Note: Descriptions are shown in the official language in which they were submitted.
CA 02369720 2002-O1-30
MAL900.171 Int'1 Filing ISURF#02653
CHEMICAL SENSOR WITH BLOCK COPOLYMER COATINGS
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention g~erally relates to systems for monitoring environmental
contaminants and, more particularly, to systems in which acoustic wave-based
chemical
sensors are provided with absorbent coatings for monitoring fugitive emissions
from process
equipment.
14 2. Backexound of the Invention
Industrial plants that handle volatile organic compounds (VOCs) typically
experience
unwanted emissions of such VOCs and other volatilized compounds into the
atmosphere
from point sources, such as smoke stacks, and non-point sources, such as
valves, pumps, and
fittings installed in pipes and vessels containing the VOCs. Such VOCs may
include, but are
15 not limited to, aromatics (e.g., benzene, toluene, ethylbenzene, and
xylenes), halogenated
hydrocarbons (e.g., carbon tetrachloride, 1,1,1-trichloroethane, and
trichloroethylene),
ketones (e.g., acetone, and methyl ethyl ketone), alcohols (e.g., methanol,
ethanol, and
propanol), ethers (e.g., dimethyl ether and methyl t-butyl ether), and
aliphatic hydrocarbons
(e.g., natural gas and gasoline). Inorganic compounds may also be carried into
the
20 atmosphere along with or separately from the organic materials.
Emissions from non-point sources, referred to as fugitive emissions, typically
occur
due to leakage of the VOCs from joints and seals. Fugitive emissions from
control valves
may occur as the result of leakage through the packing between various parts
of the valve,
25 such as between the valve stem and the body'or bonnet of the valve or from
damaged or worn
areas within the transport system for the VOCs. Valves employed under
demanding service
conditions involving frequent movement of the valve stem, intense vibration,
and large
temperature fluctuations typically suffer accelerated deterioration of the
valve stem packing,
which results in statistically higher levels of fugitive emissions than valves
employed in less
30 demanding service.
CA 02369720 2002-O1-30
t
While improvem~ts in valve stem packing materials and designs have reducai
fugitive emissions and lengthened the life of valve packing, the monitoring of
fugitive
emissions has become important as a means of id~tifying and reducing fugitive
emissions,
and facilitates the ability of the systems to comply with more stringent
regulations of
emissions. For example, the Environmental Protection Agency (EPA) has
promulgated
regulations for specifying the maximum permitted emission of certain hazardous
air
pollutants from control valves, and requires periodic surveys of emissions
from control
valves. Where particularly noxious or toxic materials are transported through
the valves,
there is almost no tolerance for any fugitive emission. It therefore becomes
important to be
able to monitor the presence of leaks of such materials in a cost effective
manner. This
monitoring must be rapid, inexpensive, and reproducible.
Current methods of monitoring fugitive emissions involve manual procedures
using a
portable organic vapor analyzer. This manual method is time consuming and
expensive to
perform, and it can also yield inaccurate results due to ineffective
collection of the fugitive
emissions from the equipment being monitored. If measurements are made on a
valve
exposed to air currents or wind, emissions from the valve may be dissipated
before the
analyzer can properly measure the concentration of the emissions. Also, if the
analyzer is not
carefully moved around the exterior of the valve to capture all the eanissions
from the valve,
an inaccurate measurement may result. Manual measur~nent methods also require
plant
personnel to dedicate a significant amount of time to making the measurem~ts,
thereby
distracting plant personnel from other duties.
Automated monitoring and detection of fugitive emissions can yield significant
advantages over existing manual methods. Some EPA regulations require surveys
of fugitive
emissions at periodic intervals. The length of the survey interval nmy be
monthly, quarterly,
semi-annually, or annually, with the required surveys becoming less frequent
if the facility
operator can document a sufficiently low percentage of control valves
exhibiting excessive
leakage. Achieving a low percentage of leaking valves may therefore reduce the
number of
surveys required per year. In a large industrial facility, where the total
number of survey
2
CA 02369720 2002-O1-30
points can range from 50,000 to 200,000, a reduced number of surveys can
result in large cost
savings. By installing automated fugitive mission-sensing systems on valves
subject to the
most demanding service conditions, and thus, monitoring those valves most
likely to develop
leaks, compliance with the EPA regulations can be more readily achieved for
the entire
facility.
However, employing chemical sensors in an. industrial environm~t requires
designing sensors that perform satisfactorily in the presence of high relative
humidity across
a broad temperature range. The sensors must be able to discriminate between
the emissions of
interest and other environmental contaminants, while retaining sufficient
sensitivity to detect
low concentrations of the fugitive emissions. A provision also must be made to
enable
periodic calibration of the chemical sensors. The output signals from the
fugitive emission
sensing system must be suitable for input into plant monitoring and control
systems typically
found in process plants. This remote sensing permits simple and inexpensive
integration of
the sensing system into existing plant process control systems.
The fugitive emission sensing system should be inexp~sive to manufacture,
provide
local notification of monitoring results (e.g., within an internal facility
network system), and
use a power source that is readily available in a typical process plant in
order to keep
installation costs to a minimum. The system should be suitable for use in
hazardous areas
subject to risk of explosion, requiring electrical equipment to be
intrinsically safe and/or of an
explosion-proof design. The system also should be able to operate in harsh
environments,
including areas subject to spray washing, high humidity, high and low
temperatures, and
vibration. The system also should be simple and reliable, to keep maintenance
costs to a
minimum.
In certain applications, the sensors used to detect fugitive emissions are
provided in
the form of piezoelectric-based sensors having high sensitivities to surface
mass changes, so
that when an alternating potential is applied across the sensors, changes in
resulting acoustic
wave characteristics in the sensors, specifically the resonant frequ~cy;
indicate the presence
3
CA 02369720 2002-O1-30
of the analyte, for example, the absorbed or adsorbed VOC. More specifically,
the sensors
typically include a quartz crystal substrate with conductive electrodes and an
outer layer (on
the electrodes) made of a material selected to most effectively absorb the
analyte. Such outer
coatings are selected to increase sensitivity, while reducing acoustic wave
damping effects.
In addition, such materials should be environmentally robust to accommodate
the
aforem~tioned wide temperature ranges, humidity ranges, and high levels of
dust particles
and other contaminants.
Various Patent literature discusses the structure of such chemical sensors and
the
compositions of the absorbing layers. U.S. Patent No. 5,883,457 describes.the
use of organic
polymeric matrices as the coatings and particularly thermoset resins that are
stable against
ambient conditions.
U.S. Patent No. 5,900,128 describes the use of a protective intermediate layer
between the absorbing layer and an electrolyte precious metal layer.
U.S. Patent No. 5,910,286 describes the use of macroporous crosslinked layers
with
steric and functional configurations specifically suited to capturing
molecular and/or ionic
species.
The prior art, including the following U.S. Patents describe various
structures and
associated aspects of chemical sensors, some particularly quartz sensor,
provides significant
background on such chemical s~sors. These patents include U.S. Patent Nos.
5,852,229;
5,936,150; 5,606,633; and 5,482,678.
4
CA 02369720 2002-O1-30
SUMMARY OF THE INVENTION
An acoustic wave-based sensor according to the present invention is provided
which
may include at least one substrate, at least two electrodes connected to the
at least one
substrate, and a layer comprising a block or graft copolymer of at least two
distinct polymer
units having differing viscoelastic characteristics. The block or graft
copolymers may be
constituted of any combination of blocks or units of polymers that can be
grafted or
combined into polymeric combinations of film forming polymeric material. At
least one unit
of the at least two distinct units in the polymer must be capable of absorbing
at least one
volatile organic compound, and preferably two of the polymer units or all of
the polymer
units are capable or absorbing one or more volatile organic compound(s). The
combination
of distinct polymer units having differing viscoelastic properties (e.g., high
and low glass
transition temperatures (Tg)) within the same molecule facilitates tailoring
of the acoustic
properties of the polymeric film materials. These materials have favorable
acoustic
properties over extended temperature ranges, resulting in a wider operating
temperature range
for acoustic wave-based sensing of VOCs: Moreover; the improved acoustic
properties
enable thicker coatings to be used, which increases the detection sensitivity
of the sensor.
The combination of distinct polymer units also facilitates tailoring of
physical absorption
characteristics. That is, the distinct polymer units can be designed for
sensitivity towards
specific different VOCs so that a single sensor may be used to sense for two
or more distinct
volatile organic compounds. These block and graft copolymers may be blended or
mixed
into combinations with other physically compatible additives, including
polymers and resins.
A method of detecting volatile organic compounds using the sensor of the
present
invention is provided. The sensor comprises a substrate, at least two
electrodes connected to
the substrate, and a coating positioned over the substrate and at least one of
the electrodes.
The coating is a block or graft polymer or polymeric mixture comprising at
least two
polymeric units, at least one unit being capable of absorbing at least one
volatile organic
compound.
CA 02369720 2002-O1-30
These and other aspects and features of the present invention will become more
apparent from the following detailed description when taken in conjunction
with the
accompanying drawings and examples.
BRIEF DESCRIPTION OF THE FIGURES
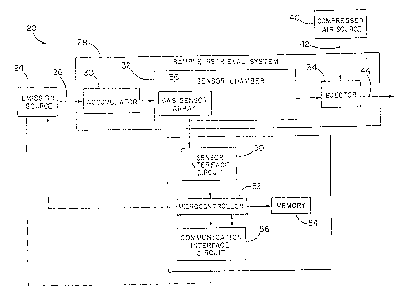
Figure 1 is a block diagram of an illustrative fugitive emissions sensing
system
employing the chemical sensor of the invention.
Figure 2 shows a chemical sensor connected to an oscillator circuit for
monitoring
emissions.
Figure 3 is a plot that compares sensitivity at 50 °C of quartz crystal
microbalance
(QCM) sensors coated with various polymeric films. The sensitivity to tolu~e,
a
representative VOC, in Hertz frequency change per ppm of toluene, is shown as
a function of
the resistance of the sensor. The varying resistance of the sensor is a result
of the different
thicknesses of the coatings.
Figure 4 is a plot that compares the effect of temperature on the resistance
of a sensor
with a representative block copolymer-based coating vs. a homopolymer coating.
Figure 5 is a plot that compares the frequency response of a QCM coated with a
representative block copolymer-based coating vs. a homopolymer coating at 50
°C. Resonant
frequency change is plotted as a function of the gas phase concentration of
the representative
VOC toluene.
Figure 6 is a plot that compares the speed of sensor response of a
representative block
copolymer-based coating vs. a homopolymer coating at -10 °C. The
responses of each sensor
have been scaled to the maximum response to 1000 ppm toluene in 15 minutes to
facilitate
comparison.
DETAILED DESCRIPTION OF THE INVENTION
A sensor is provided which may include at least a substrate, at least two
electrodes
connected to the substrate, and a layer of a block or graft copolymer having
at least a first
polymeric unit and a second polymeric unit with differing viscoelastic
properties (such as
6
CA 02369720 2002-O1-30
differing Tgs), wherein at least one of the first and second polymeric units
absorb at least one
volatile organic compound.
One aspect of the present invention includes a chemical sensor comprising; a
substrate; at least two electrodes connected to the substrate; and a layer of
polymer positioned
over the substrate and at least one of the electrodes, wherein the layer of
polymer comprises a
polymeric layer selected from the group consisting of block copolymers, graft
copolymers,
and star copolymers. The block copolymer may preferably comprise at least 20%
by weight
of a block copolymer having at least two different blocks or a graft copolymer
having at least
two different graft units, and the star copolymer contains at least two
different monomer
dived units, wherein different blocks or units within the block copolymer,
graft copolymer
or star copolymer have at least one property selected from the group
consisting of volatile
organic compound absorption characteristics, film strength and film
brittleness different from
the same property of at least one other block, star or graft unit in the
layer. For example, the
block copolymer may comprise at least some units of a polymer derived from
monomers
having at least one ethylenically unsaturated polymerizable group. In
particular, it is one
embodiment to use a graft copolymer that comprises at least some units of a
polymer derived
from monomers having at least one ethylenically unsaturated polymerizable
group, such as
polymers with at least one monomer selected from the group consisting of
olefins; styrenes,
butadienes, and vinyl resins. This invention enables the use of a layer of
polymer with a
thickness of betwe~ l and 10 micrometers. An example of the class of block
copolymers
comprises a block copolymer comprising AB diblock copolymer, ABA triblock
copolymer,
ABC triblock copolymer, and (ABA, copolymer. The chemical sensor may have the
block
copolymer comprise a combination of high Tg (e.g., above 40°C, above 50
°C, above 60°C,
and above 70°C or I00 °C or more) and low Tg (e.g., Less than
25°C, less than 20°C, less than
15°C, less than 10°C or less than 0 °C) polymeric blocks,
preferably with a difference of at
least 25°C between the high and the low Tg polymeric blocks. These
types of chemical
sensors using the block, graft or star copolymer coating may provide a rapid
response time of
less than 3 minutes for 90% response at -10 °C. The coatings may also
provide the chemical
sensor with thermally stable response characteristics within the range of
temperatures from
7
CA 02369720 2002-O1-30
less than -10 °C to greater than 50 °C. The chemical sensor may
be manufactured where the
polymer layer was formed by spin coating: The chemical sensor may comprise a
mass-
sensitive acoustic wave chemical sensor or a sensor comprising a sensor
selected from the
group consisting of quartz crystal microbalances, surface acoustic wave
devices, flexural
mode sensors, optical sensors, electrochemical sensors and non-acoustic wave
sensors.
The block copolymers may, for example, comprise elastomeric materials such as
those known to those skilled in the art as A B, A-B-A, A-B-C, or (A-B~, block
copolymers.
These block copolymers are described, for example, in U.S. Pat. Nos.
3,265,765; 3,562,356;
3,700,633; 4,116,917 and 4,156,673, the substance ofwhich is incorporated
herein by
refer~ce. The block copolymers preferably comprise at least one polymeric unit
derived
from an ethylenically unsaturated monomer. Non-limiting examples of such block
copolymers comprise styrene, isoprene, butadi~e, ethylene-styr~e, halog~ated
polyvinyl
aromatics), and/or butylenes based (SIS, SBS or SEBS) block copolymers,
acrylate
(including methacrylate) block copolymers, urethane block copolymers, silicone
resin block
copolymers, and mixed resin system block copolymers. Other useful elastomeric
compositions can include elastomeric block copolymers of polyurethanes,
polyamides,
poly(N,N-dialkylacrylamides), poly(vinylpyridines), ethylene copolymers such
as ethylene
vinyl acetates, ethylene/propylene copolymer elastomers or
~hylene/propylene/diene
terpolymer elastom~s. Blends of these elastomers with each other or with
modifying non-
elastomers are also contemplated. The properties of block copolymers can be
tailored by
incorporating additives that preferentially associate with one of the
polymeric units. With
regard to acoustic properties, the effect of the additive depends on whether
the additive has a
higher or lower softening point than the associated polymeric unit. For
example, additives
that associate with a high Tg unit and have a lower Tg will lower the
softening point of that
unit. Conversely, an additive with a higher softening point will raise the
softening point of
the unit. For example, up to 50 weight percent, but preferably less than 30
weight percent, of
polymers can be added as stiffening aids, such as polyvinylstyrenes,
polystyrenes such as
poly(alpha-methyl)styrene, polyesters, epoxies, polyolefins, e.g.,
polyethylene or certain
ethylene/vinyl acetates, preferably those of higher molecular weight, or
coumarona-indene
8
CA 02369720 2002-O1-30
resin. The ability to use these types of elastomers and blends provides the
invention film
material with significant flexibility in tailoring the absorption and acoustic
properties of the
film.
As noted, acrylic (including meethacrylic) polymers, conjugated or not
conjugated
block copolymers, including dime polymer blocks, such as a polymer block
comprising an
olefmic monomer and a polymer block comprising at least one constitutional
unit selected
from the group consisting of an acrylic, methacrylic, (meth)acrylic acid,
(m~h)acrylate and
(meth)acrylonitriles such as those selected from the group consisting of
acrylic acid,
methacrylic acid, ethyl acrylate, ethyl acrylate, propyl acrylate, butyl
acrylate, 2-ethylhexyl
acrylate, dodecyl acrylate, 2-hydroxy ethyl acrylate, methyl methacrylate,
ethyl methacrylate,
propyl methacrylate, butyl rnethacrylate, 2-ethylhexyl methacrylate, dodecyl
methacrylate, 2-
hydroxy ethyl methacrylate, glycidyl acrylate, glycidyl methacrylate,
dimethylaminoethyl
acrylate and quaternary salts thereof dimethylaminoethyl methacrylate and
quaternary salts
thereof, acrylonitril~ and methacrylonitrile, and mixtuxes thereof.
Hydrogenated or non-
hydrogenated derivatives may be used. An example of a thermoplastic polymer
composition
of this class might have a degree of hydrogenation of the hydrogenated
conjugated dime
polymer block (B) not smaller than 50 mol%. Typically, the average molecular
weight
(number average value M~ of each block or graft unit present is within the
range from 3000
to 500,000, preferably from 10,000 to 200,000 g/mol.
Viscosity reducing polymers and plasticizers can also be blended with the
elastomers
such as low molecular weight polyethylene and polypropylene polymers and
copolymers, or
tackifying resins such as Wingtack~, aliphatic hydrocarbon tackifiers
available from
Goodyear Chemical Company. Tackifiers can also be used to increase the
adhesiveness of an
elastomeric cores) to the matrix material. Examples of tackifiers include
aliphatic or
aromatic liquid tackifiers, aliphatic hydrocarbon resins, polyterpene resin
tackifiers, and
hydrogenated tackifying resins. Aliphatic hydrocarbon resins are preferred.
Additives such
as dyes; pigments, antioxidants, antistatic agents, bonding aids, antiblocking
agents, slip
agents, heat stabilizers, photostabilizers, foaming agents, glass bubbles,
starch and metal salts
9
CA 02369720 2002-O1-30
for degradability or microfibers can also be used in the elastomeric core
layer(s). Suitable
antistatic aids include ethoxylated amines or quaternary amines such as those
described, for
example, in U.S. Pat. No. 4,386,125 (Shiraki), who also describes suitable
antiblocking
agents, slip agents and lubricants. Soft~ing agents, tackifiers or lubricants
are described, for
S example, in U.S. Pat. No. 4,813,947 (Korpman) and include coumarona-indene
resins,
terpene resins, hydrocarbon resins and the like. These agents can also
function as viscosity
reducing aids. Conventional heat stabilizers include organic phosphates,
trihydroxy
butyrophenone or zinc salts of alkyl dithiocaxbonate. Oxidation is an
important issue when
the acoustic wave sensing devices are placed in the presence of strong
oxidizing compounds
such as ozone, sometimes found in industrial environments. Suitable
antioxidants include
hindered phenolic compounds and amines possibly, with thiodipropionic acid or
aromatic
phosphates or tertiary butyl cresol, see also U.S. Pat. No. 4,476,180 (Wnuk)
for suitable
additives and percentages. The use of chemical additives should be minimized
to maintain a
high level of absorbency, so that plasticizers and chemical additives in
addition to the block
1S copolymer itself should be maintained below 2%, preferably below 1.S%,
below 1%, below
0.5%, below 0.1%, for example.
Graft copolymers may also be used, including those prepared by such diverse
methods as shown In U.S. Patent No. 6,114,460; 6,114,440; 6,107,440; 6,100,33
l; 6,096,827;
6,060,566; 6,054,539; and the like. The graft or block copolymers are used in
essentially
identical manners after being prepared, being coated onto the surface of the
sensor, using
such coating methods that provide an appropriately uniform coating, for
example, dip
coating, spray coating, evaporative coating methods, sputter coating, spin
coating and the
like.
Short fibers or microfibers can be used to reinforce the block or graft
copolymer for
certain applications. These fibers are well known and include polymeric
fibers, mineral
wool, glass fibers, carbon fibers, silicate fibers and the like. Further,
certain particles can be
used, including carbon and pigments.
10
CA 02369720 2002-O1-30
Glass bubbles or foaming ag~ts are used to lower the density of the
elastomexic layer
and can be used to reduce cost by decreasing the eIastom~ contest. These
agents can also be
used to increase the bulk of the elastomer. Suitable glass bubbles are
described in U. S. Pat.
Nos. 4,767,726 and 3,365,315. Foaming ag~ts used to generate bubbles in the
elastomer
include azodicarbonamides. Fillers can also be used to some extent to reduce
costs. Fillers,
which can also function as antiblocking agents, include titanium dioxide and
calcium
carbonate.
According to the present invention, a polymer layer comprising a mixture of at
least
one block or graft copolymer (hereafter referred to as a "polymeric mixture")
is positioned
over the substrate and at least one of the electrodes of the sensor. The
polymer may comprise
any form of mixture of the block or graft copolymer and additives (whether a
mixture, blend,
solid state solution, suspension, dispersion, interpenetrating network or the
like), alone or in
admixture with other physically compatible polymers. The polymeric mixture
should be able
to absorb (or less preferably adsorb) volatile organic compounds onto its
surface or into pores
within its macro-structure or molecular structure. An increase in the total
mass of the coating
on the sensor from absorption of the VOCs causes a change (typically a
decrease) in the
resonant frequency of the sensor according to conventional mathematic and
physics
principles relating to the effect of mass increases on resonant frequencies.
This change in
resonant frequency provides a detectablesignal to the sensing device that VOCs
are present.
Both the pore size of the bladed coating, additives provided within the
polymer (including
polymeric plasticizers or solid additives with specific absorption
characteristics), its surface
energy, oleophilicity/hydrophilicity, oleophobicity/hydrophobicity, and even
active groups in
the polymer may be altered to tailor the absorption of the unit to more
specific VOCs or to
keep the absorption of VOCs more general.
11
CA 02369720 2002-O1-30
Referring now to the drawings, and with specific reference to Fig. l, a
fugitive
emissions sensing system utilizing the present inv~tion is generally depicted
by reference
numeral 20. However, it is to be understood that the present invention is
primarily directed to
a chemical sensor 22 (Fig. 2) which can be employed in a variety of
applications, including
applications separate from the fugitive emissions sensing system 20.
By way of overview, Figure 1 is a block diagram of an illustrative fugitive
emissions
sensing system 20 employing the chemical sensor 22. An emission source 24 is
shown, from
which a sample stream 26 is drawn into sample retrieval system 28. The sample
retrieval
syst~n 28 includes an accumulator 30, a sensor chamber 32, and an ejector 34.
A chemical
sensor array 36 is located within the sensor chamber 32. The sample stream 26
is drawn from
the accumulator 30 into the sensor chamber 32, exposing the chemical sensor
array 36 to the
sample stream 26. The chemical sensor array 36 contains one or more of the
chemical .
sensors 22. The sample stream 26 the passes into the ejector 34. A compressed
air source
40 provides compressed air 42 to the ejector 34, creating a pressure drop
within the ejector 34
that draws a sample stream 26 through the sensor chamber 32 and into the
ejector 34. The
compressed air 42 and sample stream 26 are mixed within the ejector 34 and
exhausted to
atmosphere as a mixture 44.
The gas sensor array 36 is connected to a sensor interface circuit 50, which
processes
the signals from the sensor array 36 and provides the process signals to a
microcontroller 52.
The microcontroller 52 stores the data from the sensors 22 in a m~nory 54, and
uses the
sensor data received from the fugitive emissions sensing system 20 to initiate
control actions
to reduce or eliminate the emissions. For example, the microcontroller 52
could close a valve
upstream from the emissions source 24 to stop the flow of fluid through the
emissions source
24 in order to stop emissions caused by the leakage of the fluid.
Alternatively, the
microcontroller 52 could alter operating conditions of the ennissions source
24 to reduce or
eliminate the fugitive emissions. The microcontroller 52 may use a
communication interface
circuit 56 to provide control signals to the upstream valve, the emission
source 24, or any
other equivalern that may be used to rexiuce or eliminate the emissions. The
microcontroller
12
CA 02369720 2002-O1-30
52 may also be able to provide signals to distal locations to provide warnings
or signals.
Such distal locations could include firefighting locations (which are often
responsible for
addressing chemical leaks) or other safety or emergency facilities.
It can therefore be seen that the fugitive emissions sensing system 20 may be
used to
detect the presence of, or measure the concentration of, various types of
fluids emitted from
the emissions source 24, and to provide controls and signals relating to the
status of the
concentrations of the fluids monitored emissions. The system may be used to
detect
hazardous, toxic or polluting substances Knitted from the source, or to detect
leakage of
non-hazardous substances, the loss of which rnay be a cause of concern. The
fugitive
emission sensing system 20 rnay be used to detect emissions from any kind of
source,
particularly industrial process equipment or hospital equipment from which
hazardous
substances may leak. Examples include control valves, block valves, pumps
installed on
lines carrying hazardous gases, sterilizers, agitators, screw conveyors, or
other equipment
installed on process vessels containing hazardous fluids, hazardous materials
that are being
treated, heat exchanges, reactors, etc. When emissions are detected by the
fugitive emissions
sensing system 20, this data may be used by the fugitive emissions sensing
system 20 to
control the process in such a way as to reduce or eliminate the emissions.
As indicated above, the chemical sensor array 36 may include one or more:
chemical
sensors 22 responsive to a particular analyte or fugitive emission being
monitored. In the
embodiment d~icted in Figure 2, the chemical sensor 22 is a quartz crystal
microbalance
(QCM) sensor, but can be another type of piezoelectric acoustic wave devices,
including
surface acoustic wave (SAW) devices, acoustic plate mode (APM) devices, and
flexural plate
wave (FPW) devices. Alternatively, fiber optic sensors and electrochemical
sensors may be
used.
As shown in Figure 2, the chemical sensor 22 may be connected to an oscillator
circuit 62 for monitoring emissions. In an alternative embodiment, the
chemical sensor 22
could be connected to a network analyzer. More specifically, the oscillator
circuit 62 may
13
CA 02369720 2002-O1-30
include NAND gates 64 and 66, and an AND gate 68, connected in series. A
resistor 70 may
be connected between the output of the NAND gate 66 and the circuit power
supply voltage
72, and a resistor 74 may be connected between the output of NAND gate 66 and
circuit
power supply voltage 72. A resistor 75 may be connected across the NAND gate
64,
connecting a first input to the output. A select signal 76 may be connected to
the second
input of the NAND gate 64, and the same select signals may also be connected
to an input of
the AND gate 68. An enable signal 78 may be connected to an input of the NAND
gate 66.
When the select signal 76 and the enable signal 78 are both high, the NAND
gates 64 and 66
ad as high-gain inverting amplifiers and cause an oscillator 80 to oscillate
between high and
low voltage, producing an oscillating square wave output. The oscillating
voltage from the
oscillator output 80 may be transferred through the AND gate 68 and applied
across the
chemical sensor 22 causing the chemical sensor 22 to physically resonate.
In order to appreciate the significance of this resonance, it is first
iriiportant to
understand that the chemical sensor 22 utilizes the converse pi~oelectric
effect. By way of
background, the piezoelectric effect holds that a mechanical stress applied to
the surfaces of
various crystals, including quartz, affords a corresponding electrical
potential across the
crystal having a magnitude proportional to the applied stress. The electrical
charge generated
in the quartz crystal under stress is due to the shift of dipoles resulting
from the displacement
of atoms in the crystalline material. The converse piezoelectric effect holds
that application
of a voltage across certain crystals, including quartz crystals, results in a
corresponding
mechanical strain in the crystal. In quartz, this strain or deformation is
elastic. It follows that
an alternating potential across the crystal causes a vibrational or vibratory
motion in the
quartz crystal, i.e., the aforementioned resonance. The chemical sensor 22
therefore includes
a crystal substrate 82 which interacts with the oscillating circuit 62, aid in
turn causes the
oscillator circuit 62 to oscillate at the resonant frequency of the chemical
sensor 22. Thus,
the frequency of the oscillator output 80 will vary as the resonant frequency
of the chemical
sensor 22 varies, and the resonant frequency of the chemical sensor varies in
proportion to the
amount of absorbed material, the absorbed material at least increasing the
mass on the
vibratory surface or vibratory elem~ts ofthe chemical sensor 22 device.
14
CA 02369720 2002-O1-30
The resonant frequency of the chemical sensor 22 can vary based on a number
parameters, including the mass, size, shape, and cut of the substrate 82. That
substrate is
preferably a crystalline substrate, such as a quartz crystal substrate 82.
Quartz crystal
exhibits a natural resonant frequency that is a function of the mass and
structure of the
crystal. The precise size, type of cut, and thickness of the quartz crystal
substrate 82 are
selected to result in a particular resonant frequency. For example, an AT-cut
crystal with a
nominal resonant frequ~cy of 8-30 megahertz is suitable for gas sensor
applications.
Suitable quartz crystal substrates may be obtained from Standard Crystal
Corporation of
California. Other types of suitable materials to serve as the substrate
include lithium niobate
(LiNb03), which is particularly suited for a surface acoustic wave (SAVi~
based-sensor; and
aluminum nitride (AII~, which is particularly suited for a thin film resonator
based-sensor.
In order to apply the alternating potential across the substrate 82, first and
second
electrodes 84 and 86 are connected to the crystal substrate 82 and may be
constructed of
gold-on-chromium, although other suitable corrosion-resistant conductors may
be used,
possibly including aluminum, palladium, gold, chromium, and graphite. The
electrodes 84
and 86 may serve as both the conductors for generating the alternating current
across the
crystal substrate 82, and as transducers for sensing parameters related to
changes in resonant
frequencies resulting in the crystal substrate 82.
As indicated above, the resonant frequency of the chemical sensor 22 is
primarily a
function of the total mass of the device (although alterations in flexibility
or stiffness of the
coating because of the effects of absorbents or temperature changes may also
similarly
influence the resonant frequency). Therefore, the mass of any coating provided
around the
crystal substrate 82 also affects the total mass of the device, and thereby
affects the resonant
frequency of the chemical sensor 22. The coatings provided about the crystal
substrate 82 are
selected to absorb molecules of the analyte. Why analyte molecules are
absorbed by the
coating, the mass of the coating is slightly increased, which in turn
increases the mass of the
entire s~sor 22, and thus changes the resonant frequency of the s~sor 22. The
resonant
CA 02369720 2002-O1-30
frequency of the chemical sensor 22 is also a function of the viscoelastic
properties of the
coatings and mechanical stresses caused by temperature effects in the sensor
mounting
structure. However, viscoelastic effects are reduced for coatings as in this
invention and
mechanical stress effects are either negligible or can be compensated for.
Thus, a very
sensitive chemical detector may be constructed by selecting a coating that has
a chemical
affinity for the particular analyte of interest. The quamity of molecules
absorbed and
deposited, and the resulting change in the operating frequency of the
oscillator circuit 62, is a
function of the concentration of the analyte being measured in the environment
surrounding
the chemical sensor 22. The frequency changes linearly with changes in analyte
concentration, within certain limits.
Thus, a change in the concentration of the analyte rnay be measured by
measuring the
change in the frequency of the oscillator output 80. The chemical sensor 22
may be
calibrated by exposing the chemical s~sor 22 to known concentrations of the
analyte and
recording the resulting frequency of the oscillator output 80. The chemical
sensor 22 may
then be used to measure the absolute concentration of the analyte by comparing
the measured
frequency to the aforementioned recorded values.
The particular coating chosen for the crystal substrate 82 should preferably
readily
absorb the molecules of the analyte, to provide fast response times and a high
degree of
sensitivity to the analyte over a broad temperature range, but do so without
damping the
generated waves. Additionally, the coating should not degrade by oxidation.
The present
invention provides such a coating in the form of a graft or block copolymer
coating 88.
Mass-sensitive acoustic chemical sensors, such as polymer-coated QCMs and
surface
acoustic wave (SAW) devices, are used for the detection and monitoring of VOCs
(J.W.
Grate, S.J. Martin and R.M. White, Anal. Chem, 1993, 65, pp. 957A-996A).
Ideally, the
sensitivity of such sensors is directly proportional to the thickness ofthe
polymeric coating.
Coating development to date has focused primarily on polymers with glass
transition
temperature (usually the first order glass transition temperature, but the
second order glass
16
CA 02369720 2002-O1-30
transition temperature may also be considered), Tg., below room temperature
because vapor
sorption in soft rubbery polymers is rapid and reversible. Such polymers,
however, are not
optimal for use in acoustic wave-based devices because they have a low shear
modulus, and
therefore poor acoustic wave properties (Kanazawa, K.K., IB~1 Research Report,
Physics,
1986, RJ 5125, 53236). As a result of these deficiencies in properties, the
usable film
thickness, and h~ce sensitivity, of soft polymers is limited by the
attenuation of the acoustic
energy. The operating temperature of the device also affects the upper limit
of the thickness.
As the temperature increases, the shear modules of the film decreases,
resulting in further
damping of the acoustic wave (J. W. Grate, supra). Stiff; high Tg, polymers
have a higher
shear modulus, but exhibit slow VOC uptake and release. Using very thin films
can reduce
the uptake and release times. At the same time, unfortunately, this results in
reduced
detection sensitivities.
It was found that coatings made from blends of low and high Tg polymers (il.S.
Patent
1 S Application, U.S. Serial No. 09/413,401, in the names of R. Shinar et al.)
can be used at
relatively large thicknesses in acoustic-wave based chemical sensors without
significantly
damping the acoustic wave. Such coatings have the advantage of combining the
preferred
properties of soft and stiff polymers, thereby creating fast-responding,
highly sensitive
coatings with improved acoustic properties, and therefore enhanced performance
at elevated
temperatures (Shinar et al., supra). Alternatively, the component polymers
ca.n be linked
together into a single polymer chain, effectively 'chemically blending' the
polymers and
h~ce avoiding issues of macroscopic phase separation. Polymers in which large
its
are composed of one type of polymer, followed by a segm~t of a different
polymer, are
called block copolymers. This inv~tion describes the use of block and graft
copolymers to
produce acoustic wave-based sensors of improved performance.
The unique physical properties of thermoplastic elastomer block copolymers
arise
because the blocks that make up the polymer molecules are chemically
incompatible.
Because of this incompatibility, there is a thermodynamic driving force toward
phase
separation. The similar. blocks from different polymer chains associate
strongly with one
17
CA 02369720 2002-O1-30
another. However, macroscopic phase separation is prevented by the chemical
bonding
between incompatible blocks in individual polymer chains. The result is the
generation of
rnicrophase domains. These domains are physically, rather than chemically
crosslinked.
Since the material is not chemically erosslinked, it can be melted and
dissolved, facilitating
thin film coating fabrication. The physical properties of block copolymers are
a combination
of the properties of the component blocks. We have found that the 'chemical
blending' of
high Tg and low Tg polymers into a single polymer chain, and the physical
crosslinking of
these chains, results in coatings with favorable acoustic properties for
acoustic wave-based
chemical sensors.
We observed also that f and R values of block and graft copolymer coatings of
component ratios of between 1%-99% and 99% to 1% for two block systems were
useful,
although performance would appear to tend towards a distinctly avwaging level.
That is, if
blocks A and Blocks B were combined in 50/50 proportions, the performance of
the system
would be the performance expected by averaging the performance characteristics
of pure
100% A polymer and 100% B polymer. It is preferred to obtain a measurable
effect of the
combination of the two blocks (as a 1 % level would provide minimum or even
unmeasurable
benefit in excess of the accuracy of the measurements from the 1 % component),
and this
would be accomplished by using proportions in two component system of between
5-
95%A/95%-5%B, 10-90%A/90-10%B, 15-85%A/85%-I5%B, 20-80%A/80-20%B, 25-
75%A/75-25%B, 30-70%A/70-30%B, 35-65%A/65-35%B, 40-60%A/60-40%B, 45-
55%A/55-45%B, and 50/50 block or graft ratios.
The polymer units in the graft or block copolymers may be selected on the
basis that
each ofthe units may have similar physical/chemical absorption properties and
different
physical structural properties so that a good balance may be achieved in the
physical
structural properties while maintaining the appropriate absorption properties.
Similarly, each
polymer unit may have good physical structural properties, but dif~'er too
extremely in their
absorption characteristics towards a specific VOC or towards a group of
distinct VOCs. For
example, although A arid B may have good VOC absorption properties, a polymer
from only
18
CA 02369720 2002-O1-30
A blocks might be too brittle, and a polymer from only B blocks may be too
soft. By forming
a graft or block copolymer from the two distinct units, the good VOC
absorption properties
may be maintained, but an acceptable intermediate structural physical property
attained.
Block A may have good VOC absorption properties towards a highly non-polar
organic and
B might have good VOC absorption towards a highly polar VOC. To have a sensor
that is
capable of responding to both types of VOCs, it would therefore be desirable
to have both A
units and B units available in the polymer coating on the sensor.
The coatings according to the present invention exhibited favorable attributes
observed in blends of high and low Tg polymers. These included reduced damping
of the
acoustic wave, which enables sensor operation at elevated temperatures (R.
Shinar et al.,
supra). Thick coatings (e.g., from 1 to 10 microm~~s, 4-6.5 mieromet~s,
especially ~5.5
pm) of the graft or block polymers or blends according to the present
invention, with
excellent performance characteristics (typical of high and low Tg polymer
blends) over a wide
temperature range of-10 to 50°C, can be used in sensor applications for
long periods
While the invention is susceptible of various modifications and alternative
constructions, certain illustrated embodiments have been shown in the drawings
and will be
described below in detail. It should be understood, however, that there is no
intention to limit
the invention to the specific form disclosed, but on the contrary, the
intention is to cover all
modifications, alternative constructions, and equivalents, falling within the
spirit and scope of
the invention as defined by the appended claims.
Example I
Experiments that demonstrate the advantages of using block copolymer filins as
sensor coatings were conducted using AT-cut quartz crystal microbalances
(QCMs) as the
acoustic wave-based sensor. Analogous results are expected using other
acoustic wave-based
sensor platforms (e.g., surface acoustic wave devices). The invention
described here has
been demonstrated using triblock copolymers having the two end blocks made
from
19
CA 02369720 2002-O1-30
polystyrene and a midblock containing a rubbery polymer component. Similar
results can be
expected using different block copolymer architectures. Table 1 lists the
copolymers tested.
Table 1
polystyrene-block polybutadiene-block-polystyrene
polystyrene-block poly(ethylene-ran-butylene)-block polystyrene blended with
poly(2,6-
dimethyl p-phenylene oxide)t2~
polystyrene-block poly(e~thyl~e-ran-butylene)-block polystyrene bladed with
coumarone-
indene resin~3~
polystyrene-block-polybutadiene-block-polystyrene blended with coumarone-
indene resin
Kraton 61652 and Kraton Gl 654 from Shell Chemical
~2~ Blend is Kraton G7723x from Shell Chemical
~3~ Resin is Cumar LX-509 from Neville Chemical
Polymer film coatings can be made by a number of techniques. Typically, the
starting
material is a solution of the block copolymer in an appropriate solvent. The
solvent can
either dissolve one of the blocks preferentially or be a good solvent for all
blocks of the
copolymer. The choice of solvent can impact the morphology of the films and
thus
potentially their acoustic properties. To date all studies have been conducted
using films
made from solutions of block copolymers dissolved in toluene, which is a good
solvent for all
of the components of the block copolymers tested. Techniques for forming thin
films of
block copolymers on the acoustic wave sensor include spin coating, spray
coating, and dip
coating. We have tested coatings formed by spin coating, spray coating, and
dip coating and
have observed comparable results. The sensors wee made by coating both sides
of ~l0 MHz
QCMs with solutions that ranged between 5% and 12% w/v and allowing the
solvent to
evaporate. A network analyzer was used to measure the resonant frequency of
the QCM as a
function of analyte concentration and temperature. The network analyzer also
measured the
dissipation of energy in the QCM, in units of resistance (Ohms), which is
related to the
acoustic loss in the device: Other types of impedance analyzers can also be
used for these
measurements. Field-deployable sensors typically incorporate the QCM in an
oscillator
circuit and employ a frequency counter to measure the resonant frequency.
These devices
cannot sustain oscillation if the resistance is too high (i.e., if the
acoustic Loss is too high).
CA 02369720 2002-O1-30
This occurs when R>1/(~oCo) where R is the resistance, ~o is the angular
resonant frequency
of the quartz crystal and Co is the static capacitance of quartz. For typical
QCMs; the
maximum resistance is therefore 1-2 kS2. We chose 100 S2 as a refer~ce
resistance level at
which to compare sensor performance. Resistances of this magnitude usually do
not prevent
resonator oscillation.
Figure 3 shows the performance at 50 °C of coatings of ABA block
copolymers and
mixtures of block copolymers and additives compared to the homopolymers
polyisobutylene
(PIB) and poly(diphenoxyphosphazene) {PDPP). This figure displays the measured
sensitivity of various coatings, defined as the change in oscillation
frequency of the sensor
per unit concentration of VOC, (parts per million toluene in this example), as
a function of
resistance. The sensitivity of a coating increases approximately linearly with
coating
thickness because the amount of VOC sorbed by the coating is proportional to
the amount of
coating on the sensor. Resistance is a measure of acoustic loss in the device,
which usually
increases with temperature. It also increases with thickness, but much more
rapidly than
sensitivity (roughly exponentially). The rnaxirnum sensitivity of a coating is
thus limited by
the maximum resistance (i.e., acoustic loss) that can be tolerated. Figure 3
displays sensor
resistance and sensitivity data measured for coatings of various thickness. It
is seen that the
block copolymers outperform the homopolymers in terms higher sensitivity at a
given level
of resistance (see Table 2 for a comparison of sensitivities at a reference
resistance of 100 S2,
interpolated or extrapolated from the data in Figure 3).
Table 2
Polymer Sensitivity (Hz/pprn toluene}
Homopolymers
PDPP 0.10
PIB 0.11
Block Copolymers and Blends
SEBS~2? 0.15
SBS 0.22
SEBS + PP0~3~ ~ 0.24
SEBS + coumarone-indene 0.48
resin~4~
~'~ Sensitivity to toluene at 50 °C at a thickness that gives a
resistance of 100 S2
~Z~ Shell Kraton 61652
~3~ Shell Kraton G7723x
21
CA 02369720 2002-O1-30
~4~ Shell ICraton 61652 + Neville Cumar LX-509
The SEBS polymers tested were Kraton 61652 and 61654x. Kraton G1654x has a
styrene content of 31 % and has a molecular weight approximately twice that of
Kraton
61652, which has a styrene contest of 30%. They dissolve readily in toluene,
producing
films with fast response times (2-3 minutes to reach 90% of full response at -
10 °C, the
lowest temperature tested) and linear calibration curves (correlation
coeffici~ts >0.99).
There is very little difference between the two in term of sensor resistance
and sensitivity.
The polystyrene-block-polybutadiene-block-polystyrene (SBS) tested had a
styrene
content of 28% and is a block copolymer with very good sensor characteristics.
A 2.5 Nxn
coating of SBS has a sensitivity comparable to a 4.9 ~n PIB film, but has a
faster response
time at -10 °C (1 minute vs. 15 minutes to reach 90% of full response).
The resistance of this
sensor was 100 S2 at 50 °C, compared to 320 S2 for the 4.9 p,m PIB
coating.
Block Copolymers Blended with Additives to Enhance Performance
The commercially available blend Kraton G7723x contains roughly 20% poly(2,6-
dimethyl p-phenylene oxide), a high Tg polymer that is miscible with
polystyrene, and 80%
24 ICraton 61652, an SEBS triblock copolymer. The poly(2,6-dim~hyl p-phenylene
oxide)
makes Kraton G7723x stiffer than Kraton G 1652. Because of its lower acoustic
loss, it was
possible to make thicker, and consequently more sensitive coatings with this
material than
with Kraton 61652.
High melting point resins can also associate with the polystyrene phase of
these block
copolymers and raise their softening point. An example is Cumar LX 509, a
coumarone-
indene resin manufactured by Neville Chemical Co. Tests were made with blends
of SEBS
and SBS block copolymers with this resin. The addition of Cumar LX-509 to the
SEBS
block copolymers enhanced their performance dramatically (Figure 3 and Table
2). The
maximum sensitivity at 100 SZ is three times greater than that obtained
without the
22
CA 02369720 2002-O1-30
coumarone-indene resin. This improvement is due to the lower acoustic loss of
this coating
material, which permits thicker coatings to be used. Importantly, rapid
response times at low
temperature were maintained.
Example II
The improvement in s~sor performance is further illustrated in Figure 4.
Figure 4
shows how the resistance, (i.e., acoustic loss) of sensors with representative
coatings is
affected by temperature changes. It is particularly noteworthy that the bled
of Kraton
61652 with Cumar LX-509 shows a decrease in acoustic loss at elevated
temperatures. This
relatively stable acoustic loss permits the use of much thicker coatings of
this material, which
sorb more VOC, and operation of sensors coated with this material over the
full temperature
range of-10 °C to 50 °C.
Example III
Figure 5 illustrates the enhanced sensitivity achievable with block copolymer
coatings
and the linearity of sensor response. For the particular coatings tested at 5b
°C, the sensitivity
of the block copolymer coating was 0.77 Hz/ppm toluene with a correlation
coefficient, r2, of
0.9997. In comparison, the PIB coating sensitivity was much lower, 0.21 I-
IzJppm with r2 =
0.9980. The thickness of the block copolymer coating ( 10 p,m) was chosen to
maintain the
resistance below 100 S2. In contrast, the lower sensitivity of the PIB coating
is in spite of a
thickness (4.9 p.m) that was too large to maintain this level of resistance.
The resistance of the
PIB-coated s~sor was 320 SZ.
Example IV
Figure 6 demonstrates that the s~sor response time can be improved through the
use
of block copolymer coatings. Shown is a plot that compares the speed of sensor
response of a
representative block copolymer-based coating vs. a homopolymer coating at -10
°C. The
responses of each sensor have been scaled to the maximum response to 1000 ppm
toluene in
15 minutes to facilitate comparison. It is seen that at -10 °C, the
block copolymer/resin
coating responds more quickly than a PIB coating of 1/2 the thickness.
23