Note: Descriptions are shown in the official language in which they were submitted.
CA 02366767 2001-09-25
WO 00/59379 PCT/US00/08558
-1 -
UNSTENTED HEART VALVE BIOPROSTHESES AND
METHODS OF MAKING THE SAME
CROSS-REFERENCE TO RELATED APPLICATION
This application is related to, and claims domestic priority benefits
under 35 USC ~119(e) from, U.S. Provisional Application Serial No.
60/127,479 filed on April 2, 1999, the entire content of which is expressly
incorporated hereinto by reference.
FIELD OF THE INVENTION
The present invention relates generally to bioprostheses and
methods of making the same. In preferred forms, the present invention is
embodied in unstented heart valve bioprostheses and methods of making
the same.
BACKGROUND AND SUMMARY OF THE INVENTION
Bioprosthetic porcine xenograft valves have been employed in the
~5 past for the successful treatment of human heart valve disease. More
specifically, atrioventricular valve replacements have occurred in the past
using stent mounting and glutaraldehyde fixation of porcine valves. More
recently, stentless porcine xenograft valves, such as the O'Brien-Angell
stentless valve, have been used with considerable success.
2o Notwithstanding the clinical successes of such stentless porcine xenograft
valves, improvements are still desirable.
For example, it would especially be desirable if stentless
bioprosthetic valves were provided with integral inflow and/or outflow
conduits. Such an improvement would thereby allow the bioprosthetic
CA 02366767 2001-09-25
WO 00/59379 PCT/US00/08558
-2-
valve to be used as a root valve to replace the entirety of a patient's
native aortic valve or the pulmonary valve and its outflow tract.
Alternatively, such a valve would be suitable for use as an inclusion-type
valve following appropriate trimming.
Furthermore, it would also be desirable to remove all vestiges of
myocardium so that only the connective tissue portions of the heart valve
remain present. In this manner, a bioprosthetic valve could be provided
which is potentially less immunogenic than other unfixed tissue grafts
since it is capable of being decellularized to leave primarily the
o extracellular matrix of the leaflets, aortic wall and mitral leaflets.
It is towards fulfilling such needs that the present invention is
directed. Broadly, therefore, the present invention is embodied in
stentless heart vaive bioprosthesis comprised of multiple noncoronary
sections sutured together along lengthwise commissure lines. These
~5 sutured noncoronary sections establish a generally tubular bioprosthetic
structure having an inflow conduit, an outflow conduit and a valve section
intermediate to the inflow and outflow sections. Most preferably three
noncoronary leaflet sections are employed to establish a trileaflet valve
section intermediate to the inflow and outflow sections.
2o Importantly, the heart valve bioprosthesis of the present invention
is a composite structure formed of the noncoronary sections of unfixed
heart valve tissue. Preferably, all myocardium is omitted from the valve
components and the finished bioprosthesis in order to produce a structure
with low antigenicity and maximal integrity of suturable tissue.
CA 02366767 2001-09-25
WO 00/59379 PCT/US00/08558
-3-
These and other aspects and advantages of the present invention
will become more clear after careful consideration is given to the following
detailed description of the preferred exemplary embodiments thereof.
BRIEF DESCRIPTION OF THE ACCOMPANYING DRAWINGS
Reference will hereinafter be made to the accompanying drawings,
wherein like reference numerals throughout the various FIGURES denote
like structural elements, and wherein,
FIGURE 1 is a perspective view of a stentless heart valve
bioprosthesis in accordance with the present invention;
o FIGURES 2A-2F represent a presently preferred technique for
fabricating the stentless heart valve bioprosthesis depicted in FIGURE 1
as viewed from the exterior of the tissue segments;
FIGURES 3A and 3B schematically depict a preferred suturing
technique using horizontal mattress sutures for joining adjacent
~5 noncoronary tissue segments during the fabrication of the bioprosthetic
heart valve of this invention;
FIGURE 4A is a photograph of one embodiment of a completed
bioprosthetic heart valve according to this invention using the mattress
sutures exemplified by FIGURES 3A and 3B;
2o FIGURE 4B is a photograph of another embodiment of a
completed bioprosthetic heart valve according to this invention using
conventional interrupted sutures; and
FIGURE 5 depicts a modification of the heart valve bioprosthesis
depicted in FIGURE 1 which is particularly useful for aortic valve repair.
CA 02366767 2001-09-25
WO 00/59379 PCT/US00/08558
-4-
DETAILED DESCRIPTION OF THE INVENTION
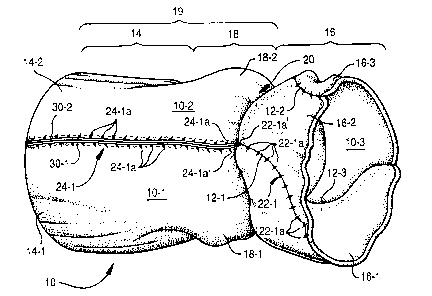
Accompanying FIGURE 1 shows a stentless bioprosthetic heart
valve 10 in accordance with the present invention. As depicted, the heart
valve 10 is fabricated from three noncoronary aortic leaflet sections 10-1,
10-2 and 10-3, most preferably dissected from porcine heart tissue.
Adjacent noncoronary sections 10-1, 10-2 and 10-3 are sutured together
along commissure lines 12-1, 12-2 and 12-3. That is, noncoronary
section 10-1 is sutured to noncoronary sections 10-2 and 10-3 along
commissure lines 12-1 and 12-3, respectively, while noncoronary sections
10-2 and 10-3 are sutured together along commissure line 12-2. The
sutured noncoronary sections 10-1, 10-2 and 10-3 thereby form a
generally tubular structure having an outflow conduit 14, an inflow conduit
16 formed of mitral leaflets 16-1, 16-2 and 16-3, and a sinus section 18,
distally of the annulus 20, intermediate to the inflow and outflow conduits
~5 14, 16, respectively. The arterial conduit 19 is thus comprised of the
outflow conduit 14 and the sinus region 18.
The sinus section 18 interiorly includes three leaflet cusps (not
shown in FIGURE 1, but see the exemplary leaflet cusps 104-1, 104-2
and 104-3 in the bioprosthetic heart valve 100 depicted in FIGURE 5)
2o which collectively form the trileaflet valve in the complete bioprosthetic
heart valve 10. The inflow section 16, on the other hand, is formed of the
individual anterior mitral leaflets associated with each of the noncoronary
sections 10-1, 10-2 and 10-3. The completed trileaflet bioprosthetic heart
valve 10 will thereby approximate a patient's natural, but diseased, heart
25 valve.
To fabricate the heart valve 10, porcine heart tissue is procured
and dissected fresh leaving only the aortic valve, the mitral leaflet and a
CA 02366767 2001-09-25
WO 00/59379 PCT/US00/08558
-5-
lengthwise segment (preferably approximately 3 mm from the leaflet
base) of myocardium. More specifically, the porcine heart tissue is first
cut longitudinally between the left and right coronary arteries and placed
flat on a dissection area with the lumenal surface of the valve tissue
facing upwardly. The myocardium is then cut away so that only about
3mm in length and width remain as well as the aortic arterial conduit,
three leaflet cusps, and the mitral leaflet. The tissue is then cut
longitudinally on either side of the noncoronary leaflet along the
commissures from the free edge of the outflow conduit to approximately
2-3 mm below the base of the noncoronary leaflet. Instead of separating
the left, right and noncoronary leaflet sections by making a straight
incision along the line of the commissure, the cut is most preferably made
slightly broader (e.g., from about 1 to about 1.5 mm wider than the natural
commissure line) near the outflow free edge in order to allow for histology
samples to be taken. The valve conduit is cut along the commissure lines
just prior to assembly. In this regard, care should be taken so that the
conduit is not more narrow than the widest portion of the leaflet.
The tissue is next cut at an angle from left to right (usually an angle
between about 30° to about 50°, and more preferably an angle of
about
20 45°, depending on the natural anatomy of the distal myocardium)
starting
from the end of the first vertical cuts (near the plane of the leaflet base)
to the free end of the mitral leaflet, thus removing most of the myocardium
as well as the right and left coronary leaflets and conduit. This trimming is
performed on either side of the noncoronary section. It should be noted
25 here that the chordae tendineae of the mitral leaflet are not removed until
the valve 10 is assembled (as will be discussed in greater detail below).
However, following assembly of the valve 10, the chordae tendineae are
cut around the circumference of the inflow region 16 about 1.5 mm
CA 02366767 2001-09-25
WO 00/59379 PCT/US00/08558
-6-
proximal to the final interrupted suture along the free edge of the mitral
valve in a manner that is parallel to the presumptive annulus of the
composite.
All myocardium and excess adventitia are removed from the
noncoronary portion that remains. Portions of the annular region 20 are
also bladed so that the tissue has no jagged areas and to assure that the
tissue displays uniform thickness.
The noncoronary tissue segment may then be subjected to a
conventional decellularization process. In this regard, following
o dissection, the tissue may be subjected to one or more of the treatments
by which the tissue may be decellularized, soluble proteins removed,
tissue constituent covalently or ionically modified, chemical or biochemical
substituents added, or tissue crosslinked. Upon completion of these
processes, the tissue is stored in a suitable medium and temperature
~5 (e.g., at about 4°C in an aqueous medium) to stabilize the tissue
and/or
modification until completion of the valve.
As shown in FIGURE 2A, the noncoronary sections 10-1, 10-2 and
10-3 are selected from tissue storage due to their approximate similar
size (e.g., ~ 2 mm) and are measured for purposes of such matching. In
2o this regard, the measurements include the distance and/or dimensions (i)
between commissures, (ii) from the anterior of the leaflet (point of
coaptation) to the posterior (base) of the leaflet, (iii) from the top of the
commissure on either side of the leaflet to the center of the base of the
leaflet, and (iv) from the free edge of the leaflet to the base of the
25 coaptive margin. The noncoronary sections 10-1, 10-2 and 10-3 are then
CA 02366767 2001-09-25
WO 00/59379 PCT/US00/08558
-7-
filled with storage solution (e.g., saline) in order to observe the shape and
extension of the leaflet.
Prior to production of the valve 10, however, the three noncoronary
sections 10-1, 10-2 and 10-3 are subjected to further dissection and
inspection to assure optimal alignment for suturing. Straight cuts are
made along each commissure line of the non-coronary segment. The
cuts should be made as close to the leaflets and commissure tips as
possible without causing any damage. The cuts are performed at a slight
angle toward the right, which should follow the angle at the point of
o coaptation (approximately 10° to 15° to the right of the
vertical
leaflet/commissure line).
Any excess adventitia must be removed. The commissures should
also not have any remaining leaflet tissue from the discarded left or right
coronary sections, and the non-coronary leaflet and commissures should
be inspected for damage after trimming this area. The free edges along
the commissure lines 12-1, 12-2 and 12-3 should also be smooth to avoid
gaps between tissue sections following suture placement. Any variance
in the thickness of the outflow free edges 10-1 a, 10-2a and 10-3a of the
outflow conduit sections 14-1, 14-2 and 14-3 (which ultimately will
2o collectively form the outflow conduit 14 of the valve 10) should be trimmed
carefully to a substantially uniform thickness without leaving any jagged
areas. This trimming should, however, only be done if the variance is less
than or equal to about 1 mm. If the variance is greater than about 1 mm,
the tissues should not be matched for assembly.
Any remaining myocardium is also removed and the annulus 20
bladed to a smooth finish of substantially constant thickness. The
noncoronary sections 10-1, 10-2 and 10-3 are frequently inspected for
CA 02366767 2001-09-25
WO 00/59379 PCT/US00/08558
-$_
any damage, such as tears, holes or cuts that rendered an area too thin,
and are discarded if any such defects are present.
The initial pair of noncoronary sections 10-1 and 10-2 are sutured
together beginning generally at the base of the sinuses 18-1, 18-2 of each
tissue segment as depicted in accompanying FIGURE 2B. In this regard,
the first joining stitch 22-1a' is a basic interrupted suture placed through
the arterial conduit with entry and exit points approximately 1.5 mm from
the free edges of the commissure lines 12-1 on the exterior of the
noncoronary sections using suitable suture material and needle (e.g., 6-0
Prolene monofilament polypropylene sterile suture with 3/8 inch tapered
needle). The depth of the suture 22-1 a' through the tissue thickness
should be about 0.5 mm from the lumenal surface of each conduit,
without penetrating any interior surface of the tissue, particularly the
leaflet. The suture 22-1 a is completed using a triple surgeon's knot and
~5 the free ends of the suture should be cut to less than about 1 mm in
length.
The next interrupted suture 22-1 a in the suture line 22-1 is placed
about 1.5 mm from the initial suture 22-1 a' in the direction of the mitral
leaflets 16-1, 16-2. Four or five of these interrupted sutures 22-1 a in the
2o suture line 22-1 should be completed for the purpose of easing the later
placement of the horizontal mattress sutures (a few of which are identified
in FIGURE 1, for example, by reference numeral 24-1a and collectively
form the suture line 24-1 ) by having a joined section to grasp and anchor
the tissue. The remainder of the interrupted sutures 22-1 a of the suture
25 line 22-1 are placed once the entire line of mattress sutures 24-1 a
forming
suture line 24-1 are completed, as will now be described.
CA 02366767 2001-09-25
WO 00/59379 PCT/US00/08558
-9-
Specifically, as shown in FIGURE 2C, the first horizontal mattress
suture 24-1 a' is positioned approximately 0.5 mm distal to the initial
interrupted suture 22-1 a' (i.e., toward the outflow) using the same Prolene
material and needle as for the interrupted sutures forming the suture line
22-1 discussed previously.
As is perhaps more clearly depicted in FIGURES 3A and 3B, the
needle is inserted approximately 1 mm from the free edge of the conduit
exterior. The depth of the suture is the same as for the interrupted
sutures (that is, less than or equal to about 0.5 mm from the lumenal
surface). The first exit point of the suture is approximately 1 mm from the
free edge of the adjacent tissue's conduit exterior. The suture is pulled
through the tissue, leaving about a 2 cm tail of suture extending out of the
entry point to allow the suture to be tied off. The needle is then inserted
between about 1 mm to about 1.5 mm from the first exit point in a
~5 direction parallel to the commissure line and towards the outflow tissue,
leaving the tissue about 0.5 mm from the lumenal surface and a distance
of between about 1 mm to about 1.5 mm from the first half-loop of the
suture. Next, the suture is placed through the thickness of the opposing
tissue and exits the tissue no more than about 1 mm from the free edge
20 12-1 of the conduit exterior. The final exit point should be 1 to 1.5 mm
from the initial entry point (where the tail of the suture is protruding). The
suture should be tied off using a triple surgeons knot and the free ends
should be trimmed to less than about 1 mm in length. The angle of the
mattress sutures 24-1 a causes a minor eversion (depicted by tissue
25 mounds 30-1 and 30-2 in FIGURE 3B) between the connected tissues.
This eversion creates a seal between the sections that substantially
reduces leakage along the suture line. However, the tissue mounds 30-1,
CA 02366767 2001-09-25
WO 00/59379 PCT/US00/08558
-10-
30-2 should not protrude more than 1 mm outwardly from the external
surface of the conduit to avoid causing an obstructive surface.
The next mattress suture 24-1a in the suture line 24-1 should be
initiated on the opposite tissue from that which had the knot for the initial
suture 24-1 a'. Each mattress suture 24-1 a is begun on the opposite
tissue from the suture 24-1 a before it. This alternating method reduces
the puckering on the lumenal side of the tissue. Each mattress suture
should be positioned approximately 0.5 mm to 1 mm from the external
loop of the previous suture. The alternating mattress sutures in the suture
line 24-1 should be completed from the base of the sinus region to the
free edge of the outflow conduit (see FIGURE 2D). The width of the
eversion mounds 30-1 and 30-2 between tissues for the entire suture line
should be no more than 3 mm for size 19 to 23 mm InOD and no more
than 4 mm for size 25 to 29 mm InOD valves.
~5 Once the mattress suture line 24-1 is complete, the interrupted
sutures forming the suture line 22-1 along the mitral leaflets should be
finished as depicted in FIGURE 2E. In this regard, the interrupted sutures
of suture line 22-1 should extend as far along the mitral leaflets 16-1, 16-2
as possible until the chordae tendineae begin to proliferate. However, the
20 length of the inflow must extend at least 4 mm beyond the base of each
leaflet. One final interrupted suture 22-1 b (see FIGURE 2E) is placed 1 to
1.5 mm from the free edge of the outflow proximal to the final mattress
suture in suture line 24-1. This procedure aids the cylindrical shaping of
the outflow region.
25 The procedures discussed above are repeated so as to join the
noncoronary sections 10-2 and 10-3 along commissure line 12-2 as
depicted in accompanying FIGURE 2F. Thereafter, the noncoronary
CA 02366767 2001-09-25
WO 00/59379 PCT/US00/08558
-11 -
sections 10-1 and 10-3 are joined to one another along the commissure
line 12-3 in a similar manner so as to form the tubular valve 10 depicted in
FIGURE 1. In this regard, when initiating the final suture lines 22-1 and
24-1 along commissure 12-3, it is sometimes necessary to insert a sizing
dilator (e.g., a suitably sized steel rod) into the inflow and sinus region
16,
18, respectively, when tying the knot of the suture. This procedure will
thereby hold the noncoronary sections 10-1, 10-2 and 10-3 in a generally
cylindrical shape, easing the knotting process. The sizing dilator may be
removed after completing each stitch.
Once the entire valve 10 has been sutured, the outflow conduit
section 14 is trimmed along the circumference of its free edges 10-1 a, 10-
2a and 10-3a so as to present a substantially level border around the
circumference of the outflow conduit section 14. The inflow region
fashioned with the mitral leaflets 16-1, 16-2 and 16-3 is also trimmed
~5 approximately 1.5 mm beyond the final interrupted mitral suture.
Specifically, the mitral leaflets 16-1, 16-2 and 16-3 should be trimmed
substantially parallel to the annulus 20 and all chordae tendineae must be
removed.
The thus assembled valve may then be placed in a specimen cup
2o containing a sufficient quantity of storage solution to fully cover the
entire
valve 10 and held at about 4°C in aqueous medium for further
processing.
In this regard, further processing may include one or more of the
treatments by which the tissue may be decellularized, soluble proteins
removed, tissue constituents covalently or ionically modified, chemical or
25 biochemical substituents added, or tissue crosslinked. Furthermore, the
tissue may be treated with suitable mammalian cells in a manner such as
to produce a recellularized tissue.
CA 02366767 2001-09-25
WO 00/59379 PCT/US00/08558
-12-
Accompanying FIGURE 4A is a photograph of an exemplary
bioprosthetic heart valve in accordance with the present invention. In this
regard, a suture line comprised of horizontal mattress sutures is clearly
visible in FIGURE 4A between the bases of the leaflet sections to the
distal outflow free edges of the joined tissue segments. Moreover,
FIGURE 4A visibly reveals a line of everted tissue which protrudes
outwardly from the noncoronary sections formed by the mattress sutures.
Although the discussion previously focused on forming the suture
line 22-1 from interrupted sutures 22-1 a, and forming the suture line 24-1
from horizontal mattress sutures 24-1 a, it should be evident that the
suture lines 22-1 and/or 24-1 can be formed from any type and/or
combination of sutures suitable for the tissue involved and/or the ultimate
placement of the bioprosthetic valve 10. In this regard, the sutures used
for the suture lines should not tear the tissue and should accommodate
relatively compliant tissue. The sutures should also form a substantially
leak-free juncture between the tissue segments. Suitable sutures that
may be employed in the practice of this invention include continuous
sutures, lock-stitch sutures, interrupted sutures, mattress and the like. By
way of example, another embodiment of a bioprosthetic heart valve in
2o accordance with the present invention is depicted in FIGURE 4B as
having noncoronary tissue sections joined together by interrupted sutures.
In use, the valve 10 may be surgically implanted as a total
replacement for a patient's native aortic valve or the pulmonary valve and
its outflow tract.
25 The attending surgeon may modify the bioprosthetic heart valve 10
to suit the particular anatomy of the patient. Thus, the inflow and/or
outflow conduits 16, 14, respectively, may be trimmed in their lengthwise
CA 02366767 2001-09-25
WO 00/59379 PCT/US00/08558
-13-
direction between adjacent sutures prior to surgical implantation so as to
provide an overall lengthwise size suitable for the patient only if a
continuous suture line has not been used to from the valve.
Accompanying FIGURE 5 depicts a modified bioprosthetic heart
valve (designated by reference numeral 100) in accordance with the
present invention. In general, the heart valve 100 depicted in FIGURE 5
is a surgically modified version of the valve 10 discussed previously in
that noncoronary tissue segments 100-1, 100-2 and 100-3 have been
sutured together to form a trileaflet valve structure. The valve 100,
however, includes scallop regions 102-1 and 102-2 defined by excised
tissue from the outflow conduit region of joined tissue segments 100-1
and 100-2. These scallop regions 102-1 and 102-2 thereby allow fluid
communication between the outflow side of the trileaflet valve structure
104 (formed by the juncture of leaflet cusps 104-1, 104-2 and 104-3) and
~5 the patient's native coronary arteries. Thus, the valve 100 shown in
FIGURE 5 is especially useful as an inclusion valve for aortic valve repair.
It will be understood that; although two such scallop regions 102-1 and
102-2 are depicted in FIGURE 5, more or less scallop regions could be
provided in the surgeon's discretion to suit particular aortic valve repairs.
2o Thus, a single scallop region, or three scallop regions in each of the
tissue segments 100-1, 100-2 and 100-3 could be provided in the valve
100.
Therefore, while the invention has been described in connection
with what is presently considered to be the most practical and preferred
25 embodiment, it is to be understood that the invention is not to be limited
to
the disclosed embodiment, but on the contrary, is intended to cover
CA 02366767 2001-09-25
WO 00/59379 PCT/US00/08558
-14-
various modifications and equivalent arrangements included within the
spirit and scope of the appended claims.