Note: Descriptions are shown in the official language in which they were submitted.
CA 02360074 2001-07-27
WO 00/44963 PCT/AU00/00042
ELECTROLYTIC CELLS SWEPT BY AN ELECTROMAGNETIC
FIELD AND PROCESS THEREFOR
The present invention relates to a molten electrolyte electrolysis
apparatus and process and their embodiments which are adapted for the
reduction of metallic minerals. The device according to the present invention
can be adapted for the production of the principle industrial metal such as
zinc,
magnesium, aluminium, titanium, and also zirconium, tungsten, lead, etcetera.
Certain of the embodiments of the invention can allow the simultaneous
separation of different metals which are common constituents of ores, therefor
producing an improved economy of production which is capable of competing
favorably with the traditional industrial technology of the prior art.
The principle ores processed to obtain metals generally comprise oxides,
sulfates or carbonates. By roasting, the sulfates are transformed to oxides
while
the carbonates generally undergo a decarbonation which also converts them to
oxides. It is the reduction of these oxides which generally produces the pure
metal. This reduction of oxides can be achieved either by thermo-metallic
reaction or by electrolysis.
Thermo-metallic reaction comprises displacing oxygen from an oxide by
another, more reductive metal, or fixation by carbon with production of carbon
containing gas, in which case the process is called a thermo-carbonic
reaction.
Electrolysis causes the decomposition of a molecule. In this case the oxide is
often transformed into chloride, such as, for example in the electrolysis of
magnesium or zinc. Alternatively, the oxide is put in solution in a molten
salt
bath, such as in case of aluminium oxide which is put in solution in a molten
cryolite at high temperature. In an electrolysis cell, the metal deposits on
the
cathode while the oxygen or halogen is released at the anode and can then
participate in the reaction with the anode.
The industry. has a particular type of treatment cells adapted for the
reduction of each type of oxide.. Although each metal corresponds 'to a
particular
type of cell, one can nevertheless classify the electrolytic cells according
to their
different types.
SUBSTITUTE SHEET (RULE 26) (ROlAU)
CA 02360074 2001-07-27
WO 00/44963 PCT/AU00/00042
-2-
To begin with, one can categorise the electrolytic cells into multi-
electrode cells and into mono-cathode cells.
In the mufti-electrodes cells, the electrodes are generally placed
vertically, alternating cathodes with anodes, while in the mono-cathode cells,
the bottom of the bath comprises the electrode and the bottom is necessarily
in a
horizontal orientation.
For example the electrolysis of, zinc chloride in aqueous media is said to
use a low temperature mufti electrode cell, and the zinc is fixed directly on
the
cathodes. Likewise, for the electrolysis of molten magnesium chloride, a mufti
electrodes cell is used. In this case, at the temperature of electrolysis, the
magnesium is liquid and being less dense, it goes up the length of the
cathodes
and floats at the surface of the bath. If diaphragms are not interposed
between
the anodes and cathodes, the chloride which is released from the anodes
recombines with the magnesium and the cell efficiency is null.
As distinct from the preceding electrolysis cells, mono-cathodic
electrolysis cells comprise only one cathode which makes up the bottom of the
cell, while one or more anodes are immersed in the electrolyte above the
cathode. One of the best known mono-cathodic cell is the aluminium
electrolysis cell. This apparatus is particularly well adapted for the
electrolysis
of aluminium at the elevated temperatures of electrolysis; the density of
aluminium is slightly greater than the density of the electrolyte in the cell,
so
that aluminium can accumulate at the bottom of the bath while the oxygen
liberated at the upper anodes forms carbon-bearing gas, without any risk of
recombining with the metal deposited at the bottom of the bath.
A priori, the arrangement comprising an anode at the bottom of the bath
and one or more cathodes in an upper position, that is to say, an inversed
arrangement, would not be possible. The problem is that when a metal is of
lower density than the density of the electrolyte, the material released at
the
anode inevitably become recombined with the metal:
According to another method of classification, it is also possible to
distinguish the electrolysis cells according to whether the electrolysis is
carried
SUBSTITUTE SHEET (RULE26) (ROlAU)
CA 02360074 2001-07-27
WO 00/44963 PCT/AU00/00042
-3-
out at low temperatures in an aqueous medium, or carried at high temperature
in
a bath of molten salt.
Alternatively, it is also possible to classify the cells according to the
nature of the electrolyte or whether or not it is in circulation. But this
last
classification can overlap with preceding classifications.
In effect, during electrolysis in an aqueous media, the electrolyte may be
put into continuous circulation with. the aid of pumps, such that the
(titrage)
baths are controlled, filtered and regulated. Furthermore the sweeping or
flowing movement of the electrolyte assists the anodic depolarization and
improves the deposition at the cathode.
By contrast, in the electrolytic cells (ignee) or molten salt baths, the
aggressiveness of the electrolyte at high temperature makes it virtually
impossible to put the electrolyte into circulation using mechanical pumps
centrifuges or other pumps. Moreover, it is undesirable to have excessive
1 S perturbation of the electrolyte baths, particularly molten salt baths, and
generally, there is no circulation of electrolyte in electrolytic cells having
a
molten electrolyte. Thus, for example, in the electrolysis of aluminium, the
densities of the different components of the bath are very similar, and
agitation
of the bath can cause collisions between the cathode and anodes. These
collisions can then cause destructive electromagnetic reactions.
It is known that a magnetic field can be used as the source of movement
of electrolyte of an electrolysis cell, but the problem is that in reality,
the
magnetic fields caused by the passage of current in industrial electrolytic
cells,
do not have perceptible effects. With respect to multi-electrode cells, the
magnetic fields are generally compensated for or negated by other effects,
while
in the mono-cathodic cells such as aluminium electrolysis cells, the passage
of
current in the electrolyte causes a vertical, axially centred magnetic
pressure
and has little effect (or causes stabilizing effects) and are incapable of
causing a
circulation of electrolyte between the electrodes.
SUBSTITUTE SHEET(RULE26) (ROlAU)
CA 02360074 2001-07-27
WO 00/44963 PCT/AU00/00042
-4-
It is has now been found, that a device and process can be provided in
which there is forced circulation of the electrolyte between anodes and
cathodes
of an electrolytic cell.
The present invention therefor provides an electromagnetic cell
comprising electrolyte, at lest one anode and at least one cathode,
characterized
in that electrolyte is caused to circulate between the cathodes) and anodes)
by
imposition of a magnetic field. Typically the magnetic field is perpendicular
to
the passage of current in the electrolyte. Typically deflectors are included
in the
apparatus to direct the flow of electrolyte.
The present Invention also provides a method of circulating electrolyte
between the cathodes) and anodes) of an electrolytic cell comprising the step
of imposing a magnetic field. Furthermore, the present invention also provides
unique methods of processing metals, metallic minerals and the like using the
apparatus and process of the present invention.
One benefit or effect resulting from the forced circulation of electrolyte or
sweeping of electrodes is an improvement of the efficiency of the electrolytic
cell due to depolarisation of the anode. A further benefit of effect is a
reduction
in wear of the anode since the sweeping reduces direct attack on the anode by
oxygen or halogen which is formed.
In the case an electrolytic cell arrangement having a horizontal lower anode,
the
circulation of electrolyte is sufficiently energetic to permit lateral
stirring. The
gas bubbles of oxygen or halogen are separated by a deflector, formed such
that
it cannot damage the metal deposit at cathode. This arrangement is
particularly
advantageous by virtue of the fact that the location of the anode in the
bottom of
a bath already naturally favours the depolarisation and the reduction of
attachment to the anode of oxygen or halogen which is formed there.
In the apparatus of the present invention, the principal feature is the
circulation of the electrolyte in a closed circuit around the electrodes. To
circulate the electrolyte; the anodes) add rhe_ cathodes) can be ~cotlsidered
to
function as electrodes of an electromagnetic conduction pump, so that in
arranging the induction conductors creating the electrode current, a magnetic
SUBSTITUTE SHEET (RULE 26) (ROlAU)
CA 02360074 2001-07-27
WO 00/44963 PCT/AU00/00042
-5-
field perpendicular to the passage of the current in the electrolyte is
created,
which forces the electrolyte to circulate between the electrodes, in
accordance
with Laplace's law. At the end of the electrodes, deflectors separate the
electrolyte flow into two flows paths : the first flow path passing the anode
and
the second flow path passing the cathode. The electrolyte flowing past the
anode
sweeps the gas bubbles, while the electrolyte flow passing the cathode is
loaded
with reduced metal.
After the two flows pass the anode and the cathode respectively, these
two flow paths rejoin between the electrodes to complete the closed circuit.
In a preferred embodiment, an arrangement is used in which an upper
anode is used and different deflectors are used according to whether the
density
of the metal deposited is greater or lesser that the density of the molten
salt bath.
In fact, these deflectors can be of particularly simple design when used for
separating a lighter metal using a cell having a cathode in the upper position
or,
alternatively, when separating a heavier metal using a cell having a cathode
in
the lower position. Conversely, in the respective opposing cases, the
deflectors
can be adapted to facilitate the meeting of the flow paths and thus provides
notable reduction to the mechanical wear caused by the circulation of the
electrolyte.
The apparatus and process of the present invention is herein described
with reference to different embodiments. The following description of
different
embodiments provides further illustrations and examples of the possible
adaptations of the process and apparatus to some well known types of
industrial
electrolysis and can even simplify the known treatment of certain minerals.
For
example, a first simple embodiment of the apparatus according to the invention
can be provided for the electrolysis (ignee) of zinc chloride.
The apparatus and process of the present invention will now be further
described with reference to the drawings.
Figure 1 is a simplified view of a' first embodiment of the apparatus of
the present invention. The cell is of a toroidal shape with a vertical axis
and the
recirculation of the electrolyte is arranged so that the electrolyte flows
SUBSTITUTE SHEET (RULE 26) (ROlAU)
CA 02360074 2001-07-27
WO 00/44963 PCT/AU00/00042
-6-
orthogonally through the toroid. The magnetic field is induced by a coil
conductor in the form of a toroidal solenoid which is fed in series by current
running to the anode or cathode. The anode is comprised of agglomerated
graphite in the form of a crown at the bottom of the cell. The catnodes
comprise
segments of solid crowns adjacent the cover of the cell. The flow deflector is
situated on the largest diameter of the toroid, since in that embodiment the
direction of the coil of the circuit is the same as between the electrodes,
thus
providing a centrifugal force in the electrolyte, whereas above the cathodes
the
circulation is necessarily in the opposite direction. The deflector has a
profile
suitable for separation of the flows and a system for collection for liquid
zinc.
Collection tubes collectively guide the liquid zinc towards a circular drain
where it may be regularly pumped. The segmentation of the cathode crown is
particularly useful for ease of location of the cell cover, but also
facilitates the
regulation, segment by segment of the distance between the electrodes. The
cover is located atop a chloride collector. In addition to the pump for zinc
collection, a conduit is provided for feeding of zinc chloride.
Obviously, by an inverting the direction of the current flow in the
associated solenoid or, by changing the direction of the winding, the force
exerted on the electrolyte can be reversed, causing centripetal flow and the
deflector must be moved or relocated accordingly.
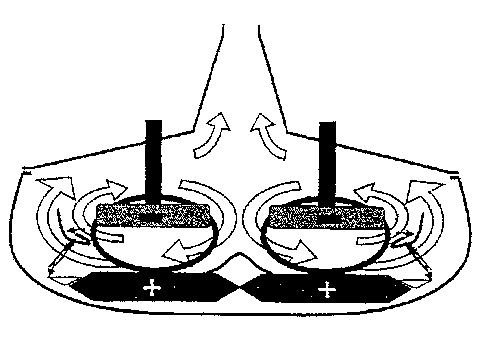
Figure 2 is a simplified depiction of an embodiment of a cell adapted for
the electrolysis of magnesium chloride. Like the preceding cell, this cell is
in the
shape of o toroid having a vertical axis and the recirculation of the
electrolyte is
in a direction orthogonal to the toroid. The magnetic field is induced by a
wound conductor in the form of a toroidal solenoid fed in series by the
current
induced at the cathode. The anode is constituted by graphite agglomerate at
the
bottom of the cell. The cathode is also in the form of a crown and overhangs
the
magnetic induction toroid. The flow deflector is reduced to a simple plate
interdependent with the cathode. The active surface of the cathode is formed
by
a grate which leaves a passage for the magnesium so that it can accumulate on
the upper part of the hollowed cathode. The profile will be adapted according
to
SUBSTITUTE SHEET (RULE 26) (RO/~1 U)
CA 02360074 2001-07-27
WO 00/44963 PCT/AU00/00042
the direction of circulation be it centrifugal or centripetal. The cover of
the cell
is mounted on a chloride collector, but also on a basket of carbo-chloride fed
continuously with magnesium oxide and a powder of carbon so as to produce
the magnesium chloride and cause the liberation of gaseous chlorine. The
magnesium pumping apparatus can be any of the many suitable pumping
apparatus known in the art and is not shown in the drawings.
In the two preceding descriptions the embodiments of the invention are very
similar and differ only slightly to the first embodiment, the metal produced
is
heavier than the molten salt bath, making it necessary to have a flow
separation
deflector passing the anode and the cathode, whereas in the second embodiment
the metal is lighter than the molten bath, the metal is caught when traversing
the
cathode, and the deflector is much simplified. This embodiment is generally
useful for the electrolysis of lower density salts and metal oxides such as:
magnesium, calcium, lithium, sodium, potassium and barium, since all these
metals are principally lighter than their salts or their oxides. These
relatively
low density metals are also the most reductive metals and these are the metals
used the most frequently in thermo-metallic reactions for the reduction of
metallic oxides, This is why, it becomes particularly advantageous to combine
the first inverse electrolytic apparatus having electromagnetic sweeping, with
a
second reduction cell which uses thermo-metallics or even electrolysis. The
combination of the two, permits the production in situ of thermo-metallic
reductors. This combination permits not only perceptible improvement of the
classic process but also permits the reduction of metal oxides, which are
particularly difficult to reduce, such as titanium oxides or zirconium oxides.
The figure 3 is a simplified depiction of a first step in the combination of
a production cell of a light metal reductor, and of a classic aluminium
electrolysis cell. In a first approach, the reductor produced is lithium which
is
compatible with aluminium. Lithium produced by the swept electrolysis cell is
injected at the bottom of the aluminium bath, located adjacent the cathode of
the
electrolysis cell. Lithium is less dense than the alurriinium and tends to
rise in
the bath and can directly enter into a thermo-metallic reduction reaction with
the
SUBSTITUTE SHEET (RULE 26) (ROlAU)
CA 02360074 2001-07-27
WO 00/44963 PCT/AU00/00042
_$_
aluminium oxide. The aluminium produced by this reaction tends to accumulate
at the bottom while the less dense lithium oxide tends to move towards the
surface where it can react with the carbon and form floating liquid lithium
carbonate which is removed by overflow. This combination naturally drives a
reduction in consumption of graphite of the anodes of the aluminium cells,
even
if these is replaced by a carbon powder consumption. Through a second
approach, we will be able to replace almost the whole cryolite bath by a
lithium
carbonate bath in which alumina will be in suspension ; by doing so, fluor
fumes will be substantially reduced while the graphite consumption will be
also
be reduced.
Figure 4 shows as indicated earlier arrangement of an electrolytic cell
with a sweeping capacity for the production of a reducing alloy, with a
metallo-
thermical cell still equipped with electrodes. Both cells are intimately
linked in
order to create only one cell. This cell could be applied to the alumina
reduction
process but we prefer to describe it hereafter in a titanium dioxide reduction
application.
The cell is presented as a cylindertype of tank comprising inside two
cores sharing the same vertical axis with in the middle a chlorination
reaction at
their centre. The lower toroid corresponds to a reducing cell having an alloy
reducer. It is constructed in a similar fashion to the cell describe in figure
2. A
toroidal solenoid induces a magnetic field perpendicular to the passage of
current between the electrodes. The bottom of the tank is comprised by the
anode, and the cathode facing it is in the form of a hollowed crown which at
the
same time performs the function of deflector.
The upper toroid which corresponds to the thermo-metallic cell, comprises
a lower half toroid which at the same time, assures the closure of the lower
toroid. It is capped by a cover which is also the closure cover of the cell.
This
upper toroid also functions in the electrolysis tank with a cathode in the
form of
a crown located at the bottom of the toroidal tank. Several immersed anodes
are
suspended from the cell cover. The electrodes of the thermo-metallic cell
permit the generation of an electric field favourable for the reduction of
titanium
SUBSTITUTE SHEET (R ULE 26) (ROlA U)
CA 02360074 2001-07-27
WO 00/44963 PCT/AU00/00042
-9-
dioxide and they are useful for starting off the cell. The cover may also be a
collector for carbon-based gas which is generated from the final step of
reduction.
The lower cell is replenished with a mixture of lithium chloride and sodium
chloride. The chlorine electrolysis product is directed towards the reaction
zone,
while the lithium and sodium are co-products and collected by the hollow
cathode, then pumped and injected into the liquid cathode of the upper cell.
The upper cell is replenished with lithium carbonate and sodium carbonate
which functions as electrolyte and dispersant for the titanium oxide. At the
bottom of the upper cell, the liquid cathode is constituted by an alloy bath.
The
alloy bath is basically heavy metals at lower melting point which is injected
to
continuously reduce the alloy at the cell base, but this liquid cathode also
continuously extracts an alloy rich in titanium. In effect, the alloy
reductant of
the cathode is oxidised in reducing the titanium oxide which is in suspension
in
the bath. The oxides formed migrate towards the surface of the bath where, in
the presence of carbon, they form liquid carbonates which move to the centre
of
the cell and the chlorination reaction zone. Accordingly this at the same
time,
causes the dissociation of carbon-bearing gas and regeneration of the
chlorides.
If the consumption of the chlorides is theoretically nil or minimal because
the chlorides are regenerated in the course of a cycle, and it is necessary to
gradually extract the titanium of the cathode alloy to refeed the thermo-
metallic
cell with titanium dioxide and carbon powder.
Figure 5 shows a cell which is similar to the preceding cell, but adapted for
the reduction of wolframite. In this double cell, the lower toroid corresponds
to
the reduction cell and is provide with an alloy reductor. The upper toroid
corresponds to the thermo-metallic cell. The lower cell is filled with a
mixture
of sodium chloride and lithium chloride. The electrolysis produces chlorine
which is directed towards the chloride regeneration zone while the sodium and
lithium are co-produced and are collected by the hollow . cathode, then pumped
and injected in the liquid electrode of the upper cell.
SUBSTITUTE SHEET (RULE 26) (RDlAU)
CA 02360074 2001-07-27
WO 00/44963 PCT/AU00/00042
- 10-
The wolframite is introduced to the heart of the bath of the upper cell. The
wolframite is decomposed to tungsten oxides and iron oxides. The tungsten
oxide and the sodium oxide form a liquid composition which the essential part
of the bath for putting the wolframite into solution. The iron oxide which is
in
suspension, is reduced. The tungsten oxide is reduce on contact with the
electrode.
conversely to the arrangement described in respect of the preceding figure
4, the electrodes to the upper tank are in this embodiment, fed by alternating
current and furthermore, an magnetic induction toroid connected in series,
creating circulation between the electrodes. This permits better decantation
of
the bath, facilitates the surface concentration of sodium oxide and also
permits
elimination of the iron slug. Furthermore, the tungsten penetrates the liquid
electrode and concentrates at the lowest point because the tungsten density
differs considerably to the densities of the most important metals present.
The sodium oxide returns to the surface, moving progressively In the
central regeneration zone, where it reacts in the presence of chlorine and
carbon
powder, to form chlorine and carbon-bearing gas. The cell cover then become a
collector for the carbon-bearing gas.
Thus, this cell permits at the same time, separation of iron and production of
a
tungsten alloy, for example : an alloy of tungsten-lead-bismuth, an alloy
which
facilitates the use of tungsten in manufactured products.
A sith embodiment of the apparatus according to the invention allows the
direct
treatment of concentrates of zinc or lead, without roasting pre-treatment, but
with separation of iron impurities present and with condensation of sulphur.
For example, figure 6 shows schematically and partially, a cell for
treatment of a concentrated mixture of zinc and lead sulphurs. The cell is of
toroidal shape having a vertical axis and the circulation of the electrolyte
is
arranged so as to have an orthogonal flow in the toroid. The electric field is
induced by a rolled conductor in the form of a toroidxl solenoid which is fed
in
series by a current to the anode or the cathode. Like. the cell depicted in
figure 1,
this cell is comprised of an upper cathode and a lower anode in the form of a
SUBSTITUTE SHEET (R ULE 26) (ROlfl U)
CA 02360074 2001-07-27
WO 00/44963 PCT/AU00/00042
-11-
crown, but in contrast to figure l, the diffuser or deflector is now central
and the
circulation is under the anode. The central diffuser has the function of a
basket
for dilution of the chloride concentrate. In the upper part of the basket the
chloride attacks the lead and zinc sulfates, while in the lower part, the
chlorides
are diluted in the electrolyte. The sulphur formed by the reaction is
condensed
by a device which is not depicted in the drawing. The chloride in circulation
are
reduced by the cathode and are deposited at the bottom of the cell.
It is also possible to conceive of a process in which zinc is drawn off,
allowing initial concentration of the lead by thermo-metallic processing of
zinc,
then leading into a first phase of lead production and enrichment of the
concentrate of zinc sulphate, and a second phase using the preceding cell for
the
production of zinc, lead of pre-treatment purity. The improvement of the type
already described allow further separation of the iron and impurities which
are
present.
A seventh embodiment of the invention concerns the treatment of dolomite.
Dolomite is a double carbonate of magnesium and calcium. Dolomite is an
abundant mineral which is can be used similarly or concurrently with
magnesium minerals, but it is necessary to perform decarbonation and a
separation of calcium oxide. One embodiment according to the present the
present invention allows the treatment of dolomite directly after
decarbonation,
but without the necessity of separating the calcium oxide by pre-treatment.
This
adaption allows isolation of magnesium and calcium . For example, a first
thermo-metallic cell or thermo-calcic cell uses magnesium of decarbonated
dolomite. It is possible to use a rotary oven comprising a lead-calcium bath
on
which the dolomite floats. This oven includes a compartment for condensation
of magnesium.
The oven can also be used for the decarbonation which then follows the
thermo-calcic treatment. After exhaustion and drawing off of the magnesium,
the lime is taken out aild the oven is reloaded for a new treatment.
The lime formed by the treatment is treated completely, or in part by a
sweep cell for calcium production. The calcium produced is alloyed with the
SUBSTITUTE SHEET (RULE 26) (RDlAU)
CA 02360074 2001-07-27
WO 00/44963 PCT/AU00/00042
- 12-
lead and returned to the rotary oven. This alloy of lead-calcium is returned
to
the rotary oven, but part can be directly used as a metalo-thermic reductor
for
other applications or again, be used directly as an alloy for the manufacture
of
electric accumulator plates.
In the previous examples, the cells have been described with a core shape
included a coiling up movement in order to create a magnetic field, but they
could as well be made in a cylinder shape, while imagining the possibility of
opening the core and uncoiling it. In this case, the magnetic field need to be
closed by a magnetic breech, but, by twinning two cylinders next to each
other,
the head breeches of the cylinders will then end up enjoying a very simplified
design.
In a same manner, and according to the same invention, it remains possible
to conceive some applications with a vertical or oblique setting of the
electrodes, while judiciously planning the electrolyte circulation and the
setting
of the deflectors.
Other embodiments can be envisaged to carry out the treatment of
ilmenite with iron separation. Numerous other variations can be described to
be
adapted for the treatment and reduction of different minerals often providing
substantial improvements in economy in comparison with existing treatment
and technologies of the prior art. The preceding description of embodiments of
the apparatus of the mineralogical processes are not exhaustive and further
embodiments apparent to the person skilled in the relevant technology are
within the scope and spirit of the present invention. The present invention is
not
to be considered to be limied to the embodiments described.
SUBSTITUTE SHEET (RULE 26) (ROlAU)