Note: Descriptions are shown in the official language in which they were submitted.
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
METHOD AND APPARATUS FOR AUTOMATED EXCISION OF SAMPLES
FROM TWO-DIMENSIONAL ELECTROPHORESIS GELS
CROSS-REFERENCE TO RELATED APPLICATION
This application claims priority based on U.S. provisional patent application
no.
60/120,471 filed February 17, 1999.
FIELD OF THE INVENTION
The present invention relates to the analysis and separation of biomolecules.
More
particularly, the present invention relates to a method and apparatus for the
automated
1 o excision of individual protein samples from two-dimensional
electrophoresis gels for
subsequent analysis of protein content.
BACKGROUND OF THE INVENTION
The method and apparatus described herein are used for the automated excision
of
individual samples from two-dimensional ("2D") electrophoresis gels for
subsequent
analysis (referred to herein as the "Invention"). The Invention may be used in
any art or
occupation where the user wishes to separate and analyze proteins or other
substances
that are identifiable by 2D gel electrophoresis techniques, or any other
technique that
results in the physical separation of substances within planar and cuttable
materials.
By way of example, one such art is "proteomics," especially in conjunction
with a
2o related art, "genomics." Proteomics is the study of the protein complement
that an
organism is capable of producing, whereas genomics is the study of
deoxyribonucleic
acid ("DNA"), its genes, and the processes that lead to the creation of
proteins.
Proteomics provides data on the outcome of gene expression. Genomics provides
the
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
comprehensive gene sequence data, often derived by microarray analysis,
required to
advance protein research.
In complex organisms, individual cells may selectively express genes in their
DNA to yield sets of proteins required for specific cell or organ functions.
Much current
scientific effort is directed to creating databases concerning how these genes
are
regulated and how this regulation may change in disease or other states,
whether before
and after treatment.
In order to evaluate the effects of gene regulation, methods must be used that
measure, separate, and qualitatively and quantitatively analyze proteins,
which are one
output of gene expression. One currently favored proteomic technique is 2D
polyacrylamide gel electrophoresis. This technique separates complex mixtures
of
proteins so that they can be isolated, quantified, identified and then
assessed for their role
in a disease process or as a target for novel drugs.
One approach to proteomic study using 2D gel techniques can be considered as
comprising eight individual operations (see Figure 1 ):
1. Solubilization 16 - The proteins in a sample 15 of cells or tissue are
released from the underlying cellular or tissue matrix by solubilizing the
proteins with
detergents.
2. Separation 17 - The solubilized proteins are then physically separated into
2o a square gel array using 2D gel electrophoresis.
2
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
3. Staining 18 - The separated proteins are demonstrated in the gel by
staining with or attaching Coomassie brilliant blue, silver staining, SYPRO
ruby,
fluorescent compounds, or by other appropriate techniques.
4. Ima in 19 - The stained 2D gels are imaged by electronic optical or other
means for resolving protein sample spots which are potentially interesting.
For example,
proteins that occur differentially in diseased but not healthy tissue could be
considered of
interest.
5. Picking 20 - The spots of gel containing the proteins of interest are
excised
from the main gel matrix.
6. Digestion of protein into peptides 21 - The proteins are broken down,
usually enzymatically, into constituent peptides whose masses can be measured
by mass
spectrometry.
7. Mass spectral analysis 22 - The size of the isolated and digested protein
peptides are measured using a matrix assisted laser desorption ionization-time
of flight
("MALDI-TOF") mass spectrometer, or analyzed by liquid chromatography-mass
spectrometry, quadropole time of flight, or other means.
8. Identification 23, 24 - The proteins are identified by matching the masses
of the set of peptide fragments to fragments predicted by public and private
databases
after similar proteolytic (enzymatic) treatment. Once identified, the role of
each protein
2o in a disease process or as a potential point of intervention in a disease
process (e.g., a
drug target) can be considered along with information from pathology,
pharmacology and
known biological pathways.
3
WO 00/49397
CA 02362984 2001-08-15
PCT/GB00/00573
In conjunction with computer databases and analysis, 2D gel electrophoresis
can
provide a means to physically resolve the proteome of a tested sample
according to each
protein's isoelectric point, reflected on one axis of the planar 2D gel
sample, and its
molecular weight or size, reflected by a corresponding perpendicular planar
axis. Thus,
2D gel analysis of any given sample may produce a "fingerprint" that reflects
an
orthogonal planar distribution of its protein complement according to
individual protein
characteristics. Once prepared, resolved 2D gels may be translated by
staining, imaging,
and bioinformatic software into high-resolution digital protein maps, which
may be
stored for future use in computer or other databases. The resulting data may
be used to
1o determine the protein profiles of different tissues in both healthy and
disease states, and
ultimately for proteome libraries.
In addition, individual proteins may be excised from 2D gels, split into
peptide
fragments, and measured using mass spectrometry or other means. However, the
large-
scale study of proteins and protein networks is currently limited in part by
the ability to
physically isolate, segregate, and study individual proteins. Currently
operations like
those in Figure 1 are done in a sequential and modular fashion. The output of
each step is
transferred manually from operation to operation. These individual unconnected
manual
operations make the technique slow and cumbersome, prone to error due to the
repetitive
nature of each manual step, and subject to contamination, for example, by
keratin
2o contamination from skin during handling.
Scientists studying proteomics and genomics, and others, are extremely
interested in rapid, accurate high throughput methods and instruments to carry
out protein
4
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
analysis. It is clear that advances in robotics and software/computing
technology could
improve the throughput and rate of the analysis.
One U.S. company, BioRad Laboratories, is developing a protein-picking system
in collaboration with a company called AARM (an Australian firm). However,
among
other distinctions, their system is only semi-automated, and the user must
manually
identify the proteins to be picked from a particular 2D gel. Furthermore, the
BioRad
system does not use information stored in 2D gel databases to identify
proteins of interest
to be excised. Finally, the BioRad system does not have the capability of
utilizing
excision tools of different sizes based upon the size of the protein in the 2D
gel.
to Although there is other information to suggest other interest in the field,
see e.g.,
Anderson, et al., U.S. Patent No. 5,993,627 at Columns 26-28, there appears to
be no
claimed invention or art providing the novel elements, means and utility of
the claimed
Invention.
SUMMARY OF THE INVENTION
The Invention offers a method and automated apparatus for the separation,
excision, and high throughput handling of protein samples demonstrated via 2D
gel for
further analysis. The Invention utilizes a laboratory-grade XYZ Gantry robot,
a novel
approach to the identification of the proteins of interest to be excised,
novel tools for the
excision of the protein samples from the 2D gels, and novel means for
controlling robot
2o and process steps to accomplish selective and automated protein sample
excision.
Currently, the process of protein excision is performed by hand, is extremely
labor-intensive, and is prone to error. The manual process is also susceptible
to
5
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
contamination, rendering the protein under analysis virtually useless. The use
of the
laboratory robot and the novel excision tools described herein will increase
the efficiency
of protein excision and will greatly reduce contamination by minimizing user
handling of
the protein samples.
BRIEF DESCRIPTION OF THE DRAWINGS
The features and inventive aspects of the present Invention will become more
apparent upon reading the following detailed description, claims, and
drawings, of which
the following is a brief description:
Figure 1 is a logic flow diagram of one approach to proteomic analysis
starting
1o with a test or control sample and continuing through intermediate steps to
data capture
and analysis.
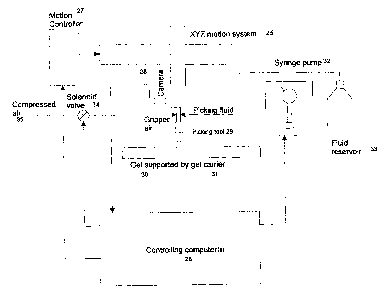
Figure 2 is a schematic diagram of the basic elements of the current
Invention.
Figure 3 is an illustration of positions of the robot arm, gel samples, and
collection trays.
Figure 4 is a top view of an arrangement of gel samples, tips, wash stations,
and
output trays, and related work areas.
Figure 5 is an illustration of a fixed cutting tool arm and tip.
Figure 6 is an illustration of a cutting tool arm and tip used with
interchangeable
or disposable tips.
2o Figure 7 is an illustration of an example of a configuration for a gel
picking run.
Figure 8 is an illustration of the sample dimensions of a cutting tip with a
configured shoulder setback.
6
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
Figure 9 is an illustration of a cutting tip with a shoulder setback and
conical
internal coring cavity.
Figure 10 is an illustration of sample plug cutting and shape using a cutting
tip
without a configured shoulder.
Figure 11 is an illustration of sample plug cutting using a cutting tip with a
configured shoulder.
Figure 12 is an illustration of alternative tip or cap insertion into
collecting tray
wells.
Figure 13 is an illustration of automated means to transport and handle
pluralities
to of gel samples and collecting trays.
DETAILED DESCRIPTION OF A PREFERRED EMBODIMENT
The basic process and elements of the Invention are to acquire an image of a
processed 2D gel sample using a CCD or other camera or imaging system, analyze
the
image to fmd regions of interest and to generate a "pick" list of spot
coordinates, sample
the selected gel regions by coring a gel plug from each of them, and deposit
the core plug
into a collection vessel. Steps in this process may include:
1 Presenting 2D gels 30, 38 to the excision working area of the machine
Presenting collection trays 40 for holding sample cores to the working area of
the
machine
~ Presenting coring tips 42 and/or tray caps to the machine
1 Illuminating the gel via a transmissive, reflective, visible, or ultraviolet
light source
7
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
Obtaining and capturing an electronic image of the gel by means of a mounted
camera 28
1 Processing the image by computer means 26 to find contrasting areas, for
example,
by commercially available software
1 Further electronic processing to identify protein spot areas of interest
1 Further processing to calibrate geometry of the gel sample and any stored
image
Further processing to compare/contrast with database
User processing to identify sampling positions
o Generating a list of physical positions to pick from and to link with
calibrated
1 o identification information
1 For each pick,
1 Optionally collecting a new (clean) coring tool or clean the (reusable) tool
o moving the picking tool 29, 37 to the required position over the gel
operating the picking tool to remove a core
I S 1 moving the core to the relevant well 79 in the output tray 40, 41
1 depositing the core in a well 79
1 disposing of coring tool (if disposable)
1 collecting cap 77 from storage area, move to well 79
1 capping the well
20 ~ Removing the output tray 88, 91 and gel from the machine at an
appropriate time
1 Downloading a log of picking information to another system to build the
results into
the (or another) database.
8
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
Gel images are usually captured initially by using an imaging system 28 and
analyzing the image quantitatively with a commercially available,
comprehensive 2-D gel
analysis software package, such as Genomic Solutions, Inc.'s InvestigatorT"''
2-D Analyzer
Software. The image acquisition hardware provides high accuracy and high
resolution
and may offer special features to image fluorescent- or radioactive-marked
gels.
Once a gel 30, 38 has been imaged and its data added to a database along with
data from other gel samples, the gel may be stored for later processing.
However, there
may be distortion and movement of the gel during storage. If the distortion is
not
excessive, then the coring can be performed, relative to mechanical
registration features
io on the gel carrier sheet. However, if the distortion is not acceptable, it
must be corrected
or accounted for prior to picking.
In one embodiment, the Invention may re-image the gel in the picking system to
enhance basic accuracy and resolution. The image is then matched to the
original stored
image within the 2-D analysis software, and calibration factors are derived to
match the
spot coordinates in the original image with the actual gel sample for spot
excision
purposes.
The software allows users to optimize automatic spot finding with adjustable
parameters. Users may perform database queries to filter information based on
existence
of spots, quantitative ratios of matched spots, spot integrated intensities,
molecular
2o weight, iso-electric point, area, and user-defined spot or image
characteristics. The
current system creates an image from the gel on the protein-picking robot.
This image is
subsequently "matched" with an image of the same gel analyzed previously. The
process
9
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
involves some user interaction to effectively "teach" the gel analysis
software where to
fmd the gel's "anchor points," which may establish a coordinate system for the
gel under
analysis.
The protein spots to be excised from the gel are identified via user-initiated
queries to the spot image database via the 2-D software. For example, if the
user desires
to pick the proteins which have been overexpressed in an experimental schema
with
respect to a control sample, the user may initiate a database query to
identify the spots
and to relay their image coordinate positions to the picking robot.
Analytical software on the market already calculates the size of the spots,
1 o typically in square millimeters. The user or the software determines which
spots are of
interest, and the software creates a picking list with the coordinates of the
spots within the
image to be excised and the size of each spot. The pick list is created
upstream from the
picking process in a database of spots, taking individual images, and matching
them
together.
Optical calibration marks can be applied to the face of the gel carrier plate
31, 39,
77. These can be imaged by a high-performance imaging system, for example, the
InvestigatorTM 2-D Analysis System, as well by lower performance cameras or
imaging
systems fitted to the picking system. Thus, the picking system can be used to
re-image
the gel sheet, and a match can be made to the "main" image, which was captured
using a
2o high-performance imager.
To further automate the protein picking process described herein, the
Invention
may use the incorporation of specific fluorophores to the proteins and
specifically to the
to
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
gel image anchor points. When excited by light of appropriate wavelength, the
fluorophores incorporated into the gel's anchor points will emit light of a
characteristic
wavelength that can be imaged separately from the "study" proteins in the same
gel. The
anchor points are then imaged using an imaging system 28, such as a CCD camera
or
other imaging system, on the picking robot, and a segmentation algorithm will
be applied
to the digital image to determine the coordinates of the anchor points.
Alternatively, the additional reference marks may show contrast in both
visible
light and by fluorescence. Using such marks, the gel may be imaged first in a
special
fluorescent imaging system, separate from the picking system. Subsequently,
the gel is
to imaged by a camera built into the picking system using visible-light
contrast rather than
fluorescent emission from the gel. This allows picking from gels stained by
fluorophores
even though the picking system is insensitive to the fluorescent emission. The
two images
(one from the separate fluorescent imaging system and the other from the
camera built
into the picking system) are matched using the reference marks since these are
visible in
I5 both images. Once matched, the locations of desired (fluorescently marked)
locations can
be translated to the visible-light image and used as coordinates from which to
pick.
At the beginning of the picking cycle (Figure 3), the operator mounts the gel
on
the gel carrier. 2D gels can be fragile and prone to tearing, creating some
difficulty in
transferring them from one substrate to another without damage or geometric
distortion.
2o In proteomic analysis, the registration of the gel must be maintained
between imaging
and picking in order to avoid degradation in accuracy. Because the imaging and
picking
may be done at different times and/or in different machines, it is important
to be able to
I1
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
transfer the gel without distortion. This may be done by supporting the gel on
a substrate
that will not stretch and which has reference points that may be used in
imaging and
picking to ensure correct positioning. The present Invention may use a simple
sheet of
acrylic or silica glass, called a gel carrier sheet. The gel sheet is loose-
laid onto a hard,
smooth support. Alternatively, the gel may be fixed to a stretch-resistant
substrate by, for
example, proprietary materials such as "Gel Bond". Immobilizing the gel in
this way
eases the handling difficulties and reduces geometric distortion
In the present embodiment, the gel carrier may be part of the robot, or an
intermediate carrier that can be detached from the robot and used to transport
the gel on
1 o the carrier. The gel carrier may be comprised of a fixture plate, a gel
carrier, and a gel
plate, all fitting on top of the other. The sheet can also have both
mechanical and optical
registration features. These are functionally transparent in order to permit
transmission
from the illumination source or have holes to permit transmission of light.
Optionally, the
substrate must also transmit UV light in order to allow UV illumination of
gels marked
i s with fluorescent dyes.
In any case, the light source can be fluorescent tubes or other suitable
source.
With the camera (or other imaging device) typically positioned above the gel,
light may
be passed upwards through the gel from beneath (transillumination) or shone
downwards
from above (epi-illumination). To aid spot finding by automatic processes, it
is important
2o that the illumination is maximally uniform. For transillumination, this is
typically
achieved with a diffusing grid or panel.
12
CA 02362984 2001-08-15
WD 00/49397 PCT/GB00/00573
The gel carrier is then transported to and mounted in the excision work area
within the illumination zone. Once the gel has been placed in the carrier and
moved to the
sampling position, a camera may be used to determine protein spot locations in
order to
align the gel carrier's coordinate system with that of the previously analyzed
image of the
gel. In one embodiment, the camera is fixed to the moving head on the robot
arm that can
be used to image part of the gel (Figure 2). The resulting images may be
processed
separately, or the individual "frames" from the camera image may be tiled to
form a
larger image. In another embodiment, the camera may be a high-resolution
camera fixed
above the gel, either above the head or not, in order to produce a single
image.
When the images are obtained, the spots of interest are located by
commercially
available software in the controlling computer or in one or more other
computers linked
to the controlling computer 26. The analytical product gives XY coordinates
for spot of
interest for excision. Once the spots are found, certain picking criteria may
be applied.
By way of example, spot locations may be known to correspond with certain
known
1s proteins, or other spots found by comparison to images in the database may
be selected
for excision. The operator may employ different selection criteria using the
images on the
controlling computer or the associated computer and translated by means of
operation of
the computer back to the controlling arm. The communication contains one or
more
coordinates from which the computer will direct the arm to pick.
The controlling computer 26 (Figure 2) performs a number of functions
electronically, including controlling the motion commands 27 for the robot,
executing tip
pick-up and eject cycles, controlling the valves 34 to operate the feed of
pressured gas or
13
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
air, controlling solenoid valves 34 or syringe pump valves 32, and controlling
the vacuum
cycles and eject cycles for the samples themselves. Means for generating and
implementing commands for such functions will be apparent to those skilled in
the art.
The controlling computer may be a single computer or a number of linked
computers that
intercommunicate so that individual tasks can be distributed 26. The camera on
the robot
may communicate with that computer, an additional computer, or an additional
image
processing system of other forms. The controlling computer may also
communicate with
another computer to control the automatic stacking and handling of plates or
carriers
(Figure 13) in and out of the robotic system itself.
to Mapping between image coordinates and robot coordinates is coordinated
through
a calibration procedure using a test target or targets. The coordinates are
translated from
stored spot image coordinates to robot coordinates by means of a mapping
translation that
performs a mathematical match between a test target position with known
physical
locations and coordinates from spot finding for that target. This is
preferably part of the
means in the controlling computer that controls the robot but may be embodied
separately.
Once picking coordinates have been established and communicated to the motion
controller, the robot has a list of coordinates to pick from and may begin the
picking
cycle. The basic cycle takes the robot head to a drain position over a waste
collection
2o trough 43 (Figure 4) 85. To achieve good performance, it is important to
prevent cross
contamination between successive coring operations. The target proteins are
normally
held within the gels, but should particles of gel be carried over from one
coring operation
14
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
to the next, then there is the potential for contamination. Fluid is
discharged through the
tip by cycling the syringe pump in order to wash out debris and to ensure that
the system
is filled with fluid. The fluid 33 used during the picking cycle must match
that used
during pretreatment of the gel so that mismatch in composition of the fluids
does not
cause shrinkage or expansion of the gel, Such fluids may be water, 10%
ethanol/water,
10% ethanol/2% glycol/water, or other compatible fluids.
In one embodiment using an interchangeable tip, the tips are held in a
separate
rack 42, 84. At the beginning of a picking run, the robot picks up a tip. With
interchangeable tips, the robot may be instructed to use one tip for the whole
picking run,
i o or to use a new tip for each picked spot during the picking run, putting
the tip away and
collecting a new one, for example, to reduce the possibility of cross-
contamination
among samples. Optionally the controlling computer may be programmed to direct
a
washing procedure so that each of the interchangeable tips are put through a
washing
procedure automatically in the absence of a gel, through optional water, other
solvent or
ultrasonic baths 43, 44, 83.
In a preferred embodiment, the gel may be irrigated during the picking. At a
predetermined interval selected by the operator, the picking tool 29, 37 may
begin an
irrigation process comprised of moving the head back and forth across the gel
in a raster
fashion, dropping fluid as it proceeds. The patterns may repeat, change
directions, or the
2o wetting pattern may be shifted by a fraction of the line pitch, for
example, to irrigate in
the gaps between previous lines in order to enhance uniform irrigation. Excess
fluid
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
during irrigation runs off the gel onto the carrier plate 39 into a waste
collection trough
85.
The robot arm may be used with a fixed tip with a semipermanent connection
(Figure 5), or an interchangeable tip that may be disposable or reusable
(Figure 6). Fixed
tips may be made of stainless steel or similar metal known to one skilled in
the art that is
low corrosion and high cleanliness, cleanable with corrosive solvents with no
leeching
from the materials. The interchangeable or disposable tips may be made of
various
polymers, such as polypropylene, nylon, or POM (acetal) materials, or other
suitable
materials.
l0 To minimize contamination, the tip may be cleaned between coring operations
or
it may be replaced (i.e. a disposable coring tip). The latter approach is
preferred for best
performance. The tips may be of the same diameter, or different diameters may
be
selected according to different spot parameters, such as spot diameter or
optical density.
A robotic manipulator 25 optionally carries a tool gripper. When
interchangeable
tips are used, the head gripper on the robot arm has means to grip, hold and
eject the tips,
an eject spring 53 with an associated sleeve 59, and an inflatable cuff 57
(Figure 6).
There are two feeds to the head gripper. One feed 54 provides fluid pressure
or vacuum
through the gripping tip to a picking tip from the syringe pump 32 and fluid
reservoir 33
to enable gel core extraction and ejection. The gripper has a cylindrical
elastic cuff 57
2o that can be expanded by internal gas or liquid pressure. The second feed
35, 55 supplies
the cavity 56 between the inflatable cuff 57 and the body of the gripper 52.
That cavity is
inflated with air, other gas or fluid to push out the cuff to grip the
internal wall of the tip.
16
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
The cuff inflation pipe 55 communicates through the body of the gripper to the
cavity 56
behind the inflatable cuff 57 for all interchangeable and disposal tips.
When no interchangeable tip is in place, the robot arm 25 with the gripper 52
may
be cycled to the tip rack, moved so that gripper 52 inserts into the cavity of
a tip 58, and
lowered to depress the eject spring 53. Pressure is then applied to the
inflatable cuff 57 so
that it inflates and grips inside of the tip. The gripper is then withdrawn
vertically with
the tip in place. The eject spring 53 remains compressed due to the insertion
into the
cutting tip 58. After the gel coring operation has been performed, the cuff
pressure may
be released, thereby releasing the gripping pressure and permitting the eject
spring (with
to a force, for example, of a range of'/2 - 1 Newton) to eject the
interchangeable tip. There is
an intermediate sleeve 59 between the eject spring and the disposable or
interchangeable
tip to bear between the spring and the end of the tip.
With a fixed picking tip (Figure 5), there are no inflatable cuffs, and the
cutting
edge 51 is built as part of the gripping tool with a single fluid way 50 and
attached to the
1 S moving head of the robot with semi-permanent means.
There are variations in configuration and dimensions of the cutting tips.
Simple
trials on 1.5 mm gels suggest the preferred tip dimensions shown in Figure 8.
In one
embodiment, the lead edge 69 of the cutting tip may have an inside diameter of
1.3
millimeters and an outside diameter of 1.5 millimeters, with a shoulder 68
setback of 0.4
20 millimeters from the lead edge 69. The internal diameter of the cutting tip
may range
from 0.5 mm up to 5 mm, with a fme cutting edge width, for example about 0.1
mm
width, and a sharpened and preferably beveled edge.
17
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
It would be beneficial to apply a radius to the outer corner of the shoulder
68 to
minimize damage to the gel in the vicinity of the pick. The setback of the
shoulder and
the outer diameter of the outer shoulder may be varied according to the gel
thickness and
mechanical properties, such as elasticity, tear and tensile strength. The
depth of the
shoulder and the overall diameter may be optimized for a particular gel
thickness and gel
properties. The above referenced dimensions are typical cutting tip dimensions
for use
with 1 mm to 1.5 mm thickness duracryl gels. With a thicker gel, the 4 mm
outside
diameter and the shoulder setback are increased. For a weaker gel with a lower
tensile
strength for a given amount of elasticity, the cutting setback shoulder depth
would be
to increased.
In one preferred embodiment (Figure 9), the internal shape of tip is optimally
conical to create a tapered core cavity 73 to the tip. This improves
reliability of ejection
of gel plugs after picking. If the cavity is cylindrical, there is a
possibility that during
ejection by fluid pressure, the plug may twist in the cavity about an axis
perpendicular to
the axis of the tool. This creates an escape path for the ejection fluid and
consequently the
plug may not eject. This mode is similar to the action of a butterfly valve so
is known as a
"butterfly valve" failure. Making the internal cavity conical restricts the
ability of the
plug to rotate so improving reliability. The dimensions optimally include a 14-
degree
taper on each side of the cavity 73 beginning at the internal edge of the
bevel. The
internal tapered cavity may be polished to avoid gripping on any rough
surface. The
depth of the cavity is matched to the depth of the thickness of the gel,
typically equal to
the thickness of the gel.
1s
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
As a plug is cut, the gel may deform in such a way that the resulting plug
shape is
"mushroom"-shaped 74 (Figure 10). This shape has two main effects: (1) during
vacuum
extraction, there is a tendency to ingest the plug into the body of the
picking tip; and (2)
the amount of material in the plug is substantially reduced, leading to a plug
sample that
is smaller yet material is still taken from a larger area, resulting in poorer
sample/background ratio or overall resolution.
The shoulder 71 on the cutting tip may be used to change the shape of the
resulting core sample (Figure 11). If one is less concerned about the shape of
plug, or if
one is cutting large sample plugs (in comparison to the thickness of the gel)
where
1 o mushrooming is less significant, one need not use the shoulder. In other
circumstances,
the shoulder tends to push material back under the tip to counteract the
distortion caused
by the cutting force. Shoulder depth and shoulder diameter are parameters that
need to be
set to match a given gel thickness, stiffness and cutting strength. The match
is not critical,
however, as variances result in relatively small changes in plug shape.
In the preferred embodiment, this sample shape is addressed by producing
"conical" plugs 75 (Figure 11 ). The degree of "conicality" depends upon the
ratio of tip
diameter to gel thickness and the cutting force relative to the gel stiffness.
The cutting
force is a function of cutting perimeter, edge sharpness and gel properties.
In practice, a
conicality ratio of around 2:1 (max diameter to min diameter) is common.
2o As the picking cycle continues, the tip is purged at the waste collection
trough 43,
85, with fluid cycled through it from the fluid reservoir 33 using the syringe
pump 32 to
ensure that the tip is clean and that the system is purged of air with a full
complement of
19
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
fluid. The robot then is commanded to the X-Y position on the gel and spaced
off the gel
by a small distance, such as 5 mm. Optionally, a small amount of fluid, such
as 40
microliters, is dispensed from the picking tip onto the gel in a prewetting
step so that the
picking target is prewetted.
Air is then aspirated back into the tip to form an air lock volume, such as
100 ul.
The picking tip is lowered onto the gel until the spring 60 supporting the
picking tip
compresses, defining the cutting force 64 and cutting through the gel to the
hard gel
support (Figure 7). The cutting tool has a hollow cutting tip 65 of selected
size and shape
that is pressed down through the gel sheet until it meets the supporting sheet
(Figure 7).
to The tip may be spring-loaded to limit the insertion force and to
accommodate
inaccuracies in the vertical registration of the tool to the supporting sheet.
A preferred
spring force is approximately 3 newtons.
The syringe pump 32 is then operated in suction mode to withdraw a small
volume of fluid, such as approximately 70 microliters, forming a partial
vacuum that is
applied through the feed line into the picking tip that has been sealed by
insertion into the
gel. The aspirated air acts like a spring to control the amount of vacuum
applied to the
plug. This aspirated airlock also acts to separate the contaminated zone in
the coring tool,
preventing gel particles or other contaminants from being taken up into the
gripper or the
feed tube. It is important that the airlock is not too large as this increases
the ejection
2o compliance that can hinder placement of the core in the well. A small
compliance is,
however, advantageous during core extraction as it helps maintain a partial
vacuum (as
the core is taken from the gel sheet) if there is a small leak around the core
in the tip.
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
To remove the core, the tool is withdrawn, taking the gel plug with it.
However,
the softness and wet state of the sheet may cause problems. Firstly, as the
tool presses in,
the gel under the cutting edge distorts and tends to move outwards (away from
the axis of
the tool). A second problem also relates to removal; as the tool is pulled
out, a vacuum
develops under the tip. This is not relieved as the wetness of the sheet
maintains a good
seal and the result may be that the core is left in the sheet. The Invention
addresses these
issues by:
1 As discussed above, by applying vacuum to the top of the gel plug via the
tool to hold
the core in the tool
l0 1 Optionally, once the core has been cut, by moving the tool laterally for
small
distances (for example, '/2 mm) before removing it from the sheet. This
overcomes
any gel adherence to the underlying carrier and breaks any vacuum that may
exist
between the plug and the gel itself by opening a small gap between the outside
of the
tool and the remainder of the sheet to allow air (or free fluid) under the
edge of the
is tool.
The tip is then lifted out of the gel and transported with the cut plug to the
collection tray 40, which is typically a ninety-six (96) well microtiter
plate. Gel plugs are
placed individually into small wells in the microtiter plates. The narrow
portion of the
picking tip is lowered partially into the well (Figure 12). A small amount of
fluid is
2o dispensed via the syringe plug, ejecting the core sample. The fluid will
include the air
lock volume, plus the backoff volume, plus a small volume, such as a net 100
microliters,
pushing the plug out of the cup in the end of the tip, capturing the plug in a
droplet, and
21
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
dropping the droplet off the tip into the well. Use of liquid in contrast to
gas pressure to
eject the plug reduces the ejection velocity, which can cause the ejected
sample to bounce
around within the collection vessel. Liquid ejection is a much slower,
controlled process
ensuring that the sample is deposited in the bottom of the well captured in
fluid to keep it
hydrated if the plate goes into storage. The plates may then be covered
manually or
automatically, with adhesive plates or otherwise fixed coverings (for example
plastic
sheet heat-sealed to the open tops.)
With interchangeable tips, the tip may be put down or disposed, and a cap that
fits
the gripper may be picked up and pushed into the collection tray with the
spring,
1o plugging the microtiter well (Figure 12).
In one embodiment, the caps are fitted into the coring tips, and the resulting
stacks
placed in the wells. In the machine, the gripper first takes hold of the inner
cap and lifts
the cap and coring tip combination out of the tray. In this embodiment, the
coring tip is
used to extract a core from the gel and deposit it back into the vacant well
in the tray. A
stripping device is provided in the machine into which the used coring tip is
inserted.
This holds onto the coring tip, and the cap is pulled out of the coring tip by
the gripper.
A flange may facilitate this operation. The coring tip falls to waste from the
stripping
device, and the robotic manipulator replaces the cap into the tray well.
If the coring tips are made so their major bores match those of the tray
wells, then
2o the caps can be fitted either into the tray wells or into the coring tips.
This allows both
the caps and coring tips to be pre-loaded into the trays before the trays are
presented to
the machine. It will be evident that the cap must have a hole to allow
pressure/vacuum to
22
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
pass to the coring tip. This may permit subsequent stages of processing where
it is
necessary to insert a probe into the well, such as to permit protein
digestion. The hole in
the cap is made to match the dimensions of the probe to provide the partial
seal around
the probe necessary for the particular fluid handling. The robot cycles to
pick up a new
tip, to perform another wash bath cycle and then the next cycle is started.
One embodiment may include an autoloader, thus permitting several picking runs
to be performed (Figure 13). Once spots are picked from a gel, the gel may be
shunted off
the bed of the machine into an automatic stacker 89, and the next gel is
placed on the
machine for picking. The existing output tray 88 may continue to be filled, or
additional
l0 output trays 91 may be loaded to match trays with gels. The gel carrier 86
moves back
and forth in the stacking system. Each gel would have a removable lid that
would be
automatically removed before the gel is placed on the robot. A separate part
of the
stacking system takes the carrier out of the stack, removes the lid,
optionally retaining the
lid or placing it back in the stack, and then places the carrier with the
exposed gel on the
bed of the robot (optionally via a vacant position in the stack). Vertical
stacks of
pigeonholes take gel carrier or sets of output plates for automatic dispersal.
Preferred embodiments of the present Invention have been disclosed. A person
of
ordinary skill in the art would realize, however, that certain modifications
would come
within the teachings of this invention, and the following claims should be
studied to
2o determine the true scope and content of the invention. In addition, the
methods and
structures of the present invention can be incorporated in the form of a
variety of
embodiments, only a few of which are described herein. It will be apparent to
the artisan
23
CA 02362984 2001-08-15
WO 00/49397 PCT/GB00/00573
that other embodiments exist that do not depart from the spirit of the
invention. Thus, the
described embodiments are illustrative and should not be construed as
restrictive.
24