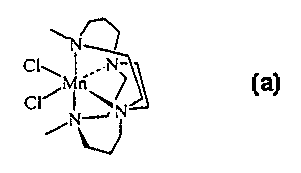Note: Descriptions are shown in the official language in which they were submitted.
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
STABILIZED BLEACH COMPOSITIONS
FIELD OF THE INVENTION
The present invention relates to stabilized bleaching and detergent
compositions which
comprise a catalytically effective amount of a transition-metal bleach
catalyst which is a complex
of a transition-metal and a cross-bridged macropolycyclic ligand and an anti-
oxidant or reducing
agent which serves to stabilize said catalyst. The present invention further
relates to a method for
bleaching/cleaning fabric with a catalytically effective amount of said
stabilized transition-metal
bleach catalyst wherein the method is performed substantially free of any
organic or inorganic
peroxygen compound or precursors to any organic or inorganic peroxygen
compound.
BACKGROUND OF THE INVENTION
Bleaching of fabric is essentially exposing soiled or stained fabric to a
chemical reaction
the purpose of which is to eliminate the soil or stain. At one point in time,
bleaching involved
exposure of fabric to a solution of hypochlorite. Therefore, fabric which was
colored or dyed via
sensitive pigments were excluded from treatment with bleach. To the benefit of
the consumer,
formulators developed various forms of bleach inter alia peroxygen bleaching
systems which
typically comprise a source of hydrogen peroxide and a bleach activator. This
combination of
source of hydrogen peroxide and activator plays a dominating role in
effective, safe bleaching
compositions. An effective example of this peroxygen bleaching system employs
perborate
(peroxygen source) and nonanoyloxybenzene sulfonate (activator).
In order to boost the performance of bleaching agents and to develop bleaching
systems
which are safe to any type of dyed or colored fabric inter alia silk,
polyester blends, cotton,
nylon, formulators have continued to develop peroxygen bleaching systems, as
well as new
methods of forming activated oxygen.
However, there still remains a need in the art for a stable bleaching system
which will
effectively bleach fabric without the need for reactive chemicals such as
peroxides, sources of
peroxide, and/or mixtures thereof. Said stable bleaching system will comprise
a transition-metal
catalyst which does not loose its effectiveness due to the instability of the
catalyst to oxidation
and/or reduction post formulation.
SUMMARY OF THE INVENTION
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
2
The present invention meets the aforementioned needs in that it has been
surprisingly
discovered that bleaching systems comprising a transition-metal catalyst which
are capable of
bleaching soils and stains in the absence of a source of hydrogen peroxide or
other peroxygen
bleaching agent can be successfully stabilized against the unwanted
degradation of the transition-
s metal catalyst. In other words, it has been surprisingly discovered that the
addition of an
effective amount of an anti-oxidant and/or free radical scavenger or an
reducing agent can
stabilize a nil hydrogen peroxide bleaching system.
A first aspect of the present invention relates to stabilized bleaching
compositions
comprising:
A) a catalytically effective amount of a transition-metal bleach catalyst
which is a
complex of a transition-metal and a cross-bridged macropolycyclic ligand;
B) an effective amount of a stabilizing agent, said agent selected from
i) one or more anti-oxidants;
ii) one or more reducing agents;
1 S iii) and mixtures thereof; and
C) the balance carriers and other adjunct ingredients;
provided said composition is substantially free of any organic or inorganic
peroxygen
compounds.
The present invention further relates to a method for cleaning and/or
bleaching soils and
stains on fabrics, said method comprising the step of contacting the fabric in
need of cleaning
and/or bleaching with an aqueous solution containing a composition which is
substantially free of
a peroxygen source and which comprises:
a) a catalytically effective amount of a transition-metal bleach catalyst
which is a
complex of a transition-metal and a cross-bridged macropolycyclic ligand;
b) an effective amount of a stabilizing agent, said agent selected from
i) one or more anti-oxidants;
ii) one or more reducing
agents;
iii)and mixtures thereof;
and
c) the balance carriers and other adjunct ingredients;
provided the concentration of said transition metal bleach catalyst in the
aqueous solution is at
least about 0.01 ppb and said composition is substantially free of any organic
or inorganic
peroxygen compounds.
The compositions and methods of the present invention are suitable for
cleaning/bleaching any surface in need of stain removal. For example, hard
surface cleaners and
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
automatic dishwashing compositions can employ the bleach catalysts of the
present invention in
applications which are substantially free of any organic or inorganic
peroxygen compounds.
These and other objects, features, and advantages will become apparent to
those of
ordinary skill in the art from a reading of the following detailed description
and the appended
claims.
All percentages, ratios and proportions herein are by weight, unless otherwise
specified.
All temperatures are in degrees Celsius (° C) unless otherwise
specified. All documents cited are
in relevant part, incorporated herein by reference.
DETAILED DESCRIPTION OF THE INVENTION
The present invention relates to the surprising discovery that transition-
metal catalysts
which are capable of bleaching of soils and stains in the absence of an added
peroxygen bleach
can be suitably stabilized by the addition of one or more stabilizing agents.
The stabilizing
agents suitable for use in the present invention are present at varying level
depending upon the
1 S formulation inter alia liquid, solid, gel, and the concentration of the
transition-metal present.
Absence of Peroxyg-en Sources
The compositions of the present invention, as well as the methods for cleaning
and/or
bleaching of fabric which utilize the compositions of the present invention
are substantially free
of any peroxygen source such as hydrogen peroxide, peroxyacid etc. The
compositions of the
present invention need only have an effective amount of the herein below
described catalyst
present for effective bleaching. For the purposes of the present invention the
term "substantially
free" is defined as "the formulator does not include in the composition any
peroxygen compound
or source of peroxygen at a level required for either effective bleaching
without a transition metal
catalyst, or which would provide an increase in effectiveness of bleaching in
the presence of a
transition metal catalyst as defined herein." Therefore, as will be further
described herein below,
effective bleaching of stains can be accomplished by simply adding an aqueous
or non-aqueous
solution of a catalyst as described herein to fabric which is stained,
preferably the fabric is in an
aqueous solution when contacted with the catalyst. However, it is recognized
that because of
factors outside the control of the formulator inter alia source of product raw
materials, unwanted
decomposition of one or more ingredients, that a source of peroxygen may be
introduced and/or
formed unknowingly in the product. The compositions of the present invention
do not require
any peroxygen source, but the presence of any minor amounts will not effect
the performance of
the bleaching compositions described herein.
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
4
Formulators may typically include a small amount of a source of hydrogen
peroxide into
compositions for the purposes of stabilizing enzymes, for example, a minor
amount of perborate
may be added. However, this-amount of perborate is typically so minor that it
has no effect on
the bleaching capacity of the compositions of the present invention. In cases
where the
formulator has added a minor amount of an oxidant, or other source of peroxide
for the purposes
of stabilizing an adjunct ingredient, for the purposes of the present
invention, those compositions
are still defined as "substantially free" of a source of peroxygen as defined
herein above if they
do not provide additional bleaching activity on stains under typical use
conditions. For example,
a "substantially free" composition can include an amount of peroxygen source
provided the
degree to which the catalyst is effective is substantially the same as if the
source of peroxygen
were absent. For the purposes of the present invention, any composition which
comprises less
than 0.1%, preferably less than 0.01% of a primary oxidant, such as a pre-
formed peracid or a
source of hydrogen peroxide is considered "substantially free" as further
defined herein above.
Additionally, any laundry liquor, laundry wash water, pre-soak bath, or other
fabric or surface
cleaning solution, wherein the present catalysts are used and which comprises
less than 0.001%
by weight of a source of peroxygen, pre-formed or otherwise formed in situ, is
defined herein as
"substantially free" as defined herein above. Stated otherwise, if the
catalysts of the present
invention are used to bleach stains on fabric, or otherwise clean/bleach a
hard surface or
dishware, and the solution containing the catalyst has a concentration of a
source of peroxygen
less than 0.001%, that solution is defined herein as "substantially free" of a
source of peroxygen.
Bleach Catalyst
The compositions of the present invention comprise an effective amount of a
bleach
catalyst. The term "an effective amount" is defined as "an amount of the
transition-metal bleach
catalyst present in the present invention compositions, or during use
according to the present
invention methods, that is sufficient, under whatever comparative or use
conditions are
employed, to result in at least partial oxidation of the material sought to be
oxidized by the
composition or method." Typically the material to be oxidized is an unwanted
substance intef~
ulia food and beverage stains. greasyioily stains. body soils on fabric.
how°ever, this is not the
limitation to which the invention is applicable. Oxidation in the absence of a
source of
peroxygen has wide applicability and the present invention is not limited
solely to bleaching
andior cleaning of fabric. For example, automatic dishwashing compositions are
an embodiment
of the present invention wherein bleaching of a stain with a composition
and~'or with a solution
which is "substantially free" of a source of pero~ygen is a pao of the present
invention. The
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
same is equally true for hard surface cleaning compositions and solutions
which comprise hard
surface cleaning compositions which are "substantially free" of a source of
peroxvgen.
Preferably the compositions of the present invention comprise from about 1 ppb
(0.0000001%), more preferably from about 100 ppb (0.00001%), yet more
preferably from about
S 500 ppb (0.00005%), still more preferably from about 1 ppm (0.0001%) to
about 99.9%, more
preferably to about 50%, yet more preferably to about 5%, still more
preferably to about 500 ppm
(0.05%) by weight of the composition, of a transition-metal bleach catalyst as
described herein
below.
In the broadest view, the transition-metal bleach catalyst of the present
invention
comprises:
i) a transition metal selected from the group consisting of Mn(II), Mn(III),
Mn(IV),
Mn(V), Fe(II), Fe(III), Fe(IV), Co(I), Co(II), Co(III), Ni(I), Ni(II),
Ni(III), Cu(I),
Cu(II), Cu(III), Cr(II), Cr(III), Cr(IV), Cr(V), Cr(VI), V(III), V(IV), V(V),
Mo(IV), Mo(V), Mo(VI), W(N), W(V), W(VI), Pd(II), Ru(II), Ru(III), and
Ru(IV), preferably Mn(II), Mn(III), Mn(IV), Fe(II), Fe(III), Fe(IV), Cr(II),
Cr(III), Cr(IV), Cr(V), Cr(VI), and mixtures thereof; and
ii) a cross-bridged macropolycyclic ligand being coordinated by four or five
donor
atoms to the same transition metal, said ligand comprising:
a) an organic macrocyele ring containing four or more donor atoms
(preferably at least 3, more preferably at least 4, of these donor atoms are
N) separated from each other by covalent linkages of 2 or 3 non-donor
atoms, two to five (preferably three to four, more preferably four) of
these donor atoms being coordinated to the same transition metal atom in
the complex;
b) a cross-bridged chain which covalently connects at least 2 non-adjacent
donor atoms of the organic macrocycle ring, said covalently connected
non-adjacent donor atoms being bridgehead donor atoms which are
coordinated to the same transmon metal in the complex, and wherein
said cross-bridged chain compnses from 2 to about 10 atoms (preferably
the cross-bridged chain is selected from 2, 3 or 4 non-donor atoms, and
4-6 non-donor atoms with a further donor atom);
c) optionally, one or more non-macropolycyclic ligands, preferably selected
from the group consisting of Ii~O, ROH, NR3, RCN, OH , OOH , RS ,
RO,RCOO,OCN,SC'.~I.~3.CN,F,CI,Br,I,02,N03,N02,
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
6
5042 , S032 , P043 , organic phosphates, organic phosphonates,
organic sulfates, organic sulfonates, and aromatic N donors such as
pyridines, pyrazines, pyrazoles, imidazoles, benzimidazoles, pyrimidines,
triazoles and thiazoles with R being H, optionally substituted alkyl,
optionally substituted aryl.
The preferred cross-bridged macropolycyclic ligands are is selected from the
group
consisting of:
a) a cross-bridged macropolycyclic ligand of formula (I) having denticity of 4
or 5:
Rn~\D/ E \ D Rn
E ~B/ E
G/ \G
Rn~/D~E~D Rn
(I);
b) a cross-bridged macropolycyclic ligand of formula (II) having denticity of
5 or 6:
Rn~ D/E\D Rn,
G Rn~~ G
E \B/ E
/G/ ~ \G~ I
D G D
Rn~/ \ ~ ~ W Rn
E~D~ E
I
Rn
(II);
c) the cross-bridged macropolycyclic ligand of formula (III) having denticity
of 6 or
7:
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
E~D~E
~G G~
E ~ / E
Rn~~D~ I ~DwRn~
Ew. D~ E
I
(IB)
wherein each E unit represents the moiety having the formula:
(CRn)a-X-(CRn)a,
wherein X is selected from the group consisting of oxygen, sulfur, -NR-,
phosphorous, or X represents a covalent bond wherein E has the formula:
(CRn)a (CRn)a~
for each E units the sum of a + a' is independently selected from 1 to 5; each
G
unit is a moiety (CRn)b; each R unit is independently selected from H, alkyl,
alkenyl, alkynyl, aryl, alkylaryl, and heteroaryl, or two or more R units are
covalently bonded to form an aromatic, heteroaromatic, cycloalkyl, or
heterocycloalkyl ring; each D unit is a donor atom independently selected from
the group consisting of nitrogen, oxygen, sulfur, and phosphorous, and at
least
1$ two atoms which comprise D units are bridgehead donor atoms coordinated to
the transition metal; B units are a carbon atom, a D unit, or a cycloalkyl or
heterocyclic ring; each n is an integer independently selected from 1 and 2,
completing the valence of the carbon atoms to which the R units are covalently
bonded; each n' is an integer independently selected from 0 and l, completing
the
valence of the D donor atoms to which the R moieties are covalently bonded;
each n" is an integer independently selected from 0, 1, and 2 completing the
valence of the B atoms to which the R moieties are covalently bonded; each a
and a' is an integer independently selected from 0 to 5, wherein the sum of
all a +
a' values in the ligand of formula (I) is within the range of from about 8 to
about
12; the sum of all a + a' values in the ligand of formula (II) is within the
range of
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
8
from about 10 to about 15; and the sum of all a + a' values in the ligand of
formula (III) is within the range of from about 12 to about 18; each b is an
integer independently selected from 0 to 9, or in any of the above formulas,
one
or more of the (CRn)b moieties covalently bonded from any D to the B atom is
absent as long as at least two (CRn)b covalently bond two of the D donor atoms
to the B atom in the formula, and the sum of all b indices is within the range
of
from about 2 to about 5.
A further description of the bleach catalysts of the present invention can be
found in WO
98/39406 A1, published September 11, 1998, WO 98/39098 A1, published September
11, 1998,
and WO 98/39335 A1, published September 11, 1998, all of which are included
herein by
reference.
The nomenclature used throughout this patent to describe the transition-metal
bleach
catalysts is the same nomenclature style used in the above-identified
references. However, the
chemical names of one or more of the herein described ligands may vary from
the chemical name
assigned under the rules of the International Union of Pure and Applied
Chemistry (IUPAC). For
example, a preferred ligand for the purposes of the present invention, 5,12-
dimethyl-1,5,8,12-
tetraaza-bicyclo[6.6.2]hexadecane, has the IUPAC name 4,11-dimethyl-1,4,8,11-
tetraaza-
bicyclo[6.6.2]hexadecane.
Transition-metal bleach catalysts useful in the invention compositions can in
general
include lrnown compounds where they conform with the invention definition, as
well as, more
preferably, any of a large number of novel compounds expressly designed for
the present laundry
or cleaning uses. Non-limiting examples of suitable catalysts according to the
present invention
include:
Dichloro-5,12-dimethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane Manganese(II)
Dichloro-4,10-dimethyl-1,4,7,10-tetraazabicyclo[5.5.2]tetradecane
Manganese(II)
Diaquo-5,12-dimethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane Manganese(II)
Hexafluorophosphate
Aquo-hydroxy-5,12-dimethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
Manganese(III)
Hexafluorophosphate
Diaquo-4,10-dimethyl-1,4,7,10-tetraazabicyclo[5.5.2]tetradecane Manganese(II)
Hexafluorophosphate
Diaquo-5,12-dimethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane Manganese(II)
Tetrafluoroborate
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
9
Diaquo-4,10-dimethyl-1,4,7,10-tetraazabicyclo[5.5.2]tetradecane Manganese(II)
Tetrafluoroborate
Dichloro-5,12-dimethyl-1,5,8;12-tetraazabicyclo[6.6.2]hexadecane
Manganese(III)
Hexafluorophosphate
Dichloro-5,12-di-n-butyl-1,5,8,12-tetraaza-bicyclo[6.6.2]hexadecane
Manganese(II)
Dichloro-5,12-dibenzyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane Manganese(II)
Dichloro-5-n-butyl-12-methyl-1,5,8,12-tetraaza- bicyclo[6.6.2]hexadecane
Manganese(II)
Dichloro-5-n-octyl-12-methyl-1,5,8,12-tetraaza-bicyclo[6.6.2]hexadecane
Manganese(II)
Dichloro-5-n-butyl-12-methyl-1,5,8,12-tetraaza- bicyclo[6.6.2]hexadecane
Manganese(II)
Dichloro-5,12-dimethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane Iron(II)
Dichloro-4,10-dimethyl-1,4,7,10-tetraazabicyclo[5.5.2]tetradecane Iron(II)
Dichloro-5,12-dimethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane Copper(II)
Dichloro-4,10-dimethyl-1,4,7,10-tetraazabicyclo[5.5.2]tetradecane Copper(II)
Dichloro-5,12-dimethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane Cobalt(II)
Dichloro-4,10-dimethyl-1,4,7,10-tetraazabicyclo[5.5.2]tetradecane Cobalt(II)
Dichloro 5,12-dimethyl--4-phenyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
Manganese(II)
Dichloro-4,10-dimethyl-3-phenyl-1,4,7,10-tetraazabicyclo[5.5.2]tetradecane
Manganese(II)
Dichloro-5,12-dimethyl-4,9-diphenyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
Manganese(II)
Dichloro-4,10-dimethyl-3,8-diphenyl-1,4,7,10-tetraazabicyclo[5.5.2]tetradecane
Manganese(II)
Dichloro-5,12-dimethyl-2,11-diphenyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
Manganese(II)
Dichloro-4,10-dimethyl-4,9-diphenyl-1,4,7,10-tetraazabicyclo[5.5.2]tetradecane
Manganese(I)7
Dichloro-2,4,5,9,11,12-hexamethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
Manganese(II)
Dichloro-2,3,5,9,10,12-hexamethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
Manganese(II)
Dichloro-2,2,4,5,9,9,11,12-octamethyl-1,5,8,12-
tetraazabicyclo[6.6.2]hexadecane Manganese(II)
Dichloro-2,2,4,5,9,11,11,12-octamethyl-1,5,8,12-
tetraazabicyclo[6.6.2]hexadecane Manganese(II)
Dichloro-3,3,5,10,10,12-hexamethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
Manganese(II)
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
Dichloro-3,5,10,12-tetramethyl-1,5,8,12-tetraazabicyclo [6.6.2]hexadecane
Manganese(II)
Dichloro-3-butyl-5,10,12-trimethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
Manganese(II)
5 Dichloro-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane Manganese(II)
Dichloro-1,4,7,10-tetraazabicyclo[5.5.2]tetradecane Manganese(II)
Dichloro-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane Iron(II)
Dichloro-1,4,7,10-tetraazabicyclo[5.5.2]tetradecane Iron(II)
Aquo-chloro-2-(2-hydroxyphenyl)-5,12-dimethyl,5,8,12-
tetraazabicyclo[6.6.2]hexadecane
10 Manganese(II)
Aquo-chloro-10-(2-hydroxybenzyl)-4,10-dimethyl-1,4,7,10-
tetraazabicyclo[5.5.2]tetradecane
Manganese(II)
Chloro-2-(2-hydroxybenzyl)-5-methy 1,5, 8,12-tetraazabicyclo [6.6.2]hexadecane
Manganese(II)
Chloro-10-(2-hydroxybenzyl)-4-methyl-1,4,7,10-
tetraazabicyclo[5.5.2]tetradecane Manganese(II)
Chloro-5-methyl-12-(2-picolyl)-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
Manganese(II) Chloride
Chloro-4-methyl-10-(2-picolyl)-1,4,7,10-tetraazabicyclo[5.5.2]tetradecane
Manganese(II) Chloride
Dichloro-5-(2-sulfato)dodecyl-12-methyl-1,5,8,12-
tetraazabicyclo[6.6.2]hexadecane Manganese(III)
Aquo-Chloro-5-(2-sulfato)dodecyl-12-methyl-1,5,8,12-
tetraazabicyclo[6.6.2]hexadecane
Manganese(II)
Aquo-Chloro-5-(3-sulfonopropyl)-12-methyl-1,5,8,12-
tetraazabicyclo[6.6.2]hexadecane
Manganese(II)
Dichloro-5-(Trimethylammoniopropyl)dodecyl-12-methyl-1,5,8,12-
tetraazabicyclo[6.6.2]hexadecane
Manganese(III) Chloride
Dichloro-5,12-dimethyl-1,4,7,10,13-pentaazabicyclo[8.5.2]heptadecane
Manganese(II)
Dichloro-14,20-dimethyl-1,10,14,20-tetraazatriyc lo( 8.6.6 ]docosa-3(8),4,6-
triene
Manganese(II)
Dichloro-4,11-dimethyl-1,4,7,11-tetraazabicyclo(6.5.2jpentadecane
Manganese(II)
Dichloro-5,12-dimethyl-1,5,8,12-tetraazabicyclo[ 7.6.'' J heptadecane
Manganese(II)
Dichloro-5,13-dimethyl-1,5,9,13-tetraazabicyclo(7.7.?jheptadecane
Manganese(II)
Dichloro-3,10-bis(butylcarboxy)-5,12-dimethyl- I , 5.8,12-
tetraazabicyclo[6.6.2]hexadecane
Manganese(II)
Diaquo-3,10-dicarboxy-5,12-dimethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
Manganese(II)
CA 02364538 2001-08-28
WO 00/52124 PCT/LJS00/05291
11
Chloro-20-methyl-1,9,20,24,25-pentaaza-tetracyclo[7.7.7.137.111,15.]pentacosa-
3,5,7(24),11,13,15(25)-hexaene manganese(II) Hexafluorophosphate
Trifluoromethanesulfono-20-methyl-1,9,20,24,25-pentaaza-tetracyclo[7.7.7.13
7.111,15,]pentacosa-
3,5,7(24),11,13,15(25)-hexaene Manganese(II) Trifluoromethanesulfonate
Trifluoromethanesulfono-20-methyl-1,9,20,24,25-pentaaza-tetracyclo[7.7.7.13
7.111,15.]pentacosa-
3,5,7(24),11,13,15(25)-hexaene Iron(II) Trifluoromethanesulfonate
Chloro-5,12,17-trimethyl-1,5,8,12,17-pentaazabicyclo[6.6.5]nonadecane
Manganese(II)
Hexafluorophosphate
Chloro-4,10,15-trimethyl-1,4,7,10,15-pentaazabicyclo[5.5.5]heptadecane
Manganese(II)
Hexafluorophosphate
Chloro-5,12,17-trimethyl-1,5,8,12,17-pentaazabicyclo[6.6.5]nonadecane
Manganese(II) Chloride
Chloro-4,10,15-trimethyl-1,4,7,10,15-pentaazabicyclo[5.5.5]heptadecane
Manganese(II) Chloride
Dichloro 5,12,15,16-tetramethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
Manganese(II)
Chloro 5-methyl-12-(2'-oxybenzyl)-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
Manganese(II)
Preferred complexes useful as transition-metal bleach catalysts more generally
include
not only monometallic, mononuclear kinds such as those illustrated hereinabove
but also
bimetallic, trimetallic or cluster kinds. Monometallic, mononuclear complexes
are preferred. As
defined herein, a monometallic transition-metal bleach catalyst contains only
one transition metal
atom per mole of complex. A monometallic, mononuclear complex is one in which
any donor
atoms of the essential macrocyclic ligand are bonded to the same transition
metal atom, that is,
the essential ligand does not "bridge" across two or more transition-metal
atoms.
Stabilization Agents
Anti-oxidants
One type of preferred stabilization agent according to the present invention
are anti-
oxidants. The compositions of the present invention will comprise an effective
amount of the
anti-oxidant, preferably from about 0.01%, more preferably from about 0.1%,
most preferably
from about 0.2% to about 10%, preferably to about 5%, more preferably to about
1% by weight
of an anti-oxidant. Preferably the anti-oxidant is a free radical scavenger.
One class of anti-oxidants suitable for use in the present invention are the
alkylated
phenols having the general formula:
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
12
OH
fR'lX
R
wherein R is C,-C2~ linear or branched alkyl, preferably methyl or branched C3-
C6 alkyl; C3-C6
alkoxy, preferably methoxy; R' is a C3-C6 branched alkyl, preferably tert-
butyl; x is 1 or 2.
Another class of anti-oxidants suitable for use in the present invention are
benzofuran
derivatives having the formula:
Rz
R'
wherein R' and Rz are each independently C,-C4 alkyl or R' and RZ can be taken
together to form
a CS-C6 cyclic hydrocarbyl moiety; R4 is C,-C6 alkyl; RS is hydrogen or -
C(O)R3 wherein R3 is
hydrogen or C,-C,9 alkyl; R6 is C,-C6 alkyl; R' is hydrogen or C,-C6 alkyl; X
is -CHZOH, or -
CHZA wherein A is a nitrogen comprising unit, phenyl, or substituted phenyl.
Preferred nitrogen
comprising A units include amino, pyrrolidino, piperidino, morpholino,
piperazino, and mixtures
thereof.
Non-limiting examples of anti-oxidants suitable for use in the present
invention include
phenols inter ulia 2,6-di-tert-butylphenol, 2,6-di-tert-butyl-4-methylphenol,
mixtures of 2 and 3-
tort-butyl-4-methoxyphenol, and other ingredients including include propyl
gallate, ter-
butylhydroquinone, .benzoic acid derivatives such as methoxy benzoic acid,
methylbenzoic acid,
dichloro benzoic acid, dimethyl benzoic acid, ~-hydroxy-2,2,4,G,7-pentamethyl-
2,3-dihydro-1-
benzofuran-3-one, 5-hydroxy-3-methylene-2,2,4,6,7-pentamethyl-2,3-dihydro-
benzofuran, 5-
benzyloxy-3-hydroxymethyl-2,2,4,6,7-pentamethyl-2,3-dihydro-1-benzofuran, 3-
hydroxymethyl-
5-methoxy-2,2,4,6,7-pentamethyl-2,3-dihydro-1-benzofuran.
Reducing~ents
One type of preferred stabilization agent according to the present invention
are reducing agents.
The compositions of the present invention will comprise an effective amount of
one or more
reducing agents, preferably from about 0.001 %, more preferably from about
0.01 %, most
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
13
preferably from about 0.02% to about 1%, preferably to about 0.5%, more
preferably to about
0.1 % by weight of a reducing agent. Preferably the reducing agent is an
inorganic compound.
Non-limiting examples of reducing agents include compounds capable of yielding
sulfite
ions such as sodium sulfite, potassium sulfite, ammonium sulfite, sodium
hydrogen sulfite,
S sodium metabisulfite, potassium metabisulfite, potassium hydrogen sulfite,
pyrosulphite salts,
sodium borohydride, lithium borohydride, lithium aluminum isopropoxide, and
mixtures thereof.
METHOD OF USE
The present invention further relates to a method for using stabilized bleach
catalysts
without the requirement of a peroxygen source of peroxygen to clean soil from
and stains from
fabric.
The present invention, therefore, relates to a method for bleaching soils and
stains on
fabric in the absence of a bleaching agent, said method comprising the step of
contacting fabric in
need of cleaning with an aqueous or non-aqueous solution containing a
composition which is
substantially free of a peroxygen source, comprising:
a) a catalytically effective amount of a transition-metal bleach catalyst
which is a
complex of a transition-metal and a cross-bridged macropolycyclic ligand;
b) an effective amount of a stabilizing agent, said agent selected from
i) one or more anti-oxidants;
ii) one or more reducing agents;
iii) and mixtures thereof; and
c) the balance carriers and other adjunct ingredients;
provided the concentration of said transition metal bleach catalyst in the
solution is at least about
0.01 ppb and said composition is substantially free of any organic or
inorganic peroxygen
compounds..
Preferably the solution which comprises the transition-metal bleach catalyst
has a
solution concentration of catalyst of from about I ppb, more preferably from
about 10 ppb, yet
more preferably from about 100 ppb. For example, 100 ppb (parts per billion)
is a solution which
comprises 0.00001% by weight, of a catalyst. As defined herein above,
solutions which
comprises less than 0.001 % of a source of peroxygen are solutions which are
"substantially free"
of any organic or inorganic peroxygen compounds.
Methods directed entirely to large scale bleaching per se, for example, an
industrial or
manufacturing process, may utilized a higher concentration of catalyst, for
example, 1 ppm or
higher in order to reduce the contact time of the fabric with the catalyst
containing solution.
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
14
ADJUNCT INGREDIENTS
The bleaching, pre-soak, pre-treatment, laundry or automatic diswashing, or
hard surface
cleaning compositions of the present invention, whether granular, solid (bar),
gel, or liquid may
S further comprise one or more carriers and adjunct ingredients.
Compositions according to the present invention may comprise:
a) a catalytically effective amount of a transition-metal bleach catalyst
which is a
complex of a transition-metal and a cross-bridged macropolycyclic ligand; and
b) optionally from about 0.001% to about 90% by weight, of one or more dye
fixing
agents;
c) optionally from about 0.01% to about SO% by weight, of one or more
cellulose
reactive dye fixing agents;
d) optionally from about 0.01% to about 15% by weight, of a chlorine
scavenger;
e) optionally about 0.005% to about 1% by weight, of one or more crystal
growth
inhibitors;
f) optionally from about 0.01 % to about 20% by weight, of a fabric abrasion
reducing polymer;
g) optionally from about 1% to about 12% by weight, of one or more liquid
Garners;
h) optionally from about 0.001 % to about 1 % by weight, of an enzyme;
i) optionally from about 0.01% to about 8% by weight, of a polyolefin emulsion
or
suspension;
j) optionally from about 0.01% to about 0.2% by weight, of a stabilizer;
k) optionally from about 1% to about 80% by weight, of a fabric softening
active;
I) optionally less than about 15% by weight, of a principal solvent; and
m) optionally from about 0.01%, preferably from about 0.1%, to about 60%,
preferably to about 30% by weight, one or more surfactants, said surfactants
selected from the group consisting of anionic, cationic, nonionic, ampholytic,
zwitterionic surfactants, and mixtures thereof.
Surfactants
The bleaching, pre-soak, pre-treatment, and laundry detergent compositions of
the
present invention may comprise at least about 0.01% by weight, preferably from
about 0.1% to
about 60%, preferably to about 30% by weight, of a detersive surfactant
system, said system is
comprised of one or more category of surfactants depending upon the
embodiment, said
categories of surfactants are selected from the group consisting of anionic,
cationic, nonionic,
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
zwitterionic, ampholytic surfactants, and mixtures thereof. Within each
category of surfactant,
more than one type of surfactant of surfactant can be selected. For example,
preferably the solid
(i.e. granular) and viscous semi-solid (i.e. gelatinous, pastes, etc.) systems
of the present
invention, surfactant is preferably present to the extent of from about 0.1%
to 60 %, preferably to
5 about 30% by weight of the composition.
Nonlimiting examples of surfactants useful herein include:
a) C"-C,8 alkyl benzene sulfonates (LAS);
b) C,o-CZO primary, branched-chain and random alkyl sulfates (AS);
c) C,o-C,8 secondary (2,3) alkyl sulfates having the formula:
IOSO3 M+ OI SO3 M+
CH3(CH2)X(CH)CH3 ar CH~(CH?)y(CH)CH2CH3
wherein x and (y + 1 ) are integers of at least about 7, preferably at least
about 9; said
surfactants disclosed in U.S. 3,234,258 Morris, issued February 8, 1966; U.S.
5,075,041
Lutz, issued December 24, 1991; U.S. 5,349,101 Lutz et al., issued September
20, 1994;
and U.S. 5,389,277 Prieto, issued February 14, 1995 each incorporated herein
by
reference;
d) C,o-C,8 alkyl alkoxy sulfates (AEXS) wherein preferably x is from 1-7;
e) C,o-C,g alkylalkoxy carboxylates preferably comprising 1-5 ethoxy units;
f) C,2-C,8 alkyl ethoxylates, C6-C,2 alkyl phenol alkoxylates wherein the
alkoxylate units
are a mixture of ethyleneoxy and propyleneoxy units, C,~-C,8 alcohol and C6-
C,~ alkyl
phenol condensates with ethylene oxideipropylene oxide block polymers inter
alia
Pluronic ex BASF which are disclosed in U.S. 3,929,678 Laughlin et al., issued
December 30, 1975, incorporated herein by reference;
g) Alkylpolysaccharides as disclosed in U.S. .1.65,647 Llenado, issued January
26, 1986,
incorporated herein by reference;
h) Polyhydroxy fatty acid amides having the formula:
O R8
R7-C- V' -Q
wherein R7 is CS-C31 alkyl; R8 is selected from the group consisting of
hydrogen, C1-C4 alkyl,
C1-C4 hydroxyalkyl, Q is a polyhydroxyalkyl moiety having a linear alkyl chain
with at least 3
hydroxyls directly connected to the chain, or an alkoxylated derivative
thereof; preferred alkoxy
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
16
is ethoxy or propoxy, and mixtures thereof; preferred Q is derived from a
reducing sugar in a
reductive amination reaction, more preferably Q is a glycityl moiety; Q is
more preferably
selected from the group consisting of -CH2(CHOH)nCH20H, -CH(CH20H)(CHOH)n-
1 CH20H, -CH2(CHOH)2-(CHOR')(CHOH)CH20H, and alkoxylated derivatives thereof,
wherein n is an integer from 3 to 5, inclusive, and R' is hydrogen or a cyclic
or aliphatic
monosaccharide, which are described in U.S. 5,489,393 Connor et al., issued
February 6, 1996;
and U.S. 5,45,982 Murch et al., issued October 3, 1995, both incorporated
herein by reference.
The bleaching, pre-soak, pre-treatment, and laundry detergent compositions of
the
present invention can also comprise from about 0.001% to about 100% of one or
more
(preferably a mixture of two or more) mid-chain branched surfactants,
preferably mid-chain
branched alkyl alkoxy alcohols having the formula:
R R' RZ
I I I
CH3CH2(CH2)H,CH(CHz),~CH(CHZ)yCH(CHZ)~(EO/PO)mOH
mid-chain branched alkyl sulfates having the formula:
R R1 RZ
I ( I
CH3CH2(CH2)WCH(CH2),~CH(CHZ)yCH(CHZ)ZOS03M
and mid-chain branched alkyl alkoxy sulfates having the formula:
R Rl RZ
I
CH3CH2(CHZ)WCH(CHZ)XCH(CHZ)yCH(CHZ)Z(EO/PO)n,OS03M
wherein the total number of carbon atoms in the branched primary alkyl moiety
of these formulae
(including the R, R1, and R2 branching, but not including the carbon atoms
which comprise any
EO/PO alkoxy moiety) is from 14 to 20, and wherein further for this surfactant
mixture the
average total number of carbon atoms in the branched primary alkyl moieties
having the above
formula is within the range of greater than 14.5 to about 17.5 (preferably
from about 15 to about
17); R, R1, and R2 are each independently selected from hydrogen, C1-C3 alkyl,
and mixtures
thereof, preferably methyl; provided R, R1, and R2 are not all hydrogen and,
when z is l, at least
R or R1 is not hydrogen. M is a water soluble cation and may comprises more
than one type of
cation, for example, a mixture of sodium and potassium. The index w is an
integer from 0 to 13:
x is an integer from 0 to 13; y is an integer from 0 to 13; z is an integer of
at least 1; provided w
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
17
x + y + z is from 8 to 14. EO and PO represent ethyleneoxy units and
propyleneoxy units having
the formula:
( H3 CH3
CHCHZO- or -CHZCHO-
respectively, however, other alkoxy units inter alia 1,3-propyleneoxy, butoxy,
and mixtures
thereof are suitable as alkoxy units appended to the mid-chain branched alkyl
moieties.
The mid-chain branched surfactants are preferably mixtures which comprise a
surfactant
system. Therefore, when the surfactant system comprises an alkoxylated
surfactant, the index m
indicates the average degree of alkoxylation within the mixture of
surfactants. As such, the index
m is at least about 0.01, preferably within the range of from about 0.1, more
preferably from
about 0.5, most preferably from about 1 to about 30, preferably to about 10,
more preferably to
about 5. When considering a mid-chain branched surfactant system which
comprises only
alkoxylated surfactants, the value of the index m represents a distribution of
the average degree
of alkoxylation corresponding to m, or it maybe a single specific chain with
alkoxylation (e.g.,
ethoxylation and/or propoxylation) of exactly the number of units
corresponding to m.
The preferred mid-chain branched surfactants of the present invention which
are suitable
for use in the surfactant systems of the present invention have the formula:
i H3
CH3(CH2)aCH(CHZ)bCH2(EO/PO)n,OS03M
or the formula:
H3 CH3
CH3(CHZ)dCH(CHZ)eCHCH2(EO/PO)n,OS03M
wherein a, b, d, and a are integers such that a + b is from 10 to 16 and d + a
is from 8 to 14; M is
selected from sodium, potassium, magnesium, ammonium and substituted ammonium,
and
mixtures thereof.
The surfactant systems of the present invention which comprise mid-chain
branched
surfactants are preferably formulated in two embodiments. A first preferred
embodiment
comprises mid-chain branched surfactants which are formed from a feedstock
which comprises
25% or less of mid-chain branched alkyl units. Therefore, prior to admixture
with any other
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
18
conventional surfactants, the mid-chain branched surfactant component will
comprise 25% or
less of surfactant molecules which are non-linear surfactants.
A second preferred embodiment comprises mid-chain branched surfactants which
are
formed from a feedstock which comprises from about 25% to about 70% of mid-
chain branched
alkyl units. Therefore, prior to admixture with any other conventional
surfactants, the mid-chain
branched surfactant component will comprise from about 25% to about 70%
surfactant molecules
which are non-linear surfactants.
The surfactant systems of the laundry detergent compositions of the present
invention
can also comprise from about 0.001%, preferably from about 1%, more preferably
from about
5%, most preferably from about 10% to about 100%, preferably to about 60%,
more preferably to
about 30% by weight, of the surfactant system, of one or more (preferably a
mixture of two or
more) mid-chain branched alkyl arylsulfonate surfactants, preferably
surfactants wherein the aryl
unit is a benzene ring having the formula:
RIR2L R3
CM q+~
b
s~3
wherein L is an acyclic hydrocarbyl moiety comprising from 6 to 18 carbon
atoms; R', R2, and R3
are each independently hydrogen or C,-C3 alkyl, provided R' and RZ are not
attached at the
terminus of the L unit; M is a water soluble cation having charge q wherein a
and b are taken
together to satisfy charge neutrality.
Builders
The compositions of the present invention, especially when comprising
surfactants,
preferably comprise one or more detergent builders or builder systems. When
present, the
compositions will typically comprise at least about 1% builder, preferably
from about 5%, more
preferably from about 10% to about 80%, preferably to about 50%, more
preferably to about
30% by weight, of detergent builder.
The level of builder can vary widely depending upon the end use of the
composition and
its desired physical form. When present, the compositions will typically
comprise at least about
1% builder. Formulations typically comprise from about 5% to about 50%, more
typically about
5% to about 30%, by weight, of detergent builder. Granular formulations
typically comprise
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
19
from about 10% to about 80%, more typically from about 15% to about 50% by
weight, of the
detergent builder. Lower or higher levels of builder, however, are not meant
to be excluded.
Inorganic or P-containing detergent builders include, but are not limited to,
the alkali
metal, ammonium and alkanolammonium salts of polyphosphates (exemplified by
the
tripolyphosphates, pyrophosphates, and glassy polymeric meta-phosphates),
phosphonates,
phytic acid, silicates, carbonates (including bicarbonates and
sesquicarbonates), sulphates, and
aluminosilicates. However, non-phosphate builders are required in some
locales. Importantly,
the compositions herein function surprisingly well even in the presence of the
so-called "weak"
builders (as compared with phosphates) such as citrate, or in the so-called
"underbuilt" situation
that may occur with zeolite or layered silicate builders.
Examples of silicate builders are the alkali metal silicates described in U.S.
4,664,839
Rieck, issued May 12, 1987. NaSKS-6 is the trademark for a crystalline layered
silicate
marketed by Hoechst (commonly abbreviated herein as "SKS-6")
Examples of carbonate builders are the alkaline earth and alkali metal
carbonates as
disclosed in German Patent Application No. 2,321,001 published on November 15,
1973.
Aluminosilicate builders are useful in the present invention. Examples of
suitable
aluminosilicate builders are described in U.S. 4,274,975 Corkhill et al.
included herein by
reference. Aluminosilicate builders are of great importance in most currently
marketed heavy
duty granular detergent compositions, and can also be a significant builder
ingredient in liquid
detergent formulations. Aluminosilicate builders include those having the
empirical formula:
[Mz(zA102)y] ~xH20
wherein z and y are integers of at least 6, the molar ratio of z to y is in
the range from 1.0 to
about 0.5, and x is an integer from about 1 S to about 264. Preferred
synthetic crystalline
aluminosilicate ion exchange materials useful herein are available under the
designations Zeolite
A, Zeolite P (B), Zeolite MAP and Zeolite X.
Organic detergent builders suitable for the purposes of the present invention
include, but
are not restricted to, a wide variety of polycarboxylate compounds. As used
herein,
"polycarboxylate" refers to compounds having a plurality of carboxylate
groups, preferably at
least 3 carboxylates. Polycarboxylate builder can generally be added to the
composition in acid
form, but can also be added in the form of a neutralized salt. When utilized
in salt form, alkali
metals, such as sodium, potassium, and lithium, or alkanolammonium salts are
preferred.
Suitable are disclosed in U.S. 3,128,287 Berg, issued April 7, 1964, U.S.
3,635,830
Lamberti et al., issued January 18, 1972, U.S. 4,663,071 Bush et al., issued
May 5, 1987, U.S.
3,923,679 Rapko, issued December 2, 1975; U.S. 4,1 S 8,635 Crutchfield et al.,
issued June 19,
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
1979; U.S. 4,120,874 Crutchfield et al., issued October 17, 1978; U.S.
4,566,984, Bush, issued
January 28, 1986, U.S. 4,144,226, Crutchfield et al., issued March 13, 1979
and in U.S.
3,308,067, Diehl, issued March 7, 1967, Diehl U.S. Patent 3,723,322, and U.S.
4,102,903
Crutchfield et al., issued July 25, 1978 and further U.S. Patents 3,159,581;
3,213,030; 3,422,021;
3,400,148 and 3,422,137.
Citrate builders, e.g., citric acid and soluble salts thereof (particularly
sodium salt), are
polycarboxylate builders of particular importance for heavy duty liquid
detergent formulations
due to their availability from renewable resources and their biodegradability.
Citrates can also
be used in granular compositions, especially in combination with zeolite
and/or layered silicate
10 builders. Oxydisuccinates are also especially useful in such compositions
and combinations.
Disgersants
A description of other suitable polyalkyleneimine dispersants which may be
optionally
combined with the bleach stable dispersants of the present invention can be
found in U.S.
4,597,898 Vander Meer, issued July 1, 1986; European Patent Application
111,965 Oh and
15 Gosselink, published June 27; 1984; European Patent Application 111,984
Gosselink, published
June 27, 1984; European Patent Application 112,592 Gosselink, published July
4, 1984; U.S.
4,548,744 Connor, issued October 22, 1985; and U.S. 5,565,145 Watson et al.,
issued October 15,
1996; all of which are included herein by reference. However, any suitable
clay/soil dispersant
or anti-redepostion agent can be used in the laundry compositions of the
present invention.
20 In addition, polymeric dispersing agents which include polymeric
polycarboxylates and
polyethylene glycols, are suitable for use in the present invention. Polymeric
polycarboxylate
materials can be prepared by polymerizing or copolymerizing suitable
unsaturated monomers,
preferably in their acid form. Unsaturated monomenc acids that can be
polymerized to form
suitable polymeric polycarboxylates include acrylic acid, malefic acid (or
malefic anhydride),
fumaric acid, itaeonic acid, aconitic acid, mesacon~c acid, citraconic acid
and methylenemalonic
acid. The presence in the polymeric polycarboxylates herein or monomeric
segments, containing
no caiboxylate radicals such as vinylmethyl ether, st<Tene, ethylene, etc. is
suitable provided that
such segments do not constitute more than about -10° o by weight.
Particularly suitable polymeric polycarboxylates can be derived from acrylic
acid. Such
acrylic acid-based polymers which are useful herein are the water-soluble
salts of polymerized
acrylic acid. The average molecular weight of such polymers in the acid form
preferably ranges
from about 2,000 to 10,000, more preferably from about -1,000 to 7,000 and
most preferably from
about 4,000 to 5,000. Water-soluble salts of such acrylm acid polymers can
include, for example,
the alkali metal, ammonium and substituted ammonium salts. Soluble polymers of
this type are
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
21
known materials. Use of polyacrylates of this type in detergent compositions
has been disclosed,
for example, in Diehl, U.S. Patent 3,308,067, issued march 7, 1967.
Acrylic/maleic-based. copolymers may also be used as a preferred component of
the
dispersing/anti-redeposition agent. Such materials include the water-soluble
salts of copolymers
of acrylic acid and malefic acid. The average molecular weight of such
copolymers in the acid
form preferably ranges from about 2,000, preferably from about 5,000, more
preferably from
about 7,000 to 100,000, more preferably to 75,000, most preferably to 65,000.
The ratio of
acrylate to maleate segments in such copolymers will generally range from
about 30:1 to about
1:1, more preferably from about 10:1 to 2:1. Water-soluble~salts of such
acrylic acid/maleic acid
copolymers can include, for example, the alkali metal, ammonium and
substituted ammonium
salts. Soluble acrylate/maleate copolymers of this type are known materials
which are described
in European Patent Application No. 66915, published December 15, 1982, as well
as in EP
193,360, published September 3, 1986, which also describes such polymers
comprising
hydroxypropylacrylate. Still other useful dispersing agents include the
maleic/acrylic/vinyl
alcohol terpolymers. Such materials are also disclosed in EP 193,360,
including, for example, the
45/45/10 terpolymer of acrylic/maleic/vinyl alcohol.
Another polymeric material which can be included is polyethylene glycol (PEG).
PEG
can exhibit dispersing agent performance as well as act as a clay soil removal-
antiredeposition
agent. Typical molecular weight ranges for these purposes range from about 500
to about
100,000, preferably from about 1,000 to about 50,000, more preferably from
about 1,500 to about
10,000.
Polyaspartate and polyglutamate dispersing agents may also be used, especially
in
conjunction with zeolite builders. Dispersing agents such as polyaspartate
preferably have a
molecular weight (avg.) of about 10,000.
Soil Release Agents
The compositions according to the present invention may optionally comprise
one or
more soil release agents. If utilized, soil release agents will generally
comprise from about
0.01%, preferably from about 0.1%, more preferably from about 0.2% to about
10%, preferably
to about 5%, more preferably to about 3% by weight, of the composition.
Polymeric soil release
agents are characterized by having both hydrophilic segments, to hydrophilize
the surface of
hydrophobic fibers, such as polyester and nylon, and hydrophobic segments, to
deposit upon
hydrophobic fibers and remain adhered thereto through completion of the
laundry cycle and, thus.
serve as an anchor for the hydrophilic segments. This can enable stains
occuring subsequent to
treatment with the soil release agent to be more easily cleaned in later
washing procedures.
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
22
The following, all included herein by reference, describe soil release
polymers suitable
for use in the present invention. U.S. 5,728,671 Rohrbaugh et al., issued
March 17, 1998; U.S.
5,691,298 Gosselink et al., issued November 25, 1997; U.S. 5,599,782 Pan et
al., issued February
4, 1997; U.S. 5,415,807 Gosselink et al., issued May 16, 1995; U.S. 5,182,043
Morrall et al.,
S issued January 26, 1993; U.S. 4,956,447 Gosselink et al., issued September
11, 1990; U.S.
4,976,879 Maldonado et al. issued December 11, 1990; U.S. 4,968,451 Scheibel
et al., issued
November 6, 1990; U.S. 4,925,577 Borcher, Sr. et al., issued May 15, 1990;
U.S. 4,861,512
Gosselink, issued August 29, 1989; U.S. 4,877,896 Maldonado et al., issued
October 31, 1989;
U.S. 4,771,730 Gosselink et al., issued October 27, 1987; U.S. 711,730
Gosselink et al., issued
December 8, 1987; U.S. 4,721,580 Gosselink issued January 26, 1988; U.S.
4,000,093 Nicol et
al., issued December 28, 1976; U.S. 3,959,230 Hayes, issued May 25, 1976; U.S.
3,893,929
Basadur, issued July 8, 1975; and European Patent Application 0 219 048,
published April 22,
1987 by Kud et al.
Further suitable soil release agents are described in U.S. 4,201,824 Voilland
et al.; U.S.
4,240,918 Lagasse et al.; U.S. 4,525,524 Tung et al.; U.S. 4,579,681 Ruppert
et al.; U.S.
4,220,918; U.S. 4,787,989; EP 279,134 A, 1988 to Rhone-Poulenc Chemie; EP
457,205 A to
BASF (1991); and DE 2,335,044 to Unilever N.V., 1974; all incorporated herein
by reference.
Enzymes
The detergent and cleaning compositions herein may also optionally contain one
or more
types of detergent enzymes. Such enzymes can include other proteases,
amylases, cellulases and
lipases. Such materials are known in the art and are commercially available
under such
trademarks as . They may be incorporated into the non-aqueous liquid detergent
compositions
herein in the form of suspensions, "marumes" or "prills". Another suitable
type of enzyme
comprises those in the form of slurries of enzymes in nonionic surfactants,
e.g., the enzymes
marketed by Novo Nordisk under the tradename "SL" or the microencapsulated
enzymes
marketed by Novo Nordisk under the tradename "LDP." Suitable enzymes and
levels of use are
described in U.S. Pat. No. 5,576,282, 5,705,464 and 5,710,115.
Enzymes added to the compositions herein in the form of conventional enzyme
prills are
especially preferred for use herein. Such prills will generally range in size
from about 100 to
1,000 microns, more preferably from about 200 to 800 microns and will be
suspended throughout
the non-aqueous liquid phase of the composition. Prills in the compositions of
the present
invention have been found, in comparison with other enzyme forms, to exhibit
especially
desirable enzyme stability in terms of retention of enzymatic activity over
time. Thus,
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
23
compositions which utilize enzyme prills need not contain conventional enzyme
stabilizing such
as must frequently be used when enzymes are incorporated into aqueous liquid
detergents.
However, enzymes added to the compositions herein may be in the form of
granulates,
preferably T-granulates.
"Detersive enzyme", as used herein, means any enzyme having a cleaning, stain
removing
or otherwise beneficial effect in a laundry, hard surface cleaning or personal
care detergent
composition. Preferred detersive enzymes are hydrolases such as proteases,
amylases and lipases.
Preferred enzymes for laundry purposes include, but are not limited to,
proteases, cellulases,
lipases and peroxidases. Highly preferred for automatic dishwashing are
amylases and/or
proteases, including both current commercially available types and improved
types which, though
more and more bleach compatible though successive improvements, have a
remaining degree of
bleach deactivation susceptibility.
Examples of suitable enzymes include, but are not limited to, hemicellulases,
peroxidases, proteases, cellulases, xylanases, lipases, phospholipases,
esterases, cutinases,
pectinases, keratanases, reductases, oxidases, phenoloxidases, lipoxygenases,
ligninases,
pullulanases, tannases, pentosanases, malanases, 13-glucanases,
arabinosidases, hyaluronidase,
chondroitinase, laccase, and known amylases, or mixtures thereof.
Examples of such suitable enzymes are disclosed in U.S. Patent Nos. 5,705,464,
5,710,115, 5,576,282, 5,728,671 and 5,707,950
The cellulases useful in the present invention include both bacterial or
fungal cellulases.
Preferably, they will have a pH optimum of between S and 12 and a specific
activity above 50
CEVU/mg (Cellulose Viscosity Unit). Suitable cellulases are disclosed in U.S.
Patent 4,435,307,
J61078384 and W096/02653 which discloses fungal cellulase produced
respectively from
Humicola insolens, Trichoderma, Thielavia and Sporotrichum. EP 739 982
describes cellulases
isolated from novel Bacillus species. Suitable cellulases are also disclosed
in GB-A-2.075.028;
GB-A-2.095.275; DE-OS-2.247.832 and W095/26398.
Examples of such cellulases are cellulases produced by a strain of Humicola
insolens
(Humicola grisea var. thermoidea), particularly the Humicola strain DSM 1800.
Other suitable cellulases are cellulases originated from Humicola insolens
having a molecular
weight of about SOKDa, an isoelech-ic point of 5.5 and containing 415 amino
acids; and a '43kD
endoglucanase derived from Humicola insolens, DSM 1800, exhibiting cellulase
activity; a
preferred endoglucanase component has the amino acid sequence disclosed in WO
91/17243.
Also suitable cellulases are the EGIII cellulases from Trichoderma
longibrachiatum described in
W094/21801 to Genencor. Especially suitable cellulases are the cellulases
having color care
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
24
benefits. Examples of such cellulases are cellulases described in European
patent application No.
91202879.2, filed November 6, 1991 (Novo). Carezyme and Celluzyme (Novo
Nordisk A/S) are
especially useful. See also W091/17244 and W091/21801. Other suitable
cellulases for fabric
care and/or cleaning properties are described in W096/34092, W096/17994 and
W095/24471.
Cellulases, when present, are normally incorporated in the cleaning
composition at levels
from 0.0001 % to 2% of pure enzyme by weight of the cleaning composition.
Other preferred enzymes that can be included in the cleaning compositions of
the present
invention include lipases. Suitable lipase enzymes for detergent usage include
those produced by
microorganisms of the Pseudomonas group, such as Pseudomonas stutzeri ATCC
19.154, as
disclosed in British Patent 1,372,034. Suitable lipases include those which
show a positive
immunological cross-reaction with the antibody of the lipase, produced by the
microorganism
Pseudomonas fluorescent IAM 1057. This lipase is available from Amano
Pharmaceutical Co.
Ltd., Nagoya, Japan, under the trade name Lipase P "Amano," hereinafter
referred to as "Amano-
P". Other suitable commercial lipases include Amano-CES, lipases ex
Chromobacter viscosum,
I S e.g. Chromobacter viscosum var. lipolyticum NRRLB 3673 from Toyo Jozo Co.,
Tagata, Japan;
Chromobacter viscosum lipases from U.S. Biochemical Corp., U.S.A. and Disoynth
Co., The
Netherlands, and lipases ex Pseudomonas gladioli. Especially suitable lipases
are lipases such as
Ml LipaseR and LipomaxR (Gist-Brocades) and LipolaseR and Lipolase
UltraR(Novo) which
have found to be very effective when used in combination with the compositions
of the present
invention. Also suitable are the lipolytic enzymes described in EP 258 068, WO
92/05249 and
WO 95/22615 by Novo Nordisk and in WO 94/03578, WO 95/35381 and WO 96/00292 by
Unilever.
Also suitable are cutinases [EC 3.1.1.50] which can be considered as a special
kind of
lipase, namely lipases which do not require interfacial activation. Addition
of cutinases to
cleaning compositions have been described in e.g. WO-A-88/09367 (Genencor); WO
90/09446
(Plant Genetic System) and WO 94/14963 and WO 94/14964 (Unilever).
Lipases and/or cutinases, when present, are normally incorporated in the
cleaning
composition at levels from 0.0001% to 2% of pure enzyme by weight of the
cleaning
composition.
In addition to the above referenced lipases, phospholipases may be
incorporated into the
cleaning compositions of the present invention. Nonlimiting examples of
suitable phospholipases
included: EC 3.1.1.32 Phospholipase Al; EC 3.1.1.4 Phospholipase A2; EC
3.1.1.5
Lysopholipase; EC 3.1.4.3 Phospholipase C; EC 3.1.4.4. Phospolipase D.
Commercially
available phospholipases include LECITASE~ from Novo Nordisk A/S of Denmark
and
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
Phospholipase A2 from Sigma. When phospolipases are included in the
compositions of the
present invention, it is preferred that amylases are also included. Without
desiring to be bound
by theory, it is believed that the combined action of the phospholipase and
amylase provide
substantive stain removal, especially on greasy/oily, starchy and highly
colored stains and soils.
5 Preferably, the phospholipase and amylase, when present, are incorporated
into the compositions
of the present invention at a pure enzyme weight ratio between 4500:1 and 1:5,
more preferably
between 50:1 and l: 1.
Suitable proteases are the subtilisins which are obtained from particular
strains of B.
subtilis and B. licheniformis (subtilisin BPN and BPN'). One suitable protease
is obtained from
10 a strain of Bacillus, having maximum activity throughout the pH range of 8-
12, developed and
sold as ESPERASE~ by Novo Industries A/S of Denmark, hereinafter "Novo". The
preparation
of this enzyme and analogous enzymes is described in GB 1,243,784 to Novo.
Proteolytic
enzymes also encompass modified bacterial serine proteases, such as those
described in European
Patent Application Serial Number 87 303761.8, filed April 28, 1987
(particularly pages 17, 24
1 S and 98), and which is called herein "Protease B", and in European Patent
Application 199,404,
Venegas, published October 29, 1986, which refers to a modified bacterial
serine protealytic
enzyme which is called "Protease A" herein. Suitable is the protease called
herein "Protease C",
which is a variant of an alkaline serine protease from Bacillus in which
Lysine replaced arginine
at position 27, tyrosine replaced valine at position 104, serine replaced
asparagine at position
20 123, and alanine replaced threonine at position 274. Protease C is
described in EP 90915958:4,
corresponding to WO 91/06637, Published May 16. 1991. Genetically modified
variants,
particularly of Protease C, are also included herein.
A preferred protease referred to as "Protease D" is a carbonyl hydrolase as
described in
U.S. Patent No. 5,677,272, and W095/10591. Also suitable is a carbonyl
hydrolase variant of
25 the protease described in W095/10591, having an amino acid sequence derived
by replacement
of a plurality of amino acid residues replaced in the precursor enzyme
corresponding to position
+210 in combination with one or more of the following residues : +33, +62,
+67, +76, +100,
+101, +103, +104, +107, +128, +129, +130, +132, - 135, +156, +158, +164, +166,
+167, +170,
+209, +21 S, +217, +218, and +222, where the numbered position corresponds to
naturally-
occurring subtilisin from Bacillus amyloliquefaciens or to equivalent amino
acid residues in other
carbonyl hydrolases or subtilisins, such as Bacillus ltntus subtilisin (co-
pending patent
application US Serial No. 60/048,550, filed June 04, 1997 and PCT
International Application
Serial No. PCT/IB98/00853).
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
26
Also suitable for the present invention are proteases described in patent
applications EP
251 446 and WO 91/06637, protease BLAP~ described in W091/02792 and their
variants
described in WO 95/23221.
See also a high pH protease from Bacillus sp. NCIMB 40338 described in WO
93/18140 A to Novo. Enzymatic detergents comprising protease, one or more
other enzymes, and
a reversible protease inhibitor are described in WO 92/03529 A to Novo. When
desired, a
protease having decreased adsorption and increased hydrolysis is available as
described in WO
95/07791 to Procter & Gamble. A recombinant trypsin-like protease for
detergents suitable
herein is described in WO 94/25583 to Novo. Other suitable proteases are
described in EP 516
200 by Unilever.
Particularly useful proteases are described in PCT publications: WO 95/30010;
WO
95/30011; and WO 95/29979. Suitable proteases are commercially available as
ESPERASE~,
ALCALASE~, DURAZYM~, SAVINASE~, EVERLASE~ and KANNASE~ all from Novo
Nordisk A/S of Denmark, and as MAXATASE~, MAXACAL~, PROPERASE~ and
MAXAPEM~ all from Genencor International (formerly Gist-Brocades of The
Netherlands).
Such proteolytic enzymes, when present, are incorporated in the cleaning
compositions
of the present invention a level of from 0.0001% to 2%, preferably from 0.001%
to 0.2%, more
preferably from 0.005% to 0.1% pure enzyme by weight of the composition.
Amylases (a and/or 13) can be included for removal of carbohydrate-based
stains.
W094/02597 describes cleaning compositions which incorporate mutant amylases.
See also
W095/10603. Other amylases known for use in cleaning compositions include both
a- and (3-
amylases. a-Amylases are known in the art and include those disclosed in US
Pat. no. 5,003,257;
EP 252,666; WO/91/00353; FR 2,676,456; EP 285,123; EP 525,610; EP 368,341; and
British
Patent specification no. 1,296,839 (Novo). Other suitable amylases are
stability-enhanced
amylases described in W094/18314 and W096/05295, Genencor, and amylase
variants having
additional modification in the immediate parent available from Novo Nordisk
A/S, disclosed in
WO 95/10603. Also suitable are amylases described in EP 277 216.
Examples of commercial a-amylases products are Purafect Ox Am~ from Genencor
and
Termamyl~, Ban~ ,Fungamyl~ and Duramyl~, all available from Novo Nordisk A/S
Denmark.
W095/26397 describes other suitable amylases : a-amylases characterised by
having a specific
activity at least 25% higher than the specific activity of Termamyl~ at a
temperature range of 25°
C to 55°C and at a pH value in the range of 8 to 10, measured by the
Phadebas~ a-amylase
activity assay. Suitable are variants of the above enzymes, described in
W096/23873 (Novo
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
27
Nordisk). Other amylolytic enzymes with improved properties with respect to
the activity level
and the combination of thermostability and a higher activity level are
described in W095/35382.
Such amylolytic enzymes, when present, are incorporated in the cleaning
compositions of
the present invention a level of from 0.0001 % to 2%, preferably from 0.00018%
to 0.06%, more
preferably from 0.00024% to 0.048% pure enzyme by weight of the composition.
The above-mentioned enzymes may be of any suitable origin, such as vegetable,
animal,
bacterial, fungal and yeast origin. Origin can further be mesophilic or
extremophilic
(psychrophilic, psychrotrophic, thermophilic, barophilic, alkalophilic,
acidophilic, halophilic,
etc.). Purified or non-purified forms of these enzymes may be used. Nowadays,
it is common
practice to modify wild-type enzymes via protein / genetic engineering
techniques in order to
optimize their performance efficiency in the laundry detergent and/or fabric
care compositions of
the invention. For example, the variants may be designed such that the
compatibility of the
enzyme to commonly encountered ingredients of such compositions is increased.
Alternatively,
the variant may be designed such that the optimal pH, bleach or chelant
stability, catalytic
activity and the like, of the enzyme variant is tailored to suit the
particular cleaning application.
In particular, attention should be focused on amino acids sensitive to
oxidation in the
case of bleach stability and on surface charges for the surfactant
compatibility. The isoelectric
point of such enzymes may be modified by the substitution of some charged
amino acids, e.g. an
increase in isoelectric point may help to improve compatibility with anionic
surfactants. The
stability of the enzymes may be further enhanced by the creation of e.g.
additional salt bridges
and enforcing calcium binding sites to increase chelant stability.
These optional detersive enzymes, when present, are normally incorporated in
the
cleaning composition at levels from 0.0001% to 2% of pure enzyme by weight of
the cleaning
composition. The enzymes can be added as separate single ingredients (prills,
granulates,
stabilized liquids, etc... containing one enzyme ) or as mixtures of two or
more enzymes ( e.g.
cogranulates ).
Other suitable detergent ingredients that can be added are enzyme oxidation
scavengers.
Examples of such enzyme oxidation scavengers are ethoxylated tetraethylene
polyamines.
A range of enzyme materials and means for their incorporation into synthetic
detergent
compositions is also disclosed in WO 9307263 and WO 9307260 to Genencor
International, WO
8908694, and U.S. 3,553,139, January 5, 1971 to McCarty et al. Enzymes are
further disclosed in
U.S. 4,101,457, and in U.S. 4,507,219. Enzyme materials useful for liquid
detergent formulations,
and their incorporation into such formulations, are disclosed in U.S.
4,261,868.
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
28
Amylase enzymes are suitable for use in the compositions of the present
invention.
Amylase enzymes and variants used in the present invention include, but are
not limited to, the
amylase enzymes described in WO 95/26397 and in WO 96/23873 (Novo). These
enzymes are
incorporated into cleaning compositions at a level of from about 0.0001%,
preferably from about
0.00018%, more preferably from about 0.00024%, most preferably from about
0.05% to about
0.1%, preferably to about 0.060%, more preferably to about 0.048% by weight of
the cleaning
compositions of pure enzyme.
The amylase variants are preferably selected from the group consisting of a-
amylase
variants.
Suitable a-amylase variants for use in the present invention include, but are
not limited to
the following a-amylases:
(i) a-amylase characterized by having a specific activity at least 25%
higher than the specific activity of Termamyl~ at a temperature range of
25°C to 55°C and at a
pH value in the range of 8 to 10, measured by Phadebas~ a-amylase activity
assay and/or;
(ii) a-amylase according to (i) comprising the amino acid sequence shown in
SEQ >D No. 1 or an a-amylase being at least 80% homologous with the amino acid
sequence
shown in SEQ m No. 1 andlor;
(iii) a-amylase according to (i) comprising the amino acid sequence shown in
SEQ lD No. 2 or an a-amylase being at least 80% homologous with the amino acid
sequence
shown in SEQ m No. 2 and/or;
(i'v) a-amylase according to (i) comprising the following amino acid sequence
N-
terminal: His-His-Asn-Gly-Thr-Asn-Gly-Thr-Met-Met-Gln-Tyr-Phe-Glu-Trp-Tyr-Leu-
Pro-Asn-
Asp (SEQ m No. 3) or an a-amylase being at least 80% homologous with the amino
acid
sequence shown (SEQ m No. 3) in the N-terminal and/or;
(v) a-amylase according to (i-iv) wherein the a-amylase is obtainable from an
alkalophilic Bacillus species and/or;
(vi) a-amylase according to (v) wherein the amylase is obtainable from any of
the strains NCIB 12289, NCIB 12512, NCIB 12513 and DSM 935 and/or;
(vii) a-amylase showing positive immunological cross-reactivity with
antibodies
raised against an a-amylase having an amino acid sequence corresponding
respectively to SEQ
>D No. 1, )D No. 2, or )T7 No. 3 and/or;
(viii) variant of a parent a-amylase, wherein the parent a-amylase (1) has one
of
the amino acid sequences shown in SEQ 1D No. 1, )D No. 2, or )D No. 4,
respectively, or (2)
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
29
displays at least 80% homology with one or more of said amino acid sequences,
and/or displays
immunological cross-reactivity with an antibody raised against an a-amylase
having one of said
amino acid sequences, and/or-is encoded by a DNA sequence which hybridizes
with the same
probe as a DNA sequence encoding an a-amylase having one of said amino acid
sequences, in
which variants: (A) at least one amino acid residue of said parent a-amylase
has been deleted;
and/or (B) at least one amino acid residue of said parent a-amylase has been
replaced by a
different amino acid residue; and/or (C) at least one amino acid residue has
been inserted relative
to said parent a-amylase; said variant having an a-amylase activity and
exhibiting at least one of
the following properties relative to said parent a-amylase: increased
thermostability; increased
I O stability towards oxidation; reduced Ca ion dependency; increased
stability and/or a-amylolytic
activity at neutral to relatively high pH values; increased a-amylolytic
activity at relatively high
temperature; and increase or decrease of the isoelectric point (pI) so as to
better match the pI
value for a-amylase variant to the pH of the medium.
A polypeptide is considered to be X% homologous to the parent amylase if a
comparison
of the respective amino acid sequences, performed via algorithms, such as the
one described by
Lipman and Pearson in Science 227, 1985, p. 1435, reveals an identity of X%.
In the context of the present invention, the term "obtainable from" is
intended not only to
indicate an amylase produced by a Bacillus strain but also an amylase encoded
by a DNA
sequence isolated from such a Bacillus strain and produced in a host organism
transformed with
the DNA sequence.
Enzyme Stabilizers
Enzymes for use in detergents can be stabilized by various techniques. Enzyme
stabilization techniques are disclosed and exemplified in U.S. 3,600,319, EP
199,405 and EP
200,586. Enzyme stabilization systems are also described, for example, in U.S.
3,519,570. A
useful Bacillus, sp. AC13 giving proteases, xylanases and cellulases, is
described in WO
9401532. The enzymes employed herein can be stabilized by the presence of
water-soluble
sources of calcium and/or magnesium ions in the finished compositions which
provide such ions
to the enzymes. Suitable enzyme stabilizers and levels of use are described in
U.S. Pat. Nos.
5,705,464, 5,710,115 and 5,576,282.
The following is a non-limiting example of the preparation of a bleach
catalyst which
effectively bleaches stains in the absence of a source of peroxygen.
EXAMPLE 1
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
Synthesis of 5,12-dimethyl-1 5 8 12-tetraaza-bicyclol6 6 2~hexadecane
manganese (II)
chloride, having the formula:
~N
Mri
Cl~ N ~N~
5 To a 250 mL, 3 necked round bottom flask, equipped with a thermometer,
nitrogen inlet,
and magnetic stirrer is added N,N'-bis(2-aminoethyl)-1,3-propanediamine
(S.OOg, 31.3 mmol)
and absolute ethanol (100 mL). The solution is stirred under argon and cooled
to 15°C using an
ice bath. Aqueous glyoxal (4.78 g., 33 mmol, 40% in water) is added dropwise
with stirring.
Upon completion of the addition, the solution is concentrated under reduced
pressure to yield a
10 clear, colorless oil. The isolated oil has the formula 1:
N N
N N
H I
H H
and is obtained in 100% yield (6.0 g).
Cyclic amine 1 (6.0 g) is suspended in acetonitrile ( 100 mL). Potassium
carbonate (25 g)
15 and 1,3-propanediol ditosylate (12.61 g, 32.8 mmol) ~s added. The solution
is stirred vigorously
at RT overnight. The reaction is then warmed to 70°C and filtered hot
with glass fiber filter paper
and vacuum filtration. The resulting solid.is washed with acetonitrile (100
mL). The acetonitrile
filtrate is concentrated under reduced pressure to geld a light green oil
having the formula 2:
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
31
N N
N N
2
and is obtained in 100% yield (7.0 g).
The tetraamine 2, (7.0 g) is dissolved in acetonitrile (150 mL). Methyl
sulfate (2.5
equiv.) is added, the reaction warmed to 65°C and stirred for 9 days.
The solvent is removed
under reduced pressure to yield a brown oil having the formula 3
~/ _
N N CH30S03
CH30S03 -/N N
3
and is obtained in approximately 85% yield.
Distilled water (25 mL) and potassium carbonate (13.8 g) are added to a 250 mL
round
bottomed flask. Absolute ethanol (75 mL) is added and the resulting biphasic
solution is stirred
and heated to 60°C with an oil bath. Sodium borohydride ( 1.60 g., 42.3
mmol) and 3 ( 10.0 g.,
21.1 mmol) was added to the solution. The reaction is stirred at 60°C
for 75 minutes. The
reaction mixture is placed in a separatory funnel and the ethanol layer
collected. The solvent is
then removed under reduced pressure, the resulting tan solid/oil is dissolved
in SN KOH (5 mL)
and extracted with toluene (2 x 50 mL). The toluene is removed under reduced
pressure to yield
5,12-dimethyl-1,5,8,12-tetraaza-bicyclo[6.6.2]hexadecane having the formula:
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
32
N N
/N N
4
as an oil, in 95% yield (5.2 g) after distillation.
To a flame dried 12 liter, 3 neck round bottom flask equipped with a heating
mantle, stir
bar, and oven dried condenser is added anhydrous acetonitrile (5 liters) and
yield 5,12-dimethyl-
1,5,8,12-tetraaza-bicyclo[6.6.2]hexadecane (484 gm., 1.9 moles). The milky
white suspension is
placed under a 10 mm vacuum until the suspension boiled and then the reaction
vessel flushed
with argon. This degassing is performed 7 times. After degassing is complete
manganese (II)
chloride (228 gm, 1.81 moles) is added. After refluxing for 4 hours with
vigorous stirring, the
suspension is immediately filtered through glass filter paper. The solvent is
removed from the
filtrate under reduced pressure at 45° C to yield a solid. The solid is
then suspended in 500 ml.
toluene, and the supernatant decanted off. This washing is repeated until the
supernatant is free
of color (typically 7 times with about 7X500 ml of toluene). The solid which
remains I dried in
vacuo to yield 575 g (84%) of 5,12-dimethyl-1,5,8,12-tetraaza-
bicyclo[6.6.2]hexadecane
magnanese (II) chloride. A second crop of product is obtained by further
washing of the solid
material and subsequent treatment of the resulting solid in a like manner.
Total yield is 636 g
(93%).
The following are non-limiting examples of Heavy Duty Liquid (HDL) laundry
detergent
compositions according to the present invention.
TABLE I
weight
Ingredients 2 3 4 5
C,4-C,5 alkyl E1.0 sulfate22.5 22.5 22.5 22.5
Linear alkyl benzene 3.0 3.0 3.0 3.0
sulfonate
C, amidopropyl DMA 1.5 1.5 1.5 1.5
C,2-C,4 alkyl E7.0 3.0 3.0 3.0 3.0
Citric Acid 2.5 2.5 2.5 2.5
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
33
C,~-C,8 alkyl fatty 3.5 3.5 3.5 3.5
acid
Rapeseed fatty acid 5.0 5.0 5.0 5.0
protease 0.8 1.57 1.57 1.57
amylase 0.055 0.088 0.088 0.088
cellulase 0.188 0.055 0.055 0.055
lipolase 0.06 -- -- --
mannanase 0.007 0.0033 0.0033 0.0033
Sodium metaborate 2.0 2.5 2.5 2.5
Ca formate/CaCh 0.02 0.10 0.10 0.10
Bleach catalyst' 0.035 0.034 0.034 0.034
Anti-oxidant '' 0.23 0.25 0.25 0.25
Reducing agent' 0.02 -- -- --
Reducing agent 4 -- -- 0.0042 0.0083
Hydrophobic dispersant 0.65 0.76 0.76 0.76
Soil release agent 6 0.147 -- -- --
Soil release agent' -- 0.10 0.10 0.10
Suds suppresser 0.60 0.60 0.60 0.60
Water and minors balance balance balance balance
1. 5,12-dimethyl-1,5,8,12-tetraaza-bicyclo[6.6.2]hexadecane manganese (II)
chloride.
2. Butylated hydroxy toluene.
3. Potassium metabisulfite.
4. Potassium sulfite.
5 5. PEI 189 E15-18 according to U.S. Patent 4,597,898 Vander Meer, issued
July 1, 1986.
6. Soil release agent according to U.S. Patent 4,702,857 Gosselink, issued
October 27, 1987.
7. Soil release agent according to U.S. Patent 4,968,451, Scheibel et al.,
issued November 6,
1990.
TABLE II
weight
Ingredients 6 7 8 9
C,4-C,5 alkyl E1.0 sulfate22.5 22.5 -- --
C,Z-C,5 alkyl EI .8 -- -- 23.5 23.5
sulfate
Linear alkyl benzene 3.0 3.0 3.0 3.0
sulfonate
CA 02364538 2001-08-28
WO 00/52124 PCT/US00/05291
34
C,~ amidopropyl DMA 1.5 1.~ -- --
C, ~-C,:, alkyl E7.0 3.0 3.0 2.0 2.0
Citric Acid 2.5 2.5 2.5 2.5
Sodium sulfate -- -- 1.75 1.75
C,,-C,8 alkyl fatty acid3.5 3.~ 5.0 5.0
Rapeseed fatty acid 5.0 5.0 6.5 6.5
protease 1.57 1.57 1.57 1.57
amylase 0.088 0.088 0.088 0.088
cellulase 0.055 0.055 0.055 0.055
mannanase 0.0033 0.0033 0.0033 0.0033
Sodium metaborate 2.5 2.5 2.5 2.5
Ca formate/CaCh 0.10 0.10 0.10 0.10
Bleach catalyst' 0.034 0.004 0.04 0.04
Anti-oxidant 2 0.25 0.025 0.25 0.25
Reducing agent 3 -- -- 0.01 --
Reducing agent 4 0.0083 0.001 -- 0.01
Hydrophobic dispersant' 0.76 0.76 1.20 1.20
Soil release agent 6 -- -- -- --
Soil release agent' 0.10 0.10 0.10 0.10
Suds suppresser 0.60 0.60 0.60 0.60
Water and minors balance balance balance balance
1. 5,12-dimethyl-1,5,8,12-tetraaza-bicyclo[6.6.2]hexadecane manganese (II)
chloride.
2. Butylated hydroxy toluene.
3. Potassium metabisulfite.
4. Potassium sulfite.
5. PEI 189 E15-18 according to U.S. Patent 4,597,898 Vander Meer, issued July
1, 1986.
6. Soil release agent according to U.S. Patent 4,702,857 Gosselink, issued
October 27, 1987.
7. Soil release agent according to U.S. Patent 4,968,451, Scheibel et al.,
issued November 6,
1990.