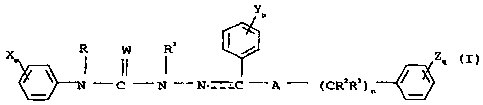Note: Descriptions are shown in the official language in which they were submitted.
CA 02367984 2001-09-11
WO 00/54591 PCT/US00/05879
-1-
SYNERGISTIC INSECTICIDAL COMPOSITIONS
BACRGROUND OF THE INVENTION
Insecticidal agents and compositions have been
developed to control insect pests such as
agrohorticultural pests, hygienic pests, or wood-eating
pests and in practice have been used as a single or a
mixed agent. However, economically efficient and
ecologically safe insect control compositions are still
being sought. Insecticidal compositions which allow
for reduced effective dosage rates, increased
environmental safety and lower incidence of insect
resistance are highly desirable. Although the
rotational application of insect control agents having
different modes of action may be adopted for good pest
management practice, this approach does not necessarily
give satisfactory insect control. Further, even though
combinations of insect control agents have been
studied, a high synergistic action has not always been
found. Obtaining an insecticidal composition which
demonstrates no cross-resistance to existing
insecticidal agents, no toxicity problems and little
negative impact on the environment is extremely
difficult.
Therefore, it is an object of this invention to
provide a synergistic insecticidal composition which
demonstrates a high controlling effect with concomittant
reduced crop production cost and reduced environmental
load.
CA 02367984 2001-09-11
WO 00/54591 PCT/US00/05879
-2-
It is another object of this invention to provide
methods for synergistic insect control and enhanced
crop protection.
SUMMARY OF THE INVENTION
The present invention provides a synergistic
insecticidal composition comprising as essential active
ingredients a synergistically effective amount of a
neuronal sodium channel antagonist in combination with
one or more compounds selected from the group
consisting of pyrethroids, pyrethroid-type compounds,
recombinant nucleopolyhedroviruses capable of
expressing an insect toxin, organophosphates,
carbamates, formamidines, macrocyclic lactones,
amidinohydrazones, GABA (gamma-aminobutyric acid)
antagonists, and acetylcholine receptor ligands.
The present invention also provides a method for
synergistic insect control which comprises contacting
said insect with a synergistically effective amount of
a neuronal sodium channel antagonist in combination
with one or more compounds selected from the group
consisting of pyrethroids, pyrethroid-type compounds,
recombinant nucleopolyhedroviruses capable of
expressing an insect toxin, organophosphates,
carbamates, formamidines, macrocyclic lactones,
amidinohydrazones, GABA antagonists and acetylcholine
receptor ligands.
The present invention further provides a method
for the enhanced protection of plants from infestation
and attack by insects.
CA 02367984 2001-09-11
WO 00/54591 PCT/USOO/05879
-3-
DETAILED DESCRIPTION OF THE INVENTION
Definitions
- Acetylcholine receptor ligand compound - as used
in this application means a compound which is capable
of binding to the acetylcholine receptor site.
,, Group A- as used in this application means
insecticidal
1) pyrethroid compounds;
2) pyrethroid-type compounds;
3) recombinant nucleopolyhedroviruses capable of
expressing an insect toxin;
4) organophosphate compounds;
5) carbamate compounds;
6) formamidine compounds;
7) macrocyclic lactone compounds;
8) amidinohydrazone compounds;
9) GABA antagonist compounds; and
10) acetylcholine receptor ligand compounds.
,, Haloalkyl" as used in this application means an
alkyl group C,H2X+1 having 1 to 2x+1 halogen atoms which
may be the same or different. Similarly, the terms
- haloalkenyl" , " haloalkynyl" , " haloalkoxy ,
- halophenyl,, and the like mean mono- to perhalogen
substitution wherein the halogens may be the same or
different.
- Halogen - as used in this application means Cl,
Br, I or F.
- Neuronal sodium channel antagonist as used in
this application means a compound which is capable of
preventing the ability of a neuron cell to transfer
sodium ions across the cell membrane.
-Pyrethroid-type compounds " as used in this
application means those compounds characterized by a
non-ester linked aryl-phenoxybenzyl moiety.
CA 02367984 2001-09-11
WO 00/54591 PCT/US00/05879
-4-
Synergism , as used in this application means a
cooperative action encountered in a combination of two
or more biologically active components in which the
combined activity of the two or more components exceeds
the sum of the activity of each component alone.
Surprisingly, it has now been found that a
composition which comprises a combination of a neuronal
sodium channel antagonist and a second insecticidal
ingredient provides superior insect control at lower
levels of the combined active agents than may be
achieved when the neuronal sodium channel antagonist or
the second insecticidal ingredient is applied alone.
As previously stated, the term neuronal sodium
channel antagonist designates a compound which is
capable of preventing the ability of a neuron cell to
transfer sodium ions across the cell membrane. A
neuron cell thus affected is unable to fire, resulting
in paralysis, and ultimately mortality, in the target
host. Descriptions of neuronal sodium channel
antagonists and their mode of action may be found in
Pesticide Biochemistry and Physiology, 60: 177-185 or
Archives of Insect Biochemistry and Physiology, 37: 91-
103.
Neuronal sodium channel antagonists include
compounds such as those described in U.S. 5,543,573;
U.S. 5,708,170; U.S. 5,324,837 and U.S. 5,462,938,
among other publications. Exemplary of the neuronal
sodium channel antagonist compounds useful in the
composition of this invention are those compounds
having the structural formula
CA 02367984 2001-09-11
WO 00/54591 PCT/US00/05879
-5-
Y
p
X m R W R1 ` Zq
N-C-N-N-C-A- (CRZR3 / ) n
(I)
X'
m
i
(CR~R8)r t
N ~R9
Ri0 Yi N W
I - p II
W=C-N N-C-N-Q
R11 R OJ G
33
(II) (III)
wherein A is CRQRS or NR6;
W is O or S;
X, Y, Z, X', Y' and Z' are each independently H;
halogen; OH; CN; NO2; Cl-C6alkyl optionally
substituted with one or more halogen, C1-
C3alkoxy, Cl-C3haloalkoxy, C3-
C6cycloalkyl, C2-C6alkenyloxy or
sulfonyloxy groups;
C1-C6alkoxy optionally substituted with one
or more halogen, C1-C3alkoxy or C3-
C6cycloalkyl groups;
C1-C6alkoxycarbonyl, C3-
C6cycloalkylcarbonyloxy, phenyl optionally
substituted with one or
more halogen, Cl-C4alkyl, or C1-C,alkoxy
groups;
CA 02367984 2001-09-11
WO 00/54591 PCT/US00/05879
-6-
aminocarbonyloxy optionally substituted with
one or more Cl-C3alkyl groups;
Cl-C6alkoxycarbonyloxy; C1-C6alkylsulfonyloxy;
C2-C6alkenyl; or NR12R13;
m, p and q are each independently an integer of 1,
2, 3, 4, or 5;
n is an integer of 0, 1 or 2;
r is an integer of 1 or 2;
t is an integer of 1, 2, 3 or 4;
R, Rl, R2, R3, R4 and RS are each independently H or
Cl-C4alkyl;
R6 is H, Cl-C6alkyl, C1-C6haloalkyl, Cl-C6alkoxyalkyl,
Cl-C6alkoxy, Cl-C6haloalkoxy, CZ-C6alkenyl,
C2-C6alkynyl, Cl-C6alkylcarbonyl, Cl-C6alkoxy-
carbonyl, Cl-C6alkylthio, or Cl-
C6haloalkylthio;
R7 and R. are each independently H; halogen;
Cl-C6alkyl; Cl-C6alkylcarbonyloxy; or phenyl
optionally substituted with one or more
halogen, CN, NO2, C,-C6alkyl, Cz-C6halo-
alkyl, Cl-C6alkoxy or Cl-C6haloalkoxy
groups;
R9 and Rlo are each independently H, or C1-C4alkyl;
Rll is H, Cl-C6alkyl, Cl-C6haloalkyl, Cl-C4alkyl-
carbonyl, C1-C6alkoxycarbonyl, or C1-C6halo-
alkoxycarbonyl;
R12 and R13 are each independently H or C1-C6alkyl;
G is H; C1-C6alkyl optionally substituted with one
or more halogen, Cl-C4alkoxy, Cl-
C6haloalkoxy, CN, NOzS (0) uR14, COR15,
C02R16 , phenyl or
C3--C6cycloalkyl groups;
C1-C6alkoxy; Cl-C6haloalkoxy; CN; NO2; S(O) õRl,;
COR1e; C02R19; phenyl optionally substituted
with one or more halogen, CN, C1-C3halo-
CA 02367984 2001-09-11
WO 00/54591 PCTIUSOO/05879
-7-
alkyl, or C1-C3haloalkoxy groups;
C3-C6cycloalkyl; or phenylthio;
Q is phenyl optionally substituted with one or
more halogen, CN, SCN, NO21 S(O) uR20, Cl-
C4alkyl,
Cl-C4haloalkyl, Cl-C4alkoxyalkyl, C1-C6alkoxy,
Cl-C6haloalkoxy, or NR21R22 groups;
u is an integer of 0, 1 or 2;
R14, R15, R16, Rla, R19, R21 and R22 are each
independently H or C1-C6alkyl;
R17 and R20 are each independently Cl-C6alkyl or
Cl-C6haloalkyl ;
R33 1.S' CO2R34 ,
R34 is H, Cl-C6alkyl, Cl-C6haloalkyl, phenyl or
halophenyl; and the dotted line configuration
C-N represents a double bond or a single bond
(i.e. C-N or C=N); or
a stereoisomer thereof.
Preferred neuronal sodium channel antagonists
suitable for use in the composition of the invention
are those compounds of formula I, II or III wherein the
dotted line configuration C=_N represents a double
bond.
More preferred neuronal sodium channel antagonists
suitable for use in the inventive composition are those
compounds of formula I or formula III wherein the
dotted line configuration represents a double bond.
Particularly preferred neuronal sodium channel
antagonists useful in the composition of the invention
are those compounds of formula I or formula III wherein
W is 0; X is trifluoromethoxy and is in the 4-position;
Y is trifluoromethyl and is in the 3-position; Z is CN
and is in the 4-position; A is CH2; n is 0; m, p and q
are each 1; R and R. are each H; Z, is Cl; R33 and G are
each CO2CH3; Q is p- (trifluoromethoxy) phenyl; and the
CA 02367984 2001-09-11
WO 00/54591 PCT/US00/05879
-8-
dotted line configuration C-=-N represents a double
bond; or a stereoisomer thereof.
Further neuronal sodium channel antagonist
compounds include those described in U.S. 5,116,850 and
U.S. 5,304,573, among other publications. Exemplary of
further neuronal sodium channel antagonist compounds
suitable for use in the composition of the invention
are those compounds having structural formula
Xii 23 W Y
m aRR2 4 ff m R2 6 w R2 7
N-C TNI R25 N-C-C-N i -4
Q,
N
G'
(IV) (V)
wherein W is 0 or S;
X" and Y" are each independently H; halogen; CN;
SCN; C1-C6alkyl optionally substituted with
one or
more halogen, NO2, CN, C1-C4alkoxy,
C1-C4alkylthio, phenyl, halophenyl,
Cl-C4alkylsulfonyl, Cl-
C4haloalkylsulfonyl, or Cl-
C4alkoxycarbonyl groups;
C2-C4alkenyl; C2-C4haloalkenyl; C2-C4alkynyl;
C2-C4haloalkynyl; C3-C6cycloalkyl; C3-
C6halocycloalkyl; phenyl optionally
substituted
with one or more halogen, CN, NO2, C1-
C4alkyl, C1-C4haloalkyl, Cl-Caalkoxy, Cl-
C4haloalkoxy, C1-C4alkylthio, C1-
C4alkylsulfonyl or Cl-C4haloalkylsulfonyl
groups;
C1-C4alkylcarbonyl; C1-C4haloalkylcarbonyl; or
CA 02367984 2001-09-11
WO 00/54591 PCT/USOO/05879
-9-
NR28R29;
m is an integer of 1, 2, 3, 4 or 5;
G' is phenyl optionally substituted with one or
more groups which may be the same or
different selected from X";
a 5-membered heteroaromatic ring containing
one or two heteroatoms selected from 0
or 1 oxygen, 0 or 1 sulfur and 0, 1 or 2
nitrogen atoms said 5-membered
heteroaromatic ring being attached via
carbon and being optionally substituted
with one or more groups which may be the
same or different selected from X"; or
a 6-membered heteroaromatic ring containing
one or two heteroatoms selected from 0
or 1 oxygen, 0 or 1 sulfur and 0, 1 or 2
nitrogen atoms said 6-membered
heteroaromatic ring being attached via
carbon and being optionally substituted
with one or more groups which may be the
same or different selected from X";
Q is H; C1-C6alkyl optionally substituted with one
or more halogen, CN, Cl-C3alkoxy, C1-
C6alkoxycarbonyl, or phenyl optionally
substituted with one or more
halogen, CN, NO2, Cl-CQalkyl, C1-
C4haloalkyl,
C1-C4alkylsulfonyl or C1-CQalkyl-
sulfinyl groups;
C2-C6alkenyl; C2-C6alkynyl; or phenyl
optionally substituted with one to three
groups, which may be the same or
different, selected from X" ;
R23, R24, RZ5, R26, R27, R28 and R29 are each
independently H or C1-C,alkyl; and the dotted
CA 02367984 2008-03-12
-10-
line configuration C.-N represents a double bond
or a single bond (i.e. C-N or C=N); or
a stereoisomer thereof.
Further preferred neuronal sodium channel
antagonist compounds of the invention are those
compounds of formula IV or V wherein the dotted line
configuration C-_N represents a double bond.
Other preferred neuronal sodium channel antagonist
compounds suitable for use in the composition of the
invention are those compounds of formula IV or V
wherein W is 0; X" and Y' are each independently H or
Cl-C,haloalkyl; m is 1; R,,, R24, R,S, R2j and R,7 are each
H; G is phenyl optionally substituted with one or more
halogen atoms; Q' is halophenyl or C1-C,alkyl optionally
substituted with one phenyl or halophenyl group; and
the dotted line configuration C-N represents a double
bond; or a stereoisomer thereof.
In the invention as claimed, the sodium channel antagonists are however
restricted to those of the formula I as defined hereinabove,
wherein
A is CR4R5 or NR6;
W is O or S;
X, Y and Z are each independently H; halogen; OH; CN; NOz;
Cl-C6-alkyl unsubstituted or substituted with one or
more halogen, Cl-C3 alkoxy, Cl-C3 haloalkoxy, C3-
C6 cycloalkyl, C2-C6-alkenyloxy or sulfonyloxy groups;
Cl-C6 alkoxy unsubstituted or substituted with one or
more halogen Cl-C3-alkoxy or C3-C6-cycloalkyl
groups;
CA 02367984 2008-03-12
-10a-
Cl-C6-alkoxycarbonyl, C3-C6-cycloalkylcarbonyloxy,
phenyl unsubstituted or substituted with one or more
halogen, C,-C4 alkyl or Cl-C4 alkoxy groups;
aminocarbonyloxy unsubstituted or substituted with
one or more C,-C3-alkyl groups;
Cl-C6-alkoxycarbonyloxy; Cl-C6-alkylsulfonyloxy; C2-
C6-alkenyl; or NR7R8;
m, p and q are each independently an integer of 1, 2, 3, 4 or 5;
n is an integer of 0, 1 or 2;
R, RI, R2, R3, R4 and R5 are each independently H or Cl-C4 alkyl;
R6 is H, C,-C6-alkyl, C,-Cs-haloalkyl, C,-C6 alkoxyalkyl,
Cl-C6-alkoxy, C,-C6-haloalkoxy, C2-C6-alkenyl,
C2-C6-alkynyl, Cl-Cs-alkylcarbonyl, CI-Cs-
alkoxycarbonyl, C,-C6-alkylthio, or Cl-C6
haloalkylthio;
Wand R8 are each independently H or C,-C6-alkyl;
and the dotted line configuration
C N
represents a double bond or a single bond.
The second active ingredient of the insecticidal
composition of the invention includes one or more
compounds selected from Group A:
1) pyrethroid compounds which are known to be
insecticidally active such as cypermethrin,
cyhalothrin, cyfluthrin, permethrin or the like;
2) pyrethroid-type compounds which are known to
be insecticidally active such as ethofenprox,
silafluofen, or the like;
3) recombinant nucleopolyhedroviruses capable of
expressing an insect toxin, preferably an insect
CA 02367984 2008-03-12
-10b-
neurotoxin such as Androctonus australis insect toxin
(AaIT), for example HzNPV-AaIT;
4) organophosphate compounds which are known to
be insecticidally active such as profenofos, acephate,
sulprofos, malathion, diazinon, methyl parathion,
terbufos, or the like;
CA 02367984 2001-09-11
WO 00/54591 PCT/US00/05879
-11-
5) carbamate compounds which are known to be
insecticidally active such as methomyl, thiodicarb,
fenothiocarb, or the like;
6) formamidine compounds which are known to be
insecticidally active such as amitraz, chlordimeform,
hydramethylnon, chlorfenamidine, or the like;
7) macrocyclic lactone compounds which are known
to be insecticidally active such as spinosad,
avermectin, emamectin, milbemectin, nemadectin,
moxidectin or the like;
8) amidinohydrazone compounds which are known to
be insecticidally active such as hydramethylnon;
9) GABA antagonist compounds which are known to
be insecticidally effective such as fipronil,
endosulfan, or the like;
10) acetylcholine receptor ligand compounds which
are known to be insecticidally effective such as
imidacloprid, acetamiprid, nitenpyram, thiamethoxam, or
the like.
Descriptions of the above-listed commercially
available compounds may be found in The Pesticide
Manual, llth Edition, British Crop Protection Council
(1997)among other publications. Descriptions of
recombinant nucleopolyhedroviruses capable of
expressing an insect toxin include Treacy et al,
Proceedings Beltwide Cotton Conference (1999), pp 1076-
1083.
Preferred compositions of the invention are those
compositions having a neuronal sodium channel
antagonist compound of formula I or formula III in
combination with one or more compounds selected from
Group A.
More preferred compositions of the invention are
those compositions having a formula I or formula III
compound wherein W is 0; X is trifluoromethoxy and is in
CA 02367984 2001-09-11
WO 00/54591 PCT/US00/05879
-12-
the 4-position; Y is trifluoromethyl and is in the 3-
position; Z is CN and is in the 4-position; A is CH3; n
is 0; m, p and q are each independently 1; R and R1 are
each independently H; Z' is Cl; R33 and G are each
independently COZCH3; Q is p- (trifluoromethoxy)phenyl;
and the dotted line configuration C=-N represents a
double bond in combination with one or more compounds
selected from Group A.
Each of the compounds of formula I, II, III, IV
and V embody assymetric centers which may be
represented in the stereoisomeric R-form or S-form.
The present invention also includes the R-form, the S-
form or mixtures comprising the R-form and the S-form
in an arbitrary ratio. For compounds of formula III,
the S-form is preferred.
Advantageously, the neuronal sodium-channel
antagonist compound of formula I, II, III, IV or V or a
mixture thereof may be formulated with a second
insecticidally effective ingredient and optionally other
customary formulation adjuvants. Said formulation may
be dispersed in a solid or liquid diluent for
application to the insect, its food supply, breeding
ground or habitat as a dilute spray or as a solid dust
or dust concentrate.
The active ingredients of the inventive
composition may also be formulated separately as a
wettable powder, emulsifiable concentrate, aqueous or
liquid flowable, suspension concentrate or any one of
the conventional formulations used for insect control
agents and tank-mixed in the field with water or other
inexpensive liquid for application as a liquid spray
mixture. The separately formulated compositions may
also be applied sequentially.
Advantageously, the composition of the invention
may be formulated as a bait composition comprising a
CA 02367984 2001-09-11
WO 00/54591 PCT/t7S00/05879
-13-
synergistically effective amount of a combination of a
neuronal sodium channel antagonist plus one or more
compounds selected from Group A and a solid or liquid
edible nutritive substance. A preferred bait
composition may contain by weight about 0.01a to 20%
active ingredients, preferably a neuronal sodium
channel antagonist in combination with hydramethylnon.
In actual practice, the composition of the
invention may be applied to the plant foliage or plant
stem or to the insect habitat or to the locus of a
hygienic pest as a dilute spray prepared from any of
the above-said formulations. The ratio of the
essential active ingredients of the composition of the
invention is about 1 weight part of a neuronal sodium
channel antagonist to about 0.01-100 weight parts of
one or more compounds selected from Group A.
The compositions of the invention are superior
insecticidal compositions and are especially useful for
the control of agrohorticultural pests, hygienic pests
or wood-eating pests. Said compositions are highly
effective for the protection of growing and harvested
plants including: leguminous crops such as soybeans,
snap beans, peas, wax beans and the like as well as
cotton, forage crops, cole crops, leafy vegetables,
tobacco, hops, tomatoes, potatoes, flowering
ornamentals such as chrysanthemums, vine crops such as
grapes, squash, pumpkin or melon and fruit trees such
as cherry, peach, apple or citrus, from the ravages of
insects.
The synergistic insecticidal composition of the
invention is found to be highly active against a wide
variety of lepidopteran and coleopteran insects such as
Helicoverpa zea (cotton bollworm), Heliothis virescens
(tobacco budworm), Leptinotarsa decemlineata(Colorado
CA 02367984 2001-09-11
WO 00/54591 PCTIUSOO/05879
-14-
potato beetle), Diabrotica spp. (corn rootworm) and the
like.
Beneficially, the composition of the invention may
be useful for the prevention and control of hygienic or
public health pests such as: Diptera, e.g. houseflies,
mosquitoes, or the like; Hymenoptera, e.g. ants,
parasitic wasps, wasps or the like; Blattaria, e.g.
cockroaches; or the like.
Further, the compositions of the invention may be
particularly useful for the prevention and control of
wood-eating insects such as termites (Isoptera),
carpenter ants (Hymenoptera), wood-destroying beetles
(Coleoptera) or the like.
These and other advantages of the invention may
become more apparent from the examples set forth herein
below. These examples are provided merely as
illustrations of the invention and are not intended to
be construed as a limitation thereof.
CA 02367984 2001-09-11
WO 00/54591 PCT/USOO/05879
-15-
E7CAMPLE 1
Evaluation of the Synergistic Insecticidal Effect
of a Combination of a Neuronal Sodium Channel
Antagonist Plus a Second Insecticide
In this evaluation, Heliothis zea (cotton
bollworm), Heliothis virescens (tobacco budworm) and
pyrethroid-resistant Heliothis virescens larvae used are
obtained from laboratory colonies. Pyrethroid-resistant
H. virescens are derived from the PEG-strain [Campannola
& Plapp, Proceedings of Beltwide Cotton Conference
(1988)].
Cotton leaves are immersed in 1:1 v/v,
acetone/water solutions of test compound, or solutions
of a combination of test compounds for a period of about
3 seconds. Following immersion, leaves are allowed to
air-dry for 2-3 hours. Plastic bioassay trays
containing multiple open-faced wells (4.0 x 4.0 x 2.5
cm) are used as the test arenas. Cut portions of a
treated leaf, a moistened cotton dental wick and a
single third-instar larva are placed into each well,
covered with an adhesive vented clear plastic sheet and
held under constant fluorescent light at about 27 C for
a predetermined period of time. Larval
mortality/morbidity is evaluated at 5 days after
treatment. All treatments are replicated 4-5 fold in a
randomized complete block design with 16-32 larvae per
treatment. Using conventional log-probit analysis, the
LCso of each treatment is determined.
Using the above protocol, a neuronal sodium channel
antagonist (Compound A) may be evaluated alone at dose
rates of 0.1 ppm, 1.0 ppm and 10.0 ppm and in
combination with 1.0 ppm of a second insecticidal
CA 02367984 2001-09-11
WO 00/54591 PCT/US00/05879
-16-
compound. Treatments which may be used are shown in
Table I.
CA 02367984 2001-09-11
WO 00/54591 PCT/US00/05879
-17-
Table I
Second Dose Compound Al
Active Rate Dose Rate
Compound (ppm) (ppm) (ppm) (ypm) (ppm)
cypermethrin 0 0 0.1 1.0 10.0
1.0 0 0.1 1.0 10.0
amitraz 0 0 0.1 1.0 10.0
1.0 0 0.1 1.0 10.0
fipronil 0 0 0.1 1.0 10.0
1.0 0 0.1 1.0 10.0
acetamiprid 0 0 0.1 1.0 10.0
1.0 0 0.1 1.0 10.0
spinosad 0 0 0.1 1.0 10.0
1.0 0 0.1 1.0 10.0
thiodicarb 0 0 0.1 1.0 10.0
1.0 0 0.1 1.0 10.0
1Compound A formula Ia neuronal sodium channel antagonist
CF3
O
/ ~ H II H ~ ~
F3CO N-C-NN=C-CHZ CN
(Ia)
CA 02367984 2001-09-11
WO 00/54591 PCT/US00/05879
-18-
EXAMPLE 2
Evaluation of the Svneraistic Insecticidal Effect Of a
Combination Of A Neuronal Sodium Channel Antagonist
Plus an Amidinohydrazone
In this evaluation, adult male German cockroaches
(Blattella germanica) are used. For each test, a 4.0 g
portion of ground Purina Dog Chow (Hi-Pro Glo ) is
treated with an acetone solution of test compound alone
or in combination with a second test compound. After
treatment, the acetone is evaporated and the treated
dog chow is placed in a 3/4 oz plastic cup which is
placed in a harborage made of folded sheets of blotter
paper placed in a plastic box (16,, L x 11 W x 6,, H) .
The plastic box (test arena) is also fitted with a 1 oz
narrow mouth bottle with 2 dental wicks inserted at the
mouth. A control box is prepared in the same manner
using ground dog chow which has been treated with
reagent grade acetone. Each treatment is replicated
three times. Into each test arena are placed 20
healthy adult male cockroaches which have been reared
in an insectary. The test arenas are then stored at
76 F and mortality is determined daily by visual
examination. The data obtained are shown in Table II.
CA 02367984 2001-09-11
WO 00/54591 PCT/US00/05879
-19-
Table II
% Mortality
Test ~ Active Days After Treatment
Compound Ingredien 3 4 5 6 7 8
A' 0.05 0 0 0 0 0 0
A 0.10 1.7 11.7 11.7 11.7 18.3 18.3
A 0.50 5.0 5.0 5.0 5.0 5.0 5.0
B2 1.00 0 5.0 28.3 71.7 90.0 93.3
A+B 0.05+1.0 0 20.0 41.7 81.7 95.0 98.3
A+B 0.10+1.0 0 21.7 51.7 88.3 95.0 95.0
A+B 0.50+1.0 16.7 58.3 80.0 95.0 98.3 100.0
Control 0 0 1.7 3.3 3.3 3.3 5.0
'Compound A = formula Ia neuronal sodium channel antagonist
2Compound B = hydramethylnon
CF3
0
~ ~ H II H ~ ~
F3C0 N-C-NN=C-CHZ CN
(Ia)
As can be seen from the data shown in Table II,
combinations of a neuronal sodium channel antagonist
plus an amidinohydrazone insecticide demonstrate
synergistic insect control.
CA 02367984 2001-09-11
WO 00/54591 PCT/US00/05879
-20-
EXAMPLE 3
Evaluation of the Synergistic Insecticidal Effect Of a
Combination Of A Neuronal Sodium Channel Antagonist
Plus A Recombinant Nucleopolyhedrovirus Capable Of
Expressincr An Insect Toxin
In this evaluation, Helicoverpa zea (cotton
bollworm) larvae are obtained from a laboratory colony.
Test compounds are dissolved in 1:1 v/v acetone/water.
Plastic bioassay trays (C-D International, Pitman, NJ)
are used as test arenas. Each tray contains 32 open-
faced wells, 4.0 x 4.0 x 2.5 cm. A portion (5 ml) of a
wheat germ-soybean flour-based artificial diet
(Southland Products, Lake Village, AR) is poured into
each well. After the diet hardened, 0.4 ml of test
solution is pipetted onto the diet surface in each
well. Test solutions are evenly spread over surfaces of
diet by picking up the tray and gently tilting it from
side to side. Trays are then held in a vented area for
about 2 h, until water is no longer pooled on diet
surfaces. A single 4-day-old H. zea larva is then
placed on the surface of diet in each well. After
larval infestation, each well is covered with an
adhesive, vented, clear plastic sheet.
All test arenas are held under constant
fluorescent light and a temperature of about 27 C for
duration of the assay. Larval mortality is determined
at 2, 3, 4 and 7 days after treatment. A larva was
considered to be dead if it exhibited little to no
movement, even after being shaken in the diet tray. A
total of 32 insects were tested for each treatment.
CA 02367984 2001-09-11
WO 00/54591
PCT/US00/05879
-21-
The data obtained are shown in Table III.
Table III
Conc. of % Mortality
Test Active Days After Treatment
Compound Inaredient 2 3 4 7
A' 0.1 ppm 43.8 46.9 53.1 53.1
B2 1000 0B3/ml 3.1 34.4 50.0 62.5
B 500 OB/ml 0.0 9.4 18.8 40.6
B 100 OB/ml 3.1 3.1 3.1 15.6
A+B 0.1+1000 87.5 90.6 93.8 96.9
A+B 0.1+500 75.0 78.1 84.4 87.5
A+B 0.1+100 62.5 75.0 75.0 78.1
Control 0 3.1 3.1 3.1 3.1
'Compound A = formula Ia neuronal sodium channel antagonist
2Compound B = HzNPV-AaIT, Helicoverpa zea Nucleopolyhedrovirus
which expresses Androctonus australis insect toxin
30B = viral occlusion bodies
CF3
0
/ \ H H / \
F3C0 N-C-NN=C-CH2 CN
(Ia)
As can be seen from the data shown in Table III,
combinations of a neuronal sodium channel antagonist
plus a recombinant nucleopolyhedrovirus which is
CA 02367984 2001-09-11
WO 00/54591 PCTIUSOO/05879
-22-
capable of expressing an insect toxin demonstrate
synergistic insect control.