Note: Descriptions are shown in the official language in which they were submitted.
CA 02360311 2002-05-22
76909-200(S)
improved Dialysis Solutions and Methods
Background of the Invention
The instant invention is in the field of dialysis methods and solutions, and
1s Advanced Glycation End-products (AGEs) inhibition and inhibitors thereof.
Protein Aging and Advanced Glycosylatioh End products
Nonenzymatic glycation by glucose and other reducing sugars is an important
post-translational modification of proteins that has been increasingly
implicated in
20 diverse pathologies. Irreversible nonenzymatic glycation and crosslinking
through a
slow, glucose-induced process may mediate many of the complications associated
with
diabetes. Chronic hyperglycemia associated with diabetes can cause chronic
tissue
damage which can lead to complications such as retinopathy, nephropathy, and
atherosclerotic disease. (Cohen and Ziyadeh, 1996, J. Amer. ,.Sot. Nephrol. 7:
i 83-190). It
25 has been shown that the resulting chronic tissue damage associated with
long-term
diabetes mellitus arise in part from in situ immune complex formation by
accumulated
ianmunoglobulins andlor antigens bound to long-lived structural proteins that
have
undergone Advanced Glycosylation End-product (AGE) formation, via non-
enzymatic
glycosyiation (Brownlee et aL, 1983, J. Exp. Med. 158:1739-1744). The primary
protein
3o target is thought to be extra-cellular matrix associated collagen.
Nonenzymatic glycation
of proteins, lipids, and nucleic acids may play an important rate in the
natural processes
of aging. Recently protein glycation has been associated with Li-amyloid
deposits and
1
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
formation of neurofibrillary tangles in Alzheimer disease, and possibly other
neurodegenerative diseases involving amyloidosis (Colaco and Harrington, 1994,
.VeuroReport ~: 859-861 ). Glycated proteins have also been shown to be toxic,
antigenic,
and capable of triggering cellular injury responses after uptake by specific
cellular
receptors (see for example, Vlassara, Bucala & Striker, 1994, Lab. Invest.
70:138-151;
Vlassara et al., 1994, PNAS(USA) 91:11704-11708; Daniels & Hauser, 1992,
Diabetes
41:1415-1421; Brownlee, 1994, Diabetes 43:836-841; Cohen et al., 1994, Kidney
Int.
45:1673-1679; Brett et al., 1993, Arn. J. Path. 143:1699-1712; and Yan et al.,
1994,
PNAS(USA) 91:7787-7791).
to The appearance of brown pigments during the cooking of food is a
universally
recognized phenomenon, the chemistry of which was first described by Maillard
in 1912,
and which has subsequently led to research into the concept of protein aging.
It is known
that stored and heat-treated foods undergo nonenzymatic browning that is
characterized
by crosslinked proteins which decreases their bioavailibility. It was found
that this
15 Maillard reaction occurred in vivo as well, when it was found that a
glucose was attached
via an Amadori rearrangement to the amino-terminal of the a-chain of
hemoglobin.
The instant disclosure teaches previously unknown, and unpredicted mechanism
of formation of post-Amadori advanced glycation end products (Maillard
products;
AGEs) and methods for identifying and characterizing effective inhibitors of
post-
2o Amadori AGE formation. The instant disclosure demonstrates the unique
isolation and
kinetic characterization of a reactive protein intermediate competent in
forming post-
Amadori AGES, and for the first time teaching methods which allow for the
specific
elucidation and rapid quantitative kinetic study of "late" stages of the
protein glycation
reaction.
25 In contrast to such "late" AGE formation, the "early" steps of the
glycation
reaction have been relatively well characterized and identified for several
proteins
(Harding, 1985, Adv. Protein Chem. 37:248-334; Monnier & Bavnes eds., 1989,
The
Maillard Reaction in Aging, Diabetes, and Nutrition (Alan R. Liss, New York);
Finot et
al., 1990, eds. The Maillard Reaction in Food Processing, Human Nutrition arid
30 Physiology (Birkhauser Verlag, Basel)). Glycation reactions are known to be
initiated by
reversible Schiff base (aldimine or ketimine) addition reactions with lysine
side-chain s-
amino and terminal a-amino groups, followed by essentially irreversible
Amadori
2
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
rearrangements to yield ketoamine products e.g. 1-amino-1-deoxy-ketoses from
the
reaction of aldoses (Bavnes et al., 1989, in The Maillard Reaction in A~in~.
Diabetes.
and Nutrition, ed. Monnier and Baynes, (Alan R. Liss, New York, pp 43-67).
Typically, sugars initially react in their open-chain (not the predominant
pyranose and
furanose structures) aldehydo or keto forms with lysine side chain E-amino and
terminal
a-amino groups through reversible Schiff base condensation (Scheme I). The
resulting
aldimine or ketimine products then undergo Amadori rearrangements to give
ketoamine
Amadori products, i.e. 1-amino-1-deoxy-ketoses from the reaction of aldoses
(Means &
Chang, 1982, Diabetes 31, Suppl. 3:1-4; Handing, 1985, Adv. Protein Chem.
37:248-
334). These Amadori products then undergo, over a period of weeks and months,
slow
and irreversible Maillard "browning" reactions; forming fluorescent and other
products
via rearrangement, dehydration, oxidative fragmentation, and cross-linking
reactions.
These post-Amadori reactions, (slow Maillard "browning" reactions), lead to
poorly
characterized Advanced Glycation End-products (AGES).
As with Amadori and other glycation intermediaries, free glucose itself can
undergo oxidative reactions that lead to the production of peroxide and highly
reactive
fragments like the dicarbonyls glyoxal and glycoaldehyde. Thus the elucidation
of the
mechanism of formation of a variety of AGES has been extremely complex since
most in
vitro studies have been carried out at extremely high sugar concentrations.
2o In contrast to the relatively well characterized formation of these "early"
products, there has been a clear lack of understanding of the mechanisms of
forming the
"late" Maillard products produced in post-Amadori reactions, because of their
heterogeneity, long reaction times, and complexity. The lack of detailed
information
about the chemistry of the "late" Maillard reaction stimulated research to
identify
fluorescent AGE chromophores derived from the reaction of glucose with amino
groups
of polypeptides. One such chromophore, 2-(2-furoyl)-4(5)-(2-furanyl)-1H
imidazole
(FFI) was identified after nonenzymatic browning of bovine serum albumin and
polylysine with glucose, and postulated to be representative of the
chromophore present
in the intact polypeptides. (Pongor et al., 1984, PNAS(USA~ 81:2684-2688).
Later studies
3o established FFI to be an artifact formed during acid hydrolysis for
analysis.
A series of U.S. Patents have issued in the area of inhibition of protein
glycosylation and cross-linking of protein sugar amines based upon the premise
that the
3
CA 02360311 2002-05-22
~769.09-200 (S)
mechanism of such glyeosylation and cross-linking occurs via saturated
glycosylation
and subsequent cross-linking of protein sugar amines via a single basic, and
repeating
reaction. These patents include h.S. Patents 4,665,192; 5,017,696; 4,758,853;
4.908,446; 4,983,604; 5,140,048; x,130,337; 5,262,152; 5,130,324; 5,272,165;
5?21,683; 5,258,381; 5,106,877; 5,128,360; 5,100,919; 5,?54,593; 5,137,916;
5;272,176; 5,175,192; 5,218,001: 5,238,963; 5,358,960; 5,318,982; and
5,334,6I7.-
The focus of these U.S. Patents, are a method for inhibition of AGE formation
focused on the carbonyl moiety of the early glycosylation Amadori product, and
in
to particular the most effective inhibition demonstrated teaches the use of
exogenousiy
administered aminowanidine. The effectiveness of aminoguanidine as an
inhibitor of
AGE formation is currently being tested in clinical trials.
Inhibition of AGE formation has utility in the areas of, for example, food
spoilage, animal protein aging, and personal hygiene such as combating the
browning of
~ 5 teeth. Some notable, though quantitatively minor, advanced glycation end-
products are
pentosidine and NE -carboxymethyllysine (Sell and Monnier, 1989, J. Biol.
Chem.
264:21597-21602; Ahmed et al., 1986, J. Biol. Chem. 261:4889-4894).
The Amadori intermediary product and subsequent post-Amadori AGE
formation, as taught by the instant invention, is not fully inhibited by
reaction with
2o aminoguanidine. Thus, the formation of post-Amadori AG)s as taught by the
instant
disclosure occurs via an important and unique reaction pathway that has not
been
previously shown, or even previously been possible to demonstrate in
isolation. It is a
highly desirable goal to have an efficient and effective method for
identifying and
characterizing effective post-Amadori AGE inhibitors of this "late" reaction.
By
i5 providing efficient screening methods and model systems, combinatorial
chemistry can
be employed to screen candidate compounds effectively, and thereby greatly
reducing
time, cost, and effort in the eventual validation of inhibitor compounds. It
would be very
useful to have in vivo methods for modeling and studying the effects of post-
Amadori
AGE formation which would then allow for the efficient characterization of
effective
30 inhibitors. -
Inhibitory compounds that are biodegradable and/or naturally metabolized are
more desirable for use as therapeutics than highly reactive compounds which
may have
4
CA 02360311 2002-05-22
76909-200(S)
toxic side effects, such as aminoguanidine.
SUMllZA,RY OF THE INVENTION
The present invention provides improved dialysis methods and compositions for
dialysis that comprise utilizing an amount effective to inhibit AGE formation
of a
compound of the general formula:
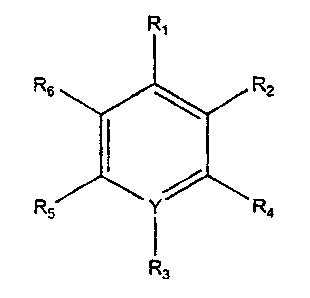
R,
Rs R2
RS r R4
i .
R3
wherein R1 is CHzNH2, CHzSH, COOH, CHzCH2NH2, CHZCHZSH, or CHzCOOH;
Rz and R6 are H, OH, SH, NH2, C 1-6 alkyl, alkoxy or alkene;
R4 and RS are H, C 1-6 alkyl, alkoxy or alkene;
Y is N or C, such that when Y is N, R3 is nothing, and when Y is
C, R3 is NO2 or another electron withdrawing group, and salts
thereof .
In further aspects, the present invention provides
methods and uses for inhibiting dialysis-related cardiac morbidity
and mortality, dialysis-related amyloidosis, limiting dialysis-
related increases in permeability of the peritoneal membrane in a
dialysis patient, inhibiting renal failure progression in a
patient, and inhibiting ultrafiltration failure and peritoneal
membrane destruction in a patient, comprising introducing into the
patient or using a dialysis solution that comprises an amount
effective to inhibit or limit the specified endpoint of a compound
of the general formula
CA 02360311 2002-05-22
76909-200(S)
R,
R2
R5 T R4
R3
wherein R1 is CHZNH2, CH2SH, COOH, CHZCHzNHz, CHZCHzSH, or CHZCOOH;
Rz and R6 are H, OH, SH, NHz, C 1-6 alkyl, alkoxy or alkene;
R4 and RS are H, C 1-6 alkyl, alkoxy or alkene;
Y is N or C, such that when Y is N, R3 is nothing, and when Y is
C, R3 is NOZ or another electron withdrawing group, and salts
thereof .
In another aspect, the present invention comprises a method for inhibiting AGE
formation in a dialysis patient comprising administering to the patient a
dialysis solution
comprising an amount effective amount to inhibit AGE formation of a compound
of the
general formula:
R,
R6 R2
Rs R4
R3
wherein R1 is CHZNH2, CHZSH, COOH, CHZCH2NH2, CHzCH2SH, or CH2COOH;
RZ and R6 are H, OH, SH, NH2, C 1-6 alkyl, alkoxy or alkene;
R4 and R5 are H, C 1-6 alkyl, alkoxy or alkene;
Y is N or C, such that when Y is N, R3 is nothing, and when Y is
C, R3 is NOZ or another electron withdrawing group, and salts
thereof .
The instant invention encompasses pharmaceutical compositions which comprise
one or more of the compounds of the present invention, or salts thereof, in a
suitable
carrier. The instant invention encompasses methods for administering
pharmaceuticals of
the present invention for therapeutic intervention of pathologies which are
related to
AGE formation if: vivo. In one preferred embodiment of the present invention
the AGE
related pathology to be treated is related to diabetic nephropathy.
6
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a series of graphs depicting the effect of vitamin B6 derivatives
on
AGE formation in bovine serum albumin (BSA). Figure 1A Pyridoxamine (PM);
Figure
1B pyridoxal phosphate (PLP); Figure 1C pyridoxal (PL); Figure 1D pyridoxine
(PN).
Figure 2 is a series of graphs depicting the effect of vitamin B 1 derivatives
and
aminoguanidine (AG) on AGE formation in bovine serum albumin. Figure 2A
Thiamine
pyrophosphate (TPP); Figure 2B thiamine monophosphate (TP); Figure 2C thiamine
(T);
l0 Figure 2D aminoguanidine (AG).
Figure 3 is a series of graphs depicting the effect of vitamin B( derivatives
on
AGE formation in human methemoglobin (Hb). Figure 3A Pyridoxamine (PM); Figure
3B pyridoxal phosphate (PLP); Figure 3C pyridoxal (PL); Figure 3D pyridoxine
(PN).
Figure 4 is a series of graphs depicting the effect of vitamin B1 derivatives
and
1 S aminoguanidine (AG) on AGE formation in human methemoglobin. Figure 2A
Thiamine
pyrophosphate (TPP); Figure 2B thiamine monophosphate (TP); Figure 2C thiamine
(T);
Figure 2D aminoguanidine (AG).
Figure 5 is a bar graph comparison of the inhibition of the glycation of
ribonuclease A by thiamine pyrophosphate (TPP), pyridoxamine (PM) and
20 aminoguanidine (AG).
Figure 6A is a graph of the kinetics of glycation of RNase A ( 10 mgimL) by
ribose as monitored by ELISA. Figure 6B is a graph showing the dependence of
reciprocal half times on ribose concentration at pH 7.5.
Figure 7 are two graphs showing a comparison of uninterrupted and interrupted
25 glycation of IZNase by glucose (7B) and ribose (7A), as detected by ELISA.
Figure 8 are two graphs showing kinetics of pentosidine fluorescence
(arbitrary
units) increase during uninterrupted and interrupted ribose glycation of
RNase. Figure
8A Uninterrupted glycation in the presence of 0.05 M ribose. Figure 8B
Interrupted
glycation after 8 and 24 hours of incubation.
30 Figure 9 is a graph which shows the kinetics of reactive intermediate
buildup.
Figure 10 are graphs of Post-Amadori inhibition of AGE formation by ribose.
Figure 10A graphs data where aliquots were diluted into inhibitor containing
buffers at
7
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
time 0. Figure lOB graphs data where samples were interrupted at 24h, and then
diluted
into inhibitor containing buffers.
Figure 11 is a graph showing dependence of the initial rate of formation of
antigenic AGE on pH following interruption of glycation.
a Figure 12 are two graphs showing the effect of pH jump on ELISA detected
AGE formation after interrupted glycation. Interrupted samples left 12 days at
37°C in
pH ~.0 buffer produced substantial AGEs (33%; Figure 12 B) when pH was changed
to
7.~, as compared to the normal control sample not exposed to low pH (Figure 12
A).
Figure 13 is a series of graphs depicting the effect of vitamin B6 derivatives
on
1 o AGE formation during uninterrupted glycation of ribonuclease A (RNase A)
by ribose.
Figure 13A Pyridoxamine (PM); Figure 13B pyridoxal-S'-phosphate (PLP); Figure
13C
pyridoxal (PL); Figure 13D pyridoxine (PN).
Figure 14 is a series of graphs depicting the effect of vitamin B1 derivatives
and
aminoguanidine (AG) on AGE formation during uninterrupted glycation of
ribonuclease
15 A (RNase A) by ribose. Figure 14A Thiamine pyrophosphate (TPP); Figure 14B
thiamine monophosphate (TP); Figure 14C thiamine (T); Figure 14D
aminoguanidine
(AG).
Figure 15 is a series of graphs depicting the effect of vitamin B6 derivatives
on
AGE formation during uninterrupted glycation of bovine serum albumin (BSA) by
2o ribose. Figure 15A Pyridoxamine (PM); Figure 15B pyridoxal-5'-phosphate
(PLP);
Figure 1 SC pyridoxal (PL); Figure 1 SD pyridoxine (PN).
Figure 16 is a series of graphs depicting the effect of vitamin B 1
derivatives and
aminoguanidine (AG) on AGE formation during uninterrupted glycation of bovine
serum
albumin (BSA) by ribose. Figure 16A Thiamine pyrophosphate (TPP); Figure 16B
25 thiamine monophosphate (TP); Figure 16C thiamine (T); Figure 16D
aminoguanidine
(AG).
Figure 17 is a series of graphs depicting the effect of vitamin B6 derivatives
on
AGE formation during uninterrupted glycation of human methemoglobin (Hb) by
ribose.
Figure 17A Pyridoxamine (PM); Figure 17B pyridoxal-5'-phosphate (PLP); Figure
17C
3o pyridoxal (PL); Figure 17D pyridoxine (PN).
Figure 18 is a series of graphs depicting the effect of vitamin B6 derivatives
on
post-Amadori AGE formation after interrupted glycation by ribose. Figure 18A
BSA and
8
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
Pyridoxamine (PM); Figure 18B BSA and pyridoxal-~'-phosphate (PLP); Figure 18C
BSA and pyridoxal (PL); Figure 18D RNase and pyridoxamine (PM).
Figure 19 are graphs depicting the effect of thiamine pyrophosphate on post-
Amadori AGE formation after interrupted glycation by ribose. Figure 19A RNase,
Figure
19B BSA.
Figure 20 are graphs depicting the effect of aminoguanidine on post-Amadori
AGE formation after interrupted glycation by ribose. Figure 20A RNase, Figure
20B
BSA.
Figure 21 is a graph depicting the effect of Na-acetyl-L-lysine on post-
Amadori
1 o AGE formation after interrupted glycation by ribose.
Figure 22 are bar graphs showing a comparison of post-Amadori inhibition of
AGE formation by thiamine pyrophosphate (TPP), pyridoxamine (PM) and
aminoguanidine (AG) after interrupted glycation of RNase (Figure 22A) and BSA
(Figure 22B) by ribose.
t5 Figure 23 is a bar graph showing the effects of Ribose treatment in vivo
alone on
rat tail-cuff blood pressure. Treatment was with 0.05 M, 0.30 M, and 1 M
Ribose (R)
injected for 1, 2 or 8 Days (D).
Figure 24 is a bar graph showing the effects of Ribose treatment in vivo alone
on
rat creatinine clearance (Clearance per 100 g Body Weight). Treatment was with
0.05 M,
20 0.30 M, and 1 M Ribose (R) injected for 1, 2 or 8 Days (D).
Figure 25 is a bar graph showing the effects of Ribose treatment in vivo alone
on
rat Albuminuria (Albumin effusion rate). Treatment was with 0.30 M. and 1 M
Ribose
(R) injected for 1, 2 or 8 Days (D).
Figure 26 is a bar graph showing the effects of inhibitor treatment in vivo,
with
25 or without ribose, on rat tail-cuff blood pressure. Treatment groups were:
25 mg/100 g
body weight aminoguanidine (AG); 25 or 250 mg/100 g body weight Pyridoxamine
(P);
250 mg/ 100 g body weight Thiamine pyrophosphate (T), or with 1 M Ribose (R).
Figure 27 is a bar graph showing the effects of inhibitor treatment in vivo,
with
or without ribose, on rat creatinine clearance (Clearance per 100 g body
weight).
30 Treatment groups were: 25 mg/100 g body weight aminoguanidine (AG); 25 or
250
mg/100 g body weight Pyridoxamine (P); 250 mg/100 g body weight Thiamine
pyrophosphate (T), or with 1 M Ribose (R).
9
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
Figure 28 is a bar graph showing the effects of inhibitor treatment i~t vivo
without ribose, and ribose alone on rat Albuminuria (Albumin effusion rate).
Treatment
Groups were: 2~ mg/100 g body weight aminoguanidine (AG); 250 mg/100 g body
weight Pyridoxamine (P); 2~0 mg/100 g body weight Thiamine pyrophosphate (T),
or
treatment with 1 M Ribose (R) for 8 days (D). Control group had no treatment.
Figure 29 is a bar graph showing the effects of inhibitor treatment iri vivo,
with 1
M ribose , on rat Albuminuria (Albumin effusion rate). Treatment groups were:
25
mg/100 g body weight aminoguanidine (AG); 25 and 250 mg/100 a body weight
Pyridoxamine (P); 250 mg/100 g body weight Thiamine pyrophosphate (T), or
treatment
1o with 1 M Ribose (R) for 8 days (D) alone. Control group had no treatment.
Figure 30A depicts Scheme 1 showing a diagram of AGE formation from
protein. Figure 30B depicts Scheme 2, a chemical structure of aminoguanidine.
Figure
30C depicts Scheme 3, chemical structures for thiamine, thiamine-~'-phosphate,
and
thiamine pyrophosphate. Figure 30D depicts Scheme 4, chemical structures of
15 pyridoxine, pyridoxamine, pyridoxal-5'-phosphate, and pyridoxal. Figure 30E
depicts
Scheme 5, kinetics representation of AGE formation. Figure 30F depicts Scheme
6,
kinetics representation of AGE formation and intermediate formation.
Figure 31 shows a 125 MHz C-13 NMR Resonance spectrum of Riobonuclease
Amadori Intermediate prepared by 24 HR reaction with 99% [2-C13]Ribose.
2o Figure 32 are graphs which show AGE intermediary formation using the
pentoses Xylose, Lyxose, Arabinose and Ribose.
Figure 33 is a graph showing the results of glomeruli staining at pH 2.~ with
Alcian blue.
Figure 34 is a graph showing the results of glomeruli staining at pH 1.0 with
25 Alcian blue.
Figure 35 is a graph showing the results of immunofluroescent glomeruli
staining for RSA.
Figure 36 is a graph showing the results of immunofluroescent glomeruli
staining for Heparan Sulfate Proteoglycan Core protein.
3o Figure 37 is a graph showing the results of immunofluroescent glomeruli
staining for Heparan Sulfate Proteoglycan side-chain.
Figure 38 is a graph showing the results of analysis of glomeruli sections for
CA 02360311 2002-05-22
76909-200 (S)
average alomerular volume.
Figure 39 is a graph demonstrating AGE formation (BSA model) aad AGE
inhibition by pyridoxamine in 4.25% DIANEAL~ + 1M glucose at pH 7.5 conducted
at
37°C for ~2 days.
Figure 40 is a graph demonstrating AGE formation (myoglobin model) and AGE
inhibition by pyridoxamine in I3IANEAL~ post-dialysis fluid for 12 hours at
60°C.
Figure 41 is a graph demonstrating AGE formation an metmyoglobin in 4.25%
DIANEALc7 post-dialysis fluid at pH 7.5 conducted at 37° for varying
time periods.
DETAILED DESCRIPTION
Aj:iniul Models for Protein Aging
AIloxan induced diabetic Lewis rats have been used as a model for protein
aging
to demonstrate the in vivo effectiveness of inhibitors of AGE formation. The
correlation
1s being demonstrated is between inhibition of late diabetes related pathology
and effective
inhibition of AGE formation (Brownlee, Cerami, and Vlassara, 1988, New Eng.
.I. Med.
318(20):1315-132I). Streptozotocin induction of diabetes in Lewis rats, New
Zealand
White rabbits with induced diabetes, and genetically diabetic BB/Worcester
rats have
also been utilized, as described in, for example, U.S. Patent 5,334,617.
2o A major problem with these model systems is tlne long time period required
to demonstrate AGE related injury, and thus to test compounds for AGE
inhibition. For
example, 16 weeks of treatment was required for the rat studies described in
U.S: Patent
5,334,617, and I2 weeks for the rabbit studies. Thus it would be highly
desirable and
useful to have a model system for AGE related diabetic pathology that will
manifest in a
25 shorter time period, allowing for more efficient and expeditious
determination of AGE
related injury and the effectiveness of inhibitors of post-Amadori AGE
formation.
Antibodies no AGEs
An important tool for studying AGE formation is the use of polyclonal and
3o monoclonal antibodies that are specific for AGEs elicited by the reaction
of several
sugars with a variety of target proteins. The antibodies are screened for
resultant
specificity for AGES that is independent of the nature of the protein
component of the
11
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
AGE (Nakayama et al., 1989, Biochern. Biophys. Res. Comm. 162: 740-745;
Nakayama et al., 1991, J. hnmienol. Methods 140: 119-125; Horiuchi et al.,
1991, J.
Biol. Chem. 266: 7329-7332; Araki et al., 1992, J. Biol. Chern. 267: 10211-
10214;
Makita et al., 1992, J. Biol. Chem. 267: 5133-5138). Such antibodies have been
used
to monitor AGE formation in vivo and in virro.
Tlticamine - vitamin BI
The first member of the Vitamin B complex to be identified, thiamine is
practically devoid of pharmacodynamic actions when given in usual therapeutic
doses;
l0 and even large doses were not known to have any effects. Thiamine
pyrophosphate is the
physiologically active form of thiamine, and it functions mainly in
carbohydrate
metabolism as a coenzyme in the decarboxylation of a-keto acids. Tablets of
thiamine
hydrochloride are available in amounts ranging from 5 to 500 mg each. Thiamine
hydrochloride injection solutions are available which contain 100 to 200
mg/ml.
For treating thiamine deficiency, intravenous doses of as high as 100 mg / L
of
parenteral fluid are commonly used, with the typical dose of 50 to 100 mg
being
administered. GI absorption of thiamine is believed to be limited to 8 to 15
mg per day,
but may be exceed by oral administration in divided doses with food.
Repeated administration of glucose may precipitate thiamine deficiency in
under
nourished patients, and this has been noted during the correction of
hyperglycemia.
Surprisingly, the instant invention has found, as shown by in vitro testing,
that
administration of thiamine pyrophosphate at levels above what is normally
found in the
human body or administered for dietary therapy, is an effective inhibitor of
post-
Amadori antigenic AGE formation, and that this inhibition is more complete
than that
possible by the administration of aminoguanidine.
Pyridoxine - Vitamin B6
Vitamin B6 is typically available in the form of pyridoxine hydrochloride in
over-the-counter preparations available from many sources. For example Beach
pharmaceuticals Beelith Tablets contain 25 mg of pyridoxine hydrochloride that
is
equivalent to 20 mg of B6, other preparations include Marlyn Heath Care Marlvn
Formula 50 which contain 1 mg of pyridoxine HCl and Marlyn Formula 50 Mega
Forte
which contains 6 mg of pyridoxine HCI, Wyeth-Ayerst Stuart Prenatal~ tablets
which
12
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
contain 2.6 mg pyridoxine HCI, J&J-Merck Corp. Stuart Formula~ tablets contain
2 mg
of pyridoxine HC1, and the CIBA Consumer Sunkist Children's chewable
multivitamins
which contain 1.05 mg of pyridoxine HCI, 150% of the U.S. RDA for children 2
to 4
years of age, and 53% of the U.S. RDA for children over 4 years of age and
adults.
(Physician's Desk Reference for nonprescription drugs, 14th edition (Medical
Economics Data Production Co., Montvale, N.J., 1993).
There are three related forms of pyridoxine, which differ in the nature of the
substitution on the carbon atom in position 4 of the pyridine nucleus:
pyridoxine is a
primary alcohol, pyridoxal is the corresponding aldehyde, and pyridoxamine
contains an
to aminomethyl group at this position. Each of these three forms can be
utilized by
mammals after conversion by the liver into pyridoxal-5'-phosphate, the active
form of the
vitamin. It has long been believed that these three forms are equivalent in
biological
properties, and have been treated as equivalent forms of vitamin B~ by the
art. The
Council on Pharmacy and Chemistry has assigned the name pyridoxine to the
vitamin.
t5 The most active antimetabolite to pyridoxine is 4-deoxypyridoxine, for
which the
antimetabolite activity has been attributed to the formation in vivo of 4-
deoxypyridoxine-
5-phosphate, a competitive inhibitor of several pyridoxal phosphate-dependent
enzymes.
The pharmacological actions of pyridoxine are limited, as it elicits no
outstanding
pharmacodynamic actions after either oral or intravenous administration, and
it has low
20 acute toxicity, being water soluble. It has been suggested that
neurotoxicity may develop
after prolonged ingestion of as little as 200 mg of pyridoxine per day.
Physiologically,
as a coenzyme, pyridoxine phosphate is involved in several metabolic
transformations of
amino acids including decarboxylation, transamination, and racemization, as
well as in
enzymatic steps in the metabolism of sulfur-containing and hydroxy-amino
acids. In the
2S case of transamination, pyridoxal phosphate is aminated to pyridoxamine
phosphate by
the donor amino acid, and the bound pyridoxamine phosphate is then deaminated
to
pyridoxal phosphate by the acceptor oc-keto acid. Thus vitamin B complex is
known to
be a necessary dietary supplement involved in specific breakdown of amino
acids. For a
general review of the vitamin B complex see The Pharmacological Basis of
3o Therapeutics, 8th edition, ed. Gilman, Rall, Nies, and Taylor (Pergamon
Press, New
York, 1990, pp. 1293-4; pp. 1523-1540).
Surprisingly, the instant invention has discovered that effective dosages of
the
13
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
metabolically transitory pyridoxal amine form of vitamin B6 (pyridoxamine), at
levels
above what is normally found in the human body, is an effective inhibitor of
post-
Amadori antigenic AGE formation, and that this inhibition may be more complete
than
that possible by the administration of aminoguanidine.
Formation of Stable AmadorilScltiff base Intermediary
The typical study of the reaction of a protein with glucose to form AGEs has
been
by ELISA using antibodies directed towards antigenic AGES, and the detection
of the
production of an acid-stable fluorescent AGE, pentosidine, by HPLC following
acid
to hydrolysis. Glycation of target proteins (i.e. BSA or RNase A) with glucose
and ribose
were compared by monitoring ELISA reactivity of polyclonal rabbit anti-Glucose-
AGE
RNase and anti-Glucose-AGE-BSA antibodies. The antigen was generated by
reacting 1
M glucose with RNase for 60 days and BSA for 90 days. The antibodies (R618 and
8479) were screened and showed reactivity with only AGEs and not the protein,
except
15 for the carrier immunogen BSA.
Example 1
Thiamine Pyrophosphate and Pyridoxamine Inhibit the Formation of Antigenic
Advanced Glycation End-Products from Glucose: Comparison with
2o Aminoguanidine
Some B6 vitamers, especially pyridoxal phosphate (PLP), have been previously
proposed to act as "competitive inhibitors" of early glycation, since as
aldehydes they
themselves can form Schiff bases adducts with protein amino groups (Khatami et
al.,
25 1988, Life Sciences 43:1725-1731 ) and thus limit the amount of amines
available for
glucose attachment. However, effectiveness in limiting initial sugar
attachment is not a
predictor of inhibition of the conversion of any Amadori products formed to
AGES. The
instant invention describes inhibitors of "late" glycation reactions as
indicated by their
effects on the in vitro formation of antigenic AGES (Booth et al., 1996,
Biochem.
3o Biophys. Res. Com. 220:113-119).
Chemicals
Bovine pancreatic ribonuclease A (RNase) was chromatographically pure,
aggregate-free grade from Worthington Biochemicals. Bovine Serum albumin (BSA;
14
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
fraction V, fatty-acid free), human methemoglobin (Hb), D-glucose, pyridoxine,
pyridoxal, pyridoxal 5'phosphate, pyridoxamine, thiamine, thiamine
monophosphate,
thiamine pyrophosphate, and goat alkaline phosphatase-conjugated anti-rabbit
IgG were
all from Sigma Chemicals. Aminoguanidine hydrochloride was purchased from
Aldrich
Chemicals.
Uninterrupted Glvcation with Glucose
Bovine serum albumin, ribonuclease A, and human hemoglobin were incubated
with glucose at 37°C in 0.4 M sodium phosphate buffer of pH 7.5
containing 0.02%
to sodium azide. The protein, glucose (at 1.0 M), and prospective inhibitors
(at 0.5, 3, 15
and 50 mM) were introduced into the incubation mixture simultaneously.
Solutions were
kept in the dark in capped tubes. Aliquots were taken and immediately frozen
until
analyzed by ELISA at the conclusion of the reaction. The incubations were for
3 weeks
(Hb) or 6 weeks (RNase, BSA).
Preparation of polvclonal antibodies to AGE proteins
Immunogen preparation followed earlier protocols (Nakayama et al., 1989,
Biochem. Biophys. Res. Comm. 162:740-745; Horiuchi et al., 1991, J. Biol.
Chem.
266:7329-7332; Makita et al., 1992, J. Biol. Chem. 267:5133-5138). Briefly,
immunogen
2o was prepared by glycation of BSA (R479 antibodies) or RNase (R618
antibodies) at 1.6
g protein in 15 ml for 60-90 days using 1.5 M glucose in 0.4 M sodium
phosphate buffer
of pH 7.5 containing 0.05% sodium azide at pH 7.4 and 37°C. New Zealand
white rabbit
males of 8-12 weeks were immunized by subcutaneous administration of a 1 ml
solution
containing 1 mg/ml of glycated protein in Freund's adjuvant. The primary
injection used
the complete adjuvant and three boosters were made at three week intervals
with
Freund's incomplete adjuvant. Rabbits were bled three weeks after the last
booster. The
serum was collected by centrifugation of clotted whole blood. The antibodies
are AGE-
specific, being unreactive with either native proteins (except for the
carrier) or with
Amadori intermediates. The polyclonal anti-AGE antibodies have proven to be a
3o sensitive and valuable analytical tool for the study of "late" AGE
formation in vitro and
in vivo. The nature of the dominant antigenic AGE epitope or hapten remains in
doubt,
although recently it has been proposed that the protein glycoxidation product
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
carboxymethyl lysine (CmL) may be a dominant antigen of some antibodies (Reddy
et
al., 1995, Biochem. 34:10872-10878). Earlier studies have failed to reveal
ELISA
reactivity with model CmL compounds (Makita et al., 1992, J. Biol. Chem.
267:5133-
5138).
ELISA detection of AGE Products
The general method of Engvall (1981, Methods Enzvmol. 70:419-439) was used
to perform the ELISA. Typically, glycated protein samples were diluted to
approximately 1.5 ug/ml in 0.1 M sodium carbonate buffer of pH 9.5 to 9.7. The
protein
l0 was coated overnight at room temperature onto 96-well polystyrene plates by
pippetting
200 u1 of the protein solution in each well (0.3 ug/well). After coating, the
protein was
washed from the wells with a saline solution containing 0.05% Tween-20. The
wells
were then blocked with 200 u1 of 1 % casein in carbonate buffer for 2 h at
37°C followed
by washing. Rabbit anti-AGE antibodies were diluted at a titer of about 1:350
in
15 incubation buffer, and incubated for 1 h at 37°C, followed by
washing. In order to
minimize background readings, antibodies 8479 used to detect glycated RNase
were
raised against glycated BSA, and antibodies 8618 used to detect glycated BSA
and
glycated Hb were raised against glycated RNase. An alkaline phosphatase-
conjugated
antibody to rabbit IgG was then added as the secondary antibody at a titer of
1:2000 or
20 1:2500 (depending on lot) and incubated for 1 h at 37°C, followed by
washing. The p-
nitrophenylphosphate substrate solution was then added (200 ul/well) to the
plates, with
the absorbance of the released p-nitrophenolate being monitored at 410 nm with
a
Dynatech MR 4000 microplate reader.
Controls containing unmodified protein were routinely included, and their
25 readings were subtracted, the corrections usually being negligible. The
validity of the use
of the ELISA method in quantitatively studying the kinetics of AGE formation
depends
on the linearity of the assay (Kemeny & Challacombe, 1988, ELISA arid Other
Solid
Phase Immtcnoassavs, John Wiley & Sons, Chichester, U.K.). Control experiments
were
carried out, for example, demonstrating that the linear range for RNase is
below a
30 coating concentration of about 0.2-0.3 mg/well.
Results
16
CA 02360311 2001-10-04
w0 00/59493 PCT/US00/09241
Figure 1 A-D are graphs which show the effect of vitamin B6 derivatives on
post-
Amadori AGE formation in bovine serum albumin glycated with glucose. BSA (10
mg/ml) was incubated with 1.0 M glucose in the presence and absence of the
various
indicated derivative in 0.4 M sodium phosphate buffer of pH 7.5 at 37°C
for 6 weeks.
Aliquots were assayed by ELISA using 8618 anti-AGE antibodies. Concentrations
of the
inhibitors were 3, 15 and 50 mM. Inhibitors used in Figures (1A) Pyridoxamine
(PM);
(1B) pyridoxal phosphate (PLP); (1C) pyridoxal (PL); (1D) pyridoxine (PN).
Figure 1 (control curves) demonstrates that reaction of BSA with 1.0 M glucose
is slow and incomplete after 40 days, even at the high sugar concentration
used to
to accelerate the reaction. The simultaneous inclusion of different
concentrations of various
B~ vitamers markedly affects the formation of antigenic AGES. (Figure lA-D)
Pyridoxamine and pyridoxal phosphate strongly suppressed antigenic AGE
formation at
even the lowest concentrations tested, while pyridoxal was effective above 15
mM.
Pyridoxine was slightly effective at the highest concentrations tested.
Figure 2 A-D are graphs which show the effect of vitamin B 1 derivatives and
aminoguanidine (AG) on AGE formation in bovine serum albumin. BSA (10 mg/ml)
was
incubated with 1.0 M glucose in the presence and absence of the various
indicated
derivative in 0.4 M sodium phosphate buffer of pH 7.5 at 37°C for G
weeks. Aliquots
were assayed by ELISA using 8618 anti-AGE antibodies. Concentrations of the
inhibitors were 3, 15 and 50 mM. Inhibitors used in Figures (2A) Thiamine
pyrophosphate (TPP); (2B) thiamine monophosphate (TP); (2C) thiamine (T); (2D)
amino guanidine (AG).
Of the various B 1 vitamers similarly tested (Figure 2A-D), thiamine
pyrophosphate was effective at all concentrations tested (Figure 2C), whereas
thiamine
and thiamine monophosphate were not inhibitory. Most significantly it is
remarkable to
note the decrease in the fcnal levels of AGES formed observed with thiamine
pyrophosphate, pyridoxal phosphate and pyridoxamine. Aminoguanidine (Figure
2D)
produced some inhibition of AGE formation in BSA, but less so than the above
compounds. Similar studies were carned out with human methemaglobin and bovine
3o ribonuclease A.
Figure 3 A-D are graphs which show the effect of vitamin B6 derivatives on
AGE formation in human methemoglobin. Hb (1 mg/ml) was incubated with 1.0 M
17
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
glucose in the presence and absence of the various indicated derivative in 0.4
M sodium
phosphate buffer of pH 7.5 at 37°C for 3 weeks. Aliquots were assayed
by ELISA using
8618 anti-AGE antibodies. Concentrations of the inhibitors were 0.5, 3, 15 and
50 mM.
Inhibitors used in Figures (3A) Pyridoxamine (PM); (3B) pyridoxal phosphate
(PLP);
(3C) pyridoxal (PL); (3D) pyridoxine (PN).
It had been previously reported that Hb of a diabetic patient contains a
component that binds to anti-AGE antibodies, and it was proposed that this
glycated Hb
(termed Hb-AGE, not to be confused with HbAlc) could be useful in measuring
long-
term exposure to glucose. The in vitro incubation of Hb with glucose produces
antigenic
to AGES at an apparently faster rate than observed with BSA. Nevertheless, the
different
B6 (Figure 3A-D) and B1 (Figure 4A-C) vitamers exhibited the same inhibition
trends in
Hb, with pyridoxamine and thiamine pyrophosphate being the most effective
inhibitors
in each of their respective families. Significantly, in Hb, aminoguanidine
only inhibited
the rate of AGE formation, and not the final levels of AGE formed (Figure 4D).
With RNase the rate of antigenic AGE formation by glucose was intermediate
between that of Hb and BSA, but the extent of inhibition within each vitamer
series was
maintained. Again pyridoxamine and thiamine pyrophosphate were more effective
that
aminoguanidine (Figure 5).
Figure 4 A-D are graphs which show the effect of vitamin B 1 derivatives and
2o aminoguanidine (AG) on AGE formation in human methemoglobin. Hb (1 mgiml)
was
incubated with 1.0 M glucose in the presence and absence of the various
indicated
derivative in 0.4 M sodium phosphate buffer of pH 7.5 at 37°C for 3
weeks. Aliquots
were assayed by ELISA using 8618 anti-AGE antibodies. Concentrations of the
inhibitors were 0.5, 3, 15 and 50 mM. Inhibitors used in Figures (4A) Thiamine
pyrophosphate (TPP); (4B) thiamine monophosphate (TP); (4C) thiamine (T); (4D)
aminoguanidine (AG).
Figure 5 is a bar graph which shows a comparison of the inhibition of the
glycation of ribonuclease A by thiamine pyrophosphate (TPP), pyridoxamine (PM)
and
aminoguanidine (AG). RNase ( 1 mg/ml) was incubated with 1.0 M glucose (glc)
in the
3o presence and absence of the various indicated derivative in 0.4 M sodium
phosphate
buffer of pH 7.5 at 37°C for 6 weeks. Aliquots were assayed by ELISA
using 8479 anti-
AGE antibodies. The indicated percent inhibition was computed from ELISA
readings in
18
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
the absence and presence of the inhibitors at the 6 week time point.
Concentrations of the
inhibitors were 0.5, 3, 15 and 50 mM.
Discussion
These results demonstrate that certain derivatives of B 1 and B~ vitamins are
capable of inhibiting "late" AGE formation. Some of these vitamers
successfully
iWibited the final levels of AGE produced, in contrast to aminoguanidine,
suggesting
that they have greater interactions with Amadori or post-Amadori precursors to
antigenic
AGES. The Amadori and post-Amadori intermediates represent a crucial juncture
where
to the "classical" pathway of nonenzymatic glycation begins to become
essentially
irreversible (Scheme I). In earlier inhibition studies "glycation" was usually
measured
either as Schiff base formed (after reduction with labeled cyanoborohydride)
or as
Amadori product formed (after acid precipitation using labeled sugar). Such
assays do
not yield information on inhibition of post-Amadori conversion steps to "late"
AGE
15 products, since such steps lead to no change in the amount of labeled sugar
that is
attached to the proteins. Other "glycation" assays have relied on the sugar-
induced
increase of non-specific protein fluorescence, but this can also be induced by
dicarbonyl
oxidative fragments of free sugar, such as glycoaldehyde or glyoxal (Hunt et
al., 1988,
Biochem. 256:205-212), independently of Amadori product formation.
20 In the case of pyridoxal (PL) and pyridoxal phosphate (PLP), the data
support the
simple mechanism of inhibition involving competitive Schiff base condensation
of these
aldehydes with protein amino groups at glycation sites. Due to internal
hemiacetal
formation in pyridoxal but not pyridoxal phosphate, stronger inhibition of
post-Amadori
AGE formation by PLP is expected by this competitive mechanism. This indeed is
25 observed in the data (Figure 1B, 1C, Figure 3B, 3C). The inhibition by
pyridoxamine is
necessarily different, as pyridoxamine lacks an aldehyde group. However,
pyridoxamine
is a candidate amine potentially capable of forming a Schiff base linkage with
the
carbonyls of open-chain sugars, with dicarbonyl fragments, with Amadori
products, or
with post-Amadori intermediates. The mechanism of inhibition of B 1 compounds
is not
30 obvious. All the forms contain an amino functionality, so that the marked
efficiency of
only the pyrophosphate form suggests an important requirement for a strong
negative
charge.
19
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
A significant unexpected observation is that the extent of inhibition by
aminoguanidine, and some of the other compounds, is considerably less at late
stages of
the reaction, than during the early initial phase. This suggests a different
mechanism of
action than that of pyridoxamine and thiamine pyrophosphate, suggesting that
the
therapeutic potential of these compounds will be enhanced by co-administration
with
amino~Tuanidine.
Example 2
Kinetics of Non-enzymatic glycation: Paradoxical Inhibition by Ribose and
Facile
1 o Isolation of Protein Intermediate for Rapid Post-Amadori AGE Formation
While high concentrations of glucose are used to cause the non-enzymatic
glycation of proteins, paradoxically, it was found that ribose at high
concentrations is
inhibitocv to post-Amadori AGE formation in ribonuclease by acting on the post-
15 Amadori "late" stages of the glycation reaction. This unexpectedly
inhibitory effect
suggests that the "early" reactive intermediates, presumably Amadori products,
can be
accumulated with little formation of "late" post-Amadori AGE products (AGES;
Maillard products). Investigation into this phenomenon has demonstrated: ( 1 )
ability to
define conditions for the kinetic isolation of Amadori (or post-Amadori)
glycated
2o intermediate(s); (2) the ability study the fast kinetics of buildup of such
an intermediate;
(3) the ability to study the surprisingly rapid kinetics of conversion of such
intermediates
to AGE products in the absence of free or reversibly bound sugcar; (4) the
ability to use
these intermediates to study and characterize inhibition of post-Amadori steps
of AGE
formation thus providing a novel system to investigate the mechanism of
reaction and the
25 efficacy of potential agents that could block AGE formation; and (5) with
this knowledge
it is also further possible to use 13C NMR to study the reactive intermediates
and
monitor their conversion to various candidate AGES (Khalifah et al., 1996,
Biochemistry
35(15):4645-4654).
30 Chemicals and Materials As in Example 1 above.
Preparation of polvclonal antibodies to AGEs
As in Example 1 above.
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
ELISA detection of AGE products As in Example 1 above.
~lnrirro Acid Analysis
Amino acid analyses were carried out at the Biotechnology Support Facility of
the Kansas University Medical Center. Analyses were performed after hydrolysis
of
glycated protein (reduced with sodium cyanoborohydride) with 6 N HCl at
110°C for 18-
24 h. Phenyl isothiocyanate was used for derivatization, and PTH derivatives
were
analyzed by reverse-phase HPLC on an Applied Biosystems amino acid analyzer
(420A
1o derivatizer, 130A separation system, 920A data analysis system).
Pentosidine Reverse-Phase HPLC Analysis
Pentosidine production in RNase was quantitated by HPLC (Sell & Monnier,
1989, J. Biol. Chem. 264:21597-21602; Odetti et al., 1992, Diabetes 41:153-
159).
15 Ribose-modified protein samples were hydrolyzed in 6 N HCl for 18 h at
100°C and then
dried in a Speed Vac. The samples were then redissolved, and aliquots were
taken into
0.1 % trifluoroacetic acid and analyzed by HPLC on a Shimadzu system using a
Vydac
C-18 column equilibrated with 0.1% TFA. A gradient of 0-6% acetonitrile (0.1%
in
TFA) was run in 30 min at a flow rate of about 1 ml/min. Pentosidine was
detected by
20 335 nm excitation/385 nm emission fluorescence, and its elution time was
determined by
running a synthesized standard. Due to the extremely small levels of
pentosidine
expected (Grandhee & Monnier, 1991, J. Biol. Chem. 266:11649-11653; Dyer et
al.,
1991, J. Biol. Chem. 266:11654-11660), no attempt was made to quantitate the
absolute
concentrations. Only relative concentrations were determined from peak areas.
Glvcation Hodifications
Modification with ribose or glucose was generally done at 37°C in
0.4 M
phosphate buffer of pH 7.5 containing 0.02% sodium azide. The high buffer
concentration was always used with 0.5 M ribose modifications. The solutions
were kept
3o in capped tubes and opened only to remove timed aliquots that were
immediately frozen
for later carrying out the various analyses. "Interrupted glycation"
experiments were
earned out by first incubating protein with the ribose at 37°C for 8 or
24 h, followed by
21
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
immediate and extensive dialysis against frequent cold buffer changes at
4°C. The
samples were then reincubated by quickly warming to 37°C in the absence
of external
ribose. Aliquots were taken and frozen at various intervals for later
analysis. Due to the
low molecular weight of RNase, protein concentrations were remeasured after
dialysis
even when low molecular weight cut-off dialysis tubing was used. An
alternative
procedure was also devised (see below) in which interruption was achieved by
simple
100-fold dilution from reaction mixtures containing 0.5 M ribose. Protein
concentrations
were estimated from UV spectra. The difference in molar extinction between the
peak
(278 nm) and trough (250 nm) was used for RNase concentration determinations
in order
1o to compensate for the general increase in UV absorbance that accompanies
glycation.
Time-dependent UV-difference spectral studies were carried out to characterize
the
glycation contributions of the UV spectrum.
Data analvsis and Numerical Simulations of Kinetics
Kinetic data were routinely fit to monoexponential or biexponential functions
using nonlinear least-squares methods. The kinetic mechanisms of Schemes 5-6
have
been examined by numerical simulations of the differential equations of the
reaction.
Both simulations and fitting to observed kinetics data were carried out with
the
SCIENTIST 2.0 software package (Micromath, Inc.). Determination of apparent
half
2o times (Figure 6B) from kinetic data fit to two-exponential functions
(Figure 6A) was
carried out with the "solve" function of MathCAD 4.0 software (MathSoft,
Inc.).
RESULTS
Comparison of Glvcation by Glucose and Ribose
The reaction of RNase A with ribose and glucose has been followed primarily
with ELISA assays, using 8479 rabbit AGE-specific antibodies developed against
glucose-modified BSA. To a lesser extent, the production of pentosidine, the
only known
acid-stable fluorescent AGE, was quantiated by HPLC following acid hydrolysis.
Preliminary studies using 0.05 M ribose at 37°C showed that the rate of
antigenic AGE
3o formation appears to be modestly increased (roughly 2-3 fold as measured by
the
apparent half time) as the pH is increased from 5.0 to 7.5, with an apparent
small
induction period at the beginning of the kinetics in all cases. The glycation
of RNase
22
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
with 0.05 M ribose at pH 7.5 (half time near 6.5 days) appears to be almost an
order of
magnitude faster than that of glycation with 1.0 M glucose (half time in
excess of 30
days; see Figure 7B, solid line). The latter kinetics also displayed a small
induction
period but incomplete leveling off even after 60 days, making it difficult to
estimate a
true half time.
When the dependence of the kinetics on ribose concentration was examined at pH
7.5, a most unexpected result was obtained. The rate of reaction initially
increased with
increasing ribose concentration, but at concentrations above 0.15 M the rate
of reaction
leveled off and then significantly decreased (Figure 6A). A plot of the
dependence of the
1o reciprocal half time on the concentration of ribose (Figure 6B) shows that
high ribose
concentrations are paradoxically inhibitory to post-Amadori antigenic AGE
formation.
This unusual but consistent effect was found to be independent of changes in
the
concentration of either buffer (2-fold) or RNase ( 10-fold), and it was not
changed by
affinity purification of the 8479 antibody on a column of immobilized AGE-
RNase. It is
also not due to effects of ribose on the ELISA assay itself. The measured
inhibitory
effect by ribose on post-Amadori AGE formation is not likely due to ribose
interference
with antibody recognition of the AGE antigenic sites on protein in the ELISA
assay.
Prior to the first contact with the primary anti-AGE antibody on the ELISA
plates,
glycated protein has been diluted over 1000-fold, washed extensively with
Tween-20
2o after adsorption, and blocked with a 1 % casein coating followed by further
washing with
Tween-20.
Kinetics of Formation of post-Amadori Antigenic AGES by "Interrupted Glycation
"
In view of the small induction period seen, an attempt was made to determine
whether there was some accumulation during the reaction, of an early precursor
such as
an Amadori intermediate, capable of producing the ELISA-detectable post-
Amadori
antigenic AGES. RNase was glycated at pH 7.5 and 37°C with a high
ribose
concentration of 0.5 M, and the reaction was interrupted after 24 h by
immediate cooling
to 4°C and dialysis against several changes of cold buffer over a
period of 24 h to
remove free and reversibly bound (Schiff base) ribose. Such a ribose-free
sample was
then rapidly warmed to 37°C without re-adding any ribose, and was
sampled for post-
Amadori AGE formation over several days. The AGE antigen production of this 24
h
23
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
"interrupted glycation" sample is shown by the dashed line and open triangles
in Figure
7A, the time spent in the cold dialysis is not included. An uninterrupted
control (solid
line and filled circles) is also shown for comparison. Dramatically different
kinetics of
post-Amadori antigenic AGE formation are evident in the two samples. The
kinetics of
AGE antigen production of the ribose-free interrupted sample now show (1)
monoexponential kinetics with no induction period, (2) a greatly enhanced rate
of
antigenic AGE formation, with remarkable half times of the order of 10 h, and
(3)
production of levels of antigen comparable to those seen in long incubations
in the
continued presence of ribose (see Figure 6A). Equally significant, the data
also
to demonstrate that negligible AGE antigen was formed during the cold dialysis
period, as
shown by the small difference between the open triangle and filled circle
points at time 1
day in Figure 7A. Verv little, if any, AGE was formed by the "interruption"
procedure
itself. These observations show that a fully competent isolatable intermediate
or
precursor to antigenic AGE has been generated during the 24 h contact with
ribose prior
15 to the removal of the free and reversibly bound sugar.
Samples interrupted after only 8 h produced a final amount of AGE antigen that
was about 72% of the 24 h interrupted sample. Samples of RNase glycated with
only
0.05 M ribose and interrupted at 8 h by cold dialysis and reincubation at
37°C revealed
less than S% production of ELISA-reactive antigen after 9 days. Interruption
at 24 h,
2o however, produced a rapid rise of ELISA antigen (similar to Figure 7A) to a
level
roughly ~0% of that produced in the uninterrupted presence of 0.05 M ribose.
The same general interruption effects were also seen with other proteins (BSA
and Hemoglobin). Except for a somewhat different absolute value of the rate
constants,
and the amount of antigenic AGEs formed during the 24 h 0.5 M ribose
incubation, the
25 same dramatic increase in the rate of AGE antigen formation was observed
after removal
of 0.5 M ribose.
Glycation is much slower with glucose than with ribose (note the difference in
time scales between Figure 7A and Figure 7B). However, unlike the case with
ribose,
interruption after 3 days of glycation by 1.0 M glucose produced negligible
buildup of
3o precursor to ELISA-reactive AGE antigens (Figure 7B, dashed curve).
Kinetics of Pentosidine Formation
24
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
The samples subjected to ELISA testing were also assayed for the production of
pentosidine, an acid-stable AGE. The content of pentosidine was measured for
the same
RNase samples analyzed for antibody reactivity by ELISA. Glycation by ribose
in 0.4 M
phosphate buffer at pH 7.5 produced pentosidine in RNase A that was
quantitated by
fluroescence after acid hydrolysis. Figure 8A shows that under uninterrupted
conditions,
0.05 M ribose produces a progressive increase in pentosidine. However, when
glycation
is carried out under "interrupted" conditions using 0.5 M ribose, a dramatic
increase in
the rate of pentosidine formation is seen immediately after removal of excess
ribose
(Figure 8B), which is similar to, but slightly more rapid than, the kinetics
of the
1o appearance of antigenic AGES (Figure 7A). A greater amount of pentosidine
was also
produced with 24 h interruption as compared with 8 h. Reproducible differences
between
the kinetics of formation of pentosidine and antigenic AGES can also be noted.
A
significant amount of pentosidine is formed during the 24 h incubation and
also during
the cold dialysis, resulting in a jump of the dashed vertical line in Figure
8B. Our
observations thus demonstrate that a pentosidine precursor accumulates during
ribose
glycation that can rapidly produce pentosidine after ribose removal (cf.
Odetti et al.,
1992, Diabetes 41:153-159).
Rate of Buildup of the Reactive Intermediates)
2o The "interrupted glycation" experiments described above demonstrate that a
precursor or precursors to both post-Amadori antigenic AGES and pentosidine
can be
accumulated during glycation with ribose. The kinetics of formation of this
intermediate
can be independently followed and quantitated by a variation of the
experiments
described above. The amount of intermediate generated in RNase at different
contact
times with ribose can be assayed by the maximal extent to which it can produce
antigenic
AGE after interruption. At variable times after initiating glycation, the free
and
reversibly-bound ribose is removed by dialysis in the cold or by rapid
dilution (see
below). Sufficient time (5 days, which represents several half lives according
to Figure
7A) is then allowed after warming to 37°C for maximal development of
post-Amadori
antigenic AGES. The ELISA readings 5 days after each interruption point,
representing
maximal AGE development, would then be proportional to the intermediate
concentration present at the time of interruption.
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
Figure 9 shows such an experiment where the kinetics of intermediate buildup
are
measured for RNase A in the presence of 0.5 M ribose (solid symbols and
curve). For
comparison, the amount of AGE present before ribose removal at each
interruption point
is also shown (open symbols and dashed lines). As expected (cf. Figure 7A),
little AGE
is formed prior to removal (or dilution) of ribose, so that ELISA readings
after the 5 day
secondary incubation periods are mostly a measure of AGE formed after ribose
removal.
The results in Figure 9 show that the rate of buildup of intermediate in 0.5 M
ribose is
exponential and very fast, with a half time of about 3.3 h. This is about 3-
fold more rapid
than the observed rate of conversion of the intermediate to antigenic AGES
after
to interruption (open symbols and dashed curve Figure 7A).
In these experiments the removal of ribose at each interruption time was
achieved
by 100-fold dilution, and not by dialysis. Simple dilution reduced the
concentration of
ribose from 0.05 M to 0.005 M. It was independently determined (Figure 6A)
that little
AGE is produced in this time scale with the residual 5 mM ribose. This
dilution approach
was primarily dictated by the need for quantitative point-to-point accuracy.
Such
accuracy would not have been achieved by the dialysis procedure that would be
carried
out independently for each sample at each interruption point. Our results show
that
dilution was equivalent to dialysis.
A separate control experiment (see Figure 10 below) demonstrated that the
2o instantaneous 100-fold dilution gave nearly identical results to the
dialysis procedure.
These control experiments demonstrate that the reversible ribose-protein
binding (Schiff
base) equilibrium is quite rapid on this time scale. This is consistent with
data of Bunn
and Higgins ( 1981, Science 213: 222-224) that indicated that the half time of
Schiff base
formation with 0.5 M ribose should be on the order of a few minutes. The 100-
fold rapid
dilution method to reduce ribose is a valid method where quantitative accuracy
is
essential and cannot be achieved by multiple dialysis of many samples.
Direct Inhibition of Post-Amadori AGE Formation from the Intermediate by
Ribose and
Glucose
The increase in the rate of AGE formation after intemzption . and sugar
dilution
suggests, but does not prove, that high concentrations of ribose are
inhibiting the reaction
at or beyond the first "stable" intermediate, presumably the Amadori
derivative (boxed in
26
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
Scheme I). A test of this was then earned out by studying the effect of
directly adding
ribose, on the post-Amadori reaction. RNase was first incubated for 24 h in
0.5 M ribose
in order to prepare the intermediate. Two protocols were then carried out to
measure
possible inhibition of the post-Amadori formation of antigenic AGES by
different
concentrations of ribose. In the first experiment, the 24 h ribated sample was
simply
diluted 100-fold into solutions containing varying final concentrations of
ribose ranging
from 0.005 M to 0.505 M (Figure 10A). The rate and extent of AGE formation are
clearly seen to be diminished by increasing ribose concentrations.
Significantly, up to the
highest concentration of 0.5 M ribose, the kinetics appear exponential and do
not show
1o the induction period that occurs with uninterrupted glycation (Figures 6A
and 7A) in
high ribose concentrations.
A second experiment (Figure 10B) was also conducted in which the 24 h
interrupted sample was extensively dialyzed in the cold to release free and
reversibly
bound ribose as well as any inhibitory products that may have formed during
the 24 h
15 incubation with ribose. Following this, aliquots were diluted 100-fold into
varying
concentrations of freshly made ribose, and the formation of antigenic AGE
products was
monitored as above. There results were nearly identical to the experiment of
Figure 10A
where the dialysis step was omitted. In both cases, the rate and extent of AGE
formation
were diminished by increasing concentrations of ribose, and the kinetics
appeared
20 exponential with no induction period.
The question of whether glucose or other sugars can also inhibit the formation
of
AGES from the reactive intermediate obtained by interrupted glycation in 0.5 M
ribose
was also investigated. The effects of glucose at concentrations of 1.0-2.0 M
were tested
(data not shown). Glucose was clearly not as inhibitory as ribose. When the 24
h ribose
25 interrupted sample was diluted 100-fold into these glucose solutions, the
amount of
antigenic AGE formed was diminished by about 30%, but there was little
decrease in the
apparent rate constant. Again, the kinetics appeared exponential.
Effect of pH on Post-Amadori Kinetics of AGE Formation
The interrupted glycation method was used to investigate the pH dependence of
the post-Amadori kinetics of AGE formation from the reactive intermediate. In
these
experiments, RNase A was first reacted for 24 h with 0.5 M ribose at pH 7.5 to
generate
27
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
the reactive intermediate. The kinetics of the decay of the intermediate to
AGES were
then measured by ELISA. Figure 11 shows that an extremely wide pH range of 5.0-
9.5
was achievable when the kinetics were measured by initial rates. A remarkable
bell
shaped dependence was observed, showing that the kinetics of antigenic AGES
formation
are decreased at both acidic and alkaline pH ranges, with an optimum near pH
8.
A single "pH jump" experiment was also carned out on the pH 5.0 sample
studied above which had the slowest rate of antigenic AGE formation. After 12
days at
37°C in pH 5.0 buffer, the pH was adjusted quickly to 7.5, and
antigenic AGE formation
was monitored by ELISA. Within experimental error, the sample showed identical
1o kinetics (same first order rate constant) of AGE formation to interrupted
glycation
samples that had been studied directly at pH 7.5 (Figure 12). In this
experiment, the
relative amounts of antigenic AGE could not be directly compared on the same
ELISA
plate, but the pH-jumped sample appeared to have formed substantial though
somehow
diminished levels of antigenic AGES. These results demonstrate that
intermediate can be
prepared free of AGE and stored at pH 5 for later studies of conversion to
AGES.
Inhibition of Post-Amadori AGE formation by Aminoguanidine
The efficacy of aminoguanidine was tested by this interrupted glycation
method,
i.e., by testing its effect on post-Amadori formation of antigenic AGES after
removal of
2o excess and reversibly bound ribose. Figure 20A demonstrates that
aminoguanidine has
modest effects on blocking the formation of antigenic AGES in RNase under
these
conditions, with little inhibition below 50 mM. Approximately 50% inhibition
is
achieved only at or above 100-250 mM. Note again that in these experiments,
the protein
was exposed to aminoguanidine only after interruption and removal of free and
?5 reversibly bound ribose. Comparable results were also obtained with the
interrupted
glycation of BSA (Figure 20B).
Amino acid analysis of Interrupted Glvcation Samples
Amino acid analysis was carried out on RNase after 24 h contact with 0.5 M
3o ribose (undialyzed), after extensive dialysis of the 24 h glycated sample,
and after S days
of incubation of the latter sample at 37°C. These determinations were
made after sodium
cyanoborohydride reduction, which reduces Schiff base present on lysines or
the
28
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
terminal amino group. All three samples, normalized to alanine ( 12 residues),
showed the
same residual lysine content (4.0 t 0.5 out of the original 10 in RNase). This
indicates
that after 24 h contact with 0.5 M ribose, most of the formed Schiff base
adducts had
been converted to Amadori or subsequent products. No arginine or histidine
residues
were lost by modification.
Discussion
The use of rapidly reacting ribose and the discovery of its reversible
inhibition of
post-Amadori steps have permitted the dissection and determination of the
kinetics of
different steps of protein glycation in RNase. Most previous kinetic studies
of protein
"glycation" have actually been restricted to the "early" steps of Schiff base
formation
and subsequent Amadori rearrangement. Some kinetic studies have been carried
out
starting with synthesized fructosylamines, i.e. small model Amadori compounds
of
glucose (Smith and Thornalley, 1992, Eur. J. Biochem. 210:729-739, and
references
cited therein), but such studies, with few exceptions, have hitherto not been
possible with
proteins. One notable exception is the demonstration by Monnier (Odetti et
al., 1992,
supra) that BSA partially glycated with ribose can rapidly produce pentosidine
after
ribose removal. The greater reactivity of ribose has also proven a distinct
advantage in
quantitatively defining the time course of AGE formation. It is noted that
glucose and
ribose are both capable of producing similar AGE products, such as pentosidine
(Grandhee & Monnier, 1991, supra; Dyer et al. 1991, supra), and some 13C NMR
model
compound work has. been done with ADP-ribose (Cervantes-Laurean et al., 1993,
Biochemists: 32:1528-1534). The present work shows that antigenic AGE products
of
ribose fully cross-react with anti-AGE antibodies directed against glucose-
modified
proteins, suggesting that ribose and glucose produce similar antigenic AGES.
The
primary kinetic differences observed between these two sugars are probably due
to
relative differences in the rate constants of steps leading to post-Amadori
AGE
formation, rather than in the mechanism.
The results presented reveal a marked and paradoxical inhibition of overall
AGE
formation by high concentrations of ribose (Figure 6) that has not been
anticipated by
earlier studies. This inhibition is rapidly reversible in the sense that it is
removed by
dialysis of initially modified protein (Figure 7A) or by simple 100-fold
dilution (as used
29
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
in Figure 11 ). The experiments of Figure 10 demonstrate that it is not due to
the
accumulation of dialyzable inhibitory intermediates during the initial
glycation, since
dialysis of 24 h modified protein followed by addition of different
concentrations of
fresh ribose induces the same inhibition. The data of Figure lOA,B show that
the
inhibition occurs by reversible and rapid interaction of ribose with protein
intermediate
containing reactive Amadori products. The inhibition is unlikely to apply to
the early
step of formation of Amadori product due to the rapid rate of formation of the
presumed
Amadori intermediate that was determined in the experiment of Figure 9. The
identification of the reactive intermediate as an Amadori product is well
supported by the
1o amino acid analysis carned out (after sodium cyanoborohydrate reduction)
before and
after dialysis at the 24 h interruption point. The unchanged residual lysine
content
indicates that any dischageable Schiff bases have already been irreversibly
converted
(presumably by Amadori rearrangement) by the 24 h time.
The secondary ribose suppression of "late" but not "early" glycation steps
significantly enhances the accumulation of a fully-competent reactive Amadori
intermediate containing little AGE. Its isolation by the interruption
procedure is of
importance for kinetic and structural studies, since it allows one to make
studies in the
absence of free or Schiff base bound sugar and their attendant reactions and
complications. For example, the post-Amadori conversion rates to antigenic AGE
and
pentosidine AGE products have been measured (Figure 7A, open symbols, and
Figure
8B), and demonstrated to be much faster (t 1/2 ~ 10 h) than reflected in the
overall
kinetics under uninterrupted conditions (Figure 6A and Figure 8A). The rapid
formation
of pentosidine that was measured appears consistent with an earlier
interrupted ribose
experiment on BSA by Odetti et al. (1992, supra). Since ribose and derivatives
such as
ADP-ribose are normal metabolites, the very high rates of AGE formation seen
here
suggest that they should be considered more seriously as sources of potential
glycation in
various cellular compartments (Cervantes-Laurean et al., 1993, supra), even
though their
concentrations are well below those of the less reactive glucose.
Another new application of the isolation of intermediate is in studying the pH
3o dependence of this complex reaction. The unusual bell-shaped pH profile
seen for the
post-Amadori AGE formation (Figure 11) is in striking contrast to the mild pH
dependence of the overall reaction. The latter kinetics reflect a composite
effect of pH on
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
all steps in the reaction, including Schiff base and Amadori product
formation, each of
which may have a unique pH dependence. This complexity is especially well
illustrated
by studies of hemoglobin glycation (Lowery et al., 1985, J. Biol. Chern.
260:11611-
11618). The bell-shaped pH profile suggests, but does not prove, the
involvement of two
ionizing groups. If true, the data may imply the participation of a second
amino group,
such as from a neighboring lysine, in the formation of dominant antigenic
AGES. The
observed pH profile and the pH-jump observations described suggest that a
useful route
to isolating and maintaining the reactive intermediate would be by the rapid
lowering of
the pH to near 5.0 after 24 h interruption.
to The kinetic studies provide new insights into the mechanisms of action of
aminoguanidine (guanylhydrazine), an AGE inhibitor proposed by Cerami and co-
workers to combine with Amadori intermediates (Brownlee et al., 1986, szspra).
This
proposed pharmacological agent is now in Phase III clinical trials for
possible
therapeutic effects in treating diabetes (Vlassara et al., 1994, supra).
However, its
15 mechanism of AGE inhibition is likely to be quite complex, since it is
multifunctional.
As a nucelophilic hydrazine, it can reversibly add to active carbonyls,
including
aldehydo carbonyls of open-chain glucose and ribose (Khatami et al., 1988,
Life Sci.
43:1725-1731; Hirsch et al., 1995, Curbohyd. Res. 267:17-25), as well as keto
carbonyls
of Amadori compounds. It is also a guanidinium compound that can scavange
highly
20 reactive dicarbonyl alycation intermediates such as glyoxal and glucosones
(Chen &
Cerami, 1993, J. Cczrbohvd. Chem. 12:731-742; Hirsch et al., 1992, Carbohvd.
Res.
232:125-130: Ou & Wolff; 1993, Biochein. Phar-macol. 46:1139-1144). The
interrupted
glycation method allowed examination of aminoguanidine efficacy on only post-
Amadori steps of AGE formation. Equally important, it allowed studies in the
absence of
25 free sugar or dicarbonyl-reactive fragments from free sugar (Wolff & Dean,
1987,
Biochent. J. 245:243-250; Wells-Knecht et al., 1995, Biochemistry 34:3702-
3709) that
can combine with aminoguanidine. The results of Figure 20 demonstrate that
aminoguanidine has, at best, only a modest effect on post-Amadori AGE
formation
reactions, achieving 50% inhibition at concentrations above 100-250 mM. The
efficacy
30 of aminoguanidine thus predominantly arises either from inhibiting early
steps of
glycation ( Schiff base formation) or from scavenging highly reactive
dicarbonyls
generated during glycation. Contrary to the original claims, it does not
appear to inhibit
31
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
AGE formation by complexing the Amadori intermediate.
The use of interrupted glycation is not limited for kinetic studies.
Interrupted
glycation can simplify structural studies of glycated proteins and identifying
unknown
AGEs using techniques such as 13C NMR that has been used to detect Amadori
adducts
a of RNase (Neglia et al., 1983, J. Biol. Chem. 258:14279-14283; 1985, J.
Biol. Chem.
260:5406-X410). The combined use of structural and kinetic approaches should
also be
of special interest. For example, although the identity of the dominant
antigenic AGEs
reacting with the polyclonal antibodies remains uncertain, candidate AGES,
such as the
recently proposed (carboxymethyl)lysine (Reddy et al., 1995, Biochemistry
34:10872-
to 10878; cf. Makita et al., 1992, J. Biol. Chem. 267:5133-5138) should
display the same
kinetics of formation from the reactive intermediate that we have observed.
The
availability of the interrupted kinetics approach will also help to determine
the
importance of the Amadori pathway to the formation of this AGE. Similarly,
monitoring
of the interrupted glycation reaction by techniques such as 13C NMR should
identify
15 resonances of other candidate antigenic AGEs as being those displaying
similar kinetics
of appearance. Table I lists the 13C NMR peaks of the Amadori intermediate of
RNase
prepared by reaction with C-2 enriched ribose. The downfield peak near 205 ppm
is
probably due to the carbonyl of the Amadori product. In all cases, the ability
to remove
excess free and Schiff base sugars through interrupted glycation will
considerably
2o simplify isolation, identification, and structural characterization.
Table I lists the peaks that were assigned to the Post-Amadori Intermediate
due to
their invariant or decreasing intensity with time. Peak positions are listed
in ppm
downfield from TMS.
Table I 125MHz C-13 NMR
Resonances of Ribonuclease
Amadori
25 Intermediate Prepared Reaction with 99% [2-C13]Ribose
by 24 HR
216.5 ppm 108.5 ppm
211.7 105.9
208 103.9
103
30 172 95.8
165
163.9 73.65
162.1 70.2
69.7
32
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
Ribonuclease A was reacted for 24 hr with 0.5 M ribose 99% enriched at C-2,
following which excess and Schiff base bound ribose was removed by extensive
dialysis
in the cold. The sample was then warmed back to 37°C immediately before
taking a 2 hr
NMR scan. The signals from RNase reacted with natural abundance ribose under
identical conditions were then subtracted from the NMR spectrum. Thus all
peaks shown
are due to enriched C-13 that originated at the C-2 position. Some of the
peaks arise from
degradation products of the intermediate, and these can be identified by the
increase in
the peak intensity over time. Figure 31 shows the NMR spectrum obtained.
l0
Example 3
In I~itro Inhibition of the Formation of Antigenic Advanced Glycation End-
Products (AGES) by Derivatives of Vitamins B1 and B( and Aminoguanidine.
Inhibition of Post-Amadori Kinetics Differs from that of Overall Glvcation
is
The interrupted glycation method for following post-Amadori kinetics of AGE
formation allows for the rapid quantitative study of "late" stages of the
glycation
reaction. Importantly, this method allows for inhibition studies that are free
of pathways
of AGE formation which arise from glycoxidative products of free sugar or
Schiff base
20 (Namiki pathway) as illustrated in Scheme I. Thus the interrupted glycation
method
allows for the rapid and unique identification and characterization of
inhibitors of "late"
stages of glycation which lead to antigenic AGE formation.
Among the vitamin B 1 and B6 derivatives examined, pyridoxamine and thiamine
pyrophosphate are unique inhibitors of the post-Amadori pathway of AGE
formation.
z5 Importantly, it was found that efficacy of inhibition of overall glycation
of protein, in the
presence of high concentrations of sugar, is not predictive of the ability to
inhibit the
post-Amadori steps of AGE formation where free sugar is removed. Thus while
pyridoxamine, thiamine pyrophosphate and aminoguanidine are potent inhibitors
of AGE
formation in the overall glycation of protein by glucose, aminoguanidine
differs from the
30 other two in that it is not an effective inhibitor of post-Amadori AGE
formation.
Aminoguanidine markedly slows the initial rate of AGE formation by ribose
under
uninterrupted conditions, but has no effect on the final levels of antigenic
AGES
produced. Examination of different proteins (RNase, BSA and hemoglobin),
confirmed
that the inhibition results are generally non-specific as to the protein used,
even though
33
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
there are individual variations in the rates of AGE formation and inhibition.
Chemicals and ~Llaterials As in Example 1 above.
Preparation of polvclonal antibodies to AGEs
:As in Example 1 above.
ELISA detection ofAGE products As in Example 1 above.
Uninterrupted ribose glvcation assays
Bovine serum albumin, ribonuclease A, and human hemoglobin were incubated
with ribose at 37°C in 0.4 M sodium phosphate buffer of pH 7.5
containing 0.02%
sodium azide. The protein ( 10 mg/ml or 1 mg/ml), 0.05 M ribose. and
prospective
inhibitors (at 0.5, 3, 15 and 50 mM) were introduced into the incubation
mixture
simultaneously. Solutions were kept in the dark in capped tubes. Aliquots were
taken and
immediately frozen until analyzed by ELISA at the conclusion of the reaction.
The
incubations were for 3 weeks (Hb) or 6 weeks (RNase, BSA). Glycation reactions
were
monitored for constant pH throughout the duration of the experiments.
Interrupted (post-Arnadori) ribose glycation assays
Glycation was first carried out by incubating protein ( 10 mg/ml) with 0.5 M
ribose at 37°C in 0.4 M phosphate buffer at pH 7.5 containing 0.2%
sodium azide for 24
h in the absence of inhibitors. Glycation was then interrupted to remove
excess and
reversibly bound (Schiff base) sugar by extensive dialysis against frequent
cold buffer
changes at 4°C. The glycated intermediate samples containing maximal
amount of
Amadori product and little AGE (depending on protein) were then quickly warmed
to
37°C without re-addition of ribose. This initiated conversion of
Amadori intermediates to
AGE products in the absence or presence of various concentrations (typically
3, 15 and
50 mM) of prospective inhibitors. Aliquots were taken and frozen at various
intervals for
later analysis. The solutions were kept in capped tubes and opened only to
remove timed
aliquots that were immediately frozen for later carrying out the various
analyses.
Nurnerical Analvsis of kinetics data
Kinetics data (time progress curves) was routinely fit to mono- or bi-
exponential
functions using non-linear least squares methods utilizing either SCIENTIST
2.0
34
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
(MicroMath, Inc.) or ORIGIN (Microcal, Inc.) sofrivare that permit user-
defined
functions and control of parameters to iterate on. Standard deviations of the
parameters
of the fitted functions (initial and final ordinate values and rate constants)
were returned
as measures of the precision of the fits. Apparent half times for bi-
exponential kinetics
fits were determined with the "solve" function of MathCad software (MathSoft,
Inc.).
RESULTS
Inhibition by vitamin B( derivatives of the overall kinetics of AGE formation
from
RlIJOSe.
1o The inhibitory effects of vitamin B1 and B6 derivatives on the kinetics of
antigenic AGE formation were evaluated by polyclonal antibodies specific for
AGEs.
Initial inhibition studies were carried out on the glycation of bovine
ribonuclease A
(RNase) in the continuous presence of 0.05 M ribose, which is the
concentration of
ribose where the rate of AGE formation is near maximal. Figure 13 (control
curves,
15 filled rectangles) demonstrates that the formation of antigenic AGES on
RNase when
incubated with 0.05 M ribose is relatively rapid, with a half time of
approximately 6
days under these conditions. Pyridoxal-5'-phosphate (Figure 13B) and pyridoxal
(Figure
13C) significantly inhibited the rate of AGE formation on RNase at
concentrations of 50
mM and 15 mM. Surprisingly, pyridoxine, the alcohol form of vitamin B6, also
20 moderately inhibited AGE formation on RNase (Figure 13D). Of the B(
derivatives
examined, pyridoxamine at 50 mM was the best inhibitor of the "final" levels
of AGE
formed on RNase over the 6-week time period monitored (Figure 13A).
Inhibition by vitamin BI derivatives of the overall kinetics of AGE formation
from
25 Ribose.
All of the B 1 vitamers inhibited antigenic AGE formation on RNase at high
concentrations. but the inhibition appeared more complex than for the B~
derivatives
(Figure 14A-C). In the case of thiamine pyrophosphate as the inhibitor (Figure
14A),
both the rate of AGE formation and the final levels of AGE produced at the
plateau
3o appeared diminished. In the case of thiamine phosphate as the inhibitor
(Figure 14B),
and thiamine (Figure 14C), there appeared to be little effect on the rate of
AGE
formation, but a substantial decrease in the final level of AGE formed in the
presence of
the highest concentration of inhibitor. In general, thiamine pyrophosphate
demonstrated
greater inhibition than the other two compounds, at the lower concentrations
examined.
Inhibition by aminoguanidine of the overall kinetics of AGE formation from
Ribose
Inhibition of AGE formation by aminoguanidine (Figure 14D) was distinctly
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
different from that seen in the B 1 and B6 experiments. Increasing
concentration of
aminoguanidine decreased the rate of AGE formation on RNase, but did not
reduce the
final level of AGE formed. The final level of AGE formed after the 6-weeks was
nearly
identical to that of the control for all tested concentrations of
aminoguanidine.
Inhibition of tlTe overall kinetics of AGE formation in serum albumin and
hemoglobin
from Ribose
Comparative studies were carried out with BSA and human methemoglobin (Hb)
to determine whether the observed inhibition was protein-specific. The
different
to derivatives of vitamin B6 (Figure 15A-D) and vitamin B1 (Figure 16A-C)
exhibited
similar inhibition trends when incubated with BSA as with RNase, pyridoxamine
and
thiamine pyrophosphate being the most effective inhibitors or each family.
Pyridoxine
failed to inhibit AGE formation on BSA (Figure 15D). Pyridoxal phosphate and
pyridoxal (Figure 15B-C) mostly inhibited the rate of AGE formation, but not
the final
1, levels of AGE formed. Pyridoxamine (Figure 15A) exhibited some inhibition
at lower
concentrations, and at the highest concentration tested appeared to inhibit
the final levels
of AGE formed more effectively than any of the other B6 derivatives. In the
case of B1
derivatives, the overall extent of inhibition of AGE formation with BSA
(Figure 16A-C),
was less than that observed with RNase (Figure 14A-C). Higher concentrations
of
2o thiamine and thiamine pyrophosphate inhibited the final levels of AGES
formed, without
greatly affecting the rate of AGE formation (Figure 16C). Aminoguanidine again
displayed the same inhibition effects with BSA as seen with RNase (Figure
16D),
appearing to slow the rate of AGE formation without significantly affecting
the final
levels of AGE formed.
25 The kinetics of AGE formation was also examined using Hb in the presence of
the B6 and B 1 vitamin derivatives, and aminoguanidine. The apparent absolute
rates of
AGE formation were significantly higher with Hb than with either RNase or BSA.
However, the tested inhibitors showed essentially similar behavior. The
effects of the
vitamin B6 derivatives are shown in Figure 17. Pyridoxamine showed the
greatest
inhibition at concentrations of 3 mM and above (Figure 17A), and was most
effective
when compared to pyridoxal phosphate (Figure 17B), pyridoxal (Figure 17C), and
pyridoxine (Figure 17D). In the case of the B 1 vitamin derivatives (data not
shown), the
inhibitory effects were more similar to the BSA inhibition trends than to
RNase. The
inhibition was only modest at the highest concentrations tested (50 mM), being
nearly
35 30-SO% for all three B 1 derivatives. The primary manifestation of
inhibition was in the
reduction of the final levels of AGE formed.
36
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
Inhibition by vitamin B6 derivatives of the kinetics of post-Amadori ribose
AGE
formation
Using the interrupted glycation model to assay for inhibition of the "late"
post-
Arnadori AGE formation, kinetics were examined by incubating isolated Amadori
intermediates of either RNase or BSA at 37°C in the absence of free or
reversibly bound
ribose. Ribose sugar that was initially used to prepare the intermediates was
removed by
cold dialysis after an initial glycation reaction period of 24 h. After AGE
formation is
allowed to resume, formation of AGE is quite rapid (half times of about 10 h)
in the
absence of any inhibitors. Figure 18 shows the effects of pyridoxamine (Figure
18A),
to pyridoxal phosphate (Figure 18B), and pyridoxal (Figure 18C) on the post-
Amadori
kinetics of BSA. Pyridoxine did not produce any inhibition (data not shown).
Similar
experiments were carried out on RNase. Pyridoxamine caused nearly complete
inhibition
of AGE formation with RNase at 15 mM and 50 mM (Figure 18D). Pyridoxal did not
show any significant inhibition at 15 mM (the highest tested), but pyridoxal
phosphate
15 showed significant inhibition at 15 ml~I. Pyridoxal phosphate is known to
be able to
affinity label the active site of RNase (Raetz and Auld, 1972, Biochemisttv
11:2229-
2236).
With BSA, unlike RNase, a significant amount of antigenic AGE formed during
the 24 h initial incubation with RNase (25-30%), as evidenced by the higher
ELISA
2o readings after removal of ribose at time zero for Figures 18A-C. For both
BSA and
RNase, the inhibition, when seen, appears to manifest as a decrease in the
final levels of
AGE formed rather than as a decrease in the rate of formation of AGE.
Inhibition by vitamin BI derivatives of the kinetics of post-Antadori ribose
AGE
3~ formation
Thiamine pyrophosphate inhibited AGE formation more effectively than the
other B 1 derivatives with both RNase and BSA (Figure 19). Thiamine showed no
effect,
while thiamine phosphate showed some intermediate effect. As with the B6
assays, the
post-Amadori inhibition was most apparently manifested as a decrease in the
final levels
30 of AGE formed.
Effects of aminoguanidine and No~-acetyl-L-Ivsine on the kinetics of post-
Amadori ribose
AGE formation
Figure 20 shows the results of testing aminoguanidine for inhibition of post-
35 Amadori AGE formation kinetics with both BSA and RNase. At 50 mM,
inhibition was
about 20% in the case of BSA (Figure 20B), and less than 15% with RNase
(Figure
?OA). The possibility of inhibition by simple amino-containing functionalities
was also
37
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
tested using Na-acetyl-L-lysine (Figure 21 ), which contains only a free Na-
amino
group. Na-acetyl-L-lysine at up to 50 mM failed to exhibit any significant
inhibition of
AGE formation.
Discussion
Numerous studies have demonstrated that aminoguanidine is an apparently potent
inhibitor of many manifestations of nonenzymatic glycation (Brownlee et al.,
1986;
Brownlee, 1992,1994, 1995). The inhibitory effects of aminoguanidine on
various
phenomena that are induced by reducing sugars are widely considered as proof
of the
to involvement of glycation in many such phenomena. Aminoguanidine has
recently
entered into a second round of Phase III clinical trials (as pimagedine) for
ameliorating
the complications of diabetes thought to be caused by glycation of connective
tissue
proteins due to high levels of sugar.
Data from the kinetic study of uninterrupted "slow" AGE formation with RNase
1 ~ induced by glucose (Example 1 ) confirmed that aminoguanidine is an
effective inhibitor,
and further identified a number of derivatives of vitamins B1 and B6 as
equally or
slightly more effective inhibitors. However, the inhibition by aminoguanidine
unexpectedly appeared to diminish in effect at the later stages of the AGE
formation
reaction. Due to the slowness of the glycation of protein with glucose, this
surprising
20 obser<~ation could not be fully examined. Furthermore, it has been
suggested that there
may be questions about the long-term stability of aminoguanidine (0u and
Wolff, 1993,
suprn ).
Analysis using the much more rapid glycation by ribose allowed for the entire
time-course of AGE formation to be completely observed and quantitated during
25 uninterrupted glycation of protein. The use of interrupted glycation
uniquely allowed
further isolation and examination of only post-Amadori antigenic AGE formation
in the
absence of free and reversibly bound (Schiff base) ribose. Comparison of the
data from
these two approaches with the earlier glucose glycation kinetics has provided
novel
insights into the mechanisms and effectiveness of various inhibitors. Figure
22 are bar
3o graphs which depict summarized comparative data of percent inhibition at
defined time
points using various concentrations of inhibitor. Figure 22A graphs the data
for
inhibition after interrupted glycation of RNase AGE formation in ribose.
Figure 22B
graphs the data for inhibition after interrupted glycation of BSA AGE
formation by
ribose.
~5 The overall results unambiguously demonstrate that aminoguanidine slows the
rate of antigenic AGE formation in the presence of sugar but has little effect
on the final
amount of post-Amadori AGE formed. Thus observations limited to only the
initial
38
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
"early" stages of AGE formation which indicate efficacy as an inhibitor may in
fact be
misleading as to the true efficacy of inhibition of post-Amadori AGE
formation. Thus
the ability to observe a full-course of reaction using ribose and interrupted
glycation
gives a more complete picture of the efficacy of inhibition of post-Amadori
AGE
formation.
Example 4
Animal model & testing of in vivo effects of AGE formation/inhibitors
Hyperglycemia can be rapidly induced (within one or two days) in rats by
administration of streptozocin (aka. streptozotocin, STZ) or alloxan. This has
become a
common model for diabetes melitus. However, these rats manifest nephropathy
only
after many months of hyperglycemia, and usually just prior to death from end-
stage renal
disease (ESRD). It is believed that this pathology is caused by the
irreversible glucose
chemical modification of long-lived proteins such as collagen of the basement
membrane. STZ-diabetic rats show albuminuria very late after induction of
hyperglycemia, at about 40 weeks usually only just prior to death.
Because of the dramatic rapid effects of ribose demonstrated in vitro in the
examples above, it was undertaken to examine the effects of ribose
administration to rats,
and the possible induction of AGES by the rapid ribose glycation. From this
study, a rat
2o model for accelerated ribose induced pathology has been developed.
E_f'fects of very short-tern: ribose administration in vivo
Phase I Protocol
Two groups of six rats each were given in one day either:
. a. 300 mM ribose (two intraperitoneal infusions 6-8 hours apart, each 5%
of body weight as ml); or
b. 50 mM ribose (one infusion)
Rats were then kept for 4 days with no further ribose administration, at which
time they were sacrificed and the following physiological measurements were
3o determined: (i) initial and final body weight; (ii) final stage kidney
weight; (iii) initial
and final tail-cuff blood pressure; (iv) creatinine clearance per 100 g body
weight.
Albumin filtration rates were not measured, since no rapid changes were
initially
anticipated. Past experience with STZ-diabetic rats shows that albuminuria
develops very
late (perhaps 40 weeks) after the induction of hyperglycemia and just before
animals
expire.
Renal Physiology Results
39
CA 02360311 2001-10-04
VVO 00/59493 PCT/US00/09241
a. Final body weight and final single kidney weight was same for low and high
ribose treatment groups.
b. Tail-cuff blood pressure increased from 66 ~ 4 to 83 ~ 3 to rats treated
with
low ribose ( 1 x ~0 mM), and from 66 t 4 to 106 ~ 5 for rats treated with high
ribose (2 x
300 mNI). These results are shown in the bar graph of Figure 23.
c. Creatinine clearance, as cc per 100 g body weight, was decreased (normal
range expected about 1.0-1.2) in a dose-dependent fashion to 0.87 t 0.15 for
the low
ribose group, and decreased still further 30% to 0.62 ~ 0.13 for the high
ribose group.
These results are shown in the bar graph of Figure 24.
l0
Phase I Conclusion
A single day's ribose treatment caused a dose-dependent hypertension and a
dose-dependent decrease in glomerular clearance function manifest 4 days
later. These
are significant metabolic changes of diabetes seen only much later in STZ-
diabetic rats.
15 These phenomenon can be hypothesized to be due to ribose irreversible
chemical
modification (glycation) of protein in vivo.
Effect of exposure to higher ribose concentrations for longer time
Phase l1 Protocol
20 Groups of rats (3-6) were intraperitoneally given 0.3 M "low ribose dose"
(LR)
or 1.0 M "high ribose dose" (HR) by twice-daily injections for either (i) 1
day, (ii) a
"short-term" (S) of 4 days, or (iii) a "long-term" (L) of 8 days.
Additionally, these
concentrations of ribose were included in drinking water.
Renal Physiology Results
a. Tail-cuff blood pressure increased in all groups of ribose-treated rats,
confirming Phase I results. (Figure 23).
b. Creatinine clearance decreased in all groups in a ribose dose-dependent and
time-dependent manner (Figure 24).
c. Albumin Effusion Rate (AER) increased significantly in a ribose-dependent
manner at 1-day and 4-day exposures. However, it showed some recovery at 8 day
relative to 4 day in the high-dose group but not in the low-dose group. These
results are
shown in the bar graph of Figure 25.
d. Creatinine clearance per 100 g body weight decreased for both low- and high-
ribose groups to about the same extent in a time-dependent manner (Figure 24).
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
Phase II Conclusion
Exposure to ribose for as little as 4 days leads to hypertension and renal
dysfunction, as manifest by both decreased creatinine clearance and increased
albumin
filtration. These changes are typical of diabetes and are seen at much later
times in STZ-
diabetic rats.
Inten~ention by two new therapeutic compounds and aminoguanidine
Phase III Protocol
Sixty rats were randomized into 9 different groups, including those exposed to
1
M ribose for 8 days in the presence and absence of aminoguanidine,
pyridoxamine, and
thiamine pyrophosphate as follows:
Control Groups:
( i ) no treatment;
ii) high dose (250 mg/kg body weight) of pyridoxamine ("compound-P");
(iii) high dose (250 mg/kg body weight of thiamine pyrophosphate ("compound-T"
or
"TPP"); and
(iv) low dose (25 mg/kg body weight) of aminoguanidine ("AG").
Test Groups:
(i) only 1 M ribose-saline (2 x 9 cc daily IP for 8 days);
z0 (ii) ribose plus low dose ("LP") of pyridoxamine (25 mg/kg body weight
injected as 0.5
ml with 9 cc ribose);
(iii) ribose plus high dose ("HP") of pyridoxamine (250 mg/kg body weight
injected as
0.5 ml with 9 cc ribose);
(iv) ribose plus high dose ("HT") of thiamine pyrophosphate (250 mg/kg body
weight
injected as 0.5 ml with 9 cc ribose); and
(v) ribose plus low dose of amino guanidine (25 mg/kg body weight injected as
0.5 ml
with 9 cc ribose).
Unlike Phase II, no ribose was administered in drinking water. Intervention
compounds were pre-administered for one day prior to introducing them with
ribose.
Renal physiology Results
a. Blood pressure was very slightly increased by the three compounds alone
(control group); ribose-elevated BP was not ameliorated by the co-
administration of
compounds. These results are shown in the bar graph of Figure 26.
b. Creatinine clearance in controls was unchanged, except for TPP which
diminished it.
41
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
c. Creatinine clearance was normalized when ribose was co-administered with
low dose (25 mgikg) of either aminoguanidine or pyridoxamine. These results
are shown
in the bar graph of Figure 27.
d. High concentrations (250 mg/kg) or pyridoxamine and TPP showed only
partial protection against the ribose-induced decrease in creatinine clearance
(Figure 27).
e. Albumin effusion rate (AER) was elevated by ribose, as well as by high dose
of pyridoxamine and TPP, and low dose of aminoguanidine in the absence of
ribose.
These results are shown in the bar graph of Figure 28.
f. Albumin effusion rate was restored to normal by the co-administration of
low
t0 dose of both aminoguanidine and pyridoxamine. These results are shown in
the bar graph
of Figure 29.
Phase III Conclusions
As measured by two indicies of renal function, pyridoxamine and
aminoguanidine, both at 25 mg/kg, were apparently effective, and equally so,
in
preventing the ribose-induced decrease in creatinine clearance and ribose-
induced mild
increase in albuminuria.
(i) Thiamine pyrophosphate was not tested at 25 mg/kg; (ii) thiamine
pyrophosphate and pyridoxamine at 250 mg/kg were partially effective in
preventing
2o creatinine clearance decreases but possibly not in preventing mild
proteinuria; (iii) at
these very high concentrations and in the absence of ribose, thiamine
pyrophosphate
alone produced a decrease in creatinine clearance, and both produced mild
increases in
albuminuria.
Summary
Renal Facnction and Diabetes
Persistent hyperglycemia in diabetes mellitus leads to diabetic nephropathy in
perhaps one third of human patients. Clinically, diabetic nephropathy is
defined by the
presence of:
3o 1. decrease in renal function (impaired glomerular clearance)
2. an increase in urinary protein (impaired filtration)
3. the simultaneous presence of hypertension
42
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
Renal function depends on blood flow (not measured) and the glomerular
clearance. which can be measured by either inulin clearance (not measured) or
creatinine
clearance. Glomerular permeability is measured by albumin filtration rate, but
this
parameter is quite variable. It is also a log-distribution function: a hundred-
fold increase
in albumin excretion represents only a two-fold decrease in filtration
capacity.
Ribose Diabetic Rat Model
By the above criteria, ribose appears to very rapidly induce manifestations of
diabetic nephropathy, as reflected in hypertension, creatinine clearance and
albuminuria,
1o even though the latter is not large. In the established STZ diabetic rat,
hyperglycemia is
rapidly established in 1-2 days, but clinical manifestations of diabetic
nephropathy arise
very late, perhaps as much as 40 weeks for albuminuria. In general,
albuminuria is highly
variable from day to day and from animal to animal, although unlike humans,
most STZ
rats do eventually develop nephropathy.
Intervention by Compounds
Using the ribose-treated animals, pyridoxamine at 25 mg/kg body weight appears
to effectively prevent two of the three manifestations usually attributed to
diabetes,
namely the impairment of creatinine clearance and albumin filtration. It did
so as
2o effectively as aminoguanidine. The effectiveness of thiamine pyrophosphate
was not
manifest, but it should be emphasized that this may be due to its use at
elevated
concentrations of 250 mg/kg body weight. Pyridoxamine would have appeared much
less
effective if only the results at 250 mg/kg body weight are considered.
Effect of Compoacnds Alone
Overall, the rats appeared to tolerate the compounds well. Kidney weights were
not remarkable and little hypertension developed. The physiological effects of
the
compounds were only tested at 250 mg/kg. Thiamine pyrophosphate, but not
pyridoxamine, appeared to decrease creatinine clearance at this concentration.
Both
3o appeared to slightly increase albuminuria, but these measurements were
perhaps the least
reliable.
43
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
Human Adrninistration
.A typical adult human being of average size weighs between 66 - 77 Kg.
Typically, diabetic patients may tend to be overweight and can be over 112 Kg.
The
Recommended dietary allowances for an adult male of between 66 - 77 Kg, as
revised in
1989, called for 1.5 mg per day of thiamine, and 2.0 mg per day of Vitamin B6
(Merck
Manual of Diagnosis and Therapy, 16th edition (Merck & Co., Rathaway, N.J.,
1992) pp
938-939).
Based upon the rat model approach, a range of doses for administration of
pyridoxamine or thiamine pyrophosphate that is predicted to be effective for
inhibiting
1 o post-Amadori AGE formation and thus inhibiting related pathologies would
fall in the
range of 1 mg/100 g body weight to 200 mg/100 g body weight. The appropriate
range
when co-administered with aminoguanidine will be similar. Calculated for an
average
adult of 75 Kg, the range (at 10 mg/1 Kg body weight) can be approximately 750
mg to
upwards of 150 g (at 2 g/1 Kg body weight). This will naturally vary according
to the
particular patient.
Example 5
In i~ivo Inhibition of the Formation of Advanced Glycation End-Products (AGEs)
by Derivatives of Vitamins B1 and B6 and Aminoguanidine. Inhibition of
diabetic
2o nephropathy.
The interrupted glycation method, as described in the examples above, allows
for
the rapid generation of stable well-defined protein Amadori intermediates from
ribose
and other pentose sugars for use in irz vivo studies.
?5 The effects of 25 mg/kg/day pyridoxamine (PM) and aminoguanidine (AG) on
renal pathology induced by injecting Sprague-Dawley rats daily with 50
mg/kg/day of
ribose-glycated Amadori-rat serum albumin (RSA), AGE-RSA, and unmodified RSA
for
6 weeks. Hyperfiltration (increased creatinine clearance) was transiently seen
with rats
receiving Amadori-RSA and AGE-RSA, regardless of the presence of PM and AG.
3o Individuals from each group receiving Amadori-RSA and AGE-RSA exhibited
microalbuminuria, but none was seen if PM was co-administered. Immunostaining
with
anti-RSA revealed glomerular staining in rats treated with AGE-RSA and with
Amadori
RSA; and this staining was decreased by treatment with PM but not by AG
treatment. A
decrease in glomerular sulfated glycosaminoglycans (Alcian blue pH 1.0 stain)
was also
44
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
found in rats treated with glycated (Amadori and AGE) RSA. This appears to be
due to
reduced heparan sulfate proteoglycans (HSPG), as evidenced by diminished
staining
with mAb JM-403 that is specific for HSPG side-chain. These HSPG changes were
ameliorated by treatment with PM, but not by AG treatment.
Thus we conclude that pyridoxamine can prevent both diabetic-like glomerular
loss of heparan sulfate and glomerular deposition of glycated albumin in SD
rats
chronically treated with ribose-glycated albumin.
Materials and methods
Chemicals
Rat serum albumin (RSA) (fraction V, essentially fatty acid-free 0.005%;
A2018), D-ribose, pyridoxamine, and goat alkaline phosphatase-conjugated anti-
rabbit
IgG were all from Sigma Chemicals. Aminoguanidine hydrochloride was purchased
from Aldrich Chemicals.
Preparcation of ribated RSA
Rat serum albumin was passed down an Affi-Gel Blue column (Bio-Rad), a
heparin-Sepharose CL-6B column (Pharmacia) and an endotoxin-binding affinity
column (Detoxigel, Pierce Scientific) to remove any possible contaminants. The
purified
2o rat serum albumin (RSA) was then dialyzed in 0.2 M phosphate buffer (pH
7.5). A
portion of the RSA (20 mg/ml) was then incubated with 0.5 M ribose for 12
hours at
37°C in the dark. After the 12 hour incubation the reaction mixture was
dialyzed in cold
0.2 M sodium phosphate buffer over a 36 hour period at 4°C (this
dialysis removes not
only the free ribose, but also the Schiff base intermediaries). At this stage
of the
?5 glycation process, the ribated protein is classified as Amadori-RSA and is
negative for
antigenic AGES, as determined by antibodies reactive with AGE protein (as
described
previously; 8618, antigen:glucose modified AGE-Rnase). The ribated protein is
then
divided into portions that will be injected either as: a)Amadori-RSA, b)NaBH4-
reduced
Amadori-RSA, c)AGE-RSA.
3o The ribated protein to be injected as Amadori-RSA is simply dialyzed
against
cold PBS at 4°C for 24 hours. A portion of the Amadori-RSA in 0.2 M
sodium
phosphate is reduced with NaBH~ to form NaBH4-reduced Amadori-RSA. Briefly,
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
aliquots were reduced by adding ~ uL of NaBH4 stock solution (100 mg/ml in 0.1
M
NaOH) per mg of protein, incubated for 1 hour at 37°C, treated with HC1
to discharge
excess NaBH,~, and then dialyzed extensively in cold PBS at 4°C for 36
hours. The AGE-
RSA was formed by reincubating the Amadori-RSA in the absence of sugar for 3
days.
The mixture was then dialyzed against cold PBS at 4°C for 24 hours. All
solutions were
filtered (22 um filter) sterilized and monitored for endotoxins by a limulus
amoebocyte
lysate assay (E-Toxate, Sigma Chemical) and contained <0.2 ng/ml before being
frozen
(-70°C) down into individual aliquots until it was time for injection.
~lninurl Studies
Male Sprague-Dawley rats (Sasco, 100g) were used. After a 1 week adaptation
period. rats were placed in metabolic cages to obtain a 24 hour urine specimen
for 2 days
before administration of injections. Rats were then divided into experimental
and control
groups and given tail vein injections with either saline, unmodified RSA (50
mg/kg),
Amadori-RSA (50 mg/kg), NaBH4-reduced Amadori-RSA (50 mg/kg), or AGE-RSA (50
mg/kg).
Rats injected with Amadori-RSA and AGE-RSA were then either left untreated,
or futher treated by the administration of either aminoguanidine (AG; 25
mg/kg),
pyridoxamine (PM; 25 mg/kg), or a combination of AG and PM (10 mg/kg each)
2o through the drinking water. Body weight and water intake of the rats were
monitored
weekly in order to adjust dosages. At the conclusion of the experimental study
the rats
were placed in metabolic cages to obtain 24 hour urine specimen for 2 days
prior to
sacrificing the animals.
Total protein in the urine samples was determined by Bio-Rad assay. Albumin in
urine was determined by competitive ELISA using rabbit anti-rat serum albumin
(Cappell) as primary antibody (1/2000) and goat anti-rabbit IgG (Sigma
Chemical) as a
secondary antibody (1/2000). Urine was tested with Multistix 8 SG (Miles
Laboratories)
for glucose, ketone, specific gravity, blook, pH, protein, nitrite, and
leukocytes. Nothing
remarkable was detected other than some protein.
3o Creatinine measurements were performed with a Beckman creatinine analyzer
II.
Blood samples were collected by heart puncture before termination and were
used in the
determination of creatinine clearance, blood glucose (glucose oxidase, Sigma
chemical),
46
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
fructosamine (nitroblue tetrazolium, Sigma chemical), and glycated Hb
(columns, Pierce
chemicals). Aorta, heart, both kidneys and the rat tail were visually
inspected and then
quickley removed after perfusing with saline through the right ventricle of
the heart. One
kidney, aorta, rat tail, and the lower 2/3 of the heart were snap-frozen and
then
permanently stored at -70°C. The other kidney was sectioned by removing
both ends
(cortex) to be snap-frozen, with the remaining portions of the kidney being
sectioned into
thirds with two portions being placed into neutral buffered formalin and the
remaining
third minced and placed in 2.5% glutaraldehyde/2% paraformaldehyde.
l0 Light Microscopy
After perfusion with saline, kidney sections were fixed in ice-cold 10%
neutral
buffered formalin. Paraffin-embedded tissue sections from all rat groups (n =
4 per
group) were processed for staining with Harris' alum hematoxylin and eosin
(H&E),
perodic acid/Schiff reagent (PAS), and alcian blue (pH 1.0 and pH 2.5) stains
for
histological examination. The alcian blue sections were scored by two
investigators in a
blinded fashion.
Electron Microscopy
Tissues were fixed in 2.5% glutaraldehyde/2% paraformaldehyde (0.1 M sodium
2o cacodylate, pH 7.4), post-fixed for 1 hour in buffered osmium tetroxide
(1.0%),
prestained in 0.5% uranyl acetate for 1 hour and embedded in Effapoxy resin.
Ultrathin
sections were examined by electron microscopy.
Immunoflzcorescence
Parrafin-embedded sections were deparaffinized and then blocked with 10% goat
serum in PBS for 30 min at room temperature. The sections were then incubated
for 2
hour at 37°C with primary antibody, either affinity purified polyclonal
rabbit anti-AGE
antibody, or a polyclonal sheep anti-rat serum albumin antibody (Cappell). The
sections
were then rinsed for 30 min with PBS and incubated with secondary antibody,
either
3o affinity purified FITC-goat anti-rabbit IgG (H+L) double stain grade
(Zymed) or a
Rhodamine-rabbit anti-sheep IgG (whole) (Cappell) for 1 hour at 37°C.
The sections
were then rinsed for 30 min with PBS in the dark, mounted in aqueous mounting
media
47
CA 02360311 2001-10-04
«'O 00/59493 PCT/US00/09241
for immunocytochemistry (Biomeda), and cover slipped. Sections were scored in
a
blinded fashion. Kidney sections were evaluated by the number and intensity of
glomerular staining in 5 regions around the periphery of the kidney. Scores
were
normalized for the immunofluorescent score per 100 glomeruli with a scoring
system of
0-3.
Preparation of Polyclonal Antibodies to AGE-Proteins
Immunogen was prepared by glycation of BSA (R479 antibodies) or Rnase
(R618 antibodies) at 1.6 g protein in 15 ml for 60 - 90 days using 1.5 M
glucose in 0.4
M phosphate containing 0.05% sodium azide at pH 7.4 and 37°C. New
Zealand white
rabbit males of 8-12 weeks were immunized by subcutaneous administration of a
1 ml
solution containing 1 mg/ml of glycated protein in Freund's adjuvant. The
primary
injection used the complete adjuvant and three boosters were made at three
week
intervals with Freund's incomplete adjuvant. The rabbits were bled three weeks
after the
last booster. The serum was collected by centrifugation of clotted whole
blood. The
antibodies are AGE-specific, being unreactive with either native proteins or
with
Amadori intermediates.
ELISA Detection of AGE Products
2o The general method of Engvall (21 ) was used to perform the ELISA. Glycated
protein samples were diluted to approximately 1.5 ug/ml in 0.1 M sodium
carbonate
buffer of pH 9.5 tQ 9.7. The protein was coated overnight at.room temperature
onto a 96-
well polystyrene plate by pippetting 200 u1 of protein solution into each well
(about .3
ug/well). After coating, the excess protein was washed from the wells with a
saline
solution containing 0.05% Tween-20. The wells were then blocked with 200 u1 of
1
casein in carbonate buffer for 2 hours at 37°C followed by washing.
Rabbit anti-AGE
antibodies were diluted at a titer of 1:350 in incubation buffer and incubated
for 1 hour at
37°C, followed by washing. In order to minimize background readings,
antibody 8618
used to detect glycated RSA was generated by immunization against glycated
Rnase. An
o alkaline phosphatase-conjugated antibody to rabbit IgG was then added as the
secondary
antibody at a titer of 1:2000 and incubated for 1 hour at 37°C,
followed by washing. The
p-nitrophenolate being monitored at 410 nm with a Dynatech MR4000 microplate
48
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
reader.
Results
The rats in this study survived the treatments and showed no outward signs of
any gross pathology. Some of the rats showed some small weight changes and
tail
scabbing.
Initial screening of kidney sections with PAS and H&E stains did not reveal
any
obvious changes, and some EM sections did not reveal any gross changes in the
glomerular basement membrane (GBM). However, upon Alcian Blue staining,
striking
to differences were discovered. Alcian blue staining is directed towards
negatively charged
groups in tissues and can be made selective via changes in the pH of staining.
At pH 1.0
Alcian blue is selective for mucopolysaccharides, and at pH ?.5 detects
glucoronic
groups. Thus negative charges are detected depending upon the pH of the stain.
At pH 2.5 Alcian blue staining showed that Amadori-RSA (p<0.05) and AGE-
15 RSA (p<0.01 ) induced increased staining for acidic glycosaminoglycans
(GAG) over
control levels (Figure 33). For both AGE-RSA and Amadori-RS A, treatment with
pyridoxamine (PM) prevented the increase in staining (p<0.05 as compared with
controls). In contrast, treatment with aminoguanidine (AG) or combined PM and
AG at
mg/kg each, did not prevent the increase.
2o At pH 1.0 Alcian blue staining was significantly decreased by AGE-RSA
(p<0.001 ) (Figure 34). However, no significant difference was seen with
Amadori-RSA.
Due to faint staining, treatment with PM, AG and combined could not be
quantitated.
Immunofluorescent glomerular staining for RSA showed elevated staining with
Amadori-RSA and AGE-RSA injected animals (Figure 35). Significant reduction of
this
25 effect was seen in the rats treated with PM, and not with AG or combined AG
& PM.
Immunofluorescent glomerular staining for Heparan Sulfate Proteoglycan Core
protein showed slightly reduced staining with Amadori-RSA and AGE-RSA injected
animals but were not statistically significant(Figure 3G). A reduction of this
effect was
seen in the rats treated with PM, and not with AG or combined AG & PM.
However,
o immunofluorescent glomerular staining for Heparan Sulfate Proteoglycan side-
chain
showed highly reduced staining with Amadori-RSA and AGE-RSA injected animals
(Figure 37) A significant reduction of this effect was seen in the rats
treated with PM,
49
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
and not with AG or combined AG & PM.
Analysis of average glomerular volume by blinded scoring showed that Amadori-
RSA and AGE-RSA caused significant increase in average glomeruli volume
(Figure
38). A significant reduction of this effect was seen with treatment of the
rats with PM.
No effect was seen with treatment with AG or combined AG and PM at 10 mg/kg
each.
Example 6
AGE Inhibitor Compounds
1 o The present invention encompasses compounds. and pharmaceutical
compositions containing compounds having the general formula:
R~
Rz
Y
R3
Formula I
wherein R, is CH~NH2, CHZSH, COOH, CH~CHZNHz, CHZCH~SH, or CHZCOOH;
R~ is OH, SH or NHZ;
Y is N or C, such that when Y is N R3 is nothing, and when Y is C, R~ is NOZ
or another
electron withdrawing group;
and salts thereof.
2o
The present invention also encompasses compounds of the general formula
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
R,
R6 Rz
R5 T R4
R3
Formula II
wherein R, is CHZNH~, CHzSH, COOH, CHzCH2NH~, CHzCHZSH, or CH~COOH;
to RZ is OH, SH or NHS;
Y is N or C, such that when Y is N R3 is nothing, and when Y is C, R~ is NOz
or another
electron withdrawing group;
R4 is H, or C 1-6 alkyl;
RS and R~ are H, C 1-6 alkyl, alkoxy or alkane;
15 and salts thereof.
51
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
In addition, the instant invention also envisions compounds of the formulas
CH~NH~
HOHZC OH
HO CH20H
CHzNH2
and
CH2NH2
HO OH
N C:HZNHz
By "alkyl" and "lower alkyl" in the present invention is meant straight or
1o branched chain alkyl groups having from 1-12 carbon atoms, such as, for
example,
methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl, tert-butyl, pentyl. 2-
pentyl, isopentyl.
neopentyl, hexyl, 2-hexyl, 3-hexyl, and 3-methylpentyl. Unless indicated
otherwisea the
alkyl group substituents herein are optionally substituted with at least one
group
independently selected from hydroxy, mono- or dialkyl amino, phenyl or
pyridyl.
1 s By "alkoxy" and "lower alkoxy" in the present invention is meant straight
or
branched chain alkoxy groups having 1-6 carbon atoms, such as, for example,
methoxy,
ethoxy, propoxy, isopropoxy, n-butoxy, sec-butoxy, tert-butoxy, pentoxv. 2-
pentyl,
isopentoxy, neopentoxy, hexoxy, 2-hexoxy, 3-hexoxy, and 3-methylpentoxy.
By "alkene" and "lower alkene" in the present invention is meant straight arid
?o branched chain alkene groups having 1-6 carbon atoms, such as, for example,
ethlene,
propylene, 1-butene, 1-pentene, 1-hexene, cis and traps 2-butene or 2-pentene,
isobutylene, 3-methyl-1-butene, 2-methyl-2-butene, and 2,3-dimethyl-2-butene.
52
The compounds of the present invention can embody one or more electron
withdrawing groups, such as and not limited to -NH2, -NHR, -NR?, -OH, -OCH3, -
OCR,
and -NH-COCH3 where R is C 1-6 alkyl.
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
By "salts thereof' in the present invention is meant compounds of the present
invention as salts and metal complexes with said compounds, such as with, and
not
limited to, AI,Zn, Mg, Cu, and Fe.
One of ordinary skill in the art will be able to make compounds of the present
invention using standard methods and techniques.
The instant invention encompasses pharmaceutical compositions which comprise
one or more of the compounds of the present invention, or salts thereof, in a
suitable
carrier. The instant invention encompasses methods for administering
pharmaceuticals of
the present invention for therapeutic intervention of pathologies which are
related to
AGE formation in vivo. In one preferred embodiment of the present invention
the AGE
related pathology to be treated is related to diabetic nephropathy.
Example 7. Improved Dialysis Solutions and Methods
15 It has also been demonstrated that formation of AGE products occurs in
dialysis
fluid in vitro (Lamb et al., Kidney Intl. 47:1768-1774 (1995)). Furthermore,
the level of
various AGE species is increased in blood of patients on either CAPD
(continuous
ambulatory peritoneal dialysis) (See, for example, Degenhardt et al., Kidney
Intl.
52:1064-1067 (1997); Shaw et al., Cellular and Molecular Biology 44:1061-1068
20 (1998)) or maintenance hemodialysis (HD) (Motomiya et al., Kidney Intl.
54:1357-1366
(1998)), regardless of whether the patient is hyperglycemic. (Miyata et al.,
Kidney Intl.
55:389-399 (1999))
CAPD involves the use of dialysis solutions containing high sugar
concentrations. while HD does not. Thus, the precipitating factor in AGE
formation in
z5 dialysis patients has been hypothesized to involve "carbonyl stress",
resulting either from
increased oxidation of carbohydrates and lipids ("oxidative stress"), or
inadequate
detoxification or inactivation of reactive carbonyl compounds derived from
both
carbohydrates and lipids by oxidative and non-oxidative chemistry. (Miyata et
al.,
Kidney Intl. 55:389-399 (1999))
30 Other studies indicate that nonenzymatic glycosylation of peritoneal
components
occurs during peritoneal dialysis. (See. for example. Friedlander et al., J.
Clin. Invest.
1996. 97:728-735; Nakayama et al., Kidney Intl. 51:182-186 (1997); and Korbet
et al.,
Am. J. Kidney Disease 22:588-591 (1993)
53
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
These various studies have implicated accumulation of AGEs in the following
pathologies in patients receiving dialysis:
(1998)
1. Increased cardiac morbidity and mortality (Korbet et al., 1993)
?. Dialysis-related amyloidosis (Motomiya et al., Kidney Intl. X4:1357-1366,
3. Increased permeability of the peritoneal membrane (Nakayama et al.,
1997)
4. Renal failure progression (Dawnay and Millar, Cell. Mol. Biol. 44:1081-
1094 ( 1998) (increased rate to end-stage renal disease)
~. Ultrafiltration failure and peritoneal membrane destruction (Linden et al.,
Perit. Dial. Int. 18:290-293 (1998)
Thus, in another aspect, the present invention provides improved dialysis
methods and compositions for dialysis that comprise utilizing an effective
amount of one
is or more of the compounds of the invention to inhibit AGE formation,
particularly due
to carbonyl stress, including the conversion of Amadori compounds to advanced
glycation endproducts and inadequate detoxification or inactivation of
reactive carbonyl
compounds.
In further aspects, the present invention provides methods for inhibiting
dialysis-
2o related cardiac morbidity and mortality, dialysis-related amyloidosis,
limiting dialysis-
related increases in permeability of the peritoneal membrane in a dialysis
patient,
inhibiting renal failure progression in a patient, and inhibiting
ultrafiltration failure and
peritoneal membrane destruction in a patient, comprising introducing into the
patient a
dialysis solution that comprises an amount of one or more of the compounds of
the
25 invention sufficient to inhibit or limit the specified endpoint.
In another aspect, the present invention comprises a method for inhibiting AGE
formation in a dialysis patient comprising administering to the patient a
dialysis solution
comprising an effective amount of a compound of the invention to inhibit AGE
formation.
3o As used herein, dialysis solutions comprise solutions for both peritoneal
dialysis
(PD) and hemodialysis (HD). As used herein, PD differs from HD in that the
patient's
peritoneum, not an artificial kidney, forms the dialyzing membrane.
As used herein, the term "osmotically active agent" refers to a substance
present
in the dialysis solution which is capable of maintaining the osmotic gradient
required to
35 cause transport of water and toxic substances across the peritoneum into
the dialysis
solution.
54
CA 02360311 2002-05-22
76909-200(S)
The normal function of the mammalian kidney includes such activity as
maintaining a constant acid-base and electrolyte balance, removing excess
fluids and
removing undesirable products of the body's metabolism from the blood (U.S.
Patent No.
5,869,444. In an individual with end
stage renal disease, this functioning of the kidney may be reduced to as low
as 5% or less
of the normal level. When renal function has decreased to this point, dialysis
is used in
an attempt to replace kidney activity. This is accomplished clinically by the
use of
dialysis. One of the most common dialysis methods is hemodialysis ("HD"), in
which the
patient's blood is passed through an artificial kidney dialysis machine,
wherein a
~ o synthetic non-permeable membrane acts as an artificial kidney with which
the patient's
blood is contacted on one side. On the opposite side of the membrane is a
dialyzing fluid
or dialvsate, the composition of which is such that the undesirable products
in the
patient's blood will naturally pass across the membrane by diffusion, into the
fluid. The
blood is thus cleansed, in essentially the same manner as the kidney would
have done,
and the blood is returned to the patient's body. Examples of 1~D solutions can
be found in
U.S. Patent Nos. 5,474,992; and 5,211,643.
The dialysis solutions for HD are manufactured in the form of a suitable
solution by standard procedures. The osmotic pressure and pH of the liquid
preparation
are preferably adjusted within the respective ranges far HD solutions in
general. The HD
2o may contain a' variety of other ingredients which are generally included in
dialysis
solutions for extracorporeai hernodialysis, for example various salts such as
sodium
'chloride, potassium chloride, calcium chloride, magnesium chloride, sodium
acetate, and
sodium hydrogen carbonate.
Altennatively, the patient's own peritoneum can be used as the required
semipermeable membrane. The peritoneum is the membranous lining of the body
cavity
that contains large numbers of blood vessels and capillaries,. thus allowing
its function as
a natural semipermeable membrane. (U.S. Patent No. 5,869,444) Dialysis
solution is
introduced into, the peritoneal cavity, via a catheter in the abdominal wall.
A suitable
period of residence time for the diaIysate is allowed to permit the exchange
of solutes
3o between it and the blood. Fluid removal is achieved by providing a suitable
osmotic
gradient, via inclusion of an osmoticaliy active agent in the dialysate. from
the blood to
the dialysate to permit water outflow from the blood. Thus, the proper acid-
base,
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
electrolyte and fluid balance is returned to the blood and the dialysis
solution is simply
drained from the body cavity through the catheter. Although more than one type
of
peritoneal dialysis exists, the technique known as continuous ambulatory
peritoneal
dialysis (CAPD) is particularly favored, since it does not require the patient
to remain
tied to machinery while the solute and fluid exchange is accomplished. The
only
sedentary period required is during infusion and draining of the dialysis
solution.
The osmotically active agent which has currently achieved the most widespread
acceptance is glucose. Glucose has the advantage of being non-toxic, and is so
readily
metabolizable if it enters the blood. However, glucose is readily taken up
into the blood
1o from the dialysate, which may lead to various complications. (U.S. Patent
No.
5,869,444) Among these complications is the build-up of advanced glycation end
products discussed above.
Therefore, in one aspect the present invention provides improved dialysis
solutions comprising an amount effective to inhibit AGE formation in a patient
who is to
receive the dialysis solution of one or more compounds or pharmaceutical
compositions
comprising a compound of the general formula:
R,
Rs Rz
t(5 Y K4
R3
wherein R, is CH~NH~, CH~SH, COOH, CHZCH?NH~, CHzCHzSH, or CHZCOOH;
RZ and R~, is H, OH, SH, NHZ, C 1-6 alkyl, alkoxy or alkene;
2o R4 and R; are H, C 1-6 alkyl, alkoxy or alkene;
Y is N or C, such that when Y is N R3 is nothing, and when Y is C, R~ is NO~
or another
electron withdrawing group, and salts thereof.
According to this aspect of the invention, the compounds) is used as an
additive
to any type of dialysis solution in which inhibiting AGE formation is
desirable, including
but not limited to hemodialysis solutions and peritoneal dialysis solutions.
In one embodiment, the dialysis solutions comprise:
56
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
a. an osmotically active agent that is capable of maintaining the osmotic
gradient required to cause transport of water and toxic substances across the
peritoneum
into the dialysis solution; and
b. an amount of the compounds of the invention effective to inhibit the
conversion of Amadori compounds to post Amadori advanced glycation endproducts
in a
patient who is to receive the solution.
In a preferred embodiment. the osmotically active agent is selected from the
group consisting of ribose, lyxose, xylose, arabinose, glucose, fructose,
maltose, lactose,
mannose, fructose, and galactose, or polymers thereof, and polyanions. (For
examples of
to polymers, see Barre et al., Adv. Perit. Dial. 15:12-16 (1999); Wang et al.,
Perit. Dial. Int.
18:193-203 (1998); Plum et al., Am. J. Kidney Dis. 30:413=422 (1997); Ho-dac-
Pannekeet et al., Kidney Intl. 50:979-986 ( 1996); Chen et al., Adv. Perit.
Dial. 14:116-
119 (1998); Dawnay et al., Perit. Dial. Int. 17:52-58 (1997); Twardowski et
al., Artif.
Organs 7:420-427 (1983))
15 In a further preferred embodiment, the dialysis solution further comprises
sodium
m a concentration that is less than a sodium plasma concentration in a renal
patient who
is to receive the solution. In another preferred embodiment, the osmotic agent
is glucose.
In a most preferred embodiment, the compound comprises pyridoxamine.
In a further aspect, the present invention comprises an improved method of
2o performing dialysis on a patient wherein the improvement comprises
introduction into
the patient in need of dialysis a dialysis solution that comprises an amount
effective to
inhibit AGE formation in the patient of one or more compounds or
pharmaceutical
compositions comprising a compound of the general formula:
R,
Rs R2
K5 Y R4
R3
?5 wherein R, is CH~NH~, CH~SH, COOH, CHzCH~NHz, CH~CHzSH, or CHZCOOH;
Rz and R~, is H, OH, SH, NHS, C 1-6 alkyl, alkoxy or alkene;
57
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
R4 and R; are H, C 1-6 alkyl, alkoxy or alkene;
Y is N or C, such that when Y is N R3 is nothing, and when Y is C, R, is NO?
or another
electron withdrawing group, and salts thereof.
In a preferred embodiment, the compound comprises pyridoxamine. The dialysis
solutions for use in this aspect of the invention are as described above.
In other aspects, the present invention provides methods for inhibiting
dialysis-
related cardiac morbidity and mortality, dialysis-related amyloidosis.
limiting dialysis-
related increases in permeability of the peritoneal membrane in a patient,
inhibiting renal
to failure progression in a patient, and inhibiting ultrafiltration failure
and peritoneal
membrane destruction in a patient, comprising introducing into the patient a
dialysis
solution that comprises an amount of one or more of the compounds of the
invention
sufficient to inhibit or limit the specified endpoint. In another aspect, the
invention
comprises a method for inhibiting AGE formation in a dialysis patient
comprising
15 administering to a patient undergoing dialysis an effective amount of one
or more of the
compounds of the invention to inhibit AGE formation. In a preferred embodiment
of
each of these methods, the compound is pyridoxamine.
The concentration of the compounds of the invention in the dialysis solutions
is
based on a variety of factors, including the composition of the dialysis
solution,
20 treatment of the dialysis solution (i.e.: sterilization, etc.), type of
dialysis (CAPD vs.
HD), type of condition, compound used, age, weight, sex, medical condition of
the
individual. and the severity of the condition. Thus, the concentration may
vary widely,
but can be determined routinely by a physician using standard methods.
Concentration
levels of the order of between 1 pM to 100 mM are useful for all methods of
use
25 disclosed herein.
Examples
Example I. Inhibition of AGE formation in peritoneal dialysis fluid
Albumin was added to DIANEAL~ peritoneal dialysis (PD) (Baxter Corp.
3o Deerfield, IL) fluid after adjustment of the PD fluid pH to 7.5. The
DIANEAL~ PD
fluid used in this experiment was composed of:
sodium = 132 mEq/ 1,
calcium = 2.5 mEq/ 1,
magnesium = 0.5 mEq/1,
58
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
chloride = 95 mEqi l,
lactate = 40 mmol/1,
4.25% dextrose
with an osmolarity = 483 mOsmol/1.
PD fluid containing glucose as an osmotic agent is generally prepared at a pH
between 5.0 and 5.5 to prevent carmelization of glucose upon heat
sterilization of the PD
fluid. (U.S. Patent No. 5,869,444). At this non-physiological pH, AGES do not
form.
.As the PD fluid enters the body, its pH changes very quickly to physiological
pH.
The PD fluid-albumin samples were then incubated at 37°C for 52 days
in the
presence and absence of pvridoxamine ( 1 mM, 3 mM, and 15 mM). In addition, 1M
glucose was added to each of the samples treated with pyridoxamine as well as
to one
control sample. Glucose addition was utilized to accelerate the process of AGE
formation. Antibodies specific for AGES (carboxymethyl-lysine) were used to
conduct
ELISA to determine the amount of albumin AGES in each sample. Figure 39
t 5 demonstrates that pyridoxamine significantly inhibits formation of protein
(albumin)
AGES in PD fluid under these conditions.
Example ~. Inhibition of AGE formation from patient post-dialysis peritoneal
dialysis
fluid
z0 Post-dialysis fluid was collected from a non-diabetic peritoneal dialysis
patient at
the University of Kansas Medical Center, and had a pH of 7.5. Prior to
dialysis, the PD
components were as described above, except that the PD solution contained 2.5%
dextrose, and no glucose was added. However, the composition of a PD solution
is
altered by exchange with the peritoneum. Thus, the exact composition of the PD
25 solution is difficult to determine.
Myoglobin was incubated with post-dialysis PD fluid for 12 hours at
60°C in the
presence and absence of pyridoxamine. (3 mM, 0.5 mM, 0.1 mM, and 0.02 mM) This
experiment was conducted at 60°C to accelerate AGE formation, which is
temperature
dependent. Antibodies were then used to conduct ELISAs as described above. The
30 results of these experiments (Figure 40) demonstrate that pyridoxamine
inhibits the
formation of myoglobin AGES in post-dialysis PD fluid under these conditions.
In a similar experiment, post-dialysis fluid was collected from a diabetic
peritoneal dialysis patient at the University of Kansas Medical Center, and
had a pH of
59
CA 02360311 2001-10-04
WO 00/59493 PCT/US00/09241
7.5. Prior to dialysis, the PD components were as described above, except that
the PD
solution contained 4.25% dextrose, and no glucose was added.
Metmyoglobin was incubated with the PD fluid at 37°C for various
periods of
time, up to 42 days. The experiments were done in the presence and absence of
3 mM
pyridoxamine). The data (Figure 41) demonstrate that pyridoxamine inhibits the
formation of protein (metmyoglobin) AGES in post-dialysis PD fluid under these
conditions.
The instant invention may be embodied in other forms or carried out in other
ways without departing from the spirit or essential characteristics thereof.
The present
1 o disclosure and enumerated examples are therefore to be considered as in
all respects
illustrative and not restrictive, the scope of the invention being indicated
by the appended
claims, and all equivalency are intended to be embraced therein. One of
ordinary skill in
the art would be able to recognize equivalent embodiments of the instant
invention, and
be able to practice such embodiments using the teaching of the instant
disclosure and
only routine experimentation.