Note: Descriptions are shown in the official language in which they were submitted.
CA 02366800 2002-O1-07
-I-
CARBO~CYLIC ACID D:ERfVATIVES THAT INRIBIT THE BINDING OF
INTEt~RINS TO THEiR: REC1EPTORS
Cross-Reference to Related Avnlications
This application l:; a continuation-in-part of U.S. Patent Application Serial
No.
091707,068 filed November b, 2000 which is a continuation-in-part of U.S.
Patent
Application Serial No. 091565,92,0, fled May 5, 2000, which claims the benefit
of U.S.
Provisional Patent Application S~.riaI No. 60/132,971, filed May 7, 1999.
Field of the .Tnventiezn
?his invention ie directed generally to the inhibition of the binding of a4~i~
integrin to
its receptors, for example VCAM-1 (vascular cell adhesion molecule-1) and
fibronectin. The
invention also relates to compounds that inhibit this binding;; to
pharmaceutically active
compositions comprising such compounds; and to the use of such compounds
either as above,
IS or in formulations for the control or prevention of disease states in which
a4(3f is involved.
l3ack ound of the lavention
When a tissue has been invaded by a microorganisnx or has been damaged, white
blood cells, also called leukocyte>, play a major role in the inflammatory
response. One of
the most important aspects of the inflammatory response involves the cell
adhesion event.
Generally, ~vhite blood cells are found circulating through the bloodstream.
However, when
a tissue is infected or becomes damaged, the white blood cells recognize ehe
invaded or
damaged tissue, bind to the wall of the capillary and migrate through the
capillary into the
affected tissue. These events are mediated by a family;of proteins called cell
adhesion
molecules.
There are three main type;; of white blood cells; granulocytes, monocytes and
lymphocytes. The intesrin er4~3, (also called VLA-4 for very late antigen-4)
is a heterodimeric
protein expressed on the surface of monocytes, Iyrnphocytes and two subclasses
of
granuiocytes: eosinophils and basophils. This protein plays a key role in cell
adhesion
through its ability to recognize and bind VCAM-1 and fibronectin, proteins
associated with
the endothelial cells that line the interior wall of capillaries.
CA 02366800 2002-O1-07
-2-
Following infection or dat~ilage of tissue surrounding a capillary,
endothelial cells
express a series of adhesion molecules, including YCAIvt-1, that are critical
for bindingthe
white blood cells that are necessary for fighting infection: Prior to binding
to VCAM~ 1 or
.~,,;,
fibronectin, the white blood cells initially bind to certain adhesion
molecules to slow their
flow and allow the cells to "roll" alc>ng the activated endothelium.
Monocytes; lymphocytes,
hasophils and eosinophils are then able to firmly bind to VCA.M-1 or
ftbronectin on the blood
vessel wall via the aapt integrin. There is evidence that such interactions
are also involved in
transmigration of these white blood cells into the damaged tissue as well as
the initial rolling
event itself.
Although white blood cell rGdgration to the site of injury helps fight
infection and
destroy foreign material, in many instances this migration can become
uncontrolled, with
white blood cells flooding to the scene, causing widespread tissue damage.
Compounds
capable of blocking this process, thc;refore, may be beneficial as therapeutic
agents. Thus, it
would be useful to develop inhibito:,rs that would prevent the binding of
white blood cells to
VCAM-l and fibronectin.
Some of the diseases that might be treated by the inhibition of a~Q ~ binding
include,
but are not limited to, atherosclerosis, rheumatoid arthritis, asthma,
allergy, multiple sclerosis,
lupus. inflammatory bowel disease, graft rejection, contact hypersensitivity,
and type I
diabetes. In addition to being found, on some white blood cells, a,~~3~ is
also found on various
cancer cells, including leukemia, melanoma, lymphoma and sarcoma cells, It has
been
suggested that cell adhesion involving a~~3~ rnay be involved i.n the
metastasis of certain
cancers. Inhibitors of a~~3, binding may, therefore, also be useful in the
treatment of some
forms of cancer.
The isolation and purification of a peptide which inhibits the binding of
a4~it to a
protein is disclosed in U.S. Fatent No. 5,510,332. Peptides which inhibit
binding are
disclosed in WO 95/15973, EP 0 341 91 S, EP 0 422 938 A1, t'J.S. Patent No.
5,192,?46 and
WO 95106208. Novel compounds which are useful for inhibition and prevention of
cell
adhesion and cell adhesion-mediated pathologies are disclosed in WO 96/22966,
WO
98/04247 and WO 98104913.
CA 02366800 2002-O1-07
-3-
It is therefore an object of the invention to provide novel compounds which
are
inhibitors of aa(3' binding, and pharraaceutical compositions including such
novel
com~unds.
Brie F Sumrnarv of the Invention
The present invention is dirrcted to compounds of Formula I
X
' ~r
as
Trt.~Ra
Formula I
30 wherein Y, at each occurrer'ce, is independently selected from the ,group
consisting
of C(O), N, CR', C(RZ )(R3 ), NRS, CH, O and 5;
q is an integer of from 3 to 10;
A is selected from the group consisting of O, S,: C(R'6 )(R1' ) and NRb:
E is selected from the group consisting of CHz, O, S, and
IS NR';
T is selected from the group consisting of O, S and NRB;
T is selected from the group consisting of C(O} and (C;HZ)b wherein b is an
integer
of from 0 to 3;
M is selected from the group consisting of C(R9)(R.~~ and
20 (CHZ)", wherein a is an integer of from 0 to 3;
L is selected from the group consisting of O, NR' ', S, and
(CHz)~ wherein n is "n integer of 0 or 1;
X is selected from the group consisting of COZB; l?O3H2, __.
S03H, SO:NH~, SO~NHCOR'2, OPO~H~, C(O)NHC(O)R",
25 C(O)NHSOZR~'', hydroxyl, tetrazoIyl and hydrogen;
W is selected from the group consisting of C, CR'5 and N; and
CA 02366800 2002-O1-07
-4-
B, Ri, RZ, R~, R4, Rs, R6, R', R8, R~, R1°, R' 1, R~~, Rt3, R'4, R'S,
R~6 and R~~ at each
occurrence are independently selected frorn the group consisting of
hydrogen, halogen, alkyl, alkenyl, alkynyl, atkoxy, aIkenoxy,
allcynoxy, thioalkoxy, hydroxyalkyl, aliphatic acyl, -CF3, -COaH, -SH,
-CN, NOZ, -NHI, -OFi, alicynylaraino, alkoxycarbonyl, heterocycloyi, carboxy,
-N(C,-C, alkyl)-C(O)(C,-C3 alkyl), -NHC(O)N(Ci-C3 alkyt)C(O)-
NH(Ci-CaalkYl), -NAC(O)NH(Ci-C6 alkyl), -NHSO2(Ci-C3 alkyl),
-NHSOZ(aryl), alkoxyalkyl, alkylamino,,alkeaylamino, di(Ci-C3)amino,
-C(O)O-(C1-C3)alkyl, -C(O)NH-(C,-C3)al,kyl, -C(O)N(Ci-C3 atkyl)2,
-CH NOH, -P03Hi, -OP03H2, haloalkyl; alkoxyalkoxy, carboxaldehyde,
carboxamide, cycloaikyl, cycioalkenyl, cy<>loalkynyl, cycloalkylalkyl, aryl,
amyl, aryloxy, arylamino, biaryl, thiaaryl, diarylamino, heteroeyalyl,
alkylaryl, .
aralkenyl, atalkyl, alkylheterocyclyl, heterocyclylalkyi, sulFonyl,
-S02-(Cs-C3 alkyl), -S03-(C1-C3 alkyl), sulfonamido, carbamate, aryloxyalkyl
and -C(O)NH(benzyl) groups;
wherein H, RI RZ R' Rø R3 R6 :Et7 Rg R9 R'° R~~ Rz2 R~3 R''r
, , , , s s , a o » , i
RCS, R~6and R'' are unsubstituted or substituted with at least one
electron donating or electron withdrawing group;
wherein when L is NR' 1, R4 and R~ ~ taken together may form a ring;
and.vherein when 1VI is C(R9)(Rt°), R9 and R~° taken togesther
may ,
form a ring;
and wherein when A is NR6 and at least one Y is CRI, R~ and R6 taken
together may form a ring;
or a pharmaceutically acceptable salt thereof;
with the proviso that when A is C(R~6)(Rt~), E is not NR7.
For Formula I, presently preferred compounds many have A as NR6; E as NR'; J
as O;
M as C(R~(Rl~; q as 4 or 5; T as (CH~b wherein b is 0; L as (CHah, wherein n
is 0; X as
CQZB; W as C or CRS; R4 as aryl, alkyIaryI, aralkyl, lieterocyclyl,
allrylheterocyclyl or w
heteracyclylalkyl; and R6, R?, R9,1R° and R'S independently as hydrogen
or lower alkyl.
More specifically, the compounds of this invention may be described by
Formula II
CA 02366800 2002-O1-07
-5-
q~ ',
~N N ~ \ Rd
Formula g
wherein Y, at each occurrence; is independently selected from the group
consisting
of C(O), N, CRS, C(Rz )(R3 ), NRS, CH, O and S;
q is an integer of from 3 to 7;
T is selected from the group consisting of C(O) and (CHZ)b wherein b is an
integer of
Oto3;
L is selected from the group consisting of O, NR~ ~, S, and
(CH2)" wherein n is un integer of 0 or 1;;;
I0 W is selected from the group consisting of C, CR~I=' and N; and
B, R', RZ, R~, R°, R'; R6, R', R9, R'°, R' a and Rl' ate
independently selected from
the group consisting of hydrogen, halogen, alkyl, alkenyl, alkynyl,
alkoxy, alkene~y, alkynoxy, thioalkoxy; hydroxyalkyl, aliphatic acyl, -CF3,
-COzH, -SH, -CN, -NOZ, -NHz, -OH, alkynylamino, alkoxycarbonyl,
IS heterocycloyl, carboxy, -N(C~-C3 alkyl)-C(O)(CS-C, alkyl),
NHC(O)N(C,-C3 aikyl)C(O}NH(Ci-C3alkyl), -NHC(O)NH(Ci-C6 alkyl),
-NHS02(C~-C3 alkyl), -NHS02(aryl), alkoxyalkyl, slkylamino, aIke~rlarnino,
di(Ct-C3)~ino, -C(O)O-(Ci-C3)alkyl; -C(O)NH-(C,-C3)alkyl, -C(O)N(Ci-Ca
alkyl)Z, -CH=NOH, -POzHl, -OP03H2, haloalkyl, alkoxyalkoxy,
ZO carboxaldehyde, carboxamide, cycloaIkyl, cycloalkerzyl, cycloalkynyl,
cycloalkylalkyl, aryl, aroyl, atyloxy, aryiannino, biaryl, thioaryl,
diarylanuno,
heterocyclyl, atkylaryl, aralkenyl, aralkyl, alkylheterocyclyl;
heterocyctyialkyi, --'
sulfonyl, -SOZ-(C~-C3 alkyl), -SO3-(C~-C3 alkyl), sulfonamido, carbamate,
aryloxyalkyl and -C(O)NH(benzyi) groups;
25 wherein B, R', R=, R3, R°, R5, R6, R7, R9, Rj°, Rl' and Rls
are unsubstltuted or
CA 02366800 2002-O1-07
~~6-
substituted with at least ane electron.donating or electron withdrawing
group;
wherein when L is NR", R4 and R" taken together may form a ring;
and wherein R9 and R'° taken together may form a ring;
and wherein wben at least one Y is CR', R' and R6 taken
together may form a ring;
or a pharmaceutically acceptable salt thereof.
For Formula II, presently preferred compounds may have q as 4 or 5; W as C or
CR'S;
T as (CHZ)b wherein b is 0; L as (CH=)" whereir n is 0; R'' as aryl,
alkylaryl, aralkyl;
heterocyclyl; alkylheterocyclyl or heterocyclylalkyl; and R6, R', R9,
R'° and R'S as
independently hydrogen or lower alkyl.
More specifically, the compounds of this invention may be described by Formula
III
0
Rs
~Y ~ q O Rj° OB
Rss.,r
-N N T R
o ~s
Formula III
I5
wherein Y, at each occurrence, is independently selected from the group
consisting of C(O), N, CR', C(R2 XR3 ), NRS, CH, O and S;
q is an integer of from 2 to 5;
T is selected from the group consisting of C(O) and {CHZjs wherein b is an
integer of
2(3 4 to 3;
L is selected from the group consisting of O, NR' ~, ~, and
(CFIZ~, wherein n is an integer of U yr 1; ' __.
Rs, R6, R', R' ~ and R'e are each independently selected from the group
consisting of
alkyl, alkenyi, alkynyl, hydroxyalkyl, aliphatic acyi, alkynylamino;
25 alkoxycarbonyl, heterocycloyl, -CH=NOH, haloalkyl, alkoxyalkoxy,
CA 02366800 2002-O1-07
_7_
carboxaldehyde, carboxamide, cycloalkyl, cycloalkenyl, cycloalkynyl,
cycloalkylalkyl, aryl, aroyl, aryloxy, arylaminu, biaryl, thioaryl,
diarylamino,
heterocyclyl, alkylaryl, aralkenyl, aralkyi, alkylheterocyelyl,
heterocyclylalkyl,
carbamate, aryloxyalkyl, hydrogen and --C(O)NFi(benzyl) groups; and
S B, R', RZ, Rj, R4, R9 and R~° are independently selected from
the
group consisting of hydrogen, halogen, alkyl, alkenyl, alkynyl,
alkoxy, alkenoxy, alkyaoxy, thioalkoxy, hydroxyalkyl, aliphatic acyl, -CF3,
-CC?zII, -SH, -CN, NO z> -NHz, -OH, allcynylamino, alkoxycarbonyl,
heterocycloyl, carboxy, -N(Ci-C3 alkyl)-C(O)(C,-C3 alkyl),
IO NHC(O)N(Ci-C3 alkyl)C(O)NH(C,-C3alkyl}, -NHC(O)NH(Ci-C6 alkyl),
-NHS02(Ci-Ca alkyl), -NH50z(aryl), alkoxyalkyl, allrylamino, alkenylamino,
di(Ct-C~)amino, -C(O)O-(C 1-C3}alkyl, -C(O)NH-
(C1-C3)alkyl, -C{O)N(C,-C3 alkyl)1, -CH=NOH, -P03HZ, -OP03HZ, haloalkyt,
slkoxyalkoxy, carboxaldehyde, carboxamide,, cycloalkyl, cycloalkenyl,
I5 cycloalkynyi, cycloalkylalkyl, aryl, aroyl, aryloxy, arylamino, biaryl,
thioaryl,
diarylamino, heterocyclyl, alkylaryl, araikenyl, aralkyl, alkylheterocyclyl,
heterocyclylalkyl, sulfonyl, -SO~-(C,-C3 alkyl}, -S03-(C~-C3 alkyl},
sulfanamido, carbamate, aryloxyalkyl and -C'.(O)NH(benzyl) groups;
wherein B, R', Rz, R3, R'~, Its, Rb, R~, R9, R'i'; R~' and Rl$ are
unsubstituted or
20 substituted with at least one electron donating or electron withdrawing
group;
wherein when L is NR' ~, R4 and R" taken together may form a ring;
and wherein R4 and R'° taken together may form a ring;
and wherein when at least one Y is CRS, R~ and R6 taken
25 together may form a ring;
or a pharmaceutically acceptable salt thereof.
For Formula IIt, presently preferred compounds may have R~e as hydrogen,
alkyl, aryl,
aralkyl, cycloalkyl, alkylhzterocyclyl, heterocyclylalkyl or heterocyclyl; T
as (CHZ)b wherein b
is 0; L as (CHZ)r wherein n is 4; Y as CR' and C(R~ ){R3 ) and q as 2 or 3.
30 In Formula fft; the portion of the molecule
CA 02366800 2002-O1-07
-g. ( .
rz: .
Rie...-
can be
~lR~al N il0.a°~ '~W .l0.ry~
.. , ~ N~/' ~ '
pra y1/ w R19/ ~ ~ . pre/N
O O Q
~ , a~Q
and pharmaceutical acceptable salts thereof and pharmaceutical acceptable
salts
thereof
wherein R~9, R'° , R'' and R''s at each occurrence are independently
selected from
the group consisting ofhalogen, alkyl. alkenyl, alkynyi, alkoxy, alkenoxy,
alkynoxy, thioalkox~r. hydroxyalfiyl, aliphatic acyl, -CF3, -OH,
-CO_H, -5H, -Chi, .VOA, -NH=, alhynylarriino, alkoxycarbonyl,
heterocycloyi, carboxy, -?vi(C~-C. alkyl)-C(O)(C,-C3 alkyl),
-NHC(O)N(Ci-C3 alkyl)C(O)NH(C,-C,alkyl); -NHC(O)NH(Ci-Cbalkyl),
;,.
-NHSOI(Ci-C. alkyl), -NHSOa(ary1), alkoxyalkyl, alkylamino, all:enylamino,
di(C~-C~)amino, -C(O)O-(Ci-C~)alkyI, -C(O)NH-(C1~'C3)alkyl, -C(O)N(Ct-C3
alkyl):, -CH=NOH, -POOH:, -OPO3HZ, haloalkyl, alkoxyalkoxy,'
carboxaldehyde, carboramide, cycloalkyl, c;ycloaikenyl, cycloalkynyl,
cycloalkylalkyl, aryl, aroyl, aryloxy, arylamino, biaryl, thioaryl,
diarytamino,
heteroeyclyl, alkylatyl, aralkenyl, aralkyl, alkylheteroeyclyl,
heteroeyclytalkyl,
sulfonyl, -SO=-(Ci-C3 alkyl), -S03-(C ~-C3.alkyl),
sulfonamido; catbamate, aryloxyalkyl and -L(O)NH(benzyl) soups;
R13 is selected from the group consisting of alkyl, alkenyl, alkynyl,
hydroxyalkyl,
aliphatic aryl. alkynylamino, alk~xycarbc~ttyl, heterocycloyl, -CH=NOH, _
CA 02366800 2002-O1-07
-g-
haloalkyl, alkoxyalkoxy, carboxaldehyde, carboxamide, cycloslkyl,
cycloalkenyl, cycloalkynyl, cycloalkylalkyl, aryl, aroyl, sryloxy, arylamino,
biaryl, thioaryl, diarylamino, heterocyelyl, alkylaryl, aralkenyl, aralkyl;
alkylhetorocyclyl, haterocyclylalkyl, carbamate, aryloxyalkyl, hydrogen and
-~(O)NH(benzYl) groups;
R~ is selected fram the group consisting of hydrogen, halogen, alkyl, alkenyl,
alkynyl, alkoxy, allcenoxy, alkynoxy, thioalkoxy, hydroxyalkyl, aliphatic
acyl, -CF3, -COzH. -SH, -CN, -NO2, -NHz, -4F3, alkYnylaraino,
alkoxycarboayl, heterocycloyl, carboxy, -N(C1-C3 alkyl)-C(O)(C~-C3
..10 alkyl), -NHC(O)N(CuC3 alkyl)C(O)NH(C~-C~~alkyl), -NFiC(O)NH(C~-Cs
alkyl), -NHSOi(C,-C3 alkyl), -NRSOz(arYl), alkoxyalkyl, alkylamino,
alkenylamino, di(Ct-C,)amino, -C(O)O-(Cl-Cj~llcyl,
-C(O)NH-(Cl-C3)alkyl, -C(O)N(Ci-Cj alkyl}2, -CH=NOH, -POsH2,
-OPOsHz, haloalkyl, alkoxyalkoxy; carboxaldehyde, carboxamide,
cycloalkyl, cycloalkenyl, cycloalkynyl, cycloalkylalkyl, aryl, amyl, aryloxy,
arylamino, biaryI, thioaryl, diarylamino, heterocyclyl, alkylaryl; aralkenyl,
aralkyl, alkylheterocyclyl, heterocyclytalkyl, sutfonyl, -SO2-(Ct-C3 alkyl),
-S03-(Ci-C, alkyl), sulfonamido, carbamate,'a:ryloxyalkyl and
-C(O)NH(benzyl) groups;
c is an integer of 2em to two;
d is an integer of zero to three;
a is an integer of zero to four, and
i is an integer of zero to two.
In one embodiment, Rlg is aralkyl; R'~ is aryl; T is (CHZ)b where b is zero; L
is (CH.,~ where
ZS n is zero; and, B, R6, R7, R9 aad Rl° are each independently
hydrogen.
CA 02366800 2002-O1-07
-1~-
lVioze specifically, the compounds of this invention nnay be described by
Formula 1V
.,,
9~R23 R9
w
'' ~ O Rio OS
r
~e:.~'N L ~.,.
R \
I ~6 E
O
Formula 1V
wherein T is selected from the group consisting of C'(O) and (CHz)b wherein b
is an
integer of from 0 to 3;
L is selected from the goup consisting of O, NR~', S, and
(CHZ)~ wherein n is an integer of 0 or 1;
g is an integer of from 0 to 7; y.
B, R~, R9, R'° and R'' at each occurrence are independently selected
from the
group con$isting of hydrogen, halogen, alkyl, atkenyl, alkynyl, atkoxy,
alkenoxy, alkynoxy, thioatkoxy, hydmxyalkyl, aliphatic acyl,
-CF3, -CO=H; -SH, -CN, -NOZ, -NHz, -OH, alkynylamino,
alkoxycatbonyl, heterocycloyl, carboxy, -N(C~-C3 alkyl)-C(O)(C~-C3
alkyl),
-NHC(O)N(C~-C3 alkyi)C(O)NH(C,-C3alkyl) -NHC(O)NH(C1-C6 alkyl),
-NHSOz(C~-C3 alkyl), -NHSOz(aryl), alkoxyalkyl, alkylamino,
alkenylamino, di(C~-C3)amino, -C(0~3-(C~-C3)alkyl, -C(O)NH-(C~-
Ca)aikyl, -C(O)N(C~-C3 alkyl)a, -CH=NOH~, -P03Hz,~-OP03Ha, hsloalkyl,
alkoxyalkoxy, carboxaldehyde, carboxamide, cycloalkyl, cycloaIkenyl,
cycloalkynyl, cycloalkylalkyl, aryl, aroyl, aryloxy, arylamino, biaryi,
thioaryl, diarylamino, heterocyclyl, allrylaryl, aralkenyl, aralityl,
aikyiheterocyclyl, heterocyclylalkyl, sulfonyl,
-SO;-(CmC3 alkyl), -S03-(C,-CJ alkyl); sulfonamido, carbamate, aryloxyalkyl
CA 02366800 2002-O1-07
-11-
and -C(O)NH(benzyl) groups; and
R6, R7, R~ t and Rte are each independently selected [from the group
consisting of alkyl,
alkenyl, alkynyl, hydroxyalkyl, aliphatic aryl" alkynylamino, alkoxycarbonyl;
heterocycloyl, -CH NOH, haloalkyl, alkoxyalkoxy, carboxaldehyds,
carboxamide, cycloalkyl, cycloalkenyl, cycloalkynyl, cycloalkylalkyt, aryl,
amyl, acyloxy, arylamiao, biaryl, thioaryl, diarylamino, heterocyclyl,
alkylaryl,
aralkenyl, aralkyl, all'ylheterocyclyl, heterocyclylalkyl, carbanlate,
aryloxyalkyl, hydtogea and --C(0}NfI(benzyl) groups;
wherein B, Ra, R6, R7, R9, R'°, R' 1, R' ' and R23 are unsubstituted or
substituted
1p with at least one electron donating or electron withdrawing group;
wherein when L is NR~ t, R° and R~ ~ taken. together may form a ring;
and wherein R9 and R'° taken together may form a ring;
or a pharmaceutically acceptable salt thereof.
Presently preferred compounds of the present invention may also be described
by
15 Formula V.
F
R25
Formula V
20 wherein h is an integer of zero to five;
B, R9, R'°, R2', and R~ are each irdependeatly selected from the group
consistins
CA 02366800 2002-O1-07
-12-
of hydrogen, halogen, alkyl, alkenyl, alkynyl, alkoxy, alkenoxy, alkynoxy,
thioalkoxy, hydroxyalkyl, aliphatic acyl,'~-CF3, -COZH, -SH, -CN, -NO=,
NH2, -0H, alkynylamino, alkoxycarbonyl, heterocycloyl, carboxy,
-N(CuC3 alkyl)-C(C3)(C,-C3 alkY1)~
-NHC(O)N(C,-C3 allcyl)C(O)NH{Ct-Cs~yl). -NHC(O)NH(Ct-Cs alkyl)>
-NHSOz(CrC3'~5'I), -NIxS02(aryl), alkoxyalkyl, alkylamino,
alkenylamino, di(C~-C3)aminn, -C(O)O-(Cu-Ca)alkyl,
-C{O)NH-(C~-C3~ikyl, -C(O)N(C~-C3 alkyl)a, -CH~NOH, -P03Fiz,
-OP03H2, haloalkyl, alkoxyalkoxy, carboxaldehyde, carboxamide,
. . 10 cycloalkyl, cycloalkenyl, cycloalkynyl, cyt:loalkylalkyl, aryl, aroyl,
aryloxy, arylatnino, biaryl, thioaryl, diarylamino, heterocyclyl; alkylaryl,
aralkenyl, aralkyi, alkylheterocyclyl, heterocycIylalkyl, sulfonyl,
-SOz-(C~-C3 alkyl), -S03-(C~-C3 alkyl), sulfonainido, carbamate,
aryloxyalkyl and -C(O)NH(benzyl) gzoups;
1g RZ', at each occurrence, is independently selected from the group
consisting of
halogen, alkyl, alkenyl, alkynyl, alkoxy, all~enoxy. alkynoxy, thioalkoxy,
hydroxyelkyl, aliphatic acyl, -CF3, -CO~H> -SH, -CN, -NO;, -NHz, -OH,
alkynylamino, alkoxycarbonyl, heterocycloyI, carboxy, -N(C~-C3 alkyl)-
C(O)(C~-C3 alkyl), -NHC(O)N(G4-Cs alky'I)C(O)NH(C~-Caalkyl),
ZO -NHC(O)NH(C1-Cs alkyl), -NHSOa(Ct-C3 alkyl}, -NHSO2(aryrl), N(C~-Cy
alkyl)SOz(Ci-C3alkyl),-N(Ct-C3alkyl)SQz(aryl); C
alkoxyalkyt,alkylsmino, alkenylamino, di(C~-C3)amino, -C(O)O-(Ci-
C3)atkyl, -C(O)NH-(C,-C~~llcyl, -C(O)N(Ct-C~ alkyls, -CH~NOH,
-PO~HZ, -OP03Ha. haloalkyl, alkoxyalkoxy, carboxaldehyde, carboxamide,
25 cycloalkyl, cycloalkenyl, cycloalkynyl, cycloalkylalkyl, aryl; aroyl,
aryloxy, arylamino, biaryl, thioaryl, diarylamiao, heterooyclyl, alkylaryl,
aralkenyl, aralkyl, alkylheterocyclyl, heterocyclylallryl, sulfonyl,
-SOz-(Ci-C3 alkyl), -SOa'(Ci-C3 alkyl) sulfonatnido, carbamate,
aryloxyalkyl and -C(O)NH(bznzyl) groups;
30 R6, R' and R~s are each independently selected from the group consisting of
alkyl, alkettyl, alkynyl, hydroxyalkyl, aTip~hatic acyl, alkynylamino,
CA 02366800 2002-O1-07
-13- ~ ;..
alkoxycarbouyl, heterocycloyl, -CH=NOH, iialoatkyl, alkoxyalkoxy,
carboxaldehyrcle, carboxamide, cycloslkyl, cycloalkenyl, cycloalkynyl,
cycloalkylalkyl, aryl, aroyl, aryloxy, arylamiao; biaryl, thioaryI,
diarylarnino,
hsterocyclyl, allcylaryl, aralkenyl, aralkyt, alkylheterocyclyl,
heterocyclylalkyl,
carbamate, arylaxyalkyl, hydrogen and-C(O)NH(benzyl) ;groups; aad,
R26 is selected from the group consisting of hydmgen, alkyl, alkenyl, alkynyl,
hydroxyalkyi, aliphatic acyl, -CF3, alkoxycaibonyl, heterocycloyl; carboxy;
-C(O)O-(Ct-C3~lkYi, -C(O)NH-(Ci'C3)81~3'I, -C(O)N(C1-C3 a~Yi)2,
P03Ha, haloalkyl, carboxamide, cycloalkyh::~ycloalkenyl, cycloalkynyl,
. 30 cycloalkyla~lcyl, aryl, aroyl, biaryl, heterocyclyl, allcylaryl,
aralkenyl,
atalkyl, aLkylhe~rocyclyl, heterocyciylaikyl, sulfonyl, -S02-(CrCs alkyl),
suifonamido, aryloxyalkyl and -C(O)NH(benzyt) groups;
wherein 8, R6, R', R9, R'°, R~s, Ray, R=s, R=6 and Ra' are
unsubstituted or
substituted urith at least one electron donating or electron withdrawing
group;
wherein Rye and Rza taken together may foitn a ring;
RZ' and Rz5 taken together may form a ring;
R'S and R'6 taken together may form a ring;
and wherein R9 and Rt° taken together rnay form a rix<g;
or a pharmaceutically acceptable salt thereof.
Presently preferred compounds of Formula V have B, Rb, Ix', R9, Rio, RZ4, Rzs
~d Rzs each
independently hydrogen and R'8 as substituted or unsubstibuted aralkyl.
Qther presently preferred compounds of the present invention tnay be described
by
Formula V3..
0
R
0 R~Q SOB
~ \' 'a _.
N N ? R
o ~e ~r
Formula V1
CA 02366800 2002-O1-07
-14-
wherein ~, at each occurrence, is independently selected from the group
consisting
of C(O), N, CR~°, C(R" )(R3z ), NR'3, CH, O and S; '
z is an integer of from 3 to 6; '
k is an intager of from 0 to 5; ,
F
S T is selected from tire group consisting of C(O) and (CHz)b wherein b is an
integer of from 0 to 3;
L is selected from the group consisting of O, NRIJI~ S, and ,
(CHZ)" wherein n is an integer of 0 or 1;
Rs, R~, Rt', Rya and R33 are each independently selected from the group
consisting of
!0 alkyl, alkenyl, alkynyl, hydroxyalkyl, aliphatic acyl, alkynylamino,
alkoxycarbonyl, heterocycloyI, -CH=NOH, haloalkyl, alkoxyalkoxy,
carboxaldehyde, earboxamide, cycloaikyi, cycloaIkeityl, cycloalkyrtyl,
cyclos?kylalkyl, aryl, amyl, aryloxy, aryl~mino, biaryl, thioaryl,
diarylamino,
:.z
heterocyclyl, alkylaryl, aralkenyl, aralkyi, alkylheterocyclyl,
heterocyolylalkyl,
1S carbamate, aryloxyalkyl, hydrogen and -C(O}NH(benzyl) groups;
B, R'', R', R'°, R3°, R3~ and R3=at each occurrence are
independently selected from
the group consisting of hydrogen, halogen; alkyl, alkerryl, alkynyl, alkoxy,
alkenoxy, alkynoxy, thioalkoxy, hydroxyall~cyl, aliphatic acyi, -CF3,
-C02H, -SH,-GN, -NOZ, -NHz, -OH, alkynylamino, alkoxycarbonyi,
20 heterocycloyl, carboxy, -N(C,-C3 alkyl)-C(O)(Ci-C3 alkyl),
-NHC(O)N(C~-Cs alkyt~(O)NH(G~.C3alkyl), NHC(O)NH(CrCsaIkyl),
-NHSO~(CaC3 alkyl); -NHSOz(aryl), alko,~xyalkyl, alkylamino;
alkenylamino; di(Ct-C3)amino, -C(O)O-(G~-Cs)aikyl,
-C(O}NH-(Cl-C3)alkyl, -C(O)N(C~-C3 alkyl)a, -CH=NOH, -F03H=,
25 -OPO3H2, haloalkyl; atkoxyalkoxy, cazboxaldehyde, carboxsmide,
cyeloa,Ikyl, cycloalkenyl, cycloalkynyl;'cycloalkylalkyl, aryl, azoyl,
aryloxy, atylamino, biaryl, thioaryl, diarylamino, heterocyclyl, atkyiaryl,
aralkenyl, aralkyl, aliylheterocyclyl, heterocycIylalkyl, sulfonyl, ~-SOz-E,C~-
C~ alkyl), -SO3-(GrC3 alkyl), sulfonamido, carbamate, aryloxyalkyl and
30 -C(O)NH(benzyl) groups; and,
Rz9, at each occurrence, is independentty selected from the group consisting
of
CA 02366800 2002-O1-07
-ls-
halogen, alkyl, alkenyl, alkynyl, alkoxy, ahenoxy, alkynoxy, thioalkoxy,
,.
hydroxyalkyl, aliphatic acyl, -CF3, -COzH, -SH, -CN, l~IOa, -NHa; -OH,
allrynylamino; alkoxycarbonyl, heterocycloyl, catboxy, -N(Ct-C3 allryl)-
C(O)(Ct-C3 alkyl), NHC(O)N(Ct-Cs slkyl)C(O)NH(C~-Csalkyl),
S NHC(O)NH(Ct-C6 alkyl), -NHSO2(C~_Cj..alkyl), -NHS02(aryl),
alkoxyalkyl, alkylamino, alkenylamino, di(C i-C3)amino, -C(O)O-(C t-
C3)alkyl, -C(O)NH-(Ct-C3)alkyl, -C(O)N(Cn-C3 alkyl, -CH=NOH,
-P03Ha, -OPOSHa. haloalkyt, alkoxyalkoxy, carboxaldehyde,
carboxamide, cycloalkyt, cycloalkenyl, cycloalkynyl, cycioalkylalkyl,
1p aryl, arvyl, atyloxy, arylamino, biaryl, thioaxyl, diarylarnino,
hetemcyclyl,
alkylaryl, aralkenyl, aralkyl, atkylheterocyclyi, heterocyelylalkyl, sulfonyl,
-SOZ-(Ct-C3 alkyl), -S03-(C~-C3 alkyl), sulfonamido, carbamate,
aryloxyalkyl and -C(O}NH(benryl) grougs~
wherein B, R4, R6, R', Rg, Rt°, Rtt, RtB, Rz9, R3°:R3t, Rsa and
R33 are
is unsubstituted or substituted with at least otxe electron donating or
electron
withdrawin; group;
wherein when L is NRt t, R~ and R't taken together may form a ring;
and wherein RQ and R'° taken together may form a ring;
or a pharmaceutically acceptable salt thereof.
20 Some compounds of Formulae I-VI can be prepared from novel intermediates of
Formula VII and Formula VIII.
X25
Rye N
34 ._.
Formula VII
wherein R2'' and R~5 are each independently selected from the group consisting
of
k
CA 02366800 2002-O1-07
-16-
F;,i,:
C.
hydrogen, halogea, allcyl, alkenyl, alkynyl, alkoxy, alkenoxy, alkynoxy,
thioaIkoxy, hydroxyalkyl, aliphatic aayla~F3, -8H, -OH,
~:.i
-C02H, -CN, NOz, NHz, alkynylarrtino~'allkoxyearbonyl, heteroeycloyl,
carboxy, -N(C~-C3 allryl)-C(O)(Ci-C3 alkyl), -NHC(O)N(Ct-C3
g alkyl)C(O)NH(C,-C3alkyl), -NI~C(O)NH(Ci-C6aIky1), alkylamino,
NHSOa(C~-Cs $lkyl), NHSOz(atyl), alkoxyalkyl, alkenylamino, di(C1-
Ca)amino, -C(O)O-(C~-C3)allryl, -C(O)NH-(Ct-C3~1kY1,
-C(O)N(CS-C3 alkyl)z, -GH=NOH, -P03H?, -OPO~Iiz, haloalkyl,
alkoxyalkoxy, carbaxaldehyde, carboxarnide, cycloalkyl, cycloalkenyl,
cycloalkynyl, cycloalkyialkyl, aryl, amyl, aryloxy, aryiamiao, biaryl,
thioaryl,
diarylainino, heterocyclyl, alkylaryl, ara~kenyl, aralkyl, alkylhetatocyclyl,
heterocyclylalkyl, sulfo~l, -SOz-(Ct-C~ alkyl), -S03-(Ct-Ca alkyl),
sulfonaniido, carbamate, aryloxyalkyl and -C(C~)NH(ber~zyl) groups; and
Ry$ and R34 are each independently selected from the group consis#ng of alkyl,
atkenyl, al cyt~tyl, hydmxyalkyl, aliphatic acyt, alkyqylamino,
alkoxycarbonyl,
heteroeyeloyl, -CH~NOH, haloalkyl, alkoxyalkoxy, carboxaldehyde,
carboxarnide, cycloalkyl, cycloalkenyl, cycloalkynyl, cycloalkylalkyl, aryl,
arayl, aryloxy, arytsmino, biaryl, thiaaryl, diarylamino, heterocyclyl,
alkyiaryI,
aralkenyl, aralkyl, alkylheterocyclyl, heterocyclylalkyl, carbamate,
aryloxyalkyl, hydrogen and --C(O)NH(bet~ayl) groups;
wherein Rig, RZ°, R''S and R34 are unsubstituted or substituted with
at least one electron doii~ting or electron withdrawing
group;
and wherein R1° and R2S taken together may fonca a ring;
with the proviso that when R'f' and R2S taken together f~zm a ring, the riag
formed is not
ben2ene. Presently preferred compounds of Formula dII have Rj" as hydrogen;
R'8 as
aralkyl; and RZ° and R2g each indpendezitty as hydrogen, Iower alkyl or
lower alkyl wherein
R24 and R'~ are taken together to form a ring. . ._.
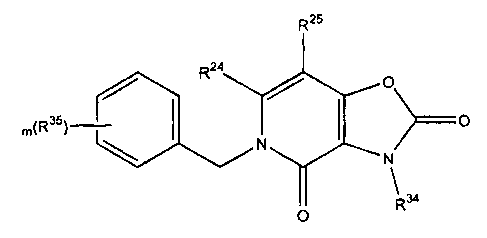
Formula VT1I shows presently preferred novel intermediates.
CA 02366800 2002-O1-07
s;::.
R25
m~~35~~ O
Formula VI>I
wherein R=4 and Rz5 are each independently selected from the group consisting
of
hydrogerC, halogen, alkyl, alkenyl, alkynyl, alko~y, alkenoxy, alkynoxy,
thioalkoxy, hydroxyalkyt, aliphatic acyl, -CFs, -SH, -OH,
-COzH; -CN, -N02, -NHi, alkynylamino, alkoxycarbonyl, heterocycloyl,
casboxy, -N(C!-C3 alkyl)-C(O)(Ci-C3 alkyl)" -NHC(O)N(C~-C3
alkyl)C(O)NH(Ci-C3alkyl), -NHC(O)NH(C ~-C6 alkyl), NHSOZ(C,-C3
atkyl), -NH50:(eryt), alkoxyalkyl, alkylamino, alkenylamino, di(C~-
p C3)amino, -C(O)O-(C,-Cs)alkyi, -C(O)N~I=(C~-C3)alkyl, -C(O)N(Ca-C3
atkylh, -CH~NO1:I; -P03Hz. - OPO~Hz, haloalkyl, alkoxyalkoxy;
carboxaldehyde, carboxamide, cycloalk~i,, cycloalkenyl, cycloalkynyl,
~,;_
cycioallcylalkyl, aryl, amyl, aryloxy, arylamino; biaryl, thioaryl,
diarylamino, hetetocyclyl, alkylaryl, arelkenyl, aralkyl, alkylheterocyclyl,
15 heterocyclylalkyl, sutfonyl, -SOa-(Ci-C~ alkyl), -S03-(Ci-C3 alkyl),
sulfonamido, carbamate, aryloxvalkyl and -~C(O)NH(ben2yl) groups;
R34 is selected from the Sroup consisting of alkyl, alkenyl, alkynyl,
hydroxyalkyl,
aliphatic acyl, alkynylamino, alkoxycarbonyi, heterocycloyl, -CH=NOH,
haloalkyl, alkoxyalkoxy, carboxaldehyde;.carboxamide, cycloallcyl,
20 cycloalkenyt, cycloalkynyl, cycloalkylalkyl, aryl, aioyl, aryloxy,
arylamino,
biaryl, thiaaryl, diarylamino, heterocyclylalkylaryi; aralkenyl, aralkyl, ___
alkylheterocyclyl, heterocyclylalhyl, carlsamate, aryloxyalkyl, hydrogen and
-C(O)NH(benzyl) groups; and,
R3S, at each occurrence, is independently selected from the group consisting
of
CA 02366800 2002-O1-07
halogen, alkyl, alkeayl, alkynyl, alko~:y, alkenoxy, alkynoxy, thioalkoxy,
hydroxyalkyl, aliphatic acyl, -CFa. -COiH, -SH, -CN, -N02, -NHze -OH,
alkynylamino, alkoxycarbonyl, heterocycloyl, carboxy, -N(Ct-C3 alkyl)-
C(O)(Ct-Cs ~kYl), -NHC(O)N(Ct-C3 ~Yl)Cl0)NH(C1'C3a1kY1),
s -rrHC(o)rrH(C,-C6 alhyn. -rlHSO2(C t-C~ a~yn, NHSO2(aryl),
alkoxyalkyl,allrylamino, alkenylamino, di(Ct-C3)~no; -C(O)O-(Ct_
C3)alkyi, -C(O)NH-(Ct-Cz)alkyl, -C(O)N(;-C3 alkyl)z, .-CH=NOH,
~:
-P03H2, -OP03Hz, haloalkyl, alkoxyalkoxy;carboxaldehyde, carboxamide,
cycloal'kyl, cy~cloalkenyl, cycloallcynyl, cycloalkylaikyl, aryl, amyl,
14 aryloxy, arylamino, biaryl, thioaryl, diarylamino, heterocyclyi, alkylaryl,
aralkeayl, arallcyl, alkylheterocyclyl, heterocyclylalkyl, sulfonyl,
-SOz-(Ct-C3 alkyl), -S03-(Ct-C3 allcyl), sutfonamido, carbamate,
eryloxyalkyi and -C(O)NH(benzyl) groups;.
wherein RZa, Ru, Rj' and R35 are ttasubstituted or substituted with
1g at least one electron donating or electron vsrithdrawing
group; and,
m is an integer of from 0 to 5, Presently preferred compounds ofFormuIa VIII
have R3'~ as hydrogen; m as an integer of one to three and Rr3 at each
occturence as alkyl,
halogen, aikoxy, haloatkyl, sulfonyl, -OH or -CN.
2O Presently preferred compounds of Formula I include;
(3S)-3-(( {[2-methyl-4-(2-methylpropyl)-6-oxo-1-(pbenylrnethyl)-1,6-dihydro-5-
pyrimidinyijemiao}carbonyl)aminoj-3-(4-methylphenyl)propaaoic acid, (3S)-3-
(1,3-
benzodioxol-5-yl)-3-[({[2-oxo-1-(Phenylmathyl)-4-propyl-1;2.dihydro-3-
pyridinyl]amino}carbonyl)amino]propanoic acid, (3S)-3-{[({1-[(2-
chlorophenyl)methyl]-4-
25 ethyl-2-oxo-1,2-dihydro-3-pyridinyl}amino)carbonyl]amino}-3-(4-
methylphenyl)propanoie
acid, (3S}-3-([({1-[(2-chlorophenyl)methylj-2-oxo-4-p~opyl-1,2-dihydro-3-
pyridinyi}aminoxarbonyljamino}-3-(4-methylphenyl)~iopamoic acid, (3S)-3-{[({I-
[(2-
chlorophenyl)methyl]-4-methyl-2-oxo-1,2-dihydro-3-
pyridinyl}amiao)carbonyl]amimo}-3-(4- __.
methylphenyl)propanoic acid, (3S)-3-{[({6-methyl-2-oxo-1-(phanylmethyl)-4-
30 ((Phenylmethyl)oxyj-1,2-dihy3ro-3-pyridinyl}aminoxarbonyl]amino}-3-(4-
znethylphenyl)pzopanaie acid, (3S)-3-{[(il-[(2-chlorophenyl)methyl]-2,4-
dimethyl-6-oxo-
CA 02366800 2002-O1-07
-19-
1,6-4ihydro-5-pyrimidinyl}amino)carbonyl]amino}-3-(4-m~thylphenyl)propanaic
acid, (35).
3-{[({4-amino-1-[(2-chlorophe~nyl)methyij-6-methyl-2-oxo l,2-dihydro-3-
pycidinyl}amino)carbonyljamino}-3-(4-methylphenyl)prop~ataoic acid, (3S)-3-
~[({1-[(2-
chlorophenylhnethyt]-4-methyl-2-oxo-1,2-dihydro-3:pyri~i~y1}
arnino~arbonyl)amino}-3-[4-
(methyloxy)phemrljprapanoic acid, (3S)-3- f [({1-[(2-chlo=bplsenyi)methylJ-4-
methyl-2-oxo-
1,2-dihydro-3-pyridinyi}amino)carbonyl]amino}-3-(3,4-dirnetbylphenyl)propanoic
said, (3S)-
3- {[( {4-amino-1-[(2-chlorophenyl)methyl]-2-oxo-1,2-dihydro-3-
pyridinyl}amino)carbonyljamino}-3-(4-methylphenyl)propanoic acid, (3S)-3-{[( f
I-[(Z-
chEorophenyI)rr~ethyl)-4-hydroxy~2-oxo-1,2-dil:,~dro-3-
pyridinyl}aminokarbonyl]amino} -3-
(4-tnethylphenyl)propanoic acid; (3S)-3-[({[1-[(Z-chlorophenyl)methyl]-4:(1,4-
oxazinan-4-
yl)-2-oxo-1,2-dihydra-3-pyridinyl]amino} caxbonyl~amino~j-3-(4-
methylphenyl]prapanoic
acid, (3S)-3-[({[1-[(2-chlorophenyl)methyl)-2-oxo-4-(propylamiuo)-1,2-dihydro-
3~
,_
pyridinyljamino}carbonyl)emiao)-3-(4-methylphenylarroic acid, (3S)-3-{[({1-[(2-
bromoghenyl)methyl]~!-methyl-2-oxo-1,2-dihydro-3-
py~idinyt)atctino)carbonyljamina}-3-(4-
methylghenyl)proganoic acid; (3S)-3-{[({1-[(Z-chlorophenyl)methyl]-4-hydroxy-2-
oxo-1,2-
dihydro-3-pyridinyl } amino)earbonyljamino } -3-{3-methyl-4-
(mechyloxy)phenyljpropanoic
acid, (35)-3-{[({1-[(2-chlorophenyl)methyl)-2-oxo-4-phen.yt-I,2-dihydro-3-
pyridinyl}amino)carbonyl]amino}-3-(~-methyiphenyl)propanoic acid, (3S)-3-{[({1-
[(2-
chloraphenyl)methyl].4-[(2-{(z-(methyloxy)ethyl]oxy} ethyl)oxy]-2-oxo-1,2-
dihydro-3-
pyridinyl}amino)carbonyl]amino}-3-(4-methylphenyl)propanoic acid, (3S)-3-([({1-
[(2-
chlorophenyl)methyl]-4-hydtoxy-6:methyl-2-oxo-1,2-dihydro-3-
yridinyl}amino)carbonyl~amino}-3-(4-methylphenyi)propanoic acid, (3S)-3-{t({1-
[(2-
chloraphenyl)methylj-4-[(1,1-ditnethyIethyl)amino]-2-.oxo-1,2-dihydro-3-
pyridiayl}amino)carbonyl]amino}-3-(4-methylphenyl~iiopanoic acid, (35)-3-{[({1-
[(2-
chlorophenyl)methyl]-~4-hydroxy-2-oxo-1,2-dihydro-3-
pytidinyl}amino)carbonyl)amino~-3-
phenylpropanoic acid, (3S)-3- f [({i-[(2-chloraphenyl)methylj-4-[4-
methyltetrahydro-1(2H)-
pyrazinylj-2-oxo-1,2-dihydso-3-pyridinyl}amino)carbonytjamino}-3-(4-
methylphenyl)propanoic acid, (3S)-3-{[([i-[(2-chloroptneayl)methyl]-4-hydmxy-2-
oxa-1,2-
dihydro-3-pyridinyl}amino)carbonyl]amino}-3-[4-(methyloxy)phenyljpropanoic
acid;
(3S)-3-{[({1-[(2-chlarophenyl)methyl]-4-hydroxy-2-oxo-1,2-dihydro-3-
pyridinyi}amino)carbonyl)amino}-3-(3,5-dimethylphej~yl)propanoic acid, (3S)-3-
{[({1-[(2-
CA 02366800 2002-O1-07
24- ~ ~<
chlorophenyl~ethyl]-4-hydroxy-Z-oxo-1,2-dihydro-3
pyti;.vinyl}amino)carbonyl]amino}-3-
(3-methylpheiryl)prapanoic aoid, (3S)-3-{[({1-[(2-chlompk~8ayt)methylJ-4-
hydroxy-2-oxo-
1,2-dihydro-3 pyridinyl}amino)carbonyl]amino}-3-[3-
(metliyloxy)phenyt]propanoio acid,
{3S)-3-(3,5 bis(methyloxy)phenyll-3-{[({1-[(2-chlorophenyl)rnathyi]-4-hydroxy-
2-oxo-1,2.
dihydro-3~yridinyl}amino)carboiryl]amino}propaaoic acid, (3S)-3-([( f i-[{Z-
chlorophenyl~nethyl]-4-hydtoxy-Z-axo-1,2-dihydro-3-
quinolinyl}amino)carbonyl]arnino}-3-
(4-mathylpheayl}propanoic acid, (3S)-3- f [({ I -[(2-chiorophenyl)methyl]-4-
hydroxy-2-oxo-
1,2-dihydro-3-pyridinyl} amino)carbonyl]amino}-3-[3-
(trifluoromethyl)phenyl]pmpanoic
acid, {3S)-3-~:[({1-[(2-chlorophenyI)n~ethylJ-4-
(({ethyl[(ettiylamino}carbonyl]
..10 amino}carbonyl)amino]-2-oxo-1,2:dihydro-3-
pyridinyI}axriir~o)carboaylJamino}-3-(4-
methylpheuyi)propanoic acid, (3S)-3-{[({4-(i-azetanyl)-~=[(z-
chlorophenyl)rnethylJ-2-oxo-
x.::
1,Z-dihydto-3-yridinyl}amino)carboxyl]amino}-3-(a-me~ylphenyl)propanoic acid,
(3S)-3-
[( ( [1-[{2-chlorophenyl)methyl]-4-( {2-j(2-{[2-(methyloxy)e~hyl]oxy}
ethyI)oxy]ethyl}
oxy)-2-oxo-1,2-dihydro-3-pytidinyl?amino} cattsonyl)aminoJ-3-(4-
methylphenyl)propanoic
acid, (3S)-3-{[({I-[(2-fluorophenyl)methylJ-4-hydroxy 2-oxo-I,2-dihydro-3-
. pyridinyl}amino)carbonyl]amino}-3-(4-methylghenyl~ropanoic acid, (3S)-3-
{[({I-[(2-
chloro-6-fluorophenyl)methyl3-~-hydroxy-2-oxo-I ,Z-dihydro-3-
pyridinyl}amino)carbonyljamino~-3-(4:methylphenyl)propanoic acid, (3S)-3-{[({1-
[(2-
chtorophenyl)methyl]-5-methyl-Z-oxo-1,2-dihydro-3-pyridinyl }
amino)carbonyi]amino}-3-(4-
methylphenyl)propaaoic acid, (3S).3-(1,3-benzodioxol-S=yl)-3-(({(2-oxo-1-((4-
(trifluoromechyl)phenyl)methyl)-1,2 dihydro-3-
py~idiny'~jamino)carbonyl)amino)propanoic
acid, (3S)-3-((((1-((2-chlorophenyl)methyl)-Z--oxo-I;Z-dihydro-3-
pyridinyl)amino)carbonyl)amino)-3-(4-methylphenyl)propanoic acid, (3S)-3-((((1-
((2-
fluoropheayl)methyl)-2-oxo-1,2-dihydro-3-pyridinyl)amino)carbonyl)amino)-
3-(4-methyIphenyl~rvpanoic acld, (3S)-3-((((1-((2-bromophenyl)methyl)-2-oxo-
1,2-dihydro-
3-pyridinyl)amino)carbonyl)amino)-3-(4-methylghenyl~ropanoic acid, (3S)-3-
((((1-((Z,4-
dichlorophenyl)methyl.2-oxo-1,2-dihydro-3-pyridinyl)amino)carbony1)
amino}-3-(4-methylpheayl}propanoic acid, (3S)-3-((((Z'=((2-chloro-6-
iluorophenyl)methyl)-2.
oxo-1,2-dihydro-3 pyridinyl)amino)carbonyl)amino}-3-(4-methyiphenyl~ropanoic
acid, (3S)
3-((((1-((Z-chlarophenyl)methyl)-4-hydroxy-2-oxo-1,2-rlihydxo-3
pyridinyl)amino)carbonyi)amino)-3-(4-trifluoromethy!)oxy)phenyl)propanoic
acid, (3S)-3-
CA 02366800 2002-O1-07
p;
-21- 'v
j({[1-(2-chloro-6-methoxybenzyl)-2-oxo-1,2-dihydropyriditi-3-
yl]amino}carbonyl)amino)-3-
(4-methytphenylic acid, 4-([3-[({[(iS)-2-carboxy-1-(4-
mathylphenyl)ethyl) amino} carbonyl)amino]-1-(2-chlombeazyl)-2-oxo-1,2-
dihydropyridin-4
yi}amino}benzoic acid, (3S~3-{j({1-(2-chlorobe~nzyl)-4-
[(2,2~~dirnethylpropanoyl)aminoJ-2
oxo-1,2-dihydropyridin-3 yl}amino)carbonyl~amino}-3-(4-methylphenyl)propanoic
acid,
(3S)-3-[( {[4- f [(test-butylamino)carbonyl)amino}-1-(2-chloxt~benzyl)-2-oxo-
1,2-
dihydropyrldia-3-yl]amino}carbonyl)amino)-3-(4-methylpher~yl)propanoic acid,
(3S)-3-[({[1-
(2-cyanobenzyl)-4 hydroxy-2-oxo-1,2-dihydropyridin-3-y1]amino}carbonyi)amino3-
3-(4-
methylpherryl)propanoic acid, (3 S)-3-[( { [ 1-{2-chlorabenzyl~~ 4-hydtoxy-2-
oxo-1,2-
dihydropyridin-3-yl]amino}carbonyl)amino]-3-(2,3-dihydro=1,~4-benzodioxin-6-
yl~ropanoic
acid, (3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-1,2-dihydropyridin-3-
yl]amino}carbonyl)amino)-3-(7-methoxy-1,3-benzodioxol-5-yl)propanoic acid,
(3S)-3-[({[1-
(2-chlotobenzyl)-4-hydroxy-2-oxo-1,2-dihydropytidin-3-yi]amino}
carbonyl)aminoJ-3-(3-
ethoxy-4~methoxyphenyl)ptopanoic acid, (3S)-3-[( { [ 1-(2-chlorobenzyl)-4-
hydro~cy 2-oxo-
1S 1,2-dihydropytidin-3-yl)amino}carbonyl)amino)-3-(3,4-
dimethoxyphenyl)propanoic acid,
(3S)-3-[( { [ 1-(4-chlorobenryl)-4-hydroxy-2-oxo-1,2-dihydTOpyridin-3-
yl]amino}carbonyl)amino]-3-(4-methylphenyl)propanoic acid, (3S)-3-[({[1-(2-
chloro-6-
methoxybenzyl)-4-hydroxy-?-oxo- I ,2-dihydropytidin-3-yl] amino }
carbonyl)amino j-3-(:1-
methylphenyl)propanoic acid, (3S)-3-[(1[1-(2-chlorobenzyl)-4-hydroxy-5-methyl-
2-oxa-i,2.
dihydropyridin-3-yl]amino}carbonyl)amino]-3-(4-xnethylpitenyl)propanoic acid,
(3S)-3-[({[1-
(2,b-difluorobenzyl)-4-hydroxy-2-oxo-1,2-dihydropyridin-3-
y1]amino}carbonyl~aninoJ-3-(4-
methylphenyl)propattoic acid, (3S)-3:[({[1-(2-chloro-6-methoxybenzyl)-4-
hydroxy-2-oxo-
1,2-dihydropyridin-3-y1]amino}carbonyl)amino)-3-(3,5-dimethoxyphebyl)propanoic
acid,
(3S)-3-[( {[I-(2-chlorobenzyl)-4-hydroxy-2-oxo-1,2-dihydropyridin-3-
yl]amino}carbonyl)amino]-3-(3,4-diethoxyphenyl)propanoic acid, (3S)-3-[(([1-(2-
chlorobenzyl)-4-hydroxy-2-oxo-I,2-dihydmpyridin-3-yl]aiilino}carbonyl)atnino]-
3-(3-
ethoxyphenyl~ropanoic acid, (3S)-3-[(([1-(2-chiorobenzyl)-4-hydroxy 2-oxo-1;2-
dihydropytidin-3-yljatnino) carbonyl)amino]-3-(3-metho~y=4-
methylphenyl)propanoic acid,
(3S)-3-[( {[ i-(Z-chiorobenzyl)-4-hydroxy-2-oxo-1,2-dihy3ircrpyridin-3-
yl]amino}carboxyl)amino]-3-(3,5-dimethoxy-4-methylphenyl)propanoic acid, (3S)-
3-[({[1-
(2-chlorobenzyl)-4-hydroxy-2-oxo-1,2-dihydropyridin-3-yl]amino}
carbonyl)arnir~o]-3-(3;4-
CA 02366800 2002-O1-07
-22-
dimethylphenyl)propanoic acid, (3S)-3-[( f [I-(2-chlorobenzyl)-S-ethyl-4-
hydroxy-2-oxo-1,2-
dr'hydropyridin-3-yl]amino}carbonyl)amino]-3-(4-methylphenyl)propanoic acid,
(3S)-3-
f(({I-[2-chloro-5-(tri~uoromethyl)benzylJ-4-hydroxY-2-oxo-1,2-dihydropyridin-3-
yl}amino)carbonyl]amino}-3-(4-methylphenyl)propanoic acid, (3S)-3~j({(1-(2-
chloro-6-
methoxybenzyl)-4-hydroxy-2-oxo-I,2-dihydropyridin-3-yljainino}carbonyl)amino]-
3-(3-
methylphenyl)propaaoic acid, (3S)-3-[( f [I-(2-chloro-6-meifijrlbenzyl)-4-
hydroxy 5-methyl-2-
oxo-I,2-dihydropyridin-3-yl]amigo}carbonyl)amino]-3-(4-
irie~thylphenyl)propanoic acid,
(3S)-3-[( ([1-(2-chlombenzylr4-hydroxy-2-oxo-2,5,6,7-tetrahydro-1Fi-
cyclopenta(b)pyridin-
3-yljarnino}carbonyl)amino]-3-(4-methylphenyl)propa>ioic acid, (3S)-3-[(([1-
(2,6-
dimethoxybenayl)-4-hydroxy-2-oxo-1,2-dihydropyridin-3-yI]amino}carbonyl)amino]-
3-(4-
methylphenyl)propanoie acid; (3S)-3-[({[1-(2-chlorobenzyl)-4~-hydroxy-2-oxo-
1,2-
dihydropyridir~-3-yl]ammo}carbonyl)amino]-3-(3-propoxyphenyl)propanoic acid,
(3S)-3-
[( f L 3 -(2-c~orobenzyl)-4-hydroxy-2-oxo-5-propyl-1,2-dihydiopyridin-3-
yl]amino}carbonyl)amino]-3-(3-ethoxyghenyl~ropanoic acid, (3S)-3-[(([1-(2-
chloroberlzyl)-
4-hydroxy-5,6-dimethyl-2-oxo-1,2-dihydropyridin-3-y1]amino}carbonyl)amino]-3-
(4-
methylghenyl)propanoic acid, (3S)-3-[(f [I-(2-chlorobenzy>;)~4~-hydroxy-2-oxo-
S-propyl-1,2-
dihydropyridin-3-yl]amino}carbonyl)amino]-3-(3,4-diethoxvphenyi}propanoic
acid, (3S)-3-
(3-batoxyphanyl)-3-[( ( [ 1-(2-chlorobenzyl)-4-hydroxy-2-oxo= Y ,2-
dihydrapyridin-3 -
yl]amino}carbonyl)amino]propanoic acid, (35)-3-([( f 1-(2-chloro-
5~(methylsulfonyl)benzyl]-
4-hydroxy-2-oxo-I,2-dihydiopyridin-3-yl; amino)carbonyl]arn,ino}-3-(4-
methyIphenyl~ropanoic acid, (3S)-3-[( f[1-(2-chlorobenryi)-4-hydraxy-2-oxo-1,2-
dihydropyridin-3-yI]amino}carbonyl)amino]-3-(3-(2-
methoxyethoxy)phenyl]propanoic acid,
(3 S)-3-[( f [ 1-(2-chlombenzyl)-4-hydroxy-2-oxo-1,2-dihydropy r; din-3-
yl]amino}carbonylamino]-3-(3,4-dipcopoxyphenyl)gropanoic acid, (3S)-3-[( f [1-
(2-
chlorobenzyl~-hydroxy-2-oxo-1,2-dihydmpyridin-3-yl]aminojcarbonyl)amino3-3-[3-
(ditluoromethoxy)phenyl]propanoic acid, (3 S)-3-(( { [ 1-(2-chlorobenzyl)-4-
hydroxy-5-methyi-
2-oxo-1,2.dihydropyridin-3-ylJamino}carbonyl)amino]-3-(3,4.-
diethoxyphenyl~ropanoic
acid, (3S)-3-[(f[1-(Z-chlorobenzyl)-4-hydroxy-5-tnethyl-2-oxo-I,2-
dihydropytidin-3-
yl]amino}carbonyl)amino]-3-(3-ethoxyphenyl)propanoic acid, (3S)-3-[({[1-(2-
chloro-6-
methylbenzyl)-4-hydroxy-5,6-dimethyl-2-oxo-1,2-dihydropyridin-3-
yl]amino}carbonyl)aminoJ-3-(3,4-diethoxyphenyl)propanoic,acid, (3S)-3-[(([1-(2-
chloro-6-
CA 02366800 2002-O1-07
-23-
cyan~benzyl}-4-hydroxy-2-oxo-I;2-dihydropyridin-3-yl] amino } carbonyl)atninoJ-
3-(4-
methylphenyl)propanoic acid, 3~[({[1-(2-chlombenzyl)-4-hyxy-2-oxo-1,2-
dihydropyridin-
3-yl]a~nino3carbonyl)amigo]-3-(2-naphthyl)propanolc acid and (3S)-3-[({[1-(2-
,;
chlorobenzyl)-4-hydroxy-5,6-dimethyl-2-oxo-1,2-dihydropy~;d~in-3-
yljamino}carbonyl)aminoj-3-(3,4-diethoxyphettyl~ropanoie'acid, (3S)-3-[({(!-(2-
chloro-b-
methoxybenzyl)-4-hydroxy 5-methyl-2-oxo-1,2-dihydropyridin-3-
ylJaxnino}carbonyl)amino]-
3-(3,4-diethoxyphenyl)propaaoic acid, (3S)-3-[({[I-(2-chlorobenxyl)-4-hydroxy-
2-oxo-1,2-
dihydropyridin-3-yljamino}carbonyl)amiroJ-3-(3-isopropoxyphenyl)propanoic
acid, (3S)-3-
[(.[[!-(2-chlorobenzyl)-4-hydroxy 5-methyl-2-onto-1,2-dihydropyridin-3-
,10 yl]amino}carbonyl)amino)-3-(4-methoxyphenyl)propanoic acid, (3S)-3-[({[1-
(2-chloro-6-
methylbenxyl)-4-hydroxy-2-oxo-2,~,6,7-tetrahydro-1H-cyclopenta[b]pyridin-3-
yljamino}carbonyl}amino)-3-(3-ethoxyphenyl)propanoicacid, (3S)-3-[({[1-(2-
chlom-6-
ethoxybenzyl~4-hydroxy-2-oxo-1,2-dihydropyridin-3-yl]amino}carboayl)amino]-3--
(3-
ethoxyphetryl)propanoic acid, (3S)-3-[({jl-(Z-chloro-6-etho~cyb~nzyl)-4-
hydroxy-5-methyl-2-
IS oxo-1,2-dihydropyridin-3-yl]amino}carbonyl)amino]-3-(3-
isaprapoxyphenyl)propanoic acid;
(3 S)-3-[( ( [ 1-(Z-cbloro-6-ethoxybenzyl)-4-hydroxy-Z-oxo-2,5,5, 7-tetrahydro-
1 H-
cyclopenta[bjpyridin-3-ylJatnino}carbonyl)amiaoj-3-(3-ethoxyphenyl)propanoic
acid, (3S)-3-
[( { [ 1-(2-chloro-6-ethoxybenzyl}-4-bydroxy-5~methyl-2-oxo-1. ;,2-
dihydropyridin-3 -
yl]amino}carbonyl)aminoj-3-(I-methyl-1H-indol-5-yl)propanoic acid, (3S)-3-
[({[1-(2-chloro-
20 6-ethoxybenzyl)-4-hydroxy 5.methyl-2-oxo-l,2-dihydropyridin-3-
yl]amiao}carbonyl)amino]-
3-(2,3-dihydro-I-benzoftcran-5-yl]propanoic acid, (3S)-3-[({[1..(2-chlo=o-6-
ethoxybenzyl)-4-
hydroacy 2-oxo-2,5,6,7-tetrahydto-1H-cyclopenta[b]pyridin-3-
yi]ami~torcarbortyl)aminoj-3-
(3,~-diethoxyphenyl)propanoic acid, (3S)-3-[({[5-chloro-1-~~~~ehloro-6-.
ethoxybenzyl)-4-
~.
hydroxy-2-oxo-1,2-dihydropyridin-3-yl]amino} carbonyl)arri'u!~o]-3-(3-
25 ethoxyphenyl~ropanoic acid, (3S)-3-[([[!-(2-ehloro-6-ethoxbbenzyl)-4-
hydroxy-2-oxo-1,2-
dihydropytidin-3-yl]amino}carbonyl)amino]-3-(3-isopropoxyphenyl)piopanoic
acid, (3S)-3-
[( j[I-(2-chioro-6-ethoxybenzyt)-4-hydroxy-2-oxo-Z,s,6,7-tetr~ahydro-iH-
cyclopentafb]pyridin-3-yl]amino}carbonyl)aminaj-3-(3-p:opoxyphenyl)propanoic
acid, (3S)- ._.
3-[( {[ 1-(2-chloro-6-ethoxybenzylr4-hydroxy-2-oxo-2,5,b,7-tetrahydro-1 H-
30 cyclopenta[b]pyridin-3-yllamino}cnrbonyl)amino]-3-phenylpropanoic acid,
(3S)-3-[({[1-(2-
chlorobenzyl)-4-hydroxy-2-oxo-2, 5,6, 7-tetrahydro-1 H-cyclogenta[bJpyridin-3 -
CA 02366800 2002-O1-07
-24-
y1]amino} carbonyl)amino]-3-( 1,3-diethyl-2-oxo-2,3-d~ydror 1 H-benzimidazol-5-
yI)pmpanoic acid, (3S)-3-[({[l-(2-chloro-6-ethoxybenryl~~aydroxy-5-methyl-2-
ova-1,2-
dihydropyridi~3-ylJamino}carbonyl)amino]-3-[3-
(trifluoroinethoxy)pher~ylJpropanoic acid,
{3 S)-3~[( {[ 1-(2-chloro-6-ethoxybenzyl)-4-hydro~cy-5, 6-dimathyl-2-oxo-1,2-
dihyd=opyridin-3-
yl]amino}carbonyl)amino]-3-(3-isopropaxypiienyl)propanoic acid, (3S)-3-[({[1-
(2-
chlorobenzyl)-4-hydroxy 2~oxo-2,5,6,7 tetrahydro-1H-cyclopenta[b~pyridin-3-
yl]amino}carbonyl)amino)-3-(1-methyl-iH-i-tdoi-S-yl~ropanoic acid, (3S)-3-
[(([1-(2-chloro-
6-athoxybenzyl)-5-cyclopropy!-4-hydroxy-2-vxo-1,2-dihydropyrictin-3-
yljsmino}carbonyl)aminoj-3-(3-isopropoxyphenyl)propant~ic acid, (3S)-3-[(([I-
(2-chloro-6-
., IO ethoxybenzyl)-5-cyclopropyl-4-hydroxy-2-oxo-1,2-dihydropyHdia-3-
yljamino}carbonyl)amino]-3-(4-methylphenyl)propanoic a~,id, (3S)-3-[({[1-(2-
chloro-5-
methoxybenzyl)-4-hydroxy-5-methyl-2-oxa-1,2-dihydropyi]din-3-yl)amino
}carbonyl~minoj-
3-(4-methytphenyl)propanoic acid, (3S)-3-[({[1-(2-chloro-6-~;thaxybenzyl)-4-
hydroxy-6-
methyl-2-oxo-1,2-dihydropyridin~3-yl]amino } carbonyl)amxno]-3-(3-
15 isopropoxyphenyl)propanoic acid, {3S)-3-[({[1-(2-chIoro-6-ethoxybenzyl)-4
hydtoxy-5-
rnethyl-2-oxo-1,2-dihydropyridin-3-yl ]amino } carbonyl)amino)-3-( 1-methyl-1
H-indol-6-
ylJpropanoic acid, (3S)-3-[(([1-(2-chloro-6-ethoxybenzyl)-4-hydroxy 2-oxo-
2,5,6,7-
tetrahydro-1 H-cyclopenta[b]pyridin-3-yt]amino } carbonyl)amino]-3-[3-
{cyclopropyloxy)phenyl]propanoic acid, (3S)-3-[(([1-(2-chl~robenzylr4-hydroxy-
3-oxo-
20 2,5,6,7-tetrahydro-1H-cyclopenta[b)pyridin-3-yI]amino}caibonyl)amino]-3-[3-
(cyclopmpylmethoxy)phenyl]propanoic acid, (3S)-3-[(([1-(2-chloro-6-
ethoxybenzyl)-4-
hydroxy-2-oxo-2,5,6,7-tartahydro-1H-cyclopenta[b~pyridin-3-
yi]amino}carbonyl)amino]-3-
[3-(cyelopropylmethoxy)phenylJpropanoic acid, (3S)-3-[( {[ l -(2-chlorobenzyt)-
4:-hydroxy-2-
oxo-2,S,G,7-tetrahydro-1 H-cyclapenta[b]pytidin-3-yl] amino } carbonyl)amino]-
3 ~(3,5-
25 dimethylphenyI)propanoic acid, (3S)-3-i[({1-[(2-chIorophenyl)methyl]-4-
hydroxy-2-oxo-
2,5,6,7-tetrahydro-1 H-cyclopenta[b]pyridin-3-yI } amino)carbonyljamino }-3-
(3-
[(dilluoromethyt)oxy]phenyl}propanoic acid, (3S)-3-{j({l;-[(2-
chlorop>zenyl)methylJ-4-
hydroxy-2-oxo-2,5,6,7-tetFahydro-1H-cyclopenta[b]pyridii~-3-
yj}aminoxarbonyl]amino} ~-
(3-[(1,I,2,2-tetrafluoroethyl)oxy]phenyl}propanoic acid, (3S)-3-{[({1-[(2-
30 chlomphenyl)methylj-4~hydroxy-2-oxa-2,5,6,7-tetrahydro-1H-
cyclopenta[b]pyrid'tn-3-
yl}amino)carbonyl]amino}-3-(1-ethyl-1H-indol-5-y1)propanoie acid and (3S)-3- f
[((1-[(2-
CA 02366800 2002-O1-07
-~' :4,ZS
~;'M:
,
chlorophenyl)methylJ-4-hydroxy 2-oxo-2,5,6,7-tetxahydm-1$-cyclopenta[bJpyridin-
3
yl}amino)carbonylJamino}-3-[3-(diethylamizio)phenylJpropanoic acid, (3S)-3-
[(~[1-(Z-
chlorobenzyl)-4-hydroxy-5-methyl-2-oxo-1,2-dihydrapyridin-3-
yiJaanino}carbonyl)aminoJ-3-
(4-methylphenyl)propanoic acid, (3Sj-3-{({[1-(2-chlorobeazyl)-4-hydroxy-2-oxo-
2,5,6,7-
tetrahydto-IH-cyclopenta[bjpyriden-3-ylJamino)carbonyl)arninoj-3-(4-
methylphertyl)propanoic acid, (3S)-3-[({[1-(2-chloro-6-ethoxybenzyl)-4-hydroxy-
5-methyl-2-
.~;a,
oxo-1,2-dihydropyridin-3-yl]amino}carbonyl)amino]-3-(4-
rriethylphenyl)propanoic acid,
(3S}-3-[({[1-(2-ehloro-6-ethoxybenzyl)-4-hydroxy-5-methyl=2~~oxo-1,2-
dihydmpyridin-3-
yl]amino}carbonyl)amino]-3-(3-ethoxyphenyl)pmpanoic ac~i~, (3S)-3-[({[1-(2-
chlorobenzyl)-
4-hydroxy-2-oxo-2,5,6,7-tetrahydro-1H-cyclopenta[b]pyridin-a-
ylJamino}carbonyl)arninoJ-3-
{3-isopropoxyphenyl)propanoic acid, (3S)-3-[({[1-(2-chlaro-6-ethoxybenzyl)-4-
hydroxy-5-
methyl-2-oxo-1,2-dihydropyridin-3-ylJamino} carbonyl}amino]-3-(6-methoxy-2-
naphthyl)propanoic acid, (3 S)-3-[( { [ 1-(2-chlorobenzyl)-4-hydroxy-2-oxo-2,
5,6,7-tetrahydro-
1H-cyclopenta[b]pyridin-3-ylJamino}carbonyl)amino]-3-(3-methylphenyl)propanoic
acid,
(3S)-3-{({[1-(2-chlorobenzyl)-4-hydroxy-5-methyl-2-oxo-1,2-dihydropyzidin-3
y1]amino}carbonyl)amino]-3-[3-(diethylamino)phenyl]propancic acid, and
{3S)-3-{[( { 1-[(2-chioro-6-metitylphenyl)methyl]-4-hydroxy-2-oxo-2,5,6,7-
tetrahydro-1 H-
cyclopanta(b~pyridin-3.y1 } arnino)carbonyl Jamino ) -3-( 1-methyl-1 H-indol-5-
yl)propanoic
acid, (3S)-3-{[({ 1-[(2-chlorophenyl)methyl]~-hydroxy-2-oxo-2,5,6,7-tetrahydro-
1H-
cyclopenta[b]pyridin-3-yl}amino)oarbonyl]amino}-3-{3-
[(methylsulfonyl)ameno)phenyi}proparaoic acid, (3S)-3-{[({1-j(2-chloro-6-
me~ylphenyl)methyl)-4-hydroxy-2-oxo-?,5,6, 7-tetrahydro-1 i~-
cyclopenta[b]pyridin-3-
yl}amino)carbonyljamino}-3-{3-[(methylsulfonyl)arninoJphenyl}propanoic acid,
(3S)-3-
{[( ( 1-[(2-chlorophenyl)methylj-4-hydroxy-2-oxo-2,5,6,7=te~rahydro-1 FI-
cyclopenta[bJpyridin-3-yl)amino)carbonyl]amino}-3-{3-
[methyl(tnethyl6ulfonyl)ammo]phenyl}propanoic acid, (3S)~3-{[({1-[(2-chloro-6-
methylphenyl)methyl]-4-hydroxy-2-oxo-2,5,6,7-tetrahydro-11-I-
cyclopetltajblPY~in-3-
yl}amino)carbonyl]amino}-3-{3-{methyl(methylsulfonyl)ainoJphenyl}propanoic
acid, (3S)~
3- { [( { 1-j(2-chlorophenyl)methyl]-4-hydroxy-2.oxo-2, 5,6,7=te;trahydto- I H-
cyclopenta(b]pyridin-3-yl}amino)cazbonyl]amino}-3-(3-
[ethyl(methylsulfonyl)amino]phenyl}propanoic acid, (3S)-3-{[({1-[(2-chloro-6-
CA 02366800 2002-O1-07
-zs-
methylphenyl)methyl-4-hydroxy-2-oxo-2,5,6,7-tetrahydm-1 H-cyclopenta(b]pyridin-
3-
yl3amino)carbonyl]amino}-3-~,3-[ethyl(methylsulfonyl)amino]phenyl}propanoic
acid, (3S)-3-
{ [( { 1-[(2-chloro-6-methylphenyl)methyl]-4-hydroxy-2-oxo-2, ,5,6, 7-
tetrahydro-1 H-
,r
cyclopenta[b]pyridin-3-yl) amino)carbonylJamino}-3-(I H-iridal-5-yl)propanoic
acid and
pharmaceutically acceptable salts thereof of the above compounds.
Presently preferred compounds of Fotmula VII include:
5-(2-ehlombenzyl)-3,5-dihydro[l,3joxazolo[4,5-cJpyiidiae=~,4~-dione, 5-(2-
chIorobenzyl)-6-
methyl-3,S-dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5=(2-fluoroben2yl)-
3,5-
dihydro[l,3joxazolo[4,5-ejpyridine-2,4-dione; 5-(2-chloro-6-fluorobenzyl)-3,5-
dihydro[l;3)oxazolo[4,5-c]pyridin~2,4-dione, S-benzyl-6-methyl-3,5-
dihydto[1,3]oxazoIo(4,S-c)pyridine-2,4-diotte, 5-benzyl-3,S-
dihydro[1,3)oxazolo[4,5-
cjpyt;diae-2,4-dione, 5-(2,5-dimethylbenzyt)-3,5-dihydrojl,3]oxaxolo[4,5-
c]pyridine-2,4-
dione, 5-(2-methylbenzyt)-3,5-dihydro[1,3)axazolo[4,5-c]pyridine-2,4-dione, 5-
(2,4-
dichlorobenzyl)-3,5-dihydro[1,3]oxazolo[4,5-cJpyridine-2,4-duone, 5-(2-
methoxybenzyl)-3,5-
dihydro[l,3joxazolo[4,5-cjpyridine-2,4-dione, S-(2,5-difluorobenzyl)-3,5-
dihydro[1,3)oxazolo[4,5-e]pycidine-2;4-dione, 5-[2-
chloro=S=(methytthio)benzyl)-3,5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 3-(4-fluorob'enzyl)-3,S~
dihydro[1,3]oxazolo{4,S-cjpyridine-2,4-dione, 5-(2-chloro-~-methoxybenzyl)-3,5-
dihydro[1,3]oxazolo[4,5-cJpyridine-2,4-dione, S-[3,5-
bis(trifluoromethyl)benzyl]-3,5-
dihydm[1,3)oxazoio[4,~-c)pyridine-2,4-dione, ~-(4-tart-burylbenzyl)-3,5-
dihydro[l,3joxazoio[4,5-c]pyridine-2,4-dione, S-(3-chlorobezizyl)-3,5-
dihydro[I,3)oxazolo[4,5-c]pyridine-2,4-dione, 5-(4-chlorobenzyl)-3,5-
dihydro[l,3joxazolo[4,5-cjpyndine-2,4-dione, 5-[3-(trifluorornethyl)benzyl]-
3,5-
dihydm[1,3]oxazolo[4,S-a]pyridine-2,4-dione, S-(2-bromobeniyl)-3,5-
dihydro[1,3]oxa2olo[4,5-c]pyridine-2,4-dione, 5-{3,4-dichlorobenzyl)-3,5-
dihydro[1,3]oxazolo[4,5-cJpyridine-2,4-dione, S-(4-methyllianzzyl}-3,5-
dihydro[l,3]oxazolo[4,5-c)pyridine-2,4-dione, S-{2-chloro-6-methoxybenzyl}-3,5-
dihydm[1,3)oxazolo[4,5-c)pyridine-z,4-dione, 5-[4-(trifluorornethyl)benzylJ-
3,5-
dihydro[1,3]oxazolo[4,S-c)pyridine-2,4-dione, S-(3-methylbenzyt)-3;5-
dihydro[1,3)oxazola[4,5-c]pyridine-2,4=dione, S-(pyridin-2-ylmethyl)-3,5-
dihydro[1,3)oxazolo[4,5-c)pyridine-2,4-dione, 5-(2-chlorobenzyl)-7-methyl-3,~-
CA 02366800 2002-O1-07
-27-
dihyciro[1,3]oxazolo[4,5-c]pyridine-2,4-diope, 5-(2,4-difluorobenzyl)-3,5-
,=a, ,
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(2,6-difluc~iobenzyl)-3,5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-[3-(trifluoromethoxy)banzyl]-
3,5-
dihydro[1,3]oxazolo[4,5-cJgyridine-2,4-diona, 5-[4-(tri#luoxomethoxy)benzylJ-
3,5-
dshydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-[2-(trifluo;omethyl)benzyl]-
3,5-
dihydro[l,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(3-methoxybenzyl)-3,5-
dihydro[I,3Joxazolo[4,5-c]pyridine-2,4-dlone, 5-(2,3-dichlorobenzyl)-3,5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(3,5-dimeth~~lbenzyl)-3,5-
dihyc~o[1,31oxazolo[4,5-c)pyrfdine-2,4-dione, ~-(2-chlorobenzyl}-7-p~ntyl-3,5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(2,4-dichlorobenzyl)-7-methyl-
3,5-
dihydroji,3]oxazolo[4,5-c]pyridine-2,4.dione, S-(2-chlorobenxyl)-7-ethyl-3,5-
dihydto[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 7-butyl-S-(2-chlorobenzyl)-3,5-
dihydro[1,3]oxazolo[4,5-cjpyridine-2,4-dione, 5-[2-chloro-5-
(tritluommethyl)benzyl~-3,5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5.(2,6-dichlorobenzyl)-3,5-
dihydzo[1,3]oxazolo[4,5-cJpyridine-2,4-dione, S-(2-chloro-5-tIuorobenzyl)-3,5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(2-chloro-6-mechylbenzyl)-7-
methyl-3,5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(4-chlorobenzyl)-7-methyl-3,5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, S-(2-chIorobenzyl)-5,6,7,8-
tetrahydro-2H-
cyelopenta[b][I,3]oxazolo[5;4-d]pyridine-2,4(3H)-dione, 7-methyl-S-[4-
(methylsulfonyl)benzyll-3,5-dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(4-
methoxybenzyl)-3,5-dihydro(1,3]oxazolo[4,5-c]pyridine-2,4-dlone, 5-(2-
chlombenzyl)-7-
prapyl-3,5-dihydco[1,3]oxazolo[4,S-c]pyridine-2,4-dione, 4-[(2,4-dioxo-2,3-
dihydrojl,3]oxazolo{4,5-c]pyridin-5(4H)-yl)methyl]-N,N-
dirnethylben2enesul.fonamide, 5-
(mositylmethyl)-3,5-dihydro(1,3]oxazolo[4,5-c]pyridine-2;4-~iione, 5-(2-
chlorobenzyl)-
3,5,6,7,8,9-hexahydro[1,3]oxazolo[4,3-c]quinoline-2,4-dione, 5-(2-
chlorobenzyl)-7-ethyl-6-
methyl-3,5-dihydro[1,3]oxezolo[4,5-a]pyridine-2,4-dione, 5-[2-
(methylthio)benzyl]-3,5-
dihydro[I,3]oxazolo[4,5-c]pytidine-2,4-dione, Z-[(2,4-dioxo-~2,3-
dihydro[I,3]oxazolo[4,5-
c]pyridin-5(41;I)-yl)methyl]-N,N-diaaethylbenzenesulfonamide, S-(2,6-
dimethoxybenryl)-3,5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-[2-(tritluvromethoxy)benzyl]-
3,5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dzone, 5-(2-chlorobenzyl)-6,7-dimethyl-
3,5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-[2-chloro-5-
{methylsulfonyl)benzyl]-3,5-
.~
CA 02366800 2002-O1-07
_2g-
dihydro(1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(4-chloro~2-methoxybenzyl)-3;5-
dihydro[1,3]oxazolo[4,5-e]pyridine-2;4-dione, 5-(Z.chloi~benzyl)-5,6,7,8,9,10-
hexahydto-
2H-cyclohepta[b][1,3]oxazolo[5,4-d]pyridine.-2,4(3Fi)-dione;, S-[2-
(ditluoromethoxy)benzyl]-
3,5-dihydro[1,3]oxazolo[4,5-c]pytidine-2,4-dione, 7-methyl-5-[(1R)-1-
phenylethyl]-3,5-
dihydro[1,3]vxazolo[4,5-c]pyridine-2,4-dione, 5-(4-chlorobenzyl)-7-propyl-3,5-
dihydto[l,3joxazolo[4,5-c]pyridine-2,4-dione, 5-[2-(methylsulfouyl)benzyl]-3,5-
di'hydro[1,3]oxazolo[4,5-a]pyridine-2,4-diore, 5-(2,6-dimethylbenzyl)-3,5-
dihydro[I,3]oxazolo[4,5-c]pyridine-?,4-dione, 3-ehlom-2-j(2,4-dioxo-2,3- V
dihydm[1,3]oxazolo[4,5-c]pyridin-5(4H)-yl)methyl]benxoaitcile, 5-(2-chloro-6-
,,.
methylbenzyl)-6,7-dimethyl-3,5-dihydro[I;3]oxazolo[4,S.e]pyridine-2,4-dione, 2-
[(2,4-dioxo-
2,3-dihydro[l,3Joxazolo[4,5-cjpyridin-5(4H)-yl)methyl]ber~zonitrile, 5-(2-
chloro-6-
tnechoxyhenzyl)-7-methyl-3,5-dihydm[l,3joxazolo[4,5-c~pyrldine-2,4-dione, 5-[3-
Y.:.
(methylthio)beazyl]-3,5-dihydro[I,3joxazolo[4,5-cjpyridine-2,4-dione, S-(2-
chlorobezszyl)-7
cyclopropyt-3,5-dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(3-
chlorobenzyl)-7-mettryl
3,5-dihydro[I,3]oxazoIo[4,5-c]pyridine-2,4-dione, 5-(2,6-dichlorobenzyl).'7-
methyl-3,5
dihydro(1;3]oxazolo[4;5-c]pyridine-2,4-dione, 7-methyl-5-(4-methylbeazyl)-3,5-
dihydro[1,3]oxazolo[4,~-cjpyridine~2,4-dione, ~-(3,~-dimethoxybettzyl)-7-
methyl-3,5-
dihydro[i,3]oxazolo[4,5-a]pyridine-2,4-dione, 5-(2,6-difluorobenzyl)-7-methyl-
3,5-
dihydm[I,3]oxazolo[4,S-c]pyridine-2,4-dione, 5-[3-(methylsulfonyl)benzyl]-3,5-
dihydro[1,3]oxa2olo[4,5-c]pyridine-2,4-dione, 5-(2-chloro-6-ethoxybenzyl)-3,5-
dihydro[1,3]oxszolo[4,5-c]pyridine-2,4-dione, 5-(2-chloxo-6-ethoxybenzyl)-7-
methyl-3,5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(2-fluoro-6-methoxybenzyl)-
7~methyl-3,5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(2-chloro- !i-methoxybenzyl)-7-
propyl-3,5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(5-chloro-3-fluorobenzyl~-7-
methyl-3,5-
2S dihydto[I,3]oxa2oto[4,5-c]pyridine-2,4-dione, 5-(2-chlorobenzyl)-7-
isopropyl-3,5-
dihydm[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-{5-fluoro-2-methylbenzyl)-7-
methyl-3,5-
dihydro[1,3]oxazolo[4,5-o]pyridine-2,4-dione, 7-methyl-5~-[{1S)-I-phenylethyl]-
3,5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(2-chloro-5-isopropoxybemzyl)-
7-methyl-
3,5-dihydro[1,~]oxazolo[4,S-c]pyridine-2,4-dione, S-(5-acetyl-2-methoxybenZyl)-
3;5-
dihydro[1,3]oxazolo[4,5-c]pyridine-2,4~dione, 5-(2-chlofobenzyl)-'7-methyl-3,S-
dihydm[1,3]oxazolo[4,5-d]pyridazine-2,4-dione, S-[2-fliforo-6-
(triiluoromethyl)benzyl]-7-
CA 02366800 2002-O1-07
-29- ;7:.
methyl-3,5-dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(2-chloro-6-
methylbenzyl)-
5,6,7,8-tei:ahydro-2H-cyclopenta[b][1,3]oxazolo[5,4~]pyridine-2,4(3H)-dione, 5-
(Z-chloro-
6-ethoxybenzyl)-7-ethyl-3,5-dihydro[1,3]oxazolo[4,S-c]pyridine-2,4-dione, 5-(2-
ohloro-6-
propoxybetszyl)-7-methyl-3,5-dihydro[1,3]oxazolo[4,5-c]pyridine-Z,4-dione, 5-
(2-chIoro-6-
isobutoxybenzyl~7 lasthyl-3,5-dihydro[l,3Joxa2oloj4,5-a3pyridine-2,4-dione, 5-
(2-chloro-6-
ethoxyben2y1~5,6,7,8-tetrahydro-2H-cyclopenta[b][1,3]oxaz~alo[5,4-d]pyridine-
2,4(3H)-
dione, 5-(2-chlom-6-isopropoxybenzyl)-7-methyl-3,5-dihyd=o[1,3]oxazolo[4,5-
c]pyridine-
2;4-dione, 5-[2-chloro-6-(2,2,2-trifluoroethoxy)benzyl]-7-methyl-3,5-
dihydra[1,3]oxazolv[4,5-c]pyridine-2,4-dione, 5-(2-chloro~6..ethoxyben~zyl)-7-
methyl-3,5-
.. 10 dihydro[I,3]oxazolo[4,5-d]pyridazine-2,4-dione, 5-[2-chloTO-6-(2-
methoxyethoxy)benzyl]-
5,6,7,8-tetrahydzo-2H-cyclopenta[b][1,33oxazolo[5,4-d]pyridine-2,4(38)-dione,
5-(2-cbloro-
6-ethoxybenzyl)-6,7-dimethyt-3,5-dihyrlro[1,3]oxazolo[4,5-c]pyridine-2,4-
dione; 5-(2-chloro-
6-ethoxybenzyl)-7-ethyl-6-methyl-3,5-dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-
dione, 5-(2-
chlombenzyl)-7-ethyl-3,5-dihydro[1,3]oxaaolo[4,5-d]pyridaxine-2,4.dioae, 5-(2-
chloro-6-
ethoxybertzyl)-7-propyl-3,5-dihydro[ 1,3]oxazolo j4,5-c]pyridine-z,4-dione, 5-
(2-chloro.:6-
ethoxybenzyl)-7-eyciopropyl-3,5-dihydro[1,3]oxazal0[4,S~;c,],pyrtdine-2,4-
dione, 5-(2-chloro-
5-propoxybenzyl)-7-methyl-3;5-dihydro[i,3]oxazolo[4,5-a]pyridine:2,4-dione, 5-
(2-chloro-5-
methoxybenzyl)-7-methyl-3,5-dihydrojl,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(2-
chloro-6-
ethoxybenzyl)-6-methyl-3,5-dihydro[1,3]oxazolo[4,5-c]pyttdine-2,4-dione, 5-(2-
chloro-5-
ethoxybenryl)-7-methyl-3,5-dihydro[1,3)oxazolo[4,5-c]pyridine-2,4-dione, 5-[2-
chloro-5-
(piperidin-i-ylsulfonyl}benzyl]-7-methyl-3,5-dihydro[1,3]oxazolo[4,5-
e]pytidine-2,4-dione,
5-[2-chIoro-5-(pyrrolidin-1-ylsulfonyl)benzylj-7-methyl-3,5~-dihydr~[
1,3]oxazolo[4,5-
c]pyridine-2,4-dione, 5-[2-chloro-6-(cyclopentylmethoxy)benzyl]-7-methyl-3,5-
dihydro[1,3]oxa2olo[4,5-c]pyridine-2,4-dione, 5-[2-(benzylaxy}-6-chlorobenzyl]-
7-methyl-
ZS 3,5-dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-dione, 5-(2,3-dichloto-6-
ethoxybenzy1~5,6,7,8-
tetrahydro-2H-ayclopanta[bj[1,3]oxazolo[5,4-d]pyridine-~4(3I-I)-dione, 5-[2-
ehloro-5-
(triflnoromethyl)benzyt]-7-methyl-3,5-dihydro[1,3~oxazolo[4,5-c}pyredine-2,4-
dione and 5-
(2-chloro-5-fIuorobenzyl)-7-methyl-3,5-dihydro[1,3]oxazolo[4,5-c]pyridine-2,4-
dione,
Derivatives such as esters, earbamates, aminals, asi~ic3es, optical isomers
and pm-drubs
are also contemplated.
CA 02366800 2002-O1-07
-30-
The present invention also relates to pharmaceutical compositions comprising a
physiologically acceptable diluent and at least one compound of the present
invention.
The present invention further relates to a process of inhibiting the binding
of
aa(3i integrin to VCAM-1 comprising exposure of a cell expressing aa~t
integrin to a cell
expressing VCAM-1 in the presence of an effective inhibiting amount of a
compound
of the present invention. The VCAM-1 may be on the surface of a vascular
endothelial cell,
an antigen presenting cell, or other cell type. The exa~il. may ~e on a white
blood cell such as a
monoeyte, lymphocyte, granulocyte; a stem cell; or any other cell that
naturally expresses
a4~1~
The invention also.provides a method for treating disease states mediated by
otd(3~ binding which comprises administration of an effective amount of a
compound of the
present invention, either alone or in formulation, to an afflicted patient.
Detest '~ sct~i lotion of t1 a Tnvention
Definitions of Terms
The term "alkyl" as used herein, alone or in combi~iat'ton, refers to C~-G~z
straight or
branched, substituted or unsubsEi2uted saturated chain radicals derived from
saturated
hydrocarbons by the removal of one hydrogen atom, unless the term alkyl is
preceded by a
Cx-CY designation. Representative examples of alkyl groups iinclude methyl,
ethyl,
n-propyl, iso-propyl, n-butyl, sec-butyl, iso-butyl, and tart-butyl among
others.
T'Itte term "alkenyl" as used herein, alone or in combination, refers to a
substituted or
unsubstituted straight-chain or substituted or unsubstituted branched-chain
alkenyl radical
containing from 2 to 10 carbon atoms: Examples of such iadicals include, but
are not limited
to, ethenyl, E- and Z-gentenyl, decenyl and the like.
The term "alkynyt" as used herein, alone or in combination, refers to a
substituted or
unsubstituted straight or substituted of unsubstituted branched chain alkynyl
radical
containing from 2 to 10 carbon atoms. Examples of such' radicals include, but
are trot limited
to ethyrtyl, propynyl, pcopargyl, butynyl, hexynyl, decyrtyl and the like.
The term "lower" modifying "alkyl", ''alkenyl", "alkyrryl" or "alkoxy" refers
to a C l-
Cbunit far a particular functionality. For example lower alkyl means C1-C6
alkyl.
CA 02366800 2002-O1-07
-31-
The term "aliphatic acyl" as used herein, alone or in combination, refers to
radicals of
formula alkyl-C(O)-, alkenyl-C(O}- and allc~myl-C(O)- derived from an alkane-,
alkene- or
alkyncarboxylic acid, wherein the terms "alkyl", ''alkenyl";F~nd
"alkynyl°' are as defined
above. Examples of such aliphatic acyl radicals include, bu,t are not limited
to, acetyl,
prapionyl, butyryl, valeryl, 4-methytvalery' l, acryloyl, crotyl, propiolyl
and methylpropiolyi,
among others.
The term "cycloalkyl" as used herein refers to an aliphatic ring systera
having 3 to 10
earban atoms and 1 to 3 rings, including, but not limited to eyclopropyl,
cyclopentyl,
cyclohexyl, rtorbornyl, and adamantyl among ornate. Cycloalkyl groups can b~
unsubstituted
. . 10 or substituted with one, two or three substituents itxdependently
selected from Lower alkyl,
haloalkyl, alkoxy, thioalkoxy, amino; alkylamino, dialkylanniao, hydroxy,
halo, mercapto,
vitro, carboxaldehyde, carboxy, alkoxycarbonyl and carboxamide.
"Cycloalkyi" includes cis or traps forms. Furthermore, the substituents may
either be
in endo or exo positions in the bridged bicyclic systems:
The term "cyeloalkenyl" as used herein alone or is combination refers to a
cyclic
earbocycle containing from ~ to 8 carbon atoms and one or more double bonds.
Examples of
such cyeloalkenyl radicals include, but are not limited to, cyclopentenyl;
cyclohexenyl,
cyelopentadienyl and the like. '
The term "cycloaikylatkyl" as used herein refers to a cycloalkyI group
appended to a
lower alkyl radical, including, but not limited to cyclohexylmethyl.
The term "halo" or "halogen" as used herein refers to I, Br, CI or F.
The term "haloalkyl" as used herein refers to a lower aIlcyl radical, to which
is
appended at least one halogen substituent, for example chloromethyl,
fluoroethyl,
trifluoromethyl and pentafluoroethyl among others.
The term "alkoxy" as used herein, alone or in combination, refers to an alkyl
ether
radical, wherein the term "alkyl" is as defined above, fixamples of suitable
alkyl ether
radicals include, but are not limited vo, methoxy, ethoxy, n-propoxy, iso
propoxy, ~butoxy,
iso-butoxy, sec-butoxy, tart-butoxy and the like.
The term "alkoxyalkyl" as used herein, refers to Ry-O-R=, wherein Ry is IoWer
alkyl as
3U defined above, and Rz is alkylene (-(C~i2),w) wherein w is an integer of
from one to six.
CA 02366800 2002-O1-07
-32-
Representative examples include methoxymethyl, rnethoxye:~hyl, and ethoxyethyI
among
others.
The term "alkenoxy" as used herein, along or in cozribination, refers to a
radical of
formula alkenyl-O, provided that the radical is got an enol ether, wherein the
term "alkenyl"
is as defined above. Examples of suitable alkenoxy radicals iaciude, but are
not limited to,
allyloxy, E- and Z- 3-methyl-2~propenoxy and the like.
The term "alkyaoxy" as used herein, alone or in combination, refers to a
radical of
formula alkynyl-O, provided that the radical is not an -yaol 'ether. Examples
of suitable
alkynoxy radicals include, but are not limited to, propargyloxy, 2-butynyloxy
aad the like.
The term "earboxy" as used herein refers to -C(O)O-.
The term "thioalkoxy" refers to a thioether radical of formula alkyl-S-,
wherein
"alkyl" is as defined above.
The term "sulfonamido" as used Drain refers to -SOzNHz.
The term "carboxaldehyde" as used herein refers to -C(O)R wherein R is
hydrogen.
The terms "carboxamide" or "amide" as used herein refer to -C(O)NRaRb wherein
R,
and Rb are each independently hydrogen, alkyl or any other suitable
substituent.
The term "alkoxyalkoxy" as used herein refers to R~O~-It,~O- wherein R~ is
tower
alkyl as defined above and R~ is allcylene wherein alkylene is -(CHo)~,-
wherein n' is an integer
from 1 to 6. Representative examples of alkoxyalkoxy groups include
methoxymethoxy,
24 ethoxymethoxy, t-butoxymethoxy among others.
'Ihe term "alkylamino" as used herein refers to ReNH- wherein Re is a lower
alkyl
group, for example, ethyiamino, burylamino, among others:
The term "alkenyIatriino" as used herein, alone or in combination, refers to a
radical of
formula alkenyl-NH-or (alkenyl)zN-, wherein the term "alkenyl°' is as
defined aboYe,
provided that the radical is not an enamine. An example of such alkenylamino
radical is the
allylamino radical.
The tetm "alkynylamino" as used herein, alone or in combination, refers to a
radical
of formula sikymyi-NH or (alkynyljtN- wherein the termw,"alkynyl" is as
defined above,
provided that the radical is not an amine. An example of such alkyaylatriino
radicals i$ the
propargyI amino radical.
The term "dialkylamino" as used herein refers to R fP;gN- wherein RP and Rs
are
CA 02366800 2002-O1-07
_33.
s .::
independently selected from lower alkyl, for example diethylatnino, and methyl
propylamino,
among others.
The term "alkoxycarbonyl" as used herein refers to an alkoxyl group as
previously
defined appended to the pmrent moiacular moiety through a carbonyl group.
Examples of
alkoxycarbonyl include methoxycarbonyl, ethoxycarbonyl; and isopropoxycarbonyl
among
others.
The term "aryl" or "aromatic" as used herein alone' or in combination refers
to a
substituted or unsubstituted carbocyciic aromatic group having about 6 to 12
carbon atoms
such as phenyl, naphthyl, indenyi, indanyl, azulenyl, tluorenyl and
anthracenyl; or a
haterocyclic aromatic group containing at least one endocyclic N, O or S atom
such as furyl,
thienyl, pyridyl, pytrolyl, oxazolyl, thiazolyl, imidazolyl, pyrazolyl, 2-
pyrazolinyl,
pyrazolidinyl, isoxazolyl, isothiazolyI, 1,2,3-oxadiazolyi, I,2,3-tdazolyl,
1,3,4-thiadiazolyl,
pyridazinyl, pyrimidinyl, pyrazinyl, 1,3,5-triazinyl, 1,3,5-trithianyl,
indolizinyl, indolyi,
isoindolyl, 3H-indolyi, indolinyl, benzo[b]furanyl, 2,3-dihydtobenzofiuar~yl,
IS ber~zo[b]thiophenyl, 1H-indazolyl, benzimidazolyl, benzthiazolyi, purinyl,
4H-quinolizinyi,
isoquinolinyl, cinnolinyl, phthalazinyl, quinazolinyl, quinoxalinyl, 1,8-
naphthxidinyl,
ptelidinyl, carbazolyl, acridinyl, pherlazinyl, phenothiazinyl,,
phenoxyazinyI, pyrazolo(1,3-
c)criazinyl and the like. "Aralkyl" and "alkylaryl°' employ the term
"alkyl" as defned above.
Rings may be multiply substituted.
2O The term "aralkyl" as used herein, alone or in combination, refers to an
aryl
substituted alkyl radical, wherein the terms "alkyl" and ''aryl" are as
defined above.
Examples of suitable aralkyl radicals include, but are not lizrAited to,
phenylmethyl, phenethyl,
phenylhexyl, diphenylmethyl, pyridylmethyl, tetrazolyl sneth,yl, furylmethyl,
inlidazolyl
methyl, indolylmethyi, thienylpmpyl and the like.
25 The term "aralkenyl" as used herein, alone or in combination; refers to an
aryl
substituted alkenyl radical, wherein the terms "aryl" and "alkenyI" are as
defined above.
The term ''arylamino" as used herein, atone or in combination, refers to a
radical of
formula aryl-NhI-, wherein "aryl' is as deftned above, Examples of arylamino
radicals
include, but are not limited to, phenylamino(anilido}, naphthlamino, 2-, 3-,
and 4-
30 pyridylatnis~o and the like.
The term "benzyi" as used herein refers to C~IS-CHz-.
CA 02366800 2002-O1-07
-34-
The term "'biaryl" as used herein, alone or in combination, refers to a
radical of
formula aryl-aryl, wherein the term "aryl" is as defined above;.
The term "thiosryl" as used herein; alone or in combiruation, refers to a
radical of
formula aryl-S-, wherein the term "aryl" is as defined above" An example of a
thioaryl radical
is the thiophenyl radical.
The term "aroyl" as used herein, alone or in combina'ti,on, refers to a
radical of
formula aryl-CO-, wherein the term "aryf° is as defined above, Examples
of suitable
aromatic acyl radicals include, but are not limited to, benzoyl, 4-
halobenzoyl, 4-
carboxybenzoyl, naphthoyl, pyridylcarbonyl and the like.
The teen "heterocyciyl'' as used herein, alone or in combination, refers to a
non-
aromatic 3- to 10- membered ring containing at least one enducyclic N, O, or S
atom. The
heterocycIe may be optionally aryl-fused. The heterocycle may also optionally
be substituted
with at least one substituent which is independently selected from the group
consisting of
hydrogen, halogen, hydroxyl, amino, vitro, trifluoromethyl,'trifluoromethoxy,
a11.y1, aralkyl,
alkettyl, alkynyl, aryl, cyano, carboxy; carboalkoxy, carboxyalkyl, oxo,
arylsulfoayl and
aralkylaminocarbonyl among others.
The team "alkylheterocyctyl" as used herein refers to an allkyl group as
previously
defined appended to the parent molecular moiety through a heterocyclyl group,
including
but not limit$d to 2-methyl-5-thiazolyl, 2-methyl-1-pyrrolyl and 5-ethyl-2-
thienyl.
The term "heterocyclylalkyl" as used herein refers to a heterocyclyl group as
previously
defined appended to the parent molecular moiety through an ;alkyl group,
including but not
limited to 2-thienylmethyl, 2-pyridinylmethyl and 2-(1-piperidinyl) ethyl.
The term "heterocycloyl" as used herein refers to radicals of the formula
heterocyclyl-
C(O)-, wherein the term "hetercyclyf° is as defined above.
The term "aminal" as used herein refers to a hemi-aGetal of she structure
RnC(NR;Ri)(NRrR~)- whereizt Rh, R;, RI, Rk and R, are each- independently
hydrogen,
alkyl or any other suitable substituent.
The term "ester-' as used herein refers to -C(O)Ra" wlherein RM is hydrogen,
alkyl or
any other suitable substituent.
The term "carbamate" as used herein refers to compo~uzads based on carhamic
acid
NHZC(O)OI-1.
CA 02366800 2002-O1-07
-3S-
The term "optical isomers" as used herein refers to compounds which differ
only in
the stereochenustry of at least one atom, including enantioiriers,
diastereorners and racemates.
Use of the above terms is meant to encompass substituted and unsubstituted
moieties. Substitution may be by one or more groups sucl~~ a~3 alcohols,
ethers, esters,
~r..
'amides, sulforses, sulfides, hydroxyl, vitro, cyano, carboxy;~y'a~mines,
heteroatoms; lower
alkyl; lower aIkoxy, lower alkoxycarbonyl, alkoxyalkoxy, ac~,yloxy, halogens,
trifluoromethoxy, trifluocomethyl, alkyl, aralkyl, alkenyl, alkynyl, aryl,
cyano, carboxy,
carboalkoxy, carboxyalkyl, cycloalkyl, cycloalkylalkyl, heterocyclyl,
alkytheterocyclyl,
hetetocyclylalkyl, oxo, arylsulfonyl and aralkylaminocarbonyl or any of the
substituents of
,. 1~ the preceding paragraphs or any of thosa substituents either attached
directly or by suitable
linkers, The linkers are typically short chains of 1-3 atoms containing any
combination of
-C-, -C(O)-, -NH-, -S-, -S(O)-, -O-, -C(O)O- or -S(O}O-. Rungs may be
substituted
multiple times.
The terms "electron-withdrawing" or "electron-donating" refer to the ability
of a
substituent to withdraw or donate electrons relative to that 'of hydrogen if
hydrogen occupied
the same position in the molecule. These terms are well-understood by one
skilled in the sit
and are discussed in Advanced OrLanic Chemistry by J. March, 1985, pp. 36-18,
incoipotated
herein by reference. Electron withdrawing groups include halo, ttitro,
carboxyl, lower
alkenyl. lower alkynyl, carboxaldehyde, carboxyamido, aryl, quaternary
ammonium,
trifluoromethyl, sulfonyl and aryl lower alkanoyl among others. Electron
donating groups
include such groups as bydroxy, lower alkyl, amino, loweralkylarriino,
di(lower alkyl)amino,
aryloxy, tnercapto, lower alkylthio, lower alkylmercapto, a.~d disulfide among
others. One
skilled in the art will appreciate that the aforesaid substitueists may have
electron donating or
electron withdrawing properties under different chemical conditions. Moreover,
the present
invention contemplates any combination of substituents selected from the above-
identified
groups,
The most preferred electron donating or electron withdrawing substituents are
halo,
vitro, alkanoyl, caacboxaldehyde, ary-lalkanoyl, aryloxy, carboxyl,
carboxamide, cyano,
sulfvnyl, sulfoxide, heterocyclyl, guanidine, quaternary ammonium, lower
alkenyl; lower
alkynyl, sulfonium salts, hydmxy, lower alkoxy, lower alkyl; amino, lower
alkylamino,
di(lower all'yl)amino, amine lower alkyl rnercapto, mereaptoalkyl, alkylthio,
czrboay lower
CA 02366800 2002-O1-07
-36-
allyl, arylalkoxy, alkanoylamitio, allanoyl(lower alkyl)amino, lower
alkylsufonylamino;
arylsuifonylamino, alkylsulfonyl(lower alkyl)amino, arylsulfon~rl(l.ower
aikyl)amino, lower
alkylcarboxamide, di(lovi!er allyl)carboxamide, sulfonamide, lower
alkylsuifonamide;
di(fower alky?)sulfonamide, lower alkylsulfonyl, arylsulfonyl and alkyldithio.
As used herein, the team "composition" is intended to encompass a product
comprising the specified ingredirrnts irr the specified amounts, as well as
any product which
results, directly or indirectly, from a combination of the specified
ingredients in the specified
amounts.
As used herein, the term "mammals" includes humans and other animals.
The ring defined by Y in Formulae I, II and ff! can be a mono-cyclic
heterocycle or
aromatic ring, or can be a bicyclic ring.
The dotted lines used in Fornnulae I; B, I>I, N and VI'indicate that the bond
at that
Ioeation can be either single or double. The bond between the atoms Yaxad W
for example
can be a single or double bond if Y and/or W is a substitutent~s,uch as N, C
or CH. Therefore,
l 5 the ring defined by Y in the Formulae can ba either saturated or
unsaturated, depending upon
which W and/or Y is selected. In Formulae N and YT, the dotted line indicates
that the
nitrogen -containing ring optionally contains double bonds at the indicated
locations.
In the Formulas, certain R erour; potentially substitute their associated
rings a
number of times. R~9, R=°, R=~, R~', R'', Ri8 , R=9 and R=$ may each
substitute their
24 associated rings rrzore than once. For example for R~9, when c is zero, the
associated ring is
unsubstituted, having hydrogens at the C-2 and C--4 positions; and for Rz3,
when g is zero,
hydrogens are at the C-2 = C-5 positions.
Suitable substituents for the aryl, alkyl, cycloaikyl, heterocyclyl groups or
the ring defined
by Y and W in the formulae described above, when present, include alcohols,
amines,
25 hetematorns, or any combination of aryl, alkoxy, alkoxyalkoxy, alkyl,
cycloalkyl or
heterocyclyl groups either attached directly, or via suitable linkers. T'he
linkers are typically
short chains of 1-3 atoms containing any combination of C, C =O, C02, O, N, S,
S=O, SO2, as
for example ethers, amides, amines, areas, sulfamides, sulfonamides, among
others. __,
For example, R~, RZ, R3, R3, R6, R7 and Rs in the above formulae may
independently
30 be, but are not limited to: hydrogen, alkyl, phenyl, thienylmethyl,
isobutyl, n-butyl, 2-
thienylmethyl, 1,3-thiazol-2-yl-methyl, benzyl, thienyl, 3-pyridinylmethyl, 3-
methyl-1-
CA 02366800 2002-O1-07
_37_
.,;
benaothiopheir2-yl, allyl, 3-methoxybenzyl, pmpyi, 2-ethoXyethyi,
cyclopropylmethyl,
benzylsulfanylmethyl, benzylsulfonylmethyl, ghenylsulfanylmethyl,
phenethylsulfanylmethyl, 3-phenyIpropylsulfatsylmethyl, 4-((2-
toiuidinocarbonyi)amino)benzyl, 2-pyridiz~ylethyl, 2-(1H-indol-3~yl)ethyl; 1Fi-
benzimidazol-2 y1, 4 piperidinylmethyl, 3-hydmxy-4-methoxybenzyl, 4-
hydroxyphenethyt, 4-aminobenzyl, phenylsulfonylmethyl, 4-(acetylamino}phenyl,
4-
methoxyphenyl, 4-aaiinopheayl, 4-chlorophenyl,
(4=(ben~ylsulfonyl)amiao)phenyl, (4-
(methylsuifonyi)aminojphenyl, 2-aminophenyl, 2-methylpilenyl, isopropyl, 2-oxo-
1-
pymolidinyl, 3-(rtaethylsulfar~yl)propyl, (propylsuifanyl)methyl,
oclylsulfanylmethyl, 3-
aminophenyl, 4-((2-toluidinocarbonyl)amino}phenyl, z-
((inethylberizyl)amino)benzyl,
methylsulfanylethyl, hydroxy, chloro, fluoro, bromo, ureido, amino,
methanesulfonyiamiuo, acetylamino, ethylsulfanylmethyl, 2-ehlorobenzyl, 2-
bromobenzyl; 2-tluorobetiayl, 2-chloro-6-$uorobenzyl, 2-chloro-4-fluorobenzyl,
2,4-
dichiorobenzyl, 2-chIoro-6-methoxybeinayl, 2-cyanobenzyl, 2,6-difluorobenzyl,
2-chIoro-
1S S-(trifluoromethyl)benzyl, 2-chloro-6-methylbenzyl, 2,6-dirnethoxybenzyl, 2-
chloro-5-
(methylsulfonyl)benzyl, 2-chioro-6-cyanobenzyl, Z-chloro-6-ethoxybenzyi, 2-
chloro-5-
methoxybenzyl, ~-chlom-5-fluorobenzyl, ~-chloro-2-flnorobenzyt, ethyl, propyl,
buryI,
penryl, cyclopropyl, tert-burylamino, propylarnino, ~t-methyl-I-piperazinyI, I-
azetidinyI,
4-morpholino, (4-carboxyphenyt)amino, pivaloylamino, ((tert-
24 butylamino)carbonyl)amino, trifluoromethyl, benzyloxy, 2-(2-
methoxyethoxy)ethoxy, 2-
(Z-(2-methoxyethoxy)etho~cy}ethoxy and 2-(2-(Z-(2-
methoxyethoxy)ethoxy)ethoxy)ethoxy.
The R' substituenc for the formulae above may be, but is not limited to 1,3-
benzodioxol-5-yl, 1-naphthyl, thienyl, A-isobutoxyphenyl, 2,b-dimethytphenyl,
,
25 allyloxyphenyi, 3-bromo-4-methoxyphenyl, 4-butoxyphenyl, I-benzofuran-2-yl;
Z-
thienylmethyl, phenyl, methylsulfanyl, phenylsulfanyl, phenethylsulfanyl,
4.bromo-2-
rhienyl, 3-methyl-2-thienyl, 4-methylphenyl, 3,5-bis(methyloxy)phenyl, 4-
(methyloxy}phenyl, 4-fluorophenyl, 3-(methyloxy~henyl, 3,4,5-
tris(methyloxy~henyl, --
2,3-dihydro:I-benzofuran-S-yl, 3-fluorophenyl, 4-(erifiuoromethyl)phenyl, 4-
fluoro-3-
30 (trifluoromethyl)phenyl, 4-(1,1-dirnethyiethyi)phenyl, 3,5-dimethylphenyl;
4-
hydroxyphenyl, 3,4-dimethylphenyl, 3-methyl-4-(methyloxy)phenyl, 4-hydroxy-3-
CA 02366800 2002-O1-07
-38-
methylphenyl, 3-methylphenyl, 2,3~dihydro-inden-5-y!, 2-methylphenyl, 2,6-
bis{methyloxy~henyl, 2;6-dihydroxypherryl, 4-chloropheayl, 3-chlorophenyl,
3,4.
dichiorophetryl, 4-((ttifluoromethyl)oxy)phenyl, 4-ethylphenyl, 4-
(ethyloxy)phenyl,
methyl, 2 propyl, 4,5-dihydro-1,3-oxazol-2-yl, 3-(trifluotomethyl)ph~nyl, 4-
(trifluoramethoxy)phenyl, 2,3-dihydro-1,4-benzodioxin~6-yl; 7-methoxy-1,3-
benzodioxol-S-yl, 3-ethoxy-4-methoxyphenyl, 3,4-dimethoxyp~henyl, 3,4-
diethoxyphenyl,
3-ethooyphenyl, 3-merhoxy-4-methylphenyl, 3,S-dimethoxy-4~methylphenyl, 3-
propoxyphenyl, 3 butoxyphenyl, 3-(2-methoxyethoxy)phe~ayl; 3,4-dipmpoxyphenyl,
3-
(diftuoromethoxy)phenyl, 2-naphthyl, 3-isopropoxyphenyl, 1-methyl-1H-indol-5-
yl, 2,3-
dihydro-I-benzofuran-5-yl, 1,3-diethyl-2-oxo-2,3-dihydro-1H-~benzimidazol-5-
yl, 3
(trifluoramethoxy)phenyl, 1-methyl-1H-indol-b-yi, 3-(cycloprapoxy)phenyl; 3
(cyclopropylmethoxy)phenyl, 3-(difluoromethoxy)phenyl, 3-(1,1,2,2-
tetrafiuoroethoxy)phenyl, 1-ethyl-IH-indol-5-yI, 3-(diethylaminaJphenyl, 6-
methoxy-2-
naphthyl, 3-[(methylsulfonyi)amino]phenyl, 3-
[methyl(methylsulfonyl)amino]phenyl, 3-
Methyl(methylsulfonyl)amino]phenyl, IH-indol-5-yl, 3-fluoro-4-methoxyphenyl
and 3-
(difluoromethyt)phenyl.
Two independent Rt, R'', Rl or R3 groups taken together may be linked to form
a
sing.
R4 and R~1 may be linked to fornt a ring such as 1-pycTOlidino, 1-piperidino,
4-methyl-1-
24 piperaaino, 4-acetyl-1-piperazino and 4-mozpholino among others.
R9 and Rl° may be linked to forth a ring such as cyclopropyl,
cyclobutyl, cyclopentyl,
and cyclohexyl among others,
Abbreviations
Abbreviations which have baen used in the schemes and the examples which
follow
ZS are: BOC for t-butyloxycarbonyl; DMF for dimethylformamide; THF far
tetrahydTO~uran;
DME for dimethoxyethane; DMSO for dimethylsulfoxide; NlvIM for N-methyl
morpholine; D1PEA for diisopropylethylamine; CDT for 1,1'-carbonyldiimida2ole;
TBS
for TRtS-buffered saline; Ms for methanesulfonyi, TMEDA for N,N,N',N'- ~--
tetrarnethylethylenediatnine, DCE for 1,2-dichloroethane, NCS for N-
ehtorosuccinimide,
30 NBS for N-bromosucciaimide; DPPA for diphenylphosphorylazide, DEAD for
diethyl
azodicarboxylate, m-CPBA for 3-chloroperoxybenzoic acid, TFAA for
trifluoroacetic
CA 02366800 2002-O1-07
-39-
anhydride, DCM for dichlororrtethaae, LHMDS for lithium
bis(trimethylsilyl)arnide and
Cbz for benzyloxycarboteyl. Amino acids are abbreviated as follows: C for L-
cysteine; D
for L-aspartic acid; B for L-glutamic acid; G fot glycine; H for L-histidine;
I for L-
isoleucine; L for L-leucine; N for L-asparagine; P for Lproline; Q far L-
glutarniae; S for
S L-serine; T for L-thseonine; V for L-valine and W for L-tryptophan.
Examples of the procedures that may be used to synthesize compounds of the
Formulae described above are shown in the Schemes which follow, A detailed
description of the representative compounds of the present invention is set
forth in the
Examples below.
. . 10
CA 02366800 2002-O1-07
Scheme 1 below illustrates the pracedure described in example 1.
~ NOZ ~ NHZ Trimethylacccyl
Hz,1PdlC _ ~ chtorid~ ''~ \(
N~ O~' MrOH N Oi NEt;, DCM ~ O
1 2 3
a) n.BuLi, TMEDA
THF ~ W w . ~ w
b) t-Butt N ,i ~ Kl, FiOA ~ HN I NH
c) Echyliodide .
i0
KHMDS, THF / I / ~ 6M HCI ~ I
N
NH .~_...,.. W N NHa
f~$r CI O Q~~ CI O
C ''t
6 7
DCE Cl'' DtPEA / COOEt a) vtaOH, TEIF,
b) COOED ' O - 1"t,
w I ~ I N-~ V -.:
b) HCI
C1 p H H I
9
O COON
\ I ~ ( N~N \
ci v ~ H I
Scheme 1
CA 02366800 2002-O1-07
-tl 1 _
Saherne 2, illustrating the procedure of Example 2, is shown below,
CH K t:0 , Bn~r
t S xn, sat.NH,~CI
RN~N02 /,c~tone, ratiux ~' ~ MeOH 0 °C
0 w
11
12
s) coCl$, otpeA,
CHadg
b) cooet \ I N~o~ cco~t
N. N
H H I
a ~
13 1d
2N NaO~ ~ / p O G00H
Tt~IEIMeON, t.t ~ I N I _
O N H I
16
Scheme 2
CA 02366800 2002-O1-07
-42-
Scheme 3, illustrating the procedure of Example 3, is shown LRelow.
i ~H NaH, DMF i / OH P0C13
t
HN NO= CI _ \ N NOZ ,~ . TO °C
o ~ W 0
11 18
I N ~ CI N~ / i NHS 7Cn, sat'd aq. NN,~CI
~1N02 MeOtl, 6S °C w N N0z Me0tl, 85 °C
f
Ci 0 C! 0
1T
t8
NHz NMM, DMf~ SD °C ,, ,, NN~
~ I o I ~ f
Ci N O NN O~N ~ ~ OJOL,N OEt CI N d N H I %
19 ~ ~ Zt
O
NaOH, H20 ' I N I NH~ OH
THF, MeOH ~
" H H I \
cr o ,.
22
Scheme 3
5
CA 02366800 2002-O1-07
-43-
Scheme 4, illustrating the procedure of Example 4, is shown below.
OH N~, DMF, 5S °~ ~ j ~ , OH 11GZC03, MeI
C1 \ N NO~ Aoeton
~NOx
p CI CI O
2S R4
W ~ N
NOz
C)
2S
Scheme 4
Scheme 5, illustrating the procedure of Example 5; is shown below.
a) ~-Bu~i! 'fMSDA, .~ F
N THF
N~ .
N fl ~ b1 ~phSOz)2NF NH
,O
3 f 2S
. Scheme 5
Scheme 6, fllustratfng the procedure of Example 6; is shows below.
a) THF 1.~' TMEDA, ~ ' C~ ._.
N~
~ N o ~ d) NCS NH
~ iD p~
n~ ~
I5 Scheme 6
CA 02366800 2002-O1-07
Scheme 7, illustrating the procedure of ExamplE 7, is shown below.
a~ THF u' TMEDA, ~
N I
~NN
.r~ p~
Z I'
8
Scheme 7
Scheme 8, illustrating tha procedure of Example 8, is shown below.
I ~ I OH Zri~ sat'd aq, NHqCI ~ I ~~OH CDl, DMF
W N TIpZ MeOH, 65 °C ~Y~'r~NH2 7~ °C
CI a CI O
24 Z~
I N I °~o °~~, ~o °c :- , ,~ I ou~ co2~t
N w
Ct O H OEt CI O H H ( r
S0 ~_~ ~ ~~ 31
g
NaOii, ifg0, ~ ~ OHO COZH
TNF~ Met~hl \ I N~N~N
t'
CI O H H I
S$
Scheme 8
CA 02366800 2002-O1-07
-45-
Scheme 9, itlustratiag the procedure of Example 9, is shown below.
~ l
r PhB(OI~I)2r KaP~a
~NH DMPr H=0; pd(PPh$)a N NHa
CI p~
S3
3b
Scheme 9
CA 02366800 2002-O1-07
-46-
Scheme 10, illustrating the ptocedure of Example 10, is shown below.
~H H=N~ NH
Me N
Me 0Me Ms0tl, 50°C N [ ~'., ;
SS 36
H~Y H
Meo ~ 0Me $s~ MeOl~t, refiWx
Me0 i~OMe EtON, pyridine,
p~pe~iaine,reflux 0 0
3T 38
N NBS, (PhCO~jZ, ~ N Na041, N~0
CCI4e KxCCl~ ' ~ N ~ ?HF
C00Me ~ C00Me
0 , O
ao
N a) DPPA, Et~~i, CsHp N C00Et
C00Et \
C00H HzN ~ ~ \' O H tNl
d4 g 42
NaoH, H=o ~. ~N p C00H
THP ~ I' [
w N H~"~~ [ ~
43
Scheme 1'4
CA 02366800 2002-O1-07
Scheme 11, illustrating the procedure of Example 1I, is shown below.
H
Et0 M~H 1 / / . N
Ms0 ~ 0Me $C ~ ' N ~ CpOMe
Et0fl, PY~'ldlne, p'
44 PlPgrldlre~ reflex 4$
Scheme 11
Scheme 12, illustratinS the procedure of Example 12, is shown below.
1
O~NH
O~N~
E , ~ NNZ Nail, EtNCO / ~ / ~ .NN
w N~NO= TNF, O °C, \ N~'NOx
G! O warm to RT C! O
48 4T
Scheme 12
Scheme 13, illustrating the procedure of Example 13, is shown below.
w ~i w CI w
i 011 ~l ~ ~ i ON
HN~ NaH, DMF, 55 °C ~ ( N
j[ N0s ~ -NOZ
0 CI O
48 d8
Scheme 13
CA 02366800 2002-O1-07
_Q.8.
Scheme 14, illustrating the procedure of Example 14, is shown below.
~NH2
I r Ct ~ I H t
~N CHO.
K0~0Et EDC~ Ct N o o OEt A'oN, EtOH
~I I~ Reflex
51 52
l l NaOH, TN_E l
w 1 N I 0Et MeOH, NZ0 ~ I N I 0H
CI 0 C( t~ 0
$$ 54
a) DPP4, o~Pt:A,
TIiF, Reflex ~ / . O C00Nt N$0ii T_HF
bj C00Bt ~, f N~N,.(~N w Me01~1, NZO
~2N l l CI O H H I r
8 55
0 COOH
w I N~N~N w
CI p H H I
S8
Scheme 14
CA 02366800 2002-O1-07
-49-
Scheme 15, illustrating the procedure of Example 15, is shown below.
s) MsCI, NEt3,
'S/ O~ CHZCI= / 1 ~ ~ Fe, A~eOH, 60 °C / ~ ~
b) ~ S N~ldOZ ~ S N~NHZ
N~ Npy O O
off 58 59
a} COCIa, DIPEA, COOEt
DCM S ~ O a) NaOH, THF,
/~ N L ~ O HZO, Me0>;
b) COOEt ~~ N N ~ b) HCi
HN O O H H
a ~ 7 61
O
COOH
S i O~~
/i ht~N~N W O
O H H
O
62
Scheme I5
Scheme 16, illustrating the procedure of Examplz I6, is shown below,
a) H=, PcVC, MeOH
O CD~F03, MeI O b) 2-Thiophenecarboxaldehyde
CbzHN~~OH CbzHN~~0141e ~'aaH(OAc)~, CtCHzCI-t~Cl
NHBoc NHHoc
63
COOEt
S ~ HCt, Dioxane a) CDI, CHZCI,, HiN
~.~..5
~N ~ /i ~ NH 60
NHHvc
O
66 ~ b) 66
COOEt COOH
O a) NaOH, THF, ~ ~ p _.
i N~N~N HzO~ i ~N ~ ~ O
IO1 H H ( ~ O) b) HCI ~I
O
67 68
5 Scheme 16
CA 02366800 2002-O1-07
-50.
Scheme 17, illustrating tha procedure of Example 17, is shown below
O s) DMSO, (COCt)a O
a) l HuOCOCt, p
HO~~OBrt 6O HO~OMe b) 69Ch. . .=."r., OHC~''~OMe
O NHBoc NHBoc c) NEt3 NHBoc
69 ~ '70
~,.~ HCt ~"~ a) CDI, CH2CtZ
NHa \ ~ OJ/ NHBoc Dinxane 5 N~~ b) COOEc
a
NaBFI(OAch, ~ O H N O
z
CtCH=CHZCt ~I ~2 O
COOEt OppH
a) NaOH, THF /''~: O
N~ HaO. MeOH 't
~~N~N ~ O
p H H I , ~ b) HCI \ O H H
O ~~ ~ O
T3 74
S Scheme Z7
..,.
CA 02366800 2002-O1-07
-51 _ '
Scheme 18, illustrating the procedure of Example 18, is sho~m below.
CI
i w KNOB, NaNOz ~ ' W
~c2o. err Hc~ w I I ~ Noz
pH OH
99
C1
z~. t~Hda ' I
MeOH, Ha0 ~a
OH
100
C1 ,
COOEt 8) CpC~2~ DtPEA , , COOEt
CICHzCH:Cl \ I \ I O
I r b) 100
OFi ~ H ~ \
=g 10:
Scheme 18
Scheme 19, illustrating the procedure of Example 19, is shown below
O 0
N i ~ NH: N
rte
~\ / o
t oz
Scheme 19
CA 02366800 2002-O1-07
-52-
Scheme 24, illustrating tha pTOCedure of Example 20, is shown beiow.
~OH / I ,
N
NOz --~-.. ~ NO=
OH CH2CIa O
103
Scheme 20
Scheme 2I, illustrating the procedure of Example 21, is shown below.
NOZ g ~' NOZ
CHO ~ TFAA,
/, TES
DCM
NH NCH{OAajs, DC$, HN
meleculer sieves
1Q4
Nvz NH2
. ,~
Fe, AcOH, EtOH ~ /
~ S
FaC N \ F9C N
0
O
Los io6
Scheme 2I
CA 02366800 2002-O1-07
-53-
Scheme 22, illustrating the procedure of Example 22, is shown below.
0
s . Merrtho! e~ LIiMDS, THF I \ ~ S ~N~ I \
/O
I b. OHC ~ p ~ ~O
108
F F CO2Et
Hr~COzEt a) Zn, THF ~ ' . TFA, MeOH
bllos 1 ~ S'H
F F
/ O.
109
F F COZEt
H2N ~
f/
O
110
Scheme 22
Scheme 23, illustrating the procedure of Example 23, is shown below:
a) n-BuLi, TMEDA, THF
b) t-BuLi \ r1
( ~ .O °) ~ 8r I ,
N p . Br ~ Op
i1!
Scheme 23
Scheme 24, illustrating the procedure of Example 24, is shown below.
COOH
~, O COOEt BBr / ~ p
\ N N ~ N \ CH_Ctz \ 1N H ~ H I \
3
Cl O H H I / C( ~ ~ OH
IlZ OMe
113
Scheme 24
CA 02366800 2002-O1-07
-54.
Scheme 25; illustrating the procedure of Example 25, is shown below.
a) NaH, TMEDA, OH O O I , ' NHa
O
~ ~~ ?IiF, -20 °C ~ ~ Cl
~~'~OMe n- a i~ ' 0'~ ONde
c) HCOOMe, -20 °C '~ MeOH Reftux
,
114
/ / OH NaNOZ, F1r103, / / t?H a) Zn, Et3N~HC1
I N~ AcOH, Ii20, R? ~ I N Il DMF, 35 °C
Cl 'O' CI O~~NO b) CDI, 80 °C
179 116
O THF, 55 °C ~ , O 0 C02Et
\ ~ N I N~O 4 \ ' N ~ N~N
CI O H H~ N , OEt C, O H H ( /
1I7 g 11.8
NaOH, HyO, / / OHO COzH
THF, MaOH II
w N ~~H I
C1 O
119
Scheme 25
CA 02366800 2002-O1-07
-SS-
Schenne 26, illustrating Example 26 is shown below.
a) KOH; DMSO,
~ci
~HCl ~/' O
O
Hs
z H H
b) 2n, Et3NHCI,
DMF, 80° C o
c) CDI; 80° C
23 ' 120
Scheme 26
Scheme 27, illustrating Example 27, is shown below,
~S ~s~
OH m-CPBA / , OH
w I IV~NO' CHaCIZ ' w j N~vTp=
Ct '~'O~ Cl IIllO
121 122
Scheme 27
CA 02366800 2002-O1-07
-56-
Scheme 28, illustrating Example 28, is shown below.
O , OHO COZt-Bu s) 2nBr2, ~ O ~ O COZH
' ~ N I ~ C~hCl2
N ~ b) HZO '~ N N
G! ~~ H I ~ Ct O H H I ,,
133 f24
Scheme 28
Scheme 29, illustrating Example 29, is shown below.
0 O
CI OH HZ, PdIC, NEt3
RCN ~HCt EtOH
0
12S
OH
~2 Ec3NHC1 ,
O
136
Scheme 29
CA 02366800 2002-O1-07
Scheme 30, illustrating Example 30, is shown below.
~ ' COOEt a) NaN, TNF, 0 °C
COOEt
Toluene, Reflux~ / ' NH b) AcCI, RT
O (-fh0)
c! lay
~COOEt
LEiMDS, THF ,,. / OH
/ 1 N ~ 0 °C, warm to RT \~ N
O
C! C1 O
1~ 1 Z9
Scheme 30
Scheme 31, illustrating Example 31, is shown below.
O~ NH
NH2 a) NaH, THF, 0 °C l ' / ' NH
W N NOz b) ~-$uNCO, warm W N~NO
to RT
c! o c! o
4G 130
Scheme 31
CA 02366800 2002-O1-07
Cl
oxo cooEt , oHO cooEt
\ '' N I ~ S02C1Z, CHzCh ~ ( ~
H I ~ \ N~H~~. I
C1 O i CI O
31 131
Scheme 3~
Phi N' Li COOt-Bu
c-Bu00C / \ Ph'J'''~ ~' \
.,~~ ~ J'',I/'
( Ph N
THF, -78 °C, Ph Br
warm to RT 13Z
Me COOL-Bu
tHOy,s w
Ph~N ~ OMc H;, Pd/C
Mz0 ~ I ~ EtOH, HOAc ~
PdCl2(PPh3h, K3POa~ Ph~ ,,~~~ (
DME, Reflux 133 . VteO
COOt-Su
~izN I ~ oMe
Me0 ( ~
134
S Scheme 33
CA 02366800 2002-O1-07
-S9-
OEt
NaOt-Bu> EtOH OEt CN BH3, TIiF
~ CN T~~ RT i ~ Reflex ~ ' ~ NH2
./ CI / Cl / Cl
135 136
OEt OEt w SOC12~
HOAo, Ac20
N3NOZ [ ~ OAo NaOH I ~ . 'OH CH2C1=
C1 ~ CI
137 138
OEt OEt O
Cl Mep HZ~ ~ ~ NHNHZ -~COOEt
/ RT '~ Cl CHCl3, MgS04, RT
CI
139 140
Et gt
O N " -COOEt _C1COCHZC_OOMa / O N~COOEt NaH, DMF
y I ~f~~ 0°Cto60°C
NH NaH, THF, 0 °C, ~ COOMe
Warm to RT
CI Cl O
141 1d2
Et Et
O N i OH HC1, H~_O, Dioxane / O N i OH
Reflex ~ '~ N
~COOMe
CI O CI O
143 144
Scheme 34
CA 02366800 2002-O1-07
-60-
a) NaH, DMF,
0 °C to RT \ N DMF, POCl3
I -~ r(~O b) BtI, RT I ~ N~O ~ ~0 °C
H
I4S
i46
OHC ~ HOOCCHyCOOH HOOC / \
\ ~~N~
~O Piperidine, Pyridine I , N/-O
N EtOH, Reflux
147 I48
Scheme 35
Et.O
Et
OH O O I ~ C1 ~ O ~ 'OH NaNOz, HN03,
\ ~ ~ 136 \ ( N ~ AcOH, H,O, RT
~~~~OMe
MeOH, Reflux Ci O
ild 149
Et Et
DMF. 55 °C
O , OH a) Zn, $t33~f~HC1 / O / ,O
N J DMF, 55 °C \ ( N I ~O OEc
NOz b) CDl, 80 °C ' H H=N 1
c~ o c! o
iso s
151
Et
p ON COZEt
I / I O NaOH, H20, ~ 0 ~ OHM COZH
w N~ ~, THF, MeOH I I'
II H H I y w .rN~N~N
Cl O / C~ O H H
152 153
Scheme 36
CA 02366800 2002-O1-07
Et fit
O , p DMF, 55 °G / O / OH D CO=Et
I N~O C02Et- ~ I N~N~N
CI O H H=N ~ ~ CI O H H ( /
Z 51 1 sg OEt
OEt
154
Et
NaOH, H O, / O ~ OliO COZH
THF,Me~H~ ~ I N~N~N
CI O H 156
OEt
Scheme 3?
Bt Et
i ~
/ O / O DMF; 55 °C ~ O / OHO COZEt
I N~N O CO=Et \ ( N~N~N \ \
H \ ! j C1 fpl H H I / / OMe
CI O H;N
15i OMe 138
157
Et
i
NaOH, HZO, / O / OF~ CO=t~
THF, MtOH \ ( N
\ \
C! O H ~ I /
139 OMe
Scheme 38
CA 02366800 2002-O1-07
-62-
NaNO , HNO;, ~,, OH , a) zn, Et3N~HCi
/ / AcOH~Hxp. RT_ / , ~ DMF, 33 °C
0
NOZ b~ CDI: 80 C
C1 60
C
129
DNff> 80 °C / OHp COZEt
O COOEt ' N ~ N W
O N
N ~ H2~ y, Cl ~ H H ~ l
C1 O ~ 163 pi-Pr
161 Oi-Pr
162
NeOH> Ha0> ~~ i ONp C02H
TNF~ ' ~
H H I,
CZ O
164
Oi-Pr
Scheme 34
COOEt
COOEt
SO=C12- HiN 1
H=N , / 0 °C to RT
C1
oiPr
OiPr
165
162
Scheme 40
CA 02366800 2002-O1-07
-63-
,. C1 / OH NaNOZ, HOAc / Cl / OH
MeOH, H O
f N 4 z ~ I N ( ~O
0°C toRT
N o I~ N o
166 ~ 167
Scheme 41
EtOOC
Br ~
~COOBt ~ I ~ (MezN)SF3
Pd(OAc)Z P(o-totyl), /
CHO NEt3, DMF> 125 °C
168 169 CHO
EtOOC. COOEt
a) s-HuLi, THF> -78 °C
b) 1?0, THF, -7B °C gh.~'~N
N-H
Ph--~ Ph~ ~ ~,,i
170 CHFZ 1~~ CFIF
COOEt
Hz, Pd/C, AcOH HEN i y
fitOH, 33 °C
171 CHF=
Scheme 42
The compounds of the present invention can be used in the form of
pharmaceutically
acceptable salts derived from inorganic or organic acids. The phrase
"pharmaceutically
14 acceptable salt" means those salts which are, within the scope of sound
medical judgement, ___
suitable for use in contact with the tissues of humans and tower animals
without undue
toxicity, irritation, allergic response and the like end are commensurate.with
a reasonable
benefit/risk ratio, Pharmaceutically acceptable salts are well-known in the
art. For example,
S. M. Berge et al, describe pharmaceutically acceptable salts in detail in J.
Pharmaceutical
CA 02366800 2002-O1-07
-64-
Sciences. 1977, 66: 1 et seq: The salts can be prepared inaicu during the
final isolation and
puriftcanon of the compounds of the invention or separately by reacting a free
base function
with a suitable organic acid. Representative acid addition'salts include, but
are not limited to
acetate; adipate, alginate, citrate, aspartate, benzoate, benzenesulfonate,
bisulfate, butyrate,
camphorate, camphorsulfonate, digluconata, glycerophosphate, hemisulfate,
heptanoate,
hexanoate, fumarate, hydrochloride, hydrobromide, hydroiodide; 2-
hydroxyethansulfonate
(isothionate), lactate, maleate, methanesulfonate, nicotinate, 2-
naphthalenesulfonate, oxalate,
palrnitoate, pectinate, persulfate, 3-phenylgropionate, pierate, pivalate,
propionate, succinate,
tattrate, thioeyanate, phosphate, glutamate, bicarbonate, p=toluenesulfonate
and undecanoate.
Also, the basic nitrogen-containing groups can be quaternized with such agents
as lower alkyl
halides such as methyl, ethyl, propyt, and butyl chlorides;, bromides and
iodides; dialkyl
sulfates like dimethyl, diethyl, dibutyl and diamyl sulfates;! long chain
halides such as decyl,
lauryl, myristyl and stearyl chlorides, bromides and iodides; arylalkyl
halides like benzyl and
phenethyl bromides and others. Water or oil-soluble or dispersible products
are thereby
obtained. Examples of acids which can be employed to form pharmaceutically
acceptable
acid addition salts include such inorganic acids as hydrochloric acid,
hydrobromic acid,
sulphuric acid and phosphoric acid and such organic acids as oxalic acid,
trtaleic acid, succinic
acid and citric acid.
Basic addition salts can be prepared in situ during the final isolation and
purification of compounds of this invention by reacting a carboxylic acid-
containing
moiety with a suitable base such as the hydroxide, carbonate or bicarbonate of
a
pharmaceutically acceptable metal canon or with ammonia or an organic primary,
secondary or tertiary amine. Pharmaceutically acceptable salts include, but
are not limited
to, canons based on alkali metals or alkaline earth metals such as lithium,
sodium,
potassium, calcium, magtesium and aluminum salts and the like and nontoxic
quaternary
amrnonia and amine canons including ammonium, tetramethylamrrionium,
tetraethylemmonium, methylammonium, dimethylammonium, trimethylammonium, ,_,
triethylammonium, diethylammonium, and ethylarnmonium among others. Other
representative organic amines useful for the formation of base addition salts
include
ethylenediamine; ethanolamine; diethanolamine, pipetidine, piperazine and the
like.
CA 02366800 2002-O1-07
-65-
Dosage forms for topical administration of a compound of this invention
include
powders, sprays, ointments and inhalants. The active compound is mixed under
sterile
conditions with a pharmaceutically acceptable carrier aad any needed
preservatives, buffers or
propellants which can be required. Opthalmic formulations, eye ointments,
powders and
solutions are also contemplated as being within the scope of this invention.
Actual dosage levels of active ingredients in the pharmaceutical compositions
of
this invention can be varied so as to obtain an arnount of the active
compound(e) which is
effective to achieve the desired therapeutic response for a, particular
patient, compositions
and mode of administration. The selected dosage level vii depend upon the
activity of
. 10 the particular compound, the route of administration, the severity of the
condition being
treated and the condition aad prioz medical history of the patient being
treated. However,
it is within the stall of the art to start doses of the compound at levels
lower than required
to achieve the desired therapeutic effect and to gradually increase the dosage
until the
desired e~Ct is achieved.
When used in the above or other treatments, a therapeutically effective amount
of
one of the compounds of the present invention can be employed in pure form or,
where
such forms exist, in pharmaceutically acceptable salt, ester or prodrug form.
Alternatively; the compound can be administered as a pharmaceutical
composition
containing the compound of interest in combination with bane or more
pharmaceutically
acceptable excipients. The phrase "therapeutically affective amount" of the
compound of
the invention means a sufficient amount of the compound to treat disorders, at
a
reasonable benefttlrisk ratio applicable to any medical treatment. It will be
understood,
however, that the total daily usage of the compounds and compositions of the
present
invention will be decided by the attending physician within the scope of sound
medical
judgement. The specific therapeutically effective dose level for any
particular patient will
depend upon a variety of faGtOTb including the disorder being treated and the
seventy of
the disorder; activity of the specific compound employed; the specific
composition __
employed; the age, body weight, general health, sex and diet of the patient;
the time of
administration, route of administration, and rate of excretion of the specific
compound
employed; the duration of the treatment; drugs used in combination or
coincidental with
the specific compound employed; and like factors well known in the medical
arts. For
CA 02366800 2002-O1-07
-66-
example, it is well within the skill of the art to start doses of the compound
at levels lower
than required to achieve the desired therapeutic effect and to gradually
increase the
dosage until the desired effect is achieved.
The total daily dos~ of the compounds of this in~'eszi:ion administered to a
human
S or lower animal may range from about 0.0001 to about 1000 mg/kg/day. For
purposes of
oral administration, more preferable doses can be in the range of from about
0.001 to
.Yu°u!
about 5 ms/kg/day. If desired, the effective daily dose can be divided into
multiple doses
for purposes of administration; consequently, single dose compositions may
contain such
amounts or submultiples thereof to make up the daily dose.
The present invention also provides pharmaceutical compositions that comprise
compounds of the present invention formulated together with one ox more non-
toxic
pharmaceutically acceptable carriers. The pharmaceutical compositions can be
speciahy
formulated for oral administration in solid or liquid form, far parenteral
injection or for rectal
administration.
The pharmaceutical compositions of this invention can be administered to
humans
and other mammals orally, rectally, parenterally , intracisternally,
intravaginally,
intraperitoneally, topically (as by powders, ointments or dFOps), bucally or
as an oral or
nasal spray. The terns "parenterally," as used herein, refers to modes of
administration
which include intravenous, intramuseular, intraperitoneal, intrasternal,
subcutaneous and
intraarticular injection and infusion.
Ts another aspect, the pzesent invention provides a pharmaceutical composition
comprising a component of the present invention and a physiologically
tolerable diluent.
The present invention irtcIudes one or more compounds' as described above
formulated
into compositions together with one or more non-toxic physiologically
tolerable or
2S acceptable diluents, carriers, adjuvants or vehicles that are collectively
referred to herein
as diluents, for parenteral injection; for intranasal delivez~i; foz oral
administration in solid
or liquid form, for rectal or topical administration, among others. ,__
The compositions can also be delivered through a catheter for local delivery
at a
target site, via an inttaeoronary scent (a tubular device composed of a fine
wire mesh); or
via a biodegradable polymer. The compounds may also be cornplexed to Iigands,
such as
antibodies, for targeted delivery.
CA 02366800 2002-O1-07
-67-
Compositions suitable for parenteral injection may'comprise physiologically
acceptable, sterile aqueous or nonaqueous solutions, dispersions, suspensions
or
emulsions and sterile powders for reconstitution into sterile injectable
solutions or
dispersions. Examples of suitable aqueous and nonaquecius carriers, diluents,
solvents or
vehicles include water, ethanol, polyols (propyleneglycol, polyethyleneglycol,
glycerol,
and the like); vegetable oils (such as olive oil), injectable organic esters
such as ethyl
oleate, and suitable mixtures thereof.
These compositions can also contain adjuvants such as preserving, wetting,
emulsifying, and dispensing agents. Prevention ~f the action of microorganisms
can be
ensured by various antibacterial and antifungal agents, for example, parabens,
chlorobutanol, phenol, sorbic acid, and the like. It may also be desirable to
include
isotonic agents, for example sugars, sodium chloride and the like. Prolonged
absorption
of the injectable pharmaceutical form can be brought about by 'the use of
agents delaying
absorption, for example, aluminum monostearate and gelatin.
Suspensions, in addition to the active compounds, may contain suspending
agents,
as for example, ethoxylated isostearyl alcohols; polyoxyethylene sorbitol and
sorbitan
esters, microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-
agar and
tragacanth, or mixtures of these substances, and the like.
In some cases, in order to prolong the effect of the drug, it is desirable to
slow the
absorption of the drug from subcutaneous or intramuscular injection. This can
be
accomplished by the use of a liquid suspension of crystalline or amorphous
material with
poor water solubility. The rate of absorption of the drug then depends upon
its rate of
dissolution which, in tum, may depend upon crystal size and crystalline form.
Alternatively, delayed absorption of a parenterally administered drug form is
accomplished by dissolving or suspending the drug in an oil vehicle.
injectable depot forms are made by forming microencapsule matrices of the drug
in biodegradable polymers such as polyIactide-polyglycolide. Depending upon
the ratio
of drug to polymer and the nature of the particular polymer employed, the rate
of drug
release can be controlled. Examples of other biodegradable polymers include
poly(orthoesters) and poIy(anhydzides). Depot injectable fbrmulations are also
prepared
CA 02366800 2002-O1-07
-68-
by entrapping the drug in liposomes or microetnulsions which are compatible
with body
tissues.
The injectable formulations can be sterilized, for example, by filtration
through a
bacterial-retaining filter or by iacorpotating sterilizing agents in the form
of sterile solid
compositions which can be dissolved or dispersed in sterile water ar other
sterile
injectable medium just prior to use.
Solid dosage forms for oral administration include capsules, tablets, pills,
powders
and ganules: In such solid dosage forms, the active compound may be mixed with
at
least one inert, pharmaceutically acceptable excipient or carrier, such as
sodium citrate or
dicalcium phosphate andlor a) fillers or extenders such as starches, lactose,
sucrose,
glucose, mannitol and silicic acid; b) binders such as
carboxyrnethylcellulose, alginates,
gelatin, ~lyvinylpyrrolidone, sucrose and acacia; c) humectants such as
glycerol; d)
disinte~aling agents such as agar-agar, calcium carbonate, potato or tapioca
starch,
alginic acid, certain silicates and sodium carbonate; e) solution retarding
agents such as
paraffin; fj absorption accelerators such' as quaternary ammonium compounds;.
g) wetting
agents such as cetyl alcohol and glycerol monostearate; h) absorbents .uch as
kaolin and
bentonite slay and l) lubricants such as talc, calcium stearate, magnesium
stearate, solid
polyethylene glycols, sodium lauryl sulfate and mixtures thereof. In the case
of capsules; .
tablets and pills, the dosage form may also comprise buffering agents.
Solid compositions of a similar type may also be employed as fillers in soft
and
hard-filled gelatin capsules using such excipients as lactose or milk sugar as
well as high
molecular weight polyethylene glycols and the like.
'the solid dosage forms of tablets, dragees, capsules, pills and granules can
be
prepared with coatings and shells such as enteric coatings and other coatirtga
well-known
in the pharmaceutical formulating art. They may optionally contain opacifying
agents and
may also be of s composition such that they release the active ingredients)
only, or
preferentially, -in a certain pact of the intestinal tract, optionally, in a
delayed manner. --
examples of embedding compositions which can be used include polymeric
substances
arid waxes.
The active compounds can also be in micro-encapsulated form, if appropriate,
with one or more of the above-mentioned excipients.
CA 02366800 2002-O1-07
~~ n
Liquid dosage forms for oral administration includ~ pharmaceutically
acceptable
emulsions, solutions, suspensions, syrups and elixirs. In addition to the
active
compounds, the liquid dosage forms may contain inert diluents commonly used in
the art
such as, for example, water or other solvents, solubilizing agents and
ernulsifiera such as
ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl
alcohol, benzyl
benzoate, propylene glycol, I,3-butylene glycol, dimethyl:.formamide, oils (in
particular,
cot~nseed, groundnut, corn, gerrm,, olive, castor sad sesame oils), glyceml,
tetrahydrofiufuryl alcohol, polyethylene glycols and fatty acid esters of
sorbitan and
raixtures thereof.
Besides inert diIueats, the oral compositions may also include adjuvants such
as
wetting agents, emulsifying and suspending agents, sweetening, flavoring and
perfuming
agents.
Compositions for rectal or vaginal administration are preferably suppositories
which can be prepared by mixing the compounds of this invention with suitable
non-
I S irritating exeipients or carriers such as cocoa butter, polyethylene
glycol or a suppository
wax which are solid at room temperature but liquid at body terx'iperature and
therefore
melt in the rectum or vaginal cavity and release the active-compound.
Compounds of the present invention can also be administered in the fomn of
liposomes. As is known in the art, liposomes are generally derived from
phospholipids or
other lipid substances. Liposomes axe formed by mono- or mufti-lamellar
hydrated liquid
crystals which are dispersed in an aqueous medium. Any non-toxic,
physiologically
acceptable and metabolizabIe lipid capable of forming liposomes can be used.
The
present compositions in liposome form can contain, in addition to a compound
of the
present invention, stabilizers, preservatives, excipients and the like.
The'preferred lipids
are natural and synthetic phospholipids and phosphatidyl cholines (lecithins)
used
separately of together.
Methods to form liposomes are known in the art.: 'See, for example, Prescott,
fid., --
Methods irlCell $ioloev: Volume XN, Academic Press; N'eW York, N.Y. (1976), p.
33 et
say.
The term "pharmaceutically acceptable prodrugs" as used herein represents
those
pmdrugs of the compounds of the present invention which are, within the scope
of sound
CA 02366800 2002-O1-07
medical judgement, suitable for use in contact with the tissues of humans and
lower
animals without undue toxicity, irritation, allergic response, and the like,
commensurate
with a reasonable benefit/rislc ratio, and effective for their intended use,
as well as the
zwitterionic fornzs, where possible, of the compounds of the invention.
Prodrugs of the
present invention may be rapidly transformed in vivo to the parent compound of
the above
formula, for example, by hydrolysis in blood. A thoro~lgh discussion is
provided in T.
Higuchi and V. Stella, Pro-drugs as Novel Delivery Svsterns, V. 14 of the
A.C.S.
Symposium Series, and in Edward B. Roche, ed:, Bioreversible Carriers a'~t~~
Designn,
American pharnnaeeutical Association and Pergamon Press (19$7), hereby
incorporated
by reference.
Compounds of the present invention that are formed by in vtvo conversion of a
different compound that was administered to a mammal are intended to be
included
within the scope of the present invention.
Compounds of the present invention rt~.ay exist as stereoisomers wherein
asymmetric or chiral centers are present. These stereoisomers are "R" or "S"
depending
on the configuration of substituents around the chiral carbon atom. The
present invention
contemplates various stereoisomers and mixtures thereof. Stereoisomers include
enantiomers and diastereomers, and mixtures of enantiomers or diastereomers.
lndividuaI
stereoisomers of compounds of the present invention may be prepared
synthetically from
commercially available starting materials which contain asymmetric or chiral
centers or
by preparation of racemic mixtures followed by resolution well-known to those
of
ordinary skill in the art. These methods of resolution are e~templified by (1)
attachment
of a mixture of enantiomers to a chiral auxiliary, separation of the resulting
rrxixture of
diastereomers by recrystallization or chromatography and liberation of the
optically pure
ZS product from the auxiliary or (2) direct separation of the ziiixture of
optical enantiomers
on chiral chromatographic columns.
The compounds of the invention can exist in unsolvated as well as solvated
forms,
including hydrated forms, such as hemi-hydrates. In general, the solvated
forms, with
pharmaceutically acceptable solvents such as water and ethanol among others
are
eguivalent to the unsolvated forms for the purposes of the invention.
CA 02366800 2002-O1-07
-71-
In another aspect, the present invention contemplates a process of inhibiting
the
s.
binding of a4/3~ integrin to VCAM-1. A process of the pr$sent invention can be
used either
in vitro or in vivo. 1n accordance with a process of the present invention, a
cell expressing
Oc4~ii integrin is exposed to a cell expressing VCAM-I iti~the presence of an
effective
S inhibiting amount of a compound of the present invention:
A cell expressing nay integzia can be a naturally occurring white blood cell,
mast
cell ar other cell type that naturally expresses a4pi on the cell surface, or
a cell
transfected with an expression vector that contains a poly-nucleotide (e.g.,
genomic DNA
or cDNA) that encodes a4~i, integtin. In an especially preferred embodiment,
vca(3lincegrin is present on the surface of a white blood cell such as a
monocyte, a
lymphocyte or a granulocyte (e.g., an eosinophil or a basophil).
A cell that expresses VCAM-1 can be a naturally occurring cell (e.g. as
endothelial cell) or a cell transfected with an expression .victor containing
a
_: ,;
palyaucleotide that encodes VCAM-1. Methods for producing transfected calls
that
I5 express VCAM-1 are well known in the art.
Where VCAM-1 exists on the surface of cell, the expression of that VCAM-1 is
preferably induced by inflammatory cytokines such as tumor necrosis factor-Oc
iaterleukin-4 and interteukzn-I Vii.
Where the cells expressing a4~3, integ~cin and YCAfrI-1 are in a Living
organism, a
compound of the present invention is administered in an effective amount to
the living
organism. Preferably, the compound is in a pharn~aceuticsl composition of this
invention.
A process of the present invention is espzcially useful in-treating eliseases
associated
with uncontrolled migration of white blood cells to damaged tissue. Such
diseases
include, but are not limited to, asthma, atheroselemsis, rheumatoid arthritis,
allergy,
multiple sclerosis, lupus, inftammatory bowel disease, graft rejection,
contact
hypersensitivity, type I diabetes, leukemia, and brain cancer. Administration
is
preferably accomplished via intravaseular, subcutaneous, intranasal,
transdermal or oral ._.
delivery.
The present invention also provides a process of selectively inhibiting the
binding
of a~~3iintegrin to a protein comprising exposing the integrin to the protein
in the
presence of an effective inhibiting amount of a compound of the present
invention. In a
CA 02366800 2002-O1-07
;v
-72-
preferred embodiment, the tray integrin is expressed on the surface of a cell,
either
~:~
naturally occurring or a cell transformed to express aa(iiii~tegrin.
The protein to which tt~e oe4(3, integrin binds can be e~cpressed either on a
eelI
surface or be part of the extracellular matrix. Especially preferred proteins
are fibronectin
or unvasin.
The ability of compounds of the pr$sent invention to inhibit binding is
described in
detail hereinafter in the Examples. These Examples are presented to describe
preferred
embodiments and utilities of the invention and are not meat to limit the
invention unless
otherwise stated in the claims appended hereto.
The ability of compounds of the present invention to inhibit binding is
described
in detail hereinafter in the Examples. These Examples are'pxesented to
describe preferred
embodiments and utilities of the invention and are not meant to limit the
invention unless
otherwise stated in the claims appended hereto.
Exam 1e
Synthesis of (3S)-3-{[( f 1-[(2-chlorophenyl)methyl]-4-ethyl-2-oxo-I,2-dihydro-
3-
pyridinyl}amino)carbonyl]amino}-3-(4-methylphenyl)propanoic acid {10):
Step One; Compound l (20.8 g, 135 ~nmol) was dissolved in methanol (270 mL)
and palladium on carbon (I0 % Pd dry weight basis, Degtfssa type 8101 NFaW;
~50%
water content, 5.75 g, 2.? mmol Pd) was added. ?he atmosphere was replaced
with
ZO hydrogen {toggle between vacuum and hydrogen from a balloon five times),
the mixture
was stirred overnight, then filtered. The filtrate was concentrated under
vacuum and the
residue was taken up in a 1:1 hexanes:ethyl acetate mixture and washed with a
4:1
mixture of water and saturated Na~iC03, saturated NaHG03 and brine. The
organic layer
was dried over MgS04 and filtered and the filtrate was concentrated under
reduced
pressure to give compound 2 (12.43 g; 74°!°) as a white solid.
This material was used
without purification.
Step Two: Compound 2 {2.64 g, 21.3 mmol) was dissolved in dichlozomethane w
(50 mL) artd chilled to 0 °C. The cold solution was treated
sequentially with
triethylamine (3.6 mL, 25.6 mmol) and trimethylacetyl chtoride (2.90 ri~L,
23.4 mmol).
The solution was stirred at room temperature for 5 hours,vthert refluxed
overnight. The
CA 02366800 2002-O1-07
-73-
mixtura was partitioned between dichloromethane and aqueous NaOH (2N). The
organic
layer was washed with brine, dried over MgS04 and filtered and the filtrate
was
concentrated to give compound 3 (3.33 g, 75%):
Sue: Compound 3 (0.50 g, 2.4 mmol) was dissolved in dry THF, (9.6 mL)
and TMEDA (I.1 mL, 7.2 mmol) under a dry nitrogen atmosphere. The resulting
solution was chilled to between -20 and -10 °C and treated'
sequentially with n-
r
butyllithium (1.6 M in hexanes 2.25 mL) and t-butyllithium (1.7 M in pentane,
2.I mL)
dmpwise via syringe. After 30 minutes the bath temperat(ite was allowed to
come to -5 to
0 °C and treated with ethyl iodide via a syringe (0.7? mL:9.6 rnmol).
?he solution was
IU stirred at 0 °C for 2 hours,thezz room tempezature overnight. The
mixture was quenched
with methanol and concentrated to dryness. The residue was purified by
filtering througl~e
silica gel, eiutiag with 3:1 hexanes:ethyl ecetate and then recrystallizing
from hexanes to
yield compound 4 (0.32 g, 56%).
Sten Four: Compound 4 (0.32 g, 1.3 mmol) was dissolved in glacial acetic acid
(4.5 taL) and treated with potassium iodide (0.65 g, 3.9 srimol). The
resulting mixture was
heated in an oil bath regulated at I 15 °C for 1.0 hour. The mixture
was cooled, diluted
with water and adjusted to pH 6 using 2N NaOH and 2N HCI. The mixture was
extracted
;~-
with chloroform: (4 times). The combined extract,; were washed with aqueous
sodium'
thiosulfate, dt'ied over MgSOa and filtered. The filtrate was coneentxated
under reduced
pressure to give compound 5 (0.25 g, 86%) as a white solid. This material was
used
without further purification.
Step Five: Compound 5 (0.25 g, 1.1 mmol) was dissolved in THF (45 mL) and
treated dropwise with a solution of potassium bis(trimethylsilyl)amide (0.5 M
in toluene,
2.7 mL) at 0 °C. ?he resulting solution was treated with 2-
chlorobenzylbromide (0.16
mL, 1.2 mmol) and the solution was allowed to warm to room temperature
overnight.
The mixture was partitioned between 2N HCl and ethyl acetate. The organic
layer was
washed with brine, dried over MgSOa and filterod. 'xhe fi~ti~ate was
concentrated under
reduced pressure and the residue was purified by chtomato~aphy (SiOz, gradient
elution
4:1 switching to 2:1 hexanes:ethyl acetate) to gave compound 6 (0.16 g, 41%).
Ste ix: Compound 6 (0.16 g, 0.46 mmol) was suspended in 1:1
water;concentrated HCl (4.6 mL). The suspension was brought to reflux for 4
hours,
CA 02366800 2002-O1-07
-74-
during which time the compound dissolved. The mixture was cooled; diluted with
water
and extracted with diethyl ether. The aqueous layer adjusted basic with excess
saturated
sodium bicarbonate solution, and the mixture was extzacted with ethyl acetate.
The
extracts were combined, washed with brine, dried over MgSOa and filtered. The
filtrate
was concentrated under reduced pressure to give compound 7 (0.081 g; 67%).
Step Seven: Compound 7 (0,080 g, 0.30 nnnol); was dissolved in I ,Z-
dichlvmethane
(1.2 mL) and DIPIrA (0.115 mL, 0,66 mmol) and chilled to 0 °C. The cold
solution was
treated rapidly with a solution ofphosgene (1.93 M in toluene, 0.170 mL, 0.33
mmol). After
30 minutes a solution of compound 8 (0.068 g, 0.33 mmol) in 1,2-dichioroethane
(0.5 mL)
was added rapidly via syringe. The resulting mixture was heated to 55
°C. for 1 hour, The
mixture was partitioned between dichloromethane and' 2N HCI. The organic layer
was
washed with saturated aqueous NaHC03 and brine, dried over MgS04 and filtered.
The
filtrate was concentrated to give compound 9(0.110 g; 74%).
Ste Ei t: Compound 9 (0.11 g, 0.22 mmol) was dissolved in 2:1 THF:HZO
1 S (0.88 mL) and treated with a solution of 2N NaOH (0.33 mL). Methanol was
added
dropwise until a horzzogeneous solution was obtained. 'The mixture was stirred
for 20
minutes, diluted with water and washed with ethyl ether. The aqueous layer was
acidified
with 2N HCl and extracted with ethyl acetate. The ethyl acetate layer was
washed with
brine, dried over MgSOa and filtered, The filtrate was concentrated to give
(3S)-3-{[(z I-
[(2-chlorophenyl)methyl]-4-ethyl-2-oxo-1,2-dihydro-3-
pyridinyl} amino)carbonyl]amino}-3-(4-methylphenyl}propanoic acid (I0, 0.095
g, 92%).
i
Example 2
Synthesis of(3S)-3-{[((6-m~thyl-2-oxo-1-(phenylmethyl)-4-[(phenylmethyl)oxy)-
1,2
dihydro-3-pyridinyl}amino)carbonyl]amino}-3-(4-methylphenyl)propanoic acid
(15),
Step One: To a suspension of compound 11 (1.0 g, 5,9 mmol) and KZC03 (2.40 g
I7.6 mmol) in acetone (50 mL) was added benzylbromide (2.31 g, 13.5 mmol).
After
refluxing overnight, the reaction was cooled and the mixture was partitioned
between
ethyl acetate and saturated NaHCOa. The organic layer was washed with dilute
HCI and
brine, dried over MgS04 and filtered and the filtrate was concentrated to give
compound
12 (1.60 g, 80%).
CA 02366800 2002-O1-07
-75-
Step Two: Compound 12 (0.30 g, 0.86 mmol), zinc powder (0.3U g, 4.6 mmol)
and saturated aqueous NFi~CI (0.30 mL) were mixed in MeOH (18 mL). This
mixture
was allowed to stir at room temperature for 1 hour before additional zinc
(0.30 g, 4.6
mmol) was added. ?lie resulting heterogeneous mixture was retluxed overnight.
After
filtration of the hot mixture and concentration of the filtrato ander reduced
pressure, the
residua was dissolved in ethyl acetate and washed with saturated aqueous
NaHC03 and
brine. 'The organic layer was dried over lVIgS04 and f lured and the filtrate
was
concentrated under reduced pressure to give compound 13 (0.18 g, 66%).
Step Three: Compound 13 (0.30 g, 0.94 mmol.) and DIPEA (0.40 mL, 2.3 mmol.)
I0 were dissolved in CH=CI2 and the mixture was cooled to 0 °C.
Phosgene (1.9 M in
toluene, 0.55 mL, 1.0 mmol) was added to the solution dropwise. The reaction
mixture
was stirred at 0 °C for 15 minutes before compound 8 (0'.19 g, 0.94
mmol) in CfIaCla (2
mL) was added. The resulting solution was stirred at rooztz temperature
overnight then
»:
poured into ethyl acetate and washed with saturated aqueous NaHC03, I N HCi
and
brine. The organic layer was dried over MgSOo and filtered and the filtrate
was
concentrated under reduced pressure. The residue was purified by flash
chromatography
on silica gel, eluting with I:I increasing to I;2 hexanes:ethyl acetate to
give compound I4
(0.33 g,.64%).
Step Four: A solution of compound 14 ( 0.33 g, 0.6 mmol) in THF (6 mL) was
treated with 2N NaOH (2 mL). MeOH was added until Homogeneous solution was
achieved. The reaction mixture was stirred at room temperature for 30 minutes
and
poured into HBO (SO mL). The aqueous layer was washed with diethyl ether
(twice), and
then acidified with 1N HCl, The aqueous layer was extracted with ethyl acetate
(twice).
The combined ethyl acetate extzacts were washed with brine (twice), dried over
Mg$04
and filtered. ?he filtrate was concentrated under reduced pressure to give
(3S)-3-{[((6
methyl-2-oxo-1-(phanylmethyl)-4-[(phenylmethyi)oxy]-I;2-dihydro-3-
pyridinyl}amino)carbonyl]amino)-3-(4-tnethylphenyl)propanoic acid (15, 0.26 g,
90%) as -w
an off white solid. Melting point: 124-126 °C.
Example 3
Synthesis of (3S)-3-{[((4-amino-I -[(2-chlorophenyl)methyl]-6-methyl~2-oxo-1,2-
CA 02366800 2002-O1-07
-76-
dihydro-3 pyridinyl}amino)carbonyl]amino}-3-(4-znethj~lghenyl)propanoic acid
(22).
~Yt
To a solution of compound 11 ( 10.00 y;' X8.8 mmol) in anhydrous DMF
(120 mL) at 0 °C was added NaH (60% dispersion in mineral oil, 5.40 g,
13 5 mmol). The
mixture was stirred at 0 °C for 15 minutes before the addition of 2-
chlorobenzylchloride
S (12.3 g, 76,4 mmol). Afar stirring at 55 °C overnight, the mixture
was poured into ice-
water aad washed with EtIO tw ice. The aqueous layer was acidified and
filtration of the
resulting precipitate gave compound 16 (14.7 g, 85%).
Sten 'hw_o: To a flask containing compound 16 (8:00 g, 28.6 mmol) sealed with
a
rubber septum and balloon at room temperature under dry nitrogen atmosphere,
POCl3
(30:0 ml, 322 mmol) was added via syringe. The nitroge~t line was removed and
the
reaction mixture was stirred overnight at 70 °C, then poured over ice
(300m1) and stirred
for 30 minutes. The resulting mixture was extracted with diehlorornethane (300
m1) and
the organic phase was dried over MgS04 and filtered. The filtrate was
concentrated under
reduced pressure to give compound I7 (7,3g, 86%) as a dark brown solid.
IS Step ?hree: To a 250 rot flask equipped wit>t condenser and robber septum
fitted
with a baboon, a solution of compound 17 (2.1 g, 7.05 mmol), methanol (SSmI)
and
aqueous ammonium hydroxide (28-30%, 70.0 ml, I .14 mvl) were added at room
temperature. The reaction mixtuze was heated to 65 °C for 60 hours open
only to the
balloon. The mixture was filtered and the filtrate was concentrated under
reduced
pressure to yield compound 18 { I .5 g, 76%) as a brown solid.
Stew Four: To a solution of compound 18 (0.3g, 1..02 mmol) in methanol {50 ml)
at room temperature, saturated aqueous amitionium chloride (2 ml) and zinc
dust (0.30 g,
4.6 mmol) were added sequentially. After stirring 30 minutes at room
temperature,
addirional zinc was added (0.30 g, 4.6 mmol) and the reaction mixture was
refluxed
2S overnight. The reaction mixture was filtered hot and the filtrate was
concentrated under
reduced pressure- The residue was partitioned between ethyl acetate and 1N
NaOH, The
solution was filtered and the aqueous phase extracted with ethyl acetate. The
combined --.
organic phases were dried over M~604 and filtered. The filtzate was
concentrated under
reduced pressure to yield compound 19 (0.21 g; 78%) as..~ brown solid.
sty rive: ~1 solution of compound Z9 (0.10 g, 0:38 mmol), NMM (0.040 mL,
0.38 mn#ol) and compound 20 (0.14 g, 0.38 mmol) in anhydrous DMF (5 mL) was
heated
CA 02366800 2002-O1-07
.i..a.,i:i
F :-..:
to 50 °C overnight. The mixture was cooled and diluted with ethyl
acetate (60 mL). The
organic layer was washed with O.SN NaOH (3 x 30 mL) and brine, dried over
MgSOa and
filtered, The filtrate was concentrated under reduced pressure and the residue
was
purified by flash chromatography on silica gel, eluting with 9:1 increasing to
17:3
CHCI3:MeQH to give compound 2Z (0.120 g, 65%) as a yellow foam.
5_ t~p Six: A solution of compound 21 (0.I20 g, 0;25 mmol) in THF (6 mL) was
seated with 2N NaOH (2 mL). Methanol was added until a homogeneous solution
was
achieved. The r~action mixture was stirred at room temperature for 30 minutes
and
poured into Hi0 (50 mL). The aqueous layer was washed with diethyl ether
(twice), and
then acidified with 1N HCl. The aqueous layer was extracted with ethyl acetate
(twice).
The combined ethyl acetate extracts were washed with brine (twice), dried over
MgSOa
and filtered. The filtrate was concentrated under reduced pressure to give (3
S)-3- ( [( {4-
amino- I -[(2-ehloropbenyl)methyl]-6-methyl-2-oxo-1;2-dihydro-3-pyridinyl }
amino)-
carbonyl]amino}-3-(4-methylphenyl)propanoic acid (22, 0.100 g, 89%) as an off
white
solid. Melting point: 145-147 °C.
Example 4
Synthesis of (3 S)-3-[ ( { [ 1-[(2-chlorophenyl)methyl]-4-(methyloxy)-2-oxo-
1,2-dihydro-
3-pyridiayl]amino)carbonyl)amino]-3-(4-methylphenyl)propanoic acid.
StegOne: To a solution ~f compound 23 (10.00 g, fr4.0 mmol) in anhydrous DMF
(130 mL) at 0 °C was added NaH (60% dispersion in mineral oil, 5.90 g,
147 mmol). The
mixture was stirred at 0 °C for 15 minutes before the addition of 2-
chlorobenzylchloride
(13.4 g, 83.3 mmoi). After stirring at 55 °C overnight, the mixture was
poured into ice
water and washed with EtzO (twice). The aqueous layer was acidified and
filtration of the
resulting precipitate gave compound 24 (13.5 g, 75%).
Step Two: A suspension of compound 24 (I .0 g, 3~ mmol), KzC03 (0.85 g, 6.2
mmol) and MeI (I.18 g, 8.3 mmol) in acetone (20 mL) was refluxed overnight.
The
reaction mixture was diluted with ethyl acetate and washed with saturated
aqueous
NaHC03, 1 N HCI and brine. The organic layer was dried over MgS04 and filtered
and
the filtrate was concentrated under reduced pressure to give Compound 2S (0.74
g, 70%).
(3S)-3-[({[I-[(2-chlorophenyl)methyl]-4-(methyloxy)-2-oxo-I;2-dih3rdro-3-
pyridinyl]amino}carbonyl)amino]-3-(4-methylphenyl)propanoic acid was pzepared
from
CA 02366800 2002-O1-07
-78.
compound 23 according to procedures described in Example 3: lvlS: Calculated:
{M+H)+
= 469.93; Found; (Ni+H)+ = 470.01.
Exa 1e
Synthesis of (3S)-3-{[({1-[(2-chlorophenyl)methyl]-4-fluoro-2-oxo-1,2-dihydro-
3-
pyridinyl}amino)catbonyl]amino}-3-(4 methylphenyl~ropanoic acid.
.';
Step One: Compound 3 (0.65 g, 3.1 mmol) was~dissolved in dry THF (12.4 mL)
and TMBDA (0.90 rnL, 6 tnmol) under a dry nitrogen atmosphere. The resulting
solution
was chilled to between -15 and -t0 °C and n-butyllithium. (1:6 M in
hexanes, 7.75 mL,
IO 12.4 mmol) was added dropwise via sytiuge. After 1.5 hours, a solution of N-
fluorobenzenesulfonimide (1.078, 3.4 mmol) in T'Hf (5 mL) was added to the
cold
solution rapidly vla syringe, The solution was stirred at 0 °C for I
hour, then room
temperature for 3 hours. The mixture was quenched with water and extracted
with
chloroform (4 times). The combined organic extracts weie washed with brine,
dried over
LS MgS04 and filtered. The filtrate was concentrated under reduced pressure
and the residue
was purified by chromatography, (Si02, plug gel, using v::L switching to 3:1
hexanes:ethyl
acetate) io yield compound 26 (0.177g, 25%).
(3S)-3-{[( { 1-[(2-Chlorophenyl)methylJ-4-fluoro-2-oxo-1,2-dihydro-3-
pyridinyl}amino)carbonyl]amino}-3-(4-methylphenyl)propanoic acid was prepared
from
2Q Compound 26 according to procedures described in Example 1. MS: Calculated:
(M+H)+ = 458.12; Found: (M+li)+ ~ 458.0I .
am 1e 6
Synthesis of (3S)-4-chloro-3-{[({1-[(2-chlorophenyl)methyl]-2-oxo-1,2-dihydro-
25 3-pyridinyl}amino)carbonyl)amino}-3-(4-methyIphenyl)propanoic acid.
Step One: Compound 3 (0.65 g, 3.1 mmol) was dissolved in THF (21 mL) and
TMEDA (1.20 mL, 7.75 mrnol) and chilled to -15 °C. The solution was
treated with n-
butyllithium (1.6 M in hexanes, 4.8 mL, 7,8 mmol). The mixture was maintained
between ._.
-20 and -10 °C for 1 hour, then cooled to -78 °C. ;>olid N-
ehlorosuecinimide
30 (U.45 g, 3.4 moral) was added while the apparatus was under a positive flow
of nitrogen.
The reaction was allowed to gradually warm to room temperature then stirred
overnight.
The mixture was quenched with water and extracted with chloroform (4 times).
The
CA 02366800 2002-O1-07
_79-
organic layers were combined, dried over MgSOa and: filtered. The filtrate was
concentrated under reduced pressure and the residue Was recrystallized from
hexanes to
give compound 27 (0.25 g, 33%).
(3 S)-4-ChIoro-3- { [( { I -[(2-chlorophenyl)methyl]- 2-oxo-1,2-dihydro-3-
pyridinyl}amino)carbonyl]amino}-3-(4-methylphenyl)propanoic acid was prepared
from
compound 27 according to procedures described in Example 1.
Example 7
Synthesis of (3S)-4-bmmo-3-{[({1-[(2-chlorophenyl)methyl}- 2-oxo-1,2-dihydro-
3-pyridinyl}amino)carbonyl]amino}-3-(4-methylphenyl)propanoic acid.
I0 Step One: Compound 3 (2.00g, 9.6 mmol) was dissolved in dry THF (32 mL) and
TMBDA (2.20 mL, 14.4 mmol) under a dry nitrogen atmosphere. The resulting
solution
was chilled to between -20 and -10 °C and n-butyl lithium (1.60 M in
hexanes, 18.0 rnL,
28.8 mmol) was added dropwise via syringe. Upon completion of the addition,
the
solutioa was chilled to -78 °C and bromine (0.49 mL, 10.5 mmol) was
added dropwise
via syringe. The solution was allowed to warm slowly to room temperature
overnight,
then was quenched with water and extracted with chlorofbrm. The organic layer
was
dried over MgS04 and filtered and the filtrate was concentrated under reduced
pressure.
The residue was recrystallized from hexanes to give compound 28 ( 1.32 g, 48%)
as a
tarnish white solid,
(3S)~F-Bromo-3-{[({1-((2-chloropheayl)methyl]-2-oxo-1,2-dihydro-3-
pytidinyl}amino)carbonyl]amino}-3-(4-methylphenyi)propanoic acid was prepared
from
compound 28 according to procedures described in Example 1.
~ple 8
Synthesis of (3 S)-3-{ [( { 1-[(2-chlorophenyl)methyl]-4-hydroxy-2-oxo-1,2-
dihydro-3-pyridinyl}amino)carbonyl]amino}-3-(4-methylphenyl}propanoic acid
(32).
t One: To a solution of compound 24 (1.5 g, 5.3 mmol) in methanol (50 ml) at
room temperature, saturated ammonium chloride ( 1.5 mL) and zinc dust ( 1, 5
g, 23 mmol) -w
were added sequentially. After stirring 30 minutes at room temperature,
additional zinc
dust (1.5 g, 23 mmol) was added and the reaction mixtuie was refluxed
overnight, The
reaction mixture was filtered while hot and the filtrate was concentrated
under reduced
pressure. HCl (1 1V) was added to the resulting residue until the pH was
approximately 4
CA 02366800 2002-O1-07
-80- ,
and the resulting precipitate was collected by filtratioriE'ro give compound
29 (0.80 g,
57%) as a brown solid.
Step Two: A solution of compound Z9 (0.26 g, 1~.0 mmol) and CDI (0:25 g, 1,6
mmol) in DMF (10 mL) was heated to 70 °C overnight. ~r8er cooling to
room
temperature, the mixture was diluted with ethyl acetate and washed with 1N ICI
(3
times) and brine. The organic layer was dried over MgSO4 and filtered and the
filtrate
was concentrated under reduced pressure to give compound 30 (0.14 g, 50%} as a
bzowri
solid,
Step Three: A solution of compound 30 (0.1 g, 0.36 mmol) and compound 8 (0.082
g,
0.40 mmol) in anhydrous DMF (5 mL) was heated to 70 ~°C overnight. The
mixture was
cooled, diluted with ethyl acetate and washed with IN HCI (3 times) and brine.
The organic
layer was dried over MgSOa ~d filtered and the filtrate was concentrated under
reduced
pressure. The residue was purified by flash chromatography (SiOz), eluting
with 9:I
CHCI3:MeOH to give compound 31 (0.17 g, 97%).
Step Four: A solution of compound 31 (0.170 g, 0.35 ntmol) in THF (3 mL) was
treated with 2N NaOH (1 mL). Methanol was added until a homogeneous solution
was
achieved. The reaction mixture was stirred at room temperature for 30 minutes
and
poured into HZO (50 mL). The aqueous layer was washed with diethyl ether
(twice), and
then acidified with 1N HCI. The aqueous layer was extracted with ethyl acetate
(twice).
The combined ethyl acetate extracts were washed with brine (twice), dried over
MgS04
and filtered. The filtrate was concentrated under reduced pressure to give
(3S)-3-{[( {1-
[(Z-chlorophenyl)methyl]-4-hydroxy-2-oxo-1,2-dihydro-3-
pyridinyl}amino)carbonyllamino}-3-(4-methylphenyl)propanoic acid (32, 0.150 g,
94%)
as an off white solid. Melting point: 1 I3-115 °C.
Exa a 9
Synthesis of(3S)-3-{[((I-[(2-chlorophenyi)methyl;~-2-oxo-4-phenyl-1,2-dihydro-
3-pyridinyl}arnina)carbonyl]amino}-3-(4-methylphenyl)propanolc acid.
Steo pna: Compound 33 (prepared from compound 28 according to procedures
described in Example 1, 0.20 g, 0.50 mmol) was dissolved in DMF (1.8 mL) and
water
(0.7 mL) and treated with K3POa (0,39 g, I.86 moral) and phenyl boronic acid
(0.113 g,
CA 02366800 2002-O1-07
-8I-
0.93 mmol). The resulting mixture was deoxygenated (switching between vacuum
and
nitrogen 5 times), then tstrakis(triphenylphosine)palladium(0) (8.7 mg, 0.050
mmol) was
added, The mixture was deoxygenated as before and heated at 90 °C
overnight. The
mixture was cooled, diluted with water and extracted with ethyl acetate (2
times). T'he
combined extracts were washed with brine, dried over MgSOa and filtered
through silica
gel and concentrated under reduced pressure. The residue was suspended in 1:1
water:concentrated HCl (2 mL) and acetonitrile (0.5 mL). The suspension was
brought to
reflex for I hour, then cooled; and partitioned between ethyl acetate and
saturated
aqueous NaHC03. The ethyl acetate layer was washedrwith brine, dried over
MgS04,
filtered, and concentrated under reduced pressure. The residue was~purified by
flash
chromatography (SiOz, 3:1 hexanes/ethyl acetate) to give compound 34 (0,115 g,
94%),
This material was used without purification.
(3 S)-3-{[( { I -[(2-Chlorophenyl)methyl]-2-oxo-4-phenyl-1,2-dihydro-3-
pyridinyl}amino)carbonyl]amino)-3-(4-methylphenyl)propanoic acid was prepared
from
Compound 34 according procedures described in Example 1. 'H NMR (400 MHz,
CD30D): 52.25 (s, 3H), 2.50 (m, 2H), 4.89 (t, J = 5:9 Hz, 1 H), x.34 (s, 2F3),
6.40 (d, J --
7.OHz, IH), 7.0 (d, J = 8.0 Hz, 2H), 7.10 (d, J = 8.0 Hz, 21~), 7.18 (m, 1H),
7.28 (m, 2IF),
~-35 (m, 3H), 7.43 (m, 1H), 7.49 (m, 3H).
Exam""ple 10
Synthesis of(3S)-3-[({[2-methyl-a-(2-methylpropyl)-6-oxo-1-(phenylmethyl)-1,6-
dihydro-5-pyrimidinyl~amino}carbonyl)amino)-3-(4-methylphenyl)propanaic acid
(43).
Step One: Compound 35 (2.00 g 18,2 mmol) was dissolved in 30 mL of dry
methanol. To this was added benzylamine ( 1.97 g 18.2 mmol) and triethylamine
(2.0 g
20.0 mmol). The reaction mixture was stirred at 50 °C for 3 hours, and
then concentrated
under reduced pressure. The residue was partitioned between HBO and CH2Clz.
The
organic layer was dried over MgSOa and filtered and the filtrate was
concentrated under
reduced pressure to give compound 36 (2.3 g, 82%). --
t Two: ?o a solution of compound 37 (3.50 g; :26.5 mmoI) in ethanol (10 mL)
and pyridine (5 mL) was added isovaleraldehyde (2.8 mh 27 mmol) and piperidine
(1
mL). The reaction mixture was heated to reflex for 3 hours and concentrated
under
reduced pressure. The residue was partitioned between 2N HCl (15 rnL) and
ethyl acetate
CA 02366800 2002-O1-07
-82-
(30 xnL). The organic layer was dried over MgS04, and filtered and the
filtrate was
concentrated under reduced pressure. The residue was purified by silica gel
chromatography, eluting with 2:1 hexanes:ethyl acetate to' give compound 38
(3.6 g,
67%).
S Steo Three: A solution of compound 38 (2.5 g, 12:48 mmol) and compound 36
(2.52 g, 13.7 mmoI) in dry methanol (2S mL) was heated to vigorous reflux for
3 hours,
cooled and concentrated under raduced pressure. The residue was
chromatographed on
silica gel eluting with 2:1 hexanes:ethylacetate to give compound 39 (2.75 g,
69%}.
Step Four: To a solution of compound 39 (2.S g, 7,9 mmol) in CC14 (1 S mL) was
,10 added NBS (I.4 g, 8.0 mmoL), KzC03 (11.0 g, 80.0 mmol}, and benzoyl
peroxide (50 mg,
0.20 mmvl). The reaction mixture was heated to reflux for 1 hour, cooled to
room
temperature, diluted with Ha0 and extracted with CHiCl2. The organic layer was
dried
over MgSOa and filtered and the filtrate was concentrated'.under reduced
pressure. The
residue was chmmatographed on silica gel eluting with 3:1 hexanes:ethyl
acetate to give
15 compound 40 (0.62 g, 25%).
Step Five: Compound 40 {0.60 g, 1.9 mrnol) was rebated with 2N NaOH (SmL)
and THF (3 mL). The resulting mixture was stirred at room: temperature for 2
hours,
acidified with 2N HCl and exaacted with ethyl acetate. The organic layer was
dried over
MgSO~and filtered and the filtrate was concentrated under Deduced pressure to
give
20 compound 41 {560 mg, 98%).
Stap Six: To a solution of compound 4I (0.56 g, 1.8b mmol) in dry benzen~ (10
mL), diphenylphosphorylazide (0.56 g, 2.0 mmol) and trietllylamine (2.02 g,
2.0 mmol)
were added. The reaction mixture was heated to 90 °C for 1 hour then a
solution of
compound 8 (0.39 g, 1.9 mmol) in benzene (2 mL) was added. The reaction was
stirred
25 at 90 °C for an additional 1 hour, cooled to room temperature,
diluted with 10% aqueous
ammonium chloride and extracted with ethyl acetate. The organic layer was
dried over
MgSOa and filtered and the filtrate was concentrated under reduced pressure.
The residue -
was chromato~aphed on silica gel, eluting with 7:3 ethyl acatate:hexane to
give
compound 42 {0.38 g, 40%).
30 t Seven: To a solution of compound 42 (0.35 g 0.7 mmoI) in I :1 mixture of
THF:MeOH (8 mL) was added 2N NaOH (8 mL). The reaction was stirred at room
CA 02366800 2002-O1-07
-83-
temperature for 3 hours, acidified with 2N HC1 ( 10 mL} and extracted with
ethyl acetate
(20 mL). The organic Layer wss dried over MgSOa and filter~d and the filtrate
was
concentrated under reduced pressure to give (3S)-3-[({[2=methyl-4-(2-
methylpropyl)-6-
oxo-1-(phenylmethyl)-I,6-dihydro-5-pyrirnidinylJamino} carbonyl)amino]-3-{4-
methylphenyl)propanoic acid (43, 250 mg; 7~%). MS: Calculated: (M+H)+ = 477.25
m/z;
Found: (IvI+H)+ = 477. I 7 m/z.
Example I1
Synthesis of (3S)-3-[([[2-methyl-6-oxo-1-(phenylmethyl)-1,6-dihydro-5-
pyrimidinyljamino'rcarbonyl)amino]-3-(4-methylphenyl}propanoic acid
Step One: A solution of compound 36 (2.3 g , 15.5 mmol) and compound 44 (3.36
g, 15.5 mmol) in absolute ethanol (35 mL) was refluxed for 3 hours and
concentrated.
The residue was chromatographed on silica gel, eluting with I :1 ethyl
acetate:hexane to
,give compound 45 {1.87 g, 55% yield).
(3S)-3-[({[2-Methyl-6-oxo-1-(phenylmethyl)-i,6-dihydro-S-
iS pyrimidinyl]amino}carbonyl)amino]-3-(4-methylphenyl)propanoic acid was
prepared
from compound 4S according to procedures described in Example 10. ~H NMR (400
MH2, CD30D) S 2.28 (s, 3H), 2.35 (s, 3H), 2.57 (m, 2H), 5.16 (m, 1H), 5.30 (s,
2H), 7.13
(m, 4H), 7.30 (m, SH), 8.50 (s, IH).
Example 12
Synthesis of (3S)-3-([( f i-[(2-ehlorophenyl)methyl]-4-[( f ethyl[(ethylamino)
carbonyl)amino } carbonyl)amino]-2-oxo-1,2-dihydro-3-
pyridinyl}amino)carbonyl]amino]-3-(4-methylphenyl)propanoic acid.
a One: To a solution of compound 46 (preparei~ according to procedures
described in Example 3, 0.50 g, 1.8 mmol) in THF (10 mL) at 0 °C was
added NaH (60%
dispersion in mineral oil, 0.23 g, 5,1 mmol). The mixture was stirred for 10
minutes at 0
°C, then ethyl isocyanate (0.65 g; 9.15 mmol) was added. ,The mixture
was stirred at
room temperature over the weekend; was quenched with 1 N HCl and extracted
with w
ethyl acetate. The organic layer was dried over MgSOa and ~Itered and the
filtrate was
concentrated under reduced pressure to give compound 47, (t1.60 g), This
material was
used without purification.
(3 S)-3- { [( { 1-[(2-Chlorophenyl)methyl]-4-[( { ethyl [{ethylamino)carbonyl]
CA 02366800 2002-O1-07
-$4-
;;~
amino) carbonyl)aminol-2-oxo-1,2-dihydro-3-pyridinyl} ammo)carbonyl]amino}-3-
(4-
Ljj;:
methylphenyl)propanoic acid was prepared from compound 4'7 according to
procedures
described in Example 3. Melting point: 128-I30 °C.
Ex~Die t 3
Synthesis of (3S)-3-{[({1-[(2-chlorophenyl~nethylj-4-hydroxy-2-oxo-1,2
dihydro~3-quiaolinyl}amino)carbonyl]amino}-3-(4-tnethylphenyl)propanoic acid:
S~s~On_e: T'o a solution of compound 48 (2.00 g, 9.70 mmol) in anhydrous DMF
(25 mL) at 0 °C was added NaFi (60% dispersion in rninera~ oil, 0.89 g,
22 mmol). The
mixture was stirred at 0 °C for L5 minutes before the addition of 2-
chlorobenzylchlotide
ZO (2.03 g, 12.6 mmol). After stirring at 55 °C overnight, the mixture
was poured into ice
water and washed with EtzO (twice). The aqueous layer was acidified and
filtration of the
resulting precipitate gave compound 49 (3.45 g). This material was used
without
purification.
(3 S)-3- { [( { I -[(2-chlorophenyl)methyl j-4-hydroxy-2-oxo-1,2-dihydro-3-
quinoIinyl}amino)carbonyl]amino}-3-(4-methylphenyl)propanoic acid was prepared
from
compound 49 according co procedures described in Example 8. Melting point: I
34-136
aC,
By
Synthesis of (3S)-3-{[({ I-[(2-chlorophenyl)methyl]-5-methyl-2-oxo-1,2-dihydro-
3-pyridinyl}amino)carbonylJamino}-3-(4-rnethylphenyl)pr~panoic acid (S6).
Stev One: To a suspension of compound 31 (1.67 g.9.81 mmol) in DMF (33 mL)
at room temperature wader a dry, nitrogen atmosphere, 2-chlorobenaylamine (I
.30 mL,
10.8 mrnol) and EDCI (2.35 g, 12.3 mmol) were added sequentially. The
resulting ,
mixture was vigorously stirred at mom temperature for 5 hours, diluted with
ethyl acetate
and washed with 2 N HCI, Hi0 (3 times), saturated aqueous I~aHC03 and brine.
The
organic layer was dried over MgS04 and filtered and the filtrate was
concentrated under
reduced pressure to give compound 5Z (2.55 8,100%) as ~ pale yellow solid.
Stew Two: A solution of compound 52 (SSS mg, 2.17 mmol) and 3-
dimethylamino-Z-methylpropenal (738 mg, 6.5 mmol) in absolute etha~oI (4.3 mL)
and
glacial acetic acid (0.22 mL) was heated ~to reflux overnight: The resulting
mixture was
cooled to room temperature; diluted with ethyl acetate and washed with 2 N HCi
(twice),
CA 02366800 2002-O1-07
-85_ :.
H_0 and brine. The atgazuc layer was dried over MgSOa and filtered and tha
filtrate was
concentrated under reduced pressure, The preasare was puzified by
chromatography on
silica gel, eluting with 7:3 inc=ensing to 1:1 hexanes:ethyl acetate and
finally 19:19;2
hexanes:ethyl acetate:methanol to yield compound 53 (182 rag, 27°r6) as
a yellow oil.
g Ste e: ?o a solution of compound S3 (167 mg, 0.55 mmol) in THE (3 mL), 2
N NaOH (1 mL) and methanol (2 mL) were added. The resulting mixture was
stirred for
15 minutes, diluted with H20 and extracted with ethyl ether. The aqueous Isyer
was
acidified with 2 N HCl and extracted with ethyl acetate. Tk~e ethyl acetate
layer was
washed with fia0 and brine, dried over MgS04 and filtered. The filtrate was
concentrated
under reduced pressure to give compound S4 (139 mg, 91%) as a white solid.
Step Four: To a suspension of compound S4 (I 75 mg, 0.63 mmol) in ThIF ($.7
mL) and DIPEA (0.23 mL, 1.34 mmol) at room temperature under a dry, nitrogen
atmosphere, DPPA (0.29 mL, 1.34 mmol) was added via syringe. 'The resulting
mixture
was stirred at room temperature for I S minutes, then heated to reflex for 3.5
hours. The
mixture was allowed to cool to room temperature and a solution of compound 8
(278 mg,
1.34 mmol) in THF (b.0 mL) was added via cannula along with a THF (0.7 mL)
rinse.
The resulting mixture was stirred at room temperature overnight, diluted with
ethyl
acetate and washed with 2 N HCl (twice), saturat~T aqueous NaHC03 and brine.
The
organic layer was dried over MgS04 and filtered and the filtrate was
concentrated under
reduced pressure. The residue was purified by silica gel chromatography,
eluting with 7:3
then 3:2 and finally 1:1 hexanes:ethyl acetate to yield compound 55 (60 mg,
20%) as a
colorless oil.
Step Five: To a solution of compound 55 (60 mg, 0.12 mmol) in T'HF (3 mL),
0.192 N NaOH (0.65 mL, 0.12 mmol) and methanol (2 mL) were added. The
resulting
mixture was stirred at room temperature for 24 hours, then was diluted with
H20. The
organic solvents were removed under reduced pressure and the resulting aqueous
mixture
was extracted with ethyl ether. The aqueous phase was lyophilized to give (3
S)-3-{ [( { 1-
[(2-chlorophenyl)methyl]-5-methyl-2-oxo.l,2-dihydro-3- v
pyridiayl~aminoxarbonyl]amino}-3-(4-methylphenyl)propanoic acid, sodium salt
(56, 56
mg, 95%) as an off white solid. MS: Calculated far (CzaH23CIN30a)': 452.14
mJz;
Found: 451.99 mlz.
CA 02366800 2002-O1-07
-86-
Ex~le 15
Synthesis of (3S)-3-(1,3-benzodioxol-5-y1~3-[( {[2-oxo-1-(2-thienylmethyl)-1,2-
dihydro-3-pyridinyl]amino)carbonyl)amino]propanoic acid (62).
Std To a solution of 2-thiophenemethanol (1.015 g, 8.89 mmot) in CHZClZ
(17.8 ml) cooled to O~C under a dry nitrogen atmosphere, triethylamine (2.98
ml, 21,4
mmol) and methanesulfonylchloride (0.69 ml, 8.9 mmol):were added sequentially
by
syringe. The resulting mixture was stirred at 0°C for 15 minutes, then
2-hydroxy-3-
nitxopyridina (1.496 g, 10.7 mmol) and 4-dimethylaminopyridine (catalytic)
were added,
The mixture was allowed to gradually warm to room temperature and then was
stirred
-ZO overnight. The mixture was diluted with ethyl acetate and washed with 2N
HCI, HaO,
saturated NaHC03 and brit:e. The organic phase was dried over MgSOa and
filtered and
the filtrate was concentrated under reduced pressure to give 58 (395 mg) as a
yellow
waxy solid. This material was u~d without purification.
Step Two: To a solution of S8 (330 mg, 1.40 mmol) in glacial acetic acid (6.6
ml)
15 at room temperature under a dry nitrogen atmosphere, irori:powder (I54 mg,
2.8 mmol,
-325 mesh) was added. The resulting solution was heated to 60°C in an
oil bath with
vigorous stirring for 20 minutes. The mixture was cooled to room temperature,
diluted
with ethyl acetate and filtered through Celite. The filtrate was washed with
H20,
saturated NaHCOs and brine. The organic phase was dried over MgSOa and
filtered and
- 20 the filtrate was concentrated under reduced pressure. The residue was
filtered through
silica. gal, eluting with 1:l hexanes:ethxl acetate increasing to 1:3
hexanes:ethyl acetate to
yield 39 (I88 mg, I2% for two steps) as a greenish solid, ,
Stev Three: To a solution of 39 (111 mg, 0.54 mmol) in CHzClz (2.7 ml) cooled
to
0°C under a dry nitrogen atmosphere, N,N-diisopropylethylamine (U.23
ral, 1.30 mmol)
25 and phosgene (0.31 ml, I .9M in toluene, 0.59 mmol) were, added
sequentially by syringe.
The resulting mixture was stirrer at 0°C for 15 minutes, theft a
solution of ~-amino ester
6fl ( 167 mg, 0.70' mmol) in CH2Cl2 (2.7 ml) was added by eanrmla along with a
CH2Ci=
rinse ( 1.0 ml). The resulting mixture was allowed to warm to room
temperature, was
stirred for 2 hours, was diluted with ethyl acetate and washed with 2N HCI,
TdzO,
30 saturated NaHC03 and brine. The organic phase vYas dried over MgS04 and
filtered and
CA 02366800 2002-O1-07
-87-
the fihrate was concentrated under reduced pressure. Tfie~zesidue was purified
by silica
gel chromatography, eluting with I:1 hexanes:ethyl acetate to yield 61 (231
mg, 91%) as
a purple foam.
S~ Four: To a solution of ester 6! (227 mg, 0.48'~~mol) in THF (6 ml) at morn
temperature, NsOH (2 ml; 2N in HsO, 4 mmol) and methanol {enough to give a
clear
solution, approximately 2 ml) were added. The resulting mixture was stirred
for 15
minutes, then was diluted with water and extracted with ether. The aqueous
phase was
acidified with HCl (2N) and extracted with ethyl acetate. ?he organic phase
was washed
with brine, dried over MgS04 and faltered and the filtrate was concentrated
under reduced
la pressure to give 62 (191 mg, 90%) as a white solid. ~H NNYR (400 MHz,
CD3SOCD3) 8
2.63 (d, J = 7.3 Hz, 2H), 4.99 (dt, J ~ 8.4, 7.3 Hz, IH), 5.3:0 (s, 2I-~, 5.98
(m, 2H), 6.21
(dd, J ~ 7.5, 7.0 Hz, IH), 6.78 (dd. 3 = 8. I, 1.6 Hz, lI~, 6:85 (d, J = 8.1
Hz, III, 6.88 (d,
J =1.6 Hz, ll~j6.97 (dd, J = 5.1, 3.5 Hz, 1H), 7.17 (dd,~J = 3.5, I .1 Hz,
lId), 7.35 (dd, J
= 7.0, I .8 Hz, I H), 7.44 (dd, J = S.I, I . I Hz, 1 H), 7.67 (d;" J = 8.4 Hz,
I H); 7.94 (dd, J
?.5, L8 Hz, IH), 8.40 (s, IH).
xaraple 16
Synthesis of (3S)-3-(1,3-benzodioxol-5-yl)-3-[(f((3S)-2-oxo~1-(2
thienylmethyl)hexahydro-3-pyridinylJamino} carbonyl)aminoJpropanoic acid (68).
t O e: To a solution of N-a-tent-butoxycarbonyl-N-S-benzyloxycarbonyl-L-
omithine 63 (1.00 g, 2.73 mmol) and cesium carbonate (I .33 g, 4.I mmol) in
DMF (10
ml) at room temperature under a dry nitrogen atmosphere; iodomechane (0.22
rnl, 3.3
mmol) was added by syringe. The resulting mixture was: stirred at room
temperature for
18 hours then was diluted with ethyl acetate and washedv~ith H=O, 10% NaaS205,
saturated NaHC03 and brine. The organic phase was dried over IvIgSO~ and
filtered and
the filtrate was concentrated under reduced pressure to give ester 64 (I.2lg)
as a pale
yellow oil. This material canta~ned DMF but was used without puri$cation.
a wo: To a solution of 64 (0.86 g of crude material prepared in previous .-.
procedure, 1.94 mmol theoretical) in methanol (10 mI) at 0°C under a
dry nitrogen
atmosphere, palladium on charcoal (300 mg,10% Pd, Degussa type fiIQI NEIW,
wet,
50% water by weight) was added. The nitrogen atmosphere was replaced by
hydrogen
(alternate five times between vacuum and hydrogen supplied by balloon) and the
mixture
CA 02366800 2002-O1-07
-88-
was stirred at 0°C for 30 miautes then filtered directly into'a flask
containing 2-
thiophenecarboxaldehyde (177 mg, I.58 nunol). fine mixture was concentrated
(water
bath at loom temperature) and the residue was taken up in dichloroethane (6
mI). To this
solution, sodium triacetoxyborohydride (479 mg, 2.26 mmol) was added and the
mixture
3 was stirred for 2 hours, diluted with ethyl acetate and washed with
saturated NaFIC03 (2
times) and brine. The organic phase was dried over MgSOa and filtered and the
filtrate
was concentrated under reduced pressur~. The residue was filtered through
silica gel,
eluting with 7:3 hexanes:ethyl acetate to yield lactam 65 (75 mg, 12°~o
for two steps) as a
colorless oil.
Step Three: To a flask containing 63 (89 mg, 0.29 mmol) sealed with a rubber
septum at room temgerature under a dry nitrogen atmosphere, HCl (7.2 ml, 4.0M
in
dioxane, 28.8 rnmoI) was added by syringe. The nitrogen needle was removed and
the
mixture in the sealed flask was stirred overnight. The mixture was diluted
with CH2Cl2
and washed with saturated NaHC03: The organic phase was dried over MgS04 and
filtered and the filtrate was concentrated under reduced pressure to give
amine 66 (60
mg, 100%) as a light yellow oil. This material was used without.purifcation_
Step Four: To a solution of a-amino ester 60 (75 mg, 0.32 mmol) in CHZCIi (0.6
mI) at room temperature under a dry nitrogen atmosphere; earbonyldiimidazdle
(S I rng,
0.32 ramol) was added. The resulting mixture was stirred at room temperature
for 5
minutes and a solution of amine 66 (60 mg, 0.29 mmol) in Cl-IZCI2 (0.6 ml) was
added by
cannula along with a CHaCl2 (0.2 ml) rinse. T'he;resulting rctixture was
stirred at room
temperature for 3 days, then was diluted with atltyl acetate anal washed with
2N HCl (2
times), H20, saturated NaHC03 and brine. The organic phase was dried over
MgS04 and
filtered and the filtrate was concentrated under reduced pressure. The residue
was filtered
through silica gel, eluting with 1:1 hexanes:ethyl acetate increasing to 2:3
hexanes:ethyl
acetate to yield urea 67 (110 mg, 80%).
Step Five: To a solution of urea 67 (108 mg, 0.23.mrnol) in THF (3 ml) at mom
temperature, NaOH (1 rnl, 2N ita HZO, 2 mmol) and rnethaaol (enough to give a
cleat
solution; approximately 2 ml) were added. The r~sulting Fnixture was stirred
for I5
minutes, then was diluted with water and extracted with ether. The aqueous
phase was
acidified with HCI (2N) and extracted with ethyl acetate. The ethyl acetate
layer was
CA 02366800 2002-O1-07
~.y,;.
washed with brine, dried over MgS04 and filtered and the filtrate was
concentrated under
reduced pressure to give 68 (92 mg, 90%) as a white foam. 'H NMR (400 MHz,
CD3SOCD3) a 1.45 (m, lI~, 1.76 (m, 2H), 2.62 (m, 2H), 3.25 (m overlapping HZO,
2H),
4.01 (m, 1 H), 4.59 (d, J =15.0 Hz, 1 H), 4.68 (d, J =15.0 Hz,. 1 H), 4.96 (m,
1 H~, 5.97 (s,
. 2H), 6.24 (d, J ~ 6.6 Fiz, 1 Hi), 6.71 (d, J = 8.4 Hz, 1 H), 6.75 (dd, J =
8.1, 1.5 Hz, I H},
6.82 (d, J = 8.1 Hz, lI~, 6.85 (d, J=1.5 Hz, l~, 6.97 (dd1= 5,1, 3,3 Hz, iH),
7.03 (dd,
J = 3.3,1.5 Hz, 1 H), 7.42 (dd, J - 5.1, 1.5 Hz, l H),12.06 (br. S, lIi}.
Example 1?
Synthesis of (3S)-3-( 1,3 benzodioxoI-5-y1~3-[( t[(3S)-2-vxo-1-(2-
. I0 thienyimethyl}tetrahydto-1H-pyrrol-3-ylJamino}carlmnyl)amino]propanoic
acid (74).
Stan One: To a solution of N-tart-butoxycarbonyl-L-aspartic acid a-benzylester
(2.10 g, b.5 mmol) in dimethoxyethaae (15 mI) cooled to -15°C (bath
temperature) under
a dry nitrogen atmosphere, 4-methylmorpholine (0.71 ml, 6.5 mmoI) and isobutyl
chloroforucate (0.84 ml, 6.S mmol) were added sequentially by syringe. 'The
resulting
mixture was stirred for Z minutes, then was filtered, washing the solid cake
with
dimethoxyethaae (I0 ml). The filtrate was recooled to -15°C (bath
temperature) and a
solution of sodium bomhydride (370 mg, 9.7 mmol) in H20 (3 ml) was added
Followed
immediately by the addition of HZO (100 m1). The mixtui~ was extracted with
ethyl
acetate (3 times) and the organic layers were combined and washed with cold
(0°C) HCl
(O.Z1V), HZO, saturated NaHC03 and brine. The resulting organic layer was
dried over
MgSOa and filtered arid the filtrate was concentrated wader reduced pressure
to give 69
(2.50 8) as a colorless oil. This material contains some of the unreduced
mixed-
anhydride but was used without purification.
Step Two: To a solution of oxalyl chloride (2.4 ml, 2.0 M in CHzCIZ, 4.8 mmol)
in
CHZC)z (30 tnl) cooled to -65°C under a dxy nitrogen atmosphere, a
solution of
methylsuifoxide (0.55 ml, 7.8 mmol) in CH=CIZ (8 ml) was added by syringe. The
resulting mixture was stirred at -65°C for I S minutes, then a solution
of alcohol 69 --
(1.00 g, 3.2 mmol) in CHZCIz (29 ml) was added by canniila along with a CHIC12
(3 ml)
rinse. The mixture was stirred at -65°C for 3 hour8, then was allowed
to warm to -20°C
(bath temperature). Triethylamine (0.96 ml, 6.9 mural} was added, followed by
HZO (20
CA 02366800 2002-O1-07
ml). The aqueous layer was extracted with CH2C12 and the combined organic
phases
were dried over MgSO~ and filtered. The filtrate was concentrated under
reduced
pressure to give aidehyde TO as a white solid. This material was used
immediately
without purification.
Step Three: To a solution of the crude aldehyde 70 (3,Z mmol theoretical) and
2-
aminomefhylthiophene (402 mg, 3.55 mmol) in dichioroettiane (13 ml) at room
temperature under a dry nitrogen atmosphere, sodium triacAtoxybomhydtide (959
mg, 4,5
nunol) was added. The resulting mixture was stirred at roorri temperature
overnight, then
was diluted with ethyl acetate and washed with saturated NapICOa and brine.
The organic
. 10 phase was dried over MgS04 and filtered and the filtrate was concentrated
under reduced
pressure. The residue was purified by silica get chromatography, eluting with
1:1
hexanes:ethyl acetate to yield laetam 71 (220 mg, 23% for 3 steps) as a white
solid.
Step Four: To a solution of 71 (220 mg, 0.74 mmoiy in dioxane ( 1.5 ml) sealed
with a
rubber septum at room temperature under a dry nitrogen atmosphere, HCl (1.50
ml, 4.0M in
dioxane, 6.0 mmol) was added by syringe. The nitrogen naedle was removed and
the mixture
in the sealed flask was 6tixred for 5 hours. The mixture was diluted with
CHaCIz and washed
with saturated NaHC03. The organic phase was dried over MgSO.~ and filtered
and the
filtrate was concentrated under reduced pressure to give amine 72 (I29 mg,
89%) as a light
yellow oil. This material was used without purification.
Step Five: To a solution of amine 72 (12? mg, 0:63 mmol) in CH2CI2 (I.5 ml) at
room temperature under a dry nitrogen atmosphere, carbonyldiimidazole ( 112
mg, 0.69
mmol) was added. The resulting mixture was stirred at room temperature for 5
minutes
and a solution of /3-amino ester 60 (164 mg, 0.69 mmol) in~'CH2C12 (0.8 ml)
was added by
cannula along with a CH=C12 (0 ? ml) rinse. The resulting mixture was stirred
at mom
temperature overnight, then was diluted with ethyl acetate and washed with 2N
HCl (2
times), HaO, saturated NaFiC03 and brine. The organic phase was dried over
Mg504 and
filtered and the filtrate was concentrated under reduced pressure. The residue
waa filtered
through silica gel, eluting with 49:1 chloroform:methanol to yield urea 73
(230 mg, 800)
as a colorless oil which slowly solidified on standing. ,
t Six: To a solution of urea 73 (230 mg, 0.50 mmol} in THF (3 ml) at room
temperature, NaOH (1 ml, 2N in H20, 2 mmot) and mettsanol (1 ml) were added.
The
CA 02366800 2002-O1-07
-91-
resulting mixture was stirredfor ! hour, then was diluted with water and
extracted with
ether. The aqueous phase was acidified with HCi (21~ and extracted with ethyl
acetate.
The ethyl acetate layer was washed with brine, dried over IVIgS04 and
filtered. and the
filtrate was concentrated under reduced pressure to give 74(181 mg, 84%) as a
white
S foam. ~H NMR (400 MZ<Iz,, CD3SOCDj) ~ 1.64 (m, 1 H), 2:30 (m, 1H), 2.64 (m,
2H),
3.20 (m, 2H), 4.17 (dd, J = 8.8, 8.4 Hz, 11~, 4.56 (s, 2H), 4.96 (zn, 1H),
5.97 (s, 2H), 6.30
(d, 3 $ 7.0 Hz, IH), 6.58 (d, J = 8.8 Hz, 1I~, 6.77 (m, 1H), 6.80-6.90 (m,
2H), 6.96-7.04
(m, 2H), 7.45 (dd; J = 5.1, 0.7 H2, 1H), 12.10 (br. s,1H).
Ex_a~ple 18
Synthesis of (3S)-3-[(t[5-chloro-2-hydroxy-3-
(phenylmethyl~henyl]amino}carbonyl)amino-3-(4-methylphenyl)propanoic acid.
Step , ne: To a mixture of 2-phe~rylmethyl-3-chloraphenol (5.00 g, 22.9 mmol)
in
EtZO (20 mL) and 6N HCl (50 mL), KN03 (2.30 g, 22.9 miizol) arid NaN02 (20 mg;
;. ;; ._
catalytic) were added sequentially. The resulting mixture vvas stirred for 2
hours, diluted
IS with water and extracted with ethyl acetate. The organic Iayer was washed
with water
and brine, dried over MgSO~ and filtered. The filtrate was concentrated under
reduced
pressure to give 99 (6.4 g, 100%).
Two: To a solution of 99 (6,0 g, 22.8 mmol) in methanol (360 mL), zinc
powder (6.0 g; 92 mrnol) and sat~.rated aqueous NH4C1 (6 m>r) were added. The
resulting
heterogeneous mixture was refluxed overnight, After filtration of the hot
mixture and
concentration of the filtrate under reduced pressure, the residue was
dissolved in ethyl
acetate and washed with saturated aqueous NaHC03 and brine. The organic layer
was
dried over MgSOa and $ltazed and the filtrate was concentrated under reduced
pressure to
give compound 100 (2.93 g, 55%).
Step Thr~g: To a solution of 25 (0.20 g, 0.96 mmol) in CH2CI2 at 0 °C,
DIP13A
(0.40 mL, 2.4 rnmol) and phosgene (1.93 M in totuene, 0.b0 mL; 1.2 mmol) were
added
sequentially. The resulting mixture was allowed to warm to room temperature,
stirred for ~ ---
20 minutes, then recooled to 0 °C. To this mixture, a solution of 100
(0.25 g~ l .1 mmol)
in CHZC12 was added dropwise. The resulting mixture was allowed to warm to
room
temperate=e overnight, was diluted with water and was extracted with CH2C12.
The
organic layer was washed with water and brine, dried oYer, MgSOa and filtered.
The
CA 02366800 2002-O1-07
:92-
filtrate was concentrated under reduced pressure and the residue was purified
by silica gel
,Sj'T
chmmatogtaphy, eluting with 9:1 and increasing to 5:1 hexanes:ethyl acetate to
give 101
(60 mg, 12a/o).
(3S)-3-[({[5-Chloro-2-hydroxy-3-(phenylmethyl)phen~yl]amino} carbonyl)amino)-
3 3-(4-methylphenyl~ropanoic acid was prepared from 101 by procedures
described in
Example 1. ~H NMR (400 MHz, CD3S0=CD3) 82.26 (s, 3H), 2,58 (dd, J = 15.8, 6.6
Hz,
iIi), 2.67 (dd, J =15.8, 8.4 Hz, 1H), 3.49 (s, 2~, 4.88 (rn, 1H), 7.00-7.70
(m, l3Fi),
11.95 (br. s, 1H).
Exam 1~ a 19
IO Synthesis of (3S)-3-(1,3-benzodioxol-5-yl)-3-[(zbutyl[2,5-dioxo-1-
{phenylmethyl)tetrahydro-1H-pyrrol-3:y1)amino}carbonyl)amino]propanoic acid.
:,
Step One: A solution ofN-beazylmaleimide (2.60 g,~ 13.9 mmol) and n-
butylamine (1.00 g, I3.7 mmol) in T'TxF (15 mL) was stirred at room
temperature
overnight and concentrated under reduced pressure. The residue was purified by
silica
IS gel chromatography, eluting with 4:1 increasing to 2:1 hexanes:ethyl
acetate to give 102
(3.25 g, 90%).
(3S)-3-(1,3-Benzodioxol-5-yl)-3-[( {butyl[2,5-dioxo=I-(phenyhnethyl)tetxahydro-
IH-
pyrrol-3-yl)amino'~carbonyl)amino)propanoic acid was prepared from 102
according to
procedures described in Exempla I. MP: 80-85 °C.
20 ~ple 20
Synthesis of (3S)-3-(1,3-benzodioxol-5-y1~3-[({[1-(cyclopentylmethyl)-2-oxo-
1,2-
dihydro-3-pyridinyl)amino}carbonyl)amino)propanoic acid.
Step One: To a solution of 2-hydroxy-3-nitropyridine (200 mg, 1.4 mmol) in
CHiCl2 (14 mi:l at 0 °C under a nitrogen atmosphere,
cyclopentanemethanol (178 mg,
25 1.78 mrnol) was added followed by triphenyiphosphine (551 mg, 2.1 mmol).
The
solution was t:drred at 0 °C for 15 minutes and diethyl
azodicarboxylate (366 mg, 2.1
mmol) was added dropwise'via syringe. The reaction was allowed to stir at 0
°C for one -w
hour aad then at room temperature overnight. The mixture was quenched with
methanol
(20 mh) and washed with water (twice). The aqueous layer was extracted with
30 dichloromethane and the combined organic layers were dried over magnesium
sulfate and
filtered. The $ltrate was concentrated and the residue wasrpurified by silica
gel
CA 02366800 2002-O1-07
-93- ,, :
chromatography, eluting with 1;i hexanes:ethyl acetate to afford 103 (299 mg,
96% yield)
as a yellow solid.
(3 S)-3-(1,3-Benzodioxol-5-yt)-3-[( { [ Z -(cycloper~tylmethyl)-Z-oxo-1,2-
dihydro-3-
pyridinyl]amino}carbonyl)amino]propanoic acid was prepared from 103 according
to
S procedures deson'bed in Example 1. ~H NMR (400 MHz, C,DC13): S i.2-I.7 (m,
8H), 2.34 (m,
1H), 2.81 (dd, 3 = , 1H), 2.95 (dd, J =, IH), 3.92 (d, J -- T.? Hz, 2H), 5.30
(m, 1H), 5.92 (m,
2H), 6.30 (t, 3 = 7.1 Hz, 1 H), 6.68-7.00 (m, 5H), 8.33 (d, J = 7.7 Hz, 1 H),
8.89 (s, 1 H).
E~Qe 21
Synthesis of (3S)-3-(1,3-benzodioxol-S-yl)-3-{j( {3 '-
((2~thiophenylrnethyl)amirto~
IO phenyl}amino)carbonyl]amino}propanoic acid.
Step One: To a solution of 2-thiophenecarboxaldehyde (0.48 g, 4.0 mmol) in
dichiorornethane was added 3 nitroaniline (U.51 g, 3.7 mmol). The solution wes
concentrated to dryness and brought up in 1,2-dichloroethane (I6 mL).
Molecular sieves
(3d, 1.1 ~ were added followed by NaHH(OAc)3 (1.01 g, 4.8 mmol). The solution
was
15 stirred overnight at room temperature, diluted with chloroform and washed
with water:
The organic Layer was dried over MgS04 and filtered and the filtrate was
concentrated
under reduced pressure to give 104 (0.72 g, 84%).
S Two: To a solution of 104 (0.30 g; I.3 mmol),.in CH2ClZ (5.2 mL) at:d
S1._
triethylamine (0.215 mL, 1:5 mmol) at 0 °C was added irifluoroacetic
anhydride (0.193
20 tnL, 1.4 mmol). The solution was stirred Z 5 minutes at 0 °C, the
ice bath was removed
and the mixture was stirred for an additional 15 minutes. The mixture was
diluted with
CHzCiz, washed with 2N HCI, water and brine. The organic layer was dried over
NazS04
and Sltered and the filUrate was concentrated under reduced pressure to give
LOS (0.38 g,
100 %) as a yellow solid.
25 Steo Three: To a solution of !OS (0.38 g, 1.4 mmot~ in ethanol (2.6 mL) and
acetic
acid (2.6 mL) at room temperature, Fe powder (0.36 g, 6.5 mmol) was added and
the
suspension was stirred vigomusIy at 40 °C until TLC indicated complete
consumption of w
LOS. The mixhtre was filtered through Celite, washing wig chloroform. The
filtrate was
diltuted with saturated sodium bicarbonate and the chloroform Layer was dried
over
30 NaZSOa and filtered. 'The filtrate was concentrated under reduced pressure
and the residue
CA 02366800 2002-O1-07
-94-
was purified by chromatography on silica gal (gradient elution 6:1 to 4:1
hexanes:ethyl
acetate) to give compound 106 (0.102 g, 25%)
{3S)-3-(1,3~Benzodioxol-5-yl)-3- f [({3-[(2-thiophenylmethyl)amino]phenyl}
amino)earbonyl]amino}propanoic acid was prepared from I06 according to
procedures
described in Example 1. 1H NMR (400 MHz, CD3SOxCD3j S 2.50 (m, 2H overlapping
DMSO), 4.37 (d, J = 5.9 Hz; 2H); 4.94 (m, 1H), 5.94 (m, 2H), 6.06 (t, J = 5.8
Hz, 1 H), 6.16
(m. 1H), 6.59 (d, J = 8.8 Hz, 1H), 6.78 (m, 3H), 6.85 (dd, J~~= 8.8, 7.7 Hz,
1H), 6.90 (s, 1H),
6.94 (dd, J = 5:2, 3.7 Hz, 1F~, 7.00 (d, J = 3.3 Hz, 1H), 7.33 (dd, 3 = 5.1,
1.1 Hz, 1 H), 8.5 (s,
1H).
E~ 22
Synthesis of 3-(1,3-benzodioxol-5-yl)-2,2-difluoro-3-[({[2-oxo-1-(2-
thiophenylmethyl)1,2-dihydro-3-pyridinyl]amino}carbanyl)amino]propanaic acid.
Stev One: To a solution of ( 1 S,2R,SS)-(+)-menthyl (F~)-p-toluenesulfinate
(3.00 g,
10.2 mmol) in THF (25.5 mL) chilled to -78 °C, lithium bis(h-
imethylsilyl)amide (1.0 M is
THF, 15.3 mL) was added dropwise over 15 minutes. The resulting mixture was
stirred at
room temperature for 6 hours, then chilled to 0 °C. Piperonal (3.06 g,
20.4 rnmol) and CsF
(3.10 g, 20.4 mmol) were added rapidly and the suspension stirred 36 hours at
room
temperature, The reaction was quenched with saturated NHaCI and extracted with
ethyl
acetate. The organic layer was washed with brine, dried over NaZSOa and
filtered and the
filtrate was concentrated under reduced pressure. The residue was
recrystahized from
hexanes and dichloromethane to give compound 108 (1.36 g, 46 %)
Step Two: Ethyl bromodifluoroacetate (0.78 mL, 6.1 rnmol) was added to a
suspension of Zn dust (2.00 g, 30.5 mmol) in THF (20.2 rnL) and retluxed for
15 minutes.
The suspension was chilled to 0 ° C and lOS (0.87 g, 3.0 mural) was
added. The suspension
was allowed to warm to room temperature and stirred overnight. The mixture was
quenched
with a minimum atrrount of saturated NH4C1 and extracted with ethyl acetate.
The organic
layer was washed with saturated aqueous NaHC03 and brine, dried over Na2S04
and filtered. -
The filtrate was concentrated under reduced pressure and the residue was
purified by
chromatography on silica gel (gradient elution 6:1 to 4:1 hexanes:ethyl
acetate to give 109
(0.607 g, 61 % at 80% conversion).
Step TMee: To a solution of 109 (0.700 g, 1.70 mmol) in methanol (4.3 mL) at 0
CA 02366800 2002-O1-07
-95-
°C, trifluoroacetic acid (0.26 mL 3:4 mmol) was added. Tlie solution
was stirred at 0 °C
for 2 hours, then concentrated to dryness under seduced pressure, while
maintaining the
external temperature below 30 °C. The residue was taken up nn diethyl
ether and washed
with 2N HCl (2 times). The combined aqueous layers wetewcarefuliy basified
with excess
saturated NaHC03 and extracted with diethyl ether. The ether layer was dried
oyez
MgSOa and filtered and the filtrate was concentrated under reduced pressure to
give 110
(0.326 g, 80 %).
3-(1,3-Benzodioxol-5-yir2,2-difluoro-3-j( f j2-oxo-1-(2-thiophenylmethyl)-1,2-
dihydro-3 pyridinyl]amino}carborryl)amirro]propanoic acid was prepared from
110
according to procedures described in Example 1. MS: Calculated (M-Hf ---
476:07;
Found (M-FI~-=476.x.
Examvle 23
Synthesis of {3S)-3-(1,3-benzodioxol-5-yI)-3-({[9-~oxo-8-(phenylmethyl)-
2,3,4,5,8,9-hexahydro-IH-pyrido[3,4-b]azepin-1-yl]carboriyI}amino)propazyoic
acid.
Step One: To a solution of 3 (0.74 g, 3.6 mmoI) in T'iiF (14.4 mL) and TMEDA
(1.b4 mL, 10.8 mmol) at -20 °C, n-butyllithium (1.6 M in hexanes, 3.4
mL, 5,4 mmol)
and tart-butyllithium (1.7M in pentane, 2.5 mL, 4.3 mmol) were sequentially
added
dropwise by syringe. 'Ihe temperature was allowed to wanes to between -10 and
0 °C and
maintained there for 2 hours. To the resulting mixture, 1,4-d~bromobutane
(z.75 mL,
ZO 14.7 mmol) was added rapidly and the solution was allowed to warm to room
temperature
and stirred for 4 days. The reaction was quenched with water and extracted
with CHCI3 (3
times). The combined extracts were washed with brine, dried over NaS04 and
filtered.
The filtrate was concentrated under reduced pressure and the residue was
purified by
chromatography on silica gel, eluting with 4;1 hexanes:ethyl acetate to give
111 (0.41 g,
44%).
(3S)-3-( 1,3-Benzodioxol-5-y1)-3-( {[9-oxo-8-(phenyirnethyl)-2,3,4,5,8,9-
hexahydro-1 H pyrido[3,4-b~azepin-1-y1]carbonyl) amino~ropstwie acid was
prepared ~--
from 111 according to the procedures described in Exaraple 4. MS: Calculated
(M-H)'=
488.18; Found (M-H)' ~ 488.21.
CA 02366800 2002-O1-07
Examrle 24
Syntheses of (3Sr3-{[({I-[(2-chloroph~nyl)taethyl]-2-oxo-1,2-dihydro~3-
pyridinyl}amino)carbonyl]amino}-3-(4-hydroxyphsnyl)propanoic acid.
Step Cyne: To a solution of I12 (prepared according to procedures described in
Example 15, 0.19 g, 0.39 mmol) in CHZCIZ at 0 °C under nitrogen, BBr3 (
1.0 M in
CHaCIz, 1.2 mL, 1.2 mmol) was added by syringe. The mixture was allowed to
gradually
warm to room tea~cperature and then stirred overnight The mixture was diluted
with
water and stirred for 30 minutes and further diluted with saturated aqueous
NaFiCOj. The
organic Isyer was washed with water and the aqueous layers were combined arid
acidified
IO with 2N ~IC1 and extracted with ethyl acetate (3 times). The combined ethyl
acetate
layers were dried over MgSOa and filtered and the filtrate eras concentrated
under
redteced pressure to yield {3S)-3-{[({1-[(2-chlorophenyl)rilethyl]-2-oxo-1,2-
dihydro-3-
pyridinyl}amino)carbonyl]amino}-3-(4-hydroxyphenyi)propanoic acid (113, 120
mg,
70%). IH IvMR;(400 MHz, CD3SOzCD3) 8 2.95 (d, J s 5.2 H2, 2H), 5.28 (s, 2H),
5.35
1S (ddd, J = 9.2, 4.8, 4.4 Hz, 1H), 6.33 (t,1 ~ 7.1 Hz, 1H), 6.60 (d9 J = 8.8
Hz, 2fi), 7.04 (rn,
5H), 7.22 (m, 3H), 7.37 (dd, J = 7.7, 1:5 Hz, 1 Fi), 8.35 {dd, J -- 7.6, 1.5
Hz, 1 H), 8.80 (s,
1 H).
Exam 1~?5
Synthesis of(3S)-3-[({[l-(2-ehlorobenzyl)-4-hydroxy-5-methyl-2-oxo-I;2-
20 dihydropyridin-3-yl]arrrino}carbonyl)amine]-3-(4-methylphenyl)propanoie
acid, I19.
t One: To a suspension of sodium hydride {3.6 g of 60% dispersion in mineral
oil,
90 mmol) in THF (300 mL) under a dry nitrogen atmosphere, TMLDA (13.2 mL, 87.5
rnmol)
was added and the mixture was cooled to -20 °C. Methyl propionylacetate
(9.60 mL, 76.5
mmol) was added dropwise and the solution was sdxsed for an additional 15
minutes. A
25 solution of n-hutyllithium (90 mL, 1.6M in hexanes, 144 mrnol) was added
dropwise and the
resulting mixture was stirred at -20 °C for 15 minutes. Methyl formate
(6.0 mL, 97 mmol) was then added rapidly and the mixture was allowed to stir
for 15 minutes
.;
before quenching with HCI (2 N, 250 mL). The reaction v~ias diluted with
diethyl ether (I50
m.L) and the organic layer was washed twice more with water. The aqueous
layers were
CA 02366800 2002-O1-07
;."
-97-
combined aad sodium chloride was added until saturated. This mixture was
extracted-with
ethyl acetate (3 times}. The original ether layer was washed with saturated
sodium
bicarbonate solution and water. The combined aqueous washes were acidified
with excess
HCi (2 N), saturated with sodium chloride and extracted with ethyl acetate (3
times). All of
the ethyl acetate extracts were combined and dried over lvlgSOø. The resulting
mixture was
vacuum filtered through coarse silica gel and the filtrate was concentrated
under reduced
pressure to give 114 (8.278, 68%) ae a light yellow oil. This material was
used without
further purification.
To a solution of 114 (3.958, 25.0 mmol) in anhydrous methanol (225mL)
at room temperature, a solution of 2-chlorobenzylamine (4.2 g, 30 mmol) in
anhydrous
methanol (25 nxL) was added drvpwise from an addition funnel. The solution was
heated at
45 °C overnight then refluxed for two hours. The reaction mixture was
cooled to room
temperature and concentrated to dryness. The residue was brought up in
dichloromethane and
filtered. The solid was collected and dried under vacuum to give 115 ( 2.20 g
35%) as a light
yellow solid.
See e: To a suspension of 11S (840 mg, 3.4 rtimol) in glacial aceric acid (11
mL)
at room temperature, NaNOZ (46 mg, 0.67 mmol), water (0.92 mL) and HN03 (70%,
0.85
mL, 13.4 mrnol) were added sequentially. The resultin8 bright yellow solution
was stirred at
room temperature overnight, then was diluted with CHZCl2 and water. The
aqueous phase
was extracted with CH2CIz, the organic layers were combined arid washed with
water (3
times} and brine. 'The organic layer was dried over MgS04 and filtered and the
filtrate was
concentrated under reduced pressure to give 116 (910 mg,.92%) as a bright
yellow solid.
This material was used without purification,
St our: To a solution of 116 (910 mg, 3.I mmol) in DMF (10 .3 mL) at room
temperature under a dry nitrogen atmosphere, Zn powder (909 mg, 13.9 mmol) and
triethylamine hydrochloride (2340 mg, 17.0 mmol) were added. The resultin8
mixture was
heated to 55 °C for 2 hours, then was cooled to room temperature. To
the resulting mixture,
CA 02366800 2002-O1-07
CDI (1002 mg, 6.18 mmol) was added as a solid. Upon addition, gas evolution
occurred.
T ha mixture was then heated to 80 °C for 1 hour, cooled to room
tempezature, and diluted
with CH2Clz and HCl (21~. The aqueous phase was exttacted with CI~CIa, the
organic
layers were cortibined and washed with water (4 times) and brine. The organic
layer was
dried aver MgS04 and filtered and the filtrate was concentrated under reduced
pressure to
g.ve 117 (920 mg) as a yellow solid. This materiel contained a small amount of
AMF and
was used without purification.
Step five: A suspension of 1I7 (970 mg coxde material, 3. I mmol t:seoretical)
and 8
(800 mg, :;:8d mmol) in 21 ml THF under a dry nitrogen atmosphere was heated
to 5s °C
i(~ overnight, cooled to room temperature and then diluted with ethyl acetate.
The resulting
mucntre was washed twice with HCl (2N} and brine and the organic layer was
dried over
PfgS04 and ftitered. The filtrate was concentrated under reduced pressure and
the resulting
;aidue was purified by silica gel chromatography, eluting with 7;3
hexanes:ethy? acetate to
give 118 (1095 mg, 71% for two steps) as a pale yellow foam,
1 S S~Six: To a solution of 118 ( t b91 rag, ~ .19 mmol) in THF ( 18 rnL) a2
Toorr:
ternperati.re, aoditlm hydroxide (2 N, 6 nL.) and methanol (12 mL) were added.
't he mixture
cLas stirred fov 2(i rrtinutes, theta :'as diluted with water and extracted
with Whvl ether. The
queous phaar was acidif'.ed with HCI (2 N) end cxtzacted with ethyl a~:etate.
The ethyl
aceta~ie layer was washed with water and brine; dried over MgSos arid
filtered. The filtrate
20 was concentrated under reduced pressure :o give (3S)-3-~(([I-(2-
chlorobea2yi)-4-hydroxy-5-
methyl-i-oxo-1,2-dihydropyridin-3-yl~amino; carbonyl)arninoj-3-(4-
mcthy~phenyl)propanui:,
acid, 119, (1045 .nb, quantitativaj as a white taam MS: Calculated (M-
'rT)'~=X68.13 mJz;
found (M-H)' = =10'1.99 mlz.
;:.xamala 16 __.
25 Synthesis :~f (3S)-3-[({[4-hydroxy-2-cxo-1-(pyridin-Z-ylmethylj-I.l-
dihydropyridin-
'~'-yi]aminojcarbuny;lamino~-3-(4-methylphenyl)propanoic acid.
Ste~(~ng: To a solution of Z3 (0.50 g, 3,.~ mmai) in DMSC c;:2.5 rr~i) at room
CA 02366800 2002-O1-07
-99-
temperature, powdered KOH (0.89g, 16 mmol) was added and the mixture was
stirred for 1.5
hours. To~ the resulting mixture, Z picolylchloride hydrochloride (0.638, 3.8
mmol) was added
as a solid axed the mixture was stirred overnight. At this.point,
triethylamine hydrochloride
(3.52 g, 25.6 mmol) and DMF (5 mL) were added followed by zinc powder ( 1,04
g, 16.0
mmol). The mixture was heated to 80 °C for 2 hours then cooled to room
temperature. To
this mixture, CDI (1.00 g, 6.2 mmol) was addad and the resulting mixture was
heated to 80
°C overnight. The mixture was diluted with ethyl acetate and saturated
aqueous NaHC03.
The organic layer was dried over MgS04 and filtered and the filtrate was
concentrated under
reduced pressure. The residue was filtered through a pad of silica gal,
eluting with 9: l
CHC13:CH30H to give 120 (0.14 g, 18%).
(3S)-3-[( {[4-Hydroxy-Z-oxo-1-(pyridin-2-ylmethyl)-1,2-dihydropyridia-3-
yl]amino}carbonyl)aminoJ-3-(4-methylphenyI)gropanoia acid was prepared from
124
according to procedures described in Example 25. MS: Calculated (M-H)' =
421.15 m/z;
Found (M-H)' = 421.06 m/z.
ExamQe 27
Synthesis of (3S)-3-{[({1-[2-chloro-5-(methytsulfoz~yl)benzyl]-4-hydroxy-2-oxo-
I,2-
dihydropyridin-3-yl}amino)carbonyl]amino}-3-(4-methylphenyI)propanoic acid.
Sten One: To a solution of 121 (prepared from 23 according to procedures
described
in Example 4, 220 rug, 0.67 mmol) in anhydrous CH,Ch (1,4 mL) cooled to 0
°C under a dry,
nitrogen atmosphere, m-CPBA (610 mg, 3.6 mmol) wasadded; The resulting mixture
was
allowed to warm to mom temperature and stirred for 4 houxs. The reaction was
diluted with
water (50 ml) and the aqueous phase was extracted with CIizCI2 (2 times). The
combined
organic layers were dried over MgS04 and filtered and the filtrate was
concentrated under
reduced pressure. The residue was purified by silica gel chromatogaphy,
eluting with 9:I
CHCI3:MeOH to give 122 (219 mg; 9I% yield) as a yellow solid.
(3S)-3-{(( ( 1-[2-Chloro-5-(methylsulfonyl)benzyl]-4-hydroxy-2-oxo-I,2-
dihydropyridin-3-yl}amino)carbonyl]arrzino}-3-(4-methylphenyl)propanoic acid
was prepared
CA 02366800 2002-O1-07
-100-
froze 122 according to procedures described in Example Z5. MS: Calculated (M-
H)- = 532,10
mlz; Found (M Iy = 531.94 mlz.
E~IZIe 28
Synthesis of (3S~-3-[({[1-(2-chloro-6-tnethoxybenzyl)-4-hydroxy-2-oxo-1,2-
dihydropyridin-3-yl)amino}carbonyl)amino]-3-(3-methylphenyl)propanoic acid.
~t ,~C>~e: To a solution ofthe 113 (70 rag, 0.13 mmol) in anhydrous CH2CI2 (3
mL),
stirring under a nitrogen atmosphere, ZnBrZ (200 mg, 0.82rnmol) was added. The
solution
was stirred at 0 °C for one hour. The reaction mixture was allowed to
wazizz to room
temperature and was stirred overnight. At this point, water (50 ml) was added
and the
I0 mixture was stirred for an additional three hours. The layers were
separated and the aqueous
Layer was extracted with CHZCI~ (2 times). The combined oxganic layers were
dried over
MgSOa and filtered and the fzlcrate was concentrated under reduced pz~essure
to give (3 S)-3-
[{ ([1-(2-chlozo-6-methoxy6enzyl)-4-hydroxy-2-oxo~1,2-dnydropyridin-3-
yl]amino}carbonyl)amino)=3-(3-znethylphenyl)propanoic acid, 124 (60 mg,
95°/a yield). MS:
Calculated (M-H)' = 484.13 mlz; Found (M-H)' = 484.00 zn/z.
Exam~e 29
Synthesis of (3S)-3-(( f [1-(2-chlorobenzyl)-4-hydroxy-2-oxo-5-propyl-1,2-
dihydropyridin-3-y1]amino}carbonyl)amino]-3-(4-methylphenyl)propanoic acid.
Stets One: A mix~re ofmalonyl dichloride (25.0 g, 177 mmol) and vaIeronitrile
(25.0
g, 300.7 znmol) under an anhydrous atmosphere was vigorously stinted at room
temperature
for 24 hours. ~ Diethyl ether,(50 mL) was added w the resulting heterogeneous
mixture. 'Ihe
precipitate was collected and Washed with diethyl ether to give 125~HCl as a
white solid (20.2
~e 64%).
,S_teo Two: To a suspension of 125~HCl {6.10 g, 27:2 znmol) in EtOH ( 100 mL),
triethylamine (5.$ g, 57.3 mmol) and palladium on carbon (I O % Pd dry weight
basis,
Degussa type E I 01 1~TE/W, -.50% water content, 3.5 g, L6 mmo3 Pd) were
added, The
atmosphere was replaced with hydrogen (toggle between vacuum and hydrogen from
a
CA 02366800 2002-O1-07
-101-
balloon five times) and the raixture was stirred overnight, then filtered. The
filtrate was
concentrated under reduced pressure to give 126~2Et3NHCi (11.0 g, 94%). This
material was
used without further purification.
(3 S)-3-[( { [ I -(2-Chlombenzyl)-4-hydroxy-2-oxo-S-pmpyl- L,2-dihydropyridin-
3-
yI]amino}carbonyl)aminoJ-3-(4-methylphenyl)propanoic abi~3 was prepared from
126~2Et3NHCi according to procedures described in Example 25. MS: Calculated
(M-H)- _
496.16 m/z; Found (M-H)- = 495.94 m/Z.
Example 30
Synthesis of (3S)-3-~({jl-(2-chlorobenzyl)-4-hydroxy-2-oxo-2,5,6,7-tetrahydro-
1H-
cyclopenta[b]pyridin-3-yl]amino}carbonyl)amizioJ-3-(4-methylphenyl)propanoic
acid.
to One: To a solution of ethyl 2-oxocyciopentanecarboxylate (3.30 g, 21.1
mrnol) in
toluene (45 m1), 4-chlorobenzylamine (2.56 mL, 21.1 mmol) was added. The
resulting
mixture was refluxed overnight with azeotropic removal of water via a Dean-
Stark trap. The
reaction mixture was concentrated under reduced pressure to give 1Z? (5.90 g,
99%) as a red
1S oil. This material was used without purification.
Step Two: To a solution of 127 (I I.O g, 39.3 mmol) in anhydrous TFiF (75 mL)
cooled to 0 'C under a dry, nitrogen atmosphere, NeH (60% dispersion in
mineral oil, 1.73 g,
43.2 mmol) was added, The reaction was stirred for 10 minutes at 0 °C,
then acetyl chloride
(3.9 tnL, 55 mrnol} was added. The reaction mixture was allawed ~o gradually
warm to zoom
temperatuze, then was stirred overnight. The resulting mixture was
concentrated under
reduced pressure and a mixture of ice water (200 mL) and HC;1 (1 N, 200 mL)
was added to
the residue. This mixture was extracted with ethyl acetate (300 mL) and the
ethyl acetate
layer was dried ever MgSOa and filtered. The filtrate was cbnceatrated under
reduced
pressure to give 129 (13.4 g) as a brown oil. This material contained mineral
oil but was used
without purification.
Sten Three: To a solution of crude 1Z8 (13.4 g, 39.3 mmol theoretical) in
anhydrous
THF (50 mI) cooled to 0 °C under a dry, nitrogen atmosphere,
lithium
CA 02366800 2002-O1-07
-102-
bis(ttirnethylsilyl)amide (L0 M in THF, 125 mL, 125 mmol) was added slowly via
syringe.
The reaction mixture was allowed to warm to room temperature, then was stirred
overnight:
The mixture was concentrated under reduced pressure and the residue was
triturated with
ethyl acetatelhexane and filtered. The solid was washed with hICI (1 N, 250
ml) and water
S (500 ml) to give 129 (5.488, 48% for two steps) as a brow~..solid,
<;.
(3S)-3-[({[I-{2-Chlorobenzyl)-4-hydra?cy-2-oxo-2;5;6,7-tetrahydro-1 H-
cyclopenta[b]pyridin-3-yl]amino}carbonyl)amino]-3-(4-methylphenyl)propanoic
acid was
synthesized from 129 according to procedures described in Example 25. MS:
Calculated
(M+H)+= 496.16 ~Iz; Found (M+H}+ = 495,99 m/z.
I0 Exam_ nle 31
Synthesis of (3S)-3-[( f [4-{[(tent-burylamino)carbonyl]amt»o}-1-(2-
chlorobenzyl)-2-
oxo-1,2-dihydropyridin-3-yl]amino}carbonyl)amino]-3-(4-methylpheayl)propanoic
acid.
S One: To a solution of 46 (500 mg, 1.79 mrnol),in anhydrous THT' (10 mL)
cooled
to 0 °C under a dry nitrogen atmosphere, NaH (60% dispersion in mineral
oil, 210 mg, 5.3?
IS mmol) was added and the resulting mixture was stirred for 20 minutes. To
this mixture, tert-
butyl isocyanate {0.3I mL, 2.68 mmol} was added and the reaction mixture was
allowed to
warm to room temperature, then was stirred for 2 days. The reaction mixture
was quenched
with water and extracted twice with ethyl acetate. The organic layers were
combined, dried
over MgSOa and filtered and the filtrate was concentrated under reduced
pressure to give 130
20 {660 mg, 9?%) as a brown solid_
(3S)-3-[( { [4-{((tert-butylamiao)carbonyl]amino} -1-(Z-chlorobenzyl)-2-oxo-
1,2-
dihydropyridin-3-yljamino}carbonyl)amino]-3-(4-methylphenyl)propanoic acid was
prepared
frora 130 according to procedures described in Example 3. MS: Calculated (M-
H)' = 552.20
m/z; Found (M-H)' -- 551.89 m/z. "'
25 Synthetic procedures similar to those described above may be utilized to
obtain the compounds of Tables 2, 3, 4 and 5.
CA 02366800 2002-O1-07
-103-
fixample 32
A. _f, .,
Synthesis of {3S)-3-[({[5-chloro-1-(2-chlorobenzyl)k-4-hydioxy-2-oxo-1,2-
dihydropyridin-3-yl]amino}carbonyl)aminoJ-3-(4-methylphenyl)propanoic acid.
Step One: To a solution of 31 (350 mg, 0.72 mmol) in CH=Cla at room
temperature
S under a dry nitrogen atmosphere, sulfurylchloride (1.0 M iri CHzCI=, 0.65
mL, 0.65 mrrtol)
was added by syringe, The resulti~ag mixture was stirred at room
ter:riperature for 1 hour, then
was partitioned between CI~2CIa aad.water. The organic layer was washed with
brine and
dried over MgSOa and the filtrate was concentrated undez reduced pressure. The
residue was
purified by silica gel chromatography, eluting with 8: I, then 4~: I and
finally 1:1 hexanes:ethyl
acetate to give 131 (240 mg; 64%).
(3S)-3-[( {[5-Chloro-1-(2-chlorobenzyl)-4-hydroxy-2-axo-1,2-dihydropyridin-3-
yL]amino}carbonyl}aminoJ-3-(4-methylphenyl)propanoic acid was synthesized from
13I
according to procedures described in Example 1. MS: Calculated (M-H)' ---
488.08; Found
(M-I-1T ~ 487.97.
Exa~ph 33
Synthesis of (3S)-3-[({[I-{2-chlorobenzyl)-4-hydroxy-5-methyl-2-oxo-1,2-
dihydropyridin-3-ylJamino)carbonyl}amino]-3-(2',6'-dimethoxy-I,1'-biphenyl-4-
yl)propanoic
acid.
to On ; To a solution of (R)-(*)-N-benzyl-a-methylbenzyl amine (5.07 g, 24
mmol)
in THF (S5 mL) under nitrogen in a flame-dried flask, cooled to -78 °C,
sec-butyllithium (I .3
M solution in cyclohexane, 18.0 mL, 23.4 mmol) was added dropwise over a 30
minute
period. The mixture was stirred an additional 30 minutes at -78 °C,
then a solution of t-butyl
4-bromocinnamate (5.1 g, 20 mmol) in THF (20 mL) was added dropwise and the
mixture
was allowed to come to room temperature overnight. The ruction was quenched by
addition
of saturated ammonium chloride (~50 mL) and the organic layer was washed with
saturated
sodium chloride, dried over MgS04 then filtered. The filtrate was concentrated
under
reduced pressure and the residue was purified by silica gel chromatography
eluting with _
hexanes and increasing to 3:1 hexanes:ethyl acetate to give 13Z (4.33 g, 47%)
as a pale
yellow oil.
Step Two: To a solution of I32 (7.4 g, I S mmol) and 2,6-dimethoxyphenyl
boronic
acid (4.9 g, 27 mmol) in DME ( 100 mL) at room temperature 'under a dry,
nitrogen
CA 02366800 2002-O1-07
atmosphere, finely-powdered potassium phosphate (8.0 g, 3'1.5 mM) and
A r::
dichlorobis(triphenylphosphiae)palladium (0) (0.5 g, 0.'l5 ~iamoi) were added.
The mixture
was deoxygenated (toggle between vacuum and nitrogen gss 5 times) and then
heated to
reflex for 8 hours The mixntre was then cooled and filtered thmugh Celite~
521; and the
filtrate was concentrated under reduced pressure. The residue was purified by
silica gel
chromatography, eluting with hexanes increasing to 3:1 hexanes;ethyt acetate
to give 133 (7.8
g, 95°r6yield).
to Three: To a solution of 133 (3.39 g, 6.1 mmol):in ethanol (80 mL) in a 250
mL
flask, acetic acid (0.5 mL) and palladium on carbon (10% Pd dry weight basis,
water content
.10 ~50%, Desussa type E101 NEIW, 2.5 g, 1.2 mmol Pd) were° added
sequentially, The mixture
was stirred under a hydrogen atmosphere from a balloon for 36 hours. The
reaction mixture
was filtered through Celite~ 521 and the filtrate was concentrated under
reduced pressure.
The residue was recrystallized from etlryl acetate to give 134~HOAc (1.0 g,
'71%) as a white
solid.
(3S)-3-[({j1-(2-chlorobenzyl)-4-hydroxy-5-methyl-2-oxo-1,2-dihydropyridin-3-
yl~amino}carbonyl)amino]-3-(2',6'-dimethoxy-1,1'-biphenyl-4-yi)propenoic acid
was
synthesized from 134~HOAc by procedures described in Example 25. MS: Measured
(M+H)'~ = 592.04; Calculated (M+H)'' = 592.19.
sample 34
Synthesis of (3S)-3-[(t[2-(2-chloro-6-ethoxybenzyl)=5-hydroxy-6-methyl-3-oxo-
2,3-
dihydropyridazin-4-yl]amino}carbonyl)amino]-3-(3-ethox~phenyl)propanoic acid.
,Step One: To a solution of sodium t-butoxide (6S g, 0.642 mot) in TFiF (1 L),
at room
temperature under a dry nitrogen atmosphere; ethanol (250. mL, 5.35 mot) was
added over a
10 minute period, To the resulting solution, 2-chloro-6-fluorobenzonitrlIe
(100 g, 0.642 mot)
was added in portions. The reaction mixture was stirred at room temperature
for 30 minutes
and then reduced to a volume of approximately 250 mL under reduced pressure.
The
resulting mixture was poured into chloroform and water and the layers
separated. The
organic layei was washed with water (twice) and brine, dried over MgSOa and
filtered. The
filtrate was concentrated under reduced pzessure to affozd at light yellow
solid. This material
was recrystallized from hexa::es to provide the 2-chloro-6-
atrioxybenzonitrile,133, (1O1 g, 87
yield) as a white crystalline solid.
CA 02366800 2002-O1-07
M', pi
-105-
c-a
S Two: To a solution of 2-ehloro-6-ethoxybenzonitrile,135, (93.2 g, 0.513 mol)
in
THF (350 mL) at room temperature under a dry nitrogen atmosphere was added
borane in
THF (1.0 M, 620 mL, 0.62 mol). The resulting mixture was heated to reflex for
3 hours and
then cooled to room tetriperature. Water (250 mL} was added very slowly to the
solution
allowing for the evolution of hydrogen. Concentrated HCI,(50 mL) was then
added over
several minutes and the solution was heated to 50 °C for Z beers. The
mixture was cooled
and partitioned between chloroform and water. The aqueous layer was washed 6
times with
chloroform. The combined organic fractions were washed'~~vith HCl (1 Nl~ and
this organic
layer was discarded. Chloroform was added to the combined aqueous layers and
solid KOH
IO was added until the aqueous phase was basic (pH > 9). The aqueous layer
washed with
chloroform an additional five times. The organic fractions were combined and
washed with
water, brine, and dried ova MgSOa and silica gei (2 g). This mixture was
filtered and the
filtrate was concentrated under reduced pressure o give Z-chloro-6-
ethoxybenzylamine,136,
{60.1 g, 64% yield) as a light yellow oil.
Step Three: To s solution of 2-chloTO-6-ethoxybenzylamine,136, (7.30 g, 39.3
mmol)
in glacial acetic acid (50 mL} and acetic anhydride (50 mL) at room
temperature, sodium
nitrite (6.00 g, 85.7 mmol} was added in small portions. The resulting mixture
was stirred at
room temperatuze overnight than was poured into ice water and extracted with
ethyl acetate.
The orgatuc layer was washed with aqueous NaOHV(1N, 2 X 100 mL) and brine
(twice). The
organic layer was dried over Na2SO.s and filtered and the filtrate was
concentrated under
reduced pressure to give 137 (9.00 g, 100%) as a light yellow solid.
St ur: To a solution of 137 (9.00 g, 39.3 nunol) and tetrabutylamrnonium
bromide (1.0 g, 3. I mmol) in THF (50 ml) ~a room temperature, aqueous NaOH
{2N, 50 mL,
100 ntmol) was slowly added and the mixture was heated to 45 °C
overnight. The reaction
mixture was cooled to room temperature, then was diluted,with water and
extracted with ethyl
acetate. The organic layer was washed with brine, dried over NaZS04 and
filtered and the
filtrate was concentrated under reduced pressure to give 138 (7.08 g, 96%
yield). --.
Step Five: ?o a solution of 138 (7.08 g, 37.9 mmol~;in CHiCl2 (55 mL) at room
temperature under a dry nitrogen atmosphere, a solution of SOCIZ (9.0 mL, 120
mmol) in
CHaCIZ (30 mL) was added dropwise. The resulting mixture was sowed at room
temperature
overnight, then was poured into ice water. The aqueous layer was extracted
with CH~CI= and
CA 02366800 2002-O1-07
-146-
the combined organic layers were washed with aqueous NaOH (1N, twice), water
(3 times)
and brine (twice). The organic layer was dried over NaZS04 and filtered and
the filtrate was
concentrated under reduced pressure to give 2-chloro-6-
ethoxybenzylchloride,139, (b.69 g,
86% yield) as a viscous, brown oil.
$ Step Six: A solution of 2-chloro-6-ethoxybenzychloride, 139, (6.90 g, 33.7
mmol) and
hydrazine (21.60 g, 673 mrnol) in MeOH (22 mL) Was stiired at room temperature
for 3
hours. The mixture was then partitioned between CH2C12 vnd water. The organic
layer was
dried over MgSOa and filtered and the filtrate was concentrated under reduced
pressure to
give 140 (6.18 g, 92%).
Steo Seven: To a suspension of ethyl pyruvate {3.85 mL, 33.7 mmol) and MgSOd
in
CHCl3 (b5 mL), a solution of 140 (6.14 g, 30.b mmol) in CHC33 (30 mL) was
slowly added.
The resulting mixture was stirred at room temperature overnight. The resulting
mixture was
filtered and the filtrate was concentrated under reduced pressure to give 141
{8.43 g, 92%).
This material was used in the next step without purification.
St ' ht: To a solution of 141 (8.43 g, 28.2 mmol) in dry THF (110 mL)cooled to
0
°C under a dry nitrogen atmosphere, sodium hydride (60%'dispersion in
mineral oil, 1.88 g,
47.1 mmol) was added in one portion. The resulting mixture was stirred at 0
°C for 30
minutes, then methyl malonylchloride (6.63 g, 47.10 mmol) 'was slowly added.
The mixture
was allowed to warn to room temperature, stirred overnight, carefully quenched
with water
then extracted with ethyl acetate (twice). The organic layers were combined,
washed with
brine, dried over MgSOa and filtered. The f ltrate was concentrated under
reduced pressure to
give I42 (14.29 g). This material was used in the next step without further
purification.
a Nine: To a sot~tion of crude 142 {14.29 g) in dry DMF (60 mL) cooled to 0
°C
under a dry nitrogen atmosphere; sodium hydride (60% dispersion in mineral
oil; 2.90 g, 72.2
mmol) was added in one portion. This solution was heated to 60 °C
overnight, cooled down
in an ice bath, then shaken with hexane. The layers weze seperrated and the
DMF layer was
poured into ice water. The mixture was acidified (pH 1) by adding HCl (2N).
The precipitate
was collected by filtration the dissolved in ethyl acetate. The organic
solution was dried over
MgS~a and filtered and the filtrate was concentrated to give 143 (8.42 g, 85%
yield for two
steps).
CA 02366800 2002-O1-07
-107-
Step Ten: A solution of 143 (8.42 g, 23:9 mriiol) in dioxane (100 mL) and
aqueous
HCl (60 mL, 5.21 was refluxed overnight. The mixture was cooled to room
temperature,
diluted with water arid extracted with ethyl acetat~. The organic layer was
washed with brine,
dried over MgSOa and filtered. The filtrate was concentrated under reduced
pressure and the
residue was purified by silica gel chromatography eluting with l :1 ethyl
acetate:hexanes,
then ethyl acetate and finally 9:I ethyl acetate:methanol to give 144 (2.0 g,
2S%).
Synthesis of (3S)-3-[({[2-(2-chloro-6-ethoxybenzyl)-~-hydroxy-6-methyl-3-oxo-
2;3-
dihydropyridazin-4-yl]amino}carbonyl)amino)-3-(3-ethoxyphenyl)prapanoic acid
was
prepar~d from 144 by procedures provided in Example 25. MS: Measured (M+H)'' =
545.05;
Calculated (M+H)t ~ 545.18.
Example
Synthesis of (3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy 2-oxo-2,5,6,7-tetrahydro-
IH-
cyclopeata[b)pyridin-3-ylJamino} carbonyl)aminoJ-3-(I,3-diethyl-2-oxo-2,3-
dihydm-1H-
benzimidazol-5-yl)propanoic acid.
IS Sten One: An ice-cold mixture of sodium hydride (8';00 g, 60% dispersion in
mineral
oil, 200 mmol) and 145 {8.948, 66,6 mmol) in DMF (250 mL) under a dry nitrogen
atmosphere was allowed to gradually warm to room temperature. To the resulting
mixture,
iodoethsne (16 mI, 200 mmol) was added and the mixture was stirred at =oom
temperature
overnight: The reaction mixtur= was poured into ice and extracted with ethyl
acetate. The
organic layer was washed with water and brine, dried over 1Va=S04 and
filtered. The filtzate
was concentrated under reduced pressure acrd the residue was taken up in
hexanes and
filtered. The resulting brown solid was dried under reduced pressure to give
146 (9.00 g,
71 % yield). This material was used' without purification.
Sten Twa: A mixture of DMp (3.6 g, 49 mmol) and~P(JC13 (9.6 mL, 100 mmol) was
stirred at room temperature under a dry nitrogen atmosphere for 1 hour. The
flask containing
this mixture was then placed in a 45 °C oil bath and 146 (7.6 g, 40
mmol) was added in small
portions. The oil bath temperature was raised to 70 °C and the mixture
was stirred overnight, --.
then cooled to room temperature. The mixture was diluted with water and
exaacted with
ethyl acetate. The organic layer was washed with water and br;ne, dried over
Na2SOa and
filtered. The filtrate was concentrated under reduced pressuie to give a 7:3
mixture of
147;146 (6.69 g). This material was used without purification.
CA 02366800 2002-O1-07
-108-
a ee: To a solution of the 14:146 mixture obtained above (2.2 g) in ethanol
::i°:,
(2.2~mL), malonic acid (i.16 g, 11.2 mmol), pyridine (0.44~mL) and piperidine
(0:99 mL)
were added sequentisily. The resulting mixture was heated tb reflux for 6
hours, then cooled
to room temperature. The mixture was diluted with aqueous NaOH (lIV) and
extracted with
ethyl acetate (4 times). The aqueous phase was acidified to pH 3 with HCl (1N)
and the
resulting suspension was filtered, washing the solid with water. The white
solid was
collected and dried under reduced pressure to give ld8 (1,69 g, 49% for twv
steps).
(3S)-3-[( {[1-(2-chlorobenayl)-4-l~ydzoxy-2-oxo-2,5,6,7-tetrahydro-1H-
cyclopenta[bJpyridin-3 yl]amino}carbonyl)amino]-3-(1,3-diethyl-2-oxo-Z,3-
dihydro-1H-
14 henzimidazol-5-yljpropax<oic acid was prepared from 148 by procedures
described is
Examples 33 and 25_ MS: Measured (M+H)* --- 594.05; Calculated (M+H)'' =
594.21.
Example 36
Synthesis of give (3Sr3-[({[1-(2-chloro-6-ethoxybenzylr4-hydroxy:5-methyl-2-
oxo-
1,2-dihydmpyridin-3-yl]amino}carbonyl)amine]-3-(4.methylphenyl)propanoic acid,
I33.
Sts re: To a solution of 114 (20.3 g, 1 z9 ntmol) in anhydrous methanol (430
naL)
at room temperature under a dry nitrogen atmosphere, 2-chloro-6-
ethoxybenrylarnine, 136,
(31.1 g, 168 mmol) was added. The solution was heated at 45 °C for 1
hour then retluxed
overnight. The reaction mixture was cooled to roam temperature and
concentrated to
dryness. The residue was brought up in dichloromethane and filtered. The solid
was
ZO collected and dried under vacuum to give I49 (I4.7 g, 39%).
Sten Two: To a suspension of I49 (1 I.02 g, 37.8 msnol) in glacial acetic acid
(126
mL) at room temperature, NaNOa (522 mg, 7.6 mmol), water (10:5 mL) and HN03
(70%, 9.6
mL, ISI.2 mmo!) were added sequentially. The resulting bright yellow solution
was stirred at
room temperature overnight; then was diluted with CHZCl= and water. The
aqueous phase
was extracted with CHzCI2, the organic layers were combined and washed with
water (3
times) and brine. The organic layer was dried over MgSOa and ftltered and the
filtrate was
concentrated under reduced pressure. The residue was recrystallized from
CFi2Ch/ethyl __,
acetate to give 1S0 (10.9 g, 85%) as a bright yellow solid. .
to Three: To a solution of 150 (10.9 g, 3Z.2 mmol) in DMF (107 mL) at room
temperature under a dry nitrogen atmosphere, Zn powder (9:a8 g, 145 mmol) and
triethylamine hydrochloride (24.4 g, 177 mmoi) were added, The resulting
mixture was
CA 02366800 2002-O1-07
-I09-
heated to 55 °C for 1 h, then was cooled to room temperature. To the
resulting mixture, CDI
(10.4 g, 64.4 nunol) was added as a solid. Upon addition, gas evolution
occurred. The
mixture was then heated to 80 °C for 2 hours, cooled to roost
temperature and poured into
HCI (2 N,1L). 'Ihe resulting suspension was stirred for 20 minutes and then
was diluted with
water (1L) and filtered. The solid was resuspended in water (1 L) and 'then
filtered, The solid
was dried under vacuum to give 151 (10.78 g, 100% yield) as a white powder.
Step Four: A mixture of 151 (10.68 g, 31.9 mmol) and 8 (8.27 g, 39.9 mmol) in
DMF
(64 mL) under a dry nitrogen atmosphere was heated to S5 °C overnight,
cooled to room
temperature and then diluted with ethyl acetate. The reaultihg mixture was
washed with HCI
(2N), water (4 times) and brine and the organic Layer was dried over Mg80a and
filtered. The
filtrate was concentrated under reduced pressure and the resulting residue was
puriRed by
silica gel chromatography, eluting with 7:3 hexartes:ethyl acetate to give lSZ
(14"2 g, g2%) as
a pate yellow foam.
Std Five: To a solution of I32 (I 1.60 g, 2I.4 mmoI) in THF (138 mL) at zoom
temperature, aqueous sodium hydroxide (2 N, 46 mL) and methanol (92 mL) were
added.
The mixture was stirred for 20 minutes, then was diluted with water and
extracted with ethyl
ether. The aqueous phase was acidified with HCI (2 N) and extracted with ethyl
acetate. The
ethyl acetate layer was washed with water and brine, dtied.-aver MgSOa and
filtered. ?he
filtrate was concentrated under reduced pressure to give (3Sj-3.[( { [ I -(2-
chloro-6-
ethoxybenzyl)-4-hydroxy-5-methyl.2-oxo-1,2-dihydropyridin-3-
yl]ammo}carbonyl)amino]-3-
{4-methylphenyl)propanoic acid,153, (10.82, 98% yield) as a light tan foam.
MS: Calculated
(M-I~' = 512.16; Measured (M-H)' = 512.03.
,BxamvIe 37
Synthesis of (3S)-3-[({[1-(2-chloro-6-ethoxybenzyl)-4-hydroxy-5-methyl-2-oxo-
1,2-
dihydropyridin-3-yl]aminof carbonyl)amino]-3-(3-ethoxypheayl)gtopanoic acid,
156.
Sleo One: A mixture of 131 (8.40 g, 28.8 mmol) arid 154 (8.2 g, 35 mmol) in
DMF
(100 mL) under a dry nitrogen atmosphere was heated to 5~, °C
overnight, cooed to room
temperature and then diluted with ethyl acetate. ?he resulting mixture was
washed with HCI
{2N), water {4 times) and brine and the organic Layer was dried over MgS04 and
filtered. The
CA 02366800 2002-O1-07
-I 10-
filtrate was concentrated under reduced pressure and the resulting residue was
purified by
silica gel chromatography, eluting with 8:2 increasing to 1:1 hexanes:ethyl
acetate to give 153
(I 1.I g, 67% yield).
Step Two: To a solution of IS5 (9.12 g, 15.9 mmol)in THF (1U0 mL) at room
~rnperature, aqueous sodiunt hydroxide (1 N, 88 mL) and methanol (63 mL) were
added.
The mixture was stirred for 20 minutes, then was diluted v~rith water and
extracted with ethyl
ether. This ether layer was discarded. The aqueous phase was acidified with
HCl (2 N) and
extracted with ethyl ether (4 times). The organic layers were washed with
water and brine,
daed over MgS04 and filtered. The.filtrate was concentrated under reduced
pressure to give
IO (3S}-3-[({[1-(2-chloro-6-ethoxybenzyl)-4-hydroxy-5-m~thyl~~2-oxo-1,2-
dihydropyridin-3-
yl]amino}carbonyl)amino]-3-(3-ethoxyphenyl)propanoic acid, 156, (8.13 g, 93%)
as a white
foam. MS: Calculated (M+H}+ -- 544.19; Measured (M+H)+ -- 544.04.
example 3 8
Synthesis of(3S)-3-[({[I-(2-chloro-6-ethoxybenzyl)-4-hydroxy-5-methyl-2-oxo-
1,2-
IS dihydropyridin-3-yl]amino}carbonyl)amino]-3-(6-methoxy-2-naphthyl)propanoic
acid, 159.
S ne: A mixture of 15I (I I O mg, 0.29 mmol), 15'7 (I30 mg, 0.34mmo1) and NMM
(0.50
mL, 4,5 mmol) in bMF (1.0 mL) under a dry nitrogen atmosphere was heated to 55
°C
overnight, cooled to room temperature and then diluted with ethyl acetate. The
resulting
mixture was washed with HCl (2N), water (4 times) and brine and the organic
layer was dried
20 over MgS04 and filtered. The filtrate was concentrated under reduced
pressure and the
resulting residue was purified by silica gel chromatography, eluting with 1:1
hexanes:ethyl
acetate to give 158 (130 mg, 73% yield).
Std Two: To a solution of 158 (130 mg, 0.2I mmol) in THF (3 mL) at room
temperature, aqueous sodium hydroxide (2 N, 1 nzl,) and methanol (2 mL) were
added. The
25 mixture was srirred for 20 minutes, then was diluted with water and
extracted with ethyl
ether. The aqueous phase was acidified with HCI (2 N) and extracted with ethyl
acetate. The
ethyl acetate layer was washed with water and brine, dried over MgSOa and
filtered. The
filtrate was concentrated under reduced pressure to give (3S)-3-[({[1-(2-
chloro-6-
ethoxybenzyl)-4-hydroxy-5-methyl-2-oxo-1,2-dihydropyridin-3-yl ) amino }
carbonyl)amino ]-3-
30 (6-methoxy-2-naphthyl)propanoic acid, 159, (90 mg, 74% yield). MS: Measured
(M+H)+ _
580.07; Calculated (M+H)' s 580,19.
CA 02366800 2002-O1-07
-111-
Synthesis of (3S)-3~[({[1-(2-chlorobenzyl)-4.-hydroxy-2-oxo-2,5,6,7-tetrahydro-
1H-
cyclopenta[b)pyridin-3-yl]amino}carbonyl)amino]-3-
(3.isopxopoxyphenyl)propanoic acid,
I64.
One: To a suspension of 1Z9 (5.30 g,19.2 mrriol) in glacial acetic acid (64
mL)
at room temperature, NaNOa (266 mg, 3.9 mrnol), water (5.3 mL) and HhI03
(?0°!°, 4.9 mL,
?7 moral) were added sequentially. The resulting bright yellow solution was
stirred at zoom
temperature overnight, then was poured into water arid filtered, washing with
water. ?he
yellow solid was dried under reduced pressure to give 160 (5.35 g, 87%).
S Two: To a solution of I60 (5.35 g, 16.? mmol) in DMF (56 mL) at room
temperature under a dry nitrogen atmosphere, Zn powder (~;.BS g, 74.7 mmol)
and
triethylamine hydrochlorida (12.6 g, 91.5 mmot) were added. The resulting
mixture was
heated to 55 °C for l h, then was cooled to room temperature. To the
resulting mixture, CDT
~~:
(5.41 g, 33.4 nunol) was added as a solid. Upon addition, gas evolution
occurred. The
mixture was then heated to 80 °C for 2 hours, cooled to room
temperature and poured into
HCI (2 N, 540 mL). The resulting suspension was stirred for 20 minutes sad
than was diluted
with water (500 mL) as'~d filtered. The solid was resuspended in water (5G0
mL) and then
filtered. The solid was dried under vacuum to give 161 (5.0 g,
95°!° yield) as a white powder.
St~Three: A mixture of 161 (6.14 g, 19.4 mmol) and 162 (5.12 g, 20.3 mmol) is
DMF (90 mL) under a dry nitrogen atmosphere was heated to 80 °C
overnight, cooled to
room temperature and then diluted with ethyl acetate. The resulting mixture
was washed with
HCi (2 N), water (4 times) and brine and the organic layer was dried over
MgS04 and filtered.
The filtrate was concentrated under reduced pressure and the resulting residue
was purified
by silica gel chromatography, eluting with ?:3 hexanes:ethyl acetate to give
163 (8.9G g, 81 %)
as a pale yello w foam.
S Four: To a solution of I63 (8.69 g, 15.3 mmol) in THF (35 mL) at mom
temperature, aqueous sodium hydroxide (2 N, 30 mL) and methanol (30 mL) were
added. ._.
The mixture was stirred overnight, then was diluted with water and extracted
with ethyl ether.
The aqueous phase was acidified with HCI {2 N) and extracted with ethyl
acetate. The ethyl
acetate Layer was washed with water and brine, dried over MgS04 and filtered:
The filtrate
was concentrated under reduced pressure to glue (3S)-3-[({(1-(2-chlorobenzyl)-
4-hydroxy-2-
CA 02366800 2002-O1-07
r:
-112-
oxo-2,5,6,7-tetrahydro-1H-cyclopenta[b]pyridin-3-yI]aminoycarbonyl)amino]-3-(3-
isopmpoxyphenyl~ropanoic acid,164, (7,50 g, 91°!o yield)..~~,MS:
Measured (M+I~*
540,03; Calculated (M+I~* = 540.19.
Example 40
Syntheses of(38)-3-[({jI-(Z~chlorobenzyl)-4-hydmxy 2-oxo-2,5,6,7-tetrahydro-1H-
cyclopenta[b]gyridin~3-ylJamir:o } carbonyl)amino]-3-(4-chloro-3-
isopropoxyphen~yl)pioganoio acid,
to One: To a mixture of 16Z (200 mg, 0.80 mmol),in glacial acetic acid (1.65
mL)
cooled to 0 °C under a dry nftrogen atmosphere, a mixture of SC7aCiz
(1.2 rnL, 1 S mmol) in
glacial acetic acid (1.0 nll,) was added dropwise by syringe. : The resulting
mixture was
stirred at 0 °C for 30 minutes then was warmed to room temperature.
After stirring for an
additional 4 hours; the mixture was recooled to 0 °C and quenched by
careful addition of
saturated aqueous NaHC03. The mixture was extracted with ethyl acetate and the
organic
layer was washed with saturated aqueous NaHC03, dried over MgSOa and filtered.
The
filtrate was concentrated under reduced pressure and the residue was purified
by silica gel
chromatography, eluting with 2:1 hexanes:ethyl acetate to give 163 (148 mg,
65%).
(3 S)-3-[( { [ 1-(2-chlorobenzyI)-4-hydroxy-2-oxo-2, 5,6, 7-tetrahydro- I H-
cyclopenta[b]pyridin-3-yl]amino } carbonyl)amino]-3-(4-chloro-3-
isopropoxyphenyl]propanoic acid was prepared from 1fi5 according to procedures
described
2Q in Examples 25 and 30. MS: Calculated (M-H)'= 586.I5~,Found (M-H)' =
585.92.
Exam a 4I
Synthesis of (3S)-3-({[(1-{[2-chloro-6-tetcahydxo-1(2H)-
pyridinylphenyl)methyi}-4-
hydroxy-5-methyl-2-oxo-1,2-dihydro-3-pyaidinyl)amino]carbonyl) amino)-3-(4-
methylphenyl)prdpanoic acid.
Ste One: To a suspension of 166 (0.35 g, 1.0b mtnol, prepared according to
procedures described in Exattiples 34 and 25) in methanol (7 mL) and water
(3.5 mL) cooled
to 0 °C, glacial acetic acid (189 p.I;, 3.2 mmol) and sodium nitrite
(178 mg, 2.65 mmol) .--
were added sequentially. The mixture was allowed to slowly warm to room
temperature
overnight and then was diluted with chloroform and water:; The pH of the
aqueous phase was
choked to ensure a pH of 4-5. The organic layer was washed with bring, dried
over MgSOa
CA 02366800 2002-O1-07
-113-
~.:::
and filtered and the filtrate was concentrated under reduced pz~essure to give
16? (0.35g, 92%)
as a yellow solid.
(3S)-3-( {j(1-{(2-ehloro-6-tetrahydro-1 (2H)-pyridinylplnenyl]methyl } -4-
hydroxy-5-
methyl-Z~oxo-1,2-dihydro-3-pyrldirtyl)amino]carbonyl}amino)-3-(4-
methylphenyl~propanoic
acid was sytsthesized from 157 according to the procedures described in
Example 25. MS:
Calculated (M~IT)' ~ 551.21; Found (M-H)' = SS I .06.
Fxam~e 42
Synthesis of (3S)-3-{j(~(I-j(2-chjorophe~yl)methyl]~-hydroxy-5-methyl-2-oxo-
1,2-
dihydro-3-pyridi tyl}aminoxarbonyljatnino}-3-[3-
(difluororiiethyl)phenyl]pmpanoic acid,
to One: To a solution of 3-bromobenzaldehyde,168, (3.00 g, 2 6.2 mmol) in DMF
(69 mL) under a dry nitrogen atmosphere, pahadium acetate (73 mg, 0.32 mmol),
tti-o-
tolylphosphine (197 mg, 0.65 mmol), ethyl acrylate (2.20 mL, 20.3 mmol) ~d
ttiethylamine
(4.50 mL, 32.4 mmol) were added, The system was deoxygenated (toggle between
vacuum
. and nitrogen five times), the mixture was heated to I25 °C for 19
hours and then cooled to
room temperature. The reaction was poured into water end extracted with ether.
Tlte organic
~,::
layer was washed with HCl (4h1) and brine, dried over MgSO, and filtered. The
filtrate was
concentrated under reduced pressure to give I69 (2.7~4g, 83%), which was used
without
further purification.
Stew Two: To a flask containing 169 (I .00 g, 4.9 mmol) under a dry nitrogen
atmosphere, (dimethylamiao)sulfur trifluoride (0.96 mL, 9.8 rntnol) was added
by syringe.
The mixture was heated to 90 °C behind a blast shield for 25 minutes
then was cooled to
room temperature: The resulting mixture was diluted with CHZCl= and washed
with saturated
aqueous NaHC03 and HzO. The organic layer was dried over MgSOa and filtered
and the
filtrate was concentrated under reduced'pressure. The residue was purified by
silica gel
chromatography, eluting with 1:5 ethyl ectetate;hexanes to give 170 (0.62 g,
56%).
t Three: To a solution of (R)-(+)_TT-benzyl-a-znethylbenZylamine (0.70 g, 3.3
mmol) in Ti3F (6.7 mL) cooled to -78 °C under a dry nitrhgen
atmosphere, sec-BuLi (4.22
mL, 1.3M in cyclohexane, 5.5 mmol) was added dropwise: The resulting mixture
was stirred
at -78 °C for 30 minutes and then a solution of 170 (0.62 g, 2.74 mmol)
in THF (3.4 mL) was
added dropwise by syringe. The mixture was. stirred at -78 °C for 5
hours and than quenched
CA 02366800 2002-O1-07
-114-
with glacial AcOH (2 mL) in THF (5 mL). The reaction mixture was warmed to
room
temperature, poured into a 1:1 mixture of saturated aqueous NaHC03:EtOAc. The
organic
layer was washed with ~O (2 times) and brine, dried ever MgSC?4 and filtered.
The filtrate
was concentrated under reduced pressure and the residue was: purified by
silica gel
chmmatograghy, eluting with t :5 ethyl actetate:hexanes to give 171 (1.2 g,
100%). This
material still contained minor impurities but was used without further
purification.
Step F,o~r: ?o a solution of 17I (0.50 g, 1.14 mmol).in EtOH (10 rte.) at room
temperature under a dry nitregen atmosphere, PdIC (I0% Pd dry weight basis,
50% water by
weight, Degttssa type ElOI NEIW, 0.25 g) and glacial AcOH (0.5 mL) were added.
The
atmosphere was replaced by hydrogen (toggle between vacuum and hydrogen from a
balloon
five tunes) and the mixture was heated to 35 °C for 6 hours. The
reaction was cooled to room
temperature, filtered through a plug of Celite's 521 and the filtrate was
concentrated under
reduced pressure. The residue was diluted with CHCl3 aad washed with saturated
aqueous
NaHCO~. The aqueous Iayer was extracted with CHC13 (2 times) and the combined
organic
layers were dried over MgS04 a. d filtered. The filtrate was concentrated
under reduced
pressure and the residue was purified by silica gel chromato'~raphy, eluting
with 1:10
MeOFi:CHCl3 to give 172 (180 mg, 67%).
(3S)-3-{(({ 1-[(2-chlorophenyl}methyl]-4-hydroxy-5-methyl-2-oxo-1,2-dihydro-3-
pyridinyl)amino)carbonyl]amino}-3-[3-(difluoromethyl)phenyl]propanoic acid was
synthesised from 172 according to procedures desczzbed in Example 25. MS:
Calculated (M-
H)" = 504. 31; Found (M-H)" = 503.96.
Example 43
?he procedures described in Examples 3, 4, 8, 25, 26, 27, 29, 30, 34, 36, 39
and 41
were utilized to synthesize several compounds of general Formla VTI and
general Fomoula
VIII, by varying starting materials. In Table 1 shown belovu, characterization
data is
provided for compounds synthesized.
CA 02366800 2002-O1-07
-lI5-
Table I
Compound 1H NMR (400 MHz)
5-(Z-chlorobenzyl)-3,5- (CD3S02CD3) 8 5.27 (s; 2H), 6.67 (d, J = 7.4
dihydro[1,3]oxazolo[4,5- Hz, iH), 6.88 (dd, J=7.g, 1.4 Hz, IH), 7.27-
c]pyridine-2,4-dione 7.37 (m, 2H), 7.51 (dd;-J s 7.9, I.5 Hz, 1H),
7.65 (d; J = 7.4 Hz, 1 H), 12.01 (br. s, 1 H).
5-(2-chlorobenzyl)-6-methyl- (CD3SOzCD3) 8 2.27 (s, 3H), 5.36 (s, 2H),
3,5-dihydro[1,3]oxazolo[4,5- 6.60 (d, J = 7.3 Hz, 1H), 6.63 (s, 1H), 7.27
c]pyridine-2,4-dione 7.37 (m, 2H); ?. S 1 (d, J = 7.7 Hz; 1 H), 11,9
(br. s, IH).
5-(2-fluorobenzyl)-3,5- , (CDjSOZCD3) S 5.26 ~s, 2H}, 6.65 (d, J = 7.3
dihydro[1,3]axazolo[4,5- Hz, 1H), 6.88, 7.I2-7.26 (m, 3H), 7.37 (m,
c]pyridine-2,4-dione 1H), 7.69 (d, J = 7.3 Hz, 1H), 11.93 (br. a,
1 H).
5-(2-chloro-6-tluorobenzyl)- (CD3SOaGD3) & 5.30 ~(s, 2H), 6.56 (d, J = 7.3
3,5-dihydro[1,3]oxazolo[4,5- Hz, IH}, 7.25 (ddd, J = 9.4, 8.9, .1.1 Hz,
cjpyridine-2,4-dione 1H},7.37 (d; J - 8.0 I-~z, IH), 7.43 (m, 2H),
11.93 (br. s, I H).
S-betizyl-6-methyl-3,5- (CD3S0=CD3) S 2.30 (s, 3H), 5.37 (s, 2H),
dihydro[I,3joxazolo[4,5- 6.55 (s, 1H), 7.10 (d, J s 7.0 Hz, 2H), 7.24-
c]pyridine-2,4-dione 7.36 (m, 3H), I 1.88 (br. s, 1H).
5-benzyl-3,5- (CD3SOZCD3) 8 5.20 (s, 2H), 6.60 (d, 3 = 7.3
dihydro[1,3]oxazolo[4,5- Hz,IH), 7.28-7.36 (m;'SH), 7.72 (d, J = 7.3
c]pyridine-2,4-dione Hz, 1H), 11.97 (br. s, 1H).
5-(2,S-dirnethylbenzyl}-3,5: (CDC13) s 2.27 (s, 3>ay, 2.32 (s, 3H), 5.27 (s,
dihydro[1,3]oxazolo[4,5- 2H), 6.42 (d, J=7.3 Hz, IH) 6.90 (s, 1H),
c]pyridine-2,4-dione 7.09 (m, 3H), 10.68 (br s, 1H).
5-(2-methylbenzyl)-3,5~ (CDC13) 8 2.30 (s, 3H), 5.28 (s, 2H), 6.39 (d, J
dihydro[1,3]oxazoto[4,5- = 7.3 I-lz, 1H), 7.06 (d, 3 = 7.3 Hz, 1H), 7.09
c]pyridine-2,4-dione (d, J = 7.7 Hz, 1H), 7.18 - 7.28 (m; 3H) 10.91 __.
(br s, IH},
S-(2,4-dichlorobenzyl)-3,5- (CDC13) 8 5.33 (s, 2H), 6.47 (d, J ~ 7.3 Hz,
dihydro[l,3joxazolo[4,5- 1H), 7.29 (rn, IH}, ?.38 (d; J = 7.3 Hz, 1I!),
c]pyridine-2;4-dione '7.42 - 7.48 (m, 2H) 10.7'7 (br s, IH).
CA 02366800 2002-O1-07
-116-
5-(2-methoxybenzyl)-3,5- (CDC13) 5 3.87 (s; 1H)~;5:24 (s, 2H), 6.36 (d, J
dihydro[1,3]oxazolo[4,5- =7:5 Hz, 1H), 6.88 (d~J = 8.1 Hz, IH), 6.97
cjpypdine-2,4-dione (m, 1H), 7.30 (m, 1H)7.45 (d, J = 7.5 Hz,
1H), 7.55 (m; 1H), 10.75 (br. s, IH).
5-(Z,5-difluorobenzyl)-3,5- (CDC13) 8 5.26 (s, 2H), ti.46 (d, J = 7.4 Hz,
dihydro[1,3]oxazolo[4,5- 1H), 6.96-7.05 (m, 2H), 7.30-7.37 (m, 1H),
c]pyridine-2,4-dione 7.39 (m, 1 H), 10.68 (br. s, 1 H).
5-[2-chloro-5- (CD3SOzCDs) 8 2.41 (s, 3H),~5,24 (s, 2H),
(methylthio)benzyl]-3,5- 6.65 (d, J = 7.2 Hz, I F~, 6.83 (d, J = 2.6 Hz,
dihydro[l,3joxazolo[4,5- IH), 7.25 (dd, J= 8.0;;2.6 Hz, 2H), 7.45 (d, J =
c]pyridine-2,4-dione 8.0 Hz, 1H), 7.62 (d, J.= 7.2 Hz, 1H), 12.01
(br: s, 1 H).
5-(4-fluorobenzyl)-3,5- (CD3SOZCD3) 8 5.18 {s, 2H), 6.61 (d, J = 7.4
dihydro[l;3joxazolo[4,5- Hz, 1H), 7.14-7.2 (m, 2H), 7.35-7.39 (m, 2H),
cjpyridine-2,4-dione 7.74 (d, J = 7.3 Hz, 1H), 11.96 (br. s, 1H),
5-(2-chloro-5-methoxybenzyl)- (CD3SOzCD3) 6 3.69 (s; 3H), 5.22 (s, 2H),
3,5-dihydro[l,3joxazolo[4,5- 6.42 (d, J = 2:9 Hz, 1H), 6.65 (d, J = 7.3 Hz,
cjpyridine-2,4-dione 1H), 6.94 (dd, J = 8.8, 2.9 Hz, 1H), 7.43 (d, J
= 8. 8 Hz, 1 H), 7.62 (d, J = 7.3 Hz, 1 H); 12.0 5
(br. s, 1 H).
5-[3,5- (CD3SOZCD3) 8 5.36 (s, 2H), 6.69 (d, 3 = 7.5
bis(trifluoromethyl)benzylj- Hz, 1H), 7.9I (d, J = 7.5 Hz, 1H), 8.08 (s,
3,5-dihydro[I;3joxazolo[4,5- 3H), 12.04 (br: S, 1H).
cjpyridine-2,4-dione
5-(4-tent butylbenzyl)-3,5- (CD3S02CD3) S I.24 (s, 9H), 5.15 (s, 2H),
dihydro[1,3]oxazolo[4,5- 6.61 (d, J = 7.3 Hz, 1H), 7.23 (d, J = 8.4 Hz,
cjpyndine-2,4-dione 2H), 7.35 (d; J = 8.4 Hz, 2H), 7.74 (d, J = 7.3
Hz, 1 H), 12.02 (br. s, 1 H).
CA 02366800 2002-O1-07
-I17-
5-(3-chiorobenzyl)-3,5- (CD3SOZCD3) & 5.20 (s, 2H), 6.63 (d, J = 7.4
di'hydro[1,3]oxazoio[4,5- Hz, IH),1.25 (m, lIi}; 7,35-7.39 (m, 3H),
cjpyridine-2,4-dione 7,76 (d, J = 7.4 Hz, 1H), 11.97 (br, s, 1H).
5-(4-chlorobenzyl)-3,5- (CD3SOaCD3) S 5.19 (s, 2H), 6.62 (d, J -- 7.3
dihydro(1,3]oxa2olo(4,5- Hz, 1H), 7:29-7.33 (m, 2H), 7.37-7.42 (m,
c]pyridine-2,4-dione 2H), 7.73 (d; J = 7.3 Hz, 1 H}, 11.97 (br. s,
1 ~,
5-[3-(trifluoromethyl)benzyl]- n.d.
3,5-dihydro[1,3]oxazolo(4,5-
c]pyridirJe-2,4-dione
5-(2 bmmobenzyi)-3,5- (CD3SOZCD3} 8 5.23 (s, 2H), 6.68 (d, J 5 7.4
dihydro[l;3]oxazolo[4,5- Hz, 1H), 6.79 (m, IH), ?.~6 (m, IH), 7.34 (m,
c]pyridine-2,4-dione 1 H), 7.6~! {d; J = 7.4 Hz, 1 H), ?.68 (m, 1 H),
Z 2.02 (br. s, i H).
5-{3,4-dichlorobenzyi)-3,5- (CD3SOzCD3) 8 5.19 (s, 2H), 6.64 (d, T = 7.3
dihydm[1,3]oxazolo(4,5- Hz, IH), 7.29 (m, IH), 7.61 (m, 2H), 7.77 (d,
c]pyridine-2,4-dione J = 7.3 Hz, I H), 11,98 (br. s, I H).
5-(4-methylbenzyl)-3,5-(GD~SOzCD3) $ 2.27 (s, 3H),
5.14 (s, 2H),
dihydro[1,3]oxazulo(4,5-6.59 {d, J = 7.5 Hz, I H),
7, I4 (d, 3 ~ 8.2 Hz,
c]pyridiae-2,4-dione 2H), 7.20 (d, J = 8.2 Hz, 2H),
7.69 (d, 3 = 7.5
Hz, 1 H), I 1.9S (6r, s1 H).
5-(2-chlozo-6-methoxybeazyl}-(CD3SOzGD3) 8 3.80 (s, 3H),
5.23 (s, 2H),
3,5-dihydro[l;3Joxazolo[4,5-6.48 (d; J = 7:4 Hz, 1 H},
7.45-7,15 (m, 3H);
c]pyridine-2,4-dione 7:42 (m, IH), 11:95 ( br. s;
1H).
5-[4-(trifluoromethyl)benzyl]- (CD3SOaCD3) S 5.30 (s, 2I-~, 6.65 (d, J = 7.3
3,5-dihydro[1,3]oxazoio[4;5- Hz, IH), 7.48 (d, J = 8,4 Hz, 2I~, 7.71 (d, J =
cjpyridine-2,4-dione 8.0 Hz, 2H),.7.76 (d, J = 7.3 Hz; IH), 1 I.96
(br, s, IH).
5-(3-mZthyibGnzyl)-3;5-(CD3SOzCD3) 8 2.27 (x, 3H), 5.15 (s, 2H),
dihydro[1,3]oxazolo[4,5-6.62 (d, J = 7.3 Hz, 1H), 7.10 (m, 4H), 7.72
c]pyridine-2,d-dione(d, J = 7.3 Hz, 1H), 12.53 (br. s, IH).
5-(gyridin-2-ylrnethyl)-3,5-(CD3SOZCD3) 0 5.29 (s, 2H), 6.62 (d, J = 7.3
dihydro(1,3]oxazolo[4,5-Hz, IH), 7.22-7.33 (m, 2H}, 7.71 (d. J = 7.3
c]pyridine-2,4-shoneHz, lI~, 7:79 (m, IH), 8.50 {m, IH}; 11.96
(lir. s, 1 H).
CA 02366800 2002-O1-07
-118-
5-(2-chlorobenzyl}-?-methyl- (CD3SC3zCD3) 8 2.I0 (s, 3H), 5,23 (s, ZH),
3,S-dihydro[1,3]oxazolo[4,5- 6.86 (dd, r = 7.7, 1.5 Hz, IH), 7.31 (m, 2H);
a~pyridine-2,4-dione 7,50 (m, 2H), I2.01 (br s, 1H). ,
5-(2,4.-difluozobenzyl)-3,5-(CD3S02CD3} 8 $,21:~s, 2H),
6.63 (d, J = 7.3
dihydm[1,3]oxazolo[4,5Hz, IH), 7.02-7.07 (m; IH),
7.20-7.29 (m,
c~pyrudine-2,4-dione 2H), 7.65 (d, J = 7.3 Hz, 1H},
11.9? (br. s,
1H). St:.
5-(2,6-difluosobenzyl)-3,5-(CD3SOZCDa) 8 5.25' (S, 2I~,
6.58 (d, J = 7.3
dihydro[1,3]oxaavlo[4,5-Hz, 1H), 7,02-7.12 (m', 2H)
7.38-7.55 (m,
a]pyridine-2,4-dione IH), 7.63 (d, J ='7~3 Hz, lFn,
11.91 (bx. s,
1H).
5-(3- (CD3SO2CD3) 8 5.24 (~, 2H),
6.64 (d, J = 7.3
(trifluoramethoxy)benzyl)-3,5-Hz, 1H), 7.22-7.35 (m, 3H),
7.46 (t, J = 7.7
dihydro[I,3]oxazolo[4,5-Hz, IH), ?.78 (d, J= 7.3 Hz,
1H),11.99 (br. s,
a)pyridine-2,4-dione 1 H). '
S-[4- (CD3SOZCD~) 8 5.23 (s, 2H),
6.63 (d, J = 7.3
(triffuoromcchaxy)benzyl]-3,5-Hz, IH), ?.29-?.45 (m, 4H},
7,7b (d, J = 7.3
dihydro[1,3]oxazolo[4,5-Hz, lI~, 11.98 (br. S;1H).
c]pyridine-2,4-dione
5-[2-(arifluoromethyl)benzyI]-(CD3SOzCDs) S 5.40 (S, 2H),
6.73 (d, J = 7.3
3,5-dihydro[1,3]oxazolo[4,5-Hz, 1H), 6.81 (d, J = 7.5 Hz,
IH); 7.51 (t, J =
c]pyridine-24-dione 7.5 Hz, 1H), 7.61 (t; J = 7,5
Hz, 1H), 7.70 (d,
1= 2.3 Hz; I H), 7. 80 (d, J
_ 'l.5 Hz, 1 H),
12.04 (br. s, 1 H).
5-(3-methoxybenzyl)-3;3- n, d.
dihydro[1,3]oxazalo[4,5-
c]pyridine-2,4-dione
5-(2,3-dichlorobenzyl)-3,5- n. d.
dihydro[1,3]oxazolo[4,S-
c]pyridine-2,4-dione
5-(3,5-dimethylbenzyl)-3,5- (CD3SC72CD3) S 2.23 (s, 6H), S.I 1 (s, 2H),
dihydro[1,3]oxazolo[4,5- 6.61 (d, J = 7.3 Hz, ;1H), 6.91 (m, 3H1; 7.59
c]pyridine-2,4-dione (d, J = 7.3 Hz, 1H)12.00 (br, s, 1H).
5-(2-cblorobet~zyl)-7~pentyl- (CD3S02CD3) 8 0.86 (t, J ~ 6.Z I3z, 3H), 1,27
3,5-dihydro[1,3)oxazolo(4,5- (m, 6H), 1.65 (t, J = 6.7 Hz, 2H}, 5.24 (s, 2H),
c]pyridine-2,4-diot~e 6.83 (d, J = 6.6 Hz, 1 H); 7.24-7.34 (m, 2H),
7.48 (s, ~ H), 7.50 (d, J = 7.7 Hz, 1 H), 12.00
(tar. s, 1 H).
CA 02366800 2002-O1-07
-119-
5-(2,4-dichlorobanzyl)-7- (CD3SOxCD3) 6 2.10 (s> 3H), 5.19 (s, 2H),
methyl-3,5- 6.87 {d, J s 8.4 Hz, 1 H), 7.3 8 (dd, J = 8.4, 2.2
dihydro[1,3]oxazolo[4,5- Hz, 1H), 7:5(~ (s, 1H), :7.69 (d, J = 2.2 Hz,
c]pyadine-2,4- ,dione 1H), 12.02 (br, s, 1H).
5-(2-chlorobenayl)-7-ethyl-3,5- (CD~SOxCD3) 8 1.17 ,(t, J = 7.5 Hz, 3H), 2.50
dihydro[1,3]oxazolo[4;5- (m, 2H overlapping DI~JfSO), 5.25 (s, 2H),
c)pyridine-2,4-diona 6.54 (m, ll~; 7.30 (m, 2H), 7.49 (m, 2H),
12.02 (br, s, ll~.
7 butyl-5-(Z-chlorobenzyt~ (CD3SOzCD3) S 0.87 (t; J ~ 7.3 Hz, 3I-~, 1:28
3,5-dihydro[1,3]oxazolo[4,5- (m, 4H), 1.54 (t, J = 7.1 Hz, 2H), 5.24 (s, 2H),
c]pyridine-2,4-dione 6.83 (d, J = 6,8 Hz, 1H), 7.24-7.34 (m, 2H),
7.48-7.ss (m, 2H), I2:QO (br. B, lid.
5-[2-chloro-5- (CD3SOzCD3) cS 5.33~(~, ZH),
6.68 (d, J = 7.3
(tritluommethyl)benzylj-3,5-Ha, 1H), 7.35 (s, 1H).; 7.69-7.79
(m, 3H),
dihydro[I,3]oxazolo[4,5-11.96 (br, s, 1H).
cjpyridine-2,4-dione
5-(2,6-dichlorobenzyl)-3,5-(CD~902CD3) 8 5.38 (s, 2H),
6.53 (d, J = 7.4
dihydm[l,3joxazolo[4,5-Hz, 1H), 7,07 (d; J=7.7 I~z,
1H), 7.45-7.50
c]pyridine-2,4-dione (m, 1H), 7.52-7.59 (m, 2H),
11.99 (br. s, 1H).
5-(2-chloro-5-fluorobecizyl)- (CD3SOaCD3) a 5.27 (s, 2H), 6.67 (d, J = 7.3
3,5-dihydro[l,3Joxazolo[4,5- Hz, 1H), b.72 (dd, J = 7.3, 3.2 Hz, 1H), 7.2I
cjpyridine-2,4-dione ?.~3 (m, 1H)> 7.55-7.59 (tn, 1H), 7.65 (d, J=
7.3 Hz, 1 H), 12.00 (br. s, I H).
5-(2-chloro-6-methylbenzyt)-7- (CDCI3) 5 2.07 (s; 3H), 2.29 (s, 3H), 5.48 (s,
methyl-3,5- 2H), 6.b3 (s, 1H), 7',.I6 (d, J = 7,7 blz, 1H),
dihydro[1,3]oxazolo[4,5- 7.Z5 (t, J ~ 7.7 Hz, 1II), 7.34 (d, J = 7.7 Hz,
c]pyridine-2;4-dione 1H), 11.33 (br. S, 1H).
5-(4-chIorobenzyt)-7-methyl- (CD3SOaCD3) 8 2:08 (s, 3H), 5.I4 (s; 2H),
3,5-dihydro[1,3]oxazolo[4,5- 7.31 (d, J = 8.4 Hz, 2H), 7.41 (d, J = 8.4 Hz,
c]pyridine-2;4-dione . 2H), 7.58 (s, 1H), 12.03 (br. s, 1H).
5-(2-ehlorobeazyl)-5,6,7,8- (CD3SOzCD3) 0 2.04 (m, 2H), 2.80 (m, 4H),
tetrahydro-2H- 5.a8 (s, 2H), 6.68 (d, J ~ 7.3 Hz, 1H), 7.18-
cyclopenta[b][1,3]oxazolo[5,4- 7.34 {m, 2H), 7.5I (dy J = 7.7 Hz, 1H), 11.92
d]pyridine-2,4(3H)-dione (br. s, 1 H).
CA 02366800 2002-O1-07
-120-
7 methyl-5-[4- . (CD3SOzCD3) 8 2.11 (s3H), 2.58 (s, 3H),
(methylsulfoayl)benzyl~~~3,5- 5.28 (s; 2H), x.58 (d, J= 7.3 Hz; 2H), 7.64 {s,
dihydro[1,3]oxa2olo[4,5- 1H), 7.91 (d, J=?.3 Hz; 2H); 12.06 {br. s,
cjpyridine-2,4-dione 1H).
5-(4-methoxybenzyl)-3,5-(CD3S02CDs) 8 3.73 (s, 3H),
5.10 (s, 2H),
dihydro[1,3]oxazolo[4,5-6.56 (6r. d, J = 5.9 Hz, 1H),
6.89 (d, J = 8.8
c]Pyridine-2,4-dione Hz, 2I~, 7.2T (d, J = 8.8 Hz,
2Fi), 7.67 (br. m,
i H), 12.06 (br. s, 1 H).
5-(2-chlorobenzyl)-7 (CD3SOZCD3) 8 0.88 (t, T = 7.4
propyl- Hz, 3H), 1.57
3,5-d~ydro[1,3]oxazolo[4,5-(m, 2H), 2.46 (rn, 2H);.5.24
(s, 2H), 6.84 (d, J
cjpyridine-2,A~-dione=6.2 Hz, IH), 7.26-7.38 (m,
2H), 7.48 (s,
1H), 7.50 (d; J = 7.7 Hz, 1H),
12.00 (br. s,
1 H}.
4-[(2,4-dioxo-2,3- (CD3SOZCD~} 8 2.55 (s, 6I~,
5.3I (s, 2H),
dihydro[1,3]oxazolo[4,5-6.67 (d, J = T.3 Hz, 1H), 7.43-7.51
(m, 2H},
c~pyridin-5(4H}-yI}methyl]-7.66-7:74 (m, 2H), 7.77 (d,
J = 7.3 Hz, IH), ,
N,N- 12,00 (br. s, 1I~.
dimethylbe~nzenesulfoaamide
5-(mesitylmethyl}-3,5- (CDCl3) 8 2,19 (s, 6H), 2.30 (x, 3H), 5.25 {s,
dihydm[1,3~oxazolo[4,5- 2H}; 6.31 (d, J = 7.3 Hz, 1H); 6,73 (d, J = 2.3
c]pyridine-2,4-dione Hz, l H), 6.94 (s, 2H); 11.01 (br. s, 1 H),
5-(2-chiarobenzyl)-3,5,6,7,8,9- (CD3SOzCD3) 8 1.64 (m, 4H), 2.50 (m, 4I~,
hexahydro[1,3]oxazolo[4,5- 5.34 (s, 2H), 6.59 (d, J = 8:1 Hz, tH); 7.25-
c]quinoline-2,4-dione 7.34 (m, 2H), 7.51 (d, J = 7.7 Hz, 1 H), 11.92
(br. s, 1H).
5-(2-chlorobenzyl)-7-ethyl-6. (CD3SOzCD3) 8 1.10 (t, J = 7.4 Hz, 3H}, 2.22
methyl-3,5- (s, 3T~, 2.56 {m, 2H}, 5.40 (s, 2H), 6.58 (d, J
dihydto[I,3]vxa2olo[4,5- = 7.0 Hz, 1H), 7.23-7.34 (rn, 2H}, 7,52 (d; J =
cJpyridine-2,4-dione 8.1 Hz, 1H), 11.92 (br. s, 1H).
5-[2-(methylthio)benzyl]-3,5- (CD3SOaCD3} S 2.52 (s, 3H), 5.19 (s, 2147,
dihydro[I,3]oxazolo[4,5- 6.63 (d, J = 7.3 Hz, 1H), 6.76 (d, J = 7.7 Hz,
c]pyridine-2,4-dione 1H}, 7.09-7.17 (rn, 1H), 7.29-7.37 (m, 2H),
7.55 (d, J = 7.3 Hz, .1 H), 11.99 (s, 1 H). __.
CA 02366800 2002-O1-07
n.
-I21-
2-[(2,4-dioxo-2,3- (CD;SOzCD3) b 2.8I (s, 6H), 5.54 (s, 2»j,
dihydxo[1,3]oxazoloj4,5- 6.71 (d, J = 7.3 Hz, lI~, 6.81 (d, J = 7.3 Hz,
c]pyxi 'din-5(4H)-yI)znethyl]- IIi), 7.49-7.61 (m, 2H), 7.69 (d, J = 7.3 Hz,
N,N- 1H), 7.85 (d, J ~ 7.3 Hz, II-~, 12.05 (br. s,
dimethylbenzenesulfonamide 1H).
5-(2,6-dimethoxybenzyl)-3,5- (CD3S02CD3) 0 3.76 (s, 6H), 5.07 (s, 2H),
dihydro[1,3]oxazolo(4,5- ' 6.43 (d, J = 7.7 Hz,11~, 6.73 (d, J = 8.4 Hz,
capyridine-2,4-dione 2I~, 7.00 (d, J = 7:7 Hz, 1H), 7.37 (t, J = 8:4
Hz, 1H), 11.92 (br. s, 1H).
5_[2_ (CD;SOaCDl) 8 5.27 (s, 2H), 6.65 (d, J ~ i .3
(trifluoromethoxy)benzyl]-3,5- HZ, IH)~ 7.08 (dd, J =.7.3, 1.4 Hz, IH), 7.30-
dihydro[1,3]oxazolo[4,5- 7.49 Cm, 3t~, 7.63 (dJ = 7.3 Hz, 1H), 1 i.99
c]pyridine-2,4-dione (br. s, 1H).
5-(2-ohlombenzyl)-6,''(CD~SOaCD31 a 2.12 (s, 3H),
2.19 {s, 3H),
dimetbyl-3,5- x.40 (s, 2H), 6.59 (d, J ~ 6.6
Hz, 1H), ~.25-
dihydro[l,3joxaz~la[4,5-7:34 (m, 2'Fi),'.52 (d, J =
7.7 Hz, IH), I 1.91
c]pyridine-2.,,~-dione(br. e, 1H).
5-[2-chloro-S~ (CD;SOaCD3) s 3.20 (s, 3H).
5.35 (s, 2H),
(tnethylstelfonyl)benzyl]-3,5-6.70 td, J ~ 7.3 Hz, IH), 7.5S
(m, 1H), ?.69
dihydro[I,3]vxazolo[4,5-(m, iH), 7 90 (m, 2H), 12.04
(br. s, 1 H).
o]pyridine-Z,4-dione
5-(4-chloro-2-methoxybenzyl)-(CD3S03CD~) 8 3.86 (s, 3H),
5.09 (s, 2H),
3,5-dihydro[1,3]oxazolo[4,5-6.f0 (d, J = 7.3 Hz, 1H), 6.90-6.98
(m, 2H),
c]pyridine-2,4-dione ', 1 2 (d, J = 2.2 Hz, 1 I~),
7.59 (d, J = 7.3 Hz,
1~I), 11.95 (br. s, 1T~.
S-(Z-chlorob$n2y1)- (CI33S02CD3) b 1.34 (m, 2H),
1.56 (m, 2H),
5,6,7,8,9,10-hexahydto-2H-1.69 (m, 2H), 2.70 (rri, 4H),
, 5.45 (s, 2H). 6.69
cyclohepta(b][1,3]oxazolo[5;4-(d, J = 6.6 Hz, IH), 7.24-7.35
(tn, 2H), 7.5?
d]pyridine-?,4(3H)-dione(d. J = 7.7 Hz, IH), 1 I.9I
(br. s, 1H).
5-[2- (CDsSOaCD3) 8 5.21 (s, 21i), 6.64 (d, 3 = 7.3
(dittuoromethoxy)benzyl]-3,5- Hz, 111), 7.02 (d, 3 - 7.3 Hz, 1 H), 7.20-7.25
dihydro[I,3~axazola[4,5- (m, 2H), 7.27 (t, J = 74.0 Hz, IH), 7.62 (d, ! _
clPYnd~e-2:4-dione 7.3 Hz, l i~, 12.00 (bx. s, 1 H).
7-methyl-S-[(1R)-1- (CD3SOrCD;) S 1.72 (d, J = 7.3 Hz, 3H), 2.07
pheaylethyl]-3,5- (s, 3H); 6.27 (q, J = 7.3 Hz, 1H), 7.27-7.40 ._.
dihydro[1,3]oxazolo[4,5- (m, 6H), 11.95 (br..s; 1H).
c]pyridine-2,4-dione
CA 02366800 2002-O1-07
-1~~
S-(4-chiorobenzyl)-7 propYl' (CD3S02CD3) s 0.89 (t, J = 7.3 Hz, 3H), 1.54
3,5-dihydro[1,3]oxazolo[4,5- (xa, 2H); 2.4A (t, J = 7.7 Hz, 2H), 5.1 S (s,
2H),
c]pyridine-2,4-diorze7.30 (d; J = 8.4 Hz, 2H), 7.39
(d, J = 8.4 Hz,
2H), 7.57 (s, l I~, 11.9'7 (br.
s, I H).
5-[2-(methylsulfonyl)benzyl]-(CDsSOaCD~) 8 3.43 (s, 3H),
5.60 (s; 2H);
3,5-dihydro[1,3]oxazolo[4,5-6:?5 (d; J = 7.3 Hz, 1H), 7.49-7.61
(m; 2H);
c]pyridine-2,4-dior~e7.65-7.70 (m, 2H) 7.89-7.91
(m, 1H), 12.02,
(br. s, 1H).
5-(2,6dimethylbenzyl)-3,5-(CD,SOZCD3) 8 2.21 (s, 6H),
5,16 (s, 2H),
dihydro[1,3]oxazolo[4,5-6.47 (d, J = 7.3 Hz, 1 H), 6.80
(d, J = 7:3 Hz,
clpyridine-2;4-dione1H), 7.09-7.22 (m, 3H), 12.0U
(br. s, IH).
3-chloro-2-[(2,4-dioxo-2,3- (CDsSOaCD3) 8 5.38 (s, 2H), 6.61 (d, 7,4 Hz,
dihydro[1,3]oxazolo[4,5- 1H), 7.55 (t, J = 8.0 Hz, 1H), 7.62 (d, J = 7.4
c]pyridia-S(4H}- Hz, 1 H), 7. 82 (d, J = 8.0 Hz, 1 H), 7.87 (d, J =
yl)methyl]beazonitrile 8.0 Hz, 1 Fn, 1 ! .96 (br. s, 1 H).
5-(2-chioro-6-methylbenryl)- {CD3SO2CD3) 8 2Ø6: (s, 3H), 2.119 (s, 3H),
6,?-dituethyl-3,5- 2.10 (s, 3H), 5,58 (s2H), 7.13 (d, J = 7.7 Hz,
dihydro[1,3]oxazolo[4,5- 1H}; 7:20 (t, J = 7.7 Ptz, 2H), 7.27 (d, J = 7.7
c]pyridine-2,4-dione Hz, 1 H), 11.84 (br. s; 1 H).
2-[(2,4.dioxo-2,3- (CD3SOZCD3) ~ 5.40 (a, 2H), 6.70 (d, J = 7.4
dihydco[ 1,3}oxazolo[4,5- Hz, 1 H), 7,11 (d, J = 7.7 Hz, l Ii), 7.50 (t, J =
c~pytidin-5(4H)- 7.7 Hz, 1 H), 7.66 (td, J = 7.7, 1, I Hz, 1 H),
yl)methyl]beazonitrile 7.74 (d, J = 7,4 Hz, 1H), 7.88 (dd, 3 = 7.7; I .1
Hz, 1H), 12.01 (br. s, 1H).
5-(2-chloro-6-methoxybenzyl)- (CD3SOZCD3) 8 2.01 {s, 3H), 3.81 (s, 3H),
7-methyl-3,5- 5.21 (s, 2H), 6.86 (s, 1H), 7.11 (m, 2H), 7.41
dihydro[1,3~oxazolo[4,5- {t, J ~ 8.2 Hz, 1H); I I.96 (br. s, IH).
c~pyridine-2,4-dione
S-[3-(methylthio)benzyl)-3,5- (CD.3SOZCD3) 8 2.45 (s, 3H), 5.1b (s, 2H), _
dihydm[1,3]oxazolo[4,5- 6.61 (d,1= 7.3 Hz, 1H); 7.04 (d, J = 7.3 Hz,
c]pyridine-2,4-done 1H), 7.16-7,34 (m, 3H), 7.?3 (d, J = 7.3 Hz,
1 H), I 1.97 {br. s, 1 H).
CA 02366800 2002-O1-07
-123-
5-(2-chlorobenzyl)~7- (CD3SOzCD~) b 0.70 Vim, 2I~, 0:87 (m, 2H),
cyclopropyl-3,5- 1.79 (m, IH), 5.22 (s,'2H), 6.79 (d, 3 = 7.3
dihydco[1,33oxazolo[4,5- Hz, 1H), 7.31 (m, 1H)~, 7.45 (s, IH), 7.50 (d, J
c]pyridine-Z,4:dione = 7.7 Hz, 1H), 12.01 {br: s; 1H).
,~=.
5-(3-cblombenzyl)-7-methyl- (CD3SOzCD3) 8 2.89'(d, J = 1.1 Hz, 3H), 5.15
3,5-dihydm[1,33oxazolo[4,5- ' (s, 2H), 7.26 (m, 1H), 7.33-7.41 (m, 3H), 7.59
c]pyridiae-2,4-dione (q, J =1. I Hz; IH),11.97 (br, s, 1 H).
5-(2,6-dichlorobenzyl)-7~ (CD3SOxCD3) b 2.03 (d, J = 1.I Hz, 3H), 5.36
methyl-3,5- (s, 2F1), 6.87 (q, J = 1.I Hz, 1 H), ?.46 (dd, J =
dihydro[1,3)oxazolo[4,5- 8.8, 7.4 Hz, 1H), 7.56 (d, J a 7,4 Hz, 1H),
c]pycidins-2,4-dione 7.57 (d, J = 8.8 Hz, 1 H), I 1' .99 (br. s, l H).
7-methyl-5-(4-methylbenzyl)- (CD3SOz~3) 8 2.07 (s, 3H), 2.27 (s, 3H),
3,5-dihydto[l,3joxazolo[4;5- 5.10 (s, 2H), 7.08-7.23 (rn, 4H); 7.52 (s, IH),
cjpyridine-2,4-dioae 11.95 (br. s, 1H).
5-(3,5-dimethoxybenzyl)-7- (CD3SO2CD3) d 2.09 (s, 3H), 3.71 (s, 6H),
methyl-3,5- 5.06 (s, 2H), 6.42 (t, J = 2.2 Hz,.IH), 6.46 (t1,
dihydro[1,3]oxazolo[4,5- J = 2.2 Hz, 2H), 7.51 (s, 1H), 11.96 (br. s,
c]pyiidine-2,4-dione 1 H).
S-(2;6-ditluorobenzyl)-7- (GD3SOaCD3) 8 2.09 (d, J = 1.1 Hz, 3H), 5.21
methyl-3,5- (s, 2~, 7.04-7.13 (m, 2H), 7.38-7,47 (m, 2H),
dihy~o[l,3joxazolo[4,5- 11.91 (br. s, 1H).
c]pyridine-2,4-dione
5-(3-(metltylsulfonyl)benzylj- (CD3SOZCD3) 8 3.20' (s, 3H), 5.31 (s, 2H),
3,5-dihydro[l,3]oxazolo[4,5- G:66 (d; J = 7.3 Hz, t:F~, T.5-7.7 (m, 2H),
7.81
c]pycidine-2;4-dione (d, J = ?,3 Ha, IH), 7.83-7:96 (m, 2H), 1
L.99
(br. s, 1H).
5-(Z-Chloro-6-ethoxybenzyl)- (CD3SOZCD3) 8 I.25 (t, J = 7:0 Hz, 3H), 4.05
3,5-dihydro[l,3joxazolo[4,5- (q, J = 7.0 Hz; 2H), 5.25 (s, ZH), 6.49 (d,
J =
c]pyridine-2;4-dione 7.3 Hz, 1H), 7.06 (d,, J = S.4 Hz, 1 H), 7.10
(d,
J = 8.1 Hz, 1H), 7.12 (d, J = 7.3 Hz, 1H),
7.37
(dd, J = 8.4, 8.1 Hz, IH), 1 I.95 (br. s, IH).
5-(2-chloro-b-ethoxybenzyl)-7- (CD3SO=CD3) 8 1.25 (t, J = 7.0 Hz, 3H), 2.02
methyl-3,5- (s, 3H), 4,04 (q, J __-- 7.0 Hz, 2H), 5.23
(s, 2H),
dihydro[1,3]oxazolo[4,5- 6.97 (s, IH), 7.04 (d, J= 8.4 Hz, 1H), 7.09
(d,
cjpyridine-2,4-dione 3 = 8:0 Hz, 1 H), 7:36 (dd, J = 8.4; 8.0 Hz,
1H),11.93 (br. s,1H).
CA 02366800 2002-O1-07
-124- ; 5
5-(2-fluoro-6-metJzoxybenzyl)- (CD3SO2CD3) 8 2.0S ~s, 3H); 3.82 (s, 3H),
7-methyl-3,5- 5.12 (s, 2H), 6.82 (ddb = 9.5, 8,4 Hz; 1H),
dihydro[l,3Joxazolo[4,5- 6.91 (d, J = 8.4 Hz,1H), 7.18 (s, 1H), 7.37
cJpyridine-2,4-dione (td, J ~ 8.4, 6.6 Hz, 1 H), 11, 89 (br, s, 1 H).
S-(2-chloro-6-methoxybenzyl)- (CD3S0=CD3) S 0;82 (t, J = 7.3 Hz, 3H), 1.47
7-prapyl-3,5- (sextet, J = 7.3 Hz, 2H), 2.38 (t, J = 7.3 Hz,
dihydro[1,3]oxazolo[4,5- 2H), 3.80 (s, 3H), 5.21 (s; 2H), 6.89 (s, IH);
cJpyridine~2,4-dione 7:08-7:13 (m, 2H), 7.40 (t, J = 8.3 Hz, I H),
11.93 (br. s, 1H).
5-(5-chlom-2-fluorobeazyl)-7- (CD3SO=CD3) 8 2.10;(s, 3Fi), 5.18 (s, 2H),
methyl-3,5- 7.20 (dd, J ~ 6.6, 3,0 Hz, 1H), 7.29 (dd, J =
dihydro[l,3Joxazolo[4,5- 9.6, 8.8 Hz, 1H), 7.42 (ddd, J ~ 8.$, 4.4, 3,0
c~pyridine-2;4-dione Hz, 1H), 7,5T (s, lHj; 11.96 (br, s, 1I-1],
S-(2-chlorobenzyl~7-(CD3SOaCD3) b 1.23 (d, J = 7.0
Hz, 6H), 2,92
isopropyl-3,5- (m, IFS, 5.25 (s, 2H), 6.83 (dd,
J = 7.4, 2.2
dihydxo[i,3]oxazolo[4,5-Hz, 1H), 7.2'7-7.35 (in, 2H),
7.49 (8, 1H), 7.51
c]pyridine-2;4-dione(dd, J = ?.3, I .8 Hz, 1 H),
12.01 (br. s, I H).
5-(5-fluoro-2-methylbenzyl)-7- (CD35OZCD3) ~ 2.10 (d, J -- I .1 Hz, 3H), 2.34
methyl-3,5- (s, 9H), 5.13 (s, 2H), 6.55 (dd; J = 9.9, 2:6
Hz,
dihydro[1,3]oxazola[4,5-1H), 7.01 (td, J ~ 8.42:6 Hz, IH), 7.25 (da,
J
c]pyridine-2,4-dione= 8.4, 5.9 Hz, 1 H), 7x:42 (q; 1.1 Hz, 1 H),
1 I .99
(br. s, 1 H).
7-methyl-5-[(IS)-I- (CD3SOzCD3) o I.72 (d, J ~ 7.3 Hz, 3H), 2.07
phenylethyl]-3,5- (s, 3H), 6.27 (q, J='7.3 Ha, IH), 7.27-7.40
dihydro[l,3Joxa2olo[4,5-(m, 6H), 11.95 (br, s, 1H):
c)pyridine-2,4-dione
5-(2-ahloro-5- (CD3SOaCD3) & 1.20 (d, J = 6.0 Hz; 6H), 2.11
isopropoxybenzyl)-7-methyl-(s, 3H), 4.50 (m, 1H), 5:16 (s, 2H), 6.34 (d,
J
3,5-dihydro[I,3Joxazolo[4,5-= 3.0 Hz, 1H), 6.91 (dd, J = 8.8, 3,0 Hz, 1H),
c]PTnd~e-2,4-dione 7.38 (d, J = 8.8 Hz, 1H), 7.47 (s, IH), 12.01
(br. S, 11~.
5-(5-acetyl-2-methoxybenzyl)-(CD~SOzCD3) 8 2.4? (s, 3H), 3.93 (s, 3H),
3,5-dihydro[1,3]oxazolo[4,5-5.16 (s, 2H), 6.62 (d, 3-7.3 H2, 1H), 7.i6
(d,
c)pyridine-2,4-dioneJ = 8,4 Hz, 1 H), 7.59 (d, J = 2.2 Hz, 1 H),
7.63
(d, J = 7.3 Hz, 1 H); 7.97 (dd; J = 8.4, 2.2
Hz,
I H), 1 I ,96 (br, s, YH).
CA 02366800 2002-O1-07
-125- 1
5-(2-chlorabenzyl)-7-methyl- (CD3SOaCDs) S 2.29 (s, 3H), 5.39 (s, 2H),
3,5-dihydro[I,3;oxazolo[4,5- 7.00 (d, J = 7.4 Hz, lIi), 7.26-7.37 (m, 2H),
d]pyridazine-2,4-dione 7.51 (d, J = 7.7 Hz, lHa, 12.80 (bx. s, lI~.
5-[2-fluoro-b- (CD3SOaCDa) s 2.04 (s, 3H),
5.33 (s, zH),
(trifluozomethyl)be~nzyl)-7-7.05 (s, 1H), 7.5I-7.72 (nt,
3H), 11.98 (br. s,
methyl-3,5- 1 H). ,
dihydro[l,3joxazolo[4,5-
e]pyridine-2,4-dione
5-(Z-chloro-6-methylbenzyl~(CD3SO2CD3) 0 2.02 (ns, 2H),
2:21 (s, 3H),
S,b,?,8-tetrahydro-2H- 2,64-2.80 (m, 4H), 5.a2 {s,
2H), 7.05-7.33 (m,
cyclopenta[bj[1,3]oxazolo[5,4-3H), 11.81 (br. s, lI~.
djpyridine-2,4(3I-17-dione
5-(2-chloro-6-ethoxybertzyl)-7-(CD3SO~CD3) 8 1,08 (t, J = 7.7
Ha, 3H), 1,25
ethyl-3,5- (t, J ~ 7.0 Hz, 3H), 2.44 (q,
J = 7.7 Hz, 2H),
dihydro[l,3joxazolo[4;5-4.05 (q, J = 7.0 Hz, 2H); 5.23
(s, 2H), b.99 (s,
cjpyridine-2,4-dione 1 H), 7:05 (d, J = 8.4 Hz, 1
H), 7.09 (d, J -- 8, I
Hz, lI~, 7.36 (dd, J = 8.4,
8.1 Hz, 1H), 11.93
(br. s, 1H).
5-(2-chloxo-6-propoxybeuzyl)-(CD3S0_CD3) 8 0.88: (t, 3 =
7.3 Hz, 3H), 1.66
7-methyl-3,5- (m, 2H), 2,0I (d, J =I .1 Hz,
3H), 3.95 (t, J =
dihydro[l,3joxazolo[4,5-6,2 Hz, 2H), 5.24 (s~2H), 5.91
(q, J = I.1 Hz,
ejpyridine-2;4-dione 1 H), 7.03 (d, J = 8.4 Hz, 1
H), 7.10 (d, J J 8.1
Hz, IH), 7.37 (dd, J = 8.4;
8.1 Hz, 1H), 11.95
{br. S, 1 H).
5-(2-chlora-b- (CD3SOaCD3) b 0.89 {d, 3 = 7.0 H2, 6H), 1.95
isobutoxybentyl)-7-methyl- (m, 1H), 2:00 (s, 3H); 3:79 (d, J ~ 6.2, 2H),
3,5-dihydro[1,3]oxazolo[4,5- 5.25 {s, 2H), 6.85 (s, llH), 7.0o (d, J = 8.4 Hz,
cJpyriditte-2,4-dione IH), 7.11 (d, T= 8;1 Hz, 1H), 7.38 (dd, J=
8.4, 8.1 Hz, 1 H), 11.97 (bx. s, I H) .
5-(2-chloro-6-ethoxybenzyl)- (CD3SOaCD3) S 1.10 (t, J= 7.0 Hz, 3H), 2.06
5;6,7,8-tetrahydro-2H- (m, 2H), 2.70-2.92 {m, 4H); 3.90 (q, J = 7,0
cyclopenta[b)[1,3]oxazolo[5,4- Hz, 2H), 5.33 (s, 2H), 6.93 (d, J = 8.4 Ha,
d]pyridine-2,4(3H~dione 1 H), 7.03 (d, J = 8. T rHz; 1 H), 7.26 (dd, J = --.
8.4, 8.1 Hz, 1H), 11.75 (br. s, 1H).
CA 02366800 2002-O1-07
-I2fi-
5-(Z-chloro6- (CD3S02CD3) 81.16 (d, I = 6.2
Hz, 6H), 2.02
isopropoxybenzyl)-7methyl-(s, 3H), 4.67 (m,1F~; 5:21 (s,
2H); 6.94 (s,
3,5-dihydro[l,3joxazolo(4,5-IH), 7.07 (d,1= 8.0 Jiz, 2H),
7.34 (t; 3 = 8:0
c]pyridine-2,4-dioae Hz, 1H), I 1.93 (br. 's, IH).
5-[2-chlom-6-(2,2,2- (CD3SO2CD3) 8 2.01 (s, 3H),
4,82 (q, T ~ 8.8
trifluoroeti~oxy)benzyl]-7-Hz, 2H), 5.24 (s, 2H), 6.94
{s, 1H), 7.19 (d, J
methyl-3,5- = 8.4 Hz, IH), 7.22 (d, J =
8.I Hz, 1H), 7,43
dihydro[I,3joxazolo[4,5-(dd, J = 8.4, 8,1 Hz; lI~, 11.92
(br. s, IH).
c]pyridine-2,4-dione
5-(2-chloro-6-ethoxybenzyl)-7-(CD~SOZCD3) s 1.I9 (t, J = 7.0
Hz, 3H), 2.19
methyl-3,5- (s, 3H), 3,99 (q, J ~ 7.0 Hz,
2H), 5.41 (s, 2H),
di'hydro[1,3]oxaaolo[4,5-6.98 (d, J = 8.4 Hz, IH}, 7.05
(d, J = 8.0 Hz,
djpyridazine-2,4-dione1 H), 7.30 (dd, J = 8.4, 8.0
Hz, 1 H}, 12.70 (br.
s, I T~,
5-[2-chloro-6-(2- (CD3SOZCD3) 8 2.06 (m, 2H),
2.74-2.90 (m,
methoxyethoxy}benzylj-4H), 3,20 (s, 3H), 3.47 (t,
J = 4.4 Hz, 2H};
5,6,7,8-tetrahydro-2H-4,01 (t, J = 4.4 Hz, 2H), 5.33
(s, 2H), 6.98 (d,
cyclopenta[b][l;3jvxazolo[5,4-J = 8.0 Hz, 1H), 7.04 .(d, J
-- 8.0 Hz, 1H), 7.27
djpyridine-2,4(3H)-dione(t, J = 8.0 Hz, IH), (br. s,
lFi}.
5-(2-chloro-6-ethoxybenzyl)-(CD35OzCD3) 8 1.03 (t, J = 7.0
Hz, 3H}, 2.06
G,7-dimethyl-3,5- (s, 3H), 2.22 (s, 3H), 3.84
' (q, J = 7.0 Hz, 2H),
dihydro[1,3]oxazolo[4,5-5.48 (s, 2H), 6.92 (d, 8.,4
Hz, IH), 7.03 (d, J =
c]pyridine-2,4-dione 8.1 Hz, I H), 7.24 (dd, J =
8.4, 8.1 Hz, I H);
1 I.76 {br.s, 1H),
5-(2-chloro-6-ethoxybenzyl)-7-(CDJSOZCD3) S I.06 (m, 6H),
2.24 {s, 3H),
ethyl-6-methyl-3,5- 2.48-2.56 (m overlapping DMSO,
2H), 3.85
dihydro[I,3]oxazolo[4,5-(q, J = 7.0 Hz, 2H), 5.48 (s;
2H), 6.92 (d, 8:4
c]pyridine-2;4-dione Hz, lI~, 7.03 (d, J = 8.1 Hz,
1H}, 7.24 (dd, J
-- 8.4, 8.1 H2, 1H); 11<.:77
(br.s, 1H).
5-(2-chlorobenzyl)-7-ethyl-3,5-(CD3SOzCD3) 8 1,18 (t, J = 7.S
Hz, 3H), 2.70
dihydro[1,3]oxazolo[4,5-(q, J = 7.5 Hz, 2H), 5.38 (s,
2H), 7Ø7.6 (m,
d)pyridazine-2,4-dioae4H), 12,77 (br. s, 1H),
5-(2-chlom-6-ethoxybenzyl)-7-(CD3SOZCD3) 8 0.82 '(t, J s
7.3 Ha, 3H), 1,24
propyl-3,5- (t, J = 7.0 Hz, 3H}, 1.48 (m,
2H), 2.3? (t, J =
dihydro[I,3]oxazolo[4;5-7,3 Hz, 2H1, 4.05 (q, J = 7.0
Hz, 2H), 5,23 (s,
c]pyridine-2,4-dione 2H), C.93 (s, 1H), 7.05 (d,
J = 8.4 Hz, 1H),
7:09 (d, J = 8.1 Hz, 1H), 7.36
(dd, J = 8.4, 8. I
Hz,1H), 11.94 (br. s; 1H).
CA 02366800 2002-O1-07
-12?-
5-(2-chloro-8-ethoxybeazyly-7- (CD3SOZCD3) s:0.55 (m, 2H), 0.8I (m, 2i~,
cyciopmpyl-3,5- I,26 (t, J = 7.O Iii, 3H), 1.72 (m; IH), 4.0S (q,
dihydro[I,3joxazolo[4,5- J = 7.0 Hx, 2,H); 5.Z2 (s; 2H), 6.95 {s, 1H),
c]pyridine-2,4-dione 7.05 (d, J ~ 8.4 Hz, 1 H), 7.09 (d, J = 8,1 IHz,
lI~, 7.36 (dd, ~n 8.4, 8.1 Hz; 1H), 11.93 (br.
s, 1 H}.
5-(2-chtoro-5-pmpoxybenzyl}- (CD3SOzCD3) b 0.92 (t, 7 = 7.3 Hz; 3H), 1.66
7-methyl-3,5- (rn, 2H), 2.10 (s, 3I~, 3.85 (m, 2H}, S:I7 (s,
d~ydso[1,3]oxazoio[4;5- 2H), 6.41 (d, J =3.3 Hz, iFi), 6.9I (dd, J =
c]gyridine-2;4-dione 8.8, 3.3 Hz, 1 H), 7.39 (d, J = 8.8 Hz, 1 H),
7.45 (s, 1H), 12.00 (bz, s, IH).
5-(2-chloro-5-methoxyber~zyl)- (CD3S0=CD') 8 2.10 (s, 3H), 3.9 (s, 3H), 5, I8
7-methyl-3,5- (s, 2H), 6.42 (d, J ~ 3.0 Hz, 1 H), b.93 {dd, I =
dihydro[1,3]oxazolo[4;5- 8,8, 3.0 H2, 1H), 7.42 {d, J = 8.8 Hz, 1:H},
c]pytidiae-2;4-dione 7.44 (s, I H), 12.00 (br. s, 1 H).
5-{2-chloro-6~ethoxybenzyl)-6= (CD~SOzCD3) 8 1.07 {t, J = 7.0 Hz, 3H), 2:32
methyl-3,5- (s, 3H}, 3,87 (q, J =7.0 Hz, 2H), 5.42 (s, 2H),
dihydro[1;3]oxazolo[4;5- 6.44 (s, lI~, 6.92 (d, J = 8.4 Hz, 1H), 7.03 (d,
c]pyridine-2,4-dione J = 8.1 Hz, 1H), 7.24 (dd, J = 8.4, 8.1 Hz,
1 H), 1 I .74 (br. s, 1 H).
5-(2-chtoro-5-ethoxybenzyI)-7- (CD3SO~CD3) 8 1,26 (t, J ~ 7.0 Hz, 3H), 2.10
rnathyl-3,5- (s, 3H), 3.94 (q, J = 7.0 Hz, 2H), 5.17 (s, 2H),
dihydro[I,3]oxa2oto[4,5- 6.38 (d, J =2:9 Hz, 1H), 6.91 {dd, J= 8.8, 2.9
c]pyridine-2,4-dione Hz, 1H), 7,39 {d, J-- 8.8 Hz, IH), 7,44 (s,
1 H), 11.99 (br. s, t Fl).
5-[2-rhloro-S-(pipexidin-1- (CD3SOaCD3) 8 1.35 (m, 2H), 1.47 (m, 4H);
ylsulfonyi)benzyl]-7-methyl- 2.10 (s, 3I-I), 2.8I (m, 4H), 5.30 (s, 2H), 7.18
3,5-dihydro[1,3)oxazvlo[4,5- (d, J = 2.2 Hz, 1H), 7.5? (s, II-~, 7.6? (dd, J
c]pyridine-2,4-dione 8,4, 2.2 Hz, IH), ?:78 (d, J = 8.4 Hz, IFi),
32.07 (br, s, 1H).
5-[2-chloro-5-(pyrrolidin-1- (CD3SOxCDa) 8 i.62 (m, 4H), 2.11 (s, 3H),
ylsulfonynbenzyi]-?-methyl- 3.05 (ra, 4H), 5.30 (s, 2H), 7.30 (s, 1H}, 7.57
3,S-dihhydro[1,3]oxazolo[4,5- (s, 1H), 7.75-7.82 (m, ZFi), 12.08 (br. s, 1H),
c]pyridine-2,4-dione
CA 02366800 2002-O1-07
-128-
1:
5-[2-chlorn-6- (CD3SOZCD3) $ I:22 (m, 2F~;
1.5I (m, 4I~,
(cyctopentylmethoxy)benzyl]-1.68 (m, 2H), 2.00(s, 3H), 2.20
(m, iH), 3.89
7-methyl-3;5- (d, J = 7.0 Hz; 2I~, 5.24 (s,
2H), 6.86 (s, 1 H),
dihydro[1,3]oxazolo[4,5-7.47 (d, J = 8.4 Hz, 1H), 7.11
(d, J = 8.1 Hz,
c]pyridine-2,4-dione l I~, 7.37 (dd, 3 = 8.4, 8.1
Hz, 1 H), I 1.97 (br.
s, 1H).
S-[2-(benzyloxy)-6- (CD3SOZCD3) & 1.90 (s, 3H),
5.15 (s, 2I~,
chlombenzyl]-7-methyl-3,5-5.25 (s, 2I~, 6:84 (s, lI~;
7. I3 (d, J = 8.1. Hz,
dihydm[1,3]oxazolo[4,5-1~T), 7,19 (d, J--- 7.7 Hz,
IH), 7.30-7.37 (m,
c]pyridine-2;4-dione SH), 7.39 (dd, J = 8.1 p 7:7
Hz, 1 T~, 11.9 I (br.
s, 1H).
5-(2,3-dichloro-6- (CD3SOaCD3) ~ I.10 (t, J = 7.0
Hz, 3H), 2.09
ethoxybenzylr5,6,7,8-(m, 2H) 2.80 (m, 2H), 2.89 (m,
2H), 3.92 (q, J
tetrahydro-2H- ~ 7.0 Hz, 2H), 5.33 (s, 2H),
6.98 (d, J = 8.8
cyclopenta(b][1,3]oxazolo[5,4-Hz, iH), ?.50 (d, J ~ 8.8 Hz,
IH), 11.71 (br, s,
d]pytldine-2,4(3~-I}-dione1H~.
~-[2-chloro-5- (CD3S02CD3) S 2.11 (s, 3H), 5.29 (s, 2H),
(trifluoromethyl)benzyl}-7- 7.34 (s, 1H), 7.54 (s, 1H), 7.72-7,79 (m, 2H);
methyl-3,5- 12.00 (br. s, lI~.
dihydro[1,3]oxazolo[4,5-
clpYri~e-2,4-dione
S.(2-chloro-5-fluorobenzyl)-7- (CD3502CD3) 8 2.11 (s, 3H), 5.20 (s, 2H),
methyl-3,5- 6.7I (dd, J - 9.4, 2.9 Hz, l I-i~, '7:22 (td, J =
dihydro[1,3]oxazolo[4,5- 8.4, 2.9 Hz, 1H), 7.49 {s, 1IH), 7.57 (dd, J s
c)pyridine-2,4-dione 8.4, 5.2 Hz, 1 H), 11.99 (br. s, 1 H).
Example 42
A procedure in which a 26-amino acid peptide containing the CS 1
sequence of fibronectin with an N-terminal Cys
(CDELPQLvTI,plipNLHGPEIt;DVP$T) was coupled to maleimide
activated ovalbumin was used to determine the efficacy of the compounds
synthesized: Bovine serum albumin (BSA) and CS1 conjugated
ovalbumin were coated onto 96-well polystyrene plates at 0.5 ~.g/ml in
TBS (50 mNt TRIS, pH 7.5; I50 mM NaCl) at 4°C for 16 hours. The
IO plates were. washed-thr~e times with TBS and blocked with THS
containing 3°lo BSA at room temperature for 4 hours. Blocked plates
were
CA 02366800 2002-O1-07
-129-
washed three times in binding buffer (TBS; 1 mM MgCla; 1 tnM CaClz; I
mM MnCI~ prior to assay. Ramos cells fluorescently labeled with calcein
AM were resuspended is binding buffer (10' cellsiml) and diluted 1:2
with same buffer with or without compound, I00 p.N! of compound was
added. The cells were added immediately to the wells (2.5 x I OS
cells/well) and incubated for 30 minutes at 37°C. Following three
washes
with-binding buffer, adherent cells were lysed and quantitated using a
fluorome~ter. TI~ results are shown in Tables 2-7. ICso is defined as the
dose required to give 50% inhibition, measatred in ,uM for Tables 2 and 4.
The lower the ICsa value and the greater the pcbrcentage of inhibition, the
more efficient the compound is at preventi~n of cell adhesion.
CA 02366800 2002-O1-07
-130-
Table 2
Name TCso Mass
Spectral Data (rt~Jz)
(3S)-3-(1,3 benzodioxol-5-y'i)-3-[({[(3S)-2-oxo-1-(2- 0.2 Calc'd (M-H)-
2444.12;
thienylmethyl)hexahydtb-3- Found (M-H)'= 444.08
pyridinyI]amino}carbonyl)amino]propanoic
acid
(3S)-3-(I,3-benzodioxol-5 yI),3-[(~[(3S)-2-oxo-1-(2-i 5 Cala'd (M-l~iy'
=430.11;
tltienylmethyl)tehahydro-lH=pyrml-3-Found (M-~ 430.06
yl]amino}carboayl)amino]propanoic
acid
(3S)-3-(1,3-benzodioxol-S-yl)-3-[({[(3R)-2-oxo-1-(2-2 Calc'd (M-H)- =444.12;
thienylmethyl)hexahydro-3- Found (M-I~~= 444.05
pyridinyl]amino}carbonyl)amino]propanoic
acid
(3S)-3-(1,3-benzodioxol-5-yl)-3-(({[2-oxo-I-(2-0.9 Calc'd (M-H)' X40.09;
thienylmethyl)-1,2-dihydzo-3- Found (M-H)'= 439.98
pyridinyl]amino}carbonyl)aminoJpropanoic
acid
(3S)-3-(1,3-benzodioxol-S-yl)-3-({[((3S)-2-oxo-1-~4-[(2-0.0003 Calc'd (M-H)-
586.23;
toluidinocarbonyl)amino]benzyl} hexahydm-3-' Found (M-H)"= 586.17
pyridinyl)amine]carbonyl}amino)gropanoic acid
(3S)-3-(1,3-benzodioxoi-5-yl)-3-[({[2-oxo-1-i4-[(2- 0.001 Calc'd (M-H)'
=582.20;
toluidinocarbonyl)aminoJbenzyl}-1,2-dihydro-3- Found (M-H)'= 582.20
pyridinyljamino}carbonyl)aminoJpropanoic acid
{3S)-3-(I,3-benzodioxol-5-yl)-3-({[((3S)-1-{4-[(2- nd nd
methylbenzyl)aminoJbenzyi}-2-axohexahydro-
pyridinyl)amino]carbonyl} amino)ptopaaoic acid
(3S)-3-11,3 bezizadioxol-5-yl)-3-[({butyl[2-oxo-I-(2- 20 Calculated (M-H)' =
496.15;
thienylra~thyl)-1,2-dihydro-3- Found (M-H)" = 49b.10
pyridinyt]amino~carbonyl)amino]propanoic acid
(3S)-3-(1,3-benzodioxol-5-yl)-3-[({[(3S)-2-oxo-1-(2- 0.015 Calculated (M-H)' =
458.13;
thienylmethyl)azepanyl]araino}carbonyl)amino]propanoic Found (M-H)' ~ 458.09
acid
s
CA 02366800 2002-O1-07
-131-
Table 3
Compound ZCso Mass Spectral Data
(~
(3S)-3-(({[2-ruethyl~-(2-methylpropyl)-6-oxo-1-I O Calculated {M-H)' =
475.23 m/z;
(IYYI~ 1,G.dihydro=5- Found (M-H)' = 4'75.02
m/z.
pyrimidinyl]amino} carbonyl)amino]-3-(4-
methylpheyl~ropanoic acid
(35)-3-(1;3-berzodioxol-S-yI)-3-[({[2-oxo-1-10 Calculated (M-H)' =
476.18 m/z;
(phenylmethyl)-4-propyl-1,2-d~'hydro-3-Found {M-H)' = 475.99 mlz.
PYn~Yllo)carbonYl)amiuvjpropanoic
acid
(3S)-3-{1,3 benzodioxol-5-yl)-3-({[9-oxo-8-4000 Calculated (M-H)'
= 485.18 m/z;
~
(phenylmethylr2,3,x,5,8;9-hexahydro-1H-Found (M-I~' = 488,19 m/z.
pyzido[3,4-b]azepin-1-
yl]carbonyl} amino)propanoic
acid
(3S)-3-{[({1-((2-chlorophenyl)methyl]-4-ethyl-2-10 Calculated (M-H)'=466.15
m/2;
oxo-1,2-dihydro-3- Found {M-H)- = 465.95 m/z.
pyridinyl}amino)carbonyl]amino}-3-{4-
methylphenyl)propanoic acid
(3S)-3-{[({1-((2chloropb~snyl)methyl]-2-oxo-4-4 Calculated (M-H)'- 480.17
mlz;
propyt-1,2-dihydro-3- Found (M-H)' = 480.00 rn/z.
pyridinyl}amino)carbonyl]emir
~}-3-(4-
methylphenyl)proganoic acid
(3S)-3-{[({1-[(2-chlorophenyl)methyl]-4..methyl-S Calculated {M+H)+--454.15
m/z;
2-oxo-1,2-dihydro-3- Found (M+H)+= 454.09 m/2.
pyridinyl} amino)carbonyljamino}-3-(4-
methylphenyl~ropanoic acid
(3 S)-3- ([( {6-methyl-2-oxo-1.(phenylmethyl)-4-S Calculated (M-H)' = 524.22
m/z;
((phenylmethyl)oxyj-1,2-dihydro-3-1<ound (M-H)' = 524.02
m/z.
pyridinyl}amino)carbonyl]amino}-3-(4-
methylphenyl)propanoic acid
(3S)-3-{[({1-j(2-chlvrophenyl)methyl]-2,4-10 Calculated (M-H)' --
46?.15 m/z;
dirnethyl-6-oxo-1,6-d~ydro-5- Found (M-H)' = 467:00 mlz.
pyrimidinyl}amino)carbonyl]amino}-3-(4-
methylphenyl)propanoic acid
CA 02366800 2002-O1-07
-132-
{3S)-3-{[({1-[(2,4-diohlorophenyl)mathyl]-4-30 Calculated (M-H)' 1486.10
mlz;
methyl-2-oxv-1,2-dihydm-3- ;Found (M-H)' = 485,95
u~/z.
pyridinyl} amino)carboayl]amino}-3-(4-
methylphenyl)prapanoic acid
{3S)-3-{[({4-amino-1-[(2-chlorophenyl~nethylJ-10 Calculated (M-H)'=467.15
m/z;
6-methyl-2-oxo-1,2-dihydro-3- Fouad (M-H)' = 467.14 m/z.
pyridinyl}amino)carbonyl]amino}-3-(4-
methylphenyl)propanoic acid
(3S}-3-j({[1-[(2-cblorophenyl)methylJ-4-20 Calculated (M-H)' =
468,13 mlz;
(methyloxy)-2-oxo-1,2-dihydro-3-Found (M-H)' = 467:97 mJz.
pyridinyl~amino} carbonyl)amino]-3-(4-
methylphenyl)propanoic acid
(3S)-3-{[{{4-chloro-I-[(2-chlorophenyl)methyl]-20 calculated (M-H)-=472.08
mlz;
2-oxo-1,2-dihydro.3- Fouad (M-H)- --- 471.91
m/2.
pycidinyl}amino)carbonyl]amino}-3-{4-
methylphenyl)propanoic acid
(3S)-3-{j({I-j(2-chlorophenyl)methyl~-4-methyl-IS Calculated{M-H)'=482.15m/z;
2-oxo-1,2-dihydro-3- Found {M-H)-= 481.93 m/z.
pyridinyl} amino)carbonyl]amino
~-3-[3-methyl-4-
(methyloxy~pheayl]propanoic acid
(3 S)-3- { [{ { 1-[(2-chlorophcnyi)methyIj-4-methyl-3 Calculated (M+H)+ -
470.15
m/z;
2-oxo-I,2-dihydro-3- Found (M+H)'" = 470,01
m/z.
pyridiayI yaztlit~o)carbonyl]amino}-3-[4-
(methyloxy)pbenyljpropanoic acid
(3S)-3-{[({1-[(2-chlorophenyl)methylJ-4-methyl-10 Calculated (M+H)+=468.17m/z;
2-oxo-1,2-dihydro-3- Found (M+H)+ - 468:05 m/z.
pyridinyl} amino)carbonyl]amino}-3-(3,4-
dimethylphenyI)propanoic acid
(3S)-3-{[({4-amino-1-[{2-chlorophenyl)methyl]-10 Ca3culated (M-I-1T =
453.13 mlz;
2-oxo-1,2-dihydro-3- ' Found (M-H}' = 453.0I m/2.
pyridinyl}amiao)carbonyl]amino}-3-(4-
methylphenyl)propanoic acid
(3S)-3-{[({1-[(2-ahloraphenyl)methyIl.4-fluoro-15 Calculated (M-H)'=456,IZm/z;
2-oxo-1,2-dihydro-3- Found (M-H)' = 455.94 ttslz.
pyridinyl} amino)carbonyl]amino}-3-(4-
methyipheny!)propanoic acid
CA 02366800 2002-O1-07
-I33-
(3S)-3-[({(1-[(2-chlotophenyl)methyl]-2-oxo-4- 20 Calculated (M-H)'= 529.16
m/z;
(pheaylamino}-1,2-d~ydro-3- Found (M-H)-= 529.02 m/z.
pyridinylJamino}carbonyl)aminoJ-3-(4-
methylphenyl)propanoic acid
(3S)-3-[({[1-[(2-chlorophenyl)methyl]-2-oxo-4- 15 Calculated (M-H)'= 530.16
m/z;
(2-pyridinylamino)-1,2=dihydro-3- Found (M-H)' =529.99 mlz.
pyridinylJamino} carbonyl)amino]-3-(4-
methylphenyl)propanoic acid
(3S)-3-{[({1-[(2-chlorophenyl)methyl]-4- I0 Calculated (M-H)'=454.11 mlz;
hydroxy-2-oxo-1,2-dihydro-3- Found (M-H)' = 454.05 m/z.
pyridinyl}amino)carbonylJamirro}-3-(4-
methylphenyl)propanoic acid
(3S)-3-([({1-[(2-chlorophenyl)methyl]-2-oxo-4- 15 Calculated (M-H)' = 544.17
m/z;
[(2-pyridinylmethyl)aminoJ-1,2-dihydro-3- Found (M-H}' a 544.03 m/z.
pyridinyl}amino)carbonyl]amino}-3-(4-
methylphenyl)propanoic acid
(35)-3-{[({1-[(2-chlorophenyl)methylJ-2-oxo-4- 20 Calculated (M-H)-= 544.17
m/z;
[(3-pyridinylmethyl)ammo]-1,2-dihydro-3- Found (M-H)' --- 544.02 m/z.
pyridinyl}amino)carbonyl]amino}-3-(4-
methylphenyl)propanoic acid
(3S)-3-[({[1-[(2-chlorophenyl)methyl]-4-(1,4- 1 Calculated (M-H)' = 523.17
m/z;
oxazinan-4-yl)-2-oxo-1;2-dihydm-3- Found (M-H}' ~ 523.02 mlz.
pyridinylJarnino } carbonyl)aminoJ-3-(4-
methylphenyl)propanoic acid
(3S)-3-[({[1-[(2-chlorophenyl)methyl]-2-oxo-4- 10 Calculated (M-H)'= 495.18
mlz;
(pmpylamino)-1,2-dihydro-3- Found (M-H)-=495.04 mlz.
pyridinyl]arnino} carbonyl)aminoj-3-(4-
methylphenyljpropanoic acid
(3S)-3-{[({1-[(2-fluoropheayl)methyi]-4-methyl- 20 Calculated (M-
H)'=436.I7m/z;
2-oxo-1,2-dihydro-3- Found (M-H)' = 435,99 mlz.
pyridinyl}aminp)carbonyl]amino}-3-(4-
methylphenyl~propanoic acid .
(3S)-3-{[({1-[(2,6-dichlorophenyl)methyl]-4- 20 Calculated (M-H)'=486.10 mlz;
methyl-2-oxo-I,2-dihydzo-3- Found (M-H)' = 485.95 m/z.
pyridinyl} amirro)carbonylJamino}-3-(4-
methylphenyl)propanoic acid
CA 02366800 2002-O1-07
-134-
(3R)-3-{[({ 1-j(2-chlorophenyl~ae~yl]-4300 Calculated (M-T~' =
methyl- 376.11 mlz;
2-oxo-1,2-dihydro-3- Found (M-H)' _ 376:00 m/z.
pyridinyl}smino)carbonyl]amisio}butanoic
acid
(3S)-3-([({1-j(2-bt'omophenyl~ethyl]-4-methyl-10 ;Calculated (M-H)'=496.09
m/z;
2-oxo-1,2-dihydro-3- .Found (M-H)' = 495:8 m/z,
PYn~YI} ~ino)carbonyl]amino}-3-(4-
rnethylphenyl)propanoic acid
(3S)-3-[({[4-methyl-2-oxo-1-(phenylmethyl)-1,2-30 'Calculated (M-H)' =
418.17 snlz;
dihydro-3-pyridinyljamino}carbonyl)amino]-3-(4-Found (M-H)' = 417.96 rn/z.
methylphenyl~ropanoic acid
(3S)-3-{[({1-[(2-chloropheayl~nethyl]-4-8 Calculated (Nt-H)'= 484.12
m/z;
hydroxy-2-oxo-1,2dihydm-3- Found (M-I~' = 484.03 m/z.
pyridiayl} amino)carboyl]amino}-3-[3-methyl-4-
(methyloxy~henyl]propanoic acid
(3S)-3-{[({1-[(Z-chlorophenyl)methyl]-2-oxo-4-I0 Calculated (M-H)'= 514.15
m/z;
phenyl-I,2-dihydro-3- Found (M-H)' = S 14.00
rn/z.
pycidinyl}aminokarbonyl]amino}-3-(4=
methylphenyl)propanoic acid
(3S)-3-{[({4-bromo-1-[(2-chlorophenyl)methyl]-20 Calculated (M-H)-= 5I6,03
mlz;
2-oxo-1,2-dihydro-3- Found (M-H)' = SI5.90 m/z.
pyridinyl} amino)carbonyl]amigo
}-3-(4-
methylphenyl)propanoic acid
(3S)-3-(I,3-benzodioxol-5-yl)-3-{[({1.[(2-20 Calculated (M-H)'=484.09m1z;
chlorophenyl)methyl)-4-hydroxy-2-oxo-Z,2-Found (M-H)' = 484.03 m/z.
dihydro-3-
pyridinyl}amino)carbonyl]amino}propanoic
acid
(35)-3-{j({I-((2-chIorophenyl)methyl]:4-[(2-{[2-2 Calculated (M-H)-= 556.18
mlz;
(rnethyloxy)ethyl]oxy}ethyl)oxy]-2-oxo-1,2-Found (M-H)'= 556.03 mlz.
dihydro-3 pyridinyl}amino)carbonyl]atttino}-3-
(4-methylphenyl)propanoie acid
CA 02366800 2002-O1-07
-135-
(3S)-3-{[({1-[(2-chlorophenyl)methyl]-4-15 Calculated (M-H)' ~ 468.13 m/z;
hydroxy-b-methyl-2-oxo-1,2-dihydro-3-Found (M-H)-= 468.05 mfz.
pyridinyl} amino)carbonyl)arnino}-3-(4-
methylphenyl)propanoic acid
(3S)-3-{[({1-[(2-chlorophenyl)methylj-4-[(1,1-3 Calculated (M-H)'=509.20 m/z;
dimethyletbyl)amino]-2-oxo-1,2-dihydro-3-Found (M-H)-= 509:06 m/z.
pyridinyl}amino)carbonyl]amino}-3-(4-
methylphenyl)propanoic acid
(3S)-3-{[({1-[(2-chlomphenyl)methylj-4-10 Calculated (M-I-~'-440.10 m/z;
hydroxy-2-oxo-I,2-dihydro-3- Found (M-H)' = 440:04 m/z.
pyridinyl}smino)carbonyljamino}-3-
phenylpropanoic acid
(3S)-3-{[({1-[(2-chlorophenyl)methyl~-4-[4-3 Calculated (M-H)'--536.20 m/z;
methyltetrahydro-1(2H)-pyraginyl]-2-oxo-I,2-Found (M-H)- = 536:12 zn/z.
dihydro-3-pyridinyl } amino)carbonyl]amino
} -3-
(4-methylphertyl~ropanoic acid
(3S)-3-{[({1-[(2-chlorophenyl)methyl]-4-5 Calculated (M-H)'--470.11 m/z;
hydroxy-2-oxo-1;2-dihydro-3- Found {M-H)- = 470:05 mlz.
pyridinyl j amino)carbonyl] amino
} -3-[4-
{methyloxy)phenyl]propanoic acid
(3S)-3-{[({1-[(2-chlorophenyl)methyl]-4-20 Calculated (M-H)'= 530.13 mlz;
hydroxy-2-oxo-1;2-dihydro-3- Found (M-H)' = 530.05 m/2.
pyridinyl}amino)carbonyl]amino}-3-[3,4,5-
tris(methyloxy)phenyl]gropanoic
acid
(3S)-3-{[({I-[{2-chlorophenyl)methyl]-4-15 Calculated (M-H)' = 468.13 mlz;
hydroxy 2-oxo-1,2-dihydro-3- Found M-H '
( ) 468.08 mlz.
pyridinyl } amino)carbonyl]amino
} -3-(3, 5-
dimethyiphenyl)pmpanoic acid
(3S)-3-{[({l-[(2-chlorophenyI)methyl]-4-j(3-15 Calculated (M-1~-= 534.15 m/z;
methyl-5-isoxazolyl)amino]-2-oxo-1,2-dihydro-3-Found (M-H)' = 534.01 m/z.
pyridinyl} amino)carbonyI]aminoj
-3-(4-
methylphenyl)propanoic acid ._.
(3 S)-3- {[( { 1-[(2-chlorophenyl)methyl]-4-20 Calculated (M-H)- = 454.17
rrt/2;
hydroxy-2-oxo-1,2-dihydro-3- Found (M-H)' = 454:04 m/z.
pyridinyl} amino}carbonyl]amino
}-3-(3-
methylphenyl)propanoic acid
CA 02366800 2002-O1-07
-136-
(3S)-3-{[({1-[(2-chloropherryl)methyl]-4-5 Calculated (M-H)' = 470.11
m/2;
hydroxy 2-oxo 1,z-dihydro-3- :Found (M-H)- = 470.03 rn/z.
P3'ndinYl}amino)carborryI~amino'~-3-[3-
(methyloxy)phenyl]propanoic acid
(3S)-3-[3,5-bis(methyloxy)phenyl]-3-{[(3 Calculated (M-H)'500,12
f l-((2- m/z;
chlorophenyl)rc~ethyl]-4-l~ydroxyFound (M-H)' = 500.07 rn/z.
2-oxo-1,2-
dihydro-3-
pyridinyl}amino)carbonyl]amino}propanoic
acid
(3S}-3-{[({1-[(2-chlorophenyt)methyl]-4-8 Calculated (M-H)'= 504.13
m/z;
hydroxy-2-oxo-1,2-dihydro-3- Found (hs-F~'= 504:06 m/z.
quinolinyl}amino}carbonylJamino}-3-(4-
methylphenyl)propaaoic acid
(3S)-3..{[({1-[(2-chlorophenyl)methylJ.4-20 C.'alculated (M-H)'=
508:04 zn/z;
hydroxy-2-oxo-1;2-d~ydr~-3- , Found (M-H)' = 508:09 mlz.
pyridiayl}amino)carbonylJaniino}-3-[3-
(trifluoromethyl)phenyl]prapanoic
acid
(3S)-3-{[({1-[(2-chlorophenyl)methylJ-4-2 Calculated (M-1~'=595,21m/z;
[({ethyl[(ethylamino)carbonyl]amino}carbonyl)Found (M-H)' = 594:97 mlz.
aminoj-2-oxo-1,2-dihydro-3-
pyridinyl}amino)carbonyljamino}-3-(4-
~nethylphenyl)propanoic acid
(3S)-3-{[({4-(1-azetanyl)-1-[(2-5 Calculated (M-H)'=493.16m/z;
chloroghenyl)metlzylJ-2-oxo-1,2-dihydro-3-Found (M-H)' = 493.05 mlz.
pyridinyl}amino)carbonyl]amino}-3-(4-
methylphenyl)propanoic acid
(3S)-3-{[({1-((2-chloropherzyl)methyl]-4-30 Calculated (M-H)'= 458.09
m/z;
hydroxy 2-oxo-1,2-dihydro-3- Found (M-H)' = 458.~3 mlz,
pyridinyl}amino)carbonyl)amino}-3-(4.
fiuorogheayl)propanoic acid
(3S)-3-{[({1-[(2-chlorophenyl)methyl]-4-40 Calculated (M-H)' = 458.09
mlz;
hydroxy=2-oxo-1,2-dihydro-3- Found
(M-I~' = 458.06 mlz.
pyridinyl}amiaokarbonyl]amino}-3-(3-
fluorophenyl)proPanaic acid
(3S)-3-[({[1-[(2-chlorophenyl)methyl]-4-({2-((2-2 Calculated (M-H)'; 600;21
m/z;
{[2-(methyloxy)ethyl]oxy}ethyl)oxy]ethyl}oxy)-Found (M-I~' = 600.10 m/z.
2-oxo-1,2-dihydro-3-
pyridinylJamino} carbonyl)amino]-3-(4-
methylphenyl)propanoic acid
CA 02366800 2002-O1-07
-137-
(3S)-3-{~[({1-[(2-chlorophenyl)methyl]-4- 25 ~~alculated (M-H)'= 508.09 m/z;
hydroxy-Z-oxo-1,2-dihydro-3- ~ Found (M-H)' -- 508.02 mlz.
pyridirryl}amino)carbonylJamina}-3-[4-
(trifluoromethyl)phenylJpropa:wic acid
(3S)-3-{(({1-[(2-fluomphenyl~nethyl]-4- 30 Calculated (M-H)' = 438.15 m/z;
hydroxy-2-oxo-I,2.dahydto-3- ' Foursd (M-F~' = 438.07 mlz.
pyridinyl}amino)carbonyl]amino}-3-(4-
mashylphenyl)propanoic acid
(3S)-3-{(({1-[(2-chloro-6-fluorophenyl)methyl]- 10 Calculated (M-H)' ~ 472.11
m/z;
4-hydroxy-2-oxo-I,2-d~ydro-3- Found (M-1"i)' = 472:06 m/z.
pyridinyl}amino)carhonyl]amino}-3-(4-
methylphenyl)propanoic acid
(3S)-3-{[({1-[(2-chloruphenyl)methyl]-4- 400 Calculated (M-H)' =496.16 mlz;
hydroxy-2-oxo-I,2-dihyam-3- Found (M-H)- = 496. I I m/z.
pyridinyl}amino)carbonyl]amino} -3-(4-( 1,1-
dimethylethyl)phenyl]pmganoia acid
(3S)-3-{[( { 1-[(2-chlorophenyl)methylJ-5-methyl- 70 Calculated (M-H)' =
452.14 m/z;
2-oxo-1,2-ds~ydro-3- Found (M-H)' -= 451:99 mlz.
pyridinyl} aminokarhonyl]amino} -3-(4-
methylphenyl)propanoic acid
3-(4-chlomphenyl)-3-{[( f 1-[(2- _ 30 Calculated (M-Fi)' = 474.06 m/2;
chloropheriyl)methyl]-4-hydroxy-2-oxo-1,2- Found (M-H)'-- 474.07 m/z.
dihydro-3-
pyridinyl}amino)carbonyl]amino}propanoic acid
CA 02366800 2002-O1-07
-138-
(3S)-3-[({[2-methyl-6-oxo-1-(phenylmethyl)-4- 25 Calculated (M+H)+= 498.22
m/z;
(Z-pyridinyl)-1,6-dihydro-5- Found (M+H)+ = 498.10 m/z.
pytimidinyl]amino} carbonyl)amino]-3-(4-
methylphenyl}propanoic acid
3-(3-chlorophenyl)-3-{[({1-[(2- 30 Calculated (M-I~)-=474.b6 m/z;
chlorophenyl)methyl]-4:hydroxy-2-vxo-1,2- Found (M-H)' = 474.03 m/z,
dihydro-3-
pyridinyl}amino)carbonyl]amino}propaaoic acid
3-{[( ( 1-[(2-chiorophenyl)methyl]-4-hyrlroxy-2- 40 Calculated (M-H)' = 508.02
nz/z;
oxo-1,2-dihydto-3- Found (M-H)' = 507.97 m/z.
pyridinyl}amino}carbonyl]amino}-3-(3,4-
dichlorophenyl)propaaoic acid
?able 4
Name ~ ICso Mass Spectral Data
(3S)-3-{1,3-benzodioxol-5-yl)-3-[({[2-oxo-1- 0.015 Calculated (M-H)' = 452.18
m/z;
(phenyimethyl)-3. F ound (M-H)- = 452, I 0 m/z.
azepanyl]amino}carbonyl)amino]propanoic acid
(3S)-3-(1,3-benzodioxol-5-yl)-3-{[({1-~(3- 0.04 Calculated (M-H)-~ 477,18 m/z;
cyanophenyl)methyl]-2-oxo-3- Found (M-H)' = 477.14 rn/z.
azepanyl } amino)carbonyl]amino } propanoic
acid
(3S)-3-(4-rn~thylphenyl)-3-[({[2-oxo=I-(2- 0.6 Calculated (M-H)' -- 410.11
m/z;
thiophenylmethyl)-1,2-dihydro-3- Found (M-H)-= 410.00 m/z.
pyridinyl)amino j~ carbonyl}amino]propanoic
acid
(3S)-3-(1,3-benzodioxol-5-yl)-3-[({[2-oxo-1- 0.5 Calculated (M-H)'=434.13 m/z;
(phenyimethyl)-i,2-dihydro-3- F'ouad (M-H)' = 434.05 m/z.
pyridinyl]amino } carbonyl)amino]propanoic
acid
(3S)-3-(1,3-benzodioxol-5-yl)-3-{[({1-[(4- I Calculated (M-H)' = 44$.14 m/z;
methylphenyl)methyl]-2-oxo-1,2-dihydto-3- Found (M-H)' = 448:02 m/z.
pyridinyl } amino)carbonyl]amino} propanoic
acid
CA 02366800 2002-O1-07
-I39-
(3S)-3-(1,3-ben2odioxol-S-yl)-3-({[(1-{[4- 3 calculated (M-H)-= 464.I4 mlz;
(methyloxy)phenyl]methyl}-2-oxo-1,2-dihydro- Found (M-H)- = 464.03 m/z.
3 pyridinyl)amino]carbonyl}amino~ropanoic
acid
(3S)-3-(1,3-benzodioxol-5-yl)-3-{[({1-[(3- 1.5 Calculated (M-H)-=448.15m1z;
methylphenyl)methyl]-2-oxo-I,2-dihydzo-3- Found (M-H)- = 448.04 mlz.
pyridinyl} amino)carbonyl]amino} propanoic
acid
(3S)-3-[3,5 bis(metlrylaxy)phenyl]-3-j({jZ-oxo- 0.7 Calculated (M-H)' =456.12
m/z;
1-(2-thiophenylmethyl)-1,2-dihydm-3- Found (M-H)' =456.00 m/z.
pytidinyl]amino} carbonyl)amino]prapanoic
acid
(3S)-3-[4~(methyloxy~henyl]-3-[({[2-oxo-I-(2- 0.8 Calculated (M-H)'=426.11m/z;
thiophenylmethyl)-1,2-dihydro-3- Found (M-H)- = 426.00 m/z.
pyridinyl]amigo} carbonyl)amino]propanoic
acid
(3S)-3-[({[2-oxo-1-(2-thiophenylmethyl)-1,2- 2.5 Calculated (M-H}-=464.09
rn/z;
dihydro-3-pyridinyl]amino}carbonyl)amino]-3- Found (M-H)- = 463:99 m/z.
[3-(trifluoromethyl)phenyl]propanoic acid
(3S)-3-(I,3-benzodioxol-5-yi)-3-[({[3- 50 Calculated (M-H)' = 419.12 m/z;
(phenyloxyrphenyt]amino} carbonyl)amino] Found (M-H)' = 418.97 m/z.
propanoic acid
(3S)-3-(1,3-benzodioxol-5-yl)-3-{[({3-[(2- S Calculated (M-H)'=438.11 mlz;
thiophenylmethyl)amino]phenyyamino)carbony Found (M-H)-= 438.00 mlz.
1] amino}propanoic acid
(3S)-3-(1,3-benzodioxol-5-yl)-3-{[({I-[(3- 0.8 Calculated (M-H)' = 468.09 m/2;
chlorophenyl)methyl]-2-oxo-1,2-dihydro-3- Faund (M-H)- = 468.01 m/z.
pyridinyl}amino)carbonyl]amino}propanoic
acid
(3S)-3-(1,3-benzodioxol-5-yl)-3-({[(2-oxo-1- 0.8 Calculated (M-H)' = 502.12
m/z;
{[3-(tritluoromethyl~henyl]methyl}-1,2- Found (M-H)' --- 502.03 mlz.
dihydro-3-
pyridinyl)amino]carbonyl} amino)propanoic
acid
(3S)-3-(4-fluorophenyl)-3-[({[2-oxo-1-(2- 1.6 Calculated (M-H)' = 414.09 m/z;
thiophenylmethyl)-1,2-dihydro-3- Faund (M-H)' = 414.01 mlz.
pyridinyl]amino} carbonyl)amino]propanoic
acid
CA 02366800 2002-O1-07
-140-
(3S)-3-(I,3-benzodioxol-5=ylr3-{[{{I-[(4- 3 Calculated (M-H)'=46$.09m1z;
chloropheuyI~nethyl]-2-oxo-1,2-dihydro-3- lFound (M-F~- = 467.99 mlz.
pyridinyl}amino)carbhnyl]amino}propanoic
acid
(3S)-3-(1,3 benzodioxol-5-yl)~3-({[(1-{(2- 0.5 Calculat~d (M-H)'=464.14 mlz;
(mechyloxy)pheiryl]me~yl}-2-oxo-1,2-dihydro- Found (M-H)-= 464.04 mlz.
3 pyridinyl)amino]carbonyl}amiino)propanoic
acid
(3S)-3-[3-(methyloxy~henyl]-3-[({[2-oxo-1-(2- 1.4 Calculated (M-H)'=426.11
m/z;
thiopheuylmethyl)-1,2-d~yd=o-3- Found (M-H)' = 426.02 m/z.
pyridinyl]amino} carbonyl)amiao]propanoic
acid
(3S)-3-[({[Z-oxo-i-(2-thiophenylmethyI)-1,2- 1 Calculated (M-H)' = 396.10 m/z;
dihydro-3-pycidinyl]amino}carbonyl)aminoJ-3- Found (M-H)' = 396.01 m/z.
pheriylpropanoic acid
(3S)-3-[({(2~oxo-1-(2,-shiophenylmathyl)-1,2- 0.3 Calculated (M-H)"=486.13
mlz;
dihydto-3~yridinyl)ammino}carbonyl)amino]-3- Found (M-H)- = 485.98 m/z.
(3,4,5-tris(methyloxy)pheaylJgropanoic acid
(3S)-3-(1,3-benzodioxol-5-yl~-3-{[({I-[(2- 0.3 Calculated (M-H)'=468.08 mlz;
chlorophenyi)methyl)-2-oxo-1,2-dihydro-3- Found (M-H)'= 468.03 m/z.
pyridinyl } amino)carbonyl ]amino} propanoic
acid
(3S}-3-(1,3-benzodioxol-5-yl)-3-([({I-[(4- 2 Calculated (M-H)'=452.12 mlz;
fluarophenyi)methyl]-2-oxo-1,2-dihydro-3- Found (M-H)- = 452.00 inlz.
pyridinyi} amino)carbonylJarnino} propanoic
acid
3-(1,3-benzodioxoi-5-yI)-2,2-difluoro-3-[( {[2- >100 Calculated (M-H)" =
476;07 mlz;
oxo-1-(2-thiophenyhnethyl)-1,2-dihydro-3- Found (M-H)- ~ 476:00 tnlz.
pyridir~yl] amino } carbonyl)amino)propanoic
acid
(3S)-3-(1,3-benzodioxol-5-yl)-3-{[({2-oxo-1- 14 Calculated (M-H)' = 478,16
m/a;
[3-(phenyloxy)propyl]-I,2-dihydro-3- Found (M-H)- = 478:09 m/z. __
pyridinylJ amino)carbonylJamino}propanoic
acid
(3S)-3-(1,3-benzodioxol-5-yl)-3- f [({1-[(3,5- 5 Calculated (M-I-17~ -- 502.05
m/z;
dichiorophenyl)methyl]-2-oxo-1,2-dihydro-3- Found (M-H)' = 501.94 mlz.
pyridinyl} amino)carbonyl]amiao)propanoic
acid
CA 02366800 2002-O1-07
-i41-
(3S)-3-(1,3-benzodioxol-5=yl)-3-[({[1-6 Calculated (M-H)'= 426.16
mlz;
(cyclopentylmethyl)-2-oxo-,1,2-dihydro-3-Fnuad (M-H)' = 426.09 m/z.
PYndinYil~ino} carbanyl)amino]propanoic
acid
(3S)-3-(1,3-benzvdioxol-5-y1)-3-{[({2-oxo-115 Calculated (M-H)' = 454.09
m/z;
[2-(2-thiophenyl~thylj-1,2-dihydro-3-Found (M-H)' = 453.99 mlz.
pyridinyl} amino)carbonyl]amino}pmpanoic
acid
(3S)-3-{[({1-[(2-chlorophenyl)mathyl]-2-oxo-O,I Ca?culated(M+H)+=440.14m/z;
1,2-dihydro-3- Found (M+H)~=440.09 m/z.
pyridinyl}aznino)carbonyl]amino}-3-(4-
methylphenyl)propanoic acid
(3S)-3-(2,3-dihydro-1-benzofuran-S-y1)-3-[({[2-O. I4 Calculated (M-H)' =
438.11 m/z;
oxo-1-(2-thiophenylmethyl}-1,2-dihydm-3-Feund (M-i-I)' = 437.99 rn/z.
pyridinyl]amino} carbonyl)amino]pmpanoic
acid
(3S)-3-(3-fluorophenyl)-3-[({[2-oxo-1-(2-3 CaIcuIated(M-H)-=414.09rn/z;
thiophenylmethyl)-1,2-dihydro-3-Found (M-H)' = 413.99 m/z.
pyridirryl]amino} carbonyl)aminoJpropanoic
acid
(3S)-3-[({(2-oxo-1-(2-thiophenylmethyl)-1,2- 1.5 Calculated (M-H)' = 464.09
m/z;
dihydro-3-pyridinyl]amino}carbonyl)amino]-3- Fnund (M-H)' = 463.99 m/z.
[4-(trifluoromethyl)phenyl]propanoic acid
(3S)-3-(I,3-benzodioxol-5-yl)-3-[({[6-oxo-1- 0.5 Calculated (M-H)' = 434.13
m/z;
(phenylmethyl)-1;6-dihydro-3- Found (M-H)' = 434:02 mlz.
pyridinylJamino} carbonyl)amino]propanoic
acid
(3S)-3-[4-fluoro-3-(trifluoromethyI}phenyll-3- 0.35 Calculated (M-H)'= 482.08
mlz;
[({[2-oxo-1-(2-thiophenylmethyl)-i,2-dihydro- Found (M-H)' = 481.97 m/z. _
3-pyridinyl]amino } carbonyl)amino]prcrpanoic
acid
(3S)-3-[4-(l,x-dimathylethyl)phenyi]-3-[({[2- 2 Calculated (M-H)' = 45x.16
m/z;
oxo-i-{2-thiophenylmethyt)-1,2-dihydro-3- Found (M-H)' = 452.02 mlz.
pyridinyl]amino} carbonyl)smino]propanoic
acid
CA 02366800 2002-O1-07
-142-
(3S)-3-(1,3-benzodioxol-S-ylr3-[({butyl[2,5-70 Calculated {M-Vii)--494.19
mlz;
d3oxo-1-(phanylmethyl)tetrahydro-IH-pyrrol-3-Found (M-H)- = 494.12 m/z.
yl]amino}carbonyl)amino]proganoic
acid
(3S)-3-{[({1-[(2-chlorophenyl)methyl]-2-oxo-0.04 Calculated (M+H)+= 516.16
m/z;
1,2dihydro-3- Found (Ivt+1~* = St6.02 m/z.
pyt;dinyl) amino)carbonyl]amigo}-3-[3,4,5-
tris(methyloxy)phenyl]prop$noic
acid
(3S)-3-{[({1-[(2,6-dichlorophenyl)methyt]-2-0.2 Calculated (M+H)+= 474.10
mlz;
oxo-1,2-dihydro-3- Found (M+H)'' = 474.04 rn/z.
pyridinyl} amino)carboayl]amino}-3-(4-
methylphenyl)gropanoic acid
{3S) 3-{[({1-[(2-chlorophenyl)methyl]-2-oxo-0.2 Calculated (M+H)~'= 512.10
m/z;
1,Z-dihydro-3-
Found (M+H)+ = S 12.04 m/z.
pyridinyl) amino)carbonyl]amino}-3-[4-fluoro-
3-(trifluoromethyl)phenyl]propanoic
acid
(3S)-3-t[((I-[(2-fluorophenyl)methyl]-2-oxo-0.1 Calculated (M-H)w-422.15
mlz;
1,2-dihydro-3- Found (M-H)- = 422.01 m12.
pyridinyl } amino)carbonyl]amino
} -3-(4-
methylphenyl)propanoic acid
CA 02366800 2002-O1-07
-143-
(3S)-3-(4-methylphenyl)-3-{[({1-[(2-0.1 t;alculated (M-H)'=418.18
m/z;
methyIphereyl)methyl]-2-oao-1,2-dihydro-3-Found (M-H}' = 418:02 m/z.
pyridinyl) amino)carbonyl]amino}
propanoic
acid
(3S)-3-{[({1-[(2-bromaphenyl)methylj-2-oxo-0.05 Calculated (M+H)+=484.09m1z;
1,2-dihydro-3- JFvund (M+H)+ = 484.03 m/z.
pyridinyl}amino)carboayl]amino}-3-(4-
methylphenyl)propanoic acid
(3S)-3-{[({1-[(2,4-dichlorophenyl)~nethyi]-2-0.4 C:alculated(M+H)t=474.1Om/z;
oxo-1,2-dihyc}ro-3- Found (M~H)* = 474.05 mlz.
pyridinyl} amino)carbonyljamino}-3-(4-
methylphenyl)propanoic acid
(3S)-3-{[({1-[(2-chlorophenyl)methyl]-2-oxo-0.04 Calculated (M-H)'=466.11.m1z;
1,2-dihydro-3- ' Found (M-H)- = 4b6.00
m/z.
pyridinyl} amino)carbonyl)amino}-3-(2,3-
dihydro-1-benzofuran-S-yt)propanoic
acid
(3R)-3-(I,3-benzodioxoi-5-yI)-3-{[({1-[(2-2 Calculated (M-H)-=468.09
m/z;
chlorophenylrnethylj-2-oxo-1,2-dihydro-3-Found (M-H)' = 467.97 m/z.
pyridinyl}amino)carbonyl]amino}prapanoic
acid
(3S)-3-(4-methylphenyl)-3-({[(2-oxo-1-{[2- 1~ Calculated (M+I~)*=474.10 mlz;
(trifluoromethyl)phenyl]methyl}-1,2-dihydro-3- Found (M+H)t ~ 474.09 mlz.
pyridinyl)aminojcarbonyl } amino)propaaoic
acid
(3S)-3-[[({1-j(2,5-dichlorophenyl)rnethyl]-2- 0.15 Calculated (M+H)+=474.10
m/z;
oxo-i,2-dihydm-3- Found (M+H)* =474.04 m/z,
Pl'nd~Yi)~ino)carbonyl]amine}-3-(4-
methylphenyl)propanoic acid
(2R)-2-{[({1-[(2-chlomphenyl)methylj-2-oxo- 50 Calculated (M-H)- ~ 424.10
rn/z;
1,2-dihydro-3- Faund (M-H)- = 423.99 m/z.
pyridinyl}amino)carbonyl]amino}-3-
phenylptopanoic acid ._.
(2it)-2-{[({1-[(2-chlorophenyl)rnethylj-Z-oxo- 80 Calculated (M-H)' = 410,08
mlz;
1,2-dihydro-3- Faund (M-H)' = 409.95 mlz.
pyridinyl}amino)carbonyljamino}-2-
phenylethanoic acid
CA 02366800 2002-O1-07
-I44-
(3S)-3-{[({1-[(2-chlorophenyl)methyl~-2-oxo-0.1 Calculated {M-H)' $
452.14 mlz;
1,2-dihydro-3- Found (M-I~' = 451.96 mlz.
pyridiztyl}aminokarbonyl]amino}-3-(3,5-
dimethylphs~nyl)propanoic acid
(3S)-3-{[({I-[{2-chloroghenyl)methyl]-2-oxo-0.1 Calculated (M-H)' ~
424.10 m/z;
1,2-dihydro-3- Found (M-H)' = 424.07 m/z.
Pyn~Yi} ~iaoxs~rbonyl]amino}-3-
phenylpropanoic acid
{3S)-3-{[{{1-[(2-chlorophanyl?methyl-2-oxo-0.1 Calculated (M-H)'=454.11
m/z;
I,2.dihydrv-3- Found (M-H)- = 454.01 m/z:
pyridinyl}amino)carbonyl~amino}-3-[4-
(methytoxy)pharyl]prapanoic
acid
(3S}-3-{[({1-[{2-chlorophenyl)methyI]-2-oxo-O.I Calculated (M-H)-=440.10
mlz;
1,2-dihydro-3- Found (M-H)' = 440.00 mlz.
pyridfayl}aminokarbonyl]amino}-3-(4-
hydroxyphenyl)propanoic acid
(3S)-3-({[(1-{[3-(methyloxy)phenyIJmethyl}-2-0.2 Calculated (M-H)-=434.17
mlz;
oxo-1,2-dihydro-3- Found (M-H)' = 434,01 m/z.
pytfdiayl)anrinojcarbonyl} atnina~3-(4-
methylphenyl~ropanoic acid
(3S)-3-{[({1-[(Z-bromophenyl)methyl]-2-oxo-0,08 Calcutated(M-H)'=558.09m/z;
1,2-dihydm-3- Found (M-H)' = 557.87 mlx.
pyridinyl}aminokarbpnyl]amino}-3-[3;4,5-
tris(methyloxy)phenyl)propanoic
acid
(3S)-3-{[({1-[(2-chlorophenyl)methyl]-z-oxo-0.09 Calculated (M+H)'~=454:15m/z;
1,2-dihydro-3- F~oured (M+H)+ ~ 454.07
m/z.
PYnd~Yl}aminoxarbonyl]amino}-3-(3,4-
dimethylphenyl)piopanoic acid
(3S)-3-[({[5-chloro-2-hydroxy-3-8 Calculated (M-H)'=437.12
m/z;
(pheaylmathyl~henyljamitto} Found (M-H)- = 437.06 m/z.
catboayl)amino)-
3-(4-methylphenyl)propanoic
acid
(3S)-3-(4-methylphenyl~3-[( 10 Calculated (M-H}- = 387.
f [3: I7 mlz;
(phenylmeihyl)phenyl)amine}carbonyl)amino].Found (M.H)- ~ 387:00 rnlz.
propanoic acid
(3S)-3-{[((Z-[(2-chlorophenyl)rr:ethyl)-2-oxo- 0.04 Calculated (M-H)'=468.13
m/z;
1,2-dihydro-3- Found (M-H)- ~ 468,01 rnJ2.
pyridinyl} amino)carbonyl]amino}-3-[3-methyl-
4-(methyioxy}phenyl)propanoic acid
CA 02366800 2002-O1-07
-145-
(3S)-3-{[({1-[(2-ehlorophenyl)metlryl~-2-oxo-0.07 Calculated (M.H)'=454.11
mlz;
1,2-dihydro-3- Found (M-H)' = 454.00 mlz:
pyridinyl}amiao)carboaylJamino}-3-{4-
hydroxy-3-iuethylpheayl~rOpanoic
acid
(3S)-3-{[({1[(2,3-dichlorophenyl)methyl]-2-0.35 ~alcuIated (M-H)'=472.08 rnlz;
oxo-I,2-d~ydro-3- Found (M-H)' = 471.94 mlz.
pyridanyl}amino)carbonyl]amino}-3-(4-
methylphenyl)propanoic acid
(3S)-3-[({[1-{[l,l'-biphenyl]-2-ylmethyl).2-2.5 Calculated (M-H)'=480.19 m/z;
oxo-1,2-dihydco-3- Found (M-H)' = 480.05 m/z.
pyzidinyl]amino}carbonyl)amino]-3-(4-
mechyipheayl)propanoie acid
(3S)-3-{[({1-[(2-chIorophenylhnethyl]-2-oxo-0.2 Calculated (M-H)' = 438.12
m/z;
I,Z-dihydro-3- Found (M-H)' ~ 438.00 mlz.
pyredinyl}amino)carbonyl]amino}-3-(3-
rnethylphenyl)propanoic acid
(3S)-3-{[{{ 1-[(2-chlorophenyl)methyl]-2-oxo:3 Calculated (M-H)' = 438.1 Z
m/z;
1,2-dihydm-3- Found (M-H)' = 437.99 mlz.
pyridinyl}amino)carbo~ryl]amino)-3-(2-
meth~Iphenylypropanoic acid
(3S)-3-{[( { 1-[(2-chlorophenyl)matltyl]-2-oxo-0.3 Calcula~~l {M-H)' = 464.I3
m/z;
1;2-dihydro-3- Found (M-H)' = 464.03 m/z.
pyridinyl} amino)carbonyl]amino
}-3-(2,3-
dihydro-1H-inden:S-yl)propanoic
acid
(3S)-3-{[({ 1-[(2-cyanophenyt)methylJ-2-oxo-0. I Calculated (M+H}+ ~ 43I ,18
m/z;
1,2-dihydro-3- ~ Found (M+~* -- 431.09 m/z.
pyridinyl } amino)carbonyl]amino
} -3-(4-
methylphe~syl)propanoic acid
(3S)-3-[2,b-bis(me~thyloxy~henyl]3-{[((I-[(2-6 Calculated{M-H)'=484.14m/z; ,__
chlorophenyl)methyl]-2-oxo-1,2-dihydro-3-Found (M-H)-=483.96 mlz.
P3'n~nYl}amino)carbor~yl]amino}Prapanoic
acid
CA 02366800 2002-O1-07
-146-
(3S)-3-{[({1-[(3-hydsoxyghenyl)methyl]-2-oxo0.2 Calculated (M+I~+=420.18
mlz;
1,2-dihydm-3- Found (M+H)+ ~ 422:05 m/z.
pyr;diayl}aminoxarbonyl]amino}-3-(4-
methylpbenyl)prop~a~wic acid _
(3S)-3-[({[2-methyl-b-oxo-1-(phenylmethyl)-0.1 Calculated (M-H)'=419.17
m12;
1,6-dihydro-5 kound (M-I~' = 419.03 m/2.
pyrimidinyl]amino} carbonyl)amino]-3-(4_
methylphenyl)propatioic acid
(3S)-3-{[{{1-[(2-chlorophenyl)methyl]-4-oxo-0.1 Calculated (M-H)' g
~ 438,12 m/z;
1,4-dihydro-3- Found (M-H)' = 438.10 m/z.
pyridinyl}amino)catbonyl)amino}-3-(4-
methylphenyl)propanoic acid.
(3S)-3-(4-methylphenyl)-3-{[({1-[(2-1 Calculated (M+1~+=451.17
m/z;
nitrophenyl)methyl]:2-oxo-1,2-dihydm-3-Found (M+I~* ~ 451.07 mlz.
pyridinyl} amino)cazbonyllamino}propanoic
acid
(3S)3-(4-methylphenyl)-3- {[( 1 Calculated (M+H)+ = 45
{ 1-[(4- 1 .17 m/z;
nitrophenyl)methyl)-2-oxo-1,2-dihydro-3-xound (M+H)'' ---451.09
m/z.
pyridinyI} amino)carbonyl~amino}propanoic
acid
(3S3-3-{[({1-[(2-chiorophenyl)methylJ-2-axo-3 Calculated (M-H)' = 456.10 m/z;
1,2-dihydtor.3- Faund (M-H)- = 456.04 m/z.
pyridinyl}aminoxarbonyl]amino}-3-(2,6-
ds'hydmxyphertyl)propanoic acid
(3S)-3-{[~ f 1-[(Z,6-difluorophenyl}methyl]-2-0,3 Calculated (M-H)' = 440.14
m/z;
oxo-1,2-dihydro-3- Found (M-H)' = 440.00 mlz.
pyridinyl} amino)carbonyl]amino
} -3-(4-
methylphenyi)propanoic acid
(3S)-3-{[({l-[(2,4-difluorophenyl)methyl]-2-1.3 Calculated (M-H)'=440.14 m/z;
oxo-1,2-dihydto-3- Found (M-H)' ~ 439.96 tnlz.
pyridinyl}amino)carbonyl]an~inoj-3-(4-
methylphenyl~propanoic acid
(3S)-3-{[({1-[(2,5-difluorophenylrnethylj-2-0.8 Calculated (M-H)-=440.14 m/z;
oxo-I,2-dihydro-3- Found (M-H)'= 439.96 mlz.
pyridinyl} aminokarbonyl]amino}-3-(4-
methylphenyl)propanoic acid .
CA 02366800 2002-O1-07
-14?-
(3S)-3-{[({1-[(2-chlorophet~yl)methy7]-2-0.09 Calculated (M-H)'=453.13 m/z;
methyl-5-oxo-1,6-dihydro-5- Found (M-I~' -- 453.00 mlz.
Pyrirnidinyl}amino)carbonyljamino}-3-(4-
methylpherryl)prapanoic acid
(3S)-3-{[({ 1-[(2-chloro-6- 0.1 Calculated (M-H)' = 456.11 mlz;
fluomphenyt)rnethylj 2-oxa Found (M-F~~ = 455.94 m/z.
1,2-dihydro-3-
pyridinyl} amino)earbonylJamino}-3-(4-
methylphenyl)prapanoic acid
(3S)-3-{[({1-[(2-bromo-5- 0.5 Calculated (M-I~' = 500.06 m/z;
fluorophenyl)methyl]-2-oxo-1,2-dihydro-3-Found (Ivi-I~' = 499.91 mlz.
pyridinyl}amino}carbonyl]amino}-3-(4-
methylphenyl)propar<oic acid
{3S)-3-{[({1-[(2-chloro-4- 0.35 Calculated (M-H)'=456.11 mlz;
fluorophenyl)methylj-2-oxo-1,2-dihydro-3-Found (M-H)' = 455.93 mlz.
pyridinyl}amino)carbonyljamino}-3-(4-
mathylphenyl)propanoic acid
(3S)-3-{[({1-[{2-bromophenyl)methyl]-2-oxo-0.2 Calculated (M-H)' = 512.08 m/z;
I,2-dihydm-3- Found (M-H)' = 511.96 m/z.
P5'n~nYl}~~o)carbonyljamino}-3-[3-methyl-
4-(metl2yloxy)phenyl]propanoic
acid
(3S)-3-{[({1-[(3,5-dimethyl-4-3 Calculated (M-H)'= 423.17 m/2;
'
isoxazolyl)methyl]-2-oxo-1,2-dihydro-3-Found (M-H)' = 423.02 m/z.
pyridinyl}amino)carbonyljamino}-3~(4-
methylpherzyl)propanoic acid
(353-(4-ruethylphe,~iryi)-3-{[({2-oxo-1-[(2,4,6-2.5 Calculated (M-H)' = 44b.21
m/z;
trimethylphenyt)methyl]-1,2-dihydro-3-Found (M-H~' = 446.08 m/z.
pyridinyl} aminokazbonyl]amino}
propanoic
acid
(3S)-3-(4-methylphenyl)-3-{[({1-((2-methyl-1 Calculated (M-H)' = 425,13 m/z;
1,3-thiazol-4-yI)methylJ-Z-oxo-1,2-dihydro-3-Found (M-H)-= 424.99 m/z.
pyridinyl}tunino)carbonyl]amir:o}propanoic
acid
(3S)-3-({((1-{[4-(1,1- 6 Calculated (M-Id)~ =460.22 mla;
,__
dimethylethyl)phenyl]rr~ethyl}-2-oxo-I,2-Found (M-1~I)-= 460.07 mlz.
dihydro-3-pyzidinyl)amino]carbonyl
} amino)-3-
(4-methylphenyl)propanoic acid
CA 02366800 2002-O1-07
-148-
(3S)-3-[(([1-(1,3-benxoxazol-2-ylmethyl)-2->10 Calculated (M-H')-=445.15 m/z;
oxo-1,2-dihydro-3- Found (M-H)' = 445.01 m/z.
pytidinyl]amino}carbonyl)amino]-3-{4-
.
methylphenyl)propanoic acid
(3S)-3-({[(I-{2-[(2-hydtoxyphenyl)amino]-2->10 Calculated {M-H)-= 463.16 m/z;
oxoethyl}-x-oxo-I,2-dihydro-3- Found (M-H)' = 463.06 m/z.
pyridinyl}amino]carbonyl}amino)-3-(4-
methyIphenyl)propanoia acid
(3S)-3-{[({I-((2-chloro-6-nitrophenyl)methyl]-4 Calculated (M-I~'=483.11 m/z;
2-oxo-1,2-dihydro-3- Found (M-H)- = 483.01 m/z.
pyridinyl}amino)carbonyl]amino}-3-(4-
mathylphenyl)prvpanoic acid
(3S)-3-{[({1-[(5-chloro-Z- 2.5 Calculated (M-H)'= 456.11 m/z;
fluorophenyl)methylJ-2-oxo-1,2-dihydro-3-Found (M-H)' = 456.00 m/z.
pyridinyl}amino)carbonyl]amino}-3-(4-
methylphenyl)propanvic acid
(3S)-3-{[({1-[(2-amino-6- 2 Calculated (M-H)-=453.13 mlz;
chiorophenyl)methyl]-2-oxo-1,2-dihydro-3-Found {M-H)' = 453.02 mlz.
pyridinyl}amino}carbonyl]arninoj-3-(4-
methylphenyl)propanoic acid
(3S)-3-({[(1-f [2-fluoro-4- 3 Calculated (M-H)'= 490.14 mlz;
(trifluoromethyl)phenyl)methyl}-2-oxo-1,2-Found (M-H)' = 489.99 mlz.
dihydro-3-pyridinyl)amino]carbonyl}amino)-3-
(4-metttylphenyl)propanoic acid
(3S)-3-{[({1-[(5-chloro-2-thiophenyl)methy!]-2-1.3 Calculated (M-H)'=444.08
m/z;
oxo-1,2-dihydro-3- Fvund (M-H)' = 443.97 m/z.
pyridinyl} amino)carbonyl]amino}-a-(4-
methylphenyi~ropanoic acid
(3S)-3-{[({1-[(2-bromo-5-nitrophenylhnethyl]-2 Calculated {M-H)'= 527,06 m/z;
2-oxo-1,2-dihydro-3- Found (M-H}-= 526.95 m/z.
pyridiny~} amino)carbonyl]aminoj-3-(4-
methylphenyl)propanoic acid
3-(4-chlorophenyl)-3-{[({1-[(2-0.03 Calculated (M-H)' = x74.06
m/z; ._.
chlorophenyl)methyl]-~l-hydroxy-2-~oxo-1,2-Found (M-H)-=474.07 m/z.
dihydro-3-
pyridinyl } amino)carbonyl)
amino } propanoic
acid
CA 02366800 2002-O1-07
-149-
(3S)-3-[({[2-methyl-6-oxo-1-(phenylmethyl)-4-0.02 Calculated (M+H)+-
498.22 m/x;
(2-pyridinyl)-1,6-dihydro-5- Found (M+H)'' = 498.10 m/z.
pyrimidinyl]amino}carbonyt)amino]-3-(4-
rnethylphenyl~ropanoic acid
(3S)-3-{[({1-[(5-amino-2- 0.08 Calculated (M-H)'=497.08
m/z;
bromaphanyl)methylj-2-oxo-1,2-dihydro-3-Found (M-H)' = 497.02 m/z.
pyridinyl}amino)carbonyl]amino}-3-(4-
methylpheayl)propanoic acid
(3S)-3-{[({1-[(2,5-dimethylphenyljmethyl]-2-0.15 Calculated (M-H)'=432.19
m/z;
oxo-1,2-dihydro-3- Found (M-H)' = 432.04 m/z.
pyridinyl}amino)carbonyl]amino}..3-(4-
tnethylphenyl~ropaaoic acid
3-(3-chlorophenyl)-3-{[(tl-[(2-0.03 Calculated (M-H)-~
4~4.Ob m/z;
chlorophenyl)rtiethyl]-4-hydroxy-2-oxo-1,2-Found (M-H)' = 474,03 mlz,
dihydro-3-pyridinyl}amino)
carbonylaamino}propanoic acid
3- f [( f 1-[(2-chlorophenyl)methyl]-4-hydroxy-2-0.04 Calculated (M-H)-=
508.02 mlz;
oxo-1,2-dihydro-3- Found (M-H)-= 507.97 m/z.
pyridinyl}amino)carbonyl)amino}-3-(3,4-
dichlorophenyl)propanoic acid
(3S)-3-({[(1-{[5-(acetylamino)-2-0.2 Calculated (M-H)'= 539.09
mlz;
bromophenyl]methyl}-2-oxo-1,2-dihydro-3-Found (M-H)' = 539.02 m/z.
pyridinyl)amino]carbonyl} amino)-3-(4-
mathylphenyl)propanoic acid
(3S)-3-[({[i-({2-bromo-5- 0.25 Calculated (M-H)-=
575.06 m/z;
[{methylsulfonyt)amino]phenyi}methyl)-2-oxo-Found (M-H)' = 575.41 m/z.
1,2-dihydro-3-
pyridinyl]amino}carbonyl)amino]-3-(4-
methylphenyl)propanoic acid
3-(4-ohlorophenyl)-3-f [({I-[{2-0.4 Caiculated {M-H)' =
458.07 m/z;
chlorophenyl)methyl]-2-oxo-I,2-dihydro-3-Found (M-H)'=457.96 m/z.
pyridinyl } amino)carbonyl]amino}propanoic
acid
3-(3-chiorophenyi)-3-{[{{1-[(2-1 Calculated (M-H)' = 458:07
m/z;
chlorophenyl)methyl)-2-oxo-1,2-dihydro-3-Found (M-H)' = 457.93 m/z,
pyridinyl}amino)cazbonyl)amino};propanoic
acid
CA 02366800 2002-O1-07
-150-
3-{[( { 1-[(2-chlorophenyl~nethylJ-2-oxo-1,2-1 Calculated (M-H)' = 492.03
m/z;
dihydro-3-pyridinyl}amino)carbonyl]amino}-3-Found (M-H)'$ 49J .85 mlz.
{3,4-dichlomphenyl~ropanoic
acid
(3S)-3-{[({1-[(2-bromo-4- 1 Calculated (M-H)' = 516.03
m/z;
'
chlorophenyl)methyl]-2-oxo-1,2-dihydm-3-= 515.91 m/z.
Found (M-H)
pytidinyl}amino)carbonyi]amino}-3-(4-
methylphenyl)propanoic acid
{3S)-3-{[({1-[(4-chlorophenyl)methyl]-2-oxo-2 Calculated (M-H)'=438.12rnIz;
1,2-dihydxo-3- Found (M-H)' = 437.88 mlz.
pyridinyl}amino)carbonyl]amino}-3-(4-
methylphenyl)ppropaaoic said
(3S)-3-{[({1-[(2-chiorophenyl)methyl]-4-0.035 Caaculated (M-I~'
= 498.14 mlz;
lrydroxy-2oxo-1,2-dihydro-3- Found (M-Fl)- = 498.05
m/z.
pyridirryl}amino)carboayljamino}-3-[2,3-
dimethyl-4-{methyloxy)phenyljpropanoic
acid
(3S)-3-{[({1-[(2-chloropbenyl)methyl]-4-0.015 Calculated (M-H)'=
524.08 m/z;
hydmxy 2-oxo-1,2-dihydro-3- Found (M-I~' = 524.03 mlz.
pyridiayl} amino)carbonylJamino
}-3- {4-
j(trifluaromethyl)oxy]phenyl}propanoic
acid
(3R)-3-[({[1-[(2-chlorophenyl)methylj-4-(1.4-0.1 Calculated (M-H)' =
489.19 m/z;
oxazinan-4-yl)-2-oxo-1;2-dihydro-3-Found (M-H)' = 489.13 m/z.
pyridinyl]amino } carbonyl)aminoj-5-
methylhexanoic acid
(3 S)-3-[( {[4-hydroxy-6-methyl-2-oxo-1-0.03 5 Calculated (M-H)'
= 434.17 rn/z;
(phenylmethyl)-1,2-dihydro-3- Found (M-H)' = 434.08 mlz.
pyridinyljamino} carbonyl)aminoj-3-(4-
rnethylpheayl)propanoic acid
(3S)-3-{[({1-[(2-chlorophenyl)methylj-2-oxo-4-0.030 Calculated (M-H)~=
559.14 m/z;
[(propylsulfonyl)amino].1,2-dihydro-3-Found (M-H)' - 559.04 mlz.
pyridiny3}amino)carboyyl]amino}-3-(4-
methylphenyl~p:opanoic acid
CA 02366800 2002-O1-07
-151-
(3S)-3-~[( f 1-[(2-chloropheayl)methylj-4- 0.025 Calculated (M-H)' = 4.68.13
mlz;
hydroxy-2-oxo-1,2-dihydro-3- Found (Ad-I~' ° 468;06 m/z.
pyridinyl) a~ino)carbonylJarnino}-3-(4-
ethylphenyl)pmpanoic acid
(3S)-3-{[({I-[(2-chlotaphenyl)rnathyl]-4- 0.02 Calculated{M-H)'=484.13m/z;
hydsoxy 2-oxo-1,2-dihyclro-3- Found (M-H)' = 484.06 m/z.
pyridinyl} amiaoxarbonyljsmino}-3-[4-
(ethyloxy)phenyl]propaaoic acid
(3S)-3-[({[4-hydmxy-2-oxo-1-(phenylmethyI)- 0.030 Calculated (M-~' ~ 420.16
mlz;
I,2-dihydro-3- Found (M-H)- = 420.08 mJz.
pyridinyl)amino} carbonyl)aminoj-3-(4-
rnethylphenyl)propanoic acid
Table S
Name ICso {1M) Mass Specttal Data
(3S)-3-[( f [I-(3-tent butyl-2-methoxybenzyl)-2-oxo- 2.5 Calculated (M-H)' =
1,2-dihydropyridin-3-yl]amino}carbonyl)amino]-3-490.23 m/z; Found (Nt-H)-
(4-methylphenyI)propanoic acid = 490.11 mlz.
(353-[( f [1-(4-fluvroban2yl)-2-oxo-1,2-2 Calculated (M-H)' _
dihydropyridin-3-yl]amino}carbonyl)amino]-3-(4-422.12 mlz; Found (M-I~'
methyIphenyl)propanvic acid = 422.00 m/z.
(3S)-3-[(f[1-(2-chlorvbenzyl)-4-hydroxy-2-oxv-0,025 Calculated (M-I~-=
I,2-dihydropysidin-3 yljamino}carbonyl)amino]-3-526.OS mlz; Found (M-H)'
[4.fluoro-3-(trifluoromethyl)phenyljpropanoic= 526.01 m/z.
acid
(3S)-3-[( f [1-(2,5-dimethylbenzyl)-4-hydroxy-2-0,02 Calculated (M-H)'
oxo-1,2-dihydropyridin-3- 448.19 m/z; Found (M-H)-
yl]amino}carbonyl)amino]-3-(4- = 448.00 mJa.
znethylphenyl)propanoic acid
(3S)-3-[( f [4-hydroxy-1-(2-methylbenzyl)-2-oxo- 0.02 Calculated (M-H)' _
1,2-dihydrapyridin-3 yl]amino}carbanyl)aminoj-3- 434.17 m/z; Found (M-I~-
(4-methylphenyl)pmpanoic acid = 434.05 m/z. - ---
(3S)-3-[( f f 1-(2-hydroxybenzyl)-2-oxo-1,2- 0.2 Calculated (M-H)-=
dihydropyridin-3-yljamino}carbonyl)amino]-3-(4- 420.16 mlz; Found (M-H)'
methylphenyl)propanoic acid = 420.09 m/z,
CA 02366800 2002-O1-07
-152-
(3S)-3-[({[1-{3-chlorobenzylr2-oxo-1,2-0,5 Calculated (M-H)-=
dihydropyridin-3-yl]amino}catbonyl)aminoJ-3-(4- 438.12 m/z; Found (M-H)-
methylphenyl)propanoic acid = 43 8:O I m/z.
(3S)-3-[({[1-(2chioro-6methoxybeszyl)-2-oxo-0.,1 Calculated (M-H)'
1,2-dihydzvpyridin-3-y1]amino}carbonyl)amino]-3- 468,13 mlz; Found (M-H)'
(4-methylphenyl)propanoic acid = 468.08 mlz.
(3S)-3-j({[1-(2-chlorobenzyl)-4-hydroxy0.035 Calculated (M-H)-=-
2-oxo
1,2-dihydropyridin-3-yl]amino}carbonyl)aminoJ-3- 498.14 m/z; Found (M-H)'
(4-methoxy-3,5-dirnethylphenyl)propanoic -- 497,94 mlz.
acid
4-{[3-[({((1S)-2-carboxy-1-(4- 0.004 Calculated (M-H)-=
methylphenyl)ethyl)amino}carbonyl)amino]-1-(2- 573.15 mlz; Found (M-H)-
chloroben:ryl)-2-oxo-1,2-dihydrapyridin-4- = 572.92 m/z.
yl]amino}benzoic acid
(3S)-3-{[{{1-(2-chlorobenzyl)-4-[(2,2-0.01 Calculated (IvI-H)'=
dimethylpropanoyl)amino]-2-oxo-1,2- 537.19 m/z; Found (M-H)-
dihydropyridin-3-yI}aminokarbonylJamino}-3-(4-, = 536.88 m/z,
methylphfsnyl)propanoic acid
(3S)-3-[([[I-(2-chloro-5-methoxybenzyl)-2-oxo-0.09 Calculated (M-H)' _
I,2-dihydropyridin-3-yl]amino}carbonyl)aminoJ-3- 468.13 m/z; Found (M-H)-
(4-methylphenyl)propartoic acid = 467.99 m/z:
(3R)-3-(({[1-(2-chlorobenzyl)-4-hydroxy-Z-oxo-0,19 Calculated(MH)'= .
1,2-dihydxapyridin-3- 378.09 m/z; Found (M-H)'
yl]amino}carbonyl)amino)butanoic _ 378.0I m/z.
acid .
(3S)-3-[(([4-{[(tent-butylamino)carbonyl]amino}-1-0,01 Calculated (M-H)'=
(2-chlorobenzyl)-2-oxo-1,2-dihydropyridin-3- 552.20 mlz; Found (M-H)-
ylJamino}carbonyl)amino]-3-(4- = 551.89 m/z:
methylphenyl}propanoic acid
(3S)-3-[({[I-(2-chloro-5-hydroxybenzyl)-2-oxo-0,25 Calculated (M-H)' _
1,2-dihydxopytidin-3-yl]amino}carbonyl)amino)-3- 454.12 m/z; Found {M-H)'
(4-methylphenyl)propaaoic acid = 454.03 m/z.
(3S)-3-[({[I-(2-cyanobenzyl)-4hydroxy-2-oxo-1,2-0.009 Calculated (M-H)'=
dihydropyridin-3-yl]amino}carbonyl)amino]-3-(4- 445,15 m/z; Found (M-H)'
methylphrnyI)propanoic acid = 445.01 mlz:
(3S)-3-[({[1-(2,4-dichlombenzyl)-4-hydroxy-2-oxo-0.06 Calculated (M-H)'= _
-.
1,2..dihydropyridin-3yl)amino}caibonyl)aminoJ-3- 488.08 m/z; Found (M-H)'
(4-methylphenyl)propaaoic acid = 487:96 mlz.
CA 02366800 2002-O1-07
-153-
(3S)-3-(({[4 hydroxy 1-(2-methoxybenzylr2-oxo-0.08 Calculated (M-H)' _
1,2-d~ydropyrid~-3-ytjanaioj~carbonyl)amino]-3- 450.1? m/z; Found (M-H)'
(4-methylpheny>)propanoic acid -- 450.02 m/z.
(3S)-3-(({[1-(2-chlorobenzyl)-4-hydmxy0.08 Calculated (M-H)'
2-oxo-
1,2-dihydropyddin-3 yljamino}carbonyl)amino]-3- 498.14 m/z; Found (M-H)'
(4-methoxy-2,5-dimethylphenyl)Ixopanoic = 497.95 mlz.
acid
(3S)-3-[(([1-(2-cbloro-6-hydmxybenzylr2-oxo-0:1 Calculated (M-H}'s
1,2-dihydmpyridin-3 yljamino}cacbonyl}aminoj-3- 454.12 rnlz; Found (M-F~-
(4 methylphenyl)proganoic acid = 454.05 m/z.
(3S)-3-[({[i-(3-tent-butyl-2-hydroxybenayl)-2-oxo-4 Calculated (M-I~'=
I,2-dihydropyridin-3-yljamino}carbonyl)amino]-3- 476.02 m/z; Found (M-H)'
(4-methylphenyl)propanoic acid _ 476:00 mlz.
(3R)-3-[( ([1-(2-cltlorobenzyl)-4-lrydmxy0.3 Calculated (M-H)' _
2-oxo-
1,2-dihydropyridin-3-yl]amino}carbonyl)aminoj-3- 454.17 m/z; Found (M-H}'
(4-methylphenyl)propanoic acid = 454,05 m/z.
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-0.015Calculated (M-H)' _
~
1,2~dihydropyridin-3-yl]amino}carbonyl)amino]-3- 468.13 m/z; Found (M-H)-
(3-ethylphenyl)propanoic acid = 467.9S m/z.
(3S}-3-[({[1-(Z-chlotobenzyl}-4-hydroxy-2-oxo-0.01 Calculated (M-H)'=
1,2-dihydropyridin-3-yl]amino}carbonyl)amino]-3- 498.10 mlz; Found (M-H)'
(2,3-dihydro-1,4 benzfldioxin-6-yl~ropanoic = 497.85 m/z.
acid
(3S)-3-[({(1-(2,5-difluorobenzyl)-4-hydroxy-2-oxo-0.015Calculated (M-H)'
1,2-dihydropyridin-3-yt]amino)carbonyl)amino]-3- 456:14 mlz; Found (M-H)-
(4-methylphenyl~ropanoic acid = 455.96 m/z.
(3S)-3-[(((1-(2-chlorobenzyl)-4-hydroxy-2-oxo-30 Calculated (M-H)'=
1,2-dihydtogyridin-3-yljamino)carbonyl)aminoj-4- 468.13 mlz; Found (M-H)
(4-methylphenyl)butanoic acid = 467.87 mlz.
(3S)-3-{[({1-(2-chloro-5-(methylthio)benzyl]-4-0.015Calculated (M-H)'=
hydroxy 2-oxo-1,2-dt'hydmpyridin-3- 500.10 mlz; Found (M-H)'
yl}amino)carbanyljamino)-3-(4- = 499.92 m/z.
methylphenyl)propanoic acid
(3S)-3-[(([1-(2-chlorobenzyl)-4-hydroxy-2-oxo-0.005Calculated (M-H)'
1,2-dihydropyridin-3-yl]amino}carbonyl)aminoj-3- 514.10 m/z;,Found (M-H)-
(7-methoxy-I,3-benzodioxol-5 yl)propanoic~ = 513.86 mlz. _.
acid
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-0,002Calculated (M-H)'=
1,2-dihytimpyridin-3-yl]amino}carbonyl}aminoj-3- 514.13 m/z; Found (M-H)'
(3-ethox~ 4-methoxyphenyl)ptnpanoic~acid = 513.90 mlz.
CA 02366800 2002-O1-07
-I54-
(38)-3-[({[l-(2-chlombanzyt)-4 hydroxy-2-oxo- 4.015 Calculated (M-H)-=
1,2-dihydrogyridin:3-yl]amino}carbonyl)amino)-3- 488:10 mlz; Found (M-
Idj'
(3-fluoro-4-methoxyphenyl~ropanoic = 487.92 mlz.
acid
(3S)-3-[({[1-(2-chlombenzyl)-4-hydroxy-2-oxo-O.Q02 Calculated (M-I~'=
1,2-dihydropyridin-3 ylJamino}carbonyl)ami~w]-3- 500.12 m!2; Found (M-H)'
(3,4-dimethoxyphenyl)prvpanoic = 500,0I mlz.
acid
(3S)-3-[({[I-(4-tluombenzyl)-4-hydroxy-2-oxo-1,2-0.022 Calculated (M-T~' _
dihydrapyridin-3-ylj~nino}carbonyl)amino]-3-(4- 438.18 m/2; Found (M-H)'
methylphenyl~ropaaoic acid . = 438.00 mlz:
(353-[{ fi[ I-(2-methoxybenzylr2-oxo-1,2-0.25 Calculated (M-H)' _
dihydropyridin-3-yl]arrtino}carbonyl)amino]-3-(4- 434.17 m/2; Found (M-
I~'
methylphenyl~rapanoic acid = 433.95 m/z:
(3Sr3-[({[1-(z-chlorobenzyl)-4-hydroxy-2-oxo-0.05 Calculated (M-H)'=
1,2-dihydropyridiu3-yl]amino}carbonyl)amino]-3- 468.13 m/z; Found (M-H)'
(Z,5-dim~thylphenyl)propaaoic = 467.94 mlz.
acid
(3S)-3-[({[t-(2-chloro-5-methoxybenzyI)-4-O.O1Z Calculated (M-H)'=
hydroxy-2-oxo-1,2-dihydtopyridin-3- 484.13 m/z; Found (M-H)-
yI]amino}carbonyl)amino]-3-(4- =484.03 rn/z.
methyIphenyl~ropanoic acid
(3S)-3-{[({1-[3,5-bis(trifluoromethyI)benzyl]-4-0.3 Calculated (M-H)' _
hydmxy-2-oxo-1,2-dzhydropyridin-3- 556.13 m/z; Found (M-H)'
yl}amino)carbonyl]amino}-3-(4- = 555.95 m/z:
methylphenyl)propanoic acid
(3S)-3-[({[1-(4-tent-butylbenxyl)-4-hydroxy-2-oxo-0.03 Calculated (M-H)' a
1,2-dihydropyridin-3-yl]amino}casbonyl)amino]-3- 476.22 m/z; Found (M-H)'
(4-methylphenyl)ptopanoic acid = 476.05 mlz.
(3S)-3-(({[1-(3-chlorobenzyl)-4-hydroxy-2-oxo-0.015 Calculated {M-H)-=
1,2-dihydropyridin-3-yl]amino) 454,12 mlz; Found (M-H)'
carbonyl)amino]:3-
(4-methylphenyl)propanoic acid = 453.99 rn/z.
(3S)-3-[({[1-(4-chlombenzylj-4-hydroxy0.007 Calculated (M-t~-=
2-oxo-
1,2-dihydropyridin-3-yl]amino}carbonyl)amino]-3- 454.12 mlz; Found (M-I~-
(4-methylphenyl)propanoic acid = 454.00
. mlz.
(3S)-3-{[({4-hydroxy-2-oxo-1-[3- 0.017 Calculated (M-H)'=
(trifluoromethyl)benzyl~-I,2-dihydmpyridin-3- 488.14 m/z; Found (M-I~'
._.
yl}amino)carbo~iyl)amino}-3-(4- ~ =.487.99 m/z.
methylphenyI)propanoic acid
CA 02366800 2002-O1-07
-155-
(3S)-3-[( f [1-(2-bromobenzyl)-4-hydroxy-2-oxo- 0.(71 5 Calculated (M-H)- =
1,2-d~ydxopyridin-3 yI]amiao}carbonyl)aminoJ-3- 498.07 m/z; Found (M-~'
(4-methylphenyl)propanoic acid = 497.97 mlz.
(3S)-3-[(~[1-(3,4-dichlorobenzyl)-4-hydroxy-2-oxo- 0.045 Calculated (M-H)' g
1,2-dihydxopyridin-3 ylJamino}carbonyl)aminoj-3- 488.08 m/z; Found (M-H)'
(4-methylpheuyl)p~anoic acid = 487.96 m/z.
(3S)-3-[( f [4-hydroxy-1-(4-methylbet~zyl)-2-oxo- 0.025 Calculated (M-H)' =
I,2-dihydropyridin-3 yljamino}carbonyl)atnino]-3- 434.17 mlz; Found (M-I~-
(4-methylphenyl)pmpaaoic acid = 434.0S m/z.
(3S)-3-(({[1-(2-chloro-6-methoxybanzyl)-4- 0.003 Calculated (M-I~' _
hydroxy 2-oxo-1,2-dihydropyridin-3- 484.13 mlz; Found (M-H)'
yl]amino}carbonyl)amino]-3-(4- = 484.02 rnlz.
methylphenyl)propanoic acid
(3S)-3-{[({4-hydroxy-2-oxo-I-[4- 0.02 Calculated (M-1~'=
(trifluommethyl)benzylj-I,2-dihydrapyridin-3-488.14 m/z; Found (M-H)'
yl}amino)carborryljamino}-3-(4- = 487.99 m/z.
methylph~nyl)ptopanoic acid
(3S)-3-[(t[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-0.02 Calculated (M-H)-=
1,2dihydropyridin-3-yljamino}carbonyl)amino]-3-524.08 tnlz; Found (M-H)'
[3-(trifluorornethoxy)phenyl]propanvic= 523.91 rn/z.
acid
(3S)-3-[(ij4-hydroxy-1-(3-methylbenzyl)-2-oxo-0.055 Calculated (M-H)'=
1,2-dihydzopyridin-3-yl]amino}carbonyt)aminoj-3-434.17 mlz; Pound (M-H)'
(4-methylphenyl)propanoic acid -- 433.99 mlz.
(3S)-3-[({[4-hydroxy-2-oxo-1-(pyridin-2-ylmethylr 0.045 Calculated (M-H)' =
1,2-dihydropyridin-3-yljamino}carbonyl)amino]-3- 421.15 mlz; Found (M-H)'
(4-methylphenyl)propanoic acid = 421.06 mlz.
(3S)-3-[(([I-(2-chlorobenzyl)-4-hydroxy-5-methyl= 0.005 Calculated (M-H)-=
2-oxo-1,2-dihydropyridin-3- 468.13 m/z; Found (M-H)'
yl]amino}carbanyl)amiao]-3-(4- = 467.99 mlz.
methylphenyl)propanoic acid
(3S)-3-[( {[1-(2,4-difluorobenzyl)-4-hydroxy-2-oxo- U.03 Calculated (M-I~' =
1,2-dihydropyt;din-3-yljamino}carbonyl)anninoJ-3- 456.14 mlz; Found (M-~'
(4-methylphenyl~propanoic acid = 456.01 mlz.
(3S)-3-[({[1-(2,6-difluorobenzyl)-4-hydroxy-2-oxo- 0.008 Calculated (M-H)'=
1,2-dihydropyridin-3-yljantrino}carbonyl)aminoj-3- 456.14 m/z; Found (M-H)-
(4-methylphenyl)propanoic acid = 456.01 mlz.
CA 02366800 2002-O1-07
-156-
(3S)-3-{[({4~hydroxy-2-oxo-I-[3- 0:045 Calculated (M-H)-=
(trifluoromethoxy)benzyl]-1,2-dihydropyridin-3- 504.14 m/z; Found (M-I~'
yl}amino~arbonyt])-3-{4- ~ 503.98 ailz.
methylphenyl)propanoic acid
(3S)-3-{[{ {4-hydroxy 2-oxo-1-[4-0.025 Calculated (M-H)'
(trifluoromethoxy)banzylJ-1,2-dihydropyddin-3- 504.14 mlz; Found
(M-H)'
yl}amino)carbonyl)amino}-3-(4- = 503.98 mlz.
methylphenyl)propanoic acid
(3Sj-3-[({[1-(2-chloro-6-methoxybenzyl)-4-O.OO1SCalculated (M-H)'
hydroxy-2-oxo-1,2-dihydtopyridin-3-, 530.13 mlz; Found
(M-I~)-
yl]amino}carbonyl)amino]-3-(3,5- = 529.91 m/z.
~
dimethoxyphenyl)propanoic acid
3-[({(1(2-chlorobenzylr4-hydxoxy-2-oxo-1,2-0.05 Calculated (M-H)-=
dihydropyridin-3-yI]amino}carbonyt)amino]-3-(2- 430.08 m/x; Found
(M-H)-
furyl)propanoic acid ~ 429.94 m/z.
(3S)-3-{[({4-hydroxy-2-oxoI-[2- 0.02 Calculated (M-H)'a
~
{trifluoromethyl)benzyl]-I,2-dihydropyridin-3- 488.14 mlz; Found
(M-H)'
yl}amino)carbonyl]amino}-3-(4- = 487.96 mlz.
methylphenyl~ropanaic acid
(3R)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-O.IS Calculated (M-H)'
=
I,2-dihydropyridin-3-yl]amino}carbonyl)amino]-4- 468.13 m/z; Found
(MH)-
(4-rnethyiphenyl)butanoic acid = 467.99 m/z.
(3S)-3-[({(1-(2-chlorobenzyl)-4-hydroxy-2-oxo-0.0008Calculated (M-H)'
=
1,2-dihydropyridin-3 yl]amino} 528.15 m/z; Found
carbonyl)amino]-3- {M-H)-
(3,4-diethoxyphenyl)gropanoic = 527.96 mlz.
acid
(3S)-3-[({[1-(2-chlorobeazyl)-4-hydroxy-2-oxo-0.003 Calculated (M-H)-=
1,2-dihydropyridin-3-yl]amino}carbonyl)amino]-3- 484.12 rrt/z; Found
(M-H)'
{3-ethoxyphenyl)propanoic acid . =483.94 mlz.
.
(3S)-3-[(t[4-hydroxy-1-(3-methoxybenzyl)-2-oxo-0.04 Calculated(NI-H)'=
1,2-dihydropyridin-3-yl]amino}carbonyl)axtiino]-3- 454.17m/z;:Found(M-~'
(4-methyIphenyl)propanoic acid =450.00 mla.
(3S)-3-[({[1-(2,3-dichloroben2yl)-4-hydroxy-2-oxo-0.13 Calculated (M-H)'=
1,2-dihydmpyridin-3-yI]amino}carbonyl)aminoj-3- 488.08 mla; Found
(Ivi-H)'
(4-methylphenyl)propaaoic acid = 487.92 rnlz.
(3S)-3-[({[1-benzyi-2-oxo-S-(trifluoromethyl)-1,2- 1.5 Calculated (M-H)' =
dihydropyridin-3-yl]amino}carbonyl)amino]-3-(4- 472.15 mlz; Found (M-H)'
methylphenyl)propanoic acid = 471.89 mlz.
CA 02366800 2002-O1-07
-I57-
(3S)-3-[((C1-(3;5-dime~tbylbenzyl)-4-hydsoxy0.06 Calculated {M-H)' s
2-
oxo-1,2-dihydropyridin-3- 448,19 m/z; Found (M-H)'
yt~amino}carbonyl)amino]-3-{4- ' = 448.02 m/z,
methylphet~yi)pmpanoic acid
(3S)-3-[({[1-(2-chlom-6-methoxybenzyl)-4-0,04 Calculated (M-F~'
hydroxy-2-oxo-1,2-d~ydropyridin-3- 554.49 aa/z; Found (M-H)'
yl]amino}carbonyl)amino]-3-(4- = 553.98 mlz.
(tritluoromethoxy]phenyl]propanoic
acid
(3S)-3-[({[I-{2-chlorobenzyl)-4-hydroxy-2-oxo-0.003 Calculated (M-I~'
1,2-dihydrvpyridin-3-y1]amino}carbonyl)amino]-3- 484.13 m/z; Found (M-H)
(3-methoxy-4-methylphenyl)propanoic = 483,95 m/z.
acid
{3S)-3-(({(1-(2-chlorobenzyl)-4-hydroxy-2-oxo-0,003 Calculated (M-H)'=
1,2-dihydtopyridin-3-yl]atuino}carbonyl~nino]-3- 514.14 m/z; Found (M-I-
iT
(3,5-dimethoxy.4-xnethyiphenyl)propanoic = 513.95 m/z:
acidv
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-S-0,04 Calculated {M-H)' =
pentyl-1,2-dihydropyridin-3- 524.20 mlz; Found (M-F~-
yl]amino}aarboiryl)amino]-?-(4- = 523.98 mlz.
methylphenyl)propanoic acid
(3S)-3-[{{(1-(2-chlorobenzyl)-4-hydroxy-2-oxo-0.005 Calculated (M+H)~ 468.13
1,2-dihyd=opyridin-3-yl]amino}carbonyl)amino]-3- m/z; Found (M+I~+=
(3,4-dimelhylphenyi)propanoic 467.99 m/z.
acid
(3S)-3-[({(1-{2,4-dichlorobenzyl)-4-hydroxy-S-0.02 Calculated (M-H)'=
methyl-2-oxo-1,2-dihydropyridin-3- 502.09 m/z; Found (M-H)"
yl]amino}carbonyl)amirto]-3-(4- = 501,89 mlz:
medrylphenyl)propanoic acid
[2-( f [1-(2-chlorobenzyl)-4-hydroxy>10 Calculated (M-H)'=
2-oxo-1,2-
dihydropyridin-3-yl]amino}carbonyl)-1-(4- 455.11 m/z; Found (M-1~'
methylphenyl)hydrazino]acetic = 454.97 m/z.
acid
(3S)-3-[({[1-(2-chlorobenzylr5-ethyl-4-hydroxy-2-0.01 Calculated (M-H)'=
oxo-I,2-dihydropyridin-3- 482.15 m/z; Found (M-H)-
yl]amino}carbonyl)amino]-3-(4- = 482.00 m/z.
methyiphenyl)propanoic acid
3-[{{[I-(2-chiorober~zyl)-4-hydroxy-2-oxo-1,2-0,05 Calculated (M-H)-=
dihydropyridin-3 yl]amino}carbonyi)aminoJ-3- 441.09 m/z; Found (M-H)-
pyridin-3-ylpropanoic acid = 441.00 mlz.
{3S}-3-[({[5-butyl=1-(2-chlorobex~zyl)-4-hydroxy-2-0.025 Calculated (M-H)-=
oxo-1,2-dihydropyridin-3- 510.18 m/z; Found (M-H)'
yl]amino}carbonyl)aminoJ-3-(4- = 509.98 m/z.
methylphenyl)propanoic acid
CA 02366800 2002-O1-07
-158-
(3S)~3-{[((1-[2-chloro-5-(trifluoromethyl)benzyl]- 0.01 Calculated (M-I-~-=
4-hydroxy 2-oxo-1,2.dihydropyridin-3- 522.10 m/z; Found (M H)'
yl}amino)carbonyl]anunv}-3-(4- = 521.97 mlz.
methylphehyl)propanoic acid
(3S)~3-[({[I~(2-chloro-6-methoxybenzyl)-4. 0.005 Calculated (M-H)' --
hydmxy-2-oxo-1,2-ds'hydropyridin-3- 484,13 m/z; Found (M-I~'
yl]amino}catbonyl)amino]-3-(3- =484.00 mlz.
methylphe~l)propaaoic acid
(3S)-3-[({[1-{2,6-dichlorobanz5rl)-4-hydroxy-2-oxo- 0.013 Calculated (M-H)-=
I,Z-dihydiopyridin-3-yl)amino3carbonyl)amino]-3- 488.08 m/z; Found (M-H)'
(4-methylphemrl)lnopanoic acid = 487.91 m/z.
(3S)-3-[(([1-{2-ehloro-S-fluombenzyl)-4-hydroxy 0,014 Calculated (M-H)' _
2-oxo-I,2~~dihydropyzidin-3- 472.1 I mlz; Found (M~I-1T
yl]amino}carbonyI)atnino]-3-(4- = 471.96 xnlz.
m~YIP~YI~oP~oie acid
(3S)-3-[(([1-(2-chloto-6-tnett~ylbenryl)-4-hydroxy- 0.01 Calculated (M-H)' _
5-methyl-2-oxo-1,2-dihydropyrtdin-3- 482.15 m/z; Found (M-H)-
yl]amino}carbonyl)amino]-3-(4- = 481.98 m/z:
methylphenyl)pmpanoic acid
(3 S)-3-[( { [ 1-(4-chlorobenzyl)-4-hydroxy-5-methyl- 0.02 Calculated (M-H)' 3
2-oxo-1,2-dihydmpyriditt-3- 468.13 mlz; Found (M-H)-
yl]amino) carbonyl)amino]-3-(4- = 467:94 m12:
methylphenyl)propanoic acid .
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-0.003 Calculated (M+H)'~
_
2,5,6,7-tetrahydro-1H-cycIopenta[b]pyridia-3-496.16 tn/x; Found
yl]amino}carbonyl)amino]-3-(4- (M+H)* = 495.99 mlz.
methylpher~yl)propaaoic acid
(3S)-3-{[({4-hydroxy 5-methyl-I-14-0.02 Calculated (M-H)'=
(methylsulfonyl)benzylJ-2-oxo-1,2-dihydropyridin-512.15 m/z; Found (M-H)'
3-yl}amino)carbonyl]amino}-3-(4- = 511.96 mlz.
methytphenyl~ropanoic acid
(3S)-3-[( {[4-hydroxy-1-(4-methoxybenzyl)-2-oxo-0.02 Calculated (M-H)-=
1,2-dihydropyridin-3-yl]amino}carbonyl)aminoJ.3-450.17 rn/z; Found (M-H)'
(4-methylphenyl)propanoic acid = 449:99 m/z.
(3S)-3-[({[1-{2-chlorobenzyl)-4-hydroxy-2-oxo-S- 0.02 Calculated (M-H)'= w
propyl-1,2-dihydrogyridirf-3- 496.16 mlz; Pound (M-H)'
yl]amino}carbonyl)amino]-3-(4- =495.94 m/z.
methylphenyl)propaaoic acid
CA 02366800 2002-O1-07
-159-
(3S)-3-({[(1-{4-[(ditnethylamino)sulfonyl]benzyl}- 0,035 Calculated (M-H)- _
4-hydroxy-2-oxo-1,2-dihydsopyridin-3- 527.16 mlz; Found (M-~'
yI)amino]carbonyljatninor3-(4- = 526.96 m/z.
methylphsayl)prapanoic acid
(3S)-3-(({[4 hydroxy-1-(mesitytmethyl)-2-oxo-1,2- 0.06 Calculated (M-F1T=
dihydropyridin-3-ylJaminojcarbonyl)aminoj-3-(4- 462.20 mlz; Found (M-H)-
rnethylphenyl}propanoic acid = 462.02 m/z.
(3S)-3-((([1=(2-chlorobenzylr4-hydroxy=2-oxo- 0.02 Calculated (M-H)' _
1,2,5,b,7,8-hexahydroquinolin-3- 508.16 m/z; Found (M-H)'
yljaminojcarbonyl)atninoJ-3-(4- = 507.96 mlz.
methylphenyi)propanoic acid
(35}-3-(({(1-(2-chlorobenzyl)-5-ethyl-4-hydroxy-6- 0.025 Calculated (M-H)'=
methyl-2=oxo-1,2-dihydmpyddin-3- 496,16 m/2; Found (M-I~'
yljaminojcarbonyl)amino]-3-(4- = 495.96 tn/z.
methylphenyl)propanoic acid
(3S)-3-[(t[1-(2-chlorobenzyl)-4-hydroxy 2-oxo- 0.4 Calculated (M-H)-=
1,2-dihydropyridin-3- ' 468,13 mlz; Found (M-H)'
ylJaminojcarbonyl)(methyl)aminoj-3-(4-=467.85 m/z.
methylphenyl)propanoic acid
(3S)-3-{j({4-hydmxy 1-[2-(methylthio)benzylJ-2-11.02 Calculated (M-H)-=
oxo-I,2-dihydropyridin-3- 468.14 mlz;'Found (M-H)'
yl}amino)carbonylJaminoj-3-(4- = 465.97 m/z.
methylphenyl)propanoic acid
(3S)-3-({((1-~(2-[(dimethylamino)sulfoayljbenzyl}-0.03 Calculated (M-H)'=
4-hydro~:y-2~xo-:1,2-dihydropyridin-3-527.16 m/z; Found (M-H)'
yl)amino]carbonyl}amino)-3-(4- = 526.97 m/z.
methylphenyl)propanoic acid
(3S)-3-[({[1-(2,6-dimethoxybenzyl)-4-hydroxy-2-0.01 Calculated (M-H)'
_
oxo-1,2-dihydropyridin-3- 480.18 m/z; Found (M-H)'
yljaminojcarbonpl)amino]-3-(4- =480.00 m/z.
.
methylphenyl)prapanoic acid
(3S)-3-{j({4-hydroxy 2-oxo-1-j2- 0.025 Calculated (M-H)' _
(trifluorotnethoxy)benzyl]-1,2-d~ydropyridin-3- 504.14 mlz; Found (M-H)'
yl}amino)carbonyl]aminoj-3-(4- = 503.96 mlz.
methylphenyl)propanoic acid ,_
(3R)-3-(({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo- 0.35 Calculated (M-H)-=
1,2-dihydropyridin-3~y1)aminojcarbonyl)amino]-4- 522.10 m/z; Found (M-H)-
(3-(trifluoromethyl)phenylJbutanoic acid = 521.95 m/z.
CA 02366800 2002-O1-07
-160-
(3S)-3-[({[1-(2-chlorobeazyl)-4-hydroxy-2-oxo- O.Q03 Calculated (M-H)'
I,2-d~ydropyridin-3-yl]amino)carboayI)aminaj-3- 498.14 tn/z; Found (M-H)'
(3.propoxypherryl~mpanoic acid ~ 497.97 mlz.
(35)~3-[( ([I-(2-chlorobgazyi)~4.hydroxy-2-oxo-5- 0:003 Calculated (M~H)+
psopyl-1,2-dihydropyridirt-3- 528.19 m/z; Found
yl3amino}carboayl)sminoj-3-(3- (M+I~'~ ~ 52802 mlz.
ethoxyphenyl)pmpsnoic acid
{3S)-3-[(([1-(2-chlorobenryl)-4-hydroxy:5,6-0.406 Calculated {M-~'
dimethyl-2-oxo-I,2-dihyd=opycidin-3- 482.15 m/z; Found (M-H)'
yljamiuo}ca~rbonyl)arni~)-3-(4- = 481.95 mlz.
methylphez~yl~rapa=wic acid
(3S)-3-[(([1-(2-chlorobenzyl)-4-hydroxy-2-oxo-5-O:OOS Calculated (M-I~'=
propyl..l,2-dihydropytidin-3- 570.20 mlz; Fouad (M-H)'
yl]amino}carbonyl)aminoj-3-(3,4- a 569.98 m/z.
diethoxyphenyl)prapanoic acid-
(3S)-3-(3 butoxypheayl)-3-[({[1-(2-chlorobenzyl)-0.005 Calculated (M+I~*
4-hydroxy2-oxo-1,2-dihydropyridin-3- 514.17 nn/z; Found
,
ytJamino}carbonyl)amirio]propanoic (M+H)~ = 514.00 m/z.
acid
(3S)-3-{[({1-[2-chloro5-(methylsulfonyl)benzylj-0.003 Calculated (M=H)'=
4-hydroxy~~2-oxo-1,2-dihydropyridin-3. 532.10 m/z; Found (M-I~'
yl}amino)carbonyljamino}-3-(4- = 531.94 rrt/a.
methylphenyl~ropanoic acid
(3R)-3-[( f [1-(2-chlorotsenzyI)-4-hydroxy.2-oxo-0.08 Calculated {M-H)" _
1,2-dihydropyridin-3-yljamino}carbonyl)amino]-4- 468.13 m/z; Round (M-I~'
(2-methylphenyl)butanoic acid ~ 468.03 mlz
(3S)-3-[( [[ I -(2-chlorobenzyl)-4-hydroxy-2-oxo-0.003 Calculated (M-H)"
1,2-dihydropy:idin-3-yl)amino}carbonyl)aminoJ-3- 514.14 m/z; Found (M-H)'
[3-(2-metl~roxyethoaty)phenyljpropanoic = 513,95 mlx.
acid
(3S)-3-(({[1-(4-chloro-2-methoxybenzyl)-4-0.025 Calculated (IVi-I~'=
hydroxy-x-oxo-I,2-dihydropyridin-3- 484.13 m/z; Found (M-H)'
yl]amino}carbonyl)aminoJ-3-(4- =483.93 mlz.
methylphenyl)propanoic acid
(3S)-3-[(~[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-0.003 Calculated (MH)'
1,2-dihydropyridin-3-yl]amino}carboayl)aminoj-3- 556.18 mlz; Found (M-H)'
~ w
(3,4-dipropoxypherryl~ropanoic = 555.94 m/z.
acid
CA 02366800 2002-O1-07
-161- .
(3S~3-[(([1-(2-chlorobenzyl)-4-hydrQxy 2-oxo- 0.12 Calculated (M-~' _
2,5,5,'7,8,9-hoxahyd=o-1H-cyclohapta[b)pyridin-3- 522.18 m/2; Found
(M-H)'
yI]amino}carboa~yl~niaoJ-3-(4- = 521.98 mlz.
methylphenyl)propanoic acid
(3S)-3-[({[I-(2-chlorobenzylr4-hydmxy-2-oxo-22 Calculated (M-H)-=
1;2-dil~ydropycidin-3-yl~amino}carbonyl)amino]- 530.15 m/z; Found
(M-H)-
4,4-diphenylbutanoic acid = 529.92 mlz.
{3S)-3-{[({1-[2-(difluoromethoxy)benzyl]-4-0.075 Calculated (M-H)'
_
hydrvxy-2-oxo-1,2-dihydtopyridin-3- 4$5.15 m/z; F.ound
(M-H)-
yl)amino)carbonyljamino}-3-(4- = 48b.00 mlz.
methylphenyl)~ropanoic acid
(3S)-3-([((4-hydmxy-5-methyl-2-oxo-1[(1R)-1-4 Calculated (M-H)'=
phenylethyl]-1;2-dihydropyridin3- 448.19 m/z; Found
(M-H)-
' yl}aminokarbonyljamitto}-3-(4- = 447.99 m/z:
methylpheryl)propanoic acid
(3S)-3-[(([1-(4-chlombenzylr4-hydroxy-2-oxo-5-0.03 Calculated (M-H)'=
propyl-1,2-dihydropyridin-3- , 496.15 m/z; Found
(M-H)'
yl]amino)uarbonyl)amiaoJ-3-(4- = 495.96 mlz.
methylpheryl)propaGnoic acid
(3S)-3-[({[I-(2-chlorobettzyl)-4-hydroxy0.05 Calculated (M-I~'=
2-oxo-
1,2-dihydropyridin-3-yljamino}carbonyl)amino]-3- 496.16 m/z; Found
(M-H)-
(3,4-diethylphenyl)propanoic acid = 495.98 m/z.
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-axo-0.05 Calculated (M-H)'--
1,2-dihydropyridin-3-ylJamino)carbonyl)amino]-3- 476,08 mlz; Found
(M-H)-
(3,5-difluorophenyl)propanoic acid = 475.93 mlz.
3-[( f [1-(2-chlorobenzyl)-4-hydroxy-2-oxo-1,2-0.02 Calculated (M-H)-=
dihydmpyridin-3-y1]amino}carbonyl)amino)-3-(2- 490.12 m/z; Found
(M-I~-
naphthyl)propanoic acid = 489.97 mlz:
3-j({[1-(Z-chlorober~zyl)-4-hydroxy-Z-oxo-1,2-0.025 Calculated (M+1~+~
dihydrogyridin-3-ylJaiaino}catbonyl)arninoJ-3-(5- 446.11 mlz; Found
methyl-2-furyl)propanoi-c acid (M+H)+ = 446.08
m/z.
(3S)-3-j({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-0.025 Calculated (M-H)'=
1,2-dihydxopyridin-3-yl]amino}carbonyl)arnino]-3- 584.21 mlz; Found
(M-H)'
(3,4-dibutoxyphenyl)propanoic acid = 583.98 m/z.
(3S)-3-{[({4-hydroxy 1-[2- 0.035 Calculated (M+H)+=
(methylsulfonyl)benzyl)-2-oxo-I,2-dihydropyridin- 500.15 mlz; Found
3-yi}amino)carbonylJamino}-3-(4- (M~-H)'' = 500.01
m/z.
methylphenyI~rapanoic acid
CA 02366800 2002-O1-07
-162-
3-[({[1-(2-chlorobenzyl)-4=hydroxy-2-oxo-I,2-0.2 Calculated (M-H)'
_
dihydropyridin-3 yi]amino} carbonyi)amino]-3-(490.12 m/z; Found (M-H)'
1-
aphthyl~roparloic acid = 489.91 m/x.
(353-[{{[1-(4-chlorobenzyl)-4-hydroxy0.03 Calculated (M-Iy
2-oxo-5- =
pmpyl-1,2-dihydropyridin-3- 526.17 mlz; Found (M-H}'
yljamino}carbonyl)amino]-3-(3- = 525.95 m/z.
ethoxyphenyl)prapaaoic acid
(3S)-3-j({[1-(4-chlorobettzyl)-4-hydroxy-2-oxo-5-0.015 Calculated (M-H)'=
propyl-1,2-dihydropyridin-3- 570.20 mlz; Found (M-H)-
yljamino)carboayl)amirio]-3-(3,4-= 569.97 tn/z.
diethoxyphenyl)propanoic acid
(3S)-3-[({jl..(2,6-dimethytbenzyl)~4-hydtoxy 2- 0.035 Calculated (M-H)'=
oxo-T,2-dihydropyridin-3- 448.19 m/z; Found (M-H)'
yl]axnino)cxrbonynaminoJ-3-(4- = 448.02 mlz.
methylphenyl}propanoic acid
(3S)-3-[3,5-bis(trifluoromethyl~henyl]-3-[({jl-(2~ 0.22 Calculated (M-H)' _
chlvrobenzyl)-4-hydroxy-2-oxo-1,2-dilnydropyridin- 576.08 mlz; Found (M-H)'
3~yl]amino}carbonyl)amino]propanoic acid = 575.91 m/z.
(3S)-3-[({[I-(2-chlorobetixyl)-4-hydroxy-2-oxo- 0,006 Calculated (M-H)'=
1,Z-dihydropyridin-3-yl]amino)carbonyl)amino]-3- 546.09 m/z; Found (M-H}'
[3-(difluoroxnethoxy~henyl]propanoic acid = 505.93 m/z.
(3R)-3-[({[1-(2-chlorober~zyl)-4-hydroxy-2-oxo- 0.225 Calculated (M-H)'=
I,2-dihydropyridin-3-yl]amino}carbonyl}amino]-4- 455.11 m/z; Found (M-H)'
pyridin-2-yIbutanoic acid = 455.09 m/z.
(3S}-3-[({[1-(2-chlvrobenzyl)-4-hydro~y-5~methyl- 0.0006 Calculated (M-H)'
2-oxo-1,2-dihydropyridin-3- 542.17 m/z; Found (M-I~'
yljannino]carbonyl)amino]-3-(3,4- = 542.06 rn/z.
diethoxyphenyl~ropaaoic acid
(3S}-3-[({[1-(2-chlorobenzyl).4-hydroxy-5-methyl- 0.002 Calculated (M-H)'=
2-oxo-1,2-dihydropyridin-3- 499.15 rnlz; Found (M-I~'
yl]amino] carbonyl}ainino]-3-(3- = 498.07 rn/z.
ethoxyphenyl)propaaoic acid
(3S)-3-[({[I-(2-chlorobenzyl)-4-hydroxy-5-methyl- O.Oe 0 Calculated (M+H)'' _
2-oxo-1,2-d~ydropyridin 3- 500.16 m/z; Found
yl]amino}carbonyl)amino]-3-(3-methoxy-4- (M+H)+m 500:02m12. --.
methylghenyl}propanoic acid
CA 02366800 2002-O1-07
-163..
3-[(~[1-(a-chlorobenzyl}-4 hyaroxy s-methyl-2- 0.03o Calculates (M-F~v
oxo-1,2-dihydropyridin-3- so4.13 m/z; Founa (M-H)'
ylJamino;carbonyl}ami~aoJ-3-(2-naphthyl)propanoic = 504.04 m/z.
acid
(3S)-3-[({[1-(2-chl4m-6-methylbanzyl)-4:hydroxy- 0.015 Calculated (M-H)'=
5,6-dimatltyl-2-oxo-1,2-dihydmpy~idin-3~ 526.17 tn/z; Fouad (M-F~'
yl)amino}carboayl)amino]-3-(3- = 525.95 zn~z.
ethoxyphanyl)propanoic acid
(3S)-3-[({[1-(2-chloro-6-rnathylbenzyl)-4-hydroxy- 0.0;X5 Calculated (M-H)'=
5,6-di~aethyl-2-oxo-t,2-dihydmpyridin-3-526.17 m/z; Found (M-H)-
yl)amino}carbanyl)amino]-3-(3-methoxy-4-= 525.97 m/z.
methylpheriyl)propanaic acid
(3S~3-[({[1-(2-chloro-6-methylbenzyl)-40:084 Calculated (M-H)-=
hydroxy-
5;6-dimethyl-2-oxo-1,2-dihydropyridin-3-570.20 m/z; Found (M-H)'
ylJamino}carbonyl)s~oJ-3-(3,4- = 570.00 rn/z.'
diethoxyphenyl)propanoic acid
(3S)-3-[({[1-(2-chlom-6-cyanobenzyi)-4hydroxy-0.007 Calculated (M-H)~=
2-oxo-1,2d~yd;opyridln-3- 479.11 mlz; Found (M-H)'
yl)amino}carbonyl)amino)-3-(4- = 478.90 n~z,
.
methylphenyl}propanoic acid
(3S)-3-[(t[1-(2-chloro-6-methylbenzyl)-4-hydroxy-0.03 Calculated (M-H)-=
~,6-dimathyl-2-oxo-1;2-dihydropyridin-3-496:16 m/z; Fovnd (M-FI)-
ylJamino}carbonyl)arnino)-3-(4- = 495.97 m/z.
methylphcnyl)propanoic acid
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-5,6-0.015 Calculated (M-H)-=
dimethyl-a-oxo-1,2-dihydropyridin-3-512.16 tn/z; Found (M-H)'
ylJamino}carbonyl)amino]-3-(3-raethoxy-4-= 511.95 m/z.
methylphenyl)propanoic acid
(3S)-3-[({(1-(Z-chlorobenzyl)-4 Mydroxy-S,6- 0.003 Calculated (M-H)-=
dimethyl-2-oxo-1,2-dihytlropyridin-3- 556.18 rn/z; Found (M-H)'
ylJamino}carbonyl)aminoJ-3-(3,4- = 555.99 rn/z.
diethoxyphenyl)propanoic acid
CA 02366800 2002-O1-07
-164-
Table 6
Compound ICso (nM) Mass Spectral Data
(m/z)
(3R)-3-[({['1-(2-chlorobenzyl)-4.-hydroxy 2-oxo-1,2- 2500 Calculated (M-H}'
dihydropyridin-3-ylJan3ino}carbonyl)aminoJ-4-(I- 504.13; Found (M-H)' _
aaphthyl}butanoic acid 503.97.
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-5,6-3~ Calculated (M-H)'=
dimethyl-2-oxo-1,2-dihydropyridin-3- 512.16; Found
(M-Fi)-
ylJamino}carbonyl)amino]-3-(3- S 1 I .99.
ethoxyphenyl~mpanoic acid
(35}-3-[({[i-(2-chlorobenzyl)-4-hydroxy40 Calculated (M-H)'
5,6- _
dimethyl-2-oxo-1,2-dihydropyridin-3- 496.16; Found
(M-H)' _
yl]amino} carbonyl)amino]-3-(3,4- 496.05.
dimethylphenyl)prapanoic acid
{3S}-3-(({[1-(2-chloro-6-methoxybenzyl)-4-~~ Calculated (M-H)'=
hydroxy 5-methyl-2-oxo-1,2-dihydropyridin-3- 498.15; Found
(M-H)'
yl]amino}carbonyl}amino]-3-(4- 497,91,
methylpherlyl)pmpanoic acid
(3S)-3-[{{[1-(2-chloro-6-methoxybenzyl)-4-2. Calculated (M-H)-=
hydroxy-5-methyl-2-oxo-1,2-dihydropyridin-3- 572.18; Found
(M-H)' _
yl]amino}carbonyl)amino]-3-(3,4- 57 I .96.
diethoxyphenyl)prapanoic acid
(3S)-3-[({[ 1-(2-chloro-6-methoxybenzyl)-4-6 Calculated (M-H)'
_
hydroxy-5-methyl-2-oxo-1,2-dihydropyridin-3- 528,15; Found
(M-H)' _
yl]amino}c;arbonyl)amino]-3-(3-methoxy-4- 527.95.
rnethyIphex~yl~ropanoic acid
(3S)-3-[( { [ 1-(2-chloro-6-methoxybeazyl)-4-3 Calculated (M-I~'
_
hydroxy-5-methyl-2-oxo-1,2-dihydropyridin-3- 528.15; Found
(M-Fib' _
yl]amino}carbonyl)aminoJ-3-(3- 527.99.
ethoxyphenyl)propanoic acid
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-1,2-15 Calculated {M-H)'=
dihydropyridin-3-yl]amino}carbonyl)amino]-3-(3- 556.09; Found
(M-H)'=
(1,1,2,2-tetrafluoroethoxy)phenyl]propanoic 555.97.
acid
(3R)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-1,2- 700 Calculated (M-H)-
dihydropyridin-3-ylJamino}carbonyl)amino]-4-(2- 488.08; Found (M-H)' _
chlorophenyl)butarroic acid 487.96.
CA 02366800 2002-O1-07
-165-
(3S)-3-{[({4-hydroxy i-[3-(methylthio)benzyl]-2-20 Calculated (M-H)-=
oxo-1,2-dihydropyridin-3- 466.14; Found
(M-H)' =
yl}amino)carbonyl]amino}-3-(4- 466.04.
methylphenyl)ptvpanoic acid
(3S)-3[({[1-(2-chlorobenzyl)-4 15 Calculated (M-H)'
hydroxy-5-methyl- _
2-oxo-1,2-dihydropyridia-3- 482.15; Found
(M-H)' _
yl]amino J carbonyl)amino]-3-(3,4- 482.02.
dimetbYlpt~nyl)propsaorc acid
(3S)-3-[({[1-(2-chloro-6-methoxybenzyl)-4-3 Calculated (M:Fn'=
hydroxy 5-methyl-2-oxo-1;2-dihydropyddin-3- 512.16; Found
(M-H)' _
yl]amino}catbonyl)mnino]-3-(3,4- 512:03.
dnnethylphenylic acid
(3S}-3-[({[1-(2-chlorobenzyl)-5-cyclopropyl~4-20 Calculated (M+H)*=
hydtoxy-2-oxo-1,2-dihydropyridia-3- 496.16; Found
(M+H)+
yl]amino)carl~nyl)~niao]-3-(4- 496.05.
methytphenyl)pmpanoic acid
(3S)-3-[({[1-(4-chlorobenzyl)-4-hydroxy-2-oxo-50 Calculated (M-H)'=
2,5,6,7-tetrahydro-IH-cyclopenta[b]pyridin-3- 494.15; Fvund
(M-H)'
y1]amino}oarbonyl)aminoj-3-(4- 494.02.
methylphenyl~ropanoic acid
(3S)-3-[({[1-(3-chlorobenzyl)-4-hydroxy-5-methyl-20 Calculated (M-I-i)'
2-oxo-1,2-dihydropyridin-3- 4b8.13; Found
(M-H)" _
yl]amino}csrbonyl)amino]-3-(4- 468.02.
methylphenyl)propanoic acid
(3S)-3-[( {{ 1-(Z,6-dichlorobenzyl)-4-hydroxy-5-20 Calculated (M-H)'
methyi-Z-oxo-1,2-cli~hydropytidin-3- 502.09; Found
(M-H)'
yl]amino}carbonyl)aminoJ-3-(4- 501.92.
methylphenyi)propanoic acid
(3S)-3-[({[4-hydroxy-5-methyl-1-(a-rnethylbenzyl)- 150 Calculated (M-~-=
2-rnco-1,2-dihydropyridin-3- 448.19; Found (M H)' =
ylJamino}carbonyl)amino]-3-(4- 448.05.
methylphe~nyl)propanoic acid
3-(1-benzofiuan-2-yl)-3-(({[1-(2-chlombenzyl)-4- 140 Calculated (M-H)-=
hydroxy-2-oxo-1,2-dihydropyridin-3- 480.10; Found (M-I~- _
yI]amino}carbonyl)amino)propanoic acid 479.96.
(3S)-3-[({[1-(2-chlombenzyl)-4-hydroxy-2-oxo- 3 Calculated (M-H)'=
2,5,6,7-tetrahydro-iH-cyclopenta[b]pyridin-3- 524.16; Found (1Vt-H)-=
yl]amino}carbonyl)amino]-3-(3- 523.95.
ethoxyphenyl)prapanoic acid
CA 02366800 2002-O1-07
-166-
3-[({[1-(2-chlorobenzyl)-4 hydroxy-2-oxo-1,2- ' 15 Calculated (M-H)'=
dihydrapyridin-3-y1)amino}catboayl)amino]-3-(6- 520,13; Faund (M-H)-=
methoxy-2-naphthyl~mpanoic acid 520.00.
353-[(([1-(3,5-diaae:hOx~~benzyl~4-hydroxy-5-70 Calculated (M-H)'
_
methyl-2oxo-I,2-dihydropyadin-3-494. I9; Found (M-H]'
_
yl]amino}carbonyljamino]-3(4- 494.04.
methylptaenyl)propanoic acid
(3S)-3-[( f [1-(2,6-difluorobenzyl)-4-hydroxy-5-2S Calculated (M-H)'
_
methyl 2-oxo-1,2-dihydropyridia-3-470.15; Found (M-H)'
=
yl]amino}carbonyl)amino]-3-(4- 470.03.
methylphenyl~ropanoic acid .
(3S)-3-[( {[ 1-(2-chlorobe~nzyl)-4-hydroxy3 Calculated (M+H)+
2-oxo- _
2,5,6,7-t~trahydro-1H-cyclopenta[b)pyridin-3-570.20; Found (M-~H)*-
yl]aminojcarbonyl)amino]-3-(3,4-570.00.
diethoxyphenyl)propanoic acid
(3S)-3-{[({4-hydroxy I-[3-(methylsulfonyl)benzyl]-25 Calculated (M-H)'
-
2-oxo-I,2-dihydropyridin-3- 498.13; Found
(M-I~'
yl}aminA)carbonyl]amino}-3-(4- 498.01.
methylphenyl~ropanoic acid
(3S)-3-[({[I-(2-chloro-6-methylbenzyl)-4-hydroxy-3 Calculated (M-H)-=
5-methyl-2-oxo-1,2-dihydmpyridin-3- 556.19; Found
(14t-H)'
ylJamina} carbonyl)amino]-3-(3,4- 556:02.
diethoxyphenyl)propanoic acid
(3S)-3-[({I1-(2-chloro-6-methyla~nzyl)-4-hydroxy-4 Calculated (M-H)'=
5-methyl:2-oxo-1,2-dihydropyridin-3- 512. I 6; Found
(M-H)' =
yl]amino}carbonyl)anuno]-3-(3- 512.02.
ethoxyphenyl)propanoic acid
(3S)-3-[({[1-(2-chloro-6-methytbenzyl)-4-hydroxy-45 Calculated (M-H)'=
5-methyl-2-oxo-1,2:dt'hydropyidirt-3- 496.16; Found
(M-H)'
yl]amino;carbonyl~mino]-3-(3,4- 496.01.
dirnethylphenyl)propanoic acid
(3S)-3-[({[1-(2-chloro-6-methylbenzyl)-~1-hydroxy-25 Calculated (M-H)'=
5-methyl-2-oxo-l,2-cfhydropyridin-3- 312.16; Found
(M-F~' _
yI]amino}carbonyl)amino]-3-(3-methoxy-4- 511.97.
tnethylphenyl)propanoic acid
3-[({[1-(2-chIorobenzyl)-4-hydroxy-2-oxo-1,2-11S Calculated (M-H)
dihydropyridin-3-yl)amino}carbonyl)aminoJ-3-(4,5- 458.11; Found
(M-I-n''
dimethyl-2-furyl)praganoic acid 457.99.
CA 02366800 2002-O1-07
-167-
3-[({[1-(2-chll)4-hydro~cy 2-oxo-l,2- 160 Calculated (M-T~-=
dihydropyridia-3-yl]amino}carbonyl)amino]-3-(4- 520.13; Found (M-H)-
methoxy~1-naph~tyl)prapanoic acid 519.97.
(3R)-3-[({[t-(2-chloroboazyl~4-hydroxy-2-oxo-1,2- 115 Calculated (M-H)'= '
dihydtopyridit~-3 yi]amino}carbonyl~mino]-5- 468.13; Found (M-H)-=
phenylpen~tanoic acid 467,98.
(353-[({{1-(2-chlorobe~zyl~4-hydroxy-2-oxo-1,2- 12 Calculated (M-H)'
dihydroquinolin-3-yljamino}carbonyl)amino]-3-(3- 534.14; Found (iVl'-I~'
ethoxyphenyl~ropaaoic acid 533.94.
(3S)-3-[({[1-(2-ahlorobenzyl)-4-hydroxy-2-oxo-18 Calculated (M+H)+=
2,5,6;7-tetrahydzo-1H-cyclopenta[bapyridin-3-510.18; Found (M+H)+=
yljamino}carbonyl)amizio]-3-(3,4-510.06.
disnethylphenyl)pra~panoic acid
(3S)-3-j({[1-(2-chloro-6-ethoxybetu'yl)-4-hydroxy-7 Calculated (M+I~+-
2-oxo-1,2-di'hydropyridin-3- 500.16; Found (M+I~*
_
yl]amino}carbonyl)amiao]-3-(4- 500.06.
methylplienyljprapanoic acid
(3S)-3-[({[1-(2-chloro-6-ethoxybenzyl)-4-hydroxy-3 Calculated (M-Id)'
_
5-methyl-2-oxo-1,2-dihydropyridin-3-S 12.16; Found (M-H)'
_
y1 jamina}carbonyl)amino]-3-(4- 512.03.
methylphrnyl)propamoic acid
(3S)-3-[( { [ 1-(2-chlorobenzyl)-5-cycloptopyl-4-14 Calculated (M+H)~'
_
hydroxy.2-oxo-1,2-d~ydropyridin-3-526.17; Found (M+H)~
-
yl]amino) carbonyl)amino]-3-(3- 526.01.
ethoxyphenyl)~ropanoic acid
(3S)-3-j({[1-(2-chlorobenzyl)-5-cyctopropyl-4-6 Calculated (M+H)''=
hydroxy-2-oxo-1,2-dihydaopyridin-3-570.20; Found (M+I-~+
yljamino} carbonyl)anvno]-3-(3,4-570.04.
diethoxyphenyl)propanoic acid
(3S)-3-[(([1-(2-chlorobenzyt)-4 hydroxy-2-oxo-1,2- 30 Calculated (M-I~'=
dihydrop~,~idin-3-yl]amuno}carbonyl)aminoj-3-[4- 506.09; Found (M-H)'
(difluoroznethoxy~henyl]propanoic acid 505.96.
3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-1,2- lOS Calculated(M-,~'=
dihydropyridin-3-yl]amino}carbonyl)amiao]-3- 491.11; Found (M-I~-=
quinolin-2-ylprapanoic acid . 490:96.
CA 02366800 2002-O1-07
-I6s-
{3S}-3-[({(1~(2-fluoro-6~methoxybenzyl)-4-hydmxy- 10 Calculated (M-H)'=
5 methyl-2-oxo-1;2-dihydrapyridin-3-482.17;
ylJamino}carbonyl}amino]-3-(4- Found (M-H)' = 482.02.
methylphenyl)prapaaoic acid
(38)-3-(({[1-(2-chloro-6-methoxyben2yl)~-1 S Calculated (M+H}'"
hydroxy 2-oxo-5-propyl-1,2-dihydropyridin-3-528.19; Found (M+I~*
=
ylJamino}carbonyl)amiaoJ-3-(4- 528.04.
methylplxenyl)pmpanoic acid
(3S)-3-[({[I-(2-chloro-6-methoxybenzyl)-4-7 Calculated (M+H)+=
hydroxy 2-oxo-5-propyl-I,Z-dihydropyridin-3-558.X0; Found (M+I~'"
_
ylJasaino)carbonyl)amino]-3-(3- 558_07,
ethoxypbenyl}propaaoic avid
(3S)-3-[({[I-(S-chlom-2-lluorobenzyl)-4-hydroxy-5-1S Calculated (M-H)'=
methyl-2-oxo-1,2-dihydropyridin-3-486.12; Found (M-H)'
=
yl]amino}cacbonyl)amino]-3-(4- 486.00.
methylphertyl)propanoic acid
(3S)-3-[({[1-(2-chlorobenzyl)4-hydroxy-2-oxo-I,2-14 Calculated (M-H)'=
dihydroquinolin-3-yl]amino}carbonyl)amino]-3-(3-534.14; Found (M-I~'
_
methoxy~4..methylphenyl}pmpanoic 533.95,
acid
(3S)-3-[({[1-{2-chlorobenzyl)-4-hydroxy-2-oxo-1,2-4 Calculated (M-H)-=
dihydroquinolin3-ylJamino}carbonyl)amiao]-3-578.17; Found (M-H}'
_
(3,4-diethoxyphenyl)propanoic acid577.99.
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-1,2- 25 Calculated (M-F~'a
dihydroquinoIin-3-ylJamino}carboreyl)amino}-3- 518.15; Found (M-H)' _
(3,4-dimethylphenyl)psopanoic acid 517.96,
(3S)-3-[({[I-(2-chlorobenzyl)-4-hydroxy-2-oxo-1,2- 150 Ca)culated (M+H)+=
dihydropyridin-3-yl]amino}carbonyl)amino]-3- 443.11; Found (M+I~+--
pyridin 2-ylpmpaaoic acid 443.03.
(3S)-3-(({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-1,2- 3 Calculated (M-H)'=
dihydropyridin-3-yI)aati~w}casbonyl)araino]-3-(3- 498.14; Found (M-H)- _
isopropoxyphenyl}propanoic acid ~ 498:04.
(3S)-3-[({jI-(2-chlombenzyl)-4-hydroxy-2-oxo-1,2- 7 Calculated (M-H)'=
dihydropyridin 3-yl]amino}carbonyl)amino]-3-(3,5- 528.15; Found (M-H)' _
diet~eoxyphenyl)propanoic acid
528.02.
CA 02366800 2002-O1-07
-169-
(3S)-3-[({[1-(2-chlombenryl)-4-hydroxyGO Calculated(11~i+F~+_
5- ~'
isopropyl-2~xo-1,2-dihydropyc'idirr3- 498.18; Found
(M+H)
yl]amino },~rbonyl~aino]-3-(q.. 498,05.
methyiphenyl)propa~aoic acid
(3S)-3-[(([i-(5-flnoro-2-methylbenxyl)-4-hydroxy-'~0 Calculated (M+H)*
+
S-methyl-2-oxo-l,?rdihydropyridin-3- =
468.19; Found
(M+H)
yl]amino} carbonyl)aminoJ-3-(4- 468,0?.
rnethylphenyl)propanoic acid
(3S)-3-{[({4-hydroxy-5'-methyl-Z-oxo-I-[(1S)-1-1500 Calculated (M+Fi)+=
phenyiethyl]-1,2-dihydropyridin 450.20; Found
3- (M+H)+
yl}amino)carbonryljamino)-3-(4- 4sa:o7. ,
methylphenyl)prop~anoic acid-
(3S)-3-[{{[1-(2-chloto-6-rnethoxybetizyl)4-3 Calculated (M+H)+=
hydroxy 2-oxo-5-propyl-1,2.~dihydmpyridiu-3- 502.23; Found
(M+H)+=
yl]amino}carbanyl)amino)-3-(3,4- 602.04.
diethoxypheayl~ropanoic acid
(3S)-3-[(~([1-(2-chloro-5-isopropoxybenzyl)-4-7 Calculated (M
H)'=
hydroxy 5-methyl-2-oxo-I,2-dihydropyridin-3- 526.17; Found
(M Fn-=
ylJaminoJ carbonyl)arrunoJ-3:(4- 526.04.
methylphenylJgropanoic acid
(3S)-3-[({[1-(2-chloro-6-methoxybenryl)-~4-15 Calculated (M+H)+=
hydroxy-2-oxo-5 prapyl-1,2-dihydropyridin-3- 558.20; Found
(M+Fi)+ -
ylJamino} carbonyl)ataino]-3-(3-methoxy-4- 55 8.45.
rnethylphenyl)propanoic acid
(3S)-3-[({[1-(2-chloro-6-ethoxybenzyl)-4-hydroxy-2 Calculated (M+H)''
' _
5-methyl-2-oxo-1,2-dihydropyridin-3- 544.19; Found
(M+H)+=
yl)amino}carbonyl)amino]-3-(3- 544.04.
ethoxyghemyl)preganoic acid
(3S)-3-[({[1-(5-acetyl-2-methaxybenzyl)-4-hydroxy-33 Calculated (M
H)-=
2-oxo-1;2-dihydropytidia-3- 492.18; Found
(M-H)'=
yl)amino} carbonyl)aminoJ-3-(4- 492.04.
methylphenyl)propanoic acid
3-[({[1-(2-chloro-6-methylber~zyl)-4-hydroxy-5-3S Calculated (M.H)'=
methyl-2-oxo-1,2-dihydropyridin-3- 548:16; Found
{M-H)-
yllamino}carbonyl)amino]-3-(6-methoxy-2- 548.01.
naghthyI)propanoic acid
(3S)-3-[({[1-(2-chloro-6-methoxybenZyl)-4-17 Calculated (M+I~+$
hydroxy-2-oxo-5-propyl-I,2-dt'hydropyridin-3- 542,21; Found
(M+H)*
yl]amino} catbonyl)amino]-3-(3,4- 542.05.
dimethylphenyi)pmpanoic acid
CA 02366800 2002-O1-07
-170-
{3S)-3-[({[1-{2-chlorobertzy!)-4-hydroxy3 Calculated (M-I~'
2-oxo-1,2-
dihydropyridia-3:ylJamino}carbonyl)amirroJ-3-(1- 493.13; Found (M-I~-=
methyl-1H-indol:5 yl)ixopanoic 492.95.
acid
(3S)-3-[{ f [2-(2-chlorobenzyl)-5-hydroxy18 Calculated (M+I~t
6-methyl- =
3-oxo-2,3-dihydropycidazin-4- 471.14; Found (M+H)*
_
y1]atitino}carbouyl)amino]-3-{4- 471.00.
methylphenyl)pm~panoic acid
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-5S Calculated (M-I~-
methyl-
2-oxo-I,Z-dihydti~yridin-3- 534.14; Found (M-I~'
yl]amino} carbonyl)aminoJ-3-(6-methoxy-2- 533.91.
naphihyl~rapanoio acid
(3S)-3-[(([2-(Z-chlorobgazyl~5-hydroxyS Calculated (M+I~*
6-methyl-
3-oxo-2,3-dihyd~pyrida2im-4- 501.15; Found (M+I~+=
yl]amine} carbonyl)aminoJ-3-(3- 501.01.
ethoxyphenyl~rapanoic acid
3-[({[1-(2-chlorobenzyl)-4-hydtoxy30 Calculated (1VI+I~+~
2-oxo-1,2-
dihydropyridin-3-yl)amino}carbonyl}amino)-3- 448.07; Fouad (M+H)+=
thien-2-ylpropanoic acid 447.97.
(3S}-3-[({[5-chloro-1-(2-chlorobenzyl)-4-hydroxy-2- 6 Calculated (M-H)-
oxo-1,2-dihydropyridin-3- 488.08; Found (M-H)- _
yl]amino}carbonyl~mino]-3-(4- 487.97.
methylphenyl)propanoic acid
(3S)-3-(3-butoxyphenylr3-[({(1-(2-chlombenzyl)-4-20 Calculated (M-H)'=
hydroxy-2-oxo-2,5,6,7-tettattydro-I552.19; Found (M-H)'
H- _
cyclopet~ta[b)pyddin-3- 552.01.
yl]amino}carboayl~nunojpropanoic
acid
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-1,2-5 Calculated (M-H)'
_
dihydropyridin 3-yl]amino}carbonyl)amino3-3-[3-524.16; Found (M-H)'
=
(cyclopetityloxy)phenyljpropanoic524:00.
acid
(3S)-3-[({[2-(2-chlombenxyl)-5-hydroxy-6-methyl-3 Calculated (M+H)t=
3-oxo-2,3-dihydrapyridazin-4- 545.18; Found (M+I~+
_
yl]amino}carbortyl)amiuo)-3-{3,4-544,98.
diethoxyphenyl)pmpar~oic acid
(3S]~3-[({[1-(2-chlorobettzyl)-4-hydtoxy-5-methyl-3 Calculated (M-H)~=
2-oxo- Z,2-dihydropyridin-3-
507.14; Found (M-H)'
_
yl]amino}carbonyl)amino)-3-{1-methyl-IH-indol-5-506.94.
yl)propanoic acid
CA 02366800 2002-O1-07
-172-
(3S)-3-[({ (2-(2-chlombenzyl}-5 10 Calculated (M+H)+
hydmxy-6-methyl- _
3-axo-2,3-dihydropyridazin-4- 545.18; Found (M+F~*
_
yI]amino}carbonyl)amino]-3-(3,S- 545.01.
diethoxyphenyl)pmpanoic acid
(3S)-3-[({,jl-(2-chlorobanzyl}-4-hydroxy>>0 Calculated (M-H)'
5-methyl- _
2-oxo-1,2-dihydropyridin-3- 538.10; Found (M-Fi)'
yI]amino} carbonyl)amino]-3-[4- 537.95.
(trifluorotnethoxy)phenyl]propanoic
acid
(3S)-3-((((1-(2-chlorobenzyl)-4-hydroxy-5-methyl-10 Calculated (M-H)'=
2-oxo-1,2-dihydropyridin-3- 538.10; Found (M-Z~'
=
yl]amino fcarbonyl)aruino]-3-[3- 537.y5.
(trifluoromethoxy)phenyl]propanoic
acid
(3S}-3-(({(1-(2-chlorobenayl)-4-hydroxy-5-methyl-4 Calculated (M+.H)+=
2-oxa-1,2-dihydropyridin-3- 48b.14; Found (M+FI)fi
=
yl]amino}carbonyl)amino]-3-(4- 486.04.
methoxyphenyl)propanaic acid
(3S)-3-[({[1-(2-chlorobenryl~4-hydroxy-2-oxo-1,2-IS Calculated (M-H)'=
dihydropyridin-3-yl]amino}carbonyl)arzzino]-3-(6-520.13; Found (M-I3)-
=
methoxy-2-naphthyl~ropanoic acid 520.03.
(3S)-3-{[({1-(2-tluoro-6-(trifluoromethyl)benrylJ-4- 100 Calculated (M-H)'=
hydroxy-S-methyl-2-oxo-1,2-dihydropyridin-3-520.15; Found (M-H)'
_
yl}amino)carbonyl]amino}-3-(4- 519.9?.
methylphenyl)propanoic acid
(3S)-3-[({( 1-(Z-chlorobenzyl)-4-bydroxy-S-methyl-10 Calculated (M-Fi)~
_
2-oxo-1,2-dihydropyridia-3- 522.10; Found (M-H)'
_
yl]arninoE carbonyl)amino]-3-[3- 521.96.
(trifluoro~nethyl)phenyl]propanoic
acid
(3S)-3-((([1-(2-chlorobenzyl)-4-hydroxy-5-methyl-3 Calculated (M-H}'
2-oxa-1,2-dihydtopyridin-3- 484.13; Found (M-I~'
=
yl]amino} carbonyl)amino]-3-(3- 484.00.
methaxyphenyl)propanoic acid
(3S}-3-[(~([1-(2-chloto-6-methylbenzyl)~-hydroxy-20 Calculated (M+H)*=
2-oxo-2,5,6,7-tetrahydro-I H-cyclopenta[b]pyridin-3-S 10,18; Found (M+H)+
-
yl]arnino~icarbonyl)amino]:3-(4- 510.05.
methylphenyi)propaat~ic acid
(35)-3-[({[I-(2-chloro-6-methylbenzyl)-4-hydroxy-4 Calculated (M+IT)+$
2-oxo-2,5,6,7-tetrahydro-1H-cyclopenta(b]pyridin-3-540.19; Found (M+H)*
_
yl]amino}carbonyl)amino]-3-(3- 540.10.
.
ethoxyghenyl~ropanoic acid
CA 02366800 2002-O1-07
-172-
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy3 Calculabad (M+H)+=
2-oxo-
2,5,6;7-r~trahydro-I H-cyclopenta[b]pyridin-3-540.19; Found (M+I~+
--
yl]amino]carbonyl)amino]-3-(3- 540,09.
isopropvxyphenyl)propanoic acid
(3S)-3-[( ([l-{2-chlorobenryl)-4-hydroxy3 Calcutaxed (M-H)-=
5-methyl-
2-oxo-1,2-dihydropyridin-3 542.17; Found (M-I-~'
_
ylJamino}carborryl)amino]-3-(3,5- 542.00.
diethoxyphenyl)propanoic acid
(3S)-3-[({[1-(2-chloro-6-ethoxybenzyl)-S-ethyl-4- 4 Calculated (M-I~'
hydroxy-2-oxo-1;2-dihydtopyridin-3- 556.19; Found (M-H)' _
yl)amin4)carbonyl)amino]-3-(3- SS6.01.
ethoxyphenyl)propanoic acid
(3Sr3-(({jl-(2-chloro-6-ethoxybenzyl)-4-hydroxy-3 Calculated (M+Id)~'=
2-oxo-1,2-dihydrvpyridin-3- 530.17; Found (Ivl+H)+=
yl]amino} carbonyl)amino)-3-{3- 530.04.
ethoxyphenyl~ropaaoic acid
(3S)-3-[({[I-(2-chlarobenayl)-4-hydmxy-5-methyl-15 Calculated (M-I~'=
2-oxo-1,2-dihydropyridin-3- ' 538.17; Found (M-H}-=
yl]amino}carbonyl)atriino]-3-[3-538.03.
(cyclopentyloxy)phenyl)propanoic
acid
3-(l,l'-biphenyl-4 yIJ-3-[({[I-(2-chlvrobenzyl)~- 130 Calculated (M-H)-=
hydroxy-5-methyl-2-oxo-1,2-dihydrvpyridin-3- 534.15; Found (M-F~-=
yl]amino}carbonyl)aminoJpropanoic acid 529.96.
{3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo- 30 Calculated (M+H)+=
2,5,6,7-tetrahydro-1H-cyclopenta[bjpyridin-3- 580.15; Found (M+F~+=
yl]amino}carbomyl)amino]-3-[3-(2,2,2- 580,02.
trifluoroethoxy)phenyl]propanoic acid
(3 S}-3-[( { [ I-{2-chlorobenzyl)-4-hydroxy-S-methyl-I S Calculated (M+H)+
_
2-oxo-1,2-dihydropyridin-3- 554.13; Found (M+I~+
_
yl]amino} carbonyI)amino]-3-[3-(2,2,2-554.00.
trifluoraethoxy)phenyljpropanoic
acid
(3S)-3-[({(1-(2-chlorobenzyl)-4-hydroxy3 Calculated (M+I~"'=
5-methyl-
2-oxo-I,2-dihydropyridin-3- 514.17; Found (M+H)+=
yl]amine} carbonyl)amino]-3-{3- 514.05.
isopropoxyphenyl)propanoic acid
(3S)-3-[{{[1-(Z-chloro-6-ethoxyben2yl)-4-hydroxy- 4 Calculated (M+H)+=
5-methyl-2-oxo-1;2-dihydrogyridin-3- SS8.20; Found (M+H)+ _
yl]amina)carbonyl)aminoJ-3-(3- 558.05.
isopropoxyphenyl)propanoic acid
CA 02366800 2002-O1-07
-1~3-
Table 7
Compound ICso Mass Spectral
(nM) Data
{mlz)
(38)-3-[({[1-(2-chlorobe~yl)-4-hydmxy-59 Calculated (M+IT)+=
methyl-2-
oxo-1,2-dihydropyridin-3-yl]amino}carbonyl)amino]- 500.16;.Found
(M+H)''
3-(4-methoxy-3-methylphenyl~ropanoic = 500.01,
acid
{3S)-3-[({[1-(2-chloro-6-methylbenzyl)-4-hydrvxy-2-14 Calculated {M+H)+
_
oxo-2,5;6,7-tstrahydro-1H-cyclopenta[bJpyridin-3- 554.21; Found
{M+~*
yl]amino}carbonyl)amiaoJ-3-(3- = 554.06.
isopropoxyphenyl)pmpanaic acid
(3S)-3-[({[1-(2-chloro-6-ethoxybenzyl)-4-hydroxy-5-3 Calculated (M+H)+=
methyl-2-oxo-1;2-dihydropyridin-3- 580.19; Found
(M+H)+
yl]amino}cesbonyl)amino]-3-(b-methoxy-2- = 580.07.
naphthyl]pmpanoic acid
(3S)-3-[({[1-(2-chlotobenzyl)-4-hydroxy-5-methyl-2-12 Calculated (M+I-~*
oxo-1,2-dihydropyridin-3-yl]amino}carbonyI)aminoJ- 530.17; Found
(M+H)+
3-(3,5-dimethoxy-4-methylphenyl)propanvic = 530.00.
acid
(3S)-3-[({[1-(2-chloro-6-methylbenzyl)-4-?eydroxy-2- 12 Calculated (M+H)+
oxo-2,5,6,7-tetrahydro-1H-cyclopenta[bJgyridin-3-554.21; Found (M+H)'"
yI]amino}carbonyl)arninoJ-3(3- = 554.05.
propoxyphenyt~rapanoic acid
(3S~3-[({[1-(Z-chloro-6-propoxybenzyl)-4-hydmxy-10 Calculated (M+H)+=
methyl-2-oxo-I,2-dihydropyridia-3-528,19; Found {M+H)~
yl]amino}carbonyl)ansino]-3-(4- = 528.06.
methylphenyl~ropanoic acid
(3Sr3-[({[1-(2-chloro-6-isobutoxybenzyl)-4-22 Calcuiated(M+H)'=
hydroxy-5-methyl-2-oxo-I,2-dihydropyridin-3-542.21; Found (M+1-~i
yl]amino}carbonyl)aminoJ-3-(4- = 542.06.
methylphenyl)propanoic acid
(35)-3-[([[1-(2-chlorobenzyl~4-hydroxy-2-oxo-15 Calculated (M+H)'"=
2,5,6,7-tetsahydro-1H-cyclopenta[b]pyridin-3-540.19; Found (M+>;I)+
~
yl]amino}carbonyl)arniaoJ3-(3- _ 540.07.
propoxyphenyl)propanoic acid
(3S)-3-[({[I-(2-chloro-6-ethoxybenryl)-4-hydroxy-2-3 Calculated (M+H)''=
oxo-2,5,6,7.:tetrahydro-1H-cyclopenta[b]pyridin-3-540.19; Found (M+H)+
yl]amino}carbonyl)amino]-3-(~i- = 540.04.
methylphenyl)propanoic acid
(3 S)-3-[( {[ 1-(2-chloro-6-ethoxybenryi)-4-hydFOxy-2-4 Calculated (M+H)+
_
oxo-2,5,6;7tetrahydro-1H-cyclopenta[b]pyridin-3-584.22; Found (M+H)+
yl]amino}carbonyl)amino]-3.(3- = 584.05.
iSOpropoxyphenyl)ptopanoic acid
CA 02366800 2002-O1-07
-I74-
(3S)-3-[({[I-{2-chlorobenzyl)-4-hydroxy40 Calculated (M+I~+
methyl-2- ~
oxo-1,2dihydropytidin-3-yl]amino}carbonyl)aminoj- 592.19; Found
(M+H)''
3-(2',6r dimethoxy-1,1'-biphenyl-4 = 592.04.
yl~ropanoic acid
(3S}-3-[{{[1-(2-chlorobenzyl)-4-hydroxy30 Calculated (M+H)+_
5-methyl-2-
oxo-1,2-dihydropyridin-3-yl]amino}carbonyl)amino]- 509:16; Found
(M+H)+
3-(1-methyl-iH-indol-7=yt)propanoic = 509.03.
acid
(3S)-3-[({[i-(2-chloro-6-ethoxybenzyl)-4-hydroxy-2-2 Calculated (M+F~+=
oxo-2,5,6,7aetrahydro-1H-cyclopenta[b]pyridin-3- 570.20; Found
(M+H)'~
yl]amino}carbonyl}amino]-3-(3- = 570.09.
ethoxyphenyl)propanoic acid
(3S)-3-[({[I~(2-chlozo-6-propoxybenzyl)-4-hydroxy-5 Calculated (M+H)+=
5-methyl-2-c~xo-1,2-dihydropyridin-3- 558.20; Found
(M+H)'
yl]amino}caxbonyl)amino]-3-(3- = 558.03.
athoxyphenyl)pmpanoic acid
(3S)-3-[({[1-(2-chloro-6-isohutoxybenxyl)-4-14 Calculated (M+I~+_
hydroxy-5-methyl-2-oxo-1;2-dihydropyridin-3- 572.22; Found
(M+H)+
yl]amino}carbonyl)amino~-3-(3- = 572.05.
ethoxyphenylJpropanoic acid
(3S)-3-[({[l-(2-chloro-6-isopropoxybenzyl)-4-7 Calculated {M+H)*
_
hydroxy-5-methyl-2-oxo-1,2-dihydropyridin-3- 558.20; Found
(MtH)+
yl]amino}carbonyl)amino]-3-(3- = 558.03.
ethoxyphenyl~ropanoic acid
(3S)-3-{[( { 1-[2-chlom6-(2,2,2- 4 Calculated (M+H)+
trifluoroethoxy)benzyl]-4-hydroxy-5-methyl-2-oxo- 598.16; Found'
(M+H)'"
1,2-dihydropyridin-3-y1}amino)carl~~nyl]amino}-3- = 597.99.
(3-ethoxyphenyl)propanoic acid
3-[({[1-(2-chlozobenzyl)-4hydroxy-5-methyl-2-oxo-la Calculated (M+H)+=
1,2-dihydropyridin-3-yl]amino}carbonyl)amino~-3- 502.12; Found
(M+H)+
[4-{methylthio)phenyl]propanoic acid = 501.98.
(3S~3-[({(I-(2-chioro-6-ethoxybenzyl)-4-hydroxy-Z-2 Calculated (M+H)+=
oxo-2,5,6,7-tetrabydro-IH-cyclopenta[b]pyridin-3- 606.20; Found
(M+H)''
yl]amino}ca~rboayl)amino]-3-(6-methoxy-2- a 606.04.
naphthyl)pmpanoic acid
{3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-5-methyl-2-6 Calculated (M+H)+~
oxo-1,2-dihydropyridin-3-yl]amino}carbonyt)amino]- 498.14; Found
(M+I~+
3-(2,3-dihydro-1-benzoft~ran 5-yl)propanoic = 498.02.
acid
(3S)-3-[({[1-(2-chloro-6-ethoxybenzyt)-4-hydroxy-5- 3 Calculated (M+H)'' _
methyl-2-oxo-1,2-dihydropyridin-3- 553.19; Found (M+H)+
yl]amino}carbonyl)amino]-3-(1-methyl-1H-indol-5- = 553.05.
yl)propanoi~~ acid
CA 02366800 2002-O1-07
-175-
(3S)-3-[({[1-(2~-chloso-6-athoxybsr~zyl)-4-hydroxy-S-2 Calculat~d (Mt~+
~
+
methyl-2-oxo-1,2-dihydropyridin-3- 542.17; Found.
(M+H)
y1]amino}carbonyl)amino]-3-(2,3-d~ydro-1- = 542.06.
benzofuran-5-yl)propanoic adid
(3S)-3-[( f [1-(2-chloro-6-ethoxybenzyl)-4:hydroxy-2-3 Calculated (M+I~''
7-tetrahydro-1H-cyclopenta[b]pyridin-3- 614.22; Found
(M+I~*
oxo-2
6
, = 614.11,
,
,
yl]amino}cazbonyl)amino]-3(3,5.
diethoxyphenyl)propaxxoic acid
(3S}-3-[({L1-(?.-chloro-6-isopmpoxybenzyl)-4-4 Calculated (M+H)+=
hydroxy-5-methyl-Z-oxo-I,Z-dihydropyridin-3- 558.20; Found
(M+H}'"
yl]amino}carbonyl)amirlo]-3-(3- = 558.02.
ethoxyphanyl)propanoic acid
(3S)-3-[( f [1-(2-chloro-6-ethaxybenzyl)-4-hydroxy-5-3 Calculated (M+~-I)+
methyl-2-oxo..l,2=dihydropyridin-3- 558.20; Found
. (M+H)+
yl]amino}carbonyl)amino]-3-(3- = 558.07.
propoxyphenyl)propanoic acid
(3S)-3-(3-butoxyphanyl)-3-(( f Ll-(2-chloro-6-4 Calculated (M+H)+
etho7cybenayl}-A-hydtoxy-5 methyl-2-oxo-1,2- 572.22; Found
(M+H)r
dihydropyridin-3- = 572.04.
yl]amino}catbonyl)amino]propanoic
acid
(3S)-3-[({[5-chloro-1-(Z-chloro-6-ethoxybenzyl)-4-3 Calculated (M+H)+=
hydroxy-2-oxo-1,2-dihydropycidin-3- 564.13; Found:
(M+1~+
yl]amino}caibonyl)arnino]-3-(3- ~ S63.99.
ethoxyphenyl)propanoic acid
(3S)-3-[(~([1-(2-chloro-6-ethoxybenzyl)-4-hydroxy-2-3 Calculated (M+H)+
axo-1,2-dihydropyridin-3-yl]amino}carbonyl)aminoj_ =544.19; Found
3-(3-isop=opoxyphenyl}gropaaoic (M+I3)* ~ 544.06.
acid
(3S)-3-[({[I-(2-chlorobenzyl)-4-hydroxy-2-oxo-l Calculated (M+I~+
2,5,6,7-tetrahydro-1H-cyclopenta[b]pyridin-3- =524.1b; Found
yI]amino}carbonyl)amino]-3-(2,3-dihydro-1- (M+H)t = 524,03.
benzofuraa-5-yI)propaaoic acid
(3S)-3-[( f [2-(2-chloro-6-ethoxybenzyl)S-hydroxy-6-7 Calculated (M+I-~*
s
methyl-3-oxo-2,3-dihydropyridazin-4- 515.19; Found
(M+I-17*
yl]amino}carbonyl)amino]-3-(4- = 515.05.
methylphenyl)gropaaoic acid
CA 02366800 2002-O1-07
-176-
(3S)-3-[({[1=(2-chlo=o-6-ethaxybenzyl)-4-hydroxy-2-3 Calculated (M+H)+=
oxo-2,5,6,7-tetrahydro-il:I-cyciopenta[b~yridin-3- 584.21; Fouad
(M+H)+
yl]amino}carbonyl)amiuo]-3-(3- - 584.10.
propoxyQhenyl~ropanoic acid
(3S)-3-{({[2-(2-chlora-6-iethoxybenzyt)-5-hydmxy-6-3 Calculated (M+I~''=
methyl-3-oxo-2,3-dihydropyridazin-4- 545.18; Found
(M+H)+
yl]amino}carbonyl)amino]-3-(3- = s45.05.
ethoxyphenyi)propanoic acid
(3S)-3-[({[2-(2-chioro-6-ethoxybenzyl)-5-hydroxy-6-2 Calculated (M+H)*
=
methyl 3-oxo-2,3-dihydropyddazin-4- 559.20; Found
(M+~I)+
yl]amino}carbonyl~mina]-3-(3- ~ 559.04.
isopzopoxyphenyi~ropanoic acid
3S)-3-[({[I-(2-chloro-6-ethoxybenzyl}-4-hydroxy-2-d Calculated (M+H);
_
oxo-2,5,6,7-tetrahydro-IH-cyclopenta[b]pyridin-3- 610.23; Found
(M+I~~'
yl]amino}carbonyl)amino]-3-[3- = 610.14.
(cyclopentyloxy)phenyl]propanoic
acid ,
(3Sr3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-'7 Calculated (M+H)+
a
2,5,6,?-tetrahydro-1H-cyclopgnta[b]pyridin-3- 566.21; Found
(M+H)+
yl]amino}carbonyi)amino]-3-[3- = 566.09.
(cyclopontyloxy)phenyljpropanoic
acid
(3S)-3-j({[1~(2-chloro-6-etttoxybenzyl)-4-hydroxy-2-2 Calculated (M+H)*
oxo-2,5,6,7-2etxahydra-1H-cyclopenta[b]pyridin-3- 526.17; Found
(M+H)+
yl]amino}carbotzyl)amino]-3-phenyipropanoic = 526.07.
acid
(3S)-3-[({[1-(2-chiorobenzyl)-4-hydroxy8 Calculated (M+H)+=
2-oxo-
2,5,6,7-tetrahydro-1H-cyclopenta[b]pyridin-3- 482.15; Found
(M+H)+
yl]amino}carbonyl)amino]-3-phenylpropanoic = 482.07,
acid
(35)-3-(({[1-(2,chloro-6-methytbenzyl)-4-hydroxy-5-5 Calculated (M+H)~'
_
'
rwethyl-2-oxo-1,2-dihydropycidin-3- 512.3.6; Found
(M+H)'
yl]amino}carbonyl)amino]-3-(2;3-dihydro-1- -- 512.03.
benzofuran-5-yl)prapanoic acid
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-4 Calculated (M+H)+=
2,5,6,7-tetrahydro-1H-cyciopenta[b]pyridin-3- 594.21; Found
(M+H)+
yI]amino}carbonyl)amino]-3-(1,3-diethyl-2-oxo-Z,3- = 594.05,
dihydro-IH-ber~zimidazol-5-yl)pmpanoic
acid
(3S)-3-[({[1-(2-chioro-6-ethoxybenzyl}-4-hydroxy-5-3 Caloulated (1VI+H)t
=
methyl-2-oxo-1,2-dihydmpyridin-3- 568,15; Found
(M+H)~
yl]amino}carbonyl)amino]-3-[3- = 568.00.
(trifluoromethyl)phenyl)propanoic acid
CA 02366800 2002-O1-07
-177-
(3S)-3-[({[1-(2-chloro-6-ethoxybenzyl)-4-hydroxy-S-4 Calculated (M+H)*--
methyl-2-oxo.l,2-dihydropyridin-3- 584.14; Found
(M+H)*
yl}amino}carbonyl)aminoj-3-[3- = 584.01.
(trifluororneihoxy)phenyllpropanoic
acid
(3S)-3-{[({1-[2-chloro.6-(2-methoxyethoxy)benzyl]-4-6 Calculated (M-Fi)-=
hydroxy-2-oxo-2,5,6,7-tetrahydro-1H= 568.18; Found
(M-H)'
cyclopenta[bjpyridia-3-yI}amino)carbonyljamino}-3- = 568:03.
(4-methylphanyl)propanoic acid
(3S)-3-{[({ 1-[2-chloro-6-(2-methoxysthoxy)bez~zyl]-4-4 Calculated (M-H)'
_
hydrQxy-2-oxo-2,5,6,7-tetrahydro-1H- 598.19; Found
(M-H}'
cyclopentatb]pyridin-3-yl}amino)carbonyl]amino}-3- = 598.01.
(3-ethoxyphenyl)propanoic acid
(3S)-3-[({[1-(2chlorobenayl)~-hydroxy-Z-oxo-2,5,6,7-4 Calculated (M+H)+=
teirahydro-1Fi-cyclopeata[bJpyridin-3- 538,17; Found
(M+I-p+
yljamino} carbonyl)aminoj-3-[3- = 538.09.
(cyclopropyloxy)phenyljpropanoic
acid
(3S)-3-[({[1-(2-chlom-6-ethoxybenzyl)~-hydroxy-5,6-4 Calculated (M-H)-=
dimethyl-2-oxo-1,2-dihydmpyridin-3- 556.19; Found
(M-H)'
yl]amuno}carbonyl)amino]-3-(3- ~ 556.02.
ethoxyphenylJpropanoic acid
(3S)-3-[({[I-(2-chloro-6-ethoxybenzyl)-4-hydroxy-5,6-4 Calculated (M-H)'-
dirnethyl-2-oxo-1,2-dihydropyridin-3- 526.17; Found
(M-H)'
yI]amino}carbonyI)amino]-3-(4- = 526.02.
methylphersyl)propanoic acid
(3S)-3-[({[1-(2-chloro-6-ethoxybenzyl)-5-ethyl-4-4 Calculated (M-H)'=
hydroxy-6-methyl-2-oxo-1,2-dihydropyridin-3-, 570.20; Found
(M-H)'
yl]amino}carbonyl)aminoj-3-(3- = 570.04.
ethoxyphenyl}propanoic acid
(3S)-3-[({[1-(2-chloro-6-ethoxybenzyl)-5-ethyl-4-4 Calculated (M-H)-=
hydroxy-6-methyl-2-oxo-1,2-dihydropyridin-3- 540.19; Found
(M-H)'
yljamino}carbonyl)amino)-3-{4- = 540.05.
methylphenyl)propanoic acid
(3S)-3-[({[I-(2-chlorvbenzyl)-4-hydzoxy-5-methyl-2-25 Calculated (M+II)*
oxo-1,2-dihydropyridin-3-yl]amino}carbonyl)amino]-3- 562,09; Found
(M+H)+
(Z'-methoxy-1,1'-biphenyl-4-yl)propanoic = 562.17.
acid
(3S)-3-[({[1-(2-chloro-6:ethoxybenzyl)-~-hydroxy 5,6- 3 Calculated (M-H)'=
dimethyl-2-vxo-1,2-dihydropyridin-3- 570.20; Found (M-H)'
yl]amino}carbonyl)amino]-3-(3- = 570.00. w
isopropoxyphenyi~ropanoic acid
(3S)-3-[({[1-(2-ehloro-6-ethoxybenzyl)-4-hydroxy-5,6- 4 Calculated (M-H)'=
dimethyl-2-oxo-1,2-dihydropyridin-3- 512.16; Found (M-H)-
yl]amino}carbonyl)amino]-3-phenylpropanoic acid = 512.01.
CA 02366800 2002-O1-07
-178-
(3S)-3-[({[l-(2-chloro-6-ethoxybenxyl)-5-ethyl-45 Calculated (M-H)-=
hydroxy-6-methyl-2-oxo-1;2-dihydropyridin-3- 584.22; Found (M-H)'
yl]amino}carbonyl)amino]-3-(3- = 584.03.
isopropoxyphenyl~ropanoic acid
(35)-3-[({[1-(2-chloro-6-athoxybenzyl)-5-ethyl-4-4 Calculated (M-H)' _
hydroxy-6-methyl-2oxo1,2-dihydropyridin-3- 526.17; Found. (M-H)'
yljatniao}carbonyl)amino]-3-phenylproganoic = 526.00.
acid
(3S)-3-[({[1-(2-chloro-6-ethoxybenzyl)-4-hydroxy-5-6 Calculated {M-H)'=
methyl2-oxo-1,2-dihydropyridin-3- 592.19; Found (M-H)'
yl]amino}carbonyl)ar~tino]-3-(6-ethoxy-2- = 592.00.
naphthyl)propanoic acid
(3S)-3-[({[2(2.chlombenzyl)-6-e~yl-522 Calculated {M-H)-=
hydroxy-3-oxo-
2,3-dihydropyridazin-4-yl]amino}oarbonyl)amino]-3- 483.14; Found (M-H)'
(4-methylphenyl)propanoic acid = 483.03.
(3S)-3-[(([1-(2-chlorobenzyl)~l-hydroxy2-oxo-2,5,6.7-I5 Calculated (M-I~'_
tetrahydm-1H-cyclopenta[b]pyridin-3- 536.20; Found {M-H}-
ylJamino}carbonyl)amino]-3-(3- = 535.99.
isohutylphc;nyl)propanoic acid '
(3S)-3-[({[I-(2-chlorobenzyl)-4-hydroxy-5-methyl-2-4 Calculated (M+H)t=
oxo-I,Z-dihydropyridira-3-yljemino}carbonyi)amino~-3- 509.16; Found (M+H)'"
1-methyl-IH-indol-6-yl)propanoic = 509,05.
acid
(3S)-3-[({[1-(2-chloro-6-methylbenzyl)-4-hydroxy-2-4 Calculated (M-H)' _
oxo-2,5,6,7-tetrahydro-IH-cyclopenta[b]pyridin-3- 550.17; Found (M-H)'
yljamino}carbonyl)amino]-3-[3- = 550.01.
{cyclopropyloxy)phenyl]propanoic
acid
(38)-3-[({[1-(2-chlorobenzyl).4-hydroxy-2-oxo-2,5,6,7-IS Calculated (M-H)' _
tetrahydro-1H-cyclopenta[bjpyridin-3- 574.17; Found (M-H)'
yljamino}carbonyl)amino]-3-(6-ethoxy-2- = 574:02.
naphthyl)propanoic acid
{3S)-3-[({[1-{2-chloro-6-ethoxybenryl)-4-hydroxy-2-23 Calculated (M-H)'=-
oxo-S-propyl-1,2-dihydropyridin-3- 526.17; Found (M-H)-
yl]amino}carbonyl)aminoj,3-phenylptopanoic = 526.04.
acid
(3 S)-3-[( {[ 1-(Z-chloro-6-e~thoxybenzyi}-4-hydroxy-2-22 Calculated (M-H)' _
oxo-5-propyl-1;2-dihydropytidin-3- 584.22; Found (M-H)'
yl]amino}carbonyl)aminoj-3-(3- = 584.09.
isopropoxyphenyl)proparjoic acid
(3S)-3-[{{[1-{2-chloro-6-ethoxybenzyl)-4-hydroxy-2-20 Calculated (M-H)'=
oxo-5-propyl-1;2-dihydropyridin-3- 540.19; Found (M-H)'
yljamino}carbonyl)amino]-3-(4- = 540:05.
rnethylpher~yl)propanoic acid
CA 02366800 2002-O1-07
-179-
(3S)-3-[({[1-(2-chlvro~6-ethoXybenzyl)-4-hydroxy-2- 6 Calculated (M-Fi)-s
oxo-S-prapyl-1,2-dihydropyridin-3- 570.20; Found .(M-H)'
yl]amino}carbvnyl)amino]-3-(3- = 570.04.
ethoxyphenyl)propanoic acid
(3S)-3-[({[1-(2-chlorobanzyl)-4-)'ydroxy-2-oxo-1,2- 40 Calculated (M-~~=
dihydropyxidin-3-ylJamino}carbonyl)aminoJ-3-(4'- 530.15; Found (M-H)-
methyt-1,1'-biphenyl-4-yl)propanoic acid = 530.02.
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-2,5,6,7-4 Calculated (M-H)'=
tatrahyd3~o-1H-cyclopeata[b]pyridin-3- 533.16; Found
(M-H)-
yl]amino}~;arbonyl)aminoj-3-(I-methyl-1H-indol-5- = 533.00.
yl)pzopaanaic acid
(3S)-3-[({~:1-(2-chloro-6-ethoxybenzyl)-5-cyclopmpyl-3 Calculated (M-H)'
4-hydroxy-2-oxo-1;2-dihydmpyridin-3- 582,20; Found
(M-H)'
ylJamino}carbonyl)aminoj-3-(3- = 582;07,
isopropoxyphenyl)propanoic acid
(3S)-3-[({[1-(2-chlom-6-etboxyber~zzyl}-5-cyclopropyl-3 Calculated (M-H)'
_
4-hydroxy-2-oxo-1,2-dihydropyridin-3- 538.17; Found
(M-H)'
yljamino}carbonyl)aminoJ-3-(4- = 538.06.
methylphe~nyl}propanoic acid
(3S)-3-[({[1-(2-chloro-5-propoxybenzyl)-4-hydroxy-5-6 Calculated (M-H)'=
methyl-2-oxo-1,2-dihydropyridin-3- 526.1?; Found
(M-H)'
yljamino}carbonyI)aminoj-3-(4- = 526.05.
methylphenyl)propanoic acid
(3S}-3-[({[1-(2-chloro-5-methoxybenzyl)-4-hydroxy-S-3 Calculated (M-H)'
_
methyl-2-axo-1,2-dihydropyridin-3- 498. I4; Found
(M-H)'
yl)amino}carbonyl)aminoJ-3-(4- = 498.01.
methylpbenyl~ropanoic acid
3-[( { [ 1-(2-chloro-6-ethvacybenzyl)-4-hydroxy-5-methyl-I Calculated (1vI-
H)-
3
2-oxo-1,2-dihydropyridin-3-yl]amino}'carbonyl)amino)- 548.Ifi; Found
(M-H)'
3-(2-naphthyI)~ropanoic acid = 548.01.
3-[({[1-(2-chtoro-6-ethoxybenzyl)-4-hydroxy-5$ Calculated (M-Fi)-=
methyl-
2-oxo-1,2-dihydropytidin-3-yt]amino)carbonyl)aminoJ- 576.12; Found
(M-H)'
3-[4-(methylsulfonyl)phenyt]propanoic = 576,00.
acid
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-1,2- 27 Calculated (M-H)-=
dihydropyridin-3-yl]amino}carbonyl)amino]-3-(3'- 560.16; Fourrd (M-H)'
ethoxy 1,1'~biphenyl-4-yl)propanoic acid = 560.04.
(3S)-3-[({[1-(2-chloro-6-mathylbenzyl)-4-hydroxy-2- 20 Calculated (M-H)'=
oxo-2,5,6,7-tetrahydro-1H-cyclopenta[bJpyridin-3- 564.19; Found (M-H)-
yl]amino}carbonyl)aminoJ-3-[3- = 564.00.
(cyclobutyloxy)phenyl)propanoic acid
CA 02366800 2002-O1-07
-I80-
(3S)-3-I({[1-(2-chtorobea~zyl~4-hydroxY-2-oxo-2,5,6,7-17 Calculated (M-H)'
'
tetrahydm1H-cyclopenta[b)pyridin-3- 550.17; Found (M-H)-
yl)amino}carbonyl]-3-(3- = 550.02.
(cyclobutyloxy)phenyl)propanoic
acid
(3S~3-j({[1-(2-ehloro-6-ethoxybenzyl)-4-hydroxy-6-3 Calculated (M-H)-$
methyl-2-oxo-1,2-dihydtopyridin-3- 556.19; Found (M-H)-
yI)amino} carbonyl)amirio)-3-(3- = 556.05.
isopropoxyphenyl~ropaaoic acid
3-[({[1-(2-chlorobenzyl)-4-hydroxy-5-methyl-2-oxo-IO Calculated (M-H)'=
I,2-dihydropyridin-3-yl)smino}carbonyt)aminoj-3-(3- 523.17; Found (M-H)-
pyrrolidin-I-ylghenyl)propanoic = 522.99.
acid
3-(({[1-(2-chlorobenzyi)-4-hydroxy.5-methyl-2-oxo-22 Calculated (M-H)-=
1,2-dihydzopyridin-3-yl]amino}carbonyl}amino]-3-(3- 537.19; Found (M-H)'
piparidin-I-ylphenyl)propanoic acid ~ 537.08.
(3S)-3-[({[1-(Z-chloro6-methylbenzyl~4-hydroxy-2-22 Calculated (M-I~'=
oxo-2,5,6,7-tetrahydro-IH-cyclopenta(b]pyridin-3- 580.22; Found (M-H)'
yi)amino}carbonyl)amino]3-[3-(1- = 580.04.
ethylpropoxy~henyl]propanoic acid
(3S)-3-[({[1-(2-chlonobenz~)-4.hydroxy2fl Calculated (M-H)'-
2-oxo-2,S,G,7-
tetrahydro-1H-cyclopenta[b)pyridin-3- 566.20; Found (M-H)'
yI]amino}carbonyl)amino)-3-(3-(1- = 566.01.
ethylpropoxy)phenyl)propanoic acid
(3S)-3-(~1-chloro-3-isopropoxyphenyl)-3-j({[1-(2-23 Calculated (M-H)-=
chloro-6-methyibenzyl)-4hydroxy-2-oxo-2,5,6,7- 586.15; Found (M-H)'
tetrahydro-1Fi-cyclopentajb]pytidin-3-~ ~ 585.92.
yl}amino}carbonyl)arnino)propanoic
acid
(35)-3-[( f [ 1-(Z-chlorobenzyl)~4-hydroxy-2-oxo-2,5,6,7-38 Calculated (M-
H)' _
tetrahydro-1H-cyclopenta[b]pyridin-3- 572.14; Found (M-H)'
yl)amino)carbonyl)amino]-3-(4-chloro-3- = 572.00.
isopropoxyphenyl~ropanoic acid
(3S)-3-[({[I-(2-chIorobenzyl)-4-hydroxy-2-oxo-I,2-30 Calculated (M-H)-=
dihydropyridin-3-yl]amino}carbonyt)amino)-3-(3'- 530.15; Found (M-I~'
methyl-1,1'-biphenyl-4-yl}lsropanoic = 530.02.
acid
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-2,5,6,7-3 Calculated (M-H)'=
teirahydrwlH-cyclopenta jb)pytidin-3- 533.16; Found (M-H)'
yl)amino)carbonyl)amino]-3-(1-methyl-1H-indol-6- = 532,97.
yl)propanoic acid ._.
(3S)-3-[({[I-(2-chloro-6-ethoxybenzyl)-4-hydroxy-5-3 Calculated (M-H)'=
methyl-2-oxo-1,2-dlhydmpyridin-3- 551.17; Found
( M-H)'
yl]amino}carbonyl)amino]-3-(1-methyl-1H-indol-6- = 551.02.
yt)propanoic acid
CA 02366800 2002-O1-07
-181-
(3S)-3-[({[1-(2-chlorobenxyl)-4-hydroxy-5-methyl-2-Calculated (Nf-H)'=
23
oxo-1,2-dihydropyridia-3-yl~amino}carbonyi)amino]-3-550.16; Found
(M-H)'
(4'-methoxy 1,1'-biphenyl-4-yl)propanoic= 560.01:
acid
(38)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-5-methyl-2-Calculated (M+H)+=
55
oxo-1,2-dihydropyridin-3-yl]amino}carbonyl)aminoJ-3-546.18; Found
(M+I~+
(2'-methyl-1,1'biphenyl-4-y1)propanoic = 546.11.
acid
(3S)-3-[({[i-(2-ohlorobenzyl)-4-hydmxy-Z-oxo-2,5,6,7-3 Calculated (M=F~'=
tetrahydro-1H-cyclopenta~b]pyridin 560.16; Found
3- (M-H)'
yl]amino}carbonyl)amino]-3-(6-methoxy-2- = 560.00.
naghthyl)propanoic acid
(3S)-3-(4-chloro-3-ethoxyphenyl)-3-[({[1-(2-chloro-6-~!5 Calculated (M-H)'=
methylbenzyl)-4-hydroxy-2-oxo-2,5,6,7-tetrahydro-1H- 572.14; Found
(M-H)'
cyclopenta[b]pyridin-3- = 571.94.
yl]amino}carbonyl)amino]propanoic
acid
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-2,5,6,7-30 Calculated (M-H)'=
tetrahydxo-1H-cyclopenta[b]pyridin-3- 558.12; Found
(M-H)-
yljamino}catbonyl)amino]-3-(4-chloro-3- = 557,77.
ethoxyphenyl)propanoic acid
(3S)-3-[({[i-(2-chloro-6-ethoxybenryl)-4-hydroxy-2-4 Calculated (M+H)+=
oxo-2,5,6,7-tetrahydro-1H-cyclogenta[b~pyridin-3- 582.24; Found
(M+H)t
yl]amino}carbonyl)amino)-3-(3- = 582.10.
isobutylphenyl)propanoic acid
(3S)-3-[({[1-(2-chloro-S-ethoxybenzyl)-4-hydroxy-5-~4 Calculat~d (M+H)+=
methyl-2-oxo-1,2-dihydropyridin-3- 514.17; Found
(M+H)*
yl]amino}carbonyl)aminoj-3-(4- = 514.08.
methylphenyl)propanoic acid
3-[({[I-(2-c:hlorobenzyl)-4-hydroxy-5-methyl-2-oxo-134 Calculated (M+H)+=
1,2-dihydro~yridin-3-yl]amino}carbonyl)amino]-3-[4- 534.11; Found
(M+H)+
(methylsulfonyl)phenyl]propanoic = 534.07.
acid
(3S)-3-[(![1-(Z-chlorobenzyl)~-hydroxy-2-oxo-2,5,6,7- 225 Calculated (M+H)* _
tetrahydro-1H~cyclopenta[b]pyridin-3- 594.09; Found (M+H)*
yl]amino}carbonyl)amino]-3-(2,4-dichIoro-3- = 593.98.
ethoxyphenyl)propanoic acid
(3S)-3-{[({1-[2-chlom-5-(piperidin-1- 27 Calculated (M-H)-=
ylsulfonyl}b~enzyl]-4-hydroxy-5-methyl-2-oxo-1,2- 615.17; Found (M-H)'
dihydropyridin-3-y1}amino)carbonyl]amino}-3-(4- x 615.04.
methylphenyl)propenoic acid .--
(3S)-3-{[({1-[2-chloro.5-(pyaolidin-1- 15 Calculated (M-H)' _-
ylsulfonyl)benzyl]-4-hydroxy 5-methyl-2-oxo-1,2- 601.15; Found (M-H)'
dihydropyridin-3-yl}amino)carbonyl]amino}-3-(4- = 601.03:
methylphenyl~ropanoic acid
CA 02366800 2002-O1-07
-182-
(3S)-3-[({[I-(2chloro-6-ethoxybenzyt)-4-hydroxy-2-Z Calculated (M+H)*
_
oxo-Z,S,6,7-tetrahydro-1H-cyclopenta[bJpyt;din-3- 582,20; Found
(M+H)*
ylJataiao}carbonyt)aznir~oJ-3-[3- = 582.10.
(cyclopropyloxy)phenylJpropanoic acid
(3S)-3-{[({I-[2-chloro-6-(cyctopentylmethoxy)benzyl]-20 Calculated (M-H)-=
4-hydroxy-S-methyl2roxo-1,2-dihydropyridin-3- 566.20; Found
(M-F>7-
yl}amino)carbonyl]amino}-3L(4- - = 566.09.
rnethylphenyl)propaaoic acid
{3S)-3-{[({1-[2-(benzyloxy)-6-chlorobenzyl]-4- 10 Calculated (M-H)'
hydroxy-S-methyl-2-oxo.l,2-dihydropyridin-3- 574.17; Found
(M-I~'
yl}atniao)carbozlylJamino}-3-(4. = 574.01,
methylphenyljpropanoic acid
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-2-oxo-2,5,6,7-3 Calculated (M+I~+=
tetrahydro 1 H-cyclopenta[bJpyridin-3- 604.16; Found
(M+I-i~*
ylJamino}carbonyl)amino]-3-(3-chloro-4,5- = 604.02.
diethoxyphenyl)propanoic acid
(3S)-3-[({[1-(2-chloro-6-methylbenzyl)-4-hydroxy-2-S00 Calculated (M+H)*=
oxo-2,5,6,7tetrahydrv1H-cyclopenta[bJpyridin-3- 652.14; Found
(M+H)*
ylJamino}carbonyl)amino]-3-(2,4-diehloro-3,S- = 651.98.
diethoxyphenyl)propanoio acid
(3S)-3-[({[1-(2-chlorobenzyi)-4-hydroxy-2-oxo-2,5,6,7-450 Calculated (M+H)+=
tetrahydro-IIi-cyclopenta[b]pyridin-3- . 638.12; Found
(M+F~''
ylJamino}carbonyl)amino]-3-(2,4-dichloro-3,5- = 637.97.
diethoxyphenyl)propanoic acid
(3S)-3-[({[1(2-chlorobenzyl)-4-hydroxy-2-oxo-2,5,6,7-,9 Calculated (M+H)+=
tetrahydro-1H-cyclopenta[bJpyridi:. 3- 552.19; Found
(M+H)'"
ylJamino}casbonyl)amino]-3-[3- = S52.10.
(cyclopropylmethoxy)phenyl]propanaic acid
(3S)-3-[({(1-(2-chloro-6-ethoxybenzyI)-4-hydroxy-2-4 Calculated (M+H)*=
oxo-2,5,6,7-tetrahydro-1H-cyclopenta[b]pyridir~-3- 596.21; Found
(M+H)*
ylJamino}carbonyl)amincJ-3-[3- = 596.11.
(cyclopropylmethoxy)phenylJpropanoic acid
(3S)-3-[({[1-(2-chloro-6-methylbeazyl)-4-hydroxy-2-10 Calculated (M+H)+
_
oxo-2,5,6,7-tetrahydro-1H-cyclopenta[bJpytidin-3- 566.20; Found
(M+H)'
ylJamino}carbonyl)amino]-3-[3- = 566.12.
(cyclopropylmethoxy)phenylJpmpanoic acid
(3S)-3-[( {jI-(2-chlorobenzyl)-4=hydroacy-5-methyl-Z- 13 Calculated (M-H)' _
oxo-1,2-dihydmpyiidin-3-yl]amino}carbonyl)aminoJ-3- 544.16; Found (M-I~-
(2,4.diethaxypyrimidin-5-yl)propanoic acid = 544.00.
CA 02366800 2002-O1-07
(3S)-3[({[1-(2,3-dichloro-6-ethoxyZyl)-4-hydroxy-5 Calculated (M-H)' =
2-oxo.z,5,6,7-tetza#~ydro-lI~-cyclopenta['b)pyridin-3- 5?2.t3; Found (MH).
yl]amino}carbonyt)atnina]-3-(4- = 571.97.
methylphsnyl)propanoic acid
(35~;3-j3-(cyoloPropYlmathoxy~henyl3-3-[({L1-(2,3-7 Calculated (M-H)-~ .
dichloro-6=ethoxybeazyl)-4-hydroxy-2-oxo-2,5,6,7- 628;16; Found (M-H)'
tetrahydro-1H-cyclopenta[b]pyridin-3 = 627.98.
yl]amino} carboayl)amzno]propairoic
acid
(3S)-3-[({[1-(2,3-dichloro-6-athoxybenzyl~3 Calculated (M-I~'=
hydroxy-
2.oxo-2,5,6,7 ydro-1H-cyclopenta[b)pyridin-3- 602.15; Found (M-H)'
yljamino} carbonyl~mir~o]-3-(3- = 601.99.
eti'oxyphenyl)propartoic acid ,
(3S)-3-j( f jl-(2.3dichloto-6-ethoxybenzyl)-4-hydtoxy-5 Calculated (M-H)'
Z-oxo-2,5,6,7-tetrahyc~ro-1H-cyclapentajb]pyridin3- 616.16; Fotund (M-H)'
yl]amino}carbonyl)aminoj-3-(3- = 616.01.
isopropoxypheayl~ropanoic acid
(3S)-3-({tjI-(2-chlarobenzyl)=4-mathoxy-2-oxo-1,2-2000Calculated (M-~I)-=
dihydmpyridit~.3-yl](methyl)amino)carbonyl}amino)-3- 482.14; Foand (M-H)'
(4-methylphenyl)propanoic acid = 482.07.
(3S)-3-[({[1-(2-chlotob~zyl~4-lzydroxy-S-methyl-2-15 Calculated (M-I~-=
.
oxo-1,2-dihydropyridia-3-yl]aasino}earbonyl)arz~ino)-3- 560.16; Found (MH)~
(2~-methoxy 1,1'-biphenyl-3-yl~ropanoio ~ 559.98.
acid
3-j({jl-(2-ehloroberazyl)-4-hydroxy-5-methyl-2-oxo-20 Calculated (M-1~'
1,2-dihydrapyddin-3-yl]amino}carboayl)amino]-3-(5- 458,11; Found (M-H)'
rrtethyl-Z-furyl)propanoic acid = 457.99, '
3-[({jl-(Z-chloro-6-metbylben2yl)-4-hydroxy43 Calculated (M~-H)+
5-methyl-
Z-oxo-l,2-dihydropyridin-3-yl]amino}carbonyl)amino]- 548.13; Found (M+F~+
3-j4-(methylsulfonyl)phenyl]propanoic ~ 548.07.
acid
3-[({[1-(2-ehlorobenzyl)-4-hydroxy-2-oxo-2,5>6,7-5 Calculated (M-H)'=
tetrahydm-1H-cyclopenta[bjpyridin-3- 470.11; Found (M-I~'
yljamino}carbpnyl)amino]-3-(2-furyl)gropanoic = 469.96.
acid
3-j({j1-(2-chtorobenzyl}-4-hydroxy-5-methyl-2-oxo-4 Calculated (M-Id's
i,2-dihydropyridin-3-yl]amino}carbonyl)amiao)-3-(2- 444.10; Found (M-H)'
fuzyl~ropano':c acid = 443 .91.
(3S)-3-j({jl-(2-chlorobenzyl).4-hydroxy.2-oxo-2,5,6,718 Calculated (M-I~' =
tetrahydm-1H-cyclopentajb]pyridin-3- 548.12; Found (M-H)' , ._.
yl]amino}carboayl)amino]-3-[4- = 548.40.
(trifluoromethyl)phenyl]propanoic
acid
CA 02366800 2002-O1-07
-1~4-
(3S)-3-[(~[jl-(2-chlorobenayl).4-hydroxy-2-a~ca-2,5,6,7-5 Calculated (M-H)'
tetrahydro-1H-cyclopenta[bjpyxidin-3- 494.15; Found (M
1i)'
yl]amino}carbonyl)amino]=3-(3_ C 494.02:
methylphenyljpropaaoic acid
(3S)-3-j({El-(2-chlombenzyl)4-hydroxyi0 Calculated (M-.~'=
2-oxo-2,5,6,7-
tetrahydro-lei-cy~clopenta{b]pyrldin-3- S48.I2; Found {M-~'
yI]amino}carbouyl)amino]-3-(3- ~ 547.99. ,
(trifluoroxnethyl~henyl]propanoip
acid
(3S)-3-[({(1-(2-chlvrobenzyl)-4-hydroxy9 Calculated (M-H)-=
2-oxo-2,5,6,?-
tetrahydro-1H-cyclopents[b]pyridin-3- 508.16; Found (M-H)'
yljamino}carbonyl)amino]-3-(3,5- = 508:02.
dimethylphenyl)propanoic acid
(3S)-3-[3,5-bis{txifluoromethyl)phenyl]-3.(({[1-(2-I30 Calculated (M-H)-=
chlorobenzyl).4 hydroxy 2oxo-2,5,6,7-tetrahydro-1H- 415.11; Found (M-H)'
cyclopenta[b]pyridin-3- = 615.99.
yl]amino}carbonyl)amino]propanoic
acid
(3S)-3:{[(I-[2-chloro-5-(trifluoromethyl)bet~aylj46 Calculated{M-H)'=
hydroxy-5-methyl-2-oxo-1,2-dihydropyidin-3- ~3b.12; Found (M-H)'
y1# amino)carbonyl]amino}-3-(4- = 535.99,
methylphenyl)prapanoic acid
(3Sr3-[({{1-(2-chloro-5-fluorobenZyl).45 Calculated (M-H)-=
hydraxy-S-
methyl-2-oxo-1,2-dihydropyridin-3- 486.12; Found (M-H)'
yljamino}carbonyl)amino]-3-(4- = 485.97.
methyiphenyl)pmpanoic acid
(3S)-3-[({[1-(2-chlorobenzyl)-4-hydroxy-5-methyl-2-2 Calculated (M-H)'
oxa-1,2-diltydropyridin-3-yI]amino}carbonyl)amino]-3- 525.19; Found (M-H)'
(3-(diethylamino)phenyl]propanoic = 525.00.
acid
3-(1,1'-biphenyl-4~yl)-3-[( f [1-(2-chlorobenayl)-4- 30 Calculated (M-H)' _
hydroxy-2-oxo-2;S,b,7-tetrahydro-1 H- 556.16; Found (M-H)'
cyclopenta(b]pyridin-3-
= 555.99.
yljamino}carbonyl~mino]pmpanoic acid
(3S)-3-j{{[1-(2-chlot~obenzyl)-4-hydroxy-2-oxo-2,5,6,7- 8 Calculated (M+H)+=
tetrahydro-1H-cyclopenta(b]pyridin-3- 522,17; Found (M+H)'~
yI]amino}carbonyl)amino]-3-(2,3-dihydro-1H-inden-S- = 522.03.
yl~rapanoic acid
(3S)-3-j(([1-(2-ehloxo-b-methylbenzyl)-4-hydroxy-2- 10 Calculated (M+H)+= -
oxo-2,5,6,7-tetrahydro-lH-cyclopenta[bapyridix~-3- 536.19; Found (M+I~+
yl]amino}carbonyl)amino]-3-(2;3-dihydro.lH-inden-5- = 536.08.
yl)propanoic acid .-.
CA 02366800 2002-O1-07
-1$5-
N-{1-[(2-ohlorophenyl)methyll-4-hydroxy-5-methyl- 6000 Calculated (M+fi)+=
2-oxa-1,2-dihydro-3-pycidinyl}-N'-((1S~I-(4- 494.17; Found (M+H)+
methylphenyl)-2-(1H-I,2,3,4-tetraszol-5-yI)ethyl]urea = 494.0I.
{3S)-3-[1,1'-biphs~nylJ-3-yI-3-{[({I-[{2-17 Calculated (M-H)-=
chlorophenyl)ttrethyl]-4-hydroxy-2-oxo-2,5,6,7- 558.16; Found
(M-H)'
tetrahydro-1Fi-cyciopenta[b~yridin-3- = 556.01.
yI}amino)oarbanyi~amino}propanoic
acid
(3S)-3-{[({1-[(2-chlorophenyl)methyl]-4-hydroxy-2-13 Caloulated(M-I~'=
axe-2,5,8,7-tet<ahydro-1H-cyclopentajb]pyridin-3- 564.11; Found
(M-fTj'
yI}amino)carbonyl]amino}-3-{4- = 564.QI.
[(trifluoromet>syl)oxy]phenyl}propanoic
acid
(3S)-3-{[({1-[(2-chlorophenyl)methyl]-4-hydroxy-2-13 Galculatsd (M-H)-=
oxo-2,5,6,7-tetrahydro-1H-cyclopentajb]pyridin 546.12; Found
3- (M-H)-
yI}amino)carbonylJamino}-3-{4- = 545.97.
[(difluorornethyl)oxyJphenyl}propanoic
acid
(3S)-3-{[({1-[(Z-chlorophenyl)methyl}-4-hydroxy-2-10 Calculated (M-Ii~'=.
oxo-2,5,6,7-tstrahydro-IH=cyclopenta[b]pyridin-3- 564.11; Found
(M-H)'
yl}aminoxarbonyl)art~ina}-3-{3- ~ 563.98.
[(trifluozomethyt)oxy)phenyl}prapanoxc
acid
(3S)-3-{(({1.:[(2-chlorophenyl)rnethyl]-4-hydroxy5 Calculated (M-H)'=
2-
oxo-2,5,8,7-~etrahydro-1H-cyclopenta[bJpyridin-3- 54b.12; Found
(M-H)-
yl} amino)caxbonyI]amino} -3- f = 546.01.
3-
j(difluoromethyl)oxyJpheayl}propanoic
acid
(35)-3-.([({I-[(2-chIoropheuyl)methyl]-4-hydroxy-2-4 Calculated (M-H)'=
oxo-2,5,6,7-tetrahydro-lI-I-cyciopenta(b)pyridin-3- 595,12; Found
(M-H)'
yI}amino)carbonyl]amino}-3-{3-[(1,I,2,2- = 596.02.
tetrafluoroethyl)oxy)phenyl}p~ropanoic
acid
(3S)-3-{[({ 1-[(2-chlorophcnyl)methyl]-4-hydroxy-Z-11 Calculated (M-H)'
oxo-2,5,6,7-tetrahydro-1H-cyciopenta[b)pyridin-3- 538.17; Foand
(M-H)-
yI}amino)carbonyl]amino}-3-[3,5-dimethyl-4- = 538.04.
(nnethyloxy)phenyl]propanoic acid
(3S)-3-{[({1-((2-chlorophenyl)methyl]-4-hydroxy-2-5 Calculated (M+H)''
_
oxo-2,5,6,?-tet:ahydro-IH-cyclogenta[bjpyridin-3- 549.19; Found
(M+H)~'
yl}amino)carbonyljamino}-3-(I-ethyl-IH-indoI-5- ~ 549,02.
yl)propanoic acid
(3S)~3-{[{{I-[(2-chlorophetryl)znetbylJ-4-hydroxy-2-7 Calculated (M-H)'=
oxo-2,5,5,7-totrahydro-3 H-cyclopenta[b]pyridin-3- S I 6.11; Found
(M-H)-
yl}amino)carbonyl]amino}-3-{3,5- ~ 516.01.
difZuoroplteayl)propanoic acid
CA 02366800 2002-O1-07
-I s5-
L3S)-3-{C({1-[(2-chloro'PIumYYI?methylJ-4-hydroxy-2-3 Calculated (M-H)'
oxa-2,S,b,7-tetc~.hydro-IH-cyclopenta[b]pyridin-3- S28.13; Faund
(M-H)'
yl~amino)carbonyl]amma}-3-[3-fiteoro-~1- = 528.00.
(methyloxy)phenylJpropanoic acid
(3S)-3- f [({1-[(2-chlorophenyl)methyl]-4-hydroxy-2-I Calculated (M-H)-
7
axo-2,5,6,7-tetrahydro-I H-cyclopenta[b]pyrydirr3- 522. l 8; Found
(M-H)'
yI}araino)carbanyl]amino}-3-(4- = 522.04.
prapylphenyl)gmpauoic acid
(3S)-3- f [({1-[(2-chlom-6-methylphenyl)methyl]-4-20 Calculated (M-H)'
_
ltydraxy-2-oxo-2,S,b,7-tetrahydro-1H- 536.20; Found
(M:H)-
cyclopenta[bjp~yridin-3-yl}atnino)carbonyl]amino}-3- = 53b.Ob.
(4-prapylphenyl~rapanoic acid
(3S~3-{[({1-[(2-chlorophenylrnethyl]-4267 Calculated (M-H)-
hydroxy-5-
mathyI-2-oxo-l,2-d~ydxo-3- 4b8.13; Found
(M-H)'
pyridinyl}amino}carbonyl]amino}-3-(2- ~ 468.00.
uu3thylphenyl)pmpanoic mid
(3$)-3-{[({I-[(2-chlorophenyl)methyl]-4-hydroxy-2-25 Catculatcd(l~i+H)+=
oxo-2,5,b,7-tetrahydro-IH-cyclopentatbJpyridin-3-
f
522.18; Found
(M+I~
yl}aminoxari~onyljamino}-3-(4- = 522.04.
cyclopropylphenyl~ropanoic acid
(3S)-3-{[({1-[(2-chlorophenyl)methyl]-4-hydroxy-5-22 Calculated (M-H)'
methyl-2-oxo.-1,2-dihydro-3- 505.13; Found
(M-H)'
pyridinyt}amitto)carbonylJamino}-3(3- = 5p4,,98,
quinolinyl)prc~panoic acid
(3S)-3-{j({I-[(2-chlorophanyl)methyl]-4.-hydroxy-2-22 Calculated (M-H)'s
oxo-2,S,b,7-tE;trahydro-IFi-cyclopemajbJpyridfn-3- 531.14; Found
(M-H)'
yl}amino)carhonylJamigo}-3-(3-quinotinyl)propanoic -- 530.99.
acid
3-({[(I-{[2-c''hloro-b-(ethyloxy)phenyi)methyl}-4-8 Calculated (M-H)-=
hydroxy-S methyl-2-oxo-1,2-dihydro-3- 488.12; Found
(M-H)'
PYna~Yl)amino]carbonyl}amino)-3-(2- = 487.98.
'
furanyl}propanoic acid
(3S)-3-[2,4-bis(ethyloxy)~5-pyriiuidirxyl]-3{[(I-[(2-1.5 Calculated (M-I~'
ehlorophenyl)methylJ-4-hydraxy-2-oxo-2,5,b,7- 570.18; Found
(M-H)'
tetrahydm-1H-cyclopentajbJpyridin-3~ ~ 570.14,
yl}amino)caxbonyi]amino}propanoic
acid
(3S)-3-{[({I-j(2.chloro-6mathylphcnyi)tnethylJ-4-19 Calculated (M+H)+_-
hydroxy 2-oxo-2,S,b,7-tetrahydro-IH- 536.20; Found
(M+F~I)i
cyclopenxa[bJpyridin-3-yt}amino)carbonyl?amino)-3- = 536.07.
(4-cycloprapylphenyl)propanoic
acid
CA 02366800 2002-O1-07
(3R)-3-{[({1-[(a-chloropheayl)methyl]-4-hydroxy-2- 15 Calculated (M-H)'
oxo-2,5,6,7-tetrahydro-1H-cyclopenta[bJpyridin-3- 4I8.12; Found (M-H)'
yI}amino)csrbonyi]amino}butanoic acid = 418.00.
(3S)-3-{[({1-[(2-ci2lorophenyl)methyl]-4-hydroxyS Calculated (M-F~'=
2-
oxo-2,5,6,7-tetrahydro-1>'I-cyclopenta[b]pyridin-3-508.16; Found (M-H)'
yl}aminokarbonyl]amino}-3-(4- = SO8.o6,
ethylphenyl)propanoic acid
(3S)-3-{[({1-[(2-chlorophenyI)methyl]-4-hydroxy-2-17 Calculated (M-H')'=
oxo-2,5,8,7-tetrahYdro-1H-cyclopenta(bJpyridin-3-522.I7; Found (1~I-H)'
yl}amino)oarb~x~yl]amino}-3-[4-(1- = 522.06.
methyletl~yl~h~nyl]propanoic acid
(3S)-3-{[({1-[(2-chlotophextyl)methyl]-4-hydro:cy-S-30 Calculated (M-H)'=
methyl-2-oxo-1.,2-dihydto-3- 482.I4; Found (M-I~'
pyridinyl} axnino)carbonyl]amino}-3:(4--- 482.00.
ethylphenyl)propanoic acid.
(3S)-3-([({1-[(2-chlompheny!)methyl]-4-hydroxy-5-I75 Calculated (M-H)'=
methyl-2-oxo-1,2-dihydro-3- ,496.16; Fonad (M-H)'
pyridiuyl}amino)carbonyl]amino}-3-[4-(I-= 496.0I.
methyleihyl)phenyl]propanoic acid
(3S)-3-{[({I-[(2-chlorophenyl)rnethyt]-4-hydroxy-5-6 Calculated (M-H)'=
methyl-2-oxo-1,2-dihydro-3- S 10.14; Found (M-H)'
pyridinyl}amino)carbonyl]amino}-3-[4- = 510.00.
(cyclvpropyloacy)phenyllpropanoic acid
(3S)-3-{[({1-[(2-chiorophenyl)methyl)-4-hydroxy-S-IZ Calculated (M-H)'
_
methyl-2-oxo-1,2-dihydro-3- 496.16; Found (M-H)'
pyridinyl}amitto)carbonyl]amino}-3-(4- = 495.99.
propylphenyl)propanoic acid
CA 02366800 2002-O1-07
-Ig~-
3-{[({1-[(2-chlorophenyl)mcthyl]-4-hydroxy35 Calculated (M-H)'
5- _
methyl-2-oxo-1,Z-dihydro-3- 494.15; Found
(M H)'
pyridinyt} amiuo)carbonyI]amino} = 494.01.
-3-(4-
cyclopropylgheayl)proganaic, acid
(3S)'3-{[({1-[(2-chlorophenyl)methyl)-4-hydroxy-5-18 Calculated (M-F~-=
methyl-2-oxo-1,2-dihydzo-3- 494.15; Found
{M-1~-
pyridirryl}amino)carbonylJamino}-3-(2,3-dibydro- = 494.02.
1H-inden-5-yl)propanoic acid
(3S)-3-{[({1-[(2-chlorophenyl)tnethylJ-4-hydroxy-2-I3 Calculated (M-H)'=
oxo-2,5,6,7-tetrahydro-1FI-cyclopenta[b]pyridin-3- 597.19; Found
(M-I~'
yl}antina)carboxyl]amino}-3-(9-ethyl-9H-carbazoi-3- = 597.01.
yt)propenoic acid
(3S)-3-{[({1-C(2-chlorophenyl)methyl]-423 Calculated (M-H)'
hydroxy-5-
methyl-2-oxo-I,2-dihydro-3- 571.17; Found
(M-H)'
pyridinyl}amine)carbonyl~amino}-3-(9-ethyl-9H- = 570.99.
carbazol-3-yl)propaaoic acid
(3S)-3-{[({I-f(2chloso-6-methylphenyl)methylJ-4-3 Calculated (M-H)'=
hydroxy 2-oxo-2,5,6,7-tetrahydro-1H- 547.17; Found
(M-H)'
cyolopenta[b)pyridin-3-yl}amino)carbonyl]amino}-3- = 547.04.
(1-methyl-1H-indol-5-yt)propanoic
acid
(3S)-3-{[({I-[(2-chloro-6-methytphenyl)methylJ-4-3 Calculated (M-H)'=
hydmxy-2-oxo-2,5,6,7-tetrahydro-1H- 560.14; Found
(M-H)'
cyclopenta[b]pyridin-3-yl}amino)carbonyl]amino}-3- = 560.03.
{3-[(difluoromethyl}oxyJphenyl}propanoic
acid
(3S)-3-{[({I-[{2-chloropheayl)methyl]-4-hydroxy-5-25 Calculated (M-H)'
methyl-2-oxo-I,2-dihydro-3- 574.17; Found
(M-H)'
pyridinyl}amino)carbonylJamino}-3-[2- = 574,00.
(ethy3oxy)[1,1'-biphenyl]-4-yllpropanoic
acid
(3S)-3-{[({1-[(2-chlorophenyl)methyl]-4-hydroxy-2-20 Calculated (M-H)'
_
oxo-2,5,6,7-tetrahydro-1H-cyclopenta[bJpyridin-3- 600.19; Found
(M-H)'
yl}amino)carbonyl]amino}'-3-[2-(ethyloxy)[1,1'- =b00.01.
,
biphenyl]-4-ylJpropanoic acid
(3S)-3-{[({1-{(2-chlorophenyl)methyl]-4-hydroxy-5-20 Calculated (1VI-H)'=
methyl-Z-oxo-1,2-dihydro-3- 544.16; Found (M-H)-
pyridinyl}amino)carbonyl]amino}-3-(Z'-methyl[1,1'-= 544.04.
biphenyl]-3-yl)propanoic acid
(3S)-3-{[({1-[(2-chlorophenyl)methyl]-4-hydroxy-S-1~ Calculated (M-H)'= ___
methyl-2-oxo-1,2-dihydm-3- 544.16; Found (M-H)'
pyridinyl}amino)carbonyl]amino}-3-(3'-methyl[i,1'-~ .--544.00.
biphenyl)-3-yl)gropanoic acid
CA 02366800 2002-O1-07
.1 R4-
(3S)-3-((tc1-rr3~lart,-f.Estral>y~o..lf2H)-90 calculated
(N~.>:1~-=
nyridiaylphcriyl]mathyh,-4-hydn~xy.5-methyl-2-oxo- 351.21; Found
(M:llj-
t,?-d~yctro-3~yridinyl)amino)c~bonyl]aluino):3-(4- ~ 551.06.
mcthylphenyl)pmpanoic arid
f3g~3-([(ft-l(2~hloroP~py1)rt~Yll-d-lrydro~y-5-23 Calcul9aad(M-ilT
cnathyl-?-oxa-1,2-d~ydro-3- 544,16; Found
(M H)'
Pyndinyltamino)ostbor~Yllamiou)-9-(4'-methyl[l.l'_ = Sd3-99.
biphenyl]-3-y1)pmpanoic acid
{3S)-3-{[(dl-[(2-chlcuc~Pt~nYl)methyi]ll-hydroxy-2-3 Calcul~ (M-I~'=
txn-2,5;5,7-rckahydt'o-1FH 351.2!; Futmd
cycl~penta[bJpyridia-3- (M-Et)-
yl}amino)rrarTwmyl)amiao}-3-[3- = 551,05.
(diathyltmnino)pheayl]pn>panaic
acid
(''3S)-3-f[((1-[(:~chtoi~apbepy!)methyl]-4-hydroxy-5-20 Catwtiatcd
(M-H)-=
methyl-2-oxu1,2-dihydm-3- 504.1 l; Fotmd
(M-H)-
pyridirtyl}otnirto)calbonyljamiru~}-3-[3- - 503.96.
{difluur~mcthyl)p6cayl]'ptopanotc
acid
{3 S)-3- f [( f 1-((2-ctrlorol'henyi)metllyl]~4-hydroxy-2-i Calculated
G (M-H)'
.
meo-2,S,G,7-G9Ztattydto:lHcyclopenta[b]pyridin-3- 498.12; Found
{M-H)-
yl}amiuu)carhonylJamino}-3-(3- -- 498.02,
fluotuphenyl)propsmuic acid
(35j-3-(L((1-[(2-chlotophrnyl)mcthyl]-4hydroxy-Z-9 Calculated{M-Hj'-
vxu-2,9,6,7~tetrehydto-1H-cyolopenta[b]pyridin-3- 498,12; Found
(M-Hj'
yl)atnitro)~bonyljarnim}-3-(4- > d9R.01.
flunrophenyl)propartoic acid
N-(1-[(2-chlenophcny!)methyl]-4-hydroxy-5-methyl->IU0!!0Cnloul3:ed
(M-Ii)'=
2-oxol,Z-dihydro-3-pyridinyl}-N'-[(R)-phenyl(1H- 464,12; Fund
(M-H)-
t,2,3,~9.~o1-5-yl)methyl]urcu =404.01.
t3s)=3-ft(~1-I(2-~taoro-c;-a~etnyl~heuyt?me~yy-4. 4 calcu>~ted (M.H)'=
hydrtlxy5':methyl-2-o~w-1,2-dihydro-j- , 521-IG;Found (M-H)'
lmiditrvl)antit~o)oarboay!!amino}-3.(1-evethyl-ill- = 521.00.
indal-5-yt)propanoic acid
{3S)-3- ([((1-r(2-chloto-6-euethylpTrenyl)mcthylj-4- 10 Calculated (M-1i)'
hydroa~y-2.axo-2,5,6,7-Dettshy~o-tH- X65.14; Found (M-I~'
eyclopente[b]pyridiu-3-yt}amino)csrbonyl]omlno}-3- - SG5.4d,
r3-{diethy1su11rb1ph~y111~P~iv; acid
(35)-3'(((;1-r(I-chluro-fi-methyiphcnyt)methylj-4- 4 Calculalcd (TW-H)'~ ._.
hydrnxy-Z-oxo-2.5.6,7 tetnthydro~1H- 508.16; Found (M-H)-
cyeloperrta[b]pyridin-3-ytjaminojcorbonyl}emiao}-3- = SOR.03.
t3-mc~y1P1~711jp1~patwic: acid
CA 02366800 2002-O1-07
-190-
(353~{(({1-((2-ektkom-6-methylPhenYl)mcthylj-4- 17 CAlculatGd (M-!~j'=
hydroxy 2-oxn 2,5,6,x-teaahydm-l 494.15; Fout>d
!i- (M-Il).
cyclvpenta[brpyridiw3-yk f = d94.O9.
amidokarbonYljamino}-3-
1~'p5'~p~"o aala
(3S)-3.{[(t1-[(2-obl~upl~anyl~net2ryl]-Q-hydroxy-?.R C~lculticed
(M-H)-=
exo-2,5,6,7-tatrahyt~O-1H-~yckope~t~,b]pyridin-3- 496.13; Found
(M-H)-
yl}xmitzokmrboryl]uuino'-3-(3- = 49$.99.
hydrnxy~,crtyk)~tvpapoic mid
(3S)-3-((({1((2-ehlomphe~dyl)methylj-4-hydrvxy-5.9 C:akeulatad(M-Fi)'=
modryl=2-oxo-1,1-~'hyclm-3- 470.i 1; r~uad
(M-H)'
Pyridltryl}~unino)corbotayk]amieo)-3-(.3- = 469.98.
hydrmiyphenyliv ncia
(3S)-3-(1({!-[(Z-chloaoplsenynmethYlj-4-hydtoxy-3-50 Calcuknit~d
(M-H)' _
methyl-3-utr4-1,2-ds~ydto-3- 55$.18; Found
(M-H)-
pyrid;ny!}aminoayc]eraina} . = sss.oo.
3-(3',5T-
dintedey![t,l~-biph~yl]-3
yI~mpanoie acid
(38)-3-t'[({1[(2-oblorophenyl)znethylj-A-hydroxyS-15 Calculated
(M.I~'=
methyl-2-exo-1,2dihydro-3- 455.12; Found
(M-1~
pyridinyl}asninokarbony!]amino)-3-phenylprnpat~oic -454.40.
acts
(38r3-t[({1-((~~chlprophet>,yT)methylj.4-hydtoay-~-3 Calculated
(M-H)-=
oxo-.~,,Ssb,7-fctrahydro-IH-cyclopcut;n[bjPyri~lin.'~. 573.!2; FoanJ
(M117-
yl}amino)oarbonyl]amlnol ~3-t3- = Sa2.9R.
[(methyk-sulfanYly3t~tu)phe~nyl}Propanoic
~scid
(3 S)-3 {[( { 1-[(2-cbioro-6 3 Calculated
merhyiphenyk)methyiJ-4- (M-Hj' =
hydroxy-~~-oxo,2,5,6,7-tenahYdro-tH. 587.14; Frnrnd
tM-I~-
cyclupenta[bjpytidin-3yl}ernitso)varbonylJam.ino)-3- ~ 5t~6.98.
{3-[(methylsulfoaylo)phenyt
Jpropanoic acid
(353..{[((k.[(2."blorophenylhnrthyl)-4-hydroxy4 Calculated
Z- (M-H)'=
oato-2,S,C,7-bef~nhydto-lFLeycloDente(b]pyridin-3- 53U.l3; Found
(M-11)-
yk}amiuo)csui~nyljamino)-3-[3. = 531t.U3,
(difli~arantathyl)phenyk~propanoic
acid
(:S,3S)-3-([( f 1-[(2-chlo~phcnyijmethyt]-4-hydroxy-I50aGaiculaved
(M-I~'
5-methyk-2-oxo-1,2.dilttydro-3- 482.15; itonad
(M-Hj-
pyridirryl f afiino)carbonyl]~iDO)-Z-tr~lhyl-3-(4- - 481,99.
medtykp'heuyl~ropanoic acid
(3S)-3-([(t1-((2-chtoro-5-m~thylphenyl)methy(]~t-15 Cakcul~ed(M-H)'=
hydroxy-2-ORO-2.3,6e7-teh'sbydrfi-1H- 522.18; Fot111d
(M N)-
cyalopeztta(bJpyridiri-3-yl}atninu)cafiopy!]amine}-3. = 522.t)'4.
(4-cthylpheoyF)propcnioic
acid
CA 02366800 2002-O1-07
~I5~1-
t3S~3tI((I-[(x-chlaropb~yl~thyll.4-hydruxy.2-3 C:aiculated (M-H)-
oxo-2,5,6;7 tetrtdtyQro.lFicyciopentu~bjpyridia-3-5S0.17; round (M
Ry.
yl}amisokarbcmylJarnino} 3-(2;2.di~aethyl-2,3-~ 550.05.
dihydru.l-bEazotlusn.5-yl~ta~anoic:
acid
(3A1')-3- f [( ( l-[ f 2-oitlom-6-mrthylpheayi)tusthyiJ-~t-3 Cslculatod (M-H)'
hyctcoxy Z-oxc~-Z,s,6,'7-~ctn<hydcoiT't.542,13: Fowul (M-H)-
oyclop~ta[bjpy:i~in-3.y1}mnhtokncbonyl}amino}-3-= sa2.uv,
[3-tluoro-4-(mdhyloxylpl~Yt]propanoic
acid
(3~~)-3-{[( f 1-[(2-chloro-ti-~ethyiphenyt)metbyl]-4-I 1 Coloulate~d
(M-H)- _-
xy 2-oxo-2,5,6,7-tetcahydro-1H-579.13; Found (M-H)-
cycioPerits[blpyridin-3 yt}~apino~rbottqi]amino}-3-= 878.02.
{3-[(hiflnorotnathyl)nxYJP~yS}Pt~~oio
acid
t3S)-3- f {( {1-((2-chlcnophea9il)mechyi].4.hydroxy1.6 CxtculatCd
2- (M-H)' l
oxo-2,5,6,7:tetrahydlp-1FIcyclopentajb'tpyrldin-3-587.14; Found (M-I~-
yl}amino)oar'bonyi]~~ninop-3-{3-~ Si46.99.
(metttyumetl~laulEorryi)ra~ninefPb~11P1T~hanoic
acid
(3S).-3-t(t{1-[(2.dyloto-6-methylphenyl~Cnethyl].4-1.3 Cctieulated
(M.11)
lrydmxy-Z-oxo-2,5 6,'7-t~ahydxo-1- 60 f .15: Found
H- (M-It)-
cyclapasntay jpyridin 3-yl}araiao)earbonylJnmino}-3-= 60i .00,
{3-[methyl(~thylauifonyl)atr~ino)phonyl}prcpanoic
acid
(3S)-3-[[(~(1-((2-ohloTOphcnyinnc~thylJ-4-lrydmxy1 CalcutstGd (M-H~'
?-
vxo-2,5,6,7-tetrahydtn-1H-cyclupentaLb]Pyrldin-3-601.15: Found (M-H)-
yl}aminu)Carbonyllarnino}-3{3- = 501.04.
(edtyl(met~ylsulfimyl)ominojphenytjpropanoiv
acid
(3S)-3:t[({1-[(?-chi~6-afetltylplrenyljmathyl]..4-t CxlctitntCd fMH)-=
hydtoxy-Z~xo-2>5,6,~-tCtrabydro6i3.i7; Fo,md (M-Fi)-
~!H-
vyclopentafbJPr'~~r-Jd})zerbonyllamiru~}-3... 615.04.
t3-[rtlryl(methylsulfonyi)a~oiPh~Yl}P~Panoic
acid
(3S)-3-{[(1-[(2.chlorophenyl}mctl~yij-425 C:alculated(M-h)'=
hyd~rixy-5-
mettryl-2-axo-l,l.dihydro-3- 548.14; Found (M:H)-
PYt't~}8m'rno)catbonylJxmina-3-(='-fluonv[I,1'-= 547.96.
6iphetiyl)-3-yt~rvpannic did
(3S)-3-(I({l-[(2-chlorophenyl,)raethyl]-4-hydroxy-3-15'7 Catculatzd
(M-H)--
methyl-2-oxo-1,2-3il~ydtn.3- 59ti.14; Found
(M-H)-
pytidirrlrl}aminoxwbonylJsmino}3-j2~-= 597.97.
(trifhuorcnnethyi)(l,l'-blpi'mryl]-3
yl]propsuwicacid
(35j-3-{[tai-t(2-chloropttenyl)mett~ylj-4-hydruxy.S-10 Galculat~ed
(M H
methyl-2-axo-1,2..di6ydro-3- 473.11; I"ound
(MH)-
pyridiuyt}arnino)cerbonyl]an~i~oj-3-(?-=411.98.
Iluorapheziyl)pnpanoic acid
CA 02366800 2002-O1-07
-19~2-
(35)-3-{[(~i'[(2-of~loto-6-mcthyiphescy't~estltyl}-4-2 l:aiculsted
(M H)'
hydtoxy y-oxw2,5,6,7-t~ahy~o.l533.16; round
H- (NI H)-
cyclopeiata[b]p~yridin.3-yi)antQtQycarbonyl]amioo}-3-~ 533.01.
(1H-atdol-5yt~propanoic acid
(3S)-3- ([,(~l-~t'Z.chloro-6-u7ed'~rl~PLarYi~methYl]'4.11 Cakuiattd [M-Fi')~
L
hydrc~xy-2-oxo-,'.,5,6,7-tet~rdto-l~i-53U.13; Found
. (MII~
cyolopetua[bJpyridint 3-~rt}aznirw)carbonYll~ina}-3-=530.OD.
(3,S~iftuoropBenyI)Pmhanoic
acid
CA 02366800 2002-O1-07
-193-
SEQUENCfl IIQTIN(3
(1) GENERAL TrTFORMATIUN:
(i) APP1.1CANT: Bisdiges, R,onsld J.; Chen, Qi; Decker. b, Radford; Holland,
C3mcuge W.;
Kasair, 3ama1 M.; Li, Wen; Marget, Robot Y.; Scott, Ian L.; Wu, f.'hengdc;
attd 1i, Jian
(ii) TITLE OF 1NYEN1'ION; Carboxylic Acid Dctivativcs that Iafhibit
the Binding of bys to their lteccptora
(iii) NUMBER of ~tffmN(:ES: 1
(iv) CORRESPONDLNCE ADDRESS:
!5 (A) ADDRESSBfi: R~ckey, Milnamow b'c Ketz. Ltd,
(B) 97R1:FT: IZ30 N. Steieon Avenue, 2 Pmdential I'1$~s,
Suite 47
(G) i.'ITY~ Chicago
(D) STA'19?: Illiaois
tE) COUNTRY: U.S,A.
~) zIP: boboi
(v) rOMI'UTBR READAHLE FORM:
(A) MEDIUM TYPE: Floppy disk
23 1,13) GOMPtITER: I$M Pt'' compatible
(C) OPERA1'iN'CJ SYSTEM: PC-DOSIM3-DCl i
~'D} SOFTWARE: T'e~entls~ Release f~t.0, Version #t_30
(vi) CURB .FNT APPLICATION DATA:
{Al APPLICATION NUM11EA:
(A) pll.EV(i 1)lITE:
(C} CLASSIFICATInN:
(viii) ATTORNIsY/ACrEN'!' INFORMAIION:
(A} NAME: KaPr, Mariin T..
($) ItECiISTRATION NUMBER: 25,011
(C) RBFEREItT~'FIDOCKI:'I' NUMBER: T'Ekd3d2P0402L19
(iz) TELt!i :nMMUNICATION INFORMATION: .
411 (A) THLHPHONE: 912-fil fi-54t)V ._.
(B)'I'LLIrFAX: 312-616-5460
(2) 1NFOR MATIUN r'UR SEQ m NO:I
(i) SEQU.INC;E CHARACTERISTICS:
CA 02366800 2002-O1-07
S
(A) LL::i~IG'IH: 2b amino acids
(8) TYPE: amino acid
(C;) STRANDBDNE~q9: single
(D) TOPULOQY: linear
(ii) MGLECtTLE TYPE: p~mcn
(xi) SEQUF.NGr DESCR>PTION: SrQ >D NU:1:
10
Cars .asp Gtu Leu Pxv G1n l.eu Ysi 'Itu Leu Pro His Pto Axn Leu His .
1 5 10 IS
Gly Pm rlu 11a Leu Asp Vel Prn Ser '1'hr
15 20 Z5
CA 02366800 2002-O1-07
l~s-
Alt refem aitod sre hereby incoporated byrererenca.
The present iorcntion is illnattated by way of the foregoing do~cription and
. examples, Tlte foc~going dcaotiptiaa is ince~ded as a non-limfting
illusn~ti~n, since
many vatiatiorte will become apparent to thuse slillcd in the aft in view
thereof: It ie
5 iutcnded th0.t atl such v~ixtiona within ine scope and apQit of the appended
~:laims bo
embraced therCby,
Changes oan be made in the compoaitlots, operation and arrangement of the
tnetitod of tDe preacnt invention described herein without departing t'TOm the
concept and
BCOpC ut the invention as deflued in the following claims.