Note: Descriptions are shown in the official language in which they were submitted.
CA 02354343 2001-10-31
INTEGRATED SELECTIVE OXIDATION REACTOR APPARATUS AND PROCESS
FIELD OF THE INVENTION
The present invention relates to a process for recovering useful heat from a
selective
oxidation apparatus and a reactor apparatus for selectively oxidizing carbon
monoxide contained
in a hydrogen-rich stream at optimally controlled temperature conditions to
produce a useful gas
for a fuel cell power system.
BACKGROUND OF THE INVENTION
Catalytic reaction apparatus and processes for converting hydrocarbon
feedstocks to
useful gases, such as hydrogen, are well known in the art. Hydrogen-rich gases
formed by these
catalytic processes, such as the sequence of steam reforming followed by high
and low
temperature shift reactions, typically contain residual carbon monoxide
concentrations in the
range of 0.5 to 1.0 volume percent. Hydrogen-rich gas streams can be
beneficially used to
generate electrical power in a fuel cell system. The use of proton exchange
membrane (PEM)
fuel cells has particular economic advantage for certain electric power
applications. However,
when hydrogen-rich fuel is produced for use in PEM fuel cells, the carbon
monoxide
concentration must be reduced to low levels, preferably below 10 ppm, in order
to prevent
poisoning of the anode electrocatalysts typically used in these fuel cell
systems.
While several methods are known in the art for purification of hydrogen-
containing gas
streams, selective catalytic oxidation of carbon monoxide by oxygen is a
favored method for use
in small fuel cell applications because of its simplicity and potentially low
cost. The principal
obj ective of a selective catalytic oxidation reactor apparatus for fuel cell
applications is to oxidize
carbon monoxide to the fullest extent possible to form carbon dioxide by
reaction with molecular
oxygen, while simultaneously minimizing the quantity of hydrogen that is
oxidized to form HZO.
The oxidation process is exothermic and the process generates useful heat.
A selective process can be quantified by a conversion efficiency factor and a
selectivity
factor. Conversion efficiency is defined herein as the ratio of the quantity
of COZ produced by
oxidation to the initial quantity of CO in the feed stream, and selectivity is
defined here as the
ratio of the quantity of oxygen consumed by CO oxidation to the total quantity
of oxygen
consumed during reaction. For PEM fuel cell applications, it is desirable that
the conversion
2
CA 02354343 2001-10-31
efficiency be at least in the range of 99% to 99.9%, in order to met PEM fuel
cell CO tolerance
limitations, and that the selectivity be at least in the range of 25% to 50%,
in order to prevent
unnecessary consumption of hydrogen that would otherwise limit fuel cell
electric power
generation efficiency. It is desirable that these objects be met when the
selective oxidation
reactor is operated in a temperature range where the exothermic heat of
reaction can be
beneficially used within the process.
Oh and Sinkevitch~'~ reported the results of CO oxidation tests conducted in
the presence
of hydrogen using a variety of catalytic materials including platinum,
palladium, rhenium,
ruthenium, silver, mixtures of cobalt and copper, and mixtures of nickel,
cobalt and iron. Both
RulAl203 and Rh/A1z03 catalysts were found to be active for CO oxidation at
temperatures above
about 200 °F, and to exhibit acceptable conversion efficiencies and
selectivities for PEM
applications at temperatures up to about 350 °F. Pt/A1203 was found to
be active only at
temperatures above about 375 °F. Other catalyst materials tested
required even higher activation
temperatures and were generally less selective than the Ru/A1z03 and Rh/A1z03
catalysts.
Yasumoto et at (U.S. Patent No. 5702838) describe a catalyst material
comprising an A-
type zeolite carrying at least one metal selected from the group consisting of
Pt, Pd, Ru, Au, Rh,
and Ir, or an alloy of two or more metals. The catalyst material is claimed to
have high activity
and selectivity suitable for PEM fuel cells in the temperature range from
about 122 °F to 392 °F.
Sato et at (U.S. Patent No. 5658681) describe a selective oxidation system
that uses
catalytic material comprising Au supported on an oxide carrier consisting of
at least one oxide
selected from Fe203, CO, NiO, A1203, TiOz, Zr02 and SiOz. The catalytic
material is claimed to
have activity and selectivity suitable for PEM fuel cells in the temperature
range of 194 °F to 284
°F. The invention teaches the use of circulating cooling water to
maintain the selective oxidation
catalyst in the desired temperature range using a reactor comprising
partitioned plates.
Vanderborgh et at (U.S. Patent No. 5271916) describe a method and apparatus
for
selectively oxidizing carbon monoxide in a hydrogen rich stream using two or
more reactors
operated at progressively higher temperatures. The main portion of oxidizing
air is fed to the
first reactor and a smaller portion of oxidizing air is fed to the second
reactor. The invention
claims to use a catalyst, for instance Pt/A1z03, in the first reactor that is
fed gases at a preferred
temperature range of 320 °F to 347 °F. The inlet temperature of
the first reactor is controlled
by exchanging heat against a two-phase fluid, such as 1,3,5 - trimethyl
benzene, that boils at
3
CA 02354343 2001-10-31
about 328 °F. The first reactor is operated adiabatically so that the
exit temperature is greater
than the inlet temperature due to the heat of oxidation. The exit gas from the
first reactor is
cooled by heat exchange to a second temperature preferably about 374 °F
before entering a
second adiabatic reactor.
Pow et at (U.S. Patent No. 5316747) describes use ofheat exchange coil
apparatus packed
with a selective oxidation catalyst, for instance Pt/A1203, for the removal of
oxidation reaction
heat to a pressurized thermal fluid that is circulated within the Apparatus.
The apparatus includes
means to introduce oxidant at a primary inlet and a secondary or multiple
secondary inlets. The
invention teaches the importance of reactor temperature control to maintain
high reaction
selectivity.
SUMMARY AND OBJECTS OF THE INVENTION
An obj ect of this invention to provide a novel catalytic reaction apparatus
and process for
the selective oxidation of carbon monoxide contained in a hydrogen-rich gas
stream, and to
control the temperature of the catalytic reaction apparatus in a temperature
range that is both
optimum for conducting the oxidation reaction at high conversion efficiency
and selectivity, and
for recovering the exothermic heat of reaction in order to generate steam that
is needed in a
process for converting hydrocarbons feedstock to useful gases, such as
hydrogen. The invention
uses an array of catalyst packed tubes that are disposed within a waste heat
steam generator that
is operated at a pressure corresponding to the optimum temperature for
conducting the catalytic
oxidation reaction. The subject invention is particularly well suited for the
production of
hydrogen for fuel cells having low tolerance to carbon monoxide.
PEM fuel cells systems based on the steam reforming of hydrocarbons to
hydrogen-rich
gases require that steam is available in sufficient quantities to conduct the
steam reforming
reactions under conditions that avoid carbon formation within the catalytic
steam reforming
apparatus. Typically, the conditions needed to avoid carbon formation within
the catalytic steam
reforming apparatus require the addition of approximately 3 moles of steam per
mole of carbon
contained in the hydrocarbon feed. If the PEM fuel cell system is to achieve
high thermal
efficiency, the required steam quantity must be generated from waste heat
recovered from the
hydrocarbon conversion process.
4
CA 02354343 2001-10-31
The oxidation of either carbon monoxide or hydrogen releases heat energy in an
amount
greater than 60,000 calorie per g mole of reactant. This represents a
substantial amount of heat
energy that can be beneficially recovered to generate steam necessary for the
conversion of
hydrocarbon feedstocks using a steam reforming process. Thus, it is desirable
that the
exothermic heat of reaction released by the selective oxidation process be
recovered at a
temperature range suitable for generating steam needed for the hydrocarbon
steam reforming
process.
For certain PEM fuel cell applications, such as the generation of residential
electrical
power, it is highly desirable that the pressure of the steam reforming process
be as low as
possible, typically in the range of 3 psig to 10 psig, for reasons of safety
and cost, and because
residential fuels, such as natural gas, are typically available only at
relatively low pressure. Also,
for reasons of safety and cost, it is preferred that the steam generator
operate at the minimum
pressure needed to supply steam to the steam reforming process. Thus, the
preferred pressure
operating range for the steam generator is about 5 psig to 10 psig,
corresponding to a steam
saturation temperature range of approximately 230 °F to 240 °F.
In the present invention, the selective oxidizer reactor apparatus is
integrated with a waste
heat recovery steam generator operating at a pressure range of 5 psig to 10
psig. The steam
generator contains a reservoir of boiling water maintained at a temperature
range of about 230
°F to 240 °F. An array of tubes are packed with a suitable
oxidation catalyst and immersed
within the boiling water reservoir of the steam generator. Since the surfaces
of the tubes are in
thermal communication with the boiling water, once thermal equilibration has
occurred between
the boiling water and the catalyst packed tubes, the temperature of the
selective oxidation
apparatus is maintained at a temperature at least equal to the saturation
temperature of the boiling
water. Thus, the present invention provides a convenient means for heating the
selective
oxidation reactor to a predetermined minimum operating temperature range
during start-up.
During normal operation, hydrogen-rich gases containing CO are mixed with a
defined
portion of oxidant and passed over the catalyst contained within the tubes of
the selective
oxidizer apparatus. As the mixture passes through the catalyst packed tubes,
oxygen reacts with
the carbon monoxide and a portion of the hydrogen to form carbon dioxide and
Hz0 thereby
releasing exothermic heat. As the heat is released by the oxidation reaction,
the gas temperature
within the catalyst bed rises until the temperature driving force is
sufficient so remove the heat
CA 02354343 2001-10-31
of reaction by heat transfer from the catalyst packed bed tube surfaces to the
boiling water
contained within the reservoir of the waste heat steam generator.
The ratio of the heat transfer surface to the catalyst volume can be
controlled by design
in order to maintain the maximum temperature differential between the catalyst
bed and the
boiling water to within a predetermined limit, such as between 10 °F
and 70 °F, corresponding
to maximum catalyst bed operating temperatures ranging from about 240
°F to 300 °F. Thus,
the present invention achieves the object of reliably controlling the
selective oxidation reactor
temperature in an optimum range of about 240 °F to 300 °F while
simultaneously recovering
useful heat.
In the present invention, the selective oxidizer uses a catalyst that achieves
high
conversion and selectivity in the optimum operating temperature range of 240
°F to 300 °F. It
is apparent from the prior art that there are several catalysts available, for
instance Ru/A1203,
Rh/A1203, Pt/A-zeolite, and Au/Fe203, that have the desired activity and
selectivity to met PEM
applications for use in a selective oxidation reactor apparatus operating at
temperatures in the
range of about 240 °F to 300 °F.
The foregoing and other objects, features, and advantages of the present
invention will
become more apparent in the light of the following detailed description of the
preferred
embodiments.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a graph showing the effect of temperature on the reduction of CO
concentration
using a selective oxidation catalyst operating at a space velocity of 3000 hr-
' and an oxygen to
CO ratio of 2:1.
FIG. 2 is a process flow diagram for recovering heat from a selective
oxidation process
and to control the selective oxidizer temperature within an optimum range to
achieve high
conversion efficiency and selectivity.
FIG. 3 is a preferred embodiment of a selective oxidation reactor apparatus
according to
the invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
A process is described that makes beneficial use of the heat of reaction from
a selective
oxidation reactor that is employed to produce hydrogen-rich gases containing
low concentrations
of carbon monoxide from hydrocarbon feedstocks.
6
CA 02354343 2001-10-31
Refernng to FIG. 1 the test results for a selective oxidation reactor
apparatus using a
commercial catalyst and operating at a space velocity of 3000 hr-' and an
oxygen to CO ratio of
2:1. The tests were conducted over a range of temperatures using a reactant
gas mixture
consisting of 1 % CO and 99% hydrogen. The test results show that the CO
concentration of the
reactor effluent was less than 10 ppm over the temperature range of
approximately 200 °F to 300
°F, corresponding to a CO conversion efficiency of greater than 99.9%
and a selectivity of at
least 25%. The test results show that a selective oxidation apparatus can be
operated within the
optimum temperature range of about 200 °F to 300 °F necessary
for the useful generation of
steam in a low pressure steam generator.
FIG. 2 illustrates a method and process for integrating a selective oxidation
reactor
apparatus in a process to beneficially recovery the exothermic heat of
reaction of a selective
oxidation reaction apparatus operating in the temperature range of about 240
°F to 300 °F for
the object of generating steam that is useful for converting hydrocarbon
feedstock to
hydrogen-rich gases and for controlling the temperature of the selective
oxidation reactor in a
predetermined range.
Refernng to FIG. 2, a reactant mixture 1 consisting of hydrocarbon feedstock
32 and
steam 11 are preheated in an exchanger 2 and introduced into a tubular
catalytic reactor 3 that
is contained within a combustion chamber 4. The tubular catalytic reactor
typically contains a
supported Ni catalyst and is commonly referred to in the industry as a steam
reformer. Fuel 5
and air 6 are combusted in the chamber to heat the reactant mixture so as to
produce a
hydrogen-rich stream 7 containing carbon monoxide concentrations typically
ranging from 5%
to 15 %.
Combustion products 8 from the combustion chamber pass through a flue gas heat
exchange coil 9 that is contained within a waste heat steam generator 10,
wherein the combustion
products are cooled and steam 11 is generated.
The hydrogen-rich stream is cooled in an exchanger 2 to a temperature
typically in the
range of 600 °F to 650 °F, whereupon the cooled stream 12 is
introduced into a fixed catalyst
bed reactor 13 to effect a water gas shift reaction that converts a portion of
the carbon monoxide
to hydrogen and carbon dioxide by reaction with steam. The fixed catalyst bed
reactor typically
contains a supported Fe/Cr catalyst and is commonly known in the industry as a
high temperature
7
CA 02354343 2001-10-31
shift reactor. The carbon monoxide concentration of the process gas 14 exiting
the high
temperature shift reactor typically ranges from 2% to 5%.
The products from the high temperature shift reactor are cooled in a process
exchange coil
15 that is contained within the waste heat steam generator 10 thereby
generating steam and
cooling the process gas to a temperature typically in the range from 350
°F to 450 °F before it
is introduced into a second fixed catalyst bed reactor 16 wherein the carbon
monoxide
concentration is further reduced by a water gas shift reaction. The fixed
catalyst bed reactor
typically contains a supported Cu/Zn catalyst and is commonly known in the
industry as a low
temperature shift reactor. The carbon monoxide concentration of the process
gas 17 exiting the
low temperature shift reactor is typically less than 1 %.
The process gas 17 is cooled in a second process exchange coil 18 that is
contained within
the waste heat steam generator 10 thereby generating steam and cooling the
process gas. The
second process exchange coil 18 is designed so that the temperature of the
process gas 19 at the
coil exit is close to the saturation temperature of the steam generated in the
waste heat steam
generator 10. Typically, the process gas temperature is 10 °F to 30
°F higher than the steam
saturation temperature.
The process gas 19 enters an air eductor 20 that induces by motive force a
flow of
ambient air 21 that is mixed with the process gas. The quantity of ambient air
introduced into
the process gas is typically controlled by design of the eductor to provide an
OZ:CO ratio in the
range of 1:1 to 2:1.
The mixture 22 is introduced into an array of catalyst packed tubes 23, also
known as the
selective oxidizer, that are disposed within the waste heat steam generator
operating at a pressure
in the range of 5 psig to 25 psig, and preferably in the range of 5 psig, to
10 psig. The surface
of the tubes are generally immersed within the water reservoir 25 of the waste
heat steam
generator. The tubes contain a selective oxidation catalyst having optimum
activity and
selectivity in the temperature range of approximately 240 °F to 300
°F. As the mixture passes
through the catalyst packed tubes, oxygen reacts with the carbon monoxide to
form carbon
dioxide to release exothermic heat. A portion of the oxygen also reacts with
hydrogen to release
additional heat. As the heat is released by the oxidation reaction, the gas
temperature within the
catalyst bed rises until the temperature driving force is sufficient to remove
the heat of reaction
by heat transfer from the catalyst packed bed tube surfaces 24 to the boiling
water 25 contained
8
CA 02354343 2001-10-31
within the waste heat steam generator. The ratio of the heat transfer surface
to the catalyst
volume can be controlled by design in order to maintain the catalyst bed in
the desired operating
temperature regime.
The process gas 26 exiting the selective oxidizer typically contains less than
10 ppm
carbon monoxide. The process gas is then cooled in an air cooler 27 wherein
excess steam is
condensed and recovered in a separator vessel 28. The hydrogen-rich gases 29,
having been
purified of carbon monoxide to low concentrations are available for use, for
instance, in fuel cells
having a low tolerance to carbon monoxide.
The condensed steam or condensate 30 is pumped by a pressurizing means 31
through
a boiler feedwater preheater that receives heat by exchange against combustion
products 10 to
heat the water before it is introduced into the waste heat steam generator.
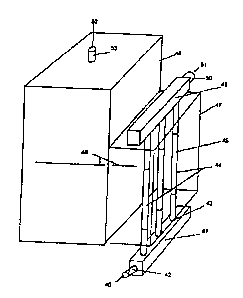
Refernng to FIG. 3 a design of a selective oxidation reactor apparatus is
given that
accomplishes the obj ect of useful energy recovery to generate steam and for
control of the reactor
apparatus temperature in an optimum regime during start-up and normal
operating states. A
process gas and oxidant mixture 40 enters an inlet manifold 41 through an
inlet means 42. The
inlet manifold evenly distributes the mixture to the inlet means 43 of an
array of tubes 44. The
tubes are packed with a selective oxidation catalyst 45 having optimum
performance in the
temperature range of 240 °F to 300 °F.
The array of tubes are disposed in a waste heat steam generator 46 or
compartment 47
thereof operating at a pressure of 5 psig to 25 psig, and preferably between S
psig and 10 psig.
The tubes are partially or fully immersed in a reservoir of boiling water 48.
The tube diameters
typically range from 3/8" to 2.0" and preferably from'/2" to 1 %2". The
catalyst particle diameters
contained in the tubes typically range from 1/32" to 1/2" and preferably 1/16"
to'/4", however,
the catalyst may take many forms including pellet, structured packings, and
monoliths. The
space velocity in the catalyst packed tubes is typically in the range of 1000
to 15000 hr -' and
preferably from 2000 hr' to 6,000 hr-'. The catalyst bed may be diluted by
inert packing to
control the heat release profile within the tubes.
As the heat is released by the oxidation reaction, the gas temperature within
the catalyst
bed rises until the temperature driving force is sufficient to remove the heat
of reaction by heat
transfer from the catalyst packed bed tube surfaces to the boiling water 48
contained within the
waste heat steam generator. The steam 52 generated from the process exits the
waste heat steam
9
CA 02354343 2001-10-31
generator at an exit means 53. The ratio of the heat transfer surface to the
catalyst volume can
be controlled by design in order to maintain the catalyst bed in the desired
operating temperature
regime of 190°F to 300°F and preferably 240 °F to 300
°F. Typically, this ratio is in the range
of 10 ft-' to 150 ft~' and preferably from 30 ft-' to 100 ft-'.
The tube array 44 is connected to an exit manifold 49 to collect product gases
that exit
the apparatus through an outlet means 50. The product gases, having a CO
concentration reduced
below 10 ppm, typically exit at a temperature in the range of 190 °F to
300°F and preferably
from 240 °F to 300 °F.
Other embodiemtns of the invention will be readily apparent to theose skilled
in the art
and are meant to be within the scope of the claims appended hereto.