Note: Descriptions are shown in the official language in which they were submitted.
CA 02352194 2001-05-25
1
AZEPINOINDOLE DERIVATIVES, THE PRODUCTION AND USE THEREOF
The present invention relates to novel azepinoindole derivatives,
their preparation and use as inhibitors of the enzyme
poly(ADP-ribose) polymerase or PARP (EC 2.4.2.30) for the
production of pharmaceuticals.
Poly(ADP-ribose) polymerase (PARP) or, as it is also called,
poly(ADP-ribose) synthase (PARS) is a regulatory enzyme which is
found in cell nuclei (K. Ikai et al., J. Histochem. Cytochem.
1983, 31, 1261-1264). It is assumed that PARP plays a role in the
repair of DNA breaks (M. S. Satoh et al., Nature 1992, 356,
356-358). Damage to or breaks in the DNA strands activate the
enzyme PARP, which, when it is activated, catalyzes the transfer
of ADP-ribose from NAD (S. Shaw, Adv. Radiat. Biol., 1984, 11,
1-69). In this process, nicotinamide is released from NAD.
Nicotinamide is converted again into NAD by other enzymes using
the energy carrier ATP. Overactivation of PARP would accordingly
have resulted in a nonphysiologically high consumption of ATP and
in the extreme case this leads to cell damage and cell death.
It is known that free radicals such as the superoxide anion, NO
and hydrogen peroxide can lead to DNA damage in cells and thus
activate PARP. The formation of large amounts of free radicals is
observed in a number of pathophysiological conditions and it is
assumed that this accumulation of free radicals leads or
contributes to the observed cell or organ damage. This counts,
for example, ischemic conditions of organs such as in stroke,
cardiac infarct (C. Thiemermann et al., Proc. Natl. Acad. Sci.
USA, 1997, 94, 679-683) or ischemia of the kidneys, but also
reperfusion damage such as occurs after the lysis of cardiac
infarct (see above: C. Thiemermann et al.). The inhibition of the
enzyme PARP could accordingly be a means of preventing or
alleviating this damage at least partially. PARP inhibitors could
thus be a novel therapy principle for the treatment of a number
of diseases.
The enzyme PARP influences the repair of DNA damage and could
thus also play a role in the therapy of carcinomatous diseases,
as a higher potential of action against tumor tissue has been
observed in combination with cytostatically active substances (G.
Chen et al. Cancer Chemo. Pharmacol. 1988, 22, 303).
Nonlimiting examples of tumors are leukemia, glioblastoma,
lymphoma, melanoma, mammary and cervical carcinoma.
CA 02352194 2001-05-25
BASF Aktiengesellschaft 992956 O.Z. 0050/51636 DE
2
Moreover, it has been found that PARP inhibitors can show
immunosuppressant action (D. Weltin et al. Int. J.
Immunopharmacol. 1995, 17, 265-271).
It has likewise been discovered that PARP is invoved in
immunological disorders or diseases in which the immune system
plays an important role, such as rheumatoid arthritis and septic
shock, and that PARP inhibitors can show a favorable effect on
the course of the disease (H. Kroger et al. Inflammation 1996,
20, 203-215; W. Ehrlich et al. Rheumatol. Int. 1995, 15, 171-172;
C. Szabo et al., Proc. Natl. Acad. Sci. USA 1998, 95, 3867-3872;
S. Cuzzocrea et al. Eur. J. Pharmacol. 1998, 342, 67-76).
pARP within the meaning of this invention is also understood as
meaning isoenzymes of the PARP enzyme described above.
The PARP inhibitor 3-aminobenzamide furthermore showed protective
effects in a model of circulatory shock (S. Cuzzocrea et al., Br.
J. Pharmacol. 1997, 121, 1065-1074).
There are likewise experimental indications that inhibitors of
the enzyme PARP could be useful as a means for the treatment of
diabetes mellitus (V. Burkart et al. Nature Med. 1999, 5,
314-319).
WO 00/42040 mentions azepinoindoles which inhibit the PARP
enzyme. In particular, derivatives are described there as active
which carry a phenyl ring in the 2 position, which can
additionally be substituted by simple substituents.
The compounds of the general formula I according to the invention
here have not been described until now and are accordingly novel.
In the present invention, novel azepinoindole derivatives of the
general formula I are described which are potent PARP inhibitors.
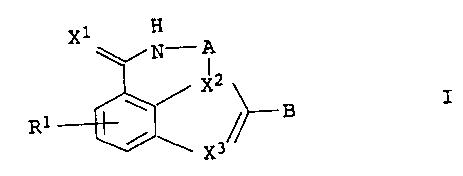
The present invention relates to substituted azepinoindole
derivatives of the general formula I
X1 H
~N-A
/ X2
I
R 1 ~ ~~ B
~ \ X3
in which
CA 02352194 2001-05-25
BASF Aktiengesellschaft 992956 o.Z. 0050/51636 DE
3
can be a C1-C3 chain, where each carbon atom can additionally
carry one or two of the following substituents: C1-C4-alkyl,
OH, O-C1-C4-alkyl, COON, C00-C1-C4-alkyl and phenyl or a C
atom can also carry an =O group and
X1 can be S, 0 or NH and
X2 can be a carbon atom which can additionally carry a Ci-C4
chain, and N and
X3 can be N or C-R2, where
R2 can be hydrogen, branched or unbranched C1-C6-alkyl,
C1-C9-alkylphenyl, phenyl and
XZ and X3 cannot simultaneously be N, and
R1 can be hydrogen, chlorine, fluorine, bromine, iodine,
branched or unbranched C1-C6-alkyl, OH, vitro, CF3, CN,
NR11R12. NH-CO-R13. O-C1-C4-alkyl, where R11 and R12
independently of one another are hydrogen or C1-C4-alkyl and
Ri3 is hydrogen, C1-C4-alkyl, C1-C4-alkylphenyl or phenyl, and
g can be an unsaturated, saturated or partially unsaturated
mono-, bi- or tricyclic ring having at most 15 carbon atoms,
an unsaturated, saturated or partially unsaturated mono-, bi-
or tricyclic ring having at most 14 carbon atoms and 0 to 5
nitrogen atoms, 0 to 2 oxygen atoms and 0 to 2 sulfur atoms,
which in each case are additionally substituted by one R4 and
at most 3 different or identical radicals R5, and can carry
one or two carbon or sulfur atoms and also one or two =O
groups such as keto groups, sulfones or sulfoxides,
R4 is -(D)p-(E)s-(F1)q-G1-(FZ)r-(GZ) G3' where G1, G2 and G3 cannot
simultaneously be hydrogen or a bond and if p = s = 0 and q
or r = 1 or p, q and r = 0, then two radicals G1, G2 and G3
cannot simultaneously be a bond or hydrogen, and
D can be S, NR43 or 0
can be phenyl,
-S02-, -SOZNH-, -NHCO-, -CONH-, NHS02-,
C=O ,
-NHCOCHZX4,
and
CA 02352194 2001-05-25
BASF Aktiengesellschaft 992956 O.Z. 0050/51636 DE
4
g4 can be S, 0 or NH, and
F1 can be a straight-chain or branched saturated or
unsaturated carbon chain of 1 to 8 C atoms and
F2 independently of F1 has the same meaning as F1.
G1 is a bond or can be an unsaturated, saturated or
partially unsaturated mono-, bi- or tricyclic ring having
at most 15 carbon atoms, an unsaturated, saturated or
partially unsaturated mono-, bi- or tricyclic ring having
at most 14 carbon atoms and 0 to 5 nitrogen atoms, 0 to 2
oxygen atoms and 0 to 2 sulfur atoms, which in each case
are additionally substituted by at most 3 different or
identical radicals R5 and can carry one or two carbon or
sulfur atoms and one or two =O groups, and
G2 is NR41Ra2 or
R~
R~
R~ R7
/ N N- R9
N N~ N
R~
R7 R7
NJ N O
N~NwR9 U
or a bond and
G3 can be an unsaturated, saturated or partially unsaturated
mono-, bi- or tricyclic ring having at most 15 carbon
atoms, an unsaturated, saturated or partially unsaturated
mono-, bi- or tricyclic ring having at most 14 carbon
atoms and 0 to 5 nitrogen atoms, 0 to 2 oxygen atoms or 0
to 2 sulfur atoms, which in each case are additionally
substituted by at most 3 different or identical radicals
g5, and one or two carbon or sulfur atoms can also carry
one or two =0 groups, or hydrogen, and
p can be 0 or 1 and
s can be 0 or 1 and
q can be 0 or 1 and
CA 02352194 2001-05-25
BASF Aktiengesellschaft 992956 O.Z. 0050/51636 DE
r can be 0 or 1 and
R41 can be hydrogen, C1-C6-alkyl, where each carbon atom can
additionally carry up to two radicals R6, phenyl which can
5 additionally carry at most two radicals R6, and (CHz)t-K and
-C6-alkyl, -CO-R8~ C02-R8. SOzNHz, SOz-R8~
Raz can be hydrogen, C1
-(C=N)-R8 and -(C=N)-NHRg and
R43 can be hydrogen or C1-C4-alkyl and
t can be 1, 2, 3 or 4 and
K can be NRllRiz, NR11-Ci-C9-alkylphenyl, pyrrolidine,
piperidine, 1,2,5,6-tetrahydropyridine, morpholine,
homopiperidine, piperazine, which can additionally be
substituted by an alkyl radical C1-C6-alkyl, and
homopiperazine which can additionally be substituted by an
alkyl radical C1-C6-alkyl, and
5 can be hydrogen, chlorine, fluorine, bromine, iodine, OH,
R
NR11R1z~ NH-CO-R13, Ci-C4-alkyl-CO-NH-R13, CORE,
nitro, CF3, CN,
Co-C4-alkyl-O-CO-R13, Ci-Ca-alkylphenyl, phenyl,
COz-Ci-Ca-alkyl, and branched or unbranched C1-C6-alkyl,
O-C1-C4-alkyl, S-C1-C4-alkyl, where each C atom of the alkyl
chains can carry up to two radicals R6 and the alkyl chains
can also be unsaturated, and
R6 can be hydrogen, chlorine, fluorine,OH ron tro, CF ,n CN,
branched or unbranched C1-C6-alkyl,
NR11R1z, NH-CO-R13, 0-C1-C4-alkyl
R~ can be hydrogen, C1-C6-alkyl, phenyl, where the ring can
additionally be substituted by up to two radicals R~1, and one
amine NR11Ri2 or a cyclic saturated amine having 3 to
7 members, which can additionally be substituted by an alkyl
radical C1-C6-alkyl, and homopiperazine which can additionally
be substituted by an alkyl radical C1-C6-alkyl, and
where the radicals R11, Riz and R13 in K, R5, R6 and R~
independently of one another can assume the same meaning as R1,
and
R~1 can be OH, C1-C6-alkyl, 0-C1-C4-alkyl, chlorine, bromine,
iodine, fluorine, CF3, nitro, NHz, and
CA 02352194 2001-05-25
BASF Aktiengesellschaft 992956 O.Z. 0050/51636 DE
6
R8 can be C1-C6-alkyl, CF3, phenyl, C1-C4-alkylphenyl, where the
ring can additionally be substituted by up to two radicals
R81, and
R81 can be OH, C1-C6-alkyl, O-C1-C4-alkyl, chlorine, bromine,
iodine, fluorine, CF3, nitro, NHz, and
R9 can be hydrogen, C1-C6-alkyl, C1-C4-alkylphenyl,
COZ-C1-C4-alkylphenyl, COZ-C1-C4-alkyl, S02-phenyl, COR$ and
phenyl, where the phenyl rings can additionally be
substituted by up to two radicals R91, and
R91 can be OH, C1-C6-alkyl, 0-C1-C4-alkyl, chlorine, bromine,
iodine, fluorine, CF3, nitro, NH2,
- and their tautomeric forms, possible enantiomeric and
diastereomeric forms, and their prodrugs.
Preferred compounds of the formula I are those where
A is a C2 chain which can be substituted, and
X1 is O and
R1 is hydrogen.
Preferred compounds of the formula I are those as indicated
above, in which
B can be an unsaturated, saturated or partially unsaturated
mono-, bi- or tricyclic ring having at most 15 carbon atoms,
an unsaturated, saturated or partially unsaturated mono-, bi-
or tricyclic ring having at most 14 carbon atoms and 0 to 5
nitrogen atoms, 0 to 2 oxygen atoms or 0 to 2 sulfur atoms,
which in each case are additionally substituted by an R4 and
at most 3 different or identical radicals R5, and can carry
one or two carbon or sulfur atoms and also one or two =0
groups.
Particularly preferred radicals for B are the following:
B is phenyl, cyclohexyl, piperidine, pyridine, pyrimidine,
pyrrole, pyrazole, thiophene, furan, oxazole, naphthalene,
piperazine, quinoline, pyrazine, which can additionally be
substituted by one R4 or at most 2 R5.
CA 02352194 2001-05-25
BASF Aktiengesellschaft 992956 O.Z. 0050/51636 DE
7
Very particularly preferred compounds of the formula I are those
where
R4 is D-Flo,1-G2-G3 where G3 is hydrogen and
D is 0 or NR93, where R43 is hydrogen or C1-C3-alkyl and
F1 is Cz-C4-alkyl.
The compounds of the formula I can be employed as racemates, as
enantiomerically pure compounds or as diastereomers. If
enantiomerically pure compounds are desired, these can be
obtained, for example, by carrying out a classical resolution
with the compounds of the formula I or their intermediates using
a suitable optically active base or acid.
Alkyl chains can in each case be branched or unbranched.
Unbranched alkyl chains are preferred.
The invention also relates to compounds which are mesomeric or
tautomeric to compounds of the formula I.
The invention further relates to the physiologically tolerable
salts of the compounds I, which can be obtained by conversion of
compounds I using a suitable acid or base. Suitable acids and
bases are listed, for example, in Fortschritte der
Arzneimittelforschung, 1966, Birkhauser Verlag, Vol. 10,
pp. 224-285. These include, for example, hydrochloric acid,
citric acid, tartaric acid, lactic acid, phosphoric acid,
methanesulfonic acid, acetic acid, formic acid, malefic acid,
fumaric acid etc. or sodium hydroxide, lithium hydroxide,
potassium hydroxide and tris.
Prodrugs are understood as meaning those compounds which are
metabolized in vivo to compounds of the general formula I.
Typical prodrugs are phosphates, carbamates of amino acids,
esters and others.
The azepinoindole derivatives I according to the invention can be
prepared in various ways, such as described in WO 00/42040.
The substituted azepinoindole derivatives I obtained in the
present invention are inhibitors of the enzyme
poly(ADP-ribose)polymerase or PARP (EC 2.4.2.30).
CA 02352194 2001-05-25
BASF Aktiengesellschaft 992956 O.Z. 0050/51636 DE
8
The inhibitory action of the substituted azepinoindole
derivatives I can be determined using an enzyme test which is
already known in the literature, the measure of activity
determined being a Ki value. The azepinoindole derivatives I were
measured in this way for inhibitory action of the enzyme
poly(ADP-ribose)polymerase or PARP (EC 2.4.2.30).
The substituted azepinoindole derivatives of the general formula
I are inhibitors of poly(ADP-ribose)polymerase (PARP) or, as it
is also called, polY(~P-ribose)synthase (PARS) and can thus be
used for the treatment and prophylaxis of diseases which are
connected with an increased enzyme activity of these enzymes.
The compounds of the formula I can be employed for the production
of pharmaceuticals for the treatment of damage after ischemia and
for prophylaxis in the case of expected ischemia of various
organs.
The present azepinoindole derivatives of the general formula I
can accordingly be used for the treatment and prophylaxis of
neurodegenerative diseases which occur after ischemia, trauma
(craniocerebral trauma), mass hemorrhages, subarachnoid
hemorrhages and stroke, and of neurodegenerative diseases.such as
multiple infarct dementia, Alzheimer's disease, Huntington's
disease and of epilepsy, in particular of generalized epileptic
attacks, such as petit mal and tonic-clonic attacks and partially
epileptic attacks, such as temporal lobe, and complex/partial
attacks, and furthermore for the treatment and prophylaxis of
damage to the heart after cardiac ischemia and damage to the
kidneys after renal ischemia, for example of acute renal
insufficiency caused by medicinal therapies such as in the case
of cyclosporin treatment, of acute kidney failure or of damage
which occurs during and after a kidney transplantation.
Furthermore, the compounds of the general formula I can be used
for the treatment of acute myocardial infarct and damage which
occurs during and after medicinal lysis thereof (for example
using TPA, reteplase, streptokinase or mechanically using a laser
or rotablator) and of microinfarcts during and after heart valve
replacement, aneurysm resection and heart transplantation.
Likewise, the present azepinoindole derivatives I can be used for
the treatment of a revascularization of critically constricted
coronary arteries, for example in PCTA and bypass operations, and
critically constricted peripheral arteries, for example leg
arteries. Moreover, the azepinoindole derivatives I can be useful
for the treatment of tumors and metastasis thereof and for the
treatment of inflammation and rheumatic disorders, such as
rheumatoid arthritis and also for the treatment of diabetes
CA 02352194 2001-05-25
BASF Aktiengesellschaft 992956 O.Z. 0050/51636 DE
9
mellitus, for the treatment of multiorgan failure, e.g. in septic
shock and for the treatment of ARDS (acute respiratory distress
syndrome, shock lung).
In addition to the customary pharmaceutical excipients, the
pharmaceutical preparations according to the invention contain a
therapeutically active amount of the compound I.
For local external application, for example in powders, ointments
or sprays, the active compounds can be present in the customary
concentrations. As a rule, the active compounds are present in an
amount from 0.001 to 1% by weight, preferably 0.001 to 0.1% by
weight.
In the case of internal administration, the preparations are
administered in individual doses. In an individual dose, 0.1 to
100 mg are given per kg of body weight. The preparations can be
administered daily in one or more doses, depending on the nature
and severity of the disorders.
Corresponding to the desired manner of administration, the
pharmaceutical preparations according to the invention contain
the customary vehicles and diluents in addition to the active
compound. For local external application, pharmaceutical
excipients, such as ethanol, isopropanol, ethoxylated castor oil,
ethoxylated hydrogenated castor oil, polyacrylic acid,
polyethylene glycol, polyethylene glycol stearate, ethoxylated
fatty alcohols, liquid paraffin, petroleum jelly and wool fat can
be used. For internal administration, for example, lactose,
30.propylene glycol, ethanol, starch, talc and polyvinylpyrrolidone
are suitable.
Antioxidants such as tocopherol and butylated hydroxyanisole and
also butylated hydroxytoluene, flavor-improving additives,
stabilizers, emulsifieres and lubricants can furthermore be
present.
The substances contained in the preparation in addition to the
active compound and the substances used in the production of the
pharmaceutical preparations are toxicologically innocuous and
compatible with the respective active compound. The
pharmaceutical preparations are produced in a customary manner,
for example by mixing the active compound with other customary
vehicles and diluents.
CA 02352194 2001-05-25
- BASF Aktiengesellschaft 992956 O.Z. 0050/51636 DE
The pharmaceutical preparations can be administered in various
modes of administration, for example perorally. parenterally,
such as intravenously, by infusion, subcutaneously,
intraperitoneally and topically. Thus, preparation forms such as
5 tablets, emulsions, infusion and injection solutions, pastes,
ointments, gels, creams, lotions, powders and sprays are
possible.
Pharmacological Example:
10 Inhibition of the enzyme poly(ADP-ribose)polymerase or PARP
(EC 2.4.2.30)
A 96-well microtiter plate (Flacon) is coated with histories (type
II-AS; SIGMA H7755). For this purpose, histories are dissolved in
carbonate buffer (0.05 M NaHC03; pH 9.4) to give a concentration
of 50 ~g/ml~ The individual wells of the microtiter plates are
incubated overnight with 100 ~1 each of this histone solution.
The histone solution is then removed and the individual wells are
incubated at room temperature for 2 hours with 200 ~1 of a 1%
strength BSA (bovine serum albumin) solution in carbonate buffer.
The wells are then washed three times with wash buffer (0.05%
Tween 10 in PBS). For the enzyme reaction, 50 ~1 of the enzyme
reaction solution (5 ~1 of reaction buffer (1 M tris HCl pH 8.0,
100 mM MgClz, 10 mM DTT), 0.5 ~1 of PARP (c = 0.22 ~g/~1). 4 ~1 of
activated DNA (SIGMA D-4522, 1 mg/ml in water), 40.5 ~1 of H20)
are preincubated with 10 ~1 of an inhibitor solution per well for
10 minutes. The enzyme reaction is started by addition of 40 ~.1
of a substrate solution (4 ~1 of reaction buffer (see above),
8 ~1 of NAD solution (100 ~M in H20), 28 ~1 of HZO). The reaction
time is 20 minutes at room temperature. The reaction is stopped
by washing three times with wash buffer (see above). A one-hour
incubation at room temperature with a specific
anti-poly-ADP-ribose antibody then follows. The antibodies used
were monoclonal anti-poly-(ADP-ribose) antibodies "lOH"
(Kawamaitsu H et al. (1984) Monoclonal antibodies to poly
(adenosine diphosphate ribose) recognize different structures.
Biochemistry 23, 3771-3777). Polyclonal antibodies can also be
used.
The antibodies were employed in a 1:5 000 dilution in antibody
buffer (1% BSA in PBS; 0.05% Tween 20). After washing three times
with wash buffer, a one-hour incubation at room temperature with
secondary antibody follows. Here, for the monoclonal antibody, an
anti-mouse IgG coupled to peroxidase (Boehringer Mannheim) and,
for the rabbit antibody, an anti-rabbit IgG coupled to peroxidase
(SIGMA A-6154), in each case in a 1:10 000 dilution in antibody
buffer, were used. After washing three times with wash buffer,
CA 02352194 2001-05-25
BASF Aktiengesellschaft 992956 O.Z. 0050/51636 DE
11
the color reaction was carried out using 100 p.l/well of color
reagent (SIGMA, TMB ready-to-use mix, T8540) for about 15 min at
room temperature. The color reaction is stopped by addition of
100 ~1 of 2 M H2S04. The color is then immediately measured
(450 nm against 620 nm; ELISA "Easy Reader" EAR340AT plate
reader, SLT-Labinstruments, Austria). The IC50 value of an
inhibitor to be measured is the inhibitor concentration where a
half-maximal color concentration change occurs.
The following compounds according to the invention can be
prepared analogously to the methods described above:
1. 2-(4-(4-n-Propylpiperazin-1-yl)phenyl)-1,3,4,5-tetrahydro-
6H-azepino[5,4,3-c,d]indol-6-one
2. 2-(4-Piperazin-1-ylphenyl)-1,3,4,5-tetrahydro-6H-azepino-
[5,4,3-c,d]indol-6-one
3. 2-(4-(4-Isopropylpiperazin-1-yl)phenyl)-1,3,4,5-tetrahydro-
6H-azepino[5,4,3-c,d]indol-6-one
4. 2-(4-(4-Benzylpiperazin-1-yl)phenyl)-1,3,4,5-tetrahydro-6H-
azepino[5,4,3-c,d]indol-6-one
5. 2-(4-(4-n-Butylpiperazin-1-yl)phenyl)-1,3,4,5-tetrahydro-
6H-azepino[5,4,3-c,d]indol-6-one
6. 2-(4-(4-Ethylpiperazin-1-yl)phenyl)-1,3,4,5-tetrahydro-6H-
azepino[5,4,3-c,d]indol-6-one
7. 2-(4-(2-N,N-Dimethylaminoeth-1-yloxy)phenyl)-1,3,4,5-
tetrahydro-6H-azepino[5,4,3-c,d]indol-6-one
8. 2-(4-(2-Pyrrolidinyleth-1-yloxy)phenyl)-1,3,4,5-tetrahydro-
6H-azepino[5,4,3-c,d]indol-6-one
9. 2-(4-(2-Piperidin-1-yleth-1-yloxy)phenyl)-1,3,4,5-tetrahydro-
6H-azepino[5,4,3-c,d]indol-6-one
10. 2-(4-(2-Piperazin-1-yleth-1-yloxy)phenyl)-1,3,4,5-tetrahydro-
6H-azepino[5,4,3-c,d]indol-6-one
11. 2-(4-(2-(4-Methylpiperazin-1-yl)eth-1-yloxy)phenyl)-
1,3,4,5-tetrahydro-6H-azepino[5,4,3-c,d]indol-6-one
CA 02352194 2001-05-25
BASF Aktiengesellschaft 992956 O.Z. 0050/51636 DE
12
12. 2-(4-(2-(4-Propyl-piperazin-1-yl)eth-1-yloxy)phenyl)-
1,3,4,5-tetrahydro-6H-azepino[5,4,3-c,d]indol-6-one
13. 2-(4-(2-(4-Ethyl-piperazin-1-yl)eth-1-yloxy)phenyl)-
1,3,4,5-tetrahydro-6H-azepino[5,4,3-c,d]indol-6-one
14. 2-(4-(2-(4-Benzylpiperazin-1-yl)eth-1-yloxy)phenyl)-
1,3,4,5-tetrahydro-6H-azepino[5,4,3-c,d]indol-6-one
15. 2-(4-(2-(4-Acetamidopiperazin-1-yl)eth-1-yloxy)phenyl)-
1,3,4,5-tetrahydro-6H-azepino[5,4,3-c,d]indol-6-one
16. 2-(4-(2-(4-Benzamidopiperazin-1-yl)eth-1-yloxy)phenyl)-
1,3,4,5-tetrahydro-6H-azepino[5,4,3-c,d]indol-6-one
17. 2-(4-Homopiperazin-1-yl-phenyl)-1,3,4,5-tetrahydro-6H-
azepino[5,4,3-c,d]indol-6-one
18. 2-(4-(4-Methylhomopiperazin-1-yl)phenyl)-1,3,4,5-tetrahydro-
6H-azepino[5,4,3-c,d]indol-6-one
19. 2-(4-(4-Benzylhomopiperazin-1-yl)phenyl)-1,3,4,5-tetrahydro-
6H-azepino[5,4,3-c,d]indol-6-one
20. 2-(4-(4-n-Butylhomopiperazin-1-yl)phenyl)-1,3,4,5-tetrahydro-
6H-azepino[5,4,3-c,d]indol-6-one
21. 2-(4-(4-Ethylhomopiperazin-1-yl)phenyl)-1,3,4,5-tetrahydro-
6H-azepino[5,4,3-c,d]indol-6-one
22. 2-Piperidin-4-yl-1,3,4,5-tetrahydro-6H-azepino[5,4,3-c,d]-
indol-6-one
23. 2-(1-Methylpiperidin-4-yl)-1,3,4,5-tetrahydro-6H-azepino-
[5,4,3-c,d]indol-6-one
24. 2-(1-n-Propylpiperidin-4-yl)-1,3,4,5-tetrahydro-6H-azepino-
[5,4,3-c,d]indol-6-one
25. 2-(1-Benzylpiperidin-4-yl)-1,3,4,5-tetrahydro-6H-azepino-
[5,4,3-c,d]indol-6-one
26. 2-(1-n-Butylpiperidin-4-yl)-1,3,4,5-tetrahydro-6H-azepino-
[5,4,3-c,d]indol-6-one
CA 02352194 2001-05-25
BASF Aktiengesellschaft 992956 O.Z. 0050/51636 DE
13
27. 2-(1-Isopropylpiperidin-4-yl)-1,3,4,5-tetrahydro-6H-azepino-
[5,4,3-c,d]indol-6-one
28. 2-(2-(N,N-Dimethylamino)eth-1-ylamino)phenyl)-1,3,4,5-
tetrahydro-6H-azepino[5,4,3-c,d]indol-6-one
29. 2-(2-(N,N-Diethylamino)eth-1-ylamino)phenyl)-1,3,4,5-
tetrahydro-6H-azepino[5,4,3-c,d]indol-6-one
30. 2-(2-Piperidin-1-yleth-1-ylamino)phenyl)-1,3,4,5-tetrahydro-
6H-azepino[5,4,3-c,d]indol-6-one
31. 2-(2-Pyrrolidin-1-yleth-1-ylamino)phenyl)-1,3,4,5-tetrahydro-
6H-azepino[5,4,3-c,d]indol-6-one
32. 2-(3-(N,N-Dimethylamino)prop-1-ylamino)phenyl)-1,3,4,5-
tetrahydro-6H-azepino[5,4,3-c,d]indol-6-one
33. 2-(3-(N,N-Diethylamino)prop-1-ylamino)phenyl)-1,3,4,5-
tetrahydro-6H-azepino[5,4,3-c,d]indol-6-one
34. 2-(3-Piperidin-1-yl-prop-1-ylamino)phenyl)-1,3,4,5-
tetrahydro-6H-azepino[5,4,3-c,d]indol-6-one
35. 2-(3-Pyrrolidin-1-yl-prop-1-ylamino)phenyl)-I,3,4,5-
tetrahydro-6H-azepino[5,4,3-c,d]indol-6-one
35
45