Note: Descriptions are shown in the official language in which they were submitted.
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
Method for Localization of Blood Clots
FIELD OF THE INVENTION
The present invention relates to a medical diagnostic
method and, in particular, to an in vivo diagnostic method for
detecting a blood clot, such as a pulmonary embolism or a
thrombus, employing a radiopharmaceutical contrast agent and
volume rendering of single photon emission computed tomography
(SPECT) images .
BACKGROUND OF THE INVENTION
Pulmonary embolism is a condition of the lung that
emerges when a portion of a blood clot (i.e., thrombus)
growing pathologically within a patient breaks off (i.e.,
embolizes) and travels to the lung. In many instances, the
condition itself is immediately life-threatening. However,
even when the condition is not immediately life-threatening, a
patient presenting with symptoms characteristic of pulmonary
embolism must be properly diagnosed to assure that the
symptoms do not represent other diseases. Accordingly,
detection and localization of pulmonary embolism are critical
to insure that the patient receives the appropriate care.
Previously, a technique for diagnosing tumors has been
developed. The technique involves .localizing a contrast agent
at the tumor and obtaining a series of image slices of the
tumor using single photon emission computed tomography
(SEECT). The image slices are then individually inspected by
a physician. As a result, the process is time consuming and
expensive.
To improve the ability to diagnose tumors using SPECT, a
volume rendering technique has been developed for displaying
SPECT data derived from a complete set of image slices through
the tumor. According to this technique, a three-dimensional
matrix of data is assembled from the image slices. The three-
dimensional matrix of data is then scanned along an array of
parallel lines at a given angle with respect to the tumor.
-1-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
For each parallel line, the value of the most intense pixel
along the parallel line is determined and assigned to a pixel
in a two-dimensional array whose position corresponds to the
position of the corresponding parallel line in the array of
parallel lines. The process is repeated for a series of
angles over 360° to produce a series of two-dimensional
images. When the series of two-dimensional images are
displayed sequentially, a rotating view of the most intense
pixels is produced.
In spite of the foregoing, the utility of SPELT as a tool
for diagnosing pulmonary embolism remained limited. The
limited use of SPELT in connection with pulmonary embolism is
due, at least in part, to the fact that the normal anatomy of
the thorax is complex. As a result, structures highlighted by
the contrast agent are variable, often in a pattern that is
unfamiliar to physicians, and without the normal identifying
landmarks. Thus, the location of the thrombus and the extent
of disease was expected to be difficult to ascertain from the
SPELT images, even if volume rendering techniques were
employed.
Accordingly, it would be highly beneficial to provide a
method for identification and localization of pulmonary
embolism using SPELT wherein a three-dimensional
representation of a thrombus is obtained. The three-
dimensional representation of the thrombus should enable a
physician to more clearly, accurately, and efficiently
determine the extent of disease. Accordingly, the present
invention should provide a significant qualitative improvement
in the ability of a naive physician to identify and localize a
thrombus.
SUi~2ARY OF THE INVENTION
The shortcomings associated with the known methods for
localization of blood clots are overcome to a large degree by
a method in accordance with the present invention. The method
according to the present invention comprises the step of
localizing a radiolabelled compound at a thrombus by
-2-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
administering a radiopharmaceutical compound to the patient.
Two-dimensional images representing a physical property
associated with the radiolabelled thrombus, such as single
photon emission computed tomography (SPELT) images, are then
acquired and assembled into a three-dimensional matrix of
data. The three-dimensional matrix of data is then scanned
along an array of parallel lines to determine a maximum value
along each line. The maximum value along each line is then
assigned to a pixel in a two-dimensional array, where the
relative position of the pixel in the two-dimensional array
corresponds to the relative position of the line in the array
of parallel lines. The three-dimensional matrix of data is
optionally scanned along additional arrays of parallel lines
to produce a series of images of the thrombus as viewed from
different angles. The series of images can be displayed
sequentially to produce a rotating view of the thrombus.
BRIEF DESCRIPTION OF THE DRAWINGS
The foregoing summary, as well as the following detailed
description of the preferred embodiments of the present
invention, will be better understood when read in conjunction
with the accompanying drawing, in which:
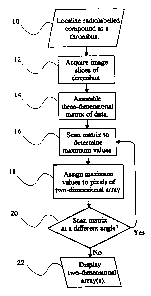
Fig. 1 is a flow chart depicting the steps of a method
for imaging a thrombus in accordance with the present
invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention relates to a method for imaging a
thrombus, the steps of which are depicted in Fig. 1. At step
10, the patient is administered a radiolabelled compound that
preferentially binds to the thrombus. For example, the
radiolabelled compound may be administered by injecting
approximately 20 mCi (740 Mbq) of the radiolabelled compound
into the venous circulation system of the patient. In one
embodiment, the radiolabelled compound comprises a
radiopharmaceutical of the type described in U.S. Patent No.
5,744,120 issued April 28, 1998 to Edwards et al., U.S. Patent
_3_
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
No. 5,879,657 issued March 9, 1999 to DeGrado et al., U.S.
Patent No. 5,879,659 issued March 9, 1999 to Edwards et al.,
and U.S. Patent No. 5,750,088 issued May 12, 1998 to Sworin et
al., all of which are incorporated herein by reference.
Specifically, a radiopharmaceutical useful as an imaging
agent in accordance with the present invention is given by
formula (I):
(Q) d' -Ln-Cn~ Ix'MT (AzO Y ~Ar.2~ Z
(I) ,
wherein d' is preferably between about 1 and 20, x is
independently 1-2; y is independently 1-2; and z is
independently 0-4.
Q is a glycoprotein IIb/IIIA binding compound selected
from the group including the cyclic IIb/IIIa, receptor
antagonist compounds described in co-pending U.S. Serial
No.08/415,908,861 (equivalent to WO 94/22494); the RGD
containing peptides described in U.S. Patent Nos. 4,578,079
and 4,792,525, the patent applications PCT US88/04403, PCT
US89/01742, PCT US90/03788, and PCT US91/02356, and by Ojima
et. al., 204th Meeting of the Amer. Chem. Soc., 1992, Abstract
44; the peptides that are fibrinogen receptor antagonists
described in European Patent Application Nos. 90202015.5,
90202030.4, 90202032.2, 90202032.0, 90311148.2, 90311151.6,
and 90311537.6; the specific binding peptides and polypeptides
described as IIb/IIIa receptor ligands, ligands for the
polymerization site of fibrin, laminin derivatives, ligands
for fibrinogen, or thrombin ligands in PCT WO 93/23085
(excluding the technetium binding groups); the oligopeptides
that correspond to the IIIa protein described in PCT WO
90/00178; the hirudin-based peptides described in PCT WO
90/03391; the IIb/IIIa receptor ligands described in PCT WO
90/15818; the thrombus, platelet or atherosclerotic plaque
binding peptides described in PCT WO 92/13572 (excluding the
technetium binding group) and GB 9313965.7; the fibrin binding
peptides described in U.S. Patent Nos. 4,427,646 and
-4-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
5,270,030; the hirudin-based peptides described in U.S. Patent
No. 5,279,812; the fibrin binding proteins described in U.S.
Patent No. 5,217,705; the guanine derivatives that bind to the
IIb/IIIa receptor described in U.S. Patent No. 5,086,069; the
tyrosine derivatives described in European Patent Application
No. 0478328A1, and Hartman et. al., J. Med. Chem., 1992, 35,
4640; or an oxidized low density lipoprotein (LDL).
In one embodiment , Q is of the formula ( I I )
/ K L \
,J M
R32 /NR2
R~ R23 C ' (C/(R2~)R~)n'
( C ) )n"\R3~/
(II)
or a pharmaceutically acceptable salt or prodrug form thereof
wherein:
R31 is a C6-C14 saturated, partially saturated, or
aromatic carbocyclic ring system substituted with 0-4 R10
or RlOa
R32 is selected from:
-C (=O) - ;
-C (=S) -
-S (=O) 2- l
-S (=O) -;
-P (=Z) (ZR13) -:
Z is S or O;
n" and n' are independently 0-2;
-5-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
R1 and R22 are independently selected from the following
groups:
hydrogen,
C1-Cg alkyl substituted with 0-2 R11;
C2-Cg alkenyl substituted with 0-2 R11;
C2-Cg alkynyl substituted with 0-2 R11;
C3-C10 cycloalkyl substituted with 0-2 R11;
aryl substituted with 0-2 R12;
a 5-10-membered heterocyclic ring system containing
1-4 heteroatoms independently selected from N, S,
and O, said heterocyclic ring being substituted with
0-2 R12;
=O, F, Cl, Br, I, -CF3, -CN, -C02R13, -C(=O)R13,
-C(=O)N(R13)2, -CHO, -CH20R13, -OC(=O)R13,
-OC (=O) ORl3a ~ _OR13 ~ -OC (=O) N (R13 ) 2 , -NR13C (--O) R13 ,
-NR14C (=O) ORl3a ~ _~13C (=O) N (R13 ) 2 , -NR14SO2N (R13 ) 2 .
-~14g02R13a~ _g03H~ _S02R13a~ _SR13~ _g(=O)Rl3a~
-S02N (R13 ) 2, -N (R13 ) 2, -NHC (=NH) NHR13 , -C (=NH) NHR13 ,
=NOR13, N02, -C(=O)NHOR13, -C(=O)NHNR13R13a~
-OCH2C02H, 2-(1-morpholino)ethoxy;
R1 and R21 can alternatively join to form a 3-7 membered
carbocyclic ring substituted with 0-2 R12;
when n' is 2, R1 or R21 can alternatively be taken
together with R1 or R21 on an adjacent carbon atom to
form a direct bond, thereby to form a double or triple
bond between said carbon atoms;
R22 and R23 can alternatively join to form a 3-7 membered
carbocyclic ring substituted with 0-2 R12;
when n" is 2, R22 or R23 can alternatively be taken
-6_
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
together with R22 or R23 on an adjacent carbon atom to
form a direct bond, thereby to form a double or triple
bond between the adjacent carbon atoms;
R1 and R2, where R21 is H, can alternatively join to form
a 5-8 membered carbocyclic ring substituted with 0-2 R12;
R11 is selected from one or more of the following:
=O, F, Cl, Br, I, -CF3, -CN, -C02R13, -C(=O)R13,
-C(=O)N(R13)2, -CHO, -CH20R13, -OC(=O)R13,
-OC (=O) ORl3a~ _OR13 ~ -pC (=O) N (R13 ) 2 ~ -~13C (=O) R13 .
_~14C (=O) ORl3a ~ _Ngl3C (=O) N (R13 ) 2 . -Ng14S02N (Rl3 ) 2 .
-~14g02R13a~ _g03H, _g02R13a~ _gRl3~ _g(=O)Rl3a~
-S02N(R13)2, -N(R13)2, -NHC(=NH)NHR13, -C(=NH)NHR13,
=NOR13, N02, -C(=O)NHOR13, -C(=O)NHNR13R13a~
-OCH2C02H, 2-(1-morpholino)ethoxy,
C1-C5 alkyl, C2-C4 alkenyl, C3-C6 cycloalkyl, C3-C6
cycloalkylmethyl, C2-C6 alkoxyalkyl, C3-C6
cycloalkoxy, C1-C4 alkyl (alkyl being substituted
with 1-5 groups selected independently from:
~~13R14~ _CF3~ N02, -S02R13a, or -S(=O)Rl3a).
aryl substituted with 0-2 R12,
a 5-10-membered heterocyclic ring system containing
1-4 heteroatoms independently selected from N, S,
and O, said heterocyclic ring being substituted with
0-2 R12;
R12 is selected from one or more of the following:
phenyl, benzyl, phenethyl, phenoxy, benzyloxy,
halogen, hydroxy, nitro, cyano, C1-C5 alkyl, C3-C6
cycloalkyl, C3-C6 cycloalkylmethyl, C~-C10
arylalkyl, C1-C5 alkoxy, -C02R13, -C(=O)NHORI3a~
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
-C(=O)NHN(R13)2~ =NOR13, -B(R34)(R35). C3_C6
cycloalkoxy, -OC(=O)R13, -C(=O)R13,-OC(=O)ORl3a~
-OR13, - (C1-C4 alkyl) -OR13, -N(R13) 2,
-OC(=O)N(R13)2. -NR13C(=O)R13, -NR13C(°O)ORl3a~
-NR13 C ( =O ) N (R13 ) 2 ~ -X13 S02N ( R13 ) 2 ~ -NR13 S02R13 a
-S03H~ _g02R13a~ -S(=O)Rl3a~ _SR13~ _g02N(R13)2.
C2-C6 alkoxyalkyl, methylenedioxy, ethylenedioxy,
C1-C4 haloalkyl, C1-C4 haloalkoxy, C1-C4
alkylcarbonyloxy, C1-C4 alkylcarbonyl, C1-C4
alkylcarbonylamino, -OCH2C02H,
2-(1-morpholino)ethoxy, C1-C4 alkyl (alkyl being
substituted with -N(R13)2; -CF3, N02, or
-S (=O) Rl3a)
R13 is selected independently from: H, C1-C10 alkyl,
C3-C10 cycloalkyl, C4-C12 alkylcycloalkyl, aryl, -(C1-C10
alkyl)aryl, or C3-C10 alkoxyalkyl;
Rl3a is C1-C10 alkyl, C3-Clp cycloalkyl, C4-C12
alkylcycloalkyl, aryl, -(C1-C10 alkyl)aryl, or C3-C10
alkoxyalkyl;
when two R13 groups are bonded to a single N, said R13
groups may alternatively be taken together to form
-(CH2)2-5- or -(CH2)O(CH2)-;
R14 is OH, H, C1-C4 alkyl, or benzyl;
R21 and R23 are independently selected from:
hydrogen;
C1-C4 alkyl, optionally substituted with 1-6
halogen;
benzyl;
R2 is H or C1-Cg alkyl;
_g_
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
R10 and RlOa are selected independently from one or more
of the following:
phenyl, benzyl, phenethyl, phenoxy, benzyloxy;
halogen, hydroxy, nitro, cyano, C1-C5 alkyl, C3-C6
cycloalkyl, C3-C6 cycloalkylmethyl, C~-C10
arylalkyl, C1-C5 alkoxy, -C02R13, -C(=O)N(R13)2,
-C(=O)NHORI3a~ _C(=0)NHN(R13)2, =NOR13,
-g(R34)(R35)~ C3-C6 cycloalkoxy, °OC(=O)R13,
-C(=O)R13~_OC(=O)ORl3a~ _OR13~ -(C1-C4 alkyl)-OR13,
-N(R13)2~ -OC(=O)N(R13)2~ _~13C(=O)R13
_~13C(=O)ORl3a~ _~13C(=O)N(R13)2, -NR13S02N(R13)2.
-~13S02R13a~ _g03H, _g02R13a~ _S(=O)Rl3a~ _gRl3~
-S02N(R13)2, C2-C6 alkoxyalkyl, methylenedioxy,
ethylenedioxy, C1-C4 haloalkyl (including -CvFw
where v = 1 to 3 and w = 1 to (2v+1)),. C1-C4
haloalkoxy, C1-C4 alkylcarbonyloxy, C1-C4
alkylcarbonyl, C1-C4 alkylcarbonylamino, -OCH2C02H,
2-(1-morpholino)ethoxy, C1-C4 alkyl (alkyl being
substituted with -N(R13)2, -CF3, N02, or
-S (=O) Rl3a) ;
J is 3-aminopropionic acid or an L-isomer or D-isomer
amino acid of structure -N(R3)C(R4)(R5)C(=O)-, wherein:
R3 is H or C1-Cg alkyl;
R4 is H or C1-C3 alkyl;
RS is selected from:
hydrogen;
C1-Cg alkyl substituted with 0-2 R11;
C2-Cg alkenyl substituted with 0-2 R11;
C2-Cg alkynyl substituted with 0-2 R11;
C3-C10 cYcloalkyl substituted with 0-2 R11;
aryl substituted with 0-2 R12;
_g_
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
a 5-10-membered heterocyclic ring system containing
1-4 heteroatoms independently selected from N, S, or
O, said heterocyclic ring being substituted with 0-2
R12
=O, F, Cl, Br, I, -CF3, -CN, -C02R13, -C(=O)R13,
-C(=O)N(R13)2, -CHO, -CH20R13, -OC(=O)R13,
-OC (=O) ORl3a~ _OR13 ~ -pC (=O) N (R13 ) 2, -NR13C (=O) R13 ,
-~14C (=O) ORl3a~ _~13C (=O) N (R13 ) 2. -NR14SO2N (R13 ) 2 .
_~14g02R13a~ _g03H~ _S02R13a~ _gRl3~ _g(=O)Rl3a~
-S02N(R13)2, -N(R13)2, -NHC(=NH)NHR13, -C(=NH)NHR13,
=NOR13, N02, -C(=O)NHOR13, -C(=O)NHNR13R13a~ =NOR13,
_B(R34)(R35)~ -OCH2C02H, 2-(1-morpholino)ethoxy,
-SC(=NH)NHR13, N3, -Si(CH3)3, (C1-C5 alkyl)NHR15;
- (Cp-C6 alkyl) X;
-(CH2)q ~ ~ (CHZ)q-X
where q is
independently 0,1;
-CHZ CHZX
-(CH2)mS(O)p~(CH2)2X, where m = 1,2 and p' - 0-2;
wherein X is defined below; and
-10-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
R3 and R4 may also be taken together to form
( ~ H2)nX
CHzCHCH2 ~ where n = 0,1 and X is
NR~3
NH-C\
N~R~a~R~s
R3 and R5 can alternatively be taken together to form
-(CH2)t- or -CH2S(O)p~C(CH3)2-, where t = 2-4 and p' -
0-2; or
R4 and R5 can alternatively be taken together to form
-(CH2)u-, where a = 2-5;
R16 is selected from:
an amine protecting group;
1-2 amino acids;
1-2 amino acids substituted with an amine protecting
group;
K is a D-isomer or L-isomer amino acid of structure
- (R6) CH(R~) C (=O) -, wherein:
R6 is H or C1-Cg alkyl;
R~ is selected from:
- (C1-C~ alkyl) X;
-11-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
(CHZ)q-X
-(CHZ)q
wherein each q is
independently 0-2 and substitution on the
phenyl is at the 3 or 4 position;
-(CHz)q (CHz)q-X
, wherein each
g is independently 0-2 and substitution on the
cyclohexyl is at the 3 or 4 position;
~C~ Cs a~~
~\~NH
\,M,/O - 3 ;
-(CH2)m0-(C1-C4 alkyl)-X, where m = 1 or 2;
-(CH2)mS(O)p~-(C1-C4 alkyl)-X, where m = 1 or 2
and p' - 0-2; and
X is selected from:
NR~3
-NH-C\
N(Rt~R~a
-12-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
-N (R13 ) R13 ; -C (=NH) (NH2) ; -SC (=NH) -NH2 ; -NH-C (=NH) (NHCN) ;
-NH-C(=NCN)(NH2); -NH-C(=N-OR13)(NH2);
R6 and R~ can alternatively be taken together to form
( ~ H2)nX '
-(CH2)qCH(CH2)q- ~ wherein each q is independently 1
or 2 and wherein n = 0 or 1 and X is -NH2 or
NR~3
-NH°C\
N(R~~R~3
i
L is -Y(CH2)vC(=O)-, wherein:
Y is NH, N(C1-C3 alkyl), O, or S; and v = 1 or 2;
M is a D-isomer or L-isomer amino acid of structure
0
-NR~~-CH-C-
i
(~H(R4))q~
Rg
wherein:
q~. is 0-2;
-13-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
R1~ is H, C1-C3 alkyl;
R8 is selected from:
-C02R13,-S03R13, -S02NHR14, -B(R34)(R35), -NHS02CF3,
-CONHNHS02CF3, -PO(OR13)2, -PO(OR13)R13~
-S02NH-heteroaryl (said heteroaryl being
5-10-membered and having 1-4 heteroatoms selected
independently from N, S, or O) , -S02NH-heteroaryl
(said heteroaryl being 5-10-membered and having 1-4
heteroatoms selected independently from N, S, or O),
-S02NHCOR13, -CONHS02R13a~ -CH2CONHS02R13a~
-NHS02NHCORI3a~ _~CONHS02R13a~ _g02NHCONHR13;
R34 and R35 are independently selected from:
-OH,
-F,
-N(R13)2, or
C1-Cg-alkoxy;
R34 and R35 can alternatively be taken together form:
a cyclic boron ester where said chain or ring
contains from 2 to 20 carbon atoms and, optionally,
1-4 heteroatoms independently selected from N, S, or
O;
a divalent cyclic boron amide where said chain or
ring contains from 2 to 20 carbon atoms and,
optionally, 1-4 heteroatoms independently selected
from N, S, or O;
a cyclic boron amide-ester where said chain or ring
contains from 2 to 20 carbon atoms and, optionally,
1-4 heteroatoms independently selected from N, S, or
O.
In another embodiment, Q is of the formula (III):
-14-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
/K L'
M
2
NR
C R~
(III)
or a pharmaceutically acceptable salt or prodrug form
thereof wherein:
the shown phenyl ring may be further substituted with 0-3
R10
R10 is selected independently from: H, C1-Cg alkyl,
phenyl, halogen, or C1-C4 alkoxy;
R1 is H, C1-C4 alkyl, phenyl, benzyl, or phenyl-(C1-
C4)alkyl;
R2 is H or methyl;
R13 is selected independently from: H, C1-C10 alkyl,
C3-C10 cycloalkyl, C4-C12 alkylcycloalkyl, aryl, -(C1-C10
alkyl)aryl, or C3-C10 alkoxyalkyl;
Rl3a is C1-C10 alkyl, C3-C10 cycloalkyl, C4-C12
alkylcycloalkyl, aryl, -(C1-C10 alkyl)aryl, or C3-C10
alkoxyalkyl;
-15-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
when two R13 groups are bonded to a single N, said Rl3
groups may alternatively be taken together to form
-(CH2)2-5- or -(CH2)O(CH2)-;
R14 is OH, H, C1-C4 alkyl, or benzyl;
J is (3-alanine or an L-isomer or D-isomer amino acid of
structure -N(R3)C(R4)(R5)C(=O)-, wherein:
R3 is H or CH3;
R4 is H or C1-C3 alkyl;
R5 is H, C1-Cg alkyl, C3-C6 cycloalkyl, C3-C6
cycloalkylmethyl, C1-C6 cycloalkylethyl, phenyl,
phenylmethyl, CH20H, CH2SH, CH20CH3, CH2SCH3, CH2CH2SCH3,
(CH2)sNH2, -(CH2)sNHC(=NH)(NH2), -(CH2)sNHRl6, where s =
3-5; or
R16 is selected from:
an amine protecting group;
1-2 amino acids; or
1-2 amino acids substituted with an amine protecting
group;
R3 and R5 can alternatively be taken together to form
-CH2CH2CH2-; or
R4 and R5 can alternatively be taken together to form
-(CH2)u-, where a = ~-5;
K is an L-isomer amino acid of structure
-N(R6)CH(R~)C(=O)-, wherein:
R6 is H or C1-Cg alkyl;
R~ is:
-16-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
/NH
-(CH~q ~ ~ NH-
NH2
NH
_ccH,n
NH2 ~ where q = 0 or 1;
-(CH2)rX, where r = 3-6;
-CHZ CHzX
;
-CHZ ~ ~ CHZ-X
- ( CH2 ) mS ( CH2 ) 2X, where m = 1 or 2 ;
-(C3-C7 alkyl)-NH-(C1-C6 alkyl);
-17-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
~C~ - C4 alky~
NH
~~O-3 .
-(CH2)m-0-(C1-C4 alkyl)-NH-(C1-C6 alkyl), where
m = 1 or 2;
-(CH2)m-S-(C1-C4 alkyl)-NH-(C1-C6 alkyl), where
m = 1 or 2; and
X is -NH2 or -NHC(=NH)(NH2), provided that X is not -NH2
when r = 4; or
R6 and R7 are alternatively be taken together to form
( ~ HZ)~X
CH2CHCH2 where n = 0 , 1 and X is -NH2 or
-NHC (=NH) (NH2) ;
L is -Y(CH2)vC(=O)-, wherein:
Y is NH, O, or S; and v = 1,2;
M is a D-isomer or L-isomer amino acid of structure
O
-NR~7-CH-C-
i
(CH(R4))q,
Re
wherein:
q' is 0-2;
-18-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
R1~ is H, C1-C3 alkyl;
RS is selected from:
-C02R13,-S03R13, -S02NHR14, -B(R34)(R35), -NHS02CF3,
-CONHNHS02CF3, -PO(OR13)2, -PO(OR13)R13,
-S02NH-heteroaryl (said heteroaryl being
5-10-membered and having 1-4 heteroatoms selected
independently from N, S, or O) , -S02NH-heteroaryl
(said heteroaryl being 5-10-membered and having 1-4
heteroatoms selected independently from N, S, or O),
-S02NHCOR13, -CONHS02R13a, _CH2CONHS02R13a,
-NHS02NHCORI3a, -~CONHS02R13a, -S02NHCONHR13,
Ch. is a radionuclide metal chelator or bonding unit bound
to the biologically active compound Q, either directly or
through the optional linking group Ln. C,,. is preferably
selected from the group consisting of: R40N=N+=, R40R41N_N=,
R40N=, and R40N=N(H)-; wherein,
R40 is independently selected at each occurrence from the
group consisting of: a bond to Ln, C1-C10 alkyl
substituted with 0-3 R52, aryl substituted with 0-3 R52,
cycloaklyl substituted with 0-3 R52, heterocycle
substituted with 0-3 R52~ heterocycloalkyl substituted
with 0-3 R52, aralkyl substituted with 0-3 R52 and
alkaryl substituted with 0-3 R52;
R41 is independently selected from the group consisting
of: hydrogen, aryl substituted with 0-3 R52, C1-C10 alkyl
substituted with 0-3 R52, and a heterocycle substituted
with 0-3 R52;
R52 is independently selected at each occurrence from the
group consisting of: a bond to Ln, =O, F, Cl, Br,
I , -CF3 , -CN, -C02R53 , -C (=0) R53 , -C (=O) N (R53 ) 2 . -CHO,
-CH20R53, -OC(=O)R53, -OC(=O)OR53a, _OR53,
-19_
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
-OC (=0) N (R53 ) 2 , -NR53C (=O) R53 ~ _~54C (=O) OR53a
-NR53C(=O)N(R53)2, -NR54S02N(R53)2, -NR54S02R53a~ -S03H
-S02R53a~ _gR53~ _g(=O)R53a~ _g02N(R53)2, -N(R53)2,
-NHC(=NH)NHR53, -C(=NH)NHR53, =NOR53, N02, -C(=O)NHOR53,
-C(=O)NHNR53R53a~ -OCH2C02H, 2-(1-morpholino)ethoxy; and
R53~ R53a~ and R54 are each independently selected at
each occurrence from the group consisting of: hydrogen,
C1-C6 alkyl, and a bond to Ln.
In order to have a chelating diazenido group (i.e., a group of
formula R"°N=N+= or R'°N= (H) -) at least one other atom of the
group located on R40 must also be bound to the radionuclide.
The atoms bound to the metal are termed donor atoms.
The optional linking group Ln is given by the formula:
Ml- ~Y1 (CR55R56) f (Z1) f"Y2~ f' -M2,
wherein:
Ml is - ~ (CH2) gZll g' - (CR55R56) g..-:
M2 is - (CR55R56) g" _ (Z1 (CH2) gl g' -:
g is independently 0-10;
g' is independently 0-1;
g" is independently 0-10;
f is independently 0-10;
f' is independently 0-10;
f" is independently 0-1;
Y1 and Y2, are independently selected at each
-20-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
occurrence from: a bond, O, NR56, C=O, C(=O)O,
OC(=0)0, C(=O)NH-, C=NR56, S, S0, 502, S03,
NHC (=0) , (NH) 2C (=O) , and (NH) 2C=S;
Z1 is independently selected at each occurrence
from a C6-C14 saturated, partially saturated,
or aromatic carbocyclic ring system,
substituted with 0-4 RS~; and a heterocyclic
ring system, substituted with 0-4 RS~;
R55 and R56 are independently selected at each
occurrence from the group consisting of:
hydrogen; C1-C10 alkyl substituted with 0-5
RS~; and alkaryl wherein the aryl is
substituted with 0-5 RS~;
R5~ is independently selected at each
occurrence from the group: hydrogen, OH, NHR58,
C(=O)R58, OC(=O)R58, OC(=O)ORSa, C(=O)OR58,
C(=O)NR58, C=N, SRSS, SOR58, S02R58,
NHC (=O) R58, NHC (=O) NHR58, NHC (=S) NHR58; or,
alternatively, when attached to an additional
molecule Q, R5~ is independently selected at
each occurrence from the group: O, NR58, C=O,
C (=0) O, OC (=O) O, C (=O) N-, C=NR58 , S , SO, S02 ,
503, NHC(=O), (NH)2C(=O), (NH)2C=S; and
R58 is independently selected at each
occurrence from the group: hydrogen; C1-C6
alkyl; benzyl, and phenyl.
The radiopharmaceutical compound used in accordance with
the present invention is radiolabelled. By "radiolabelled",
it is meant that the compound contains a radioisotope which is
suitable for administration to a mammalian patient. Suitable
radioisotopes are known to those skilled in the art and
include, for example, isotopes of halogens (such as chlorine,
-21-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
fluorine, bromine and iodine), technetium and indium.
Preferable radioisotopes include 123I~ 1252 131I~ 99mTc, and
111In, more preferably 111In~ 1231 and 99mTc, and most
preferably 99mTc. Radiolabelled compounds of the invention
may be prepared using standard radiolabelling procedures well
known to those skilled in the art. The glycoprotein IIb/IIIa
binding compound, Q, is radiolabelled indirectly (that is, by
incorporating the radiolabel into the compound through the
chelating agent Ch,). Such radiolabelling should also be
reasonably stable, both chemically and metabolically, applying
recognized standards in the art. Also, although the
radiolabelled compound may be labeled in a variety of fashions
with a variety of different radioisotopes, as those skilled in
the art will recognize, such radiolabelling should be carried
out in a manner such that the high binding affinity and
specificity of the unlabeled glycoprotein IIb/IIIa binding
compound to the glycoprotein IIb/IIIa receptor is not
significantly affected. By not significantly affected, it is
meant that the binding affinity and specificity is not
affected more than about 50%, preferably not more than about
40%, more preferably not more than about 30%, even more
preferably not more than about 20%, and still even more
preferably not more than about 10%, and most preferably the
binding affinity and specificity is not affected at all.
Referring again to formula (I), MT is a transition metal
radionuclide which is attached to the biologically active
compound Q via the chelator Ch.. Preferred radiolabelled
compounds of the invention are radiolabelled compounds wherein
the radiolabel is located on the carbocyclic ring system of
R31 of formula (II). Even more preferred radiolabelled
compounds of the invention are those of formula (III), wherein
the radiolabel is located at position R10 or RlOa substituted
on the benzene ring.
The coordination sphere of the radionuclide includes all
the ligands or groups bound to the radionuclide. For a
transition metal radionuclide, Mt, to be stable it typically
has a coordination number comprised of an integer greater than
-22-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
or equal to 5 and less than or equal to 7; that is there are 5
to 7 atoms bound to the metal and it is said to have a
complete coordination sphere. If the chelator or bonding unit
does not provide all of the atoms necessary to stabilize
the metal radionuclide by completing its coordination sphere,
the coordination sphere is completed by donor atoms from other
ligands, termed ancillary or co-ligands, which can be either
terminal or chelating.
A large number of ligands can serve as ancillary or
co-ligands, the choice of which is determined by a variety of
considerations such as the ease of synthesis of the
radiopharmaceutical, the chemical and physical properties of
the ancillary ligand, the rate of formation, the yield, the
number of isomeric forms of the resulting
radiopharmaceuticals, the ability to administer said ancillary
or co-ligand to a patient without adverse physiological
consequences to said patient, and the compatibility of the
ligand in a lyophilized kit formulation. The charge and
lipophilicity of the ancillary ligand will effect the charge
~ and lipophilicity of the radiopharmaceuticals. For example,
the use of 4,5-dihydroxy-1,3-benzene disulfonate results in
radiopharmaceuticals with an additional two anionic groups
because the sulfonate groups will be anionic under
physiological conditions. The use of N-alkyl substituted
3,4-hydroxypyridinones results in radiopharmaceuticals with
varying degrees of lipophilicity depending on the size of the
alkyl substituents.
The radiopharmaceuticals prepared from the reagents of
the present invention can be comprised of one or two ancillary
or co-ligands, designated AL1, in a binary ligand system. The
one or two ancillary or co-ligands, AL1, comprising the
radiopharmaceuticals can be independently selected from the
group consisting of: dioxygen ligands, functionalized
aminocarboxylates and halides; provided that the coordination
sphere of the radionuclide is complete.
Ancillary dioxygen ligands include ligands that
coordinate to the metal ion through at least two oxygen donor
-23-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
atoms. Examples include but are not limited to:
glucoheptonate, gluconate, 2-hydroxyisobutyrate, lactate,
tartrate, mannitol, glucarate, maltol, Kojic acid,
2,2-bis(hydroxymethyl)propionic acid,
4,5-dihydroxy-1,3-benzene disulfonate, or substituted or
unsubstituted 1,2 or 3,4 hydroxypyridinones, or
pharmaceutically acceptable salts thereof.
Functionalized aminocarboxylates include ligands that
have a combination of nitrogen and oxygen donor atoms.
Examples include but are not limited to: iminodiacetic acid,
2,3 diaminopropionic acid, nitrilotriacetic acid,
N,N'-ethylenediamine diacetic acid, N,N,N'-ethylenediamine
triacetic acid, hydroxyethylethylenediamine triacetic acid,
N,N'-ethylenediamine bis-hydroxyphenylglycine, or the ligands
described in Eur. Pat. Appl. No. 93302712.0, or
pharmaceutically acceptable salts thereof.
Halides which are suitable for use as the ancillary
ligand ALlcan be chloride, bromide, fluoride, iodide, or
pharmaceutically acceptable salts thereof.
Of particular utility are radiopharmaceuticals prepared
from the reagents of the present invention comprised of two
different types of ancillary or co-ligands, one or two ligands
designated the .first ancillary or co-ligand or ligands, AL1.
and independently selected from the group: dioxygen ligands,
functionalized aminocarboxylates and halides; and one to four
ligands designated the second ancillary or co-ligand or
ligands, AL2, selected from the group: trisubstituted
phosphines, trisubstituted arsines, tetrasubstituted
diphosphines and tetrasubstituted diarsines, in a ternary
ligand system. Radiopharmaceuticals comprised of one or more
ancillary or co-ligands AL2 are more stable compared to said
radiopharmaceuticals that are not comprised of one or more
ancillary ligands, AL2; that is, they have a minimal number of
isomeric forms, the relative ratios of which do not change
significantly with time, and remain substantially intact upon
dilution.
In one particular embodiment, the radiopharmaceutical
-24-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
comprises a compound of the formula (IV):
NH O
O
H2N ~ N N
H H
O N~ HN OH
~~~\\
J O O
03S
S03
0
'pig \ P O ~ NH
HO ~ N
\_ H
NH~
J o
~O _ I N-H N
O
(IV) .
In another particular embodiment, the radiopharmaceutical
comprises a compound of the formula (V)
NH O
~ O
HZN- 'N N
H H
O N~ HN OH
O O
., ,.~\\
OH
O
HO
O
NH//~~ ~
H ~ _
O
HO O~T ~ N'-N N~ O
NH O
O
(V) .
-25-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
After the radiopharmaceutical has been administered, a
series of image slices of the thrombus are acquired at step 12
of Fig. 1. The image slices reflect the concentration of
radioactivity within the thrombus. Each image slice is
composed of a two-dimensional array of pixels, wherein each
pixel comprises an intensity value representative of the
concentration of radioactivity at the particular position
within the thrombus which corresponds to the pixel. In one
embodiment, the image slices are obtained using a gamma camera
to record single photon emission computed tomography (SPELT)
images.
When the method of the present invention is used to
detect an arterial thrombus, for example, data should be
acquired using parameters that enhance the sensitivity of the
technique for small lesions. In particular, when SPELT images
are acquired, the parameters should include a 3 mm or smaller
digital sampling (i.e., pixel size), and a minimum of 90 views
over a 360 degree rotation or 45 views over a 180 degree
rotation. Further, very high-resolution collimators and
multiple heads should be used to increase the resolution and
sensitivity, respectively. In addition, the acquired data
should be reconstructed using a spatial filter with a
relatively high frequency cutoff (i.e., approximately 3.00 or
higher) to minimize resolution loss due to smoothing. A
minimum of thirty angles ensures a visually smooth effect when
the views are intended to be displayed sequentially to produce
a rotating view of the thrombus, as described below.
At step 14, the image slices are reconstructed and
assembled into a three-dimensional matrix of data. The
three-dimensional matrix of data is assembled by stacking the
individual image slices in sequential order. When the image
slices are collected perpendicularly to the long axis of the
patient, the three-dimensional matrix of data is organized as
a series of transaxial image slices.
Once the three-dimensional matrix of data has been
assembled, the matrix of data is scanned along an array of
parallel lines (usually perpendicular to the vertical axis of
-26-
CA 02364753 2001-08-20
WO 00/57787 PCTlUS00/07891
the body), at step 16, to determine the maximum intensity
value along each of the parallel lines. The maximum intensity
value along a parallel line is equivalent to the most intense
pixel within the matrix of data encountered along that
parallel line. The most intense pixel is defined as the pixel
corresponding to the position within the lesion where the
radioactivity is most intense.
At step 18,, the maximum value along each parallel line is
assigned to a pixel in a two-dimensional image array. The
relative position of the pixel in the two-dimensional image
array corresponds to the relative position of the line in the
array of parallel lines. The resulting two-dimensional image
array therefore represents an image of the most intense pixels
as viewed by an observer viewing the thrombus along the array
of parallel lines.
The three-dimensional matrix of data can be scanned along
additional arrays of parallel lines in order to produce image
arrays of the thrombus from different angles. At step 20, it
is determined whether the three-dimensional matrix of data is
to be scanned at a different angle. If the matrix of data is
to be scanned at a different angle, the matrix of data is
scanned along the new angle at step 16. Preferably, the
lesion is scanned along a minimum of 90 views over a series of
angles over 360° or a minimum of 45 views over a series of
angles over 180°.
If additional views of the thrombus are not desired, the
results are displayed at step 22. The results can be
displayed as individual views of the thrombus by displaying
one or more of the individual two-dimensional image arrays.
Alternatively, when two-dimensional image arrays at more than
one angle have been obtained, the individual two-dimensional
image arrays can be displayed sequentially by angle to produce
a rotating view of the most intense pixels.
Although the above discussion has focused primarily on
localization of pulmonary embolism, the present invention is
not intended to be so limited. Instead, the present invention
is intended to relate to any medical condition capable of
-27-
CA 02364753 2001-08-20
WO 00/57787 PCT/US00/07891
diagnosis using a clot-binding radiopharmaceutical contrast
agent and SPECT. For example, it is recognized that the
present invention is equally applicable to localization of
thrombii in general and arterial coronary thrombii in
particular.
It will be recognized by those skilled in the art that
changes or modifications may be made to the above-described
embodiments without departing from the broad inventive
concepts of the invention. It should therefore be understood
that this invention is not limited to the particular
embodiments described herein, but is intended to include all
changes and modifications that are within the scope and spirit
of the invention as set forth in the claims.
-28-