Note: Descriptions are shown in the official language in which they were submitted.
SEP. id.2001 11: d8AM f_'Rni~IELL & MnRINr LLP ~-q~~' d
CA 02367848 2001-09-18
Now preteJ, a 1
Process for produoina a c:dta~lYtic convwrter
The ~ nverztion relato~s to a proc:ees fox produri ng a
catalytic coziverter.
The diACloaurC JP-A-08 Z34 ~R2 has dQacribod as
eleotroplafiing procQaa for coatirxg a metallto substxa'Ce
with a smooth p.~waioua metal Zayer, in which an ~.xon~-
conzaining auhatrat~ ire provzd~d with a platinutri
aov~ring. The patent pL~ x97 3Z 170 C:2 has d'i,aeloaed a~
process for cov~r~inc~ a a~x'amia 8iC substrate in a
locally selective manner wlLh a p~.at3num aovwtine~, th~
10 surtr~ua of tnlhich m~tvhea the xough oQramic surtave, as
a r~oult of a direct voltac~a being applied b~el:ween the
aubat.cw~e and s countern7.eantrode. Th~ ooatcd aub~tsata
i.s thQn troated at elevated tampdi~atu=e Of over 4UU'~.
J~P-A-106 1.97, which formp the atartirrg point Zor thA
invention, has d:Cacloped a process for th~a el~etroahemical
deposition n~ a pozrou.a platinum layer . T'tie layer is
depoaitod on a ca~:bom ~r semioonduaror. aLtbatratQ. Tn thi,a
process. s otua-off high volta~go puls~o i~a applied between
substrate and couriterelaatrode. so that nueloi ax'!
deposited on the a~ohstrate. There thcn follows a prvlongsd
pulse at a lower voltage, during Whioh groral-.h of th~
nuclei l,r~kes place. US-A-4,273,624 hac disclosed au
cl~ctroplating proacaa in which a thin, oontinuo»R layax
3 0 of platinum is deposited on an SnOa substrate . JP-R-'7 80
has diaa~,oeed a catalytic wnverter i=i which a
metallic subt~i;rate is coated with a thizi, rion-porous layer
of precicu~ metal. Thha laclc of poroa u~saxzs that the
catalytic conv~swl.er is distinguished tay i high reoiotunce
3 3 t0 OXy!~e11.
AM~1~TDED Sl'ih7~T
SEP.ia.200i li:a8~7M CRftLIELL & Mf.IR.ING LLP Nf_1.928 P.5
CA 02367848 2001-09-18
74.~Irlitioasl pn$e 1a
The obj~et of t:h.R invention ie to pzw~i.de a procea~a ~or
vva~.Szlg n metallic cub~tratn whieh all.owa the
depoei.t-.i.nn o~ a precious m~~a~l with. a large aur~aae
aLwa nizd good ndhcaion to a aubetrzte .
Thin object is achi~ved by t-.r~ teaturee of the
independprit claim.
A~c:nrdiag to the inverit;:~oil, a layer of catalyt3.enlly
active metallic mat~ri.~.l. is dey~o~ized on ~, v~~a1
substrate key meanp of electrochemival de8or~ition, the
oubEtrat~ being imnte~.r..~ed in an electzolyl.r~ which
1~ coriCain~s tam catalytically active rnota~l.lic material
AMENDED SH81ET
Nn.928~ sZ
SEP.ia.200i ii:a4AM CR~LIELL & MnRiNG LLP
CA 02367848 2001-09-18
aubatxate ass a porous or non-cohceive layer.
Ii.-. is part3cula~:ly advantageouo for the eubatraZ~ to ba
provic~.ed, on itA murfaac which :La Zo be routed, with a
prR~letermined aurfr~ce roughness prior I:o the
deposition, they surfs~ce roughness preferably lying in
the rang~ from 0, 3 bra to lU ~.un. A further preferred
range ~c~r the surface roughne~a is k~etWeen U . 3 ~tm and
3 ym. The surface rouc~hnesl~ is e~~cpadi~ntly generated by
to thermal ~xnd/or mechr~nieal and/or chemi~:r~l Zreatment .
The catalyLieally active mat~xial is preferably foamed
from metal clusters with a dla,mezer of hetw~ex~ a run arid
1 Nm, preferably between 2 nm and 300 nm.
18
Th~ pe,rticula~r advantage of the process ie that it
possible to depoaoit arrta,lytically active a ayerc with a
v~xy large surfaaa area and x~ relatively low level of
aatalytically ~aative ma~tcrial. They layers exhibit good
a.~lheaion and acre etable at high tAmpQrxturoo even aster
prolonged use.
Preferred catalytiaallly aotivc materai.a~ls are pr~cioua
m~talp. on~ au.itab~.e oat~xlytically avtive material l~s
35 platinum. A pxeterrwc~l eounterclectrode is formed by
platinum-coat~d titan~.um sheet . A ti~rthar profexred
countcrelecLrode consi~st.9 of platinum-coated nickel.
In a particularly pretwrred refinement of the
30 inven'Cion, platinum ire depvs~.zed with differcat
morpholog~.eo. Thla provides r~atalytio eonvQ~rtezw whioh
haves different. ael~vtivitie~s ~ow conver8ion of nwtter.
For o~.at~plo, it ire posa3lble fio produaa a preferred
platiixum o~talytir. aoavartex for a weforrrtir~ ra~.ctor
35 wh~lah, d~apito a high carbon monexi.d! aoneentration J.u
an Ha/CO mixture, bass a vontiauvualy high activity for
hydrogAn. ooriv~xaion iz~ operation.
Further advantages arid conf;iguraLionag of the invention
SEP.ia.200i ii:aSpM rRnIAIELL & MnRiN~ LLP Nn.928 P.iO
CA 02367848 2001-09-18
will emerge from the fiurt-.hAr. claimw aid tho
description, Th~ ir~.vention io deocribnd in moms c~dt~til
below wll:~l reference to a drawing, in wh:i.ch:
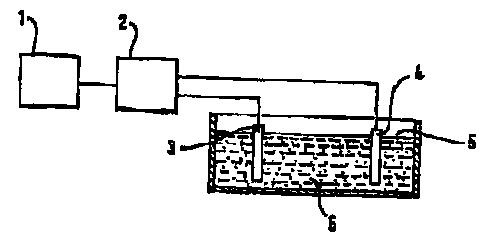
Figure 1 shows au outline view of a structure i~or
carrying out the pros~ps aocording to tho irxvention,
higure 2 diagrammatically depicts a a0vtioa thx'ough a
coated surfiaee,
io
Figure~a 3a, b ahoo~r two acaiulliig-elnctromt~.orolscOpe
images of the surrace of a, or~t.alytie convert~r w~.th
dondritio platinum clusters, ,
Figure 4 shows a acsnniag electron microacoge image of
the ~urLt~ee of a catalytic converrwr w3.th spherical
platinum olu~t~ra,
Fir.~urQO Sa, b show two eoatmnir~g-clcctroa mivroacope
2 o images of tlzs auwr~c~r~ of a catalytic convertor with
dendritic plxti m.~m c.lustarm before (Figure sa) and
after (Figure 5b) a long-l,~,wu Lest,
Figures 6a, b chow a cornpa~~:ir~on of rc::uWti (Figure 6a)
arid smooth (Figure Rb) r~urfaes profile: of atoQl
eubatrateo prior to the coatirsg with catalyst nu~l.at~ial,
Figur~ '7 ahowp a meaa~uremerxt curve iridicating tam
lxyc3x~c~ett conversion achieved by a catalytic eonvextwr
with sph~rioal platir~,um cluotcra in e~ CO-containing
al:n:c~b~xieiw, and
Figure 8 a~aowd a measurement Curve iiluecrating the
rnnvPrr~-ton of hydrogen achi~vod by a catalytic
30 converter witlZ 3exidill.ic: platinum cluezere in a GO-
containing atmosphRr~.
b'igure 1 shows an arrangame~nt for carxyincg out the
proooco sccordirLg to the inver~,tion. A function
j
Nra . ~ca . .
SEp.ia.2001 li:a~M rRnLIELL & MIRING LLP
CA 02367848 2001-09-18
generator l ,generates a modulated voltage which i~
' amplifinrl in as anipli~ier x arid i,s appli~d betw~ea an
ar~ode 3 and a nuk~strate 4 which iE to be corxi:vd in
depoeizion rath 8. A direct vu~.l;aae Vas is prs~~rably
superimposed with art alterriati ng voltage V~. Tl~s sum
voltage of direst voltage V~ a~c~d alternating voltage
V,~" ie also ye~erx;c~d zo below e9 the tnodul,atod woli.uge
V,~. The alLernat.inc~ voltage ie expediaiitly eirusoidal,
but may also adapt uLher forms, tar. example oawtooth ow
ZO scdu,~xwe-wave form.
The caLdlytioally active matorial 6 is depoa5.lted on the
substr.ats 4 in the form of vluete7co. Aee~rdirxg to the
311VCIltit711, the Cluagterra may hav~ different fvrrns wlzioh
can hR prs~dot4x~nir~cd by the deposition paremetera . The
oubatrate 4 which is rented with er~,tn7,ytically acCive
material 6 the~s forma the catalyl.ic converter_
The direac voltago V~ io prefera~rly apt least aae great
as the dopoaition pvtezztisl of the eatalytically activa~
nu~terial 6 on the substrate 4, particularly preferably
at ~nopt SO% higher. The precise levQl of thm direvt
voltage Vas is dependent on the conat~.tuemi;s and proeea~a
cond3tior~.a employed and may. ~'or exs~t~ple, adopt
2~ diL,Cerent values f.~r. differently pretreata~d substrates,
a7.t-.hough th4ae vahzea dv not u~ua~.ly differ greatly
from o~m another. When depositing rn~.xed syetomo as
catalytirally active material, it is also pnseibla for
the pre~erze~d direct voltage Vac to lie below Ltr,is
depos~i.tion pot~sitial.
Stain7.ws~s Ate~1, particularly C,r-N~, steel 1..A.~a41 or
CR-Ni eteol 1.4871 or Gr'Al stRwl. 1.4'767 io used as a
particularly a»i tablra substrate. Tt is ~xpedieriz fox'
tho a~otrdtQ to pe r~and'blasted or. roughen~d in some
ul.tier way, for ~xamplQ chQeni,eally, and i,o 'undergo
a11ca1inn degrcanixig prior Co the coatl.rag. This improves
thd adhesion of thw catalytically active mate~ri.al 6 zo
fibs substrate 4.
4
SEP.ia.200i ii:a4AM CR.OLIELL & MnpiNr LLP Nr~.~28 P.13
CA 02367848 2001-09-18
=n a preferred embodiment, the altexia,atiisg voltatge V~
ha.t~ s maxi mum lroltagc awing Vap between minimum and
maximum which io lowex than the direct voltage V~, ao
that overall the sign of the cum voltage between
substrate ~4 and counterelectrode 3 doo~ not chango.
The current betw~~z~. substrate 4, whioh c~rvas as the
cathode during the depoe~.tivn, and anode ~ is recorded
and, xn accordance with Farada~r~ g law, is! used a: a
measure for determining the caudutll:y ~f c;uLalyt,'i.c$lly
aetiv~ mat~rial 6 dapoa~.tad, this material being
cont~.inda l.i~ l.h~s deposition oath; cu~:rent contxibutiona
which flow for thQ purpo~s of building up pnc7. brw~ek9ng
tit~wtl the eleotrolytic double layer on account of the
modul~.tien are expediAntJ.y ~1 i m5.nate~d, since they do
not originate from the reduction or oxidction o~
cations Or aniOnS.
zt is preferable zo deposit precious metals 1n order to
produce the aatalytio aoav~rter. Howev~r, it is alAv
possible to deposit mixtures of prev~.ous metall~. An
axpcdiont, ~.nexpenaive anodes il~ platirzum-eeatrd
titanium instead vt a c~tr~=ia~.z'c:~ r~acriticial anode made
2a~ trom solid platinum, which can be used to gaztieul.s~.rly
good erLeul, if platinum is to be depos~,ted ae
oatalytically active mat~rial. HOwevRr, nth.~r, pr.~aeioue
metals and ~l~r~ other metals can be depo'ited ire thxe
invvat itrs way .
The frA~mnry and/or the amplitude v~,r and/or the
voltage offset V~ of the modulated voltago caxi be
~d~umted in order to optimize the deposition pax'ameters
for the p~rtioular cyst~m. Th~ va~luos affect both the
~S size of the elusze~ag which are deposited vu Lhe
metallic ac~thode and th~aix morphology. Overall, the
elust~rs on the substrata 4 c;~~lai::Ltuta a large active
our~aa~s area for catalytic raactionn. The optimum
cluster size c;du Le stet im each cases for diifereat
5
5EP . i a . 2001 11: 49RM rROLIELL & htOR I1VG LLP NC1. 928 P . i a
CA 02367848 2001-09-18
applieaticns by ~ami.fi.ably s~tleoting the deposit3os~
parameters end the duration of coe~tirig.
It is particularly etdvantageous for the surfaces of l,xid
substrate 4 which is to be coated to be roug~wttAra. prior
to the coating, for e~ampl.e by pickling ox oaad-
blasting. other methods for increasing the surface
roughness ar~ also potasibl~. This is illuotratod in
Figure z with reference to a diagrammatic side view of
1,0 a coated surface. A sub>ztrat~ 4 hats st rough~ned ~urf;ee
4.1 on which spherical metal cl.usz~rs 6.1 arc arranged
in reessa~sef . The metal clusters 6 .1 may a.lsao be
deposited o>,z the peaks or the flaizk~ ut Lk~d wraugtidand
armas_
i~
ThR inr..rw~pR~c1 pm.rt~~:A .ro~.tghnees has th9 advRntxtrds ths<t
deposited cluatexe G.x adhere mono auceessiully to the
substrate surface, arid and~sirable combining of the
clusters 6.1 ie ouppreaAed. A catalytically active
3u layer of individual clusters 6.1 is form~dr the layer
i~ preferably not aontinuouc, but rather is formed from
xsolat~d clusters 6.1.
Ttie ~ur.Cdc;d iwuc~lmaa~ is ptwfert~k~ly bwl.wdnri 0.3 ~t arsd
25 10 pm, particularly preferably bwtwewa 0.3 pm and 3 pn.
A large active surface eras. is formed from thr~ firmly'
distributed elustsrsa 6.1. A further advantage is that
the imcr~cascd aurfaee rougluzeass even also coi~tribut~ss
t~ i nrre~~r,~1 ng 1-.hw >;nrf~arr~ ~re~~ of rh~ t~mh~~hrate 4 and
30 therefore also the chemivally act~.ve surface area, At
the game time, the clusters 6.1 can be very small, so
that overall only a r:mall quantity of the expensive
catalytically active material 6 has to b~ deposited,
yet at the same time the eatalytio converter ice
35 distinguished, by a high catalytic atctivity.
The adheaivrz ofi the metallic c;lusLera~ 6.1 to the
substrat~ surfao~ 4.1 is v~ry good. This mak~s the
udt,alyal, layex mole resistanC to aro~siort,.
H
SEP.ia.200i ii:50RM CRnLIELL & MORING LLP t~.928 P.is
CA 02367848 2001-09-18
A particular advantage over convv~emtioizal c:~t;alya~i.
- layer8 ie that, aceordtng t~ ~:na invention, good host
traastcr ~rom the cs~talyat layer to the substrate 4 ins
possible, since metallic clusters 6.1 are ~oinac7
metallic oubotrato 4, ey contrast, known, conventional
catalyst 7.ayers are produced, for exan~l~, with
supported catalyst matsxials, in which ceramic support
particles are coated with a precious metal. 1n this
ease, the heat trax~afQr b~tw~on aata~lytiaally act3.ve
p~.wc;iou~ u~at;dl a,rsd a substrate is siqnitioarttly worse,
r~ince the ceramic part~teles axrarlgQd bstw~.n them haves
only a low thermal conductivity. 2iz addltl~m, iu a
c:r~i-.a'l,yt-.i c! o~nvRrtRx which is produced according to the
1b invention, it is also possible to diepaaso with
standard adhesion promoter l~syaa~~, whi r..h have an
additional r~dverae e~~ect on the heat transfer
properties between eacalytically active material 6, 6.1
a.nd ssubatrate 4.
A subAtratw 4 which i~o coated according to the
invention is therefore particularly suitable for use as
an oxidation catalytic corm~rt~x for t~rextisig ~xhaust
gases iia tual cell syr~te~ys . A tu.~~l,xa~w expedient
application ip for various heterogenQOUSly catalysed
proceaaea . The catalytic comvexta~r accrrr~tirzr~ t;o l:~m
invention and the pros~r~a ~,ceord~,ruJ to the invention
are particularly advantageous iiz exhaust,gaa c$tal~r~lc
converters for vehicles.
35
r~igure 3 a~hows searuzing-electron microscope images of
prcfcrrcd catalytic converters With de~ndxitaLc platinum
clusters (Figures 3a, 3b), and w~.ch rhodium clusters
which are of substantially dendritic form.
Derldr~.tic platirmm clustexs ar~ profQrably dQpoeitnd oxz
a t~tr~~.mldd~s t~taal aub~rtrate a~ a result of a~ d~.reez
voltage V~" of 1.4 volts being superi~poR~d with axe
altornatizzg voltage V8e with Vpa~0.7S volt (peak-peals
7
SEP.ia.2001 1i:50AM CRnLIELL & MnRING LLP NO.S28 P.i6
CA 02367848 2001-09-18
voltag~ swzr~g) and the modulated voltage b~slng applied
between a ataialeaa ote~1 Aubatrate and a
coaster~leCtrOGl9. The frequency ~t the alternating
voltage Vsc is ZO IIa. Tho eloetxolyta u~m~d i ~ platiniC
acid, in particular hexachloroplatiri,ic acid, r~r3.tk~, a
pl~~iylz,~,m content o~ 0 .1 g/l . The depr~r~i r.i.na preferably
takA~ place az room tampowa~uze.
platinum clusters praaesizt der~,dr~.tic growth, arid no
spherical platinum clu~t~rr~ axe observed. The prerd~wacl
Ga~.t~.:lyt~LC converter h~.p a high lovel of aotivity for
hydrogca aonvers.ion wen i n the nreserlce of c;arl~on
monoxide, carkwii monoxide alAO be~.ng aenvert~rd w~.th a
good yield at the samw time.
zn th~ selleted say~tem, dendritic; platinum cludtero are
tiepor~~.l.ad with frequcncieo of betw4en 5 ~vncl 15 Hz atld
an altern2~h ing voltage V,~ with a voltages awing Vry of
betwee~u 0.3-1 volt.
A further prcferrod catalytic aonve~rter is Droduced bY
depc~pi.t ion of x'hodium ors ataiialesa oteel , prei~rab~.Y
compriving etaitllaar~-steel ~aheet 1.4541 or 1.437x or
b'7. It is also podaible ~or mixed oatalycts of
z5 platinum to b4 deposited. ixi this waY or to c,~~podit
eatalyBts compriblng mixtures of precious metals and/or
catalytieally native matRrialg, such as for example
Ptlih, PtRu, PdPL .
3U Dep06itivti takes place fro~nn a anolution of 0.2 g/1 of
rhodium i.x~ c1.1 M lizbOa at room ~ampaxaturo with n sir~ct
voltac~~ Y,Ia of 1.4-1.6 volts and a super~.tctpoa~c.~
altarnar i ng voltage V~,o wi l.h Vap-1 volt (peak-p~a,k
amplitude) and a frecjuency of ~ ~ Hz . 'fhe formation c;W
derLdritic~ growth of zhe 'cluc~tera can be improvod ;still
further by vax~ying the voltage parameters.
A aatalytio co~n~rort~r of this tyre has a good activity
for methanol. A catalytic converter of thi.R type is
a
Str. sa. ~Idld1 11: ~eRM rRnI~IELL & MORINr LLP Nf_1.928 P.17
CA 02367848 2001-09-18
thereforA particularly suitable for use in fuel cell
vehicles operated with methanol, partivul~trly
pr~gprably in catalytic burners.
r'iguxe 4 shows a acanning~el~ci;;~'om ~tviw~oscope image of
c~ further pre~~rred ca,talytie oozwextar with aphRriQal
platinum clusters . 'Txm d~poaition takes place uair~g a
dir~ct voltage Vas of 1.2-1.56 v~lt~ and an alternating
voltage Via with VBp~O.~ volt a,nd 100 Hx; the deposition
~.0 ba.th othsrw~.sa! corresponds fi.n t-.r~t used for b'igure 3.
In this r~yAtAm, xn alternating voltage v°Q with
frequencina of more thar~ 50 Hz, preierubly up to
150 i~~, iR preferably used for the deposition of
~.5 spherical platinum eluctero . The dir~cst volt:ge V~
which i ~ applied varies with the substrata pweLi'ea1;11~1'~t
arid, for the dopos3tiorx of epho~'ioa,l alusot~rp, is
substantially equal to or tends to be all~x~t,~.y lower
than that used ~or bhe deposition of dendrit3e
U c~.uscera .
Figure 5 ahewr~ a ~:omparison of acanning,clectx'vn
micro:cop~ i.ma,g~s of s rr~ta:lytic converter with
deudrlt,i.c platinum clusters before (Figure 5a) and
aft~r (Figure 5b) a 7.o~lg-1'.e~rm test, iri whioh ~Che
c;~xl.a~lytie corsvextey ways eapoaed to an ~I,/GO mixture at
high temperatux.'A for. more than 200 h. Although eXposed
to high tempcraturaa of up to 600°C, the porous ox rrorr-
aohesive platinum layer ae catalyst remains stdblc~; th8
30 cluatexa Yemc~ia ~ixad in posit~.on and do not conv~re'~e.
The layer which has been deposited lii accordanoe with
the 3r~vet~tion domonot~'ates th~.t the elustox's is
practioe do riot change before z~nd af~~sr long-term uea.
35 Figure 6 shows a compariaroxi of rough (Figure 6a) and
smooth (Figures 6b) surface pr~fi l.ws et steel a9ubetraLes
whicxi have been used for the dopooition of th~
platinum. The adhAr~i.on et the platinum clusters to the
roughened e~ubatrnte is aignificaatly bettor than th~
9
5EP.14.2001 11:~M CRnhIELL & MnRIIVr LLP NQ.928
CA 02367848 2001-09-18
adhesion to the smooth subl~trats ~ the layers era wipe-
reair~tant, while the layers on ars ur~.tLented, smooth
Aubatr~.tR era nit wi pR-r~~i ~t~nt . They ~xm.rf~rp .r~mghnRaA
is preferobly botweea 0.3 dun and 10 ~.m, particulaxly
preferably between 0.3 dun and 3 pm.
'the catalytic activity and/or aeleotivity of the
catalytic convert~r diff4rs d~p~ading on the paxtiou~.ar
form of the clusters. A preferred catalytic converter
to with sph~ricxl platinum eluleters converts hydrogen in a
substantially selective manner. The catalytic converter
is poiaos~ed and has m greatly reduced activity in the
preseizce of carbon monoxide . A cectalytic converter of
thin type is preferalaly u.r~sr~. ~.mc3Rr ~arhnn mnn~xi dw-l~rwA
1S conditioni!t.
8y contraot~ a prefcryed catalytic converter with
dendritic platinum clusters tirezly has a hiqh
toley sate to carbon morxo~cide during the hydrogen
20 convsrs~.on and secondly has a ha.~h selectivity and high
activity both for the hydrogen vonv~r~aior~ and for the
carbon monoxide conversion.
Figure 7 shows a. ~ua~bu.~wusrs~~l. cu;we illu~strat:Lnq the
95 hydrogoa aonv~raion achieved by a pr~ferred catalytic
aonvortet~ w~,~l~ aplzerics,l platinum aluotcra. The
catalytic convartQx is~ exposed to an Hz/CO mixturA
u.czder standard operating cvnditxons. After even, a few
mi m~t:e~~, the hydrogen conversion in the pre9ence of CU
30 ~allcs to low levels.
Figure a shows a meas~uremo~nt curve illustrating the
hydrogen conversion achieved by a preferred catalytic
converter with dendtitia platinum oluAtQx~a. In thisc
35 case too, the catalytic converter i~ exposed to an
I~l/CO m~Lxtura and~r standard op~rating conditioaca.
However, the ntc~ar~u~~~u~smL cuzvrs slZOws tlu~t the
convwrsion of hydrog~n and for carbon monoxide remains
al; ~aWat,~xllLla~,ly coizstant high lo~rele~ over more than
l0
SEP.ia.200i 11:50AM CRnLIELL & MnRINr LLP NO.=~28 P.19
CA 02367848 2001-09-18
200 hours. The conversion can in each caQO bo unproved
furth.~r, since th~ operati~.g conditioxut have not been
_ optima.zed.
1i catalytic coizve,rl,er which has beAn produe~d in
accordance wi.rh. the inwr~tioa is particularly tw~istant
to erooioa, and itn prvc3uctivn sari be surc~es~sfully
reproduced. The clRposition para~metors of the
catalyt~,aally active mat~.c~l~l on Che substrate ea.n
3,0 easily be optimized for. diffaxent matsri~.lo which are
to be deposited by suitak~ly selecting the d~ rPat
vvltaQe vd~. alzet-nating voltac~! amplitude V~~ and/or
.frAquency. proceae control im simple, uzic3 the catalytic
comver~~i~ proper'Cies cats be resproduoibly sot by Aimple
modifi.oatiox~.~a to the depopition proceao, Thd yield oZ
material is qooa. so 'Chat, for examplQ fox highly
aetivp platir~um catalytic oonverter~s, relat.lvely small
quantities of they preciou' metal have to ha used.
30 A preferred use few a eat.~,lytie convertAr produced
according to thw inverstioxi is ucc in a CO-rich
~avironmcnt, is partivuldx~ in e.n exhaust-gas a~wr~nin~
system in a motor vehinl.e. A further prwferxcd uoe of a~
catalytic corivort~r according to L~ae i~ivenLion is~ a.ts
use in a fuel Cell syst~tn.
11