Note: Descriptions are shown in the official language in which they were submitted.
CA 02369137 2008-04-24
CA 02369137 2001-10-09
WO 00/61370 PCTIEP00102666
Description
MULTILAYER CONTAINERS EXHIBITING AN IMPROVED ADHERENCE BETWEEN
POLYMER LAYERS AS WELL AS EXCELLENT BARRIER CHARACTERISTICS
The present invention relates to multilayer containers comprising at least
one cycloolefin polymer, to a process for producing these multilayer con-
tainers and to the use of these multilayer containers.
JP-A-4 276 253 discloses multifayer containers comprising a layer of a
thermoplastic saturated norbornene polymer which has been prepared by
ring-opening metathesis polymerization and a further layer of a thermo-
plastic polymer having good gas barrier properties.
JP-A-9 239 909, JP-A-7 171 858 and JP-A-9 011 416 disclose
polyolefin/polycycloolefin multilayer containers which are suitable for the
packaging of foods due to a further layer of a thermoplastic polymer having
good oxygen barrier properties. The use of a bonding layer which improves
adhesion between the different polymer layers is also described.
EP-A-824 067 and JP-A-10 059 343 likewise describe muitilayer containers
which are made up of polyolefin layers and polycycloolefin layers and are
able to keep in flavors and are thus suitable, for example, for the packaging
and storage of toothpaste. In these patent publications, it is stated that the
layers of various polymers are joined by means of bonding layers.
It is an object of the present invention to provide multilayer containers
having improved adhesion between the layers and also an economical and
environmentally friendly process for producing multilayer containers. For
the purposes of the present invention, multilayer containers are containers
which are made up of two or more layers of polymers.
The object of the present invention is achieved by multilayer containers
comprising suitable cycloolefin polymers.
CA 02369137 2007-10-02
1A
Brief Description of the Drawings
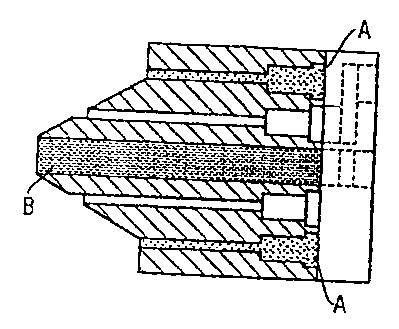
Figure 1 shows an injection head used in accordance to produce a multilayer
container in an embodiment of this disclosure;
Figure 2 shows an injection head used in accordance to produce a multilayer
container in an embodiment of this disclosure;
Figure 3a and 3b show the injection profile for Polyester/Topas 6013; and
Figure 4a and 4b shows the injection profile for Trogamid/Topas 6013.
The multilayer containers of the invention comprise at least one cycloolefin
polymer which comprises polymerized units derived from at least one cyclic,
in particular polycyclic, olefin and, if desired, from at least one
CA 02369137 2001-10-09
2
acyclic olefin. The term cycloolefin polymer encompasses cycloolefin
copolymers as well as cycloolefin homopolymers.
The multilayer containers of the invention comprise two or more polymer
layers of which at least one polymer layer comprises at least one
cycloolefin polymer comprising from 0.1 to 100% by weight, preferably from
0.1 to 99.9% by weight, particularly preferably from 3 to 75 mol%, based on
the total mass of the cycloolefin polymer, of polymerized units derived from
at least one polycyclic olefin of the formulae I, II, II', III, IV, V or VI
R
CH
I~H , CH CH R- C i
CH
IH
C C H C H \
I~H I CH
. I CH
(11)~
CHR'-C -R
CH
CHS
IH
C H C H,
I~H I CH
~ C H : (11),
CH3CR,
CH- ~
CIH
R
C H C H
I~H CH CH
(111)
CH R C- R' I RS- C-R6 I H CH C H C H R
R'
CH CH ~ ff C H CH CH CH
CH
H R- C- R' R C-Rf I R- I -Ra I (IV),
C
/CH\' CH CH
CH CH CH R
CA 02369137 2001-10-09
3
R
R'
CH C H I
C I H R'- I_ R' IH iH (V),
CH C \\ CH \ R'
CH CFt~
R2
R'
(
CH CH CH \.' R,
I~H CH CH CH
CH R C- R' H H R'_ C-Rb I (VI),
CH CH CH I CH \R,
R
where R1, R2, R3, R4, R5, R6, R' and R8 are identical or different and are
each a hydrogen atom or a Cl-C20-hydrocarbon radical such as a linear or
branched Cl-C8-alkyl radical, C6-C18-aryl radical, C7-C20-alkylenaryl
radical, a cyclic or acyclic C2-C20-alkenyl radical or form a saturated,
unsaturated or aromatic ring, where identical radicals R1 to R8 in the
various formulae I to VI may have different meanings and n is from 0 to 5,
and from 0 to 99.9% by weight, preferably from 0.1 to 99.9% by weight,
particularly preferably from 5 to 80 mol /a, based on the total mass of the
cycloolefin polymer, of polymerized units derived from one or more acyclic
olefins of the formula VII
Rs R;o
õ-- _ ~~~ l= I I),
R R,2
where R9, R10~ R11 and R12 are identical or different and are each a
hydrogen atom or a linear, branched, saturated or unsaturated
Cl-C20-hydrocarbon radical such as a Cl-C8-alkyl radical or a C6-C18-aryl
radical.
CA 02369137 2001-10-09
4
The cyclic olefins also include derivatives of these cyclic olefins containing
polar groups such as halogen, hydroxyl, ester, alkoxy, carboxy, cyano,
amido, imido or silyl groups.
In addition, the cycloolefin polymers used according to the invention for
microstructured components can further comprise from 0 to 45% by weight,
based on the total mass of the cycloolefin polymer, of polymerized units
derived from one or more monocyclic olefins of the formula VIII
HC CH (Vlll),
~ 210
where m is from 2 to 10.
For the purposes of the present invention, preference is given to cycloolefin
polymers which comprise polymerized units derived from polycyclic olefins
of the formula I or III and polymerized units derived from acyclic olefins of
the formula VII.
Particular preference is given to cycloolefin polymers which comprise
polymerized units derived from olefins having a norbornene skeleton, very
particularly preferably from norbornene and tetracyclododecene and, if
desired, vinyinorbornene or norbornadiene. Particular preference is also
given to cycloolefin polymers which comprise polymerized units derived
from acyclic olefins having terminal double bonds, e.g. a-olefins having
from 2 to 20 carbon atoms, very particularly ethylene or propylene.
Exceptional preference is given to norbornene-ethylene and
tetracyclododecene-ethylene copolymers.
The preparation of the cycloolefin polymers can be carried out by means of
heterogeneous or homogeneous catalysis by organometallic compounds,
as described in many patents.
The cycloolefin polymers used according to the invention can be prepared
at temperatures of from -78 to 200 C and a pressure of from 0.01 to
200 bar in the presence of one or more catalyst systems which comprise at
least one transition metal compound and, if appropriate, a cocatalyst and, if
CA 02369137 2007-10-02
appropriate, a support material. Suitable transition metal compounds are
metallocenes, in particular stereorigid metallocenes. Examples of catalyst
systems which are suitable for the preparation of the cycloolefin polymers
used according to the invention are described in EP-A-407 870,
5 EP-A-485 893 and EP-A-503 422.
The cycloolefin polymers used according to the invention can be prepared
using a metallocene as transition metal compound and an aluminoxane of
the formula IXa
R13
R13 ( R13 IX a
~I--O Ai-O AI~
R13 P R,3
for the linear type and/or the formula lXb
R1a
I IX b
AJ-0
J F =2
for the cyclic type, where, in the formulae lXa and lXb, R13 is a CI-C6-alkyl
group or phenyl or benzyl and p is an integer from 2 to 50.
The transition metal component can be a metallocene of the formula X
/R1E
R14
R'a M' x
~17
where
M1 is a metal of groups 3 to 10 or the lanthanide series of the Periodic
Table of the Elements, preferably titanium, zirconium, hafnium, vanadium,
CA 02369137 2001-10-09
6
niobium or tantalum,
R14 and R15 are identical or different and are each a hydrogen atom, a
halogen atom, a Cl-Clp-alkyl group, a Cl-Clp-alkoxy group, a C6-Clp-aryI
group, a C6-Clp-aryloxy group, a C2-Clp-alkenyl group, a C7-C40-arylalkyl
group, a C7-C40-alkylaryl group or a C8-C40-arylalkenyl group,
R16 and R17 are identical or different and are each a monocyclic or
polycyclic hydrocarbon radical which can form a sandwich structure, with
the central atom M1,
Rie R19 Ri9 Rs R19 R's n
~ 2 I NR-- CR22, ~
I I f 1 1 11i
R~0 RZ0 R21 R= R20 R2o R21
BR , AIR ,
= 1919, -Ge-, -Sn-, -0-, -S-, SO2, NR~9, CO, PR~9 or P(O)R~9
where R19, R20 and R21 are identical or different and are each a hydrogen
atom, a halogen atom, a Cl-Clp-alkyl group, a Cl-Clp-fluoroalkyl group, a
C6-Clp-fluoroaryl group, a C6-Clp-aryl group, a Cg-Clp-aryloxy group, a
Cl-Clp-alkoxy group, a C2-Clp-alkenyl group, a C8-C40-arylalkyl group, a
C7-C40-alkylaryl group or a C8-C40-arylalkenyl group or R19, R20 and R21
in each case together with the atoms connecting them form a ring, and
M2 is silicon, germanium or tin.
Examples of transition metal compounds used are:
rac-dimethylsilylbis(1-indenyl)zirconium dichloride,
rac-dimethylgermylbis(1-indenyl)zirconium dichloride,
rac-phenylmethylsilylbis(1-indenyl)zirconium dichloride,
rac-phenylvinylsilylbis(1-indenyl)zirconium dichloride,
1-silacyclobutylbis(1-indenyl)zirconium dichloride,
rac-diphenylsilylbis(1-indenyl)hafnium dichloride,
rac-phenylmethylsilylbis(1-indenyl)hafnium dichloride,
rac-diphenylsilylbis(1-indenyl)zirconium dichloride,
rac-ethylene-1,2-bis(1-indenyl)zirconium dichloride,
dimethylsilyl(9-fluorenyl)(cyclopentadienyl)zirconium dichioride,
diphenyisilyl(9-fluorenyl)(cyclopentadienyl)zirconium dichloride,
bis(1-indenyl)zirconium dichloride,
diphenylmethylene(9-fluorenyl)cyclopentadienylzirconium dichioride,
CA 02369137 2001-10-09
7
isopropylene(9-fluorenyl)cyclopentadienylzirconium dichloride,
rac-isopropylidenebis(1-indenyl)zirconium dichloride,
phenylmethylmethylene(9-fluorenyl)cyclopentadienylzirconium dichloride,
isopropylene(9-fluorenyl)(1-(3-isopropyl)cyclopentadienyl)zirconium
dichloride,
isopropylene(9-fluorenyl)(1 -(3-methyl)cyclopentadienyl)zirconium
dichioride,
diphenylmethylene(9-fluorenyl)(1-(3-methyl)cyclopentadienyl)zirconium
dichloride,
methylphenylmethylene(9-fluorenyl)(1-(3-methyl)cyclopentadienyl)-
zirconium dichloride,
dimethylsilyl(9-fluorenyl)(1-(3-methyl)cyclopentadienyl)zirconium dichloride,
diphenylsilyl(9-fluorenyl)(1-(3-methyl)cyclopentadienyl)zirconium dichloride,
diphenylmethylene(9-fluorenyl)(1-(3-tert-butyl)cyclopentadienyl)zirconium
dichloride,
isopropylene(9-fluorenyl)(1-(3-tert-butyl)cyclopentadienyl)zirconium
dichloride,
isopropylene(cyclopentadienyl)(1-indenyl)zirconium dichloride,
diphenylcarbonyl(cyclopentadienyl)(1-indenyl)zirconium dichloride,
dimethylsilyl(cyclopentadienyl)(1-indenyl)zirconium dichloride,
isopropylene(methylcyclopentadienyl)(1-indenyl)zirconium dichloride,
4-(rI5-cyclopentad ienyl)-4,7,7-trimethyl(,q5-4,5,6,7-tetrahyd roindenyl-
zirconium dichloride,
[4-(115-cyclopentad ienyl)-4,7,7-triphenyl(,q 5-4, 5,6,7-tetrahyd roindenyl)]-
zirconium dichloride,
[4-(rl5-cyclopentad ienyl)-4,7-d imethyl-7-phenyl(r15-4,5,6,7-tetrahydro-
indenyl)]zirconiurn dichloride,
[4-(ri5-3'-tert-butylcyclopentad ienyl)-4,7,7-triphenyl(r15-4,5,6,7-tetra-
hydroindenyl)]zirconium dichloride,
[4-(r15-3'-tert-butylcyclopentadienyl)-4,7-dimethyl-7-phenyl(,n 5-4,5,6,7-
tetra-
hydroindenyl)]zirconium dichloride,
[4-(r15-3'-methylcyclopentad ienyl)-4,7, 7-trimethyl(r15-4,5,6,7-tetrahydro-
indenyl)]zirconium dichloride,
[4-r15-3'-methylcyclopentadienyl)-4,7,7-trip henyl(,q 5-4,5,6, 7-tetrahydro-
indenyl)]zirconium dichioride,
[4-(rl 5-3'-methytcyclopentadienyl)-4,7-d imethyl-7-phenyl(r1 5-4,5,6,7-tetra-
hydroindenyl)]zirconium dichloride,
[4-(ri5-3'-isopropylcyclopentad ienyl)-4,7,7-trimethyl(,q 5-4,5,6,7-tetrahyd
ro-
indenyl)]zirconium dichloride,
CA 02369137 2007-10-02
8
[4-(r15-3'-isopropylcyclopentadienyl)-4,7,7-triphenyl(r15-4,5,6,7-tetrahydro-
indenyl)]zirconium dichloride,
[4-(r15-3'-isopropylcyclopentadienyl)-4,7-dimethyl-7-phenyl(r15-4,5,6,7-tetra-
hydroindenyl)]zirconium dichloride,
[4-(-q 5-cyclopentadienyl)(r15-4,5-tetrahydropentalene)]zirconium dichloride,
[4-(rl -3'-cyclopentadienyl)-4-methyl(ri 5-4,5-tetrahydropentalene)]zirconium
dichloride,
[4-(r1 5-cyclopentadienyl)-4-phenyl(rt5-4, 5-tetrahyd ropentalene)]zirconium
dichioride,
[4-(rl5-cyclopentadienyl)-4-phenyl(,15-4,5-tetrahydropentalene)]zirconium
dichloride,
[4-(r15-3'-methylcyclopentadienyl)(r15-4,5-tetrahydropentalene)]zirconium
dichloride,
[4-(r15-3'-isopropylcyclopentadienyl)(-q 5 -4,5-tetra hyd rope ntalene)]zircon
iu m
dichloride,
[4-(115-3'-benzylcyclopentadienyl)(r15-4,5-tetrahydropentalene)]zirconium
dichloride,
[2,2,4-trimethyl-4-rl5-cyclopentadienyl)(r15-4, 5-tetrahydropentalene)J-
zirconium dichloride
[2,2,4-trimethyl-4-(rlt-3,4-diisopropyl)cyclopentadienyl)(-q 5-4,5-tetrahydro-
pentalene)]zirconium dichloride.
The preparation of the cycloolefin polymers can also be carried out in other
ways which are briefly outlined below. Catalyst systems based on mixed
catalysts comprising titanium salts and organoaluminum compounds are
described in DD-A-109 224 and DD-A-237 070. EP-A-156 464 describes
the preparation of cycloolefin polymers using catalysts based on vanadium.
EP-A-283 164, EP-A-407 870, EP-A-485 893 and EP-A-503 422 describe
the preparation of cycloolefin polymers using catalysts based on soluble
metallocene complexes.
The cycloolefin polymers used according to the invention can be prepared
by homopolymerization and/or copolymerization of cyclic, preferably
polycyclic, olefins with retention of the rings.
The cycloolefin polymers can also be prepared by ring-opening polymeriza-
tion of at least one of the monomers of the formulae I to VI and subsequent
CA 02369137 2007-10-02
9
hydrogenation of the products obtained. The cycloolefin polymers may also
be prepared by ring-opening copolymerization of at least one of the
monomers of the formulae I to VI with further monomers, e.g. monocyclic
monomers of the formula Vlll, and subsequent hydrogenation of the
products obtained. The preparation of cycloolefin polymers is described in
the Japanese patents JP-B-3-14882, JP-B-3-122137, JP-B-4-63807,
JP-B-2-27424 and JP-B-2-276842. Derivatives of these cyclic olefins
containing polar groups such as halogen, hydroxyl, ester, alkoxy, carboxy,
cyano, amido, imido or silyl groups are likewise included.
Hydrogenated polymers and copolymers, e.g. those of styrene and dicyclo-
pentadiene, are likewise suitable and are likewise referred to as cycloolefin
polymers for the purposes of the present application.
The polymerization can also be carried out in a plurality of stages, with
block copolymers also being able to be formed (DE-A-42 05 416),
Cycloolefin polymers are preferably amorphous, transparent and colorless
materials. The heat distortion resistances of the cycloolefin polymers can
be set within a wide range. For cycloolefin polymers, the glass transition
temperature can be employed as an indication of the heat distortion
resistance, as can be determined in accordance with ISO 75 part 1 and
part 2 on injection-molded bodies. The cycloolefin polymers described have
glass transition temperatures in the range from -50 to 220 C. Preference is
given to glass transition temperatures in the range from 0 to 180 C,
particularly preferably from 40 to 180 C.
The mean molar mass of the cycloolefin polymers can be controlled in a
known manner by addition of hydrogen, variation of the catalyst concentra-
tion or variation of the temperature. The cycloolefin polymers present in the
microstructured components according to the present invention have mass
average molar masses MW of from 1 000 to 10 000 000 g/mol. Preference
is given to mass average molar masses Mw in the range from 5 000 to
5 000 000 g/mol, particularly preferably from 10 000 to 1. 200 000 g/mol.
The cycloolefin polymers present in the multilayer containers of the inven-
tion have viscosity numbers in the range from 5 to 1 000 mi/g. Preference
= CA 02369137 2001-10-09
is given to viscosity numbers in the range from 20 to 500 ml/g, particularly
preferably from 30 to 300 milg.
The multilayer containers of the invention can also contain blends of at
5 least one cycloolefin polymer and at least one further polymer in any mixing
ratios in one or more layers.
For the blends with cycloolefin polymers, preference is given to using the
following polymers: polyethylene, polypropylene, ethylene-propylene
10 copolymers, polybutylene, poly(4-methyl-1 -pentene), polyisoprene,
polyisobutylene, natural rubber, poly(1-methyl methacrylate), further
polymethacrylates, polyacrylate, acrylate-methacrylate copolymers,
polystyrene, styrene-acry lonitrile copolymers, bisphenol A polycarbonate,
further polycarbonates, aromatic polyester carbonates, polyethylene
terephthalate, polybutylene terephthalate, amorphous polyacrylate, nylon 6,
nylon 66, further polyamides, polyaramides, polyether ketones,
polyoxymethylene, polyoxyethylene, polyurethanes, polysulfones, polyether
sulfones, polyvinylidene fluoride.
For blends of cycloolefin polymers and polyolefins, preference is given to
using the following polyolefins: homopolymers of ethylene and propylene
and copolymers of these two monomers, copolymers based on ethylene
together with linear or branched olefins such as butene, pentene, hexene,
heptene, octene, nonene, decene, undecene and dodecene, copolymers
based on propylene together with linear or branched olefins such as
butene, pentene, hexene, heptene, octene, nonene, decene, undecene and
dodecene, terpolymers of ethylene, propylene and linear or branched
olefins such as butene, pentene, hexene, heptene, octene, nonene,
decene, undecene and dodecene.
The blends can be produced by customary methods, e.g. by coextrusion of
the polymer components from the melt, with or without use of further
additives, and subsequent granulation.
Cycloolefin polymers can be processed from the melt or from solution.
Suitable solvents are aprotic nonpolar hydrocarbons such as decalin or
mixtures of linear or branched hydrocarbons.
The multilayer containers of the invention comprise at least one layer
CA 02369137 2001-10-09
11
comprising at least one cycloolefin polymer together with at least one
further layer of another polymer or a blend of further polymers. Suitable
polymers are, for example, polyethylene, polypropylene, ethylene-
propylene copolymers, polybutylene, poly(4-methyl-1 -pentene),
polyisoprene, polyisobutylene, natural rubber, poly(1-methyl methacrylate),
further polymethacrylates, polyacrylate, acrylate-methacrylate copolymers,
polystyrene, styrene-acrylonitrile copolymers, polyacrylonitrile, bisphenol A
polycarbonate, further polycarbonates, aromatic polyester carbonates,
polyethylene terephthalate, polybutylene terephthalate, further polyesters,
amorphous polyacrylates, nylon 6, nylon 66, further polyamides,
polyaramides, polyether ketones, polyoxymethylene, polyoxyethylene,
polyurethanes, polysulfones, polyether sulfones, polyvinyl chloride,
polyvinylidene chloride, polyvinylidene fluoride.
Particularly useful polymers are ones which have good gas barrier
properties, e.g. polyesters such as polyethylene terephthalate, polyethylene
naphthalate and also liquid-crystalline polyesters, polyacrylonitrile,
polyvinyl
chloride, polyvinylidene chloride, ethylvinyl alcohol, polyamides and others.
The production of the multilayer containers of the invention can be carried
out by multicomponent injection molding, multicomponent injection blow
molding and multicomponent injection stretch blow molding.
The production of preforms for multicomponent injection blow molding and
multicomponent injection stretch blow molding by multicomponent injection
molding leads to multilayer preforms. Multilayer preforms can be produced,
for example, by coinjection of two or more materials or by injecting one or
more further materials over the first layer of a material.
The production of the multilayer containers of the invention can be carried
out using customary amounts of additives such as plasticizers, UV
stabilizers, optical brighteners, antioxidants, antistatics, heat stabilizers
or
reinforcing additives such as glass fibers, carbon fibers or high-modulus
fibers such as polyaramides or liquid-crystalline polyesters or the like.
Furthermore, fillers such as inorganic materials, talc, titanium dioxide or
the
like can be used. The abovementioned additives and fillers can have been
added before the processing of the polymer materials or else can be added
during processing.
CA 02369137 2001-10-09
12
It is possible to join the various polymer layers to one another by use of
bonding layers. Materials suitable for this purpose are, for example,
polyolefins such as the materials Tafiner AdmerG from Mitsui Chemicals.
However, it has surprisingly been found that the cycloolefin polymers used
according to the invention display excellent adhesion to the other layers.
Use of bonding layers can therefore be dispensed with, which is of great
economic advantage owing to the lower material costs and the greater
simplicity of the production of the multilayer containers.
The cycloolefin copolymers used according to the invention surprisingly
display a significant improvement in the mechanical properties in the
production of multilayer systems according to the invention without bonding
layers.
The adhesion between the various layers of the multilayer containers of the
invention can be optimized by matching the shrinkage behavior of the
different materials used according to the invention.
Compared to containers produced from only cycloolefin polymers, the
multilayer containers of the invention have improved resistance to impacts
and also display improved resistance to oils and fats.
Due to their high purity, extraordinarily low water absorption, excellent
barrier properties toward gases and moisture, their good blood
compatibility, excellent biocompatibility, good sterilizability by means of
hot
steam, hot air, ethylene oxide gas and high-energy radiation (gamma-rays
and electron beams), high resistance to acids, alkalis and polar solvents,
the multilayer containers of the invention are very suitable for packaging
and storage in the medical, pharmaceutical, cosmetic and food sectors,
e.g. in the form of syringes, injectors, cartridges, vials and bottles, tubes
and other containers.
Owing to their particular properties, the multilayer containers of the
invention are extremely well suited to hot packaging of liquids in these
containers, e.g. in the food sector.
The invention is illustrated by the following examples.
CA 02369137 2007-10-02
13
Examples
Example 1- 2-component injection molding
Inlay injection molding of Topas on a tensile bar tool having two
cavities
The experiments were carried out using a Krauss Maffei model
KM 90-210B injection molding machine and a tool for producing DIN
standard tensile bars having two cavities in an S shape.
To prepare for the experiments, tensile bars were produced from all
materials and were halved. The part of the tensile bar farthest from the gate
was used as inlay for the 2-component injection molding procedure. For
this purpose, the halved tensiie bar (inlay) was placed in the tool in its
original position and the missing half of the tensile bar was in each case
injected onto it using the second polymer (over-injected polymer; see
table 1). Tensile tests were then carried out on the resulting tensile bars
with joining seam in accordance with ISO 527-1 to determine the rupture
stress and elongation at break (see table 1).
The processing conditions are shown in tables 2 and 3.
CA 02369137 2007-10-02
14
Table I
Over-injected polymer Test specimen initially Rupture Elongation
placed in the tool stress at break
MPa %
LLDPE Innovex To as 6013 Lot 74093 3.9 11.8
HostalenO PPR 1042 Topas 6013 Lot 74093 3.7 3.6
Im etO PET TS6 To as 6013 Lot 74093 8.3 4.4
Nylon MXD6 To as 6013 Lot 74093 - -
Ba er PC CD 2005 To as 6013 Lot 74093 - -
DSM Stanylan LDPE- To as@ 8007 Lot 54028 2.4 8.1
Hostalen PPR 1042 To asO 8007 Lot 54028 9.2 7.0
Im et@ PET T86 To asO 8007 Lot 54028 8.7 5.3
Nylon MXD6 To asO 8007 Lot 54028 990 6.3
Ba er@ PC CD 2005 To as@ 8007 Lot 54028 - -
TopasO 6013 Lot 74093 DSM Stanylan LDPE 6.8 53.1
To as 6013 Lot 74093 Hostaten PPR 1042 1.0 0.85
To asO 6013 Lot 74093 Im et PET T86 3.3 2.6
To as 6013 Lot 74093 Nylon MXD6 3.7 2.7
To as@ 6013 Lot 74093 Ba er PC CD 2005 - -
To as 8007 Lot 54028 DSM Stanylan LDPE 4.4 16.9
To asO 8007 Lot 54028 Hostalen PPR 1042 8.5 6.6
To asg 8007 Lot 54028 Im et(D PET T86 4.8 3.3
To as@ 8007 Lot 54028 Nylon MXD6 5.0 3.2
To asO 8007 Lot 54028 Ba er@ PC CD 2005 6.0 3.9
CA 02369137 2007-10-02
c
O
~ E co U) tn U)
LO 10 d
rn
O O U O Q C) O 0 C) C) O C) O C) O
0 E (D co Ln U) v LO cD c0 Cfl c0 c0 LO
A A A A A A A A A A A A
o
v~ *
U (n a) ~ ,Q m a) O O (0 Q) ~ M
O- IT LO M N f'') c7 M 'IT cY) Cr)
N N
LO
cn ~- LO C) O LO tC'>
N U M ' ~ \ O p \ ~ O O O
2 Q E (M M 00
q- N ~- (Y) 00 m M OO 0p Op
O
N O O O O O 0 O 0 O O O O O
~p o O O O O O O 0 O 0 O O 0
O)
C
O
O
0- M CO Cfl ~ l 0 (O U") ~ t~f
~
O O
C
O
CL)
Or ~ ~ ~ ~ t. 0 !) ~ tpf) ~ tn 0 ~ 0 l ~
lf) v
~-- O
~ N N N N N (Y) ce) ('M c'') M cY) cr)
m 4 -
O
~ a O N 0
M ~ (N
O E Q U") O O U~ O M U) C) o o O LO
~ ~ M tf ) (p M M M lf ) ~ M ~T M
Q) E U 0 O 0 O 0 ln O 0 m 0 O 0
~ M I~ f~ 00 f~ ~ M I~ f~ 00 f~
N N N N N N N N N N N N
:_.
N
~Z a)
_ .-. .~ ..-r-+ . .r .-. ~ ..-. .
0 C O 0 0 0 0 0 0 0 0 0 O 0 O fn
0 J J .J J J J J J J J J J ~
C~) M M C'~) M C~) f- t~ t~ f~ I-
O O O O 0
O O
= O w O O O O 0 O p O O 0 O O
U N C (O Cfl CO (D CO (O 00 00 00 OO 00 OO
~ ~ ~ ~ ~ U) ~ ~ w cn
= O (~ RS N f6 (a tII N f0 (0 (B (0 (0 cu
~ V Q Q Q Q Q Q Q Q Q Q Q Q
4) Co 0 O O O O 0 O O 0 O O O~
o t- Q f- ~ r-- ~ t- -- F- r- rF- r E E
QE
L O
~OOON
V Q CL O~O Op) m 00 pOj M~ t
F-
_ -o o a ~ ~ ~ r
ca a F co 'n o ~
0
I- - {- (1)
~ ~ n X ~ ~ ~ W L 00
N N a ~ C- a
~
O_ ~ Q ~ E
cn W O O (Q ~ O 0 N (0
F- 0 0 E Z m v) O E cd
- (7 0 2 - Z m U' ~
CA 02369137 2007-10-02
C
ci ~ LO Lo co cfl (P 1 v v U) 0
rn
o(L) 0 O O O 0 0 O o C) 0 0 0 O
E y to lh to tn t() Lo tn Ln tn tn l,()
VLr- A A A A A A n A A A A A
c
o c
c L O N
:-. U) O m ~f V' '~fi ~ U M M M (y) M O
U) ~
O
O U .- 0 O 0 O O O C) 0 0
O N O
Z a E N (7 ~ ~ ~ =~ M C7 M M c7
+_~. r 00 CO 00 CO r r r r r pp C
O
O O O O 0 O O O O 0 0 C)
C IO o 0 O O O O O O O O O O O
CT)
C
U O
2
v t() N N N ~A M M M tl) u~'~ M
~ 0
C
O
t~ f0A lf) ll) lo Lo ln lf) tn l lO Lo U') lf)
CQ
c- O
y
(V Cc
C7 CO M M M M M M M M CM C'M
m f2 s- r r r e- s- ~-- r r ~- ~-- r
O
O Q T-
E ~n O o o e-~ ~n O O
CM d r r r M cr ~f ~ ~f V
O O O O O O O O O O O C)
Cfl ~U W Cfl CO <O N N N N N M
_ N N N N N N CV N N N N N
Q ~ ~ i ~ ~ ~ ~ i ~
E V O C) O 0 0 O O O O 0 O O
f- 1- f~ !- 1~ 1- c'') C'M Cr) CM M N
~ ~ N N N N N N N N N N N N
W u) W ~ Ln Nj
C C _ a O O d O O
0 O r (O N O Q O
r Cfl N O U)
o C a F- ~ O C 00 O) V)
~ N c0 p ~ ~p C~ Q' tp O U R~
O W p V ~- c W p Q 0- a
X a (9)
N E~ iv c ~ tU
~
S n
in , O ' a)
~
o~ M Q c~ _OE z Q
m c~ o i~ z m E
E c~ c~) M M M c'M oo Co Cp op ap ~~ O
O O O O O O N N N N N ln
0 O 0 0 O 0 0 O O O O 0 O O f/) ~ N
E V d 'V d d V d ~7 ~ ~ ~ d N
C~ _A t' 1~ f- 1~ f~ 1+ t1~ tn tl) ln U) f~ ~ t
~ O 0 0 0 0 0 0 0 0 0 0 0 O
Cl ~ J J J J J J J J J J J~ p~
O r ~~ i- r- O O p 0 0 O ~ O C)
M 0 0 O O 0 0 0 C) O 0 0 O O aLf) r'
4) O CO CO CO f0 (O Cfl 00 00 OO 00 00 O
o o
~
F~- > a a a a a Q. a ~ a Q a a
~ F - 1~ 1- 10 F - 1- H F - F- F0 - F - F- *~*
CA 02369137 2007-10-02
-17-
Example 2 - 2-component injection molding
Coinjection of TopasO by injection molding using a multi-part mold
This was carried out using a 3-component Kl'ockner Ferromatik injection
molding
machine and a centrally gated plate (300 x 215 x 3 mm).
Using a special injection head having controlled and sprung needle valves, the
injection head nozzle was selected by means of a preceding 2-component
distributor
disk so that the core component was injected centrally into the cavity and the
second
melt exiting coaxially around it was injected simultaneously or, if desired,
after an
interval (see fig. 1).
Mixing of the two streams of material was avoided by selection of the
respective
fluidities and, in particular, by selection of the injection sequence/process
conditions.
Formation of the core layer, here component B, was monitored in the case of
transparent or slightly colored materials. As colorant, a TopasO 5013
(cycloolefin
copolymer/Ticona ; HDT/B (0.45 N/mm2) = 130 C; melt index MVR at 260 C, 2.16
kg,.
= 56 ml/min)/Sandoplast BIauCD B masterbatch was added in a concentration of
about 2%.
Selection of the component A of the outer layer and the component B of the
core
layer is summarized in table 4.
CA 02369137 2007-10-02
Table 4: Material combinations:
Component B Component A
Topas Cycloolefin copolymer/Ticona HDT/B (0.45 Metocen(D PP X50081
Transparent
8007 N/mm2) = 75 C melt index MVR at 260 C, 94-2710/Tagor polypropylene
2.16 kg = 30 mI/min
Topas Cycloolefln copolymer/Ticona HDT/B (0.45 K-Resin KR-01/ Phillips
Styrene-
8007 N/mm2) = 75 C melt index MVR at 260 C, butadiene
2.16 kg = 30 mi/min copolymer
TopasU Cycloolefin copolymer/Ticona HDT/B (0.45 PET Polyester T86 Transparent
6013 N/mm2) = 130 C melt index MVR at 260 C, W03/Kosa polyester
2.16 kg = 13 ml/min
Topas Cycloolefin copolymer/Ticona HDT/B (0.45 Grilamid TR90/ Transparent
6013 N/mm2) = 130 C melt index MVR at 260 C, EMS-Chemie polyamide
2.16 kg = 13 mi/min
Topas Cycloolefin copolymer/Ticona HDT/B (0.45 Nylon MXD 6/Mitsui
Transparent
6013 N/mm2) = 130 C melt index MVR at 260 C, polyamide
2.16 kg = 13 ml/min
Topas Cycloolefin copolymer/Ticona HDT/B (0.45 Trogamid CX7323/ Transparent
6013 N/mm) = 130 C melt index MVR at 260 C, Degussa-Huls polyamide
2.16 kg = 13 mI/min
Topas Cycloolefin copolymer/Ticona HDT/B (0.45 Metocen(ED PP X50081
Transparent
6013 N/mm2) = 130 C melt index MVR at 260 C, 94/2710/Tagor polypropylene
2.16kg=13mI/min
Procedure:
-~-,-.-r-_--
The various material combinations were each processed for two different
injection profiles.
The profiles differed in the sequence of injection of the enveloping component
(component
A) and the core component (component B).
CA 02369137 2007-10-02
19
Example 2a:
In the case of injection profile 2a, the time delay between commencement of
injection of the component B and the component A was about two seconds, at
which
time 82 percent of the amount of component A had already been injected. The
injection profile 2a is shown by way of example in figs. 3a and 3b for
polyester/Topas 6013.
Example 2b:
In the case of the injection profile 2b, the time delay between commencement
of
injection of component B and component A was only about 0.5 seconds. After
this
time, about 90 percent of the remaining amounts were injected simst(taneQusly.
The
injection profile 2b is shown by way of example in figs. 4a and 4b for
Trogamid /Topas 6013.
In both cases, the sprue is sealed with the component A at the end of the
respective
injection procedure. Further processing parameters for the experiments are
shown in
table 6.
Breakthrough of the enveloping layer through the core component was not
observed,
even when using very fluid enveloping material such as Metocen PP X50081.
The layer thickness distribution can be influenced via the respective
injection
volumes, injection pressures and injection rates of the 2 independently
regulatable
injection molding units, while the position of the core layer can be shifted
by means
of the temperature of the tool.
Uniform layer thickness distributions and good transparency of the plates can
be
achieved under the above-described process conditions. In addition, the plates
were
assessed in respect of adhesion of the layers and distortion tendency (see
table 5).
CA 02369137 2007-10-02
Table 5: Experimental results:
Injection Material combination Adhesion Clouding Distortion
molding Component A/ between tendency
profile layers
Component B
2a I Metocen PP / Topas 8007 ++ o -
2b I Metocen PP / Topas 8007 ++ +0 -
2a II K-Resin / Topas@ 8007 + +0 +0
2b II K-Resin / Topas 8007 + + +0
2a III Metocen PP / Topas@ 6013 + o -
2b III Metocen PP / Topas@ 6013 + +0 -
2a IV Trogamid / Topas@ 6013 +0 + +0
2b IV Trogamid / Topas 6013 +0 ++ +0
2a V GrilamidO / Topas@ 6013 0 + +
2b V Grilamid / Topas 6013 0 + +
2a VI PET Polyester / Topas 6013 - 0- +
2b VI PET Polyester / Topas 6013 - o +
2a VII Nylon MXD6 / Topas 6013 0- o- +
2b VII Nylon MXD6 / Topas@ 6013 0- 0 +
CA 02369137 2001-10-09
00
~ d ~ 0 O 0 OV ~ 0 ~ ~ 0 ~ O 0 0 0 C) LO
d X == CO M N N N N N N N ~ qT CD N
-o
m M
~ U) 2 X ~ O O ~ 0) 0) O 0 d ~ ) O O O 0 O O LO
H U '. Cfl cM N N N M M N c'M CO CO ~ r t0 N
Cfl N
~
C _ p
0 cl a M 0 0 0
>+ 0 O c ~ ~ ti 0 0 000 0 a0
)( 0 U-) lf) M tn
Z ~ ~(fl ce) N N N N N N N fY) Cr) LO ~ ~- N
i f)
N
(D
~ f=M- ~ C) (M N N N N N N N OV (V LO ~ COO N
~ N
E 0) 0 Q ~
CY)
12 3 ~ M N N N N N N 0) -O [') ~ L[) 0 N
O O O 0 0 O 00 00 C) OM) O O O L1')
~ (p cf) cY) M co co M m N ~ U3 U) N N
a
a
c
~
o
"
~ ~
~
~ C) 0 O 0 t~ ~ 0 0 ~ 0 O O O 0 COO N
N X ~ ~O M N N N N N N N ~t tt
i
f6
a
O O ~ ~ ~ ~ v~i ~ O O LO LO
Y Y ~ QO Ce) N N N N N N LO LO N
cn
o 0 0 ~ n ~ n ~ n ~ c) l n ~ n 0 0 C) o 0 LO
F- 00 ~ M N N N N N N U) Lf) V) N (O N
Q
~ uUi r- N c~) 'It ~ p) N 0
C ~ ~ C C X ~
0 4m, 0
ti N N N N N~~ C7 W
a
M
E
EL N
=H ~
~-.
N
~ E UN
Q (,D FL
a~
a N
~ o o Q.~ o a~
a a c = Z
~ o E ai U 1 , u~i m d ~ U
1- U U F- U~ ,...