Note: Descriptions are shown in the official language in which they were submitted.
CA 02351953 2001-06-29
t
Clariant GmbH 2000DE123 Dr. HU/sch
Description
Use of iron azo complex compounds as charge control agents
The present invention is described in the German priority application No.
10032138,
filed 1 July 2000, which is hereby incorporated by reference as is fully
disclosed
herein.
The present invention lies within the field of charge control agents, i.e.,
components
which selectively influence electrostatic charging in a matrix.
In electrophotographic recording processes a latent charge image is produced
on a
photoconductor. This latent charge image is developed by applying an
electrostatically charged toner which is then transferred to, for example,
paper,
textiles, foils or plastic and is fixed by means, for example, of pressure,
radiation,
heat or the action of solvent. Typical toners are one- or two-component powder
toners (also known as one- or two-component developers); also used are
specialty
toners, such as magnetic toners, liquid toners or polymerization toners, for
example.
By polymerization toners are meant those toners which are formed by, for
example,
suspension polymerization (condensation) or emulsion polymerization and lead
to
improved particle properties in the toner. Also meant are those toners
produced
basically in nonaqueous dispersions.
One measure of the quality of a toner is its specific charge q/m (charge per
unit
mass). In addition to the sign and level of the electrostatic charge, the
principal,
decisive quality criteria are the rapid attainment of the desired charge
level, the
constancy of this charge over an extended activation period and the
insensitivity of
the toner to climatic effects, such as temperature and atmospheric humidity.
Both positively and negatively chargeable toners are used in copiers and laser
printers, depending on the type of process and type of apparatus.
CA 02351953 2001-06-29
2
To obtain electrophotographic toners or developers having either a positive or
negative charge, it is common to add charge control agents. Since the charge
of
toner binders is often heavily dependent on the activation period, the
function of a
charge control agent is, on the one hand, to set the sign and level of the
toner
charge and, on the other hand, to counteract the charge drift of the toner
binder and
to provide for constancy of the toner charge. Another important practical
requirement
is that the charge control agents should have sufficient thermal stability and
good
dispersibility. Typical temperatures at which charge control agents are
incorporated
into the toner resins, when using kneading apparatus or extruders, are between
100°C and 200°C. Accordingly, thermal stability at 200°C
is of great advantage. It is
also important for the thermal stability to be ensured over a relatively long
period
(about 30 minutes) and in a variety of binder systems.
For effective dispersibility it is of advantage for the charge control agent
not to
exhibit any waxlike properties or any tackiness and to have a melting or
softening
point of >150°C, better still >200°C. Tackiness frequently leads
to problems in the
course of metered addition to the toner formulation, and low melting or
softening
points may result in failure to achieve homogeneous distribution in the course
of
incorporation by dispersion, since the material amalgamates in the form of
droplets
in the carrier material.
Typical toner binders are addition polymerization, polyaddition and
polycondensation
resins, such as styrene, styrene-acrylate, styrene-butadiene, acrylate,
polyester and
phenol-epoxy resins, and also cycloolefin copolymers, individually or in
combination,
which may also include further components, examples being colorants, such as
dyes
and pigments, waxes or flow assistants, or may have these components added
subsequently, such as highly disperse silicas.
Charge control agents may also be used to improve the electrostatic charge of
powders and coating materials, especially in triboelectrically or
electrokinetically
sprayed powder coating materials as are used to coat surfaces of articles made
from, for example, metal, wood, plastic, glass, ceramic, concrete, textile
material,
CA 02351953 2001-06-29
3
paper or rubber. The powder coating material, or the powder, receives its
electrostatic charge, in general, by one of the two following methods:
In the case of the corona method, the powder coating material or powder is
guided
past a charged corona and is charged in the process; in the case of the
triboelectric
or electrokinetic method, the principle of frictional electricity is utilized.
It is also
possible to combine the two methods. The powder coating material or powder in
the
spray apparatus receives an electrostatic charge which is opposite to the
charge of
its friction partner, generally a hose or spray pipe made, for example, from
polytetrafluoroethylene.
Typical powder coating resins employed are epoxy resins, carboxyl- and
hydroxyl-
containing polyester resins, polyurethane resins and acrylic resins, together
with the
customary hardeners. Resin combinations are also used. For example, epoxy
resins
are frequently employed in combination with carboxyl- and hydroxyl-containing
polyester resins.
It has additionally been found that charge control agents are able to improve
considerably the charging and the charge stability properties of electret
materials,
especially electret fibers (DE-A-43 21 289). Typical electret materials are
based on
polyolefins, halogenated polyolefins, polyacrylates, polyacrylonitriles,
polystyrenes or
fluoropolymers, for example polyethylene, polypropylene,
polytetrafluoroethylene
and perfluorinated ethylene and propylene, or on polyesters, polycarbonates,
polyamides, polyimides, polyether ketones, on polyarylene sulfides, especially
polyphenylene sulfides, on polyacetals, cellulose esters, polyalkylene
terephthalates,
and mixtures thereof. Electret materials, especially electret fibers, can be
used, for
example, to filter (very fine) dusts. The electret materials can receive their
charge by
corona or triboelectric charging.
Additionally, charge control agents can be used in electrostatic separation
processes, especially in processes for the separation of polymers. For
instance,
using the example of the externally applied charge control agent
trimethylphenylammonium tetraphenylborate, Y. Higashiyama et al. (J.
Electrostatics
30 (1993) 203 - 212) describe how polymers can be separated from one another
for
CA 02351953 2001-06-29
4
recycling purposes. Without charge control agents, the triboelectric charging
characteristics of low-density polyethylene (LDPE) and high-density
polyethylene
(HDPE) are extremely similar. Following the addition of charge control agent,
L_DPE
takes on a highly positive and HDPE a highly negative charge, and the
materials can
thus be separated easily. In addition to the external application of the
charge control
agents it is also possible to incorporate them into a polymer in order, for
example, to
shift the position of the polymer within the triboelectric voltage series and
to obtain a
corresponding separation effect. In this way it is possible to separate other
polymers
as well, such as polypropylene (PP) and/or polyethylene terephthalate (PET)
and/or
polyvinyl chloride (PVC), from one another.
Salt minerals can likewise be separated if they are admixed beforehand
(surface
conditioning) with an agent which improves the substrate-specific
electrostatic
charging (A. Singewald et al., Zeitschrift fur Physikal. Chem. 124 (1981 ) 223
- 248).
Charge control agents are employed, furthermore, as "electroconductivity
providing
agents" (ECPAs) in inks for inkjet printers (JP-05-163 449).
JP-A-62-129 358 discloses iron azo complex compounds containing unsubstituted
naphthyl radicals, but having poor light stability and a level of charging
which is in
need of improvement.
The object of the present invention was to find effective and
ecotoxicologically
compatible charge control agents, containing in particular no toxic heavy
metals.
Furthermore, these compounds should be readily dispersible, without
decomposition, in various toner binders employed in practice, such as
polyesters,
polystyrene-acrylates or polystyrene-butadienes/epoxy resins and also
cycloolefin
copolymers. Furthermore, their action should be largely independent of the
resin/carrier combination, in order to open up broad applicability. They
should
likewise be readily dispersible, without decomposition, in common powder
coating
binders and electret materials, such as polyester (PES), epoxy, PES-epoxy
hybrid,
polyurethane, acrylic systems and polypropylenes.
CA 02351953 2001-06-29
In terms of their electrostatic efficiency the charge control agents should be
active
even at very low concentration (1 % or less) and should not lose this
efficiency when
in conjunction with carbon black or other colorants. It is known of colorants
that they
can affect - in some cases lastingly - the triboelectric charging of toners.
5
Furthermore, the compounds used in accordance with the invention ought to be
suitable for use as colorants in inkjet inks, so that good water solubility
and high light
stability are desirable.
Surprisingly it has now become evident that iron azo complex compounds
described
below have advantageous charge control properties, especially a high negative
charge, and high thermal stabilities, the charge control property being lost
neither by
combination with carbon black nor by combination with other colorants.
Furthermore,
the compounds are readily compatible with the customary toner, powder coating
and
electret binders and are easy to disperse. Moreover, the compounds are readily
water-soluble and possess high light stability.
The present invention provides for the use of iron azo complex compounds of
the
formula (1 ) as charge control agents in electrophotographic toners and
developers,
in powder coating materials, electret materials and in electrostatic
separation
processes, in inkjet inks and in color filters,
- CA 02351953 2001-06-29
6
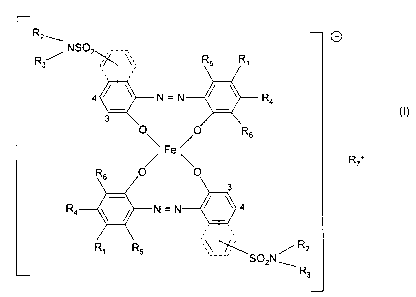
R2w
NS02 ,____,\
R3 y ~~, Rs R~
,,
,,
N=N ~ ~ R
4
3 (I)
O R6
Fe /
R+
R4 I =
R
R~ R5 ____. S02N'
R3
in which
R~ is hydrogen or a radical of the formula
Alkyl;
- N. ~ / ~ ~ oder
N
R2 and R3 are identical or different and are hydrogen, alkyl, alkoxyalkyl,
cycloalkyl
or aryl;
R4 is hydrogen or hydroxyl;
R5 is hydrogen, alkyl, alkoxyalkyl or cycloalkyl; and
R6 is hydrogen or a group of the formula (2)
CA 02351953 2001-06-29
7
,___ (2)
';
-N =
R2
H 2 3~ S02N/ and
~R
3
R~+ is ammonium or aliphatic, alicyclic or heterocyclic ammonium.
In the above definitions of the radicals R' to R6, "alkyl" is preferably (C~-
C4)-alkyl,
especially methyl, ethyl, n-propyl, i-propyl, n-butyl and t-butyl.
"Alkoxyalkyl" is
preferably (C~-C4)-alkoxy-(C~-C4)-alkyl, especially methoxy(C~-C4)-alkyl, such
as
methoxypropyl for example.
"Cycloalkyl" is preferably C5-C6-cycloalkyl.
"Aryl" is preferably unsubstituted C6-Coo-aryl or C6-C,o-aryl substituted by
1, 2 or 3 of
the following substituents:
halogen, preferably CI and Br, OH, C~-C4-alkyl, C~-C4-alkoxy, cyano, N02,
C~-C4-alkylcarbonyl, SCN, C~-C4-alkoxycarbonyl, benzoyl, phenoxycarbonyl,
C~-C4-alkylcarbonyloxy, aminocarbonyl, mono-(C,-C4-alkyl)-aminocarbonyl,
di-(C~-C4-alkyl)-aminocarbonyl, mono-(C~-C4-alkoxy-C2-C4-alkyl)-aminocarbonyl,
di-(C~-C4-alkoxy-C2-C4-alkyl)-aminocarbonyl, aminosulfonyl, mono-(C~-C4-alkyl)-
aminosulfonyl, di-(C~-C4-alkyl)-aminosulfonyl, mono-(C~-C4-alkoxy-C2-C4-alkyl)-
aminosulfonyl, di-(C~-C4-alkoxy-C2-C4-alkyl)-aminosulfonyl and
phenylaminosulfonyl.
R~+ is preferably ammonium, mono-(C5-C2o-alkyl)-ammonium, di-(C5-C2o-alkyl)-
ammonium, tri-(C5-C2o-alkyl)ammonium, tri-(C5-C2o-alkyl-)methyl-ammonium,
preferably mono-C$-Cis-alkylammonium, di-(C8-C~6-alkyl)-ammonium, tri-
(C$-C~6-alkyl)-ammonium, with particular preference 4-amino-2,2,6,6-tetra-
(C~-CZ-alkyl)-piperidinium, 4-hydroxy-2,2,6,6-tetra(C~-C2-alkyl)-piperidinium,
4-keto-
2,2,6,6-tetra(C~-C2)alkyl-piperidinium and tri-(C$-C~$-alkyl-)methyl-ammonium.
Very particular preferred groups R~+ are monooctylammonium, 2-ethylhexyl-
ammonium, 4-amino-2,2,6,6-tetramethylpiperidinium, 4-hydroxy-2,2,6,6-
tetramethyl-
piperidinium, 4-keto-2,2,6,6-tetramethylpiperidinium and tri-(C~o-C~6-alkyl)-
methyl-
ammonium.
CA 02351953 2001-06-29
8
Preferably, the sulfamoyl groups are located in position 4 or 5 on the phenyl
ring, or
in position 4 or 6 on the naphthyl ring system.
Particularly preferred compounds in the context of the present invention are
those of
the formula (la)
R3R2NS02 ,---
<,
6 5
N=N ~ ~ ~ OH
_ 4
(la)
R6
Fe
R6 O O R
3 2 2 3
HO 4~ ~1 N-N
5 6
8 , ,,
;5
7 s S02NR2R3
or mixtures thereof, in which R2, R3 and R6 are as defined above and the group
-S02NR2R3 is in position 4 or 5 if the ring is a phenyl ring or is in position
4 or 6 if the
ring system is a naphthyl ring system.
The preparation of the compounds of the formula (I) is described in WO
98/05717.
The compounds of the formula (I) may be obtained as symmetrical compounds or
asymmetrical compounds. By symmetrical compounds are meant those in which
both radicals R6 are hydrogen or both radicals R6 are azosulfamoylphenyl of
the
formula (2). Asymmetrical compounds are those in which the radicals R6 each
have
different meanings.
CA 02351953 2001-06-29
9
Preference is given in the context of the present invention to the
asymmetrical
compounds, or to mixtures of asymmetrical with symmetrical compounds.
The iron azo complex compounds used in accordance with the invention can be
matched precisely to the particular resin/toner system. A further technical
advantage
of these compounds is that they are inert toward the various binder systems
and can
therefore be employed widely, it being particularly significant that they are
not
dissolved in the polymer matrix but rather are present as small, very finely
divided
solid structures. Furthermore, they exhibit high and generally constant charge
control
properties and also good thermal stabilities. Moreover, the Fe azo complex
compounds used in accordance with the invention are free-flowing and possess
good dispersibility.
Dispersion means the distribution of one substance within another, i.e. in the
context
of the invention the distribution of a charge control agent in the toner
binder, powder
coating binder or electret material.
It is known that crystalline substances in their coarsest form are present as
agglomerates. To achieve homogeneous distribution within the binder, these
agglomerates must be disrupted by the dispersing operation into smaller
aggregates
or, ideally, into primary particles. The particles of charge control agent
present in the
binder following dispersion should be smaller than 1 Nm, preferably smaller
than
0.5 pm, with a narrow particle size distribution being of advantage.
For the particle size, defined by the d5o value, there are optimum ranges of
activity
depending on the material. For instance, coarse particles (1 mm) can in some
cases
not be dispersed at all or can be dispersed only with considerable investment
of time
and energy, whereas very fine particles in the submicron range harbor a
heightened
safety risk, such as the possibility of dust explosion.
The particle size and form is established and modified either by the synthesis
and/or
by aftertreatment. The required property is frequently possible only through
controlled aftertreatment, such as milling and/or drying. Various milling
techniques
are suitable for this purpose. Examples of advantageous technologies are
airjet
mills, cutting mills, hammer mills, bead mills and impact mills.
CA 02351953 2001-06-29
The binder systems mentioned in connection with the present invention are,
typically, hydrophobic materials. High levels of water in the charge control
agent can
either oppose wetting or else promote dispersion (flushing). The practicable
moisture
content is therefore specific to the particular material.
5
The compounds employed in accordance with the invention feature the following
chemical/physical properties:
The water content, determined by the Karl-Fischer method, is between 0.001 %
and
30%, preferably between 0.01 and 25% and, with particular preference, between
0.1
10 and 15%, it being possible for the water to be in adsorbed and/or bonded
form, and
for its proportion to be adjusted by the action of heat at up to 200°C
and reduced
pressure down to 10-8 torr or by addition of water, or by storage under
defined air
humidity conditions.
The particle size, determined by means of evaluation by light microscope or by
laser
light scattering, and defined by the d5o-value, is between 0.01 pm and 1000
pm,
preferably between 0.1 and 500 Nm, and with very particular preference between
0.5
and 400 Nm. It is particularly advantageous if milling results in a narrow
particle size.
Preference is given to a range 0 (d95-d5o) of less than 500 Nm, in particular
less than
400 Nm.
The iron azo complex compounds employed in accordance with the invention can
also be combined with further positive or negative charge control agents in
order to
obtain good performance chargeabilities, the overall concentration of the
charge
control agents being judiciously between 0.01 and 50% by weight, preferably
between 0.05 and 20% by weight, with particular preference between 0.1 and 5%
by
weight, based on the overall weight of the electrophotographic toner,
developer,
powder or powder coating material.
Examples of suitable further charge control agents are:
triphenylmethanes; ammonium and immonium compounds, iminium compounds;
fluorinated ammonium and fluorinated immonium compounds; biscationic acid
amides; polymeric ammonium compounds; diallylammonium compounds; aryl
CA 02351953 2001-06-29
11
sulfide derivatives, phenol derivatives; phosphonium compounds and fluorinated
phosphonium compounds; calix(n)arenes, cyclically linked oligosaccharides
(cyclodextrins) and their derivatives, especially boric ester derivatives,
interpolyelectrolyte complexes (IPECs); polyester salts; metal complex
compounds,
especially salicylate metal complexes and salicylate nonmetal complexes,
hydroxycarboxylic acid metal complexes and hydroxycarboxylic acid nonmetal
complexes, benzimidazolones; azines, thiazines or oxazines, which are listed
in the
Colour Index as Pigments, Solvent Dyes, Basic Dyes or Acid Dyes.
Particular preference is given to the charge control agents specified below,
which
can be combined individually or in combination with one another with the iron
azo
complex compounds:
triphenylmethanes, as described for example in US-A-5 051 585;
ammonium and immonium compounds, as described for example in
US-A-5 051 676; fluorinated ammonium and fluorinated immonium compounds, as
described for example in US-A-5 069 994; biscationic acid amides, as described
for
example in WO 91/10172; diallylammonium compounds, as described for example
in DE-A-4 142 541, DE-A-4 029 652 or DE-A-4 103 610; alkyl sulfide
derivatives, as
described for example in DE-A-4 031 705; phenol derivatives, as described for
example in EP-A-0 258 651; phosphonium compounds and fluorinated
phosphonium compounds, as described for example in US-A-5 021 473 and US-A-5
147 748;
calix(n)arenes, as described for example in EP-A-0 385 580;
benzimidazolones, as described for example in EP-A-0 347 695;
cyclically linked oligosaccharides, as described for example in DE-A-4 418
842;
polyester salts, as described for example in DE-A-4 332 170;
cyclooligosaccharide compounds, as described for example in DE-A-197 11 260;
interpolyelectrolyte complexes, as described for example in DE-A-197 32 995;
salt-like structured silicates, as described for example in DE-A-199 57 245.
Also suitable, especially for liquid toners, are surface-active, ionic
compounds and
those known as metal soaps.
CA 02351953 2001-06-29
12
Particularly suitable are alkylated arylsulfonates, such as barium petronates,
calcium
petronates, barium dinonylnaphthalenesulfonates (basic and neutral), calcium
dinonylsulfonate or Na dodecylbenzenesulfonate, and
polyisobutylenesuccinimides
(Chevron's Oloa 1200).
Also suitable are soya lecithin and N-vinylpyrrolidone polymers.
Also suitable are sodium salts of phosphated monoglycerides and diglycerides
with
saturated and unsaturated substituents, AB diblock copolymers of A: polymers
of
2-(N;N)di-methylaminoethyl methacrylate quaternized with methyl p-toluene-
sulfonate, and B: poly-2-ethylhexyl methacrylate.
Also suitable, especially in liquid toners, are divalent and trivalent
carboxylates,
especially aluminum tristearate, barium stearate, chromium stearate, magnesium
octoate, calcium stearate, iron naphthalite and zinc naphthalite.
Also suitable are chelating charge control agents (EP 0 636 945 A1 ), metallic
(ionic)
compounds (EP 0 778 501 A1 ), phosphate metal salts, such as described in
JP-9-106107. Also suitable are azines of the following Colour Index Numbers:
C.I. Solvent Black 5, 5:1, 5:2, 7, 31 and 50; C.I. Pigment Black 1, C.I. Basic
Red 2
and C.I. Basic Black 1 and 2.
The iron azo complex compounds used in accordance with the invention are
incorporated individually or in combination with one another or with further
charge
control agents, mentioned above, in a concentration of from 0.01 to 50% by
weight,
preferably from 0.05 to 20% by weight, with particular preference from 0.1 to
5.0%
by weight, based on the overall mixture, into the binder of the respective
toner,
developer, coating material, powder coating material, electret material or of
the
polymer which is to be electrostatically separated, said incorporation being
homogeneous and taking place, for example, by means of extrusion or kneading,
beadmilling or using an Ultraturrax (high-speed stirrer). In this context the
compounds employed in accordance with the invention can be added as dried and
milled powders, dispersions or solutions, presscakes, masterbatches,
preparations,
made-up pastes, as compounds applied from aqueous or nonaqueous solution to
appropriate carriers such as silica gel, Ti02, AI203 or carbon black, for
example, or
mixed with such carriers, or added in some other form. Similarly, the
compounds
used in accordance with the invention can also in principle be added even
during the
CA 02351953 2001-06-29
13
preparation of the respective binders, i.e., in the course of their addition
polymerization, polyaddition or polycondensation.
In order to prepare electrophotographic color toners it is possible to add
further
colorants, such as organic color pigments, inorganic pigments or dyes. The
organic
color pigments may be from the group of the azo pigments or polycyclic
pigments or
mixed crystals (solid solutions) of such pigments.
The mixtures may be prepared in the form of the powders, by mixing of
presscakes,
spray-dried presscakes, masterbatches, and by dispersing (extrusion, kneading,
roll-
mill methods, beadmills, Ultraturrax) in the presence of a carrier material in
solid or
liquid form (aqueous and nonaqueous inks) and also by flushing in the presence
of a
carrier material. Where the colorant is used with high water or solvent
fractions
(> 5%), mixing may also take place in the presence of elevated temperatures
and
with vacuum assistance. The flushing operation may take place in the presence
or
absence of organic solvents and of waxes.
Especially for increasing the brilliance, but also for shading the hue,
mixtures with
organic dyes are appropriate, especially those dyes which have or which give a
black color. Preferred such dyes include:
water-soluble dyes, such as Direct, Reactive and Acid Dyes, and also solvent-
soluble dyes, such as Solvent Dyes, Disperse Dyes and Vat Dyes. Examples that
may be mentioned include: C.I. Solvent Black 45, 27; C.I. Reactive Black 31,
C.I. Direct Black 168, C.I. Solubilized Sulfur Black 1.
Inorganic pigments, such as Ti02 or BaS04, for example, are used in mixtures
for
brightening. Also suitable are mixtures comprising effect pigments, such as
pearlescent pigments, Fe203 pigments (~Paliocroms) and also pigments based on
cholesteric polymers, which give colors that differ depending on the viewing
angle.
Further inorganic pigments, such as carbon black, for example, especially
C.I. Pigment Black 7, are used to prepare black toners.
CA 02351953 2001-06-29
14
The present invention additionally provides an electrophotographic toner,
powder or
powder coating containing from 30 to 99.99% by weight, preferably from 40 to
99.5%
by weight, of customary binder, for example a styrene, styrene-acrylate,
styrene-
butadiene, acrylate, urethane, acrylic, polyester or epoxy resin or a
combination of
the last two, from 0.01 to 50% by weight, preferably from 0.05 to 20% by
weight, with
particular preference from 0.1 to 5% by weight of at least one iron azo
complex
compound, and, if desired, from 0.001 to 50% by weight, preferably from 0.05
to
20% by weight, of a colorant, based in each case on the overall weight of the
electrophotographic toner, powder or powder coating material.
Furthermore, the compounds described in accordance with the invention
comprising
"free-flow agents" may be applied as an additional charge control element in
suspended form or as a dry blend. The compounds described in accordance with
the invention may also be used for a carrier coating.
It has additionally been found that the iron azo complex compounds of the
formula
(I) are suitable as colorants in inkjet inks on an aqueous basis
(microemulsion inks)
and on a nonaqueous (solvent-based) basis, and also in inks which operate in
accordance with the hot-melt technique, and also in UV (ultraviolet)-curing
inks.
The present invention additionally provides inkjet recording liquids which
comprise
one or more of the iron azo complex compounds. The finished recording liquids
generally contain a total of from 0.5 to 15% by weight, preferably from 1.5 to
8% by
weight (calculated on a dry basis) of one or more, e.g., 2 or 3, of the
compounds of
the formula (I).
Microemulsion inks are based on organic solvents, water and, if desired, an
additional hydrotropic substance (interface mediator). Nonaqueous inks contain
substantially organic solvents and, if desired, a hydrotropic substance.
Microemulsion inks contain preferably from 0.5 to 15% by weight, in particular
from
1.5 to 8% by weight, of a compound of the formula (I), from 5 to 99% by weight
of
CA 02351953 2001-06-29
water and from 0.5 to 94.5% by weight of organic solvent and/or hydrotropic
compound.
Solvent-based inkjet inks contain preferably from 0.5 to 15% by weight of one
or
5 more compounds of the formula (I), from 85 to 99.5% by weight of organic
solvent
and/or hydrotropic compounds.
Hot-melt inks are based mostly on waxes, fatty acids, fatty alcohols or
sulfonamides
which are solid at room temperature and which become liquid on heating, the
10 preferred melting range being between about 60°C and about
140°C. The invention
additionally provides a hot-melt inkjet ink consisting substantially of from
20 to 90%
by weight of wax and from 1 to 10% by weight of the iron azo complex
compounds.
It is also possible for from 0 to 20% by weight of an additional polymer (as
"dye
dissolver"), from 0 to 5% by weight of dispersing auxiliary, from 0 to 20% by
weight
15 of viscosity modifier, from 0 to 20% by weight of plasticizer, from 0 to
10% by weight
of tack additive, from 0 to 10% by weight of transparency stabilizer
(prevents, for
example, crystallization of the waxes), and from 0 to 2% by weight of
antioxidant to
be present. Typical additives and auxiliaries are described, for example, in
US-A 5,560,760.
UV inks contain preferably from 0.5 to 15% by weight of one or more compounds
of
the formula (I), from 50 to 99.5% by weight of photopolymerizable monomers
(containing for example acyl, epoxy, vinyl groups), photoinitiators and/or
further
additives, as described for example in US 5,275,646).
Water used to prepare the recording liquids is preferably employed in the form
of
distilled water or deionized water.
The solvents present in the recording liquids may comprise an organic solvent
or a
mixture of such solvents. Examples of suitable solvents are monohydric or
polyhydric alcohols, their ethers and esters, e.g., alkanols, especially those
having 1
to 4 carbon atoms, such as methanol, ethanol, propanol, isopropanol, butanol,
isobutanol; dihydric or trihydric alcohols, especially those having 2 to 5
carbon
CA 02351953 2001-06-29
16
atoms, examples being ethylene glycol, propylene glycol, 1,3-propanediol, 1,4-
butanediol, 1,5-pentanediol, 1,6-hexanediol, 1,2,6-hexanetriol, glcyerol,
diethylene
glycol, dipropylene glycol, triethylene glycol, polyethylene glycol,
tripropylene glycol,
polypropylene glycol; lower alkyl ethers of polyhydric alcohols, such as
ethylene
glycol monomethyl, monoethyl or monobutyl ether, triethylene glycol monomethyl
or
monoethyl ether; ketone and ketone alcohols such as acetone, methyl ethyl
ketone,
diethyl ketone, methyl isobutyl ketone, methyl pentyl ketone, cyclopentanone,
cyclohexanone, diacetone alcohol; amides, such as dimethylformamide,
dimethylacetamide, N-methylpyrrolidone, toluene, and n-hexane.
As hydrotropic compounds, which may if desired also serve as solvents, it is
possible to use, for example, formamide, urea, tetramethylurea, E-caprolactam,
ethylene glycol, diethylene glycol, triethylene glycol, polyethylene glycol,
butyl glycol,
methylcellosolve, glycerol, N-methylpyrrolidone, 1,3-diethyl-2-
imidazolidinone,
thiodiglycol, sodium benzenesulfonate, Na xylenesulfonate, Na
toluenesulfonate,
sodium cumenesulfonate, Na dodecylsulfonate, Na benzoate, Na salicylate or
sodium butyl monoglycol sulfate.
Furthermore, the recording liquids of the invention may comprise customary
additives, examples being preservatives, cationic, anionic or nonionic surface-
active
substances (surfactants and wetting agents), and also viscosity regulators,
e.g.,
polyvinyl alcohol, cellulose derivatives, or water-soluble natural or
synthetic resins as
film formers and/or binders for increasing the adhesion and abrasion
resistance.
Amines, such as ethanolamine, diethanolamine, triethanolamine, N,N-dimethyl-
ethanolamine or diisopropylamine, for example, serve primarily to increase the
pH of
the recording liquid. They are normally present at from 0 to 10% by weight,
preferably from 0.5 to 5% by weight, in the recording liquid.
The inkjet inks of the invention may be prepared by dispersing the iron azo
complex
compounds of the formula (I) as powders, as a preparation, as a suspension or
as
presscakes into the microemulsion medium or into the nonaqueous medium or into
CA 02351953 2001-06-29
17
the wax for preparing a hot-melt inkjet ink. The presscake may also be a
highly
concentrated presscake, in particular a spray-dried presscake.
Furthermore, compounds of the formula (I) are also suitable as colorants for
color
filters, both for subtractive and for additive color production (P. Gregory
"Topics in
Applied Chemistry: High Technology Application of Organic Colorants" Plenum
Press, New York 1991, pp. 15 - 25).
In the examples below, parts and percentages are by weight.
Preparation Example 1
a) 60.7 g (0.3 mol) of 2-amino-5-(methylaminosulfonyl)phenol were stirred into
a
mixture of 450 g of water and 120 g of 30% strength by weight aqueous HCI.
Following the addition of 75 g of ice, the amine was diazotized by adding 79 g
of 4N
sodium nitrite solution. The suspension obtained was stirred at 0°C for
3 hours.
Then a solution of 22.2 g of resorcinol (0.2 mol) in 100 g of water and 21.2 g
of
sodium carbonate was slowly added dropwise. The mixture obtained was stirred
at
room temperature for 8 hours and brought to a pH of 1.5 by adding 30% strength
by
weight aqueous HCI. The precipitate was filtered off, washed with 4 000 g of
water
and dried.
b) 80 g of the monoazo dye prepared in a) were suspended in a mixture of 300 g
of water, 15 g of dipropylene glycol monomethyl ether and 39.4 g of sodium
carbonate. After heating at 98°C for 1 hour, a solution of 25.2 g of
FeCl3 x 6Hz0 in
130 g of water were slowly added dropwise, in the course of which a bulky
precipitate of the iron azo complex was formed. Over the course of 2 hours,
the
temperature was lowered to 30°C with vigorous stirring and the
suspension was
slowly reacted with a solution of 186 parts of °Primene 81 R (C~2-C~4-t-
alkylamines,
Rohm & Haas) in 80 g of water and 12 g of 30% strength by weight aqueous HCI.
The resulting precipitate was adjusted to a pH of 6.5 by adding about 23 g of
30% by
weight aqueous HCI.
CA 02351953 2001-06-29
18
The mixture was stirred at room temperature for 1 hour and filtered, and the
residue
was washed with a solution of 100 g of acetic acid and 900 g of water and then
dried. This gave an iron azo complex dye of the formula
H3C ~
NH
O=~ / \ N= OH
I I
O
O + alkyl(C12 C14)
CH3
OH \ O H3N alkyl(C -C )
N H \\~ 12 14
alkyl(C12 C14)
O II ~ ~ N N O O
II
O HO ~ ~ N=N ~ ~ S=O
HN
\CH3
Characterization
Brown, water-insoluble powder, readily soluble in ethanol (to 400 g/liter)
pH: 5
Conductivity 410 ,uS/cm
Residual moisture content 0.6% (Karl-Fischer method)
X-ray diffraction: There is no reflexion peak between two theta 4.0
and two theta 36.0, i.e., the compound is
x-ray-amorphous
DTA: Decomposition begins from 200°C (at
3°C/min heating rate)
Particle size distribution: d5o < 71 ,um
Use Example 1.1
1 part of the compound from Preparation Example 1 is incorporated
homogeneously
using a kneading apparatus over the course of 30 minutes into 95 parts of a
toner
CA 02351953 2001-06-29
19
binder 60:40 styrene-n-butyl methacrylate (~Dialec S309). The composition is
then
ground on a universal laboratory mill and subsequently classified in a
centrifugal
classifier. The desired particle fraction (4 to 25 Nm) is activated with a
carrier
comprising styrene-acrylate-coated magnetite particles of size 50 to 200 pm.
Measurement is carried out on a customary g/m measurement stand. By using a
sieve having a mesh size of 25 pm it is ensured that no carrier is entrained
when the
toner is blown out. The measurements are made at about 50% relative
atmospheric
humidity. As a function of the activation period, the following q/m values
[NC/g] are
measured:
Activation periodCharge q/m [,uC/g]
10 min - 22
30 min - 30
2 h - 35
24 h -35
Use Example 1.2
6 parts of the compound from Preparation Example 1 are dissolved with stirring
(paddle stirrer or dissolver) in 94 parts of methyl ethyl ketone. The inkjet
ink thus
obtained shows good to very good fastness properties on inkjet paper
(lightfastness:
5-6, evaluated in accordance with the blue scale (ISO 12040/DIN 16525), where
the
lowest score, 1, denotes very poor lightfastness and the highest score, 8,
denotes
very high lightfastness.
1 = very low, 2 = low, 3 = moderate, 4 = fairly good, 5 = good, 6 = very good,
7 = excellent, 8 = outstanding.
CA 02351953 2001-06-29
Lightfastness comparative examples:
In accordance with the method described above, the lightfastness of the
compounds
described in JP-A-62-129 358, Examples 8, 9 and 14, was measured. For all
three
5 compounds, the lightfastness was poor to moderate (2-3).
Use Example 1.3
5 parts of the compound from Preparation Example 1 are dissolved with stirring
in 30
parts of glycol ether (~Dowanol EPh, Dow Chemical). This solution is
subsequently
10 added with stirring to a solution of 50 parts of deionized water with 15
parts of
xylenesulfonate.
The microemulsion ink thus obtained has the following composition:
parts of glycol ether,
5 parts of compound from Preparation Example 1,
15 15 parts of xylenesulfonate (interface mediator, hydrotropic substance),
50 parts of deionized water.
This gives an inkjet ink having high lightfastness (5-6) and good passage
through
the nozzles.
Preparation Example 2
a) 78.1 g (0.3 mol) of 2-amino-4-(3'-methoxypropylaminosulfonyl)phenol were
stirred into a mixture of 600 g of water and 120 g of 30% strength by weight
aqueous
HCI. Following the addition of 75 g of ice, the amine was diazotized by adding
79 g
of 4N sodium nitrite solution. The suspension obtained was stirred at
0°C for
3 hours. Then a solution of 22.2 g (0.2 mol) of resorcinol and 100 g of water
and
21.2 g of sodium carbonate was slowly added dropwise. The mixture obtained was
stirred at room temperature for 8 hours and brought to a pH of 1.5 by adding
30%
strength by weight aqueous HCI. The precipitate was filtered off, washed with
4 000 g of water and dried.
CA 02351953 2001-06-29
21
b) 96.2 g of the monoazo dye prepared in a) were suspended in a mixture of
300 g of water, 15 g of dipropylene glycol monomethyl ether and 39.4 g of
sodium
carbonate. After heating at 98°C for 1 hour, a solution of 25.2 g of
FeCl3 x 6H20 in
130 g of water was slowly added dropwise, during which a bulky precipitate of
the
iron azo complex was formed. Over the course of 2 hours, the temperature was
reduced to 30°C with vigorous stirring and the suspension was slowly
reacted with a
solution of 186 g of ~Primene 81 R (C~2-C~4-t-alkylamines, Rohm & Haas) in 80
g of
water and 12 g of 30% strength by weight aqueous HCI. The resulting
precipitate
was adjusted to a pH of 6.5 by adding about 23 g of 30% strength by weight
aqueous HCI.
The mixture was stirred at room temperature for 1 hour and filtered and the
residue
was washed salt-free with water and then dried. This gave a compound of the
formula
O
/~~ N H
Oy /
i
O ~ \ N=N ~ ~ OH
O O~ O/O H N alkyl(C~2 C~a)
OH Fe 3 ~~ alkyl(C~2 C~a)
alkyl(C~2 C~4)
N-N O
Hag HO / \ N = N
\O
~O~~N~ ~O
H
Characterization
pH: 6.4
Conductivity 610 ,uS/cm
Residual moisture content 0.8% (Karl-Fischer method)
X-ray diffraction: There is no reflexion peak between two theta 4.0
and two theta 36.0, i.e., the compound is
CA 02351953 2001-06-29
22
x-ray-amorphous
DTA: Decomposition begins from 200°C (at 3°C/min
heating rate)
Particle size distribution: d5o = 78,um
Use Example 2.1
The procedure of Use Example 1.1 is repeated but incorporating now 1 part of
the
compound from Preparation Example 2 rather than 1 part of the compound from
Preparation Example 1.
As a function of the activation period, the following q/m values are measured:
Activation periodq/m [,uC/g]
10 min -11
30 min -19
2 h -29
24 h -29
Use Example 2.2
1 part of the compound from Preparation Example 2 is incorporated
homogeneously
as described in Use Example 1.1 into 99 parts of a powder coating binder based
on
a carboxyl-containing polyester resin, e.g., ~Crylcoat 430 (UCB, Belgium).
To determine the deposition rate, 50 g of the test powder coating material are
sprayed with a defined pressure through a triboelectric spray gun. By
differential
weighing it is possible to determine the amount of powder coating deposited
and to
define a deposition rate in %, and also, by means of the charge transfer, to
derive a
current flow [pA].
Pressure [bar] Current [NA] Deposition rate [%]
5 0.7 80
3 0.4 55
CA 02351953 2001-06-29
23
Use Example 3 (comparative)
To determine the deposition rate of the straight powder coating binder
°Crylcoat
430, the procedure described above is repeated but without incorporation of an
additive.
Pressure [bar] Current [NA] Deposition rate [%]
3 0.1 5
Use Example 4 (comparative)
The procedure of Use Example 1.1 was repeated, using solely the straight toner
binder without additions.
As a function of the activation period, the following q/m values are measured:
Activation period~~Charge q/m [NC/g]
10 min -8
30 min -12
2 h -18
24 h -19