Note: Descriptions are shown in the official language in which they were submitted.
CA 02352660 2001-07-09
BYK-Chemie GmbH
Process for preparing a broad-compatibility, storage-
stable, rheologically active urea urethane solution
The invention relates to a process for preparing a
solution which comprises urea urethanes and is
effective as a thixotropic agent, which involves
reacting monohydroxy compounds with an excess of
toluylene diisocyanate, removing the unreacted portion
of the toluylene diisocyanate from the reaction
mixture, and further reacting the resultant
monoisocyanate adducts with diamines in an aprotic
solvent in the presence of a lithium salt to give urea
urethanes. The invention additionally relates to the
use of the solution to receive thixotropic coating
compositions.
In order to control the rheology of liquid coating
systems, predominantly organically modified bentonites,
silicas, hydrogenated castor oil and polyamide waxes
are used. A disadvantage of these substances is that
they are mostly dry solids which have to be brought
into the form of a semi-finished product using solvents
and shear forces, and incorporated into the liquid
coating system under careful temperature control.
Failure to observe such temperatures results in
crystallites in the finished coating system, which may
lead to defects in the coating.
The general disadvantage of these presently used
rheological auxiliaries is that they lead to
turbidities and haze in clear, transparent coatings.
Moreover, handling dry pulverulent products which give
rise to dusts in the course of processing is
undesirable.
Different solutions for rheology control have been
described in European Patent Application EP-A-0 198
519. There, an isocyanate is reacted with an amine in
CA 02352660 2001-07-09
- 2 -
the presence of solutions of film-forming resin to give
a urea which forms acicular crystals in very finely
.disperse form. The film-forming binders thus modified
are sold as rheology-controlling and anti-sagging
binders, referred to as sag control agents. The
disadvantage of these products is that they are always
bound to the binders in wh,ich they were prepared, and
there is no possibility of subsequent, universal
correction of finished coating compositions.
European Patent EP-B-0 006 252 describes a process for
preparing a thixotropic agent that removes some of the
abovementioned disadvantages, describing urea urethanes
which are prepared by reaction of isocyanate adducts
with polyamines in aprotic solvents in the presence of
lithium salts. The products thus prepared, however,
have two significant disadvantages. Firstly, these
thixotropic agents are characterized by an undefined
structure owing -to the preparation process. Although
monoisocyanate adducts are described, the actual
products are in fact not monoadducts at all, as is
clearly evident from the example, but rather mixtures
of different adducts. In the process described, one
mole of a diisocyanate is first reacted with one mole
of a monoalcohol. By this process the desired NCO-
functional monoadducts are partially formed, but also
non-NCO-functional diadducts, which in the course of
subsequent reaction with polyamines in the presence of
lithium chloride leads to uncontrolled chain extension
of the urea urethane and to polymeric ureas. These
products then tend to display precipitation phenomena
and are extremely difficult to keep in solution. A
further disadvantage of the thixotropic agents prepared
by this process can be recognized in the fact that
always only monoisocyanate adducts with the same
structure are reacted with the diamine. This leads
firstly to limited compatibility in the coating systems
used, manifested in gel structures or severe
CA 02352660 2003-10-09
_ 3 -
turbidities, and secondly to a poorer rheological
effectiveness.
It is therefore an object of the present invention to
find thixotropic agents which on the one hand have a
defined structure and therefore permit relatively high
storage stability of the solution thus prepared, over
several months, and on the other are characterized by a
relatively broad compatibility in binders, thereby
permitting reliable use of the products. This is of
particular interest in modern coating systems which are
relatively polar formulations, such as waterborne
coating materials or high-solids systems, for example.
Surprisingly it has been found that this object can be
achieved in a process of the type specified at the
outset by reacting at least two structurally different
monoisocyanate adducts, which differ in their alcohol
component, with the diamines to give urea adducts.
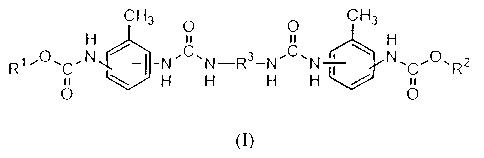
The present invention accordingly provides a process in
which at least two structurally different monohydroxy
compounds of the general structure R-OH, in which R
represents an n-alkyl radical or an iso-alkyl radical
having from 4 to 22 carbon atoms, an alkenyl radical
having from 3 to 18 carbon atoms, a cycloalkyl radical,
an aralkyl radical, or a radical of the formula
CmH2m+1 (O-CnH2n) x, CmH2m+i (OOC-CvH2v) x or Z-C6H4 (O-CnH2n) xr in
which m is 1-22, n is 2-4, x is 1-15, v is 4 or 5 and Z
is an alkyl radical having from 1 to 12 carbon atoms,
are reacted with a from 1.5- to 5-fold excess of
toluylene diisocyanate to give monoisocyanate adducts
of the general structure (I)
CH3
H
I \ N~ "o, ~
C R
11
OCN O
CA 02352660 2003-10-09
- 4 -
and the unreacted portion of the toluylene diisocyanate
is removed from the reaction mixture, and the resultant
monoisocyanate adducts are reacted with diamines of the
formula H2N-R'-NH2, in which R' is a radical
-CoH2o- where o = 2-12, -(CFH2P-O-CPH2p) q where p = 2-4 and
q = 1-10,
CH2 CH2
R"
R"
CH 2 where R" = CH3 or H, or
or mixtures thereof in an aprotic solvent in the
presence of a lithium salt to give urea adducts of the
general structure'(II)
CH3 O O CH3
H 11 11 H
(II) R1O.C.N ~-I -N'C'N-R3-Ne C, N-~-\ N%C~O'R2
u \ H H H H / n
O O
in which the radicals R' and R2 satisfy the conditions
for the radical R.
In the process of the invention, the abovementioned
diamines may be replaced in whole or in part by para-
xylylenediamine of the formula
H2N CH2 CH2 NH2
CA 02352660 2001-07-09
- 5 -
By the process of the invention, the solution which
comprises urea urethanes and is active as a thixotropic
agent may be obtained in principle by two different
routes:
a) on the one hand, it is possible first to mix at
least two structurally different alcohols R-OH and
then to react the mixture with a from 1.5- to 5-
fold excess of toluylene diisocyanate. The
unreacted portion of the toluylene diisocyanate is
subsequently removed from the reaction mixture
under gentle conditions, in accordance with the
prior art, and the resultant mixture of the
structurally different monoisocyanate adducts is
then reacted with the diamines in an aprotic
solvent in the presence of a lithium salt to give
urea urethanes of the general structure (II).
b) on the other hand, it is possible first to react
at least two structurally different alcohols R-OH
separately from one another with a from 1.5- to 5-
fold excess of toluylene diisocyanate. The
unreacted portion of the toluylene diisocyanate is
removed from the respective reaction mixture under
gentle conditions, in accordance with the prior
art, and the resultant, structurally different
monoisocyanate adducts are mixed with one another.
The resultant mixture of structurally different
monoisocyanate adducts is then reacted with the
diamines in an aprotic solvent in the presence of
a lithium salt to give urea urethanes of the
general structure (II).
The molar fraction of the respective monoisocyanate
adducts in the mixture of structurally different
monoisocyanate adducts is between 20 and 80%,
preferably between 35 and 65%, with particular
CA 02352660 2001-07-09
- 6 -
preference between 45 and 55%, the sum of the molar
fractions of the monoisocyanate adducts being 100%.
The molar excess of tolylene diisocyanate is preferably
from 1.5 to 5.0 mol, with particular preference from
2.0 to 4.0 mol.
The solids content of the urea urethane solutiorns thus
produced is from 5 to 80%, preferably from 20 to 60%,
with particular preference from 25 to 50%. The reaction
of the monoisocyanate adduct mixtures with the diamine
takes place in polar aprotic solvents, such as dimethyl
sulphoxide, N,N-dimethylformamide, N,N-dimethyl-
acetamide, N-methylpyrrolidone, N-butylpyrrolidone or
comparable alkylpyrrolidones, or mixtures thereof, for
example.
The fraction of lithium compounds is from 0.2 to 2 mol,
preferably from 0.5 to 1.5 mol, with particular
preference from 0.75 to 1.25 mol of lithium based on
the amine equivalent of the diamine used.
Particularly advantageous is the use of LiNO3 rather
than LiCl, since chloride ions have adverse effects in
coating systems and may promote the corrosion of the
metallic substrates to which the thixotropic coating
systems are applied.
The alcohols R-OH used to prepare the monoisocyanate
adducts preferably comprise linear or branched primary
alcohols which may be saturated or unsaturated, such as
n-butanol, 2-ethylhexanol, isotridecyl alcohol, Guerbet
alcohols of chain length Clo to C20, oleyl alcohol,
linoleyl alcohol, lauryl alcohol, stearyl alcohol, for
example, but cycloaliphatic alcohols, such as
cyclohexanol or its alkyl-substituted derivatives, for
example, and, additionally, aromatically substituted
alkanols such as benzyl alcohol, are also suitable.
CA 02352660 2007-12-06
- 7 -
Particularly suitable for adjusting the polarity are
the alkoxylated derivatives of the abovementioned
alcohols, in which case lower alcohols such as methanol
or allyl alcohol, for example, may also be used as
starting components for the alkoxylation. The products
thus prepared include preferably ethylene oxide and;or
propylene oxide units in the chain and may contain
these units in alternation or in blocks. For the
alkoxylation it is also possible to use aromatic
alcohols such as phenols or alkylphenols, for example,
as starting components.
In order to adjust the compatibility of the urea
urethanes of the invention to modern binder systems, it
is also possible to incorporate ester groups or
polyester groups into the alcohol component, for
example by addition reaction of lactones, such as s-
caprolactone, for example, with the abovementioned
alcohols or alcohol alkoxylates, or by the use of
hydroxy-functional (rneth)acrylates.
The diisocyanates which are used to form the
monoisocvanate adducts substantially comprise tolylene
diisocyanates in the known and customary isomer
distribution - in the course of the distillation of the
excess fractions of diisocyanate, shifts may occur in
the proportion of isomers, so that higher proportions
of 2,6-toluylene diisocyanate than commonly available
commercially may also be formed. These distillates may
be used again in the preparation of further
monoadducts. Preference is given to toluylene
diisocyanate isomers having a 2,4-isomer fraction of
from 50 to 100%, most preferably from about 65% and
still more preferably from about 80%. All percentages
expressed on the total weight of toluylene diisocyanate.
The diamines of the formula H2N-R'-NHZ substantially
comprise linear diamines of chain length C2 to C12 which
may be straight-chain or branched, such as 1,3-
propanediamine, hexamethylenediamine, octamethylene-
diamine, diaminododecane or neopentanediamine, for
CA 02352660 2001-07-09
- 8 -
example. Likewise suitable are. cyclic diamines such as
4,4'-diamin-odicyclohexylmethane or 3,3'-dimethyl-4,4'-
diaminodicyclohexylmethane, for example. Particular
preference is given to aromatic-aliphatic diamines such
as meta-xylylenediamine or para-xylylenediamine, for
example. The diamines may also be used as a.mixture in
order to form the urea, since by this means the
crystallization tendency of the urea urethane in
solution is reduced.
The urea urethanes prepared by the process of the
invention contain neither free isocyanate nor free
amino groups. Accordingly, they are physiologically
acceptable. Moreover, there are no adverse side-
reactions with binders or fillers. The storage
stability of the urea urethane solutions prepared in
this way is extremely high and at normal storage
temperatures is easily 6 months or more. Furthermore,
the urea urethane solutions possess broad compatibility
in binders and therefore permit reliable use of the
thixotropic agents.
The present invention additionally provides for the use
of the urea urethane solution prepared by the process
of the invention to render coating compositions
thixotropic. The coating compositions comprise,
preferably, aqueous, solvent-borne and solvent-free
coating materials, PVC plastisols, epoxy-based
coatings, and those based on unsaturated polyester
resins.
The main features of the process of the invention are
illustrated by the following working examples.
CA 02352660 2001-07-09
- 9 -
EXAMPLES
Comparative example in accordance with EP-B=0 006 252
( rion-inventive )
1 mol (174 g) of toluylene diisocya.nate (65% 2,4-
isomer, called T65 below) is charged to a reaction
vessel, 1 mol of inethoxypolyethylene glycol (average
MW: 350 g/mol) is slowly added dropwise, with stirring,
and the reaction is completed in accordance with a
known process. During the reaction, the temperature is
held below 40 C. The isocyanate adduct thus prepared
still has a free TDI content of 6.3%; the total NCO
content is 8.05%. This reaction mixture is added to a
solution of 0.5 mol of xylylenediamine (68 g) and
0.75 mol of LiCl, based. on amine equivalent, in N-
methylpyrrolidone (NMP). The SC is 50%. The reaction is
exothermic. The initially clear product tends to form
gel after storage for 2 months.
Preparation of the monoadducts (inventive)
Examples 1-7:
Example 1:
0.5 mol (37 g) of n-butanol is added over 2 hours at
30 C to 1.25 mol (217.5 g) of toluylene diisocyanate
(80% 2,4-isomer, called T80 below). The temperature is
held below 45 C. After the end of the addition,
stirring is continued for 2 h until the theoretical NCO
content of 33.0% has been reached. The excess
isocyanate is removed by vacuum (0.1 mbar) distillation
at from 150 to 170 C. The NCO content is 16.9%, the
free TDI content < 0.5%.
Example 2:
0.25 mol (53 g) of butyl triglycol is added over
2 hours at RT to 0.625 mol (108.75 g) of toluylene
diisocyanate (T65). The temperature is held below 45 C.
After the end of the addition, stirring is continued
for 2.5 h until the theoretical NCO content of 25.8%
CA 02352660 2007-12-06
- 10 -
has been reached. The excess isocyanate is removed by
vacuum (0.1 mbar) distillation at from 150 to 170 C.
The NCO content is 10.9%, the free TDI content < 0.5%.
Example 3:
0.25 mol (50 g) of iso-tridecanol is added over 2 hours
at 40 C to 0.75 mol (130.5 g) of toluylene diisocyanate
(T65) . The temperature is held below 60 C. After the
end of the addition, stirring is continued for 2 h
until the theoretical NCO content of 29.1% has been
reached. The excess isocyanate is removed by vacuum
(0.1 mbar) distillation at from 150 to 170 C. The NCO
content is 11.3%, the free TDI content < 0.5%.
Example 4:
0.25 mol (18 g) of butanol is reacted with 0.5 mol
(57 g) of caprolactone and 0.1% (0.075 g) of DBTL at
160 C for 6 h and then cooled to 50 C. The hydroxy
ester thus prepared (BuCP2) (hydroxyl number 186) is
metered at 40 C over 2 h into 0.75 mol (130.5 g) of
toluylene diisocvanate. The temperature is held below
60 C. After the end of the addition, stirring is
continued for 2 h until the theoretical NCO content of
26.2% has been reached. The excess isocyanate is
removed by vacuum (0.1 mbar) distillation at from 150
to 170 C. The NCO content is 9.2%, the free TDI content
< 0.5%.
Example 5:
0.2 mol (70 g) of methyoxypolyethylene glycol 350' is
added over 2 hours at 50 C to 0.6 mol (104.4 g) of
toluylene diisocyanate (T80). The temperature is held
between 50 C and 55 C. After the end of the addition,
stirring is continued for 3 h until the theoretical NCO
content of 24.1% has been reached. The excess
isocyanate is removed by vacuum (0.1 mbar) distillation
at from 150 to 170 C. The NCO content is 8.0%, the free
TDI content < 0.5%.
CA 02352660 2007-12-06
- 11 -
Example 6:
0.2 mol (100 g) of inethyoxypolyethylene glycol 500T'" is
added over 2 hours at 50 C to 0.6 mol (104.4 g) of
toluylene diisocyanate (T80). The temperature is held
between 50 C and 55 C. After the end of the addition,
stirring is continued for 3 h until the theoretical NCO
content of 20.5% has been reached. The excess
isocyanate is removed by vacuum (0.1 mbar) distillation
at from 150 to 170 C. The NCO content is 6.2%, the free
TDI content < 0.50.
Example 7:
A mixture of 0.2 mol (100 g) of inethyoxypolyethvlene
glycol 500TM and 0.2 mol (70 g) of inethoxypolyethylene
glycolTM 350 is added over 2 hours at 50 C to 0.8 mol
(139.2 g) of toluylene diisocyanate (T80). The
temperature is neld between 50 C and 55 C. After the
end of the addition, stirring is continued for 3 h
until the theoretical NCO content of 16.3% has been
reached. The excess isocyanate is removed by vacuum
(0.1 mbar) distillation at from 150 to 170 C. The NCO
content is 7.0%, the free TDI content < 0.5%.
Table 1: Monoadducts
Example Alcohol NCO Equivalent Molar
content weight ratio TDI
alcohol
1 Butanol 16.9% 248 2.5 : 1
2 Butyl 10.9% 392 2.5 : 1
triglycol
3 Iso- 11.3% 372 3 1
tridecanol
4 BuCP2 9.2% 457 3 1
5 MPEG 350 8.0% 525 3 1
6 MPEG 500 6.2% 675 3 1
7 MPEG 7.0% 600 2 1
350/MPEG 500
1:1
CA 02352660 2001-07-09
- 12 -
Table 2: Mixtures of the monoadducts from Table l
Mixture Monoadduct mixture Molar ratio
A Butyl triglycol/iso-tridecyl 1 : 1
(from Ex. 2 + 3)
B Butyl triglycol/MPEG 500 1 1.75
(from Ex. 2 + 5)
C Butyl triglycol/MPEG 350/MPEG 1 1 : 1
500 (from Ex. 2+ 5+ 6)
D Butyl triglycol/BuCP2 2 1
from Ex. 2 + 4)
E Butyl triglycol/MPEG 500 1 2
(from Ex. 2 + 6)
F MPEG 350/MPEG 500 1 1
(from Ex. 7)
Preparation of the urea urethanes (inventive)
Examples 8-14:
Example 8:
15.9 g of LiCl and 68 g(0.5 mol) of xylylenediamine
are dissolved in 403 g of N-methylpyrrolidone at 80 C.
Then 320 g of the mixture A are added in 1 h. When
addition is complete, stirring is continued for 30
minutes and then the solution is cooled to RT. The
resulting urea urethane solution has a solids content
of 50%. The product is clear and remains stable for a
relatively long time without forming gel.
Example 9:
15.9 g of LiCl and 68 g(0.5 mol) of xylylenediamine
are dissolved in 656 g of N-methylpyrrolidone at 80 C.
Then 572 g of the mixture B are added in 1 h. When
addition is complete, stirring is continued for 30
minutes and then the solution is cooled to RT. The
resulting urea urethane solution has a solids content
CA 02352660 2001-07-09
- 13 -
of 50%. The product is clear and remains stable for a
relatively long time.
Example 10:
25.8 g of LiN03 and 68 g(0.5 mol) of xylylenediamine
are dissolved in 690 g of N-methylpyrrolidone at 80 C.
Then 472 g of the mixture C are added in 1 h. When
addition is complete, stirring is continued for 30
minutes and then the solution is cooled to RT. The
resulting urea urethane solution has a solids content
of 45%. The product is clear over a prolonged period.
Example 11:
25.8 g of LiN03 and 68 g(0.5 mol) of xylylenediamine
are dissolved in 760 g of dimethylacetamide at 80 C.
Then 413 g of the mixture D are added in 1 h. When
addition is complete, stirring is continued for 30
minutes and then the solution is cooled to RT. The
resulting urea urethane solution has a solids content
of 40%. The product is clear and remains stable for a
relatively long time.
Example 12:
15.9 g of LiCl and 84 g (0.5 mol) of
hexamethylenediamine are dissolved in 830 g of N-
methylpyrrolidone at 80 C. Then 580 g of the mixture E
are added in 1 h. When addition is complete, stirring
is continued for 30 minutes and then the solution is
cooled to RT. The resulting urea urethane solution has
a solids content of 45%. The product is clear and
remains stable for a relatively long time.
Example 13:
15.9 g of LiCl and 84 g (0.5 mol) of
hexamethylenediamine are dissolved in 630 g of N-
methylpyrrolidone at 80 C. Then 320 g of the mixture A
are added in 1 h. When addition is complete, stirring
is continued for 30 minutes and then the solution is
cooled to RT. The resulting urea urethane solution has
CA 02352660 2001-07-09
- 14 -
a solids content of 40%. The product is clear and
remains stable for a relatively long time without
forming gel.
Example 14:
15.9 g of LiCl and 68 g(0.5 mol) of xylylenediamine
are dissolved in 497 g of N-methylpyrrolidone at 80 C.
Then 413 g of the mixture F are added in 1 h. When
addition is complete, stirring is continued for 30
minutes and then the solution is cooled to RT. The
resulting urea urethane solution has a solids content
of 50%. The product is clear and remains stable for a
relatively long time without forming gel.
Example 15:
11.1 g of LiCl and 47.6 g (0.5 mol) of para-
xylylenediamine are dissolved in 460 g of N-
methylpyrrolidone at 80 C. Then 400.4 g of the mixture
B are added in 1 h. When addition is complete, stirring
is continued for 30 minutes and then the solution is
cooled to RT. The resulting urea urethane solution has
a solids content of 50%. The product is clear and
remains stable for.a relatively long time.
Example 16:
15.5 g of LiN03 and 68 g (0.5 mol) of para-
xylylenediamine are dissolved in 414 g of N-
methylpyrrolidone at 80 C. Then 283.2 g of the mixture
C are added in 1 h. When addition is complete, stirring
is continued for 30 minutes and then the solution is
cooled to RT. The resulting urea urethane solution has
a solids content of 45%. The product is clear over a
prolonged period.
Application Examples:
The urea urethane solutions prepared by the process of
the invention were tested in water and in water/solvent
mixtures for their compatibility and for their
rheological effectiveness.
CA 02352660 2001-07-09
- 15 -
To examine the compatibility and the rheological
effectiveness in water and in water/solvent mixtures,
the urea urethanes are incorporated into water or into
the water/solvent mixtures with stirring for 2'minutes
at a shear rate of 1 m/sec. Assessment is made after
4 hours.
Evaluation of the rheological effectiveness: 1 = thick
gel, 6 = no gel.
Evaluation of the compatibility: 1= clear solution,
6 = precipitation of the urea urethanes
Table 3: Application results I
Additives Water/butyl glycol 95 : 5 Water/methoxypropanol 95 : 5
Rheological Compatibility Rheological Compatibility
effectiveness effectiveness
Compar. Ex. 4 5 3-4 4-5
Example 10 2-3 2-3 2 3
Example 9 3 1-2 1 3-4
Example 12 3 1-2 1 3'
Additives Water/methoxypropanol Water/butyl glycol/
90 : 10 methoxypropanol
95 : 2.5 : 2.5
Rheological Compatibility Rheological Compatibilit
effectiveness effectiveness y
Compar. Ex. 4 5 3 4
Example 10 1 2-3 1-2 2
Example 9 1-2 2-3 2 1-2
Example 12 2 2-3 2 1-2
To examine the anti-settling effect of the urea
urethanes of the invention, pigment slurries were
prepared and the sedimentation behaviour was examined
after 3 weeks' storage.
CA 02352660 2007-12-06
- 16 -
To prepare the pigment slurries, first of all a mixture
of water, butyl glycol and Disperbyk 192TM is prepared.
This mixture is then added with stirring to the
pigment, Iriodin 9303 Royal Gold WR IITM from Merck.
Thereafter, the urea urethanes of the invention are
incorporated likewise with stirring using the
Dispermat, for 2 minutes at a shear rate of 2 m/s. To
assess the sedimentation behaviour, these slurries are
introduced into a glass tube (10 cm high, 1.5 cm 0) to
a height of 7.5 cm. The syneresis is determined after 3
weeks' storage at RT.
Table 4: Application results II
Control Compar. Example Example
Ex. 10 12
Iriodin 9303 30.0 30.0 30.0 30.0
Royal Gold WR II""
Water 64.5 64.5 64.5 64.5
Butyl glycol 4.0 4.0 4.0 4.0
Disperbyk 192'" 1.5 1.5 1.5 1.5
Urea urethane 1.0 1.0 1.0
(Table 4 indicates the relative fractions of the
individual components in the mixture)
Table 5: Anti-settling effect
Control Compar. Example Example
Ex. 10 12
Total height 7.5 cm 7.5 cm 7.5 cm 7.5 cm
Syneresis 4.2 cm 1.7 cm 0.1 cm 0 cm