Note: Descriptions are shown in the official language in which they were submitted.
CA 02356969 2001-06-27
WO 00/40553 . PCT/US99/28234
-1-
3-SUBSTITUTED PYRROLIDINES USEFUL AS INHIBITORS
OF MATRIX METALLO-PROTEINASES
BACKGROUND OF THE INVENTION
1o The matrix metalloproteinases (MMPs) are a family of zinc containing
endopeptidases
which are capable of cleaving large biomolecules such as the collagens,
proteoglycans and
gelatins. Expression is upregulated by pro-inflammatory cytokines and/or
growth factors.
The MMP's are secreted as inactive zymogens which, upon activation, are
subject to control
by endogenous inhibitors, for example, tissue inhibitor of metalloproteinases
(TIMP) and a~-
15 macroglobulin. Chapman, K.T. et al., J. Med. Chem. 36, 4293-4301 ( 1993);
Beckett, R.P. et
al., DDT 1, 16-26 ( I996). The characterizing feature of diseases involving
the enzymes
appears to be a stoichiometric imbalance between active enzymes and endogenous
inhibitors,
leading to excessive tissue disruption, and often degradation. McCachren,
S.S., Arthritis
Rheum. 34, 1085-1093 ( 1991 ).
The discovery of different families of matrix metalloproteinase, their
relationships, and
their individual characteristics.have been categorized in several reports.
Emonard, H. et al.,
Cell Molec. Biol. 36, 13I-153 (1990); Birkedal-Hansen, H., J. Oral Pathol. 17,
445-451
(1988); Matrisian, L.M., Trends Genet. 6, 121-125 (1990); Murphy, G.J.P. et
al., FEBS Lett.
289, 4-7 (1991); Matrisian, L.M., Bioessays 14, 455-463 (1992). Three groups
of MMPs have
been delineated: the collagenases which have triple helical interstitial
collagen as a substrate,
the gelatinases which are proteinases of denatured collagen and Type IV
collagen, and the
stromelysins which were originally characterized as proteoglycanases but have
now been
identified to have a broader proteolytic spectrum. Examples of specific
collagenases include
fibroblast collagenase (MMP-1 ), neutrophil collagenase (MMP-8), and
collagenase 3 (MMP-
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-2-
identified to have a broader proteolytic spectrum. Examples of specific
collagenases include
fibroblast collagenase (MMP-1), neutrophil collagenase (MMP-8), and
collagenase 3 {MMP-
13). Examples of gelatinases include 72 kDa gelatinase (geIatinase A; MMP-2)
and 92 kDa
gelatinase (gelatinase B; MMP-9). Examples of stromelysins include stromelysin
1 (MMP-3),
stromeiysin 2 (MMP-10) and matrilysin (MMP-7). Other MMPs which do not fit
neatly into
the above groups include metalloelastase (MMP-12), membrane-type MMP (MT-MMP
or
MMP-14) and stromelysin 3 (MMP-11 ). Beckett, R.P. et al., su ra.
1o Over-expression and activation of MMPs have been linked with a wide range
of
diseases such as cancer; rheumatoid arthritis; osteoarthritis; chronic
inflammatory disorders,
such as emphysema and smoking-induced emphysema; cardiovascular disorders,
such as
atherosclerosis; corneal ulceration; dental diseases such as gingivitis and
periodontal disease;
and neurological disorders, such as multiple sclerosis. For example, in
adenocarcinoma,
15 invasive proximal gastric cells express the 72 kDa form of collagenase Type
IV, whereas the
noninvasive cells do not. Schwartz, G.K. et al., Cancer 73, 22-27 (1994). Rat
embryo cells
transformed by the Ha-ras and v-myc oncogenes or by Ha-ras alone are
metastatic in nude
mice and release the 92 kDa gelatinase/collagenase (MMP-9). Bernhard, E.J. et
al., Proc.
Natl. Acad. Sci. 91, 4293-4597 (1994). The plasma concentration of MMP-9 was
2o significantly increased (P < 0.01 ) in 122 patients with gastrointestinal
tract cancer and breast
cancer. Zucker, S. et al., Cancer Res. 53, 140-146 (1993). Moreover,
intraperitoneal
administration of batimastat, a synthetic MMP inhibitor, gave significant
inhibition in the
growth and metastatic spread and number of lung colonies which were produced
by
intravenous injection of the B16-BL6 marine melanoma in C57BL/6N mice.
Chirivi, R.G.S.
25 et al., Int. J. Cancer 58, 460-464 (1994). Over-expression of TIMP-2, the
endogenous tissue
inhibitor of MMP-2, markedly reduced melanoma growth in the skin of
immunodeficient
mice. Montgomery, A.M.P. et al., Cancer Res. 54, 5467-5473 ( 1994).
Accelerated breakdown of the extracelIular matrix of articular cartilage is a
key
3o feature in the pathology of both rheumatoid arthritis and osteoarthritis.
Current evidence
suggests that the inappropriate synthesis of MMPs is the key event. Beeley,
N.R.A. et al.,
Curr. Opin. Ther. Patents, 4(1), 7-16 (1994). The advent of reliable
diagnostic tools have
allowed a number of research groups to recognize that stromelysin is a key
enzyme in both
arthritis and joint trauma. Beeley, N.R.A. et al., Id.; Hasty. K.A. et al.,
Arthr. Rheum. 33,
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 . PCT/US99/28234
-3-
388-397 (1990). It has also been shown that stromelysin is important for the
conversion of
procollagenase to active collagenase. Murphy, G. et al., Biochem. J. 248, 265-
268 ( 1987).
Furthermore, a range of MMPs can hydrolyse the membrane-bound precursor of the
pro-inflammatory cytokine tumor necrosis factor a (TNF-a). Gearing, A.J.H. et
al., Nature
370, 555-557 (1994). This cleavage yields mature soluble TNF-a and the
inhibitors of MMPs
can block production of TNF-a both in vitro and in vivo. Gearing, A.J.H. et
al., Id.; Mohler,
K.M. et al., Nature 370, 2I8-220 (1994); McGeehan, G.M. et al., Nature 370,
558-56I (1994).
This pharmacological action is a probable contributor to the antiarthritic
action of this class of
io compounds seen in animal models. Beckett, R.P. et al., supra.
Stromelysin has been observed to degrade the a,-proteinase inhibitor which
regulates
the activity of enzymes such as elastase, excesses of which have been linked
to chronic
inflammatory disorders such as emphysema and chronic bronchitis. Beeley,
N.R.A. et al.,
15 supra.; Wahl, R.C. et al., Annual Reports in Medicinal Chemistry 25, 177-
184 (1990). In
addition, a recent study indicates that MMP-I2 is required for the development
of smoking-
induced emphysema in mice. Science, 277, 2002 ( 1997). Inhibition of the
appropriate MMP
may thus potentiate the inhibitory activity of endogenous inhibitors of this
type.
2o High levels of mRNA corresponding to stromelysin have been observed in
atherosclerotic plaques removed from heart transplant patients. Henney, A.M.,
et al., Proc.
Natl. Acad. Sci. 88, 8154-8158 (1991). It is submitted that the role of
stromelysin in such
plaques is to encourage rupture of the connective tissue matrix which encloses
the plaque.
This rupture is in turn thought to be a key event in the cascade which leads
to clot formation
25 of the type seen in coronary thrombosis. MMP inhibition is thus a
preventive measure for
such thromboses.
Collagenase, stromelysin and gelatinase have been implicated in the
destruction of the
extracellular matrix of the cornea. This is thought to be an important
mechanism of morbidity
3o and visual loss in a number of ulcerative ocular diseases, particularly
those following infection
or chemical damage. Burns, F.R. et al., Invest. ~pthalmol. and Visual Sci. 32,
1569-1575
(1989). The MMPs present in the eye during ulceration are derived either
endogenously from
infiltrating leucocytes or fibroblasts, or exogenously from microbes.
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-4-
Collagenase and stromelysin activities have been identified in fibroblasts
isolated from
inflamed gingiva and the levels of enzyme have been correlated with the
severity of the
gingivitis observed. Beeley, N.R.A. et al., su ra.; Overall, C.M. et al., J.
Periodontal Res. 22,
81-88 (1987).
Excessive levels of gelatinase-B in cerebrospinal fluid has been linked with
incidence
of multiple sclerosis and other neurological disorders. Beeley, N.R.A. et al.,
s_u~ra.; Miyazaki,
K. et al., Nature 362, 839-841 (1993). The enzyme may play a key role in the
demyelination
of neurones and the breakdown of the blood brain barrier which occurs in such
disorders.
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PGT/US99I28234
-5-
SUMMARY OF THE INVENTION
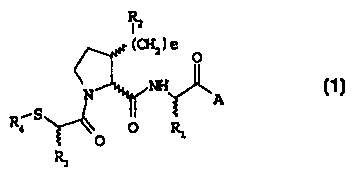
The present invention provides novel 3-substitutedpyrrolidines of formula ( 1
):
Rz
I
( CHZ ) a
O
N NH
I A
~S
R4 ~~~ O R1
O
R3
formula ( 1 )
wherein
a is an interger from 0 to 2;
_ _. A_ is selected from the group consisting of -OH and -NRR';
wherein
R and R' are independently selected from the group consisting of hydrogen and
C,-Cb
alkyl or R and R' taken together with the nitrogen atom to which they are
attached
form a N-morpholino, N-piperidino, N-pyrrolidino, or N-isoindolyl;
R, is selected from the group consisting of hydrogen, C,-C6 alkyl, -(CHz)a-
COZRS,
-(CHZ)e C(O)NH2, -(CHz)aNHz, -(CHi)3-NH-C(NH)NHz, -(CHZ)2-S(O)b-CH3, -CHZ-OH,
-CH(OH)CH3, -CHz-SH, -(CHZ)d-Ar,, and -CHz-Ar2;
wherein
a is 1 or 2;
bis0, l,or2;
d is an integer from 0 to 4;
RS is selected from the group consisting of hydrogen, C, _Ca alkyl, and
benzyl;
Are is a radical selected from the group consisting of
and
R6 R~
wherein
R~ is from 1 to 2 substituents independently selected from the group
consisting
ofhydrogen, halogen, C,_Ca alkyl, hydroxy, and C,_C:~ alkoxy;
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28Z34
-6-
R7 is selected from the group consisting of hydrogen, halogen, C,_C~ alkyl,
and
C ~ _C4 alkoxy;
Are is a radical selected from the group consisting of
N
~y
I ~~ and NH ~
NH
RZ is a radical selected from the group consisting of
I ~ and I
R ~ R ~ _..._ _ ..
z z
1 o wherein
wherein
Rz= is from 1 to 2 substituents selected from the group consisting of
hydrogen, halogen,
C,-Ca alkyl, and C,-C4 alkoxy;
R3 is selected from the group consisting of Ci-C6 alkyl, -(CHZ)m W, -(CHZ)p-
Ar3,
-(CHZ)k-COzR9, -(CH2)m-NRg'S02-Y> > ~d -(CHZ)rmZ-Q
wherein
m is an integer from 2 to 8;
p is an integer from 0-10;
k is an integer from 1 to 9;
W is phthalimido;
Ar3 is selected from the group consisting of
N
Rz3 \ I ~ ,
NH
N
and
S p N
wherein
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PGT/US99/28Z34
-7-
Rz, is from 1 to 2 substituents independently selected from the group
consisting
of hydrogen, halogen, C,-C.~ alkyl, and C,-C., alkoxy;
RR~ is hydrogen or C,-C~ alkyl;
R~ is hydrogen or C~-C~ alkyl;
Y, is selected from the group consisting of hydrogen, -(CHz)~-Are, and -
N(Rz,~)z
wherein
jis0orl;
Rza each time selected is independently hydrogen or C,-C6 alkyl or are taken
together with the nitrogen to which they are attached to form N-morpholino, N-
1o piperidino, N-pyrrolidino, or N-isoindolyl;
Ar.~ is
f / RZs
wherein
Rzs is from 1 to 3 substituents independently selected from the group
consisting
of hydrogen, halogen, C~-Ca alkyl, and C,-C4 alkoxy;
Z is selected from the group consisting of -O-, -NRg-, -C(O)NRs-, -NRBC(O)-,
-NRBC(O)NH-, -NR8C(O)O -, and -OC(O)NH-;
wherein
R8 is hydrogen or Cl-C6 alkyl;
Q is selected from the group consisting of hydrogen, -(CHz)n-Yz, and -(CHz),~
Y3;
wherein
n is an integer from 0 to 4;
x is an integer from 2 to 4;
Yz _is selected from the group consisting of hydrogen, -(CHz),,-Ar; and
-(CHz)~-C(O)ORz~
wherein
Ar; is selected from the group consisting of
i
R26 and ~ /
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28Z34
_$_
wherein
Rz6 is from 1 to 3 substituents independently selected from the
group consisting of hydrogen, halogen, C,-C., alkyl, and Ci-C.~
alkoxy;
h is an integer from 0 to 6;
t is an integer from 1 to.6;
RZ~ is hydrogen or C~-C6 alkyl;
Yz is selected from the group.consisting of -N(Rz$)Z, N-morpholino, N-
piperidino, N-pyrrolidino, and N-isoindolyl;
1 o wherein
Rz$ each time taken is independently selected from the group consisting
of hydrogen and C,-C~ alkyl;
R4 is selected from the group consisting of hydrogen, -C(O)R~o, -C(O)-(CHz)q-K
and -S-G
wherein
R,o is selected from the group consisting of hydrogen, C,_Ca alkyl, phenyl,
and benzyl;
q is 0, 1, or 2;
K is selected from the group consisting of
-N~V J -
r
Rm
" and -a
Rll
N~Rll
Rll ~
2o wherein
V is selected from the group consisting of a bond, -CHI-, -O-, -S(O),.-, -NRZ,-
,
and -NC(O)R22-;
wherein
ris0, l,or2;
RZ, is selected from the group consisting of hydrogen, Ci_Ca alkyl, and
benzyl;
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
_g_
R>; is selected from the group consisting of hydrogen, -CFz, C,-C,~,
alkyl, phenyl , and benzyl;
R,~ is selected from the group consisting of hydrogen, Ci_Ca alkyl, and
benzyl;
Ri ~ ~ is selected from the group consisting of hydrogen, C~_C.~ alkyl, and
benzyl;
G is selected from the group consisting of
/ ( CHz )~ Rlz V
(CHz)~
R13 Vz
/ (CHz)w \~ / (CHz)w /~
~N V~~N
V4
( CHz ) w \
Rl9 / ( CHz )~ NHRl s
COzRls
and
N /
wherein
w is an integer from 1 to 3;
1o R,Z is selected from the group consisting of hydrogen, C,-C6 alkyl,
-CHzCH2S(O)rCH3, and benzyl;
wherein f is 0,1, or 2;
R~3 is selected from the group consisting of hydrogen, hydroxy, amino, C~-C6
alkyl, N-methylamino, N,N-dimethylamino, -COZR~7, and -OC(O)R,g;
wherein
R,7 is hydrogen, -CHZO-C(O)C(CH3)3, C~_C.~ alkyl, benzyl, or
diphenylmethyl;
R~g is hydrogen, C,-C~ alkyl or phenyl;
R,4 is 1 or 2 substituents independently selected from the group consisting of
2o hydrogen, Ci_Ca alkyl, C,_C.~ alkoxy, or halogen;
V, is selected from the group consisting of -O-, -S-, and -NH-;
Vz is selected from the group consisting of -N- and -CH-;
SUBST1TUTE SHEET (RULE 2~
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-10-
Vz is selected from the group consisting of a bond and -C(O}-;
V:~ is selected from the group consisting of -O-, -S-, -NR,9-, and -NC(O)RZO-;
wherein
R,9 is hydrogen, C,_C4 alkyl, or benzyl;
RZO is hydrogen, -CF3, C~-C,~ alkyl, or benzyl;
R,; is selected from the group consisting of hydrogen, C~-C~ alkyl and benzyl;
R,~ is selected from the group consisting of hydrogen and C,_C4 alkyl;
and stereoisomers, pharmaceutically acceptable salt, and hydrate thereof.
to The present invention further provides a method of inhibiting matrix
metallo-
proteinases (MMPs) in a patient in need thereof comprising administering to
the patient an
effective matrix metalloproteinase inhibiting amount of a compound of formula
( 1 ). As such
the present invention provides a method of treating a neoplastic disease state
or cancer;
rheumatoid arthritis; osteoarthritis; osteoporosis; cardiovascular disorders,
such as
Z5 atherosclerosis; corneal ulceration; dental diseases, such as gingivitis or
periodontal disease;
and neurological disorders, such as multiple sclerosis; chronic inflammatory
disorders, such as
emphysema and especially smoking-induced emphysema.
In addition, the present invention provides a composition comprising an
assayable
20 amount of a compound of formula (1) in admixture or otherwise in
association with an inert
carrier. The present invention also provides a pharmaceutical composition
comprising an
effective MMP inhibitory amount of a compound of formula ( 1 ) in admixture or
otherwise in
association with one or more pharmaceutically acceptable Garners or
excipients.
25 As is appreciated by one of ordinary skill in the art the compounds of
formula ( 1 ) exist
as stereoisomers. Specifically, it is recognized that they exist as
stereoisomers at the point of
attachment of the substituents R,, -(CHZ)e-RZ, R3, and -SR.,, -C(O}NH-CHR,-
C(O)A, R~2, and
-NHR,;. Where indicated the compounds follow either the (+)- and (-)-
designation for
optical rotation, the (D)- and (L)- designation of relative stereochemistry,
or the Cahn-Ingold-
3o Prelog designation of (R)-and (S)- for the stereochemistry of at specific
postions in the
compounds represented by formula ( 1 ) and intermediates thereof. Any
reference in this
application to one of the compounds of the formula ( 1 ) is meant to encompass
either specific
stereoisomers or a mixture of stereoisomers.
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-11-
The specific stereoisomers can be prepared by stereospecific synthesis using
enantiomerically pure or enantiomerically enriched starting materials which
are well known in
the art. The specific stereoisomers of amino acid starting materials are
commercially
available or can be prepared by stereospecific synthesis as is well known in
the art or
analogously known in the art, such as D. A. Evans, et al. J. Am. Chem. Soc.,
112, 4011-4030
(1990); S. Ikegami et al. Tetrahedron, 44, 5333-5342 (1988); W.Oppolzer et al.
Tet. Lets. 30,
6009-6010 (1989); Synthesis of Optically Active a-Amino-Acids, R. M. Williams
(Pergamon
Press, Oxford 1989); M. J. O'Donnell ed.: a-Amino-Acid S~mthesis, Tetrahedron
Symposia in
print, No. 33, Tetrahedron 44, No. 17 (1988); U. Schollkopf, Pure Appl. Chem.
55, 1799
to (1983); U. Hengartner et al. J. Ors. Chem., 44. 3748-3752 (1979); M. J.
O'Donnell et al. Tet.
Lets., 2641-2644 (1978); M. J. O'Donnell et al. Tet. Lets. 23, 4255-4258
(1982); M. J.
O'Donnell et al. J. Am. Chem. Soc., 1 I0, 8520-8525 ( 1988).
The specific stereoisomers of either starting materials or products can be
resolved and
recovered by techniques known in the art, such as chromatography on chiral
stationary phases,
enzymatic resolution, or fractional recrystallization of addition salts formed
by reagents used
for that purpose. Useful methods of resolving and recovering specific
stereoisomers are
known in the art and are described in Stereochemistry of Organic Compounds, E.
L. Eliel and
S. H. Wilen, Wiley (1994) and Enantiomers. Racemates. and Resolutions, J.
Jacques, A.
2o Collet, and S. H. Wilen, Wiley (1981).
As used in this application:
a) the tenor "halogen" refers to a fluorine atom, chlorine atom, bromine atom,
or iodine atom;
b) the term "C~-C~ alkyl" refers to a branched or straight chained alkyl
radical containing
from 1 to 6 carbon atoms, such as methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, t-butyl,
pentyl, hexyl, etc.;
c) the term "C,-C.~ alkyl" refers to a saturated straight or branched chain
alkyl group
containing from 1-4 carbon atoms and includes methyl, ethyl, propyl,
isopropyl, n-butyl, s-
butyl, isobutyl, and t-butyl;
SUBSTITUTE SHEET (RULE 2~
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-12-
d) the term "C,-C.~ alkoxy" refers to a straight or branched alkoxy group
containing from 1 to
4 carbon atoms, such as methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy,
isobutoxy, t-
butoxy, etc.;
e) the designation '' '~~~~'' " refers to a bond for which the stereochemistry
is not designated;
f) the designation " "'- " refers to a bond that protrudes forward out of the
plane of the
page.
g) the designation " °°°°°' " refers to a
bond that protrudes backward out of the plane of the
page.
h) as used in the examples and preparations, the following terms have the
meanings indicated:
"g" refers to grams, "mg" refers to milligrams, "pg" refers to micrograms,
"mol" refers to
moles "mmol" refers to millimoles "nmole" refers to nanomoles "L" refers to
liters "mL" or
> >
"ml" refers to milliliters, "pL" refers to microliters, "°C" refers to
degrees Celsius, "Rf' refers
to retention factor, "mp" refers to melting point, "dec" refers to
decomposition, "bp" refers to
boiling point, "mm of Hg" refers to pressure in millimeters of mercury, "cm"
refers to
centimeters, "nm" refers to nanometers, "brine" refers to a saturated aqueous
sodium chloride
2o solution, "M" refers to molar, "mM" refers to millimolar, "pM" refers to
micromolar, "nM"
refers to nanomolar, "HPLC" refers to high performance liquid chromatography,
"HRMS"
refers to high resolution mass spectrum, "DMF" refers to dimethylformamide,
"pCi" refers to
microcuries, "i.p." refers to intraperitoneally, "i.v." refers to
intravenously, and "DPM" refers
to disintegrations per minute;
i) for substituent Z, the designations -C{O)NR8-, -NRgC(O}-, -NRBC(O)NH-. -
NRgC(O)O-,
and -OC(O)NH- refer to the functionalities represented, respectively, by the
following
formulae showing the attachment of the group (Q):
SUBST'TTUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCTNS99/28234
-13-
0 0 °
Q) \ ~ . \N~Ni (Q)
N . N (Q) , I H
I ~ '
RB
Re RB
0 O
~N~°, (Q) ~d \ ~ ~ (Q)
0 N
H
Re
these designations are referred to hereinafter as amido, amide, urea, N-
carbamoyl, and
O-carbamoyl, respectively;
j) the term "pharmaceutically acceptable salts" thereof refers to either an
acid addition salt or
a basic addition salt.
The expression "pharmaceutically acceptable acid addition salts" is intended
to apply
to any non-toxic organic or inorganic acid addition salt of the base compounds
represented by
to formula (1) or any of its internlediates. Illustrative inorganic acids
which form suitable salts
include hydrochloric, hydrobromic, sulphuric, and phosphoric acid and acid
metal salts such
as sodium monohydrogen orthophosphate, and potassium hydrogen sulfate.
Illustrative
organic acids which form suitable salts include the mono-, di-, and
tricarboxylic acids.
Illustrative of such acids are for example, acetic, glycolic, lactic, pyruvic,
malonic, succinic,
15 glutaric, fumaric, malic, tartaric, citric, ascorbic, malefic,
hydroxymaleic, benzoic,
hydroxybenzoic, phenylacetic, cinnamic, salicyclic, 2-phenoxybenzoic, p-
toluenesulfonic
acid, and sulfonic acids such as methane sulfonic acid and 2-hydroxyethane
sulfonic acid.
Such salts can exist in either a hydrated or substantially anhydrous form. In
general, the acid
addition salts of these compounds are soluble in water and various hydrophilic
organic
2o solvents, and which in comparison to their free base forms, generally
demonstrate higher
melting points.
The expression "pharmaceutically acceptable basic addition salts" is intended
to apply
to any non-toxic organic or inorganic basic addition salts of the compounds
represented by
25 formula ( 1 ) or any of its intermediates. Illustrative bases which form
suitable salts include
alkali metal or alkaline-earth metal hydroxides such as sodium, potassium,
calcium,
SUBSTTTUTE SHEET (RULE Z6)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-14-
magnesium, or barium hydroxides; ammonia, and aliphatic, alicyclic, or
aromatic organic
amines such as methylamine, dimethylamine, trimethylamine, and picoline.
As with any group of structurally related compounds which possess a particular
utility,
certain groups and configurations of substituents are preferred for the
compounds of formula
( 1 ). Preferred embodiments are given below:
The compounds in which R~ is selected from the group consisting of C~-C6 alkyl
and
-(CHZ)d-Ar, are preferred;
The compounds in which R, is -(CHZ)d-Ar, are more preferred;
The compounds in which R, is -(CHZ)d-Are in which d is 1 or 2 and Ari is
phenyl are most
preferred;
Compounds in which R4 is selected from the group consisting of hydrogen, -
C(O)R~o and -SG
are preferred;
Compounds in which RQ is selected from the group consisting of -C(O)R,o and
R1o is C,_C4
alkyl more preferred;
Compounds in which A is -OH are preferred; and
Compounds in which A is -NRR' wherein R is hydrogen and R' is methyl are
preferred.
Examples of compounds encompassed by the present invention include the
following.
It is understood that the examples encompass all of the isomers of the
compound and mixtures
thereof. This list is meant to be representative only and is not intended to
limit the scope of
the invention in any way:
The compounds of formula ( 1 ) can be prepared by a variety of procedures
readily
known to those skilled in the art. Such procedures include, peptide counlin~,
such as solid
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-15-
phase sequential procedures and solution phase sequential procedures using
suitable amino
acids and substituted acids and displacement. modification, and
functionalization procedures,
as required, utilizing suitable protecting groups and deprotection procedures.
As used herein the term "amino acid" refers to naturally occurring amino acids
as well
as non-naturally occurnng amino acids having substituents encompassed by Ri
and RZ as
described above. The naturally occurring amino acids included are glycine,
alanine, valine,
leucine, isoleucine, serine, methionine, threonine, phenylalanine, tyrosine,
tryptophan,
cysteine, histidine, aspartic acid, asparagine, glutamic acid, glutamine,
arginine, ornithine, and
lysine. Non-naturally occurring amino acids within the term "amino acid,"
include without
limitation, the D-isomers of the naturally occurring amino acids, norleucine,
norvaline,
alloisoleucine; t-butylglycine, methionine sulfoxide, and methionine sulfone.
Other non-
naturally occurring amino acids within the term "amino acid," include without
limitation
phenylalanines, phenylglycines, homophenylalanines, 3-phenylpropylglycines, 4-
phenylbutylglycines; each including those substituted by R6 and R6- as
described above; and
1-naphthylalanines and 2-naphthylalanines; including those substituted by R~
and R~- as
described above.
The compounds of formula (1) can be prepared by utilizing techniques and
procedures
well known and appreciated by one of ordinary skill in the art. To illustrate,
general synthetic
schemes for preparing intermediates and the compounds of formula (1) are set
forth below. In
the reaction schemes below, the reagents and starting materials are readily
available to one of
ordinary skill in the art and all substituents are as previously defined
unless otherwise
indicated.
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-16-
Reaction Scheme A
Rz Rz
( CHz ) a gte 1 ( CHz ) a O
P
N OH N NH
I I O l I A
pgl O Hz Pgi O R1
(2 ) A,
(2b)
R1 (3a)
step 2
Rz
I
( CHz ) a O Ste 3 Rz
p ( CHz ) a
N NH ' O
I A O N NH
\\~O O R1 Y H I A
R , X O R1
3
R'
(4) 3 (3b) (2c)
In Scheme A, step 1, an appropriate protected compound of the formula (2a) is
coupled with an appropriate compound of formula (3a) to give a compound of
formula (2b).
An appropriate protected compound of the formula (2a) is one in which RZ is as
desired in the
final compound of formula ( 1 ) or gives rise after deprotection to R~ as
desired in the final
compound of formula (I), a is as desired in the final product of formula (1),
and Pg, is an
amine protecting group. In addition, an appropriate compound of formula (2a)
may also be
one in which the stereochemistry at the carboxy and -(CHZ)e RZ bearing carbons
is as desired
to in the final product of formula (I ). In Reaction Scheme A the protecting
group, Pg,, is one in
which the can be removed in the presence of the amide formed in this step. The
use and
removal of amine protecting groups is well known and appreciated in the art
and described in
Protective Groups in Ors;anic S my hesis, Theodora W. Greene (Wiley-
Interscience, 2nd
Edition, I 991 ). In Reaction Scheme A, the use of t-Boc and F-moc for Pg, is
preferred.
An appropriate compound of the formula (3a) is one in which R, is as desired
in the
final compound of formula ( 1 ) or gives rise after deprotection to R, as
desired in the final
SUBSTITUTE SHEET (RULE Z6)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-17-
compound of formula ( 1 ) and A' is -NRR' as desired in the final product of
formula ( I ) or a
protected carboxy group which gives rise to -OH as desired in the final
product of formula ( 1 ).
A' may also be an attachment to a suitable resin. Such a protected carboxy or
resin is chosen
so that it does not interfere with subsequent deprotection, displacement,
derivitivization,
functionalization, or modification reactions, as are required. The use and
removal of carboxy
protecting groups is well known and appreciated in the art and described in
Protective Groups
in Org-anic Synthesis, Theodora W. Greene (Whey-Interscience, 2nd Edition,
1991 ). In
addition, an appropriate compound of formula (3a) may also be one in which the
stereochemistry at the R, bearing carbon is as desired in the final product of
formula { 1 ).
to
Such coupling reactions are carried out by a variety of procedures readily
known to
those skilled in the art. Such procedures include, peptide coupling, such as
solid phase
sequential procedures and solution phase sequential procedures using suitable
amino acids and
substituted acids followed by displacement, modification, and
functionalization procedures, as
15 required, utilizing suitable protecting groups and deprotection procedures.
As used herein the term "amino acid" refers to naturally occurring amino acids
as well
as non-naturally occurring amino acids having substituents encompassed by Ri
and
-(CHZ)e RZ as described above. The naturally occurring amino acids included
are glycine,
20 alanine, valine, leucine, isoleucine, serine, methionine, threonine,
phenylalanine, tyrosine,
tryptophan, cysteine, histidine, aspartic acid, asparagine, glutamic acid,
glutamine, arginine,
ornithine, and lysine. Non-naturally occurring amino acids within the term
"amino acid,"
include without limitation, the D-isomers of the naturally occurring amino
acids, norleucine,
norvaline, alloisoleucine, t-butylglycine, methionine sulfoxide, and
methionine sulfone. Other
25 non-naturally occurring amino acids within the term "amino acid," include
without limitation
phenylalanines substituted by R6 as described above; phenylglycines,
homophenylalanines, 3-
phenylpropylglycines, 4-phenyIbutylglycines; including those substituted by
R~, as described
above; and 2-naphthylalanines, including those substituted by R7 as described
above. The
preparation of amino acids bearing -(CHz)e-Rz are knkown in the art and
described herein.
Solid phase sequential procedures can be performed using established methods,
including automated methods such as by use of an automated peptide
synthesizer. Steward
and Young, Solid Phase Peptide Synthesis (Freeman 1969) and B. Merrifield,
Peptides:
Smthesis, Structures, and Applications (B. Gutte, Ed., Acedemic Press 1995).
In this
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-18-
procedure a protected amino acid bearing R, or protected R, is bound to a
resin support. The
resin support employed can be any suitable resin conventionally employed in
the art for the
solid phase preparation of poly-peptides, preferably polystyrene which has
been crossed away
with about 0.5 to about 3 percent divinyl benzene, which has been either in
chloromethylated
or hydroxymethylated to provide sites for ester formation with the initially
introduced
protected amino acid. Suitable resins are well known and appreciated in the
art, including
those described in Rink, Tet. Let., 28, 3787 (1987) and Sieber, Tet. Let., 28,
2107 (1987).
Included within the solid phase methods are combinatorial methods which are
known in the
art. K. S. Lam, Chem. Rev., 97, 411-448 (1997).
In a subsequent step the resin-bound protected amino acid bearing R, is
sequentially
amino deprotected and coupled with a protected amino acids bearing -(CHZ)e-Rz
to give a
resin-bound protected dipeptide. This resin bound protected dipeptide is
sequentially amino
deprotected and coupled with a protected amino acid bearing R3 or protected R3
to give a
is protected tripeptide. Alternately, an appropriate protected dipeptide may
be coupled by the
solution method prior to coupling with the resin-bound amino acid.
Each protected amino acids or amino acid sequence is introduced into the solid
phase
reactor and about a two-fold to four-fold excess. The coupling is carried out
in a suitable
2o medium, for example dimethylformamide, dichloromethane, or mixtures of
dimethylformamide and dichloromethane. As is well known and appreciated in the
art,
wherein complete coupling occurs, the coupling in procedure is repeated before
removal of
the protecting group, prior to the coupling of the next amino acids in the
solid phase reactor.
25 After the compound of formula (1 ) or protected compound of formula (1 )
has been
obtained it is removed from the resin under conditions well known in the art
which are
appropriate for the resin selected.
Compounds of formula ( 1 ) obtained by solid phase sequential procedures can
be
3o purified by procedures well known and appreciated in the art, such as
chromatography,
lyophilzation, trituration, salt formation, and crystallization.
The compounds of formula (1) can also be prepared by solution phase sequential
procedures well known and appreciated in the art. Accordin~lv. suitably
protected amino
SUBSTITZJZ'E SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/Z8234
-19-
acids, substituted acids or dipeptides are coupled by procedures requiring
activation of the
carbonyl group and coupling reaction with amine function of an appropriate
protected amino
acid or dipeptide. These procedures are well known appreciated in the art.
The selection of an appropriate coupling reagent is within the skill of the
art.
Particularly suitable coupling reagents include N-((dimethylamino)-IH-1,2,3-
triazolo[4,5-
b]pyridin-1-ylmethylene)-N-methylmethanaminium hexafluororphosphate N-oxide
{HATU),
I-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 1-hydroxy-
benzotriazole
or N,N'-diisopropylcarbodiimide and 1-hydroxy-benzotriazole. Other coupling
agents are
l0 pyridine benzotriazol-1-yloxytris(dimethylamino)phosphonium
hexafluorophosphate complex
carbodiimides (e.g., N,N'-dicyclohexylcarbodiimide); cyanamides (e.g.; N,N-
dibenzylcyanamide); {3b) ketenimines; isoxazolium salts (e.g., N-ethyl-5-
phenyl-isoxazolium-
3'-sulfonate; monocyclic nitrogen containing heterocyclic amides of aromatic
character
containing one through four nitrogens in the ring such as imidazolides,
pyrazolides, and 1,2,4-
15 triazolides. Specific heterocyclic amides that are useful include N,N'-
carbonyldiimidazole and
N,N-carbonyl- di-1,2,4-triazole; alkoxylated acetylene (e.g.,
ethoxyacetylene); reagents
which form a mixed anhydride with the carboxyl moiety of the amino acid (e.g.,
ethylchloroformate and isobutylchloroformate). Other activating reagents and
their use in
peptide coupling are described by Kapoor, J. Pharm. Sci., 59, 1-27 (1970).
Such coupling reactions to form amides are carried out in suitable solvents,
such as
dichloromethane, tetrahydrofuran, diethyl ether, chloroform, and the like, and
using suitable
bases, such as triethylamine, N-methylmorpholine, N,N-disopropylethylamine,
pyridine, and
the like, and coupling reagents, as required, and are well known and
appreciated in the art.
The reactions are generally carried out at -10°C to the refluxing
temperature of the solvent and
generally require form 1 hour to 2 days. The product can be isolated and
purified by
techniques well known in the art, such as extraction, evaporation,
trituration, lyophilization,
chromatography, and recrystallization.
3o In Reaction Scheme A, step 2, the amine protecting group, Pg,, of the
compound of
formula (2b) is selectively removed to give the compound of fotTnula (2c).
Such selective
amine deprotection reactions are well known and appreciated in the art. The
product can be
isolated and purified by techniques well known in the art, such as extraction,
evaporation, salt
formation. trituration, lvovhilization. chromatoeranhv. and recrvstaltization.
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCTNS99/28234
-2a-
In Reaction Scheme A, step 3, a compound of formula (2c) coupled with an
appropriate acid derivative bearing R3~ and Y (compound of formula (3b)) to
give a compound
of formula (4). Such coupling reactions are well known and appreciated in the
art and
discussed above. The product can be isolated and purified by techniques well
known in the art
such as extraction, evaporation, salt formation, trituration, lyophilization,
chromatography,
and recrystallization.
An appropriate compound of formula (3b) is one in which R3- is R3 as desired
in the
1o final product of formula (1) or gives rise after deprotection to R3 as
desired in the final product
of formula ( 1 ) and Y is a protected thio substituent or Y may be a protected
hydroxy
substituent or bromo which gives rise upon selective deprotection and
displacement or
displacement and further deprotection and/or elaboration, if required, to -SR4
as desired in the
final product of formula ( 1 ). Alternately, an appropriate compound of
formula (3b) may also
15 be one in which R3~ gives rise to R3~~ which, upon derivatization, gives
rise R3 as desired in the
final product of formula (1) and Y is a protected thin substituent. In
addition, an appropriate
compound of formula (3b) may also be one in which the stereochemistry at the
R3~ and Y
bearing carbon is as desired in the final product of formula ( 1 ) or gives
rise after displacement
to the stereochemistry as desired at that carbon in the final product of
formula ( 1 ). The
2o activating group (A) is one which undergoes an amidation reaction. As is
well known in the
art an amidation reaction may proceed through an acid, X is -OH; or an acid
may be first
converted to an acid chloride, X is -Cl; or an activated intermediate; such as
an anhydride;
a mixed anhydride of aliphatic carboxylic acid, such as formic acid, acetic
acid, propionic
acid, butyric acid, isobutyric acid, pivalic acid, 2-ethylbutyric acid,
trichloroacetic acid,
25 trifluoroacetic acid, and the like; of aromatic carboxylic acids, such as
benzoic acid and the
like; of an activated ester, such as phenol ester, p-nitrophenol ester, 2,4-
dinitrophenol ester,
pentafluorophenol ester, pentachlorophenol ester, N-hydroxysuccinimide ester,
N-
hydroxyphthalimide ester, 1-hydroxy-1 H-benztriazole ester, and the like;
activated amide,
such as imidazole, dimethyipyrazole, triazole, or tetrazole; or an
intermediate formed in the
3o presence of coupling agents, such as dicyclohexylcarbodiimide or 1-(3-
dimethyaminopropyl)-
3- ethylcarbodiimide. Acid chlorides and activated intermediates may be
prepared but are not
necessarily isolated before the addition of a compound of formula (3b).
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCTNS99/28234
-21-
The use and selection of appropriate protecting groups is within the ability
of those
skilled in the art and will depend upon compound of formula (3b) to be
protected, the presence
of other protected amino acid residues, other protecting groups, and the
nature of the
particular R3 and/or R~ group(s) ultimately being introduced. Compounds of
formula (3b) in
which Y is bromo and protected thio are commercially available or can be
prepared utilizing
materials, techniques, and procedures well known and appreciated by one of
ordinary skill in
the art or described herein. See PCT Application WO 96/11209, published 18
April 1996.
Examples commercially available compounds of formula (3b) in which Y is bromo
include 2-
bromopropionic acid, 2-bromobutyric acid, 2-bromovaleric acid, 2-bromohexanoic
acid, 6-
(benzoylamino)-2-bromohexanoic acid, 2-bromoheptanoic acid, 2-bromooctanoic
acid, 2-
bromo-3-methylbutyric acid, 2-bromoisocaproic acid, 2-bromo-3-(5-
imidazoyl)proionic acid,
(R)-(+)-2-bromopropionic acid, (S)-(-)-2-bromopropionic acid.
In Reaction Scheme B a final product of formula (1) is prepared from a
compound of
formula (4) (prepared as described in Reaction Scheme A) in which R3~ is R3 as
desired in the
final product of formula (1) or gives rise after deprotection to R; as desired
in the final product
of formula ( 1 ) and Y is a protected thio substituent or hydroxy or bromo.
Reaction Scheme B
Rz Rz
(CHz) a (CHz) a
step 1
N NH A. N NH A
y ..~~~~\\ ~ HS
\\~O O R1 \~O O R1
Ra (4) Ra (5)
step 3 step 2
Rz
I
( CHz ) a
O
N NH
A
R4 ~~~ O R1
O
R3
(formula ( 1 ) or
protected formula ( 1 ))
SUBSTITUTE SHEET (RULE 26~
CA 02356969 2001-06-27
WO 00/40553 PCT/US99128234
-22-
In Reaction Scheme B, step 1, a compound of formula (4) in which Y is
protected thio
gives rise upon selective deprotection to give a compound of formula (5).
For example, compounds of formula (4) in which Y is a protected thio
substituents are
selectively deprotected to give a thiol of formula (5). Protected thio
substituents include
thioesters, such as thioacetyl or thiobenzoyl, thioethers, such as thiobenzyl,
thio-4-
methoxybenzyl, thiotriphenylmethyl, or thio-t-butyl, or unsymmetrical
sulfides, such as
dithioethyl or dithio-t-butyl. The use and selective removal of such thio
protecting groups is
well known and appreciated in the art and described in Protective Groups in
Oceanic
t0 S tin hesis, Theodora W. Greene (Wiley-Interscience, 2nd Edition, 1991).
In Reaction Scheme B, step 2, a compound of formula (S) undergoes modification
reaction to give a compound of formula (6). Such modification reactions
include, thiol
esterification and disulfide formation.
Compounds of formula (6) in which R4 is -C(O)R,o or -C(O)-(CHZ)q-X group can
be
synthesized by thiol esterifications according to techniques well known and
appreciated by
one of ordinary skill in the art, such as those disclosed in U.S. Pat. Nos.
5,424,425, issued Jun.
13, 1995.
For example, in a thiol esterification a compound of formula (5) is contacted
with
about an equimolar amount of an appropriate acid, such as HO-C(O)R~o or
HO-C(O)-(CHZ)q-K in the presence of a suitable coupling agent to give a
compound of
formula (6) in which R4 is -C(O)R~o or -C(O)-(CH2)q-K. The reaction is carried
out in the
presence of a coupling agent such as 2-fluoro-1-methylpyridinium p-
toluenesulfate, 1-(3-
dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, carbonyldiimidazole, 1-
ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline, or diethylcyanophosphonate in a
suitable
aprotic solvent such as methylene chloride. The reaction is generally carned
out at
temperature of between -20°C and the boiling point of the solvent.
Generally, the reaction
requires 1 to 24 hours. The product can be isolated and purified by techniques
well known in
the art, such as extraction, evaporation, trituration, lyophilization,
chromatography, and
recrystallization.
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-23-
Compounds of formula (6) in which R.~ is -S-G group can be synthesized
according to
techniques well known and appreciated by one of ordinary skill in the art, as
disclosed in PCT
Application No. WO 95/21839, published 17 August 1995 and U.S. Patent Nos.
5,491,143,
issued February 13, 1996, and 5,731,306, issued March 24, 1998, and Roques,
B.P. et al., J.
Med. Chem. 33, 2473-2481 ( 1992).
For example, in a disulfide formation a compound of formula (5) is contacted
with an
appropriate compound of formula (7).
y
G~S~S N
(7)
A.n appropriate eompound-of formula (7) is one which gives G as desired in the
final
product of formula ( 1 ) or gives rise upon deprotection to G as is desired in
the final product of
formula (1). In addition, the compound of formula (7} may have stereochemistry
as desired in
the final product of formula (1). The reaction is carried out in a suitable
solvent, such as
ethanol, methanol, dichloromethane, or mixtures of ethanol or methanol and
dichloromethane.
15 The solvent is degassed by passing a stream of nitrogen gas through it for
15 minutes before
the reaction is carried out. The reaction is carried out using from 1.0 to 4.0
molar equivalents
of an appropriate compound of formula (7). The reaction is carned out at
temperatures of
from 0°C to the refluxing temperature of the solvent, with a
temperature of 10°C to 30°C
being preferred. The reaction generally requires from 1 to 48 hours. The
product can be
2o isolated by techniques well known in the art, such as extraction,
evaporation, and precipitation
and can be purified by chromatography and recrystallization.
In Reaction Scheme B, step 3, a compound of formula (4) in which Y is hydroxy
or
bromo can be displaced by an appropriate thiol, HSR4, to give a compound of
formula (1) or a
25 protected compound of formula ( 1 ). In Reaction Scheme B, step 3, an
appropriate thiol HSR.
is one which gives R4 as desired in the final product of formula (1) or gives
rise to R.~ as
desired in the final product of formula ( 1 ).
In Reaction Scheme B, step 3, a compound of formula (4) in which Y is hydroxy
30 (obtained from protected hydroxy compounds of formula (4)) undergoes a
displacement
SUBS11TUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99l28234
-24-
reaction with an appropriate thio introducing reagent by the method of
Mitsunobu to give a
compound of formula (4) in which Y is a protected thio substituent or
-SR., as desired in the final compound of formula (1 ). For example, a
compound of formula
(4) in which Y is hydroxy reacts with thioacetic acid or thiobenzoic acid,
triphenylphosphine,
and diethylazodicarboxylate in a suitable aprotic solvent, such as
tetrahydrofuran to give a
compound of formula (4) in which Y is thioacetyl or thiobenzoyl. Selective
removal of the
thioacetic acid or thiobenzoic acid moiety gives the desired compound of
formula (5). The
product can be isolated and purified by techniques well known in the art, such
as extraction,
evaporation, trituration, lyophilization, chromatography, and
recrystallization.
to
Also, in Reaction Scheme B, step 3, a compound of formula (4) in which Y is
bromo
undergo a displacement reaction with an appropriate thio introducing reagent
to give a
compound of formula (4) in which Y is protected thio substituent which gives
rise upon,
deprotection and subsequent elaboration, if desired, the -SR4 as desired in
the final compound
t5 of formula (1). An appropriate thio introducing reagent is also one which
introduces a group
-SR.~ as desired in the final compound of formula ( 1 ).
Far example, a solution of p-methoxybenzylmercaptan in a suitable organic
solvent
such as dimethylformamide is degassed and treated with a suitable base such as
sodium
20 hydride, sodium hydroxide, or cesium carbonate. After about 1 to 2 hours, a
solution of a
compound of formula (4) in which Y is bromo is added. The reaction may benefit
from the
addition of a suitable catalyst; such as tetra-n-butylammonium iodide. The
reaction mixture
is carried out for 1 to 25 hours at temperatures ranging form 0°C to
about 100°C. Selective
removal of the 4-methoxybenzyl moiety gives the desired compound of formula (
1 ). The
25 product can be isolated and purified by techniques well known in the art,
such as extraction,
evaporation, trituration, lyophilization, chromatography, and
recrystallization.
In addition, in Reaction Scheme B, step 3, a compound of formula (4) in which
Y is
bromo can be displaced by an appropriate thio ester, Ph3S-C(O)-(GH~)q-X by
techniques well
30 known and appreciated in the art, as disclosed in U.S. Pat. No. 5,424,425,
issued Jun. 13,
1995.
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCTNS99/28234
-25-
In Reaction Scheme B, in an optional step, a protected compound of formula (1
) is
deprotected to give a compound of formula (1 ). Such deprotection reactions
are well known
appreciated in the art and may include selective deprotections.
In Reaction Scheme C a final product of formula ( 1 ) is prepared from a
compound of
formula (4) (prepared as described in Reaction Scheme A) in which R3- gives
rise to R3-~ and Y
is -SR4 as is desired in the final product of formula ( 1 } or a protected
thio substituent gives a
compound of formula (I).
Reaction Scheme C
Rz Rz
(CHz) a O (CHz) a O
N NH A' step 1 N NH A
y ,~ I y ~(I I
\\\G O O Rl \\ ~G O O Rl
R3 ~ (4) R3 ~~ (4a)
step 2
Rz
I
( CHz ) a O Rz
(CHz) a
N NH ~ step 3
HS I A - N NH A.
O R1 y ~
O ,E~\'~ O R1
R \\~O
s $ R3 (4
Step 4
step 5
Rz
I
(CHz) a
O
N NH
( A'
S
R4 ~~~ O R~
O
R3
(formula ( 1 ) or
protected formula ( 1 ))
to In Reaction Scheme C, step l, an appropriate compound of formula (4) is
deprotected,
hydrolyzed, or reduced to give a compound of formula (4a). In Reaction Scheme
C, step 1, an
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-26-
appropriate compound of formula (4) is one in which R;~ gives rise to a
compound of formula
(4a) in which R;w undergoes further derivitization (step 2) to give a compound
of formula (4)
in which R: is -(CHz)m-NRg~-SOz-Y~ or -(CHz)m-Z-Q as desired in the final
product of
formula ( 1 ). In Reaction Scheme C, step 1, an appropriate compound of
formula (4) is one in
which Y is -SR.~ as desired in the final compound of formula ( 1 ) or Y is
protected thio which
gives rise upon deprotection or deprotection and further functionalization to
give
-SR4, as desired, in the final product of formula ( 1 ) as described in
Reaction Scheme B, step 2,
above.
l0 For example, in a deprotection a compound of formula (4) in which R3~ is -
(CHz)m-W
(W is a phthalimido group) is contacted with a molar excess of hydrazine
monohydrate to give
a compound of formula (4a) in which R3- is -(CHz)m NHR$ in which R8 is
hydrogen. The
reaction is typically carned out in a protic organic solvent, such as methanol
or ethanol. The
reaction is generally carned out at room temperature for a period of time
ranging from 5-24
15 hours. The product can be isolated by techniques well known in the art,
such as extraction,
evaporation, and precipitation and can be purified by chromatography and
recrystallization.
Alternately, for example, in a deprotection a compound of formula {4) in which
R3~ is
-(CHz)m NRg-t-Boc is contacted with a molar excess of a suitable acid to give
a compound of
20 formula (4a) in which R3~~ is -(CHz)m-NHRg. The reaction is typically
carned out in a organic
solvent, such as methanol, ethanol, ethyl acetate, diethyl ether, or dioxane.
Suitable acids for
this reaction are well known in the art, including hydrochloric acid,
hydrobromic acid,
trifluoroacetic acid, and methanesulfonic acid. The reaction is generally
carned out at room
temperature for a period of time ranging from 1-10 hours. The product can be
isolated by
25 techniques well known in the art, such as extraction, evaporation, and
precipitation and can be
purified by chromatography and recrystallization.
For example, in a hydrolysis a compound of formula (4) in which R3' is
-(CHz)m C(O)OPg~ and Pg~ is methyl or ethyl is contacted with about 1 to 2
molar equivalents
30 of lithium hydroxide, sodium hydroxide, or potassium hydroxide, to give a
compound of
formula (4a) in which R;~- is -(CHz)m COzH. The reaction is carried out in a
suitable solvent,
such as methanol, ethanol methanol/water mixtures, ethanol/water mixtures, or
tetrahydrofuran/water mixtures and generally requires 1 to 24 hours. The
reaction is carried
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/Z8234
-27-
out at temperatures of from about 0°C to the refluxing temperature of
the solvent. The
resulting acid is isolated and purified by techniques well known in the art,
such as
acidification, extraction, evaporation, and precipitation and can be purified
by trituration,
precipitation, chromatography, and recrystallization.
For example, in a reduction a compound of formula (4a) in which R3~ is
-(CH2)"~,-COZPg3 in which Pg3 is methyl or ethyl is contacted with a suitable
reducing agent,
such as lithium borohydride, diisobutylaluminum hydride, 9-
borabicyclo[3.3.1]nonane,
preferably lithium borohydride to provide a compound of formula (4a) in which
R3- is -
lo (CH2)m_,-CH~OH. The reaction is carried out in a suitable solvent, such as
dichlorornethane,
tetrahydrofuran, or toluene, with tetrahydrofuran being preferred. The
reaction is carned out
at a temperature of from about -30°C to about 50°C and generally
requires from 2 to 12 hours.
The product can be isolated by quenching, extraction, evaporation, and
precipitation and can
be purified by trituration, chromatography, and recrystallization.
In Reaction Scheme C, step 2, a compound of formula (4a) undergoes a
derivitization
reaction to give a compound of formula (5) in which R3 is as desired in the
final product of
formula (1). Such derivitization reactions include hydrolysis of esters and
ester formations as
are well known in the art, ether formation, amine aIkylation, formation of
amides, urea
2o formation, carbamate formation, and formation of sulfonamide. In Reaction
Scheme C, step
2, the compound of formula (4a) is one in which Y is a protected thio group,
such as
thioacetyl, thiobenzoyl, 4-methoxybenzyl thiol or t-butylthiol.
For example, in an ether formation a compound of formula (4a) in which R3- is
-(CHZ)m_i-CHZOH is contacted with 1 to 10 molar equivalents of a suitable
alkylating agent to
give a compound of formula (5) in which R3 is -(CH2)m Z-Q in which Z is -O-. A
suitable
alkylating agent is one which transfers Q or protected Q as desired in the
final product of
formula (I), such as benzyl bromide, benzyl chloride, substituted benzyl
bromide, substituted
benzyl chloride, ethyl bromoacetate, t-butyl bromoaceate, ethyl 3-
chloropropionate, ethyl 3-
3o bromopropionate, ethyl 5-bromovalerate, ethyl 4-bromobutyrate, 3-
chloropropionamide, 2-
bromoethylbenzene, substituted 2-bromoethylbenzene, 1-chloro-3-phenylpropane,
1-bromo-4-
phenylbutane, and the like, or nitrogen mustards, including 2-
dimethylaminoethyl chloride, 2-
diethylaminoethyl chloride, and 3-dimethylaminopropyl chloride. The reaction
is carried out
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
_28_
in a suitable solvent, such as diethyl ether, tetrahydrofuran,
dimethylformamide, dimethyl
sulfoxide, or acetonitrile and using a suitable base, such as sodium hydride,
potassium
hydride, potassium t-butoxide, and lithium diisopropylamide. The reaction is
generally carned
out at temperatures of -70°C and room temperature and require from
about 1-24 hours. The
product can be isolated by techniques well known in the art, such as
extraction, evaporation,
and precipitation and can be purified by chromatography and recrystallization.
Alternately, as appreciated by those skilled in the art, an ether formation
can also be
carried out by a procedure similar to the one above using a compound of
formula (4a) in
which R3w is -(CH2)m_,-CHZOH in which the hydroxy group is first converted to
a leaving
group, such as chloro, bromo, or mesylate and a suitable alcohol which
transfers Q or
protected Q as desired in the final product of formula ( 1 ), such as benzyl
alcohol, substituted
benzyl alcohol, phenol, substituted phenol, and the like. The conversion of
hydroxy to leaving
groups, such as chloro, bromo, and mesylate are well known and appreciated in
the art.
For example, in an amine alkylation a compound of formula (4a) in which R3- is
-(CHZ)m NHRB is contacted with 1 to 10 molar equivalents of a suitable
alkylating agent to
give a compound of formula (5) in which R3 is -(CHZ)m-Z-Q in which Z is -NR8-.
The
reaction may be carried out after protection of the amine function of R3~- in
which R8 is
2o hydrogen by a suitable protecting group, such as benzyl or t-Boc. For an
amine alkylation a
suitable alkylating agent is one as described above for the ether fonmation
and also includes
alkylhalides, such as methyl iodide, methyl bromide, ethyl bromide, propyl
bromide, propyl
chloride, butyl bromide, butyl chloride, and the like. The reaction is carned
out in a suitable
solvent, such as methanol, ethanol, dimethylformamide, or pyridine and using a
suitable base,
such as sodium carbonate, triethylamine, N,N-diisopropylethylamine or
pyridine. The
reaction is generally carried out at temperatures of room temperature to the
refluxing
temperature of the solvent and require from about 1-24 hours. The product can
be isolated by
techniques well known in the art, such as extraction, evaporation, and
precipitation and can be
purified by chromatography and recrystallization.
Alternately, for example, in an amine alkylation a compound of formula (4a) in
which R3» is -(CHZ)m NHRR is contacted in a reductive alkylation with a
suitable aldehyde
to give a compound of formula (5) in which R3 is -(CHZ)"; Z-Q in which Z is -
NRg-. A
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-29-
suitable aldehyde is one which transfers Q or protected' Q as desired in the
final product of
formula ( 1 ), such as benzaldehyde and substituted benzaldehydes. The
reaction is carried
out in a suitable solvent, such as methanol, ethanol, tetrahydrofuran, or
mixtures of
methanol or ethanol and tetrahydrofuran. The reaction may be carried out in
the presence
of a drying agent, such as sodium sulfate or molecular sieves. The reaction is
carried out in
the presence of from 1.0 to 6.0 molar equivalents of a suitable reducing
agent, such as,
sodium borohydride or sodium cyanoborohydride with sodium cyanoborohydride
being
preferred. It may be advantageous to maintain the pH in the range of about 4
to 6. The
reaction is generally carried out at temperatures of from 0°C to the
refluxing temperature of
to the solvent. Generally, the reactions require 1 to 72 hours. The product
can be isolated by
techniques well known in the art, such as extraction, evaporation, and
precipitation and can
be purified by chromatography and recrystallization.
For example, in an amido formation a compound of formula (4a) in which R3~~ is
is -(CHz)m-COZH is contacted with a suitable amine in an amide formation to
give a
compound of formula (5) in which R3 is -(CH2)m-Z-Q in which Z is amido. Such
amide
formation reactions using carboxy activation or suitable coupling agents are
well known in the
art and described above. A suitable amine, HNRBQ, gives rise to RR and Q as
desired in the
final product of formula (1), such as methylamine, ethylamine, propylamine,
butylamine, N-
2o methyl benzylamine, benzyl ~i-alanine, 4-(3-aminopropyl)morpholine, and the
like.
For example, in an amide formation a compound of formula (4a) in which R3~~ is
is -(CHz)m NHRB is contacted with a suitable carboxylic acid in an amide
formation to give a
compound of formula (5) in which R3 is -(CHZ)m-Z-Q in which Z is amide. Such
amide
formation reactions using carboxy activation or suitable coupling agents are
well known in the
art and described above. Suitable carboxylic acids, QC(O)-OH, are ones give
rise to Q as
desired in the final product of formula ( 1 ), such as benzoic acid,
substituted benzoic acids,
phenyl acetic acids, substituted phenylacetic acids, mono-t-butyl malonate,
and the like.
3o For example, in a urea formation a compound of formula (4a} in which R3w is
is -(CHZ)m NHRB is contacted with an appropriate isocyanate, O=C=N-Q, to give
a compound
of fomlula (5) in which R~ is -(CHZ)m-Z-Q in which Z is urea. An appropriate
isocyanate is
one which gives rise to Q as desired in the final product, such as phenyl
isocyanate,
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-30-
substituted phenyl isocyanate, napthyl isocyanate, ethyl isocyanatoacetate,
and the like. The
reaction is carned out by adding an equivalent of, or a slight molar excess
of, an appropriate
isocyanate is added to a solution of a compound of formula (4a) in which R3~~
is -(CHZ)m-
NHRg in a suitable solvent, such as diethyl ether, benzene, or toluene. The
reaction is carried
out at temperature of from about 0°C to the refluxing temperature of
the solvent and require
about 1-24 hours. The product can be isolated and purified by techniques well
known in the
art, such as filtration, extraction, evaporation, trituration, chromatography,
and
recrystallization.
For example, in an N-carbamoyl formation a compound of formula (4a) in which
R3..
is -(CHZ)m-NHRg is contacted with an appropriate chloroformate to give a
compound of
formula (5) in which R3 is -(CH2)m Z-Q in which Z is N-carbamoyl. An
appropriate
chloroformate is one which gives rise to Q as desired in the final product of
formula ( 1 ).
Examples of chloroformates include benzyl chloroformate, naphthyl
chloroformate, phenyl
chloroformate, and substituted phenyl chloroformates, such as 4-chlorophenyl
chloroformate,
4-methylphenyl chloroformate, 4-bromophenyl chloroformate, 4-fluorophenyl
chloroformate,
4-methoxyphenyl chlorofotrnate and the like. The reaction is carried out by
adding an
equivalent of, or a slight molar excess of, an appropriate chloro formate to a
solution of a
compound of formula (4a) in which R3- is -(CHZ)m NHRg in a suitable solvent,
such as
toluene, tetrahydrofuran, dimethylformamide, dichloromethane, pyridine, or
chloroform. The
reaction is carried out in the presence of an excess of a suitable base, such
as triethylamine,
sodium carbonate, potassium bicarbonate, pyridine or N,N-
diisopropylethylamine. The
reaction is carned out at a temperature of fram -70°C to the refluxing
temperature of the
solvent and generally requires from 30 minutes to 24 hours. The product can be
isolated and
purified by techniques well known in the art, such as extraction, evaporation,
chromatography,
and recrystallization.
For example, in an O-carbamoyl formation a compound of formula (4a) in which
R3~
is -(CH~)~,_~-CH~OH is contacted with an appropriate isocyanate, as defined
above for urea
formation, to give a compound of formula (5) in which R3 is -(CHZ)m Z-Q in
which Z is O-
carbamoyl. The reaction is carried out in a suitable solvent, such as diethyl
ether,
tetrahydrofuran, dimethylformamide, or acetonitrile. The reaction may be
facilitated by the
use of catalytic amount of a suitable base, such as sodium hydride, potassium
hydride, or
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCTNS99/28234
-31-
potassium t-butoxide. The reaction is generally carried out at temperatures of
from -20°C to
room temperature and require from about 1-24 hours. The product can be
isolated by
techniques well known in the art, such as extraction, evaporation, and
precipitation and can be
purified by chromatography and recrystallization.
For example, in a sulfonamide formation to prepare a compound in which R3 is
-(CHa)m SO~NRB-Y,, a compound of formula (4a} in which R3w is -(CH2)m NHRB is
contacted
with an appropriate sulfonamide forming reagent. An appropriate sulfonamide
forming
reagent, such as a sulfonyl chloride, YiS(O)ZCI, or sulfonyl anhydride,
Y,(O)ZS-O-S(O)Z Y,,
is one which gives rise to Y~ as desired in the final product. Examples of
appropriate
sulfonamide forming reagents are, benzenesulfonyl chloride, 1-
napthalenesulfonyl chloride, 2-
napthalenesuIfonyl chloride, dansyl chloride, N-morpholinylsulfonyl chloride,
N-
piperidinylsulfonyl chloride, 2,4,5-trichlorobenzenesulfonyl chloride, 2,5-
dichlorobenzenesulfonyl chloride, 2,4,6-triisopropylbenzenesulfonyl chloride,
2-
mesitylenesulfonyl chloride, 4-bromobenzenesulfonyl chloride, 4-
fluorobenzenesulfonyl
chloride, 4-chlorobenzenesulfonyl chloride, 4-methoxybenzenesulfonyl chloride,
4-t-
butylbenzenesulfonyl chloride, p-toluenesulfonyl chloride, 2,3,4-
trichlorobenzenesulfonyl
chloride, 2,5-dimethoxybenzenesulfonyl chloride, 4-ethylbenzenesulfonyl
chloride, 3,4-
dimethoxybenzenesulfonyl chloride, 2,6-dichlorobenzenesulfonyl chloride, 3-
bromobenzenesulfonyl chloride, 4-n-butylbenzenesulfonyl chloride,
benzenesulfonic
anhydride. 4-toluenesulfonic anhydride, and 2-mesitylenesulfonic anhydride.
The reaction is
carried out in a suitable solvent, such as tetrahydrofuran, dichloromethane,
pyridine, or
chloroform and in the presence of an excess of a suitable base, such as
triethylamine, sodium
carbonate, pyridine, or N,N-diisopropylethylamine. The reaction is carried out
at a
temperature of from -50°C to the refluxing temperature of the solvent.
The reaction generally
requires from 30 minutes to 24 hours. The product can be isolated and purified
by techniques
well known in the art, such as extraction, evaporation, chromatography, and
recrystallization.
In Reaction Scheme C, step 3, a compound of formula (5} in which R3 is as
desired in
the final product of formula (1) undergoes a selective thiol deprotection to
give a compound of
formula (5). Such selective thiol deprotections using suitable protecting
groups are well
known and appreciated in the art as discussed in Reaction Scheme B, step 1,
above.
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-32-
In Reaction Scheme C, step 4, a compound of formula (5) undergoes a
modification
reaction to give a compound of formula ( 1 ) or protected compound of formula
( I ) as described
in Reaction Scheme B, step 2, above.
In Reaction Scheme C, step 5, a compound of formula (4) in which Y is
protected thin
is deprotected to give a compound of formula ( 1 ) or to a protected compound
of formula ( 1 ).
In Reaction Scheme C, in an optional step, a protected compound of formula ( 1
) is
deprotected to give a compound of formula ( 1 ). Such deprotection reactions
are well known
1o appreciated in the art and may include selective deprotections.
Alternate routes for preparing the compounds of formula (3b) in which Y is
bromo
are presented in Reaction Schemes F.1 and F.2.
Reaction Scheme F.1
0 0
Ra R
3,
OH - ~--~1 OH
NH2 Br
(8) ((3) in which Y1
is bromo and X is -OH)
15 In Reaction Scheme F.1, an appropriate a-amino carboxylic acid of formula
(8) is
deaminobrominated to give a compound of formula (3b) in which Y is bromo and X
is -OH.
An appropriate a-amino carboxylic acid of formula (8), and protected forms
thereof, is one
which is one in which R3~ is R3 as desired in the final product of formula ( 1
) or gives rise after
deprotection to R3 as desired in the final product of formula ( 1 ) In
addition, a-amino
20 carboxylic acid of formula (8) may also be one in which the stereochemistry
at the R3' bearing
carbon gives rise after displacement to the stereochemistry as desired at that
carbon in the
final product of formula ( 1 ). Such appropriate a-amino carboxylic acid of
formula (8), are
commercially available or may be readily prepared by techniques and procedures
well known
and appreciated by one of ordinary skill in the art. For example, L-alanine, D-
alanine, L-
25 valine, D-valine, D-norvaline, L-leucine, D-leucine, D-isoleucine, D-tert-
leucine, glycine, L-
glutamic acid, D-glutamic acid, L-glutamine, D-glutamine, L-lysine, D-lysine,
L-ornithine, D-
ornithine, (D)-(-)-2-aminobutyric acid. D-threonine, D-homoserine, D-
allothreonine, D-serine,
D-2-aminoadipic acid, D-aspartic acid, D-glutamic acid, D-lysine hydrate, 2,3-
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-33-
diaminopropionic acid monohydrobromide, D-ornithine hydrochloride, D,L-2,4-
diaminobutyric acid dihydrochloride, L-meta-tyrosine, D-4-
hydroxyphenylglycine, D-
tyrosine, L-phenylalanine, D-phenylalanine, D,L-2-fluorophenylalanine, beta-
methyl-D,L-
phenylalanine hydrochloride, D,L-3-fluorophenylaIanine, 4-bromo-D,L-
phenylalanine, L-
phenylalanine, L-phenylglycine, D-phenylglycine, D,L-4-fluorophenylalanine, 4-
iodo-D-
phenylalanine, D-homophenylalanine, D,L-2-fluorophenylglycine, D,L-4-
chlorophenylalanine, and the like, are ail commercially available and the
methods in D. A.
Evans, et al. J. Am. Chem. Soc., 112, 4011-4030 (1990); S. Ikegami et al.
Tetrahedron, 44,
5333-5342 (1988); W. Oppolzer et a1. Tet. Lets. 30, 6009-6010 (1989);
Synthesis of Optically
1o Active a-Amino-Acids, R. M. Williams (Pergamon Press, Oxford 1989); M. J.
O'Donnell ed.:
a-Amino-Acid Synthesis, Tetrahedron Symposia in print, No. 33, Tetrahedron 44,
No. 17
(1988); U. Schollkopf; PureAppl. Chem. 55, 1799 (1983); U. Hengartner et al.
J. Ors. Chem.,
44, 3748-3752 (1979); M. J. O'Donnell et al. Tet. Lets., 2641-2644 (1978); M.
J. O'Donnell et
al. Tet. Lets. 23, 4255-4258 (1982); M. J. O'Donnell et al. J. Am. Chem. Soc.,
I 10, 8520-
is 8525 (1988).
The deaminobromination described in Reaction Scheme F.1 can be performed
utilizing
conditions described in Compagnone, R.S. and Rapoport, H., J. Ors. Chem., 51,
1713-1719
(1986); U.S. Pat. No. 5;322,942, issued June 21, 1994; Overberger, C.G. and
Cho, L, J. Ora.
2o Chem., 33, 3321-3322 (1968); or Pfister, K. et al., J. Am. Chem. Soc., 71,
1096-1100 (1949).
For example, an a-amino carboxylic acid of formula (8) and a suitable bromide,
such
as hydrogen bromide or potassium bromide in acidic solution, such as sulfuric
acid, is treated
with sodium nitrite. The reaction temperature is carned out at temperatures of
from about
25 25°C to about ambient temperature and require about 1 to 5 hours.
The product can be isolated
and purified by techniques well known in the art, such as acidification,
extraction,
evaporation, chromatography, and recrystallization to give the compound of
formula (3b) in
which Y is bromo and X is -OH. The product can be isolated and purified by
techniques well
known and appreciated in the art, such as acidification, basification,
filtration, extraction,
3o evaporation, trituration, chromatography, and recrystallization.
SUBSTITUTE SHEET (RULE Z6)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-34-
Reaction Scheme F.2
0 0
R3'~ R3.
\OH ~OH
(9) Br
((3) in which Y1
is bromo and X is -OH)
In Reaction Scheme F.2, an appropriate carboxylic acid of formula (9) is
brominated
to give compound of formula (3b) in which Y is bromo and X is -OH. An
appropriate
carboxylic acid of formula (9), and protected forms thereof, is one which is
one in which R3~ is
R3 as desired in the final product of formula ( 1 ) or gives rise after
deprotection to R3 as
desired in the final product of formula ( 1 }.
For example, a mixture of a carboxylic acid of formula (9) and dry red
phosphorous
are treated dropwise with bromine at temperature ranging from about -
20° to about 10°C. The
reaction mixture is then warmed to room temperature and then heated to about
80°C for about
2-5 hours. The reaction mixture is then cooled to room temperature, poured
into water
containing sodium bisulfate, and neutralized using solid sodium carbonate. The
aqueous layer
is extracted and acidified with a suitable acid, such as concentrated
hydrochloric acid. The
is precipitate is collected by filtration and dried to give the compound of
formula (3b) or formula
(3b2)in which Y is bromo and X is -OH. The product can be isolated and
purified by
techniques well known and appreciated in the art, such as acidification,
basification, filtration,
extraction, evaporation, trituration, chromatography, and recrystallizatian.
Compounds of formula (8} and (9) in which R3~ is a -(CHZ)m-W for use in
Reaction
Schemes F.I and F.2 are prepared according to Reaction Scheme G.1 and G.2.
SUBSTTTUTE SHEET (RULE 2~
CA 02356969 2001-06-27
WO 00/40553 PCTNS99/28234
-35-
Reaction Scheme G.1
0
0 0
___.... _ ~~)m i ~ \ __.__._..__..._.___.__~ f~~~~\~ N __ (~)m _.~
_~ ~ 'i
:W v
O
(11) (9) in which R3. is
W_(CHZ)m
In Reaction Scheme G.1 an appropriate w-amino carboxylic acid of formula (11)
is
converted to an compound of formula (9) in which R3' is W-(CHZ)m . An
appropriate w-
amino carboxylic acid of formula (11) is one in which m is as desired in the
anal product of
formula (1) and are readily available in the art. For example, the reaction is
carried out in a
suitable polar solvent, such as water, ethanol, diethyl ether,
tetrahydrofuran, or a water/ethanol
solvent mixture using a suitable base, such as sodium carbonate and N-
carbethoxyphthalimide. The reaction mixture is typically stirred at about
ambient temperature
for 1-5 hours. The product can be isolated and purified by techniques well
known in the art,
1o such as acidification, extraction, evaporation, chromatography, and
recrystallization to give
the desired compound of formula (9) in which R3~ is W-(CHZ)m .
suas~~s sM~~r c~u~ 2s~
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-36-
Reaction Scheme G.2
0 0
H2N- ( CHZ ) m HEN- ( CHz ) m
(12) off step 1 off
NHz (13) NHPg9
Step 2
O
O
\N- (CHZ) m
OH
o ( 14) NxPg;
step 3
0
0
\ N- ( CHz ) m
OH
0
(8) in which R3, is
W-(CH2)rri
Reaction Scheme G.2, step 1, an appropriate a,cu-diamino acid of formula (12)
undergoes a selective N-a-protection to give an N-a-protected-r~-diamino acid
of formula
(13). An appropriate a,w-diamino acid of formula (12) is one in which m is as
desired in the
final product of formula ( 1 ).
For example, a selective N-a-protection of a suitable a,c~-diamino acid, such
as L-
lysine (formula 22 in which m is 4), is accomplished by masking the w-amino
group by
to formation of a benzylidene imine. The benzylidene imine is formed by
dissolving L-lysine
monohydrochloride in lithium hydroxide and cooling the solution to a
temperature ranging
from about 0° to 10°C. Freshly distilled benzaldehyde is then
added and the solution is
shaken. N-w-benzylidene-L-lysine is recovered by filtration and evaporation.
The a-amino
group of the N-c~-benzvlidene-L-lysine then undergoes protection. such as the
introduction of
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCTNS99/28234
-37-
a Cbz or t-Boc group, followed by hydrolytic cleavage of the imine in situ to
give N-a-
benzyloxy-carbonyl-L-lysine. Accordingly, N-w-benzylidene-L-lysine is added to
a mixture
of sodium hydroxide and ethanol, cooled to a temperature of from about -
5°C to about
-25°C. Then, precooled solutions of benzyloxycarbonyl chloride in a
solvent, such as ethanol,
is added to the reaction mixture. The temperature is maintained in a range of
from about
-10°C to about -25°C during the course of addition, and may
allowed to rise afterwards. The
reaction mixture is then acidified using a suitable acid, such as precooled
hydrochloric acid,
and N-a-benzyloxycarbonyl-L-lysine, which corresponds to formula (13) where m
is 4, is
recovered by filtration evaporate and recrystallization.
In Reaction Scheme G.2, step 2, N-a-benzyloxycarbonyl-L-lysine or other
compounds
of formula (13) is converted.to w-phthalimido-a-benzyloxycarbonyl-L-lysine or
other co-
phthalimido-a-aminoprotected carboxylic acid of formula ( 14) by the method
described in
Reaction Scheme G.1, above.
IS
In Reaction Scheme G.2, step 3, the w-phthalimido-a-aminoprotected carboxylic
acid
of formula ( 14) is deprotected to give compound of formula (8) in which R3~
is W-(CHZ)m .
For example, w-phthalimido-a-benzyloxycarbonyl-L-lysine is contacted with
2o hydrogen in the presence of a hydrogenation catalyst, such as 10%
palladium/carbon. The
reactants are typically contacted in a suitable solvent mixture such as
ethanol, methanol,
water, ethanol/water mixtures, or methanol/water mixtures. The reactants are
typically shaken
under a hydrogen atmosphere of 35-45 psi at room temperature for a period of
time ranging
from 5-24 hours. The product is typically recovered by filtration and
evaporation of the
25 solvent.
A route for preparing the compounds of formula (3b) and formula (3b2) in which
Y~ is protected thio is presented in Reaction Scheme H. The reagents and
starting materials
are readily available to one of ordinary skill in the art. In Reaction Scheme
H all
30 substituents, unless otherwise indicated, are as previously defined.
SUBSTITUTE SHEET (RULE 2~
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-38-
Reaction Scheme H
0 0
Br~ Y
o-Pgs step 1 o-pgs
(15) (17)
step 2
0 0
y Step 3 y
OH O pgs
R3~ Ra
((3b) ~ which Y is _ . _ . _ _ ( 1 g). _. .
protected thio and X is -OH)
In Reaction Scheme H, step 1, a bromoacetate of formula (15) is contacted with
an
appropriate thiol to give a protected acetic acid ester of formula (17). In a
bromoacetate of
formula ( 15) Pg5 is a protecting group, such as methyl, ethyl, t-butyl, and
benzyl. An
appropriate thioI is one which gives rise to a protected thin group, Y, in the
product of formula
(3b). In Reaction Scheme H, step 1, the use of 4-methoxybenzylmercaptan is
preferred.
For example, a bromoacetate of formula (IS) is contacted with an appropriate
thiol in a
suitable organic solvent, such as dimethylformamide. Advantageously, the
solvent is
io degassed. The reaction is carried out using a suitable base, such as sodium
hydroxide,
triethylamine, or N,N-diisopropylethylamine. The reaction is carried out at
temperatures of
from about -50°C to about ambient temperature and requires about 1 to
72 hours. The
protected acetic acid ester of formula ( 17) can be isolated and purified by
methods well known
and appreciated in the art, such as extraction, evaporation, chromatography,
and distillation,
and recrystallization.
In Reaction Scheme H, step 2, the protected acetic acid ester of formula ( 17)
is
alkylated with an appropriate akylating agent to give a compound of formula
(18). In
Reaction Scheme H, step 2, an appropriate alkylating agent is one which
transfers R;~ which is
2o Rz as desired in the final product of formula ( 1 ) or gives rise after
deprotection to R3 as
desired in the final product of formula (1) or gives rise to R;-~ as defined
in Reaction Scheme
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28Z34
-39-
C, step 1. Appropriate alkylating agents include alkylhalides, such as methyl
iodide, methyl
bromide, ethyl bromide, propyl bromide, propyl chloride, butyl bromide, butyl
chloride, and
the like; benzyl bromide, benzyl chloride, substituted benzyl bromide,
substituted benzyl
chloride, ethyl bromoacetate, t-butyl bromoaceate, ethyl 3-chloropropionate,
ethyl 3-
bromopropionate, ethyl 5-bromovalerate, ethyl 4-bromobutyrate, 3-
chloropropionamide, 2-
bromoethylbenzene, substituted 2-bromoethylbenzene, 1-chloro-3-phenylpropane,
1-bromo-4-
phenylbutane, and the like, N-(2-bromoethyl)phthalimide, , N-(3-
bromopropyl)phthalimide,
N-(4-bromobutyl}phthalimide, and the like; 1-bromo-2-phenylethane, 1-bromo-3-
phenylpropane, 1-bromo-4-phenylbutane, and the like.
For example, a protected acetic acid ester of formula (17) is alkylated with
an
appropriate alkylating agent. The reaction is carned out in a suitable
solvent, such as diethyl
ether, tetrahydrofuran, dimethylformamide, and toluene using a suitable base,
such as sodium
hydride, potassium hydride, potassium t-butoxide, lithium
bis(trimethylsilyl}amide, sodium
bis(trimethylsilyl)amide, potassium bis(trimethylsilyl)amide, or lithium
diisopropylamide. The
reaction is generally canned out at temperatures of about -70°C to
about room temperature and
require from about 1-24 hours. The product can be isolated by techniques well
known in the
art, such as extraction, evaporation, and precipitation and can be purified by
chromatography
and recrystallization.
In Reaction Scheme H, step 3, the compound of formula (18) the carboxy
protecting
group Pg5 is selectively removed to give a compound of formula (3b) in which Y
is protected
thio. Such deprotection of esters to acids in the presence of suitable thio
protecting groups are
well known and appreciated in the art.
Reaction Scheme I describes the preparation of a specific diastereomer of the
compounds of formula (2a).
SUBSTT>rLfTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-40-
Reaction Scheme I
Rz IRz
( CHz ) a ~ ~ ( CHz ) a
step 1
0 0
(20) (21 )
OPg
Rz step 2
/ ( CHz ) a Rz
( CHz ) a
p ( step 3
O
- N~O
0 off (22)
m",
(23)
step 4
Rz Rz
( CHz ) a
( CHz ) a
O O
step 5 Na
- N~O -
N~O
~ ii~" ~,
O ~ ~ ~i~"~~ ~O
(23a) (23b)
step 6
Rz
I ~ Rz
,~~~ ( CHZ ) a Step 7
( CHz ) a
N OCH3 O
I N3
H o (25)
ocH3 (24)
step 8
Rz Rz
I
~~~ ( CHz ) a
step 9 ,,.v ( cHz ) a
N~OH
P N~OH
III
~I I(!
gl O Pgl O
(25a) (2a as a specific diastereomer)
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PC'T/US99/28234
-41-
In Reaction Scheme I, step 1, an appropriate aldehyde of formula (20) is
converted to a
compound of formula (21 ) in which Pg is a protecting group. Such conversions
can be
accoplished by Aldol-type condensation reactions or Wittig-type olefination
reactions, each of
which are well known in the art. An appropriate aldehyde of formula (20) is
one in which a
and R~ are as desired in the final product of formula ( 1 ). Appropriate
aldehydes of formula
(20) include, benzaldehyde, substituted benzaldehydes, 1-naphthaldehyde,
substitued 1-
naphthaldehydes, 2-naphthaldehyde, substitued 2-naphthaldehydes,
phenylacetaldehyde,
substituted phenylacetaldehydes, hydrocinnamaldehyde, and substituted
hydrocinnamaldehyde.
to
Suitable Aldol-type condensations include the Claisen-Schmidt and Knoevenaglel
reactions. Modern Synthetic Reactions, H.O. House (2"d Ed. The
Benjamin/Cummings
Publishing Co. 1972). As is appreciated by one of skill in the art the Claisen-
Schmidt reaction
using malonic acid, or esters thereof, give compounds of formula (22) upon
decarboxylation
1~5 or hydrolysis and decarboxylation.
Sutiable Wittig-type reacations include the Wittig and Wadswoth-Edmonds
reactions.
For example, an appropriate aldehyde of formula (20) is reacted with an
appropriate reagent,
such as (carbethoxymethylene)triphenylphosphorane or dimethyl
20 trimethylsilyloxycarbonylmethyl phosphonate. The reaction is carried out in
solvent, such as
ethanol, benzene, toluene, or tetrahydrofuran. Typically the reaction is
carried out at
temperature of from about -20° to reflux and require about 4 to 48
hours. The product can be
isolated and purified by techniques well known and appreciated in the art,
such as quenching,
acidification, filtration, extraction, evaporation, trituration,
chromatography, and
25 recrystallization.
Alternately, for example, an appropriate aldehyde of formula (20) is reacted
with an
appropriate reagent, such as dimethyl trimethylsilyloxycarbonylmethyl
phosphonate. The
reaction is carned out in solvent, such as benzene, toluene, diethyl ether, or
tetrahydrofuran.
3o The reaction is carried out using a suitable base, such as potassium t-
butoxide, sodium
hydride, lithium diisopropylamide, or sodium or potassium
bis(trimethylsilyl)amide.
Typically the reaction is carried out at temperature of from about -70°
to ambient temperature
and require about 1 to 48 hours. The product can be isolated and purified by
techniques well
SUBSTI1'~JTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-42-
known and appreciated in the art, such as quenching, acidification,
filtration, extraction,
evaporation, trituration, chromatography, and recrystallization.
In Reaction Scheme I, step 2, a compound of formula (21 ) is hydrolysed to
give a
compound of formula (22). Such hydrolysis of esters under acidic or basic
conditions is well
known and appreciated in the art and described in Protective Groups in Organic
Synthesis,
Theodora W. Greene (Wiley-Interscience, 2nd Edition, 1991 ).
For example, a compound of formula (21 ) is reacted with a suitable
hydrolyzing
1o agent, such as sodium hydroxide, potassium hydroxide, lithium hydroxide, or
sodium
carbonate to give an acid. The hydrolysis reaction is carned out in a suitable
solvent, such as
water, ethanol, methanol, or water/methanol mixtures, water/ethanol mixtures,
water/tetrahydrofuran mixtures. The reactions are carried out at temperatures
of from 0°C to
the refluxing temperature of the solvent and generally require from 30 minutes
to 48 hours.
15 The acid produced in the hydrolysis reaction can be isolated using
techniques well known
in the art, such as acidification, extraction, and evaporation. The acid may
be used after
isolation without further purification or may be purified by chromatography,
tritruration, and
recrystallization as is known in the art.
2o As is appreciated by the skilled person, some of the compounds of formual
(22) are
readily available and may be available in activated form, such trans-cinnamic
acid, substituted
trans-cinnamic acids, cinnamoyl chloride, and substituted cinnamoyl chlorides.
In Reaction Scheme I, step 3, a compound of formula (22) is activated and
reacted
25 with a lithiated 4-substituted-oxazolidin-5-one to give a compound of
formula (23). Suitable
4-substituted-oxazolidin-5-ones include 4-phenyl-2-oxazolidinone, (R)-4-phenyl-
2-
oxazolidinone, (S)-4-phenyl-2-oxazolidinone, 3,3-dimethyl-4-phenyl-2-
oxazolidinone, (R)-
3,3-dimethyl-4-phenyl-2-oxazolidinone, and (S)-3,3-dimethyl-4-phenyl-2-
oxazolidinone. The
use of (R)-4-phenyl-2-oxazolidinone is depicted in Reaction Scheme I.
For example, the compound of formula (22) in a suitable organic solvent, such
as
tetrahydrofuran diethyl ether, is treated with a suitable tertiary organic
amine such as
triethylamine or N-methylmorpholine and cooled to -78°C. A suitable
acid halide such as
SUBSTITUTE SHEET (RULE Z~
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-43-
trimethylacetyl chloride is added and the mixture is transferred to an ice
bath for 0.5 to 1.0
hours. then recooled to -78°C. The resulting slurry is treated with
lithiated (R)-4-phenyl-5-
oxazolidinone, prepared by adding n-butyllithium to (S)-4-phenyl-2-
oxazoIidinone in
tetrahydrofuran, and allowed to warm gradually to ambient temperature over a
period of time
ranging from about 10 to 20 hours. The product can be isolated by methods well
known and
appreciated in the art, such as extraction and evaporation. The product can be
purified by
methods well known and appreciated in the art, such as flash chromatography.
In Scheme I, step 4, a compound of formula (23) undergoes a 1,4-addition of a
vinyl
l0 group to give a compound of formula (23a).
For example, a compound of formula (23) and trimethylsilyl chloride in a
suitable
solvent, such as tetrahydrofuran is added to a prepared solution of copper (I)
iodide and
N,N,N', N'-tetramethylethylenediamine and vinylmagnesium bromide in
tetrhydrofuran. The
15 reaction is carried out at temperatures of form about -78°C to about
0°C and requires form
about 1 to 12 hours. The product can be isolated and purified by techniques
well known and
appreciated in the art, such as quenching, acidification, filtration,
extraction, evaporation,
trituration, chromatography, and recrystallization.
20 In Scheme I, step 5, a compound of formula (23a) undergoes an azide
introduction
reaction with a suitable azide transfer agent to give a compound of formula
(23b). Such azide
introductions are described in the art in J. Am. Chem. Soc., 112, 401 I -4030
( 1990).
For example, a solution of a suitable amide such as potassium
bis(trimethylsilyl)amide
25 in a suitable organic solvent, such as tetrahydrofuran, is cooled to -
78°C and treated with a
solution of a compound of formula (32a) in tetrahydrofuran, precooled to -
78°C. A solution
of a suitable azide transfer agent, such as trisyl azide, prepared by the
method described in J.
Ors. Chem., 38, 11-16 (1973), in a suitable organic solvent, such as
tetrahydrofuran,
precooled to -78°C is then added. The solution is stirred, quenched
with acetic acid. After a
3o period of time ranging from about 12 to 48 hours, the product is isolated
by methods well
known and appreciated in the art, such as extraction and evaporation. The
product can be
purified by methods well known in the art, such as flash chromatography.
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-44-
In Reaction Scheme I, step 6, a compound of formla (23b) is hydrolyzed and
esterified
to give a compound of formula (24}.
For example, a compound of formula (23b) is reacted with a suitable
hydrolyzing
agent, such as lithium hydroxide and hydrogen peroxide. The hydrolysis
reaction is carried out
in a suitable solvent, such as water/tetrahydrofuran mixtures. The reactions
are carried out at
temperatures of from 0°C to the refluxing temperature of the solvent
and generally require
from 30 minutes to 48 hours. The acid produced in the hydrolysis reaction can
be isolated
using techniques well known in the art, such as quenching of peroxides,
acidification,
extraction, and evaporation. The acid may be used after isolation without
further purification
or may be purified by chromatography, tritruration, and recrystallization as
is known in the
art. The acid is then esterified to give a compound of formula (24). For
example, to give the
methyl ester depicted in Reaction Scheme I, the acid is contacted with a ester
forming reagent,
such as (trimethylsilyl)diazomethane. This reaction is carried out in a
suitable solvent, such as
methanol or methanol/tetrahydrofuran mixtures. The reactions are carried out
at temperatures
of from 0°C to the refluxing temperature of the solvent and generally
require from 12 to 48
hours. The product can be isolated and purified techniques well known in the
art, such as
acidification, extraction, evaporation, chromatography, tritruration, and
recrystallization.
Alternately, for example, to give the methyl ester depicted in Reaction Scheme
I, the acid is
2o contacted with methanol under acidic conditions. The reactions are carried
out at
temperatures of from 0°C to the refluxing temperature of methanol and
generally require from
1 to 48 hours. The product can be isolated and purified techniques well known
in the art, such
as acidification, extraction, evaporation, chromatography, tritruration, and
recrystallization.
In Reaction Scheme I, step 7, a compound of formula (24) is reduced and
cyclized to
give a compound of formula (25).
For example, a compound of formula (24) is contacted with a suitable recucing
agent,
such as dicyclohexylborane. The reaction is carried out in a suitable solvent,
such
3o tetrahydrofuran. The reactions are carried out at temperatures of from -
20°C to ambient
temperature and generally require from 1 to 48 hours. The product can be
isolated and purified
techniques well known in the art, such as quenching, extraction, evaporation.
chromatography,
tritruration, and recrystallization.
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-45-
In Reaction Scheme I, step 8, a compound of formula (25) is protected to give
a
compound of formula (2a). The use of amine protecting groups is well known and
appreciated
in the art and described in Protective Groups in Oreanic Synthesis, Theodora
W. Greene
(Wiley-Interscience, 2nd Edition, 1991 ).
The following examples present typical syntheses as described in the Reaction
Schemes above. These examples and preparations are understood to be
illustrative only and
are not intended to limit the scope of the invention in any way.
io PREPARATION 1
Synthesis of 2-bromo-6-phthalimidohexanoic acid
Combine 6-aminohexanoic acid (6-aminocaproic acid) (8.0 g, 60 mmol) and water
(100 mL). Add sodium carbonate (6.84 g, 64 mmol) and N-carbethoxyphthalimide
(14.0 g, 64
mmol). After 1.5 hours, extract the reaction mixture with ethyl acetate ( 100
mL). Cool the
is aqueous layer in an ice bath and acidify using concentrated hydrochloric
acid to give a solid.
Collect the solid by filtration, rinse with water, and dry to give 6-
phthalimidohexanoic acid
(12.7 g, 80% yield).
Combine 6-phthalimidohexanoic acid (12.7 g, 48 mmol) and dry red phosphorous
(1.95 g, 63 mmol). Cool in an ice bath and add dropwise bromine (12.7 mL, 246
mmol).
2o Warm to room temperature and then heat to 80°C. After 3 hours, cool
the reaction mixture to
ambient temperature, pour into water (300 mL) containing sodium bisulfate, and
neutralize
using solid sodium bicarbonate and extract with diethyl ether (about 150 mL).
Acidify the
aqueous layer with concentrated hydrochloric acid give a solid. Collect the
solid by filtration
and dry to give the title compound (15 g, 91.5% yield, 73.2% for both steps).
PREPARATION 2
Synthesis of (R)-2-bromo-6-nhthalimidohexanoic acid
Combine (R)-2-N-carbobenzyloxy-6-aminohexanoic acid ((R)-Noc-Cbz-lysine) (14.0
g, 50 mmol) and water (500 mL). Add sodium carbonate (5.65 g, 53 mmol) and N-
3o carbethoxyphthalimide (13.15 g, 60 mmol). After 1.5 hours, acidify using
concentrated
hydrochloric acid to give a solid. Collect the solid by filtration, rinse with
water, and dry to
give (R)-2-N-carbobenzyloxy-6-phthalamidohexanoic acid.
Combine (R)-2-N-carbobenzyloxy-6-phthalamidohexanoic acid obtained above,
methanol (200 mL), 10% palladium-on-carbon ll el and treat with hvdroeen at
atmospheric
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28Z34
-46-
pressure. After 18 hours, filter, add to the filtrate a solution of
hydrochloric acid in methanol
(50 mL, I M, 50 mmol), and evaporate in vacuo to give (R)-2-amino-6-
phthalamidohexanoic
acid hydrochloric acid salt.
Combine (R)-2-amino-6-phthalamidohexanoic acid hydrochloric acid salt (12.5 g,
40
mmol) and a 2.5 M aqueous sulfuric acid solution (40 mL). Cool in a salt-ice
bath. Add 49%
aqueous hydrobromic acid solution ( 13.2 g). Add dropwise over about 20
minutes, an
aqueous solution of sodium nitrite (2.8g, 40 mmol, in 20 mL of water). After 3
hours, warm
to ambient temperature. After 18 hours, collect the resultant solid by
filtration, rinse with
water and dry in vacuo to give a residue. Chromatograph the residue on silica
gel eluting with
1/1 ethyl acetate/dichloromethane containing 5% acetic acid to give the title
compound.
PREPARATION 3
Synthesis of 1-Fmoc-traps-3 j R( )-(naphth-2-yl)-2(S)-carboxynyrrolidine
Combine 2-naphthaldehyde (7.8 g, 50 mmol) and
(carbethoxymethylene)triphenylphosphorane (18.3 g, 52.5 mmol) in 50 mL ethanol
(50 mL).
After 18 hours, the reaction mixture was diluted with diethyl ether (500 mL)
and washed with
aqueous I M phosphoric acid solution (2 x 100 mL), saturated sodium
bicarbonate 100 mL),
water (100 mL), and then brine ( 100 mL). Dry the organic layer over Mho:,,
filter, and
concentrate in vacuo to give a residue. Chromatograph the residue on silica
gel eluting with
9:1 hexane:ethyl acetate to give ethyl traps-3-(naphth-2-yl)-propenoate as an
85:15 mixture of
geometric isomers (favoring traps by nmr). Recrystallize from hexane/ethyl
acetate to give
ethyl traps-3-(naphth-2-yl)-propenoate as a 97:3 mixture of isomers (favoring
traps by nmr).
Concentrate the mother liquor and recrystallize to recover an additional 2.9
g. (total yield
65%). NMR (CDCI3) 8 7.93 (s, 1 H); 7.88-7.83 (c, 4 H); 7.67 (dd, 1 H, J = 1.6,
8.6 Hz); 7.53-
7.50 {c, 2 H); 6.55 (d, 1 H, J =16.0 Hz); 4.30 (q, 2 H, J = 7.1 Hz); 1.42 (t,
3 H, J = 7.1 Hz).
Combine ethyl traps-3-(naphth-2-yl)-propenoate (4.24 g, 18.8 mmol) and
tetrahydrofuran (75 mL}. Add litium hydroxide hydrate (2.36 g, 56.3 mmol) in
water (19
mL). After 18 hours, acidified to a pHof about 2 with aqueous 12 M
hydrochloric acid
solution to give a precipitate. Extract with ethyl acetate (3 x I50 mL). Dry
the combined
3o extracts over M~oa, filter, and concentrated in vacuo to give traps-3-
(naphth-2-yl}-propenoic
acid as a vrhite solid (3.66 g, 98% yield). NMR (CDC13) b 7.97 (d, 1 H, 3 =
15.7 Hz); 7.90 (d,
1 H, J = 15.3 Hz); 7.90-7.83 (c, 3 H); 7.70 (dd, 1 H, J = 1.6, 8.6 Hz); 7.57-
7.50 (c, 2 H); 6.58
(d, 1 H, J = 16.0 Hz).
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-47-
Combine traps-3-{naphth-2-yl)-propenoic acid (3.66 g, 18.5 mmol) and
triethylamine
(1.87 g, 2.56 mL, 18.5 mmol) in tetrahydrofuran (74 mL). Cool to -78°C.
Add pivaloyl
chloride (2.35 g, 2.40 mL, 19.4 mmol). After 10 minutes. warm in an ice-bath.
After 10
minutes, cool to -78°C. Prepare 1-lithio-4-phenyl-2-oxazolidinone in a
separate flask by the
addition ofn-butyl lithium (1.6 M in hexane, 11.6 mL, 18.~ mmol) to 4-phenyl-2-
oxazolidinone (3.31 g, 20.3 mmol) in anhydrous tetrahydrofuran (74 mL) at -
78°C. After 1.5
hours, add the mixed anhydride prepared abpve via cannula and place the
reaction mixture in
an ice-bath. After 1 hour, warm to ambient temperature. After 18 hours, quench
with a
saturated aqueous ammonium chloride soluiton (50 mL) and evaprorate to remove
most of the
tetetrahydrofuran. Extract with dichloromethane (3 x 75 mL), combine the
organic layers,
extract an aqueous 1 M sodium hydroxide solution (2 x 50 mL), dried dry over
Mgsoa~ filter,
and concentrate in vacuo to give a residue. Recrystallize the residue from
ethyl
acetate/hexane to give traps-3-(naphth-2-yl)-propenoic acid' as a white solid
(61 %). NMR
(CDCl3) 8 8.05 (d, 1 H, J =15.7 Hz); 7.94 (d, 1 H, J = 15.4 Hz); 7.87-7.81 (c,
3 H); 7.76 (dd,
1 H, J = 1.5, 8.6 Hz); 7.53-7.47 (c, 2 H); 7.41-7.34 (c, 5 H); 5.58 (dd, 1 H,
J = 8.7, 3.9 Hz);
4.76 (t, 1 H, J = 8.7 Hz); 4.33 (dd, 1 H, J = 8.8, 3.9 Hz).
!3'R4S)-3-(3'-(2"-Naphthyl)-4'-nentenoyl)-4-phenyl-2-oxazolidinone (4)
To a solution of CuI (3.96 g, 20.9 mmol) and N,N.N'. N'-
tetramethylethylenediamine
(2.66 g, 3.46 mL, 22.9 mmol) in anhydrous tetrahydrofuran (92 mL) at -
78°C was added
vinylmagnesium bromide (1.0 M in tetrahydrofuran, 20.9 mL, 20.9 mmol). The
mixture was
stirred for 15 minutes. In a separate flask trimethylsilyl chloride (5.69 g,
6.64 mL, 52.2 mmol)
was added to a solution of unsaturated imide 3 (3.87 g, 11.3 mmol) in
anhydrous
tetrahydrofuran (42 mL). Owing to insolubility of the imide, the septum of the
flask
containing the cuprate reagent was removed and the slurried imide added in one
portion
rinsing quickly with a small amount of tetrahydrofuran. The bath temperature
was raised to -
30°C and stirring continued for i h. The reaction mixture was poured
into 250 mL of a 3:2
mixture of saturated Ammonium chlroide:concentrated NH40H. The layers were
separated
and the aqueous layer extracted with ethyl acetate (3 x 200 mL). The combined
organic layers
3o were washed sequentially with saturated Ammonium chlroide (1 x 100 mL) and
water (1 x
100 mL). The organic layer was dried dry over M&so,~, and concentrated under
reduced
pressure. The residue was purified by passage through a plug of Si02 eluting
with 4:1
hexane:ethyl acetate. The eluant was concentrated in vacuo to recover a white
solid (3.64 g,
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-48-
9.81 mmol, 87% yield). NMR (CDC13) 8 7.87-7.82 (c, 3 H); 7.72 (s, 1 H); 7.54-
7.27 (c, 8 H);
6.11 (ddd, 1 H, J = 6.7, 10.4, 17.0 Hz); 5.34 (dd, I H, J = 8.6, 3.5 Hz); 5.10
(d, 1 H, J = 8.2
Hz); 5.08 (d, 1 H, J = 17.2 Hz); 4.56 (t, 1 H, J = 8.8 Hz); 4.26 (dd, 1 H, J =
8.8, 3.5 Hz); 4.16
(ddd, I H, J = 8.1, 7.0, 6.9 Hz); 3.68 (dd, 1 H, J = 8.4, 16.5 Hz); 3.50 (dd,
1 H, J = 6.5, 16.5
Hz).
(2'S3'R4S)-3-(2'-Azido-3'-(2"-naphthyl)-4'-nentenoyl)-4-phenyl-2-oxazolidinone
(5)
Potassium hexamethyidisilazide (0.5 M in toluene, 25.5 mL, 12.8 mmol) was
added in
one portion to anhydrous tetrahydrofuran (34 mL) at -78°C. Imide 4
(3.64 g, 9.81 mmol) was
to slurned in tetrahydrofuran (34 mL) and added via cartnula, rinsing with
tetrahydrofuran (2 x
11 mL} to complete the transfer. After 30 min, trisylazide (4.40 g, 14.2 mmol)
was dissolved
in tetrahydrofuran (34 mL), cooled to -78°C, and added via cannula.
Thirty minutes later,
AcOH ( 1.41 g, 1.34 mL, 23.4 mmol) was added to quench the reaction. The
mixture was
stirred at room temperature overnight. The mixture was partitioned between
dichloromethane
(300 mL) and dilute brine (150 mL). The layers were separated and the aqueous
phase
extracted with dichloromethane (3 x 150 mL). The combined organic layers were
dried dry
over Mgsoa, and concentrated under reduced pressure.The residue was purified
by flash
chromatography to recover the product (3.41 g, 8.28 mmol, 84 % yield). Proton
nmr indicated
that a byproduct derived from trisyl azide was also present. NMR (CDCI~} 8
7.85-7.82 (c, 3
2o H); 7.72 (s, 1 H}; 7.53-7.47 (c, 2 H); 7.42 (dd, 1 H, J = 1.7, 8.5 Hz);
7.37-7.31 (c, 3 H); 7.18-
7.15 (c, 2 H); 6.28 (ddd, 1 H, J = 8.2, 10.2, 17.1 Hz); 5.63 (d, 1 H, J = 10.2
Hz); 5.37 (d, 1 H, J
= 17.0 Hz); 5.34 (d, 1 H, J = 10.2 Hz); 4.83 (dd, 1 H, 3 = 3.0, 8.3 Hz); 4.14
(t, 1 H, J = 7.2
Hz); 4.07 (dd, 1 H, J = 9.3, 17.9 Hz); 3.94 (dd, 1 H, J = 3.0, 5.8 Hz); 3.68
(t, 1 H, J = 8.6 Hz).
Notes: Trisylazide is not commercially available. Sulfonyl azides can be
prepared according to
J. Org. Chem. 1973, 38, I I-16. The azide transfer can be difficult. See J.
Am. Chem. Soc.
1990, 112, 4011-4030 for a full discussion. After the addition of the
trisylazide, an
intermediate that is more polar than starting material is rapidly formed.
After addition of
AcOH, the polar intermediate slowly disappears and the product azidoimide spot
begins to
3o form. It is only slightly less polar than the starting imide. A
decomposition product of
trisylazide nearly coelutes with the product. Timing of this reaction is
critical as the yield
erodes with increasing time between addition of the trisyl azide and the
acetic acid.
SUBSTTrUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PGT/LJS99/28234
-49-
Methyl (2S3R1-2-Azido-3-(naphth-2-yl)-4-pentenoate (6)
To a solution of imide 5 (3.41 g, 8.28 mmol) in tetrahydrofuran (62 mL) was
added
water (21 mL), 35% H2O2 (2.7 mL), and LiOH~H20 (695 mg, 16.6 mmol). After 2
hours
sodium sulfite (4.17 g, 33.1 mmol) was added as a solution in water (41 mL).
The mixture was
stirred for 15 minutes and tetrahydrofuran removed under reduced pressure. The
aqueous
solution was acidified with hydrochloric acid and extracted with ethyl acetate
(2 x 150 mL).
The combined extracts were dried dry over Mgsoa, and concentrated under
reduced pressure.
The residue was passed through a Si02 plug column eluting with 1:1
hexane:ethyl acetate to
recover, after concentration, a white solid that was presumably a mixture of
the carboxylic
1o acid and chiral auxiliary. Recrystallization from hexane/ethyl acetate
yielded the chiral
auxiliary as needles. The mother liquor was concentrated and carried on to the
esterification
step.
The residue containing the crude carboxylic acid was dissolved in anhydrous
MeOH
(46 mL) and cooled to 0°C. Thionyl chloride ( 1.18 g, 0.725 mL, 9.94
mmol) was added and,
after 10 minutes, the mixture heated at reflux for 2 hours. Water (1.0 mL) was
added to the
mixture, stirred for I O minutes, and the contents of the flask concentrated
under reduced
pressure. The residue was partitioned between ethyl acetate ( 150 mL) and
brine ( 100 mL).
The layers were separated and the organic layer was dried dry over M~oa, and
concentrated
under reduced pressure. The residue was purified by flash chromatography (19:1
hexane:ethyl
2o acetate) to recover the methyl ester (1.54 g, 5.48 mmol, 66% yield). NMR
(CDC13) b 7.84-
7.80 (c, 3 H); 7.71 (s, 1 H); 7.50-7.46 (c, 2 H); 7.39 (dd, 1 H, J = 1.8, 8.5
Hz); 6.23 (ddd, 1 H,
J = 8.3, 10.9, 17.6 Hz); 5.30 (d, 1 H, J = 9.9 Hz); 5.28 (d, 1 H, J = 17.7
Hz); 4.22 (d, 1 H, J =
7.5 Hz); 4.06 (t, 1 H, J = 7.9 Hz).
Notes: The intermediate carboxylic acid and chiral auxiliary tend to coelute
by flash
chromatography and the indicated recrystallization only removed a layer of the
auxiliary. The
auxiliary was thus present for the esterification step without complication.
However under
these conditions it has been observed that the auxiliary can ring open and
subsequently
decarboxylate leaving a primary amine which can attack the ester. If the
esterification is run
3o with the auxiliary present, the reaction should be monitored carefully.
Trans-3-(naphth-2-yl)-L-proline methyl ester (7)
SUBS1TTUTE SHEET (RULE ZG)
CA 02356969 2001-06-27
WO 00/40553 PC'T/US99/28234
-50-
Borane-methyl sulfide complex (2.0 M in tetrahydrofuran, 6.~7 mL, 13.1 mmol)
was
diluted with anhydrous tetrahydrofuran (26 mL) and cooled to 0°C.
Cyclohexene (2.16 g, 2.66
mL, 26.3 mmol) was added cautiously via syringe. After 30 minutes a white
precipitate had
formed. Stirnng was continued for three hours. The contents of the flask were
concentrated in
vacuo. Note: Care should be taken to minimize air exposure as dicyclohexyl
borane is
extremely moisture sensitive. The reagent was slurned in dichloromethane (36
mL) and
cooled to 0°C. Vinyl azide 6 (1.23 g, 4.38 mmol) was dissolved in
dichIoromethane (9 mL)
and added via cannula. The reaction mixture became pale yellow and gas
evolution was
evident. The mixture was warmed to room temperature overnight. Added MeOH (26
mL) and
1o stirred for an additional 15 minutes. The mixture was concentrated under
reduced pressure.
The residue was taken up in diethyl ether (25 mL) and extracted with 0.1 M
hydrochloric acid
(5 x 25 mL). The aqueous extracts were basicified with saturated sodium
bicarbonate and
extracted with dichloromethane (3 x 100 mL). The organic extracts were dried
dry over M~,
and concentrated in vacuo to recover the cyclized product along with some
dicyclohexyl
borane derived contaminants (974 mg, 3.82 mmol, 87% yield of crude material).
NMR
(CDCl3) = 7.84-7.78 (c, 3 H); 7.71 (s, 1 H); 7.49-7.41 (c, 3 H); 3.91 (d, 1 H,
J = 6.9 Hz); 3.69
(s, 3 H); 3.63 (m, 1 H), 3.48 (dd, 1 H, J = 8.2, 15.4 Hz); 3.27 (d, 1 H, J =
7.8 Hz); 3.25 (d, 1 H,
J = 7.8 Hz); 2.33 (m, 1 H), 2.09 (m, 1 H).
2o Note: The cyclization can be capricious. The best results were obtained
when fresh bottles of
borane were employed. A suggestion is to make the dicylcohexylborane reagent
in dry diethyl
ether using neat borane-methyl sulfide complex (approximately 10 M).
N-Fmoc-trans-3-(naQhth-2-yl)-L-proline (8)
A solution of amino ester 7 (4.31 mmol) in 5 M hydrochloric acid (20 mL) was
heated
at 100°C overnight. The reaction mixture was concentrated in vacuo to
recover the amino
acid.
To a solution of the crude amino acid in acetone (22 mL) was added 20% aqueous
Na2C03 until the pH of the mixture was 9 - 10 (pH paper). Fmoc-O-succinimide
(1.60 g, 4.74
3o mmol) was added, the pH readjusted, and the reaction mixture stirred
overnight. The mixture
was carefully acidified with concentrated hydrochloric acid to about pH = 2
and extracted
with ethyl acetate (3 x 100 mL). The combined organic layers were dried dry
over Mgsoa, and
concentrated under reduced pressure. The residue was purified by flash
chromatography (97:3
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-51-
dichloromethane:MeOH). By tlc some impurities remained. These could be removed
by
boiling the residue in a small amount of dichloromethane, filtering, and
washing the tan solid
with hexane to deliver clean product (930 mg, 2.00 mmol, 46% yield) as judged
by tlc, HPLC,
and nmr. NMR (d~-DMSO) b 7.95-7.80 (c, 6 H); 7.68 (d, 1 H, J = 7.3 Hz); 7.60
(d, 1 H, J =
7.4 Hz); 7.50-7.34 (c, 6 H); 7.25 (m, 1 H), 4.39-4.15 (c, 4 H); 3.70-3.48 (c,
3 H); 2.29 (m, 1
H); 2.I4 (m, 1 H).
Note: The final target can also be recrystallized from hexane/ethyl acetate.
1o The present invention provides a method of inhibiting matrix
metalloproteinase
(MMP) to a patient in need thereof comprising administering to the patient an
effective matrix
metalloproteinase inhibiting amount of a compound of formula (1).
As used herein, the term "patient'' refers to warm-blooded animals or mammals,
t5 including guinea pigs, dogs, cats, rats, mice, hamsters, rabbits and
primates, including
humans. A patient is in need of treatment to inhibit MMP when it would be
beneficial to the
patient to reduce the physiological effect of active MMP. For example, a
patient is in need of
treatment to inhibit MMP when a patient is suffering from a disease state
characterized by
excessive tissue disruption or tissue degradation, such as, but not limited
to, a neoplastic
20 disease state or cancer; rheumatoid arthritis; osteoarthritis;
cardiovascular disorders, such as
atherosclerosis; corneal ulceration; dental diseases, such as gingivitis or
periodontal disease;
and neurological disorders, such as multiple sclerosis; chronic inflammatory
disorders, such as
emphysema and especially smoking-induced emphysema.
25 The identification of those patients who are in need of treatment to
inhibit MMP is
well within the ability and knowledge of one skilled in the art. A clinician
skilled in the art
can readily identify, by the use of clinical tests, physical examination and
medical/family
history, those patients who are suffering from disease states characterized by
excessive tissue
disruption or tissue degradation.
An "effective matrix metalloproteinase inhibiting amount" of a compound of
formula
( 1 ) is an amount which is effective, upon single or multiple dose
administration to the patient,
in providing relief of symptoms associated with MMP and is thus effective in
inhibiting
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-52-
MMP-induced tissue disruption and/or MMP-induced tissue degradation. As used
herein,
"relief of symptoms" of MMP-mediated conditions refers to decrease in severity
over that
expected in the absence of treatment and does not necessarily indicate a total
elimination or
cure of the disease. Relief of symptoms is also intended to include
prophylaxis.
An effective matrix metalloproteinase inhibiting dose can be readily
determined by the
use of conventional techniques and by observing results obtained under
analogous
circumstances. In determining the effective. dose, a number of factors are
considered
including, but not limited to: the species of the patient; its size, age, and
general health; the
1o specific disease involved; the degree of involvement or the severity of the
disease; the
response of the individual patient; the particular compound administered; the
mode of
administration; the bioavailability characteristics of the preparation
administered; the dose
regimen selected; and the use of concomitant medication.
15 An effective matrix metalloproteinase inhibiting amount of a compound of
formula ( 1 )
will generally vary from about 0.1 milligram per kilogram of body weight per
day
(mg/kg/day) to about 300 milligrams per kilogram of body weight per day
(mg/kg/day). A
daily dose of from about 1 mg/kg to about 100 mg/kg is preferred.
2o A neoplastic disease state refers to an abnormal state or condition
characterized by
rapidly proliferating cell growth or neoplasm. Neoplastic disease states for
which treatment
with a compound of formula (1) will be particularly useful include: Leukemias,
such as, but
not limited to, acute lymphoblastic, chronic lymphocytic, acute myeloblastic
and chronic
myelocytic; Carcinomas and adenocarcinomas, such as, but not limited to, those
of the cervix,
25 oesophagus, stomach, small intestines, colon, lungs (both small and large
cell), breast and
prostate; Sarcomas, such as, but not limited to, oesteroma, osteosarcoma,
lipoma, liposarcoma,
hemangioma and hemangiosarcoma; Melanomas, including amelanotic and melanotic;
and
mixed types of neoplasias such as, but not limited to carcinosarcoma, lymphoid
tissue type,
follicullar reticulum, cell sarcoma and Hodgkin's Disease. Neoplastic disease
states for which
3o treatment with a compound of formula ( 1 ) will be particularly preferred
include carcinomas
and adenocarcinomas, particularly of the breast, prostate and lung.
Atherosclerosis is a disease state characterized by the development and growth
of
atherosclerotic lesions or plaque. The identification of those patients who
are in need of
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-53-
treatment for atherosclerosis is well within the ability and knowledge of one
of ordinary skill
in the ari. For example, individuals who are either suffering from clinically
significant
atherosclerosis or who are at risk of developing clinically significant
atherosclerosis are
patients in need of treatment for atherosclerosis. A clinician of ordinary
skill in the art can
readily determine, by the use of clinical tests, physical examination and
medical/family
history, if an individual is a patient in need of treatment for
atherosclerosis.
The term "chronic inflammatory disease" refers to diseases or conditions
characterized
by persistent inflammation in the absence of an identifiable irritant or
microbial pathogen.
Inflammatory diseases for which treatment with a compound of formula (1) will
be
particularly useful include: emphysema, chronic bronchitis, asthma, and
chronic
inflammation, and especially smoking-induced emphysema.
In effecting treatment of a patient, a compound of formula ( 1 ) can be
administered in
any form or mode which makes the compound bioavailable in effective amounts,
including
oral and parenteral routes. For example, the compound can be administered
orally,
subcutaneously, intramuscularly, intravenously, transdermally, topically,
intranasally, rectally,
inhalation, and the like. Oral and inhalation administration is generally
preferred. One skilled
in the art of preparing formulations can readily select the proper form and
mode of
2o administration depending upon the disease state to be treated, the stage of
the disease, and
other relevant circumstances. Remin on's Pharmaceutical Sciences, 18th
Edition, Mack
Publishing Co. (1990).
A compound of formula ( 1 ) can be administered in the form of pharmaceutical
compositions or medicaments which are made by combining a compound of formula
(1) with
pharmaceutically acceptable Garners or excipients, the proportion and nature
of which are
determined by the chosen route of administration, and standard pharmaceutical
practice.
The pharmaceutical compositions or medicaments are prepared in a manner well
known in the pharmaceutical art. The carrier or excipient may be a solid, semi-
solid, or liquid
material, which can serve as a vehicle or medium for the active ingredient.
Suitable Garners
or excipients are well known in the art. The pharmaceutical composition may be
adapted for
oral or parenteral use and may be administered to the patient in the foam of
tablets, capsules,
suppositories, solution, suspensions, eels, ointments. aerosol or the like.
SUBSTT>l'UTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-54-
The pharmaceutical compositions may be administered orally, for example, with
an
inert diluent or with an edible carrier. They may be enclosed in gelatin
capsules or
compressed into tablets. For the purpose of oral therapeutic administration, a
compound of
formula ( 1 ) may be incorporated with excipients and used in the form of
tablets, troches,
capsules, elixirs, suspensions, syrups, wafers, chewing gums and the like.
These preparations
should contain at least 4% of a compound of formula ( 1 ), the active
ingredient, but may be
varied depending upon the particular form and may conveniently be between 4%
to about
70% of the weight of the unit. The amount of the active ingredient present in
compositions is
to such that a unit dosage form suitable for administration will be obtained.
The tablets, pills, capsules, troches and the like may also contain one or
more of the
following adjuvants: binders such as microcrystalline cellulose, gum
tragacanth or gelatin;
excipients such as starch or lactose, disintegrating agents such as alginic
acid, Primogel, corn
starch and the like; lubricants such as magnesium stearate or Sterotex;
glidants such as
colloidal silicon dioxide; and sweetening agents such as sucrose or saccharin
may be added or
a flavoring agent such as peppermint, methyl salicylate or orange flavoring.
When the dosage
unit form is a capsule, it may contain, in addition to materials of the above
type, a liquid
carrier such as polyethylene glycol or a fatty oil. Other dosage unit forms
may contain other
various materials which modify the physical form of the dosage unit, for
example, as coatings.
Thus, tablets or pills may be coated with sugar, shellac, or other enteric
coating agents. A
syrup may contain, in addition to the present compounds, sucrose as a
sweetening agent and
certain preservatives, dyes and colorings and flavors. Materials used in
preparing these
various compositions should be pharmaceutically pure and non-toxic in the
amounts used.
For the purpose of parenteral therapeutic administration, the compounds of the
present
invention may be incorporated into a solution or suspension. These
preparations should
contain at least 0.1 % of a compound of the invention, but may be varied to be
between 0.1
and about 50% of the weight thereof. The amount of the active ingredient
present in such
3o compositions is such that a suitable dosage will be obtained. Preferred
compositions and
preparations are able to be determined by one skilled in the art.
The solutions or suspensions may also include one or more of the following
adjuvants:
sterile diluents such as water for infection. saline solution. fixed oils.
nolvethvlene glvcols.
SUBSTITUTE SHEET (RULE Z6)
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28234
-55-
glycerine, propylene glycol or other synthetic solvents; antibacterial agents
such as benzyl
alcohol or methyl paraben; antioxidants such as ascorbic acid or sodium
bisulfate; chelating
agents such as ethylene diaminetetraacetic acid; buffers such as acetates,
citrates or
phosphates and agents for the adjustment of toxicity such as sodium chloride
or dextrose. The
parenteral preparation can be enclosed in ampules, disposable syringes or
multiple dose vials
made of glass or plastic.
The compounds of the present invention may also be administered by inhalation,
such
as by aerosol or dry powder. Delivery may be by a liquefied or compressed gas
or a suitable
pump system which dispenses the compounds of the present invention or a
formulation
thereof. Formulations for administration by inhalation of compounds of formula
(1) rnay be
delivered in single phase, bi-phasic, or tri-phasic systems: A variety of
systems are available
for the administration by aerosol of the compounds of formula ( 1 ). Dry
powder formulations
are prepared by either pelletizing or milling the compound of formula ( 1 ) to
a suitable particle
size or by admixing the pelletized or milled compound of formula (I ) with a
suitable carrier
material, such as lactose and the like. Delivery by inhalation includes the
necessary container,
activators, valves, subcontainers, and the like. Preferred aerosol and dry
powder formulations
for administration by inhalation can be determined by one skilled in the art.
The MMP inhibitors of the present invention can be evaluated by the procedures
that
follow.
EXAMPLE A
Source and Activation of nroMMP-12
ProMMP-1 (EC 3.4.24.7; interstitial collagenase) was purified from culture
medium of
human rheumatoid synovial fibrobiasts stimulated with macrophage-conditioned
medium
according to Okada, Y. et al., J. Biol. Chem. 261, 14245-14255 (1986). The
active MMP-1
was obtained by treatment of proMMP-1 with trypsin (5 p.g/mL) at 37°C
for 30 minutes,
followed by addition of soybean trypsin inhibitor (SO p,g/mL).
Determination of Inhibition Constant ~K;) for MMP-1
The activated MMP-1 is assayed using a fluorogenic substrate, Mca-Pro-Leu-Gly-
Leu-
Dpa-Ala-Arg-NHZ, Knight, C.G. et al., FEBS Lett. 296, 263-266 (1992), at
37°C in 2.0 mL of
SUBSTTrUTE SHEET (RULE 2~
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/28Z34
-56-
assay buffer containing 50 mM Tris, pH 7.6, 0.2 M sodium chloride, 50 mM
calcium chloride,
and 0.02% Brij-35. The increase in fluorescence due to cleavage of Gly-Leu
peptide bond by
MMP-3 was monitored with Perkin-Elmer LSSOB Fluorimeter (i-e~ 328 nm, ?.em 393
nm,
excitation slit 2.5, emission slit 10). Substrate and inhibitor stock
solutions were made in
DMF. For determination of K; values for MMP-1 inhibitors, a series of
intermediate inhibitor
solutions were prepared in DMF and 1 or 2 pL of the diluted inhibitor solution
was mixed
with 1 ~L of 2 mM substrate solution in DMF in a quartz cuvette containing 2
mL of assay
buffer. The enzyme (10 pL of 0.2 pM MMP-3 dilution in assay buffer) was added
at the last
to start the reaction. For routine measurement of a K; value for a reversible,
competitive
io inhibitor, the initial rates in the presence of at least four inhibitor
concentrations (two
concentrations above K; and two concentrations below K;) were measured using
[S] = 1 pM
(« Km) and [MMP-1 ] = 0.8 nM. Under these conditions, the measured K;, apP is
close to true
K;.
Calculation of K; Values
The K; for a competitive inhibitor is calculated using:
vo/v; _ ( 1 + [I]/K;, apP) and K; = K;, ep~/( 1 + [S]/Km), where vo is the
initial rate in the absence of
inhibitor, v; is the initial rate in the presence of inhibitor at the
concentration of [I], [S] is the
substrate concentration, and Km is the Michaelis constant. If slow binding is
observed (i.e. if
zo the approach to the binding equilibrium is slow), the final steady-state
rate rather than the
initial rate is taken as v;.
EXAMPLE B
Source and Activation of proMMP-2
Recombinant MMP-2 was purified from the fermentation broth of yeast Pichia
pastoris that carnes the integrated MMP-2 gene into its chromosome. In brief,
the full-length
cDNA for MMP-2 was obtained by reverse transcription of RNA from human
melanoma
A375M cell line by the reverse transcriptase polymerase chain reaction (RT-
PCR) using
sequence specific oligonucleotides. The nucleotide sequence was confirmed by
Taq cycle
3o sequencing. The cDNA was ligated into the Pichia pastoris expression vector
pHIL-D2 in
such a way that the expression of pro-MMP-2 is under the control of the
methanol inducible
alcohol oxidase promoter. The expression construct was digested with either
SaII or NsiI and
used to transform the Pichia pastoris strains KM71 and SMD1168. A large-scale
culture of a
SUBSTTTUTE S»ET (RULE 2~
CA 02356969 2001-06-27
WO 00/40553 PCT/US99/Z8234
-57-
selected clone designated 24S was performed in a high cell density fermentor
and the
recombinant MMP-2 was purified from the culture supernatant by gelatin-
sepharose 4B
(Pharmacia). The enzyme is sufficiently pure at this stage for routine
measurement of
inhibition. If desired, however, the enzyme may be further purified by AcA 44
gel filtration
(Spectra).
Deterniination of Inhibition Constant (K;~ for MMP-2
The active MMP-2 was obtained by activation of proMMP-2 at 37°C for 1 h
with 4-
aminophenylmercuric acetate which was then removed by a Sephadex G-50 spin
column. The
io enzyme is assayed using a fluorogenic substrate, Mca-Pro-Leu-Gly-Leu-Dpa-
Ala-Arg-NHZ, at
37°C in 2.0 mL of assay buffer containing ~0 mM Tris, pH 7.6, 0.2 M
sodium chloride, 50
mM calcium chloride, 0.02% Brij-35, and 50 ~M [i-mercaptoethanol. The increase
in
fluorescence is monitored (~.ex 328 nm, ~,~m 393 nm). Substrate and inhibitor
stock solutions
are made in DMF. The enzyme is added at the Iast to start the reaction. For
routine
15 measurement of a K; value for a reversible, competitive inhibitor, the
initial rates in the
presence of at least four inhibitor concentrations (two inhibitor
concentrations above K; and
two below K;) are measured using [S] = 1 p.M («Km) and [MMP-2] = 0.4 nM. Under
these
conditions, the measured K;, a~ is close to true K;.
2o EXAMPLE C
Source and Activation ofproMMP-3
ProMMP-3 (EC 3.4.24.17; Stromelysin-1) was purified from culture medium of
human rheumatoid synovial fibroblasts stimulated with macrophage-conditioned
medium
according to Okada, Y. et al., J. Biol. Chem. 261, 14245-14255 (1986). The
active MMP-3
25 was obtained by treatment of proMMP-3 with trypsin (5 ~g/mL) at 37°C
for 30 minutes,
followed by addition of soybean trypsin inhibitor (50 ~g/mL). Aliquots of the
activated
MMP-3 were stored at
-20°C.
30 Determination of Inhibition Constant (K;~ for MMP-3
The activated MMP-3 is assayed using a fluorogenic substrate, Mca-Pro-Leu-Gly-
Leu-
Dpa-Ala-Arj NHS, Knight, C.G. et al., FEBS Lett. 296, 263-266 (1992), at
37°C in an assay
buffer containing 50 mM Tris, pH 7.6, 0.2 M sodium chloride, 50 mM calcium
chloride, and
SUBSTITUTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 PCT/US991Z8234
-58-
0.02% Brij-35. The increase in fluorescence due to cleavage of Gly-Leu peptide
bond by
MMP-3 was monitored with Perkin-Elmer LS50B Fluorimeter (~.e,~ 328 nm, ~,em
393 nm,
excitation slit 2.5, emission slit 10). Substrate and inhibitor stock
solutions were made in
DMF and 0.1 % HCl-DMF, respectively. For determination of K; values for MMP-3
inhibitors, a series of intermediate inhibitor solutions were prepared in 0.1
% HCl-DMF and 1
or 2 pL of the diluted inhibitor solution was mixed with 1 pL of 2 mM
substrate solution in
DMF in a quartz cuvette containing 2 mL of assay buffer. The enzyme (10 p,L of
0.2 ~M
MMP-3 dilution in assay buffer) was added at the last to start the reaction.
For routine
measurement of a K; value for a reversible, competitive inhibitor, the initial
rates in the
to presence of at least four inhibitor concentrations (two concentrations
above K; and two
concentrations below K;) were measured using [S] = 1 ~M (« Km) and [MMP-3] = 1
nM.
Under these conditions, the measured K;, apP is close to true K;.
Calculation of K; Values
The K; for a competitive inhibitor is calculated using: Vo/v; _ ( 1 + [I]/K;,
~,) and K; _
K;, aP~/( 1 + [S]/Km), where vo is the initial rate in the absence of
inhibitor, v; is the initial rate in
the presence of inhibitor at the concentration of [I], [S] is the substrate
concentration, and K,°
is the Michaelis constant. If slow binding is observed (i.e. if the approach
to the binding
equilibrium is slow), the final steady-state rate rather than the initial rate
is taken as v;.
EXAMPLE D
Source of MMP-12 (macroph~e metalloelastase)
MMP-12 (EC 3.4.24.65) was cloned, expressed and purified according to Shapiro,
S.D. et al., J Biol. Chem. 268, 23824-23829 (1993). Autoactivation resulted in
the fully
processed active form of the enzyme. Aliquots of MMP-12 were stored at -70C.
Determination of the inhibition constant (K~ for MMP-12.
The potency of inhibitors of MMP-12 was measured using either quartz cuvettes
or
microtiter plates. The activity of MMP-12 was measured using a fluorogenic
substrate, Mca-
3o Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2, Knight, C.G. et al., FEBS Lett. 296, 263-
266 (1992), at
25°C in an assay buffer containing 50 mM Tris, pH 7.6, 0.2 M sodium
chloride, 50 mM
calcium chloride, and 0.02% Brij-35. The increase in fluorescence due to
cleavage of Gly-
Leu peptide bond by MMP-12 was monitored with a Perkin-Elmer LS50B Fluorimeter
(lex
SUBSTTTiTTE SHEET (RULE 26)
CA 02356969 2001-06-27
WO 00/40553 ~ PCT/US99/28234
-59-
328 nm, lem 393 nm, excitation slit 2.5, emission slit 10) for the cuvette
assay and with a
Molecular Devices Fmax fluorescence plate reader (~,e~ 320 nm, ~,em 405 nm)
for the
microtiter plate assay. Substrate and inhibitor stock solutions were made in
N,N-
dimethylformamide (DMF) and 0.1% HCl-DMF, respectively.
K; values were determined using the cuvette method by preparing a series of
intermediate inhibitors solutions in 0.1 % HCl-DMF and mixing the inhibitor
with substrate
(final concentration 2 mM) in a quartz cuvette containing 2 ml of assay
buffer. MMP-12 was
added to start the reaction at a concentration of 2 nM and progress curves
were generated. For
routine measurement of a K; value for a reversible competitive inhibitor, the
initial rates in the
presence of at least four inhibitor concentrations ( two concentrations above
and two
concentrations below the K;) were measured [S] = 2 mM («Km) and [MMP-12] = 2
nM.
Under these conditions, the measured K;,BpP is close to the true K;.
K; values were determined using the microtiter plate method in a manner
similar to
that described for the cuvette method with same modifications. Four different
inhibitor
concentrations (50 ml in assay buffer)of each compound were added to separate
wells of a
microtiter plate and substrate was added ( 100 ml) to get a final
concentration of 4 mM.
MMP-12 was added to a final concentration of 2 nM (50 ml) to start the
reaction. Cleavage of
substrate was recorded every 30 seconds for 30 minutes and progress curves
were generated.
Calculation of K; values
The K; for a competitive inhibitor is calculated using: V~/v; _ ( 1 + [I]/K;,
~P) and K; _
K;, ate,/( 1 + [S]/Km), where vo is the initial rate in the absence of
inhibitor, v; is the initial rate in
the presence of inhibitor at the concentration of [I], [S] is the substrate
concentration, and Km
is the Michaelis constant. If slow binding is observed (i.e. if the approach
to the binding
equilibrium is slow), the final steady-state rate rather than the initial rate
is taken as v;.
SUBSTITUTE SHEET (RULE 26)