Note: Descriptions are shown in the official language in which they were submitted.
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
MINIMALLY INVASIVE MEDICAL DEVICE DEPLOYMENT AND
RETRIEVAL SYSTEM
FIELD OF THE INVENTION
The present invention generally relates to minimally invasive surgical
procedures, e.g., angioplasty and atherectomy procedures, and has particular
utility in connection with retrieving a medical device which has already been
deployed. In one embodiment, the invention provides a vascular filter which
can
be retrieved with minimal risk of dumping the entrained contents back into the
patient's bloodstream.
BACKGROUND OF THE INVENTION
In some medical procedures, a minimally invasive medical device is used
to capture or dislodge material from within a patient's vascular system or
other
body vessel. For example, in certain procedures, balloon catheters are
positioned such that the deflated balloon is placed distally of a vascular
occlusion. Typically these vascular occlusions are relatively soft,
uncalcified
deposited along the walls of an artery. The balloon then may be inflated and
drawn proximally. This will tend to dislodge any atheromatous material and
withdraw it proximally with the balloon. In current procedures, an aspiration
catheter will be moved distally into position adjacent the balloon and will be
used
to aspirate the dislodged material from the vessel.
A number of other minimally invasive surgical procedures are being used
to treat vascular occlusions. These procedures include rotational atherectomy
and balloon angioplasty. With the increasing use of vascular stents, it has
been
discovered that tissue or other material may build up inside a stent, reducing
the
patency of the vessel through the stent. In the course of improving the
patency
of the blood vessel utilizing these techniques, there is a risk that the
material
which was formally causing the obstruction within the vessel can simply float
downstream with the flow of blood to the vessel. Accordingly, there is an
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
2
increasing recognition of the value of taking steps to capture the dislodged
material.
A number of researchers have proposed various traps or filters for
capturing the particulate matter or other embolic particles let loose in such
s procedures. Some filters are permanently implanted within the vessel. Emboli
trapped within the filter are either aspirated out of the interior of the
filter or are
dissolved using drugs. Other filters are intended to be temporary in nature,
typically being removed after the angioplasty, atherectomy or other procedure
is
complete. Generally, the goal is to retract the filter with the thrombi
trapped
1o therein. Unfortunately, many designs of such temporary filters may get
relatively
difficult or complex to retract the trap back in to the catheter through which
it was
delivered without simply dumping the trapped thrombi back in to the
bloodstream.
One particularly advantageous vascular filter is shown in co-pending U.S.
Is Patent Application No. 08/272,425, and International Patent Application No.
PCT/US95/08613, which was published as International Publication No. WO
96/01591, the teachings of which are specifically incorporated herein by
reference.
Figures 11-16 of WO 96/01591 are attached hereto as Figures 1-6 of the
?o present application. Figure 1 is a vascular trap which is suitable for use
in
temporarily filtering embolic particles and the like from blood passing
through a
patient's vascular system. This device would most frequently be used to filter
emboli from a patient's blood when another medical procedure is being
performed, such as by using the trap in conjunction with a rotating cutting
blade
2s during an atherectomy, with a balloon catheter during angioplasty, or with
a
device used to clear the lumen of a stent during a stent cleaning procedure.
It is
to be understood, though, that the trap could also be used in other similar
applications, such as in channels in patient's bodies other than their
vascular
systems.
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
3
The vascular trap 250 of Figures 1A and 1B comprises a generally
umbrella-shaped basket 270 carried adjacent a distal end of a guidewire 260.
The guidewire in this embodiment includes a tapered distal section 262 with a
spirally wound coil 264 extending a distal length of the wire. Guidewires
having
such a distal end are conventional in the art. The basket 270 is positioned
generally distally of the coil 264, and is desirably attached to the guidewire
approximately with the proximal end of the tapered section as shown in these
drawings.
The basket 270 of the device shown in WO 96/01591 (shown in its
1o collapsed configuration in Figure 1A) includes a distal band 272 and a
proximal
band 274. The distal band may be made of a radiopaque material, such as
gold, platinum or tungsten, and is affixed directly to the shaft of the
guidewire
260. This attachment may be made by any suitable means, such as by welding,
brazing or soldering. Alternatively, the distal band 272 may comprise a bead
of
a biocompatible cementitious material, such as a curable organic resin. WO
96/01591 teaches that a radiopaque metal or the like can be imbedded in the
cementitious material to increase the visibility of the band for fluoroscopic
observation. The proximal band 274 may be formed of a hypotube sized to
permit the tube to slide along the guidewire during deployment. The inventors
of
2o that prior application suggest that the hypotube be made of a metallic
material; a
thin-walled tube of a NiTi alloy should suffice. If so desired, the proximal
band
may be formed of a more radiopaque metal, or a NiTi alloy band can have a
radiopaque coating applied to its surface.
As taught in some detail in WO 96/01591, the basket 270 taught therein
is formed of a metal fabric. The metal fabric of this embodiment is optimally
initially formed as a tubular braid and the ends of the wires forming the
braid can
be attached together by means of the bands 272, 274 before the fabric is cut
to
length. These bands 272, 274 will help prevent the metal fabric from
unraveling
during the forming process. (The method of forming the basket 270 is described
3o in great detail in WO 96/01591 and this process is still believed to
provide a
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
4
suitable means for creating such a basket. The process is also discussed
briefly
below in connection with Figure 6.)
When the device is in its collapsed state for deployment in a patient's
vessel (as illustrated in Figure 1A), the basket 270 of this device is said to
be
collapsed toward the axis of the guidewire 260. The distal 272 and proximal
274
bands are spaced away from one another along the length of the guidewire, with
the fabric of the device extending therebetween. This publication teaches it
is
preferred that the basket is in its collapsed engages the outer surface of the
guidewire to permit the device to be deployed through a relatively small lumen
of
To a catheter or another medical device.
When the device is deployed in a patient's vascular system, the basket
will take on an expanded configuration wherein it extends outwardly of the
outer
surface of the guidewire. As best seen in Figure 1 B, the shape of the basket
270 when deployed may generally resemble a conventional umbrella or
parachute, having a dome-like structure curving radially outwardly from the
guidewire moving proximally from the distal band 272. It is to be understood
that other suitable shapes could easily perform the desired filtering
function,
such as a conical shape wherein the slope of the device changes more linearly
than the smooth, rounded version shown in Figure 1 B. A relatively flat, disc
2o shape may also suffice, but it is preferred that the device have a cavity
or recess
(discussed below) to better retain emboli or other material captured thereby.
In
this expanded configuration, the two bands 272, 274 are closer together, with
the distal band 272 optimally being spaced only a short distance from the
proximal band 274, as illustrated.
In moving from its collapsed state (Figure 1A) to its expanded state
(Figure 1 B), the metal fabric of this device turns in on itself, with a
proximal
portion 282 of the collapsed basket being received within the interior of a
distal
portion 284 of the collapsed basket. This produces a two-layered structure
having a proximal lip 286 spaced radially outwardly of the guidewire, defining
a
3o proximally-facing cup-shaped cavity 288 of the basket. When blood (or any
other fluid) flows through the basket in a distal direction, any particulate
matter in
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
the blood, e.g. emboli released into the bloodstream during atherectomy or
angioplasty procedures, will tend to be trapped in the cavity 288 of the
basket.
WO 96/01591 teaches that the precise dimensions of the metal fabric can
be varied as desired for various applications. If the device 250 is to be used
as
s a vascular filter to trap emboli released into the blood, for example, this
reference teaches that the pores (i.e. the openings between the crossing metal
strands) of the fabric are desirably on the order of about 1.0 mm. These
inventors deemed this to be the minimum size of any particles which are likely
to
cause any adverse side effects if they are allowed to float freely within a
blood
1o vessel. They teach that the pores should not be too small, though, because
the
blood (or other fluid) should be free to pass through the wall of the basket
270.
If so desired, the basket may be coated with a suitable anti-thrombogenic
coating to prevent the basket from occluding a blood vessel in which it is
deployed.
When a fabric having 1.0 mm pores is used to form this basket 270, the
forming process reorients the wires relative to one another and in some areas
(e.g. adjacent the proximal lip 286) the pores tend to be larger than 1.0 mm.
However, because the basket's walls are formed of essentially two thicknesses
282, 284 of the fabric, the effective pore size of the device may be
significantly
2o reduced even at these locations.
The device 250 of Figures 11 is also provided with tethers 290 for
collapsing the basket 270 during retraction. The basket may include four
independent tether wires, each of which extends proximally from the proximal
lip
286 of the deployed basket. The authors suggested, though, that the four
tether
wires illustrated in the drawings be formed of two longer wires, with each
wire
extending peripherally about a portion of the proximal lip of the basket.
These
tether wires may be intertwined with the wires of the metal fabric to keep the
tethers in place during use. When such tethers are retracted or drawn down
toward the guidewire, the wires extending along the proximal lip of the basket
3o will tend to act as drawstrings, drawing the proximal end of the basket
radially
inwardly toward the guidewire. This tends to close the basket and entrap any
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
6
material caught in the cavity 288 of the basket during use so that the basket
can
be retracted without the use of a cover.
The tether wires 290 may extend along much of the length of the
guidewire so that they will extend outside the patient's body during use of
the
device 250. When it is desired to collapse the basket for retrieval, the
operator
can simply hold the guidewire 260 steady and retract the tethers with respect
to
the guidewire. This can tend to be relatively cumbersome, though, and may be
too difficult to effectively accomplish without breaking the tethers if the
device is
deployed at a selective site reached by a tortuous path, such as in the brain.
1o To address this issue, the authors suggest, as shown in Figures 1A and
1 B, that the tethers 290 be attached to the guidewire 260 at a position
spaced
proximally of the basket. The tethers may, for example, be attached to a metal
strap 292 or the like and this strap 292 may be affixed to the shaft of the
guidewire. When it is desired to close the aroximal end of the basket fnr
retraction, they suggest urging an external catheter (not shown) distally
toward
the basket 270. When the catheter encounters the radially extending tethers,
the distal end of the catheter will tend to draw the tethers toward the
guidewire
as the catheter is advanced, which will, in turn, tend to draw the proximal
end of
the basket closed.
2o Figures 2A and 2B illustrate an alternative embodiment of the device
shown in Figures 1 A and 1 B, also in accordance with the teachings of WO
96/01591. Figure 2A shows the device collapsed in a catheter C for deployment
while Figure 2B shows the device in its deployed configuration. In Figures 2A
and 2B, the basket 270 is much the same as that outlined above in connection
with Figures 1A and 1B. In the embodiment of Figures 12, though, the distal
band 272 is affixed to the guidewire 260' at the distal tip of the guidewire.
The
guidewire 260' is of the type referred to in the art as a "movable core"
guidewire.
In such guidewires, a core wire 265 is received within the lumen of a
helically
wound wire coil 266 and the core wire 265 extends distally beyond the distal
end
of the coil 266. A thin, elongate safety wire 268 may extend along the entire
lumen of the coil 266 and the distal end of the safety wire may be attached to
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
7
the distal end of the coil to prevent loss of a segment of the coil if the
coil should
break.
In the embodiment of Figures 1 A and 1 B, the proximal ends of the
tethers 290 are attached to a metal strap 292 which is itself attached the
shaft of
the guidewire 260. In Figures 2A and 2B, the tethers are not attached to the
core wire 265 itself. Instead, the tethers are attached to the coil 266 of the
guidewire. The tethers may be attached to the coil by any suitable means, such
as by means of laser spot welding, soldering or brazing. The tethers 290 may
be attached to the coil 266 at virtually an spot along the length of the coil.
As
1o illustrated in these drawings, for example, the tethers may be attached to
the coil
adjacent the coil's distal end. However, if so desired the tethers may be
attached to the coil at a location space more proximally from the basket 270.
An external catheter such as that referred to in the discussion of Figures
1A, but not shown in that figure, is illustrated in Figures 2A and 2B. Once
the
basket 270 is deployed in a patient's vessel to substantially reach the
expanded
configuration shown in Figure 2B and the basket has performed its intended
filtration function, the external catheter C can be urged distally toward the
basket
270. As this catheter is urged forward, the tethers will tend to be drawn into
the
distal end of the catheter, which is substantially narrower than the proximal
lip
286 of the basket. This will tend to draw the tethers down toward the
guidewire
and help close the basket, as explained above.
Figures 3-5 illustrate yet another alternative embodiment of a vascular
trap in accordance with WO 96/01591. This vascular trap 300 includes a basket
320 received over a guidewire 310. In most respects, the basket 320 is
directly
analogous to the basket 270 illustrated in Figures 1-2. The basket 320
includes
a proximal band 322 and a distal band 324. As in the device of Figures 2A and
2B, the distal band may be attached to the guidewire adjacent its distal end.
If
so desired, though, a structure such as is shown in Figures 1 A and 1 B,
wherein
the guidewire extends distally beyond the basket, could instead be used.
As best seen in its collapsed state (shown in Figure 3), the basket
includes a distal segment 325 and a proximal segment 326, with the distal end
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
8
of the distal segment being attached to the distal band 324 and the proximal
end
of the proximal segment being attached to the proximal band 322. When the
basket 320 is in it expanded configuration (shown in Figure 4), the proximal
segment 326 is received within the distal segment 325, defining a proximal lip
s 328 at the proximal edge of the device. The wall of the basket thus formed
also
includes a cavity 329 for trapping solids entrained in a fluid, such as emboli
in a
patient's blood stream.
The basket 320 of Figures 3-5 is also shaped a little bit differently than
the basket 270 of the previous drawings. The primary difference between these
1o two baskets is that the basket 320 is a little bit shorter along its axis
that is the
basket 270. This different basket shape is simply intended to illustrate that
the
basket of a vascular trap in accordance with the invention can have any of a
wide variety of shapes and no particular significance should be attached to
the
slightly different shapes shown in the various drawings.
IS In the vascular traps 250 and 250' of Figures 1 and 2, respectively,
tethers were used to draw down the proximal end of the basket 270 to close the
basket for retraction. In the embodiment shown in Figures 3-5, though, the
trap
300 includes a basket cover 340 positioned proximally of the basket 320. The
basket cover may also be formed of a metallic tubular braid and is also
adapted
2o to be collapsed to lay generally along the outer surface of the guidewire
310.
The cover 340 is not directly affixed to the guidewire at any point, though,
but is
instead intended to be slidable along the guidewire. As best seen in Figures 3
and 4 wherein the cover is in its collapsed state, the cover 340 includes a
distal
hypotube 342 and a proximal control hypotube 344, with the distal hypotube
25 being attached to the distal end of the cover 340 and the proximal control
hypotube 344 being attached to the proximal end of the cover.
The cover 340 is shown in its deployed, expanded configuration in Figure
5. As shown in that figure, the cover has a similar structure to that of the
basket
320, but is oriented to be open distally rather that proximally, as is the
basket.
3o As best seen in Figures 3 and 4 wherein the cover is in its collapsed
state, the
cover has a distal segment 352 and a proximal segment 354. When the cover is
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
9
deployed by urging it distally out of the distal end of the deployment
catheter C,
the cover 340 will tend to resiliently return to its expanded configuration
and the
distal hypotube 342 will slide axially proximally along the guidewire toward
the
proximal control hypotube 344. This will invert the collapsed cover so that
the
distal section 352 is generally received within the proximal section 354,
defining
a distal lip 358 of the cover.
WO 96/01591 teaches that the proximal control hypotube 344 of this
cover may extend along a substantial portion of the length of the catheter 310
so
that it extends out of the patient's body when the device 300 is in place. By
1o grasping the control hypotube and moving it relative to the guidewire 310,
an
operator can control the position of the cover 340 with respect to the basket
320,
which is affixed to the guidewires. As explained in more detail below in
connection with the use of the device 300, once the basket has been deployed
and has been used to filter objects entrained in the fluid (e.g. emboli in
blood),
the cover 340 may be deployed and the trap may be drawn proximally toward
the cover by moving the guidewire proximally with respect to the control
hypotube 344.
The inner diameter of the distal lip 358 of the cover is desirably slightly
larger than the outer diameter of the proximal lip 328 of the basket. Hence,
2o when the basket is drawn proximally toward the cover it will be
substantially
enclosed therein. The cover will therefore tend to trap any emboli (not shown)
or
other particulate matter retained within the cavity 330 of the basket. A
retrieval
sheath S may then be urged distally to engage the outer surface of the cover
340. This will tend to cause the cover to collapse about the basket, tightly
engaging the outer surface of the basket. This somewhat collapsed structure
can then be withdrawn from the patient's channel and removed from the
patient's body. By enclosing the basket within the cover, the likelihood of
any
filtered debris within the basket being lost as the basket is retrieved will
be
substantially eliminated
3o Figure 6 illustrates the molding element 370 suggested in WO 96/01591
for use in making a basket 270. Although the basket 320 and cover 340 of the
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
trap 300 are shaped somewhat differently, an analogous molding element can
be used for these portions of the trap 300 as well by simply modifying some of
the dimensions of the molding element 370, but retaining the basic shape and
structure of the molding element. It also should be understood that the
molding
s element 370 is merely one possible molding element for forming a shape such
as that of the basket 270 and WO 96/01591 teaches a variety of different
molding elements and notes that other designs will be apparent to those
skilled
in the art.
The molding element 370 of Figure 6 has an outer molding section 372
10 defining a curved inner surface 374 and an inner molding section 376 having
an
outer surface 378 substantially the same shape as the curved inner surface 374
of the outer molding section. The inner molding section 376 should be sized to
be received within the outer molding section, with a piece of the metal fabric
(not
shown) being disposed between the inner and outer molding sections. In a
preferred embodiment, the inner surface 374 of the outer molding element and
the outer surface 378 of the inner molding section each include a recess (375
and 379, respectively) for receiving an end of the braid. The molding surface
of
this molding element 370, to which the fabric will generally conform, can be
considered to include both the inner surface 374 of the outer molding section
2o and the outer surface 378 of the inner molding section.
WO 96/01591 teaches that the two molding sections 372, 376 are spaced
apart from one another and a length of a tubular braid of metal fabric (not
shown
in Figure 6) is disposed between these molding sections. Optimally, one end of
the fabric is placed in the recess 375 of the outer molding section and the
other
end of the fabric is placed in the recess 379 in the inner molding section. As
noted above, the ends of the tubular fabric can be clamped prior to this
molding
process to limit the likelihood that the fabric will unravel. The inner and
outer
molding sections can then be urged generally toward one another. As the ends
of the wire approach one another, the tubular braid will tend to invert upon
itself
3o and a surface of the tubular braid will generally conform to either the
inner
surtace 374 of the outer molding section or the outer surface 378 of the inner
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
11
molding section, arriving at a shape analogous to that of the basket 270 of
the
traps 250, 250'. The two molding sections can them be locked in place with
respect to one another and the metal fabric may be heat treated to set the
wires
in this deformed configuration.
s This published international application also teaches how one may use
the traps 250, 250' and 300 taught therein. It suggests that these traps be
deployed for use in conjunction with another medical device and that they will
most frequently be retracted from the patient's body after use. WO 96/01591
uses a balloon angioplasty procedure and an atherectomy procedures as
contexts for illustrating a method of using such traps. In balloon
angioplasty,
balloon catheters having inflatable balloons at their ends are positioned
within a
blood vessel so that the balloon is positioned within a stenosis. These
balloons
are positioned by tracking the balloon catheter along a guidewire or the like;
the
balloons typically have a central bore therethrough. Once the balloon is
properly
positioned, it is inflated and urges radially outwardly against the stenosis.
This
will tend to squeeze the stenosis against the walls of the vessel, improving
patency of the vessel.
When the stenosis is treated in this fashion, though, there is a risk that
some debris will break free and enter the blood flowing through the vessel. If
left
2o unchecked, this embolus can drift downstream and embolize a distal portion
of
the vessel. Depending on where the embolus comes to rest, the embolization
can result in significant tissue or organ damage. In order to prevent, or at
least
substantially limit, such embolization, WO 96/01591 suggests the use of a
vascular trap 250, 250' or 300 of with the balloon catheter. The device should
be sized to permit it to be passed through the lumen of the particular balloon
catheter to be used in the angioplasty.
In one method taught in WO 96/01591, the trap is deployed first. The
basket (270 or 320) of the trap is guided to a position located downstream of
the
desired treatment site through an introduction catheter (e.g. the catheter C
in
3o Figures 12-15). The basket is then urged distally beyond the end of the
catheter, which permits the basket to resiliently substantially return to its
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
12
expanded configuration from its collapsed configuration within the catheter.
Once the trap is in place, the balloon catheter can be exchanged for the
introduction catheter, and the balloon catheter can track the guidewire (260
or
310) of the vascular trap. The balloon can then be positioned within the
stenosis
s and expanded, as outlined above. Once the angioplasty has been completed,
the balloon can be deflated again and withdrawn proximally out of the patient.
WO 96/01591 also explains that the balloon catheter can be used to
perform the same function as performed by the introduction catheter in the
preceding embodiment. In this embodiment, the balloon catheter is positioned
Zo in the patient's vessel so that the distal end of the balloon catheter is
located
downstream of the stenosis. The vascular trap (250, 250' or 300) of the
invention is then passed through the lumen of the balloon catheter and the
basket is urged out of the distal end of the catheter. The basket will
resiliently
substantially return to its preferred expanded configuration, whereupon the
15 balloon catheter can be retracted along the shaft of the device's guidewire
until
the balloon is properly positioned within the stenosis.
If so desired, the balloon catheter can instead be provided with a length
of standard catheter extending distally beyond the distal end of the balloon.
The
balloon can then be positioned within the stenosis and the basket can be urged
20 out of the distal end of the distal extension of the catheter. In such an
embodiment, the length of the distal extension of the catheter should be
sufficient to properly position the basket with respect to the balloon when
the
basket exits the distal end of the catheter. This will eliminate the need to
perform the separate step of retracting the balloon into position within the
25 stenosis after the basket is deployed. The balloon can then be expanded,
deflated and withdrawn as described above.
WO 96/01591 teaches that much the same procedure can be used to
deploy a vascular trap for use in an atherectomy procedure. In such
procedures, a cutting head is positioned at the distal end of an elongate,
hollow
30 shaft and the cutting head has a bore extending therethrough. The trap can
be
deployed in either of the methods outlined above, but it is anticipated that
in
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
13
most instances the first procedure will be used, i.e. the basket will be
deployed
with an introduction catheter, which will be removed so that the cutting
device
can be guided over the guidewire of the vascular trap. This publication also
stresses that the device 250, 250' and 300 could be used in other medical
procedures in other bodily channels besides a patient's vascular system.
Since the trap is positioned downstream of the stenosis, any debris
released during one of these procedures will tend to drift distally toward the
basket and be caught therein. In order to prevent any emboli from simply
floating past the trap, it is preferred that the proximal lip (288 or 328) of
the
To basket be at least as large as the lumen of the vessel. WO 96/01591
suggests
that the natural dimension of the proximal lip (i.e. where the basket has
fully
returned to its expanded configuration) be made somewhat greater than the
vessel's inner diameter so the basket will firmly engage the wall of the
vessel.
The method of retracting the basket will depend on which embodiment of
IS the vascular trap is used, namely whether or not the device includes a
cover
340. The device 250 or 250' of Figures 1 or 2, respectively, do not include
such
a cover. However, they do include tethers 290 which extend proximally from the
proximal lip 288 of the basket to an attachment to the guidewire. In either of
these embodiments, a retrieval catheter can be introduced over the guidewire
2o and urged distally toward the basket. As explained above in connection with
Figures 1 and 2, this will tend to draw the tethers down toward the guidewire,
effectively closing the proximal end of the basket 270. Once the basket is
sufficiently closed, such as when the proximal lip of the basket engages the
distal tip of the retrieval catheter, the catheter and the vascular trap can
be
25 retracted together from the patient's body. By substantially closing the
proximal
end of the basket in such a fashion, any emboli which are captured in the
basket
when it is deployed can be retained within the basket until it is removed from
the
patient's body.
If so desired, a balloon catheter or like device can instead be used, with
3o the balloon catheter being used to draw down the tethers 290 and collapse
the
basket. The vascular trap can then be withdrawn with the balloon catheter
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
14
rather than having to separately introduce a removal catheter to remove the
trap.
In withdrawing the embodiment illustrated in Figures 3-5, the cover 340 is
positioned over the proximal lip of the basket before the vascular trap 300 is
retracted. Once the medical procedure is completed and any debris has been
captured in the basket, the cover 340 is allowed to resiliently substantially
return
to its expanded configuration. Once it is deployed proximally of the basket,
the
basket 320 can be drawn proximally toward the cover 340 until it engages or is
received within the cover, as noted above in connection with Figure 5.
In actuality, the cover 340 of Figures 3-5 may be unable to return to its full
expanded configuration due to the confines of the vessel in which it is
deployed.
As explained previously, the cover 340 is desirably larger than the basket 320
so
that the basket can be received within the cover. However, the basket is
optimally sized to engage the walls of the vessel to prevent the unwanted
passage of emboli or other debris around the edges of the basket. Accordingly,
the distal lip 358 of the cover will engage the wall of the channel before it
expands to its full size. The walls of most bodily channels, such as blood
vessels, tend to be somewhat elastic, though. The cover 340 will 'therefore
tend
to urge harder against the wall of the vessel than the smaller basket and may
2o stretch the vessel a little bit more than will the basket. In this fashion,
the cover
may still be able to expand to a dimension large enough to permit the basket
to
be received in the cavity 356 of the cover. If not, the distal lip 358 of the
cover
can simply be brought into close engagement with the proximal lip 328 of the
basket to generally seal the basket.
Once the cover 340 is brought into engagement with the basket 320,
whether by receiving the basket within the cover or, less preferably, by
engaging
the lips 358, 328 of the cover and the basket, the device can be withdrawn
proximally from the patient's vascular system. The cover will tend to prevent
any
emboli caught in the basket during deployment from being inadvertently lost
3o during withdrawal.
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
The vascular traps 250, 250' and 300 shown in Figures 1-6 represent a
significant advancement over previously available devices. The embodiment of
Figures 3-5 shows particular promise in that the cover permits the user to
withdraw the basket with the emboli entrained therein without having to take
any
5 additional precautions to minimize the chances that these emboli will be
accidentally dumped back into the bloodstream.
SUMMARY OF THE INVENTION
The present invention provides a medical device retrieval system and a
method of retrieving a medical device. In accordance with one embodiment of
1o the invention, a medical device retrieval system includes a medical device
and a
retrieval cover. The medical device comprises a working element carried by a
flexible, elongate shaft. The working element has a proximal profile and the
shaft extends proximally from the working element. The retrieval cover is
slidably carried along the shaft of the medical device. The cover has a
deployed
15 configuration and is capable of being compressed in a compressed
configuration
for deployment, yet resiliently substantially returned to the deployed
configuration. The cover in its deployed configuration has a radially reduced
proximal portion. A distally open distal end defining a distal opening having
a
maximum dimension at least as great as the maximum dimension on the
2o proximal profile of the working element of the medical device, and an
elongate
internal recess defined between the proximal portion on the distal end. The
cover in its compressed configuration is radially compressed inwardly toward
the
shaft and is distally open, with the distal end defining the distal-most
portion of
the cover. Optimally, the retrieval cover is designed to maintain this general
orientation wherein the distal end of the device is always the distal-most
portion
of the cover, regardless of the configuration of the device.
This medical device retrieval system may further include a retrieval
sheath which is slidable along the shaft of the medical device. Such a sheath,
if
included, is desirably positioned distally on the cover when the cover is in
its
3o deployed configuration. This retrieval sheath may have an inner diameter
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
16
smaller than the outer diameter of the cover in its deployed configuration.
This
sheath is adapted to slide distally along the cover to compress the cover
about
the medical device.
In accordance with a further embodiment, the present invention provides
s a medical device retrieval system which comprises a medical device, a
retrieval
sheath, a deployment stylet and a retrieval cover. The medical device
comprises a working element carried by a flexible, elongate shaft having an
outer diameter. The working element has a proximal profile and the shaft
extends proximally from the working element. The retrieval sheath is slidable
1o along the shaft of the medical device and optimally has a beveled distal
end with
a distal lumen. The deployment stylet is slidable along the shaft of the
medical
device and has a distal tip. This distal tip tapers distally from a first
diameter
approximating the diameter of the distal lumen of the sheath to a second
diameter more closely approximating the outer diameter of the medical device
15 shaft. This provides a transition between the shaft of the medical device
and the
distal end of the retrieval sheath when the deployment stylet is positioned
such
that a distal tip extends distally beyond the distal end of the retrieval
sheath.
The retrieval cover is slidable along the shaft of the medical device and is
exchangeable for the stylet along that shaft. The cover has a deployed
2o configuration and is capable of being compressed into a compressed
configuration for sliding within the lumen of the retrieval sheath yet
resiliently
substantially return to the deployed configuration. In its deployed
configuration,
the cover has a radially reduced proximal portion, a distally open distal end,
and
an elongate internal recess defined between the proximal portion and the
distal
25 end. The distal end defines a distal opening having a maximum dimension at
least as great as the maximum dimension of the proximal profile of the working
element of the medical device. In its compressed configuration, the cover is
radially compressed inwardly toward the shaft and is distally open, with the
distal
end defining the distal-most portion of the cover.
3o Another embodiment of the invention provides a retractable medical
device system including a medical device, a retrieval cover and a retrieval
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
17
sheath. The medical device comprises a working element carried by a flexible,
elongate shaft. The retrieval cover is slidable along the shaft of the medical
device. The cover has a radially reduced proximal portion, a distally open
distal
end an elongate tubular wall extending therebetween and defining a recess.
s The working element of the medical device is completely retained within the
recess of the cover such that the tubular wall extends distally beyond the
medical device. The retrieval sheath has a lumen and is slidable with respect
to
both the medical device and the cover. At least a proximal length of the
working
element of the medical device and the cover are retained within the lumen of
the
retrieval sheath, with the retrieval sheath regularly compressing the proximal
length of the cover such that an intermediate portion of the wall tightly
engages
the surface of the medical device. This will tend to effectively trap any
emboli or
other materials retained by the medical device.
As noted above, the present invention contemplates a method. One such
method involves receiving particulate or other form material within a channel
of a
patient's body. As a first step in performing this method, one provides a
medical
device having a working element and a flexible, elongate shaft adapted to
follow
a path within the channel; a distally open cover slidable with respect to the
shaft;
and a retrieval sheath movable with respect to the cover on the shaft. The
2o medical device is positioned within the vessel to engage a wall of the
channel
and trap the material within the channel. Either during such positioning or
after
the medical device has been positioned and while it is trapping material
within
the channel, the cover and the retrieval sheath may be positioned so they are
spaced proximally of the working element along the shaft of the medical
device.
The cover is radially compressed within the lumen of the retrieval sheath such
that it has a distally open distal end and a wall defining a recess, the wall
engaging an inner surface of the retrieval sheath. The cover is moved distally
with respect to the retrieval sheath, thereby permitting the cover to radially
expand into a deployed configuration wherein the distal end remains distally
open and the enclosure is radially expanded. The cover expands radially
outwardly into the deployed configuration without having to invert on itself.
The
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
18
cover is then moved distally into engagement with a surface of the medical
device to form therebetween an enclosure. The retrieval sheath may then be
moved distally with respect to the cover to urge to cover to collapse about
the
medical device and tightly engage the surface of the medical device.
S BRIEF DESCRIPTION OF THE DRAWINGS
Figures 1A is a schematic side view in accordance with WO 96/01591,
showing a vascular trap in a collapsed state for deployment in a patient's
vascular system;
Figure 1B is a schematic side view of the medical device of Figure 1A in
1o an expanded state for deployment in a patient's vascular system;
Figure 2A is a schematic side view in accordance with WO 96/01591,
showing an alternative vascular trap in a collapsed state within a catheter
for
deployment;
Figure 2B is a schematic side view of the device of Figure 2A, showing
15 the device deployed distally of the catheter;
Figure 3 is a schematic perspective view in accordance with WO
96/01591 showing a vascular trap and a cover, both of which are collapsed
within a catheter for deployment in a channel in a patient's body;
Figure 4 is a schematic side view of the device of Figure 3 in a partially
2o deployed state, wherein the vascular trap has been deployed, but the cover
is
still collapsed within the catheter;
Figure 5 is a schematic side view of the device of Figure 3 in a fully
deployed state;
Figure 6 illustrates one embodiment of a molding element which may be
25 used in making a portion of the vascular traps shown in Figures 1-5;
Figure 7 is a schematic illustration of a retrieval sheath catching on a
vascular obstruction proximally of the desired distal deployment site;
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
19
Figure 8 is a schematic side view of a device in accordance with the
present invention with both the trap and the cover fully deployed;
Figure 9 is a schematic cross sectional view showing the device of Figure
8 wherein the trap has been deployed but the cover has retained within the
retrieval sheath;
Figure 10 is a schematic illustration showing the invention deployed within
a patient's vessel and having emboli retained therein;
Figure 11 is view similar to Figure 10, but showing the trap being
retracted into the confines of the cover;
1o Figure 12 is a schematic, partially cut away view of the device of Figures
and 11 showing the cover being retracted within the retrieval sheath;
Figure 13 is a schematic partial cross sectional view of a distal portion of
a medical device retrieval system of the invention utilizing a deployment
stylet;
and
Figure 14 is a schematic side view of a distal length of an alternative
retrieval sheath for use with the invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
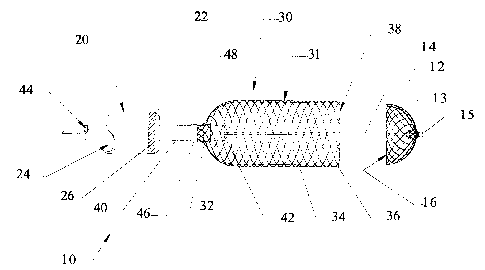
Figures 8-12 schematically illustrate the operation of one embodiment of
the present invention. Figure 8 illustrates certain operative portions of the
2o medical device retrieval system 10 of the invention in a fully deployed
state. As
noted above, the retrieval system of the invention is intended to be used in
connection with a medical device having a working element carried by flexible,
elongate shaft. In these drawings, the medical device is typified as a
vascular
trap similar to the vascular trap 250 of Figure 11, but omitting the tethers
290.
The working element of this medical device is a basket 12, which may be
substantially as outlined above in connection with the description of the
basket
270. The shaft in this design may simply comprise a guidewire 14. While the
construction and operation of the basket 12 may be substantially the same as
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
that outline for the baskets shown in Figures 1-5, it is generally preferred
that the
proximal band 13 of this basket be attached to the guidewire while the distal
band 15 be permitted to slide along the guidewire. Hence, when the basket 12
is released from a delivery catheter and the basket is allowed to achieve a
s radially expanded configuration, the distal end of the collapsed device (272
in
Figure 1A) will slide approximately toward the proximal end (274) of the
collapsed device.
It should be recognized that the medical device can be varied as desired.
For example, the medical device used in connection with the present retrieval
1o system could instead by a balloon catheter, wherein the working element
would
be the balloon portion of the catheter and the shaft would comprise the body
of
the catheter extending proximally of the balloon.
The other elements of the retrieval system 10 generally comprise a
retrieval sheath 20 and a cover 30. It is to be understood that these drawings
15 are intended merely for illustrative purposes and are not drawn to scale.
In
actual operation, the retrieval sheath 20 and the shaft 40 of the cover would
likely be much smaller. These elements are simply drawn larger to make the
various components easier to see in the attached illustrations.
The cover 30 includes a radially expandable body 31 carried by a shaft
20 40. The body has a proximal portion 32 which is radially compressed into
close
proximity with the shaft 40 and is desirably attached directly thereto. A
tubular
wall 34 extends distally from the proximal portion and terminates in a
distally
open end 36. The body 31 defines a recess 38 within which the working
element of the medical device may be retracted, as explained more fully below.
The majority of the length of this recess is defined by the generally tubular
wall
34.
This radially expandable body 31 can be formed of any suitable material.
As explained more fully below, it is preferred that this body be capable of
being
collapsed within the retrieval sheath 20 for deployment, radially expand into
a
3o deployed configuration, yet be readily collapsed by the retrieval sheath to
tightly
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
21
engage the working element of the medical device. Any material which achieve
this function may be used.
In one embodiment (not shown), the body 31 is formed of a flexible plastic
material, which may be reinforced with one or more flexible metal hoops or the
like to bias the tubular plastic member into a funnel-like configuration.
The illustrated embodiment is shown as comprising a series of flexible
metal wires. As explained in some detail in International Publication No.
WO 96/01591, such a radially expandable device may be made rather
conveniently utilizing a metal fabric having strands formed of a material
which is
1o both resilient and which can be heat treated to substantially set a desired
shape.
Materials such as elgiloy, hastelloy, incoloy, certain grades of stainless
steel and
shape memory alloys. Of these materials, shape memory alloy such as nitinol
are particularly preferred.
In one useful embodiment, the radially expandable body 31 is formed
using the techniques outlined in WO 96/01591, starting with a metal fabric
comprising both nitinol and platinum. For example, the fabric may be a
generally tubular fabric formed of 48 wires having a diameter on the order of
about 0.0015 inches and a pic rate of about 80-100 pics per inch. Of the 48
wires used to form this metal fabric, a relatively small percentage of the
wires
(e.g. 4-6 wires) may be formed of platinum or some other relatively radiopaque
material to enhance visibility of the device on a fluoroscope without unduly
affecting the resiliency of the fabric. If so desired, the wires can be coated
with a
therapeutic agent or with an antithrombogenic material. For example, the wires
may be coated with heparin or with a known platelet-deactivating drug, e.g., a
2s 2B-3A antagonist.
This radially expandable body 31 is carried by a axially slidable shaft 40.
This shaft may take the form of a metallic hypotube, such as that discussed in
connection with the embodiment of Figures 3-5. More preferably, though, the
shaft 40 comprises a flexible plastic material of the type that is commonly
used
3o in forming medical catheters. If friction of this shaft 40 with the
retrieval sheath
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
22
20 and/or the shaft 14 of the medical device is anticipated to present a
problem,
this shaft 40 of the cover may be formed of polytetrafluoroethylene or another
suitable low-friction material.
The radially expandable body 31 may be attached to the shaft 40 in any
s suitable manner. Presumably, the ends of the wires defining the body 31
could
be simply cast into the plastic defining the flexible shaft 40. However, the
embodiment shown in the drawings is somewhat easier to make, utilizing a pair
of marker bands 46 and 48 to attach the body to the shaft by clamping the
proximal end about the exterior of the sheath. Forming these clamps of a
1o radiopaque material will make it easier to track the position of the cover
30 as it
is deployed. In the illustrated embodiment, the cover comprises an exterior
layer
and an interior layer of the metal fabric, much like the basket 270 described
above in connection with Figures 1-5. In this configuration, the proximal
marker
band 46 may be used to clamp the exterior layer of the metal fabric to the
15 exterior of the shaft 40 while the distal marker band 48 is used to clamp
the
interior layer of the fabric to the shaft.
In the illustrated embodiment, the shaft 40 includes a lumen 44 through
which the shaft 14 of the medical device is received, thereby permitting the
cover 30 to track that shaft for deployment. The shaft 40 shown in Figures 8
2o and 9 extends distally beyond the distal marker band 48 such that the
distal tip
42 of the shaft is received within the recess 38 of the cover. Not only will
this
make manufacturing easier, but it will reduce the likelihood that any
guidewire or
other device passing through the lumen 44 of the shaft 40 will get caught up
in
the metal fabric defining the radially expandable body 31.
25 The retrieval sheath 20 may simply take the form of a standard medical
catheter, with a tip as described below. This sheath has a generally tubular
wall
defining a lumen 24 within which the shaft 14 of the medical device and the
shaft
40 of the cover may be slidably received. The differences in the diameters of
these three elements 20, 40 and 14 are exaggerated in Figures 8 and 9 to
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
23
illustrate operation of the device. In reality, these diameters would likely
be
substantially closer than those shown.
The distal tip 22 of the retrieval sheath 20 may be beveled to produce a
smoother tip. (The advantage of this tip construction will be highlighted
below in
connection with the discussion of Figures 7, 13 and 14.) If so desired, a
marker
band 26 may be incorporated into the wall of the retrieval sheath 20 adjacent
the
distal tip 22. This will help an operator visualize the relative position of
the
retrieval sheath 20, the cover 30 and the basket 12 during operation.
Figure 9 is a schematic cross sectional view of the device illustrated in
Figure 8 prior to deployment of the cover. In operation, the medical device
will
typically be put in place first. As outlined above in connection with Figures
1 and
2, the basket 12 may be positioned distally of a particular treatment site and
the
treatment device (e.g. a balloon catheter or an atherectomy device) can be
guided over the shaft 14 of the trap to perform the intended procedure. In the
use of the retrieval system of the invention with such a trap, one would
typically
deploy the retrieval sheath 20 and the cover 30 after the basket 12 has been
in
place for some time rather than deploying all three elements at substantially
the
same time. It should be understood, though, that simultaneous deployment may
be appropriate in other circumstances, such as when a cover 30 and retrieval
2o sheath 20 are used in connection with a Foley catheter or the like.
Whereas Figure 8 illustrates the cover in its deployed configuration,
Figure 9 illustrates the cover in a compressed configuration which is suitable
for
deployment. Even in its compressed configuration, it can be seen that the body
31 of the catheter generally includes a radially reduced proximal portion 32,
an
elongate tubular wall 34 and a distally open distal end 36 which defines the
distal-most portion of the cover. This is indirect contrast to the structure
shown
in Figures 3 and 4, which show the cover 340 of that device in its collapsed
state. In this collapsed state, the cover 340 has a distal segment 352 and a
proximal segment 354, both of which are generally tubular in shape and lie
proximate the exterior surface of the guide wire 310. Once this cover is
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
24
deployed as shown in Figure 5, though, the cover must invert on itself to
position
the distal section 352 generally within the proximal section 354 to define a
distal
lip 358 of the cover. This distal lip 358 is merely an intermediate point
along the
longer, axially expanded configuration of the device when it is collapsed, as
s shown in Figures 3 and 4.
There are a number of advantages of the structure of the present cover
30 over the mechanically more complex design of Figures 3-5. In the cover 340
of Figures 3-5, the cover must invert on itself before it can be used to
enclose
the basket 320. The resilient nature of the metal fabric used to form the
cover
340 will tend to resiliently draw the distal hypotube 342 proximally toward
the
proximal control hypotube 344 once the constraint of the deployment catheter C
has been removed.
The walls of the vessel can hinder complete inversion of the cover 340,
though. In particular, if the inner diameter of the vessel within which the
cover is
IS to be deployed is significantly smaller than the outer diameter of the
fully
deployed cover, the cover may take on a sausage-like configuration, with the
distal and proximal segments 352, 354 of the cover expanding into engagement
with the wall of the vessel, but being unable to expand sufficiently to allow
the
distal hypotube to invert the distal segment 352 so that it may be received
within
2o the proximal section 354. In such a circumstance, the cover will not define
a
suitable recess for receiving the basket 320 therein.
The design shown in Figures 8-12 does not require that the radially
expandable body 31 invert on itself to reach its fully deployed configuration.
Instead, the recess 38 will always remain in place. Deployment of the body 31
25 distally beyond the distal tip 22 of the retrieval sheath will simply allow
this
recess to expand to a size wherein it may readily receive the working element
of
the medical device with which the cover is used.
While Figures 8 and 9 schematically illustrate the structure of the device
and its various elements, Figures 10-12 are intended to schematically
illustrate
3o the manner in which the cover 30 may be used to retrieve a basket 12 which
is
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
full of emboli or other particular material. In Figure 10, the deployment
catheter
C (discussed above in connection with Figures 1-5) is shown extending into the
lumen of the vessel and terminating proximally of the position of the basket
12.
The cavity of the basket 12 is filled with emboli E. If one were to simply
pull the
s guidewire 12 proximally, this will tend to evert the generally umbrella-
shaped
basket 12, raising the possibility that the emboli E could be dumped into the
bloodstream of the vessel.
In Figure 10, the retrieval sheath 20 is positioned proximally of the basket
12, leaving a space between the distal tip 22 of the sheath 20 and the basket
10 12. In this Figure, the cover 30 is still within the lumen 24 of the
retrieval sheath
20, much as in the configuration shown in cross section in Figure 9.
Once the retrieval sheath, with the cover retained therein, is properly
positioned, the shaft 40 of the cover 30 may be advanced distally with respect
to
the sheath 20. This may be accomplished either by holding the sheath 20
15 stationary and advancing the shaft 40 of the cover distally or by holding
the shaft
40 of the cover relatively stationary and withdrawing the retrieval sheath 20
proximally to expose the readily expandable body 31 beyond the distal tip 22
of
the sheath 20.
When the body 31 of the cover exits the distal end of the retrieval sheath
20 20, it will tend to resiliently substantially return to the configuration
schematically
illustrated in Figure 8. Unlike the cover 340 of Figures 3-5, the body 31 of
the
present invention will begin to radially expand into its final shape as soon
as the
distal end 36 clears the distal tip 22 of the sheath 20. Accordingly, there is
no
need to deploy the cover 30 so that even the proximal marker band 46 is
2s positioned distally of the distal tip 22 of the retrieval sheath as shown
in Figure
8. Instead, the proximal portion 32 of the body 31 may remain within the lumen
of the retrieval sheath 20, as suggested in Figure 11, without compromising
operation of the cover 30.
Figure 11 illustrates the device wherein the 'cover has been sufficiently
deployed to define a recess large enough to receive the body of the basket 12
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
26
therein. To achieve the configuration shown in Figure 11, the shaft 14 of the
vascular trap is withdrawn proximally, drawing the basket 12 within the
enclosure 38.
As noted above in connection with Figure 8, the presently preferred
s embodiment of such vascular trap employs a proximal band 13 which attaches a
proximal end of the metal fabric defining the basket directly to the shaft of
the
guidewire 14 while the distal connector 15 is allowed to slide along the
length of
the shaft 14. Accordingly, when the operator pulls proximally on the guidewire
14, this will tend to elongate the trap and cause it to evert. In the absence
of
1o aspiration or a cover 30, this could present some difficulties.
Prior to withdrawing the shaft 14 proximally, the distal end 36 of the cover
is desirally brought immediately adjacent the basket 12. In a preferred
embodiment, the distal end 36 of the body 31 of the cover defines a distal
opening having a maximum dimension which is at least as great as the
IS maximum dimension of the proximal profile of the basket 12, i.e., the
maximum
dimension of the proximal projection of the deployed basket. If the vessel is
large enough, this would permit the cover to simply slide around the basket 12
without significantly stressing the basket and causing it to collapse in any
way.
More likely than not, though, there will be insufficient clearance between the
2o basket 12 and the wall of the vessel to permit the cover to readily slide
between
the vessel and the basket. Accordingly, the distal end of the cover will
typically
be brought into engagement with a surface of the basket 12. This will form
between the cover and the basket and enclosure that includes both the cavity
of
the basket and the recess 38 of the cover. This movement of the cover distally
25 into engagement with the medical device may be achieved either by actually
physically moving the cover distally in an absolute sense, or simply
withdrawing
the basket 12 toward the cover which will effectively move the cover distally
with
respect to the medical device.
Figure 11 illustrates the relative positions of the elements of the invention
3o if the operator continues to withdraw the guidewire 14 proximally after the
cover
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
27
initially engages the surface of the basket 12. The basket has started to
evert
into a more oblong shape rather than the umbrella-shape shown in Figure 10.
Nonetheless, the emboli still are retained within the enclosure defined by the
cover and the basket.
In one preferred embodiment, the body 31 of the cover is at least as long
as the working element of the medical device which is to be retrieved
therewith.
This permits the working element to be entirely enclosed by the cover during
the
retrieval process, enhancing the likelihood of a successful retrieval without
inadvertent dumping of the matter captured by the medical device back into the
patient's body. While the cover can be little longer than the working element
of
the medical device, it is anticipated that the cover may be significantly
longer
than that working element. This will permit an operator greater flexibility in
using
the device without adding unduly to the cost.
Figure 12 schematically illustrates the next stage of the method of
IS removing the medical device from the patient's vascular system. In this
view,
the retrieval sheath has been moved distally with respect to the cover. As
suggested above, this may be achieve either by moving the retrieval sheath
distally along the cover or by withdrawing the cover (and, optimally, the
medical
device) proximally while holding the retrieval sheath 20 stationary. Urging
the
retrieval sheath distally with respect to the cover urges the cover to
collapse
about the medical device received therein. This causes the cover to tightly
engage the surface of the medical device, helping better encase any particular
matter received within the enclosure and limit the likelihood that it may
spill back
into the patient's vascular system. It also presents the device with a
radially
reduced profile, making it easier to withdraw the device from the patient's
body
without undue trauma.
Looking at the device in Figure 12, the system has a particular
configuration which is unique to the present invention. In this configuration,
the
working element of the medical device is completely retained within the recess
38 of the body 31 of the cover such that the distal end 36 of the cover 30 is
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
28
positioned distally beyond the distal end of the working element 12. In Figure
12, at least a proximal length of the basket 12 and the body 31 of the cover
are
retained within the lumen of the retrieval sheath 20. This retrieval sheath
radially
compresses the proximal length of the cover such that an intermediate portion
of
s the generally tubular wall 34 of the body 31 tightly engages a surface of
the
basket 12.
If so desired, the cover 30 and basket 12 may be further retracted so that
they are both completely enclosed within the lumen of the retrieval sheath 20
prior to withdrawing the device from the patient's vessel. This is not
necessary
to for effective operation of the current device, though, and may be left up
to the
physician's choice during the procedure. It should also be noted that the
configuration shown in Figure 12 may be further collapsed by withdrawing the
basket 12, cover 30 and retrieval sheath 20 proximally into the deployment
catheter C, thereby further encasing the emboli and making it easier to
withdraw
1s the device from the vascular system.
Figure 7 illustrates one problem which could be encountered in deploying
a medical device retrieval system 10 of the invention across a vascular
obstruction. The vascular obstruction in Figure 7 is typified as a stent 4
having a
stenotic lesion 6 partially occluding the lumen thereof, but this is selected
merely
2o for illustration. Much the same problem could also be encountered with a
variety
of other vascular obstructions.
The illustrated deployment sheath 20 has a blunt distal tip 22'. Due to
the curvature of the vessel where the stent is located, the retrieval sheath
tends
to drift upwardly toward the outside of the curve rather than easily tracking
the
25 shaft 14 of the medical device through the center of the vessel. This
problem
becomes even more pronounced if the retrieval sheath is made stiffer, such as
by incorporating metallic braid into the wall of the sheath, to improve
pushability.
In some instances, it can take undue time and effort to manipulate the distal
tip
of the retrieval sheath to clear the obstruction. In addition, use of excess
force
30 or movement of the sheath to clear the obstruction risks displacing the
working
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
29
element (not shown) of the medical device from the treatment site where it has
been deployed.
Figures 13 and 14 illustrate two proposed solutions to ameliorate these
deployment difficulties. A first solution is illustrated in Figure 13 while
Figure 14
s illustrates another improvement which may be used alone or in conjunction
with
the device of Figure 13.
Turning first to Figure 13, the retrieval sheath 20 shown therein includes a
deployment stylet 70 slidably received in the lumen 24 thereof. This stylet
has a
lumen 75 within which the shaft 14 of the medical device is received,
permitting
1o the stylet to slide along that shaft 15 with the retrieval sheath 20. The
stylet 70
is provided with an elongate tubular body 72 and a tapering distal tip 74. In
use,
the body 72 of the stylet desirably extends along the entire length of the
retrieval
sheath so that the proximal end of the sheath (not shown) extends proximally
beyond the proximal end of the retrieval sheath so an operator may selectively
15 control the stylet independently of the guide wire and of the retrieval
sheath.
The distal tip 74 of the stylet tapers from its proximal end 76 to its distal
end 78. At its proximal end, the distal tip has an outer diameter which
approximates the diameter of the lumen 24 of the retrieval sheath at the
distal
end 22 thereof. As illustrated, it is not intended that the stylet 70
completely fill
2o the lumen 24 of the sheath as that would lead to undue friction in moving
the
stylet relative to the sheath. The outer diameter of the sheath at the
proximal
end 76 of the tip 74 need only be close enough to the diameter of the distal
lumen of the sheath 20 to avoid a sharp, traumatic change in diameter which
would be likely to catch on vascular obstructions and hinder deployment of the
25 sheath 20 in the vessel. The transition from the distal tip 74 of the
stylet to the
outer diameter of the sheath 20 can be further eased by proving the distal tip
22
of the sheath 20 with a beveled distal end.
The distal end 78 of the stylet's distal tip 74 has an outer diameter which
more closely approximates the outer diameter of the medical device shaft 14.
It
3o is not expected that this distal end 78 be infinitely thin and track
directly against
the surface of the shaft 14. Again, it is sufficient that the distal end 78 of
the
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
stylet be close enough to the diameter of the shaft 14 of the medical device
to
avoid a sharp, traumatic change in diameter which would be likely to catch on
vascular obstructions and hinder deployment of the sheath 20 in the vessel.
When the stylet is deployed such that its distal tip 74 extends distally
s beyond the distal tip 22 of the retrieval sheath, the stylet provides a
transition
between the shaft 14 of the medical device and the distal end of the retrieval
sheath 20. This makes it easier to track the shaft 14 and guide the device
into
position across a vascular obstruction. Figure 13 illustrates the stylet
positioned
such that the proximal end 76 of the distal tip 74 is positioned immediately
to adjacent the distal tip 22 of the retrieval sheath, but this is not
necessary. If the
body 72 of the stylet has a substantially constant diameter over the relevant
length, the stylet can be moved distally relative to the sheath 20 such that
the
body extends beyond the distal end of the sheath. This will not case any undue
problem as the outer diameter of the body is desirably substantially the same
as
15 the outer diameter of the proximal end 76 of the distal tip.
Use of the retrieval sheath 20 with the stylet 70 can be varied. If so
desired, one can use the stylet in each and every deployment of the retrieval
system of the invention. However, as outlined below, use of the stylet adds an
additional step to the retrieval process and its use may be reserved for those
2o circumstances where the operator either expects to encounter a vascular
obstruction or has already encountered such an obstruction.
In use, the stylet 70 and the cover 30 are exchangeable for one another,
i.e., either the stylet or the cover may track along the shaft 14 within the
lumen
24 of the retrieval sheath, but both cannot be used at the same time. Instead,
25 one must be removed and replaced with the other. If the operator
anticipates a
vascular obstruction (or he or she wants to avoid exchanging devices twice if
an
obstruction is encountered), he or she can initially deploy the sheath 20 with
the
stylet. This may be accomplished by positioning the stylet 70 with respect to
the
sheath 20 such that the distal tip 74 of the stylet extends distally beyond
the
3o distal tip 22 of the sheath. Optimally, both the stylet and the sheath are
advanced together along the shaft 14 until the distal tip 22 of the sheath is
in a
CA 02364136 2001-09-04
WO 00/53120 PCT/US00/06212
31
desired position with respect to the working element of the medical device.
(In
most circumstances, this will be at a location wherein the distal tip of the
sheath
is near the working element, but spaced proximally therefrom, as discused
above in connection with Figure 11.)
s Once the sheath is in position, the stylet 70 may be exchanged for the
cover 30. This may be done in much the same fashion that catheters are
exchanged in a typical balloon angioplasty procedure or the like. In most
circumstances, an exchange wire will be attached to the proximal end of the
shaft 14 of the medical device and the stylet 70 can be retracted proximally
onto
1o the exchange wire. Thereafter, the exchange wire can be disconnected and
the
cover may be advanced along the shaft 14 through the lumen 24 of the retrieval
sheath. Using the marker band 26 of the retrieval sheath and the marker band
13 of the basket 12 (for example), any final adjustments to the position of
the
sheath with respect to the working element of the medical device can be made
TS prior to deployment of the cover.
The cover may then be moved distally with respect to the sheath 20,
either by distally advancing the cover or proximally retracting the sheath. As
noted above, this permits the body 31 of the cover to radially expand into a
deployed configuration wherein the distal end remains distally open and the
2o enclosure is radially expanded. The cover may then be moved distally with
respect to the working element of the medical device and into engagement with
a surface of the medical device to form therebetween an enclosure. Optimally
(but not necessarily, depending on the configuration of the medical device and
the shape of the cover), the cover is advanced further with respect to the
25 working element until the entire working element is effectively received in
the
recess 38 of the cover. Thereafter, the retrieval sheath is moved distally
with
respect to the cover to urge the cover to collapse about the working element
and
tightly engage the surface of the working element to retain any debris in the
enclosure.
3o Figure 14 illustrates another improvement of the sheath 20 of the
invention. In this embodiment, a distal length 21 of the sheath 20 is bent at
an
CA 02364136 2001-09-04
WO 00/53120 PCT/CTS00/06212
32
angle with respect to the body of the sheath. If a vascular obstruction is
encountered, this distal bend will permit the operator to clear the
obstruction by
reorienting the sheath so that the distal tip 22 thereof is spaced toward the
center of the vessel and away from the obstruction, whereupon the sheath can
be further advanced. An angle of between about 5 and about 30° is
believed to
be sufficient for most purposes without unduly interfering with the proper
deployment and retrieval of the cover 30. The length of the distal length 21
can
be varied as needed. In most circumstances, it is envisioned that the distal
length 21 will be 5 cm or less, with a length of 1 cm to 3 cm being most
likely.
As noted previously, the sheath 20 of Figure 14 with its bent distal length 21
may be used instead of or in conjunction with the stylet 70 shown in Figure
13.
While a preferred embodiment of the present invention has been
described, it should be understood that various changes, adaptations and
modifications may be made therein without departing from the spirit of the
invention and the scope of the appended claims.