Note: Descriptions are shown in the official language in which they were submitted.
CA 02355346 2001-05-30
-1-
ELECTROLYTIC APPARATUS, METHODS FOR PURIFICATION
OF AQUEOUS SOLUTIONS AND SYNTHESIS OF CHEMICALS
FIELD OF THE INVENTION
The present invention relates generally to the purification
of aqueous solutions and preparation of useful chemical products,
and more specifically, to electrochemical methods and more
efficient, economic and safer electrolytic apparatus for both the
electropurification of drinking water, industrial waste waters
and contaminated ground water, as well as the.electrochemical
synthesis (electrosynthesis) of useful products, e.g., organic
and inorganic chemicals.
BACKGROUND OF THE INVENTION
Wastewater can be a valuable resource in cities and towns
where population is growing and water supplies are limited. In
addition to easing the strain on limited fresh water supplies,
the reuse of wastewater can improve the quality of streams and
lakes by reducing the effluent discharges they receive.
Wastewater may be reclaimed and reused for crop and landscape
irrigation, groundwater recharge, or recreational purposes.
The provision of water suitable for drinking is another
essential of life. The quality of naturally available water
varies from location-to-location, and frequently it is necessary
to remove microorganisms, such as bacteria, fungi, spores and
other organisms like crypto sporidium; salts, heavy metal ions,
organics and combinations of such contaminants.
Over the past several years, numerous primary, secondary and
tertiary processes have been employed for the decontamination of
industrial wastewater, the purification of ground water and
treatment of municipal water supplies rendering them safer for
drinking. They include principally combinations of mechanical and
biological processes, like comminution, sedimentation, sludge
digestion, activated sludge filtration, biological oxidation,
nitrification, and so on. Physical and chemical processes have
also been widely used, such as flocculation and coagulation with
CA 02355346 2001-05-30
-2-
chemical additives, precipitation, filtration, treatment with
chlorine, ozone, Fenton's reagent, reverse osmosis, W
sterilization, to name but a few.
Numerous electrochemical technologies have also been
proposed for the decontamination of industrial wastewater and
ground water, including treatment of municipal water supplies for
consumption. While growing in popularity, the role of
electrochemistry in water and effluent treatment heretofore has
been relatively small compared to some of the mechanical,
biological and chemical processes previously mentioned. In some
instances, alternative technologies were found to be more
economic in terms of initial capital costs, and in the
consumption of energy. Too often, earlier electrochemical methods
were not cost competitive, both in initial capital costs and
operating costs with more traditional methods like chlorination,
ozonation, coagulation, and the like.
Earlier electrochemical processes required the introduction
of supporting electrolytes as conductivity modifiers which adds
to operating costs, and can create further problems with the
disposal of by-products. Electrochemical processes in some
instances have been ineffective in treating solutions by reducing
concentrations of contaminants to levels permitted under
government regulations. Heretofore, such electrochemical
processes have often lacked sufficient reliability for
consistently achieving substantially complete mineralization of
organic contaminants, as well as the ability to remove sufficient
color from industrial waste waters in compliance with government
regulations.
Notwithstanding the foregoing shortcomings associated with
earlier electrochemical technologies, electrochemistry is still
viewed quite favorably as a primary technology in the
decontamination of aqueous solutions. Accordingly, there is a
need for more efficient and safer electrochemical cell
configurations and processes for more economic treatment of large
volumes of industrial waste waters, effluent streams and
CA 02355346 2001-05-30
-3-
contaminated ground water, including the decontamination of
municipal water supplies making them suitable for drinking. Such
electrochemical cell configurations should also be useful in the
electrosynthesis of chemical products.
SUMMARY OF THE INVENTION
The present invention relates to improved means for
electropurification of aqueous solutions, particularly effluent
streams comprising waste waters polluted with a broad spectrum
of chemical and biological contaminants, including members from
such representative groups as organic and certain inorganic
chemical compounds. Representative susceptible inorganic
pollutants include ammonia, hydrazine, sulfides, sulfites,
nitrites, nitrates, phosphates, metal ions, and so on. Included
as organic contaminants are organometallic compounds; dyes from
textile mills; carbohydrates, fats and proteinaceous substances
from food processing plants; effluent streams, such as black
liquor from pulp and paper mills containing lignins and other
color bodies; general types of water pollutants, including
pathogenic microorganisms, i.e., bacteria, fungi, molds, spores,
cysts, protozoa and other infectious agents like virusesl oxygen-
demanding wastes, and so on.
While it is impractical to specifically identify by name all
possible contaminants which may be treated successfully according
to the claimed methods, it will be understood that language
appearing in the claims, namely "contaminated aqueous electrolyte
solution", or variations thereof is intended to encompass all
susceptible pollutants whether organic, inorganic, metal ions or
biological.
The electropurification methods and apparatus for practicing
this invention are particularly noteworthy in their ability to
effectively purify virtually any aqueous solution comprising one
or more organic, certain inorganic, including hazardous metal
ions and biological contaminants present in concentrations
ranging from as low as <1 ppm to as high as >300,000 ppm.
CA 02355346 2001-05-30
-4-
Only electricity is required to achieve the desired chemical
change in the composition of the contaminant(s), in most cases.
The conductivity of tap water is sufficient for operation of the
improved cell design. Hence, it is neither required, nor
necessarily desirable to incorporate additives into the
contaminated aqueous solutions to modify the conductivity of the
solution being treated to achieve the desired decomposition of
the pollutant/contaminant. Advantageously, in most instances
solid by-products are not produced in the electropurification
reactions as to create costly disposal problems. The improved
electrochemical processes of the invention are able to achieve
complete or virtually complete color removal; complete
mineralization of organic contaminants and total destruction of
biological pollutants even in the presence of mixed contaminants,
and at a cost which is competitive with traditional non-
electrochemical methods, such as chlorination, ozonation and
coagulation, and thereby meet or exceed government regulations.
Accordingly, it is a principal object of the invention to
provide an electrolysis cell which comprises at least one anode
and at least one cathode as electrodes positioned in an
electrolyzer zone. The electrodes are preferably spaced
sufficiently close not only to provide an interelectrode gap
capable of minimizing cell voltage and IR loss, but also to
achieve conductivity without the need for extra supporting
electrolytes or current carriers. Means are provided for directly
feeding electrolyte solutions to the electrodes for distribution
through the interelectrode gap(s). Means are provided for
regulating the residency time of the electrolyte solution in the
electrolyzer zone. When the electrolysis cell is employed in
electropurification the electrolyte would remain in the
electrolyzer zone for a sufficient time interval for modification
of contaminants to occur, ether electrochemically by direct means
and/or by chemical modification of contaminants to less hazardous
substances during residency in the cell. Additional means are
provided for collecting treated electrolyte solution descending
CA 02355346 2001-05-30
-5-
from the electrolyzer zone. It is also significant, the
electrolysis cell according to the invention has an "open
configuration".
In addition to the electrochemical cell of this invention,
further means are provided for practical and efficient operation,
directly feeding contaminated aqueous electrolyte solution to the
cell by pump means or by gravity; pretreatment means for the
contaminated aqueous electrolyte solutions, for example, means
for aeration, pH adjustment, heating, filtering of larger
particulates; as well as means for post-treatment, for example,
pH adjustment and cooling, or chlorination to provide residual
kill for drinking water applications. In addition, the invention
contemplates in-line monitoring with sensors and microprocessors
for automatic computer-assisted process control, such as pH
sensors, UV and visible light, sensors for biological
contaminants, temperature, etc.
It is still a further object of the invention to provide a
system for purification of aqueous solutions, which comprises:
(i) an electrolysis cell comprising at least one
anode and at least one cathode as electrodes positioned in an
electrolyzer zone. The electrodes are spaced sufficiently close
to one another to provide an interelectrode gap capable of
minimizing cell voltage and IR loss. Also included is a conduit
means for directly feeding a contaminated aqueous electrolyte
solution to the electrodes in the electrolyzer zone. The
electrolysis cell is characterized by an open configuration.
(ii) A control valve means for regulating the flow
of contaminated aqueous electrolyte solution to the electrodes
directly via the conduit means of (i) above.
(iii) Means are included for pumping contaminated
aqueous electrolyte solution through the conduit means, and then
(iv) rectifier means are included for providing
a DC power supply to the electrolysis cell.
The purification system may also include sensor means and
computerized means for receiving input from the sensor means and
CA 02355346 2001-05-30
-6-
providing output for controlling at least one operating condition
of the system selected from the group consisting of current
density, flow rate of contaminated aqueous solution to the
electrolysis cell, temperature and pH of the contaminated aqueous
electrolyte solution. Optional components include exhaust means
for further handling of electrochemically produced gaseous by-
products; means for pretreatment of the contaminated aqueous
electrolyte solution selected from the group consisting of
filtration, pH adjustment and temperature adjustment.
As previously discussed, the electrochemical cells of this
invention are especially novel in their "open configuration." As
appearing in the specification and claims, the expression "open
configuration" or variations thereof are defined as
electrochemical cell designs adapted for controlled leakage or
discharge of treated and decontaminated aqueous electrolyte
solution and gaseous or volatile by-products. The above
definition is also intended to mean the elimination or exclusion
of conventional closed electrochemical cells and tank type cell
designs utilizing conventional indirect means for feeding
electrolyte to electrodes. Closed flow type electrochemical
cells, for example, are often fabricated from a plurality of
machined and injection molded cell frames which are typically
joined together under pressure into a non-leaking sealed stack
with gaskets and 0-rings to avoid any leakage of electrolyte from
the cell. This type of sealed electrochemical cell is typically
found in closed plate and frame type cells. Very close fitting
tolerances for cell components are required in order to seal the
cell and avoid leakage of electrolyte and gases to the
atmosphere. Consequently, initial capital costs of such
electrochemical cells, refurbishment costs, including replacement
costs for damaged cell frames and gasketing from disassembly of
closed plate and frame type cells are high.
Because the configuration of the electrochemical cells of
this invention is "open", and not sealed, allowing for controlled
leakage of aqueous electrolyte solution and gaseous by-products,
CA 02355346 2001-05-30
-7_
sealed cell designs, including gaskets, O-rings and other sealing
devices are eliminated. Instead, cell component parts are
retained together in close proximity by various mechanical means
when needed, including, for instance, clamps, bolts, ties,
straps, or fittings which interact by snapping together, and so
on. As a result, with the novel open cell concept of this
invention initial cell costs, renewal and maintenance costs are
minimized.
In the open configuration cells of this invention,
electrolyte is fed directly to the electrodes in the electrolyzer
zone from a feeder which may be positioned centrally relative to
the face of the electrodes, for example, where the contaminated
solution engages with the electrodes by flowing through very
narrow interelectrode gaps or spaces between the electrodes.
During this period the contaminants in the aqueous solution are
either directly converted at the electrodes to less hazardous
substances and/or through the autogenous generation of chemical
oxidants or reductants, such as chlorine, bleach, i.e.,
hypochlorite; hydrogen, oxygen, or reactive oxygen species, like
ozone, peroxide, e.g., hydrogen peroxide, hydroxy radicals, and
so on, chemically modified to substances of lesser toxicity, like
carbon dioxide, sulfate, hydrogen, oxygen and nitrogen. In some
instances, depending on the compositional make-up of pollutants
in the solution being treated, it may be desirable to add certain
salts like sodium chloride, iron salts or other catalytic salts
at low concentration to the solution before or during treatment
in the cell. For example, this could be used to generate some
active chlorine to provide a residual level of sterilant in the
treated water, or to produce ferrous iron to promote the
formation of Fenton's reagent with added or electrogenerated
hydrogen peroxide. Likewise, oxygen or air may be introduced
into the feed stream to enhance peroxide generation.
Because electrolyte is fed directly to the electrode stack
usually under positive pressure, gases such as hydrogen and
oxygen generated during electrolysis are less prone to accumulate
CA 02355346 2001-05-30
_g_
over electrode surfaces by forming insulative blankets or pockets
of bubbles. Gas blinding of electrodes produces greater internal
resistance to the flow of electricity resulting in higher cell
voltages and greater power consumption. However, with direct flow
of electrolyte to the cell, the dynamic flow of solution in
interelectrode gaps, according to this invention, minimizes gas
blanketing, and therefore, minimizes cell voltages.
The aqueous solution entering the cell by means of pumping
or gravity feed, cascades over and through available
interelectrode gaps, and on exiting the electrolyzer zone of the
cell through gravitational forces, descends downwardly into a
reservoir, for post treatment, for example, or discharged, such
as into a natural waterway. Any undissolved gases generated by
electrolysis, in contrast, are vented upwardly from the cell to
the atmosphere or may be drawn into a fume collector or hood, if
necessary, for collection or further processing.
While the direct feed "open configuration" electrochemical
cells, as described herein, preferably provide for the
elimination of conventional cell housings or tanks, as will be
described in greater detail below, the expression "open
configuration" as appearing in the specification and claims, in
addition to the foregoing definition, is also intended to include
electrochemical cell designs wherein the directly fed electrodes
are disposed in the interior region of an open tank or open cell
housing. A representative example of an open tank electrochemical
cell is that disclosed by U.S. Pat. 4,179,347 (Krause et al) used
in a continuous system for disinfecting wastewater streams. The
cell tank has an open top, a bottom wall, sidewalls and spaced
electrodes positioned in the tank interior. Instead of feeding
the contaminated aqueous solution directly to the electrodes
positioned in the tank the electrolyte, according to Krause et
al, is initially fed to a first end of the tank where interior
baffles generate currents in the wastewater causing it to
circulate upwardly and downwardly through and between the
parallel electrodes. Hence, instead of delivering electrolyte
CA 02355346 2001-05-30
-9-
directly to the electrode stack where under pressure it is forced
through interelectrode gaps between adjacent anodes and cathodes
according to the present invention, the electrolyte in the open
tank cell of Krause et al indirectly engages with the electrodes
through a flooding effect by virtue of the positioning of the
electrodes in the lower region of the tank where the aqueous
solution resides. This passive, flooding effect is insufficient
to achieve the mass transport conditions necessary for efficient
destruction particularly of contaminants when present in low
concentrations. Consequently, gaseous by-products of the
electrolysis reaction can and often do result in the development
of a blanket of gas bubbles on electrode surfaces. This
generates elevated cell voltages and greater power consumption
due to higher internal resistances.
Accordingly, for purposes of this invention the expression
"open configuration" as appearing in the specification and claims
is also intended to include open tank type electrochemical cells
wherein the electrode stack is positioned in the interior of an
open tank/housing and includes means for directly feeding
contaminated aqueous solutions to the electrodes. With direct
feeding the housing does not serve as a reservoir for the
contaminated aqueous solution which otherwise would passively
engage the electrodes indirectly by a flooding effect.
For purposes of this invention, it is to be understood the
expression "open configuration" is also intended to allow for
safety devices positioned adjacent to the electrochemical cells
and purification systems, such as splash guards, shields and
cages installed for minimizing the potential for injuries to
operators. Hence, the confinement of the electrolysis cells or
an entire water purification system of this invention inside a
small room, for example, is also intended to be within the
meaning of "open configuration" as appearing in the specification
and claims.
A further type of electrochemical cell design is disclosed
by Beck et al in U.S. Pat. 4,048,047. The Beck et al cell design
CA 02355346 2001-05-30
-10-
comprises a bipolar stack of circular electrode plates separated
by spacers to provide interelectrode gaps ranging from 0.05 to
2.0 mm. Liquid electrolyte is fed directly to the electrode
plates through a pipeline into a central opening in the electrode
stack and then outwardly so it runs down the outside of the
stack. However, the electrode stack is placed in a conjoint
closed housing with a covering hood to avoid loss of gaseous
reactants, vapors or reaction products. Thus, the closed
configuration of the Beck et al cell does not meet the criteria
of an "open configuration" cell according to this invention.
While it has been pointed out the "open configuration" of
the improved, highly economic electrochemical cell designs of
this invention are based on the elimination of traditional closed
cell designs, including plate and frame type cells and
conventional tank type cells, as well as traditional partially
open tank type cell designs, whether batch or continuous, it is
to be understood, the expression "open configuration", as
appearing in the specification and claims, also contemplates
electrochemical cells which may be modified with various inserts,
~0 barriers, partitions, baffles, and the like, in some instances
positioned adjacent to cell electrodes, or their peripheral
edges. Such modifications can have the effect of altering
electrolyte circulation and direction, and increase
residency/retention time, and therefore, affect the residency
time and rate of discharge of electrolyte from the cell.
Notwithstanding, such modified electrochemical cells which are
partially open do fall within the intended meaning of "open
configuration" when the electrodes per se remain substantially
accessible. Representative modified electrochemical cell designs
with electrodes which remain substantially accessible that are
included within the definition for "open configuration" as
appearing in the claims include modified, so called "Swiss roll
cell" designs wherein, for example, the closed tubular
containment for ~he electrodes, which are superimposed onto one
another and rolled up concentrically, is removed, thereby forming
CA 02355346 2001-05-30
-11-
an "open type Swiss roll cell".
It is yet a further object of the invention to provide a
more efficient electrochemical cell design which can be used in
effectively treating a wide spectrum of both chemical and
biological contaminants in aqueous media, but also of varying
concentration (from less than a few ppm to several thousand ppm)
which is both economically competitive in capital costs and power
consumption to more conventional water purification systems. The
electrochemical systems and methods of the invention have such
significantly improved economics, as to be readily adaptable to
treating via continuous processes, large volumes of industrial
waste waters from manufacturing facilities, such as chemical
plants, textile plants, paper mills, food processing plants, and
so on.
Lower cell voltages and higher current densities are
achieved with the highly economic, open configuration, especially
when configured as monopolar electrochemical cells equipped with
electrodes having narrow capillary interelectrode gaps.
Generally, the width of the gap between electrodes is
sufficiently narrow to achieve conductivity without extra
supporting electrolytes or current carriers being added to the
contaminated aqueous solutions. Thus, the need for adding
supporting electrolyte to the contaminated aqueous electrolyte
solution as supporting current carrier can be avoided.
It is thus a further object of the invention to provide for
improved, more economic and safer continuous, semi-continuous or
batch methods for electropurification of contaminated aqueous
solutions by the steps of:
(i) providing an electrolysis cell comprising at least one
anode and at least one cathode as electrodes positioned in an
electrolyzer zone. The electrodes are spaced sufficiently close
to one another to provide an interelectrode gap capable of
minimizing cell voltage and IR loss. Means are provided for
direct feeding a contaminated aqueous solution to the electrodes
in the electrolyzer zone. Means are provided for regulating the
CA 02355346 2001-05-30
-12-
residency time of the electrolyte solution in the electrolyzer
zone during electrolysis for modification of the contaminants.
The electrolysis cell is characterized by "open configuration"
as previously described;
(ii) directly feeding into the electrolyzer zone of the
electrolysis cell a contaminated aqueous electrolyte solution,
and
(iii) imposing a voltage across the electrodes of the
electrolysis cell to modify, and preferably destroy the
contaminants in the aqueous electrolyte solution.
It will be understood that generally the process will
include the step of recovering a purified electrolyte solution
from the electrolysis cell. However, the invention contemplates
direct delivery of purified aqueous solutions to a watershed, for
example, or optionally to other post-treatment stations.
As previously mentioned, the methods are performed in an
open configuration electrolysis cell which may be either
monopolar or bipolar configuration. Because of the open
configuration, as defined herein, the electrochemical cells of
this invention can be readily configured to a monopolar design.
This is especially advantageous since higher current densities
would be desirable in electrolyzing contaminated aqueous
solutions having relatively low conductivities while still also
maintaining low cell voltages. Likewise, the improved
electrochemical cells of this invention may have a bipolar
configuration, especially for large installations to minimize
busbar and rectifier costs.
Typically, in the monopolar open cell design electrical
connections are made to each electrode. Whereas in bipolar
configurations electrical connections are made to the end
electrodes. However, many applications require increased
electrode surface area, especially in scaling up from a
laboratory scale electrochemical cells to pilot scale, and
finally to commercial size open cells. It would be advantageous
if in scaling up cells one could achieve a more efficient cell
CA 02355346 2001-05-30
-13-
design for performing the processes of this invention, and
minimize capital and operating costs even further.
It is therefore still another principal object to provide
alternative, more economic embodiments of the open electrolysis
cell concept of this invention, wherein faces of multiple porous
electrodes are positioned adjacent to one another and arranged
either in a vertical plane or superimposed horizontally relative
to one another in the format of a stack. The porous electrodes,
usually meshes or screens, are in electrical contact with one
another, with each electrode stack requiring but a single feeder
electrode for introducing a voltage thereto. Hence, by arranging
the electrodes in these representative formats, the effective
electrode surface area is significantly increased without also
increasing the number of external electrical contacts otherwise
required to the power source. By layering the electrodes
connection costs are minimized, while also realizing capital
savings in electrode purchases. Other benefits include improved
efficiency of operation with the open cell configuration, reduced
power consumption and lower operating costs, as a result of lower
cell voltages.
Accordingly, the invention contemplates an embodiment of the
"open cell" configuration wherein the electrolysis cell comprises
at least one anode and at least one cathode as electrodes
positioned in an electrolyzer zone. At least one of the
electrodes comprises a plurality of conductive porous elements
positioned adjacent to and in electrical contact with one
another. Means are provided for directly feeding an aqueous
electrolyte solution to the electrodes in the electrolyzer zone,
and for regulating the residency time of the electrolyte solution
in the electrolyzer zone.
As an alternative, the electrode consisting of a plurality
of conductive porous elements may be in combination with a solid,
non-porous conductive electrode element.
Also included is a method for electropurification of
contaminated aqueous solutions by the steps of:
CA 02355346 2001-05-30
-14-
(i) providing an open configuration electrolysis cell
with at least one anode and at least one cathode as electrodes
positioned in an electrolyzer zone. At least one of the
electrodes comprises a plurality of conductive porous elements,
as for example, mesh or screen, positioned adj acent to and in
electrical contact with one another. Means are provided for
direct feeding a contaminated aqueous electrolyte solution to the
electrodes in the electrolyzer zone. Means are also provided for
regulating the residency time of the aqueous electrolyte solution
in the electrolyzer zone for modification of contaminants
therein;
(ii) a contaminated aqueous electrolyte solution is
introduced into the electrolysis cell of (i), and
(iii) a voltage applied across the electrodes of the
electrolysis cell to modify the contaminants in the electrolyte
in the aqueous electrolyte solution.
The improved electropurification methods of the invention
also contemplate the treatment of aqueous solutions contaminated
with metal ions. Often, they are toxic substances from plating
bath effluents, metal stripping baths, biocide formulations and
paints, and may be sequestered by a complexing agent, surfactant
or reducing agent. The electropurification methods of the
invention destroy the complexing agent, surface active agent or
reducing agent to release the hazardous metal for further
treatment in the electrolysis cell, or alternatively, for
instance, transferred to a metal recovery cell for plating out
metals from solution.
While the electrolysis cells disclosed herein have as a
principal utility the electropurification of contaminated
solutions, the "open" cell configuration of this invention can
be readily employed in other useful applications. Representative
examples include the electrochemical synthesis of inorganic and
organic compounds, such as iodate and periodate salts, chlorine
dioxide, persulfate salts, and dimers via the Kolbe method of
electrolysis of carboxylic acids, or by electrohydrodimerization
CA 02355346 2001-05-30
-15-
of activated olefins, the electrolysis of water to form hydrogen
and oxygen, and so on.
It is therefore yet a further principal object of the
invention to provide electrosynthesis processes in the production
of useful products, by the steps of:
(i) providing an electrolysis cell with an open
configuration wherein the cell is equipped with at least one
anode and at least one cathode as electrodes positioned in an
electrolyzer zone. At least one of the electrodes comprises a
plurality of conductive porous elements, for example, mesh or
screens, positioned adjacent to and in electrical contact with
one another. Means are provided for direct feeding of an
electrolyte solution to the electrodes in the electrolyzer zone.
Also included are means for regulating the residency time of the
electrolyte solution in the electrolyzer zone;
(ii) introducing into the electrolysis cell of (i) an
electrolyte comprising a solution of an electroactive substrate,
such as an inorganic salt, e.g., aqueous solution of an alkali
metal chloride when making bleach, an iodate salt when making
periodate, an aqueous solution of an acid, etc., and
(iii) imposing a voltage across the electrodes of the
electrolysis cell to electrolyze the electrolyte solution to form
a useful chemical product.
This embodiment of the invention includes methods for
electrosynthesis of a useful product wherein the electrolysis
cell is equipped with a porous diaphragm or permselective
membrane.
BRIEF DESCRIPTION OF THE DRAWINGS
For a further understanding of the invention and its
characterizing features reference should now be made to the
accompanying drawings wherein:
FIG. 1 is a side elevational view illustrating a first
embodiment of a direct feed, open configuration, controlled
leakage electrochemical cell of the invention wherein the
CA 02355346 2001-05-30
-16-
electrodes are positioned above a water collection vessel in a
horizontal orientation;
FIG. 2 is a side elevational view of the electrochemical
cell of Fig. ?. except the electrodes are in a vertical
orientation;
FIG. 3 is a side elevational view illustrating a second
embodiment of a direct feed, open configuration, controlled
leakage electrochemical cell of the invention wherein the
electrodes are positioned in the interior of an open cell
housing;
FIG. 4 is an exploded view of the electrode cell stack of
Fig. 1 .
FIG. 5 is a side elevational view of an electrode stack of
the invention connected in a monopolar configuration;
FIG. 6 is a side elevational view of an electrode stack of
the invention connected in a bipolar configuration;
FIG. 7 is an elevational view of an electrode stack
compartmentalized with a separator;
FIG. 8 is a side elevational view of an open electrochemical
cell with stacks of porous electrodes connected in a monopolar
configuration;
FIG. 9 is a side elevational view of an open electrochemical
cell with stacks of porous electrodes connected in a bipolar
configuration, and
FIG. 10 illustrates the results of electropurification of
an aqueous solution of phenol decontaminated according to the
methods of the invention, as performed in Example I
DESCRIPTION OF THE PREFERRED EMBODIMENTS
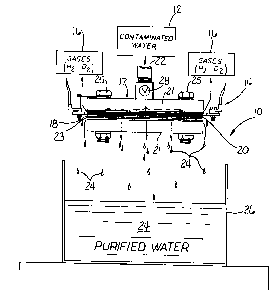
Turning first to Fig. 1 there is illustrated an
electrochemical cell 10 for purification of contaminated aqueous
solutions, as previously discussed, represented by contaminated
water 12 passing through inlet 22. The contaminated water 12 is
treated in the electrolyzer zone 14 of cell 10 which is
illustrated in a fully open configuration allowing gaseous by
CA 02355346 2001-05-30
-17-
products of the electrolysis reaction, such as oxygen and
hydrogen 16 to be released to the atmosphere. It may be desirable
in some instances to collect certain potentially hazardous gases
generated during the electrolysis reaction to avoid discharging
to the atmosphere. Chlorine, for example, may be generated at the
anode during electrolysis of aqueous effluent streams containing
brine or sea water. Such gases can be recovered, for instance,
by a vacuum powered hood device of conventional design (not
shown) positioned adjacent to electrochemical cell 10.
The electrolyzer zone 14 includes an electrode stack 17
shown in a horizontal orientation in Figs. 1 and 4, and comprises
at least one cathode 18 and at least one anode 20. Anodes 20, for
example, may also serve as end plates 21 for holding an assembly
of electrodes, spacers, and separators, whenever used, into an
assembled electrode stack 17. Non-conductive electrode spacers
23 positioned between electrodes provide the desired
interelectrode gap or spacing between adjacent anodes and
cathodes. While Figs. 1 & 4 of the drawings may be shown with
only a central cathode with anodes on opposite sides of cathode
18, for example, it is to be understood the electrode stacks may
be formed from several alternating anodes, spacers, cathodes, and
so on, with bolting means 25 running through the stack and end
plates for maintaining the components in a structurally stable
assembly.
The end plates, electrodes and spacers may have a generally
rectangular geometry. However, any number of possible alternative
geometrical shapes and sizes are within the purview of the
invention, including square, round or circular configurations,
to name but a few. Contaminated aqueous electrolyte solutions are
fed directly to the electrodes in electrolyzer zone 14 via supply
line 22. Supply line 22 is shown centrally positioned relative
to anode/end plate 21. The electrodes, which may be solid and
planar, are preferably mesh/screen-type materials. This enables
the aqueous electrolyte solutions entering the electrode stack
to directly engage with the electrodes, and in so doing flow
CA 02355346 2001-05-30
-18-
radially across the face of the individual electrode surfaces
within the stack toward their peripheral edges. In addition, the
entering solution usually flows axially, or normal to the
longitudinal axis of the plane of the electrodes, so the
contaminated aqueous solution simultaneously cascades over and
through the electrode stack in a fountain-like effect to maximize
contact with electrode surfaces in the process. Purified water
24, free or virtually free of contaminants exiting electrolyzer
zone 14, can be collected in an open tank 26, or funneled into
a discharge line (not shown) for emptying into a natural
watershed, etc.
It will be understood the direct feed of contaminated
aqueous solutions to the electrolyzer zone need not be centrally
positioned relative to the electrode stack, as illustrated in
Figs. 1-4. Alternative direct feed routes include inverting the
point of feed, so that contaminated aqueous solutions are fed
from the bottom of the electrode stack, or at an oblique or
obtuse angle to the planar surface of the electrodes. In
addition, the direct feed entry point may also be axial with the
edge of the planar surface of the electrodes wherein contaminated
solution is delivered to the peripheral edge of an electrode
stack.
A convenient means for regulating the residency time of the
contaminated aqueous solution in electrolyzer zone 14 and for
controlling leakage of decontaminated and purified water 24
therefrom can be through valve 28 and/or pumping means of
conventional design (not shown). The flow rate of contaminated
water directly entering the electrode stack and exiting the stack
as decontaminated water can be regulated through manual or
automated flow control valve 28 of standard design. The flow rate
(liters/minute)is adjusted, so it is sufficient to provide
effective destruction of pollutants by the time the treated
solution exits the electrolyzer zone. Persons of ordinary skill
in the art having the benefit of this disclosure will also
recognize the performance of the electrochemical cells of this
CA 02355346 2001-05-30
-19-
invention may be optimized by alternative means, such as
increasing the path of the solution in the electrolyzer zone. The
installation of baffles, for instance, can increase the dwell
time of the solution in the electrolyzer zone. Alternative means
include enlarging the surface area of the electrodes for reducing
the residency time in the electrolysis zone. In practice,
electrochemists skilled in the art will also recognize the
performance of the cell can be increased with higher current
densities.
Because of cell geometry, and the ability to conveniently
use both monopolar and bipolar configurations, practically any
electrode material can be employed, including metals in the form
of flat sheet, mesh; foam or other materials, such as graphite,
vitreous carbon, reticulated vitreous carbon and particulate
carbons. This also includes combinations of electrode materials,
such as bilayer elements comprising two metal layers separated
by appropriate insulating or conductive materials, and so on.
Representative examples of useful anodes would include
those generally known as, noble metal anodes, dimensionally
stable anodes, carbon, vitreous carbon and graphite-containing
anodes, doped diamond anodes, substoichiometric titanium oxide-
containing anodes and lead oxide-containing anodes. More specific
representative examples include platinized titanium noble metal
anodes; anodes available under the trademark DSA-02, and other
anodes, such as high surface area type anodes like felts, foams,
screens, and the like available from The Electrosynthesis Co,
Inc., Lancaster, New York. Other anode materials comprise
ruthenium oxide on titanium, platinum/iridium on titanium,
iridium oxide on titanium, silver oxide on silver metal, tin
oxide on titanium, nickel III oxide on nickel, gold,
substoichiometric titanium oxides, and particularly the so called
Magneli phase titanium oxides having the formula TiOx wherein x
ranges from about 1.67 to about 1.9. A preferred specie of
substoichiometric titanium oxide is Ti40~. Magneli phase titanium
oxides and methods of manufacture are described in U.S. Pat.
CA 02355346 2001-05-30
-20-
4,422,917 (Hayfield) which teachings are incorporated-by-
reference herein. They are also commercially available under the
trademark Ebonex~. Where electrocatalytic metal oxides, like
Pb02, Ru02, Ir02, Sn02, Ag20, Ti40~ and others are used as anodes,
doping such oxides with various cations or anions has been found
to further increase the electrocatalytic oxidation behavior,
stability, or conductivity of the decontamination reactions of
this invention. ~he selection of appropriate anode materials is
made by considering such factors as cost, stability of the anode
material in the solutions being treated and its electrocatalytic
properties for achieving high efficiencies.
Suitable cathode materials include metals, such as lead,
silver, steel, nickel, copper, platinum, zinc, tin, etc., as well
as carbon, graphite, Ebonex, various alloys, and so on. Gas
diffusion electrodes are also useful in the methods of this
invention. In this regard, they may be used as cathodes in
converting oxygen or air to useful amounts of peroxide,
minimizing hydrogen evolution and/or for lowering cell voltages.
The electrode material, whether anode or cathode, may be coated
with an electrocatalyst, either low or high surface area. Higher
surface area electrodes, for example, expanded metal screens,
metal or graphite beads, carbon felts, or reticulated vitreous
carbon are especially useful in achieving higher efficiencies for
destruction of toxic or hazardous substances when present at low
concentrations in the aqueous electrolyte.
Specific anode and cathode materials are selected on the
basis of cost, stability and electrocatalytic properties. For
example, persons of ordinary skill in the art of electrochemistry
will recognize which electrode material to select when it is
desired to convert chloride to chlorine; water to ozone, hydroxyl
radicals or other reactive oxygen species; oxygen or air to
hydrogen peroxide or hydroxyl radicals via electrochemically
generated Fenton's reagent using for instance, a slowly
dissolving iron-containing metal anode; and catalytic reduction
of nitrate to nitrogen or of organohalogen compounds to halide
CA 02355346 2001-05-30
-21-
ions and organic moieties of lesser toxicity.
Of special importance in the selection of electrocatalytic
anode and cathode materials occurs when treating aqueous
solutions comprising complex mixtures of pollutants wherein
electrode materials may be selected for paired destruction of
pollutants. For example, an aqueous stream contaminated with
organics, microorganisms and nitrate pollutants may be treated
simultaneously in the same electrochemical cell using paired
destruction methods with a reactive oxygen species generating
anode, such as platinum on niobium or Ebonex for destruction of
microorganism and oxidation of organics. In addition, the same
cell could also be equipped with a lead or other electrocatalytic
cathode material designed for nitrate destruction.
As previously mentioned, non-conductive electrode spacers
23 provide the desired interelectrode gap or spacing between
adjacent anodes and cathodes. The thickness of spacers 23, which
are non-conductive, insulative porous mesh screens fabricated
from polymeric materials, such as polyolefins, like polypropylene
and polyethylene, determines the width of the interelectrode gap.
Alternatively, it is permissible to employ ionic polymer spacers
which can effectively increase the ionic conductivity of the
cell, so as to reduce cell voltage and operating costs further.
Ion-exchange resins of suitable dimensions, like cation and anion
exchange resin beads are held immobile within the gap between
electrodes.
For most ap~~lications, the interelectrode gap ranges from
near zero gap, to avoid electrode shorting, to about 2 mm. More
specifically, this very small capillary size gap is preferably
less than a millimeter, ranging from 0.1 to <1.0 mm. The very
small interelectrode gap makes possible the passage of current
through relatively non-conductive media. This is the case, for
example, in water contaminated with organic compounds. Thus, with
the present invention it is now possible to destroy contaminants
in solution without adding any current carrying inorganic salts
to increase the ionic conductivity of the aqueous media.
CA 02355346 2001-05-30
-22-
Furthermore, the very narrow interelectrode gap provides the
important advantage of lower cell voltages which translates into
reduced power consumption and lower operating costs. Hence, the
combination of open configuration electrochemical cells and very
narrow interelectrode gaps of this invention provide for both
lower initial capital costs, as well as lower operating costs.
This achievement is especially important in large volume
applications, as in the purification of drinking water, and
wastewater, according to the claimed processes.
Fig. 2 represents a further embodiment of the
electrochemical cells of this invention wherein the electrolyzer
zone 30 is also in an open configuration. The electrolyte 32 is
fed directly to the electrode stack 34 which is in a vertical
orientation. As a result, treated aqueous solution 36 is shown
exiting mainly from both the top and bottom peripheral edges of
electrode stack 34. This may be altered further depending on the
use of baffles, for instance, in controlling residency time for
the solution being treated. Purified solution is collected in
vessel 38 below electrolyzer zone 30.
Fig. 3 represents still a third embodiment of the invention
wherein the electrolyzer zone 40 comprises an electrode stack 42,
as discussed above, positioned in the interior of an open
housing/tank 44. Housing 44 is open at the top allowing gaseous
by-products of the electrolysis reaction, like hydrogen and
oxygen, for instance, to be readily discharged into the
atmosphere or collected through aid of an appropriate device,
such as a hood (not shown). Aqueous contaminated electrolyte
solution 46 is fed directly to electrode stack 42 positioned in
open housing 44, unlike other tank cells wherein the electrodes
3.0 receive solution indirectly as a result of their immersion in
the solution delivered to the tank. Purified water 48 cascading
downwardly as a result of gravitational forces collects at the
bottom of the interior of housing 44, and is withdrawn.
An important advantage of the open configuration
electrochemical cells of this invention resides in their ability
CA 02355346 2001-05-30
-23-
to be readily adaptable to either a monopolar or bipolar
configuration. In this regard, Fig. 5 illustrates a monopolar
open configuration electrochemical cell. In the monopolar cell
of Fig. 5, anodes 52, 54 and 56 each require an electrical
connector as a current supply, in this case through a bus 58 as
a common "external" supply line. Similarly, cathodes 60 and 62
each require an electrical connection shown through a common bus
64. It is also characteristic of the monopolar cell design that
both faces of each electrode are active, with the same polarity.
Because water purification for a municipality, in general,
is a large volume application, lowest possible cell voltages are
essential in order to minimize power consumption. The open
configuration, monopolar cell design of the present invention in
combination with very narrow interelectrode gaps offers not only
the benefits of lower initial capital costs, but also low
operating costs, due to lower internal resistances, lower cell
voltages and higher current densities. This combination is
especially desirable when treating contaminated aqueous media of
relatively low conductivity without the addition of inorganic
salts as current carriers in accordance with certain embodiments
of this invention; e.g., aqueous solutions contaminated with non-
polar, organic solvents.
The open configuration, monopolar, controlled leakage
electrochemical cells with very narrow interelectrode gaps of
this invention are particularly unique in light of the Beck et
al cells of U.S. pat. 4,048,047. The closed configuration of the
electrochemical cells of Beck et al make it very difficult and
costly to achie~°e a monopolar connection with high current
densities associated with external electrical contacts to each
electrode. By contrast, with the open configuration of the
electrochemical cells of this invention electrical connections
to individual electrodes are facilitated, irrespective of whether
the cell is a monopolar or bipolar design. Thus, the closed,
bipolar electrochemical cell configuration of Beck et al would
not be economic and cost competitive with the improved
CA 02355346 2001-05-30
-24-
electrochemical cells of the present invention, or with other
non-electrochemical technologies used in high volume water
purification processes.
As previously indicated, the open configuration, controlled
leakage electrochemical cells of this invention having very
narrow capillary interelectrode gaps are also readily adaptable
to bipolar configuration. Fig. 6 illustrates open configuration
bipolar cell 70, according to the present invention, requiring
only two "external" electrical contacts 72 and 74 through two end
electrodes /end plates 76 and 78. Each of inner electrodes 80,
82 and 84 of the bipolar cell has a different polarity on
opposite sides. While the bipolar cell can be quite economic in
effectively utilizing the same current in each cell of the
electrode stack, one important aspect of the invention relates
to treating solutions by passing a current through relatively
non-conductive media using very narrow interelectrode gaps. That
is, the contaminated aqueous solutions can have relatively low
conductivities, about equivalent to that of tap water. In order
to efficiently treat such solutions it would be desirable to
operate at higher current densities. The monopolar cell
configurations of the invention enable operating at desired low
cell voltages and high current density. While not specifically
illustrated, it will be understood standard power supplies are
utilized in the electrolysis cells of the invention, including
DC power supply, AC power supply, pulsed power supply and battery
power supply.
The invention also contemplates open configuration
electrochemical cells with distributor means for contaminated
aqueous electrolyte solutions, such as a length of pipe 81 with
multiple openings or pores, or a feeder tube extending from the
contaminated aqueous electrolyte feed inlet through the depth of
the electrode stack in the electrolyzer zone. This can provide
more uniform flow of solution to the electrode elements.
Especially useful for stacks containing many electrode elements,
these porous tubes of metal or plastic material, of sufficient
CA 02355346 2001-05-30
-25-
porosity, diameter and length, are applicable to monopolar,
bipolar, and for example, Swiss roll cells of open configuration.
For deep cell stacks with electrode elements, each of larger
surface area, more than one porous feeder tube may be provided,
manifolded together with the feed inlet conduit.
The open configuration, bipolar type, controlled leakage
electrochemical cells of the present invention can be most
effectively used in the purification of aqueous solutions
possessing greater ionic conductivities than those previously
discussed, allowing for economical operation at lower current
densities. In each instance, the open configuration of the
electrochemical cells of this invention facilitates their
electrical connection, whether the cell is a monopolar or bipolar
design.
Most desirably, large volume applications like water
purification require low capital and operating costs in order to
be economically attractive. These inventors found that capital
costs are largely reduced by eliminating the need for precision
machined components, gasketing, costly membranes and cell
separators. Lower operating costs can be achieved through lower
cell voltages from narrow interelectrode gaps and lower IR from
elimination of cell membranes and separators, i.e., undivided
electrochemical cells. The smaller interelectrode gap, however,
also makes possible the operation of the cells of this invention
in an organic media, for example, containing low concentrations
of supporting electrolyte, with a variety of electrode, insulator
materials, and so on. Many of such applications would be readily
adaptable to the open cell configuration of this invention, but
with use of a cell divider forming anolyte and catholyte
compartments, such as membranes or cell separators. Examples of
useful processes for the electrochemical cells of this invention
would include mediated reactions in electrochemical synthesis in
which the objective of the membrane or separator would be to
prevent reduction of anodically produced species at the cathode,
and/or oxidation of cathodically produced species at the anode.
CA 02355346 2001-05-30
-26-
Fig. 7 is a representative example of an open configuration
electrochemical 90 having anode/end plates 92 and 94 with central
cathode 96 and cation exchange membranes 98 and 100 positioned
between the electrodes. Membranes 98 and 100 prevent mixing of
the anolyte and catholyte in the cell while the solution is
allowed to flow through opening 102 in. the center of the
membrane.
Those embodiments of the electrochemical cells employing
a diaphragm or separator are preferably equipped with ion
exchange membranes, although porous diaphragm type separators can
be used. A broad range of inert materials are commercially
available based on microporous thin films of polyethylene,
polypropylene, polyvinylidene-difluoride, polyvinyl chloride,
polytetrafluoro-ethylene (PTFE), polymer-asbestos blends and so
on, are useful as porous diaphragms or separators.
Useful cationic and anionic type permselective membranes are
commercially available from many manufacturers and suppliers,
including such companies as RAI Research Corp., Hauppauge, NY,
under the trademark Raipore; E.I. DuPont, Tokuyama Soda, Asahi
Glass, and others. Generally, those membranes which are
fluorinated are most preferred because of their overall
stability. An especially useful class of permselective ion
exchange membranes are the perfluorosulfonic acid membranes, such
as those available from E.I. DuPont under the Nafion~ trademark.
The present invention also contemplates membranes and electrodes
formed into solid polymer electrolyte composites. That is, at
least one of the electrodes, either anode or cathode or both
anode and cathode, are bonded to the ion exchange membrane
forming an integral component.
While embodiments of the invention previously discussed
mention electrode stacks, e.g., 17 and 34 of Figs. 1 and 2,
respectively, etc., such electrode stacks are comprised of
individual, single anode and single cathode elements spaced from
one another by narrow interelectrode gaps. Fig. 4 illustrates
representative electrode stacks in exploded view comprising a
CA 02355346 2001-05-30
-27-
cathode 18 consisting of a single planar screen element having
its own external electrical contact 19. Non-conductive porous
spacers 23 on each side of cathode 18 provide the desired
interelectrode gaps separating the cathode element from adjacent
end anodes 20. While Fig. 4 illustrates an electrode stack with
a single cathode screen positioned between end anodes 20, it is
to be understood that larger capacity commercial and semi-
commercial pilot scale cells of this invention will usually have
cell stacks comprising a multiplicity of alternating anodes and
cathodes each having an external electrical contact when in a
monopolar configuration.
However, those scaled up versions of the electrolysis cells
of this invention requiring increased electrode surface area can
achieve this result more economically by stacking a plurality of
individual porous electrode elements as illustrated by Figs. 8
and 9. Multiple electrode elements consisting of conductive
porous elements, for example, meshes or screens are positioned
adjacent to and in electrical contact with one another in either
monopolar (Fig. 8) and bipolar (Fig. 9) open cell configurations.
Either or both the anodes and cathodes of the open cell
embodiment may have the multiple electrode element design. That
is, an anode stack consisting of multiple electrode elements are
held together in electrical contact, and may be positioned
adjacent to a cathode consisting of a single electrode element,
and vice versa. This is best illustrated by Fig. 8, which
consists of monopolar open cell 104 held between end plates 105
with multiple porous anode elements 106 positioned between single
element cathodes 108, illustrated as porous cathodes, but may
also be non-porous plate electrodes. Anodes 106 are separated
from cathodes 108 by means of porous non-conductive spacers 107.
Advantageously, anode stacks 106 need only a single "feeder"
electrode 110 for transferring a voltage on either side to other
electrode elements of the same stack in contact therewith. By
. stacking electrode elements in this manner, the effective
electrode surface can be significantly increased without
CA 02355346 2001-05-30
-28-
increasing the number of external electrical contacts 112 to the
power source 113, which would otherwise be required. This not
only minimizes costs for external electrical connectors and
capital costs for electrodes, but also improves efficiency of
operation resulting in lower cell voltages and reduced power
consumption for lower operating costs.
The conductive porous elements of the electrodes may be
fabricated from metal or carbon, for instance, and can be in the
form of perforated metal plates, welded wire cloth, woven wire
cloth, expanded metal, carbon felts, woven carbon cloth,
reticulated vitreous carbon, including metallic foams, such as
nickel foam having sponge-like characteristics. Representative
examples of comzr,ercially available perforated metal plates are
low carbon steel sheets and micro-etched type 316 stainless steel
sheets with hole patterns which are uniform and accurate in size.
Welded wire cloth includes type 304 stainless steel cloth and
stainless steel knitted wire mesh. Wire cloth is a woven or
welded material formed from metal wire, and is available in a
variety of mesh sizes. Also available is type 304 stainless steel
grade. Expanded metal consists of plates which have been slit
and stretched. The plates/sheets are lightweight, yet strong due
to the diamond truss pattern of their openings. They are
commonly fabricated from carbon steel and type 304 stainless
steel.
The invention contemplates a combination of different porous
materials for use as the electrode elements in a single cell to
achieve a combination oxidation/reduction effect, for example.
The pore density of the conductive porous elements of the
electrodes may range from 1 to about 500 mesh/linear inch. The
conductive porous elements may also have an open area ranging
from about 10 to about 90 percent. Some elements, such as foams
may have porosities ranging from 1 to 1000 pores/lineal inch and
a densities ranging from 5 to about 85 percent. The electrode
elements may simply be stacked in close electrical contact or
welded together and to the feeder electrode, as appropriate, to
CA 02355346 2001-05-30
-29-
ensure electrical connectivity through all members of the stack.
Fig. 9 also shows an open electrolysis cell design 114
similar to Fig. 8, but in a bipolar configuration shown with all
intermediate electrode stacks 116 consisting of a plurality of
porous electrode elements. Individual elements of each electrode
stack are in electrical contact with other members of the same
stack. Power is routed to the cell through end plate anodes 118.
Stacks 116 are spaced from one another by porous spacers 120.
The optimum number of electrode elements which may be used
with or without a "feeder" electrode is a function of a number
of variables, including the thickness of each porous electrode
element, the conductivity of the solution being treated and the
overall optimum cell design. The number of electrode elements,
in addition to the feeder electrode (Fig. 8) may range from 1 to
100, and more specifically, 1 to 10 electrode elements. The
"feeder" electrode may be the same material of construction as
the individual electrode elements, or different, provided the
feeder is stable under electrolysis conditions, and is
electrically conductive.
In the purification of solutions the invention provides for
the treatment of low conductivity media. However, it may be
necessary to add very low concentrations of inert, soluble salts,
such as alkali metal salts, e.g. sodium or potassium sulfate,
chloride, phosphate, to name but a few. Stable quaternary
ammonium salts may also be employed. As previously mentioned, ion
exchange resin beads of appropriate size can be inserted in the
spaces between the electrodes to increase conductivity. This
will provide further reductions in cell voltage and total
operating costs.
Contaminated solutions entering the cell can range in
temperature from near freezing to about boiling, and more
specifically from about 40° to about 90°C. Higher temperatures
can be beneficial in lowering cell voltages and increase rates
of contaminant destruction. Such higher temperatures can be
achieved, if needed, by preheating the incoming solution, heating
CA 02355346 2001-05-30
-30-
the electrodes, or through IR heating in the cell, especially
when solution conductivities are low, as for example in
purification of drinking water. By suitably adjusting the cell
voltage and residence time in the cell, beneficial temperatures
in the above rang?s are possible.
As a preferred embodiment of the invention, as an undivided
cell, for the purification of contaminated aqueous solutions a
variety of useful anode and cathode species can be generated
during electrolysis which in turn aid in the chemical destruction
of contaminants and the purification of the aqueous solutions.
They include such species as oxygen, ozone, hydrogen peroxide,
hydroxyl radical, and other reactive oxygen species. Less
preferred species, although useful in the process include the
generation of chlorine or hypochlorite (bleach) through the
electrolysis of brine or sea water. While not wishing to be held
to any specific mechanism of action for the success of the
processes in the decontamination, decolorization and
sterilization of aqueous solutions contaminated with toxic
organics and microorganisms, several processes, including those
previously mentioned, may be occurring simultaneously. They
include, but are not limited to the direct oxidation of
contaminants at the anode: destruction of contaminants by direct
reduction at the cathode; oxygenation of the feed stream by micro
bubbles of oxygen produced at the anode; degasification of
volatiles in the feed stream by oxygen and hydrogen micro
bubbles; IR heating in the cell; aeration of the water stream
exiting the open cell, and so on.
A broad ra.zge of compounds, microorganisms and other
hazardous substail.ces, such as metal ions as previously discussed
are successfully destroyed or removed in the open cell
configuration of the invention employing the processes as
described herein. Representative examples include aliphatic
alcohols, phenols, nitrated or halogenated aromatic compounds,
and so on. Color reduction or complete elimination of color can
also be achieved, along with disinfection, including the
CA 02355346 2001-05-30
-31-
destruction of viruses.
There are many kinds of metal salts in aqueous solutions,
including toxic metals in ionic form in plating bath effluents,
metal stripping baths, biocide formulations, paints, etc., which
are difficult to remove or recover by ion exchange or by
conventional chemical or electrochemical means. Such metals
include the precious metals, like platinum, silver and gold, as
well as non-precious metals, such as copper, nickel, cobalt and
tin, to name but a few. Government regulations are becoming
increasingly strict, as to maximum acceptable levels of these
metals which may be discharged into our waterways. These
solubilized metal solutions are often difficult to treat because
of other components which may be present, typically complexing
agents, surfactants, reducing agents, and other similar type
materials.
Accordingly, the present invention also contemplates
electropurification of aqueous solutions contaminated with
hazardous metal ions by treating in the open electrolysis cells
disclosed herein using the methods previously discussed. This
includes decontaminating the solutions by metal reduction at the
cathodes of the open cell, as well as treating metal ions from
plating bath effluents, metal stripping baths, biocide
formulations, paints, and other contaminated industrial aqueous
solutions, wherein the metals are sequestered by various
complexing agents, surfactants or reducing agents, for instance.
Solution components, including complexing agents are initially
destroyed electrochemically, greatly facilitating recovery/
removal of metals from the solutions. Representative complexing
agents may include cyanides, ferricyanides, thiosulfates, imides,
hydroxycarboxylic acids, like tartaric, citric and lactic acids,
and so on. This method effectively releases the ionized metal
for reduction in the cell or for removal/recovery. Alternatively,
the partially treated aqueous solution may be treated further
outside the open cell using such methods as ion exchange,
precipitation with base, by electrolysis in a metal recovery
CA 02355346 2001-05-30
-32-
electrochemical cell, such as a Renocell''T' manufactured by
Renovare International. This latter method allows metal to be
plated onto a high surface area cathode.
The following specific examples demonstrate the various
embodiments of the invention, however, it is to be understood
they are for illu:>trative purposes only and do not purport to be
wholly definitive as to conditions and scope.
EXAMPLE I
A monopolar electrochemical cell having an open
configuration was set up with an electrode stack comprising 316
stainless steel end plates each with a diameter of 12.065
centimeters and a thickness of 0.95 centimeters. The end plates
were connected as cathodes. A central cathode was also assembled
into the stack and consisted of 316 stainless steel mesh with 7.8
x 7.8 openings/linear centimeter, 0.046 centimeter wire diameter,
0.081 centimeter opening width and 41 percent open area. The
anodes consisted of two platinum clad niobium electrodes
manufactured by Blake Vincent Metals Corp. of Rhode Island. The
anodes which were clad on both sides of the niobium substrate had
a thickness of 635 micrometers, were expanded into a mesh with
a thickness of about 0.051 centimeters, with 0.159 centimeter
diamond shaped interstices. The spacers positioned between
adjacent electrodes were fabricated from polypropylene mesh with
8.27 x 8.27 openings/linear centimeter, 0.0398 centimeter thread
diameter, 0.084 centimeter opening and a 46 percent open area was
supplied by McMaster-Carr of Cleveland, Ohio. The gap between the
electrodes was approximately 0.04 centimeters, determined by the
thickness of the polypropylene mesh. A schematic of the
electrochemical cell corresponds to Fig. 1 of the drawings,
except a hood was omitted. Recirculation of the aqueous solution
between the glass collection tank and the cell was effected by
means of an AC-3C-MD March centrifugal pump at a flow rate of
about 1 liter/minute. A Sorensen DCR 60-45B power supply was used
to generate the necessary voltage drop across the cell.
CA 02355346 2001-05-30
-33-
A test solution was prepared containing 1 g of phenol in 1
liter of tap water. The solution was recirculated through the
cell while a constant current of 25 amps was passed. The
solution which was initially clear turned red after about 2-3
minutes into the treatment process, possibly indicating the
presence of quinone-type intermediates. The initial cell voltage
of 35 V decreased rapidly to 8-9 V, and the temperature of the
solution stabilized at about 56-58°C. Samples taken were
analyzed periodically for total organic carbon (TOC). The
results, which are shown in Fig.lO, appear to suggest the
decrease in TOC is from the phenol probably undergoing complete
oxidation to carbon dioxide which is then eliminated as gas from
the solution.
EXAMPLE II
In order to demonstrate color reduction in a textile
effluent 1 liter of solution was prepared with tap water
containing 0.1 g of the textile dye Remazol''M Black B (Hoechst
Celanese), 0.1 g of the surfactant Tergitol'~' 15-S-5 (Union
Carbide) and 1 g of NaCl.
The composition of the test solution was similar to that of
typical effluents produced in textile dyeing processes where even
very low concentrations of Remazol Black impart very strong
coloration to solutions. Remazol Black is a particularly
difficult to treat textile dye. Heretofore, other methods used
to treat Remazol Black, such as by ozonation or with hypochlorite
bleach have failed to produce satisfactory color reduction.
The above solution containing Remazol Black was electrolyzed
in the monopolar cell set up of Example I above, at a constant
current of 25 amps. The cell voltage was about 25 V, and the
temperature of the solution reached 52°C. The initial color of
the solution was dark blue. After 10 minutes of electrolysis the
color of the solution turned to pink, and after 30 minutes the
solution was virtually colorless.
CA 02355346 2001-05-30
-34-
EXAMPLE III
A further experiment was conducted in order to demonstrate
the decontamination of ground water. Humic acids are typical
contaminants of ground water, produced by the decomposition of
vegetable matter. Water containing humic acids is strongly
colored even at low concentrations, and the elimination of the
color can be difficult.
A dark brown solution in tap water was prepared containing
30 ppm of the sodium salt of humic acid (Aldrich) without any
additives to increase the electrical conductivity of the
solution. The solution was recirculated through a monopolar
electrochemical cell similar to that used in Example I, but
equipped with only one anode and two cathodes. A constant
current of 10 amps was passed for 2.5 hours. The cell voltage was
24-25 volts, and the temperature reached 58°C. At the end of the
experiment the solution was completely clear, demonstrating the
effective destruction of humic acid.
EXAMPLE IV
A further experiment was conducted to demonstrate the
effectiveness of the electrolysis cells and methods of this
invention in the sterilization and chemical oxygen demand (COD)
reduction in effluents from food processing plants.
250 ml of wastewater from a Mexican malt manufacturing
company was treated using a monopolar, open electrochemical cell
similar to that employed in Example I, except the total anode
area of 6 cm2. The objectives were to reduce the COD, partial or
total reduction of the color, elimination of microorganisms and
odor.
A current of 1 amp was passed for 150 minutes; the initial
cell voltage of 22 V dropped to 17.5 V, and the temperature of
the solution reached 44°C.
The results are shown in the following Table:
CA 02355346 2001-05-30
-35-
TABLE
Initial Final
COD 1700 ppm 27 ppm
Color Yellow-Orange Clear
Microorganisms Active Sterilized
Odor Yes No
EXAMPLE V
A further experiment was conducted to demonstrate the
effectiveness of the electrolysis cells and methods of this
invention in the removal of color in a single-pass configuration.
A dark purple solution containing methyl violet dye in tap
water at a concentration of 15 ppm was circulated through a
monopolar, open electrochemical cell similar to that employed in
Example 1, in single-pass mode, at a flow rate of 250 ml/minute.
The objective was to achieve total reduction of the color.
A current of 25 amp was passed; the cell voltage was 25 V,
and the temperature of the solution reached 65°C.
After a single pass through the cell a clear solution was
obtained.
EXAMPLE VI
An experiment can be conducted to demonstrate the utility
of the open configuration electrochemical cell in the
electrosynthesis of chemicals, in this instance sodium
hypochlorite.
The electrochemical cell of Example I is modified by
replacing the anodes with catalytic chlorine evolving anodes,
such as DSA~ anodes manufactured by Eltech Systems. A solution
of brine containing lOg of sodium chloride per liter is
introduced into the electrolyzer zone wherein chlorine is
generated at the anode and sodium hydroxide is produced at the
cathode. The chlorine and caustic soda are allowed to react in
the cell to produce a dilute aqueous solution of sodium
CA 02355346 2001-05-30
-36-
hypochlorite bleach.
EXAMPLE VII
To demonstrate the open cell configuration employing
electrodes comprising a plurality of conductive porous elements
positioned adjacent to and in electrical contact with one
another, an experiment is performed using the monopolar cell
configuration illustrated in Fig. 8. The cell is equipped with
a Pt/Nb woven mesh anode having 10 strands per linear inch. Two
of the Pt/Nb screens are in electrical contact and stacked on
top of a third Ft/Nb screen, as the feeder electrode, which in
turn is connected to the positive terminal of a DC power supply.
The cathode element is a single nickel screen connected to the
negative terminal of the DC power supply.
The electrolyte to be treated consists of 5 g of sodium
chloride added to one liter of an aqueous electroless nickel
plating effluent at 60°C, containing 60 g of nickel salt, 25 g
sodium hypophosphite, and a COD of 20,000 ppm. Electrolysis is
conducted at 55mA/cm2, at a cell voltage of 5.5V until the COD
of the effluent drops to about 10 percent of its initial value.
The effluent is then treated in an electrochemical cell
containing a high surface area carbon cathode to plate out most
of the nickel remaining in solution.
This demonstrates the destruction of complexing agents in
electroless plating bath effluent and release of metal ions for
recovery by plating means.
While the invention has been described in conjunction with
various embodiments, they are illustrative only. Accordingly,
many alternatives, modifications and variations will be apparent
to persons skilled in the art in light of the foregoing going
detailed description, and it is therefore intended to embrace all
such alternatives and variations as to fall within the spirit and
broad scope of the appended claims.