Note: Descriptions are shown in the official language in which they were submitted.
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
TITLE: REMOTELY INTERROGATED DIAGNOSTIC IMPLANT DEVICE
WITH ELECTRICALLY PASSIVE SENSOR
Technical Field
The present invention relates generally to medical implant devices, and
more particularly to devices which may be interrogated remotely from outside
the body.
Background of the Invention
Various types of medical implant devices have been developed over the
years. In many instances, such devices enable humans to live longer, more
comfortable lives. Implant devices such as pacemakers, artificial joints,
valves, grafts, stents, etc. provide a patient with the opportunity to lead a
normal life even in the face of major heart, reconstructive, or other type
surgery, for example.
It has been found, however, that the introduction of such implant
devices can sometimes lead to complications. For example, the human body
may reject the implant device which can ultimately lead to infection or other
types of complications. Alternatively, the implant device may malfunction or
become inoperative. Therefore, it is desirable to be able to monitor the
condition of the implant device. On the other hand, it is highly undesirable
to
have to perform invasive surgery in order to evaluate the condition of the
device.
Still further, it is desirable to be able to monitor conditions related to
the use of implant devices. For example, in heart patients it may be helpful
to
know the amount of blood flowing through a stent or graft in order to
evaluate the health of the patient. Again, however, it is undesirable to have
to perform invasive surgery in order to evaluate such conditions.
Techniques have been developed which enable the function of an
implant device to be monitored remotely from outside the body of the patient.
These techniques involve including one or more sensors in the device for
sensing the condition of the device. The device further includes a small
transceiver for processing the output of the sensors and transmitting a signal
1
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
based on the output. Such signal typically is a radio frequency signal which
is
received by a receiver from outside the body of the patient. The receiver then
processes the signal in order to monitor the function of the device.
While such conventional techniques may be effective in avoiding the
need to perform invasive surgery, there are however several drawbacks
associated therewith. For example, the transceiver included in the implant
device typically includes complex electrical circuitry such as mixers,
amplifiers, microprocessors, etc. for receiving an interrogation signal and
for
transmitting a response signal based on the output of the sensors. Such
complex circuitry has a relatively high cost associated therewith. In
addition,
the complexity of the circuitry increases the likelihood that the device
itself
may be defective. This would then require further invasive surgery and could
even result in physical harm to the patient.
In view of the aforementioned shortcomings associated with
conventional implant devices, there is a strong need in the art for a medical
implant device which can be remotely interrogated but which does not require
complex electrical circuitry such as a transceiver with mixers, amplifiers,
microprocessors, etc. There is a strong need for a medical implant device
which carries out a function within a human or other living animal, and can be
remotely interrogated simply and reliably. There is a strong need for such an
implant device which permits most or all of the sensor circuitry to be
embedded directly within the device.
Summary of the Invention
The present invention is responsive to the aforementioned
shortcomings with conventional devices, and is directed towards an implant
device to be implanted within a living animal and responsive to an
interrogation circuit having an exciter/interrogator element which is located
outside the living animal. The implant device includes a structure implantable
within the living animal and operatively configured to carry out or assist in
carrying out a function within the living animal. The implant device further
includes an electrically passive sensing circuit integral with the structure
for
2
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
sensing a parameter associated with the function, the sensing circuit
including
an inductive element wherein the sensing circuit has a frequency dependent
variable impedance loading effect on the interrogation circuit in response to
an
interrogation signal provided by the exciter/interrogator element, the
impedance loading effect varying in relation to the sensed parameter. In
another embodiment, active elements are combined to produce a varying
impedance loading effect.
To the accomplishment of the foregoing and related ends, the
invention, then, comprises the features hereinafter fully described and
particularly pointed out in the claims. The following description and the
annexed drawings set forth in detail certain illustrative embodiments of the
invention. These embodiments are indicative, however, of but a few of the
various ways in which the principles of the invention may be employed.
Other objects, advantages and novel features of the invention will become
apparent from the following detailed description of the invention when
considered in conjunction with the drawings.
Brief Description of the Drawings
Fig. 1 is an environmental view illustrating a system including a
remotely interrogated medical implant device and exciter/interrogator unit in
accordance with the present invention;
Fig. 2 is a simplified block diagram of the system of Fig. 1;
Fig. 3 is a schematic diagram of the system including the remotely
interrogated medical implant device and exciter/interrogator unit in
accordance
with the present invention;
Fig. 4 is a more detailed schematic diagram representing the remotely
interrogated medical implant device and exciter/interrogator unit in
accordance
with the present invention;
Fig. 5 is a representative graph of primary current (as detected by
voltage across a sense resistor) vs. excitation frequency for the circuit of
Fig.
4;
3
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
Fig. 6a is a partial cut-away side view of a remotely interrogated stent
in accordance with a first embodiment of the present invention;
Figs. 6b and 6c illustrate different equivalent circuits for the stent in
accordance with the present invention;
Fig. 7a is a side view of a remotely interrogated stent in accordance
with a second embodiment of the present invention;
Figs. 7b and 7c are partial cross-sectional views illustrating possible
configurations of the stent in accordance with the present invention;
Fig. 7d represents the equivalent circuit of the stent in Fig. 7a;
Fig. 8a is a side view of a remotely interrogated stent in accordance
with a third embodiment of the present invention;
Fig. 8b is a simplified electrical diagram of the stent shown in Fig. 8a;
Fig. 9a is a side view of a remotely interrogated stent in accordance
with a fourth embodiment of the present invention;
Fig. 9b is a simplified electrical diagram of the stent shown in Fig. 9a;
Fig. 10 is a partial cut-away side view of a remotely interrogated graft
in accordance with a fifth embodiment of the present invention;
Fig. 1 1 a is a side view of a remotely interrogated graft in accordance
with a sixth embodiment of the present invention;
Figs. 1 1 b and 1 1 c are partial cross-sectional views illustrating possible
configurations of the graft in accordance with the present invention;
Fig. 12 is a is a side view of a remotely interrogated graft in
accordance with a seventh embodiment of the present invention;
Fig. 13 is a perspective view of a remotely integrated graft in
accordance with an eighth embodiment of the present invention;
Fig. 14a and 14b represent a side view and end view, respectively, of a
remotely interrogated graft in accordance with a ninth embodiment of the
present invention;
Fig. 14c is a perspective view of a strain gaged annulus included in the
graft represented in Figs. 14a and 14b;
Fig. 14d represents an equivalent circuit of the graft in Figs. 14a and
14b;
4
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
Fig. 15a is a side view, partially cut away, of a remotely interrogated
graft in accordance with a tenth embodiment of the present invention;
Fig. 15b represents an equivalent circuit of the graft in Fig. 15a.
Figs. 16a, 16b and 16c represent a remotely interrogated graft which
combines impedance loading and active electronics according to another
aspect of the invention;
Fig. 17 represents an alternate embodiment of a sensing circuit which
is provided in accordance with the present invention;
Fig. 18 represents another embodiment of a system including remotely
interrogated implant device in accordance with the present invention;
Fig. 19 illustrates a further embodiment of a remotely interrogated graft
in accordance with the present invention; and
Fig. 20 is a cross-section of the graft shown in Fig. 19.
Description of the Preferred Embodiments
The present invention will now be described with reference to the
drawings, wherein like reference numerals are used to refer to like elements
throughout.
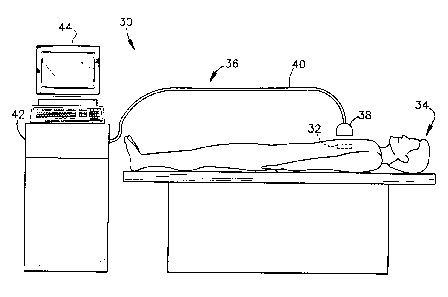
Referring initially to Fig. 1, a system for remotely interrogating a
medical implant device in accordance with the invention is generally
designated 30. The system 30 includes a medical implant device 32 which is
implanted in a living animal such as a human patient 34. As is discussed in
more detail below, the medical implant device 32 can be any of a wide variety
of different types of devices including, for example, a stent, graft,
artificial
joint, etc.
The device 32 is configured to carry out or assist in carrying out a
function within the patient 34. For example, in the case of a stent the device
32 prevents the closing of an arterial wall and permits the flow of blood
therethrough. In the case of a graft, the device 32 serves to couple blood
flow between two separate ends of an artery. The device 32 may instead
consist of an artificial hip or knee which facilitates movement of the leg of
the
5
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
patient 34. Other functions include, but are not limited to, a hemodialysis
shunt and spinal brace, for example.
The device 32 includes a sensing circuit (not shown in Fig. 1 ) which
serves to sense a parameter associated with the function performed by the
device. For example, in the case of a stent or graft the sensor may be used
to detect the degree of restenosis which occurs within the device 32.
Alternatively, for example, the sensing circuit may detect an amount of strain
or displacement which occurs in an artificial hip or knee. Still further, the
sensor may serve to sense the condition of the implant device in carrying out
its intended function. For example, in the case of a pacemaker the sensor
may detect the pulse rate.
The system 30 further includes interrogation instrumentation 36 for
remotely interrogating the implant device 32 in order to evaluate the device
function. The instrumentation 36 includes an exciter/interrogator unit 38
which is positioned outside the patient 34 in close proximity to the implant
device 32. As will be discussed in more detail below, the exciter/interrogator
unit 38 serves to excite the sensing circuit within the device 32. The sensing
circuit is designed to have a variable impedance loading effect on the
exciter/interrogator unit 38, which varies in relation to the sensed parameter
(e.g., blood flow, amount of restenosis, etc.).
The exciter/interrogator unit 38 is coupled via an electrical cable 40 to
the main circuitry 42 included in the interrogation instrumentation 36. The
main circuitry 42 includes suitable circuits for driving the
exciter/interrogator
unit 38 as described below, and for processing the output of the
exciter/interrogator unit 38 in order to provide an output to an operator
(e.g.,
display 44). In particular, the variable impedance loading effect of the
device
32 on the exciter/interrogator unit 38 is detected at different frequencies
and
processed to produce a display or the like indicative of the function
performed
using the device 32.
As will be better understood based on the description which follows,
the present invention preferably utilizes magnetic coupling between the
exciter/interrogator unit 38 and the implant device 32. The sensing circuit in
6
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
the device 32 is a passive circuit designed to have an impedance loading
effect on the exciter/interrogator unit 38. In this manner, the sensing
circuit
can be a very simple, low cost circuit which is less prone to failure. The
device 32 does not require an active transmitter, mixer, amplifier, etc. as in
other conventional devices. Moreover, the sensing circuit can be embedded
within the device structure to reduce the amount of obstruction which occurs
in the device and, for example, to increase performance.
Fig. 2 represents a simplified block diagram showing the positional
relationship between the implant device 32 and the exciter/interrogator unit
38. The exciter/interrogator unit 38 preferably is a hand-held sized device
which is held by a doctor, nurse or medical assistant in close proximity to
the
implant device 32. Since the system 30 is non-invasive, the
exciter/interrogator unit 38 may be placed adjacent the implant device 32
with the body of the patient (e.g., skin, muscle tissue, etc.), designated 50,
disposed therebetween. The preferred embodiment of the present invention
relies on magnetic and/or electromagnetic coupling (represented by field lines
52) between the exciter/interrogator unit 38 and the implant device 32 to
interrogate the device 32 non-invasively.
More particularly, the preferred embodiment of the present invention
introduces sensor technology developed in the aerospace industry into
medical implant devices. Commonly owned U.S. Patent No. 5,581,248
describes in detail how magnetic coupling between an interrogation circuit and
a sensor coil, based on an impedance loading effect, can be used to
interrogate an embedded sensor. Heretofore, however, no one has thought to
utilize such technology in medical implant devices. The entire disclosure of
U.S. Patent No. 5,581,248 is incorporated herein by reference.
Fig. 3 illustrates the electrical configuration of the exciter/interrogator
unit 38 and implant device 32 in more detail. The exciter/interrogator unit 38
includes an exciter/interrogator coil 52, a voltage controlled oscillator 54,
and
a load sensing resistor 56. The oscillator 54 provides an excitation signal to
the exciter/interrogator coil 52 and the load sensing resistor 56 which are
coupled in series. The exciter/interrogator unit 38 is coupled via the cable
40
7
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
to the main circuitry 42 which includes signal conditioning electronics 58 and
a data processing and control section 60. The data processing and control
section 60 produces a control signal on line 62 for controlling the frequency
and the magnitude of the excitation signal that the oscillator 54 applies to
the
exciter/interrogator coil 52. The exciter/interrogator coil 52, sensing
resistor
56 and oscillator 54 provide a resonant exciter/interrogator circuit that is
used
to induce currents in a coil within the implant device 32 in order to perform
interrogation.
More specifically, the implant device 32 includes a sense coil 64 which
is embedded in the structure of the implant device. As is discussed in more
detail below in connection with Figs. 6a, 7a, 8a, etc., the implant device 32
may be any type of implant such as a stent or graft. The sense coil 64 may
be integrally secured to a surface of the stent or graft, for example, or even
formed directly within the structure. The sense coil 64 is part of a passive
resonant sensing circuit 65 which includes, for example, a capacitor 66 and a
sensing element 68 in electrical series with the sense coil 64. The sensing
element 68 can be any sensor which produces a variable impedance (e.g.,
resistance, capacitance or inductance), or which produces an output that can
be converted into a variable impedance that can change or modulate the
impedance of one or more of the resonant circuit components.
As shown in Fig. 3, the sensing element 68 is represented by a variable
resistance which varies based on a sensed parameter. In an alternative
embodiment, the sensing element 68 may provide a capacitance, inductance
and/or resistance which varies based on a sensed parameter. As long as the
sensing element 68 in combination with the sense coil 64 alone or together
with one or more elements (e.g., capacitor 66) form a resonant sensing circuit
65 (e.g., LC or LRC), the benefits of the invention may be obtained.
The sensing element 68 can be any of a variety of known types of
sensors which may be used to sense a functional parameter within the living
body. Such parameters may include, but are not limited to, vascular
parameters such as blood flow rate, blood pressure, oxygen content,
cholesterol, restenosis, glucose level, temperature, etc.; hemotology
8
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
parameters such as blood gases, blood chemistry, hemoglobin content, etc.,
and skeletal/muscular parameters such as force, strain, displacement, etc. As
mentioned above, the sensing element 68 itself may be characterized as an
impedance based sensor whose resistance, capacitance and/or inductance
varies directly with respect to frequency as a function of the sensed
parameter, or another type sensor whose output can be converted into a
variable impedance. Exemplary sensor types include electrical, piezoelectric,
sonic optical, microfluidic, chemical, membrane, thermal,
magnetohydrodynamic, an NMR varient, magnetic, magnetostrictive,
biological, microelectromechanical sensors (MEMsI, etc.
In the particular examples discussed below, the sensing element 68
may be a MEMs device whose impedance varies as a function of the amount
or rate of blood flow through a stent or graft. Alternatively, the sensing
element 68 may be a surface acoustic wave (SAW) device which can detect
blood flow. In yet another alternative, the sensing element 68 may be a
piezoelectric device within a stent or graft for detecting blood pressure.
According to yet another embodiment discussed below, the sensing
element 68 may be included within the sense coil 64 itself. For example, the
embodiments of Figs. 7a, 8a, 9a, etc. as described below incorporate the
sense coil 64 within the tubular housing of a stent or graft. Changes in the
amount of blood flow through the stent or graft and/or the occurrence of
restenosis therein affect the overall inductance of the sense coil 64. Hence,
the sense coil 64 alone or in combination with one or more other sensing
elements 68 may be used to vary the impedance of the resonant sensing
circuit based on the sensed parameter.
As is explained more fully in the aforementioned '248 patent, the basic
operation of the system 30 of Fig. 3 according to the invention is as follows.
The sensing circuit 65 exhibits a resonant frequency which is defined as the
frequency which is the point of maximum sensitivity to changes in the
excitation current IP for a given change in the impedance of the sensing
element 68. The resonant frequency fs is determined by the sum total of the
reactive elements of the circuit which includes the inductance of the sense
9
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
coil 64 and the exciter/interrogator coil 52, as well as the capacitance 66
(and
parasitic capacitances CP, and CPZ shown in Fig. 4) and the value of a
coupling constant K. The amplitude of the current through the coil 64 is also
a function of the sensing element 68, particularly at the resonant frequency
of
the sensing circuit 65. When the exciter/interrogator coil 52 has an AC signal
applied, current in the primary or exciter/interrogator coil 52 induces
current in
the secondary or sense coil 64, as in an air gap transformer. This current in
the sense coil 64, however, is reflected back to the exciter/interrogator coil
52 by the mutual coupling of the two coils. The sensing resistor 56 is used to
detect the current in the exciter/interrogator coil 52.
When the excitation frequency is approximately at the resonant
frequency of the sensing circuit 65, the current in the exciter/interrogator
coil
52 changes maximally in relation to the value of the sensing element 68.
Thus, the condition of the sensing element 68 can be determined as a
function of the detected current in the exciter/interrogator coil 52. Using an
amplifier 72, the signal conditioning electronics 58 amplifies the voltage
developed across the sensing resistor 56 by the exciter/interrogator circuit
current IP. This amplified voltage is then rectified and low pass filtered via
a
rectifier and low pass fitter circuit 74 to provide a DC voltage output Vd~.
The
control circuit 60 then uses the DC value to determine the state or output of
the sensing element 68.
Fig. 4 provides a more detailed circuit model of an exciter/interrogator
unit 38 and the implant device 32. As shown, the exciter/interrogator unit 38
includes the exciter/interrogator coil 52 that has a determinable inductance
LP. The coil 52 and associated components of the exciter/interrogator unit 38
also will exhibit an overall parasitic capacitance, CP,, that appears in
parallel
with the coil inductance. The exciter/interrogator unit 38 further includes
the
variable frequency oscillator 54 and the sensing resistor 56 used to sense the
primary or excitation current IP. Thus, all components in the
exciter/interrogator unit 38 are known quantities for each application.
The resonant sensing circuit 65 includes the sense coil 64 which has a
determinable inductance, LS, in one embodiment; or in another embodiment an
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
inductance which varies in relation to the sensed parameter. In such
embodiment, the sense coil 64 itself forms part of the sensing element 68.
The sense coil 64 also has an associated parasitic capacitance, which
parasitic capacitance is in effect part of the capacitance CP2 which is a
discrete capacitor selected to optimize the sensitivity of the device 32 to
changes in the value of the sensing element 68. In other words, the value of
CP2 can be selected, such as based on experimental data for specific circuits,
to maximize the current IP induced in the exciter/interrogator unit 38 as a
function of changes in the resistance of the sensing element 68. The sensing
circuit 65 also includes the additional discrete capacitor 66 which is
selected
to adjust the frequency at which the change in current vs. change in sensing
element resistance ratio is optimized.
Thus, for the sensing circuit 65, all of the component parameters are
known quantities except the coupling constant, K, and the value of the
sensing element 68 output. Accounting for the coupling constant K as
described more fully in the '248 patent, the DC output of the signal
conditioning electronics 58 is indicative of the sensed parameter of the
implant device 32.
Fig. 5 is a graph showing in a representative manner a typical
frequency response characteristic of the circuit of Fig. 4. By comparing a
family of curves determined by monitoring the primary current IP vs.
excitation
frequency for different K values (in this example for K = 0.1, K = 0.5 and
K=0.9) and different resistance values for the sensing element 68, the
sensed parameter (e.g., blood flow rate, degree of restenosis, etc.) may be
determined.
Fig. 6a presents a first embodiment of the present invention in which
the medical implant device 32 is a stent. As is known, a stent is a round,
spring-like device that provides mechanical support to the wall of a blood
vessel such as an artery. As is shown in Fig. 6a, the stent 32 is inserted
within a blood vessel 80. The stem 32 is a tube shaped structure made up of
a generally helical formed wall 82. The stent 32 prevents the walls of the
11
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
blood vessel 80 from collapsing while providing a path 84 through which
blood may flow.
The wall 82 typically is formed of stainless steel or some other material
(e.g., a composite and/or plastic material) which is biocompatible within the
body. Depending on the embodiment, the wall 82 preferably is made of a
non-conductive material or materials in one case, or a conductive material in
another case. In this particular embodiment, the wall 82 preferably is made
of a non-conductive material such as plastic. The sense coil 64 is formed on
an outer (or inner surface) of the tube shaped structure. Alternatively, the
sense coil 64 may be embedded within the wall 82. The sense coil 64 is
coupled via electrical conductors 86 and one or more through holes 87 to the
remainder of the sensing circuit 65 which is formed on an inner surface of the
wall structure 82. The sensing element 68 in such an embodiment may be a
MEMs device whose capacitance and/or resistance varies as a function of the
amount of restenosis which forms on the element 68 within the stent 32.
Alternatively, the sensing element 68 may be a piezoelectric device which
produces an impedance output which varies as a function of the pressure of
the blood flowing within the stent 32. If desirable, the sense coil 64 and all
or part of the remainder of the sensing circuit 65 may be covered with a
protective coating material to avoid corrosion or other related problems.
Upon being implanted within the vessel 80, the exciter/interrogator unit
38 (Fig. 3) can be positioned outside the body of the patient in close
proximity to the stent 32. The exciter/interrogator unit 38 serves to excite
the sense coil 64 which in turn induces a current in the load resistor 56
which
varies as a result of the variable impedance loading effect of the sensing
circuit 65 with respect to frequency. Thus, as the output of the sensing
element 68 varies based on the build up of restenosis, change in blood
pressure, or other desired parameter, such variation may be detected
remotely.
Fig. 6b illustrates the equivalent circuit for the sensing circuit 65 in an
embodiment where the sensing element 68 provides a resistance which varies
in response to a sensed parameter. Fig. 6c illustrates an equivalent circuit
for
12
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
the sensing circuit 65 in an embodiment where the sensing element 68'
produces an output which varies in capacitance based on the sensed
parameter. In each case, the impedance loading effect of the sensing circuit
65 varies in accordance with the sensed parameter by virtue of the resonance
of the circuit being affected.
An alternative embodiment for a stent 32 is shown in Fig. 7a. In this
particular embodiment, the helical shaped wall 82 preferably is made of a
molded plastic. The sense coil 64 is made up of a conductive wire 92
embedded through several turns in the wall of the helix 82 as shown in cross-
section in Fig. 7b. Return wires 94 embedded in and traversing the helix 82
are provided to connect the respective ends of the coil 64 to the remainder of
the resonant sensing circuit 65 mounted on the helix 82 as in the previous
embodiment. During manufacture, the sense coil 64 may serve as the frame
about which the molded plastic helix 82 is formed.
The embodiment of Fig. 7c varies slightly from that shown in Figs. 7a
and 7b. In this particular embodiment, the return wires 94 are formed on the
inner surface of the helix 82. Such embodiment simplifies the manufacturing
process by allowing the helix 82 to be formed without the return wires 94
traversing the helical turns in an embedded manner.
Fig. 7d illustrates generally the equivalent circuit for the stent 32
shown in Figs. 7a thru 7c. As will be appreciated, the sensing element 68
may be a resistive device as before, or some other type of sensor. In each
case, the sense coil 64 provides a means for magnetic coupling between the
exciter/interrogator coil 52 and the resonant sensing circuit 65. As blood
flow, restenosis, etc. varies within the stent 32, the impact of such
variation
on the impedance loading effect of the resonant sensing circuit 65 on the
exciter/interrogator unit 38 may be detected with respect to frequency. Such
information can then be utilized in ascertaining the precise rate of blood
flow,
degree of restenosis, etc. via the data processing and control 60. As will be
appreciated, in each of the embodiments discussed herein the particular type
of sensing element 68 will be dictated, of course, by the particular parameter
13
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
of interest and the manner in which the output of the exciter/interrogator
unit
38 is processed.
Fig. 8a illustrates another embodiment of a stent 32 which utilizes the
conductive properties of a metal-type helix wall 82. The helix wall 82 is
made of metal and therefore can itself form the sense coil 64. The metal
helix is electrically isolated via a non-conductive coating, for example. Each
end 96 of the helix is connected to the remainder of the resonant sensing
circuit 65 via return wires 94 as shown in phantom in Fig. 8a. As in the
previous embodiments, the resonant sensing circuit with the sensing element
68 may be mounted on the inner surface of the stent 32. Fig. 8b
diagrammatically represents the electrical circuit of this particular
embodiment.
In each of the embodiments which utilize the body 82 of the stent 32
to form the sense coil 64, e.g., the embodiments of Figs. 7a, 7c and 8a, it
will be appreciated the inductance of the sense coil 64 may itself vary as a
function of the sensed parameter. In such instance, the sense coil 64 serves
as a sensing element in addition and/or in place a discrete sensing element
68. More particularly, the sense coil 64 formed within the helix may be
considered an inductive element. It is combined with a discrete capacitor 66
and resistance 68 to form an LRC resonant sensing circuit 65.
The inductance of the sense coil 64 depends directly on the magnetic
permeability of the material inside it. Since iron strongly affects
permeability,
the amount of blood in the stent 32 as a fraction of the available volume
(reduced by restenosis~ will modulate the permeability and hence the resonant
frequency of the sensing circuit 65. The resonant frequency can be
determined by inductively coupling the stent 32 to the exciter/interrogator
unit
38 via the externally generated swept frequency magnetic field. Knowledge
of the resonant frequency then allows a determination of the inductance of
the coil 64. Since the value of inductance depends on the degree of
restenosis, an estimate of its occlusion of the stent 32 can be made.
The embodiments of Figs. 7c and 8a each include some type of direct
linear connection via the return wires 94 between the sense coil 64 and the
14
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
remainder of the resonant sensing circuit 65: Such design may not be
optimum from a biocompatibility standpoint or manufacturing standpoint.
Figs. 9a and 9b represent an embodiment which eliminates the need for such
return wires 94. In this case, a double helix configuration is used to
complete
the resonant circuit.
As is shown more clearly in Fig. 9b, the helix wall 82 is made of
conductive metal and from one end to the other forms part of the coil 64.
The return wire 94 is a second helix with the same pitch as the helix 82 but
having an axial direction which is reversed relative to the helix 82. The
return
wire 94 is connected to one end of the helix 82 and returns to the other end
where the resonant sensing circuit 65 can be closed with the capacitance 66
and resistance 68. Electrically, such configuration doubles the inductance L
of the coil 64, and currents in the two helical sections 82 and 94 will
produce
magnetic fields which add rather than cancel. In the presence of a changing
magnetic field, conversely, the current in the circuit 65 is doubled.
Other embodiments may include a stent 32 which has a uniform wall
rather than a helix shaped wall. In such case, the sense coil 64 may be
formed on a surface as in the embodiment of Fig. 6a. Alternatively, the sense
coil 64 may be embedded in the structure as in the embodiments of Figs. 7b
and 7c, for example.
Fig. 10 illustrates an embodiment of the invention wherein the implant
device 32 comprises a graft for joining separate ends 100 of a blood vessel.
The graft 32 is a tube shaped structure 102 made up of metal such as
stainless steel, or a composite and/or plastic material. Using known
techniques, the graft 32 is implanted within the patient by securing
respective
ends 100 of a blood vessel to corresponding ends of the graft 32.
Consequently, blood will flow through the interior of the graft 32 as
represented by arrow 84.
As in the case of the stent described above, the resonant sensing
circuit 65 can be any combination of a sense coil 64, a capacitor 66, a
resistor 68, etc. One or more of these components presents an impedance
which varies as a function of the parameter to be sensed. Similar to the
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
stent, it is desirable with the graft 32 to sense remotely the degree of
restenosis and/or blood flow in the device. By using impedance-based
sensing devices, the frequency dependent impedance loading effect of the
sensing circuit may be detected externally using the exciter/interrogator unit
38 as previously described.
The embodiment of Fig. 10 is similar to that of Fig. 6a where the sense
coil 64 is mounted on a surface of the tube structure 100. The sensing
element 68 and capacitor 66, for example, are mounted on an interior surface
of the structure 100. Electrical connections to the coil 64 are provided by
conductors 86 and vias 87. Operation is fundamentally the same as
described above in relation to the stent embodiment.
Figs. 1 1 a thru 1 1 c illustrate an embodiment of a graft 32 analogous to
the stent of Figs. 7a thru 7c. The structure 100 is made of a non-conductive
material and the windings of the coil 64 are embedded directly within the
tube. Again, for example, the structure 100 may be molded plastic or the like
with the coil 64 serving as a skeletal support.
Fig. 12 represents an embodiment of a graft 32 which uses a double
helix structure similar to the stent in Fig. 9a. In this case, however, since
the
structure 100 is uniform rather than helical, two separate helical wires 104
and 106 are embedded along the length of the tube 102. Electrically
speaking, the circuit is identical to that shown in Fig. 9b. As the amount of
blood/restenosis varies in the graft 32, the inductance of the helical wires
104
varies which changes the impedance loading effect on the exciter/interrogator
unit 38.
Fig. 13 illustrates yet another embodiment of a graft 32 (or stent)
which is remotely interrogated in accordance with the present invention. In
the case of a tube shaped structure 102 serving as the body of the graft or
stent, a conventional device may be modified by placing a desired number of
windings around the outer surface of the structure 102 to form the sense coil
64. The capacitor 66 or other. fixed components may similarly be mounted on
the outer surface. The sensing element 68 is mounted on the inside surface
and connected through vias 87 to the coil 64 and capacitor 66 to form the
16
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
LRC resonant sensing circuit 65. Alternatively, the sensing element 68 may
be mounted on the outer surface also, provided the sensing element is
capable of sensing the desired parameter through the structure 102.
Subsequently, a laminate sheath 1 10 is applied over the outer surface
of the structure 102 and heated to form an integrated graft 32. The sensing
circuit 65 can then be interrogated in the same manner described above in
connection with the other embodiments.
Figs. 14a and 14b illustrate another embodiment of the invention
represented by a remotely interrogated graft 32, for example. The graft 32
again includes a tube shaped structure 102 as in the previous embodiments,
and is inserted between separate ends of a blood vessel. The graft 32 also
includes a resonant sensing circuit 65 as in the previous embodiments. For
example, the resonant sensing circuit 65 includes a sense coil 64 and
capacitance 66 mounted on or within the tube 102 as in any of the previous
embodiments described herein. In the present embodiment, however, the
sensing element 120 in the sensing circuit 65 includes an annulus 122 which
is fitted around the outer circumference of the tube 102. As is shown in Fig.
14c, the annulus 122 serves as a carrier for one or more strain gage elements
124 which are mounted to the inner surface of the annulus 122. In the case
of more than one strain gage element 124, the elements may be distributed
about the inner circumference of the annulus 122. In another embodiment,
for example, the strain gage elements 124 may be mounted on the outer
surface of the annulus 122 or on both the inner and outer surfaces.
The annulus 122 with the strain gage elements 124 is designed to
sense changes in pressure within the graft 32. As the diameter of the tube
102 expands or contracts in the direction noted by arrow P in Fig. 14a, such
changes are detected by the strain gage elements 124 on the annulus 122.
An output produced by the strain gage elements 124 varies in impedance as a
function of the change in pressure, and the output is coupled to the remainder
of the sensing circuit 65 via electrical lines 126. Consequently, a change in
pressure within the graft 32 results in a frequency dependent variation in the
17
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
impedance loading effect of the sensing circuit 65 similar to the embodiments
discussed above.
It has been shown that a change in pressure exerted by the blood
within a graft or shunt is a reliable indicator of vascular problems. Such
problems may include clogging within the graft or shunt, for example, or
clogging within the vessel. Thus, the sensing circuit 65 can be interrogated
remotely by sweeping the frequency of the excitation signal in order to detect
changes in the pressure, and hence potential vascular problems.
In the preferred embodiment, the tube 102 is made of a thin, compliant
material which tends to deform measurably as a result of changes in blood
pressure within the graft 32. As a particular example, the tube 102 is a
conventional graft made of a compliant woven fabric. The tube 102 may
include the sense coil 64 (shown diagrammatically in Figs. 14a and 14b)
mounted on the side of the tube 102 similar to the embodiment of Fig. 10.
Alternatively, for example, the sense coil 64 may be formed within a helical
tube 102 similar to the embodiments of Figs. 1 1 a and 12. The capacitance
66 is mounted on the tube 102 and is electrically connected to the sense coil
64 and the sensing element 120 as shown in Fig. 14d, for example.
The annulus 122 is made of a thin, flexible material such as a polyimide
film or a polyimide film mounted on a metal annulus 122, for example. The
annulus 122 includes the strain gage elements 124 formed on the inner
and/or outer surface of the annulus. In the exemplary embodiment, the strain
gage elements 124 are piezoresistive devices whose resistance changes as a
function of mechanical strain of the annulus in the direction of its
circumference. The piezoresistive devices are formed using MEMs
technology, patterned lithography, etc. either directly on the annulus 122
material, or are subsequently mounted to the annulus via adhesive, etc. The
outputs of the strain gage elements 124 are combined in parallel, series, as a
Wheatstone bridge, etc., via conductive lines 130 formed on the annulus 122,
for example. The conductive lines 130 are coupled to lines 126 to produce a
resistance across lines 126 which consequently varies as a function of the
pressure exerted on the annulus 122 by the tube 102.
18
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
The material of which the annulus 122 is made is preferably stiffer than
the woven fabric or other compliant material making up the tube 102. Prior
to the graft 32 being installed, the annulus 122 is formed around the
circumference of the tube 102 so as to slightly compress the tube 102. As a
result, any expansion which occurs in the tube 102 due to blood flowing
therethrough will result in an expansion of the annulus 122, and hence a
change in the output resistance of the strain gage elements 124. Experience
has shown that with a 6 millimeter ID teflon graft, there is approximately a
0.004 inch increase in diameter of the graft 102, for example, when blood is
introduced therethrough at a pressure of 100 millimeters of mercury. Thus,
by forming the annulus 122 around the circumference of the tube 102 to be
slightly compressed, e.g., on the order of 0.004 inch, the annulus 122 will be
subjected to the majority of the load due to blood pressure.
The annulus 122 is adhered to the outer circumference of the tube 102
according to any of a number of suitable techniques. For example, the
annulus 122 may be joined with the tube 102 via ultrasonic welding, an
adhesive, friction fit, etc. Moreover, the tube 102 may include one or more
walls or protrusions on each side of the annulus 122 to prevent movement of
the annulus 122 in the axial direction.
Although not shown in the figures, the annulus 122 may include one or
more flat spots at which the strain gage elements 124 are located for
concentrating the strain forces in the regions) at which the elements 124 are
located. In addition, the flat spots may include small cuts, etc. designed to
focus further the strain on the respective elements.
An advantage of the embodiment of Fig. 14a is that the annulus 122
and strain gage elements 124 do not come into direct contract with the
flowing blood. Moreover, the annulus 122 makes it possible to measure
pressure independent of any shear force the flowing blood may otherwise
introduce to a sensing element.
According to one variation of the graft 32 shown in Fig. 14a, the strain
gage elements may be made up of other types of strain gages. For example,
the elements 124 may be conventional foil type strain gages formed on the
19
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
annulus 122. Although the exemplary embodiment involves a strain gage
element 124 having a resistance which changes as a function of the amount
of strain, another embodiment can incorporate a strain gage which produces a
capacitance or inductance that changes as a function of strain as will be
appreciated.
In another embodiment, the tube 102 is made of a relatively less
compliant material such as stainless steel, or a composite and/or plastic
material. For such case, the annulus 122 may be omitted and the strain gage
elements 124 may be formed directly on the surface of the tube 102.
Fig. 15a illustrates another embodiment of a graft 32 in accordance
with the present invention. The graft 32 again includes a tube shaped body
102 as shown. In this particular example, the tube 102 includes an aperture
140 in which a small, flexible diaphragm 142 is inserted. The diaphragm 142
includes a piezoresistive strain gage element 144 formed thereon whose
resistance changes as a function of the deformation of the diaphragm 142.
The strain gage element 144 may be a MEMs type device formed on the
diaphragm 142 via lithography or the like. Alternatively, the strain gage
element 144 may be a foil type element or other type which presents an
impedance which varies as a function of deformation of the diaphragm 142.
The output of the strain gage element 144 is coupled to the remainder of a
sensing circuit 65 formed on or within the tube 102 similar to the previous
embodiments discussed above.
The diaphragm 142 with the strain gage element 144 is responsive to
changes in pressure P exerted by the blood flowing through the graft 32. As
is represented in Fig. 15b, the diaphragm 142 and strain gage element 144
form the sensing element in a sensing circuit 65. As in the previous
embodiments, the sensing circuit 65 includes a sense coil 64 and capacitance
66. The sensing circuit 65a has a frequency dependent variable impedance
loading effect on the interrogation circuit in response to the interrogation
signal provided by the exciter/interrogation unit 38. Such loading effect thus
will be indicative of the pressure component P exerted within the graft 32.
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
Referring now to Fig. 16a, a hemodialysis shunt (or graft) 200 in
accordance with another embodiment of the invention is illustrated. In this
particular embodiment, an ultrasound transit time or phase shift is used to
measure blood flow is the shunt 200. As is described in U.S. Patent No.
4,227,407, the entire disclosure of which is incorporated herein by reference,
an ultrasonic transit time or phase shift can be used to measure fluid flow.
According to the preferred embodiment of the present invention, the
shunt 200 includes a tube-shaped shunt body 202 with a sleeve 204 which
fits over the body 202. The sleeve 204 is made of a relatively rigid material
such as plastic, stainless steel, etc., which serves as a reflecting surface
for
ultrasonic waves which are created within the shunt 200. More particularly,
the sleeve 204 includes a pair of piezoelectric crystals 206a and 206b
mounted on an inner surface as shown in Figs. 16a and 16b. The
piezoelectric crystals 206a and 206b are separated by a distance L along the
axis of the shunt body 202. The sleeve 204 is shaped as a polygon with an
oppositely facing side 208 relative to the crystals 206a and 206b which
serves to reflect an ultrasound wave which is transmitted by one crystal
through the shunt body 202 back towards the other crystal as represented by
signal path 210. In the exemplary embodiment, the shunt body 202 is made
of plastic, woven fabric, or any other material which permits ultrasonic waves
to pass through the respective walls and through the blood in order to be
reflected by the opposite side 208.
Fig. 16c shows the sensing circuit 220 included in the shunt 200 for
measuring relative transit times which relate to the flow rate of blood
through
the shunt 200, and for permitting the shunt to be interrogated remotely based
on the aforedescribed impedance loading effect. More particularly, the
sensing circuit 220 includes a sense coil 64 which receives an excitation
signal from the external exciter/interrogator coil 52 by way of magnetic
coupling as in the previous embodiments. The sensing circuit 220 further
includes a power supply 222 in this embodiment. The power supply 222 may
be a battery which is replaced periodically. Alternatively, and more
preferably, the power supply 222 includes a bridge circuit and capacitor.
21
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
Immediately prior to measuring the blood flow rate, the
exciter/interrogator coil 52 is driven at a predefined frequency. Energy from
the exciter/interrogator coil 52 is magnetically coupled to the sense coil 64
which produces an AC voltage at across the terminals 224 of the coil 52 and
the input terminals of the power supply 222. The power supply 222 converts
the AC voltage into a DC voltage which is stored as charge in a capacitor
within the power supply 222. The output of the power supply serves to
provide an operating voltage to the various active electronics included in the
sensing circuit 220 as further discussed below.
The blood flow rate is measured by providing an excitation signal to the
exciter/interrogator coil 52 which consists of bursts of a high frequency
signal
at a predefined rate. Each burst is magnetically coupled to the sense coil 64
through the body of the patient to produce a voltage spike across the
terminals 224 of the sense coil 64. The piezoelectric crystals 206a and 206b
are coupled across the terminals 224 by wires 228, and the voltage spike
produced by a given burst of the exciter/interrogator coil 52 results in each
of
the crystals 206a and 206b emitting an ultrasonic pulse. The pulse
transmitted by the crystal 206a passes through the shunt body 202 and the
blood therethrough so as to reflect off the opposite face 208 and
subsequently be received by the crystal 206b. Conversely, the pulse
transmitted by the crystal 206b in the opposite direction so as to pass
through the shunt body 202 and the blood therethrough in order to be
reflected off the opposite face 208 and subsequently received by the crystal
206a.
Since the blood flowing through the shunt 200 will be flowing in a
particular direction, the transit time for an ultrasonic pulse emitted from a
first
one of the crystals 206 to the second will be delayed relative to the transit
time of the ultrasonic pulse emitted from the second crystal 206 to the first.
Accordingly, there will be a time difference between the time when the first
crystal 206 receives a corresponding pulse and the second crystal receives a
corresponding pulse. This time difference, as will be appreciated, is related
to
the blood flow rate.
22
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
Such time difference is measured as follows. Following each burst
from the exciter/interrogator coil 52, the crystals 206a and 206b will each
receive the reflected ultrasonic pulse from the other. The received ultrasonic
pulses result in the crystals 206a and 206b producing corresponding electrical
pulses, or "echo pulses" following the excitation pulse, across lines 228. The
sensing circuit 220 includes a pulse width decoder 230 configured to produce
an output pulse having a width which is based on the time period between
the two echo pulses. The output pulse from the decoder 230 is provided to a
charge accumulator 232 which produces an output voltage which varies as a
function of the pulse width of the pulse width decoder 230 output. For
example, the charge accumulator 232 may be an RC circuit whose charge
builds up as a function of the pulse width. The output of the charge
accumulator 232 is provided to a varactor diode 234 included in the sensing
circuit 220. The main terminals of the varactor 234 are coupled across lines
228, and the output of the accumulator 232 serves as a control voltage to
the varactor 234 to effect a change in the impedance (e.g., capacitance) of
the varactor 234 across lines 228 as a function of the accumulator 232
output.
Consequently, the time period between echo pulses produced by the
crystals 206a and 206b following an excitation pulse received from the
exciter/interrogator coil 52 affects the impedance which appears across the
terminals 224 of the sense coil 64. This impedance can then be detected by
the exciter/interrogator coil 52 based on the amount of loading which occurs
on the exciter/interrogator coil 52 as a result of the impedance of the
varactor
234. Accordingly, the impedance loading effect of the sensing circuit 220 on
the exciter/interrogator coil 52 is indicative of the flow rate of the blood
through the graft 200. The main circuitry 42 (Fig. 1 ) can then process such
information in order to produce an output representing the measured flow
rate.
The sensing circuit 220 as described above will essentially produce a
time averaged flow rate based on a sequence of excitation pulses each
followed by corresponding echo pulses. The pulse width decoder 230
23
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
discriminates between the excitation pulses and the echo pulses based on
fact that the echo pulses will be several orders of magnitude lower in
amplitude. The pulse width decoder 230 is reset by each excitation pulse,
and then provides a pulse output having edges which are set by the echo
pulses produced across lines 228.
In an embodiment in which the instantaneous blood flow rate is
desired, the charge accumulator 232 includes reset terminals (not shown)
coupled across lines 228. The charge accumulator reset terminals are
configured like the pulse width decoder 230 to discriminate between the
excitation pulses and the echo pulses. Upon the occurrence of each
excitation pulse the charge accumulator 232 is effectively "zeroed". The
resultant pulse produced by the pulse width decoder 230 then serves to
charge the charge accumulator 232 to provide a controlled impedance across
lines 228. The loading effect of the sensing circuit 220 can then be sensed
by sweeping the excitation frequency of the exciter/interrogator coil 52 at a
low amplitude prior to the next excitation pulse.
Although the exemplary embodiment relies on transit times to
determine flow rate, a different configuration of the piezoelectric crystals
206
can be provided which utilizes a phase difference as will be appreciated.
Furthermore, more than one pair of crystals 206 can be used on the sleeve
204 by placing additional pairs on another face and reflecting the ultrasonic
pulses off of a different oppositely disposed surface of the sleeve 204. Yet
further, in the exemplary embodiment the sleeve 204 is hexagonal in cross
section, with mirror like reflection of the ultrasonic pulses occurring at the
flat
facets. However, cylindrically or spherically curved facets could also be used
as well as other shapes, and could proved additional focusing power on the
ultrasonic waves.
in addition, an embodiment is also within the scope of the invention
whereby the sleeve 204 is omitted and the crystals 206 are disposed along
the axis of the shunt 200. The ultrasonic pulses in such case may be
transmitted directly between the two crystals without reflection. The crystals
206 may be affixed directly to the body 202 in such case.
24
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
While the embodiment of Figs. 16a thru 16c incorporates some active
components and possibly a discrete power supply, it still offers advantages
over conventional devices which require a radio transmitter or transceiver to
provide for remote interrogation. Again, the complexity of a radio transmitter
or transceiver increases the cost of the implant device and provides more
opportunity for operational problems. The impedance loading effect of the
present invention enables the implant device to be interrogated without the
need for such a radio transmitter or transceiver.
Fig. 17 represents a sensing circuit 240 which can be used in a stent,
graft or other implant device in accordance with another embodiment of the
invention. The sensing circuit 240 incorporates a microprocessor or other
digital circuitry for processing information which is obtained from one or
more
biological measurand transducers. The microprocessor provides an output
signal indicative of a desired property which is converted into a
corresponding
impedance value that can be ascertained via the exciter/interrogator coil 52
by
virtue of the impedance loading effect of the sensing circuit 240. In this
manner, the microprocessor can perform any complex computations,
analyses, etc. on the data obtained from the transducer(sl, and output a
simplified (e.g., positive/negative) response which is interrogated remotely
from outside the body.
As specifically shown in Fig. 17, the sensing circuit 240 includes one
or more transducers 242 which serve to measure one or more properties
within the stent, graft, etc. For example, the transducers) 242 may be strain
gage, pressure sensor, and/or different types of biosensors. The output of
the transducer 242 is provided to an analog-to-digital converter 244 which
converts the output of the transducer to a digital signal that is input to a
microprocessor 246 included in the sensing circuit 240. The microprocessor
246 executes a program stored internally in non-volatile memory so as to
condition and/or analyze the output of the transducer 242 in accordance with
a preprogrammed routine. The microprocessor 246 outputs a digital signal
indicative of the result of the conditioning and/or analysis to an impedance
conversion circuit 248. The output signal may indicate measurands such a
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
blood pressure, flow rate, biological composition, etc. The value of the
output signal is varied by the microprocessor 246 as a function of the
measurand.
The conversion circuit 248 includes a switched capacitor network or
other circuitry which converts a digital signal into a corresponding impedance
value. In the exemplary embodiment, the impedance presented by the
conversion circuit 248 is placed across the sense coil 64. Thus, the
impedance loading effect of the sensing circuit 248 on the
exciter/interrogator
coil 52 will vary as a function of the output of the microprocessor 246. A
power supply 222 such as the one discussed above in connection with the
embodiment of Fig. 16c provides the necessary operating power to the
respective circuit elements.
Thus, the present invention as represented in Fig. 17 provides another
alternative to an implant device requiring a radio transmitter/transceiver.
Such
embodiment enjoys a reduction in power consumption, circuit complexity and
size, for example.
Fig. 18 illustrates a wireless system 30' which may be used in
accordance with the present invention. In this case, the exciter/interrogator
unit 38 houses all of the electronics shown in Fig. 3, together with a
wireless
transmitter (not shown) used to transmit the output from the data processing
and control section 60 to main circuitry 42. For example, the unit 38 includes
a radio frequency (RF) transmitter which transmits data output from the data
processing and control section 60 to an RF receiver (not shown) included in
the main circuitry 42 (e.g., using antennas 260). Alternatively, an optical
link
or other wireless connection may be utilized. A rechargeable power supply
(not shown) included in the unit 38 provides the appropriate operating power.
The system 30' provides flexibility which enables one or more units 38
to be networked into a centralized main circuitry 42. For example, in a
hospital or other health care facility the unit 38 may be carried about to
different patients where an implant device is interrogated. The results of the
interrogation are then transmitted to the main circuitry 42 for storage and/or
further processing, analysis by an expert, etc. Each unit 38 may include a
26
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
keypad or other input means which allows the operator to input the identity of
the patient. Such identity is transmitted together with the output of the data
processing and control section 60 to the main circuitry 42.
Figs. 19 and 20 illustrate yet another embodiment of a graft 32 which
utilizes a concentric cylinder arrangement to provide a capacitance which
varies in relation to the pressure of the blood flowing therethrough. More
specifically, the tube structure 102 is made up of an inner tube 102a and an
outer tube 102b. The inner tube 102a is made of a generally compliant such
as thin plastic, whereas the outer tube 102b is made of a relatively rigid
material such as a less compliant plastic.
A surface of the inner tube 102a includes an electrically conductive
layer 280, and a surface of the outer tube 102b includes an electrically
conductive layer 282. Such layers 280 and 282 may be formed via
deposition, etc. The inner tube 102a and outer tube 102b are separated by a
compliant foam material 284 having a dielectric constant e. Compliant seals
286 are provided at the ends of the tube structure 102 to prevent blood from
entering the region between the inner tube 102a and outer tube 102b.
As blood flows through the central passageway 288 of the graft 32,
the pressure of the blood will cause the inner tube 102a to expand radially
towards the outer tube 102b. This results in the foam 284 compressing and
the distance D between the inner and outer tubes decreasing as a function of
the blood pressure. In the meantime, the conductive layers 280 and 282 with
the foam 284 act as a capacitor having a capacitance which varies as a
function of the distance D. Thus, the capacitance of the tube structure 102
as measured across conductive layers 280 and 282 will vary as a function of
the blood pressure within the graft 32.
Accordingly, the tube structure 102 will serve as a capacitive sensing
element included in the sensing circuit together with the sense coil 64 and
discrete capacitor 66 (optional). The conductive layers 280 and 282 are
connected to the sense coil 64 and capacitor 66 by wires 290 to form a
resonant LC or LRC sensing circuit. Since the capacitance of the tube
27
CA 02364869 2001-09-21
WO 00/56210 PCT/US00/07694
structure 102 will vary as a function of blood pressure, so will the impedance
loading effect of the sensing circuit with respect to frequency.
The tube structure 102 in Figs. 19 and 20 therefore provides a manner
in which the blood pressure within the graft 32 can be interrogated according
to the above-described principles.
Although the invention has been shown and described with respect to
certain preferred embodiments, it is obvious that equivalents and
modifications will occur to others skilled in the art upon the reading and
understanding of the specification. For example, various other types of
implant devices can benefit from the present invention and the invention is
not intended to be limited only to stents and grafts in its broadest
application.
The present invention includes all such equivalents and modifications, and is
limited only by the scope of the following claims.
28