Note: Descriptions are shown in the official language in which they were submitted.
CA 02358506 2001-07-09
UB
Translation
A Process and a Device for the Differential Determination of the Amount of at
Least One Analyte
The subject matter of the present invention is a process for the differential
determination of the amount of at least one analyte in a sample with the use
of
a solid phase and a device for carrying out said process.
In today's analytics, test strips have frequently been used. Test strips
meeting
the demands of a short-term test are basically based on the principle of
fixing
a reagent to a solid matrix, with said reagent reacting with the analyte to be
detected. If required, an alteration of color, a change in color or another
detectable property can be measured. Thus, for example, a coloration serves
for detecting the presence of an agent to be identified.
Here, only WO-A-95/13542, WO-A-96/09546, EP-A-0 492 326 will be mentioned
as examples of a very extensive state of the art.
WO-A-98/22824 pertains to a so-called inhibitor assay. This assay
configuration
is based on labeled compounds which correspond to the analyte or which are
identical therewith. The labeled compound is provided on the test strip and
competes with the analyte existing in a sample. The labeled compound leaves
the initial zone and can be detected. In this process a linear way of
proceeding
is basically not possible.
US-A-5,527,509 pertains to an assay determining the analyte by an enzymatic
reaction of a low-molecular analyte and a subsequent color reaction of the
CA 02358506 2001-07-09
-2_
anaiyte. So-called capture assays being the subject matter of the present
invention are not mentioned in said publication. A linear embodiment of said
assay is not possible.
US-A-5,252,496 pertains to a color-forming reaction. Basically, it discloses
only
qualitative assays. If the assay is performed with two zones, one zone only
serves as a control.
US-A-5,229,073 pertains to a non-linear competitive immunoassay.
WO-A-98/36278 pertains to a capture immunoassay having various detection
zones comprising capture reagents having different capture concentrations each
such that a linear realization of the assay described in said publication is
not
possible.
Similar circumstances exist in the processes described in WO-A-96/34287, WO-
A-96/34271, US-A-4,059,407, US-A-4,042,329, and EP-A-0 833 159 which are
either not capture assays or not configured in a linear manner.
Many test strips or similar systems have in common that often only one single
kind of dyeing is performed which excludes any quantification or such a test.
Said test is merely used to enable a yes/no statement. Either the analyte
exists
in a detectable amount resulting in the formation of a color or the analyte
does
not exceed a previously defined limit, i.e. it falls below the limit of
detection of
the respective test strip or test system such that a coloration is not formed.
Moreover, an operational check has to be provided by introducing an additional
reaction-independent parameter in order to exclude systematic errors. Although
this test strip design is sufficient for many applications, questions such as
the
degree of severity of an exposure or a disease or the change of the amount of
analyte during a rehabilitation process or a therapy trial cannot be answered
satisfactorily.
CA 02358506 2001-07-09
-3-
Another drawback of the common test strips or assay devices is the necessity
to indicate limit exceeds in a safe manner. This means that often only a very
small concentration interval between a base value, where the coloration
already
begins, and a maximum coloration exists. Actually, however, it is possible
that
large variations in concentration exist between the limit of detection for an
analyte representing the sensitivity of the test system and the actual value
to
be measured. Thus, e.g., in the field of environmental analysis ratios of
1/105
frequently exist, and even in the medical field analyte ratios of 1/103 are
not
unusual.
Therefore, the technical problem forming the basis of the invention is to
avoid
the above-mentioned drawbacks of the system existing in the art and to provide
quantifying short-time tests.
The technical problem forming the basis of the invention is solved by a
process
for the differential determination of the amount of at least one analyte in a
liquid
sample with the use of a test strip. A differential determination has to be
interpreted as an at least semiquantitative determination, that is an analysis
exceeding a merely qualitative determination. Said test strip must have at
least
two zones and ensure a lateral flow of the liquid sample or a sample
proportion
through the test strip and said at least two zones. At least two zones are
configured such that they are capable of enabling a statement on the amount
of said at least one analyte due to the fact that a first zone has a
previously
defined capacity for binding said at least one analyte and a bonding of said
at
least one analyte takes place in at least one second zone if this capacity is
exceeded by an amount of said at least one analyte being too large for this
capacity, wherein the capacity of said at least two zones is essentially
equal,
respectively, and the bonding of said at least one analyte in said at least
first and
at least second zones is measured by a detectable property. Preferably, the
amount of said at least one analyte is determined by an activity of said at
least
one analyte.
CA 02358506 2001-07-09
-4-
The analyte can be bonded to the test strip in a specific and/or unspecific
manner. Preferably, the specific bonding of said at least one analyte takes
place
by an affinity to another ligand or an affinity to the material used for the
test
strip. In this case an immunoaffinity bonding by bonding partners bonded to
the
test strip surface in a specific or unspecific manner is possible. The
precipitation
of said at least one analyte on the used test strip illustrates the unspecific
bonding of said at least one analyte to the test strip. The specific bonding
between said at least one analyte and an affinity bonding partner is
accomplished
by, e.g., receptor/ligand interaction, enzyme/substrate interaction, anti-
gen/antibody interaction and other affinity bonds such as avidin,
streptavidin,
protein A, IgG, and other systems known to the skilled person.
When carrying out the process of the invention, it is preferred to specify the
defined capacity for the bonding of said at least one analyte on the test
strip as
a function of the amount of said at least one analyte expected in the sample
to
be analyzed in order to be able to determine said at least one analyte in the
second zone if the capacity of said at least first zone is exceeded.
Preferably, the capacity difference of the capture component applied in the
first
and second zones of the inventive capture assay does not exceed f 10 %, in
particular t 5 %. Generally, it has to be remarked that the linearity of the
inventive assay is the more precise the more accurate the capacity of the
capturer in said at least two zones has been adjusted. According to the circum-
stances, when using more than two zones, it may be advantageous to provide
the additional zone with a higher amount of capturer, f.e. to increase the
capacity
of this zone in order to improve the detection. When using several zones,
preferably the last respective zone is provided with a higher capturer
concentra-
tion.
The advantage of the present invention is that essentially equal amounts of
bonding agent (capturer) are applied. Thus, a linearity is achieved enabling
the
safe rating also of intermediate colors. Therefore, different from the other
known multizone assays one does not depend on a titration (i.e. in fact a
CA 02358506 2001-07-09
-5-
logarithmic representation) of an antibody, see, e.g., WO-A-98/36278. Also in
displacement reactions, which basically are not linear due to the labeled
analyte
to be charged previously, linearity can be maintained by applying the labeled
analyte outside the bonding zone. For example, this is not possible according
to US-A-5,229,073.
Figure i exemplifies the operating principle of the inventive process.
Figure 2 illustrates a result of the inventive process if three zones having
respective defined capacities are provided.
Figure 3 pertains to a device with the test strip according to Fig. 4.
Figure 4 pertains to the inventive test strip.
Figure 1 schematically illustrates the fixation of an antibody on a solid
matrix,
e.g., cellulose nitrate. Then, in ib) the so-coated phase is treated with the
analyte. The analyte bonds to the respective antibody by immunoaffinity.
Subsequently, in ic) another antibody which bonds to said antibody and is also
labeled, in particular with a gold colloid, is added to said antigen for
detection.
This indicates the presence of the analyte. If the capacity of the first zone
is not
sufficient to bond all analytes in the sample, said analytes together with
other
sample components diffuse on the solid porous support further towards said at
least second zone, where the analytes are bonded to the antibodies immobilized
there.
Hence, according to the invention it can be differentiated whether the amount
of analyte in a sample is below the limit of detection, exceeds the limit of
detection or distinctly exceeds the limit of detection. Therefore, the first
zone
only bonds the maximally bondable amount of analyte, and with the present
amount of analyte being exceeding a further bonding in said at least second
zone
or additional zones will occur.
CA 02358506 2001-07-09
-6-
In this context, the previous determination of the capacity to bond the
analyte
in the respective zone is important. The skilled person is aware that said
capacity is determined by the respective problem to be solved. If, e.g., in
the
field of environmental analytics a pollutant has to be determined, it may be
advisable to choose the capacity of said at least first zone such that the
limit
exceeding results in a weak coloration of said at least first zone. If the
sample
contains a distinctly greater amount of pollutant, also said at least second
zone
and optionally additional zones on the used test strip will be calored as a
function
of the corresponding pollutant concentration. In the medical field one may
proceed in a similar manner by choosing the capacity of the respective zones
such that information on a particular analyte titer becomes ascertainable. If
a
particular value (threshold value) of an analyte is indicative for a
pathologic
condition and said value has to be determined, it may be advantageous also in
this case to choose the capacity of said first zone to be within the
concentration
range of this threshold value. If, however, for example an operational check
by
determining an analyte is desired, instead of exceeding a threshold value
which
could be detected in the first zone the presence of the analyte at all can be
indicated such as, e.g., in the case of the determination of C-reactive
protein.
In this case, preferably anti CRP antibody is immobilized in the first zone in
an
amount enabling the detection of a CRP normal value. An analytical objective
can be attained in various ways by choosing the capacity of the inJividual
zones
appropriately.
A fine graduation of the measuring range to be detected can be attained by
increasing the number of zones. However, for practical reasons the number of
zones will regularly be between three and ten. In this case, the upper limit
will
regularly be determined by the readability or Interpretability of the results.
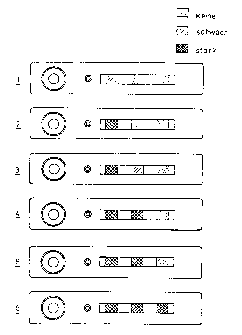
Figure 2 shows the example of a medicinal immunotest. Thus, e.g., in humans
the amount of C-reactive plasma-bonded proteins, a common marker for
bacterial inflammations, is about between 0.2 and 200 mg/I with values of up
to 10 mg/I being regarded as normal. Concentrations exceeding this value
indicate different forms of bacterial inflammations. Since, moreover, the
plasma
CA 02358506 2001-07-09
_ 7 _
concentration of C-reactive proteins very rapidly reacts to a positive
therapy, in
the practice actually all concentrations fall in the mentioned range.
The situation schematically depicted in figure 2 shows three different zones
which
may immunologically interact with C-reactive proteins. Regularly, the
following
three conditions can visually be read off: - no coloration; (+) a weak; and +
a
strong coloration. If a test strip reveals a situation indicated as situation
1 in
figure 2, namely a weak coloration of the first zone and no coloration of the
second and third zones, the amount of C-reactive proteins is within the range
of 0.2 to 5 mg/I. In this case, the capacity of the respective zones has been
adapted to these amounts. Situation 2 shows an increased plasma concentration
of C-reactive protein, since the coloration is stronger. This situation
corresponds
to a content of 5 to 10 mg/I. In situation 3 the first zone shows the
maximally
possible coloration and the coloration of the second zone is weaker, whereas
zone 3 is not colored at all, which indicates an amount of 1l to 15 mg/I.
Situation 4 shaves a strong coloration of the first and second zones, whereas
the
third zone is not colored, which indicates a plasma level of 16 to 25 mg/I.
Situation 5 shows a maximum coloration of the first two zones and a weak
coloration of zone 3, which corresponds to a plasma level of 25 to 50 mg/I.
Situation 6 shows a strong coloration of all three zones enabling one to
conclude
that >50 mg/I is present. Thus, this exemplifying three-zone model overall
enables a six-step differentiation by a simple combination of the number of
colored zones and the color intensities. In addition, an operational check is
inherently present by providing the first zone with a capacity between 0.2 and
mg/I since the first zone practically has to be weakly positive with any
patient
(basal level). In principle, with N zones it will be possible to distinguish a
number
of conditions corresponding to twice the number of N of in a safe manner with
very varying graduations being possible by applying different reagent amounts
to the respective zones, as has been exemplified above.
According to the invention, e.g., C-reactive protein (CRP), rheumatoid
factors,
troponin I, creatinine kinase (CK-MB), myoglobin; antigens of the organisms of
Salmonella, Legionella, E. coli (EHEC), Aspergillus flavus and fumigatus;
glucose,
CA 02358506 2001-07-09
dioxin, PCB, cocaine (ecgonine), heroin, amphetamine, and other pathologically
indicative or relevant in environmental analytics can be measured as an
analyte.
Advantageously, also two or more analytes can be determined in parallel by
covering said at least two zones appropriately. For example, the following
pairs
of analytes being relevant in the clinical practice or in environmental
analytics
can be determined: CRP and antistreptolysin; troponin I and CK-MB; myoglobin
and CK-MB; CK-MB, myoglobin, and troponln I; cocaine, heroin, and amphet-
amine; Salmonella and E. coli.
In principle, any material having a certain porosity such as porous supports
comprising cellulose nitrate, nylon, PTFE, polystyrene, latex, glass fibers,
silica
gel, aluminium oxide, cellulose, dextran, agarose, polyvinyl alcohol, also in
the
form of micro-beads or "magnetobeads", optionally preactivated by BrCN, NPCF,
NHS chloroformate, tresyl chloride, squaric acid esters, and/or corresponding
chlorosilanes may be employed for the test strip advantageously used in the
inventive process.
For that reason the inventive process is particularly user-friendly since
individual
analytical problems can be solved by choosing the concentrations which are to
be detected in the separate zones. Thus, e.g., the user himself can solve
individual analytic problems by employing for the zones an agent interacting
with
the analyte.
The inventive test strip suitable for performing the inventive process has an
essentially porous support 2 being penetrable for liquids and at least two
zones
3, 4 having at least one capturer for at least one analyte, wherein said at
least
one analyte is indirectly or directly bonded to the capturer and the zones 3,
4
each have previously defined bonding capacities for said at least one analyte
which are essentially equal and the capturers for the respective analytes are
identical. A typical test strip is illustrated in Figure 4.
CA 02358506 2001-07-09
_g_
The inventive device for performing the inventive process accommodates the
inventive test strip. The device has a housing wherein the test strip is
arranged.
The housing consists of a top side having at least one opening at the sites of
said
at least two zones of the test strip, said top side extending over the area of
said
at least two zones of the test strip, and at least one additional opening for
applying at least one sample to be tested and/or auxiliary agents for
pertorming
an assay, said additional opening enabling the liquid to contact the test
strip.
Preferably, the device has an additional opening for taking up auxiliary
reagents.
In another embodiment of the inventive device the device has a number of
openings corresponding to the number of zones of the test strip instead of
said
one opening 12 extending over the complete area of zones 3, 4 of the test
strip.
To be able to read off the result in the test strip zones, the openings in the
housing have essentially to be congruent with the test strip zones.
Figure 3 illustrates a preferred embodiment of the inventive device. Here, the
top side 11 of the housing 10 has openings at several sites. At the sites of
said
at least two zones 3, 4 of the test strip 1 an opening 12 is provided in the
top
side il of the housing 10 extending over the area of at least two zones 3, 4
of
the test strip 1. Furthermore, there is provided an opening 14 through which
the application of the sample to be investigated is effected. Optionally, also
auxiliary agents for pertorming the inventive process can be added through
opening 14. The opening 14 has a wall and is connected to the test strip 1.
After being applied, the sample soaks in the test strip due to the porosity
thereof
and migrates in lateral direction through the test strip and consequently
through
zones 3, 4 due to capillary forces. The size of the opening 14 determines the
volume the opening can take up. The sample volume is precisely defined by the
exact diameter and the height of the opening. If the volume provided by
opening 14 is too small for a sample application, the sample uptake volume is
increased by a tube which can be inserted into the opening. In particular, by
inserting a tube having a defined volume (defined wall thickness and length)
it
is possible to provide a defined volume which is matched to the quantitative
or
semiquantitative assay which can be performed using the inventive device. The
additional opening 15 is suitable for taking up auxiliary agents.
CA 02358506 2001-07-09
- i l) -
The opening 14 can be adapted to the respective requirements by additional
supplements. If, e.g., a commercial membrane being capable of separating
blood cells from plasma due to the defined pure size thereof is positioned
directly
beneath the opening 14 and in contact therewith, an analysis of plasma parame-
ters can directly be performed in the test strip. This membrane may be, e.g.,
a glass fiber filter having a suitable density or a plastic membrane having
narrow
channels, preferably asymmetric channels, enabling the blood to soak in. Thus,
the demands on a short-time assay in on-site analytics are met.
In this manner impairing parameters can selectively be filtered off. If the
opening 14 is filled with, e.g., aluminium oxide, a sample can directly be
degreased, which is very important in food analytics. In a similar manner,
particles can be removed by the use of a filter membrane or soil samples can
directly be investigated by the use of a filter together with an absorbing
medium
(for example activated carbon).
The inventive test strip can be prepared by procedures well-known in the art
(WO-A-95/13542). Also WO-A-96/09546 and EP-A-0 291 194, which are
incorporated herein by reference, pertain to the preparation of test strips
which
may also be employed according to the present invention. The system provided
by the invention is advantageous in that it permits the preparation ~ of test
strips
which may be coated by the customer himself. For this, the zones on the test
strip serving for the detection of the analyte are merely preactivated, e.g.,
by
a chemical activation or the application of immunoreactive substances, e.g.,
protein A, protein G, streptavidin, etc. Then, the user has only to modify hls
analyte by complementary structures or to bond antibodies for the analyte in
the
zones in order to use the test strips for his purposes.
The invention will be described more detailed by the following examples:
CA 02358506 2001-07-09
-11-
Example 1
The quantitative determination of C-reactive protein is performed as follows:
the
reactive zones 3, 4 and a respective third zone are charged with 2.3 ug each
of
an anti-human CRP rabbit antibody on a cellulose nitrate strip 4 cm in width.
Subsequently, the surplus reactive groups of the cellulose nitrate are
saturated
with skimmed milk. Subsequently, a test strip consisting of said cellulose
nitrate,
thereon a porous glass fiber strip for taking up the anti-CRP gold conjugate
and
a bfood/plasma-separating glass fiber membrane are mounted on a self-adhesive
plastic sheet. After the application of thick paper strips for taking up the
required
washing liquids, strips 5 mm in width are placed in a housing according to
figure
3.
In order to perform the analysis, the opening 14 is filled with 20 ul of blood
(this
reproducibly corresponds to an plasma amount reaching the test strip suited
for
carrying out the test). After 2 min opening 15 is filled with buffer.
In this embodiment, the plasma is passed through the three reactive zones. The
buffer first passes the glass fiber filter comprising the lyophilized gold-
antibody
conjugate and from there through the zones to which CRP is already bonded.
The gold conjugate bonds to the sites where CRP is present and tl-us generates
a coloration.
Surplus reagent and other substances are washed out by the buffer which is
added subsequently. The result can be read off after 5 min.
Example 2
Also troponin I and CK-MB can be measured in parallel in a manner analogous
to that of example 1. Although both parameters are important indicators of a
cardiac infarction, the amounts thereof increase differently in time according
to
the reason of the myocardial injury such that the parallel determination
thereof
provides important diagnostic information. For the determination thereof, two
CA 02358506 2001-07-09
-12-
zones each containing a monoclonal murine anti-human troponin I and a CK-MB
antibody are applied. The difference with respect to the test according to
example 1 is that lyophilized antibody enzyme conjugates are used instead of
the antibody gold conjugate. Said antibodies are directed against a second
domain of the analyte. In this case anti-troponin I is conjugated with
peroxidase, anti CK-MB with alkaline phosphatase.
Subsequent to the reaction BCIP/INT (a phosphatase reagent leaving an orange
precipitate at the phosphatase site) and TMB (a peroxidase reagent, a blue
colorant) are introduced in opening 15. Subsequently, the test may be analyzed
according to the description of example i.