),
4.84 (d,
J = 3.7 Hz, 1H), 4.87 (d, J = 2.9 Hz, 1H), 4.94-5.01 (m, 2H), 5.07 (dd, J =
2.0, 17.4
Hz, 1H), 5.21 (t, J = 9.7 Hz, 1H), 5.27 (d, J = 3.7 Hz, 1H), 5.31 (d, J = 8.8
Hz, IH),
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-79-
5.80-5.91 (m, 1H), 7.08 (dd, J = 2.0, 8.3 Hz, 1H), 7.46 (d, J = 8.1 Hz, 1H),
7.61 (s,
1H), 9.49 (s, 1H); IR (KBr) 3400, 2950, 1755, 1690, 1630, 1590, 1525, 1420,
1370,
1235, and 1050 cm'; mass spectrum [(+) FAB], m/z 858/860 (M + H)', 880/882 (M
+
Na)', Anal. Calcd. for C38H48C1NO~9 - 0.5 HZO: C, 52.63; H, 5.69; N, 1.62,
Found:
S C, 52.65; H, 5.66; N, 1.59.
step 2
N {2-Chloro-5-[(p-D-maltosyl)-oxy-methyl]-phenyl}-pent-4-enylamide
The title compound was prepared as an off-white solid (0.0614 g, 93%) from
N [2-chloro-5-[[[2,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-«-D-
glucopyranosyl)-
p-D-glucopyranosyl]oxy]methyl]phenyl]-4-pentenamide and a procedure similar to
step 4 of Example 1, mp >103 °C (decomp.); 'H NMR (DMSO-d6) s 2.30-2.37
(m,
2H), 2.43-2.49 (m, 2H), 3.02-3.10 (m, 2H), 3.19-3.28 (m, 2H), 3.30-3.49 (m,
5H),
3.54-3.63 (m, 2H), 3.72 (d, J = 10.8 Hz, 1H), 4.28 (d, J = 7.7 Hz, 1H), 4.40-
4.67 (m,
2H), 4.54 (d, J = 12.3 Hz, 1H), 4.72-4.96 (m, 2H), 4.80 (d, J = 12.3 Hz, 1H),
4.96-
5.04 (m, 2H), 5.08 (dd, J = 1.5, 17.1 Hz, 1H), 5.13-5.33 (bs, 1H), 5.33-5.59
(bs, 2H),
5.80-5.92 (m, 1H), 7.22 (dd, J = 1.5, 8.1 Hz, 1H), 7.44 (d, J = 8.1 Hz, IH),
7.63 (s,
IH), 9.51 (s, 1H); IR (KBr) 3400, 2910, 1665, 1590, 1530, 1440, 1420, 1370,
1310,
1140, 1070, and 1035 cm-'; mass spectrum [(+) FAB], m/z 564/566 (M + H)',
586/588 (M + Na)', Anal. Calcd. for C~,H34C1NO,2: C, 51.11; H, 6.06; N, 2.48,
Found: C, 51.17; H, 6.06; N, 2.36.
step 3
N {2-Chloro-5-[(4',6'-O-benzylidene-~-D-maltosyl)-oxy-methyl]-phenyl}-pent-4-
enamide
The title compound was prepared as a white powder (0.102 g, 88%) from N
{ 2-chloro-5-[(p-D-maltosyl)-oxy-methyl]-phenyl }-pent-4-enylamide using a
procedure similar to Example 24, mp 191-193 °C; 'H NMR (DMSO-db) 8 2.30-
2.37
(m, 2H), 2.43-2.49 (m, 2H), 3.06-3.13 (m, 1H), 3.28-3.33 (m, 1H), 3.34-3.41
(m,
3H), 3.43-3.49 (m, 1H), 3.51-3.60 (m, 2H), 3.64-3.75 (m, 3H), 4.11 (dd, J =
2.9, 8.1
Hz, 1H), 4.30 (d, J = 7.7 Hz, 1H), 4.67 (t, J = 5.9 Hz, 1H), 4.68 (ABq, J =
12.3 Hz,
os = 0.22, 2H), 4.99 (dd, J = 2.0, 10.3 Hz, IH), 5.08 (dd, J = 1.8, 17.1 Hz,
IH), 5.14
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
- 80 -
(d, J = 3.7 Hz, 1H), 5.25 (d, J = 5.3 Hz, 1H), 5.30 (d, J = 5.3 Hz, IH), 5.51
(d, J = 3.3
Hz, 1H), 5.57 (s, 1H), 5.63 (d, J = 6.6 Hz, 1H), 5.81-5.91 (m, 1H), 7.23 (dd,
J = 2.0,
8.3 Hz, 1H), 7.34-7.38 (m, 3H), 7.42-7.47 (m, 3H), 7.64 (d, J = 1.5 Hz, 1H),
9.51 (s,
1H); IR {KBr) 3410, 2900, 2870, 1670, 1640, 1590, 1535, 1445, 1420, 1375,
1370,
1325, 1310, 1270, 1150, 1070, and 1030 cm-'; mass spectrum [(+) FAB], m/z
652/654
(M + H)', 674/676 (M + Na)'; Anal. Calcd. for C3,H,8C1NO,2: C, 57.10; H, 5.87;
N,
2.15, Found: C, 56.76; H, 5.81; N, 2.31.
Example 66
N (2-Chloro-S-f!6-O-benzoyl-4'.6'-O-benzilidene-d-D-maltosyl)-ox~-methvll-
phen,~pent-4-enamide
The title compound was prepared as a white solid (1.10 g, 84%) from N {2-
chloro-5-[ (4' , 6' -O-benzylidene-~-D-maltosyl)-oxy-m ethyl]-phenyl }-pent-4-
enamide
using a procedure similar to Example 2, mp >110 °C (decomp.); 'H NMR
(DMSO-
d6) s 2.28-2.35 (m, 2H), 2.41-2.46 (m, 2H), 3.16-3.23 (m, 1H), 3.28-3.43 (m,
2H),
3.48-3.64 (m, 4H), 3.68-3.73 (m, 1 H), 3.73-3.79 (m, 1 H), 4.03-4.08 (m, 1H),
4.33-
4.38 (m, 1H), 4.39 (d, J = 7.7 Hz, 1H), 4.58-4.63 (m, IH), 4.65 (ABq, J = I2.5
Hz,
es = 0.14, 2H), 4.95-4.99 (m, 1H), 5.06 (dd, J = 2.0, 17.4 Hz, 1H), 5.13 (d, J
= 4.0
Hz, 1H), 5.35 (t, J = 4.8 Hz, 2H), 5.52 (s, IH), 5.57 (d, J = 2.9 Hz, 1H),
5.80 (d, J =
6.4 Hz, 1H), 5.81-5.90 (m, IH), 7.20 (dd, J = 2.0, 8.1 Hz, IH), 7.33-7.37 (m,
3H),
7.38-7.43 (m, 3H), 7.50-7.55 (m, 2H), 7.62 (d, J = 1.5 Hz, 1H), 7.63-7.68 (m,
1H),
7.99 (dd, J = 1.1, 8.3 Hz, 2H), 9.49 (s, 1H); IR (KBr) 3400, 3270, 3080, 2910,
2880,
1725, 1660, 1590, 1530, 1445, 1425, 1375, 1325, 1275, 1140, 1070, 1025, 990,
and
715 cm'; mass spectrum [(+) FAB], m/z 756/758 (M + H)', 7781780 (M + Na)';
Anal.
Calcd. for C3gH4,C1N0,3 ~ 0.5 H20: C, 59.65; H, 5.66; N, 1.83, Found: C,
59.73; H,
5.64; N, 1.75.
Example 67
5-!6-O-Benzoyl-4'.6'-O-benzylidene-Q-D-maltos 1~)-ox,y-methyl-2-chloro-
phenvlamine
To a stirred solution of N {2-chloro-5-[(6-O-benzoyl-4',6'-O-benzilidene-p-
D-maltosyl)-oxy-methyl]-phenyl }-pent-4-enamide (0.681 g, 0.901 mmol) in
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-81-
THF:H20 (1:1, 50 mL) at rt was added iodine (0.685 g, 2.70 mmol). After 5 min.
at
this temperature, it was quenched with solid Na2S203 until brown color went
clear.
The mixture was diluted with EtOAc ( 100 mL), washed with brine ( 10 mL), and
then
dried (NaZSO,). After concentration, the oilly residue was purified by flash
chromatography (1:99 to 13:87 MeOH:CHC13 gradient) to afford the product
(0.483
g, 80%o) as a white solid, mp 168-171 °C; 'H NMR (DMSO-d6) 8 3.17 (t, J
= 8.3 Hz,
1H), 3.31-3.43 (m, 2H), 3.48-3.63 (m, 4H), 3.68-3.77 (m, 2H), 4.05 (dd, J =
4.8, 9.9
Hz, 1H), 4.32-4.38 (m, 2H), 4.52 (ABq, J = 11.9 Hz, e8 = 0.18, 2H), 4.61 (d, J
=
10.5 Hz, 1H), 5.14 (d, J = 3.7 Hz, 1H), 5.22-5.32 (bs, 3H), 5.32-5.39 (bs,
1H), 5.52
(s, 1H), 5.57 (s, 1H), 5.76-5.83 (bs, 1H), 6.53 (dd, J = 1.8, 8.1 Hz, 1H),
6.75 (d, J =
2.0 Hz, 1H), 7.08 (7.9 Hz, 1H), 7.33-7.37 (m, 3H), 7.38-7.44 (m, 2H), 7.51-
7.56 (m,
2H), 7.63-7.68 (m, 1H), 8.00 (dd, J = 0.7, 7.9 Hz, 2H); IR (ICBr) 3390, 2920,
2860,
1730, 1620, 1590, 1495, 1440, 1430, 1370, 1315, 1270, 1070, 1025, 1000, and
710
cni'; mass spectrum [(+) FAB], m/z 674/676 (M + H)', 696/698 (M + Na)'; Anal.
Calcd. for C,3H36C1NO,2 ~ 1.0 HZO: C, 57.27; H, 5.53; N, 2.02, Found: C,
57.28; H,
5.39; N, 1.99.
Example 68
(4-Chlorol-benzyl-4'.6'-O-benzylidene-a-D-maltoside
step 1
(4-Chloro-benzyl)-2,2',3,3',4',6,6'-hepta-O-acetyl-p-n-maltoside
The title compound was prepared as white needles (3.96 g, 73%) from
acetobrornomaltose using 4-chloro-benzyl alcohol and a procedure similar to
step 1 of
Example 1, mp 138-141 °C;'H NMR (DMSO-d6) s 1.994 (s, 3H), 1.999 (s,
3H), 2.00
(s, 3H), 2.025 (s, 3H), 2.029 (s, 3H), 2.10 (s, 3H), 2.16 (s, 3H), 3.66 (ddd,
J = 2.9,
4.4, 9.9 Hz, 1H), 3.96 (ddd, J = 2.4, 4.0, 10.1 Hz, 1H), 4.00-4.07 (m, 2H),
4.21-4.28
(m, 2H), 4.51 (dd, J = 2.9, 12.3 Hz, 1H), 4.56 (d, J = 2.4 Hz, 1H), 4.58 (d, J
= 6.8 Hz,
1H), 4.80-4.92 (m, 3H), 5.05 (t, J = 9.9 Hz, 1H), 5.22 (t, J = 9.2 Hz, 1H),
5.35 (dd, J
= 9.4, 10.3 Hz, 1H), 5.41 (d, J = 4.0 Hz, 1H), 7.21 (d, J = 8.6 Hz, 2H), 7.32
(d, J =
8.3 Hz, 2H); IR (KBr) 3480, 2960, 2880, 1755, 1650, 1610, 1495, 1440, 1375,
1335,
1240, 1170, 1135, 1050, 935, 910, 820, and 615 cm~'; mass spectrum ((+) FAB],
m/z
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-82-
761/763 (M + H)', 783/785 (M + Na)', Anal. Calcd. for C33Ha,C10,g: C, 52.08;
H,
5.43; N, 0.00, Found: C, 51.88; H, 5.37; N, 0.01.
step 2
(4-Chloro-benzyl)-p-n-maltoside
The title compound was prepared as a white foam (1.55 g, 95%) from (4-
chloro-benzyl)-2,2',3,3',4',6,6'-hepta-O-acetyl-p-D-maltoside using a
procedure
similar to step 4 of Example 1, mp >102 °C (decomp.); 'H NMR (DMSO-db)
s 3.02-
3.11 (m, 2H), 3.19-3.26 (m, 2H), 3.29-3.36 (m, 2H), 3.37-3.49 (m, 3H), 3.54-
3.64
(m, 2H), 3.72 (d, J = 11.0 Hz, 1H), 4.27 (d, J = 7.7 Hz, 1H), 4.39-4.65 (bs,
2H), 4.69
(ABq, J = 12.5 Hz, es = 0.20, 2H), 4.76-5.03 (bs, 1H), 5.01 (d, J = 3.7 Hz,
1H), 5.10-
5.63 (bs, 4H), 7.37-7.43 (m, 4H); IR (ICBr) 3340, 2920, 2890, 1625, 1600,
1490,
1450, 1400, 1365, 1150, 1075, 1030, and 820 cm~'; mass spectrum [(+) ESI], m/z
484.4/486.4 (M + NH,)', Anal. Calcd. for C,9HZ,C10" ~ 0.5 H20: C, 47.96; H,
5.93;
N, 0.00, Found: C, 47.62; H, 5.82; N, 0.24.
step 3
(4-Chloro)-benzyl-4',6'-O-benzylidene-p-D-maltoside
The title compound was prepared as a white foam (1.20 g, 65%) from (4-
chloro-benzyl)-p-D-maltoside using a procedure similar to Example 24, mp 187-
188°C; 'H NMR (DMSO-db) 8 3.10 (t, J = 8.3 Hz, 1H), 3.27-3.41 (m, 4H),
3.46 (t, J =
8.8 Hz, 1H), 3.51-3.59 (m, 2H), 3.64-3.75 (m, 3H), 4.12 (dd, J = 3.1, 8.1 Hz,
1H),
4.29 (d, J = 7.7 Hz, 1H), 4.62-4.71 (bs, 1H), 4.70 (ABq, J = 12.5 Hz, es =
0.20, 2H),
5.14 (d, J = 3.7 Hz, 1H), 5.21-5.36 (bs, 2H), 5.47-5.55 (bs, IH), 5.57 (s,
1H), 5.59-
5.67 (bs, 1H), 7.34-7.39 (m, 3H), 7.39-7.46 (m, 6H); IR (KBr) 3570, 3430,
3080,
2870, 1615, 1495, 1450, 1435, 1375, 1360, 1340, 1255, 1160, 1120, 1070, 1030,
1000, and 755 cm~'; mass spectrum [(+) ESI], m/z 555/557 (M + H);, 572/574 (M
+
NH,)', I 126/1128 (2M + NH,)i; Anal. Calcd. for C26H3,C1O": C, 56.27; H, 5.63;
N,
0.00, Found: C, 56.09; H, 5.73; N, 0.23.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-83-
Example 69
Benzoic acid 1-O-l4-chloro)-benzyl-4',6'-O-benzylidene-6-deox~fi-D-malto-6-yl
ester
The title compound was prepared as a white solid (0.800 g, 66%) from (4
chloro)-benzyl-4',6'-O-benzylidene-p-D-maltoside using a procedure similar to
Example 2, mp >110 °C (decomp.); 'H NMR (DMSO-d6) 8 3.16-3.23 (m,
1H), 3.27
3.43 (m, 2H), 3.49-3.64 (m, 4H), 3.68-3.78 (m, 2H), 4.02-4.08 (m, 1H), 4.35
(dd, J =
5.5, 12.5 Hz, 1H), 4.39 (d, J = 7.9 Hz, 1H), 4.57-4.62 (m, 1H), 4.67 (ABq, J =
12.5
Hz, es = 0.14, 2H), 5.14 (d, J = 3.7 Hz, 1H), 5.36 (dd, J = 5.1, 10.5 Hz, 2H),
5.52 (s,
1H), 5.58 (d, J = 2.9 Hz, 1H), 5.80 (d, J = 6.2 Hz, 1H), 7.33-7.43 (m, 9H),
7.53 (t, J =
7.5 Hz, 2H), 7.66 (td, J = 1.1, 7.7 Hz, 1H), 7.99 (dd, J = 0.9, 8.1 Hz, 2H);
IR (KBr)
3410, 2890, 1725, 1630, 1610, 1495, 1440, 1380, 1320, 1275, 1075, 1025, and
710
cm-'; mass spectrum [(-) FAB], m/z 657/659 (M - H)-; Anal. Calcd. for
C33H35C1O,2
1.0 H20: C, 58.54; H, 5.51; N, 0.00, Found: C, 58.75; H, 5.36; N, 0.14.
Example 70
4-Benzovl-N ( 5-f l4'.6'-O-benzvlidene-a-D-maltosyl)-oxv-methyll-2-chloro-
phenvll-
benzamide
step 1
4-Benzoyl-N-{2-chloro-5-[(2,2',3,3',4',6,6'-hepta-O-acetyl-~-n-maltosyl)-oxy-
rnethyl]-phenyl }-benzamide
The title compound was prepared as a white foam (0.240 g, 94%) from 2-
chloro-5-(hepta-O-acetyl-p-D-maltosyl-oxymethyl)-phenylamine using p-benzoyl-
benzoic acid and a procedure similar to step 1 of Example 65, mp >84 °C
(decomp.);
'H NMR (DMSO-d6) s 1.93 (s, 3H), 1.94 (s, 6H), 1.97 (s, 6H), 2.01 (s, 3H),
2.08 (s,
3H), 3.93-4.03 (m, 4H), 4.15 (dd, J = 4.6, 12.3 Hz, 1H), 4.21 (dd, J = 4.6,
12.1 Hz,
1H), 4.39 (dd, J = 2.2, 11.9 Hz, 1H), 4.70 (ABq, J = 12.7 Hz, es = 0.14, 2H),
4.74
(dd, J = 8.1, 9.7 Hz, 1H), 4.86 (dd, J = 4.0, 10.5 Hz, 1H), 4.90 (d, J = 8.1
Hz, 1H),
4.98 (t, J = 9.7 Hz, 1H), 5.21 (dd, J = 9.7, 10.5 Hz, 1H), 5.28 (d, J = 4.0
Hz, 1H),
5.31 (dd, J = 8.6, 9.4 Hz, 1H), 7.22 (dd, J = 2.0, 8.3 Hz, 1H), 7.52 (d, J =
2.0 Hz,
1H), 7.55-7.62 (m, 3H), 7.69-7.74 (m, 1H), 7.76-7.80 (m, 2H), 7.85-7.88 (m,
2H),
8.11-8.14 (m, 2H), 10.30 (s, 1H); IR (KBr) 3400, 3010, 2950, 1755, 1675, 1650,
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
- 84 -
1590, 1530, 1440, 1420, 1370, 1230, 1130, and 1040 cm-'; mass spectrum [(+)
FAB],
m/z 984/986 (M + H)', 1006/1008 (M + Na)+, Anal. Calcd. for C"HSOCINO2o: C,
57.35; H, 5.12; N, 1.42, Found: C, 57.11; H, 5.03; N, 1.32.
step 2
4-Benzoyl-N-{2-chloro-5-[3,4-dihydroxy-6-hydroxymethyl-5-(3,4,5-trihydroxy-6-
hydroxymethyl-tetrahydro-pyran-2-yloxy)-tetrahydro-pyran-2-yloxyrnethyl]-
phenyl}-benzamide
The title compound was prepared as a off-white glassy solid (1.50 g, 95%)
from 4-benzoyl-N { 2-chloro-5-[(2,2',3,3',4',6,6'-hepta-O-acetyl-p-D-maltosyl)-
oxy-
methyl]-phenyl }-benzamide using a procedure similar to step 4 of Example 1,
mp
>131 °C (decomp.); 'H NMR (DMSO-d6) s 3.02-3.32 (m, 4H), 3.32-3.40 (m,
2H),
3.40-3.50 (m, 3H), 3.55-3.64 (m, 2H), 3.73 (d, J = 10.8 Hz, 1H), 4.31 (d, J =
7.9 Hz,
1H), 4.48-4.53 (bs, 1H), 4.53-4.59 (bs, 1H), 4.61 (d, J = 12.5 Hz, IH), 4.83-
4.92 (m,
3H), 5.02 (d, J = 4.0 Hz, 1H), 5.21-5.31 (bs, IH), 5.36-5.48 (bs, 1H), 5.48-
5.56 (bs,
1H), 7.32 (dd, J = 2.0, 8.3 Hz, 1H), 7.53 (d, J = 8.1 Hz, 1H), 7.56-7.62 (m,
3H), 7.69-
7.74 (m, 1H), 7.78 (dd, J = 1.3, 8.3 Hz, 2H), 7.86 (d, J = 8.6 Hz, 2H), 8.14
(d, J = 8.6
Hz, 2H), 10.33 (s, IH); IR (KBr) 3410, 2910, 1660, 1590, 1530, 1440, 1420,
1370,
1325, 1275, 1140, 1100, 1080, 1030, 910, and 710 cm-'; mass spectrum [(+)
FAB],
m/z 690/692 (M + H)', 712/714 (M + Na)', Anal. Calcd. for C33H36CINO'3 ~ 1.0
HzO:
C, 55.97; H, 5.41; N, 1.98, Found: C, 55.98; H, 5.36; N, 1.97.
step 3
4-Benzoyl-N-{5-[(4',6'-O-benzylidene-p-v-maltosyl)-oxy-methyl]-2-chloro-
phenyl}-benzamide
The title compound was prepared as a white solid (1.07 g, 66%) from 4-
benzoyl-N-{ 2-chloro-5-[3,4-dihydroxy-6-hydroxymethyl-5-(3,4,5-trihydroxy-6-
hydroxymethyl-tetrahydro-pyran-2-yloxy)-tetrahydro-pyran-2-yloxymethyl]-phenyl
}-
benzamide using a procedure similar to Example 24, mp 208-211 °C; 'H
NMR
(DMSO-db) s 3.09-3.15 (m, 1H), 3.29-3.42 (m, 4H), 3.47 (td, J = 3.1, 8.8 Hz,
1H),
3.52-3.60 (m, 2H), 3.64-3.76 (m, 3H), 4.12 (dd, J = 3.1, 8.3 Hz, 1H), 4.34 (d,
J = 7.7
Hz, 1H), 4.68 (t, J = 7.5 Hz, 1H), 4.75 (ABq, J = 12.5 Hz, os = 0.22, 2H),
5.14 (d, J =
CA 02350066 2001-05-09
WO OOJ31096 PCT/US99/27828
-85-
4.0 Hz, 1H), 5.30 (dd, J = 1.8, 5.3 Hz, 2H), 5.52 (d, J = 3.3 Hz, 1H), 5.57
(s, IH),
5.63 (d, J = 6.6 Hz, 1H), 7.34-7.38 (m, 4H), 7.42-7.46 (m, 2H), 7.55 (d, J =
8.1 Hz,
1H), 7.56-7.62 (m, 3H), 7.69-7.74 (m, 1H}, 7.76-7.80 (m, 2H), 7.87 (d, J = 8.3
Hz,
2H), 8.14 (d, J = 8.3 Hz, 2H), 10.33 (s, 1H); IR (KBr) 3410, 3070, 2920, 2860,
1655,
1590, 1530, 1445, 1425, 1380, 1330, 1275, 1145, 1070, 1030, 1000, 910, and 700
cm~
'; mass spectrum [(+) FAB], m/z 778/780 (M + H)+, 800/802 (M + Na)+; Anal.
Calcd.
for C,oH,aCINO,3 ~ 0.5 HZO: C, 61.03; H, 5.25; N, 1.78, Found: C, 61.11; H,
4.86; N,
1.74.
Example 71
4-Benzovl-N- ~ 5-f (6-benzovl-oxy-4' ,6'-O-benzylidene-a-D-maltosyl)-oxy-
methyll-2-
chloro-phenyl)-benzoic acid amide
The title compound was prepared as a white solid (0.352 g, 63%) from 4-
benzoyl-N-{ 5-[(4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-chloro-phenyl
x-
benzamide using a procedure similar to Example 2, mp >135 °C (decomp.);
'H NMR
(DMSO-d6) 8 3.18-3.24 (m, 1H), 3.32-3.43 (m, 2H), 3.50-3.65 (m, 4H), 3.72 (td,
J =
5.3, 10.1 Hz, 1H), 3.77-3.82 (m, 1H), 4.05 (dd, J = 4.8, 10.1 Hz, 1H), 4.36
(dd, J =
4.8, 12.1 Hz, 1H), 4.43 (d, J = 7.9 Hz, 1H), 4.62 (d, J = 10.3 Hz, 1H), 4.72
(ABq, J =
12.7 Hz, es = 0.14, 2H), 5.14 (d, J = 3.7 Hz, 1H), 5.32-5.37 (m, 1H), 5.40 (d,
J = 4.4
Hz, 1H), 5.52 (s, 1H), 5.59 (s, 1H), 5.80 (d, J = 5.5 Hz, 1H), 7.31-7.37 (m,
4H), 7.39-
7.43 (m, 2H), 7.48-7.53 (m, 3H}, 7.56-7.66 (m, 4H), 7.71 (tt, J = 1.1, 6.8 Hz,
1H),
7.77 (dd, J = 1.1, 7.9 Hz, 2H), 7.85 (d, J = 8.6 Hz, 2H), 7.99 (dd, J = 0.9,
8.1 Hz,
2H), 8.12 (d, J = 8.6 Hz, 2H), 10.33 (s, 1H); IR (KBr) 3420, 3080, 2850, 1720,
1660,
1600, 1530, 1440, 1420, 1370, 1320, 1275, 1140, 1070, 1030, and 7I5 cni'; mass
spectrum [(+) FAB], m/z 882 (M + H)', 904/906 (M + Na)'; Anal. Calcd. for
C"H~C1N0" ~ 1.0 H20: C, 62.70; H, 5.15; N, 1.56, Found: C, 62.83; H, 5.02; N,
1.70.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
- 86 -
Example 72
4-Benzoyl-N- ( 5-f (4' .6'-D-benzylidene-6-O-f 2-iodo)-benzoyl-a-D-rnaltosyl)-
oxy-
methvll-2-chloro-phenyl ~-benzamide
The title compound was prepared as a white solid (0.145 g, 32%) from 4-
benzoyl-N {5-[(4',6'-O-benzylidene-~-D-maltosyl)-oxy-methyl]-2-chloro-phenyl}-
benzamide using o-I-BzCI and a procedure similar to Example 2, mp >122
°C
(decomp.); 'H NMR (DMSO-d6) s 3.16-3.23 (m, lH), 3.28-3.45 (m, 2H), 3.49-3.67
(m, 4H), 3.75 (dd, J = 4.8, 7.5 Hz, 1H), 3.78-3.84 (m, 1H), 4.14 (dd, J = 4.6,
9.7 Hz,
1H), 4.36 (dd, J = 5.5, 12.1 Hz, 1H), 4.44 (d, J = 7.7 Hz, 1H), 4.61-4.67 (m,
1H),
4.73 (ABq, J = 12.7 Hz, es = 0.15, 2H), 5.16 (d, J = 4.0 Hz, 1H), 5.38 (dd, J
= 5.3,
10.5 Hz, 2H), 5.55 (s, IH), 5.60 (d, J = 2.6 Hz, 1H), 5.88 (d, J = 6.2 Hz,
IH), 7.26
(td, J = 1.8, 7.9 Hz, 1H), 7.31-7.38 (m, 4H), 7.40-7.45 (m, 2H), 7.48-7.53 (m,
2H),
7.56-7.62 (m, 3H), 7.71 (tt, J = 1.3, 6.8 Hz, 1H), 7.75-7.79 (m, 3H), 7.85
(dd, J = 1.8,
6.6 Hz, 2H), 8.00 (dd, J = 1.1, 8.1 Hz, 1H), 8.12 (dd, J = 1.8, 6.6 Hz, 2H),
10.30 (s,
1H); IR (KBr) 3410, 3070, 2850, 1730, 1655, 1590, 1525, 1440, 1420, 1375,
1280,
1250, 1140, 1070, 1025, and 705 cm '; mass spectrum [(+) FAB], m/z 1008 (M +
H)',
1030 (M + Na)'; Anal. Calcd. for C4,H,3C1IN0,4 ~ 0.5 H20: C, 55.50; H, 4.36;
N,
1.38, Found: C, 55.14; H, 4.22; N, 1.36.
Example 73
4-Benzoyl-N-15-f (4'.6'-O-benzylidene-6-O-(3-iodo-benzovl)-a-D-maltosvl)-oxy-
methyll-2-chloro-phenxl l-benzamide
The title compound was prepared as a white solid (0.244 g, 54%) from 4-
benzoyl-N {5-[(4',6'-O-benzylidene-~-D-maltosyl)-oxy-methyl]-2-chloro-phenyl}-
benzamide using m-I-BzCI (prepared from m-I-benzoic acid and oxalyl chloride)
and
a procedure similar to Example 2, mp 185-188.5 °C; 'H NMR (DMSO-db) s
3.18-
3.24 (m, 1H), 3.27-3.43 (m, 2H), 3.50-3.63 (m, 4H), 3.71 (td, J = 4.6, 9.9 Hz,
1H),
3.77-3.82 (m, 1H), 4.06 (dd, J = 4.8, 9.9 Hz, 1H), 4.35 (dd, J = 5.5, 12.1 Hz,
1H),
4.44 (d, J = 7.7 Hz. 1H), 4.61-4.67 (m, 1H), 4.73 (ABq, J = 12.5 Hz, es =
0.13, 2H),
5.14 (d, J = 4.0 Hz. 1 H), 5.34 (d, J = 5.3 Hz, 1 H), 5.39 (d, J = 5.1 Hz, 1
H), 5.53 (s,
1H), 5.58 (d, J = 2.9 Hz, 1H), 5.79 (d, J = 6.2 Hz, 1H), 7.31 (d, J = 7.9 Hz,
1H),
7.33-7.37 (m, 4H), 7.38-7.43 (m, 2H), 7.51 (d, J = 8.1 Hz, 1H), 7.56-7.62 (m,
3H),
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
_ 87 -
7.71 (tt, J = 1.3, 6.8 Hz, 1H), 7.76-7.80 (m, 2H), 7.85 (dd, 3 = 1.8, 6.6 Hz.
2H), 7.99
(dt, J = 1.5, 7.7 Hz, 2H), 8.11 (dd, J = 1.8, 6.8 Hz, 2H), 8.25 (t, J = 1.8
Hz, 1H),
10.29 (s, 1H); IR (KBr) 3410, 3080, 2910, 2856, 1725, 1650, 1590, 1570, 1530,
1440. 1420. 1375, 1280, 1255, 1140, 1070, 1030, 750, and 700 cm-'; mass
spectrum
[(+) FAB], m/z 1030 (M + Na)'; Anal. Calcd. for C,~H;3C1IN0,4 ~ 0.5 H20: C,
55.50;
H, 4.36; N, 1.38. Found: C, 55.13; H, 4.15; N, 1.38.
Example 74
4-Benzovl-N (5-((4'.6'-O-benzylidene-6-(4-iodo-benzoyl)-oxy-f3-D-maltosvl)-
oxy.
methvll-2-chloro-phenyl ~-benzoic acid amide
The title compound was prepared as a white solid (0.378 g, 59%) from 4-
benzoyl-N-{ 5-[(4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-chloro-phenyl
}-
benzamide using p-iodobenzoyl chloride and a procedure similar to Example 2,
mp
,151 °C (decomp.); 'H NMR (DMSO-db) s 3.18-3.24 (m, 1H), 3.32-3.42 (m,
2H),
3.49-3.63 (m, 4H), 3.71 (td, J = 4.8, 9.9 Hz, 1H), 3.78 (ddd, J = 1.1, 4.6,
9.2 Hz, 1H),
4.04 (dd, J = 4.8, 9.9 Hz, 1H), 4.35 (dd, J = 4.8, 12.1 Hz, 1H), 4.42 (d, J =
7.7 Hz,
1H), 4.59 (d, J = 10.8 Hz, 1H), 4.72 (ABq, J = 12.7 Hz, 08 = 0.13, 2H), 5.13
(d, J =
4.0 Hz, 1 H), 5.34 (d, J = 5.3 Hz, 1 H), 5.39 (d, J = 5.1 Hz, 1H), 5.52 (s, 1
H), 5.59 (d,
J = 2.4 Hz. 1H), 5.82 (d, J = 5.9 Hz, 1H), 7.31-7.37 (m, 4H), 7.38-7.42 (m,
2H), 7.51
(d, J = 8.3 Hz, 1H), 7.56-7.61 (m, 3H), 7.69-7.75 (m, 3H), 7.76-7.79 (m, 2H),
7.85
(d, J = 8.6 Hz, 2H), 7.89 (d, J = 8.6 Hz, 2H), 8.12 (d, J = 8.6 Hz, 2H), 10.30
(s, 1H);
IR (KBr) 3420, 3080, 2890, 2840, 1725, 1655, 1590, 1530, 1440, 1420, 1380,
1365,
1325, 1280. 1160. 1120, 1070, 1030, 1005, 750, and 700 cm-'; mass spectrum
[(+)
FAB], mlz 1030/1032 (M + Na)'; Anal. Calcd. for C;,H43C1INO,4: C, 55.01; H,
4.42;
N, 1.36, Found: C, 54.99; H, 4.38; N, 1.40.
CA 02350066 2001-05-09
WO 00/31096 PCTNS99/27828
_88_
Example 75
(1-(5-f(4'.6'-O-Benzylidene-a-D-maltosyl)-ox -~yli-2-chloro-phenK-
carbamo I~?ethyl)-carbamic acid 9H-fluoren-9vlmethyl ester
step 1
N-{2-Chloro-5-[(2,2',3,3',4',6,6')-hepta-O-acetyl-~-n-maltosyl-oxymethyl]-
phenyl}-(9H-fluoren-9-ylmethoxycarbonyl)-L-alaninamide
The title compound was prepared as a white foam (2.50 g, 36%) from 2-
chloro-5-(hepta-O-acetyl-~-D-maltosyl-oxymethyl)-phenylamine using N-(9H-
fluoren-9-ylmethyoxycarbonylamino)-L-alanine and a procedure similar to step 1
of
Example 65, mp >96 °C (decomp.); 'H NMR (DMSO-d6) s 1.33 (dd, J = 7.2
Hz, 3H),
1.918 (s, 3H), 1.919 (s, 3H), 1.94 (s, 3H), 1.966 (s, 3H), 1.97 (s, 3H), 2.01
(s, 3H),
2.07 (s, 3H), 3.91-4.02 (m, 4H), 4.12-4.24 (m, 3H), 4.24-4.34 (m, 3H), 4.34-
4.40 (m,
1H), 4.53 (d, J = 12.7 Hz, 1H), 4.68-4.75 (m, 2H), 4.84 (d, J = 4.0 Hz, 1H),
4.86 (d, J
= 2.6 Hz, 1 H), 4.97 (t, J = 9.7 Hz, 1 H), 5.21 (t, J = 9.7 Hz, 1 H), 5.27 (d,
J = 3.7 Hz,
IH), 5.27-5.32 (m, 1H), 7.08 (dd, J = 1.8, 8.1 Hz, 1H), 7.32 (t, J = 7.2 Hz,
2H), 7.40
(t, J = 7.5 Hz, 2H), 7.47 (d, J = 8.1 Hz, 1H), 7.69-7.78 (m, 4H), 7.88 (d, J =
7.5 Hz,
2H}, 9.42 (s, 1H); IR (KBr) 3360, 3010, 2950, 1755, 1590, 1535, 1440, 1420,
1370,
1230. 1050, and 755 cm-'; mass spectrum [(+) ESI], m/z 1069.2 (M + H)',
1086.2/1088.2 (M + NH4)+, Anal. Calcd. for CS,HS,CIN,Oz, ~ 3.5 H,O: C, 54.09;
H,
5.70; N. 2.47. Found: C, 53.67; H, 5.1 I ; N, 2.34.
step 2
N-[2-Chloro-5-(R-D-maltosyl-oxymethyl)-phenyl]-(9H-fluoren-9-
ylmethoxycarbonyl)-L-alaninamide
To a stirred solution of KCN (0.032 g, 0.491 mmol) in MeOH (10 mL) at
0°C
was added (I-{5-[(4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-chloro-
phenyl-
carbamoyl}ethyl)-carbamic acid 9H-fluoren-9ylmethyl ester (1.05 g, 0.982
mmol).
The reaction mixture was stirred at this temperature for 24 h and then
concentrated.
The resulting residue was diluted with THFaat. aq. NaHC03 ( 1:1. 20 mL)
followed
by addition of Fmoc-Cl (0.170 g, 0.658 mmol). This solution was stirred at rt
for 0.5
h, and the resulting mixture filtered to remove the solid that formed. The
filtrate was
concentrated, and the residue was purified by flash chromatgraphy (80:2:1 to
4:2:1
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-89-
EtOAc:EtOH: H20) to afford the product (0.600 g, 79%) which was used in the
next
step without further purification.
step 3
(1-{5-[(4',6'-O-Benzylidene-p-n-maltosyl)-oxy-methyl]-2-chloro-phenyl-
carbamoyl}ethyl)-carbamic acid 9H-fluoren-9ylmethyl ester
The title compound was prepared as a off-white solid (0.295 g, 41 %) from N-
[2-chloro-5-(p-D-maltosyl-oxymethyl)-phenyl]-(9H-fluoren-9-ylmethoxycarbonyl)-
L-
alaninamide using a procedure similar to Example 24, mp >190 °C
(decomp.); 'H
NMR (DMSO-d6) s 1.34 (d, J = 7.0 Hz, 3H), 3.06-3.14 (m, 1H), 3.28-3.42 (m,
3H),
3.41 (td, J = 2.2, 9.2 Hz, 1H), 3.51-3.6U (m, 2H}, 3.64-3.76 (m, 4H), 4.11
(dd, J =
2.4, 7.7 Hz, IH), 4.22 (t, J = 6.6 Hz, 1H), 4.26-4.35 (m, 4H), 4.67 (t, J =
5.7 Hz, 1H),
4.69 (ABq, J = 12.5 Hz, es = 0.22, 2H), 5.14 (d, J = 3.7 Hz, IH), 5.26 (d, J =
4.8 Hz,
1H), 5.31 (d, J = 5.1 Hz, 1H), 5.52 (d, J = 2.6 Hz, 1H), 5.57 (s, IH), 5.64
(d, J = 6.2
Hz, 1H), 7.24 (d, J = 7.7 Hz, 1H), 7.28-?.48 (m, lOH), 7.69-7.79 (m, 4H}, 7.88
(d, J
= 7.5 Hz, 2H), 9.46 (s, IH); IR (KBr) 3390, 3080, 2920, 2870, 2350, 1705,
1590,
1525, 1445, 1420, 1375, 1340, 1310, 1255, 1140, 1070, 1030, and 740 cm'; mass
spectrum [(+) ESI], m/z 880 (M + NH4)'; Anal. Calcd. for C,4H"ClNzO" ~ I.0
H20:
C, 59.96; H, 5.6U; N, 3.18, Found: C, 60.23; H, 5.53; N, 3.45.
Example 76
N l9H-Fluoren-9ylmethoxvcarhonyl)-N'-15-ff6-O-benzoyl-4'.6'-O-benzvlidene-a-D-
maltos l~v-methyll-2-chloro-phenyll-L-alaninamide
The title compound was prepared as a white solid (0.083 g, 62%) from (I-{5-
[(4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-chloro-
phenylcarbamoyl}ethyl)-
carbamic acid 9H-fluoren-9ylmethyl ester using a procedure similar to Example
2,
mp 224-226 °C; 'H NMR (DMSO-d6) s 1.32 (d, J = 7.2 Hz, 3H), 3.19 (t, J
= 8.3 Hz,
1H), 3.26-3.37 (m, 4H), 3.48-3.63 (m, 3H}, 3.70 (dd, J = 5.1, 9.9 Hz, 1H),
3.73-3.78
(m, 1H), 4.05 (dd, J = 4.8, 9.9 Hz, 1H), 4.21 (t, J = 6.8 Hz, 1H), 4.24-4.38
(m, 3H),
4.40 (d, J = 7.7 Hz, IH), 4.56-4.63 (m, 1H), 4.65 (ABq. J = 12.5 Hz, es =
0.15, 2H),
5.13 (d, J = 3.7 Hz, 1H), 5.27-5.41 (bs, 2H), 5.52 (s, 1H), 5.54-5.61 (bs,
IH), 5.75-
5.84 (bs, 1H), 7.21 (d, J = 8.6 Hz, 1H), 7.28-7.37 (m, 5H), 7.37-7.45 (m, 5H),
7.51 (t,
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-90-
J = 7.9 Hz, 2H), 7.63 (t, J = 7.5 Hz, 1H), 7.68-7.77 (m, 4H), 7.88 (d, J = 7.7
Hz, 2H),
7.98 (d, J = 7.2 Hz, 2H), 9.43 (s, 1H); IR (KBr) 3400, 3080, 2920, 2850, 1725,
1590,
1530, 1440, 1420. 1375, 1320, 1275, 1070, 1025, 745, and 715 cm~'; mass
spectrum
[(+) FAB], m/z 989/991 (M + Na)'; Anal. Calcd. for CS,HS,C1N20,5 ~ 1.0 H20: C,
62.16; H, 5.42; N, 2.84, Found: C, 61.99; H, 5.23; N, 3.06.
Example 77
N'-( 5-((6-O-benzoyl-4'.6'-O-benzylidene-a-D-maltosyl)-oxy-methyl]/-2-chloro-
phen.~L-alaninamide
To a stirred solution of 20% piperidine (2.00 mL, 20.2 mrnol) in DMF ( 10
mL) at rt was added N-(9H-fluoren-9ylmethoxycarbonyl)-N'-{5-[(6-O-benzoyl-
4',6'-
O-benzylidene-p-~-maltosyl)-oxy-methyl]-2-chloro-phenyl}-L-alaninamide (0.300
g,
0.256 mmol). After 2 h at this temperature, the solution was concentrated on
the high
vacuum. The residue was purified by preparatory plate chromatography ( 10:2:1
EtOAc:EtOH:H,O) to afford the product (0.018 g, 78%) as an off-white solid, mp
131-133 °C; 'H NMR (DMSO-db) s 1.26 (d, J = 7.0 Hz, 3H), 3.15-3.22 (m,
1H),
3.26-3.42 (m, 5H), 3.47-3.64 (m, 4H), 3.70 (dd, J = 4.6, 9.7 Hz, 1H), 3.?3-
3.79 (m,
1 H), 4.05 (dd, J = 4.8, 9.9 Hz, 1 H), 4.35 (dd, J = 5.1, 12.1 Hz, I H), 4.40
(d, J = 7.7
Hz, 1H), 4.60 (d, J = 12.7 Hz, 1H), 4.66 (ABq, J = 12.3 Hz, os = 0.14, 2H),
5.13 (d, J
= 3.7 Hz, 1H), 5.34 (dd, J = 0.9, 5.3 Hz, 2H), 5.52 (s, IH), 5.57 (d, J = 2.9
Hz, 1H),
5.80 (d, J = 6.2 Hz, 1H), 7.15 (dd, J = 2.0, 8.1 Hz, IH), 7.33-7.38 (m, 3H),
7.38-7.45
(m, 3H), 7.53 (t, J = 7.7 Hz, 2H), 7.63-7.68 (m, 2H), 7.98 (d, J = 1.3 Hz,
1H), 8.00 (s,
1H), 8.20 (s, 1H); IR (KBr) 3410, 2920, 2850, 1720, 1625, 1590, 1525, 1445,
1420,
1375, 1275, 1070, 1025, and 715 em-'; mass spectrum [(+) FAB], m/z 745 (M +
H)',
?67 (M + Na)'; Anal. Calcd. for C36H"CIN~O,3 ~ 2.0 H20: C, 55.35; H, 5.81; N,
3.59,
Found: C, 55.63; H, 5.77; N, 3.23.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-91 -
Example 78
N ( 5-f (4'.6'-O-Benzylidene-a-D-maltos l~vmethvll-2-chloro-ohenvl ~-N methyl-
acetamide
step 1
N-[5-(2,2',3,3',4',6,6'-Hepta-O-acetyl-p-n-maltosyl)-oxy-methyl-2-chloro-
phenyl]-N-methyl-acetamide
To a stirred solution of N-[2-chloro-5-(hepta-O-acetyl-p-D-maltosyl-
oxymethyl)-phenyl]-acetamide (0.100 g, 0.122 mmol) in THF (2.0 mL) at -78
°C was
added NaHMSA (0.183 mL, 1.0 M in THF). After 0.5 h at this temperature, methyl
iodide (0.0152 mL, 0.244 mmol) was added, and the reaction was warmed to rt
for 2
h. At this point, the reaction was diluted with EtOAc ( 100 mL), washed with 1
N HCl
( 10 mL), sat. aq. NaHC03 ( 10 mL), and brine ( 10 mL), and then dried
(MgSO,).
After concentration, the resulting oilly residue was purified by preparatory
plate
chromatography using 50:50 EtOAc:petroleum ether as the eluant to afford the
i5 product (0.0408 g, 40%) as a white foam, mp >252 °C (decomp.); 'H
NMR (CDCl3) s
1.81 (s, 3H), 2.00 (s, 3H), 2.01 (s, 6H), 2.02 (s, 3H), 2.04 (s, 3H), 2.10 (s,
3H), 2.15
(s, 3H), 3.19 (d, J = 2.9 Hz, 3H}, 3.67-3.72 (m, 1H}, 3.94-3.99 (m, 1H), 4.03
(t, J =
9.4 Hz, 1H), 4.07 (d, J = 2.2 Hz, 1H), 4.21-4.28 (m, 2H), 4.54 (dd, J = 2.9,
12.3 Hz,
IH), 4.59 (d. J = 11.6 Hz, 1H}, 4.64 (d> J = 7.5 Hz, 1H)> 4.83-4.93 (m, 3H),
5.06 (t, J
= 10.1 Hz, IH), 5.26 (td, J = 3.3> 9.0 Hz, 1H), 5.36 (t, J = 9.7 Hz, 1H), 5.42
(d, J =
4.0 Hz, 1 H), 7.20 (d, J = 2.0 Hz, 1 H), 7.24 (dt, J = 2.0, 8.1 Hz, 1 H), 7.47
(dd, J = 1.1,
8.1 Hz, 1H); IR (KBr) 3470, 2950, 1755, 1620, 1480, 1420, 1380, 1230, 1140,
and
1045 cm-'; mass spectrum [(+) ESI], m/z 832 (M + H)~, Anal. Calcd. for
Cj6H,6C1NO~9
1.5 H=O: C, 50.32; H, 5.75; N, 1.63, Found: C, 50.17; H, 5.38; N, I.67.
step 2
N-{2-Chloro-5-[(p-u-maltosyl)-oxymethylJ-phenyl}-N-methyl-acetamide
The title compound was prepared as a white solid (0.475 g, 99%) from N-[5-
(2,2',3,3' ,4' ,6,6' -hepta-O-acetyl-~-D-maltosyl)-oxy-methyl-2-chloro-phenyl]-
N-
methyl-acetamide using a procedure similar to step 4 of Example 1, mp >96
°C
(decomp.); 'H NMR (DMSO-db) s 1.67 (s, 3H), 3.05 (d, J = 1.1 Hz, 3H), 3.06-
3.14
(m, IH), 3.18-3.28 (m, 2H), 3.30-3.38 (m, 2H), 3.38-3.49 (m, 4H), 3.53-3.63
(m,
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-92-
2H), 3.68-3.?5 (m, 1H), 4.28 (dd, J = 7.9, 9.7 Hz, 1H), 4.48-4.53 (m, 2H),
4.62 (dd, J
= 3.3, 13.2 Hz, 1H), 4.84 (d, J = 13.0 Hz, 1H), 4.89 (dd, J = 5.5, 7.7 Hz,
2H), 5.01 (d,
J = 3.7 Hz, 1H), 5.30 (dd, J = 4.8, 7.7 Hz, 1H), 5.42 (dd, J = 2.4, 6.2 Hz,
1H), 5.52
(d, J = 3.1 Hz, 1H), 7.43 (dt, J = 1.8, 8.1 Hz, 1H), 7.57 (s, 1H), 7.60 (d, J
= 8.3 Hz,
5 1H); IR (ICBr) 3400, 3000, 2910, 1645, 1580, I480, 1420, 1385, /320, 1255,
1195,
1145, 1120, 1075, 1035, and 755 cm~'; mass spectrum [(+) FAB], m/z 538 (M +
H)',
560 (M + Na)', Anal. Calcd. for C2zH3,CINO,~ ' 1.0 H~O: C, 47.53; H, 6.16; N,
2.52,
Found: C, 47.18; H, 6.01; N, 2.38.
step 3
N-{5-[(4',6'-O-Benzylidene-p-n-maltosyl)-oxymethyl]-2-chloro-phenyl}-N-
methyl-acetamide
The title compound was prepared as a white foam (0.315 g, 63%) from N-{2-
chloro-5-[(p-D-maltosyl)-oxymethyl]-phenyl }-N-methyl-acetamide using a
procedure
similar to Example 24, mp >125 °C (decomp.); 'H NMR (DMSO-db) s 1.68
(d, J =
1.3 Hz, 3H), 3.06 (d, J = 1.3 Hz, 3H), 3.09-3.16 (m, 1H), 3.28-3.42 (m, 4H),
3.42-
3.50 (m, IH), 3.50-3.60 (m, 2H), 3.64-3.75 (m, 3H), 4.09-4.14 (m, IH), 4.30
(dd, J =
7.7, 10.3 Hz, IH), 4.60-4.69 (m, 2H), 4.85 (d, J = 13.2 Hz, 1H}, 5.14 (d, J =
4.0 Hz,
IH), 5.30 (d, J = 5.1 Hz, 1H), 5.33 (dd, J = 5.1, 7.~) Hz, 1H), 5.53 (d, J =
3.3 Hz, 1H),
5.57 (s, 1H), 5.63 (dd, J = 2.9, 6.8 Hz, 1H), 7.34-7.38 (m, 3H), 7.41-7.46 (m,
3H),
7.58 (s, 1H), 7.60 (d, J = 8.3 Hz, IH); IR (KBr) 3410, 2920, 2860, 1640, 1610,
1580,
1480, 1440, 1410, 1380, 1320, 1185, 1150, 1070, 1030, 955, and 755 cm~'; mass
spectrum [(-) FAB), rn/z 624 (M - H)-; Anal. Calcd. for CZ9H36C1NO'= ' 2.5
HZO: C,
51.90; H, 6.16; N, 2.09, Found: C, 51.92; H, 5.48; N, 2.09.
Example 79
N-( 5-f (6-O-Benzoyl-4',6'-O-benzylidene-t~-D-maltos~rl)-oxpmethyll-2-chloro-
phenyl 1-N-methyl-acetamide
The title compound was prepared as a white solid (0.087 g, 37%) from N-{ 5-
[(4',6'-O-benzylidene-~-D-maltosyl)-oxymethyl]-2-chloro-phenyl}-N-methyl-
acetamide using a procedure similar to Example 2, mp 180-183 °C; 'H NMR
(DMSO-db) s 1.64 (d, J = 4.0 Hz, 3H), 3.02 (s, 3H)> 3.17-3.25 (m, 1H), 3.35
(d, J =
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-93-
9.4 Hz, 1H), 3.37-3.43 (m, 1H), 3.49-3.65 (m, 4H), 3.70 (dd, J = 4.6, 9.9 Hz,
1H),
3.73-3.79 (m, 1H), 4.04 (d, J = 5.1, 9.9 Hz, 1H), 4.34 (ddd, J = 2.4, 4.8,
12.1 Hz,
1H), 4.41 (dd, J = 7.9, 10.1 Hz, 1H), 4.58 (d, J = 11.4 Hz, 1H), 4.72 (ABq, J
= 12.5
Hz, os = 0.12, 2H), 5.14 (d, J = 3.7 Hz, 1H), 5.34 (d, J = 4.8 Hz, 1H), 5.43
(dd, J =
5.3, 7.2 Hz, 1H), 5.52 (s, 1H), 5.59 (d, J = 2.6 Hz, 1H), 5.79 (dd, J = 2.0,
5.7 Hz,
1H), 7.33-7.37 (m, 3H), 7.38-7.43 (m, 3H), 7.50-7.55 (m, 3H}, 7.56 (d, J = 8.3
Hz,
1H), 7.63-7.68 (m, 1H), 7.96-8.00 (m, 2H); IR (KBr) 3495, 3400, 3090> 2930,
2890,
1730, 1645, 1600, 1575, 1480, 1445, 1420, 1385, 1360, 1320, 1270, 1200, 1160,
1110, 1070, 1050, 1020, 985, and 715 cm~'; mass spectrum [(+) FAB], m/z 730 (M
+
H)', 752 (M + Na)'; Anal. Calcd. for C36HQOC1N0,3 ~ H,=O: C, 59.22; H, 5.52;
N, 1.92,
Found: C, 59.02; H, 5.50; N, 1.79.
Example 80
N 15-f(4'.6'-O-Benzyidene-D-D-maltos lv )-oxv-methvll-2-chloro-phenvll-
carbamic
acid methyl ester
step 1
N-{5-[(2,2',3,3',4',6,6'-Hepta-O-acetyl-p-D-maltosyl)-oxymethyl]-2-chloro-
phenyl}-carbamic acid methyl ester
To a stirred solution of 2-chloro-5-(hepta-O-acetyl-p-D-maltosyl-oxymethyl)
phenylamine (1.40 g, 1.80 mmol) in THF (18 mL) at 0 °C was added NaH
(0.108 g,
2.70 mmol). After 10 min. at this temperature, methyl chloroformate (0.167 mL,
2.16
mmol) was added, and then the reaction was warmed to rt for 3 h. At this
point, the
reaction was concentrated, and the residue was diluted with EtOAc (300 mL).
This
solution was washed with 1N HCl (30 mL), sat. aq. NaHCO, (30mL), and brine (30
mL) and then dried (MgSO,). After concentration, the resulting oilly residue
was
purified by flash chromatography (2:98 to 10:90 acetone:CHCl3 gradient) to
afford
the product (1.33 g, 88%) as a white foam, mp >79 °C (decomp.); 'H NMR
(DMSO-
db) s 1.93 (s, 3H), 1.94 (s, 6H), 1.970 (s, 3H), 1.973 (s, 3H), 2.01 (s, 3H),
2.08 (s,
3H), 3.64 (s, 3H), 3.91-4.03 (m, 4H), 4.12-4.23 (m, 2H), 4.38 (dd, J = 1.8,
11.9 Hz,
1H), 4.54 (d, J = 12.? Hz, 1H), 4.69-4.75 (m, 2H), 4.83-4.88 (m, 2H), 4.97 (t,
J = 9.7
Hz, 1H), 5.21 (dd, J = 9.7, 10.3 Hz, 1H), 5.27 (d, J = 3.7 Hz, 1H), 5.30 (dd,
J = 8.6,
9.2 Hz, 1H), 7.07 (dd, J = 2.0, 8.3 Hz, 1H), 7.44 (d> J = 8.1 Hz, 1H), 7.48
(d, J = 1.8
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-94-
Hz, 1H), 9.08 (s, IH); IR (KBr) 3420, 2950, 1755, 1590, 1530, 1450, 1420,
1375,
1230, 1130, and 1040 cm-'; mass spectrum [(+) FAB], m/z 834 (M + H)+, 856 (M +
Na)', Anal. Calcd. for C35H44C1NOZO ~ 0.5 H,O: C, 49.86; H, 5.38; N, 1.66,
Found:
C, 49.68; H, 5.14; N, 1.58.
step 2
{2-Chloro-5-[(p-D-maltosyl)-oxy-methyl]-phenyl}-carbamic acid methyl ester
The title compound was prepared as a white foam (0.753 g, 99%) from N-{5-
[(2,2',3,3',4',6,6'-hepta-O-acetyl-~-D-maltosyl)-oxymethyl]-2-chloro-phenyl }-
carbamic acid methyl ester using a procedure similar to step 2 of Example 63,
mp
>109 °C (decomp.); 'H NMR (DMSO-ds) s 3.01-3.11 (m, 2H), 3.19-3.27 (m,
2H),
3.28-3.38 (m, 2H), 3.38-3.50 (m, 3H), 3.52-3.64 (in, 2H), 3.64 (s, 3H), 3.72
(d, J =
11.2 Hz, 1H), 4.28 (d, J = 7.9 Hz, 1H), 4.44-4.57 (m, 2H), 4.67 (ABq, J = 12.5
Hz, es
= 0.22, 2H), 4.83-4.96 (bs, 2H), 5.01 (d, J = 4.0 Hz, 1H), 5.16-5.32 (bs, 1H),
5.34-
5.58 (bs, 2H), 7.21 (dd, J = 2.0, 8.1 Hz, 1H), 7.43 (d, J = 8.1 Hz, 1H), 7.53
(d, J = 1.8
Hz, 1H), 9.07 (s> 1H); IR (KBr) 3420, 2920, 1725, 1590. 1530, 1450, 1425,
1370,
1310, 1255, 1230. 1140, 1070, and 1030 cm '; mass spectrum [(+) FAB], m/z
562/564
(M + Na)', Anal. Calcd. for CZ,H3oCINO'3 ~ 0.5 H20: C, 45.95; H, 5.69; N,
2.55,
Found: C, 45.81; H, 5.82; N, 2.39.
step 3
N-{5-[(4',6'-O-Benzylidene-p-n-maltosyl)-oxy-methyl]-2-chloro-phenyl}-
carbamic acid methyl ester
The title compound was prepared as a white solid (0.552 g, 71 %) from { 2-
chloro-5-[(p-D-maltosyl)-oxy-methyl]-phenyl }-carbamic acid methyl ester using
a
procedure similar to Example 24, mp 142-145 °C; 'H NMR (DMSO-d6) s 3.06-
3.13
(m, 1H), 3.28-3.41 (m, 4H), 3.46 (td, J = 2.4, 8.8 Hz, 1H), 3.50-3.61 (m, 2H),
3.65 (s,
3H), 3.65-3.75 (m, 3H), 4.11 (dd, J = 3.1. 8.1 Hz, 1H), 4.30 (d, J = 7.7 Hz,
IH), 4.64-
4.69 (m, 1H), 4.68 (ABq, J = 12.5 Hz, es = 0.22, 2H), 5.14 (d, J = 3.7 Hz,
1H), 5.26
(d, J = 5.1 Hz. 1H), 5.30 (d, J = 4.8 Hz, 1H), 5.51 (d, J = 2.9 Hz, 1H), 5.57
(s, 1H),
5.63 (d, J = 6.4 Hz, 1H), 7.22 (dd, J = 2.0, 8.1 Hz, 1H), 7.34-7.38 (m, 3H),
7.41-7.46
(m, 3H), 7.54 (d, J = 1.8 Hz, 1H)> 9.07 (s, 1H); IR (KBr) 3530, 3410, 2920,
2850,
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-95-
1730, 1590, 1535, 1450, 1420, 1375, 1310, 1250, 1230, l I45, 1075, 1030, and
1000
cm-'; mass spectrum [(+) FAB], m/z 650/652 (M + Na)'; Anal. Calcd. for
C~8H3,C1N0" ~ 0.5 H20: C, 52.79; H, 5.54; N, 2.20, Found: C, 52.85; H, 5.77;
N,
2.11.
Example 81
N-15-f(6-O-Benzovl-4'.6'-O-benzvlidene-f3-D-maltosyl)-ox -~methyll-2-chloro-
phenyl?-carbamic acid methyl ester
The title compound was prepared as a white solid (0.407 g, 71 %) from N-{ 5-
[(4',6'-O-benzylidene-~-D-maltosyl)-oxy-methyl]-2-chloro-phenyl }-carbamic
acid
methyl ester using a procedure similar to Example 2, mp >103 °C
(decomp.); 'H
NMR (DMSO-db) s 3.16-3.22 (m, 1H), 3.27-3.42 (m, 2H), 3.48-3.63 (m, 4H), 3.63
(s, 3H), 3.70 (dd, J = 5.1, 10.1 Hz, 1H), 3.73-3.79 (m, 1H), 4.05 (dd, J =
4.8, 9.9 Hz,
1H), 4.35 (dd, J = 5.1, 12.1 Hz, 1H), 4.40 (d, J = 7.7 Hz, 1H), 4.58-4.63 (m,
1H),
4.66 (ABq, J = 12.5 Hz, es = 0.I4, 2H), 5.14 (d, J = 4.0 Hz, 1H), 5.35 (dd, J
= 5.3,
8.3 Hz, 2H), 5.52 (s, 1H), 5.57 (d, J = 3.1 Hz, 1H), 5.80 (d, J = 6.2 Hz, 1H),
7.19 (dd,
J = 2.0, 8.1 Hz, 1H), 7.33-7.43 (m, 6H), 7.50-7.55 (m, 3H), 7.63-7.68 (m, 1H),
8.00
(dd, J = 1.1, 8.1 Hz, 2H), 9.05 (s, 1 H); IR (KBr) 3420, 3080, 2920, 2860,
1725, 1640,
1590, 1530, 1445, 1425, 1370, 1320, 1275, 1220, 1140, 1070, 1025, and 720 cm-
';
mass spectrum [(+) ESI], m/z 7321734 (M + H)+, 754/756 (M + Na)'; Anal. Calcd.
for
C35H,8C1N0": C, 57.42; H, 5.23; N, 1.91, Found: C, 57.17; H, 5.26; N, 1.81.
Example 82
N-15-f (6-O-l3-Benz~l-1-oxo-propyl)-4',6'-O-benzYlidene-a-D-maltosyl)-oxy
methvll-2-chloro-nhenyl }-carbamic acid methyl ester
The title compound was prepared as a white solid (0.152 g, 42%) from N-{5-
[(4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-chloro-phenyl}-carbamic acid
methyl ester using hydrocinnamoyl chloride and a procedure similar to Example
2,
mp >93 °C (decomp.); 'H NMR (DMSO-db) s 2.66 (t, J = 7.7 Hz, 2H), 2.84
(t, J = 7.7
Hz, 2H), 3.08-3.16 (m, 1H), 3.27-3.49 (m, 4H), 3.53-3.60 (m, 2H), 3.62-3.72
(m,
2H), 3.63 (s, 3H), 4.05-4.13 (m> 2H), 4.31-4.37 (nu, 2H), 4.63 (ABq, J = 12.5
Hz, es
= 0.15, 2H), 5.09 (d, J = 3.7 Hz> 1H), 5.34 (t, J = 5.5 Hz, 2H), 5.55-5.58 (m,
2H),
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-96-
5.82 (d, J = 6.2 Hz, 1H), 7.12-7.18 (m, 1H), 7.18-7.27 (m, SH), 7.34-7.39 (m,
3H),
7.41-7.46 (m, 3H), 7.54 (d, J = 1.8 Hz, 1H), 9.07 (s, 1H); IR (KBr) 3410,
3080, 3030,
2920, 2850, 1735, 1590, 1530, 1450, 1425, 1375, 1310, 1250, 1220, 1145, 1070,
1030, 750, and 700 cm~'; mass spectrum [(-) ESI], m/z 758 (M - H)-; Anal.
Calcd. for
C3,H;_C1N0,4 ~ 1.0 H20: C, 57.11; H, 5.70; N, 1.80, Found: C, 57.30; H, 5.52;
N,
1.77.
Example 83
N-(5-f(4',6'-O-Benzvlidene-B-D-maltos ly )-ox -y methyll-2-chloro-phenyll-
methanesulfonamide
step 1
N-{5-[(2,2',3,3',4',6,6'-Hepta-O-acetyl-p-n-maltosyl)-oxy-methyl)-2-chloro-
phenyl}-methanesulfonamide
To a stirred solution of NaH (0.0467 g, 1.17 mmol) and CH2C12 (10 mL) at rt
was added 2-chloro-5-(hepta-O-acetyl-p-D-maltosyl-oxymethyl)-phenylamine
{0.755
mg, 0.973 mmol). After 10 min., MsCI (0.0906 mL, 1.17 mmol) was added to this
solution dropwise, and the reaction was stirred at rt for 18 h. Another 2.4
eq. MsCI
added and continued stirring at rt for 144 h. Since reaction was only about
25%
complete, it was refluxed for 24 h. Then another 2.4 eq. MsCI added and
continued
refluxing for 12U h. The resulting solution was concentrated and then diluted
with
EtOAc (200 mL). This layer was washed with 1 N HCl (20 mL), sat. NaHC03 (20
mL), and brine (20 mL) and then dried (MgS04). After concentration, the oilly
residue was purified by flash chromatography ( 10:90 to 60:40 acetone:hexane
gradient) to afford the product (0.423 g, S 1 %) as a white foam, mp >73
°C
(decomp.); 'H NMR (DMSO-db) s 1.93 (d, J = 1.8 Hz, 3H), 1.94 (d, J = 1.8 Hz,
3H),
1.95 (d, J = 1.5 Hz, 3H), 1.97 (d, J = 0.9 Hz, 6H), 2.01 (d, J = 1.5 Hz, 3H),
2.08 (d, J
= 1.5 Hz, 3H), 3.02 (d, J = 1.3 Hz, 3H), 3.90-4.04 (m, 4H), 4.11-4.23 (m, 2H),
4.38
(d, J = 11.6 Hz. 1H), 4.56 (d, J = 12.7 Hz, 1H), 4.68-4.77 (m, 2H), 4.82-4.89
(m,
2H), 4.97 (t, J = 9.7 Hz, 1H), 5.20 (t, J = 9.4 Hz, IH), 5.27 (d, J = 3.1 Hz,
1H), 5.27-
5.34 (m, 1H), 7.15 (d, J = 8.3 Hz, 1H), 7.35 (s, 1H), 7.50 (dd, J = 1.5, 8.3
Hz, 1H),
9.47 (s, IH); IR (KBr) 3480, 3260, 3010, 2950, 1755, 1590, 1495, 1420, 1375,
1355,
1230. 1140, 1045, 975, 900, and 755 cm-'; mass spectrum [(+) FAB], m/z 854 (M
+
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-97-
H)', 876 (M + Na)', Anal. Calcd. for C3,H4,C1NO,~S ~ 1.25 HBO: C, 46.58; H,
5.35; N,
1.60, Found: C, 46.22; H, 4.93; N, 1.49.
step 2
N-{5-[(p-~-Maltosyl)-oxy-methyl]-2-chloro-phenyl}-methanesulfonamide sodium
salt
The title compound was prepared as a white solid (0.310 g, 71 %) from N { 5-
[(2,2',3,3',4',6,6'-hepta-O-acetyl-p-D-maltosyl)-oxy-methyl]-2-chloro-phenyl }-
methanesulfonamide using 1.3 eq. 25 weight % NaOMe in MeOH (because of
sulfonamide acidity) and a procedure similar to step 4 of Example 1, mp ,189
°C
(decomp.);'H NMR (DMSO-db) s 2.54 (s, 3H), 3.05 (t, J = 8.1 Hz, 2H), 3.1b (s,
1H),
3.18-3.24 (m, 2H), 3.27-3.42 (m, 2H), 3.42-3.51 {m, 2H), 3.53-3.63 (m, 2H),
3.72 (d,
J = 11.2 Hz, 1H), 4.24 (d, J = 7.7 Hz, 1H), 4.44-4.54 (bs, 1H), 4.50 (ABq, J =
11.6
Hz, os = 0.24, 2H), 4.54-4.60 (bs, 1H), 4.80-4.93 (bs, 2H), 5.01 (d, J = 3.7
Hz, 1H),
5.09-5.18 (bs, 1H), 5.27-5.55 (bs, 2H), 6.47 (dd, J = 2.0, 7.9 Hz, 1H), 7.05
(d, J = 7.9
Hz, 1H), 7.19 (d, J = 2.0 Hz, 1H); IR (KBr) 3410, 2920, 1630, 1590, 1475,
1420,
1375, 1310, 1215, 1140, 1110, 1075, and 1020 cm'''; mass spectrum [(+) FAB],
m/z
582 (M + Na)', Anal. Calcd. for CZOH29C1NO,3S ~ Na ~ 3.5 H20: C, 37.24; H,
5.63; N,
2.17, Found: C, 37.00; H, 5.10; N, 2.13.
step 3
N-{5-[(4',6'-O-Benzylidene-(3-D-maltosyl)-oxy-methyl]-2-chloro-phenyl}-
methanesulfonamide
The title compound was prepared as a white solid (0.198 g, 55%) from N {5-
[(p-D-maltosyl)-oxy-methyl]-2-chloro-phenyl }-methanesulfonamide sodium salt
using a procedure similar to Example 24, mp >93 °C (decomp.); 'H NMR
(DMSO-
db} s 3.05 (s, 3H), 3.07-3.14 (m, 1H), 32.6-3.42 (m, 4H), 3.42-3.48 (m, 1H),
3.50-
3.59 (m, 2H), 3.63-3.75 (m, 3H), 4.l I (d, J = 5.4 Hz, 1H), 4.30 (d, J = 7.7
Hz, IH),
4.66 (t, J = 6.6 Hz, IH), 4.71 (ABq, J = 12.7 Hz, es = 0.19, 2H), 5.14 (d, J =
3.7 Hz,
1H), 5.29 (t, J = 4.2 Hz, 2H), 5.51 (d, J = 3.1 Hz, 1H), 5.57 (s, 1H), 5.63
(d; J = 6.6
Hz, 1H), 7.30 (dd, J = 1.8, 8.1 Hz, 1H), 7.34-7.39 (m, 3H), 7.42-7.47 (m, 3H),
7.49
(d, J = 8.1 Hz, 1H), 9.44 (s, 1H); IR (KBr) 3410, 2910, 2840, 1630, 1590,
1495,
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-98-
1445, 1385, 1345, 1235, 1160, 1070, 1025, 990, and 755 cm-'; mass spectrum [(-
)
FAB], m/z 646 (M - H)-; Anal. Calcd. for CZ~H3;C1N0,3S ~ 2.0 HzO: C, 47.40; H,
5.60; N, 2.05, Found: C, 47.09; H, 4.99; N, 1.98.
Example 84
N-15-f(6-O-Benzovl-4',6'-O-benzvlidene-(~-D-maltosvl)-oxv-methyll-2-cyano-
phenyl ~-acetamide
step 1
«-Bromo-2-vitro p-tolunitrile
A stirred mixture containing 4-methyl-2-nitrobenzonitrile (2.04 g, 12.6
mmol), N-bromosuccinimide (2.24 g, 12.6 mmol) and azobisisobutyronitrile
(0.103
g, 0.630 mmol) in CCI, (50 mL) was irradiated with a 300 watt flood light for
2 h.
The reaction was diluted with CH2C12 (50 ml), filtered and concentrated.
Purification
by flash chromatography (35 and 40% ether/pet. ether gradient) gave 1.44 g
(47%) of
the title compound as a yellow oil. 'H NMR (DMSO-db) s 4.90 (s, 2 H), 8.05
(dd, J =
8.0, 1.5 Hz, 1 H), 8.18 (d, J = 8.0, 1 H), 8.52 (s, 1 H).
step 2
«-Hydroxy-2-vitro p-tolunitrile
A stirred solution containing «-bromo-2-vitro p-tolunitrile (1.228 g, 5.095
mmol) and sodium formate (0.8664 g, 12.74 mmol) in ethanol:water (4:1, 25 mL)
was refluxed for 2 h. The reaction was cooled to room temperature, diluted
with 20%
CHZClz/EtOAc, washed with H20 (3x), dried (MgS04) and concentrated.
Purification
by flash chromatography (1,2 and 3% MeOH/CHCI, gradient) gave 0.695 g (77%) of
the title compound as a white solid. 'H NMR (DMSO-db) s 4.71 (d, 2 H), 5.75
(t, 1
H), 7.89 (dd, J = 7.9 Hz, 1 H), 8.14 (d, J = 7.9 Hz, l H), 8.32 (s, 1 H).
step 3
5-[(Hepta-O-acetyl-p-D-maltosyl)-oxy-methyl]-2-cyano-1-nitrobenzene
At ambient temperature, to a stirred solution of acetobromomaltose (2.39 g,
3.41 mmol), a-hydroxy-2-vitro p-tolunitrile (0.789 g, 4.43 mmol) and HgBr2
(1.60 g,
4.43 mmol) in freshly distilled CH3CN (34 mL) was added in one portion Hg(CN)
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
-99-
( 1.12 g, 4.43 g, mmol). After 16 h, brine (SU mL) was added and the mixture
was
extracted with 10% CHZCh/EtOAc. The combined organic extracts were washed
with brine (3x), dried (MgS04) and concentrated. Purification by flash
chromatography (1,2 and 3% MeOH/CHC13 gradient) gave 1.941 g (71%) of the
title
compound as a foam. An analytical sample was obtained by cyrstallization from
EtOAc/Hexane followed by recyrstallization from EtOH to give a colorless
solid, mp
155 - 157 °C; 'H NMR (DMSO-db) s 1.93 (s, 3 H), 1.94 (s, 3 H), 1.97 (s,
3 H), 1.99
(s, 3 H), 2.01 (s, 3 H}, 2.06 (s, 3 H), 3.93 - 4.01 (m, 4 H), 4.36 (d, J =
11.0 Hz, 1 H),
4.77 (dd, J = 9.6, 8.0 Hz, 1 H), 4.83 - 4.88 (m, 2 H}, 4.93 - 5.00 (m, 3 H),
5.21 (dd, J
= 10.3, 9.7 Hz, 1 H), 5.27 (d, J = 3.7 Hz, 1 H), 5.30 - 5.34 (m, 1 H), 7.84
(dd, J = 7.9,
1.5 Hz. 1 H), 8.18 {d, J = 7.9 Hz, 1 H), 8.27 (s, 1 H). IR (KBr) 3450, 2950,
2225,
1750, 1225 and 1050 cm-'. mass spectrum [(+) FAB] m/z 797 (M + H)'. Anal.
Caled.
for C3,H,oN~O~o: C, 51.26; H, 5.06; N, 3.52. Found: C, 51.06; H, 5.02; N,
3.31.
step 4
5-[(Hepta-O-acetyl-p-maltosyl)-oxy-methyl]-2-cyano-phenylamine
A stirred mixture containing 5-[(hepta-O-acetyl-p-D-rnaltosyl)-oxy-methyl]-2-
cyano-1-nitrobenzene (1.491 g, 1.872 mmol) iron powder (0.3658 g, 6.550 mmol)
and glacial acetic acid (7 mL) in 2-propanol (7 mL) was heated at 75°C
for 2 h. To
the reaction was added activated charcoal and the hot solution was filtered
through a
sulka ploc pad, rinsing with EtOAc. The filtrate was washed with H20 (3x),
with sat.
aq. NaHC03 (3x), dried (NazS04) and concentrated. Purification by flash
chromatography (1,2 and 3% MeOH/CHCI3 gradient) gave 1.04 g (72%) of the title
compound. 'H NMR (DMSO-db) s 1.94 (s, 3 H), 1.95 (s, 3 H), 1.96 (s, 3 H}, 1.98
(s,
6 H), 2.02 (s, 3 H), 2.09 (s> 3 H), 3.95 - 4.U2 (m, 4 H), 4.14 - 4.22 (m, 2
H), 4.36 -
4.4U (m, I H}. 4.Sb (ABq, J = 13.2 Hz, es = O.U9, 2 H}, 4.72 (dd, J = 9.4, 8.2
Hz, 1
H), 4.98 (t, J = 9.7 Hz, 1 H), 5.19 - 5.37 (m, 3 H), 6.06 (s, 1 H), 6.49 {dd,
J = 8. l, 1.0
Hz, I H), 6.66 (s, 1 H), 7.36 (d, J = 8.1 Hz, 1 H). mass spectrum [(+)FAB],
m/z 767
(M + H)'.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
- 100 -
step 5
N-{5-[(Hepta-O-acetyl-p-D-maltosyl)-oxy-methyl]-2-cyano-phenyl}-acetamide
At ambient temperature, to a solution of 5-[(hepta-O-acetyl-p-maltosyl)-oxy-
methyl]-2-cyano-phenylamine (0.280 g, 0.365 mmol) in CHZCh (3.6 mL) was added
60% NaH/mineral oil ( 14.6 mg, 0.365 mmol) and the reaction was stirred for
0.5 h.
To the reaction was added acetyl chloride (31.3 ~L, 0.438 mmol) and the
reaction
was stirred for 16 h. The reaction was quenched with sat. aq. NaHC03 (25 mL)
and
extracted with EtOAc. The combined organic extracts were dried (Na2S0,) and
concentrated. Purification by flash chromatography (1,2 and 3% MeOH/CHCI,
gradient) gave 0.249 g (84%) of the title compound. An analytical sample was
obtained by cyrstallization from EtOAc/Hexane to give a colorless solid, mp 85
-
95°C; 'H NMR (DMSO-d6) s 1.93 (s, 3 H), 1.94 (s, 3 H), 1.95 (s, 3 H),
1.97 (s, 6 H),
2.01 (s, 3 H), 2.07 (s, 3 H), 2.08 (s, 3 H), 3.92 - 4.01 (m, 4 H), 4.13 - 4.21
(m, 2 H),
4.37 (dd, J = 12.0, 2.1 Hz, 1 H), 4.73 (ABq, J = 13.8 Hz, es = 0.07, 2 H),
4.73 (dd, J
= 9.5, 8.0 Hz, 1 H), 4.84 - 4.89 (m, 2 H), 4.97 (t, J = 9.8 Hz, 1 H), 5.21
(dd, J = 10.3,
9.7 Hz, 1 H), 5.27 - 5.33 (m, 2 H), 7.21 (dd, J = 8.0, 1.4 Hz, 1 H), 7.48 (s,
1 H), 7.78
(d, J = 8.0 Hz, 1 H), 10.15 (s, 1 H). IR (KBr) 3400, 2950, 2225, 1750, 1240
and 1050
cm'. mass spectrum [(+) ESI], m/z 809 (M + H)'. Anal. Calcd. for C36H44N2019'
C,
53.47; H, 5.84; N, 3.46. Found: C, 53.55; H, 5.41; N, 3.40.
step 6
N-{5-[(p-v-Maltosyl)-oxy-methyl]-2-cyano-phenyl}-acetamide
At ambient temperature, to a stirred solution of N { 2-cyano-[5-
(2,2',3,3',4',6,6'-hepta-O-acetyl-p-D-maltosyl)-oxy-methyl]-phenyl}-acetamide
(2.31
g, 2.86 mmol) in MeOH (70 mL) was added in one portion potassium cyanide (92.9
mg, 1.43 mmol). After 3 h, the reactions was concentrated in vactco.
Purification by
preparative HPLC (C18, 20% CH3CN:HzO) and gave 1.18 g (80%) of the title
compound; 'H NMR (DMSO-db) s 2.08 (s, 3H), 3.03-3.17 (m, 2H), 3.20-3.49 (m,
7H), 3.50-3.64 (m, 2H), 3.71-3.75 (m, 1H), 4.31 (d, J = 7.6 Hz, 1H), 4.51-4.55
(m,
2H), 4.64-4.78 (m, 3H), 4.88-5.00 (m, 2H), 5.02 (d, J = 3.7 Hz, 1H), 5.29-5.53
(m,
3H), 7.38 (dd, J = 8.1, 1.1 Hz, 1H), 7.56 (s, 1H), 7.77 (d, J = 8.1 Hz, 1H),
7.80 (s,
1H).
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
- 101 -
step 7
N-{5-[(4',6'-O-Benzylidene-p-D-maltosyl)-oxy-methyl]-2-cyano-phenyl}-
acetamide
The title compound was prepared as a solid (0.682 g, 57%) from N-{5-[(p-D-
maltosyl)-oxy-methyl]-2-cyano-phenyl }-acetamide using a procedure similar to
Example 24; 'H NMR (DMSO-db) s 2.09 (s, 3H), 3.13-3.16 (m, 2H), 3.35-3.73 (m,
9H), 4.12-4.13 (m, 1 H), 4.34 (d, J = 7.8 Hz, 1 H), 4.65-4.70 (m, 2H), 4.91
(d, J = 13.6
Hz, 1H), 5.15 (d, J = 3.8 Hz, 1H), 5.32 (m, 2H), 5.55-5.58 (m, 3H), 7.36-7.47
(m,
6H), 7.56 (s, 1H), 7.78 (d, J = 8.2 Hz, 1H), 10.17 (s, 1H).
step 8
N-{5-[(6-O-Benzoyl-4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-cyano-
phenyl}-acetamide
The title compound was prepared as a white solid (0.173 g, 49%) from N-{5-
[(4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-cyano-phenyl}-acetamide
using
a procedure similar to Example 2, mp 122-129 °C'.; 'H NMR (DMSO-db) s
2.05 (s,
3H), 3.21 (t, 1H), 3.34-3.41 (m, 2H), 3.53-3.64 (m, 4H), 3.71-3.77 (m, 2H),
4.03 (dd,
1H), 4.34 (dd, J = 12.2, 4.9 Hz, 1H), 4.42 (d, J = 7.7 Hz, 1H), 4.59 (d, 1H),
4.75
(ABq, J = 13.7 Hz, e~ = 0.06, 2H), 5.14 (d, J = 4.0 Hz, 1H), 5.35 (br. s> 1H),
5.41 (br.
s, 1H), 5.52 (s, 1H), 5.58 (br. s> 1H), 5.82 (br. s, 1H), 7.34-7.37 (m, 4H),
7.40-7.42
(m, 2H), 7.51-7.54 (m, 3H), 7.65 (t, 1H), 7.73 (d, 3 = 7.9 Hz, 1H), 7.97-8.00
(m, 2H),
10.13 (s, 1H); IR (KBr) 3400, 2900, 2200, 1710, 1275 and 1065 cm~'; mass
spectrum
[(+) ESI], m/z 724 (M + NH4)'; Anal. Calcd. for C36H3HNzO~3 ~ 0.5 H20: C,
60.42; H,
5.49 N, 3.91, Found: C, 60.36; H, 5.22; N, 3.91.
Example 85
N-(5-f(6-O-Benzoyl-4',6'-O-benzylidene-D-D-maltosyl)-ox -~yll-2-methyl-
phenyl }-acetamide
The title compound was prepared as a colorless solid (1.30 g, 60%) from N-
{ 5-[(4',6'-O-benzylidene-p-D-maltosyl)-oxy-methyl]-2-methyl-phenyl }-
acetamide
using a procedure similar to Example 2, mp 193-198 °C; 'H NMR (DMSO-
~l6) s 2.00
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
- 1 U2 -
(s, 3H), 2.15 (s, 3H), 3.17 (t, J = 8.4 Hz, IH), 3.34-3.40 (m, 2H), 3.48-3.62
(m, 4H),
3.69-3.77 (m, 2H), 4.06 (dd, 1H), 4.33-4.38 (m, 2H), 4.60 (ABq, J = 11.9 Hz,
ns =
O.UB, 2H), 4.61 (d, J = 1U.5 Hz, IH), 5.14 (d, J = 4.0 Hz, IH), 5.33 (br. s,
2H), 5.52
(s, 1H), 5.56 (br. s, IH), 5.79 (br. s, 1H), 7.06 (dd, 1H), 7.11 (d, J = 7.9
Hz, 1H),
7.34-7.37 (m, 4H), 7.40-7.43 (m, 2H), 7.51-7.55 (m, 2H), 7.63-7.68 (m, 1H),
7.99-
8.01 (m, 2H), 9.26 (s, 1H); IR (KBr) 3250, 2900, 1725, 1650, 1275 and 1070 cm-
';
mass spectrum [(+) ESI], m/z 696 (M + H)'; Anal. Calcd. for C36H4tN013' C,
62.15;
H, 5.94; N, 2.01, Found: C, 62.20; H, 6.02; N, 2.04.
Example 86
6-f-6-(4-Chloro-3-nitro-benzylsulfanyl)-4,5-dihvdroxy-2-hvdroxymeth,yl-
tetrahydro-
p~rran-3-vloxyl-2-phenyl-hexahydro-pyranof3,2-dlf 1,31dioxine-7.8-diol
step 1
(4-Chloro-3-nitro-benzyl) -hepta-O-acetyl-1-thio-(3-D-maltoside
To a stirred solution of hepta-O-acetyl-1-thio-(3-maltose (2.0 g, 3.065 mmol)
in acetone (20 ml) were added 4-chloro-3-nitrobenzyl bromide (0.844 mg, 3.37
mmol) and a solution of potassium carbonate (0.423 mg, 3.065 mmol) in water
(I0
ml). The mixture was boiled under reflux for 30 min, cooled and concentrated.
The
residue was extracted with dichloromethane, and the combined extracts were
washed
with water, and brine, dried (MgSO,) and concentrated. Purification by flash
chromatography (40%-60% EtOAc/petroleum ether gradient) afforded 1.588 g (63%)
of the title compound as a white solid, mp 73-75 °C; 'H NMR (CDCl3) s
1.99 (s, 3
H), 2.00 (s, 3 H), 2.02 (s, 3 H), 2.03 (s, 6 H), 2.11 (s, 3 H), 2.15 (s, 3 H),
3.61 - 3.64
(m, I H), 3.80 (d, J = 13.6 Hz, 1H), 3.94 - 4.U0 (m, 3 H), 4.08 (dd, J = 12.3,
2.4 Hz, 1
H), 4.18 - 4.27 (m, 2 H), 4.36 (d, J = 9.9 Hz, 1H), 4.50 (dd, 3 = 12.1, 2.6
Hz, 1 H),
4.85 (dd, 3 = 10.5. 4.0 Hz, 1 H), 4.90 (apparant t, J = 9.9 Hz, 1 H), 5.05
(apparant t, J
= 9.9 Hz, 1 H), 5.23 (apparant t, J = 9.2 Hz, 1 H), 5.34 (apparant t, J = 9.7
Hz, 1 H),
5.40 (d, J = 4.0 Hz. 1 H), 7.47 (dd, J = 8.4, 2.0 Hz, 1 H), 7.51 (d, J = 8.4
Hz, 1H},
7.87 (d, J = 2.0, Hz, 1 H). IR (KBr) 3500, 2950, 1750, 125U and 1050 cm-',
mass
spectrum [(+) FAB], m/z 822 (M + H)', 844 (M + Na)'. Anal. Calcd. for
C33H,oCINO,~S: C, 48.21; H, 4.90; N, 1.70. Found: C, 47.75; H, 4.86; N, 1.65.
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
- 103 -
step 2
(4-Chloro-3-nitro-benzyl)-1-deoxy-1-thio-p-D-maltoside
The title compound was prepared as a white solid (0.513 g, 99%) from (4-
chloro-3-nitro-benzyl) -hepta-O-acetyl-1-thio-(3-D-maltoside using a procedure
similar to step 4 of Example 1, mp 90-93 °C; 'H NMR (DMSO-d6) s 3.03 -
3.74 (m, ,
11 H), 3.80 (d, J = 6.2 Hz, 1 H), 3.86 (d, J = 13.4 Hz, 1 H), 4.01 - 4.08 (m,
2 H), 4.58
(bd, 2 H), 4.98 (bd, 3 H), 5.20 - 5.67 (bs, 3 H), 7.65 - 7.72 (m, 2 H), 8.03
(d, J = 1.76
Hz, 1 H).IR (KBr) 3400, 2930, 1550, 1300 and1075 cm~', mass spectrum [(-)
FAB],
m/z 526 (M - H)-.Anal. Calcd. for C,9HZ6C1NO,~S ~ HZO: C, 41.80; H, 5.13; N,
2.56.
Found: C, 41.35; H, 4.89; N, 2.40.
step 3
6-[-6-(4-Chloro-3-nitro-benzylsulfanyl)-4,5-dihydroxy-2-hydroxymethyl-
tetrahydro-pyran-3-yloxy]-2-phenyl-hexahydro-pyrano[3,2-d][1,3]dioxine-7,8-
diol
The title compound was prepared as a white solid from (4-chloro-3-nitro-
benzyl)-1-deoxy-1-thio-~-D-maltoside using a procedure similar to Example 24,
mp
120-122 °C; 'H NMR (DMSO-db) s 3.07-3.24 (m, 2H), 3.24-3.43 (m, 3H),
3.47-3.58
(m, 3H), 3.64-3.75 (m, 3H), 3.95 (ABq, J = 13.4 Hz, es = 0.12, 2H), 4.08-4.13
{m,
2H), 4.77 (t, J = 5.5 Hz, 1H), 5.12 (d, J = 3.95 Hz, 1 H), 5.28 (d, J = 5.3,
Hz, 1H),
5.31 (d, J = 5.3, Hz, 1H), 5.56 (m, 2H), 5.65 (d, J = 6.4, Hz, 1H), 7.35-7.40
(m, 3H),
7.42-7.46 (m, 2H), 7.66-7.71 (m, 2H), 8.04 (d, J = 1.76 Hz, 1H); IR (KBr)
3450,
2930, 1550, 1300 and1075 cm-'; mass spectrum [(-) FAB], m/z 614 (M - H)~;
Anal.
Calcd. for C~6H3oC1NO,2S: C, 49.96; H, 5.1; N,2.24, Found: C, 49.42; H, 4.78;
N,
2.26.
Example 87
(4-Chloro-3-nitro-benzyl) 6-O-benzoyl-4',6'-O- benzoyl-4',6',-O-benzylidene-1-
thio-a-D-maltoside
The title compound was prepared as a white solid from 6-[-6-(4-chloro-3-
nitro-benzylsulfanyl)-4,5-dihydroxy-2-hydroxymethyl-tetrahydro-pyran-3-yloxy]-
2-
phenyl-hexahydro-pyrano[3,2-d][1,3]dioxine-7,8-diol using a procedure similar
to
CA 02350066 2001-05-09
WO 00/31096 PCT/US99/27828
- 104 -
Example 2, mp 105-107 °C; 'H NMR (DMSO) s 3.17-3.23 (m, 1H), 3.27-
3.42 (m,
2H), 3.46-3.51 (m, 1H), 3.53-3.62 (m, 3H}, 3.69-3.76 (m, 2H), 3.91 (q, J =
14.1 Hz,
2H), 4.06 (dd, J = 10.3, 4.8 Hz, 1H), 4.28 - 4.34 (m, 2H), 4.62 (d, J = 10.5
Hz, 1H),
5.13 (d, J = 3.7 Hz, 1H), 5.34 (d, J = 5.3 Hz, IH), 5.41 (d, J = 6.2 Hz, 1H),
5.53 (s,
1 H), 5.64 (d, J = 2.9 Hz, 1 H), 5.80 (d, J = 6.1 Hz, 1 H), 7.31 (m, 3H), 7.40-
7.43 (m,
2H), 7.47 (t, J = 5.7 Hz, 2H), 7.59-7.65 (m, 3H), 7.95-7.98 (m, 3H); IR (KBr)
3400,
2930, 1745, 1550, 1255 and1075 cm-'; mass spectrum [(+) FAB], m/z 720 (M +
H)y,
742 (M + Na)'; Anal. Calcd. for C3,H3,C1NO,3S: C, 55.04; H, 4.76; N, 1.95,
Found:
C, 55.36; H, 4.89; N, 1.91.