Note: Descriptions are shown in the official language in which they were submitted.
CA 02354197 2001-07-27
1
IDENTIFICATION OF NUCLEOTIDE SEQUENCES SPECIFIC FOR MYCOBACTERIA AND
DEVELOPMENT OF DIFFERENTIAL DIAGNOSIS STRATEGIES FOR MYCOBACTERIAL SPECIES.
FIELDS OF THE INVENTION.
The present invention refers to new genetic sequences, diagnostic and/or
quantification
methods and devices using said sequences for the identification of various
types of
Mycobacterium strains
BACKGROUND TO THE INVENTION.
Classification
The mycobacterial diseases may be divided in three categories: tuberculosis
which is
caused either by M. tuberculosis or M. bovis, both belonging to the M.
tuberculosis-complex
(TUB), leprosy caused by M. leprae, and diseases caused by nontuberculous
mycobacteria
(NTM), which cause all mycobacterial diseases other than tuberculosis and
leprosy.
Historically, tuberculosis and leprosy are the two preponderant human
mycobacterial
diseases. In recent years however, NTM have become more frequent in developed
countries, partly because of the development of Acquired Immunodeficiency
Syndrome
(AIDS) (1). Tuberculosis occurs only in humans and animals, no other reservoir
has been
2o found to date. Conversely, the reservoir of mycobacteria responsible for
NTM disease is
mainly environmental and the majority of these mycobacteria are naturally
resistant to known
antimycobacterial drugs, making eradication unfeasible. Identification of
mycobacteria
species by rapid and specific methods is now mandatory. In particular, there
is a need for
rapid differentiation of strict human and animal pathogenic mycobacteria (such
as, for
example, M. tuberculosis-complex, M. paratuberculosis, M. leprae) from
potentially
pathogenic mycobacteria (such as, for example, M. avium-complex; M. chelonae,
M.
kansasii, M. xenopi, M. simiae, M. malmoense) and from normally saprophytic
species (such
as, for example, M. gordonae; M. terrae; M. nonchromogenicum; M. flavescens;
M. gastri; M.
smegmatis). Except for the strict pathogens, the majority of mycobacteria can
indeed be
3o found everywhere in the nature (soil, fresh and seawater) and can colonise
temporary or
permanently the human respiratory or digestive tract after ingestion or
inhalation.
a) Members of the tuberculosis-complex (TUB):
- This group comprises M. tuberculosis, M. bovis, M. africanum, M. microti.
b) Non tuberculous mycobacteria (NTM)
- Members of the M. avium-complex (MAC-complex) includes several
environmental species that resemble M. avium and M. intracellulare or that
have
CA 02354197 2001-07-27
2
intermediate characteristics common to these species (M. avium; M.
intracellulare; M. paratuberculosis, M. scrofulaceum).
- Mycobacteria other than TUB (also named MOTT) and not belonging to the MAC-
complex (such as, for example, M. malmoense, M. sulzgai, M. kansasii, M.
xenopi, M. chelonae, M. simiae, M. marinum, M. gordonae, M. fortuitum).
Epidemiolo~y
Tuberculosis infects one-third of the world's population and kills more than 3
million of
people each year. Tuberculosis, caused by mycobacteria of the M. tuberculosis
group
(TUB), is still highly endemic in large parts of the world and also the
potential for introduction
into and further transmission within countries where it has become rare (2).
Tuberculosis has
indeed re-emerged in many industrialised nations in recent years as a results
of AIDS and
changing population dynamics characterised by increased numbers of migrant
workers,
immigrants, and homeless people (3). Of particular concern is the spread of
drug-resistant
strains (4). Cases of tuberculosis are more and more often difficult to treat
because of the
increasing prevalence of TUB strains that are resistant to all front-line
antituberculous drugs.
In HIV-1 infected people, TUB is the most common opportunistic bacterial
infections. In
advanced stages of AIDS, mycobacterial infections due to members of the M.
avium-
intracellulare complex (MAC) are the most common systemic bacterial
opportunistic infection
(1 ). Moreover, reduced and compromised immune function as found in new-borns,
infants,
and immune-suppressed individuals allows opportunistic infections caused by
mycobacteria
other than M. tuberculosis (NTM) including M, avium-intracellulare, M.
chelonae, M.
fortuitum, M. kansasii, M. xenopi, M. marinum, M. ulcerans, M. scrofulaceum,
and M.
szulgai.
The increasing number of mycobacterial infections has made it clinically
important to quickly
identify mycobacteria at the species level. The diagnosis of a pathogenic
versus a non-
pathogenic species not only has epidemiological implications but is also
relevant to the
demands of patient management. Individuals with highly contagious infections
may be
3o isolated to prevent the spread of the disease. As a matter of fact,
antibiotic treatments may
vary according to the species encountered. Today, the number of non-
tuberculous infections
is difficult to assess because there is no system for notification as it
exists for M.
tuberculosis. The currently reported frequency of these species is likely to
be an
underestimation due to the lack of additional testing in cases of minimal
disease or
misidentification as M. tuberculosis. Despite the likely underestimation of
NTM diseases, a
growing number of NTM isolates are submitted to laboratories for
identification. This may
CA 02354197 2001-07-27
3
reflect an increase in the prevalence of opportunistic mycobacterial disease
(notably in the
AIDS context), or it may also reflect an increase in the number and nature of
specimens
submitted for culture brought about by a greater awareness of tuberculosis.
The ability to detect and identify these mycobacterial diseases in an early
stage would
potentially decrease morbidity and mortality and lessen its socio-economic
impact. The
«gold standard» for definite diagnosis has remained culturing the
mycobacterium combined
with phenotypic tests (for example, the analysis of fatty acid and mycolic
acid contents).
However, traditional culture methods for human specimens can require up to 8
weeks (this
1o varies according to the mycobacterial species). For instances, primary
culture of M.
ulcerans under optimal conditions may take several months (5).
Other methods based on molecular biology technologies have allowed the
development of
genetic tests for the identification of some mycobacterial species. In the
last decade, the use
of restriction fragment length polymorphism (RFLP) analysis, oligotyping, and
nucleic acid
probes have been proposed as a mean for detecting and definitively identifying
infectious
agents (6). These tests are highly specific and rapid when compared to
conventional culture
method and have been applied to a wide variety of pathogens, especially to
fastidious micro-
organisms like mycobacteria. To date, the design of molecular tests has
speeded up the
2o diagnosis of this fastidious genus but still suffers some drawbacks. For
species identification,
a highly polymorphic region of the 16S rRNA gene has been shown to contain
species-
specific polymorphisms. This region is currently used in several commercially
available
assays, applicable either directly on a specimen (Accu-Probe; Gen-Probe) or
after enzymatic
amplification of the target for improved sensitivity (AMTD; Gen-Probe;
Amplicor MTB,
Roche). However, these commercial kits are mostly designed for the diagnosis
of the most
common disease-causing mycobacterial species, i.e. M. tuberculosis and MAC
strains, and
each kit has been designed to identify only one mycobacterial species per test
which makes
it a very expensive and limitative way of identifying mycobacterial species.
CA 02354197 2001-07-27
4
SUMMARY OF THE INVENTION
The newly identified and characterised sequences described in the present
invention enable
the design of differential diagnosis strategies. These diagnosis strategies
are able to identify,
in one single assay, a wide range of mycobacterial species that include TUB
and NTM. In
addition, within the NTM group, it is even possible to differentiate between
the members of
the MAC-complex. Moreover, these molecular targets open new perspectives for
the
quantification of mycobacteria strains in clinical samples.
to The present invention aims to provide new genetic sequences, methods and
devices for the
improvement of the identification and/or the quantification of various types
of mycobacteria
species through their upstream-p34 (us-p34) determinants, which allow by a
rapid molecular
screening, their epidemiological study as well as their rapid characterisation
in clinical
human, animal and/or environmental samples.
Another aim of the invention is to identify similar genetic sequences which
may exist in
known and yet unknown Mycobacteria species.
DETAILED DESCRIPTION OF THE INVENTION
2o The present inventors for the first time recognised, by sequencing the
upstream part of the
p34 gene, species-specific differences in the sequences between various
mycobacterial
strains, allowing determination of the presence and identification of specific
Mycobacterium
strains or species in various samples.
Therefore, according to a first embodiment the present invention relates to a
method for
detecting at least one Mycobacterium strain in a sample, comprising:
(i) providing at least one Mycobacterium species-specific upstream p34 gene
region (us-
p34) nucleotide probe,
(ii) reacting said us-p34 nucleotide probe with said sample under conditions
that allow for
3o the selective formation of nucleotide duplexes between said us-p34
nucleotide probe
and a corresponding Mycobacterium nucleic acid target present in said sample,
and,
(iii) detecting any nucleotide duplexes containing said us-p34 nucleotide
probe.
Said probes may comprise the whole or only parts of species-specific us-p34
gene region
sequences, some of which are disclosed in Figure 3 which the skilled man has
aligned in
Figures 1 and 8 to 10. On the basis of such an alignments it is within the
skilled man's
knowledge to actually design suitable probes to be used in said method.
CA 02354197 2001-07-27
In this method more than one Mycobacterium strain may be detected, provided
that the
species-specific probes can be distinguished from each other. Numerous well-
known
methods are described in the art to come to this result. As an example, the
species-specific
probes can be labelled with different markers which can be detected by various
methods or
5 devices after hybridisation with the target as to distinguish between the
nucleotide duplexes
formed.
It should be understood that the above described method is not limited to the
us-p34
sequences actually provided in Figure 3. The us-p34 sequences envisaged in the
invention
1o encompass the complete us-p34 sequences and also all the corresponding
sequences of
the various Mycobacerium species which are shown to contain species-specific
differences.
Said differences not only comprise point mutations but also include deletion
and/or insertion
of several nucleotide bases. These deletions and/or insertions may result in
different lengths
of the us-p34 upstream region between various species.
According to a preferred embodiment the present invention more particularly
relates to a
method as defined above, wherein said Mycobacterium species-specific us-p34
nucleotide
probe specifically hybridizes with at least part of a sequence selected from
Figure 3, or the
complement thereof, or the corresponding sequences wherein T has been replaced
by U.
The specific or non-specific hybridization of the species-specific us-p34
probes is further
illustrated in Figure 5.
In Tables 3 (a) to (c), nucleotide sequences are represented for use as probe
or primer in
2s various methods for detection, identification, hybridisation to, capture,
amplification of
Mycobacterium species-specific nucleic acids.
The present invention more particularly relates to a method as defined above,
wherein said
Mycobacterium species-specific us-p34 nucleotide probe is selected from the
group of
3o sequences represented by SEQ ID NOs 8 to 54 (Tables 3 (a) to (c)) and SEQ
ID NOs 57 to
74 (Figure 3), or the corresponding sequences wherein T has been replaced by
U.
According to yet another embodiment, the invention relates to a method for the
differential
detection of Mycobacteria in a sample, comprising:
35 (i) providing at least two distinct Mycobacterium species-specific us-p34
nucleotide
probes,
(ii) reacting said us-p34 nucleotide probes with said sample under conditions
that allow
for the selective formation of nucleotide duplexes between said us-p34
specific
nucleotide probe and a Mycobacterium nucleic acid present in said sample,
40 (iii) detecting any nucleotide duplexes containing said us-p34 nucleotide
probe, and,
CA 02354197 2001-07-27
6
(iv) inferring from the nucleotide duplex formed, the presence and the
identification of a
specific Mycobacterium strain.
In a preferred embodiment, said species-specif us-p34 probes are chosen from
the group of
sequences represented by SEQ ID NOs 8 to 54 and 57 to 74
The present invention also relates to a method for detecting at least one
Mycobacteria strain
in a sample, comprising:
(i) providing at least one suitable us-p34 primer pair comprising a sense and
anti-sense
1o us-p34 primer,
(ii) reacting said us-p34 primer pair with said sample under conditions that
allow for the
selective amplification of us-p34 sequences of at least one Mycobacterium
nucleic
acid present in said sample,
(iii) detecting the amplified product of step (ii), and,
(iv) inferring from the amplified product formed, the presence and the
identification of at
least one (specific) Mycobacterium strain or species.
It should be clear to the one skilled in the art and from the information
provided in the
Figures, especially Figure 4, that the above described method does not
specifically require
2o the use of a Mycobacterium species-specific us-p34 primers to be able to
identify specific
Mycobacterium strans or species from the amplification product formed. It has
been found by
the present inventors that the differences in the us-p34 region of various
species results in
different lengths of the amplification products when amplified for instance
with the universal
primers provided in Tables 1 and 2 (SEQ ID NOs 1 to 7). The detection of
Mycobacterium
species in a sample is therefore possible through means of non-specific or
specific
amplification of the us-p34 regions. Said universal primers are especially
designed by the
present inventors to amplify us-p34 sequences of most if not all Mycobacterium
species. In
Tables 1 and 2 the skilled man will find several universal sense and antisense
primers which
can be used according to the sheme represented in figure 2 to form a suitable
primer pair for
3o amplifying the desired us-p34 region in the above method.
Alternatively also species-specific primers can be used in such a method,
which may lead to
the species-specific identification of the Mycobacterium present in the
sample. Species-
specific us-p34 primers are derived from us-p34 sequences (parts of the
sequences as given
in Figure 3) which are present in one specific species but not in the others.
On the basis of
the alignment of the sequences as given in Figure 3 (see Figures 8 to 10), it
is within the
knowledge of the skilled man to design actual primer sequences which will
selectively
amplify one species (type) of Mycobacterium. Nucleotides having a sequence as
CA 02354197 2001-07-27
7
respresented in any of SEQ ID NOs 8 to 54 (Table 3 (a) to (c)) are used as a
species-
specific primer in a such a method.
The invention thus also relates to a method for detecting at least one
Mycobacterium strain
in a sample, comprising:
(i) providing at least one suitable primer pair comprising a sense or
antisense
Mycobacterium species-specific us-p34 primer selected from the group of
sequences
represented in SEQ ID NOs 8 to 54,
(ii) reacting said us-p34 primer pair with said sample under conditions that
allow for the
to selective amplification of an us-p34 sequence in a Mycobacterium nucleic
acid
present in said sample, and,
(iii) detecting the amplified product of step (ii), and,
(iv) inferring from the amplification product the presence and the
identification of at least
one specific Mycobacterium strain.
It should be obvious for the skilled in the art that the second primer
constituting said primer
pair can be chosen from the sequences provided in Table 3 (a) to (c) where
sequences of a
sense and antisense primer are provided for a specific Mycobacterium species.
Alternatively,
the skilled in the art can choose the second primer from the universal primers
presented in
2o Tables 1 and 2 and represented by SEQ ID NOs 1 to 7. As such, the
amplification products
resulting from one universal and one species-specific primer are still species-
specific.
The invention also relates to a method for the differential detection of
mycobacteria in a
sample, comprising:
(i) providing at least one suitable us-p34 primer pair containing a sense and
anti-sense
us-p34 primer,
(ii) reacting said us-p34 primer pair with said sample under conditions that
allow for the
selective amplification of us-p34 sequences of at least one Mycobacterium
nucleic
acid present in said sample,
(iii) detecting the amplified product of step (ii), and,
(iv) inferring from the amplified product formed, the presence and the
identification of at
least one (specific) Mycobacterium
As described above, already the length of the amplification products may be
indicative for
the presence of a specific Mycobacterium species. Therefore according to a
preferred
embodiment, the primers to be used in the above method are selected from the
sequences
represented in SEQ ID NOs 1 to 7 and presented as universal primers in Tables
1 and 2.
Alternatively, also species-specific us-p34 primers are prefebly used in the
above method.
Therefore the invention also related to a method as described above wherein
the primers are
selected from the species-specific us-p34 primers represented by SEQ ID NOs 8
to 54. The
invention also relates to a method as described above wherein a first primer
constituting the
CA 02354197 2001-07-27
8
primer pair is selected from the universal primers represented by SEQ ID NOs 1
to 7 and a
second primer is selected from the species-specific primers represented by SEQ
ID NOs 8
to 54.
According to a preferred embodiment the invention relates to a method for the
detection of
MAC complex Mycobacterium species in a sample, comprising:
(i) providing at least one us-p34 probe selected from the group of sequences
represented in SEQ ID NOs 8, 14, 15, 22, 27, 28, 29, 34, 35, 50, 51, 57, 68,
and 73,
(ii) reacting said us-p34 probe with said sample under conditions that allow
for the
Io selective formation of nucleotide duplexes between said us-p34 nucleotide
probe and
a MAC complex Mycobacterium nucleic acid target in said sample, and,
(iii) detecting any nucleotide duplexes containing said us-p34 nucleotide
probe.
Members belonging to the MAC comples Mycobacterium species are presented in
Table 4.
In an alternative method also embodied by the present invention, step (iii) of
the above
described method can be replaced by the step of determining the us-p34
sequence of said
MAC Mycobacterium nucleic acid in said sample wherein the with the expression
"determining the us-p34 sequence" is meant, for instance, direct sequencing to
confirm the
presence of said specific MAC complex us-p34 sequence. Other methods can be by
mass
spectrometry, capillary electrophoresis or HPLC.
According to a preferred embodiment the invention relates to a method for the
detection of
MOTT Mycobacterium species in a sample, comprising:
(i) providing at least one us-p34 probe selected from the group of sequences
represented in SEQ ID NOs 9 to 13, 16 to 21, 24, 25, 26, 30 to 33, 36 to 47,
49, 53,
54, 59 to 64, 67, 69 to 72 and 74,
(ii) reacting said us-p34 primer probe said sample under conditions that allow
for the
selective formation of nucleotide duplexes between said us-p34 nucleotide
probe and
a MOTT Mycobacterium nucleic acid target in said sample, and,
(iii) detecting any nucleotide duplexes containing said us-p34 nucleotide
probe.
Members belonging to the MOTT comples Mycobacterium species are presented in
Table 4.
In an alternative method also embodied by the present invention, step (iii) of
the above-
described method can be replaced by the step of determining the us-p34
sequence of said
MOTT Mycobacterium nucleic acid in said sample wherein the with the expression
"determining the us-p34 sequence" is meant, for instance, direct sequencing to
confirm the
presence of said specific MOTT complex us-p34 sequence. Other methods can be
by mass
spectrometry, capillary electrophoresis or HPLC.
CA 02354197 2001-07-27
9
The invention further relates to a method for detecting new us-p34 sequences
in a sample,
comprising:
(i) providing at least one suitable primer pair comprising a sense and anti-
sense us-p34
primer selected from the sequences represented in SEQ ID NOs 1 to 7,
(ii) reacting said us-p34 primer pair with said sample under conditions that
allow for the
amplification of an us-p34 sequence in a Mycobacterium nucleic acid target in
said
sample, and,
(iii) determining the sequence of the amplification product obtained in (ii).
to The expression "new us-p34 sequences" refers to new (not yet identified)
mycobacterium
species sequences. Some of these new sequences are disclosed in Figure 3.
The present invention further relates to a method for the differential
detection of
mycobacteria in a sample, comprising:
(i) providing at least one suitable primer pair comprising a sense and anti-
sense us-p34
primer selected from SEQ ID NOs 1 to 7,
(ii) reacting said us-p34 primer pair with said sample under conditions that
allow for the
amplification of an us-p34 sequence in a Mycobacterium nucleic acid target in
said
sample,
(iii) selectively hybridizing the amplification products obtained in (ii) with
at least one
Mycobacterium species-specific us-p34 nucleotide probe selected from the group
of
sequences represented in SEQ ID NOs 8 to 74,
(iv) detecting any nucleotide duplexes containing said Mycobacterium species-
specific
us-p34 nucleotide probe, and,
2s (v) inferring from the nucleotide duplex formed the presence of a specific
Mycobacterium
species.
Different examples of how universal primers and species-specific and probes
are used in the
above method are shown in Table 3.
The present invention also relates to a Mycobacterium species-specific us-p34
nucleotide
probe or primer comprising at least 8 contiguous nucleotides from one of the
nucleic acid
sequences represented in SEQ ID NOs 57 to 74, or the complement thereof, or
the
corresponding sequences wherein T has been replaced by U.
The present invention also relates to the Mycobacterium species-specific us-
p34 nucleotide
probe or primer as represented in SEQ ID NOs 8 to 54.
CA 02354197 2001-07-27
to
The invention also relates to the Mycobacterium us-p34 universal primers as
represeted in
SEQ ID NOs 1 to 7.
The present invention also relates to the non-tuberculosis Mycobacteria
species-specific us-
p34 nucleotide probe as defined above selected from Table 3.
The present invention also relates to a nucleic acid comprising a sequence
selected from
Table 3 and Figure 3 and having a sequence as represented by SEQ ID NOs 8 to
54.
Preferably said sequence is a continuous sequence of any of said SEQ ID Nos
having a
length from 8 to maximally the full length of said SEQ ID NOs.
The present invention also relates to a composition comprising at least one
nucleotide
probe, primer or sequence as defined above. Said composition preferably
contains two,
three or more of said components.
The present invention also relates to a diagnostic kit comprising a probe,
primer or sequence
as defined above or a composition as defined above.
The present invention also relates to a solid support for the detection of
(specific)
2o mycobacteria comprising fixed to said support at least two capture probes
selected from
Table 3 (SEQ ID NOs 8 to 54) or from the sequences represented by SEQ ID NOs
57 to 74.
The invention also relates to a solid support for the detection of
mycobacteria comprising
fixed to said support at least one nucleic acid having a sequence as
represented in SEQ ID
NOs 1 to 7.
The present invention also relates to a solid support as defined above for use
in a method as
defined above.
Different techniques can be applied to perform the methods of the present
invention. These
3o techniques may comprise immobilizing the target polynucleic acids, after
amplification, on a
solid support and performing hybridization with labelled oligonucleotide
probes.
Alternatively, the probes of the invention may be immobilized on a solid
support (covalently
or non-covalently) and hybrdization may be performed with labelled target
polynucleic acids,
possibly after amplification. This technique is called reverse hybridization.
Said techniques
are well-known to the man skilled in the art.
It is to be understood that also any other technique for detection of the
above-mentioned
amplified target sequences are also covered by the present invention. Such a
technique can
involve sequencing or other micro-array methods known in the art.
CA 02354197 2001-07-27
11
In preferred methods according to the invention it is possible to
differentiate between two
Mycobacterium species.
Therefore in a preferred embodiment, the invention relates to a method for
differentiating
between Mycobacterium bovis and Mycobacterium tubercolusosis in a sample,
comprising:
(i) providing at least one us-p34 probe selective for Mycobacterium bovis or
Mycobacterium tuberculosis wherein said probe is SEQ ID NO 66 for
Mycobacterium
bovis or SEQ ID NO 65 for Mycobacterium tuberculosis,
to (ii) reacting said us-p34 nucleotide probe with said sample under
conditions that allow
for the selective formation of nucleotide duplexes between said us-p34
nucleotide
probe and a corresponding Mycobacterium bovis or tuberculosis nucleic acid
target
present in said sample, and,
(iii) detecting any nucleotide duplexes containing said Mycobacterium bovis or
tuberculosis specifc us-p34 nucleotide probe.
The invention also relates to a method for differentiating between
Mycobacterium bovis and
Mycobacterium tubercolusosis in a sample, comprising:
providing at least one suitable primer pair comprising at least one sense or
antisense
2o us-p34 primer selective for Mycobacterium bovis or Mycobacterium
tuberculosis
wherein said primer is SEQ ID NO 66 for Mycobacterium bovis or SEQ ID NO 65
for
Mycobacterium tuberculosis,
(ii) reacting said us-p34 primer pair with said sample under conditions that
allow for the
selective amplification of Mycobacterium bovis andlor Mycobacterium
tuberculosis
nucleic acid target present in said sample, and,
(iii) inferring from the reaction products) the presence of Mycobacterium
bovis and/or
Mycobacterium tuberculosis in said sample.
The invention further relates to a method for differentiating between
Mycobacterium avium
3o and Mycobacterium avium subspecies paratuberculosis in a sample,
comprising:
(i) providing at least one us-p34 probe selective for Mycobacterium avium and
Mycobacterium avium subspecies paratuberculosis wherein said probe is selected
from the sequences represented in SEQ ID NOs 8, 27 to 29, 50 or 58 for
Mycobacterium avium or SEQ ID NO 68 for Mycobacterium avium subspecies
paratuberculosis ;
(ii) reacting said us-p34 nucleotide probe with said sample under conditions
that allow
for the selective formation of nucleotide duplexes between said us-p34
nucleotide
probe and a corresponding Mycobacterium avium and Mycobacterium avium
subspecies paratuberculosis nucleic acid target present in said sample, and,
CA 02354197 2001-07-27
12
(iii) detecting any nucleotide duplexes containing said Mycobacterium avium
and
Mycobacterium avium subspecies paratuberculosis specifc us-p34 nucleotide
probe
The invention also relates to a method for differentiating between
Mycobacterium avium and
Mycobacterium avium subspecies paratuberculosis in a sample, comprising:
(i) providing at least one suitable primer pair comprising at least one sense
or antisense
us-p34 primer selective for Mycobacterium avium and Mycobacterium avium
subspecies paratuberculosis wherein said primer is selected from the sequences
represented in SEQ ID NOs 8, 27 to 29, 50 or 58 for Mycobacterium avium or SEQ
1o ID NO 68 for Mycobacterium avium subspecies paratuberculosis,
(ii) reacting said us-p34 primer pair with said sample under conditions that
allow for the
selective amplification of Mycobacterium avium and Mycobacterium avium
subspecies paratuberculosis nucleic acid target present in said sample, and,
(iii) inferring from the reaction products) the presence of Mycobacterium
avium and
Mycobacterium avium subspecies paratuberculosis in said sample.
The following definitions and explanations will permit a better understanding
of the present
invention.
2o The target material in the samples to be analysed may either be DNA or RNA,
e.g. genomic
DNA, messenger RNA or amplified versions thereof. These molecules are in this
application
also termed "polynucleic acids" or "nucleic acids".
Well-known extraction and purification procedures are available for the
isolation of RNA or
DNA from a sample (e.g. in Sambrook et al., 1989).
The term "probe" according to the present invention refers to a single-
stranded
oligonucleotide which is designed to specifically hybridize to the target
polynucleic acids.
According to the invention those probes can be as long as the complete us-p34
sequences
3o the Mycobacterium p34 gene. Some examples of those sequences are provided
in the
figures and in the nucleic acids having a sequence as represented in SEQ ID
NOs 57 to 74.
Also preferred according to the invention are probes having a sequence which
is part of the
us-p34 upstream sequences of about 5 to 50 nucleotides long, more preferably
from about
10 to 25 nucleotides. Particularly preferred lengths of probes include 10, 11,
12, 13, 14, 15,
16, 17, 18, 19, 20, 21, 22, 23, 24 or 25 nucleotides. The nucleotides as used
in the present
invention may be ribonucleotides, deoxyribonucleotides and modified
nucleotides such as
CA 02354197 2001-07-27
13
inosine or nucleotides containing modified groups which do not essentially
alter their
hybridization characteristics.
Probes may be labelled with a label of choice (e.g. biotin, fluoresceine,
radioactive labels)
according to methods well known in the art.
The term "primer" according to the present invention refers to a single
stranded
oligonucleotide sequence capable of acting as a point of initiation for
synthesis of a primer
extension product which is complementary to the nucleic acid strand to be
copied. The
1o length and the sequence of the primer must be such that they allow to prime
the synthesis of
the extension products. Preferably the primer is about 5-50 nucleotides long.
Specific length
and sequence will depend on the complexity of the required DNA or RNA targets,
as well as
on the conditions at which the primer is used, such as temperature and ionic
strength. It is
to be understood that the primers of the present invention may be used as
probes and vice
versa, provided that the experimental conditions are adapted.
The expression "suitable primer pair" in this invention refers to a pair of
primers allowing
specific amplification of a us-p34 target polynucleic acid fragment as defined
above. Said
amplification can be species-pecific or not, dependending on the use of
universal or species
2o specific primers as indicated in the description.
The term "target region" of a probe or a primer according to the present
invention is a
sequence within the polynucleic acids to be detected to which the probe or the
primer is
completely complementary or partially complementary (i.e. with some degree of
mismatch).
It is to be understood that the complement of said target sequence is also a
suitable target
sequence in some cases.
"Specific hybridization" of a probe to a target region of a polynucleic acid
means that said
probe forms a duplex with part of this region or with the entire region under
the experimental
3o conditions used, and that under those conditions said probe does not form a
duplex with
other regions of the polynucleic acids present in the sample to be analysed.
"Specific hybridization" of a primer to a target region of a polynucleic acid
means that, during
the amplification step, said primer forms a duplex with part of this region or
with the entire
region under the experimental conditions used, and that under those conditions
said primer
does not form a duplex with other regions of the polynucleic acids present in
the sample to
CA 02354197 2001-07-27
14
be analysed. It is to be understood that "duplex" as used hereby, means a
duplex that will
lead to specific amplification.
The fact that amplification primers do not have to match exactly with the
corresponding
target sequence in the template to warrant proper amplification is amply
documented in the
literature. However, when the primers are not completely complementary to
their target
sequence, it should be taken into account that the amplified fragments will
have the
sequence of the primers and not of the target sequence. Primers may be
labelled with a
label of choice (e.g. biotin). The amplification method used can be either
polymerase chain
1o reaction (PCR), ligase chain reaction (LCR), nucleic acid sequence-based
amplification
(NASBA), transcription-based amplification system (TAS), strand displacement
amplification
(SDA) or amplification by means of Qf3 replicase or any other suitable method
to amplify
nucleic acid molecules known in the art.
Probes and primer sequences are represented throughout the specification as
single
stranded DNA oligonucleotides from the 5' to the 3' end. It is obvious to the
man skilled in
the art that any of the below-specified probes can be used as such, or in
their
complementary form, or in their RNA form (wherein T is replaced by U).
2o The probes, primer and other nucleotide sequences according to the
invention can be
prepared by cloning of recombinant plasmids containing inserts including the
corresponding
nucleotide sequences, if need be by excision of the latter from the cloned
plasmids by use of
the adequate nucleases and recovering them, e.g. by fractionation according to
molecular
weight. The probes and primers according to the present invention can also be
synthesized
chemically, for instance by the conventional phospho-triester method.
The oligonucleotides used as primers or probes may also comprise nucleotide
analogues
such as phosphorothiates, alkylphosphorothiates or peptide nucleic acids or
may contain
intercalating agents. As most other variations or modifications introduced
into the original
3o DNA sequences of the invention these variations will necessitate
adaptations with respect to
the conditions under which the oligonucleotide should be used to obtain the
required
specificity and sensitivity. However the eventual results of hybridization
will be essentially the
same as those obtained with the unmodified oligonucleotides. The introduction
of these
modifications may be advantageous in order to positively influence
characteristics such as
hybridization kinetics, reversibility of the hybrid-formation, biological
stability of the
oligonucleotide molecules, etc.
CA 02354197 2001-07-27
I5
The term "solid support" can refer to any substrate to which an
oligonucleotide probe can be
coupled, provided that it retains its hybridization characteristics and
provided that the
background level of hybridization remains low. Usually the solid substrate
will be a microtiter
plate, a membrane (e.g. nylon or nitrocellulose) or a microsphere (bead) or a
chip (biochip).
Prior to application to the membrane or fixation it may be convenient to
modify the nucleic
acid probe in order to facilitate fixation or improve the hybridization
efficiency. Such
modifications may encompass homopolymer tailing, coupling with different
reactive groups
such as aliphatic groups, NH2 groups, SH groups, carboxylic groups, or
coupling with biotin,
to haptens or proteins.
The term "labelled" refers to the use of labelled nucleic acids. Labelling may
be carried out
by the use of labelled nucleotides incorporated during the polymerase step of
the
amplification or labelled primers, or by any other method known to the person
skilled in the
art. The nature of the label may be isotopic (32p, sSS, etc.) or non-isotopic
(biotin,
digoxigenin, etc.).
The term "sample" refers any sample of biological or non-biological origin.
The term
"biological sample or sample" refers to for instance naso-pharyngeal
aspirates, throat or
2o nasopharyngeal swabs, nasopharyngeal washes or tracheal aspirates or other
respiratory
tract sample comprising DNA or RNA. Samples of non-biological origin can be
from any
organic or inorganic material which can be suspected to contain microbial
material.
For designing probes with desired characteristics, the following useful
guidelines known to
the person skilled in the art can be applied.
Because the extent and specificity of hybridization reactions such as those
described herein
are affected by a number of factors, manipulation of one or more of those
factors will
determine the exact sensitivity and specificity of a particular probe, whether
perfectly
complementary to its target or not. The importance and effect of various assay
conditions
3o are explained further herein.
The stability of the [probe : target] nucleic acid hybrid should be chosen to
be compatible
with the assay conditions. This may be accomplished by avoiding long AT-rich
sequences,
by terminating the hybrids with G:C base pairs, and by designing the probe
with an
appropriate Tm. The beginning and end points of the probe should be chosen so
that the
length and %GC result in a Tm about 2-10°C higher than the temperature
at which the final
assay will be performed. The base composition of the probe is significant
because G-C base
CA 02354197 2001-07-27
16
pairs exhibit greater thermal stability as compared to A-T base pairs due to
additional
hydrogen bonding. Thus, hybridization involving complementary nucleic acids of
higher G-C
content will be more stable at higher temperatures.
Conditions such as ionic strength and incubation temperature under which a
probe will be
used should also be taken into account when designing a probe. It is known
that the degree
of hybridization will increase as the ionic strength of the reaction mixture
increases, and that
the thermal stability of the hybrids will increase with increasing ionic
strength. On the other
hand, chemical reagents, such as formamide, urea, DMSO and alcohols, which
disrupt
1o hydrogen bonds, will increase the stringency of hybridization.
Destabilization of the hydrogen
bonds by such reagents can greatly reduce the Tm. In general, optimal
hybridization for
synthetic oligonucleotide probes of about 10-50 bases in length occurs
approximately 5°C
below the melting temperature for a given duplex. Incubation at temperatures
below the
optimum may allow mismatched base sequences to hybridize and can therefore
result in
reduced specificity.
It is desirable to have probes which hybridize only under conditions of high
stringency. Under
high stringency conditions only highly complementary nucleic acid hybrids will
form; hybrids
without a sufficient degree of complementarity will not form. Accordingly, the
stringency of
2o the assay conditions determines the amount of complementarity needed
between two
nucleic acid strands forming a hybrid. The degree of stringency is chosen such
as to
maximize the difference in stability between the hybrid formed with the target
and the non-
target nucleic acid.
2s Regions in the target DNA or RNA which are known to form strong internal
structures
inhibitory to hybridization are less preferred. Likewise, probes with
extensive self-
complementarity should be avoided. As explained above, hybridization is the
association of
two single strands of complementary nucleic acids to form a hydrogen bonded
double
strand. It is implicit that if one of the two strands is wholly or partially
involved in a hybrid that
3o it will be less able to participate in formation of a new hybrid. There can
be intramolecular
and intermolecular hybrids formed within the molecules of one type of probe if
there is
sufficient self complementarity. Such structures can be avoided through
careful probe
design. By designing a probe so that a substantial portion of the sequence of
interest is
single stranded, the rate and extent of hybridization may be greatly
increased. Computer
35 programs are available to search for this type of interaction. However, in
certain instances, it
may not be possible to avoid this type of interaction.
CA 02354197 2001-07-27
17
Standard hybridization and wash conditions are disclosed in the Materials &
Methods section
of the Examples. Other conditions are for instance 3X SSC (Sodium Saline
Citrate), 20%
deionized FA (Formamide) at 50°C. Other solutions (SSPE (Sodium saline
phosphate
EDTA), TMAC (Tetramethyl ammonium Chloride), etc.) and temperatures can also
be used
provided that the specificity and sensitivity of the probes is maintained.
When needed, slight
modifications of the probes in length or in sequence have to be carried out to
maintain the
specificity and sensitivity required under the given circumstances.
1o The term "hybridization buffer" means a buffer allowing a hybridization
reaction between the
probes and the polynucleic acids present in the sample, or the amplified
products, under the
appropriate stringency conditions.
The term "wash solution" means a solution enabling washing of the hybrids
formed under
the appropriate stringency conditions.
The invention, now being generally described, will be more readily understood
by reference
to the following examples and figures, which are included merely for the
purposes of
illustration of certain aspects and embodiments of the present invention and
are in no way to
2o be construed as limiting the present invention. All of the references
mentioned herein are
incorporated by reference.
CA 02354197 2001-07-27
Ig
FIGURE LEGENDS
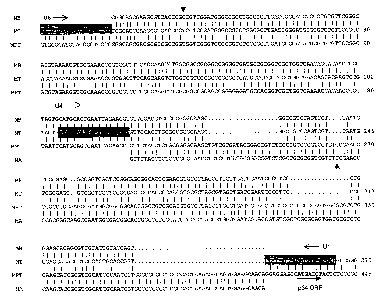
Figure 1.
Multiple nucleotide sequences alignment of M. bovis (MB), M. tuberculosis
(MT), M. avium
subsp. paratuberculosis (MPT) and M. avium ss (MA) us-p34 genes. Gaps between
sequences are indicated by dots (.). Vertical bar (I) indicates identity
across sequences. The
start codon (ATG) for the p34 ORF is in bold. Primer sequences are indicated
by shaded
boxes. The arrows indicate point mutations between M. tuberculosis and M.
bovis and M.
avium subsp. paratuberculosis and M. avium ss.
1o Figure 2.
Amplifications of us-p34 regions with primers U1, U2, U3, U4, U6 and U9.
Figure 3.
New us-p34 sequences (5' to 3'). Primers used to obtain the sequence (either
U2-U1 ; 03-
U1 ; U4-U1 ; U2-U9 ; U3-U9 or U4-U9) and the amplicon size are as indicated.
Sequence
variations (point mutations) found in the same species (for instance M.
ulcerans) are also
indicated when known.
Figure 4.
2o U1-U4 consensus amplification of us-p34 regions of different mycobacterial
species.
Figure 5.
Specific and non-specific hybridization.
Figure 6.
Differential reverse hybridization of mycobacteria target amplicons on a nylon
membrane
disclosing species-specific mycobacteria probes.
a) Unlabeled amplified DNA segments specific for various mycobacteria species
were first
transferred on nylon membrane (M. tuberculosis (TB), M. avium (AV), M. szulgai
(SZ), M.
3o kansasii (KA), M. xenopi (XE), M. simiae (S1) and M. malmoense (ML)).
b) Digoxigenin-labeled amplicons from M. tuberculosis (TB*), M. avium (AV*),
M. szulgai
(SZ*), M. kansasii (iCA*), M. xenopi (XE*) and M. simiae (SI*) were hybridized
on the nylon
membrane. Specific differential hybridization is obtained.
CA 02354197 2001-07-27
19
Figure 7.
Example of biochips detecting specifically M. gordonae
Figure 8.
Alignment of several Mycobacterial us-p34 sequences.
Figure 9.
Alignment of three Mycobacterial us-p34 sequences (M. tuberculosis, M. avium
and M.
intracellulare.
to
Figure 10.
Alignment of several pairs of Mycobacterial us-p34 sequences.
Figure 11.
A. Organisation of the rrn operon of Pseudomonas aeruginosa (Ps.ae),
Burkholderia cepacia
(Bu.ce) and Stenotrophomonas maltophilia (St.ma).
B. Comparison of the sequences flanking the regions encoding 16S and 23S RNA
from
Pseudomonas aeruginosa (Ps.ae) (SEQ ID NO 76), Burkholderia cepacia (Bu.ce)
(SEQ ID
NO 77). CNS: consensus.
Figure 12.
Discrimination by multiplex PCR.
The DNA from Ps. aeruginosa (1), Bu. cepacia (2) or St. maltophilia (3) was
amplified using
a conserved primer and a set of specific primers.
A . Multiplex PCR theoretical scheme.
B. Agarose Gel allowing the visualization of the amplified fragments of
different sizes. In a,
molecular weight marker (scale 123 bp).
Figure 13.
3o Multiplex PCR sensibility validation
The DNA from clinical strains (1 to 10) and from Ps. aeruginosa (A), Bu.
cepacia (B) or St.
maltophilia (C) were amplified using PsMulti, Paer, Bcep and Sma1 primers. The
amplified
fragments (20 NI) are visualized after agarose gel (2 %) migration.
In a, molecular weight marker (scale 123 bp). In b, c and d are control
fragments amplified
from Ps. aeruginosa, Bu. cepacia and St. maltophilia respectively.
CA 02354197 2001-07-27
Figure 14.
Multiplex PCR specificity validation
In a, molecular weight marker (scale 123 bp). In b, c and d are control
fragments amplified
from Ps. aeruginosa, Bu. cepacia and St. maltophilla respectively.
5 The DNA from clinical strains of Ps. putida (1 ), Ps. fluorescens (2) or Ps.
alcaligenes (3), Ps.
pseudoalcaligenes (4) and Ps. stuzeri (5) were amplified using PsMulti, Paer,
Bcep and
Sma1 primers. The absence of amplification products (20 NI) was visualized
after agarose
gel (2 %) migration.
Inset: The DNA from clinical strains of Ps. putida (1 ), Ps. fluorescens (2)
or Ps. alcaligenes
10 (3), Ps. pseudoalcaligenes (4) and Ps. stuzeri (5) was amplified using
ProUni1 and ProUni2
primers (universal primers amplifying part of the 16S RNA gene). The
amplification products
(20 NI) were visualized after agarose gel (2 %) migration.
Figure 15.
15 Reverse hybridization for the discrimination between Ps. aeruginosa, Bu.
cepacla and St.
maltophilia
2 and 5 NI of probes corresponding to the three species where sequentially
immobilized on
Hybond N+ nylon membrane (from top to bottow, 2 NI and 5 NI of the probe
specific for Ps.
aeruginosa, 2 NI and 5 NI of the probe corresponding to Bu. cepacla and 2 NI
and 5 NI of
2o probe specific for St. maltophllia). Biotinylated amplicons were produced
from the genomic
DNA of each species and were hybridized with a membrane containing the
different probes.
The hybrids were colorimetrically detected.
Figure 16.
Visualization of the second rrn operon from Ps. putida
A. Amplification products (20 NI) from DNA of Ps. aeruginosa (1), Bu. cepacla
(2), St.
maltophilia (3), Ps. putida (4), Ps. alcallgenes (5), Ps. pseudoalcaligenes
(6), Ps. fluorescens
(7) using PsSeq1 and PsSeq2 primers, visualized on agarose gel (2 %).
B. The DNA was transferred from the gel A to a nylon membrane, and hybridized
with a
3o biotiylated probe (DNA from Ps. aeruginosa amplified with PsSeq1 and
psSep2b primers).
The hybrids were revealed by colorimetry (streptavidine, DAB).
PM = molecular weight marker
Figure 17.
CA 02354197 2001-07-27
21
Alignment of the two rrn operon sequences from Ps. putida. First (top)
sequence is SEQ ID
NO 78, second (bottom) sequence is SEQ ID NO 79.
The sequence of the shortest operon is boxed, the double-underlined sequence
correspond
to the missing region of the shortest operon.
Figure 18.
Alignment and consensus sequence between Pseudomonas aeruginosa
(ps.msf{padfc}),
Burkholderia cepacia (ps.msf{pcdfg} and Stenotrophomonas maltophilia
(ps.msf{xmdfa}).
The underlined sequences correspond to the regions where the primers for
amplification
to were chosen.
Figure 19.
Alignment without consensus sequence between Pseudomonas aeruginosa
(ps.msf{padfc})
(SEQ ID NO 80), Burkholderia cepacia (ps.msf{pcdfg} (SEQ ID NO 81) and
Stenotrophomonas maltophilia (ps.msf{xmdfa}) (SEQ ID NO 82).
CA 02354197 2001-07-27
22
EXAMPLES
Example 1: Differential diagnosis of Mycobacterial species
9. Preparation of mycobacterial DNA.
a) short protocol (mycobacteria obtained from the Pasteur Institute)
DNA has been obtained by boiling the samples in order to put the mycobacterial
DNA into
solution.
1o b) long protocol (mycobacteria obtained from the Institute of Tropical
Medicine):
Mycobacteria (10 mg [wet weight]) were suspended in 200 p1 of lysis solution
(0.1 M NaOH,
1 M NaCI, and 5% sodium dodecyl sulfate [SDS]) and heated (100°C) for
20 min. The
suspension was then cooled, neutralized with 3 volumes of 0.1 M Tris-HCI (pH
7.4) buffer,
and centrifuged (5,000 x g, 5 min). Supernatants were extracted with phenol-
chloroform, and
1s DNA was precipitated with ethanol, collected by centrifugation, dissolved
in 50 NI of H20,
and stored at 20°C.
2. PCR amplifications.
For amplification, an aliquot (10 NI) of the DNA samples was added to 90 NI of
PCR mixture
2o consisting of 10 mM Tris-HCI (pH 8.8), 1.5 mM MgCl2, 50 mM KCI, 0.1 %
Triton X-100, 0.25
mM (each) deoxynucleoside triphosphates, 10 pmol of each primer (Tables 1 and
2) and
0.625 U of DyNAzyme DNA polymerase (Finnzymes Inc., Espoo, Finland). After an
initial
denaturation step (3 min at 96°C), 30 cycles of amplification were
performed as follows:
denaturation at 96°C for 30 s, annealing at 58°C for 45 s, and
DNA extension at 72°C for 30
2s s, with an increment of 1 s per cycle for the denaturation and extension
segments. A final
extension was performed at 72°C for 15 min. Amplifications were carried
out in a DNA 2400
thermocycler (Perkin-Elmer Applied Biosystems, Foster City, Calif.). PCR
products were
checked by loading 5 ~I on a 2% (wt/vol) agarose gel. Electrophoresis is
performed in 0.1 M
Tris HCI (pH 8.6), 80 mM boric acid, 1 mM EDTA buffer containing 0.5 pg of
ethidium
3o bromide per ml. DNA fragments are visualized on a UV transilluminator at
300 nm. PCR
products were cloned using the TOPO XL PCR cloning kit (Invitrogen, Carlsbad,
Calif.),
according to the manufacturer's protocol. The clones were further sequenced
with the Taq
Dye Deoxy Terminator Cycle sequencing kit and an ABI 377 DNA sequences (Perkin-
Elmer
Applied Biosystems).
3s
3. DNA seauence analysis.
CA 02354197 2001-07-27
23
Nucleotide sequence analyses were performed with the Genetics Computer Group
software
obtained from the University of Wisconsin, through the use of the Belgian
EMBnet Node
facility. Sequences were aligned by the Pileup program and paired comparisons
were
realised with the Bestfit program.
4. Reverse hybridization analysis.
Mycobacterial species-specific capture probes were produced by amplification
of the us-P34
cloned-DNA from the reference strains with primers U1 and U4. Amplified DNA
fragments
were sequentially transferred onto nylon membranes (Hybond-N+; Amersham,
Little
to Chalfont, United Kingdom) according to the Southern blot method. After 2 h
of
prehybridization, membranes were hybridized with us-p34 digoxigenin-labeled
target probes,
obtained by PCR amplification of reference or clinical mycobacterial genomic
DNA with
primers U1 and U4 (Table 1), in the presence of DIG-11-dUTP. Hybridization of
heat
denatured target probes (5 min at 95 °C) was performed at 50 °C
for 4 h in 2x SSC (1x SSC
is 0.15M NaCI and 0.015 M sodium citrate), 1 % blocking reagent, 0.1 % SDS,
0.1 % N-
Lauroylsarcosine, 5 mg/ml of salmon sperm DNA and 5% formamide. Filters were
then
washed twice with 2x SSC (1X SSC is 0.15 M NaCI plus 0.015 M sodium citrate)-
0.1% SDS
at 37°C for 5 min, and twice with 0.2x SSC-0.1 % SDS at 50°C for
5 min. Hybridized
digoxigenin-labeled DNA fragments were detected through alkaline-phosphatase-
labeled
2o anti-DIG Fab fragments. Colorimetric detection was performed with Nitro
Blue Tetrazolium
Chlorure and 5,3-bromo-4-indolylphosphate (BCIP) (Boerhinger Mannheim)
according to the
manufacturer's instructions.
5. Analysis on "Mvcobacteria Biochips".
Design of the biochips. Mycobacterium micro-arrays have been developed by AAT
(Namur,
Belgium). This array is a glass slide bearing on its surface different
mycobacterial species-
specific capture nucleotide sequences (Table 3). Fixation, positive and
negative
hybridization controls capture probes are also included in the array and each
capture probe
of the array is composed of four replicates.
Production of biotinylated am,vlicons. Amplification of the mycobacterial us-
p34 sequences is
performed in a final volume of 50 NI containing 2.5 mM MgCl2, 75 mM Tris-HCI,
pH 9.0, 50
mM KCI, 20 mM (NH4)2S04, 0.5 NM of primers U4 and U5, 200 NM of dATP, 200 NM
of
dCTP, 200 NM of dGTP, 150 NM of dTTP, 50 NM of biotin-16-dUTP, 0.5 U of uracil-
DNA-
Glycosylase (Boehringer Mannheim, Germany), 1.25 U of Taq DNA polymerase
(Biotools,
Madrid, Spain) and 10 p1 of DNA template. The reagents are first incubated at
94°C for 5 min
CA 02354197 2001-07-27
24
and then cycled 40 times in a DNA 9600 thermocycler (Perkin Elmer, Foster
City, CA, USA)
using the following temperatures and cycle times: 94°C for 30 s,
49°C for 45 s, 72°C for 30
s. A final extension step of 10 min at 72°C is performed. PCR products
are directly used or
stored at -20°C.
Hybridization and colorimetric detection. The procedure for detecting the PCR
products is
carried out according to the manufacturer's instructions (AAT, Namur, Belgium)
as follows: 5
NI of PCR product and 5 NI of the positive control, provided in the kit, are
denatured with
fresh NaOH 0.05N for 5 min at room temperature. This solution is then mixed
with 35 NI of
1o hybridization solution and loaded on the array framed by a hybridization
chamber (Biozym,
Landgraaf, The Netherlands). The chamber is closed with a plastic coverslip
and
hybridization is carried out for 30 min at 53°C. Slides are washed four
times 1 min with
washing buffer. The glass samples are then incubated 45 min at room
temperature with 800
NI of streptavidin-conjugate 1000 times diluted in blocking buffer. After 5
washes, the slides
are incubated 3 times 10 min with 800 NI of revelation mixture, then rinsed
with water, dried
and imaged using a colorimetric microarray reader (AAT, Namur, Belgium). While
a quick
visual inspection already yields most of the images relevant informations, the
image
obtained is analysed by an algorythm allowing the quantification of the spots
intensities
values and by a pattern recognition algorithm.
6. Cloning and seguencing of the upstream-P34 region of mycobacterial species.
Comparison of homologous p34 genes and its regulatory sequences (upstream p34
or us-
p34) from M. tuberculosis (GenBank accession no. 279700) and M. avium subsp.
paratuberculosis (GenBank accession no. X68102) showed the presence of
deletions in the
M. tuberculosis region upstream of the p34 protein start codon (Figures 1 and
2). To confirm
and extend these findings, the us-p34 sequence of M. bovis BCG and M. avium D4
were
amplified and sequenced. Multiple sequences alignment of this region revealed
interspecies
polymorphisms specific for both tuberculous (M. tuberculosis and M. bovis
Bacile de
Calmette et Guerin [BCG]) and non-tuberculous (M. avium sp and M. avium subsp.
3o paratuberculosis) mycobacteria, i.e. the us-p34 fragment was 79 bases
shorter in the
tuberculous species. Conversely, us-p34 appeared to be highly conserved within
each
group: differentiation between M. tuberculosis and M. bovis relied on a single
T to C
transition at position 41 of us-p34, and a single C to G transversion at
position 264 of us-p34
in M. avium ss and M. avium subsp. paratuberculosis (Figure 1 ).
CA 02354197 2001-07-27
Based on sequence homology between the M. tuberculosis l M. bovis and M. avium
l M.
avium subsp. paratuberculosis, several oligonucleotides, U1, U2, U3, U4 and U9
(Tables 1
and 2), matching conserved sequences of the polymorphic us-p34 region, were
designed to
amplify the corresponding region of others reference bacteria from the
mycobacterial genus,
5 M. szulgai, M. africanum, M. gordonae, M. gastri, M. kansasii, M. marinum,
M. ulcerans, M.
intracellulare, M. scrofulaceum, M. xenopi, M. malmoense and M. simiae (Table
3). These
fragments have been cloned and sequenced (Figure 3).
7. Development of a consensus PCR amplicafion of mycobacterial species.
1o Based on the mycobacterial sequences of us-p34, a consensus PCR assay was
developed.
Primers U1 and U4 have allowed to amplify the corresponding fragments, ranging
from 163
by and 298 by in size, for M. szulgai (163 bp), M. non chromogenicum (169 bp),
M.
tuberculosis, M. bovis, M. africanum (178 pb), M. gordonae (182 bp), M. gastri
(223 bp), M.
kansasii (225 bp), M. marinum, and M. ulcerans (236 pb), M. intracellulare, M.
avium, M.
15 paratuberculosis (256 bp), M. scrofulaceum (259 bp), M. xenopi (265 bp), M.
leprae (269
bp), M. malmoense (290 bp) and M. simiae (298 bp) (Figure 4; Table 4).
Sequences
alignments indicate both size and nucleotide sequence polymorphisms, as
illustrated by
some pair-wised alignments of all mycobacterial sequences with M. tuberculosis
us-p34
region.
8. Validation of the consensus mycU1-mvcU4 PCR on clinical isolates.
The reliability of this consensus amplification strategy was tested on a range
of clinical
mycobacterial isolates (Table 4). The strategy is characterized by a good
sensitivity for most
mycobacteria. The lower sensitivity displayed for some species is probably
related rather to
the DNA sample than to a lack of sensitivity of the strategy. As a matter of
fact, M. marinum-
M. ulcerans amplifications show a better sensitivity for "long protocol" DNA
preparations
(20/20) than for the "short protocol" ones (4/10). The quality of these "short
protocol" DNA
preparations have to be checked before running further conclusions. This could
indeed
influence further identification of potentially specific amplicons in other
mycobacterial species
(such as, for example, M. phlei, M. flavescens, M. nonchromogenicum, M.
chelonae)
9. Development of species-specific PCR amplifications.
Based on alignment of all upstream p34 sequences between U1 and U4 (which
contain most
inter-species differences) or at the broadest between U9 and U2 (which also
may contain
specific inter-strain differences), primers which will selectively amplify one
strain (two new
CA 02354197 2001-07-27
26
primers or a combination of one new primer and one primer presented in Tables
1 and 2) are
designed. This strategy enables the differentiation of the mycobacterial
species by PCR.
10. Reverse hybridization strateav.
Based on homologous and non-homologous hybridization of amplicons from the
same
species and amplicons from two different species, respectively (Figure 5), a
reverse
hybridization assay was set up. In a first step, species-specific probes
corresponding to each
mycobacteria species, were produced by amplification of reference strains us-
p34 cloned
DNA with the two consensus primers U1 and U4 and were immobilized on nylon
strips. DNA
to of species to be identified were amplified in the presence of the same
primers, biotinylated at
their 5' ends, hybridized with the immobilized probes and colorimetrically
assayed. An assay,
allowing a correct discrimination between M. tuberculosis, M. avium, M.
szulgai, M. xenopi,
M. simiae and M. malmoense is presented in Figure 6.
I5 11. Development of a "Mycobacteria Biochips".
The polymorphic us-p34 sequences can also be applied on low-density microarray
technology in order to develop simultaneous species-specific detection of
mycobacteria. In a
preliminary study, mycobacteria microarrays, bearing different mycobacterial
species-
specific capture probes, have been developed by AAT (Namur, Belgium). These
probes
2o were hybridized with the us-p34 biotinylated amplicons from the different
mycobacterial
species. Illustration of a specific detection of M. gordonae versus M.
tuberculosis, M. gastri,
M.kansasii, M. intracellulare, M. leprae, M. avium, M, marinum, M. simiae, M.
xenopi, M.
malmoense, M. szulgai and M. scrofulaceum specific capture probes is presented
in
Figure 7.
CA 02354197 2001-07-27
27
Example 2: Differential diagnosis of Pseudomonas species
1. The Pseudomonas
The Pseudomonas belong to the family of non-fermenting bacteria (7, 8). They
are divided
into 5 groups based on the homology level of their ribosomal RNA (Table 5).
From a clinical point of view, the three species most frequently found are:
Pseudomonas
aeruginosa, Burkholderia cepacia, Stenotrophomonas maltophilia.
2. Differential diagnosis
1o The present inventors developed a method of differential identification
allowing the
discrimination of three species belonging to the same family of Pseudomonads:
Pseudomonas aeruginosa, Burkholderia cepacia, Stenotrophomonas maltophilia.
The
identification method is based on the rRNA operon (rrn), and more particularly
on the
variable region (spacer) which separates the different genes coding for 16S
and 23S rRNA
and the Isoleucine and Alanine tRNA.
Some studies have been published related to the amplification of the gene
coding for 16S
and 23S ribosomal RNA (ref 7 to 9, non-exhaustive).
3. Organization of the rrn operon of "Pseudomonas"
2o The bacteria belonging to the "Pseudomonas" group have an operon coding for
the three
ribosomal RNA, 16S, 23S and 5S. The organization of this operon is relatively
conserved
(Figure 11A). Between the sequences encoding 16S and 23S rRNA, one finds
sequences
coding for two tRNA (Isoleucine and Alanine tRNA) separated by non-coding
regions of
variable length and composition (Figure 11A and 11 B). It is also noticed that
these two tRNA
have the following order in P. aeruginosa and 8. cepacia: Isoleucine tRNA
followed by
Alanine tRNA and that this order is reversed in S. maltophilia.
The first developed molecular strategy was an amplification by multiplex PCR
allowing the
production of fragments of different sizes according to the species from which
DNA was
3o amplified (Figure 12). The sensibility and the specificity of this first
strategy was validated on
clinical strains (Figure 13 and 14).
The second strategy comprised the development of a reverse hybridization
system wherein
the labeled and amplified DNA is specifically hybridized to probes
corresponding to the
different species (Figure 15). The feasibility of this strategy allows to
consider bioships /
microarray application.
CA 02354197 2001-07-27
28
4. Organization of the rrn gene in Pseudomonas putida
In order to extend the amplification strategies by multiplex PCR and reverse
hybridization to
other species from the Ps. aeruginosa group, an amplification of the 3' end of
16S tRNA to
the 5' end of 23S tRNA was performed with DNA from control strains of Ps.
putida, Ps.
fluorescens, Ps. alcaligenes and Ps. pseudoalcaligenes, using PsSeq1 and
PsSeq2 primers.
Surprisingly the inventors noticed that for Ps. putida two bands of equal
intensity but of
different sizes were obtained (Figure 16A). The presence of this second band
of smaller size
was confirmed for two other isolates of Ps. putida (clinical samples form
different origin). A
to hybridization was performed using a probe produced by DNA amplification of
Ps. aeruginosa
(primers PsSeq1 and PsSeq2). The colorimetric detection of the hybrids
confirmed that this
smaller band was specific of the rrn gene (Figure 16B). Two hypotheses were
considered to
explain the presence of this band:
- the presence of a second hybridization site for one of the primers
- the presence of two rrn operon of different sizes;
To check these two hypotheses, the two fragments were cloned and sequenced.
The
sequences confirmed that in Ps. putida, two rrn operons are present, one of
which has a
deletion. This deletion covers the region coding for the two tRNA
corresponding to Alanine
and Isoleucine amino acids (Figure 17). Based on this knowledge, methods and
primers for
2o differential diagnosis are designed.
5.Ali~nment of the seauences of the three studied species
a) Primers used for the multiplex PCR:
The multiplex PCR amplifies distinctly Pseudomonas aeruginosa, Burkholderia
cepacia and
Stenotrophomonas maltophilia.
The primers represented in Table 6 have been designed by the present
inventors.
« Psmulti(PsSeq1 ) » and « PsSeq2 » are universal primers for Pseudomonas
(hybridizing
effectively to a consensus region determined by the alignment of the three
sequences). The
three other primers « Bcep », « Paer » and « Sma1 » are species-specific (c.f.
Figure 18 and
3o Figure 12A).
The amplification of the conserved region on the 16S rRNA gene was used as a
control in
most of the experiments. These sequences are represented in Table 7 and are
known in the
art.
b) Length of the amplified fra4ments (c.f. Fipure 12)
CA 02354197 2001-07-27
29
PsSeq1-PsSeq2: 726 by
Bu. cepacia: 209 by
Ps. aeruginosa: 427 by
St. Maltophilia: 612 by
ProUn1-ProUn2: 211 by
c) Code used for the alignments
ps.msf(padfc) = Pseudomonas aeruginosa
ps.msf(pcdfg) = Burkholderia cepacia
to ps.msf(xmdfa) = Stenotrophoromonas maltophilia
d) Parameters used for the alignments in Fictures 18 and 19
Plurality: 3.00 Threshold: 1 AveWeight: 1.00 AveMatch: 1.00 AvMisMatch: 0.00
CA 02354197 2001-07-27
REFERENCES
1. Portaels f. Epidemiology of mycobacterial diseases. Clinics in Dermatology
1995 ;13:
207-222.
5
2. Raviglione MC, Snider DE, Kochi A. Global epidemiology of tuberculosis :
morbidity and
mortality of a wordlwide epidemic. JAMA 1995 ;273 :220-26.
3. Cobelens FG, van Deutekom H, Draayer-Jansen IW, et al. Risk of infetion
with
to Myvccobacterium tuberculosis in travellers to areas of high tuberculosis
endemicity=.
Lancet 2000 ;356 :461-465.
4. Pablos-Mendez A, raviglione MC, Laszlo A, et al ; Global surveillance for
anti-
tuberculosis-drug resistance, 1994-1997. New Engl J Med 1998;338 :1641-49.
5. Tsang AY and Farber ER. The primary isolation of Mycobacterium ulcerans.
Am. J. Clin.
Pathol. 1973 ; 59 : 688-692.
6. Portaels F, Fonteyne PA, De Beenhouwer H, de Rijk P, Guedenon A, Hayman J,
Meyers
2o WM. Variability in 3' end of 16S rRNA sequence of Mycobacterium ulcerans is
related to
geographic origin of isolates. J. Clin Microbiology 1996 ;34 :962-965.
7. O'Callaghan EM, Tanner MS, Boulnois GJ. Developpement of a PCR. probe test
for
identifying P.aeruginosa and P.(8) cepacia. Clin Pathol 1994 ; 47:222-226.
8. Karpati F and Jonasson J. Polymerase chain reaction for the detection of
Pseudomonas
aeruginosa, Stenotrophomonas maltophilia and Burkholderia cepacia in sputum of
patients with cystic fibrosis. Molecular and Cellular probes, 1996, 10 :397-
403.
9. Tyler SD, Strathdee CA, Rozee KR and Johnson WM. Oligonucleotide primers
designed
to differentiate pathogenic pseudomonads on the basis of the sequencing of
genes
coding for 16S-23S rRNA internal transcribed spacers. Clinical and Diagnostic
Laboratory Immunology 1995 448-453.
CA 02354197 2001-07-27
31
Table 1. Oli~onucleotide primers
These consensus primers have been successfully used to amplify a wide panel of
reference
and clinical mycobacterial strains (see Table 4)
U1: 5'-GAGTAGGTCATGGCTCCTCC-3' (antisense) (SEQ ID NO 1)
U6: 5'-GTGCGCATATAGCGGTCGTC-3' (sense) (SEQ ID NO 2)
U4: 5'-CATGCAGCGAATTAGAACGT-3' (sense) (SEQ ID NO 3)
to
Table 2. Olistonucleotide primers
U2: 5'-AACTTGACGAACTCGCCG-3' (sense) (SEQ ID
NO 4)
U3: 5'-AGGTATTCGCGCAGCATG-3' (sense) (SEQ ID
NO 5)
U5 : 5'-GTASGTCATRRSTYCTCC-3' (antisense) (SEQ
ID NO 6)
U9: 5'-GGTGAACATTGGGCCGAA-3' (antisense) (SEQ
ID NO 7)
S=GorC; R=GorA;Y=TorC
2o U5 is an example of a degenerated primer that in combination with U4, as a
consensus or
universal primer, was used to amplify DNA fragments of various mycobacterial
species.
These mycobacterial species could subsequently be identified by reverse
hybridization on a
biochips
It is obvious that the above-mentioned nucleic acids can also be used as
probes in other
experiments.
Table 3. Oliaonucleotide probes and primers
(a) Example of oligonucleotides used as capfure probes that can specifically
hybridize
Mycobacteria strains on a biochips after DNA amplifrcafion using U4 and U5
consensus primers
For some species, several potential capture probes have been designed. Further
testing
may reveal advantages of some with respect to the others.
Avium 1 : 5'-CGGTCGTCTCCGAAGCCCGCG-3'(SEQ ID NO 8)
Gastrii 1 : 5'-GATCGGCAGCGGTGCCGGGG-3'(SEQ ID NO 9)
Gastrii 2 : 5'-GTATCGCGGGCGGCAAGGT-3'(SEQ ID NO 10)
4o Gastrii 3 5'-TCTGCCGATCGGCAGCGGTGCCGG-3'(SEQ ID NO 11)
:
Gastrii 4 : 5'-GCCGGGGCCGGTATTCGCGGGCGG-3'(SEQ ID NO 12)
Gordonae 1 : 5'-GACGGGCACTAGTTGTCAGAGG-3'(SEQ ID NO 13)
CA 02354197 2001-07-27
32
Intracellulare 5'-GGGCCGCCGGGGGCCTCGCCG-3'(SEQ ID NO 14)
1:
Intracellulare 5'-GCCTCGCCGCCCAAGACAGTG-3'(SEQ ID NO 15)
2 :
Leprae 1: 5'-GATTTCGGCGTCCATCGGTGGT-3'(SEQ ID NO 16)
Kansasi 1: 5'-GATCGTCGGCAGTGGTGACGG-3'(SEQ ID NO 17)
Kansasi 2 : 5'-TCGTCGGCAGTGGTGAC-3'(SEQ ID NO 18)
Kansasi 3 : 5'-ATCCGCCGATCGTCGGCAGTGGTGACG-3'(SEQ ID NO
19)
Malmoense 1 : 5'-GACCCACAACACTGGTCGGCG-3'(SEQ ID NO 20)
Marinum 1 : 5'-CGGAGGTGATGGCGCTGGTCG-3'(SEQ ID NO 21)
Scrofulaceum 1 5'-CGGCGGCACGGATCGGCGTC-3'(SEQ ID NO 22)
:
to Simiae 1: 5'-ATCGCTCCTGGTCGCGCCTA-3' (SEQ ID NO 23)
Szulgai 1 : 5'-CCCGGCGCGACCAGCAGAACG-3'(SEQ ID NO 24)
Tuberculosis 1: 5'-GCCGTCCAGTCGTTAATGTCGC-3'(SEQ ID NO 25)
Xenopi 1: 5'-CGGTAGAAGCTGCGATGACACG-3'(SEQ ID NO 26)
It is obvious that the above-mentioned nucleic acids can also be used as
primers in other
experiments.
(b) Example of oligonucleotides used as capture probes thaf can specifically
hybridize Mycobacteria strains on a biochips after DNA amplihcafion using U1
2o and U4 consensus primers
For some species, several potential capture probes have been designed. Further
testing
may reveal advantages of some with respect to the others.
Avium 2 : 5'-GCGCGGTCGTCTCCGAAGCCC-3'(SEQ ID NO 27)
Avium 3 : 5'-CCGCTCGGCACTAAAAGGCAGTGGAAGC-3'(SEQ ID NO 28)
Avium 4 : 5'-GAAGCCCGCGGGCAAGCCAAT-3'(SEQ ID NO 29)
Gastrii 1 : 5'-GATCGGCAGCGGTGCCGG-3'(SEQ ID NO 30)
Gastrii 5 : 5'-GCGGTGCCGGGGCCGGTA-3'(SEQ ID NO 31)
3o Gastrii 6 : 5'-CGGTATCGCGGGCGGCAAGGT-3'(SEQ ID NO 32)
Gordonae 2 : 5'-GGCGACGGGCACTAGTTGTCAGAGGTG-3'(SEQ ID NO 33)
Intracellulare 5'-CCGCCGGGGGCCTCGC-3'(SEQ ID NO 34)
3 :
Intracellulare 5'-TCGCCGCCCAAGACAGTGGCG-3' (SEQ ID NO 35)
4 :
Kansasii 4 : 5'-ATCCGCCGATCGTCGGCAGTGGT-3'(SEQ ID NO 36)
Kansasii 5 5'-GATCGTCGGCAGTGGTGACGGGG-3'(SEQ ID NO 37)
:
Kansasii 6 : 5'-GGGCCGGTATCACGGGGGCAA-3'(SEQ ID NO 38)
Leprae 2: 5'-GATTTCGGCGTCCATCGGTGGTAG-3'(SEQ ID NO 39)
Malmoense 2 : 5'-AACGCAAGATCTCGAAGGTGTTTTCAAAGGCG-3'(SEQ ID NO
40)
Malmoense 3 : 5'-GACCCACAACACTGGTCGGCGCC-3'(SEQ ID NO 41)
4o Marinum 2 : 5'-GCCAATCGGCTCGGCGGGA-3'(SEQ ID NO 42)
Marinum 3 : 5'-ATCGACGGAGGTGATGGCGCTG-3'(SEQ ID NO 43)
Simiae 2 : 5'-CGATCGCTCCTGGTCGCGCCT-3'(SEQ ID NO 44)
Simiae 3 : 5'-CCGGCGCACCCGCTCGAAC-3'(SEQ ID NO 45)
CA 02354197 2001-07-27
33
Szulgai 2 : 5'-CTGCGATGAGCAAGCGGCCCG-3'(SEQ ID NO 46)
Szulgai 3 : 5'-GCGGCCCGGTCGGCCG-3'(SEQ ID NO 47)
Tuberculosis 2 : 5'-CGGCCGTCCAGTCGTTAATGTCGC-3' (SEQ ID NO 48)
Xenopi 2 : 5'-CGGTAGAAGCTGCGATGACACGCCA-3'(SEQ ID NO 49)
s It is obvious that the above-mentioned nucleic acids can also be used as
primers in other
experiments.
Table 3 (c) Example of oligonucleotides used as species specibc primers
to The specific primer pair includes an universal (consensus) antisense primer
(for instance U1,
U5 or U9) and one of the following primers. This can be used for amplifying
mycobacterial
DNA in a multiplex diagnostic strategy.
Theoretical annealing temperature (Tm) of each primer as well as the expected
size of the
amplicon are given.
Is
U1 5'-GAGTAGGTCATGGCTCCTCC -3' Tm 63,7 (see table
1)
Avium 5 : 5'- GCAAGCCAATGGCGA -3' (SEQ ID NO Tm 64 ,8 166
50)
20 by
Intracellulare 5'- CTCGCCGCCCAAGA -3'(SEQ ID NO Tm 65,1 172
: 51 )
by
Tuberculosis 5'- CCGTCCAGTCGTTAATGTC -3'(SEQ ID Tm 61,6 134
3 : NO 52)
by
2s Simiae 4 : 5'- ACGATCGCTCCTGGTCG -3'(SEQ ID Tm 66,6 219
NO 53)
by
Malmoense 4 : 5'- AAGATCTCGAAGGTGTTTTCAA -3'(SEQ 54) Tm 119
ID NO 62,5
by
Mycobacterium avium can be further distinguished from Mycobacterium avium
subspecies
paratuberculosis by co-amplification with F57 gene specific primers.
A specific primer pair for amplification of at least part of the F57 gene of
Mycobacterium
avium subspecies paratuberculosis is:
3s
F57a 5'-GGTCGCGTCATTCAGAATC-3'(SEQ ID NO 55) Tm 64,4 439
by
F57b 5'-TCTCAGACAGTGGCAGGTG-3'(SEQ ID NO 56) Tm 64,1
Other F57 primers can be used such as MPT1 (internal primer), which in
combination with
F57b generates a fragment of 618 by with a sequence represented in SEQ ID NO
75.
CA 02354197 2001-07-27
34
It is obvious that the above-mentioned nucleic acids can also be used as
probes in other
experiments.
CA 02354197 2001-07-27
Table 4. Validation of the consensus U1-U4 amplification on clinical
mycobacterial
strains.
Mycobacteria size (bp) Nbr of + amplif. /Nbr of samples
5
Tuberculosiss Groua (TUB)
M. tuberculosis 177 15/15
M. bovis 177 9/10
M. africanum 177 5/5
1o Non Tuberculosis Group
(NTB)
M. avium Comalex (MAC)
M. avium 256 16/17
M. paratuberculosis 256 21/21
M. intracellulare 256 9/9
15 M. scrofulaceum 259 4/5
MOTT (Myc. Other Then Tub
- MOTT)
M. szulgai 163 9/9
M. kansasii 225 10/11
M. gastri 223 2/2
2o M. xenopi 265 12/12
M. ulcerans 211 20/20
M. leprae 269 1 /1
M. non chromogenicum 169 2/2
M. simiae 298 6/11
25 M. malmoense 290 4/7
M. gordonae 182 4/10
M. marinum 236 5/5
CA 02354197 2001-07-27
36
Table 5. Classification of Pseudomonas
Group Pseudomonas fluorescens (P. aeruginosa; P. fluorescens;
I P. putida)
Pseudomonas stutzeri
Pseudomonas pseudoalcaligenes; Ps. alcaligenes
Group Burkholderia cepacia; B. mallei; B. pseudomallei;
II 8. pickettii; 8. gladioli
Group Comanomas testosterone; C. acidovrans
III
Group Brevendimonas diminuta; 8. vesicularis
IV
Group Stenotrophomonas maltophilia
V
Table 6. Species specific and universal probes
Name Sequence Tm
PsMulti 5' ACGTCACACCATGGGAGT 3' (SEQ ID NO Sense 62.8C
PsSe 1 83)
Bcep 5' CCCTGAGTCTGTCTTTAATTTAC 3' (SEQ ID Antisense58.3C
NO 84)
Paer 5' CTTTCGACGATTGTTTTAGT 3' (SEQ ID NO Antisense56.8C
85)
Smal 5' TCAATAAAAGAGACTTGCGTC 3' (SEQ ID Antisense57.9C
NO 86)
PsSeq2 5' GATTGCCAAGGCATCCAC 3' (SEQ ID NO Antisense65.4C
87)
Table 7. Control probes
CA 02354197 2001-11-29
36a
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT: UNIVERSITE CATHOLIQUE DE LOUVAIN
(ii) TITLE OF INVENTION: IDENTIFICATION OF NUCLEOTIDE SEQUENCES
SPECIFIC FOR MYCOBACTERIA AND DEVELOPMENT OF DIFFERENTIAL
DIAGNOSIS STRATEGIES FOR MYCOBACTERIAL SPECIES
(iii) NUMBER OF SEQUENCES: 89
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: SMART & BIGGAR
(B) STREET: 650 WEST GEORGIA STREET, SUITE 2200
(C) CITY: VANCOUVER
(D) STATE: BRITISH COLUMBIA
(E) COUNTRY: CANADA
(F) ZIP: V6B 4N8
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: PatentIn version 3.1 (converted to PatentIn 1.30)
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER: CA 2,354,197
(B) FILING DATE: 27-JUL-2001
(C) CLASSIFICATION:
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: KINGWELL, BRIAN G
(C) REFERENCE/DOCKET NUMBER: 81906-12
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: (604) 682-7780
(B) TELEFAX: (604) 682-0274
(2) INFORMATION FOR SEQ ID NO.: 1
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 20
(ii) MOLECULAR TYPE: DNA
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Mycobacterium sp.
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 1
GAGTAGGTCA TGGCTCCTCC 20
(2) INFORMATION FOR SEQ ID NO.: 2
(A) LENGTH: 20
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
CA 02354197 2001-11-29
36b
(A) ORGANISM: Mycobacterium sp.
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 2
CATGCAGCGA ATTAGAACGT 20
(2) INFORMATION FOR SEQ ID NO.: 3
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 20
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: Mycobacterium sp.
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 3
CATGCAGCGA ATTAGAACGT 20
(2) INFORMATION FOR SEQ ID NO.: 4
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 18
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: Mycobacterium sp.
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 4
AACTTGACGA ACTCGCCG 18
(2) INFORMATION FOR SEQ ID NO.: 5
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 18
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: Mycobacterium sp.
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 5
AGGTATTCGC GCAGCATG 18
(2) INFORMATION FOR SEQ ID NO.: 6
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 18
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: Mycobacterium sp.
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 6
GTASGTCATR RSTYCTCC 18
CA 02354197 2001-11-29
36c
(2) INFORMATION FOR SEQ ID NO.: 7
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 18
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: Mycobacterium sp.
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 7
GGTGAACATT GGGCCGAA 1g
(2) INFORMATION FOR SEQ ID NO.: 8
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 21
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: Mycobacterium avium
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 8
CGGTCGTCTC CGAAGCCCGC G 21
(2) INFORMATION FOR SEQ ID NO.: 9
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 20
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM GASTRI
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 9
GATCGGCAGC GGTGCCGGGG 20
(2) INFORMATION FOR SEQ ID NO.: 10
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 19
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM GASTRI
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 10
GTATCGCGGG CGGCAAGGT 19
(2) INFORMATION FOR SEQ ID NO.: 11
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 24
CA 02354197 2001-11-29
36d
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM GASTRI
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 11
TCTGCCGATC GGCAGCGGTG CCGG 24
(2) INFORMATION FOR SEQ ID NO.: 12
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 24
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM GASTRI
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 12
GCCGGGGCCG GTATTCGCGG GCGG 24
(2) INFORMATION FOR SEQ ID NO.: 13
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 22
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM GORDONAE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 13
GACGGGCACT AGTTGTCAGA GG 22
(2) INFORMATION FOR SEQ ID NO.: 14
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 21
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM INTRACELLULARE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 14
GGGCCGCCGG GGGCCTCGCC G 21
(2) INFORMATION FOR SEQ ID NO.: 15
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 21
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM INTRACELLULARE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 15
GCCTCGCCGC CCAAGACAGT G 21
CA 02354197 2001-11-29
36e
(2) INFORMATION FOR SEQ ID NO.: 16
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 22
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM LEPRAE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 16
GATTTCGGCG TCCATCGGTG GT 22
(2) INFORMATION FOR SEQ ID NO.: 17
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 21
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM KANSASII
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 17
GATCGTCGGC AGTGGTGACG G 21
(2) INFORMATION FOR SEQ ID NO.: 18
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 17
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM KANSASII
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 18
TCGTCGGCAG TGGTGAC 17
(2) INFORMATION FOR SEQ ID NO.: 19
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 27
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM KANSASII
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 19
ATCCGCCGAT CGTCGGCAGT GGTGACG 27
(2) INFORMATION FOR SEQ ID NO.: 20
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 21
CA 02354197 2001-11-29
36f
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM MALMOENSE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 20
GACCCACAAC ACTGGTCGGC G 21
(2) INFORMATION FOR SEQ ID NO.: 21
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 21
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM MARINUM
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 21
CGGAGGTGAT GGCGCTGGTC G 21
(2) INFORMATION FOR SEQ ID NO.: 22
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 20
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM SCROFULACEUM
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 22
CGGCGGCACG GATCGGCGTC 20
(2) INFORMATION FOR SEQ ID NO.: 23
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 20
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM SIMIAE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 23
ATCGCTCCTG GTCGCGCCTA 20
(2) INFORMATION FOR SEQ ID NO.: 24
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 21
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM SZULGAI
CA 02354197 2001-11-29
36g
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 24
CCCGGCGCGA CCAGCAGAAC G 21
(2) INFORMATION FOR SEQ ID NO.: 25
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 22
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM TUBERCULOSIS
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 25
GCCGTCCAGT CGTTAATGTC GC 22
(2) INFORMATION FOR SEQ ID NO.: 26
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 22
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM XENOPI
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 26
CGGTAGAAGC TGCGATGACA CG 22
(2) INFORMATION FOR SEQ ID NO.: 27
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 21
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: Mycobacterium avium
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 27
GCGCGGTCGT CTCCGAAGCC C 21
(2) INFORMATION FOR SEQ ID NO.: 28
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 28
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: Mycobacterium avium
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 28
CCGCTCGGCA CTAAAAGGCA GTGGAAGC 28
(2) INFORMATION FOR SEQ ID NO.: 29
CA 02354197 2001-11-29
36h
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 21
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: Mycobacterium avium
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 29
GAAGCCCGCG GGCAAGCCAA T 21
(2) INFORMATION FOR SEQ ID NO.: 30
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 18
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM GASTRI
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 30
GATCGGCAGC GGTGCCGG 18
(2) INFORMATION FOR SEQ ID NO.: 31
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 18
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM GASTRI
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 31
GCGGTGCCGG GGCCGGTA 18
(2) INFORMATION FOR SEQ ID NO.: 32
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 21
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM GASTRI
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 32
CGGTATCGCG GGCGGCAAGG T 21
(2) INFORMATION FOR SEQ ID NO.: 33
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 27
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
CA 02354197 2001-11-29
36i
(A) ORGANISM: MYCOBACTERIUM GORDONAE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 33
GGCGACGGGC ACTAGTTGTC AGAGGTG 27
(2) INFORMATION FOR SEQ ID NO.: 34
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 16
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM INTRACELLULARE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 34
CCGCCGGGGG CCTCGC 16
(2) INFORMATION FOR SEQ ID NO.: 35
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 21
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM INTRACELLULARE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 35
TCGCCGCCCA AGACAGTGGC G 21
(2) INFORMATION FOR SEQ ID NO.: 36
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 23
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM KANSASII
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 36
ATCCGCCGAT CGTCGGCAGT GGT 23
(2) INFORMATION FOR SEQ ID NO.: 37
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 23
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM KANSASII
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 37
GATCGTCGGC AGTGGTGACG GGG 23
CA 02354197 2001-11-29
36j
(2) INFORMATION FOR SEQ ID NO.: 38
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 21
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM KANSASII
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 38
GGGCCGGTAT CACGGGGGCA A 21
(2) INFORMATION FOR SEQ ID NO.: 39
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 24
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM LEPRAE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 39
GATTTCGGCG TCCATCGGTG GTAG 24
(2) INFORMATION FOR SEQ ID NO.: 40
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 32
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM MALMOENSE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 40
AACGCAAGAT CTCGAAGGTG TTTTCAAAGG CG 32
(2) INFORMATION FOR SEQ ID NO.: 41
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 23
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM MALMOENSE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 41
GACCCACAAC ACTGGTCGGC GCC 23
(2) INFORMATION FOR SEQ ID NO.: 42
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 19
(ii) MOLECULAR TYPE: DNA
CA 02354197 2001-11-29
36k
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM MARINUM
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 42
GCCAATCGGC TCGGCGGGA 19
(2) INFORMATION FOR SEQ ID NO.: 43
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 22
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM MARINUM
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 43
ATCGACGGAG GTGATGGCGC TG 22
(2) INFORMATION FOR SEQ ID NO.: 44
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 21
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM SIMIAE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 44
CGATCGCTCC TGGTCGCGCC T 21
(2) INFORMATION FOR SEQ ID NO.: 45
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 19
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM SIMIAE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 45
CCGGCGCACC CGCTCGAAC 19
(2) INFORMATION FOR SEQ ID NO.: 46
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 21
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM SZULGAI
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 46
CTGCGATGAG CAAGCGGCCC G 21
CA 02354197 2001-11-29
361
(2) INFORMATION FOR SEQ ID NO.: 47
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 16
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM SZULGAI
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 47
GCGGCCCGGT CGGCCG 16
(2) INFORMATION FOR SEQ ID NO.: 48
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 24
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM TUBERCULOSIS
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 48
CGGCCGTCCA GTCGTTAATG TCGC 24
(2) INFORMATION FOR SEQ ID NO.: 49
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 25
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM XENOPI
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 49
CGGTAGAAGC TGCGATGACA CGCCA 25
(2) INFORMATION FOR SEQ ID NO.: 50
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 15
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: Mycobacterium avium
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 50
GCAAGCCAAT GGCGA 15
(2) INFORMATION FOR SEQ ID NO.: 51
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 14
CA 02354197 2001-11-29
36m
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM INTRACELLULARE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 51
CTCGCCGCCC AAGA 14
(2) INFORMATION FOR SEQ ID NO.: 52
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 19
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM TUBERCULOSIS
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 52
CCGTCCAGTC GTTAATGTC 19
(2) INFORMATION FOR SEQ ID NO.: 53
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 17
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM SIMIAE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 53
ACGATCGCTC CTGGTCG 17
(2) INFORMATION FOR SEQ ID NO.: 54
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 22
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM MALMOENSE
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 54
AAGATCTCGA AGGTGTTTTC AA 22
(2) INFORMATION FOR SEQ ID NO.: 55
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 19
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM AVIUM SUBSPECIES PARATUBERCULOSIS
CA 02354197 2001-11-29
36n
(XI) SEQUENCE DESCRIPTION: SEQ ID 55
NO.:
GGTCGCGTCA TTCAGAATC 19
(2) INFORMATION FOR SEQ ID NO.:
56
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 19
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM AVIUM
SUBSPECIES PARATUBERCULOSIS
(XI) SEQUENCE DESCRIPTION: SEQ ID 56
NO.:
TCTCAGACAG TGGCAGGTG 19
(2) INFORMATION FOR SEQ ID NO.:
57
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 216
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM INTRACELLULARE
(XI) SEQUENCE DESCRIPTION: SEQ ID 57
NO.:
GTTCTACCTG TGCTGAGCAA GCTCCGGTGA CTCGCCGGAG GGCCGCCGGG60
TACCGACCGT
GGCCTCGCCG CCCAAGACAG TGGCGGCGCC GCACGTGCGC TAGCGTGGGT120
ACCGGTTCCC
GATCGACCGC GTCGCAATGC GGTGACGCGC AGCGTCGCAT CGCCACCGCG180
CTGCAAGCAC
GCGCCCGCTC GGCACTTAAA GGCACTGGTA 216
GCAACA
(2) INFORMATION FOR SEQ ID NO.: 58
(I) SEQUENCE
CHARACTERISTICS
(A) LENGTH:
881
(ii) MOLECULAR
TYPE:
DNA
(VI) ORIGINAL
SOURCE:
(A) ORGANISM:
Mycobacterium
avium
(XI) SEQUENCE 58
DESCRIPTION:
SEQ ID
NO.:
TCGTAGCTGGCTTCCTCGTCGGTCCACAGC GCCCGCATCGCTTCCAGGTA TTCGCGCAGC60
ATGGTGCGGCGCCGGCCCGCCGGCACGCCG TGGTCGGCGAGTTCGTCGGT GTTCCAGCCG120
AACCCGACGCCGAGGCTGACCCGGCCGCCG GACAGATGGTCAAGGGTGGC AATACTTTTC180
GCCAGCGTGATCGGGTCGTGTTCGACCGGC AGGGCCACCGCGGTGGACAG CCGCACCCGC240
GAGGTGACGGCACAGGCCGCGCCCAGACTG ACCCACGGGTCCAGGGTGCG CATGTAGCGG300
TCGTCGGGCAGCGACGCGTCGCCGGTGGTC GGGTGCGCGGCCTCCCGCTT GATCGGGATA360
TGCGTGTGTTCCGGCACGTAGAAGGTCGCA AACCCGTGGTCGTCGGCAAG CTTCGCGGCC420
GCAGCCGGAGAGATGCCACGGTCGCTGGTG AAAAGCACAAGCCCGTAATC CATGCAGTGA480
ATTAGAACGTGTTCTACCTCTGCGGGGCAA GCTGTCGTGATACGGACCGT CTCGCCGCGC540
GGTCGTCTCCGAAGCCCGCGGGCAAGCCAA TGGCGACGGCACCGGCCGTC GCACGTGCGC600
TAGCGTGGGTGATCGACCGTGTCGCTCGCG CAGTGACGCGCCTGCAAGCA CCGCGTCGCA660
TCGCAACCGTGGCGCCCGCTCGGCACTAAA AGGCAGTGGAAGCAACAGGA GGAGCCATGA720
CCTACTCTCCCGGCAGCCCCGGATATCCAC CGGCGCAGTCTGGCGGCACC TATGCAGGCG780
CCACACCATCTTTCGCCAAAGACGACGACG GCAAGAGCAAACTCCCGCTC TACCTCAACA840
TCGCCGTGGTCGCCCTGGGTTTCGCGGCCT ACCTGCTGAAT 881
CA 02354197 2001-11-29
360
(2) INFORMATION FOR SEQ 59
ID NO.:
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 642
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM
GASTRI
(XI) SEQUENCE DESCRIPTION: 59
SEQ ID NO.:
GTGCGCCGGC GCCCCGGCGG CACGCCATGGTCGGCGAGTTCGTGCGCCCG GCGGCACGCC60
ATGGTCGGCG AGTTCGTCGG TGTTCCAGCCGAATCCGACGCCGACGCTGA CCCGGCCCCC120
GGATAGTGGT CCAGCGTGGC AATGCTTTTGGCCAGCGTGATCGGGTCATG CTCCACCGCA180
GCGCAACCGC GGTTGACAGC CTGACTCGGGAGGTGACCGCTGAAGCCGCA CCCAAGCTCA240
CCCACGGGTC CAGGGTGCGC ATATAGCGGTCGTCCGGCAGCGACGCGTCA CCCGTCGTGG300
GATGGGCGGC TTCCCGTTTG ACCGGGATATGCGTGTGTTCGGGCACGTAG AGAGTGCGAA360
AGCCATGGTC GTCGGCCAGT TTCGCGGCTGCCGCCGGGGAGATCCCACGG TCGCTGGTGA420
AAAGGACAAG CCCGTAATCC ATGAACAGAATTAGAACGTGTTCTACCTCC GCCGGGCAAG480
CGGCTCATCT GCCGATCGGC AGCGGTGCCGGGGCCGGTATCGCGGGCGGC AAGGTCGCCA540
CGGCGTGAGT ACCCGGCCGT GCGCTAGCGTGGGTCATCGAATTGTGTCGC AGGGAGCAAT600
CGTCGCATTG CAGCAGGCGT AGCGACGGCACCGGAGGTAACA 642
(2) INFORMATION FOR SEQ 60
ID NO.:
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 745
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM
GORDONAE
(XI) SEQUENCE DESCRIPTION: 60
SEQ ID NO.:
GTGCGACGAC GGCCGGCCAG CACGTTATGGTCGGCGAGCTCGTCGGTGTT CCAGCCGAAC60
CCGACGCCGA GGCTAACTCG CCCGCCGGACAGGTGATCCAGCGTGGCGAT GCTTTTCGCC120
AAGGTGATCG GGTCATGCTC GACCGGCAACGCGACTGCCGTCGACAGCCG CACCCGCGAC180
GTCACAGCAC ACGCCGCGCC CAGGCTCACCCAGGGATCCAGGGTGCGCAT ATAACGGTCG240
TCGGGCAGCG TCTCGTCTCC GGTGGTGGGATGAGCCGCCTCGCGTTTGAT CGGGATATGC300
GTGTGTTCGG GTACGTAGAA GGTGTGAAAACCATGTGTGTCGGCAAGTTT CGCTGCTGCC360
GCAGGGGAAA TACCGCGATC GCTGGTGAACAGAACGAGGCTGTAGTCCAT GCCCCAATTT420
AGAACGTGTT CTACTTTTGG CCGCAGCCGACCCCCTGCGGCGACGGGCAC TAGTTGTCAG480
AGGTGCGCTA GCGTGGTTGA TCGAATGCGTCGCAGGCCGTACCGCGTCGT GCCGAAGCAG540
AGGGGCCGTG ACGGCACCGG AAGCAACAGGAGGACTTATGACCTACCCGC CCGGTAGTCC600
CGGATATCCA TCCGCCCAGC AGTCGGCCGGCAACTACGGCAGCTCCGCTC CCGCCGCCGG660
CCAGTCCGAG CCGGGTGAAA GCAAGCTGGGACTGTACCTGGCCATCGCGG TGGCGGCCCT720
GGGCCTACTG GCGTACCTCT TCAGC 745
(2) INFORMATION FOR SEQ 61
ID NO.:
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 785
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM
KANSASII
(XI) SEQUENCE DESCRIPTION: 61
SEQ ID NO.:
CA 02354197 2001-11-29
36p
GTGCGCCGGCGCGCCGGCGGCACGCCATGGTCAGCGAGTTCGTCGGTGTTCCAGCCGAAT60
CCGACGCCGACGCTGACCCGCCCCCCGGATAGGTGGTCCAGCGTGGCAATGCTTTTGGCC120
AGCGTGATCGGGTCATGCTCGACCGGCAACGCAACCGCTGTTGACAGTCGGACCCGGAAG180
GTGACCGCTGAAGCCGCGCCCAAACTCACCCACGGGTCCAGCGTGCGCATATAGCGGTCG240
TCCGGCAGCGACGCGTCACCCGTCGTGGGATGGCGGCCTCCCGTTTGACCGGGATGTGCG300
TGTGTTCGGGCACGTAGAAAGTGCGAAAGCCATGGTCGTCGGCCAGTTTCGCGGCTGCCG360
CGGGAGAAATGCCACGGTCGCTGGTGAAAAGGACAAGCCCGTAATCCATGAACAGAATTA420
GAACGTGTTCTACCTCAGCCGGGCAAGCGGCTCATCCGCCGATCGTCGGCAGTGGTGACG480
GGGCCGGTATCACGGGGGCAAGGTCGCCACGGCGCGAGTACCAGGCCGTGCGCTAGCGTG540
GGTCATCGAATCGTGTCGCAGGGAGCAATCGTCGCATTGCAGCAGGCGTAGCGACGGCAC600
TGGAGGTAACAGGAGGAGCCATGACCTACTCACCAGGTAGTCCCGGATATCCGCCCGCGC660
AATCGGCCGGCTCCTACGGAGCCGCCACACCGTCTTTCGCCAAGGCCGACGACGGTGTCA720
GCAAGCTTCCGATGTACCTGAGCATGGCGGTTGCCGCGCTCGGGCTGCTGGCGTATCTGG780
ccagc 785
(2) INFORMATION SEQ ID 62
FOR NO.:
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 691
(ii) MOLECULAR E: DNA
TYP
(VI) ORIGINAL
SOURCE:
(A) ORGANISM: YCOBACTERIUM
M MALMOENSE
(XI) SEQUENCE 62
DESCRIPTION:
SEQ ID NO.:
TCGTAGGCCG CTTCCTCCTGGGTCCACAGCGCCCGCATTGCCTCGATGTA TTCACGCAGC60
ATGGTGCGAC GGCGCCCGGCCGGCACGCCGTGGTCGGCGAGCTCGTCGGT GTTCCAGCCA120
AACCCAACGC CGAGGCTGACCCGGCCGCCGGACAGGTGGTCCAAGGTGGC AATACTTTTC180
GCCAGCGTGA TCGGGTCGTGCTCGACGGGCAGCGCCACCGCGGTAGACAG CCGCACCCGC240
GACGTCACGG CGCACGCCGCGCCCAGGCTCACCCACGGGTCTAGCGTGCG CATATAGCGG300
TCGTCCGGCA AGCGACGCGCCACCCGTCGTCGGATGGGCCGCCTCGCGCT TGACCGGGAT360
ATGGGTGTGT TCCGGCACGTAGAACGTCTGGAAGCCGTGGTCGTCGGCAA GTTTGGCGGC420
TGCCGCCGGG GAGATGCCGCGGTCGCTGGTGAAAAGTACAAGCCCGTAAT CCATGGACAG480
AATTAGAACG TGTTCTACCGGCGGTGGGCAAGCCGCTGCGCCGCCGAGGA TCTCGACTCG540
GACCCACAAC ACTGGTCGGCGCCGGGCGCGCCGACAGGTCGGTCGGCCCG GCACGGGCGG600
CCGAACGTGC GCTAGCGTGGGTGATCGATCGCGTCGCAACGCAAGATCTC ATGCGGCGTC660
GCTGAGGGTC TTGAAGGCACTGGAAGCAATA 691
(2) INFORMATION FOR SEQ ID NO.: 63
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 698
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM SIMIAE
(XI) SEQUENCE DESCRIPTION: SEQ ID 63
NO.:
TCGTATTGGG CTTCTTCCTG CGTCCACAGC CTTCCAGGTACTCGCGCAGC60
GCCCGCATGG
ATGGTCCGCC GGCGCGCCGG CGGCACGTTG GTTCGTCGGTGTTCCAACCG120
TGGTCGGCCA
AACCCGACGC CCACACTGAC CCGTCCGCCG CCAGGGTGGCGATGCTTTTC180
GACAGATGGT
GCCAGCGTGA TCGGGTCGTG CTCGACGGGC CGGTGGACAGTCGCACCCGC240
AGCGCGACCG
GAGGTGACCG CGCACGCCGC GCCCAGACTG CCAGCGTGCGCATGTAGCGG300
ACCCACGGGT
TCGTCGGGCA GCGATTCGTC GCCCGTCGTG CCTCGCGCTTGATCGGGATG360
GGATGGGCCG
TGAGTGTGTT CTGGCACGTA GAACGTTGTG CGTCGGCGAGTTTGGCCGCG420
AAGCCATGGT
GCCGCCGGGG CGATGCCCCG ATCACTGGTG GCCCGTAATCCATGCACAGA480
AAAAGCACGA
ATTAGAACGT GTTCTACCTC TGTGGAGCAA CTACGTCGACCCGCAGACGG540
GCGGCCCCCG
GCCGCTGAGA CGATCGCTCC TGGTCGCGCC TCGCTCCCGCGCACCCGCTC600
TAGGGGCCGG
CA 02354197 2001-11-29
36q
GAACGTGCGC TAGCGTGGTT GATCGGTCGCGCGTAACGCAAACGCGGGCA AGCAGTGACG660
TCGCGCCCGA CGAGGTCTTG AAGGCACTGGAAGCAACA Egg
(2) INFORMATION FOR SEQ 64
ID NO.:
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 712
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM
SZULGAI
(XI) SEQUENCE DESCRIPTION: 64
SEQ ID NO.:
GTGCGGCGGC GCCCGGCCGG GACGCCGTGATCAGCGAGCTCGTCGGTATT CCAGCCGAAG60
CCGACGCCGA GGCTGACCCG GCTGCCGGACAGATGATCCAGCGTGGCAAT GCTTTTGGCC120
AGCGTGATCG GATCATGCTC GACCGGCAGCGCCACCGCGGTGGACAACCG GACCCGAGAC180
GTCACCGCGG CCGCAGCACC CAAACTCACCCACGGGTCCAGCGTGCGCAT GTAGCGGTCA240
TCGGGCAGCG ACGCGTCACT CGTAGTGGGATGGGCAGCCTCCCGCTTGAT CGGGATGTGG300
GTGTGTTCAG GCACGTAGAA CGTCTGAAAACCGTGGTCGTCGGCCAGCTT TGCGGCCGCC360
GCCGGGGCAA TGCCGCGATC GCTGGTGAAAAGTACAAGCCCGTAATCCAT GCACCGAATT420
AGAACGTGTT CTACCTGCGA TGAGCAAGCGGCCCGGTCGGCCGACGAGCA GGTCGGCCCG480
GCGCGACCAG CAGAACGTGC GCTAGCGTGGTTGATCGAGTCGCGCACCGG AAAGCAACCG540
GAAGTAATCA GGAGGAGCCA TGACCTACTCGACCGGCAGCCCCGGATATC CGCCTGCGCA600
GCAGCCCGGG GGGTCGTACG GCGGCGCCACTCCTGGTGACGCTCAGAGCA AGCTTCCGCT660
GTACCTCAGC ATGGCGGTGG CCGCCCTCGGCCTGGCCGCGTATCTCGCCA GC 712
(2) INFORMATION FOR SEQ ID NO.: 65
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 802
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM
TUBERCULOSIS
(XI) SEQUENCE DESCRIPTION: 65
SEQ ID NO.:
TCATAGCAGG CCTCCTCTTG GGTCCACAACGCCCGCATCGCCTCGAGGTA TTCGCGCAGC60
ATGGTGCGGC GGCGTCCGGG TGGCACACCATGATCGACGAGCTCGTCGGT GTTCCAGCCG120
AACCCGACCC CGACGCTGAC CCGGCCGTGCGACAAATGATCCAGCGTCGC AATGCTTTTC180
GCCAGCGTGA TCGGATCATG CTCGACCGGCAGCGCCACCGCGGTGGCAAG CCGGATCCGC240
GACGTCACCG CCGATGCTGC TCCCAGGCTCACCCACGGGTCCAACGTGCG CATATAGCGG300
TCGTCCGGCA GCGAAGCGTC ACCCGTCGTCGGATGGGCCGCCTGGCGCTT GACCGGGATG360
TGGGTGTGTT CGGGCACGTA AAACGTGCGAAACCCGTGGCTTTCAGCAAG TCTGGCGGCC420
GCGGCCGGGG TGATGCCGCG GTCGCTGGTGAACAGCACAAGTCCGTAGTG CATGCACCGA480
ATTAGAACGT GTTCCACCTG CGCCGGGCAAGCGGCCGTCCAGTCGTTAAT GTCGCGAGCG540
CCGGTCGCTC CGGCAGCGGC ACCCGAACGTGCGCTAGCGTGGTTGATCGA ATCGCGTCGC600
CGGGAGCACA GCGTCGCACT GCACCAGTGGAGGAGCCATGACCTACTCGC CGGGTAACCC660
CGGATACCCG CAAGCGCAGC CCGCAGGCTCCTACGGAGGCGTCACACCCT CGTTCGCCCA720
CGCCGATGAG GGTGCGAGCA AGCTACCGATGTACCTGAACATCGCGGTGG CAGTGCTCGG780
CCTGGCTGCG TACTTCGCCA GC 802
(2) INFORMATION FOR SEQ 66
ID NO.:
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 628
(ii) MOLECULAR TYPE: DNA
CA 02354197 2001-11-29
36r
(VI) ORIGINAL SOURCE:
(A) ORGANISM: Mycobacterium bovis
(XI) SEQUENCE 66
DESCRIPTION:
SEQ ID
NO.:
TCATAGCAGGCCTCCTCTTGGGTCCACAAC GCCCGCATCGCCTCGAGGTATTCGCGCAGC60
ATGGTGCGGCGGCGTCCGGGTGGCACACCA TGATCGACGAGCTCGTCGGTGTTCCAGCCG120
AACCCGACCCCGACGCTGACCCGGCCGTGC GACAAATGATCCAGCGTCGCAATGCTTTTC180
GCCAGCGTGATCGGATCATGCTCGACCGGC AGCGCCACCGCGGTGGCAAGCCGGATCCGC240
GACGTCACCGCCGATGCTGCTCCCAGGCTC ACCCACGGGTCCAACGTGCGCATATAGCGG300
TCGTCCGGCAGCGAAGCGTCACCCGCCGTC GGATGGGCCGCCTGGCGCTTGACCGGGATG360
TGGGTGTGTTCGGGCACGTAAAACGTGCGA AACCCGTGGCTTTCAGCAAGTCTGGCGGCC420
GCGGCCGGGGTGATGCCGCGGTCGCTGGTG AACAGCACAAGTCCGTAGTGCATGCACCGA480
ATTAGAACGTGTTCCACCTGCGCCGGGCAA GCGGCCGTCCAGTCGTTAATGTCGCGAGCG540
CCGGTCGCTCCGGCAGCGGCACCCGAACGT GCGCTAGCGTGGTTGATCGAATCGCGTCGC600
CGGGAGCACAGCGTCGCACTGCACCAGT 628
(2) INFORMATION FOR SEQ 67
ID NO.:
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 400
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM
XENOPI
(XI) SEQUENCE DESCRIPTION: 67
SEQ ID NO.:
GTTCACCCAC CGCGAGCAAG CGGCGCCGGTAGAAGCTGCGATGACACGCC AGTCGCCGCG60
AGACCCCCGC CGCCAGGTGC GCTAGCGTGGATGGTCGAATCGCGTCGCAA CGCCTGCCCT120
GACAAGTCAC GGCGTTAATG GAGCGGTCCACGCAGCGTCGCGCGGAAGCG GCGCCCTGGG180
GATACAGCGT CGCAACACAG TGGCGCCCCAACGGCACTGATGCACAGGAG AAGCCATGAC240
GTACTCGCCC GGTAGCCCCG GATATCCACCCGCGCAGTCCCCCGGTTCCT ACGGCGGCTC300
CCCACAGTCG TTCGCCAAAT CCGATGACGGCGCCAGCAAGCTGCAGCTGT ATCTGACCGT360
CGCGGTGGTG GCGCTCGGCC TGGCGGCCTACCTGGCGAGT 400
(2) INFORMATION FOR SEQ ID NO.: 68
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 707
(ii) MOLECULAR
TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM
PARATUBERCULOSIS
(XI) SEQUENCE DESCRIPTION: 68
SEQ ID NO.:
TCGTAGCTGG CTTCCTCGTCGGTCCACAGC GCCCGCATCGCTTCCAGGTATTCGCGCAGC60
ATGGTGCGGC GCCGGCCCGCCGGCACGCCG TGGTCGGCGAGTTCGTCGGTGTTCCAGCCG120
AACCCGACGC CGAGGCTGACCCGGCCGCCG GACAGATGGTCAAGGGTGGCAATACTTTTC180
GCCAGCGTGA TCGGGTCGTGTTCGACCGGC AGGGCCACCGCGGTGGACAGCCGCACCCGC240
GAGGTGACGG CACAGGCCGCGCCCAGACTG ACCCACGGGTCCAGGGTGCGCATGTAGCGG300
TCGTCGGGCA GCGACGCGTCGCCGGTGGTC GGGTGCGCGGCCTCCCGCTTGATCGGGATA360
TGCGTGTGTT CCGGCACGTAGAAGGTCGCA AACCCGTGGTCGTCGGCAAGCTTCGCGGCC420
GCAGCCGGAG AGATGCCACGGTCGCTGGTG AAAAGCACAAGCCCGTAATCCATGCAGTGA480
ATTAGAACGT GTTCTACCTCTGCGGGGCAA GCTGTCGTGATACGGACCGTCTCGCCGCGC540
GGTCGTCTGC GAAGCCCGCGGGCAAGCCAA TGGCGACGGCACCGGCCGTCGCACGTGCGC600
TAGCGTGGGT GATCGACCGTGTCGCTCGCG CAGTGACGCGCCTGCAAGCACCGCGTCGCA660
TCGCAACCGT GGCGCCCGCTCGGCACTAAA AGGCAGTGGAAGCAACA 707
CA 02354197 2001-11-29
36s
(2) INFORMATION FOR SEQ ID NO.: 69
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 686
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM
MARINUM
(XI) SEQUENCE DESCRIPTION: 69
SEQ ID NO.:
TCGTAGGCGG CTTCCTCCTG CGTCCACAGTCGCCCGCATCGCCTCGAGGT ATTCACGCAA60
CATCGTGCGG CGCCGTCCGG GTGGAACGCCATGGTCGGCGAGTTCGTCGG TGTTCCAACC120
GAACCCCACG CCGAGGCTGA CCCGTCCGCCGGACAGATGATCCAGCGTGG CAATGCTCTT180
GGCCAGGGTG ATCGGGTCAT GCTCGACGGGCAGCGCCACCGCAGTCGACA GCCGTACCCG240
CGAGGTCACC GCCGATGCCG CGCCCAAACTCACCCAGGGGTCCAGCGTGC GCATATAACG300
ATCGTCGGGA AGCGAGGAAT CGCCCGTCGTTGGATGAGCGGCTTCTCGCT TGATTGGGAT360
ATGGGTGTGC TCAGGCACGT AGAAGGTGTGAAAGCCGTGGTCGTCAGCGA GTCTCGCCGC420
CGCCGCCGGA GCGATGCCGC GGTCGCTGGTGAAAAGCACAAGCCCATAGT CCATAACAGA480
ATTAGAACGT GTTCTACCTC GGCCGGGCAAGCGCCCCCCGCGCCAATCGG CTCGGCGGGA540
TCGACGGAGG TGATGGCGCT GGTCGAGCGGGGGCAGGTCGCCGCGGCGCG AGCACCGGAA600
CGTGCGCTAG CGTGGTTGTT CGAATCGCGTCGCAGGGACCAAGCGTCGCA ATGCAGCAGC660
GGCGCCGCGA CGGCGCGCAA GTAACA 686
(2) INFORMATION FOR SEQ ID NO.: 70
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 685
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM ULCERANS
(XI) SEQUENCE DESCRIPTION: SEQ ID 70
NO.:
TCGTAGGCGG CTTCCTCCTG CGTCCACAGC CCTCGAGGTA TTCACGCAAC60
GCCCGCATCG
ATCGTGCGGC GCCGTCCGGG TGGAACGCCA GTTCGTCGGT GTTCCAACCG120
TGGTCGGCGA
AACCCCACGC CGAGGCTGAC CCGTCCGCCG CCAGCGTGGC AATGCTCTTG180
GACAGATGAT
GCCAGGGTGA TCGGGTCATG CTCGACGGGC CAGTCGACAG CCGTACCCGC240
AGCGCCACCG
GAGGTCACCG CCGATGCCGC GCCCAAACTC CCAGCGTGCG CATATAACGA300
ACCCAGGGGT
TCGTCGGGAA GCGAGGAATC GCCCGTCGTT CTTCTCGCTT GATTGGGATA360
GGATGAGCGG
TGGGTGTGCT CAGGCACATA GAAGGTGTGA CGTCAGCGAG TCTCGCCGCC420
AAGCCGTGGT
GCCGCCGGAG CGATGCCGCG GTCGCTGGTG GCCCATAGTC CATAACAGAA480
AAAAGCACAA
TTAGAACGTG TTCTACCTCG GCCGGGCAAG GCCAATCGGC TTGGCGGGAT540
CGCCCCCCGC
CGACGGAGGT GATGGCGCTG GTCGAGCGGG CGCGGCGCGA GCACCGGAAC600
GGCAGGTCGC
GTGCGCTAGC GTGGTTGTTC GAATCGCGTC AGCGTCGCAA TGCAGCAGCG660
GCAGGGACCA
GCGCCGCGAC GGCGCGCAAG TAACA 685
(2) INFORMATION FOR SEQ ID NO.:
71
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 729
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM LEPRAE
(XI) SEQUENCE DESCRIPTION: SEQ ID 71
NO.:
CA 02354197 2001-11-29
36t
TCATATAACG GCTTCATTCTTGTGTCCATAATGCCTGCATTGCTTCGAGGCATTCGTACA60
CCATGGTGCG GCGCCGCCCGGATGGCACATCGTGATCGGTGAGCTCGTTGGTCTTCCAAC120
CGAACCCGAC GCCGAAGTTCACTCACTCGCCGGACAAATTATCCAGGTTGACAATACTTT180
TCGCAAGTGT GATTGGGTCATGTTAGACGGGCAGCGCCACCACCATGAACAGTCGTAGCC240
TGCCGATATA ACCCGCATGTCGCGCCCAAACTTACCCATGAGTCATAGGTACGCATCGCA300
TATAGCTGTC GTCACTGGACAGTGATACTCATCCGTAACCAGGTAGTGGGGTCTGAGTGG360
CAATGGCATA TGGGTGTGTTCGGGCACATAGAACTTGCGGAAGCCGTGGCTCTCCGCAAG420
CTTGACTGCT GCCGCGGGGGTGATGCCGCGGTCGTTGGTTAAAAGCGCAATCCCGTAGCC480
CATACCAAGA ATTTAGAGCGTGTTCCACCTGCGACGGCCAAGCGGTCGTGCCGACGATTT540
CGGCGTCCAT CGGTGGTAGGCGAGCTGACACGCAGGTCGTGCCGGCGCGGTCGCCCTAAC600
GTGCGCTAGC GTTGATGATCGAATGCGCCGCAACGTAAGCGCTGCCAATTTGGGCGTTTA660
TCCAACGGTG CGCATGGGAGCACAGCGTTGCACTGCAGCAGTGGCGCCGTGACGGCACTG720
gaaataaca 729
(2) INFORMATION SEQ ID 72
FOR NO.:
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 129
(ii) MOLECULAR
TYPE: DNA
(VI) ORIGINAL
SOURCE:
(A) ORGANISM:
MYCOBACTERIUM
NONCHROMOGENICUM
(XI) SEQUENCE 72
DESCRIPTION:
SEQ ID NO.:
GTTCCTGTTC GGCGGGCAACGGGGGGGTCCTTGTCGCGCAGTGTTGACCCACCGACTCGG60
CCCGCAAGTG CGCTAGCGTGGATGGTCGAAGCGCGCCGCACCGCCCACCAGCGCCCTGCC120
acaagcaca 129
(2) INFORMATION SEQ ID 73
FOR NO.:
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 219
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM SCROFULACEUM
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 73
GTTCTACCTC CGGTGAGCAA GCTGCCGCCG CGGCGGCACG GATCGGCGTC CAAGCCGGTC 60
GCGACGGCAC GCCCGTCCCG AAGTGCGCTA GCGTGGTTGA TCGATCGCGT CGCAACGCAA 120
CCGCCGGGCA CGGCATTCGT GGAACGGCGC GCCCGCACGC ACAGCGCCGC GACGCAACTG 180
TGGCGCCCGC AAAGGCACTT CACGGCACTG GAAGCAACA 219
(2) INFORMATION FOR SEQ ID NO.: 74
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 116
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM TRIPLEX
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 74
GTTCTACCTT GGTCGGCAAG CGGCGCGGGA ACGGCCCCGG CACCGGCTCC CCGACGTGCG 60
CTAGCGTGGT TGTTCGAATC GCGTCGCAAC GCAAGCGCGG CGAGCCTGGA AAAACA 116
CA 02354197 2001-11-29
36u
(2) INFORMATION FOR SEQ ID NO.: 75
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 568
(ii) MOLECULAR
TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: MYCOBACTERIUM
PARATUBERCULOSIS
(XI) SEQUENCE DESCRIPTION: 75
SEQ ID NO.:
GATCTCAGAC AGTGGCAGGTGGCGGCTCCG AAGCTGGCGTCAGCTATTGGTGTACCGAAT60
GTTGTTGTCA CCGAGCCGGTCCCAGGTGTG TTCGAGTTGCAGCTGAGAATTGTCGATCCG120
CTTAGTTCGC CGCTTGAATGGTCGTCTGTG CCAGCCGCCCACTCGTGGTCTCTGAGTTTG180
GGTATCGATG AAATGGGCGTCTACCAGTCG CTCCCGTTGGCGAACGTATCGGGCGTTGTA240
GTGGGAGGCG TACCAGGGTCGGGGAAAACC GCGTGGCTGACGAGTGCTCTGGGGTCGTTC300
GGTGCGTCAG CGGCGGTCCAGTTCGCTGTC ATCGACGGGAAGGGTGGTCAGGACTTGGAA360
TGCCTGCGTG CTCGTAGCTGCCGATTCATG AATGACGATCTGGAGCTGCCTGAGATTGCA420
GCGATTCTGA ATGACGCGACCGGTCTAGTC CGTGATCGAATTAGACAGGGCAACAACATA480
TTCGGATCGT CCAACTTTTGGGATCGCGGC CCGACGCCGCAGGTTCCGCTGGTGTTCGTG540
GTGATTGACG GCTATCGGGGCCGAGATC 568
(2) INFORMATION FOR SEQ ID NO.: 76
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 715
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: PSEUDOMONAS
AERUGINOSA
(XI) SEQUENCE DESCRIPTION: 76
SEQ ID NO.:
GCCCGTCACA CCATGGGAGT GGGTTTTACCAGAAGTGGCTAGTCTAACCG CAAGGAGGAC60
GGTCACCACG GTAGGATTCA TGACTGGGGTGAAGTCGTAACAAGGTAGCC GTATCGGAAG120
GTGCGGCTGG ATCACCTCCT TTCCAGAGCTTCTCGCACAAGTTGAGCGCT CACGCTTATC180
GGCTGTAAAT TAAAGACAGA CTCAGGGGTCTGTAGCTCAGTCGGTTAGAG CACCGTCTTG240
ATAAGGCGGG GGTCGTTGGT TCGAATCCAACCAGACCCACCATTGTCTGT CGGTAACACA300
CCTGAGGCAA ATCTGTACAT GGGGGCATAGCTCAGCTGGGAGAGCACCTG CTTTGCAAGC360
AGGGGTCGTC GGTTCGATCC CGTCTGCCTCCACCAATCACCAACGCTAAG GGCTTGGTTC420
AGACACTGAA CCGAGAATTT TGCATTGGCGATTGAGCCAGTCAGAGGATA TCAACAGATA480
TCGGCTGTCG TTCTTTAACA ATCTGGAAGAAGTAAGTAATTTGGATAGCG GAAGCGTCTT540
GAGATGGACG TGGAAACTAT CCGGGTTGTGATTGTATCGATGTATCTCAA GATGATTCGA600
ACTCTAAGTT TGACTCAATT GGAATACGGCACAACGCGAGAACTCAACCT GTAACGAGAC660
AGACTCGTTA TAGGGTCAAG CGAACAAGTGCATGTGGTGGATGCCTTGGC RRTCA 715
(2) INFORMATION FOR SEQ 77
ID NO.:
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 653
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: BURKHOLDERIACEPACIA
(XI) SEQUENCE DESCRIPTION: 77
SEQ ID NO.:
GCCCGTCACA CCATGGGAGT GGGTTGCTCCAGAAGTAGCTAGTCTAACCG CAAGGGGGAC60
GGTTACCACG GAGTGATTCA TGACTGGGGTGAAGTCGTAACAAGGTAGCC GTAGGGGAAC120
CA 02354197 2001-11-29
36v
CTGCGGCTGGATCACCTCCTTAATCGAAGATCTCAGCTTCTTCATAAGCT CCCACACGAA180
TTGCTTGATTCACTGGTTAGACGATTGGGTCTGTAGCTCAGTTGGTTAGA GCGCACCCCT240
GATAAGGTGAGGTCGGCAGTTCGAATCTGCCCAGACCCACCAATTGTTGG TGTGCTGCGT300
GATCCGATACGGGCCATAGCTCAGCTGGGAGAGCGCCTGCTTTGCACGCA GGAGGTCAGG360
AGTTCGATCCTCCTTGGCTCCACCATCTAAAACAATCGTCGAAAGCTCAG AAATGAATGT420
TCGTGAATGAACATTGATTTCTGGTCTTTGCACCAGAACTGTTCTTTAAA AATTCGGGTA480
TGTGATAGAAGTAAGACTGAATGATCTCTTTCACTGGTGATCATTCAAGT CAAGGTAAAA540
TTTGCGAGTTCAAGCGCGAATTTTCGGCGAATGTCGTCTTCACAGTATAA CCAGATTGCT600
TGGGGTTATATGGTCAAGTGAAGAAGCGCATACGGTGGATGCCTTGGCRR TCA 653
(2) INFORMATION FOR SEQ 78
ID NO.:
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 600
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: PSEUDOMONAS
PUTIDA
(XI) SEQUENCE DESCRIPTION: 78
SEQ ID NO.:
GGGTTCCCCG AAGTAGCTAG TCTAACCTTCGGGAGGACGGTTACCACGGTGTGATTCATG60
ACTGGGGTGA AGTCGTAACA AGGTAGCCGTAGGGGAACCTGCGGCTGGATCACCTCCTTA120
ATCGACGACA TCAGCCTGCT GATGAGCTCCCACACGAATTGCTTGATTCATTGTCGAAGA180
CGATCAAGAC CCTATATAGG TCTGTAGCTCAGTTGGTTAGAGCGCACCCCTGATAAGGGT240
GAGGTCGGCA GTTCAAATCT GCCCAGACCTACCAATATGCGGGGCCATAGCTCAGCTGGG300
AGAGCGCCTG CCTTGCACGC AGGAGGTCAGCGGTTCGATCCCGCTTGGCTCCACCACTCG360
CTTTACTTGA TCAGAACTTA GAAATGAACATTCGTTGATGAATGTTGATTTCTGACTTTT420
GTCAGATCGT TCTTTAAAAA TTCGGATATGTGATAGAAATAGACTGAACACCAGTTTCAC480
TGCTGGTGGA TCAGGCTAAG GTAAAATTTGTGAGTTCTGCTCGAAAGAGCAACGTGCGAA540
TTTTCGGCGA ATGTCGTCTT CACAGTATAACCAGATTGCTTGGGGTTATATGGTCAAGTG600
(2) INFORMATION FOR SEQ ID NO.: 79
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 446
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: PSEUDOMONAS PUTIDA
(XI) SEQUENCE DESCRIPTION: 79
SEQ ID NO.:
GGTTCACCAG AAGTAGCTAG TCTAACCTTCGGGAGGACGGTTACCACGGT GTGATTCATG60
ACTGGGGTGA AGTCGTAACA AGGTAGCCGTAGGGGAACCTGCGGCTGGAT CACCTCCTTA120
ATCGACGACA TCAGCCTGCT GATGAGCTCCCACACGAATTGCTTGATTCT TTGTAAAAGA180
CGATCAAGGC CTTGTGCAGG CCTCGCGTTGTTCCTGATCAGAACTTGGAA ATGAGCATTC240
GCTTCGAATG TTGATTTCTG GCTTTTGTCAGATCGTTCTTTAAAAATTCG GATATGTGAT300
AGAAATAGAC TGAACACCAG TTTCACTGCTGGTGGATCAGGCTAAGGTAA AATTTGTGAG360
TTCTGCTCGA AAGAGCAACG TGCGAATTTTCGGCGAATGTCGTCTTCACA GTATAACCAG420
ATTGCTTGGG GTTATATGGT CAAGTG 446
(2) INFORMATION FOR SEQ 80
ID NO.:
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 660
(ii) MOLECULAR TYPE: DNA
CA 02354197 2001-11-29
36w
(VI) ORIGINAL
SOURCE:
(A) ORGANISM:
PSEUDOMONAS
AERUGINOSA
(XI) SEQUENCE 80
DESCRIPTION:
SEQ ID
NO.:
GCCCGTCACACCATGGGAGT GGGTTGCTCCAGAAGTAGCTAGTCTAACCGCAAGGGGGAC60
GGTTACCACGGAGTGATTCA TGACTGGGGTGAAGTCGTAACAAGGTAGCCGTAGGGGAAC120
CTGCGGCTGGATCACCTCCT TAATCGAAGATCTCAGCTTCTTCATAAGCTCCCACACGAA180
TTGCTTGATTCACTGGTTAG ACGATTGGGTCTGTAGCTCAGTTGGTTAGAGCGCACCCCT240
GATAAGGTGAGGTCGGCAGT TCGAATCTGCCCAGACCCACCAATTGTTGGTGTGCTGCGT300
GATCCGATACGGGCCATAGC TCAGCTGGGAGAGCGCCTGCTTTGCACGCAGGAGGTCAGG360
AGTTCGATCCTCCTTGGCTC CACCATCTAAAACAATCGTCGAAAGCTCAGAAATGAATGT420
TCGTGAATGAACATTGATTT CTGGTCTTTGCACCAGAACTGTTCTTTAAAAATTCGGGTA480
TGTGATAGAAGTAAGACTGA ATGATCTCTTTCACTGGTGATCATTCAAGTCAAGGTAAAA540
TTTGCGAGTTCAAGCGCGAA TTTTCGGCGAATGTCGTCTTCACAGTATAACCAGATTGCT600
TGGGGTTATATGGTCAAGTG AAGAAGCGCATACGGTGGATGCCTTGGCRRTCASAGGCGA660
(2) INFORMATION FOR SEQ ID NO.: 81
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 722
(ii) MOLECULAR
TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: BURKHOLDERIA CEPACIA
(XI) SEQUENCE DESCRIPTION: 81
SEQ ID NO.:
GCCCGTCACA CCATGGGAGTGGGTTTTACCAGAAGTGGCTAGTCTAACCGCAAGGAGGAC60
GGTCACCACG GTAGGATTCATGACTGGGGTGAAGTCGTAACAAGGTAGCCGTATCGGAAG120
GTGCGGCTGG ATCACCTCCTTTCCAGAGCTTCTCGCACAAGTTGAGCGCTCACGCTTATC180
GGCTGTAAAT TAAAGACAGACTCAGGGGTCTGTAGCTCAGTCGGTTAGAGCACCGTCTTG240
ATAAGGCGGG GGTCGTTGGTTCGAATCCAACCAGACCCACCATTGTCTGGCGGTAACACA300
CCTGAGGCAA ATCTGTACATGGGGGCATAGCTCAGCTGGGAGAGCACCTGCTTTGCAAGC360
AGGGGTCGTC GGTTCGATCCCGTCTGCCTCCACCAATCACCAACGCTAAGGGCTTGGTTC420
AGACACTGAA CCGAGAATTTTGCATTGGCGATTGAGCCAGTCAGAGGATATCAACAGATA480
TCGGCTGTCG TTCTTTAACAATCTGGAAGAAGTAAGTAATTTGGATAGCGGAAGCGTCTT540
GAGATGGACG TGGAAACTATCCGGGTTGTGATTGTATCGATGTATCTCAAGATGATTCGA600
ACTCTAAGTT TGACTCAATTGGAATACGGCACAACGCGAGAACTCAACCTGTAACGAGAC660
AGACTCGTTA TAGGGTCAAGCGAACAAGTGCATGTGGTGGATGCCTTGGCRRTCASAGGC720
ga 722
(2) INFORMATION FOR SEQ 82
ID NO.:
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 725
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: STENOTROPHOMONAS ILIA
MALTOPH
(XI) SEQUENCE DESCRIPTION: 82
SEQ ID NO.:
GCCCGTCACA CCATGGGAGT TTGTTGCACCAGAAGCAGGTAGCTTAACCTTCGGGAGGGC60
GCTTGCACGG TGCTGCGATG ACTGGGGTGAAGTCGTAACAAGGTAGCCGTATCGGAAGGT120
GCGGCTGGAT CACCTCCTTT TGAGCAAAGACAGCATCGTCCTGTCGGGCGTCTTCACAAA180
GTACCTGCAT TCAGAGAATC ACAACGGCCAGGCCGATGTGAGAGTCCCTTTTGGGCCTTA240
GCTCAGCTGG GAGAGCACCT GCTTTGCAAGCAGGGGTCGTCGGTTCGATCCCGACAGCTC300
CACCATGTTC GAGCTGTATA CCGAAGTCCCTTTCGAAGAGCCCGCACATCCATGTGCTAC360
TTTTTGAAAA AGCCTTTCGG GTCTGTAGCTCAGGTGGTTAGACGCACCCTGATAAGGGTG420
AGGTCGGTAG TTCGAGTCTA CCCAGACCCACCATTCTCTGAATGACGCATACATTCGATC480
CA 02354197 2001-11-29
36x
TTTATACGCA TCAGCACTGT GGCTGGTACG TGTTCTTTTA AAACTTGTGA CGTAGCGAGC 540
GTTTGAGATG TTCTATCAGA CGTGTCGTGA GGCTAAGGCG AGAGACGCAA GTCTCTTTAT 600
TGATTGAGTC GTTATATTCG TATCCGGGCT TTGTACCCCC GGGTCGTGTG TAACCCAAGG 660
CAACTTGCGG TTATATGGTC AAGCGAATAA GCGCACACGG TGGATGCCTT GGCRRTCASA 720
ggcga 725
(2) INFORMATION FOR SEQ ID NO.: 83
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 18
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: Pseudomonas sp.
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 83
ACGTCACACC ATGGGAGT 18
(2) INFORMATION FOR SEQ ID NO.: 84
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 23
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: BURKHOLDERIA CEPACIA
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 84
CCCTGAGTCT GTCTTTAATT TAC 23
(2) INFORMATION FOR SEQ ID NO.: 85
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 20
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: PSEUDOMONAS AERUGINOSA
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 85
CTTTCGACGA TTGTTTTAGT 20
(2) INFORMATION FOR SEQ ID NO.: 86
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 21
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: STENOTROPHOMONAS MALTOPHILIA
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 86
TCAATAAAAG AGACTTGCGT C 21
CA 02354197 2001-11-29
36y
(2) INFORMATION FOR SEQ ID NO.: 87
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 18
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: Pseudomonas sp.
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 87
GATTGCCAAG GCATCCAC 18
(2) INFORMATION FOR SEQ ID NO.: 88
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 18
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: Pseudomonas sp.
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 88
GAGGAAGGTG GGGATGAC 18
(2) INFORMATION FOR SEQ ID NO.: 89
(I) SEQUENCE CHARACTERISTICS
(A) LENGTH: 18
(ii) MOLECULAR TYPE: DNA
(VI) ORIGINAL SOURCE:
(A) ORGANISM: Pseudomonas sp.
(XI) SEQUENCE DESCRIPTION: SEQ ID NO.: 89
TGGGAACGTA TTCACCGT 18