Some of the information on this Web page has been provided by external sources. The Government of Canada is not responsible for the accuracy, reliability or currency of the information supplied by external sources. Users wishing to rely upon this information should consult directly with the source of the information. Content provided by external sources is not subject to official languages, privacy and accessibility requirements.
Any discrepancies in the text and image of the Claims and Abstract are due to differing posting times. Text of the Claims and Abstract are posted:
| (12) Patent Application: | (11) CA 2352428 |
|---|---|
| (54) English Title: | PACKING MEANS FOR MEDICAMENTS IN THE FORM OF A FOLDED BOX COMBINATION FOR AT LEAST TWO DIFFERENT PRIMARY PACKINGS |
| (54) French Title: | SYSTEME D'EMBALLAGE POUR MEDICAMENTS SOUS FORME DE COMBINAISON DE BOITES PLIANTES POUR AU MOINS DEUX EMBALLAGES PRIMAIRES DIFFERENTS |
| Status: | Deemed Abandoned and Beyond the Period of Reinstatement - Pending Response to Notice of Disregarded Communication |
| (51) International Patent Classification (IPC): |
|
|---|---|
| (72) Inventors : |
|
| (73) Owners : |
|
| (71) Applicants : |
|
| (74) Agent: | MCCARTHY TETRAULT LLP |
| (74) Associate agent: | |
| (45) Issued: | |
| (86) PCT Filing Date: | 1999-11-24 |
| (87) Open to Public Inspection: | 2000-06-08 |
| Availability of licence: | N/A |
| Dedicated to the Public: | N/A |
| (25) Language of filing: | English |
| Patent Cooperation Treaty (PCT): | Yes |
|---|---|
| (86) PCT Filing Number: | PCT/EP1999/009071 |
| (87) International Publication Number: | WO 2000032484 |
| (85) National Entry: | 2001-05-28 |
| (30) Application Priority Data: | ||||||
|---|---|---|---|---|---|---|
|
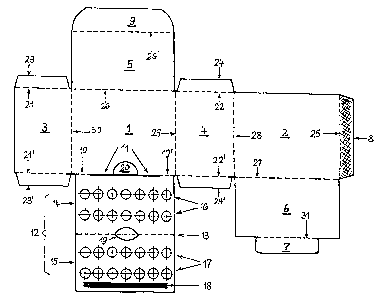
The invention relates to a packing means for medicaments in the form of a
folded box combination for at least two different primary packings, containing
transdermal plasters and capsules, in the form of a cardboard folded box
accommodating both medicaments. Said folded box comprises a receptacle for
individual rectangular plasters to be stacked in bulk in said receptacle. Said
receptacle has a structure according to DIN 55 429 with a front wall (1) and a
back wall (2) with joints (26 to 30) for a folding lid (6), a folding bottom
(5) and lateral walls (3, 4). The inventive packing means is characterized in
that the folded box further has a punch blank part (12) which is joined by
means of joints (10, 10') to the front wall (1) at both sides of a push-in
slot (11) formed in between. Said punch blank part (12) comprises two flat
sections (14, 15) which can be folded on top of each other along a folding
line (13). A number of holes (16, 17) is arranged in said flat sections. Said
punch blank part is configured to accommodate a tablet blister unit with deep-
drawn tablet cups containing the capsules. Said tablet blister unit can be
inserted between the flat sections (14, 15).
L'invention concerne un système d'emballage pour des médicaments, se présentant sous forme d'une combinaison de boîtes pliantes pour au moins deux emballages primaires différents, qui contient d'une part un emplâtre transdermique et d'autre part, des capsules, sous forme de boîte pliante en carton contenant les deux médicaments. Ce système comprend un espace collecteur approprié pour loger des emplâtres rectangulaires individuels pouvant être empilés de manière lâche. Ledit espace collecteur présente une structure de type DIN 55 429, avec une paroi avant (1) et une paroi arrière (2), avec des articulations (26 à 30) pour un couvercle à emboîter (6), un fond à emboîter (5), ainsi que des faces latérales (3, 4). L'invention se caractérise en ce que la boîte pliante comprend en outre une partie découpée matricée (12) montée par des articulations (10, 10') au niveau de la paroi avant (1) des deux côtés d'une fente d'emboîtement formée entre eux. Cette partie découpée matricée comprend deux parties en nappe (14, 15) pouvant être rabattues l'une sur l'autre le long d'une ligne de pliage (13) et comportant dans chaque cas, une pluralité de perforations (16, 17) pratiqués dedans. Cette partie découpée matricée est munie, pour recevoir une unité de plaquette alvéolaire pour comprimés, insérable entre les parties en nappe, de godets emboutis contenant les capsules.
Note: Claims are shown in the official language in which they were submitted.
Note: Descriptions are shown in the official language in which they were submitted.
2024-08-01:As part of the Next Generation Patents (NGP) transition, the Canadian Patents Database (CPD) now contains a more detailed Event History, which replicates the Event Log of our new back-office solution.
Please note that "Inactive:" events refers to events no longer in use in our new back-office solution.
For a clearer understanding of the status of the application/patent presented on this page, the site Disclaimer , as well as the definitions for Patent , Event History , Maintenance Fee and Payment History should be consulted.
| Description | Date |
|---|---|
| Application Not Reinstated by Deadline | 2003-11-24 |
| Time Limit for Reversal Expired | 2003-11-24 |
| Inactive: Agents merged | 2003-02-07 |
| Deemed Abandoned - Failure to Respond to Maintenance Fee Notice | 2002-11-25 |
| Letter Sent | 2001-10-17 |
| Inactive: Cover page published | 2001-09-27 |
| Inactive: Single transfer | 2001-09-06 |
| Inactive: First IPC assigned | 2001-08-29 |
| Inactive: Courtesy letter - Evidence | 2001-08-14 |
| Inactive: Notice - National entry - No RFE | 2001-08-07 |
| Application Received - PCT | 2001-07-30 |
| Application Published (Open to Public Inspection) | 2000-06-08 |
| Abandonment Date | Reason | Reinstatement Date |
|---|---|---|
| 2002-11-25 |
The last payment was received on 2001-05-28
Note : If the full payment has not been received on or before the date indicated, a further fee may be required which may be one of the following
Please refer to the CIPO Patent Fees web page to see all current fee amounts.
| Fee Type | Anniversary Year | Due Date | Paid Date |
|---|---|---|---|
| MF (application, 2nd anniv.) - standard | 02 | 2001-11-26 | 2001-05-28 |
| Basic national fee - standard | 2001-05-28 | ||
| Registration of a document | 2001-05-28 |
Note: Records showing the ownership history in alphabetical order.
| Current Owners on Record |
|---|
| LTS LOHMANN THERAPIE-SYSTEME AG |
| Past Owners on Record |
|---|
| CORNELIA WALTHER |