Note: Descriptions are shown in the official language in which they were submitted.
CA 02362840 2001-08-30
WO 00/52053 PCT/US00/05279
ANTIBODIES AGAINST PHOSPHOMANNAN
THAT ARE PROTECTIVE AGAINST CANDIDIASIS
FIELD OF THE INVENTION
The present invention relates to antibodies of the IgG class that protect a
host
against candidiasis, particularly to antibodies that specifically bind to a
carbohydrate
antigen of the cell wall of a yeast from the Candida genus. The invention
further relates
to pharmaceutical compositions and therapeutic methods useful in the treatment
of
candidiasis and diagnostic methods useful in diagnosing candidiasis and
monitoring the
course of treatment of candidiasis.
ACKNOWLEDGMENT OF FEDERAL SUPPORT
The disclosed invention was supported by the National Institute of Allergy and
Infectious Diseases Grants RO1 AI24912 and PO1 AI37194. The United States
government has certain rights in the invention.
BACKGROUND OF THE INVENTION
The yeast genera, Candida, can cause a variety of clinical syndromes that are
generically termed candidiasis and are usually categorized by the
physiological site of
involvement. The two most common syndromes are mucocutaneous candidiasis
(e.g., stomatitis or thrush, esophagitis and vaginitis) and invasive or deep
organ
candidiasis (e.g., fungemia, endocarditis, and endophthalmitis). These
syndromes are
discussed in Dismukes, Candidiasis, IN CECIL'S TEXTBOOK OF MEDICINE 1827-1830
(Bennett et al. eds., 1996).
Patients suffering from mucocutaneous infections may be treated with any one
of
several topical preparations including nystatin, clotrimazole, econazole,
ketoconazole,
butoconazole, terconazole, and miconazole. Id. For the treatment of more
clinically
serious Candida related disease (e.g., candidemia or disseminated candidiasis)
CA 02362840 2001-08-30
WO 00/52053 PCT/US00/05279
-2-
amphotericin B formulations, both the deoxycholate version and the newer
liposomal
product, are administered only intravenously. Fluconazole is oral. Therapy for
Candida
peritonitis involves either intravenous amphotericin B or oral gluconazole.
Id.
The medical literature also reported that various classes of antibodies (IgA,
IgG
and IgM) directed against several C. albicans antigens were of experimental
interest and
of diagnostic or therapeutic value. See, e.g., Torres-Rodriguez et al., 1997
Mycoses
40:439-44 with respect to C. albicans germ tube; and Reboli, 1993 J. Clin.
Microbiol. 31:
518-23 with respect to a dot immunobinding assay involving total Candida
protein.
Monoclonal antibodies specific to an iC3b receptor, which is an integrin that
has
antigenic and structural homology with a Candida surface antigen, were
demonstrated to
increase survival of mice with disseminated candidiasis. Lee et al., 1997
Immunology
92: 104-110. Similarly, antibodies to mannoprotein (MP) and aspartyl
proteinase (Sap)
have been shown to protect against vaginitis in rats. De Bernardis et al.,
1997 Infect.
Immun. 65: 3399-3405.
U.S. Patents No. 4,670,382 and 4,806,465 to Buckley et al. (1989) describe IgG
monoclonal antibodies against a set of closely related cytoplasmic antigens of
C.
albicans, but present no therapeutic data showing efficacy against Candida
infection.
U.S. Patent No. 5,288,639 to Burnie et al. (1994) describes monoclonal
antibodies
against stress or heat shock proteins of Candida, which were shown to produce
33
survival at 24 hours in animals challenged with a lethal dose of C. albicans.
Also, U.S.
Patent No. 5,641,760 to Yu et al. (1997) discloses monoclonal antibodies
against C.
albicans fimbrial subunits that are said to be useful for treating C. albicans
infections.
However, although this patent identifies antibodies as members of the IgG2
isotype, no in
vivo data showing protection against Candida infection were provided.
Certain immunogenic phosphomannan preparations of C. albicans, which is
known to contain adhesins, have been used to prepare vaccines for the
treatment of, and
elicit antibodies against, disseminated candidiasis due to infection by C.
albicans. For
example, European Patent No. 344,320 to Kawamura et al. (1989) describes human
monoclonal antibodies of IgG and IgM classes that were raised against mannan
extracted
from Candida. Although antibodies of the IgG class are said to be preferred
and
CA 02362840 2001-08-30
WO 00/52053 PCT/US00/05279
-3-
agglutinating activity is discussed, the skilled artisan will understand that
agglutination is
distinct from protective effect and no therapeutic data against Candida
infection were
provided by Kawamura et al..
Therapeutic efficacy was shown in U.S. Patent No. 5,578,309 to Cutler et al.
(1996) described the immunization of mice with liposome-encapsulated Candida
phosphomannoprotein and obtained several monoclonal antibodies specific for
that
fraction. In addition, mice were immunized with a liposome encapsulated mannan
adhesin extracted from the cell wall ("L-adhesin" or "L-mannan" or "L-mann"),
and two
IgM class monoclonal antibodies specific for yeast surface epitopes were
described in
Cutler et al. Although both antibodies (B6.1 and B6) were strong agglutinins,
only one
(B6.1) was shown to protect naive mice against disseminated candidiasis. Each
antibody
recognizes a distinct G albicans mannan cell wall determinant, and the MAb
B6.1
recognized a carbohydrate antigen. See, also, Han et al., 1997 Infect. Immun.
65: 4100-
07. The B6.1 antibody also enhanced ingestion and killing of yeast cells by
polyrnorphonuclear leukocytes (PMNs) in the presence of serum complement.
Caesar-
TonThat et al., 1997 Infect. Immun. 65: 5354-57.
Thus, unlike the disclosure of the B6.1 antibody, which is an IgM class
antibody,
the other literature citations discussed above apparently did not demonstrate
protection
against candidiasis by antibodies of the IgG class that were specific for the
phosphomannan antigen of the Candida cell wall. Notwithstanding the work by
Cutler et
al., it was surprising that a protective IgG class antibody has been found by
the present
inventors that is specific for a carbohydrate antigen.
With respect to antibody class, class switching does not guarantee similar
levels
of effectiveness. Thus, the reported effectiveness of IgM antibodies such as
the B6.1
antibody does not mean that antibodies of a different class even when directed
against the
same antigen will be similarly effective in a clinical setting. For example,
in Group B
streptococcal disease, an IgM specific for a cell wall carbohydrate was much
more
effective than an IgG2a of the same specificity as the IgM. Hill et al., 1992
Clin.
Immunol. Immunopathol. 62: 87-91. Similarly, isotype switching can convert
protective
antibodies to nonprotective antibodies. For example, with respect to
Cryptococcus
CA 02362840 2001-08-30
WO 00/52053 PCT/US00/05279
-4-
neoformans, isotype switching from IgG3 to IgGl, while maintaining identical
epitope
specificity, converted a nonprotective antibody to a protective one in
experimental
cryptococcosis. Yuan et al., 1995 J. Immunol. 154: 1810-1816; and Yuan et al.,
1998
Infect. Immun. 66: 1057-1062.
As is known in the art, antibodies of the IgM class can fix complement and
directly activate macrophages. Because IgG class antibodies can activate
complement as
well as bind to macrophages and neutrophils, they are able to enhance immunity
by
antibody mediated and cellular responses. IgG antibodies also may have higher
binding
affinities than do IgM antibodies. The immune responses mediated by IgM may
have
quite different outcomes in terms of how a mammalian host infected with
Candida is able
to recognize and respond to this pathogen, because both antibody and cellular
responses
are known to be important in responding to Candida infection. Thus, the use of
both IgG
and IgM antibodies, administered together or perhaps sequentially, may help to
boost an
infected mammalian host's immune response. Although the B6.1 IgM antibody is
known
to recognize a phosphomannan adhesin and is protective, no monoclonal IgG
antibodies,
until the invention described herein, had been described in the literature
that recognized a
Candida adhesin and were therapeutically effective in inhibiting or preventing
candidiasis.
SUMMARY OF THE INVENTION
The present invention relates to antibodies that protect a host against
candidiasis,
particularly disseminated candidiasis, mucocutaneous candidiasis (e.g.,
stomatitis or
thrush, esophagitis and vaginitis or vaginal candidiasis) and invasive or deep
organ
candidiasis (e.g., fungemia, endocarditis, and endophthalmitis). More
particularly, the
invention relates to antibodies of the IgG class and IgG3 isotype. The
invention also
relates to therapeutic methods useful in the treatment of candidiasis and
diagnostic
methods useful in diagnosing candidiasis and monitoring the course of
treatment of
candidiasis.
It is an object of the present invention to provide a purified or isolated IgG
antibody that specifically binds to a carbohydrate antigen of the cell wall of
a yeast from
CA 02362840 2001-08-30
WO-00/52053 PCT/US00/05279
-5-
the Candida genus, where the antibody is protective against infection of a
mammalian
host by the yeast. More particularly, it is an object of the invention to
provide an
antibody that specifically binds to an acid-labile component of the
phosphomannan
complex, particularly a ~i-1,2-linked oligomannosyl or a (3-1,2-mannotriose
residue, of
the.cell wall of the yeast.
The foregoing antibodies are protective against a several types of
candidiasis,
including disseminated candidiasis and mucocutaneous candidiasis. The antibody
may
be of any IgG isotype, including IgGl, IgG2 and IgG3 and their various sub-
isotypes.
Also contemplated are human antibodies, chimeric antibodies or humanized
antibodies.
It is a further object of the invention to provide pharmaceutical compositions
comprising the foregoing antibodies, formulated with pharmaceutically
acceptable
carriers and excipients as appropriate. Contemplated yeast infections
treatable by such
pharmaceutical compositions include C. albicans, C. glabrata and C. tropicalis
and
strains thereof. Topical, systemic and aerosol formulations are expressly
contemplated,
as are formulations in unit dose form and in formulations containing one or
more other
anti-fungal, antibody or other therapeutic agents. In a preferred formulation,
both IgG
and IgM antibodies are administered against the same yeast, either at about
the same time
or at different times.
Another object of the invention is to utilize the foregoing pharmaceutical
compositions in methods to treat disseminated candidiasis and mucocutaneous
candidiasis. Diagnostic kits comprising the antibodies described above,
together with a
reagent for detecting binding of the antibody to a carbohydrate antigen of the
cell wall of
a yeast from the Candida genus also are contemplated, as are hybridoma cells
that
express these antibodies.
BRIEF DESCRIPTION OF THE DRAWING FIGURES
Figs. lA and 1B show that passive transfer of MAb C3.1 protects against
disseminated candidiasis in BALB/c mice.
Figs. 2A and 2B show that MAb C3.1 has a prophylactic effect against Candida
vaginal infection in pseudoestrus mice.
CA 02362840 2001-08-30
WO-00/52053 PCT/US00/05279
-6-
DETAILED DESCRIPTION OF THE INVENTION
1. De anitions
The term "antibody" as used herein, unless indicated otherwise, is used
broadly to
refer to both antibody molecules and a variety of antibody-derived molecules.
Such
antibody-derived molecules comprise at least one variable region (either a
heavy chain or
light chain variable region) and include molecules such as Fab fragments, Fab'
fragments,
F(ab')Z fragments, Fv fragments, Fabc fragments, single chain Fv (scFv)
antibodies,
individual antibody light chains, individual antibody heavy chains, chimeric
fusions
between antibody chains and other molecules, and the like.
Antibodies of the invention may be isolated from a hybridoma cell, the serum
of a
vertebrate, recombinant eukaryotic or prokaryotic cells transfected with a
nucleic acid
encoding the antibody, which may include plant cells, ascites fluid, or the
milk of
transgenic animals.
The term "antigen" means a molecule that is specifically recognized and bound
by
an antibody. The specific portion of the antigen that is bound by the antibody
is termed
the "epitope".
The term "humanized antibody" refers to an antibody which is substantially
human in structure; that is, it derives at least substantially all of its
constant regions from
a human antibody even though all or a part of its variable regions are derived
from some
other species. "Human antibody" refers to an antibody which is encoded by a
nucleotide
of human origin and such nucleotides may be modified by the skilled artisan by
known
nucleotide manipulation techniques.
Antibodies described herein also may contain alterations of the amino acid
sequence compared to a naturally occurnng antibody. In other words, the
antibodies of
the invention need not necessarily consist of the precise amino acid sequence
of their
native variable region or constant region framework, but contain various
substitutions
that improve the binding properties of the antibody to its cognate antigen or
change the
binding of the antibody to effector molecules such as complement or the Fc
receptor. In
another format, a minimal number of substitutions are made to the framework
region in
order to ensure reduced, and preferably, minimal immunogenicity of the
antibody in
CA 02362840 2001-08-30
WO 00/52053 PCT/US00/05279
humans. In preferred embodiments of recombinant antibodies of the invention,
any non-
human framework regions used may be altered with a minimal number of
substitutions to
the framework region in order to avoid large-scale introductions of non-human
framework residues.
The term "conventional molecular biology methods" refers to techniques for
manipulating polynucleotides that are well known to the person of ordinary
skill in the art
of molecular biology. Examples of such well known techniques can be found in
MOLECULAR CLONING: A LABORATORY MANUAL 2ND EDITION, Sambrook et al., Cold
Spring Harbor, N.Y. (1989) and in CURRENT PROTOCOLS IN MOLECULAR BIOLOGY,
Ausebel et al., 4 vols. John Wiley & Sons, NY. Examples of conventional
molecular
biology techniques include, but are not limited to, in vitro ligation,
restriction
endonuclease digestion, polymerase chain reaction (PCR), cellular
transformation,
hybridization, electrophoresis, DNA sequencing, cell culture, and the like.
The term "isolated" or "substantially pure" as used herein refers to an
antibody or,
for example, a fragment thereof, which is substantially free of other
antibodies, proteins,
lipids, carbohydrates or other materials with which it is naturally
associated. One skilled
in the art would be able to isolate or to substantially purify MAb C3.1
antibodies using
conventional methods for antibody or protein purification
The terms "protective" or "therapeutically effective" generally mean that the
antibody is effective to block attachment of a yeast cell to its target tissue
or cells in a
host, or to decrease or prevent the increase in fungal cell levels in the
bloodstream or at
an organ site or other site of infection. More specifically, the phrase
"protective" or
"therapeutically effective" means that the antibodies or pharmaceutical
compositions
according to the present invention are able to opsonize Candida pathogens to
facilitate
macrophage, monocyte or neutrophil phagocytosis and killing, or can activate
the
macrophages that can amplify the cellular and immune responses. Preferably,
the
treatment methods of the present invention are effective to kill at least
about 20 %, more
preferably 40%, even more preferably 60% and most preferably 90% or more of
the
Candida organisms in an infected mammalian host in a therapeutic course of
treatment.
CA 02362840 2001-08-30
WO-00/52053 PCT/US00/05279
_g_
The terms "variable region" and "constant region" as used herein in reference
to
antibody and immunoglobulin molecules have the ordinary meaning given to the
term by
a person of ordinary skill in the art of immunology. Both antibody heavy
chains and
antibody light chains may be divided into a "variable region" and a "constant
region."
The point of division between a variable region and a contrast region may be
determined
by the person of ordinary skill in the art by reference to standard texts
describing
antibody structure. See, e.g., Kabat et al., "Sequences of Proteins of
Immunological
Interest: 5th Edition" U.S. Department of Health and Human Services, U.S.
Government
Printing Office (1991).
2. Candida Related Conditions
Among the more than 150 recognized species of Candida, C. albicans is the most
commonly identified pathogen in humans. Other clinically important species
include C.
guilliermondi, C. krusei, C. parapsilosis, C. pseudotropicalis and G
tropicalis.
Mucocutaneous infections include thrush or oropharyngeal candidiasis,
cheilosis,
esophagitis, gastrointestinal candidiasis, intertrigo, paronychia,
vulvovaginitis, balanitis,
Candida cystitis, and chronic mucocutaneous candidiasis. Numerous diagnostic
categories exist for serious or deep Candida infection including candidemia,
disseminated candidiasis, systemic candidiasis, invasive candidiasis, visceral
candidiasis
and terms indicating involvement of specific organs such as hepatosplenic
candidiasis
and ocular candidiasis. See, e.g., Dismukes, 1996. Serious or deep Candida
infections
are frequently observed in immunodeficient or immune compromised patients,
such as in
patients with Acquired Immundeficiency Syndrome (AIDS).
3. Preparation o~vbridomas that Produce Monoclonal Antibodies Against
Candida antigens.
To produce antibodies, various species of host animals may be immunized by
injection with the L-mane antigen or with appropriately prepared Candida
extracts or
whole cells. Appropriate animals for this purpose include, but are not limited
to rabbits,
mice, and rats, etc. Various adjuvants may be used to increase the
immunological
CA 02362840 2001-08-30
WO 00/52053 PCT/US00/05279
-9-
response, depending on the host species, including but not limited to Freund's
(complete
and incomplete), mineral gels such as aluminum hydroxide, surface active
substances
such as lysolecithin, pluronic polyols, polyanions, peptides, oil emulsions,
keyhole limpet
hemocyanin, dinitrophenol, and potentially useful human adjuvants such as BCG
(bacilli
Calmette-Guerin) and Corynebacterium parvum.
Monoclonal antibodies to Candida antigens may be prepared by using any
technique which provides for the production of antibody molecules by
continuous cell
lines in culture. These include but are not limited to the hybridoma technique
originally
described by Kohler and Milstein (Nature, 1975, 256:495-497), the human B-cell
hybridoma technique (Kosbor et al., 1983, Immunology Today, 4:72; Cote et al.,
1983,
Proc. Natl. Acad. Sci., 80:2026-2030) and the EBV- hybridoma technique (Cole
et al.,
1985, Monoclonal Antibodies and Cancer Therapy, Alan R. Liss, Inc., pp. 77-
96). In
addition, techniques developed for the production of "chimeric antibodies"
(Morrison et
al., 1984, Proc. Natl. Acad. Sci., 81:6851-6855; Neuberger et al., 1984,
Nature,
312:604-608; Takeda et al., 1985, Nature, 314:452-454) by splicing the genes
from a
mouse antibody molecule of appropriate antigen specificity together with genes
from a
human antibody molecule of appropriate biological activity can be used.
Alternatively,
techniques described for the production of single chain antibodies (U.S. Pat.
No.
4,946,778) can be adapted to produce Candida specific single chain antibodies.
4. Isolation o~'Candida antigen specific B cells.
Antigen specific B cells may be isolated from convenient samples, such as
peripheral blood lymphocytes from a human patient infected with Candida, by
techniques known and available in the art. For instance, fusion proteins of
the invention
may be used to detect and isolate B cells which express immunoglobulin which
specifically binds to the phosphomannan antigen by affinity chromatography,
fluorescent
activated cell sorting (FACS) and other commonly used techniques such as Zn-
chelating
sepharose or protein-A sepharose (see Harlow et al., ANTIBODIES: A LABORATORY
MANUAL, Cold Spring Harbor Laboratory, 1988).
CA 02362840 2001-08-30
WO-00/52053 PCT/US00/05279
-10-
As another example, lymph nodes obtained from a candidiasis patient may be cut
into fine pieces and meshed through a wire gauze using a rubber policeman.
Pure B cells
may be isolated using CD19 coated immunomagnetic beads. Antigen specific B
cells
may be isolated using the appropriate fusion protein by affinity
chromatography or
fluorescent activated cell sorting. The Candida antigen specific B cells may
then be
immortalized using known techniques such as immortalization by EBV. Any
effective
lymphotropic virus or other transforming agent able to transform the B-cells
to grow in
continuous culture and still produce monoclonal antibodies specific for the
Candida
associated antigens can be used.
5. Isolation o antigen specific immunoglobulin heavy and light chain
sequences.
In addition to providing Candida phosphomannan (and ~3-1,2-mannotriose)
specific antibodies, the subject invention provides for polynucleotides
encoding Candida
specific antibodies. The polynucleotides may have a wide variety of sequences
because
of the degeneracy of the genetic code. A person of ordinary skill in the art
may readily
change a given polynucleotide sequence encoding a Candida specific antibody
according
to the present invention into a different polynucleotide encoding the same
antibody. For
example, the polynucleotide sequence encoding the antibody may be varied to
take into
account factors affecting expression such as codon frequency, RNA secondary
structure,
and the like.
6. Production o~recombinant human antibodies
The antibodies of the subject invention may be produced by a variety of
methods
useful for the production of polypeptides, e.g., in vitro synthesis,
recombinant DNA
production, and the like. Preferably, humanized antibodies are produced by
recombinant
DNA technology. The antigen specific antibodies of the invention may be
produced
using recombinant immunoglobulin expression technology. The recombinant
production
of immunoglobulin molecules, including humanized antibodies is described in
U.S.
Patent No. 4,816,397 (Boss et al.), U.S. Patent No. 4,816,567 (Cabilly et
al.), U.K. patent
GB 2,188,638 (Winter et al.), and U.K. patent GB 2,209,757 (Winter et al.).
Techniques
CA 02362840 2001-08-30
WO-00/52053 PCT/US00/05279
-11-
for the recombinant expression of immunoglobulins, including humanized
immunoglobulins, can also be found, among in Goeddel et al., "Gene Expression
Technology Methods" IN ENZYMOLOGY Vol. 185 Academic Press (1991), and
Borreback,
ANTIBODY ENGINEERING, W. H. Freeman ( 1992). Additional information concerning
the
generation, design and expression of recombinant antibodies can be found in
Mayforth,
DESIGNING ANTIBODIES, Academic Press, San Diego (1993).
As an example, the recombinant antibodies of the present invention may be
produced by the following process:
a) constructing, by conventional molecular biology methods, an expression
vector comprising a nucleotide sequence that encodes an antibody heavy chain
in which
the CDRs and a minimal portion of the variable region framework that are
required to
retain donor antibody binding specificity are derived from the human
immunoglobulin,
and the remainder of the antibody is derived from another human
immunoglobulin,
thereby producing a vector for the expression of a humanized antibody heavy
chain;
b) constructing, by conventional molecular biology methods, an expression
vector
comprising a nucleotide sequence that encodes an antibody light chain in which
the
CDRs and a minimal portion of the variable region framework that are required
to retain
donor antibody binding specificity are derived from the human immunoglobulin,
and the
remainder of the antibody is derived from another human immunoglobulin,
thereby
producing a vector for the expression of humanized antibody light chain;
c) transferring the expression vectors to a host cell by conventional
molecular
biology methods to produce a transfected host cell; and
d) culturing the transfected cell by conventional cell culture techniques so
as to
produce recombinant antibodies.
Host cells may be cotransfected with two expression vectors of the invention,
the
first vector encoding a heavy chain derived polypeptide and the second
encoding a light
chain derived polypeptide. The two vectors may contain different selectable
markers but,
with the exception of the heavy and light chain coding sequences, are
preferably
identical. This procedure provides for equal expression of heavy and light
chain
polypeptides. Alternatively, a single vector may be used which encodes both
heavy and
CA 02362840 2001-08-30
WO-00/52053 PCT/US00/05279
-12-
light chain polypeptides. The coding sequences for the heavy and light chains
may
comprise cDNA or genomic DNA or both.
The host cell used to express the recombinant antibody of the invention may be
a
bacterial cell such as Escherichia coli, or antigen binding fragments may be
expressed in
S available phage display systems (see Winter et al. (1994) Ann. Rev. Immunol.
12: 433
455 and Little et al. (1995) J. Biotechnol. 41(2-3): 187-195). Preferably a
eukaryotic cell
or most preferably a mammalian cell, such as a Chinese hamster ovary cell, may
be used.
The choice of expression vector is dependent upon the choice of host cell, and
may be
selected by a person skilled in the art so as to have the desired expression
and regulatory
characteristics in the selected host cell.
The general methods for construction of the vector of the invention,
transfection
of cells to produce the host cell of the invention, culture of cells to
produce the antibody
of the invention are all conventional molecular biology methods. Likewise,
once
produced, the recombinant antibodies of the invention may be purified by
standard
procedures of the art, including cross-flow filtration, ammonium sulphate
precipitation,
affinity column chromatography, gel electrophoresis and the like.
7. Preparation o~~nostic~. thera,Peutic and proPhvlactic compositions
The antibodies of the present invention may be used in conjunction with, or
attached to other antibodies (or parts thereof) such as human or humanized
monoclonal
antibodies. These other antibodies may be reactive with other markers
(epitopes)
characteristic for the disease against which the antibodies of the invention
are directed or
may have different specificities chosen, for example, to recruit molecules or
cells of the
human immune system to the diseased cells. The antibodies of the invention (or
parts
thereof) may be administered with such antibodies (or parts thereof) as
separately
administered compositions or as a single composition with the two agents
linked by
conventional chemical or by molecular biological methods. Additionally the
diagnostic
and therapeutic value of the antibodies of the invention may be augmented by
labeling
the humanized antibodies with labels that produce a detectable signal (either
in vitro or in
vivo) or with a label having a therapeutic property. Some labels, e.g.,
radionuclides may
CA 02362840 2001-08-30
WO 00/52053 PCT/US00/05279
-13-
produce a detectable signal and have a therapeutic property. Examples of
radionuclide
labels include'ZSI and'3'I. Examples of other detectable labels include a
fluorescent
chromophore such as fluorescein, phycobiliprotein or tetraethyl rhodamine for
fluorescence microscopy, an enzyme which produces a fluorescent or colored
product for
detection by fluorescence, absorbance, visible color or agglutination, which
produces an
electron dense product for demonstration by electron microscopy; or an
electron dense
molecule such as ferritin, peroxidase or gold beads for direct or indirect
electron
microscopic visualization. Labels having therapeutic properties include drugs
for the
treatment of candidiasis such as are described below.
The subject invention also provides for a variety of methods for treating
and/or
detecting Candida cells. These methods involve the administration to a patient
of
Candida specific antibodies, either labeled or unlabeled. One method of
detecting
Candida cells in a human involves the step of administering a labeled Candida
specific
antibody (labeled with a detectable label) to a human and subsequently
detecting bound
labeled antibody by the presence of the label. Alternatively, the Candida
specific
antibodies may be linked or conjugated to a therapeutic molecule such as ricin
or other
toxins.
The recombinant antibodies of this invention may also be used for the
selection
and/or isolation of human monoclonal antibodies, and the design and synthesis
of peptide
or non-peptide compounds (mimetics) which would be useful for the same
diagnostic and
therapeutic applications as the antibodies (e.g., Saragovi et al., 1991
Science 253:
792-795).
When the Candida specific antibodies of the invention are used in vivo, the
antibodies are typically administered in a composition comprising a
pharmaceutical
Garner. A pharmaceutical carrier can be any compatible, non-toxic substance
suitable for
delivery of the monoclonal antibodies to the patient. Sterile water, alcohol,
fats, waxes,
and inert solids may be included in the Garner. Pharmaceutically accepted
buffering
agents or dispersing agents may also be incorporated into the pharmaceutical
composition.
CA 02362840 2001-08-30
WO 00/52053 PCT/US00/05279
-14-
The antibody compositions of the invention may be administered to a patient in
a
variety of ways. Preferably, the compositions may be administered
parenterally, i.e.,
subcutaneously, intramuscularly or intravenously. Aerosol formulations are
also
expressly contemplated. Injectable forms of administration are sometimes
preferred for
S maximal systemic effect against systemic infections and infections of the
respiratory tract
and the deep tissues. When long term administration by injection is necessary,
medi-
ports, in-dwelling catheters or automatic pumping mechanisms may be used.
Thus, this
invention provides compositions for parenteral administration which comprise a
solution
of the human antibody or a cocktail thereof dissolved in an acceptable
carrier, preferably
an aqueous Garner. A variety of aqueous carriers can be used, e.g., water,
buffered water,
0.4% saline, 0.3% glycine and the like. These solutions are sterile and
generally free of
particulate matter. These compositions may be sterilized by conventional, well-
known
sterilization techniques.
The compositions may contain pharmaceutically acceptable auxiliary substances
as required to approximate physiological conditions such as pH adjusting and
buffering
agents, toxicity adjusting agents and the like, for example sodium acetate,
sodium
chloride, potassium chloride, calcium chloride, sodium lactate, etc. The
concentration of
antibody in these formulations can vary widely, e.g., from less than about
0.5%, or at
least about 1 % to as much as 1 S or 20% by weight and will be selected
primarily based
on fluid volumes, viscosities, etc., in accordance with the particular mode of
administration selected.
A preferred dose of antibody for systemic administration of the antibodies of
the
present invention are in the range of about 0.1 to about 5 mg/kg of body
weight. A more
preferred dose is in the range of about 0.5 to about 2.0 mg/kg, most
preferably about 1.0
to about 1.5 mg/kg. Human or other mammalian subjects are treated with
multiple doses
of antibody pharmaceuticals on an appropriate schedule, for example, a
schedule that
results in and maintains substantially saturating antibody levels or
significant
opsonization levels in the blood or infected tissue of a patient undergoing
treatment
according to the methods of the present invention. For example, a one-time
dose of a
chimeric antibody may be administered as described in Clark et al., " Effect
of a chimeric
CA 02362840 2001-08-30
WO 00/52053 PCT/US00/05279
-15-
antibody to tumor necrosis factor-(alpha) on cytokine and physiologic
responses in
patients with severe sepsis - A randomized, clinical trial" in Crit. Care Med.
26:1650-59
(1998).
Local or mucocutaneous infections would be treated by topical application of
the
therapeutic antibody compositions of the present invention. For oral delivery,
for
example, the pharmaceutical compositions may be administered in the form of a
cream or
a wash that can be applied by, e.g., swab or by rinsing at period intervals.
These
compositions also may be formulated into buccal suppositories for release,
e.g., from the
oral region over an extended period of time. In an alternative embodiment,
tablets or oral
insert or gum may be utilized as delivery vehicles. For vaginal delivery, the
composition
may be administered in a cream formulation, vaginal suppository or insert, as
is well
known in the art.
Pharmaceutically effective amounts would be those amounts of the proposed
pharmaceutical compositions required to yield a positive effect. Positive
effects include a
reduction of organism load in the subject, death or inactivation of the
organism, or
complete or nearly complete elimination of the infecting organism from the
body.
Preferably, the patient has an infection as measured by any appropriate
testing parameter,
which is reduced at least 100-fold, more preferably 1,000-fold, and even more
preferably
is undetectable after treatment.
Yet other embodiments of the invention are directed to compositions of the
invention which can be used in combination with other agents (e.g., anti-
fungal agents) to
maximize the effect of the compositions in an additive or synergistic manner.
Agents
that may be effective in combination with the compositions of the invention
include other
drugs and treatments which are known or suspected to have a positive effect
against a
Candida organism. Such agents include, but are not limited to, flucytosine,
mycoconazole, fluconazole, itraconazole, ketoconazole, griseofulvin,
amphotericin B and
derivatives, modifications and combinations of these agents. Other agents are
described,
for example, in U.S. Patent No. 5,679,648 to McCaffrey et al. (1977).
Actual methods for preparing parenterally administrable compositions and
adjustments necessary for administration to subjects will be known or apparent
to those
CA 02362840 2001-08-30
WO -00/52053 PCT/US00/05279
-16-
skilled in the art and are described in more detail in, for example,
REMINGTON'S
PHARMACEUTICAL SCIENCE, 15th Ed., Mack Publishing Company, Easton, Pa. (1980),
which is incorporated herein by reference.
8. Diagnostic kits for detecting diseased tissues and Candida cells
A kit can be prepared that comprises an antibody according to the present
invention capable of binding to a diseased tissue or to Candida. These kits
can be used in
conjunction with existing histological staining techniques to determine more
quickly, as
well as more accurately, what disease is present and the extent of infection
or stage of
disease. This would be useful for purposes of diagnosing, detecting and/or
determining
what therapy or therapies may be appropriate in treating a particular
subject's disease.
The preferred kit would have the antibody prepared for contact with a tissue
or
biological fluid sample, for example. The sample then would be incubated with
the
antibody, as would be known for conventional methods used in the art. After
incubation
with kit antibody, the cells and/or tissue would be examined for the presence
or absence
of binding. Standard assays to be used in such kits include, but are not
limited to latex
agglutination, radio immunoassay (RIA), enzyme-linked immunosorbent assay
(ELISA)
or other suitable antigen detection system.
In light of the foregoing general discussion, the specific examples presented
below are illustrative only and are not intended to limit the scope of the
invention. Other
generic configurations will be apparent to one skilled in the art.
EXAMPLES
Example l: Materials and Methods.
The organisms and culture conditions for preparing the cell line which
produces
the C3.1 antibody is as follows. C. albicans CA-1 was started from frozen
glycerol
stocks as previously described (Han et al., 1995 Infect. Immun. 63: 2714-9;
Han et al.,
1998 Infect. Immun. 66: 5771-5776; and Kanbe et al., 1993 Infect. Immun. 61:
2578-
2584) and was grown as hydrophilic stationary-phase yeast cells in glucose-
yeast extract-
CA 02362840 2001-08-30
WO 00/52053 PCT/US00/05279
-17-
peptone (GYEP) broth at 37°C. Hydrophilic yeast cells were washed,
suspended to the
desired yeast cell concentration in Dulbecco's phosphate-buffered saline
(DPBS, Sigma
Chemical Co., St. Louis, MO.), and used to infect mice.
The BALB/c female mice obtained from Charles River Laboratories (Kingston,
N.Y.), were 6 to 8 weeks old and were used throughout this study.
The MAb C3.1 was one of several MAbs, including the protective MAb B6.1,
that were isolated through hybridoma techniques from the L-mane-vaccinated
mice (Han
et al., 1995). MAb C3.1 was produced in serum-free medium and ammonium sulfate
precipitated by LigoCyte Pharmaceuticals, Inc. (Bozeman, MT). MAb B6.1 served
as a
positive control antibody and was characterized as described previously (Han
et al., 1997
J. Infect. Dis. 175: 1169-1175; Han et al., 1998). MAb B6.1 was produced in
serum-free
medium and ammonium sulfate precipitated by LigoCyte Pharmaceuticals, Inc.
(Bozeman, MT). Antibody titers were measured by agglutination with either
whole C.
albicans yeast cells or mannan coated latex beads (see Han et al., 1995; and
Han et al.,
1998).
Isotyping of the MAb C3.1 was performed as follows. The MAb C3.1 isotype
was detected by capture enzyme-linked immunosorbent assay (ELISA), and was
confirmed by immuno-double diffusion (Ouchterlony) techniques. All anti-mouse
immunoglobulins were purchased from Sigma.
Mannan extraction and acid hydrolysis. C. albicans yeast cells, grown as
described above, were treated with (3-mercaptoethanol to yield a mannan
extract, which
was further fractionated on a concanavalin A affinity column as before (Kanbe
et al.,
1993 Infect. Immun. 61: 2578-2584). The extract was hydrolyzed by boiling in
10 mM
HCI, neutralized, and applied onto a P-2 (Bio-Rad, Richmond, CA) column as
described
(Han et al., 1997 Infect. Immun. 4100-4107). The various fractions eluted from
the P-2
column were tested for their ability to react with MAb C3.1 in the same manner
as
previously described for identification of the epitope specificity of MAb B6.1
(Id.).
CA 02362840 2001-08-30
WO fl0/52053 PCT/US00/05279
-18-
Fluorescence Microscopy. Distribution of the C3.1 epitope on yeast cells was
determined by an indirect immunofluorescence (IFA). MAb C3.1 was reacted with
yeast
cells, washed, and interacted with fluorescein-labeled anti-mouse IgG or anti-
mouse IgM.
The cells were observed by fluorescence and by phase-contrast microscopy.
Example 2: MAb C3.1 Transfers Protection Against Disseminated
Candidiasis.
Protection against Candida infections by passive transfer. MAb C3.1 was
immediately used or stored at -20°C, treated at 56°C for 30 min
before use, or absorbed
with C. albicans yeast cells (Hen et al., 1995; Han et al., 1998; Hen et al.,
1998 J. Infect.
Dis. (in press)). The prophylactic effect of MAb C3.1 was tested against
experimental
disseminated candidiasis and Candida vaginal infection as follows:
1 ) Against disseminated candidiasis:
0 h, Treat with MAb C3.1, infra peritoneally (i.p.)
4 h, Challenge with C. albicans (5x105 yeast cells), intravenously
(i.v.)
48 h, Determine kidney colony forming units (CFL~ or continue to
measure survival times.
2) Against vaginal infection:
0 h, Inject estradiol (0.5 mg/mouse), subcutaneously (s.c.)
72 h, Treat with MAb C3.1 (0.5 ml/mouse), i.p. or i.vg. (For i.vg.,
100 wl of Mab C3.1 was given).
76 h, Challenge with C. albicans (5x105 yeast cells), i.vg.
120 h, Determine vaginal CFU (Hen et al., 1998 Infect
Immun 66:5771-5776).
Statistical significance of differences in survival times was calculated by
use of
the Kaplan-Meier test (Systat 7.0, SPSS Inc. Chicago, IL). For other analyses,
the
Student's t-test was used. P-values were considered statistically significant
if they were
less than 0.05.
CA 02362840 2001-08-30
WO 00/52053 PCT/US00/05279
-19-
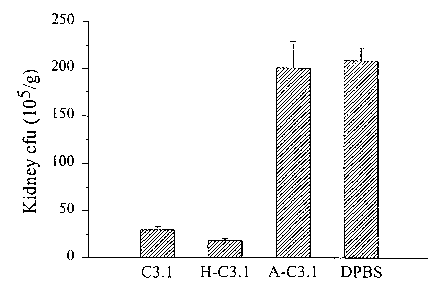
Figure 1 demonstrates that MAb C3.1 has a prophylactic effect against Candida.
In
Panel (A), BALB/c mice were given unheated MAb C3.1 (0-1), MAb heated at
56°C for
30 min (H-C3.1), C. albicans-absorbed MAb C3.1 (A-C3.1) or DPBS (buffer
diluent) i.p.
In Panel B of Figure 1, mice were given MAb C3.1, MAb B6.1 or DPBS as a
control.
In both panels, the animals were challenged i.v with 5x105 viable yeast cells
and
susceptibility to disseminated disease was assessed by determining kidney CFU
(A) and
by survival curves (B).
Mice that received the unheated or heated MAb C3.1 (Figure lA) had 86% and
88% fewer CFU, respectively, than mice that received DPBS (P < 0.001). Mice
given the
absorbed serum developed almost the same number of CFU as control mice that
received
the DPBS. Bars show standard errors. Mice that received MAb C3.1 had survival
times
similar to animals given MAb B6.1. Their mean survival times were
significantly longer
than animals given DPBS (P<0.05). The conclusion is that MAb C3.1 enhances
resistance of mice against disseminated candidiasis.
Example 3: MAb C3.1 Has Prophylactic Effect against Candida Vaginal
Infection.
Pseudoestrus mice were given MAb C3.1 infra peritoneally (i.p.) (Figure 2A) or
infra vaginally (i.vg.) (Figure 2B) before an i.vg. challenge with yeast cells
(5x105).
Vaginal CFUs were compared with CFU from animals that were given unheated MAb
C3.1 (C3.1), heat treated at 56°C for 30 min (H-C3.1), C. albicans-
absorbed C3.1 (A-
0.1), or DPBS (diluent) as described in Figure 2A.
In Figure 2A, mice that received the unheated (C3.1) or heated (H-C3.1)
developed 60% and 49% fewer CFU, respectively, than DPBS-control mice. Mice
that
received the absorbed C3.1 (A-C3.1) or DPBS developed similar CFU.
In Figure 2B, mice that received the unheated or heated MAb C3.1 developed
approximately 86% fewer CFU than DPBS control mice. This CFU reduction is
similar
to that observed due to administration of MAb B6.1. Mice given the C. albicans-
absorbed C3.1 resulted in similar CFU as the DPBS-control mice.
CA 02362840 2001-08-30
WO-00/52053 PCT/US00/05279
-20-
In both panels, significant differences were found between mice that received
either the unheated MAb C3.1 or heated MAb C3.1 and DPBS controls (P < 0.05).
Bars
show standard errors.
Example 4: Antibodies of the IgG Class that Are Protective
Against C. Tropicalis
The techniques described in Example 1 are followed using yeast cells of the C.
tropicalis species to prepare an L-mane immunogen. BALB/c female mice obtained
from Charles River Laboratories (Kingston, N.Y.), that are 6 to 8 weeks old
are
immunized. Hybridomas are produced and screened through hybridoma techniques
from
the L-mane-vaccinated mice per the procedures of Han et al., 1995. Antibody
titers are
measured by agglutination with either whole C. tropicalis yeast cells or
mannan coated
latex beads by the techniques of Han et al., 1995; and Han et al., 1998.
Selected
antibodies to be isotyped are detected by capture enzyme-linked immunosorbent
assay
(ELISA), and isotype is confirmed by immuno-double diffusion (Ouchterlony)
techniques. Protection against Candida infections by passive transfer is
determined by
the technique shown in Example 3.
Example 5: Additional IgG Antibodies Against Cahdida albicahs
The techniques described in Example 1 are followed using yeast cells of the C.
albicans species to prepare an L-mann immunogen. BALB/c female mice obtained
from
Charles River Laboratories (Kingston, N.Y.), that are 6 to 8 weeks old are
immunized.
Hybridomas are produced and screened through hybridoma techniques from the L-
mann-
vaccinated mice per the procedures of Han et al., 1995. Antibody titers are
measured by
agglutination with either whole C. albicans yeast cells or mannan coated latex
beads by
the techniques of Han et al., 1995; and Han et al., 1998. Selected antibodies
to be
isotyped are detected by capture enzyme-linked immunosorbent assay (ELISA),
and
isotype is confirmed by immuno-double diffusion (Ouchterlony) techniques.
Protection
against Candida infections by passive transfer is determined by the technique
shown in
Example 3.
CA 02362840 2001-08-30
WO 00/52053 PCT/US00/05279
-21-
Example 6: Treatment of Candida Infection in Human Patients
For the treatment of disseminated disease, patients who develop evidence of
disseminated disease should receive the antibody i.v. or i.p. or i.m., alone
or in
combination with other antifungal agents. For the prevention of candidiasis,
high-risk
patients should be identified (e.g., those who will undergo abdominal surgery,
open heart
surgery, kidney transplants, bone marrow transplants, receive indwelling
catheters,
corticosteriods, broad spectrum antibiotics), and the antibody pharmaceutical
compositions described above should be administered i.v. or i.m. prior to the
procedure.
For the treatment of vaginal Candida infections, the antibody pharmaceutical
compositions described above are administered intravaginally, as well as i.v.
or i.p. or
i.m., alone or in combination with other antifungal agents.
Example 7: Use of Test Kits to Detect Candida Infection
The antibodies as described above are used in a capture antigen format to
capture
Candida antigen in the serum or vaginal secretions from an infected patient.
Such kits
are further prepared with agents to detect the binding of the antibody to such
antigens.
It should be understood that the foregoing discussion and examples present
merely present a detailed description of certain preferred embodiments. It
therefore
should be apparent to those of ordinary skill in the art that various
modifications and
equivalents can be made without departing from the spirit and scope of the
invention. All
articles, patents and patent applications that are identified above are
incorporated by
reference in their entirety.
CA 02362840 2001-08-30
W O-00/52053 PCT/US00/05279
-22-
REFERENCES:
1. Shibata, N., K. Iuta, T. Imai, Y. Satoh, R. Satoh, A. Suzuki, C. Kojima, H.
Kobayashi, K. Hisamichi and S. Suzuki, 1995. Existence of branched side chains
in
the cell wall mannan of pathogenic yeast, Candida albicans. Structure-
antigenicity
relationship between the cell wall mannans of Candida albicans and Candida
parapsilosis. J.Biol.Chem. 270:1113-1122.
2. Han, Y. and J.E. Cutler, 1995. Antibody response that protects against
disseminated candidiasis. Infect.lmmun. 63:2714-2719.
3. Han, Y. and J.E. Cutler, 1997. Assessment of a mouse model of neutropenia
and the effect of an anti-candidiasis monoclonal antibody in these animals.
J.Infect.Dis.
175:1169-1175.
4. Han, Y., T. Kanbe, R. Cherniak and J.E. Cutler, 1997. Biochemical
characterization of Candida albicans epitopes that can elicit protective and
nonprotective
antibodies. Infect.Immun. 65:4100-4107.
5. Han, Y., R.P. Morrison and J.E. Cutler, 1998. A vaccine and monoclonal
antibodies that enhance mouse resistance to Candida albicans vaginal
infection.
Infect.lmmun. 66:5771-5776.
6. Han, Y., M.A. Ulrich and J.E. Cutler, 1998. Candida albicans mannan
extract-protein conjugates induce a protective immune response against
experimental
candidiasis. J.Infect.Dis. In Press.
7. Hill, H.R., D.K. Kelsey, L.A. Gonzales and H.V. Raff, 1992. Monoclonal
antibodies in the therapy of experimental neonatal group B streptococcal
disease.
Clin.lmmunol.lmmunopathol. 62:87-91.
8. Kanbe, T., Y. Han, B. Redgrave, H.H. Riesselman and J.E. Cutler, 1993.
Evidence that mannans of Candida albicans are responsible for adherence of
yeast forms
to spleen and lymph node tissue. Infect.Immun. 61:2578-2584.
9. Shibata, N., K. Hisamichi, T. Kikuchi, H. Kobayashi, Y. Okawa and S.
Suzuki, 1992. Sequential nuclear magnetic resonance assignment of (3-1,2-
linked
mannooligosacharides isolated from the phosophomannan of the pathogenic yeast
Candida albicans NIH B-792 strain. Biochemistry 31: 5680-5686.
CA 02362840 2001-08-30
WO UO/52053 PCT/US00/05279
-23-
10. Yuan, R., A. Casadevall, G. Spira, and M.D. Scharff, 1995. Isotype
switching from IgG3 to IgGI converts a nonprotective marine antibody to
Cryptococcus
neoformans into a protective antibody. J.Immunol. 154:1810-1816.
11. Yuan, R.R., G. Spira, J. Oh, M. Paizi, A. Casadevall and M.D. Scharff,
1998. Isotope switching increases efficacy of antibody protection against
Cryptococcus
neoformans infection in mice. Infect.Immun. 66:1057-1062.