Note: Descriptions are shown in the official language in which they were submitted.
CA 02366400 2004-04-02
IMPROVED MULTI-LAYER GOLF BALL
Field of the Invention
The present invention relates to golf balls and, more particularly, to
improved golf
balls comprising multi-layer covers which have a hard inner layer and a
relatively soft outer
layer.
Background of the Invention
Traditional gotf ball covers have been comprised of balata or blends of balata
with
elastomeric or plastic materials. The traditional balata covers are relatively
soft and
flexible. Upon impact, the soft balata covers compress against the surface of
the club
producing high spin. Consequentty, the soft and flexible balata covers provide
an
experienced golfer with the ability to apply a spin to control the bail in
flight in order to
pnoduce a draw or a fade, or a backspin whkh r.auses the baii to "bite" or
stop abnlptiy on
contact with the green. Moreover, the soft balata covers produce a soft "feel"
to the low
handicap player. Such playabirity properties (workability, feel, etc.) are
particulariy
important in short iron play with low swing speeds and are exploited
signifccantty by
relatively skltied players.
Despite all the bBnefds of balata, balata covered golf balls are easity cut
andlor
damaged if mis-hit Golf balls produced with balata or baiata-containing cover
-compositions therefore have a relatively short lifespan.
CA 02366400 2004-04-02
As a result of this negative property, balata and its synthetic substitutes,
trans-
polybutadiene and transpolyisoprene, have been essentially replaced as the
cover
materials of choice by new cover materials comprising ionomeric resins.
lonomeric resins are polymers containing interchain ionic bonding. As a result
of
their toughness, durability and flight characteristics, various ionomeric
resins sold by E.1.
DuPont de Nemours & Company under the trademark "Suriyn ' and more recently,
by the
Exxon Corporation (see U.S. Patent No. 4,911,451) under the trademarks "ESCOR
" and
the trade name "IotekT"'", have become the materials of choice for the
construction of golf
ball covers over the traditional "balata" (transpolyisoprene, natural or
synthetic) rubbers.
As stated, the softer balata covers, although exhibiting enhanced playability
properties,
lack the durability (cut and abrasion resistance, fatigue endurance, etc.)
properties
required for repetitive play.
lonomeric resins are generally ionic copolymers of an olefin, such as
ethylene, and
a metal salt of an unsaturated carboxylic acid, such as acrylic acid,
methacrylic acid, or
maleic acid. Metal ions, such as sodium or zinc, are used to neutral'cze some
portion of
the acidic group in the copolymer resulting in a thermoplastic elastomer
exhibiting
enhanced properties, i.e. durability, etc., for golf ball cover construction
over balata.
However, some of the advantages gained in inawsed durability have been offset
to some
degnep by the deaeases=pnodueed in playability. This is because atthough the
ionomeric
resins are very durable, they tend to be very hard when utitized for golf ball
cover
construcdon, and=thus lack the degree of softness required to impart the spin
necessary
to control the ball in ftight. Since the ionomeric resins are harder tttian
balata, the
ionorneric resin covers do not compress as much agalnst the face of the dub
upon impact,
thereby producing less spin. In addition, the harder and more durable
tonomeric resins
lack the "feel" characteristic associated with the softer balata.related
covers.
As a result, while there are currently more than fifty (50) cocrunercial
grades of
ionomers available both from DuPont and Exocon, with a wide range of
properties which
vary according to the type and amount of metal cations, molecular weight,
composition of
the base resin (i.e., retative eontent of ethylene and mettracrytic and/or
acrylic acid groups)
2
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
and additive ingredients such as reinforcement agents, etc., a great deal of
research
continues in order to develop a golf ball cover composition exhibiting not
only the improved
impact resistance and carrying distance properties produced by the "hard"
ionomeric
resins, but also the playability (i.e., "spin", "feeP', etc.) characteristics
previously associated
with the "soft" balata covers, properties which are still desired by the more
skilled golfer.
Consequently, a number of two-piece (a solid resilient center or core with a
molded
cover) and three-piece (a liquid or solid center, elastomeric winding about
the center, and
a molded cover) golf balls have been produced to address these needs. The
different
types of materials utilized to formulate the cores, covers, etc. of these
balls dramatically
alters the balls' overall characteristics. In addition, multi-layered covers
containing one or
more ionomer resins have also been formulated in an attempt to produce a golf
ball having
the overall distance, playability and durability characteristics desired.
This was addressed by Spalding & Evenflo companies, Inc., the assignee of the
present invention, in U.S. Patent No. 4,431,193 where a multi-layered golf
ball is produced
by initially molding a first cover layer on a spherical core and then adding a
second layer.
The first layer is comprised of a hard, high flexural modulus resinous
material such as type
1605 Surlyn (now designated Surlyn 8940). Type 1605 Surlyn (Surlyn 8940)
is a
sodium ion based low acid (less than or equal to 15 weight percent methacrylic
acid)
ionomer resin having a flexural modulus of about 51,000 psi. An outer layer of
a
comparatively soft, low flexural modulus resinous material such as type 1855
Surlyn (now
designated Surfyn 9020) is molded over the inner cover layer. Type 1855
Suriyn
(Surlyn(& 9020) is a zinc ion based low acid (10 weight percent methacrylic
acid) ionomer
resin having a flexural modulus of about 14,000 psi.
The'193 patent teaches that the hard, high flexural modulus resin which
comprises
the first layer provides for a gain in coefficient of restitution over the
coefficient of
restitution of the core. The increase in the coefficient of restitution
provides a ball which
serves to attain or approach the maximum initial velocity limit of 255 feet
per second as
provided by the United States Golf Association (U.S.G.A.) rules. The
relatively soft, low
3
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
flexural modulus outer layer provides for the advantageous "feel" and playing
characteristics of a balata covered golf ball.
In various attempts to produce a durable, high spin ionomer golf ball, the
golfing
industry has blended the hard ionomer resins with a number of softer ionomeric
resins.
U.S. Patent Nos. 4,884,814 and 5,120,791 are directed to cover compositions
containing
blends of hard and soft ionomeric resins. The hard copolymers typically are
made from
an olefin and an unsaturated carboxylic acid. The soft copolymers are
generally made
from an olefin, an unsaturated carboxylic acid, and an acrylate ester. It has
been found
that golf ball covers formed from hard-soft ionomer blends tend to become
scuffed more
readily than covers made of hard ionomer alone. It would be useful to develop
a golf ball
having a combination of softness and durability which is better than the
softness-durability
combination of a golf ball cover made from a hard-soft ionomer blend.
Most professional golfers and good amateur golfers desire a golf ball that
provides
distance when hit off a driver, control and stopping ability on full iron
shots, and high spin
on short "touch and feel" shots. Many conventional two-piece and thread wound
performance golf balls have undesirable high spin rates on full shots. The
excessive spin
on full shots is a sacrifice made in order to achieve more spin which is
desired on the
shorter touch shots. It would be beneficial to provide a golf ball which has
high spin for
touch shots without generating excessive spin on full shots.
Summary of the Invention
An object of the invention is to provide a golf ball with a soft cover which
has good
scuff resistance.
Yet another object of the invention is to provide a golf ball having a
favorable
combination of spin rate and durability.
A further object of the invention is to provide a golf ball having a soft
cover made
from a cover material which is blended with minimal mixing difficulties.
Another object of the invention is to provide a method of making a golf ball
which
has a soft cover with good scuff resistance and cut resistance.
4
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Another object of the invention is to provide a golf ball which has a high
spin on
shots of 250 feet or less and an average spin on full shots using a 9 iron.
Yet another object of the invention is to provide a method of making a durable
golf
ball with a relatively high spin rate.
A further object of the invention is to provide a multi-layer golf ball having
exceptionally soft feel and high spin rates on short shots while maintaining
good distance
on full shots.
Yet another object of the invention is to provide a multi-layer golf ball
having a high
spin rate on short shots and not having an excessive spin rate on long shots.
Other objects will be in partC obvious and in part pointed out more in detail
hereafter.
The invention in a preferred form is a golf ball, comprising: a core, an inner
cover
layer formed over the core, the inner cover layer having a Shore D hardness of
at least 60
as measured on the curved surface thereof, and an outer cover layer formed
over the inner
cover layer, the outer cover layer having a Shore D hardness of no more than
53 as
measured on the curved surface thereof, at least one of the inner and outer
cover layers
comprising at least one member selected from the group consisting of
polycarbonates,
reaction-injection-molded polyurethanes, and styrene-butadiene elastomers, the
golf ball
having a PGA compression of 100 or less and a coefficient of restitution of at
ieast 0.750.
The outer cover layer preferably has a Shore D hardness of no more than 50.
The
ball preferably has a PGA compression of 90 or less. In a particularly
preferred form of the
invention, the outer cover layer comprises a thermoplastic material.
In a preferred form of the invention, the inner cover layer comprises reaction-
injection molded polyurethane. In another preferred form, the inner cover
layer comprises
polycarbonate. The inner cover layer preferably has a Shore D hardness of 60 -
85, and
more preferably 65 - 85.
The outer cover layer preferably has a Shore D hardness of 30 - 50.
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Another preferred form of the invention incorporates polycarbonates in the
outer
cover layer.
The golf ball of the invention preferably has a core which is a solid or is
filled with
liquid. The core can be wound or non-wound. A wound core may comprise a
liquid, solid,
gel or multi-piece center. In one preferred form of the invention, at least
one of the inner
and outer cover layers has a thickness of 0.01 - 0.150 inches, more preferably
0.02 - 0.10
inches, and even more preferably 0.03 - 0.07 inches.
The outer cover layer preferably has a Shore D hardness of 45 - 53 as measured
on the curved surface thereof. The inner cover layer preferably has a Shore D
hardness
of 60 -85 as measured on the curved surface thereof.
Yet another preferred form of the invention is a golf ball comprising: a core,
an
inner cover layer formed from a composition which includes at least 50 weight
% of at least
one material selected from the group consisting of polycarbonates, reaction-
injection-
molded polyurethanes, and styrene-butadiene elastomers, and an outer cover
layer formed
over the inner cover layer, the outer cover layer having a Shore D hardness of
no more
than 53 as measured on the curved surface thereof, the golf ball having a PGA
compression of 100 or less and a coefficient of restitution of at least 0.750.
The invention accordingly comprises the several steps and the relation of one
or
more of such steps with respect to each of the others and the article
possessing the
features, properties, and the relation of elements exemplified in the
following detailed
disclosure.
Brief Description of the Drawings
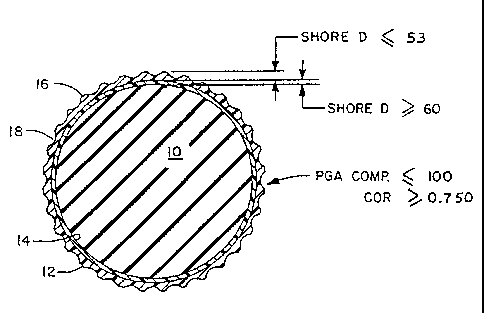
FIG. 1 is a cross-sectional view of a golf ball embodying the invention
illustrating
a core 10 and a cover 12 consisting of an inner layer 14 and an outer layer 16
having
dimples 18; and
FIG. 2 is a diametrical cross-sectional view of a golf ball of the invention
having a
core 10 and a cover 12 made of an inner layer 14 and an outer layer 16 having
dimples
18.
6
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Detailed Description of the Invention
The present invention relates to improved multi-layer golf balls, particularly
a golf
ball comprising a multi-layered cover 12 over a core 10, and method for making
same.
The golf balls of the invention, which can be of a standard or enlarged size,
have a unique
combination of high coefficient of restitution and a high spin rate on short
shots.
The core 10 of the golf ball can be formed of a solid, a liquid, or any other
substance which will result in an inner ball, i.e. core and inner cover layer,
having the
desired COR, compression and hardness. The multi-layered cover 12 comprises
two
layers: a first or inner layer or ply 14 and a second or outer layer or ply
16. The inner
layer 14 can be ionomer, ionomer blends, non-ionomer, non-ionomer blends, or
blends of
ionomer and non-ionomer. The outer layer 16 is softer than the inner layer and
can be
ionomer, ionomer blends, non-ionomer, non-ionomer blends or blends of ionomer
and non-
ionomer.
In a first preferred embodiment, the inner layer 14 is comprised of a high
acid (i.e.
greater than 16 weight percent acid) ionomer resin or high acid ionomer blend.
Preferably,
the inner layer is comprised of a blend of two or more high acid (i.e. at
least 16 weight
percent acid) ionomer resins neutralized to various extents by different metal
cations. The
inner cover layer may or may not include a metal stearate (e.g., zinc
stearate) or other
metal fatty acid salt. The purpose of the metal stearate or other metal fatty
acid salt is to
lower the cost of production without affecting the overall performance of the
finished golf
ball. In a second embodiment, the inner layer 14 is comprised of a low acid
(i.e: 16 weight
percent acid or less) ionomer blend. Preferably, the inner layer is comprised
of a blend
of two or more low acid (i.e. 16 weight percent acid or less) ionomer resins
neutralized to
various extents by different metal cations. The inner cover layer may or may
not include
a metal stearate (e.g., zinc stearate) or other metal fatty acid salt. The
purpose of the
metal stearate or other metal fatty acid salt is to lower the cost of
production without
affecting the overall performance of the finished golf ball.
It has been found that a hard inner layer provides for a substantial increase
in
resilience (i.e., enhanced distance) over known multi-layer covered balls. The
softer outer
7
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
layer provides for desirable "feel" and high spin rate while maintaining
respectable
resiliency. The soft outer layer allows the cover to deform more during impact
and
increases the area of contact between the club face and the cover, thereby
imparting more
spin on the ball. As a result, the soft cover provides the ball with a balata-
like feel and
playability characteristics with improved distance and durability.
Consequently, the overall
combination of the inner and outer cover layers results in a golf ball having
enhanced
resilience (improved travel distance) and durability (i.e. cut resistance,
etc.) characteristics
while maintaining and in many instances, improving the playability properties
of the ball.
The combination of a hard inner cover layer with a soft, relatively low
modulus
ionomer, ionomer blend or other non-ionomeric thermoplastic elastomer outer
cover layer
provides for excellent overall coefficient of restitution (i.e., excellent
resilience) because
of the improved resiliency produced by the inner cover layer. While some
improvement
in resiliency is also produced by the outer cover layer, the outer cover layer
generally
provides for a more desirable feel and high spin, particularly at lower swing
speeds with
highly lofted clubs such as half wedge shots.
Inner Cover Layer
The inner cover layer is harder than the outer cover layer and generally has a
thickness in the range of 0.01 to 0.150 inches, preferably 0.02 - 0.10 inches,
and more
preferably 0.03 to 0.06 inches for a 1.68 inch ball and 0.03 to 0.07 inches
for a 1.72 inch
(or more) ball. The core and inner cover layer together form an inr.er ball
preferably having
a coefficient of restitution of 0.770 or more and more preferably 0.780 or
more, and a
diameter in the range of 1.48 - 1.66 inches for a 1.68 inch ball and 1.50 -
1.70 inches for
a 1.72 inch (or more) ball. The inner cover layer has a Shore D hardness of 60
or more.
It is particularly advantageous if the golf balls of the invention have an
inner layer with a
Shore D hardness of 65 or more. The above-described characteristics of the
inner cover
layer preferably provide an inner ball having a PGA compression of 100 or
less. lt is found
that when the inner ball has a PGA compression of 90 or less, excellent
playability results.
8
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2004-04-02
The inner layer compositions of the first and third embodiments include the
high acid
ionomers such as those developed by E.I. DuPont de Nemours & Company under the
trademark "Surlyn " and by Exxon Corporation under the trademark "Escor "
ortradename
"lotek", or blends thereof. Examples of compositions which may be used as the
inner layer
herein are set forth in detail in U.S. patent No. 5,688,869. Of course, the
inner layer high
acid ionomer compositions are not limited in any way to those compositions set
forth.
The high acid ionomers which may be suitable for use in formulating the inner
layer
compositions of the subject first and third embodiments of the invention are
ionic
copolymers which are the metal, i.e., sodium, zinc, magnesium, etc., salts of
the reaction
product of an olefin having from about 2 to 8 carbon atoms and an unsaturated
monocarboxylic acid having from about 3 to 8 carbon atoms. Preferably, the
ionomeric
resins are copolymers of ethylene and either acrylic or methacrylic acid. In
some
circumstances, an additional comonomer such as an acrylate ester (i.e., iso-
or n-
butyiacryiate, etc.) can also be included to produce a softer terpolymer. The
carboxylic
acid groups of the copolymer are partially neutralized (i.e., approximately 10
- 100%,
preferably 30 - 70%) by the metal ions. Each of the high acid lonomer resins
which may
be induded iin the inner layer cover compositions of the invention contains
greater than
about 16% by weight of a carboxytic acid, preferably from about 17% to about
25% by
weight of a carboxylic acid, more preferably from about 18.5% to about 21.5%
by weight
of a carboxylic acid.
Afthough the inner layer cover composition of the first and third embodiments
of the
invention preferably includes a high acid ionorneric resin and the scope of
the patent
embraces ali icnown high add ionomeric resins faeing within the parameters set
forth
above, only a relatively limited number of these high acid ionomeric resins
have recently
become commercially available.
9
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
The high acid ionomeric resins available from Exxon under the designation
"EscorO" and or "lotek", are somewhat similar to the high acid ionomeric
resins available
under the "Surlyn " trademark. However, since the Escor /lotek ionomeric
resins are
sodium or zinc salts of poly(ethylene-acrylic acid) and the "Surlyn " resins
are zinc,
sodium, magnesium, etc. salts of poly(ethylene-methacrylic acid), distinct
differences in
properties exist.
Examples of the high acid methacrylic acid based ionomers found suitable for
use
in accordance with this invention include Surlyn 8220 and 8240 (both formerly
known as
forms of Surlyn AD-8422), Surtyn 9220 (zinc cation), Surlyn SEP-503-1 (zinc
cation),
and Surlyn SEP-503-2 (magnesium cation). According to DuPont, all of these
ionomers
contain from about 18.5 to about 21.5% by weight methacrylic acid.
More particularly, Surlyn AD-8422 is currently commercially available from
DuPont
in a number of different grades (i.e., AD-8422-2, AD-8422-3, AD-8422-5, etc.)
based upon
differences in melt index. According to DuPont, Surlyn 8422, which is
believed recently
to have been redesignated as 8220 and 8240, offers the following general
properties when
compared to Surlyn 8920, the stiffest, hardest of all on the low acid grades
(referred to
as "hard" ionomers in U.S. Patent No. 4,884,814):
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE I
LO4I ACID IIIGII ACID
(15 wtt Acid) (>20 wtt Acid)
SURLYII SURLYN SURLYti
8920 8422-2 48 22-3
IONOtIER
cation t(a tla tla
hielt Index 1.2 2.8 1.0
Sodium, Wt$ 2.3 1.9 2.4
Base Resin MI 60 60 60
MP" C 88 86 85
FP', C 47 48.5 45
COMPRESSION MOLDING1
Tensile Break,
psi 4350 4190 5330
Yield, psi 2880 3670 3590
Elongation, 1 315 263 289
F'_ex Mod,
K psi 53.2 76.4 88.3
Shore D
hardness 66 67 68
1 DSC second heat, 10 C/min heatinq rate.
1 Samples compression molded at 150=C annealed 24
hours at 60 C. 8422-2, -3 were homogenized at
190 C before molding.
11
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2004-04-02
in comparing SurlyrO 8920 to Surtyn 8422-2 and Surlyn(D 8422-3, it is noted
that
the high acid Surlyn 8422-2 and 8422-3 ionomers have a higher tensile yield,
lower
elongation, slightly higher Shore D hardness and much higher flexural modulus.
Surlyn
8920 contains 15 weight percent methacrylic acid and is 59% neutralized with
sodium.
In addition, SurlynO SEP-503-1 (zinc cation) and Sur(yn SEP-503-2 (magnesium
cation) are high acid zinc and magnesium versions of the Surlyn AD 8422 high
acid
ionomers. When compared to the Surlyn AD 8422 high acid ionomers, the Surlyn
SEP-
503-1 and SEP-503-2 ionomers can be defined as follows:
Surlvn lonomer ion Melt- Index Neutratization %
AD 8422-3 Na 1.0 45
SEP 503-1 Zn 0.8 38
SEP 503-2 Mg 1.8 43
Fclrthermore, Surfyn 8162 is a zinc cation ionomer resin containing
approximately
20% by weight (i.e. 18.5 - 21.5 /a weight) methacrylic acid copolymer that has
been 30 -
70 /a neutralized. Surlyn 8162 is currently commercially available from
DuPont.
Examples of the high acid acrylic acid based lonomers suitable for use in the
present invention also include the EscorO or lotek high acid ethylene acryiic
acid ionomers
produced by Exxon such as ExTM 1001, 1002, 959, 960, 989, 990, 1003, 1004,
993, 994. In
this regard, EscotO or lotek 959 is a sodium ion neutralized ethylene-acrylic
neutrattzed
ethylene-acrylic acid copolymer. According to ExxoN loteks 959 and 960 contain
from
about 19.0 to about 21.0 k by weight acrylic acid with approximately 30 to
about 70
percent of the acid groups neutr$Czed with sodium and zinc ions, respectively.
The
physical properties of these high acid acrylic acid based ionomers are as
follows:
12
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE 2
Exxon High Acid lonomers
ESCOR ESCOR
(IOTEK) (IOTEK)
Property Ex 1001 Ex 1002 959 Ex 1003 Ex 1004 960
Melt index, g/10 min. 1.0 1.6 2.1 1.1 2.0 1.8
Cation Na Na Na Zn " Zn Zn
Melting Point, C 83.7 83.7 -- 82 82.5 79
Vicat Softening Point, C 51.5 51.5 58 56 55 55
Tensile @ Break 34.4 MPa 31.7 MPa 34 MPa 24.8 MPa 20.6 MPa 24 MPa
Elongation @ Break, % 341 348 280 387 437 430
Hardness, Shore D 63 62 65 54 53 57
Flexural Modulus 365 MPa 380 MPa 480 MPa 147 MPa 130 MPa 170 MPa
13
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE 3
Additional Exxon High Acid lonomers
Property Unit EX 989 EX 993 EX 994 EX 990
Melt Index g/10 min. 1.30 1.25 1.32 1.24
Moisture ppm 482 214 997 654
Cation Type -- Na Li K Zn
M+ content by AAS wt % 2.74 0.87 4.54 0
Zn content by AAS wt % 0 0 0 3.16
Density kg/m3 959 945 976 977
Vicat softening point C 52.5 51 50 55.0
Crystallization point C 40.1 39.8 44.9 54.4
Melting point C 82.6 81.0 80.4 81.0
Tensile at yield MPa 23.8 24.6 22 16.5
Tensile at break MPa 32.3 31.1 29.7 23.8
Elongation at break % 330 260 340 357
1 /a secant modulus MPa 389 379 312 205
Flexural modulus MPa 340 368 303 183
Abrasion resistance mg 20.0 9.2 15.2 20.5
Hardness Shore D -- 62 62.5 61 56
Zwick Rebound % 61 63 59 48
Furthermore, as a result of the development by the assignee of this
application of
a number of new high acid ionomers neutralized to various extents by several
different
types of metal cations, such as by manganese, lithium, potassium, calcium and
nickel
14
SUBSTITUTE SHEET (RULE 26)
. ;'
CA 02366400 2004-04-02
cations, several new high acid ionomers and/or high acid ionomer blends
besides sodium,
zinc and magnesium high acid ionomers or ionomer blends are now available for
golf ball
cover production. It has been found that these new cation neutralized high
acid ionomer
blends produce inner cover layer compositions exhibiting enhanced hardness and
resilience due to synergies which occxJr during processing. Consequently, the
metal cation
neutralized high acid ionomer resins recently produced can be blended to
produce
substantially higher C.O.R.'s than those produced by the low acid ionomer
inner cover
compositions presentiy commercially avaiiabie.
More particularly, several new metal cation neutralized high acid ionomer
resins
have been produced by the inventor by neutralizing, to various extents, high
acid
copolymers of an alpha-olefin and an alpha, beta-unsaturated carboxylic acid
with a wide
variety of different metal cation salts. This discovery is the subject matter
of U.S.
patent No. 5,688,869. It has been found that numerous new metal cation
neutralized high
acid ionomer resins can be obtained by reacting a high acid copolymer (i.e. a
copolymer
containing greater than 16% by weight acid, preferably from about 17 to about
25 weight
percent acid, and more preferably about 20 weight percent acid), with the
metal cation salt
capable of ionizing or neutralizing the copolymer.to the extent desired (i.e.
from about 10%
to 90%).
The base copolymer is made up of greater than 16% by weight of an alpha, beta-
unsaturated carboxyrtc acid and an alpha-olefin. Optionally, a softening
comonomer can
be induded In the copolymer. Generally, the alpha-olefin has from 2 to 10
carbon atoms
and Is preferably ethylene, and the unsah.aated carboxylic add is a carboxylic
acid having
from about 3 to 8 cxrbons. Examples of such acids indude acrylic acid,
methaca'ylic acid,
ethacryiic acid, chloroacrylic acid, crotonic acid, maleic acid, fumaric acid,
and Itaconic
acid, with acrylic acid being prefemed.
The softening comonomer that can be optionally included In the Inner cover
layer
for the golf ball of the invention may be selected from the group consisting
of vinyl esters
of aliphatic cartfioxylic acids wherein the acids have 2 to 10 carbon atoms,
vinyl ethers
wherein the alkyl groups oontains I to 10 carbon atoms, and alkyl acaylates or
CA 02366400 2004-04-02
methacrylates wherein the alkyl group contains 1 to 10 carbon atoms. Suitable
softening
comonomers include vinyl acetate, methyl acrylate, methyl methacrylate, ethyl
acrylate,
ethyl methacrylate, butyl acryiate, butyi methaaylate, or the like.
Consequently, examples of a number of copolymers suitable for use to produce
the
high acid ionomers included in the present invention include, but are not
limited to, high
acid embodiments of an ethyfene/acry(ic acid copolymer, an
ethylenelmethacrylic acid
copolymer, an ethylenertaconic acid copolymer, an ethylene/maleic acid
copolymer, an
ethylene/methacrylic acid/vinyl acetate copolymer, an ethy(ene/acrylic
aGd/vinyl alcohol
copolymer, etc. The base copolymer broadly contains greater than 16% by weight
unsaturated carboxylic acid, from about 39 to about 83% by weight ethylene and
from 0
to about 40% by weight of a softening comonomer. Preferably, the copolymer
contains
about 20% by weight unsaturated carboxyiic acid and about 80% by weight
ethylene. Most
preferably, the copolymer contains about 20% acrylic acid with the remainder
being
ethytene.
Along these lines, examples of the preferred high acid base copolymers which
fulfill
the criteria set forth above, are a series of ethylene-acrylic copolymers
which . are
commercially available from The Dow Chemical Company, Midland, Michigan, under
the
"PrimacorTM" designation. These high acid base copolymers exhibit the typical
properties
set forth below in Table 4.
16
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE 4
Typical Properties of Primacor
Ethylene-Acrylic Acid Copolymers
GRADE PERCENT DENSITY MELT 'I'ENSII..E FLEXURAL VICAT SHORE D
ACID G/CC INDEX YD ST MODULUS SOFT PT HARDNESS
G/10 MIN (PSI) ( PSI) ( C)
ASTM D-792 D-1238* D-630 D-790 D-1525 D-2240
5980 20.0 0.958 300.0 -- 4800 43 50
5990 20.0 0.955 1300.0 650 40 42
5981 20.0 0.960 300.0 900 3200 46 48
5983 20.0 0.958 500.0 850 3100 44 45
5991 20.0 0.953 2600.0 635 2600 38 40
* 190 C
Due to the high molecular weight of the Primacor 5981 grade of the ethylene-
acrylic acid copolymer, this copolymer is the more preferred grade utilized in
the invention.
The metal cation salts utilized in the invention are those salts which provide
the
metal cations capable of neutralizing, to various extents, the carboxylic acid
groups of the
high acid copolymer. These include acetate, oxide or hydroxide salts of
lithium, calcium,
zinc, sodium, potassium, nickel, magnesium, and manganese.
Examples of such lithium ion sources are lithium hydroxide monohydrate,
lithium
hydroxide, lithium oxide and lithium acetate. Sources for the calcium ion
include calcium
hydroxide, calcium acetate and calcium oxide. Suitable zinc ion sources are
zinc acetate
dihydrate and zinc acetate, a blend of zinc oxide and acetic acid. Examples of
sodium ion
sources are sodium hydroxide and sodium acetate. Sources for the potassium ion
include
potassium hydroxide and potassium acetate. Suitable nickel ion sources are
nickel
17
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2004-04-02
acetate, nickel oxide and nickel hydroxide. Sources of magnesium include
magnesium
oxide, magnesium hydroxide, magnesium acetate. Sources of manganese include
manganese acetate and manganese oxide.
The new metal cation neutralized high acid ionomer resins are produced by
reacting the high acid base copolymer with various amounts of the metal cation
salts
above the crystalline melting point of the copolymer, such as at a temperature
from about
200 F to about 500 F, preferably from about 250 F to about 350 F under high
shear
conditions at a pressure of from about 10 psi to 10,000 psi. Other well known
blending
techniques may also be used. The amount of metal cation salt utilized to
produce the new
metal cation neutralized high acid based ionflmer resins is the quantity which
provides a
sufficient amount of the metal cations to neutralize the desired percentage of
the
carboxyiic acid groups in the high acid copolymer. The extent of
neutralization is generally
from about 10% to about 90%.
As indicated below in Table 5 and more specifically in Example 1 in U.S.
patent No. 5,688,869, a number of new types of metal cation neutralized high
acid ionomers can be obtained from the above indicated process. These indude
new high
acid ionomer resins neutraiized to various extents with manganese, lithium,
potassium,
calcium and nickel cations. In addition, when a high acid ethylene/acrylic
acid copolymer
is utilized as the base copolymer component of the invention and this
component is
subsequently neutralized to various extents with the metal cation salts
producing acrylic
acid based high acid ionomer resins neutralized with cations such as sodium,
potassium,
lithium, zinc, magneskm, manganese, calcium and nickel, several new cation
neutralized
acrylic acid based high acid ionomer resins are produced.
18
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE 5
Ut-X 1!t-X Ilelt Shore 0
forrtulstlon Ilo, Cet(on Selt ftutrellietl M jndex C.O.R. lerdne
1(14.011) 6.98 67.5 0.9 .804 71
2(14e011) 5.66 54.0 2.4 .808 T3
3(4011) 3.84 35.9 12.2 .812 69
40e011) 2.91 2T.0 17.5 .012 (brlttle)
5(HnAc) 19.6 71.7 7.5 .809 73
6(14nAc) 23.1 88.3 3.5 .814 TT
T(HnAc) 15.3 53.0 7.5 .810 72
8(Hwlc) 26.5 106 0.7 .813 (brlttle)
9(1.1011) 4.54 71.3 0.6 .810 T4
10(11011) 3.38 52.5 4.2 .818 72
11(L1011) 2.34 35.9 10.6 .e15 72
12(K011) 5.30 36.0 19.3 Broke 70
13(K011) 8.26 57.9 7.18 .504 70
14 (K011) 10.7 77.0 4.3 .801 6T
15(ZnAc) 17.9 71.5 0.2 .806 71
16(ZnAc) 13.9 53.0 0.9 .797 69
17(2nAc) 9.91 36.1 3.4 .793 67
18(HpAc) 17.4 TO.T 2.8 .81'4 74
19(HpAc) 20.6 87.1 1.5 .815 76
20(HpAc) 13.5 53.8 4.1 .814 TS
2I(CsAc) 13.2 69.2 1.1 .813 74
22(CeAc) T.12 34.9 10.1 .BoB T0
Controls: 50/50 Ylend ot loteks 8000/7030 C.O.R.*.810/6S Sllore 0 Ilsrdness
OuPont High Acid SurlyrM 5422 (Me) CØR.=.811/70 Share 0 Ilsrdneas
OuPont Mlyh Acid Surlyne 8162 (Zn) C.o.ll.=.80T/65 S)wre 0 llerdness
Exxon 11(9h Acid lotek EX-960 (in) C.O.Jt.~.T96/65 shere 0(lerdness
19
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE 5 (cont.)
4t-X Ut-X Halt Shore 0
rm1 (Ltlon No, Catlon Sau Neutrsli=etlon I[dex C.O.R. ardneas
23(HCO) .2.91 53.5 2.5 .813
2t(Hga) 3.85 71.5 2.e eae
25(HVa) 4.76 89.3 1.1 .a09
26(HOa) 1.96 75.7 T.S .815
Contral (or farnulatlona 23-26 Is $0/50 lotak 8000/7030,
C.O.R.=.81~, foraulatlors 26 C.O.R. vaa rw- lI:ad to that control accordlnClY
t!t-X Nt X Hqlt
fornulletIon No. Cat(on Salt Neutrati=atlon jridex ~.R,.
27(HIAc) 13.04 61.1 0.2 :E02 71
2E(HIAe) 10.71 4a.9 0.! .799 72
29(HIAe) E.26 36.7 1.E .796 69
30(HIAc) 5.66 24.4 7.5 .786 64
Control (or foroulatlon Hos. 2T-30 Is SO/SO latak E000/7030, C.O.R.=.807
When compared to low acid versions of similar cation neutralized ionomer
resins, the new
metal cation neutralized high acid ionomer resins exhibit enhanced hardness,
modulus and
resilience characteristics. These are properties that are particularly
desirable in a number
of thermoplastic fields, including the field of golf ball manufacturing.
When utilized in the construction of the inner layer of a multi-layered golf
ball, it
has been found that the new acrylic acid based high acid ionomers extend the
range of
hardness beyond that previously obtainable while maintaining the beneficial
properties (i.e.
durability, click, feel, etc.) of the softer low acid ionomer covered balls,
such as balls
produced utilizing the low acid ionomers disclosed in U.S. Patent Nos.
4,884,814 and
4,911,451.
Moreover, as a result of the development of a number of new acrylic acid based
high acid ionomer resins neutralized to various extents by several different
types of metal
cations, such as manganese, lithium, potassium, calcium and nickel cations,
several new
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
ionomers or ionomer blends are now available for production of an inner cover
layer of a
multi-layered golf ball. By using these high acid ionomer resins, harder,
stiffer inner cover
layers having higher C.O.R.s, and thus longer distance, can be obtained.
More preferably, it has been found that when two or more of the above-
indicated
high acid ionomers, particularly blends of sodium and zinc high acid ionomers,
are
processed to produce the covers of multi-layered golf balls, (i.e., the inner
cover layer
herein) the resulting golf balls will travel further than previously known
multi-layered golf
balls produced with low acid ionomer resin covers due to the balls' enhanced
coefficient
of restitution values.
The low acid ionomers which may be suitable for use in formulating the inner
layer
compositions of the second and third embodiments of the subject invention are
ionic
copolymers which are the metal, i.e., sodium, zinc, magnesium, etc., salts of
the reaction
product of an olefin having from about 2 to 8 carbon atoms and an unsaturated
monocarboxylic acid having from about 3 to 8 carbon atoms. Preferably, the
ionomeric
resins are copolymers of ethylene and either acrylic or methacrylic acid. In
some
circumstances, an additional comonomer such as an acrylate ester (i.e., iso-
or n-
butylacrylate, etc.) can also be included to produce a softer terpolymer. The
carboxylic
acid groups of the copolymer are partially neutralized (i.e., approximately 10
- 100%,
preferably 30 - 70%) by the metal ions. Each of the low acid ionomer resins
which may be
induded in the inner layer cover compositions of the invention contains 16% by
weight or
less of a carboxylic acid.
The inner layer compositions indude the low acid ionomers such as those
developed and sold by E.I. DuPont de Nemours & Company under the trademark
"Surlyn " and by Exxon Corporation under the trademark "Escor " or tradename
"lotek",
or blends thereof.
The low acid ionomer resins available from Exxon under the designation "Escor
"
and/or "lotek", are somewhat similar to the low acid ionomeric resins
available under the
"Surlyn " trademark However, since the Escor /Iotek ionomeric resins are
sodium or
zinc salts of poly(ethylene-acrylic acid) and the "Surlyn " resins are zinc,
sodium,
21
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
magnesium, etc. salts of poly(ethylene-methacrylic acid), distinct differences
in properties
exist.
When utilized in the construction of the inner layer of a multi-layered golf
ball, it
has been found that the low acid ionomer blends extend the range of
compression and
spin rates beyond that previously obtainable. More preferably, it has been
found that
when two or more low acid ionomers, particularly blends of sodium and zinc
ionomers, are
processed to produce the covers of multi-layered golf balls, (i.e., the inner
cover layer
herein) the resulting golf balls will travel further and at an enhanced spin
rate than
previously known multi-layered golf balls. such an improvement is particularly
noticeable
in enlarged or oversized golf balls.
As shovm in the Examples, use of an inner layer formulated from blends of
lower
acid ionomers produces multi-layer golf balls having enhanced compression and
spin
rates. These are the properties desired by the more skilled golfer.
In a third embodiment of the inner cover layer, a blend of high and low acid
ionomer resins is used. These can be the ionomer resins described above,
combined in
a weight ratio which preferably is within the range of 10 - 90 to 90 - 10 high
and low acid
ionomer resins.
A fourth embodiment of the inner cover layer is primarily or fully non-
ionomeric
thermoplastic material. Suitable non-ionomeric materials include metallocene
catalyzed
polyolefins or polyamides, polyamide/ionomer blends, polycarbonates,
polyphenylene
etherronomer blends, etc., which have a Shore D hardness of z 60 and
preferably have
a flex modulus of greater than about 30,000 psi, or other hardness and flex
modulus
values which are comparable to the properties of the ionomers described above.
Other
suitable materials include but are not limited to thermoplastic or
thermosetting
polyurethanes/polyureas, including castable polyurethanes/polyureas, reaction
injection
moldable polyurethanes/polyureas and injection moldable
polyurethanes/polyureas,
thermoplastic block polyesters, such as a polyester elastomer marketed by
DuPont under
the trademark Hytrel , thermoplastic block poolyamides, such as a polyester
amide
marketed by Elf Atochem S.A. under the trademark Pebax , a blend of two or
more non-
22
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2004-04-02
ionomeric themtioplastic elastomers, or a blend of one or more ionomers and
one or more
non-ionomeric thermoplastic elastomers. These materials can be blended with
the
ionomers described above in order to reduce cost relative to the use of higher
quantities
of ionomer.
Outer Cover Layer
While the core with the hard inner cover layer formed thereon provides the
multi-
layer golf ball with power and distance, the outer cover layer 16 is
comparatively softer
than the inner cover layer. The softness provides for the feel and playability
characteristics typically associated with balata or balata-blend balls. The
outer cover layer
or ply is comprised of a relatively soft, low modulus (about 1,000 psi to
about 10,000 psi)
and, in one embodiment, low acid (less than 16 weight percent acid) ionomer,
an lonomer
blend, a non-ionomeric themloplastic or thermosetting materia( such as, but
not limited to,
a metallocene catalyzed polyolefin such as EXACTTM material available from
EXXON, a
thermoplastic or thermoset polyurethane/polyurea, including castable
polyurethaneslpolyureas, reaction injection moldable polyurethaneslpotyureas,
and
injection moldable polyurethaneslpolyureas, polycarbonates, thesmoplastic
block
polyesters, such as a potyester elastomer marketed by DuPont under the
trademark
Hytrei , thermoplastic block polyarriides, such as a polyester amide marketed
by Elf
Atochem S.A. under the trademark Pebax@, a blend of two or more non-ionomeri.c
themioplastic or themlosetting materials, or a blend of one or more lonomers
and one or
more non-ionomeric thermoplas6c materials. The outer layer Is from about 0.010
to about
0.150 inches In tiiidcness, preferably 0.02 - 0.10 inches, more desirably 0.03
to 0.06
inches in thickness for a 1.680 inch ball and 0.03 to 0.07 inctm In tfii*ness
for a 1.72
inch or more ball), but thick enough to achieve desired playability
c~aaracteristics while
minimizing expense. Thidvtess is defined as the average thidvtiess of the non-
dimPled
areas of the outer cover layer. The otiter cover layer 16 has a Shore 0
hardness of 55 or
less, more preferably 53 or less, and even more preferably 50 or less.
23
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
In one embodiment, the outer cover layer preferably is formed from an ionomer
which constitutes at least 75 weight % of an acrylate ester-containing ionic
copolymer or
blend of acrylate ester-containing ionic copolymers. This type of outer cover
layer in
combination with the core and inner cover layer described above results in
golf ball covers
having a favorable combination of durability and spin rate. The one or more
acrylate ester-
containing ionic copolymers each contain an olefin, an acrylate ester, and an
acid. In a
blend of two or more acrylate ester-containing ionic copolymers, each
copolymer may
contain the same or a different olefin, acrylate ester and acid than are
contained in the
other copolymers. Preferably, the acrylate ester-containing ionic copolymer or
copolymers
are terpolymers, but additional monomers can be combined into the copolymers
if the
monomers do not substantially reduce the scuff resistance or other good
playability
properties of the cover.
For a given copolymer, the olefin is selected from the group consisting of
olefins
having 2 to 8 carbon atoms, including, as non-limiting examples, ethylene,
propylene,
butene-1, hexene-1 and the like. Preferably the olefin is ethylene.
The acrylate ester is an unsaturated monomer having from 1 to 21 carbon atoms
which serves as a softening comonomer. The acrylate ester preferably is
methyl, ethyl,
n-propyl, n-butyl, n-octyl, 2-ethylhexyl, or 2-methoxyethyl 1-acrylate, and
most preferably
is methyl acrylate or n-butyl acrylate. Another suitable type of softening
comonomer is an
alkyl vinyl ether selected from the group consisting of n-butyl, n-hexyl, 2-
ethylhexyl, and
2-methoxyethyl vinyl ethers.
The acid is a mono- or dicarboxylic acid and preferably is selected from the
group
consisting of methacrylic, acrylic, ethacrylic, a-chloroacrylic, crotonic,
maleic, fumaric, and
itaconic acid, or the like, and half esters of maleic, fumaric and itaconic
acid, or the like.
The acid group of the copolymer is 10 - 100% neutralized with any suitable
cation, for
example, zinc, sodium, magnesium, lithium, potassium, calcium, manganese,
nickel,
chromium, tin, aluminum, or the like. It has been found that particularly good
results are
obtained when the neutralization level is about 50 - 100%.
24
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2004-04-02
The one or more acrylate ester-containing ionic coa olymers each has an
individual
Shore D hardness of about 5- 64. The overall Shc: e D hardness of the outer
cover is 55
or less, and generally is 40 - 55. It is preferred that the overall Shore D
hardness of the
outer cover is in the range of 40 - 50 in order to impart particularly good
playability
characteristics to the ball.
The outer cover layer of the-invention is formed over a core to result in a
golf ball
having a coefficient of restitution of at least 0.770, more preferably at
least 0.780, and
most preferably at least 0.790. The coefficient of restitution of the ball
will depend upon
the properties of both the core and the cover. The PGA compression of the golf
ball is 100
or less, and preferably is 90 or less.
The acrylate ester-containing ionic copolymer or copolymers used in the outer
cover layer can be obtained by neutralizing commercially available acrylate
ester-
oontaining acid copolymers such as polyethylene-methyi acrylate-acrytic acid
terpolymers,
including ESCOR ATXT"' (Exxon Chemical Company) or poly (ethylene-butyl
acrylate-
methacrylic acid) terpolymers, including NUCRELT"' (DuPont Chemical Company).
Particularly preferred commercially available materials include ATXT"4 320,
ATX 325, ATX
310, ATX 350, and blends of these materials with NUCREL 010 and NUCREL 035.
The
acid groups of these materials and blends are neutralized with one or more of
various
cation salts including zirtc, sodium, magnesium, lithium, potassium, calcium,
manganese,
nickel, etc. The degree of neutralization ranges from 10 - 100%. Generally, a
higher
degree of neutrsrmtion resutts in a harder and tougher cover material. The
properties of
nonAaniting examples of commercially available un-neutralized acid terpolymers
which can
be used to form the golf ball outer cover layers of the invention are provided
below In
Table 6.
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Table 6
Trade Name Melt Index Acid No. Flex modulus Hardness
dg/min % KOH/g MPa (Shore D)
ASTM D1238 (ASTM D790)
ATX 310 6 45 80 44
ATX 320 5 45 50 34
ATX 325 20 45 9 30
ATX 350 6 15 20 28
Nucrel 010 11 60 40 40
Nucrel 035 35 60 59 40
The ionomer resins used to form the outer cover layers can be produced by
reacting the acrylate ester-containing acid copolymer with various amounts of
the metal
cation salts at a temperature above the crystalline melting point of the
copolymer, such as
a temperature from about 200 F to about 500 F, preferably from about 250 F to
about
350 F, under high shear conditions at a pressure of from about 100 psi to
10,000 psi.
Other well known blending techniques may also be used. The amount of metal
cation salt
utilized to produce the neutralized ionic copolymers is the quantity which
provides a
sufficient amount of the metal cations to neutralize the desired percentage of
the
carboxylic acid groups in the high acid copolymer. When two or more different
copolymers
are to be used, the copolymers can be blended before or after neutralization.
Generally,
it is preferable to blend the copolymers before they are neutralized to
provide for optimal
mixing.
The compatibility of the acrylate ester-containing copolymers with each other
in
a copolymer blend produces a golf ball outer cover layer having a surprisingly
good scuff
resistance for a given hardness of the outer cover layer. The golf ball
according to the
invention has a scuff resistance of no higher than 3Ø It is preferred that
the golf ball has
a scxrff resistance of no higher than about 2.5 to ensure that the golf ball
is scuff res.istant
when used in conjunction with a variety of types of clubs, including sharp-
grooved irons,
26
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2004-04-02
which are particularly inciined, to result in scuffing of golf ball covers.
'The best results
acoording to the invention are obtained when the outer cover layer has a scuff
resistance
of no more than about 2Ø The scuff resistance test is described in detail
below.
Additional materials may also be added to the inner and outer cover layer of
the
present invention as long as they do not substantially reduce the playability
properties of
the ball. Such materials include dyes (for example, Ultramarine BIueTM sold by
Whitaker,
Clark, and Daniels of South Plainsfield, N_J.) (see U.S. Pat. No. 4,679,795),
pigments such
as titanium dioxide, zinc oxide, barium sulfate and zinc suffate; UV
absorbers; antioxidants;
antistatic agents; and stabilizers. Moreover, the cover compositions of the
present
invention may also contain softening agents such as those disclosed in
U.S..Patent Nos.
5,312,857 and 5,306,760, including plasticizers, metal stearates, processing
acids, etc.,
and reinforcing materials such as glass fibers and inorganic filters, as long
as the desired
properties produced by the golf ball covers of the invention are not impaired.
The outer layer in another embodiment of the invention includes a blend of a
soft
(low acid) ionomer resin with a small amount of a hard (high acid) ionomer
resin. A low
modulus ionomer suitable for use in -the outer layer blend has a flexural
modulus
measuring from about 1,000 to about 10,000 psi, witfi a hardness of about 20
to about 40
on the Shore D scale. A high moduius ionomer herein is one which measures from
about
15,000 to about 70,000 psi as measured in aocordance with ASTM method D-790.
The
hardness may be defined as at least 50 on the Shore D scale as measured in
accordance
with ASTM method D-2240.
Soft lonomers primarily are used in formulating the hard/soft blends of the
cover
compositions. These ionomers include acrylic acid and methacrylic acid based
soft
ionomers. They are generally dharacterized-as comprising sodium, zinc, or
other mono-
or d'nralent metal cation salts of a terpolymer of an olefin having from about
2 to 8 carbon
atoms, metharxyiic add, acrylic acid, or another a, li- unsaturated carboxylic
acid, and an
unsatixated monomer of the aciyiate ester class having from I to 21 carbon
atoms. The
soft lonomer is preferably made from an acrylic acid base polymer in an
unsaturated
monomer of the acxylate ester dass.
27
CA 02366400 2004-04-02
Certain ethylene-acrylic acid based soft ionomer resins developed by the Exxon
Corporation under the designation "lotek 7520" (referred to experimentally by
differences
in neutralization and melt indexes as LDXTM 195, LDX 196, LDX 218 and LDX 219)
may be
combined with known hard ionomers such as those indicated above to produce the
inner
and outer cover layers. The combination produces higher C.O.R.s at equal or
softer
hardness, higher melt flow (which corresponds to improved, more efficient
molding, i.e.,
fewer rejects) as well as significant cost savings versus the outer layer of
multi-layer balls
produced by other known hard-soft ionomer blends as a result of the lower
overall raw
materials costs and improved yields.
Test data collected by the inventor indicates that lotek 7520 resins have
Shore D
hardnesses of about 32 to 36 (per'ASTM D-2240), melt flow indexes of 3t0.5
g/10 min (at
190 C. per ASTM D-1288), and a flexural modulus of about 2500 - 3500 psi (per
ASTM
0-790). Furthermore, -testing by an independent testing laboratory by
pyrolysis mass
spectrometry indicates that lotek 7520 resins are generally zinc salts of a
terpolymer of
ethylene, acrylic acid, and methyl acrylate.
Furthermore, the inventor has found that a grade of an acrylic acid based soft
ionomer available from the Exxon Corporation under the designation lotek 7510
is also
effective when combined with the hard-ionomers indicated above in producing,
golf ball
covers exhibifing higher C.O.R. values at equal or softer hardness than those
produced
by known hard-soft ionomer blends. In this regard, lotek 7510 has the
advantages (i.e.
improved flow, higher C.O.R. values at equal hardness, increased darity, etc.)
produced
by the lotek 7520 resin when compared to the methacryiic acid base soft
lonomers known
in the art (such as the Suriyr-M 8625 and the Suriyn 8629 combinations
disclosed in U.S.
Patent No. 4,884,814).
In addition, lotek 7510, when compared to lotek 7520, produces slightly higher
C.O.R. values at equal softness/hardness due to the lotek 7510's higher
hardness and
neutralization. Simiiariy, lotek 7510 produces better release properties (from
the moid
cavities) due to its slightly higher stiffness and lower flow rate than lotek
7520. This is
28
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
important in production where the soft covered balls tend to have lower yields
caused by
sticking in the molds and subsequent punched pin marks from the knockouts.
According to Exxon, lotek 7510 is of similar chemical composition as lotek
7520
(i.e. a zinc salt of a terpolymer of ethylene, acrylic acid, and methyl
acrylate) but is more
highly neutralized. Based upon FTIR analysis, lotek 7520 is estimated to be
about 30 -
40 wt.-% neutralized and lotek 7510 is estimated to be about 40 - 60 wt.-%
neutralized.
The typical properties of lotek 7510 in comparison of those of lotek 7520 in
comparison
of those of lotek 7520 are set forth below:
TABLE 7
Physical Properties of lotek 7510 in Comparison to lotek 7520
Property Unit IOTEK 7520 IOTEK 7510
Melt Index g/10 min. 2.0 0.8
Density g/cc 0.96 0.97
Melting Point F 151 149
Vcat Softening Point F 108 109
Flex Modulus psi 3800 5300
Tensile Strength psi 1450 1750
Elongation % 760 690
Hardness, Shore D -- .32 35
The hard ionomer resins utilized to produce the outer cover layer composition
hard/soft blends include ionic copolymers which are the sodium, zinc,
magnesium, lithium,
etc. salts of the reaction product of an olefin having from 2 to 8 carbon
atoms and an
unsaturated monocarboxylic acid having from 3 to 8 carbon atoms. The
carboxylic acid
groups of the copolymer may be totally or partially (i.e. approximately 15 -
75 percent)
neutralized.
29
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
The hard ionomeric resins are likely copolymers of ethylene and acrylic and/or
methacrylic acid, with copolymers of ethylene and acrylic acid being the most
preferred.
Two or more types of hard ionomeric resins may be blended into the outer cover
layer
compositions in order to produce the desired properties of the resulting golf
balls.
As discussed earlier herein, the hard ionomeric resins introduced under the
designation Escor and sold under the designation "lotek" are somewhat similar
to the
hard ionomeric resins sold under the Surlyn trademark. However, since the
"lotek"
ionomeric resins are sodium or zinc salts of poly(ethylene-acrylic acid) and
the Surlyn
resins are zinc or sodium salts of poly(ethylene-methacrylic acid) some
distinct differences
in properties exist. As more specifically indicated in the data set forth
below, the hard
'9otek" resins (i.e., the acrylic acid based hard ionomer resins) are the more
preferred hard
resins for use in formulating the layer blends for use in the present
invention. In addition,
various blends of "lotek" and Surlyn hard ionomeric resins, as well as other
available
ionomeric resins, may be utilized in the present invention in a similar
manner.
Examples of commercially available hard ionomeric resins which may be used in
the present invention in formulating the outer cover blends include the hard
sodium ionic
copolymer sold under the trademark Surlyn 8940 and the hard zinc ionic
copolymer sold
under the trademark Surlyn 9910. Surlyn 8940 is a copolymer of ethylene
with
methacrylic acid and about 15 weight percent acid which is about 29 percent
neutralized
with sodium ions. This resin has an average melt flow index of about 2.8.
Surlyn 9910
is a copolymer of ethylene and methacrylic acid with about 15 weight percent
acid which
is about 58 percent neutralized with zinc ions. The average melt flow index of
Surlyn
9910 is about 0.7. The typical properties of Suriyn 9910 and 8940, as well as
other
Surlyn resins, are set forth below in Tables 8 and 9:
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE 8
Typical Properties of Commercially Available Hard Surlyn Resins
Suitable for Use in the Present Invention
ASTM D 8940 9910 8920 8528 9970 9730
Cation type Sodum Z'inc Sodium Sodium Zinc Zinc
tL{ett flow index, pmaJ10 min. D-1238 2.8 0.7 0.9 1.3 14.0 1.6
SPecific 9r+itY. OY-' D-792 0.95 0.97 o.95 0.94 0.95 0.95
Hardness.Sfare 0 D-2240 65 64 66 60- 62 63
Tensite strenQth. (ipm) D-638 (4.8) (3.6) (5.4) (41) (3.2) (4.1)
mlj'+ 33.1 24.8 37.2 29.0 22.1 28.3
Elanpation. % 0.638 470 290 350 450 460 460
Fbxural Modulus. (Ipsl) 0-790 (51) (48) (~) (32) (28) (30)
mPa 350 330 380 220 190 210
Tensile Impaot (23=C), K.YmO D-1822S 1020 1020 865 1160 760 1240
(R.-Ibstn~ (485) (485) (410) (550) m (590)
Vicat Temperature, =C D-1525 63 62 58 73 61 73
31
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Table 9
Properties of Additional Hard Surlyrg Resins
SURLYNO SURLYN SURLYN
IONOMER 8920 8140 9120
Cation, Na Na Zn
Melt Fiow Index gms/10 min. 0.9 2.6 1.3
MP C 84 88 85
FP C 52 49 50
Tensile Strength kpsi 5.4 5.0 3.8
Yield Strength kpsi 2.2 2.8 2.4
Elongation % 350 340 280
Flex Modulus kpsi 55 71 64
Shore D Hardness 66 70 69
Examples of the more pertinent acrylic acid based hard ionomer resin suitable
for
use in the cover compositions sold under the "lotek" tradename by the Exxon
Corporation
include lotek, but are not limited to, 8000, 8010, 8020, 8030, 7030, 7010,
7020, EX 1001 -
1009, lotek 959 and lotek 960, as well as the materials listed above on Tables
2 and 3.
The typical properties of the remainder of these and other lotek ionomers
suited for use
in formulating the cover compositions are set forth below in Tables 10 and 11:
32
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE 10
Typical Properties of lotek lonomers
ASTM
Resln Properties Method Unfts 7010 7020 7030 8000 8020 8030
Cation ~ anc pnc zinc sodium sodium sodium
Metl Index D-1238 9/10min 0.8 1S 2S 0.8 1.6 2.8
Density D-1505 kp/m' 968 966 964 957 956 956
Metdnp Point 0J417 C 83.5 84 85 83 84 87
Crystsll'ugtion Point D-3117 C 55 56 58 45 47 49
Vkat Softening Point D-1525 C 60 60 60 54 64.5 55.5
Tensile strength at break D-638 MPa 24.5 23.5 22.6 33 32.5 32
yiey Wongth 0.638 wo 14 13 12 19 18.5 18
Elunpatlon at break D4538 16 440 450 460 370 380 410
1 % Seoantniodulus D-638 MPa 150 135 125 280 280 280
Shore Hardnesa D D-2240 -- 54 53 52 60 60 60
Flex modulus (3mm) D 790 MPa 190 175 155 320 340 355_jj
33
SUBSTITUTE SHEET (RULE 26)
I 3
CA 02366400 2004-04-02
TABLE 11
Examples of Exxon High Molecular Weight lonorners
PROPERTY Ex1005 Ex1006 Ex1007 Ex1008 Ex1009 7310
Melt Index, g/10 min. 0.7 1.3 1.0 1.4 0.8 1.0
Cation Na Na Zn Zn Na Zn
Melting Point, C 85.3 86 85.8 86 91.3 91
Vicat Softening Point, C 54 57 60.5 60 56 69
Tensile @ Break, MPa 33.9 33.5 24.1 . 23.6 32:4 24
Elongation @ Break, % 403 421 472 427 473 520
Hardness, Shore D 58 58 51 50 56 52
Flexural Modulus, MPa 289 290 152 141 282 150
It has been determined that when hard/soft ionomer blends are used for the
outer
cover layer, good results are achieved when the relative combination is in a
range of about 3- 25 percent hard ionomer and about 75 - 97 percent soft
ionomer.
Moreover, in altemative embodiments, the Inner and/or outer cover layer
formulation may also comprise up to 100 wt % of a non-ionomeric thermoptastic
or
themzoset materiat inGuding a polyester polyurethane such as B.F. Goodrich
Company's
Estane polyester polyurethane X-4517 or a reas;tion-iNection motded material
such as
one or more of the BayflexTM RIM polyurethanes from Bayer. The non-ionomeric
thermoplastic material may be blended with a soft iQnomqr. For example,
polyamides
blend well with soft ionomer. According to B.F. Gooddch, Estane X-4517 has
the
following properties:
34
CA 02366400 2004-04-02
Properties of Estane X-4517
Tensile 1430
100% 815
200% 1024
300% 1193
Elongation 641
Youngs Modulus 1826
Hardness A/D 88139
Dayshore Rebound 59
Solubility in Water (nsoluble
Melt processing temperature >350 F (>177 C)
Specific Gravity (H20=1) 1.1 -1.3
Other soft, relatively low modulus non-ionomeric thermoplastic or thermoset
materials may also be utilized to produce the inner and/or outer cover layers
as long as
the non-ionomeric materials produce the playability and durability
characteristics desired
without adversely affecting the enhanced travel distance characteristic
produced by the
high acid lonomer resin composition. These include, but are not (imited to
thenmoplastic
polyurethanes such as TexinTM thermoplastic polyurethanes from Mobay Chemical
Co. and
the PellethaneTM thermoplastic polyurethanes from Dow Chemical Co.; non-
ionomeric
thermoset polyurethanes including but not limited to those disclosed In U.S.
Patent
5,334,673; cross-tinked metallocene catalyzed polyolefins; lonomer/cubber
blends. such
as fhose In Spalding U.S. Patents 4,986,545; 5,098,105 and 5,187,013; stynene-
butadiene-
styrene block copolymers, inciuding functionalized styrene-butadiene-styrene
block
copolymers, styrene-ethylene-butadime-styrene (SEBS) bloolc copolymers such as
KRATONT"" materials from Shell Chemical Co., including functionalized SEBS
block
copolymers; and, Hytrel polyester elastomers from DuPont and Pebax
polyesteramfdes
from Elf Atochem SA
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Core
The cores of the inventive golf balls typically have a coefficient of
restitution of
about 0.750 or more, more preferably 0.770 or more and a PGA compression of
about 90
or less, and more preferably 70 or less. The core used in the golf ball of the
invention
preferably is a solid and can be wound or liquid filled. The term "solid
cores" as used
herein refers not only to one piece cores but also to those cores having a
separate solid
layer beneath the covers and over the central core. The cores have a weight of
25 - 40
grams and preferably 30 - 40 grams. When the golf ball of the invention has a
solid core,
this core can be compression molded from a slug of uncured or lightly cured
elastomer
composition comprising a high cis content polybutadiene and a metal salt of an
a, P,
ethylenically unsaturated carboxylic acid such as zinc mono- or diacrylate or
methacrylate.
To achieve higher coefficients of restitution and/or to increase hardness in
the core, the
manufacturer may include a small amount of a metal oxide such as zinc oxide.
In addition,
larger amounts of metal oxide than are needed to achieve the desired
coefficient may be
included in order to increase the core weight so that the finished ball more
closely
approaches the U.S.G.A. upper weight limit of 1.620 ounces. Non-limiting
examples of
other materials which may be used in the core composition including compatible
rubbers
or ionomers, and low molecular weight fatty acids such as stearic acid. Free
radical
initiator catalysts such as peroxides are admixed with the core composition so
that on the
application of heat and pressure, a curing or cross-linking reaction takes
place.
As indicated above, a thread wound aore may comprise a liquid, solid, gel or
multi-
piece center. The thread wound core is typically obtained by winding a thread
of natural
or synthetic rubber, or thermoplastic or thermosetting elastomer such as
polyurethane,
polyester, polyamide, etc. on a solid, liquid, gel or gas filled center to
form a thread rubber
layer that is then covered with one or more mantle or cover layers.
Additionally, prior to
applying the cover layers, the thread wound core may be further treated or
coated with an
adhesive layer, protective layer, or any substance that may improve the
integrity of the
wound core during application of the cover layers and ultimately in usage as a
golf ball.
Since the core material is not an integral part of the present invention,
further detailed
36
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
discussion conceming the specific types of core materials which may be
utilized with the
cover compositions of the invention are not specifically set forth herein.
Method of Making Golf Ball
In preparing golf balls in accordance with the present invention, a hard inner
cover
layer is molded (by injection molding or by compression molding) about a core
(preferably
a solid core). A comparatively softer outer layer is molded over the inner
layer.
The solid core for the multi-layer ball is about 1.2 - 1.6 inches in diameter,
although
it may be possible to use cores in the range of about 1.0 - 2.0 inches.
Conventional solid
cores are typically compression or injection molded from a slug or ribbon of
uncured or
lightly cured elastomer composition comprising a high cis content
polybutadiene and a
metal salt of an a, f3, ethylenically unsaturated carboxylic acid such as zinc
mono or
diacrylate or methacrylate. To achieve higher coefficients of restitution in
the core, the
manufacturer may include fillers such as small amounts of a metal oxide such
as zinc
oxide. In addition, larger amounts of metal oxide than those that are needed
to achieve
the desired coefficient are often included in conventional cores in order to
increase the
core weight so that the finished ball more closely approaches the U.S.G.A.
upper weight
limit of 1.620 ounces. Other materials may be used in the core composition
including
compatible rubbers or ionomers, and low molecular weight fatty acids such as
stearic acid.
Free radical initiators such as peroxides are admixed with the core
composition so that on
the application of heat and pressure, a complex curing cross-linking reaction
takes place.
The inner cover layer which is molded over the core is about 0.01 inches to
about
0.10 inches in thickness, preferably about 0.03 - 0.07 inches thick. The inner
ball which
includes the core and inner cover layer preferably has a diameter in the range
of 1.25 to
1.60 inches. The outer cover layer is about 0.01 inches to about 0.10 inches
in thickness.
Together, the core, the inner cover layer and the outer cover layer combine to
form a ball
having a diameter of 1.680 inches or more, the minimum diameter permitted by
the rules
of the United States Golf Association and weighing no more than 1.62 ounces.
37
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2004-04-02
In a particularly preferred embodiment of the invention, the golf ball has a
dimple
pattem which provides coverage of 65% or more. The golf ball typically is
coated with a
durable, abrasion-resistant, relatively non-yellowing finish coat.
The various cover composition layers of the present invention may be produced
according to conventional melt blending procedures. Generally, the copolymer
resins are
blended in a BanburyTM type mixer, two-roll mill, or extruder prior to
neutralization. After
blending, neutralization then occurs in the melt or molten state in the
Banbury mixer.
Mixing problems are minimal because preferably more than 75 wt %, and more
preferably
at least 80 wt % of the ionic copolymers in the mixture contain acrytate
esters, and in this
respect, most of the polymer chains in the mixture are similar to each other.
The blended
composition is then formed into slabs, pellets, etc.; and maintained in such-
a state until
molding is desired. Altemat'rvely, a simple dry blend of the peltet~zed or
granulated resins
which have previously been neutralized to a desired extent and colored
masterbatch may
be prepared and fed directly into the injed.ion molding machine where
homogenization
occurs in the mixing section of the barrel prior to injection into the mold.
If necessary,
further additives such as an inorganic filler, etc., may be added and
uniformly mixed befor.e
initiation of the molding process. A similar process is utillzed to formulate
the high acid
ionomer resin compositions used to produce the inner cover layer. In one
embodiment of
the irrrention, a masterbatch of non-acrylate ester-containing ionomer with
pigments and
other additives Incorporated ttierein is mixed with the aaytate ester-
contairiing eopolymers
in a ratio of about 1- 7 weight % masterbatch and 93 - 99 weight % acxylate
ester-
containing copolymer.
The gotf balls of the present irmntion can be produaed by molding processes
which
indude but are not limited to those which are cxtrrently well known in the
gotf ball art. For
example, the gotf balls can be produced by Injection molding or comptession
molding the
novel cover axnpositions around a wound or solid molded core to produce an
inner ball
which typically has a diameter of about 1.50 to 1.67 Inches. The outer layer
is
subsequentty molded over the iruter layer to produce a golf ball having a
diameter of 1.620
inches or more, preferably about 1.680 inches or more. Aithough either solid
cores or
38
CA 02366400 2001-09-21
WO 00/57963
PCT/US99/06748
wound cores can be used in the present invention, as a result of their lower
cost and
superior performance, solid molded cores are preferred over wound cores. The
standards
for both the minimum diameter and maximum weight of the balls are established
by the
United States Golf Association (U.S.G.A.).
In compression molding, the inner cover composition is formed via injection at
about
380 F to about 450 F into smooth surfaced hemispherical shells which are then
positioned
around the core in a mold having the desired inner cover thickness and
subjected to
compression molding at 200 to 300 F for about 2 to 10 minutes, followed by
cooling at
50 to 70 F for about 2 to 7 minutes to fuse the shells together to form a
unitary
intermediate ball. In addition, the intermediate balls may be produced by
injection molding
wherein the inner cover layer is injected directly around the core placed at
the center of
an intermediate ball mold for a period of time in a mold temperature of from
50 -to about
100 F. Subsequently, the outer cover layer is molded about the core and the
inner layer
by similar compression or injection molding techniques to form a dimpled golf
ball of a
diameter of 1.680 inches or more.
After molding, the golf balls produced may undergo various further processing
steps
such as buffing, painting and marking as disclosed in U.S. Patent No.
4,911,451.
The resulting golf ball produced from the hard inner layer and the relatively
softer,
low flexural modulus outer layer provide for an improved multi-layer golf ball
which
provides for desirable coefficient of restitution and durability properties
while at the same
time offering the feel and spin characteristics associated with soft balata
and balata-like
covers of the prior art.
Unique Spin Characteristics
As indicated above, the golf ball of the invention is unique in that it
provides good
distance when hit with a driver, good control off of irons, and excellent spin
on short chip
shots. This golf ball is superior to conventional soft covered two-piece or
wound balls in
that it has lower spin off of a driver and higher spin on short shots.
39
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2004-04-02
The spin factor of the baQ of the invention may be specified in the manner
described
below.
Step 1. A golf bali testing machine is set up in order that it meets the
following
conditions for hitting a 1995 Top-FliteT"' TourTM' Z-balata 90 ball produced
by Spalding &
Evenflo Companies.
Club Launch Angle Ball Speed Spin Rate
9 iron 21 t 1.5 160.5 t 9.0 9925 t 600
The machine is set up such that the above conditions are met for each test
using 10 Z-
balata 90 golf balls which are hit 3 times each at the same machine setting.
The thirty
measurements of spin rate are averaged to obtain Ngzs.
Step 2. Ten goff balls of the invention (Ball X) are hit 3 times each using
the same
machine setting as was used for the Z-balata balls and spin data is collected.
Any clearly
erratic spin test result is eliminated and replaced by a new test with the
same ball. The
thirty measurements of spin rate are averaged to obtain Nw-,L
Step 3. The machine is set up in order that it meets the following conditions
for
hitting a 1995 Z-balata 90 ball, the condifions being intended to replicate a
30 yard chip
shot
Club Launch Angie Ball Speed Spin Rate
Sand Wedge 28 t 4.5 58.0 t 4.0 4930 t 770
The machine is set up such that the above conditions are met for each test
using 10 Z
balata 90 golf balls which are hit 3 times each at the same machine setting.
The thirty
measurements of spin rate are averaged to obtain Nz,,,,n. -
Step 4. The 10 gotf balls used in Step 2 are hit ttuee times each using the
same
madNne setting as was used in Step 3 and spin data Is collected Any dearly
erratic spin
test result is eliminated and replaced by a new test wtth the same ball. The
thirty
measurements of spin rate are averaged to obtain N.
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Step 5. The numerical values of N91z8, N91_x, Nsw_ze and Nsw_x are inserted
into the
following formula to obtain a spin factor.
Nsw-x Nswze
Spin factor = - x 100
Nsi-x Nsi-ze
The golf ball of the invention has a spin factor of 3.0 or more, more
preferably 5.0
or more, and most preferably 8.0 or more.
Definitions
Coefficient Of Restitution
As is apparent from the above discussions, two principal properties involved
in golf
ball performance are resilience and PGA compression. The resilience or
coefficient of
restitution (COR) of a golf ball is the constant "e," which is the ratio of
the relative velocity
of an elastic sphere after direct impact to that before impact. As a result,
the COR ("e")
can vary from 0 to 1, with I being equivalent to a perfectly or completely
elastic collision
and 0 being equivalent to a perfectly or completely inelastic collision.
COR, along with additional factors such as club head speed, club head mass,
ball
weight, ball size and density, spin rate, angle of trajectory and surface
configuration (i.e.,
dimple pattem and area of dimple coverage) as weli as environmental conditions
(e.g.
temperature, moisture, atmospheric pressure, wind, etc.) generally determine
the distance
a ball will travel when hit. Along this line, the distance a golf ball will
travel under
controlled environmental conditions is a function of the speed and mass of the
club. and
size, density and resilience (COR) of the ball and other factors. The initial
velocity of the
club, the mass of the club and the angle of the ball's departure are
essentially provided by
the golfer upon striking. Since club head, club head mass, the angle of
trajectory and
environmental conditions are not determinants controllable by golf ball
producers and the
ball size and weight are set by the U.S.G.A., these are not factors of concem
among golf
41
SUBSTITUTE SHEET (RULE 26)
CA 02366400y2004-04-02
ball manufacturers. The factors or determinants of interest with respect to
improved
distance are generally the coefficient of restitution (COR) and the surface
configuration
(dimple pattem, ratio of land area to dimple area, etc.) of the ball.
The COR of solid core balls is a function of the composition of the core and
of the
cover. The core and/or cover may be comprised of one or more layers such as in
multi-
layered balls. In balls containing a wound core (i.e., balls comprising a
liquid or solid
center, elastic windings, and a cover), the coefficient of restitution is a
function of not only
the composition of the center and cover, but also the composition and tension
of the
elastomeric windings. As in the solid core balls, the center and cover of a
wound core ball
may also consist of one or more layers. The COR of the golf balls of the
present invention
is a function of the composition and physical properfies of the core and cover
layer
materials such as flex modulus, hardness and particularly, their resilience,
i_e. ability to
quickly recover from a high impact defortnatiori.
The coefficient of restitution is the ratio of the* outgoing velocity to the
incoming
velocity. In the examples of this application, the coefficient of restitution
of a golf ball was
measured by propelling a ball horizontally at a speed of 125t 5 feet per
second (fps) and
convded to 125 fps against a generally vertical, hard, flat steel plate and
measuring the
ball's incoming and outgoing velocity electronically. Speeds were measured
with a pair
of Oehler Mark 55T " ballistic screens available from Oehler Research, Inc.,
P.O. Box 9135,
Austin, Texas 78766, which provide a timing pulse when an object passes
ftuough them.
The screens were separated by 36" and are located 2525" and 61.25" from the
rebound
wall. The ball speed was measured by timing the pulses from screen I to screen
2 on the
way into the rebound wall (as the average speed of the ball over 36"), and
then the exit
speed was timed from saeen 2 to screen I over the same distanoe. The rebound
watl was
tilted 2 degrees from a vertical plane to allow the ball to rebound slightly
downward In
order to miss the edge of the cannon that fired it., The rebound wall Is solid
steel 2.0
Inches thick.
Ap indicated above, tlw inooming speed should be 125 t5 fps but emected to 125
fps. The correlation between COR and forward or incoming speed has been
studied and
42
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
a correction has been made over the 5 fps range so that the COR is reported
as if the ba{I
had an incoming speed of exactly 125.0 fps.
The coefficient of restitution must be carefully controlled in all commercial
golf batls
if the ball is to be within the specifications regulated by the United States
Golf Association
(U.S.G.A). As mentioned to some degree above, the U.S.G.A. standards indicate
that a
"regulation" ball cannot have an initial velocity exceeding 255 feet per
second in an
atmosphere of 75 F. when tested on a U.S.G.A. machine. Since the coefficient
of
restitution of a ball is related to the ball's initial velocity, it is highly
desirable to produce
a ball having sufficiently high coefficient of restitution to closely approach
the U.S.G.A. limit
on initial velocity, while having an ample degree of softness (i.e.,_
hardness) to produce
enhanced playability (i.e., spin, etc.).
PGA Compression
PGA compression is another important property involved in the performance of a
golf ball. The compression of the ball can affect the playability of the ball
on striking and
the sound or "click" produced. Similarly, compression can affect the "feeP' of
the ball (i.e.,
hard or soft responsive feel), particularly in chipping and putting.
Moreover, while compression itself has little bearing on the distance
performance
of a ball, compression can affect the playability of the ball on striking. The
degree of
compression of a ball against the club face and the softness of the cover
strongly
influences the resultant spin rate. Typically, a softer cover will produce a
higher spin rate
than a harder cover. Additionally, a harder core will produce a higher spin
rate than a
softer core. This is because at impact a hard core serves to compress the
cover of the ball
against the face of the club to a much greater degree than a soft core thereby
resulting in
more "grab" of the ball on the clubface and subsequent higher spin rates. in
effect the
cover is squeezed between the relatively incompressible core and clubhead.
When a
softer core is used, the cover is under much less compressive stress than when
a harder
core is used and therefore does not contact the clubface as intimately. This
results in
lower spin rates. The term "compression" utilized in the golf ball trade
generally defines
the overall deflection that a golf ball undergoes when subjected to a
compressive load.
43
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
For example, PGA compression indicates the amount of change in golf ball's
shape upon
striking.
In the past, PGA compression related to a scale of from 0 to 200 given to a
golf ball.
The lower the PGA compression value, the softer the feel of the ball upon
striking. In
practice, tournament quality balls have compression ratings around 70 - 110,
preferably
around 80 to 100.
In determining PGA compression using the 0 - 200 scale, a standard force is
applied to the external surface of the ball. A ball which exhibits no
deflection (0.0 inches
in deflection) is rated 200 and a ball which deflects 2/10th of an inch (0.2
inches) is rated
0. Every change of .001 of an inch in deflection represents a 1 point drop in
compression.
Consequently, a ball which deflects 0.1 inches (100 x .001 inches) has a PGA
compression value of 100 (i.e., 200 -100) and a ball which deflects 0.110
inches (110 x
.001 inches) has a PGA compression of 90 (i.e., 200 -110).
In order to assist in the determination of compression, several devices have
been
employed by the industry. For example, PGA compression is determined by an
apparatus
fashioned in the form of a small press with an upper and lower anvil. The
upper anvil is
at rest against a 200-pound die spring, and the lower anvil is movable through
0.300
inches by means of a crank mechanism. In its open position the gap between the
anvils
is 1.780 inches allowing a clearance of 0.100 inches for insertion of the
ball. As the lower
anvil is raised by the crank, it compresses the ball against the upper anvil,
such
compression occurring during the last 0:200 inches of stroke of the lower
anvil, the ball
then loading the upper anvil which in tum loads the spring. The equilibrium
point of the
upper anvil is measured by a dial micrometer if the anvil is deflected by the
ball more than
0.100 inches (less deflection is simply regarded as zero compression) and the
reading on
the micrometer dial is referred to as the compression of the ball. In
practice, toumament
quality balls have compression ratings around 80 to 100 which means that the
upper anvil
was deflected a total of 0.120 to 0.100 inches.
An example to determine PGA compression can be shown by utilizing a golf ball
compression tester produced by Atti Engineering Corporation of Newark, N.J.
The value
44
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2004-04-02
obtained by this tester relates to an arbitrary value expressed by a number
which may
range from 0 to 100, although a value of 200 can be measured as indicated by
two
revolutions of the dial indicator on the apparatus. The value obtained defines
the
deflection that a golf balt undergoes when subjected to compressive loading.
The Atti test
apparatus consists of a lower movable platform and an upper movable spring-
loaded anvil.
The dial indicator is mounted such that it measures the upward movement of the
springloaded anvil. The golf ball to be tested is placed in the lower
platform, which is then
raised a fixed distance. The upper porti:on of the golf ball comes in contact
with and exerts
a pressure on the springloaded anvil. Depending upon the distance of the golf
ball to be
compressed, the upper anvil is forced upward against the spring.
Attetnative devices have also been employed to determine compression. For
example, Applicant also utilizes a modified RiehleTM Compression Machine
originally
produced by Riehle Bros. Testing Machine Company, Phil., PA to evaluate
compression
of the various components (i.e., cores, mantle cover balls, finished balls,
etc.) of the golf
balls. The Riehle compression device determines deformation in thousandths of
an inch
under a fixed initialized load of 200 pounds. Using such a device, a Riehie
compression
of 61 corresponds to a deflection under load of 0.061 inches.
Additionally, an approximate relationship between Riehle compression and PGA
compression exists for balls of the same size. It has been detecmined by
Applicant that
Riehle compression corresponds to PGA compression by the general formula PGA
compression = 160 - Riehle compression. Consequentiy, 80 Riehie compression
corresponds to 80 PGA compression. 70 Riehle compression cortesponds to 90 PGA
compression, and 60 Riehle compression corresponds to 100 PGA compression. For
reporting purposes, ApplicanYs compression values are usually measured as
Riehie
compression and converted to PGA compression.
Furthermore, additionai compression devices may also be utilized to monitor
golf
ball compression so tong as the correlation to PGA oompress.ton Is know. These
devices
have been designed, such as a Whitney Tester, to correlate or ooarespond to
PGA
compression through g set relationship or formula.
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Shore D Hardness
As used herein, "Shore D hardness" of a cover is measured generally in
accordance
with ASTM D-2240, except the measurements are made on the curved surface of a
molded
cover, rather than on a plaque. Furthermore, the Shore D hardness of the cover
is
measured while the cover remains over the core. When a hardness measurement is
made
on a dimpled cover, Shore D hardness is measured at a land area of the dimpled
cover.
Fillers
In a particularly preferred form of the invention, at least one layer of the
golf ball
contains at least 0.01 parts by weight of a filler. Fillers preferably are
used to adjust the
density, flex modulus, mold release, and/or melt flow index of a layer. More
preferably, at
least when the filler is for adjustment of density or flex modulus of a layer,
it is present in
an amount of at least five parts by weight based upon 100 parts by weight of
the layer
composition. With some fillers, up to about 200 parts by weight probably can
be used.
A density adjusting filler according to the invention preferably is a filler
which has
a specific gravity which is at least 0.05 and more preferably at least 0.1
higher or lower
than the specific gravity of the layer composition. Particularly preferred
density adjusting
fillers have specific gravities which are higher than the specific gravity of
the resin
composition by 0.2 or more, even more preferably by 2.0 or more.
A flex modulus adjusting filler according to the invention is a filler which,
when used
in an amount of e.g. 1 - 100 parts by weight based upon 100 parts by weight of
resin
composition, will raise or lower the flex modulus (ASTM D-790) of the resin
composition
by at least 1% and preferably at least 5% as compared to the flex modulus of
the resin
composition without the inclusion of the flex modulus adjusting filler.
A mold release adjusting filler is a filler which allows for the easier
removal of a part
from a mold, and eliminates or reduces the need for extemal release agents
which
otherwise could be applied to the mold. A mold release adjusting filler
typically is used in
an amount of up to about 2 wt% based upon the total weight of the layer.
A melt flow index adjusting filler is a filler which increases or decreases
the melt
flow, or ease of processing of the composition.
46
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
The layers may contain coupling agents that increase adhesion of materials
within
a particular layer e.g. to couple a filler to a resin composition, or between
adjacent layers.
Non-limiting examples of coupling agents include titanates, zirconates and
silanes.
Coupling agents typically are used in amounts of 0.1 - 2 wt /a based upon the
total weight
of the composition in which the coupling agent is included.
A density adjusting filler is used to control the moment of inertia, and thus
the initial
spin rate of the ball and spin decay. The addition in one or more layers, and
particularly
in the outer cover layer of a filler with a lower specific gravity than the
resin composition
results in a decrease in moment of inertia and a higher initial spin rate than
would result
if no filler were used. The addition in one or more of the cover layers, and
particularly in
the outer cover layer of a filler with a higher specific gravity than the
resin composition,
results in an increase in moment of inertia and a lower initial spin rate.
High specific
gravity fillers are preferred as less volume is used to achieve the desired
inner cover total
weight. Nonreinforcing fillers are also preferred as they have minimal effect
on COR.
Preferably, the filler does not chemically react with the resin composition to
a substantial
degree, although some reaction may occur when, for example, zinc oxide is used
in a shell
layer which contains some ionomer.
The density-increasing fillers for use in the invention preferably have a
specific
gravity in the range of 1.0 - 20. The density-reducing fillers for use in the
invention
preferably have a specific gravity of 0.06 - 1.4, and more preferably 0.06 -
0.90. The flex
modulus increasing fillers have a reinforcing or stiffening effect due to
their morphology,
their interaction with the resin, or their inherent physical properties. The
flex modulus
reducing fillers have an opposite effect due to their relatively flexible
properties compared
to the matrix resin. The melt flow index increasing fillers have a flow
enhancing effect due
to their relatively high melt flow versus the matrix. The melt flow index
decreasing fillers
have an opposite effect due to their relatively low melt flow index versus the
matrix.
Fillers which may be employed in layers other than the outer cover layer may
be or
are typically in a finely divided form, for example, in a size generally less
than about 20
mesh, preferably less than about 100 mesh U.S. standard size, except for
fibers and flock,
47
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
which are generally elongated. Flock and fiber sizes should be small enough to
facilitate
processing. Filler partide size will depend upon desired effect, cost, ease of
addition, and
dusting considerations. The filler preferably is selected from the group
consisting of
precipitated hydrated silica, day, talc, asbestos, glass fibers, aramid
fibers, mica, calcium
metasilicate, barium suffate, zinc sulfide, lithopone, silicates, silicon
carbide, diatomaceous
earth, polyvinyl chloride, carbonates, metals, metal alloys, tungsten carbide,
metal oxides,
metal stearates, particulate carbonaceous materials, micro balloons, and
combinations
thereof. Non-limiting examples of suitable fillers, their densities, and their
preferred uses
are as follows:
48
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Filier Table
Filler Type Spec. Grav. Comments
Precipitated hydrated silica 2.0 1,2
Clay 2.62 1,2
Talc 2.85 1,2
Asbestos 2.5 1,2
Glass fibers 2.55 1,2
Aramid fibers (KEVLAR ) 1.44 1,2
Mica 2.8 1,2
Calcium metasilicate 2.9 1,2
Barium sulfate 4.6 1,2
Zinc sulfide 4.1 1,2
Lithopone 4.2 - 4.3 1,2
Silicates 2.1 1,2
Silicon carbide platelets 3.18 1,2
Silicon carbide whiskers 3.2 112
Tungsten carbide 15.6 1
Diatomaceous earth 2.3 1,2
Polyvinyl chloride. 1.41 1,2
Carbonates
Calcium carbonate 2.71 1,2
Magnesium carbonate 2.20 1,2
Metals and Alloys (powdersl
Titanium 4.51 1
Tungsten 19.35 1
Aluminum 2.70 1
49
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Bismuth 9.78 1
Nickel 8.90 1
Molybdenum 10.2 1
Iron 7.86 1
Steel 7.8 - 7.9 1
Lead 11.4 1,2
Copper 8.94 1
Brass 8.2 - 8.4 1
Boron 2.34 1
Boron carbide whiskers 2.52 1,2
Bronze 8.70 - 8.74 1
Cobalt 8.92 1
Beryllium 1.84 1
Zinc 7.14 1
Tin 7.31 1
Metal Oxides
Zinc oxide 5.57 1,2
Iron oxide 5.1 1,2
Aluminum oxide 4.0
Titanium oxide 3.9 - 4.1 1,2
Magnesium oxide 3.3 - 3.5 1,2
Zirconium oxide 5.73 1,2
Metal Stearates
Zinc stearate 1.09 3,4
Calcium stearate 1.03 3,4
Barium stearate 1.23 3,4
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
F Lithium stearate 1.01 3,4
Magnesium stearate 1.03 3,4
Particulate carbonaceous materials
Graphite 1.5-1.8 1,2
Carbon black 1.8 1,2
Natural bitumen 1.2 -1.4 1,2
Cotton flock 1.3 - 1.4 1,2
Cellulose flock 1.15-1.5 1,2
Leather fiber 1.2-1.4 1,2
Micro balloons
Glass 0.15 -1.1 1,2
Ceramic 0.2 - 0.7 1,2
Fly ash 0.6 - 0.8 1,2
Coupling Agents Adhesion Promoters
Titanates 0.95-1.17
Zirconates 0.92-1.11
Silane 0.95 -1.2
COMMENTS:
1 Pardculariy useful for adjusting density of the cover layer.
2 Particularly useful for adjus6ng flex modulus of the cover layer.
3 Particulariy useful for adjusting mold release of the cover layer.
4 Particularty useful for increasing melt flow index of the cover layer.
AII fillers except for metal stearates would be expected to reduce the melt
flow index
of the cover layer.
The amount of filler employed is primarily a function of weight requirements
and
distribution.
51
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
The present invention is further illustrated by the following examples in
which the
parts of the specific ingredients are by weight. It is to be understood that
the present
invention is not limited to the examples, and various changes and
modifications may be
made in the invention without departing from the spirit and scope thereof.
Example 1: Ionic Terpolymer-Containing Cover
A set of two-piece golf balls was made with solid cores and a cover
composition
of 75 weight % NUCREL 035, which is an acrylate ester-containing acid
terpolymer,
and 25 weight % of a masterbatch containing 4.5 weight % MgO in Surlyn 1605
("MgO Masterbatch"). The terpolymer was reacted with the masterbatch at a
temperature of about 250 F under high shear conditions at a pressure of about
0 to
100 psi. The magnesium in the masterbatch neutralized acid groups of the
terpolymer
at a level of about 62% neutralization. The molded balls were finished with
polyurethane primer and top coats. The PGA compression, coefficient of
restitution,
Shore C hardness, scuff resistance, spin rate and cold crack of the golf balls
were
determined. The results are shown on Table 12 below.
To measure cold crack, the finished golf balls were stored at -10 F for at
least
24 hours and were then subjected to 5 blows in a coefficient machine at 165
ft/sec.
The balls were allowed to return to room temperature and were then visually
inspected
for cover cracking. None of the golf balls experienced cracking.
Coefficient of restitution (C.O.R.) was measured by firing the resulting golf
ball in
an air cannon at a velocity of 125 feet per second against a steel plate which
was
positioned 12 feet from the muzzle of the cannon. The rebound velocity was
then
measured. The rebound velocity was divided by the forward velocity to give the
coefficient of restitution. Shore hardness was determined in general
accordance with
ASTM Test 2240, but was measured on a non-dimpled area of the surface of the
golf
ball.
52
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2006-11-21
Comparative Example 1: Ionic Copolymer Cover (Non-Terpolymer)
A set of 12 two-piece golf balls was made according to the same
procedure as that of Example 1 with the exception that NUCRELT"' 925, a non-
acrylate ester-containing acid copolymer was substituted for NUCREL 035. The
resulting golf ball cover was too hard. resulting in four breaks during cold
crack
testing. The results are shown on Table 12.
Comparative Example 2: lonomer - Non-Ionic Terpolymer Blend
The procedure of Example 1 was repeated with the exception that the
MgO Masterbatch was replaced by pure Surlyn 1605. All of the golf ball
covers broke during cold crack testing. The results are shown on Table 12.
Comparative Example 3: lonomer - Non-Ionic Copolymer Blend
The procedure of Comparative Example 1 was repeated with the
exception that the MgO Masterbatch was replaced by pure Surlyn 1605. The
results are shown on Table 12. When subjected to cold crack testing, all of
the
golf ball covers broke.
As can be seen from the results of Example 1 and Comparative Examples
1 - 3, inferior golf balls are obtained when a hard, non-acrylate ester-
containing
copolymer is used instead of a softer, acrylate ester-containing terpolymer,
and
when either an acrylate ester-containing acid terpolymer or a non-acrylate
ester-
containing acid copolymer is not neutralized with metal ions.
53
CA 02366400 2001-09-21
WO 00/57963
PCT/US99/06748
TABLE 12
Experiment Cover Weight PGA COR Shore C Cold
No. Material Comp. (x1000) Hardness Crack
1-1 75% Nucrel 0351 45.2 104 .783 80 No breaks
25% MgO MB in
Surlyn 1605
Comp. 1 75% Nucrel 925/ 45.1 111 .798 90 4 breaks
25% MgO MB in
Surlyn 1605
Comp. 2 75% Nucrel 0351 45.1 99 .774 70 All broke
25% Surlyn 1605
Comp. 3 75% Nucrel 925/ 45.2 106 .790 75 All broke
25% Surlyn 1605
Example 2: Ionic Terpolymers
An acrylate ester-containing terpolymer sold as ESCOR ATX 325 (Exxon
Chemical Co.) was 57% neutralized with lithium cations. The ionomeric
material,
which also contained titanium dioxide, brightener, etc. from a white
masterbatch, was
placed over a solid golf ball core and the golf ball was primed and top
coated. The
properties of the resulting goif ball are shown on Table 13. This procedure
was
repeated using different combinations of terpolymers with cations and cation
blends at
the degrees of neutralization which are shown on Table 11. In the cation
blends, mole
ratios were about 1:1:1. All of the ATX materials shown on Table 13 are ESCOR
ATX
materials available from Exxon Chemical Co. The Nucrel materials are available
from
DuPont Chemical Co. Primacor 3440 is available from Dow Chemical Co.
54
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2006-11-21
The spin rate of the golf ball was measured by striking the
resulting golf balls with a pitching wedge or 9-iron wherein the club-head
speed
is about 80 feet per second and the ball was launched at an angle of 26 to 34
degrees with an initial velocity of 100 - 1 15 feet per second. The spin rate
was
measured by observing the rotation of the ball in flight using stop action
Strobe
photography or via the use of a high speed video system.
The scuff resistance test was conducted in the following manner: a Top-
Flite tour pitching wedge (1994) with box grooves was obtained and was
mounted in a MiyamaeTM driving machine. The club face was oriented for a
square hit. The forward/backward tee position was adjusted so that the tee was
four inches behind the point in the downswing where the club was vertical. The
height of the tee and the toe-heel position of the club relative to the tee
were
adjusted in order that the center of the impact mark was about 3/4 of an inch
above the sole and was centered toe to heel across the face. The machine was
operated at a club head speed of 125 feet per second. A minimum of three
samples of each ball were tested. Each ball was hit three times.
After testing, the balls were rated according to the following chart:
Rating Type of damage
1 Little or no damage (groove markings or dents)
2 Small cuts and/or ripples in cover
3 Moderate amount of material lifted from ball surface
but still attached to ball
4 Material removed or barely attached
The balls that were tested were primed and top coated.
As shown on Table 13, many of the cover materials resulted in golf balls
having a scuff resistance of 1.5 or less, and others had a scuff resistance
rating
of 1.5 - 2.5.
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Comparative Example 4: Hard/Soft lonomer Blend
A golf ball with a cover formed from a blend of a commercially available hard
sodium ionomer and a commercially available soft acrylate ester-containing
zinc
ionomer in which the blend contains less than 60 wt % soft ionomer was
subjected to
the same testing as the golf balls of Example 2. The results are shown on
Table 13.
56
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE 13
Experiment Cover Cation y, PGA COR Shore D Scuff Spin Rate
No. Material Neutratization Comp. (x1000)Hardnes Resist- (#9 Iron at
s 105 ft/sec)
Comp.4 hard-soft Zn/Na 60% 90 787 68 4.0 9,859
ionomer
blend I
(control)
2-1 ATX 325 Li 570/0 86 787 61 1.0 10,430
2-2 ATX 325 LUZn/K 65% 86 787 50 1.0 10,464
2-3 ATX 320 Li 57% N.T. N.T. 56 1.0 10,299
2-4 ATX 320 LilZn/K 65% 87 790 55 1.5 10,355
2-5 Nucre1010 Li - 89 803 65 3.0 7,644
2-6 Nucrel 010 LifZn/K - 89 802 65 4.0 7,710
2-7 Nucre1035 Li - 87 801 62 3.0 8,931
2-8 Nucre1035 L'UZnlK - 87 798 62 3.0 8,915
2-9 ATX 310 Li 53% 88 802 62 2.5 8,892
2-10 ATX 310 L1JZn/K 60% 88 801 63 2.5 8,244
2-11 ATX 325 Li 570/, 83 797 65 1.5 -
2-12 ATX 325 Li/Zn/K 65 /a 82 796 63 1.5 -
2-13 50% ATX (L.i1 28.5b/o 89 777 60 1.6 -
325-Li
50% ATX
320-unneuL
2-14 76%A1X320 (LVZn/K) 49% 87 776 64 1.6
-
-L.i/Zn/K
25%ATX320
-unneut.
2-16 60%ATX325 (LvZn/K) 39% 88 779 64 1.5 -
-LiIZJt/K
40 /,Primacor
3440-unneut.
2-16 ATX 320 UnneuL - 88 776 45 2.0 -
2-17 ATX 325 Unneut - 98 - 42 1.5 -
2-18 ATX 325 u 60% 95 795 60 1.0 -
2-19 ATX 325 Lj 30% 96 791 46 1.6 -
2-20 ATX 325 LVZn/K 60% 91 791 48 1.0 -
2-21 ATX 325 LVZn/K 30% 90 N.T. 45 1.0 -
2-22 ATX 325 LLrZrdK 50% 91 N.T. 47 1.0 -
57
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Example 3: Ionic Terpolymers
The procedure of Example 2 was repeated with the exception that single cations
of lithium, magnesium, sodium and potassium were used in the cover material.
The
results are shown on Table 14.
As indicated on Table 14, the scuff resistance of the golf balls was 3.0 or
better.
The scuff resistance of the balls with covers made of an acrylic acid
terpolymer was
1Ø For a given terpolymer, the scuff resistance did not change when
different cations
were used for neutralization.
TABLE 14
Experiment Cover Catlon 9'e PGA COR Shore D Scuff
No. Material Neutralization Comp. (x1000) Hardness Resistance
3-1 Nucre1035 U 100 90 792 62 3.0
3-2 Nucre1035 Mg 100 89 792 62 3.0
3-3 ATX 325 U 100 86 790 51 1.0
3-4 ATX 325 Mg 100 85 791 51 1.0
3-5 ATX 325 Na 81 85 790 51 1.0
3-6 ATX 325 K 95 85 791 51 1.0
Comparative Example 5:
Several intermediate balls (cores plus inner cover layers) were prepared in
accordance with conventional molding procedures described above. The inner
cover
compositions were molded around 1.545 inch diameter cores weighing 36.5 grams
with
a specific gravity of about 1.17 such that the inner cover had a wall
thickness of about
58
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2004-04-02
0.0675 inches and a specific gravity of about 0.95, with the overall ball
measuring
about 1.680 inches in diameter.
The cores utilized in the examples were comprised of the following
ingredients:
100 parts by weight high cis-polybutadiene, 31 parts by weight zinc
diacrylate, about 6
parts by weight zinc oxide, 20 parts by weight zinc stearate, 17 - 18 parts by
weight
calcium carbonate, and small quantities of peroxide, coloring agent and a
polymeric
isocyanate sold as PapiTM 94 (Dow Chemical Co.). The molded cores exhibited
PGA
compressions of about 100 and C.O.R. values of about.800.
The inner cover compositions designated herein as compositions A - E utilized
to formulate the intermediate balls are set forth in Table 15 below. The
resulting
molded intermediate balls were tested to determine the individual compression
(Riehie), C.O.R., Shore C hardness, spin rate and cut resistance properties.
These
results are also set forth in Table 15 below.
The data of these examples are the average of twelve intermediate balls
produced for each example. The properties were measured according to the
following
parameters:
Cut resistance was measured in accordance with the following procedure: A golf
ball was fired at 135 feet per second against the leading edge of a pitching
wedge
wherein the leading edge radius is 1f32 inch, the loft angle is 51 degrees,
the sole
radius is 2.5 inches and the bounce angle is 7 degrees.
The cut resistance of the balis tested herein was evaluated on a scale of I to
5.
The number I represents a cut that extends completely tfirough the cover to
the core.
A 2 represents a cut that does not extend completely through the cover but
that does
break the surface. A 3 does not break the surface of the cover but does leave
a
permanent dent. A 4 leaves only a slight cxease which Is permanent but not as
severe
as 3. A 5 represents virtually no visible indentation or damage of any sort
The spin rate of the golf ball was measured by striking the resulting golf
balls
with a pitctftng wedge or 9 Iron wherein the club head speed Is about 105 feet
per
second and the ball is launched at an angle of 26 to 34 degrees with an
initial velocity
59
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
of about 110 to 115 feet per second. The spin rate was measured by observing
the
rotation of the ball in flight using stop action Strobe photography.
Initial velocity is the velocity of a ball when struck at a hammer speed of
143.8
feet per second in accordance with a test as prescribed by the U.S.G.A.
As will be noted, compositions A, B and C include high acid ionomeric resins,
with composition B further including zinc stearate. Composition D represents
the inner
layer (i.e. Surlyn(D 1605) used in U.S. Patent No. 4,431,193. Composition E
provides a
hard, low acid ionomeric resin.
The purpose behind producing and testing the balls*of Table 15 was to provide
a
subsequent comparison in properties with the multi-layer golf balls of the
present
invention.
Table 15
Molded Intermediate Golf Balls
Ingredients of
Inner Cover Compositions A B C D E
lotek 959 50 50 - - -
lotek 960 50 50 - - -
Zinc Stearate - 50 - - -
Surlyn 8162 - - 75 - -
Suriyn 8422 - - 25 - -
Surlyn 1605 - - - 100 --
lotek 7030 - - - - 50
lotek 8000 - - - - 50
Properties of Molded
Intermediate Balls
Compression 58 58 60 63 62
C.O.R. .811 .810 .807 .793 .801
Shore C Hardness 98 98 97 96 96
Spin Rate (R.P.M.) 7,367 6,250 7,903 8,337 71956
CutResistance 4-5 4-5 4-5 4-5 4-5
As shown in Table 15 above, the high acid ionomer resin inner cover layer
(molded intermediate balls A - C) have lower spin rates and exhibit
substantially higher
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
resiliency characteristics than the low acid ionomer resin based inner cover
layers of
balls D and E.
Example 4
Multi-layer balls in accordance with the present invention were then prepared.
Specifically, the inner cover compositions used to produce intermediate golf
balls from
Table 15 were molded over the solid cores to a thickness of about 0.0375
inches, thus
forming the inner layer. The diameter of the solid core with the inner layer
measured
about 1.620 inches. Altematively, the intermediate golf balls of Table 15 were
ground
down using a centeriess grinding machine to a size of 1.620 inches in diameter
to
produce an inner cover layer of 0.0375 inches.
The size of 1.620 inches was determined after attempting to mold the outer
cover layer to various sizes (1.600", 1.610", 1.620", 1.630" and 1.640") of
intermediate
(core plus inner layer) balls. It was determined that 1.620" was about the
largest
"intermediate" ball (i.e., core plus inner layer) which could be easily molded
over with
the soft outer layer materials of choice. The goal herein was to use as thin
an outer
layer as necessary to achieve the desired playability characteristics while
minimizing
the cost of the more expensive outer materials. However, with a larger
diameter final
golf ball and/or if the cover is compression molded, a thinner cover becomes
feasible.
With the above in mind, an outer cover layer composition was blended together
in accordance with conventional blending techniques. The outer layer
composition
used for this portion of the example is a relatively soft cover composition
such as those
listed in U.S. Patent No. 5,120,791. An example of such a soft cover
composition is a
45% soft/55% hard low acid ionomer blend designated by the inventor as'TE-90".
The
composition of TE-90 is set forth as follows:
61
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2004-04-02
Outer Cover Layer Composition TE-90
lotek 8000 22.7 weight %
lotek 7030 22.7 weight %
lotek 7520 45.0 weight %
White MB' 9.6 weight %
'White MB consists of about 23.77 weight percent TiO.; 0.22 weight percent
UvitexT"' QB, 0.03 weight percent Santonoxn" R, 0.05
weight percent Ultramarine blue and 75.85 weight percent lotek 7030.
The above outer layer composition was molded around each of the 1.620
diameter intermediate balls comprising a core plus one of compositions A - D,
respectively. In addition, for comparison purposes, Surlyr* 1855 (new Surlyn
9020),
the cover composition of the'193 patent, was molded about the inner layer of
composition D (the intermediate ball representative of the'193 patent). The
outer layer
TE-90 was molded to a thickness of approximately 1.680 inches in diameter. The
resulting balls (a dozen for each example) were tested and the various
properties
thereof are set forth in Table 16 as follows:
TABLE 16
Finished.Balls
Inaredt-=ncs: _!_ ~ ~- ~ -~-
tlwn=r Cewe co"lclen A i c
p,icer Cavac Co.pestttan TE-44 i~-90 iE-40 ZE-44 fwlYM 4020
~irop~cttes eE
Retded Ft fshed lsitst
Ctoapresslan 63 63 69 T0 d1
C.ti.>l. .~at .77a .750 .770 .7ST
fhora C Ysrdness s3 !d da 94 99
spin R.!?S i.854 4,414 8.990 l,8L6
Cvt seststanes 3-4 3-~ 7-4 3-4 1-2
62
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
As it will be noted in finished balls 1 - 4, by creating a multi-layer cover
utilizing
the high acid ionomer resins in the inner cover layer and the hard/soft low
acid ionomer
resin in the outer cover layer, higher compression and increased spin rates
are noted
over the single layer covers of Table 12. In addition, both the C.O.R. and the
Shore C
hardness are reduced over the respective single layer covers of Table 12. This
was
once again particularly true with respect to the multi-layered balls
containing the high
acid ionomer resin in the inner layer (i.e. finished balls 1- 5). In addition,
with the
exception of prior art ball 5 (i.e. the'193 patent), resistance to cutting
remains good but
is slightly decreased.
Furthermore, it is also noted that the use of the high acid ionomer resins as
the
inner cover material produces a substantial increase in the finished balls
overall
distance properties. In this regard, the high acid ionomer resin inner covers
of balls 1-
3 produce an increase of approximately 10 points in C.O.R. over the low acid
ionomer
resin inner covers of balls 4 and about a 25 point increase over the prior art
balls 5.
Since an increase in 3 to 6 points in C.O.R. results in an average increase of
about I
yard in distance, such an improvement is deemed to be significant.
Several other outer layer formulations were prepared and tested by molding
them around the core and inner cover layer combination to form balls each
having a
diameter of about 1.68 inches. First, B.F. Goodrich Estane X-4517 polyester
polyurethane was molded about the core molded with inner layer cover
formulation A.
DuPont Surlyn 9020 was molded about the core which was already molded with
inner
layer D. Similar properties tests were conducted on these golf balls and the
results are
set forth in Table 17 below:
63
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE 17
Finished Balls
Ingredients: 6 7
Inner Cover Layer
Composition A D
Outer Cover Layer
Composition Estane 4517 Surlyn 9020
Properties of
Molded Finished Balls:
Compression 67 61
C.O.R. .774 .757
Shore C Hardness 74 89
Spin (R.P.M.) 10,061 8,846
Cut Resistance 3-4 1-2
64
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
The ball comprising inner layer formulation D and Surlyn 9020 identifies the
ball in the Nesbitt 4,431,193 patent. As is noted, the example provides for
relatively
high softness and spin rate though it suffers from poor cut resistance and low
C.O.R.
This ball is unacceptable by today's standards.
As for the Estane X-4517 polyester polyurethane, a significant increase in
spin
rate over the TE-90 cover is noted along with an increase in spin rate over
the TE-90
cover is noted along with an increased compression. However, the C.O.R. and
Shore
C values are reduced, while the cut resistance remains the same. Furthermore,
both
the Estane X-4517 polyester polyurethane and the Surlyn 9020 were relatively
difficult to mold in such thin sections.
Example 5
In order to analyze the change in characteristics produced by multi-layer golf
balls (standard size) having inner cover layers comprised of ionomer resin
blends of
different acid levels, a series of experiments was run. A number of tests were
performed, varying the type of core, inner cover layer and outer cover layer.
The
results are shown below on Table 18:
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE 18
fNNER COMP/ MffER COHP SHORE
Semole CORE LAYER THICKHESS COR roVER THICXNESS Rhiele COR 0 SPIN
8 1042 YELLOW HONE SEc BELCM TOP GRADE 0.055" 61 .800 68 T331
9 1042 YELLOW NONE SEE BELON 959/960 0.055" 56 .808 73 6516
SPECIAL 1.47" 959/960 0.050" 65/.805 959/960 0.055" 48 .830 73 6258
11 1042 YELLOW NCME - SEE BELCM SO 90 0.055" 62 .792 63 8421
12 SPECIAL 1.47" TOP GRADE 0.050" 66/.799 50 90 0.055" 55 .511 63 8265
13 SPECIAL 1.47" 959/960 0.050" 65/.805 mW 0.055" 53 .813 63 8254
14 SPECIAL 1.47" TOP GRAOE 0.050" 66/.799 TOP GRADE 0.055" 51 .819 68 7390
1042 YELLOW NC+NE _ SEE BELOU 2'BAl-ATA 0.055" 67 .782 55 9479
16 SPECIAL 1.47" 9591960 0.050" 65/.805 Z-BALATA 0.055" 61 .800 55 9026
17 SPECIAL 1.47" TOP GRAOE 0.050" 66/.799 Z"BALATA 0.055" 60 .798 55 9262
1042 YELLCM>C'OHP~72, COR=.780
SPECIAL 1.47" CORE>COt4P-67, CDR=.782
In this regard, 'Top Grade" or'TG" is a low acid inner cover ionomer resin
blend
comprising of.70.6% lotek 8000, 19.9% lotek 7010 and 9.6% white masterbatch.
"959/960" is a 50/50 wtJwt blend of lotek 959/960. In this regard, EscorO or
lotek 959
is a sodium ion neutralized ethylene-acrylic neutralized ethylene-acrylic acid
copolymer. According to Exxon, loteks 959 and 960 contain from about 19.0 to
about
21.0% by weight acrylic acid with approximately 30 to about 70 percent of the
acid
groups neutralized with sodium and zinc ions, respectively.
66
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Furthermore, the low acid ionomer formulation for "SD 90" and "Z-Balata" are
set
forth below:
SD Cover ZB Cover
17.2% Surlyn 8320 19% lotek 8000
7.5% Surlyn 8120 19% lotek 7030
49% Surlyn 9910 52.5% lotek 7520
16.4% Surlyn 8940 9.5% white MB
9.7% white MB
The data clearly indicates that higher C.O.R. and hence increase travel
distance
can be obtained by using multi-layered covered balls versus balls covered with
single
layers. However, some sacrifices in compression and spin are also noted.
Further, as
shown in comparing Example Nos. 12 vs. 13, Example Nos. 17 vs. 16, etc. use of
lower
acid level inner cover layers and relatively soft outer cover layers (i.e., 50
wt.% or more
soft ionomer) produces softer compression and higher spin rates than the golf
balls
comprised of high acid inner cover layers. Consequently, use of blends of.low
acid
ionomer resins to produce the inner layer of a multi-layer covered golf ball,
produces
not only enhanced travel distance but also enhanced compression and spin
properties.
Example 6
Multi-layer oversized golf balls were produced utilizing different ionoriier
resin
blends as the inner cover layer (i.e., core plus inner cover layer is defined
as "mantel").
The "ball data" of the oversized multi-layer golf balls in comparison with
production
samples of 'Top-Flite XL" and Top-Flite Z-Bafata" is set forth below.
67
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Top-Flltea Top-Fliteo
T8 19 20 Z-Belete 90
Core oata
Size 1.43 1.43 1.43 1.545 1.545
COR .787 .787 .787 , -
Mantel Data
Material TG TG TG - _,
Size .161 1.61 1.61 ~ -
Thickness .090 .090 .090 ~ -
Hantel Data
cont'd
Sliore 0 68 68 68
Compression 57 57 57
COR .815 .815 .815
Bail Data
Cover TG Z8 50 TG zB
Size 1.725 1.723 1.726 1.681 1.683
ueiglit 45.2 45.1 45.2 45.3 45.5
Shore 0 1 68 56 63 68 56
Compression 45 55 49 53 77
COR .820 .800 .810 .809 .797
Spin 7230 9268 8397 7133 9287
The results indicate that use of multi-layer covers enhances C.O.R. and travel
distance. Further, the data shows that use of a blend of low acid ionomer
resins (i.e.,
"Top Grade") to form the inner cover layer in combination with a soft outer
cover ("ZB"
or "SD") produces enhanced spin and compression characteristics. The overall
combination results in a relatively optimal golf ball with respect to
characteristics of
travel distance, spin and durability.
Example 7
Golf balls 7 - 1, 7- 2, 7 - 3 and 7- 4 having the formulations shown on Table
19
were formed.
68
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Table 19
Chemical Component 7-1 7-2 7-3 7-4
Core Data
Size 1.4T' 1.47" 1.47" 1.47"
Weight 32.7g 32.7g 32.7g 32.7g
PGA Compression 70 60 70 60
COR 780 770 780 770
Composition
High cis polybutadiene 100 100 100 100
Zinc obde 30.5 31.6 30.5 31.6
Core regrind 16 16 16 16
Zinc Stearate 16 16 16 16
Zinc Diacryiate 22 20 22 20
Initiator 0.9 0.9 0.9 0.9
Inner Cover Layer
Size 1.57" 1.57" 1.57" 1.57"
Weight 38.4g 38.4g 38.4g 38.4g
PGA Compression 83 75 83 75
COR 801 795 801 795
Thickness 0.050" 0.050" 0.050" 0.050"
Hardness (Shore C1D) 97/70 97/70 97l70 97l70
Composition
lotek 1002 50% 50% 50% 50%
lotek 1003 50% 50% 50% 50%
69
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2004-04-02
Chemical Component 7-1 7-2 7-3 7-4
Outer Cover Layer
Hardness (Shore C1D) 71146 71146 71146 71146
Thickness 0.055" 0.055" 0.055' 0.055"
Composkion
lotek 7510 92.8% 928 ,G 42%. 42%
lotek 7520 42% 42%
lotek 7030 7.2% 7.2% 7.3% 7.3%
latek 8000 8.7% 8.7%
Wtrtener Package
UnitaneT"' 0-110 23 phr 2.3 phr 2.3 phr 2.3 pM
Eastobrite TM OB1 0.025 ptir 0.025 phr 0.025 ptir 0.025 phr
Utira Marine Bhx 0.042 phr 0.042 ptir 0.042 phr 0.042 phr
Santanox R 0.004 ptu 0.004 phr 0.004 ptu 0.004 ptir
Final Bati Data
Size 1.68" 1.68" 1.68' 1.68"
Weight 45.4g 45.4g 45.4g 45.40
PGA Compre.ssion 85 78 85 78
COR 783 785 793 785
The balls of Example 7- 2 were tested by a number of professional quality
gotfers using a driver, 5-iron, 9-iron,_ and sand wedge or pitching wedge.
Each player
used his own clubs and hit both the ball of Example 7- 2 and a control ball,
Which Was'
the 1995 two-piece Top-Fiite Tour Z-balata 90. The Z-balata 90 has a 1.545"
core of
about 36.8g with a PGA compression of about 80 and a COR of about.794. The
cover
of the Z-balata 90 is about 0.068 in. thidc, and is a blend of lotek 8000 and
lotek 7510
with or without masterbatch containing lotek 7030. The cover has a Shore D
hardness
of about 55. The ball has a PGA compression of about 79 and a COR of about
0.788.
Each player hit six of the balls of Example 7- 2 and six Z-balata control
balls one time
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
each. For each shot, measurements were made of the initial launch conditions
of the
golf ball, including launch angle and ball speed. Furthermore, spin rates at
initial
launch, carry distance, and total distance were measured. The players hit full
shots
with the driver (1 W), 5-iron (51) and 9-iron (91). With the sand wedge or
pitching wedge
(SW), the players hit about 30 yard chip shots. Data points were removed if
determined to be "wild points." A point was said to be wild if it fell outside
2 standard
deviations of the 6-hit average. Initial launch conditions were determined
using a
highly accurate high speed stop action video photography system. The results
are
shown on Table 21.
As shown on Table 21, multi-layer ball 7 - 2 was longer than the Z-balata
control
when hit with a 5-iron but only slightly longer than the Z-balata ball using a
driver and
9-iron. The multi-layer ball 7 - 2 and the two-piece control were generally
the same in
overall distance using a driver. In each case, the multi-layer ball 7 - 2 had
a higher
spin rate off the 30-yard chip shot than the Z-balata. The spin rate of the
ball of
Example 7 - 2 was an average of 11.6% higher than the spin rate of the Z-
balata
control in the 30 yard chip shot.
71
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE 20
2-Piece Control 7-2
Player Club LA B.S. Spin Cerry Total LA B S. Spn Carty Total
(da9) (fps) (rpm) (Yds) (yds) (deg.) (tps) (rPrt+) (Yds) (yds)
1 1W 10.4 262.2 3537 272.5 288.9 10.0 262.3 3247 271.6 292.2
2 1 W 9.5 240.1 3124 238.1 253.6 119 238.3 2935 236.3 257.4
3 1 W 8.6 258.8 3695 254.1 259.9 6.3 251.2 3357 247.6 260.8
4 1 W 10.9 2526 2639 271.6 289.8 125 251.4 3066 279.0 296.7
1W 9.5 211.7 3627 237.2 255.2 9.4 208.7 3415 235.0 259.8
6 1W 10.2 2420 3105 363.8 283.2 11.0 243.9 2903 267.6 288.4
7 1 W 11.5 214.9 3089 265,4 279.0 11.6 212.6 3165 262.9 274.4
8 1W 9.7 239.5 3129 263.6 288.8 9.3 235.3 2884 257.2 276.8
9 1 W 11.7 211.2 2939 231.4 255.8 11.3 208.5 2032 2222 244.3
1W 10.2 244.0 2797 243.3 250.2 9.7 239.6 3072 236.8 251.1
11 1W 247.4 263.8 13.8 215.8 3916 245.4 266.8
AVE 10.2 237.7 3168 253.5 269.8 10.3 233.4 3090 251.1 270.1
1 51 12.4 207.3 5942 198.3 209.8 11.8 206.3 5507 196.2 207.8
2 51 178.3 184.2 14.9 199.4 5094 1822 187.8
3 51 10.9 196.8 6462 185.2 188.9 11.5 197.0 6009 187.4 193.4
4 51 14.4 205.5 6683 207.8 213.7 14.7 208.3 6601 207.5 217.8
5 51 13.6 163.3 6734 1829 189.4 14.2 180.9 6380 184.2 190.7
6 51 124 204.5 5771 201.0 210.5 12.9 208.4 5414 208.0 218.3
7 51 14.1 184.3 6013 194.8 198.1 13.1 1827 6000 1929 200.0
8 51 128 187.2 6149 188.0 200.3 13.1 191.6 6183 191.7 2020
9 51 13.2 176.5 6000 168.2 173.7 13.6 172.5 6166 169.7 174.3
10 51 13.9 199.9 7214 175.2 178.2 14.9 199.1 6237 169.0 170.2
11 51 14.2 179.5 6669 181.9 187.8 15.7 181.2 5338 184.0 190.7
AVE 13.2 1925 6364 187.4 194.1 13.7 193.4 5903 188.4 195.7
72
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Player CluD LA B.S. Spln Carry Total L.A. B.S Spn C.a.rry Total
(deg) (fps) (rpm) (yds) (Yds) (deg) (fps) (rWn) (yds) (Yds)
1 91 20.0 168.1 9865 152.5 159.5 20.4 172.2 9210 153.4 159.6
2 91 21.8 165.9 9770 1327 137.0 23.0 164.7 8949 132.7 134.6
3 91 19.9 154.3 10764 128.8 134.3 19.9 156.5 10161 129.8 135.0
4 91 22.7 166.4 10551 146.0 148.8 23.9 165.7 9990 150.3 154.2
91 22.1 147.4 9682 137.1 138.1 222 148.5 9324 139.3 141.7
6 91 19.4 169.7 8939 153.3 158.0 19.7 168.2 8588 156.2 163.5
7 91 20.4 151.1 9899 147.5 150.0 21.6 150.3 9084 148.6 151.3
8 91 18.5 143.0 9408 142.0 147.5 18.3 141.8 9038 141.2 144.8
9 91 20.0 134.5 9124 124.9 128.8 20.1 132.9 8834 125.0 128.9
91 23.2 156.1 10603 1227 124.1 23.2 155.6 11017 116.2 116.3
11 91 21.5 149.4 9729 131.0 134.5 23.4 151.7 6666 133.3 136.8
AVE 20.9 155.1 9649 138.0 141.9 21.4 155.3 9353 138.7 142.4
1 SW 29.2 56.4 5647 24.8 58.9 6679
2 SW 26.6 57.4 5446 25.2 57.8 5647
3 SW 25.8 64.1 4925 24.3 63.5 5550
4 SW 30.9 60.9 5837 31.1 57.9 6158
5 SW 20.3 56.7 4152 19.0 56.3 4288
6 SW 34.3 57.1 3798 324 61.5 4700
7 b1W 30.5 51.5 4712 29.3 52.3 5374
AVE 28.2 67.7 4931 26.6 58.3 6485
73
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2004-04-02
Example 8
The ball of Example 7 - 2 was compared to a number of competitive products in
distance testing using a driving machine in which the bail was struck with a
club. The
results are shown on Table 19 below. The distance test shows that Example 7- 2
is
about the same distance as the Z-balata 90 control and longer than the
TitleistT"' HP-2 Tour
(soft covered two-piece) and Titleist Tour Balata 100 ball (Balata covered
wound ball). The
other balls that were tested include the MaxfliT"~ (Dunlop) XS100, Maxfli
(Dunlop) XF100, and
the GIGAT"' Top-Flite goif ball sold by Spalding in Japan. In Table 19, the
ball of Example
7-2 is the longest ball.
TABLE 21
pistance Regort
Test Number. 131951 Club Head Speed: 157.35
Club Name: TFT 10.5 DEG MW (DRIVER) No. BallslType: 10
Average Test Conditions:
I.aunch Angles (Deg.): 9.6
Ball Speed (fps): 217.8
Spin Rate (rpm): 3390
Turf Condition: FiRM
Wind (mph/dir): ' 2.55 135.20
TenipJRH (degN/o): 0.61 91.59
P-Bar (mbar): 1015
Ba11 Tvae r~' PTime ~gy Car Diff Ctr Dev Roll T Dist T Diff
HP2TOUR 8.7 6.0 230.4 -4.1 3.0 9.9 240.3 -4.3
ZB90 9.0 6.1 231.8 -2.7 5.4 9.1 241.0 -3.6
GIGA 8.8 6.0 234.5 0.0 5.7 10.2 244.6 0.0
Example 7-2 8.3 5.9 229.6 -4.9 3.8 11.1 240.7 -3.9
T'itleist Tour
Balat.a 100 9.2 6.2 229.2 -5.3 7.8 7.8 236.9 -7.7
74
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Distance Report
Test Number: 0203963 Club Head Speed: 126.18
Club Name: TFT 5 IRON No Balls/Type: 12
Average Test Conditions:
Launch Angles (Deg.): 14
Ball Speed (fps): 180.1
Spin Rate (rpm): 5424
Turf Condition: FIRM
Wind (mph/dir): 6.23 171.38
Temp./RH (degN/o): 62.20 98.16
P-Bar (mbar): 1015
Ball Type Traj PTime Carry Car Diff Ctr Dev Roll T Dist T Diff
HP2TOUR 25.3 6.0 156.0 -7.4 -3.0 1.5 157.5 - 9.5
ZB90 25.2 6.0 157.1 -6.3 -3.3 2.2 159.3 -7.7
GIGA 25.0 6.0 162.2 -1.2 -3.1 2.9 165.1 -1.9
Example 7-2 23.5 6.0 163.4 0.0 -3.3 3.7 167.0 0.0
Titleist Tour
Balata 100 23.9 6.0 158.7 -4.7 -2.3 2.5 161.2 -5.8
ZB 100 26.1 6.0 155.6 -7.8 -4.5 2.0 157.6 -9.4
XS 100 23.9 6.0 161.3 -2.3 -5.6 2.6 163.9 -3.1
XF 100 24.5 6.0 152.0 -11.4 -6.2 1.6 153.7 -13.3
Example 9
A number of golf ball cores having the following formulation were made:
PARTS
High-cis polybutadiene 100
Zinc oxide 30
Core regrind 16
Zinc stearate 16
Zinc diacrylate 21
Peroxide (231 xl) 0.9
The cores had a diameter of 1.470", a weight of 32.5 g, a PGA compression of
57 and
a COR of 0.768.
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
The cores were divided into four sets and each set was covered with one of the
mantle formulations shown below on Table 22.
TABLE 22
MANTLE FORMULATIONS
Mantle Type A B C D(control)
Surlyn 8940 (g) 656 880 1610 -
Surlyn 9910 (g) 1964 2180 535 -
Surlyn 8120 (g) 300 160 475 -
Surlyn 8320 (g) 700 400 1000 -
lotek 7030 (g) 380 380 380 -
lotek 1002 (g) - - - 2000
lotek 1003 (g) - - - 2000
The mantle covered cores had the following physical properties:
TABLE 23
MANTLE-COVERED CORES
A B C D
Size (Pole) (Inches) 1.577 1.576 1.572 1.573
Weight (g) 38.6 38.5 38.3 38.4
PGA Compression 71 74 70 76
COR .7795 .7831 .7768 .7946
Std. Dev. COR .0051 .0026 .0016 .0012
Shore C 92 94 90 97
Shore D 62 65 61 70
76
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Each set of mantle-covered cores was divided into three subsets and a cover
layer
having one of the cover formulations shown below on Table 24 was formed over
the
mantled cores. The "whitener package" on Table 24 has the same formulation as
that
shown above on Table 19.
TABLE 24
COVER FORMULATIONS
CoverType x Y Z
lotek 7520 (g) 1660 1480 1300
lotek 7510 (g) 1660 1480 1300
lotek 8000 (g) 304 664 1024
lotek 7030 (g) 282 282 282
Whitener package (g) 94 94 94
The balls had the mantle and cover combinations and properties shown below
on Table 25.
77
SUBSTITUTE SHEET (RULE 26)
TABLE 25
BALL PROPERTIES Example No. 9-1 9-2 9-3 9-4 9-5 9-6 9-7 9-8 9-9 9-10 9-11 9-12
Mantle A A A B B B C C C D D D
Cover X Y Z X Y Z X Y Z X Y z
Size (inch) 1.6820 1.6810 1.6820 1.6820 1.6820 1.6820 1.6820 1.6820 1.6810
1.6820 1.6820 1.6810
Weight (g) 45.68 45.57 45.58 45.77 45.62 45.58 45.63 45.58 45.48 45.67 45.65
45.57
PGA Comp. 73.5 74.3 74.7 77.4 76.7 76.3 70.8 71.9 73.3 79.5 80 82.5 W
,1
H o,
COR .7639 .7665 .7680 .7701 .7703 .7704 .7607 .7630 .7661 .7771 .7798 .7839 0
0
Std. Dev. COR .0041 .0027 .0037 .0077 .0034 .0023 .0037 .0030 .0028 .0034
.0028 .0020 0
Shore C 71 76 81 71 76 81 70 76 80 71 76 81 ~
o
Shore D 46 50 53 46 50 53 46 49 52 47 51 53
ro
00
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Ball 9-10 was the control.
The results from Table 25 demonstrate that a multi-layer ball having a mantle
hardness of 60D or greater (Ex. 9-7, 9-8, 9-9), and preferably 63D (Ex. 9-1, 9-
2, 9-3) or
greater give a ball having a COR of at least 0.761 (Ex. 9-7) and while a
harder mantle
(Ex. 9-4, 9-5, 9-6, 9-10, 9-11, 9-12) will generaly give higher COR, the
mantle also
contributes to a harder PGA compression. Versus the control ball (Ex. 9-10) it
is
demonstrated that softer compressions can be obtained with slightly softer
mantles
while maintaining a good COR. Likewise versus the control, higher COR balls
may be
designed (Ex. 9-11, 9-12) that still have a relatively soft compression for
good feel.
Example 10
A number of golf balls were made having the core and cover formulations and
the physical properties shown on Tables 26 and 27. The balls of Examples 10-1,
10-2
and 10-5 are part of the invention. The balls of Examples 10-3, 10-4 and 10-6
are
controls based upon the cover layer chemistry of comparative Example 2 of U.S.
Patent
No. 5,586,950. The balls of Example 10-4 are replicas of comparative Example 2
of
U.S. Patent No. 5,586,950.
For all of the balls, the cores were molded and centeriess ground to the
appropriate size. The mantles of Examples 10-1 to 10-4 were injection molded
in a
1.63" injection mold. The mantles for the balls of Examples 10-5 and 10-6 also
were
injection molded. All of the outer cover layers were injection molded.
79
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE 26
EX. EX. EX. EX. EX. EX.
Core Data 10-1 10-2 10-3 10-4 10-5 10-6
Core Type (see Table 2) A A A A B B
Core Size (in.) 1.50 1.50 1.50 1.50 1.47 1.47
Mantle Data
Ingredients phr phr phr phr phr phr
lotek 1002 50 --- 50 --- 50 50
lotek 1003 50 -- 50 -- 50 50
Surlyn 9910 --- 50 - 50 --- ---
Suriyn 8940 --- 35 - 35 Surlyn 8920 --- 15 -- 15 -- ---
TiOZ 2 2 2 2 --
Diameter (in.) 1.625 1.625 1.625 1.625 1.57 1.57
Thickness (in.) 0.063 0.063 0.063 0.063 0.050 0.050
Shore C/D Hardness 97/70 96/68 97/70 96/68 97/70 97/70
(measured on ball)
Cover Data
Cover Type (see Table 2) #1 #1 #2 #2 #1 #2
Size (in.) 1.70 1.70 1.70 1.70 1.68 1.68
Thickness (in.) 0.038 0.038 0.038 0.038 0.055 0.055
Shore C/D Hardness 75/49 75/49 84/57 83/57 72/48 83/56
(measured on ball)
Compression (Riehle) 63 66 60 63 83 80
COR 800 795 805 798 779 787
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2004-04-02
TABLE 27
Qore Formulations Cover Forn,ulations
Materials (phr) A B Materials (phr) #1 #2
BR 1220 73 70 lotek 8000 8.5 ---
(High cis polybutadiene)
Taktene 220 27 30 lotek 7510 41 -
(High cis polybutadiene)
Zinc Oxide 22.3 31.5 totek 7520 41 --
TG Regrind 10 16 Masterbatch C 9.5 ---
Zinc Stearate 20 16 Suriyn 1557 - 10
Zinc Diacrylate 26 20 Surlyn 1855 - 20
Masterbatch A 0.15 - Surlyn 8265 - 20
Masterbatch B - 0.15 Surlyn 8269 - 50
LupercoTM 231 XL 0.9 0.9 TiOz - 2
peroxide
81
.,: . ,.... _. _ ., . .. ..__ .._ _....~.._ _,.,_.. .. , , ~_._.. , . , ..
.._....__ ..._ _.... _ _.
CA 02366400 2004-04-02
Example 11
A number of golf balls were made with mantles comprising a blend of
polycarbonate and metallocene catalyzed polyolefin.
Cores having the formulation shown below on Table 30 were obtained and
covered with a mantle layer formed from 83 parts by weight of Lexan TM ML 5776-
7539 (GE)
and 17 parts by weight of a maleic anhydride modified metallocene catalyzed
polyolefin
obtained from Exxon Chemical Co. and designated by Exxon as MDX-96-2r""
According to Exxon, MDX-96-2 contains 1.7 wt k maleic anhydride. This material
was
added as an impact modifier. The mantles were then covered with the same cover
fonnulation as was used in Examptes 7-3 and 7-4. The results are shown below
on
Table 28.
Example 12
A number of golf balls were made using a nylon-ionomer graft copolymer as the
mantle layer. The fomiulations and physical properties of the balls are shown
below on
Table 30.
Example 13
The procedure of Example 11 was repeated with the exception that Lexan
SP131 OR-112 was used. This Lexan material was found to have a plaque Shore D
hardness of 85 when tested in the lab. The plaque was stored at room
temperature for
several weeks before the Shore D hardness measurement was made.
Example 14
The procedure of Example 11 was repeated with the exception of CapronTM 8351,
a nylon-ionomer gmft copolymer available from Allied-Signal, was used In the
mantle
layer in place of the polycarbonate and a different maeic anhydride modified
metallocene catalyzed potyo{efin was used, MDX 95-2. The formuiations and
physical
properties of the balls are shown below on Table 30.
The properties of Capron 8351 are shown below on Table 28.
82
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE 28
CAPRON 8351
Flexurals at 23 C
Modulus, kpsi 235
Strength, kpsi 9.7
Notched Izod
at 23 C, ft-lb/in 22.9
Failure Mode NB
at -40 C, ft-lb/in 4.9
Failure Mode CB
Drop Weight at 23 C
Impact Strength, ft-lbs 148
Failure Mode D
Impact Strength, -40 C
Failure Mode
Tensiles at 23 C
Strength at Break, kpsi 9.4
Strength at Yield, kpsi 7.3
Yield Elongation 3%
Ultimate Elongation 277%
Example 15
The procedure of Example 14 was repeated with the exception that Capron
Ultratough, also known as Capron XA-2571, was used in place of Capron 8351.
The
properties of Capron XA-2571 are shown beiow on Table 29.
83
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE 29
CAPRON XA-2571
Fiexurals at 23 C
Modulus, kpsi 240
Strength, kpsi 9.7
Notched Izod
at 23 C, ft-Ib/in 20
Failure Mode NB
at -40 C, ft-Ib/in 20.0
Failure Mode CB
Drop Weight at 23 C
Impact Strength, ft-lbs 114
Failure Mode D
Impact Strength, -40 C 160
Failure Mode D
Tensiles at 23 C
Strength at Break, kpsi 8.4
Strength at Yield, kpsi 6.9
Yield Elongation 7%
Ultimate Elongation 200%
84
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
TABLE 30
Chemical Component EXAMPLE 11 EXAMPLE 12 EXAMPLE 13 EXAMPLE 14
Core Data
Size 1.47" 1.47" 1.47" 1.47"
Weight 32.7g 32.7g 32.7g 32.7g
PGA Compression 78 78 78 78
COR (x 1000) 764 764 764 764
Composition (pph)
High cis pofybutadiene 100 100 100 100
Zinc o)ade 6 6 6 6
Core regrind 10 10 10 10
Zinc Stearate 20 20 20 20
Zinc Diacryiate 20.5 20.5 20.5 20.5
IniGator 0.9 0.9 0.9 0.9
Inner Cover Layer
Size 1.64" 1.64" 1.64" 1.64"
Weight (g) 39.2g 39.2g 38.7 39.7
PGA Compression 95 97 96 97
COR (x 1000) 636 748 605 771
Thickness .100" .100" .100" .100'
Hardness (Shore C) 95 98 95 95
(Shore D) 80 78 75 70
Composibon (wt ,6)
MDX 96-2 17% 17% - -
MDX 95-2 - - 17% 17%
LN SP131OR -112 - 83% - -
LN ML 5776-7539 83% - - -
CAPRON 8351 - - 83% -
CAPRON XA 2571 - - - 83%
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
Chemical Component EXAMPLE 11 EXAMPLE 12 EXAMPLE 13 EXAMPLE 14
Outer Cover Layer
Hardness (Shore C/D) 71/46 71/46 71/46 71/46
Thickness 0.055" 0.055" 0.055" 0.055"
Composition (wt ,6)
lotek 7510 42% 42% 42% 42%
lotek 7520 42% 42% 42% 42%
lotek 7030 7.3 ,6 7.3 ,6 7.3% 7.3%
lotek 8000 8.7% 8.7% 8.7% 8.7%
Whitener Package
Unitane 0-110 2.3 phr 2.3 phr 2.3 phr 2.3 phr
Eastobrite 0B1 0.025 phr 0.025 phr 0.025 phr 0.025 phr
Uttra Marine Blue 0.042 phr 0.042 phr 0.042 phr. 0.042 phr
Santanox R 0.004 phr 0.004 phr 0.004 phr 0.004 phr
Final Ball Data
Size 1.73" 1.73" 1.73" 1.73"
Weight 46.7g 46.7g 46.3g 46.2g
PGA Compression 93 94 100 95
COR X 1000) 746 719 672 753
86
SUBSTITUTE SHEET (RULE 26)
CA 02366400 2001-09-21
WO 00/57963 PCT/US99/06748
The invention has been described with reference to the preferred embodiment.
Obviously, modifications and alterations will occur to others upon reading and
understanding the proceeding detailed description. It is intended that the
invention be
construed as including all such modifications and alterations insofar as they
come
within the scope of the appended claims or the equivalents thereof.
87
SUBSTITUTE SHEET (RULE 26)