Note: Descriptions are shown in the official language in which they were submitted.
CA 02355187 2001-06-14
WO 00/35351 PCT/US99/3006
METHOD AND APPARATUS FOR PRODUCING HOMOGENOUS
CAVITAT10N TO ENHANCE TRANSDERMAL TRANSPORT
FIELD OF THE INVENTION
This invention relates to transdermal molecular transportation More
specifically, this invention relates to methods and apparatus for producing
homogenous
cavitation in a transdermal transport system.
BACKGROUND OF THE INVENTION
Drugs are routinely administered either orally or by injection. The
effectiveness of most drugs reifies on achieving a certain concentration in
the
bloodstream. Although some drugs have inherent side effects which cannot be
eliminated in any dosage form, many drugs exhibit undesirable behaviors that
are
specifically related to a particular route of administration. For example,
drugs may be
degraded in the GI tract by the low gastric pH, local enzymes or interaction
with food
l 5 or drink within the stomach. The drug or disease itself may forestall or
compromise
drug absorption because of vomiting or diarrhea. if a drug entity survives its
trip
through the GI tract, it may face rapid metabolism to pharmacologically
inactive forms
by the liver, the first-pass effect Sometimes the drug itself has inherent
undesirable
attributes such as a short half life, high potency or a narrow therapeutic
blood level
range
Recently, efforts aimed at eliminating some of the problems of traditional
dosage forms involve transdermal delivery of the drubs (TDD). Topical
application has
been used for a very long time, mostly in the treatment of localized skin
diseases. Local
treatment, however, only require that the drug permeate the outer layers of
the skin to
treat the diseased state, with little or no systemic accumulation.
Transderrnal delivery
systems are designed for, inter alia, obtaining systemic blood levels, and
topical drug
application For purposes of this application, the word "transdermal" is used
as a
generic term to describe the passage of substances to and through the skin.
TDD offers several advantages over traditional delivery methods including
injections and oral delivery. When compared to oral delivery, TDD avoids
gastrointestinal drug metabolism, reduces first-pass ef~'ects, and provides
sustained
CA 02355187 2001-06-14
WO 00135351 PCT/US99/30067
2
release of drugs for up to seven days. as reported by Elias in I'ercutarre«u.s
Ah.srnption:
lLlecharrisnr.s-Mcthndnl~y; u-I~ru~,~ I~clivcry, Bronaugh, R. L. Maibach, N 1.
(Ed), pp 1-
12, Marcel Dekker, New York, 1989.
The transport of drugs through the skin is complex since many factors
influence their permeation. These include the skin structure and its
properties, the
penetrating molecule and its physical-chemical relationship to the skin and
the delivery
matrix, and the combination of the skin, the penetrant, and the delivery
system as a
whole. Particularly, the skin is a complex structure. There are at least four
distinct
layers of tissue: the nonviable epidermis (stratum corneum, SC) the viable
epidermis, the
viable dermis, the subcutaneous connective tissue. Located within these layers
are the
skin's circulatory system, the arterial plexus, and appendages, including hair
follicles,
sebaceous glands, and sweat glands. The circulatory system lies in the dermis
and
tissues below the dermis. The capillaries do not actually enter the epidermal
tissue but
come within 150 to 200 microns of the outer surface of the skin.
In comparison to injections, TDD can reduce or eliminate the associated
pain and the possibility of infection. Theoretically, the transdermal route of
drug
administration could be advantageous in the delivery of many therapeutic
drugs,
including proteins, because many drugs, including proteins, are susceptible to
gastrointestinal degradation and exhibit poor gastrointestinal uptake,
proteins such as
interferon are cleared rapidly from the blood and need to be delivered at a
sustained rate
in order to maintain their blood concentration at a high value, and
transdermal devices
are easier to use than injections.
In spite of these advantages, very few drugs and no proteins or peptides are
currently administered transdermally for clinical applications because of the
low skin
permeability to drugs. This low permeability is attributed to the SC, the
outermost skin
layer which consists of flat, dead cells filled with keratin fibers
(keratinocytes)
surrounded by lipid bilayers. The highly-ordered structure of the lipid
bilayers confers
an impermeable character to the SC (Flynn, G.L., in I'ercutaneous Ahsnrptivn:
~Llechani.sms-il~lcthndolo~;y~-Orrr~,~l)clivcry.; Bronaugh, R.L., Maibach, H.
1. (Ed), pages
27-53, Marcel Dekker, New York 1989). Several methods have been proposed to
CA 02355187 2001-06-14
WO 00/35351 PCT/US99/30067
enhance transdermal drug transport, including the use of chemical enhancers,
i.e. the use
of chemicals to either modif~~ the skin structure or to increase the drug
concentration in
a transdermal patch (Burnette, R. R., in I~evcln~mentall.ss7~c.5 and
IZe.scarch lnitia~rves;
Hadgraft J., Guy, R H., Eds., Marcel Dekker: 1989; pp 247-288; Junginger, et
al. in
S l)ru~~l'ermeal~nn I:nhanccment; Hsieh, D.S., Eds., pp. 59-90, Marcel Dekker,
Inc. New
York l 994) and the use of applications of electric fields to create transient
transport
pathways [electroporation] or to increase the mobility of charged drugs
through the skin
(iontophoresis) (Prausnitz I'rnc. Natl. Acad. Sci. USA 90, )0504-10508 (1993);
Waiters, K. A., in %ransdermal Drug Delevery: Developmental Lrsucs and
IZescarch
Initiatives, Ed. Hadgraft J., Guy, R.H., Marcel Dekker, 1989). Another
approach that
has been explored is the application of ultrasound.
Ultrasound has been shown to enhance transdermal transport of low-
molecular weight drugs (molecular weight less than 500) across human skin, a
phenomenon referred to as sonophoresis (Levy, J. Clin. Invest. 1989, 83, 2974-
2078;
l 5 Kost and Langer in "7bpical drug I3inavailability, l3ioeyuivalencc, and
l'cnctration ";
pp. 91-103, Shah V. P., Maibach H.I., Eds. (Plenum: New York, 1993); Frideman,
R.M., "Inlerferon.s~: A Primer ", Academic Press, New York, 1981 ). For
example, U.S.
Patent No. 4,309,989 to Fahim and U. S. Patent No. 4,767,402 issued to Kost et
al. both
describe the use of ultrasound in conjunction with transderrnal drug delivery.
U.S.
Patent No. 4,309,989 discloses the topical application of a medication using
ultrasound
with a coupling agent such as oil. Ultrasound at a frequency of at least l 000
kHz and
a power of one to three W/cmz was used to cause selective localized
intracellular
concentration of a zinc containing compound for the treatment of herpes
simplex virus.
U.S. Patent No. 4,309,989, the disclosure of which is specifically
incorporated by reference, discloses the use of ultrasound for enhancing and
controlling
transdermal permeation ofa molecule, including drugs, antigens, vitamins,
inorganic and
organic compounds, and various combinations ofthese substances, through the
skin and
into the circulatory system. Ultrasound having a frequency between about 20
kHz. and
10 MHz. and having an intensity between about 0 and 3 W/cm2 is used
essentially to
drive molecules through the skin and into the circulatory system. A
significant drawback
CA 02355187 2001-06-14
WO 00!35351 PCT/US99/30067
4
to this system is that the resultant enhanced permeability only occurs while
the
ultrasound is being applied to the skin Thus, the skin is often damaged due to
over
exposure to the ultrasound.
Although a variety of ultrasound conditions have been used for
sonophoresis, the most commonly used conditions correspond to therapeutic
ultrasound
(frequency in the range of betv,~een one MHz and three I\9Hz, and intensity in
the range
ofbetwecn above zero and two W/cm~) (such as that described in the Kost et al.
patent).
It is a common observation that the typical enhancement induced by therapeutic
ultrasound is less than ten-fold. In many cases, no enhancement of transdermal
drug
transport has been observed upon ultrasound application. Accordingly, a better
selection
of ultrasound techniques is needed to induce a higher enhancement of
transdermal drug
transport by sonophoresis.
Application of low-frequency (between approximately 20 and 200 kHz}
ultrasound can dramatically enhance transdermal transport of drugs, as
described in
PCT/US96/12244 by Massachusetts Institute of Technology. Transdermal transport
enhancement induced by low-frequency ultrasound was found to be as much as
1000-
fold higher than that induced by therapeutic ultrasound. Another advantage of
low-
frequency sonophoresis as compared to therapeutic ultrasound is that the
former can
induce transdermal transport of drugs which do not passively permeate across
the skin.
In addition to there being a need to deliver drugs through the skin, there
is a major medical need to extract analytes through the skin. For example, it
is desirable
for diabetics to measure blood glucose several times per day in order to
optimize insulin
treatment and thereby reduce the severe long-term complications of the
disease.
Currently, diabetics do this by pricking the highly vascularized fingertips
with a lancet
to perforate the skin, then milking the skin with manual pressure to produce a
drop of
blood, which is then assayed for glucose using a disposable diagnostic strip
and a meter
into which this strip fits. This method of glucose measurement has the major
disadvantage that it is painful, so diabetics do not like to obtain a glucose
measurement
as often as is medically indicated.
CA 02355187 2001-06-14
WO 00/35351 PCT/US99/3006 i
Therefore, many groups are working on non-invasive and less invasive
means to measure glucose, such as micro lancets that are very small in
diameter, very
sharp. and penetrate only to the interstitium (not to the blood vessels of the
dermis). A
small sample, from about 0.1 to two ~l, of interstitial fluid is obtained
through capillary
S forces for glucose measurements. Other groups have used a laser to breach
the integrity
of the stratum corneum and thereby make it possible for blood or interstitial
fluid to
diffuse out of such a hole or to be obtained through such a hole using
pneumatic force
(suction) or other techniques. An example of such a laser based sampling
device is
disclosed in US Patent No. 5,165,418 to Tankovich and WPl ACC No: 94-167045/20
by Budnik (assigned to Venisect, Inc.).
A problem with methods that penetrate the skin to obtain interstitial fluid
is that interstitial fluid occurs in the body in a gel like form with little
free fluid and in
fact is even negative pressure that limits the amount of free interstitial
fluid that can be
obtained When a very small hole is made in the skin, penetrating to a depth
such that
interstitial fluid is available, it takes a great deal of mechanical force
(milking, vacuum,
or other force) to obtain the quantity of blood used in a glucose meter.
Thus, there has been described methods for application of ultrasound and
extraction of analyte that rely on techniques known in the art such as are
disclosed in
United States Patent Application No. 08/885,93 J filed June 30, 1997, the
disclosure of
which is hereby incorporated by reference. The methods described therein
channel or
focus an ultrasound beam onto a small area of skin. In some embodiments,
methods and
devices utilizing a chamber and ultrasound probe disclosed can be used to non-
invasively
extract anaiyte and deliver drugs (i.e., broadly transdermally transport
substances). This
provides many advantages, including the ability to create a small puncture or
localized
erosion of the skin tissue, without a large degree of concomitant pain. The
number of
pain receptors within the ultrasound application site decreases as the
application area
decreases. Thus, the application of ultrasound to a very small area will
produce less
sensation and allow ultrasound and/or its local effects to be administered at
higher
intensities with little pain or discomfort. Channeling of ultrasound
geometrically is one
way to apply ultrasound to a small area The oscillation of a small element
near or in
CA 02355187 2001-06-14
WO 00/35351 PCT/US99!3006 i
6
contact with the surface of the skin is another way to apply ultrasound to a
small area
Large forces can be produced locally, resulting in cavitation, mechanical
oscillations in
the skin itself, and lame localized shearing forces near the surface of the
skin The
element can also produce acoustic streaming, which refers to the large
connective flows
produced by ultrasound. This appears to aid in obtaining a sample of blood or
interstitial
fluid without having to "milk" the puncture site. Ultrasound transducers are
known to
rapidly heat under continuous operation, reaching temperatures that can cause
skin
damage. Heat damage to the skin can be minimized by using a transducer that is
located
away from the skin to oscillate a small element near the skin. In the case of
analyte
extraction, compounds present on the surface of and/or in the skin can
contaminate the
extracted sample. The level of contamination increases as skin surface area
increases.
Surface contamination can be minimized by minimizing the surface area of
ultrasound
application. Thus, skin permeability can be increased locally, and transiently
through the
use of the methods and devices described herein, for either drug delivery or
measurement of analyte.
Moreover, it has been disclosed that the application of ultrasound is only
required once for multiple deliveries or extractions over an extended period
of time
rather than prior to each extraction or delivery. That is, it has been shown
that if
ultrasound having a particular frequency and a particular intensity of is
applied, multiple
analyte extractions or drug deliveries may be performed over an extended
period oftime.
For example, if ultrasound having a frequency of 20 kHz. and an intensity of I
0 VV/cm2
is applied, the skin retains an increased permeability for a period of up to
four hours.
This is described more particularly in United States Provisional Patent
Application No.
60/070,813 filed on January 8, l 998, the disclosure ofwhich is specifically
incorporated
by reference herein.
Nevertheless, the amount (e ~, duration, intensity, duty cycle etc.) of
ultrasound necessary to achieve this permeability enhancement varies widely
Several
factors on the nature of skin must be considered For example, the type of skin
which
the substance is to pass through varies from species to species, varies
according to age,
with the skin of an infant having a greater permeability than that of an older
adult, varies
CA 02355187 2001-06-14
WO 00/35351 PCT/US99/30067
7
according to local composition, thickness and density, varies as a function of
injury or
exposure to agents such as organic solvents or surfactants, and varies as a
function of
some diseases such as psoriasis or abrasion.
Vvhen cavitation is relied upon to enhance transdermal transport, care must
be taken to avoid excessive cavitation which can do damage to the skin through
the
localized increases ofheat and pressure characteristic with cavitation
phenomena. Ifthe
cavitation produced is sporadic or nonuniform, it very difficult to prevent
the localized
heat and pressure increases.
SUMMARY OF THE 1NVENT10N
Therefore, a need has arisen for a method and apparatus that provides
homogenous cavitation for use in a transdcrmal transport system.
According to one embodiment, the present invention comprises an
improved ultrasound source. The ultrasound source comprises an ultrasound
transmitting element having an axis and a first cross-section along said axis.
The
5 ultrasound transmitting element also has a first axial end operable to
produce ultrasonic
waves and a second axial end. The first axial end comprises a matrix of
ultrasound
producing portions.
According to another embodiment, the present invention comprises an
ultrasound source. The ultrasound souce comprises an ultrasound transmitting
element
having an axis and a cross-section along the axis. The ultrasound transmitting
element
- also has a first axial end and a second axial end operable to produce
ultrasonic waves.
The cross-section has an area having a maximum value at the first axial end
and a
minimum value at the second axial end.
According to another embodiment, the present invention comprises a
method for producing homogenous cavitation at an area of skin. The method
comprises
creating a volume offluid having a uniformly dispersed concentration of
cavitation nuclei
adjacent the area of skin. Ultrasound is then applied to the volume of fluid
and causes
cavitaiion at the cavitation nuclei.
According to another embodiment, the present invention comprises a
method for producing homogenous cavitation at an area of skin. The method
comprises
CA 02355187 2001-06-14
WO 00/35351 PCT/US99!30067
8
creating a volume of fluid having a uniformly dispersed concentration of a
first substance
adjacent the area of skin. The first substance is a substance that facilitates
the
production of cavitation. Ultrasound is then applied to the volume of fluid to
cause
cavitation.
According to another embodiment, the present invention comprises a
method for producing homogenous cavitation at an area of skin. An ultrasound
source
is provided to apply an ultrasonic wave to the area of skin. A screen having a
number
of opening therein is positioned between the area of skin and the ultrasound
source.
Finally, ultrasound is applied to the area of skin through the screen The
openings in the
l 0 screen nucleate cavitation and control the size of cavitation bubbles
produced
According to another embodiment, the present invention comprises an
ultrasound device. The ultrasound device includes an ultrasound horn and a
housing for
the ultrasound horn. The housing has a portion with a reduced inside diameter
relative
to a diameter of the horn. The reduced inside diameter focuses ultrasonic
energy on a
l 5 small area of skin.
BRIEF DESCRIPTION OF THE DRAWINGS
The features and objects of the present invention, and the manner of
attaining them is explained in detail in the following DETAILED DESCRIPTION OF
THE PREFERRED EMBODIMENTS ofthe invention when taken in conjunction with
20 the accompanying drawings wherein:
Figures 1 a and l b depict an ultrasonic horn configuration according to one
embodiment of the present invention.
Figures 2a-2d depict an ultrasonic horn configuration according to another
embodiment of the present invention.
25 Figures 3a and 3b depict an ultrasonic horn configuration according to
another embodiment of the present invention.
Figure 4 depicts an ultrasound configuration according to another
embodiment of the present invention.
CA 02355187 2001-06-14
WO 00/35351 PCT/US99/3006 i
9
DETAILED DESCRIPTION OF THE PREFERRED EMBOD1~~IENTS
The use of ultrasound to facilitate transdermal transport is known The
mechanism by which ultrasound is used to facilitate transdermal transport has
differed.
In the context of transdermal delivery systems, ultrasound was initially used
as a driving
force that essentially pushed drugs through the skin and into the circulatory
system.
Ultrasound is also used to increase the permeability of the skin. That is,
application of
ultrasound having a particular frequency will disorganize the lipid bilayer in
the skin and
thus increase the permeability of the skin In this context, either drugs can
be delivered
through the skin to the body or analyte can be extracted through the skin from
the body.
A driving force of some type is still required, but the required intensity of
the driving
force is decreased For example, a concentration gradient is generally
sufficient driving
force for transdermal transport through skin whose permeability has been
enhanced
usin~n ultrasound.
The permeability enhancement that results from the application of
ultrasound is due, at least in pan, to cavitation that is caused by the
ultrasound When
used to irradiate a liquid medium such as the coupling medium used in
conjunction with
the present invention, certain ultrasonic fields will cause cavitation in the
liquid. Broadly
defined, cavitation is the formation of vapor- or gas-filled cavities in
liquids when
subjected to mechanical forces. One problem with being able to effectively use
cavitation to enhance skin permeability is that cavitation is not readily
predictable or
controllable. In the context of a transdermal delivery system, cavitation that
is
inconsistent and unevenly dispersed is not as effective at enhancing skin
permeability as
cavitation that is consistent and evenly dispersed. Moreover, cavitation that
is highly
localized may cause skin damage. This application describes various apparatus
and
methods the inventors have found to produce consistent, evenly dispersed
cavitation.
Ultrasound is created and transmitted using a combination of a transducer
and horn. The transducer, converts an electrical impulse into a mechanical
vibration and
the horn transmits that mechanical vibration to a medium. The configuration
ofthe horn
determines the wave pattern of the ultrasound being transmitted to the medium.
Moreover, the wave pattern of the ultrasound is, at least in part, responsible
for the
CA 02355187 2001-06-14
WO 00/35351 PCT/US99r30067
cavitation Therefore, the horn configuration directly affects the amount and
dispersement of the cavitation caused by an ultrasonic wave. The inventors
have found
a number of horn configurations that produce a wave pattern that causes evenly
dispersed and consistent cavitation.
5 According to one embodiment, the present invention comprises an
ultrasonic horn configuration including a number of ultrasound producing
portions or
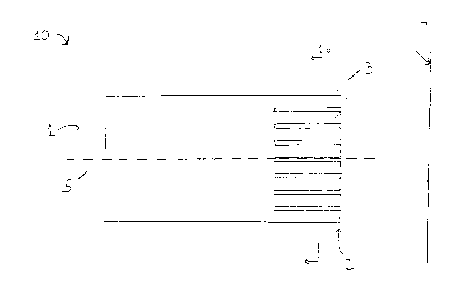
"fingers" that produce evenly dispersed cavitation As shown in Figures 1,
cylindrical
shaped ultrasound horn 10 having an axis S comprises a first axial end 1, a
second axial
end 2 and a plurality of ultrasound producing portions 3. Ultrasound horn 10
is
l0 generally connected to a transducer at its first axial end 1. The
transducer transmits a
vibration to horn 10 and the vibration is, in turn, transmitted to a fluid
medium at second
axial end 2 of horn 10.
Second axial end 2 of horn l0 is configured to include a plurality of
ultrasound producing portions or fingers 3. Each ultrasound producing portion
3
produces a separate ultrasonic wave and therefore a separate cavitation
source.
Moreover, in operation the ultrasonic wave produced by each finger 3 is in
phase with
and overlaps with the ultrasonic waves produced by its neighboring fingers.
This
overlap results in more evenly distributed ultrasound that in turn leads to
more evenly
distributed cavitation.
In the environment of an apparatus used to enhance the permeability of the
skin, ultrasound horn l0 is preferably configured so that the more evenly
distributed
cavitation occurs at or near the surface of the skin. This is accomplished by
controlling
the width of each finger, WF, the width of the gaps between the fingers, WG,
and the
distance, D, between the second axial end of the horn and the skin surface 4.
Ultrasound producing portions 3 can be fabricated on the end of horn 10
in a number of ways depending on the material used for horn l 0. For example,
if horn
10 is made of metal, fingers 3 may be configured on the second axial end of
horn 10 by
making a number of cuts through horn 10 in parallel with axis 5 These cuts can
be
made, for example, by and electrical discharge manufacturing process. This can
be used
to produce a matrix of ultrasound producing portions such as is shown in
Figures 1. In
CA 02355187 2001-06-14
w0 00/35351 PCT/US99/3006 i
other embodiments, ultrasound producing portions 3 are affixed to second axial
end 2
of horn l 0 by for example by press fitting the fingers into the end of horn l
0 The
fingers are preferably made from a hard and durable material such as titanium,
and
carbide steel. Other materials such as, stainless steel, aluminum, ceramic and
glass could
S be used
Horn J O is shown as a cylindrical horn having ultrasound producing
portions having a square cross-section along the horn axis. But, the horn and
ultrasound
producing portions could have many different shapes and many difTerent
combinations
of shapes. For example, the horn could be a bar shaped horn having a square
cross-
section and the fingers could be cylindrical with a circular cross-section.
Further, the
number of fingers configured on the end of the horn can vary. The number of
fingers
will determine the necessary dimensions WG and WF.
According to another embodiment, the present invention comprises an
ultrasonic horn having a "bullet" configuration that produces a cavitation
effect that
l S spreads out over the surface of the skin 24 As shown in Figures 2, bullet
shaped
ultrasound horn 20 having an axis 2S comprises a first axial end 2l , a second
axial end
22 having a tapered or bullet shaped configuration. Ultrasound horn 20 is
generally
connected to a transducer at its first axial end 21. The transducer transmits
a vibration
to horn 20 and the vibration is, in turn, transmitted to a fluid medium at
second axial end
22 of horn 20.
Second axial end 22 of horn 20 is configured to include a bullet shape.
That is, the cross-section along axis 2S of horn 20 varies in size between
first axial end
2l and second axial end 22. More specifically, the axial cross-section has an
area having
a maximum value at first axial end 21 and a minimum value at second axial end
22.
2S Referring particularly to Figures 2b, 2c and 2d, various cross sections of
horn 20 are
shown. As is readily apparent, the area A is greater than the area A 1, and
the area A 1
is greater than the area A2; A2 being the area of the cross-section nearest
the second
axial end of horn 20 and A being the area of the cross-section nearest the
first axial end
of horn 20. In operation, the ultrasonic wave produced by this bullet shaped
CA 02355187 2001-06-14
WO 00/35351 PCT/US99/30067
l2
configuration gradually spreads out as the distance from second axial end 22
increases
and Leads to cavitation that spreads out over skin surface 24.
This extent of the spreading out effect can be optimized somewhat by
controlling the rate of decrease of the cross-sectional area of horn 20. In
general, as the
rate of area reduction increases, that is, horn 20 becomes more tapered, the
spreading
effect becomes greater up to the point where second axial end 22 has a
spherical
configuration.
Horn 20 can be fabricated from any suitable material. The bullet
configuration can be formed at second axial end of horn 20 using any suitable
machininb
process. For example, second axial end 22 can be turned on a lathe to the
bullet
configuration.
Norn 20 is shown as a cylindrical horn. Nevertheless, a similar spreading
effect can be obtained by machining the bullet configuration at the second
axial end of
any horn. For example, a bar shaped horn having a square cross-section along
the horn
axis could be configured with a bullet shaped end.
According to another embodiment, the present invention comprises an
ultrasonic horn that combines the beneficial features of the finger horn and
bullet horn
described in conjunction with Figures 1 and 2. As shown in Figures 3,
ultrasound horn
30 having an axis 35 comprises a first axial end 31, a second axial end 32,
and a plurality
of ultrasound producing portions 33. Ultrasound horn 30 is generally connected
to a
transducer at its first axial end 31. The transducer transmits a vibration to
horn 30 and
the vibration is, in turn, transmitted to a fluid medium at second axial end
32 of horn 30.
Second axial end 32 of horn 30 is configured to include a plurality of
ultrasound producing portions or fingers 33. Each ultrasound producinf;
portion 33 has
a tapered or bullet shaped configuration and generates a separate ultrasonic
wave that
produces a cavitation effect that spreads out over the surface of the skin 34.
In
operation the ultrasonic wave produced by each finger 33 is in phase with and
overlaps
with the ultrasonic waves produced by its neighboring fingers This overlap
results in
more evenl~~ distributed ultrasound that in turn leads to more evenly
distributed
cavitation.
CA 02355187 2001-06-14
WO 00/35351 PCT/US99/30067
l3
Each bullet shaped finer 33 has an axis 33_5 and a cross-section that varies
in size between a first axial end 331 and a second axial end 332. More
specifically, the
axial cross-section has an area having; a maximum value at first axial end 331
and a
minimum value at second axial end 332. Horn 30 is depicted as having eighteen
fingers
only for ease of illustration In a preferred embodiment, horn 30 has a number
of figures
necessary to produce a desired cavitation pattern. According to one
embodiment, horn
30 is configured to have about 60 fingers.
In the environment of an apparatus used to enhance the permeability of the
skin, ultrasound horn 30 is preferably configured so that the more evenly
distributed
cavitation occurs at or near the surface ofthe skin This is accomplished by
controlling;
the width of each finger, WF, the width of the gaps between the fingers, WG,
and the
distance, D, between the second axial end of the horn and the skin surface 34.
Horn 30 is shown as a cylindrical horn. Nevertheless, horn 30 may have
many difl:'erent configurations. For example, bullet shaped fingers could be a
incorporated into a bar shaped horn having a square cross-section. Further,
the number
of fingers configured on the end of horn 30 can vary. The number of fingers
will
determine the necessary dimensions WG and WF.
Ultrasound transducers endure a great stress in normal operation. For
example, cavitation can cause localized hot spots and hiSh pressure gradierns.
Extended
exposure to ultrasound and cavitation can cause pitting of the ultrasound.
Pitting; of an
ultrasound horn quickly leads to accelerated decay, because the
nonuniformities in the
horn act as cavitation nuclei and therefore lead to cavitation occurring at
the surface of
the horn. Moreover, when cavitation occurs at the surface of the horn, it
interrupts
further transmission of the ultrasonic wave and therefore diminishes the
amount of
2S cavitation occurring elsewhere. In the context of an apparatus for
enhancing skin
permeability, this is disadvantageous because it reduces the efhectiveness of
the
ultrasound. Exposure times need to be increased to enhance permeability, thus
increasing; the chance of over exposure to ultrasound.
Therefore, according to another embodiment, the present invention
comprises a highly durable ultrasound horn. According to one embodiment the
present
CA 02355187 2001-06-14
WO 00/35351 PCT/US99/30067
14
invention comprises an ultrasound horn comprised of a carbide steel tip. In
another
embodiment, the present invention comprises an ultrasound horn that has an
anodized
hard coating. The use of carbide steel is generally limited to the tip of the
horn to
minimize losses. An anodized coating can be used on the entire horn or simply-
the
ultrasound radiating portion. The teachings ofthis embodiment ofthe present
invention
could be applied to any configuration of ultrasound horn including any of the
horns
shown and described in Figures J-4. For example, in the context of Figure l,
an
improved ultrasound horn 10 is formed by fabricating ultrasound radiating
portions 3
from carbide steel According to another example, an improved ultrasound horn
10 is
formed by anodizing the entire horn to after fabrication. Both the use of an
anodized
coating or carbide steel provide an ultrasound horn having enhanced durability
and
resistance to pitting.
Similarly, according to another embodiment, the present invention
comprises a highly polished ultrasound horn. For reasons discussed above, a
highly
polished ultrasound horn produces more consistent and homogenous cavitation.
By
polishing the ultrasound horn, nonuniformities are removed from the surface
ofthe horn.
This, in turn, limits the chance of sporadic cavitation at the horn surface.
According to another embodiment, the present invention comprises a
method of producing consistent and evenly dispersed cavitation using a
cavitation
screen. Structurally, the cavitation screen is a screen as that term is
conventionally used.
That is, a cavitation screen according to embodiments of the present invention
is a flat,
planar object having a matrix of openings therein. The cavitation screen is
preferably
formed from a durable and non-corrosive material such as metal. The cavitation
screen
may also be treated or coated with durable coating so that it is more
resistant to the
effects of ultrasound. For example, the screen may be anodized.
Operationally, the cavitation screen is positioned between an ultrasound
horn and the object to which ultrasound is to be applied The cavitation screen
enables
transmission and growth of consistent bubbles. The openings in the screen
nucleate
cavitation and filter the bubbles produced by cavitation. That is, cavitation
bubbles may
still be produced throughout the liquid, but the screen acts to break the
bubbles that are
CA 02355187 2001-06-14
WO OOI35351 PCT/l,'S99/30067
larger than the size of the openings in the screen. The size of the openings
can be
adjusted to produce the cavitation desired Further, in the context of an
apparatus for
enhancing skin permeability, the screen may be positioned anywhere between the
horn
and the skin if the screen is positioned close to the horn, the cavitation
will be
5 somewhat separated from the skin surface and have a lesser effect If the
screen is
moved closer to the skin, the cavitation also occurs closer to the skin and
therefore will
have a more pronounced effect on skin permeability
According to another embodiment, the present invention comprises a
method of producing consistent and evenly dispersed cavitation by "seeding"
the
10 coupling medium with cavitation nuclei. More, specifically, it has been
found that the
addition of particles to the coupling medium used in an apparatus for
enhancing skin
permeability leads to more consistent cavitation. Each particle dispersed
within the
coupling medium acts as a cavitation nuclei. Therefore, if particles are
evenly dispersed
throughout the coupling medium, more consistent and evenly dispersed
cavitation
l 5 results The particles may be formed from ceramics, polystyrene, titanium
dioxide or any
other metal or polymer The particles are sized appropriately for dispersion in
the
coupling medium. In one embodiment, the particles are I -20pm in diameter.
Smaller
or larger sizes are possible. The concentration of particles used should be
appropriate
for dispersion in the coupling medium. In one embodiment 5-10 mg/ml of
panicles are
used. The concentration of particles used varies depending on the type of
particles used
and the coupling medium.
In a related embodiment, dissolved gas, such as OZ is used in the coupling
medium to "seed" cavitation. if the dissolved gas is in the form ofbubbles,
these bubbles
act as cavitation nuclei If the dissolved gas exists at the molecular level,
it diffuses into
cavitation bubbles and enhances growth The cavitation enhancement is directly
proportional to the amount of dissolved gas in the medium Therefore, by
controlling
the dissolved gas concentration in the medium, the amount of cavitation
produced by
ultrasound can be controlled. Any suitable gas may be used to enhance
cavitation
Suitable gasses include, for example, oxygen, zenon, neon, argon, krypton and
helium.
Ifoxygen is used as the gas, a concentration ofabout 5 mg/dl is provided in
the coupling
CA 02355187 2001-06-14
WO 00/35351 PCT/US99/30067
16
medium. Other concentrations are possible and within the scope of the present
mvent~on.
In another embodiment, the present invention comprises a method for
producing consistent and evenly dispersed cavitation by dissolving chemicals
in the
coupling medium. Certain chemicals have properties that are helpful for
producing
consistent cavitation. Jn one embodiment, fluorocarbons are added to the
coupling
medium in an attempt to produce more consistent cavitation. Fluorocarbons have
a very
low boiling point. Therefore, when fluorocarbons are subjected to ultrasound
they tend
to evaporate. This evaporation causes gas bubbles in the coupling medium.
These gas
bubbles, in turn, act as cavitation nuclei and thus produce consistent
cavitation. The
amount of fluorocarbon added to the coupling medium can be adjusted based on
the
desired amount of cavitation. Suitable fluorocarbons include, for example,
perfluoropentane, perfluorohexane and similar molecules. In one embodiment,
the
fluorocarbons are used at a concentration of 5-10 ml;lml. Other concentrations
are
1 S possible and within the scope of the present invention.
Similarly, surfactants can be added to the coupling medium to produce
more consistent cavitation by a different mechanism. The use of surfactants in
the
coupling medium does not "seed" cavitation as the above methods do Rather, by
adding surfactant to the coupling medium, the surface tension of the coupling
medium
is reduced. This reduced surface tension makes it easier for cavitation to
occur by
making it easier for bubbles to form in the medium. Suitable surfactants
include sodium
lauryl sulfate and fatty alcohols, for example, dodecanol.
In another embodiment, the present invention comprises a method for
producing consistent and evenly dispersed cavitation by pretreating the skin
with
chemicals or cavitation nuclei. In one embodiment, the skin surface to be
subjected to
ultrasound is wiped with a chemical cleansing agent that removes
inhomogeneities from
the skin surface. The removal of inhomogeneities from the skin surface leads
to more
consistent cavitation by removing substances that could act as cavitation
nuclei and
cause sporadic, localized cavitation that could damage the skin. Alhocols such
as
ethanol and isopropyl alcohol are suitable for use to pretreat the skin.
CA 02355187 2001-06-14
WO 00/35351 PCT/US99l30067
17
In another embodiment, the skin to be treated with ultrasound is presoaked
with cavitation nuclei to produce more consistent cavitation The cavitation
nuclei could
be in any of the forms discussed above According to one embodiment, the skin
is
presoaked with solution having evenly dispersed and very fine particles The
particles
evenly distribute themselves on the surface of the skin. This results in
consistent and
evenly dispersed cavitation when ultrasound is applied In another embodiment,
the skin
is presoaked with a liquid having a high dissolved gas content. Similar to
above, when
ultrasound is applied, the dissolved gas acts as cavitation nuclei and thus
produces
consistent cavitation.
l0 Referring to Figure 4, an ultrasound configuration accordinb to another
embodiment of the present invention is provided. Ultrasonic horn 40 may be
used in
conjunction with transducer housing 42 that has a reduced inside diameter,
relative to
horn 40, where housing 42 is in contact with skin 44. Ultrasonic horn may be
coupled
with skin 44 through coupling medium 46. The walls of reduced diameter housing
42
mask a significant portion of skin 44, and expose only a fraction of skin 44
to
ultrasound.
The cavitation effect on the skin is generally most pronounced in the center.
Therefore, through this configuration, the level of permeability enhancement
achieved
is centralized of the treated skin
Other methods, such as a pin horn and accoustic channeling, may be used
to produce a similar efFect on the skin
The above embodiments focus on methods and apparatus used to produce
consistent and homogenous cavitation As v,~ill be apparent to one of ordinary
skill in
the art, these methods are not mutually exclusive. The methods and apparatus
can be
combined to provide even greater control of cavitation. For example, any of
the horns
shown in Figures 1-4 can be used in conjunction with the addition of
cavitation nuclei
to the coupling medium. Similarly, both chemicals and cavitation nuclei could
be added
to the coupling medium for an enhanced effect The area of skin can be
pretreated in
conjunction with any of the above apparatus and methods.
CA 02355187 2001-06-14
WO 00/35351 PCT/US99,'3006 i
18
Although the present invention has been described in detail, it should be
understood that various changes, substitutions, and alterations can be made
without
departing from the intended scope as defined by the appended claims.