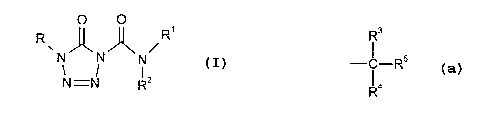Note: Descriptions are shown in the official language in which they were submitted.
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
_1_
Novel Tetrazolinone derivatives
The present invention relates to novel tetrazolinone derivatives, to a process
for their
preparation and to their use as herbicides.
In Japanese Laid-Open Patent Publications No. 82258/1995, No. 97372/1995 and
No. 118246/1995 the preparation of certain tetrazolinone derivatives and 1-
substi-
tuted-5(4H)-tetrazolinones, the intermediates thereof is disclosed. Moreover,
in EP-
A-146 279 it is shown that some tetrazolinone derivatives have herbicidal
activities.
However, the herbicidal activity of these compounds is not satisfying in every
re-
spect.
There have now been found novel tetrazolinone derivatives of the formula (I)
O O
Rw ~N~ /R~
N I N2
N =N R
in which
R represents a group
3
R
C s
- - R
1 4
R
and
R3 and R4 each independently represent a hydrogen atom, C,_~ alkyl, C,_6
haloalkyl,
C3_g cycloalkyl, C~_9 aralkyl or optionally substituted phenyl (substituents
to
the phenyl being 1 to 5 groups selected from the group consisting of halogen,
C,_6 alkyl, C,_6 haloalkyl, C,_6 alkoxy, C,_6 haloalkoxy, C,_6 alkylthio, C,_6
halo-
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-2-
alkylthio, phenyl, phenoxy and nitro),
RS represents optionally substituted phenyl, optionally substituted C;_,,
aralkyl,
optionally substituted phenoxy-C,_4 alkyl or optionally substituted phenylthio-
C,_4 alkyl (substituents to these groups being 1 to 5 groups selected from the
group consisting of halogen, C,_6 alkyl, C,_6 haloalkyl, C,_~ alkoxy, C,_~
halo-
alkoxy, C,_6 alkylthio, C,_6 alkylsulfinyl, C,_~ alkylsulphonyl, C,_~ halo-
alkylthio, phenyl, phenoxy, C,_4 alkoxycarbonyl, nitro and cyano),
R' and RZ each independently represent C,_,o alkyl, C,_6 haloalkyl, C;_8
cycloalkyl,
C~_,o alkenyl, Cz_6 alkinyl, optionally substituted C,_~ aralkyl or optionally
sub-
stituted phenyl (substituents to these groups being 1 to 5 groups selected
from
the group consisting of halogen, C,_6 alkyl, C,_6 haloalkyl, C,_6 alkoxy, C,_6
haloalkoxy, C,_6 alkylthio, C,_6 haloalkylthio, phenyl, phenoxy and vitro), or
R' and R', together with the nitrogen atom to which they are bonded, form a 5-
or 6-
membered heterocyclic group, which may be optionally condensed with
cyclohexane or benzene or may be optionally substituted by C,_4 alkyl or
halogen,
provided that in case R represents non-substituted C;_9 aralkyl or substituted
benzyl
(substituents to the benzyl being 1 to 5 groups selected from the group
consisting of halogen, C,_6 alkyl, C,_6 haloalkyl, C,_~ alkoxy, C,_~
haloalkoxy,
C,_6 alkylthio, C,_6 alkylsulfinyl, C,_6 alkylsulphonyl, C,_~ haloalkylthio,
phenyl, phenoxy, C,_4 alkoxycarbonyl, vitro and cyano),
R' represents C,_6 alkyl, C,_6 haloalkyl, C3_8 cycloalkyl, C,_~ alkenyl, Cz_6
alkinyl,
optionally substituted C;_~ aralkyl or optionally substituted phenyl (substitu-
ents to these groups being 1-5 groups selected from the group consisting of
halogen, C,_6 alkyl, C,_6 haloalkyl, C,_6 alkoxy, C,_~ haloalkoxy, C,_~
alkylthio,
C,_6 haloalkylthio, phenyl, phenoxy, methoxycarbonyl, ethoxy, carboxy,
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-3-
cyano and nitro) and
R'- represents C,_,o alkyl, C,_6 haloalkyl, C3 or C,_g cycloalkyl, C,_,o
alkenyl or CZ_6
alkinyl,
or
R' and RZ, together with the nitrogen atom to which they are bonded, form a 5-
or 6-
membered heterocyclic group, which are condensed with cyclohexane or ben-
zene and may be optionally substituted by C,_4 alkyl or halogen.
The compounds of the formula (I), according to the invention, can be obtained
by a
process in which
a) compounds of the formula (II)
O
R\
N NH (II)
N=N
in which R is defined as mentioned above,
are reacted with compounds of the formula (III)
O
M-~CsN~R~
~R2
(III)
in which R' and RZ are defined as mentioned above, and
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-4-
M represents a commonly known leaving group such as halogen (e.g. chloro,
bromo or iodo), sulfonate (e.g. triflate, mesylate, toluenesulfonate) or
alkoxy
etc., with chloro, bromo being particularly preferred, in the presence of
inert
solvents, and if appropriate, in the presence of an acid binding agent.
The compounds of the formula (I), according to the present invention have
strong
herbicidal activities and especially exhibit an excellent herbicidal action
combined
with a good compatibility with the crops. In this regard they constitute an
improve-
ment with regard to the known compounds described in the aforementioned EP-A-
146 279 which are similar to the compounds of the formula (I). Therefore, the
com-
pounds of the present invention posses a significant value as selective
herbicides.
In the formulae:
Halogen in "halogen", "haloalkyl", "haloalkoxy" and "haloalkylthio" represents
fluoro, chloro, bromo or iodo, and preferably is fluoro, chloro or bromo.
"Alkyl" may be straight chain or branched chain and there may be mentioned,
for
example, methyl, ethyl, n- or iso-propyl, n-, iso-, sec- or tert-butyl, n- or
iso-pentyl,
tert-amyl, pentan-3-yl, neopentyl, n-hexyl.
"Alkoxy" may be straight chain or branched chain and there may be mentioned,
for
example, methoxy, ethoxy, propoxy, iso-propoxy, n-, iso-, sec- or tert-butoxy,
n-
pentyloxy, n-hexyloxy.
"Alkylthio" may be straight chain or branched chain and there may be
mentioned, for
example, methylthio, ethylthio, propylthio, isopropylthio, n-butylthio.
"Alkylsulfinyl" may be straight chain or branched chain and there may be
mentioned,
for example, methylsulfinyl, ethylsulfinyl, propylsulfmyl, isopropylsulfmyl, n-
butyl-
sulfmyl.
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-5-
"Alkylsulfonyl" may be straight chain or branched chain and there may be men-
tioned, for example, methylsulfonyl, ethylsulfonyl, propylsulfonyl,
isopropylsulfo-
nyl, n-butylsulfonyl.
"Cycloalkyl" includes, for example, cyclopropyl, cyclopentyl, cyclohexyl,
cyclo-
heptyl and cyclooctyl.
As "alkenyl" there may be mentioned, for example, allyl, 1-methyl-allyl, l,l-
dimeth-
ylallyl.
As "alkinyl" there may be mentioned, for example, propargyl, 1-methyl-
propargyl,
1,1-dimethylpropargyl.
As "aralkyl" there may be mentioned, for example, benzyl, phenethyl, 1-
phenylethyl,
3-phenylpropyl, 1-methyl-2-phenylethyl, 2-methyl-2-phenylethyl, a,a-dimethyl-
benzyl, 1-methyl-3-phenylpropyl, a-propylbenzyl, a-isopropylbenzyl, 1-ethyl-2-
phenylethyl, ,-butylbenzyl, 1-ethyl-3-phenylpropyl.
"Haloalkyl" may be straight chain or branched chain and there may be
mentioned, for
example, difluoromethyl, trifluoromethyl, 2-fluoroethyl, 2-chloroethyl, 2-
bromo-
ethyl, 2,2,2-trifluoroethyl, 1,1,2,2-tetrafluoroethyl, 1,1,2,2,2-
pentafluoroethyl,
2,2,3,3,3-pentafluoropropyl, 3-chloropropyl, 1,3-difluoropropan-2-yl, 1,1,1-
trifluoro-
propan-2-yl, 2,2,3,3,4,4,4-heptafluorobutyl, 3-bromopropyl.
Haloalkyl part in "haloalkoxy" and "haloalkylthio" may be as defined in the
above-
mentioned "haloalkyl".
As "alkoxycarbonyl" there may be mentioned, for example, methoxycarbonyl, eth-
oxycarbonyl, propoxycarbonyl, isopropoxycarbonyl, butoxycarbonyl.
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
_6_
"5- or 6-membered heterocyclic group" may be optionally condensed with cyclo-
hexane or benzene and there may be mentioned, for example, pyrrol-1-yl,
pyrrolidin-
1-yl, piperidin-1-yl, indol-1-yl, indolin-1-yl, octahydroindol-1-yl, 1,2-
dihydro-
quinolin-1-yl, 1,2,3,4-tetrahydroquinolin-1-yl, decahydroquinolin-1-yl.
Moreover,
these heterocyclic groups may be optionally substituted by C,_4 alkyl such as
methyl,
ethyl, propyl, isopropyl, n-, sec-, iso- or tert-butyl etc., or halogen such
as fluoro,
chloro etc. and in case that a plurality of substituents exist, they may be
identical or
different each other.
As a preferable group of compounds of the present invention there can be
mentioned
the compounds of the formula (I)
in which
R represents a group
R3
-C-Rs
Ra
in which
R~ and R4 each independently represent a hydrogen atom, C,_5 alkyl, C,_;
haloalkyl,
C3_~ cycloalkyl, C,_g aralkyl or optionally substituted phenyl (substituents
to
the phenyl being 1 to 3 groups selected from the group consisting of halogen,
C,_~ alkyl, C,_4 haloalkyl, C,~, alkoxy, C,_4 haloalkoxy, C,_4 alkylthio, C,_4
halo-
alkylthio, phenyl, phenoxy and nitro),
RS represents optionally substituted phenyl, optionally substituted C,_,o
aralkyl,
optionally substituted phenoxy-C,_3 alkyl or optionally substituted phenylthio-
C,_3 alkyl (substituents to these groups being 1 to 4 groups selected from the
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
group consisting of halogen, C,_; alkyl, C,_; haloalkyl, C,_; alkoxy, C,_;
halo-
alkoxy, C,_; alkylthio, C,_; alkylsulfinyl, C,_; alkylsulphonyl, C,_; halo-
alkylthio, phenyl, phenoxy, C,_3 alkoxycarbonyl, vitro and cyano),
R' and R'- each independently represent C,_; alkyl, C,_; haloalkyl, C~_,
cycloalkyl, Cz_;
alkenyl, CZ_; alkinyl, optionally substituted C~_~ aralkyl or optionally sub-
stituted phenyl (substituents to these groups being 1 to 4 groups selected
from
the group consisting of halogen, C,_; alkyl, C,_; haloalkyl, C,_; alkoxy, C,_;
haloalkoxy, C,_; alkylthio, C,_; haloalkylthio, phenyl, phenoxy and vitro), or
R' and R', together with the nitrogen atom to which they are bonded, form a 5-
or 6-
membered heterocyclic group, which may be optionally condensed with
cyclohexane or benzene or may be optionally substituted by methyl, ethyl, n-
propyl, isopropyl, fluoro, chloro or bromo
provided that in case R represents non-substituted C,_~ aralkyl or optionally
substituted benzyl (substituents to the benzyl being 1 to 4 groups selected
from the group consisting of halogen, C,_; alkyl, C,_; haloalkyl, C,_; alkoxy,
C,_; haloalkoxy, C,_; alkylthio, C,_; alkylsulfinyl, C,_; alkylsulphonyl, C,_;
haloalkylthio, phenyl, phenoxy, C,_, alkoxycarbonyl, vitro and cyano),
R' represents C,_; alkyl, C,_;haloalkyl, C3_, cycloalkyl, Cz_; alkenyl, C,_;
alkinyl,
optionally substituted C,_~ aralkyl or optionally substituted phenyl (substitu-
ents to these groups being 1 to 4 groups selected from the group consisting of
halogen, C,_; alkyl, C,_; haloalkyl, C,_; alkoxy, C,_; haloalkoxy, C,_;
alkylthio,
C,_; haloalkylthio, phenyl, phenoxy, methoxy carbonyl, ethoxy carbonyl,
cyano and vitro) and
R' represents C,_; haloalkyl, C3 or C, cycloalkyl or C,_; alkinyl, or R' and
R',
together with the nitrogen atom to which they are bonded, may form a 5- or 6-
membered heterocyclic group, which are condensed with cyclohexane or ben-
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
_g_
zene and may be optionally substituted by methyl, ethyl, n-propyl, isopropyl,
fluoro, chloro or bromo.
Furthermore, as a more preferable series of compounds there can be mentioned
the
compounds of the aforementioned formula (I)
in which
R represents a group
R3
-~-Rs
1 4
R
in which
R~ and R4 each independently represent a hydrogen atom, methyl, ethyl, n-
propyl,
iso-propyl, n-butyl, iso-butyl, sec-butyl, chloromethyl, trifluoromethyl,
cyclopropyl, cyclopentyl, cyclohexyl, cycloheptyl, benzyl or optionally sub-
stituted phenyl (substituents to the phenyl being 1 to 2 groups selected from
the group consisting of fluoro, chloro, methyl, trifluoromethyl, methoxy, tri-
fluoromethoxy, difluoromethoxy, methylthio, trifluoromethylthio and nitro),
R' represents phenyl, benzyl, phenethyl, 1-phenylethyl, 3-phenylpropyl, 1-
methyl-2-phenylethyl, 2-methyl-2-phenylethyl, a,a-dimethylbenzyl, phenoxy-
methyl, 1-phenoxyethyl, 2-phenoxyethyl, phenylthiomethyl, 1-phenyl-
thioethyl or 2-phenylthioethyl (where these groups may be substituted by 1 to
3 groups selected from the group consisting of fluoro, chloro, bromo, methyl,
ethyl, n-propyl, iso-propyl, tert-butyl, trifluoromethyl, methoxy, ethoxy, iso-
propoxy, trifluoromethoxy, difluoromethoxy, 2,2,2-trifluoroethoxy, methyl-
thio, ehtylthio, iso-propylthio, methylsulfinyl, methylsulfonyl, trifluorometh-
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-9-
R1 and RZ each independently represent methyl, ethyl, n-propyl, iso-propyl, n-
butyl,
iso-butyl, sec-butyl, tent-butyl, n-pentyl, 2-chloroethyl, cyclopropyl, cyclo
pentyl, cyclohexyl, cycloheptyl, allyl, propargyl, 1-methyl-3-propinyl or l,l
dimethyl-3-propinyl, or
R' and RZ each independently represent benzyl, phenethyl, 1-phenylethyl, 3-
phenyl-
propyl, 1-methyl-2-phenylethyl, 2-methyl-2-phenylethyl, a,a-dimethylbenzyl
or phenyl (where these groups may be optionally substituted by 1 to 3 groups
selected from the group consisting of fluoro, chloro, bromo, methyl, ethyl, n-
propyl, iso-propyl, tert-butyl, trifluoromethyl, methoxy, ethoxy, iso-propoxy,
trifluoromethoxy, difluoromethoxy, 2,2,2-trifluoroethoxy, methylthio, eth-
ylthio, iso-propylthio, methylsulfinyl, methylsulfonyl, trifluoromethylthio,
2,2,2-trifluoroethylthio, phenyl, phenoxy, methoxycarbonyl, ethoxycarbonyl,
nitro and cyano), or
Rl and Rz, together with the nitrogen atom to which they are bonded, may form
pyr-
rolidin-1-yl, 2-methylpyrrolidin-1-yl, 2,5-dimethylpyrrolidin-1-yl, piperidin-
1-yl, 2-methylpiperidin-1-yl, 2,6-dimethylpiperidin-1-yl, octahydroindol-1-yl,
2-methyloctahydroindol-1-yl, indolin-1-yl, 2-methylindolin-1-yl, 5-fluoro-2-
methylindolin-1-yl, 2,3-dimethylindolin-1-yl, decahydroquinolin-1-yl, 2-
methyldecahydroquinolin-1-yl, 1,2,3,4-tetrahydroquinolin-1-yl, 2-methyl-
1,2,3,4-tetrahydroquinolin-1-yl, 6-fluoro-2-methyl-1,2,3,4-tetrahydroquino-
lin-1-yl, 6-chloro-2-methyl-1,2,3,4-tetrahydroquinolin-1-yl, 2,6-dimethyl-
1,2,3,4-tetrahydroquinolin-1-yl, 2,2-dimethyl-1,2,3,4-tetrahydroquinolin-1-yl,
6-fluoro-2,2-dimethyl-1,2,3,4-tetrahydroquinolin-1-yl, 2,2,4-trimethyl-
1,2,3,4-tetrahydroquinolin-1-yl, 6-chloro-2,2,4-trimethyl-1,2,3,4-tetrahydro-
quinolin-1-yl, 2,2-dimethyl-1,2-dihydroquinolin-1-yl, 6-fluoro-2,2-dimethyl-
1,2-dihydroquinolin-1-yl, 2,2,4-trimethyl-1,2-dihydroquinolin-1-yl or 2,2,4,6-
tetramethyl-1,2-dihydroquinolin-1-yl,
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-10-
provided that in case R represents benzyl, phenethyl, 1-phenylethyl, 3-
phenylpropyl,
1-methyl-2-phenylethyl, 2-methyl-2-phenylethyl, a,a-dimethylbenzyl or
substituted benzyl (substituents to the benzyl are 1 to 3 groups selected from
the group consisting of fluoro, chloro, bromo, methyl, ethyl, n-propyl, iso-
propyl, tert-butyl, trifluoromethyl, methoxy, ethoxy, iso-propoxy,
trifluoromethoxy, difluoromethoxy, 2,2,2-trifluoroethoxy, methylthio,
ehtylthio, methylsulfinyl, methylsulfonyl, iso-propylthio,
trifluoromethylthio,
2,2,2-trifluoroethylthio, phenyl, phenoxy, methoxycarbonyl, ethoxycarbonyl,
nitro and cyano),
Rl represents methyl, ethyl, n-propyl, iso-propyl, n-butyl, iso-butyl, sec-
butyl,
tert-butyl, n-pentyl, 1-chloroethyl, cyclopropyl, cyclopentyl, cyclohexyl, cy-
cloheptyl, allyl, propargyl, 1-methyl-3-propinyl or l,l-dimethyl-3-propinyl,
benzyl, phenethyl, 1-phenylethyl, 3-phenylpropyl, 1-methyl-2-phenylethyl, 2-
methyl-2-phenylethyl, a,a-dimethylbenzyl or phenyl (where these groups
may be optionally substituted by 1 to 3 groups selected from the group
consisting of fluoro, chloro, bromo, methyl, ethyl, n-propyl, iso-propyl, tert-
butyl, trifluoromethyl, methoxy, ethoxy, iso-propoxy, trifluoromethoxy,
difluoromethoxy, 2,2,2-trifluoroethoxy, methylthio, ethylthio, iso-propylthio,
methylsulfmyl, methylsulfonyl, trifluoromethylthio, 2,2,2-trifluoroethylthio,
phenyl, phenoxy, methoxycarbonyl, ethoxycarbonyl, nitro and cyano), and
RZ represents 1-chloroethyl, cyclopropyl, cycloheptyl or propargyl, or
Rl and R2, together with the nitrogen atom to which they are bonded, may form
octa-
hydroindol-1-yl, 2-methyloctahydroindol-1-yl, indolin-1-yl, 2-methylindolin-
1-yl, 5-fluoro-2-methylindolin-1-yl, 2,3-dimethylindolin-1-yl, decahydro-
quinolin-1-yl, 2-methyldecahydroquinolin-1-yl, 1,2,3,4-tetrahydroquinolin-1-
yl, 2-methyl-1,2,3,4-tetrahydroquinolin-1-yl, 6-fluoro-2-methyl-1,2,3,4-tetra-
hydroquinolin-1-yl, 6-chloro-2-methyl-1,2,3,4-tetrahydroquinolin-1-yl, 2,6-
dimethyl-1,2,3,4-tetrahydroquinolin-1-yl, 2,2-dimethyl-1,2,3,4-tetrahydro-
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-11-
quinolin-1-yl, 6-fluoro-2,2-dimethyl-1,2,3,4-tetrahydroquinolin-1-yl, 2,2,4-
trimethyl-1,2,3,4-tetrahydroquinolin-1-yl, 6-chloro-2,2,4-trimethyl-1,2,3,4-
tetrahydroquinolin-1-yl, 2,2-dimethyl-1,2-dihydroquinolin-1-yl, 6-fluoro-2,2-
dimethyl-1,2-dihydroquinolin-1-yl, 2,2,4-trimethyl-1,2-dihydroquinolin-1-yl
or 2,2,4,6-tetramethyl-1,2-dihydroquinolin-1-yl.
The radical definitions listed above, whether general or listed in ranges of
preference
can be combined as desired with one another, thus including combinations
between
the preferred ranges cited.
The compounds of the above formula (I) contain an asymmetric carbon atom, as
shown by the following formula
R3 O O
RS W ~ ~ ,R,
R N N R2
In which, the carbon atom represented as C* is asymmetric carbon atom. The com-
pounds of the formula (I) which are meant according to the invention are the
pure,
optically active enantiomers as well as mixtures in appropriate ratio of
optically ac-
tive enantiomers (including the racemate).
Therefore, the compounds of the above formula (I), according to the invention
are
optically active enantiomers or mixtures thereof.
Furthermore, as specific examples of the compounds of the formula (I),
according to
the invention, there may be shown the following compounds in Table 1.
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-12-
Table 1
R5
~C\N~N~N/R~
/ N N RZ
R3 R4 RS R 1 R?
H H phenyl ethyl cyclopropyl
H H phenyl ethyl cycloheptyl
H H phenyl 2-chloroethyl2-chloroethyl
H H 2-methylphenylethyl cycloheptyl
H H 2-methylphenylethyl cyclooctyl
H H 2-methylphenyln-propyl cyclopropyl
H H 2-methylphenyl2-chloroethyl2-chloroethyl
H H 3-methylphenylethyl cyclopropyl
H H 4-methylphenylethyl cyclopropyl
H H 4-methylphenylethyl cycloheptyl
H H 2-fluorophenylethyl cycloheptyl
H H 3-fluorophenylethyl cycloheptyl
H H 2-chlorophenylethyl cyclopropyl
H H 3-chlorophenyl2-chloroethyl2-chloroethyl
H H 4-chlorophenyl2-chloroethyl2-chloroethyl
H H 2,3-dimethyl-ethyl cyclopropyl
phenyl
H H 2,4-dichlorophenylethyl cyclopropyl
methyl H phenyl ethyl cyclopropyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-13-
Table 1 (continued)
R3 R4 RS R1 R2
methyl H phenyl ethyl cycloheptyl
methyl H phenyl n-propyl cyclopropyl
methyl H phenyl 2-chloroethyl2-chloroethyl
methyl H 2-methylphenyl methyl methyl
methyl H 2-methylphenyl methyl ethyl
methyl H 2-methylphenyl methyl n-propyl
methyl H 2-methylphenyl ethyl ethyl
methyl H 2-methylphenyl ethyl n-propyl
methyl H 2-methylphenyl ethyl isopropyl
methyl H 2-methylphenyl ethyl n-butyl
methyl H 2-methylphenyl ethyl sec-butyl
methyl H 2-methylphenyl ethyl tent-butyl
methyl H 2-methylphenyl ethyl n-pentyl
methyl H 2-methylphenyl ethyl cyclopropyl
methyl H 2-methylphenyl ethyl cyclopentyl
methyl H 2-methylphenyl ethyl cyclohexyl
methyl H 2-methylphenyl ethyl cycloheptyl
methyl H 2-methylphenyl n-propyl n-propyl
methyl H 2-methylphenyl n-propyl isopropyl
methyl H 2-methylphenyl n-propyl n-butyl
methyl H 2-methylphenyl n-propyl sec-butyl
methyl H 2-methylphenyl n-butyl n-butyl
methyl H 2-methylphenyl isobutyl isobutyl
methyl H 2-methylphenyl 2-chloroethyl2-chloroethyl
methyl H 2-methylphenyl ally) allyl
methyl H 2-methylphenyl ethyl benzyl
methyl H 2-methylphenyl ethyl 3-methylbenzyl
methyl H 2-methylphenyl n-propyl 4-fluorobenzyl
methyl H 2-methylphenyl ethyl phenethyl
methyl H 2-methylphenyl ethyl 3-phenylpropyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
- 14-
Table 1 (continued)
R3 R4 RS RI R2
methyl H 2-methylphenyln-propyl 1-methyl-2-phenylethyl
methyl H 2-methylphenylethyl 2-methyl-2-phenylethyl
methyl H 2-methylphenylethyl a,a-dimethylbenzyl
methyl H 2-methylphenylethyl phenyl
methyl H 2-methylphenylisopropyl phenyl
methyl H 2-methylphenylisopropyl 2-methylphenyl
methyl H 2-methylphenylisopropyl 3-methylphenyl
methyl H 2-methylphenylisopropyl 4-methylphenyl
methyl H 2-methylphenylisopropyl 4-methoxyphenyl
methyl H 2-methylphenylisopropyl 4-ethoxyphenyl
methyl H 2-methylphenylisopropyl 4-isopropoxy-phenyl
methyl H 2-methylphenylisopropyl 4-fluorophenyl
methyl H 2-methylphenylisopropyl 4-trifluoromethylphenyl
methyl H 2-methylphenylisopropyl 2,4-dimethylphenyl
methyl H 2-methylphenylisopropyl 3,4-dimethylphenyl
methyl H 2-methylphenylisopropyl 2,4-difluorophenyl
methyl H 2-methylphenylisopropyl 2,4-dichlorophenyl
methyl H 2-methylphenylpyrrolidin-1-yl
methyl H 2-methylphenyl2-methylpyrrolidin-1-yl
methyl H 2-methylphenyl2,5-dimethylpyrrolidin-1-yl
methyl H 2-methylphenylpiperidin-1-yl
methyl H 2-methylphenyl2-methylpiperidin-1-yl
methyl H 2-methylphenyl2,6-dimethylpiperidin-1-yl
methyl H 2-methylphenyloctahydroindol-1-yl
methyl H 2-methylphenyl2-methyloctahydroindol-1-yl
methyl H 2-methylphenylindolin-1-yl
methyl H 2-methylphenyl2-methylindolin-1-yl
methyl H 2-methylphenyl5-fluoro-2-methylindolin-1-yl
methyl H 2-methylphenyl2,3-dimethylindolin-I-yl
methyl H 2-methylphenyldecahydroquinolin-I-yl
methyl H 2-methylphenyl2-methyl-decahydroquinolin-I-yl
methyl H 2-methylphenyl1,2,3,4-tetrahydroquinolin-I-yl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-15-
Table 1 (continued)
R3 R4 RS _ R1 R2
methyl H 2-methylphenyl 2-methyl-1,2,3,4-tetrahydroquinolin-1-yl
methyl H 2-methylphenyl 6-fluoro-2-methyl-1,2,3,4-tetrahydroquinolin-1-
Yl
methyl H 2-methylphenyl 6-chloro-2-methyl-1,2,3,4-tetrahydroquinolin-1-
YI
methyl H 2-methylphenyl 2,6-dimethyl-1,2,3,4-tetrahydroquinolin-1-yl
methyl H 2-methylphenyl 2,2-dimethyl-1,2,3,4-tetrahydroquinolin-1-yl
methyl H 2-methylphenyl 6-fluoro-2,2-dimethyl-1,2,3,4-tetrahydroquin-
olin-I-yl
methyl H 2-methylphenyl 2,2,4-trimethyl-1,2,3,4-tetrahydroquinolin-1-yl
methyl H 2-methylphenyl 6-chloro-2,2,4-trimethyl-1,2,3,4-tetrahydro-
quinolin-1-yl
methyl H 2-methylphenyl 2,2-dimethyl-1,2-dihydroquinolin-1-yl
methyl H 2-methylphenyl 6-fluoro-2,2-dimethyl-1,2-dihydroquinolin-I-yl
methyl H 2-methylphenyl 2,2,4-trimethyl-1,2-dihydroquinolin-1-yl
methyl H 2-methylphenyl 2,2,4,6-tetramethyl-1,2-dihydroquinolin-1-yl
methyl H 2-methylphenyl propargyl propargyl
methyl H 2-methylphenyl 1-methyl-3- n-propyl
propinyl
methyl H 2-methylphenyl I,1-dimethyl-3-n-propyl
propinyl
methyl H 3-methylphenyl ethyl isopropyl
methyl H 3-methylphenyl ethyl cyclohexyl
methyl H 3-methylphenyl isopropyl isopropyl
methyl H 3-methylphenyl isopropyl cyclopentyl
methyl H 3-methylphenyl isopropyl cyclohexyl
methyl H 3-methylphenyl isopropyl phenyl
methyl H 3-methylphenyl isopropyl 4-ethylphenyl
methyl H 3-methylphenyl isopropyl 4-n-propylphenyl
methyl H 3-methylphenyl isopropyl 4-isopropylphenyl
methyl H 3-methylphenyl isopropyl 4-tert-butylphenyl
methyl H 3-methylphenyl isopropyl 2-chlorophenyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
- 16-
Table 1 (continued)
R4 R5 - R 1
methyl H 3-methylphenyl isopropyl 3-bromophenyl
methyl H 3-methylphenyl isopropyl 4-trifluoromethoxyphenyl
methyl H 3-methylphenyl isopropyl 4-difluoromethoxyphenyl
methyl H 3-methylphenyl isopropyl 4-(2,2,2-trifluoroeth-
oxy)phenyl
methyl H 3-methylphenyl isopropyl 4-methylthiophenyl
methyl H 3-methylphenyl isopropyl 4-ethylthiophenyl
methyl H 3-methylphenyl isopropyl 4-isopropylthiophenyl
methyl H 3-methylphenyl isopropyl 4-methylsulfinylphenyl
methyl H 3-methylphenyl isopropyl 4-methylsulfonylphenyl
methyl H 3-methylphenyl isopropyl 4-trifluoromethylthio-
phenyl
methyl H 3-methylphenyl isopropyl 4-difluoromethylthio-
phenyl
methyl H 3-methylphenyl isopropyl 4-(2,2,2-trifluoroethyl-
thio)phenyl
methyl H 3-methylphenyl isopropyl 4-phenylphenyl
methyl H 3-methylphenyl isopropyl 4-phenoxyphenyl
methyl H 3-methylphenyl isopropyl 4-cyanophenyl
methyl H 3-methylphenyl isopropyl 4-nitrophenyl
methyl H 3-methylphenyl isopropyl 2-methoxycarbonylphenyl
methyl H 3-methylphenyl isopropyl 2-ethoxycarbonylphenyl
methyl H 4-methylphenyl ethyl ethyl
methyl H 4-methylphenyl ethyl n-pentyl
methyl H 4-methylphenyl ethyl cyclohexyl
methyl H 4-methylphenyl isopropyl phenyl
methyl H 4-methylphenyl isopropyl 2-methylphenyl
methyl H 4-methylphenyl isopropyl 3-chlorophenyl
methyl H 4-methylphenyl isopropyl 2,4-difluorophenyl
methyl H 4-methylphenyl ethyl cyclohexyl
methyl H 4-methylphenyl isopropyl phenyl
methyl H 4-n-propylphenylethyl cyclohexyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
- 17-
Table 1 (continued)
R3 R4 RS R1 R2
methyl H 4-n-propylphenylisopropyl phenyl
~
methyl H 4-isopropylphenylethyl cyclohexyl
methyl H 4-isopropylphenylisopropyl phenyl
methyl H 4-tert-butylphenylmethyl methyl
methyl H 4-tert-butylphenylethyl ethyl
methyl H 4-tert-butylphenylisopropyl phenyl
methyl H 2-methoxyphenylisopropyl phenyl
methyl H 3-methoxyphenylethyl cyclohexyl
methyl H 3-methoxyphenylisopropyl phenyl
methyl H 4-methoxyphenylisopropyl phenyl
methyl H 2-ethoxyphenyl isopropyl phenyl
methyl H 4-ethoxyphenyl isopropyl phenyl
methyl H 4-isopropoxyphenylisopropyl phenyl
methyl H 4-methylthiophenylisopropyl phenyl
methyl H 4-ethylthiophenylisopropyl phenyl
methyl H 4-isopropylthiophenylisopropyl phenyl
methyl H 4-methylsulfinylmethylisopropyl phenyl
methyl H 4-methylsulfonylmethylisopropyl phenyl
methyl H 2-fluorophenyl ethyl ethyl
methyl H 2-fluorophenyl ethyl cyclohexyl
methyl H 2-fluorophenyl allyl allyl
methyl H 2-fluorophenyl isopropyl phenyl
methyl H 3-fluorophenyl ethyl ethyl
methyl H 3-fluorophenyl ethyl isopropyl
methyl H 3-fluorophenyl ethyl cyclohexyl
methyl H 3-fluorophenyl 2-chloroethyl2-chloroethyl
methyl H 3-fluorophenyl isopropyl phenyl
methyl H 4-fluorophenyl ethyl ethyl
methyl H 4-fluorophenyl ethyl isopropyl
methyl H 4-fluorophenyl ethyl cyclohexyl
methyl H 4-fluorophenyl isopropyl cyclohexyl
methyl H 4-fluorophenyl isopropyl phenyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-18-
Table 1 (continued)
3 R4 RS RI R' _
methyl H 4-fluorophenyl isopropyl 4-chlorophenyl
methyl H 4-fluorophenyl isopropyl 2,4-difluorophenyl
methyl H 4-fluorophenyl pyrrolidin-I-yl
methyl H 4-fluorophenyl 2-methylpyrrolidin-I-yl
methyl H 4-fluorophenyl 2,~-dimethylpyrrolidin-I-yl
methyl H 4-fluorophenyl piperidin-1-yl
methyl H 4-fluorophenyl 2-methylpiperidin-1-yl
methyl H 4-fluorophenyl 2,6-dimethylpiperidin-1-yl
methyl H 4-fluorophenyl octahydroindol-1-yl
methyl H 4-fluorophenyl 2-methyloctahydroindol-1-yl
methyl H 4-fluorophenyl indolin-1-yl
methyl H 4-fluorophenyl 2-methylindolin-I-yl
methyl H 4-fluorophenyl 5-fluoro-2-methylindolin-1-yl
methyl H 4-fluorophenyl 2,3-dimethylindolin-1-yl
methyl H 4-fluorophenyl decahydroquinolin-1-yl
methyl H 4-fluorophenyl 2-methyl-decahydroquinolin-1-yl
methyl H 4-fluorophenyl 1,2,3,4-tetrahydroquinolin-I-yl
methyl H 4-fluorophenyl 2-methyl-1,2,3,4-tetrahydroquinolin-1-yl
methyl H 4-fluorophenyl 6-fluoro-2-methyl-1,2,3,4-tetrahydroquinolin-1-
YI
methyl H 4-fluorophenyl 6-chloro-2-methyl-1,2,3,4-tetrahydroquinolin-1-
Yl
methyl H 4-fluorophenyl 2,6-dimethyl-1,2,3,4-tetrahydroquinolin-1-yl
methyl H 4-fluorophenyl 2,2-dimethyl-1,2,3,4-tetrahydroquinolin-1-yl
methyl H 4-fluorophenyl 6-fluoro-2,~-dimethyl-1,2,3,4-tetrahydro-
quinolinon-1-yl
methyl H 4-fluorophenyl 2,2,4-trimethyl-1,2,3,4-tetrahydroquinolin-I-yl
methyl H 4-fluorophenyl 6-chloro-2,2,4-trimethyl-1,2,3,4-tetrahydro-
quinolin-I-yl
methyl H fluorophenyl 2,2-dimethyl-1,2-dihydroquinolin-1-vl
methyl H 4-fluorophenyl 6-fluoro-2,2-dimethyl-1,2-dihydroquinolin-1-yl
methyl H 4-fluorophenyl 2,2,4-trimethyl-1,2-dihydroquinolin-1-yl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-19-
Table 1 (continued)
R4 RS R 1 R2 _.
methyl H 4-fluorophenyl 2,2,4,6-tetramethyl-1,2-dihydroquinolin-1-yl
methyl H 4-fluorophenyl propargyl propargyl
methyl H 4-fluorophenyl 1-methyl-3- n-butyl
propinyl
methyl H 4-fluorophenyl l,l-dimethyl-3-n-butyl
propinyl
methyl H 2-chlorophenyl methyl isopropyl
methyl H 2-chlorophenyl ethyl ethyl
methyl H 2-chlorophenyl ethyl sec-butyl
methyl H 2-chlorophenyl ethyl cyclohexyl
methyl H 2-chlorophenyl n-propyl n-propyl
methyl H 2-chlorophenyl isopropyl phenyl
methyl H 2-chlorophenyl isopropyl 3-methoxyphenyl
methyl H 2-chlorophenyl isopropyl 3,4-dimethylphenyl
methyl H 3-chlorophenyl ethyl ethyl
methyl H 3-chlorophenyl ethyl n-propyl
methyl H 3-chlorophenyl ethyl cyclopropyl
methyl H 3-chlorophenyl ethyl cyclohexyl
methyl H 3-chlorophenyl 2-chloroethyl2-chloroethyl
methyl H 3-chlorophenyl allyl allyl
methyl H 3-chlorophenyl isopropyl phenyl
methyl H 3-chlorophenyl isopropyl 4-fluorophenyl
methyl H 3-chlorophenyl isopropyl 3-bromophenyl
methyl H 4-chlorophenyl methyl isopropyl
methyl H 4-chlorophenyl ethyl ethyl
methyl H 4-chlorophenyl ethyl iso-butyl
methyl H 4-chlorophenyl ethyl cyclohexyl
methyl H 4-chlorophenyl n-propyl isopropyl
methyl H 4-chlorophenyl n-propyl iso-butyl
methyl H 4-chlorophenyl isopropyl phenyl
methyl H 4-chlorophenyl isopropyl 3-trifluoromethylphenyl
methyl H 3-bromophenyl isopropyl phenyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-20-
Table 1 (continued)
R; R4 RS R 1 R2
methyl H 4-bromophenyl ethyl ethyl
methyl H 4-bromophenyl ethyl cyclohexyl
methyl H 4-bromophenyl isopropyl phenyl
methyl H 2-trifluoromethylphenylethyl cyclohexyl
methyl H 2-trifluoromethylphenylisopropyl phenyl
methyl H 3-trifluoromethylphenylisopropyl phenyl
methyl H 4-trifluoromethylphenylisopropyl phenyl
methyl H 4-trifluoromethylphenylethyl cyclohexyl
methyl H 4-trifluoromethylphenylisopropyl phenyl
methyl H 4-difluoromethoxy-isopropyl phenyl
phenyl
methyl H 4-(2,2,2-trifluoroeth-isopropyl phenyl
oxy)phenyl
methyl H 4-trifluoromethylthio-ethyl cyclohexyl
phenyl
methyl H 4-trifluoromethylthio-isopropyl phenyl
phenyl
methyl H 4-difluoromethylthio-isopropyl phenyl
phenyl
methyl H 4-(2,2,2-trifluoroethyl-isopropyl phenyl
thio)phenyl
methyl H 2-methoxycarbonyl-isopropyl phenyl
phenyl
methyl H 2-ethoxycarbonylphenylisopropyl phenyl
methyl H 4-cyanophenyl isopropyl phenyl
methyl H 4-nitrophenyl isopropyl phenyl
methyl H 2,3-dimethylphenylethyl cyclohexyl
methyl H 2,3-dimethylphenylisopropyl phenyl
methyl H 2,4-dimethylphenylethyl ethyl
methyl H 2,4-dimethylphenylethyl isopropyl
methyl H 2,4-dimethylphenylallyl allyl
methyl H 2,4-dimethylphenylethyl phenyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-21 -
Table 1 (continued)
R3 R4 R5 R1 R2
methyl H 2,5-dimethylphenylethyl ethyl
methyl H 2,5-dimethylphenyln-propyl phenyl
methyl H 2,6-dimethylphenylethyl ethyl
methyl H 2,6-dimethylphenylethyl cyclopentyl
methyl H 3,4-dimethylphenylethyl ethyl
methyl H 3,4-dimethylphenylethyl cyclopropyl
methyl H 3,4-dimethylphenylethyl cyclohexyl
methyl H 3,4-dimethylphenylisopropyl benzyl
methyl H 3,4-dimethylphenylisopropyl phenyl
methyl H 3,5-dimethylphenylethyl ethyl
methyl H 3,5-dimethylphenylethyl 4-fluorophenyl
methyl H 3,5-dimethylphenylisopropyl phenyl
methyl H 2-methyl-6- isopropyl phenyl
chloro-phenyl
methyl H 2,4-difluorophenylisopropyl phenyl
methyl H 3,4-difluorophenylisopropyl phenyl
methyl H 3-fluoro-4-methoxy-isopropyl phenyl
phenyl
methyl H 2,3-dichlorophenylethyl ethyl
methyl H 2,3-dichlorophenylethyl cyclohexyl
methyl H 2,3-dichlorophenylisopropyl phenyl
methyl H 2,4-dichlorophenylethyl ethyl
methyl H 2,4-dichlorophenylethyl cyclopentyl
methyl H 2,4-dichlorophenylethyl cyclohexyl
methyl H 2,4-dichlorophenylethyl phenyl
methyl H 2,4-dichlorophenylisopropyl phenyl
methyl H 2,6-dichlorophenylisopropyl phenyl
methyl H 3,4-dichlorophenylisopropyl phenyl
methyl H 3,5-ditrifluoromethyl-isopropyl phenyl
phenyl
methyl H 2,4,5-trimethylphenylethyl cyclohexyl
methyl H 2,4,5-trimethylphenylisopropyl phenyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-22-
Table 1 (continued)
R3 R4 R5. R1 R2 -
methyl H 2,4,6-trimethylphenylethyl cyclohexyl
methyl H 2,4,6-trimethylphenylisopropyl phenyl
methyl H 2,3,4-trichlorophenylethyl ethyl
methyl H 2,3,4-trichlorophenylethyl isopropyl
methyl H 2,3,4-trichlorophenylethyl cyclohexyl
methyl H 2,3,4-trichlorophenylethyl phenyl
methyl H 2,3,4-trichlorophenylisopropyl phenyl
ethyl H phenyl ethyl cyclopropyl
ethyl H phenyl 2-chloroethyl2-chloroethyl
ethyl H 3-methylphenyl isopropyl phenyl
ethyl H 4-methylphenyl isopropyl 4-fluorophenyl
ethyl H 3-methoxyphenylisopropyl phenyl
ethyl H ' 2-fluorophenyl isopropyl phenyl
ethyl H 4-fluorophenyl isopropyl phenyl
ethyl H 3-chlorophenyl isopropyl phenyl
ethyl H 3-bromophenyl isopropyl phenyl
ethyl H 2-trifluoromethylphenylisopropyl phenyl
ethyl H 3,4-dimethylphenylisopropyl phenyl
ethyl H 3,4-dichlorophenylisopropyl phenyl
n-propyl H phenyl ethyl cyclohexyl
n-propyl H phenyl isopropyl phenyl
n-propyl H 3-methylphenyl isopropyl phenyl
n-propyl H 3-fl uorophenylisopropyl phenyl
n-propyl H 3-chlorophenyl isopropyl phenyl
isopropylH phenyl ethyl ethyl
isopropylH phenyl ethyl isopropyl
isopropylH phenyl ethyl c~~clohexyl
isopropylH phenyl n-propyl n-propyl
isopropylH phenyl isopropyl phenyl
isopropylH 2-fl uorophenylisopropyl 4-fl uorophenyl
isopropylH 2,4-dimethylphenylisopropyl phenyl
n-butyl H phenyl isopropyl phenyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
- 23 -
Table 1 (continued)
R3 R4 RS RI R2
sec-butyl H phenyl isopropyl phenyl
cyclopropylH phenyl' isopropyl phenyl
cyclopropylH 4-methoxyphenylisopropyl phenyl
cyclopentylH phenyl isopropyl phenyl
cyclopentylH phenyl ethyl ethyl
cyclohexylH phenyl ethyl cyclohexyl
cyclohexylH phenyl isopropyl phenyl
cyclohexylH 4-chlorophenyl isopropyl phenyl
cycloheptylH phenyl isopropyl phenyl
benzyl H phenyl isopropyl phenyl
2-chloro- H phenyl isopropyl phenyl
benzyl
phenyl H phenyl isopropyl phenyl
3-methyl- H phenyl isopropyl phenyl
phenyl
3-methoxy-H phenyl isopropyl phenyl
phenyl
4-fl uorophenylH phenyl isopropyl phenyl
4-chloro- H phenyl isopropyl phenyl
phenyl
3-trifluoro-H phenyl isopropyl phenyl
methylphenyl
3,4-dimethyl-H phenyl isopropyl phenyl
phenyl
3,4-dichloro-H phenyl isopropyl phenyl
phenyl
3-methyl- H 3-methylphenyl isopropyl phenyl
phenyl
3-methyl- H 3,4-dimethylphenylisopropyl phenyl
phenyl
2-fluorophenylH 3-methylphenyl isopropyl phenyl
4-fl uorophenylH 4-fluorophenyl isopropyl phenyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-24-
Table 1 (continued)
I R2
4-chloro- H 3-methylphenyl isopropyl phenyl
phenyl
chloromethylH phenyl isopropyl phenyl
chloromethylH phenyl isopropyl 4-fluorophenyl
trifluoro-H phenyl isopropyl phenyl
methyl
trifluoro-H phenyl isopropyl 4-fluorophenyl
methyl
methyl methylphenyl ethyl cyclopropyl
methyl methylphenyl ethyl cycloheptyl
methyl methyl2-methylphenyl isopropyl phenyl
methyl methyl3-methylphenyl isopropyl phenyl
methyl methyl4-methylphenyl isopropyl phenyl
methyl methyl4-methoxyphenylisopropyl phenyl
methyl methyl3-fluorophenyl isopropyl phenyl
methyl methyl4-fluorophenyl isopropyl phenyl
methyl methyl2-chlorophenyl isopropyl phenyl
methyl methyl3-chlorophenyl ethyl ethyl
methyl methyl3-chlorophenyl ethyl cyclohexyl
methyl methyl3-chlorophenyl isopropyl phenyl
methyl methyl3,5-dimethylphenylisopropyl phenyl
methyl methyl3,5-dichlorophenylisopropyl phenyl
ethyl methylphenyl isopropyl phenyl
ethyl methyl3-methylphenyl isopropyl phenyl
ethyl ethyl 3-chlorophenyl isopropyl phenyl
ethyl ethyl phenyl isopropyl 2-methylphenyl
ethyl n-propylphenyl isopropyl phenyl
n-propyl methylphenyl isopropyl phenyl
n-propyl methyl3-chlorophenyl isopropyl phenyl
isopropyl methylphenyl isopropyl phenyl
isopropyl methyl3-fluorophenyl isopropyl phenyl
H H benzyl ethyl cyclopropyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
- 25 -
Table 1 (continued)
R3 R4 RS R1 R2
H H 3-methylbenzyl ethyl ~ cyclopropyl
H H 3-methoxybenzylisopropyl phenyl
H H 3-chlorobenzyl isopropyl 4-fluorophenyl
H H 1-phenylethyl ethyl - cyclopropyl
H H 1-phenylethyl 2-chloroethyl2-chloroethyl
H H 1-(3-methylphenyl)ethylisopropyl phenyl
H H I-(4-fluorophenyl)ethylethyl cyclohexyl
H H a,a-dimethylbenzylisopropyl phenyl
methyl H benzyl ethyl cyclopropyl
methyl H benzyl 2-chloroethyl2-chloroethyl
methyl H 2-methylbenzyl isopropyl phenyl
methyl H 3-fluorobenzyl isopropyl 2-chlorophenyl
methyl H 3-fluorobenzyl isopropyl 2,4-difluorophenyl
m ethyl H 3-chlorobenzyl isopropyl phenyl
methyl H 3-trifluoromethylbenzylisopropyl phenyl
methyl H I-phenylethyl isopropyl phenyl
methyl H I-(3-fluorophenyl)ethylisopropyl phenyl
methyl methylbenzyl isopropyl phenyl
methyl methyl3-chlorobenzyl isopropyl phenyl
m ethyl H a,a-dimethylbenzylisopropyl phenyl
ethyl H benzyl isopropyl phenyl
ethyl H 4-methylbenzyl isopropyl phenyl
ethyl H 2,4-dimethylbenzylisopropyl phenyl
n-propyl H benzyl ethyl cyclohexyl
n-propyl H benzyl isopropyl 4-fl uorophenyl
isopropylH benzyl ~ ethyl ethyl
isopropylH benzyl ethyl isopropyl
isopropylH benzyl ethyl cyclopentyl
isopropylH benzyl ethyl cyclohexyl
isopropylH benzyl isopropyl isopropyl
isopropylH benzy isopropyl phenyl
isopropylH 4-fl uorobenzylisopropyl phenyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-26-
Table 1 (continued)
R' R4 RS RI R2
isopropylH 4-fluorobenzyl isopropyl 4-fluorophenyl
H H phenethyl ethyl cyclopropyl
H H 3-methylphenethylethyl cyclohexyl
H H 4-fluorophenethylisopropyl phenyl
H H 2-chlorophenethylethyl 2-chlorophenyl
methyl H phenethyl methyl methyl
methyl H phenethyl ethyl ethyl
methyl H phenethyl ethyl isopropyl
methyl H phenethyl ethyl cyclopentyl
methyl H phenethyl ethyl cyclohexyl
methyl H phenethyl ethyl phenyl
methyl H phenethyl n-propyl n-propyl
methyl H phenethyl isopropyl phenyl
methyl H phenethyl isopropyl 2-methylphenyl
methyl H phenethyl isopropyl 3-chlorophenyl
methyl H 3-methylphenethylisopropyl phenyl
methyl H 3-methoxyphenethylisopropyl phenyl
methyl H 3-fluorophenethylisopropyl phenyl
methyl H 3-trifluoromethylphen-isopropyl phenyl
ethyl
methyl H 3,5-dimethylphenethylisopropyl phenyl
methyl H 3,5-difluorophenethylisopropyl phenyl
methyl H 2-methyl-2-phenylethylisopropyl phenyl
methyl H 1-methyl-2-phenylethylisopropyl phenyl
methyl H 1,2-dimethyl-2-phenyl-isopropyl 4-methoxyphenyl
ethyl
methyl methylphenethyl isopropyl phenyl
methyl ethyl phenethyl isopropyl phenyl
ethyl H phenethyl isopropyl phenyl
ethyl H 2-methylphenethylisopropyl phenyl
ethyl H 3-fluorophenethylisopropyl phenyl
ethyl H 2-chlorophenethylisopropyl 3-fluorophenyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-27-
Table 1 (continued)
R~ R4 RS Rl R2
ethyl H 3,5-dimethylphenethylisopropyl phenyl
ethyl H 3,5-difluorophenethylisopropyl phenyl
n-propyl H phenethyl isopropyl phenyl
n-propyl H 3-fluorophenethylisopropyl phenyl
isopropyl H phenethyl isopropyl phenyl
isopropyl H 3-methylphenethylisopropyl phenyl
n-butyl H phenethyl isopropyl phenyl
n-butyl H 3-chlorophenethylisopropyl phenyl
methyl H 3-phenylpropyl isopropyl phenyl
methyl H phenoxymethyl ethyl ethyl
methyl H phenoxymethyl ethyl cyclohexyl
methyl H phenoxymethyl isopropyl phenyl
methyl H 4-methylphenoxymethylisopropyl phenyl
methyl H 3-fluorophenoxymethylisopropyl phenyl
methyl H 3,4-dimethylphenoxy-isopropyl phenyl
methyl
methyl H 3,4-difluorophenoxy-isopropyl phenyl
methyl
methyl H 1-phenoxyethyl isopropyl phenyl
methyl H 1-(3-fluorophenoxy)-isopropyl phenyl
ethyl
methyl methylphenoxymethyl isopropyl phenyl
methyl ethyl phenoxymethyl isopropyl phenyl
methyl H 2-phenoxyethyl isopropyl phenyl
methyl H phenylthiomethylethyl ethyl
methyl H phenylthiomethylethyl cyclhexyl
methyl H phenylthiomethylisopropyl phenyl
methyl H 4-methylphenylthio-isopropyl phenyl
methyl
methyl H 4-chlorophenylthio-isopropyl phenyl
methyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-28-
Table 1 (continued)
R~ R4 RS R1 R2
methyl H 3,4-dimethylphenylthio-isopropyl phenyl
methyl
methyl H 1-phenylthioethylisopropyl phenyl
methyl methylphenylthiomethylisopropyl phenyl
methyl ethyl phenylthiomethylisopropyl phenyl
methyl methylphenoxymethyl isopropyl phenyl
methyl ethyl phenoxymethyl isopropyl phenyl
methyl H 2-phenoxyethyl isopropyl phenyl
methyl H phenylthiomethylethyl ethyl
methyl H phenylthiomethylethyl cyclohexyl
methyl H phenylthiomethylisopropyl phenyl
methyl H 4-methylphenylthio-isopropyl phenyl
methyl
methyl H 4-chlorophenylthio-isopropyl phenyl
methyl
methyl H 3,4-dimethylphenylthio-isopropyl phenyl
methyl
methyl H I-phenylthioethylisopropyl phenyl
methyl H 2-phenylthioethylisopropyl phenyl
methyl methylphenylthiomethylisopropyl phenyl
methyl ethyl phenylthiomethylisopropyl phenyl
R' R4 RS R I R2 R/S
methyl H 2-methylphenylisopropyl phenyl R
methyl H 2-methylphenylisopropyl phenyl S
methyl H 2-methylphenylisopropyl 4-fluorophenylR
methyl H 2-methylphenylisopropyl phenyl R
methyl H 3-methylphenylisopropyl phenyl S
~
methyl H 3-methoxyphenylisopropyl phenyl R
methyl H 3-methoxyphenylisopropyl phenyl S
methyl H 2-fluorophenylisopropyl phenyl R
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-29-
R' R4 RS R 1 R2 R/S
methyl H 2-fluorophenylisopropyl phenyl S
methyl H 3-fl uorophenylisopropyl phenyl R
methyl H 3-fluorophenylisopropyl phenyl S
methyl H 4-fluorophenylisopropyl phenyl R
methyl H 4-fluorophenylisopropyl phenyl S
methyl H 2-chlorophenylisopropyl phenyl R
methyl. H 2-chlorophenylisopropyl phenyl S
methyl H 3-chlorophenylisopropyl phenyl R
methyl H 3-chlorophenylisopropyl phenyl S
methyl H 4-chlorophenylisopropyl 3-trifluoro- R
methylphenyl
methyl H 4-chlorophenylisopropyl 3-trifluoro- S
methylphenyl
methyl H 3-trifluoromethyl-isopropyl phenyl R
phenyl
methyl H 3-trifluoro- isopropyl phenyl S
methylphenyl
methyl H 2,4-difluorophenylisopropyl phenyl R
methyl H 2,4-difluorophenylisopropyl phenyl S
ethyl H 2-fl uorophenylisopropyl phenyl R
ethyl H 2-fluorophenylisopropyl phenyl S
n-propyl H phenyl isopropyl phenyl R
n-propyl H phenyl isopropyl phenyl S
methyl H phenethyl isopropyl phenyl R
methyl H phenethyl isopropyl phenyl S
ethyl H phenethyl isopropyl phenyl R
ethyl H phenethyl isopropyl phenyl S
methyl H phenoxymethylisopropyl phenyl R
methyl H phenoxymethylisopropyl phenyl S
Using, for example, 1-(1-methyl-3-phenylpropyl)-5(4H)-tetrazolinone and N-
ethyl-
N-cyclohexylcarbamoyl chloride as starting materials in the preparation
process a) of
the compounds of the aforementioned formula (I), said preparation process can
be
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-30-
illustrated by the following reaction formula:
CH3 O O
CHZCH2~H~ ~ +; CI/ ' ~CZHs + base
\ / H N ---
N=N
H
CHs O O
CH2CHZ~Hw ~ ~ CZHs
/ ~ ~ N/
N=N
H
The starting materials (compounds of the formula (II)) in the above-mentioned
prepa-
ration process a) are, except in case in which R represents non-substituted
C,_~ aralkyl
or substituted benzyl (substituents to the benzyl being 1 to 5 groups selected
from the
group consisting of halogen, C,_~ alkyl, C,_6 haloalkyl, C,_~ alkoxy, C,_~
haloalkoxy,
C,_6 alkylthio, C,_6 alkylsulfinyl, C,_6 alkylsulphonyl, C,_6 haloalkylthio,
phenyl,
phenoxy, C,_4 alkoxycarbonyl, nitro and cyano), novel compounds which were not
described in the literature.
In the especially preferred compounds of formula (II) the substituent R has
the same
meaning as described for the preferred or more preferred definition of R of
com-
pounds according to formula (I).
The compounds of the formula (II) can be in general prepared, for example, by
the
following preparation processes b) or c).
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-31 -
Preparation process b):
Compounds of the formula (IV)
R-N=C=O (I V )
in which R is as defined above,
are reacted with trimethylsilyl azide in the presence of a catalytic quantity
of boron
trifluoride-ether-complex.
Preparation process c):
Compounds of the above-mentioned formula (IV) are reacted with sodium azide in
a
polar solvent in the presence of a catalytic quantity of aluminium chloride.
The compounds of the formula (IV) which are used as starting materials in the
above-mentioned preparation processes b) and c) include isocyanates known in
the
area of organic chemistry and can be easily obtained, for example, by reacting
amines represented by the formula (V)
R-NHZ (V)
in which R is as defined above,
with, for example, phosgene according to the method described .in "SHIN JIKKEN
KAGAKU KOUZA" (New experimental chemistry lecture) Vol. 14, III, pp.1490-
1496 (published by Maruzen Ltd. on February 20, 1978).
Compounds of the above-mentioned formula (V) can be synthesized, for example,
similarly to the methods described in "SHIN JIKKEN KAGAKU KOUZA" (New
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-32-
experimental chemistry lecture) Vol. 14, III, pp.1332-1398 (published by
Maruzen
Ltd. on February 20, 1978) or Organic Reactions, Vol. 5, 1949, 301-330 (John
Wiley
& Sons, Inc.).
As typical examples of the compounds of the formula (II) the following
compounds
can be mentioned:
1-( 1-(2-methylphenyl)ethyl)-5(4H)-tetrazolinone,
1-( 1-(3-methylphenyl)ethyl)-5 (4H)-tetrazolinone,
1-( 1-(4-methylphenyl)ethyl)-5(4H)-tetrazolinone,
1-( 1-(3-fluorophenyl)ethyl)-5 (4H)-tetrazolinone,
1-( 1-(4-chlorophenyl)ethyl)-5 (4H)-tetrazolinone,
1-( 1-(3-trifluoromethylphenyl)ethyl)-5 (4H)-tetrazolinone,
1-( 1-(3,4-dimethylphenyl)ethyl)-5 (4H)-tetrazolinone,
1-( 1-(2,4-dichlorophenyl)ethyl)-5 (4H)-tetrazolinone,
1-( 1-(2,3,4-trichlorophenyl)ethyl)-5(4H)-tetrazolinone,
1-( 1-(4-fluorophenyl)propyl)-5 (4H)-tetrazolinone,
1-( 1-(3-methoxyphenyl)propyl)-5 (4H)-tetrazolinone,
1-( 1-(3-fluorophenyl)butyl)-5 (4H)-tetrazolinone,
1-(2-methyl-1-phenylpropyl)-5 (4H)-tetrazolinone,
1-(.-cyclohexylbenzyl)-5 (4H)-tetrazolinone,
1-(diphenylmethyl)-5 (4H)-tetrazolinone,
1-benzyl-2-methylpropyl)-5 (4H)-tetrazolinone,
1-( 1-methyl-3-phenylpropyl)-5(4H)-tetrazolinone,
1-(.,.-dimethyl-4-fluorobenzyl)-5(4H)-tetrazolinone,
1-( 1-methyl-2-phenoxyethyl)-5 (4H)-tetrazolinone,
1-( 1-methyl-phenylthioethyl)-5 (4H)-tetrazolinone
1-( 1-(R)-(2-methylphenyl)ethyl)-5(4H)-tetrazolinone,
1-( 1-(S)-(3-methylphenyl)ethyl)-S (4H)-tetrazolinone,
3 0 1-( 1-(R)-(3-fluorophenyl)ethyl)-5 (4H)-tetrazolinone,
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
- 33 -
1-( 1-(S)-(3-fluorophenyl)ethyl)-5(4H)-tetrazolinone,
1-( 1-(R)-(2,4-difluorophenyl)ethyl)-5(4H)-tetrazolinone,
1-( 1-(R)-(4-fluorophenyl)propyl)-5 (4H)-tetrazolinone,
1-( 1-(S)-(4-fluorophenyl)propyl)-5 (4H)-tetrazolinone,
1-(2-methyl-1-(R)-phenylpropyl)-5 (4H)-tetrazolinone,
1-( 1-(R)-methyl-3-phenylpropyl)-5 (4H)-tetrazolinone,
1-( 1-(R)-methyl-2-phenoxyethyl)-5 (4H)-tetrazolinone
and the like.
As typical examples of the compounds of the formula (III) to be reacted with
the
compounds of the above-mentioned formula (II) are carbamoyl chlorides which
are
well known in the area of organic chemistry the following compounds can be men-
tioned:
N,N-diethylcarbamoyl chloride,
N-cyclohexyl-N-ethylcarbamoyl chloride,
N,N-di-n-propylcarbamoyl chloride,
N-cyclopropyl-N-n-propylcarbamoyl chloride,
N-cyclopentyl-N-isopropylcarbamoyl chloride,
N,N-diallylcarbamoyl chloride,
N,N-dipropargylcarbamoyl chloride,
N-isopropyl-N-phenylcarbamoyl chloride,
N-2-chlorophenyl-N-isopropylcarbamoyl chloride,
N-3-bromophenyl-N-isopropylcarbamoyl chloride,
N-4-fluorophenyl-N-isopropylcarbamoyl chloride,
N-(4-methylphenyl)-N-isopropylcarbamoyl chloride,
N-benzyl-N-ethylcarbamoyl chloride,
1-indolinylcarbonyl chloride,
1,2,3,4-tetrahydroquinolin-1-ylcarbonyl chloride,
2-methyl-1,2,3,4-tetrahydroquinolin-1-ylcarbonyl chloride,
2,2-dimethyl-1,2,3,4-tetrahydroquinolin-1-ylcarbonyl chloride,
N-1,1-dimethylpropargyl-N-phenylcarbamoyl chloride,
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-34-
N-methyl-N-phenylcarbamoyl chloride,
N-ethyl-N-phenylcarbamoyl chloride,
N-n-propyl-N-phenylcarbamoyl chloride,
N-cyclohexyl-N-isopropylcarbamoyl chloride,
2-methyl-1,2-dihydroquinolin-1-ylcarbonyl chloride,
2,2-dimethyl-1,2-dihydroquinolin-1-ylcarbonyl chloride
and the like.
The reaction of the preparation process a) are conducted usually in an inert
organic
solvent. As examples of such inert organic solvents in said reaction there can
be
mentioned aliphatic, alicyclic and aromatic hydrocarbons (which may optionally
be
chlorinated), for example, pentane, hexane, cyclohexane, petroleum ether,
ligroine,
benzene, toluene, xylene, dichloromethane, chloroform, carbon tetetrachloride,
1,2-
dichloroethane, chlorobenzene, dichlorobenzene; ethers, for example, diethyl
ether,
methyl ethyl ether, diisopropyl ether, dibutyl ether, dioxane, dimethoxyethane
(DME), tetrahydrofuran (THF), diethylene glycol dimethyl ether (DGM);
nitriles, for
example, acetonitrile, propionitrile; acid amides, for example,
dimethylformamide
(DMF), dimethylacetamide (DMA), N-methylpyrrolidone, 1,3-dimethyl-2-imidazol-
idinone, hexamethylphosphoric triamide (HMPA) etc.
The preparation process a) may be conducted in the presence of an acid binding
agent. As a preferable example of the usable base 4-dimethylaminopyridine
(DMAP) can be mentioned.
In case of using DMAP as an acid binding agent, the reaction of the
preparation
process a) may be conducted usually at about -10 to about 200°C,
preferably about
25 to about 140°C under normal pressure. Optionally it is possible to
conduct it
under elevated pressure or under reduced pressure.
Moreover, it is possible to conduct the reaction of the preparation process a)
using
other acid binding agents than DMAP. As such acid binding agents there can be
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-35-
mentioned inorganic salts (for example, sodium carbonate, potassium carbonate
etc.),
alkyl alcoholates (for example, sodium methoxide, sodium ethoxide, potassium
tert-
butoxide), sodium hydroxide, potassium hydroxide, lithium hydroxide, organic
bases
(for example, triethylamine, 1,1,4,4-tetramethylethylenediamine, N,N-
dimethylani-
line, pyridine etc.).
In case of conducting said reaction using these acid binding agents, the
compounds
of the formula (I) can be selectively obtained by using DMAP as a catalyst.
Reaction temperature in this case may be in a range of usually about 0 to
about
150°C, preferably about 25 to about 100°C. Said reaction is
conducted desirably
under normal pressure. Optionally, however, it is possible to conduct it under
ele-
vated pressure or under reduced pressure.
The compounds of the formula (I), according to the present invention can be
pre-
pared, for example, by reacting 1 mole of the compound of the formula (II)
with
about 1 mole to about 1.5 moles of the compound of the formula (III) in the
presence
of about 1 mole to about 1.5 moles of DMAP as a base and in an above described
inert solvent.
The compounds of the formula (I) can also be prepared by reacting 1 mole of
the
compound of the formula (II) with about 1 mole to about 1.5 moles of the
compound
of the formula (III) in the presence of about 0.01 mole to about 0.3 moles of
DMAP
as a catalyst and, for example, about 1 mole to about 1.5 moles of potassium
carbon-
ate as a base and in such an inert solvent as described above.
The compounds of the formula (I), according to the present invention, thus
obtained
can be isolated and purified, for example, by means of crystallization,
chromato-
graphy etc.
The reaction of the aforementioned preparation process b) may be conducted
using a
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-36-
boron trifluoride-ether-complex as a catalyst. The reaction temperature may be
usu-
ally about 0 to about 200°C, preferably about 50 to about 150°C.
The reaction is
conducted preferably under normal pressure.
Optionally, however, it is possible to conduct it under elevated pressure or
under
reduced pressure.
The preparation process b) may be conducted by reacting 1 mole of the compound
of
the formula (IV) with about 1 mole to about 2 moles of trimethylsilyl azide in
the
presence of about 0.005 moles to about 0.01 mole of boron trifluoride-ether-
complex
as a catalyst.
The reaction of the preparation process c) may be conducted usually in a polar
sol-
vent. As usable polar solvents there can be mentioned, for example, acid
amides
such as dimethylformamide, dimethylacetamide etc. and sufoxides such as
dimethyl-
sulfoxide, sulfolane etc. The reaction temperature may be generally about 0 to
about
200°C, preferably about 20 to about 150°C. The reaction is
conducted preferably
under normal pressure. Optionally, however, it is possible to conduct it under
ele-
vated pressure or under reduced pressure.
The preparation process c) may be conducted by reacting usually 1 mole of the
com-
pound of the formula (IV) with about 1 mole to about 1.5 moles of sodium azide
in
the presence of about 0.05 moles to about 1 mole of aluminium chloride as a
catalyst
and in a polar solvent, for example, dimethylformamide.
The active compounds of the formula (I), according to the present invention.
have, as
shown in the test examples, which are described later, excellent herbicidal
activities
and can be used as herbicidal agents for controlling weeds. In this regard
"Weeds"
mean, in the broadest sense, all plants which grow in locations where they are
unde
sired.
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-37-
The compounds, according to the present invention, act as total or selective
herbi-
cides depending upon the applied concentration. The active compounds of the
pres-
ent invention can be used, for example, as selective herbicides between the
following
weeds and cultures.
Dicotyledon weeds of the genera: Sinapis, Lepidium, Galium,
Stellaria, Chenopodium, Urtica, Senecio, Amaranthus, Portulaca, Xanthium, Ipo-
moea, Polygonum, Ambrosia, Cirsium, Sonchus, Solanum, Rorippa, Lamium, Ve-
ronica, Datura, Viola, Galeopsis, Papaver, Centaurea, Galinsoga, Rotala,
Lindernia
etc.
Dicotyledon cultures of the genera: Gossypium, Glycine, Beta, Daucus,
Phaseolus,
Pisum, Solanum, Linum, Ipomoea, Vicia, Nicotiana, Lycopersicon, Arachis, Bras-
sica, Lactuca, Cucumis, Cucurbita etc.
Monocotyledon weeds of the genera: Echinochloa, Setaria, Panicum, Digitaria,
Phleum, Poa, Festuca, Eleusine, Lolium, Bromus, Avena, Cyperus, Sorghum, Agro-
pyron, Monochoria, Fimbristylis, Sagittaria, Eleocharis, Scirpus, Paspalum,
Ischae-
mum, Agrostis, Alopecurus, Cynodon etc.
Monocotyledon cultures of the genera: Oryza, Zea, Triticum, Hordeum, Avena, Se-
cafe, Sorghum, Panicum, Saccharum, Ananas, Asparagus, Allium etc.
The use of the active compounds of the formula (I), according to the present
inven-
2~ tion, is not restricted to the above-mentioned plants, but may be applied
to other
plants in the same manner.
According to the invention all plants and plant parts can be treated. The term
plants
includes all plants and plant populations, such as desired or undesired wild
plants and
cultivated plants (including naturally occurring cultivated varieties).
Cultivated
plants can be plant varieties that were obtained by conventional breeding and
opti-
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
mizing processes or by biotechnological and genetic engineering methos or a
combi-
nation of such processes and methods, including transgenic plants and
including
plant varieties that cannot or can be protected by plant patents or plant
variety rights.
Plant parts are all parts and organs of plants occurring above or below the
surface of
the soil, e.g. shoots, leaves, needles, stalks and stems, trunks, flowers,
fruits and
seeds as well as roots, tubers, bulbs and rhizomes. The term plant parts also
includes
harvested corps and propagation material, e.g. cuttings, tubers, bulbs,
rhiozomes,
shoots and seeds.
According to the invention the plants and plant parts are treated using the
usual
methods by applying the active ingredients or compositions containing them
directly
to the plants or plant parts or to their surroundings (including the soil) or
storeroom,
e.g. by dipping, spraying, dusting, fogging, spreading and in the case of
propagation
material also by coating using one or multiple layers.
The active compounds of the present invention can, depending upon the applied
con-
centration, non-selectively control weeds and may be used, for example, on
industrial
terrain, rail tracks, paths, places with or without tree plantings.
Moreover, the active compounds, according to the present invention, can be
used for
controlling weeds in perennial cultures and applied in, for example,
afforestations,
decorative tree plantings, orchards, vineyards, citrus groves, nut orchards,
banana
plantations, coffee plantations, tea plantations, rubber plantations, oil palm
planta-
tions, cocoa plantations, soft fruit plantings, hopfields etc. and can be
applied for the
2~ selective controlling of weeds in annual cultures.
The active compounds of the formula (I), according to the present invention,
can be
made into the customary formulations, when they are applied. As such
formulations
there can be mentioned, for example, solutions, emulsions, wettable powders,
sus-
pensions, powders, soluble powders, granules, tablets, suspension-emulsion
concen-
trates, microcapsules, in polymeric substances, jumbo formulations etc.
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-39-
These formulations can be prepared according to per se known methods, for
example,
by mixing the active compound with extenders, namely liquid diluents and/or
solid
diluents or carriers, optionally with surface-active agents, namely
emulsifiers and/or
dispersants and/or foam-forming agents. When water is used as extender, for ex-
ample, organic solvents can be used as auxiliary solvents.
As liquid diluents or carriers there can be mentioned generally aromatic
hydrocar-
bons (for example, xylene, toluene, alkylnaphthalene etc.), chlorinated
aromatic or
chlorinated aliphatic hydrocarbons (for example, chlorobenzenes, ethylene
chlorides,
methylene chloride etc.), aliphatic hydrocarbons [for example, cyclohexane
etc. or
paraffins (for example, mineral oil fractions etc.)], alcohols (for example,
butanol,
glycols and their ethers and esters etc.), ketones (for example, acetone,
methyl ethyl
ketone, methyl isobutyl ketone, cyclohexanone etc.), strongly polar solvents
(for ex-
ample, dimethylformamide, dimethylsulphoxide etc.) and water.
As solid diluents there can be mentioned, for example, ground natural minerals
(for
example, kaolin, clay, talc, chalk, quartz, attapulgite, montmorillonite,
diatomaceous
earth etc.), ground synthetic minerals (for example, highly dispersed silicic
acid,
alumina, silicates etc.) etc.
As solid carriers for granules there can be mentioned, for example, crushed
and frac-
tionated rocks (for example, calcite, marble, pumice, sepiolite, dolomite
etc.), syn-
thetic granules of inorganic and organic meals, granules of organic material
(for ex-
ample, sawdust, coconut shells, maize cobs and tobacco stalks etc.) etc.
As emulsifier and/or foam-forming agents there can be mentioned nonionic and
ani-
onic emulsifiers [for example, polyoxyethylene fatty acid esters,
polyoxyethylene
fatty alcohol ethers (for example, alkylaryl polyglycol ethers,
alkylsulphonates,
alkylsulphates, arylsulphonates etc.)], albumin hydrolysis products etc.
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-40-
As dispersants include, for example, ligninsulphite waste liquor and methyl
cellulose.
Tackifiers may also be used in formulations (powders, granules, emulsions). As
us-
able tackifiers there can be mentioned, for example, carboxymethyl cellulose,
natural
and synthetic polymers (for example, gum arabic, polyvinyl alcohol, polyvinyl
ace-
tate etc.).
Colorants may also be used. As said colorants there can be mentioned, for
example,
inorganic pigments (for example, iron oxide, titanium oxide, Prussian Blue
etc.),
organic dyestuffs such as alizarin dyestuffs, azo dyestuffs or metal
phthalocyanine
dyestuffs, and further trace nutrients such as salts of metals such as iron,
manganese,
boron, copper, cobalt, molybdenum, zinc etc.
Said formulations can contain in a range of generally 0.1-95 % by weight,
preferably
0.5-90 % by weight of the compounds of the aforementioned formula (I).
The active compounds of the present invention can be used as such or in their
for-
mutation forms for controlling weeds. They can be used also as a mixed agent
with
known herbicides. Such mixed agent can be previously prepared as a final
formula-
tion form or can be prepared by tank-mixing on occasion of application.
The active compounds of the formula (I) of the present invention can be used
also
with a safener and their application as a selective herbicide may be broadened
by
such a mixing. As an example of the safener 1-(a,a-dimethylbenzyl)-3-p-
tolylurea
can be mentioned.
As possible combinations as the above-mentioned mixed agents there can be men-
boned, for example, the following known herbicides:
4-amino-6-(1,1-dimethylethyl)-3-ethylthio-1,2,4-triazin-5(4H)-one, 1-amino-6-
ethyl-
thio-3-(2,2-dimethylpropyl)-1,3,5-triazin-2,4(1H,3H)-dione, or N-(2-
benzothiazolyl)-
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-41 -
N,N'-dimethylurea etc. for controlling weeds in cereals culture;
4-amino-3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one etc. for controlling weeds
in
sugar cane culture;
4-amino-6-(1,1-dimethylethyl)-3-methylthio-1,2,4-triazin-5(4H)-one etc. for
control-
ling weeds in soybean culture;
Surprisingly, mixed agents of some of the compounds of the formula (I) of the
pres-
ent invention show synergistic effects.
In case of using the active compounds of the formula (I) of the present
invention they
can be directly used as such or used in formulation forms such as ready-to-use
solu-
tions, emulsions, suspensions, powders, granules or used in the use forms
prepared
by further dilution.
The active compounds of the formula (I) of the present invention can be
applied by
watering, spraying, atomizing, dusting or granule application etc.
The active compounds of the formula (I) of the present invention can be
applied at
any stages before and after germination of plants. They may also be mixed into
the
soil before sowing.
The application amount of the active compounds may be varied in a substantial
range
and are fundamentally different according to the nature of the desired effect.
If used
as herbicides, as the application amount there can be mentioned, for example,
ranges
of about 0.01 to about 5 kg, preferably about 0.1 to about 3 kg of active
compounds
per hectare.
Then the preparations and applications of the compounds of the present
invention
will be described more specifically by the following examples. However, the
present
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-42-
invention should not be restricted to them in any way. "Parts" mean "parts by
weight" unless specified.
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
- 43 -
Preparation examples of the compounds
Synthesis Example 1
CH3 ~ p
CHZCH2~f-Iw ~ ~CI \ CZHs
j N~
N=N
1-( 1-Methyl-3-phenylpropyl)-5(4H)-tetrazolinone ( 1.0 g),
dimethylaminopyridine
(0.67 g) and N-cyclohexyl-N-ethylcarbamoyl chloride (1.04 g) are dissolved in
tolu-
ene (20 ml) and stirred at 80°C for 8 hours on heating. After naturally
cooling the
reaction mixture is washed with water (2 times x 10 ml), dried with anhydrous
so-
dium sulfate and then the solvent is distilled off under reduced pressure. The
residue
is treated by silica gel column chromatography (eluant: hexane: ethyl acetate
= 3:1 )
to obtain 1-(1-methyl-3-phenylpropyl)-4-(N-cyclohexyl-N-ethylcarbamoyl)-5(4H)-
tetrazolinone (1.65 g).
nDZO 1.5215
The compounds obtained in the same manner as the above-mentioned Svnthesis Ex-
ample 1 are shown in the following Table 2 together with the compound obtained
in
Synthesis Example 1.
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-44-
Table 2
R3 O O
Rs N~N~N~R,
R4
N N R2
Com-
pound R3 R4 R5 R1 ~2 Property
No.
1 methylH 2-methylphenyl ethyl ethyl n201.5289
2 methylH 2-methylphenyl ethyl cyclohexyln20 1.5282
3 methylH 2-methylphenyl isopropylphenyl mp.65-69C
4 methylH 3-methylphenyl isopropylisopropyln20 1.5148
methylH 3-methylphenyl isopropylcyclohexyln ~ 1.5139
6 methylH 3-methylphenyl isopropylphenyl n20 1.5494
7 methylH 4-methylphenyl ethyl ethyl n20 1.5293
8 methylH 4-methylphenyl ethyl cyclohxyln20 1.5263
9 methylH 4-methylphenyl isopropylphenyl n20 1.5431
methylH 4-tert-butylphenylmethyl methyl mp.123-125C
11 methylH 4-tert-butylphenylethyl ethyl n20 1.5201
12 methylH 2-fluorophenyl ethyl ethyl n20 1.5194
13 methylH 2-fluorophenyl ethyl cyclohexyln20 1.5204
14 methylH 2-fluorophenyl isopropylphenyl mp.60-62C
methylH 3-fluorophenyl ethyl ethyl n ~ 1.5199
16 methylH 3-fluorophenyl ethyl cyclohexyln 0 1.5170
17 methylH 3-fluorophenyl isopropylphenyl n ~ 1.5422
18 methylH 4-fluorophenyl ethyl ethyl n ~ 1.5170
19 methylH 4-fluorophenyl ethyl cyclohexyln20 1.5147
methylH 4-fluorophenyl isopropylphenyl n ~ 1.5402
21 methylH 2-chlorophenyl ethyl ethyl n20 1.5339
22 methylH 2-chlorophenyl ethyl cyclohex n20 1.5314
23 methylH 2-chlorophenyl isopropylphenyl mp.92-96C
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-45-
Tabelle 2 (continued)
Com-
poundR3 R4 R5 RI R2 Property
No.
24 methyl H 3-chlorophenylethyl ethyl n20 1.5372
25 methyl H 3-chlorophenylethyl cyclohexyl n20 1.5343
26 methyl H 3-chlorophenylisopropylphenyl n201.5608
27 methyl H 4-chlorophenylethyl ethyl n20 1.5375
28 methyl H 4-chlorophenylethyl cyclohexyl n20 1.5378
29 methyl H 4-chlorophenylisopropylphenyl n20 1.5559
30 methyl H 4-bromophenylethyl ethyl n20 1.5443
31 methyl H 4-bromophenylethyl cyclohexyl n20 1.5519
32 methyl H 4-bromophenylisopropylphenyl mp.87-89C
33 methyl H 2-trifluorometh-isopropylphenyl mp.80-86C
ylphenyl
34 methyl H 3-trifluorometh-isopropylphenyl n20 1.5183
ylphenyl
35 methyl H 4-trifluorometh-isopropylphenyl mp.lll-113C
ylphenyl
36 methyl H 3,4-dimethyl-ethyl ethyl n20 1.5271
phenyl
37 methyl H 3,4-dimethyl-ethyl cyclohexyl n2~ 1.5254
phenyl
38 methyl H 3,4-dimethyl-isopropylphenyl n2~ 1.5480
phenyl
39 methyl H 2,4-dichloro-ethyl ethyl n2~ 1.5381
phenyl
40 methyl H 2,4-dichloro-ethyl cyclohexyl n20 1.5422
phenyl
41 methyl H 2,4-dichloro-isopropylphenyl mp.62-70C
phenyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-46-
Tabelle 2 (continued)
Com-
pound R3 R4 R5 RI R2 Property
No.
42 methyl H 2,3,4-trichloro-ethyl ethyl mp.97-99C
phenyl
43 methyl H 2,3,4-trichloro-ethyl cyclohexyln20 1.5430
phenyl
44 methyl H 2,3,4-trichloro-isopropylphenyl mp.99-100C
phenyl
45 ethyl H phenyl ethyl cyclopropyln2~ 1.5242
46 isopropylH phenyl ethyl ethyl n20 1.5233
47 isopropylH phenyl ethyl cyclohexyln20 1.5203
48 isopropylH phenyl isopropylphenyl mp.81-92C
49 cyclo- H phenyl ethyl ethyl n2~ 1.5320
hexyl
50 cyclo- H phenyl ethyl cyclohexylmp.107-112C
hexyl
51 cyclo- H phenyl isopropylphenyl mp.166-168C
hexyl
52 phenyl H phenyl isopropylphenyl mp.189-190C
53 isopropylH benzyl ethyl ethyl n20 1.5139
54 isopropylH benzyl ethyl cyclohexyln ~ 1.5165
55 isopropylH benzyl isopropylphenyl mp.63-66C
56 methyl H phenethyl ethyl ethyl n2~ 1.5219
57 methyl H phenethyl ethyl cyclohexyln2~ 1.5215
58 methyl H phenethyl ethyl phenyl n ~ 1.5440
59 methyl H phenylethylisopropylphenyl n p 1.5411
60 methyl H phenoxymethylethyl ethyl n p 1.5266
61 methyl H phenoxymethylethyl cyclohexylmp.92-94C
62 methyl H phenoxymethylisopropylphenyl mp.89-96C
63 methyl H phenylthiomethylethyl ethyl n ~ 1.5545
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-47-
Tabelle 2 (continued)
Com-
poundR3 R4 R5 Rl R2 Property
No.
64 methyl H phenylthiomethylethyl cyclohexyl n20 1.5502
65 methyl H phenylthiomethylisopropylphenyl n2p 1.5703
66 methyl H 3,4-difluoro-isopropylphenyl mp.70-72C
phenyl
67 methyl H 2,4-difluoro-isopropylphenyl n20 1.5371
phenyl
68 methyl H 2-fluorophenylisopropyl3-fluorophenyln2~ 1.5403
69 ethyl H 3-fluorophenylisopropylphenyl mp.110-112C
70 methyl H 2-fluorophenylisopropyl4-fluorophenylmp.96-99C
71 methyl H 3-fluorophenylisopropyl2-fluorophenyln20 1.5334
72 methyl H 3-fluorophenylisopropyl3-fluorophenylmp.51-53C
73 methyl H 3-fluorophenylisopropyl4-fluorophenylmp.85-87C
74 methyl H 4-fluorophenylisopropyl2-fluorophenyln ~ 1.5326
75 methyl H 4-fluorophenylisopropyl3-fluorophenylmp.63-68C
76 methyl H 4-fluorophenylisopropyl4-fluorophenyln2 1.5309
77 methyl H 2-chlorophenylisopropyl4-fluorophenylmp.96-99C
78 methyl H 2-bromophenylisopropylphenyl mp.110-113C
79 methyl H 3-bromophenylisopropylphenyl n20 1.5681
80 methyl H 3-methylphenylisopropyl4-fluorophenyln20 1.5398
81 methyl H 4-methylphenylisopropyl4-fluorophenylmp. 79-81
C
82 methyl H 2-methoxyphenylisopropylphenyl mp.72-76C
83 methyl H 3-methoxyphenylisopropylphenyl n20 1.5552
84 methyl H 4-methoxyphenylisopropylphenyl n~0 1.5442
85 n-propylH phenyl isopropylphenyl n~0 1.5469
86 isobutylH phenyl isopropylphenyl mp.84-90C
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
- 48 -
Synthesis Example 2 (Intermediate)
CH3 O
CHZCHZ~H~N~
/ NH
N=N
1-Methyl-3-phenylpropyl isocyanate (7.68 g), trimethylsilyl azide (7.58 g) and
a
catalytic amount of boron trifluoride-ether-complex are mixed and refluxed for
16
hours on heating. Excess amount of trimethylsilyl azide is distilled off under
reduced
pressure and the residue is treated by silica gel column chromatography
(eluant:
hexane : ethyl acetate = 1:1) to obtain 1-(1-methyl-3-phenylpropyl)-5(4H)-
tetrazoli
none (8.70 g).
n 2° 1.5372
Synthesis Example 3 (Intermediate)
CH3 O
CHZCHZ~H~
/ N NH
N=N
Sodium azide (1.95 g) is suspended in anhydrous dimethylformamide (18 ml) and
anhydrous aluminium chloride (0.2 g) is added in argon stream under ice
cooling and
stirred for 15 minutes. Then 1-methyl-3-phenylpropyl isocyanate (5.25 g) is
added
drop by drop and the reaction mixture is stirred for 3 hours in argon stream
on heat-
ing at 70-75°C. After natural cooling, the reaction mixture is added to
a mixture of
sodium sulfite (0.5 g), water (100 ml) and ice (50 g) on stirring, acidified
with 10%
hydrochloric acid and extracted ethyl acetate. After drying the organic layer
with
anhydrous sodium sulfate, the solvent is distilled off under reduced pressure
and the
residue is treated by silica gel column chromatography (eluant: hexane : ethyl
acetate
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-49-
= l:l) to obtain 1-(1-methyl-3-phenylpropyl)-5(4H)-tetrazolinone (4.82 g).
nD2° 1.5372
Specific examples of the compounds obtainable in the same manner as the above-
mentioned Synthesis Examples 2 or 3 are shown in the following Table 3
together
with the compounds obtained in Synthesis Examples 2 and 3.
Table 3
R3
R~ ~ O
C* ~
R4~ ~N~NH
N N
Com-
pound R3 R4 RS Property
No.
IL 1 methyl H 2-methylphenyl
IL2 methyl H 3-methylphenyl mp.69-73C
IL3 methyl H 4-methylphenyl mp.154-159C
IL4 methyl LI 4-tert-butylphenyl
IL5 methyl H 3-methoxyphenyl
IL6 methyl H 4-methylthiophenyl
II.7 methyl H 4-methylsulfinylphenyl
IL8 methyl H 4-methylsulfonylphenyl
IL9 methyl H 2-fluorophenyl mp.100-106C
IL10 methyl H 3-fluorophenyl mp.116-120C
IL 11 methyl H 4-fluorophenyl
I1.12 methyl H 2-chlorophenyl mp.112-I14C
IL13 methyl H 3-chlorophenyl mp.110-113C
I1.14 methyl H 4-chlorophenyl
1L15 methyl H 4-bromophenyl mp.143-146C
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-50-
Tabelle 3 (continued)
Com-
pound R3 R4 R5 Property
No.
IL16 methyl II 2-trifluoromethylphenylmp.121-131C
11.17 methyl H 3-trifluoromethylphenylmp.90-93C
11.18 methyl H 4-trifluoromethylphenylmp.112-115C
11.19 methyl H 4-trifluoromethoxyphenyl
IL20 methyl II 4-trifluoromethylthiophenyl
IL21 methyl H 2-methoxycarbonylphenyl
IL22 methyl H 2,4-dimethylphenyl
IL23 methyl II 3,4-dimethylphenyl
IL24 methyl H 3,4-difluorophenyl
IL25 methyl H 2,4-dichlorophenyl mp.137-139C
IL26 methyl H 3,4-dichlorophenyl
IL27 methyl H 2,3,4-trichlorophenyl
1L28 ethyl H 3-methylphenyl
11.29 ethyl H 2-fluorophenyl
IL30 ethyl H 4-fluorophenyl
IL31 ethyl H 3-methoxyphenyl
IL32 n-propyl H phenyl
IL33 n-propyl H 3-fluorophenyl
IL34 isopropyl H phenyl mp.114-118C
IL35 isopropyl H 2-fluorophenyl
IL36 n-butyl H 4-fluorophenyl
IL37 sec-butyl H phenyl
11.38 cyclopropyl H phenyl
IL39 cyclopentyl H phenyl
IL40 cyclohexyl H phenyl mp.131-135C
IL41 cycloheptyl H phenyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
- Sl -
Tabelle 3 (continued)
Com-
pound R3 ' R4 RS Property
No.
I1.42 benzyl H phenyl
IL43 phenyl H phenyl
11.44 3-methylphenylH phenyl
11.45 4-fluorophenylH phenyl
IL46 2-fluorophenylH 3-methoxyphenyl
IL47 chloromethylH phenyl
I1.48 trifluoromethylH phenyl
IL49 methyl methyl3-methylphenyl
IL50 methyl methyl4-fluorophenyl
IL51 ethyl ethyl phenyl
IL52 ethyl n-propylphenyl
1L53 n-propyl methylphenyl
IL54 isopropyl methylphenyl
IL55 methyl H 3-fluorobenzyl
IL56 methyl H I-phenylethyl
IL57 methyl methylbenzyl
IL58 H H a,a-dimethylbenzyl
IL59 methyl H a,a-dimethylbenzyl
IL60 ethyl H benzyl
IL61 n-propyl H benzyl
11.62 isopropyl H benzyl mp.125-126C
IL63 methyl H phenethyl n20 1.5372
IL64 methyl H 3-fluorophenethyl
11.65 methyl H 2-methyl-2-phenylethyl
IL66 methyl H I-methyl-2-phenylethyl
IL67 methyl methylphenethyl
11.68 ethyl H phenethyl
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-52-
Tabelle 3 (continued)
Com-
pound R3 " R4 RS Property
No.
IL69 n-propyl H phenethyl
IL70 isopropyl H phenethyl
IL71 n-butyl H phenethyl
IL72 methyl H 3-phenylpropyl
IL73 methyl H 1-phenoxyethyl
IL74 methyl H I-phenoxyethyl
IL75 methyl methylphenoxymethyl
IL76 methyl ethylphenoxymethyl
IL77 methyl H 2-phenoxyethyl
IL78 methyl H phenylthiomethyl mp.97-102C
IL79 methyl H 1-phenylthioethyl
IL80 methyl H 2-phenylthioethyl
IL81 methyl methylphenylthiomethyl
IL82 methyl ethylphenylthiomethyl
IL83 methyl H 2,4-difluorophenyl
Com-
pound R3 R4 RS ~S
No.
11.84 methyl H 2,4-difluorophenyl R
IL85 methyl H 2,4-difluorophenyl S
IL86 methyl H 2-methylphenyl R
IL87 methyl H 2-methylphenyl S
IL88 methyl H 3-methylphenyl R
IL89 methyl H 3-methylphenyl S
IL90 methyl H 3-methoxyphenyl R
11.91 methyl H 3-methoxyphenyl S
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-53-
Com-
pound R3 R4 R5 ~S
No.
11.92 methyl H 2-fluorophenyl R
11.93 methyl H 2-fluorophenyl S
IL94 methyl H 3-fluorophenyl R
IL95 methyl H 3-fluorophenyl S
IL96 methyl H 4-fluorophenyl R
IL97 methyl H 4-fluorophenyl S
IL98 methyl H 2-chlorophenyl R
IL99 methyl H 2-chlorophenyl S
IL100 methyl H 3-chlorophenyl R
IL101 methyl H 3-chlorophenyl S
IL102 methyl H 4-chlorophenyl R
IL103 methyl H 4-chlorophenyl S
IL 104 methyl H 3-trifluoromethylphenylR
IL 105 methyl H 3-trifluoromethylphenylS
IL 106 ethyl H 2-fluorophenyl R
IL 107 ethyl H 2-fluorophenyl S
IL108 n-propyl H phenyl R
11.109 n-propyl H phenyl S
IL110 methyl H phenethyl R
IL 111 methyl H phenethyl S
IL112 ethyl H phenethyl R
IL113 ethyl H phenethyl S
IL114 methyl H phenoxymethyl R
IL 115 methyl H phenoxymethyl S
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-54-
Biological test examples
Test Example 1: Test for herbicidal effect against paddy field weeds
Preparation of formulation of the active substances:
Carrier: Acetone 5 parts by weight
Emulsifier: Benzyloxypolyglycolether 1 part by weight
A formulation of the active substances is obtained as emulsion by mixing 1
part by
weight of an active substance with the above-mentioned amount of carrier and
emul-
sifter. A prescribed amount of said formulation is diluted with water to
prepare a
formulation for testing.
Test method
In a greenhouse 3 seedlings of paddy rice (cultivar: Nipponbare) of 2.5
leafstage (15
cm tall) were transplanted in a 500 cmz pot filled with paddy field soil. Then
seeds
of barnyard grass, cow hairs, smallflower, bulrush, monochoria and broad-
leaved
weeds (common false pimpernel, Indian toothcup, long stemmed water wort, Am-
manrtia multiflora Roxb., Dopatrium junceum Hammilt etc.) were sown and water
was poured on the soil to a depth of about 2-3 cm.
5 days after the rice transplantation a formulation of each active compound
prepared
according to the above-mentioned preparation method was applied to the surface
of
the water. The herbicidal effect was examined on the day after 3 weeks from
the
treatment during which period the water depth of 3 cm was maintained. The
herbi-
cidal effect was rated as 100% in the case of complete death and as 0% in the
case of
no herbicidal effect.
As a result, the compounds of the present invention Nos. 6, 8, 18, 26, 27, 38,
46 and
48 showed at the chemical amount of 0.5 kg/ha sufficient herbicidal effect
against
paddy field weeds and showed safety to the transplanted paddy rice.
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-SS-
Formulation Example 1 (granule)
To a mixture of 10 parts by weight of the compound 6, 30 parts by weight of
bento-
nite (montmorilonite), 58 parts by weight of talc and 2 parts by weight of
ligninsul-
phonate salt, 25 parts by weight of water are added, well kneaded, made in
granules
of 10-40 mesh by extrusion granulation and dried at 40-50°C to obtain
granules.
Formulation Example 2 (granule)
95 parts by weight of clay mineral particles having a particle size
distribution of 0,2-
2 mm are put in a rotary mixer. While rotating it, 5 parts by weight of
compound 18
are sprayed together with a liquid diluent in the mixer, wetted uniformly and
dried at
40-50°C to obtain granules.
Formulation Example 3 (Emulsifiable concentrate)
30 parts by weight of the compound 26, 5 parts by weight of xylene, 8 parts by
weight of polyoxyethylenealkyl phenyl ether and 7 parts by weight of calcium
alkyl-
benzenesulphonate are mixed and stirred to obtain an emulsion.
Formulation Example 4 (Wettable powder)
15 parts by weight of the compound 38, 80 parts by weight of a mixture of
white
carbon (fine particles of hydrous amorphous silicon oxide) and powder clay
(1:5), 2
parts by weight of sodium alkylbenzenesulphonate and 3 parts by weight of
sodium
alkylnaphthalenesulphonate-formalin-polymer are mixed in powder form to obtain
a
wettable powder.
CA 02357867 2001-07-05
WO 00/40568 PCT/IB00/00008
-56-
Formulation Example 5 (Water dispersible granule)
20 parts by weight of the compound 46, 30 parts by weight of sodium
ligninsulpho-
nate, 15 parts by weight of bentonite and 35 parts by weight of calcined
diatoma-
ceous earth powder are well mixed, added with water, well kneaded and then ex-
truded with a 0.3 mm screen and dried to obtain a water dispersible granule.