Note: Descriptions are shown in the official language in which they were submitted.
CA 02356578 2001-09-05
Organic amines as additives in electrochemical cells
The invention relates to the use of additives to reduce
the acid content in aprotic electrolyte systems in
electrochemical cells, particularly in lithium ion
batteries, and to an electrochemical cell containing an
aprotic electrolyte system which comprises an additive
to reduce the acid content of the electrolyte system.
Electrochemical cells are used to convert chemical
energy into electrical energy or electrical energy into
chemical energy. In both cases, chemical reactions take
place which involve the migration of electrical charges
or cause electrical potentials to arise. Examples of
electrochemical cells in which the energy conversion
can be carried out reversibly comprise secondary
batteries (or accumulators) and double-layer
capacitors.
Secondary batteries are used mainly in portable
electronic appliances such as e.g. in mobile phones,
computers, CD players and camcorders. Secondary
batteries for this type of application must be
distinguished by high energy density, high capacity, a
high cell voltage, low self-discharge, long service
life, low weight and excellent charging and discharging
characteristics. In recent years, lithium ion batteries
have been developed which meet the abovementioned
requirements.
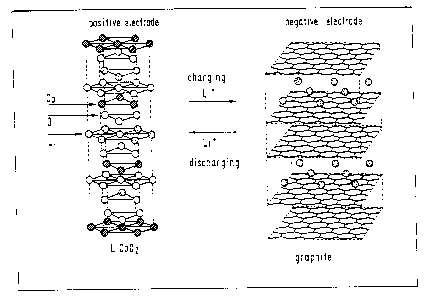
Lithium ion batteries comprise a negative electrode
with a material which is capable o~ reversible uptake
and re-release of lithium ions, graphite being one
example, a positive electrode with a lithium
transition-metal oxide such as e.g. lithium manganese
oxide, lithium cobalt oxide or lithium nickel oxide,
and also comprise an aprotic electrolyte system.
Lithium ion batteries, which are typical 4 V systems,
do not, in contrast to lithium batteries belonging to
the 3 V systems, comprise any metallic lithium.
CA 02356578 2001-09-05
- 2 -
The chemical reactions which proceed at the positive
electrode and at the negative electrode when a lithium
ion battery is being charged or discharged can be
stated as follows, e.g. for a cell which comprises
LiCo02 as a material for the positive electrode and
graphite as a material for the negative electrode:
Charging
Positive Electrode: LiCo02 -----~ Li~_xCo02 t xL1' + xe'
Discharging
Charging
Negative Electrode: C + xLi' + xe --~ CLEx
f -
Discharging
Charging
Overall balance: LiCo02 + C -.-a Lip-"Co02 + CLtx
.__
Discharging
When the lithium ion battery is being charged, lithium
is intercalated reversibly in the graphite material.
This process is shown in Figure 1.
The electrolyte systems used in lithium ion batteries
generally comprise an aprotic organic solvent such as
e.g. ethylene carbonate, propylene carbonate, dimethyl
carbonate, 'y-butyrolactone, DMF or THF, or a solvent
mixture such as e.g. ethylene carbonate/dimethyl
carbonate (Ec~/DMC, and a conducting salt such as e.g.
LiPFb, which is soluble therein.
Owing to the preparation process of the components of
the electrolyte system, such systems generally contain
trace amounts of water which reacts with the conducting
salt to form acid. LiPF6, for example, is decomposed as
follows:
LiPF,; + Hz0 ~ LiF + POF3 + 2 HF
CA 02356578 2001-09-05
_ 3 _
In addition to this there is a preparation-related acid
content in the ppm range, such as e.g. an HF content of
at least 50 ppm in LiPF6.
The acid formed enters into a further reaction with the
components of the electrochemical cell and e.g.
decomposes the surface layer of the electrodes in the
electrochemical cell.
On graphite electrodes, the surface layer mainly
consists of alkyl carbonates, LizC03, LiOH and Li20. HF,
for example, reacts with this film as follows:
Li2C03 + 2HF -~ 2LiF + H2C03
LiOH + HF -~ LiF + H~0
Li20 + 2HF ~ 2LiF + H20
The original film is therefore replaced by an LiF-
containing film which, in contrast to the original
film, impairs or prevents the passage of lithium ions,
resulting in an increase in battery impedance and
consequently in reduced performance of the battery.
Moreover, the acid, such as e.g. H:F, leaches metals
from highly oxidizing electrode materials such as e.g.
from LiMn20q, leading to a steady loss of active
electrode material and consequently to a steady loss in
capacity, thereby reducing the number of possible
charge/discharge cycles of the battery:
4H+ + 2LiMn3~Mn4+O9 -~ 3Mn0z + Mn'+ + 2Li+ +2H20
The above-described problems resulting from the
increase in the acid content occur not only in lithium
ion batteries but also in all other electrochemical
cells comprising an aprotic electrolyte system, and do
so independently of the type of the conducting salt
used and the type of electrodes.
CA 02356578 2001-09-05
- 4 -
US Patent 5 316 876 describes a lithium ion battery
containing an aprotic electrolyte system which
comprises a cyclic ester and a tertiary amine. The
amine is added to prevent decomposition of the cyclic
ester when the battery is being charged.
EP-A-785 586 describes a lithium ion battery which
comprises a negative electrode containing an amorphous
chalcogen compound and/or an amorphous oxide, a
positive electrode containing a material which is
capable of reversible absorption and release of
lithium, and an aprotic electrolyte system. The
electrolyte system comprises a nitrogen-containing
compound such as e.g. an amine, which improves the
charging and discharging characteristics of the
battery. The electrodes may comprise a constituent
which increases the conductivity of the electrodes,
carbon being one example.
US Patent 5 846 673 describes a lithium ion battery
which comprises an aprotic electrolyte system including
a fluoride-containing conducting lithium salt such as
e.g. LiBFq, LiAsF6 or LiPF6, and a basic compound such
as e.g. an alkylamine, preferably tributylamine. The
basic compound is added to reduce the HF content which
is produced upon hydrolysis of the fluoride-containing
conducting lithium salt.
Whilst tributylamine is capable of effective reduction
of the HF content, it is not electrochemically
oxidation-resistant and is irreversibly decomposed from
about 3.3 V against Li/Li+. This potential is clearly
too low for use in 4 V systems, in which highly
oxidizing electrode materials such as LiMn204, LiCoOz,
LiNi02 or Li(CoNi)OZ are used.
It is therefore a requirement to replace the basic
compound such as e.g. the alkylamine by additives which
CA 02356578 2001-09-05
- 5 -
are oxidation-resistant in 4 V systems. These additives
. should meet the following criteria:
1) Additives that bind acid in the electrolyte system
should be Lewis bases (electron pair donors). A
criterion for the strength of a Lewis base is the
pKa value. The pKa of the additives should be above
10;
2) Additives that bind acid in the electrolyte system
must not introduce any erotic impurities (such as
water) into the electrolyte system;
3) The additives should be soluble in the electrolyte
system; and
4) Additives that bind acid in the electrolyte system
must be electrochemically ~>table. In the
cyclovoltammogram, the electrolyte system should
be stable in the reductive and oxidative range. A
cyclovoltamogram at a Pt electrode therefore must
not show any additional peak in the range from 0
to 4.3 V (against lithium).
It is an object of the present invents_on to improve the
performance, particularly the service life and the
charging and discharging characteristics, of
electrochemical cells such as e.g. lithium ion
batteries which comprise an aprctic electrolyte system
wi~h a conducting salt.
This object is achieved, according to the invention, by
the use of additives of the general formula (I)
NRIRzR3 ( I )
where
CA 02356578 2001-09-05
- 6 -
R1 and R2 are H, CyF~y+i-ZHZ or (CnF2n_mHm) X, X being an
aromatic or heterocyclic radical, and
R3 is (CnFzr-a,Hm) y. Y being a het=erocyclic radical,
or is (CoF2o_pH~) Z, S being an aromatic: radical,
and where n, m, o, p, y and z satisfy the following
conditions:
<_n <_ 6,
0
0 <_m <_ 2n,
2 < o < 6,
0 <_p <_ 20,
1 <_v <_ 8
and
<_z <_ 2y+1,
0
to reduce the acid content in aprotic electrolyte
systems in electrochemical cells.
The invention also provides an electrochemical cell
comprising an anode, a cathode and an apro~tic
electrolyte system which comprises an additive of the
general formula (I).
Said electrochemical cell can be used e.g. as a
building block of a lithium ion battery or as a
building block of a double-layer capacitor.
The additives of the general formula (I) satisfy the
abovementioned criteria (1) to (4).
It was found, surprisingly, that the inventively
employed organic amines having aromatic or heterocyclic
groups are distinctly more oxidation-resistant than
aliphatic amines. Thus 4-(N, N'-dimethylamino)pyridine
(DMAP), for example, has an oxidation potential which
is about 0.9 to 1.0 V higher than tributylamine as
described in US Fatent 5 846 673. The inventively used
CA 02356578 2001-09-05
amines are capable of quantitative scavenging of acids
in the electrolyte system.
The groups represented by R', R' and R3 in the general
formula (I) ca:i be substituted or unsubsti tuted.
R1 and R' preferably are H, an alkyl group (such as e.g.
a methyl group, an ethyl group, an n-propyl group, an
isopropyl group, an n-butyl group, a pentyl group, a
hexyl group, a heptyl group or an octyl group), a
fluorinated alkyl group (such as e.g. a monofluoroethyl
group, a difluoroethyl group, a trifluoroethyl group or
a trifluoropropyl group), an aralkyl group (such as
e.g. a benzyl group) or an aryl group (such as e.g. a
phenyl group or a naphthyl group). The heterocyclic
group represented by R1, R2 and R3 is preferably a
pyridyl group or a pyrazyl group. Examples of
fluorinated groups comprise a fluoropyridyl group, a
fluorobenzyl group and a perfluoropyri-dyl group.
Examples of the additives comprise 2-phenylethylamine,~
2-phenylpropylamine, 3-phenylpropyl<~mine, 2-phenyl-
butylamine, 3-phenylbutylamine, 4-phenylbutylamine,
2-phenylethyl-N-methylamine, 2-phenylpropyl-N-methyl-
amine, 3-phenylpropyl-N-methylamine, 2-phenylbutyl-N-
methylamine, 3-phenylbutyl-N-methylamine, 4-phenyl-
butyl-N-methylamine, 2-phenylethyl-N-ethylamine, 2-
phenylpropyl-N-ethylamine, 3-phenylpropyl-N-ethylamine,
2-phenylbutyl-r1-ethylamine, 3-phenylbutyl-N-ethylamine,
4-phenylbutyl-rl-ethylamine, 2-phenylethyl-N,N-dimethyl-
amine, 2-phenylpropyl-N,N-dimethylamine, 3-phenyl-
propyi-N,N-dimethylamine, 2-phenylbutyl-N,N-dimethyl-
amine, 3-phenylbutyl-N,N-dimethylamine, 4-phenylbutyl-
N,N-dimethylamine, 2-phenylethyl-N,N--diethylamine, 2-
phenylpropyl-N,N-diethylamine, 3-phenylpropyl-N,N-
diethylamine, 2-phenylbutyl-N,N-diethylamine, 3-phenyl-
butyl-N,N-diethylami.ne, 4-phenylbutyl-N,N-diethylamine,
N,N-dimethylaminopyridine, N,N-dimethylaminopyrazine,
CA 02356578 2001-09-05
- g
N,N-diethylaminopyridine and N,N-diethylaminopyrazine.
Particular preference is given to the use of 4-
phenylbutylamine and N,N-dimethylaminopyridine.
The additives are preferably employed in an amount of
up to 10 wto and particularly preferably in an amount
in the range of from 0.01 to 5 wto, based on the weight
of the electrolyte system.
The electrolyte system of the electrochemical cell
further comprises an aprotic solvent and a conducting
salt dissolved therein.
Examples of preferred solvents comprise ethylene
carbonate, propylene carbonate, but,ylene carbonate,
dimethyl carbonate, diethyl carbonate, methyl ethyl
carbonate, y-butyrolactone, methyl formate, methyl
acetate, 1,2-dimethoxyethane, tetrahydrofuran,
2-methylte trahydrofuran, dimethyl sulfoxide, 1,3-
dioxolane, formamide, dimethylformamide, dioxolane,
dioxane, acetonitril.e,
nitromethane,
a phosphoric
acid
triester, trimethoxymethane, dioxolane derivatives,
sulfolane, 3-methyl-2-oxazolidinone, propylene
carbonate derivatives and tetrahydrofuran derivatives.
Solvent
mixtures such
as e.g. ethylene
carbonate/
dimethyl carbonate
(EC/DMC) can
also be used.
Examples of suitable conducting salt=s comprise salts
with an anion, selected from the group consisting of
BFq , PF6 , As F6 , SbF6 , ClOq , N (SO~CnF~~m-mHm) 2 i
C ( SC'zC~F2nm-mHm) 3 i 0 ( SO~CnFzn+~-mHm) arid [ PF6_p (C.,F2"+~_-mHm) o~ -~
where 0 <_ n <_ 6, 0 _<_ m <_ 2n+1 and 0 <_ p _< 5. The canon
of the conducting salt can be an alkali metal ion such
as a . g . Li~, Na+, K+, Rb+ or Cs+, or an onium ion such as
e.g. NHq+.
The conducting salts are preferably employed in a
concentration in the range of from 0.01 to 3 mol/1 and
CA 02356578 2001-09-05
- 9 -
particularly preferably in a concentration in the range
of from 0.1 to 2 mold.
The additives of the general formula (I) can e.g. be
employed to reduce the acid content in the electrolyte
system of a lithium ion battery.
The negative electrode of a lithium ion battery
comprises a material which is capable of reversible
uptake and re-release of lithium ions, graphite being
an example of such a material.
The positive electrode of a lithium ion battery
comprises a lithium transition-metal oxide. Preferred
transition metals comprise Ti, V, Cr, Mn, Fe, Co, Ni,
Mo and W. Preferred lithium transition-metal oxides
comprise LiCoOz, Li NiOz, LiMnOz, LiMnz04, Li (CoNi) Oz,
Li (CoV) Oz and Li (CoFe) Oz.
The electrodes of the lithium ion battery can comprise
further additives such as e.g. conductivity-enhancing
constituents, binders, dispersants, fillers and ion-
cor.ducting materials. For example, those additives can
be employed which are described in EP--A-785 586.
The additives can be employed in electrolytes
comprising conventional conducting salts. Suitable, for
example, are electrolytes comprising conducting salts
selected from the group consisting of LiPF6, LiBF4,
LiClOq, LiAsF6, LiCF 3S03, LiN (CF3SOz) z or LiC (CF3SOz) 3 and
mixtures of these. The electrolytes may also comprise
organic isocyanates (DE 199 44 603) to reduce the water
content. Equally, the electrolytes can comprise organic
alkali metal salts (DE 199 10 968) as an additive. A
suitable example is that of alkali metal borates of the
general formula
Li+B- ( ~R1 ) m ( ~R' ) p
CA 02356578 2001-09-05
- 10 -
where
m and p are 0, i, 2, 3 or 4, with m+p=4, and
R1 and R' are identical or different,
are linked or not linked directly to one another via a
single or double bond,
each, individually or jcintly, have she meaning of an
aromatic or aliphat;~c carboxylic, dicarboxylic or
sulf:onic acid radical, or
each, individually or jointly, have the meaning of an
aromatic ring from the group consisting of phenyl,
naphthyl, anthracenyl or phenanthrenyl which can be
unsubstituted or mono- to tetrasubstituted by A or Hal,
or
each, individually or jointly, have the meaning of a
heterocyclic aromatic ring from the group consisting of
~0 pyridyl, pyrazyl or bipyridyl, which can be
unsubstituted or mono- to trisubstituted by A or Hal,
or
each, individually or jointly, have the meaning of an
aromatic hydroxy acid from the group consisting of
aromatic hydroxy carboxylic acids or aromatic hydroxy
sulfonic acids, which can be unsubstituted or mono- to
tetrasubstituted by A or Hal,
and
Hal is F, Cl or Br
anc
A is alkyl which has from 1 to 6 C atoms and can
be mono- to trihalogenated. Equally suitable are alkali
metal alcoholates of the general formula
Li+OR-
CA 02356578 2001-09-05
- 11 -
where R
has the meaning of an aromatic or aliphatic carboxylic,
dicarboxylic or sulf.onic acid radical, or
has the meaning of an aromatic ring from the group
consisting of phenyl, naphthyl, anthracenyl or
phenanthrenyl which can be unsubstit:uted or mono- to
tetrasubstituted by A or Hal, or
has the meaning of a heterocyclic aromatic ring from
the group consisting of pyridyl, pyrazyl or bipyridyl,
which can be unsubstituted cr mono- to trisubstituted
by A or Hal, or_
has the meaning of an aromatic hydra xy acid from the
group consisting of aromatic hydroxy carboxylic acids
or aromatic hydrox y sulfonic acids, which can be
unsubstituted or mono- to tetrasubstit:uted by A cr Hal,
and
Hal is F, C1 or Br
and
A is alkyl which has from 1 to 6 C atoms and can
be mono- to trihalogenated.
The electrolyte ca'n also comprise lithium complex salts
of the formula
Ra
O,~S~ O
I ~' Q,oR~
B 2
OR
R~
where
CA 02356578 2001-09-05
- 1G
R1 and R2 are identical or different, are directly
linked or not directly linked to one another via a
single or double bond, each, individually or jointly,
have the meaning of an aromatic ring from the group
consisting of phenyl, naphthyl, anthracenyl or
phenanthrenyl, which can be unsubstit.uted or mono- to
hexasubstituted by alkyl (C-_ to CE), alkoxy groups (Ci
to C6) or halogen (F, C1, Br) , or each, individually or
jointly, have =he meaning of an aromatic heterocyclic
ring from the group consisting of pyridyl, pyrazyl or
pyr~~midyl, which can be unsubstituted or mono- to
tetrasubstituted. by alkyl (Ci to C6) , alkoxy groups (C1
to C6) or halogen (F, C1, Br),
or each, individually or jointly, have the meaning of
an aromatic ring from the group hydroxybenzenecarboxyl,
hydroxynaphthalenecarboxyl, hydroxybenzenesulfonyl and
hydroxynaphthalenesulfonyl, which can be unsubstituted
or mono- to tetrasubstituted by alkyl (C1 to Cb), alkoxy
groups (C1 to C~) or ha 1 ogen ( F , C1, Br) ,
R3-R.~' can each, individually or pairwise, being directly
linked or not directly linked to one another via a
single or double bond, have the following meaning:
1. Alkyl (C1 to Co), alkyloxy (C1 to C6) or halogen
(F, C1, Br)
2. An aromatic ring from the groups consisting of
Phenyl, napthyl, anthracenyl
or phenanthrenyl, which
can be unsubstituted or mono- to hexasubstituted by
alkyl (C1 to C6), alkoxy groups (C, to C5) or halogen
( F , C1, Br) ,
Pyri.dyl, pyrazyl or pyrimidyl, which can be
unsubstituted or mono- to tetrasubstituted by alkyl
(C,_ to C6), alkoxy groups (CL to C6) or halogen (F, Ci,
Br) ,
which are prepared via the following method
(DE 199 32 317)
CA 02356578 2001-09-05
- 13 -
a) 3-, 4-, 5-, 6-substituted phenol in a suitable
solvent is admixed with chlorosulfonic acid,
b) the intermediate from a) is reacted with
chlorotrimethylsilane, filtered and subjected to
fractional distillation,
c) the intermediate from b) is reacted with lithium-
tetramethanolate borate (1-) in a suitable solvent and
t~_e end product is isolated therefrom.
Equally, the electrolytes can comprise compounds of the
following formula (DE 199 4i 566)
C ( LR1 (CRzRs) ;~] iAX) YKt; r -N (CF3) z
where
Kt = N, P, As, Sb, S, Se
A = N, P, P (0) , 0, S, S (0) , SOz, As, As (0) , Sb,
Sb (0)
R1, R2 and R3, identically or differently, are
H, halogen, substituted and/or unsubstituted alkyl
CaH=n+i. substituted and/or u~:substit:uted alkenyl havi ng
2-18 carbon atoms and one or more double bonds,
substituted and/or unsubstituted alkynyl having 2-18
carbon atoms and one or more triple bonds, substituted
and/ or unsubstituted cycl oalkyl CmH2m-i. mono- or
polysubstituted and/or unsubstituted phenyl,
substituted and/or unsubstituted het:eroaryl,
A can be included in various positions in R1, R2 and/or
R3
Kt can be included in cyclic or heterocyclic rings,
the groups bound to Kt can be identical or different,
and
n = 1-18
m = 3-7
k = 0, 1-6
1 - i or 2 in the case of x=1 and 1 in the case x=0
x = 0, 1
y = 1-4.
CA 02356578 2001-09-05
- 14 -
The method for preparing these compounds is
characterized in that an alkali metal salt of the
gene:=a1 formula
D+ -N (CF3) z
D+ being selected from the group consisting of the
alkali metals, is reacted in a polar organic solvent
with a salt of the general formula
[ ( [R1 (CR'R3) x] iAx) vKt]+ E
where
Kt, A, Ri, R', P.3., k, 1, x and y have the abovementioned
meanings and
E iS F-, C1 , Br , I , BFI , C104 , ASF,~-, SbF~ Or
PF6 .
It is equally possible, however, to employ electrolytes
comprising compounds of the general formula
(DE 199 53 638)
X- ( CYZ ) mS02N ( CR1RZR3 ) 2
where
2 5 X i S H, F, C1, CnFzn+1 i CnF2n-~ i ( S02 ) xN ( CR1RZR3 )
Y is H, F, C1
Z is H, ~', C1
R1, R', RJ are H and/or alkyl, fluoroalkyl, cycloalkyl
m is 0-9 and if X=H, m~0
n is 1-9
k is 0, if m=0, and k=1 if m=1-9,
prepared by reacting partially fluorinated or
perfluorinated alkylsulfonyl fluorides with
dimethylamine in organic solvents and complex salts of
the general formula (DE 199 51 804)
M"+[EZ]r" by
CA 02356578 2001-09-05
- 15 -
where
x, y are i, 2, 3, 4, 5, 6
M"+ is a metal ion
E is a Lewis acid selected from the group
consisting of
BR1R'R3, A1 R1RZR3, PR1RZR3R9R5, ASRIRzR3R4R~', VR1R~R3R4R5,
R' to RS are identical or different and are directly
linked or are not directly linked to one another via a
sine=Le or double bond, each, individually or jointly,
have the meaning of a halogen (F, C1, Br),
of an alkyl or alkoxy radical (C1 to Ce) which may be
partially or ccmpletely substituted by F, Cl, Br,
an aromatic ring which is bound or not bound via
oxygen, is selected from the group consisting of
phenyl, naphthyl, anthracenyl or phenanthrenyl, and can
be unsubstituted or mono- to hexasubstituted by alkyl
(C, to Ca) or F, C1, Br,
an aromatic heterocyclic ring which is bound or not
bound via oxygen, is selected from they group consisting
of pyridyl, pyrazyl or pyrimidyl, and can be
unsubstituted or mono- to tetrasubstit:uted by alkyl (C1
to Ce) or F, Cl, Br, and .
2 is OR°, NRc;~~, CRSRlRE3, OSOzR', N ( SO~Rj) ( SO2R' ) ,
C ( S02R6 ) ( SO~R~ ) ( SORB ) , OCORb, where
R° tc~ Re are identical or different, ar_e directly linked
or not directly linked to cne another via a single or
double bond, and each, individually or jointly, have
the meaning
of a hydrogen or the meaning as Rl to R5, prepared by
reacting an appropriate boron or phosphorus Lewis acid
solvent adduct with a lithium or tetraalkylammonium
imide, methanide or triflate.
Also present can be borate salts (DE L99 59 722) of the
general formula
CA 02356578 2001-09-05
- 16 -
R4 R, v.
/ ~
Ra Rz x~Y
where
M is a metal ion or tetraalkylam:-nenium ion
x, y are l, 2, 3, 4, 5 or 6,
R~' to R4 are identical or different alkexy or carboxy
radicals (C1 to C$) which are directly linked or are net
directly linker to one another via a single or double
bond. These borate salts are prepared by reacting
lithium tetraalcoholate borate or a l:i mixture of
lithium alcoholate with a boric acid ester in an
aprotic solvent with a suitable hydroxyl or carboxyl
compound in a ratio of 2:1 or 4:i.
The additives can also be employed in electrolytes
which comprise lithium fluoroalkyl phosphates of the
general formula,
Li+[PF;~ (C"F2,~+i-~H~) 6-:~l
where
1 <_ x <_ 5
3 <_ y <_ 8
0 <_ z _< 2y + 1
and the ligands ~ (CyF~y+i-zH~) can be identical or
different, with the exception of the compounds of the
general formula,
Li+ [ PFa (CHbF~ (CF3) d) e~
where a ,~s an integer from 2 to 5, b =- 0 or 1, c = 0 or
l, d - 2 and a is an integer from ~ to 4, with the
conditions that b and c are not simultaneously - 0 and
the sum a + a equals 6 and the ligands (CHbF~ (CF3) d) can
CA 02356578 2001-09-05
- 17 -
be identical or different (DE 100 08G 55).
The method
for preparing lithium fluoroa~~kyl phosphates of the
general formula is characterized in that at least one
compound of the general formula
HmP ( CnH2n+1 ) 3-mr
0 P ( CI;H~n+1 ) 3 r
ClmP ( CnH2n+1 ) 3-mi
F'mP ( CnH2n+1 ) 3-mr
Clop (CnH?n+1) S-or
FoP ( CnHzn+1 ) 5-o r
in each of which
0 <_ m <_ 2, 3 <_ n <_ 8 and 0 <_ o <_ is fluorinated by
4,
electrolysis in. hydrogen fluoride, the fluorinat ion
product mixture thus obtained is separated by
extraction, phase separatio:~: and/or distillation, and
the fluorinated alkylphosphcrane tnus obtained is
reacted with lithium fluoride in an aprotic solventor
solvent mixture with the exclusion of moisture, the
and
sale thus obtained is purified and isolated in
accordance with standard procedures.
The additives can be employed in electrolytes .for
electrochemical cells which comprise anode material
consisting of coated metal cores selected from the
group Sb, Bi, Cd, Ir_, Pb, Ga and tin or alloys of these
(DE 100 I6 024). The method for prex>aring said anode
material is characterized in that
a) a suspension or a sot of the metal core or alloy
core is prepared in urotropine,
b) the suspension is emulsified with CS-C,~ hydro-
carbons,
c) the emulsion is precipitated on to the metal or
allc>y cores, and
d) the metal hydroxides or oxihydroxi.des are converted
into the corresponding oxide by annealing the system.
The additives can also be used in electrolytes for
electrochemica~y cells comprising cathodes of standard
CA 02356578 2001-09-05
- 18 -
The additi~res can also be used in electrolytes for
electrochemical cel:Ls comprising cathodes of standard
lithium intercalation and insertion compounds, but
alternatively comprising cathode materials which
consist of lithium mixed oxide particles which are
coated with one or more metal oxides (DE 199 22 522),
by suspending the particles in an organic solvent,
admixing the suspension with a solution of a
hydrolyzable metal compound and a hydrolysis solution
and then filtering off the coated particles, drying
them and calcining them if required. Alternatively,
they can consist of lithium mixed oxide particles which
are coated with, one or more polymers (DE 199 46 066),
obtained by a method in which the particles are
suspended in a solvent and the coated particles are
ther_ filtered off, dried and calcined if required.
Equally, the additives according to the invention can
be employed in systems comprising cathodes which
consist of lithium mixed oxide particles, which are
singly or multiply coated with alkali metal compounds
and metal oxides (DE 100 14 884). The method for
preparing these materials is characterized in that -the
particles are suspended in an organic solvent, an
alkali metal salt compound suspended in an organic
solvent is added, metal oxides dissolved in an organic
solvent are added, the suspension is admixed with a
hydrolysis solution, and the coated particles are then
filtered off, dried and calcined.
The following examples illustrate the invention.
EXAMPLE 1
Preparation of electrolyte solutions comprising amines
2 or 5 wto of the amines listed in Table 1 were
dissolved in an initial electrolyte (1M LiPF~ in EC:DMC
(1:1); HF content of the initial electrolyte: 100 ppm).
CA 02356578 2001-09-05
- 19 -
The solutions were stirred for 3 hours at roan
temperature, after which the HF content of each
elect=rolyte was measured.
Table 1
Additive HF content Oxidation
after stirring resistance
for 3 hours against Li/Li'
4-Phenylbutylamine (5 wto) < 5 ppm 4.8 V
4-(N,N'-Dimethyl.amino) < 5 ppm 4.4 V
pyridine (2 wto)
Tributylamine (5 wt%) < 5 ppm 3.4 V
~vnnnDT ~
Oxidation resistance of the additives from Example 1
A measuring cell comprising a platinum working
electrode, lithium counter-electrode and lithium
reference electrode was used to record, in each case, 5
i5 cyclovoltammograms successively. To do this, 'the
potential, starting from the rest potential, was first
increased at a rate of 10 mV/s ro 6.0 V against Li/Li+
and subsequently run back to the rest potential.
Table 1 shows the oxidative decomposition potentials
20~ cbtained, Figures 2 to 4 show the c yclovoltammograms
obtained for 4-(N, N'-dimethylamino)pyridine, 4-phenyl-
butylamine and tributylamine (described in US Patent
5 896 673).
25 As can be gathered from the results shown in Table 1,
the additives used according to the ,invention are more
resistant to oxidation than aliphatic amines such as
e.g. rributylamine and can be employed e.g. in lithium
ion batteries, i . a . in 4 V systems, t;o reduce the acid
30 content in aprotic electrolyte systems.